- Department of Thoracic Surgery, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, University of Electronic Science and Technology of China, Chengdu, China
Lung cancer-associated obstructive pneumonia and pulmonary abscess represent critical oncologic complications. Effective management hinges on dynamically balancing primary tumor control with secondary infection management. Recent major advances in targeted therapy, immunotherapy, and novel anti-infective technologies have driven a shift towards integrated, multidimensional clinical strategies.This review details diagnostic and therapeutic progress for these conditions, emphasizing the critical balance between controlling severe infections and managing antitumor therapy toxicities, alongside recent advances in nanomedicine for pulmonary infections.
Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide. According to the latest statistics from the China National Cancer Center (2022), approximately 1,060,600 new lung cancer cases were diagnosed, accounting for 22.0% of all malignant tumors, with roughly 733,300 deaths (Zheng et al., 2024). Notably, advanced lung cancer often causes bronchial obstruction due to tumor progression, leading to distal lung parenchymal infection, termed post-obstructive pneumonia (POP). POP incidence reaches 45-55%, with 10-15% of cases progressing to pulmonary abscess (Valvani et al., 2019; Moretti et al., 2023). Central lung cancer, particularly squamous cell carcinoma, exhibits a significantly higher incidence of obstructive pneumonia than peripheral types. This results from bronchial obstruction and impaired mucociliary clearance, leading to secretions retention and bacterial colonization (Wei et al., 2024). Furthermore, the immunosuppressive state in advanced lung cancer patients may substantially elevate the risk of pulmonary abscess formation.
Postobstructive pneumonia exerts a significant adverse impact on the quality of life and prognosis of lung cancer patients. Studies indicate that patients with concurrent postobstructive pneumonia often experience severe limitations in daily activities due to persistent dyspnea, recurrent fever, and purulent sputum production. Frequent hospitalizations and prolonged use of broad-spectrum antibiotics further contribute to treatment-related complications (e.g., nephrotoxicity, antibiotic-associated diarrhea) while concurrently impeding the delivery of antitumor therapies (Jo, 2022). A study revealed that patients with stage I/II lung cancer comorbid with chronic obstructive pulmonary disease (COPD) were less likely to undergo surgery (56.8% vs. 65.9%) or surgery with adjuvant chemotherapy (15.4% vs. 17.1%), but more likely to receive radiotherapy (26.0% vs. 21.8%) (all p<0.001). Among stage III/IV patients, those with COPD showed lower rates of chemotherapy (55.9% vs. 64.4%) or radiotherapy (42.5% vs. 47.5%) (all p<0.001) (Goffin et al., 2021). Current evidence primarily consists of sporadic retrospective studies with limited sample sizes. A retrospective analysis of 408 stage III/IV lung cancer patients revealed a 30-day mortality rate of 30% in those with obstructive pneumonia. Multivariate logistic regression identified CURB-65 score (OR: 73.20, p = 0.001) and smoking status (OR: 0.009, p = 0.015) as significant predictors of 30-day mortality (Moretti et al., 2023). Conversely, a separate study on surgico-pathological features of superficial endobronchial lung cancer (SELC) reported no significant prognostic impact (P = 0.96) from tumor-associated obstructive atelectasis or pneumonia (Chen et al., 2010). Therefore, it remains challenging to draw definitive conclusions regarding whether obstructive pneumonia or lung abscess impacts the prognosis of lung cancer. Prospective studies are warranted to address this clinical question. Furthermore, existing research has demonstrated the specific pro-tumorigenic role of inflammation in lung cancer progression. Notably, IL-6 has been identified as a key mediator that facilitates both tumor initiation and advancement, thereby establishing a vicious cycle (Ochoa et al., 2011; Chen et al., 2020). Based on the evidences, infectious complications should be considered as an independent prognostic factor in lung cancer management strategies, with early intervention demonstrating potential to enhance quality of life and prolong survival.
Pathophysiological mechanisms
The direct mechanisms of bronchial obstruction caused by lung cancer primarily involve tumor-related mechanical compression or infiltration: centrally located tumors (e.g., squamous cell carcinoma or small cell carcinoma) may grow intraluminally, resulting in bronchial stenosis or complete occlusion, while extrinsic compression from mediastinal lymph node metastases can similarly obstruct airways, ultimately leading to distal atelectasis (Moretti et al., 2023). Indirect mechanisms involve tumor-associated inflammation and immune dysregulation: The tumor microenvironment secretes pro-inflammatory factors (e.g., IL-8, TNF-α), inducing bronchial mucosal edema and mucus hypersecretion, which leads to mucus plug formation and exacerbates airway obstruction (Oliviero et al., 2016; Cazzola et al., 2017; Ragnoli et al., 2024). Concurrently, immunosuppressive factors (e.g., IL-10, TGF-β) impair local macrophage function, increasing susceptibility to opportunistic infections (e.g., Pseudomonas aeruginosa or Aspergillus) (Meziani et al., 2021; Wei et al., 2022; Cui et al., 2023). Subsequent infectious inflammation further disrupts airway architecture. Treatment-related injuries also contribute to indirect obstructive mechanisms, such as post-radiotherapy fibrosis causing loss of bronchial elasticity (Ozkok et al., 2008),while chemotherapeutic agents (e.g., gemcitabine) may induce mucositis, exacerbating cicatricial stenosis (Lee et al., 2008), as illustrated in Figure 1.
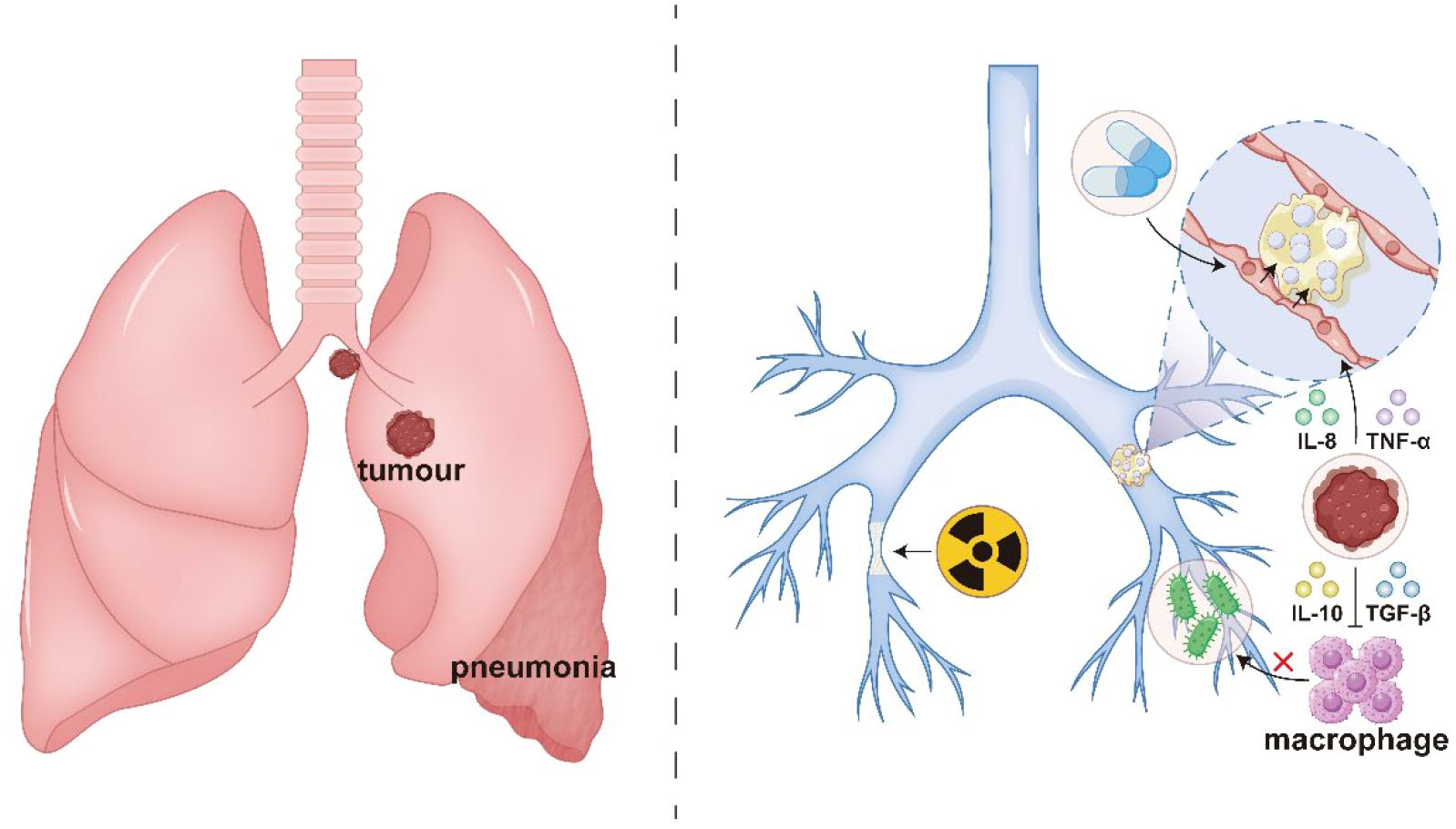
Figure 1. Direct and indirect mechanisms in the pathogenesis of obstructive pneumonia: tumor-induced bronchial stenosis and obliteration (direct); and tumor-associated inflammation and immune dysregulation (indirect).
