- 1Medical Science Research and Innovation Institute, Prince of Songkla University, Hat Yai, Thailand
- 2Research Center of Excellence for Oral Health, Faculty of Dentistry, Prince of Songkla University, Hat Yai, Thailand
- 3Department of Oral Diagnostic Sciences, Faculty of Dentistry, Prince of Songkla University, Hat Yai, Thailand
Background: A previous study indicated that poly L-lysine-glycerol monolaurate mouthwash reduced the virulence of Helicobacter pylori; however, these compounds are derivatives. Thus, this study aimed to compare the effects of postbiotics, postbiotic-glycerol monolaurate, and poly L-lysine-glycerol monolaurate mouthwashes against clinical H. pylori strains.
Methods: Postbiotics, Lacticaseibacillus paracasei SD1, L. rhamnosus SD4, and L. rhamnosus SD11 were examined for anti-bacterial activity and synergistic effects. Subsequently, mouthwashes containing postbiotics, postbiotic-glycerol monolaurate, and poly L-Lysine-glycerol monolaurate were prepared and evaluated for their ability to reduce H. pylori adhesion to host cells, suppress inflammation induced by H. pylori, eradicate biofilm, decrease cagA expression, and assess epithelial cell viability. The stability of the mouthwashes was evaluated every 4 weeks up to 24 weeks for their efficacy against H. pylori growth, biofilm eradication, and epithelial cell viability.
Results: The postbiotics, L. paracasei SD1 and L. rhamnosus SD11, demonstrated significant anti-H. pylori activity, with synergistic effects observed in combinations with derivative compounds. Postbiotic-glycerol monolaurate mouthwashes exhibited higher efficacy in reducing H. pylori adhesion to host cells (42.64-43.83%), suppressing pro-inflammatory cytokines, eradicating biofilm (82.62% at 24 h), and reducing cagA expression (112.60 fold) compared to others. Such mouthwashes also displayed low cytotoxicity (< 30% for 15 min) to all cells tested. The stability was observed up to 24 weeks.
Conclusion: This in vitro study demonstrated that postbiotic-glycerol monolaurate mouthwash revealed the highest efficacy against H. pylori with low cytotoxicity to host cells. The stability lasted for 24 weeks.
1 Introduction
Helicobacter pylori is found in more than 50% of the global population and has been linked with gastritis and gastric cancer (Zamani et al., 2018). In our previous study, the prevalence of H. pylori in the oral cavity of participants ranged from 83.92% to 86.73%, which was detected by a polymerase chain reaction (PCR). Among patients with gastritis, the prevalence was observed to be between 93.33% and 100.00%, while in gastric cancer patients, it ranged from 80.00% to 86.67% as determined by a PCR (Wongsuwanlert et al., 2024a). The results suggested that the oral cavity may serve as a reservoir for H. pylori or a means of transmission to other sites. Furthermore, studies found that oral H. pylori infections are associated with a high gastritis recurrence rate (13.20%-18.40%) (Yee, 2017; Sheu et al., 2007). Thus, combining oral and gastrointestinal therapies enhances the effective treatment of H. pylori infection (Wang et al., 2014).
Standard treatment for H. pylori infection is triple therapy, which combines two antibiotics (clarithromycin and either amoxicillin or metronidazole) with a proton pump inhibitor (Chey and Wong, 2007). H. pylori strains have demonstrated significant antibiotic resistance, particularly to metronidazole, while bismuth compounds exhibit high toxicity to host cells, which complicates treatment further (Wongsuwanlert et al., 2024a). Additionally, treatment of H. pylori infection focuses on removing H. pylori from the stomach, but there is limited data on eliminating H. pylori in the mouth. A previous study by Wang et al. (2014) demonstrated that a mouthwash containing poly L-lysine and glycerol monolaurate reduced oral H. pylori levels in volunteers. Specifically, the mouthwash decreased cagA mRNA expression, reduced bacterial adhesion, inhibited H. pylori growth, and lowered interleukin-8 expression (Wongsuwanlert et al., 2024b). Glycerol monolaurate is a derivative compound recognized for its beneficial effects for food preservation and oral hygiene products (Wang et al., 2014; Wongsuwanlert et al., 2024b). In contrast, poly L-lysine has several limitations, including its high cost, significant toxicity to epithelial cells, reliance on chemical synthesis, lack of biodegradability under all environmental conditions, and complex polymerization processes (Wang et al., 2014; Chen et al., 2021). There is significant interest in researching the effects of organic substances as alternatives to replace derivative compounds to develop safer and more effective therapeutic agents targeting H. pylori. Meta-analyses of systematic reviews have shown that probiotic supplementation was significantly associated with increased H. pylori eradication rates by 10% compared to the control group and showed a lower risk of overall side effects by 46% compared to standard therapy (Yang et al., 2024; McFarland et al., 2016). In addition, consuming probiotics before or after triple treatment can enhance the therapeutic effects for individuals infected with H. pylori (Wang et al., 2023). Postbiotics have recently gained considerable popularity. They are preparations of inanimate microorganisms or their components including cell-free supernatants, cell walls, and metabolites (Salminen et al., 2021). Postbiotics from Lacticaseibacillus paracasei SD1 and L. rhamnosus SD11 have been shown to inhibit pathogen growth and reduce pro-inflammatory cytokines with effects comparable to those of live probiotics in vitro and in vivo (Wanitsuwan et al., 2024; Pahumunto and Teanpaisan, 2023). Postbiotics from L. paracasei SD1, L. rhamnosus SD4, and L. rhamnosus SD11 have been shown to inhibit H. pylori adhesion to oral epithelial cells (Juntarachot et al., 2023). The inactivated Limosilactobacillus reuteri DSM17648 improves H. pylori eradication in patients with functional dyspepsia and decreases digestive adverse effects (Ivashkin et al., 2024). However, Yang et al. (2021) found that non-viable L. reuteri DSM17648 with triple therapy did not improve eradication rates; however, it promoted a beneficial microbial profile and a decline in gastrointestinal symptoms. Thus, it remains necessary to identify the most effective postbiotics for H. pylori treatment. This study aimed to examine the anti-bacterial ability of selected postbiotic strains against oral Helicobacter pylori strains. Subsequently, this study compared the effects of postbiotics-, postbiotic-glycerol monolaurate-, and poly L-Lysine-glycerol monolaurate- mouthwashes against oral H. pylori strains.
2 Materials and methods
2.1 Bacterial preparation
2.1.1 Probiotic cultivation
The selected probiotic strains were Lacticaseibacillus paracasei SD1, L. rhamnosus SD4, and L. rhamnosus SD11, which were isolated from the oral cavity of individuals with a caries-free status. These strains were cultured on de Man, Rogosa, and Sharpe (MRS) agar (Difco™, Sparks, MD, USA) at 37°C for 24 h under anaerobic conditions (10% CO2, 10% H2, 80% N2). These optimal conditions were observed in the log phase of growth for the probiotic strains (Juntarachot et al., 2023; Wannun et al., 2014). Probiotic cells were collected using centrifugation at 10,000 rpm for 10 min at 20°C, and the cells were adjusted to 8 log CFU/mL before testing.
2.1.2 Postbiotic preparation
Probiotic strains (8 log CFU/mL) were cultured in de Man, Rogosa, and Sharpe (MRS) broth (Difco™, Sparks, MD, USA) at 37°C for 24 h under anaerobic conditions (10% CO2, 10% H2, 80% N2) based on our previous studies that tested incubation times at 12, 24, 48, and 72 h (Thananimit et al., 2022). A 24 h incubation was identified as the optimal condition for promoting the production of bioactive compounds including anti-oxidant, short-chain fatty acids, and antimicrobial proteins (Chooruk et al., 2017; Thananimit et al., 2022; Wannun et al., 2014). The postbiotics were collected by centrifugation at 10,000 rpm for 10 min at 4°C. They were then filtered using a 0.45 µm syringe filter (Sartorius stedium, Germany) and lyophilized using a freeze dryer. The postbiotic powder was kept at -80°C until usage.
2.1.3 Helicobacter pylori cultivation
The study was approved by the Ethical Committee of the Faculty of Dentistry, Prince of Songkla University (EC6410-066). H. pylori ATCC43504 and nineteen clinical strains of H. pylori isolated from the oral cavity (saliva and plaque) were used throughout the study. Clinical strains were confirmed using a polymerase chain reaction with specific primers (Wongsuwanlert et al., 2024a). H. pylori strains were cultured on brain heart infusion (BHI) agar (Difco™, Sparks, MD, USA) supplemented with 5% blood at 37°C for 48 h in anaerobic conditions (Juntarachot et al., 2023; Perez-Perez et al., 2016). H. pylori strains (3–5 colonies) were picked up to re-streak a full plate on BHI agar, and H. pylori cells were collected for culture in BHI broth (Difco™, Sparks, MD, USA) for 48 h at 37°C in anaerobic conditions (Juntarachot et al., 2023; Wongsuwanlert et al., 2024b). Then H. pylori cells were collected using centrifugation at 10,000 rpm for 5 min and adjusted to 8 log CFU/mL to be used in all experiments.
