- 1Department of Breast Medicine 1, Cancer Hospital of China Medical University, Liaoning Cancer Hospital, Shenyang, China
- 2Department of Pharmacology, Cancer Hospital of China Medical University, Liaoning Cancer Hospital, Shenyang, China
- 3Department of Breast Medicine, Cancer Hospital of Dalian University of Technology, Liaoning Cancer Hospital, Shenyang, China
Background: Hepatitis A Inactivated and Hepatitis B (Recombinant) Vaccine (Hep AB) was approved for use in 2001. Hep AB demonstrates satisfactory efficacy in protecting the public from hepatitis virus infections. However, there is a lack of recent real-world report on its adverse events (AEs).
Methods: We retrieved US AE reports related to Hep AB vaccination from VAERS for the period 2020-2024. We used four algorithms: Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN) and Multi-Item Gamma-Poisson Shrinkage (MGPS) to examine AE signals. The ROR and PRR algorithms have higher sensitivity but lower specificity. However, BCPNN and MGPS compensate for this limitation. Combining all four algorithms helps reduce false-positive signals. In addition to the general population, we also focused on reports stratified by gender.
Results: We retrieved 1,640 eligible reports from VAERS. In the general population, we identified two AE signals at the System Organ Classification (SOC) level. Additionally, we found 39 AE signals at the Preferred Term (PT) level. Among these, endocrine disorders were identified for the first time as AE signals. In the subsequent gender stratified analysis, more AE signals were identified in females compared to males. Notably, signals for endocrine disorders (autoimmune thyroiditis and Graves’ disease) were detected in females, whereas no such signals were found in males.
Conclusions: We conducted a comprehensive examination of the recent AE reports for Hep AB and identified unexpected AEs, particularly in females. These findings will provide valuable insights into future evidence-based surveillance strategies of Hep AB.
1 Introduction
Hepatitis is a common liver disease, with viral hepatitis being one of its primary types (Cooke et al., 2024). Hepatitis A virus (HAV) and hepatitis B virus (HBV) are two important pathogens responsible for viral hepatitis. HAV is transmitted through the fecal-oral route, commonly through contaminated water, food or daily contact (Cuthbert, 2001). HBV is transmitted through blood and sexual contact (Daka et al., 2022; Fu et al., 2024). The symptoms of HAV infection manifest in the short term, including jaundice, abdominal pain and hyperbilirubinemia (Abutaleb and Kottilil, 2020). However, HBV infection causes chronic liver inflammation, which may eventually progress to cirrhosis and liver cancer (Trépo et al., 2014). In order to ensure public health, the HAV and HBV vaccines were introduced. They will prevent HAV and HBV infections by stimulating the body to produce specific antibodies. To meet broader health needs, the first Hepatitis A Inactivated and Hepatitis B (Recombinant) vaccine (Hep AB) was licensed for use in the United States in 2001 (Advisory Committee on Immunization Practices (ACIP) et al., 2006). Previous analysis showed that routine childhood vaccination would prevent 322 million cases of disease and approximately 732,000 premature deaths among children born between 1994 and 2013 (Hill et al., 2015). Viral hepatitis can also reduce its incidence through vaccination. The World Health Organization (WHO) has stated that there has been a notable decline in infection rates since the introduction of HAV and HBV vaccination (WHO position paper on hepatitis A vaccines: June 2012-recommendations, 2013; World Health Organization, 2019).
Like medications, vaccination can also induce a range of adverse events (AEs) and sometimes serious AEs (SAEs) (Li et al., 2024f; Zhang et al., 2024; Li et al., 2025e). Then, the safety of Hep AB is often a concern for vaccine recipients. The most commonly reported AEs in clinical trials were general fatigue, injection site pain, erythema and headache (Murdoch et al., 2003). Safety analysis of vaccination during pregnancy indicated that Hep AB vaccination did not show any patterns of adverse pregnancy outcomes (Celzo et al., 2020). Moreover, AEs after Hep AB vaccination were found to be similar to those reported after single-dose HAV and HBV vaccinations (Woo et al., 2006). These reports indicate a safety profile for Hep AB. The safety of Hep AB has been comprehensively evaluated prior to its market launch. Nevertheless, its long-term safety has still attracted widespread attention. Over the past decade, the safety of Hep AB vaccination has continued to be assessed. However, there has been a lack of real-world report of Hep AB vaccination in the past five years.
AE related studies in vaccine informatics have attracted significant public attention, as evidenced by recent research advancements (Li et al., 2024a, 2024b, 2025a; Pan et al., 2024; Huffman et al., 2025). These studies are essential for improving the safety monitoring of vaccines and understanding potential risks. One of the key systems used to monitor AEs is the Vaccine Adverse Event Reporting System (VAERS). This system plays a crucial role in collecting and analyzing reports of vaccine AEs, contributing to a better understanding of vaccine safety. VAERS is a passive reporting system, meaning that it relies on healthcare providers and the public to voluntarily report AEs (Li et al., 2024e, 2024c, 2024d, 2025e, 2025c, 2025b, 2025d; Moro et al., 2024; Oliveira et al., 2025). In this study, we aim to collect recent reports for Hep AB to identify AE signals. Four disproportionate analysis methods will be employed to examine AE signals. Besides the general population, we also focused on reports fstratified by gender. Overall, this study will provide valuable insights for the future evidence-based surveillance strategies of Hep AB.
2 Method
The data in VAERS includes demographic details of the vaccinated individuals, types of vaccines administered and information on AE reports (Shimabukuro et al., 2015). AEs and their related signs and symptoms are coded using the Medical Dictionary for Regulatory Activities (MedDRA, version 27.0). Each report may include one or more MedDRA Preferred Terms (PTs). PTs are not necessarily medically confirmed diagnoses. This study adheres to the REporting of A Disproportionality Analysis for DrUg Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV) guidelines (Fusaroli et al., 2024).
