- 1Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Kattankulathur, Chengalpattu District, Tamil Nadu, India
- 2University at Buffalo, State University of New York, Buffalo, NY, United States
This succinct article addresses the multifaceted interactions between the fungal organism Candida albicans and the Gram-positive bacterium Streptococcus mutans in the development of oral biofilms and pathobiology of oral diseases. S. mutans is considered to be a major pathogen in the development of dental caries. It is often found to interact with C. albicans in oral infection settings. The interaction of these organisms is often mediated via the binding of Glucosyltransferase (GtfB) enzyme secreted by S. mutans to C. albicans surface proteins Als1 and Hwp1. During these interactions, both C. albicans and S. mutans exhibit increased gene regulatory activity, leading to the modulation of virulence attributes and adaptation to environmental changes. This results in the strong attachment of the species to tooth surfaces and increased resistance of the mixed species biofilms to external factors. Mechanistically, intercellular communication between these species in mixed biofilms through quorum sensing and production of exoenzymes such as glucosyltransferases account for the synergy and modulation of their virulence attributes. Specifically, these mixed-species biofilms exhibit increased acid production and enhanced resistance to antimicrobial agents. Understanding these complex interkingdom pattern of interactions is essential to develop efficient therapeutic approaches against biofilm-associated oral infections. The review also highlights probiotic strategies to interfere with these interkingdom interactions to combat oral diseases like early childhood caries (ECC).
Introduction
The majority infectious diseases worldwide develop from the formation of biofilms on mucosal surfaces by specific organisms. Early Childhood Caries (ECC) being one of the most common biofilms associated tooth infection, notably increasing among preschool children. In ECC, S. mutans and C. albicans are oral pathogens which form mixed species polymicrobial biofilms over the enamel and dental surfaces (Shirtliff et al., 2009). S. mutans has been recognized as one of the major etiologic agents of caries. It can rapidly form plaque biofilms