2.2 Screening of anti-H. pylori activity of probiotics and postbiotics using agar well diffusion and agar overlay methods
2.2.1 Agar well diffusion method
An H. pylori strain at 8 log CFU/mL was added to 20 mL of melted BHI agar and poured on a plate with metal cups. After solidifying, the metal cup was removed and postbiotic solutions (postbiotic powder 1 mg in 1 mL distilled water) or derivative compounds (1 mg of poly L-Lysine or glycerol monolaurate in 1 mL distilled water) (Sigma, St. Louis, MO, USA) were added to each well. The plate was incubated overnight at 37°C in anaerobic conditions. The inhibition zone was measured and reported as mm. The experiment was performed in triplicate and distilled water was used as a negative control.
2.2.2 Agar overlay method
Probiotic microorganisms (10 µL of 8 log CFU/mL) were dotted on MRS agar and incubated at 37°C overnight in anaerobic conditions. Afterward, the plate was poured with 5 mL of melted BHI agar containing H. pylori cells (8 log CFU/mL) and incubated overnight in anaerobic conditions. The inhibition zone was measured and reported as mm. The experiment was performed in triplicate.
2.3 Minimum inhibitory concentration, minimum bactericidal concentration, and fractional inhibitory concentration of postbiotics and derivative compounds
2.3.1 Broth microdilution assay
As mentioned above, postbiotic solutions and derivative compounds (poly L-Lysine or glycerol monolaurate) were prepared. A compound of 100 µL (at a concentration of 1.2 mg/mL) was diluted by 2-fold dilution with 100 µL of BHI broth in a 96-well plate, and 100 µL of H. pylori cells (8 log CFU/mL) were added to the well. The plate was incubated overnight at 37°C in anaerobic conditions, and the last well to be inhibited (clear under vision) was recorded as the MIC value. All wells were dotted on BHI agar (Difco™, Sparks, MD, USA) to check H. pylori growth, and the last well showed no H. pylori growth, so it was recorded as the MBC value. As some strains did not exhibit a measurable MBC value, although inhibitory zones were observed, MBC values were determined using the following concentration series: 1.2, 1.1, 1.0, 0.9, 0.8, 0.7, and 0.6 mg/mL, respectively. The experiment was performed in triplicate.
2.3.2 Checkerboard assay
Postbiotics derived from L. paracasei SD1 (postbiotic SD1), L. rhamnosus SD4 (postbiotic SD4), L. rhamnosus SD11 (postbiotic SD11), as well as poly L-Lysine and glycerol monolaurate, were tested for synergistic or antagonistic effects using a checkerboard assay. Each postbiotic solution, poly L-Lysine or glycerol monolaurate, was prepared at the concentrations of 1/12X, 1/6X, 1/4X, 1/2X, 1X, 2X, and 3X MIC, and 100 µL of each concentration was added into the 96-well plate. Then 100 µL of H. pylori (8 log CFU/mL) was added and the plate was incubated overnight at 37°C in anaerobic conditions. The lowest concentration of the combination of postbiotics, poly-L-lysine, or glycerol monolaurate showed growth inhibition compared to the untreated control. The accompanying equation calculated the fractional inhibitory concentration index (FIC index): FIC index = (MIC of solvent A in combination)/(MIC of solvent A alone) + (MIC of solvent B in combination)/(MIC of solvent B alone). The experiment was performed in triplicate.
2.4 Postbiotics, postbiotic-glycerol monolaurate, and poly L-Lysine-glycerol monolaurate mouthwash preparation
Mouthwashes were prepared in 3 formulations as follows: (i) postbiotic mouthwash containing 0.03 mg/mL postbiotic SD1 and 0.03 mg/mL postbiotic SD11, (ii) postbiotic-glycerol monolaurate mouthwash containing 0.02 mg/mL glycerol monolaurate along with 0.03 mg/mL postbiotic SD1 and 0.03 mg/mL postbiotic SD11, and (iii) poly L-lysine-glycerol monolaurate mouthwash containing 0.03 mg/mL poly L-lysine and 0.02 mg/mL glycerol monolaurate. All types of mouthwashes were used throughout the study and were freshly prepared before use. The mouthwash base comprising glycerol, kolliphor RH40, peppermint oil, saccharin sodium salt hydrate, methyl 4-hydroxybenzoate, and water (Wongsuwanlert et al., 2024b) was prepared and used as a negative control in all experiments.
2.5 Cell viability assay after being treated with postbiotics and postbiotic-glycerol monolaurate mouthwashes
H357, AGS, and PDL cells were used in this experiment. The cells were subcultured using the trypsinization method, and then the cells (3 x 104 cells/mL) were seeded into 96-well plates. The culture plate was incubated for 72 h in a CO2 incubator and the cells showed approximately 99% cell confluence. An amount of 100 µL of postbiotic mouthwash or postbiotic-glycerol monolaurate mouthwash or poly L-Lysine-glycerol monolaurate mouthwash was added to monolayer cells for 5 min, 15 min, 1 h, 2 h, 6 h, and 24 h in a CO2 incubator. Cell viability was assessed using a MTT assay, and the percentage of cell viability was calculated by (OD 570 of the treated wells/OD 570 of non-treated wells) × 100.
2.6 Anti-adhesion ability of postbiotics and postbiotic-glycerol monolaurate mouthwashes
2.6.1 Cell cultivation
Human oral squamous cell carcinoma (H357 was kindly derived from Professor Paul Speight of the University of Sheffield, UK) and human gastric adenocarcinoma cells (AGS, CRL-1739) were used in this study. H357 or AGS cells were cultured in cell culture flasks with Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Grand Island, NY, USA), 1% Penicillin-Streptomycin (Thermo Fisher Scientific, Grand Island, NY, USA), and 1% amphotericin B (Thermo Fisher Scientific, Grand Island, NY, USA) at 37°C in 5% CO2. The cells were subcultured using the trypsinization method, and then the cells (105 cells/mL) were seeded into 24-well plates. The plate was incubated at 37°C in a CO2 incubator for 3 days, or until 95% cell confluence was reached, for the anti-adhesion study.
2.6.2 Anti-adhesion assay
H. pylori cells (8 log CFU/mL) or a combination of H. pylori cells and postbiotic mouthwash or a combination of H. pylori cells and postbiotic-glycerol monolaurate mouthwash or a combination of H. pylori cells and poly L-Lysine-glycerol monolaurate mouthwash were added to the monolayer cells (Wongsuwanlert et al., 2024b). The plate was incubated at 37°C for 1 h and washed twice with PBS (pH 7.0). Trypsin-EDTA (0.25%) (Thermo Fisher Scientific, Canada) was added to remove adherent cells, and bacterial adhesion was counted by the plate count method. The percentage of adhesion ability was calculated using 100 x adherence bacteria/bacteria at the beginning. The experiment was conducted in triplicate, and the mouthwash base was used as the negative control.
2.7 Pro-inflammatory cytokines suppression after being treated with postbiotics and postbiotic-glycerol monolaurate mouthwashes
AGS and human periodontal ligament (PDL) cells were subcultured by the trypsinization method, and the cells (105 cells/mL) were seeded in 6-well plates for 3 days or until 90% confluence. The cells were treated with an H. pylori strain and a combination of H. pylori and postbiotic mouthwash or H. pylori and postbiotic-glycerol monolaurate mouthwash or H. pylori and poly L-Lysine-glycerol monolaurate mouthwash for 24 h in a CO2 incubator. The cells were extracted for RNA using an RNA extraction kit (Thermo Fisher Scientific, CA, USA), and 2 µg RNA was synthesized for cDNA using a cDNA synthesis kit (Thermo Fisher Scientific, MA, USA). cDNA was measured for interleukin (IL)-1β, IL-6, IL-8, and TNF-α mRNA expression using the CFX96 TouchTM Real-Time PCR detection system (BioRad, Foster, CA, USA), and its conditions were followed according to Wongsuwanlert et al. (2024b). Glyceraldehyde 3-phosphate dehydrogenase was used as a housekeeping gene, and untreated cells were used as the control. The cytokine expression was normalized to the control (set to 1.0) and reported as a fold of induction. The experiment was performed in triplicate and the mouthwash base was used as a negative control.
2.8 Biofilm eradication of postbiotics and postbiotics-glycerol monolaurate mouthwashes using a microtiter biofilm formation assay
An H. pylori strain (8 log CFU/mL) of 200 µL was added to a 96-well plate and incubated at 37°C for 24 h in anaerobic conditions. The biofilm was washed twice with 200 µL PBS (pH 7.0) and exposed to 200 µL of postbiotics, postbiotic-glycerol monolaurate, and poly L-Lysine-glycerol monolaurate mouthwashes at 37°C for 5 min, 15 min, 1 h, 2 h, 6 h, and 24 h in anaerobic conditions. Biofilm eradication (%) was evaluated using a MTT assay and was confirmed using Live/Dead staining (Thermo Fisher Scientific, Eugene, OR, USA). The experiment was performed in triplicate, and the mouthwash base was used as a negative control.
