- 1Peking University China-Japan Friendship School of Clinical Medicine, China-Japan Friendship Hospital, Beijing, China
- 2Laboratory of Clinical Microbiology and Infectious Diseases, Department of Pulmonary and Critical Care Medicine, Beijing Key Laboratory of Surveillance, Early Warning and Pathogen Research on Emerging Infectious Diseases, National Center for Respiratory Medicine, China-Japan Friendship Hospital, Beijing, China
- 3Institute of Respiratory Medicine, Chinese Academy of Medical Sciences, Beijing, China
- 4China-Japan Friendship Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, Beijing, China
- 5Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 6Yanjing Medical College, Capital Medical University, Beijing, China
Background: Carbapenem-resistant Pseudomonas aeruginosa (CRPA) poses a significant global health risk, particularly for immunocompromised individuals. This study documents an outbreak of CRPA strains co-harboring blaVIM-1 and blaIMP-45 on IncP-2 plasmids in a Chinese tertiary hospital, resulting in poor outcomes for transplant patients.
Methods: 17 ST313 VIM-1-IMP-45 CRPA strains were collected from transplant patients, and antibiotic susceptibility was tested via microbroth dilution. Whole genome sequencing (WGS) identified drug resistance and virulence mechanisms, analyzed ST313 P. aeruginosa phylogeny, and traced blaVIM-1 and blaIMP-45 origins. Conjugation experiments were conducted to assess the conjugative potential of the IncP-2 plasmid co-harboring blaVIM-1 and blaIMP-45. Structural and molecular docking studies explored the PBP3 (P527S) mutation’s role in aztreonam resistance.
Results: From February 2022 to July 2024, 17 ST313 VIM-1-IMP-45 CRPA strains from 10 transplant patients were identified. All strains were extensively drug-resistant but sensitive to colistin and cefiderocol. WGS showed blaIMP-45 and blaVIM-1 on an IncP-2 megaplasmid. Phylogenetic analysis indicated high homology with plasmids carrying blaIMP-45. Further analysis of the genetic environment showed that the IncP-2 plasmid co-harboring blaVIM-1 and blaIMP-45 was formed by the insertion of a Tn3-family transposon carrying blaVIM-1 into the IncP-2 plasmid carrying blaIMP-45. In addition aztreonam-resistant strains (14/15) had a PBP3 (P527S) mutation, with molecular docking studies suggesting reduced aztreonam binding.
Conclusions: This study reports a clonal outbreak of ST313 P. aeruginosa strains co-producing IMP-45 and VIM-1 carbapenemases in a tertiary hospital. The evolutionary path of the IncP-2 plasmid co-harboring blaIMP-45 and blaVIM-1 was elucidated.
Introduction
Carbapenem-resistant Pseudomonas aeruginosa (CRPA) is a major global public health threat, significantly increasing morbidity and mortality rates, especially among immunocompromised individuals (Azam and Khan, 2019). The treatment of CRPA infections is challenging due to its remarkable ability to resist multiple antibiotics (Pang et al., 2019). One of the mechanisms for carbapenem resistance is the production of metallo-β-lactamases (MBLs), mainly consisting of Verona integron-encoded metallo-β-lactamase (VIM) and imipenemase (IMP) (Cornaglia et al., 2011). Typically, VIM-1 is commonly found in Enterobacteriaceae (Walsh et al., 2005; Vatopoulos, 2008). Additionally, P. aeruginosa isolates with VIM-1 have been identified in Spain and Turkey (Malkoçoğlu et al., 2017; PÉrez-VÁzquez et al., 2020), but are rarely reported in China (Hong et al., 2015). Currently, only a few sporadic studies of VIM-1-producing P. aeruginosa have been reported in China (Kong et al., 2015; Cai et al., 2016; Jing et al., 2018; Kang et al., 2023).
In China, the presence of MBL genes such as blaIMP-1, blaIMP-4, blaIMP-6, blaIMP-8, blaIMP-9, blaIMP-10, and blaIMP-45 (Hong et al., 2015) has been documented. Initially, blaIMP-9 was detected in P. aeruginosa isolates from Guangzhou (Xiong et al., 2006), with subsequent outbreaks occurring in 2000 and between 2005 and 2007, respectively (Xiong et al., 2006; Chao et al., 2008). IMP-45, distinguished by a single amino acid substitution (G214S) in IMP-9, was first reported in 2014 in a P. aeruginosa strain of canine originated from Beijing, demonstrating increased resistance to meropenem compared to imipenem (Wang et al., 2014).
In this study, we report an outbreak of CRPA strains co-harboring blaVIM-1 and blaIMP-45 on IncP-2 plasmids in a tertiary hospital in China, leading to poor prognosis in transplant patients.
Material and methods
Bacterial isolation and clinical data collection and antimicrobial susceptibility testing
During February 2022 to July 2024, 17 nonduplicated CRPA strains co-harboring blaVIM-1 and blaIMP-45, collected from 10 patients, were enrolled in the study. Demographics and relevant clinical data of the patients were obtained through review of medical records. Susceptibility to ticarcillin-clavulanic acid, ceftazidime, cefoperazone-sulbactam, cefepime, meropenem, imipenem, amikacin, tobramycin, ciprofloxacin, levofloxacin, and colistin were measured using VITEK®2 system (BioMérieux, Marcy l’Étoile, France) following the manufacturer’s instructions. Minimal inhibitory concentrations (MICs) were determined for cefiderocol, piperacillin-tazobactam, aztreonam (AZT) and ceftazidime/avibactam (CZA) by broth microdilution method and interpretation was according to recommendations of the CLSI (Lewis and James, 2022), P. aeruginosa ATCC27853 served as quality control strains.
Comparative genomic analysis of blaIMP-45 carrying plasmids
To better understand the features of genetic environments surrounding blaIMP-45 and blaVIM-1 genes, we searched the Genbank database on NCBI and obtained 15 intact plasmids harboring blaIMP-45 worldwide from China as of October 1, 2024.
Phylogenetic analysis
As of October 1, 2024, 53 P. aeruginosa genomes assigned to ST313 were downloaded from the RefSeq database on NCBI, We excluded strains with unknown country of origin and collection time, retaining 46 ST313 P. aeruginosa isolates and 17 ST313 VIM-1-IMP-45 CRPA strains for phylogenetic analysis. The phylogenetic tree for all ST313 P. aeruginosa genomes was constructed according to their SNPs by using Snippy version 4.6.0 (https://github.com/tseemann/snippy). ST313 P. aeruginosa genomes’ SNPs were filtered to remove recombination using Gubbins v3.3.5 (Croucher et al., 2015). The phylogenetic tree of the IncP-2 plasmids carrying blaIMP-45 and the IncP-2 plasmids co-harboring blaVIM-1 and blaIMP-45 were also constructed based on their SNPs using Snippy version 4.6.0. All maximum likelihood phylogenetic trees were inferred from a core genome alignment by using Raxml v8.2.12 (Stamatakis, 2014). The specific parameters for Snippy were: alignment coverage threshold –minfrac = 0.9 and minimum depth –mincov = 10. For Gubbins, we used maximum iterations = 5 and P-value threshold = 0.05. All trees were visualized in iTOL (https://itol.embl.de).
Cefiderocol induction resistance experiments and conjugation assay of plasmid co-carrying blaVIM-1 and blaIMP-45
To induce cefiderocol resistance in P. aeruginosa, we followed a previously reported method (Nurjadi et al., 2022) with slightly modifications. Briefly, The amikacin-sensitive clinical single colony of P. aeruginosa AS01 strain grown on Columbia agar supplemented with 5% sheep blood was inoculated into 5 mL of cation-adjusted Mueller-Hinton broth (CA-MHB) (BD Diagnostics, Germany) and incubated at 37°C with constant shaking at 200 rpm for 18 hours. After overnight incubation, 100 μL of the culture was transferred into a fresh 5 mL of CA-MHB, and cefiderocol (Shionogi, Japan) was added to achieve a final concentration of 0.5 mg/L. The culture was then incubated under the same conditions as the initial culture. This process was repeated daily, with the cefiderocol concentration doubled at each passage, until no visible turbidity or bacterial growth was observed after overnight incubation, or until the cefiderocol concentration reached 64 mg/L. For population analysis profiling, 10 μL of the bacterial suspension from the overnight culture was plated onto Columbia blood agar, and 10 single colonies were randomly selected for cefiderocol susceptibility testing using broth microdilution. Conjugation experiments were performed with strain AS02, which exhibited resistance to cefiderocol but remained susceptible to amikacin and was selected as the recipient strain, while strain CJ05 served as the donor strain. Donor and recipient bacteria were separately cultured in LB broth at 37°C until reaching logarithmic growth phase (OD600 = 0.6-0.8), then mixed in a 1:1 ratio and incubated at 37°C overnight for conjugation. The conjugation mixture was subjected to 10-fold serial dilution and plated on Mueller-Hinton agar containing cefiderocol (4 mg/L) and amikacin (16 mg/L), followed by incubation at 37°C for 18–24 hours. Putative transconjugants were confirmed by whole-genome sequencing. Additional conjugation experiments were conducted using Escherichia coli J53 as an alternative recipient strain. Each conjugation experiment was repeated three times. The antimicrobial susceptibility tests mentioned above were performed on strains AS01, AS02, and transconjugant TC01 using the VITEK®2 system and broth microdilution.
