- 1Department of Gastroenterology, Wuxi No. 2 People’s Hospital, Affiliated Wuxi Clinical College of Nantong University, Wuxi, Jiangsu, China
- 2School of Medicine, Nantong University, Nantong, Jiangsu, China
- 3Wuxi School of Medicine, Jiangnan University, Wuxi, Jiangsu, China
Recent studies have deepened our understanding on gut microbiota alterations and the interaction with intestinal barrier impairments, which play a crucial role in the etiology and pathophysiology of Inflammatory bowel disease (IBD). The intestinal microbiota dysbiosis in IBD including the altered microbiota composition, decreased beneficial species and increased harmful species. The disturbed gut microbiota results in the aggravation of intestinal barrier dysfunction through regulation of antimicrobial substances in mucus layer, tight junction protein in mechanical layer and inflammatory response in immune layer. The therapeutic options targeted on the microbiota including antibiotics, probiotics and fecal microbiota transplantation (FMT) exhibit efficacies and limitations in the treatment of IBD. Reasonable single or combined use of these treatments can restore intestinal microecological homeostasis, which further contributes to the treatment of IBD. This review analyzes the underlying mechanisms for the interaction between microbiota alterations and gut barrier dysfunction in IBD; meanwhile, it provides new insights into the microbiota-targeted therapeutic options IBD, including the benefits, risks and limitations of antibiotic and probiotic therapies, unresolved clinical application strategies for FMT, and combination administrations of antibiotics and FMT.
1 Introduction
Inflammatory bowel disease (IBD), a complicated group of diseases mainly including Crohn’s disease (CD) and ulcerative colitis (UC), is characterized by the chronic mucosal or transmural inflammation of the gastrointestinal tract with notable impairment of intestinal barrier (Ungaro et al., 2017; Dolinger et al., 2024). The main symptoms of IBD, including diarrhea, hematochezia, abdominal pain, fever and malnutrition (Steinwurz et al., 2023), seriously reduce the quality of life and work ability. Due to the early disease onset and high incidence in recent years, IBD is predicted to present a high burden with continued growth until 2050 (Wang et al., 2024).
It has been demonstrated that gut dysbiosis, mainly referring to the altered composition, decreased diversity and shifted functional capacities of gut microbiota and proliferation of pathogens, are highly associated with the etiology and pathology of IBD (Schaubeck et al., 2016; Weingarden and Vaughn, 2017; Lopetuso et al., 2023). Compared to the healthy population, the diversity and stability of the gut microbiota in IBD patients are significantly reduced (Hu et al., 2022). The gut microbiota dysbiosis, exhibiting an interaction with the intestinal barrier dysfunction, contributes to the intestinal inflammation in IBD. The gut microbiota-targeted therapy in IBD, including antibiotics, probiotics and fecal microbiota transplantation (FMT), seems to show divergent outcomes on gut microbiota. Antibiotics, commonly used to treat bacterial infections, can lead to a decreased abundance and an altered composition of intestinal microbiota. Contrarily, treatments of probiotics and FMT, respectively supplementing exogenous beneficial bacteria and transferring fecal microbiota from healthy donors to the digestive tract of recipients, restore the intestinal microecology (Lopetuso et al., 2023).
This review aims to explore: 1) gut microbiota dysbiosis-related pathology in IBD, focusing on the interaction between gut microbiota dysbiosis and intestinal barrier impairment; 2) therapeutic strategies targeting gut microbiota in IBD, focusing on the indications, benefits and risks of treatment of antibiotics, probiotics, FMT, and their combination.
2 The interaction of intestinal microbiota dysbiosis and intestinal barriers in IBD
The human digestive tract contains a vast array of gut microbiota, exhibiting interactions with intestinal barrier, which are essential for multiple physiological functions including colonization resistance to pathogenic infection, regulations of the metabolites and modulations of the mucosa immune response (Morrison and Preston, 2016; Rowland et al., 2018).
It is still controversial whether the state of microecological disorder is the one of the triggers or secondary manifestation of IBD. However, as evidenced by multiple studies (Qiu et al., 2022; Yadegar et al., 2024), the gut microbiota of IBD patients is characterized by a notable reduction in diversity and a profound alteration in bacterial construction (Nishida et al., 2018; Fu et al., 2024), including proliferation of pathogenic or other harmful species, and decreased abundance of beneficial species. Furthermore, The interaction between the gut microbiota dysbiosis and the impaired intestinal barrier, including dysfunction of chemical barrier, mechanical barrier, and immune barrier (Qiu et al., 2022), is highly involved in pathophysiology of IBD (Yue et al., 2019) (Figure 1).
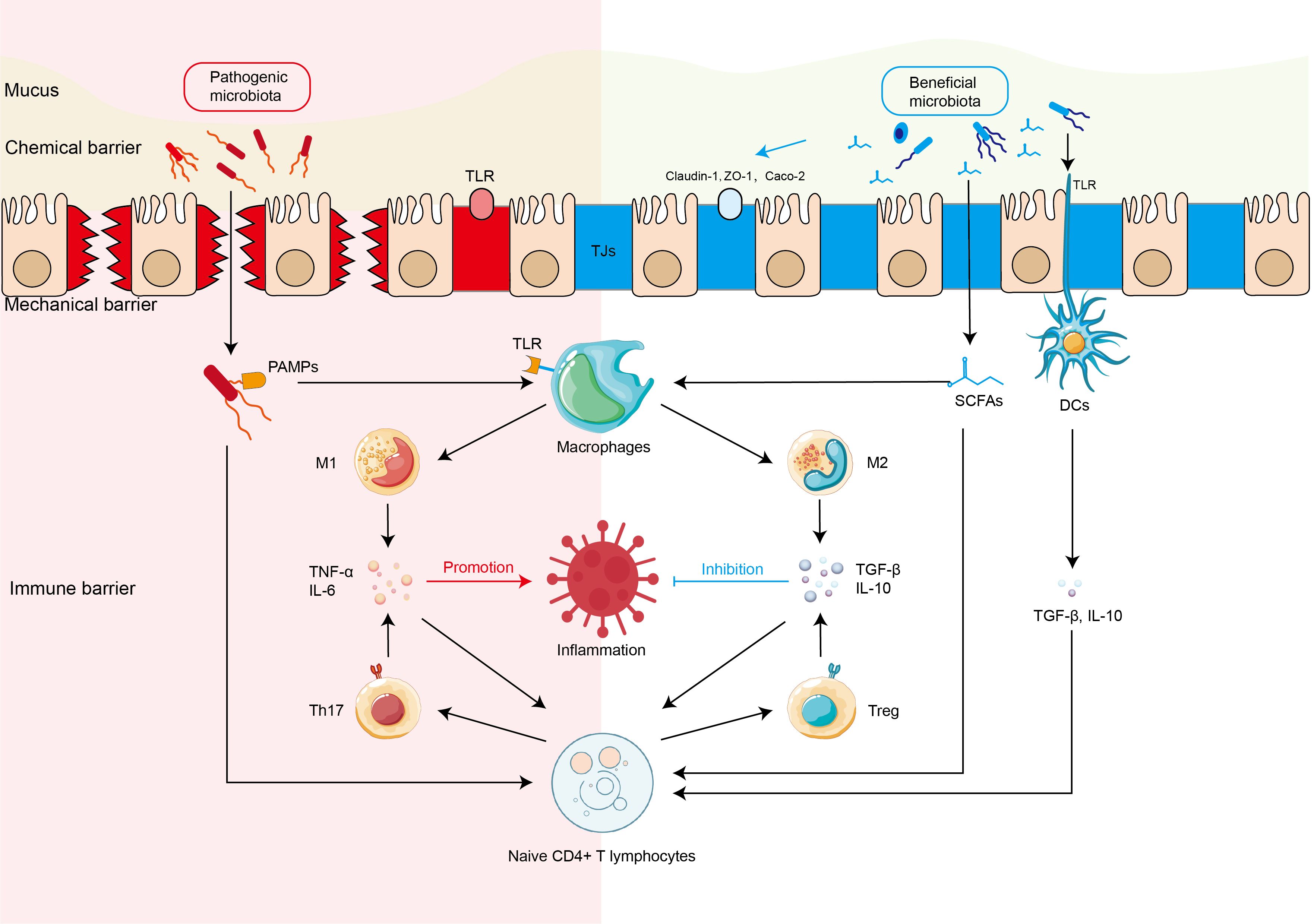
Figure 1. Interaction between intestinal microbiota and intestinal barriers in the IBD Harmful and beneficial bacteria regulate the expression of tight junction proteins of mechanical barrier and activation of intestinal immune cells (immune barrier) including macrophages, Th17 cells, dendritic cells (DCs) directly or indirectly through microbiota-derived short chain fat acids (SCFAs), which further regulate the production of inflammatory or anti-inflammatory cytokines in the pathophysiology of inflammatory bowel disease (IBD). DCs, dendritic cells; M1, M1 macrophage; M2, M1 macrophage; SCFAs, short-chain fatty acids; Tregs, regulatory T cells; TJs, tight junctions.
2.1 Alteration of gut microbiota in IBD
The stable community of gut microbiota, also known as the intestinal microbial barrier, plays multiple physiological roles, such as preventing the colonization and proliferation of harmful microorganisms, producing beneficial metabolites and vitamins, degrading nutrients, and regulating the intestinal immune and inflammatory reactions (Sugihara and Kamada, 2024). The gut microbiota, engaging in competition with pathogens for nutrients such as amino acids, sugars, metals and respiratory electron acceptors, exerts an effect of colonization resistance to pathogenic microorganisms (Stecher, 2021). For instance, commensal Escherichia coli (E. coli) competes with pathogenic E. coli for carbohydrates, amino acids, organic acids, and other nutrients (Kamada et al., 2012). Similarly, Phascolarctobacterium competitively inhibits Clostridioides difficile (C. difficile) by reducing the availability of luminal succinate, a key metabolite necessary for the growth of C. difficile (Nagao-Kitamoto et al., 2020).
The diversity of both the mucosa and fecal microbiota reveals significant reductions in IBD. Investigation of microbiomes via a metagenomic sequencing revealed that mucosal microbial genes of IBD patients reduced by 25% in comparison with those of healthy controls (Qin et al., 2010). The fecal microbiota analyses, mainly representing those of resident luminal bacteria, have exhibited the altered composition and abundance of gut flora involved in the microbiota dysbiosis in IBD. At the phylum level, the predominant gut bacteria are Firmicutes and Bacteroidetes, which comprise 80–90% of the population, and secondary ones include Actinobacteria, Proteobacteria, and Fusobacteria (Clemente et al., 2012). However, IBD patients have been reported to show a marked decrease in the abundance of Firmicutes and an increased abundance of Proteobacteria (Nishino et al., 2018; Yang et al., 2021). The alteration of Bacteroidetes in IBD patients remains controversial (Walker et al., 2011; Xu et al., 2022; Fu et al., 2024), which may be attributed to variations in samples, and activities of IBD (Matsuoka and Kanai, 2015).
At the genus level, IBD patients exhibit a reduced abundance of beneficial bacteria, including Faecalibacterium and Roseburia (Morgan et al., 2012; He et al., 2019), which are known as key producers of short-chain fatty acids (SCFAs) (Rossi et al., 2016; Lloyd-Price et al., 2019) with the anti-inflammatory properties (Machiels et al., 2014; Nie et al., 2021) It has also been indicated a notable decrease in mucosa-associated Bifidobacterium in UC patients (Mylonaki et al., 2005) as well as a significant reduction in some special species of Bifidobacterium in CD patients, including Bifidobacterium bifidum (B. bifidum), Bifidobacterium longum, Bifidobacterium adolescentis, and Bifidobacterium dentium (Gevers et al., 2014). IBD patients show lower abundance of Lactobacillus, which exhibits weaker adhesion to epithelial cells (Najafi et al., 2022). Supplementation with these reduced beneficial bacteria can be used as a treatment option for IBD, as detailed in the Probiotic treatment section (Section 3.2). Examples of the well-recognized probiotic treatments are as follows: Bifidobacterium and Lactobacillus are administrated as traditional probiotics to provide therapeutic benefits in the treatment of IBD (Jakubczyk et al., 2020; Roy and Dhaneshwar, 2023). In addition, treating Dextran Sulfate Sodium (DSS)-induced UC in mice model with Akkermansia muciniphila (Akk), another species of gut bacteria reduced in IBD (Png et al., 2010), could alleviate the colonic inflammation (Bian et al., 2019).
The abundance of pro-inflammatory bacteria is increased in IBD patients (Tsai et al., 2025). Several pathogenic microorganisms are associated with the aggravation or progression of IBD, such as C.difficile (Bosca-Watts et al., 2015), E. Coli (Rahman et al., 2014), Mycobacterium avium (M.avium) (Feller et al., 2007), Campylobacter and Salmonella enterica (Gradel et al., 2009). Additionally, Ruminococcus gnavus (R.gnavus) is a resident bacterium in healthy individuals, whereas is particularly enriched in CD patients. It has been reported to induce the dendritic cells (DCs) to produce pro-inflammatory cytokines in CD (Joossens et al., 2011; Crost et al., 2023).
2.2 Gut microbiota alteration and chemical barrier impairment
The intestinal chemical barrier is mainly composed of mucus layer, including various chemical substances, such as gastric acid, bile, digestive enzymes, lysozyme and mucins (MUCs) produced by cells of host’s GI tract, and antimicrobial substances produced by the gut microbiota (Ren et al., 2019). The outer mucus layer serves as a nurturing habitat for the gut microbiota; meanwhile, the inner mucus layer acts as a shield, keeping microorganisms away from the intestinal epithelial cells (IECs). In addition to the competition with pathogens, several beneficial bacteria produce small antimicrobial molecules called bacteriocins, which can eliminate specific pathogenic microorganisms (Dobson et al., 2012). The bacteriocins produced by lactobacilli and/or bifidobacteria, including H2O2, acetic and lactic acids and biosurfactants, show benefits in inhibiting the overgrowth of Gram-positive bacteria and pathogenic microorganisms by disrupting the cell membrane and interfering with enzyme activity (Servin, 2004). However, the reduced abundance of lactobacilli and bifidobacteria has been widely reported (Mylonaki et al., 2005; Gevers et al., 2014; Najafi et al., 2022), which might further result in the dysfunction of chemical barrier.
2.3 Gut microbiota alteration and mechanical barrier impairment
The intestinal mechanical barrier is mainly based on the integrity of the IECs and the tight junctions (TJs) between IECs (Goto, 2013; Kurashima and Kiyono, 2017). It contributes significantly to defending against pathogens.
