- 1Department of Radiology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
- 2Intervention Ward One, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
- 3The State Key Laboratory of Neurology and Oncology Drug Development, Jiangsu Simcere Diagnostics Co., Ltd, Nanjing, China
- 4Department of Medicine, Nanjing Simcere Medical Laboratory Science Co., Ltd., Nanjing, China
- 5Tianjin Second People’s Hospital, Tianjin, China
- 6Tianjin Institute of Hepatology, Tianjin, China
Helicobacter pylori (H. pylori) is a Group 1 gastric carcinogen increasingly implicated in extragastric digestive malignancies. This review synthesizes evidence on its role in liver, biliary, esophageal, colorectal, and pancreatic cancers. Based on its unique spiral morphology, flagellar motility bundle, and urease activity-mediated acidic niche adaptation, H. pylori disrupts host cellular homeostasis through multifactorial virulence mechanisms involving CagA/VacA synergy, and exploits antigenic variation and immunomodulatory strategies to achieve persistent gastric mucosal colonization and chronic infection. Emerging evidence suggests associations between H. pylori infection and nongastric digestive cancers, though relationships vary by site. For hepatocellular carcinoma (HCC), epidemiological studies indicate increased risk (OR 4.75), particularly with HCV coinfection, but mechanistic and cohort data remain conflicting. Biliary tract cancer (BTC) shows stronger epidemiological links, especially for cholangiocarcinoma (OR 4.18), supported by virulence factor detection. In esophageal cancer, H. pylori particularly CagA+ strains demonstrates a protective effect against adenocarcinoma but no significant association with squamous cell carcinoma. Colorectal cancer exhibits complex associations, with meta-analyses suggesting increased risk in East Asian populations and potential benefits from eradication therapy. Pancreatic cancer links remain inconsistent. Proposed mechanisms of H. pylori in extragastric cancers include chronic inflammation, virulence factor activity and microbiome disruption. This comprehensive review synthesizes contemporary evidence on the bacterium’s role in non-gastric digestive malignancies, examines pathways underlying its oncogenicity, and outlines translational implications for risk stratification and therapeutic innovation.
1 Introduction
Since Marshall and Warren’s pioneering isolation of Helicobacter pylori (H. pylori) from gastric mucosa in 1982 (Marshall and Warren, 1984), this spiral-shaped gram-negative bacterium has revolutionized our understanding of chronic gastritis and peptic ulcer pathogenesis. Its microaerophilic growth requirements and persistent colonization of over 40% of the global adult population have established H. pylori as a formidable clinical challenge (Li et al., 2023). Notably, the bacterium’s carcinogenic potential was formally recognized by the International Agency for Research on Cancer (IARC) in 1994, classifying it as a Group 1 carcinogen (Schistosomes, liver flukes and Helicobacter pylori, 1994).
A 2017 meta analysis that looked into the link between 31 infections and cancer found that around 4.3% of adult cancers were due to eight particular infections, with H. pylori being a significant one (Volesky-Avellaneda et al., 2023). It is well - known that H. pylori is connected to gastric cancer (GC), and the overall occurrence of GC in people with H. pylori infection was determined to be 17.4%. Interestingly, GC rates among H. pylori - infected individuals differ greatly from country to country, with Asian nations having the highest rates (Pormohammad et al., 2019). Such pathophysiological complexity underscores the need for region-specific prevention strategies addressing this persistent global health burden. Beyond its established gastric tropism, emerging evidence implicates H. pylori in extragastric oncogenesis, including hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), colorectal cancer (CRC), pancreatic malignancies, and esophageal carcinoma (EC) (de Martel et al., 2020). This expanded oncogenic spectrum appears modulated through both direct microbial interactions and systemic inflammatory cascades, with risk stratification influenced by strain-specific virulence factors, host polymorphisms, and environmental cofactors (Waldum and Fossmark, 2021). Wu et al. found that H. pylori increases expression of protein arginine deiminase type 4 (PAD4) by stabilizing hypoxia-inducible factor 1α (HIF-1α), worsening rheumatoid arthritis (Wu et al., 2024). Additionally, some preliminary studies have revealed a connection between H. pylori infection and the occurrence of nonthyroidal illness syndrome, anemia, and iron deficiency (Hou et al., 2019; Sun et al., 2021; Lee et al., 2022).
Understanding the connection between H. pylori and non - gastric digestive cancers is very important, as it may reveal new strategies for prevention and treatment. Knowing how H. pylori might contribute to these cancers could help create targeted treatments, especially in areas where H. pylori infection is common. Also, what we learn from this connection could help make public health strategies to reduce cancer cases around the world.
This comprehensive analysis synthesizes current evidence elucidating H. pylori’s emerging oncogenic potential beyond gastric malignancies, with particular focus on digestive cancers. Through systematic evaluation of molecular epidemiological data and experimental models, we delineate pathogen-host interactions driving extragastric tumorigenesis while critically assessing translational implications for early detection and therapeutic intervention.
2 Biological properties of H. pylori
2.1 Morphological and physiological features of H. pylori
The spiral morphology and polar flagellar bundle of H. pylori confer exceptional motility through viscous mucus, enabling strategic positioning at the epithelial interface where pH gradients stabilize (Marshall and Warren, 1984). This ecological specialization is augmented by urease-mediated microenvironment remodeling - enzymatic conversion of urea generates localized ammonia buffers that transiently elevate peri-bacterial pH to 6.0-7.0, facilitating survival despite luminal acidity (Weeks et al., 2000; Malfertheiner et al., 2023). Genomic plasticity further enhances ecological persistence, with phase-variable expression of outer membrane proteins creating antigenic mosaicism that confounds immune surveillance (Suerbaum and Michetti, 2002).
2.2 Pathogenic mechanisms of H. pylori
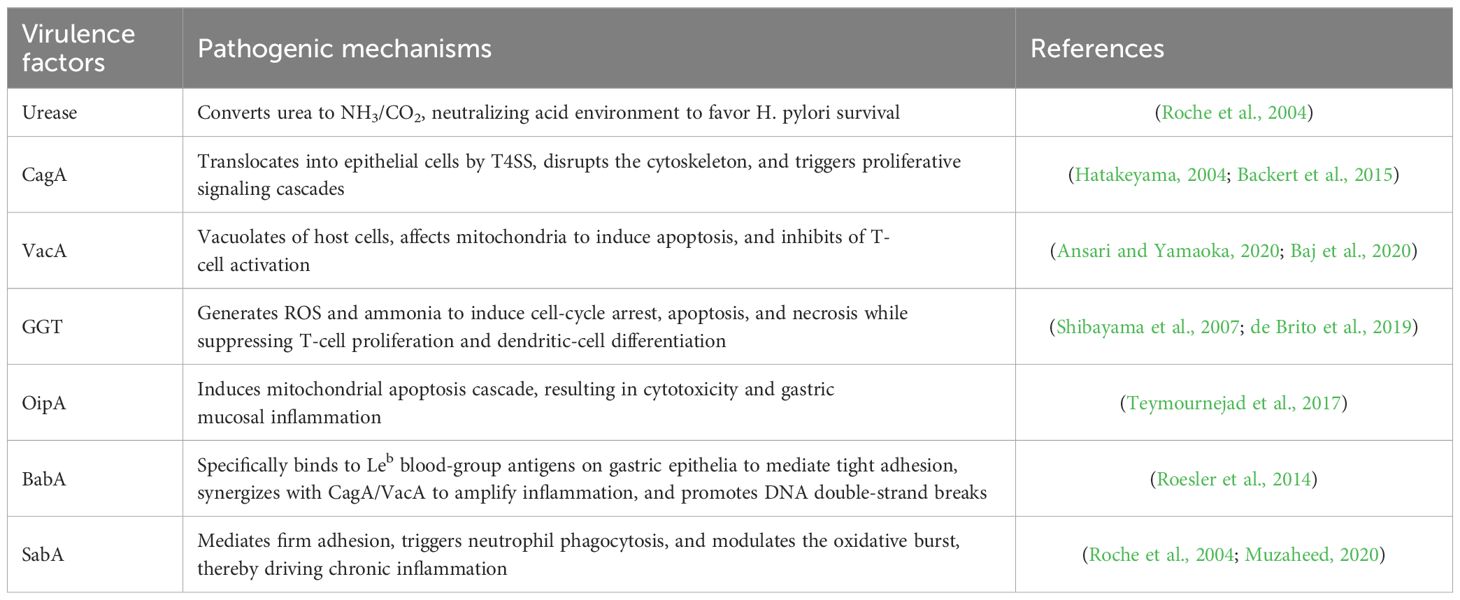
The pathogenicity of H. pylori is multifactorial and involves a complex interplay of bacterial factors and host responses. Its pathogenicity is mainly due to its virulence factors, such as urease, adhesins, toxins, and secretion systems (Suerbaum and Michetti, 2002). Urease is capable of hydrolyzing urea to produce ammonia and carbon dioxide, neutralizing gastric acid and providing an optimal pH environment for H. pylori survival (Debowski et al., 2017). The bacterium secretes a variety of virulence factors, including cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA), which play a key role in causing gastric inflammation and epithelial cell damage (de Brito et al., 2019). The Cag pathogenicity island-encoded T4SS injects phosphorylatable CagA into parietal cells, inducing cytoskeletal rearrangement via SHP-2/Rho GTPase pathways while activating β-catenin-mediated proliferative signaling (Hatakeyama, 2004; Backert et al., 2015). VacA exhibits pleiotropic toxicity, with inducing acidic vacuolation in host cell and its pore-forming capacity disrupting mitochondrial membrane potentials to inhibit apoptosis, while simultaneously blocking T-cell activation through CD25/LFA-1 interference (Ansari and Yamaoka, 2020; Baj et al., 2020). Recent single-cell transcriptomic studies reveal these virulence factors synergistically induce epithelial-mesenchymal transition (EMT) signatures and cancer stem cell marker expression in preneoplastic lesions (Zhu et al., 2017).By catalyzing the hydrolysis of glutamine and glutathione, γ-glutamyl-transpeptidase (GGT) facilitates the generation of ROS and ammonia. This process induces cell cycle arrest, apoptosis, and necrosis while concurrently suppressing T-cell proliferation and dendritic cell differentiation, consequently promoting H. pylori-associated ulceration and inflammation (Roche et al., 2004). The outer membrane proteins of H. pylori that bind to host epithelial cell receptors, such as OipA, BabA, and SabA, promote bacterial adhesion and induce chronic inflammation through IL-8 secretion (Duan et al., 2025) (Table 1).
2.3 Immune response to H. pylori infection
The immune response to H. pylori infection is intricate and involves both innate and adaptive immunity. The body’s innate immune defense recognizes H. pylori by utilizing pattern recognition receptors (PRRs), like Toll-like receptors (TLRs), which activate the generation of proinflammatory signaling proteins and chemotactic factors (Nagashima and Yamaoka, 2019). However, H. pylori has developed strategies to modulate this response, including the downregulation of TLR signaling and the inhibition of antigen presentation, thereby dampening the immune response and facilitating persistent infection (Bergman et al., 2004). H. pylori infection triggers innate immune responses, yet it suppresses immune-cell activation, thereby reducing the efficiency of macrophages and neutrophils in clearing the pathogen (Karkhah et al., 2019; Fuchs et al., 2023). T cell and B cell activation form the core of the adaptive immune response, culminating in the creation of antibodies specific to H. pylori antigens (Reyes and Peniche, 2019; Sirit and Peek, 2025) (Figure 1). Despite this robust immune response, H. pylori’s ability to express antigenic variation and implement strategies to evade immune clearance often result in chronic infection (El-Omar et al., 2000). Infection with H. pylori might increase the expression of proliferating cell nuclear antigen (PCNA) while simultaneously decreasing the expression of Bcl-2-associated X protein (Bax). This modulation promotes tumor cell proliferation and inhibits apoptosis (Wang et al., 2015).