on tooth surfaces when exposed to sucrose and induce pathogenicity. S. mutans-secreted glucosyltransferases (Gtfs) can utilize sucrose to produce an insoluble exopolysaccharide (EPS) matrix, which constitutes the primary building block of biofilms over the enamel (Bowen and Koo, 2011) (Figure 1). The glucan polymer formation increases cohesion between S. mutans and Candida and additionally present binding sites for other microorganisms to adhere and colonize (Falsetta et al., 2014). Specifically, in the presence of sucrose S. mutans adheres strongly to fungal cells. Such interactions lead to virulent forms of organisms due to the formation of hypervirulent biofilms on oral surfaces, leading to decaying of tooth surfaces (Peleg et al., 2010). Moreover, C. albicans by adhering to organisms increases its carriage and infectivity.
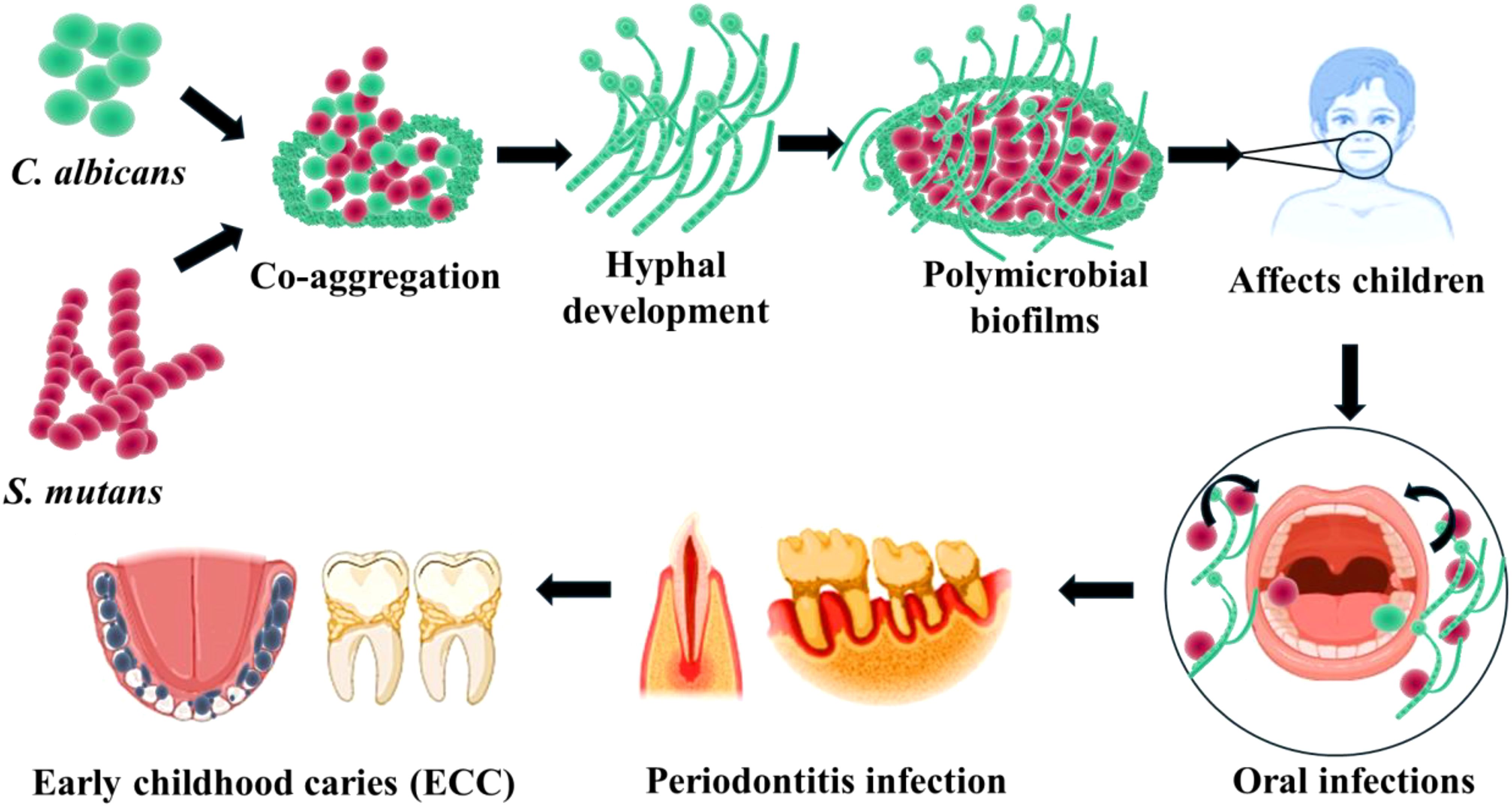
Figure 1. Cross-kingdom interactions of S. mutans and C. albicans in oral microenvironment impact the development of Early childhood caries and various oral infections.
Role of oral pathogens in Early childhood caries
Early childhood caries (ECC) is one of the common diseases affecting children worldwide (Lu et al., 2023). While acid-producing S. mutans is the primary etiological agent in initiating caries, C. albicans is often observed coexisting in the dental plaque of children with ECC, particularly in cases of Severe ECC. In these dental plaques, Candida and Streptococcus spp. form mixed biofilms on the surface of teeth, forming a thin layer of organic membrane. In addition, the surface proteins of S. mutans can also efficiently bind to other microbes, enhancing their colonization and initiating proliferation and the formation of complex multispecies polymicrobial biofilms. The process of multi-species biofilm formation is thought to be initiated by S. mutans via two different pathways: sucrose dependent and sucrose independent. In this, the sucrose-dependent pathway includes the production of glucosyl transferases GtfB, GtfC, and GtfD. In the presence of sucrose, the glucosyltransferases produce water-soluble or water-insoluble glucans the Gtfs possess sucrose-dependent activity that causes glycosidic bond breakage and releases fructose and glucose. The glucose is then linked to a developing glucan polymer (Lemos et al., 2019).
