- 1Department of Biomedical Laboratory Science, College of Medical Sciences, Soonchunhyang University, Asan, Chungnam, Republic of Korea
- 2Department of Medical Sciences, Graduate School, Soonchunhyang University, Asan, Chungnam, Republic of Korea
Candida auris has emerged as a critical nosocomial pathogen that particularly affects immunocompromised and critically ill patients in intensive care units, and it is associated with high mortality. The robust biofilm-forming ability and inherent fluconazole resistance of C. auris pose considerable treatment challenges. Consequently, novel therapeutic strategies are urgently needed to treat fluconazole-resistant C. auris (FRCA). This study investigated the antifungal effect of the combination of fluconazole with fingolimod against FRCA. The antifungal activity of fingolimod and resistance of C. auris to fluconazole were assessed using a minimum inhibitory concentration assay, and their interaction was evaluated through a checkerboard synergy assay. The combination treatment effectively inhibited early biofilm formation and eradicated mature biofilms, as demonstrated by the biofilm inhibition concentration and biofilm eradication concentration assays, respectively. The XTT reduction assay revealed a marked reduction in the metabolic activity of C. auris, which was further corroborated by confocal laser-scanning microscopy. Quantitative polymerase chain reaction revealed the downregulation of key virulence-associated genes, including ERG11 (azole resistance), CDR1 (efflux pump), and KRE6 (extracellular matrix). Collectively, these findings indicate that the combination of fingolimod and fluconazole inhibits biofilm formation, fungal metabolic activity, and virulence-related gene expression. This study suggests that fingolimod could serve as an adjuvant to improve the efficacy of fluconazole against FRCA.
Introduction
Each year, approximately 1.9 million cases of invasive fungal infections are reported globally. Among these, invasive candidiasis accounts for approximately 70% of infections, and these infections are primarily attributable to Candida spp., which are major opportunistic fungal pathogens (Fang et al., 2023). Candida auris was first discovered in Japan in 2009, and it has subsequently emerged as a major invasive pathogen with a global mortality rate of 30%–72% (Pappas et al., 2018; De Gaetano et al., 2024). C. auris colonizes the skin, wounds, and catheter surfaces, leading to catheter-related infections and severe complications in immunocompromised individuals and patients in the intensive care unit (Jeffery-Smith et al., 2018; Arensman et al., 2020). Because of its multidrug resistance, high mortality rate, and outbreak potential, the World Health Organization has classified C. auris as a critical priority pathogen in its Fungal Priority Pathogens List, highlighting the need for global surveillance and action (Kim et al., 2024).
This clinical threat of C. auris is closely linked to its biofilm-forming ability, which is facilitated by its strong adherence to indwelling medical devices such as central venous catheters (Malinovská et al., 2023). Biofilms, structured microbial communities encased in an extracellular matrix, provide protection against antifungal agents and host immune responses (Estivill et al., 2011; Tuon et al., 2023). The high biofilm-forming capacity of C. auris is well established (Kean and Ramage, 2019). This characteristic contributes to its increased resistance by limiting the effectiveness of current antifungal therapies (Horton and Nett, 2020).
Antifungal resistance of C. auris is associated with multiple mechanisms, including mutations in azole resistance genes, efflux pump activity, and extracellular matrix (ECM) production (Kean et al., 2018). Azole-class drugs exert their effect by inhibiting essential enzymes involved in ergosterol biosynthesis, a key component of the fungal cell membrane, thereby impeding its formation. However, mutations in ERG11 reduce the binding affinity of azole drugs, leading to resistance (Kelly et al., 1995).
In addition, efflux pumps play a critical role in expelling toxic compounds, including antifungals, from the cell (Chaabane et al., 2019). Overexpression of CDR1, which encodes an ABC-type efflux pump recognized as a major transporter in Candida species, increases the efflux of antifungals and contributes to multidrug resistance (Rybak et al., 2022). Meanwhile, the ECM is a complex polysaccharide structure that provides structural stability and cellular adhesion within the biofilm. Therefore, the ECM limits the penetration of antifungal agents and enhances biofilm persistence (Mitchell et al., 2016). The expression of KRE6, a gene involved in ECM production, confers additional resistance to osmotic stress and disinfectants commonly used in healthcare settings (Rybak et al., 2022). These mechanisms collectively reduce the efficacy of conventional treatments, highlighting the need for new therapeutic strategies in clinical management.
Fingolimod, a derivative of the immunosuppressive metabolite myriocin, is a Food and Drug Administration-approved treatment for multiple sclerosis (Aktas et al., 2010; Castro-Borrero et al., 2012). A previous study reported that fingolimod exhibits antibacterial effect (Jin et al., 2022). However, a few studies have investigated the antifungal activity of fingolimod against C. auris. Furthermore, its potential as an adjuvant to existing antifungal agents remains largely unexplored. Therefore, we evaluated the antifungal activity of fingolimod and its combined effects with fluconazole against fluconazole-resistant C. auris (FRCA).
Materials and methods
Organism, growth conditions, and reagents
Clinical isolates were obtained from the National Center Collection for Pathogens (Seoul, Korea) and the Korea Biobank Network (Daegu, Korea). All strains were subcultured on Sabouraud dextrose agar (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and then inoculated into Sabouraud dextrose broth (SDB; Becton, Dickinson, and Company) at 37°C. Fingolimod and fluconazole were purchased from TCI (Tokyo, Japan). Both compounds were dissolved in dimethyl sulfoxide (DMSO), and the final DMSO concentration did not exceed 2% in any assay.
Minimum inhibitory concentration assay
MIC assays were performed to assess fluconazole resistance in clinical isolates and evaluate the antifungal efficacy of fingolimod. The assays were conducted using a broth microdilution method, with slight modifications based on the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines. Fungal suspensions (1 × 106 CFU/mL) were treated with fingolimod, dispensed into 96-well plates (BD Falcon™, Becton, Dickenson and Company), and incubated at 37°C for 24 h. Absorbance was measured at 600 nm using a Multiskan™ GO Microplate Reader (Thermo Fisher Scientific, Waltham, MA, USA). The MIC was defined as the lowest concentration that inhibited ≥98% of fungal growth compared with the untreated control.
Checkerboard synergy assay
The synergistic effect of fluconazole and fingolimod against FRCA was evaluated using a checkerboard assay with a 2-fold serial dilution method (Kim and Eom, 2021a). Fluconazole (16–256 µg/mL) and fingolimod (0.125–16 µg/mL) were distributed in ascending concentrations along the rows and columns of a 96-well plate to create combination matrices, with the highest concentration corresponding to their respective MICs. After incubation at 37°C for 24 h, absorbance was measured at 600 nm using a Multiskan™ GO Microplate Reader. The fractional inhibitory concentrations (FICs) of fluconazole and fingolimod were calculated using the following formulas: FIC of fluconazole = MIC of fluconazole in combination/MIC of fluconazole alone; and FIC of fingolimod = MIC of fingolimod in combination/MIC of fingolimod alone. The sum of the individual FICs was defined as the fractional inhibitory concentration index (FICI). Based on the FICI, the interactions were classified as synergistic (FICI ≤ 0.5), indifferent (0.5 < FICI ≤ 4.0), or antagonistic (FICI > 4.0).