Clinical manifestations and differential diagnosis
Clinical presentation and differential diagnosis of lung cancer-associated obstructive pneumonia require integrated analysis of symptoms, imaging, and microbiological features.Obstructive Pneumonia: Characterized by persistent cough, purulent sputum, fever (>38.5°C), and dyspnea. High-resolution CT typically shows localized consolidation with proximal bronchial obstruction or mucus plugging.Pulmonary Abscess: Presents with high fever (>39°C) and copious foul-smelling purulent sputum (producing 300–500 mL/day). CT reveals thick-walled cavities (>2 cm diameter) with air-fluid levels and surrounding inflammatory infiltration.Elevated infectious biomarkers (e.g., Procalcitonin/PCT, C-reactive Protein/CRP) and positive microbiological identification of pathogens (e.g., Klebsiella pneumoniae, Pseudomonas aeruginosa) in respiratory samples (sputum/bronchoalveolar lavage fluid) support the diagnosis of bacterial pneumonia while ruling out alternative causes. The differential diagnosis should encompass the following conditions:Infectious pneumonia: Non-obstructive pneumonia typically presents with multifocal distribution, a broader spectrum of pathogens (e.g., viruses, Mycoplasma), and no direct evidence of tumor compression.Radiation pneumonitis: Confined to the radiation field, early-stage manifestations include ground-glass opacities with traction bronchiectasis, progressing to fibrosis in later stages, and absence of purulent sputum.Immune checkpoint inhibitor (ICI)-associated pneumonitis: Predominantly manifests as interstitial or organizing pneumonia, with histopathological findings of CD8+ T-cell infiltration and temporal association with ICI administration (Zhang et al., 2021), (Table 1). Pulmonary tumor metastasis: Characterized by multiple nodules or diffuse miliary lesions without elevated infectious inflammatory markers (Oliaro et al., 2008). Additionally, metagenomic next-generation sequencing (mNGS) of bronchoalveolar lavage fluid (BALF) enhances detection rates for mixed infections (e.g., bacterial-fungal coinfection) (Jiang et al., 2024), while bronchoscopic biopsy can exclude tumor progression or foreign body obstruction.
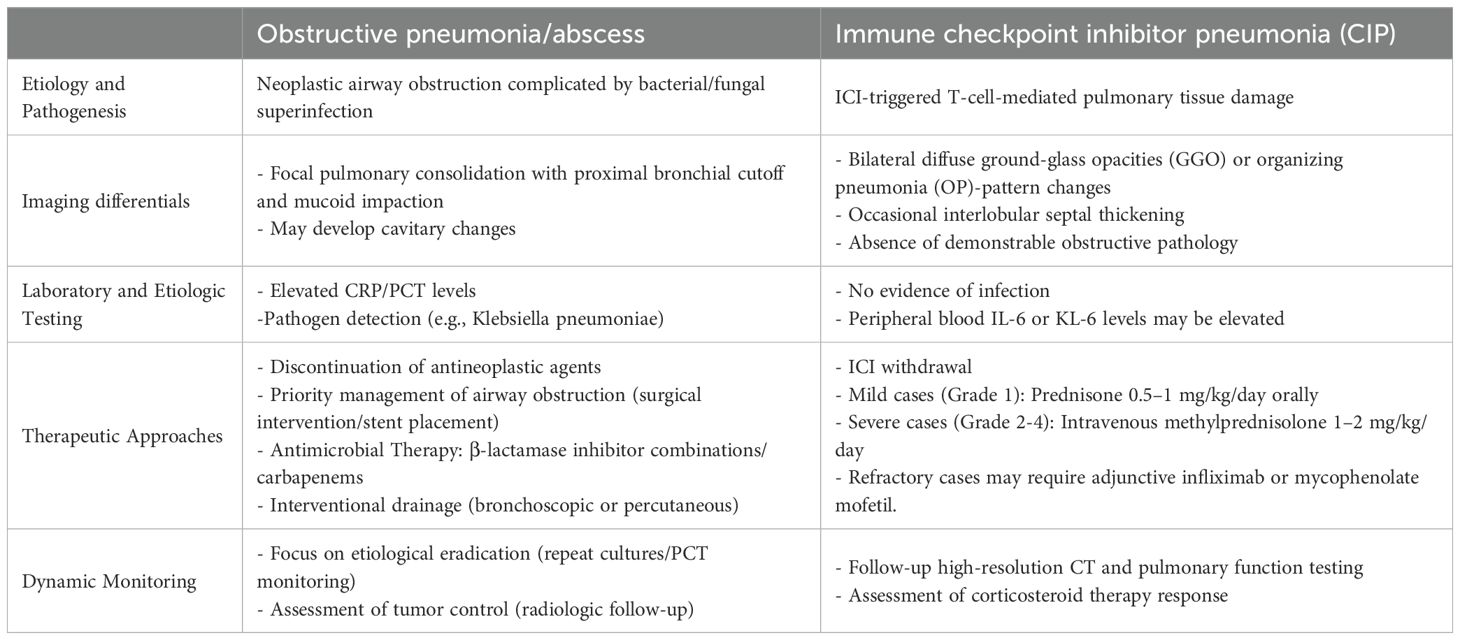
Table 1. Differentiation and management of lung cancer-associated obstructive pneumonia/abscess versus immune checkpoint inhibitor pneumonia (CIP).
Current therapeutic strategies
At present, the treatment of lung cancer-related obstructive pneumonia and lung abscess mainly combines anti-infection treatment with non-anti-infection treatment. Non-anti-infection treatment strategies include relieving airway obstruction, surgical resection and local thoracic drainage, enhancing airway clearance, oral hygiene care, preventing reflux and aspiration, symptomatic supportive treatment, oxygen therapy and respiratory support, etc. It should be noted that if pneumonia occurs during tumor treatment, it is recommended to suspend anti-tumor treatment. Anti-tumor treatment can be considered to be restarted after the patient’s acute infection symptoms have completely resolved.