Whole-genome sequencing and bioinformatics analysis
Genomic DNA from VIM-1-IMP-45-CRPA strains CJ01-CJ17, and transconjugant strain TC01 was extracted and were sequenced using an Illumina NovaSeq PE150 at the Beijing Novogene Bioinformatics Technology Co., Ltd. Raw reads were filtered to remove low-quality sequences and adaptors. De novo assembly was conducted using SOAP de novo 2.04 (Li et al., 2008; Li et al., 2010), SPAdes 3.10.0 (Bankevich et al., 2012), and ABySS 1.3.7 (Simpson et al., 2009). The assembly results were integrated with CISA 1.3 software. The gap in preliminary assembly results was filled using Gapcloser 1.12. In addition, whole-genome sequences for CJ05, AS01 and AS02 were obtained through Illumina NovaSeq PE150 and nanopore sequencing on MinION flow cells. Hybrid assemblies of Illumina short reads and MinION long reads were prepared with Unicycler version 0.4.8 (Wick et al., 2017). Multilocus sequence typing (MLST) was performed by using an MLST tool (https://github.com/tseemann/mlst). Antimicrobial drug resistance and virulence genes were identified with ABRicate version 1.0.0 (https://github.com/tseemann/abricate) according to the National Center for Biotechnology Information (NCBI) AMRFinderPlus (https://www.ncbi.nlm.nih.gov) and virulence factor (http://www.mgc.ac.cn/VFs) databases. Pairwise single-nucleotide polymorphism (SNP) distance was evaluated with Snp-dists (https://github.com/tseemann/snp-dists). Sequence comparisons were performed by using Easyfig version 2.2.3 and annotated by Prokka and Bakta (Seemann, 2014; Schwengers et al., 2021). Alignments and visualization of plasmids were generated by BLAST Ring Image Generator (BRIG) software version 0.95 (https://sourceforge.net/projects/brig). Linear alignments of blaIMP-45-bearing structures were generated using ggplot2 and gggenes in R-4.4.2. The heatmap was translated using TBtools-II (Chen et al., 2023). Platon (Schwengers et al., 2020) was used to predict plasmids in transconjugant TC01 and all ST313 VIM-1-IMP-45-CRPA except CJ05.
Structure modeling and molecular docking
Retrieving the RCSB Protein Data Bank (PDB), the crystal structures of PBP3 complexed with aztreonam (PDB DOI: https://doi.org/10.2210/pdb3PBS/pdb) was applied as the structure template in the present study (Han et al., 2010). After PSI-BLAST (Altschul et al., 1997) and MUSCLE alignment (Edgar, 2022), the homology models of PBP3(P527S) were both built in Schrödinger. The protein structure was refined and preprocessed using the OPLS4 force field (Lu et al., 2021), following the ligands preparing for diverse ionization states and isomers. In the covalent docking, the aztreonam of PBP3 complexed with aztreonam was set as the centroid of grid box, and the β-lactam was chosen as reaction type for pose prediction. After completion of docking, the Prime MM-GBSA scores of the top one output poses for each ligand was calculated for comparing the binding affinities of the ligands (Li et al., 2011).
Result
Outbreak of ST313 P. aeruginosa co-harboring blaIMP-45 and blaVIM-1
During 2022-2024, 17 ST313 P. aeruginosa strains co-harboring blaIMP-45 and blaVIM-1 were identified, including 2 isolates from 2022, 11 from 2023, and 4 from 2024. Following comprehensive disinfection measures implemented in the ward, no further occurrences of VIM-1-IMP-45-CRPA strains were detected. The VIM-1-IMP-45-CRPA strains were isolated from 10 immunocompromised patients, all of whom had undergone lung or kidney transplants (Table 1). The cohort consisted of 9 males and 1 female, aged between 40 and 70 years, all of whom presented with complex medical histories. Among the 10 patients, 8 had VIM-1-IMP-45-CRPA detected in their bronchoalveolar lavage fluid (BALF), of whom 7 received ceftazidime/avibactam combined with aztreonam (CZA+AZT) treatment. Another 2 patients had VIM-1-IMP-45-CRPA detected in their blood but did not receive CZA+AZT treatment. Among patients who received CZA+AZT treatment, the mortality rate was 12.5% (1/8), while among patients who did not receive CZA+AZT treatment, the mortality rate was 100% (3/3), with 66.7% (2/3) of the deaths associated with bloodstream infections (Table 1). Through phylogenetic analysis of 63 ST313 genomes, (46 from the NCBI Reference Sequence database and 17 from this study), using the midpoint rooting method, we found that ST313 P. aeruginosa can be divided into two subgroups (Figure 1), revealing contrasting evolutionary trajectories and transmission dynamics. Subgroup 2 demonstrated tight clonal clustering with multiple closely related strains predominantly originating from China during 2017-2024, indicating recent clonal expansion and suggesting a single-source outbreak with rapid person-to-person transmission capabilities. In contrast, subgroup 1 exhibited greater phylogenetic diversity with strains distributed across multiple countries and spanning a broader temporal range (1997-2022), reflecting longer evolutionary timescales and endemic circulation patterns. The transmission timeline analysis revealed two distinct epidemiological phases: a recent epidemic emergence in subgroup 2 concentrated within a 2-year period, and historical global circulation in subgroup 1 spanning over two decades. Notably, the evolutionary relationship analysis demonstrated that while Subgroup 1 maintained ancestral ecological flexibility with 27.5% (11/40) environmental or animal isolates (Supplementary Table S3) distributed throughout the phylogeny, subgroup 2 exhibited marked anthropocentric specialization with only a single animal isolate among an otherwise exclusively human-derived population.
