- 1Office of Research Administration, Chiang Mai University, Chiang Mai, Thailand
- 2Center of Excellence in Microbial Diversity and Sustainable Utilization, Chiang Mai University, Chiang Mai, Thailand
- 3Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
Fungal polysaccharides have demonstrated significant biological potential, including immune stimulation, antioxidant activity, anticancer properties, and anti-inflammatory effects. These properties hold considerable promise for innovative applications across various fields. This study provides a brief review of current findings, based on literature published over the past 15 years on fungal polysaccharides. This includes the production process and various extraction methods, highlighting their distinct advantages and limitations. Additionally, we summarize techniques for purification and characterization, elucidating their biological properties and practical applications in medicine, pharmacology, the food industry, agriculture, and environment. Global patent trends related to fungal polysaccharides are also reviewed. Finally, we discuss challenges and future perspectives related fungal polysaccharides. This article offers valuable insights and enhances the understanding of fungal polysaccharides for researchers, paving the way for further research and applications.
1 Introduction
Fungi have played an important role in human history for millions of years. Terrestrial fossil evidence indicates that fungi first appeared around 400 million years ago (Brundrett, 2002). An updated estimation of the global fungi species has been revised and raised to 2.5 million worldwide according to updated molecular data, but only about 155,000 species have been officially identified and described, highlighting the ongoing research in this field (Antonelli et al., 2023). Belonging to the kingdom of Fungi, they are vital in decomposing organic matter within nutrient cycles and are associated with various organisms that help maintain ecosystems (Niego et al., 2023). Certain edible fungi serve as a crucial food source, providing high nutritional value with proteins, vitamins, and essential minerals, making them suitable for consumption to enhance health. Additionally, some of them exhibit medicinal properties, including antimicrobial, anticancer, and immunomodulatory effects (Valverde et al., 2015). In the industrial sector, extracts from edible fungi are utilized in food and beverage production, such as functional foods, brewing beer, fermenting wine, and making bread (Lorenzo et al., 2018). The cultivation of edible fungi holds significant economic value, providing farmers with income opportunities due to the high market demand (Zhang et al., 2014). Edible fungi can also serve as meat substitutes and are a rich protein source in various dishes, making them an attractive option for those reducing meat consumption or following a vegetarian diet (Ayimbila and Keawsompong, 2023). Interestingly, many edible fungi produce bioactive compounds, which are chemicals that exert biological effects on the human body or other living organisms. Examples of bioactive compounds derived from fungi include polysaccharides, triterpenoids, ergosterol, polyphenols, flavonoids, vitamins, minerals, and antibiotic compounds (Valverde et al., 2015; Antonelli et al., 2023).
One of the most popular and widely recognized fungal bioactive compounds is polysaccharides. Fungal polysaccharides are complex carbohydrates composed of multiple monosaccharide units linked by glycosidic bonds, and they possess biological properties (Hamidi et al., 2022; Stoica et al., 2023). From 2000 to 2024, a search using the keyword “fungal polysaccharide” retrieved 8,138 titles of documents published over the last 25 years in the Scopus database (https://www.scopus.com, accessed 20 March 2025). It was found that the trend in research on fungal polysaccharides is expected to increase in the future (Figure 1A).
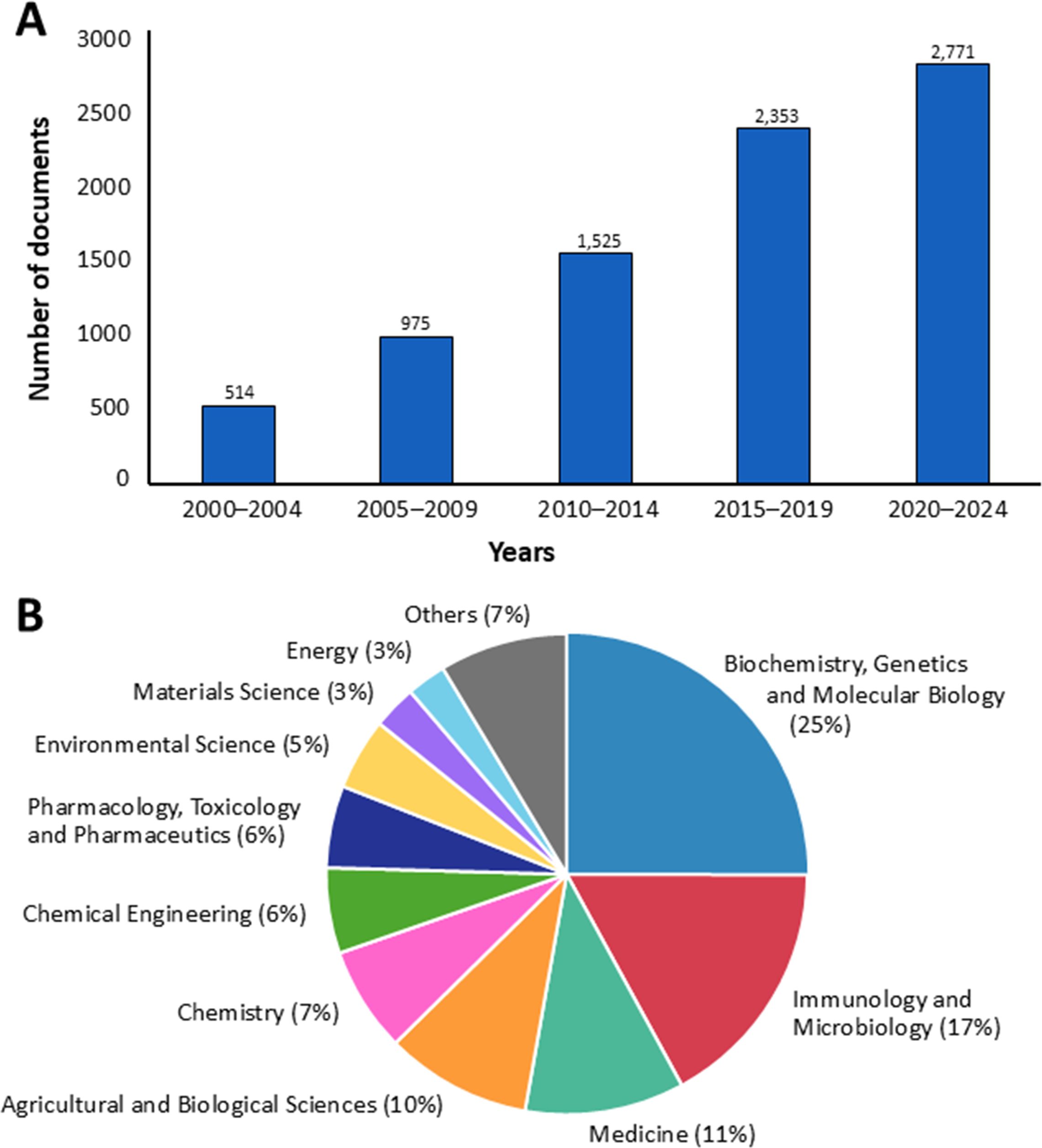
Figure 1. The number of documents (A) and the distribution of published research across various subject areas (B) on fungal polysaccharides in the Scopus database from 2000 to 2024, based on data from Scopus.
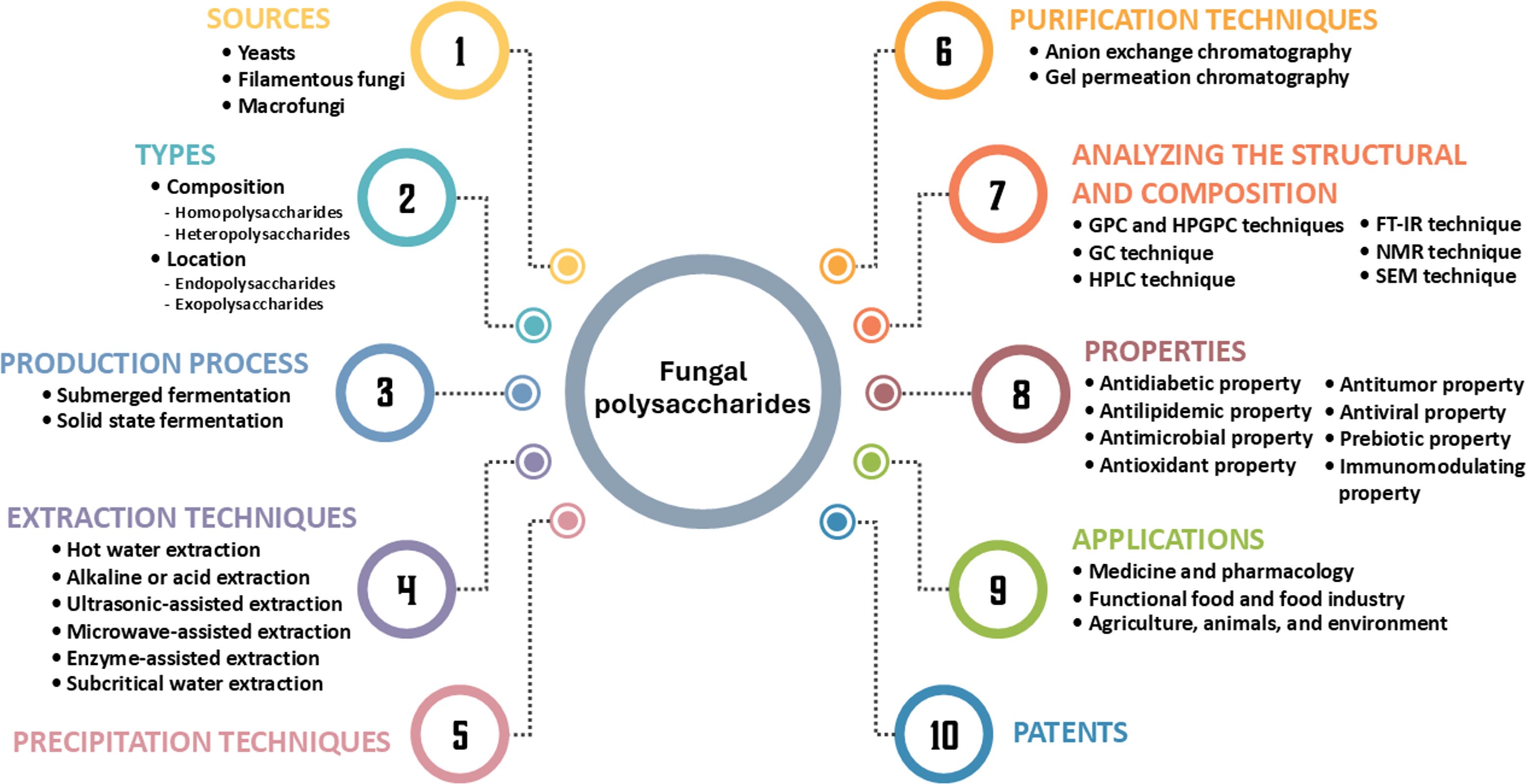
Additionally, the majority of applications for fungal polysaccharides have been reported in the fields of biochemistry, genetics, and molecular biology (25%), followed by immunology and microbiology (17%), medicine (11%), agricultural and biological sciences (10%), and chemistry (7%) (Figure 1B). In this study, we summarize current findings on fungal polysaccharides, including type, production processes, extraction methods, characterization, biological properties, and their applications in various fields from 2010 to 2024 (a 15-year period). Moreover, we summarize trends in patents related to fungal polysaccharides. The overview of this review article is presented in Figure 2.
2 Source of fungal polysaccharides
Fungal polysaccharides can be produced by yeasts (single cells), filamentous fungi (mycelia), and macrofungi (mycelia and fruiting bodies). Notably, basidiomycetous fungi have attracted significant research attention, particularly for their polysaccharide production and diverse industrial applications. Additionally, the diversity of fungal species and the types of polysaccharides they produce highlight the significant potential of fungi as a source of bioactive compounds for various applications. Examples of fungal species reported to produce polysaccharides are compiled and presented in Table 1.
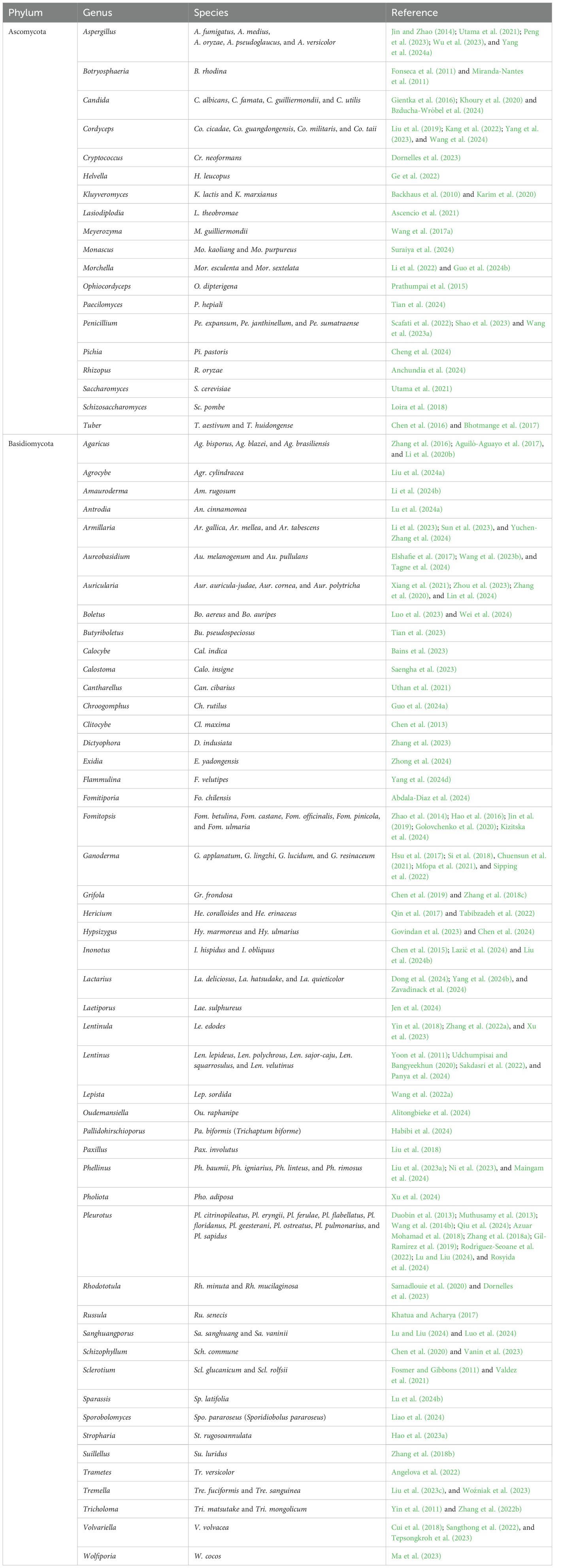
Table 1. Some fungal phylum, genus and species used for polysaccharide production over a 15-year period (2010 to 2024).
3 Types of fungal polysaccharides
Fungal polysaccharides are categorized primarily into two types based on their composition: homopolysaccharides, composed of a single type of monosaccharide, and heteropolysaccharides, which include various monosaccharides (Murphy et al., 2023a). Homopolysaccharides are polysaccharides composed of only one type of monosaccharide unit repeated in long chains, e.g., glucans (polymers of glucose), chitin (a polymer of N-acetylglucosamine), and mannans (polymers of mannose) (De Felice et al., 2020). Conversely, heteropolysaccharides are polysaccharides composed of two or more different types of monosaccharide units, e.g., galactomannans (composed of mannose and galactose), pectins (composed of galacturonic acid and rhamnose), and glycosaminoglycans (e.g., hyaluronic acid) (Kadooka et al., 2023). Additionally, fungal polysaccharides are broadly divided into two forms based on their cellular location: endopolysaccharides (EnPs) or intracellular polysaccharides, and exopolysaccharides (ExPs) or extracellular polysaccharides (Zhang et al., 2016). These types differ considerably in terms of structure, biological activities, and extraction methods, offering distinct benefits and applications in biotechnological fields.
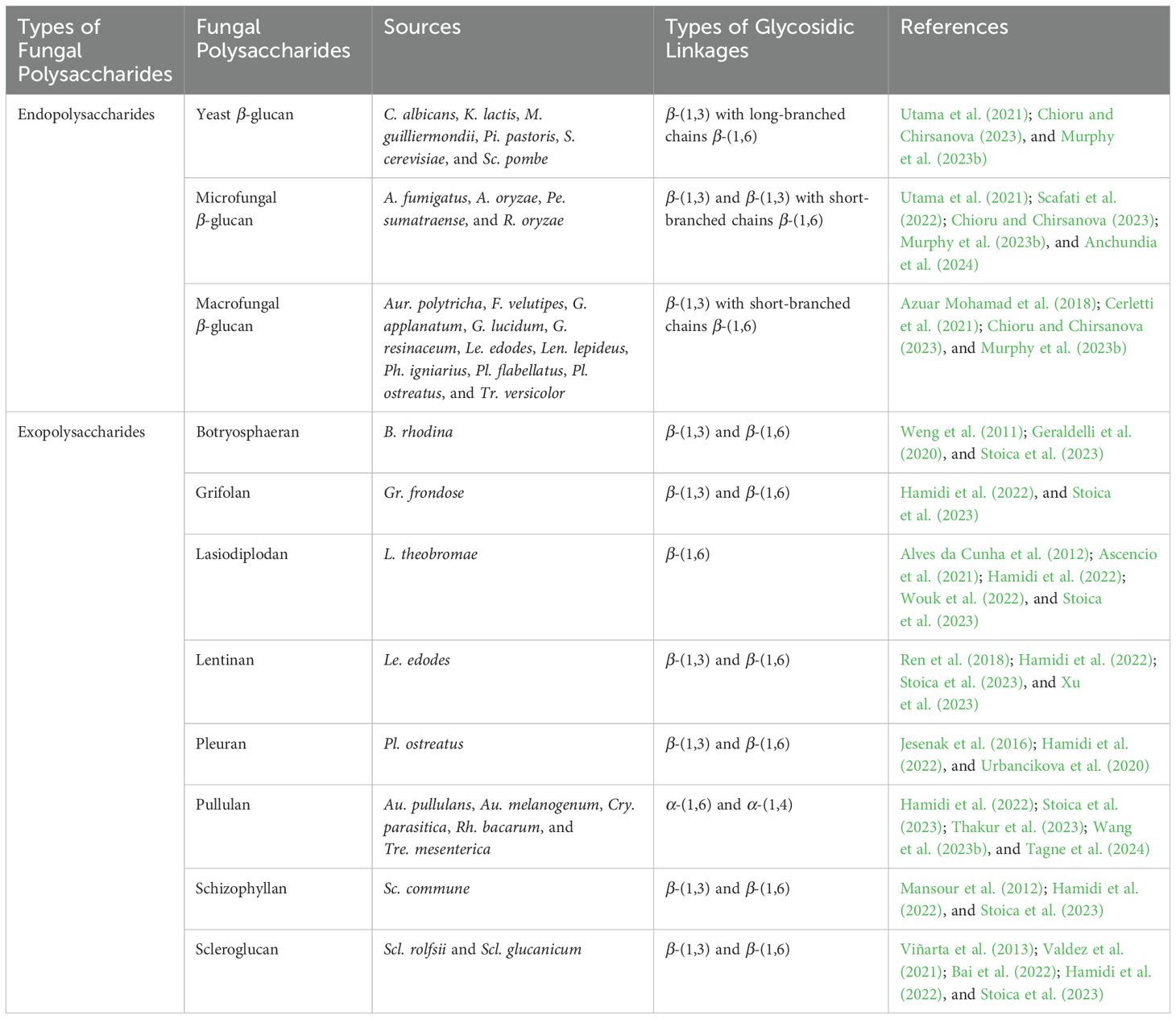
Both fungal EnPs and ExPs are crucial for fungal growth and adaptation to environmental conditions. Fungal EnPs are located within cells and often have complex, branched structures. They are crucial components of cell walls or are stored in the cytoplasm, providing structural support to the cell. They are typically composed of chitin and monosaccharides such as glucose, mannose, and galactose (Wang et al., 2017b; Fernando et al., 2021). β-glucan is well-known fungal EnPs, particularly in fungi belonging to the basidiomycetes and ascomycetes groups. In contrast, fungal ExPs are secreted by cells into their surrounding environment, where they generally form linear or mildly branched structures. These structures are typically simpler than those of EnPs. Fungal ExPs contribute to the extracellular matrix, forming gels that facilitate adhesion and biofilm formation, and providing structural support and protection to the fungi under adverse conditions (Mohamed et al., 2024). Primarily composed of glucose, fructose, and other simple monosaccharides, these polysaccharides typically lack complex functional groups and are valued for their gelling and viscosity-enhancing properties (Wang et al., 2020a). Fungal ExPs are often characterized by favorable physical properties such as high-water solubility and significant viscosity, though some types may be insoluble or display unique physical traits based on their structural makeup (Guo et al., 2017). Furthermore, extraction methods for fungal ExPs are usually more straightforward than those for fungal EnPs, as they do not require cell disruption (Jeong et al., 2013; Miao et al., 2020). The most well-known fungal polysaccharides, including both EnPs and ExPs (e.g., botryosphaeran, grifolan, lasiodiplodan, lentinan, pleuran, pullulan, schizophyllan, and scleroglucan), are listed in Table 2, and their chemical structures are presented in Figure 3.
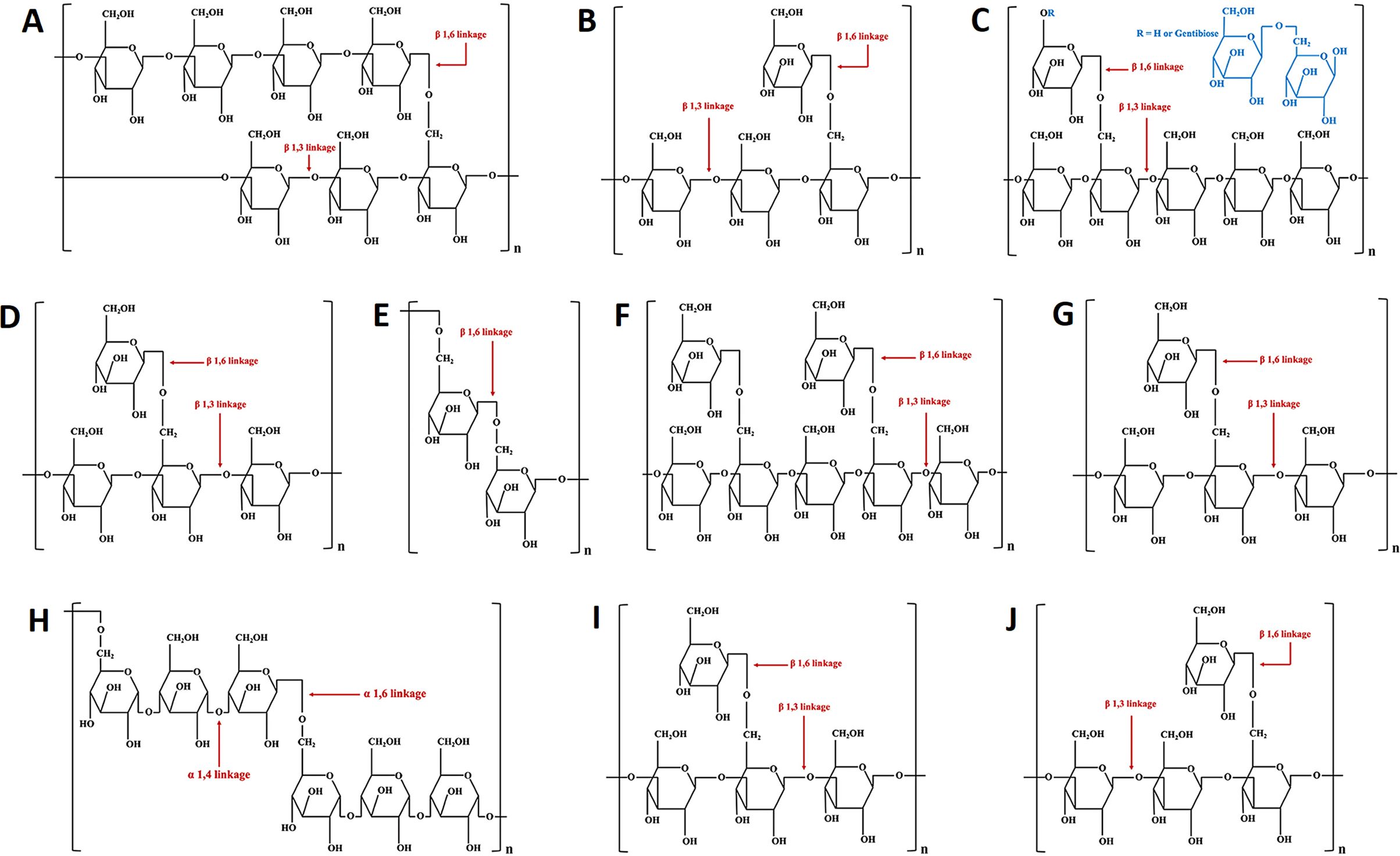
Figure 3. The chemical structures of yeast β-glucan (A), fungal β-glucan (B), botryosphaeran (C), grifolan (D), lasiodiplodan (E), lentinan (F), pleuran (G), pullulan (H), schizophyllan (I), and scleroglucan (J).
4 Production process of fungal polysaccharides
The industrial-scale production of fungal polysaccharides from fungi is a process that requires precise control and high efficiency to ensure the desired quantity and quality of the product. The production process begins with the selection of fungal strains capable of producing large amounts of polysaccharides (Sánchez et al., 2015; Junior Letti et al., 2018). Additionally, inoculum preparation under optimal conditions is crucial to maximize fungal biomass before transferring to the fermentation system. The choice of fermentation method is critical, with the primary approaches being submerged fermentation and solid state fermentation (Bakratsas et al., 2021). Solid state fermentation occurs on solid substrates or non-soluble support materials and is particularly suitable for certain fungal fruit bodies (Junior Letti et al., 2018), utilizing agricultural residues as growing substrates (Pérez-Chávez et al., 2019). In contrast, submerged fermentation, a common industrial method, involves liquid-based fermentation requiring the careful control of parameters such as pH, temperature, agitation, and aeration (Sánchez et al., 2015). The enhancement of polysaccharide production from both fermentation processes requires optimal conditions for fungal growth specific to each fungal species and strain (Montoya et al., 2013).
4.1 Solid state fermentation
Solid state fermentation (SSF) involves the cultivation of fungi on solid substrates or low-moisture materials, typically derived from agricultural or industrial residues such as wheat straw, rice straw, coffee pulp, wood logs, sawdust, bran, husks, and sugarcane bagasse (Junior Letti et al., 2018; Yafetto, 2022), due to the ability of fungi to break down and utilize lignocellulosic materials as a source of nutrients to support their growth and development. Additionally, the selection and utilization of agricultural or industrial residues in SSF is contingent on their availability in each country. SSF is particularly suitable for cultivating fungi to produce their fruiting bodies, commonly known as mushrooms (Junior Letti et al., 2018). The cultivation of mushrooms using SSF has been studied worldwide because it allows the use of various agricultural or industrial residues as substrates, promotes the recycling of these residues into materials for fungal growth, and facilitates their conversion into fruiting bodies (Grimm and Wösten, 2018; Junior Letti et al., 2018). For example, Sánchez and Montoya (2020) found that the cultivation of Pl. ostreatus, Le. edodes, and Tr. versicolor under SSF conditions using oak sawdust revealed that the highest polysaccharide production was recorded for the growing substrate of Tr. versicolor (96.09 mg/g of solid substrate), followed by Pl. ostreatus (90.78 mg/g) and Le. edodes (87.45 mg/g). The research conducted by Xu et al. (2019) found that the optimal conditions for polysaccharide production from Co. militaris using rice under SSF resulted in a maximum yield of 68.3 mg/g of dry substrate. Shi et al. (2012) used soybean curd residue as a substrate in SSF to produce polysaccharides from the fruiting bodies of F. velutipes, yielding 106.74 mg/g of dried fruiting bodies. Additionally, Le. edodes fruiting bodies cultivated under SSF using beechwood sawdust yielded a maximum polysaccharide production of 67.33 mg/g of dry weight (Reza et al., 2024). SSF of Gr. frondosa using corn bran and oak sawdust resulted in a polysaccharide yield of 60.5 mg/g of dry weight (Montoya Barreto et al., 2011). Thus, SSF can be used for the production of fungal polysaccharides, demonstrating its economic viability as well as its potential for further pilot research and large-scale industrial applications. Remarkably, the carbon/nitrogen (C/N) ratio and the composition of cellulose, hemicellulose, and lignin in agricultural or industrial substrates vary across different types, making them crucial factors in SSF. Large-scale SSF, particularly in industrial settings, remains challenging due to the need to carefully control factors such as pH, heat and mass transfer, water activity, fungal strain selection, substrate heterogeneity, C/N ratio, and optimal moisture levels for fungal growth.
