- 1Department of Emergency medical center, The First Affiliated Hospital of University of Science and Technology of China (Anhui Provincial Hospital), Hefei, Anhui, China
- 2Department of Emergency Medicine, The Third People’s Hospital of Hefei, Hefei, Anhui, China
- 3Intensive Care Unit (ICU), The Third Affiliated Hospital of Anhui Medical University (The First People’s Hospital of Hefei), Hefei, Anhui, China
- 4Department of Emergency Medicine, Suzhou Hospital of Anhui Medical University (Suzhou Municipal Hospital of Anhui Province), Suzhou, Anhui, China
Objective: To assess the diagnostic performance and clinical utility of metagenomic next-generation sequencing (mNGS) in patients with suspected central nervous system (CNS) infections.
Methods: prospective study was conducted from December 2019 to January 2024, enrolling 110 patients with suspected CNS infections. Cerebrospinal fluid (CSF) samples were subjected to mNGS, conventional biochemistry, and culture. Clinical features and outcomes were compared between patients confirmed with CNS infections and those without.
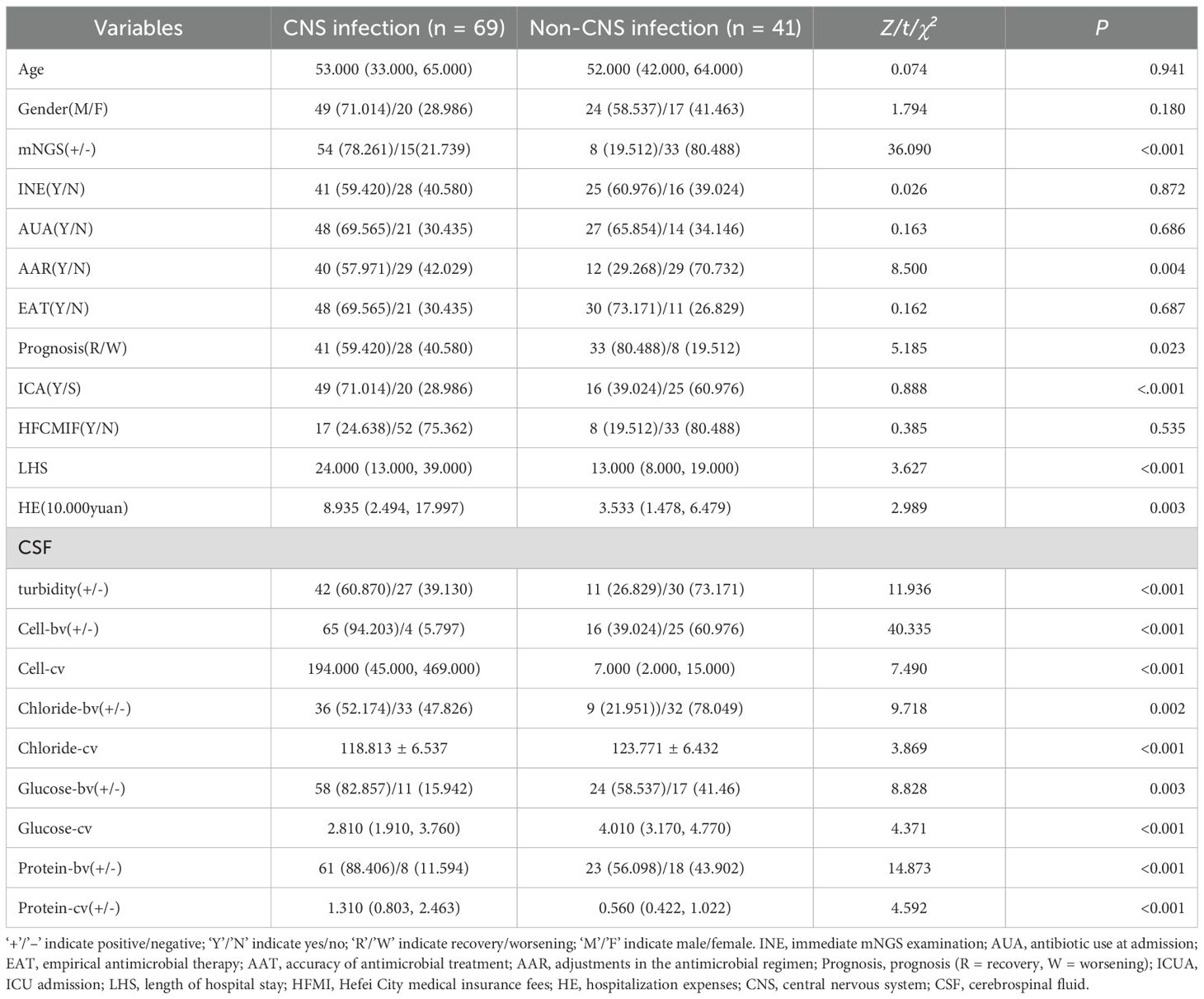
Results: Of the enrolled patients, 69 were diagnosed with CNS infections. mNGS identified pathogens in 62 cases (77.11%), including 54 clinically confirmed true positives (49.09%), significantly surpassing traditional CSF culture (6.36%). mNGS reported results within 24 hours, considerably shorter than the 72~120 hours required for culture. Compared to the non-infection group, patients with CNS infections had significantly higher ICU admission(ICUA) rates, prolonged hospital stays, increased healthcare costs, and elevated rates of antibiotic adjustment and mNGS positivity (P<0.05). CSF turbidity, cell count, and protein levels were significantly elevated, while glucose and chloride levels were reduced. Logistic regression identified mNGS, CSF protein, and glucose levels as independent predictors of CNS infection. Receiver operating characteristic (ROC) analysis demonstrated superior diagnostic accuracy for continuous CSF variables over binary ones, with mNGS showing robust performance [area under the curve (AUC) = 0.794].
Conclusion: mNGS offers rapid and accurate pathogen detection, outperforming conventional methods in sensitivity and turnaround time, and provides valuable guidance for individualized antimicrobial treatment in CNS infections.
1 Introduction
Sepsis is a common condition that is associated with unacceptably high mortality, according to the global burden of disease analysis, sepsis remains a life-threatening clinical syndrome with an alarming case-fatality rate of 26.7%, accounting for 19.7% of global mortality, predominantly driven by preventable infections in low-resource settings (Cecconi et al., 2018; Fleischmann-Struzek et al., 2020; Rudd et al., 2020). Although CNS infections account for a smaller proportion of sepsis cases, they are among its important causes. When CNS infections progress to sepsis or septic shock, patient prognosis worsens significantly, including increased mortality and longer hospital stays. These risks highlight the critical need for early and accurate pathogen detection in CNS infections (Hernandez Ortiz et al., 2018; Zhou et al., 2022). Conventional diagnostic methods, such as body fluid cultures and molecular assays, have limitations that frequently lead to diagnostic errors and delays (Filkins et al., 2020). mNGS, an emerging diagnostic tool, enables unbiased pathogen detection—including bacteria, viruses, fungi, and parasites—thus offering a rapid and accurate diagnostic approach. However, the current absence of large-scale, multicenter clinical trials results in heterogeneity of interpretation criteria and insufficient evidence-based support for routine clinical practice (Zhang et al., 2020). Consequently, this retrospective study analyzed suspected CNS infection cases in our province, aiming to evaluate the diagnostic value of mNGS in CNS infections and provide evidence to optimize the clinical management of these patients. To evaluate the diagnostic value of systemic infectious diseases and provide evidence for optimizing clinical management of these patients.
