- 1Department of Nephrology, Hangzhou Traditional Chinese Medicine (TCM) Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2Department of Nephrology, Sichuan Second Hospital of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 3Xingqiao Street Community Health Service Center, Hangzhou, Zhejiang, China
- 4Department of General Medicine, Hangzhou Xihu District Zhuantang Street Community Health Service Centre, Hangzhou, Zhejiang, China
- 5Tianshui Wulin Street Community Heal Care Centre, Hangzhou, Zhejiang, China
- 6Donghu Street Community Health Service Center, Hangzhou, Zhejiang, China
- 7Key Laboratory of Kidney Disease Prevention and Control Technology, Hangzhou, Zhejiang, China
Background: Gastrointestinal (GI) disturbances are prevalent in maintenance hemodialysis (MHD) patients and are closely associated with gut microbiota dysregulation. Tongue coating thickness, a key diagnostic feature in traditional Chinese medicine, may reflect systemic and microbial health. This study aimed to explore the relationship between tongue coating phenotype and gut microbiota composition in MHD patients.
Methods: A matched case-control study was conducted involving 30 MHD patients divided into thick (HTZ, n = 15) and thin (BTZ, n = 15) tongue coating groups, along with 15 healthy controls (DZZ). Fecal samples were analyzed via 16S rRNA sequencing to assess microbial diversity, taxonomic profiles, and predicted functional pathways.
Results: Alpha-diversity indices were significantly lower in BTZ than in DZZ (q < 0.05), while no difference was found between HTZ and BTZ. Beta-diversity showed closer clustering between HTZ and BTZ than with DZZ. Compared to DZZ, both HTZ and BTZ exhibited reduced levels of genera typically associated with health or commensal functions (Romboutsia, Subdoligranulum) and increased abundances of taxa often linked to inflammation or disease (Escherichia-Shigella, Ruminococcus gnavus). Functional predictions indicated that HTZ was enriched in pathways related to disease processes and showed diminished cellular and metabolic functions.
Conclusion: Tongue coating thickness in MHD patients reflects underlying gut microbial composition. Thick tongue coatings indicate a state of dysbiosis with potential health implications, whereas thin coatings are associated with a microbiota profile that may be more favorable. These findings support the potential use of tongue coating thickness as a noninvasive biomarker for gut health assessment in clinical nephrology.
1 Introduction
Hemodialysis is a life-sustaining treatment for patients with end-stage renal disease (ESRD), yet it is often accompanied by a range of complications that significantly impact patients’ quality of life and prognosis. Gastrointestinal (GI) problems have emerged as a prevalent and complex issue. Studies have consistently shown that hemodialysis patients frequently suffer from various GI symptoms. For instance, research by Doğu Karahan and İdris Şahin indicated that dyspepsia, nausea, and epigastric pain were highly common, affecting 50%, 45%, and 44% of the patients in their study cohort respectively (Karahan and Şahin, 2022). Additionally, Sang Cheol Park et al. found that constipation was present in 25.9% of hemodialysis patients and was associated with an increased risk of cardiovascular events and all-cause mortality (Park et al., 2025). These GI manifestations not only disrupt patients’ daily lives but also pose challenges to the effectiveness of hemodialysis treatment. A recent systematic review involving over 5,000 dialysis patients confirmed that constipation, indigestion, abdominal pain, and reflux are among the most prevalent GI symptoms in this population, and highlighted their adverse effects on quality of life and clinical outcomes (Zuvela et al., 2018).
Hemodialysis patients exhibit significant gut microbiota dysregulation. Key findings include reduced α-diversity (e.g., Shannon and Chao1 indices) and altered microbial composition, with depletion of beneficial bacteria such as short-chain fatty acid (SCFA)-producing Faecalibacterium, Bifidobacterium, and Akkermansia muciniphila, and overgrowth of pathobionts like Enterobacteriaceae and Klebsiella (Wang et al., 2025). Metabolic dysfunction drives excessive production of uremic toxins (indoxyl sulfate, p-cresyl sulfate, trimethylamine N-oxide) from tryptophan and choline metabolism, while SCFA deficiency impairs intestinal barrier integrity and triggers systemic inflammation via TLR4/NF-κB signaling (Regis et al., 2025). A recent review emphasized how these microbiota alterations contribute to systemic inflammation, cardiovascular risk, and malnutrition in ESRD patients, and noted that microbiome-targeted therapies (e.g., probiotics, prebiotics) may mitigate dialysis-related complications (Velasquez et al., 2018). Clinically, this dysregulation correlates with increased cardiovascular risk (vascular calcification, atherosclerosis), malnutrition, sarcopenia, and gut motility disorders (e.g., constipation), with severity linked to dialysis duration and toxin levels (Shao et al., 2025).
Our team’s previous research discovered significant differences in the oral microbiota between maintenance hemodialysis (MHD) patients with thick and thin tongue coatings (Zhu et al., 2025). The overall microbial species richness in MHD patients with thick tongue coatings is higher compared to those with thin tongue coatings, with distinct differences in microbiota abundance at various taxonomic levels. For example, the abundance of genera such as Prevotella and Megasphaera is significantly higher in patients with thick tongue coatings, suggesting that tongue coating thickness (TCT) may be related to the composition and diversity of the oral microbiome in MHD patients.