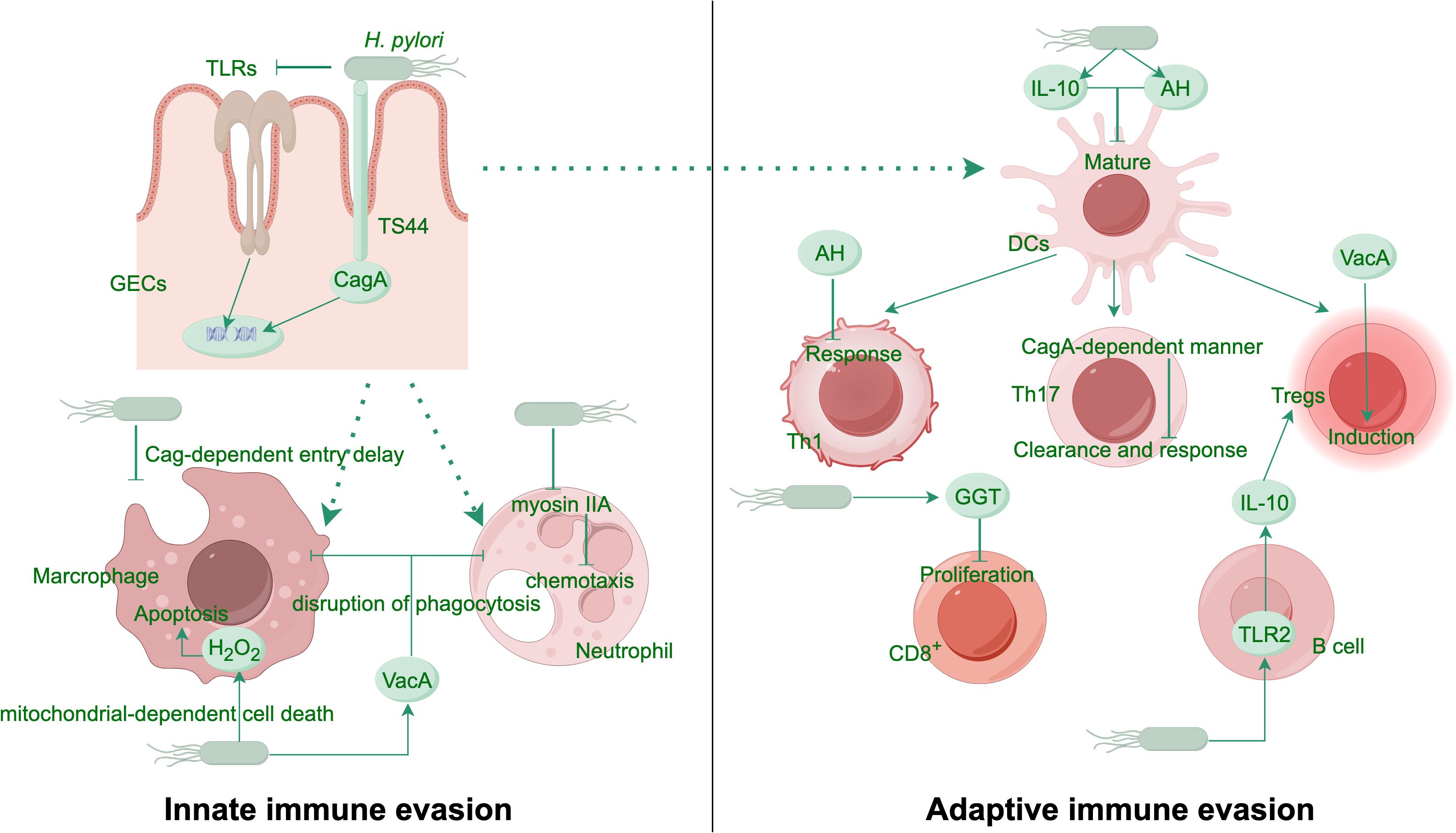
Figure 1. H. pylori evasion mechanisms from host innate and adaptive immune responses. H. pylori evades immune recognition by modifying its PAMPs and manipulating the antigen-presenting capacity of phagocytes. In a Cag-dependent manner, the bacterium delays entry into macrophages and promotes macrophage apoptosis through H2O2 production. It inhibits the myosin IIA protein in neutrophils, thereby disrupting their chemotaxis. The virulence factor VacA blocks phagosome-lysosome fusion, resisting phagocytosis by innate immune cells. The bacterial metabolite AH attenuates mature of DCs and suppresses Th1 responses. In a CagA-dependent manner, H. pylori also inhibits Th17-mediated clearance. Another virulence factor GGT induces G1 cell cycle arrest in T cells, curbing their proliferation. VacA promotes the expansion of Tregs, while H. pylori further enhances the differentiation of immunosuppressive Tregs via the TLR2/MYD88 pathway by stimulating B cells to secrete more IL-10. AH, ADP-heptose; DCs, dendritic cells; GECs, gastric epithelial cells; GGT, g-glutamyl-transpeptidase; PAMPs, pathogen-associated molecular patterns; TLRs, Toll like receptors; Tregs, regulatory T cells.
3 Association of H. pylori with nongastric digestive cancers
3.1 Hepatocellular carcinoma
3.1.1 Associated evidence
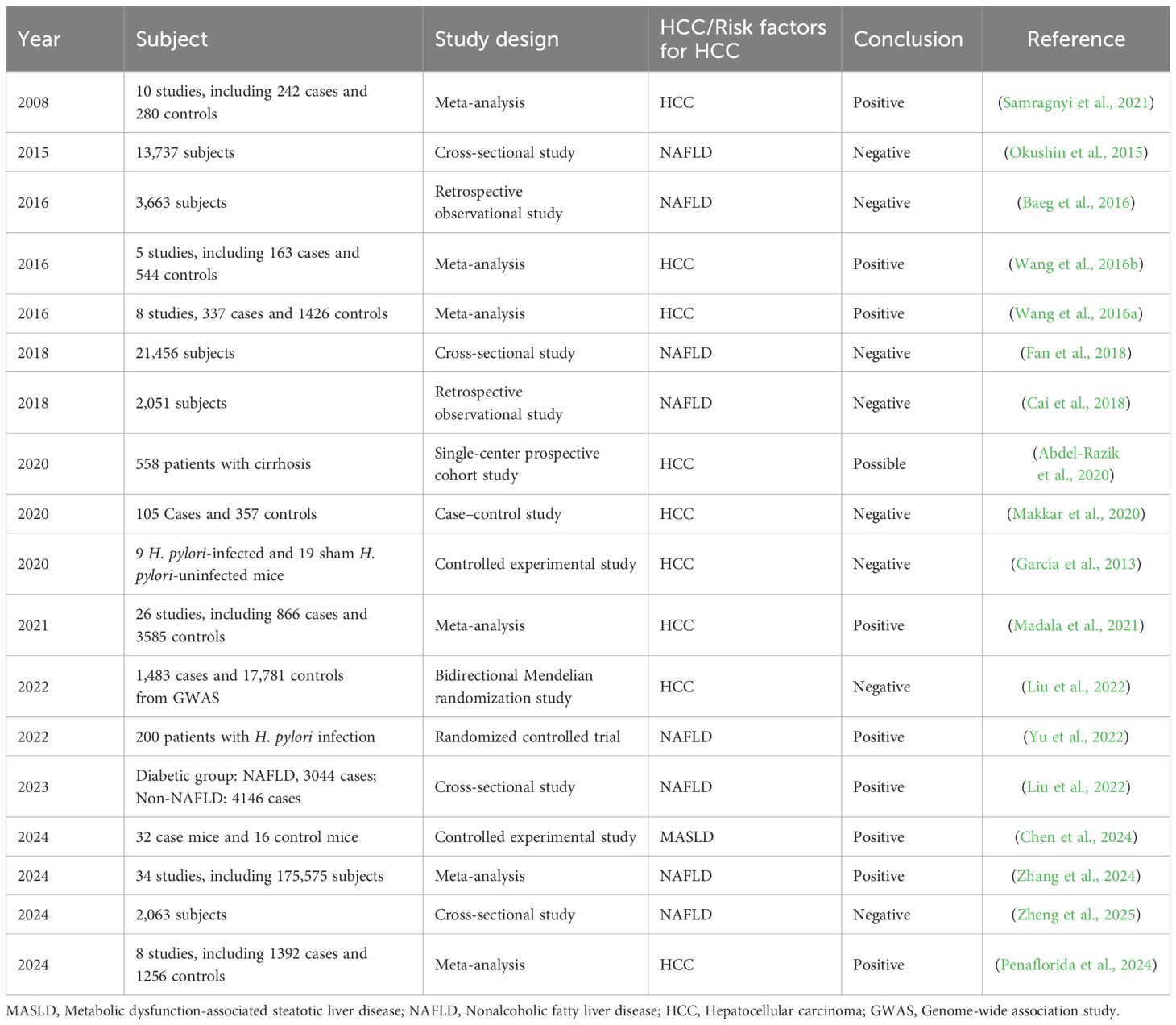
Globally, primary liver cancer represents a major contributor to both cancer incidence and mortality. It ranks as the sixth most common cancer worldwide and the third leading cause of cancer-related deaths (Bray et al., 2024). A pronounced geographic disparity exists in HCC incidence, with high frequency observed across Asia and Africa but low occurrence in Europe and North America. Regardless of regional incidence rates, virtually all areas report male incidence rates that are two- to three-fold higher than those in femaless (Altekruse et al., 2009; Bray et al., 2024). A growing body of epidemiological evidence suggests a potential association between H. pylori infection and the onset of HCC. Studies have detected H. pylori and related species in liver samples from patients afflicted with HCC and CCA, indicating a possible association (Esmat et al., 2012; Waluga et al., 2015). A single-center observational study identified H. pylori as an initiating factor for complications in cirrhotic patients that can potentially increase the incidence of HCC, with the eradication of H. pylori infection possibly reducing the incidence of such complications (Abdel-Razik et al., 2020). Furthermore, a comprehensive meta-analysis from 1994–2023 across key literature databases revealed a substantial increase in the risk of HCC associated with H. pylori infection, with an overall odds ratio of 4.75 (95% CI, 3.06–7.37). This risk is exacerbated when H. pylori infection is combined with hepatitis C virus (HCV) infection (Madala et al., 2021). The existing evidence predominantly linking HP infection to HCC is largely derived from epidemiological studies and the presence of HP at the tumor site.
However, in a nested case–control study evaluating the association between seropositivity for H. pylori proteins and the subsequent development of HCC or biliary cancers, no significant association was detected between seropositivity for these 15 proteins and the subsequent incidence of HCC or biliary cancers. Despite the conclusions of this study suggesting a potential possible association, the findings are limited by the study population, which consisted exclusively of white individuals, and assessed only antigens specific to H. pylori. Notably, in this study, H. pylori seropositivity was not found to be associated with liver cancer. This lack of association remained consistent even upon subgroup analysis of CagA+ strains (Makkar et al., 2020). Similarly, in a mouse model study examining the role of H. pylori infection in promoting HCV-associated HCC, it was determined that H. pylori does not facilitate the progression of HCC. Furthermore, research has indicated that the presence of the HCV transgene might contribute to improvements in certain liver and gastric lesions observed in mice concurrently infected with H. pylori (García et al., 2013). According to meta-analysis results published in the past 20 years, H. pylori infection and HCC occurrence are positively correlated, and the correlation is stronger in specific subgroups, including patients with hepatitis B, hepatitis C, cirrhosis, etc. Patient bile and serum samples are particularly useful in the diagnosis of H. pylori infection (Wang et al., 2016b; Wang et al., 2016a; Madala et al., 2021; Samragnyi et al., 2021; Penaflorida et al., 2024).
The development of HCC can also be attributed to chronic viral hepatitis and nonalcoholic fatty liver disease (NAFLD). In the context of chronic viral hepatitis, a comprehensive search performed across multiple databases suggests that H. pylori infection may significantly influence the progression of chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, as well as its association with HBV-associated cirrhosis and hepatocellular carcinoma (HCC) (Wang et al., 2016b; Wang et al., 2016a).