4.2 Submerged fermentation
Submerged fermentation involves the cultivation of fungal mycelium in a liquid medium, where nutrients are dissolved, and agitation is used to facilitate aeration within the fermenter or bioreactor. The primary advantages of submerged fermentation include efficient oxygen transfer and homogeneous distribution of the liquid medium. However, this technique requires meticulous control of various factors such as nutrient (carbon and nitrogen sources), temperature, aeration, agitation, pH, medium composition, and the type and amount of inoculum. Consequently, submerged fermentation is a reproducible technique for the continuous cultivation of fungal mycelium, enabling controlled production of metabolites (Elisashvili, 2012). Nonetheless, prolonged cultivation in liquid media can lead to increased medium viscosity due to the growth and accumulation of fungal mycelium, potentially impacting oxygen distribution, carbon dioxide removal, and product dilution (Bentil et al., 2018). Recently, edible fungi, particularly those from the basidiomycetes and ascomycetes genera, including Agaricus, Cordyceps, Ganoderma, Lentinus, and Pleurotus, have been successfully cultivated using bioreactors (Bakratsas et al., 2021). For example, Wang et al. (2019a) achieved the highest extracellular polysaccharide yield (5.713 g/L) of Co. militaris in a 5-L bioreactor using glucose and yeast extract as carbon and nitrogen sources, with conditions of 25°C, 150 rpm shaking speed, and 1.5 vvm aeration. The most suitable submerged fermentation conditions for Le. crinitus to achieve the highest yield of ExPs at 0.65 g/L in a 5-L bioreactor, using Kirk’s liquid medium, were 30°C, pH 4.5, 300 rpm stirring, and a 1.5 vvm aeration rate for four days (López-Legarda et al., 2020). The fed-batch submerged fermentation process for EnP production from G. lucidum was successfully scaled up in stages, from 7.5 L to 20 L, and finally to a 200-L stirred-tank reactor, where maintaining a low impeller tip speed of 1.234 m/s resulted in a maximum EnP production of 4.74 g/L (Tang et al., 2011). Moreover, Wu et al. (2013) studied the effect of different light wavelengths on ExPs production from Pl. eryngii in submerged cultivation and found that ExPs production was highest under blue light (455 mg/L) condition, compared to green (425 mg/L), red (217 mg/L), yellow (314 mg/L), white (50 mg/L), and dark (59 mg/L) conditions. In a recent study by Bakratsas et al. (2024), the cultivation of Pl. ostreatus was successfully scaled up in a 3.5 L bioreactor under optimal conditions, resulting in a maximum biomass of 12.6 g/L, the highest ExPs production of 3.7 g/L.
Overall, previous studies highlight several key factors that significantly enhance the production efficiency of polysaccharides from selected fungi. Among these factors, the selection of suitable carbon sources, such as glucose and yeast extract, plays a crucial role in stimulating fungal growth and the production of desired compounds. Optimal concentrations of nutrients should be maintained to support maximum biomass development and polysaccharide yield. Additionally, controlling fermentation parameters such as temperature, pH, agitation, and aeration is crucial for optimizing polysaccharide production. Furthermore, the use of specific light wavelengths during cultivation has been shown to enhance the production of fungal ExPs.
5 Extraction techniques for fungal polysaccharides
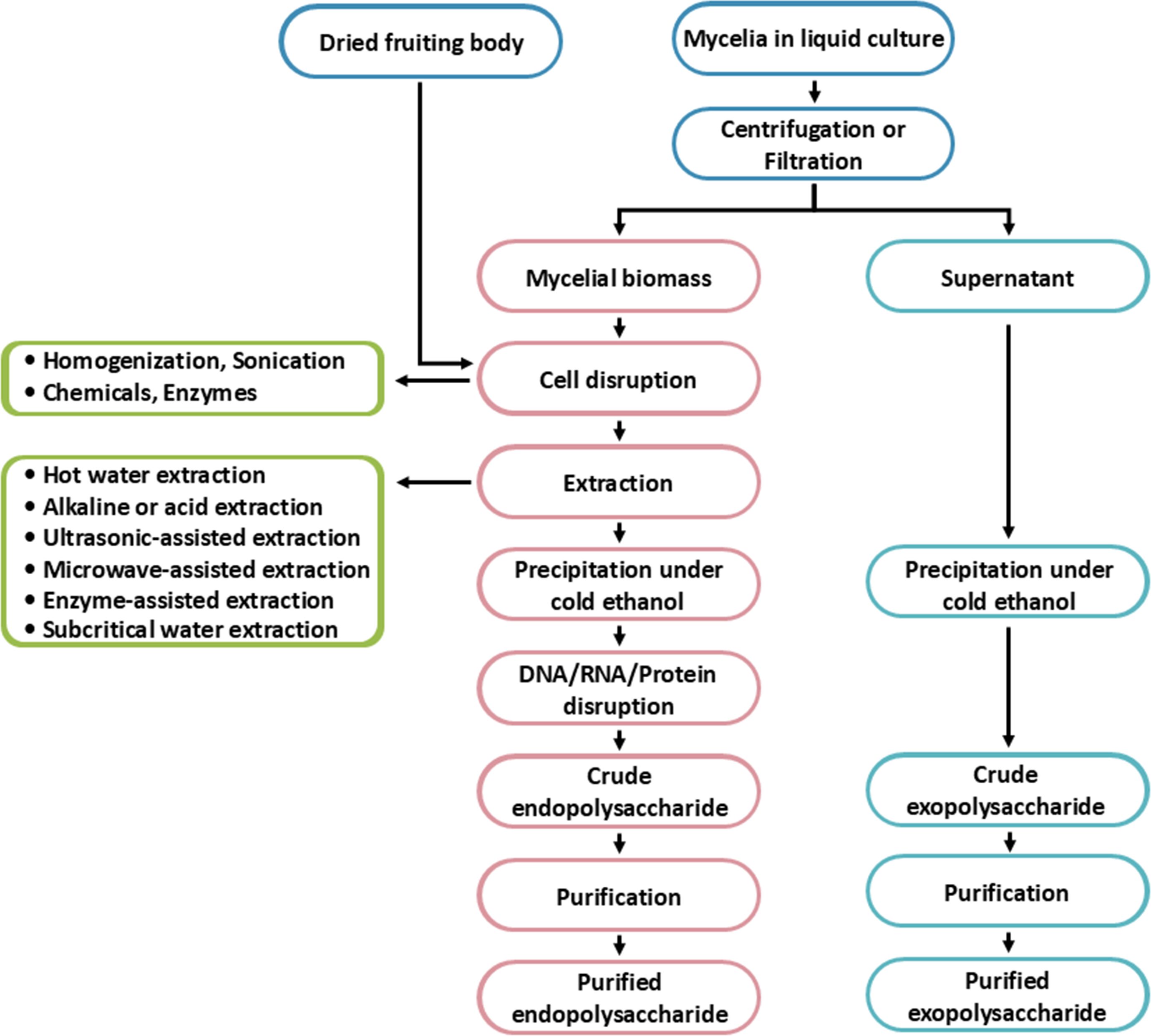
The processes for obtaining fungal EnPs and ExPs involve several steps, as shown in Figure 4. The extraction of polysaccharides from fungi primarily differs in the locations of the polysaccharides and the methods employed for extraction. For EnP extraction, the process begins with harvesting the fungal mycelia or fruiting bodies, followed by cell disruption, which can be achieved either chemically or mechanically to break the cell walls (Wang et al., 2015b; Liu et al., 2022b). On the other hand, ExP is secreted outside the fungal cells and accumulates in the liquid culture or surrounding fluid. The extraction of ExPs typically involves filtering or separating the liquid culture from the fungal biomass. The ExP extraction is simpler because it does not require cell disruption. As a result, ExPs tend to be purer since they are directly extracted from the fungal supernatant (Jeong et al., 2013). However, EnP extraction could be contaminated by other intracellular substances, requiring further purifying processes.
5.1 Extraction of fungal exopolysaccharides
Generally, submerged fermentation is used for fungal ExP production. The extraction of ExP typically involves straightforward methods. Fungal ExP is separated from the liquid culture medium by first removing the fungal mycelia and cells through filtration or centrifugation and then collecting the supernatant (Stoica et al., 2023). The ExP present in the supernatant is then precipitated with organic solvents (e.g., acetone, ethanol, propanol, or isopropyl alcohol) under cold conditions (Maziero et al., 1999; Elisashvili et al., 2009). After precipitation, centrifugation or filtration is used to collect the crude fungal ExP.
5.2 Extraction of fungal endopolysaccharides
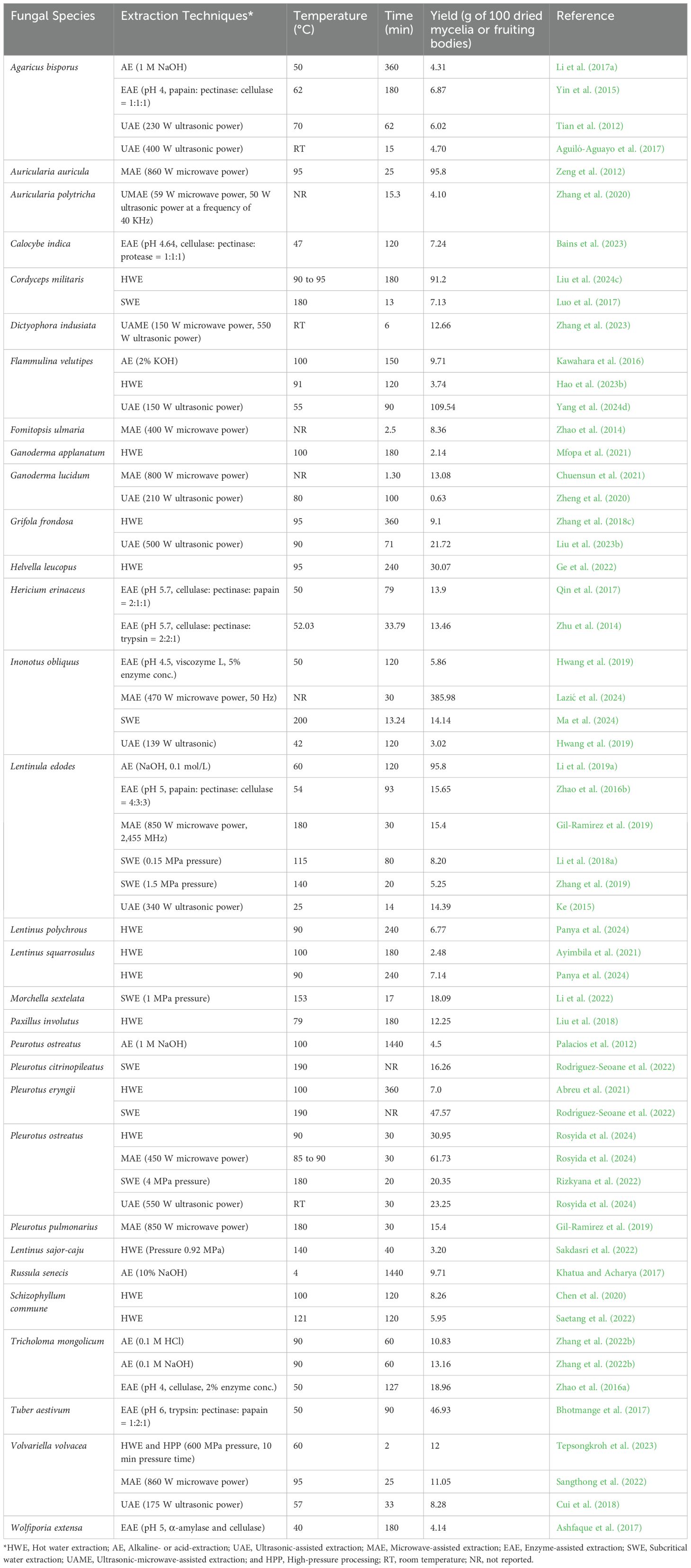
The EnP extraction from fungi is a more complex procedure than ExP extraction and requires specific methods to isolate EnP from the fungal cells (Jeong et al., 2013). After the fungus cells or fruiting bodies have been collected, the cell walls must be broken down in order to release the EnP. This can be achieved through chemical techniques, such as using lysozyme enzymes or solvents that degrade the fungal cell walls, or mechanical techniques like grinding or ultrasonic waves to break down the cell walls (Ferraboschi et al., 2021; Larrañaga-Ordaz et al., 2022). After the cell walls are disrupted, the extraction process can be carried out using a variety of techniques, including hot water extraction, alkaline- or acid-extraction, ultrasonic-assisted extraction, microwave-assisted extraction, enzyme-assisted extraction, or subcritical liquid extraction. Following extraction, EnP is collected, purified, and freeze-dried for subsequent application (Leong et al., 2021; Zhao et al., 2023b). Examples of fungi used for EnP extraction through various techniques are shown in Table 3.
5.2.1 Hot water extraction
The hot water extraction method is a popular technique for EnP extraction from fungi due to its simplicity, safety, and the availability of common materials (Dai et al., 2023). The fungal cells or fruiting bodies are collected, dried, and ground into a fine powder to enhance extraction efficiency. By boiling the powdered fungal samples in hot water at temperatures between 50°C and 121°C for 30 min to 10 h, the cell walls are broken down, releasing the EnPs (Saetang et al., 2022). The resulting solution is then filtered to remove impurities, and alcohol is added to concentrate and precipitate the EnP. The EnP is subsequently collected and dried (Leong et al., 2021). This method is suitable for various fungi and can be carried out in a standard laboratory, provided that temperature and extraction time are carefully controlled to achieve optimal results (Parniakov et al., 2014; Leong et al., 2021). The advantages of this method include the absence of toxic chemicals and low cost; however, some loss of EnP may occur due to heat, and the extraction may be incomplete if the cell walls are not fully broken down.
5.2.2 Alkaline or acid extraction
The alkaline or acid extraction technique involves using alkaline or acidic solutions to break down the cell walls of fungi and release the EnP. In alkaline extraction, an alkaline solution, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH), is used to break down the fungal cells. In contrast, an acidic solution, such as hydrochloric acid (HCl) or ammonium oxalate [(NH4)2C2O4], is used in acid extraction. After the extraction process, the extracted samples must be pH-neutralized before filtration and precipitation of the polysaccharide (Yi et al., 2020; Leong et al., 2021). Alkaline extraction, using NaOH as the extracting agent, is more commonly employed than acid extraction for isolating fungal polysaccharides. NaOH solutions are typically used in concentrations ranging from 0.1 M to 1 M, with extraction ratios varying from 1:5 to 1:20 (w/v) (Palacios et al., 2012; Yang et al., 2019). Nevertheless, acid extraction methods remain an alternative, with previous reports highlighting the use of 0.1 M HCl (1:10 w/v) and 1% (w/v) (NH4)2C2O4 for extraction (Sermwittayawong et al., 2018; Zhang et al., 2022a). This technique is highly effective, especially for extracting EnPs from fungi with robust cell walls. However, careful control of extraction conditions is necessary to prevent EnP degradation and avoid side effects from the use of highly concentrated alkaline or acidic solutions (Wang et al., 2015b, c).
5.2.3 Ultrasonic-assisted extraction
Ultrasonic-assisted extraction is a method that uses ultrasonic waves to enhance the extraction of EnP from fungal cells. This technique involves using high-frequency sound waves to create cavitation, a phenomenon where small bubbles in the liquid rapidly collapse, producing energy that can break down the cell walls of mycelia or mushrooms and facilitate the release of EnP into the extraction solvent. The ultrasonic technique can be divided into two types: low-intensity and high-intensity ultrasound. The low-intensity ultrasonic technique uses frequency waves ranging from 5 to 10 MHz and energy levels of less than 1 W/cm². On the other hand, the high-intensity ultrasonic technique uses frequencies ranging from 20 to 100 kHz and higher energy levels from 10 to 1,000 W/cm². In comparison to the low-intensity technique, the high-intensity ultrasonic method has a higher destructive capability (Charoux et al., 2017; Li et al., 2024a). In general, EnP extraction using this technique typically involves ultrasonic treatment for 14 to 180 min at temperatures ranging from approximately 25°C to 95°C (Ke, 2015). The process begins by preparing the fungal mycelia or mushroom samples, grinding or cutting them into small pieces, immersing them in an appropriate solvent, and applying ultrasonic waves to facilitate the extraction. Subsequently, the extracted sample is filtered to separate the solid residues, and the solution containing EnP is then precipitated and the EnP is harvested. The advantages of this technique include increased extraction efficiency and reduced solvent usage (Leong et al., 2021; Shen et al., 2023).
5.2.4 Microwave-assisted extraction
Microwave-assisted extraction is a technique that uses microwave energy to accelerate the extraction process. Microwave energy causes water molecules or solvents within the sample to vibrate, generating heat that helps break down the cell structure and release the desired compounds more efficiently (Hu et al., 2021). Key factors influencing EnP yield using this method include microwave power, typically ranging from 400 to 1200 W, extraction temperature, maintained between 85°C and 180°C, and extraction time, which ranges from 1 to 30 min (Zhao et al., 2014; Gil-Ramírez et al., 2019; Chuensun et al., 2021). This technique is a highly efficient and fast technique that reduces solvent and energy usage (Chen et al., 2013). However, it has limitations, including high initial costs, restrictions on solvent selection, risks of unwanted reactions, and challenges in controlling various parameters (Xu et al., 2018).
5.2.5 Enzyme-assisted extraction
Enzyme-assisted extraction is a technique that uses enzymes to extract EnP from fungi. This method leverages the ability of enzymes to break down the cell walls of fungi (Leong et al., 2021; Bains et al., 2023). Several cellulolytic enzymes and proteolytic enzymes have been used, including cellulase, papain, pectinase, protease, and trypsin (Yin et al., 2015; Bhotmange et al., 2017; Bains et al., 2023). The process begins with sample preparation, where the fungal materials are ground or milled into a fine powder. The powder is then mixed with an enzyme solution under optimized conditions to maximize enzyme activity, typically at a pH of 4 to 6, a temperature of 40°C to 60°C, and an incubation time of 30 to 180 min (Zhu et al., 2014; Yin et al., 2015; Ashfaque et al., 2017; Bhotmange et al., 2017). The enzyme is allowed to act for a period during which the cell walls are degraded, and the polysaccharides are released into the solution. After extraction, the solution is filtered, and the polysaccharides are precipitated and harvested (Qin et al., 2017; Bains et al., 2023). This technique offers advantages such as increased extraction efficiency and reduced use of harsh chemicals, making it an environmentally friendly method (Chen et al., 2013; Zhao et al., 2016b). However, it also has disadvantages, including high costs, enzyme stability that depends on environmental conditions, challenges in selecting appropriate enzymes, potentially longer extraction times, and the complexity of process optimization (Yin et al., 2011; You et al., 2013).
5.2.6 Subcritical water extraction
Subcritical water extraction is a technique that uses subcritical fluids to extract polysaccharides from fungi. A subcritical fluid is a liquid heated above its boiling point (100 to 374°C) but remains in liquid form due to the increased pressure (1 to 22.1 MPa) (Gbashi et al., 2017). This technique typically uses water, ethanol-water mixtures, or other solvents (ethanol, methanol, or acetone) under subcritical conditions for extraction (Herrero et al., 2006). The process begins by preparing the fungal material, which is then mixed with the subcritical fluid in a closed system where both temperature and pressure are carefully controlled (Morales et al., 2019; Leong et al., 2021). In subcritical conditions, the physical properties of the fluid, such as its solubility and diffusivity, change, which enhances the efficiency of polysaccharide extraction from fungal cells. After extraction, the solution is cooled and separated to isolate the desired polysaccharides, while the remaining fluid can be reused in subsequent processes (Zhang et al., 2022a). IPS extraction using this technique requires optimization of conditions, typically involving pressures ranging from 0.15 to 5 MPa, temperatures between 115°C and 210°C, and extraction durations of approximately 13 to 80 min (Luo et al., 2017; Li et al., 2018a; Zhang et al., 2019). This technique offers advantages, including shorter extraction times, reduced solvent usage, and better control over the quality of the extracted polysaccharides (Huber et al., 2021). Despite its benefits, this technique has some limitations. One challenge is the need for equipment that can withstand high pressure and temperature, resulting in higher installation costs. Furthermore, operating at high pressure requires specialized expertise to manage the process effectively (Yabalak et al., 2024).
Several previous studies have compared different extraction techniques to obtain the highest yield of fungal EnPs. For example, Sangthong et al. (2022) compared ultrasonic-assisted extraction (sonication at 95°C for 5 h), and microwave-assisted extraction (860 W for 25 min) with hot water extraction (95°C for 5 h) and found that hot water extraction yielded higher EnP levels from V. volvacea (15.58% of dried fruiting bodies) compared to microwave-assisted extraction (11.05%) and ultrasonic-assisted extraction (9.06%). Similarly, Chen et al. (2020) compared ultrasonic-assisted extraction (450 W for 20 min) and microwave-assisted extraction (550 W for 5 min) with hot water extraction (100°C for 2 h) and found that hot water extraction yielded the highest polysaccharide content from Sch. commune (8.26% of dried fruiting bodies), higher than both ultrasonic-assisted extraction (5.07%) and microwave-assisted extraction (4.98%). Additionally, Zhang et al. (2022b) conducted a comparative study on five extraction methods for isolating EnP from fruiting bodies of Tri. mongolicum, including hot water extraction (85°C for 3 h), ultrasonic-assisted extraction (300 W power at 60°C for 35 min), enzyme-assisted extraction (using 5% cellulase and 2% pectinase at pH 5 and 50°C for 100 min), alkaline extraction (0.1 M NaOH at 90°C for 1 h), and acid extraction (0.1 M HCl at 90°C for 1 h). Among these five methods, alkaline extraction yielded the highest polysaccharide content at 13.16% of dried fruiting body, followed by acid extraction (10.83%), hot water extraction (6.64%), enzyme-assisted extraction (5.87%), and ultrasonic-assisted extraction (4.41%). Therefore, the selection of an extraction method for polysaccharides from fungi depends on the characteristics of the fungal samples, the equipment, and the limitations of each extraction technique. Each extraction method has its own distinct advantages and limitations, as summarized in Table 4. Hot water extraction is a convenient and safe method, although it may result in partial loss of polysaccharides due to the heat and may not efficiently extract them if the fungal cell walls are not adequately disrupted. For fungi with hard cell walls, alkaline or acid extraction works quite well. However, extreme acidity or alkalinity must be avoided to prevent polysaccharide degradation. Ultrasonic-assisted and microwave-assisted extractions enhance efficiency by utilizing energy from sound waves or microwaves; however, these methods require careful control of energy and extraction time, as they are costly and may limit solvent selection. In addition, enzyme-assisted extraction is effective for breaking down fungal cell walls, but it requires costly enzymes and may involve longer process optimization times. Lastly, subcritical water extraction, which operates at temperatures below the boiling point of water, is efficient and fast; however, it is costly because it requires specialized equipment capable of withstanding high temperatures and pressures.

Table 4. Comparison of the principles, key factors, advantages, and limitations of fungal polysaccharide extraction techniques.
Interestingly, the combination of extraction techniques can enhance polysaccharide yields, resulting in higher yields compared to using a single technique. For instance, Zhang et al. (2023) demonstrated that the combination of microwave-assisted and ultrasonic-assisted extraction was the most effective method for extracting polysaccharides from D. indusiata, yielding 12.66%, compared to other methods, including ultrasonic extraction (11%), microwave extraction (10%), and hot water extraction (8.5%). A combination of pressure and hot water extraction was used to extract β-glucan from Len. sajor-caju under conditions of 140°C and 0.92 MPa for 40 min, resulting in a high yield of 3.20 g per 100 g of dry fruiting body (Sakdasri et al., 2022). Similarly, Tepsongkroh et al. (2023) applied high-pressure processing at 600 MPa for 10 min, combined with hot water extraction at 60°C for 2 h to extract polysaccharides from V. volvacea, resulting in a 12% increase in crude polysaccharide yield and a 20% increase in β-glucan yield. The combined extraction of polysaccharides from G. lucidum under optimal conditions (ultrasonic power of 240 W, enzyme concentration of 0.5 mg/mL, pH 7.9, solvent-to-material ratio of 50:1 mL/g, temperature of 55°C, and extraction time of 144 min) resulted in a yield of 3.72% of dried fruiting bodies (Hoa, 2018). The combination of enzyme-assisted extraction (1:1:1 ratio of papain, pectinase, and cellulase, a solvent-to-material ratio of 1:30, pH 5, a temperature of 48°C), and microwave-assisted extraction (440 W for 10 min) for the extraction of EnPs from Le. edodes resulted in a high yield of 9.79% of dried fruiting bodies (Yin et al., 2018). Recently, Yang et al. (2024d) found that the enzyme-assisted extraction technique, combined with ultrasonic-assisted extraction, effectively extracted polysaccharides from F. velutipes under optimal conditions (ultrasonic power of 150 W, snailase enzyme concentration of 1%, solvent-to-material ratio of 10:1 mL/g, temperature of 55°C, and extraction time of 90 min), resulting in a polysaccharide yield of 109.54 mg/g of dried fruiting bodies.
Thus, the selection of an extraction method of fungal polysaccharide must consider multiple factors, including the type of sample, extraction efficiency, cost, time, and environmental impact, in order to achieve the highest yield of fungal polysaccharides. Additionally, treatment temperature, solvent-to-sample ratio, and energy used in the extraction process all significantly affect polysaccharide yields. It is essential to optimize these variables to efficiently extract fungal polysaccharides and achieve high yields, along with the combination of extraction techniques.
6 Precipitation techniques for fungal polysaccharides
The precipitation of fungal polysaccharides is a process used to isolate these polysaccharides from a solution by adding a chemical agent, such as ethanol, propanol, or isopropyl alcohol, in an appropriate ratio. Ethanol is typically used to directly precipitate crude polysaccharides from the fungal extract solution (Moradi and Kalanpour, 2019; Miao et al., 2020). The use of ethanol for precipitation reduces the solubility and hydrophilic properties of polysaccharides in water (Monroy et al., 2016; Meng et al., 2023). Furthermore, precipitation at lower temperatures, such as incubation at 4°C, enhances polysaccharide precipitation (Wang et al., 2022b, 2023a, b). The precipitation of fungal polysaccharides using ethanol involves varying concentrations and volumes, depending on the type of crude polysaccharide and the extraction method employed. Previous studies reported that ethanol concentrations used for fungal polysaccharide precipitation typically range from 80% to absolute ethanol, with ethanol-to-sample ratios varying from 1:1 (v/v) to 1:5 (v/v), usually under cold conditions (4°C) and over an overnight period (Liu et al., 2018; Gao et al., 2022; Sakdasri et al., 2022; Liu et al., 2024a; Panya et al., 2024; Sahib et al., 2024). After precipitation, the polysaccharides can be separated by filtration or centrifugation and then dried by either conventional drying or lyophilization to obtain a crude solid form. The crude polysaccharides are usually kept dry to prevent moisture and microbial contamination and are used for further studies.
7 Purification techniques for fungal polysaccharides
For research and biological applications, it is essential to purify crude fungal polysaccharides in order to maintain the greatest biological properties and achieve high purity. Crude fungal polysaccharides contain a variety of contaminants, including pigments, proteins, monosaccharides, and other compounds, as a result of the limitations of extraction techniques (Wang et al., 2022b). Protein removal and decolorization of crude polysaccharides are commonly achieved using the sevage method and hydrogen peroxide treatment, respectively (Li et al., 2019b; Hu et al., 2022b). Generally, purification methods for polysaccharides include anion exchange chromatography and gel permeation chromatography. Following purification, the polysaccharides are concentrated, dialyzed, and freeze-dried. Finally, the polysaccharide and protein contents in the purified polysaccharides are quantified using the dinitrosalicylic acid, Folin-Ciocalteu, or phenol-sulfuric acid methods (Wood et al., 2012; Saravanakumar et al., 2021; Gao et al., 2022).
7.1 Anion exchange chromatography
Anion exchange chromatography is a crucial technique for purifying fungal polysaccharides, particularly those with a negative charge, such as acidic polysaccharides. This method relies on the principle of ion exchange, where the sample is passed through a column containing a medium capable of binding anions, such as DEAE-cellulose 52 or DEAE-Sephadex A-25, which have positively charged functional groups on their surfaces. When the polysaccharide is introduced into the column, negatively charged polysaccharides are adsorbed onto the medium via ion exchange. Subsequently, the bound polysaccharides are eluted from the column using a buffer solution with increasing salt concentration, which helps release the retained polysaccharides. This technique is well-suited for separating polysaccharides with varying structures and charges, enhancing extraction efficiency, and significantly reducing impurities in fungal polysaccharides (Ren et al., 2019; Wang et al., 2022b).
