- Department of Hematology, Nanfang Hospital, Southern Medical University, Clinical Medical Research Center of Hematological Diseases of Guangdong Province, Guangzhou, China
Background: Letermovir (LTV) is an effective strategy for cytomegalovirus (CMV) reactivation prophylaxis and is increasingly used for allogeneic hematopoietic stem cell transplantation. However, it carries the risk of delayed immune reconstitution. This retrospective study assessed the impact of primary LTV prophylaxis on viral infections, disease relapse, and immune reconstitution in haploidentical hematopoietic stem cell transplantation (haplo-HSCT) recipients.
Methods: Among 462 patients from Nanfang Hospital, propensity score matching created two cohorts: 106 with LTV prophylaxis and 212 without LTV prophylaxis. EBV/CMV infection, relapse, and survival were analyzed by competing risk models and Cox regression. Immune reconstitution and function were assessed by flow cytometry.
Results: LTV prophylaxis had protective effects against CMV viremia, with a 1-year incidence of 32.1% in the LTV group compared with 46.2% in the non-LTV group (P = 0.009). However, the 1-year cumulative incidence of EBV viremia was significantly higher in the LTV group than in the non-LTV group (38.7% vs.13.7%, P<0.001). On multivariate analysis, LTV prophylaxis was a protective factor for CMV viremia (HR = 0.54, P = 0.014) but a risk factor for EBV viremia (HR = 2.69, P<0.001). Additionally, the 1-year cumulative incidence of relapse post-HSCT was notably higher in the LTV group than in the non-LTV group (13.2% vs. 6.1%, P = 0.032). In multivariate analysis, LTV prophylaxis was an independent risk factor for relapse (HR = 2.56, P = 0.024). Lymphocyte subset counts and functions post-transplantation were significantly lower in the LTV group than in the non-LTV group.
Conclusion: LTV prophylaxis might play a dual role in haplo-HSCT recipients, reducing CMV infection but increasing EBV infection and relapse.
Introduction
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is widely used to treat hematologic malignancies and achieves similar outcomes compared with human leukocyte antigen (HLA)-matched sibling donor stem cell transplantation (Guo et al., 2021; Zheng and Tian, 2021). However, the strategies for graft-versus-host disease (GVHD) prophylaxis in haplo-HSCT, which mainly include ex vivo and in vivo T-cell depletion (TCD) (Mohty et al., 2024; Giardino et al., 2024), are considered to increase the risk of opportunistic infections especially viral reactivations including Epstein–Barr virus (EBV) and cytomegalovirus (CMV) (Ru et al., 2022).
The novel CMV DNA terminase inhibitor-letermovir (LTV) has been shown to be effective for prophylaxis of CMV reactivation in HSCT recipients (Zhang et al., 2024; Marty et al., 2017; Muhsen et al., 2024). LTV has consistently demonstrated efficacy in reducing the risk of clinically significant CMV infection and CMV disease across various transplantation settings, including matched sibling donors, unrelated donors, and haploidentical donors (Vyas et al., 2023). Historically, the incidence of CMV reactivation has reached 75% in haplo-HSCT recipients (Wang et al., 2019; Huang et al., 2022). With the use of LTV, less than 20% of haplo-HSCT recipients suffer CMV reactivation (Ma et al., 2023; Freyer et al., 2022; Lin et al., 2021; Terao et al., 2021).
CMV reactivation after allo-HSCT has been reported to induce long-lasting expansion of memory-like NK cells, CMV-adapted NK cells and circulating Vδ2negγδ T cells, which might benefit immune reconstruction after HSCT (Litjens et al., 2018; Jang et al., 2019). However, delayed immune reconstitution, including CMV-specific immune reconstitution, was observed among patients receiving LTV prophylaxis in several studies (Zamora et al., 2021; Sperotto et al., 2021; Orofino et al., 2023). Therefore, concern has emerged with respect to the effect of less CMV exposure on other infections especially viral infections (Ito et al., 2013; Elmaagacli et al., 2011; Takenaka et al., 2015). Several studies have reported a greater incidence of EBV reactivation in patients who underwent umbilical cord blood transplantation or haplo-HSCT when LTV was used for CMV prophylaxis (Yan et al., 2024; Kong et al., 2024).
Here, we conducted a retrospective propensity score (PS)-matched cohort study to mainly compare the incidence of EBV reactivation and relapse of disease with and without LTV implementation for CMV prophylaxis after haplo-HSCT, and then to evaluate the impact of LTV on immune reconstruction and function post-transplant.
Methods
Study design and patients
Prophylactic LTV was implemented at Nanfang Hospital, Southern Medical University from March 2022 for CMV-seropositive allo-HSCT recipients. LTV prophylaxis was started on the second day of neutrophil engraftment and was continued until day 100 with a dosage of 480 mg daily or 240 mg if concurrent cyclosporin A was used. Consecutive CMV-seropositive haplo-HSCT recipients who received LTV primary prophylaxis for CMV prophylaxis from March 2022 to December 2023 were analyzed in this retrospective study as the LTV cohort. For the non-LTV cohort, propensity score matching (PSM) for baseline variables was used and CMV-seropositive haplo-HSCT recipients without LTV between January 2020 and November 2023 were included. This study was approved by the Medical Ethics Committee of Nanfang Hospital.
Virus monitoring and GVHD prophylaxis
For all the recipients, the CMV and EBV-DNA loads in the blood were measured regularly by real-time quantitative polymerase chain reaction weekly for the first 3 months after transplantation, once every 2 weeks from the 4th to the 9th month post-transplantation and then once per month from the 10th to the 12th month. Once CMV or EBV-DNA in the blood was positive, the viral loads were detected once again the next day. If positive, viral loads were monitored twice a week. The preemptive treatment threshold is defined as either two consecutive positive CMV PCR results within one week or a single result >500 copies/mL. First-line therapies include ganciclovir and valganciclovir, while second-line options consist of foscarnet, cidofovir, immunoglobulin and cytotoxic T lymphocytes (CTLs) (2022;Xuan et al., 2012). Upon initial detection of positive EBV-DNA in blood (>500 copies/mL), repeat viral load testing was performed the following day. For patients with two consecutive positive EBV-DNA results, the EBV preemptive strategy was initiated, including antiviral therapy (ganciclovir, acyclovir, or foscarnet), intravenous immunoglobulin (0.4 g/kg/day × 3 days), or immunosuppression reduction. In cases of persistently positive EBV-DNA across four consecutive tests with an upward trend, weekly rituximab (375 mg/m²) was administered until viral clearance or for a maximum of 4 weeks (Xuan et al., 2012). ATG (ImtixSangstat, Lyon, France) was administered at 2.5 mg/kg/day from days -3 to -1.
Flow cytometry analysis
T cell and NK cell subset reconstitution was analyzed using flow cytometry at +1, +2, and +3 months after allo-HSCT in the PSM-matched population to evaluate the overall immune reconstitution process. Besides, functional analysis of cellular immunity was performed to assess the potential impact of LTV prophylaxis on immune function.
Peripheral blood mononuclear cells were isolated from fresh anticoagulated blood via Ficoll density gradient centrifugation and assayed via a FACS CANTO II flow cytometer (BD Biosciences). T cell phenotyping was performed using directly conjugated monoclonal antibodies CD3 (PerCP), CD8 (FITC-A), and CD4 (FITC-A). For NK cell surface staining to identify cell subsets, CD3 (PerCP), CD56 (PE-A), and CD16 (FITC) were used. Concurrently with T cell and NK cell phenotyping, monoclonal antibodies PD-1 (BV421), TIM-3 (PE-Cy-7-A), and CTLA-4 (APC) were used to detect T cell and NK cell exhaustion markers.
T cell and NK cell functional assays involved non-specific stimulation with phorbol myristate acetate (PMA, 50 ng/mL) and ionomycin (Ino, 1 μg/mL) to induce the secretion of intracellular cytokines. After permeabilization, intracellular cytokines interferon-γ (IFN-γ, PE-Cy-7-A), tumor necrosis factor-α (TNF-α, APC), granzyme B (APC), and perforin (PE-Cy-7-A) were detected. The acquired data were further analyzed using BD-FACSDiva™ software. The flow cytometric results are presented as the percentage of positive cells (Additional File: Figures S1, S2).
Definitions
CMV and EBV viremia were both defined as the presence of more than 500 copies/mL in the blood twice consecutively (Lin et al., 2019). The diagnosis of EBV- and CMV- associated diseases were according to the guidelines and our previous description (Lin et al., 2019; Ljungman et al., 2002). Late-onset CMV reactivation was defined as CMV reactivation occurred after 100 days after transplantation (Rowe et al., 2013; Liu et al., 2022). Breakthrough CMV infection was defined as an infection occurring during LTV administration (Perchetti et al., 2023). Patients’ COVID-19 status before HSCT is determined by pre-transplant serological antibody testing and nucleic acid testing (Nuccetelli et al., 2020). Engraftment, relapse, non-relapse mortality (NRM), overall survival (OS), and disease-free survival (DFS) were assessed as previously described (Zeng et al., 2021; Hu et al., 2024). GVHD-free and relapse-free survival (GRFS) was defined as survival without the following events: grade III-IV acute GVHD (aGVHD), severe chronic GVHD (cGVHD), disease relapse, or death from any cause after haplo-HSCT (Li et al., 2023). Organ scoring and global assessment of cGVHD were performed according to the 2014 National Institutes of Health consensus criteria (Filipovich et al., 2005), while aGVHD was evaluated based on the criteria established by the Mount Sinai Acute GVHD International Consortium (Schoemans et al., 2018).
