- 1Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- 2Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- 3Center of Excellence in Microbial Diversity and Sustainable Utilization, Chiang Mai University, Chiang Mai, Thailand
- 4Office of Research Administration, Chiang Mai University, Chiang Mai, Thailand
- 5Multidisciplinary Research Institute, Chiang Mai University, Chiang Mai, Thailand
- 6Department of Microbiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
- 7Academy of Science, The Royal Society of Thailand, Bangkok, Thailand
The discovering new fungal strains, optimal production, and understanding the fundamental aspects of exopolysaccharides (EPs) are important to utilize them in an industrial, medical, and biotechnological perspective. In this study, the optimal conditions for EP production from seven basidiomycetous fungal strains were investigated. The results indicated that six fungal species, Schizophyllum commune, Ganoderma fornicatum, G. williamsianum, Earliella scabrosa, Favolus tenuiculus, and Pycnoporus sanguineus, produced the highest EP yield in potato dextrose broth. The highest yield of EPs produced by Lentinus sajor-caju was obtained in mushroom complete medium broth. It was found that a pH value between 6 and 8 in the liquid culture media promoted EP production. The highest EP yield was obtained for 10 to 14 days which depends on fungal strain. Interestingly, this present study revealed the first report of EP production from G. fornicatum, G. williamsianum, E. scabrosa, F. tenuiculus, and P. sanguineus, including the genera Earliella and Favolus. The obtained crude EPs showed water solubilization ability. The Fourier-transform infrared spectroscopy spectra exhibited typical carbohydrate patterns in all crude EPs. Monosaccharide composition analysis revealed that the crude EPs were primarily composed of glucose, followed by fructose, allose, and allulose, with variations depending on the fungal strain. Additionally, crude EPs demonstrated positive antioxidant potential. Finally, we determined the anti-Salmonella and immunomodulatory effects of crude EPs from S. commune, G. fornicatum, and L. sajor-caju due to their high EP yield. Pretreatment of mouse macrophages with these fungal EPs enhanced the phagocytic killing activity of Salmonella-infected macrophages. Upregulations of pro-inflammatory cytokine expression in macrophages were detected in the fungal EPs-treated groups. Our study reported the optimizing conditions for EP production from several strains of Basidiomycetous fungi and their potential as an alternative to antibiotics for multidrug-resistant Salmonella infection.
1 Introduction
Fungi are a diverse group of microorganisms that can produce a wide range of biologically active compounds, including extracellular polysaccharides or exopolysaccharides (EPs). EPs are complex carbohydrates that are secreted by fungi into their environment, forming a protective matrix around the cells (Valasques et al., 2020). Mushrooms, which mostly belong to the phylum Basidiomycota, are fungal fruiting bodies that are well known for their nutritional and medicinal properties (Miles and Chang, 2004). Notably, mushrooms contain diverse polysaccharides, including schizophyllan, ganoderan, grifolan, pleuran, and lentinan, which are recognized as valuable biomacromolecules (Mahapatra and Banerjee, 2013; Nguyen et al., 2024; Prathumpai et al., 2025). However, cultivating mushrooms as a source of polysaccharides requires a large volume of substrate and space, several months, and the quality of the final product can be relatively low depending on the substrate and growing conditions (Gong et al., 2020; Leong et al., 2021). As an alternative approach, liquid and submerged cultivation of mushroom mycelia has emerged as a promising method for producing polysaccharides (Mao et al., 2014). Some mushroom mycelia can produce and secrete EPs during liquid and submerged cultivation, offering an attractive means of obtaining high-value EPs. EPs provide several advantages over intracellular and cell wall polysaccharides, including massive production, easy isolation, and purification in a short time (Yan et al., 2010; Liu et al., 2024). Remarkably, the production of EPs from fungal mycelia is influenced by a variety of factors, including genetic factors (such as species and strains), physical or environmental conditions (such as pH, temperature, and aeration) during cultivation, and nutrient availability (including carbon and nitrogen sources, as well as trace minerals) in the culture medium. Several studies have focused on the optimization of culture conditions for the production of fungal EPs, with the aim of improving yields and reducing production costs (Kim et al., 2006, 2010; Meng et al., 2010; Patel et al., 2014; Tabibzadeh et al., 2022; Thai and Keawsompong, 2019). Understanding these factors is essential for producing and utilizing fungal EPs to their full potential in a variety of applications. This knowledge could lead to the development of new, sustainable, and cost-effective methods for EP production.
Exopolysaccharides (EPs) have various applications in industries, pharmaceuticals, medicines, and foods. The production of EPs from mushroom mycelia through liquid and submerged cultures has been widely studied, including genera Agaricus, Cordyceps, Ganoderma, Grifola, Lentinus, Pleurotus, Schizophyllum, Trametes, Tricholoma and others (Mahapatra and Banerjee, 2013). Recent research has unveiled the link between mushroom EPs and human health. A number of EPs from the culture filtrate of mushroom mycelia have been exhibited with a range of interesting biological and pharmacological activities, such as anti-inflammatory, antitumor, antimicrobial, immunomodulatory, and antioxidant activities (Kheni and Vyas, 2017; Muszyńska et al., 2018; Ghosh et al., 2021). However, research on EP production in Thailand remains limited. Therefore, this study aimed to screen and select basidiomycetous fungal strains for EP production. The optimal conditions (type of liquid culture medium, pH, and cultivation period) for EP production in each fungal strain were investigated. Subsequently, the antioxidant activities, major structural characteristics, and chemical compositions of the obtained crude EPs were analyzed. The immunomodulatory effects of the selected crude fungal EPs were evaluated by assessing the mRNA expression of pro-inflammatory and chemo-attractant cytokine genes in RAW264.7 macrophage cells, as well as the macrophage intracellular survival of Salmonella enterica serovar Typhimurium (STM). The information obtained from this study can be valuable for enhancing EP production and developing crude fungal EPs for medicinal and other applications.
2 Materials and methods
2.1 Source of fungal strains
A total of seven fungal strains (including Earliella scabrosa NK0461, Favolus tenuiculus NK0550, Ganoderma fornicatum NK0524, Ganoderma williamsianum NK0540, Lentinus sajor-caju NK0427, Pycnoporus sanguineus NK0189, and Schizophyllum commune CMU-01) in this study were obtained from the culture collection of the Research Center of Microbial Diversity and Sustainable Utilization, Faculty of Science, Chiang Mai University, Thailand. The fungal mycelia were cultured on potato dextrose agar (PDA; Conda, Madrid, Spain) and kept in incubator at 30°C for 7 days.
2.2 EP production
The ability of each fungal strain to produce EPs was investigated in liquid culture. The cultivation was carried out in a 125-mL Erlenmeyer flask containing 40 mL of mushroom complete medium broth (MCMB; containing glucose 20 g/L, peptone 2 g/L, yeast extract 2 g/L, K2HPO4–1 g/L, MgSO4•7H2O 0.5 g/L and KH2PO4 0.46 g/L) with initial pH value at 6.0 (Jeong et al., 2009). Three mycelial plugs (5 mm in diameter) of each fungal strain obtained from a colony growing on PDA at 30°C for 7 days were inoculated into MCMB. The inoculated flasks were incubated at room temperature (28 ± 2°C) on a reciprocal shaker (NR-10 Bioshaker®, Japan) at 110 rpm. After 7 days, the fungal mycelia were filtered through a 25-µm membrane filter (Miracloth, Merck Millipore, USA), and the culture supernatant was collected for crude EP extraction. The fungal strains positive for EP production were selected and used for further experiments.
2.3 Precipitation of EPs
The fungal EPs were precipitated using 75% ethanol and the fungal culture supernatant in ratios of 2:1 (v/v), sealed with parafilm, and kept at 4°C according to the method described by Long et al. (2021) and Yurnaliza et al. (2021). After 24 h, EPs were collected by centrifuged at 10,000×g for 15 minutes and washed with 95% ethanol three times. Then, the obtained crude EPs were dissolved in deionized (DI) water and freeze-dried by a lyophilizer at -50°C (FreeZone 2.5 Liter Benchtop Freeze Dryer, Labconco) until completely dry. The crude fungal EPs were weighed and stored at -20°C until further experiments.
2.4 Optimization of culture conditions for enhanced EP production
The EP production of each fungal strain was investigated in 250-mL Erlenmeyer flasks containing 80 mL of the tested liquid culture under various conditions (type of liquid culture media, initial pH values, and incubation time). Five mycelial plugs of each fungal strain were inoculated into each flask. The inoculated flasks were incubated at 28 ± 2°C on a reciprocal shaker at 110 rpm. The EPs were precipitated and collected using the method described above.
2.4.1 Type of liquid culture media
Three different types of liquid culture media including MCMB, potato dextrose broth (PDB; Conda, Madrid, Spain) and yeast-malt extract broth (YMB; glucose 10 g/L, peptone 5 g/L, malt extract 3 g/L and yeast extract 3 g/L) with an initial pH level of 6.0 were used in this experiment. Mycelial plugs of each fungal strain were inoculated into each flask and cultivated on a shaker for two weeks. The EPs were precipitated and collected. The culture liquid medium of each fungal strain that provided the maximum EP yield was selected and used for further experiments.
2.4.2 Initial pH values of optimal liquid media
Pure cultures of each fungal strain were inoculated into the selected liquid culture medium with six different initial pH values (4.0, 5.0, 6.0, 7.0, 8.0, and 9.0). The cultivation was performed for two weeks, and the EPs were precipitated and collected. The initial pH value of the liquid medium that resulted in the highest EP yield for each fungal isolate was selected for further experiments.
2.4.3 Incubation time
The influence of the incubation time on EP production for each fungal strain was evaluated in the optimal liquid culture medium with an optimal initial pH value. After inoculation with pure culture, flasks were placed on a reciprocal shaker at 28 ± 2°C. The EP production yield was examined after 6, 8, 10, 12, 14, and 16 days of incubation.
2.5 Fourier transform-infrared spectroscopy (FTIR) analysis
FT-IR analysis was performed at Science and Technology Service Center, Faculty of Science, Chiang Mai University, Thailand. The FT-IR spectrum was used to elucidate the structural analysis of the crude EPs as described by Tabibzadeh et al. (2022) with some modification. The samples were prepared by mixing 2 mg of grinded EPs with 200 mg of spectroscopic-grade potassium bromide (KBr) powder, which was pulverized and compressed into 1 mm pellets for FT-IR determination. The absorption was measured and analyzed using a FT-IR spectrophotometer (Thermo Fisher Scientific, USA) in the wavenumber region of 400 to 4,000 cm-1 for detecting the presence of major structural and functional groups of EPs along with their substitutes. Schizophyllan was used as the standard for comparison.
2.6 Chemical characterization for sugar compositions of seven fungal EPs
Monosaccharide compositions of crude fungal EPs were determined by high performance liquid chromatography (HPLC) according to the method of Du et al. (2017) and Long et al. (2021). The crude EPs were prepared by dissolving 1 g of them in 10 mL of distilled water. The solution was then digested using 1M HCl, with the pH adjusted to 2, followed by heating to 97°C and maintaining it for 5 min. Subsequently, the pH was adjusted to 7 using 1M NaOH, and the mixture was centrifuged at 8,000 rpm at 25°C for 10 min. The clear supernatant was subjected to analysis for sugar compositions using a Shimadzu-brand high-performance liquid chromatography machine with an isocratic technique, utilizing a Shodex HILICpak VG-50 4E column and a refractive index (RI) detector. The column temperature was maintained at 50°C. For the analysis, the mobile phase comprised a mixture of acetonitrile, methanol, and water in a ratio of 85:10:5 (solvent A), HPLC-grade water (solvent B), and 100% acetonitrile (solvent C), with a flow rate set at 0.6 mL/min. The injection volume was 10 μL. The presence of monosaccharides was identified by comparing the retention time of allose, allulose, fructose, mannose, glucose, rhamnose, and xylose standards. The amount of each monosaccharide was quantified with the calibration curve constructed with each standard.
2.7 Antioxidant assays
Evaluation of the antioxidant activities of EPs was determined in a 96-well microplate by two methods, including 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) diammonium salt (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assays. For all assays, the EP samples were dissolved in deionized water. Gallic acid was used as a reference compound (positive control) both ABTS and DPPH assay. All samples were evaluated in triplicate. The ABTS and DPPH radical scavenging activities were expressed as the IC50 value (half maximal inhibitory concentration) in mg/mL of the EP sample required to inhibit 50% of ABTS or DPPH radical scavenging. The IC50 values were obtained by interpolation from linear regression analysis. A lower IC50 value corresponds to higher antioxidant activity.
2.7.1 ABTS radical scavenging activity
The ABTS radical scavenging activities of EPs were performed spectrophotometrically by decolorization assay according to the methods of Prajapati et al. (2023) and Wangsawat et al. (2021) with slight modifications. An ABTS radical cation solution was prepared by reacting equal volumes (500 µl) of 7 mM ABTS solution with 2.45 mM potassium persulfate (K2S2O8) solution. Then, the mixture containing free radicals was kept in the dark for 16 hours at room temperature to yield a dark-colored solution before use. After that, the stock solution was diluted with deionized water to give an absorbance of 0.70 ± 0.02 at 734 nm. The ABTS working solution (195 μL) was added to 5 μL of the EP solution with different concentrations. A mixture of deionized water and ABTS solution was used as negative control. After incubation for 10 minutes in the dark, the final absorbance of the reaction was measured at 734 nm with a microplate reader spectrophotometer (Spectra MR, Dynex Technologies, Virginia). The inhibition of the ABTS radical was calculated as IC50 and expressed in mg/mL.
2.7.2 DPPH radical scavenging activity
The DPPH radical scavenging activities of EPs were carried out by the methods described by Debnath et al. (2021) and Xu et al. (2015) with minor modifications. In brief, 50 µL of each EP sample at different EP concentrations in deionized water was mixed with DPPH radical solution in 95% ethanol (0.1 mM, 150 µL). The mixture was shaken vigorously are allowed to stand for 30 minutes in the dark. A mixture of deionized water and DPPH solution was used as negative control. Then, the absorbance of each mixture solution was spectrometrically measured at 517 nm using a microplate reader spectrophotometer (Spectra MR, Dynex Technologies, Virginia). The DPPH radical scavenging ability of EPs was calculated as the IC50 value and expressed in mg/mL.
2.8 Effect of crude fungal EPs on the cell viability of RAW264.7 cells
Murine macrophage cell lines (RAW264.7 cells) purchased from the American Type Culture Collection (ATCC® TIB-71™) were used in this study. RAW264.7 cells were cultured in Dulbecco’s modified Eagle medium (DMEM; HyClone, United States) high glucose containing 4mM L-glutamine, 4.5 g/L glucose, and sodium pyruvate supplemented with 10% fetal bovine serum (FBS; Hyclone, United States) and 1% penicillin/streptomycin (Gibco, United States). The cells were changed to antibiotic‐free media before using in an assay. These cells were maintained in an incubator with 5% CO2-humidified air at 37°C. The cell culture media were changed every 2–3 days.
The crude EPs from S. commune, G. fornicatum, and L. sajor-caju were selected for this study based on their high production yields. To assess the cytotoxicity of fungal EPs on RAW264.7 cells, cell viability was determined using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) assay following He et al. (2019) with slight modifications. Initially, 100 μL of RAW264.7 cells were seeded into a 96-well plate at a density of 1.0×104 cells/well and incubated at 37°C with 5% CO2 for 24 hours to achieve stable growth. The cells were then cultured with antibiotic-free media for an additional 24 hours. Subsequently, the cells were treated with various final concentrations of crude fungal EPs in PBS solution at concentrations of 25, 50, 100, 200, 400, 500, and 1000 µg/mL. PBS was used as the solvent for EP dilution and for the EPs-untreated control group. After 24 hours of incubation, the treatments were discarded, and 300 µL of MTT solution (1 mg/mL in PBS) was added to each well. The plates were then incubated for an additional 3 hours at 37°C. Formazan crystals formed in viable cells were dissolved by adding 150 µL of DMSO/well, and the supernatant was gently shaken on a plate shaker for 10 minutes. Absorbance values (Abs) were determined with a reference wavelength of 490 nm using a microplate reader (BioTek Synergy H4 Hybrid Multi-Mode Microplate Reader, Marshall Scientific). Increased production of formazan directly indicates the presence of a higher number of metabolically active cells. The percentage of cell viability was calculated using the formula:
Where Abs control represents the absorbance of the control reaction (without EP treatment), and Abs sample represents the absorbance of the EP treatment. Cell viability was expressed as a percentage of the control, represented by EPs-untreated cells, which were set at 100%. Due to the known variability of the MTT assay, experiments were conducted with eight replicates to ensure statistical robustness.
2.9 Effect of fungal EPs on the macrophage intracellular survival of Salmonella
2.9.1 Bacterial strain
Salmonella enterica serovar Typhimurium strain IR715 (ATCC 14028 derivative with nalidixic acid resistance; STM) was used. STM was grown at 37°C aerobically with shaking in Luria-Bertani (LB) broth (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl; Difco, United States) for 16–18 hours. STM was sub-cultured for 3 hours in LB broth before treating RAW264.7 cells. Nalidixic acid (0.05 mg/mL; AppliChem, Germany) was used as a selective antibiotic for STM in liquid and solid media.
2.9.2 Invasion assay
The effect of fungal EPs on murine macrophages to eliminate invasive STM was adapted and determined using the invasion assay previously described by Birhanu et al. (2018) with some modifications. Briefly, 500 µL of RAW264.7 cells suspended in a complete medium were seeded into 24-well plates at a density of 1.0×105 cells/well and incubated at 37°C with 5% CO2 for 24 hours. The complete growth medium was then replaced with DMEM without FBS or antibiotics for an additional 24 hours to synchronize the cells. Subsequently, RAW 264.7 cells were pre-treated with different final concentrations of the three fungal EPs in PBS solution at 25, 50, 100, and 200 µg/mL for 24 hours, with PBS being used for the EPs-untreated control. Simultaneously, overnight cultures of STM were sub-cultured in LB with 0.3 M NaCl and incubated at 220 rpm, 37°C with 5% CO2 for 3 hours. Next, the cell lines were gently treated and infected by STM adding at the multiplicity of infection (MOI) of 5 at 37°C for 1 hour. The cell culture medium in each well was removed, and the bacterial cells were subsequently rinsed twice with Dulbecco’s phosphate-buffered saline (DPBS; Hyclone, Singapore) and treated with 500 μL of 100 μg/mL gentamicin sulfate (AppliChem, Germany) in DMEM, and incubated at 37°C for a further 90 minutes. The supernatant in each well was then removed, and the RAW264.7 cells were washed twice with DPBS before adding 500 µL of 1% Triton-X-100 (Thermo Fisher Scientific, United States) in PBS to lyse the cells. Subsequently, 500 µL of PBS was repeatedly used to collect the remaining bacterial cells in each well. The cell suspensions were ten-fold serially diluted and enumerated on LB agar supplemented with nalidixic acid to determine the viable numbers of macrophage intracellular STM. The experiment was performed with six replicates per group due to limitations in the available quantity of the EP sample.
