- 1Section of Preventive and Public Health Dentistry, Division of Oral Health, Growth and Development, Faculty of Dental Science, Kyushu University, Fukuoka, Japan
- 2Section of Oral and Maxillofacial Oncology, Division of Maxillofacial Diagnostic and Surgical Sciences, Faculty of Dental Science, Kyushu University, Fukuoka, Japan
- 3Center for Cohort Studies, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- 4Department of Epidemiology and Public Health, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
Altered salivary microbiota due to the progression of periodontitis may serve as a marker for simple and accurate identification of periodontitis. In this study, we examined saliva samples collected from 2,050 community-dwelling adults using 16S rRNA gene sequencing and verified the predictive performance of salivary microbiota in detecting periodontitis using a light gradient boosting machine algorithm. Five-fold stratified cross-validation was applied with 10 iterations, and the predictive performance was evaluated using the mean area under the receiver operating characteristic curve (AUC) value. In detecting periodontitis defined by number of teeth with probing depth ≥4 mm, localized (≥2 teeth), intermediate (≥4 teeth), and generalized (≥6 teeth) cases were detected with mean AUC values of 0.81 (95% confidence intervals, 0.80–0.81), 0.85 (0.84–0.86), and 0.87 (0.87–0.88), showing an increasing trend with extent. According to the Shapley additive explanation analysis, Porphromonas gingivalis, Tannerella forsythia, Mycoplasma faucium, Treponema species HMT-237, and Fretibacterium species HMT-362 were identified as important features for the detection of periodontitis. Our study presents the potential of salivary microbiota as a tool for mass screening of periodontitis and provides information on novel and important targets, including taxa other than known periodontal pathogens, to establish salivary screening tests.
1 Introduction
Periodontitis is an inflammatory oral disease that arises from complex interactions between the host immune response and dental plaque microorganisms (Pihlstrom et al., 2005; Papapanou et al., 2018). Clinically, it is characterized by the resorption of the alveolar bone and formation of a deep periodontal pocket, eventually resulting in tooth loss. Periodontitis is also known to be associated with various systemic diseases, such as cardiovascular disease, rheumatoid arthritis, and respiratory disease (Pihlstrom et al., 2005; Hajishengallis, 2014; Angjelova et al., 2024; Dolcezza et al., 2024). Therefore, early detection and intervention in periodontitis are crucial for maintaining oral and systemic health. However, it often remains undetected until it progresses to a severe state owing to its asymptomatic nature in the early stages. Periodontal examinations by dental professionals, either dentists or hygienists, are required for the detection and diagnosis of periodontitis, which is a technical, time-consuming, and invasive process. Therefore, there is an urgent need to develop a novel approach for accurate and simple detection of periodontitis without specialized training.
Saliva is a promising specimen for the detection of periodontitis because it can be easily and noninvasively collected. Various salivary components, such as occult blood, enzymes, cytokines, and proteins, have been investigated for their potential to detect periodontal disease (Lamster et al., 2003; Nomura et al., 2006; Shimazaki et al., 2011; Maeng et al., 2016; Wu et al., 2018; Liaw et al., 2023; Lu et al., 2023). However, no definitive conclusions or methodologies have been established. In particular, we focused on salivary microbiota as a reasonable biomarker. Briefly, the progression of periodontitis increases the subgingival space of the periodontal pocket, which is occupied by obligate anaerobic and proteolytic bacteria. In parallel, salivary microbiota contains bacteria shed from the subgingival space as a minor component and their occupancy in salivary microbiota increases with the progression of periodontitis (Umeda et al., 1998; He et al., 2012; Haririan et al., 2014; Belstrøm et al., 2017; Kageyama et al., 2017; Jung et al., 2024). Considering these factors, it is reasonable to predict periodontal condition by examining the salivary microbiota. In line with these findings, we examined the salivary microbiota, focusing on subgingival bacteria, and demonstrated that it can be used for the identification of periodontitis with high predictive performance (Ma et al., 2021). However, although this performance was remarkable in generalized cases of periodontitis, it was limited in the detection of localized cases.
In this study, we used a light gradient boosting machine (LightGBM) based on salivary microbiota data to predict periodontitis. LightGBM is a high-performance machine learning algorithm based on gradient boosting decision trees designed for efficiency and scalability, enabling fast and accurate analysis of numerous variables with nonlinearity and complex feature interactions (Ke et al., 2017). In this study, we aimed to investigate the predictive performance of salivary microbiota in the detection of periodontitis, including localized cases, using this machine learning approach and to identify the key bacterial species in the prediction model.
2 Materials and methods
2.1 Study participants
The participants in this study were community-dwelling adults in Hisayama town, Japan (Hata et al., 2013). As a part of the health examination of Hisayama residents, we conducted dental examinations and saliva sampling of participants aged ≥39 years in 2012. Of the 2,654 participants who underwent dental examination, saliva samples sufficient for microbiota analysis were collected from 2,100 participants. After excluding 50 participants with <2 teeth (the required minimum for definition of outcomes, n=49) and those with missing probing depth (PD) data (n=1), 2,050 participants were finally included in the analysis. Written informed consent was obtained from all participants. The Ethics Committee of Kyushu University approved the present study and the procedure for obtaining informed consent (approval number: 23092).
2.2 Dental examination and saliva sample collection
Dental examinations and sample collection were conducted according to a previously described protocol (Takeshita et al., 2016). Briefly, the periodontal condition was evaluated by PD and bleeding on probing at two sites for all teeth except the third molars (mesio- and mid-buccal sites) based on the NHANES III method. Following the dental examination, we instructed the participants to chew gum for 2 min and collected their whole stimulated saliva in sterile plastic tubes. The collected saliva samples were stored at -80°C until analysis.