2 Materials and methods
2.1 Date collection
This study included 135 specimens collected between December 2019 to January 2024 in the Anhui Provincial Hospital. Ultimately, 110 patients were selected. All patient data were independently collected by two doctors and reviewed by a chief physician. The baseline data consist of age, gender, cerebrospinal fluid cell count, albumin, chloride, glucose levels, cerebrospinal fluid culture, and other pertinent indicators. This encompasses immediate mNGS examination(INE), antibiotic use at admission(AUA), empirical antimicrobial therapy(EAT), the accuracy of antimicrobial treatment(AAT), adjustments in the antimicrobial regimen(AAR), prognosis (recovery/worsening), ICUA, length of hospital stay(LHS), Hefei City medical insurance fees(HFMI), and hospitalization expenses(HE) extracted from the Donghua Electronic System.
2.2 Inclusion and exclusion criteria
The inclusion criteria for this study are as follows:( (1) Clinical suspicion of intracranial infection, defined by a sustained body temperature above 38°C for more than 48 hours, the presence of meningeal signs or other suspicious clinical manifestations, and abnormal CSF test results (2); History of invasive procedures: Patients who have undergone invasive procedures such as neurosurgery, lumbar puncture, or ventricular drainage in their medical history. Exclusion criteria include (1): The diagnosis opinions of the three chief doctors are inconsistent (2); Incomplete data (3). Patients with clinically confirmed intracranial infection or a clear history of central nervous system infections within the past 6 months. All patients participating in the study, as well as their families, have been fully informed about the testing procedures and have provided informed consent (Figure 1).
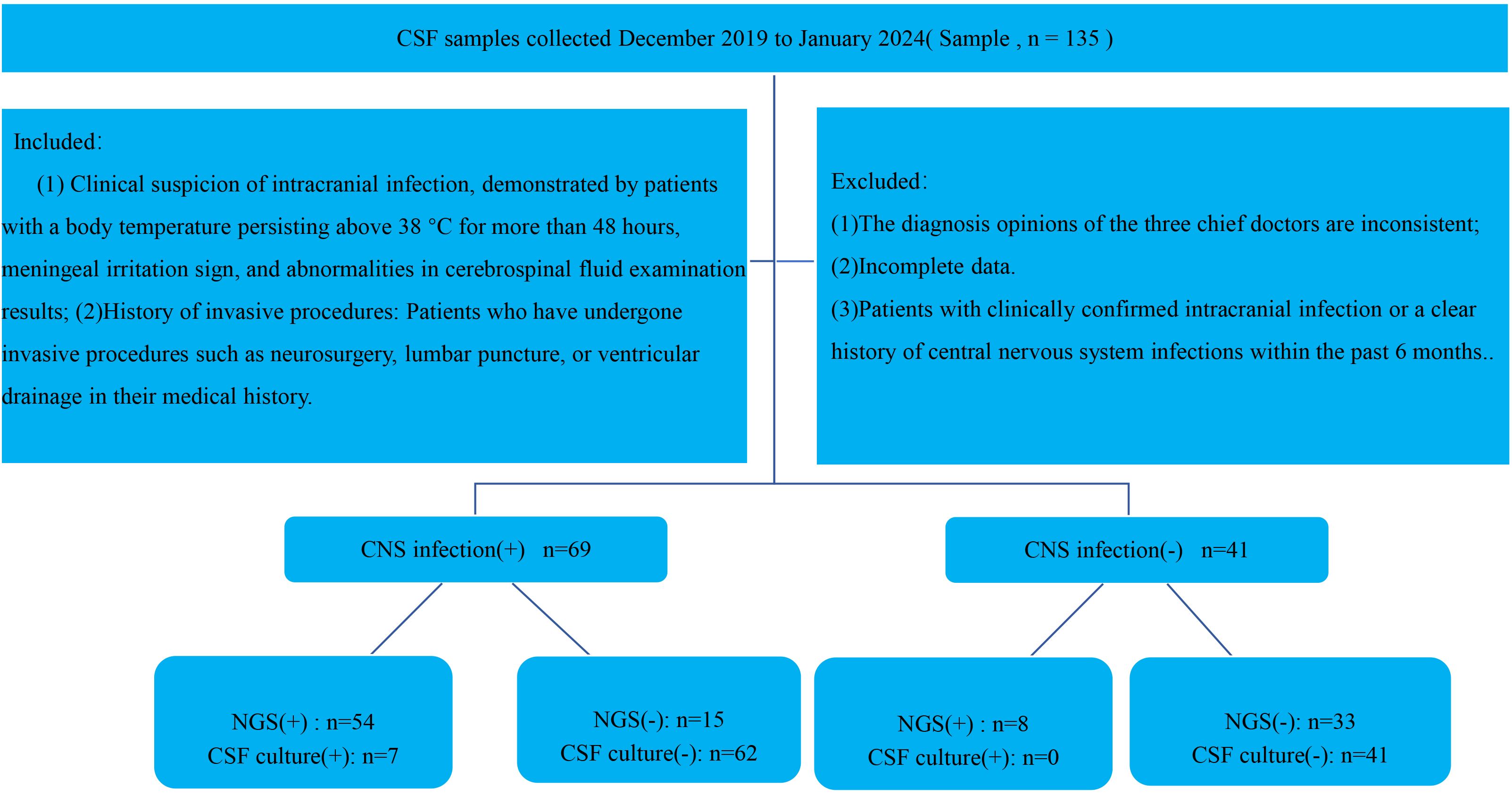
Figure 1. Flowchart illustrating patient enrollment, diagnostic grouping, and metagenomic next-generation sequencing (mNGS) results for suspected central nervous system (CNS) infections.
2.3 Diagnostic criteria of CNS infection
Clinical Manifestations:1. The patient exhibits clinical signs and symptoms, including changes in consciousness and mental state, increased symptoms and signs of intracranial pressure, positive meningeal irritation signs, and focal symptoms resulting from intracranial inflammatory reactions. Systemic infection symptoms may include abnormal body temperature (>38°C or <36°C), increased white blood cell count, elevated heart rate, and altered breathing;2 (Tunkel et al., 2017 2017; McGill et al., 2016; Chinese Neurosurgical Society et al., 2021; Long et al., 2016). Imaging Findings: Inflammatory changes in brain tissue are observed on head CT and/or MRI scans, indicating the presence of intracranial inflammation;3. CSF Testing: Abnormalities in biochemical and routine CSF examinations include changes in appearance, elevated white blood cell counts (>10×106/L), reduced glucose levels (<2.60 mmol/L), or a CSF-to-plasma glucose ratio of <2/3. Additionally, a CSF protein level exceeding 0.6 g/L is considered abnormal;4. For cases with atypical symptoms and signs, a comprehensive diagnosis is reached through the review and analysis of clinical data by three experts from ICU, neurosurgery, and neurology. The diagnosis takes into account the disease’s progression and laboratory examination results.
