- 1Department of Pathogen Biology, College of Basic Medical Sciences, Jilin University, Changchun, China
- 2Department of Histology and Embryology, College of Basic Medical Sciences, Jilin University, Changchun, China
- 3Department of Laboratory Medicine, China-Japan Union Hospital of Jilin University, Changchun, China
- 4The Medical Basic Research Innovation Center of Airway Disease in North China, Ministry of Education, Changchun, China
- 5Jilin Provincial Key Laboratory of Precision Infectious Diseases, Jilin University, Changchun, China
- 6Jilin Provincial Engineering Laboratory of Precision Prevention and Control for Common Diseases, Jilin University, Changchun, China
The gut microbiota constitutes a vital ecosystem within the human body playing a pivotal role in immune regulation and metabolic homeostasis. Emerging research underscores a sophisticated interplay between the gut and lungs, termed the “gut-lung axis.” Gut microbes exert influence over pulmonary immunity and metabolism via immune mediators (e.g., cytokines and interleukins), metabolites (e.g., short-chain fatty acids) and direct microbial translocation. Dysbiosis of the gut microbiota has been implicated in a spectrum of respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), acute lung injury (ALI), Coronavirus Disease 2019 (COVID-19), lung cancer, idiopathic pulmonary fibrosis (IPF), pulmonary arterial hypertension (PAH), acute lower respiratory infection (ALRI) and tuberculosis (TB). Although multi-omics technologies have elucidated certain mechanisms underlying the gut-lung axis, numerous pathways remain to be fully delineated. This review synthesizes current knowledge on the role of gut microbiota and their metabolites in respiratory diseases and assesses their therapeutic potential. Future investigations should prioritize strategies to restore and maintain microbial homeostasis, such as dietary modifications, probiotic supplementation and fecal microbiota transplantation to pioneer novel preventive and therapeutic approaches. These summaries of advances in gut microbiology research promise better management and exploration of therapeutic strategies for respiratory diseases.
1 Introduction
The human body harbors a diverse array of microorganisms with the gut being the most densely colonized organ, hosting up to 100 trillion (1014) microorganisms (Ley et al., 2006; Grice and Segre, 2012; Dang and Marsland, 2019) including bacteria, fungi, and viruses (Parker et al., 2020). These gut microbiota have co-evolved with the host, establishing a complex and symbiotic relationship (Wei et al., 2024). Their relative stability is critical for maintaining the body’s immune system and metabolic balance (Bock et al., 2024; Ullah et al., 2024). Additionally, gut microbes influence systemic homeostasis by modulating metabolite levels (Canfora et al., 2019). Emerging evidence highlighted the pivotal role of gut microbiota in regulating the local immune system, particularly through the modulation of neutrophil responses and pro-inflammatory signaling pathways (Danne et al., 2024; Ye et al., 2025). Consequently, ecological dysregulation of the gut microbiota may contribute to the pathophysiology of lung diseases by disrupting immune homeostasis, impairing nutrient absorption and altering metabolic equilibrium (Chu and Mazmanian, 2013; Kamada et al., 2013; Zheng et al., 2020).
Although the lungs and the gut are anatomically distinct organs, accumulating evidence suggests a close and dynamic interaction between them (Cheng et al., 2024; Kim et al., 2024), which is essential for maintaining organismal stability (Enaud et al., 2020). This interplay has led to the proposal of a novel concept: the “ gut-lung axis” (Jia et al., 2024; Sun et al., 2024). The gut-lung axis encompasses the bidirectional communication and interaction pathways between the lungs and the gut, mediated through various mechanisms including the immune system, the nervous system and metabolic substances (Budden et al., 2017; Chiu et al., 2022). Gut microbiota play a critical role in regulating the immune system influencing not only intestinal immunity (Zhou et al., 2021) but also pulmonary immune responses through the modulation of cytokines, interleukins and other signaling molecules (McAleer and Kolls, 2018; Espírito Santo et al., 2021). These interactions can potentially trigger lung infections and inflammatory responses. For instance, gut microbial dysbiosis can lead to abnormal immune system activation, resulting in the excessive release of inflammatory mediators such as TNF-γ and IL-6 (Wu Y. et al., 2022; Wu R. et al., 2024). Furthermore, gut microorganisms produce a wide range of metabolites including short-chain fatty acids, amino acids and vitamins, which can enter systemic circulation and influence host metabolic homeostasis (Hays et al., 2024; Li TT. et al., 2024; Zhan et al., 2024). Furthermore, recent research by our research group has found that succinate produced by the gut microbiota can act on the lungs, affecting protein post-translational modifications and metabolic homeostasis in the lungs (Wang et al., 2025). This result further provides evidence for the existence of the gut-lung axis. In some cases, gut microorganisms may translocate directly to the lungs via the bloodstream or lymphatic system, potentially causing lung infections or inflammation (Dickson and Huffnagle, 2015; Schuijt et al., 2016). This mechanism provides new insights into the intricate relationship between gut microbiota and lung homeostasis, highlighting the importance of the gut-lung axis in health and disease (Felix et al., 2018).
Respiratory diseases as prevalent conditions capable of triggering systemic reactions have long been a focal point of research (Zhao et al., 2023). Notably, patients with gastrointestinal disorders often exhibit disruptions in pulmonary homeostasis, which are associated with an elevated incidence of respiratory diseases (Massart and Hunt, 2020). Recent advancements in microbiomics and metagenomics have deepened our understanding of the gut-lung axis by uncovering potential connections between gut and lung microbiota (Song et al., 2020; Boesch et al., 2021; Eladham et al., 2024; Li R. et al., 2024). This review examines the latest findings on the role of gut microbiota and their metabolites in common respiratory diseases, including chronic obstructive pulmonary disease (COPD), asthma, acute lung injury (ALI), Coronavirus Disease 2019 (COVID-19), lung cancer, idiopathic pulmonary fibrosis (IPF), pulmonary arterial hypertension (PAH), acute lower respiratory infection (ALRI), and tuberculosis (TB), as well as the underlying mechanisms. Additionally, it summarizes emerging therapeutic strategies targeting gut microbiota offering new insights into the management of respiratory diseases.
2 The role of gut microbes in specific respiratory diseases
Despite the anatomical distance separating the lungs and the gut within the human body, advancements in multi-omics technologies have increasingly demonstrated a significant correlation between alterations in gut microbiota composition and the immune response of the respiratory system, as well as the maintenance of pulmonary homeostasis. Experimental studies and epidemiological data have substantiated a critical connection between the gut microbiota and lung health, conceptualized as the “gut-lung axis.” Nonetheless, the precise mechanisms and pathways underpinning this relationship warrant further elucidation. Dysbiosis is characterized by disruptions in the composition and functionality of the gut microbiota plays a pivotal role in modulating the body’s immune responses and has been implicated in the pathogenesis of various pulmonary diseases. Table 1 presents the specific changes in the gut and lung microbiota of patients with respiratory diseases. However, the existing body of research in this domain remains limited. Future investigations are anticipated to yield more comprehensive insights into the gut-lung axis, as illustrated in Figure 1, offering promising avenues for exploration.
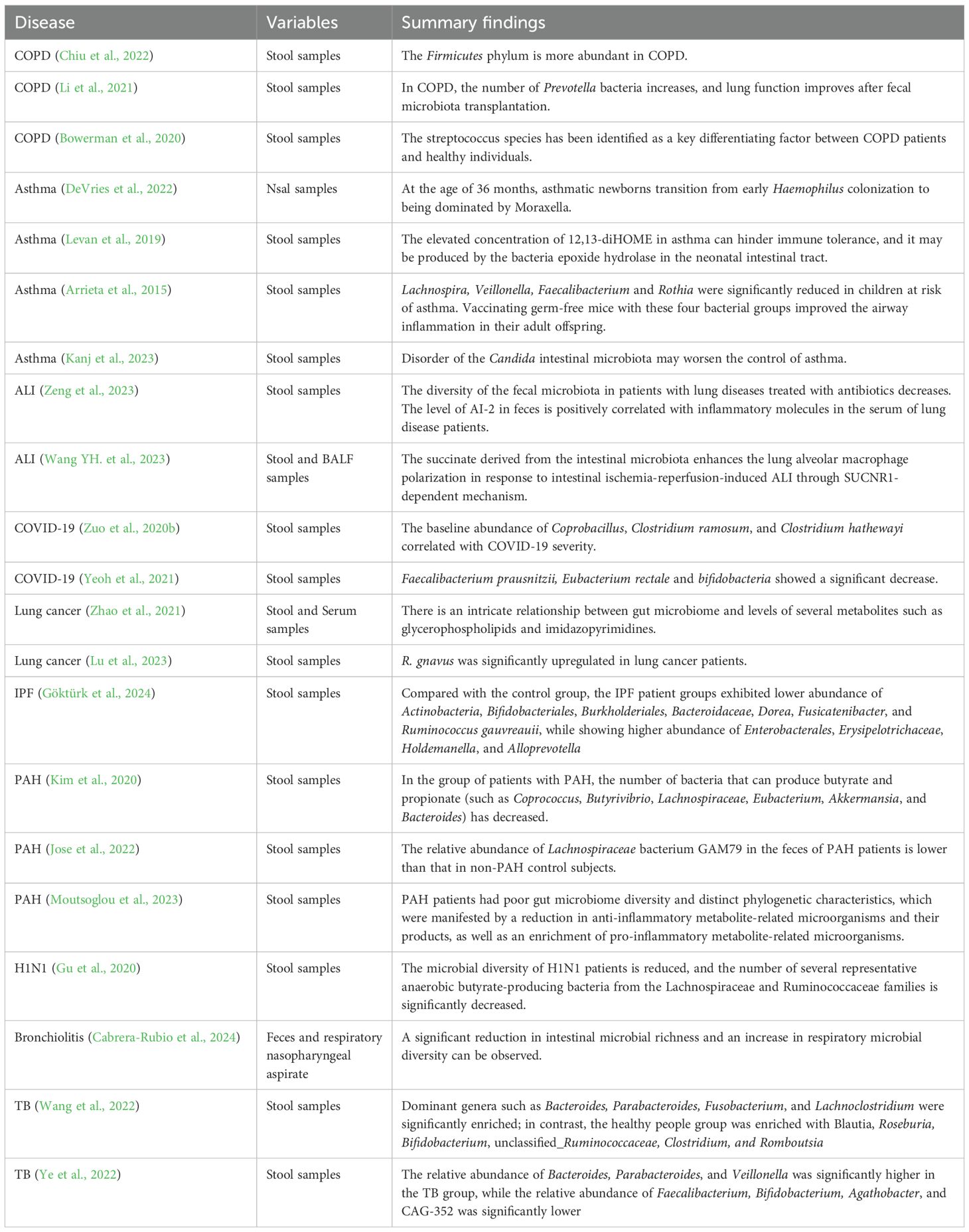
Table 1. Summary of specific microbial taxa involved in various respiratory diseases (clinical cohorts).
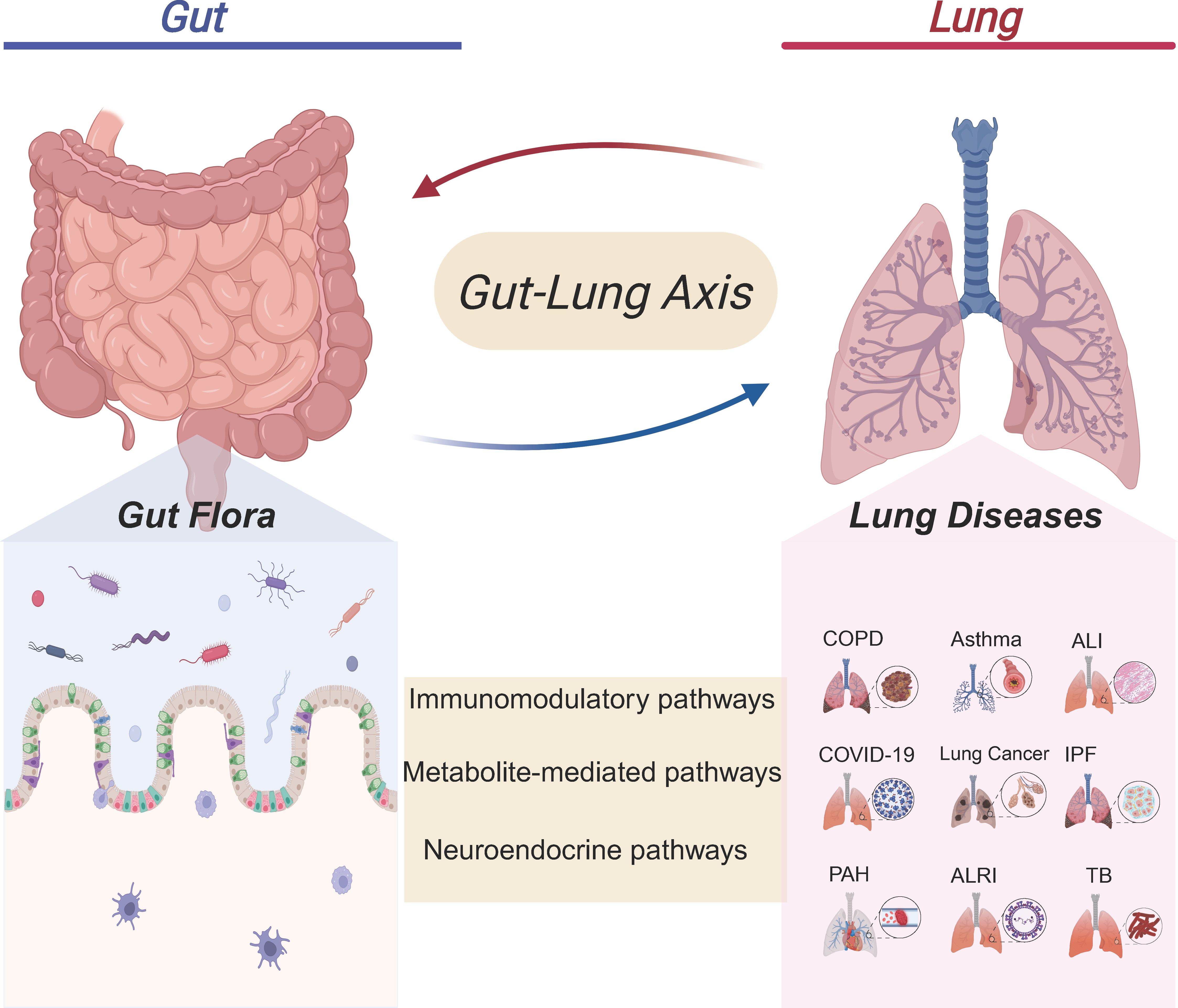
Figure 1. Crosstalk between the gut microbiota and pulmonary diseases. The gut microbiota in the Gut can interact with the Lung through immunomodulatory pathways, metabolite- mediated pathways, and neuroendocrine pathways. This gut - lung axis association involves multiple lung diseases such as chronic obstructive pulmonary disease (COPD), Asthma, Acute lung injury (ALI), coronavirus disease 2019 (COVID-19), Lung cancer, Idiopathic pulmonary fibrosis (IPF), Pulmonary arterial hypertension (PAH), Acute lower respiratory infection (ALRI), and Tuberculosis (TB). It suggests that the gut microbiota may affect lung health through the gut-lung axis, providing a new gut-related perspective for the research and intervention of lung diseases.