Biofilm inhibition concentration assay
The BIC assay was performed to evaluate the combined inhibitory effect of fluconazole and fingolimod on early biofilm formation by FRCA (Kim and Eom, 2021b). Fungal suspensions (1 × 106 CFU/mL), treated with the drugs alone (16 µg/mL fluconazole, 2 µg/mL fingolimod) or in combination, were dispensed into 96-well plates and incubated at 37°C for 24 h. After incubation, the supernatant was removed, and each well was washed twice with phosphate-buffered saline (PBS). The plate was then dried in a 60°C oven for 1 h to fix the biofilm, followed by staining with 0.5% crystal violet for 7 min. The excess stain was removed by washing with distilled water, and the plate was further dried for 30 min. Subsequently, 30% acetic acid was added to each well, followed by incubation at room temperature for 20 min to solubilize the biofilm. Finally, absorbance was measured at 595 nm to assess biofilm inhibition.
Biofilm eradication concentration assay
The BEC assay was conducted to evaluate the combined ability of fluconazole and fingolimod to eradicate preformed biofilms of FRCA (Kim et al., 2019). Untreated fungal suspensions (1 × 106 CFU/mL) were dispensed into 96-well plates and incubated at 37°C for 24 h to permit biofilm formation. After incubation, the supernatant was removed, and each well was washed twice with PBS. The preformed biofilms were then treated with the drugs alone (16 µg/mL fluconazole, 2 µg/mL fingolimod) or in combination for 24 h. Biofilm staining was performed using the same protocol described for the BIC assay.
XTT reduction assay
The metabolic activity of fungal cells within the biofilm was quantitatively assessed using the XTT reduction assay (Kim et al., 2020). Untreated fungal suspensions (1 × 106 CFU/mL) were dispensed into 96-well plates and incubated at 37°C for 24 h. After incubation, the supernatant was removed, and each well was washed twice with PBS. The preformed biofilms were then treated with the drugs alone (16 µg/mL fluconazole, 2 µg/mL fingolimod) or in combination, followed by an additional 24-h incubation. Subsequently, the wells were washed twice with PBS, and 50 µL of the XTT reagent mixture were added to each well. The plates were incubated for 3 h, and absorbance was measured at 475 nm and 660 nm using a Multiskan™ GO Microplate Reader.
Confocal laser-scanning microscopy
The metabolic activity within the biofilm was visually assessed using CLSM. Fungal suspensions (1 × 106 CFU/mL) were dispensed into 24-well glass-bottom imaging plates and incubated at 37°C for 24 h to permit biofilm formation (Kim et al., 2022). Following incubation, the supernatant was removed, and the plate was washed twice with PBS. The preformed biofilms were then treated with either fingolimod (2 µg/mL) or fluconazole (16 µg/mL) alone or both drugs together for 24 h. Subsequently, the wells were washed twice with 0.85% NaCl, and the biofilms were stained using the LIVE/DEAD™ BacLight™ Bacterial Viability Kit (Thermo Fisher Scientific). Live cells were stained with SYTO9, and dead cells were stained with propidium iodide. Imaging was conducted at ×40 magnification, with fluorescence measured at 525 nm for SYTO9 and 640 nm for propidium iodide. The relative fluorescence intensity was quantified using ImageJ (RRID: SCR_003070) software.
RNA isolation and quantitative polymerase chain reaction
For the transcriptomic analysis of FRCA, total RNA was extracted following treatment. Fungal suspensions (1 × 106 CFU/mL) were inoculated into SDB and incubated at 37°C for 10 h. Subsequently, subinhibitory concentrations of the drugs in combination were administered, followed by incubation for 18 h. After incubation, the cultures were centrifuged at 10,000 rpm for 15 min at 4°C to collect the cell pellets. RNA extraction and purification were performed using the NucleoSpin RNA Mini Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. The purity and concentration of the extracted RNA were assessed using a μDrop™ plate (Thermo Fisher Scientific).
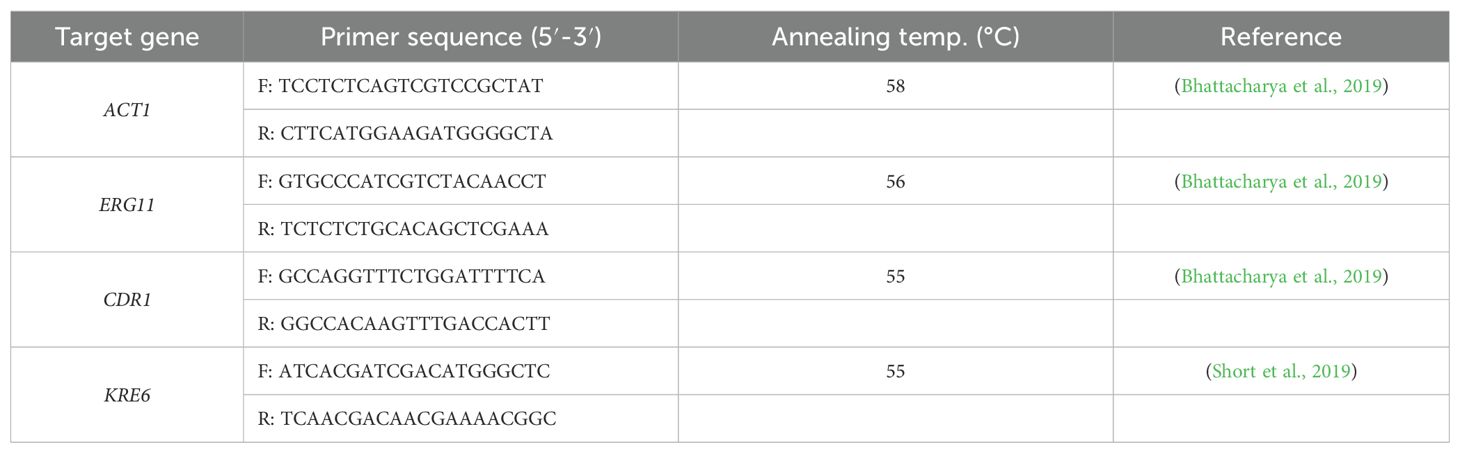
The effects of treatment on the expression of virulence-related genes in FRCA were evaluated using qPCR. cDNA was synthesized using ReverTraAce qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan) following the manufacturer’s protocol. qPCR was conducted using TOPreal™ qPCR 2× PreMIX (Enzynomics, Daejeon, Korea) on a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). ACT1 was used as the endogenous control gene. The qPCR thermal cycling conditions included initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 15 s, and extension at 72°C for 30 s. Melting curve analysis was performed at the end of the amplification under the following conditions: 95°C for 15 s, 58°C for 1 min, and 95°C for 15 s (Jin and Eom, 2025). Relative gene expression was normalized to ACT1 expression and calculated using the 2−ΔΔCT method. The primer sequences and annealing temperatures used in the analysis are listed in Table 1.
Statistical analysis
All analytical data are presented as the mean ± standard deviation, and statistical significance compared with the control group was evaluated using one-way ANOVA. Graphs were generated using GraphPad Prism version 10 (GraphPad Prism, Boston, MA, USA).