Multiple studies have highlighted the relationships between the tongue coating microbiota and gut microbiota. In healthy individuals, there is a positive correlation in the relative abundance of Prevotella in both the tongue coating and intestines (Guo et al., 2022). In disease states, such as IBS-D and MAFLD, changes in tongue coating microbiota are accompanied by alterations in gut microbiota (Lu et al., 2022; Tang et al., 2023). Systemic diseases like COVID-19 and schizophrenia also lead to concurrent changes in oral and gut microbiomes (Cui et al., 2022; Ling et al., 2023), and age-related increases in oral bacteria translocation to the gut have been observed (Iwauchi et al., 2019). A recent comprehensive review on the oral–gut axis described mechanisms including microbial migration, metabolite signaling, and immune modulation, and linked these findings to diseases such as IBD, colorectal cancer, and cardiometabolic disorders (Park et al., 2021). Another narrative review emphasized that oral pathogens (e.g., Fusobacterium, Streptococcus) may translocate and colonize the gut, contributing to gastrointestinal and systemic pathologies (Sulaiman et al., 2024). These findings imply that the oral-gut axis plays a crucial role in microbial interactions, with potential implications for disease mechanisms.
Given the established differences in oral microbiota between thick and thin tongue coatings in hemodialysis patients, and the reported connections between oral and gut microbiota, a critical question remains: do hemodialysis patients with thick and thin tongue coatings exhibit differences in gut microbiota composition and function? This study aims to investigate the structural and functional disparities in gut microbiota between these two groups, exploring whether tongue coating thickness, as a marker of oral microecology, is associated with gut microecological variations. This question is clinically relevant because recent intervention trials targeting gut microbiota (e.g., high-fiber diets, synbiotics) in ESRD patients have demonstrated reductions in uremic toxins and inflammation, suggesting potential synergy if oral markers could guide interventions (Kuskunov et al., 2023). By addressing this gap, we seek to deepen our understanding of the oral-gut axis in hemodialysis patients and provide a scientific basis for microecology-targeted interventions to improve GI outcomes in this population.
2 Methods
2.1 Study design and sample selection
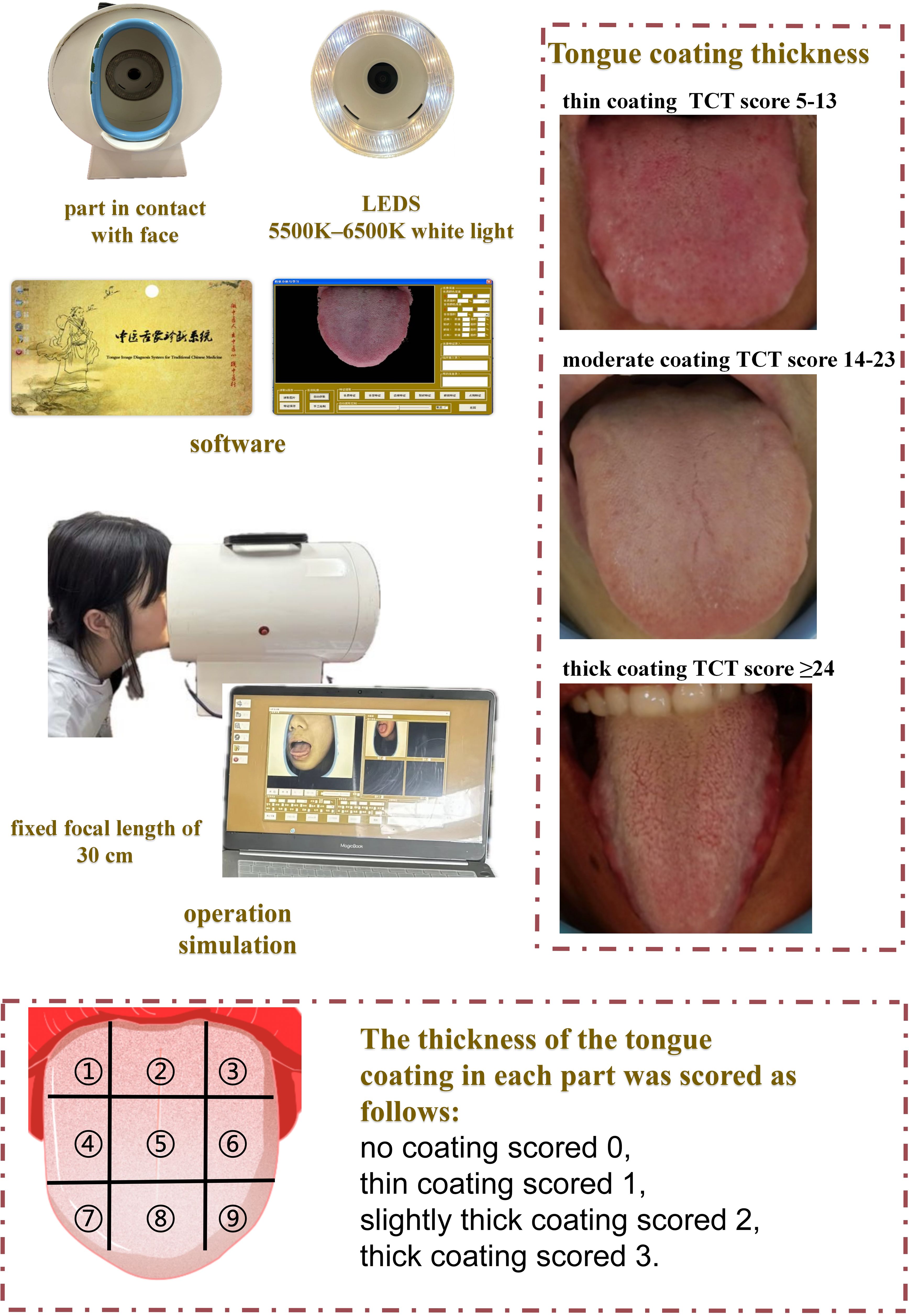
This study employed a matched case-control design, including 30 maintenance hemodialysis (MHD) patients (15 with thick tongue coating [HTZ], 15 with thin tongue coating [BTZ]) and 15 healthy controls (DZZ). Stool samples were collected concurrently with tongue coating assessments between December 2022 and June 2023 at Hangzhou TCM Hospital of Zhejiang Chinese Medical University, following the same inclusion/exclusion criteria as the original study (Zhang et al., 2024), following the inclusion criteria of ≥ 3 months of stable MHD treatment, age > 18 years, and no antibiotic use within the past month. Exclusion criteria included organic gastrointestinal disorders, recent probiotic use, or acute infections. Specifically, HTZ was defined as a tongue coating thickness (TCT) score ≥ 24 (thick coating), BTZ as 5-13 (thin coating), and DZZ as healthy volunteers with TCT scores within the moderate range (14-23). These healthy volunteers were specifically selected to have tongue coating thickness within the moderate range, in order to represent typical healthy conditions and to avoid including extremely thin or thick coatings. We did not further stratify the healthy control group into thick or thin coating subgroups because such extreme tongue coatings are rarely observed in healthy populations. This approach helps to avoid potential interference from atypical conditions in the interpretation of results.