2.1 Data source and processing
We extracted AE reports submitted to VAERS from 2020 to 2024 in the US (https://vaers.hhs.gov/). Then, we conducted the following procedures to process the data: (1) We ensured that all reports originated from the US by excluding entries labeled as “Foreign”; (2) Duplicate reports were excluded; (3) We filtered the target vaccine by restricting the “VAX_NAME” field to “HEP A + HEP B”; (4) We also excluded entries labeled as “NO BRAND NAME”; (5) Reports of vaccination errors (such as incomplete vaccination courses) without describing AEs were excluded. All other AE reports in VAERS will be used as the reference group for comparison. All PTs will be mapped to their respective System Organ Classifications (SOCs) for further exploration.
2.2 Statistical analysis
Disproportionality analysis has been widely used in pharmacovigilance to detect AE signals. This method detects signals by comparing the difference in the occurrence frequency of specific event combinations with the background frequency. The 2 × 2 contingency table used in the analysis is detailed in Supplementary Table 1. The total for each PT or SOC constitutes the value of “A”. Moreover, the values of “B”, “C” and “D” are calculated for all PTs or SOCs based on different vaccines. Reporting Odds Ratio (ROR) and Proportional Reporting Ratio (PRR) are classified as frequency-based methods, indicating high sensitivity but low specificity. Bayesian Confidence Propagation Neural Network (BCPNN) and Multi-Item Gamma-Poisson Shrinkage (MGPS) are Bayesian algorithms capable of handling complex data, but they exhibit lower sensitivity in data detection. To reduce the false positive rate of AE signals, an AE signal is generated when all four methods detect a positive result. Supplementary Table 2 presents the four algorithms and their respective thresholds for positive results.
3 Results
3.1 Descriptive characteristics
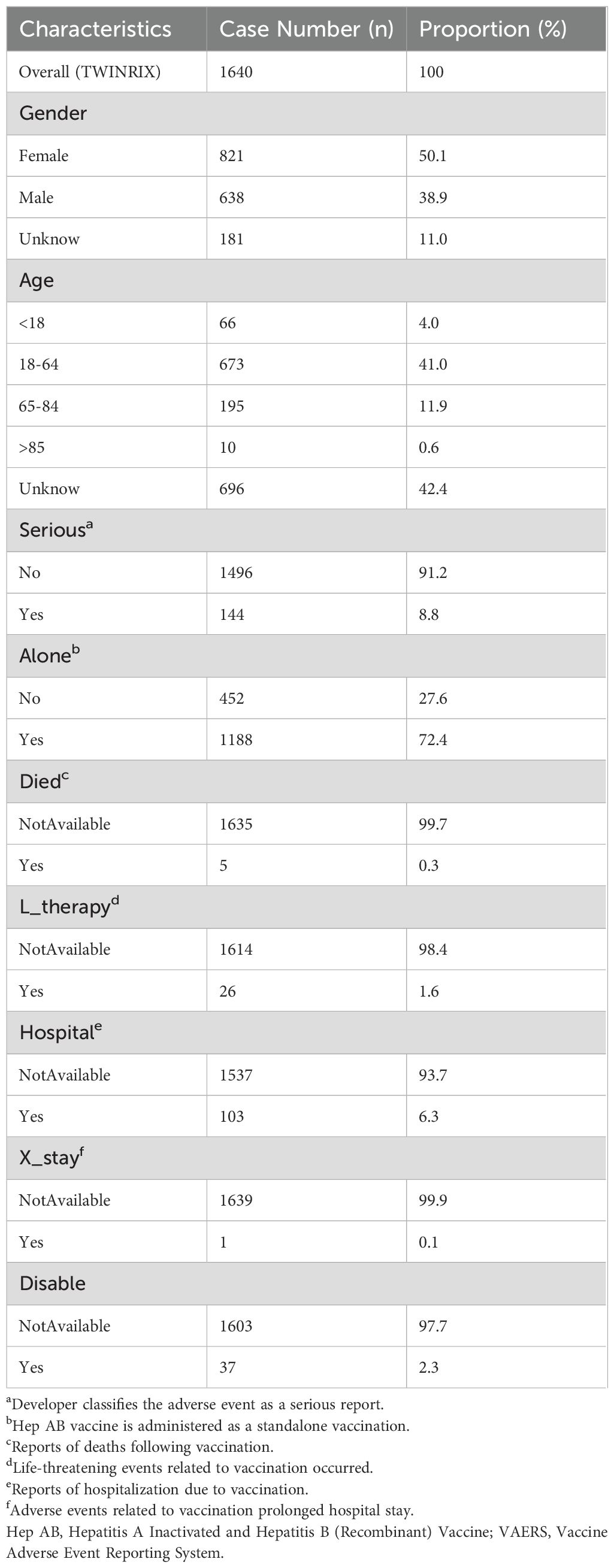
A total of 1,640 reports were retrieved during the period from 2020 to 2024 (Table 1). Compared to males (38.9%), the majority of reports came from females (50.1%), with the remaining reports not specifying gender. Reports from the 18-64 age (41.0%) were significantly higher than those from other age. However, 696 reports (42.4%) did not record the age. Non-serious reports accounted for the majority (91.2%). Subsequently, we will conduct further exploration of the population with serious reports (8.8%). The majority of reports indicated vaccination with a single dose (72.4%). In addition, only five reports indicated deaths following vaccination. The number of life-threatening events occurring after vaccination increased to 26 cases. There have been 103 reports of hospitalizations due to vaccination. However, only one report indicated vaccination prolonged hospital stay. There were 37 reports indicating disability caused by vaccination. Regarding reporting years, there were 159 reports in 2020, 224 reports in 2021, 395 reports in 2022, 475 reports in 2023 and 387 reports in 2024. The historical trend in the number of reports is depicted in Figure 1.