The impairments of intestinal mechanical barrier, in particular the apoptosis of IECs and the destruction of TJs, are widely reported in IBD (Coskun, 2014). B. bifidum has been reported to enhance the TJs through a Toll-like receptor-2 (TLR-2) and p38 kinase-dependent pathway (Al-Sadi et al., 2021a). However, Bifidobacteria, especially B. bifidum, exhibited a significant decrease in IBD patients (Duranti et al., 2016). Furthermore, the abundance of SCFA-derived beneficial bacteria reduced in IBD patients (Sokol et al., 2008). SCFAs have been demonstrated to be a key issue in the restore of the intestinal barrier through regulation of TJ proteins and protection of IECs (Wang et al., 2012). SCFAs could upregulate the expression of TJ protein including claudin-1 and Zonula Occludens-1 (ZO-1), and promote the redistribution of occludin (Wang et al., 2012). Meanwhile, butyrate, an important type of SCFAs acting as an energy source, contributes to the proliferation of IECs and reducing their apoptosis (Tremaroli and Bäckhed, 2012). In addition, succinate produced by gut microbiota can promote the specification of tuft cells, which further inhibits the chronic intestinal inflammation in mice (Banerjee et al., 2020). Tuft cells, a rare type of chemosensory epithelial cell in the gut and other mucosal tissues (Strine and Wilen, 2022), play a role in the repair of intestinal epithelium during chronic colitis (Yi et al., 2019). However, several studies reported that tuft cells were significantly reduced in the intestines of UC and CD patients (Banerjee et al., 2020; Kjærgaard et al., 2021).
Contrarily, the increases in harmful and pathogenic microbiota may lead to intestinal mechanical barrier dysfunction via the following pathways: 1) The adherent-invasive E. coli and Fusobacterium nucleatum could attach to IECs and invade mucosal tissue, and further result in excessive intestinal inflammation and intestinal barrier impairment (Darfeuille-Michaud et al., 2004; Brennan and Garrett, 2019; Liu et al., 2020). 2) Pathogen-associated molecular patterns (PAMPs), such as the lipopolysaccharide (LPS) of E. coli (Heimesaat et al., 2007) and the flagellin of Salmonella (Salazar-Gonzalez and McSorley, 2005), are highly conserved structures of microbes (Lim and Staudt, 2013). PAMPs-induced excessive activation of TLRs can result in an increased intestinal barrier permeability (Guo et al., 2013). 3) The harmful microbiota can inhibit the localization and expression of TJ proteins, which further lead to the deterioration of TJs and activation of pro-inflammatory signaling (Jergens et al., 2021). Subsequently, the increased pro-inflammatory cytokines trigger apoptosis of IECs by activating intracellular apoptotic signaling pathways, leading to disruption of the intestinal epithelial integrity and intestinal barrier dysfunction (Iwamoto et al., 1996; Di Sabatino et al., 2003). For example, Salmonella typhimurium induces a strong inflammatory response and disrupts epithelial TJs through its outer proteins such as Salmonella outer protein (Sop) B, SopE, and SopE2 (Jepson et al., 1995; Khan, 2014). 4) The harmful bacteria can activate the apoptosis pathways of IECs, which disrupts the integrity of intestinal barrier. For instance, C.difficile can secrete exotoxin A, which causes colonic epithelial cells to turn round, detach from the basement membrane, and undergo apoptosis (Mahida et al., 1998).
2.4 Gut microbiota alteration and immune barrier impairment
The intestinal immune barrier is composed of a vast array of immune cells located within the gut or dispersed across the lamina propria and intestinal epithelium (Perez-Lopez et al., 2016). In IBD patients, pathogens, such as Salmonella and Shigella, or opportunistic pathogens, such as adherent-invasive Escherichia coli (AIEC), pass through a compromised intestinal epithelial barrier, penetrate into the lamina propria (Tawfik et al., 2014), and further activate pattern recognition receptors (PRRs) of immune cells.
PAMPs primarily trigger macrophages and DCs through PRRs, including TLRs and NOD-like receptors (NLRs), and subsequently initiate the pro-inflammatory responses (Walsh et al., 2013). In detail, in the case of gut microbiota dysbiosis, certain pathogens or their products, such as LPS, can excessively activate TLRs on immune cells, especially for TLR4 (Stephens and von der Weid, 2020). The activation of TLRs initiates the downstream signaling pathways, including the MyD88-dependent and TRIF-dependent pathways. The MyD88-dependent pathway activated NF-κB, which upregulates the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and ultimately results in the amplifying of the inflammatory response. The TRIF-dependent pathway induces the production of type I interferons through interferon regulatory factor 3 (IRF3), which plays a significant role in promoting inflammatory responses (Lawrence, 2009; Lu et al., 2018; Liu et al., 2020).
As pivotal elements of the adaptive immune system, T cells differentiated from naive CD4+ T lymphocytes, such as T helper cells 17 (Th17) and regulatory T cells (Treg), are essential in the progression of IBD (Geremia et al., 2014). Th17 cells aggravate the intestinal inflammation in IBD by secreting IL-17 and IL-22, which are key pro-inflammatory cytokines for activating innate immune cells, promoting neutrophil recruitment, disrupting the intestinal epithelial barrier, and aggravating intestinal inflammation (Rutz et al., 2013; Wu et al., 2016). Gut microbiota contributes to the differentiation and activation of intestinal mucosal immunocytes. A rodent study has demonstrated that transplantation of disorganized gut flora from IBD patients to the germ-free mice resulted in an increase in Th17 cells (Britton et al., 2019). PAMPs from harmful bacteria, such as LPS and flagellin, activate DCs and macrophages, secreting IL-6 and IL-23 (Sica and Mantovani, 2012; Xu et al., 2022), which further promote the differentiation of CD4+ T-cell into Th17 cells via activation of the STAT3 pathway (Yan et al., 2020).
Gut microbiota regulates the polarization of monocytes toward different phenotypes of macrophages. On one hand, the harmful microbiota, including the pathogenic ones, promote the polarization of monocytes toward M1 phenotype macrophages through above-mentioned MyD88/NF-κB and TRIF/IRF3 pathway (Figure 2A) (Baker et al., 2011). On the other hand, the beneficial bacteria, such as Lactobacillus and Bifidobacterium, play a crucial anti-inflammatory role by maintaining immune homeostasis in IBD, which is mainly mediated via the regulation of SCFAs (Roy and Dhaneshwar, 2023). SCFAs, acting as natural histone deacetylase (HDAC) inhibitors, increase histone acetylation levels, and further induce the polarization of monocytes to M2 phenotype macrophages (Chang et al., 2014). Meanwhile, Butyrate facilitates the IL-4 induced phosphorylation of STAT6, and subsequently increases the mRNA expression of M2-associated genes, including Arg1, Fizz1, and Ym1 (Ji et al., 2016), resulting in the polarization of M0 macrophages toward the M2 phenotype. (Figure 2B). M2 phenotype macrophages secrete anti-inflammatory cytokines including TGF-β, which contribute to maintaining an immunosuppressive state (Cutolo et al., 2022). Additionally, TGF-β facilitates the differentiation of naive CD4+Tcells to Tregs, which further amplifies anti-inflammatory responses and promotes homeostasis (Hadis et al., 2011).
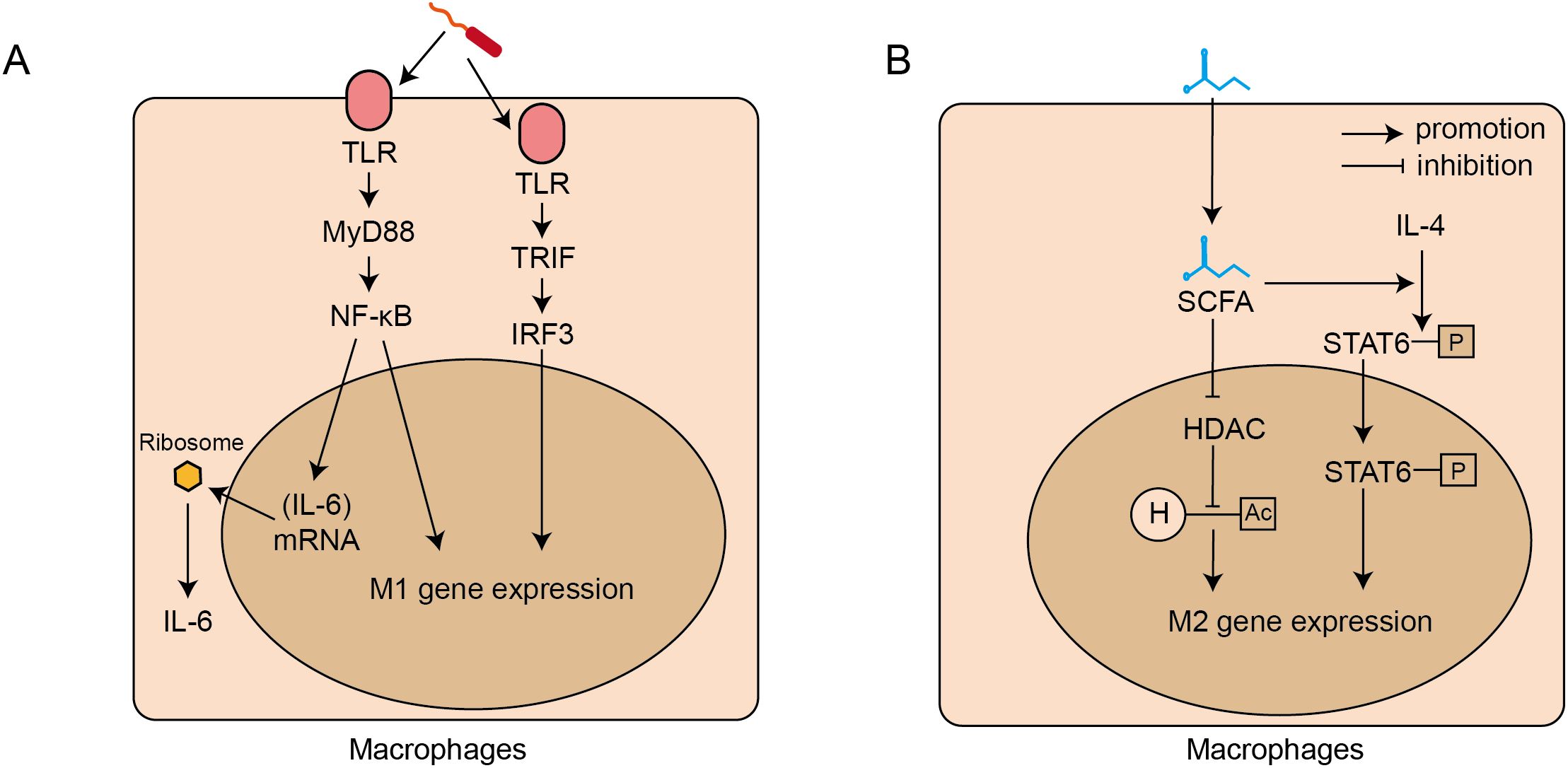
Figure 2. Signaling pathways for intestinal microbiota regulating the polarization and activation of intestinal macrophages. (A). Harmful bacteria-induced M1 polarization and IL-6 secretion: through TLR-MyD88-NF-κb and TLR-TRIF-IF3 signalling pathways. (B). Beneficial bacteria induced M2 polarization: through short chain fat acids (SCFAs) which inhibit HDAC activity and activate the STAT6 signalling pathway. TLRs, toll-like receptors; MyD88, Myeloid Differentiation Primary Response 88; NF-κB, Nuclear Factor kappa-light-chain-enhancer of Activated B cells; TRIF, TIR-domain-containing adapter-inducing interferon-β; IRF3, Interferon Regulatory Factor; M1, macrophage 1 phenotype macrophages; SCFAs, short-chain fatty acids; STAT, Signal Transducer and Activator of Transcription; HDAC, Histone Deacetylase; H, Histone; Ac, Acetyl group; M2, macrophage 2 phenotype macrophages.
In addition to the M2 type macrophage, activated DCs produce immune-suppressive cytokines, including TGF-β and IL-10, when beneficial bacteria interacts with PRRs of DCs (Ghavami et al., 2020).The maturation and activation of DCs in the mesenteric lymph nodes enhance their antigen-presenting properties, which supports immune tolerance and promote differentiation of naive T cells into Tregs (Coombes et al., 2007). For example, Faecalibacterium prausnitzii (F. prausnitzii) interacts with TLR2/6 receptors of DCs, activate the MAPK-JNK signaling pathway and enhances the expression of anti-inflammatory factors, IL-10 and IL-27, which promotes the generation/differentiation of Tregs (Alameddine et al., 2019; Amoroso et al., 2020). In IBD patients, a decrease in the abundance of F. prausnitzii leads to a notable reduction in intestinal Tregs, which subsequently diminishes the inhibition on the colonic Th17 cells (Ohnmacht et al., 2015). IL-25, another anti-inflammatory factor mainly secreted by Tuft cells, could inhibit the activation of CD4 + T cell and inhibit their differentiation into T helper 1 (Th1)/Th17 cells via an IL-10-dependent pathway in IBD (Su et al., 2013). However, both tuft cells and IL-25 are significantly reduced in IBD patients.
3 Microbiota-targeted treatment options for inflammatory bowel disease
The interaction between gut microbiota dysbiosis and the impaired intestinal barrier plays the crucial roles in the pathophysiology of IBD.As the microbiota-targeted treatment for the improvement or restoration of the intestinal microecology in IBD, antibiotics, probiotics and FMT exhibit their advantages, deficiencies and synergistic effects (see Table 1) when used alone or in combination.