2.9.3 Pro-inflammatory and chemo-attractant cytokine genes expression of EPs-treated and STM-infected RAW264.7 cells
The expression of pro-inflammatory and chemo-attractant cytokine genes of EPs and STM-infected RAW264.7 cells was performed by reverse transcription and qualitative polymerase chain reaction (RT-qPCR), as previously described by Winter et al., 2009, and Buddhasiri et al., 2021, with some modifications. In brief, 2 mL of RAW264.7 cells suspended in complete medium were seeded into 6-well plates at a density of 1.2×106 cells/well and incubated at 37°C with 5% CO2 for 24 hours. The complete medium was replaced 24 hours prior to the assay with DMEM without FBS and antibiotics. Subsequently, RAW264.7 cells were pre-treated with EP solution in PBS at two final concentrations of 50 and 200 µL/mL for 24 hours. PBS and STM suspended in PBS were used for the EPs-untreated and positive control groups, respectively. Following pretreatment, RAW264.7 cells were treated with STM at an MOI of 5 at 37°C for 1 hour. After two rounds of washing the cells with DPBS, they were lysed and collected by adding 1 mL of TRIzol® reagent (Invitrogen Life Technologies, USA) into each well, then stored at -20°C prior to subsequent use.
The total RNA of RAW264.7 cells was extracted using TRIzol® reagent following the manufacturer’s protocol recommendations. DNase treatment was carried out using a DNA clean-up and inactivation kit (Ambion, Life Technologies). RNA samples were quantified using a NanoDrop Spectrophotometer (Thermo Fisher Scientific, United States) and stored at -80°C until use. To ensure good quality and integrity, extracted RNA was evaluated by measuring the absorbance ratio of 260/280 (1.6-1.9) and running on a 1.5% agarose gel stained with SYBR Safe DNA Gel Stain (Thermo Fisher Scientific, USA). Subsequently, complementary DNA (cDNA) was synthesized by reverse transcription polymerase chain reaction (RT-PCR) using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). RT-PCR was performed by mixing extracted RNA (3 µg), Random Hexamer Primer, 10 mM dNTP mix, 5X-Reaction Buffer, RiboLock RNase Inhibitor, and RevertAid M-MuLV Reverse transcriptase (200µ/µL) in a total volume of 20 µL under the following conditions: incubation at 25°C for 5 minutes, 42°C for 60 minutes, with a termination step of 5 minutes at 70°C using a thermocycler (Labcycler, SensoQuest).
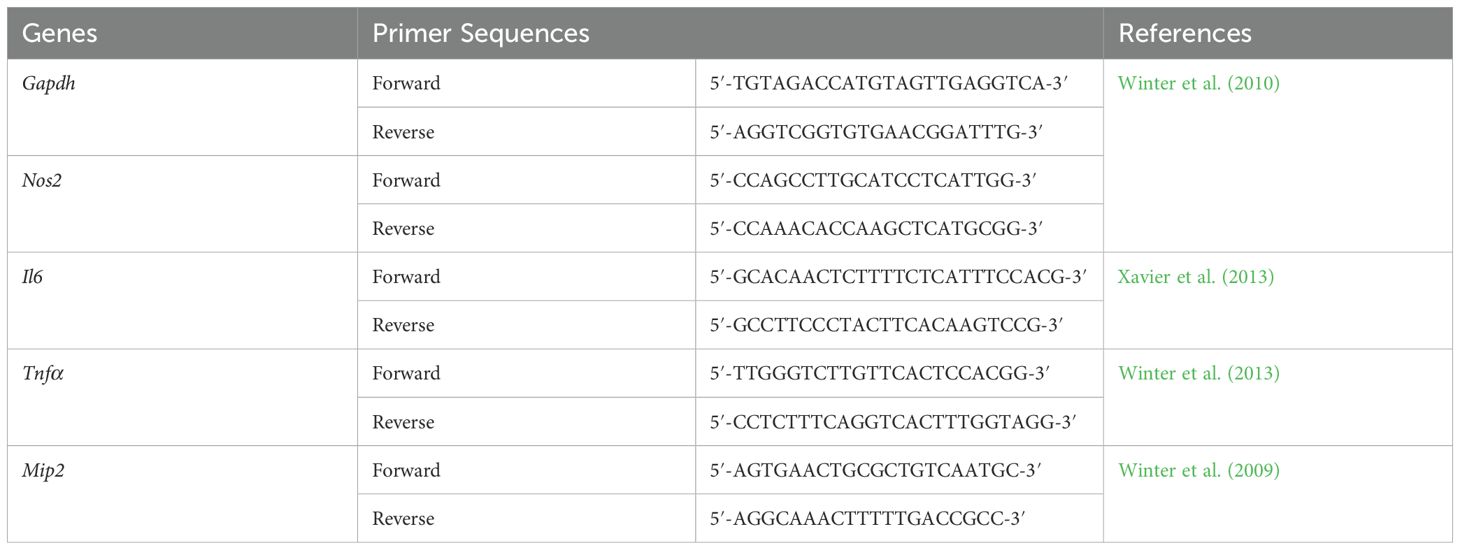
The quantitative PCR (qPCR) was performed using a reaction mixture consisting of 2X-SensiFAST™ SYBR® Lo-ROX mix (Bioline), 10 µM of forward and reverse primers, 2 µL of 50 ng/µL cDNA template, and nuclease-free water to a final volume of 20 µL. The amplification program involved an initial polymerase activation at 95°C for 2 minutes, followed by 40 cycles of denaturation at 95°C for 5 seconds and an annealing step at 60°C for 30 seconds, as developed by Boonloh et al. (2015), using the ViiA7 Real‐Time PCR system (Applied Biosystems, United States). The threshold cycle (CT) values were exported to Microsoft Excel for further analysis. Melting curve analyses were utilized to confirm the positive sample specificity of each amplified PCR product. Positive and negative controls were included with each PCR run. The relative fold change of mRNA expressions was calculated using the comparative CT (2−ΔΔCT) method (Schmittgen and Livak, 2008). The expression levels of pro-inflammatory cytokine genes, including Il6, Mip2, Nos2, and Tnfα, encoding interleukin (IL)‐6, macrophage inflammatory protein (MIP)‐2 or chemokine (C-X-C motif) ligand 2 (CXCL2), inducible nitric oxide synthase (iNOS), and tumor necrosis factor (TNF)‐α, were normalized with the stably expressed housekeeping gene (Gapdh). The primer pairs used for the qPCR gene expression in this study are presented in Table 1.
2.10 Statistical analysis
The results are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was conducted to compare the mean values using IBM SPSS Statistics for Windows, Version 26 (SPSS Inc., USA). Significant differences in EP production and antioxidant activity were determined by Duncan’s multiple range test, with p-values less than 0.05 (p<0.05) considered statistically significant. In addition, the student’s t-test was used to assess the statistical significance of differences between treatment and control groups regarding the effect of EPs on the cell viability of RAW264.7 cells, invasion assays, and the expression of pro-inflammatory and chemo-attractant cytokine genes.
3 Results
3.1 Production of fungal EPs
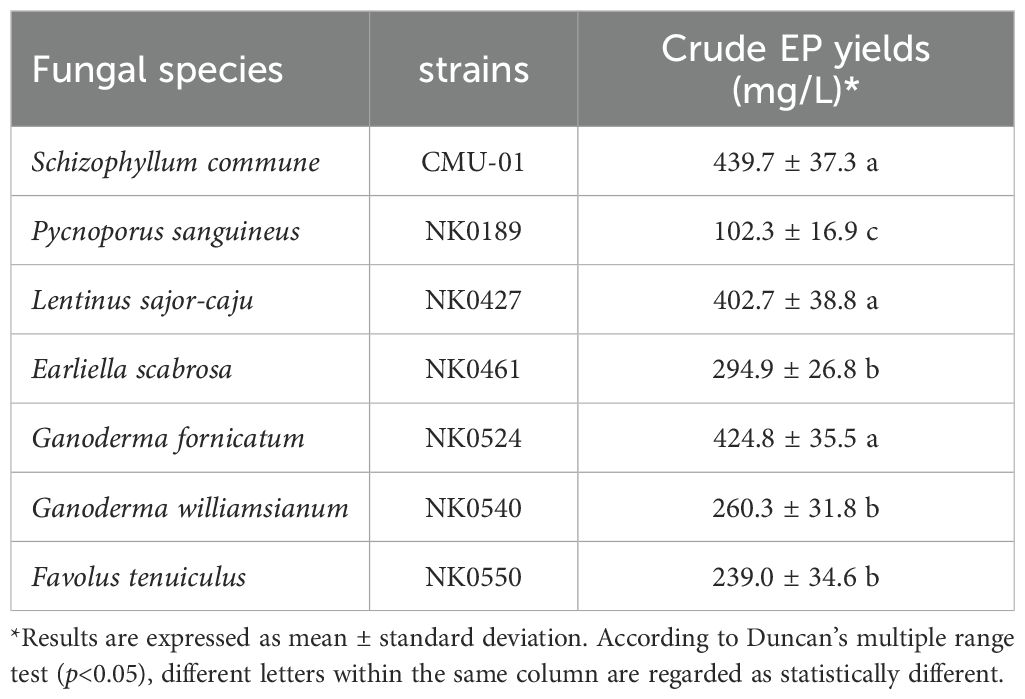
The production of EPs was investigated in MCMB with an initial pH of 6. The results indicated that the seven fungal strains could produce EPs, but at different yields (Table 2). Among the strains, the highest yield of EPs was 439.7 ± 37.3 mg/L from S. commune, which did not show significant differences compared to G. fornicatum (424.8 ± 35.5 mg/L) and L. sajor-caju (402.7 ± 38 mg/L). On the other hand, P. sanguineus exhibited the lowest EP yield at 102.3 ± 16.9 mg/L. The obtained all crude fungal EPs showed water solubilization ability and non-solubilization in ethanol and methanol.
3.2 Optimization of culture conditions for enhanced EP production
3.2.1 Type of liquid culture media
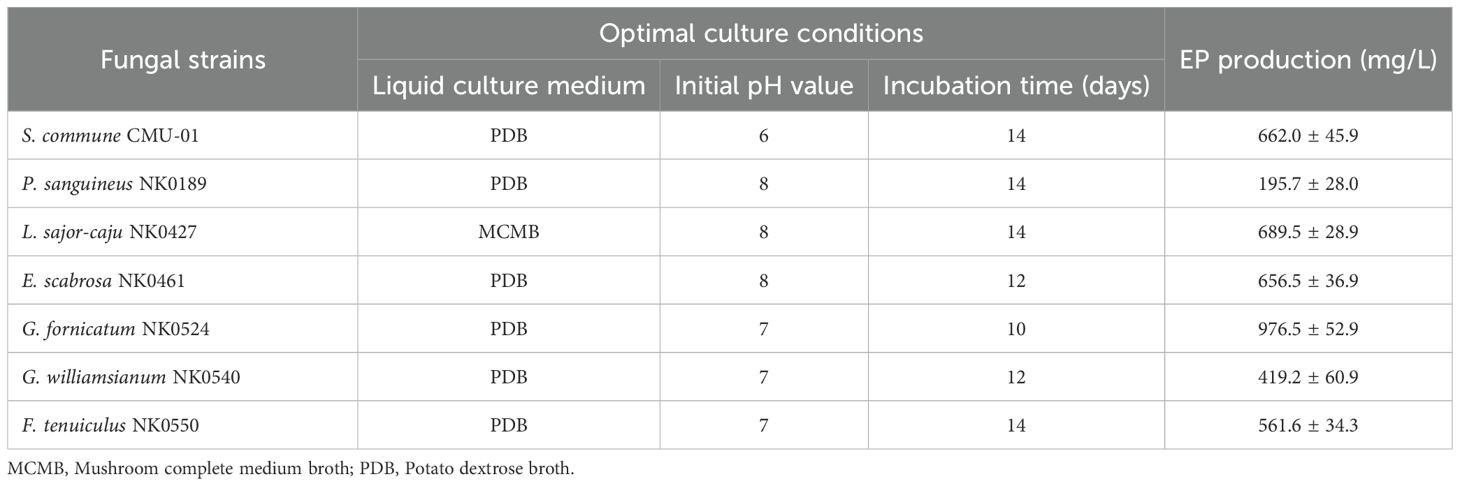
Three different types of liquid culture media (MCMB, PDB, and YMB) were used for the production of EPs of the seven fungal strains. The results of the study indicated that six fungi, S. commune, G. fornicatum, E. scabrosa, F. tenuiculus, G. williamsianum, and P. sanguineus, exhibited the highest yield of EPs when cultivated in PDB with respective yields of 533.2 ± 37.5, 496.6 ± 23.9, 439.6 ± 27.0, 427.8 ± 29.3, 341.6 ± 33.0, and 144.0 ± 20.3 mg/L (Figure 1A). Schizophyllum commune could produce the highest yield of EPs compared to the other fungi. However, only L. sajor-caju demonstrated the highest yield of EPs in MCMB, with a yield of 413.7 ± 26.7 mg/L.
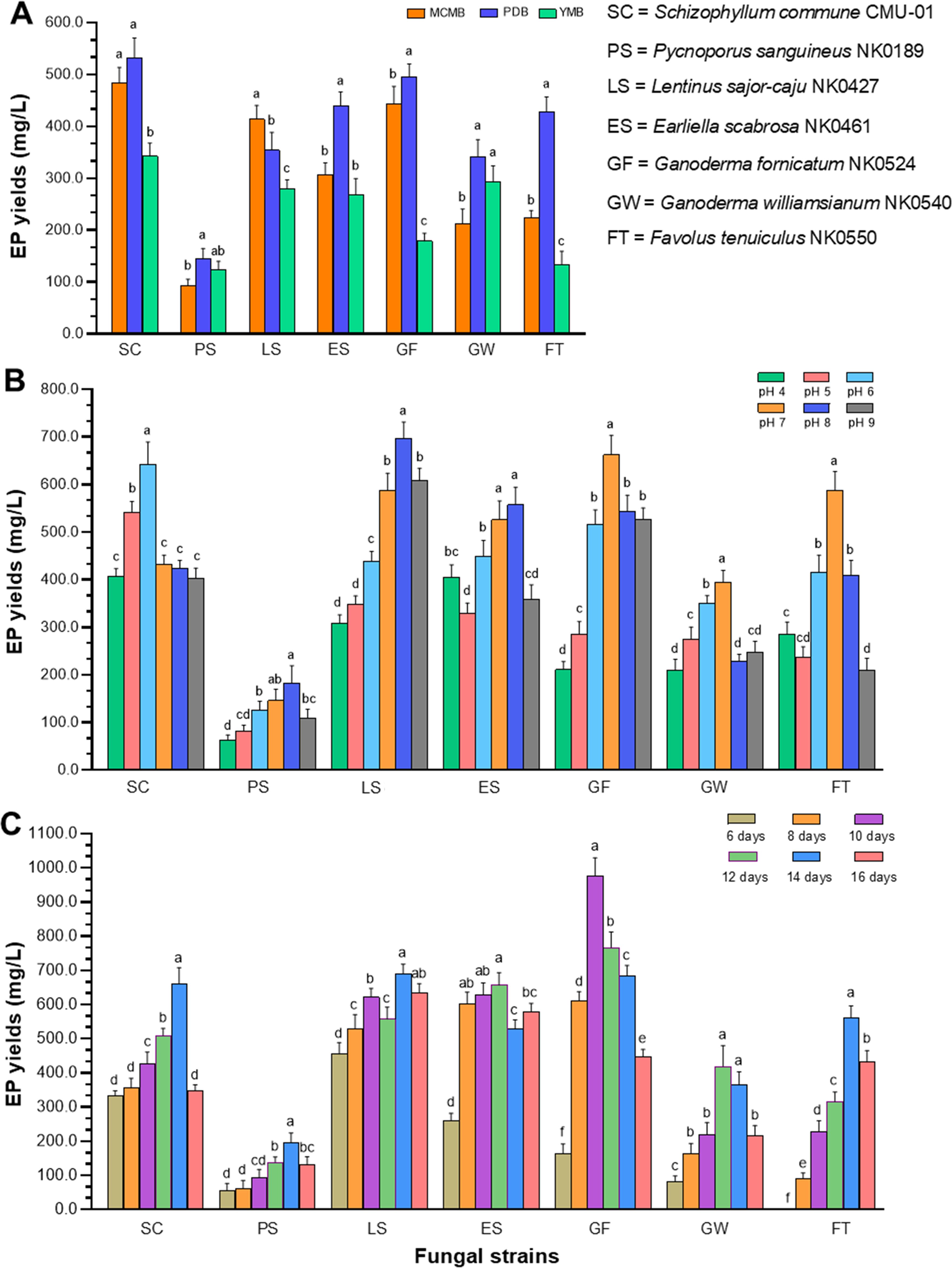
Figure 1. Optimization of culture conditions for enhanced EP production of seven fungal strains. (A) EP yields at different liquid culture media. (B) EP yields at different initial pH values of the optimal liquid media. (C) EP yields at different incubation times. Data are presented as means, and the error bars in each graph indicate the standard deviation (+ SD). Different letters within each graph indicate statistically significant differences by Duncan’s multiple range test (p<0.05) in each fungal strain.
3.2.2 Initial pH values of optimal liquid media
The optimal initial pH values of the culture liquid medium for each fungus, ranging from 4.0 to 9.0, were determined for EP production. The results indicated variations in EP yield among the different pH levels and fungi (Figure 1B). Schizophyllum commune produced the highest EP yield (643.5 ± 46.1 mg/L) at pH 6 in PDB. Three fungi, including G. fornicatum, F. tenuiculus and G. williamsianum, produced the highest yield of EPs at pH 7 in PDB. Meanwhile, E. scabrosa and P. sanguineus demonstrated their highest yields of EPs at pH 8.0 in PDB. It was found that the highest EP yield produced from L. sajor-caju was observed at pH 8.0 in MCMB.
