- 1Department of Environmental Health Sciences, Faculty of Public Health, College of Medicine, University of Ibadan, Ibadan, Oyo, Nigeria
- 2Nanobios Lab, Department of Biosciences and Bioengineering, Indian Institute of Technology (IIT), Bombay, India
- 3Department of Microbiology, University of Ibadan, Ibadan, Nigeria
- 4Edo State Ministry of Agriculture and Food Security, Benin, Edo, Nigeria
- 5Edo State Ministry of Agriculture and Food Security Animal Ethics Committee, Benin, Nigeria
- 6Institute for Advanced Medical Research and Training (IAMRAT), College of Medicine, University of Ibadan, Ibadan, Nigeria
Multidrug-resistant (MDR) bacterial infections represent a growing global health emergency, driven by interconnected human, animal, and environmental factors. This review adopts a One Health perspective to explore the transmission dynamics, operational integration, and innovative responses to antimicrobial resistance (AMR). Case studies from China, India, Nigeria, Thailand, and Brazil underscore the effectiveness of regulatory reforms, surveillance networks, and public engagement campaigns. Notably, Artificial Intelligence (AI) is transforming MDR management through real-time diagnostics and resistance prediction, though ethical concerns and infrastructure deficits in Low- and Middle-Income Countries (LMICs) remain barriers. Community-led initiatives, gender-sensitive education, and policy reforms are vital to curbing misuse and closing equity gaps. Despite successes, challenges such as fragmented governance, underfunded labs, and limited longitudinal research persist. A proactive, integrated One Health approach—linking clinical, environmental, and policy actions—is essential for reducing MDR burden. Investment in intersectoral surveillance, equitable AI deployment, and community empowerment is imperative for safeguarding antibiotics and ensuring global health resilience.
Introduction
The increasing threat of Multidrug-resistant (MDR) bacterial infections poses a global health emergency, with about 4.95 million deaths recorded annually linked to antimicrobial resistance (AMR) (Murray et al., 2022). This is driven by the interconnected dynamics of human, animal, and environmental health factors (Berendonk et al., 2015; WHO, 2023; Nigeria Centre for Disease Control, 2024). It is projected that without strategic interventions, deaths due to AMR infections could rise to 10 million per year with up to $100 trillion in economic losses by 2050 (O'Neill, 2016; Murray et al., 2022).
Clinically, the misuse and over-prescription of antibiotics, alongside rising hospital-acquired infections, drive MDR proliferation, leading to increased healthcare costs and poorer patient outcomes. While Antimicrobial Stewardship Programs (ASPs) offer some mitigation, they remain insufficient without broader systemic integration. In the environment, resistant bacteria persist and spread through agricultural runoff, industrial waste, and untreated sewage. This emphasizes the urgent need for enhanced waste management and stricter controls on antibiotic use in agriculture.
On a global scale, MDR transmission is exacerbated by international travel, trade, and healthcare inequities, particularly in LMICs (Patel et al., 2022; Yopa et al., 2023). Although global initiatives by WHO, FAO, and OIE have laid a foundation for action, significant gaps in policy, surveillance, and cross-sector collaboration remain. Hence, addressing this complex crisis requires a holistic approach that encompasses and integrates the drivers of MDR.
The aim of this paper is to describe the different drivers of MDR, explore the critical need for an integrated One Health approach and proffer solutions in combating MDR bacterial infections across LMICs.
Methodology
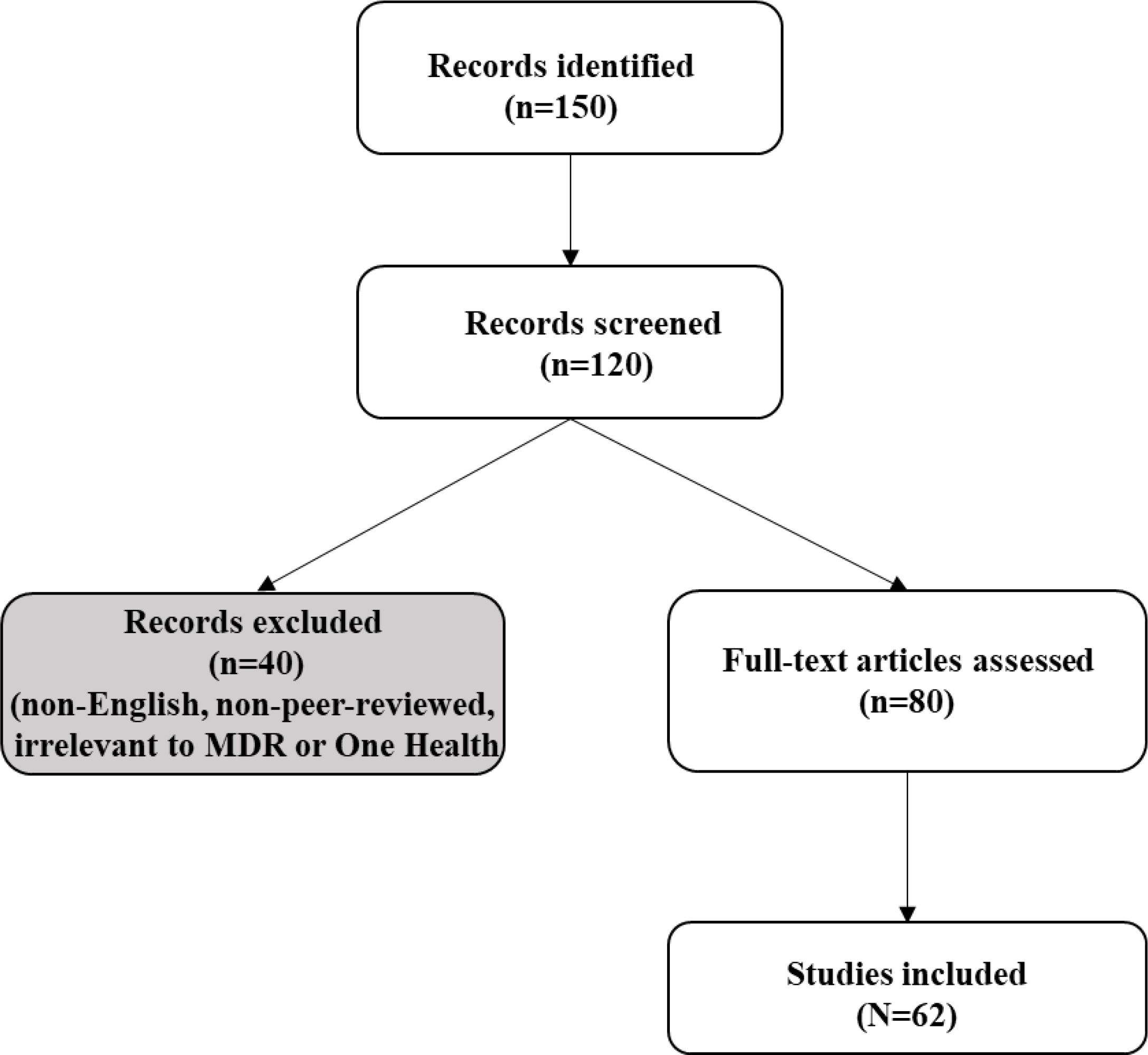
A preliminary search of literature was carried out to determine the need for the study. This was used to refine the topic within the broader concept of MDR infections. The literature searches were conducted using PubMed, Scopus, and Google Scholar (2015–2025) using keywords: “Multidrug-resistant bacteria”, “One Health”, “antimicrobial resistance”, “environmental AMR”, “AI in AMR” (Table 1). Inclusion criteria targeted English-language studies on MDR drivers, interventions, or case studies; non-peer-reviewed sources were excluded. Two authors independently screened 150 articles, resolving discrepancies via consensus, with 62 included after full-text review (Figure 1). Thematic synthesis categorized data into clinical, environmental, global, and One Health perspectives, prioritizing case studies and surveillance data. Independent screening minimized bias, ensuring a reproducible synthesis. Furthermore, the snowballing technique was used to retrieve other relevant published articles from references obtained from the databases.
Discussion
Clinical perspective
Antibiotic misuse and hospital-acquired infections
The prevalence of MDR bacterial infections is majorly influenced by human healthcare practices, societal behaviors, and veterinary contributions. Central to this crisis is the overuse and misuse of antibiotics in both clinical and agricultural settings. In human healthcare, antibiotics are often prescribed inappropriately, such as for viral infections where they have no efficacy, leading to the proliferation of resistant bacterial strains (Patel et al., 2022; Ventola, 2015). In addition, the prevalence of Hospital-Acquired Infections (HAIs) in long-term healthcare facilities (Yopa et al., 2023), with MDR pathogens like Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa demonstrating resistance to last-line antibiotics such as carbapenems (Mbimenyuy et al., 2023; Nigeria Centre for Disease Control, 2024). Resistance to other classes of antibiotics, including polymyxins and aminoglycosides, has further limited treatment options, leaving clinicians with fewer effective therapies (Sekar and Mythreyee, 2012; Tran et al., 2023). Compounding this issue are patient expectations, inadequate and sometimes lack of rapid diagnostic tools to aid in the proper use of antibiotics increasing acceptance of telemedicine by both healthcare providers and patients and systemic inefficiencies that pressure healthcare providers into unnecessary antibiotic prescriptions (WHO, 2017a; Sekar and Mythreyee, 2012; WHO, 2017b; World Health Organization (WHO), 2019).