Results
MIC of clinical C. auris isolates
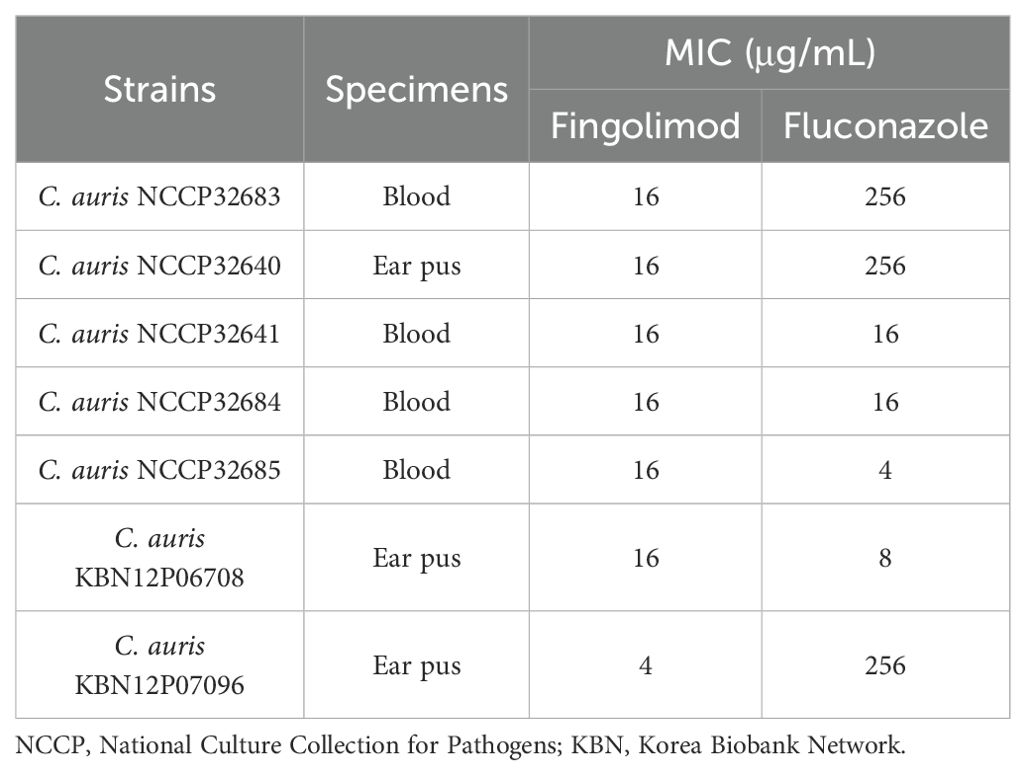
Fluconazole resistance and the antifungal activity of fingolimod were evaluated in clinical C. auris isolates (Table 2). Fingolimod exhibited strong antifungal activity, inhibiting fungal growth by more than 98% at concentrations of 4 and 16 µg/mL. The MICs of fluconazole ranged from 4 to 256 µg/mL, exhibiting considerable variability. According to the EUCAST guidelines, C. auris NCCP32683, C. auris NCCP32640, and C. auris KBN12P07096 were classified as fluconazole-resistant strains.
Checkerboard assay of fluconazole and fingolimod
A checkerboard assay was conducted to investigate the combined effect of fluconazole and fingolimod against FRCA strains (Table 3). Synergy was defined as inhibition of fungal growth by ≥90% by combinations of subinhibitory concentrations. Fluconazole and fingolimod displayed synergy at concentrations of 16 µg/mL + 2 µg/mL for C. auris NCCP32640, 16 µg/mL + 4 µg/mL for C. auris NCCP32683, and 16 µg/mL + 1 µg/mL for C. auris KBN12P07096. The FICIs for these combinations were 0.1875, 0.3125, and 0.3125, respectively, including synergy. Based on these results, the biofilm assay was performed using C. auris NCCP32640, which had the lowest FICI.
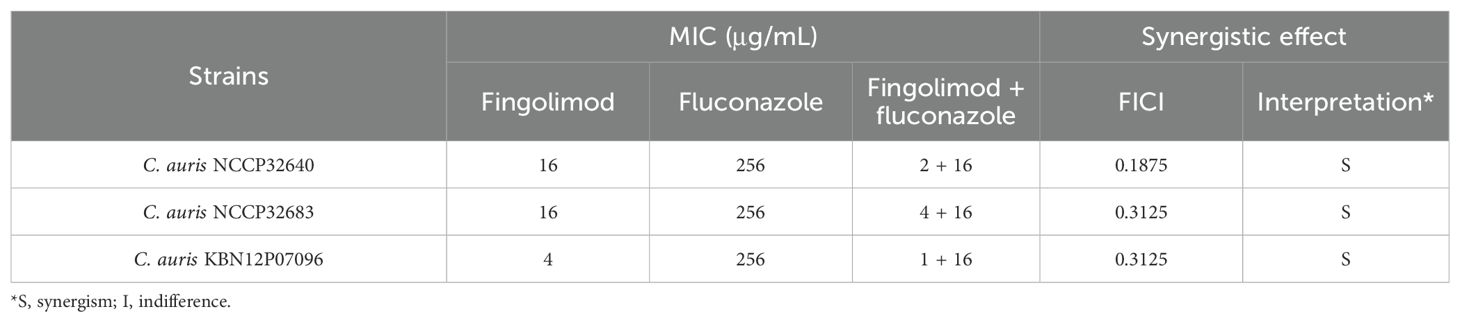
Table 3. Synergistic effects of fingolimod and fluconazole against fluconazole-resistant C. auris strains.
The inhibitory effect of fluconazole and fingolimod on early biofilm formation by FRCA
The inhibitory effect of the synergistic combination of fluconazole and fingolimod on early biofilm formation was evaluated using C. auris NCCP32640 (Figure 1). Individual treatments with fluconazole and fingolimod were administered at the concentrations used in the synergistic combination. When applied alone, fluconazole and fingolimod inhibited biofilm formation by approximately 17% and 33%, respectively. By contrast, their combination significantly inhibited early biofilm formation by up to 78%.
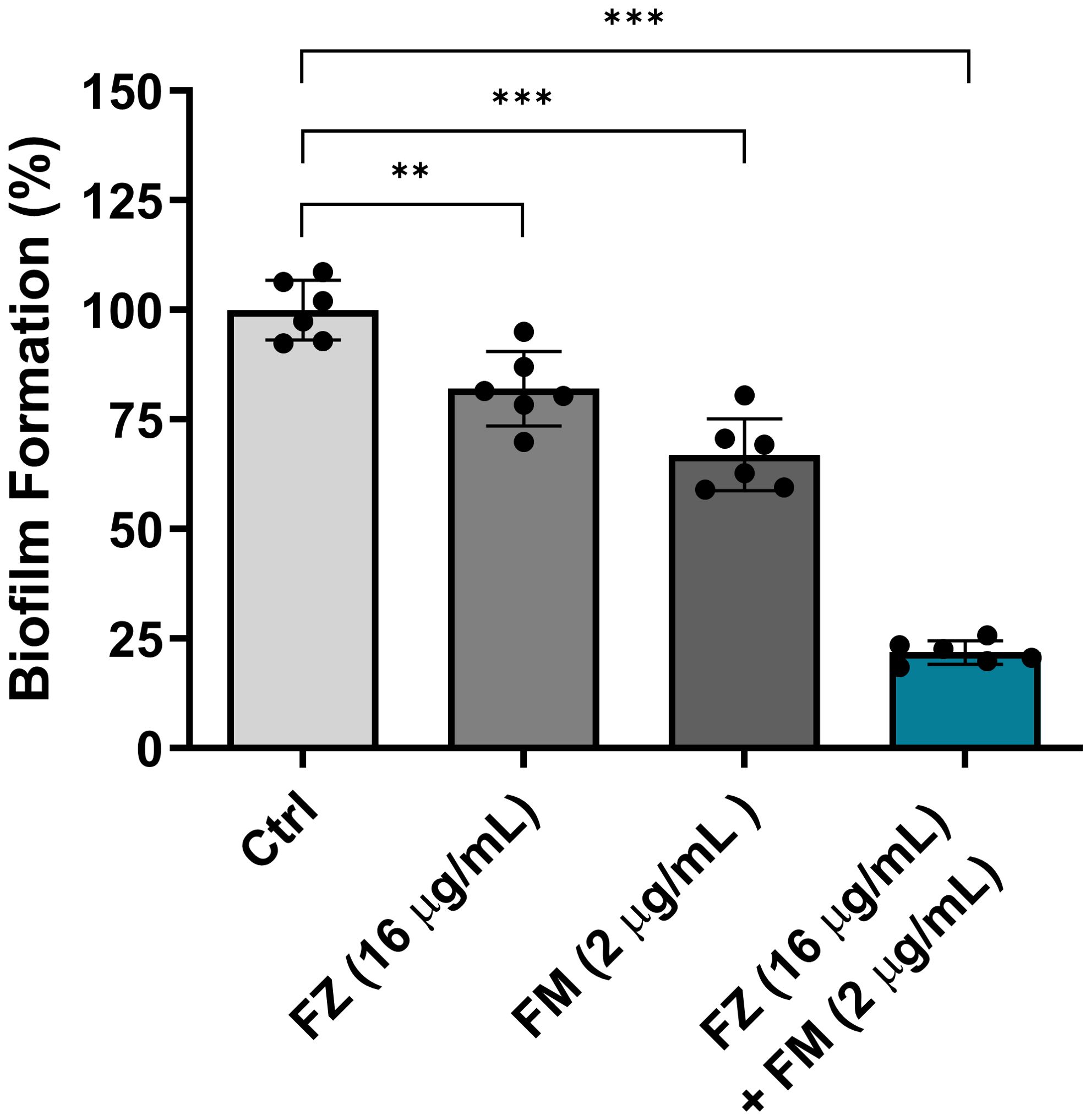
Figure 1. Inhibitory effect of fluconazole (FZ) and fingolimod (FM) on biofilm formation by C. auris NCCP32640. Biofilm formation was evaluated following treatment with FM and/or FZ. The biofilms were stained with 0.5% crystal violet, and absorbance was measured at 595 nm. Positive control (Ctrl) contains 2% DMSO. Statistical significance was assessed relative to the untreated control, with significant differences indicated by asterisks (**p ≤ 0.01, ***p ≤ 0.001). Data are presented as the mean ± standard deviation (SD), and error bars represent the SD.