Statistics
PSM for baseline variables (recipient age and sex, disease, graft type, conditioning regimen, human leukocyte antigen, pretransplant remission status, donor age and sex, CMV/EBV serostatus, mononuclear cells graft) used Logistic regression with the nearest-neighbor method. Caliper settings were set to less than 0.2 to restrict the distance between matched units. Matching ratio was 1:2 for each LTV-non-LTV cohort. Postmatching balance was evaluated with the standardized mean difference, and the optimal balance was considered as <0.2. The chi-square test and Fisher’s exact test were used for categorical or hierarchical features, and Wilcoxon rank-sum test was used to compare continuous variables. Relapse, non-relapse mortality and viral infections were evaluated using the Fine-Gray method (Austin and Fine, 2017) (package cmprsk of R), taking into account cumulative incidence. Correlated risks were estimated with Competing risk model (package FGR of R). Competing events were defined as follows: for relapse, death without relapse; for non-relapse mortality, relapse/progression; for viral infection, death without viral infection. The Kaplan-Meier survival curve and Log-rank test are used for OS, DFS and GRFS. A Cox proportional hazards model was used to evaluate the associations of patient and transplant characteristics with outcomes in a multivariate analysis. The level of statistical significance was set at P < 0.05. Statistical analyses were performed using R version 4.3.3 and SPSS 26.0.
Results
Patient characteristics
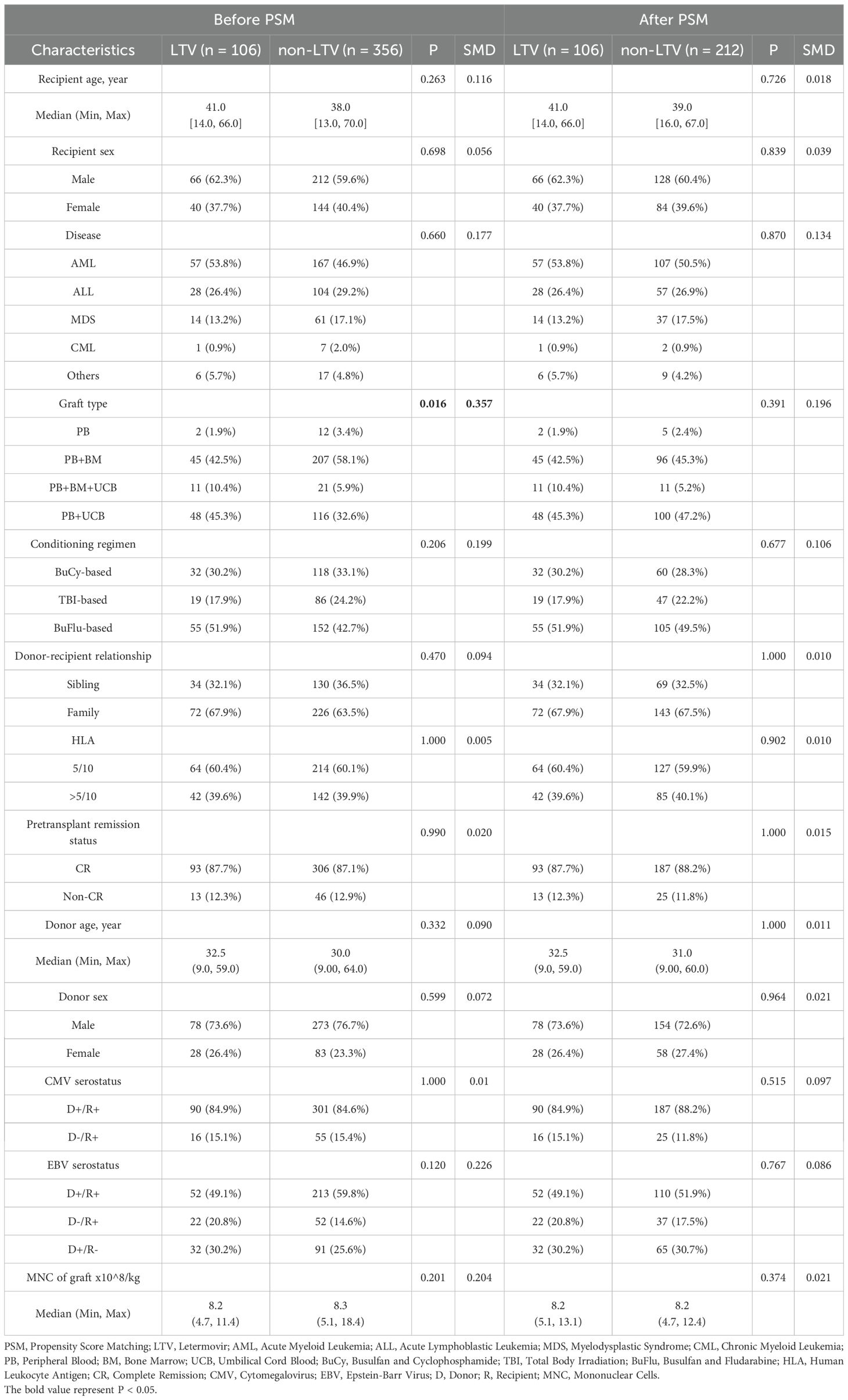
A total of 462 patients were screened in this study including 106 in the LTV group and 356 in the non-LTV group. The clinical characteristics are shown in Table 1. Among the overall cohort, the patient and transplant characteristics were comparable between the LTV group and the non-LTV group, except for differences in graft type (P = 0.016). After PSM, 212 of the 356 patients in the non-LTV group were randomized. The patient characteristics were well-balanced with PSM, and there were no significant differences between the groups (Table 1; all P > 0.05, SMD < 0.2). In the LTV group, LTV was started at a median of 13 days (range, 9-24) after haplo-HSCT and was administered for a median duration of 84 days (range, 58-103) post-HSCT.
CMV viremia and CMV-associated diseases
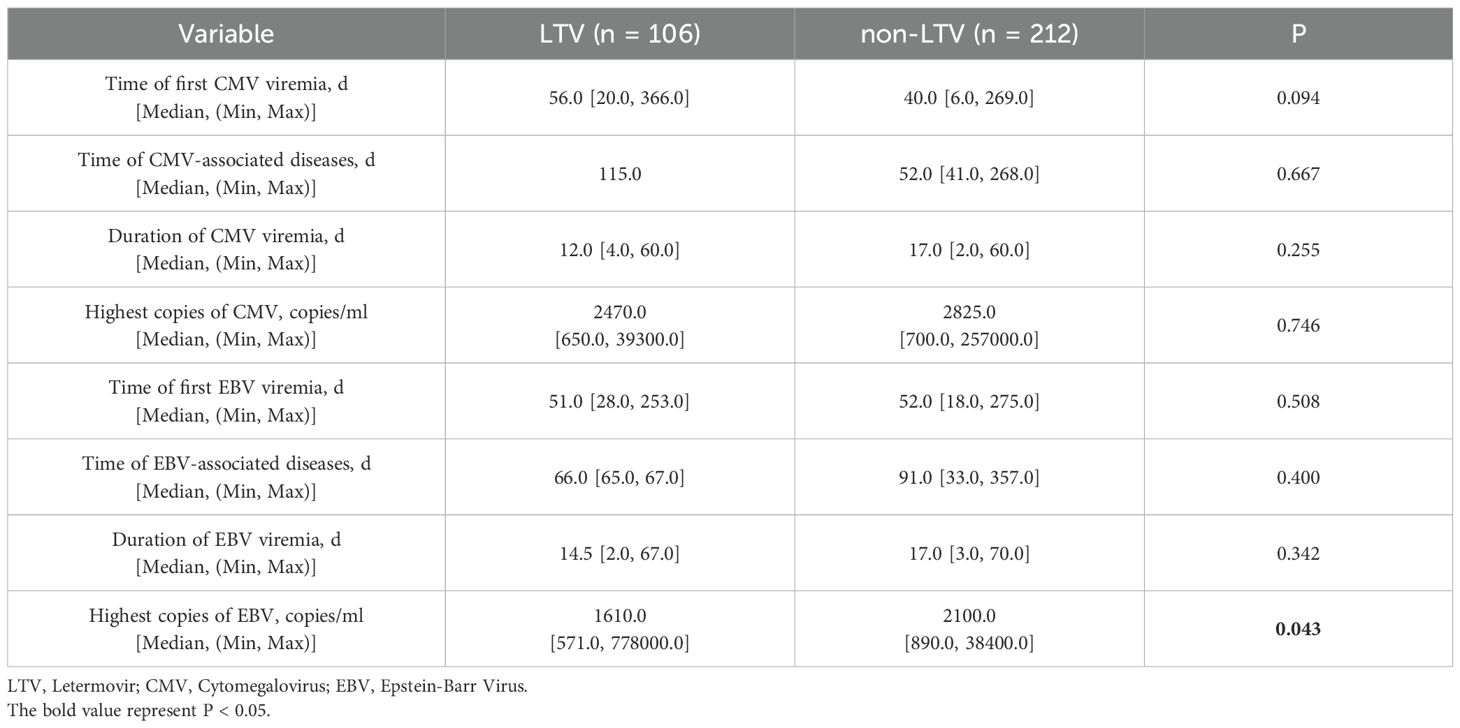
The median follow-up was 761.5 days (range, 1–1807) after transplantation. A total of 133 recipients (41.8%) experienced CMV viremia at a median of 40 days (range, 6–366) following haplo-HSCT. The 100-day cumulative incidence of CMV viremia was 24.5% (95% confidence interval [CI], 16.8–33.1%) in the LTV group and 45.8% (95% CI, 38.9–52.3%) in the non-LTV group (P < 0.001). The 1-year cumulative incidence of CMV viremia was significantly lower in the LTV group than in the non-LTV group [32.1% (95% CI, 23.4–41.1%) vs 46.2% (95% CI, 39.4–52.8%); P= 0.009] (Figure 1A). Sixteen patients experienced breakthrough CMV viremia at a median of 29.5 days (range, 20-68) after the initiation of LTV prophylaxis. Following first-line treatment, 12 patients (75.0%) achieved CMV-DNA negativity. The remaining 4 patients (25.0%) had persistent CMV infection but successfully cleared the virus after second-line therapy. No patients developed CMV disease or died with sustained CMV-DNA positivity. The 1-year cumulative incidences of late-onset CMV viremia after HSCT in the LTV and non-LTV groups were 8.5% (95% CI, 4.2-14.9%) and 0.5% (95% CI, 0-2.4%), respectively (P <0.001).