7.2 Gel permeation chromatography
Gel permeation chromatography is a widely used technique for purifying fungal polysaccharides, particularly for separating substances according to molecular size. The principle of gel permeation chromatography involves the use of a gel with a porous structure within the column, which facilitates the separation of sample molecules based on size. The polysaccharides are injected into a column packed with gel, such as Sephadex G-100 or Sephadex G-200. As the solution flows through the column, larger molecules are unable to penetrate the pores of the gel effectively, allowing them to move through the column more quickly and elute first. In contrast, smaller molecules can penetrate the pores, causing them to migrate more slowly and elute later. Gel permeation chromatography is particularly suitable for isolating polysaccharides with varying molecular weights, effectively reducing contaminants and resulting in purer, more homogeneous polysaccharides (Ren et al., 2019; Wang et al., 2022b).
8 Techniques for analyzing the structural and compositional characteristics of fungal polysaccharides
Fungal polysaccharides are complex compounds with diverse molecular weights, monosaccharide compositions, types of glycosidic bonds, and backbone structures, all of which vary significantly across species and growth conditions. These polysaccharides typically consist of monosaccharide units, such as glucose, fructose, mannose, and galactose, linked by glycosidic bonds, and may adopt either linear or branched structural configurations (Sun et al., 2022b; Yang et al., 2022a). Advanced analytical techniques, including gel permeation chromatography (GPC), high-pressure gel permeation chromatography (HPGPC), gas chromatography-mass spectrometry (GC-MS), high-performance liquid chromatography (HPLC), Fourier transform infrared (FT-IR) spectroscopy, nuclear magnetic resonance (NMR), and scanning electron microscopy (SEM), are employed to investigate the specific structural characteristics of fungal polysaccharides (Wang et al., 2022c; Zhao et al., 2023a). Thus, understanding these structural and functional properties is critical for advancing their potential applications and can lead to innovative product development that maximizes the benefits of fungal polysaccharides. Examples of monosaccharide composition, structure, and characterization methods of fungal polysaccharides are compiled and presented in Table 5.
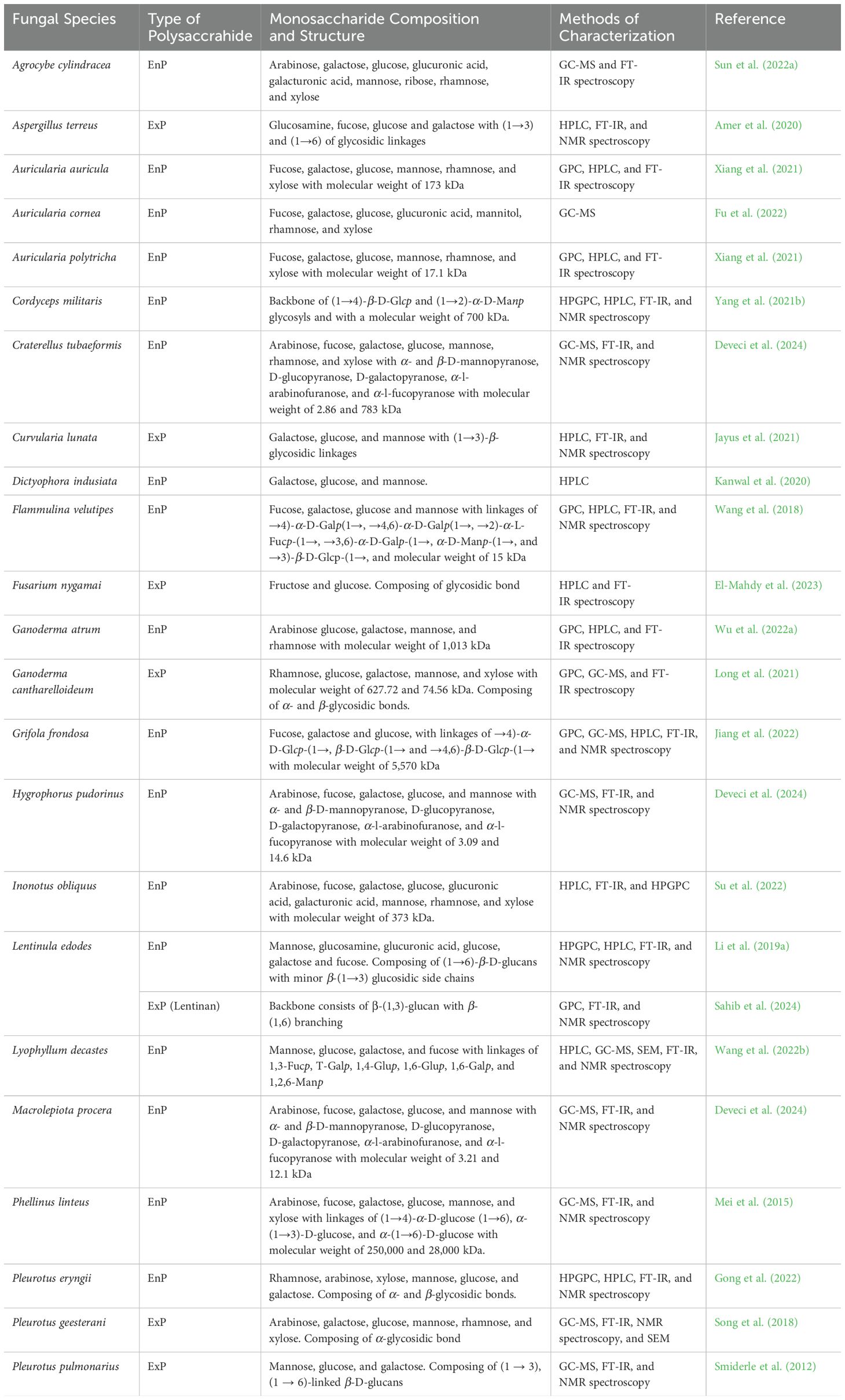
Table 5. Examples of the monosaccharide composition of fungal polysaccharides, structure, and methods of characterization.
8.1 GPC and HPGPC
GPC and HPGPC have been used to separate and analyze molecular sizes based on the principle of size exclusion. Larger molecules elute earlier from the column, while smaller molecules, which can penetrate the pores of the gel more effectively, elute later. GPC is particularly suited for determining average molecular weights and molecular weight distributions in polymers, proteins, and polysaccharides. In contrast, HPGPC is an advanced version of GPC, utilizing high-pressure systems and columns with smaller particle sizes to enhance resolution, speed, and accuracy (Ren et al., 2019). Both GPC and HPGPC are techniques that have been used to analyze polysaccharides from fungi with diverse molecular weights. For example, previous studies on the molecular weight of EnPs and ExPs from Co. militaris found that the HPGPC separates low-molecular-weight polysaccharides more effectively than the GPC, which can only obtain molecular weights lower than 100 kDa (Wang et al., 2015a; Huang et al., 2018; Kang et al., 2022; Liu et al., 2024; Wang et al., 2024). Similarly, studies on the molecular weight of EnPs and ExPs from Le. edodes found that HPGPC is more efficient than GPC at separating low-molecular-weight polysaccharides (Li et al., 2018a; Ren et al., 2018; Zhang et al., 2022a). Moreover, molecular weight analysis of both EnPs and ExPs from He. erinaceus revealed that HPGPC is more effective at separating low-molecular-weight polysaccharides below 10 kDa compared to GPC (Wang et al., 2015b; Wu et al., 2017, 2018). Based on the previous studies mentioned, HPGPC demonstrates the capability to separate smaller fungal polysaccharides with higher effectiveness than GPC.
8.2 GC-MS
GC is a technique used to separate components of a mixture in the gas phase based on differences in solubility and adsorption properties of each substance. This technique involves of two phases: a mobile phase, which is a carrier gas, and a stationary phase, which can be a liquid, solid, or semi-solid material contained within a column. Substances with different chemical characteristics move through the column at varying speeds, depending on their properties, such as solubility or interaction with the stationary phase. This technique allows for detailed analysis of monosaccharide composition in fungal polysaccharides with exceptional accuracy (Millette et al., 2023). For example, GC analysis revealed that arabinose, galactose, glucose, mannose, and xylose are the monosaccharides present in the composition of EnPs extracted from Co. militaris (Luo et al., 2017; He et al., 2020a; Liu et al., 2024c). Studies on the monosaccharide profile of EnPs from Le. edodes revealed the composition of rhamnose, arabinose, xylose, mannose, galactose, and glucose through GC analysis (Li et al., 2018a; Zhang et al., 2022a). The monosaccharide composition of the EnPs from G. resinaceum consists of glucose, galactose, mannose, fucose, and xylose, as determined by GC analysis (Bleha et al., 2022). Additionally, GC techniques have also been employed to determine the monosaccharide composition in ExPs. For instance, in the study by Vanin et al. (2023), the monosaccharide composition of ExPs from Sch. commune was found to be primarily composed of glucose, followed by xylose, mannose, galactose, and arabinose in smaller amounts. Furthermore, the study by Ren et al. (2018) investigating lentinan, an ExPs from Le. edodes, using GC analysis revealed that its monosaccharide composition consists of glucose, mannose, and galactose.
8.3 HPLC
HPLC is used to analyze and separate the components of fungal polysaccharides through precise chromatographic techniques. Prepared samples are introduced into an HPLC column packed with specialized separation media. As the solution flows through the column under high pressure, molecules interact with the column material according to their chemical characteristics and molecular size, enabling efficient separation. Upon elution, a detector captures the concentration of each component, yielding data for detailed analysis of monosaccharide composition, concentration quantification, and the assessment of polysaccharide degradation across various processes (Ingale et al., 2023). Using HPLC, Liu et al. (2018) examined the monosaccharide composition of EnPs isolated from Pax. involutus and found that glucose was the main component, followed by galactose, mannose, and fucose. Wang et al. (2024) investigated the monosaccharide composition of four EnPs isolated from Co. militaris using HPLC, finding that glucose and galactose were present, with glucose being the predominant component. In the study by Chen et al. (2020), HPLC was used to analyze the monosaccharide composition of four different EnPs extracted from Sch. commune obtained through various extraction methods. The results revealed that mannose, ribose, rhamnose, glucuronic acid, galacturonic acid, glucose, galactose, xylose, arabinose, and fucose were present in varying ratios, with glucuronic acid being the main component. Additionally, Ayimbila et al. (2021) analyzed the monosaccharide composition of EnPs extracted from Len. squarrosulus using HPLC, revealing that the polysaccharides were primarily composed of glucose, followed by galactose, mannose, and fucose. The HPLC technique has also been employed to analyze the monosaccharide composition of ExPs. For instance, a study reported that lentinan, an ExP derived from Le. edodes, predominantly consists of glucose as the main monosaccharide, with minor amounts of galactose and mannose detected (Xu et al., 2023). Additionally, the study by Kang et al. (2022) also revealed that the monosaccharide composition of ExPs from Co. militaris consists primarily of glucose, with smaller amounts of galactose and mannose. Comparative studies using HPLC and GC to analyze the monosaccharide composition of fungal polysaccharides have shown no significant differences, with the primary components being glucose, mannose, and galactose. However, arabinose, fucose, ribose, rhamnose, and xylose can also be found in some fungal polysaccharides.
8.4 FT-IR spectroscopy
FT-IR spectroscopy is an essential analytical method for studying fungal polysaccharides. This technique functions by inducing vibrational modes in molecular bonds via infrared radiation, which is then absorbed by the sample. The resulting absorption data is processed into a spectrum, presenting the frequency and absorption intensity relationship. This enables detailed structural analysis and functional group identification, such as glucose and fructose residues, allowing for precise assessments of sample purity and detection of potential impurities within the polysaccharide material (Hong et al., 2021). The study by He et al. (2020a) analyzed EnPs from Co. militaris using FT-IR spectroscopy, revealing a strong and broad absorption peak at 3392.8 cm−1, attributed to O-H stretching vibrations of hydroxyl groups due to intramolecular or intermolecular interactions, along with a weaker absorption band at 2930.6 cm−1, corresponding to C-H stretching vibrations in -CH3 or -CH2 groups. Similar to the study by Wang et al. (2024), which analyzed EnPs from Co. militaris to examine organic functional groups, the broad absorption peak at 3400 cm−1 was attributed to O-H stretching vibrations from intermolecular or intramolecular interactions within the polysaccharide, while the weaker absorption band at 2925 to 2931 cm−1 corresponded to asymmetric C-H stretching vibrations in sugar groups. In the study by Zhang et al. (2022a), the functional groups in Le. edodes EnPs were observed, with the strong absorption peak near 3300 cm-1 attributed to O-H stretching vibrations, and the signal at 2922 cm-1 resulting from C-H stretching vibrations; strong absorptions around 1600 cm-1 indicated C=O stretching, while the absorption near 1400 cm-1 was due to C-H deformation vibrations; C-O-C group signals between 1000–1100 cm-1 confirmed pyranose ring linkages, and the weaker signal at 890 cm-1 reflected β-glycosidic bonds. Ascencio et al. (2021) investigated the functional groups of lasiodiplodan from L. theobromae and revealed distinct spectral characteristics, including broadband with high intensity at 3274 cm-1 corresponding to the stretching vibration of R-OH groups, a band at 1650 cm-1 associated with the glucose ring structure, symmetric vibrations of the C-O-C bond characteristic of carbohydrates exhibiting absorption at 1075 cm-1, and a low-intensity band at 890 cm-1 indicative of glycosidic bonds representing the β-configuration arrangement. Based on the previous research examples provided, it can be observed that the strong and broad absorption peak is commonly attributed to the O-H hydroxyl group, which is frequently found in fungal polysaccharides. However, other functional groups, including C-H, C=O, and C-O-C, also appear, emphasizing the chemical diversity in fungal polysaccharides and reflecting the unique properties and structures found across different fungal species (He et al., 2020a; Zhang et al., 2022a).
8.5 NMR spectroscopy
NMR spectroscopy is a valuable tool for analyzing the structural properties of fungal polysaccharides by exploiting the behavior of atomic nuclei in a powerful magnetic field. Nuclei such as hydrogen (¹H) and carbon (¹³C) resonate at characteristic frequencies, absorbing energy in ways that reveal precise structural details. This technology allows for accurate determination of polysaccharide structure, including the sequence of monosaccharide units and the types of glycosidic linkages present (Yao et al., 2021). NMR spectroscopy was used to investigate the glycosidic bond structure of EnPs derived from Mor. sextelata, revealing that the EnPs contain glycosidic linkages similar to (1→4)-linked-α-Glcp suggesting a specific type of linkage in its structure Li et al. (2022). In the study by Tepsongkroh et al. (2023), NMR spectroscopy was used to examine the glycosidic bonds of polysaccharides from V. volvacea, revealing that the polysaccharide consists of (1→3)-linked-β-D-Glcp glycosidic linkages, forming a β-glucan (1,3/1,6) structure. Some studies have revealed the complexity of glycosidic bond structures, such as a research by Ayimbila et al. (2021), where NMR spectroscopy was used to analyze the glycosidic bond structure of EnPs derived from Len. squarrosulus. The analysis identified a complex structure composed of (1→4,6)-linked-β-D-Glcp, (1→4)-linked-β-D-Glcp, (1→6)-linked-β-D-Glcp, (1→4)-linked-β-D-Manp, α-L-fucose, and (1→6)-linked-α-galactosyl, highlighting the structural diversity of these EnPs. Zhang et al. (2023) found that glycosidic bond structure of EnPs derived from D. indusiata revealed a complex structure consisting of (1→6)-linked-β-Glcp, (1→2,6)-linked-α-Glcp, (1→3)-linked-α-Manp, (1→6)-linked-β-Manp, and (1→6)-linked-β-Galp. Additionally, lentinan from Le. edodes, analyzed for glycosidic bond structure using NMR spectroscopy, revealed that its backbone consists of β-(1,3)-glucan with β-(1,6) branching (Sahib et al., 2024). The NMR spectroscopy technique aids in identifying the types of glycosidic linkages, such as β-D-glucan and α-D-glucan, by detecting specific signals in the spectra. The most commonly observed glycosidic linkages include (1→3)-linked-β-Glcp, (1→6)-linked-β-Glcp, (1→3)-linked-α-Glcp, and (1→6)-linked-α-Glcp (Ayimbila et al., 2021; Gao et al., 2022; Li et al., 2022; Tepsongkroh et al., 2023; Zhang et al., 2023). This technique is crucial for understanding the types of glycosidic bonds and the backbone structures of fungal polysaccharides, which in turn influence the potential applications.
8.6 SEM
SEM is employed to examine the physical characteristics of fungal polysaccharides by using a high-energy electron beam to capture highly detailed surface images at the nanometer scale. This process begins with sample preparation, which typically includes applying a metallic coating to enhance conductivity. When the electron beam interacts with the sample surface, it generates scattered electrons and photons that are detected to produce a three-dimensional representation. SEM allows for precise analysis of structural features, including particle size, surface morphology, and distribution within the polysaccharide matrix (Fellak et al., 2022). In the study by Li et al. (2022), SEM analysis of EnPs from Mor. sextelata revealed the sponge-like porous structure with a smooth surface. Similarly, the study by Qiu et al. (2024) reported that the surface morphology of EnPs extracted from Pl. eryngii exhibited a sponge-like porous structure. A comparable sponge-like porous structure was also observed in pullulan, an exopolysaccharide extracted from Au. pullulans (Maia et al., 2023). Additionally, different extraction methods influence the surface morphology and structure of fungal polysaccharides. Each method affects the integrity and arrangement of molecules within the polysaccharide structure (Deng et al., 2015; Chemat et al., 2017). In the study by Zhang et al. (2022a), SEM was used to examine the surface morphology of EnPs from Le. edodes extracted using different methods. The polysaccharide extracted via subcritical water extraction with a deep eutectic solvent showed a loose, rough, and porous structure. The polysaccharide from subcritical water extraction alone exhibited a leaf-like surface with uneven distribution but a smooth and fine texture. The polysaccharide extracted by hot water extraction displayed a sheet-like structure with irregular aggregation. Moreover, in the study by Rosyida et al. (2024), EnPs from Pl. ostreatus were extracted using various techniques. The results revealed that polysaccharides extracted through kinetic-assisted hot extraction exhibited fewer pores and a smoother surface. In contrast, those extracted by microwave and ultrasound-assisted extraction displayed puffed structures, numerous open cavities, and collapsed surfaces. Furthermore, SEM has been employed to examine the surface morphology of exopolysaccharides, such as pullulan derived from Au. pullulans, which exhibits a smooth surface structure (Tagne et al., 2024), and lentinan from Le. edodes, characterized by a rough surface and irregular pores resembling a honeycomb-like porous structure (Xu et al., 2023).
9 Properties of fungal polysaccharides
Fungal polysaccharides have garnered significant interest in recent years due to their diverse and potent properties. These polysaccharides exhibit a wide range of beneficial effects, including antidiabetic, antioxidant, antiviral, antilipidemic, antitumor, and immunomodulating properties. An example of the biological properties of fungal polysaccharides is shown in Table 6.
9.1 Antidiabetic property
Diabetes, a non-communicable disease characterized by chronic hyperglycemia, is classified into Type 1 and Type 2. Type 1 occurs when the pancreas fails to produce insulin, while Type 2, the most common form, results from the body’s inability to effectively use insulin, often linked to factors like being overweight and lack of physical activity (American Diabetes Association, 2009). Natural supplements derived from plants and fungi, along with medications used to treat diabetes, can help control blood glucose levels (Liu et al., 2022a). According to previous research, fungal polysaccharides could beneficially affect people with diabetes by inhibiting glucose absorption efficacy, gastrointestinal viscosity, inhibition of α-amylase and α-glucosidase activity to control hyperglycemia, improving pancreatic β-cell mass, and enhancing insulin signaling (Jovanović et al., 2021; Fu et al., 2022; Liu et al., 2022a; Figueroa et al., 2023; Ji et al., 2023; Li et al., 2023; Yu et al., 2024). Generally, Streptozotocin-induced diabetic mice are frequently used to assess a compound’s ability to reduce blood glucose levels. This method yields consistent results, demonstrating the compound’s capability to lower blood glucose. Several previous studies demonstrated polysaccharides from Aur. auricula, Aur. polytricha, Co. taii, D. indusiate, G. frondose, Pl. eryngii, Pl. geesterani, Pl. ostreatus, and Su. luridus can effectively reduce blood glucose levels in Streptozotocin-induced diabetic mice (Duobin et al., 2013; Zhang et al., 2018a; Chen et al., 2019; Liu et al., 2019; Xiang et al., 2021) (Table 6). These findings indicate that fungal polysaccharides could be used as supplements for diabetes. However, future studies must evaluate the effectiveness of these fungal polysaccharides in diabetic patients to assess their true efficacy, safety, and potential side effects. If these polysaccharides can be developed into a treatment for diabetes, they could greatly benefit both medical practice and pharmacology by offering patients a more effective and safer treatment option. Moreover, the development of drugs based on fungal polysaccharides could offer new opportunities for treating diabetes.
9.2 Antilipidemic property
Hyperlipidemia is a condition characterized by elevated levels of total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) in the blood, exceeding established thresholds. It is a significant risk factor for various disorders, including diabetes, hypertension, stroke, and cardiovascular disease. In addition to conventional medications, dietary supplements provide an alternative for individuals with hyperlipidemia, with fungal polysaccharides emerging as a promising option due to their antilipidemic properties (De Costa and Park, 2017). Prior research employing mouse models has frequently shown that fungal polysaccharides can successfully reduce blood levels of LDL-C, TC, and TG (Table 6) (Miranda-Nantes et al., 2011; Zeng et al., 2013; Wang et al., 2019b; Ge et al., 2022). Although animal studies have demonstrated the efficacy of fungal polysaccharides in significantly reducing TC, TG, and LDL-C, further clinical trials in patients with hyperlipidemia are required to confirm their effectiveness.
9.3 Antibacterial property
The antibacterial effects refer to the ability of substances or materials to inhibit the growth of various bacteria through mechanisms such as disrupting the bacterial cell wall, inhibiting enzymes essential for bacterial growth, or preventing bacteria from adhering to host cells (Di Martino, 2022). Previous studies have focused on exploring the antibacterial properties of fungal polysaccharides, revealing that these polysaccharides have the potential to inhibit the growth of human pathogenic bacteria, including genera Bacillus, Escherichia, Klebsiella, Listeria, Micrococcus, Pseudomonas, Proteus, Shigella, Salmonella, and Staphylococcus, as well as methicillin-susceptible Staphylococcus aureus (Skalicka-Woźniak et al., 2012; Mahendran et al., 2013; Osińska-Jaroszuk et al., 2014; Wan-Mohtar et al., 2016; Ayimbila et al., 2021) (Table 6). The effectiveness of these polysaccharides varies depending on the type of fungal polysaccharide used. Thus, fungal polysaccharides exhibit promising bacterial properties and show potential for development as components in products for preventing and treating bacterial infections. However, further research is required to confirm their efficacy and safety for human use.
9.4 Antioxidant property
Free radicals, generated in the body through normal metabolism or external factors like UV radiation, pollution, or chemicals, are unstable and stabilize by stealing electrons from other molecules. They can lead to oxidative stress, which damages cells and tissues, contributing to various diseases such as heart disease, cancer, and accelerated aging (Phaniendra et al., 2015). However, the human body has compounds that protect against oxidative stress, called antioxidants, which help neutralize free radicals and reduce the damage they may cause. Antioxidants can be obtained from external sources such as food and supplements (Pham-Huy et al., 2008). Interestingly, certain fungi can produce polysaccharides with antioxidant properties, adding another potential source of these beneficial compounds (Bai et al., 2022). Previous studies have reported that fungal polysaccharides possess antioxidant properties, as demonstrated using various methods, including ABTS, DPPH, hydroxyl radical, and superoxide radical assays (Table 6) (Liu et al., 2018; Si et al., 2018; Ayimbila et al., 2021; Deng et al., 2021; Lu et al., 2022; Tabibzadeh et al., 2022). These assays help evaluate the potential of fungal polysaccharides in protecting cells from damage caused by free radicals, providing researchers with deeper insights into their role in mitigating cellular damage. However, one concern with fungal polysaccharides is the variability in their antioxidant efficacy. Thus, optimizing cultivation conditions, standardizing extraction methods, using specific fungal strains, and implementing rigorous testing and quality control can help control and reduce the variability in the antioxidant efficacy of fungal polysaccharides.
9.5 Anticancer property
Cancer is currently one of the leading causes of death worldwide. Despite significant advancements in research and treatment, including immunotherapy, targeted therapy, and proton therapy, challenges persist. Certain types of cancer remain complex and show limited response to treatment. In addition, side effects from therapies such as chemotherapy and radiation can negatively impact a patient’s quality of life (Christyani et al., 2024; Tufail et al., 2024). The alternative therapeutic strategies are being explored, including the potential of polysaccharides derived from fungi. Research has increasingly focused on the anticancer properties of fungal polysaccharides, which have shown promise in combating cancer through various mechanisms. Current studies have shown that fungal polysaccharides possess anticancer properties, with experiments conducted both in vitro using cell lines and in vivo in animal models (Table 6). However, while they induce apoptosis in cancer cells and inhibit cancer cell growth, the results obtained are still in the preliminary stages and have limitations (Mao et al., 2016; Su et al., 2018; Geraldelli et al., 2020; Udchumpisai and Bangyeekhun, 2020; Wu et al., 2022b; Abdala-Díaz et al., 2024). Although initial findings show promising potential, further long-term studies and clinical trials in humans are necessary to confirm the effectiveness and safety of using fungal polysaccharides as a therapeutic approach to cancer treatment.
9.6 Antiviral property
Developing antiviral substances holds significant importance for advancements in medicine and public health (Gonçalves et al., 2021). Antiviral properties are one of the key characteristics of fungal polysaccharides that have been extensively studied. Research has demonstrated that fungal polysaccharides can inhibit viral replication, including against herpes simplex virus type I (Sacchelli et al., 2019), hepatitis B virus (Jiao et al., 2018), and influenza A virus (Vlasenko et al., 2020) (Table 6). Although current research has yet to definitively demonstrate that fungal polysaccharides inhibit several viruses, ongoing studies are required to fully realize their potential in antiviral therapeutics. Further studies should address important constraints, including their narrow spectrum, unknown mechanisms, low bioavailability, and the need for additional clinical evidence.
9.7 Immunomodulating property
Fungal polysaccharides are known for their immunomodulatory properties, with low toxicity, high molecular mass, and diverse branching structures that may trigger appropriate immune responses in humans (Zhao et al., 2020; Yin et al., 2021). Fungal polysaccharides possess notable properties in modulating and enhancing immune system function. The key mechanism involves activating immune cells, such as macrophages, and stimulating cytokine secretion, which further supports immune system activity (Table 6). These polysaccharides hold the potential for development into immune-boosting supplements or therapeutic agents to prevent and alleviate the effects of infections, chronic diseases, or immune deficiencies (Murphy et al., 2023a, b). However, most of the research to date has been limited to in vitro and in vivo studies, which may not be sufficient for the development of clinical-grade therapeutics. Therefore, further clinical studies in humans are necessary to confirm the effectiveness of fungal polysaccharides in immune modulation, as well as to evaluate any potential side effects, ensuring safety and reliability for practical use.
9.8 Prebiotic property
Prebiotic property is another important characteristic of polysaccharides, as previous research has shown that polysaccharides from edible fungi are resistant to human digestive enzymes and serve as a crucial energy source for the gut microbiome, promoting the growth of beneficial bacteria (Uthan et al., 2021; Zhao et al., 2023b). Bifidobacterium, Lacticaseibacillus, and Lactobacillus are key probiotic bacteria in the human gastrointestinal system, playing an essential role in promoting intestinal health, supporting immune function, and maintaining the balance of gut microbiota (Wang et al., 2022b; Panya et al., 2024). Recent studies have highlighted the potent prebiotic properties of fungal polysaccharides, which can enhance the growth of probiotics both in vitro and in animal models (Table 6). These findings suggest that fungal polysaccharides hold significant potential for development as functional ingredients in prebiotic products that support gastrointestinal health (Barcan et al., 2024). Despite their potential as prebiotic agents, fungal polysaccharides have a number of limitations that require more further research and development. Unlocking their full potential as functional components in prebiotic products will require increasing their bioavailability, understanding their mechanisms of action, establishing clinical evidence, and resolving issues with cost and production scalability.