2.9 Determination of cagA suppression of postbiotics and postbiotics-glycerol monolaurate mouthwashes
Nineteen H. pylori strains (8 log CFU/mL) were incubated with a 50% lethal dose of postbiotic mouthwash or postbiotic-glycerol monolaurate mouthwash or poly L-Lysine-glycerol monolaurate mouthwash at 37°C for 24 h in anaerobic conditions. H. pylori cells were extracted for RNA using an RNA extraction kit (Thermo Fisher Scientific, CA, USA) following the instruction guidelines, and 100 ng/mL RNA was mixed with One Step real-time PCR solution (Meridian Bioscience, Memphis, TN, USA), nucleotide sequence primers (Macrogen, Korea), (Wongsuwanlert et al., 2024b) and distilled water. The reaction was used to determine cagA expression using the CFX96 TouchTM Real-Time PCR detection system (BioRad, Foster, CA, USA). The real-time PCR conditions were 40 cycles with a denaturing temperature of 95°C for 20s, annealing temperatures at 44°C for 20s, and a polymerizing temperature of 72°C for 25s. The ureA gene was used as a housekeeping gene in this study. H. pylori ATCC43504 was used as a control and the expression was set to 1.0. The experiment was performed in triplicate, and the mouthwash base was used as a negative control.
2.10 Stability test
Postbiotic mouthwash and postbiotic-glycerol monolaurate mouthwash were kept at 4°C and were tested for anti-H. pylori, biofilm eradication, and cell viability using the agar well diffusion method and MTT assay every 4 weeks for 24 weeks.
2.11 Statistical analysis
The results were expressed as mean ± SD for antibacterial activity, biofilm removal, cell viability, and pro-inflammatory cytokine suppression. A scatter plot with a mean value was displayed for the cagA mRNA expression and adhesion ability data. The differences between the groups were evaluated using the Mann-Whitney U test. A p-value < 0.05 indicated a significant difference in the data.
3 Results
3.1 Anti-H. pylori activity, MIC, MBC, and the FIC index of postbiotics and derivative compounds (poly L-Lysine and glycerol monolaurate)
An inhibition zone around the probiotic cells and postbiotics against H. pylori was observed, with the probiotic cells displaying a larger inhibition zone (28.61-31.21 mm) than postbiotics (15.21-20.00 mm, Table 1). Postbiotic SD11 (19.41 ± 0.83 mm for ATCC43504 and clinical strains) showed a greater zone than postbiotic SD1 (16.42 ± 0.40 mm for ATCC43504 and clinical strains) and postbiotic SD4 (15.61 ± 0.56 mm for ATCC43504 and clinical strains). Regarding the MIC value of postbiotics, the results showed that 0.20-0.33 mg/mL of postbiotic SD1, postbiotic SD4, and postbiotic SD11 inhibited the growth of H. pylori (ATCC43504 and clinical strains) while poly L-Lysine and glycerol monolaurate revealed 0.30 and 0.20 mg/mL, respectively. The results of H. pylori ATCC43504 showed alignment with the oral clinical strains. A synergistic effect (FIC index ≤ 0.5) was observed with postbiotic SD1 and postbiotic SD11, as well as between postbiotic SD1 and glycerol monolaurate and between postbiotic SD11 and glycerol monolaurate (Table 2). Therefore, two types of mouthwashes were used throughout the study: one containing postbiotic SD1 and postbiotic SD11 (postbiotic mouthwash) and another combining postbiotic SD1, postbiotic SD11, and glycerol monolaurate (postbiotic-glycerol monolaurate mouthwash). Additionally, poly L-lysine-glycerol monolaurate mouthwash was used for comparison.
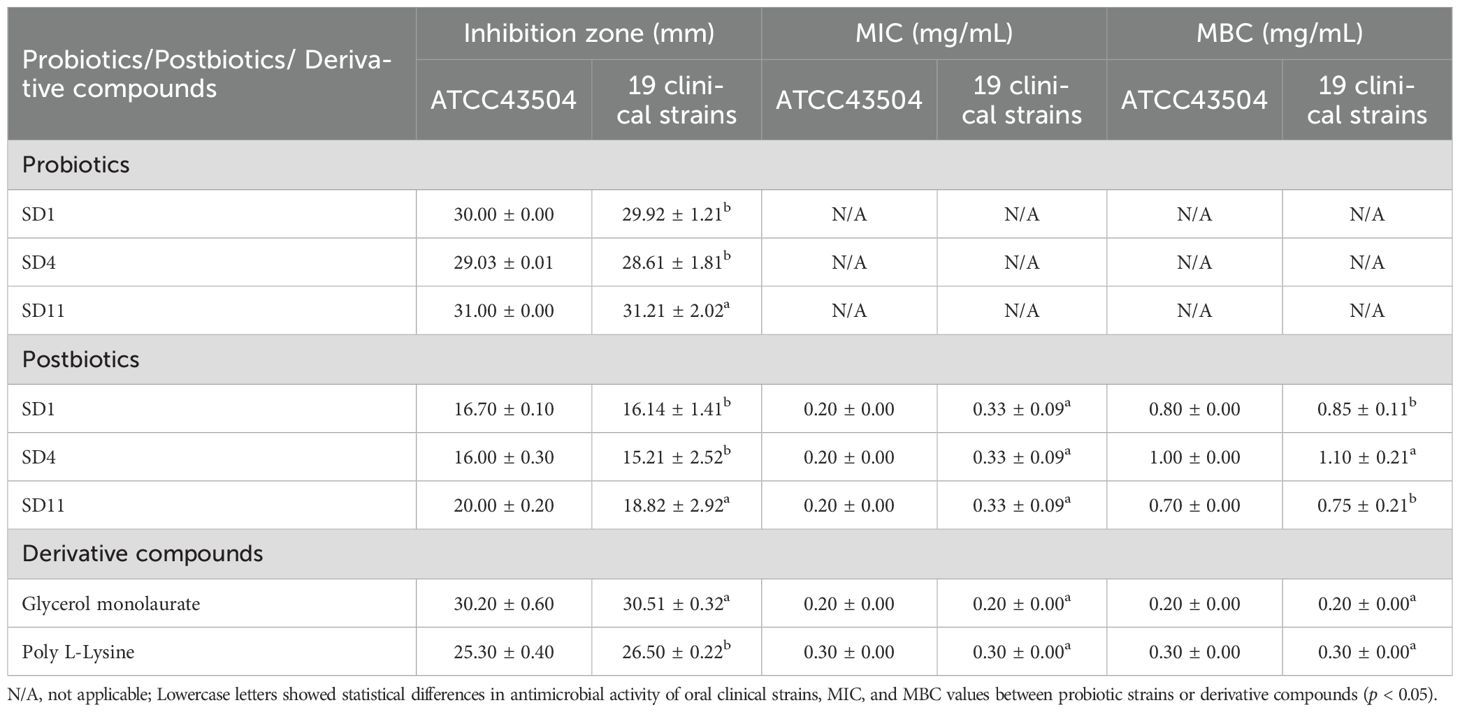
Table 1. Mean ± SD of antimicrobial activity of probiotics and postbiotics against Helicobacter pylori ATCC43504 and 19 strains of oral H. pylori.