Furthermore, a wide range of opinions exist regarding the association between the development of NAFLD and H. pylori. A recent transcriptomic study in a murine model demonstrated that H. pylori infection exacerbates hepatic steatosis associated with metabolic dysfunction by modulating the lipid metabolic pathway, with the H. pylori virulence factor CagA playing a crucial role in this regulatory mechanism (Chen et al., 2024). However, a bidirectional Mendelian randomization study using the GWAS database to evaluate the causal relationship between H. pylori infection and NAFLD suggests that there is currently insufficient genetic evidence to support a causative association between H. pylori infection and NAFLD. This study also indicates that eradicating or preventing H. pylori infection may not confer any discernible benefits for individuals with NAFLD (Liu et al., 2022). Conversely, a randomized controlled trial investigating the impact of H. pylori eradication on patients with NAFLD revealed that after one year of treatment, metabolic parameters associated with NAFLD were further reduced, and liver steatosis was ameliorated, suggesting that screening individuals at risk for NAFLD for H. pylori infection may be advantageous (Yu et al., 2022). A meta-analysis of 34 studies with a total of 175,575 subjects up to January 2024 revealed significantly higher rates of NAFLD among individuals positive for H. pylori than among those negative for H. pylori in 14 studies. In 17 studies (74,928 cases), the prevalence of H. pylori infection was significantly greater in NAFLD patients than in patients without NAFLD, providing compelling evidence supporting a strong correlation between H. pylori infection and susceptibility to NAFLD (Zhang et al., 2024). This association has been shown to be significant in specific populations, such as individuals with diabetes and females, on the basis of multiple cross-sectional studies (Wang et al., 2021; Chen et al., 2023). Despite these findings, multiple clinical studies have shown no significant correlation between H. pylori infection and the development of HCC. A cross-sectional study in Japan involving 13,737 participants who received medical checkups revealed no significant association between H. pylori infection status and the presence of fatty liver disease, including NAFLD (Okushin et al., 2015). A two-year cohort study in China and a study in Korea involving 3663 healthy individuals reported no significant association between H. pylori infection and NAFLD (Baeg et al., 2016; Zheng et al., 2025). Similarly, several single-center, cross-sectional studies have shown no significant association between H. pylori infection and NAFLD (Cai et al., 2018; Fan et al., 2018).
In summary, the association between H. pylori infection and HCC is inconclusive. While some epidemiological studies and meta-analyses suggest a potential link between an increased risk of HCC and infection with H. pylori, especially in the context of HCV coinfection, other research, including a nested case–control study and a mouse model, revealed no significant correlation (Table 2). A bidirectional Mendelian randomization study also revealed insufficient genetic evidence to support a causative link between H. pylori and NAFLD, a condition related to HCC. Despite some studies showing a higher prevalence of NAFLD in H. pylori-positive individuals, multiple cross-sectional studies reported no significant association between H. pylori infection and NAFLD, emphasizing the need for further research to clarify these relationships.
3.1.2 Possible mechanisms
The potential mechanisms by which H. pylori infection may promote HCC are multifaceted and not yet fully understood. On the basis of the available literature, the following mechanisms have been proposed. First, H. pylori infection has been shown to promote liver injury through an exosome-mediated mechanism, which may contribute to hepatocellular carcinoma development (Mohammadi Azad et al., 2024). Specifically, H. pylori infection activates the NF-κB and PI3K/AKT signaling pathways, both of which are intricately linked to hepatic inflammation and tumorigenesis. This activation enhances the proliferation, migration, and invasion capabilities of HepG2 and Hep3B cells, providing a mechanistic explanation for liver damage caused by H. pylori infection (Zhang et al., 2024). Emerging evidence suggests a pathogenic synergy between H. pylori colonization and chronic hepatotropic viral infections (HBV/HCV) in HCC development. This could be due to chronic portal hypertension-induced gastric mucosal barrier compromise facilitates bacterial translocation via portosystemic shunting (Madala et al., 2021). Moreover, CagA+ strains induce mitochondrial-dependent apoptosis in HepG2 cells through ROS-mediated p38 MAPK/JNK activation, while concurrently upregulating pro-survival Bcl-xL expression (Mohammadi et al., 2023). Bile duct-ligated murine models reveal H. pylori’s capacity to colonize peribiliary glands, triggering TLR4/MyD88-dependent IL-6 production that activates quiescent hepatic stellate cells into collagen-secreting myofibroblasts (Goo et al., 2009; Huang et al., 2009). Furthermore, H. pylori lysates impair SMAD2/3 nuclear translocation in LX-2 cells, creating TGF-β1 signaling paradox characterized by simultaneous fibrogenesis promotion and growth inhibition escape (Ki et al., 2010).
In summary, H. pylori infection may play a role in the development and progression of HCC through various mechanisms. These include directly infecting liver tissues, interacting with chronic liver diseases, altering the balance between hepatocyte proliferation and apoptosis, and potentially affecting HCC cell proliferation, invasion, and metastasis. These findings necessitate re-evaluation of antimicrobial eradication protocols in cirrhotic populations and development of microbiome-targeted adjuvant therapies.
3.2 Biliary tract cancer
3.2.1 Associated evidence
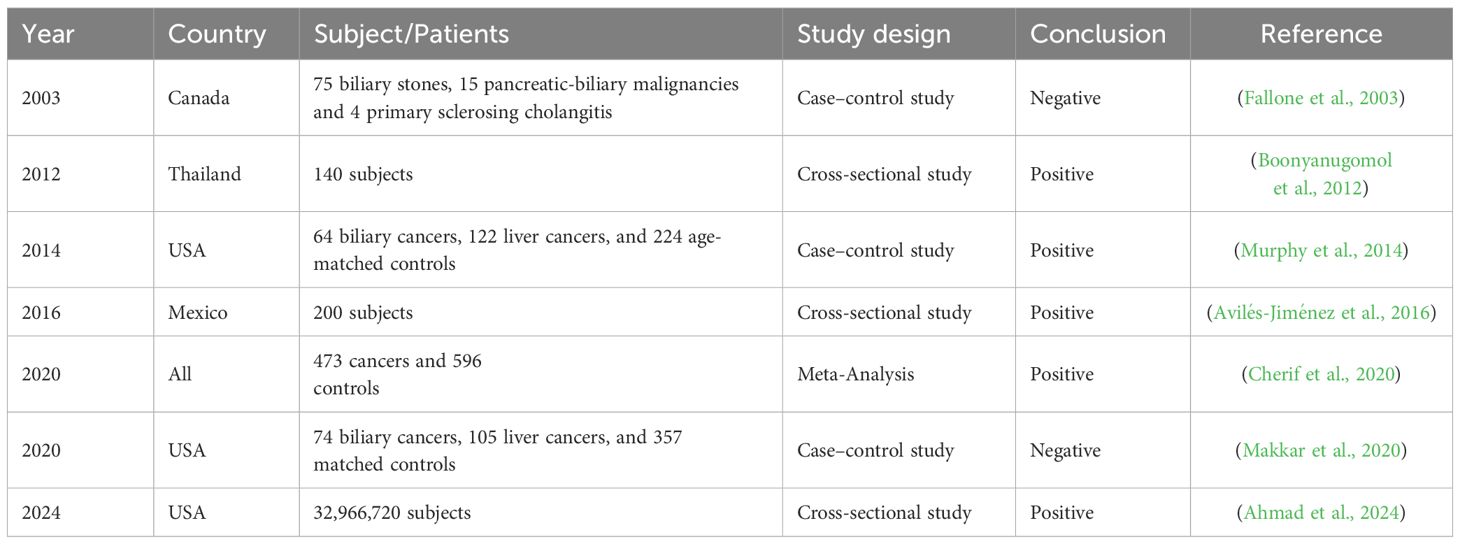
Biliary tract cancer (BTC) is a group of tumors including gallbladder cancer (GBC), extrahepatic cholangiocarcinoma (ECC), and intrahepatic cholangiocarcinoma (ICC).The incidence and mortality of biliary tract cancer (BTC) are rising worldwide, with the sharpest increases observed in South America and Asia. Epidemiologic studies show the disease peaks between ages 50 and 70. Intrahepatic and perihilar cholangiocarcinomas are about 1.5-fold more common in men, whereas gallbladder carcinoma is two- to six-fold more frequent in women (Ghidini et al., 2019). Retrospective studies show higher H. pylori detection in cholangiocarcinoma (CCA) patients than in healthy controls (Boonyanugomol et al., 2012; Avilés-Jiménez et al., 2016), with subgroup odds ratios (ORs) of 4.18 (CCA), 1.36 (GBC), and 5.93 (other BTC) (Cherif et al., 2020). The enrichment of H. pylori virulence factors (CagA, VacA) in ECC further supports this association (Avilés-Jiménez et al., 2016). Additionally, H. pylori has been associated with cholelithiasis and chronic cholecystitis but not gallbladder polyps (Lim et al., 2023). A U.S. national study (2016–2020) confirmed these findings, with chronic cholecystitis exhibiting the highest risk (Ahmad et al., 2024). In the Finnish ATBC study, a positive correlation was observed between seropositivity for H. pylori protein and an elevated risk of BTC, with an OR for all BTC combined of 5.47 (95% CI: 1.17–25.65) (Murphy et al., 2014). However, some studies, including a nested case-control analysis, found no significant link, possibly due to population differences or detection methods (Fallone et al., 2003; Makkar et al., 2020).
In a study evaluating the impact of coinfection on hepatobiliary abnormalities in hamsters, concurrent infection with H. pylori and Opisthorchis viverrini led to the most severe hepatobiliary lesions, characterized by periductal fibrosis, cholangitis, and bile duct hyperplasia (Dangtakot et al., 2017). L-fucose may serve as a viable receptor for Helicobacter pylori, facilitating its colonization in the gut environment of Opisthorchis viverrini (Penaflorida et al., 2024). Moreover, O. viverrini, which acts as a carrier of CagA+ H. pylori, comigrates to the bile duct, facilitating the colonization of H. pylori and exacerbating both pathogenesis and carcinogenesis in the biliary duct (Suyapoh et al., 2021).
Most evidence supports a H. pylori-BTC association, but inconsistencies highlight the need for further research to clarify mechanisms and guide clinical strategies (Table 3).
3.2.2 Possible mechanisms
H. pylori contributes to biliary tract cancer (BTC) through multiple interconnected mechanisms. Chronic inflammation driven by persistent infection creates a pro-carcinogenic microenvironment, promoting genomic instability when combined with other carcinogens (Mishra et al., 2019; Dangtakot et al., 2021). The bacterium may colonize biliary tissues via duodenobiliary reflux (Krasinskas et al., 2007), with clinical studies linking its presence to gallbladder cancer and cholangiocarcinoma (CCA) development, potentially through stone formation (Cherif et al., 2019; Grigor’eva and Romanova, 2020) or direct epithelial transformation (Thanaphongdecha et al., 2020). Specifically, this study investigated the impact of H. pylori-GGT on the induction of apoptosis and the stimulation of IL-8 production in a human bile duct cancer cell line (KKU-100) (Boonyanugomol et al., 2012). Key virulence factors like GGT induce IL-8 production and apoptotic dysregulation in bile duct cells, while CagA+ strains modulate proliferation and inflammatory responses in CCA (Boonyanugomol et al., 2011; Boonyanugomol et al., 2013). Emerging evidence indicates that H. pylori infection exerts significant oncogenic effects in BTC. In vitro studies using human cholangiocarcinoma models have revealed that H. pylori infection induces the activation of NF-κB signaling pathway, which subsequently stimulates VEGF upregulation and facilitates tumor-associated angiogenesis. This pathogenic cascade may substantially accelerate the progression of cholangiocellular carcinoma through enhanced vascularization (Takayama et al., 2010). Notably, the bacterium’s virulence factors appear to participate in the pathogenesis of GBC. Experimental evidence indicates that gallbladder-derived H. pylori strains exhibit marked cytotoxic potential against primary gallbladder epithelium in cell culture systems, initiating a characteristic sequence of cellular degeneration progressing through cytoplasmic vacuolization, membrane disintegration, necrotic changes, and ultimately culminating in programmed cell death (Zhao et al., 2024).
3.3 Esophageal carcinoma
3.3.1 Associated evidence
Globally, esophageal cancer ranks as the seventh most frequently diagnosed malignancy and the sixth leading cause of cancer death, accounting for an estimated 604–000 new cases and 544–000 deaths in 2020. The burden is markedly skewed toward men, who represent roughly 70% of diagnoses and experience incidence and mortality rates two- to three-fold higher than women (Obermannová et al., 2022). Oesophageal cancer is broadly classified into two clinicopathologically distinct entities: esophageal squamous-cell carcinoma (OSCC) and esophageal adenocarcinoma (OAC). OSCC dominates the global landscape, representing approximately 90% of all cases and showing pronounced geographic clustering in East Asia, East Africa and South America (Smyth et al., 2017). Recent research has explored the complex relationship between H. pylori infection and EC, revealing both protective and promotional effects. Esophageal carcinoma (EC) is a significant global health concern originating from the esophageal epithelium, with four main subtypes: esophageal squamous cell carcinoma (ESCC), esophageal adenocarcinoma (EAC), undifferentiated carcinoma, and rarer forms. ESCC and EAC account for over 95% of cases. Recent research has explored the complex relationship between H. pylori infection and EC, revealing both protective and promotional effects.