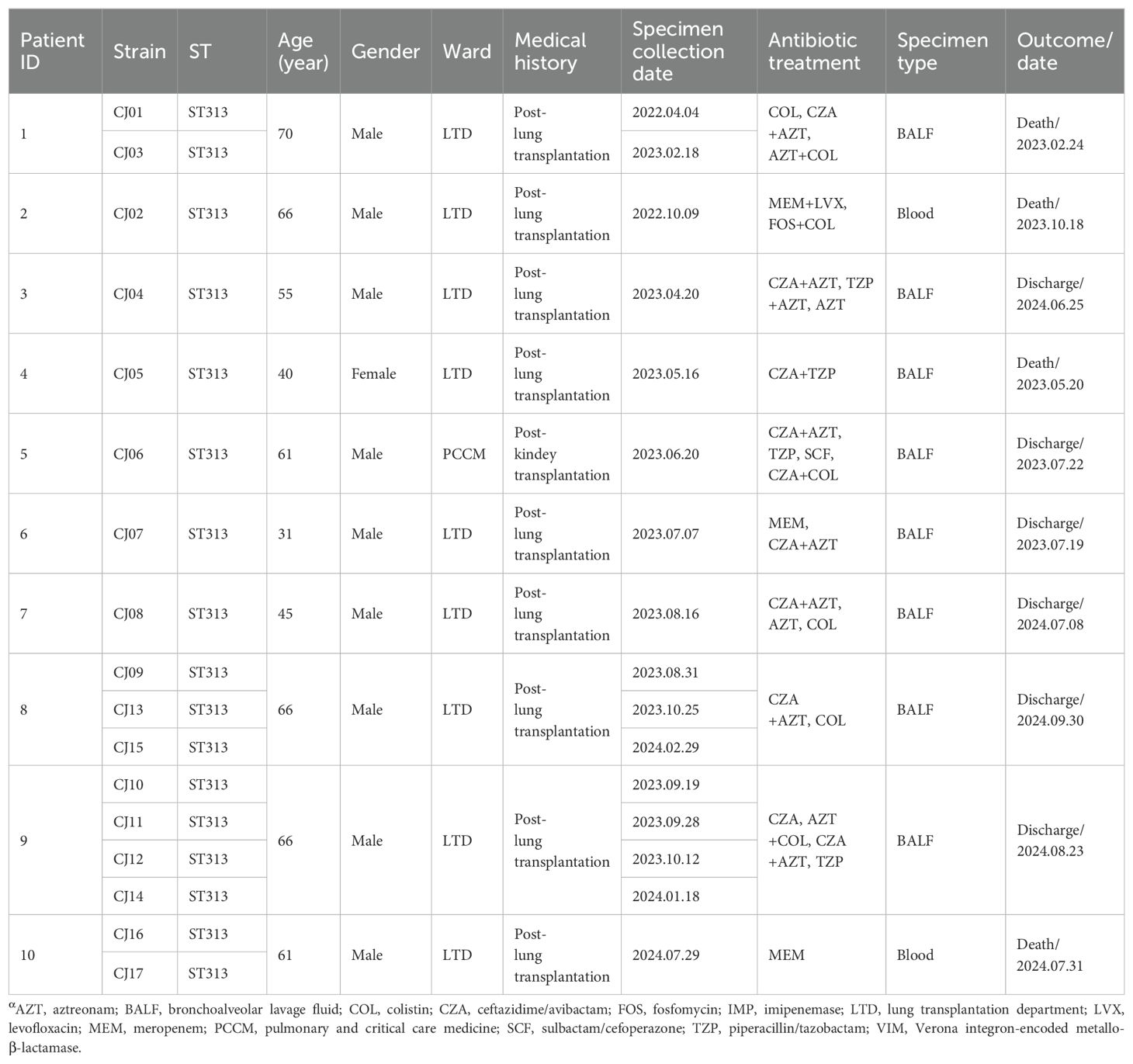
Table 1. Clinical data of 10 patients infected by Pseudomonas aeruginosa co-producing VIM-1 and IMP-45 carbapenemases, 2022–2024α.
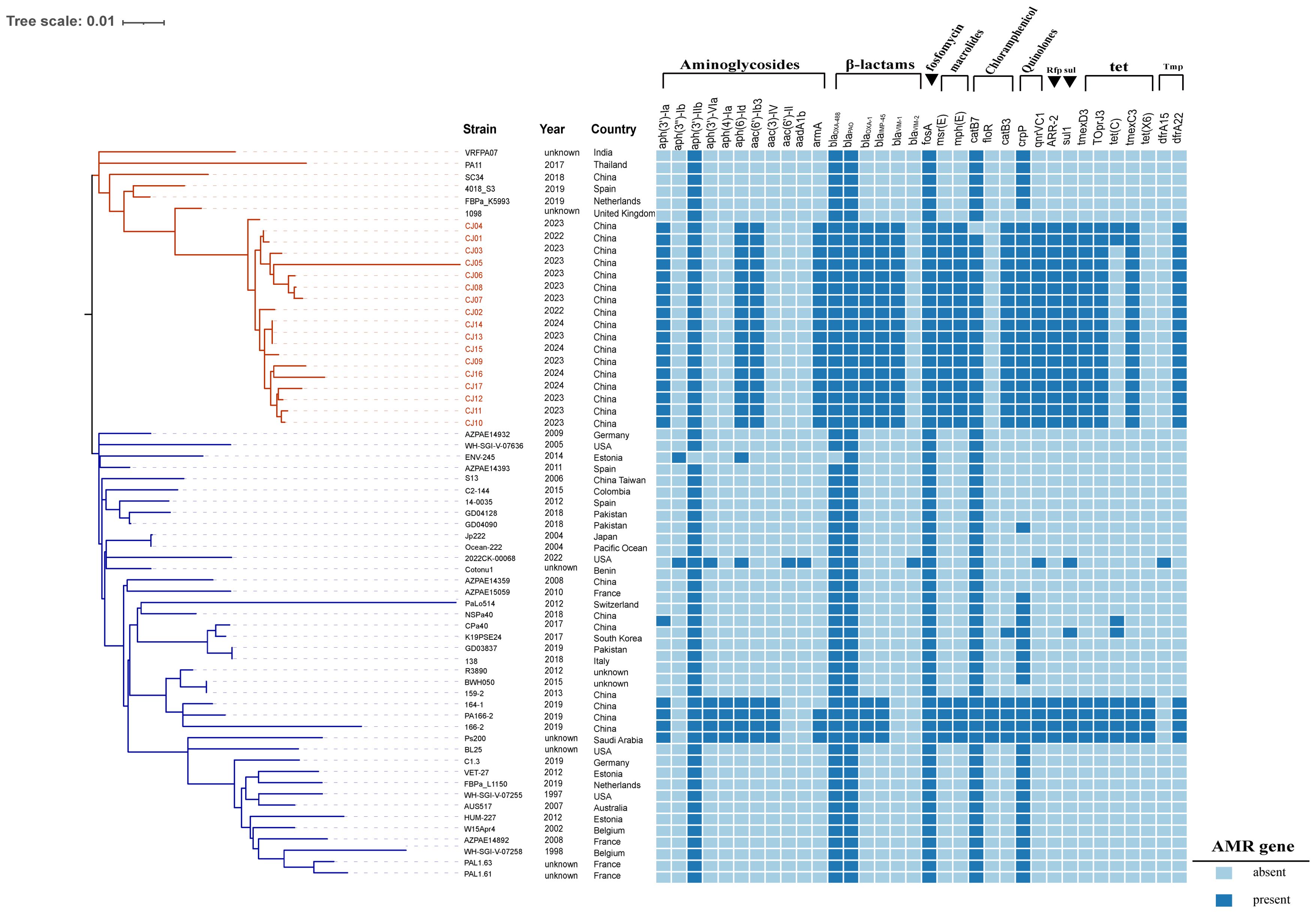
Figure 1. Phylogenetic analysis and epidemic distribution of ST313 P. aeruginosa. 63 ST313 P. aeruginosa phylogenetic analysis, blaVIM-1 and blaIMP-45 resistance genes and strains carrying blaVIM-1 and blaIMP-45 genes are marked in red, AMR gene: Antimicrobial resistance gene. In the strains, red markings indicate VIM-1–IMP-45 CRPA strain. In the phylogenetic tree, the blue branches represent subgroup1, and the red branches represent subgroup2.
Characteristics of VIM-1-IMP-45-CRPA strains
All 17 VIM-1-IMP-45-CRPA strains isolates shared almost identical resistance genes, differing by 0–192 SNPs. Except for strain CJ05, the differences among the remaining strains range from 0 to 79 SNPs, demonstrating a high degree of relatedness among those isolates and clonal transmission of -producing P. aeruginosa ST313 in China (Supplementary Figure S1). Notably, all 17 ST313 VIM-1-IMP-45-CRPA strains possessed virulence genes similar to the highly virulent strain PA14, including the type III secretion system genotypes exoU+, exoS+ and exoY+(Supplementary Figure S2).
Drug susceptibility testing showed that, except for the CJ13 and CJ04, intermediate and sensitive to aztreonam, respectively, all ST313 VIM-1-IMP-45-CRPA strains were resistant to carbapenems (meropenem and imipenem), several β-lactams (ceftazidime, cefepime, piperacillin/tazobactam, cefoperazone/sulbactam, ceftazidime/avibactam, aztreonam), quinolones (ciprofloxacin, levofloxacin), and aminoglycosides (amikacin, tobramycin). But all strains remained sensitive to colistin and cefiderocol (Table 2).