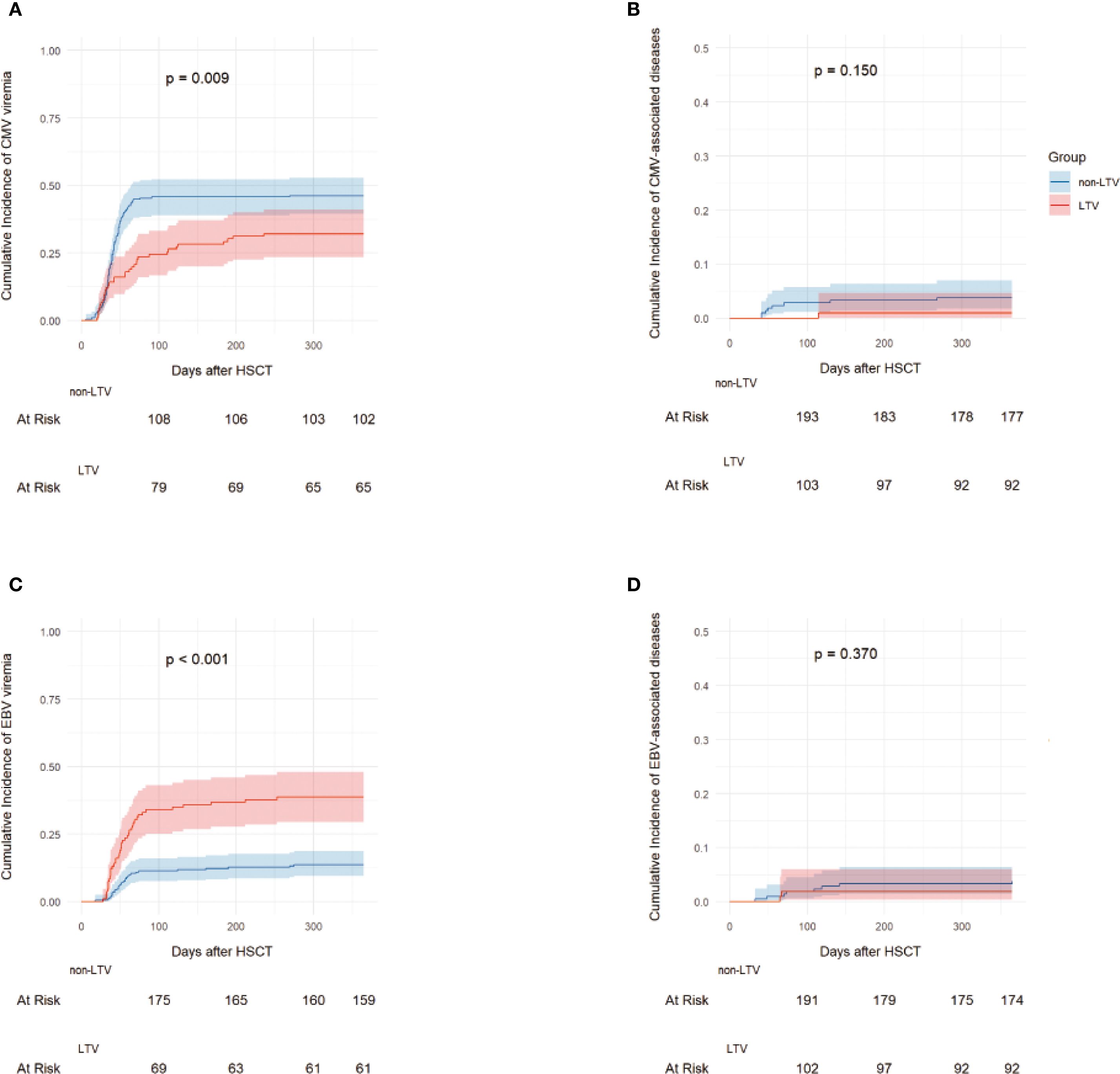
Figure 1. The incidence of CMV and EBV infection. 1-year Cumulative incidences of CMV viremia (A), CMV-associated diseases (B), EBV viremia (C) and EBV-associated diseases (D) in the LTV and non-LTV groups. LTV, Letermovir; CMV, Cytomegalovirus; EBV, Epstein-barr virus.
During the follow-up period, one patient in the LTV group developed CMV pneumonia at 115 days after haplo-HSCT. In the non-LTV group, eight patients developed CMV-associated diseases including 4 with enteritis, 3 with pneumonia, and 1 with encephalitis (Figure 1B). The median time to onset of CMV-associated diseases in the non-LTV group was 52 days (range, 41–268) after haplo-HSCT. The 1-year cumulative incidence of CMV-associated diseases was 0.9% (95% CI: 0.1–4.7%) and 3.8% (95% CI: 1.8–7.0%) in the LTV and non-LTV groups, respectively (P = 0.150). The detailed characteristics of CMV infection are summarized in Table 2.
EBV viremia and EBV-associated diseases
A total of 70 recipients (22.0%) experienced EBV viremia at a median of 51.5 days (range, 18–275) following haplo-HSCT. The 100-day cumulative incidence of EBV viremia was 34.0% (95% CI, 25.1–43.0%) in the LTV group and 11.3% (95% CI, 7.5–16.0%) in the non-LTV group (P < 0.001). The 1-year cumulative incidence of EBV viremia was significantly higher in the LTV group than in the non-LTV group [38.7% (95%CI, 29.4-47.9%) vs. 13.7% (95%CI, 9.5-18.7%), P < 0.001] (Figure 1C). In the LTV group, 16 patients with EBV viremia received rituximab preemptive therapy and 1 received EBV-CTL therapy. In the non-LTV group, 15 patients with EBV viremia received rituximab pre-emptive therapy and 6 received EBV-CTL therapy. The overall response rates to therapy were 86.7% and 72.2% for patients with EBV viremia in the LTV and non-LTV groups, respectively (P = 0.413).
During the follow-up period, two patients in the LTV group developed EBV-associated diseases including 1 hemophagocytic lymphohistiocytosis (HLH) and 1 with encephalitis while 8 in the non-LTV group developed EBV-associated diseases including 3 posttransplant lymphoproliferative disorder (PTLD), 3 with enteritis, 1 with encephalitis and 1 with pneumonia. The median time to onset of EBV-associated diseases was 66 days (range, 65-67) in the LTV group and 91 days (range, 33–357) in the non-LTV group. The 1-year cumulative incidence of EBV-associated diseases was 1.9% (95% CI: 0.4–6.1%) and 3.3% (95% CI: 1.5–6.4%) in the LTV and non-LTV groups, respectively (P = 0.370) (Figure 1D). Among the 2 patients who developed EBV- associated disease in LTV group, both received rituximab and EBV-CTL therapy but ultimately died due to disease severity. In contrast, although 8 patients experienced EBV- associated disease in non-LTV group, outcomes were comparatively better—only 1 patient with EBV pneumonia died from sepsis, while the remaining cases were controlled or cured. The detailed characteristics of the EBV infection are summarized in Table 2.
Risk factors for CMV and EBV infections
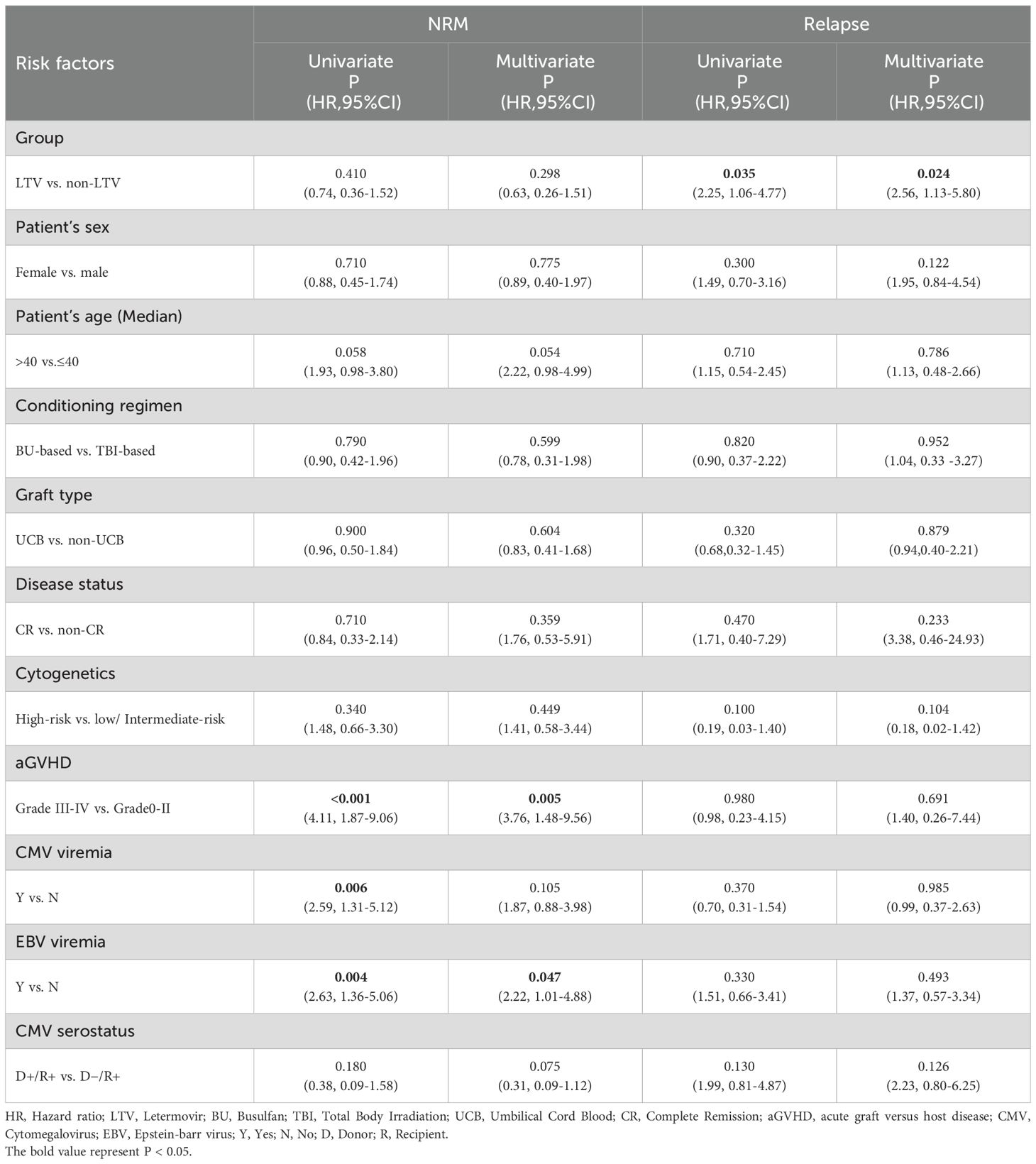
Univariate and multivariate analyses of the risk factors for EBV and CMV infections post-transplantation are shown in Table 3. On multivariate analysis, LTV prophylaxis was a protective factor for CMV viremia (HR = 0.54, 95%CI, 0.33-0.88, P = 0.014) but a risk factor for EBV viremia (HR = 2.69, 95%CI, 1.56–4.64, P<0.001). The female donor served as a protective factor for EBV viremia. Grade III-IV aGVHD was identified as a risk factor for CMV viremia, CMV-associated diseases and EBV-associated diseases. Patient sex and age, donor age, conditioning regimen, graft type, HLA typing, patients’ COVID-19 status pre-HSCT and EBV/CMV serostatus did not show any significant influence on the risk of EBV and CMV infections.