The eradication effect of fluconazole and fingolimod on the mature biofilms of FRCA
The efficacy of the synergistic combination of fluconazole and fingolimod in eradicating the preformed biofilms of C. auris NCCP32640 was evaluated (Figure 2). Individual treatments with fluconazole and fingolimod were applied at concentrations used in their synergistic combination. When applied separately, fluconazole and fingolimod achieved inhibition rates of approximately 11% and 26%, respectively. However, combination treatment eradicated approximately 85% of the preformed biofilms.
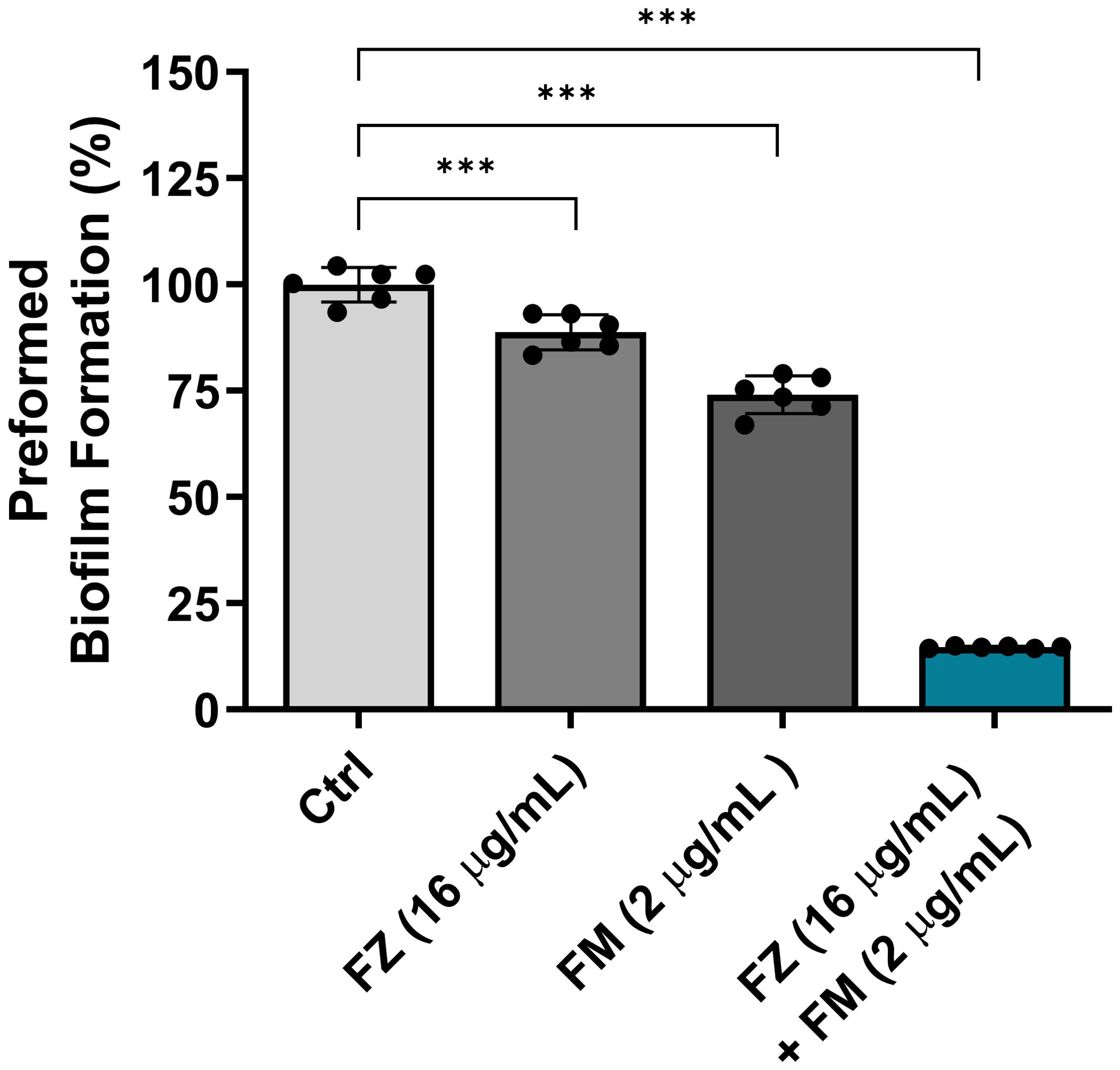
Figure 2. Eradication effect of fluconazole (FZ) and fingolimod (FM) on preformed biofilms of C. auris NCCP32640. The fungal suspensions were incubated for 24 h to allow biofilm formation, washed with PBS and treated with FZ and/or FM. The remaining biofilms were stained with 0.5% crystal violet, and absorbance was measured at 595 nm. Positive control (Ctrl) contains 2% DMSO. Statistical significance was assessed relative to the untreated control, with significant differences indicated by asterisks (***p ≤ 0.001). Data are presented as the mean ± standard deviation (SD), and error bars represent the SD.
The effect of the synergistic combination on the metabolic activity of FRCA
We confirmed that fluconazole and fingolimod inhibited the metabolic activity of fungal cells within the biofilms (Figure 3). When used individually, fluconazole and fingolimod inhibited the metabolic activity of FRCA by approximately 20% and 32%, respectively. In comparison, when applied in combination, a significant reduction in metabolic activity of approximately 66% was observed.