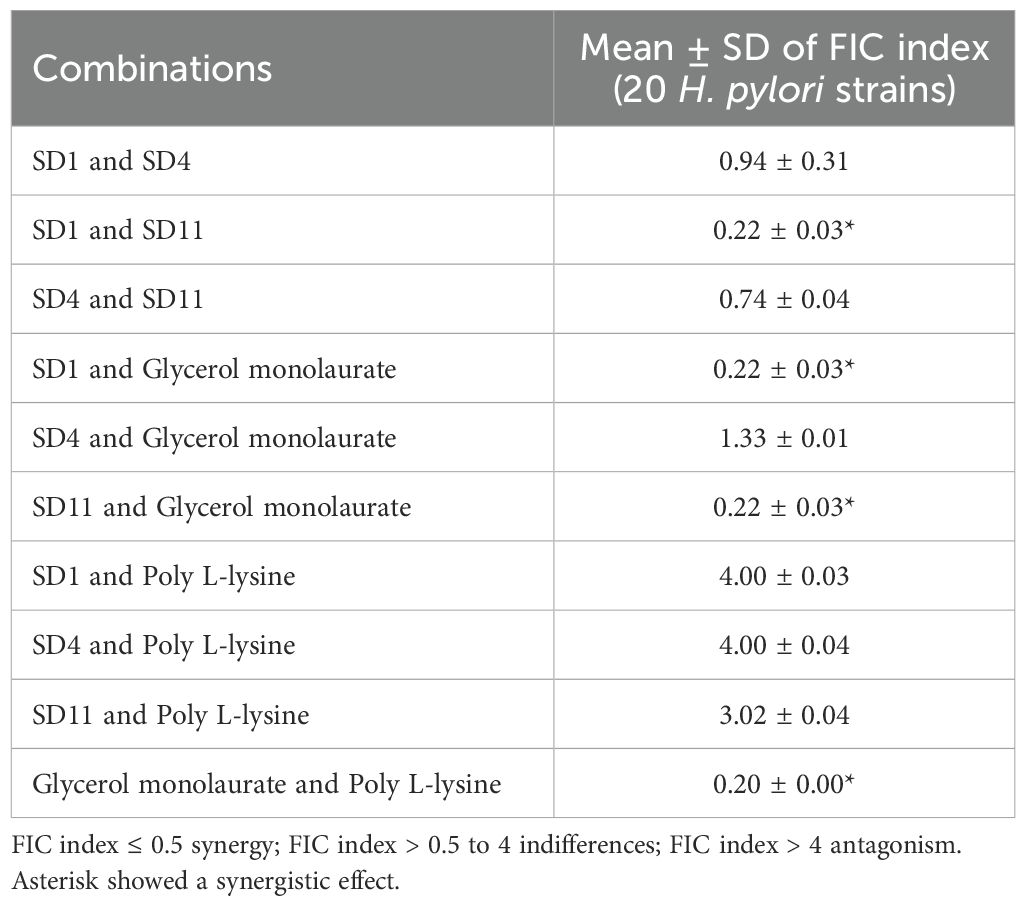
Table 2. Mean ± SD of the synergistic effect of postbiotics and derivative compounds against Helicobacter pylori strains.
3.2 Epithelial cell viability after exposure to mouthwashes
Epithelial cell viability depended on the incubation time, with 5 min (79.78%-91.56% for H357, 100.00%-100.00% for PDL, and 77.23%-80.21% for AGS) and 15 min (53.89%-83.93% for H357, 100.00%-100.00% for PDL, and 61.20%-75.23% for AGS) showing the highest cell viability. PDL cells showed 100.00% cell viability until 15 min compared to other cells, followed by H357 and AGS cells (Table 3). Postbiotic-glycerol monolaurate mouthwash showed the highest cell viability compared to the postbiotic- and poly L-Lysine-glycerol monolaurate mouthwashes at all times and cells tested.
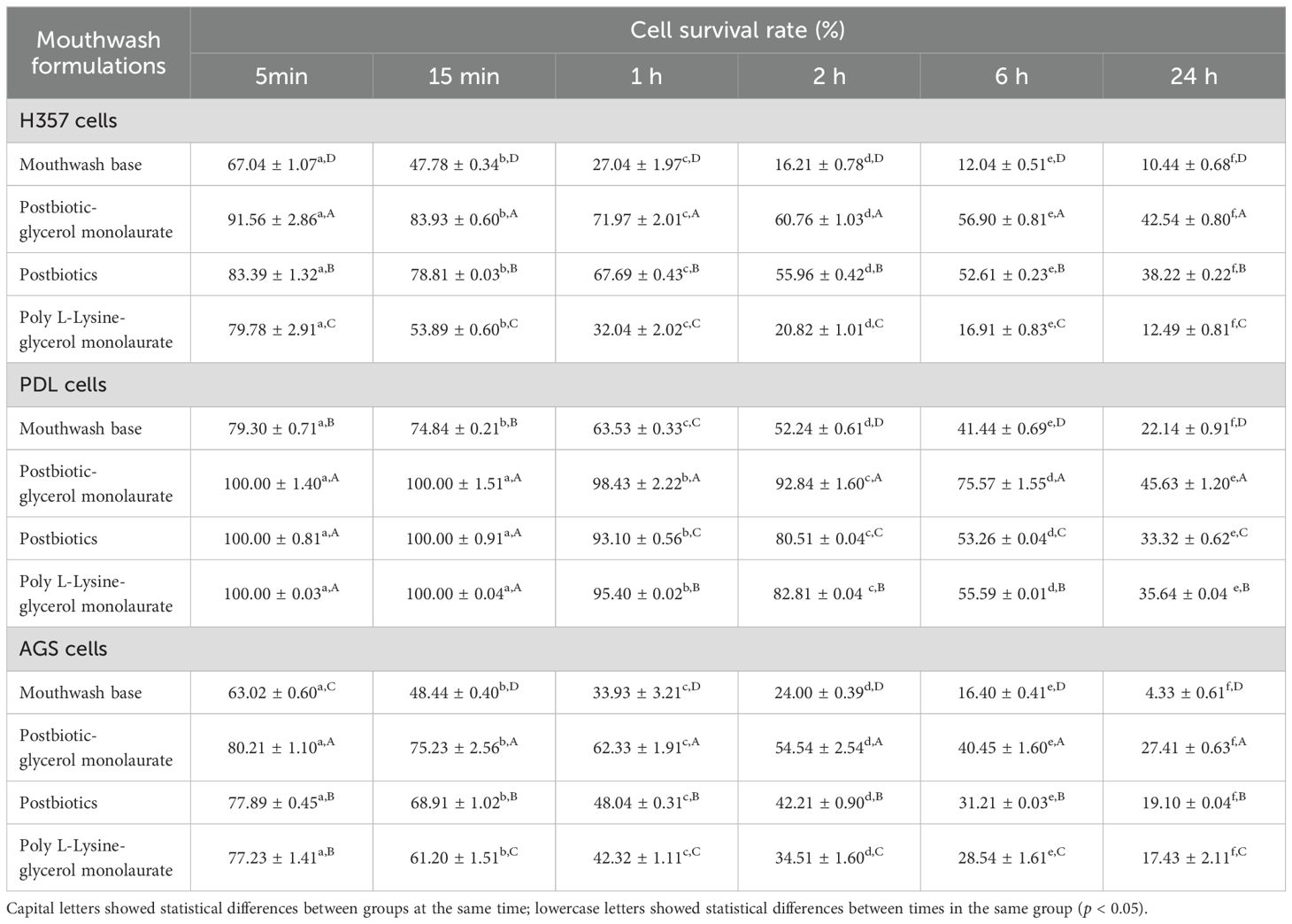
Table 3. Mean ± SD of cell viability of human oral epithelial cells (H357), human periodontal ligament cells (PDL), and human gastric cancer cells (AGS) after being treated with postbiotic-, postbiotic-glycerol monolaurate-, and poly L-Lysine-glycerol monolaurate- mouthwashes.
3.3 Anti-adhesion effects by mouthwashes
No cell cytotoxicity was found after testing with either H. pylori or the mouthwashes. A decrease in the adhesion of H. pylori strains was observed on H357 and AGS cells, particularly with the postbiotic-glycerol monolaurate mouthwash, which demonstrated the highest adhesion reduction (12.25% for H357 cells and 25.82% for AGS cells; Figure 1A). The postbiotic mouthwash resulted in an adhesion reduction of 8.47% for H357 cells and 22.44% for AGS cells, while the poly L-Lysine-glycerol monolaurate mouthwash showed a decrease of 9.88% for H357 cells and 23.72% for AGS cells. Both mouthwashes showed no significant differences, but both resulted in adhesion reductions that were significantly lower than that of H. pylori alone (p < 0.05).