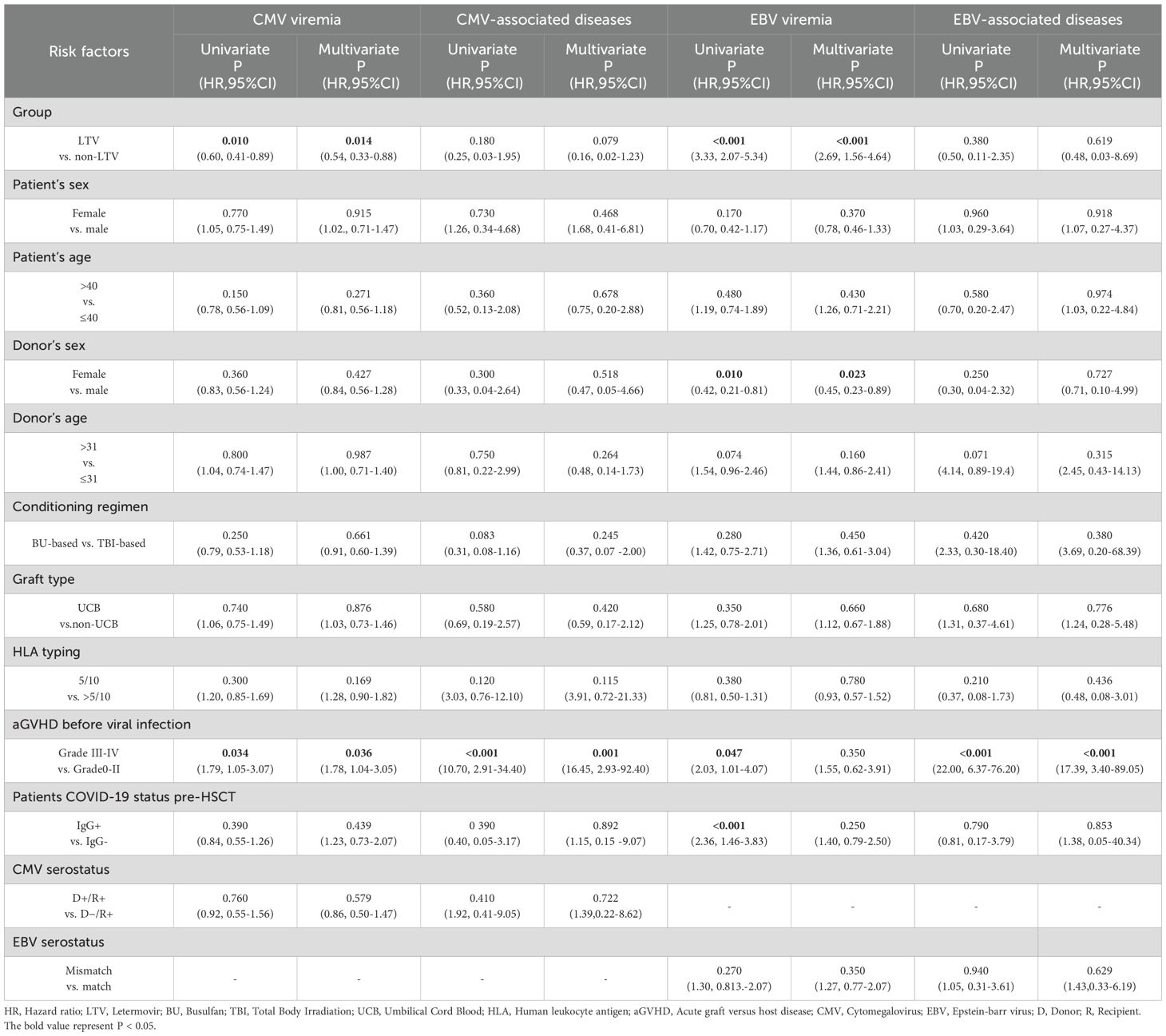
Table 3. Univariate and multivariate analyses of risk factors for EBV and CMV infections with 1-year after HSCT.
Subgroup analysis
To further examine the influence of LTV prophylaxis on EBV viremia post-transplantation, we performed subgroup analyses stratified by patient sex, patient age, donor sex, donor age, condition regimen, graft type, HLA typing, aGVHD before EBV viremia, patients COVID-19 status pre-HSCT and EBV serostatus. The 1-year incidence of EBV viremia was significantly higher in the LTV cohort than in the non-LTV cohort across all subgroups except for patients with Grade III-IV aGVHD before EBV viremia (Additional File: Figure S3).
GVHD
There were no significant differences in the cumulative incidence of grades I to IV aGVHD between the LTV group and the non-LTV group within 100 days [42.5% (95%CI, 32.9-51.7%) vs. 41.5% (95%CI, 34.8-48.1%), P = 0.780]. The cumulative incidence of grades II to IV acute GVHD for patients with LTV and patients without LTV were 22.8% (95%CI, 15.3-31.2%) and 19.4% (95%CI, 14.4-25.0%), respectively (P = 0.450). No significant differences in grades III and IV acute GVHD were detected between LTV group and non-LTV group [5.7% (95%CI, 2.3-11.2%) vs. 6.6% (95%CI, 4.0-10.5%), P = 0.738]. The cumulative incidence of moderate to severe cGVHD by 1-year post-HSCT was similar between patients with and without LTV, with rates of 17.9% (95%CI, 11.3-25.8%) and 16.5% (95%CI, 11.9-21.8%), respectively (P = 0.730).
Survival
During the follow-up period, 272 patients survived and 49 died, of whom 15 were in the LTV group and 34 were in the non-LTV group. The causes of death are presented in Additional File: Figure S4. The 1-year incidence of NRM was 9.4% (95% CI, 4.8–15.9%) in the LTV group and 12.3% (95% CI, 8.3–17.2%) in the non-LTV group (P = 0.410, Figure 2A). The 1-year incidence of relapse was 13.2% (95% CI, 7.6–20.4%) in the LTV group and 6.1% (95% CI, 3.4–9.9%) in the non-LTV group (P = 0.032, Figure 2B). The 1-year incidence of OS was 85.9% (95% CI, 78.0–91.2%) and 85.4% (95% CI, 80.0–89.5%), DFS was 77.4% (95% CI, 68.5–84.3%) and 82.1% (95% CI, 76.4–86.7%), and GRFS was 74.5% (95% CI, 65.5–81.9%) and 76.9% (95% CI, 70.8–82.1%), respectively, in the LTV and non-LTV groups (OS: P = 0.826, Figure 2C; DFS: P = 0.439, Figure 2D; GRFS: P = 0.759, Figure 2E). In multivariate analysis, LTV prophylaxis was the independent risk factor for relapse (HR, 2.56; 95% CI, 1.13-5.80 P = 0.024). EBV viremia was an independent risk factor for NRM, while Grade III–IV aGVHD was an independent risk factor for NRM, OS, and DFS (Table 4; Supplementary Table S1).
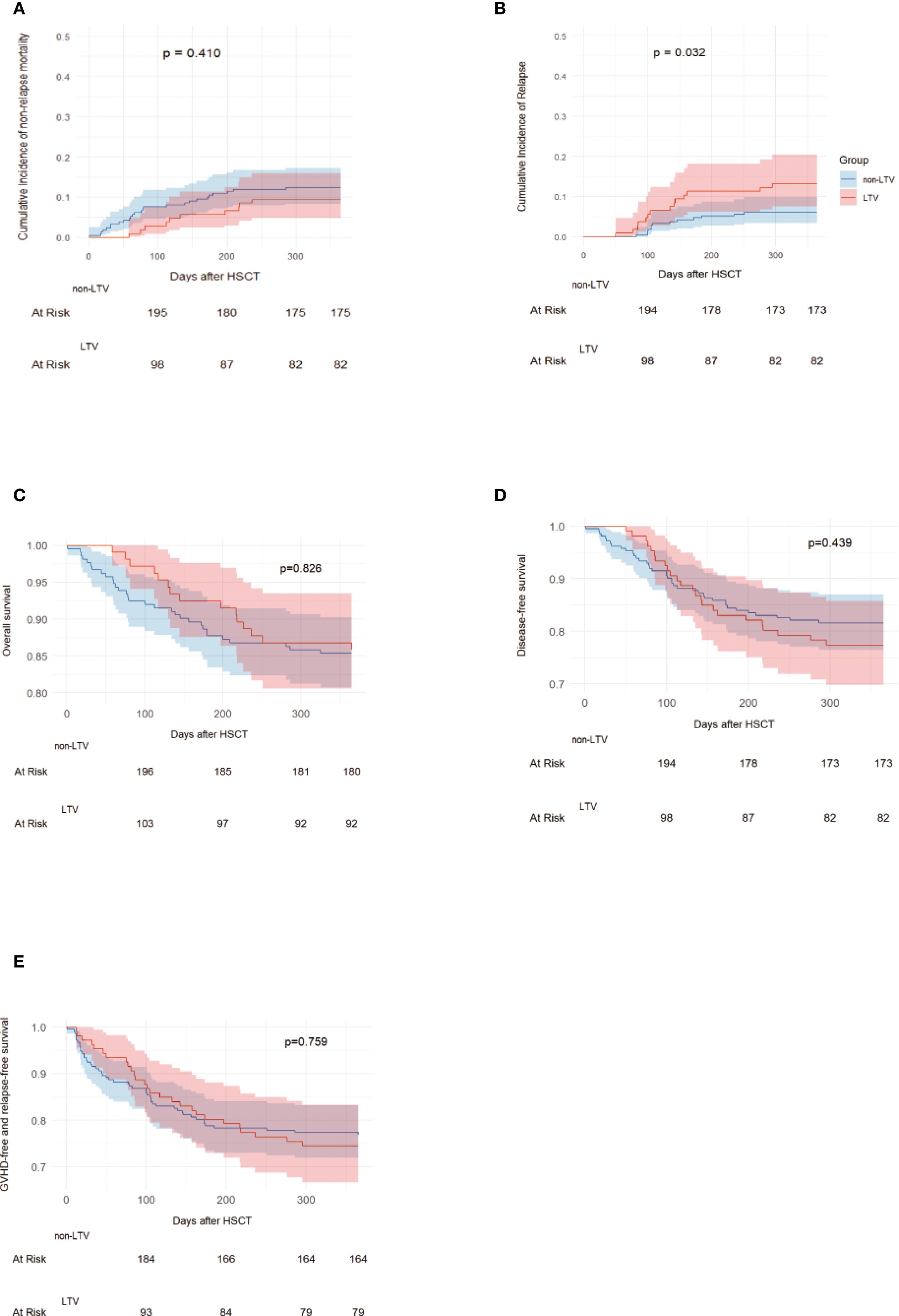
Figure 2. NRM, Relapse, OS, DFS and GRFS in LTV and without LTV groups. Non-relapse mortality (A), Relapse (B), Overall survival (C), Disease-free survival (D), GVHD free, relapse free survival (E). LTV, Letermovir; HSCT, Hematopoietic stem cell transplantation; GVHD, graft versus host disease.