Limitations of antimicrobial stewardship programs
Antimicrobial stewardship (ASP) refers to interventions designed to promote the optimal use of antibiotic agents, including drug choice, dosing, route, and duration of administration. ASPs are critical initiatives designed to optimize the use of antibiotics within healthcare settings, with the overarching goal of curbing the rise of AMR. Although, these have been documented to reduce resistance by 11–38% in high-income countries, there remain significant hurdles in LMICs (Baur et al., 2017). In sub-Saharan Africa, only 30% of countries have functional ASPs, constrained by diagnostic shortages and counterfeit drugs, which comprise 32% of antibiotics in Cameroon (Basco, 2004; Laxminarayan et al., 2013). In a rural Kenyan hospital, ASP implementation failed when clinicians, lacking resistance testing, reverted to outdated protocols, leading to treatment failures (Shamas et al., 2023). Weak enforcement, limited clinician training, and resource constraints underscore the need for a broader coordination framework to bolster stewardship efforts.
Veterinary contributions to MDR infections
The role of veterinary practices in the MDR crisis is a critical but often underappreciated component of the broader clinical perspective. Antibiotics are widely used in animal husbandry, aquaculture, and veterinary medicine, not only for treating infections but also as a routine measure to enhance productivity. Veterinary antibiotic use, particularly as growth promoters, fosters resistant Escherichia coli, transferring to humans via food chains or direct contact (Van Boeckel et al., 2015). In Nigeria, livestock antibiotic use surged 40% from 2010–2012, driven by lax regulations, with farmers purchasing over-the-counter drugs without veterinary oversight (Adesokan et al., 2015). Manure runoff contaminates water sources, creating a critical One Health interface where veterinary practices amplify environmental resistance (Woolhouse et al., 2015).
Resistance mechanisms
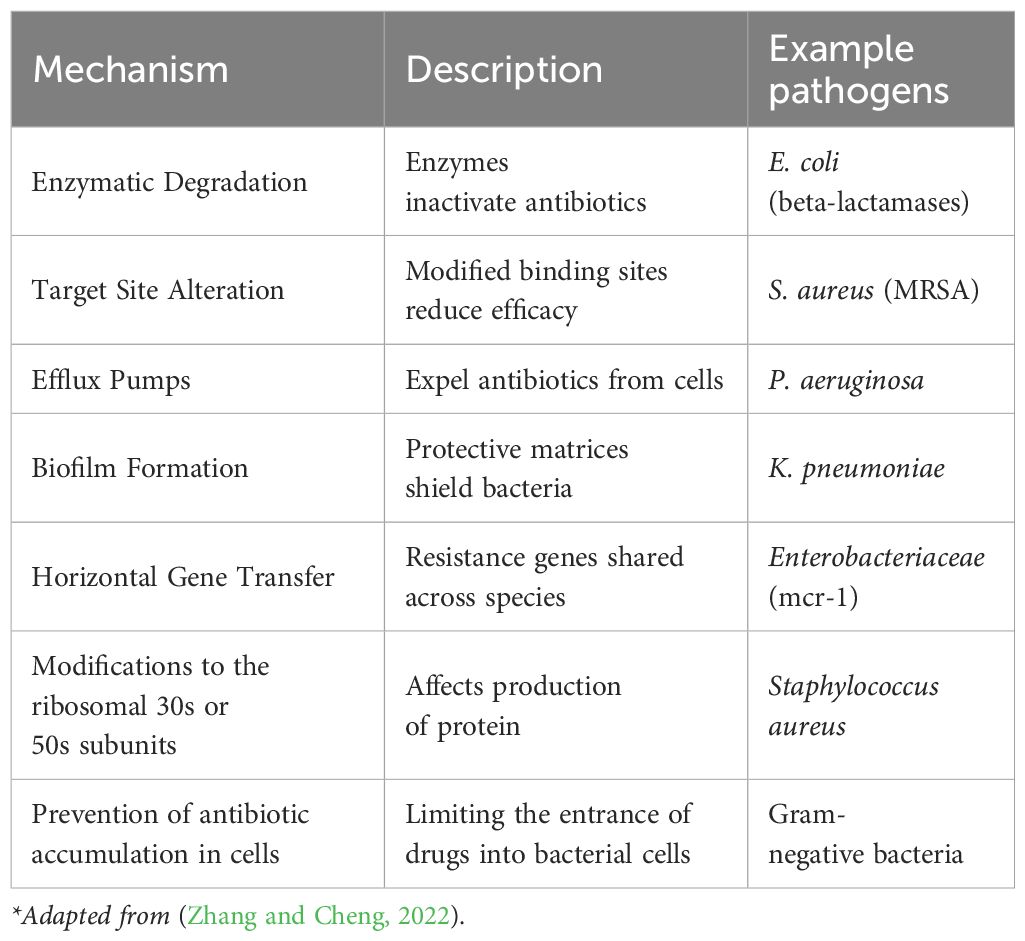
Bacteria employ different mechanisms in achieving resistance (Table 2). These include enzymatic degradation (e.g., beta-lactamases), target site alterations, efflux pumps, biofilms, and horizontal gene transfer, rendering treatments ineffective (Zhang and Cheng, 2022). Bacteria prevent antibiotic accumulation in their cells through limiting the entrance of drugs into bacterial cells. The porin channels in the outer membrane of the Gram-negative bacteria enables them to allow certain antibiotics such as like B-lactams and quinolones to penetrate the bacterial cells. Reduction in the number of bacterial porins could serve as means of hindrance against the antibiotics entering the cell thereby increasing the rate of resistance to the drugs (Darby et al., 2023). In addition, when the bacteria develop resistance to antibiotics through the modification of the ribosomal 30S or 50S subunits which in turn affects the production of protein. Some of the antibiotics that are resisted by this mechanism include: aminoglycosides, tetracycline, macrolides, chloramphenicol, lincosamides, and streptogramin (Sodhi and Singh, 2022; Sodhi et al., 2023).
These mechanisms of resistance driven by clinical and veterinary misuse, require integrated solutions. It is important to note that although clinical practices drive MDR, environmental reservoirs amplify its spread, necessitating an exploration of ecological pathways.
Environmental perspective
Pathways and hotspots
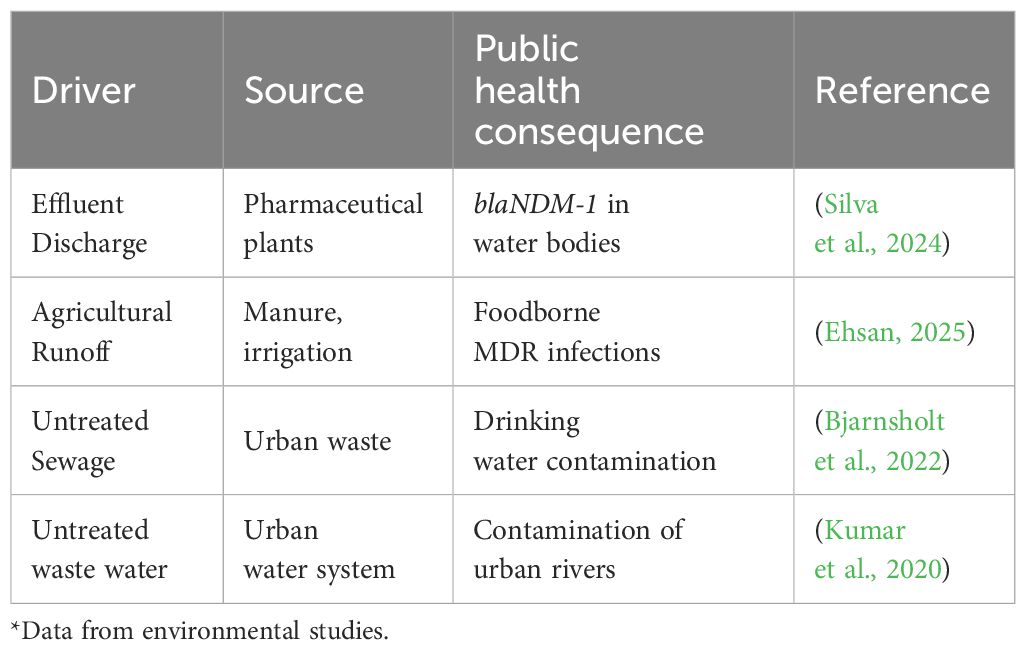
The environment functions both as a reservoir and a conduit for resistant bacteria and resistance genes. It provides a complex interface where microbial evolution, anthropogenic pollution, and public health intersect (Table 3). However, the environment is often overlooked with very deleterious repercussions to public health. An exploration of the environmental dimensions of MDR bacterial infections emphasizes the pathways of the introduction of resistance, its maintenance and transmission throughout the environment. Within an integrated framework, it is very important to highlight environmental hotspots of AMR, the associated risks to human and animal health and their implications for surveillance and policy development.