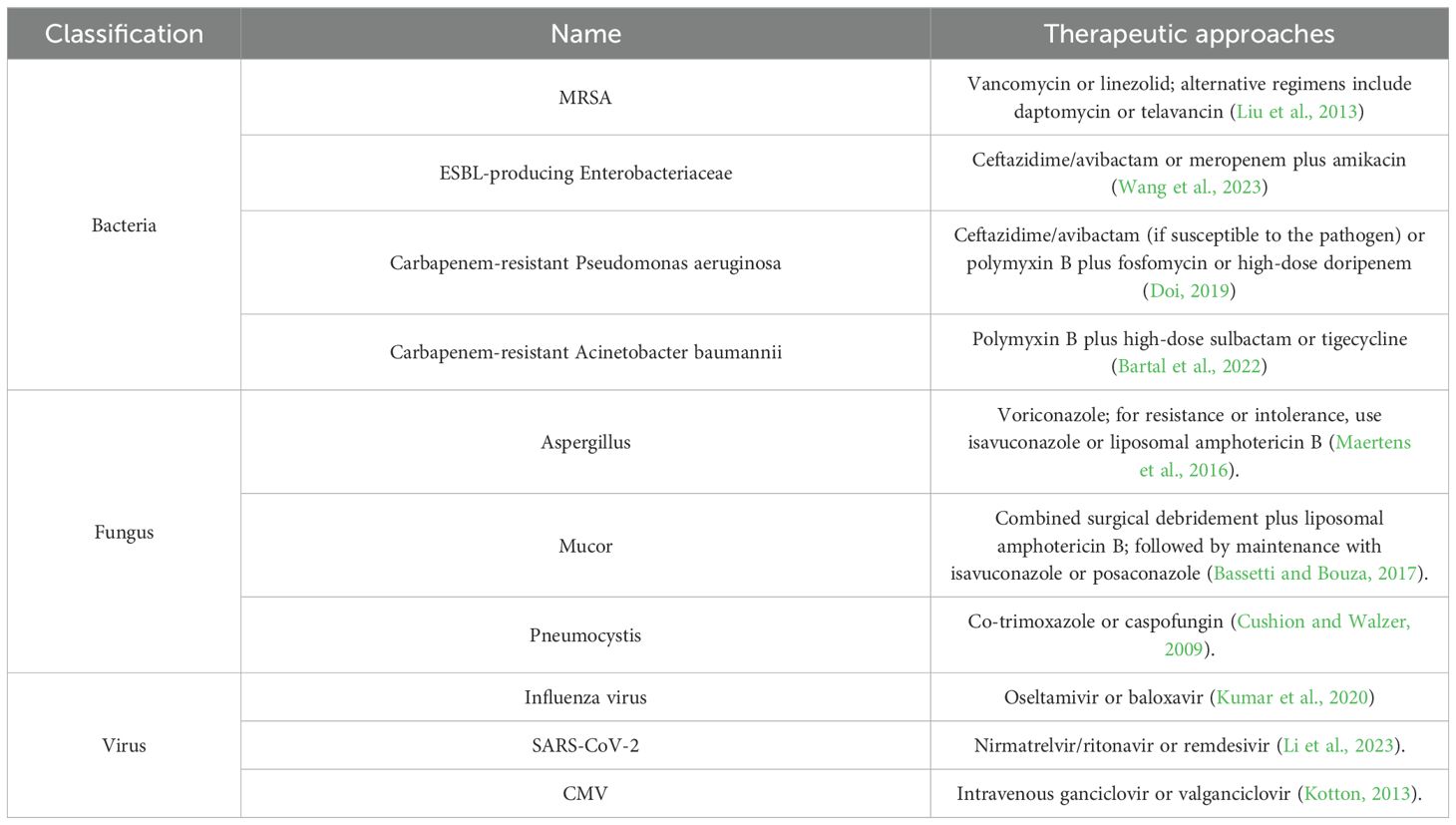
Research on the microbiological characteristics of patients with lung cancer complicated by obstructive pneumonia is currently limited. However, existing evidence indicates that the infectious microorganisms in these patients show significant diversity, mainly influenced by tumor-related immune deficiencies and treatment intervention factors (Zhao et al., 2023). The pathogen spectrum shows the coexistence of multiple pathogens, including bacteria, viruses and fungi (Meng et al., 2023; Shin et al., 2023). In patients who have not received systemic treatment and whose immune function is relatively intact, the main pathogenic bacteria are similar to those found in community-acquired pneumonia, mainly including Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and respiratory viruses (Yamada et al., 2010). However, for patients receiving multi-line treatment (especially those in the advanced stage with induced multiple immunosuppression), there is a phenomenon of opportunistic pathogen colonization. The detection rates of Pseudomonas aeruginosa, Staphylococcus aureus, Enterobacter cloacae, and Acinetobacter species have significantly increased. It is worth noting that such patients often have concurrent oral anaerobic bacterial infections (such as Bacteroides species, Peptostreptococcus species, Fusobacterium species, and Actinomyces species) (Dong et al., 2021). Furthermore, the patient’s immune status plays a decisive role in the composition of pathogens: patients with a compromised cellular immune system are prone to concurrent infections by cytomegalovirus/herpesvirus and bacteria (Suri et al., 2024); Humoral immunosuppression significantly increases the risk of invasive fungal infections (including Candida, Aspergillus, Histoplasma, and Coccidioides species) and Pneumocystis jirovecii pneumonia. Consequently, initial antibiotic selection should typically cover common Gram-negative bacteria and include anaerobic activity. Suitable options include β-lactam/β-lactamase inhibitor combinations (e.g., amoxicillin/clavulanate 1.2 g every 8 hours, piperacillin/tazobactam 4.5 g every 8 hours, or cefoperazone/sulbactam 3.0 g every 8 hours). Subsequently, therapy should be refined based on microbiological identification and antimicrobial susceptibility testing results. We summarize recommended antimicrobial regimens for specific types of infectious pneumonia requiring particular attention in Table 2.
Non-anti-infective therapy centers on: 1. relieving airway obstruction via bronchoscopic interventions—including electrocautery, argon plasma coagulation, laser therapy, radiotherapy, and cryotherapy—as well as stent placement or mechanical debridement to restore ventilation, supplemented with aerosolization, postural drainage, and chest physiotherapy to promote airway clearance (Steinfort et al., 2021; Yang et al., 2024). 2.Surgical and local interventions: For refractory lung abscesses (failed medical therapy >6 weeks, cavity >6 cm, or suspected malignancy) or acute complications (massive hemoptysis, sepsis), perform lobar/wedge resection. Combine with imaging-guided percutaneous drainage(for peripheral abscesses) (Lee et al., 2022) or bronchoscopic drainage(for central abscesses) (Herth et al., 2005). 3.Stage-directed management of empyema: Conservative therapy for acute exudative phase; video-assisted thoracoscopic debridement for fibrinopurulent phase; decortication required in chronic phase. 4.Supportive care: Elevation of the head ≥30° to prevent aspiration, maintenance of fluid/electrolyte balance and nutritional support, with early fluid resuscitation for hypotension.5.Respiratory support: Select nasal cannula/facemask oxygen (for SpO2 ≥90% or 88%-92% with hypercapnia risk), prioritizing high-flow humidified oxygen therapy or non-invasive ventilation to reduce intubation rates.Therapy must balance antitumor and anti-inflammatory requirements, optimizing individualized regimens through minimally invasive techniques (navigational bronchoscopy, targeted ablation) and ongoing reassessment of infection-tumor interactions.