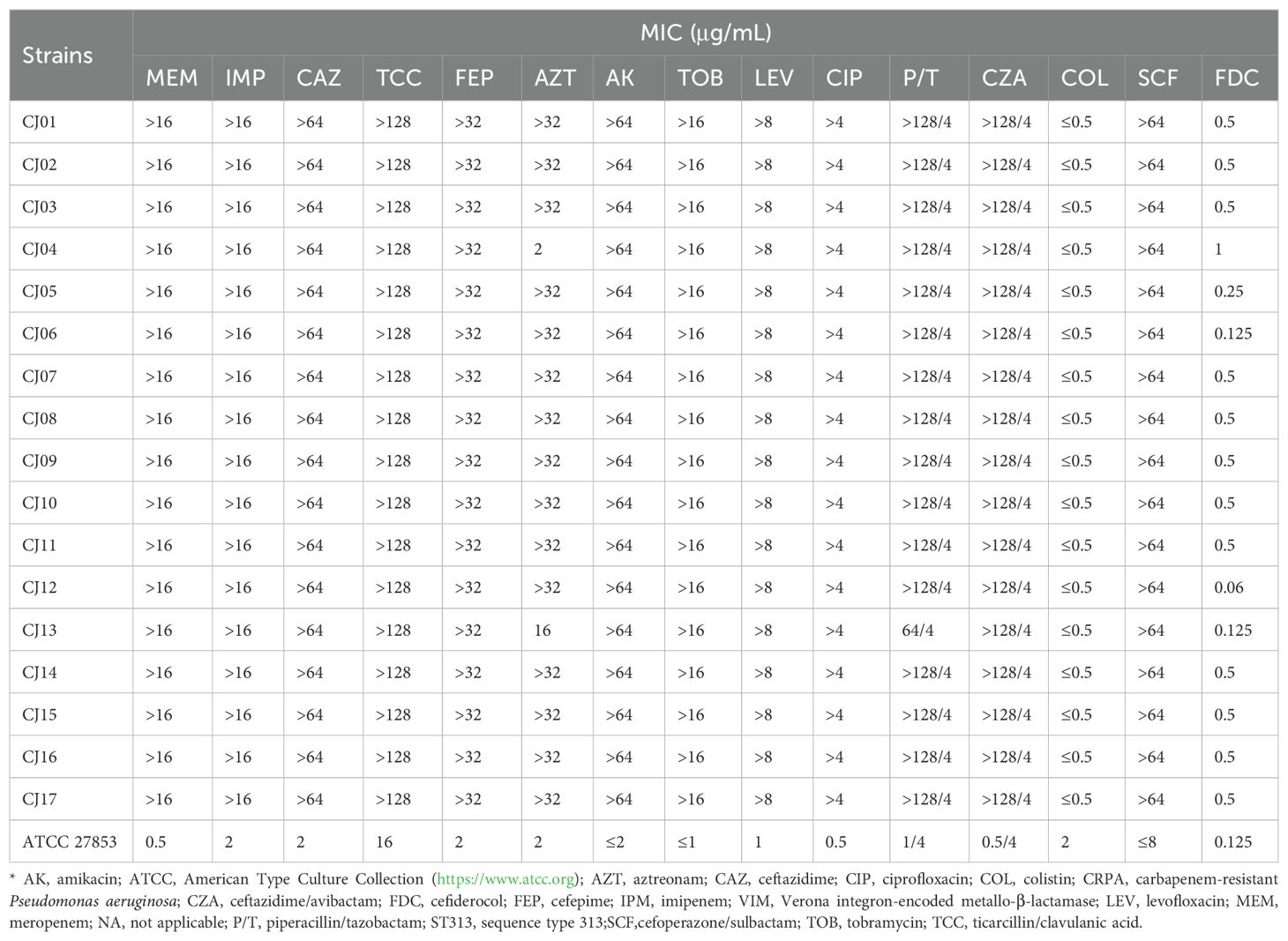
Table 2. Antimicrobial drug susceptibility profiles for the 17 VIM-1–IMP-45–CRPA strains from China*.
Interestingly, almost all aztreonam-resistant VIM-1-IMP-45-CRPA strains (14/15) harbored a mistranslation mutation (P527S) in PBP3. The non-mutated PBP3 protein was consistent with the PBP3 protein of the standard strain PAO1.Based on the PBP3(PAO1) structure in the RCSB PDB database, we used Schrödinger to predict the structure of the point-mutated PBP3 (P527S) to analyze interactions with aztreonam. Subsequently, molecular docking of aztreonam with both PBP3(PAO1) and PBP3(P527S) was performed using Schrodinger software (Supplementary Figure S4). The docking score of the PBP3(P527S) protein decreased (docking score: -9.911kcal/mol to -8.770kcal/mol). Subsequently the MM/GBSA binding free energy from the top docking pose of aztreonam to PBP3 was calculated. From our analysis, PBP3(P527S) exhibited less negative binding free energy values for aztreonam (-21.36 kcal/mol for PBP3(PAO1), -20.06 kcal/mol for PBP3(P527S)), suggesting the weakened binding affinity to PBP3(P527S).
Evaluating the transferability of the IncP-2 plasmid co-harboring blaVIM-1 and blaIMP-45
To assess plasmid transferability, we selected strain CJ05 for further experiment. CJ05 strain contains an IncP-2 megaplasmid co-harboring blaVIM-1 and blaIMP-45 by whole-genome sequencing. Given all VIM-1-IMP-45-CRPA strains were resistance to amikacin but sensitive to cefiderocol, we chosen a clinical amikacin-susceptible P. aeruginosa strain AS01 for cefiderocol induction resistance experiments. An cefiderocol induced resistance P. aeruginosa strain, AS02, was used for conjugation experiments. The AS01 strain was identified as ST463 P. aeruginosa. The whole-genome sequencing revealed that it harbors a plasmid of 41,101 bp in size carrying the blaKPC-2 gene. Other resistance genes, including aph(3’)-IIb, blaPAO, blaOXA-486, fosA, catB7, and crpP, are located on the chromosome. Antibiotic susceptibility testing showed that the strain is resistant to carbapenems (meropenem and imipenem), several β-lactams (ceftazidime, cefepime, piperacillin/tazobactam, cefoperazone/sulbactam, ceftazidime/avibactam, aztreonam), and quinolones (ciprofloxacin, levofloxacin). However, it remains sensitive to aminoglycosides (amikacin, tobramycin), colistin, and cefiderocol (Supplementary Table S2). By analyzing the genome of the AS02 strain, we found that the blaKPC-2 gene on its plasmid had evolved into blaKPC-33, while other resistance genes remained identical to those in the AS01 strain. The antibiotic susceptibility profile of AS02 was similar to that of AS01, except that AS02 exhibited resistance to cefiderocol (Supplementary Table S2). Additionally, we observed mutations in certain genes in the AS02 strain, including hasR. Furthermore, conjugation experiments were conducted using E. coli J53 as the recipient strain. Conjugation experiments showed that the IncP-2 plasmid could be transferred within P. aeruginosa, but not to E. coli I53. Using Platon to analyze the plasmid sequence of the transconjugant TC01 revealed the presence of resistance genes, including aac(6’)-Ib3, armA, blaKPC-33, blaIMP-45 and blaVIM-1. Drug susceptibility test showed that it was resistant to amikacin and cefiderocol, but only sensitive to colistin (Supplementary Table S2).
Evolutionary trajectory of the IncP-2 plasmid co-harboring blaVIM-1 and blaIMP-45
Using Platon predict plasmids in transconjugant TC01 and all ST313 VIM-1-IMP-45-CRPA strains except CJ05. Phylogenetic analysis of the system shows high homology in the IncP-2 plasmids of ST313 VIM-1-IMP-45-CRPA and transconjugant TC01 in the VIM-1-IMP-45-CRPA plasmids (Supplementary Figure S3).