2.4 Research methods
2.4.1 CSF detection
Routine and mNGS Testing for Intracranial Infections: The following procedures were performed for routine and mNGS testing: a total of 3~5 ml of CSF was collected via lumbar puncture. Various tests were subsequently conducted, including routine analysis, biochemical analysis, bacterial smear, and culture. For the culture, 3 ml of CSF was either directly sent for testing or inoculated into aerobic blood culture bottles, which were then transported to the laboratory within 2 hours at room temperature.
2.4.2 DNA extraction, library preparation, and mNGS of CSF
1.5–3 ml CSF sample was collected from each patient by lumbar puncture in accordance with standard procedures. A 1.5 ml microcentrifuge tube with 0.6 ml sample and 250 μl, 0.5 mm glass bead were attached to a horizontal platform on a vortex mixer and agitated vigorously at 2800–3200 rpm for 30 min (O’Leary et al., 2016). Then 7.2 μl lysozyme was added for wall-breaking reaction. 0.3 ml sample was separated into a new 1.5 ml microcentrifuge tube and DNA was extracted using the TIANamp Micro DNA Kit (DP316, Tiangen Biotech) according to the manufacturer’ s recommendation. Nucleic acids RNA extracted from the clinical samples using the TIANamp Micro RNA Kit (DP431, Tiangen Biotech, Beijing, China) in accordance with the manufacturer’s standard protocols. The reverse transcription reaction was performed to generate single-strand cDNA, followed by the synthesis of double-strand cDNA using the PMseq™ RNA Infection Pathogen High-throughput Detection Kit according to the manufacturer’s instructions. Then, DNA libraries were constructed by enzymatic fragmentation for 20 minutes at 37°C, followed by end repair, adapter ligation, and PCR amplification using the PMseq™ RNA Infection Pathogen High-throughput Detection Kit (BGI-Shenzhen, China), according to the manufacturer’s instructions. Each library was uniquely barcoded to distinguish individual patient samples. The resulting libraries were purified using magnetic beads and assessed for quality using an Agilent 2100 Bioanalyzer. Quality-approved libraries were pooled in equimolar amounts, converted into DNA nanoballs (DNBs), and sequenced on the BGISEQ-50/MGISEQ-2000 platform (MGI Tech Co., Ltd, China). A negative control was included in each sequencing run to monitor for potential contamination. Sequencing runs typically included 10 to 20 samples per batch to ensure efficient throughput while minimizing the risk of cross-contamination.
2.4.3 Interpretation of mNGS data
Raw sequence data were first filtered by removing common background microorganisms and low-quality reads. Then the filtered sequences were mapped to the human reference database (hg38) using Burrows-Wheeler alignment to computationally subtract the human sequence. The remaining data were further classified by simultaneously alignment to Pathogens Metagenomics Database (Refseq). The classification reference databases were downloaded from the National Center Biotechnology Information (NCBI) (ftp://ftp.ncbi.nlm.nih.gov/genomes/). RefSeq contains 4945 whole genome sequence of viral taxa, 6350 bacterial genomes or scaffolds, 1064 fungi and 234 parasites related to human infections (Li and Durbin, 2009; O’Leary et al., 2016; Miao et al., 2018).
2.4.4 Criteria for a positive mNGS result
The criteria for positive results (Li and Durbin, 2009; Infectious Diseases and Cerebrospinal Fluid Cytology Group and Neurology Branch of Chinese Medical Association, 2021; Zhang S. et al., 2022) were as follows: 1) mNGS identified bacteria (mycobacteria and nocardia excluded), virus and parasites when the coverage rate was 10-fold greater than that of any other microorganisms. 2) mNGS identified Mycobacterium tuberculosis when the genus-specific read number ≥ 1. 3) mNGS identified nontuberculous mycobacteria and nocardia when the mapping read number (genus or species level) was in the top 10 in the bacteria list. 4) mNGS identified fungi when the coverage rate was 5-fold greater than that of any other microorganisms.
2.5 Statistical analysis methods
Data processing and analysis were performed using SPSS 22.0 statistical software. The analysis methods used were as follows: continuous variables(CV) with a normal distribution were presented as mean ± standard deviation (SD), while those with non-normal distribution were expressed as M (P25 and P75). Binary variables(BV) were reported as numbers (percentage). Variable comparisons were conducted using appropriate statistical tests, including the t-test, the non-parametric Mann–Whitney U test, or the χ2 test. The diagnostic accuracy of clinical features and laboratory characteristics was assessed and compared using the AUC of ROC. Data analysis was performed using SPSS 22.0 and Medcala 18.11.3 software. Statistical significance was set at P < 0.05. All Figures were created using the R programming language.
3 Result
3.1 Patient baseline characteristics
A total of 135 patients were enrolled, and 110 patients were ultimately included in the study, comprising 73males and 37 females. Based on the final diagnosis, patients were divided into two groups: 69 patients with CNS infections (observation group) and 41 patients without CNS infections (control group). Pathogens were identified in 7 patients through routine culture and other diagnostic methods, while mNGS detected pathogens in 62 patients.
In the CNS infection patients, the ICU, LHS, HE, AAR, mNGS positivity rate, and the proportion of CSF with cloudy appearance, as well as CSF cell count and albumin levels, were all significantly higher than those in non-CNS infection patients; In contrast, the prognosis improvement rate and CSF chloride and glucose levels were lower (P<0.05) (Table 1).
3.2 Correlation and concordance between mNGS and CSF culture
Among the 110 CSF culture samples, only 7 were positive (6.36%), including Klebsiella pneumoniae (1 case), Acinetobacter baumannii (2 cases), Escherichia coli (1 case), Staphylococcus aureus (1 case), Pseudomonas aeruginosa (1 case), and Candida parapsilosis (1 case). No sample cultured multiple pathogens. In contrast, mNGS identified 80 pathogens in 62 positive samples, with 6 samples containing two or more pathogens. The most common pathogens identified were Klebsiella pneumoniae (4 cases), Acinetobacter baumannii (8 cases), Human herpesvirus 5 (4 cases), and Human herpesvirus 4 (11 cases) (see Figure 2). Furthermore, the average turnaround time for pathogen identification by mNGS was approximately 24 hours, whereas the CSF culture had a turnaround time of 72 to 120 hours.