10 Applications for fungal polysaccharides
This research presents the application of polysaccharides derived from fungi in the fields of medicine, pharmacology, food, agriculture, animal, and environmental applications as shown in Figure 5.
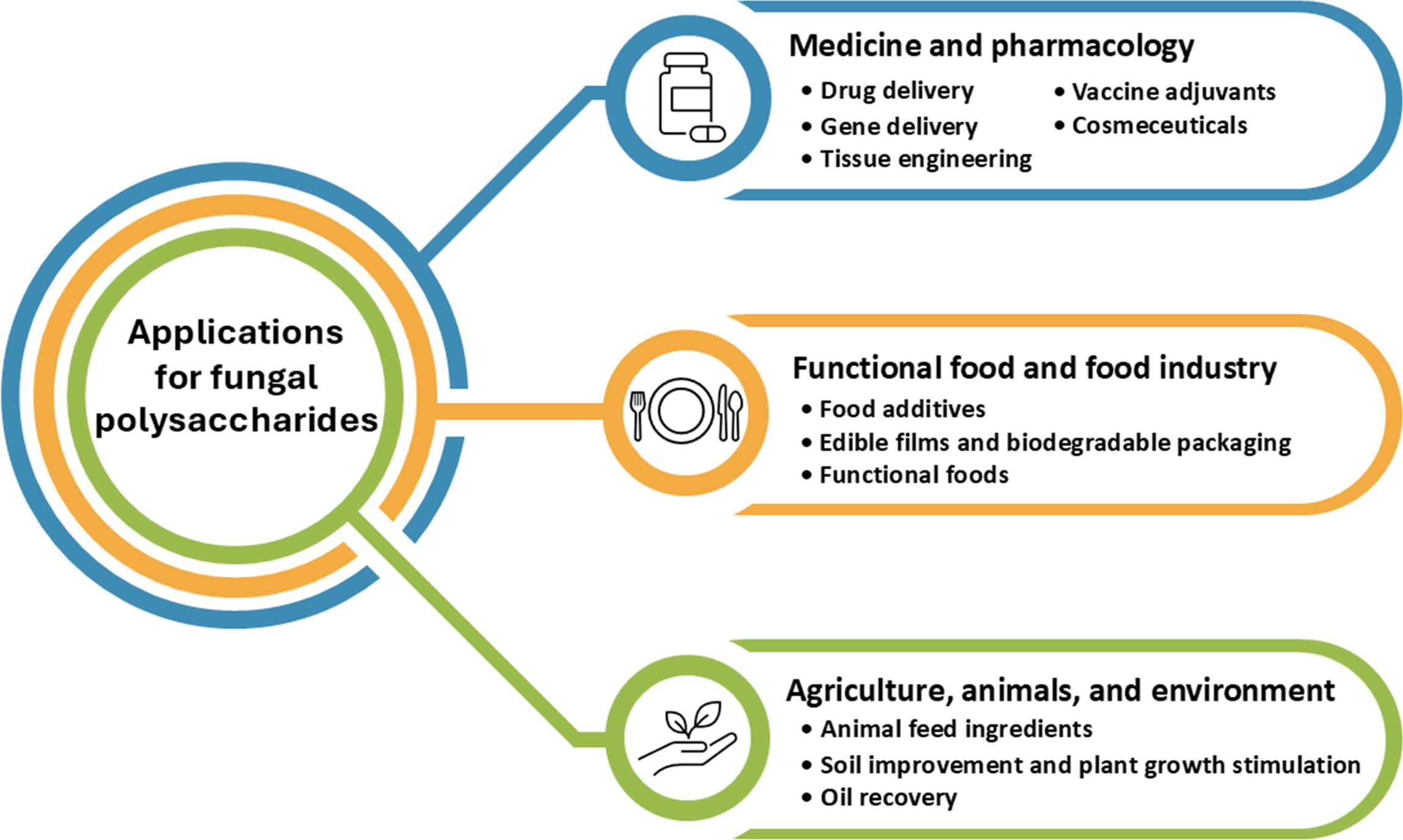
Figure 5. Applications of fungal polysaccharides in medicine, pharmacology, food industry, agriculture, animals, and environment.
10.1 Applications in medicine and pharmacology
10.1.1 Drug delivery
Considerable advancements have been made in drug delivery systems, enhancing the precision of drug release, stability against degradation, and extending the duration of drug action. Innovations such as microcapsules, nanoparticles, and polysaccharide-based coatings have been developed to improve the accuracy of drug targeting (Ezike et al., 2023). The incorporation of polysaccharide-based coatings in drug delivery systems offers several advantages, including safety, stability, controlled release, biodegradability, and natural origin, making these materials highly compatible. This versatility, especially when combined with other delivery platforms, broadens the potential applications of polysaccharides in drug administration (Visan and Cristescu, 2023). Fungal polysaccharides, particularly pullulan, play a crucial role in drug delivery due to their unique beneficial properties. As noted by Thakur et al. (2023), pullulan-based polymeric drug delivery systems offer a pioneering approach for respiratory disease treatment, featuring a sustained release profile, biocompatibility, non-immunogenicity, chemical modifiability, and superior permeability through lung mucosa compared to other polysaccharides. Gehrcke et al. (2022) have developed a bilayer film composed of pullulan, encapsulating silibin-loaded nanocapsules for the treatment of atopic dermatitis. In vitro, results demonstrated that pullulan enhances the film’s adhesion to skin tissue, and the film possesses antioxidant properties without inducing hemolysis. Interestingly, in vivo experiments, the film regulated inflammation and oxidative parameters as effectively as, or even better than, silibinin solution and hydrocortisone, which are conventional treatments for atopic dermatitis. In addition, Balasso et al. (2017) used pullulan in a bioconjugate specifically designed for targeted drug delivery to hepatocellular carcinoma. The results indicated that the PreS1-Pullulan-Doxorubicin polymer enhanced anticancer efficacy against HepG2/SERPINB3 cells twofold compared to the control pullulan-Doxorubicin. This finding highlights the potential of enhancing polysaccharides as selective therapeutic agents for hepatocellular carcinoma by promoting targeted accumulation within cancerous liver cells. Furthermore, research has been conducted on drug delivery using schizophyllan, which forms stearic acid-schizophyllan micelles, a novel type of drug carrier (Negahban et al., 2021). Paclitaxel loaded into these micelles exhibited higher anticancer activity against MCF-7 cells compared to free paclitaxel. Notably, schizophyllan also possesses unique anti-inflammatory and immune-enhancing properties, which could potentially synergize with the therapeutic effects of the loaded drug.
10.1.2 Gene delivery
Gene delivery refers to the process of delivering genetic materials, such as DNA or RNA, to various cells or tissues in the body to modify, enhance, or alter gene function. This technique is widely used in genetic research, vaccine development, gene therapy, and studies related to various diseases (Sung and Kim, 2019). Gene delivery can be achieved through several methods, including the use of viruses that have been modified to be non-pathogenic, such as adenoviruses, lentiviruses, and adeno-associated viruses (AAV), to deliver genetic materials into cells. Additionally, non-viral approaches utilize nanocarriers made from synthetic materials or natural substances to facilitate gene delivery (Pan et al., 2021). These include polymeric nanoparticles or polysaccharides, providing alternative methods for introducing genetic material without the use of viruses (Li et al., 2018b). Previous research by Kang et al. (2010) incorporated the polysaccharide pullulan into polyethyleneimine (PEI) and small interfering RNA (siRNA) to create a pullulan-modified PEI/siRNA complex for targeted delivery to the liver, comparing it with the PEI/siRNA complex. After the injection into mice, siRNA was detected using fluorescence techniques. The results revealed that the PEI-pullulan/siRNA complex exhibited higher fluorescence in the liver compared to the PEI/siRNA complex, while the PEI/siRNA complex demonstrated greater fluorescence in the lungs. The PEI-pullulan/siRNA complex significantly reduced mouse mortality and could serve as an effective, low-toxicity strategy for delivering siRNA to the liver. Similar to other targeted delivery systems for plasmid DNA (pDNA) and siRNA directed at folate receptors on cancer cells, Wang et al. (2014a) synthesized an enhanced gene delivery carrier using folate-polyethyleneimine-modified pullulan for the delivery of pDNA and siRNA. The results indicated that the folate-polyethyleneimine-modified pullulan effectively encapsulates pDNA and siRNA, with efficient delivery occurring through the folate receptor. This suggests that folate-polyethyleneimine-modified pullulan is a promising, safe, and precise gene delivery system suitable for targeting cancer cells. Additionally, the study by Liu et al. (2014) developed an innovative nanoparticle system with a core-shell structure, utilizing pullulan and poly(β-amino) ester (PBAE) conjugated to methotrexate (anti-cancer drug) and green fluorescent protein (pEGFP). This methotrexate-pullulan/PBAE/pEGFP nanoparticle was specifically designed for the targeted delivery of gene and chemotherapeutic agents to liver cancer cells. This nanoparticle exhibited strong hepatoma-targeting capability, primarily accumulating in liver cancer cells within 24 hours post-intravenous injection. It successfully facilitated the co-delivery of gene and chemotherapy agents to tumor sites at both the cellular and animal levels, offering a promising approach to liver cancer treatment.
10.1.3 Tissue engineering
Tissue engineering is an emerging field that plays a crucial role in promoting the regeneration of damaged tissues that are unable to self-repair. It enhances the body’s natural healing potential, supporting recovery in a way that complements the patient’s healing process. Injured tissue repair can be stimulated through the use of synthetic polymer scaffolds, which serve as structural supports that facilitate efficient tissue regeneration (Singh et al., 2016). In a prior study by Schlaubitz et al. (2014), pullulan combined with dextran and reinforced with nanocrystalline hydroxyapatite was developed into highly porous scaffold beads to evaluate cellular growth and new bone formation in a rat model. The findings indicated effective cellular penetration into the scaffold spaces and new tissue formation around the beads. Additionally, calcium and mineral deposition within the structure increased over time, with no inflammation observed at the implantation site. Thangavel et al. (2020) used pullulan gel to evaluate its wound-healing efficacy on open excision wounds made on the dorsum of rats. The study found that pullulan gel significantly stimulated collagen, hexosamine, protein, and DNA synthesis compared to the untreated and povidone-iodine ointment-treated groups, demonstrating its potential as a wound-healing agent. The effects of β-glucan from S. cerevisiae on wound healing in venous ulcers in humans were evaluated by Medeiros et al. (2012) through histopathological analysis. The findings revealed that β-glucan significantly accelerated wound healing by promoting epithelial hyperplasia, stimulating angiogenesis, increasing plasmocyte numbers, and enhancing fibroblast proliferation.
10.1.4 Vaccine adjuvants
Vaccine adjuvants are substances or components added to vaccines to enhance the immune response’s effectiveness. Typically, vaccines contain immunogenic agents such as antigens; however, the incorporation of adjuvants intensifies and prolongs the immune stimulation. Therefore, the use of adjuvants in vaccines enables the development of more effective vaccines and enhances their ability to prevent various diseases more effectively (Zhao et al., 2023c). A study by Zhang et al. (2017) found that lentinan, tremella, pachymaran, and their combination with the H1N1 influenza vaccine in mice enhanced the vaccine’s efficacy. These findings suggest that fungal polysaccharides could help improve the effectiveness of influenza vaccines. He et al. (2020b) used calcium carbonate-lentinan loaded with the H5N1 antigen to develop an adjuvant for the H5N1 vaccine, aimed at preventing the avian influenza virus. After injection into mice, the calcium carbonate-lentinan/H5N1 complex significantly enhanced the expression of MHC-II and CD86 in dendritic cells from lymph nodes. It also unexpectedly led to elevated hemagglutination inhibition (HI) titers and stimulated the secretion of IgG subtypes (IgG1 and IgG2b), along with T-helper-associated cytokines (TNF-α, IFN-γ, and IL-4) in vaccinated mice. Additionally, Liu et al. (2019) developed a vaccine by encapsulating polysaccharides from G. lucidum and inactivated porcine circovirus type II (PCV-II) into liposomes. When administered to mice, this vaccine induced stronger PCV-II-specific immune responses, including higher titers of PCV-II-specific IgG antibodies, increased cytokine levels, and splenocyte activation, compared to other single-component formulations.
10.1.5 Cosmeceuticals
Cosmeceuticals are products that combine the properties of cosmetics and pharmaceuticals to enhance the effectiveness of skincare and beauty treatments. These products typically contain active ingredients that nourish or treat the skin, such as vitamins, minerals, antioxidants, and natural substances. They are designed to improve skin health, prevent deterioration, and effectively reduce the appearance of wrinkles (Singh et al., 2024). Kanlayavattanakul and Lourith (2023) found that the addition of polysaccharide extract from Co. militaris to a skincare cream formulation increased the cream’s stability. A study by Woźniak et al. (2023) revealed that polysaccharide extract from Tre. fuciformis serves as a natural alternative to hyaluronic acid in cosmetic formulations, significantly improving skin hydration without causing irritation or erythema, as confirmed by dermatological evaluations. A study by Sangthong et al. (2022) demonstrated that a cosmetic gel cream containing polysaccharides from V. volvacea could enhance skin hydration, elasticity, and firmness, while reducing skin roughness, dryness, wrinkles, and melanin content, with no cytotoxic effects on human dermal fibroblasts. Additionally, a study conducted by Jesenak et al. (2016) demonstrated that the application of Imunoglukan P4H® cream, containing β-glucan (pleuran isolated from Pl. ostreatus), significantly reduced the frequency and severity of atopic dermatitis in patients.
10.2 Applications in functional food and food industry
10.2.1 Food additives
Fungal polysaccharides can be applied in the food industry as additives to improve food quality, such as gelling agents, stabilizers, thickeners, or texture modifiers. These polysaccharides do not affect the taste of food, which is a critical aspect to consider in food products, while also enhancing sensory properties. Furthermore, polysaccharides help extend the shelf life of food (Jindal and Singh Khattar, 2018). Pullulan has been used in the food industry for over 20 years and has been recognized as Generally Recognized as Safe (GRAS) in the USA (Oğuzhan and Yangılar, 2013). This is due to its characteristics as a colorless, odorless, edible polysaccharide that is non-toxic and non-carcinogenic (Prajapati et al., 2013). Pullulan has been utilized as a thickening agent in foods such as soups, sauces, and various beverages (Yatmaz and Turhan, 2012). According to Singh et al. (2019), pullulan has been used as a stabilizer and texture modifier in mayonnaise, and it has also been employed to enhance the adhesion of nuts in cookies. Furthermore, pullulan has low viscosity and remains stable when exposed to sodium chloride and heat, making it an ideal thickening agent for food products with high salt concentrations, such as barbecue sauces, soy sauces, pickled fruits, and vegetables. In addition, scleroglucan can serve as a gelling agent, stabilizer, or thickener, offering an alternative to xanthan gum in the food industry. It improves the quality of heat-treated and frozen foods, such as steamed foods, rice crackers, Japanese cakes, and bread (Jindal and Singh Khattar, 2018). Pleuran and lentinan can be used to produce hydrogels for yogurt products without affecting the flavor or texture while enhancing health benefits (Vetter, 2023).
10.2.2 Edible films and biodegradable packaging
Fungal polysaccharides can be utilized in the production of edible or biodegradable films (Cazón et al., 2017). Pullulan is widely used in the fabrication of edible films due to its strength, flexibility, and excellent oxygen barrier properties, which prevent food oxidation from air exposure (Oğuzhan and Yangılar, 2013). Edible films made from pullulan have also been employed in film formation and packaging for various foods, including vegetables, fruits, grains, snacks, and dried goods (Singh et al., 2016, 2019). Previous studies have shown that pullulan, when combined with other substances such as chitosan, alginate, or shellac, can create films with oxygen, water, and UV protection, making them suitable for food packaging (Zhou et al., 2021; Mugnaini et al., 2024; Tang et al., 2025). Additionally, pullulan has been incorporated with propolis to develop antifungal food packaging, which protects against pathogenic fungi, thus preventing fungal growth in food and extends food shelf life (Gniewosz et al., 2022). A study by Viñarta et al. (2013) revealed that scleroglucan derived from Scl. rolfsii demonstrated superior performance, particularly in enhancing pseudoplastic behavior, and showed excellent compatibility with corn starch, xanthan, pectin, and carboxymethylcellulose, making it highly suitable for applications in the food industry. Beyond pullulan, scleroglucan, and schizophyllan can be used to form edible films for dietary supplement packaging due to their chemical stability and natural biodegradability (Rahman et al., 2021; Ghosh et al., 2022).
10.2.3 Functional foods
Edible mushrooms are recognized as health-promoting foods for humans. In recent years, bioactive polysaccharides extracted from edible mushrooms have been developed into functional foods that are more easily and rapidly absorbed by the body, resulting in faster bioactivity. In addition to functional foods for human health, polysaccharides from mushrooms can also be used as animal feed (Osińska-Jaroszuk et al., 2020). β-glucan, a polysaccharide derived from mushrooms, is well-known for its diverse biological and pharmacological properties, including immunomodulatory, antioxidant, antimicrobial, anticancer, cardioprotective, and hepatoprotective activities. β-glucan from mushrooms can enhance both innate and cell-mediated immune responses. It has demonstrated varying degrees of antitumor activity in humans due to differences in structure, water solubility, size, and molecular mass (Khan et al., 2018). Furthermore, previous studies have shown that polysaccharides from several mushrooms, e.g. G. lucidum, Le. edodes, Tre. fuciformis, Au. pullulans, Pleurotus spp., and Ag. bisporus (Cheng et al., 2011; Giannenas et al., 2011; Delzenne and Bindels, 2015) exhibit prebiotic properties, promoting the growth of beneficial bifidobacteria, a probiotic in the human gut.
Currently, polysaccharide extracts from mushrooms are commercially available as dietary supplements and functional foods. For instance, Immune-Assist™ Critical Care Formula, produced by Aloha Medicinals Inc., contains extracts from various mushrooms, including Ag. blazei (58.5% β‐glucan), Co. sinensis (30% β‐glucan), Gr. frondosa (28% β‐glucan), Le. edodes (40% β‐glucan, lentinan, and α‐glucan), Tr. versicolor (40% β‐glucan), and G. lucidum (40% β‐glucan and triterpenoids). This product has been shown to reduce the side effects of chemotherapy and radiotherapy in cancer patients. Super Reishi®, a β‐glucan extract from G. lucidum produced by Mushroom Wisdom Inc., is known for modulating and supporting multiple bodily systems, including the heart, lungs, liver, nervous system, and brain. Maitake®, developed by Pharmaceutical Mushrooms Inc., is a β‐glucan-based product derived from Gr. frondosa that demonstrates strong immunomodulatory effects, notably enhancing T-cell production, and is specifically recommended for managing immunodeficiency conditions. Fine‐Agaricus® Gold, produced by FineCo. Ltd. is a 100% polysaccharide extract from Agaricus that has demonstrated efficacy against various cancers through immune system enhancement, while also balancing physiological functions and providing benefits for the treatment of chronic diseases. Additionally, Transfer Factor Plus® Tri‐Factor® Formula, produced by Product 4life Inc., contains extracts from Le. edodes, Gr. frondosa, and Cordyceps sp., comprising β‐glucans, hexaphosphate inositol, β‐sitosterol, and an extract of olive leaves. This formula has been shown to stimulate the immune system by enhancing the activity of NK cells in the body (Morris et al., 2016).
10.3 Applications in agriculture, animals, and environment
10.3.1 Animal feed ingredients
Polysaccharides from fungi, in addition to being used as functional food for humans, can also be applied as animal feed ingredients to enhance the nutritional value of livestock (Osińska-Jaroszuk et al., 2020). Research by Muthusamy et al. (2013) demonstrated that β-glucan derived from Pl. floridanus could be incorporated into poultry feed, significantly enhancing the immune response in broiler chickens. Similar to the study by Gao et al. (2024), which found that supplementation of polysaccharides from G. lucidum increased HDL-C levels in the serum, reduced TG levels, enhanced antioxidant activity, increased antioxidant enzyme levels in broiler chickens, and contributed to the improvement of gut microbiota composition. In addition, β-glucan from Pl. floridanus and Pl. ostreatus has been used in fish feed to enhance immune function in fish (Dobšíková et al., 2013). Furthermore, β-glucan has been combined with milk to serve as a dietary supplement in newly weaned piglets, promoting growth performance and gut health (Mukhopadhya et al., 2019).
10.3.2 Soil improvement and plant growth stimulation
Fungal polysaccharides can be applied in agriculture, particularly ExP, which are mucilaginous and carry ionic charges, allowing them to bind soil particles together and enhance soil fertility (Costa et al., 2018). Previous research introduced the use of ExPs in soil, revealing that ExPs improve the aggregate stability of soil particle aggregation in water, thereby enhancing water retention and increasing soil porosity (Costa et al., 2018; Angulo et al., 2024). ExPs produced by basidiomycetes and Trichocomacea can bind soil particles, promoting soil aggregate formation and stability (Daynes et al., 2012; Angulo et al., 2024). Polysaccharides from G. lucidum have been shown to increase seed germination rates and seedling heights when combined with chemical fungicides in seed-coating formulations, effectively controlling soil-borne diseases. Furthermore, polysaccharides from G. lucidum enhance the expression of genes associated with disease resistance in maize and wheat, enabling their use alongside chemical fungicides in the prevention of maize root rot, wheat root rot, and sharp eyespot diseases (Yang et al., 2022a, b). Application of polysaccharides isolated from the fruiting body of Pl. ferulae exhibited antifungal activity against Rhizoctonia solani, promoted cucumber plant growth by enhancing root length and fresh weight of cucumber seedlings, and stimulated the activities of enzymes such as superoxide dismutase, peroxidase, and polyphenol oxidase for disease resistance (Yang et al., 2024c).
10.3.3 Oil recovery
Oil recovery refers to the process of increasing the extraction rate of crude oil from underground reservoirs (Jafarinejad, 2017). After conventional extraction methods, substantial amounts of oil often remain trapped within the rock formations, necessitating enhanced techniques such as enhanced oil recovery (EOR). EOR methods utilize chemical agents, thermal injections, or biotechnological solutions to improve the recovery of this residual oil (Rizvi, 2024). Fungal polysaccharides are particularly promising for EOR applications. These polysaccharides can enhance the viscosity of injected solutions, effectively displacing oil trapped within the pore spaces of rock formations, thus facilitating oil movement toward the extraction wellbore. Additionally, fungal polysaccharides exhibit high stability under extreme temperature and pressure conditions, making them well-suited for EOR in the challenging underground environments of oil reservoirs (Xia et al., 2020). For example, scleroglucan can be modified to acquire hydrophobic properties by grafting stearate groups, along with the addition of ionic-sulfonic groups to enhance the viscosity of the compound. This modification alters the adsorption behavior of scleroglucan on oil reservoir rock surfaces, with greater stearate grafting density resulting in increased adsorption (Bakhshi et al., 2017). Schizophyllan is used in EOR processes and has been shown to increase the recovery of crude oil by up to 28% over the residual oil saturation (Joshi et al., 2016). Additionally, the use of pullulan in microbial-enhanced oil recovery experiments with Berea sandstone core samples resulted in a 9.4% increase in the recovery of medium-heavy oil (Elshafie et al., 2017).
11 Patent search
A search for patents using the keyword “fungal polysaccharides” in the Espacenet database (https://worldwide.espacenet.com, accessed 25 March 2025) reveals significant changes in patent filing trends between 2010 and 2024. The data can be categorized into three periods, as presented in Figure 6A. The number of patent filings increased significantly from approximately 9,972 between 2010 and 2014 to around 12,550 from 2015 to 2019, indicating substantial growth in interest. However, this trend reversed from 2020 to 2024, with filings decreasing to 9,488. This decline may be influenced by factors such as economic conditions, policy shifts, and evolving technological advancements during that period, as well as delays in research and patent applications resulting from the COVID-19 pandemic.
Figure 6. The number of patents (A), the top ten IPC category codes (B), and publication locations (C) between 2010 and 2024 of fungal polysaccharides. The search was conducted using the European database Espacenet (accessed on 25 March 2025).
Based on the International Patent Classification (IPC) codes, the top ten patent classifications for fungal polysaccharides revealed that category A61K31, which covers organic compounds for medicinal and cosmetic preparations, had the highest total with 6,685 patents (Figure 6B). This was followed by category C12N15 (genetic modification, vectors, and genetic material manipulation) with 6,183 patents, C12N1 (microorganisms and biotechnological processes) with 4,880 patents, A61K9 (pharmaceutical formulations) with 4,635 patents, and A61K47 (drug carriers and excipients) with 4,382 patents. Other notable categories included A61P31 (anti-infectives), C12N9 (enzymes and proenzymes), A61P35 (antineoplastic agents), and A61K39 (medicinal preparations containing antigens or antibodies). This indicates that categories involving organic compounds for pharmaceutical and cosmetic formulations were particularly popular, reflecting the strong potential of fungal polysaccharides to meet competitive market demands with value-added products. The top ten countries for fungal polysaccharide-related patent filings are shown in Figure 6C. The majority of patent filings were made through the World Intellectual Property Organization (WIPO) with 18,520 patents, followed by China with 18,137 patents, the United States with 16,038 patents, and the European Patent Office (EU) with 13,325 patents. Japan ranked fifth with 8,912 patents, followed by Canada (7,740 patents), Australia (6,488 patents), South Korea (6,150 patents), Brazil (5,070 patents), and Mexico (4,321 patents), rounding out the list.
12 Conclusions and future perspectives
Fungal polysaccharides are derived from a wide variety of fungal species, including yeasts, filamentous fungi, and mushrooms. The types of polysaccharides vary in composition and structure. To obtain the highest yield of fungal polysaccharides, the processes of fermentation, extraction, precipitation, and purification must be optimized for each fungal species and strain. Each step requires careful consideration.
In recent years, significant advancements have been made in the study of fungal polysaccharides, particularly in their structural characterization, bioactivities, and biotechnological applications. Despite this progress, notable deficiencies remain in our fundamental understanding, especially regarding structure–function and size–function relationships, as well as biosynthetic pathways. Fungal polysaccharides possess diverse biological properties and potential therapeutic benefits. These polysaccharides are used in agriculture, medicine, food, cosmetics, and biotechnology industries. The trend in research on fungal polysaccharides has been growing significantly in recent years. Future research may focus on discovering new fungal species and strains to enhance the optimization of production processes, reduce costs, and support large-scale industrial applications, along with the integration of advanced technologies. Advances in extraction, purification and characterization techniques will further enhance the quality and functional properties of fungal polysaccharides, enabling more precise applications in pharmaceuticals, food, cosmetics, and biotechnology. Moreover, challenges persist in the standardization of extraction and purification methods, which continues to be a major limitation in the field. Although fungal polysaccharides exhibit great potential in medicine and the food industry, their practical applications are still in the experimental and clinical testing stages. Furthermore, continued further research into the biomedical applications of fungal polysaccharides, such as drug delivery systems, immune modulation, and tissue engineering, will expand their impact in healthcare. The biological responses of the human body to fungal polysaccharides are not yet fully understood, highlighting the need for further studies on their safety and health impacts. Finally, to advance both the fundamental understanding and practical applications of fungal polysaccharides, these gaps must be addressed through advanced integrative techniques, including omics (genomics, transcriptomics, proteomics, and metabolomics), CRISPR-based genome editing, bioinformatics, and systems biology, combined with interdisciplinary collaboration.
Author contributions
JK: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Resources, Software. ST: Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing, Data curation. AK: Data curation, Formal Analysis, Validation, Writing – original draft. NS: Data curation, Formal Analysis, Validation, Writing – original draft, Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors sincerely appreciate the financial support provided by Chiang Mai University, Chiang Mai, Thailand.