2.2 Tongue coating image acquisition and assessment
Tongue images were obtained using the TCM Tongue Diagnostic Expert System with standardized lighting (5500–6500K white light) and a fixed focal length of 30 cm (Figure 1). All subjects were imaged in the morning before eating or oral hygiene to minimize dietary effects. Each participant sat upright and extended the tongue naturally (avoiding curling or strain) for the photograph. Two licensed TCM practitioners, blinded to each other’s ratings, independently assessed tongue coating thickness (TCT) on these images. Following Shimizu et al. (2007) (Shimizu et al., 2007) and the Japanese “Shimada” scheme, we divided the tongue dorsum into nine regions (three transverse zones: anterior, middle, posterior; each subdivided into left, center, and right). Each region was scored on an ordinal 0–3 scale (a modified version of Shimada’s criteria): 0 = no visible coating; 1 = thin coating (papillae clearly visible through the coating); 2 = moderate coating; 3 = thick coating (papillae not visible). The total TCT score for each subject was calculated as the sum of all nine regional scores (Zhu et al., 2025).
This scoring system has empirical support: Shimizu et al. demonstrated that their Tongue Coating Index (a similar 0–2 scale) correlates with tongue microbial burden. In other words, higher coating scores correspond to greater anaerobic bacterial counts on the tongue surface. To ensure reproducibility, we trained the practitioners in the above criteria before scoring and calculated inter-rater reliability by weighted Cohen’s kappa. In our study, the overall κ was 0.817 (regional κs ranged 0.631–0.876), indicating substantial-to-almost-perfect agreement. These values confirm that the nine-region TCT scoring is both scientifically grounded and consistent between observers.
2.3 Stool sample retrieval
Frozen stool samples stored at −80 °C since the original study (Zhang et al., 2024) will be utilized. Sample collection mirrored the parent study’s protocols: fasting participants provided stool samples in sterile tubes within 2 hours of collection, prior to dialysis or oral hygiene, to minimize contamination.
2.4 Microbiome analysis
2.4.1 DNA extraction
Genomic DNA was extracted using the CTAB method (Simpson, 2010). Briefly, fecal samples were lysed with CTAB buffer and glass beads, followed by phenol-chloroform extraction (Sambrook and Russell, 2006) and ethanol precipitation. DNA purity (A260/A280 = 1.8–2.0) and concentration (> 10 ng/μL) were verified by agarose gel electrophoresis and Nanodrop (Glasel, 1995).
2.4.2 16S rRNA sequencing
The V3-V4 hypervariable regions of the 16S rRNA gene were amplified using barcoded primers 338F and 806R. Each PCR reaction utilized 50 ng of template DNA under the following cycling conditions: initial denaturation at 95 °C for 3 min, 25 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s, and a final extension at 72 °C for 10 min. Amplified products were purified with AMPure XT beads and subsequently sequenced on an Illumina NovaSeq 6000 platform (2×250 bp paired-end configuration) by Hangzhou Lianchuan Biotechnology (Matsumoto and Sugano, 2013).
2.4.3 Bioinformatics pipeline
Raw sequencing reads were processed in QIIME 2 (Bolyen et al., 2019) using DADA2 (Callahan et al., 2016) to remove low-quality sequences (Q < 20), denoise, and generate ASVs. For taxonomic annotation, ASVs were compared against the SILVA database (version 138) at 97% identity, with chimeric sequences removed. Due to limited resolution of species-level annotation based on the V3-V4 region of the 16S rRNA gene, species names used in this study represent putative assignments based on the best available database match and should be interpreted with caution.
In diversity analysis, α-diversity was assessed using Chao1, Observed ASVs, Shannon, and Simpson indices to evaluate richness and evenness, with group comparisons conducted via Wilcoxon tests. For beta diversity, PCoA and NMDS were performed based on Bray-Curtis and Unweighted Unifrac distances to visualize compositional differences, with statistical significance tested using PERMANOVA (999 permutations) (Kelly et al., 2015).
Differential abundance of taxa between groups was identified using LEfSe (Segata et al., 2011) with a logarithmic LDA score threshold > 2.0 and Wilcoxon tests, applying FDR correction for multiple comparisons. Functional prediction of KEGG pathways from ASV data was carried out using PICRUSt2 (Douglas et al., 2020), with STAMP (Parks and Beiko, 2010) software comparing pathway abundances (t-test, FDR < 0.05).
2.5 Statistical analysis
Clinical demographic variables (age, sex) and clinical parameters (Kt/V, hs-CRP) were compared using t-tests (parametric) or Wilcoxon tests (non-parametric). For microbiome analyses conducted in R (v4.3.0) with phyloseq (McMurdie and Holmes, 2013) and ggplot2 (Wen et al., 2023), species differences were assessed using Fisher’s exact test (no biological replicates), Mann-Whitney U test (two-group comparisons with replicates), or Kruskal-Wallis test (multi-group comparisons with replicates). Differential abundance tests report both p and FDR-adjusted q values; statistical significance was defined as q < 0.05. Nominal (unadjusted) p-values are reported only where noted.