Epidemiological and molecular studies suggest that chronic H. pylori infection, particularly with CagA-positive strains, may protect against esophageal carcinogenesis through immunomodulation. This contrasts with other findings indicating that H. pylori either promotes cancer or has no significant impact. A recent study reported a significantly lower rate of H. pylori infection (4.5%) in patients with EC than in the general population in a cohort from Spain (López-Gómez et al., 2024). This finding challenges the established negative correlation between H. pylori and gastric malignancies and suggests that H. pylori may have a potential protective effect against EC, especially considering the widespread use of proton pump inhibitors (Omer and Habtemariam, 2024).
Meta-analyses consistently demonstrate H. pylori’s inverse association with EAC and Barrett’s esophagus (BE), attributed to reduced gastric acid secretion and GERD mitigation (Rokkas et al., 2007; Permuth et al., 2018). Via a subgroup analysis, Xie et al. also reported there was no correlation between HP and ESCC in the overall population (Xie et al., 2013). Similarly, colonization by H. pylori, particularly CagA+ strains, was found to be negatively correlated with the incidence of BE. This negative correlation may be mediated through the effects of gastroesophageal reflux disease (GERD) (Wang et al., 2018). Furthermore, Nie demonstrated a negative association between CagA+ HP colonization and ESCC in Asian populations (Nie et al., 2014). A summary of 5 meta-analyses of the occurrence factors of EAC and ESCC, which is consistent with most of the previous results, revealed that H. pylori may play a protective role in EAC and has no correlation with ESCC (Castro et al., 2018). These developments seem to suggest a potential protective association between H. pylori infection and ESCC in Asian and Middle East populations (Gao et al., 2019). A review of the association between H. pylori and EC in Asian populations revealed that there was no significant association between H. pylori and EC in Asian populations but that there was significant heterogeneity across studies, possibly due to differences in population characteristics, the number of cases and controls, H. pylori testing methods, and overall study design. Thus, the “protective” nature of this association may be overestimated in Asian populations (Liu and Liu, 2024).
In summary, the association between H. pylori and EC appears to be subspecies specific (Figure 2). The majority of evidence supports a protective effect of H. pylori in EAC, whereas its association with ESCC is less pronounced. Stratified analysis suggested that the link between H. pylori and ESCC may be overestimated in Eastern populations, contributing to the high heterogeneity observed across studies. Further research is warranted to elucidate the relationship between H. pylori and ESCC (Omer and Habtemariam, 2024).
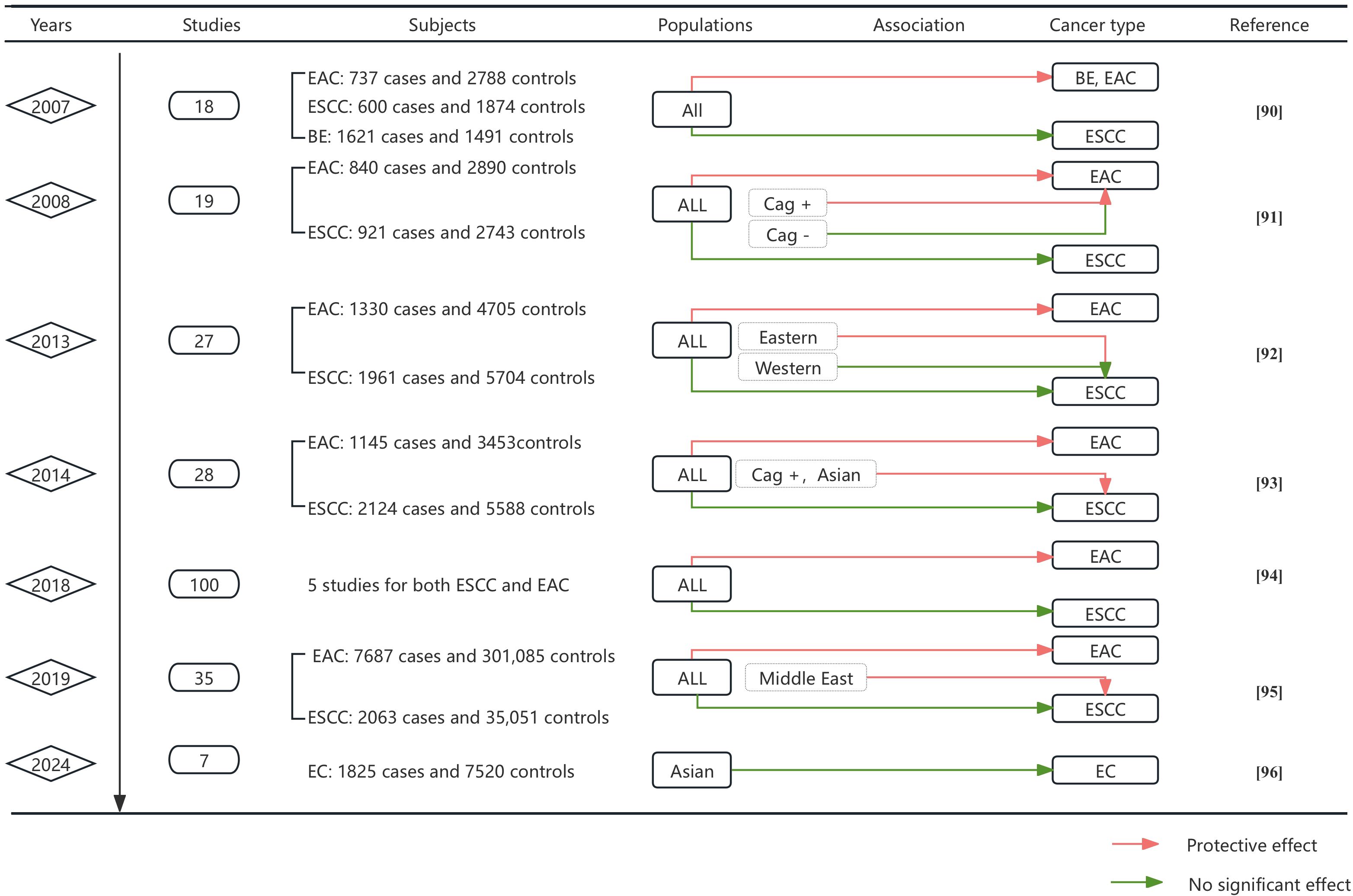
Figure 2. Meta-analysis by time progression of the association between H. pylori and EC. This figure synthesizes key findings from 11 meta-analyses evaluating H. pylori infection and esophageal cancer risk. Data include publication year, number of studies analyzed, subject counts (cases/controls), population characteristics, association direction, cancer subtypes, and references. Color-coded association indicators: Green lines denote statistically significant protective effects of H. pylori infection against cancer development, Red lines indicate no significant association between infection and cancer risk.
3.3.2 Possible mechanisms
The relationship between H. pylori and esophageal cancer (EC) remains complex. While many studies highlight a protective effect—linked to reduced gastric acid secretion (via atrophy or PPIs) and lower GERD risk (Maity et al., 2024; Zhang et al., 2024)—some suggest H. pylori may promote Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC) through COX-2 upregulation via microRNAs (Kountouras et al., 2012; Teng et al., 2018).
In the specific context of BE, H. pylori infection has the potential to induce apoptosis in gastric adenocarcinoma cells that are evolving from BE. This process is mediated through the Fas apoptotic pathway, thereby curbing the progression of these cells into EC. Moreover, H. pylori infection can influence the synthesis of ghrelin, a hormone that plays a key role in regulating appetite and energy balance. Alterations in ghrelin levels may indirectly affect obesity and GERD, both of which are established risk factors for EC (Murphy et al., 2012).
3.4 Colorectal cancer
3.4.1 Associated evidence
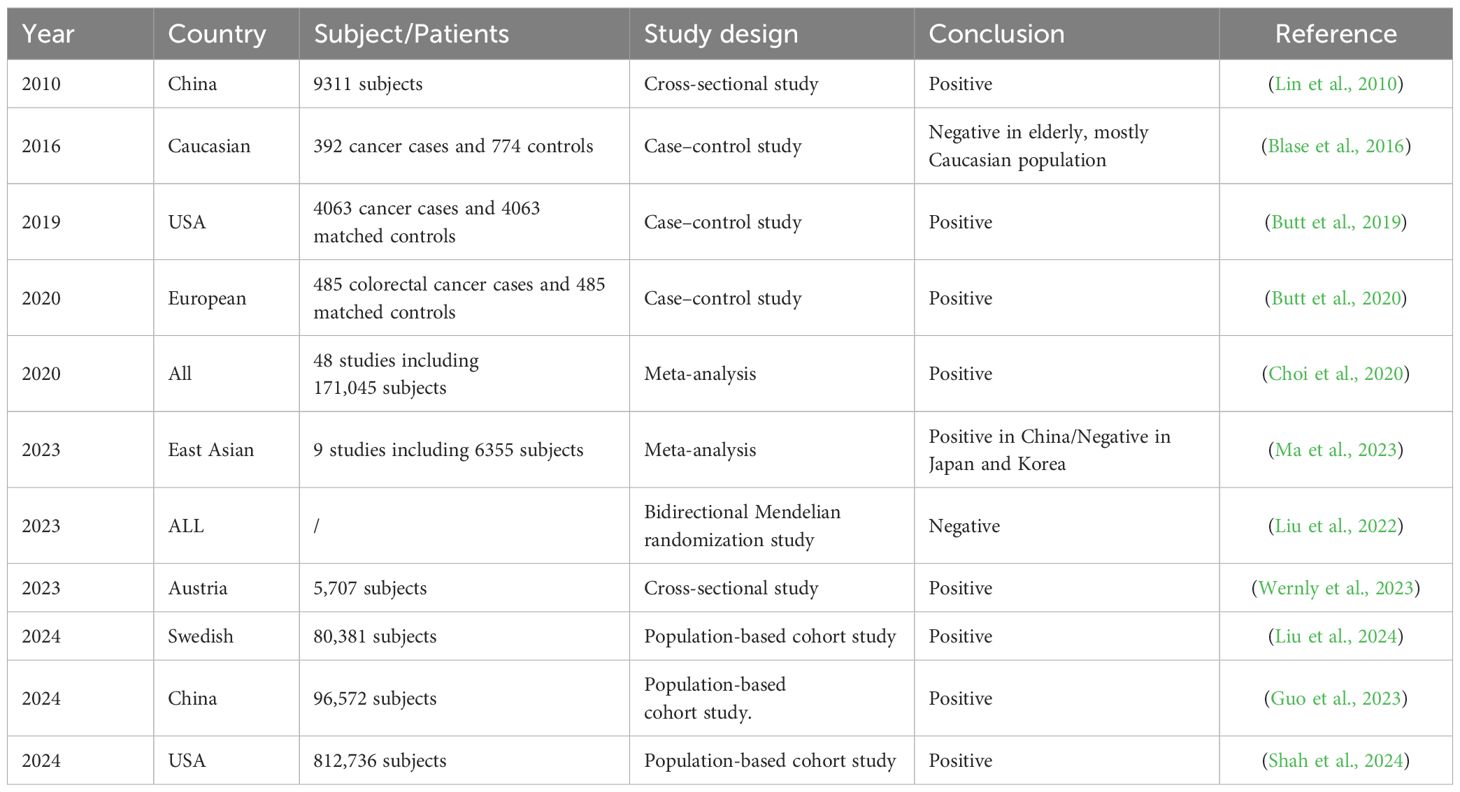
CRC ranks as the third most frequently diagnosed malignancy and the second leading cause of cancer-related death worldwide, with its incidence and mortality rates steadily rising (Abedizadeh et al., 2024). Arising from neoplastic transformation of colonic glandular epithelium, CRC develops exclusively in the colon or rectum and is broadly categorized into three major forms: sporadic, hereditary, and colitis-associated (Hossain et al., 2022). The relationship between H. pylori infection and colorectal cancer (CRC) remains complex and controversial. While CRC is a leading cause of cancer mortality worldwide with multifactorial etiology, numerous studies have investigated H. pylori’s potential role with conflicting results. Several lines of evidence suggest a positive association, particularly seropositivity for virulence factors like HcpC and VacA which correlate with increased CRC risk (Butt et al., 2020). Meta-analyses demonstrate stronger associations in East Asian populations and links with colorectal adenomas and advanced lesions (Choi et al., 2020; Ma et al., 2023). The link between H. pylori infection and an increased risk of CRC is further supported by the majority of published studies, which suggest that chronic atrophic gastritis, induced by H. pylori, enhances susceptibility to colorectal tumors and that the presence of H. pylori infection in individuals with metabolic syndrome further increases this risk (Lin et al., 2010; Kountouras et al., 2017; Venerito et al., 2017). Moreover, the role of H. pylori in the development of CRC is evident in studies examining H. pylori-related metabolic syndrome, which may precipitate the formation of digestive tract tumors. In specific subpopulations, such as those with elevated glycated hemoglobin or NAFLD, the eradication of H. pylori has shown potential in curbing these carcinogenic properties (Kountouras et al., 2018). Importantly, however, the susceptibility to the association between H. pylori and CRC may differ among various ethnic groups (Blase et al., 2016; Butt and Epplein, 2019; Butt et al., 2019). A study examining the risk of colorectal adenoma (CRA) following H. pylori eradication in a Swedish national population revealed that overall H. pylori eradication was associated with an increased risk of CRA. The risk of CRA was elevated 1–2 years after eradication but decreased to below baseline levels at 2–4 years and remained low at 4–6 years. Overall, H. pylori eradication was not consistently associated with a significant reduction in CRC incidence in the Swedish cohort (Liu et al., 2024). A comparable association was observed in the Chinese population, where the initial incidence of CRC was notably higher among individuals infected with H. pylori and subsequently declined to levels below those of the general population, particularly for rectal cancer, approximately 10 years after successful eradication of H. pylori (Guo et al., 2023). The cooccurrence of CRC with H. pylori infection has been extensively documented in numerous studies. Although the specific clinical benefits of H. pylori eradication therapy have yet to be conclusively established, the simplicity of detecting and treating H. pylori suggests that eradication may still represent a potentially beneficial intervention (Wernly et al., 2023). A retrospective cohort study conducted among veterans in the United States revealed that H. pylori positivity was significantly associated with increased CRC incidence and mortality. Over a 15-year follow-up period, individuals who received H. pylori eradication therapy presented a lower CRC incidence and mortality than those who did not receive treatment. Specifically, untreated H. pylori-infected individuals had CRC incidence and mortality rates that were 23% and 40% higher, respectively, than those of H. pylori-infected individuals who underwent eradication therapy. These findings provide further evidence that H. pylori eradication therapy may be beneficial in preventing CRC (Shah et al., 2024).