Acknowledgments
The authors would like to thank Russell Kirk Hollis for his helpful assistance with the English correction.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdala-Díaz, R. T., Casas-Arrojo, V., Castro-Varela, P., Riquelme, C., Carrillo, P., Medina, M.Á., et al. (2024). Immunomodulatory, antioxidant, and potential anticancer activity of the polysaccharides of the fungus Fomitiporia chilensis. Molecules 29, e3628. doi: 10.3390/molecules29153628
Abreu, H., Zavadinack, M., Smiderle, F. R., Cipriani, T. R., Cordeiro, L. M. C., and Iacomini, M. (2021). Polysaccharides from Pleurotus eryngii: Selective extraction methodologies and their modulatory effects on THP-1 macrophages. Carbohyd. Polym. 15, e117177. doi: 10.1016/j.carbpol.2020.117177
Aguiló-Aguayo, I., Walton, J., Viñas, I., and Tiwari, B. K. (2017). Ultrasound assisted extraction of polysaccharides from mushroom by-products. LWT-Food Sci. Technol. 77, 92–99. doi: 10.1016/j.lwt.2016.11.043
Alitongbieke, G., Zhang, X., Zhu, F., Wu, Q., Lin, Z., Li, X., et al. (2024). Glucan from Oudemansiella raphanipes suppresses breast cancer proliferation and metastasis by regulating macrophage polarization and the WNT/β-catenin signaling pathway. J. Cancer 15, 1169–1181. doi: 10.7150/jca.89873
Alves da Cunha, M. A., Turmina, J. A., Ivanov, R. C., Barroso, R. R., Marques, P. T., Fonseca, E. A. I., et al. (2012). Lasiodiplodan, an exocellular (1→6)-β-d-glucan from Lasiodiplodia theobromae MMPI: production on glucose, fermentation kinetics, rheology and anti-proliferative activity. J. Ind. Microbiol. Biot. 39, 1179–1188. doi: 10.1007/s10295-012-1112-2
Amer, M. S., Zaghloul, E. H., and Ibrahim, M. I. A. (2020). Characterization of exopolysaccharide produced from marine-derived Aspergillus terreus SEI with prominent biological activities. Egypt. J. Aquat. Res. 46, 363–369. doi: 10.1016/j.ejar.2020.08.008
American Diabetes Association (2009). Diagnosis and classification of diabetes mellitus. Diabetes Care 32, 62–67. doi: 10.2337/dc09-s062
Anchundia, M., León-Revelo, G., Santacruz, S., and Torres, F. (2024). Polyphasic identification of Rhizopus oryzae and evaluation of physical fermentation parameters in potato starch processing liquid waste for β-glucan production. Sci. Rep. 14, e14913. doi: 10.1038/s41598-024-66000-5
Angelova, G., Brazkova, M., Mihaylova, D., Slavov, A., Petkova, N., Blazheva, D., et al. (2022). Bioactivity of biomass and crude exopolysaccharides obtained by controlled submerged cultivation of medicinal mushroom Trametes versicolor. J. Fungi 8, e738. doi: 10.3390/jof8070738
Angulo, V., Bleichrodt, R., Dijksterhuis, J., Erktan, A., Hefting, M. M., Kraak, B., et al. (2024). Enhancement of soil aggregation and physical properties through fungal amendments under varying moisture conditions. Environ. Microbiol 26, e16627. doi: 10.1111/1462-2920.16627
Antonelli, A., Fry, C., Smith, R. J., Eden, J., Govaerts, R. H. A., Kersey, P., et al. (2023). State of the world’s plants and fungi 2023 (Royal Botanic Gardens, Kew). doi: 10.34885/wnwn-6s63
Ascencio, J. J., Philippini, R. R., Gomes, F. M., Pereira, F. M., da Silva, S. S., Kumar, V., et al. (2021). Comparative highly efficient production of β-glucan by Lasiodiplodia theobromae CCT 3966 and its multiscale characterization. Fermentation 7, e108. doi: 10.3390/fermentation7030108
Ashfaque, A. K., Shahzor, G. K., Ying, L., Saghir, A. S., Yan-Feng, W., Aijaz, H. S., et al. (2017). Optimization of enzyme assisted extraction of polysaccharides from Poria cocos. J. Med. Plant Res. 11, 331–337. doi: 10.5897/jmpr2017.6366
Ayimbila, F. and Keawsompong, S. (2023). Nutritional quality and biological application of mushroom protein as a novel protein alternative. Curr. Nutr. Rep. 12, 290–307. doi: 10.1007/s13668-023-00468-x
Ayimbila, F., Siriwong, S., and Keawsompong, S. (2021). Structural characteristics and bioactive properties of water-soluble polysaccharide from Lentinus squarrosulus. Bioact. Carbohydr. Diet. Fibre. 26, e100266. doi: 10.1016/j.bcdf.2021.100266
Azuar Mohamad, S., Abd Halim, N. H., Keong, C. Y., Mohd Dzomir, A. Z., Yazid, H., Hamzah, M. Y., et al. (2018). Local β-glucan source from oyster mushroom (Pleurotus flabellatus) as potential usage in medical applications. Front. Pharmacol. 9, e112.
Backhaus, K., Heilmann, C. J., Sorgo, A. G., Purschke, G., de Koster, C. G., Klis, F. M., et al. (2010). A systematic study of the cell wall composition of Kluyveromyces lactis. Yeast 27, 647–660. doi: 10.1002/yea.1781
Bai, L., Xu, D., Zhou, Y. M., Zhang, Y.-B., Zhang, H., Chen, Y.-B., et al. (2022). Antioxidant activities of natural polysaccharides and their derivatives for biomedical and medicinal applications. Antioxidants 11, e2491. doi: 10.3390/antiox11122491
Bains, A., Sridhar, K., Kaushik, R., Chawla, P., and Sharma, M. (2023). Enzyme-assisted polysaccharides extraction from Calocybe indica: Synergistic antibiofilm and oxidative stability of essential oil nanoemulsion. Int. J. Biol. Macromol. 242, e124843. doi: 10.1016/j.ijbiomac.2023.124843
Bakhshi, M., Ozeiri, M., Sharif, A., and Aalaie, J. (2017). Effect of hydrophobic modification on the structure and rheology of aqueous and brine solutions of scleroglucan polymer. Korean J. Chem. Eng. 34, 903–912. doi: 10.1007/s11814-016-0322-0
Bakratsas, G., Polydera, A., Katapodis, P., and Stamatis, H. (2021). Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods. 4, e100086. doi: 10.1016/j.fufo.2021.100086
Bakratsas, G., Tsoumanis, C., Stamatis, H., and Katapodis, P. (2024). Exopolysaccharide production in submerged fermentation of Pleurotus ostreatus under red and green light. Fermentation 10, e313. doi: 10.3390/fermentation10060313
Balasso, A., Salmaso, S., Pontisso, P., Rosato, A., Quarta, S., Malfanti, A., et al. (2017). Re-programming pullulan for targeting and controlled release of doxorubicin to the hepatocellular carcinoma cells. Eur. J. Pharm. Sci. 103, 104–115. doi: 10.1016/j.ejps.2017.02.016
Barcan, A. S., Barcan, R. A., and Vamanu, E. (2024). Therapeutic potential of fungal polysaccharides in gut microbiota regulation: implications for diabetes, neurodegeneration, and oncology. J. Fungi 10, e394. doi: 10.3390/jof10060394
Bentil, J. A., Thygesen, A., Mensah, M., Lange, L., and Meyer, A. S. (2018). Cellulase production by white-rot basidiomycetous fungi: solid-state versus submerged cultivation. Appl. Microbiol. Biotechnol. 102, 5827–5839. doi: 10.1007/s00253-018-9072-8
Bhotmange, D. U., Wallenius, J. H., Singhal, R. S., and Shamekh, S. S. (2017). Enzymatic extraction and characterization of polysaccharide from Tuber aestivum. Bioact. Carbohydr. Diet. Fibre. 10, 1–9. doi: 10.1016/j.bcdf.2017.02.001
Bleha, R., Třešnáková, L., Sushytskyi, L., Capek, P., Čopíková, J., Klouček, P., et al. (2022). Polysaccharides from basidiocarps of the polypore fungus Ganoderma resinaceum: isolation and structure. Polym. 14, 255. doi: 10.3390/polym14020255
Brundrett, M. C. (2002). Coevolution of roots and mycorrhizas of land plants. New Phytal. 154, 275–304. doi: 10.1046/j.1469-8137.2002.00397.x
Bzducha-Wróbel, A., Farkaš, P., Bieliková, S., Čížová, A., and Sujkowska-Rybkowska, M. (2024). How do the carbon and nitrogen sources affect the synthesis of β-(1,3/1,6)-glucan, its structure and the susceptibility of Candida utilis yeast cells to immunolabelling with β-(1,3)-glucan monoclonal antibodies? Microb. Cell Fact. 23, 28. doi: 10.1186/s12934-024-02305-4
Cazón, P., Velazquez, G., Ramírez, J. A., and Vázquez, M. (2017). Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 68, 136–148. doi: 10.1016/j.foodhyd.2016.09.009
Cerletti, C., Esposito, S., and Iacoviello, L. (2021). Edible mushrooms and beta-glucans: impact on human health. Nutrients. 13, e2195. doi: 10.3390/nu13072195
Charoux, C. M. G., Ojha, K. S., O’Donnell, C. P., Cardoni, A., and Tiwari, B. K. (2017). Applications of airborne ultrasonic technology in the food industry. J. Food Eng. 208, 28–36. doi: 10.1016/j.jfoodeng.2017.03.030
Chemat, F., Rombaut, N., Sicaire, A.-G., Meullemiestre, A., Fabiano-Tixier, A.-S., and Abert-Vian, M. (2017). Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 34, 540–560. doi: 10.1016/j.ultsonch.2016.06.035
Chen, Y., Huang, Y., Cui, Z., and Liu, J. (2015). Purification, characterization and biological activity of a novel polysaccharide from Inonotus obliquus. Int. J. Biol. Macromol. 79, 587–594. doi: 10.1016/j.ijbiomac.2015.05.016
Chen, Y. F., Jiang, W. W., Zhang, S. Q., Kan, J. Q., and Liang, Y. (2016). Antioxidant activity and characterization of one new polysaccharide obtained from Perigord Truffle (Tuber huidongense). Evid. Based Complementary Altern. Med. 2016, 3537193. doi: 10.1155/2016/3537193
Chen, J., Lai, P., Shen, H., Zhen, H., and Fang, R. (2013). Effect of extraction methods on polysaccharide of Clitocybe maxima Stipe. Adv. J. Food Sci. Technol. 5, 370–373. doi: 10.19026/ajfst.5.3273
Chen, Y., Liu, Y., Sarker, M. M. R., Yan, X., Yang, C., Zhao, L., et al. (2018). Structural characterization and antidiabetic potential of a novel heteropolysaccharide from Grifola frondosa via IRS1/PI3K-JNK signaling pathways. Carbohydr. Polym. 198, 452–461. doi: 10.1016/j.carbpol.2018.06.077
Chen, Y., Liu, D., Wang, D., Lai, S., Zhong, R., Liu, Y., et al. (2019). Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem. Toxicol. 126, 295–302. doi: 10.1016/j.fct.2019.02.034
Chen, Z., Yin, C., Fan, X., Ma, K., Yao, F., Zhou, R., et al. (2020). Characterization of physicochemical and biological properties of Schizophyllum commune polysaccharide extracted with different methods. Int. J. Biol. Macromol. 156, 1425–1434. doi: 10.1016/j.ijbiomac.2019.11.183
Chen, J., Yu, M., Yang, C., Huang, Z., He, L., Bian, J., et al. (2024). Study on the nutritional relationships in mycelia and fruiting bodies of Hypsizygus marmoreus under defined nutrient conditions. Food Chem. 467, 142323. doi: 10.1016/j.foodchem.2024.142323
Cheng, K. C., Demirci, A., and Catchmark, J. M. (2011). Pullulan: biosynthesis, production, and applications. Appl. Microbiol. Biotechnol. 92, 29–44. doi: 10.1007/s00253-011-3477-y
Cheng, B., Yu, K., Weng, X., Liu, Z., Huang, X., Jiang, Y., et al. (2024). Impact of cell wall polysaccharide modifications on the performance of Pichia pastoris: novel mutants with enhanced fitness and functionality for bioproduction applications. Microb. Cell Fact. 23, e55. doi: 10.1186/s12934-024-02333-0
Chioru, A. and Chirsanova, A. (2023). β-Glucans: characterization, extraction methods, and valorization. Food Nutr. Sci. 14, 963–983. doi: 10.4236/fns.2023.1410061
Christyani, G., Carswell, M., Qin, S., and Kim, W. (2024). An overview of advances in rare cancer diagnosis and treatment. Int. J. Mol. Sci. 25, e1201. doi: 10.3390/ijms25021201
Chuensun, T., Chewonarin, T., Laopajon, W., Kawee-ai, A., Pinpart, P., and Utama-ang, N. (2021). Comparative evaluation of physicochemical properties of Lingzhi (Ganoderma lucidum) as affected by drying conditions and extraction methods. Int. J. Food Sci. Technol. 56, 2751–2759. doi: 10.1111/ijfs.14906
Costa, O. Y. A., Raaijmakers, J. M., and Kuramae, E. E. (2018). Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 9, e1636. doi: 10.3389/fmicb.2018.01636
Cui, F. J., Qian, L. S., Sun, W. J., Zhang, J. S., Yang, Y., Li, N., et al. (2018). Ultrasound-assisted extraction of polysaccharides from Volvariella volvacea: Process optimization and structural characterization. Molecules 23, e1706. doi: 10.3390/molecules23071706
Dai, Y., Wang, L., Chen, X., Song, A., He, L., Wang, L., et al. (2023). Lentinula edodes Sing polysaccharide: extraction, characterization, bioactivities, and emulsifying applications. Foods 12, e3289. doi: 10.3390/foods12173289
Daynes, C. N., Zhang, N., Saleeba, J. A., and McGee, P. A. (2012). Soil aggregates formed in vitro by saprotrophic Trichocomaceae have transient water-stability. Soil Biol. Biochem. 48, 151–161. doi: 10.1016/j.soilbio.2012.01.010
De Costa, G. and Park, A. (2017). Hyperlipidaemia. Medicine 45, 579–582. doi: 10.1016/j.mpmed.2017.06.002
De Felice, B., Damiano, S., Montanino, C., Del Buono, A., La Rosa, G., Guida, B., et al. (2020). Effect of beta- and alpha-glucans on immune modulating factors expression in enterocyte-like Caco-2 and goblet-like LS 174T cells. Int. J. Biol. Macromol. 153, 600–607. doi: 10.1016/j.ijbiomac.2020.03.046
Delzenne, N. M. and Bindels, L. B. (2015). Gut microbiota: Ganoderma lucidum, a new prebiotic agent to treat obesity?: Gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 12, 553–554. doi: 10.1038/nrgastro.2015.137
Deng, Y., Huang, Q., Hu, L., Liu, T., Zheng, B., Lu, D., et al. (2021). Enhanced exopolysaccharide yield and antioxidant activities of Schizophyllum commune fermented products by the addition of Radix Puerariae. RSC Adv. 11, 38219–38234. doi: 10.1039/d1ra06314f
Deng, Q., Zinoviadou, K. G., Galanakis, C. M., Orlien, V., Grimi, N., Vorobiev, E., et al. (2015). The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: extraction, degradation, and applications. Food Eng. Rev. 7, 357–381. doi: 10.1007/s12393-014-9104-9
Deveci, E., Tel-Cayan, G., Altinok, B. Y., and Aktas, S. (2024). Characterization of polysaccharide extracts of four edible mushrooms and determination of in vitro antioxidant, enzyme inhibition and anticancer activities. ACS Omega 9, 25887–25901. doi: 10.1021/acsomega.4c00322
Di Martino, P. (2022). Antimicrobial agents and microbial ecology. AIMS Microbiol. 8, 1–4. doi: 10.3934/microbiol.2022001
Dobšíková, R., Blahová, J., Mikulíková, I., Modrá, H., Prášková, E., Svobodová, Z., et al. (2013). The effect of oyster mushroom β-1.3/1.6-D-glucan and oxytetracycline antibiotic on biometrical, haematological, biochemical, and immunological indices, and histopathological changes in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 35, 1813–1823. doi: 10.1016/j.fsi.2013.09.006
Dong, X., Sun, S., Wang, X., Yu, H., Dai, K., Jiao, J., et al. (2024). Structural characteristics and intestinal flora metabolism mediated immunoregulatory effects of Lactarius deliciosus polysaccharide. Int. J. Biol. Macromol. 278, e135063. doi: 10.1016/j.ijbiomac.2024.135063
Dong, Y., Zhang, J., Gao, Z., Zhao, H., Sun, G., Wang, X., et al. (2019). Characterization and anti-hyperlipidemia effects of enzymatic residue polysaccharides from Pleurotus ostreatus. Int. J. Biol. Macromol. 129, 316–325. doi: 10.1016/j.ijbiomac.2019.01.164
Dornelles, G., Araújo, G. R., de, S., Rodrigues, M., Alves, V., Almeida-Paes, R., et al. (2023). Comparative analysis of capsular and secreted polysaccharides produced by Rhodotorula mucilaginosa and Cryptococcus neoformans. J. Fungi 9, e1124. doi: 10.3390/jof9111124
Duobin, M., Yuping, M., Lujing, G., Aijing, Z., Jianqiang, Z., and Chunping, X. (2013). Fermentation characteristics in stirred-tank reactor of exopolysaccharides with hypolipidemic activity produced by Pleurotus geesteranus 5#. An. Acad. Bras. Cienc. 85, 1473–1481. doi: 10.1590/0001-3765201320130037
Elisashvili, V. (2012). Submerged cultivation of medicinal mushrooms: Bioprocesses and products (review). Int. J. Med. Mushrooms 14, 211–239. doi: 10.1615/intjmedmushr.v14.i3.10
Elisashvili, V. I., Kachlishvili, E. T., and Wasser, S. P. (2009). Carbon and nitrogen source effects on basidiomycetes exopolysaccharide production. Appl. Biochem. Microbiol. 45, 531–535. doi: 10.1134/s0003683809050135
El-Mahdy, O. M., Mohamed, H. I., and El-Ansary, A. E. (2023). Optimizations of exopolysaccharide production by Fusarium nygamai strain AJTYC1 and its potential applications as an antioxidant, antimicrobial, anticancer, and emulsifier. BMC Microbiol. 23, e345. doi: 10.1186/s12866-023-03100-8
Elshafie, A., Joshi, S. J., Al-Wahaibi, Y. M., Al-Bahry, S. N., Al-Bemani, A. S., Al-Hashmi, A., et al. (2017). Isolation and characterization of biopolymer producing Omani Aureobasidium pullulans strains and its potential applications in microbial enhanced oil recovery. Soc Pet. Eng. 2017, 583–593. doi: 10.2118/185326-MS
Ezike, T. C., Okpala, U. S., Onoja, U. L., Nwike, C. P., Ezeako, E. C., Okpara, O. J., et al. (2023). Advances in drug delivery systems, challenges and future directions. Heliyon 9, e17488. doi: 10.1016/j.heliyon.2023.e17488
Fellak, S., Rafik, M., Haidara, H., Taybi, H., Boukir, A., and Lhassani, A. (2022). Scanning electron microscopy examination of the surface of softwood attacked by fungus. MATEC Web Conf. 360, e00007. doi: 10.1051/matecconf/202236000007
Fernando, L. D., Dickwella Widanage, M. C., Penfield, J., Lipton, A. S., Washton, N., Latgé, J. P., et al. (2021). Structural polymorphism of chitin and chitosan in fungal cell walls from solid-state NMR and principal component analysis. Front. Mol. Biosci. 8, e727053. doi: 10.3389/fmolb.2021.727053
Ferraboschi, P., Ciceri, S., and Grisenti, P. (2021). Applications of lysozyme, an innate immune defense factor, as an alternative antibiotic. Antibiotics 10, e1534. doi: 10.3390/antibiotics10121534
Figueroa, G. M., Costa, D. N., Miranda, A. C. A., Junior, G. L. V., Mendes, T. P. S., and Cedro, P. E. P. (2023). Fungal polysaccharides and their hypoglycemic potential to act as an adjuvant in the treatment of diabetes mellitus. Evidência 23, 47–62. doi: 10.18593/evid.32561
Fonseca, P. R. M. S., Dekker, R. F. H., Barbosa, A. M., Silveira, J. L. M., Vasconcelos, A. F. D., Monteiro, N. K., et al. (2011). Thermal and rheological properties of a family of botryosphaerans produced by Botryosphaeria rhodina MAMB-05. Molecules 16, 7488–7501. doi: 10.3390/molecules16097488
Fosmer, A. and Gibbons, W. (2011). Separation of scleroglucan and cell biomass from Sclerotium glucanicum grown in an inexpensive, by-product based medium. Int. J. Agric. Biol. Eng. 4, e52. doi: 10.3965/j.issn.1934-6344.2011.01.0-0
Fu, Y., Shi, L., and Ding, K. (2019). Structure elucidation and anti-tumor activity in vivo of a polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Int. J. Biol. Macromol. 141, 693–699. doi: 10.1016/j.ijbiomac.2019.09.046
Fu, Y., Wang, L., Jiang, G., Ren, L., Wang, L., and Liu, X. (2022). Anti-diabetic activity of polysaccharides from Auricularia cornea var. Li. Food 11, e1464. doi: 10.3390/foods11101464
Gao, L., Wang, Y., Zhang, F., Li, S., Zhao, J., Zhang, Q., et al. (2022). A standardized method for the quantification of polysaccharides: An example of polysaccharides from Tremella fuciformis. LWT- Food Sci. Technol. 167, e113860. doi: 10.1016/j.lwt.2022.113860
Gao, Y. Y., Zhou, Y. H., Liu, X. P., Di, B., He, J. Y., Wang, Y. T., et al. (2024). Ganoderma lucidum polysaccharide promotes broiler health by regulating lipid metabolism, antioxidants, and intestinal microflora. Int. J. Biol. Macromol. 280, e135918. doi: 10.1016/j.ijbiomac.2024.135918
Gbashi, S., Adebo, O. A., Piater, L., Madala, N. E., and Njobeh, P. B. (2017). Subcritical water extraction of biological materials. Sep. Purif. Rev. 46, 21–34. doi: 10.1080/15422119.2016.1170035
Ge, Y., Qiu, H., and Zheng, J. (2022). Physicochemical characteristics and anti-hyperlipidemic effect of polysaccharide from BaChu mushroom (Helvella leucopus). Food Chem. 15, e100443. doi: 10.1016/j.fochx.2022.100443
Gehrcke, M., Martins, C. C., de Bastos Brum, T., da Rosa, L. S., Luchese, C., Wilhelm, E. A., et al. (2022). Novel pullulan/gellan gum bilayer film as a vehicle for silibinin-loaded nanocapsules in the topical treatment of atopic dermatitis. Pharmaceutics 14, e2352. doi: 10.3390/pharmaceutics14112352
Geraldelli, D., Ribeiro, M. C., Medeiros, T. C., Comiran, P. K., Martins, K. O., Oliveira, M. F., et al. (2020). Botryosphaeran, a (1→3)(1→6)-β-D-glucan, reduces tumor development and cachexia syndrome in obese male rats by increasing insulin sensitivity and FOXO3a activity. Int. J. Biol. Macromol. 165, 985–994. doi: 10.1016/j.ijbiomac.2020.09.168
Ghosh, T., Priyadarshi, R., Krebs de Souza, C., Angioletti, B. L., and Rhim, J. W. (2022). Advances in pullulan utilization for sustainable applications in food packaging and preservation: A mini-review. Trends Food Sci. Technol. 125, 43–53. doi: 10.1016/j.tifs.2022.05.001
Giannenas, I., Tsalie, E., Chronis, E., Mavridis, S., Tontis, D., and Kyriazakis, I. (2011). Consumption of Agaricus bisporus mushroom affects the performance, intestinal microbiota composition and morphology, and antioxidant status of Turkey poults. Anim. Feed Sci. Technol. 165, 218–229. doi: 10.1016/j.anifeedsci.2011.03.002
Gientka, I., Bzducha-Wróbel, A., Stasiak-Różańska, L., Bednarska, A. A., and Błażejak, S. (2016). The exopolysaccharides biosynthesis by Candida yeast depends on carbon sources. Electron. J. Biotechnol. 22, 31–37. doi: 10.1016/j.ejbt.2016.02.008
Gil-Ramírez, A., Smiderle, F. R., Morales, D., Iacomini, M., and Soler-Rivas, C. (2019). Strengths and weaknesses of the aniline-blue method used to test mushroom (1→3)-β-d-glucans obtained by microwave-assisted extractions. Carbohydr. Polym. 217, 135–143. doi: 10.1016/j.carbpol.2019.04.051
Gniewosz, M., Pobiega, K., Kraśniewska, K., Synowiec, A., Chaberek, M., and Galus, S. (2022). Characterization and antifungal activity of pullulan edible films enriched with Propolis extract for active packaging. Foods 11, 2319. doi: 10.3390/foods11152319
Golovchenko, V. V., Naranmandakh, S., Ganbaatar, J., Prilepskii, A. Y., Burygin, G. L., Chizhov, A. O., et al. (2020). Structural investigation and comparative cytotoxic activity of water-soluble polysaccharides from fruit bodies of the medicinal fungus quinine conk. Phytochemistry 175, e112313. doi: 10.1016/j.phytochem.2020.112313
Gonçalves, B. C., Lopes Barbosa, M. G., Silva Olak, A. P., Belebecha Terezo, N., Nishi, L., Watanabe, M. A., et al. (2021). Antiviral therapies: advances and perspectives. Fundam. Clin. Pharmacol. 35, 305–320. doi: 10.1111/fcp.12609
Gong, P., Long, H., Guo, Y., Wang, S., Chen, F., and Chen, X. (2022). Isolation, structural characterization, and hypoglycemic activities in vitro of polysaccharides from Pleurotus eryngii. Molecules 27, e7140. doi: 10.3390/molecules27207140
Govindan, S., Shanmugam, J., Rajendran, G., Ramani, P., Unni, D., Venkatachalam, B., et al. (2023). Antidiabetic activity of polysaccharide from Hypsizygus ulmarius in streptozotocin-nicotinamide induced diabetic rats. Bioact. Carbohydr. Diet. Fibre 29, 100350. doi: 10.1016/j.bcdf.2023.100350
Grimm, D. and Wösten, H. A. B. (2018). Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 102, 7795–7803. doi: 10.1007/s00253-018-9226-8
Guo, C., Guo, D., Fang, L., Sang, T., Wu, J., Guo, C., et al. (2021). Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 267, e118231. doi: 10.1016/j.carbpol.2021.118231
Guo, M. Q., Hu, X., Wang, C., and Ai, L. (2017). “Polysaccharides: structure and solubility,” in Solubility of polysaccharides. Ed. Xu, Z. (InTech, London, UK). doi: 10.5772/intechopen.71570
Guo, L., Yao, Q., Lv, J., Li, Z., Wang, L. A., and Zhang, J. (2024a). Anti-hyperglycemic effect of the brown slime cap mushroom Chroogomphus rutilus (Agaricomycetes) crude polysaccharide in vitro and in vivo. Int. J. Med. Mushrooms 26, 1–12. doi: 10.1615/IntJMedMushrooms.2024053173
Guo, W., Yun, J., Wang, B., Xu, S., Ye, C., Wang, X., et al. (2024b). Comparative study on physicochemical properties and hypoglycemic activities of intracellular and extracellular polysaccharides from submerged fermentation of Morchella esculenta. Int. J. Biol. Macromol. 278, e134759. doi: 10.1016/j.ijbiomac.2024.134759
Habibi, E., Hemmati, P., Arabnozari, H., Khalili, H. A., Sharifianjazi, F., Enderami, S. E., et al. (2024). Phytochemical analysis and immune-modulatory potential of Trichaptum biforme polysaccharides: Implications for cancer. Int. J. Biol. Macromol. 280, e135691. doi: 10.1016/j.ijbiomac.2024.135691
Hamidi, M., Okoro, O. V., Milan, P. B., Khalili, M. R., Samadian, H., Nie, L., et al. (2022). Fungal exopolysaccharides: properties, sources, modifications, and biomedical applications. Carbohydr. Polym. 284, e119152. doi: 10.1016/j.carbpol.2022.119152
Hao, H., Cui, C., Xing, Y., Jia, X., Ma, B., Kang, W., et al. (2023a). Sulfation of the extracellular polysaccharide from the edible fungus Stropharia rugosoannulata with its antioxidant activity. J. Future Foods 3, 37–42. doi: 10.1016/j.jfutfo.2022.09.006
Hao, L., Sheng, Z., Lu, J., Tao, R., and Jia, S. (2016). Characterization and antioxidant activities of extracellular and intracellular polysaccharides from Fomitopsis pinicola. Carbohydr. Polym 141, 54–59. doi: 10.1016/j.carbpol.2015.11.048
Hao, R., Zhou, X., Zhao, X., Lv, X., Zhu, X., Gao, N., et al. (2023b). Flammulina velutipes polysaccharide counteracts cadmium-induced gut injury in mice via modulating gut inflammation, gut microbiota and intestinal barrier. Sci. Total Environ. 877, e162910. doi: 10.1016/j.scitotenv.2023.162910
He, J., Liu, Z., Jiang, W., Zhu, T., Wusiman, A., Gu, P., et al. (2020b). Immune-adjuvant activity of lentinan-modified calcium carbonate microparticles on a H5N1 vaccine. Int. J. Biol. Macromol. 163, 1384–1392. doi: 10.1016/j.ijbiomac.2020.08.005
He, B. L., Zheng, Q. W., Guo, L. Q., Huang, J. Y., Yun, F., Huang, S. S., et al. (2020a). Structural characterization and immune-enhancing activity of a novel high-molecular-weight polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 145, 11–20. doi: 10.1016/j.ijbiomac.2019.12.115
Herrero, M., Cifuentes, A., and Ibanez, E. (2006). Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae A review. Food Chem. 98, 136–148. doi: 10.1016/j.foodchem.2005.05.058
Hoa, T. Q. (2018). Extraction of polysaccharides from Lingzhi by ultrasonic-assisted enzymatic method. Vietnam J. Sci. Technol. 56, e171. doi: 10.15625/2525-2518/56/4a/12879
Hong, T., Yin, J. Y., Nie, S. P., and Xie, M. Y. (2021). Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem.: X 12, e100168. doi: 10.1016/j.fochx.2021.100168
Hsu, K. D., Wu, S. P., Lin, S. P., Lum, C. C., and Cheng, K. C. (2017). Enhanced active extracellular polysaccharide production from Ganoderma formosanum using computational modeling. J. Food Drug Anal. 25, 804–811. doi: 10.1016/j.jfda.2016.12.006
Hu, Q., He, Y., Wang, F., Wu, J., Ci, Z., Chen, L., et al. (2021). Microwave technology: a novel approach to the transformation of natural metabolites. Chin. Med. 16, e87. doi: 10.1186/s13020-021-00500-8
Hu, Y., Xu, J., Sheng, Y., Liu, J., Li, H., Guo, M., et al. (2022a). Pleurotus ostreatus ameliorates obesity by modulating the gut microbiota in obese mice induced by high-fat diet. Nutrients 14, e1868. doi: 10.3390/nu14091868
Hu, Z., Yu, R., Sun, J., Duan, Y., Zhou, H., Zhou, W., et al. (2022b). Static decolorization of polysaccharides from the leaves of Rhododendron dauricum: Process optimization, characterization and antioxidant activities. Proc. Biochem. 121, 113–125. doi: 10.1016/j.procbio.2022.06.025
Huang, Z. F., Zhang, M. L., Zhang, S., Wang, Y. H., and Jiang, X. W. (2018). Structural characterization of polysaccharides from Cordyceps militaris and their hypolipidemic effects in high fat diet fed mice. RSC Adv. 8, 41012–41022. doi: 10.1039/c8ra09068h
Huber, V., Muller, L., Degot, P., Touraud, D., and Kunz, W. (2021). NADES-based surfactant-free microemulsions for solubilization and extraction of curcumin from Curcuma Longa. Food Chem. 355, e129624. doi: 10.1016/j.foodchem.2021.129624
Hwang, A. Y., Yang, S. C., Kim, J., Lim, T., Cho, H., and Hwang, K. T. (2019). Effects of non-traditional extraction methods on extracting bioactive compounds from chaga mushroom (Inonotus obliquus) compared with hot water extraction. LWT-Food Sci. Technol. 110, 80–84. doi: 10.1016/j.lwt.2019.04.073
Ina, K., Furuta, R., Kataoka, T., Kayukawa, S., Yoshida, T., Miwa, T., et al. (2011). Lentinan prolonged survival in patients with gastric cancer receiving S-1-based chemotherapy. World J. Clin. Oncol. 2, 339–343. doi: 10.5306/wjco.v2.i10.339
Ingale, A., Wadher, D. S. J., Bagul, S., Gujrati, P., and Govande, A. (2023). High performance liquid chromatography: An overview. Int. J. Pharm. Sci. Rev. Res. 3, 18–24. doi: 10.47583/ijpsrr.2023.v82i02.003
Jafarinejad, S. (2017). “Chapter 7,” in Petroleum waste treatment and pollution control (Butterworth Heinemann, Amsterdam, Netherlands), 269–270.