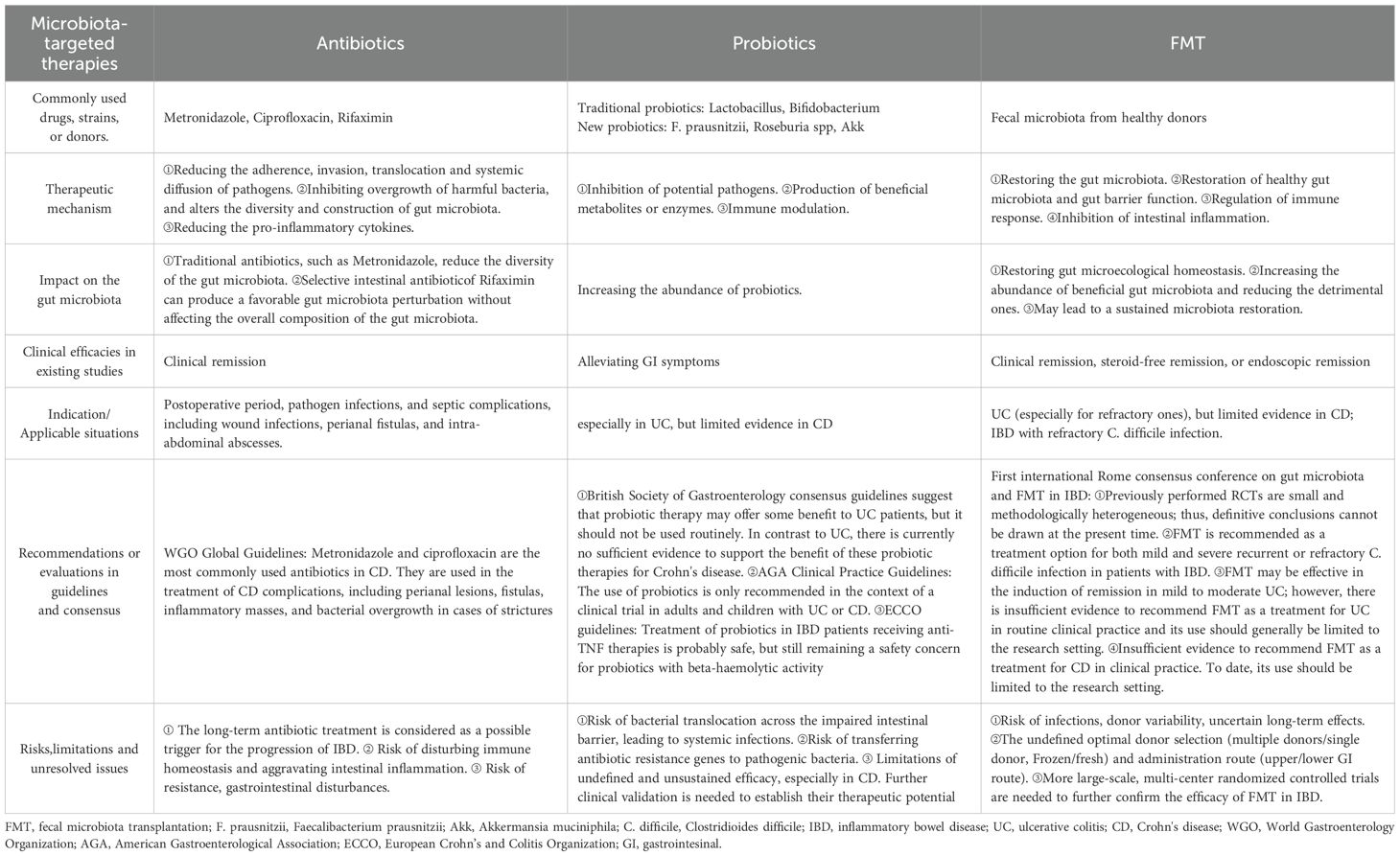
Table 1. Comparison of microbiota-targeted therapies in Inflammatory bowel disease: antibiotics, probiotics and fecal microbiota transplantation.
3.1 Antibiotic therapy in IBD
The antibiotics are applied as a reliable therapy in IBD patients (Khan et al., 2011), although they are described as “deep modulators of gut microbiota between good and evil” (Ianiro et al., 2016). The antibiotics exhibit benefits through various potential mechanisms. Firstly, antibiotics can reduce the adherence, invasion, translocation and systemic diffusion of pathogens (Ledder and Turner, 2018). Secondly, antibiotic treatment alters the diversity and construction of gut microbiota. It inhibits the overgrowth of harmful bacteria, and indirectly provide a favorable environment for the survival of beneficial bacteria. Thirdly, antibiotics can reduce the pro-inflammatory cytokines which might due to both antibacterial and immunoregulatory properties (Garrido-Mesa et al., 2011). The common antibiotics used in IBD include systemic antibiotics such as ciprofloxacin and metronidazole, as well as the intestinal-selective antibiotic rifaximin (Biancone et al., 2017). For instance, metronidazole, one of the narrow spectrum antibiotics, has been applied in CD patients for eliminating the bacterial overgrowth and blocking the bacterially mediated antigenic triggers (Lichtenstein et al., 2018). The typical prescription involves 500mg of metronidazole administrated orally or intravenously every 8 hours for a duration of 7–10 days. Rifaximin is another key antibiotic used in the treatment of IBD (Biancone et al., 2017). In addition to its direct bactericidal activity, Rifaximin has also been shown to inhibit intestinal bacterial translocation, reduce the adhesion and internalization of pathogenic bacteria (Fiorucci et al., 2002; Brown et al., 2010), and inhibit the expression of pro-inflammatory factors (Cheng et al., 2010). A clinical trial has shown that Rifaximin can induce and maintain remission in patients with moderate active Crohn’s disease (Prantera et al., 2012). Interestingly, Maccaferri S et al. showed that Rifaximin did not affect the overall composition of the gut microbiota, but it did lead to an increase in the concentration of Bifidobacterium, Atopobium, and F. prausnitzii (Maccaferri et al., 2010). This finding differentiates Rifaximin from other common antibiotics. By producing a favorable gut microbiota perturbation, Rifaximin holds the potential to open new horizons for its use in a specific group of CD patients (Lopetuso et al., 2018). Additionally, as a selective intestinal antibiotic, Rifaximin acts locally in the gut, resulting in fewer side effects and helping to avoid systemic resistance issues, making it particularly advantageous in the treatment of IBD (Biancone et al., 2017). Although widely administrated in IBD, antibiotics cannot replace first-line anti-inflammatory agents such as amino salicylates, corticosteroids, or immunosuppressants in the treatment of IBD patients (Lichtenstein et al., 2018; Rubin et al., 2019). The more widely accepted view is that antibiotic treatments might benefits IBD patients in certain circumstances such as postoperative period, pathogen infections, and septic complications including wound infections, perianal fistulas and intra-abdominal abscesses (Jha et al., 2024).
Most of the evidence suggests that short-term microbiota-targeted therapeutic agents trigger rapid alterations in the diversity and construction of microbiota, whereas the altered gut microbiota restores to its pre-treatment state once treatment is completed (Berg et al., 2015).However, the long-term antibiotic treatment is considered as a possible trigger for the progression of IBD (Theochari et al., 2018). It has been demonstrated that patients who previously received treatments of three or more antibiotics presented a 55% increased risk of developing IBD compared to those who never used antibiotics (Sokol, 2020). Based on early data from 24,000 IBD patients, a national population-based study in Sweden showed a significant increased risk of IBD which is associated with high cumulative exposure to systemic antibiotic therapy, particularly applications of broad-spectrum antibiotics (Nguyen et al., 2020). Antibiotic-related risk of IBD further increases in individuals aged 40 years or more and during cumulative antibiotic exposure or 1–2 years after antibiotic exposure (Faye et al., 2023).
Antibiotic treatments, especially long-term and/or broad-spectrum antibiotic treatments reduce the abundance of various gut microbiota, including beneficial bacteria (Jernberg et al., 2007; Kim et al., 2017), which subsequently disturbs immune homeostasis and aggravates intestinal inflammation (Yoon and Yoon, 2018). The antibiotics significantly alter the diversity of the gut microbiota, hinder the long-term reconstruction of the gut microbiota, and promote the differentiation of CD4+ T cells toward a Th17 pro-inflammatory phenotype (Burrello et al., 2018). Meanwhile, antibiotics induced disruption of the gut microbiota results in an overreaction of intestinal macrophages which produce excess pro-inflammatory cytokines (Scott et al., 2018); Further, re-exposure of antibiotic-treated mice to common microbiota induces an increased susceptibility of macrophage-dependent inflammatory Th1 cells and persistent dysbiosis in the colon (Scott et al., 2018). Accordingly, antibiotics serve as a double-edged tool in the treatment of IBD, further researches with high-level evidences are warranted for more reasonable antibiotic therapy in IBD.
3.2 Probiotic treatment in IBD
Probiotics are live microorganisms that provide a health benefit to the host when taken in sufficient quantities. According to the previous reports, when Lactobacillus and Bifidobacterium were delivered in food at a level of 1 × 10^9 colony-forming units (CFU) per serving, they were accepted and recognized as probiotics by Health Canada and the Italian Ministry of Health (Hill et al., 2014). Alternatively, the expert panel of the International Scientific Association for Probiotics and Prebiotics indicated that certain potential mechanisms of probiotics, such as the inhibition of potential pathogens or the production of beneficial metabolites or enzymes, are widespread among various strains, while other effects, such as immune modulation, are only present in specific strains (Hill et al., 2014). Probiotics, as a potential adjunctive therapy, have garnered increasing attention in the treatment of IBD, particularly in alleviating symptoms, reducing intestinal inflammation, and restoring gut microbial homeostasis (Bjarnason et al., 2019; Jakubczyk et al., 2020; Martín et al., 2023). Additionally, the application of probiotics carries potential safety concerns. probiotics are considered to have the potential for bacterial translocation across the intestinal barrier. When intestinal barrier is severely damaged in IBD, long-term colonized probiotics may enter the bloodstream, leading to systemic infections (Rouanet et al., 2020). European Crohn’s and Colitis Organization (ECCO) guidelines demonstrates that treatment of probiotics in IBD patients receiving anti-TNF therapies is probably safe, but still remaining a safety concern for probiotics with beta-haemolytic activity (Kucharzik et al., 2021). Furthermore, probiotics may carry antibiotic resistance genes, which can be transferred to pathogenic bacteria through horizontal gene transfer, thereby complicating treatment (Merenstein et al., 2023).
Lactobacillus (e.g. strains of acidophilus, casei, fermentum, gasseri, johnsonii, paracasei, plantarum, rhamnosus and salivarius) is widely recognized as one of the representative strains of probiotics in the adjuvant treatment of IBD, which plays a vital role in alleviating intestinal injury, strengthening intestinal barrier function, and regulating immune responses (Li et al., 2023). Lactobacillus can secrete antimicrobial substances, such as bacteriocins and hydrogen peroxide, which inhibit the proliferation of pathogens (Dempsey and Corr, 2022) and other harmful bacteria, such as Campylobacter jejuni, Salmonella Enteritidis, and E.coli (Carter et al., 2017; Chingwaru and Vidmar, 2017; Saint-Cyr et al., 2017). A specific strain of Lactobacillus acidophilus has been reported to strengthen the TJs of intestinal mechanical barrier through TLR-2 (Al-Sadi et al., 2021b). Lactobacillus rhamnosus has been shown to promote the expression of Muc2 and Muc3 in goblet cells in murine intestinal inflammation models, thereby enhancing mucus production to strengthen the intestinal chemical barrier (Martín et al., 2019). Additionally, Lactobacillus casei has been shown to strengthen the intestinal immune barrier, mediated via the activation of Treg cells, upregulation of IL-10, and reduction of TNF-α and IL-12, thus reflecting an anti-inflammatory effect in IBD (Liu et al., 2021).
Bifidobacteriaceae (e.g. strains of adolescentis, animalis, bifidum, breve and longum) has been well recognized as a beneficial microbiota in IBD patients. Probiotic therapy with Bifidobacteria alleviates symptoms in IBD patients (O’Callaghan and van Sinderen, 2016). Several studies reported that Bifidobacteria play an important role in maintaining the integrity of intestinal epithelial barrier (Khailova et al., 2009; Bergmann et al., 2013; Hsieh et al., 2015; Martín et al., 2016).A recent rodent study on trinitrobenzene sulfonic acid induced colitis further indicated that Bifidobacteria alleviate the colitis by upregulating the expression of indoleamine 2,3-dioxygenase, which further increases the Treg cells (Zhao et al., 2018). Bifidobacteria can produce health-promotors including vitamins and SCFAs, which strengthen the intestinal barrier and modulate the host’s immune response (Al-Sadi et al., 2021a). One study in Caco-2 monolayers showed that B. bifidum contributed to a marked, sustained enhancement on the intestinal TJs of mechanical barrier and protected against intestinal inflammation through targeting TLR-2 and via an NF-κB-independent pathway (Al-Sadi et al., 2021a). Meanwhile, it was indicated that B. bifidum regulated the host’s immune response and reduces the expression of TNF-α, exerting an anti-inflammatory effect (Chae et al., 2018).In addition to traditional probiotics including Lactobacillus and Bifidobacteria, new generation of probiotics, such as F. prausnitzii, Roseburia spp. and Akk, has being developed for the treatment of IBD (Al-Fakhrany and Elekhnawy, 2024). F. prausnitzii belongs to family Oscillospiraceae, works as the key bacteria that produce butyrate, an important SCFAs (Louis and Flint, 2017).Several researches suggest that butyrate plays a crucial role in maintaining gut homeostasis, including defense against pathogen colonization, restoration of the TJs, and modulation of the immunoreaction (Plöger et al., 2012; Miquel et al., 2013). Meanwhile, F. prausnitzii is also well known for its immunomodulatory properties, exerting an anti-inflammatory effect by upregulating the expression of Dact3, a gene linked to the WntJNK pathway (den Besten et al., 2013; Lenoir et al., 2020). Meanwhile, F. prausnitzii might promote the Tregs differentiation/expansion via an IL-10-dependent pathway, which mediate the tolerance to inflammation signals (Sarrabayrouse et al., 2014). It has been widely shown that the abundance of intestinal F. prausnitzii is significantly reduced in both CD and UC patients compared to healthy controls; meanwhile, IBD patients in active stage showed a significant lower abundance of F. prausnitzii than those in remission stage (Zhao et al., 2021). FMT therapy can partially reverse the abundance of F. prausnitzii, which also aids in modulating the intestinal Th17/Treg balance and alleviates intestinal inflammation (Huang et al., 2022b). Roseburia is known for its positive effects on IBD, particularly as a butyrate-derived bacterium (Vacca et al., 2020). In addition, Roseburia intestinalis contributes to restoration of the gut microbiota through upregulated expression of IL-22 and restoration of the intestinal barrier integrity through upregulation of the Occludin, one of the important TJ proteins. Accordingly, the abundance of Roseburia is negatively correlated with the activity of UC (Machiels et al., 2014). Akk is a new probiotic with great potential in the treatment of IBD through various mechanisms, including modulation of gut microbial homeostasis, immune response, inhibition of pathogen colonization, and enhancement of intestinal barrier function (Zheng et al., 2022). Studies have shown that both live Akk and inactivated Akk can alleviate DSS-induced colitis in mice (Bian et al., 2019; Qian et al., 2022; Zheng et al., 2023). Inactivated Akk acts through several components including outer membrane protein Amuc_1100, enzyme Amuc_2109 and extracellular vesicles (AmEVs). AmEVs has been reported to regulate intestinal barrier permeability by regulating the expression of TJ proteins (Chelakkot et al., 2018). Akk can regulate the differentiation of Tregs, increase production of SCFA, downregulate the expression of pro-inflammatory cytokines (including TNF-α and IFN-γ) in the colon of mice, and promote the restoration of the gut microbiota (Zhai et al., 2019). Emerging researches also highlights other promising probiotics like Christensenella minuta, Anaerostipes spp., Oscillospira spp., and Saccharomyces boulardii in reducing IBD risk and improving gut barrier function, supported by their anti-inflammatory butyrate production and microbiota-modulating effects. However, further clinical validation is needed to establish their therapeutic potential (Jan et al., 2024).
Overall, existing studies have demonstrated the therapeutic potential of probiotics in the treatment of IBD. However, probiotic therapy may only serve as a complementary treatment for IBD. According to the American Gastroenterological Association (AGA) Clinical Practice Guidelines, the use of probiotics is only recommended in the context of a clinical trial in adults and children with UC or CD (Su et al., 2020). The British Society of Gastroenterology consensus guidelines suggest that probiotic therapy may offer some benefit to UC patients, but it should not be used routinely. In contrast to UC, there is currently no sufficient evidence to support the benefit of these probiotic therapies for Crohn’s disease (Lamb et al., 2019). High-quality, well-designed, multi-center and large sample studies are needed to provide high-level evidence for the application of probiotics in the future.