2.6 Ethical considerations
The study was approved by the Research Ethics Committee of Hangzhou Hospital of TCM (approval number: 2023ZL110), with all participants providing written informed consent. Data were anonymized, and sample reanalysis followed the original study’s ethical guidelines.
3 Results
3.1 Species annotation analysis
According to the ASV abundance tables obtained from sequencing fecal samples of three groups, there were 2,970 ASVs in the HTZ group, 2,487 ASVs in the BTZ group, and 2,964 ASVs in the DZZ group. Notably, HTZ fecal samples contained the highest number of ASVs, while BTZ had the lowest, highlighting individual differences in the intestinal microbiota. The Venn diagram showed that HTZ and DZZ shared 928 ASVs, with HTZ having 2,042 unique ASVs and DZZ having 2,036 unique ASVs. BTZ and DZZ shared 899 ASVs, with BTZ having 1,588 unique ASVs and DZZ having 2,065 unique ASVs. HTZ and BTZ shared 955 ASVs, with HTZ having 2,015 unique ASVs and BTZ having 1,532 unique ASVs. All three groups (HTZ, BTZ, and DZZ) shared 667 ASVs. Preliminary results indicated significant differences in intestinal microbiota composition among the thick tongue coating group (HTZ), thin tongue coating group (BTZ), and healthy control group (DZZ) of hemodialysis patients. Specifically, the HTZ group had a greater variety of intestinal microbiota, while the BTZ group and healthy control group had relatively fewer. Notably, the intestinal microbiota of the HTZ group shared more common species with the BTZ group (Figure 2).
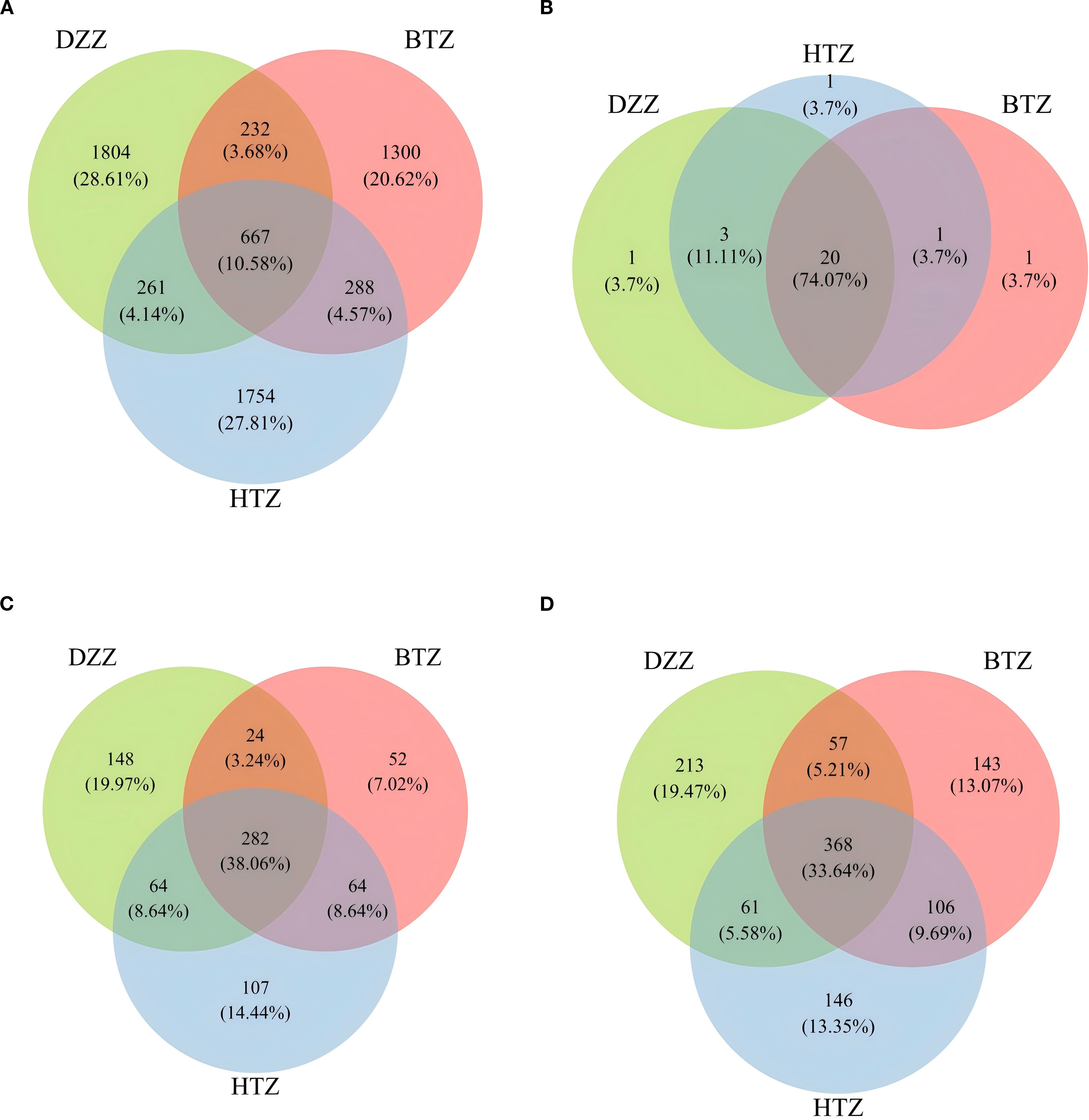
Figure 2. (A) Venn diagram of ASVs among DZZ, BTZ, and HTZ groups. A total of 6306 ASVs were detected. Among them, 667 (10.6%) were shared by all three groups, while DZZ, BTZ, and HTZ uniquely contributed 1804 (28.6%), 1300 (20.6%), and 1754 (27.8%) ASVs, respectively. Shared ASVs between two groups only accounted for a small proportion. (B) Venn diagram of bacterial phyla among DZZ, BTZ, and HTZ groups. A total of 23 phyla were detected. 20 (87.0%) were shared by all groups, and each group had one unique phylum (4.3%). No phylum was shared between only two groups, indicating high similarity at the phylum level. (C) Venn diagram of bacterial genera among DZZ, BTZ, and HTZ groups. A total of 589 genera were detected. 282 (47.9%) were shared by all groups, while DZZ, BTZ, and HTZ had 148 (25.1%), 52 (8.8%), and 107 (18.2%) unique genera, respectively. This reflects both shared and group-specific microbial features at the genus level. (D) Venn diagram of bacterial species among DZZ, BTZ, and HTZ groups. A total of 870 species were detected. 368 (42.3%) were shared by all groups, while DZZ, BTZ, and HTZ had 213 (24.5%), 143 (16.4%), and 146 (16.8%) unique species, respectively. This indicates high species-level diversity and group specificity.