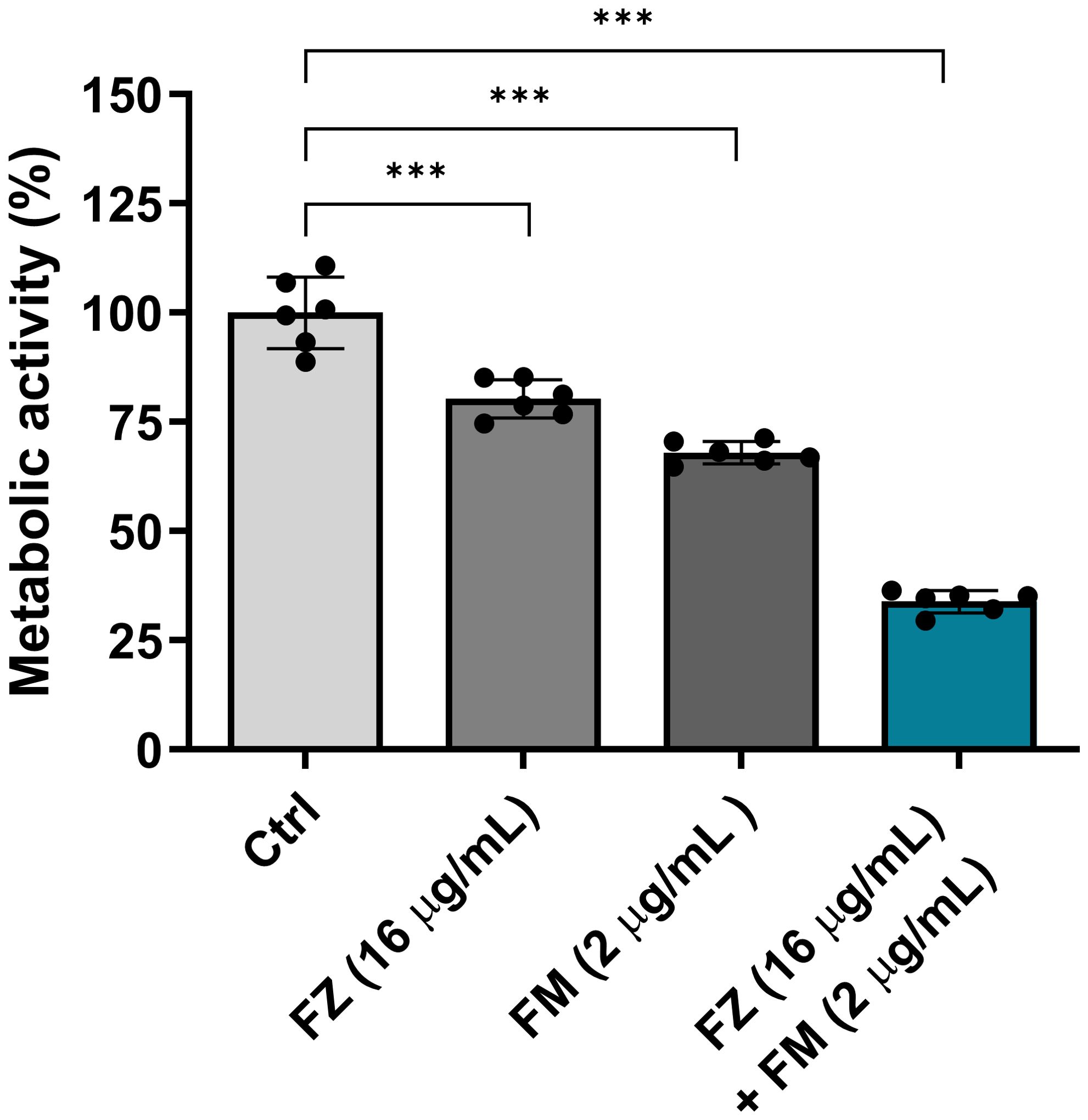
Figure 3. Antifungal effect of fluconazole (FZ) and fingolimod (FM) on the metabolic activity of C. auris NCCP32640 in biofilms. Metabolic activity was quantitatively analyzed by measuring the color changes resulting from the reduction of XTT, which reflects cell viability. Absorbance was measured at 475 and 660 nm, and values were calculated using the following formula: A475 nm (test) − A475 nm (blank) − A660 nm (test). A decrease in absorbance indicates reduced metabolic activity in C. auris and an enhanced antifungal effect. Positive control (Ctrl) contains 2% DMSO. Statistical significance was assessed relative to the untreated control, with significant differences indicated by asterisks (***p ≤ 0.001). Data are presented as the mean ± standard deviation (SD), and error bars represent the SD.
Visualization of the metabolic activity of fungal cells within biofilms
As presented in Figure 4A, strong green fluorescence, indicating living cells, was observed in the control group, whereas an increase in red fluorescence, which is associated with dead cells, was detected for the synergistic combination. Fluorescence analysis (Figure 4B) further confirmed that the combination treatment increased the number of dead cells and decreased the number of live cells compared with the findings in the control group.
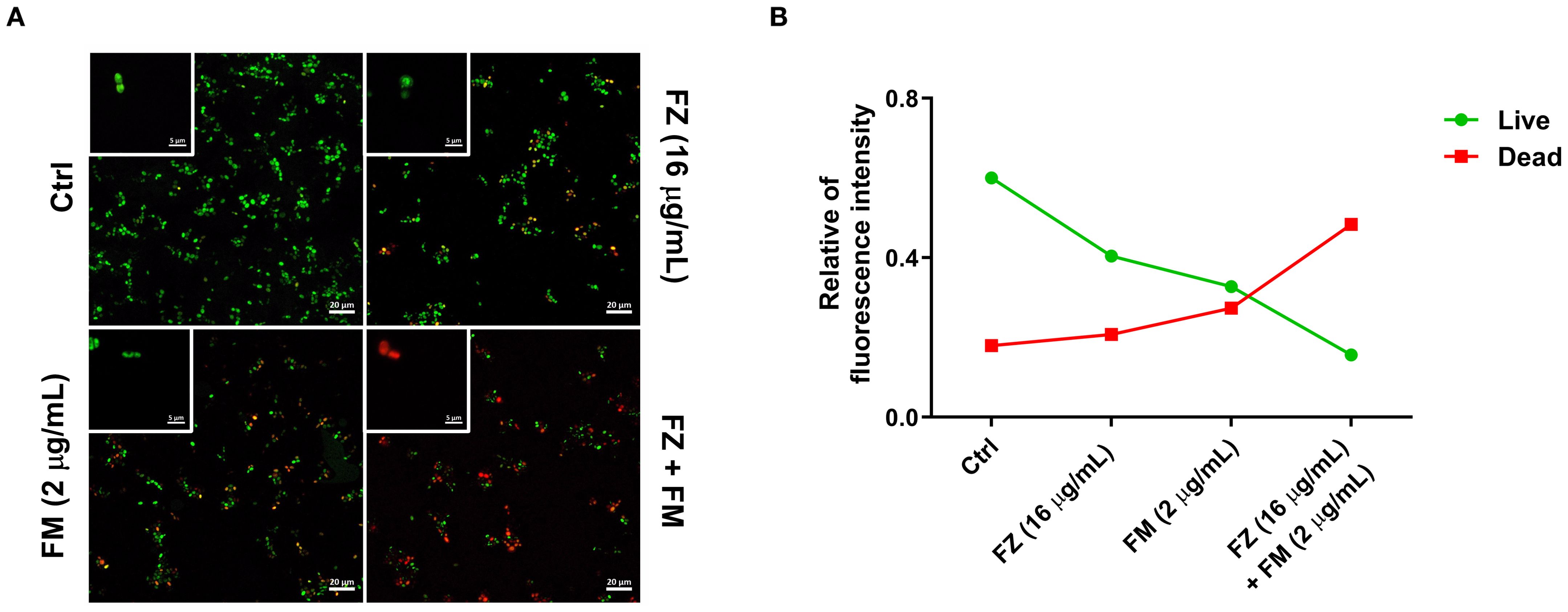
Figure 4. (A) CLSM images presenting cell viability within C. auris NCCP32640 biofilms after treatment with fluconazole (FZ) and fingolimod (FM). Live cells (green) and dead cells (red) were distinguished using LIVE/DEAD staining with SYTO9 and propidium iodide, respectively. Images were captured at ×40 and ×1000 magnification (scale bar, 20 µm). (B) Quantitative analysis of the relative fluorescence intensity comparing the ratio of live cells to dead cells based on the CLSM images.