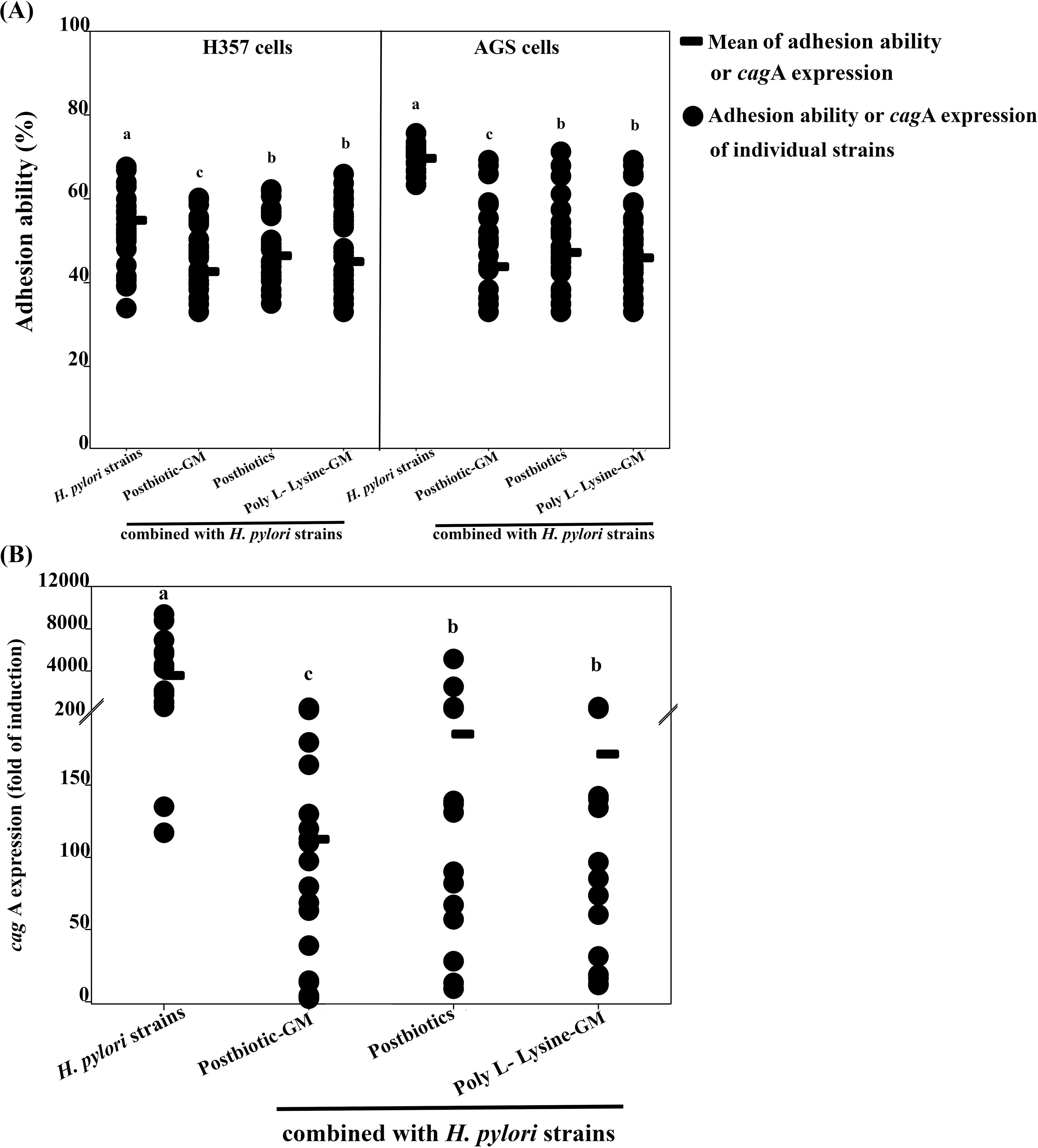
Figure 1. Graph (A) shows the adhesion ability of H357 and AGS cells to Helicobacter pylori strains and their combinations with postbiotic-glycerol monolaurate (Postbiotic-GM), postbiotics, and poly L-Lysine-glycerol monolaurate (Poly L-Lysine-GM). Each group shows the mean adhesion and individual data points. Graph (B) depicts cagA expression (fold of induction) with the same combinations, showing mean expression and individual data points. Each category has letters a, b, or c indicating significant differences.
3.4 Pro-inflammatory cytokines suppression by mouthwashes
Neither H. pylori nor the mouthwashes showed cytotoxicity against the cells tested in the experiment. The inflammation results indicated that AGS cells expressed more pro-inflammatory cytokines than PDL cells. All mouthwashes showed low levels of pro-inflammation stimulation in PDL (IL-1β at 0.53-0.91 fold, IL-6 at 0.61-1.02 fold, IL-8 at 3.42-5.71 fold, and TNF-α at 0.43-0.63 fold) and AGS cells (IL-1β at 1.37-1.62 fold, IL-6 at 1.87-2.27 fold, IL-8 at 7.42-8.51 fold, and TNF-α at 1.31-1.45 fold), whereas H. pylori strains (H. pylori ATCC43504 and 19 clinical strains) stimulated both cells to express high levels of pro-inflammatory cytokines (IL-1β at 2.04-3.24 fold, IL-6 at 3.08-4.63 fold, IL-8 at 20.23-46.51 fold, and TNF-α at 3.54-5.45 fold). Among the pro-inflammatory cytokines, IL-8 showed the highest cytokine expression, followed by TNF-α, IL-6, and IL-1β. However, pro-inflammatory cytokines were reduced after being combined with the mouthwashes. The postbiotic mouthwash revealed a high reduction of pro-inflammatory cytokines compared to others (Table 4), and IL-8 showed the highest significant decrease in both cell types (3.03-5.21 fold for PDL cells and 16.79-28.51 fold for AGS cells, p < 0.05).
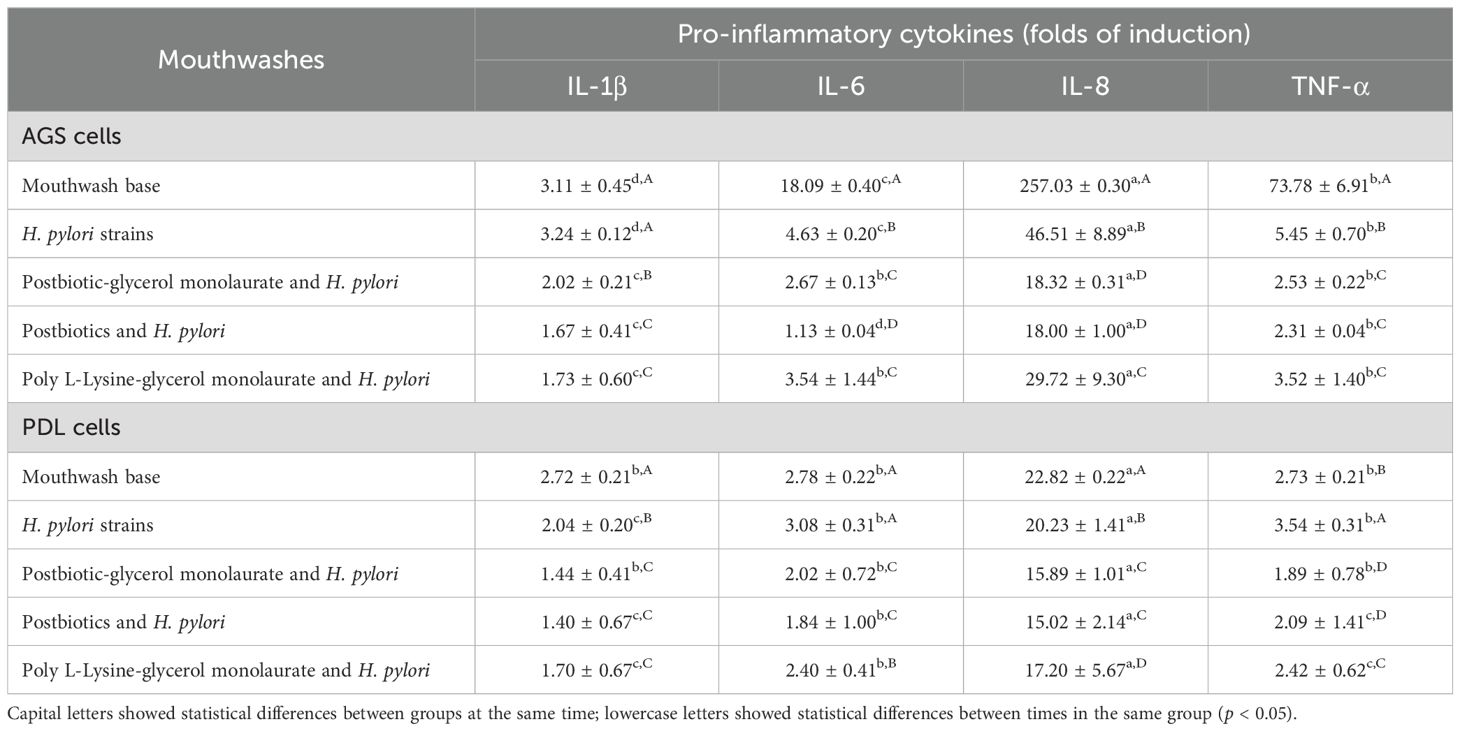
Table 4. Mean ± SD of pro-inflammatory cytokine stimulation by Helicobacter pylori (ATCC43504 and 19 strains of oral H. pylori) or by each H. pylori strain and mouthwash on AGS and PDL cells.
3.5 Biofilm eradication by mouthwashes
Figure 2A demonstrated that the biofilm was effectively removed after treatment with all mouthwashes. Importantly, postbiotic-glycerol monolaurate mouthwash showed a higher efficacy (21.30%-84.37%) in biofilm removal compared to postbiotic mouthwash (17.43%-80.51%) and poly L-Lysine-glycerol monolaurate mouthwash (18.03%-82.59%). Biofilm eradication was time-dependent, with the most effective eradication observed after 24 h of incubation with all mouthwashes (84.44%, 80.52%, and 82.57% for postbiotics-glycerol monolaurate mouthwash, postbiotic mouthwash, and poly L-Lysine-glycerol monolaurate mouthwash, respectively). Biofilm removal was confirmed with live/dead staining (Figures 2B–D). After treating the biofilm with postbiotics-glycerol monolaurate mouthwash for 24 h, a significant proportion of the biofilm was found dead (red color) compared to the untreated cells (green color).