3.2 Species diversity analysis
3.2.1 α-diversity analysis
According to the results shown in the violin plot, there was no statistically significant difference in the Chao1 index among the three groups (p > 0.05). The Observed species (q = 0.04), Shannon (q = 0.03), and Simpson indices (q = 0.03) showed that the DZZ values were higher than those of BTZ, while no significant difference was observed between HTZ and BTZ (p > 0.05). This indicates that the intestinal microbiota richness and evenness of the healthy control group were higher than those of the thin tongue coating group of hemodialysis patients. The lack of significant differences between HTZ and DZZ in α-diversity may reflect preserved overall microbial diversity in some HD patients with thick tongue coating. However, as shown in subsequent β-diversity and taxonomic composition analyses, the microbiota community structure and dominant taxa differed between HTZ and DZZ, suggesting compositional alterations despite similar richness and evenness (Figure 3).
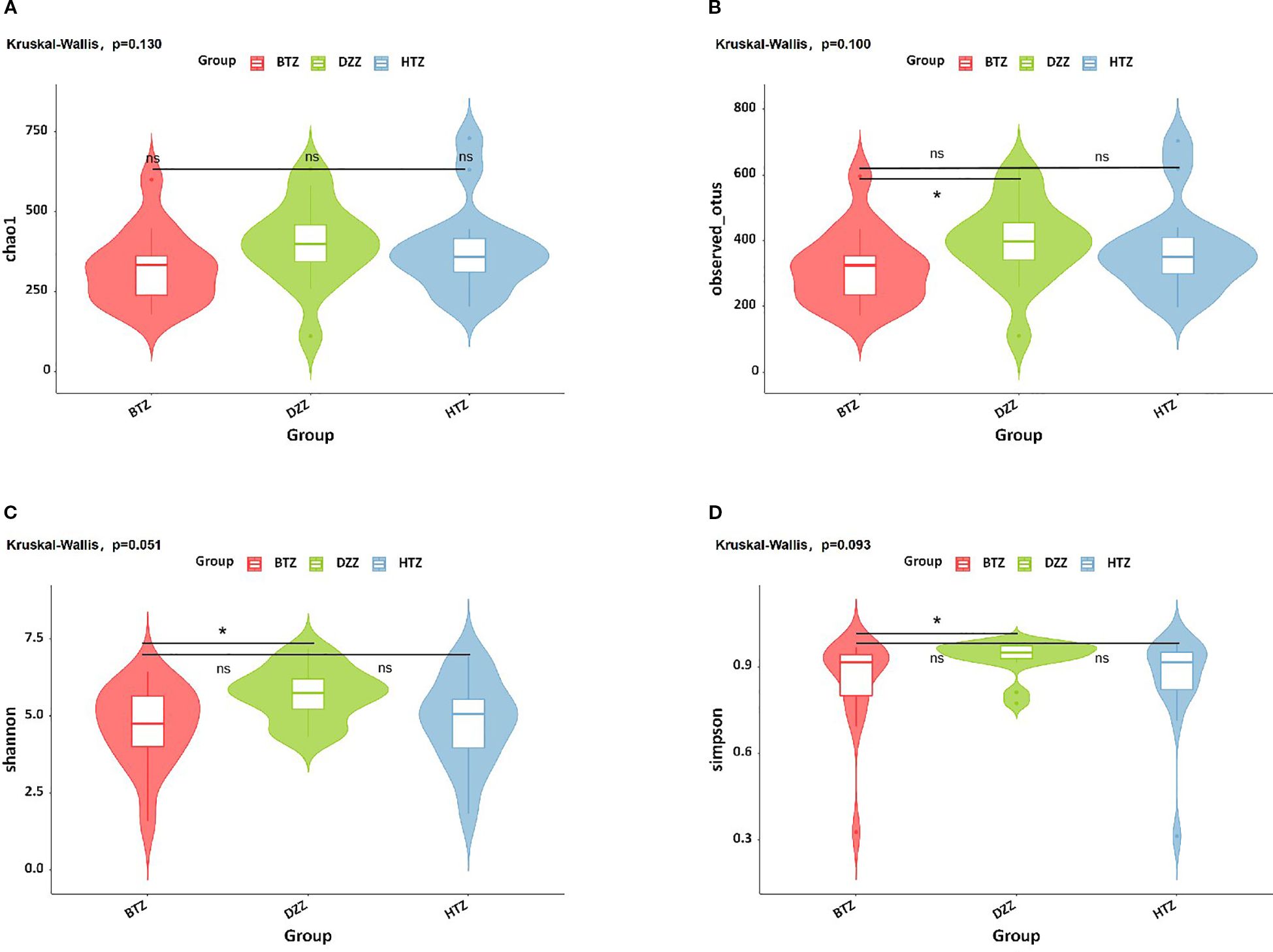
Figure 3. α-diversity. Violin plots show (A) Chao1, (B) Observed species, (C) Shannon, and (D) Simpson indices. No significant difference in Chao1 index was found among groups. DZZ had higher Observed species, Shannon, and Simpson indices than BTZ, with no difference between HTZ and BTZ. Healthy controls (DZZ) exhibited higher microbiota richness and evenness than the thin tongue coating group (BTZ) of hemodialysis patients. (ns = not significant; * indicates q < 0.05).