3.3 FMT treatment in IBD
FMT, a new therapeutic option for IBD, has attracted increasing attention for its potential benefits in IBD patients by restoring gut microecological homeostasis (Imdad et al., 2023). FMT refers to the transfer of healthy donor feces into the recipient’s GI tract, in order to restoring the gut microbiota to treat intestinal and extra-intestinal diseases. FMT is recommended in the guidelines for treating recurrent C. difficile infection (CDI) (Cammarota et al., 2017; Kucharzik et al., 2021), and is also considered to reveal therapeutic potential in the treatment of other diseases, such as IBD, irritable bowel syndrome (IBS), and functional constipation (Cammarota et al., 2017). A large number of evidences establish the beneficial efficacies of FMT in patients with IBD by restoring the disordered gut microbiota, increasing the abundance of beneficial gut microbiota and reducing the detrimental ones (Pai et al., 2021; Huang et al., 2022a; Kedia et al., 2022).
Recent randomized controlled trials (RCTs) have indicated that FMT exhibited a therapeutic property for the remission of UC (see Table 2). Most of these RCTs (5/6) demonstrated that FMT is more effective in inducing clinical and/or endoscopic remission in UC patients compared to the control group. Brezina et al (Březina et al., 2021). and Fang et al (Fang et al., 2021). indicated that FMT therapy resulted in noninferior or higher clinical remission rate compared to the traditional treatment of 5-aminosalicylic acid (5-ASA) and/or steroid. Compared to those treated with water enema (Sham-FMT) in placebo group, Moayyedi et al (Moayyedi et al., 2015). showed that 6 times of FMT treatments from single donor (FMT-SDN) resulted in a significant higher clinical remission rate (24% vs. 7%, P=0.03). Meanwhile, Costello et al (Costello et al., 2019). and Paramsothy et al (Paramsothy et al., 2017). revealed significant higher rate of Steroid-free clinical and/or endoscopic remission though FMT from multiple donors (FMT-MDN) in comparison with FMT with autologous feces (FMT-A) or Sham-FMT (isotonic saline enema). However, Rossen et al (Rossen et al., 2015). showed that 2 times of FMT-SDN (n=15) showed no significant difference on clinical remission rate in UC patients in comparison with that of FMT-A (n=8), which might be attributed to the limited sample size and FMT administration frequency.
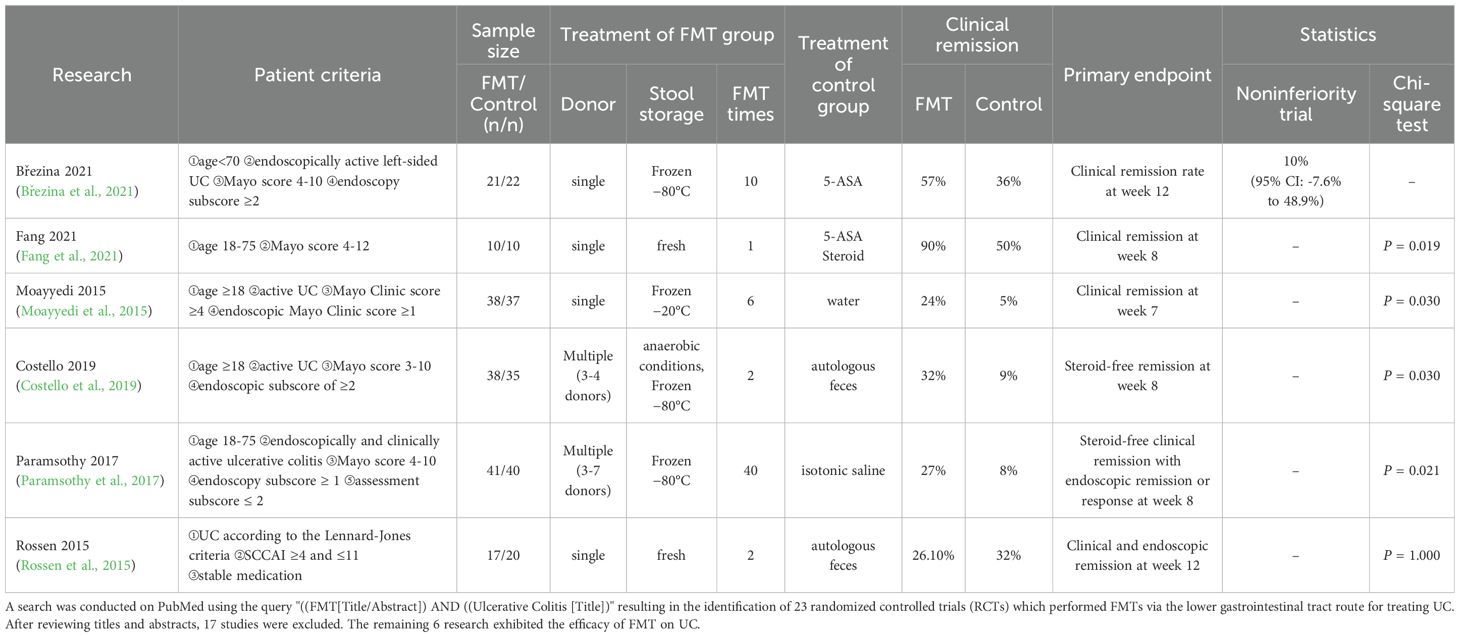
Table 2. Randomized controlled trials (RCTs) of Fecal microbiota transplantation (FMT) treatment for ulcerative colitis (UC) patients.
The variations in donor selection and treatment intensity may contribute to the different clinical outcomes. The higher bacterial species richness in donors are associated with successful transplantation in FMT treatment in UC patients (Vermeire et al., 2016). In the above-mentioned RCTs in Table 2, 3 of them used FMT-SDN, whereas Costello et al. and Paramsothy et al. applied FMT-MDN. Compared to patients received FMT-SDN, patients received FMT-MDN achieved steroid-free remission at the primary endpoint. A meta-analysis that included 14 studies indicated that FMT-MDN showed superior treatment response in IBD compared to placebo or FMT-SDN (Levast et al., 2023). Another study also revealed that pooling stools from multiple donors to increase microbial diversity could enhance remission rates in UC patients (Kazerouni and Wein, 2017). FMT-MDN in IBD patients offers broader microbial diversity that aids in restoring the gut microbial flora homeostasis and improving treatment efficacy. However, FMT-MDN results in complicated procedures and increased resource consumption, while FMT-SDN is more stable, with simple procedure and easy storage. In addition, a systematic review and meta-analysis showed that FMT-MDN and FMT-SDN were similar in therapeutic safety; meanwhile, FMT-MDN treatment demonstrated a better efficacy in UC patients, thus FNT-MDN indicated a greater benefit-risk ratio than FMT-SDN (Laperrousaz et al., 2024).
The Rome consensus indicates that donor feces, whether fresh or frozen, can be used in FMT. The frozen FMT samples are preferred over fresh preparations, primarily due to safety concerns (Lopetuso et al., 2023). However, it is still controversial whether fresh or frozen feces provides superior efficacy in FMT for IBD patients. A systematic review, which included a total of 14 trials, indicated no significant difference in clinical remission rate between FMT using fresh stool (40.9%) and frozen stool (32.2%) (Tan et al., 2022). However, Cheng et al. showed that fresh stool FMT yields a higher clinical remission rate than frozen stool FMT (73% vs. 43%, P < 0.05) (Cheng et al., 2021). Agarwal et al. indicated that larger volumes of fresh stool may be more effective than smaller amounts of frozen stool for treating recurrent or refractory C. difficile infection (rCDI) (Agarwal et al., 2021).
The acceptable and effective routes of administration are also an important issue in FMT (Gulati et al., 2020). The delivery of donor’s fecal microbiota to recipient’s GI tract can be carried out through the upper, middle, and lower GI routes (Cui et al., 2015; Zhang et al., 2020; Crothers et al., 2021). A meta-analysis of 14 trials showed that the clinical remission rate for FMT through the lower GI tract was similar to that through the upper GI tract (38.2% [151/395] vs. 31.2% [15/48]), P>0.05), indicating no significant difference in remission rate due to the different routes of administration (Tan et al., 2022). However, another meta-analysis containing 7 studies on treatment of UC revealed FMT-MDN using frozen donor stool delivered through the lower GI tract was more effective than placebo; whereas no significant difference on efficacy was recorded between FMT transplanted via the upper GI tract and placebo (Tang et al., 2020). Although it still remains unclear which route acts as optimal one regarding the efficacy of FMT, physicians typically take into account additional key factors such as patient compliance, cost-effectiveness, comfort of administration, invasiveness, risk of aspiration and infection, the number of drugs to be administered, and relapse rate when selecting the route of FMT administration (Gulati et al., 2020). For example, the upper and middle GI route, including infusion through nasogastric, nasojejunal, gastrostomy or jejunostomy tube, might result in psychological difficulties in IBD patients (Peng et al., 2016; Chen et al., 2025). However, encapsulated oral FMT for a long-term therapy in UC is considered to be safe and well accepted (Crothers et al., 2021). The lower GI routes encompass enemas, colonoscopy, colostomy, and transendoscopic enteral tubing (Peng et al., 2016). Notably, FMT through transendoscopic enteral tubing (TET) for IBD has been reported to have a high acceptance (Lin et al., 2024). Alternatively, there is growing evidence that colonic administration, rather than nasoduodenal administration, may be safer and more effective (Kelly and Ananthakrishnan, 2019).
Compared to the higher acceptance of FMT in the treatment of UC, the efficacy of FMT in CD needs to be confirmed by further research. A small-sample study indicated the more than 40%higher steroid-free clinical remission rate at week10 in FMT group compared to that of control group (Sham-FMT), but the difference did not reach statistical significance, which might be due to the limited sample size (Sokol et al., 2020). However, another research showed that multiple FMTs (repeated FMTs every 3 months) can induce and maintain clinical remission in CD with complication of inflammatory mass (He et al., 2017).
By introducing a diverse and balanced array of microbial species, FMT has been shown to significantly increase the abundance of beneficial gut microbiota (Zhi-Ning et al., 2024), which plays a key role in restoring the gut microecological homeostasis in IBD patients. FMT induced remission in IBD patients is highly associated with the increased abundance of beneficial gut microbiota, such as families Oscillospiraceae, Clostridiaceae and Lachnospiraceae of the phylum Bacillota, as well as Coriobacteriaceae and Bifidobacteriaceae of the phylum Actinobacteria (Březina et al., 2021).
In comparison with the probiotics which are typically limited in establishing a long-term colonization in the gut, FMT can result in a sustained microbiota restoration, with microbial communities from the donor to the recipient’s GI tract persisting for extended periods, potentially contributing to a long-term therapeutic effect (Weingarden et al., 2015). FMT is potential to restore the diversity of the gut microbiota, which is often compromised in IBD patients, by reintroducing a wide array of microbial species with the corresponding metabolites and functions (Hourigan et al., 2015). One study indicated that certain probiotic strains might become undetectable within two weeks after cessation of supplementation (Maldonado-Gómez et al., 2016). However, FMT has been shown to facilitate long-term engraftment of microbial species in the recipients’ gut.
3.4 Combined therapy of antibiotic and FMT in IBD patients
3.4.1 Antibiotic pretreatment of FMT
During the preparation process for FMT recipients, some researchers employ broad-spectrum antibiotics in conjunction with classic colon cleansing with polyethylene glycol as a pretreatment strategy (Pigneur and Sokol, 2016). This procedure aims to reduce the existing gut microbial load and create a favorable environment for the transplanted microbiota (Suez et al., 2018). In a recent RCT, 2-week of antibiotics including amoxicillin, metronidazole, and doxycycline were administrated in active UC patients, followed by 8-week treatment of oral lyophilised FMT or placebo. It indicated that FMT with antibiotic pretreatment showed a significant higher corticosteroid-free clinical remission rate in comparison with those with placebo pretreatment (Haifer et al., 2022). Furthermore, several studies indicated that a single FMT with the pre-treatment of combined antibiotics resulted in a notable higher clinical remission rate in comparison with multiple FMT without antibiotic pre-treatment in IBD (Moayyedi et al., 2015; Ishikawa et al., 2022; Zhang et al., 2022). The pre-treatment of antibiotic might enhance the efficacy of FMT through reducing the luminal microbial colonies and aiding in microbial restoration (Ishikawa et al., 2017; Kump et al., 2018). The pretreatment of antibiotics facilitates the successful transfer and colonization of donor microbiota which enhance the overall therapeutic effectiveness of FMT (Ji et al., 2017; Smith et al., 2022). Although these findings highlight the importance of antibiotic pretreatment in improving FMT outcomes, further RCTs are warranted to confirm the necessity and procedure of the antibiotic pretreatment of FMT.
3.4.2 FMT reverse antibiotic- induced dysbiosis
FMT contributes to the reconstruction of the gut microbiota which was disturbed by antibiotics. A rodent study showed that both antibiotics and chemotherapy treatments significantly altered the gut microbiota, reflecting as the reduced varieties and abundance of microbial species, which were quickly restored 1 week after FMT (Le Bastard et al., 2018). Further, antibiotic exposures, especially long-term and broad-spectrum ones, can lead to an imbalanced symbiotic bacteria in the colon, further resulting in gut dysbiosis and potentially CDI (Kim and Gluck, 2019). FMT not only improves the intestinal microecology but also restores the intestinal function in patients with CDI (Brandt et al., 2012). FMT treatment on CDI is mainly attributed to its direct and indirect suppression of C. difficile. The restored gut microbiota after FMT treatment directly competes with C. difficile for nutrients (Choi and Cho, 2016), and resists against its colonization (Britton and Young, 2012). Meanwhile, FMT indirectly suppresses C. difficile by reviving the metabolism of secondary bile acids, which subsequently inhibit the germination of C. difficile spores (Heath et al., 2018).
Moreover, the repeated cycles of antibiotic treatments for rCDI continuously aggravates the colonic microbiota dysbiosis, which results in an increased risk of rCDI-associated diarrhea. However, FMT has been identified as an effective and safe treatment for rCDI (Cammarota et al., 2014). In addition to reversing disturbed gut microbiota after antibiotic treatment, FMT may also play a crucial role in restoring the injured intestinal barrier in rCDI (Samarkos et al., 2018).
4 Conclusion
Gut microbiota dysbiosis and associated intestinal barrier damage play key roles in the pathophysiology of IBD. The microbiota-targeted treatment options for IBD, including antibiotics, probiotics, and FMT alone and in combination in the treatment of IBD, contribute to the restoration of healthy gut microbiota and gut barrier function, regulation of immune response, and inhibition of intestinal inflammation.
These microbiota-targeted interventions might provide new insights for IBD management. Furthermore, well-designed RCTs and animal experiments involving mechanism of maintaining intestinal micro homeostasis are essential to optimize microbiota-targeted interventions, aiming to provide safer and effective treatment options for IBD.