Wastewater treatment plants, agricultural soils, and aquatic ecosystems serve as MDR reservoirs, perpetuating resistance cycles (Wang et al., 2022). In Brazil, pharmaceutical effluents contaminate rivers with blaNDM-1, while untreated irrigation water in the Middle East spreads resistant bacteria to crops (Alaali and Thani, 2020; Silva et al., 2024). Agricultural runoff, laden with antibiotics from manure, fosters resistance genes, impacting food and water safety across ecosystems (Ehsan, 2025). The aquatic ecosystem (rivers, lakes, ponds etc.) serves as reservoir of resistant pathogens with very high potential as re-entry points of these pathogens into human populations through irrigation, domestic water supply for drinking, recreational activities and food production lines. In addition, contaminated drinking water, crops, and aerosols expose humans to these MDR bacteria, particularly in LMICs with poor sanitation (Bjarnsholt et al., 2022). In India, 60% of urban water sources harbor resistant bacteria, directly linking environmental contamination to clinical infections (Kumar et al., 2020; Hamer and Coffin, 2021).
Mitigation strategies such as surveillance of resistance genes, stricter waste discharge regulations, and composting practices can reduce environmental AMR (Tsekleves et al., 2023). Public education campaigns on exposure risks, such as avoiding contaminated water sources, further strengthen prevention efforts (WHO, 2023).
Global health perspective
Global spread via travel and migration
Environmental spread of MDR bacterial infection is compounded by global dynamics, where travel (both human and animal migration) and inequities accelerate MDR’s reach. For instance, in 2018, 1.4 billion tourists facilitated the global spread of NDM-1 and mcr-1, with Saudi Arabia’s medical tourism linked to hospital-acquired infections (Payne et al., 2016; Arcilla et al., 2017). Cross-border patient transfers, such as between the Netherlands and Germany, face hygiene discrepancies, complicating infection control (Müller et al., 2015).
Inequities in healthcare and antibiotic access
LMICs grapple with diagnostic shortages and counterfeit drugs, for example, 32% of antibiotics in Cameroon being substandard (Basco, 2004). Women, often caregivers or livestock handlers, face higher MDR exposure due to limited healthcare access, exacerbating gender disparities in treatment outcomes (Iskandar et al., 2020). In Rwanda, 55% of farmers use over-the-counter antibiotics, driven by poverty and lack of veterinary oversight (Manishimwe et al., 2017).
Role of international organizations
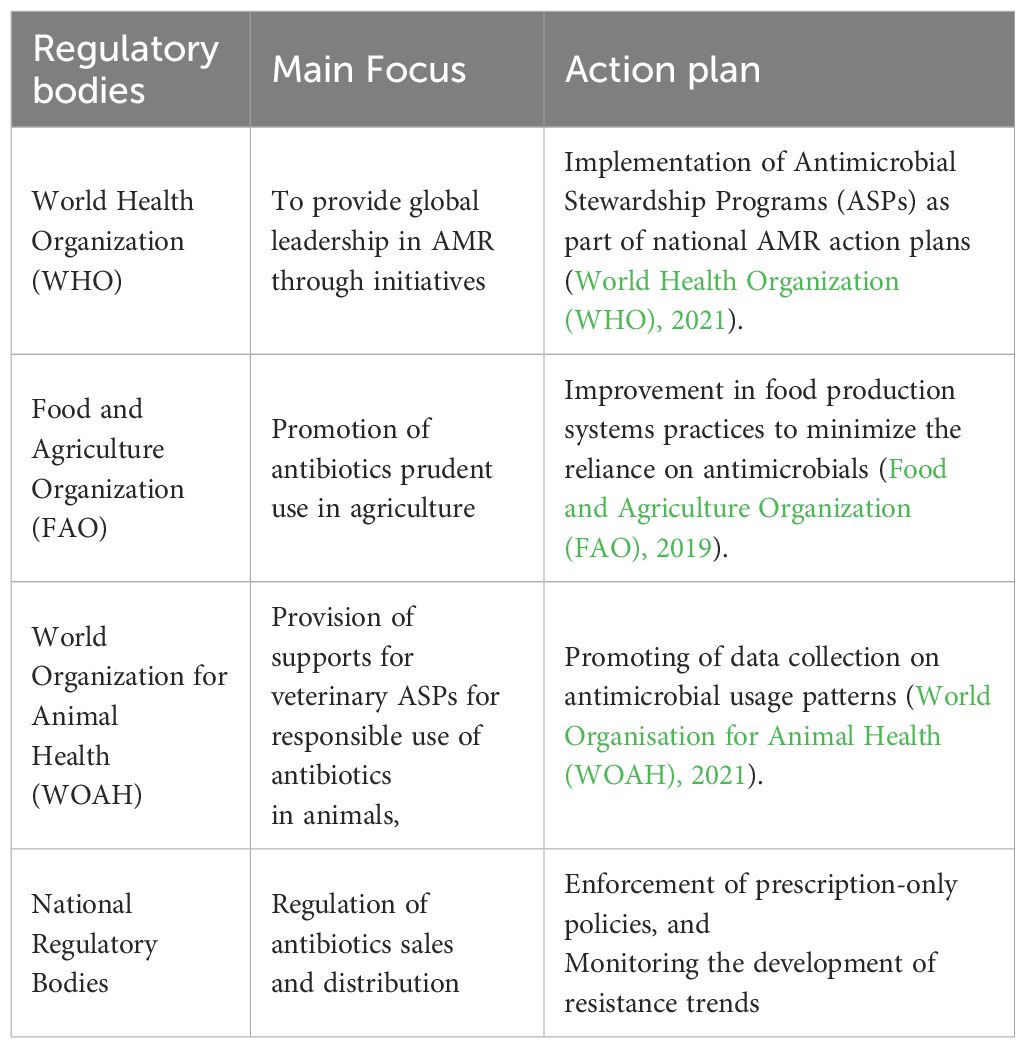
The main functions expected to be performed by the global health systems include: management of cross-border externalities, mobilizing global support for disadvantaged populations, performing oversight functions on the overall performance of the system and ensuring that adequate global public goods are provided (Moon et al., 2017) (Table 4).
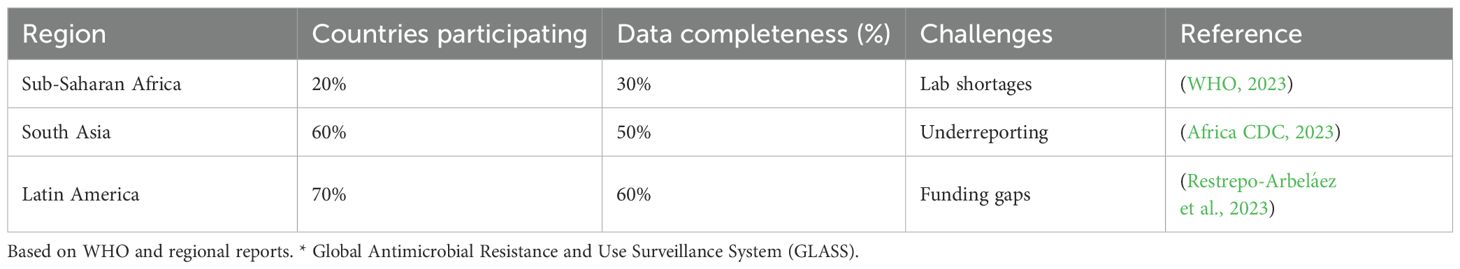
The WHO’s Global Antimicrobial Resistance and Use Surveillance System (GLASS) tracks resistance patterns, but only 20% of sub-Saharan African nations fully participate due to resource constraints (WHO, 2023). FAO and WOAH advocate phasing out antibiotic growth promoters, yet enforcement varies, with 50% of LMICs lacking national action plans (FAO, 2024) (Table 5).
Surveillance bottlenecks such as underreporting, limited laboratory capacity (only 25% of African hospitals are equipped for resistance testing), and poor data interoperability hamper global surveillance efforts (WHO, 2023). Nigeria’s National Action Plan for AMR (NAP-AMR) highlights that 50% of its labs lack resistance testing capabilities, delaying timely interventions (Nigeria Centre for Disease Control, 2023a). These global challenges underscore the need for a One Health approach, integrating human, animal, and environmental strategies to combat MDR effectively.
One health perspective
Transmission cycles
MDR bacteria cycle bidirectionally between humans, animals, and environments, with 60% of human pathogens being zoonotic, such as Escherichia coli and Salmonella (Klous et al., 2016). For example, in Ghana, ESBL-producing E. coli shared between poultry and farmers highlights occupational risks, driven by close contact in agricultural settings (Falgenhauer et al., 2019).