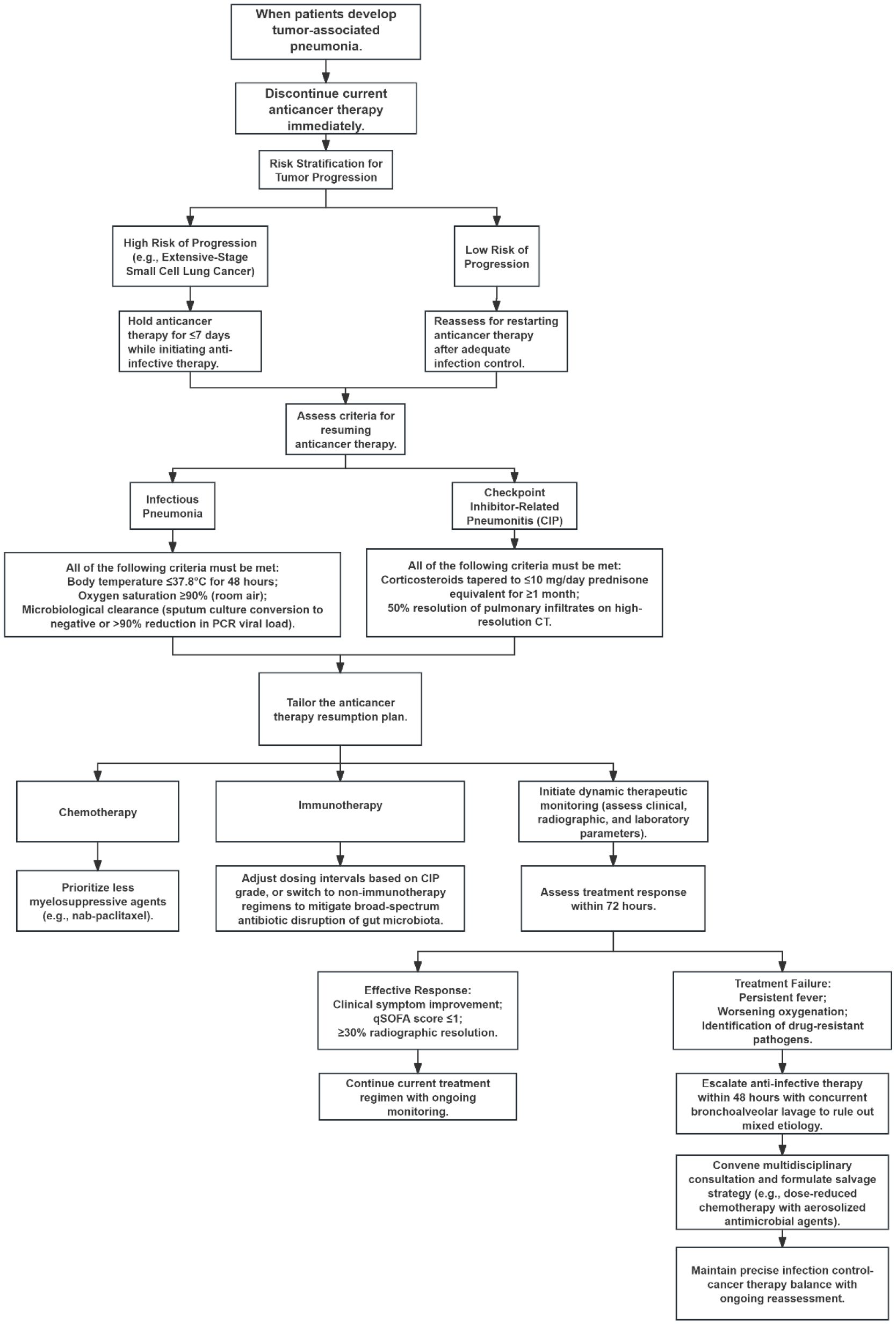
In managing tumor-associated pneumonia, the primary principle is stratified decision-making regarding antineoplastic therapy interruption and resumption, based on pneumonia type and cancer progression risk. Upon pneumonia onset, immediately suspend current antineoplastic regimens: For high-progression-risk malignancies (e.g., extensive-stage SCLC), limit interruption to ≤7 days with concurrent antimicrobial initiation; for low-risk tumors, defer resumption until infection control is achieved. Antineoplastic therapy restart requires strict clinical and laboratory criteria: For infectious pneumonia, patients must maintain temperature ≤37.8°C for 48 hours, SpO2; ≥90% on room air, and microbiological clearance (negative sputum culture or >90% reduction in PCR load) (Baden et al., 2012). For checkpoint inhibitor pneumonia (CIP), glucocorticoid tapering to prednisone <10 mg/day must be maintained for ≥1 month, with concurrent >50% infiltrate resolution on high-resolution CT. Regimen adjustments should prioritize: chemotherapy agents with minimal myelosuppression (e.g., nab-paclitaxel) (Chen Y. et al., 2022), immunotherapy modification via extended dosing intervals or transition to non-immunotherapeutic agents based on CIP grade; and avoidance of broad-spectrum antibiotics to prevent gut microbiota disruption. Therapeutic efficacy requires dynamic monitoring of clinical, radiological, and laboratory parameters: Improvement within 72 hours (clinical symptom resolution, qSOFA score ≤1, and ≥30% radiographic absorption) indicates treatment response. For persistent fever, worsening oxygenation, or detected drug-resistant pathogens, escalate antimicrobial therapy within 48 hours. Perform bronchoscopic alveolar lavage to exclude mixed etiologies and convene multidisciplinary consultation to establish salvage strategies (e.g., dose-reduced chemotherapy with aerosolized antimicrobials), achieving precision balance between infection control and oncologic management, as illustrated in Figure 2.
Case presentation
A 51-year-old male was admitted to Sichuan Cancer Hospital on October 10, 2024, with a chief complaint of “non-small cell lung cancer (NSCLC) detected more than 20 days earlier, accompanied by productive cough and fever for 2 days.” PET-CT imaging revealed: 1. a soft tissue nodular mass in the left hilum with increased metabolism, consistent with lung cancer; 2. a mass in the anterior segment of the left upper lobe with ring-shaped metabolic activity, suggestive of a lung abscess; and 3. obstructive pneumonia in the left upper lobe. Laboratory tests on admission showed elevated inflammatory markers. Initial treatment with cefoperazone-sulbactam was ineffective, with persistent recurrent fever. Antibiotic therapy was escalated to imipenem-cilastatin plus vancomycin. Follow-up CT showed poor response to anti-infective therapy. Emergency radical resection of the left upper lobe lung cancer was performed on October 17, 2024, as shown in Figure 3. The procedure was successful, and previous antibiotic regimens were continued postoperatively. The patient recovered well, with normalized body temperature and inflammatory markers, and was discharged on October 24, 2024. Pathology confirmed left lung squamous cell carcinoma, pT3N1M0 Stage IIIA. This case highlights that bronchial obstruction relief is necessary when obstructive pneumonia is refractory to conventional management.