5 Perspective
Dysbiosis of the gut microbiota contributes to the progression of IBD via the alteration of metabolism, impairment of intestinal barrier, and dysregulation of immune responses. However, several items involving the mechanism and therapeutic options targeted to gut microbiota in IBD requires further researches. 1) whether gut dysbiosis acting as a trigger or aggravator for IBD or the secondary alteration of intestinal inflammation remains ambiguous. Existing studies suggest that there is a bidirectional interaction between the gut microbiota and IBD. On one hand, dysbiosis of the gut microbiota may trigger or aggravate IBD by initiating abnormal immune responses and disrupting the intestinal barrier (Brand, 2009; Stange and Schroeder, 2019). On the other hand, the inflammatory state of the intestinal microenvironment in IBD may further alter the structure and function of the gut microbiota, creating a vicious cycle (Stange, 2024). For example, research has found that early-stage IBD patients already exhibit microbiota imbalance, suggesting that microbiota dysbiosis may play a triggering role in the pathogenesis of IBD. However, there is also evidence indicating that inflammation itself can significantly alter the intestinal microbial ecosystem, exacerbating microbiota dysbiosis (Khan et al., 2019). Therefore, conducting longitudinal studies to track the dynamic changes in microbiota structure in high-risk populations and patients is of great significance in clarifying the temporal sequence and causal relationship between dysbiosis and IBD progression, which is crucial for the formulation of precise prevention and treatment strategies in the future. Additionally, the altered gut microbiota varies among individuals and various stages of IBD. Future research is highlighted to explore key microbial species or core indicators of healthy microbiota, and to study the pivotal pathways by which the key microbiota acts on the regulation of intestinal barriers and immune response in IBD. 2) Further studies on pathophysiology of IBD might focus on a multidisciplinary perspective, integrating microbiota studies with genomics, metabolomics, and immunology, in order to establish and optimize the new therapeutic regimens. 3) Multicentral, well-designed RCTs are warranted for the guidelines of antibiotic, probiotic and FMT applications in IBD, involving the reasonable indication, optimal types, dosages, course and combined applications with other treatments. 4) The important role of the gut microbiota in host immune regulation, metabolic function, and intestinal barrier function has made it a crucial biomarker for predicting disease risk, treatment response, and disease progression (Alexandrescu et al., 2024b; Dumitru et al., 2024). Alexandrescu et al. indicated that the notable altered composition of the gut microbiota in active phase of IBD patients may be highly related to the severity of the disease and treatment response (Alexandrescu et al., 2024a). Analyzing the composition of the gut microbiota in conjunction with clinical data might offer a foundation for personalized treatment (Alexandrescu et al., 2024a). A systemic review indicated that the IBD patients who responded to treatment of anti-interleukin or anti-tumor necrosis factor reflecting constantly (both at baseline and throughout the therapy) higher α-diversity and increased relative abundances of certain genera such as Faecalibacterium, Roseburia, or Clostridium (Estevinho et al., 2020). Accordingly, prospective studies are warranted to determine the key species of microbiota and the crucial parameters regarding their composition, function, or metabolites which could be used as the biomarker for diagnosis and prediction of prognosis, risk and treatment response.
Author contributions
HX: Software, Visualization, Writing – original draft, Writing – review & editing. SY: Writing – review & editing. MT: Writing – review & editing. YX: Writing – review & editing. QS: Writing – review & editing. GW: Funding acquisition, Software, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (grant no. BJ2023031) and Wuxi innovation team construction program of Wuxi Health Committee (grant no. CXTD2021020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, A., Maheshwari, A., Verma, S., Arrup, D., Phillips, L., Vinayek, R., et al. (2021). Superiority of higher-volume fresh feces compared to lower-volume frozen feces in fecal microbiota transplantation for recurrent clostridioides difficile colitis. Dig Dis. Sci. 66, 2000–2004. doi: 10.1007/s10620-020-06459-0
Alameddine, J., Godefroy, E., Papargyris, L., Sarrabayrouse, G., Tabiasco, J., Bridonneau, C., et al. (2019). Faecalibacterium prausnitzii skews human DC to prime IL10-producing T cells through TLR2/6/JNK signaling and IL-10, IL-27, CD39, and IDO-1 induction. Front. Immunol. 10, 143. doi: 10.3389/fimmu.2019.00143
Alexandrescu, L., Nicoara, A. D., Tofolean, D. E., Herlo, A., Nelson Twakor, A., Tocia, C., et al. (2024a). Healing from within: how gut microbiota predicts IBD treatment success-A systematic review. Int. J. Mol. Sci. 25, 8451. doi: 10.3390/ijms25158451
Alexandrescu, L., Suceveanu, A. P., Stanigut, A. M., Tofolean, D. E., Axelerad, A. D., Iordache, I. E., et al. (2024b). Intestinal insights: the gut microbiome’s role in atherosclerotic disease: A narrative review. Microorganisms 12, 2341. doi: 10.3390/microorganisms12112341
Al-Fakhrany, O. M. and Elekhnawy, E. (2024). Next-generation probiotics: the upcoming biotherapeutics. Mol. Biol. Rep. 51, 505. doi: 10.1007/s11033-024-09398-5
Al-Sadi, R., Dharmaprakash, V., Nighot, P., Guo, S., Nighot, M., Do, T., et al. (2021a). Bifidobacterium bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κB-Independent Manner. Int. J. Mol. Sci. 22, 8070. doi: 10.3390/ijms22158070
Al-Sadi, R., Nighot, P., Nighot, M., Haque, M., Rawat, M., and Ma, T. Y. (2021b). Lactobacillus acidophilus induces a strain-specific and toll-like receptor 2-dependent enhancement of intestinal epithelial tight junction barrier and protection against intestinal inflammation. Am. J. Pathol. 191, 872–884. doi: 10.1016/j.ajpath.2021.02.003
Amoroso, C., Perillo, F., Strati, F., Fantini, M. C., Caprioli, F., and Facciotti, F. (2020). The role of gut microbiota biomodulators on mucosal immunity and intestinal inflammation. Cells 9, 1234. doi: 10.3390/cells9051234
Baker, R. G., Hayden, M. S., and Ghosh, S. (2011). NF-κB, inflammation, and metabolic disease. Cell Metab. 13, 11–22. doi: 10.1016/j.cmet.2010.12.008
Banerjee, A., Herring, C. A., Chen, B., Kim, H., Simmons, A. J., Southard-Smith, A. N., et al. (2020). Succinate produced by intestinal microbes promotes specification of tuft cells to suppress ileal inflammation. Gastroenterology. 159, 2101–2115.e5. doi: 10.1053/j.gastro.2020.08.029
Berg, D., Clemente, J. C., and Colombel, J. F. (2015). Can inflammatory bowel disease be permanently treated with short-term interventions on the microbiome Expert Rev. Gastroenterol. Hepatol. 9, 781–795. doi: 10.1586/17474124.2015.1013031
Bergmann, K. R., Liu, S. X., Tian, R., Kushnir, A., Turner, J. R., Li, H. L., et al. (2013). Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am. J. Pathol. 182, 1595–1606. doi: 10.1016/j.ajpath.2013.01.013
Bian, X., Wu, W., Yang, L., Lv, L., Wang, Q., Li, Y., et al. (2019). Administration of akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front. Microbiol. 10, 2259. doi: 10.3389/fmicb.2019.02259
Biancone, L., Annese, V., Ardizzone, S., Armuzzi, A., Calabrese, E., Caprioli, F., et al. (2017). Safety of treatments for inflammatory bowel disease: Clinical practice guidelines of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD). Dig Liver Dis. 49, 338–358. doi: 10.1016/j.dld.2017.01.141
Bjarnason, I., Sission, G., and Hayee, B. (2019). A randomised, double-blind, placebo-controlled trial of a multi-strain probiotic in patients with asymptomatic ulcerative colitis and Crohn’s disease. Inflammopharmacology. 27, 465–473. doi: 10.1007/s10787-019-00595-4
Bosca-Watts, M. M., Tosca, J., Anton, R., Mora, M., Minguez, M., and Mora, F. (2015). Pathogenesis of Crohn’s disease: Bug or no bug. World J. Gastrointest Pathophysiol. 6, 1–12. doi: 10.4291/wjgp.v6.i1.1
Brand, S. (2009). Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 58, 1152–1167. doi: 10.1136/gut.2008.163667
Brandt, L. J., Aroniadis, O. C., Mellow, M., Kanatzar, A., Kelly, C., Park, T., et al. (2012). Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am. J. Gastroenterol. 107, 1079–1087. doi: 10.1038/ajg.2012.60
Brennan, C. A. and Garrett, W. S. (2019). Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 17, 156–166. doi: 10.1038/s41579-018-0129-6
Březina, J., Bajer, L., Wohl, P., Ďuricová, D., Hrabák, P., Novotný, A., et al. (2021). Fecal microbial transplantation versus mesalamine enema for treatment of active left-sided ulcerative colitis-results of a randomized controlled trial. J. Clin. Med. 10, 2753. doi: 10.3390/jcm10132753
Britton, G. J., Contijoch, E. J., Mogno, I., Vennaro, O. H., Llewellyn, S. R., Ng, R., et al. (2019). Microbiotas from humans with inflammatory bowel disease alter the balance of gut th17 and RORγt(+) regulatory T cells and exacerbate colitis in mice. Immunity. 50, 212–224.e4. doi: 10.1016/j.immuni.2018.12.015
Britton, R. A. and Young, V. B. (2012). Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol. 20, 313–319. doi: 10.1016/j.tim.2012.04.001
Brown, E. L., Xue, Q., Jiang, Z. D., Xu, Y., and Dupont, H. L. (2010). Pretreatment of epithelial cells with rifaximin alters bacterial attachment and internalization profiles. Antimicrob. Agents Chemother. 54, 388–396. doi: 10.1128/AAC.00691-09
Burrello, C., Garavaglia, F., Cribiù, F. M., Ercoli, G., Bosari, S., Caprioli, F., et al. (2018). Short-term oral antibiotics treatment promotes inflammatory activation of colonic invariant natural killer T and conventional CD4(+) T cells. Front. Med. (Lausanne). 5, 21. doi: 10.3389/fmed.2018.00021
Cammarota, G., Ianiro, G., and Gasbarrini, A. (2014). Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J. Clin. Gastroenterol. 48, 693–702. doi: 10.1097/MCG.0000000000000046
Cammarota, G., Ianiro, G., Tilg, H., Rajilić-Stojanović, M., Kump, P., Satokari, R., et al. (2017). European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 66, 569–580. doi: 10.1136/gutjnl-2016-313017
Carter, A., Adams, M., La Ragione, R. M., and Woodward, M. J. (2017). Colonisation of poultry by Salmonella Enteritidis S1400 is reduced by combined administration of Lactobacillus salivarius 59 and Enterococcus faecium PXN-33. Vet. Microbiol. 199, 100–107. doi: 10.1016/j.vetmic.2016.12.029
Chae, J. M., Heo, W., Cho, H. T., Lee, D. H., Kim, J. H., Rhee, M. S., et al. (2018). Effects of Orally-Administered Bifidobacterium animalis subsp. lactis Strain BB12 on Dextran Sodium Sulfate-Induced Colitis in Mice. J. Microbiol. Biotechnol. 28, 1800–1805. doi: 10.4014/jmb.1805.05072
Chang, P. V., Hao, L., Offermanns, S., and Medzhitov, R. (2014). The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. U.S.A. 111, 2247–2252. doi: 10.1073/pnas.1322269111
Chelakkot, C., Choi, Y., Kim, D. K., Park, H. T., Ghim, J., Kwon, Y., et al. (2018). Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 50, e450. doi: 10.1038/emm.2017.282
Chen, S., Zhang, D., Li, D., Zeng, F., Chen, C., and Bai, F. (2025). Microbiome characterization of patients with Crohn disease and the use of fecal microbiota transplantation: A review. Med. (Baltimore). 104, e41262. doi: 10.1097/MD.0000000000041262
Cheng, F., Huang, Z., Wei, W., and Li, Z. (2021). Fecal microbiota transplantation for Crohn’s disease: a systematic review and meta-analysis. Tech Coloproctol. 25, 495–504. doi: 10.1007/s10151-020-02395-3
Cheng, J., Shah, Y. M., Ma, X., Pang, X., Tanaka, T., Kodama, T., et al. (2010). Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J. Pharmacol. Exp. Ther. 335, 32–41. doi: 10.1124/jpet.110.170225
Chingwaru, W. and Vidmar, J. (2017). Potential of Zimbabwean commercial probiotic products and strains of Lactobacillus plantarum as prophylaxis and therapy against diarrhoea caused by Escherichia coli in children. Asian Pac J. Trop. Med. 10, 57–63. doi: 10.1016/j.apjtm.2016.12.009
Choi, H. H. and Cho, Y. S. (2016). Fecal microbiota transplantation: current applications, effectiveness, and future perspectives. Clin. Endosc. 49, 257–265. doi: 10.5946/ce.2015.117
Clemente, J. C., Ursell, L. K., Parfrey, L. W., and Knight, R. (2012). : The impact of the gut microbiota on human health: an integrative view. Cell. 148, 1258–1270. doi: 10.1016/j.cell.2012.01.035
Coombes, J. L., Siddiqui, K. R., Arancibia-Cárcamo, C. V., Hall, J., Sun, C. M., Belkaid, Y., et al. (2007). A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764. doi: 10.1084/jem.20070590
Coskun, M. (2014). Intestinal epithelium in inflammatory bowel disease. Front. Med. (Lausanne). 1, 24. doi: 10.3389/fmed.2014.00024
Costello, S. P., Hughes, P. A., Waters, O., Bryant, R. V., Vincent, A. D., Blatchford, P., et al. (2019). Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: A randomized clinical trial. Jama 321, 156–164. doi: 10.1001/jama.2018.20046
Crost, E. H., Coletto, E., Bell, A., and Juge, N. (2023). Ruminococcus gnavus: friend or foe for human health. FEMS Microbiol. Rev. 47, fuad014. doi: 10.1093/femsre/fuad014