Immune reconstitution and function
Immune reconstitution was analyzed in 272 patients who had continuous and complete immune reconstitution data including 95 in the LTV group and 177 in the non-LTV group (Figure 3 and Additional File: Table S2). At 1 and 2 months post-transplant, the median percentage of CD8 T cells was significantly lower in the LTV group compared with the non-LTV group (both P < 0.001, Figure 3A). No significant difference was observed in the median percentage of CD4 T cells and NK cells between the two groups at 1, 2, 3 months post-transplant. (Figure 3A; C). At 2 month post-transplant, the median counts of lymphocytes were significantly lower in the LTV group compared to the non-LTV group (P < 0.001, Figure 3B). At 1 and 2 months post-transplant, the median counts of CD8 T cells were significantly lower in the LTV group compared to the non-LTV group (both P < 0.001, Figure 3B). At 2 month post-transplant, the median counts of CD4 T cells were significantly lower in the LTV group compared to the non-LTV group (P < 0.001, Figure 3B).
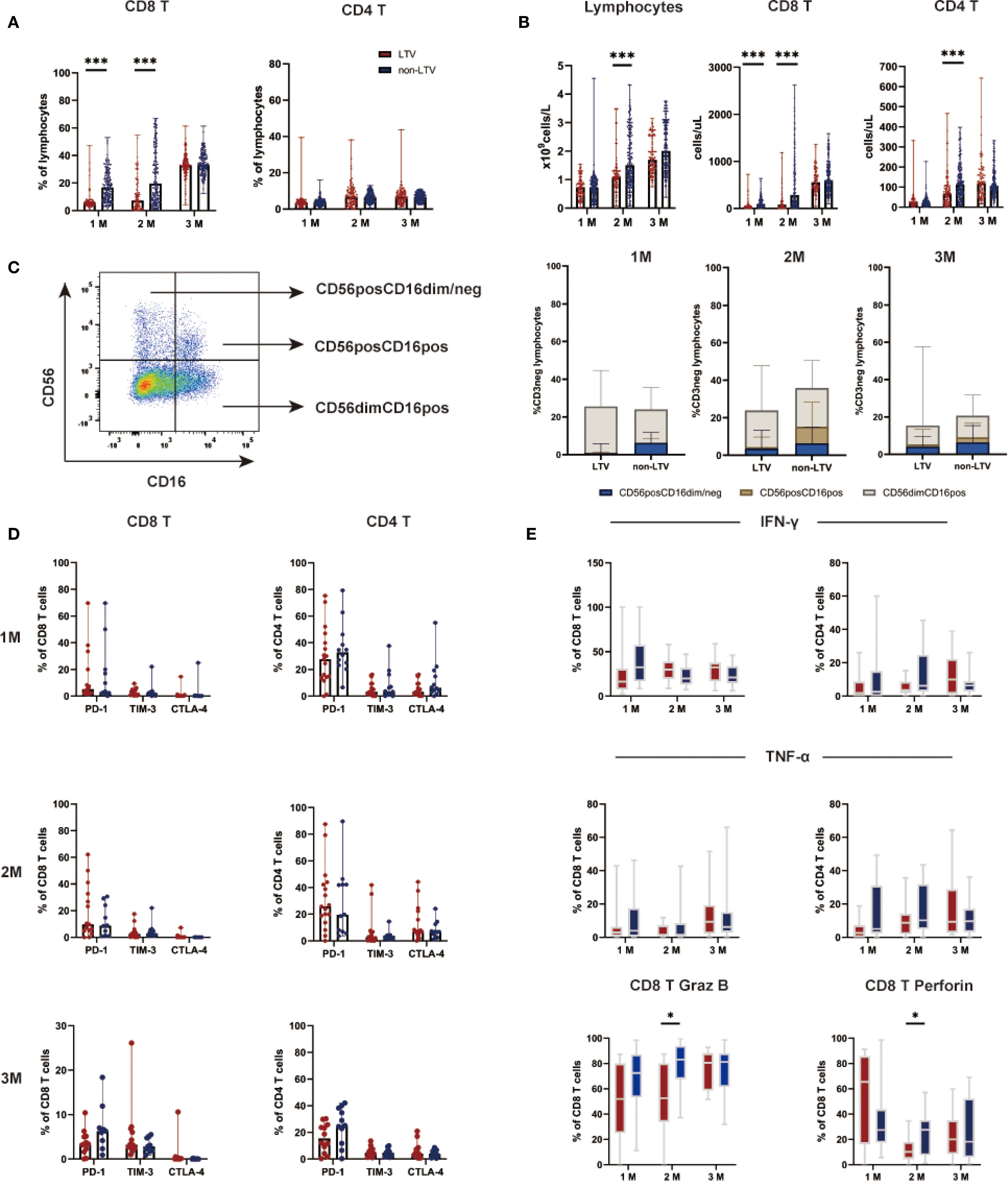
Figure 3. Lymphocyte subsets and functions within three months post-transplantation. CD8 and CD4 T lymphocyte subsets percentages (A), CD8 and CD4 lymphocyte subsets counts (B), NK lymphocyte subsets percentages (C), Expression of exhaustion markers on lymphocyte subsets (D), Function of lymphocyte subsets (E) within three months post-transplantation in the LTV and non-LTV groups. LTV, Letermovir; M, Month; *P < 0.05, ***P < 0.001.
Further analysis of T cell function was performed in 29 patients including 17 LTV group and 12 in non-LTV group. The results revealed that, at 2 months post-transplant, the Granzyme B (P = 0.014; Figure 3E) and perforin (P = 0.040; Figure 3E] expression in CD8 T cells in the LTV group was significantly lower than in the non-LTV group. The expression of PD-1, TIM-3, and CTLA-4 on CD8 and CD4 T cell subsets were similar between the two groups at 1, 2, 3 months post-transplant (Figure 3D).
Among patients with immune reconstitution cell count analysis, 51 (18.75%) developed EBV reactivation (EBV+ group); while among patients with immune function assessment, 16 (55.17%) experienced reactivation. Comparative analysis revealed that 1 month post-transplant, the EBV+ group exhibited significantly lower absolute counts of lymphocytes (P <0.001)), CD8 T cells (P <0.001), and CD4 T cells (P = 0.037) compared to the EBV- group, with CD8 T cell counts remaining significantly lower at 2 month post-transplant (P <0.001). Regarding immune function, the EBV+ group showed markedly reduced expression of granzyme B (P = 0.013) and perforin (P = 0.028) by CD8+ T cells at month 1 post-transplant relative to the EBV- group (Supplementary Figure S5).
Discussion
In the current study, LTV prophylaxis, although reducing the risk for CMV viremia, was associated with a significantly increased incidence of EBV viremia and primary disease relapse. Besides, our results demonstrated that LTV may delay T-lymphocyte reconstitution and impair its function, which could potentially contribute to these outcomes.
haplo-HSCT has become widely adopted worldwide, particularly through T-cell-replete strategies, such as those involving post-transplantation cyclophosphamide (PTCy) or ATG-based protocols. The ATG-based regimen is one of the most commonly used GVHD prophylaxis strategies for haplo-HSCT in China but is associated with a relatively high risk of CMV and EBV infection after HSCT (Wang et al., 2023; Ru et al., 2020; Yang et al., 2019). LTV has now been recommended for preventing CMV infection in CMV-seropositive recipients (Kong et al., 2024; Cesaro et al., 2023; Febres-Aldana et al., 2024). In several retrospective and observational studies on the efficacy of LTV, which included patients who underwent haplo-HSCT with ATG prophylaxis, the results showed that the incidence of post-transplant CMV viremia decreased to 20%-35% following LTV prophylaxis (Toya et al., 2024; Hopff et al., 2024). In addition, we found that the incidence of late-onset CMV and breakthrough CMV infections was also consistent with those in previous studies (Włodarczyk et al., 2024; Khawaja et al., 2023).