Operational integration of one health
operates through national task forces, as exemplified by Nigeria’s NAP-AMR, where human, veterinary, and environmental ministries collaborate on surveillance and policy (Nigeria Centre for Disease Control, 2023a). Inter-ministerial platforms facilitate data-sharing, reducing response times and aligning interventions across sectors (Liu et al., 2016).
Case study of colistin resistance in China
In 2015, China’s identification of the mcr-1 gene in livestock sparked global concern, as it spread via international trade (Charoenboon et al., 2019). A 2017 ban on colistin as a growth promoter reduced its prevalence by 40%, demonstrating the power of One Health-driven policy interventions (Charoenboon et al., 2019). Operational integration paves the way for comprehensive One Health strategies and this is evidenced by global successes.
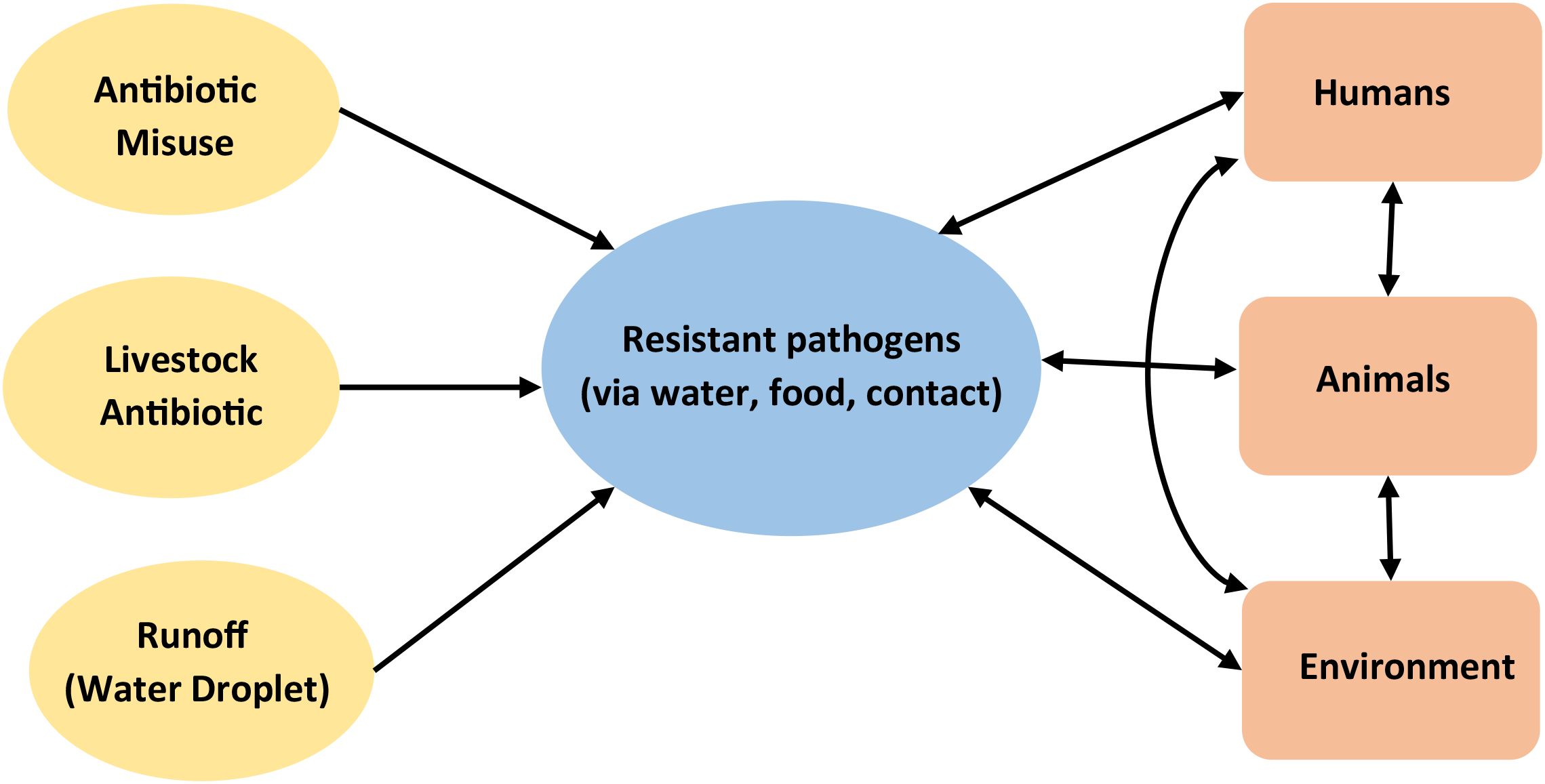
The dynamic relationships within the ecosystem (Figure 2) depicts the interactions between humans, animals and the environment and how any misuse of or release of antibiotics (inputs such as antibiotic misuse, livestock antibiotics, runoff (water droplet) disrupts the equilibrium the entire system through the release of deleterious outputs in this case-resistant pathogens via water, food and contact with negative impact.
Case studies of successful interventions
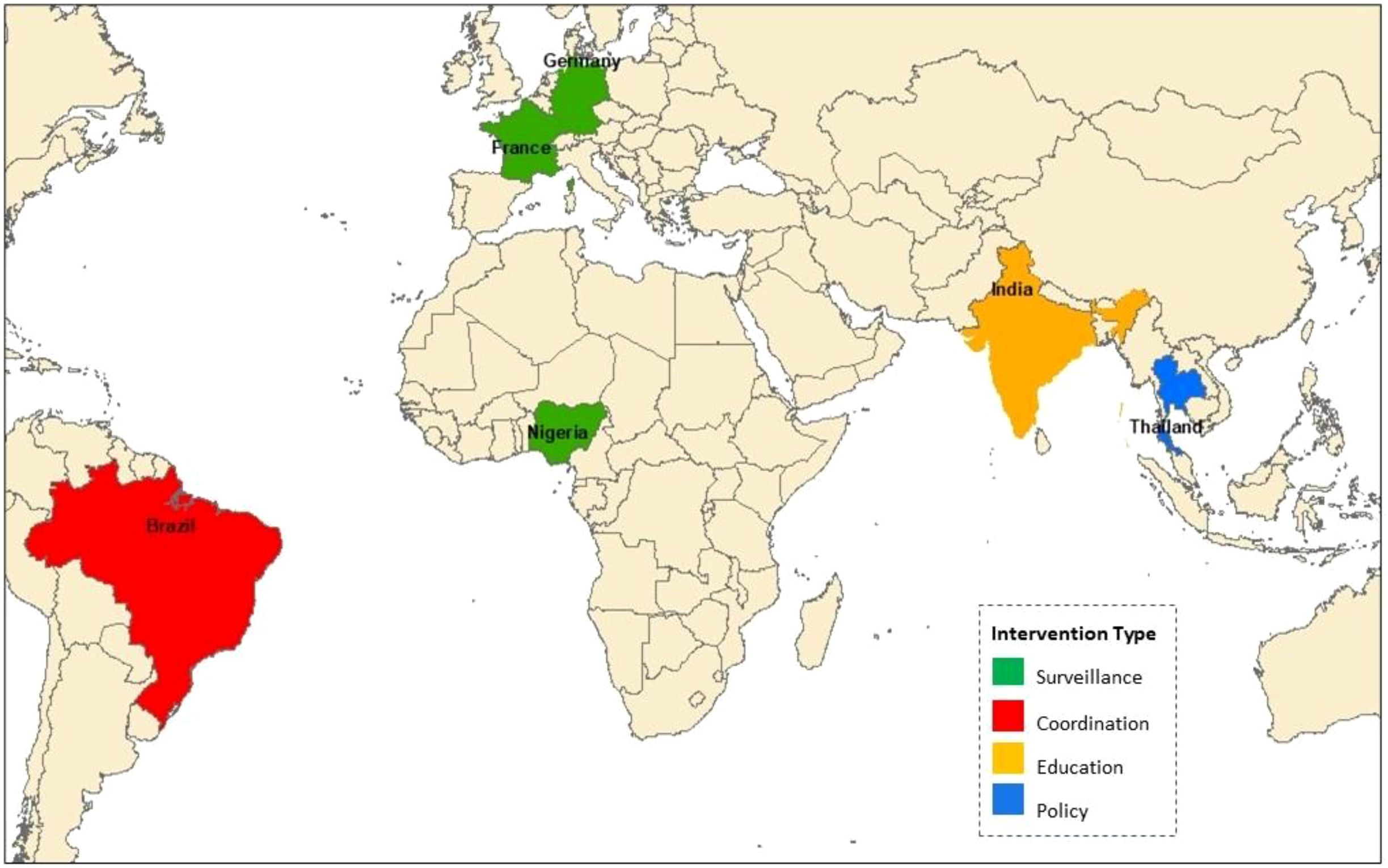
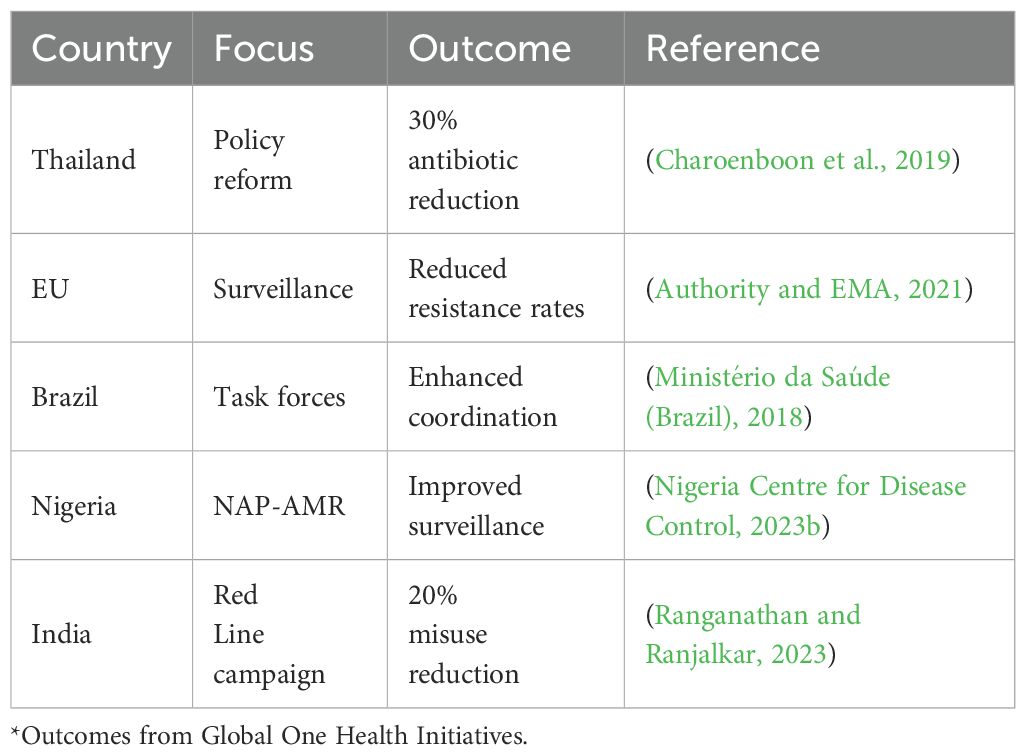
Thailand’s 2017–2021 National Strategic Plan reduced antibiotic use by 30% through regulatory reforms (Charoenboon et al., 2019). The EU’s surveillance network lowered resistance rates by enhancing data-sharing (Authority and EMA, 2021). Brazil’s 2024 AMR plan strengthened intersectoral coordination (Ministério da Saúde (Brazil), 2018). Nigeria’s NAP-AMR improved surveillance coverage (Nigeria Centre for Disease Control, 2023b). And India’s Red Line campaign cut antibiotic misuse by 20% (Ranganathan and Ranjalkar, 2023) (Figure 3).