Crothers, J. W., Chu, N. D., Nguyen, L. T. T., Phillips, M., Collins, C., Fortner, K., et al. (2021). Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: results of a single-center, prospective, randomized pilot study. BMC Gastroenterol. 21, 281. doi: 10.1186/s12876-021-01856-9
Cui, B., Feng, Q., Wang, H., Wang, M., Peng, Z., Li, P., et al. (2015). Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: safety, feasibility, and efficacy trial results. J. Gastroenterol. Hepatol. 30, 51–58. doi: 10.1111/jgh.2015.30.issue-1
Cutolo, M., Campitiello, R., Gotelli, E., and Soldano, S. (2022). The role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Front. Immunol. 13, 867260. doi: 10.3389/fimmu.2022.867260
Darfeuille-Michaud, A., Boudeau, J., Bulois, P., Neut, C., Glasser, A. L., Barnich, N., et al. (2004). High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 127, 412–421. doi: 10.1053/j.gastro.2004.04.061
Dempsey, E. and Corr, S. C. (2022). Lactobacillus spp. for gastrointestinal health: current and future perspectives. Front. Immunol. 13, 840245. doi: 10.3389/fimmu.2022.840245
den Besten, G., van Eunen, K., Groen, A. K., Venema, K., Reijngoud, D. J., and Bakker, B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340. doi: 10.1194/jlr.R036012
Di Sabatino, A., Ciccocioppo, R., Luinetti, O., Ricevuti, L., Morera, R., Cifone, M. G., et al. (2003). Increased enterocyte apoptosis in inflamed areas of Crohn’s disease. Dis. Colon Rectum. 46, 1498–1507. doi: 10.1007/s10350-004-6802-z
Dobson, A., Cotter, P. D., Ross, R. P., and Hill, C. (2012) Bacteriocin production: a probiotic trait Appl. Environ. Microbiol. 78, 1–6. doi: 10.1128/AEM.05576-11
Dolinger, M., Torres, J., and Vermeire, S. (2024). Crohn’s disease. Lancet 403, 1177–1191. doi: 10.1016/S0140-6736(23)02586-2
Dumitru, A., Matei, E., Cozaru, G. C., Chisoi, A., Alexandrescu, L., Popescu, R. C., et al. (2024). Endotoxin inflammatory action on cells by dysregulated-immunological-barrier-linked ROS-apoptosis mechanisms in gut-liver axis. Int. J. Mol. Sci. 25, 2472. doi: 10.3390/ijms25052472
Duranti, S., Gaiani, F., Mancabelli, L., Milani, C., Grandi, A., Bolchi, A., et al. (2016). Elucidating the gut microbiome of ulcerative colitis: bifidobacteria as novel microbial biomarkers. FEMS Microbiol. Ecol. 92, fiw191. doi: 10.1093/femsec/fiw191
Estevinho, M. M., Rocha, C., Correia, L., Lago, P., Ministro, P., Portela, F., et al. (2020). Features of fecal and colon microbiomes associate with responses to biologic therapies for inflammatory bowel diseases: A systematic review. Clin. Gastroenterol. Hepatol. 18, 1054–1069. doi: 10.1016/j.cgh.2019.08.063
Fang, H., Fu, L., Li, X., Lu, C., Su, Y., Xiong, K., et al. (2021). Long-term efficacy and safety of monotherapy with a single fresh fecal microbiota transplant for recurrent active ulcerative colitis: a prospective randomized pilot study. Microb. Cell Fact. 20, 18. doi: 10.1186/s12934-021-01513-6
Faye, A. S., Allin, K. H., Iversen, A. T., Agrawal, M., Faith, J., Colombel, J. F., et al. (2023). Antibiotic use as a risk factor for inflammatory bowel disease across the ages: a population-based cohort study. Gut. 72, 663–670. doi: 10.1136/gutjnl-2022-327845
Feller, M., Huwiler, K., Stephan, R., Altpeter, E., Shang, A., Furrer, H., et al. (2007). Mycobacterium avium subspecies paratuberculosis and Crohn’s disease: a systematic review and meta-analysis. Lancet Infect. Dis. 7, 607–613. doi: 10.1016/S1473-3099(07)70211-6
Fiorucci, S., Distrutti, E., Mencarelli, A., Barbanti, M., Palazzini, E., and Morelli, A. (2002). Inhibition of intestinal bacterial translocation with rifaximin modulates lamina propria monocytic cells reactivity and protects against inflammation in a rodent model of colitis. Digestion. 66, 246–256. doi: 10.1159/000068362
Fu, Q., Ma, X., Li, S., Shi, M., Song, T., and Cui, J. (2024). New insights into the interactions between the gut microbiota and the inflammatory response to ulcerative colitis in a mouse model of dextran sodium sulfate and possible mechanisms of action for treatment with PE&AFWE. Anim. Model. Exp. Med. 7, 83–97. doi: 10.1002/ame2.12405
Garrido-Mesa, N., Camuesco, D., Arribas, B., Comalada, M., Bailón, E., Cueto-Sola, M., et al. (2011). The intestinal anti-inflammatory effect of minocycline in experimental colitis involves both its immunomodulatory and antimicrobial properties. Pharmacol. Res. 63, 308–319. doi: 10.1016/j.phrs.2010.12.011
Geremia, A., Biancheri, P., Allan, P., Corazza, G. R., and Di Sabatino, A. (2014). Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 13, 3–10. doi: 10.1016/j.autrev.2013.06.004
Gevers, D., Kugathasan, S., Denson, L. A., Vázquez-Baeza, Y., Van Treuren, W., Ren, B., et al. (2014). The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 15, 382–392. doi: 10.1016/j.chom.2014.02.005
Ghavami, S. B., Yadegar, A., Aghdaei, H. A., Sorrentino, D., Farmani, M., Mir, A. S., et al. (2020). Immunomodulation and generation of tolerogenic dendritic cells by probiotic bacteria in patients with inflammatory bowel disease. Int. J. Mol. Sci. 21, 6266. doi: 10.3390/ijms21176266
Goto, Y. (2013). II: Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunol. Cell Biol. 91, 204–214. doi: 10.1038/icb.2012.80
Gradel, K. O., Nielsen, H. L., Schønheyder, H. C., Ejlertsen, T., Kristensen, B., and Nielsen, H. (2009). Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis Gastroenterol. 137, 495–501. doi: 10.1053/j.gastro.2009.04.001
Gulati, M., Singh, S. K., Corrie, L., Kaur, I. P., and Chandwani, L. (2020). Delivery routes for faecal microbiota transplants: Available, anticipated and aspired. Pharmacol. Res. 159, 104954. doi: 10.1016/j.phrs.2020.104954
Guo, S., Al-Sadi, R., Said, H. M., and Ma, T. Y. (2013). Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 182, 375–387. doi: 10.1016/j.ajpath.2012.10.014
Hadis, U., Wahl, B., Schulz, O., Hardtke-Wolenski, M., Schippers, A., Wagner, N., et al. (2011). Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 34, 237–246. doi: 10.1016/j.immuni.2011.01.016
Haifer, C., Paramsothy, S., Kaakoush, N. O., Saikal, A., Ghaly, S., Yang, T., et al. (2022). Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 7, 141–151. doi: 10.1016/S2468-1253(21)00400-3
He, Z., Li, P., Zhu, J., Cui, B., Xu, L., Xiang, J., et al. (2017). Multiple fresh fecal microbiota transplants induces and maintains clinical remission in Crohn’s disease complicated with inflammatory mass. Sci. Rep. 7, 4753. doi: 10.1038/s41598-017-04984-z
He, C., Wang, H., Liao, W. D., Peng, C., Shu, X., Zhu, X., et al. (2019). Characteristics of mucosa-associated gut microbiota during treatment in Crohn’s disease. World J. Gastroenterol. 25, 2204–2216. doi: 10.3748/wjg.v25.i18.2204
Heath, R. D., Cockerell, C., Mankoo, R., Ibdah, J. A., and Tahan, V. (2018). Fecal microbiota transplantation and its potential therapeutic uses in gastrointestinal disorders. North Clin. Istanb 5, 79–88. doi: 10.14744/nci.2017.10692
Heimesaat, M. M., Fischer, A., Jahn, H. K., Niebergall, J., Freudenberg, M., Blaut, M., et al. (2007). Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 56, 941–948. doi: 10.1136/gut.2006.104497
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. . 11, 506–514. doi: 10.1038/nrgastro.2014.66
Hourigan, S. K., Chen, L. A., Grigoryan, Z., Laroche, G., Weidner, M., Sears, C. L., et al. (2015). Microbiome changes associated with sustained eradication of Clostridium difficile after single faecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment Pharmacol. Ther. 42, 741–752. doi: 10.1111/apt.2015.42.issue-6
Hsieh, C. Y., Osaka, T., Moriyama, E., Date, Y., Kikuchi, J., and Tsuneda, S. (2015). Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol. Rep. 3, e12327. doi: 10.14814/phy2.12327
Hu, Y., Chen, Z., Xu, C., Kan, S., and Chen, D. (2022). Disturbances of the gut microbiota and microbiota-derived metabolites in inflammatory bowel disease. Nutrients 14, 5140. doi: 10.3390/nu14235140
Huang, C., Huang, Z., Ding, L., Fu, Y., Fan, J., Mei, Q., et al. (2022a). Fecal microbiota transplantation versus glucocorticoids for the induction of remission in mild to moderate ulcerative colitis. J. Transl. Med. 20, 354. doi: 10.1186/s12967-022-03569-3
Huang, C., Mei, Q., Lou, L., Huang, Z., Fu, Y., Fan, J., et al. (2022b). Ulcerative colitis in response to fecal microbiota transplantation via modulation of gut microbiota and th17/treg cell balance. Cells, 11, 1851. doi: 10.3390/cells11111851
Ianiro, G., Tilg, H., and Gasbarrini, A. (2016). Antibiotics as deep modulators of gut microbiota: between good and evil. Gut . 65, 1906–1915. doi: 10.1136/gutjnl-2016-312297
Imdad, A., Pandit, N. G., Zaman, M., Minkoff, N. Z., Tanner-Smith, E. E., Gomez-Duarte, O. G., et al. (2023). Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst. Rev. 4, Cd012774. doi: 10.1002/14651858.CD012774.pub3
Ishikawa, D., Sasaki, T., Osada, T., Kuwahara-Arai, K., Haga, K., Shibuya, T., et al. (2017). Changes in intestinal microbiota following combination therapy with fecal microbial transplantation and antibiotics for ulcerative colitis. Inflammation Bowel Dis. 23, 116–125. doi: 10.1097/MIB.0000000000000975
Ishikawa, D., Zhang, X., Nomura, K., Seki, N., Haraikawa, M., Haga, K., et al. (2022). A randomized placebo-controlled trial of combination therapy with post-triple-antibiotic-therapy fecal microbiota transplantation and alginate for ulcerative colitis: protocol. Front. Med. (Lausanne). 9, 779205. doi: 10.3389/fmed.2022.779205
Iwamoto, M., Koji, T., Makiyama, K., Kobayashi, N., and Nakane, P. K. (1996). Apoptosis of crypt epithelial cells in ulcerative colitis. J. Pathol. 180, 152–159. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y
Jakubczyk, D., Leszczyńska, K., and Górska, S. (2020). The effectiveness of probiotics in the treatment of inflammatory bowel disease (IBD)-A critical review. Nutrients 12, 1973. doi: 10.3390/nu12071973
Jan, T., Negi, R., Sharma, B., Kumar, S., Singh, S., Rai, A. K., et al. (2024). Next generation probiotics for human health: An emerging perspective. Heliyon. 10, e35980. doi: 10.1016/j.heliyon.2024.e35980
Jepson, M. A., Collares-Buzato, C. B., Clark, M. A., Hirst, B. H., and Simmons, N. L. (1995). Rapid disruption of epithelial barrier function by Salmonella typhimurium is associated with structural modification of intercellular junctions. Infect. Immun. 63, 356–359. doi: 10.1128/iai.63.1.356-359.1995
Jergens, A. E., Parvinroo, S., Kopper, J., and Wannemuehler, M. J. (2021). : rules of engagement: epithelial-microbe interactions and inflammatory bowel disease. Front. Med. (Lausanne). 8, 669913. doi: 10.3389/fmed.2021.669913
Jernberg, C., Löfmark, S., Edlund, C., and Jansson, J. K. (2007). Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. Isme J. . 1, 56–66. doi: 10.1038/ismej.2007.3
Jha, D. K., Mishra, S., Dutta, U., and Sharma, V. (2024). Antibiotics for inflammatory bowel disease: Current status. Indian J. Gastroenterol. 43, 145–159. doi: 10.1007/s12664-024-01537-x
Ji, J., Shu, D., Zheng, M., Wang, J., Luo, C., Wang, Y., et al. (2016). Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 6, 24838. doi: 10.1038/srep24838
Ji, S. K., Yan, H., Jiang, T., Guo, C. Y., Liu, J. J., Dong, S. Z., et al. (2017). Preparing the gut with antibiotics enhances gut microbiota reprogramming efficiency by promoting xenomicrobiota colonization. Front. Microbiol. 8, 1208. doi: 10.3389/fmicb.2017.01208
Joossens, M., Huys, G., Cnockaert, M., De Preter, V., Verbeke, K., Rutgeerts, P., et al. (2011). Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut. 60, 631–637. doi: 10.1136/gut.2010.223263
Kamada, N., Kim, Y. G., Sham, H. P., Vallance, B. A., Puente, J. L., Martens, E. C., et al. (2012). Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 336, 1325–1329. doi: 10.1126/science.1222195
Kazerouni, A. and Wein, L. M. (2017). Exploring the Eficacy of pooled stools in fecal microbiota transplantation for microbiota-associated chronic diseases. PloS One 12, e, e0163956. doi: 10.1371/journal.pone.0163956
Kedia, S., Virmani, S., Vuyyuru, K S., Kumar, P., Kante, B., Sahu, P, et al. (2022). Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut. 71, 2401–2413. doi: 10.1136/gutjnl-2022-327811
Kelly, C. R. and Ananthakrishnan, A. N. (2019). Manipulating the microbiome with fecal transplantation to treat ulcerative colitis. Jama. 321, 151–152. doi: 10.1001/jama.2018.20397
Khailova, L., Dvorak, K., Arganbright, K. M., Halpern, M. D., Kinouchi, T., Yajima, M., et al. (2009). Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest Liver Physiol. 297, G940–G949. doi: 10.1152/ajpgi.00141.2009
Khan, C. M. (2014). The dynamic interactions between salmonella and the microbiota, within the challenging niche of the gastrointestinal tract. Int. Sch Res. Notices. 2014, 846049. doi: 10.1155/2014/846049
Khan, I., Ullah, N., Zha, L., Bai, Y., Khan, A., Zhao, T., et al. (2019). Alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 8, 126. doi: 10.3390/pathogens8030126
Khan, K. J., Ullman, T. A., Ford, A. C., Abreu, M. T., Abadir, A., Marshall, J. K., et al. (2011). Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am. J. Gastroenterol. 106, 661–673. doi: 10.1038/ajg.2011.72
Kim, S., Covington, A., and Pamer, E. G. (2017). The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 279, 90–105. doi: 10.1111/imr.2017.279.issue-1