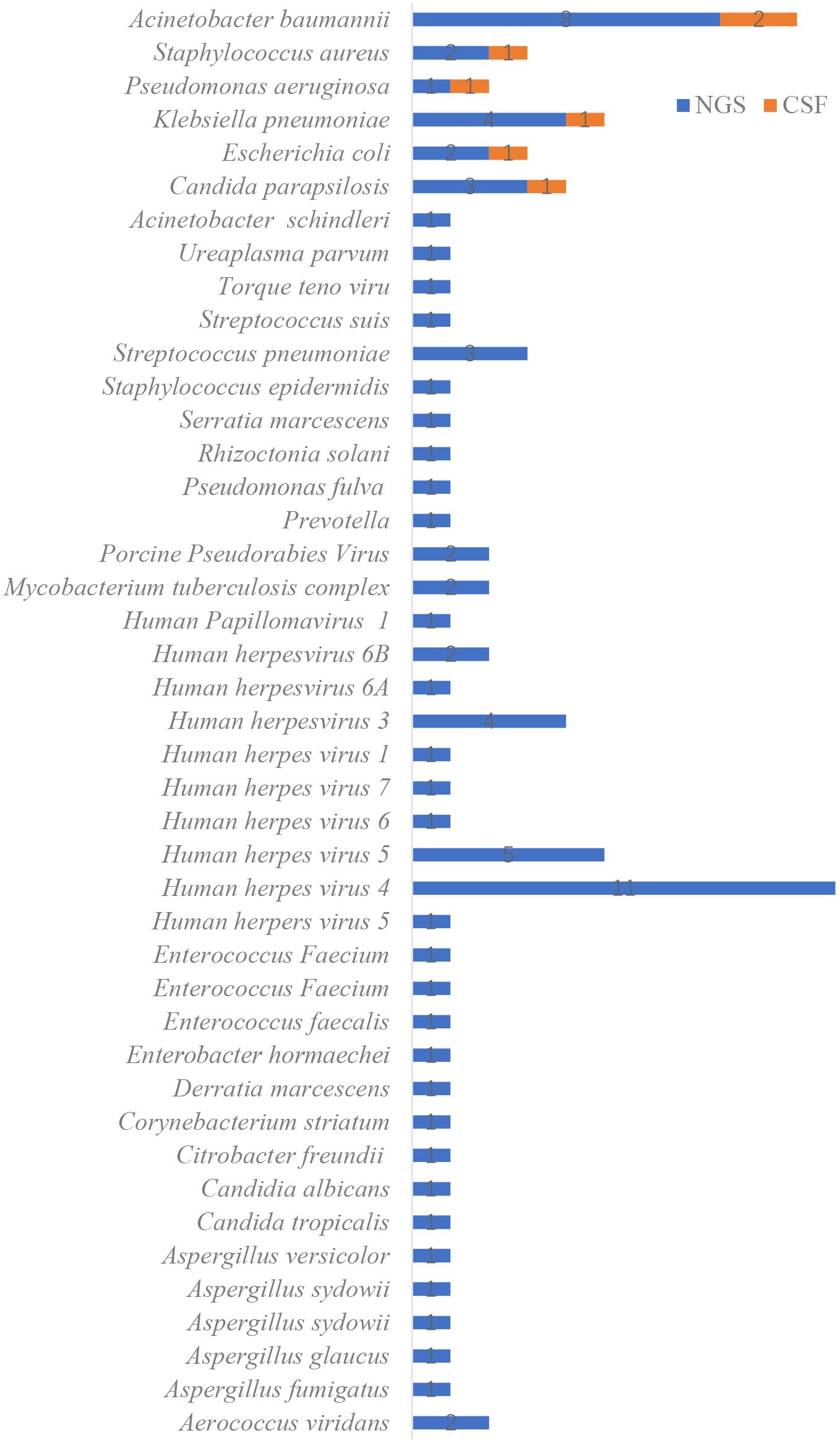
Figure 2. Distribution of pathogens identified by metagenomic next-generation sequencing (mNGS) compared with conventional cerebrospinal fluid (CSF) culture methods.
3.3 Multifactor logistic regression analysis
Logistic regression analysis indicated that mNGS was independently associated with CSF protein, glucose levels, and CNS infections (P<0.05) (Figure 3).
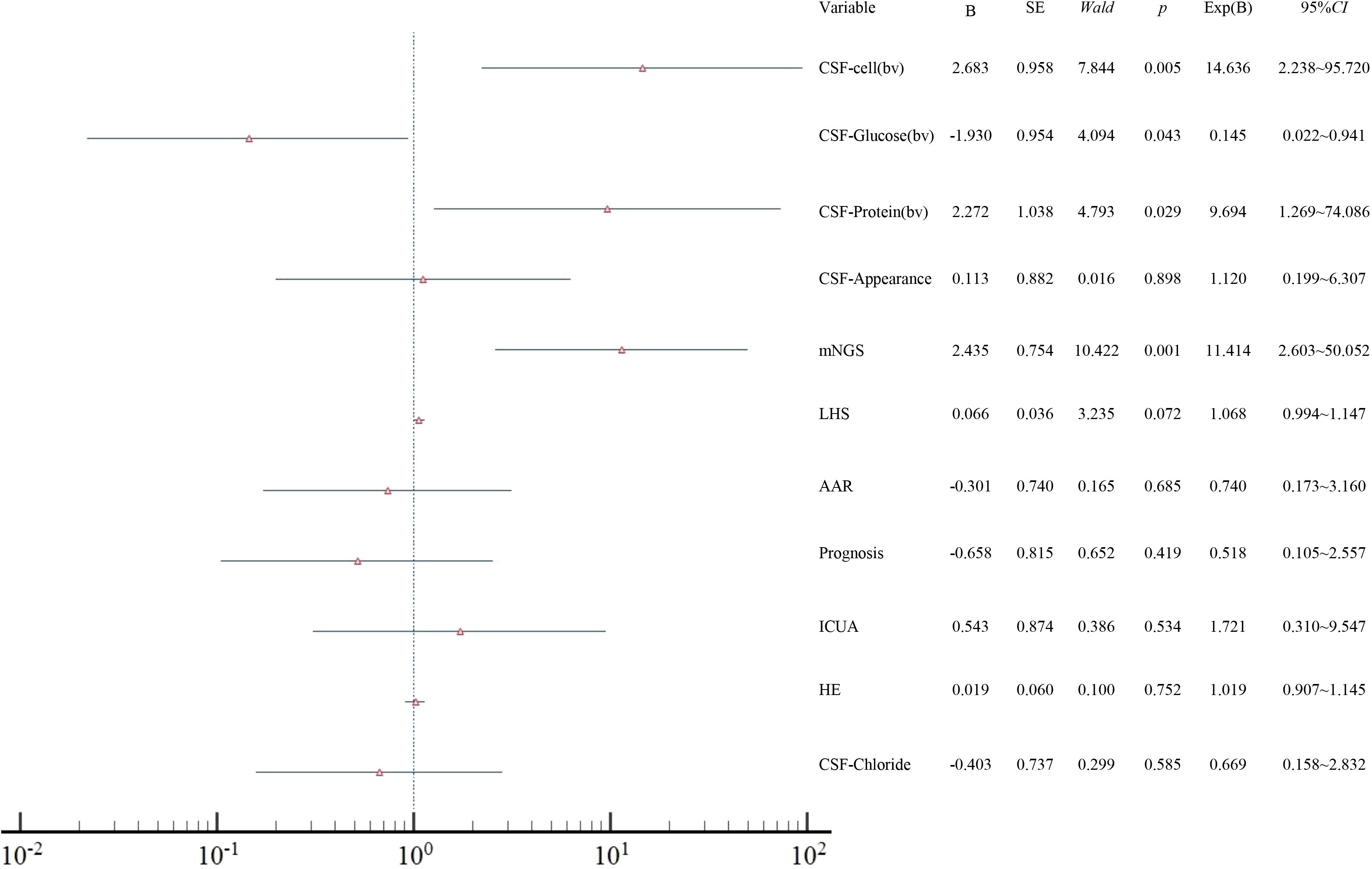
Figure 3. Forest plot of multivariate logistic regression analysis showing independent factors associated with CNS infection. AAR, adjustments in the antimicrobial regimen; ICUA, ICU admission; LHS, length of hospital stay; HE, hospitalization expenses; CSF, cerebrospinal fluid; bv, binary variables.