Jayus, J., Akroman, R., Nugraha, A. S., Piluharto, B., and Seviour, R. J. (2021). Structural elucidation of the exopolysaccharide produced by Curvularia lunata isolate RJ01. Biodiversitas 22, 2699–2705. doi: 10.13057/biodiv/d220530
Jen, C. I., Lu, M. K., Lai, M. N., and Ng, L. T. (2024). Sulfated polysaccharides of Laetiporus sulphureus fruiting bodies exhibit anti-breast cancer activity through cell cycle arrest, apoptosis induction, and inhibiting cell migration. J. Ethnopharmacol. 321, e117546. doi: 10.1016/j.jep.2023.117546
Jeong, S. C., Koyyalamudi, S. R., Hughes, J., Khoo, C., Bailey, T., Marripudi, K., et al. (2013). Antioxidant and immunomodulating activities of exo-and endopolysaccharide fractions from submerged mycelia cultures of culinary-medicinal mushrooms. Int. J. Med. Mushrooms 15, 251–266. doi: 10.1615/intjmedmushr.v15.i3.30
Jesenak, M., Urbancek, S., Majtan, J., Banovcin, P., and Hercogova, J. (2016). β-Glucan-based cream (containing pleuran isolated from Pleurotus ostreatus) in supportive treatment of mild-to-moderate atopic dermatitis. J. Dermatolog. Treat. 27, 351–354. doi: 10.3109/09546634.2015.1117565
Ji, X., Guo, J., Cao, T., Zhang, T., Liu, Y., and Yan, Y. (2023). Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Wellness 12, 1969–1980. doi: 10.1016/j.fshw.2023.03.017
Jiang, X., Hao, J., Zhu, Y., Liu, Z., Li, L., Zhou, Y., et al. (2022). The anti-obesity effects of a water-soluble glucan from Grifola frondosa via the modulation of chronic inflammation. Front. Immunol. 3, e962341. doi: 10.3389/fimmu.2022.962341
Jiao, F., Li, D., Yang, S., Zhang, J., Zhang, C., and Jia, L. (2018). Inhibition effects of polysaccharides on HBV replication and cell proliferation from Lentinus edodes waste material. Micro. Pathog. 123, 461–466. doi: 10.1016/j.micpath.2018.08.004
Jin, J., Nguyen, T. T. H., Kim, C., and Kim, D. (2019). Antimelanogenesis effects of fungal exopolysaccharides prepared from submerged culture of Fomitopsis castanea mycelia. J. Microbiol. Biotechnol. 29, 1204–1211. doi: 10.4014/jmb.1905.05037
Jin, X. and Zhao, S. (2014). Extraction optimization and bioactivities of an extracellular polysaccharide produced by Aspergillus fumigatus. Int. J. Biol. Macromol. 68, 13–17. doi: 10.1016/j.ijbiomac.2014.04.024
Jindal, N. and Singh Khattar, J. (2018). “Microbial polysaccharides in food industry”, in Biopolymers for Food Design, e.d. Grumezescu, A. M. and Holban, A. M. (Cambridge: Elsevier Inc.), 95–123. doi: 10.1016/B978-0-12-811449-0.00004-9
Joshi, S. J., Al-Wahaibi, Y. M., Al-Bahry, S., Elshafie, A., Al-Bemani, A. S., Al-Hashmi, A., et al. (2016). Production and application of schizophyllan in microbial enhanced heavy oil recovery. SPE EOR Conference at Oil and Gas West Asia. Oman: Society of Petroleum Engineers.
Jovanović, J. A., Mihailović, M., Uskoković, A., Grdović, N., Dicnić, S., and Vidaković, M. (2021). The effects of major mushroom bioactive compounds on mechanisms that control blood glucose level. J. Fungi 7, e58. doi: 10.3390/jof7010058
Junior Letti, L. A., Destéfanis Vítola, F. M., Vinícius de Melo Pereira, G., Karp, S. G., Pedroni Medeiros, A. B., Ferreira da Costa, E. S., et al. (2018). “Solid-state fermentation for the production of mushrooms,” in Current developments in biotechnology and bioengineering (Elsevier, Amsterdam, The Netherlands), 285–318. doi: 10.1016/B978-0-444-63990-5.00014-1
Kadooka, C., Tanaka, Y., Hira, D., Maruyama, J.-I., Goto, M., and Oka, T. (2023). Identification of galactofuranose antigens such as galactomannoproteins and fungal-type galactomannan from the yellow koji fungus (Aspergillus oryzae). Front. Microbiol. 14, e1110996. doi: 10.3389/fmicb.2023.1110996
Kang, J. Y., Lee, B., Kim, C. H., Choi, J. H., and Kim, M. S. (2022). Enhancing the prebiotic and antioxidant effects of exopolysaccharides derived from Cordyceps militaris by enzyme-digestion. LWT-Food Sci. Technol. 167, e113830. doi: 10.1016/j.lwt.2022.113830
Kang, J. H., Tachibana, Y., Kamata, W., Mahara, A., Harada-Shiba, M., and Yamaoka, T. (2010). Liver-targeted siRNA delivery by polyethylenimine (PEI)-pullulan carrier. Bioorg. Med. Chem. 18, 3946–3950. doi: 10.1016/j.bmc.2010.04.031
Kanlayavattanakul, M. and Lourith, N. (2023). Cordyceps militaris polysaccharides: preparation and topical product application. Fungal Biol. Biotechnol. 10, e3. doi: 10.1186/s40694-023-00150-5
Kanwal, S., Aliya, S., and Xin, Y. (2020). Anti-obesity effect of Dictyophora indusiate mushroom polysaccharide (DIP) in high fat diet-induced obesity via regulating inflammatory cascades and intestinal microbiome. Front. Endocrinol. 11, e558874. doi: 10.3389/fendo.2020.558874
Karim, A., Gerliani, N., and Aïder, M. (2020). Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 333, e108818. doi: 10.1016/j.ijfoodmicro.2020.108818
Kawahara, H., Matsuda, Y., Sakaguchi, T., Arai, N., and Koide, Y. (2016). Antifreeze activity of xylomannan from the mycelium and fruit body of Flammulina velutipes. Biocontrol Sci. 21, 153–159. doi: 10.4265/bio.21.153
Ke, L. Q. (2015). Optimization of ultrasonic extraction of polysaccharides from Lentinus edodes based on enzymatic treatment: Extraction of Lentinus edodes polysaccharides. J. Food Proces. Preserv. 39, 254–259. doi: 10.1111/jfpp.12228
Khan, A. A., Gani, A., Khanday, F. A., and Masoodi, F. A. (2018). Biological and pharmaceutical activities of mushroom β-glucan discussed as a potential functional food ingredient. Bioact. Carbohydr. Diet. Fibre 16, 1–13. doi: 10.1016/j.bcdf.2017.12.002
Khatua, S. and Acharya, K. (2017). Alkaline extractive crude polysaccharide from Russula senecis possesses antioxidant potential and stimulates innate immunity response. J. Pharm. Pharmacol. 69, 1817–1828. doi: 10.1111/jphp.12813
Khoury, Z. H., Vila, T., Puthran, T. R., Sultan, A. S., Montelongo-Jauregui, D., Melo, M. A. S., et al. (2020). The role of Candida albicans secreted polysaccharides in augmenting streptococcus mutans adherence and mixed biofilm formation: in vitro and in vivo studies. Front. Microbiol. 11, e307. doi: 10.3389/fmicb.2020.00307
Kizitska, T., Barshteyn, V., Sevindik, M., and Krupodorova, T. (2024). Evaluation of Fomitopsis betulina strains for growth on different media and exopolysaccharide production. Arch. Biol. Sci. 76, 257–265. doi: 10.2298/ABS240523018K
Larrañaga-Ordaz, D., Martínez-Maldonado, M., Millán-Chiu, B. E., Fernández, F., Castaño-Tostado, E., Gómez-Lim, M.Á., et al. (2022). Effect of shock waves on the growth of Aspergillus niger conidia: Evaluation of germination and preliminary study on gene expression. J. Fungi 8, e1117. doi: 10.20944/preprints202208.0455.v1
Lazić, V., Klaus, A., Kozarski, M., Doroški, A., Tosti, T., Simić, S., et al. (2024). The effect of green extraction technologies on the chemical composition of medicinal Chaga mushroom extracts. J. Fungi 10, e225. doi: 10.3390/jof10030225
Leong, Y. K., Yang, F.-C., and Chang, J.-S. (2021). Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 251, e117006. doi: 10.1016/j.carbpol.2020.117006
Leung, P. H., Zhao, S., Ho, K. P., and Wu, J. Y. (2009). Chemical properties and antioxidant activity of exopolysaccharides from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 114, 1251–1256. doi: 10.1016/j.foodchem.2008.10.081
Li, J., Cai, C., Zheng, M., Hao, J., Wang, Y., Hu, M., et al. (2019a). Alkaline extraction, structural characterization, and bioactivities of (1→6)-β-d-glucan from Lentinus edodes. Molecules 24, e1610. doi: 10.3390/molecules24081610
Li, J., Gu, F., Cai, C., Hu, M., Fan, L., Hao, J., et al. (2020a). Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 143, 806–813. doi: 10.1016/j.ijbiomac.2019.09.141
Li, F., Lei, H., and Xu, H. (2022). Influences of subcritical water extraction on the characterization and biological properties of polysaccharides from Morchella sextelata. J. Food Process. Preserv. 46, e9. doi: 10.1111/jfpp.16024
Li, S., Li, J., Zhang, J., Wang, W., Wang, X., Jing, H., et al. (2017a). The antioxidative, antiaging, and hepatoprotective effects of alkali-extractable polysaccharides by Agaricus bisporus. Evid. Based Complement. Alternat. Med. 2017, e7298683. doi: 10.1155/2017/7298683
Li, W. J., Li, L., Zhen, W. Y., Wang, L. F., Pan, M., Lv, J. Q., et al. (2017b). Ganoderma atrum polysaccharide ameliorates ROS generation and apoptosis in spleen and thymus of immunosuppressed mice. Food Chem. Toxicol. 99, 199–208. doi: 10.1016/j.fct.2016.11.033
Li, Y., Sheng, Y., Lu, X., Guo, X., Xu, G., Han, X., et al. (2020b). Isolation and purification of acidic polysaccharides from Agaricus blazei Murill and evaluation of their lipid-lowering mechanism. Int. J. Biol. Macromol. 157, 276–287. doi: 10.1016/j.ijbiomac.2020.04.190
Li, L., Su, Z., He, Y., Zhong, X., Fu, C., Zou, L., et al. (2024b). Physicochemical characterization and anti-angiogenesis activity of polysaccharides from Amauroderma rugosum, a medicinal and edible mushroom. Int. J. Biol. Macromol. 274, e133478. doi: 10.1016/j.ijbiomac.2024.133478
Li, Y., Thambi, T., and Lee, D. S. (2018b). Co-delivery of drugs and genes using polymeric nanoparticles for synergistic cancer therapeutic effects. Adv. Healthc. Mater. 7, 1. doi: 10.1002/adhm.201700886
Li, W., Wang, J., Chen, Z., Gao, X., Chen, Y., Xue, Z., et al. (2018a). Physicochemical properties of polysaccharides from Lentinus edodes under high pressure cooking treatment and its enhanced anticancer effects. Int. J. Biol. Macromol. 115, 994–1001. doi: 10.1016/j.ijbiomac.2018.04.094
Li, S., Wang, A., Liu, L., Tian, G., and Xu, F. (2019b). Effect of deproteinization methods on the antioxidant activity of polysaccharides extracted from Lentinus edodes stipe. J. Food Meas. Charact. 13, 1382–1389. doi: 10.1007/s11694-019-00054-2
Li, B., Zhong, M., Sun, Y., Liang, Q., Shen, L., Qayum, A., et al. (2024a). Recent advancements in the utilization of ultrasonic technology for the curing of processed meat products: A comprehensive review. Ultrason. Sonochem. 103, e106796. doi: 10.1016/j.ultsonch.2024.106796
Li, X., Zhu, J., Wang, T., Sun, J., Guo, T., Zhang, L., et al. (2023). Antidiabetic activity of Armillaria mellea polysaccharides: Joint ultrasonic and enzyme assisted extraction. Ultrason. Sonochem. 95, e106370. doi: 10.1016/j.ultsonch.2023.106370
Liao, T., Shen, F., Zhu, H., Mu, W., Qian, H., and Liu, Y. (2024). Extracellular polysaccharides from Sporidiobolus pararoseus alleviates rheumatoid through ameliorating gut barrier function and gut microbiota. Int. J. Biol. Macromol. 260, e129436. doi: 10.1016/j.ijbiomac.2024.129436
Lin, N., Li, M., Han, Z., Liu, X., Qu, J., Wang, X., et al. (2024). Polysaccharides from Auricularia cornea var. Li.: Structural alterations during in vitro digestion and its potent immunomodulatory properties on macrophages. Food Biosci. 59, e104014. doi: 10.1016/j.fbio.2024.104014
Liu, X., Chen, S., Liu, H., Xie, J., Hasan, K. M. F., Zeng, Q., et al. (2023b). Structural properties and anti-inflammatory activity of purified polysaccharides from Hen-of-the-woods mushrooms (Grifola frondosa). Front. Nutr. 10, e1078868. doi: 10.3389/fnut.2023.1078868
Liu, J. J., Chen, S. K., Wang, X., He, W. W., Song, X. X., Huang, X. J., et al. (2024a). Changes of the physicochemical properties and structural characteristics of alkali-extracted polysaccharides from Agrocybe cylindracea across the growth process. J. Agric. Food Chem. 72, 12810–12821. doi: 10.1021/acs.jafc.4c02218
Liu, Y., Chen, S., Zhang, J., Gao, M., and Li, L. (2023c). Purification of polysaccharide produced by the haploid yeast strain of Tremella sanGuinea and its antioxidant and prebiotic activities. Molecules 28, e5391. doi: 10.3390/molecules28145391
Liu, R. M., Dai, R., Luo, Y., and Xiao, J. H. (2019). Glucose-lowering and hypolipidemic activities of polysaccharides from Cordyceps taii in streptozotocin-induced diabetic mice. BMC Complement. Altern. Med. 19, e230. doi: 10.1186/s12906-019-2646-x
Liu, Y., Liu, X., Cui, Y., and Yuan, W. (2022b). Ultrasound for microalgal cell disruption and product extraction: A review. Ultrason. Sonochem. 87, e106054. doi: 10.1016/j.ultsonch.2022.106054
Liu, X., Luo, D., Guan, J., Chen, J., and Xu, X. (2022a). Mushroom polysaccharides with potential in anti-diabetes: Biological mechanisms, extraction, and future perspectives: A review. Front. Nutr. 9, e1087826. doi: 10.3389/fnut.2022.1087826
Liu, X., Wang, Q., Wang, J., Guo, L., Chu, Y., Ma, C., et al. (2024b). Structural characterization, chain conformation and immunomodulatory activity of a heteropolysaccharide from Inonotus hispidus. Int. J. Biol. Macromol. 260, e129187. doi: 10.1016/j.ijbiomac.2023.129187
Liu, Y., Wang, Y., Zhang, C., Zhou, P., Liu, Y., An, T., et al. (2014). Core-shell nanoparticles based on pullulan and poly(β-amino) ester for hepatoma-targeted codelivery of gene and chemotherapy agent. ACS Appl. Mater. Interfaces 6, 18712–18720. doi: 10.1021/am504203x
Liu, J., Wu, D., Leng, Y., Li, Y., and Li, N. (2023a). Dietary supplementation with selenium polysaccharide from selenium-enriched Phellinus linteus improves antioxidant capacity, immunity and production performance of laying hens. J. Trace Elem. Med. Biol. 77, e127140. doi: 10.1016/j.jtemb.2023.127140
Liu, Y., Yang, J., Guo, Z., Li, Q., Zhang, L., Zhao, L., et al. (2024c). Immunomodulatory effect of Cordyceps militaris polysaccharide on RAW 264.7 macrophages by regulating MAPK signaling pathways. Molecules 29, e3408. doi: 10.3390/molecules29143408
Liu, Y., Zhou, Y., Liu, M., Wang, Q., and Li, Y. (2018). Extraction optimization, characterization, antioxidant and immunomodulatory activities of a novel polysaccharide from the wild mushroom Paxillus involutus. Int. J. Biol. Macromol. 112, 326–332. doi: 10.1016/j.ijbiomac.2018.01.132
Loira, I., Morata, A., Palomero, F., González, C., and Suárez-Lepe, J. A. (2018). Schizosaccharomyces pombe: A promising biotechnology for modulating wine composition. Fermentation 4, e70. doi: 10.3390/fermentation4030070
Long, Z., Xue, Y., Ning, Z., Sun, J., Li, J., Su, Z., et al. (2021). Production, characterization, and bioactivities of exopolysaccharides from the submerged culture of Ganoderma cantharelloideum M. H. Liu. Biotech. 11, e145. doi: 10.1007/s13205-021-02696-w
López-Legarda, X., Arboleda-Echavarría, C., Parra-Saldívar, R., Rostro-Alanis, M., Alzate, J. F., Villa-Pulgarín, J. A., et al. (2020). Biotechnological production, characterization and in vitro antitumor activity of polysaccharides from a native strain of Lentinus crinitus. Int. J. Biol. Macromol. 164, 3133–3144. doi: 10.1016/j.ijbiomac.2020.08.191
Lorenzo, J. M., Munekata, P. E., Dominguez, R., Pateiro, M., Saraiva, J. A., Franco, D., et al. (2018). “Main groups of microorganisms of relevance for food safety and stability,” in Innovative technologies for food preservation. Eds. Barba, F. J., Sant’Ana, A. S., and Orlien, V. (Academic Press, Cambridge, Massachusetts, United States), 53–107. doi: 10.1016/B978-0-12-811031-7.00003-0
Lu, M. K., Lee, M. H., Chao, C. H., and Hsu, Y. C. (2024a). Sodium sulfate addition increases the bioresource of biologically active sulfated polysaccharides from Antrodia cinnamomea. Int. J. Biol. Macromol. 257, e128699. doi: 10.1016/j.ijbiomac.2023.128699
Lu, Y. and Liu, D. (2024). Optimization of polysaccharide conditions and analysis of antioxidant capacity in the co-culture of Sanghuangporus vaninii and Pleurotus sapidus. PeerJ 12, e17571. doi: 10.7717/peerj.17571
Lu, X., Wang, C., Li, Y., and Liu, P. (2022). Improved production and antioxidant activity of exopolysaccharides by submerged culture of Lentinula edodes by the addition of lignocellulose. J. Biosci. Bioeng. 134, 162–166. doi: 10.1016/j.jbiosc.2022.05.003
Lu, X., Wu, S., Ai, H., Wu, R., Cheng, Y., Yun, S., et al. (2024b). Sparassis latifolia polysaccharide alleviated lipid metabolism abnormalities in kidney of lead-exposed mice by regulating oxidative stress-mediated inflammation and autophagy based on multi-omics. Int. J. Biol. Macromol. 278, e134662. doi: 10.1016/j.ijbiomac.2024.134662
Luo, Y., Cao, N., Huang, L., Tang, L., Liu, X., Zhang, W., et al. (2024). Structural characterization, and antioxidant, hypoglycemic and immunomodulatory activity of exopolysaccharide from Sanghuangporus sanghuang JM-1. Molecules 29, e4564. doi: 10.3390/molecules29194564
Luo, X., Duan, Y., Yang, W., Zhang, H., Li, C., and Zhang, J. (2017). Structural elucidation and immunostimulatory activity of polysaccharide isolated by subcritical water extraction from Cordyceps militaris. Carbohydr. Polym. 157, 794–802. doi: 10.1016/j.carbpol.2016.10.066
Luo, Q., Li, X., Li, H., Kong, K., Li, C., Fang, Z., et al. (2023). Effect of in vitro simulated digestion and fecal fermentation on Boletus auripes polysaccharide characteristics and intestinal flora. Int. J. Biol. Macromol. 249, e126461. doi: 10.1016/j.ijbiomac.2023.126461
Ma, K. L., Kei, N., Yang, F., Lauw, S., Chan, P. L., Chen, L., et al. (2023). In vitro fermentation characteristics of fungal polysaccharides derived from Wolfiporia cocos and their effect on human fecal microbiota. Foods 12, e4014. doi: 10.3390/foods12214014
Ma, Y., Zheng, X., Chu, Z., Nan, W., Zhao, Y., Bai, Y., et al. (2024). Polysaccharides from Inonotus obliquus employing subcritical water extraction: Extraction optimization, physiochemical properties and bioactivities analysis. Ind. Crop Prod. 222, e119638. doi: 10.1016/j.indcrop.2024.119638
Mahendran, S., Saravanan, S., Vijayabaskar, P., Anandapandian, K. T. K., and Shankar, T. (2013). Antibacterial potential of microbial exopolysaccharide from Ganoderma lucidum and Lysinibacillus fusiformis. Int. J. Recent Sci. Res. 4, 501–505.