Kim, K. O. and Gluck, M. (2019). Fecal microbiota transplantation: an update on clinical practice. Clin. Endosc . 52, 137–143. doi: 10.5946/ce.2019.009
Kjærgaard, S., Jensen, T. S. R., Feddersen, U. R., Bindslev, N., Grunddal, K. V., Poulsen, S. S., et al. (2021). Decreased number of colonic tuft cells in quiescent ulcerative colitis patients. Eur. J. Gastroenterol. Hepatol. 33, 817–824. doi: 10.1097/MEG.0000000000001959
Kucharzik, T., Ellul, P., Greuter, T., Rahier, J. F., Verstockt, B., Abreu, C., et al. (2021). ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J. Crohns Colitis. 15, 879–913. doi: 10.1093/ecco-jcc/jjab052
Kump, P., Wurm, P., Gröchenig, H. P., Wenzl, H., Petritsch, W., Halwachs, B., et al. (2018). The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment Pharmacol. Ther. 47, 67–77. doi: 10.1111/apt.2018.47.issue-1
Kurashima, Y. and Kiyono, H. (2017). Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu. Rev. Immunol. 35, 119–147. doi: 10.1146/annurev-immunol-051116-052424
Lamb, C. A., Kennedy, N. A., Raine, T., Hendy, P. A., Smith, P. J., Limdi, J. K., et al. (2019). British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68, s1–s106. doi: 10.1136/gutjnl-2019-318484
Laperrousaz, B., Levast, B., Fontaine, M., Nancey, S., Dechelotte, P., Doré, J., et al. (2024). Safety comparison of single-donor and pooled fecal microbiota transfer product preparation in ulcerative colitis: systematic review and meta-analysis. BMC Gastroenterol. 24, 402. doi: 10.1186/s12876-024-03487-2
Lawrence, T. (2009). The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1, a001651. doi: 10.1101/cshperspect.a001651
Le Bastard, Q., Ward, T., Sidiropoulos, D., Hillmann, B. M., Chun, C. L., Sadowsky, M. J., et al. (2018). Fecal microbiota transplantation reverses antibiotic and chemotherapy-induced gut dysbiosis in mice. Sci. Rep. 8, 6219. doi: 10.1038/s41598-018-24342-x
Ledder, O. and Turner, D. (2018). Antibiotics in IBD: still a role in the biological era. Inflammation Bowel Dis. 24, 1676–1688. doi: 10.1093/ibd/izy067
Lenoir, M., Martín, R., Torres-Maravilla, E., Chadi, S., González-Dávila, P., Sokol, H., et al (2020). Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3. Gut Microbes 12, 1–16. doi: 10.1080/19490976.2020.1826748
Levast, B., Fontaine, M., Nancey, S., Dechelotte, P., Doré, J., and Lehert, P. (2023). Single-donor and pooling strategies for fecal microbiota transfer product preparation in ulcerative colitis: A systematic review and meta-analysis. Clin. Transl. Gastroenterol. 14, e00568. doi: 10.14309/ctg.0000000000000568
Li, C., Peng, K., Xiao, S., Long, Y., and Yu, Q. (2023). The role of Lactobacillus in inflammatory bowel disease: from actualities to prospects. Cell Death Discov. 9, 361. doi: 10.1038/s41420-023-01666-w
Lichtenstein, G. R., Loftus, E. V., Isaacs, K. L., Regueiro, M. D., Gerson, L. B., and Sands, B. E. (2018). ACG clinical guideline: management of crohn’s disease in adults. Am. J. Gastroenterol. 113, 481–517. doi: 10.1038/ajg.2018.27
Lim, K. H. and Staudt, L. M. (2013). Toll-like receptor signaling. Cold Spring Harb. Perspect. Biol. 5, a011247. doi: 10.1101/cshperspect.a011247
Lin, J., Xiong, J., Jin, Y., Wang, H., Wu, L., Chen, L., et al. (2024). Fecal microbiota transplantation through transendoscopic enteral tubing for inflammatory bowel disease: High acceptance and high satisfaction. J. Gastroenterol. Hepatol. 39, 328–336. doi: 10.1111/jgh.16435
Liu, H., Hong, X. L., Sun, T. T., Huang, X. W., Wang, J. L., and Xiong, H. (2020). Fusobacterium nucleatum exacerbates colitis by damaging epithelial barriers and inducing aberrant inflammation. J. Dig Dis. 21, 385–398. doi: 10.1111/1751-2980.12909
Liu, Y., Li, Y., Yu, X., Yu, L., Tian, F., Zhao, J., et al. (2021). Physiological Characteristics of Lactobacillus casei Strains and Their Alleviation Effects against Inflammatory Bowel Disease. J. Microbiol. Biotechnol. 31, 92–103. doi: 10.4014/jmb.2003.03041
Liu, Y., Mo, C. F., Luo, X. Y., Li, H., Guo, H. J., Sun, H., et al. (2020). Activation of toll-like receptor 3 induces interleukin-1 receptor antagonist expression by activating the interferon regulatory factor 3. J. Innate Immun. 12, 304–320. doi: 10.1159/000504321
Lloyd-Price, J., Arze, C., Ananthakrishnan, A. N., Schirmer, M., Avila-Pacheco, J., Poon, T. W., et al. (2019). Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 569, 655–662. doi: 10.1038/s41586-019-1237-9
Lopetuso, L. R., Deleu, S., Godny, L., Petito, V., Puca, P., Facciotti, F., et al. (2023). The first international Rome consensus conference on gut microbiota and faecal microbiota transplantation in inflammatory bowel disease. Gut. 72, 1642–1650. doi: 10.1136/gutjnl-2023-329948
Lopetuso, L. R., Napoli, M., Rizzatti, G., and Gasbarrini, A. (2018). The intriguing role of Rifaximin in gut barrier chronic inflammation and in the treatment of Crohn’s disease. Expert Opin. Investig. Drugs 27, 543–551. doi: 10.1080/13543784.2018.1483333
Louis, P. and Flint, H. J. (2017). Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 19, 29–41. doi: 10.1111/emi.2017.19.issue-1
Lu, Y., Li, X., Liu, S., Zhang, Y., and Zhang, D. (2018). Toll-like receptors and inflammatory bowel disease. Front. Immunol. 9, 72. doi: 10.3389/fimmu.2018.00072
Maccaferri, S., Vitali, B., Klinder, A., Kolida, S., Ndagijimana, M., Laghi, L., et al. (2010). Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: an in vitro approach using a continuous culture colonic model system. J. Antimicrob. Chemother. 65, 2556–2565. doi: 10.1093/jac/dkq345
Machiels, K., Joossens, M., Sabino, J., De Preter, V., Arijs, I., Eeckhaut, V., et al. (2014). A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 63, 1275–1283. doi: 10.1136/gutjnl-2013-304833
Mahida, Y. R., Galvin, A., Makh, S., Hyde, S., Sanfilippo, L., Borriello, S. P., et al. (1998). Effect of Clostridium difficile toxin A on human colonic lamina propria cells: early loss of macrophages followed by T-cell apoptosis. Infect. Immun. 66, 5462–5469. doi: 10.1128/IAI.66.11.5462-5469.1998
Maldonado-Gómez, M. X., Martínez, I., Bottacini, F., O’Callaghan, A., Ventura, M., van Sinderen, D., et al. (2016). St able Engraftment of Bifidobacterium longum AH1206 in the Human Gut Depends on Individualized Features of the Resident Microbiome. Cell Host Microbe. 20, 515–526. doi: 10.1016/j.chom.2016.09.001
Martín, R., Chamignon, C., Mhedbi-Hajri, N., Chain, F., Derrien, M., Escribano-Vázquez, U., et al. (2019). The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci. Rep. 9, 5398. doi: 10.1038/s41598-019-41738-5
Martín, R., Laval, L., Chain, F., Miquel, S., Natividad, J., Cherbuy, C., et al. (2016). Bifidobacterium animalis ssp. lactis CNCM-I2494 Restores Gut Barrier Permeability in Chronically Low-Grade Inflamed Mice. Front. Microbiol. . 7, 608. doi: 10.3389/fmicb.2016.00608
Martín, R., Rios-Covian, D., Huillet, E., Auger, S., Khazaal, S., Bermúdez-Humarán, L. G., et al. (2023). Faecalibacterium: a bacterial genus with promising human health applications. FEMS Microbiol. Rev. 47, fuad039. doi: 10.1093/femsre/fuad039
Matsuoka, K. and Kanai, T. (2015). The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 37, 47–55. doi: 10.1007/s00281-014-0454-4
Merenstein, D., Pot, B., Leyer, G., Ouwehand, A. C., Preidis, G. A., Elkins, C. A., et al. (2023). Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 15, 2185034. doi: 10.1080/19490976.2023.2185034
Miquel, S., Martín, R., Rossi, O., Bermúdez-Humarán, L. G., Chatel, J. M., Sokol, H., et al. (2013). Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 16, 255–261. doi: 10.1016/j.mib.2013.06.003
Moayyedi, P., Surette, M. G., Kim, P. T., Libertucci, J., Wolfe, M., Onischi, C., et al. (2015). Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 149, 102–109.e6. doi: 10.1053/j.gastro.2015.04.001
Morgan, X. C., Tickle, T. L., Sokol, H., Gevers, D., Devaney, K. L., Ward, D. V., et al. (2012). Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 13, R79. doi: 10.1186/gb-2012-13-9-r79
Morrison, D. J. and Preston, T. (2016). Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 7, 189–200. doi: 10.1080/19490976.2015.1134082
Mylonaki, M., Rayment, N. B., Rampton, D. S., Hudspith, B. N., and Brostoff, J. (2005). Molecular characterization of rectal mucosa-associated bacterial flora in inflammatory bowel disease. Inflammation Bowel Dis. 11, 481–487. doi: 10.1097/01.MIB.0000159663.62651.4f
Nagao-Kitamoto, H., Leslie, J. L., Kitamoto, S., Jin, C., Thomsson, K. A., Gillilland, M. G., 3rd, et al. (2020). Interleukin-22-mediated host glycosylation prevents Clostridioides difficile infection by modulating the metabolic activity of the gut microbiota. Nat. Med. 26, 608–617. doi: 10.1038/s41591-020-0764-0
Najafi, S., Sotoodehnejadnematalahi, F., Amiri, M. M., Pourshafie, M. R., and Rohani, M. (2022). Decreased mucosal adhesion of Lactobacillus species in patients with inflammatory bowel disease. Caspian J. Intern. Med. 13, 713–720. doi: 10.22088/cjim.13.4.71
Nguyen, L. H., Örtqvist, A. K., Cao, Y., Simon, T. G., Roelstraete, B., Song, M., et al. (2020). Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol. Hepatol. 5, 986–995. doi: 10.1016/S2468-1253(20)30267-3
Nie, K., Ma, K., Luo, W., Shen, Z., Yang, Z., Xiao, M., et al. (2021). Roseburia intestinalis: A beneficial gut organism from the discoveries in genus and species. Front. Cell Infect. Microbiol. 11, 757718. doi: 10.3389/fcimb.2021.757718
Nishida, A., Inoue, R., Inatomi, O., Bamba, S., Naito, Y., and Andoh, A. (2018). Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 11, 1–10. doi: 10.1007/s12328-017-0813-5
Nishino, K., Nishida, A., Inoue, R., Kawada, Y., Ohno, M., Sakai, S., et al. (2018). Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 53, 95–106. doi: 10.1007/s00535-017-1384-4
O’Callaghan, A. and van Sinderen, D. (2016). Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 7, 925. doi: 10.3389/fmicb.2016.00925
Ohnmacht, C., Park, J. H., Cording, S., Wing, J. B., Atarashi, K., Obata, Y., et al. (2015). MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORγt+ T cells. Science. 349, 989–993. doi: 10.1126/science.aac4263
Pai, N., Popov, J., Hill, L., Hartung, E., Grzywacz, K., and Moayyedi, P. (2021). : results of the first pilot randomized controlled trial of fecal microbiota transplant in pediatric ulcerative colitis: lessons, limitations, and future prospects. Gastroenterology. 161, 388–393.e3. doi: 10.1053/j.gastro.2021.04.067
Paramsothy, S., Kamm, M. A., Kaakoush, N. O., Walsh, A. J., van den Bogaerde, J., Samuel, D., et al. (2017). Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 389, 1218–1228. doi: 10.1016/S0140-6736(17)30182-4
Peng, Z., Xiang, J., He, Z., Zhang, T., Xu, L., Cui, B., et al. (2016). Colonic transendoscopic enteral tubing: A novel way of transplanting fecal microbiota. Endosc Int. Open. 4, E610–E613. doi: 10.1055/s-0042-105205
Perez-Lopez, A., Behnsen, J., Nuccio, S. P., and Raffatellu, M. (2016). Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 16, 135–148. doi: 10.1038/nri.2015.17
Pigneur, B. and Sokol, H. (2016). Fecal microbiota transplantation in inflammatory bowel disease: the quest for the holy grail. Mucosal Immunol. 9, 1360–1365. doi: 10.1038/mi.2016.67
Plöger, S., Stumpff, F., Penner, G. B., Schulzke, J. D., Gäbel, G., Martens, H., et al. (2012). Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann. N Y Acad. Sci. 1258, 52–59. doi: 10.1111/j.1749-6632.2012.06553.x
Png, C. W., Lindén, S. K., Gilshenan, K. S., Zoetendal, E. G., McSweeney, C. S., Sly, L. I., et al. (2010). Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 105, 2420–2428. doi: 10.1038/ajg.2010.281
Prantera, C., Lochs, H., Grimaldi, M., Danese, S., Scribano, M. L., and Gionchetti, P. (2012). Rifaximin-extended intestinal release induces remission in patients with moderately active Crohn’s disease. Gastroenterology. 142, 473–481.e4. doi: 10.1053/j.gastro.2011.11.032
Qian, K., Chen, S., Wang, J., Sheng, K., Wang, Y., and Zhang, M. (2022). A β-N-acetylhexosaminidase Amuc_2109 from Akkermansia muciniphila protects against dextran sulfate sodium-induced colitis in mice by enhancing intestinal barrier and modulating gut microbiota. Food Funct. 13, 2216–2227. doi: 10.1039/D1FO04094D
Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K. S., Manichanh, C., et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 464, 59–65. doi: 10.1038/nature08821
Qiu, P., Ishimoto, T., Fu, L., Zhang, J., Zhang, Z., and Liu, Y. (2022). The gut microbiota in inflammatory bowel disease. Front. Cell Infect. Microbiol. 12, 733992. doi: 10.3389/fcimb.2022.733992
Rahman, K., Sasaki, M., Nusrat, A., and Klapproth, J. M. (2014). Crohn’s disease-associated Escherichia coli survive in macrophages by suppressing NFκB signaling. Inflammation Bowel Dis. 20, 1419–1425. doi: 10.1097/MIB.0000000000000096
Ren, Z., Guo, C., Yu, S., Zhu, L., Wang, Y., Hu, H., et al. (2019). Progress in mycotoxins affecting intestinal mucosal barrier function. Int. J. Mol. Sci. 20, 2777. doi: 10.3390/ijms20112777
Rossen, N. G., Fuentes, S., van der Spek, M. J., Tijssen, J. G., Hartman, J. H., Duflou, A., et al. (2015). Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 149, 110–118.e4. doi: 10.1053/j.gastro.2015.03.045
Rossi, O., van Berkel, L. A., Chain, F., Tanweer Khan, M., Taverne, N., Sokol, H., et al. (2016). Faecalibacterium prausnitzii A2–165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci. Rep. 6, 18507. doi: 10.1038/srep18507