Several studies have explored the impact of LTV prophylaxis on other members of the herpesvirus family, such as HHV-6 (Kampouri et al., 2023; Terao et al., 2024) and EBV (Yan et al., 2024; Kong et al., 2024; Pei et al., 2024). Up to now, research has not revealed that using LTV increases the risk of HHV-6 reactivation or HHV-6 encephalitis (Kampouri et al., 2023; Terao et al., 2024). Studies have shown that the significant increase in EBV infection following LTV prophylaxis is primarily observed in allo-HSCT patients including both adult and pediatric populations (Zhen et al., 2025; Oikonomopoulou et al., 2025). A real-world experience demonstrated that EBV reactivation was more frequent in patients receiving umbilical cord blood transplantation with LTV prophylaxis (Yan et al., 2024). Kong et al. conducted a study involving 230 patients received haplo-HSCT, and the results showed that the incidence of EBV reactivation after HSCT was significantly higher in the patients who received LTV prophylaxis than in those who did not (Kong et al., 2024). Furthermore, a recent study indicated that haplo-HSCT recipients receiving LTV had higher risk of PTLD compared to those who did not receive LTV (Pei et al., 2024). In our study, a higher incidence of EBV viremia in the LTV group was found compared to the non-LTV group. Multivariate analysis showed that LTV prophylaxis was the only risk factor for EBV reactivation after haplo-HSCT. Subgroup evaluations further confirmed that the use of LTV increased the incidence of EBV viremia in all subgroups, except for the grade III-IV aGVHD subgroup.
Previous studies have shown that LTV prophylaxis might be associated with a delay in polyfunctional CMV-specific cellular immune reconstitution (Zamora et al., 2021; Giménez et al., 2023). LTV prophylaxis was associated with delayed polyfunctional CMV-specific T-cell subsets reconstitution and decreased CMV antigens responses at 3 months after HSCT compared with preemptive antiviral therapy (Zamora et al., 2021). Sperotto et al. (2021) conducted a study involving 110 HSCT patients with 55 receiving preemptive antiviral treatment and 55 receiving LTV, and the results showed that the LTV group experienced impaired recovery of CD4 and CD8 T cells at days +60 and +90 after HSCT. A recent study including allo-HSCT patients aged 14 years and older, of whom 96.5% received ATG as GVHD prophylaxis followed by LTV, found that compared to non-LTV patients, the incidence of EBV viremia was significantly higher at 200 days post-transplant. Additionally, the LTV group showed a reduction in lymphocytes and CD8 T cells after transplantation (Zhen et al., 2025). Consistent with these findings, our study also observed that patients in the LTV group exhibited lower counts of total lymphocytes, CD4, and CD8 T cells compared to those who did not receive LTV. Furthermore, in our study, patients receiving LTV prophylaxis had reduced Granzyme B and perforin secretion by CD8 T cells at +2 months post-transplant compared to those not receiving LTV. These results suggests that LTV may lead to delayed immune reconstitution and impaired lymphocyte function. CMV reactivation was considered to drive posttransplant T-cell reconstitution (Scheper et al., 2013; Schäfer et al., 2024). While our study did not directly assess CMV-CTL subset reconstitution, CMV-CTLs are a critical component of CD8 T cells, and their quantitative and functional recovery may contribute to overall cellular immune reconstitution. we also conducted an exploratory analysis comparing immune reconstitution and function between patients with and without EBV reactivation. The results demonstrated that patients experiencing EBV reactivation exhibited impaired lymphocytes, CD8 and CD4 T cells reconstitution and CD8 T cells functional deficits. Therefore, we presumed that the impairment of immune reconstitution and function due to the reduce of exposure of CMV following LTV prophylaxis may be attribute to increased EBV reactivation.
In our study, despite a higher incidence of EBV viremia in the LTV group, the median copy peak of EBV viremia was lower than that in non-LTV group, and LTV did not significantly affect the incidence of EBV disease. This is consistent with findings from Kong’s study (Kong et al., 2024) which reported that the proportion of patients in the LTV group with low EBV-DNA loads (≥ 5 × 10² to < 1 × 104 copies/mL) was significantly higher than that in the control group. This may be attributed to early-stage pre-emptive therapy.
Interestingly, we observed a significantly higher relapse rate in the LTV group compared to the non-LTV group, and LTV prophylaxis was identified as an independent risk factor for relapse in this study. Research from Japan also found that the use of LTV prophylaxis was associated with an increased risk of relapse after transplantation (Akahoshi et al., 2022). In 1986, Swedish research found that patients with CMV infection after HSCT had a lower relapse rate than those without the infection (Lönnqvist et al., 1986). Subsequent studies confirmed that CMV reactivation protects against relapse in acute leukemia (Elmaagacli et al., 2011; Manjappa et al., 2014), with a major Japanese study of 3,539 allo-HSCT patients demonstrating that post-transplant CMV reactivation is an independent protective factor for relapse (Takenaka et al., 2015). Recent findings suggest that CD57+/CD27- CD4+ cells expanded during CMV exposure may eliminate CMV-infected leukemic cells (Yeh et al., 2021), potentially explaining the increased relapse rate following LTV treatment.
This study has certain limitations. First, although the cohorts were well matched, other variables (such as the use of corticosteroids and other immunosuppressive agents) may influence immune reconstitution and the probability of EBV reactivation after HSCT. Additionally, the immune reconstitution of EBV-CTL, which might better reflect the immune status for EBV, in the two groups also needs further study. LTV primary prophylaxis may exert a dual effect in ATG-based haplo-HSCT recipients—reducing CMV infection while increasing the risk of EBV infection and disease relapse. This phenomenon may be attributed to the impact of LTV on post-transplant lymphocyte reconstitution and function. These findings suggest the need for further investigation into virus-specific immune reconstitution following LTV administration and highlight the potential necessity for personalized prophylactic and monitoring strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Nanfang Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YH: Data curation, Methodology, Software, Writing – original draft, Formal Analysis, Validation. SZ: Data curation, Formal Analysis, Methodology, Validation, Writing – original draft. ZF: Data curation, Writing – original draft. FH: Data curation, Writing – original draft. NX: Data curation, Writing – original draft. HJ: Data curation, Writing – original draft. MD: Data curation, Writing – original draft. LX: Writing – original draft. HL: Writing – original draft, Data curation. ZW: Data curation, Writing – original draft. JS: Data curation, Writing – original draft. QL: Data curation, Writing – review & editing. RL: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82070190), President Fund of Nanfang Hospital, Southern Medical University (2024A018), Clinical Research Project of Nanfang Hospital, Southern Medical University (2024CR012).
Acknowledgments
We thank all the faculty members that participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1639463/full#supplementary-material
Abbreviations
LTV, Letermovir; haplo-HSCT, Haploidentical hematopoietic stem cell transplantation; ATG, Antithymocyte globulin; PSM, Propensity score matching; HLA, Human leukocyte antigen; GVHD, Graft-versus-host disease; aGVHD, Acute GVHD; EBV, Epstein-Barr virus; CMV, Cytomegalovirus; PTLD, Posttransplant lymphoproliferative disorder; AML, Acute Myeloid Leukemia; ALL, Acute Lymphoblastic Leukemia; MDS, Myelodysplastic Syndrome; CML, Chronic Myeloid Leukemia; PB, Peripheral Blood; BM, Bone Marrow; UCB, Umbilical Cord Blood; BuCy, Busulfan and Cyclophosphamide; TBI, Total Body Irradiation; BuFlu, Busulfan and Fludarabine; CR, Complete Remission; MNC, Mononuclear cells; OS, Overall survival; DFS, Disease-free survival; GRFS, GVHD free, relapse free survival; T-cells, T lymphocytes; NK-cells, Natural Killer cells; CI, Confidence interval; HR, Hazard ratio.