2.1 COPD
COPD is clinically characterized by chronic inflammation and obstructive lung pathology (Song et al., 2023). Globally recognized risk factors for COPD include smoking and environmental pollution (Jiang et al., 2024). There is increasing evidence highlighting a significant interplay between gut microbiota composition and lung immunity. Peiju Fang et al. demonstrated that exposure to cigarette smoke and particulate matter disrupts the intestinal microbiota, suggesting that gut microbiota disturbances may contribute to COPD progression (Huang et al., 2024; Fang et al., 2025). A retrospective analysis of 1,228 COPD patients revealed that a majority exhibited at least one gastrointestinal symptom with a notably higher prevalence of inflammatory bowel disease and irritable bowel syndrome compared to the healthy population (Rutten et al., 2014). In a study involving 55 patients with COPD, it was found that at the phylum level, the Bacteroidetes phylum was more abundant in the control group, while the Firmicutes phylum was more abundant in the COPD group. At the genus level, the Alloprevotalla genus was more abundant in the control group than in the COPD group (Chiu et al., 2022). Naijian Li et al. demonstrated in a mouse model that alterations in the gut microbiota of COPD patients are linked to disease progression. Mice receiving fecal transplants from COPD patients exhibited significant lung pathology, reduced lung function and increased airway mucus production (Li et al., 2021). Kate L. Bowerman et al. identified distinct differences in gut microbiota composition and metabolomics between COPD patients and healthy individuals with Streptococcus spp. being a key differentiating factor. Certain members of this genus were strongly associated with reduced lung function (Bowerman et al., 2020). Dysbiosis in the gut microbiota leads to increased production of harmful bacterial metabolites and decreased short-chain fatty acid synthesis, exacerbating intestinal permeability and facilitating bacterial translocation. Immune cells in the gut interact with these metabolites and microbiota, resulting in the release of pro-inflammatory cytokines into the bloodstream, which further exacerbates COPD progression via the gut-lung axis (Li N. et al., 2023). Collectively, these findings suggest that disruption of the intestinal barrier may promote the translocation of dysregulated microbiota to the airways, thereby affecting pulmonary homeostasis (Giron et al., 2021; Ruan et al., 2022; Lim et al., 2023). Translocated pathogens and their virulence factors may also infiltrate the gastrointestinal tract, triggering intestinal inflammation and related disorders (Dong et al., 2024).
2.2 Asthma
Asthma is a chronic inflammatory disease characterized by a complex and diverse pathophysiology. Substantial research confirms that alterations in the microbiota during early life are closely associated with the onset and progression of asthma (Liu C. et al., 2022; Alhasan et al., 2023; Kahhaleh et al., 2024). The colonization of the gut microbiota is crucial for immune system development, and disruptions in this process may increase susceptibility to asthma in children (Casali and Stella, 2024; Ke et al., 2025). Studies have shown that the characteristics of newborns of mothers with asthma are that they are colonized by Haemophilus bacteria in the early stage of life, and at the age of 36 months, the microbial community shifts to being dominated by Moraxella bacteria (DeVries et al., 2022). In neonates at high risk of asthma, the concentration of 12,13-diHOME, which is produced by intestinal bacteria, is elevated in their feces (Levan et al., 2019). This substance can induce long-term immune effects, leading to CD4+ T cell dysfunction [59,60], thereby increasing susceptibility to asthma (Nilsen et al., 2024; Sanidad et al., 2024). As children grow, gut microbial diversity gradually increases, maturing into an adult-type microbiota composition by age three, characterized by elevated levels of Firmicutes and Proteobacteria and reduced levels of Actinobacteria and Bacteroidetes (Ximenez and Torres, 2017), Notably, asthmatic individuals exhibit increased diversity of Aspergillus spp (Celik et al., 2024). Polysaccharide A from Bacteroides fragilis has been shown to promote IL-10 secretion by CD4+ T cells (Zhong et al., 2024), while extracellular polysaccharides from Bifidobacterium longum inhibit Th17 responses in both the gut and lungs (Li D. et al., 2025), thereby reducing asthma susceptibility. The role of gut microbiome dysregulation in asthma progression has been extensively investigated in both human patients and mouse models (Smulders et al., 2024; Liu et al., 2025). Experiments involving germ-free mice have demonstrated that microbial exposure is critical for the morphological and functional development of the immune system (Anandakumar et al., 2024). Arrieta et al. revealed significant reductions in the abundance of Lachnospira, Veillonella, E. faecalis, and Rothia in the gut microbiota of infants with asthma. Inoculation of these bacteria into germ-free mice alleviated airway inflammation and prevented asthma development (Arrieta et al., 2015). Amjad N. Kanj’s study further confirmed that dysbiosis of the gut fungal microbiota, particularly involving Candida, enhances Th2 responses in mice sensitized with house dust mite, exacerbating asthmatic airway inflammation (Kanj et al., 2023). Collectively, these studies demonstrated that gut microorganisms influence the host immune status by modulating immune cell activity and regulating inflammatory factor production, thereby impacting the onset and progression of asthma. These findings underscored the importance of focusing on the gut-lung axis during early life and developing effective asthma prevention strategies. Examples include antibiotic use during pregnancy and infancy as well as probiotic therapies to promote gut homeostasis and reduce the likelihood of asthma development (Lee et al., 2023).
2.3 ALI
ALI is a severe respiratory condition triggered by various etiological factors (Ren Z. et al., 2024; Zhang et al., 2025). Disruptions in the gut microbiota can activate the systemic immune system and facilitate bacterial translocation to the lungs, thereby exacerbating the pathological progression of lung injury (Wang Q. et al., 2024). An imbalance in the gut microbiota modulates the TLR4/NF-κB signaling pathway within the lung immune system and induces oxidative stress, contributing to lung injury (Hua et al., 2023). Xianghao Zeng et al. observed reduced fecal microbiota diversity in pneumonia patients treated with antibiotics compared to healthy volunteers. They identified gut microbiota-derived autoinducer-2 (AI-2), a metabolite derived from the gut microbiota, as positively correlated with inflammatory molecules in fecal samples. Intraperitoneal administration of AI-2 in a mouse model of acute lung injury resulted in heightened lung inflammation, characterized by elevated levels of IL-6, IL-1β, and chemokines (Zeng et al., 2023). Zhengjian Wang demonstrated that modulating the gut microbiota composition in mice—by reducing the Firmicutes/Bacteroidetes ratio and increasing the relative abundance of short chain fatty acids (SCFAs)-producing bacteria—activated the AMPK/NF-κB/NLRP3 signaling pathway, offering a therapeutic approach for ALI (Wang Z. et al., 2023). Similarly, succinate as a metabolite produced by the gut microbiota was shown to exacerbate the inflammatory response in acute lung injury through succinate receptor 1 (SUCNR1)-dependent polarization of alveolar macrophages (Wang YH. et al., 2023).
Consequently, the gut microbiota has emerged as a promising therapeutic target for the treatment of ALI. For instance, Wei Song et al. demonstrated that the protective effects of cymbopogonin against septic acute lung injury are likely mediated through the regulation of gut microbiota and restoration of the intestinal barrier. The underlying mechanism appears to involve the aryl hydrocarbon receptor (AHR)/Nrf2 signaling pathway (Song et al., 2021). Additionally, acetic acid as a metabolite of the gut microbiota has been shown to mitigate septic ALI by modulating the MAPK pathway. This intervention improves alveolar permeability, reduces the levels of inflammatory factors, and suppresses the production of oxygen radicals (Xu et al., 2019). These findings collectively highlight the critical role of gut microbiota dysbiosis in the pathogenesis of ALI, emphasizing the potential for therapeutic interventions targeting gut-lung axis interactions.
2.4 COVID-19
COVID-19, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is primarily a respiratory illness (Zuo et al., 2020b). Previous studies have revealed a significant reduction in bacterial diversity within the fecal microbiome of COVID-19 patients (Mizutani et al., 2022; Zhang et al., 2023), alongside a decreased abundance of SCFAs-producing bacteria from the family Lachnospiraceae (Xu et al., 2021). Emerging evidence indicates that SARS-CoV-2 not only infects the lungs but also targets the gastrointestinal tract (Pagnini et al., 2020; Uno, 2020; Xiao et al., 2020). Following COVID-19 infection, patients exhibit distinct alterations in their gut microbiome, characterized by reduced levels of Ruminococcus gnavus, Bacteroides vulgatus, and Faecalibacterium prausnitzii, alongside an increase in the phyla Firmicutes and Actinobacteria (de Oliveira et al., 2021). This dysbiosis is marked by the enrichment of opportunistic pathogens and the depletion of beneficial microorganisms (Zuo et al., 2020b). Clinical data have demonstrated a negative correlation between the abundance of Bifidobacterium adolescentis, Bifidobacterium bifidum, Bifidobacterium longum, and Bifidobacterium pseudocatenulatum and SARS-CoV-2 viral loads in patients’ fecal samples (Zuo et al., 2020b; Zuo et al., 2020a; Peng et al., 2021). These findings suggest that gut microbiota alterations may play a critical role in the persistence of respiratory symptoms post-infection (Bernard-Raichon et al., 2022; Liu Q. et al., 2022). The gut microbiota may influence SARS-CoV-2 replication (Xu et al., 2021; Nagai et al., 2023) through the migration of immune cells across mucosal surfaces, interactions between the gut and lungs, and the production of cytokines on the intestinal mucosa (Nagata et al., 2023). Yun Kit Yeoh et al. analyzed fecal samples from 100 patients with confirmed SARS-CoV-2 infection and observed reduced levels of immunomodulatory bacteria such as Eubacterium rectale, Faecalibacterium prausnitzii, and Bifidobacterium bifidum. Concurrently, these patients exhibited elevated concentrations of inflammatory cytokines, which correlated with disease severity. This suggests that the gut microbiota may modulate the host immune response during COVID-19 infection (Yeoh et al., 2021). Furthermore, COVID-19 patients experienced significant and persistent changes in their fecal microbiome throughout hospitalization, characterized by the enrichment of opportunistic pathogens and the depletion of beneficial microbes. Notably, this dysbiosis persisted even after SARS-CoV-2 clearance (as confirmed by pharyngeal swabs) and the resolution of respiratory symptoms, highlighting the long-term impact of COVID-19 on gut microbiota composition and function.
The Syrian hamster model can recapitulate some pulmonary features of human COVID-19, and gastrointestinal changes during infection occur concomitantly with alterations in gut microbiota composition, characterized by increased relative abundance of deleterious bacterial taxa such as Enterobacteriaceae and Desulfovibrionaceae, whereas bacteria capable of producing SCFAs (e.g., Ruminococcaceae and Lachnospiraceae) show a decreased relative proportion (Sencio et al., 2022b). Findings from metagenomic and metabolomic studies demonstrated that: sustained decreases in phenylalanine, tryptophan, glutamate, and indoleacetic acid in elderly hamsters were positively correlated with poor weight recovery and/or pulmonary fibrosis during the recovery phase of SARS-CoV-2 infection; whereas in young hamsters, bacterial taxa such as Eubacterium, Oscillospiraceae, and Lawsonibacter, as well as plasma metabolites including carnosine and cis-aconitic acid, were all associated with mild disease outcomes (Brito Rodrigues et al., 2025). These findings indicate that developing age-specific microbiome-targeted strategies would contribute to more effective management of acute viral pneumonia and its long-term prognosis. Another study also revealed that changes in the gut microbial community were characterized by increased abundance of several genera previously linked to intestinal inflammation and disease (Haemophilus, Fusobacterium, Streptococcus, Campylobacter, and Johnsonella) (Seibert et al., 2022). Obese patients with non-alcoholic steatohepatitis (NASH) are highly susceptible to COVID-19. In obese NASH hamsters, certain taxa such as Blautia and Peptococcus were associated with pro-inflammatory parameters in the lung and liver; these taxonomic profiles and their associations with specific disease markers suggested that microbial patterns may influence COVID-19 outcomes (Sencio et al., 2022a). Additionally, oral administration of the gut microbes Oribacterium sp. GMB0313 and Ruminococcus sp. GMB0270 to hamsters conferred complete protection against SARS-CoV-2 infection by activating CD8+T cell-mediated immunity, with preventive efficacy comparable to or even superior to that of mRNA vaccines (Wang M. et al., 2024).
2.5 Lung cancer
Lung cancer remains one of the most prevalent malignant tumors globally, with its incidence and mortality rates consistently ranking among the highest for cancers (Sun et al., 2023). Microorganisms colonizing the human body can significantly influence tumor cell metabolism, growth patterns, and functions, as well as shape the tumor microenvironment (El Tekle and Garrett, 2023). Emerging evidence highlights the gut microbiota and their associated metabolites as critical factors contributing to and regulating the development of lung cancer (Zhao et al., 2021). Using Mendelian randomization analysis with the inverse variance weighted (IVW) method as the primary approach, Ying chen Li et al. identified 40 distinct groups of gut microbiota in lung cancer patients, establishing a causal relationship between specific gut microbial communities and lung cancer (Li Y. et al., 2023). Further studies have analyzed 16S rRNA gene sequencing and metabolomics data from stool and serum samples of lung cancer patients and healthy controls. These investigations revealed that specific gut microbes, such as Clostridium difficile and Bacillus augmentans, along with their associated metabolites, could serve as potential diagnostic biomarkers and therapeutic targets for lung cancer (Zhao et al., 2021). Notably, the gut microbiome composition varies among different subtypes of lung cancer. For instance, patients with squamous cell carcinoma exhibit a higher abundance of Aspergillus, Gammaproteobacteria, Enterobacteriaceae, and Firmicutes, while those with adenocarcinoma show increased levels of Clostridium and Roseburia species (Lu et al., 2023). These findings underscore the role of gut microbiota in lung cancer pathogenesis and progression. Researchers have explored the therapeutic potential of modulating the gut microbiota to influence lung cancer outcomes. For example, traditional herbal formulations such as Zeng sheng Ping have been shown to boost immunity, protect the intestinal mucosa, and regulate gut microbiota, thereby impacting lung cancer progression (Sun et al., 2023). Additionally, ginseng polysaccharides have demonstrated the ability to enhance the antitumor response to αPD-1 monoclonal antibody therapy by increasing the microbial metabolite valeric acid and reducing the ratio of L-kynurenine to tryptophan (Kyn/Trp) (Huang et al., 2022). These approaches represent innovative strategies in the prevention and treatment of lung cancer, highlighting the pivotal role of the gut-lung axis in oncology.
2.6 Idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is the most common type of idiopathic interstitial pneumonia, accounting for approximately one-third of all interstitial lung disease cases (Maher, 2024).Compared with the control group, the IPF patient groups exhibited lower abundance of Actinobacteria, Bifidobacteriales, Burkholderiales, Bacteroidaceae, Dorea, Fusicatenibacter, and Ruminococcus gauvreauii, while showing higher abundance of Enterobacterales, Erysipelotrichaceae, Holdemanella, and Alloprevotella (Göktürk et al., 2024). A two-sample Mendelian randomization analysis showed that Order Bifidobacteriales, Family Bifidobacteriaceae, and Genus RuminococcaceaeUCG009 exerted protective effects on IPF, while Genus Coprococcus2 promoted the development of IPF (Ren Y. et al., 2024). In the pulmonary fibrosis mouse model established by bleomycin injection, both the diversity and richness of gut microbiota were significantly altered (Luo et al., 2025). Xuanfei Baidu Decoction can alleviate bleomycin-induced pulmonary fibrosis by regulating gut microbiota, with AKK being the core bacterium (Jia et al., 2024).
2.7 Pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is a malignant pulmonary vascular disease, characterized by increased pulmonary vascular resistance, vasoconstriction, and right ventricular hypertrophy, ultimately leading to right heart failure and death (McLaughlin and McGoon, 2006). Researchers are increasingly focusing on the interaction between intestinal dysbiosis and PAH, and the gut microbiota may play an important role in PAH (Chen et al., 2022; Wu P. et al., 2022). A small-scale clinical cohort study showed that in the PAH patient cohort, bacteria producing butyrate and propionate—such as Coprococcus, Butyrivibrio, Lachnospiraceae, Eubacterium, Akkermansia, and Bacteroides—were reduced (Kim et al., 2020). Unlike healthy participants, this may be associated with elevated inflammatory cytokines and endotoxins (Ikubo et al., 2022). Another study found that the relative abundance of Lachnospiraceae bacterium GAM79 in the feces of PAH patients is lower than that in non-PAH control subjects (Jose et al., 2022). Fecal microbiota transplantation (FMT) can affect PAH phenotypes, and that multi-kingdom markers are more accurate in diagnosing PAH (Chen et al., 2025). Moutsoglou DM et al. also indicated that PAH patients had poor gut microbiome diversity and distinct phylogenetic characteristics, which were manifested by a reduction in anti-inflammatory metabolite-related microorganisms and their products, as well as an enrichment of pro-inflammatory metabolite-related microorganisms (Moutsoglou et al., 2023). Animal experiments have demonstrated that PAH rats exhibit significant changes in intestinal pathology and gut microbiota, as well as increased sympathetic nerve activity (Sharma et al., 2020). Moreover, supplementation of lactobacillus in PAH mice can restructure the intestinal microflora/mycobiome, restore intestinal health, inhibit systemic inflammation, reduce GP130 ligands and related right ventricular cardiomyocyte microtubule remodeling, thereby improving PAH (Prisco et al., 2025).