2.3 DNA extraction and 16S rRNA gene analysis
DNA was extracted from the saliva samples using the bead-beating method described previously (Kageyama et al., 2022, 2023). The V1–V2 regions of 16S rRNA gene were amplified using the following primers: 8F (5′-AGA GTT TGA TYM TGG CTC AG-3′) with the sample-specific tag sequence and 338R (5′-TGC TGC CTC CCG TAG GAG T-3′). Polymerase chain reaction amplification and purification were performed as described previously (Takeshita et al., 2016). The purified amplicons were pooled and sequenced using an Ion PGM Hi-Q Sequencing kit (Thermo Fisher Scientific) on an Ion PGM (Thermo Fisher Scientific). Quality filtering of all raw sequence reads was performed using a script manually written in R software (version 4.2.3). The reads that exhibited <200 bases, had an average quality score ≤25, or did not include the correct forward and reverse primer sequences were excluded from the analysis. The remaining reads were demultiplexed by examining the tag sequence at the forward end and the forward and reverse primer sequences were trimmed. The quality-checked reads (fastq.gz) were imported into QIIME 2 (version 2023.2.0) and directly clustered against 16S rRNA gene sequences in eHOMD (version 15.22) with a minimum identity of 97% using the vsearch cluster-features-closed-reference plugin in QIIME 2 (Chen et al., 2010; Rognes et al., 2016; Bolyen et al., 2019). Finally, an abundance table of salivary microbiota, including 802 taxa, was generated.
2.4 Outcomes
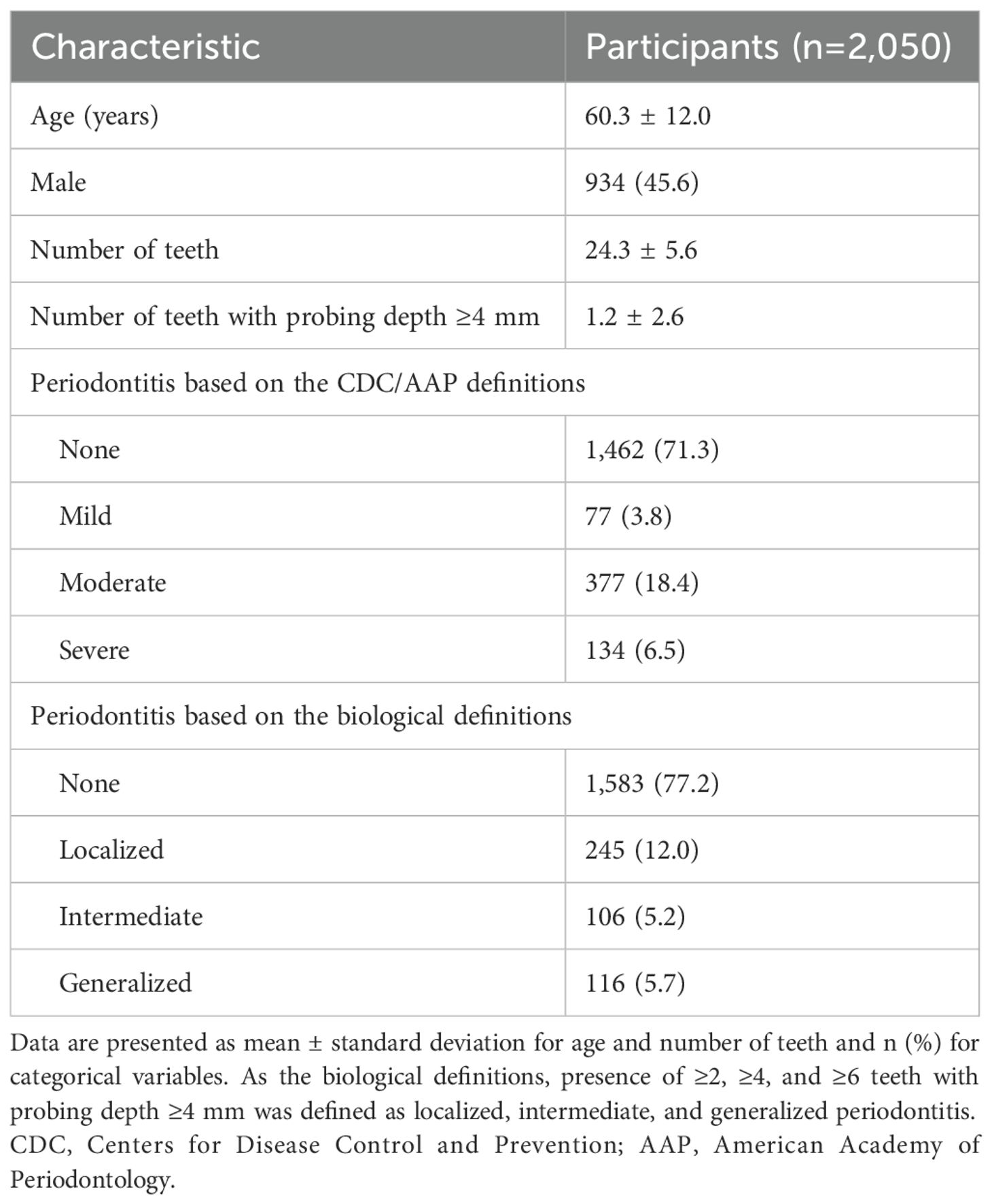
The severity of periodontitis was defined by the Centers for Disease Control and Prevention (CDC) and the American Academy of Periodontology (AAP) case definitions (Eke et al., 2012) or the biological definitions based on the number of teeth with PD ≥4 mm. As the biological definitions, we defined presence of ≥2, ≥4, and ≥6 teeth with PD ≥4 mm (top 5th, 10th, and 20th percentiles for number of teeth with PD ≥4 mm) as localized, intermediate, and generalized periodontitis. The outcomes of this study were mild, moderate, and severe periodontitis based on the CDC and AAP case definitions and localized, intermediate, and generalized periodontitis based on the biological definition, as binary classifications (such as severe and non-severe).