Maia, L. S., de Bomfim, A. S. C., de Oliveira, D. M., Pinhati, F. R., da Conceição, M. O. T., Barud, H. S., et al. (2023). Tuning of renewable sponge-like polyurethane physical-chemical and morphological properties using the pullulan as a reactive filler. J. Appl. Polym. Sci. 140, e53619. doi: 10.1002/app.53619
Maingam, C., Kanchanarach, W., Chutiman, N., Wanthong, A., Srivilai, P., and Loutchanwoot, P. (2024). Implications of ultrasonication-assisted extraction with response surface methodology on phytochemical compositions and antioxidant activity of polysaccharide extract from Phellinus rimosus (Berk.) Pilát cultivated mycelia in northeastern Thailand. Nat. Prod. J. 14, 67–83. doi: 10.2174/0122103155293542240118063111
Mansour, A., Daba, A., Baddour, N., El-Saadani, M., and Aleem, E. (2012). Schizophyllan inhibits the development of mammary and hepatic carcinomas induced by 7,12 dimethylbenz(α)anthracene and decreases cell proliferation: comparison with tamoxifen. J. Cancer Res. Clin. Oncol. 138, 1579–1596. doi: 10.1007/s00432-012-1224-0
Mao, G. H., Ren, Y., Feng, W. W., Li, Q., Wu, H. Y., Jin, D., et al. (2015). Antitumor and immunomodulatory activity of a water-soluble polysaccharide from Grifola frondosa. Carbohydr. Polym. 134, 406–412. doi: 10.1016/j.carbpol.2015.08.020
Mao, G. H., Ren, Y., Li, Q., Wu, H. Y., Jin, D., Zhao, T., et al. (2016). Anti-tumor and immunomodulatory activity of selenium (Se)-polysaccharide from Se-enriched Grifola frondosa. Int. J. Biol. Macromol. 82, 607–613. doi: 10.1016/j.ijbiomac.2015.10.083
Maziero, R., Cavazzoni, V., and Bononi, V. L. R. (1999). Screening of basidiomycetes for the production of exopolysaccharide and biomass in submerged culture. Rev. Microbiol. 30, 77–84. doi: 10.1590/s0001-37141999000100015
Medeiros, S. D. V., Cordeiro, S. L., Cavalcanti, J. E. C., Melchuna, K. M., Lima, A. M., da, S., et al. (2012). Effects of purified Saccharomyces cerevisiae (1→3)-β-glucan on venous ulcer healing. Int. J. Mol. Sci. 13, 8142–8158. doi: 10.3390/ijms13078142
Mei, Y., Zhu, H., Hu, Q., Liu, Y., Zhao, S., Peng, N., et al. (2015). A novel polysaccharide from mycelia of cultured Phellinus linteus displays antitumor activity through apoptosis. Carbohydr. Polym. 124, 90–97. doi: 10.1016/j.carbpol.2015.02.009
Meng, Y., Sui, X., Pan, X., Zhang, X., Sui, H., Xu, T., et al. (2023). Density-oriented deep eutectic solvent-based system for the selective separation of polysaccharides from Astragalus membranaceus var. Mongholicus under ultrasound-assisted conditions. Ultrason. Sonochem. 98, e106522. doi: 10.1016/j.ultsonch.2023.106522
Mfopa, A., Mediesse, F. K., Mvongo, C., Nkoubatchoundjwen, S., Lum, A. A., Sobngwi, E., et al. (2021). Antidyslipidemic potential of water-soluble polysaccharides of Ganoderma applanatum in MACAPOS-2-induced obese rats. Evid. Based Complement. Alternat. Med. 2021, e2452057. doi: 10.1155/2021/2452057
Miao, J., Regenstein, J. M., Qiu, J., Zhang, J., Zhang, X., Li, H., et al. (2020). Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from Auricularia-A review. Int. J. Biol. Macromol. 150, 102–113. doi: 10.1016/j.ijbiomac.2020.02.054
Millette, P. G., Chabot, J., Sheppard, D. C., and Le Mauff, F. (2023). Identification and quantification of monosaccharides from fungal cell walls and exopolysaccharides by gas chromatography coupled to mass spectrometry. Curr. Protoc. 3, e853. doi: 10.1002/cpz1.853
Minari, M. C., Rincão, V. P., Soares, S. A., Ricardo, N. M., Nozawa, C., and Linhares, R. E. (2011). Antiviral properties of polysaccharides from Agaricus brasiliensis in the replication of bovine herpesvirus 1. Acta Virol. 55, 255–259. doi: 10.4149/av_2011_03_255
Miranda-Nantes, C. C. B. O., Fonseca, E. A. I., Zaia, C. T. B. V., Dekker, R. F. H., Khaper, N., Castro, I. A., et al. (2011). Hypoglycemic and hypocholesterolemic effects of botryosphaeran from Botryosphaeria rhodina MAMB-05 in diabetes-induced and hyperlipidemia conditions in rats. Mycobiology 39, 187–193. doi: 10.5941/MYCO.2011.39.3.187
Mohamed, H. I., Basit, A., and Abdallah, W. E. (2024). “Exopolysaccharides produced by fungi and their environmental applications,” in Fungal secondary metabolites. Eds. Abd-Elsalam, K. A. and Mohamed, H. I. (Elsevier), 219–240. doi: 10.1016/B978-0-323-95241-5.00028-9
Monroy, Y. M., Rodrigues, R. A. F., Sartoratto, A., and Cabral, F. A. (2016). Influence of ethanol, water, and their mixtures as co-solvents of the supercritical carbon dioxide in the extraction of phenolics from purple corn cob (Zea mays L.). J. Supercrit. Fluids 118, 11–18. doi: 10.1016/j.supflu.2016.07.019
Montoya, S., Sanchez, O. J., and Levin, L. (2013). Polysaccharide production by submerged and solid-state cultures from several medicinal higher basidiomycetes. Int. J. Med. Mushrooms 15, 71–79. doi: 10.1615/intjmedmushr.v15.i1.80
Montoya Barreto, S., Orrego Alzate, C. E., and Levin, L. (2011). Modeling Grifola frondosa fungal growth during solid-state fermentation. Eng. Life Sci. 11, 316–321. doi: 10.1002/elsc.201000087
Moradi, Z. and Kalanpour, N. (2019). Kefiran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohydr. Polym. 223, e115100. doi: 10.1016/j.carbpol.2019.115100
Morales, D., Smiderle, F. R., Villalva, M., Abreu, H., Rico, C., Santoyo, S., et al. (2019). Testing the effect of combining innovative extraction technologies on the biological activities of obtained β-glucan-enriched fractions from. Lentinula edodes. J. Funct. Foods 60, e103446. doi: 10.1016/j.jff.2019.103446
Morris, H. J., Llauradó, G., Beltrán, Y., Lebeque, Y., Bermúdez, R. C., García, N., et al. (2016). “The use of mushrooms in the development of functional foods, drugs, and nutraceuticals: functional food properties and applications,” in Wild plants, mushrooms and nuts. Eds. Ferreira, I. C. F. R., Morales, P., and Barros, L. (John Wiley, Sons, Ltd), 123–157. doi: 10.1002/9781118944653.Ch5
Mugnaini, G., Bonini, M., Gentile, L., Panza, O., Del Nobile, M. A., Conte, A., et al. (2024). Effect of design and molecular interactions on the food preserving properties of alginate/pullulan edible films loaded with grape pomace extract. J. Food Eng. 361, e111716. doi: 10.1016/j.jfoodeng.2023.111716
Mukhopadhya, A., O’Doherty, J. V., and Sweeney, T. (2019). A combination of yeast beta-glucan and milk hydrolysate is a suitable alternative to zinc oxide in the race to alleviate post-weaning diarrhoea in piglets. Sci. Rep. 9, e616. doi: 10.1038/s41598-018-37004-9
Murphy, E. J., Fehrenbach, G. W., Abidin, I. Z., Buckley, C., Montgomery, T., Pogue, R., et al. (2023a). Polysaccharides-naturally occurring immune modulators. Polymers 15, e2373. doi: 10.3390/polym15102373
Murphy, E. J., Rezoagli, E., Collins, C., Saha, S. K., Major, I., and Murray, P. (2023b). Sustainable production and pharmaceutical applications of β-glucan from microbial sources. Microbiol. Res. 274, e127424. doi: 10.1016/j.micres.2023.127424
Muthusamy, G., Joardar, S. N., and Samanta, I. (2013). β-Glucan from edible mushroom (Pleurotus forida) enhances mucosal immunity in poultry. Adv. Anim. Vet. Sci. 1, 116–119.
Negahban, Z., Shojaosadati, S. A., and Hamedi, S. (2021). A novel self-assembled micelles based on stearic acid modified schizophyllan for efficient delivery of paclitaxel. Colloids Surf. B Biointerfaces 199, e111524. doi: 10.1016/j.colsurfb.2020.111524
Ni, Z., Li, J., Qian, X., Yong, Y., Wu, M., Wang, Y., et al. (2023). Phellinus igniarius polysaccharides ameliorate hyperglycemia by modulating the composition of the gut microbiota and their metabolites in diabetic mice. Molecules 28, e7136. doi: 10.3390/molecules28207136
Niego, A. G. T., Rapior, S., Thongklang, N., Raspé, O., Hyde, K. D., and Mortimer, P. (2023). Reviewing the contributions of macrofungi to forest ecosystem processes and services. Fungal Biol. Rev. 44, e100294. doi: 10.1016/j.fbr.2022.11.002
Oğuzhan, P. and Yangılar, F. (2013). Pullulan: Production and usage in food industry. Afr. J. Food Sci. Technol. 4, 57–63.
Osińska-Jaroszuk, M., Jaszek, M., Mizerska-Dudka, M., Błachowicz, A., Rejczak, T. P., Janusz, G., et al. (2014). Exopolysaccharide from Ganoderma applanatum as a promising bioactive compound with cytostatic and antibacterial properties. BioMed. Res. Int. 2014, e743812. doi: 10.1155/2014/743812
Osińska-Jaroszuk, M., Sulej, J., Jaszek, M., and Jaroszuk-Ściseł, J. (2020). “Applications of fungal polysaccharides” in Encyclopedia of mycology. Eds. Zaragoza, Ó. and Casadevall, A. (Elsevier, Oxford), 613–628. doi: 10.1016/B978-0-12-809633-8.21092-3
Palacios, I., García-Lafuente, A., Guillamón, E., and Villares, A. (2012). Novel isolation of water-soluble polysaccharides from the fruiting bodies of Pleurotus ostreatus mushrooms. Carbohydr. Res. 358, 72–77. doi: 10.1016/j.carres.2012.06.016
Pan, X., Veroniaina, H., Su, N., Sha, K., Jiang, F., Wu, Z., et al. (2021). Applications and developments of gene therapy drug delivery systems for genetic diseases. Asian J. Pharm. Sci. 16, 687–703. doi: 10.1016/j.ajps.2021.05.003
Panya, M., Kaewraemruaen, C., Saenwang, P., and Pimboon, P. (2024). Evaluation of prebiotic potential of crude polysaccharides extracted from wild Lentinus polychrous and Lentinus squarrosulus and their application for a formulation of a novel lyophilized synbiotic. Foods 13, e287. doi: 10.3390/foods13020287
Parniakov, O., Lebovka, N. I., Van Hecke, E., and Vorobiev, E. (2014). Pulsed electric field assisted pressure extraction and solvent extraction from mushroom (Agaricus bisporus). Food Bioprocess Technol. 7, 174–183. doi: 10.1007/s11947-013-1059-y
Peng, B., Liu, Y., Lin, Y., Kraithong, S., Mo, L., Gao, Z., et al. (2023). A new exopolysaccharide of marine coral-associated Aspergillus pseudoglaucus SCAU265: Structural characterization and immunomodulatory activity. J. Fungi 9, e1057. doi: 10.3390/jof9111057
Pérez-Chávez, A. M., Mayer, L., and Albertó, E. (2019). Mushroom cultivation and biogas production: A sustainable reuse of organic resources. Energy Sustain. Dev. 50, 50–60. doi: 10.1016/j.esd.2019.03.002
Pham-Huy, L. A., He, H., and Pham-Huy, C. (2008). Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 4, 89–96. doi: 10.59566/IJBS.2008.4089
Phaniendra, A., Jestadi, D. B., and Periyasamy, L. (2015). Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 30, 11–26. doi: 10.1007/s12291-014-0446-0
Prajapati, V. D., Jani, G. K., and Khanda, S. M. (2013). Pullulan: An exopolysaccharide and its various applications. Carbohydr. Polym. 95, 540–549. doi: 10.1016/j.carbpol.2013.02.082
Prathumpai, W., Rachtawee, P., and Khajeeram, S. (2015). Potential of fungal exopolysaccharide as novel source for prebiotic supplement to broiler chicken diet. Indian J. Anim. Sci. 85, e12. doi: 10.56093/ijans.v85i12.54403
Qin, Y., Zhang, Z., Song, T., and Lv, G. (2017). Optimization of enzyme-assisted extraction of antitumor polysaccharides from Hericium erinaceus mycelia. Food Sci. Technol. Res. 23, 31–39. doi: 10.3136/fstr.23.31
Qiu, J., Zheng, P., Dai, W., Zheng, Z., Lin, X., Hu, J., et al. (2024). Steam explosion-assisted extraction of polysaccharides from Pleurotus eryngii and its influence on structural characteristics and antioxidant activity. Foods 13, e1229. doi: 10.3390/foods13081229
Rahman, H. U. U., Asghar, W., and Khalid, N. (2021). “Scleroglucan and schizophyllan: microbial polysaccharides of functional importance,” in Polysaccharides of microbial origin. Eds. Oliveira, J. M., Radhouani, H., and Reis, R. L. (Springer, Cham), 1–28. doi: 10.1007/978-3-030-35734-4_16-1
Ren, Y., Bai, Y., Zhang, Z., Cai, W., and Del Rio Flores, A. (2019). The preparation and structure analysis methods of natural polysaccharides of plants and fungi: A review of recent development. Molecules 24, e3122. doi: 10.3390/molecules24173122
Ren, G., Xu, L., Lu, T., and Yin, J. (2018). Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int. J. Biol. Macromol. 115, 1202–1210. doi: 10.1016/j.ijbiomac.2018.04.132
Reza, M. A. S., Rasouli, A., Vahidi, H., and Kobarfard, F. (2024). Molecular identification of Shiitake (Lentinula edodes), analysis and production of beta-glucan using beech wood sawdust waste. Int. J. Biol. Macromol. 280, e135539. doi: 10.1016/j.ijbiomac.2024.135539
Rizkyana, A. D., Ho, T. C., Roy, V. C., Park, J. S., Kiddane, A. T., Kim, G. D., et al. (2022). Sulfation and characterization of polysaccharides from Oyster mushroom (Pleurotus ostreatus) extracted using subcritical water. J. Supercrit. Fluids 179, e105412. doi: 10.1016/j.supflu.2021.105412
Rizvi, S. M. H. (2024). Nanotechnology applications in enhanced oil recovery (EOR). Int. J. Sci. Res. Manage. 12, 135–143. doi: 10.18535/ijsrm/v12i06.c03
Rodríguez-Seoane, P., Torres Perez, M. D., Fernández de Ana, C., Sinde-Stompel, E., and Domínguez, H. (2022). Antiradical and functional properties of subcritical water extracts from edible mushrooms and from commercial counterparts. Int. J. Food Sci. Technol. 57, 1420–1428. doi: 10.1111/ijfs.15383
Rosyida, V. T., Hayati, S. N., Wiyono, T., Darsih, C., and Ratih, D. (2024). Effect of aqueous extraction method on total water-soluble polysaccharides content and phytochemical properties of white oyster mushroom (Pleurotus ostreatus). IOP Conf. Ser. Earth Environ. Sci. 1377, e012064. doi: 10.1088/1755-1315/1377/1/012064
Sacchelli, B. A. L., Faccin-Galhardi, L. C., Ito, V. Y., Lopes, J. L., Dekker, R. F. H., Barbosa-Dekker, A. M., et al. (2019). Botryosphaeran and sulfonated derivatives as novel antiviral agents for herpes simplex and dengue fever. Int. J. Biol. Macromol. 138, 334–339. doi: 10.1016/j.ijbiomac.2019.07.084
Saengha, W., Karirat, T., Pitisin, N., Plangklang, S., Butkhup, L., Udomwong, P., et al. (2023). Exploring the bioactive potential of Calostoma insigne, an endangered culinary puffball mushroom, from northeastern Thailand. Foods 13, e113. doi: 10.3390/foods13010113
Saetang, N., Rattanapot, T., Manmai, N., Amornlerdpison, D., Ramaraj, R., and Unpaprom, Y. (2022). Effect of hot water extraction process on schizophyllan from split gill mushroom. Biomass Conv. Bioref. 14, 1017–1026. doi: 10.1007/s13399-021-02286-z
Sahib, R. S., Shafiq, S. A., and Chechan, R. A. (2024). Isolation, purification and structural characterization of lentinan from a newly discovered Iraqi strain of Lentinula edodes RSR. Glob. Sci. J. Biol. 9, 119–127. doi: 10.5281/zenodo.10693647
Sakdasri, W., Arnutpongchai, P., Phonsavat, S., Bumrungthaichaichan, E., and Sawangkeaw, R. (2022). Pressurized hot water extraction of crude polysaccharides, β-glucan, and phenolic compounds from dried gray oyster mushroom. LWT-Food Sci. Technol. 168, e113895. doi: 10.1016/j.lwt.2022.113895
Samadlouie, H. R., Jahanbin, K., and Jalali, P. (2020). Production, medium optimization, and structural characterization of an extracellular polysaccharide produced by Rhodotorula minuta ATCC 10658. Food Sci. Nutr. 8, 4954–4964. doi: 10.1002/fsn3.1792
Sánchez, Ó.J. and Montoya, S. (2020). Assessment of polysaccharide and biomass production from three white-rot fungi by solid-state fermentation using wood and agro-industrial residues: A kinetic approach. Forests 11, e1055. doi: 10.3390/f11101055
Sánchez, Ó.J., Montoya, S., and Vargas, L. M. (2015). “Polysaccharide production by submerged fermentation,” in Polysaccharides. Eds. Ramawat, K. and Mérillon, J. M. (Springer, Cham), 451–473. doi: 10.1007/978-3-319-16298-0_39
Sang, T., Guo, C., Guo, D., Wu, J., Wang, Y., Wang, Y., et al. (2021). Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr. Polym. 256, e117594. doi: 10.1016/j.carbpol.2020.117594
Sangthong, S., Pintathong, P., Pongsua, P., Jirarat, A., and Chaiwut, P. (2022). Polysaccharides from Volvariella volvacea mushroom: extraction, biological activities and cosmetic efficacy. J. Fungi 8, e572. doi: 10.3390/jof8060572
Saravanakumar, K., Park, S., Sathiyaseelan, A., Mariadoss, A. V. A., Park, S., Kim, S. J., et al. (2021). Isolation of polysaccharides from Trichoderma harzianum with antioxidant, anticancer, and enzyme inhibition properties. Antioxidants 10, e1372. doi: 10.3390/antiox10091372
Scafati, V., Troilo, F., Ponziani, S., Giovannoni, M., Scortica, A., Pontiggia, D., et al. (2022). Characterization of two 1,3-β-glucan-modifying enzymes from Penicillium sumatraense reveals new insights into 1,3-β-glucan metabolism of fungal saprotrophs. Biotechnol. Biofuels Bioprod. 15, e138. doi: 10.1186/s13068-022-02233-8
Schlaubitz, S., Derkaoui, S. M., Marosa, L., Miraux, S., Renard, M., Catros, S., et al. (2014). Pullulan/dextran/nHA macroporous composite beads for bone repair in a femoral condyle defect in rats. PloS One 9, e110251. doi: 10.1371/journal.pone.0110251
Sermwittayawong, D., Patninan, K., Phothiphiphit, S., Boonyarattanakalin, S., Sermwittayawong, N., and HutadilokTowatana, N. (2018). Purification, characterization, and biological activities of purified polysaccharides extracted from the gray oyster mushroom [Pleurotus sajor-caju (Fr.) Sing.]. J. Food Biochem. 42, e12606. doi: 10.1111/jfbc.12606
Shao, Z., Tian, Y., Liu, S., Chu, X., and Mao, W. (2023). Anti-diabetic activity of a novel exopolysaccharide produced by the mangrove endophytic fungus Penicillium janthinellum N29. Mar. Drugs 21, e270. doi: 10.3390/md21050270
Shen, L., Pang, S., Zhong, M., Sun, Y., Qayum, A., Liu, Y., et al. (2023). A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 101, e106646. doi: 10.1016/j.ultsonch.2023.106646
Shi, M., Yang, Y., Guan, D., Zhang, Y., and Zhang, Z. (2012). Bioactivity of the crude polysaccharides from fermented soybean curd residue by Flammulina velutipes. Carbohydr. Polym. 89, 1268–1276. doi: 10.1016/j.carbpol.2012.04.047
Si, J., Meng, G., Wu, Y., Ma, H. F., Cui, B. K., and Dai, Y. C. (2018). Medium composition optimization, structural characterization, and antioxidant activity of exopolysaccharides from the medicinal mushroom Ganoderma lingzhi. Int. J. Biol. Macromol. 124, 1186–1196. doi: 10.1016/j.ijbiomac.2018.11.274
Singh, R. S., Kaur, N., and Kennedy, J. F. (2019). Pullulan production from agro-industrial waste and its applications in food industry: A review. Carbohydr. Polym. 217, 46–57. doi: 10.1016/j.carbpol.2019.04.050
Singh, R. S., Kaur, N., Rana, V., and Kennedy, J. F. (2016). Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 153, 455–462. doi: 10.1016/j.carbpol.2016.07.118
Singh, S., Singh, V., and Patel, S. (2024). Cosmeceuticals; The fusion of cosmetics and pharmaceuticals. J. Community Pharm. Pract. 4, 16–27. doi: 10.55529/jcpp.42.16.27
Sipping, M. T. K., Mediesse, F. K., Kenmogne, L. V., Kanemoto, J. E. N., Njamen, D., and Boudjeko, T. (2022). Polysaccharide-rich fractions from Ganoderma resinaceum (ganodermataceae) as chemopreventive agents in N-diethylnitrosamine-induced hepatocellular carcinoma in wistar rats. Evid. Based Complement. Altern. Med. 2022, e8198859. doi: 10.1155/2022/8198859
Skalicka-Woźniak, K., Szypowski, J., Łoś, R., Siwulski, M., Sobieralski, K., Głowniak, K., et al. (2012). Evaluation of polysaccharides content in fruit bodies and their antimicrobial activity of four Ganoderma lucidum (W Curt.: Fr.) P. Karst. strains cultivated on different wood type substrates. Acta Soc Bot. Pol. 81, 17–21. doi: 10.5586/asbp.2012.001
Smiderle, F. R., Olsen, L. M., Ruthes, A. C., Czelusniak, P. A., Santana-Filho, A. P., Sassaki, G. L., et al. (2012). Exopolysaccharides, proteins and lipids in Pleurotus pulmonarius submerged culture using different carbon sources. Carbohydr. Polym 87, 368–376. doi: 10.1016/j.carbpol.2011.07.063
Song, X., Liu, Z., Zhang, J., Yan, Q., Ren, Z., Zhang, C., et al. (2018). Anti-inflammatory and hepatoprotective effects of exopolysaccharides isolated from Pleurotus geesteranus on alcohol-induced liver injury. Sci. Rep. 8, e10493. doi: 10.1038/s41598-018-28785-0
Stoica, R. M., Moscovici, M., Lakatos, E. S., and Cioca, L. I. (2023). Exopolysaccharides of fungal origin: properties and pharmaceutical applications. Processes 11, e335. doi: 10.3390/pr11020335
Su, J., Su, L., Li, D., Shuai, O., Zhang, Y., Liang, H., et al. (2018). Antitumor activity of extract from the sporoderm-breaking spore of Ganoderma lucidum: Restoration on exhausted cytotoxic T cell with gut microbiota remodeling. Front. Immunol. 9, e1765. doi: 10.3389/fimmu.2018.01765
Su, L., Xin, C., Yang, J., Dong, L., Mei, H., Dai, X., et al. (2022). A polysaccharide from Inonotus obliquus ameliorates intestinal barrier dysfunction in mice with type 2 diabetes mellitus. Int. J. Biol. Macromol. 214, 312–323. doi: 10.1016/j.ijbiomac.2022.06.071
Sun, Y., He, H., Wang, Q., Yang, X., Jiang, S., and Wang, D. (2022b). A review of development and utilization for edible fungal polysaccharides: extraction, chemical characteristics, and bioactivities. Polymers 14, e4454. doi: 10.3390/polym14204454
Sun, H., Shu, F., Guan, Y., Kong, F., Liu, S., Liu, Y., et al. (2023). Study of anti-fatigue activity of polysaccharide from fruiting bodies of Armillaria gallica. Int. J. Biol. Macromol. 241, e124611. doi: 10.1016/j.ijbiomac.2023.124611
Sun, W., Zhang, Y., and Jia, L. (2022a). Polysaccharides from Agrocybe cylindracea residue alleviate type 2-diabetes-induced liver and colon injuries by P38 MAPK signaling pathway. Food Biosci. 47, e101690. doi: 10.1016/j.fbio.2022.101690
Sung, Y. K. and Kim, S. W. (2019). Recent advances in the development of gene delivery systems. Biomater. Res. 23, e8. doi: 10.1186/s40824-019-0156-z
Suraiya, S., Jang, W. J., Haq, M., and Kong, I. S. (2024). Isolation and characterization of β-glucan containing polysaccharides from Monascus spp. using Saccharina japonica as submerged fermented substrate. Polysaccharides 5, 435–449. doi: 10.3390/polysaccharides5030027
Tabibzadeh, F., Alvandi, H., Hatamian-Zarmi, A., Kalitukha, L., Aghajani, H., and Ebrahimi-Hosseinzadeh, B. (2022). Antioxidant activity and cytotoxicity of exopolysaccharide from mushroom Hericium coralloides in submerged fermentation. Biomass Convers. Biorefin. 14, 26953–26963. doi: 10.1007/s13399-022-03386-0
Tagne, R. F. T., Cruz-Santos, M. M., Antunes, F. A. F., Shibukawa, V. P., Miano, S. B., Kenfack, J. A. A., et al. (2024). Pullulan production from sugarcane bagasse hemicellulosic hydrolysate by Aureobasidium pullulans ATCC 42023 in bubble column reactor. Fermentation 10, e322. doi: 10.3390/fermentation10060322
Tang, B., Ma, J., Liu, L., Xu, J., Zhang, H., Li, K., et al. (2025). Preparation of pullulan-shellac edible films with improved water-resistance and UV barrier properties for Chinese cherries preservation. J. Future Foods 5, 107–118. doi: 10.1016/j.jfutfo.2024.01.010
Tang, Y. J., Zhang, W., Liu, R. S., Zhu, L. W., and Zhong, J. J. (2011). Scale-up study on the fed-batch fermentation of Ganoderma lucidum for the hyperproduction of ganoderic acid and Ganoderma polysaccharides. Process Biochem. 46, 404–408. doi: 10.1016/j.procbio.2010.08.013
Tepsongkroh, B., Thaihuttakij, C., Supawong, S., and Jangchud, K. (2023). Impact of high pressure pre-treatment and hot water extraction on chemical properties of crude polysaccharide extract obtained from mushroom (Volvariella volvacea). Food Chem.: X 19, e100864. doi: 10.1016/j.fochx.2023.100864
Thakur, A., Sharma, S., Naman, S., and Baldi, A. (2023). Pullulan based polymeric novel drug delivery systems: a review on current state of art and prospects. J. Drug Deliv. Sci. Technol. 90, e105117. doi: 10.1016/j.jddst.2023.105117
Thangavel, P., Vilvanathan, S. P., Kuttalam, I., and Lonchin, S. (2020). Topical administration of pullulan gel accelerates skin tissue regeneration by enhancing collagen synthesis and wound contraction in rats. Int. J. Biol. Macromol. 149, 395–403. doi: 10.1016/j.ijbiomac.2020.01.187
Tian, B., Jiang, Y., Liu, R., Hamed, Y. S., Rayan, A. M., Xu, S., et al. (2024). Positive effects of extracellular polysaccharides from Paecilomyces hepiali on immune-enhancing properties by regulating gut microbiota in cyclophosphamide-induced mice. Int. J. Biol. Macromol. 274, e133390. doi: 10.1016/j.ijbiomac.2024.133390
Tian, R., Wu, L. L., Li, H. F., Liang, Z. Q., Li, P. H., Wang, Y., et al. (2023). Purification and structure characterization of the crude polysaccharide from the fruiting bodies of Butyriboletus pseudospeciosus and its modulation effects on gut microbiota. Molecules 28, e2679. doi: 10.3390/molecules28062679
Tian, Y., Zeng, H., Xu, Z., Zheng, B., Lin, Y., Gan, C., et al. (2012). Ultrasonic-assisted extraction and antioxidant activity of polysaccharides recovered from white button mushroom (Agaricus bisporus). Carbohydr. Polym. 88, 522–529. doi: 10.1016/j.carbpol.2011.12.042
Tufail, M., Jiang, C. H., and Li, N. (2024). Altered metabolism in cancer: insights into energy pathways and therapeutic targets. Mol. Cancer 23, e203. doi: 10.1186/s12943-024-02119-3
Udchumpisai, W. and Bangyeekhun, E. (2020). Purification, structural characterization, and biological activity of polysaccharides from Lentinus velutinus. Mycobiol. 48, 51–57. doi: 10.1080/12298093.2019.1693482
Urbancikova, I., Hudackova, D., Majtan, J., Rennerova, Z., Banovcin, P., and Jesenak, M. (2020). Efficacy of pleuran (β-glucan from Pleurotus ostreatus) in the management of herpes simplex virus type 1 infection. Evid. Based Complement. Alternat. Med. 2020, e8562309. doi: 10.1155/2020/8562309
Utama, G. L., Dio, C., Sulistiyo, J., Yee Chye, F., Lembong, E., Cahyana, Y., et al. (2021). Evaluating comparative β-glucan production aptitude of Saccharomyces cerevisiae, Aspergillus oryzae, Xanthomonas campestris, and Bacillus natto. Saudi J. Biol. Sci. 28, 6765–6773. doi: 10.1016/j.sjbs.2021.07.051
Uthan, E. T., Senturk, H., Uyanoglu, M., and Yamaç, M. (2021). First report on the in vivo prebiotic, biochemical, and histological effects of crude polysaccharide fraction of golden chantharelle mushroom, Cantharellus cibarius (Agaricomycetes). Int. J. Med. Mushrooms 23, 67–77. doi: 10.1615/IntJMedMushrooms.2021038233