These diverse, well-documented successes (Table 6) inform scalable One Health models. Brazil’s intersectoral task forces exemplify effective coordination, sharing data through joint risk assessments (Ranganathan and Ranjalkar, 2023). Ghana’s Antimicrobial Awareness Week, reducing misuse by 15%, demonstrates the power of community-driven initiatives to complement policy efforts (Tsekleves et al., 2023).
Challenges and limitations
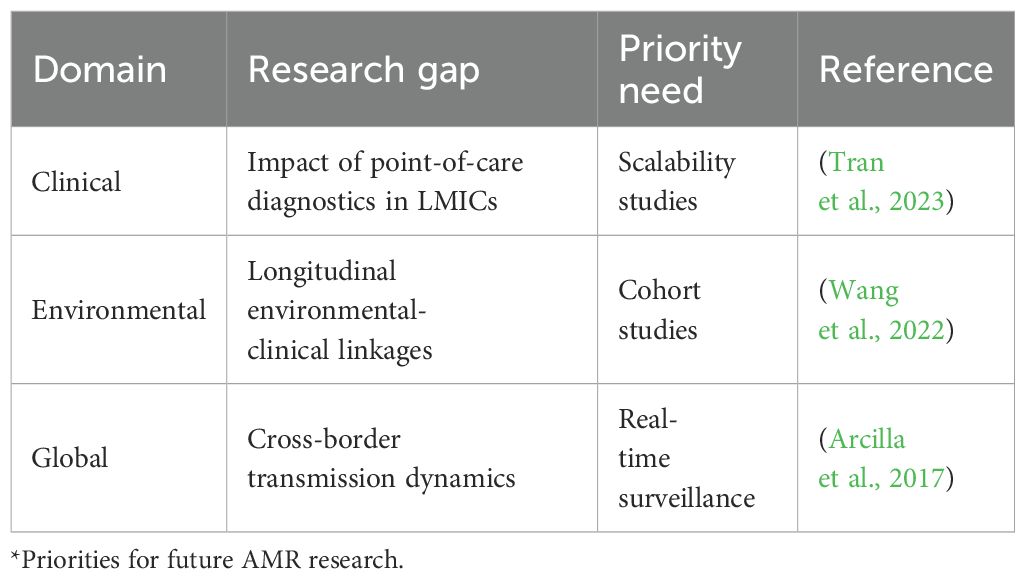
Despite the successes of One-Health interventions, persistent challenges in coordination and research gaps necessitate innovative solutions. Bureaucratic fragmentation often divides human and veterinary health agencies, delaying coordinated responses to MDR outbreaks (Masud et al., 2023; Sanga et al., 2024). In LMICs, only 25% of hospitals have laboratories equipped for resistance testing, severely limiting surveillance capabilities (WHO, 2023). Limited political will and insufficient funding exacerbate these issues, with global surveillance systems like GLASS suffering from underreporting, particularly in sub-Saharan Africa (WHO, 2023). A critical research gap lies in the paucity of longitudinal studies linking environmental contamination to clinical infections, hindering evidence-based policy development (Wang et al., 2022). Table 7 summarizes these gaps, highlighting priority areas for future investigation to strengthen One Health approaches. Addressing these challenges requires forward-thinking strategies, leveraging technology and community action to drive progress.
Integrated one health approach
Collaboration is a participatory process of engaging key actors in addressing complex problems that cannot be handled by a single entity. Intersectoral collaborations creates the needed opportunity to strengthen the health system through increasing coverage, expanding access and improving the comprehensive availability of MDR services across communities.
With respect to MDR infections, an integrated One Health approach implies collaboration across human, animal, and environmental health sectors to address the multifaceted challenges of MDR bacterial infections.