2.8 Acute lower respiratory infection
The microbial diversity of H1N1 patients is reduced, and the number of several representative anaerobic butyrate-producing bacteria from the Lachnospiraceae and Ruminococcaceae families is significantly decreased (Gu et al., 2020). In infants with bronchiolitis, a significant reduction in intestinal microbial richness and an increase in respiratory microbial diversity can be observed. This phenomenon is also present in infants with the most severe symptoms and those who experience recurrent wheezing episodes after discharge, and is associated with respiratory morbidity (Cabrera-Rubio et al., 2024). Animal experiments have confirmed that in the RSV-infected group, the genus Aggregatibacter is enriched while the genus Proteus is reduced, resulting in impaired development of intestinal Th17/Treg (Liu J. et al., 2024). Compared with the control group, the gut microbiota of neonates with neonatal acute respiratory distress syndrome (NARDS) undergoes significant changes, and these changes are associated with alterations in lung microbiota, as well as tryptophan metabolites in the lungs and plasma (Yang J. et al., 2024).
2.9 Tuberculosis
Tuberculosis (TB) is a highly contagious disease caused by Mycobacterium tuberculosis. The occurrence of intestinal dysbiosis in extraintestinal diseases suggests that native intestinal bacteria may affect trans-organ diseases, especially tuberculosis (Lin et al., 2024; Yuan et al., 2024). There is a close association between changes in the intestinal microbiota and Mycobacterium tuberculosis infection (Chai et al., 2025). A cross-sectional study showed that in the TB group, dominant genera such as Bacteroides, Parabacteroides, Fusobacterium, and Lachnoclostridium were significantly enriched; in contrast, the healthy people group was enriched with Blautia, Roseburia, Bifidobacterium, unclassified_Ruminococcaceae, Clostridium, and Romboutsia (Wang et al., 2022), and certain anaerobic bacteria in feces may be associated with the upregulation of pro-inflammatory immune pathways (Naidoo et al., 2021). At the genus level, compared with the healthy control group, the relative abundance of Bacteroides, Parabacteroides, and Veillonella was significantly higher in the TB group, while the relative abundance of Faecalibacterium, Bifidobacterium, Agathobacter, and CAG-352 was significantly lower (Ye et al., 2022). A preliminary study in China also confirmed that tuberculosis patients have reduced diversity of intestinal microbiota and lower abundance of short-chain fatty acid-producing genera (Shi et al., 2021), and gut microbial dysbiosis is strongly correlated with changes in IL-17 and IFN-γ (Han et al., 2024). Systematic review and analysis found significant differences in gut microbiota status between the tuberculosis group and healthy controls, characterized by excessive enrichment of Proteobacteria and depletion of some short-chain fatty acid-producing bacterial genera such as Bifidobacteria, Roseburia, and Ruminococcus, with anti-tuberculosis treatment exacerbating this dysbiosis (Baral et al., 2023; Li W. et al., 2024). The study found that broad-spectrum antibiotic treatment increases the susceptibility of mice to Mycobacterium tuberculosis, while Bacteroides fragilis can enhance anti-tuberculosis immunity by regulating lncRNA-CGB (which regulates IFN-γ expression through interaction with EZH2), revealing the role of the intestinal bacteria-related axis in tuberculosis immune protection and a new paradigm for treatment (Yang et al., 2022).
3 Metabolites of intestinal microbiota
Metabolites derived from gut microbiota exhibit distinct characteristics in the context of respiratory disease development. Recent advancements in the study of host-microbe interactions have highlighted the critical role of gut microbial metabolites in maintaining tissue and immune homeostasis (Rastogi et al., 2022), as illustrated in Figure 2.
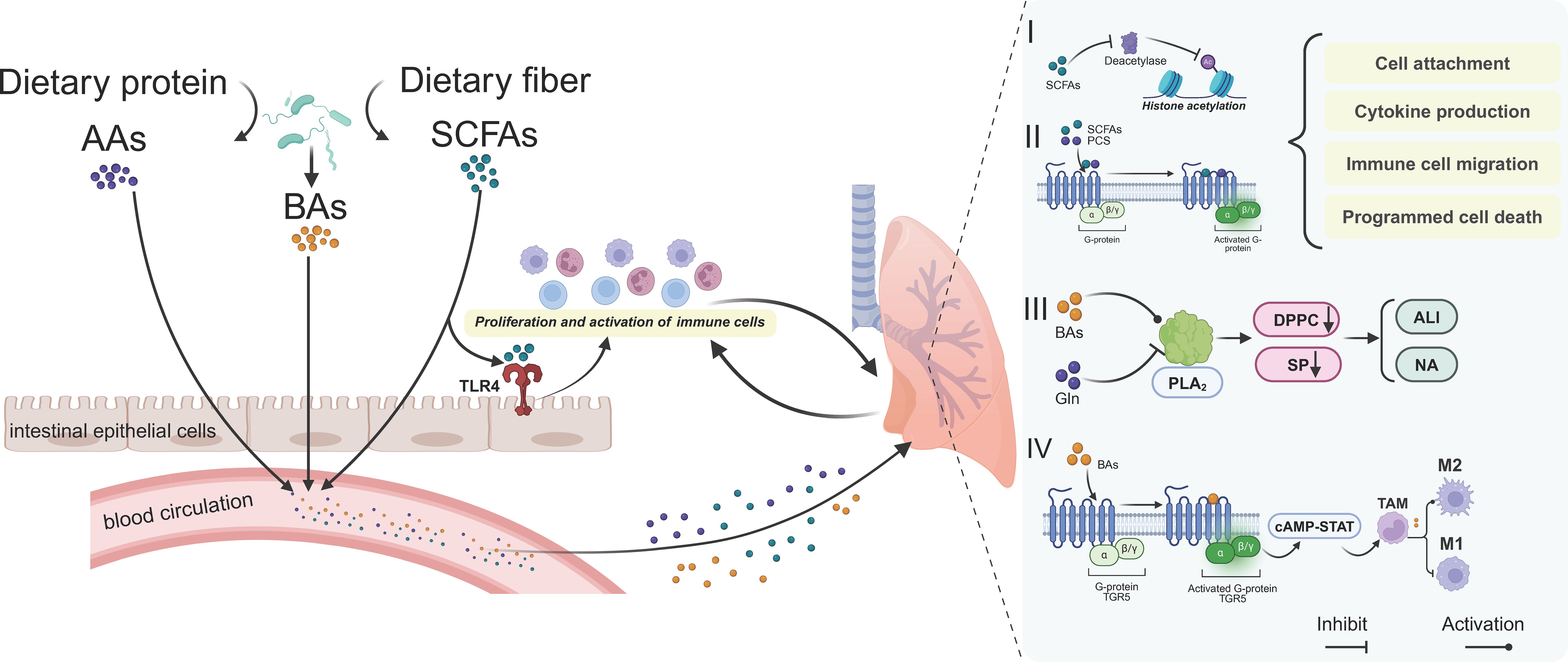
Figure 2. The role of gut microbiota metabolites in pulmonary diseases. Gut microbiota-derived metabolites can affect lung homeostasis through the bloodstream mainly based on four pathways. I) SCFAs act as deacetylase inhibitors and II) activate downstream pathways by binding to G-protein-coupled receptors. Together, they jointly regulate cell adhesion, cytokine production, immune cell migration and programmed cell death, etc. III) Metabolites such as BAs regulate PLA2 to affect pulmonary surfactant proteins and thus influence the progress of ALI and NA diseases. IV) In tumor tissues, metabolites such as BAs affect tumor immunity by regulating the polarization of TAMs. AAs, amino acids; BAs, bile acids; SCFAs, short-chain fatty acids; Gln, glutamine; ALI, acute lung injury; NA, non-eosinophilic asthma; TAM, tumor-associated macrophage.
3.1 Short-chain fatty acids
SCFAs including acetate, propionate and butyrate (Ney et al., 2023) are produced by gut bacteria through the fermentation of indigestible dietary fibers (Liu M. et al., 2024). These metabolites serve as a crucial link between the gut microbiota and the immune system, playing a vital role in maintaining immune homeostasis. Accumulating evidence indicates that SCFAs regulate the activities of various cell types within the body, including but not limited to inhibition of Th1 cells by SCFAs (Che et al., 2024), the effect of SCFAs activate the NLRP3 inflammasome in human macrophages upon TLR stimulation (Wang W. et al., 2024), and it can also effects on the B cells and plasma cells (Mann et al., 2024) and so on. Although the underlying mechanisms are not fully elucidated, G-protein coupled receptor (GPCR) activation and histone deacetylase (HDAC) inhibition are believed to play significant roles (Mann et al., 2024). SCFAs are essential for cellular homeostasis. They are through β-oxidation and regulate macromolecular synthesis, GPCR and HDAC activities, protein modifications, signaling pathways, and gene expression in cells within the tumor microenvironment, particularly in tumor and immune cells (Li S. et al., 2025). As a result, manipulating SCFAs levels by altering gut microbiota composition holds potential for cancer prevention and treatment. Among the components involved in the gut-lung axis, SCFAs are the most important immunomodulatory metabolites, exhibiting protective effects in patients with airway inflammation (Cait et al., 2018). The severe eosinophil inflammation cluster characterized by lower SCFAs levels and higher mucus plug scores (Tanabe et al., 2025). SCFAs enhance the proliferation and activation of immune cells by activating the Toll-like receptor (TLR) signaling pathway in intestinal epithelial cells (Ruan et al., 2022), thereby bolstering the body’s defense against respiratory pathogens. Additionally, SCFAs may induce myelopoiesis and contribute to an anti-inflammatory environment (Trompette et al., 2014), further underscoring their therapeutic potential (Chen et al., 2024). In conclusion, SCFAs help to maintain gut barrier integrity, reduce systemic inflammation and regulate immune responses.
3.2 Bile acids
Primary bile acids are produced by the host in the liver to solubilize dietary lipids and fat-soluble vitamins in the small intestine. The primary bile acid pool is largely recycled back to the liver, but a small proportion of these bile acids escapes to the large intestine where they are readily deconjugated and further metabolized by the microbiota into secondary bile acids, which have important effects on the host (Nicolas and Chang, 2019). BAs as critical intestinal metabolites are involved in numerous signaling pathways associated with respiratory diseases (Yin et al., 2023). Yuqiong He et al. verified BAs alleviated sepsis-induced lung injury through blocking PANoptosis-like cell death via STING pathway (He et al., 2023). Furthermore, the signaling effects of nitroolefins can mitigate allergic airway disease by modulating bile acid metabolism (Manni et al., 2021). Additionally, microaspiration of BAs may promote pulmonary fibrosis by stimulating the expression of fibrotic mediators and activating the TGF-β1/Smad3 signaling pathway (Chen et al., 2017). Jose A. Caparrós-Martín et al. investigated the role of bile acids in bronchoalveolar lavage fluid (BALF) in relation to inflammation and microbiota establishment in early cystic fibrosis lung disease (Caparrós-Martín et al., 2023). Secreted phospholipase A2 (sPLA2) as a key enzyme in the acute lung injury pathway (Nakos et al., 2005) is significantly influenced by bile acids. High concentrations of BAs (5 μmol/L) have been shown to markedly increase sPLA2 activity, leading to enhanced surfactant catabolism and exacerbation of acute lung injury (De Luca et al., 2009). Furthermore, the bile acid-responsive G-protein-coupled receptor (TGR5) has been implicated in tumor-associated macrophage (TAM) polarization. TGR5 activation converts TAMs into a tumor-promoting M2-like phenotype by activating the cAMP-STAT3/STAT6 signaling pathway. This mechanism highlights the clinical relevance of TGR5, as its co-expression with high TAM infiltration is significantly associated with prognosis and overall survival in non-small cell lung cancer patients (Zhao et al., 2022).
3.3 Tryptophan
Tryptophan is an essential aromatic amino acid acquired through common diet sources and it plays a significant role in respiratory health (Hou et al., 2023). Tryptophan metabolites may modulate asthma through the gut microbiota (Wang H. et al., 2024). Giorgia Renga confirmed that the Tph isoform 1 (Tph1)/5-hydroxytryptamine (5-HT) metabolic pathway of tryptophan plays a key role in regulating pulmonary inflammation (Renga et al., 2024). Tryptophan metabolism in the gut includes the kynurenine (Kyn), 5-HT and indole pathways (Xue et al., 2023), and they have been confirmed to be associated with a variety of inflammatory responses in the lungs. Abbas F Almulla confirmed that the serum Kyn/tryptophan ratio was significantly increased in COVID-19 patients compared to controls (Almulla et al., 2022). Kyn suppresses excessive inflammation by activating the AHR and regulating Th17/Tregs balance (de Araújo et al., 2017). A recent study showed that patients with untreated pulmonary hypertension maintained lower levels of tryptophan and higher concentrations of Kyn compared to controls (Yang C. et al., 2024). Indoleamine 2,3-dioxygenase (IDO) as the enzyme of Tryptophan, reduced IDO activity and expression in sputum of COPD patients is negatively correlated with clinical severity (Naz et al., 2019). During respiratory infection, fungi, bacteria or viruses induce IDO1 expression and enzymatic activity in epithelial, immune or even endothelial cells (Pamart et al., 2024). Xue Lu suggested that 5-HT upregulated airway responsiveness in ovalbumin (OVA)-induced allergic asthma (Lu et al., 2025). 5-HT promotes fibroblast proliferation leading to increased lung fibrosis through activation of TGF-β signaling (Xie et al., 2023). 5-HT activates proliferation (ERK1/2 pathway) and vasoconstriction of pulmonary artery smooth muscle cells via 5-HT2A/2B receptors (Yun and Park, 2025). Yumeng Huang’s study uncovers a mechanism by which peripheral 5-HT aggravated sepsis-induced ALI by promoting neutrophil extracellular trap (NET) formation in the lung of septic mice (Huang et al., 2021).
Based on the above insights, we believe that gut microbial metabolites can have complex regulatory effects on the lungs to a large extent. How to make gut metabolites achieve an immune homeostasis state in regulating lung diseases and its underlying mechanisms still need to be further elaborated in the future studies.
4 Interventions based on gut microbiota
As a critical immune barrier for the organism, the gut not only regulates the absorption of nutrients and electrolytes but also plays a pivotal role in preventing the translocation of pathogens and their toxins into the bloodstream. In this review, we specifically highlight the function of microbial metabolites and their essential contributions to maintaining homeostasis and immune tolerance within the organism. With the continuous advancement of technology, accumulating evidence demonstrates that gut-associated microorganisms exert profound effects on immune responses in distal tissues. Expanding our understanding of individual bacterial species and their metabolites will be fundamental to the future development of microbiota-targeted therapies aimed at preventing or treating systemic inflammatory diseases and infections, as illustrated in Figure 3.