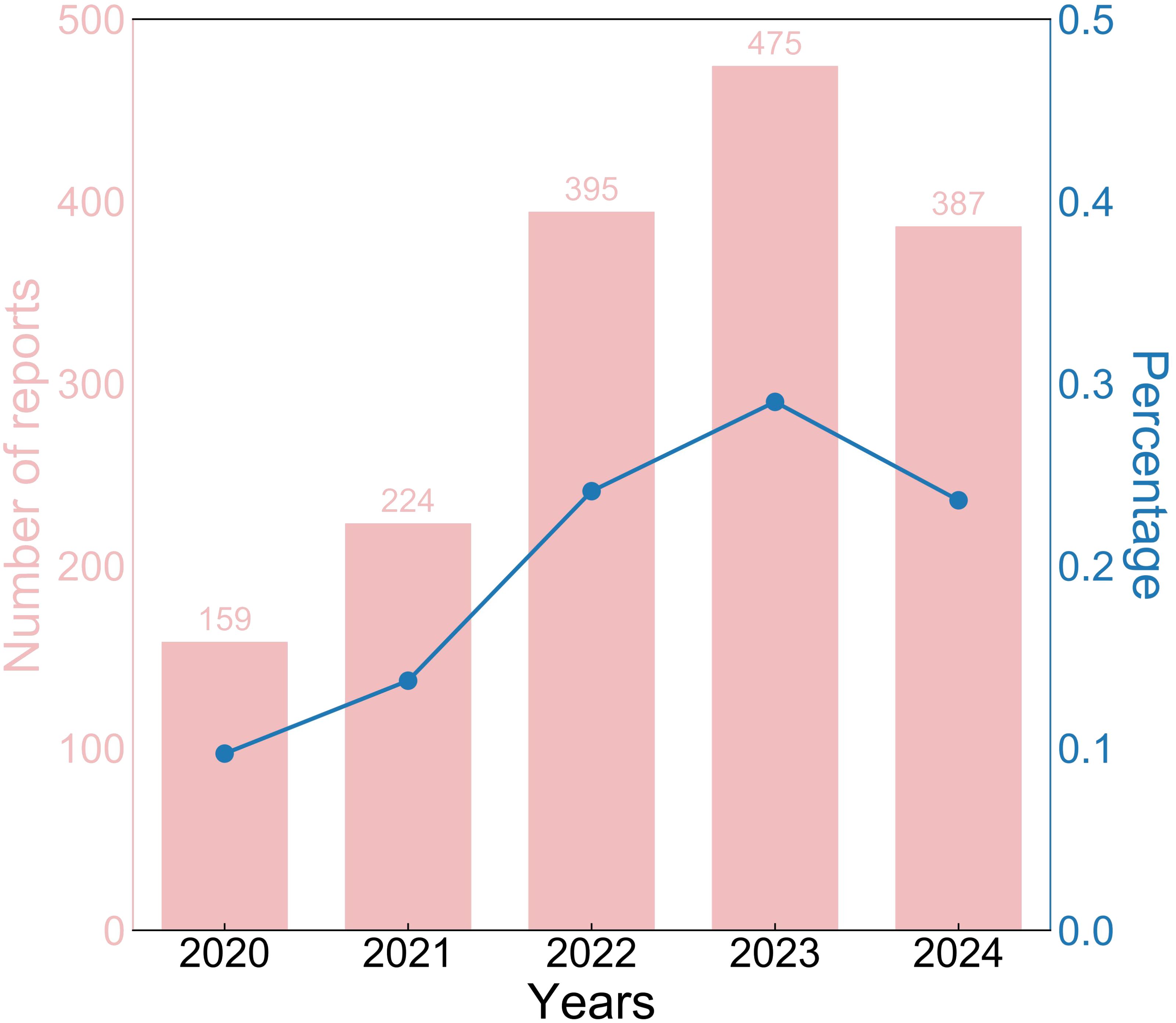
Figure 1. The historical trend of Hep AB-related AE reports from 2020 to 2024. Hep AB, Hepatitis A Inactivated and Hepatitis B (Recombinant) Vaccine; AE, adverse event.
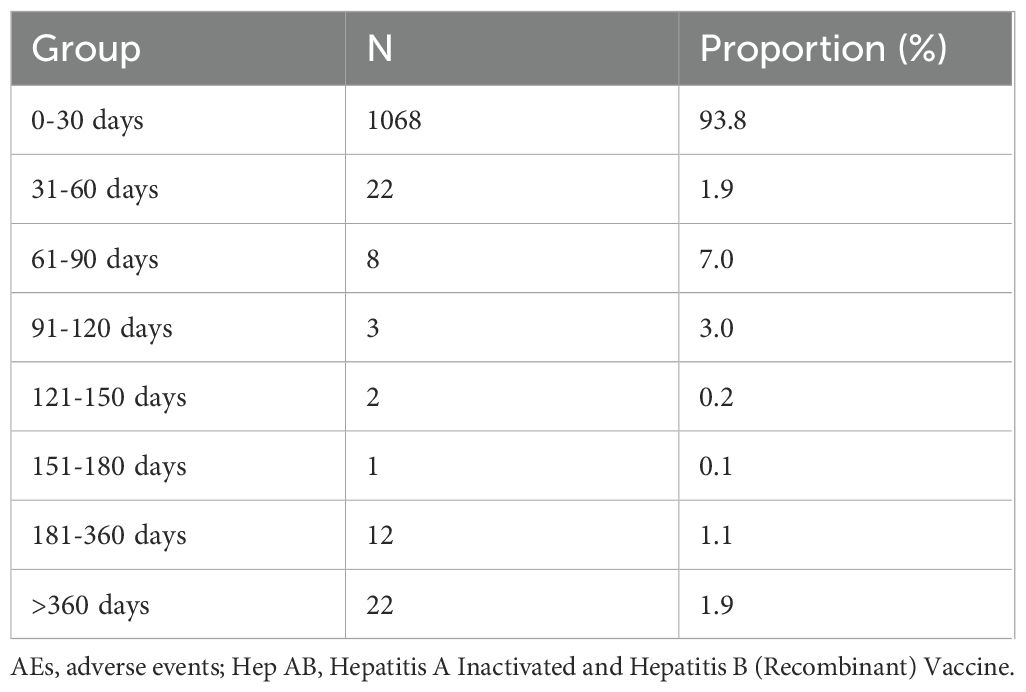
Subsequently, we compiled a total of 1,138 reports with precise onset times of AEs (Table 2). The majority of reports (93.8%) indicated that the onset times of AEs within the first month following vaccination. However, AEs were still reported one year after vaccination (1.9%).