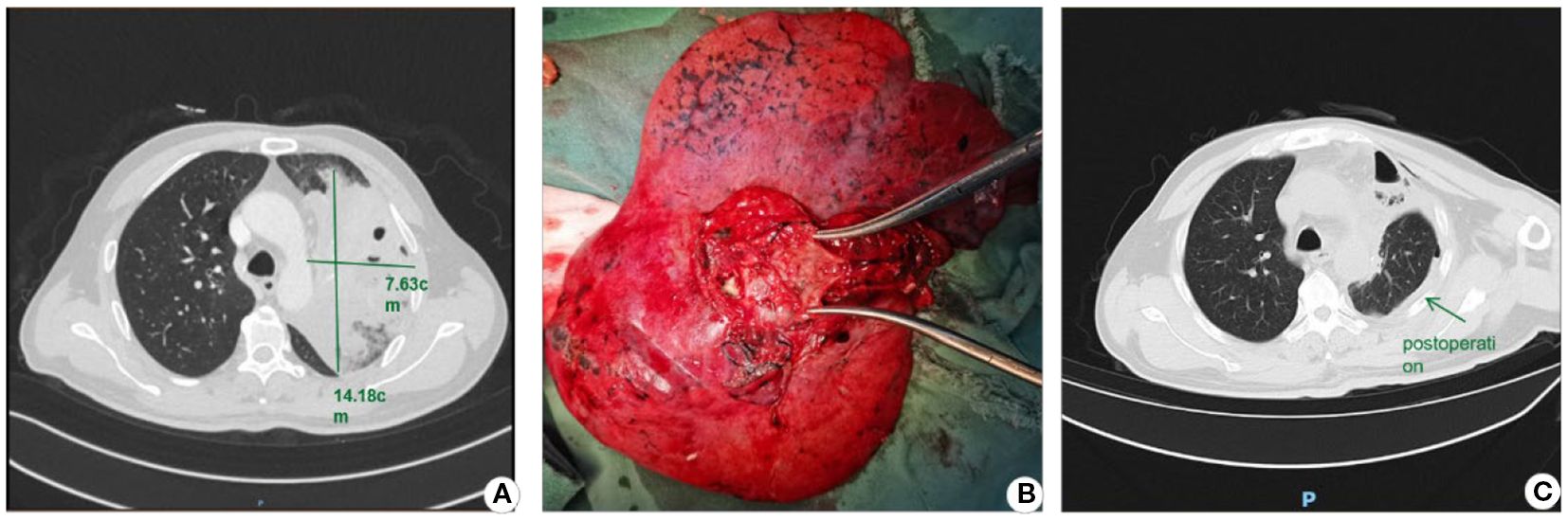
Figure 3. Lobectomy for lung cancer-associated lung abscess. (A) Preoperative CT image; (B) Resected lobe demonstrating the abscess cavity upon dissection; (C) Postoperative CT image.
Research advances and frontier technologies
Novel Diagnostic Technology: Metagenomic next-generation sequencing (mNGS) significantly enhances detection of polymicrobial coinfections (e.g., bacterial-fungal-viral) through unbiased pathogen nucleic acid analysis in bronchoalveolar lavage fluid (BALF), proving particularly valuable for immunocompromised patients (Chen S. et al., 2022). Droplet digital PCR (ddPCR) quantifies low-abundance resistance genes [e.g., blaKPC (Wu et al., 2024), ;mecA (Luo et al., 2017)] to guide precision antimicrobial therapy Concurrently, deep learning-based CT analysis systems (e.g., AI-Rad Companion) now automate detection of obstructive pneumonia features like mucoid impaction and abscess cavitation (Niehoff et al., 2023), while natural language processing (NLP) models integrate electronic health records with microbiological data to predict resistance risks and recommend personalized regimens (Mora et al., 2022).
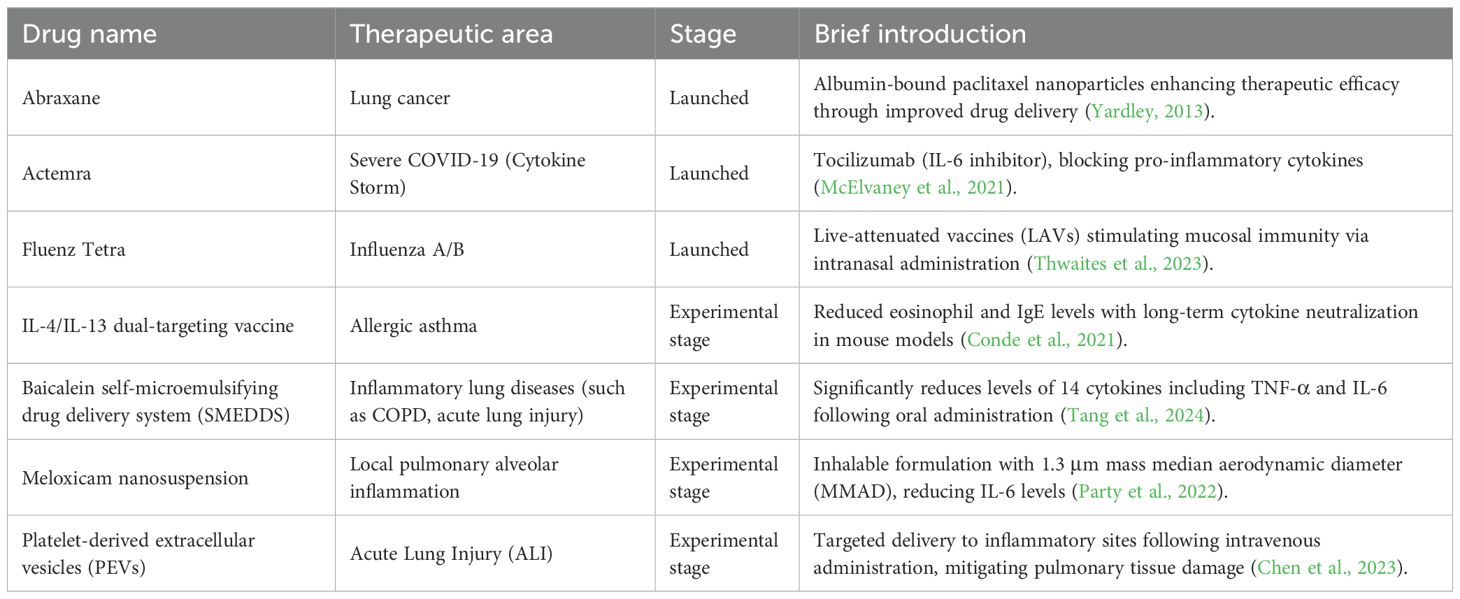
Novel Therapeutic Technologies: Nanomedicine has demonstrated significant progress in anti-inflammatory applications, exhibiting unique advantages for modulating pulmonary inflammatory disorders (e.g., pneumonia, asthma, acute lung injury, and COVID-19-associated cytokine storms) (Constantin et al., 2021; Ahmad, 2022; Zoulikha et al., 2022; Hu et al., 2023). Detailed information can be found in Table 3. Clinically relevant nanocarriers include: lipid nanoparticles and albumin-based nanovehicles enabling targeted delivery of chemotherapeutic/anti-inflammatory agents (e.g., paclitaxel, dexamethasone) to precisely inhibit inflammatory pathways (Hegeman et al., 2011; Fu et al., 2022); monoclonal antibodies (e.g., tocilizumab) neutralizing key cytokines like IL-6 to control hyperinflammation in severe pneumonia (McElvaney et al., 2021); and investigational IL-4/IL-13 dual-acting vaccines with platelet-mimetic nanovehicles advancing long-term immunomodulation and precision targeting (Karo-Atar et al., 2018; Hu et al., 2023). Core advantages include: 1. Targeted delivery: Enhanced drug accumulation at inflammatory sites via surface modification or biomimetic design reduces systemic exposure [e.g., platelet vesicles targeting acute lung injury (Chen et al., 2023)]; 2. Stability and controlled release: Nanostructures protect payloads from degradation while enabling sustained or stimuli-responsive release [e.g., PLGA nanoparticles (Guan et al., 2024)]; Overcoming conventional limitations: Improved bioavailability of poorly soluble drugs [e.g., baicalein (Wang et al., 2015)] or direct alveolar delivery via inhalable formulations (e.g., meloxicam nanosuspensions (Party et al., 2022)]. Recent advances focus on combination therapies (anti-infection + immunomodulation) (Zhang et al., 2024), biomimetic carriers (e.g., cell membrane-coated nanoparticles) (Krishnan et al., 2024), and combatting drug-resistant infections (e.g., nanoformulated ceftazidime/avibactam) (Wang et al., 2022). Despite challenges in long-term safety evaluation and uniformity of pulmonary deposition, nanomedicine is emerging as a pivotal strategy for respiratory diseases through precise modulation of inflammatory microenvironments, with multidisciplinary convergence accelerating clinical translation.