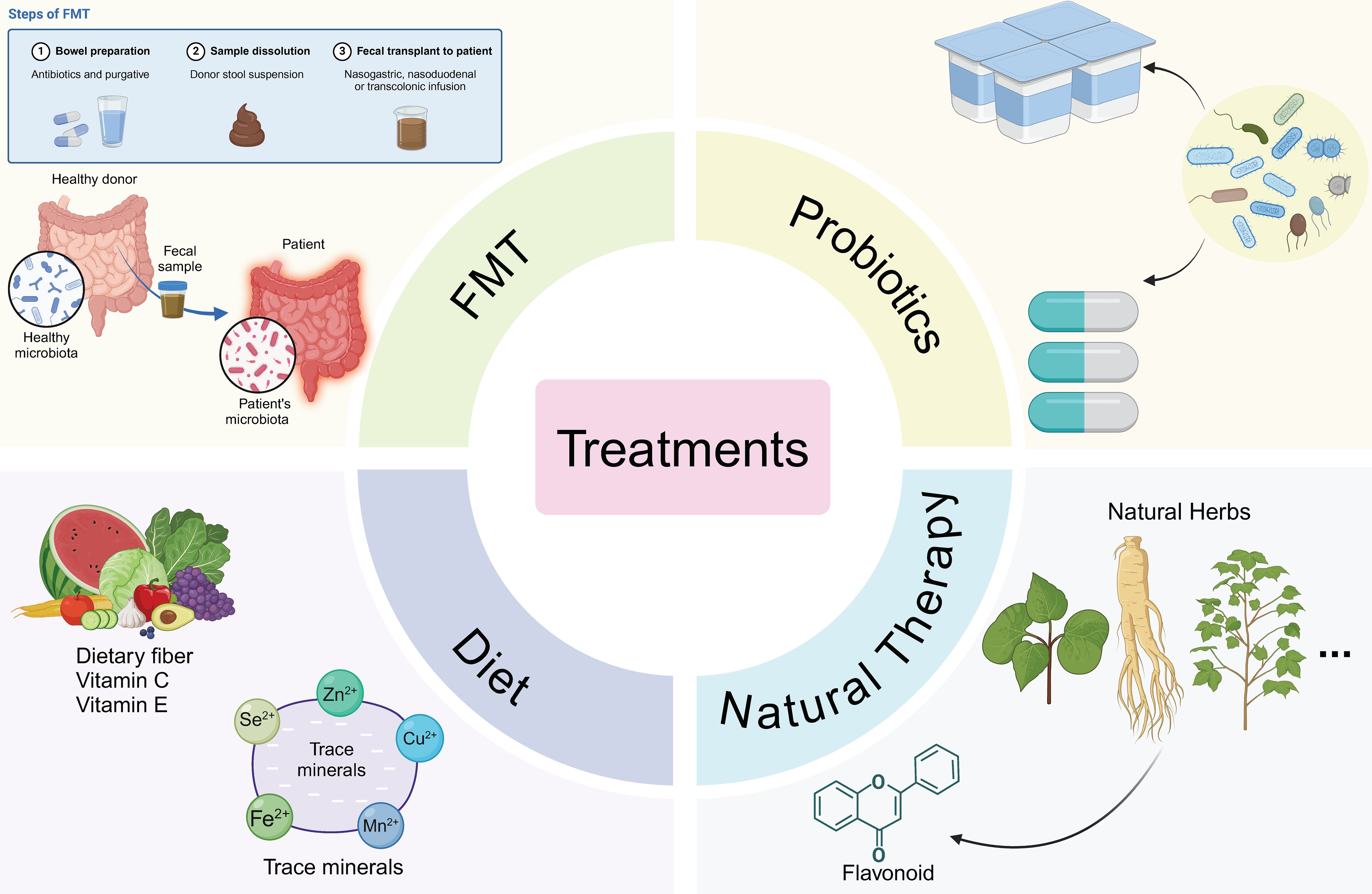
Figure 3. Therapies targeting pulmonary diseases based on the gut microbiome. Fecal microbiota transplantation (FMT) operates by rectifying the dysbiosis of the gut microbiota in patients, thereby fulfilling its therapeutic function with a gut-targeted approach. Administration of probiotic formulations or live-culture-containing yogurts is conducive to ameliorating the pulmonary immune status. Dietary fiber supplementation as well as trace element supplementation also play a part in optimizing the disease condition. Natural botanicals exert their efficacy through anti-inflammatory and antioxidant mechanisms.
4.1 Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) has garnered significant attention as an innovative therapeutic approach for inflammation and has been extensively investigated in both preclinical and clinical settings. In animal studies, fecal grafts are administered directly via gavage to mice to assess their potential to ameliorate pathophysiological conditions (Li et al., 2021). Accumulating evidence highlights the therapeutic efficacy of FMT in various respiratory diseases. For example, Tim J. Schuijt et al. utilized FMT in a mouse model of gut microbiota depletion and demonstrated that lung bacterial counts, as well as levels of tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10), returned to normal levels in FMT-colonized mice within six hours following pneumococcal infection (Schuijt et al., 2016). Similarly, Jia Tang’s research revealed that fecal microbiota transplantation inhibits NF-κB phosphorylation and reduces the release of inflammatory cytokines in the lungs, further underscoring its anti-inflammatory potential (Tang et al., 2021). In the context of the recent COVID-19 pandemic, FMT has also been explored as a novel therapeutic strategy. Experimental studies have confirmed its effectiveness in treating COVID-19, offering a promising avenue for managing this disease (Kazemian et al., 2021; Biliński et al., 2022). Collectively, these findings provide robust evidence supporting the role of FMT in treating inflammation-induced infections, highlighting its potential as a valuable intervention for respiratory and systemic inflammatory conditions.
4.2 Probiotics
Probiotics are recognized as functional foods that play a significant role in enhancing host health (Lee C. et al., 2024). When combined with prebiotics (indigestible dietary fibers or carbohydrates), they confer additional benefits to the host through anaerobic fermentation (Sasaki et al., 2025). Numerous studies have demonstrated that probiotics have dual effects of immunomodulation and infection inhibition in pulmonary diseases (Bezemer et al., 2024; Hu et al., 2024). For instance, researchers have shown that Bifidobacterium longum demonstrated the ability to inhibit the transfer of drug resistance plasmids among Carbapenem-resistant Klebsiella pneumoniae (CRKP) strains, thus limiting the horizontal spread of resistance genes (Wang J. et al., 2024). It demonstrated that Bifidobacterium longum mitigates CRKP-induced infections. In a multicenter retrospective study, Kazuki Takada et al. demonstrated that probiotic supplementation is associated with improved clinical outcomes in patients with advanced or recurrent non-small cell lung cancer (NSCLC) receiving anti-PD-1 monotherapy (Takada et al., 2021). Additionally, Lactobacillus rhamnosus has been shown to penetrate hypoxic tumor regions, enabling efficient delivery of the clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) system to tumor sites (Zhong et al., 2023). Lactobacillus rhamnosus modulates the STAT signaling-associated immune response to control the lung inflammatory insult and gut dysbiosis in a murine model of asthma-COPD overlap syndrome (ACOS) (Vasconcelos et al., 2025). The relative abundance of AKK, a minor component of the gut microbiota, has been linked to enhanced human responses to anti-PD-1 or anti-PD-L1 immunotherapy (Derosa et al., 2022). Furthermore, studies have reported that airway inflammation and early asthma-like symptoms in COPD mice can be prevented by supplementation with Bifidobacterium breve and Lactobacillus rhamnosus (Uwaezuoke et al., 2022). Hsin-Chih Lai et al. isolated Parabacteroides goldsteinii from the gut microbiota of COPD mice and demonstrated its ability to ameliorate COPD-related inflammation by reducing intestinal inflammation, enhancing mitochondrial and ribosomal activity in colon cells, and restoring aberrant host amino acid metabolism in the serum (Lai et al., 2022). Bifidobacterium infantis subsp. Infantis YLGB-1496 exhibited good safety and tolerability in young children, and could effectively alleviate gastrointestinal discomfort associated with respiratory diseases (Li P. et al., 2025). In contrast, a trial involving individuals with long-term respiratory symptoms after COVID-19 (without asthma or COPD) showed that after 12 weeks of intervention with Lactobacillus plantarum GCWB1001, there was no significant difference in the total score of the primary outcome compared with the placebo group (Kang et al., 2025). The numerical improvements in secondary outcomes were also not statistically significant after correction, and no serious adverse events were reported. Thus, its efficacy required verification in larger-scale trials. Overall, the risk of infection caused by probiotic administration is extremely low, comparable to the risk of infection from commensal bacterial strains. In general, the benefits of probiotic therapy far outweigh the potential risks. Although probiotics are generally proven to be safe, caution must be exercised when using them in specific patient populations, especially those with immunocompromised status (Tsai et al., 2019).
4.3 Postbiotics
Postbiotics, together with probiotics, have become widely recognized terms, and interest in them is constantly growing (Swanson et al., 2020; Kumar et al., 2024). As a class of substances with multiple advantages, postbiotics generally exhibit more prominent characteristics compared to probiotics: they have higher safety (Yelin et al., 2019; Vandenplas et al., 2020), usually longer shelf life, and simpler production processes; they pose no risk of antibiotic resistance gene transfer and can be used in immunocompromised populations; they are easy to encapsulate and can be targeted to specific sites (Puccetti et al., 2018), while exerting beneficial effects similar to or even better than those of probiotics (Zhang et al., 2022). In addition, postbiotics also have the feature of being easy to obtain patents, and relevant trials can be conducted in accordance with existing standard pharmacological guidelines by recording pharmacodynamic and pharmacokinetic data (Salminen et al., 2021).
In recent years, postbiotics, as inactivated products and metabolites of probiotics, have shown significant potential in the intervention of various diseases, with their mechanisms closely related to immune regulation and improvement of microecological balance. Studies have demonstrated that extracellular vesicles (EcO83-EVs) from Escherichia coli A0 34/86 can produce nitric oxide by activating the NF-κB signaling pathway, effectively regulating inflammatory activity both in vivo and in vitro, thus providing a new direction for replacing live probiotics (Razim et al., 2024). Pasteurized Weissella cibaria can improve intestinal mucosal barrier function and reverse gut microbiota dysbiosis (increasing the abundance of anti-inflammatory bacteria and reducing LPS-producing bacteria), thereby improving the survival rate of mice with sepsis-induced ALI and alleviating lung damage (Li Y. et al., 2025).
In the field of cancer treatment, the adjuvant role of postbiotics is particularly prominent. When postbiotic MS-20 is used in combination with anti-PD1 antibodies, it can reshape the tumor immune microenvironment through gut microbiota (increasing effector CD8+ T cells and downregulating PD1 expression), inhibiting the growth of colorectal and lung cancers in mice, and the increased abundance of Ruminococcus bromii may be involved in this process (Lee PJ. et al., 2024). Although postbiotic JK5G does not significantly improve the objective response rate of advanced non-small cell lung cancer (NSCLC) patients receiving PD-1 inhibitor combined with chemotherapy, it can reduce adverse reactions (such as anemia and nausea) and improve quality of life. Its mechanism is related to regulating gut microbiota, reducing inflammation, and optimizing immune cell subsets, which provides support for enhancing the tolerance of immune checkpoint inhibitor (ICIs) therapy (Chen et al., 2023).
In addition, postbiotics have also shown promising performance in the intervention of inflammatory diseases and oxidative damage. Heat-killed Lactiplantibacillus plantarum BGPKM22 and its cell-free supernatant exhibit antioxidant activity in bronchial epithelial cells exposed to cigarette smoke, which can exert protective effects by inhibiting the expression of AHR and Nrf2 genes (Babic et al., 2023). Postbiotics from Saccharomyces cerevisiae can regulate systemic and mucosal immune responses in calves, enhancing their resistance to bovine respiratory diseases (Maina et al., 2024). Pasteurized yogurt containing heat-killed Bifidobacterium longum BBMN68 can effectively alleviate mugwort pollen-induced allergic airway inflammation by regulating gut microbiota structure (increasing related beneficial bacteria) and maintaining Th1/Th2 immune balance (Niu et al., 2023).
In summary, different types of postbiotics, through mechanisms such as regulating signaling pathways, improving gut microbiota, and balancing immune responses, show broad application prospects in the fields of inflammation regulation, infection prevention, and adjuvant cancer treatment, providing new ideas for the intervention of related diseases.
4.4 Dietary fibers
Dietary influences particularly dietary fiber has long been widely recognized as having a significant impact on the abundance of the human gut microbiota (Sinha et al., 2024). McLoughlin et al. utilized a prebiotic-free inulin formulation to isolate the effects of dietary fiber from those of prebiotics and observed a significant improvement in asthma symptoms in mice after seven days of treatment (McLoughlin et al., 2019). Magdalena Prochazkova et al. conducted a cross-sectional study using multi-omics analyses (16S rRNA sequencing and metabolomics) to compare vegetarians and omnivores, revealing that vegetarians exhibit more favorable glucose and lipid homeostatic profiles. The study also highlighted that increasing vitamin intake, lowering Low-density lipoprotein (LDL) cholesterol levels and limiting the consumption of oxidized lipids can help reduce asthma risk and mitigate symptom exacerbations (Oliver et al., 2021).Flavonoids is an important class of dietary fibers which have also been shown to correlate with the prevalence of chronic respiratory diseases in adults (Wu R. et al., 2024). Resveratrol (RES) is a natural compound produced by many plants in response to stress has been found to induce a dose-dependent upregulation of PD-L1 expression in lung cancer cells. This mechanism is critical for suppressing T-cell-mediated immune responses, as demonstrated in the study (Xin et al., 2024). Furthermore, RES can alleviate asthma symptoms by inducing beneficial microbiota in the gut-lung axis and promoting the normal barrier function of the lungs (Alharris et al., 2022). Isochlorogenic acid C can exert anti-asthmatic effects by promoting the production of SCFAs and other substances by the gut microbiota, regulating immunity, lipid metabolism, and antioxidant responses through the gut-lung axis, and its mechanism is closely related to the gut microbiota (Xu et al., 2025). The use of dietary bioactive compounds, such as phenolic compounds (PC), has emerged as a potential nutritional or therapeutic adjunct for COVID-19 (Augusti et al., 2021). As a representative PC, RES has the potential to serve as a functional supplement to reduce the severity of COVID-19 in patients with poor prognosis due to cardiovascular complications (Gligorijević et al., 2021). A study has shown that supplementation with RES and copper can halve the mortality rate in severe COVID-19 patients (Mittra et al., 2020). In addition, RES acts on the gastrointestinal tract and can alter the gene expression of the gut microbiota (Inchingolo et al., 2022), which may help improve obesity, diabetes and other diseases related to dysbiosis, thereby reducing the impact of COVID-19 and subsequent syndromes.
The research team led by Song et al. found that fermented black barley exerts multiple protective effects through antioxidant activity and regulation of intestinal microbiota: it can not only improve cooking oil fume-induced lung injury in mice, but also correct smoking-induced intestinal dysbiosis by regulating the structure of intestinal microbiota (such as reducing the abundance of Lactobacillus and other genera, and increasing the abundance of Oscillospira and other genera), thereby alleviating smoking-induced damages such as lung and testis injuries as well as metabolic disorders (Ding et al., 2020; Zhong et al., 2022). Quercetin can effectively regulate intestinal dysbacteriosis, and in particular, significantly increase the abundance of AKK, thereby exerting a protective effect on rats with pulmonary fibrosis (Wu W. et al., 2024). Epigallocatechin gallate (EGCG) from green tea can alleviate obesity-aggravated lung cancer progression by modifying the intestinal microbiome, specifically by increasing the abundance of Clostridium and decreasing the abundance of Deltaproteobacteria and Epsilonproteobacteria, which effect may be closely related to the STAT1/SLC7A11 pathway (Li F. et al., 2023). These findings underscore the profound influence of diet on gut microbiota composition and its subsequent effects on respiratory health, highlighting the potential of dietary interventions in managing chronic respiratory conditions.
5 Discussion
Although a wide variety of factors in vivo and in vitro drive the progression of respiratory diseases (Ma et al., 2022), the balance of gut microorganisms has long been recognized as one of the most significant contributors to or exacerbators of such conditions. The composition and function of the gut microbiota are influenced by numerous intrinsic and extrinsic factors, necessitating further research to elucidate the causes that shape its composition. This article reviews the current state-of-the-art research on respiratory diseases and gut microbes, emphasizing the critical role of the gut-lung axis in respiratory conditions. This includes the composition of gut microbes and the diverse immunomodulatory functions of their metabolites within the organism. However, clinical data on the application of novel therapeutic strategies targeting the gut microbiota remain limited and require further exploration in the future.