3.2 Disproportionality analysis
3.2.1 General population
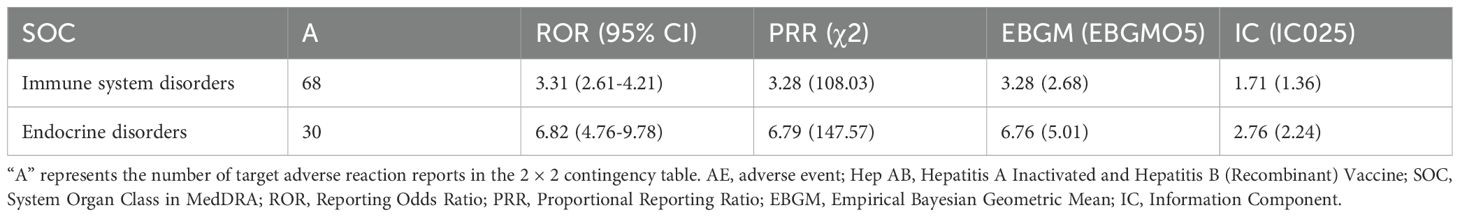
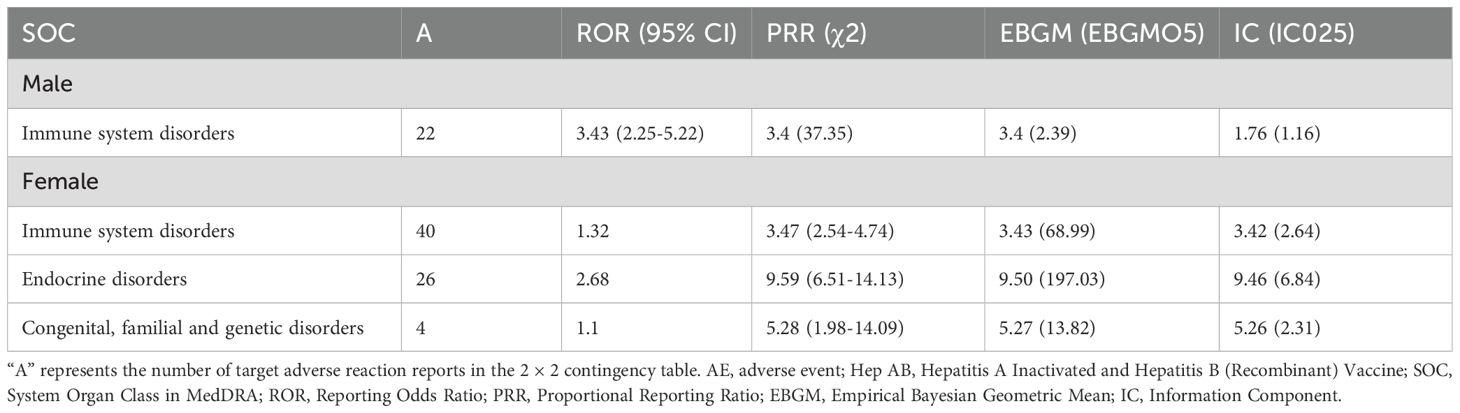
To reduce the false positive rate, we only examined AE signals where all four algorithms met the threshold. We identified two AE signals at the SOC level in the general population (Table 3), including immune system disorders and endocrine disorders. Immune system disorders have already been reported in the package insert. However, endocrine disorders are being reported for the first time.
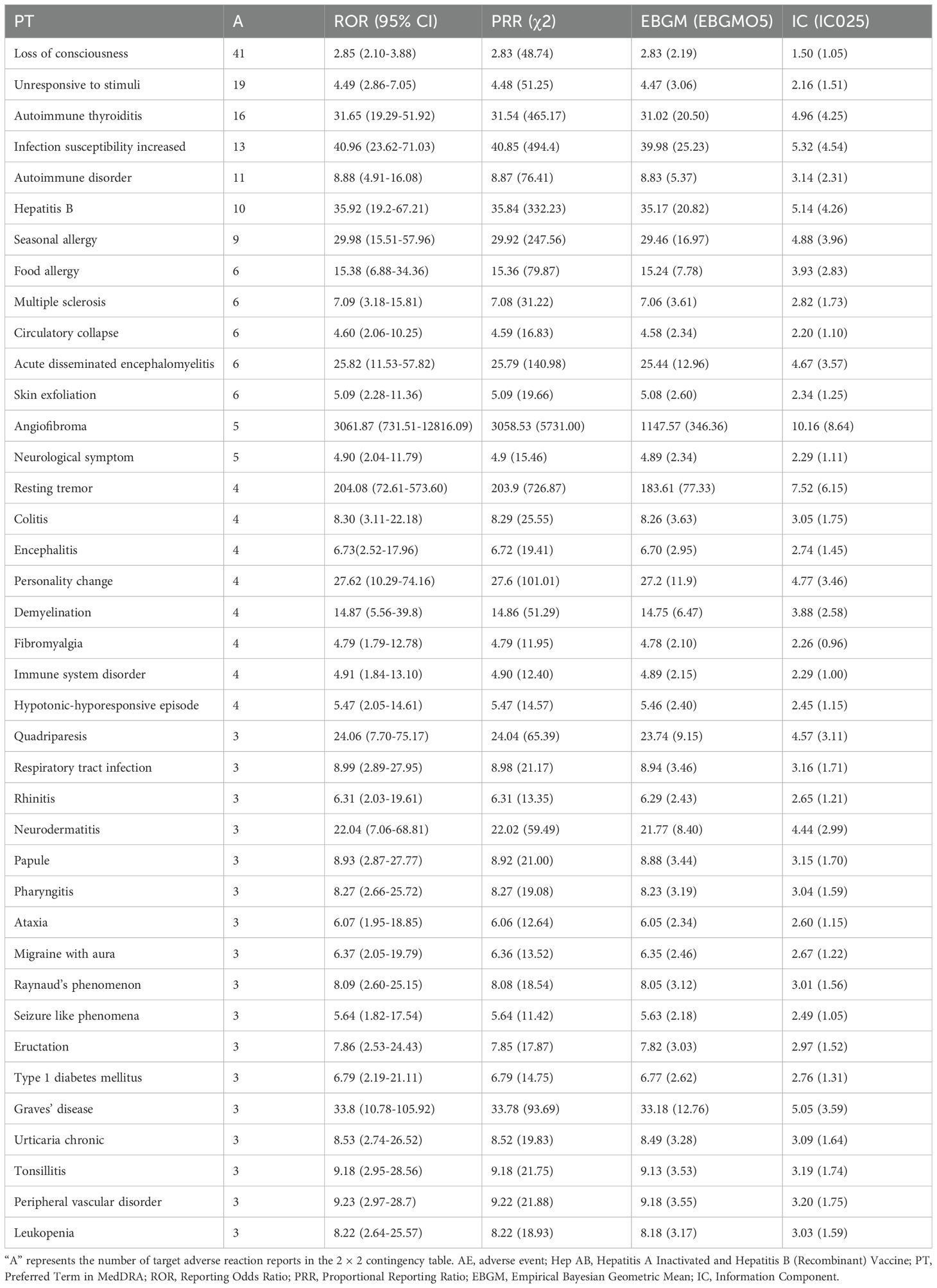
After excluding SOCs and their PT signals unrelated to vaccination (including Injury, poisoning and procedural complications, General disorders and administration site conditions, Investigations, Product issues, and Social circumstances), 39 AE signals were detected at the PT level (Table 4). Under endocrine disorders, two PTs were identified as AE signals, including