Here, GtfB is the major producer of α-1, 3 linked insoluble polysaccharide glucan, the major component of biofilm matrix, which provides adhesion sites and accumulation sites for other microbes to attach and initiate the formation of polymicrobial biofilms (Figure 2) (Cui et al., 2021, Hwang et al., 2017). The glucosyltransferase GtfB, produced by S. mutans, produces glucans from sucrose. The role of GtfB is to promote the adhesion of the EPS matrix over the enamel, causing the formation of biofilm. Gtfs will also be adsorbed to the surface of other microorganisms and converted into glucan producers (Bowen and Koo, 2011). The production of glucans on the tooth surface further promotes biofilm formation by increasing the adherence of S. mutans mediated by glucan binding proteins expressed by S. mutans (Matsumoto-Nakano, 2018). GtfB also promotes the aggregation of bacterial species and enables its growth in the hostile oral environment. Moreover, GtfB fosters coaggregation with other pathogenic species, including C. albicans, thus causing the formation of dual-species biofilm (Kulshrestha and Gupta, 2022). S. mutans also possesses multiple high affinity adhesins that enable the organism to adhere to tooth surfaces in the absence of sucrose. In this regard, the dual antigen I/II (also known as P1, SpaP, or Pac) is a multifunctional adhesin that can mediate bacterial attachment to the salivary pellicle formed on tooth surfaces and other bacteria (Brady et al., 2010).
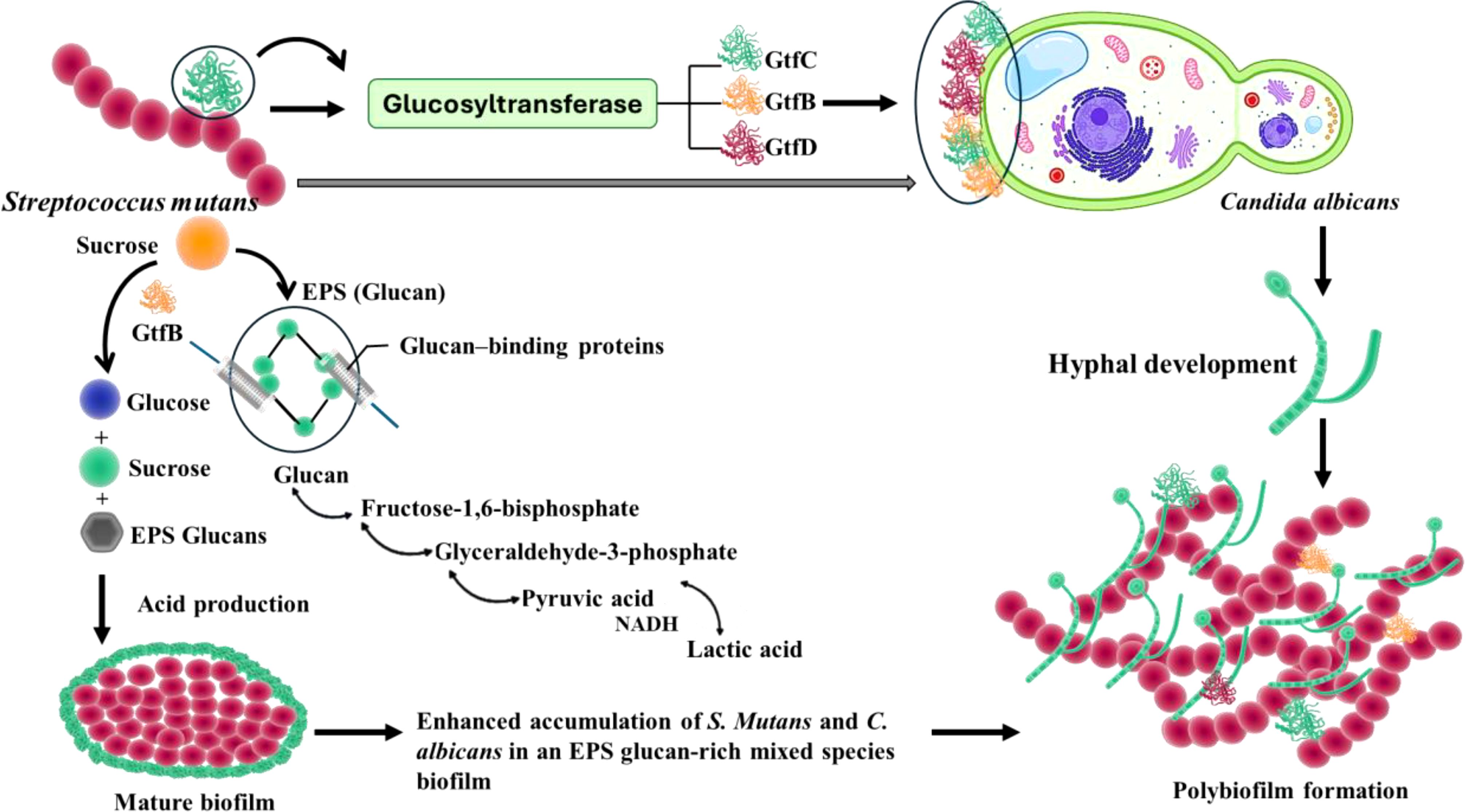
Figure 2. The interaction of S. mutans with C. albicans influences the virulence of C. albicans, by the secreted molecules and cell surface molecules by S. mutans, which also secretes Gtfs that will be attached to C. albicans and plays a crucial role in pathogenesis.