3.2.3 Incubation time
The influence of incubation time on EP production was completely examined under controlled conditions, including the suitable culture liquid medium and initial pH value. Notably, G. fornicatum exhibited the highest EP yield after 10 days of incubation time, reaching an impressive quantity of 976.5 ± 52.9 mg/L. On the other hand, E. scabrosa and G. williamsianum, displayed their highest EP yield after 12 days of incubation time, with values of 656.5 ± 36.9 and 419.2 ± 60.9, respectively. Additionally, four fungal strains, namely L. sajor-caju, S. commune, F. tenuiculus, and P. sanguineus, demonstrated their highest EP yield after 14 days of incubation (Figure 1C). The results of the optimal culture conditions and maximum EP yield for all fungal strains used in this study are presented in Table 3.
3.3 Structural characterization of crude fungal EPs by FT-IR spectroscopy
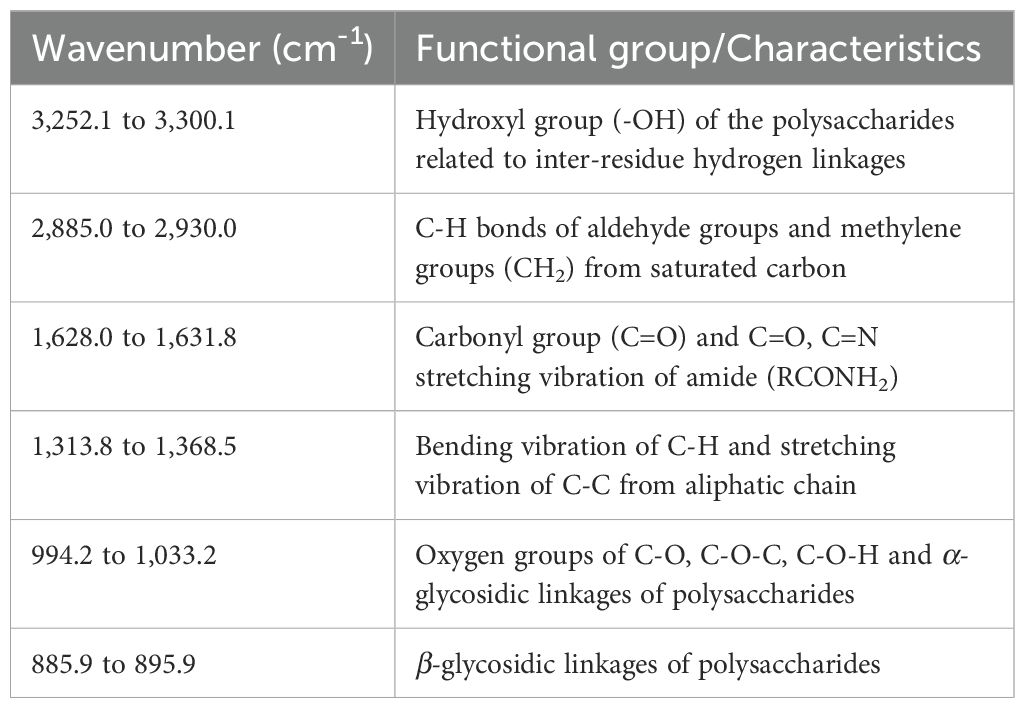
FT-IR analysis was conducted to identify the chemical functional groups present in the crude EPs of the seven fungi. The FT-IR spectra of the crude fungal EPs and the schizophyllan standard are shown in Figure 2 and Table 4. The results indicated that the FT-IR spectra of all crude fungal EPs exhibited typical carbohydrate patterns similar to those of schizophyllan. The broad peak in the range of 3,252.1 to 3,300.1 cm-1 was attributed to the hydroxyl (-OH) stretching vibration of the EPs and the main functional group of polysaccharides (Radzki et al., 2016). The peak in the region of 2,885.0 to 2,930.0 cm-1 corresponded to the stretching vibration of C-H of the aldehyde and methylene group (CH2) from saturated carbon (Tabibzadeh et al., 2022). An absorption peak recorded in the range of 1,628.0 to 1,631.8 cm-1 was assigned to carbonyl (C=O) stretching and C=O, C=N stretching vibration of amide (RCONH2) (Lima et al., 2016; Sountharavallee et al., 2023). The bands between 1,313.8 and 1,368.5 cm-1 indicated bending vibration of C-H and C-C stretching of aliphatic chains (Zhang et al., 2017). Additionally, the strong absorbance in the range of 994.2 to 1,033.2 cm-1 in the spectra of all crude fungal EPs represented the characteristic absorption peak of polysaccharides. These strong absorption bands were attributed to the stretching vibration of C-O, C-O-C, and C-O-H linkages corresponding to the α-glycosidic linkages (Ren et al., 2015; Li et al., 2017). Furthermore, the distinct characteristic absorption at approximately 885.9 to 895.9 cm-1 indicated the β-glycosidic bonds from sugar components (Du et al., 2017; Jaroszuk-Ściseł et al., 2020).
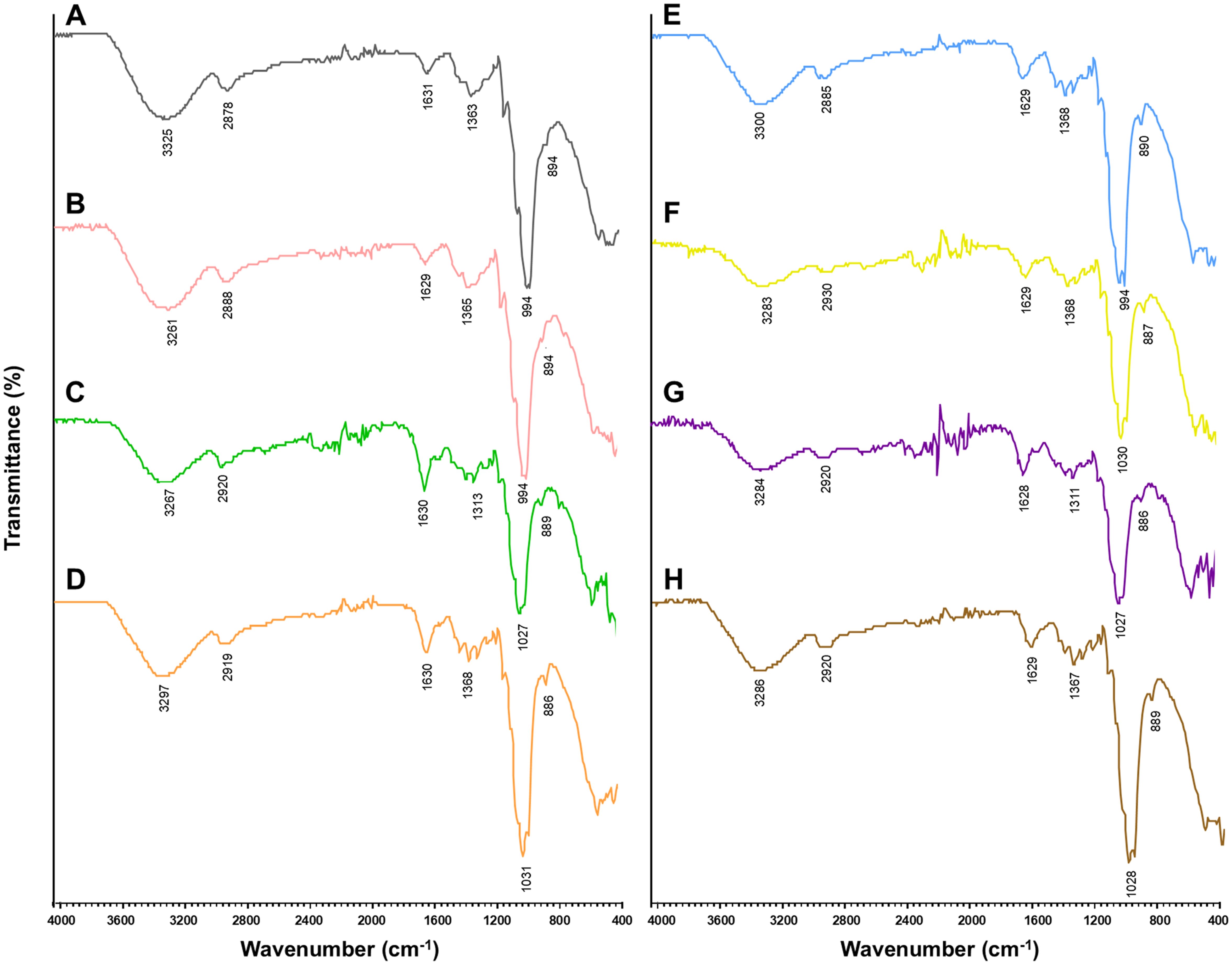
Figure 2. Structural characteristics by FT-IR spectroscopy of standard EPs (A) schizophyllan and crude fungal EPs; (B) S. commune CMU-01, (C) P. sanguineus NK0189, (D) L. sajor-caju NK0427, (E) E. scabrosa NK0461, (F) G. fornicatum NK0524, (G) G. williamsianum NK0540, and (H) F. tenuiculus NK0550.
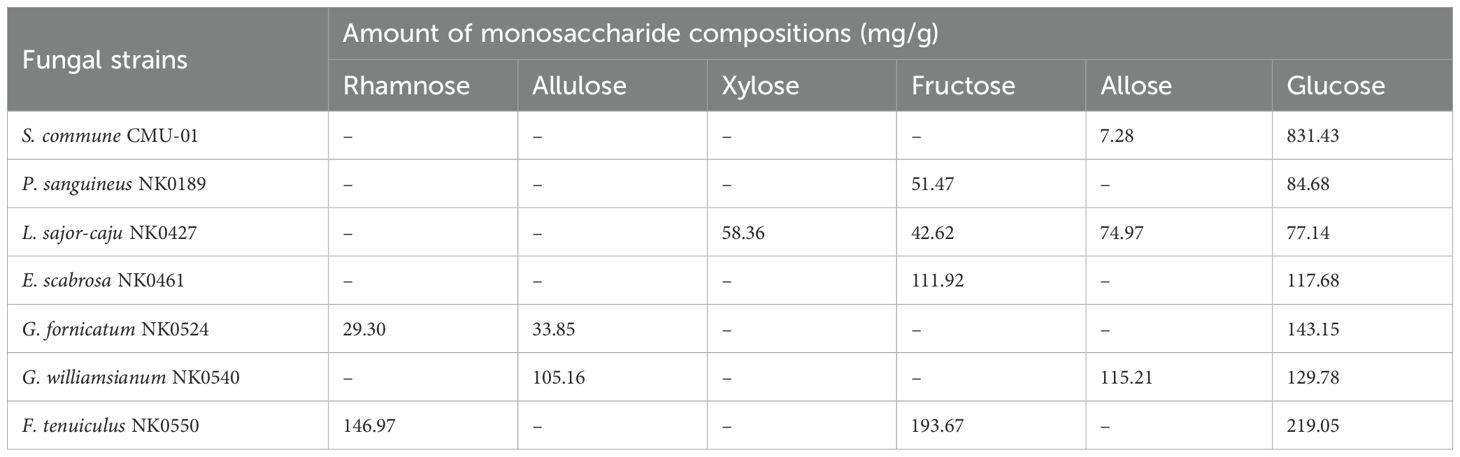
3.4 Monosaccharide compositions
The monosaccharide compositions of the crude fungal EPs were identified through the HPLC. The relation time of allose, allulose, fructose, mannose, glucose, rhamnose, and xylose were 9.8, 11.4, 12.4, 13.6, 14.7, 17.2, and 33.2 min. Our findings revealed that these seven fungal EPs are heteropolysaccharides. The monosaccharide compositions in each fungal EPs differed between fungal species (Table 5). The result indicated that glucose was found to be the main monosaccharide composition of all fungal EPs. In addition, fructose was the minor composition of the fungal EPs obtained from P. sanguineus, E. scabrosa, and F. tenuiculus. Whereas, allose was found as a minor component in the EPs obtained from L. sajor-caju and G. williamsianum. Allulose was found as a minor component in the EPs obtained from G. fornicatum. Xylose was only found in EPs obtained from L. sajor-caju. Rhamnose was found in EPs obtained from F. tenuiculus and G. fornicatum. EPs obtained from G. fornicatum and G. williamsianum were comprised of allulose. However, mannose was not found in all fungal EPs.
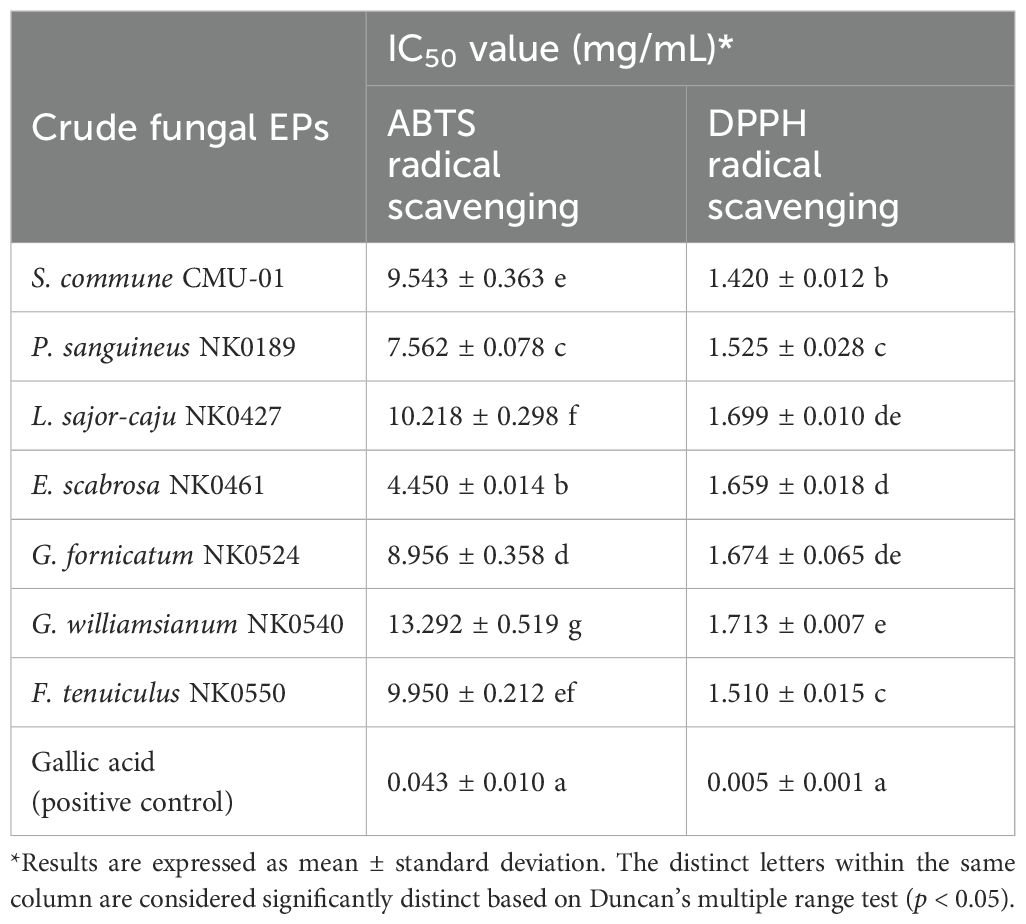
3.5 Antioxidant activities of crude EPs
The antioxidant activities of crude EPs obtained from seven fungal strains were evaluated using two assays, including ABTS and DPPH radical scavenging activities. The result demonstrated that EPs from all fungi have positive antioxidant potential activity. In this study, the IC50 value was used to express the radical scavenging abilities of DPPH and ABTS as presented in Table 6. The lower IC50 value indicated the higher antioxidant activity. In terms of the ABTS assay, the crude EPs of E. scabrosa exhibited the highest ABTS free radical scavenging activity, with the IC50 value of 4.450 ± 0.014 mg/mL. The crude EPs from P. sanguineus, G. fornicatum, S. commune, F. tenuiculus, and L. sajor-caju exhibited IC50 values of 7.562 ± 0.078, 8.956 ± 0.358, 9.543 ± 0.363, 9.950 ± 0.212, and 10.218 ± 0.298 mg/mL, respectively. On the other hand, the crude EPs obtained from G. williamsianum displayed the lowest ABTS free radical scavenging activity, with an IC50 value of 13.292 ± 0.519 mg/mL.
Regarding the DPPH assay, the crude EPs produced from S. commune exhibited the highest DPPH free radical scavenging activity, with an IC50 value of 1.420 ± 0.012 mg/mL. In addition, the crude EPs from F. tenuiculus, P. sanguineus, E. scabrosa, G. fornicatum, and L. sajor-caju exhibited IC50 values of 1.510 ± 0.015, 1.525 ± 0.028, 1.659 ± 0.018, 1.674 ± 0.065, and 1.699 ± 0.010 mg/mL, respectively. Conversely, the crude EPs obtained from G. williamsianum displayed the lowest DPPH free radical scavenging activity, with an IC50 value of 1.713 ± 0.007 mg/mL. However, it was found that gallic acid exhibited greater ABTS and DPPH radical scavenging activities than the crude fungal EPs, with exceptional IC50 values of 0.043 ± 0.010 and 0.005 ± 0.001 mg/mL, respectively.
3.6 Effect of crude fungal EPs on the cell viability of RAW264.7 cells
Next, we investigated the role of fungal EPs on the macrophage, the important innate immune cell in mammals; the cytotoxicity of fungal EPs on murine macrophage (RAW264.7) was performed by the MTT assay. The three selected crude fungal EPs from S. commune (SC-EPs), G. fornicatum (GF-EPs), and L. sajor-caju (LS-EPs) at various concentrations (25–1000 µg/mL) were incubated for 24 h. Three selected fungal EPs revealed non-cytotoxicity to RAW264.7 cells at concentrations in the range of 25-1,000 μg/mL as compared with the EPs-untreated control group (Figure 3). Interestingly, RAW264.7 cells treated with SC-EPs at concentrations of 500 and 1,000 µg/mL showed a significant enhancement of cell viability (p < 0.01), as shown in Figure 3A. Similarly, LS-EPs-treated RAW264.7 cells showed increasing effects on cell viability with concentrations of the polysaccharides at 500 and 1,000 µg/mL, in a dose-dependent manner, with p-values of < 0.05 and < 0.01, respectively (Figure 3C). However, there were no significant differences in the RAW264.7 cells treated with GF-EPs compared to the EPs-untreated control group (Figure 3B).