Autoimmune thyroiditis (ROR = 31.65; PRR = 31.54; EBGM05 = 20.50; IC025 = 4.25) and Graves’ disease (ROR = 33.80; PRR = 33.78; EBGM05 = 12.76; IC025 = 3.59) (Figure 2). Supplementary Table 3 presents detailed information on the PT-level AE signals.
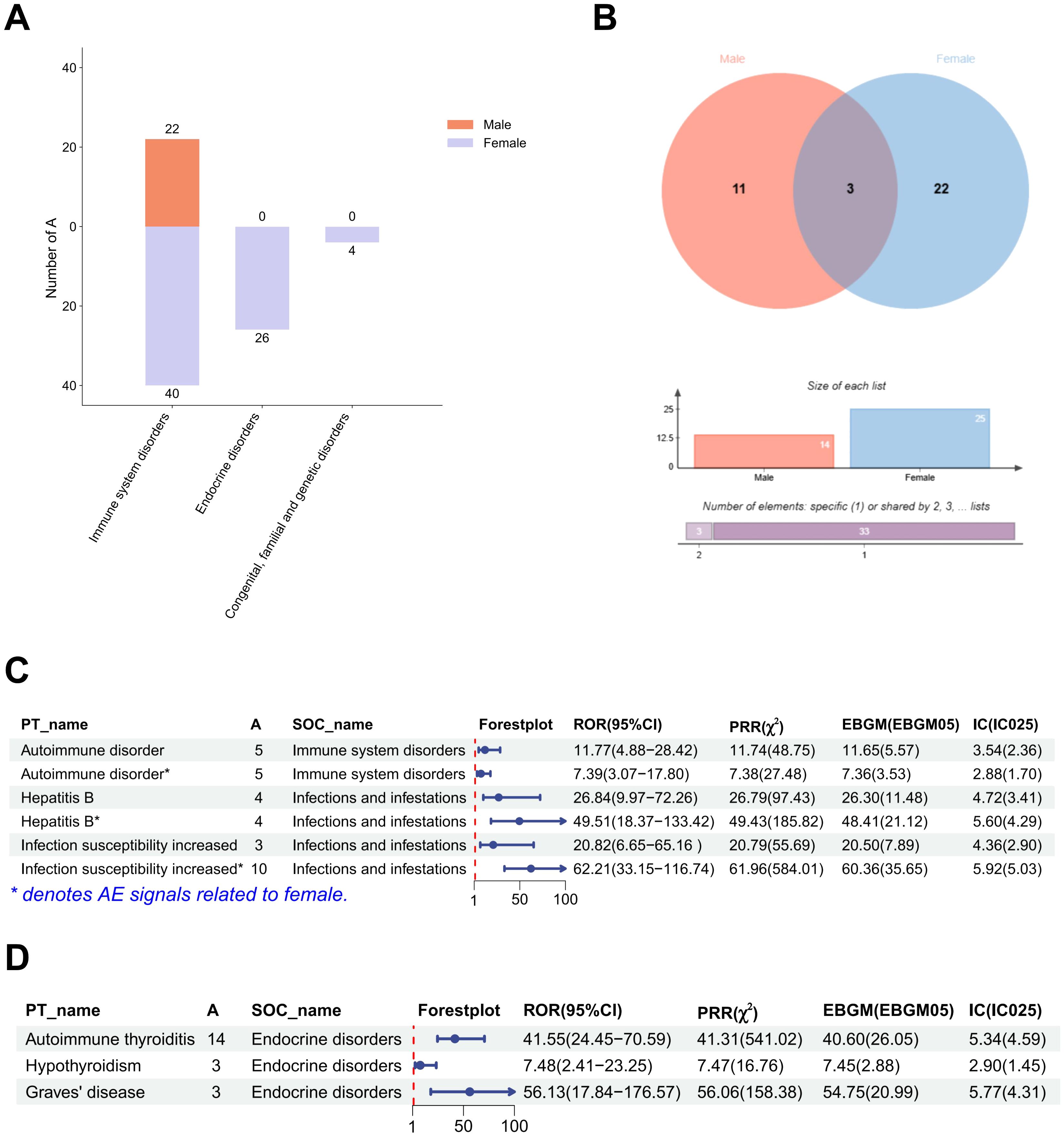
Figure 2. Gender stratified differences in AE signals at the SOC and PT levels. (A) The report number of AE signals at the SOC level. (B) Differences in AE signals between males and females at the PT level. (C) The strength of AE signals at the SOC level in males and females. (D) The strength of AE signals at the PT level under endocrine disorders in females. (A) represents the number of target adverse reaction reports in the 2 × 2 contingency table. PT, Preferred Term; SOC, System Organ Classification; AE, adverse event; ROR, Reporting Odds Ratio; PRR, Proportional Reporting Ratio; EBGM, Empirical Bayesian Geometric Mean; IC, Information Component.