S. mutans acts as one of the leading causing factors of ECC by producing organic acids via carbohydrate metabolism. Oligosaccharides from the breakdown of carbohydrate polymers are primarily transported into the cells by ATP-binding cassette (ABC) transporters, whereas monosaccharides (glucose and fructose) and disaccharides (sucrose) are predominantly taken up by the phosphoenolpyruvate dependent phosphotransferase transporters (PTS). In the cytoplasm, phosphorylated sugars are then processed to fructose-6-phosphate (Fru-6-P) and fermented into organic acids, mainly lactic acid, via glycolysis. The accumulation of these acids in the oral microenvironment reduce the pH, which leads to the major cause of demineralisation of the tooth surface. Acid resistance therefore is an immense attribute and survival tool of S. mutans (Lemos et al., 2019). It has also been shown that C. albicans can also produces acids by metabolizing carbohydrates, further exacerbating low pH of the oral environment.
Bacteria-yeast interplay
Several adhesion proteins (adhesins) mediate the attachment of microbial species to the surfaces of the oral cavity, especially to the tooth surface for the development of pathogenic biofilms of C. albicans and S. mutans. C. albicans possesses key adhesins, such as Als1, Als3, and Hwp1 that are involved in the attachment to host tissues and formation of hyphal structures that allow the invasion and development of biofilm. C. albicans interacts with bacterial species such as S. mutans through these adhesins to establish mixed-species biofilms and ensure the persistence of infection. In the process, S. mutans promotes biofilm thickening and complexity (Lu et al., 2023). Here, S. mutans adhesins, such as SpaP and Epa1, are involved in adhesion of the bacterium to the tooth surface and assembly of the biofilm. Ultimately, the biofilm community surrounded by a protective EPS meshwork induces dental caries by promoting acid production.
Interestingly, the expression of adhesin-expressing genes in S. mutans is upregulated by C. albicans, suggesting that the presence of fungal cells might enhance the capacity of S. mutans cells to colonize and form biofilms. Further, the interaction between these species occurs through a process called quorum sensing, which is a chemical communication system that induces the gene expression of adhesive molecules in C. albicans and S. mutans (Sztajer et al., 2014). Still, the protein family of Als continues to be central to the pathogenicity of C. albicans, which causes tissue invasion, immune evasion, and biofilm formation. Those interactions between adhesion proteins of S. mutans, such as SpaP and Epa1 enhance the complexity of oral biofilms and make it tough to disrupt the targeted synergistic interactions and prevention of biofilm diseases (Kriswandini and Almas, 2023). During such interaction, these S. mutans and C. albicans will evolve as a complex network of regulatory mechanisms to boost cariogenic virulence and will modulate tolerance upon stress changes in the external environment (Li et al., 2023).