3.2.2 β-diversity analysis
PCoA based on unweighted UniFrac distances was used to visualize differences in microbial community composition among the three groups. The PCoA plot showed that the HTZ and BTZ groups clustered closely together, while both were clearly separated from the DZZ group, indicating distinct differences in intestinal microbiota composition between dialysis patients and healthy controls (PCoA1 = 14.56%, PCoA2 = 8.4%, q = 0.01). These results suggest that the overall microbial communities of HTZ and BTZ patients were more similar to each other and differed markedly from those of healthy individuals (Figure 4).
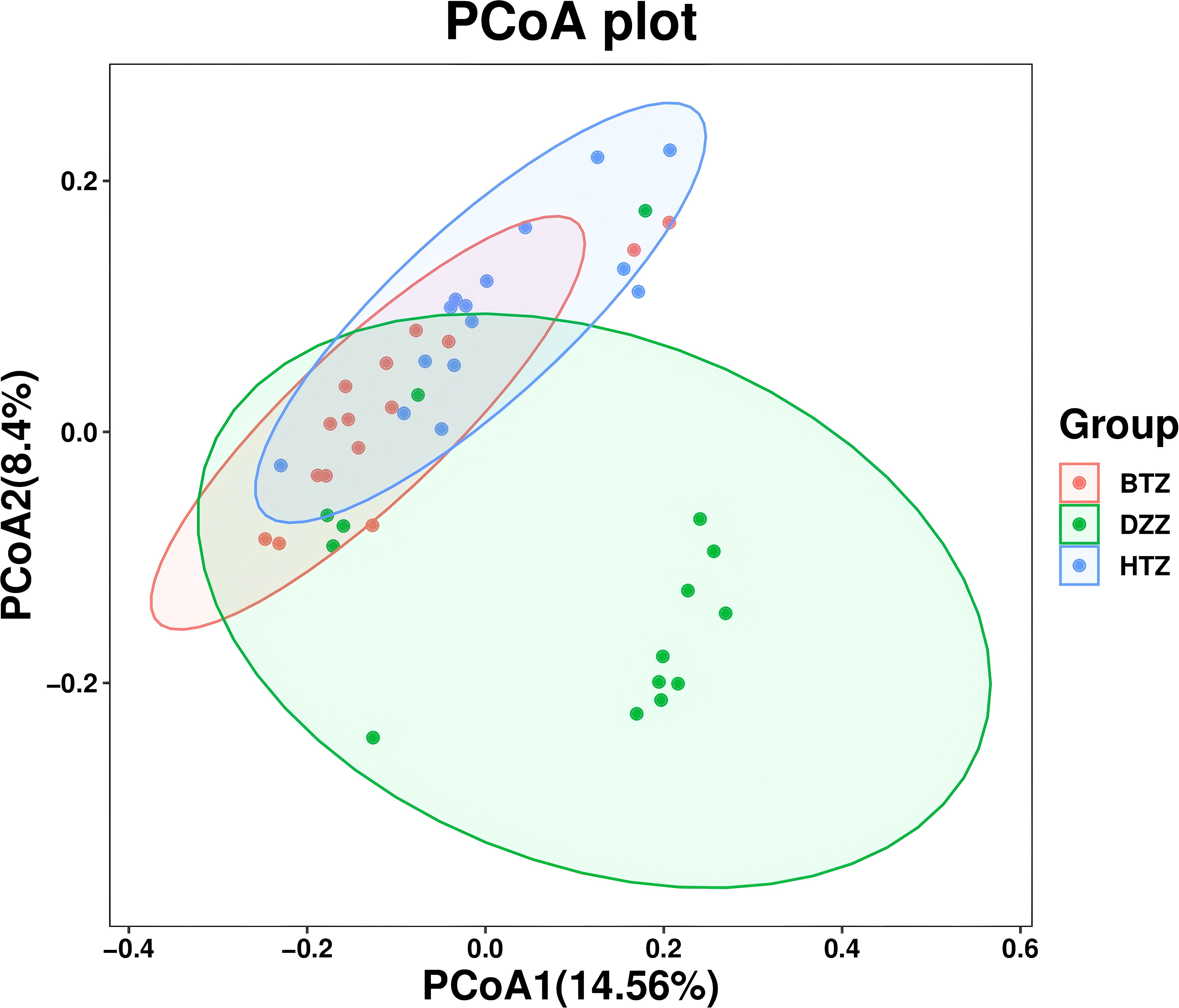
Figure 4. β-diversity. PCoA plot based on unweighted UniFrac distances. The PCoA plot illustrates the microbial community structure among HTZ, BTZ, and DZZ groups. HTZ and BTZ samples cluster closely, indicating similar microbiota composition, while both are clearly separated from the DZZ group (healthy controls), suggesting marked compositional differences (q = 0.01). (PCoA1 = 14.56%, PCoA2 = 8.4%).
3.3 Species difference analysis
3.3.1 Composition heatmap
Clustering heatmaps visually demonstrated the similarities and differences in microbiota composition among groups at various taxonomic levels. Figure 5 shows significant differences in the composition and distribution of intestinal microbiota at both the genus and phylum levels among the HTZ, BTZ, and DZZ groups.