3.4 Analysis of diagnostic-effectiveness of different indexed
Using the final clinical diagnosis of CNS infection as the reference standard, ROC curves were plotted for mNGS and CSF parameters, including cell count, glucose, and protein. Each variable was assessed in both binary (bv) and continuous (cv) forms (Figure 4). The AUC for mNGS and for CSF cell count, glucose, and protein were 0.794; 0.776 (bv)/0.982 (cv) (P = 0.225, Z = 1.214); 0.628 (bv)/0.750 (cv) (P = 0.005, Z = 2.826); and 0.662 (bv)/0.709 (cv) (P < 0.001, Z = 4.328), respectively (Figure 4). Continuous cell count demonstrated the highest diagnostic accuracy; additionally, when treated as binary variables, both mNGS and CSF cell count showed significantly better diagnostic performance than CSF glucose and protein (P < 0.05).
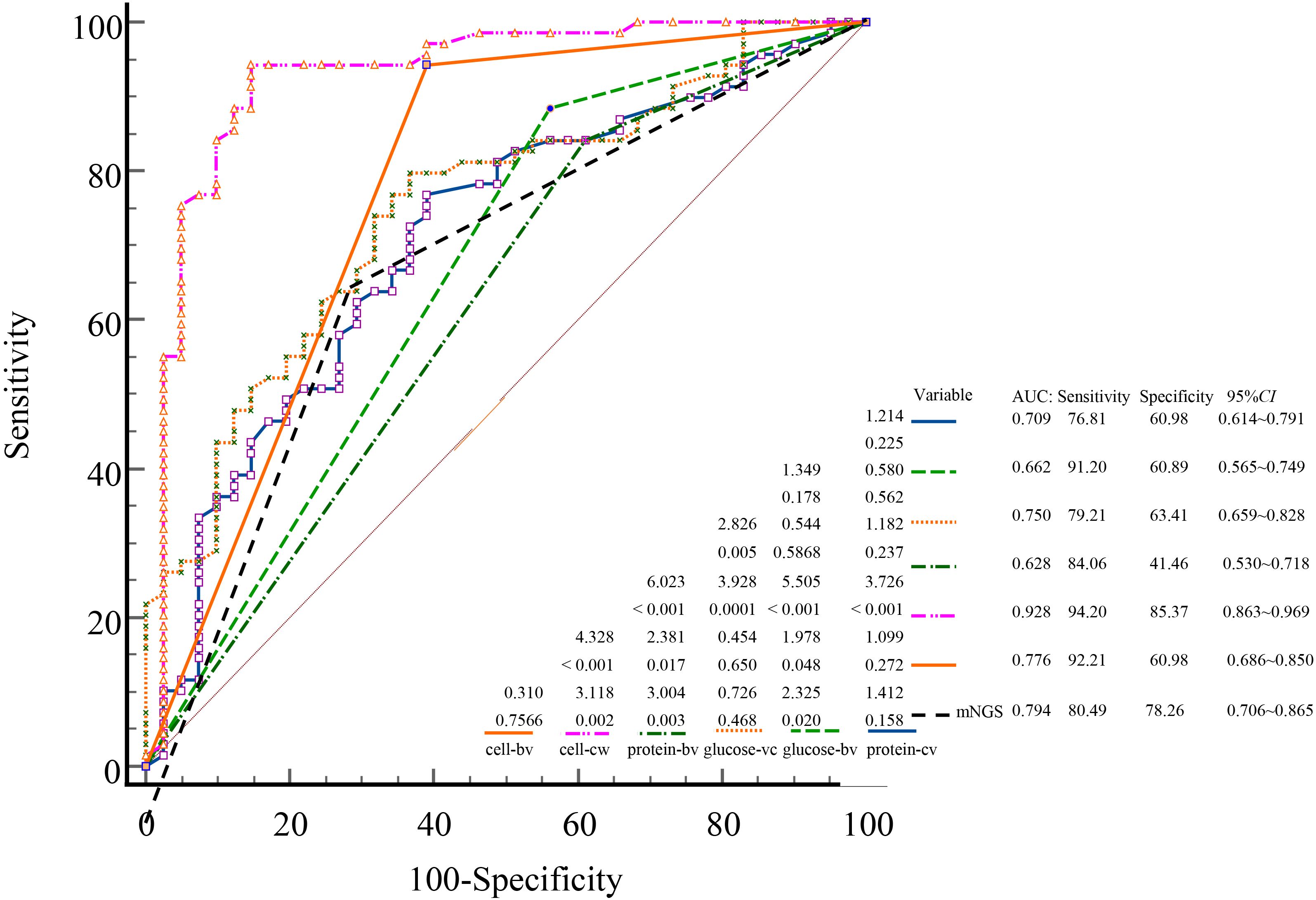
Figure 4. ROC curves comparing the diagnostic performance of mNGS and conventional CSF indicators for CNS infections. ROC, receiver operating characteristic; AUC, area under the curve; mNGS, metagenomic next-generation sequencing; CSF, cerebrospinal fluid; CNS, central nervous system; cv, continuous variables; bv, binary variables.
4 Discussion
CNS infections are a common cause of neurological disease and remain a major clinical challenge (Simner et al., 2018; GBD 2016 Meningitis Collaborators, 2018). Due to limitations in current diagnostic approaches patients often experience delayed treatment, prolonged hospitalization, increased healthcare costs, and even death (Brouwer et al., 2012; Acuña et al., 2022). In our study, patients with CNS infections had significantly longer hospital stays, higher ICU admission rates, and greater medical expenses compared to those without infection (P < 0.05). These differences may be attributed to the nonspecific clinical presentation of CNS infections, delays in etiological diagnosis, and reliance on empiric therapy in the absence of rapid confirmatory testing. These findings further highlight the urgent need for timely and accurate diagnostic strategies to improve clinical outcomes (Posadas and Fisher, 2018; Andrade et al., 2024).