Polymicrobial biofilm infections
S. mutans and C. albicans form dual-species biofilms that share a common matrix, influencing pathogenicity and virulence. For structural integrity and resilience of biofilms, extracellular polymeric substances provide support and stability. It has been shown that production the rate of synthesis of the extrapolysaccharide glucan substance (EPS) is significantly higher in mixed dual-species biofilms than in mono-species biofilms (Falsetta et al., 2014). Such increased production of EPS promotes the retention of nutrients and assistance in metabolic cross-feeding, causing an increased pathogenic capacity of both organisms. The extracellular polysaccharides matrix also allows each resident to collaborate and interact with its neighbouring species so that it can survive in such a hostile environment (Weiland-Bräuer, 2021)Nutrients are the major determining component in the colonization of microbial species. Metabolic communication involving excretion by one organism and utilization by another is a common feature in mixed species oral biofilms. For instance, early colonizer species can secrete short-chain acids such as lactate, pyruvate, and acetate through sugar metabolism, which can form a high energy source for the late colonizers. In addition to the contact-dependent microbial communication (signalling), intercellular communication (signalling) can occur via secreted diffusible molecules (Guo et al., 2024) (Table 1). C. albicans can induce several genes in S. mutans, such as gtfB responsible for glucan synthesis, a major component of insoluble EPS. This improves antimicrobial resistance and protects C. albicans from antifungal agents like fluconazole (Li et al., 2023). This environment improves metabolic interactions between species, where S. mutans could influence the growth of C. albicans in a similar way pathogenicity and virulence of these biofilms increased making it difficult to treat (Li et al., 2023).
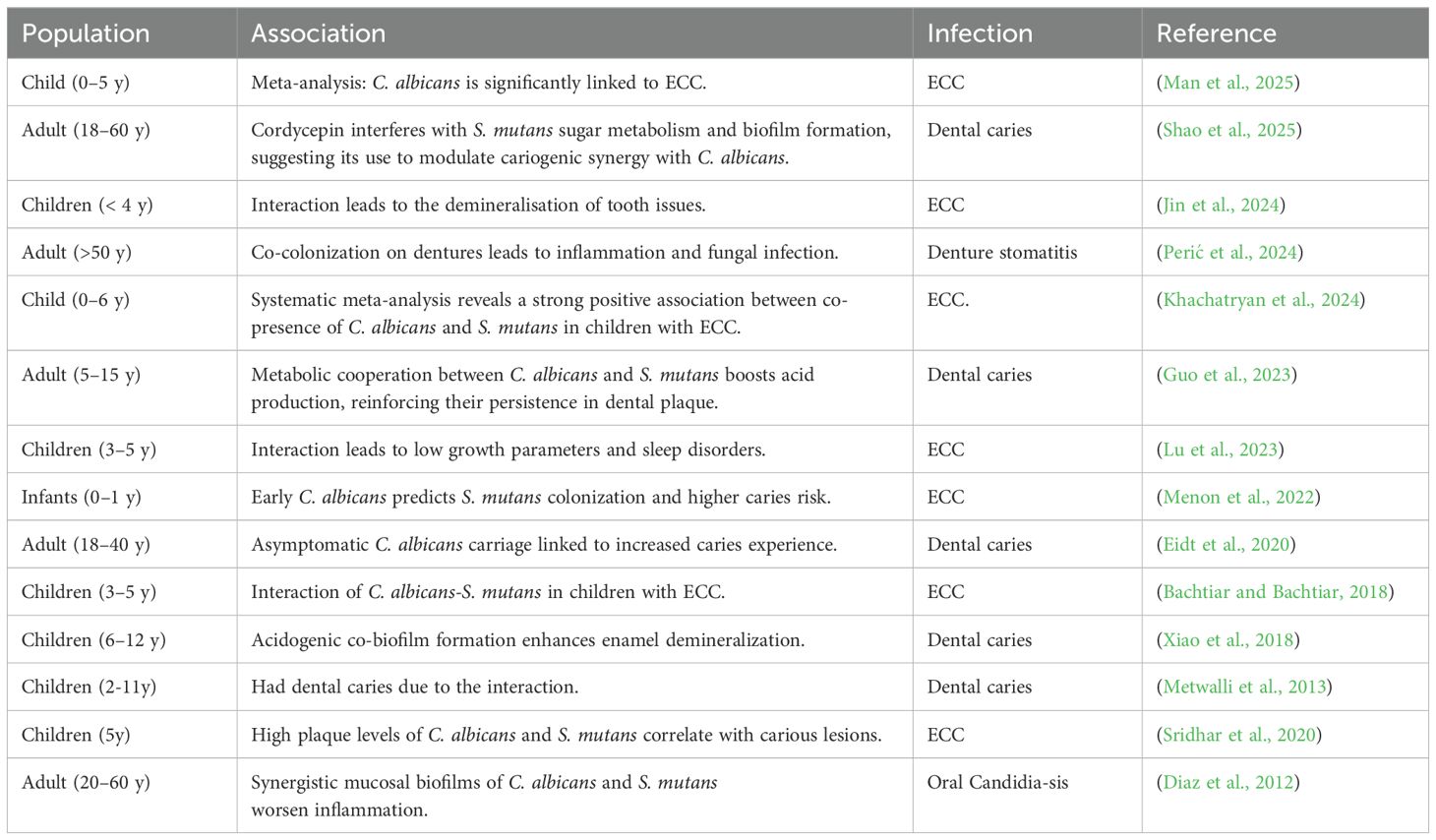
Table 1. Infections caused in diverse population by the pathogenic bacteria and yeast in oral microbiome due to their interactions.