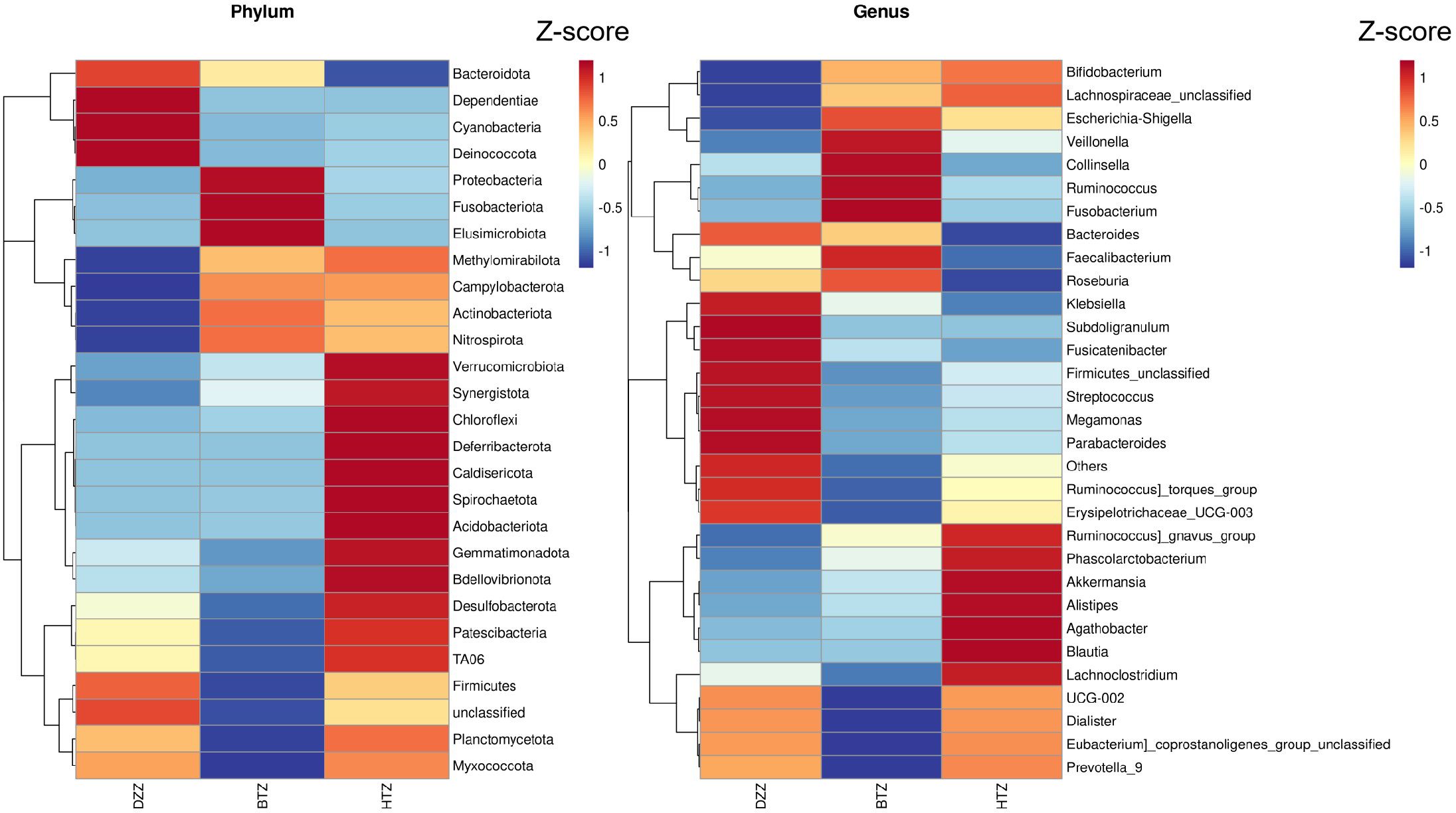
Figure 5. Phylum-level and genus-level composition heatmap. Heatmap of the top 30 most abundant bacterial phylum and genera across the HTZ, BTZ, and DZZ groups. Colors represent Z-score normalized relative abundances. Differences in phylum and genus distribution patterns indicate compositional divergence between groups.
3.3.2 Comparison of taxonomic abundance
Sample clustering was performed using Bray-Curtis distances, and phylum-level stacked plots were drawn to better illustrate the differences and similarities among HTZ, BTZ, and DZZ. Additionally, cluster analysis revealed that the phylum-level stacked plots showed the dominant phyla in the intestinal microbiota of the three groups were Firmicutes, Bacteroidota, Actinobacteriota, and Proteobacteria (q < 0.05). The microbiota compositions of the HTZ and BTZ groups were more similar (Supplementary Figure S1). Through intergroup difference analysis, nominal differences (unadjusted p < 0.05) were observed for the phyla Patescibacteria (q = 0.28), Deferribacterota (q = 0.28), Planctomycetota (q = 0.28), and Bdellovibrionota (q = 0.28), which had higher relative abundances in the HTZ group compared to the BTZ group. However, these differences did not remain statistically significant after FDR correction (q > 0.05) and should be interpreted as potential trends warranting further investigation. Although the relative abundances of these phyla were low (< 2%), the biological relevance of these nominal findings remains uncertain. The phyla Cyanobacteria (q = 0.02), and Deinococcota (q = 0.02), exhibited statistically significant higher abundances in the DZZ group compared to the BTZ group. Furthermore, a nominal reduction (p = 0.019) in the abundance of Bacteroidota (q = 0.12) was observed in the HTZ group compared to the healthy control group, but this difference was not significant after multiple testing correction (Figures 6).