Challenges and future perspectives
Lung cancer-associated obstructive pneumonia and pulmonary abscess management face two core challenges: First, balancing infection control with antineoplastic therapy toxicity. Broad-spectrum antibiotics may suppress infections but exacerbate chemotherapy/radiation-induced myelosuppression and mucosal injury, increasing secondary resistance risks. Second, compromised performance status (ECOG ≥2 or KPS ≤70) often precludes intensive therapies, forcing 30%-40% of patients into palliative approaches with significant survival implications.Future research priorities to optimize management include:Precision anti-infective/immunomodulatory integration: Rapid pathogen identification via metagenomic NGS (mNGS) to guide narrow-spectrum antibiotics, reducing resistance risks. Concurrently explore synergy between immune checkpoint inhibitors (ICIs) and antimicrobials—e.g., nano carrier-mediated local antibiotic delivery to minimize systemic toxicity, or ICI reintroduction post-infection control to delay tumor progression.Targeted antineoplastic-interventional synergy: Develop biodegradable stents with drug-eluting coatings (e.g., paclitaxel/anti-inflammatory nanoparticles) to relieve obstruction while suppressing local tumor growth and inflammation. For abscesses, investigate bronchoscopic localized antimicrobial sustained-release systems (e.g., lipid-encapsulated vancomycin) to enhance intralesional drug concentration.Personalized regimens for frail patients: Stratify by ECOG/KPS to implement low-intensity chemotherapy (e.g., metronomic dosing) with supportive care (e.g., G-CSF prophylaxis), or leverage nanotechnology (e.g., albumin-bound nanodrugs) to enhance targeting and reduce systemic exposure.Resistance/recurrence mitigation: Implement dynamic resistance gene surveillance; develop nanozyme or phage therapies against biofilm infections; optimize radiotherapy planning (e.g., SBRT) to minimize normal lung injury and secondary infection risks.Multidimensional data-driven decisions: Integrate radiomics, ctDNA, and inflammatory biomarkers (e.g., IL-6, PCT) to build predictive models dynamically assessing infection-tumor interplay, guiding therapeutic timing adjustments.Clinical translation of these strategies may overcome current limitations, achieving dual objectives of infection eradication and tumor control to ultimately improve survival quality and outcomes.
Author contributions
PC: Writing – original draft, Data curation, Visualization. LC: Investigation, Visualization, Writing – review & editing. SX: Data curation, Visualization, Writing – review & editing. BH: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Natural Science Foundation of Sichuan Province (2025ZNSFSC1536). Sichuan Provincial Science and Technology Department's Provincial-University-School Cooperation Project No. 2025YFHZ0072.Research Project of the Medical and Health Science Development Research Center of the National Health Commission No. WKZX2023WK0104.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, A. (2022). Pharmacological strategies and recent advancement in nano-drug delivery for targeting asthma. Life (Basel. Switzerland). 12(4), 596. doi: 10.3390/life12040596
Baden, L. R., Bensinger, W., Angarone, M., Casper, C., Dubberke, E. R., Freifeld, A. G., et al. (2012). Prevention and treatment of cancer-related infections. J. Natl. Compr. Cancer Netw.: JNCCN. 10, 1412–1445. doi: 10.6004/jnccn.2012.0146
Bartal, C., Rolston, K. V. I., and Nesher, L. (2022). Carbapenem-resistant acinetobacter baumannii: colonization, infection and current treatment options. Infect. Dis. Ther. 11, 683–694. doi: 10.1007/s40121-022-00597-w
Bassetti, M. and Bouza, E. (2017). Invasive mould infections in the ICU setting: complexities and solutions. J. Antimicrob. Chemother. 72, i39–i47. doi: 10.1093/jac/dkx032
Cazzola, M., Calzetta, L., Facciolo, F., Rogliani, P., and Matera, M. G. (2017). Pharmacological investigation on the anti-oxidant and anti-inflammatory activity of N-acetylcysteine in an ex vivo model of COPD exacerbation. Respir. Res. 18, 26. doi: 10.1186/s12931-016-0500-y
Chen, C., Zheng, H., Gao, W., Zhou, Y., Jiang, S., and Suen, H. C. (2010). Prognosis and staging of superficial endobronchial lung cancer: the impact of invasion depth, tumor diameter, and coexistent pneumonitis or atelectasis. Chin. Med. J. 123, 1505–1509.
Chen, J., Li, X., Huang, C., Lin, Y., and Dai, Q. (2020). Change of serum inflammatory cytokines levels in patients with chronic obstructive pulmonary disease, pneumonia and lung cancer. Technol. Cancer Res. Treat 19, 1533033820951807. doi: 10.1177/1533033820951807
Chen, K., Zhang, Z., Fang, Z., Zhang, J., Liu, Q., Dong, W., et al. (2023). Aged-signal-eliciting nanoparticles stimulated macrophage-mediated programmed removal of inflammatory neutrophils. ACS Nano. 17, 13903–13916. doi: 10.1021/acsnano.3c03815
Chen, S., Kang, Y., Li, D., and Li, Z. (2022). Diagnostic performance of metagenomic next-generation sequencing for the detection of pathogens in bronchoalveolar lavage fluid in patients with pulmonary infections: Systematic review and meta-analysis. Int. J. Infect. Dis. 122, 867–873. doi: 10.1016/j.ijid.2022.07.054
Chen, Y., Han, L., Qiu, X., Wang, M., Chen, Z., Cai, Y., et al. (2022). Reassembling of albumin-bound paclitaxel mitigates myelosuppression and improves its antitumoral efficacy via neutrophil-mediated targeting drug delivery. Drug Deliv. 29, 728–742. doi: 10.1080/10717544.2022.2046892
Conde, E., Bertrand, R., Balbino, B., Bonnefoy, J., Stackowicz, J., Caillot, N., et al. (2021). Dual vaccination against IL-4 and IL-13 protects against chronic allergic asthma in mice. Nat. Commun. 12, 2574. doi: 10.1038/s41467-021-22834-5
Constantin, C., Pisani, A., Bardi, G., and Neagu, M. (2021). Nano-carriers of COVID-19 vaccines: the main pillars of efficacy. Nanomed. (London England). 16, 2377–2387. doi: 10.2217/nnm-2021-0250
Cui, Z., Ruan, Z., Li, M., Ren, R., Ma, Y., Zeng, J., et al. (2023). Obstructive sleep apnea promotes the progression of lung cancer by modulating cancer cell invasion and cancer-associated fibroblast activation via TGFβ signaling. Redox Rep. 28, 2279813. doi: 10.1080/13510002.2023.2279813
Cushion, M. T. and Walzer, P. D. (2009). Preclinical drug discovery for new anti-pneumocystis compounds. Curr. Med. Chem. 16, 2514–2530. doi: 10.2174/092986709788682038
Doi, Y. (2019). Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin. Infect. Dis. 69, S565–s575. doi: 10.1093/cid/ciz830