Third-generation sequencing reveals that the genome of the CJ05 strain contains a chromosome and an IncP-2 megaplasmid (Figure 2A). This plasmid carries blaVIM, blaIMP, and other resistance genes like tmexCD3-toprJ3. The blaVIM-1 gene is located on a Tn3 family transposon (Figure 2C), including a complete Tn402 module. The genetic background of blaIMP-45 is similar to previously reported IncP-2 plasmids carrying blaIMP-45(Supplementary Table S1), situated on a Tn6485-like transposon (Figures 2B and 3). The blaIMP-45 gene is found directly downstream of the transposable element TnAs1 and within a class 1 integron, In786, with the gene arrangement intI1-aacA4-blaIMP-45-blaOXA-1-catB3. In786 is located within a Tn6485-like transposon. Similar genetic contexts have been detected or reported in several other IncP-2 plasmids, including pPA166-2-MDR, pR31014-IMP, pNF143349, and pPAHT-1 (Figure 2B). Subsequent snp-based phylogenetic analysis of the blaIMP-45-carrying IncP-2 plasmid revealed that pCJ05 is homologous to other blaIMP-45-carrying IncP-2 plasmids (Figure 3). Based on this observation, we hypothesize that pCJ05 acquired blaVIM-1 through horizontal gene transfer (HGT) from a blaIMP-45-carrying IncP-2 plasmid. Further analysis of the genetic context surrounding blaVIM-1 revealed direct evidence of transposition. Specifically, we identified both direct repeat (DR) and inverted repeat (IR) sequences flanking blaVIM-1. We discovered that blaVIM-1 is carried by a Tn402-like transposon, which subsequently inserted into a Tn3-family transposon harboring partial mer operon genes, resulting in the formation of a novel Tn3-family transposon (Figure 4).The Tn402-like transposon was found to carry a complete tniABQR transposition module, while the Tn3-family transposon with partial mer operon contained tnpA but lacked tnpR. Comparative analysis using the NCBI database identified two plasmids carrying these transposons, pVIM-1-ZDHY316(CP064944) and pTTS12(CP009975), respectively. Based on these findings, we propose an evolutionary trajectory for the co-harboring blaVIM-1 and blaIMP-45 IncP-2 plasmid. First of all, the Tn402-like transposon carrying blaVIM-1 transposed into the Tn3-family transposon containing partial mer operon genes, forming a novel blaVIM-1-carrying Tn3-family transposon. This composite transposon was subsequently mobilized onto the blaIMP-45-harboring IncP-2 plasmid, ultimately resulting in the emergence of an IncP-2 plasmid co-harboring both blaVIM-1 and blaIMP-45 (Figure 4).
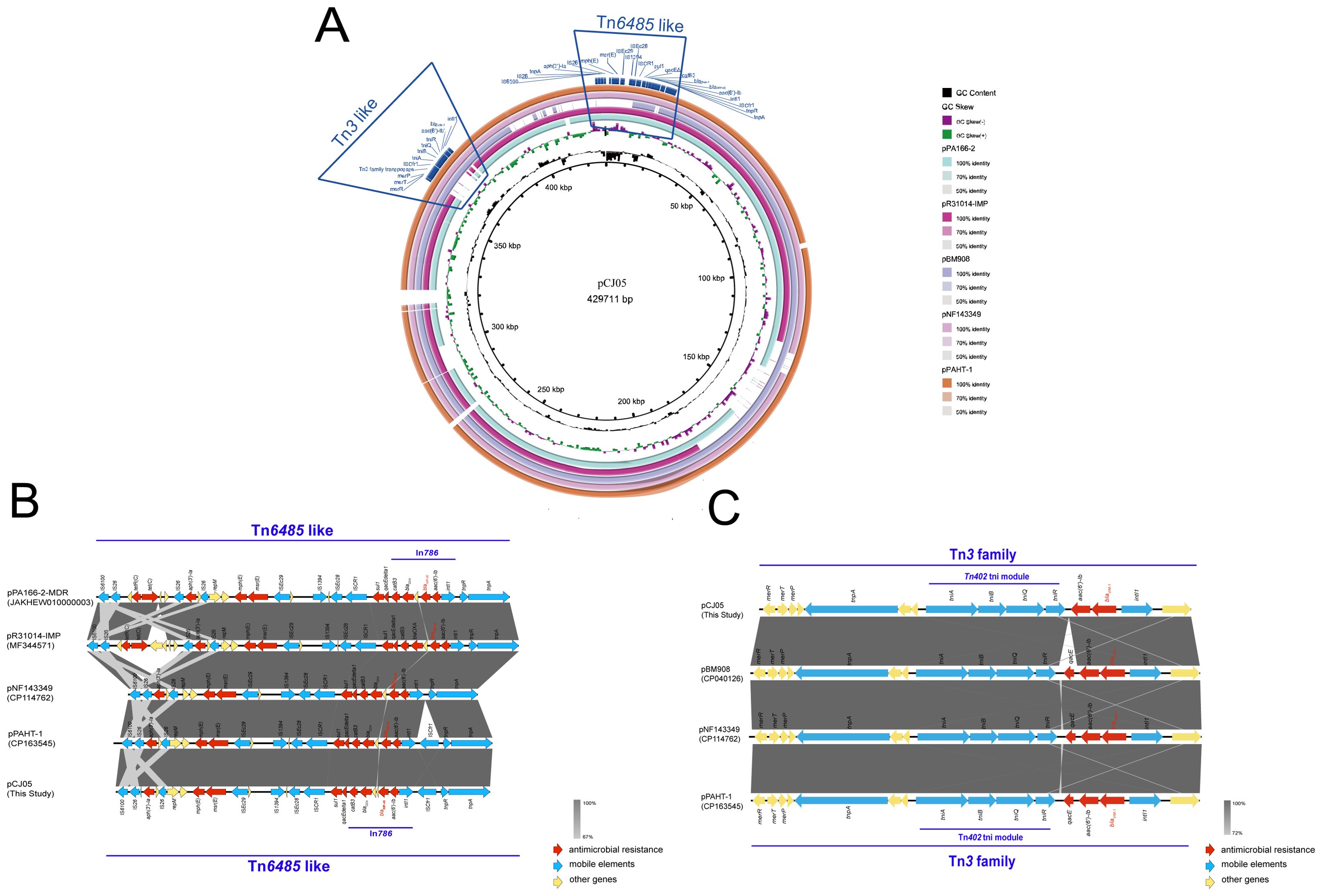
Figure 2. Genetic context of blaIMP-45 and blaVIM-1. (A) Genome comparison of plasmid pCJ05 co-harboring blaVIM-1 and blaIMP-45. Schematic map of plasmid pCJ05, this plasmid sequence was compared with plasmids pPA166-2(accession number:JAKHEW010000003), pR31014-IMP(accession number:MF344571), pNF143349(accession number:CP114762), and pPAHT-1(accession number:CP163545); (B, C) Linear characterization of Tn6485-like transposon carrying blaIMP-45 and Tn3-family transposon carrying blaVIM-1 in pCJ05 plasmid with similar sequence. Red, blue, and yellow arrows denote antimicrobial resistance genes, mobile elements, and other protein-encoding genes, respectively. The Δ symbol indicates that the gene is truncated.