2.5 Machine learning analysis
All machine learning analyses were performed using the Python software (version 3.12.5). For testing intermediate and generalized periodontitis according to the biological definitions, 10 and 24 participants with <4 and <6 teeth (the required minimum for definitions), respectively, were excluded. To focus on the predictive performance of the salivary microbiota, the dataset was composed only of age, sex, and the relative abundance of each taxon in the salivary microbiota. We applied a five-fold stratified cross-validation using the StratifiedKFold function from the scikit-learn library (version 1.5.1) (Pedregosa et al., 2011) (Figure 1). Over all five-fold training/validation splits, the model was fitted to the training set using LightGBM (version 4.5.0). Performance metrics, including the area under the receiver operating characteristic curve (AUC), sensitivity, and specificity, were assessed using the validation set. The optimal cut-off values for determining sensitivity and specificity were calculated based on the Youden index, which maximizes the sum of sensitivity and specificity (Youden, 1950). This cross-validation process was iterated 10 times, and 50 values were obtained for each performance metric. Hyperparameters were primarily set as objective=binary, metric=auc, is_unbalance=True, and force_col_wise=True, and further tuned using the LightGBMTunerCV function (n_splits=3) from the Optuna library (version 4.0.0) (Akiba et al., 2019). The best parameters obtained were used to fit the model using the training set. To interpret the model, we computed the Shapley additive explanation (SHAP) values (Lundberg et al., 2020). The SHAP framework assigns each feature an importance value for prediction, enabling interpretation of the predictions of complex models. We computed the SHAP values of all the features in each trained model (50 models) fitted to the training set, and we calculated the mean of the absolute SHAP values for each feature (version 0.46.0).
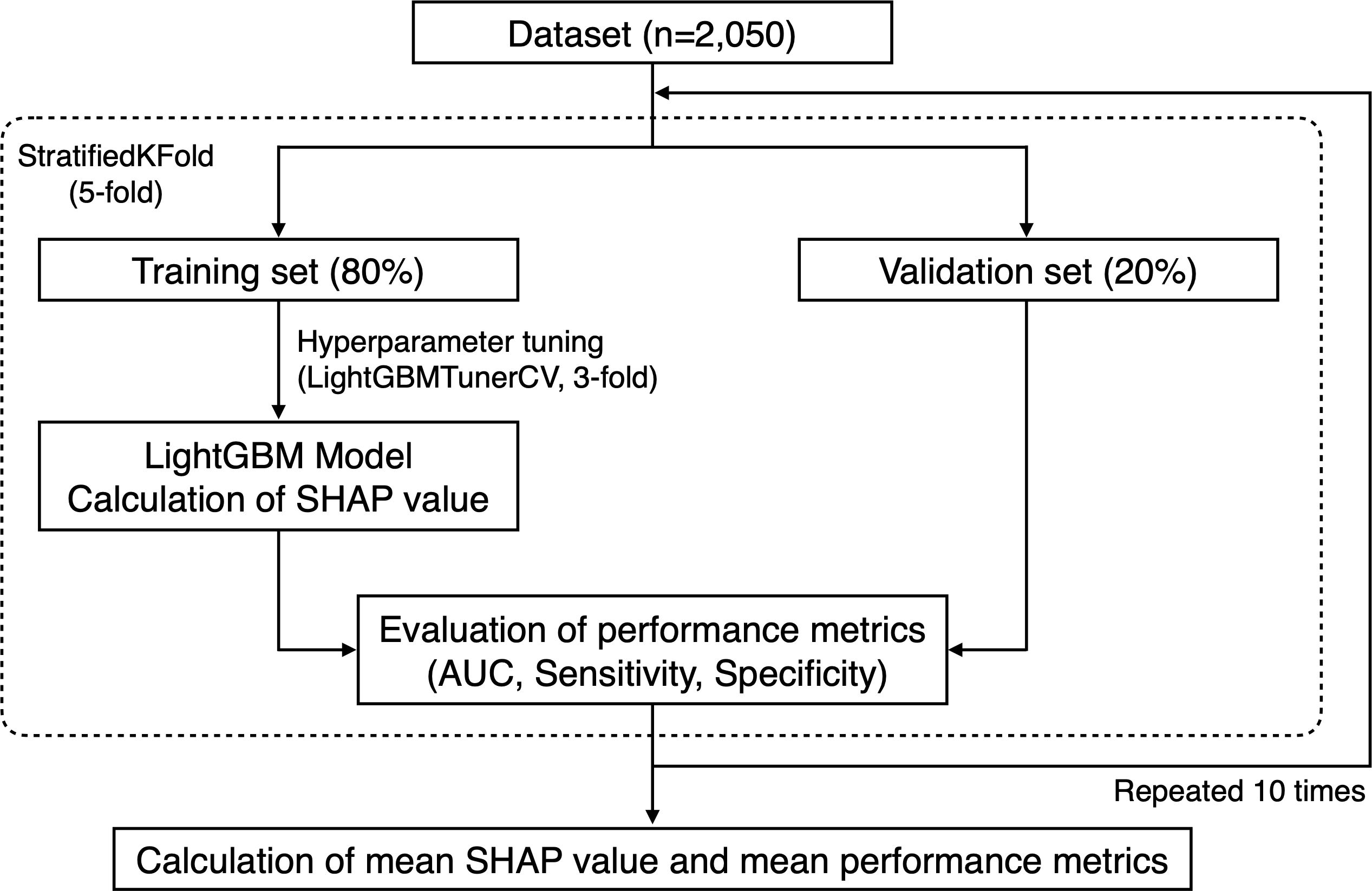
Figure 1. Flow chart of machine learning procedures. For testing intermediate and generalized periodontitis according to the biological definitions, 10 and 24 participants without the required minimum number of teeth for definitions were excluded, respectively.