Expression of virulence-related genes in FRCA
qPCR was conducted to examine the changes in the expression of virulence-related genes in C. auris NCCP32640 after treatment with fluconazole and fingolimod (Figure 5). The synergistic combination considerably reduced the expression of the azole resistance gene ERG11 by approximately 75% compared to the control group. Additionally, the expression of the efflux pump gene CDR1 and ECM-associated gene KRE6 was suppressed by approximately 81% and 87%, respectively. These results suggest that the combination of fluconazole and fingolimod effectively downregulates key virulence-related genes.
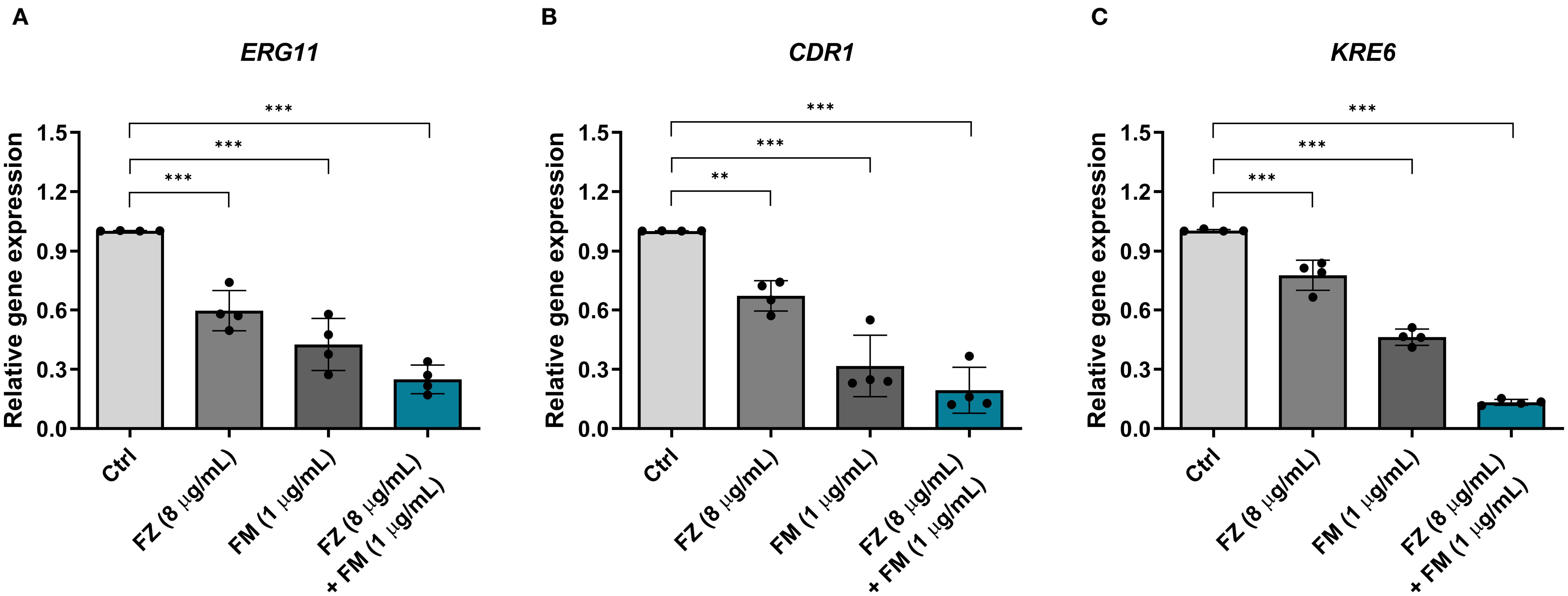
Figure 5. Relative expression of virulence-related genes in C. auris NCCP32640 treated with subinhibitory concentrations of fluconazole (FZ) and fingolimod (FM). The relative expression of (A) the azole resistance gene ERG11, (B) efflux pump gene CDR1, and (C) ECM-associated gene KRE6 was analyzed. The expression of target genes was normalized to the expression of ACT1 as an internal control. Positive control (Ctrl) contains 2% DMSO. Asterisks (*) indicate statistically significant differences compared with the control (**p < 0.01, ***p < 0.001). Data are presented as the mean ± standard deviation (SD), and error bars represent the SD.
Discussion
C. auris is a major invasive fungal pathogen responsible for invasive candidiasis because of its strong biofilm-forming ability, leading high mortality rates (Lockhart, 2019; Du et al., 2020). This issue is particularly critical in healthcare settings, in which the limitations of current treatment options have become increasingly apparent, underscoring the urgent need for novel therapeutic strategies (Cristina et al., 2023). Fingolimod, a clinically approved drug for the treatment of multiple sclerosis, has a well-established safety profile. Repurposing already approved therapeutics could offer a more efficient approach than developing entirely new drugs (Gilbert-Girard et al., 2020).
Fingolimod has been reported to exhibit antibacterial and antifungal activities, with effective concentrations of 3.125 µg/mL against Staphylococcus aureus, 4 μg/mL against Clostridium perfringens, and 0.25 mg/mL (MIC99) against C. albicans (Rumah et al., 2017; Najarzadegan et al., 2022; Shang et al., 2024). However, antifungal research related to C. auris remains limited. Previous studies have demonstrated that fingolimod did not cause cytotoxicity in the normal human colon mucosal epithelial cell line (NCM460) at concentrations below 11 mg/L (Wei et al., 2021). In this study, fingolimod exhibited antifungal activity at concentrations of 4 and 16 μg/mL against clinical C. auris isolates obtained from patients (Table 2). These findings suggest that fingolimod exhibits not only antibacterial effects but also antifungal effects, even at concentrations similar to or significantly lower than those used in previous studies.
Numerous studies have reported that various drugs can serve as adjuvants to fluconazole. In one study, the MIC of fluconazole for C. albicans remained at 0.5 μg/mL even when combined with the calcineurin inhibitor cyclosporine. However, the researchers introduced a new concept, “MIC-0,” to show a strong synergistic effect with the combinations of fluconazole (0.5 μg/mL) and cyclosporine (0.625 μg/mL) (Marchetti et al., 2000). Another study reported that the combination of fluconazole (32 μg/mL) and cyclosporine A (75 μg/mL) significantly inhibited C. albicans biofilm growth and suppressed the expression of resistance-associated genes (CDR1, MDR1, ERG11) (Jia et al., 2016). Similarly, the combination of the fluconazole (1 μg/mL) and Hsp90 inhibitor ganetespib (0.5 μg/mL) exhibited remarkable antifungal activity against azole-resistant C. albicans. At higher concentrations (8 μg/mL each), this combination further downregulated the expression of key resistance genes (CDR1, CDR2, MDR1, ERG11) (Yuan et al., 2021).
Based on this, we investigated whether fingolimod and fluconazole can exert synergistic effects. A synergistic effect was observed with the combination of 1–4 μg/mL fingolimod and 16 μg/mL fluconazole against FRCA (Table 3). In a previous study, a synergistic effect against C. albicans was reported for fingolimod combined with amphotericin B, resulting in approximately 80% growth inhibition (Wei et al., 2021). By contrast, our study demonstrated a more potent inhibitory effect, with C. auris growth reduced by more than 90% at concentrations 2–4-fold lower than the individual MICs of the two drugs. Considering that fluconazole is a first-line treatment for fungal infections, the combined use of fluconazole and fingolimod might have significant therapeutic implications in treating drug-resistant C. auris infections.