3.2.2 Gender stratification
After gender stratification, only immune system disorder was identified as AE signal in the male SOC level. However, three AE signals were identified at the SOC level in females, namely immune system disorders, endocrine disorders and congenital, familial and genetic disorders (Table 5). Reports of immune system disorders were fewer in males than in females (Figure 2A). A greater number of PT-level AE signals were detected in females. We examined the PT-level AE signals that were identified in both males and females, including autoimmune disorder, hepatitis B and increased infection susceptibility (Figure 2B). Except for increased infection susceptibility (Male: ROR = 20.82; PRR = 20.79; EBGM05 = 7.89; IC025 = 2.90 and Female: ROR = 62.21; PRR = 61.96; EBGM05 = 35.65; IC025 = 5.03), the signal strength of autoimmune disorder and hepatitis B did not show significant differences (Figure 2C). Subsequently, we conducted further investigation into the PT signals under the two SOC-level AE signals that were exclusive to females. Notably, congenital, familial and genetic disorders did not exhibit any PT-level AE signals. Three PT-level AE signals were identified under endocrine disorders, including autoimmune thyroiditis (ROR = 41.55; PRR = 41.31; EBGM05 = 26.05; IC025 = 4.59), hypothyroidism (ROR = 7.48; PRR = 7.47; EBGM05 = 2.88; IC025 = 1.45), and Graves’ disease (ROR = 56.13; PRR = 56.06; EBGM05 = 20.99; IC025 = 4.31) (Figure 2D). Supplementary Tables 4 and 5 present detailed information on the PT-level AE signals for males and females, respectively.
4 Discussion
In this study, we conducted a comprehensive analysis of AE signals following Hep AB vaccination using reports from VAERS between 2020 and 2024. Our findings indicate that the AEs observed following Hep AB vaccination are generally consistent with those listed in the package insert, including immune system disorders. In addition, we still identified unanticipated AEs, including endocrine disorders. Under gender stratification, no signals of endocrine disorders were found in males. In females, three PT-level AE signals under endocrine disorders (autoimmune thyroiditis, hypothyroidism, and Graves’ disease) were detected. Considering the continued administration of Hep AB, our findings will provide valuable insights for future evidence-based surveillance strategies of Hep AB.
Our findings suggest that females may be more susceptible to the occurrence of autoimmune thyroiditis and Graves’ disease following Hep AB vaccination. Both AEs are associated with autoimmune attacks. The signal strength is stronger in females, suggesting a potential association with gender. Gender, as a biological variable, influences the immune response to vaccination (Klein et al., 2010). Previous study has indicated that adult females exhibit stronger innate and adaptive immune responses compared to males (Klein and Flanagan, 2016). For example, plasmacytoid dendritic cells (pDCs) exposed to the TLR7 ligand in vitro resulted in higher production of interferon-α (IFN-α) in female cells compared to males (Berghöfer et al., 2006). In addition, following viral attack in adult rats, the expression of TLR pathways and pro-inflammatory genes was higher in the tissues of female rats compared to males (Hannah et al., 2008). Vaccines, as exogenous antigens, may activate a more sensitive immune response in females. Therefore, vaccination may exacerbate local or systemic inflammatory responses in females, potentially leading to autoimmune thyroiditis and Graves’ disease. Besides our research, studies on COVID-19 vaccine related AEs have also yielded similar findings (Li et al., 2024e, 2024c, 2025c, 2025b).
Epidemiological study indicates that the prevalence of autoimmune thyroid diseases in females (such as: Hashimoto’s thyroiditis and Graves’ disease) is significantly higher than in males (Vanderpump, 2011). Vaccination may act as a triggering factor, thereby enhancing susceptibility to autoimmune thyroiditis and Graves’ disease. A previous study has suggested that a positive result for anti-thyroid microsomal antibodies was observed after the second dose of the HAV vaccine (Karali et al., 2011). It is necessary to investigate whether the antigens of the hepatitis B vaccine (HBsAg) or the inactivated virus particles of the hepatitis A vaccine may have potential molecular mimicry or cross-reactivity with thyroid tissue, leading to immune system-induced attacks on thyroid cells.
In the reports we reviewed, 1,138 documented the complete onset time of vaccine-induced AEs. Almost all reports were submitted within the first month following vaccination. However, some reports indicated that AEs occurred one year after vaccination. Therefore, it is crucial to strengthen long-term surveillance of AEs following Hep AB vaccination.
In this study, although we identified unexpected AE signals, it must be acknowledged that there are certain limitations within this study. The AE signals obtained through disproportionality analysis did not include some of the common AEs in previous Hep AB clinical trial records. This suggests that future discussions on the safety of Hep AB vaccination should incorporate more real-world data and prospective evidence. Additionally, as VAERS is a passive reporting system, our results may have been influenced by the quality of AE reports, indicating that greater caution should be exercised when interpreting the findings. Finally, disproportionality analysis only detects AE signals and cannot be interpreted as indicating causality following vaccination. This requires further observational and experimental studies in the future to establish causality.