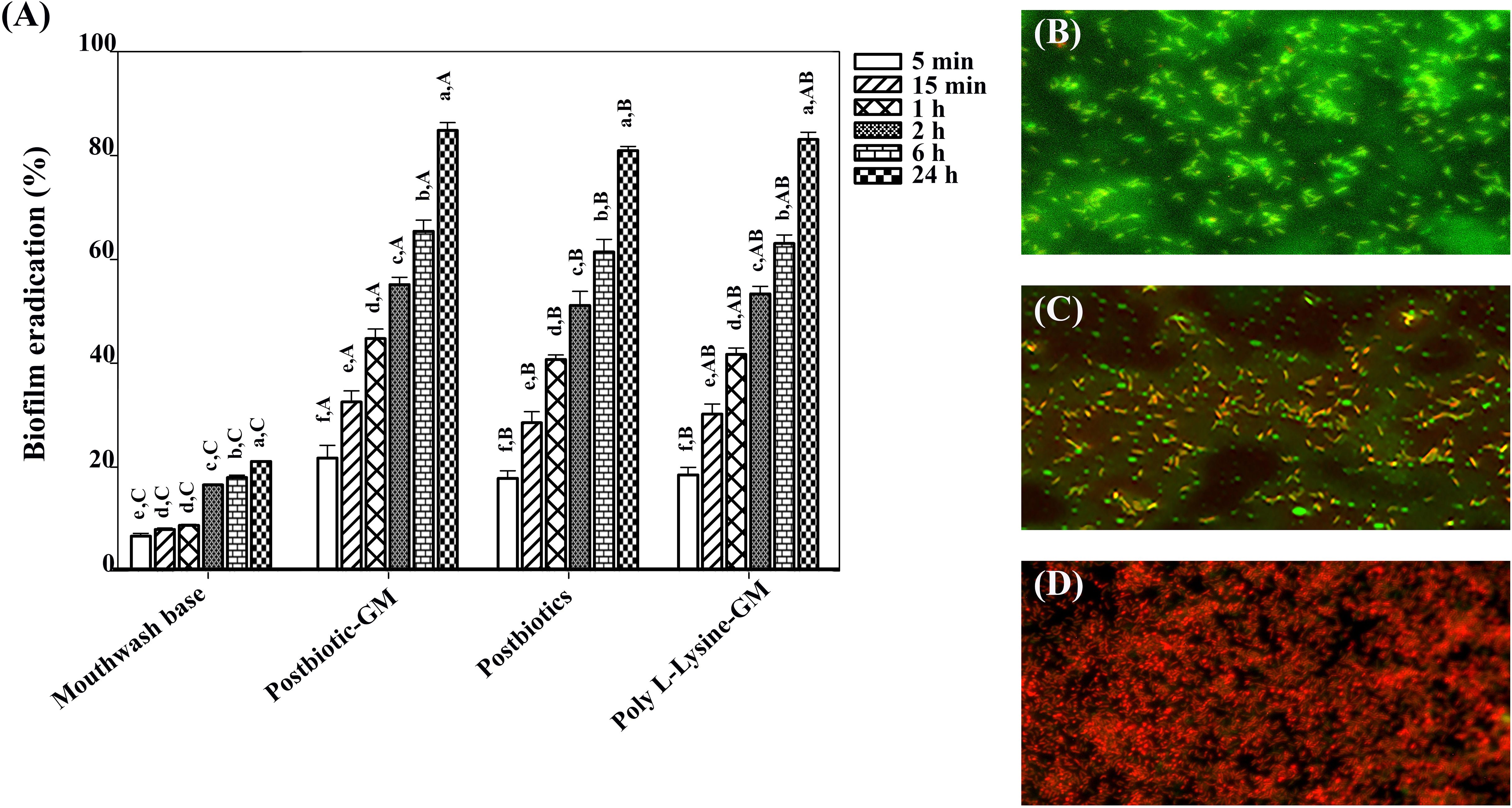
Figure 2. (A) Bar chart showing biofilm eradiation percentages for different treatments over various time intervals. Capital letters indicate significant differences between groups at the same time point, while lowercase letters indicate significant differences between time points within the same group (p < 0.05). (B) Micrograph with green fluorescence indicating biofilm presence. (C) Micrograph showing red and green fluorescence demonstrating biofilm disruption. (D) Micrograph with dominant red fluorescence, suggesting extensive biofilm eradication.
3.6 cagA suppression by mouthwashes
The study results indicate that H. pylori treated with the mouthwashes demonstrated a notable decrease in cagA expression compared to the strain without treatment (Figure 1B). Among the mouthwashes tested, the postbiotic-glycerol monolaurate mouthwash resulted in the highest reduction of cagA expression, achieving a remarkable 3467.45-fold decrease. This was closely followed by the poly L-Lysine-glycerol monolaurate mouthwash, which resulted in a 3408.50-fold reduction, and the postbiotic mouthwash, which showed a 3394.61-fold reduction in cagA expression.
3.7 Stability of postbiotic mouthwash and postbiotic-glycerol monolaurate mouthwash
The effects of both types of mouthwashes after 24 weeks showed a decrease compared to baseline levels. At the baseline, the anti-H. pylori activity, biofilm eradication, and epithelial cell viability for the postbiotic mouthwash were 20.04 mm, 78.03%, and 83.33%, respectively, while the postbiotic-glycerol monolaurate mouthwash had the corresponding values of 30.01 mm, 80.03%, and 91.58%, respectively. However, after 24 weeks, these parameters declined to 14.67 mm and 16.31 mm for anti-H. pylori activity, with biofilm eradication and cell viability dropping to 32.11% and 34.92%, as well as 35.92% and 39.45%, respectively, for both mouthwashes.
4 Discussion
Helicobacter pylori infection in the stomach is usually treated with a combination of drugs (clarithromycin and either amoxicillin or metronidazole) along with bismuth (Chey and Wong, 2007). The oral cavity may serve as a potential reservoir for H. pylori, allowing transmission to other sites in the human body and leading to the recurrence of H. pylori gastritis (Wongsuwanlert et al., 2024). Therefore, a comprehensive treatment aimed at eliminating H. pylori in both the stomach and oral cavity may help more effectively eradicate H. pylori gastric infection. The study found that postbiotic mouthwash and postbiotic-glycerol monolaurate mouthwash reduced virulence factors of H. pylori including its adhesion to host cells, pro-inflammatory cytokine production, biofilm formation, and cagA expression, while also demonstrating lower toxicity to host cells. The reduction effects of mouthwashes against clinical oral H. pylori were found for up to 24 weeks of storage.
After mouthwash formulation, the mouthwashes were assessed for toxicity on host cells, showing a time-dependent toxic effect. At 24 h of exposure, all tested cell types presented more than 50% toxicity, which based on the ISO 10993-5:2009 standard, can be classified as highly cytotoxic. However, typical daily mouthwash use does not exceed 5 min, indicating that the observed cytotoxicity at 24 h may not accurately reflect the in vivo exposure conditions during regular oral use. Our study revealed that postbiotic-glycerol monolaurate exhibited low cytotoxicity, with 0-17% cytotoxicity observed in oral cells (keratinocyte and periodontal ligament cells) and 25% in gastric epithelial cells after 15 min of incubation. According to the standard, reports cell cytotoxicity as < 30%, is classified as slightly cytotoxic. This level is generally considered acceptable for biomaterial applications, particularly when the exposure is short-term or in direct or prolonged contact with sensitive tissues. Conversely, mouthwash base displayed high cell cytotoxicity (26-53%), despite the mouthwash base adding glycerol to maintain hydration, enhancing barrier integrity, and reducing cytotoxic responses (Kvalheim et al., 2019). Postbiotics can inhibit the death of gut epithelial cells and promote their growth. They assist in tissue repair and enhance goblet cell development, which produces mucus to protect intestinal epithelial cells and maintain their integrity. A previous study showed that postbiotics exhibited low toxicity even after 15 days of use, with no significant changes in liver enzyme levels or intestinal histopathology in mice (Pesarico et al., 2023). Studies revealed that both glycerol monolaurate (at concentrations less than 640 µM) and postbiotics (at concentrations below 1000 µg/mL) exhibited no cytotoxicity toward critical immune cells including dendritic cells, human gingival fibroblast cells, and human peripheral blood mononuclear cells (Poulsen et al., 2015; Silva et al., 2018; Pahumunto et al., 2020). Notably, glycerol monolaurate has been shown to stabilize eukaryotic cell membranes, neutralize bacterial exotoxin toxicity, and affect anti-bactericidal activity. With such evidence, integrating postbiotic-glycerol monolaurate mouthwash into oral care routines is a promising approach for promoting oral health (Pesarico et al., 2023).