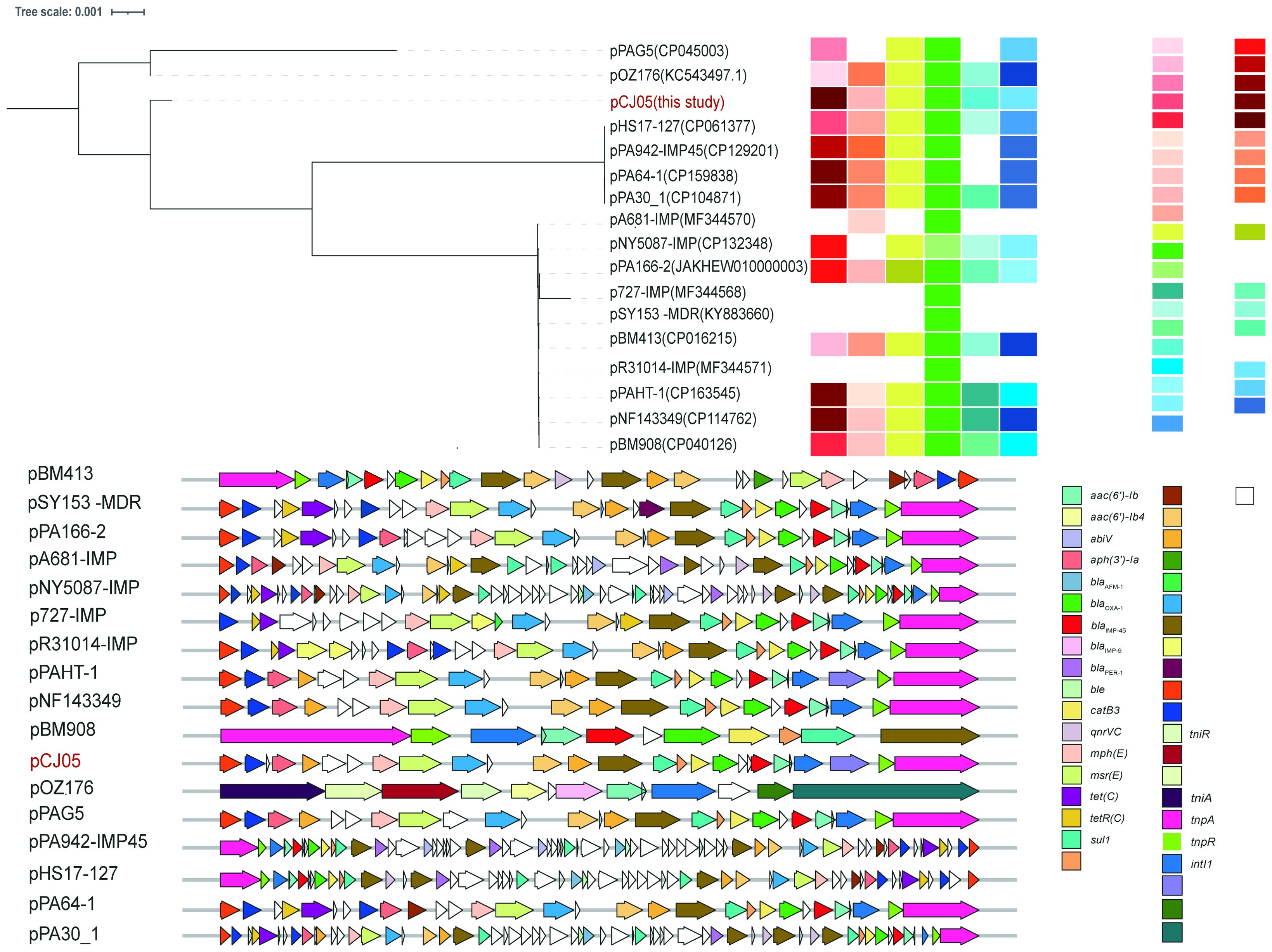
Figure 3. Evolution relationship and comprehensive information among IncP-2 plasmids carrying blaIMP-45. Plasmids were colored according to year, STs, host, strains, sample, country/province, and genetic context of blaIMP-45. The snp-based maximum likelihood phylogenetic tree was built by RaxML with pOZ176 as the reference. The Interactive Tree of Life (https://itol.embl.de) was used for visualization.
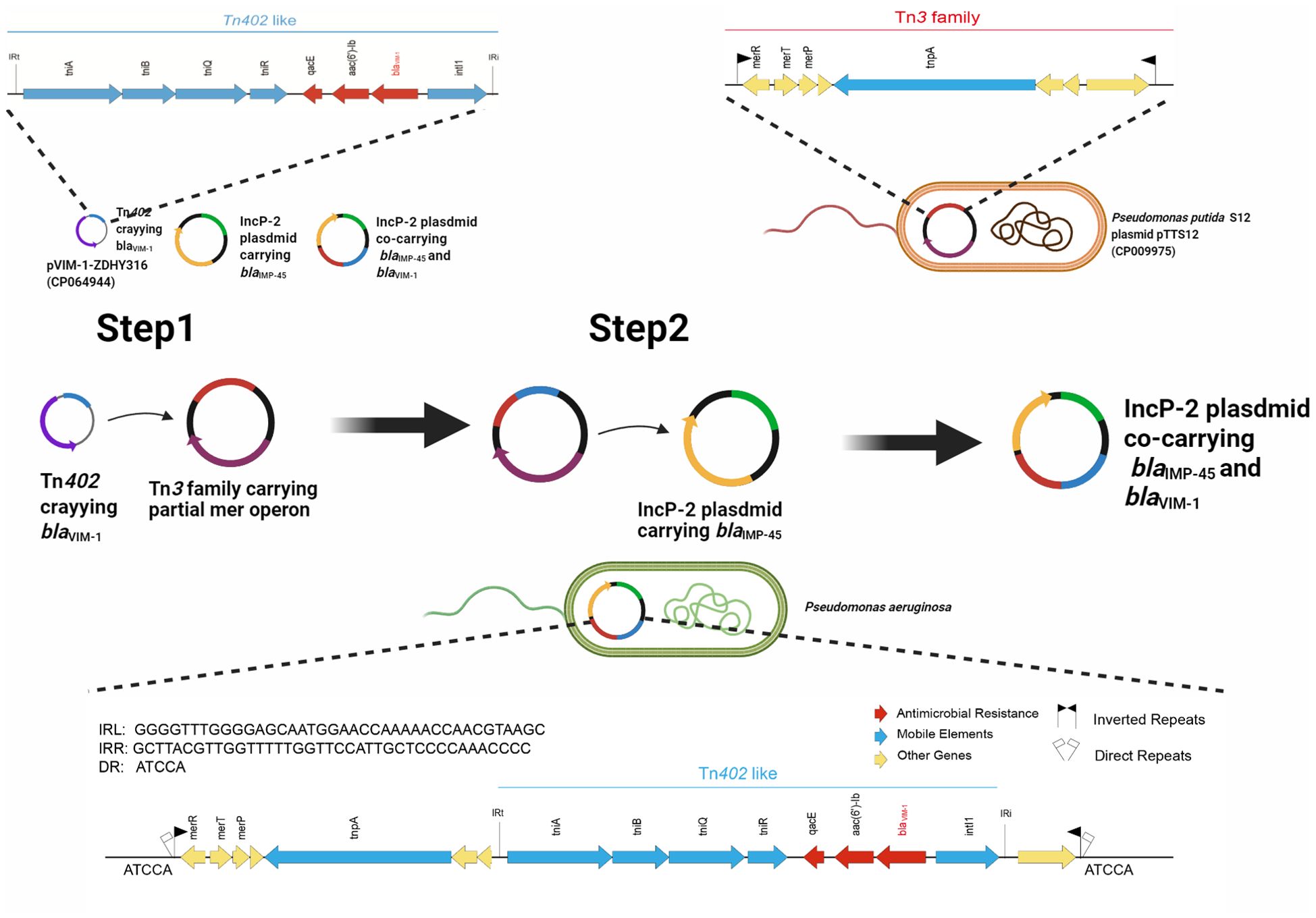
Figure 4. Evolutionary trajectory for the co-harboring blaVIM-1 and blaIMP-45 IncP-2 plasmids. Proposed formation mechanism of co-harboring blaVIM-1 and blaIMP-45 IncP-2 plasmids is as follows by sequence alignment. Step 1: Tn402-like transposon carrying blaVIM-1 was infused to Tn3-family transposon containing partial mer operon genes to generate a new Tn3-family transposon carrying blaVIM-1. Step 2: The new Tn3-family transposon mobilized onto the blaIMP-45-harboring IncP-2 plasmid, resulting in the emergence of the IncP-2 plasmid co-harboring both blaVIM-1 and blaIMP-45. Figure was created with BioRender.
Discussion
CRPA carrying carbapenemases is an opportunistic Gram-negative pathogen and a common cause of nosocomial infections, particularly respiratory tract infections. The IncP-2 plasmid harboring the blaIMP-45 gene was initially identified in China and subsequently disseminated rapidly across the country. In 2014, canine-derived P. aeruginosa carrying blaIMP-45 on IncP-2 plasmids was first reported in Beijing (Wang et al., 2014). Subsequently, the IncP-2 plasmids harboring blaIMP-45 was identified in P. aeruginosa isolates from various regions in China, spanning multiple sequence types (STs), including high-risk STs such as ST277 and ST463, indicating that P. aeruginosa with IncP-2 plasmids carrying blaIMP-45 has been disseminated in the country. IncP-2 plasmid subtypes facilitate the spread of blaIMP-45 among genetically diverse P. aeruginosa and have incorporated various resistance genes during their evolution, such as blaAFM-1, blaPER-1, tmexCD-oprJ, armA, and qnrVC1 (Zhang et al., 2021; Dong et al., 2022; Zhou et al., 2023). In 2018, the ST277 P. aeruginosa strain PA298 (CP040127), co-harboring blaVIM-1 and blaIMP-45, was identified in Guangzhou, China, with both resistance genes located on the IncP-2 plasmid pBM908 (CP040126). Later, in 2022, VIM-1–IMP-45-CRPA strains NF143349 (CP114761) and PAHT-1 (CP163544) were detected in Guangzhou, Zhuhai, and Shanxi, belonging to MLST types ST277 and ST188, respectively. Comparative analysis revealed that their plasmids shared 97% and 98% coverage, with 99.1% and 100% identity to pCJ05, respectively. A recent study also revealed that IncP-2 plasmids carrying blaNDM-1 and blaVIM-2 have caused neonatal sepsis in Morocco (Daaboul et al., 2024). IncP-2 plasmids are increasingly becoming reservoirs for metallo-β-lactamases in Pseudomonas spp worldwide.