3 Results
3.1 Characteristics of participants and 16S rRNA gene sequencing
We examined the salivary microbiota of 2,050 participants (934 male and 1,116 female) aged 39–90 years (median: 61 years). The median number of teeth present was 26 (interquartile range [IQR]: 22–28) and 33.9% of participants had ≥28 teeth (Table 1). According to the CDC and AAP case definitions, 3.8%, 18.4%, and 6.5% of the participants had mild, moderate, and severe periodontitis, respectively. Regarding the biological definitions, 12.0%, 5.2%, and 5.7% of the participants had localized, intermediate, and generalized periodontitis, respectively. Their saliva samples were analyzed using 16S rRNA gene amplicon analysis and finally 21,796,606 reads (9534.8 ± 3219.8 reads per sample) were obtained to determine the bacterial composition of salivary microbiota. The salivary microbiota of each participant comprised a median of 198 (IQR: 172–223) bacterial species and was dominated by Rothia mucilaginosa, Prevotella melaninogenica, Neisseria subflava, Streptococcus salivarius, and Granulicatella adiacens.
3.2 Predictive performance of salivary microbiota in detecting periodontitis
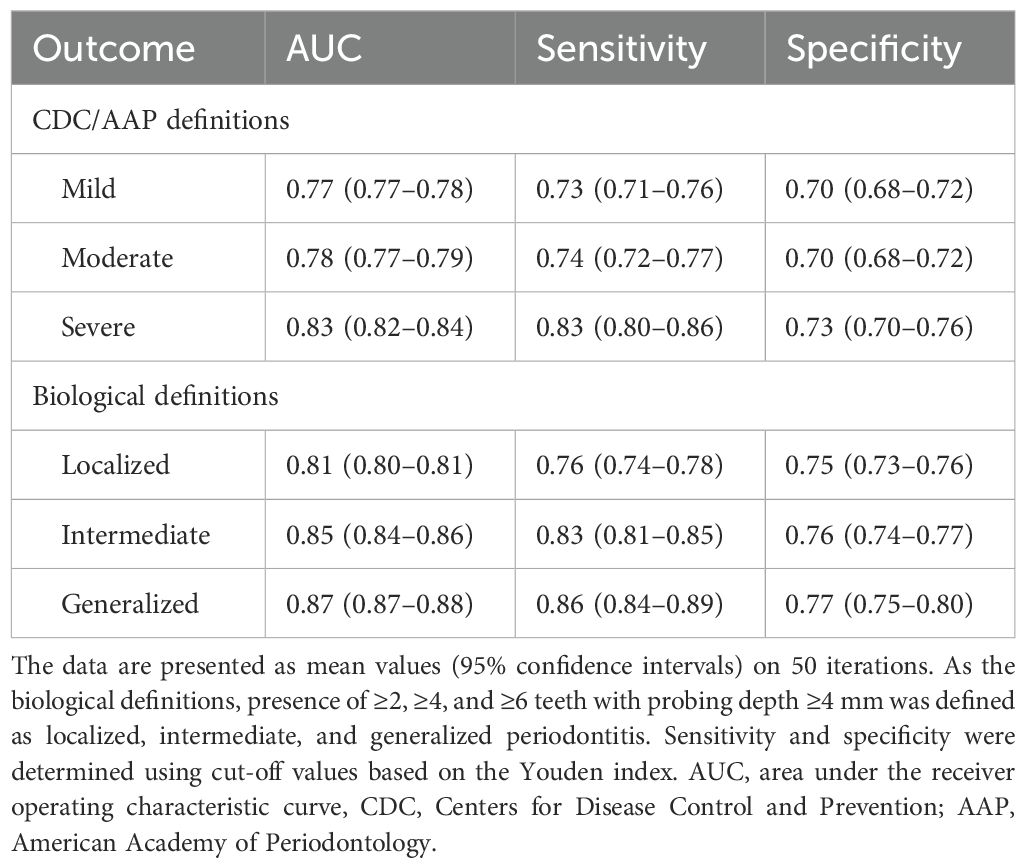
Prediction models were constructed using LightGBM with the bacterial composition data of the salivary microbiota. The predictive performance in detecting periodontitis according to each definition is presented in Table 2. The mean AUC values for detecting localized, intermediate, and generalized periodontitis defined by the biological definitions were 0.81 (95% confidence intervals [CI], 0.80–0.81), 0.85 (0.84–0.86), and 0.87 (0.87–0.88), respectively, showing an increasing trend with severity. Although severe periodontitis according to CDC and AAP case definitions were detected with an AUC value of 0.83 (0.82–0.84), the performance for detecting mild and moderate periodontitis were lower than those when using the biological definitions, with AUC values of 0.77 (0.77–0.78) and 0.78 (0.77–0.79).
3.3 Important features for detecting periodontitis
To identify important features for detecting periodontitis, we calculated the mean SHAP value of each feature in the 50 models. Figure 2 shows the top 20 most important features for detecting periodontitis based on the biological definitions (see Supplementary Table 1 for the results by definition). Porphromonas gingivalis and Tannerella forsythia demonstrated the highest and second-highest SHAP values, respectively, for localized to generalized periodontitis. These were followed by sex, Fusobacterium nucleatum subspecies vincentii, and Mycoplasma faucium for the detection of localized periodontitis. For the detection of intermediate and generalized periodontitis, M. faucium, Treponema species HMT-237, and Fretibacterium species HMT-362 were particularly important, after P. gingivalis and T. forsythia. The relative abundances of Cardiobacterium hominis, Lautropia mirabilis, and Streptococcus salivarius negatively contributed to the detection of periodontitis.