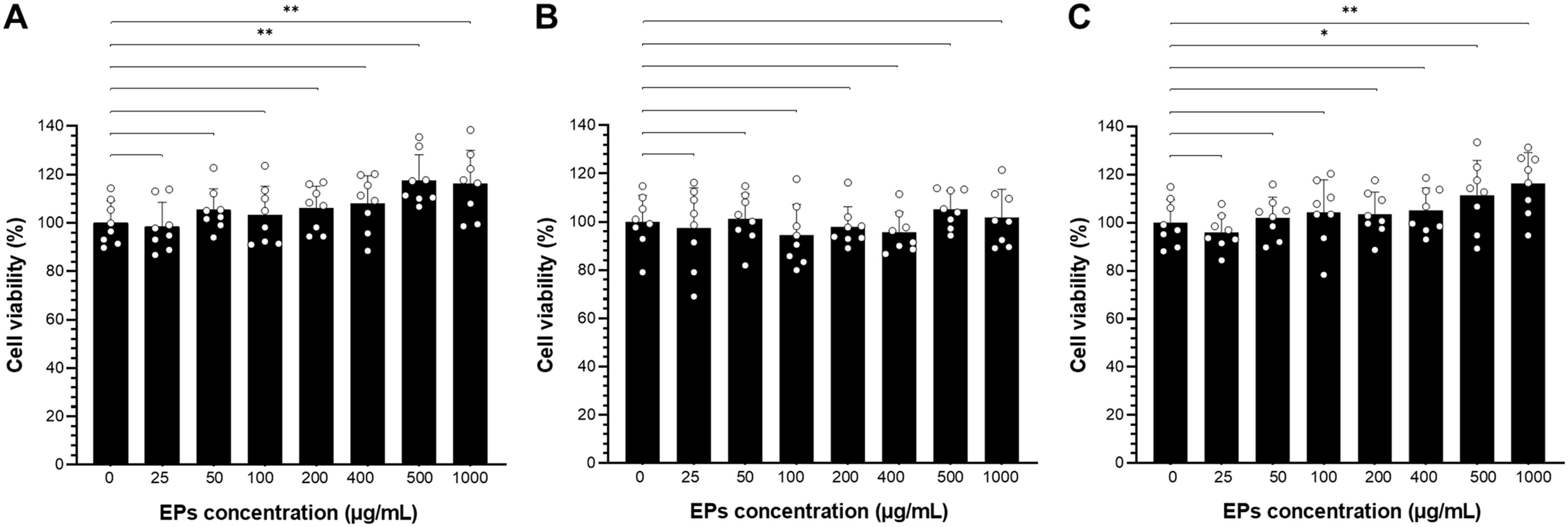
Figure 3. The cell viability of fungal EPs-treated RAW264.7 cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Murine macrophage cell lines were treated with the fungal EPs of S. commune CMU-01; SC-EPs (A), G. fornicatum NK0524; GF-EPs (B), and L. sajor-caju NK0427; LS-EPs (C) at different final concentrations (25, 50, 100, 200, 400, 500, and 1,000 μg/mL) for 24 hours exposure. Data are presented as means, and the error bars in each graph indicate the standard deviation (+ SD). One scatter dot represents each replicate. The * and ** represent significant differences with p-values < 0.05 and 0.01, respectively, compared to the EPs-untreated control group (0 μg/mL), as determined by Student’s t-test.
3.7 Pretreatment with fungal EPs increased macrophage-killing activity against Salmonella enterica serovar Typhimurium infection in a dose-dependent manner
3.7.1 Invasion assay
We investigated the effect of fungal EPs on the immunomodulatory effect of fungal EPs on macrophage against Salmonella infection. Macrophages were pre-treated with three selected fungal EPs (S. commune CMU-01, G. fornicatum NK0524, and L. sajor-caju NK0427) at concentrations of 25, 50, 100, and 200 µg/mL for 24 hours prior to infection with Salmonella enterica serovar Typhimurium. After pretreatment with fungal EPs, RAW264.7 cells were infected with STM (MOI 5) for 1 h. After the elimination of extracellular bacteria by gentamicin sulfate treatment, the macrophage cells were collected and lysed. Then, the viable number of recovered STM from the intracellular macrophages was enumerated on LB agar supplemented with nalidixic acid. The results indicated that the three fungal EPs promoted the elimination of foreign bacteria entering macrophage cells and attenuated the survival of Salmonella in intracellular macrophage by reducing the viable number of recovered STM in a dose-dependent manner (Figure 4).
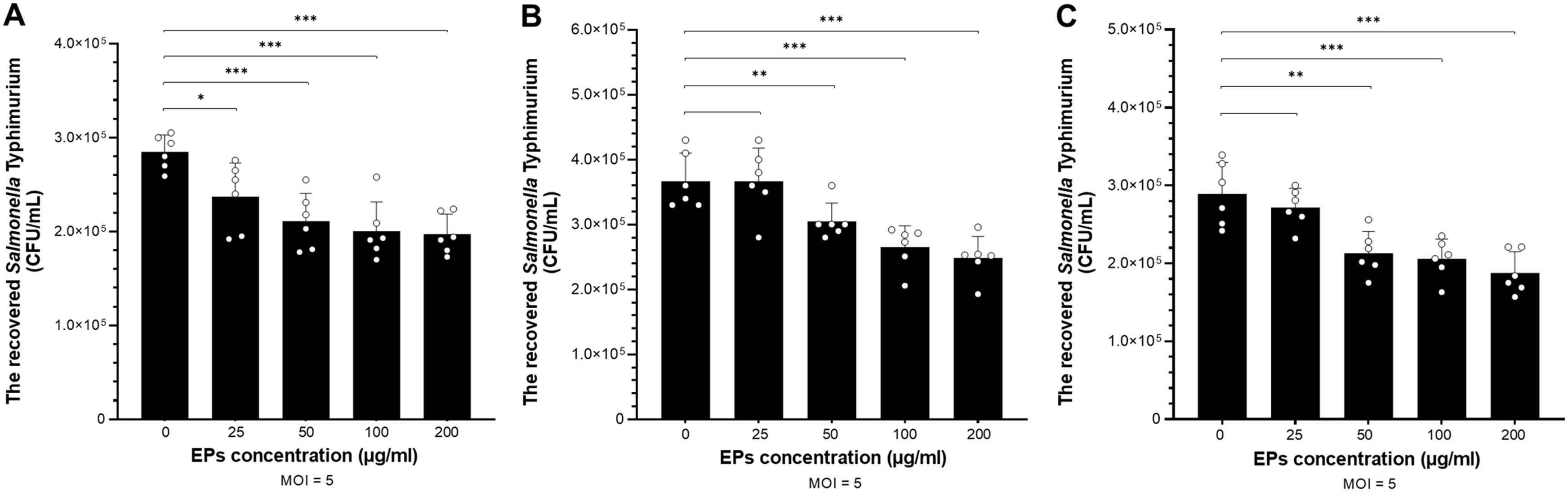
Figure 4. The viable number of STM recovered from macrophages was enumerated to assess the immunomodulatory effect of fungal EPs on the macrophage against STM. The invasion (gentamicin protection) assay was conducted in RAW264.7 murine macrophage cell lines. The cells were pre-treated with the fungal EPs of S. commune CMU-01; SC-EPs (A), G. fornicatum NK0524; GF-EPs (B), and L. sajor-caju NK0427; LS-EPs (C) at different final concentrations of 25, 50, 100, and 200 μg/mL. After 24 hours of fungal EP treatment, the cells were exposed to STM with an MOI of 5 for 1 hour. Data are presented as means, and the error bars in each graph indicate the standard deviation (+ SD). The *, **, and *** represent significant differences with p-values < 0.05, 0.01, and 0.001, respectively, compared to the EPs-untreated control group (0 μg/mL), as determined by Student’s t-test.
Compared with the EPs-untreated group, SC-EPs-treated RAW264.7 cells were the only group in which the number of recovered STM from intracellular macrophage had significantly decreased (p<0.05) at a low concentration (25 µg/mL), as shown in Figure 4A. At higher concentrations for treatment of SC-EPs (50–200 µg/mL), RAW264.7 cells exhibited a significant reduction in the number of recovered STM (p<0.001). Additionally, RAW264.7 macrophages treated with GF-EPs and LS-EPs also showed a significantly decreased viable number of survived STM in intracellular macrophages with a dose-dependent relationship. In other words, GF-EPs and LS-EPs-treated RAW264.7 cells at the concentration of 50 µg/mL significantly reduced the number of recovered STM (p<0.01). Meanwhile, pretreatment of GF-EPs and LS-EPs (100 and 200 µg/mL) inhibited the macrophage intracellular survival of STM and decreased the number of recovered STM significantly (p<0.001), as shown in Figures 4B, C. These results indicated that fungal EPs enhance the killing activity of RAW264.7 in a concentration-dependent manner. The fungal EPs from all mushroom strains at concentrations of 50 µg/mL significantly reduced the number of invaded STM.
3.7.2 Pro-inflammatory and chemo-attractant cytokine genes expression of EPs-treated and STM-infected RAW264.7 cells
The immunomodulatory effect of fungal EP treatment on RAW264.7 murine macrophages was determined by the expression of pro-inflammatory and chemo-attractant cytokine genes. After pretreatment with three selected fungal EPs at concentrations of 50 and 200 µg/mL for 24 hours, RAW264.7 cells were treated and infected by STM with an MOI of 5 for 1 hour. Then, the cells were collected and extracted for total RNA. The mRNA of pro-inflammatory cytokine genes was amplified and evaluated for expression compared with EPs-untreated and STM-treated control groups in the four genes of interest (Il6, Mip2, Nos2, and Tnfα) by RT-qPCR. As depicted in Figures 5A, B, RAW264.7 macrophages pre-treated with three fungal EPs and then infected with STM exhibited enhanced expression of cytokines IL-6, MIP-2, iNOS, and TNF-α compared to those from the EPs-untreated and STM-infected control group. These results suggest that the presence of fungal EPs can enhance the production of pro-inflammatory cytokines. However, this enhancement depends on the source of fungal EPs and the final concentration of fungal EPs in cell culture.
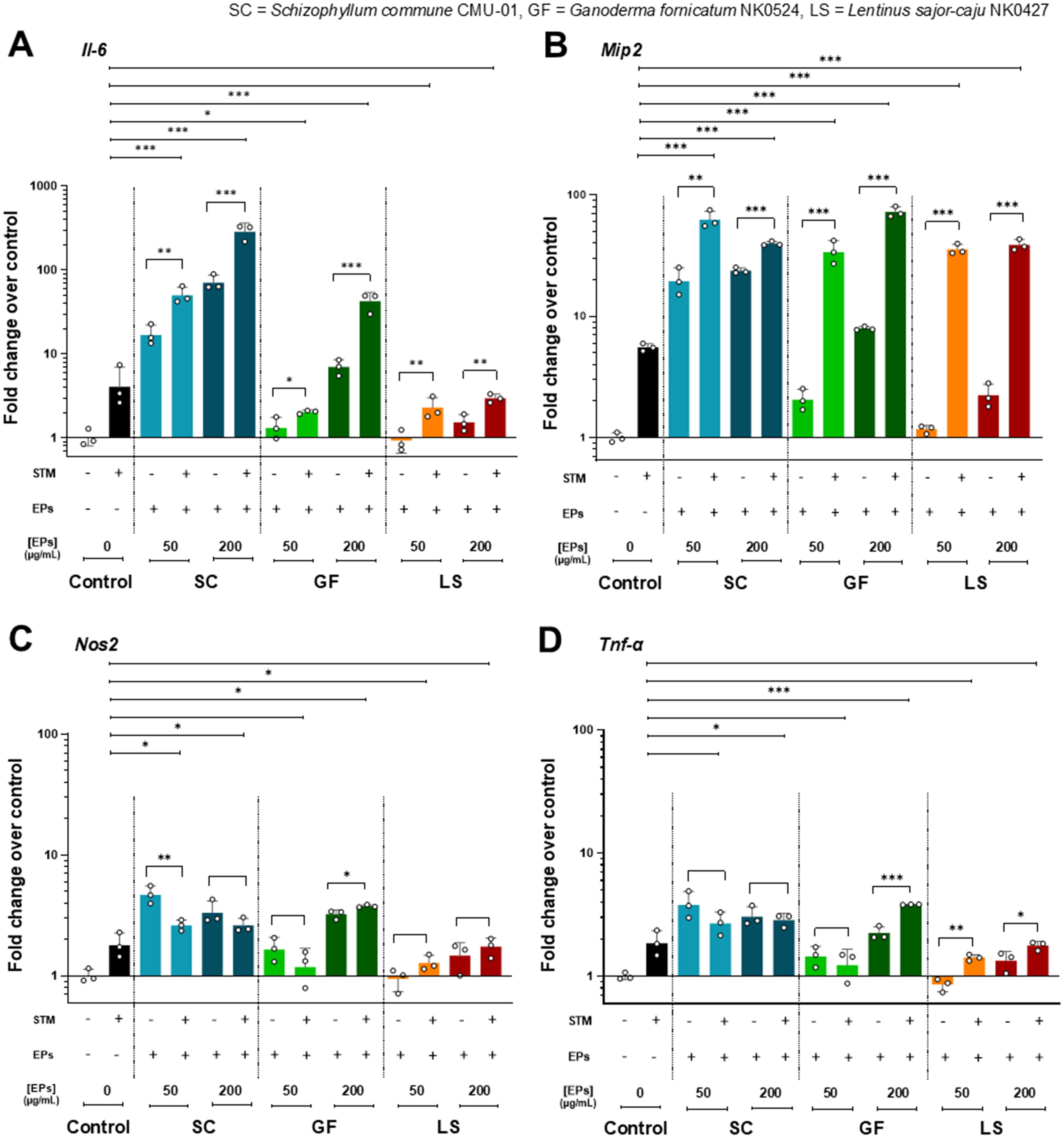
Figure 5. The pro-inflammatory cytokine gene expressions in the EPs-pretreated and STM-infected RAW264.7 cells. Fold change of gene expression of RAW264.7 pretreated with fungal EPs of S. commune CMU-01 (SC-EPs), G. fornicatum NK0524 (GF-EPs), and L. sajor-caju NK0427 (LS-EPs) at 24 hours and infected by STM for 1 hour compared to the untreated cells. The mRNA expression of (A) Il6, (B) Mip2, (C) Nos2, and (D) Tnfα. All genes were normalized with the PBS control (EPs and STM-untreated RAW264.7 cells) and analyzed with a comparative threshold cycle (CT) method over the housekeeping gene (Gapdh). Data are presented as geometric means, and the error bars in each graph indicate the geometric standard deviation (+ SD). The *, **, and *** represent significant differences with p-values < 0.05, 0.01, and 0.001, respectively, compared with the control (EPs-untreated and STM-infected RAW264.7 cells) and during the same group, as determined by Student’s t-test.
RAW264.7 cells pre-treated with SC-EPs and infected with STM showed significantly enhanced (p<0.001) expression of two pro-inflammatory cytokine genes (Il6 and Mip2) in a dose-dependent manner compared to the STM-infected and EPs-untreated control group (Figures 5A, B). Similarly, they exhibited a significant increase (p<0.05) in the expression of two pro-inflammatory cytokine genes (Nos2 and Tnfα), as shown in Figures 5C, D. The expression of Il6, Mip2, and Tnfα in GF-EPs-pretreated RAW264.7 cells at a concentration of 200 µg/mL with STM infection significantly increased (p<0.001) compared to the STM-infected and EPs-untreated control group, as shown in Figures 5A, B, D. Additionally, RAW264.7 cells pre-treated with 50 µg/mL of GF-EPs and infected with STM exhibited significantly enhanced expression of Il6 (p<0.05) and Mip2 (p<0.001), as shown in Figure 5A. Likewise, pretreatment of RAW264.7 macrophages with GF-EPs at concentrations of 50 and 200 µg/mL resulted in mRNA expression of Il6, Mip2, Nos2, and Tnfα in a concentration-dependent manner.
Pretreatment of RAW264.7 cells with 50 and 200 µg/mL of LS-EPs, followed by STM infection, significantly increased (p<0.001) the expression of Mip2 compared to the STM-infected and EPs-untreated control group, as shown in Figure 5B. The expression of Il6, Nos2, and Tnfα in LS-EPs-pretreated RAW264.7 cells with STM infection was lower than in the STM-infected control group but higher than in the EPs-untreated control group, as shown in Figures 5A, C, D. However, the expression of pro-inflammatory cytokine and chemo-attractant cytokine genes in LS-EPs-treated RAW264.7 cells increased in a dose-dependent relationship. Overall, pretreatment of RAW264.7 cells with fungal EPs at concentrations of 50 or 200 µg/mL without STM infection induced higher expression of the cytokine genes compared to EPs-untreated RAW264.7 cells. Notably, RAW264.7 cells pretreated with EPs and infected with STM exhibited a higher expression of pro-inflammatory cytokine genes than RAW264.7 macrophages treated only with EPs. These data exhibited the immunomodulatory effects of fungal EPs obtained from three fungi (S. commune, G. fornicatum, and L. sajor-caju). Pretreatment of macrophage with fungal EPs significantly stimulated the expression of pro-inflammatory genes when exposed to STM infection and reduced intracellular STM population.