However, significant limitations and contradictory evidence temper these conclusions. No association was found in elderly Caucasian populations (Blase et al., 2016), and Mendelian randomization analysis failed to establish genetic evidence for causality (Luo et al., 2023). This lack of a genetically - established association, despite some observational correlations, underscores the intricate and potentially variable nature of H. pylori’s role in CRC.
The presence of H. pylori has been identified in both CRA and CRC tissues (Kapetanakis et al., 2012), hinting at a possible biological interaction. However, as reported in a European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, the biological mechanisms that potentially underlie the causal role of H. pylori in the development of CRC remain unclear. Moreover, it is uncertain whether subsequent eradication of H. pylori can effectively reduce CRC incidence (Butt et al., 2020). This uncertainty emphasizes the necessity for further research to elucidate the relationship between H. pylori infection and colorectal neoplasm development, as the existing evidence does not consistently support a direct causal connection (Table 4).
3.4.2 Possible mechanisms
H. pylori contributes to colorectal carcinogenesis through multiple interconnected mechanisms. Recent studies have elucidated several key pathways. Direct interaction with colonic mucosa is evidenced by bacterial detection in polyps (Soylu et al., 2008; Li et al., 2025). The infection dysregulates critical cellular processes by increasing proliferation (elevated Ki-67) and inhibiting apoptosis (reduced Bax/elevated Bcl-2) (Kapetanakis et al., 2012; Kountouras et al., 2017), while also activating cancer stem cells and inducing oncogenes (Kountouras et al., 2017).
The bacterium significantly alters gut microbiota homeostasis, promoting gastrin release that stimulates colorectal cell growth (Fireman et al., 2000; Georgopoulos et al., 2006). Moreover, infection may contribute to cancer progression by disrupting the homeostasis of the gut microbiota, an essential factor in maintaining intestinal health (Kienesberger et al., 2016; Dash et al., 2019). Concurrently, H. pylori triggers chronic inflammation through inflammatory factor release (Jackson et al., 2009; Tuomisto et al., 2019), establishing a tumor-favorable microenvironment. Furthermore, H. pylori infection alters the gut microbiota, influencing intestinal immunity and potentially increasing the risk of CRC (Engelsberger et al., 2024). Key immunomodulatory effects include reduction of regulatory T cells and activation of pro-carcinogenic STAT3 signaling pathways, leading to goblet cell depletion (Ralser et al., 2023). These changes collectively promote epithelial transformation and tumor progression.
3.5 Pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) has already eclipsed breast cancer as the third deadliest malignancy in the United States, and current trajectories suggest it will surpass colorectal cancer before 2040 to rank second only to lung cancer in cancer-related mortality (Halbrook et al., 2023). In the U.S., the disease is typically diagnosed at a median age of 71 years, with incidence modestly higher in men than in women (5.5 versus 4.0 cases per 100,000) (Park et al., 2021). Emerging evidence suggests H. pylori may contribute to pancreatic carcinogenesis, though findings remain inconsistent. While some serological studies demonstrate positive associations (Raderer et al., 1998; Risch et al., 2010; Osaki et al., 2022). However, it is important to acknowledge that the relationship is not consistent, as other large cohort studies have shown either no correlation or an inverse correlation between H. pylori infection and pancreatic cancer (de Martel et al., 2008; Hirabayashi et al., 2019; Permuth et al., 2021). This highlighting the complexity of H. pylori’s oncogenic potential.
Mechanistically, H. pylori infection promotes secretion of proinflammatory cytokines (IL-8, VEGF) and activates oncogenic pathways (NF-κB) in pancreatic cells (Chen et al., 2025), providing biological plausibility for epidemiological observations. These findings underscore the need for further investigation into H. pylori’s role in pancreatic cancer development.
4 Limitations and future directions
Current evidence for H. pylori’s role in extragastric malignancies remains limited by methodological inconsistencies, including geographic/ethnic variability in study populations, divergent diagnostic approaches (serological vs molecular), and inadequate control for confounders like smoking or viral coinfections. These limitations, compounded by heterogeneous study designs, hinder reliable extrapolation of findings. Mechanistic insights are particularly constrained by the lack of translational validation through appropriate animal models or 3D organoid systems that could better recapitulate human tumorigenesis. Moving forward, resolving these uncertainties will require large-scale multicenter cohorts employing standardized diagnostic protocols, coupled with advanced molecular profiling to elucidate strain-specific effects. Simultaneously, genetically engineered models must be developed to validate proposed oncogenic pathways, while interdisciplinary collaboration will be essential for comprehensively characterizing host-pathogen interactions. Such integrated approaches promise to clarify H. pylori’s potential etiological contributions while identifying clinically relevant biomarkers and therapeutic targets for extragastric cancers associated with this persistent infection.
5 Conclusion
Our review demonstrates that H. pylori exhibits distinct associations with extragastric digestive cancers, extending beyond its established gastric carcinogenicity. Compelling epidemiological and mechanistic evidence supports a significant association with increased risk of biliary tract cancers (particularly cholangiocarcinoma, OR 4.18) and, to a more variable extent, hepatocellular carcinoma (OR 4.75, especially with HCV coinfection). In contrast, H. pylori (notably CagA+ strains) confers a protective effect against esophageal adenocarcinoma. Associations with colorectal cancer show population-specific patterns (increased risk in East Asia), while links to pancreatic cancer remain inconsistent. Proposed oncogenic mechanisms involve chronic inflammation, direct virulence factor activity (CagA/VacA), and microbiome dysbiosis. These findings highlight the potential for targeted therapeutic strategies, including eradication in high-risk populations.
Author contributions
HX: Data curation, Writing – original draft. ChiZ: Conceptualization, Writing – original draft. ChaZ: Conceptualization, Writing – original draft. XZ: Methodology, Writing – original draft. DC: Methodology, Writing – original draft. QW: Funding acquisition, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by Hospital Management Research Project of Tianjin Hospital Association (2024zc147)
Conflict of interest
Authors ChiZ, ChaZ, XZ and DC were employed by the companies Jiangsu Simcere Diagnostics Co., Ltd and Nanjing Simcere Medical Laboratory Science Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Razik, A., Mousa, N., Elhelaly, R., Elzehery, R., Hasan, A. S., Abdelsalam, M., et al. (2020). Helicobacter pylori as an initiating factor of complications in patients with cirrhosis: A single-center observational study. Front. Med. (Lausanne). 7. doi: 10.3389/fmed.2020.00096
Abedizadeh, R., Majidi, F., Khorasani, H. R., Abedi, H., and Sabour, D. (2024). Colorectal cancer: a comprehensive review of carcinogenesis, diagnosis, and novel strategies for classified treatments. Cancer Metastasis Rev. 43, 729–753. doi: 10.1007/s10555-023-10158-3
Ahmad, S. O., AlAmr, M., Taftafa, A., AlMazmomy, A. M., Alkahmous, N., Alharran, A. M., et al. (2024). Exploring the relationship between Helicobacter pylori infection and biliary diseases: A comprehensive analysis using the United States national inpatient sample (2016-2020). Cureus 16, e61238. doi: 10.7759/cureus.61238
Altekruse, S. F., McGlynn, K. A., and Reichman, M. E. (2009). Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. 27, 1485–1491. doi: 10.1200/JCO.2008.20.7753
Ansari, S. and Yamaoka, Y. (2020). Role of vacuolating cytotoxin A in Helicobacter pylori infection and its impact on gastric pathogenesis. Expert Rev. Anti Infect. Ther. 18, 987–996. doi: 10.1080/14787210.2020.1782739
(1994). Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr. Eval. Carcinog Risks Hum. 61, 1–241.