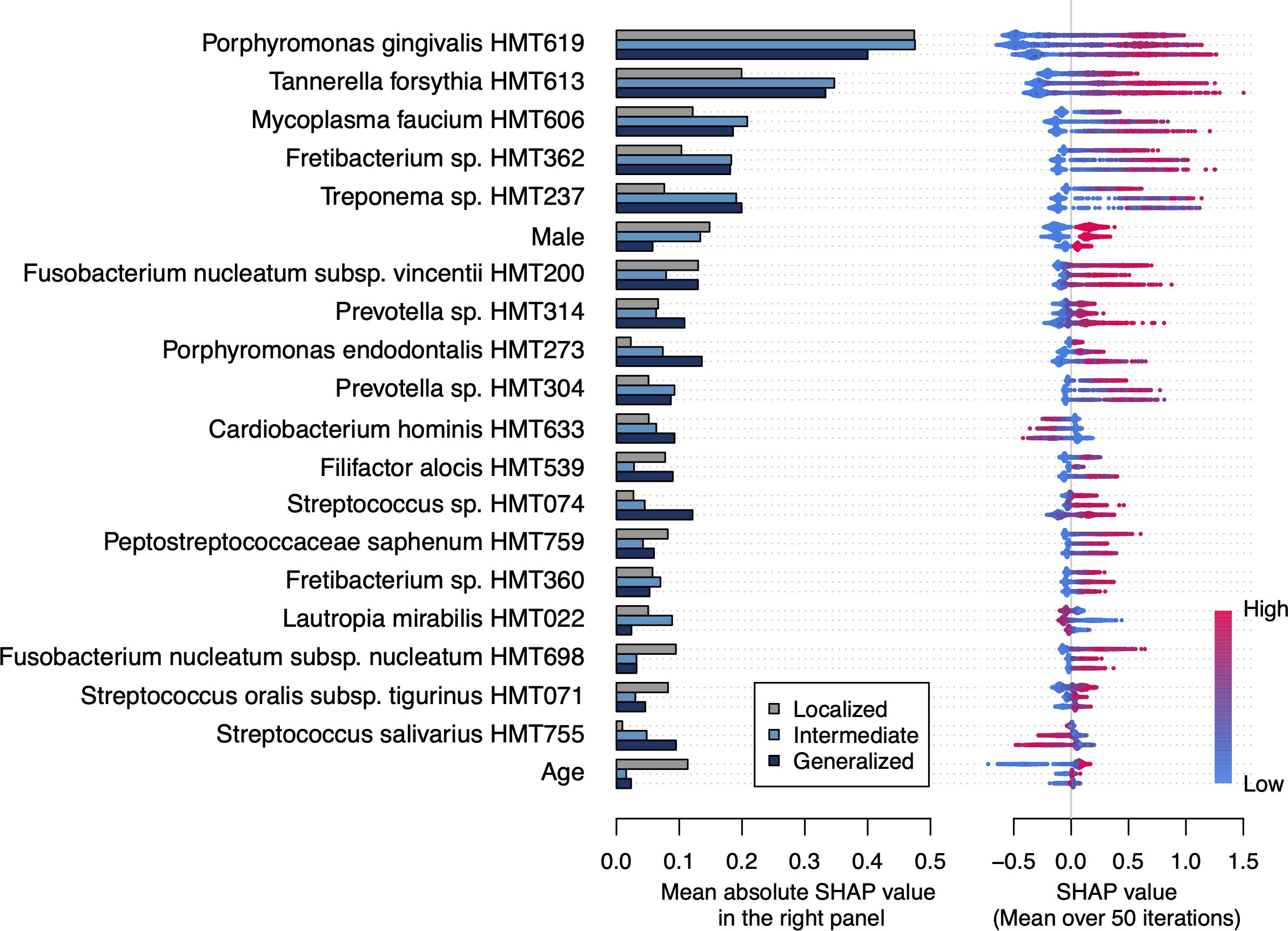
Figure 2. Important features for detecting periodontitis based on the biological definitions The bar plot shows the mean absolute Shapley additive explanation (SHAP) value on 50 iterations for each definition. A higher absolute SHAP value indicates a greater impact on the prediction. The features are ordered according to the mean absolute SHAP value among all definitions and the top 20 features are shown. Each dot represents each participant and the red color indicates that they are male, older, and have higher relative abundance of each taxon. The positive and negative values mean that the feature increases or decreases the probability of periodontitis for each participant, respectively.
4 Discussion
This study determined the salivary microbiota composition of 2,050 participants using 16S rRNA gene amplicon analysis, and verified its predictive performance in the detection of periodontitis using a machine learning approach. This approach demonstrated high performance in detecting periodontitis based on number of teeth with PD ≥4 mm, achieving an AUC value ≥0.80 not only in generalized cases but also in localized cases whose detection was limited in our previous study focusing on only subgingival-plaque specific bacteria in saliva (Ma et al., 2021). This result emphasizes the potential of whole salivary microbiota as a screening tool for periodontitis. Unlike dental examinations, which are time-consuming, invasive, and require technical tests, saliva collection is easy and noninvasive and does not require the expertise of dentists and hygienists. Furthermore, this type of salivary bacterial test is expected to contribute to the reassessment and improvement of oral health conditions. We believe that salivary microbiota has the potential to be used for extensive and non-burdensome screening that can estimate the necessity for visiting a dental office simply through collection and mailing of saliva.