Dong, J., Li, W., Wang, Q., Chen, J., Zu, Y., Zhou, X., et al. (2021). Relationships between oral microecosystem and respiratory diseases. Front. Mol. Biosci. 8. doi: 10.3389/fmolb.2021.718222
Fu, Y., Yang, S., Liu, Y., Liu, J., Wang, Q., Li, F., et al. (2022). Peptide modified albumin-paclitaxel nanoparticles for improving chemotherapy and preventing metastasis. Macromol. Biosci. 22, e2100404. doi: 10.1002/mabi.202100404
Goffin, J. R., Corriveau, S., Tang, G. H., and Pond, G. R. (2021). Management and outcomes of patients with chronic obstructive lung disease and lung cancer in a public healthcare system. PloS One 16, e0251886. doi: 10.1371/journal.pone.0251886
Guan, X., Xu, X., Tao, Y., Deng, X., He, L., Lin, Z., et al. (2024). Dual targeting and bioresponsive nano-PROTAC induced precise and effective lung cancer therapy. J. Nanobiotechnol. 22, 692. doi: 10.1186/s12951-024-02967-7
Hegeman, M. A., Cobelens, P. M., Kamps, J., Hennus, M. P., Jansen, N. J., Schultz, M. J., et al. (2011). Liposome-encapsulated dexamethasone attenuates ventilator-induced lung inflammation. Br. J. Pharmacol. 163, 1048–1058. doi: 10.1111/j.1476-5381.2011.01314.x
Herth, F., Ernst, A., and Becker, H. D. (2005). Endoscopic drainage of lung abscesses: technique and outcome. Chest 127, 1378–1381. doi: 10.1378/chest.127.4.1378
Hu, H., Hua, S. Y., Lin, X., Lu, F., Zhang, W., Zhou, L., et al. (2023). Hybrid biomimetic membrane coated particles-mediated bacterial ferroptosis for acute MRSA pneumonia. ACS Nano. 17, 11692–11712. doi: 10.1021/acsnano.3c02365
Jiang, L., Han, L., Zhong, Y., Zhang, M., Li, J., Rao, G., et al. (2024). High utility of bronchoalveolar lavage fluid metagenomic next-generation sequencing approach for etiological diagnosis of pneumonia. BMC Infect. Dis. 24, 1232. doi: 10.1186/s12879-024-10108-6
Jo, Y. S. (2022). Long-term outcome of chronic obstructive pulmonary disease: A review. Tuberculosis. Respir. Dis. 85, 289–301. doi: 10.4046/trd.2022.0074
Karo-Atar, D., Bitton, A., Benhar, I., and Munitz, A. (2018). Therapeutic targeting of the interleukin-4/interleukin-13 signaling pathway: in allergy and beyond. BioDrugs 32, 201–220. doi: 10.1007/s40259-018-0280-7
Kotton, C. N. (2013). CMV: prevention, diagnosis and therapy. Am. J. Transplant. 13 Suppl 3, 24–40. doi: 10.1111/ajt.12006
Krishnan, N., Jiang, Y., Zhou, J., Mohapatra, A., Peng, F. X., Duan, Y., et al. (2024). A modular approach to enhancing cell membrane-coated nanoparticle functionality using genetic engineering. Nat. Nanotechnol. 19, 345–353. doi: 10.1038/s41565-023-01533-w
Kumar, S., Goicoechea, S., Kumar, S., Pearce, C. M., Durvasula, R., Kempaiah, P., et al. (2020). Oseltamivir analogs with potent anti-influenza virus activity. Drug Discov Today 25, 1389–1402. doi: 10.1016/j.drudis.2020.06.004
Lee, J. H., Hong, H., Tamburrini, M., and Park, C. M. (2022). Percutaneous transthoracic catheter drainage for lung abscess: a systematic review and meta-analysis. Eur. Radiol 32, 1184–1194. doi: 10.1007/s00330-021-08149-5
Lee, J. O., Kim, D. Y., Lim, J. H., Seo, M. D., Yi, H. G., Kim, Y. J., et al. (2008). Risk factors for bacterial pneumonia after cytotoxic chemotherapy in advanced lung cancer patients. Lung Cancer (Amsterdam Netherlands). 62, 381–384. doi: 10.1016/j.lungcan.2008.03.015
Li, G., Hilgenfeld, R., Whitley, R., and De Clercq, E. (2023). Therapeutic strategies for COVID-19: progress and lessons learned, Nature reviews. Drug Discov 22, 449–475. doi: 10.1038/s41573-023-00672-y
Liu, X., He, Y., Xiao, K., White, J. R., Fusco, D. N., and Papanicolaou, G. A. (2013). Effect of linezolid on clinical severity and pulmonary cytokines in a murine model of influenza A and Staphylococcus aureus coinfection. PloS One 8, e57483. doi: 10.1371/journal.pone.0057483
Luo, J., Li, J., Yang, H., Yu, J., and Wei, H. (2017). Accurate detection of methicillin-resistant staphylococcus aureus in mixtures by use of single-bacterium duplex droplet digital PCR. J. Clin. Microbiol. 55, 2946–2955. doi: 10.1128/jcm.00716-17
Maertens, J. A., Raad, I. I., Marr, K. A., Patterson, T. F., Kontoyiannis, D. P., Cornely, O. A., et al. (2016). Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet (London England). 387, 760–769. doi: 10.1016/s0140-6736(15)01159-9
McElvaney, O. J., Curley, G. F., Rose-John, S., and McElvaney, N. G. (2021). Interleukin-6: obstacles to targeting a complex cytokine in critical illness. Lancet Respir. Med. 9, 643–654. doi: 10.1016/s2213-2600(21)00103-x
Meng, Y., Mao, Y., Tang, Z., Qiu, X., Bajinka, O., Tan, Y., et al. (2023). Crosstalk between the lung microbiome and lung cancer. Microbial. Pathogene. 178, 106062. doi: 10.1016/j.micpath.2023.106062
Meziani, L., Robert, C., Classe, M., Da Costa, B., Mondini, M., Clémenson, C., et al. (2021). Low doses of radiation increase the immunosuppressive profile of lung macrophages during viral infection and pneumonia. Int. J. Radiat. Oncol. Biol. Phys. 110, 1283–1294. doi: 10.1016/j.ijrobp.2021.03.022
Mora, S., Attene, J., Gazzarata, R., Giacobbe, D. R., Blobel, B., Parruti, G., and Giacomini, M. (2022). A NLP pipeline for the automatic extraction of a complete microorganism’s picture from microbiological notes. J. Personalized. Med. 12(9), 1424. doi: 10.3390/jpm12091424
Moretti, M., Wellekens, S., Dirkx, S., Vekens, K., Van Laethem, J., Ilsen, B., et al. (2023). Features of post-obstructive pneumonia in advanced lung cancer patients, a large retrospective cohort. Infect. Dis. (London England). 55, 149–157. doi: 10.1080/23744235.2022.2143888
Niehoff, J. H., Kalaitzidis, J., Kroeger, J. R., Schoenbeck, D., Borggrefe, J., and Michael, A. E. (2023). Evaluation of the clinical performance of an AI-based application for the automated analysis of chest X-rays. Sci. Rep. 13, 3680. doi: 10.1038/s41598-023-30521-2
Ochoa, C. E., Mirabolfathinejad, S. G., Ruiz, V. A., Evans, S. E., Gagea, M., Evans, C. M., et al. (2011). Interleukin 6, but not T helper 2 cytokines, promotes lung carcinogenesis. Cancer Prev Res. (Philadelphia Pa.) 4, 51–64. doi: 10.1158/1940-6207.Capr-10-0180
Oliaro, A., Filosso, P. L., Cavallo, A., Giobbe, R., Mossetti, C., Lyberis, P., et al. (2008). The significance of intrapulmonary metastasis in non-small cell lung cancer: upstaging or downstaging? A re-appraisal for the next TNM staging system. Eur. J. Cardio-thoracic. Surg 34, 438–443. doi: 10.1016/j.ejcts.2008.03.070
Oliviero, M., Romilde, I., Beatrice, M. M., Matteo, V., Giovanna, N., Consuelo, A., et al. (2016). Evaluations of thyme extract effects in human normal bronchial and tracheal epithelial cell lines and in human lung cancer cell line. Chemico-biol. Interact. 256, 125–133. doi: 10.1016/j.cbi.2016.06.024