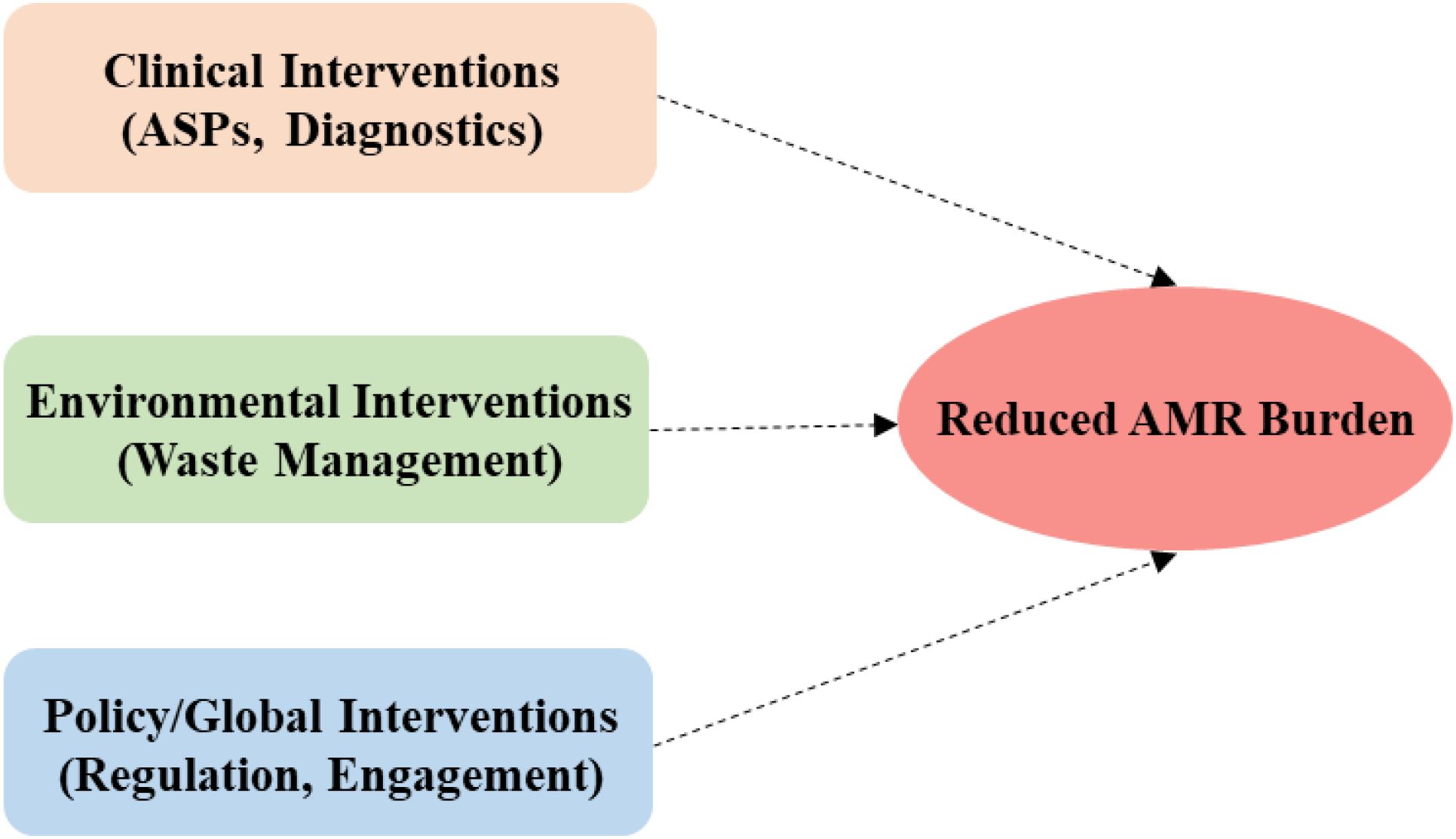
Figure 4 depicts a One Health Framework based on interconnected interventions, critical for reducing MDR burden holistically across domains. The focus is on intentional linking of clinical (ASPs, diagnostics), environmental (waste management), and policy/global (regulation, engagement) interventions with a clear convergence with reduced AMR burden as the output. This Framework projects that effective partnerships involving veterinarians, environmental health scientists, public health officials, and clinicians working together to develop targeted interventions that address resistance at its sources.
Future directions and recommendations
AI-driven solutions
The application of AI to manage AMR is fast gaining ground (Mansur et al., 2023). The potential for integrating technology (e.g., AI, Machine Learning (ML)) for real-time MDR monitoring and tailored interventions can be achieved through various ways to enhance effective healthcare delivery. The integration of AI and ML in diagnostics and treatment planning revolutionizing patient care, particularly ML, in medical diagnostics is ushering in a new era of healthcare. This revolutionary approach is characterized by increased accuracy, efficiency, and speed, surpassing traditional human capabilities in certain aspects.
Giving practical examples, in Ethiopia’s rural clinics, a young mother’s life was saved when WHO’s 2024 AMASS tool identified a resistant Escherichia coli infection in hours, cutting diagnostic time by 50% and guiding precise therapy (Martin, 2021). AI’s Machine Learning capabilities, as demonstrated by IDseq’s global deployment, analyze genomic and clinical data to predict resistance patterns with 90% accuracy, revolutionizing MDR management (Luca, 2024).
However, challenges persist: algorithm bias from unrepresentative datasets, non-standardized data inputs, and infrastructure gaps in LMICs—where only 10% of laboratories have cloud connectivity—limit scalability (Tran et al., 2023). Ethical concerns, such as patient data privacy and equitable access, further complicate adoption, necessitating robust governance frameworks (Schar et al., 2021) Despite these hurdles, AI’s transformative potential, when paired with investments in LMIC infrastructure, offers a pathway to precision interventions and global health equity.
Community engagement
Community-driven initiatives, such as Ghana’s Antimicrobial Awareness Week (15% misuse reduction) and India’s Red Line campaign (20% reduction), demonstrate the power of public education (Tsekleves et al., 2023; Sanga et al., 2024). Women, often primary caregivers and livestock handlers, face heightened MDR exposure due to limited healthcare access, requiring targeted education to address gender disparities and reduce transmission risks (Iskandar et al., 2020).
Policy reforms
Global bans on antibiotic growth promoters, public-private funding models, and standardized surveillance systems, as implemented in Brazil’s 2024 AMR plan, are essential for sustainable progress (Ranganathan and Ranjalkar, 2023). Nigeria’s NAP-AMR, emphasizing laboratory upgrades, provides a replicable model for LMICs to strengthen diagnostic capacity (Ministério da Saúde (Brazil), 2018). Clinical misuse, environmental reservoirs, and global spread, particularly in LMICs, thrive in the absence of coordinated responses. Thailand’s 30% reduction in antibiotic use through policy reform and AI’s 50% improvement in diagnostic speed offer beacons of hope, yet gender inequities and surveillance gaps persist (Authority and EMA, 2021; Martin, 2021).
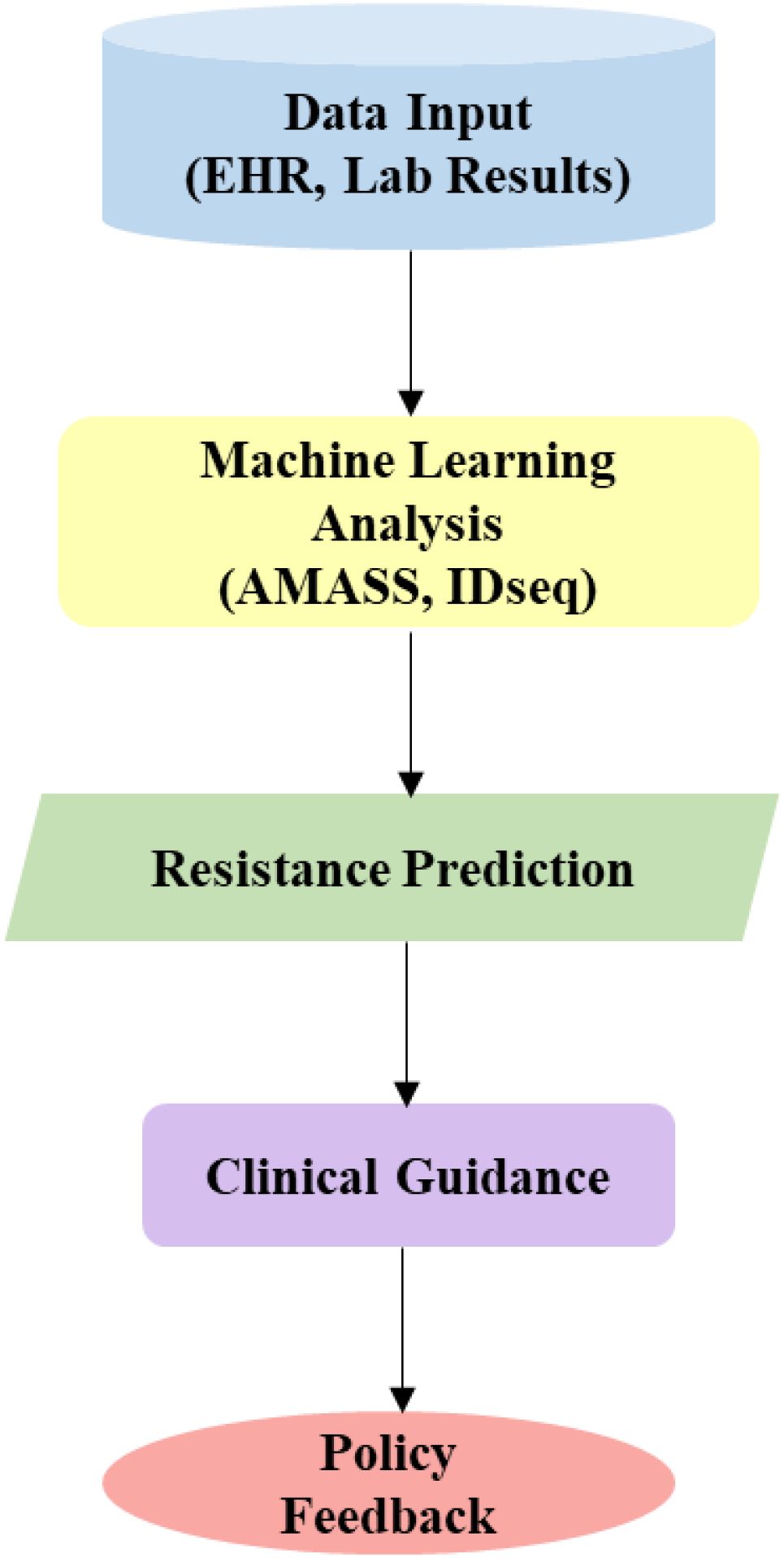
We present a flowchart of an AI workflow from data input (EHR, lab results) to ML analysis (AMASS, IDseq), resistance prediction, clinical guidance, and policy feedback (Figure 5). This will enable rapid MDR detection, critical for precision interventions, prompt community engagement and policy development.