The robust biofilm formation of C. auris is associated with over 80% resistance to fluconazole, highlighting biofilms as an important therapeutic target (Sati et al., 2022). Accordingly, we assessed the antibiofilm effects of the synergistic combination using BIC and BEC assays. Previous studies reported that 50 μM fingolimod inhibits biofilm formation and reduces biofilm thickness in Pseudomonas aeruginosa (Niazy et al., 2024). In this study, the combination therapy suppressed biofilm formation by 78% and effectively eradicated mature biofilms by 85%. (Figures 1, 2). These findings support the notion that fingolimod has the potential to disrupt biofilms in both bacterial and fungal pathogens.
Crystal violet, used in BIC/BEC assays, both viable and non-viable cells as well as the ECM. Although this enables quantification of the total biofilm biomass, it does not accurately reflect cell viability (Xu et al., 2016). Therefore, we employed an XTT reduction assay to specifically assess the metabolic activity of the living cells within the biofilm. The results revealed a significant reduction in metabolic activity following treatment with the synergistic combination, which was further supported by CLSM (Figures 3, 4). These findings indicate a fungicidal effect on FRCA with combination treatment as opposed to perhaps fungistasis in the singular treatments.
The antifungal resistance of C. auris is attributed to the interplay of multiple resistance mechanisms. Accordingly, we conducted qPCR analysis to assess the effect of combination therapy on the expression of key virulence-associated genes implicated in resistance, namely ERG11, CDR1, and KRE6 (Figure 5). The combination treatment significantly decreased the expression of all three genes, with the most pronounced reduction observed for KRE6. These findings suggest that the synergistic combination disrupts both structural and functional resistance mechanisms by reducing the expression of resistance-related genes.
However, as this study was conducted in vitro, it does not fully reflect the complex architecture of the biofilms formed in vivo. Therefore, additional in vivo studies are required to validate the clinical applicability of this combination therapy, its long-term efficacy within the host, and the potential for resistance development. This study did not elucidate the molecular pathways underlying fingolimod’s antifungal synergy or the mechanistic modeling by which it enhances fluconazole efficacy, underscoring the need for further investigation, including evaluation of its synergistic activity with other antifungals such as amphotericin B, particularly against the highly resistant C. auris.
Conclusion
This study provides experimental evidence supporting the synergistic effect of fingolimod and fluconazole against FRCA. We demonstrated that the combination therapy exhibits antibiofilm effects, inhibits fungal metabolic activity, and decreases virulence-related gene expression. These findings suggest a promising approach for developing therapeutic strategies to address the limitations of current antifungal treatments.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Requests to access the datasets should be directed to Y-BE, b21uaWJpbkBzY2guYWMua3I=.
Author contributions
J-HB: Conceptualization, Data curation, Investigation, Validation, Visualization, Writing – original draft. Y-BE: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Soonchunhyang University research fund and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (RS-2023-NR076438 (NRF-2023R1A2C1003486)).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
FRCA, fluconazole-resistant C. auris; ECM, extracellular matrix; MIC, minimum inhibitory concentration; EUCAST, European Committee on Antimicrobial Susceptibility Testing; FIC, fractional inhibitory concentration; FICI, fractional inhibitory concentration index; BIC, biofilm inhibition concentration; BEC, biofilm eradication concentration; PBS, phosphate-buffered saline; CLSM, confocal laser-scanning microscopy; qPCR, quantitative polymerase chain reaction.
References
Aktas, O., Küry, P., Kieseier, B., and Hartung, H. P. (2010). Fingolimod is a potential novel therapy for multiple sclerosis. Nat. Rev. Neurol. 6, 373–382. doi: 10.1038/nrneurol.2010.76
Arensman, K., Miller, J. L., Chiang, A., Mai, N., Levato, J., LaChance, E., et al. (2020). Clinical outcomes of patients treated for Candida auris infections in a multisite health system, Illinois, USA. Emerg. Infect. Dis. 26, 876–880. doi: 10.3201/eid2605.191588
Bhattacharya, S., Holowka, T., Orner, E. P., and Fries, B. C. (2019). Gene duplication associated with increased fluconazole tolerance in Candida auris cells of advanced generational age. Sci. Rep. 9, 5052. doi: 10.1038/s41598-019-41513-6
Castro-Borrero, W., Graves, D., Frohman, T. C., Flores, A. B., Hardeman, P., Logan, D., et al. (2012). Current and emerging therapies in multiple sclerosis: a systematic review. Ther. Adv. Neurol. Disord. 5, 205–220. doi: 10.1177/1756285612450936
Chaabane, F., Graf, A., Jequier, L., and Coste, A. T. (2019). Review on antifungal resistance mechanisms in the emerging pathogen Candida auris. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02788
Cristina, M. L., Spagnolo, A. M., Sartini, M., Carbone, A., Oliva, M., Schinca, E., et al. (2023). An overview on Candida auris in healthcare settings. J. Fungi 9, 913. doi: 10.3390/jof9090913
De Gaetano, S., Midiri, A., Mancuso, G., Avola, M. G., and Biondo, C. (2024). Candida auris outbreaks: current status and future perspectives. Microorganisms 12, 927. doi: 10.3390/microorganisms12050927
Du, H., Bing, J., Hu, T., Ennis, C. L., Nobile, C. J., and Huang, G. (2020). Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PloS Pathog. 16, e1008921. doi: 10.1371/journal.ppat.1008921
Estivill, D., Arias, A., Torres-Lana, A., Carrillo-Muñoz, A., and Arévalo, M. (2011). Biofilm formation by five species of Candida on three clinical materials. J. Microbiol. Methods 86, 238–242. doi: 10.1016/j.mimet.2011.05.019
Fang, W., Wu, J., Cheng, M., Zhu, X., Du, M., Chen, C., et al. (2023). Diagnosis of invasive fungal infections: challenges and recent developments. J. Biomed. Sci. 30, 42. doi: 10.1186/s12929-023-00926-2
Gilbert-Girard, S., Savijoki, K., Yli-Kauhaluoma, J., and Fallarero, A. (2020). Screening of FDA-approved drugs using a 384-well plate-based biofilm platform: The case of fingolimod. Microorganisms 8, 1834. doi: 10.3390/microorganisms8111834
Horton, M. V. and Nett, J. E. (2020). Candida auris infection and biofilm formation: going beyond the surface. Curr. Clin. Microbiol. 7, 51–56. doi: 10.1007/s40588-020-00143-7
Jeffery-Smith, A., Taori, S. K., Schelenz, S., Jeffery, K., Johnson, E. M., Borman, A., et al. (2018). Candida auris: a review of the literature. Clin. Microbiol. Rev. 31, e00029-17. doi: 10.1128/cmr.00029-17