Routine CSF biochemical analysis remains the primary diagnostic method due to its efficiency and cost-effectiveness (Hrishi and Sethuraman, 2019; Singh, 2024). Inflammatory responses from infections alter CSF parameters, including abnormal appearance, glucose, protein levels, and elevated white cell count, making these indicators key references in diagnosing CNS infections (Subspecialty Group of Neurology tSoPCMA, 2019; Zhang et al., 2022). This aligns with our findings, where CNS infection patients showed significantly higher CSF white cell counts, protein levels, and turbidity, and lower glucose levels than non-infection groups (P < 0.05). Logistic regression analysis indicated these parameters independently correlated with CNS infections. Additionally, we observed lower chloride levels in CNS infection patients, potentially due to pathogen-related chloride homeostasis disturbances (Wen et al., 2022), enterovirus-induced diarrhea (Koopmans et al., 2023), antidiuretic hormone dysregulation, or diuretic use (Johanson et al., 1992; Kumar et al., 2021).
In current clinical practice, laboratory indicators are often dichotomized as “normal” or “abnormal,” which may lead to loss of diagnostic detail and clinical nuance (Altman and Royston, 2006; Shinkins and Perera, 2013). In this study, CSF cell count analyzed as a continuous variable yielded a markedly higher AUC (0.982) than its binary counterpart (0.776). Similar patterns were observed for glucose (0.750 vs. 0.628) and protein (0.709 vs. 0.662). These results indicate that continuous variables retain more diagnostic information, allow finer resolution of disease severity, and improve the sensitivity and precision of ROC-based analysis. This highlights the importance of preserving quantitative data in clinical diagnostics rather than relying on simplified threshold-based classification. Future multicenter, prospective studies are warranted to validate these observations.
Although routine CSF biochemical analysis remains a useful tool in the initial assessment of CNS infections, its lack of pathogen specificity limits its diagnostic value. CSF culture, while considered the diagnostic gold standard, suffers from low sensitivity (Mengistu et al., 2013); in our study, only 6.36% (7/110) of cultures were positive, each identifying a single pathogen. This low yield may result from sampling challenges, prior antibiotic use, or low microbial load (Calderaro et al., 2016; Pope et al., 2023; Su et al., 2024; Wang et al., 2024). In contrast, mNGS is less affected by these limitations and has emerged as a valuable diagnostic tool owing to its high throughput, broad-spectrum pathogen detection, and rapid turnaround time (Wilson et al., 2019; Lin et al., 2024; Streithorst et al., 2024). In this study, mNGS achieved a significantly higher overall detection rate (77.11%), with 54 cases confirmed as true positives (49.09%), markedly outperforming conventional diagnostic methods. The mNGS positivity rate and the proportion of patients requiring antibiotic adjustment were both significantly higher in the CNS-infected group compared to the non-CNS group (P < 0.05), underscoring the clinical value of mNGS in distinguishing infectious from non-infectious etiologies and in guiding individualized antimicrobial therapy. ROC curve analysis further confirmed the diagnostic performance of mNGS, yielding an AUC of 0.794—second only to CSF cell count analyzed as a continuous variable. This result is consistent with previous reports, which documented AUC values for mNGS ranging from 0.659 to 0.846 depending on pathogen type (Xing et al., 2020; Yuan et al., 2024). Notably, the positivity criteria used in our study were based on internationally and domestically recognized expert consensus (Li and Durbin, 2009; O’Leary et al., 2016; Miao et al., 2018), which has been widely endorsed by clinicians in China, ensuring consistency in interpretation and methodological comparability. Collectively, these findings highlight the importance of mNGS in achieving rapid and accurate pathogen identification, particularly in patients with complex CNS infections.
Despite its high diagnostic yield, mNGS is not without limitations. In our cohort, 15 patients were clinically diagnosed with CNS infection despite negative mNGS results. These patients exhibited typical features of CNS infection—such as fever, altered mental status, meningeal signs, CSF pleocytosis, and hypoglycorrhachia—and showed clinical improvement following empirical antimicrobial therapy. These false-negative findings may be attributed to low pathogen burden, atypical organisms, prior antibiotic exposure, or limitations in sequencing depth and bioinformatic filtering. Conversely, 8 patients in the non-CNS group yielded low-abundance mNGS-positive results for background or latent organisms such as Torque teno virus and Human herpesvirus 4 virus. None of these patients had clinical symptoms or laboratory evidence suggestive of CNS infection, and all were ultimately diagnosed with non-infectious etiologies. These cases underscore the necessity of interpreting mNGS results in conjunction with clinical context to avoid overdiagnosis due to contaminants or colonizing organisms. Viral CNS infections further complicate the diagnostic landscape. Conventional methods such as PCR and serology are highly pathogen-specific and prone to false negatives or missed diagnoses (Agut et al., 2016; Khan et al., 2024; Marvik and Dudman, 2024). In clinical practice, CSF volume limitations often restrict parallel molecular testing. Unlike these targeted assays, mNGS offers broad-spectrum detection without prior assumptions, enabling the identification of unexpected or coexisting viral pathogens, including mixed infections.
Taken together, mNGS should be regarded as a powerful adjunct rather than a stand-alone tool in the diagnostic workup of CNS infections, especially in culture-negative or diagnostically uncertain cases where conventional methods fall short. However, the interpretation of negative or low-abundance results requires careful clinical correlation. Therefore, further prospective, multicenter studies are warranted to refine mNGS-based pathogen detection strategies and to guide individualized antimicrobial and antiviral decision-making.
4.1 Limitations
First, the single-center design and relatively modest sample size may limit the generalizability of our findings. Larger, multicenter studies are warranted to validate the diagnostic performance and clinical utility of mNGS across diverse patient populations. Second, due to the retrospective nature of data extraction from the hospital information system, certain clinical variables—such as detailed antibiotic history, time interval between symptom onset and sampling, and severity scoring—were inconsistently recorded or unavailable, potentially affecting the interpretation of mNGS results. Third, although mNGS demonstrated a higher detection rate compared to conventional methods, this study lacked comprehensive comparison with pathogen-targeted PCR and other molecular diagnostic tools, which may provide additional diagnostic context for clinical decision-making. Fourth, limited CSF volume precluded complete virological serology and parallel validation testing in some cases, particularly for patients with suspected viral or mixed infections. Finally, this study primarily focused on the diagnostic value of mNGS; future investigations should evaluate the impact of mNGS-guided antimicrobial therapy on clinical outcomes and cost-effectiveness in patients with central nervous system infections.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Although this retrospective study involving human participants required an ethical exemption under Chinese legal and institutional policies, all procedures were conducted in compliance with the ethical waiver (Approval Code: 20240409111200027209) granted by the Ethics Committee of the First Affiliated Hospital of USTC (Anhui Provincial Hospital). Patients or their legal guardians were informed about the research during their hospitalization, and written informed consent was obtained from all participants. Separate consent for publishing potentially identifiable information was waived as no such data (e.g., facial features, genetic sequences, or medical images) were included in the study.