For clinical application, it is necessary to set an appropriate cutoff value. Particularly, in the screening test, minimizing false negatives (individuals with periodontitis who test negative) is prioritized to reduce overlooking cases that require early intervention or urgent treatment. In detecting intermediate and generalized cases, the sensitivities were high (0.83 and 0.86, respectively), even when a cutoff value based on the Youden index was used, which considers a balance between sensitivity and specificity (Youden, 1950). Meanwhile, it seemed difficult to distinguish localized cases from healthy cases, as expected, and the sensitivity of localized cases (0.76) was lower than that of intermediate and generalized cases. When we recalculated the cutoff value based on an F2 score, which is a form of the F score calculated by sensitivity and precision and prioritizes sensitivity, the specificity declined to 0.60 but the sensitivity improved to 0.87 (Chinchor and Sundheim, 1993; Sasaki, 2007). In this case, false positives (individuals without periodontitis who test positive) may increase; however, false negatives will decrease. Such a trade-off and an appropriate cutoff value should be carefully considered in further investigations of independent populations.
During the prediction process, P. gingivalis and T. forsythia were identified as the most important features for the detection of periodontitis. They are classically well-known as the ”red complex” along with Treponema denticola because of their co-aggregation characteristics and strong association with periodontitis (Socransky et al., 1998; Holt and Ebersole, 2005). In addition, subgingival bacteria, such as Fretibacterium species HMT-362, F. nucleatum, Porphyromonas endodontalis, Filifactor alocis, and Eubacterium saphenum, have also been identified as important features (Pérez-Chaparro et al., 2014; Kageyama et al., 2017; Ma et al., 2021). These findings are consistent with our concept that the salivary microbiota contains bacteria shed from the subgingival space, which expands with the progression of periodontitis, and their abundance in the salivary microbiota can be used to detect periodontitis. This study also identified M. faucium as a critical feature, following P. gingivalis and T. forsythia. Although a few studies reported the detection of M. faucium from subgingival plaque in patients with periodontitis (Abusleme et al., 2013; Camelo-Castillo et al., 2015; Chen et al., 2018), M. faucium is considered as a member of microbiota on human oropharynx including palatine tonsils and might be involved in periodontitis in a manner different from subgingival bacteria (Freundt et al., 1974; Escapa et al., 2018). Further studies focusing on M. faucium may provide novel information to enhance predictive performance or understand periodontitis.
Aging is a known risk factor for periodontitis and was identified as the sixth important feature in detecting localized periodontitis. However, it was not listed in the top 20 features for intermediate and generalized periodontitis. These results suggest that an alteration in the bacterial composition of salivary microbiota by the progression of periodontitis occurs regardless of age and support the utility of salivary microbiota for detecting periodontitis.
In the present approach, the predictive performance was lower in detecting periodontitis based on the CDC and AAP case definitions compared with the biological definitions. This is partly because the former considers the clinical attachment loss (AL). For instance, the moderate definition includes cases with ≥2 interproximal sites with AL ≥4 mm. Although AL can be used to evaluate the degree of alveolar bone resorption and prior periodontitis, it is not necessarily accompanied by deep periodontal pockets. Therefore, there are many cases with no increase in subgingival bacteria in the oral cavity, and an accurate prediction may not be possible.
This study had some limitations. First, the species-level taxonomic assignment was based on the sequencing of the 16S rRNA gene V1–V2 regions. Although these regions are recommended for oral microbiota analysis because of their ability to discriminate oral streptococci from the V3–V4 regions (Wade and Prosdocimi, 2020), they might be insufficient to distinguish bacterial species with similar base sequences. Second, the present approach incurs costs for molecular analyses and sequencing. Although sequencing costs have drastically decreased over the past few decades, we should consider a cost-saving scheme such as simultaneous analysis of a large number of samples for social applications. Third, there is a need for further examination of the selection of machine learning models, input features, and outcome variables. Although we performed logistic regression analysis as a supplementary analysis, the predictive performance was lower than the present results (mean AUC values of 0.75, 0.76, and 0.77 for localized, intermediate, and generalized cases; Supplementary Table 2), suggesting the validity of a complex model, such as LightGBM. We further explored the community periodontal index (CPI) as an outcome, and the performances were mean AUC values of 0.77 and 0.78 for detecting participants with CPI scores ≥3 (with PD ≥4 mm) and 4 (with PD ≥6 mm), showing that screening results by CPI can also serve as a gold standard for future research (Supplementary Table 2). Fourth, this study included a dataset of Japanese adults, and the generalizability is limited. External validation using an independent dataset is required. Fifth, as the present results were based on a cross-sectional design, the potential utility of salivary microbiota for assessing the risk of future onset or progression of periodontitis should be further studied.
In conclusion, this study employed a machine learning approach using salivary microbiota data and highlighted the potential utility of salivary microbiota in the screening of periodontitis. Furthermore, some taxa have been identified as notable biomarkers for screening periodontitis. Further analyses assessing global generalizability, practicality, and costs would be required to support the development of a novel screening test based on salivary microbiota.
Data availability statement
The sequence data presented in the study are deposited in the DDBJ BioProject database, accession number PRJDB35955.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Kyushu University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SKag: Conceptualization, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. SH: Investigation, Methodology, Writing – review & editing. MF: Investigation, Writing – review & editing. MA: Funding acquisition, Investigation, Methodology, Writing – review & editing. SKaw: Supervision, Writing – review & editing. TN: Conceptualization, Investigation, Supervision, Writing – review & editing. TT: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by JSPS KAKENHI Grant Numbers: JP24K02660, JP25K02838, JP24K22192, and JP25K13317, and the Kakihara Science and Technology Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1631798/full#supplementary-material.