Jia, W., Zhang, H., Li, C., Li, G., Liu, X., and Wei, J. (2016). The calcineruin inhibitor cyclosporine a synergistically enhances the susceptibility of Candida albicans biofilms to fluconazole by multiple mechanisms. BMC Microbiol. 16, 113. doi: 10.1186/s12866-016-0728-1
Jin, H. W. and Eom, Y. B. (2025). Potent anti-biofilm properties of plumbagin against fluconazole-resistant Candida auris. J. Tradit. Complement. Med. 15, 140–146. doi: 10.1016/j.jtcme.2024.06.005
Jin, H. W., Kim, H. R., and Eom, Y. B. (2022). Fingolimod promotes antibacterial effect of doripenem against carbapenem-resistant Escherichia coli. Antibiotics (Basel) 11, 1043. doi: 10.3390/antibiotics11081043
Kean, R., Delaney, C., Sherry, L., Borman, A., Johnson, E. M., Richardson, M. D., et al (2018). Transcriptome assembly and profiling of Candida auris reveals novel insights into biofilm-mediated resistance. mSphere 3, e00334–e00318. doi: 10.1128/msphere.00334-18
Kean, R. and Ramage, G. (2019). Combined antifungal resistance and biofilm tolerance: the global threat of Candida auris. mSphere 4, e00458–e00419. doi: 10.1128/mSphere.00458-19
Kelly, S. L., Lamb, D. C., Corran, A. J., Baldwin, B. C., and Kelly, D. E. (1995). Mode of action and resistance to azole antifungals associated with the formation of 14α-methylergosta-8, 24 (28)-dien-3β, 6α-diol. Biochem. Biophys. Res. Commun. 207, 910–915. doi: 10.1006/bbrc.1995.1272
Kim, H. R. and Eom, Y. B. (2021a). Synergistic activity of equol and meropenem against carbapenem-resistant Escherichia coli. Antibiotics (Basel) 10, 161. doi: 10.3390/antibiotics10020161
Kim, H. R. and Eom, Y. B. (2021b). Antifungal and anti-biofilm effects of 6-shogaol against Candida auris. J. Appl. Microbiol. 130, 1142–1153. doi: 10.1111/jam.14870
Kim, H. R., Han, M. S., and Eom, Y. B. (2022). Anti-bacterial and anti-biofilm effects of equol on yersinia enterocolitica. Indian J. Microbiol. 62, 401–410. doi: 10.1007/s12088-022-01020-1
Kim, H. Y., Nguyen, T. A., Kidd, S., Chambers, J., Alastruey-Izquierdo, A., Shin, J. H., et al. (2024). Candida auris—A systematic review to inform the world health organization fungal priority pathogens list. Med. Mycol. 62, myae042. doi: 10.1093/mmy/myae042
Kim, H. R., Rhee, K. J., and Eom, Y. B. (2019). Anti-biofilm and antimicrobial effects of zerumbone against Bacteroides fragilis. Anaerobe 57, 99–106. doi: 10.1016/j.anaerobe.2019.04.001
Kim, H. R., Shin, D. S., Jang, H. I., and Eom, Y. B. (2020). Anti-biofilm and anti-virulence effects of zerumbone against Acinetobacter baumannii. Microbiology 166, 717–726. doi: 10.1099/mic.0.000930
Lockhart, S. R. (2019). Candida auris and multidrug resistance: defining the new normal. Fungal Genet. Biol. 131, 103243. doi: 10.1016/j.fgb.2019.103243
Malinovská, Z., Čonková, E., and Váczi, P. (2023). Biofilm formation in medically important Candida species. J. Fungi 9, 955. doi: 10.3390/jof910095
Marchetti, O., Moreillon, P., Glauser, M. P., Bille, J., and Sanglard, D. (2000). Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 44, 2373–2381. doi: 10.1128/aac.44.9.2373-2381.2000
Mitchell, K. F., Zarnowski, R., and Andes, D. R. (2016). The extracellular matrix of fungal biofilms. Adv. Exp. Med. Biol. 931, 21–35. doi: 10.1007/5584_2016_6
Najarzadegan, N., Madani, M., Etemadifar, M., and Sedaghat, N. (2022). Immunomodulatory drug fingolimod (FTY720) restricts the growth of opportunistic yeast Candida albicans in vitro and in a mouse candidiasis model. PloS One 17, e0278488. doi: 10.1371/journal.pone.0278488
Niazy, A. A., Lambarte, R. N. A., Sumague, T. S., Vigilla, M. G. B., Bin Shwish, N. M., Kamalan, R., et al. (2024). FTY720 reduces the biomass of biofilms in pseudomonas aeruginosa in a dose-dependent manner. Antibiotics (Basel) 13, 621. doi: 10.3390/antibiotics13070621
Pappas, P. G., Lionakis, M. S., Arendrup, M. C., Ostrosky-Zeichner, L., and Kullberg, B. J. (2018). Invasive candidiasis. Nat. Rev. Dis. Primers 4, 1–20. doi: 10.1038/nrdp.2018.26
Rumah, K. R., Vartanian, T. K., and Fischetti, V. A. (2017). Oral multiple sclerosis drugs inhibit the in vitro growth of epsilon toxin producing gut bacterium, Clostridium perfringens. Front. Cell. Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00011
Rybak, J. M., Cuomo, C. A., and Rogers, P. D. (2022). The molecular and genetic basis of antifungal resistance in the emerging fungal pathogen Candida auris. Curr. Opin. Microbiol. 70, 102208. doi: 10.1016/j.mib.2022.102208
Sati, H., Alastruey-Izquierdo, A., Beardsley, J., Morrissey, O., Alffenaar, J. W., Howard, K., et al (2022). WHO fungal priority pathogens list to guide research, development and public health action. Geneva, World Health Organization.
Shang, Y., Huang, Y., Meng, Q., Yu, Z., Wen, Z., and Yu, F. (2024). Targeting Phospholipids: Fingolimod’s Antibacterial Mechanism Against Staphylococcus aureus. Available online at: https://doi.org/10.21203/rs.3.rs-5223352/v1 (Accessed October 18, 2024).
Short, B., Brown, J., Delaney, C., Sherry, L., Williams, C., Ramage, G., et al. (2019). Candida auris exhibits resilient biofilm characteristics in vitro: implications for environmental persistence. J. Hosp. Infect. 103, 92–96. doi: 10.1016/j.jhin.2019.06.006
Tuon, F. F., Suss, P. H., Telles, J. P., Dantas, L. R., Borges, N. H., and Ribeiro, V. S. T. (2023). Antimicrobial treatment of Staphylococcus aureus biofilms. Antibiotics (Basel) 12, 87. doi: 10.3390/antibiotics12010087
Wei, L. Q., Tan, J. C., Wang, Y., Mei, Y. K., Xue, J. Y., Tian, L., et al. (2021). Fingolimod potentiates the antifungal activity of amphotericin B. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.627917
Xu, Z., Liang, Y., Lin, S., Chen, D., Li, B., Li, L., et al. (2016). Crystal violet and XTT assays on Staphylococcus aureus biofilm quantification. Curr. Microbiol. 73, 474–482. doi: 10.1007/s00284-016-1081-1
Keywords: fingolimod, antifungal, anti-biofilm, synergistic effect, fluconazole-resistant Candida auris
Citation: Bae J-H and Eom Y-B (2025) Fingolimod potentiates the antifungal activity of fluconazole against fluconazole-resistant Candida auris. Front. Cell. Infect. Microbiol. 15:1631780. doi: 10.3389/fcimb.2025.1631780
Received: 20 May 2025; Accepted: 08 September 2025;
Published: 23 September 2025.
Edited by:
Bishnu Joshi, Baylor College of Medicine, United StatesReviewed by:
Jintae Lee, Yeungnam University, Republic of KoreaMichael S. Price, Liberty University, United States
Copyright © 2025 Bae and Eom. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Bin Eom, b21uaWJpbkBzY2guYWMua3I=
†Present address: Yong-Bin Eom, Department of Biomedical Laboratory Science, College of Medical Sciences, Soonchunhyang University, Asan, Chungcheongnam, Republic of Korea
‡ORCID: Yong-Bin Eom, orcid.org/0000-0002-1569-7248
 Ji-Hui Bae1
Ji-Hui Bae1 Yong-Bin Eom
Yong-Bin Eom