Postbiotics-glycerol monolaurate mouthwash demonstrated strong anti-adhesion activity on oral keratinocyte cells (H357) and gastric epithelial cells (AGS). Our study found that the mouthwash inhibited the adhesion of H. pylori strains to AGS cells more effectively than to H357 cells, which was likely due to H. pylori stronger tendency to adhere to gastric epithelial cells compared to H357 cells. The anti-adhesion effect is likely attributed to the ability of postbiotics or probiotics to inhibit the initial colonization of pathogens by enhancing antimicrobial peptides in the body and forming protective layers that prevent pathogen adhesion and invasion (Song et al., 2019). Previous studies reported that live lactobacilli, L. acidophilus, L. bulgaricus, L. paracasei SD1, L. rhamnosus SD4, and L. rhamnosus SD11, and dead lactobacilli could inhibit H. pylori adherence to gastric epithelial cell line-1, AGS cells, and human gastric adenocarcinoma cell lines (Che et al., 2024; Xu et al., 2022; Song et al., 2019). For instance, certain Lactobacillus strains can bind to toll-like receptors on host cells, thereby inhibiting the adhesion of pathogenic bacteria to gastric mucosal epithelial cells (Song et al., 2019). Specifically, L. reuteri secretes adhesion factors that competitively block H. pylori adhesion by binding to the receptor sites on gastric mucosal epithelial cells (Song et al., 2019). The evidence clearly shows that probiotics and postbiotics are crucial in preventing pathogens from adhering and invading host cells. Nonetheless, the mechanism by which the mouthwash inhibits H. pylori adhesion remains unclear and requires further clarification.
Virulence genes of H. pylori, especially cagA and vac genes, are associated with the severity of gastritis or gastric cancer. The most studied virulence-associated component of H. pylori is the cagA protein, which enters host gastric epithelial cells through the type 4 secretion system (Azizimoghaddam et al., 2023). Our study observed variability in cagA expression among H. pylori strains, potentially linked to differences in pathological outcomes; however, the underlying mechanisms remain unclear. This suggests that strain-specific factors may contribute to the upregulation of cagA expression. A previous study showed that a mouthwash combining poly L-Lysine and glycerol monolaurate successfully reduced cagA gene expression in H. pylori strains (Wongsuwanlert et al., 2024b). However, the impact of postbiotics on cagA suppression remains unexplored. Our study demonstrates that postbiotics and postbiotic-glycerol monolaurate mouthwashes significantly inhibit cagA expression in H. pylori strains following co-incubation. Additionally, lactobacilli cell-free supernatants effectively diminished the expression of the primary exotoxins produced by Aggregatibacter actinomycetemcomitans (leukotoxin and CDT) and downregulated katA, thereby decreasing the bacterium’s survival in the presence of H2O2 (Ishikawa et al., 2021). This compelling evidence highlights the potential of postbiotics in combating harmful bacterial strains. The reduction in cagA expression was associated with a decrease in pro-inflammatory cytokines, particularly IL-8. Our study also found that postbiotics and postbiotic-glycerol monolaurate mouthwashes can downregulate pro-inflammatory cytokines in oral and gastric cells after stimulation with H. pylori strains. This finding aligns with a previous study showing that the cell-free supernatant of these strains can suppress IL-8 stimulation in oral and intestinal cells exposed to Porphyromonas gingivalis (Pahumunto and Teanpaisan, 2023). This suppression may be due to the crucial role of postbiotics in inhibiting the phosphorylation and translocation of cagA (Ma et al., 2023; Lin et al., 2009). Additionally, glycerol monolaurate can modulate the production of pro-inflammatory cytokines in eukaryotic cells (Wongsuwanlert et al., 2024b).
Growth inhibition and biofilm eradication are recommended to eliminate or reduce H. pylori in the body; these functions are important to protect against infection by this organism. Previous studies have shown that poly-L-lysine and glycerol monolaurate, in mouthwash form, inhibited the growth of H. pylori and decreased the number of H. pylori in the oral cavity of volunteers (Wongsuwanlert et al., 2024a; Wang et al., 2014). However, controlling the quality of these compounds can be challenging. This study examined postbiotics as alternatives to chemical compounds and found that postbiotic mouthwash and postbiotic-glycerol monolaurate mouthwash showed the highest capacity to eliminate H. pylori in both planktonic and biofilm forms. Surprisingly, postbiotics exhibited close inhibitory effects against both H. pylori ATCC 43504 and clinical strains, possibly due to their shared mechanisms of action in eradicating H. pylori. The mechanisms and capacities of postbiotics, specifically cell-free supernatants, containing various bioactive compounds such as antimicrobial proteins (Wannun et al., 2014), antioxidants (Chooruk et al., 2017), and short-chain fatty acids, specifically acetic acid, propionic acid, and butyric acid, have been identified (Thananimit et al., 2022). These substances can destroy bacterial cell walls, lead to bacterial death, and interfere with bacterial adhesion to host cells (Song et al., 2019). While research on postbiotics is still emerging, a few studies have specifically investigated their impact on H. pylori infection (Ma et al., 2023; Yang et al., 2024). In contrast, numerous studies have focused on the effects of probiotics on H. pylori strains. These studies have shown that probiotics such as Lactobacillus helveticus, L. acidophilus, L. brevis, L. casei, L. johnsonii, L. rhamnosus, Bifidobacterium, Streptococcus thermophilus, S. faecalis, Lactococcus, and S. intermedia can inhibit H. pylori growth, alleviate clinical symptoms, enhance the effectiveness of H. pylori eradication therapy, and reduce adverse drug reactions (McFarland et al., 2016; Yang et al., 2024). Although these studies primarily examine probiotics, recent reports suggest that postbiotics may have similar effects against pathogens (Porphyromonas gingivalis, Fusobacterium nucleatum, Salmonella enterica), including H. pylori (Juntarachot et al., 2023; Pahumunto and Teanpaisan, 2023).
A stability test of the mouthwashes was modified to allow storage at 4°C for 6 months, which is applicable to aqueous-based pharmaceutical formulations. After 24 weeks of storage, postbiotic-glycerol monolaurate mouthwashes demonstrated a notable decline in biological activities (anti-H. pylori, anti-biofilm, and cell viability) compared to their baselines. The reduction of biological activities may be the result of a loss of active antimicrobial metabolites, likely due to degradation over time, and the functional integrity of key postbiotic components, such as short-chain fatty acids and anti-microbial proteins, may have declined. These results emphasize the importance of optimizing formulation stability to preserve biological efficacy over extended storage periods.
In conclusion, the present study found that postbiotics-glycerol monolaurate mouthwash effectively reduced H. pylori adhesion to host cells and diminished pro-inflammatory cytokine stimulation. This mouthwash also demonstrated the ability to remove biofilm and regulate cagA expression. Mouthwash containing postbiotics shows promise as a preventative strategy against H. pylori infection and may serve as adjuvant therapy for gastrointestinal disorders linked to H. pylori infection, considering their low toxicity and health benefits. Further research is needed to investigate the impact of postbiotic-glycerol monolaurate mouthwash on reducing H. pylori and its virulence factors in a clinical study. In addition, the formulation should be further optimized to improve its stability during long-term storage, ensuring the preservation of its beneficial properties and therapeutic efficacy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Faculty of Dentistry, Prince of Songkla University (EC6410-066). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RT: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. NP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was granted by the Faculty of Dentistry, Prince of Songkla University.