Valdez, A. L., Delgado, O. D., and Fariña, J. I. (2021). Cost-effective optimized scleroglucan production by Sclerotium rolfsii ATCC 201126 at bioreactor scale. A quantity-quality assessment. Carbohydr. Polym. 260, e117505. doi: 10.1016/j.carbpol.2020.117505
Valverde, M. E., Hernández-Pérez, T., and Paredes-López, O. (2015). Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, e376387. doi: 10.1155/2015/376387
Vanin, A. P., Visentin, E. Z., Fontana, R. C., di Medeiros Leal, M. C. B., de Avila E Silva, S., Stokke, B. T., et al. (2023). β-(1→3)(1→6)glucan from schizophyllum commune 227E.32: high yield production via glucose/xylose co-metabolization. Carbohydr. Polym. 320, e121176. doi: 10.1016/j.carbpol.2023.121176
Vetter, J. (2023). The mushroom glucans: molecules of high biological and medicinal importance. Foods 12, e1009. doi: 10.3390/foods12051009
Viñarta, S. C., Yossen, M. M., Vega, J. R., Figueroa, L. I. C., and Fariña, J. I. (2013). Scleroglucan compatibility with thickeners, alcohols and polyalcohols and downstream processing implications. Carbohydr. Polym. 92, 1107–1115. doi: 10.1016/j.carbpol.2012.10.065
Visan, A. I. and Cristescu, R. (2023). Polysaccharide-based coatings as drug delivery systems. Pharmaceutics 15, e2227. doi: 10.3390/pharmaceutics15092227
Vlasenko, V. A., Ilyicheva, T. N., Svyatchenko, S. V., Asbaganov, S. V., Zmitrovich, I. V., and Vlasenko, A. V. (2020). Antiviral activity of total polysaccharide fraction of water and ethanol extracts of Pleurotus pulmonarius against the influenza A virus. Curr. Res. Environ. Appl. Mycol. J. Fungal. Biol. 10, 224–235. doi: 10.5943/cream/10/1/22
Wang, Y., Azhar, S., Lindström, M. E., and Henriksson, G. (2015c). Stabilization of polysaccharides during alkaline pre-treatment of wood combined with enzyme-supported extractions in a biorefinery. J. Wood Chem. Technol. 35, 91–101. doi: 10.1080/02773813.2013.875041
Wang, J., Dou, B., and Bao, Y. (2014a). Efficient targeted pDNA/siRNA delivery with folate-low-molecular-weight polyethyleneimine-modified pullulan as non-viral carrier. Mat. Sci. Eng. C Mater. Biol. Appl. 34, 98–109. doi: 10.1016/j.msec.2013.08.035
Wang, M., Gao, Y., Xu, D., and Gao, Q. (2015b). A polysaccharide from cultured mycelium of Hericium erinaceus and its anti-chronic atrophic gastritis activity. Int. J. Biol. Macromol. 81, 656–661. doi: 10.1016/j.ijbiomac.2015.08.043
Wang, T., Han, J., Dai, H., Sun, J., Ren, J., Wang, W., et al. (2022b). Polysaccharides from Lyophyllum decastes reduce obesity by altering gut microbiota and increasing energy expenditure. Carbohyd. Polym. 295, e119862. doi: 10.1016/j.carbpol.2022.119862
Wang, K., Huai, S., Tan, Z., Ngea, G. L. N., Godana, E. A., Shi, J., et al. (2023a). A first expression, purification and characterization of endo-β-1,3-glucanase from Penicillium expansum. J. Fungi 9, e961. doi: 10.3390/jof9100961
Wang, Y., Jin, H., Yu, J., Qu, C., Wang, Q., Yang, S., et al. (2020b). Quality control and immunological activity of lentinan samples produced in China. Int. J. Biol. Macromol. 159, 129–136. doi: 10.1016/j.ijbiomac.2020.05.050
Wang, J., Li, J., Li, J., Li, J., Liu, S., and Gao, W. (2017a). LSP1, a responsive protein from Meyerozyma guilliermondii, elicits defence response and improves glycyrrhizic acid biosynthesis in Glycyrrhiza uralensis Fisch adventitious roots. J. Cell. Physiol. 232, 3510–3519. doi: 10.1002/jcp.25811
Wang, L., Lian, J., Zheng, Q., Wang, L., Wang, Y., and Yang, D. (2022a). Composition analysis and prebiotics properties of polysaccharides extracted from Lepista sordida submerged cultivation mycelium. Front. Microbiol. 13, e1077322. doi: 10.3389/fmicb.2022.1077322
Wang, Q. Q., Lin, J., Zhou, Q. Z., Peng, J., Zhang, Q., and Wang, J. H. (2023b). Hyper-production of pullulan by a novel fungus of Aureobasidium melanogenum ZH27 through batch fermentation. Int. J. Mol. Sci. 25, e319. doi: 10.3390/ijms25010319
Wang, W., Liu, H., Zhang, Y., Feng, Y., Yuan, F., Song, X., et al. (2019b). Antihyperlipidemic and hepatoprotective properties of alkali- and enzyme-extractable polysaccharides by Dictyophora indusiata. Sci. Rep. 9, e14266. doi: 10.1038/s41598-019-50717-9
Wang, W., Tan, J., Nima, L., Sang, Y., Cai, X., and Xue, H. (2022c). Polysaccharides from fungi: A review on their extraction, purification, structural features, and biological activities. Food Chem.: X 15, e100414. doi: 10.1016/j.fochx.2022.100414
Wang, Q., Wang, F., Xu, Z., and Ding, Z. (2017b). Bioactive mushroom polysaccharides: A review on monosaccharide composition, biosynthesis and regulation. Molecules 22, e955. doi: 10.3390/molecules22060955
Wang, C. C., Wu, J. Y., Chang, C. Y., Yu, S. T., and Liu, Y. C. (2019a). Enhanced exopolysaccharide production by Cordyceps militaris using repeated batch cultivation. J. Biosci. Bioeng. 127, 499–505. doi: 10.1016/j.jbiosc.2018.09.006
Wang, J., Wu, C., Chen, Y., Chen, C., Hu, S., and Chang, S. (2014b). Antihyperglycemic activity of exopolysaccharide produced by mushroom Pleurotus ferulae with submerged liquid culture on streptozotocin-induced diabetic rats. J. Food Nutr. Res. 2, 419–424. doi: 10.12691/jfnr-2-7-15
Wang, L., Xu, N., Zhang, J., Zhao, H., Lin, L., Jia, S., et al. (2015a). Antihyperlipidemic and hepatoprotective activities of residue polysaccharide from Cordyceps militaris SU-12. Carbohydr. Polym. 131, 355–362. doi: 10.1016/j.carbpol.2015.06.016
Wang, Y. X., Yin, J. Y., Huang, X. J., and Nie, S. P. (2020a). Structural characteristics and rheological properties of high viscous glucan from fruit body of Dictyophora rubrovolvata. Food Hydrocoll. 101, e105514. doi: 10.1016/j.foodhyd.2019.105514
Wang, W. H., Zhang, J. S., Feng, T., Deng, J., Lin, C. C., Fan, H., et al. (2018). Structural elucidation of a polysaccharide from Flammulina velutipes and its immunomodulation activities on mouse B lymphocytes. Sci. Rep. 8, e3120. doi: 10.1038/s41598-018-21375-0
Wang, X., Zhang, J., Zhang, K., Guo, Z., Xu, G., Huang, L., et al. (2024). Ultrasound-assisted enzyme extraction, physicochemical properties and antioxidant activity of polysaccharides from Cordyceps militaris solid medium. Molecules 29, e4560. doi: 10.3390/molecules29194560
Wan-Mohtar, W. A. A. Q. I., Young, L., Abbott, G. M., Clements, C., Harvey, L. M., and McNeil, B. (2016). Antimicrobial properties and cytotoxicity of sulfated (1,3)-β-D-glucan from the mycelium of the mushroom Ganoderma lucidum. J. Microbiol. Biotechnol. 26, 999–1010. doi: 10.4014/jmb.1510.10018
Wei, S., Wang, L., Chen, X., Wang, Y., Tong, L., Han, Q., et al. (2024). Anti-inflammatory activity of Boletus aereus polysaccharides: Involvement of digestion and gut microbiota fermentation. Food Chem.: X 21, e101052. doi: 10.1016/j.fochx.2023.101052
Weng, B. B. C., Lin, Y. C., Hu, C. W., Kao, M. Y., Wang, S. H., Lo, D. Y., et al. (2011). Toxicological and immunomodulatory assessments of botryosphaeran (β-glucan) produced by Botryosphaeria rhodina RCYU 30101. Food Chem. Toxicol. 49, 910–916. doi: 10.1016/j.fct.2010.10.036
Wood, I. P., Elliston, A., Ryden, P., Bancroft, I., Roberts, I. N., and Waldron, K. W. (2012). Rapid quantification of reducing sugars in biomass hydrolysates: Improving the speed and precision of the dinitrosalicylic acid assay. Biomass Bioenergy 44, 117–121. doi: 10.1016/j.biombioe.2012.05.003
Wouk, J., Celestino, G. G., Rodrigues, B. C. D., Malfatti, C. R. M., Cunha, M. A. A., Orsato, A., et al. (2022). Sulfonated (1→6)-β-d-glucan (lasiodiplodan): A promising candidate against the acyclovir-resistant herpes simplex virus type 1 (HSV-1) strain. Biomacromolecules 23, 4041–4052. doi: 10.1021/acs.biomac.2c00156
Woźniak, B., Chudzińska, J., Szczyglewska, P., Nowak, I., and Feliczak-Guzik, A. (2023). Optimization of the composition of a cosmetic formulation containing Tremella fuciformis extract (fungi). Cosmetics 10, e82. doi: 10.3390/cosmetics10030082
Wu, J. Y., Chen, H. B., Chen, M. J., Kan, S. C., Shieh, C. J., and Liu, Y. C. (2013). Quantitative analysis of LED effects on edible mushroom Pleurotus eryngii in solid and submerged cultures: LED effect on cultivating P. eryngii. J. Chem. Technol. Biotechnol. 88, 1841–1846. doi: 10.1002/jctb.4038
Wu, Y., Jiang, H., Zhu, E., Li, J., Wang, Q., Zhou, W., et al. (2018). Hericium erinaceus polysaccharide facilitates restoration of injured intestinal mucosal immunity in Muscovy duck reovirus-infected Muscovy ducklings. Int. J. Biol. Macromol. 107, 1151–1161. doi: 10.1016/j.ijbiomac.2017.09.092
Wu, K., Li, Y., Lin, Y., Xu, B., Yang, J., Mo, L., et al. (2023). Structural characterization and immunomodulatory activity of an exopolysaccharide from marine-derived Aspergillus versicolor SCAU141. Int. J. Biol. Macromol. 227, 329–339. doi: 10.1016/j.ijbiomac.2022.12.127
Wu, D., Tang, C., Liu, Y., Li, Q., Wang, W., Zhou, S., et al. (2019). Structural elucidation and immunomodulatory activity of a β-D-glucan prepared by freeze-thawing from Hericium erinaceus. Carbohydr. Polym. 222, e114996. doi: 10.1016/j.carbpol.2019.114996
Wu, R. T., Wang, L. F., Yao, Y. F., Sang, T., Wu, Q. L., Fu, W. W., et al. (2022a). Activity fingerprinting of polysaccharides on oral, gut, pancreas and lung microbiota in diabetic rats. BioMed. Pharmacother. 155, e113681. doi: 10.1016/j.biopha.2022.113681
Wu, S., Yang, X., and Yang, X. (2022b). Methotrexate and 10-hydroxycamptothecine loaded pullulan nanoparticles with the targeting property for efficient cancer therapy. Mater. Technol. 37, 2777–2784. doi: 10.1080/10667857.2022.2075079
Wu, F., Zhou, C., Zhou, D., Ou, S., and Huang, H. (2017). Structural characterization of a novel polysaccharide fraction from Hericium erinaceus and its signaling pathways involved in macrophage immunomodulatory activity. J. Funct. Foods 37, 574–585. doi: 10.1016/j.jff.2017.08.030
Xia, S., Zhang, L., Davletshin, A., Li, Z., You, J., and Tan, S. (2020). Application of polysaccharide biopolymer in petroleum recovery. Polymers 12, e1860. doi: 10.3390/polym12091860
Xiang, H., Sun-Waterhouse, D., and Cui, C. (2021). Hypoglycemic polysaccharides from Auricularia auricula and Auricularia polytricha inhibit oxidative stress, NF-κB signaling and proinflammatory cytokine production in streptozotocin-induced diabetic mice. Food Sci. Hum. Wellness 10, 87–93. doi: 10.1016/j.fshw.2020.06.001
Xu, J., Liu, Z., Zhang, S., Xiang, J., Lan, H., and Bao, Y. (2024). Anti-hepatoma immunotherapy of Pholiota adiposa polysaccharide-coated selenium nanoparticles by reversing M2-like tumor-associated macrophage polarization. Int. J. Biol. Macromol. 277, e133667. doi: 10.1016/j.ijbiomac.2024.133667
Xu, M., Qu, Y., Li, H., Tang, S., Chen, C., Wang, Y., et al. (2023). Improved extraction yield, water solubility, and antioxidant activity of lentinan from Lentinula edodes via Bacillus subtilis natto fermentation. Fermentation 9, e333. doi: 10.3390/fermentation9040333
Xu, N., Sun, Y. H., Guo, X. L., Liu, C., Mao, Q., and Hou, J. M. (2018). Optimization of ultrasonic-microwave synergistic extraction of polysaccharides from Morchella conica. J. Food Process. Preserv. 42, e13423. doi: 10.1111/jfpp.13423
Xu, L., Wang, F., Zhang, Z., and Terry, N. (2019). Optimization of polysaccharide production from Cordyceps militaris by solid-state fermentation on rice and its antioxidant activities. Foods 8, e590. doi: 10.3390/foods8110590
Xu, D., Wang, H., Zheng, W., Gao, Y., Wang, M., Zhang, Y., et al. (2016). Charaterization and immunomodulatory activities of polysaccharide isolated from Pleurotus eryngii. Int. J. Biol. Macromol. 92, 30–36. doi: 10.1016/j.ijbiomac.2016.07.016
Yabalak, E., Aminzai, M. T., Gizir, A. M., and Yang, Y. (2024). A review: Subcritical water extraction of organic pollutants from environmental matrices. Molecules 29, e258. doi: 10.3390/molecules29010258
Yafetto, L. (2022). Application of solid-state fermentation by microbial biotechnology for bioprocessing of agro-industrial wastes from 1970 to 2020: A review and bibliometric analysis. Heliyon 8, e09173. doi: 10.1016/j.heliyon.2022.e09173
Yan, J. K., Wang, W. Q., Ma, H. L., and Wu, J. Y. (2012). Sulfation and enhanced antioxidant capacity of an exopolysaccharide produced by the medicinal fungus Cordyceps sinensis. Molecules 18, 167–177. doi: 10.3390/molecules18010167
Yang, Q., Chang, S. L., Tian, Y. M., Li, W., and Ren, J. L. (2024b). Glucan polysaccharides isolated from Lactarius hatsudake Tanaka mushroom: Structural characterization and in vitro bioactivities. Carbohydr. Polym. 337, e122171. doi: 10.1016/j.carbpol.2024.122171
Yang, X., Gao, Y., Reyimu, M., Zhang, G., Wang, C., Yang, D., et al. (2024c). Structural analysis of Pleurotus ferulae polysaccharide and its effects on plant fungal disease and plant growth. Int. J. Biol. Macromol. 282, e137396. doi: 10.1016/j.ijbiomac.2024.137396
Yang, M., Hu, D., Cui, Z., Li, H., Man, C., and Jiang, Y. (2021a). Lipid-lowering effects of Inonotus obliquus polysaccharide in vivo and in vitro. Foods 10, e3085. doi: 10.3390/foods10123085
Yang, X., Lin, P., Wang, J., Liu, N., Yin, F., Shen, N., et al. (2021b). Purification, characterization and anti-atherosclerotic effects of the polysaccharides from the fruiting body of Cordyceps militaris. Int. J. Biol. Macromol 181, 890–904. doi: 10.1016/j.ijbiomac.2021.04.083
Yang, J., Liu, J., Kuang, W., Lin, Y., Zhong, S., Kraithong, S., et al. (2024a). Structural characterization and ferroptosis-related immunomodulatory of a novel exopolysaccharide isolated from marine fungus Aspergillus medius. Int. J. Biol. Macromol. 265, e130703. doi: 10.1016/j.ijbiomac.2024.130703
Yang, M., Ren, W., Li, G., Yang, P., Chen, R., and He, H. (2022a). The effect of structure and preparation method on the bioactivity of polysaccharides from plants and fungi. Food Funct. 13, 12541–12560. doi: 10.1039/d2fo02029g
Yang, X., Sun, S., Chen, Q., Zhang, Z., Wang, J., Liu, Y., et al. (2022b). A polysaccharide of Ganoderma lucidum enhances antifungal activity of chemical fungicides against soil-borne diseases of wheat and maize by induced resistance. Agriculture 12, e55. doi: 10.3390/agriculture12010055
Yang, X. M., Wang, S. Q., Chen, L. S., and Zhu, Z. Y. (2023). Isolation and structural characterization of exopolysaccharide from the Cordyceps cicadae and the immunomodulatory activity on RAW264.7 cells. Biotechnol. Appl. Biochem. 70, 1925–1940. doi: 10.1002/bab.2500
Yang, S., Yan, J., Yang, L., Meng, Y., Wang, N., He, C., et al. (2019). Alkali-soluble polysaccharides from mushroom fruiting bodies improve insulin resistance. Int. J. Biol. Macromol. 126, 466–474. doi: 10.1016/j.ijbiomac.2018.12.251
Yang, W., Zhang, H., Ji, M., Pei, F., and Wang, Y. (2016). Antitumor effect of a polysaccharide isolated from Phellinus pullus as an immunostimulant. Biomed. Rep. 4, 361–364. doi: 10.3892/br.2016.587
Yang, Y., Zhang, X., Zhang, J., Wang, T., Liu, S., Ma, H., et al. (2024d). Green ultrasonic-assisted enzymatic extraction of polysaccharides from Flammulina velutipes residues by response surface methodology. Sustain. Chem. Pharm. 41, e101690. doi: 10.1016/j.scp.2024.101690
Yao, H. Y. Y., Wang, J. Q., Yin, J. Y., Nie, S. P., and Xie, M. Y. (2021). A review of NMR analysis in polysaccharide structure and conformation: progress, challenge and perspective. Food Res. Int. 143, e110290. doi: 10.1016/j.foodres.2021.110290
Yatmaz, E. and Turhan, İ. (2012). Pullulan production by fermentation and usage in food industry. Food 37, 95–102.
Ye, J., Wang, X., Wang, K., Deng, Y., Yang, Y., Ali, R., et al. (2020). A novel polysaccharide isolated from Flammulina velutipes, characterization, macrophage immunomodulatory activities and its impact on gut microbiota in rats. J. Anim. Physiol. Anim. Nutr. 104, 735–748. doi: 10.1111/jpn.13290
Yi, Y., Xu, W., Wang, H. X., Huang, F., and Wang, L. M. (2020). Natural polysaccharides experience physiochemical and functional changes during preparation: A review. Carbohydr. Polym. 234, e115896. doi: 10.1016/j.carbpol.2020.115896
Yin, C., Fan, X., Fan, Z., Shi, D., and Gao, H. (2018). Optimization of enzymes-microwave-ultrasound assisted extraction of Lentinus edodes polysaccharides and determination of its antioxidant activity. Int. J. Biol. Macromol. 111, 446–454. doi: 10.1016/j.ijbiomac.2018.01.007
Yin, Z., Liang, Z., Li, C., Wang, J., Ma, C., and Kang, W. (2021). Immunomodulatory effects of polysaccharides from edible fungus: a review. Food Sci. Hum. Wellness 10, 393–400. doi: 10.1016/j.fshw.2021.04.001
Yin, X., You, Q., and Jiang, Z. (2011). Optimization of enzyme assisted extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr. Polym. 86, 1358–1364. doi: 10.1016/j.carbpol.2011.06.053
Yin, X., You, Q., and Zhou, X. (2015). Complex enzyme-assisted extraction, purification, and antioxidant activity of polysaccharides from the button mushroom, Agaricus bisporus (higher basidiomycetes). Int. J. Med. Mushrooms 17, 987–996. doi: 10.1615/intjmedmushrooms.v17.i10.80
Yoon, K. N., Alam, N., Lee, K. R., Shin, P. G., Cheong, J. C., Yoo, Y. B., et al. (2011). Antioxidant and antityrosinase activities of various extracts from the fruiting bodies of Lentinus lepideus. Molecules 16, 2334–2347. doi: 10.3390/molecules16032334
You, Q., Yin, X., and Zhao, Y. (2013). Enzyme assisted extraction of polysaccharides from the fruit of Cornus officinalis. Carbohydr. Polym. 98, 607–610. doi: 10.1016/j.carbpol.2013.06.036
Yu, R., Luo, J., Liu, L., and Peng, X. (2024). Hypoglycemic effect of edible fungi polysaccharides depends on their metabolites from the fermentation of human fecal microbiota. Food 13, e97. doi: 10.3390/foods13010097
Yuchen-Zhang, Du, M. R., Zhang, Q. Y., Yang, S. Y., Chen, J. Q., Dan, C. M., et al. (2024). Armillariella tabescens-derived polysaccharides alleviated D-Gal-induced neuroinflammation and cognitive injury through enterocerebral axis and activation of keap-1/Nrf2 pathway. Int. J. Biol. Macromol. 273, e133035. doi: 10.1016/j.ijbiomac.2024.133035
Zavadinack, M., de Lima Bellan, D., Fernandes Bonaldi, M. P., da Silva Milhorini, S., Cordeiro, L. M. C., Fogagnoli Simas, F., et al. (2024). Polysaccharide fractions extracted from Lactarius quieticolor mushroom exhibit immune stimulatory activities on macrophages. Food Res. Int. 197, e115205. doi: 10.1016/j.foodres.2024.115205
Zeng, W. C., Zhang, Z., Gao, H., Jia, L. R., and Chen, W. Y. (2012). Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydr. Polym. 89, 694–700. doi: 10.1016/j.carbpol.2012.03.078
Zeng, F., Zhao, C., Pang, J., Lin, Z., Huang, Y., and Liu, B. (2013). Chemical properties of a polysaccharide purified from solid-state fermentation of Auricularia auricular and its biological activity as a hypolipidemic agent. J. Food Sci. 78, H1470–H1475. doi: 10.1111/1750-3841.12226
Zhang, Z., Ding, X., Xu, Z., Wu, X., Yang, C., Hao, J., et al. (2020). Optimization of ultrasonic/microwave assisted extraction (UMAE) and rheological properties of polysaccharides from Auricularia polytricha. Am. J. Biochem. Biotechnol. 16, 112–124. doi: 10.3844/ajbbsp.2020.112.124
Zhang, J., Gao, Z., Li, S., Gao, S., Ren, Z., Jing, H., et al. (2016). Purification, characterization, antioxidation, and antiaging properties of exopolysaccharides and endopolysaccharides of the royal sun medicinal mushroom, Agaricus brasiliensis (Agaricomycetes). Int. J. Med. Mushrooms 18, 1071–1081. doi: 10.1615/IntJMedMushrooms.v18.i12.20
Zhang, Y., Geng, W., Shen, Y., Wang, Y., and Dai, Y. C. (2014). Edible mushroom cultivation for food security and rural development in China: Bio-innovation, technological dissemination and marketing. Sustainability 6, 2961–2973. doi: 10.3390/su6052961
Zhang, Q., Hu, M., Xu, L., Yang, X., Chang, Y., and Zhu, Y. (2017). Effect of edible fungal polysaccharides on improving influenza vaccine protection in mice. Food Agric. Immunol. 28, 981–992. doi: 10.1080/09540105.2017.1323326
Zhang, W., Jiang, X., Zhao, S., Zheng, X., Lan, J., Wang, H., et al. (2018c). A polysaccharide-peptide with mercury clearance activity from dried fruiting bodies of maitake mushroom Grifola frondosa. Sci. Rep. 8, e17630. doi: 10.1038/s41598-018-35945-9
Zhang, Y., Lei, Y., Qi, S., Fan, M., Zheng, S., Huang, Q., et al. (2023). Ultrasonic-microwave-assisted extraction for enhancing antioxidant activity of Dictyophora indusiata polysaccharides: The difference mechanisms between single and combined assisted extraction. Ultrason. Sonochem. 95, e106356. doi: 10.1016/j.ultsonch.2023.106356
Zhang, L., Liu, Y., Ke, Y., Liu, Y., Luo, X., Li, C., et al. (2018b). Antidiabetic activity of polysaccharides from Suillellus luridus in streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 119, 134–140. doi: 10.1016/j.ijbiomac.2018.07.109
Zhang, J., Wen, C., Gu, J., Ji, C., Duan, Y., and Zhang, H. (2019). Effects of subcritical water extraction microenvironment on the structure and biological activities of polysaccharides from Lentinus edodes. Int. J.Biol. Macromol. 123, 1002–1011. doi: 10.1016/j.ijbiomac.2018.11.194
Zhang, N., Yang, B., Mao, K., Liu, Y., Chitrakar, B., Wang, X., et al. (2022b). Comparison of structural characteristics and bioactivity of Tricholoma mongolicum Imai polysaccharides from five extraction methods. Front. Nutr. 9, e962584. doi: 10.3389/fnut.2022.962584
Zhang, J., Ye, Z., Liu, G., Liang, L., Wen, C., Liu, X., et al. (2022a). Subcritical water enhanced with deep eutectic solvent for extracting polysaccharides from Lentinus edodes and their antioxidant activities. Molecules 27, e3612. doi: 10.3390/molecules27113612
Zhang, C., Zhang, L., Liu, H., Zhang, J., Hu, C., and Jia, L. (2018a). Antioxidation, anti-hyperglycaemia and renoprotective effects of extracellular polysaccharides from Pleurotus eryngii SI-04. Int. J. Biol. Macromol. 111, 219–228. doi: 10.1016/j.ijbiomac.2018.01.009
Zhao, T., Cai, Y., Jiang, Y., He, X., Wei, Y., Yu, Y., et al. (2023c). Vaccine adjuvants: mechanisms and platforms. Signal Transduction Targeting Ther. 8, e283. doi: 10.1038/s41392-023-01557-7
Zhao, S., Gao, Q., Rong, C., Wang, S., Zhao, Z., Liu, Y., et al. (2020). Immunomodulatory effects of edible and medicinal mushrooms and their bioactive immunoregulatory products. J. Fungi 6, e269. doi: 10.3390/jof6040269
Zhao, Q., Jiang, Y., Zhao, Q., Patrick Manzi, H., Su, L., Liu, D., et al. (2023b). The benefits of edible mushroom polysaccharides for health and their influence on gut microbiota: a review. Front. Nutr. 10, e1213010. doi: 10.3389/fnut.2023.1213010
Zhao, M., Kuang, F., Zhang, Y., and Lv, G. (2023a). Effects of hydrolysis condition and detection method on the monosaccharide composition analysis of polysaccharides from natural sources. Separations 11, e2. doi: 10.3390/separations11010002
Zhao, Y. M., Song, J. H., Wang, J., Yang, J. M., Wang, Z. B., and Liu, Y. H. (2016a). Optimization of cellulase-assisted extraction process and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. J. Sci. Food Agric. 96, 4484–4491. doi: 10.1002/jsfa.7662
Zhao, Y. T., Tang, Y. Q., Liu, K. Z., and Zhang, Y. (2014). A study about microwave-assisted extraction of polysaccharides form medicinal mushrooms Fomitopsis ulmaria (Sor.: For.) bond. Et sing. Adv. Mater. Res. 1073–1076, 1837–1840. doi: 10.4028/www.scientific.net/amr.1073-1076.1837
Zhao, Y. M., Wang, J., Wu, Z. G., Yang, J. M., Li, W., and Shen, L. X. (2016b). Extraction, purification and anti-proliferative activities of polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 93, 136–144. doi: 10.1016/j.ijbiomac.2016.05.100
Zheng, S., Zhang, W., and Liu, S. (2020). Optimization of ultrasonic-assisted extraction of polysaccharides and triterpenoids from the medicinal mushroom Ganoderma lucidum and evaluation of their in vitro antioxidant capacities. PloS One 15, e0244749. doi: 10.1371/journal.pone.0244749
Zhong, J., Fang, L., Chen, R., Xu, J., Guo, D., Guo, C., et al. (2021). Polysaccharides from sporoderm-removed spores of Ganoderma lucidum induce apoptosis in human gastric cancer cells via disruption of autophagic flux. Oncol. Lett. 21, e425. doi: 10.3892/ol.2021.12686
Zhong, M., Miao, Y., Lan, Y., Ma, Q., Li, K., and Chen, W. (2024). Effects of Exidia yadongensis polysaccharide as emulsifier on the stability, aroma, and antioxidant activities of fat-free stirred mango buffalo yogurt. Int. J. Biol. Macromol. 276, e133785. doi: 10.1016/j.ijbiomac.2024.133785
Zhou, W., He, Y., Liu, F., Liao, L., Huang, X., Li, R., et al. (2021). Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 256, e117579. doi: 10.1016/j.carbpol.2020.117579
Zhou, Y., Jia, Y., Xu, N., Tang, L., and Chang, Y. (2023). Auricularia auricula-judae (Bull.) polysaccharides improve obesity in mice by regulating gut microbiota and TLR4/JNK signaling pathway. Int. J. Biol. Macromol. 250, e126172. doi: 10.1016/j.ijbiomac.2023.126172
Keywords: bioactive compound, biological properties, bioprocesses, edible fungi, natural polysaccharide
Citation: Kumla J, Thangrongthong S, Kaewnunta A and Suwannarach N (2025) Research advances in fungal polysaccharides: production, extraction, characterization, properties, and their multifaceted applications. Front. Cell. Infect. Microbiol. 15:1604184. doi: 10.3389/fcimb.2025.1604184
Received: 01 April 2025; Accepted: 12 May 2025;
Published: 09 June 2025.
Edited by:
Allen Grace Tandog Niego, Iloilo Science and Technology University, PhilippinesReviewed by:
Yuwei Hu, Chinese Academy of Sciences (CAS), ChinaBernadeth Ticar, Iloilo Science and Technology University, Philippines
Copyright © 2025 Kumla, Thangrongthong, Kaewnunta and Suwannarach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nakarin Suwannarach, c3V3YW5fNDYxQGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Jaturong Kumla
Jaturong Kumla Suppasin Thangrongthong
Suppasin Thangrongthong Atsadawut Kaewnunta
Atsadawut Kaewnunta Nakarin Suwannarach
Nakarin Suwannarach