6 Conclusion
In conclusion, the gut-lung axis plays a pivotal role in the pathogenesis and progression of respiratory diseases, with gut microbiota composition and its metabolites significantly influencing immune responses and disease outcomes. While advancements in multi-omics technologies have deepened our understanding of this relationship, further research is needed to explore the underlying mechanisms and therapeutic potential of targeting the gut microbiota. Strategies such as dietary interventions, probiotics, and FMT hold promise for maintaining gut homeostasis and enhancing immunity, offering novel approaches for the prevention and treatment of respiratory diseases. Future studies should focus on translating these findings into effective clinical applications to improve patient outcomes.
Author contributions
XinY: Writing – original draft. XiaY: Data curation, Writing – review & editing. YW: Methodology, Writing – original draft. XG: Investigation, Writing – original draft. CW: Validation, Writing – review & editing. FW: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the following grants: (1) The Jilin Provincial Key Laboratory of Precision Infectious Diseases (Grant No. 20200601011JC); (2) The Jilin Provincial Engineering Laboratory of Precision Prevention and Control for Common Diseases (Jilin Province Development and Reform Commission Grant No. 2022C036); (3) The Medical Basic Research Innovation Center of Airway Disease in North China, Ministry of Education; and (4) Jilin Provincial Department of Education Doctoral Student Research and Innovation Ability Enhancement Project (Grant No. JJKH20250165BS).
Acknowledgments
Many thanks to biorender for the technical support of the image design.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
5-HT, 5-hydroxytryptamine; ACOS, Asthma-COPD Overlap Syndrome; AHR, Aromatic Hydrocarbon Receptor; AKK, Akkermansia; ALI, Acute Lung Injury; AI-2, Auto-inducer-2; BALF, Bronchoalveolar Lavage Fluid; COPD, Chronic Obstructive Pulmonary Disease; CRISPR/Cas9, Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated Protein 9; CRKP, Carbapenem-resistant Klebsiella pneumoniae; COVID-19, Coronavirus Disease 2019; FMT, Fecal Microbiota Transplantation; GPCR, G-protein Coupled Receptor; HDAC, Histone Deacetylase; IDO, Indoleamine 2,3-dioxygenase; IPF, Idiopathic Pulmonary Fibrosis; IVW, Inverse Variance Weighted; Kyn, Kynurenine; Trp, tryptophan; LDL, Low-Density Lipoprotein; NET, Neutrophil Extracellular Trap; NSCLC, Non-Small Cell Lung Cancer; OVA, Ovalbumin; PD-1, Programmed Cell Death Protein 1; PD-L1, Programmed Death-Ligand 1; SCFAs, Short-Chain Fatty Acids; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; sPLA2, secreted Phospholipase A2.
Microbiota, The collection of all microorganisms in a specific environment (e.g., gut microbiota); Prebiotic, Indigestible substances that promote the growth of beneficial gut bacteria (e.g., oligosaccharides); Probiotic, Live microorganisms that confer health benefits to the host when consumed in adequate amounts (e.g., lactobacilli); Postbiotics, Metabolites or inactivated cells of probiotics with health benefits; Dietary fibers, Indigestible carbohydrates from plants, aiding gut health; FMT (Fecal Microbiota Transplantation), Transplanting healthy fecal microbiota to patients to regulate gut microbiota.
References
Alharris, E., Mohammed, A., Alghetaa, H., Zhou, J., Nagarkatti, M., and Nagarkatti, P. (2022). The ability of resveratrol to attenuate ovalbumin-mediated allergic asthma is associated with changes in microbiota involving the gut-lung axis, enhanced barrier function and decreased inflammation in the lungs. Front. Immunol. 13. doi: 10.3389/fimmu.2022.805770
Alhasan, M. M., Hölsken, O., Duerr, C., Helfrich, S., Branzk, N., Philipp, A., et al. (2023). Antibiotic use during pregnancy is linked to offspring gut microbial dysbiosis, barrier disruption, and altered immunity along the gut-lung axis. Eur. J. Immunol. 53, e2350394. doi: 10.1002/eji.202350394
Almulla, A. F., Supasitthumrong, T., Tunvirachaisakul, C., Algon, A. A. A., Al-Hakeim, H. K., and Maes, M. (2022). The tryptophan catabolite or kynurenine pathway in COVID-19 and critical COVID-19: a systematic review and meta-analysis. BMC Infect. Dis. 22, 615. doi: 10.1186/s12879-022-07582-1
Anandakumar, H., Rauch, A., Wimmer, M. I., Yarritu, A., Koch, G., McParland, V., et al. (2024). Segmental patterning of microbiota and immune cells in the murine intestinal tract. Gut Microbes 16, 2398126. doi: 10.1080/19490976.2024.2398126
Arrieta, M. C., Stiemsma, L. T., Dimitriu, P. A., Thorson, L., Russell, S., Yurist-Doutsch, S., et al. (2015). Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Trans. Med. 7, 307ra152. doi: 10.1126/scitranslmed.aab2271
Augusti, P. R., Conterato, G. M. M., Denardin, C. C., Prazeres, I. D., Serra, A. T., Bronze, M. R., et al. (2021). Bioactivity, bioavailability, and gut microbiota transformations of dietary phenolic compounds: implications for COVID-19. J. Nutr. Biochem. 97, 108787. doi: 10.1016/j.jnutbio.2021.108787
Babic, M., Veljovic, K., Popović, N., Golic, N., Radojkovic, D., and Stankovic, M. (2023). Antioxidant effect of lactic acid bacteria in human bronchial epithelial cells exposed to cigarette smoke. J. Appl. Microbiol. 134, lxad257. doi: 10.1093/jambio/lxad257
Baral, T., Kurian, S. J., Thomas, L., Udyavara Kudru, C., Mukhopadhyay, C., Saravu, K., et al. (2023). Impact of tuberculosis disease on human gut microbiota: a systematic review. Expert Rev. Anti Infect. Ther. 21, 175–188. doi: 10.1080/14787210.2023.2162879
Bernard-Raichon, L., Venzon, M., Klein, J., Axelrad, J. E., Zhang, C., Sullivan, A. P., et al. (2022). Gut microbiome dysbiosis in antibiotic-treated COVID-19 patients is associated with microbial translocation and bacteremia. Nat. Commun. 13, 5926. doi: 10.1038/s41467-022-33395-6
Bezemer, G. F. G., Diks, M. A. P., Mortaz, E., van Ark, I., van Bergenhenegouwen, J., Kraneveld, A. D., et al. (2024). A synbiotic mixture of Bifidobacterium breve M16-V, oligosaccharides and pectin, enhances Short Chain Fatty Acid production and improves lung health in a preclinical model for pulmonary neutrophilia. Front. Nutr. 11. doi: 10.3389/fnut.2024.1371064
Biliński, J., Winter, K., Jasiński, M., Szczęś, A., Bilinska, N., Mullish, B. H., et al. (2022). Rapid resolution of COVID-19 after faecal microbiota transplantation. Gut 71, 230–232. doi: 10.1136/gutjnl-2021-325010
Bock, P. M., Martins, A. F., and Schaan, B. D. (2024). Understanding how pre- and probiotics affect the gut microbiome and metabolic health. Am. J. Physiol. Endocrinol. Metab. 327, E89–e102. doi: 10.1152/ajpendo.00054.2024
Boesch, M., Baty, F., Albrich, W. C., Flatz, L., Rodriguez, R., Rothschild, S. I., et al. (2021). Local tumor microbial signatures and response to checkpoint blockade in non-small cell lung cancer. Oncoimmunology 10, 1988403. doi: 10.1080/2162402x.2021.1988403
Bowerman, K. L., Rehman, S. F., Vaughan, A., Lachner, N., Budden, K. F., Kim, R. Y., et al. (2020). Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat. Commun. 11, 5886. doi: 10.1038/s41467-020-19701-0
Brito Rodrigues, P., de Rezende Rodovalho, V., Sencio, V., Benech, N., Creskey, M., Silva Angulo, F., et al. (2025). Integrative metagenomics and metabolomics reveal age-associated gut microbiota and metabolite alterations in a hamster model of COVID-19. Gut Microbes 17, 2486511. doi: 10.1080/19490976.2025.2486511
Budden, K. F., Gellatly, S. L., Wood, D. L., Cooper, M. A., Morrison, M., Hugenholtz, P., et al. (2017). Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 15, 55–63. doi: 10.1038/nrmicro.2016.142
Cabrera-Rubio, R., Calvo, C., Alcolea, S., Bergia, M., Atucha, J., Pozo, F., et al. (2024). Gut and respiratory tract microbiota in children younger than 12 months hospitalized for bronchiolitis compared with healthy children: can we predict the severity and medium-term respiratory outcome? Microbiol. Spectrum 12, e0255623. doi: 10.1128/spectrum.02556-23
Cait, A., Hughes, M. R., Antignano, F., Cait, J., Dimitriu, P. A., Maas, K. R., et al. (2018). Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 11, 785–795. doi: 10.1038/mi.2017.75
Canfora, E. E., Meex, R. C. R., Venema, K., and Blaak, E. E. (2019). Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 15, 261–273. doi: 10.1038/s41574-019-0156-z
Caparrós-Martín, J. A., Saladie, M., Agudelo-Romero, S. P., Reen, F. J., Ware, R. S., Sly, P. D., et al. (2023). Detection of bile acids in bronchoalveolar lavage fluid defines the inflammatory and microbial landscape of the lower airways in infants with cystic fibrosis. Microbiome 11, 132. doi: 10.1186/s40168-023-01543-9
Casali, L. and Stella, G. M. (2024). The microbiota in children and adolescents with asthma. Children (Basel) 11, 1175. doi: 10.3390/children11101175
Celik, E., Kocacik Uygun, D., Kaya, M. A., Gungoren, M. S., Keven, A., and Bingol, A. (2024). Aspergillus-sensitized asthma in children. Pediatr. Allergy Immunol. 35, e14212. doi: 10.1111/pai.14212
Chai, Y., Li, M., Deng, X., Ma, C., Zhou, N., Chen, Y., et al. (2025). Gut microbiota and tuberculosis infection: interaction and therapeutic potential. Gut Microbes 17, 2531201. doi: 10.1080/19490976.2025.2531201
Che, S., Qin, B., Wu, K., Zhu, M., Hu, H., Peng, C., et al. (2024). EGCG drives gut microbial remodeling-induced epithelial GPR43 activation to lessen Th1 polarization in colitis. Redox Biol. , 75:103291. doi: 10.1016/j.redox.2024.103291
Chen, Y., Chen, Z., Liang, L., Li, J., Meng, L., Yuan, W., et al. (2025). Multi-kingdom gut microbiota dysbiosis is associated with the development of pulmonary arterial hypertension. EBioMedicine 115, 105686. doi: 10.1016/j.ebiom.2025.105686
Chen, Y. J., Ho, H. J., Tseng, C. H., Chen, Y. F., Wang, S. T., Shieh, J. J., et al. (2024). Short-chain fatty acids ameliorate imiquimod-induced skin thickening and IL-17 levels and alter gut microbiota in mice: a metagenomic association analysis. Sci. Rep. 14, 17495. doi: 10.1038/s41598-024-67325-x
Chen, M., Ma, L., Yu, H., Huang, S., Zhang, J., Gong, J., et al. (2023). JK5G postbiotics attenuate immune-related adverse events in NSCLC patients by regulating gut microbiota: a randomized controlled trial in China. Front. Oncol. 13. doi: 10.3389/fonc.2023.1155592
Chen, B., You, W. J., Liu, X. Q., Xue, S., Qin, H., and Jiang, H. D. (2017). Chronic microaspiration of bile acids induces lung fibrosis through multiple mechanisms in rats. Clin. Sci. (London England: 1979) 131, 951–963. doi: 10.1042/cs20160926
Chen, Y. H., Yuan, W., Meng, L. K., Zhong, J. C., and Liu, X. Y. (2022). The role and mechanism of gut microbiota in pulmonary arterial hypertension. Nutrients 14 (20), 4278. doi: 10.3390/nu14204278
Cheng, Y., Hu, G., Deng, L., Zan, Y., and Chen, X. (2024). Therapeutic role of gut microbiota in lung injury-related cognitive impairment. Front. Nutr. 11. doi: 10.3389/fnut.2024.1521214
Chiu, Y. C., Lee, S. W., Liu, C. W., Lan, T. Y., and Wu, L. S. (2022). Relationship between gut microbiota and lung function decline in patients with chronic obstructive pulmonary disease: a 1-year follow-up study. Respir. Res. 23, 10. doi: 10.1186/s12931-022-01928-8
Chu, H. and Mazmanian, S. K. (2013). Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat. Immunol. 14, 668–675. doi: 10.1038/ni.2635
Dang, A. T. and Marsland, B. J. (2019). Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 12, 843–850. doi: 10.1038/s41385-019-0160-6
Danne, C., Skerniskyte, J., Marteyn, B., and Sokol, H. (2024). Neutrophils: from IBD to the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 21, 184–197. doi: 10.1038/s41575-023-00871-3
de Araújo, E. F., Feriotti, C., Galdino, N. A. L., Preite, N. W., Calich, V. L. G., and Loures, F. V. (2017). The IDO-ahR axis controls th17/treg immunity in a pulmonary model of fungal infection. Front. Immunol. 8. doi: 10.3389/fimmu.2017.00880
De Luca, D., Minucci, A., Zecca, E., Piastra, M., Pietrini, D., Carnielli, V. P., et al. (2009). Bile acids cause secretory phospholipase A2 activity enhancement, revertible by exogenous surfactant administration. Intensive Care Med. 35, 321–326. doi: 10.1007/s00134-008-1321-3
de Oliveira, G. L. V., Oliveira, C. N. S., Pinzan, C. F., de Salis, L. V. V., and Cardoso, C. R. B. (2021). Microbiota modulation of the gut-lung axis in COVID-19. Front. Immunol. 12. doi: 10.3389/fimmu.2021.635471
Derosa, L., Routy, B., Thomas, A. M., Iebba, V., Zalcman, G., Friard, S., et al. (2022). Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 28, 315–324. doi: 10.1038/s41591-021-01655-5
DeVries, A., McCauley, K., Fadrosh, D., Fujimura, K. E., Stern, D. A., Lynch, S. V., et al. (2022). Maternal prenatal immunity, neonatal trained immunity, and early airway microbiota shape childhood asthma development. Allergy 77, 3617–3628. doi: 10.1111/all.15442
Dickson, R. P. and Huffnagle, G. B. (2015). The lung microbiome: new principles for respiratory bacteriology in health and disease. PloS Pathogens. 11, e1004923. doi: 10.1371/journal.ppat.1004923
Ding, X., Yang, L., Guan, Q., Zeng, H., Song, C., Wu, J., et al. (2020). Fermented black barley ameliorates lung injury induced by cooking oil fumes via antioxidant activity and regulation of the intestinal microbiome in mice. Ecotoxicol. Environ. Safety 195, 110473. doi: 10.1016/j.ecoenv.2020.110473
Dong, L. L., Liu, Z. Y., Chen, K. J., Li, Z. Y., Zhou, J. S., Shen, H. H., et al. (2024). The persistent inflammation in COPD: is autoimmunity the core mechanism? Eur. Respir. Rev.: An Off. J. Eur. Respir. Soc. 33, 230137. doi: 10.1183/16000617.0137-2023