Avilés-Jiménez, F., Guitrón, A., Segura-López, F., Méndez-Tenorio, A., Iwai, S., Hernández-Guerrero, A., et al. (2016). Microbiota studies in the bile duct strongly suggest a role for Helicobacter pylori in extrahepatic cholangiocarcinoma. Clin. Microbiol. Infect. 22, 178.e11–178.e22. doi: 10.1016/j.cmi.2015.10.008
Backert, S., Tegtmeyer, N., and Fischer, W. (2015). Composition, structure and function of the Helicobacter pylori cag pathogenicity island encoded type IV secretion system. Future Microbiol. 10, 955–965. doi: 10.2217/fmb.15.325
Baeg, M. K., Yoon, S. K., Ko, S. H., Noh, Y. S., Lee, I. S., and Choi, M. G. (2016). Helicobacter pylori infection is not associated with nonalcoholic fatty liver disease. World J. Gastroenterol. 22, 2592–2600. doi: 10.3748/wjg.v22.i8.2592
Baj, J., Forma, A., Sitarz, M., Portincasa, P., Garruti, G., Krasowska, D., et al. (2020). Helicobacter pylori virulence factors-mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells 10, 27. doi: 10.3390/cells10010027
Bergman, M. P., Engering, A., Smits, H. H., van Vliet, S. J., van Bodegraven, A. A., Wirth, H. P., et al. (2004). Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 200, 979–990. doi: 10.1084/jem.20041061
Blase, J. L., Campbell, P. T., Gapstur, S. M., Pawlita, M., Michel, A., Waterboer, T., et al. (2016). Prediagnostic Helicobacter pylori antibodies and colorectal cancer risk in an elderly, Caucasian population. Helicobacter 21, 488–492. doi: 10.1111/hel.12305
Boonyanugomol, W., Chomvarin, C., Baik, S. C., Song, J. Y., Hahnvajanawong, C., Kim, K. M., et al. (2011). Role of cagA-positive Helicobacter pylori on cell proliferation, apoptosis, and inflammation in biliary cells. Dig Dis. Sci. 56, 1682–1692. doi: 10.1007/s10620-010-1512-y
Boonyanugomol, W., Chomvarin, C., Hahnvajanawong, C., Sripa, B., Kaparakis-Liaskos, M., and Ferrero, R. L. (2013). Helicobacter pylori cag pathogenicity island (cagPAI) involved in bacterial internalization and IL-8 induced responses via NOD1- and MyD88-dependent mechanisms in human biliary epithelial cells. PloS One 8, e77358. doi: 10.1371/journal.pone.0077358
Boonyanugomol, W., Chomvarin, C., Sripa, B., Bhudhisawasdi, V., Khuntikeo, N., Hahnvajanawong, C., et al. (2012). Helicobacter pylori in Thai patients with cholangiocarcinoma and its association with biliary inflammation and proliferation. HPB (Oxford). 14, 177–184. doi: 10.1111/j.1477-2574.2011.00423.x
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263. doi: 10.3322/caac.21834
Butt, J. and Epplein, M. (2019). Helicobacter pylori and colorectal cancer-A bacterium going abroad? PloS Pathog. 15, e1007861. doi: 10.1371/journal.ppat.1007861
Butt, J., Jenab, M., Pawlita, M., Tjønneland, A., Kyrø, C., Boutron-Ruault, M. C., et al. (2020). Antibody responses to Helicobacter pylori and risk of developing colorectal cancer in a European cohort. Cancer Epidemiol. Biomarkers Prev. 29, 1475–1481. doi: 10.1158/1055-9965.EPI-19-1545
Butt, J., Varga, M. G., Blot, W. J., Teras, L., Visvanathan, K., Le Marchand, L., et al. (2019). Serologic response to Helicobacter pylori proteins associated with risk of colorectal cancer among diverse populations in the United States. Gastroenterology 156, 175–186.e2. doi: 10.1053/j.gastro.2018.09.054
Cai, O., Huang, Z., Li, M., Zhang, C., Xi, F., and Tan, S. (2018). Association between Helicobacter pylori infection and nonalcoholic fatty liver disease: A single-center clinical study. Gastroenterol. Res. Pract. 2018, 8040262. doi: 10.1155/2018/8040262
Castro, C., Peleteiro, B., and Lunet, N. (2018). Modifiable factors and esophageal cancer: a systematic review of published meta-analyses. J. Gastroenterol. 53, 37–51. doi: 10.1007/s00535-017-1375-5
Chen, X., Peng, R., Peng, D., Liu, D., and Li, R. (2024). Helicobacter pylori infection exacerbates metabolic dysfunction-associated steatotic liver disease through lipid metabolic pathways: a transcriptomic study. J. Transl. Med. 22, 701. doi: 10.1186/s12967-024-05506-y
Chen, X., Sun, F., Wang, X., Feng, X., Aref, A. R., Tian, Y., et al (2025). Inflammation, microbiota, and pancreatic cancer. Cancer Cell Int. 25, 62. doi: 10.1186/s12935-025-03673-6
Chen, Y., You, N., Shen, C., Wu, J., and Zhang, J. (2023). Helicobacter pylori infection increases the risk of nonalcoholic fatty liver disease in diabetic population. Front. Nutr. 10. doi: 10.3389/fnut.2023.1076579
Cherif, S., Bouriat, K., Rais, H., Elantri, S., and Amine, A. (2020). Helicobacter pylori and biliary tract cancers: A meta-analysis. Can. J. Infect. Dis. Med. Microbiol. 2020, 1–7. doi: 10.1155/2020/9287157
Cherif, S., Rais, H., Hakmaoui, A., Sellami, S., Elantri, S., and Amine, A. (2019). Linking Helicobacter pylori with gallbladder and biliary tract cancer in Moroccan population using clinical and pathological profiles. Bioinformation 15, 735–743. doi: 10.6026/97320630015735
Choi, D. S., Seo, S. I., Shin, W. G., and Park, C. H. (2020). Risk for colorectal neoplasia in patients with Helicobacter pylori infection: A systematic review and meta-analysis. Clin. Transl. Gastroenterol. 11, e00127. doi: 10.14309/ctg.0000000000000127
Dangtakot, R., Intuyod, K., Chamgramol, Y., Pairojkul, C., Pinlaor, S., Jantawong, C., et al. (2021). CagA+ Helicobacter pylori infection and N-nitrosodimethylamine administration induce cholangiocarcinoma development in hamsters. Helicobacter 26, e12817. doi: 10.1111/hel.12817
Dangtakot, R., Pinlaor, S., Itthitaetrakool, U., Chaidee, A., Chomvarin, C., Sangka, A., et al. (2017). Coinfection with Helicobacter pylori and Opisthorchis viverrini Enhances the Severity of Hepatobiliary Abnormalities in Hamsters. Infect. Immun. 85, e00009–e00017. doi: 10.1128/IAI.00009-17
Dash, N. R., Khoder, G., Nada, A. M., and Al Bataineh, M. T. (2019). Exploring the impact of Helicobacter pylori on gut microbiome composition. PloS One 14, e0218274. doi: 10.1371/journal.pone.0218274
Debowski, A. W., Walton, S. M., Chua, E. G., Tay, A. C., Liao, T., Lamichhane, B., et al. (2017). Helicobacter pylori gene silencing in vivo demonstrates urease is essential for chronic infection. PloS Pathog. 13, e1006464. doi: 10.1371/journal.ppat.1006464
de Brito, B. B., da Silva, F. A. F., Soares, A. S., Pereira, V. A., Santos, M. L. C., and Sampaio, M. M. (2019). Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol. 25, 5578–5589. doi: 10.3748/wjg.v25.i37.5578
de Martel, C., Georges, D., Bray, F., Ferlay, J., and Clifford, G. M. (2020). Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 8, e180–e190. doi: 10.1016/S2214-109X(19)30488-7
de Martel, C., Llosa, A. E., Friedman, G. D., Vogelman, J. H., Orentreich, N., Stolzenberg-Solomon, R. Z., et al. (2008). Helicobacter pylori infection and development of pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 17, 1188–1194. doi: 10.1158/1055-9965.EPI-08-0185
Duan, Y., Xu, Y., Dou, Y., and Xu, D. (2025). Helicobacter pylori and gastric cancer: mechanisms and new perspectives. J. Hematol. Oncol. 18, 10. doi: 10.1186/s13045-024-01654-2
El-Omar, E. M., Carrington, M., Chow, W. H., McColl, K. E., Bream, J. H., Young, H. A., et al. (2000). Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404, 398–402. doi: 10.1038/35006081
Engelsberger, V., Gerhard, M., and Mejías-Luque, R. (2024). Effects of Helicobacter pylori infection on intestinal microbiota, immunity and colorectal cancer risk. Front. Cell Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1339750
Esmat, G., El-Bendary, M., Zakarya, S., Ela, M. A., and Zalata, K. (2012). Role of Helicobacter pylori in patients with HCV-related chronic hepatitis and cirrhosis with or without hepatocellular carcinoma: possible association with disease progression. J. Viral Hepat. 19, 473–479. doi: 10.1111/j.1365-2893.2011.01567.x
Fallone, C. A., Tran, S., Semret, M., Discepola, F., Behr, M., and Barkun, A. N. (2003). Helicobacter DNA in bile: correlation with hepato-biliary diseases. Aliment Pharmacol. Ther. 17, 453–458. doi: 10.1046/j.1365-2036.2003.01424.x
Fan, N., Peng, L., Xia, Z., Zhang, L., Wang, Y., and Peng, Y. (2018). Helicobacter pylori infection is not associated with non-alcoholic fatty liver disease: A cross-sectional study in China. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.00073
Fireman, Z., Trost, L., Kopelman, Y., Segal, A., and Sternberg, A. (2000). Helicobacter pylori: seroprevalence and colorectal cancer. Isr. Med. Assoc. J. 2, 6–9.
Fuchs, S., Gong, R., Gerhard, M., and Mejías-Luque, R. (2023). Immune biology and persistence of Helicobacter pylori in gastric diseases. Curr. Top. Microbiol. Immunol. 444, 83–115. doi: 10.1007/978-3-031-47331-9_4
Gao, H., Li, L., Zhang, C., Tu, J., Geng, X., Wang, J., et al. (2019). Systematic review with meta-analysis: association of Helicobacter pylori infection with esophageal cancer. Gastroenterol. Res. Pract. 2019, 1953497. doi: 10.1155/2019/1953497
García, A., Feng, Y., Parry, N. M., McCabe, A., Mobley, M. W., Lertpiriyapong, K., et al. (2013). Helicobacter pylori infection does not promote hepatocellular cancer in a transgenic mouse model of hepatitis C virus pathogenesis. Gut Microbes 4, 577–590. doi: 10.4161/gmic.26042
Georgopoulos, S. D., Polymeros, D., Triantafyllou, K., Spiliadi, C., Mentis, A., Karamanolis, D. G., et al. (2006). Hypergastrinemia is associated with increased risk of distal colon adenomas. Digestion 74, 42–46. doi: 10.1159/000096593
Ghidini, M., Pizzo, C., Botticelli, A., Hahne, J. C., Passalacqua, R., Tomasello, G., et al. (2019). Biliary tract cancer: current challenges and future prospects. Cancer Manag Res. 11, 379–388. doi: 10.2147/CMAR.S157156
Goo, M. J., Ki, M. R., Lee, H. R., Yang, H. J., Yuan, D. W., Hong, I. H., et al. (2009). Helicobacter pylori promotes hepatic fibrosis in the animal model. Lab. Invest. 89, 1291–1303. doi: 10.1038/labinvest.2009.90
Grigor’eva, I. N. and Romanova, T. I. (2020). Gallstone disease and microbiome. Microorganisms 8, 835. doi: 10.3390/microorganisms8060835
Guo, C. G., Zhang, F., Jiang, F., Wang, L., Chen, Y., Zhang, W., et al. (2023). Long-term effect of Helicobacter pylori eradication on colorectal cancer incidences. Therap Adv. Gastroenterol. 16, 17562848231170943. doi: 10.1177/17562848231170943
Halbrook, C. J., Lyssiotis, C. A., Pasca di Magliano, M., Maitra, A., et al. (2023). Pancreatic cancer: Advances and challenges. Cell 186, 1729–1754. doi: 10.1016/j.cell.2023.02.014
Hatakeyama, M. (2004). Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer. 4, 688–694. doi: 10.1038/nrc1433
Hirabayashi, M., Inoue, M., Sawada, N., Saito, E., Abe, S. K., Hidaka, A., et al. (2019). Helicobacter pylori infection, atrophic gastritis, and risk of pancreatic cancer: A population-based cohort study in a large Japanese population: the JPHC Study. Sci. Rep. 9, 6099. doi: 10.1038/s41598-019-42365-w