Role of S. mutans Glucosyl transferases Gtfs in triggering C. albicans virulence
Glucosyl transferases (GtfB, GtfC, and GtfD) expressed by S. mutans play critical roles in C. albicans-S. mutans mixed species biofilm formation and caries pathogenesis (Figure 2). As indicated above, the biofilm formation and plaque development due to S. mutans and C. albicans are influenced by the exoenzymes such as GtfB, and the adhesins such as Als1 and Als3. The role of Gtf enzymes is to assist in enhanced biofilm formation by enabling S. mutans to colonize by accumulating EPS. Gtfs share some similarities in structure, but they possess various unique functions. Among these Gtfs, GtfB produces glucans, which facilitate the attachment of microbes and alter the biofilm structure by enhancing the interaction with various oral microbes (Ellepola et al., 2019). In such dual biofilm formation, S. mutans is involved in the metabolism of carbohydrates, degradation of pyruvate, also the production of acetate and ethanol and influences the electron transport chain and the tricarboxylic acid cycle. During the interaction of S. mutans and C. albicans, C. albicans utilizes glucose predominantly but it can’t metabolize sucrose efficiently. But S. mutans is able to convert the remaining sucrose into glucans through the Gtf system. This influences the growth of C. albicans and improves the production of excess acids in the oral cavity. Further, the exoenzyme GtfB from S. mutans can bind to the mannan layer of the cell wall of C. albicans. Such interactions affect the colonization of C. albicans in the oral cavity.
Conclusion
From this review, we conclude that the oral opportunistic pathogen C. albicans and S. mutans, due to their interaction, play a major role in the pathogenesis and formation of various oral diseases through distinct mechanisms. Also, the interaction between these species proves that it is a major cause for the formation of ECC worldwide. This observation serves a critical role in proving that the extracellular matrix plays a crucial role in the formation of biofilm and leads to the formation of mixed-species poly-biofilms due to the interaction of S. mutans and C. albicans. In addition, further studies are exploring the anti-Candida therapies possible in the treatment of ECC and involving the need to develop inhibition agents that inhibit GTFs production during the interaction with S. mutans.