In the study, we documented an outbreak of P. aeruginosa co-producing the VIM-1 and IMP-45 in the IncP-2 plasmid among transplant patients in a tertiary hospital in China. Our study documented the persistent clonal dissemination of VIM-1–IMP-45 CRPA strains in a tertiary hospital and investigates a possible evolutionary trajectory for the emergence of these strains. In our study, among 10 patients infected with the VIM-1-IMP-45-CRPA strain, two patients who did not receive CZA+AZT combination therapy developed bloodstream infections caused by the VIM-1-IMP-45-CRPA strains. This suggests that CZA+AZT combination therapy may reduce the risk of bloodstream infections caused by the VIM-1-IMP-45-CRPA strains in transplant patients. Our research also indicates that cefiderocol could be a better treatment option for VIM-1–IMP-45 CRPA strains. Furthermore, PBP3 has been shown to be a common adaptive target among P. aeruginosa isolates from patients treated with β-lactams (Clark et al., 2019). In addition, the mechanisms of aztreonam resistance in P. aeruginosa are diverse, including the overexpression of the mexAB-oprM efflux system, alterations in ftsI (PBP3) leading to disrupted drug binding, and mutations or overexpression of chromosomal ampC β-lactamase or changes in its coding sequence, which enhance active drug efflux. Previous studies (Jorth et al., 2017; Clark et al., 2019; McLean et al., 2019), as well as our research, have identified the PBP3 (P527S) mutation in clinical P. aeruginosa strains resistant to aztreonam. The P527S mutation is hypothesized to contribute to resistance, not conclusively demonstrated. Through molecular docking analysis, structural bioinformatics revealed that the PBP3 (P527S) mutation results in reduced binding affinity to aztreonam. However, whether this slight reduction in binding affinity is sufficient to cause a significant increase in aztreonam MIC values requires further investigation to confirm. To our knowledge, this is the first report of an outbreak of VIM-1–IMP-45 CRPA strains among transplant patients.
In summary, the VIM-1–IMP-45 CRPA strains exhibit extensive antimicrobial resistance and resulted in a high mortality rate, enabling them to withstand host defenses and clinical interventions in transplant patients, thereby facilitating their sustained transmission. Infections caused by these strains are associated with high mortality rates, particularly among immunocompromised transplant recipients, indicating the critical need for effective infection prevention and control strategies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, BioProject: PRJNA1222284.
Ethics statement
The studies involving humans were approved by the ethics committee of the China-Japan Friendship Hospital (2022-KY-054). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YM: Writing – review & editing, Conceptualization, Formal analysis, Writing – original draft, Data curation, Investigation. ZCL: Data curation, Writing – review & editing, Formal analysis. YZ: Data curation, Formal analysis, Writing – review & editing. QL: Writing – review & editing, Data curation. FZ: Formal analysis, Writing – review & editing. HZ: Writing – review & editing, Formal analysis. XY: Writing – review & editing, Formal analysis. ZYL: Writing – review & editing, Data curation. BL: Writing – review & editing, Conceptualization, Formal analysis, Funding acquisition, Resources, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (grant number CIFMS 2021-I2M-1-030).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1623241/full#supplementary-material
References
Altschul, S. F., Madden, T.L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. doi: 10.1093/nar/25.17.3389
Azam, M. W. and Khan, A. U. (2019). Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov. Today 24, 350–359. doi: 10.1016/j.drudis.2018.07.003
Bankevich, A., Nurk, S., Antipov, D., Gurevich, A. A., Dvorkin, M., Kulikov, A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477. doi: 10.1089/cmb.2012.0021
Cai, S., Chen, Y., Song, D., Kong, J., Wu, Y., and Lu, H. (2016). Study on the resistance mechanism via outer membrane protein OprD2 and metal β-lactamase expression in the cell wall of Pseudomonas aeruginosa. Exp. Ther. Med. 12, 2869–2872. doi: 10.3892/etm.2016.3690
Chao, Z., Xiao-Feng, W., Dan-Hong, S., Jin-Ping, Y., and Nan-Shan, Z. (2008). Outbreak of Pseudomonas aeruginosa producing IMP-9-type metallo-beta-lactamase in Guangzhou, China. Int. J. Antimicrob. Agents 32, 363–365. doi: 10.1016/j.ijantimicag.2008.04.008
Chen, C., Wu, Y., Li, J., Wang, X., Zeng, Z., Xu, J., et al. (2023). TBtools-II: A "one for all, all for one" bioinformatics platform for biological big-data mining. Mol Plant. 16(11), 1733–1742. doi: 10.1016/j.molp.2023.09.010
Clark, S. T., Sinha, U., Zhang, Y., Wang, P. W., Donaldson, S. L., Coburn, B., et al. (2019). Penicillin-binding protein 3 is a common adaptive target among Pseudomonas aeruginosa isolates from adult cystic fibrosis patients treated with β-lactams. Int. J. Antimicrob. Agents 53, 620–628. doi: 10.1016/j.ijantimicag.2019.01.009
Cornaglia, G., Giamarellou, H., and Rossolini, G. M. (2011). Metallo-β-lactamases: a last frontier for β-lactams? Lancet Infect. Dis. 11, 381–393. doi: 10.1016/S1473-3099(11)70056-1
Croucher, N.J., Page, A. J., Connor, T. R., Delaney, A. J., Keane, J. A., Bentley, S. D., et al. (2015). Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 43, e15. doi: 10.1093/nar/gku1196
Daaboul, D., Osman, M., Kassem, I. I., Yassine, I., Girlich, D., Proust, A., et al. (2024). Neonatal sepsis due to NDM-1 and VIM-2 co-producing Pseudomonas aeruginosa in Morocco. J. Antimicrob. Chemother. 79, 1614–1618. doi: 10.1093/jac/dkae153
Dong, N., Liu, C., Hu, Y., Lu, J., Zeng, Y., Chen, G., et al. (2022). Emergence of an extensive drug resistant Pseudomonas aeruginosa strain of chicken origin carrying bla(IMP-45), tet(X6), and tmexCD3-toprJ3 on an Inc(pRBL16) plasmid. Microbiol. Spectr. 10, e0228322. doi: 10.1128/spectrum.02283-22
Edgar, R. C. (2022). Muscle5: High-accuracy alignment ensembles enable unbiased assessments of sequence homology and phylogeny. Nat. Commun. 13, 6968. doi: 10.1038/s41467-022-34630-w
Han, S., Zaniewski, R. P., Marr, E. S., Lacey, B. M., Tomaras, A. P., Evdokimov, A., et al. (2010). Structural basis for effectiveness of siderophore-conjugated monocarbams against clinically relevant strains of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 107, 22002–22007. doi: 10.1073/pnas.1013092107
Hong, D. J., Bae, I. K., Jang, I. H., Jeong, S. H., Kang, H. K., and Lee, K. (2015). Epidemiology and characteristics of Metallo-β-Lactamase-producing Pseudomonas aeruginosa. Infect. Chemother. 47, 81–97. doi: 10.3947/ic.2015.47.2.81
Jing, X., Zhou, H., Min, X., Zhang, X., Yang, Q., Du, S., et al. (2018). The Simplified Carbapenem Inactivation Method (sCIM) for simple and accurate detection of carbapenemase-producing gram-negative bacilli. Front. Microbiol. 9, 2391. doi: 10.3389/fmicb.2018.02391
Jorth, P., McLean, K., Ratjen, A., Secor, P. R., Bautista, G. E., Ravishankar, S., et al. (2017). Evolved aztreonam resistance is multifactorial and can produce hypervirulence in Pseudomonas aeruginosa. mBio 8(5), e00517–17. doi: 10.1128/mBio.00517-17
Kang, Y., Xie, L., Yang, J., and Cui, J. (2023). Optimal treatment of ceftazidime-avibactam and aztreonam-avibactam against bloodstream infections or lower respiratory tract infections caused by extensively drug-resistant or pan drug-resistant (XDR/PDR) Pseudomonas aeruginosa. Front. Cell Infect. Microbiol. 13, 1023948. doi: 10.3389/fcimb.2023.1023948
Kong, L. B., Ma, Q., Gao, J., Qiu, G. S., Wang, L. X., Zhao, S. M., et al. (2015). Effect of Qiguiyin Decoction on multidrug-resistant Pseudomonas aeruginosa infection in rats. Chin. J. Integr. Med. 21, 916–921. doi: 10.1007/s11655-015-2089-2
Lewis, I. and James, S. J. (2022). Performance standards for antimicrobial susceptibility testing. (Malvern, Pennsylvania, USA: Clinical and Laboratory Standards Institute (CLSI)).