Acknowledgments
The authors would like to thank the Faculty of Dentistry at Prince of Songkla University for facilitating the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Azizimoghaddam, Y., Kermanpour, S., Mirzaei, N., Houri, H., Nabavi-Rad, A., Aghdaei, H. A., et al. (2023). Genetic diversity of Helicobacter pylori type IV secretion system cagI and cagN genes and their association with clinical diseases. Sci. Rep. 13, 10264. doi: 10.1038/s41598-023-37392-7
Che, J., Shi, J., Fang, C., Zeng, X., Wu, Z., Du, Q., et al. (2024). Elimination of pathogen biofilms via postbiotics from lactic acid bacteria: a promising method in food and biomedicine. Microorganisms 12, 704. doi: 10.3390/microorganisms12040704
Chen, S., Huang, S., Li, Y., and Zhou, C. (2021). Recent advances in epsilon-poly-L-Lysine and L-Lysine-based dendrimer synthesis, modification, and biomedical applications. Front. Chem. 9. doi: 10.3389/fchem.2021.659304
Chey, W. D. and Wong, B. C. (2007). American college of gastroenterology guideline on the management of Helicobacter pylori infection. Am. J. Gastroenterol. 102, 1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x
Chooruk, A., Piwat, S., and Teanpaisan, R. (2017). Antioxidant activity of various oral Lactobacillus strains. J. Appl. Microbiol. 123, 271–279. doi: 10.1111/jam.13482
Ishikawa, K. H., Bueno, M. R., Kawamoto, D., Simionato, M. R. L., and Mayer, M. P. A. (2021). Lactobacilli postbiotics reduce biofilm formation and alter transcription of virulence genes of Aggregatibacter actinomycetemcomitans. Mol. Oral. Microbiol. 36, 92–102. doi: 10.1111/omi.12330
Ivashkin, V., Maev, I., Poluektova, E., Sinitsa, A., Avalueva, E., Mnatsakanyan, M., et al. (2024). Efficacy and safety of postbiotic contained inactivated Lactobacillus reuteri (Limosilactobacillus reuteri) DSM 17648 as adjuvant therapy in the eradication of Helicobacter pylori in adults with functional dyspepsia: A randomized double-blind placebo-controlled trial. Clin. Transl. Gastroenterol. 15, 1–10. doi:10.14309/ctg.0000000000000750
Juntarachot, N., Sunpaweravong, S., Kaewdech, A., Wongsuwanlert, M., Ruangsri, P., Pahumunto, N., et al. (2023). Characterization of adhesion, anti-adhesion, co-aggregation, and hydrophobicity of Helicobacter pylori and probiotic strains. J. Taibah Univ. Med. Sci. 18, 1048–1054. doi: 10.1016/j.jtumed.2023.02.017
Kvalheim, S. F., Xenaki, V., Kvalheim, A., Lie, S. A., Marthinussen, M. C., Strand, G. V., et al. (2019). Effect of glycerol on reconstructed human oral mucosa. Eur. J. Oral. Sci. 127, 19–26. doi: 10.1111/eos.12590
Lin, Y. C., Schlievert, P. M., Anderson, M. J., Fair, C. L., Schaefers, M. M., Muthyala, R., et al. (2009). Glycerol monolaurate and dodecylglycerol effects on Staphylococcus aureus and toxic shock syndrome toxin-1 in vitro and in vivo. PloS One 4, 7499. doi: 10.1371/journal.pone.0007499
Ma, L., Tu, H., and Chen, T. (2023). Postbiotics in human health: a narrative review. Nutrients 15, 291. doi: 10.3390/nu15020291
McFarland, L. V., Huang, Y., Wang, L., and Malfertheiner, P. (2016). Systematic review and meta-analysis: Multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United Eur. Gastroenterol. J. 4, 546–561. doi: 10.1177/2050640615617358
Pahumunto, N., Basic, A., Östberg, A. K., Teanpaisan, R., and Dahlen, G. (2020). Oral Lactobacillus strains reduce cytotoxicity and cytokine release from peripheral blood mononuclear cells exposed to Aggregatibacter actinomycetemcomitans subtypes in vitro. BMC Microbiol. 20, 279. doi: 10.1186/s12866-020-01959-5
Pahumunto, N. and Teanpaisan, R. (2023). Anti-cancer properties of potential probiotics and their cell-free supernatants for the prevention of colorectal cancer: an in vitro study. Probiotics Antimicrob. Proteins 15, 1137–1150. doi: 10.1007/s12602-022-09972-y
Perez-Perez, G. I., Van, T. N., Thu Huong, D., Zhan, G., Nguyet Anh, D., Nguyet, N. T., et al. (2016). Isolation and characterization of Helicobacter pylori recovered from gastric biopsies under anaerobic conditions. Diagn. Microbiol. Infect. Dis. 86, 136–140. doi: 10.1016/j.diagmicrobio.2016.07.009
Pesarico, A. P., Alves de Jesus, G. F., Yuri, N., Córneo, E. D. S., Dias, R., Rocha, L. B., et al. (2023). Effects of repeated low and high dosage postbiotics administration in toxicity and inflammatory responses in mice model. Braspen J. 38, 152–159. doi: 10.37111/braspenj.2023.38.2.07
Poulsen, C., Mehalick, L. A., Fischer, C. L., Lanzel, E. A., Bates, A. M., Walters, K. S., et al. (2015). Differential cytotoxicity of long-chain bases for human oral gingival epithelial keratinocytes, oral fibroblasts, and dendritic cells. Toxicol. Lett. 237, 21–29. doi: 10.1016/j.toxlet.2015.05.012
Salminen, S., Collado, M. C., Endo, A., Hill, C., Lebeer, S., Quigley, E. M. M., et al. (2021). The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649–667. doi: 10.1038/s41575-021-00440-6
Sheu, B. S., Cheng, H. C., Yang, Y. J., Yang, H. P., and Wu, J. J. (2007). The presence of dental disease can be a risk factor for recurrent Helicobacter pylori infection after eradication therapy: a 3year follow-up. Endoscopy 39, 942–947. doi: 10.1055/s-2007-966787
Silva, V. O., Pereira, L. J., Pasetto, S., da Silva, M. P., Meyers, J. C., and Murata, R. M. (2018). Effects of monolaurin on oral microbe-host transcriptome and metabolome. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.02638
Song, H., Zhou, L., Liu, D., Ge, L., and Li, Y. (2019). Probiotic effect on Helicobacter pylori attachment and inhibition of inflammation in human gastric epithelial cells. Exp. Ther. Med. 18, 1551–1562. doi: 10.3892/etm.2019.7742
Thananimit, S., Pahumunto, N., and Teanpaisan, R. (2022). Characterization of short chain fatty acids produced by selected potential probiotic Lactobacillus strains. Biomolecules 12, 1829. doi: 10.3390/biom12121829
Wang, X. M., Yee, K. C., Hazeki-Taylor, N., Li, J., Fu, H. Y., Huang, M. L., et al. (2014). Oral Helicobacter pylori, its relationship to successful eradication of gastric H. pylori and saliva culture confirmation. J. Physiol. Pharmacol. 65, 559–566.
Wang, Y., Wang, X., Cao, X. Y., Zhu, H. L., and Miao, L. (2023). Comparative effectiveness of different probiotics supplements for triple Helicobacter pylori eradication: a network meta-analysis. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1120789
Wanitsuwan, W., Pahumunto, N., Surachat, K., Thananimit, S., Wonglapsuwan, M., Laohawiriyakamol, S., et al. (2024). Comparison of the effects of postbiotics and live-probiotics containing Lacticaseibacillus paracasei SD1 and Lacticaseibacillus rhamnosus SD11 in patients with previous colorectal cancer: A randomized controlled trial. J. Funct. Foods 123, 106576. doi: 10.1016/j.jff.2024.106576
Wannun, P., Piwat, S., and Teanpaisan, R. (2014). Purification and characterization of bacteriocin produced by oral Lactobacillus paracasei SD1. Anaerobe 27, 17–21. doi: 10.1016/j.anaerobe.2014.03.001
Wongsuwanlert, M., Teanpaisan, R., Pahumunto, N., Kaewdech, A., Ruangsri, P., and Sunpaweravong, S. (2024a). Prevalence and virulence factors of Helicobacter pylori isolated from oral cavity of non-disease, gastritis, and gastric cancer patients. J. Dent. Sci. 19, 1036–1043. doi: 10.1016/j.jds.2023.06.024
Wongsuwanlert, M., Teanpaisan, R., Ruangsri, P., Kaewdech, A., Sunpaweravong, S., and Pahumunto, N. (2024b). Effect of mouthwash containing poly L-Lysine and glycerol monolaurate on oral Helicobacter pylori relating to biofilm eradication, anti-adhesion, and pro-inflammatory cytokine suppression. J. Dent. Sci. 19, 1748–1757. doi: 10.1016/j.jds.2023.10.010
Xu, W., Xu, L., and Xu, C. (2022). Relationship between Helicobacter pylori infection and gastrointestinal microecology. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.938608
Yang, C., Liang, L., Lv, P., Liu, L., Wang, S., Wang, Z., et al. (2021). Effects of non-viable Lactobacillus reuteri combining with 14-day standard triple therapy on Helicobacter pylori eradication: A randomized double-blind placebo-controlled trial. Helicobacter 26, e12856. doi: 10.1111/hel.12856
Yang, Z., Zhou, Y., Han, Z., He, K., Zhang, Y., Wu, D., et al. (2024). The effects of probiotics supplementation on Helicobacter pylori standard treatment: an umbrella review of systematic reviews with meta-analyses. Sci. Rep. 14, 10069. doi: 10.1038/s41598-024-59399-4
Yee, J. K. C. (2017). Are the view of Helicobacter pylori colonized in the oral cavity an illusion? Exp. Mol. Med. 49, 1–13. doi: 10.1111/apt.14561
Keywords: postbiotics, mouthwash, derivative compound, glycerol monolaurate, Helicobacter pylori
Citation: Teanpaisan R and Pahumunto N (2025) Postbiotics enhance the efficacy of derivative compound mouthwash against clinical Helicobacter pylori strains. Front. Cell. Infect. Microbiol. 15:1629106. doi: 10.3389/fcimb.2025.1629106
Received: 15 May 2025; Accepted: 27 June 2025;
Published: 23 July 2025.
Edited by:
Ashu Sharma, University at Buffalo, United StatesReviewed by:
Eduardo Costa, Universidade Católica Portuguesa, PortugalMichael Lynch, University at Buffalo, United States
Copyright © 2025 Teanpaisan and Pahumunto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nuntiya Pahumunto, bnVudGl5YS5wQHBzdS5hYy50aA==
 Rawee Teanpaisan
Rawee Teanpaisan Nuntiya Pahumunto
Nuntiya Pahumunto