5 Conclusions
We conducted a comprehensive examination of the recent AE reports for Hep AB and identified unexpected AEs (autoimmune thyroiditis and Graves’ disease), particularly in females. These findings will provide valuable insights into future evidence-based surveillance strategies of Hep AB, ultimately facilitating more informed decisions in vaccine safety and public health policy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YZ: Conceptualization, Formal Analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. YW: Formal Analysis, Methodology, Software, Writing – original draft. YF: Formal Analysis, Methodology, Software, Writing – original draft. HZ: Formal Analysis, Methodology, Software, Writing – original draft. TS: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. JX: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Nature Science Foundation of China (82373113, XJ), Shenyang Breast Cancer Clinical Medical Research Center (2020-48-3-1, ST), Shenyang Public Health R&D Special Project (22-321-31-04,ST), LiaoNing Revitalization Talents Program (XLYC1907160, XJ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1609409/full#supplementary-material
References
Abutaleb, A. and Kottilil, S. (2020). Hepatitis A: epidemiology, natural history, unusual clinical manifestations, and prevention. Gastroenterology Clinics North America 49, 191. doi: 10.1016/j.gtc.2020.01.002
Advisory Committee on Immunization Practices (ACIP), Fiore, A. E., Wasley, A., and Bell, B. P. (2006). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 55, 1–23.
(2013). WHO position paper on hepatitis A vaccines: June 2012-recommendations. Vaccine 31, 285–286. doi: 10.1016/j.vaccine.2012.10.102
Berghöfer, B., Frommer, T., Haley, G., Fink, L., Bein, G., and Hackstein, H. (2006). TLR7 ligands induce higher IFN-alpha production in females. J. Immunol. 177, 2088–2096. doi: 10.4049/jimmunol.177.4.2088
Celzo, F., Buyse, H., Welby, S., and Ibrahimi, A. (2020). Safety evaluation of adverse events following vaccination with Havrix, Engerix-B or Twinrix during pregnancy. Vaccine 38, 6215–6223. doi: 10.1016/j.vaccine.2020.07.041
Cooke, G. S., Flower, B., Cunningham, E., Marshall, A. D., Lazarus, J. V., Palayew, A., et al. (2024). Progress towards elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission update. Lancet Gastroenterol. Hepatol 9, 346–365. doi: 10.1016/S2468-1253(23)00321-7
Cuthbert, J. A. (2001). Hepatitis A: old and new. Clin. Microbiol Rev. 14, 38–58. doi: 10.1128/CMR.14.1.38-58.2001
Daka, D., Hailemeskel, G., and Fenta, D. A. (2022). Prevalence of Hepatitis B Virus infection and associated factors among female sex workers using respondent-driven sampling in Hawassa City, Southern Ethiopia. BMC Microbiol 22, 37. doi: 10.1186/s12866-022-02444-x
Fu, M. X., Simmonds, P., Andersson, M., and Harvala, H. (2024). Biomarkers of transfusion transmitted occult hepatitis B virus infection: Where are we and what next? Rev. Med. Virol 34, e2525. doi: 10.1002/rmv.2525
Fusaroli, M., Salvo, F., Begaud, B., AlShammari, T. M., Bate, A., Battini, V., et al. (2024). The REporting of A disproportionality analysis for drUg safety signal detection using individual case safety reports in pharmacoVigilance (READUS-PV): explanation and elaboration. Drug Saf. 47, 585–599. doi: 10.1007/s40264-024-01423-7
Hannah, M. F., Bajic, V. B., and Klein, S. L. (2008). Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway rats. Brain Behav. Immun. 22, 503–516. doi: 10.1016/j.bbi.2007.10.005
Hill, H. A., Elam-Evans, L. D., Yankey, D., Singleton, J. A., and Kolasa, M. (2015). National, state, and selected local area vaccination coverage among children aged 19-35 months - United State. MMWR Morb Mortal Wkly Rep. 64, 889–896. doi: 10.15585/mmwr.mm6433a1
Huffman, A., Gautam, M., Gandhi, A., Du, P., Austin, L., Roan, K., et al. (2025). Systematic collection, annotation, and pattern analysis of viral vaccines in the VIOLIN vaccine knowledgebase. Front. Cell Infect. Microbiol 15. doi: 10.3389/fcimb.2025.1509226
Karali, Z., Basaranoglu, S. T., Karali, Y., Oral, B., and Kilic, S. S. (2011). Autoimmunity and hepatitis A vaccine in children. J. Invest. Allergol Clin. Immunol. 21, 389–393.