References
Abusleme, L., Dupuy, A. K., Dutzan, N., Silva, N., Burleson, J. A., Strausbaugh, L. D., et al. (2013). The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 7, 1016–1025. doi: 10.1038/ismej.2012.174
Akiba, T., Sano, S., Yanase, T., Ohta, T., and Koyama, M. (2019). “Optuna: A next-generation hyperparameter optimization framework,” in Proceedings of the ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. New York, NY, USA: Association for Computing Machinery. 2623–2631. doi: 10.1145/3292500.3330701
Angjelova, A., Jovanova, E., Polizzi, A., Laganà, L., Santonocito, S., Ragusa, R., et al. (2024). Impact of periodontitis on endothelial risk dysfunction and oxidative stress improvement in patients with cardiovascular disease. J. Clin. Med. 13, 3781. doi: 10.3390/jcm13133781
Belstrøm, D., Sembler-Møller, M. L., Grande, M. A., Kirkby, N., Cotton, S. L., Paster, B. J., et al. (2017). Microbial profile comparisons of saliva, pooled and site-specific subgingival samples in periodontitis patients. PloS One 12, e0182992–e0182992. doi: 10.1371/journal.pone.0182992
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Camelo-Castillo, A. J., Mira, A., Pico, A., Nibali, L., Henderson, B., Donos, N., et al. (2015). Subgingival microbiota in health compared to periodontitis and the influence of smoking. Front. Microbiol. 6. doi: 10.3389/fmicb.2015.00119
Chen, W. P., Chang, S. H., Tang, C. Y., Liou, M. L., Tsai, S. J. J., and Lin, Y. L. (2018). Composition analysis and feature selection of the oral microbiota associated with periodontal disease. BioMed. Res. Int. 2018, 3130607. doi: 10.1155/2018/3130607
Chen, T., Yu, W. H., Izard, J., Baranova, O. V., Lakshmanan, A., and Dewhirst, F. E. (2010). The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database 2010, baq013. doi: 10.1093/database/baq013
Chinchor, N. and Sundheim, B. M. (1993). “MUC-5 evaluation metrics,” in Proceedings of the 5th Conference on Message Understanding. Stroudsburg, PA, USA: Association for Computational Linguistics. doi: 10.3115/1072017.1072026
Dolcezza, S., Flores-Fraile, J., Lobo-Galindo, A. B., Montiel-Company, J. M., and Zubizarreta-Macho, Á. (2024). Relationship between rheumatoid arthritis and periodontal disease-systematic review and meta-analysis. J. Clin. Med. 14, 10. doi: 10.3390/jcm14010010
Eke, P. I., Page, R. C., Wei, L., Thornton-Evans, G., and Genco, R. J. (2012). Update of the case definitions for population-based surveillance of periodontitis. J. Periodontol. 83, 1449–1454. doi: 10.1902/jop.2012.110664
Escapa, I. F., Chen, T., Huang, Y., Gajare, P., Dewhirst, F. E., and Lemon, K. P. (2018). New insights into human nostril microbiome from the expanded human oral microbiome database (eHOMD): a resource for the microbiome of the human aerodigestive tract. mSystems 3, e00187-18. doi: 10.1128/msystems.00187-18
Freundt, E. A., Taylor Robinson, D., and Purcell, R. H. (1974). Proposal of Mycoplasma buccale nom. nov. and Mycoplasma faucium nom. nov. for Mycoplasma orale ‘types’ 2 and 3, respectively. Int. J. Syst. Bacteriol. 24, 252–255. doi: 10.1099/00207713-24-2-252
Hajishengallis, G. (2014). Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 15, 30–44. doi: 10.1038/nri3785
Haririan, H., Andrukhov, O., Bertl, K., Lettner, S., Kierstein, S., Moritz, A., et al. (2014). Microbial analysis of subgingival plaque samples compared to that of whole saliva in patients with periodontitis. J. Periodontol. 85, 819–828. doi: 10.1902/jop.2013.130306
Hata, J., Ninomiya, T., Hirakawa, Y., Nagata, M., Mukai, N., Gotoh, S., et al. (2013). Secular trends in cardiovascular disease and its risk factors in Japanese. Circulation 128, 1198–1205. doi: 10.1161/CIRCULATIONAHA.113.002424
He, J., Huang, W., Pan, Z., Cui, H., Qi, G., Zhou, X., et al. (2012). Quantitative analysis of microbiota in saliva, supragingival, and subgingival plaque of Chinese adults with chronic periodontitis. Clin. Oral. Investig. 16, 1579–1588. doi: 10.1007/s00784-011-0654-4
Holt, S. C. and Ebersole, J. L. (2005). Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000 38, 72–122. doi: 10.1111/j.1600-0757.2005.00113.x
Jung, J.-S., Kook, J.-K., Park, S.-N., Lim, Y. K., Choi, G. H., Kim, S., et al. (2024). Salivary microbiota reflecting changes in subgingival microbiota. Microbiol. Spectr. 12, e0103024. doi: 10.1128/spectrum.01030-24
Kageyama, S., Furuta, M., Takeshita, T., Ma, J., Asakawa, M., and Yamashita, Y. (2022). High-level acquisition of maternal oral bacteria in formula-fed infant oral microbiota. mBio 13, e0345221. doi: 10.1128/mbio.03452-21
Kageyama, S., Sakata, S., Ma, J., Asakawa, M., Takeshita, T., Furuta, M., et al. (2023). High-resolution detection of translocation of oral bacteria to the gut. J. Dent. Res. 102, 752–758. doi: 10.1177/00220345231160747
Kageyama, S., Takeshita, T., Asakawa, M., Shibata, Y., Takeuchi, K., Yamanaka, W., et al. (2017). Relative abundance of total subgingival plaque-specific bacteria in salivary microbiota reflects the overall periodontal condition in patients with periodontitis. PloS One 12, e0174782. doi: 10.1371/journal.pone.0174782
Ke, G., Meng, Q., Finley, T., Wang, T., Chen, W., Ma, W., et al. (2017). LightGBM: A highly efficient gradient boosting decision tree. Adv. Neural Inf. Process. Syst. 30, 3147–3155.