These strategies converge in a bold vision for global health security, as outlined in our concluding call to action.
Conclusion
This review has given deep insight into the problems associated with antimicrobial resistance and the various drivers of MDR bacterial infections. We reiterated that the One Health approach is important for combating MDR bacterial infections because it recognizes the interconnectedness of human, animal, and environmental health. With respect to MDR infections, an integrated One Health approach implies collaboration across human, animal, and environmental health sectors to address the multifaceted challenges of MDR bacterial infections. In tackling the identified gaps, proactively incorporating AI technology for real-time drug resistance monitoring will go a long way to help in combating the problems of MDR infections in communities. Although the use of antibiotics for bacterial infection control is still very important, considering reduction in its use in all facets of One Health and focusing on alternative strategies to limit the proliferation of drug-resistant pathogens is highly recommended. Since antibiotic resistance is as a result of abuse, antibiotic-free technique is an alternative approach to dealing with the present antibacterial challenges.
In summary, a One Health approach that integrates human, animal, and environmental based strategies, is not merely an option but an imperative. A proactive paradigm shift focusing on transformative actions to:
● Fund intersectoral surveillance: Expand the WHO GLASS participation and laboratory capacity to track resistance in real time.
● Scale AI equitably: Invest in LMICs infrastructure to ensure inclusive, ethical technology deployment.
● Empower communities: Prioritize gender-focused education to curb antibiotic misuse and reduce exposure.
These steps, grounded in One Health principles, are critical to preserving antibiotics and securing a resilient global health future.
Author contributions
CO: Writing – original draft, Writing – review & editing, Resources, Conceptualization, Validation, Methodology, Data curation. OF: Data curation, Writing – original draft, Validation, Writing – review & editing. LA: Validation, Writing – review & editing, Writing – original draft, Data curation. AB: Data curation, Validation, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adesokan, H. K., Akanbi, I. M., Akanbi, I. O., and Obaweda, R. A. (2015). Pattern of antimicrobial usage in livestock animals in south-western Nigeria: The need for alternative plans. Onderstepoort J. Veterinary Res. 82, 1–6. doi: 10.4102/ojvr.v82i1.816, PMID: 26016985
Africa CDC (2023). Antimicrobial resistance surveillance in Africa: 2023 report (Addis Ababa: Africa CDC).
Alaali, Z. and Thani, A. S. B. (2020). Patterns of antimicrobial resistance observed in the Middle East: Environmental and health care retrospectives. Sci. Total Environ. 740, 140089. doi: 10.1016/j.scitotenv.2020.140089, PMID: 32559543
Arcilla, M. S., van Hattem, J. M., Haverkate, M. R., Bootsma, M. C., van Genderen, P. J., Goorhuis, A., et al. (2017). Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect. Dis. 17, 78–85. doi: 10.1016/S1473-3099(16)30319-X, PMID: 27751772
Authority, E. F. S. and EMA, E. M. A. (2021). Third joint inter-agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the EU/EEA: JIACRA III 2016-2018. EFSA J. 19, e06712. doi: 10.2903/j.efsa.2021.6712, PMID: 34221148
Basco, L. K. (2004). Molecular epidemiology of malaria in Cameroon. XIX. Quality of antimalarial drugs used for self-medication. Am. J. Trop. Med. Hygiene 70, 245–250. doi: 10.4269/ajtmh.2004.70.245, PMID: 15031511
Baur, D., Gladstone, B. P., Burkert, F., Carrara, E., Foschi, F., Döbele, S., et al. (2017). Effect of antibiotic stewardship on the incidence of infection and colonization with antibiotic-resistant bacteria and Clostridium difficile infection: A systematic review and meta-analysis. Lancet Infect. Dis. 17, 990–1001. doi: 10.1016/S1473-3099(17)30325-0, PMID: 28629876
Berendonk, T. U., Manaia, C. M., Merlin, C., Fatta-Kassinos, D., Cytryn, E., Walsh, F., et al. (2015). Tackling antibiotic resistance: the environmental framework. Nat. Rev. Microbiol. 13, 310–317. doi: 10.1038/nrmicro3439, PMID: 25817583
Bjarnsholt, T., Whiteley, M., Rumbaugh, K. P., Stewart, P. S., Jensen, P. Ø., and Frimodt-Møller, N. (2022). The importance of understanding the infectious microenvironment. Lancet Infect. Dis. 22, e88–e92. doi: 10.1016/S1473-3099(21)00122-5, PMID: 34506737
Charoenboon, N., Haenssgen, M. J., Warapikuptanun, P., Xayavong, T., and Khine Zaw, Y. (2019). Translating antimicrobial resistance: a case study of context and consequences of antibiotic-related communication in three northern Thai villages. Palgrave Commun. 5, 1–24. doi: 10.1057/s41599-019-0226-9
Darby, E. M., Trampari, E., Siasat, P., Gaya, M. S., Alav, I., Webber, M. A., et al. (2023). Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 21, 280–295. doi: 10.1038/s41579-022-00820-y, PMID: 36411397
Ehsan, H. (2025). Antibiotic resistance in developing countries: emerging threats and policy responses. Public Health Challenges 4, e70034. doi: 10.1002/puh2.70034
Falgenhauer, L., Imirzalioglu, C., Oppong, K., Akenten, C. W., Hogan, B., Krumkamp, R., et al. (2019). Detection and characterization of ESBL-producing Escherichia coli from humans and poultry in Ghana. Front. Microbiol. 9, 3358. doi: 10.3389/fmicb.2018.03358, PMID: 30697208
FAO (2024). Antimicrobial resistance in agrifood systems: 2024 technical report (Rome: Food and Agriculture Organization).
Food and Agriculture Organization (FAO) (2019). Prudent and efficient use of antimicrobials in agriculture (Geneva: World Health Organisation).
Hamer, D. H. and Coffin, S. E. (2021). Burden of neonatal sepsis in low-resource settings: high risk, high reward. Clin. Infect. Dis. 73, 281–282. doi: 10.1093/cid/ciaa550, PMID: 32421766
Iskandar, K., Molinier, L., Hallit, S., Sartelli, M., Catena, F., Coccolini, F., et al. (2020). Drivers of antibiotic resistance transmission in low-and middle-income countries from a “one health“ perspective—a review. Antibiotics 9, 372. doi: 10.3390/antibiotics9070372, PMID: 32630353
Klous, G., Huss, A., Heederik, D. J., and Coutinho, R. A. (2016). Human–livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Health 2, 65–76. doi: 10.1016/j.onehlt.2016.03.001, PMID: 28616478
Kumar, M., Chaminda, T., Patel, A. K., Sewwandi, H., Mazumder, P., Joshi, M., et al. (2020). Prevalence of antibiotic resistance in the tropical rivers of Sri Lanka and India. Environ. Res. 188, 109765. doi: 10.1016/j.envres.2020.109765, PMID: 32554273
Laxminarayan, R., Duse, A., Wattal, C., Zaidi, A. K., Wertheim, H. F., Sumpradit, N., et al. (2013). Antibiotic resistance—the need for global solutions. Lancet Infect. Dis. 13, 1057–1098. doi: 10.1016/S1473-3099(13)70318-9, PMID: 24252483
Liu, Y. Y., Wang, Y., Walsh, T. R., Yi, L. X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168. doi: 10.1016/S1473-3099(15)00424-7, PMID: 26603172
Luca, L. (2024). Detection of carbapenem-resistant Enterobacterales in food producing animals and human patients: A "One Health" perspective. Available online at: https://tesidottorato.depositolegale.it/handle/20.500.14242/192993 (Accessed August 28, 2025).
Manishimwe, R., Nishimwe, K., and Ojok, L. (2017). Assessment of antibiotic use in farm animals in Rwanda. Trop. Anim. Health Production 49, 1101–1106. doi: 10.1007/s11250-017-1290-z, PMID: 28526987
Mansur, A., Saleem, Z., Elhakim, T., and Daye, D. (2023). Role of artificial intelligence in risk prediction, prognostication, and therapy response assessment in colorectal cancer: current state and future directions. Front. Oncol. 13, 1065402. doi: 10.3389/fonc.2023.1065402, PMID: 36761957
Martin, O. (2021). Artificial intelligence in drug discovery and development. Advanced Sci. 3, 1–10.
Masud, R. I., Fahim, N. A., Rana, M. L., Islam, M. S., and Tanvir, M. (2023). Artificial intelligence, a powerful tool to combat antimicrobial resistance: an update. J. Adv. Biotechnol. Exp. Ther. 6, 711. doi: 10.5455/jabet.2023.d161
Mbimenyuy, C. M., Cho, J. F., Mugyia, A. E., Ikomey, G. M., Tebit, D. M., and Nota, D. A. (2023). Comparative HPV genotype distribution among women with normal and abnormal cervical cytology in Yaoundé, Cameroon. Afr. J. Clin. Exp. Microbiol. 24, 158–167. doi: 10.4314/ajcem.v24i2.5
Ministério da Saúde (Brazil) (2018). National action plan for the prevention and control of antimicrobial resistance (PAN-BR). (Brasília: Ministério da Saúde). 25pp.