Eladham, M. W., Selvakumar, B., Saheb Sharif-Askari, N., Saheb Sharif-Askari, F., Ibrahim, S. M., and Halwani, R. (2024). Unraveling the gut-Lung axis: Exploring complex mechanisms in disease interplay. Heliyon 10, e24032. doi: 10.1016/j.heliyon.2024.e24032
El Tekle, G. and Garrett, W. S. (2023). Bacteria in cancer initiation, promotion and progression. Nat. Rev. Cancer 23, 600–618. doi: 10.1038/s41568-023-00594-2
Enaud, R., Prevel, R., Ciarlo, E., Beaufils, F., Wieërs, G., Guery, B., et al. (2020). The gut-lung axis in health and respiratory diseases: A place for inter-organ and inter-kingdom crosstalks. Front. Cell. Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00009
Espírito Santo, C., Caseiro, C., Martins, M. J., Monteiro, R., and Brandão, I. (2021). Gut microbiota, in the halfway between nutrition and lung function. Nutrients 13, 1716. doi: 10.3390/nu13051716
Fang, P., Konyali, D., Fischer, E., Mayer, R. P., Huang, J., Elena, A. X., et al. (2025). Effects of cigarette-derived compounds on the spread of antimicrobial resistance in artificial human lung sputum medium, simulated environmental media, and wastewater. Environ. Health Perspect. 133, 47003. doi: 10.1289/ehp14704
Felix, K. M., Jaimez, I. A., Nguyen, T. V., Ma, H., Raslan, W. A., Klinger, C. N., et al. (2018). Gut microbiota contributes to resistance against pneumococcal pneumonia in immunodeficient rag(-/-) mice. Front. Cell. Infect. Microbiol. 8. doi: 10.3389/fcimb.2018.00118
Giron, L. B., Dweep, H., Yin, X., Wang, H., Damra, M., Goldman, A. R., et al. (2021). Plasma markers of disrupted gut permeability in severe COVID-19 patients. Front. Immunol. 12. doi: 10.3389/fimmu.2021.686240
Gligorijević, N., Stanić-Vučinić, D., Radomirović, M., Stojadinović, M., Khulal, U., Nedić, O., et al. (2021). Role of resveratrol in prevention and control of cardiovascular disorders and cardiovascular complications related to COVID-19 disease: mode of action and approaches explored to increase its bioavailability. Molecules 26, 2834. doi: 10.3390/molecules26102834
Göktürk, K., Tülek, B., Kanat, F., Maçin, S., Arslan, U., Shahbazova, M., et al. (2024). Gut microbiota profiles of patients with idiopathic pulmonary fibrosis. Exp. Lung Res. 50, 278–289. doi: 10.1080/01902148.2024.2437377
Grice, E. A. and Segre, J. A. (2012). The human microbiome: our second genome. Annu. Rev. Genomics Hum. Genet. 13, 151–170. doi: 10.1146/annurev-genom-090711-163814
Gu, S., Chen, Y., Wu, Z., Chen, Y., Gao, H., Lv, L., et al. (2020). Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis. 71, 2669–2678. doi: 10.1093/cid/ciaa709
Han, M., Wang, X., Zhang, J., Su, L., Ishaq, H. M., Li, D., et al. (2024). Gut bacterial and fungal dysbiosis in tuberculosis patients. BMC Microbiol. 24, 141. doi: 10.1186/s12866-024-03275-8
Hays, K. E., Pfaffinger, J. M., and Ryznar, R. (2024). The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes 16, 2393270. doi: 10.1080/19490976.2024.2393270
He, Y. Q., Deng, J. L., Zhou, C. C., Jiang, S. G., Zhang, F., Tao, X., et al. (2023). Ursodeoxycholic acid alleviates sepsis-induced lung injury by blocking PANoptosis via STING pathway. Int. Immunopharmacol. 125, 111161. doi: 10.1016/j.intimp.2023.111161
Hou, Y., Li, J., and Ying, S. (2023). Tryptophan metabolism and gut microbiota: A novel regulatory axis integrating the microbiome, immunity, and cancer. Metabolites 13, 1166. doi: 10.3390/metabo13111166
Hu, T., Zhu, Y., Zhou, X., Ye, M., Wang, X., Lu, C., et al. (2024). Baicalein ameliorates SEB-induced acute respiratory distress syndrome in a microbiota-dependent manner. Phytomedicine 135, 156049. doi: 10.1016/j.phymed.2024.156049
Hua, F., Cui, E., Lv, L., Wang, B., Li, L., Lu, H., et al. (2023). Fecal microbiota transplantation from HUC-MSC-treated mice alleviates acute lung injury in mice through anti-inflammation and gut microbiota modulation. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1243102
Huang, Y., Ji, Q., Zhu, Y., Fu, S., Chen, S., Chu, L., et al. (2021). Activated platelets autocrine 5-hydroxytryptophan aggravates sepsis-induced acute lung injury by promoting neutrophils extracellular traps formation. Front. Cell Dev. Biol. 9. doi: 10.3389/fcell.2021.777989
Huang, H., Li, S. J., Zeng, L. F., Zhang, Y., Chen, Y., Ma, Y. B., et al. (2024). Inhibition of the NF-κB signaling pathway improves cigarette mainstream smoke-induced lung injury and gut microbiota disturbance. BioMed. Environ. Sci. 37, 676–681. doi: 10.3967/bes2024.075
Huang, J., Liu, D., Wang, Y., Liu, L., Li, J., Yuan, J., et al. (2022). Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 71, 734–745. doi: 10.1136/gutjnl-2020-321031
Ikubo, Y., Sanada, T. J., Hosomi, K., Park, J., Naito, A., Shoji, H., et al. (2022). Altered gut microbiota and its association with inflammation in patients with chronic thromboembolic pulmonary hypertension: a single-center observational study in Japan. BMC Pulm. Med. 22, 138. doi: 10.1186/s12890-022-01932-0
Inchingolo, A. D., Malcangi, G., Inchingolo, A. M., Piras, F., Settanni, V., Garofoli, G., et al. (2022). Benefits and implications of resveratrol supplementation on microbiota modulations: A systematic review of the literature. Int. J. Mol. Sci. 23, 4027. doi: 10.3390/ijms23074027
Jia, M., Liu, Y., Liu, J., Meng, J., Cao, J., Miao, L., et al. (2024). Xuanfei Baidu decoction ameliorates bleomycin-elicited idiopathic pulmonary fibrosis in mice by regulating the lung-gut crosstalk via IFNγ/STAT1/STAT3 axis. Phytomedicine 135, 155997. doi: 10.1016/j.phymed.2024.155997
Jiang, X., Peng, Z., He, B., Li, S., and Huang, Q. (2024). A comprehensive review of ferroptosis in environmental pollutants-induced chronic obstructive pulmonary disease. Sci. Total Environ. 957, 177534. doi: 10.1016/j.scitotenv.2024.177534
Jose, A., Apewokin, S., Hussein, W. E., Ollberding, N. J., Elwing, J. M., and Haslam, D. B. (2022). A unique gut microbiota signature in pulmonary arterial hypertension: A pilot study. Pulm. Circ. 12, e12051. doi: 10.1002/pul2.12051
Kahhaleh, F. G., Barrientos, G., and Conrad, M. L. (2024). The gut-lung axis and asthma susceptibility in early life. Acta Physiol. (Oxford England) 240, e14092. doi: 10.1111/apha.14092
Kamada, N., Seo, S. U., Chen, G. Y., and Núñez, G. (2013). Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 13, 321–335. doi: 10.1038/nri3430
Kang, M. G., Choi, J. H., Kim, S. H., Jeong, T. B., Kim, J. Y., Kim, J. W., et al. (2025). Efficacy and safety of Lactobacillus plantarum GCWB1001 for respiratory health in a double blind randomized placebo controlled trial. Sci. Rep. 15, 22700. doi: 10.1038/s41598-025-04612-1
Kanj, A. N., Kottom, T. J., Schaefbauer, K. J., Choudhury, M., Limper, A. H., and Skalski, J. H. (2023). Dysbiosis of the intestinal fungal microbiota increases lung resident group 2 innate lymphoid cells and is associated with enhanced asthma severity in mice and humans. Respir. Res. 24, 144. doi: 10.1186/s12931-023-02422-5
Kazemian, N., Kao, D., and Pakpour, S. (2021). Fecal microbiota transplantation during and post-COVID-19 pandemic. Int. J. Mol. Sci. 22, 3004. doi: 10.3390/ijms22063004
Ke, H., Yao, H., and Wei, P. (2025). Advances in research on gut microbiota and allergic diseases in children. Curr. Res. Microb. Sci. 8, 100362. doi: 10.1016/j.crmicr.2025.100362
Kim, S., Rigatto, K., Gazzana, M. B., Knorst, M. M., Richards, E. M., Pepine, C. J., et al. (2020). Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension 75, 1063–1071. doi: 10.1161/hypertensionaha.119.14294
Kim, Y. C., Sohn, K. H., and Kang, H. R. (2024). Gut microbiota dysbiosis and its impact on asthma and other lung diseases: potential therapeutic approaches. Korean J. Intern. Med. 39, 746–758. doi: 10.3904/kjim.2023.451
Kumar, A., Green, K. M., and Rawat, M. (2024). A comprehensive overview of postbiotics with a special focus on discovery techniques and clinical applications. Foods 13, 2937. doi: 10.3390/foods13182937
Lai, H. C., Lin, T. L., Chen, T. W., Kuo, Y. L., Chang, C. J., Wu, T. R., et al. (2022). Gut microbiota modulates COPD pathogenesis: role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut 71, 309–321. doi: 10.1136/gutjnl-2020-322599
Lee, P. J., Hung, C. M., Yang, A. J., Hou, C. Y., Chou, H. W., Chang, Y. C., et al. (2024). MS-20 enhances the gut microbiota-associated antitumor effects of anti-PD1 antibody. Gut Microbes 16, 2380061. doi: 10.1080/19490976.2024.2380061
Lee, C., Kim, S. W., Verma, R., Noh, J., Park, J. C., Park, S., et al. (2024). Probiotic consortium confers synergistic anti-inflammatory effects in inflammatory disorders. Nutrients 16, 790. doi: 10.3390/nu16060790
Lee, E., Park, Y. M., Lee, S. Y., Lee, S. H., Park, M. J., Ahn, K., et al. (2023). Associations of prenatal antibiotic exposure and delivery mode on childhood asthma inception. Ann. Allergy Asthma Immunol. 131, 52–58. doi: 10.1016/j.anai.2023.03.020
Levan, S. R., Stamnes, K. A., Lin, D. L., Panzer, A. R., Fukui, E., McCauley, K., et al. (2019). Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat. Microbiol. 4, 1851–1861. doi: 10.1038/s41564-019-0498-2
Ley, R. E., Peterson, D. A., and Gordon, J. I. (2006). Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124, 837–848. doi: 10.1016/j.cell.2006.02.017
Li, T. T., Chen, X., Huo, D., Arifuzzaman, M., Qiao, S., Jin, W. B., et al. (2024). Microbiota metabolism of intestinal amino acids impacts host nutrient homeostasis and physiology. Cell Host Microbe 32, 661–675.e10. doi: 10.1016/j.chom.2024.04.004
Li, N., Dai, Z., Wang, Z., Deng, Z., Zhang, J., Pu, J., et al. (2021). Gut microbiota dysbiosis contributes to the development of chronic obstructive pulmonary disease. Respir. Res. 22, 274. doi: 10.1186/s12931-021-01872-z
Li, S., Duan, Y., Luo, S., Zhou, F., Wu, Q., and Lu, Z. (2025). Short-chain fatty acids and cancer. Trends Cancer 11, 154–168. doi: 10.1016/j.trecan.2024.11.003
Li, F., Hao, S., Gao, J., and Jiang, P. (2023). EGCG alleviates obesity-exacerbated lung cancer progression by STAT1/SLC7A11 pathway and gut microbiota. J. Nutr. Biochem. 120, 109416. doi: 10.1016/j.jnutbio.2023.109416
Li, W., Huang, Y., Tong, S., Wan, C., and Wang, Z. (2024). The characteristics of the gut microbiota in patients with pulmonary tuberculosis: A systematic review. Diagn. Microbiol. Infect. Dis. 109, 116291. doi: 10.1016/j.diagmicrobio.2024.116291
Li, D., Sorkhabi, S., Cruz, I., Foley, P. L., and Bellanti, J. A. (2025). Studies of methylated CpG ODN from Bifidobacterium longum subsp. infantis in a murine model: Implications for treatment of human allergic disease. Allergy Asthma Proc. 46, e13–e23. doi: 10.2500/aap.2025.46.240100
Li, P., Uma Mageswary, M., Taib, F., Koo, T. H., Yusof, A., Hamid, I. J. A., et al. (2025). Safety and Tolerance of Bifidobacterium longum subsp. Infantis YLGB-1496 in Toddlers with Respiratory Symptoms. Nutrients 17, 2127. doi: 10.3390/nu17132127
Li, N., Wang, N., and Ran, P. (2023). Gut microbiota dysbiosis and chronic obstructive pulmonary disease: A question of chicken and egg. Am. J. Respir. Crit. Care Med. 208, 1238–1240. doi: 10.1164/rccm.202307-1285LE
Li, Y., Wang, K., Zhang, Y., Yang, J., Wu, Y., and Zhao, M. (2023). Revealing a causal relationship between gut microbiota and lung cancer: a Mendelian randomization study. Front. Cell. Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1200299
Li, R., Yang, H., Xue, Y., and Xianqin, L. (2024). Yemazhui () ameliorates lipopolysaccharide-induced acute lung injury modulation of the toll-like receptor 4/nuclear factor kappa-B/nod-like receptor family pyrin domain-containing 3 protein signaling pathway and intestinal flora in rats. J. Traditional Chin. Med. 44, 303–314. doi: 10.19852/j.cnki.jtcm.20230510.001
Li, Y., Yang, D., Zhao, H., Dou, L., Chen, Q., Cheng, Y., et al. (2025). The pasteurized Weissella cibaria alleviates sepsis-induced acute lung injury by modulation of intestinal mucus barrier and gut microbiota. J. Trans. Med. 23, 661. doi: 10.1186/s12967-025-06674-1
Lim, E. Y., Song, E. J., and Shin, H. S. (2023). Gut microbiome as a possible cause of occurrence and therapeutic target in chronic obstructive pulmonary disease. J. Microbiol. Biotechnol. 33, 1111–1118. doi: 10.4014/jmb.2301.01033
Lin, J., Chen, D., Yan, Y., Pi, J., Xu, J., Chen, L., et al. (2024). Gut microbiota: a crucial player in the combat against tuberculosis. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1442095
Liu, Y., Dai, J., Zhou, G., Chen, R., Bai, C., and Shi, F. (2025). Innovative therapeutic strategies for asthma: the role of gut microbiome in airway immunity. J. Asthma Allergy 18, 257–267. doi: 10.2147/jaa.S504571
Liu, J., Huang, Y., Liu, N., Qiu, H., Zhang, X., Liu, X., et al. (2024). The imbalance of pulmonary Th17/Treg cells in BALB/c suckling mice infected with respiratory syncytial virus-mediated intestinal immune damage and gut microbiota changes. Microbiol. Spectrum 12, e0328323. doi: 10.1128/spectrum.03283-23
Liu, M., Lu, Y., Xue, G., Han, L., Jia, H., Wang, Z., et al. (2024). Role of short-chain fatty acids in host physiology. Anim. Model. Exp. Med. 7, 641–652. doi: 10.1002/ame2.12464
Liu, Q., Mak, J. W. Y., Su, Q., Yeoh, Y. K., Lui, G. C., Ng, S. S. S., et al. (2022). Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 71, 544–552. doi: 10.1136/gutjnl-2021-325989
Liu, C., Makrinioti, H., Saglani, S., Bowman, M., Lin, L. L., Camargo, C. A., Jr., et al. (2022). Microbial dysbiosis and childhood asthma development: Integrated role of the airway and gut microbiome, environmental exposures, and host metabolic and immune response. Front. Immunol. 13. doi: 10.3389/fimmu.2022.1028209
Lu, X., Tan, Z. X., Yao, Y. X., Li, Z. Y., Zhu, Y. Y., Yang, Q. Q., et al. (2025). Inhaling arsenic aggravates airway hyperreactivity by upregulating PNEC-sourced 5-HT in OVA-induced allergic asthma. Ecotoxicol. Environ. Safety 290, 117764. doi: 10.1016/j.ecoenv.2025.117764