4 Discussion
Nowadays, research aimed at enhancing the production of fungal EPs from each fungal species and strain focuses on optimizing conditions to achieve the highest yield. In this present study, seven fungal strains (S. commune CMU-01, P. sanguineus NK0189, L. sajor-caju NK0427, E. scabrosa NK0461, G. fornicatum NK0524, G. williamsianum NK0540, and F. tenuiculus NK0550) were found to produce EPs in MCMB with a pH of 6. The highest EP yield was produced by S. commune. This study is consistent with previous research, which indicates that fungi in the genera Ganoderma, Lentinus, Pycnoporus, and Schizophyllum have the ability to produce EPs in liquid cultivation (Jeong et al., 2009; Hao et al., 2010; Cao et al., 2014; Osińska-Jaroszuk et al., 2015). Interestingly, this study is the first to report EP production in the genera Earliella and Favolus. Our results found that the optimal conditions for EP production in each fungal strain varied based on the type of liquid culture medium. Among the three different liquid media (MCMB, PDB, and YMB), the maximum EP yield produced by S. commune, G. fornicatum, G. williamsianum, E. scabrosa, F. tenuiculus, and P. sanguineus was obtained in PDB, while the maximum EP yield of L. sajor-caju was found in MCMB. This result is supported by the findings of several previous studies, which reported that the production of fungal EPs from each strain in liquid cultivation is influenced by the type of cultivation medium (Elisashvili et al., 2009, 2012; Mahapatra and Banerjee, 2013). For example, Kim et al. (2002) reported that MCMB served as the optimal culture medium for the highest EP production in Agrocybe cylindracea, Collybia maculata, and G. lucidum, while PDB was the optimal medium for the highest EP production in Trametes versicolor, Phellinus linteus, Phellinus pini, and Pleurotus ostreatus. A study by Kizitska et al. (2024) highlighted that PDB was suitable for maximizing EP yield of Fomitopsis betulina. In contrast, Tavares et al. (2005) and Ahmed et al. (2011) found that YMB was found to be the most effective for EP production from Trametes versicolor. Therefore, the optimization of the medium type should be taken into consideration in order to achieve the highest yield of fungal EPs.
The pH value of the liquid medium and the incubation period had a significant effect on EP production in fungi. In this study, it was observed that the pH of the liquid medium has a significant effect on the production of EPs. The highest EP yield for each fungal strain varied among the different pH levels. Schizophyllum commune produced the highest EP yield at pH 6 in PDB. Three fungi, including G. fornicatum, F. tenuiculus and G. williamsianum, produced the highest yield of EPs at pH 7. Meanwhile, E. scabrosa, L. sajor-caju and P. sanguineus demonstrated their highest yields of EPs at pH 8.0. These results were in accordance with the findings of previous studies which had reported that the optimal pH value of the liquid medium range from 4.0–9.0 depending on both the fungal species and on the fungal strain (Chen et al., 2008; Osman et al., 2009; Mahapatra and Banerjee, 2013; Osińska-Jaroszuk et al., 2015). For example, the optimal pH for achieving the highest EP production from Lignosus rhinoceros and G. resinaceum was pH 6 (Lai et al., 2014; Kim et al., 2006). Hamedi et al. (2007) found that Agaricus blazei produced the highest EPs at a pH of 7. In addition, Thai and Keawsompong (2019) reported that a pH value of 6.5 supported the maximum EP production yield in Tricholoma crassum. Cordyceps militaris and Tremella fuciformis produced the highest EPs at a pH of 8 (Kim and Yun, 2005; Cho et al., 2006). In this study, the incubation period for the fungal EP production was investigated. After 10 days of incubation, G. fornicatum produced the highest yield of EPs. However, after 12 days of incubation, G. williamsianum and E. scabrosa exhibited their highest EP production. The largest yield of EPs was observed by F. tenuiculus, L. sajor-caju, P. sanguineus, and S. commune after 14 days of incubation. The results of this study are consistent with previous research, which found that the optimal incubation period for achieving the highest fungal EP production varied depending on the fungal species and strains (Nehad and El-Shamy, 2010; Mahapatra and Banerjee, 2013; Jia et al., 2015; Osińska-Jaroszuk et al., 2015). Rosado et al. (2003) and Long et al. (2021) reported that Pleurotus ostreatoroseus and G. cantharelloideum yielded the highest amounts of EP production after 7 days of incubation. A study of Jeong et al. (2009) found that the maximum yield of EPs from G. applanatum was achieved after 12 days. Sasidhara and Bakki (2014) investigated the production of EPs in G. lucidum and discovered that the maximum yield of EPs was produced after 14 days of incubation. Therefore, our findings highlight the optimal conditions required for each fungal strain to achieve its maximum EP yield. Knowing the optimal cultivation period for fungal EP production helps maximize yield, improve cost efficiency, and develop effective strategies for industrial production. However, several other factors, including aeration, temperature, carbon sources, and nitrogen sources, significantly influence fungal EP production. These parameters can affect not only the yield but also the composition and bioactivity of the produced EP. Future research should focus on optimizing these factors for each specific fungal strain to enhance EP production and biological efficiency.
The functional groups of the seven fungal EPs in this study were identified through FT-IR spectrum analysis. Their spectra unveiled functional groups, confirming the typical patterns of polysaccharides. This included broad peaks indicating hydroxyl (-OH) groups, C-H bonds from aldehyde groups, methylene (CH2) groups, carbonyl (C=O) groups, and C-H and C-C bonds from aliphatic hydrocarbons. Strong peaks were observed for polysaccharide features, including C-O, C-O-C, and C-O-H linkages. A comparison of the obtained peaks indicated the presence of both α and β glycosidic bonds, further confirming the classification of these fungal EPs as carbohydrates. Moreover, the presence of carbonyl groups in these EPs suggests their potential inclusion as part of amide (RCONH2) groups, indicating that the fungal crude EPs are protein-complex polysaccharides. These characteristic properties of the IR spectrum of these fungal EPs were similar to those of previous reports on the properties of fungal EPs (Zheng et al., 2014; Hu et al., 2017; Usuldin et al., 2020; Chen et al., 2023a; Fornal et al., 2023). For monosaccharide composition analysis, the obtained fungal EPs were heteropolysaccharides, with glucose as the major monosaccharide and minor amounts of fructose, allose, rhamnose, allulose, and others. Similarly, previous studies have reported that glucose is the most common monosaccharide in several fungal EPs, along with other monosaccharides, including arabinose, galactose, fucose, mannose, rhamnose, and xylose (Peng et al., 2015; Cao et al., 2019; Mehmood et al., 2019; Xu et al., 2019a; Long et al., 2021). Moreover, several previous studies reported that the monosaccharide composition of EPs can be significantly influenced by nutrient (carbon and nitrogen sources) availability and liquid culture conditions, especially the carbon source (Chen et al., 2015; Wu and Liang, 2018; Wu et al., 2021).
In this study, the EPs obtained from the seven fungal strains demonstrated positive antioxidant activity in both the DPPH and ABTS radical scavenging assays, which are commonly used to assess antioxidant activity in vitro. Several studies have documented the antioxidant activities of fungal EPs, quantifying these properties through IC50 values employing DPPH and ABTS radical scavenging activity assays. The IC50 value represents the concentration required to scavenge 50% of free radicals. Our study demonstrated that the fungal EPs exhibited antioxidant activity with IC50 values ranging from 4.45 to 13.29 mg/mL for ABTS assay and 1.42 to 1.71 mg/mL for DPPH assay. These findings are consistent with previous studies reporting that EPs from basidiomycetous fungal species in genera such as Agrocybe, Ganoderma, Hygrophoropsis, Inonotus, Laetiporus, Nothophellinus, Pleurotus, Pseudoinonotus, Rigidoporus, Trametes, and Tremella exhibit antioxidant activities, playing a significant role as free radical scavengers, with the activity of EPs varying according to the fungal species and strains (Lung & Huang, 2012; Zhang et al., 2015; Zhao et al., 2017; Jia et al., 2018; Xu et al., 2019b; Bai et al., 2020; Huang et al., 2020; Zheng et al., 2020; Debnath et al., 2021; Zhang et al., 2023; Gallo et al., 2024). For instance, EPs obtained from C. sinensis showed IC50 values for ABTS activity ranging from 1.027 to 2.953 mg/mL (Ho-Thi et al., 2016; Nguyen et al., 2021). In contrast, the IC50 values for ABTS activity of EPs obtained from C. cicadae and C. gracilis were 6.38 and 7.08 mg/mL, respectively (Sharma et al., 2015a, b). The EPs of C. cicadae, Hericium coralloides, P. flabellatus, and Tricholoma crissum had IC50 values for DPPH activity of 7.32, 6.59, 0.93 to 2.25, and 0.338 mg/mL, respectively (Sharma et al., 2015a; Thai and Keawsompong, 2019; Nguyen et al., 2021; Tabibzadeh et al., 2022). Prior to this present study, the antioxidant activities of EPs obtained from several basidiomycetous fungi (e.g. Fomitopsis meliae, G. lucidum, Inonotus obliquus and Stropharia rugosoannulata) have been reported from a variety of assays employing different mechanisms including lipid peroxidation, metal chelation, reducing power and scavenging activity, among others (Xu et al., 2014; Liu and Zhang, 2018; Hao et al., 2023; Prajapati et al., 2023). However, variations in the assays themselves, and the results they express, make it difficult to compare the outcomes obtained in this study with those of previous studies. Several previous studies have reported that EPs exhibit potent antioxidant activities, which are associated with their structural and chemical characteristics, including the presence of hydroxyl groups (-OH) and other specific functional groups in the structure of EPs, such as -COOH, C=O, and -O-. These groups can donate hydrogen ions or electrons to reduce free radicals, leading to the formation of more stable compounds, react with free radicals to terminate radical chain reactions, and stabilize free radicals through direct binding (Leung et al., 2009; He et al., 2012; Mao et al., 2014; Sun et al., 2015; Chen et al., 2017). Further studies are needed to elucidate the detailed chemical structure and molecular characteristics of the EPS investigated in this study. Advanced analytical techniques such as nuclear magnetic resonance (NMR) spectroscopy and size-exclusion chromatography should be employed to determine glycosidic linkages, molecular weight, and branching patterns.
Fungal EPs are present as alternative substances that have no toxicity for the activation of macrophages. These EPs obtained from different species of fungi exhibit properties that can modulate the immune response, thereby stimulating the activation of macrophages without causing any harmful effects on their function or viability. In this study, the effect of fungal EPs on the cell viability of RAW264.7 cells was examined by exposing the cells to three selected fungal EPs at different concentrations. The MTT assay indicated that the fungal EPs displayed no cytotoxic effects on the cells when present at concentrations ranging from 25 to 1,000 μg/mL, with some outstanding promotion of cell proliferation. In consistent with Zhang et al. (2022), the polysaccharides of Morchella sextelata showed no cytotoxicity at concentrations of 12.5 to 800 μg/mL and had a positive effect on the cell viability of RAW264.7 cells by significantly promoting cell proliferation. Similarly, crude and fractions of S. commune polysaccharides also significantly improved the proliferation activity of RAW264.7 cells, indicating that these certain concentrations of fungal polysaccharides were nontoxic to macrophages (Yelithao et al., 2019). Many studies have investigated the effect of fungal EP treatment on the RAW264.7 cells. It was found that fungal EPs not only promote cell viability, but they also differentiate the macrophage cells. Consistent with a study by Yang et al. (2023), fungal EPs derived from Cordyceps cicadae were found to stimulate growth, increase the density, and alter the morphology of the RAW264.7. The cells were larger and formed clusters at a higher concentration of EPs compared to the EPs-untreated control group. In addition, crude polysaccharides from Calocybe indica induced morphological changes in RAW264.7 macrophage cells, affecting treated cells to exhibit dendritic extensions in a dose-dependent manner (Ghosh et al., 2021). Fungal polysaccharides extracted from Dictyophora rubrovolvata have been found to promote the differentiation of RAW264.7 cells (Bao et al., 2023). Moreover, polysaccharides derived from Pleurotus tuber-regium were found to activate enzymes accountable for the maturation and proliferation of mice peritoneal macrophages. The treatment of fungal polysaccharides resulted in an augmentation of primary and secondary lysosomes in the cytoplasm, along with an increased abundance of ribosomes and dense chromatin in the nucleus of polysaccharide-treated cells (Wu et al., 2014). Interestingly, Inonotus obliquus polysaccharides have been found to promote cell proliferation in mouse splenocytes. This indicates that the fungal polysaccharides activate not only RAW264.7 macrophage cells but also other immune cells (Won et al., 2011). For a more thorough understanding of the cellular responses and possible underlying mechanisms of EPs in this study, future research should include further investigations into morphological alterations and stress markers, such as reactive oxygen species (ROS) production and apoptosis-related proteins, in macrophages.
Fungal polysaccharides are complex biomacromolecules and have been the focus of scientific investigations for their remarkable capacity to augment the process of phagocytosis in macrophage. Macrophages are the important innate immune cells in mammals. Upon encountering pathogenic triggers or suffering injury, the process of phagocytosis serves as the early stage in the immune reaction of macrophages toward several pathogens, especially bacteria (Weiss and Schaible, 2015). Phagocytosis is a crucial function of macrophages wherein they engulf and digest the pathogens. The effectiveness of macrophages in engulfing particles was assessed based on their uptake of neutral red or fluorescein isothiocyanate (FITC)-dextran after prior treatment with fungal polysaccharides (Zhang et al., 2008; Wan et al., 2023). Several studies have demonstrated that fungal EPs can boost the phagocytic function of RAW264.7 in vitro. Pretreatment with fungal EPs isolated from Cordyceps militaris and Paecilomyces cicadae TJJ1213 has been shown to increase the ingestion step in macrophages (Lee et al., 2015; Tian et al., 2022), as well as fungal EPs from a liquid culture of L. edodes were reported to be implicated in the enhancement of phagocytosis (Lee et al., 2008). Markedly, the phagocytosis activities of macrophages induced by fungal polysaccharides showed a concentration-dependent manner in the range of 50 to 200 µg/mL (Zhang et al., 2022).
In our study, we examined the impact of fungal EPs on the intracellular survival of Salmonella within macrophage cells. The RAW264.7 cells were pre-treated with three selected fungal EPs at various concentrations before being infected with STM. Salmonella is a diverse species of Gram-negative bacteria that can cause acute gastroenteritis in humans and animals. Salmonella infections have a significant impact on public health and the food industry globally due to their potential virulence factors and the increase in mutlidrug-resistant (MDR) strains. The bacteria have evolved mechanisms to subvert the host’s immune system and colonize the host, employing the specialized structure named “Type III secretion systems (T3SS)” to inject effector proteins into the gut epithelium and macrophage cytosol. These proteins manipulate host cell functions, aiding the establishment of intracellular niches and enhancing bacterial survival (Buckner et al., 2011). Salmonella resides within modified phagosomes known as Salmonella-containing vacuoles (SCVs), preventing bacterial degradation and enabling survival within phagosomal compartments (Kombade and Kaur, 2021). Additionally, Salmonella-induced filaments (SIFs) are membranous structures formed by the bacterium, contributing to a sheltered intracellular environment for replication within macrophages and evading host immune responses by altering host cell membrane trafficking pathways (Schroeder et al., 2011). Our study found that fungal EPs could enhance the phagocytic-killing activity of macrophages against STM infection in a concentration-dependent manner. Interestingly, all selected three fungal EPs at a concentration of 50 µg/mL significantly reduced the number of Salmonella present within macrophage cells. Notably, pretreatment of SC-EPs at a concentration of 25 µg/mL specifically demonstrated the reduction of the recovered number of intracellular STM. Cheng et al. (2019) also reported that pretreatment of the polysaccharide fraction extracted from the wild Lactarius deliciosus at least 25 µg/mL significantly enhanced the phagocytosis activity of RAW264.7. Moreover, polysaccharides from the mycelium of Cordyceps sinensis UM01 (15 µg/mL) were found to enhance the phagocytic activity of macrophages by 3.3-fold. This was evidenced by the increased uptake of FITC-dextran by RAW264.7 macrophages compared to the untreated control group (Cheong et al., 2016). The fungal EPs significantly promote the phagocytic function of macrophage cells by enhancing phagocytic uptake, which is important for boosting the immunomodulatory potential. This study focused on the phagocytic-killing activity of macrophages in response to STM. It involves subjecting macrophages to a preliminary treatment with fungal EPs, known for their immunomodulatory capabilities, followed by deliberate infection with STM. Thus, fungal EPs can improve the functioning and killing activity of RAW264.7, resulting in reducing the number of STM in intracellular macrophages during phagocytosis activity. These findings suggest that fungal EPs from mycelial culture broth have an immunomodulatory effect on the macrophage in STM infection.
Several studies have determined the inflammatory cytokines and inducible nitric oxide synthase (iNOS) production of RAW264.7 cells in cell culture supernatants by enzyme-linked immunosorbent assay (ELISA) and Western blot (Xiang et al., 2015; Zhang et al., 2022). Macrophages can be stimulated by several factors, such as pathogen-associated molecular patterns (PAMPs), including bacterial lipopolysaccharide (LPS), peptidoglycan, and lipoteichoic acid, as well as various pro-inflammatory cytokines. One of the most extensively studied PAMPs is bacterial LPS, which is found in the outer membrane of Gram-negative bacteria such as Salmonella (Rutledge et al., 2011; Wang et al., 2019a; Yamamoto et al., 2020; Chen et al., 2023b). Recognition of LPS occurs through the Toll-like receptor 4 and myeloid differentiation factor 2 complex (TLR4-MD2) mainly on macrophages (Park and Lee, 2013). When stimulated with LPS, macrophages show increased production of nitric oxide (NO) and elevated expression levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 (Facchin et al., 2022). According to our findings, the production of pro-inflammatory and chemo-attractant cytokines was assessed by the expression of Il6, Mip2, Nos2, and Tnfα using RT-qPCR. Pretreatment of the RAW264.7 cells with fungal EPs significantly increased the upregulation of these pro-inflammatory genes in a dose-dependent manner. Our results suggest that these three fungal EPs can stimulate the innate immune response against STM infection. Additionally, specific assays (such as the Limulus amebocyte lysate (LAL) assay or ELISA for LPS detection) should be incorporated to confirm the absence of LPS or any other endotoxin contamination in the EPs derived from different fungal strains, ensuring that the observed macrophage stimulation is attributable to the fungal EPs (Domingues et al., 2012; He et al., 2019; Li et al., 2020).