Author contributions
AF: Writing – original draft, Writing – review & editing, Formal Analysis. RS: Writing – original draft, Writing – review & editing, Funding acquisition. MJ: Writing – original draft, Writing – review & editing. VN: Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing, Funding acquisition, Project administration. SR: Project administration, Writing – original draft, Writing – review & editing, Conceptualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Acknowledgments
The research was supported by grants R03DE032392 (SRP) by the National Institute of Dental and Craniofacial Research (NIDCR). The financial support provided to ABF in the form of the postgraduate (M. Tech Biotechnology) fellowship by SRMIST is thankfully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bachtiar, E. W. and Bachtiar, B. M. (2018). Relationship between Candida albicans and Streptococcus mutans in early childhood caries, evaluated by quantitative PCR. F1000Research 7, 1645. doi: 10.12688/f1000research.16275.2
Bowen, W. H. and Koo, H. (2011). Biology of streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 45, 69–86. doi: 10.1159/000324598
Brady, L. J., Maddocks, S. E., Larson, M. R., Forsgren, N., Persson, K., Deivanayagam, C. C., et al. (2010). The changing faces of Streptococcus antigen I/II polypeptide family adhesins. Mol. Microbiol. 77, 276–286. doi: 10.1111/j.1365-2958.2010.07212.x
Cui, Y., Wang, Y., Zhang, Y., Pang, L., Zhou, Y., Lin, H., et al. (2021). Oral mycobiome differences in various spatial niches with and without severe early childhood caries. Front. Pediatr. 9. doi: 10.3389/fped.2021.748656
Diaz, P. I., Xie, Z., Sobue, T., Thompson, A., Biyikoglu, B., Ricker, A., et al. (2012). Synergistic interaction between candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect. Immun. 80, 620–632. doi: 10.1128/IAI.05896-11
Eidt, G., Waltermann, E. D. M., Hilgert, J. B., and Arthur, R. A. (2020). Candida and dental caries in children, adolescents and adults: A systematic review and meta-analysis. Arch. Oral. Biol. 119, 104876. doi: 10.1016/j.archoralbio.2020.104876
Ellepola, K., Truong, T., Liu, Y., Lin, Q., Lim, T. K., Lee, Y. M., et al. (2019). Multi-omics Analyses Reveal Synergistic Carbohydrate Metabolism in Streptococcus mutans-Candida albicans Mixed-Species Biofilms. Infect. Immun. 87, e00339–e00319. doi: 10.1128/IAI.00339-19
Falsetta, M. L., Klein, M. I., Colonne, P. M., Scott-Anne, K., Gregoire, S., Pai, C.-H., et al. (2014). Symbiotic Relationship between Streptococcus mutans and Candida albicans Synergizes Virulence of Plaque Biofilms In Vivo. Infect. Immun. 82, 1968–1981. doi: 10.1128/IAI.00087-14
Guo, M., Yang, K., Zhou, Z., Chen, Y., Zhou, Z., Chen, P., et al. (2023). Inhibitory effects of Stevioside on Streptococcus mutans and Candida albicans dual-species biofilm. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1128668
Guo, T., Zhou, L., Xiong, M., Xiong, J., Huang, J., Li, Y., et al. (2024). N-homocysteinylation of DJ-1 promotes neurodegeneration in Parkinson’s disease. Aging Cell 23, e14124. doi: 10.1111/acel.14124
Hwang, G., Liu, Y., Kim, D., Li, Y., Krysan, D. J., and Koo, H. (2017). Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PloS Pathog. 13, e1006407. doi: 10.1371/journal.ppat.1006407
Jin, P., Wang, L., Chen, D., and Chen, Y. (2024). Unveiling the complexity of early childhood caries: Candida albicans and Streptococcus mutans cooperative strategies in carbohydrate metabolism and virulence. J. Oral. Microbiol. 16, 2339161. doi: 10.1080/20002297.2024.2339161
Khachatryan, L. G., Allahbakhsi, F., Vadiyan, D. E., and Mohammadian, M. (2024). Investigating the association between Candida albicans and early childhood dental caries: A comprehensive systematic review and meta-analysis. PloS One 19, e0315086. doi: 10.1371/journal.pone.0315086
Kriswandini, I. L. and Almas, R. (2023). Review: Quorum sensing mechanism between Streptococcus mutans and Candida albicans in the pathogenesis of dental caries. World J. Adv. Res. Rev. 17, 1079–1082. doi: 10.30574/wjarr.2023.17.1.0066