Li, R., Li, Y., Kristiansen, K., and Wang, J. (2008). SOAP: short oligonucleotide alignment program. Bioinformatics 24, 713–714. doi: 10.1093/bioinformatics/btn025
Li, R., Zhu, H., Ruan, J., Qian, W., Fang, X., Shi, Z., et al. (2010). De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 20, 265–272. doi: 10.1101/gr.097261.109
Li, J., Abel, R., Zhu, K., Cao, Y., Zhao, S., and Friesner, R. A. (2011). The VSGB 2.0 model: a next generation energy model for high resolution protein structure modeling. Proteins 79, 2794–2812. doi: 10.1002/prot.v79.10
Lu, C., Wu, C., Ghoreishi, D., Chen, W., Wang, L., Damm, W., et al. (2021). OPLS4: improving force field accuracy on challenging regimes of chemical space. J. Chem. Theory Comput. 17, 4291–4300. doi: 10.1021/acs.jctc.1c00302
Malkoçoğlu, G., Aktaş, E., Bayraktar, B., Otlu, B., and Otlu, M. E. (2017). VIM-1, VIM-2, and GES-5 carbapenemases among Pseudomonas aeruginosa isolates at a tertiary hospital in Istanbul, Turkey. Microb. Drug Resist. 23, 328–334. doi: 10.1089/mdr.2016.0012
McLean, K., Lee, D., Holmes, E. A., Penewit, K., Waalkes, A., Ren, M., et al. (2019). Genomic analysis identifies novel Pseudomonas aeruginosa resistance genes under selection during inhaled aztreonam therapy in vivo. Antimicrob. Agents Chemother. 63(9), e00866–19. doi: 10.1128/AAC.00866-19
Nurjadi, D., Kocer, K., Chanthalangsy, Q., Klein, S., Heeg, K., and Boutin, S. (2022). New Delhi metallo-beta-lactamase facilitates the emergence of cefiderocol resistance in enterobacter cloacae. Antimicrob. Agents Chemother. 66, e0201121. doi: 10.1128/aac.02011-21
Pang, Z., Raudonis, R., Glick, B. R., Lin, T. J., and Cheng, Z. (2019). Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 37, 177–192. doi: 10.1016/j.biotechadv.2018.11.013
Pérez-Vázquez, M., Sola-Campoy, P. J., Zurita, M. Á., Ávila, A., Gómez-Bertomeu, F., Solís, S., et al. (2020). Carbapenemase-producing Pseudomonas aeruginosa in Spain: interregional dissemination of the high-risk clones ST175 and ST244 carrying bla(VIM-2), bla(VIM-1), bla(IMP-8), bla(VIM-20) and bla(KPC-2). Int. J. Antimicrob. Agents 56, 106026. doi: 10.1016/j.ijantimicag.2020.106026
Schwengers, O., Barth, P., Falgenhauer, L., Hain, T., Chakraborty, T., and Goesmann, A. (2020). Platon: identification and characterization of bacterial plasmid contigs in short-read draft assemblies exploiting protein sequence-based replicon distribution scores. Microb. Genom. 6(10), mgen000398. doi: 10.1099/mgen.0.000398
Schwengers, O., Jelonek, L., Dieckmann, M. A., Beyvers, S., Blom, J., and Goesmann, A. (2021). Bakta: rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. Genom. 7(11), 000685. doi: 10.1101/2021.09.02.458689
Simpson, J. T., Wong, K., Jackman, S. D., Schein, J. E., Jones, S. J., and Birol, I. (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069. doi: 10.1093/bioinformatics/btu153
Simpson, J. T., Wong, K., Jackman, S. D., Schein, J. E., Jones, S. J., and Birol, I. (2009). ABySS: a parallel assembler for short read sequence data. Genome Res. 19, 1117–1123. doi: 10.1101/gr.089532.108
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Vatopoulos, A. (2008). High rates of metallo-beta-lactamase-producing Klebsiella pneumoniae in Greece–a review of the current evidence. Euro Surveill. 13(4):8023. doi: 10.2807/ese.13.04.08023-en
Walsh, T. R., Toleman, M. A., Poirel, L., and Nordmann, P. (2005). Metallo-beta-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18, 306–325. doi: 10.1128/CMR.18.2.306-325.2005
Wang, Y., Wang, X., Schwarz, S., Zhang, R., Lei, L., Liu, X., et al. (2014). IMP-45-producing multidrug-resistant Pseudomonas aeruginosa of canine origin. J. Antimicrob. Chemother. 69, 2579–2581. doi: 10.1093/jac/dku133
Wick, R. R., Judd, L. M., Gorrie, C. L., and Holt, K. E. (2017). Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PloS Comput. Biol. 13, e1005595. doi: 10.1371/journal.pcbi.1005595
Xiong, J., Hynes, M. F., Ye, H., Chen, H., Yang, Y., M'Zali, F., et al. (2006). bla(IMP-9) and its association with large plasmids carried by Pseudomonas aeruginosa isolates from the people’s republic of China. Antimicrob. Agents Chemother. 50, 355–358. doi: 10.1128/AAC.50.1.355-358.2006
Zhang, X., Wang, L., Li, D., Li, P., Yuan, L., Yang, F., et al. (2021). An IncP-2 plasmid sublineage associated with dissemination of bla(IMP-45) among carbapenem-resistant Pseudomonas aeruginosa. Emerg. Microbes Infect. 10, 442–449. doi: 10.1080/22221751.2021.1894903
Zhou, L., Yang, C., Zhang, X., Yao, J., Chen, L., Tu, Y., et al. (2023). Characterization of a novel Tn6485h transposon carrying both blaIMP-45 and blaAFM-1 integrated into the IncP-2 plasmid in a carbapenem-resistant Pseudomonas aeruginosa. J. Glob. Antimicrob. Resist. 35, 307–313. doi: 10.1016/j.jgar.2023.10.010
Keywords: carbapenem-resistant Pseudomonas aeruginosa, CRPA, horizontal gene transfer (HGT), nosocomial infection, metallo-beta-lactamases (MBL), resistance dissemination
Citation: Ma Y, Lei Z, Zhang Y, Liu Q, Zhang F, Zu H, Yang X, Li Z and Lu B (2025) Tracing the evolutionary trajectory of the IncP-2 plasmid co-harboring blaIMP-45 and blaVIM-1: an outbreak of Pseudomonas aeruginosa co-producing IMP-45 and VIM-1 carbapenemases in China. Front. Cell. Infect. Microbiol. 15:1623241. doi: 10.3389/fcimb.2025.1623241
Received: 05 May 2025; Accepted: 02 June 2025;
Published: 19 June 2025.
Edited by:
Elijah Ige Ohimain, Niger Delta University, NigeriaReviewed by:
Ben Pascoe, University of Oxford, United KingdomShijun Sun, First Affiliated Hospital of Zhengzhou University, China
Copyright © 2025 Ma, Lei, Zhang, Liu, Zhang, Zu, Yang, Li and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binghuai Lu, enMyNTA0MUAxMjYuY29t
 Yiqun Ma
Yiqun Ma Zichen Lei2,3,4
Zichen Lei2,3,4 Yulin Zhang
Yulin Zhang Feilong Zhang
Feilong Zhang Ziyao Li
Ziyao Li Binghuai Lu
Binghuai Lu