References
Akahoshi, Y., Kimura, S. I., Tada, Y., Matsukawa, T., Tamaki, M., Doki, N., et al. (2022). Cytomegalovirus gastroenteritis in patients with acute graft-versus-host disease. Blood Adv 6, 574–584. doi: 10.1182/bloodadvances.2021005885, PMID: 34788389
Austin, P. C. and Fine, J. P. (2017). Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 36, 4391–4400. doi: 10.1002/sim.7501, PMID: 28913837
Cesaro, S., Ljungman, P., Tridello, G., Mikulska, M., Wendel, L., Styczynski, J., et al. (2023). New trends in the management of cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a survey of the Infectious Diseases Working Pary of EBMT. Bone Marrow Transplant 58, 203–208. doi: 10.1038/s41409-022-01863-8, PMID: 36396949
Elmaagacli, A. H., Steckel, N. K., Koldehoff, M., Hegerfeldt, Y., Trenschel, R., Ditschkowski, M., et al. (2011). Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 118, 1402–1412. doi: 10.1182/blood-2010-08-304121, PMID: 21540462
Febres-Aldana, A., Khawaja, F., Morado-Aramburo, O., Shigle, T. L., Rondon, G., Sassine, J., et al. (2024). Mortality in recipients of allogeneic haematopoietic cell transplantation in the era of cytomegalovirus primary prophylaxis: a single-centre retrospective experience. Clin. Microbiol. Infect 30, 803–809. doi: 10.1016/j.cmi.2024.03.001, PMID: 38460821
Filipovich, A. H., Weisdorf, D., Pavletic, S., Socie, G., Wingard, J. R., Lee, S. J., et al. (2005). National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Marrow Transplant. 11, 945–956. doi: 10.1016/j.bbmt.2005.09.004, PMID: 16338616
Freyer, C. W., Carulli, A., Gier, S., Ganetsky, A., Timlin, C., Schuster, M., et al. (2022). Letermovir vs. high-dose valacyclovir for cytomegalovirus prophylaxis following haploidentical or mismatched unrelated donor allogeneic hematopoietic cell transplantation receiving post-transplant cyclophosphamide. Leukemia Lymphoma 63, 1925–1933. doi: 10.1080/10428194.2022.2042686, PMID: 35188052
Giardino, S., Eikema, D. J., Piepenbroek, B., Algeri, M., Ayas, M., Faraci, M., et al. (2024). HLA-haploidentical stem cell transplantation in children with inherited bone marrow failure syndromes: A retrospective analysis on behalf of EBMT severe aplastic Anemia and pediatric diseases working parties. Am. J. Hematol 99, 1066–1076. doi: 10.1002/ajh.27293, PMID: 38497679
Giménez, E., Guerreiro, M., Torres, I., Aguilar, C., Albert, E., Hernández-Boluda, J. C., et al. (2023). Features of cytomegalovirus DNAemia and virus-specific T-cell responses in allogeneic hematopoietic stem-cell transplant recipients during prophylaxis with letermovir. Transplant. Infect. Dis. 25, e14021. doi: 10.1111/tid.14021, PMID: 36748748
Guo, H., Chang, Y. J., Hong, Y., Xu, L. P., Wang, Y., Zhang, X. H., et al. (2021). Dynamic immune profiling identifies the stronger graft-versus-leukemia (GVL) effects with haploidentical allografts compared to HLA-matched stem cell transplantation. Cell. Mol. Immunol. 18, 1172–1185. doi: 10.1038/s41423-020-00597-1, PMID: 33408344
Hopff, S. M., Wingen-Heimann, S. M., Classen, A. Y., Blau, I. W., Bug, G., Hebermehl, C., et al. (2024). Real-world experience with letermovir for cytomegalovirus-prophylaxis after allogeneic hematopoietic cell transplantation: A multi-centre observational study. J. Infect 89, 106220. doi: 10.1016/j.jinf.2024.106220, PMID: 38960103
Hu, Z., Feng, Z., Liu, S., He, H., Dong, Y., Fan, Z., et al. (2024). Intensified conditioning containing decitabine versus standard myeloablative conditioning for adult patients with KMT2A-rearranged leukemia: a multicenter retrospective study. BMC Med. 22, 605. doi: 10.1186/s12916-024-03830-0, PMID: 39736728
Huang, Z., Yan, H., Teng, Y., Shi, W., and Xia, L. (2022). Lower dose of ATG combined with basiliximab for haploidentical hematopoietic stem cell transplantation is associated with effective control of GVHD and less CMV viremia. Front. Immunol. 13. doi: 10.3389/fimmu.2022.1017850, PMID: 36458000
Ito, S., Pophali, P., Co, W., Koklanaris, E. K., Superata, J., Fahle, G. A., et al. (2013). CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant 48, 1313–1316. doi: 10.1038/bmt.2013.49, PMID: 23562969
Jang, J. E., Hwang, D. Y., Chung, H., Kim, S. J., Eom, J. I., Jeung, H. K., et al. (2019). Early cytomegalovirus reactivation and expansion of CD56(bright)CD16(dim/-)DNAM1(+) natural killer cells are associated with antileukemia effect after haploidentical stem cell transplantation in acute leukemia. Biol. Blood Marrow Transplant. 25, 2070–2078. doi: 10.1016/j.bbmt.2019.06.008, PMID: 31212079
Kampouri, E., Zamora, D., Kiem, E. S., Liu, W., Ibrahimi, S., Blazevic, R. L., et al. (2023). Human herpesvirus-6 reactivation and disease after allogeneic haematopoietic cell transplantation in the era of letermovir for cytomegalovirus prophylaxis. Clin. Microbiol. Infect 29, 1450.e1–.e7. doi: 10.1016/j.cmi.2023.07.026, PMID: 37532126
Khawaja, F., Spallone, A., Kotton, C. N., and Chemaly, R. F. (2023). Cytomegalovirus infection in transplant recipients: newly approved additions to our armamentarium. Clin. Microbiol. Infect 29, 44–50. doi: 10.1016/j.cmi.2022.07.001, PMID: 35843567
Kong, X., Xu, Z., Wu, Y., Tang, X., Xue, S., Miao, M., et al. (2024). Increased Epstein–Barr virus reactivation following prophylaxis for cytomegalovirus infection after haploidentical haematopoietic stem cell transplantation. J. Hematol. Oncol. 17, 94. doi: 10.1186/s13045-024-01612-y, PMID: 39396017
Li, X., Yang, J., Cai, Y., Huang, C., Xu, X., Qiu, H., et al. (2023). Low-dose anti-thymocyte globulin plus low-dose post-transplant cyclophosphamide-based regimen for prevention of graft-versus-host disease after haploidentical peripheral blood stem cell transplants: a large sample, long-term follow-up retrospective study. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1252879, PMID: 37954615
Lin, A., Flynn, J., DeRespiris, L., Figgins, B., Griffin, M., Lau, C., et al. (2021). Letermovir for prevention of cytomegalovirus reactivation in haploidentical and mismatched adult donor allogeneic hematopoietic cell transplantation with post-transplantation cyclophosphamide for graft-versus-host disease prophylaxis. Transplant. Cell. Ther. 27, 85.e1–85.e6. doi: 10.1016/j.bbmt.2020.10.009, PMID: 33053449
Lin, R., Wang, Y., Huang, F., Fan, Z., Zhang, S., Yang, T., et al. (2019). Two dose levels of rabbit antithymocyte globulin as graft-versus-host disease prophylaxis in haploidentical stem cell transplantation: a multicenter randomized study. BMC Med. 17, 156. doi: 10.1186/s12916-019-1393-7, PMID: 31401973
Litjens, N. H. R., van der Wagen, L., Kuball, J., and Kwekkeboom, J. (2018). Potential beneficial effects of cytomegalovirus infection after transplantation. Front. Immunol. 9. doi: 10.3389/fimmu.2018.00389, PMID: 29545802
Liu, L. W., Yn, A., Gao, F., Olson, M., Crain, M., Abboud, R., et al. (2022). Letermovir discontinuation at day 100 after allogeneic stem cell transplant is associated with increased CMV-related mortality. Transplant. Cell. Ther. 28, 510.e1–510.e9. doi: 10.1016/j.jtct.2022.05.020, PMID: 35598841
Ljungman, P., Griffiths, P., and Paya, C. (2002). Definitions of cytomegalovirus infection and disease in transplant recipients. Clin. Infect. Dis. 34, 1094–1097. doi: 10.1086/339329, PMID: 11914998
Lönnqvist, B., Ringdèn, O., Ljungman, P., Wahren, B., and Gahrton, G. (1986). Reduced risk of recurrent leukaemia in bone marrow transplant recipients after cytomegalovirus infection. Br. J. Haematol 63, 671–679. doi: 10.1111/j.1365-2141.1986.tb07551.x, PMID: 3015193
Ma, R., He, Y., Wang, H. F., Bai, L., Han, W., Cheng, Y. F., et al. (2023). Clinical analysis of the usefulness of letermovir for prevention of cytomegalovirus infection after haploidentical hematopoietic stem cell transplantation. Zhonghua nei ke Za zhi 62, 826–832. doi: 10.3760/cma.j.cn112138-20221204-00904, PMID: 37394853
Manjappa, S., Bhamidipati, P. K., Stokerl-Goldstein, K. E., DiPersio, J. F., Uy, G. L., Westervelt, P., et al. (2014). Protective effect of cytomegalovirus reactivation on relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia patients is influenced by conditioning regimen. Biol. Blood Marrow Transplant. 20, 46–52. doi: 10.1016/j.bbmt.2013.10.003, PMID: 24120526
Marty, F. M., Ljungman, P., Chemaly, R. F., Maertens, J., Dadwal, S. S., Duarte, R. F., et al. (2017). Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. New Engl. J. Med. 377, 2433–2444. doi: 10.1056/NEJMoa1706640, PMID: 29211658
Mohty, R., Al Kadhimi, Z., and Kharfan-Dabaja, M. (2024). Post-transplant cyclophosphamide or cell selection in haploidentical allogeneic hematopoietic cell transplantation? Hematol. (Amsterdam Netherlands) 29, 2326384. doi: 10.1080/16078454.2024.2326384, PMID: 38597828
Muhsen, I. N., Shaver, K. E., Wang, T., Wu, M., Lulla, P., Ramos, C. A., et al. (2024). Efficacy of letermovir for cytomegalovirus prophylaxis following alemtuzumab T-cell depleted allogeneic hematopoietic stem cell transplant. Transplant. Cell. Ther. 30, 1193.e1–.e8. doi: 10.1016/j.jtct.2024.09.009, PMID: 39277112
Nuccetelli, M., Pieri, M., Grelli, S., Ciotti, M., Miano, R., Andreoni, M., et al. (2020). SARS-CoV-2 infection serology: a useful tool to overcome lockdown? Cell Death Discov. 6, 38. doi: 10.1038/s41420-020-0275-2, PMID: 32501411
Oikonomopoulou, C., Paisiou, A., Kaisari, A., Ioannidou, E. D., Komitopoulou, A., Letsiou, M., et al. (2025). Clinically significant EBV infection in allogeneic stem cell transplanted children receiving letermovir as primary CMV prophylaxis. Transplant. Infect. Dis. 27, e70032. doi: 10.1111/tid.70032, PMID: 40254977