Lamster, I. B., Kaufman, E., Grbic, J. T., Winston, L. J., and Singer, R. E. (2003). β-glucuronidase activity in saliva: relationship to clinical periodontal parameters. J. Periodontol. 74, 353–359. doi: 10.1902/jop.2003.74.3.353
Liaw, A., Liu, C., Bartold, M., Ivanovski, S., and Han, P. (2023). Salivary histone deacetylase in periodontal disease: A cross-sectional pilot study. J. Periodontal Res. 58, 433–443. doi: 10.1111/jre.13104
Lu, X., Li, P., Li, J., Hu, J., and Tian, R. (2023). Clinical diagnostic value of IL-14, 1L-16 and SAA in periodontitis. Clin. Oral. Investig. 27, 6627–6635. doi: 10.1007/s00784-023-05269-8
Lundberg, S. M., Erion, G., Chen, H., DeGrave, A., Prutkin, J. M., Nair, B., et al. (2020). From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2, 56–67. doi: 10.1038/s42256-019-0138-9
Ma, J., Kageyama, S., Takeshita, T., Shibata, Y., Furuta, M., Asakawa, M., et al. (2021). Clinical utility of subgingival plaque-specific bacteria in salivary microbiota for detecting periodontitis. PloS One 16, e0253502. doi: 10.1371/journal.pone.0253502
Maeng, Y.-J., Kim, B.-R., Jung, H.-I., Jung, U.-W., Kim, H. E., and Kim, B.-I. (2016). Diagnostic accuracy of a combination of salivary hemoglobin levels, self-report questionnaires, and age in periodontitis screening. J. Periodontal Implant Sci 46, 10–10. doi: 10.5051/jpis.2016.46.1.10
Nomura, Y., Tamaki, Y., Tanaka, T., Arakawa, H., Tsurumoto, A., Kirimura, K., et al. (2006). Screening of periodontitis with salivary enzyme tests. J. Oral. Sci. 48, 177–183. doi: 10.2334/josnusd.48.177
Papapanou, P. N., Sanz, M., Buduneli, N., Dietrich, T., Feres, M., Fine, D. H., et al. (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions: Classification and case definitions for periodontitis. J. Clin. Periodontol. 45 Suppl 20, S162–S170. doi: 10.1111/jcpe.12946
Pedregosa, F., Varoquaux, G., Gramfort, A., Michel, V., Thirion, B., Grisel, O., et al. (2011). Scikit-learn: machine learning in python. J. Mach. Learn. Res. 12, 2825–2830. doi: 10.5555/1953048.2078195
Pérez-Chaparro, P. J., Gonçalves, C., Figueiredo, L. C., Faveri, M., Lobão, E., Tamashiro, N., et al. (2014). Newly identified pathogens associated with periodontitis: a systematic review. J. Dent. Res. 93, 846–858. doi: 10.1177/0022034514542468
Pihlstrom, B. L., Michalowicz, B. S., and Johnson, N. W. (2005). Periodontal diseases. Lancet 366, 1809–1820. doi: 10.1016/S0140-6736(05)67728-8
Rognes, T., Flouri, T., Nichols, B., Quince, C., and Mahé, F. (2016). VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, e2584–e2584. doi: 10.7717/PEERJ.2584
Shimazaki, Y., Akifusa, S., Takeshita, T., Shibata, Y., Doi, Y., Hata, J., et al. (2011). Effectiveness of the salivary occult blood test as a screening method for periodontal status. J. Periodontol. 82, 581–587. doi: 10.1902/jop.2010.100304
Socransky, S. S., Haffajee, A. D., Cugini, M. A., Smith, C., and Kent, R. L. (1998). Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25, 134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x
Takeshita, T., Kageyama, S., Furuta, M., Tsuboi, H., Takeuchi, K., Shibata, Y., et al. (2016). Bacterial diversity in saliva and oral health-related conditions: the Hisayama Study. Sci. Rep. 6, 22164. doi: 10.1038/srep22164
Umeda, M., Contreras, A., Chen, C., Bakker, I., and Slots, J. (1998). The utility of whole saliva to detect the oral presence of periodontopathic bacteria. J. periodontology 69, 828–833. doi: 10.1902/jop.1998.69.7.828
Wade, W. G. and Prosdocimi, E. M. (2020). Profiling of oral bacterial communities. J. Dent. Res. 99, 621–629. doi: 10.1177/0022034520914594
Wu, Y. C., Ning, L., Tu, Y. K., Huang, C. P., Huang, N. T., Chen, Y. F., et al. (2018). Salivary biomarker combination prediction model for the diagnosis of periodontitis in a Taiwanese population. J. Formos. Med. Assoc. 117, 841–848. doi: 10.1016/j.jfma.2017.10.004
Keywords: oral microbiota, saliva, LightGBM, SHAP, screening
Citation: Kageyama S, Hama S, Furuta M, Asakawa M, Kawano S, Ninomiya T and Takeshita T (2025) Performance of salivary microbiota in detecting periodontitis using a machine learning approach. Front. Cell. Infect. Microbiol. 15:1631798. doi: 10.3389/fcimb.2025.1631798
Received: 20 May 2025; Accepted: 03 September 2025;
Published: 18 September 2025.
Edited by:
Bastiaan P. Krom, VU Amsterdam, NetherlandsCopyright © 2025 Kageyama, Hama, Furuta, Asakawa, Kawano, Ninomiya and Takeshita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toru Takeshita, dGFrZXRvb29AZGVudC5reXVzaHUtdS5hYy5qcA==
†These authors have contributed equally to this work
 Shinya Kageyama
Shinya Kageyama Shion Hama
Shion Hama Michiko Furuta
Michiko Furuta Mikari Asakawa
Mikari Asakawa Shintaro Kawano2
Shintaro Kawano2 Toru Takeshita
Toru Takeshita