Author contributions
X-gC: Writing – original draft, Writing – review & editing. X-fZ: Writing – original draft, Writing – review & editing. ZY: Investigation, Methodology, Writing – review & editing. C-yW: Investigation, Methodology, Writing – review & editing. C-jH: Writing – review & editing, Software, Investigation, Conceptualization. MS: Data curation, Methodology, Writing – review & editing, Formal analysis. H-dM: Formal analysis, Visualization, Resources, Data curation, Validation, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acuña, M., Benadof, D., Yohannessen, K., Leiva, Y., and Clement, P. (2022). FilmArray® Meningoencephalitis panel in the diagnosis of central nervous system infections: stewardship and cost analysis in a paediatric hospital in Chile. BMC Pediatr. 22, 182. doi: 10.1186/s12887-022-03241-1
Agut, H., Bonnafous, P., and Gautheret-Dejean, A. (2016). Human herpesviruses 6A, 6B, and 7. Microbiol. Spectr. 4 (3). doi: 10.1128/microbiolspec.DMIH2-0022-2015
Altman, D. G. and Royston, P. (2006). The cost of dichotomising continuous variables. BMJ. 332, 1080. doi: 10.1136/bmj.332.7549.1080
Andrade, H. B., da Silva, I. R. F., Espinoza, R., Ferreira, M. T., da Silva, M. S. T., Theodoro, P. H. N., et al. (2024). Clinical features, etiologies, and outcomes of central nervous system infections in intensive care: A multicentric retrospective study in a large Brazilian metropolitan area. J. Crit. Care 79, 154451. doi: 10.1016/j.jcrc.2023.154451
Brouwer, M. C., Thwaites, G. E., Tunkel, A. R., and van de Beek, D. (2012). Dilemmas in the diagnosis of acute community-acquired bacterial meningitis. Lancet 380, 1684–1692. doi: 10.1016/S0140-6736(12)61185-4
Calderaro, A., Martinelli, M., Montecchini, S., Motta, F., Covan, S., Larini, S., et al. (2016). Higher recovery rate of microorganisms from cerebrospinal fluid samples by the BACTEC culture system in comparison with agar culture. Diagn. Microbiol. Infect. Dis. 84, 281–286. doi: 10.1016/j.diagmicrobio.2015.12.016
Cecconi, M., Evans, L., Levy, M., and Rhodes, A. (2018). Sepsis and septic shock. Lancet. 392, 75–87. doi: 10.1016/S0140-6736(18)30696-2
Chinese Neurosurgical Society, Neurocritical Care Committee, Beijing Society of Neurosurgery, and Department of Critical Neurosurgery (2021). Chinese expert consensus on the diagnosis and treatment of central nervous system infections in neurosurgery (2021). Chin. J. Neurosurg. 37, 2–15. doi: 10.3760/cma.j.cn112050-20200831-00480
Filkins, L. M., Bryson, A. L., Miller, S. A., and Mitchell, S. L. (2020). Navigating clinical utilization of direct-from-specimen metagenomic pathogen detection: clinical applications, limitations, and testing recommendations. Clin. Chem. 66, 1381–1395. doi: 10.1093/clinchem/hvaa183
Fleischmann-Struzek, C., Mellhammar, L., Rose, N., Cassini, A., Rudd, K. E., Schlattmann, P., et al. (2020). Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 46, 1552–1562. doi: 10.1007/s00134-020-06151-x
GBD 2016 Meningitis Collaborators (2018). Global, regional, and national burden of meningitis, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 1061–1082. doi: 10.1016/S1474-4422(18)30387-9
Hernandez Ortiz, O. H., Garcia Garcia, H. I., Munoz Ramirez, F., Cardona Florez, J. S., Gil Valencia, B. A., Medina Mantilla, S. E., et al. (2018). Development of a prediction rule for diagnosing postoperative meningitis: a cross-sectional study. J. Neurosurg. 128, 262–271. doi: 10.3171/2016.10.JNS16379
Hrishi, A. P. and Sethuraman, M. (2019). Cerebrospinal fluid (CSF) analysis and interpretation in neurocritical care for acute neurological conditions. Indian J. Crit. Care Med. 23, S115–S119. doi: 10.5005/jp-journals-10071-23187
Infectious Diseases and Cerebrospinal Fluid Cytology Group and Neurology Branch of Chinese Medical Association (2021). Expert consensus on the application of metagenomic next-generation sequencing in cerebrospinal fluid for central nervous system infectious diseases. Chin. J. Neurol. 54, 1234–1240. doi: 10.3760/cma.j.cn113694-20210730-00532
Johanson, C. E., Murphy, V. A., and Dyas, M. (1992). Ethacrynic acid and furosemide alter Cl, K, and Na distribution between blood, choroid plexus, CSF, and brain. Neurochem. Res. 17, 1079–1085. doi: 10.1007/BF00967284
Khan, S., Khadri, S., Haque, L. A., Anderson, M., and Witkowska, M. (2024). Seronegative herpes simplex virus (HSV) encephalitis causing temporal lobe epilepsy resulting in new-onset psychosis: a case report and literature review. AME Case Rep. 8, 100. doi: 10.21037/acr-24-69
Koopmans, M. M., Brouwer, M. C., Vázquez-Boland, J. A., and van de Beek, D. (2023). Human listeriosis. Clin. Microbiol. Rev. 36, e0006019. doi: 10.1128/cmr.00060-19
Kumar, A., Singh, J., Hashmat, O., Ameet, P., Budhrani, N., and Sher, K. (2021). Frequency of hyponatremia in patients of tuberculosis bacterial meningitis in a tertiary care hospital. Cureus. 13, e13888. doi: 10.7759/cureus.13888
Li, H. and Durbin, R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 25, 1754–1760. doi: 10.1093/bioinformatics/btp324
Lin, L., Fang, J., Li, J., Tang, Y., Xin, T., Ouyang, N., et al. (2024). Metagenomic next-generation sequencing contributes to the early diagnosis of mixed infections in central nervous system. Mycopathologia. 189, 34. doi: 10.1007/s11046-024-00837-2
Long, Y., Zhang, Y., Gong, Y., Sun, R., Su, L., Lin, X., et al. (2016). Diagnosis of sepsis with cell-free DNA by next-generation sequencing technology in ICU patients. Arch. Med. Res. 47, 365–371. doi: 10.1016/j.arcmed.2016.08.004
Marvik, Å. and Dudman, S. G. (2024). Clinical evaluation of the virClia igM/igG chemiluminescence tests for the diagnosis of tick-borne encephalitis in an endemic part of Norway. Viruses. 16, 1505. doi: 10.3390/v16091505
McGill, F., Griffiths, M. J., Solomon, T., Jones, L., Turtle, L., Marshall, J., et al. (2016). The UK joint specialist societies guideline on the diagnosis and management of acute meningitis and meningococcal sepsis in immunocompetent adults. J. Infect. 72, 405–438. doi: 10.1016/j.jinf.2016.01.007
Mengistu, A., Gaeseb, J., Uaaka, G., Ndjavera, C., Kambyambya, K., Indongo, L., et al. (2013). Antimicrobial sensitivity patterns of cerebrospinal fluid (CSF) isolates in Namibia: implications for empirical antibiotic treatment of meningitis. J. Pharm. Policy Pract. 6, 4. doi: 10.1186/2052-3211-6-4
Miao, Q., Ma, Y., Wang, Q., Pan, J., Zhang, Y., Jin, W., et al. (2018). Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin. Infect. Dis. 67, S231–S240. doi: 10.1093/cid/ciy693
O’Leary, N. A., Wright, M. W., Brister, J. R., Ciufo, S., Haddad, D., McVeigh, R., et al. (2016). Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44, D733–D745. doi: 10.1093/nar/gkv1189
Pope, C. E., Whitlock, K. B., Hodor, P., Limbrick, D. D., Jr, McDonald, P. J., Hauptman, J., et al. (2023). A refined, controlled 16S rRNA gene sequencing approach reveals limited detection of cerebrospinal fluid microbiota in children with bacterial meningitis. Microbiol. Spectr. 11, e0036123. doi: 10.1128/spectrum.00361-23
Posadas, E. and Fisher, J. (2018). Pediatric bacterial meningitis: an update on early identification and management. Pediatr. Emerg. Med. Pract. 15, 1–20.