Rouanet, A., Bolca, S., Bru, A., Claes, I., Cvejic, H., Girgis, H., et al. (2020). Live biotherapeutic products, A road map for safety assessment. Front. Med. (Lausanne). 7, 237. doi: 10.3389/fmed.2020.00237
Rowland, I., Gibson, G., Heinken, A., Scott, K., Swann, J., Thiele, I., et al. (2018). Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 57, 1–24. doi: 10.1007/s00394-017-1445-8
Roy, S. and Dhaneshwar, S. (2023). Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J. Gastroenterol. 29, 2078–2100. doi: 10.3748/wjg.v29.i14.2078
Rubin, D. T., Ananthakrishnan, A. N., Siegel, C. A., Sauer, B. G., and Long, M. D. (2019). ACG clinical guideline: ulcerative colitis in adults. Am. J. Gastroenterol. . 114, 384–413. doi: 10.14309/ajg.0000000000000152
Rutz, S., Eidenschenk, C., and Ouyang, W. (2013). IL-22, not simply a Th17 cytokine. Immunol. Rev. 252, 116–132. doi: 10.1111/imr.2013.252.issue-1
Saint-Cyr, M. J., Haddad, N., Taminiau, B., Poezevara, T., Quesne, S., Amelot, M., et al. (2017). Use of the potential probiotic strain Lactobacillus salivarius SMXD51 to control Campylobacter jejuni in broilers. Int. J. Food Microbiol. 247, 9–17. doi: 10.1016/j.ijfoodmicro.2016.07.003
Salazar-Gonzalez, R. M. and McSorley, S. J. (2005). Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol. Lett. 101, 117–122. doi: 10.1016/j.imlet.2005.05.004
Samarkos, M., Mastrogianni, E., and Kampouropoulou, O. (2018). The role of gut microbiota in Clostridium difficile infection. Eur. J. Intern. Med. 50, 28–32. doi: 10.1016/j.ejim.2018.02.006
Sarrabayrouse, G., Bossard, C., Chauvin, J. M., Jarry, A., Meurette, G., Quévrain, E., et al. (2014). CD4CD8αα lymphocytes, a novel human regulatory T cell subset induced by colonic bacteria and deficient in patients with inflammatory bowel disease. PloS Biol. 12, e1001833. doi: 10.1371/journal.pbio.1001833
Schaubeck, M., Clavel, T., Calasan, J., Lagkouvardos, I., Haange, S. B., Jehmlich, N., et al. (2016). Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut. 65, 225–237. doi: 10.1136/gutjnl-2015-309333
Scott, N. A., Andrusaite, A., Andersen, P., Lawson, M., Alcon-Giner, C., Leclaire, C., et al. (2018). Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci. Transl. Med. 10, eaao4755. doi: 10.1126/scitranslmed.aao4755
Servin, A. L. (2004). Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 28, 405–440. doi: 10.1016/j.femsre.2004.01.003
Sica, A. and Mantovani, A. (2012). Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795. doi: 10.1172/JCI59643
Smith, B. J., Piceno, Y., Zydek, M., Zhang, B., Syriani, L. A., Terdiman, J. P., et al. (2022). Strain-resolved analysis in a randomized trial of antibiotic pretreatment and maintenance dose delivery mode with fecal microbiota transplant for ulcerative colitis. Sci. Rep. . 12, 5517. doi: 10.1038/s41598-022-09307-5
Sokol, H. (2020). Antibiotics: a trigger for inflammatory bowel disease Lancet Gastroenterol. Hepatol. 5, 956–957. doi: 10.1016/S2468-1253(20)30208-9
Sokol, H., Landman, C., Seksik, P., Berard, L., Montil, M., Nion-Larmurier, I., et al. (2020). Fecal microbiota transplantation to maintain remission in Crohn’s disease: a pilot randomized controlled study. Microbiome . 8, 12. doi: 10.1186/s40168-020-0792-5
Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermúdez-Humarán, L. G., Gratadoux, J. J., et al (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U.S.A. 105, 16731–16736. doi: 10.1073/pnas.0804812105
Stange, E. F. (2024). Dysbiosis in inflammatory bowel diseases: egg, not chicken. Front. Med. (Lausanne). 11, 1395861. doi: 10.3389/fmed.2024.1395861
Stange, E. F. and Schroeder, B. O. (2019). Microbiota and mucosal defense in IBD: an update. Expert Rev. Gastroenterol. Hepatol. 13, 963–976. doi: 10.1080/17474124.2019.1671822
Stecher, B. (2021). Establishing causality in Salmonella-microbiota-host interaction: The use of gnotobiotic mouse models and synthetic microbial communities. Int. J. Med. Microbiol. 311, 151484. doi: 10.1016/j.ijmm.2021.151484
Steinwurz, F., MaChado, M. B., Veitia, G., De Paula, J. A., Bautista Martinez, S., Vergara, B. I., et al. (2023). Latin America consensus statement inflammatory bowel disease: importance of timely access to diagnosis and treatment. Therap Adv. Gastroenterol. 16, 17562848231207312. doi: 10.1177/17562848231207312
Stephens, M. and von der Weid, P. Y. (2020). Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 11, 421–432. doi: 10.1080/19490976.2019.1629235
Strine, M. S. and Wilen, C. B. (2022). Tuft cells are key mediators of interkingdom interactions at mucosal barrier surfaces. PloS Pathog. 18, e1010318. doi: 10.1371/journal.ppat.1010318
Su, J., Chen, T., Ji, X. Y., Liu, C., Yadav, P. K., Wu, R., et al. (2013). IL-25 downregulates Th1/Th17 immune response in an IL-10-dependent manner in inflammatory bowel disease. Inflammation Bowel Dis. 19, 720–728. doi: 10.1097/MIB.0b013e3182802a76
Su, G. L., Ko, C. W., Bercik, P., Falck-Ytter, Y., Sultan, S., Weizman, A. V., et al. (2020). AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology 159, 697–705. doi: 10.1053/j.gastro.2020.05.059
Suez, J., Zmora, N., Zilberman-Schapira, G., Mor, U., Dori-Bachash, M., Bashiardes, S., et al. (2018). Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 174, 1406–1423.e16. doi: 10.1016/j.cell.2018.08.047
Sugihara, K. and Kamada, N. (2024). Metabolic network of the gut microbiota in inflammatory bowel disease. Inflammation Regener. 44, 11. doi: 10.1186/s41232-024-00321-w
Tan, X. Y., Xie, Y. J., Liu, X. L., Li, X. Y., and Jia, B. (2022). A systematic review and meta-analysis of randomized controlled trials of fecal microbiota transplantation for the treatment of inflammatory bowel disease. Evid Based Complement Alternat Med. 2022, 8266793. doi: 10.1155/2022/8266793
Tang, L. L., Feng, W. Z., Cheng, J. J., and Gong, Y. N. (2020). Clinical remission of ulcerative colitis after different modes of faecal microbiota transplantation: a meta-analysis. Int. J. Colorectal Dis. 35, 1025–1034. doi: 10.1007/s00384-020-03599-7
Tawfik, A., Flanagan, P. K., and Campbell, B. J. (2014). Escherichia coli-host macrophage interactions in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 20, 8751–8763. doi: 10.3748/wjg.v20.i27.8751
Theochari, N. A., Stefanopoulos, A., Mylonas, K. S., and Economopoulos, K. P. (2018). : Antibiotics exposure and risk of inflammatory bowel disease: a systematic review. Scand. J. Gastroenterol. 53, 1–7. doi: 10.1080/00365521.2017.1386711
Tremaroli, V. and Bäckhed, F. (2012). Functional interactions between the gut microbiota and host metabolism. Nature . 489, 242–249. doi: 10.1038/nature11552
Tsai, Y. C., Tai, W. C., Liang, C. M., Wu, C. K., Tsai, M. C., Hu, W. H., et al. (2025). Alternations of the gut microbiota and the Firmicutes/Bacteroidetes ratio after biologic treatment in inflammatory bowel disease. J. Microbiol. Immunol. Infect. 58, 62–69. doi: 10.1016/j.jmii.2024.09.006
Ungaro, R., Mehandru, S., Allen, P. B., Peyrin-Biroulet, L., and Colombel, J. F. (2017). Ulcerative colitis. Lancet. 389, 1756–1770. doi: 10.1016/S0140-6736(16)32126-2
Vacca, M., Celano, G., Calabrese, F. M., Portincasa, P., Gobbetti, M., and De Angelis, M. (2020). The controversial role of human gut lachnospiraceae. Microorganisms 8, 573. doi: 10.3390/microorganisms8040573
Vermeire, S., Joossens, M., Verbeke, K., Wang, J., Machiels, K., Sabino, J., et al. (2016). Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease. J. Crohns Colitis. 10, 387–394. doi: 10.1093/ecco-jcc/jjv203
Walker, A. W., Sanderson, J. D., Churcher, C., Parkes, G. C., Hudspith, B. N., Rayment, N., et al. (2011). High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 11, 7. doi: 10.1186/1471-2180-11-7
Walsh, D., McCarthy, J., O’Driscoll, C., and Melgar, S. (2013). Pattern recognition receptors–molecular orchestrators of inflammation in inflammatory bowel disease. Cytokine Growth Factor Rev. 24, 91–104. doi: 10.1016/j.cytogfr.2012.09.003
Wang, S., Dong, Z., and Wan, X. (2024). Global, regional, and national burden of inflammatory bowel disease and its associated anemia, 1990 to 2019 and predictions to 2050: An analysis of the global burden of disease study 2019. Autoimmun Rev. 23, 103498. doi: 10.1016/j.autrev.2023.103498
Wang, H. B., Wang, P. Y., Wang, X., Wan, Y. L., and Liu, Y. C. (2012). Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis. Sci. 57, 3126–3135. doi: 10.1007/s10620-012-2259-4
Weingarden, A., González, A., Vázquez-Baeza, Y., Weiss, S., Humphry, G., Berg-Lyons, D., et al. (2015). Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome 3, 10. doi: 10.1186/s40168-015-0070-0
Weingarden, A. R. and Vaughn, B. P. (2017). Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes 8, 238–252. doi: 10.1080/19490976.2017.1290757
Wu, W., Chen, F., Liu, Z., and Cong, Y. (2016). Microbiota-specific th17 cells: yin and yang in regulation of inflammatory bowel disease. Inflammation Bowel Dis. 22, 1473–1482. doi: 10.1097/MIB.0000000000000775
Xu, Y. D., Cheng, M., Shang, P. P., and Yang, Y. Q. (2022). Role of IL-6 in dendritic cell functions. J. Leukoc. Biol. 111, 695–709. doi: 10.1002/JLB.3MR0621-616RR
Xu, X., Ocansey, D. K. W., Hang, S., Wang, B., Amoah, S., Yi, C., et al. (2022). The gut metagenomics and metabolomics signature in patients with inflammatory bowel disease. Gut Pathog. 14, 26. doi: 10.1186/s13099-022-00499-9
Yadegar, A., Salahi-Niri, A., Dai, C., and Shen, J. (2024). Editorial: Recent advances in understanding the role and mechanisms of gut microbiota in inflammatory bowel disease and irrita ble bowel syndrome. Front. Cell Infect. Microbiol. 14, 1521676. doi: 10.3389/fcimb.2024.1521676
Yan, J. B., Luo, M. M., Chen, Z. Y., and He, B. H. (2020). The function and role of the th17/treg cell balance in inflammatory bowel disease. J. Immunol. Res. 2020, 8813558. doi: 10.1155/2020/8813558
Yang, M., Gu, Y., Li, L., Liu, T., Song, X., Sun, Y., et al. (2021). Bile acid-gut microbiota axis in inflammatory bowel disease: from bench to bedside. Nutrients, 22, 3143. doi: 10.3390/nu13093143
Yi, J., Bergstrom, K., Fu, J., Shan, X., McDaniel, J. M., McGee, S., et al. (2019). Dclk1 in tuft cells promotes inflammation-driven epithelial restitution and mitigates chronic colitis. Cell Death Differ 26, 1656–1669. doi: 10.1038/s41418-018-0237-x
Yoon, M. Y. and Yoon, S. S. (2018). Disruption of the gut ecosystem by antibiotics. Yonsei Med. J. 59, 4–12. doi: 10.3349/ymj.2018.59.1.4
Yue, B., Luo, X., Yu, Z., Mani, S., Wang, Z., and Dou, W. (2019). Inflammatory bowel disease: A potential result from the collusion between gut microbiota and mucosal immune system. Microorganisms 7, 440. doi: 10.3390/microorganisms7100440
Zhai, R., Xue, X., Zhang, L., Yang, X., Zhao, L., and Zhang, C. (2019). Strain-Specific Anti-inflammatory Properties of Two Akkermansia muciniphila Strains on Chronic Colitis in Mice. Front. Cell Infect. Microbiol. 9, 239. doi: 10.3389/fcimb.2019.00239
Zhang, X., Ishikawa, D., Ohkusa, T., Fukuda, S., and Nagahara, A. (2022). Hot topics on fecal microbiota transplantation for the treatment of inflammatory bowel disease. Front. Med. (Lausanne). 9, 1068567. doi: 10.3389/fmed.2022.1068567
Zhang, T., Long, C., Cui, B., Buch, H., Wen, Q., Li, Q., et al. (2020). Colonic transendoscopic tube-delivered enteral therapy (with video): a prospective study. BMC Gastroenterol. 20, 135. doi: 10.1186/s12876-020-01285-0
Zhao, L., Suolang, Y., Zhou, D., Tang, Y., and Zhang, Y. (2018). Bifidobacteria alleviate experimentally induced colitis by upregulating indoleamine 2, 3-dioxygenase expression. Microbiol. Immunol. 62, 71–79. doi: 10.1111/1348-0421.12562
Zhao, H., Xu, H., Chen, S., He, J., Zhou, Y., and Nie, Y. (2021). Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J. Gastroenterol. Hepatol. 36, 320–328. doi: 10.1111/jgh.15222
Zheng, M., Han, R., Yuan, Y., Xing, Y., Zhang, W., Sun, Z., et al. (2022). The role of Akkermansia muciniphila in inflammatory bowel disease: Current knowledge and perspectives. Front. Immunol. 13, 1089600. doi: 10.3389/fimmu.2022.1089600
Zheng, T., Hao, H., Liu, Q., Li, J., Yao, Y., Liu, Y., et al. (2023). Effect of extracelluar vesicles derived from akkermansia muciniphila on intestinal barrier in colitis mice. Nutrients, 15 (22), 4722. doi: 10.3390/nu15224722
Keywords: inflammatory bowel disease, gut microbiota, intestinal barrier, fecal microbiota transplantation, antibiotics, probiotics
Citation: Xie H, Yu S, Tang M, Xun Y, Shen Q and Wu G (2025) Gut microbiota dysbiosis in inflammatory bowel disease: interaction with intestinal barriers and microbiota-targeted treatment options. Front. Cell. Infect. Microbiol. 15:1608025. doi: 10.3389/fcimb.2025.1608025
Received: 08 April 2025; Accepted: 05 June 2025;
Published: 27 June 2025.
Edited by:
Eugenia Bezirtzoglou, Democritus University of Thrace, GreeceReviewed by:
Akhter Rasool, Tamil Nadu Veterinary and Animal Sciences University, IndiaApurva Jadhav, Bharati Vidyapeeth Deemed University, India
Luana Alexandrescu, County Clinical Emergency Hospital of Constanta, Romania
Copyright © 2025 Xie, Yu, Tang, Xun, Shen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaojue Wu, d3VnYW9qdWVAYWxpeXVuLmNvbQ==
†These authors share first authorship
 Hongjun Xie
Hongjun Xie Siyan Yu2,3†
Siyan Yu2,3† Gaojue Wu
Gaojue Wu