Consistent with fungal EPs from various fungal species, they have been shown to activate macrophages. For example, EPs from Paecilomyces lilacinus PH0016 have been found to possess immunomodulatory properties on RAW264.7 cells. Treatment with these fungal EPs resulted in increased expression of inducible nitric oxide synthase (iNOS) and secretion of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and nitric oxide (NO) in macrophages (He et al., 2019). Similarly, EPs from culture broth Trichoderma pseudokoningii stimulate the activation of macrophages and induces the production of NO, IL-1β, and TNF-α (Wang et al., 2016). Purified polysaccharides from Cordyceps cicadae also exhibit good immunomodulatory activity on RAW264.7 macrophages in a concentration range of 50–400 µg/mL by enhancing the iNOS production and the secretion of the major inflammatory cytokines (IL-1β, IL-6, IL-12, and TNF-α) (Gao et al., 2023). Moreover, fungal EPs isolated from the culture broth of Cordyceps cicadae, Aspergillus aculeatus, A. terreus, and Trichoderma sp. KK19L1 showed a potent immunomodulatory effect by enhancing the production of NO, TNF-α, and IL-6 in a concentration-dependent manner from RAW264.7 (Li et al., 2020; Yang et al., 2023). Furthermore, some fungal EPs not only enhance the immunomodulatory functions of innate immunity but also boost the adaptive immune response. EPs from the culture supernatant of Phellinus linteus mycelium induced effective production of TNF-α, monocyte chemo-attractant protein (MCP)-1, IL-6, and NO in RAW264.7 and stimulated the Th1-cytokine interferon (IFN)-γ production in splenocytes (Shin et al., 2022). The mRNA expression levels of several cytokines (TNF-α, IFN-γ, and IL-2) in splenocytes and thymocytes were increased after the treatment with EPs from the cultured supernatant of Cordyceps sinensis (Sheng et al., 2011). However, further research on the effects of fungal EPs on anti-inflammatory cytokines (e.g., IL-10, and transforming growth factor-beta; TGF-β) in macrophages will be considered to provide a more comprehensive understanding of their immunomodulatory properties. Several previous studies have reported that crude EPs, isolated from liquid cultures via ethanol precipitation, can effectively activate and modulate immune responses. These findings support the potential use of crude fungal EPs as immunostimulants for future biomedical applications (Li et al., 2018; Ghosh et al., 2019, 2021; Santra and Banerjee, 2022; Zhong et al., 2023). However, ethanol precipitation may also co-precipitate fungal cell wall components such as chitin, α- and β-glucans, and glycoprotein contaminants (Złotko et al., 2019; Garcia-Rubio et al., 2020; Osibe et al., 2020; Fornal et al., 2023), which themselves possess immunomodulatory properties. Therefore, to confirm that the observed immunostimulatory effects are truly attributable to fungal EPs, future studies should incorporate specific immunoassays, such as ELISA, immunofluorescence, and β-D-glucan assays, to detect potential cell wall contaminants in crude EP preparations. Moreover, the use of purification techniques including dialysis, deproteination, enzymatic degradation with polysaccharide-degrading enzymes (e.g., endo-β-glucanase, chitinase), ion-exchange chromatography, and size-exclusion chromatography can help determine whether the observed immunostimulatory effects are truly attributable to the fungal EPs themselves rather than residual fungal cell wall components (Zheng et al., 2014; Sun et al., 2022; Fornal et al., 2023; Yang et al., 2023).
Besides, the RT-qPCR results of our study showed the downregulation of iNOS and TNF-α mRNA expression in STM-infected RAW264.7 cells when the concentration of SC-EPs was increased from 50 µg/mL to 200 µg/mL for pretreatment. In agreement with a study by Wang et al. (2019b), pretreatment of EPs from L. edodes inhibited mRNA expression levels of iNOS and TNF-α in a concentration-effect manner. Similarly, the anti-inflammatory activity of EPs from S. commune was assessed to significantly inhibit iNOS expression levels in LPS-induced RAW264.7 cells at a higher concentration (Du et al., 2017). Moreover, treatment of L. edodes polysaccharide to RAW264.7 at high concentration decreased the secretion of NO, but the production of IL-6 remained stable (Xu et al., 2012). Our findings suggest that fungal EPs from S. commune may have anti-inflammatory activity on RAW264.7 when the expression of other inflammatory cytokines (IL-6 and MIP-2) is excessive.
Overall, RAW264.7 macrophages are involved in a series of intricate steps that play a vital role in the immune response against pathogens. Treatment of RAW264.7 cells with our fungal EPs stimulated the robust expression of pro-inflammatory and related cytokines (IL-6, TNF-α, MIP-2, and iNOS) in a dose-dependent manner through several pathways, mainly involving MAPKs and NF-κB signaling during macrophage activation. Activated macrophages can also secrete ROS and reactive nitrogen species (RNS) in response to pathogenic infection (Subbian et al., 2007). RNS are a family of antimicrobial molecules derived from NO and superoxide produced via the enzymatic activity of iNOS and NADPH oxidase. Fungal EPs activate macrophages and up-regulate the expression of iNOS leading to enhanced levels of NO. The production of NO and TNF-α is an important part of the cytotoxic activity of macrophages. NO is recognized as an intercellular messenger and participates in signal transduction for immune regulation (Wu et al., 2013). TNF-α can induce the expression of inflammatory cytokines and other mediators (Glucksam-Galnoy et al., 2013). Likewise, MIP-2 and IL-6 assist in the recruitment of immune cells to the infection site, thereby modulating immune and inflammatory responses (Qin et al., 2017; Coperchini et al., 2021). When Salmonella infection occurs, PRRs/PAMPs interactions activate the phagocytic function of macrophages. This recognition triggers transduction signaling pathways, ultimately leading to the high production of inflammatory cytokines and mediators. Inflammatory responses are initiated upon exposure to certain cytokines, recruiting macrophages and other immune cells to the site of infection (Mogensen, 2009). These cytokines and inflammatory mediators serve as essential messengers both intracellularly and extracellularly for macrophages, playing a crucial role in immune defense against pathogenic bacteria (Nworu et al., 2015; Liu et al., 2021). Activated macrophages treated with fungal EPs exhibit an elongated and spindle-shaped appearance, increasing the area of contact with outer substances, which aids in phagocytic uptake in a concentration-dependent manner (Cheng et al., 2019; Yang et al., 2023). This morphological change is associated with an enhanced phagocytosis activity of the macrophages, enabling them to engulf pathogenic bacteria. Following phagocytosis, macrophages activate enzymes and antimicrobial molecules to degrade and neutralize the bacteria. Additionally, fungal EPs-treated macrophages may boost the production of ROS, NO, and several related cytokines (Hirayama et al., 2017). The heightened production of ROS and RNS plays a crucial role in effectively killing phagocytosed bacteria (Iovine et al., 2008; Didier et al., 2010). Furthermore, macrophages treated with fungal EPs not only produce pro-inflammatory cytokines, RNS, and ROS, but they also generate the production of cellular acid phosphatase (ACP) and lysozyme (Yang et al., 2023). ACP is a marker enzyme that participates in immune regulation, enhances the rates of phagocytosis, and aids in pathogenic clearance (Chen et al., 2021). Additionally, lysozyme can hydrolyze bacterial cell wall peptidoglycan, leading to bacterial cell lysis and death (Ragland and Criss, 2017). Taken together in our present study, all these related and complex mechanisms promote the full activation of macrophages ready for bacterial infection defense, resulting in the inhibition of intracellular survival and reduction of the number of Salmonella recovered within macrophages. These properties of fungal EPs could achieve the ability to limit the survival of pathogenic bacteria in intracellular macrophages. Investigating the interplay between Salmonella infection and the inflammatory cytokine production of fungal EPs-treated RAW264.7 macrophages offers insights into immunomodulation mechanisms and unveils potentially synergistic implications for defending against bacterial infection, especially for MDR STM.
5 Conclusion
The type of liquid media, initial pH value, incubation time, and fungal strain all influence the attainment of maximum EP yield. The findings in this study showed that S. commune, G. fornicatum, G. williamsianum, E. scabrosa, F. tenuiculus, and P. sanguineus produced the maximum EP yield in PDB. However, the maximum EP yield from L. sajor-caju was obtained in MCMB. Remarkably, this is the first report for EP production from the genera Earliella and Favolus. The slightly alkaline conditions of the liquid culture media could enhance EP production. The highest EP yield from the selected fungi was obtained after 10 to 14 days, depending on the fungal strain. The FT-IR spectroscopy analysis detected the major characteristics and functional groups of the crude fungal EPs, including hydroxyl, methyl, methylene, and carbonyl groups, as well as glycosidic bonds. This confirmed the typical carbohydrate patterns. Additionally, the crude fungal EPs were primarily comprised of glucose, followed by fructose, allose, and allulose, with differences based on the fungal strain. All crude fungal EPs demonstrated positive antioxidant potential in both the ABTS and DPPH assays. The immunomodulatory effects of selected crude EPs from S. commune, G. fornicatum, and L. sajor-caju against Salmonella infection were investigated. These EPs showed no cytotoxicity on cell viability and increased the proliferation of RAW264.7 macrophages at concentrations ranging from 25 to 1,000 μg/mL. Interestingly, the fungal EPs could enhance the phagocytic-killing activity of macrophages to inhibit the intracellular survival of STM. In addition, fungal EPs-pretreated RAW264.7 cells significantly upregulated the mRNA expression levels of several pro-inflammatory, and chemo-attractant cytokines in STM-infected macrophages. The results suggest that these three fungal EPs have a potential to be an alternative to antibiotics for STM infection by the modulation of host innate immune response. However, further in vivo studies for anti-Salmonella and immunomodulatory effects of these three fungal EPs are essential to aid in developing potential applications of fungal EPs in food, therapeutics, and pharmaceuticals.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
TC: Data curation, Methodology, Writing – review & editing, Software, Writing – original draft, Investigation, Formal analysis, Visualization. NS: Formal analysis, Project administration, Visualization, Methodology, Supervision, Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing, Resources, Investigation. JK: Formal analysis, Project administration, Writing – review & editing, Methodology, Writing – original draft, Visualization, Conceptualization, Investigation, Resources, Validation, Data curation, Funding acquisition. RP: Investigation, Software, Writing – review & editing, Validation, Methodology, Formal analysis. SC: Software, Methodology, Investigation, Writing – review & editing, Formal analysis. PW: Writing – review & editing, Supervision. AT: Investigation, Writing – review & editing, Formal analysis, Visualization, Methodology. SoL: Methodology, Formal analysis, Writing – review & editing, Investigation. PT: Writing – review & editing, Formal analysis, Writing – original draft, Visualization, Software, Methodology, Supervision, Investigation, Validation, Conceptualization. SaL: Writing – review & editing, Supervision, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors sincerely appreciate the TA & RA Scholarship, Multidisciplinary and Interdisciplinary School, Chiang Mai University, Thailand and Thailand and National Research Council of Thailand (N42A650198). Nakarin Suwannarach gratefully acknowledges funding support from the Fundamental Fund 2023 (Grant Number FF66/065), Chiang Mai University, Thailand.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, S., Anwar, A., Haider, A., Saeed, M. A., Nadeem, M., Nasreen, Z., et al. (2011). Selection of culture medium for exopolysaccharides production by Coriolus versicolor. Pak. J. Phytopathol. 23, 1–4.
Bai, J., Jia, X., Chen, Y., Ning, Z., Liu, S., and Xu, C. (2020). Effect of carbon source on properties and bioactivities of exopolysaccharide produced by Trametes ochracea (Agaricomycetes). Int. J. Med. Mushrooms. 22, 289–297. doi: 10.1615/intjmedmushrooms.2020033984
Bao, K., Song, M., Wang, S., Li, T., Wang, J., Cheng, X., et al. (2023). Isolation, purification, characterization and immunomodulatory effects of polysaccharides from Dictyophora rubrovalvata waste. Ind. Crops Prod. 206, e117754. doi: 10.1016/j.indcrop.2023.117754
Birhanu, B. T., Park, N., Lee, S., Hossain, M. A., and Park, S. (2018). Inhibition of Salmonella Typhimurium adhesion, invasion, and intracellular survival via treatment with methyl gallate alone and in combination with marbofloxacin. Vet. Res. 49, e101. doi: 10.1186/s13567-018-0597-8
Boonloh, K., Kukongviriyapan, V., Kongyingyoes, B., Kukongviriyapan, U., Thawornchinsombut, S., and Pannangpetch, P. (2015). Rice bran protein hydrolysates improve insulin resistance and decrease pro-inflammatory cytokine gene expression in rats fed a high carbohydrate-high fat diet. Nutrients 7, 6313–6329. doi: 10.3390/nu7085292
Buckner, M. M., Croxen, M., Arena, E. T., and Finlay, B. B. (2011). A comprehensive study of the contribution of Salmonella enterica serovar Typhimurium SPI2 effectors to bacterial colonization, survival, and replication in typhoid fever, macrophage, and epithelial cell infection models. Virulence 2, 208–216. doi: 10.4161/viru.2.3.15894
Buddhasiri, S., Sukjoi, C., Kaewsakhorn, T., Nambunmee, K., Nakphaichit, M., Nitisinprasert, S., et al. (2021). Anti-inflammatory effect of probiotic Limosilactobacillus reuteri KUB-AC5 against Salmonella infection in a mouse colitis model. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.716761
Cao, H., Ma, S., Guo, H., Cui, X., Wang, S., Zhong, X., et al. (2019). Comparative study on the monosaccharide compositions, antioxidant and hypoglycemic activities in vitro of intracellular and extracellular polysaccharides of liquid fermented Coprinus comatus. Int. J. Biol. Macromol. 139, 543–549. doi: 10.1016/j.ijbiomac.2019.08.017
Cao, J., Zhang, H., and Xu, C. (2014). Culture characterization of exopolysaccharides with antioxidant activity produced by Pycnoporus sanguineus in stirred-tank and airlift reactors. J. Taiwan. Inst. Chem. Eng. 45, 2075–2080. doi: 10.1016/j.jtice.2014.05.005
Chen, J., Liu, N., Li, B., Zhang, H., Zhao, Y., and Cao, X. (2021). The effects of fipronil exposure on oxidative stress, non-specific immunity, autophagy, and apoptosis in the common carp. Environ. Sci. pollut. Res. 28, 27799–27810. doi: 10.1007/s11356-021-12573-x
Chen, C., Nargotra, P., Kuo, C., and Liu, Y. (2023a). High-molecular-weight exopolysaccharides production from Tuber borchii cultivated by submerged fermentation. Int. J. Mol. Sci. 24, e4875. doi: 10.3390/ijms24054875
Chen, S., Saeed, A. F., Liu, Q., Jiang, Q., Xu, H., Xiao, G. G., et al. (2023b). Macrophages in immunoregulation and therapeutics. Transduct. Target. Ther. 8, e207. doi: 10.1038/s41392-023-01452-1
Chen, X., Wu, J., and Gui, X. (2015). Production and characterization of exopolysaccharides in mycelial culture of Cordyceps sinensis fungus Cs-HK1 with different carbon sources. Chin. J. Chem. Eng. 24, 158–162. doi: 10.1016/j.cjche.2015.06.016
Chen, T., Xu, P., Zong, S., Wang, Y., Su, N., and Ye, M. (2017). Purification, structural features, antioxidant and moisture-preserving activities of an exopolysaccharide from Lachnum YM262. Bioorg. Med. Chem. Lett. 27, 1225–1232. doi: 10.1016/j.bmcl.2017.01.063
Chen, W., Zhao, Z., Chen, S., and Li, Y. (2008). Optimization for the production of exopolysaccharide from Fomes fomentarius in submerged culture and its antitumor effect in vitro. Bioresour. Technol. 99, 3187–3194. doi: 10.1016/j.biortech.2007.05.049
Cheng, X., Wu, Q., Zhao, J., Su, T., Lu, Y., Zhang, W., et al. (2019). Immunomodulatory effect of a polysaccharide fraction on RAW 264.7 macrophages extracted from the wild Lactarius deliciosus. Int. J. Biol. Macromol. 128, 732–739. doi: 10.1016/j.ijbiomac.2019.01.201
Cheong, K., Meng, L., Chen, X., Wang, L., Wu, D., Zhao, J., et al. (2016). Structural elucidation, chain conformation and immuno-modulatory activity of glucogalactomannan from cultured Cordyceps sinensis fungus UM01. J. Funct. Foods. 25, 174–185. doi: 10.1016/j.jff.2016.06.002
Cho, E. J., Oh, J. Y., Chang, H. Y., and Yun, J. W. (2006). Production of exopolysaccharides by submerged mycelial culture of a mushroom Tremella fuciformis. J. Biotechnol. 127, 129–140. doi: 10.1016/j.jbiotec.2006.06.013
Coperchini, F., Chiovato, L., and Rotondi, M. (2021). Interleukin-6, CXCL10 and infiltrating macrophages in COVID-19-related cytokine storm: not one for all but all for one! Front. Immunol. 12. doi: 10.3389/fimmu.2021.668507
Debnath, S., Debnath, B., Saha, R., Dutta, A., Das, P., and Saha, A. K. (2021). Production of exopolysaccharides (EPSs) and evaluation of biological properties of Pleurotus flabellatus (Berk and Br.) Sacc. Proc. Natl. Acad. Sci. India. Sect. B. Biol. Sci. 91, 581–591. doi: 10.1007/s40011-021-01261-y
Didier, E. S., Bowers, L. C., Martin, A. D., Kuroda, M. J., Khan, I. A., and Didier, P. J. (2010). Reactive nitrogen and oxygen species, and iron sequestration contribute to macrophage-mediated control of Encephalitozoon cuniculi (Phylum Microsporidia) infection in vitro and in vivo. Microbes Infect. 12, 1244–1251. doi: 10.1016/j.micinf.2010.09.010
Domingues, M. M., Inácio, R. G., Raimundo, J. M., Martins, M., Castanho, M., and Santos, N. C. (2012). Biophysical characterization of polymyxin B interaction with LPS aggregates and membrane model systems. Biopolymers 98, 338–344. doi: 10.1002/bip.22095
Du, B., Yang, Y., Bian, Z., and Xu, B. (2017). Characterization and anti-inflammatory potential of an exopolysaccharide from submerged mycelial culture of Schizophyllum commune. Front. Pharmacol. 8. doi: 10.3389/fphar.2017.00252
Elisashvili, V. (2012). Submerged cultivation of medicinal mushrooms: bioprocesses and products. Int. J. Med. Mushrooms. 14, 211–239. doi: 10.1615/intjmedmushr.v14.i3.10
Elisashvili, V. I., Kachlishvili, E. T., and Wasser, S. P. (2009). Carbon and nitrogen source effects on basidiomycetes exopolysaccharide production. Appl. Biochem. Microbiol. 45, 531–535. doi: 10.1134/s0003683809050135
Facchin, B. M., Reis, G. O. D., Vieira, G. N., Mohr, E. T. B., Da Rosa, J. S., Kretzer, I. F., et al. (2022). Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: a systematic review and meta-analysis. Inflamm. Res. 71, 741–758. doi: 10.1007/s00011-022-01584-0
Fornal, M., Osińska-Jaroszuk, M., Jaszek, M., Stefaniuk, D., Wiater, A., Komaniecka, I., et al. (2023). A new exopolysaccharide from a wood-decaying fungus Spongipellis borealis for a wide range of biotechnological applications. Molecules 28, e6120. doi: 10.3390/molecules28166120
Gallo, A. L., Marfetán, J. A., and Vélez, M. L. (2024). Antioxidant activities of exopolysaccharides extracts from two endemic fungi from Patagonia. Curr. Microbiol. 81, e361. doi: 10.1007/s00284-024-03883-7
Gao, F., Luo, L., and Zhang, L. (2023). A new galactoglucomannan from the mycelium of the medicinal parasitic fungus Cordyceps cicadae and its immunomodulatory activity in vitro and in vivo. Molecules 28, e3867. doi: 10.3390/molecules28093867
Garcia-Rubio, R., De Oliveira, H. C., Rivera, J., and Trevijano-Contador, N. (2020). The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02993
Ghosh, S., Khatua, S., and Acharya, K. (2019). Crude polysaccharide from a wild mushroom enhances immune response in murine macrophage cells by TLR/NF-κB pathway. J. Pharm. Pharmacol. 71, 1311–1323. doi: 10.1111/jphp.13104
Ghosh, S., Khatua, S., Dasgupta, A., and Acharya, K. (2021). Crude polysaccharide from the milky mushroom, Calocybe indica, modulates innate immunity of macrophage cells by triggering MyD88-dependent TLR4/NF-κB pathway. J. Pharm. Pharmacol. 73, 70–81. doi: 10.1093/jpp/rgaa020
Glucksam-Galnoy, Y., Sananes, R., Silberstein, N., Krief, P., Kravchenko, V. V., Meijler, M. M., et al. (2013). The bacterial quorum-sensing signal molecule N-3-oxo-dodecanoyl-L-homoserine lactone reciprocally modulates pro- and anti-inflammatory cytokines in activated macrophages. J. Immunol. 191, 337–344. doi: 10.4049/jimmunol.1300368
Gong, P., Wang, S., Liu, M., Chen, F., Yang, W., Chang, X., et al. (2020). Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: a mini-review. Carbohydr. Res. 494, e108037. doi: 10.1016/j.carres.2020.108037
Hamedi, A., Vahid, H., and Ghanati, F. (2007). Optimization of the medium composition for production of mycelial biomass and exo-polysaccharide by Agaricus blazei Murill DPPh 131 using response-surface methodology. Biotechnology 6, 456–464. doi: 10.3923/biotech.2007.456.464
Hao, H., Cui, C., Xing, Y., Jia, X., Ma, B., Kang, W., et al. (2023). Sulfation of the extracellular polysaccharide from the edible fungus Stropharia rugosoannulata with its antioxidant activity. J. Future Foods. 3, 37–42. doi: 10.1016/j.jfutfo.2022.09.006
Hao, L., Xing, X., Li, Z., Zhang, J., Sun, J., Jia, S., et al. (2010). Optimization of effect factors for mycelial growth and exopolysaccharide production by Schizophyllum commune. Appl. Biochem. Biotechnol. 160, 621–631. doi: 10.1007/s12010-008-8507-6
He, P., Geng, L., Mao, D., and Xu, C. (2012). Production, characterization and antioxidant activity of exopolysaccharides from submerged culture of Morchella crassipes. Bioprocess. Biosyst. Eng. 35, 1325–1332. doi: 10.1007/s00449-012-0720-6
He, C., Lin, H., Wang, C., Zhang, M., Lin, Y., Huang, F., et al. (2019). Exopolysaccharide from Paecilomyces lilacinus modulates macrophage activities through the TLR4/NF κB/MAPK pathway. Mol. Med. Rep. 20, 4943–4952. doi: 10.3892/mmr.2019.10746
Hirayama, D., Iida, T., and Nakase, H. (2017). The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 19, e92. doi: 10.3390/ijms19010092
Ho-Thi, P. T., Vu, T. T., Huynh, T., Tran, M. T., Le-Thi, T. H., and Dinh, M. H. (2016). Comparison of radicals scavenging activity of exopolysaccharides from cultured Cordyceps spp. isolated in Vietnam. J. Sci. Technol. 54, 148–155.