Lu, X., Xiong, L., Zheng, X., Yu, Q., Xiao, Y., and Xie, Y. (2023). Structure of gut microbiota and characteristics of fecal metabolites in patients with lung cancer. Front. Cell. Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1170326
Luo, Y., Zhou, S., Zhang, X., Lin, Y., Liu, J., Cheng, W., et al. (2025). The role of the microbiota and metabolites in the treatment of pulmonary fibrosis with UC-MSCs: Integrating fecal metabolomics and 16S rDNA analysis. PloS One 20, e0313989. doi: 10.1371/journal.pone.0313989
Ma, P. J., Wang, M. M., and Wang, Y. (2022). Gut microbiota: A new insight into lung diseases. Biomed. Pharmacother. Biomed. Pharmacother. 155, 113810. doi: 10.1016/j.biopha.2022.113810
Maher, T. M. (2024). Interstitial lung disease: A review. Jama 331, 1655–1665. doi: 10.1001/jama.2024.3669
Maina, T. W., McDonald, P. O., Rani Samuel, B. E., Sardi, M. I., Yoon, I., Rogers, A., et al. (2024). Feeding Saccharomyces cerevisiae fermentation postbiotic products alters immune function and the lung transcriptome of preweaning calves with an experimental viral-bacterial coinfection. J. Dairy Sci. 107, 2253–2267. doi: 10.3168/jds.2023-23866
Mann, E. R., Lam, Y. K., and Uhlig, H. H. (2024). Short-chain fatty acids: linking diet, the microbiome and immunity. Nat. Rev. Immunol. 24, 577–595. doi: 10.1038/s41577-024-01014-8
Manni, M. L., Heinrich, V. A., Buchan, G. J., O’Brien, J. P., Uvalle, C., Cechova, V., et al. (2021). Nitroalkene fatty acids modulate bile acid metabolism and lung function in obese asthma. Sci. Rep. 11, 17788. doi: 10.1038/s41598-021-96471-9
Massart, A. and Hunt, D. P. (2020). Pulmonary manifestations of inflammatory bowel disease. Am. J. Med. 133, 39–43. doi: 10.1016/j.amjmed.2019.07.007
McAleer, J. P. and Kolls, J. K. (2018). Contributions of the intestinal microbiome in lung immunity. Eur. J. Immunol. 48, 39–49. doi: 10.1002/eji.201646721
McLaughlin, V. V. and McGoon, M. D. (2006). Pulmonary arterial hypertension. Circulation 114, 1417–1431. doi: 10.1161/circulationaha.104.503540
McLoughlin, R., Berthon, B. S., Rogers, G. B., Baines, K. J., Leong, L. E. X., Gibson, P. G., et al. (2019). Soluble fibre supplementation with and without a probiotic in adults with asthma: A 7-day randomised, double blind, three way cross-over trial. EBioMedicine 46, 473–485. doi: 10.1016/j.ebiom.2019.07.048
Mittra, I., de Souza, R., Bhadade, R., Madke, T., Shankpal, P. D., Joshi, M., et al. (2020). Resveratrol and Copper for treatment of severe COVID-19: an observational study (RESCU 002). medRxiv. doi: 10.1101/2020.07.21.20151423
Mizutani, T., Ishizaka, A., Koga, M., Ikeuchi, K., Saito, M., Adachi, E., et al. (2022). Correlation analysis between gut microbiota alterations and the cytokine response in patients with coronavirus disease during hospitalization. Microbiol. Spectrum 10, e0168921. doi: 10.1128/spectrum.01689-21
Moutsoglou, D. M., Tatah, J., Prisco, S. Z., Prins, K. W., Staley, C., Lopez, S., et al. (2023). Pulmonary arterial hypertension patients have a proinflammatory gut microbiome and altered circulating microbial metabolites. Am. J. Respir. Crit. Care Med. 207, 740–756. doi: 10.1164/rccm.202203-0490OC
Nagai, M., Moriyama, M., Ishii, C., Mori, H., Watanabe, H., Nakahara, T., et al. (2023). High body temperature increases gut microbiota-dependent host resistance to influenza A virus and SARS-CoV-2 infection. Nat. Commun. 14, 3863. doi: 10.1038/s41467-023-39569-0
Nagata, N., Takeuchi, T., Masuoka, H., Aoki, R., Ishikane, M., Iwamoto, N., et al. (2023). Human gut microbiota and its metabolites impact immune responses in COVID-19 and its complications. Gastroenterology 164, 272–288. doi: 10.1053/j.gastro.2022.09.024
Naidoo, C. C., Nyawo, G. R., Sulaiman, I., Wu, B. G., Turner, C. T., Bu, K., et al. (2021). Anaerobe-enriched gut microbiota predicts pro-inflammatory responses in pulmonary tuberculosis. EBioMedicine 67, 103374. doi: 10.1016/j.ebiom.2021.103374
Nakos, G., Kitsiouli, E., Hatzidaki, E., Koulouras, V., Touqui, L., and Lekka, M. E. (2005). Phospholipases A2 and platelet-activating-factor acetylhydrolase in patients with acute respiratory distress syndrome. Crit. Care Med. 33, 772–779. doi: 10.1097/01.ccm.0000158519.80090.74
Naz, S., Bhat, M., Ståhl, S., Forsslund, H., Sköld, C. M., Wheelock, ÅM., et al. (2019). Dysregulation of the tryptophan pathway evidences gender differences in COPD. Metabolites 9, 212. doi: 10.3390/metabo9100212
Ney, L. M., Wipplinger, M., Grossmann, M., Engert, N., Wegner, V. D., and Mosig, A. S. (2023). Short chain fatty acids: key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 13, 230014. doi: 10.1098/rsob.230014
Nicolas, G. R. and Chang, P. V. (2019). Deciphering the chemical lexicon of host-gut microbiota interactions. Trends Pharmacol. Sci. 40, 430–445. doi: 10.1016/j.tips.2019.04.006
Nilsen, M., Nygaard, U. C., Brodin, P., Carlsen, K. C. L., Fredheim, C., Haugen, G., et al. (2024). Gut bacteria at 6 months of age are associated with immune cell status in 1-year-old children. Scand. J. Immunol. 99, e13346. doi: 10.1111/sji.13346
Niu, X., Yin, X., Wu, X., Zhang, Q., Jiang, Y., He, J., et al. (2023). Heat-Killed Bifidobacterium longum BBMN68 in Pasteurized Yogurt Alleviates Mugwort Pollen-Induced Allergic Airway Responses through Gut Microbiota Modulation in a Murine Model. Foods 12, 2049. doi: 10.3390/foods12102049
Oliver, P. J., Arutla, S., Yenigalla, A., Hund, T. J., and Parinandi, N. L. (2021). Lipid nutrition in asthma. Cell Biochem. Biophysics 79, 669–694. doi: 10.1007/s12013-021-01020-w
Pagnini, C., Urgesi, R., Di Paolo, M. C., and Graziani, M. G. (2020). Fighting the battle against SARS-coV-2 as gastroenterologists in Italy. Gastroenterology 159, 1619. doi: 10.1053/j.gastro.2020.03.067
Pamart, G., Gosset, P., Le Rouzic, O., Pichavant, M., and Poulain-Godefroy, O. (2024). Kynurenine pathway in respiratory diseases. Int. J. Tryptophan Res. 17, 11786469241232871. doi: 10.1177/11786469241232871
Parker, A., Fonseca, S., and Carding, S. R. (2020). Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 11, 135–157. doi: 10.1080/19490976.2019.1638722
Peng, Y., Zhao, J., and Tun, H. M. (2021). The new foe and old friends: are we ready for microbiota-based therapeutics in treating COVID-19 patients? Gastroenterology 160, 2192–2193. doi: 10.1053/j.gastro.2020.08.048
Prisco, S. Z., Blake, M., Kazmirczak, F., Moon, R., Kremer, B. P., Hartweck, L. M., et al. (2025). Lactobacillus restructures the micro/mycobiome to combat inflammation-mediated right ventricular dysfunction in pulmonary arterial hypertension. Circ. Heart Fail. 18, e012524. doi: 10.1161/circheartfailure.124.012524
Puccetti, M., Giovagnoli, S., Zelante, T., Romani, L., and Ricci, M. (2018). Development of novel indole-3-aldehyde-loaded gastro-resistant spray-dried microparticles for postbiotic small intestine local delivery. J. Pharm. Sci. 107, 2341–2353. doi: 10.1016/j.xphs.2018.04.023
Rastogi, S., Mohanty, S., Sharma, S., and Tripathi, P. (2022). Possible role of gut microbes and host’s immune response in gut-lung homeostasis. Front. Immunol. 13. doi: 10.3389/fimmu.2022.954339
Razim, A., Zabłocka, A., Schmid, A., Thaler, M., Černý, V., Weinmayer, T., et al. (2024). Bacterial extracellular vesicles as intranasal postbiotics: Detailed characterization and interaction with airway cells. J. Extracell. Vesicles 13, e70004. doi: 10.1002/jev2.70004
Ren, Y., Zhang, Y., Cheng, Y., Qin, H., and Zhao, H. (2024). Genetic liability of gut microbiota for idiopathic pulmonary fibrosis and lung function: a two-sample Mendelian randomization study. Front. Cell. Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1348685
Ren, Z., Zheng, Z., and Feng, X. (2024). Role of gut microbes in acute lung injury/acute respiratory distress syndrome. Gut Microbes 16, 2440125. doi: 10.1080/19490976.2024.2440125
Renga, G., D’Onofrio, F., Pariano, M., Galarini, R., Barola, C., Stincardini, C., et al. (2024). Author Correction: Bridging of host-microbiota tryptophan partitioning by the serotonin pathway in fungal pneumonia. Nat. Commun. 15, 3541. doi: 10.1038/s41467-024-48040-7
Ruan, G., Chen, M., Chen, L., Xu, F., Xiao, Z., Yi, A., et al. (2022). Roseburia intestinalis and Its Metabolite Butyrate Inhibit Colitis and Upregulate TLR5 through the SP3 Signaling Pathway. Nutrients 14 (15), 3041. doi: 10.3390/nu14153041
Rutten, E. P., Spruit, M. A., Franssen, F. M., Buurman, W. A., Wouters, E. F., and Lenaerts, K. (2014). GI symptoms in patients with COPD. Chest 145, 1437–1438. doi: 10.1378/chest.14-0285
Salminen, S., Collado, M. C., Endo, A., Hill, C., Lebeer, S., Quigley, E. M. M., et al. (2021). The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649–667. doi: 10.1038/s41575-021-00440-6
Sanidad, K. Z., Rager, S. L., Carrow, H. C., Ananthanarayanan, A., Callaghan, R., Hart, L. R., et al. (2024). Gut bacteria-derived serotonin promotes immune tolerance in early life. Sci. Immunol. 9, eadj4775. doi: 10.1126/sciimmunol.adj4775
Sasaki, H., Masutomi, H., Kobayashi, Y., Toyohara, K., Imaizumi, K., Nakayama, Y., et al. (2025). Evaluation of the fermentation characteristics of prebiotic-containing granola and short-chain fatty acid production in an in vitro gut microbiota model. Food Sci. Nutr. 13, e70252. doi: 10.1002/fsn3.70252
Schuijt, T. J., Lankelma, J. M., Scicluna, B. P., de Sousa e Melo, F., Roelofs, J. J., de Boer, J. D., et al. (2016). The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65, 575–583. doi: 10.1136/gutjnl-2015-309728
Seibert, B., Cáceres, C. J., Carnaccini, S., Cardenas-Garcia, S., Gay, L. C., Ortiz, L., et al. (2022). Pathobiology and dysbiosis of the respiratory and intestinal microbiota in 14 months old Golden Syrian hamsters infected with SARS-CoV-2. PloS Pathogens. 18, e1010734. doi: 10.1371/journal.ppat.1010734
Sencio, V., Benech, N., Robil, C., Deruyter, L., Heumel, S., Machelart, A., et al. (2022a). Alteration of the gut microbiota’s composition and metabolic output correlates with COVID-19-like severity in obese NASH hamsters. Gut Microbes 14, 2100200. doi: 10.1080/19490976.2022.2100200
Sencio, V., Machelart, A., Robil, C., Benech, N., Hoffmann, E., Galbert, C., et al. (2022b). Alteration of the gut microbiota following SARS-CoV-2 infection correlates with disease severity in hamsters. Gut Microbes 14, 2018900. doi: 10.1080/19490976.2021.2018900
Sharma, R. K., Oliveira, A. C., Yang, T., Kim, S., Zubcevic, J., Aquino, V., et al. (2020). Pulmonary arterial hypertension-associated changes in gut pathology and microbiota. ERJ Open Res. 6, 00253–02019. doi: 10.1183/23120541.00253-2019
Shi, W., Hu, Y., Ning, Z., Xia, F., Wu, M., Hu, Y. O. O., et al. (2021). Alterations of gut microbiota in patients with active pulmonary tuberculosis in China: a pilot study. Int. J. Infect. Dis. 111, 313–321. doi: 10.1016/j.ijid.2021.08.064
Sinha, A. K., Laursen, M. F., Brinck, J. E., Rybtke, M. L., Hjørne, A. P., Procházková, N., et al. (2024). Dietary fibre directs microbial tryptophan metabolism via metabolic interactions in the gut microbiota. Nat. Microbiol. 9, 1964–1978. doi: 10.1038/s41564-024-01737-3
Smulders, T., van der Schee, M. P., Maitland-Van Der Zee, A. H., Dikkers, F. G., and Van Drunen, C. M. (2024). Influence of the gut and airway microbiome on asthma development and disease. Pediatr. Allergy Immunol. 35, e14095. doi: 10.1111/pai.14095
Song, P., Yang, D., Wang, H., Cui, X., Si, X., Zhang, X., et al. (2020). Relationship between intestinal flora structure and metabolite analysis and immunotherapy efficacy in Chinese NSCLC patients. Thorac. Cancer. 11, 1621–1632. doi: 10.1111/1759-7714.13442
Song, W., Yang, X., Wang, W., Wang, Z., Wu, J., and Huang, F. (2021). Sinomenine ameliorates septic acute lung injury in mice by modulating gut homeostasis via aryl hydrocarbon receptor/Nrf2 pathway. Eur. J. Pharmacol. 912, 174581. doi: 10.1016/j.ejphar.2021.174581
Song, W., Yue, Y., and Zhang, Q. (2023). Imbalance of gut microbiota is involved in the development of chronic obstructive pulmonary disease: A review. Biomed. Pharmacother. , 165:115150. doi: 10.1016/j.biopha.2023.115150
Sun, M., Lu, F., Yu, D., Wang, Y., Chen, P., and Liu, S. (2024). Respiratory diseases and gut microbiota: relevance, pathogenesis, and treatment. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1358597
Sun, E., Meng, X., Kang, Z., Gu, H., Li, M., Tan, X., et al. (2023). Zengshengping improves lung cancer by regulating the intestinal barrier and intestinal microbiota. Front. Pharmacol. 14. doi: 10.3389/fphar.2023.1123819
Swanson, K. S., Gibson, G. R., Hutkins, R., Reimer, R. A., Reid, G., Verbeke, K., et al. (2020). The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 17, 687–701. doi: 10.1038/s41575-020-0344-2
Takada, K., Shimokawa, M., Takamori, S., Shimamatsu, S., Hirai, F., Tagawa, T., et al. (2021). Clinical impact of probiotics on the efficacy of anti-PD-1 monotherapy in patients with nonsmall cell lung cancer: A multicenter retrospective survival analysis study with inverse probability of treatment weighting. Int. J. Cancer. 149, 473–482. doi: 10.1002/ijc.33557
Tanabe, N., Matsumoto, H., Morimoto, C., and Hirai, T. (2025). Sputum short-chain fatty acids, microbiome, inflammation, and mucus plugging in obstructive airway disease. J. Allergy Clin. Immunol. 155, 1675–1680. doi: 10.1016/j.jaci.2025.01.031
Tang, J., Xu, L., Zeng, Y., and Gong, F. (2021). Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 91, 107272. doi: 10.1016/j.intimp.2020.107272
Trompette, A., Gollwitzer, E. S., Yadava, K., Sichelstiel, A. K., Sprenger, N., Ngom-Bru, C., et al. (2014). Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166. doi: 10.1038/nm.3444