Klein, S. L. and Flanagan, K. L. (2016). Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638. doi: 10.1038/nri.2016.90
Klein, S. L., Jedlicka, A., and Pekosz, A. (2010). The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 10, 338–349. doi: 10.1016/S1473-3099(10)70049-9
Li, Y., Li, J., Dang, Y., Chen, Y., and Tao, C. (2024c). Adverse events of COVID-19 vaccines in the United States: temporal and spatial analysis. JMIR Public Health Surveill 10, e51007. doi: 10.2196/51007
Li, Y., Li, J., He, J., and Tao, C. (2024d). AE-GPT: Using Large Language Models to extract adverse events from surveillance reports-A use case with influenza vaccine adverse events. PloS One 19, e0300919. doi: 10.1371/journal.pone.0300919
Li, Y., Li, J., Li, M., Yu, E., Rhee, D., Amith, M., et al. (2025a). VaxBot-HPV: a GPT-based chatbot for answering HPV vaccine-related questions. JAMIA Open 8, ooaf005. doi: 10.1093/jamiaopen/ooaf005
Li, J., Li, Y., Pan, Y., Guo, J., Sun, Z., Li, F., et al. (2024a). Mapping vaccine names in clinical trials to vaccine ontology using cascaded fine-tuned domain-specific language models. J. BioMed. Semantics 15, 14. doi: 10.1186/s13326-024-00318-x
Li, Y., Lundin, S. K., Li, J., Tao, W., Dang, Y., Chen, Y., et al. (2024e). Unpacking adverse events and associations post COVID-19 vaccination: a deep dive into vaccine adverse event reporting system data. Expert Rev. Vaccines 23, 53–59. doi: 10.1080/14760584.2023.2292203
Li, Y., Tao, W., Dang, Y., Chen, Y., and Tao, C. (2025b). Exploring temporal and spatial characteristics of serious adverse event reports following COVID-19 bivalent vaccines. Res. Sq, rs.3.rs–6096098. doi: 10.21203/rs.3.rs-6096098/v1
Li, Y., Tao, W., Li, Z., Sun, Z., Li, F., Fenton, S., et al. (2024f). Artificial intelligence-powered pharmacovigilance: A review of machine and deep learning in clinical text-based adverse drug event detection for benchmark datasets. J. BioMed. Inform 152, 104621. doi: 10.1016/j.jbi.2024.104621
Li, Y., Tao, W., Lundin, S. K., Sun, Z., Dang, Y., Chen, Y., et al. (2025c). Exploring associations between COVID-19 bivalent vaccines and their related adverse events: A correlational study. Res. Sq, rs.3.rs–6152825. doi: 10.21203/rs.3.rs-6152825/v1
Li, Y., Viswaroopan, D., He, W., Li, J., Zuo, X., Xu, H., et al. (2025d). Enhancing relation extraction for COVID-19 vaccine shot-adverse event associations with large language models. Res. Sq, rs.3.rs–6201919. doi: 10.21203/rs.3.rs-6201919/v1
Li, Y., Viswaroopan, D., He, W., Li, J., Zuo, X., Xu, H., et al. (2025e). Improving entity recognition using ensembles of deep learning and fine-tuned large language models: A case study on adverse event extraction from VAERS and social media. J. BioMed. Inform 163, 104789. doi: 10.1016/j.jbi.2025.104789
Li, X., Zheng, Y., Hu, J., Zheng, J., Wang, Z., and He, Y. (2024b). VaxLLM: Leveraging Fine-tuned Large Language Model for automated annotation of Brucella Vaccines. bioRxiv 2024, 11.25.625209. doi: 10.1101/2024.11.25.625209
Moro, P. L., Ennulat, C., Brown, H., Woody, G., Zhang, B., Marquez, P., et al. (2024). Safety of simultaneous administration of bivalent mRNA COVID-19 and influenza vaccines in the vaccine adverse event reporting system (VAERS). Drug Saf. 47, 487–493. doi: 10.1007/s40264-024-01406-8
Murdoch, D. L., Goa, K., and Figgitt, D. P. (2003). Combined hepatitis A and B vaccines: a review of their immunogenicity and tolerability. Drugs 63, 2625–2649. doi: 10.2165/00003495-200363230-00008
Oliveira, M., Marquez, P., Ennulat, C., Blanc, P., Welsh, K., Nair, N., et al. (2025). Post-licensure safety surveillance of 20-valent pneumococcal conjugate vaccine (PCV20) among US adults in the vaccine adverse event reporting system (VAERS). Drug Saf. 48, 279–286. doi: 10.1007/s40264-024-01498-2
Pan, Y., Han, Y., Zhou, C., Zheng, J., Zhao, L., Ye, X., et al. (2024). Assessing acute kidney injury risk after COVID vaccination and infection in a large cohort study. NPJ Vaccines 9, 213. doi: 10.1038/s41541-024-00964-3
Shimabukuro, T. T., Nguyen, M., Martin, D., and DeStefano, F. (2015). Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine 33, 4398–4405. doi: 10.1016/j.vaccine.2015.07.035
Trépo, C., Chan, H. L., and Lok, A. (2014). Hepatitis B virus infection. Lancet (London England) 384 (9959), 2053–2063. doi: 10.1016/S0140-6736(14)60220-8
Vanderpump, M. P. J. (2011). The epidemiology of thyroid disease. Br. Med. Bull. 99, 39–51. doi: 10.1093/bmb/ldr030
Woo, E. J., Miller, N. B., Ball, R., and VAERS Working Group (2006). Adverse events after hepatitis A B combination vaccine. Vaccine 24, 2685–2691. doi: 10.1016/j.vaccine.2005.10.049
World Health Organization (2019). Hepatitis B vaccines: WHO position paper, July 2017 - Recommendations. Vaccine 37, 223–225. doi: 10.1016/j.vaccine.2017.07.046
Keywords: hepatitis AB vaccine, vaccine safety, surveillance, pharmacovigilance, VAERS
Citation: Zhou Y, Wang Y, Feng Y, Zhang H, Sun T and Xu J (2025) Real-world pharmacovigilance reports of hepatitis A inactivated and hepatitis B (recombinant) vaccine: insights from disproportionality analysis of the vaccine adverse event reporting system. Front. Cell. Infect. Microbiol. 15:1609409. doi: 10.3389/fcimb.2025.1609409
Received: 10 April 2025; Accepted: 20 May 2025;
Published: 10 June 2025.
Edited by:
Yongqun Oliver He, University of Michigan, United StatesReviewed by:
Mustafa Kursat Sahin, Ondokuz Mayıs University, TürkiyeYiming Li, Harvard Medical School, United States
Copyright © 2025 Zhou, Wang, Feng, Zhang, Sun and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junnan Xu, eGpuMDAyQDEyNi5jb20=; Tao Sun, amlhbm9uZ0AxMjYuY29t
 Yuhang Zhou
Yuhang Zhou Yue Wang1,2
Yue Wang1,2 Yun Feng
Yun Feng Tao Sun
Tao Sun Junnan Xu
Junnan Xu