Hu, T., Huang, Q., Wong, K., Yang, H., Gan, J., and Li, Y. (2017). A hyperbranched β-d-glucan with compact coil conformation from Lignosus rhinocerotis sclerotia. Food Chem. 225, 267–275. doi: 10.1016/j.foodchem.2017.01.034
Huang, L., Li, C., Sun, N., Wang, Y., Yang, H., Li, Y., et al. (2020). Optimization of liquid culture condition of a novel fungus Hygrophoropsis sp. and antioxidant activity of extracts. Biochem. Res. Int., 2020, e7403257. doi: 10.1155/2020/7403257
Iovine, N. M., Pursnani, S., Voldman, A., Wasserman, G., Blaser, M. J., and Weinrauch, Y. (2008). Reactive nitrogen species contribute to innate host defense against Campylobacter jejuni. Infect. Immun. 76, 986–993. doi: 10.1128/iai.01063-07
Jaroszuk-Ściseł, J., Nowak, A., Komaniecka, I., Choma, A., Jarosz-Wilkołazka, A., Osińska-Jaroszuk, M., et al. (2020). Differences in production, composition, and antioxidant activities of exopolymeric substances (EPS) obtained from cultures of endophytic Fusarium culmorum strains with different effects on cereals. Molecules 25, e616. doi: 10.3390/molecules25030616
Jeong, Y., Jeong, S., Yang, B., Islam, R., and Song, C. (2009). Optimal culture conditions for mycelial growth and exo-polymer production of Ganoderma applanatum. Mycobiology 37, 89–93. doi: 10.4489/myco.2009.37.2.089
Jia, X., Qu, L., Panpan, R., Liu, S., Wu, Y., and Xu, C. (2018). Characterization and antioxidant activity of an exopolysaccharide produced by Rigidoporus microporus (Agaricomycetes). Int. J. Med. Mushrooms. 20, 311–320. doi: 10.1615/intjmedmushrooms.2018025808
Jia, X., Zheng, K., Liu, S., and Xu, C. (2015). Optimization, purification, characterization, and antioxidant activity of exopolysaccharide produced by the northern tooth mushroom, Climacodon septentrionalis (Basidiomycota). Int. J. Med. Mushrooms. 17, 857–866. doi: 10.1615/intjmedmushrooms.v17.i9.60
Kheni, K. and Vyas, T. K. (2017). Characterization of exopolysaccharide produced by Ganoderma sp. TV1 and its potential as antioxidant and anticancer agent. J. Biol. Act. Prod. Nat. 7, 72–80. doi: 10.1080/22311866.2017.1306461
Kim, S., Hwang, H., Park, J., Cho, Y., Song, C., and Yun, J. (2002). Mycelial growth and exo-biopolymer production by submerged culture of various edible mushrooms under different media. Lett. Appl. Microbiol. 34, 56–61. doi: 10.1046/j.1472-765x.2002.01041.x
Kim, S. S., Lee, J. S., Cho, J. Y., Kim, Y. E., and Hong, E. K. (2010). Effects of C/N ratio and trace elements on mycelial growth and exo-polysaccharide production of Tricholoma matsutake. Biotechnol. Bioprocess. Eng. 15, 293–298. doi: 10.1007/s12257-008-0226-x
Kim, H. M., Paik, S. Y., Ra, K. S., Koo, K. B., Yun, J. W., and Choi, J. W. (2006). Enhanced production of exopolysaccharides by fed-batch culture of Ganoderma resinaceum DG-6556. J. Microbiol. 44, 233–242.
Kim, H. and Yun, J. (2005). A comparative study on the production of exopolysaccharides between two entomopathogenic fungi Cordyceps militaris and Cordyceps sinensis in submerged mycelial cultures. J. Appl. Microbiol. 99, 728–738. doi: 10.1111/j.1365-2672.2005.02682.x
Kizitska, T., Barshteyn, V., Sevindik, M., and Krupodorova, T. (2024). Evaluation of Fomitopsis betulina strains for growth on different media and exopolysaccharide production. Arch. Biol. Sci. 76, 257–265. doi: 10.2298/abs240523018k
Kombade, S. and Kaur, N. (2021). “Pathogenicity island in salmonella,” in Salmonella spp.-A Global Challenge. Eds. Lamas, A., Regal, P., and Franco, C. M. (London, United Kingdom: IntechOpen Limited). doi: 10.5772/intechopen.96443
Lai, W. H., Salleh, S. M., Daud, F., Zainal, Z., Othman, A. M., and Saleh, N. M. (2014). Optimization of submerged culture conditions for the production of mycelial biomass and exopolysaccharides from Lignosus rhinocerus. Sains. Malaysiana. 43, 73–80.
Lee, J. Y., Kim, J. Y., Lee, Y. G., Rhee, M. H., Hong, E. K., and Cho, J. Y. (2008). Molecular mechanism of macrophage activation by exopolysaccharides from liquid culture of Lentinus edodes. J. Microbiol. Biotechnol. 18, 355–364. doi: 10.1007/s00253-008-1439-9
Lee, J. S., Kwon, D. S., Lee, K. R., Park, J. M., Ha, S., and Hong, E. K. (2015). Mechanism of macrophage activation induced by polysaccharide from Cordyceps militaris culture broth. Carbohydr. Polym. 120, 29–37. doi: 10.1016/j.carbpol.2014.11.059
Leong, Y. K., Yang, F., and Chang, J. (2021). Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Res. 251, e117006. doi: 10.1016/j.carbpol.2020.117006
Leung, P. H., Zhao, S., Ho, K. P., and Wu, J. Y. (2009). Chemical properties and antioxidant activity of exopolysaccharides from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 114, 1251–1256. doi: 10.1016/j.foodchem.2008.10.081
Li, X., He, Y., Zeng, P., Liu, Y., Zhang, M., Hao, C., et al. (2018). Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 23, 4–20. doi: 10.1111/jcmm.13564
Li, Q., Wang, W., Zhu, Y., Chen, Y., Zhang, W., Yu, P., et al. (2017). Structural elucidation and antioxidant activity a novel Se-polysaccharide from Se-enriched Grifola frondosa. Carbohydr. Polym. 161, 42–52. doi: 10.1016/j.carbpol.2016.12.041
Li, H., Xie, W., Sun, H., Cao, K., and Yang, X. (2020). Effect of the structural characterization of the fungal polysaccharides on their immunomodulatory activity. Int. J. Biol. Macromol. 164, 3603–3610. doi: 10.1016/j.ijbiomac.2020.08.189
Lima, A. T., Santos, M. N., De Souza, L. A., Pinheiro, T. S., Paiva, A. A., Dore, C. M., et al. (2016). Chemical characteristics of a heteropolysaccharide from Tylopilus ballouii mushroom and its antioxidant and anti-inflammatory activities. Carbohydr. Res. 144, 400–409. doi: 10.1016/j.carbpol.2016.02.050
Liu, J., Geng, X., Hou, J., and Wu, G. (2021). New insights into M1/M2 macrophages: key modulators in cancer progression. Cancer Cell Int. 21, 1–7. doi: 10.1186/s12935-021-02089-2
Liu, Y., Shen, L., Yang, M., Yang, K., and Cheng, F. (2024). Extraction and chemical composition analyses of intracellular and extracellular polysaccharides from Trametes lactinea liquid fermentation. Fermentation 10, e76. doi: 10.3390/fermentation10020076
Liu, S. and Zhang, W. (2018). Hyperproduction of exopolysaccharides by submerged mycelial culture of Ganoderma lucidum using a solid seed grown in fine-powder of wheat bran and in vitro evaluation of the antioxidant activity of the exopolysaccharides produced. Food Sci. Biotechnol. 27, 1129–1136. doi: 10.1007/s10068-018-0343-z
Long, Z., Xue, Y., Ning, Z., Sun, J., Li, J., Su, Z., et al. (2021). Production, characterization, and bioactivities of exopolysaccharides from the submerged culture of Ganoderma cantharelloideum M.H. Liu. 3. Biotech. 11, e145. doi: 10.1007/s13205-021-02696-w
Lung, M. Y. and Huang, W. Z. (2012). Antioxidant properties of polysaccharides from Laetiporus sulphureus in submerged cultures. Afr. J. Biotechnol. 11, 6350–6358. doi: 10.5897/ajb11.1668
Mahapatra, S. and Banerjee, D. (2013). Fungal exopolysaccharide: production, composition and applications. Microbiol. Insights 6, 1–16. doi: 10.4137/mbi.s10957
Mao, D., Shi, C., Wu, J., and Xu, C. (2014). Optimization of exopolysaccharide production in submerged culture of Daedalea dickinsii and its antioxidant activity. Bioprocess. Biosyst. Eng. 37, 1401–1409. doi: 10.1007/s00449-013-1111-3
Mehmood, S., Zhou, L., Wang, X., Cheng, X., Meng, F., Wang, Y., et al. (2019). Structural elucidation and antioxidant activity of a novel heteroglycan from Tricholoma lobayense. J. Carbohydr. Chem. 38, 192–211. doi: 10.1080/07328303.2019.1582659
Meng, F., Liu, X., Jia, L., Song, Z., Deng, P., and Fan, K. (2010). Optimization for the production of exopolysaccharides from Morchella esculenta SO-02 in submerged culture and its antioxidant activities in vitro. Carbohydr. Res. 79, 700–704. doi: 10.1016/j.carbpol.2009.09.032
Miles, P. G. and Chang, S. (2004). Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact. 2nd ed. (Florida, United States: CRC Press). doi: 10.1201/9780203492086
Mogensen, T. H. (2009). Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22, 240–273. doi: 10.1128/cmr.00046-08
Muszyńska, B., Grzywacz-Kisielewska, A., Kała, K., and Gdula-Argasińska, J. (2018). Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 243, 373–381. doi: 10.1016/j.foodchem.2017.09.149
Nehad, E. and El-Shamy, A. (2010). Physiological studies on the production of exopolysaccharide by fungi. Agric. Biol. J. North Am. 1, 1303–1308. doi: 10.5251/abjna.2010.1.6.1303.1308
Nguyen, H., Pham, T., Nguyen, P., Le-Buanec, H., Rabetafika, H. N., and Razafindralambo, H. L. (2024). Advances in microbial exopolysaccharides: present and future applications. Biomolecules 14, e1162. doi: 10.3390/biom14091162
Nguyen, Q., Vu, T., Tran, M., Thi, P. T. H., Thu, H., Thi, T. H. L., et al. (2021). Antioxidant activity and hepatoprotective effect of exopolysaccharides from cultivated Ophiocordyceps sinensis against CCL4-induced liver damages. Nat. Prod. Commun. 16, 1–9. doi: 10.1177/1934578x21997670
Nworu, C. S., Ihim, S. A., Okoye, F. B. C., Esimone, C. O., Adikwu, M. U., and Akah, P. A. (2015). Immunomodulatory and immunorestorative activities of β-D-glucan-rich extract and polysaccharide fraction of mushroom Pleurotus tuber-regium. Pharm. Biol. 53, 1555–1566. doi: 10.3109/13880209.2014.991838
Osibe, D. A., Lei, S., Wang, B., Jin, C., and Fang, W. (2020). Cell wall polysaccharides from pathogenic fungi for diagnosis of fungal infectious disease. Mycoses 63, 644–652. doi: 10.1111/myc.13101
Osińska-Jaroszuk, M., Jarosz-Wilkołazka, A., Jaroszuk-Ściseł, J., Szałapata, K., Nowak, A., Jaszek, M., et al. (2015). Extracellular polysaccharides from Ascomycota and Basidiomycota: production conditions, biochemical characteristics, and biological properties. World J. Microbiol. Biotechnol. 31, 1823–1844. doi: 10.1007/s11274-015-1937-8
Osman, M. E., Hassan, F. R. H., Khattab, O. H., Ahmed, W. A., and El-Henawy, H. E. (2009). Physiological studies on growth of two different strains of Lentinus edodes. Aust. J. Basic. Appl. Sci. 3, 4094–4103.
Park, B. S. and Lee, J. (2013). Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 45, e66. doi: 10.1038/emm.2013.97
Patel, M., Patel, U., and Gupte, S. (2014). Production of exopolysaccharide (EPS) and its application by new fungal isolates SGMP1 and SGMP2. Int. J. Agric. Environ. Biotechnol. 7, 511–523.