Hossain, M. S., Karuniawati, H., Jairoun, A. A., Urbi, Z., Ooi, J., John, A., et al. (2022). Colorectal cancer: A review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers (Basel). 14, 1732. doi: 10.3390/cancers14071732
Hou, B., Zhang, M., Liu, M., Dai, W., Lin, Y., Li, Y., et al. (2019). Association of active Helicobacter pylori infection and anemia in elderly males. BMC Infect. Dis. 19, 228. doi: 10.1186/s12879-019-3849-y
Huang, Y., Tian, X. F., Fan, X. G., Fu, C. Y., and Zhu, C. (2009). The pathological effect of Helicobacter pylori infection on liver tissues in mice. Clin. Microbiol. Infect. 15, 843–849. doi: 10.1111/j.1469-0691.2009.02719.x
Jackson, L., Britton, J., Lewis, S. A., McKeever, T. M., Atherton, J., Fullerton, D., et al. (2009). A population-based epidemiologic study of Helicobacter pylori infection and its association with systemic inflammation. Helicobacter 14, 108–113. doi: 10.1111/j.1523-5378.2009.00711.x
Kapetanakis, N., Kountouras, J., Zavos, C., Polyzos, S. A., Kouklakis, G., Venizelos, I., et al. (2012). Helicobacter pylori infection and colorectal carcinoma: pathologic aspects. J. Gastrointest Oncol. 3, 377–379. doi: 10.3978/j.issn.2078-6891.2012.041
Karkhah, A., Ebrahimpour, S., Rostamtabar, M., Koppolu, V., Darvish, S., Vasigala, V. K. R., et al. (2019). Helicobacter pylori evasion strategies of the host innate and adaptive immune responses to survive and develop gastrointestinal diseases. Microbiol. Res. 218, 49–57. doi: 10.1016/j.micres.2018.09.011
Ki, M. R., Goo, M. J., Park, J. K., Hong, I. H., Ji, A. R., Han, S., et al. (2010). Helicobacter pylori accelerates hepatic fibrosis by sensitizing transforming growth factor-β1-induced inflammatory signaling. Lab. Invest. 90, 1507–1516. doi: 10.1038/labinvest.2010.109
Kienesberger, S., Cox, L. M., Livanos, A., Zhang, X. S., Chung, J., Perez-Perez, G. I., et al. (2016). Gastric Helicobacter pylori infection affects local and distant microbial populations and host responses. Cell Rep. 14, 1395–1407. doi: 10.1016/j.celrep.2016.01.017
Kountouras, J., Zavos, C., Polyzos, S. A., and Katsinelos, P. (2012). Helicobacter pylori infection might contribute to esophageal adenocarcinoma progress in subpopulations with gastroesophageal reflux disease and Barrett’s esophagus. Helicobacter 17, 402–403. doi: 10.1111/j.1523-5378.2012.00963.x
Kountouras, J., Kapetanakis, N., Polyzos, S. A., Katsinelos, P., Gavalas, E., Tzivras, D., et al. (2017). Active Helicobacter pylori infection is a risk factor for colorectal mucosa: early and advanced colonic neoplasm sequence. Gut Liver. 11, 733–734. doi: 10.5009/gnl16389
Kountouras, J., Polyzos, S. A., Doulberis, M., Zeglinas, C., Artemaki, F., Vardaka, E., et al. (2018). Potential impact of Helicobacter pylori-related metabolic syndrome on upper and lower gastrointestinal tract oncogenesis. Metabolism 87, 18–24. doi: 10.1016/j.metabol.2018.06.008
Krasinskas, A. M., Yao, Y., Randhawa, P., Dore, M. P., and Sepulveda, A. R. (2007). Helicobacter pylori may play a contributory role in the pathogenesis of primary sclerosing cholangitis. Dig Dis. Sci. 52, 2265–2270. doi: 10.1007/s10620-007-9803-7
Lee, J. Y., Kim, S. E., Park, S. J., Park, M. I., Moon, W., Kim, J. H., et al. (2022). Helicobacter pylori infection and iron deficiency in non-elderly adults participating in a health check-up program. Korean J. Intern. Med. 37, 304–312. doi: 10.3904/kjim.2020.433
Li, Y., Choi, H., Leung, K., Jiang, F., Graham, D. Y., and Leung, W. K. (2023). Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 8, 553–564. doi: 10.1016/S2468-1253(23)00070-5
Li, X., Tao, H. Q., Zhao, J. E., Zhu, J., Du, L. B., Gerhard, M., et al. (2025). Helicobacter pylori infection, anti-Helicobacter pylori treatment and risk of colorectal cancer and adenoma: an observational study and a meta-analysis. EClinicalMedicine 84, 103299. doi: 10.1016/j.eclinm.2025.103299
Lim, K. P. K., Lee, A. J. L., Jiang, X., Teng, T. Z. J., and Shelat, V. G. (2023). The link between Helicobacter pylori infection and gallbladder and biliary tract diseases: A review. Ann. Hepatobiliary Pancreat Surg. 27, 241–250. doi: 10.14701/ahbps.22-056
Lin, Y. L., Chiang, J. K., Lin, S. M., and Tseng, C. E. (2010). Helicobacter pylori infection concomitant with metabolic syndrome further increase risk of colorectal adenomas. World J. Gastroenterol. 16, 3841–3846. doi: 10.3748/wjg.v16.i30.3841
Liu, J. and Liu, Y. L. (2024). Should we pay more attention to the potential link between Helicobacter pylori and esophageal cancer in Asian countries. World J. Gastroenterol. 30, 4958–4963. doi: 10.3748/wjg.v30.i46.4958
Liu, Q., Sadr-Azodi, O., Engstrand, L., Fall, K., and Brusselaers, N. (2024). Helicobacter pylori eradication therapy and the risk of colorectal cancer: A population-based nationwide cohort study in Sweden. Helicobacter 29, e70001. doi: 10.1111/hel.70001
Liu, Y., Xu, H., Zhao, Z., Dong, Y., Wang, X., and Niu, J. (2022). No evidence for a causal link between Helicobacter pylori infection and nonalcoholic fatty liver disease: A bidirectional Mendelian randomization study. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1018322
López-Gómez, M., Morales, M., Fuerte, R., Muñoz, M., Delgado-López, P. D., Gómez-Cerezo, J. F., et al. (2024). Prevalence of Helicobacter pylori infection among patients with esophageal carcinoma. World J. Gastroenterol. 30, 3479–3487. doi: 10.3748/wjg.v30.i29.3479
Luo, F., Zhou, P., Ran, X., Gu, M., and Zhou, S. (2023). No evident causal association between Helicobacter pylori infection and colorectal cancer: a bidirectional mendelian randomization study. Sci. Rep. 13, 18544. doi: 10.1038/s41598-023-45545-x
Ma, L., Guo, W., Zeng, Z., Yang, F., Tang, S., and Ling, Y. (2023). Colorectal cancer risk in East Asian patients with Helicobacter pylori infection: A systematic review and meta-analysis. Med. (Baltimore). 102, e33177. doi: 10.1097/MD.0000000000033177
Madala, S., MacDougall, K., Surapaneni, B. K., Park, R., Girotra, M., and Kasi, A. (2021). Coinfection of Helicobacter pylori and hepatitis C virus in the development of hepatocellular carcinoma: A systematic review and meta-analysis. J. Clin. Med. Res. 13, 530–540. doi: 10.14740/jocmr4637
Maity, R., Dhali, A., and Biswas, J. (2024). Is Helicobacter pylori infection protective against esophageal cancer? World J. Gastroenterol. 30, 4168–4174. doi: 10.3748/wjg.v30.i38.4168
Makkar, R., Butt, J., Huang, W. Y., McGlynn, K. A., Koshiol, J., Pawlita, M., et al. (2020). Seropositivity for Helicobacter pylori and hepatobiliary cancers in the PLCO study. Br. J. Cancer. 123, 909–911. doi: 10.1038/s41416-020-0961-0
Malfertheiner, P., Camargo, M. C., El-Omar, E., Liou, J. M., Peek, R., Schulz, C., et al. (2023). Helicobacter pylori infection. Nat. Rev. Dis. Primers. 9, 19. doi: 10.1038/s41572-023-00431-8
Marshall, B. J. and Warren, J. R. (1984). Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1, 1311–1315. doi: 10.1016/s0140-6736(84)91816-6
Mishra, S. K., Kumari, N., and Krishnani, N. (2019). Molecular pathogenesis of gallbladder cancer: An update. Mutat. Res. 816-818, 111674. doi: 10.1016/j.mrfmmm.2019.111674
Mohammadi, M., Attar, A., Mohammadbeigi, M., Peymani, A., Bolori, S., and Fardsanei, F. (2023). The possible role of Helicobacter pylori in liver diseases. Arch. Microbiol. 205, 281. doi: 10.1007/s00203-023-03602-z
Mohammadi Azad, Z., Moosazadeh Moghaddam, M., Fasihi-Ramandi, M., Haghighat, S., and Mirnejad, R. (2024). Evaluation of the effect of Helicobacter pylori -derived OMVs and released exosomes from stomach cells treated with OMVs on the expression of genes related to the TGF-β/SMAD signaling pathway in hepatocellular carcinoma. J. Recept Signal Transduct Res. 44, 181–190. doi: 10.1080/10799893.2024.2436461
Murphy, G., Kamangar, F., Albanes, D., Stanczyk, F. Z., Weinstein, S. J., Taylor, P. R., et al. (2012). Serum ghrelin is inversely associated with risk of subsequent oesophageal squamous cell carcinoma. Gut 61, 1533–1537. doi: 10.1136/gutjnl-2011-300653
Murphy, G., Michel, A., Taylor, P. R., Albanes, D., Weinstein, S. J., Virtamo, J., et al. (2014). Association of seropositivity to Helicobacter species and biliary tract cancer in the ATBC study. Hepatology 60, 1963–1971. doi: 10.1002/hep.27193
Muzaheed (2020). Helicobacter pylori oncogenicity: mechanism, prevention, and risk factors. Scientific World Journal 2020, 3018326. doi: 10.1155/2020/3018326
Nagashima, H. and Yamaoka, Y. (2019). Importance of toll-like receptors in pro-inflammatory and anti-inflammatory responses by Helicobacter pylori infection. Curr. Top. Microbiol. Immunol. 421, 139–158. doi: 10.1007/978-3-030-15138-6_6
Nie, S., Chen, T., Yang, X., Huai, P., and Lu, M. (2014). Association of Helicobacter pylori infection with esophageal adenocarcinoma and squamous cell carcinoma: a meta-analysis. Dis. Esophagus. 27, 645–653. doi: 10.1111/dote.12194
Obermannová, R., Alsina, M., Cervantes, A., and Guidelines Committee, E. S. M. O. (2022). Electronic address:Y2xpbmljYWxndWlkZWxpbmVzQGVzbW8ub3Jn. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33, 992–1004. doi: 10.1016/j.annonc.2022.07.003
Okushin, K., Takahashi, Y., Yamamichi, N., Shimamoto, T., Enooku, K., Fujinaga, H., et al. (2015). Helicobacter pylori infection is not associated with fatty liver disease including non-alcoholic fatty liver disease: a large-scale cross-sectional study in Japan. BMC Gastroenterol. 15, 25. doi: 10.1186/s12876-015-0247-9
Omer, J. I. and Habtemariam, A. H. (2024). Revaluation of Helicobacter pylori’s role in esophageal carcinoma: A call for comprehensive research. World J. Gastroenterol. 30, 5194–5197. doi: 10.3748/wjg.v30.i48.5194
Osaki, T., Lin, Y., Sasahira, N., Ueno, M., Yonezawa, H., Hojo, F., et al. (2022). Prevalence estimates of Helicobacter species infection in pancreatic and biliary tract cancers. Helicobacter 27, e12866. doi: 10.1111/hel.12866
Park, W., Chawla, A., and O’Reilly, E. M. (2021). Pancreatic cancer: A review. JAMA 326, 851–862. doi: 10.1001/jama.2021.13027
Penaflorida, J. L. G. R., Requesto, J. R. U., Romero, K. Y. B., et al. (2024). Association between Helicobacter pylori and hepatobiliary cancer: A meta-analysis and systematic review. Asian Pac J. Cancer Prev. 25, 3363–3370. doi: 10.31557/APJCP.2024.25.10.3363
Permuth, J. B., Rahman, S., Chen, D. T., Waterboer, T., Giuliano, A. R., et al. (2018). Helicobacter pylori infection and esophageal adenocarcinoma: a review and a personal view. Ann. Gastroenterol. 31, 8–13. doi: 10.20524/aog.2017.0213