Moon, S., Røttingen, J. A., and Frenk, J. (2017). Global public goods for health: weaknesses and opportunities in the global health system. Health Economics Policy Law 12, 195–205. doi: 10.1017/S1744133116000451, PMID: 28332461
Müller, J., Voss, A., Köck, R., Sinha, B., Rossen, J. W., Kaase, M., et al. (2015). Cross-border comparison of the Dutch and German guidelines on multidrug-resistant Gram-negative microorganisms. Antimicrobial resistance infection control 4, 7. doi: 10.1186/s13756-015-0047-6, PMID: 25763183
Murray, C. J., Ikuta, K. S., Sharara, F., Swetschinski, L., Aguilar, G. R., Gray, A., et al. (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655. doi: 10.1016/S0140-6736(21)02724-0, PMID: 35065702
Nigeria Centre for Disease Control (2023a). National Action Plan for AMR (NAP-AMR): 2023–2027 (Abuja: NCDC).
Nigeria Centre for Disease Control (2023b). Progress on NAP-AMR implementation: 2023 update (Abuja: NCDC).
O'Neill, J. (2016). Tackling drug-resistant infections globally: Final report and recommendations. Rev. Antimicrobial Resistance, 84pp.
Patel, S., Preuss, C. V., and Bernice, F. (2022). Vancomycin (Treasure Island, FL, USA: StatPearls).
Payne, M., Croxen, M. A., Lee, T. D., Mayson, B., Champagne, S., Leung, V., et al. (2016). mcr-1–positive colistin-resistant Escherichia coli in traveler returning to Canada from China. Emerging Infect. Dis. 22, 1673. doi: 10.3201/eid2209.160177, PMID: 27533019
Ranganathan, R. and Ranjalkar, J. (2023). Potential role of civil society organizations (CSOs) in antimicrobial resistance (AMR) mitigation efforts in low-and middle-income countries: a report from India. J. Infection Public Health 16, 125–128. doi: 10.1016/j.jiph.2023.10.043, PMID: 37973495
Restrepo-Arbeláez, N., Garcia-Betancur, J. C., Pallares, C. J., and Villegas, M. V. (2023). Antimicrobial stewardship programs in Latin America and the caribbean: a story of perseverance, challenges, and goals. Antibiotics 12, 1342. doi: 10.3390/antibiotics12081342, PMID: 37627762
Sanga, V. T., Karimuribo, E. D., and Hoza, A. S. (2024). One Health in practice: Benefits and challenges of multisectoral coordination and collaboration in managing public health risks: A meta-analysis, Int. J. One Health 10, 26–36. doi: 10.14202/IJOH.
Schar, D., Zhao, C., Wang, Y., Larsson, D. G. J., Gilbert, M., and Van Boeckel, T. P. (2021). Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat. Commun. 12, 5384. doi: 10.1038/s41467-021-25655-8, PMID: 34508079
Sekar, R. and Mythreyee, M. (2012). Carbapenem resistant enterobacteriaceae–A growing threat to public health. Open Access Sci. Rep. 1, 324.
Shamas, N., Stokle, E., Ashiru-Oredope, D., and Wesangula, E. (2023). Challenges of implementing antimicrobial stewardship tools in low to middle income countries (LMICs). Infection Prev. Pract. 5, 100315. doi: 10.1016/j.infpip.2023.100315, PMID: 38107237
Silva, J. T. P., Santos, F. F., Valiatti, T. B., Valêncio, A., da Silva Ribeiro, Á.C., Oliveira, L. F. V., et al. (2024). Unravelling the genomic characteristics of a Klebsiella quasipneumoniae clinical isolate carrying blaNDM-1. J. Global Antimicrobial Resistance 38, 302–305. doi: 10.1016/j.jgar.2024.05.022, PMID: 38852850
Sodhi, K. K. and Singh, C. K. (2022). Recent development in the sustainable remediation of antibiotics: a review. Total Environ. Res. Themes 3, 100008. doi: 10.1016/j.totert.2022.100008
Sodhi, K. K., Singh, C. K., Kumar, M., and Singh, D. K. (2023). Whole-genome sequencing of Alcaligenes sp. strain MMA: Insight into antibiotic heavy metal resistant genes. Front. Pharmacol. 14, 1144561. doi: 10.3389/fphar.2023.1144561, PMID: 37251338
Tran, N. K., Kretsch, C., LaValley, C., and Rashidi, H. H. (2023). Machine learning and artificial intelligence for the diagnosis of infectious diseases in immunocompromised patients. Curr. Opin. Infect. Dis. 36, 235–242. doi: 10.1097/QCO.0000000000000935, PMID: 37284773
Tsekleves, E., De Souza, D., Pickup, R., Ahorlu, C., and Darby, A. (2023). Developing home cleaning intervention through community engagement to reduce infections and antimicrobial resistance in Ghanaian homes. Scientific Reports. 13 (1), 10505. doi: 10.1038/s41598-023-37317-4, PMID: 37380793
Van Boeckel, T. P., Brower, C., Gilbert, M., Grenfell, B. T., Levin, S. A., Robinson, T. P., et al. (2015). Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. 112, 5649–5654. doi: 10.1073/pnas.1503141112, PMID: 25792457
Ventola, C. L. (2015). The antibiotic resistance crisis: part 1: causes and threats. Pharm. Ther. 40, 277–283., PMID: 25859123
Wang, Y., Yu, Y., Zhang, H., Huo, Y., Liu, X., Che, Y., et al. (2022). The phytotoxicity of exposure to two polybrominated diphenyl ethers (BDE47 and BDE209) on photosynthesis and the response of the hormone signaling and ROS scavenging system in tobacco leaves. J. Hazardous Materials 426, 128012. doi: 10.1016/j.jhazmat.2021.128012, PMID: 34923383
WHO. (2017). Critically important antimicrobials for human medicine – 5th rev. (Geneva: World Health Organization). 48pp. Available online at: http://who.int/foodsafety/publications/antimicrobials-fifth/en/.
WHO. (2017). Roadmap to implement the 2030 Agenda for Sustainable Development, building on Health 2020, the European policy for health and well-being (EUR/ RC67/9) (Copenhagen, Denmark: World Health Organization). Available online at: https://www.euro.who.int/en/health-topics/health-policy/sustainable-development-goals/publications/2017/eurrc679-roadmap-to-implement-the-2030-agenda-for-sustainable-development,-building-on-health-2020,-the-european-policy-for-health-and-well-being.
WHO (2023). Global action plan on antimicrobial resistance: 2023 update (Geneva: World Health Organization).
Woolhouse, M., Ward, M., Van Bunnik, B., and Farrar, J. (2015). Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. B: Biol. Sci. 370, 20140083. doi: 10.1098/rstb.2014.0083., PMID: 25918441
World Health Organization (WHO) (2019). No Time to Wait: Securing the Future from Drug-Resistant Infections (Geneva: World Health Organisation).
World Health Organization (WHO) (2021). Global Action Plan on Antimicrobial Resistance (Geneva: World Health Organisation).
World Organisation for Animal Health (WOAH) (2021). Antimicrobial resistance and the role of veterinary services (Geneva: World Health Organisation).
Yopa, D. S., Massom, D. M., Kiki, G. M., Sophie, R. W., Fasine, S., Thiam, O., et al. (2023). Barriers and enablers to the implementation of one health strategies in developing countries: a systematic review. Front. Public Health 11, 1252428. doi: 10.3389/fpubh.2023.1252428, PMID: 38074697
Keywords: multidrug-resistant (MDR) bacteria, one health, antimicrobial stewardship, environmental reservoirs, AI technologies, inter-sectoral collaborations, zoonotic MDR outbreaks, global health
Citation: Ohia CMD, Falodun OI, Adebudo LI and Bakarey AS (2025) A one health perspective on multidrug-resistant bacterial infections: integrated approaches for surveillance, policy and innovation. Front. Cell. Infect. Microbiol. 15:1614232. doi: 10.3389/fcimb.2025.1614232
Received: 22 April 2025; Accepted: 04 August 2025;
Published: 03 September 2025.
Edited by:
Mithun Rudrapal, Vignan’s Foundation for Science, Technology and Research, IndiaReviewed by:
Samiksha Garse, DY Patil Deemed to be University, IndiaKratika Singh, Centre of Bio-Medical Research (CBMR), India
Copyright © 2025 Ohia, Falodun, Adebudo and Bakarey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chinenyenwa M. D. Ohia, b2hpYWNtZEBnbWFpbC5jb20=
 Chinenyenwa M. D. Ohia
Chinenyenwa M. D. Ohia Olutayo Israel Falodun
Olutayo Israel Falodun Lucky Icomiare Adebudo4,5
Lucky Icomiare Adebudo4,5