Peng, L., Qiao, S., Xu, Z., Guan, F., Ding, Z., Gu, Z., et al. (2015). Effects of culture conditions on monosaccharide composition of Ganoderma lucidum exopolysaccharide and on activities of related enzymes. Carbohydr. Res. 133, 104–109. doi: 10.1016/j.carbpol.2015.07.014
Prajapati, D., Bhatt, A., and Gupte, A. (2023). Evaluation of bioactive attributes and emulsification potential of exopolysaccharide produced by a brown-rot fungus Fomitopsis meliae AGDP-2. Appl. Biochem. Biotechnol. 195, 2974–2992. doi: 10.1007/s12010-022-04257-0
Prathumpai, W., Pinruan, U., Sommai, S., Komwijit, S., and Malairuang, K. (2025). Exopolysaccharide (EPS) production by endophytic and basidiomycete fungi. Fermentation 11, e183. doi: 10.3390/fermentation11040183
Qin, C., Liu, Y., Hu, Y., Yang, Y., and Chen, Z. (2017). Macrophage inflammatory protein-2 as mediator of inflammation in acute liver injury. World J. Gastroenterol. 23, e3043. doi: 10.3748/wjg.v23.i17.3043
Radzki, W., Ziaja-Sołtys, M., Nowak, J., Rzymowska, J., Topolska, J., Sławińska, A., et al. (2016). Effect of processing on the content and biological activity of polysaccharides from Pleurotus ostreatus mushroom. LWT-Food. Sci. Technol. 66, 27–33. doi: 10.1016/j.lwt.2015.10.016
Ragland, S. A. and Criss, A. K. (2017). From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PloS Pathog. 13, e1006512. doi: 10.1371/journal.ppat.1006512
Ren, L., Edwards, P. J., Perera, C. O., and Hemar, Y. (2015). Structural features of a novel polysaccharide isolated from a New Zealand Maori mushroom Iliodiction cibarium. Carbohydr. Res. 406, 19–26. doi: 10.1016/j.carres.2014.12.011
Rosado, F. R., Germano, S., Carbonero, E. R., Da Costa, S. M. G., Iacomini, M., and Kemmelmeier, C. (2003). Biomass and exopolysaccharide production in submerged cultures of Pleurotus ostreatoroseus Sing. and Pleurotus ostreatus “florida” (Jack.: Fr.) Kummer. J. Basic. Microbiol. 43, 230–237. doi: 10.1002/jobm.200390026
Rutledge, H. R., Jiang, W., Yang, J., Warg, L. A., Schwartz, D. A., Pisetsky, D. S., et al. (2011). Gene expression profiles of RAW264.7 macrophages stimulated with preparations of LPS differing in isolation and purity. Innate Immun. 18, 80–88. doi: 10.1177/1753425910393540
Santra, H. K. and Banerjee, D. (2022). Production, optimization, characterization and drought stress resistance by β-glucan-rich heteropolysaccharide from an endophytic fungi Colletotrichum alatae LCS1 isolated from clubmoss (Lycopodium clavatum). Front. Fungal Biol. 2. doi: 10.3389/ffunb.2021.796010
Sasidhara, R. and Bakki, V. (2014). Screening of mushrooms for polysaccharides. Iran. J. Biotechnol. 12, 73–76. doi: 10.15171/ijb.1089
Schmittgen, T. D. and Livak, K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. doi: 10.1038/nprot.2008.73
Schroeder, N., Mota, L. J., and Méresse, S. (2011). Salmonella-induced tubular networks. Trends Microbiol. 19, 268–277. doi: 10.1016/j.tim.2011.01.006
Sharma, S. K., Gautam, N., and Atri, N. S. (2015a). Optimized extraction, composition, antioxidant and antimicrobial activities of exo and intracellular polysaccharides from submerged culture of Cordyceps cicadae. BMC Complement. Altern. Med. 15, e446. doi: 10.1186/s12906-015-0967-y
Sharma, S. K., Gautam, N., and Atri, N. S. (2015b). Optimization, composition, and antioxidant activities of exo- and intracellular polysaccharides in submerged culture of Cordyceps gracilis (Grev.) Durieu & Mont. Evid. Based. Complement. Alternat. Med. 2015, e462864. doi: 10.1155/2015/462864
Sheng, L., Chen, J., Li, J., and Zhang, W. (2011). An exopolysaccharide from cultivated Cordyceps sinensis and its effects on cytokine expressions of immunocytes. Appl. Biochem. Biotechnol. 163, 669–678. doi: 10.1007/s12010-010-9072-3
Shin, H. Y., Kim, H., Jeong, E., Kim, H., Shin, J., Choi, S., et al. (2022). Immunostimulatory polysaccharide fractionated from a liquid culture by Phellinus linteus mycelium. J. Korean. Soc Food Sci. Nutr. 51, 19–27. doi: 10.3746/jkfn.2022.51.1.19
Sountharavallee, V. S., Kumar, M. S., Kathiresan, K., and Kavitha, D. (2023). Biosynthesis and delineate industrially prime exopolysaccharide (MYCtPs) from marine yeast Candida tropicalis (MYCt). J. Coast. Life Med. 11, 275–285.
Subbian, S., Mehta, P. K., Cirillo, S. L. G., Bermudez, L. E., and Cirillo, J. D. (2007). A Mycobacterium marinum mel2 mutant is defective for growth in macrophages that produce reactive oxygen and reactive nitrogen species. Infect. Immun. 75, 127–134. doi: 10.1128/iai.01000-06
Sun, Y., He, H., Wang, Q., Yang, X., Jiang, S., and Wang, D. (2022). A review of development and utilization for edible fungal polysaccharides: extraction, chemical characteristics, and bioactivities. Polymers 14, e4454. doi: 10.3390/polym14204454
Sun, Y., Yang, B., Wu, Y., Liu, Y., Gu, X., Zhang, H., et al. (2015). Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods. Food Chem. 178, 311–318. doi: 10.1016/j.foodchem.2015.01.105
Tabibzadeh, F., Alvandi, H., Hatamian-Zarmi, A., Kalitukha, L., Aghajani, H., and Ebrahimi-Hosseinzadeh, B. (2022). Antioxidant activity and cytotoxicity of exopolysaccharide from mushroom Hericium coralloides in submerged fermentation. Biomass Conv. Bioref. 14, 26953–26963. doi: 10.1007/s13399-022-03386-0
Tavares, A. P. A., Agapito, M. S. M., Coelho, M. A. M., Da Silva, J. A. L., Barros-Timmons, A., Coutinho, J. A. J., et al. (2005). Selection and optimization of culture medium for exopolysaccharide production by Coriolus (Trametes) versicolor. World J. Microbiol. Biotechnol. 21, 1499–1507. doi: 10.1007/s11274-005-7370-7
Thai, L. Q. and Keawsompong, S. (2019). Production of exopolysaccharide from Tricholoma crassum in submerged culture and its antioxidant activities. Int. J. Agric. Technol. 15, 141–156.
Tian, J., Tang, C., Wang, X., Zhang, X., Xiao, L., and Li, W. (2022). Supramolecular structure features and immunomodulatory effects of exopolysaccharide from Paecilomyces cicadae TJJ1213 in RAW264.7 cells through NF-κB/MAPK signaling pathways. Int. J. Biol. Macromol. 207, 464–474. doi: 10.1016/j.ijbiomac.2022.03.029
Usuldin, S. R. A., Mahmud, N., Ilham, Z., Ikram, N. K. K., Ahmad, R., and Wan-Mohtar, W. A. A. Q. I. (2020). In-depth spectral characterization of antioxidative (1,3)-β-D-glucan from the mycelium of an identified tiger milk mushroom Lignosus rhinocerus strain ABI in a stirred-tank bioreactor. Biocatal. Agric. Biotechnol. 23, e101455. doi: 10.1016/j.bcab.2019.101455
Valasques, G. L. J., Cedro, P. É. P., Mendes, T. P. S., Miranda, A. C. D. A., Filho, A. B. C., Lima, D. M, et al. (2020). Characterization and biological activities of polysaccharides extracted from the filamentous fungal cell wall: an updated literature review. Res. Soc Dev. 9, e62191110217. doi: 10.33448/rsd-v9i11.10217
Wan, J., Zhu, Y., Jiang, X., Wang, F., Zhou, Z., Wang, J., et al. (2023). Immunomodulation of RAW264.7 cells by CP80-1, a polysaccharide of Cordyceps cicadae, via Dectin-1/Syk/NF-κB signaling pathway. Food Agric. Immunol. 34, e2231172. doi: 10.1080/09540105.2023.2231172
Wang, T., He, H., Liu, X., Liu, C., Liang, Y., and Mei, Y. (2019b). Mycelial polysaccharides of Lentinus edodes (shiitake mushroom) in submerged culture exert immunoenhancing effect on macrophage cells via MAPK pathway. Int. J. Biol. Macromol. 130, 745–754. doi: 10.1016/j.ijbiomac.2019.03.023
Wang, L., Zhao, X., Xia, X., Zhu, C., Zhang, H., Qin, W., et al. (2019a). Inhibitory effects of antimicrobial peptide JH-3 on Salmonella enterica serovar Typhimurium strain CVCC541 infection-induced inflammatory cytokine release and apoptosis in RAW264.7 cells. Molecules 24, e596. doi: 10.3390/molecules24030596
Wang, G., Zhu, L., Yu, B., Chen, K., Liu, B., Liu, J., et al. (2016). Exopolysaccharide from Trichoderma pseudokoningii induces macrophage activation. Carbohydr. Polym. 149, 112–120. doi: 10.1016/j.carbpol.2016.04.093
Wangsawat, N., Nahar, L., Sarker, S. D., Phosri, C., Evans, A. R., Whalley, A. J. S., et al. (2021). Antioxidant activity and cytotoxicity against cancer cell lines of the extracts from novel Xylaria species associated with termite nests and LC-MS analysis. Antioxidants 10, e1557. doi: 10.3390/antiox10101557
Weiss, G. and Schaible, U. E. (2015). Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 264, 182–203. doi: 10.1111/imr.12266
Winter, S. E., Thiennimitr, P., Nuccio, S., Haneda, T., Winter, M. G., Wilson, R. P., et al. (2009). Contribution of flagellin pattern recognition to intestinal inflammation during Salmonella enterica serotype typhimurium infection. Infect. Immun. 77, 1904–1916. doi: 10.1128/iai.01341-08
Winter, S. E., Thiennimitr, P., Winter, M. G., Butler, B. P., Huseby, D. L., Crawford, R. W., et al. (2010). Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467, 426–429. doi: 10.1038/nature09415
Winter, S. E., Winter, M. G., Xavier, M. N., Thiennimitr, P., Poon, V., Keestra, A. M., et al. (2013). Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339, 708–711. doi: 10.1126/science.1232467
Won, D. P., Lee, J. S., Kwon, D. S., Lee, K. E., Shin, W. C., and Hong, E. K. (2011). Immunostimulating activity by polysaccharides isolated from fruiting body of Inonotus obliquus. Mol. Cells 31, 165–174. doi: 10.1007/s10059-011-0022-x
Wu, J., Kaewnarin, K., Nie, X., Li, Q., He, N., Huang, J., et al. (2021). Biological activities of a polysaccharide from the coculture of Ganoderma lucidum and Flammulina velutipes mycelia in submerged fermentation. Process. Biochem. 109, 10–18. doi: 10.1016/j.procbio.2021.06.017
Wu, J., Li, M., Liu, L., An, Q., Zhang, J., Zhang, J., et al. (2013). Nitric oxide and interleukins are involved in cell proliferation of RAW264.7 macrophages activated by viili exopolysaccharides. Inflammation 36, 954–961. doi: 10.1007/s10753-013-9626-y
Wu, C. and Liang, Z. (2018). Characteristics of exopolysaccharides from the citrine oyster mushroom, Pleurotus citrinopileatus (Agaricomycetes), depend on the nitrogen source in the medium. Int. J. Med. Mushrooms. 20, 647–655. doi: 10.1615/intjmedmushrooms.2018026571
Wu, G., Lu, C., Jiang, J., Li, Z., and Huang, Z. (2014). Regulation effect of polysaccharides from Pleurotus tuber-regium (Fr.) on the immune activity of mice macrophages. Food Funct. 5, 337–344. doi: 10.1039/c3fo60410a
Xavier, M. N., Winter, M. G., Spees, A. M., Nguyen, K., Atluri, V. L., Silva, T. M. A., et al. (2013). CD4+ T cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PloS Pathog. 9, e1003454. doi: 10.1371/journal.ppat.1003454
Xiang, P., Chen, T., Mou, Y., Wu, H., Xie, P., Lu, G., et al. (2015). NZ suppresses TLR4/NF-κB signalings and NLRP3 inflammasome activation in LPS-induced RAW264.7 macrophages. Inflamm. Res. 64, 799–808. doi: 10.1007/s00011-015-0863-4
Xu, X., Hu, Y., and Quan, L. (2014). Production of bioactive polysaccharides by Inonotus obliquus under submerged fermentation supplemented with lignocellulosic biomass and their antioxidant activity. Bioprocess. Biosyst. Eng. 37, 2483–2492. doi: 10.1007/s00449-014-1226-1
Xu, L., Wang, Q., Wang, G., and Wu, J. (2015). Contents and antioxidant activities of polysaccharides in 14 wild mushroom species from the forest of northeastern China. Int. J. Med. Mushrooms. 17, 1161–1170. doi: 10.1615/intjmedmushrooms.v17.i12.60
Xu, X., Wu, P., Wang, T., Yan, L., Lin, M., and Chen, C. (2019b). Synergistic effects of surfactant-assisted biodegradation of wheat straw and production of polysaccharides by Inonotus obliquus under submerged fermentation. Bioresour. Technol. 278, 43–50. doi: 10.1016/j.biortech.2019.01.022
Xu, X., Yan, H., and Zhang, X. (2012). Structure and immuno-stimulating activities of a new heteropolysaccharide from Lentinula edodes. J. Agric. Food Chem. 60, 11560–11566. doi: 10.1021/jf304364c
Xu, Y., Zhang, X., Yan, X., Zhang, J., Wang, L., Xue, H., et al. (2019a). Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 135, 706–716. doi: 10.1016/j.ijbiomac.2019.05.166
Yamamoto, K., Mizobuchi, H., Yamashita, M., Inagawa, H., Kohchi, C., and Soma, G. (2020). Attempt to construct an in vitro model of enhancement of macrophage phagocytosis via continuous administration of LPS. Anticancer Res. 40, 4711–4717. doi: 10.21873/anticanres.14472
Yan, J., Li, L., Wang, Z., and Wu, J. (2010). Structural elucidation of an exopolysaccharide from mycelial fermentation of a Tolypocladium sp. fungus isolated from wild Cordyceps sinensis. Carbohydr. Polym. 79, 125–130. doi: 10.1016/j.carbpol.2009.07.047
Yang, X., Wang, S., Chen, L., and Zhu, Z. (2023). Isolation and structural characterization of exopolysaccharide from the Cordyceps cicadae and the immunomodulatory activity on RAW264.7 cells. Biotechnol. Appl. Biochem. 70, 1925–1940. doi: 10.1002/bab.2500
Yelithao, K., Surayot, U., Lee, C., Palanisamy, S., Prabhu, N. M., Lee, J., et al. (2019). Studies on structural properties and immune-enhancing activities of glycomannans from Schizophyllum commune. Carbohydr. Polym. 218, 37–45. doi: 10.1016/j.carbpol.2019.04.057
Yurnaliza, Y., Jamilah, I., Hartanto, A., and Lutfia, A. (2021). Screening of endophytic fungi from oil palm (Elaeis guineensis) in producing exopolysaccharides. Biodiversitas 22, 1467–1473. doi: 10.13057/biodiv/d220350
Zhang, W., Chen, D., Chen, J., Xu, W., Chen, Q., Wu, H., et al. (2023). D-allulose, a versatile rare sugar: recent biotechnological advances and challenges. Crit. Rev. Food Sci. Nutr. 63, 5661–5679. doi: 10.1080/10408398.2021.2023091
Zhang, W., Li, J., Qiu, S., Chen, J., and Zheng, Y. (2008). Effects of the exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis on immunocytes of H22 tumor bearing mice. Fitoterapia 79, 168–173. doi: 10.1016/j.fitote.2007.09.001
Zhang, Z., Lv, G., Song, T., Jin, Q., Huang, J., Fan, L., et al. (2015). Comparison of the preliminary characterizations and antioxidant properties of polysaccharides obtained from Phellinus baumii growth on different culture substrates. Carbohydr. Polym. 132, 397–399. doi: 10.1016/j.carbpol.2015.06.006
Zhang, Z., Shi, M., Zheng, H., Ren, R., Zhang, S., and Ma, X. (2022). Structural characterization and biological activities of a new polysaccharide isolated from Morchella sextelata. Glycoconj. J. 39, 369–380. doi: 10.1007/s10719-022-10058-8
Zhang, H., Wang, X., Li, R., Sun, X., Sun, S., Li, Q., et al. (2017). Preparation and bioactivity of exopolysaccharide from an endophytic fungus Chaetomium sp. of the medicinal plant Gynostemma pentaphylla. Pharmacogn. Mag. 13, e477. doi: 10.4103/0973-1296.211033
Zhao, H., Li, J., Zhang, J., Wang, X., Hao, L., and Jia, L. (2017). Purification, in vitro antioxidant and in vivo anti-aging activities of exopolysaccharides by Agrocybe cylindracea. Int. J. Biol. Macromol. 102, 351–357. doi: 10.1016/j.ijbiomac.2017.04.039
Zheng, Q., He, B., Wang, J., Huang, S., Zou, Y., Wei, T., et al. (2020). Structural analysis and antioxidant activity of extracellular polysaccharides extracted from culinary-medicinal white jelly mushroom Tremella fuciformis (Tremellomycetes) conidium cells. Int. J. Med. Mushrooms. 22, 489–500. doi: 10.1615/intjmedmushrooms.2020034764
Zheng, J., Wang, J., Shi, C., Mao, D., He, P., and Xu, C. (2014). Characterization and antioxidant activity for exopolysaccharide from submerged culture of Boletus aereus. Process. Biochem. 49, 1047–1053. doi: 10.1016/j.procbio.2014.03.009
Zhong, X., Wang, G., Li, F., Fang, S., Zhou, S., Ishiwata, A., et al. (2023). Immunomodulatory effect and biological significance of β-glucans. Pharmaceutics 15, e1615. doi: 10.3390/pharmaceutics15061615
Keywords: antioxidant potential, fungal exopolysaccharide, immunomodulatory, liquid cultivation, mushroom mycelium, MDR Salmonella
Citation: Chotmanee T, Suwannarach N, Kumla J, Phongphisutthinant R, Chaipoot S, Wiriyacharee P, Tantibhadrasapa A, Li S, Thiennimitr P and Lumyong S (2025) Exopolysaccharide production by seven basidiomycetous fungi and their antioxidant and immunomodulatory activities against Salmonella infection. Front. Cell. Infect. Microbiol. 15:1610403. doi: 10.3389/fcimb.2025.1610403
Received: 11 April 2025; Accepted: 26 May 2025;
Published: 16 June 2025.
Edited by:
Steven L. Stephenson, University of Arkansas, United StatesReviewed by:
Prashant R. Desai, University of Wisconsin-Madison, United StatesAtif Khurshid Wani, Lovely Professional University, India
Copyright © 2025 Chotmanee, Suwannarach, Kumla, Phongphisutthinant, Chaipoot, Wiriyacharee, Tantibhadrasapa, Li, Thiennimitr and Lumyong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saisamorn Lumyong, c2Nib2kwMDlAZ21haWwuY29t; Parameth Thiennimitr, cGFyYW1ldGgudEBjbXUuYWMudGg=
 Thanawut Chotmanee
Thanawut Chotmanee Nakarin Suwannarach
Nakarin Suwannarach Jaturong Kumla
Jaturong Kumla Rewat Phongphisutthinant3,5
Rewat Phongphisutthinant3,5 Arishabhas Tantibhadrasapa
Arishabhas Tantibhadrasapa Songbo Li
Songbo Li Parameth Thiennimitr
Parameth Thiennimitr Saisamorn Lumyong
Saisamorn Lumyong