Kulshrestha, A. and Gupta, P. (2022). Polymicrobial interaction in biofilm: mechanistic insights. Pathog. Dis. 80, ftac010. doi: 10.1093/femspd/ftac010
Lemos, J. A., Palmer, S. R., Zeng, L., Wen, Z. T., Kajfasz, J. K., Freires, I. A., et al. (2019). The biology of streptococcus mutans. Microbiol. Spectr. 7, 7.1.03. doi: 10.1128/microbiolspec.GPP3-0051-2018
Li, Y., Huang, S., Du, J., Wu, M., and Huang, X. (2023). Current and prospective therapeutic strategies: tackling Candida albicans and Streptococcus mutans cross-kingdom biofilm. Front. Cell. Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1106231
Lu, Y., Lin, Y., Li, M., and He, J. (2023). Roles of Streptococcus mutans-Candida albicans interaction in early childhood caries: a literature review. Front. Cell. Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1151532
Man, V. C. W., Manchanda, S., and Yiu, C. K. (2025). Oral candida-biome and early childhood caries: A systematic review and meta-analysis. Int. Dent. J. 75, 1246–1260. doi: 10.1016/j.identj.2024.08.020
Matsumoto-Nakano, M. (2018). Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 54, 22–29. doi: 10.1016/j.jdsr.2017.08.002
Menon, L. U., Scoffield, J. A., Jackson, J. G., and Zhang, P. (2022). Candida albicans and early childhood caries. Front. Dent. Med. 3. doi: 10.3389/fdmed.2022.849274
Metwalli, K. H., Khan, S. A., Krom, B. P., and Jabra-Rizk, M. A. (2013). Streptococcus mutans, Candida albicans, and the Human Mouth: A Sticky Situation. PloS Pathog. 9, e1003616. doi: 10.1371/journal.ppat.1003616
Peleg, A. Y., Hogan, D. A., and Mylonakis, E. (2010). Medically important bacterial–fungal interactions. Nat. Rev. Microbiol. 8, 340–349. doi: 10.1038/nrmicro2313
Perić, M., Miličić, B., Kuzmanović Pfićer, J., Živković, R., and Arsić Arsenijević, V. (2024). A systematic review of denture stomatitis: predisposing factors, clinical features, etiology, and global candida spp. Distribution. J. Fungi 10, 328. doi: 10.3390/jof10050328
Shao, Y., Zhu, W., Liu, S., Zhang, K., Sun, Y., Liu, Y., et al. (2025). Cordycepin affects Streptococcus mutans biofilm and interferes with its metabolism. BMC Oral. Health 25, 25. doi: 10.1186/s12903-024-05355-7
Shirtliff, M. E., Peters, B. M., and Jabra-Rizk, M. A. (2009). Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 299, 1–8. doi: 10.1111/j.1574-6968.2009.01668.x
Sridhar, S., Suprabha, B. S., Shenoy, R., Suman, E., and Rao, A. (2020). Association of streptococcus mutans, candida albicans and oral health practices with activity status of caries lesions among 5-year-old children with early childhood caries. Oral. Health Prev. Dent. 18, 911–919. doi: 10.3290/j.ohpd.a45411
Sztajer, H., Szafranski, S. P., Tomasch, J., Reck, M., Nimtz, M., Rohde, M., et al. (2014). Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. ISME J. 8, 2256–2271. doi: 10.1038/ismej.2014.73
Weiland-Bräuer, N. (2021). Friends or foes—Microbial interactions in nature. Biology 10, 496. doi: 10.3390/biology10060496
Keywords: biofilm, ECC, polybiofilms, Candidia albicans, Streptococcus mutans
Citation: Francis AB, Settem RP, Jeyamoorthy M, Nuthangi VH, Sharma A and Rajasekharan SK (2025) Multifaceted roles of Candida albicans and Streptococcus mutans in contributing to polybiofilm infections in early childhood caries. Front. Cell. Infect. Microbiol. 15:1625103. doi: 10.3389/fcimb.2025.1625103
Received: 08 May 2025; Accepted: 09 June 2025;
Published: 27 June 2025.
Edited by:
Yao Xia, University of Western Australia, AustraliaReviewed by:
Kan Yu, University of Western Australia, AustraliaCopyright © 2025 Francis, Settem, Jeyamoorthy, Nuthangi, Sharma and Rajasekharan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashu Sharma, c2hhcm1hYUBidWZmYWxvLmVkdQ==; Satish Kumar Rajasekharan, c2F0aXNoa3IyQHNybWlzdC5lZHUuaW4=
†These authors have contributed equally to this work
 Anto Benignus Francis1†
Anto Benignus Francis1† Rajendra Prasad Settem
Rajendra Prasad Settem Ashu Sharma
Ashu Sharma Satish Kumar Rajasekharan
Satish Kumar Rajasekharan