Permuth, J. B., Rahman, S., Chen, D. T., Waterboer, T., and Giuliano, A. R. (2021). A case control study of the seroprevalence of Helicobacter pylori proteins and their association with pancreatic cancer risk. J. Pancreat Cancer. 7, 57–64. doi: 10.1089/pancan.2021.0010
Pormohammad, A., Mohtavinejad, N., Gholizadeh, P., Dabiri, H., Salimi Chirani, A., Hashemi, A., et al. (2019). Global estimate of gastric cancer in Helicobacter pylori-infected population: A systematic review and meta-analysis. J. Cell Physiol. 234, 1208–1218. doi: 10.1002/jcp.27114
Raderer, M., Wrba, F., Kornek, G., Maca, T., Koller, D. Y., Weinlaender, G., et al. (1998). Association between Helicobacter pylori infection and pancreatic cancer. Oncology 55, 16–19. doi: 10.1159/000011830
Ralser, A., Dietl, A., Jarosch, S., Engelsberger, V., Wanisch, A., Janssen, K. P., et al. (2023). Helicobacter pylori promotes colorectal carcinogenesis by deregulating intestinal immunity and inducing a mucus-degrading microbiota signature. Gut 72, 1258–1270. doi: 10.1136/gutjnl-2022-328075
Reyes, V. E. and Peniche, A. G. (2019). Helicobacter pylori deregulates T and B cell signaling to trigger immune evasion. Curr. Top. Microbiol. Immunol. 421, 229–265. doi: 10.1007/978-3-030-15138-6_10
Risch, H. A., Yu, H., Lu, L., and Kidd, M. S. (2010). ABO blood group, Helicobacter pylori seropositivity, and risk of pancreatic cancer: a case-control study. J. Natl. Cancer Inst. 102, 502–505. doi: 10.1093/jnci/djq007
Roche, N., Angström, J., Hurtig, M., Backert, S., Clyne, M., Tegtmeyer, N., et al. (2004). Helicobacter pylori and complex gangliosides. Infect. Immun. 72, 1519–1529. doi: 10.1128/IAI.72.3.1519-1529.2004
Roesler, B. M., Rabelo-Gonçalves, E. M., and Zeitune, J. M. (2014). Virulence factors of helicobacter pylori: A review. Clin. Med. Insights Gastroenterol. 7, 9–17. doi: 10.4137/CGast.S13760
Rokkas, T., Pistiolas, D., Sechopoulos, P., Robotis, I., and Margantinis, G. (2007). Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin. Gastroenterol. Hepatol. 5, 1413–1417, 1417.e1-2. doi: 10.1016/j.cgh.2007.08.010
Samragnyi, M., Kira, M., Balarama, K. S., Robin, P., Anup, K., Mohit, G., et al. (2021). Relationship between Helicobacter pylori and development of hepatocellular carcinoma: A systematic review and meta-analysis. JCO 39, 4091–4091. doi: 10.1200/JCO.2021.39.15_suppl.4091
Shah, S. C., Camargo, M. C., Lamm, M., Bustamante, R., Roumie, C. L., Wilson, O., et al. (2024). Impact of Helicobacter pylori infection and treatment on colorectal cancer in a large, nationwide cohort. J. Clin. Oncol. 42, 1881–1889. doi: 10.1200/JCO.23.0070310.1016/0002-9610(75)90174-9
Shibayama, K., Wachino, J., Arakawa, Y., Saidijam, M., Rutherford, N. G., Henderson, P. J., et al. (2007). Metabolism of glutamine and glutathione via gamma-glutamyltranspeptidase and glutamate transport in Helicobacter pylori: possible significance in the pathophysiology of the organism. Mol. Microbiol. 64, 396–406. doi: 10.1111/j.1365-2958.2007.05661.x
Sirit, I. S. and Peek, R. M., Jr. (2025). Decoding the ability of Helicobacter pylori to evade immune recognition and cause disease. Cell Mol. Gastroenterol. Hepatol. 19, 101470. doi: 10.1016/j.jcmgh.2025.101470
Smyth, E. C., Lagergren, J., Fitzgerald, R. C., Lordick, F., Shah, M. A., Lagergren, P., et al. (2017). Oesophageal cancer. Nat. Rev. Dis. Primers. 3, 17048. doi: 10.1038/nrdp.2017.48
Soylu, A., Ozkara, S., Alis, H., Dolay, K., Kalayci, M., Yasar, N., et al. (2008). Immunohistochemical testing for Helicobacter Pylori existence in neoplasms of the colon. BMC Gastroenterol. 8, 35. doi: 10.1186/1471-230X-8-35
Suerbaum, S. and Michetti, P. (2002). Helicobacter pylori infection. N Engl. J. Med. 347, 1175–1186. doi: 10.1056/NEJMra020542
Sun, B., Wang, X., McLarnon, M. E. D., Ding, Y., Liu, M., Dai, W., et al. (2021). Higher prevalence of non-thyroidal-illness syndrome in elderly male patients with active Helicobacter pylori infection. Front. Med. (Lausanne). 8. doi: 10.3389/fmed.2021.682116
Suyapoh, W., Tirnitz-Parker, J. E. E., Tangkawattana, S., Suttiprapa, S., and Sripa, B. (2021). Biliary Migration, Colonization, and Pathogenesis of O. viverrini Co-Infected with CagA+ Helicobacter pylori. Pathogens 10, 1089. doi: 10.3390/pathogens10091089
Takayama, S., Takahashi, H., Matsuo, Y., Okada, Y., and Takeyama, H. (2010). Effect of Helicobacter bilis infection on human bile duct cancer cells. Dig Dis. Sci. 55, 1905–1910. doi: 10.1007/s10620-009-0946-6
Teng, G., Dai, Y., Chu, Y., Li, J., Zhang, H., Wu, T., et al. (2018). Helicobacter pylori induces caudal-type homeobox protein 2 and cyclooxygenase 2 expression by modulating microRNAs in esophageal epithelial cells. Cancer Sci. 109, 297–307. doi: 10.1111/cas.13462
Teymournejad, O., Mobarez, A. M., Hassan, Z. M., and Talebi Bezmin Abadi, A. (2017). Binding of the Helicobacter pylori OipA causes apoptosis of host cells via modulation of Bax/Bcl-2 levels. Sci. Rep. 7, 8036. doi: 10.1038/s41598-017-08176-7
Thanaphongdecha, P., Karinshak, S. E., Ittiprasert, W., Mann, V. H., Chamgramol, Y., Pairojkul, C., et al. (2020). Infection with Helicobacter pylori induces epithelial to mesenchymal transition in human cholangiocytes. Pathogens 9, 971. doi: 10.3390/pathogens9110971
Tuomisto, A. E., Mäkinen, M. J., and Väyrynen, J. P. (2019). Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 25, 4383–4404. doi: 10.3748/wjg.v25.i31.4383
Venerito, M., Vasapolli, R., Rokkas, T., Delchier, J. C., and Malfertheiner, P. (2017). Helicobacter pylori, gastric cancer and other gastrointestinal Malignancies. Helicobacter 22, e12413. doi: 10.1111/hel.12413
Volesky-Avellaneda, K. D., Morais, S., Walter, S. D., O'Brien, T. R., Hildesheim, A., Engels, E. A., et al. (2023). Cancers attributable to infections in the US in 2017: A meta-analysis. JAMA Oncol. 9, 1678–1687. doi: 10.1001/jamaoncol.2023.4273
Waldum, H. and Fossmark, R. (2021). Gastritis, gastric polyps and gastric cancer. Int. J. Mol. Sci. 22, 6548. doi: 10.3390/ijms22126548
Waluga, M., Kukla, M., Żorniak, M., Bacik, A., and Kotulski, R. (2015). From the stomach to other organs: Helicobacter pylori and the liver. World J. Hepatol. 7, 2136–2146. doi: 10.4254/wjh.v7.i18.2136
Wang, J., Chen, R. C., Zheng, Y. X., Zhao, S. S., Li, N., Zhou, R. R., et al. (2016a). Helicobacter pylori infection may increase the risk of progression of chronic hepatitis B disease among the Chinese population: a meta-analysis. Int. J. Infect. Dis. 50, 30–37. doi: 10.1016/j.ijid.2016.07.014
Wang, J., Dong, F., Su, H., Zhu, L., Shao, S., Wu, J., et al. (2021). H. pylori is related to NAFLD but only in female: A Cross-sectional Study. Int. J. Med. Sci. 18, 2303–2311. doi: 10.7150/ijms.50748
Wang, J., Li, W. T., Zheng, Y. X., Zhao, S. S., Li, N., Huang, Y., et al. (2016b). The association between Helicobacter pylori infection and chronic hepatitis C: A meta-analysis and trial sequential analysis. Gastroenterol. Res. Pract. 2016, 8780695. doi: 10.1155/2016/8780695
Wang, Z., Shaheen, N. J., Whiteman, D. C., Anderson, L. A., Vaughan, T. L., Corley, D. A., et al. (2018). Helicobacter pylori infection is associated with reduced risk of Barrett’s esophagus: an analysis of the Barrett’s and esophageal adenocarcinoma consortium. Am. J. Gastroenterol. 113, 1148–1155. doi: 10.1038/s41395-018-0070-3
Wang, J., Wang, X., Tang, N., Chen, Y., and She, F. (2015). Impact of Helicobacter pylori on the growth of hepatic orthotopic graft tumors in mice. Int. J. Oncol. 47, 1416–1428. doi: 10.3892/ijo.2015.3107
Weeks, D. L., Eskandari, S., Scott, D. R., and Sachs, G. (2000). A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science 287, 482–485. doi: 10.1126/science.287.5452.482
Wernly, S., Semmler, G., Flamm, M., Rezar, R., Aigner, E., Datz, C., et al. (2023). The association between Helicobacter pylori and colorectal neoplasia. Med. Princ Pract. 32, 77–85. doi: 10.1159/000528794
Wu, H., Yuan, H., Zhang, J., He, T., Deng, Y., Chen, Y., et al. (2024). Helicobacter pylori upregulates PAD4 expression via stabilising HIF-1α to exacerbate rheumatoid arthritis. Ann. Rheum Dis. 83, 1666–1676. doi: 10.1136/ard-2023-225306
Xie, F. J., Zhang, Y. P., Zheng, Q. Q., Jin, H. C., Wang, F. L., Chen, M. L., et al. (2013). Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J. Gastroenterol. 19, 6098–6107. doi: 10.3748/wjg.v19.i36.6098
Yu, Y. Y., Tong, Y. L., Wu, L. Y., and Yu, X. Y. (2022). Helicobacter pylori infection eradication for nonalcoholic fatty liver disease: a randomized controlled trial. Sci. Rep. 12, 19530. doi: 10.1038/s41598-022-23746-0
Zhang, J., Ji, X., Liu, S., Sun, Z., Cao, X., Liu, B., et al. (2024). Helicobacter pylori infection promotes liver injury through an exosome-mediated mechanism. Microb. Pathog. 195, 106898. doi: 10.1016/j.micpath.2024.106898
Zhang, D., Wang, Q., and Bai, F. (2024). Bidirectional relationship between Helicobacter pylori infection and nonalcoholic fatty liver disease: insights from a comprehensive meta-analysis. Front. Nutr. 11. doi: 10.3389/fnut.2024.1410543
Zhang, F., Zhang, H., Liu, Y. M., and Tang, F. S. (2024). Helicobacter pylori, esophageal precancerous lesions, and proton pump inhibitor overuse. World J. Gastroenterol. 30, 4591–4596. doi: 10.3748/wjg.v30.i42.4591
Zhao, S. Q., Zheng, H. L., Zhong, X. T., Wang, Z. Y., Su, Y., and Shi, Y. Y. (2024). Effects and mechanisms of Helicobacter pylori infection on the occurrence of extra-gastric tumors. World J. Gastroenterol. 30, 4090–4103. doi: 10.3748/wjg.v30.i37.4090
Zheng, H., Guo, T., Zhao, X., Wang, K., Shan, S., Xie, S., et al. (2025). Helicobacter pylori infection is not associated with nonalcoholic fatty liver disease: A two-year cohort study. Dig Dis. 43, 75–83. doi: 10.1159/000542180
Keywords: Helicobacter pylori, hepatocellular carcinoma, cholangiocarcinoma, colorectal cancer, esophageal carcinoma
Citation: Xin H, Zhu C, Zhu C, Zhang X, Chen D and Wang Q (2025) Beyond the stomach: the association between Helicobacter pylori and the spectrum of digestive cancers. Front. Cell. Infect. Microbiol. 15:1633227. doi: 10.3389/fcimb.2025.1633227
Received: 22 May 2025; Accepted: 04 August 2025;
Published: 02 September 2025.
Edited by:
Asghar Ali, Jamia Hamdard University, IndiaReviewed by:
Mohammad Tahir Siddiqui, Indian Institute of Technology Delhi, IndiaMohammad Abid, Jamia Millia Islamia, India
Copyright © 2025 Xin, Zhu, Zhu, Zhang, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingping Wang, cWluZ3BpbmdhbmVza3lAMTYzLmNvbQ==; Dongsheng Chen, ZG9uZ3NoZW5nLmNoZW5Ac2ltY2VyZWR4LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 He Xin
He Xin Chi Zhu3,4†
Chi Zhu3,4† Chan Zhu
Chan Zhu Xing Zhang
Xing Zhang Dongsheng Chen
Dongsheng Chen Qingping Wang
Qingping Wang