Orofino, G., Xue, E., Doglio, M., Noviello, M., Tassi, E., Cristante, M., et al. (2023). Dynamics of polyclonal immuno-reconstitution after allogeneic transplant with post-transplant cyclophosphamide and letermovir. Bone Marrow Transplant 58, 1104–1111. doi: 10.1038/s41409-023-02046-9, PMID: 37468541
Pei, X. Y., Huang, Q., Luo, L. J., Sun, H. L., Liu, J., Sun, Y. Q., et al. (2024). Letermovir prophylaxis for cytomegalovirus is associated with risk of post-transplant lymphoproliferative disorders after haploidentical stem cell transplantation. Haematologica. 110, 1005–1009. doi: 10.3324/haematol.2024.286265, PMID: 39605206
Perchetti, G. A., Biernacki, M. A., Xie, H., Castor, J., Joncas-Schronce, L., Ueda Oshima, M., et al. (2023). Cytomegalovirus breakthrough and resistance during letermovir prophylaxis. Bone Marrow Transplant 58, 430–436. doi: 10.1038/s41409-023-01920-w, PMID: 36693927
Rowe, J., Grim, S. A., Peace, D., Lai, C., Sweiss, K., Layden, J. E., et al. (2013). The significance of cytomegalovirus viremia at day 100 or more following allogeneic hematopoietic stem cell transplantation. Clin. Transplant 27, 510–516. doi: 10.1111/ctr.12128, PMID: 23621704
Ru, Y., Zhang, X., Song, T., Ding, Y., Zhu, Z., Fan, Y., et al. (2020). Epstein-Barr virus reactivation after allogeneic hematopoietic stem cell transplantation: multifactorial impact on transplant outcomes. Bone Marrow Transplant 55, 1754–1762. doi: 10.1038/s41409-020-0831-7, PMID: 32066862
Ru, Y., Zhu, J., Song, T., Ding, Y., Zhu, Z., Fan, Y., et al. (2022). Features of epstein-barr virus and cytomegalovirus reactivation in acute leukemia patients after haplo-HCT with myeloablative ATG-containing conditioning regimen. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.865170, PMID: 35651756
Schäfer, A., Calderin Sollet, Z., Hervé, M. P., Buhler, S., Ferrari-Lacraz, S., Norman, P. J., et al. (2024). NK- and T-cell repertoire is established early after allogeneic HSCT and is imprinted by CMV reactivation. Blood Adv 8, 5612–5624. doi: 10.1182/bloodadvances.2024013117, PMID: 39047210
Scheper, W., van Dorp, S., Kersting, S., Pietersma, F., Lindemans, C., Hol, S., et al. (2013). γδT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia 27, 1328–1338. doi: 10.1038/leu.2012.374, PMID: 23277330
Schoemans, H. M., Lee, S. J., Ferrara, J. L., Wolff, D., Levine, J. E., Schultz, K. R., et al. (2018). EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant 53, 1401–1415. doi: 10.1038/s41409-018-0204-7, PMID: 29872128
Sperotto, A., Candoni, A., Gottardi, M., Facchin, G., Stella, R., De Marchi, R., et al. (2021). Cytomegalovirus Prophylaxis versus Pre-emptive Strategy: Different CD4(+) and CD8(+) T Cell Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Transplant. Cell. Ther. 27, 518.e1–518.e4. doi: 10.1016/j.jtct.2021.03.003, PMID: 33812803
Takenaka, K., Nishida, T., Asano-Mori, Y., Oshima, K., Ohashi, K., Mori, T., et al. (2015). Cytomegalovirus Reactivation after Allogeneic Hematopoietic Stem Cell Transplantation is Associated with a Reduced Risk of Relapse in Patients with Acute Myeloid Leukemia Who Survived to Day 100 after Transplantation: The Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol. Blood Marrow Transplant. 21, 2008–2016. doi: 10.1016/j.bbmt.2015.07.019, PMID: 26211985
Terao, T., Matsuoka, K. I., Fuji, S., Kawamura, S., Toya, T., Doki, N., et al. (2024). Association between human herpesvirus-6 encephalitis and antiviral prophylaxis after allogeneic hematopoietic stem cell transplantation in the letermovir era. Bone Marrow Transplant 59, 1224–1231. doi: 10.1038/s41409-024-02313-3, PMID: 38796633
Terao, T., Matsuoka, K. I., Narita, K., Tsushima, T., Yuyama, S., Kuzume, A., et al. (2021). Letermovir administration to prevent cytomegalovirus reactivation is the potential risk of chronic graft-versus-host disease in patients who received haploidentical stem-cell transplantation with post-transplant cyclophosphamide. Front. Oncol. 11. doi: 10.3389/fonc.2021.666774, PMID: 33996594
Toya, T., Mizuno, K., Sakurai, M., Kato, J., Mori, T., Doki, N., et al. (2024). Differential clinical impact of letermovir prophylaxis according to graft sources: a KSGCT multicenter retrospective analysis. Blood Adv 8, 1084–1093. doi: 10.1182/bloodadvances.2023010735, PMID: 38330190
Vyas, A., Raval, A. D., Kamat, S., LaPlante, K., Tang, Y., and Chemaly, R. F. (2023). Real-world outcomes associated with letermovir use for cytomegalovirus primary prophylaxis in allogeneic hematopoietic cell transplant recipients: A systematic review and meta-analysis of observational studies. Open Forum Infect. Dis. 10, ofac687. doi: 10.1093/ofid/ofac687, PMID: 36726548
Wang, H., Wang, N., Wang, L., Du, J., Li, F., Shao, Y., et al. (2023). Targeted dosing of anti-thymocyte globulin in adult unmanipulated haploidentical peripheral blood stem cell transplantation: A single-arm, phase 2 trial. Am. J. Hematol 98, 1732–1741. doi: 10.1002/ajh.27068, PMID: 37706580
Wang, Y., Wu, D. P., Liu, Q. F., Xu, L. P., Liu, K. Y., Zhang, X. H., et al. (2019). Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J. Hematol. Oncol. 12, 88. doi: 10.1186/s13045-019-0781-y, PMID: 31481121
Włodarczyk, M., Wieczorkiewicz-Kabut, A., Białas, K., Koclęga, A., Noster, I., Zielińska, P., et al. (2024). Real-life data on the efficacy and safety of letermovir for primary prophylaxis of cytomegalovirus in allogeneic hematopoietic stem cell recipients: A single-center analysis. Turkish J. Haematol 41, 9–15. doi: 10.4274/tjh.galenos.2024.2024.0026, PMID: 38345092
Wu, J., Lu, A. D., Zhang, L. P., Zuo, Y. X., and Jia, Y. P. (2022). The Chinese consensus on the management of cytomegalovirus infection in allogeneic hematopoietic stem cell transplantation patients, (2022)]. Zhonghua Xue Ye Xue Za Zhi = Zhonghua Xueyexue Zazhi 43, 617–623. doi: 10.3760/cma.j.issn.0253-2727.2022.08.001, PMID: 36709144
Xuan, L., Huang, F., Fan, Z., Zhou, H., Zhang, X., Yu, G., et al. (2012). Effects of intensified conditioning on Epstein-Barr virus and cytomegalovirus infections in allogeneic hematopoietic stem cell transplantation for hematological Malignancies. J. Hematol. Oncol. 5, 46. doi: 10.1186/1756-8722-5-46, PMID: 22856463
Yan, B., Sun, G., Wu, Y., Wu, W., Song, K., Cheng, Y., et al. (2024). Letermovir prophylaxis reduced cytomegalovirus reactivation and resistance post umbilical cord blood transplantation. Br. J. Haematol 204, 2378–2389. doi: 10.1111/bjh.19451, PMID: 38581290
Yang, J., Jiang, J., Cai, Y., Li, S., Wan, L., Zhu, J., et al. (2019). Low-dose anti-thymocyte globulin plus low-dose posttransplant cyclophosphamide as graft-versus-host disease prophylaxis in haploidentical peripheral blood stem cell transplantation combined with unrelated cord blood for patients with hematologic Malignancies: a prospective, phase II study. Bone Marrow Transplant 54, 1049–1057. doi: 10.1038/s41409-018-0382-3, PMID: 30446741
Yeh, A. C., Varelias, A., Reddy, A., Barone, S. M., Olver, S. D., Chilson, K., et al. (2021). CMV exposure drives long-term CD57+ CD4 memory T-cell inflation following allogeneic stem cell transplant. Blood 138, 2874–2885. doi: 10.1182/blood.2020009492, PMID: 34115118
Zamora, D., Duke, E. R., Xie, H., Edmison, B. C., Akoto, B., Kiener, R., et al. (2021). Cytomegalovirus-specific T-cell reconstitution following letermovir prophylaxis after hematopoietic cell transplantation. Blood 138, 34–43. doi: 10.1182/blood.2020009396, PMID: 33657225
Zeng, X., Xuan, L., Fan, Z., Zhang, Y., Zhao, K., Zhou, Y., et al. (2021). Allogeneic stem cell transplantation may overcome the adverse impact of myelofibrosis on the prognosis of myelodysplastic syndrome. Exp. Hematol. Oncol. 10, 44. doi: 10.1186/s40164-021-00238-x, PMID: 34391477
Zhang, Y., Chen, X., Zhou, M., Zhang, Y., Chen, C., Zhou, R., et al. (2024). Letermovir effectively prevents cytomegalovirus infection in patients with aplastic anemia after hematopoietic stem cell transplantation: A real-world retrospective cohort study. Infect. Dis. Ther. 13, 345–359. doi: 10.1007/s40121-024-00917-2, PMID: 38265628
Zhen, S., Liu, L., Zhang, X., Wang, J., Sun, J., Liang, C., et al. (2025). Increased Epstein-Barr virus reactivation but similar incidence of post-transplant lymphoproliferative disorders due to pre-emptive rituximab therapy following allogeneic hematopoietic stem cell transplantation in the letermovir era for cytomegalovirus prophylaxis. Bone Marrow Transplant. 60, 721–724. doi: 10.1038/s41409-025-02542-0, PMID: 40033131
Keywords: haploidentical donor hematopoietic cell transplantation, letermovir prophylaxis, Epstein-Barr virus infection, relapse, immune reconstitution and function
Citation: Huang Y, Zhang S, Fan Z, Huang F, Xu N, Jin H, Dai M, Xuan L, Liu H, Wang Z, Sun J, Liu Q and Lin R (2025) Increased EBV infection and relapse following haploidentical hematopoietic cell transplantation in the era of letermovir for cytomegalovirus prophylaxis: a propensity score matching analysis. Front. Cell. Infect. Microbiol. 15:1639463. doi: 10.3389/fcimb.2025.1639463
Received: 02 June 2025; Accepted: 22 September 2025;
Published: 06 October 2025.
Edited by:
Eleni Gavriilaki, Aristotle University of Thessaloniki, GreeceReviewed by:
Jose Camargo, University of Miami Health System, United StatesAsmaa Mohsen, Mansoura University, Egypt
Copyright © 2025 Huang, Zhang, Fan, Huang, Xu, Jin, Dai, Xuan, Liu, Wang, Sun, Liu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qifa Liu, bGl1cWlmYTYyOEAxNjMuY29t; Ren Lin, bGFuc2luZ2xpbnJlbkAxNjMuY29t
†These authors have contributed equally to this work
‡ORCID: Yifei Huang, orcid.org/0009-0003-7369-7144
 Yifei Huang
Yifei Huang Shanyu Zhang†
Shanyu Zhang† Fen Huang
Fen Huang Na Xu
Na Xu Hua Jin
Hua Jin Min Dai
Min Dai Li Xuan
Li Xuan Qifa Liu
Qifa Liu Ren Lin
Ren Lin