Tsai, Y. L., Lin, T. L., Chang, C. J., Wu, T. R., Lai, W. F., Lu, C. C., et al. (2019). Probiotics, prebiotics and amelioration of diseases. J. BioMed. Sci. 26, 3. doi: 10.1186/s12929-018-0493-6
Ullah, H., Arbab, S., Tian, Y., Chen, Y., Liu, C. Q., Li, Q., et al. (2024). Crosstalk between gut microbiota and host immune system and its response to traumatic injury. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1413485
Uno, Y. (2020). Why does SARS-coV-2 invade the gastrointestinal epithelium? Gastroenterology 159, 1622–1623. doi: 10.1053/j.gastro.2020.04.006
Uwaezuoke, S. N., Ayuk, A. C., Eze, J. N., Odimegwu, C. L., Ndiokwelu, C. O., and Eze, I. C. (2022). Postnatal probiotic supplementation can prevent and optimize treatment of childhood asthma and atopic disorders: A systematic review of randomized controlled trials. Front. Pediatr. 10. doi: 10.3389/fped.2022.956141
Vandenplas, Y., de Halleux, V., Arciszewska, M., Lach, P., Pokhylko, V., Klymenko, V., et al. (2020). A partly fermented infant formula with postbiotics including 3’-GL, specific oligosaccharides, 2’-FL, and milk fat supports adequate growth, is safe and well-tolerated in healthy term infants: A double-blind, randomised, controlled, multi-country trial. Nutrients 12, 3560. doi: 10.3390/nu12113560
Vasconcelos, J. A., Mota, A. S., Olímpio, F., Rosa, P. C., Damaceno-Rodrigues, N., de Paula Vieira, R., et al. (2025). Lactobacillus rhamnosus modulates lung inflammation and mitigates gut dysbiosis in a murine model of asthma-COPD overlap syndrome. Probiot. Antimicrob. Proteins 17, 588–605. doi: 10.1007/s12602-023-10167-2
Wang, W., Dernst, A., Martin, B., Lorenzi, L., Cadefau-Fabregat, M., Phulphagar, K., et al. (2024). Butyrate and propionate are microbial danger signals that activate the NLRP3 inflammasome in human macrophages upon TLR stimulation. Cell Rep. 43, 114736. doi: 10.1016/j.celrep.2024.114736
Wang, J., Fan, D. C., Wang, R. S., Chang, Y., Ji, X. M., Li, X. Y., et al. (2024). Inhibitory Potential of Bifidobacterium longum FB1–1 Cell-Free Supernatant against Carbapenem-Resistant Klebsiella pneumoniae Drug Resistance Spread. Microorganisms 12, 1203. doi: 10.3390/microorganisms12061203
Wang, H., He, Y., Dang, D., Zhao, Y., Zhao, J., and Lu, W. (2024). Gut microbiota-derived tryptophan metabolites alleviate allergic asthma inflammation in ovalbumin-induced mice. Foods 13, 1336. doi: 10.3390/foods13091336
Wang, Z., Liu, J., Li, F., Ma, S., Zhao, L., Ge, P., et al. (2023). Mechanisms of qingyi decoction in severe acute pancreatitis-associated acute lung injury via gut microbiota: targeting the short-chain fatty acids-mediated AMPK/NF-κB/NLRP3 pathway. Microbiol. Spectrum 11, e0366422. doi: 10.1128/spectrum.03664-22
Wang, M., Lkhagva, E., Kim, S., Zhai, C., Islam, M. M., Kim, H. J., et al. (2024). The gut microbe pair of Oribacterium sp. GMB0313 and Ruminococcus sp. GMB0270 confers complete protection against SARS-CoV-2 infection by activating CD8+ T cell-mediated immunity. Gut Microbes 16, 2342497. doi: 10.1080/19490976.2024.2342497
Wang, Y. H., Yan, Z. Z., Luo, S. D., Hu, J. J., Wu, M., Zhao, J., et al. (2023). Gut microbiota-derived succinate aggravates acute lung injury after intestinal ischaemia/reperfusion in mice. Eur. Respir. J. 61, 2200840. doi: 10.1183/13993003.00840-2022
Wang, S., Yang, L., Hu, H., Lv, L., Ji, Z., Zhao, Y., et al. (2022). Characteristic gut microbiota and metabolic changes in patients with pulmonary tuberculosis. Microb. Biotechnol. 15, 262–275. doi: 10.1111/1751-7915.13761
Wang, Q., Yu, Z. H., Nie, L., Wang, F. X., Mu, G., and Lu, B. (2024). Assessing the impact of gut microbiota and metabolic products on acute lung injury following intestinal ischemia-reperfusion injury: harmful or helpful? Front. Cell. Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1491639
Wang, C., Yu, X., Yu, X., Xiao, H., Song, Y., Wang, X., et al. (2025). Gut flora-derived succinate exacerbates Allergic Airway Inflammation by promoting protein succinylation. Redox Biol. 82, 103623. doi: 10.1016/j.redox.2025.103623
Wei, J., Liu, C., Qin, D., Ren, F., Duan, J., Chen, T., et al. (2024). Targeting inflammation and gut microbiota with antibacterial therapy: Implications for central nervous system health. Ageing Res. Rev. 102, 102544. doi: 10.1016/j.arr.2024.102544
Wu, Y., Pei, C., Wang, X., Wang, Y., Huang, D., Shi, S., et al. (2022). Probiotics ameliorates pulmonary inflammation via modulating gut microbiota and rectifying Th17/Treg imbalance in a rat model of PM2.5 induced lung injury. Ecotoxicol. Environ. Safety 244, 114060. doi: 10.1016/j.ecoenv.2022.114060
Wu, W., Wu, X., Qiu, L., Wan, R., Zhu, X., Chen, S., et al. (2024). Quercetin influences intestinal dysbacteriosis and delays alveolar epithelial cell senescence by regulating PTEN/PI3K/AKT signaling in pulmonary fibrosis. Naunyn Schmiedebergs Arch. Pharmacol. 397, 4809–4822. doi: 10.1007/s00210-023-02913-8
Wu, R., Zhu, X., Guan, G., Cui, Q., Zhu, L., Xing, Y., et al. (2024). Association of dietary flavonoid intakes with prevalence of chronic respiratory diseases in adults. J. Trans. Med. 22, 205. doi: 10.1186/s12967-024-04949-7
Wu, P., Zhu, T., Tan, Z., Chen, S., and Fang, Z. (2022). Role of gut microbiota in pulmonary arterial hypertension. Front. Cell. Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.812303
Xiao, F., Tang, M., Zheng, X., Liu, Y., Li, X., and Shan, H. (2020). Evidence for gastrointestinal infection of SARS-coV-2. Gastroenterology 158, 1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055
Xie, X., Wu, X., Zhao, D., Liu, Y., Du, Q., Li, Y., et al. (2023). Fluvoxamine alleviates bleomycin-induced lung fibrosis via regulating the cGAS-STING pathway. Pharmacol. Res. 187, 106577. doi: 10.1016/j.phrs.2022.106577
Ximenez, C. and Torres, J. (2017). Development of microbiota in infants and its role in maturation of gut mucosa and immune system. Arch. Med. Res. 48, 666–680. doi: 10.1016/j.arcmed.2017.11.007
Xin, Y., Liu, C. G., Zang, D., and Chen, J. (2024). Gut microbiota and dietary intervention: affecting immunotherapy efficacy in non-small cell lung cancer. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1343450
Xu, R., Lu, R., Zhang, T., Wu, Q., Cai, W., Han, X., et al. (2021). Temporal association between human upper respiratory and gut bacterial microbiomes during the course of COVID-19 in adults. Commun. Biol. 4, 240. doi: 10.1038/s42003-021-01796-w
Xu, J. Y., Rong, X. J., Shen, Z., Guo, Y. D., Zhang, Y. X., Ding, C. C., et al. (2025). Isochlorogenic acid C alleviates allergic asthma via interactions between its bioactive form and the gut microbiome. Int. J. Mol. Sci. 26, 4864. doi: 10.3390/ijms26104864
Xu, M., Wang, C., Li, N., Wang, J., Zhang, Y., and Deng, X. (2019). Intraperitoneal injection of acetate protects mice against lipopolysaccharide (LPS)−Induced acute lung injury through its anti-inflammatory and anti-oxidative ability. Med. Sci. Monitor: Int. Med. J. Exp. Clin. Res. 25, 2278–2288. doi: 10.12659/msm.911444
Xue, C., Li, G., Zheng, Q., Gu, X., Shi, Q., Su, Y., et al. (2023). Tryptophan metabolism in health and disease. Cell Metab. 35, 1304–1326. doi: 10.1016/j.cmet.2023.06.004
Yang, J., He, Y., Ai, Q., Liu, C., Ruan, Q., and Shi, Y. (2024). Lung-gut microbiota and tryptophan metabolites changes in neonatal acute respiratory distress syndrome. J. Inflammation Res. 17, 3013–3029. doi: 10.2147/jir.S459496
Yang, C., Liu, Y. H., and Zheng, H. K. (2024). Identification of metabolic biomarkers in idiopathic pulmonary arterial hypertension using targeted metabolomics and bioinformatics analysis. Sci. Rep. 14, 25283. doi: 10.1038/s41598-024-76514-7
Yang, F., Yang, Y., Chen, L., Zhang, Z., Liu, L., Zhang, C., et al. (2022). The gut microbiota mediates protective immunity against tuberculosis via modulation of lncRNA. Gut Microbes 14, 2029997. doi: 10.1080/19490976.2022.2029997
Ye, H., Wang, H., Han, B., Chen, K., Wang, X., Ma, F., et al. (2025). Guizhi Shaoyao Zhimu decoction inhibits neutrophil extracellular traps formation to relieve rheumatoid arthritis via gut microbial outer membrane vesicles. Phytomedicine 136, 156254. doi: 10.1016/j.phymed.2024.156254
Ye, S., Wang, L., Li, S., Ding, Q., Wang, Y., Wan, X., et al. (2022). The correlation between dysfunctional intestinal flora and pathology feature of patients with pulmonary tuberculosis. Front. Cell. Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.1090889
Yelin, I., Flett, K. B., Merakou, C., Mehrotra, P., Stam, J., Snesrud, E., et al. (2019). Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med. 25, 1728–1732. doi: 10.1038/s41591-019-0626-9
Yeoh, Y. K., Zuo, T., Lui, G. C., Zhang, F., Liu, Q., Li, A. Y., et al. (2021). Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70, 698–706. doi: 10.1136/gutjnl-2020-323020
Yin, T., Liu, K., Shen, Y., Wang, Y., Wang, Q., Long, T., et al. (2023). Alteration of serum bile acids in non-small cell lung cancer identified by a validated LC-MS/MS method. J. Cancer Res. Clin. Oncol. 149, 17285–17296. doi: 10.1007/s00432-023-05434-2
Yuan, Z., Kang, Y., Mo, C., Huang, S., Qin, F., Zhang, J., et al. (2024). Causal relationship between gut microbiota and tuberculosis: a bidirectional two-sample Mendelian randomization analysis. Respir. Res. 25, 16. doi: 10.1186/s12931-023-02652-7
Yun, H. M. and Park, K. R. (2025). ST1936 stimulates osteoclastogenesis and suppresses osteoclastic apoptosis through the ERK1/2 signaling pathway in bone marrow-derived macrophages. Int. Immunopharmacol. 157, 114753. doi: 10.1016/j.intimp.2025.114753
Zeng, X., Yue, H., Zhang, L., Chen, G., Zheng, Q., Hu, Q., et al. (2023). Gut microbiota-derived autoinducer-2 regulates lung inflammation through the gut-lung axis. Int. Immunopharmacol. 124, 110971. doi: 10.1016/j.intimp.2023.110971
Zhan, Q., Wang, R., Thakur, K., Feng, J. Y., Zhu, Y. Y., Zhang, J. G., et al. (2024). Unveiling of dietary and gut-microbiota derived B vitamins: Metabolism patterns and their synergistic functions in gut-brain homeostasis. Crit. Rev. Food Sci. Nutr. 64, 4046–4058. doi: 10.1080/10408398.2022.2138263
Zhang, F., Lau, R. I., Liu, Q., Su, Q., Chan, F. K. L., and Ng, S. C. (2023). Gut microbiota in COVID-19: key microbial changes, potential mechanisms and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 20, 323–337. doi: 10.1038/s41575-022-00698-4
Zhang, M., Shang, L., Zhou, F., Li, J., Wang, S., Lin, Q., et al. (2025). Dachengqi decoction dispensing granule ameliorates LPS-induced acute lung injury by inhibiting PANoptosis in vivo and in vitro. J. Ethnopharmacol. 336, 118699. doi: 10.1016/j.jep.2024.118699
Zhang, T., Zhang, W., Feng, C., Kwok, L. Y., He, Q., and Sun, Z. (2022). Stronger gut microbiome modulatory effects by postbiotics than probiotics in a mouse colitis model. NPJ Sci. Food. 6, 53. doi: 10.1038/s41538-022-00169-9
Zhao, F., An, R., Wang, L., Shan, J., and Wang, X. (2021). Specific gut microbiome and serum metabolome changes in lung cancer patients. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.725284
Zhao, L., Luo, J. L., Ali, M. K., Spiekerkoetter, E., and Nicolls, M. R. (2023). The human respiratory microbiome: current understandings and future directions. Am. J. Respir. Cell Mol. Biol. 68, 245–255. doi: 10.1165/rcmb.2022-0208TR
Zhao, L., Zhang, H., Liu, X., Xue, S., Chen, D., Zou, J., et al. (2022). TGR5 deficiency activates antitumor immunity in non-small cell lung cancer via restraining M2 macrophage polarization. Acta Pharm. Sin. B. 12, 787–800. doi: 10.1016/j.apsb.2021.07.011
Zheng, D., Liwinski, T., and Elinav, E. (2020). Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506. doi: 10.1038/s41422-020-0332-7
Zhong, Y., Chang, X., Zhao, Z., Zheng, L., Kuang, G., Li, P., et al. (2024). Bacteroides fragilis capsular polysaccharide A ameliorates ulcerative colitis in rat by recovering intestinal barrier integrity and restoring gut microbiota. Front. Pharmacol. 15. doi: 10.3389/fphar.2024.1402465
Zhong, Z., Liu, G., Tang, Z., Xiang, S., Yang, L., Huang, L., et al. (2023). Efficient plant genome engineering using a probiotic sourced CRISPR-Cas9 system. Nat. Commun. 14, 6102. doi: 10.1038/s41467-023-41802-9
Zhong, L., Qin, L., Ding, X., Ma, L., Wang, Y., Liu, M., et al. (2022). The regulatory effect of fermented black barley on the gut microbiota and metabolic dysbiosis in mice exposed to cigarette smoke. Food Res. Int. 157, 111465. doi: 10.1016/j.foodres.2022.111465
Zhou, A., Lei, Y., Tang, L., Hu, S., Yang, M., Wu, L., et al. (2021). Gut microbiota: the emerging link to lung homeostasis and disease. J. Bacteriol. 203 (4), e00454-20. doi: 10.1128/jb.00454-20
Zuo, T., Zhan, H., Zhang, F., Liu, Q., Tso, E. Y. K., Lui, G. C. Y., et al. (2020a). Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology 159, 1302–1310.e5. doi: 10.1053/j.gastro.2020.06.048
Keywords: gut microbiota, respiratory diseases, gut-lung axis, short-chain fatty acids, fecal microbiota transplantation, probiotics, postbiotics, dietary fibers
Citation: Yu X, Yu X, Wang Y, Guo X, Wang C and Wang F (2025) Respiratory diseases and the gut microbiota: an updated review. Front. Cell. Infect. Microbiol. 15:1629005. doi: 10.3389/fcimb.2025.1629005
Received: 15 May 2025; Accepted: 28 July 2025;
Published: 11 August 2025.
Edited by:
Susanta Pahari, Texas Biomedical Research Institute, United StatesReviewed by:
François Trottein, Institut Pasteur de Lille, FranceDany Mesa, Instituto de Pesquisa Pelé Pequeno Príncipe, Brazil
Xin Sun, Air Force Medical University, China
Copyright © 2025 Yu, Yu, Wang, Guo, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Wang, d2ZAamx1LmVkdS5jbg==
†These authors have contributed equally to this work
 Xin Yu
Xin Yu Xiao Yu2†
Xiao Yu2† Xiaoping Guo
Xiaoping Guo Chao Wang
Chao Wang Fang Wang
Fang Wang