Ozkok, S., Karakoyun-Celik, O., Goksel, T., Mogulkoc, N., Yalman, D., Gok, G., et al. (2008). High dose rate endobronchial brachytherapy in the management of lung cancer: response and toxicity evaluation in 158 patients. Lung Cancer (Amsterdam Netherlands). 62, 326–333. doi: 10.1016/j.lungcan.2008.03.018
Party, P., Kókai, D., Burián, K., Nagy, A., Hopp, B., and Ambrus, R. (2022). Development of extra-fine particles containing nanosized meloxicam for deep pulmonary delivery: In vitro aerodynamic and cell line measurements. Eur. J. Pharm. Sci. 176, 106247. doi: 10.1016/j.ejps.2022.106247
Ragnoli, B., Fusco, F., Pignatti, P., Cena, T., Valente, G., and Malerba, M. (2024). Bronchial progenitor cells in obstructive and neoplastic lung disease: A pilot study. J. Clin. Med. 13(2), 609. doi: 10.3390/jcm13020609
Shin, D., Kim, J., Lee, J. H., Kim, J. I., and Oh, Y. M. (2023). Profiling of microbial landscape in lung of chronic obstructive pulmonary disease patients using RNA sequencing. Int. J. Chronic. Obstruct. Pulmonary. Dis. 18, 2531–2542. doi: 10.2147/copd.S426260
Steinfort, D. P., Christie, M., Antippa, P., Rangamuwa, K., Padera, R., Müller, M. R., et al. (2021). Bronchoscopic thermal vapour ablation for localized cancer lesions of the lung: A clinical feasibility treat-and-resect study. Respiration 100, 432–442. doi: 10.1159/000514109
Suri, C., Pande, B., Sahithi, L. S., Sahu, T., and Verma, H. K. (2024). Interplay between lung diseases and viral infections: A comprehensive review. Microorganisms 12(10), 2030. doi: 10.3390/microorganisms12102030
Tang, L., Zhang, Z., Ding, W., Tang, J., Deng, X., He, Q., et al. (2024). Preparation, characterization, and Staphylococcus aureus biofilm elimination effect of baicalein-loaded tyrosine/hyaluronic acid/β-cyclodextrin-grafted chitosan nano-delivery system. Int. J. Biol. Macromol. 254, 128066. doi: 10.1016/j.ijbiomac.2023.128066
Thwaites, R. S., Uruchurtu, A. S. S., Negri, V. A., Cole, M. E., Singh, N., Poshai, N., et al. (2023). Early mucosal events promote distinct mucosal and systemic antibody responses to live attenuated influenza vaccine. Nat. Commun. 14, 8053. doi: 10.1038/s41467-023-43842-7
Valvani, A., Martin, A., Devarajan, A., and Chandy, D. (2019). Postobstructive pneumonia in lung cancer. Ann. Trans. Med. 7, 357. doi: 10.21037/atm.2019.05.26
Wang, Z., Liu, X., Duan, Y., and Huang, Y. (2022). Infection microenvironment-related antibacterial nanotherapeutic strategies. Biomaterials 280, 121249. doi: 10.1016/j.biomaterials.2021.121249
Wang, W., Xi, M., Duan, X., Wang, Y., and Kong, F. (2015). Delivery of baicalein and paclitaxel using self-assembled nanoparticles: synergistic antitumor effect in vitro and in vivo. Int. J. Nanomed. 10, 3737–3750. doi: 10.2147/ijn.S80297
Wang, X., Xiong, L., Wang, Y., Yang, K., Xiao, T., Chi, X., et al. (2023). Comparison of the inoculum effect of in vitro antibacterial activity of Imipenem/relebactam and Ceftazidime/avibactam against ESBL-, KPC- and AmpC-producing Escherichia coli and Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob. 22, 107. doi: 10.1186/s12941-023-00660-5
Wei, Q., Chen, X., Chen, X., Yuan, Z., and Wang, C. (2022). Contribution of IL-38 in lung immunity during pseudomonas aeruginosa-induced pneumonia. Shock. (Augusta Ga.) 57, 703–713. doi: 10.1097/shk.0000000000001919
Wei, Y., Yang, L., and Wang, Q. (2024). Analysis of clinical characteristics and prognosis of lung cancer patients with CPFE or COPD: a retrospective study. BMC Pulmonary. Med. 24, 274. doi: 10.1186/s12890-024-03088-5
Wu, Z., Yao, Y., Li, X., Cai, H., Wang, G., Yu, W., et al. (2024). Sensitive and rapid identification of pathogens by droplet digital PCR in a cohort of septic patients: a prospective diagnostic study. Infect. Dis. (London England). 56, 830–841. doi: 10.1080/23744235.2024.2354312
Yamada, Y., Sekine, Y., Suzuki, H., Iwata, T., Chiyo, M., Nakajima, T., et al. (2010). Trends of bacterial colonisation and the risk of postoperative pneumonia in lung cancer patients with chronic obstructive pulmonary disease. Eur. J. Cardio-thoracic. Surg 37, 752–757. doi: 10.1016/j.ejcts.2009.05.039
Yang, Q., Lv, S., Li, Q., Lan, L., Sun, X., Feng, X., et al. (2024). Bronchoscopic holmium laser ablation continuous cryoablation for the treatment of airway stenosis caused by tissue hyperplasia after tracheal intubation: clinical case observation. J. Thorac. Dis. 16, 4693–4701. doi: 10.21037/jtd-24-67
Yardley, D. A. (2013). nab-Paclitaxel mechanisms of action and delivery. J. Controlled Release. 170, 365–372. doi: 10.1016/j.jconrel.2013.05.041
Zhang, Q., Tang, L., Zhou, Y., He, W., and Li, W. (2021). Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer: current understanding in characteristics, diagnosis, and management. Front. Immunol. 12. doi: 10.3389/fimmu.2021.663986
Zhang, J., Wan, S., Zhou, H., Du, J., Li, Y., Zhu, H., et al. (2024). Programmed nanocloak of commensal bacteria-derived nanovesicles amplify strong immunoreactivity against tumor growth and metastatic progression. ACS Nano. 18, 9613–9626. doi: 10.1021/acsnano.3c13194
Zhao, L., Luo, J. L., Ali, M. K., Spiekerkoetter, E., and Nicolls, M. R. (2023). The human respiratory microbiome: current understandings and future directions. Am. J. Respir. Cell Mol. Biol. 68, 245–255. doi: 10.1165/rcmb.2022-0208TR
Zheng, R. S., Chen, R., Han, B. F., Wang, S. M., Li, L., Sun, K. X., et al. (2024). Cancer incidence and mortality in China, 2022. Zhonghua. Zhong. Liu. Za. Zhi. [Chinese J. oncology] 46, 221–231. doi: 10.3760/cma.j.cn112152-20240119-00035
Keywords: advanced lung cancer, obstructive pneumonia, pulmonary abscess, nanomedicine, antimicrobial therapy
Citation: Cao P, Chen L, Xie S and Hu B (2025) Advances in diagnosis and treatment of lung cancer-associated obstructive pneumonia and lung abscess: synergistic strategies for infection control and antitumor therapy. Front. Cell. Infect. Microbiol. 15:1638997. doi: 10.3389/fcimb.2025.1638997
Received: 01 June 2025; Accepted: 24 September 2025;
Published: 14 October 2025.
Edited by:
Meng Qin, Beijing University of Chemical Technology, ChinaCopyright © 2025 Cao, Chen, Xie and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaohua Xie, eGllc2hhb2h1YTIwMDlAMTYzLmNvbQ==; Bin Hu, aHViaW5Ac2Nzemx5eS5vcmcuY24=
†These authors have contributed equally to this work{sp}
 Peijun Cao
Peijun Cao Li Chen
Li Chen Shaohua Xie*
Shaohua Xie* Bin Hu
Bin Hu