Rudd, K. E., Johnson, S. C., Agesa, K. M., Shackelford, K. A., Tsoi, D., Kievlan, D. R., et al. (2020). Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 395, 200–211. doi: 10.1016/S0140-6736(19)32989-7
Shinkins, B. and Perera, R. (2013). Diagnostic uncertainty: dichotomies are not the answer. Br. J. Gen. Pract. 63, 122–123. doi: 10.3399/bjgp13X664090
Simner, P. J., Miller, S., and Carroll, K. C. (2018). Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin. Infect. Dis. 66, 778–788. doi: 10.1093/cid/cix881
Singh, N. (2024). Inside the brain: cerebrospinal fluid insights in meningitis. Cureus. 16, e67008. doi: 10.7759/cureus.67008
Streithorst, J., Hillberg, M., Ingebrigtsen, D., Zorn, K., Wilson, M. R., Blicharz, T., et al. (2024). Seven-year performance of a clinical metagenomic next-generation sequencing test for diagnosis of central nervous system infections. Nat. Med. 30, 3522–3533. doi: 10.1038/s41591-024-03275-1
Su, L. D., Chiu, C. Y., Gaston, D., Hogan, C. A., Miller, S., Simon, D. W., et al. (2024). Clinical metagenomic next-generation sequencing for diagnosis of central nervous system infections: advances and challenges. Mol. Diagn. Ther. 28, 513–523. doi: 10.1007/s40291-024-00727-9
Subspecialty Group of Neurology tSoPCMA (2019). Expert consensus on diagnosis and treatment of community acquired bacterial meningitis in children. Zhonghua Er Ke Za Zhi 57, 584–591. doi: 10.3760/cma.j.issn.0578-1310.2019.08.003
Tunkel, A. R., Hasbun, R., Bhimraj, A., Byers, K., Kaplan, S. L., Scheld, W. M., et al. (20172017). Infectious Diseases Society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin. Infect. Dis. 64, e34–e65. doi: 10.1093/cid/ciw861
Wang, X., Guo, X., Liu, H., Wang, B., Wu, J., Chen, S., et al. (2024). Augmented pathogen detection in brain abscess using metagenomic next-generation sequencing: a retrospective cohort study. Microbiol. Spectr. 12, e0032524. doi: 10.1128/spectrum.00325-24
Wen, A., Liu, S. M., Cao, W. F., Zhou, Y. L., Luo, C. Q., Xiang, Z. B., et al. (2022). A new scoring system to differentially diagnose and distinguish tuberculous meningitis and bacterial meningitis in south China. Front. Neurol. 13. doi: 10.3389/fneur.2022.830969
Wilson, M. R., Sample, H. A., Zorn, K. C., Arevalo, S., Yu, G., Neuhaus, J. F., et al. (2019). Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl. J. Med. 380, 2327–2340. doi: 10.1056/NEJMoa1803396
Xing, X. W., Zhang, J. T., Ma, Y. B., He, M. W., Yao, G. E., Wang, W., et al. (2020). Metagenomic next-generation sequencing for diagnosis of infectious encephalitis and meningitis: A large, prospective case series of 213 patients. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00088
Yuan, L., Zhu, X. Y., Lai, L. M., Chen, Q., Liu, Y., and Zhao, R. (2024). Clinical application and evaluation of metagenomic next-generation sequencing in pathogen detection for suspected central nervous system infections. Sci. Rep. 14, 16961. doi: 10.1038/s41598-024-68034-1
Zhang, Y., Cui, P., Zhang, H. C., Wu, H. L., Ye, M. Z., Zhu, Y. M., et al. (2020). Clinical application and evaluation of metagenomic next-generation sequencing in suspected adult central nervous system infection. J. Transl. Med. 18, 199. doi: 10.1186/s12967-020-02360-6
Zhang, S., Wu, G., Shi, Y., Liu, T., Xu, L., Dai, Y., et al. (2022). Understanding etiology of community-acquired central nervous system infections using metagenomic next-generation sequencing. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.979086
Zhang, Y., Zeng, Q., Fang, Y., Wang, W., and Chen, Y. (2022). Risk prediction of central nervous system infection secondary to intraventricular drainage in patients with intracerebral hemorrhage: development and evaluation of a new predictive model nomogram. Ther. Innov. Regul. Sci. 56, 651–658. doi: 10.1007/s43441-022-00403-2
Keywords: CNS infections, mNGS, CSF analysis, diagnostic accuracy, pathogen detection
Citation: Cao X-g, Zhu X-f, Yu Z, Wang C-y, Shao M, Meng H-d and Huang C-j (2025) Diagnostic performance and clinical utility of metagenomic next-generation sequencing in suspected central nervous system infections: a prospective comparative study. Front. Cell. Infect. Microbiol. 15:1612628. doi: 10.3389/fcimb.2025.1612628
Received: 16 April 2025; Accepted: 17 July 2025;
Published: 11 August 2025.
Edited by:
Anupam Jyoti, Parul University, IndiaReviewed by:
Lia Van Der Hoek, University of Amsterdam, NetherlandsHiroshi Takashima, Kagoshima University, Japan
Copyright © 2025 Cao, Zhu, Yu, Wang, Shao, Meng and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua-dong Meng, NDcxOTg5MzI1QHFxLmNvbQ==; Chong-jian Huang, b3Vyc291bEAxMjYuY29t
†These authors share first authorship
 Xiao-guang Cao
Xiao-guang Cao Xiong-feng Zhu2†
Xiong-feng Zhu2† Hua-dong Meng
Hua-dong Meng