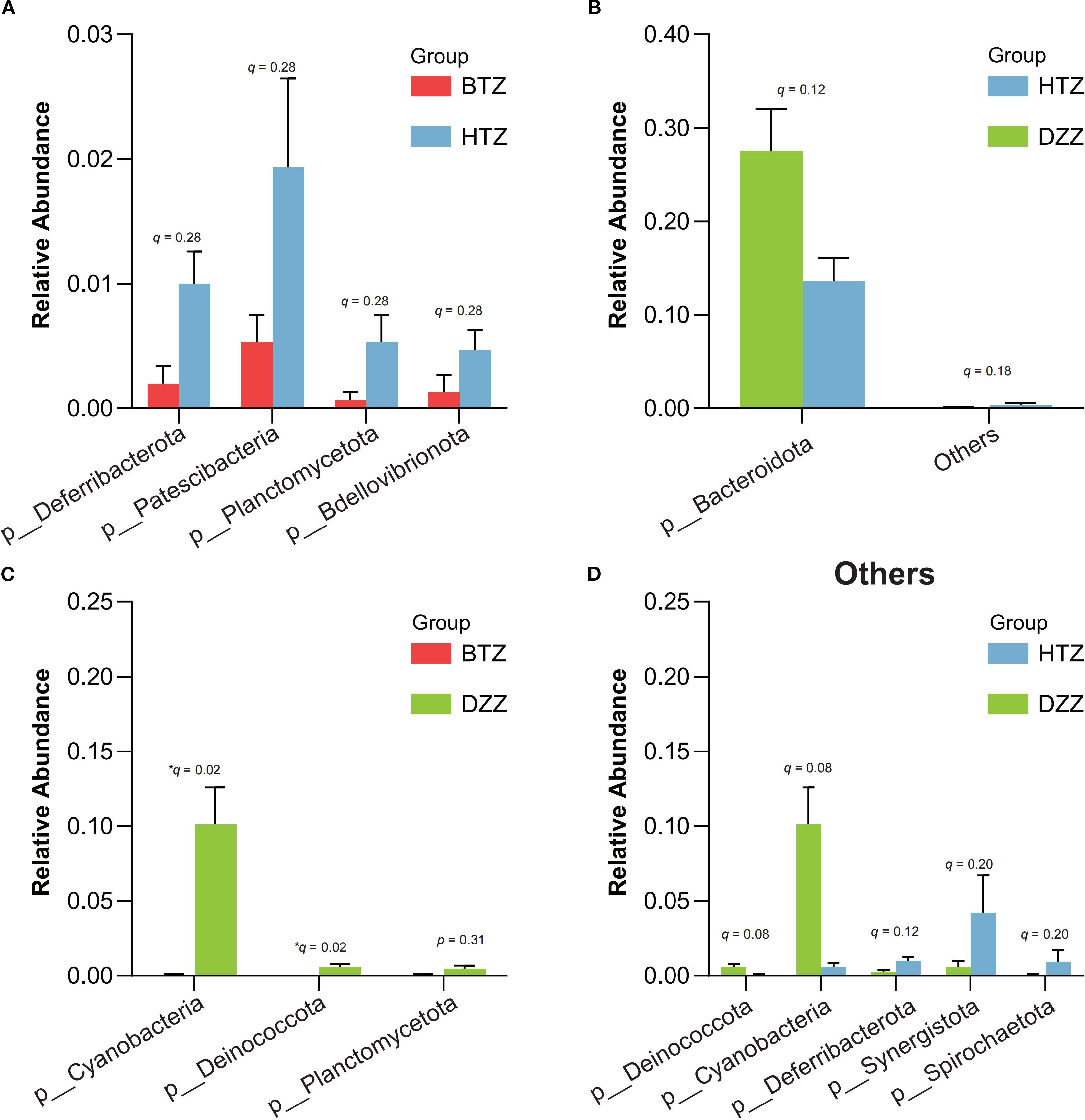
Figure 6. Comparison of microbial abundance at the phylum level. Error bars represent the standard error of the mean (SEM). Key findings are based on nominal p-values and should be interpreted with caution pending further validation: (A) HTZ had higher abundances of Patescibacteria, Deferribacterota, Planctomycetota, and Bdellovibrionota compared vs. BTZ. (B, D) Bacteroidota was lower in HTZ compared vs. DZZ. (C) DZZ had higher abundances of Cyanobacteria and Deinococcota compared vs. BTZ.
At the genus level, the Top 10 dominant genera in the intestinal microbiota of the HTZ, BTZ, and DZZ groups were: Bacteroides, Bifidobacterium, Faecalibacterium, Escherichia-Shigella, Akkermansia, Ruminococcus gnavus group, Streptococcus, Megamonas, Klebsiella, and Ruminococcus. Intergroup difference analysis using Kruskal-Wallis/Dunn’s test revealed that compared to DZZ, the relative abundances of genera such as Subdoligranulum (q = 0.03), Lachnospira (q = 0.01), Romboutsia (q = 0.01), Haemophilus (q = 0.01), and Erysipelotrichaceae UCG-003 (q = 0.01) were significantly lower in HTZ and BTZ, while the relative abundances of genera such as UBA1819 (q=0.01), Escherichia-Shigella (q = 0.08) and Ruminococcus gnavus group (q = 0.07) were nominally higher (unadjusted p < 0.05). Additionally, HTZ showed nominally higher abundances of genera Erysipelatoclostridium (q = 0.07) and Ruminococcus gnavus group (q = 0.12) compared to DZZ (unadjusted p < 0.05), while BTZ showed significantly lower abundances of genera Romboutsia (q = 0.02), Erysipelotrichaceae UCG-003 (q = 0.03), and Haemophilus (q = 0.04) compared to DZZ. Between HTZ and BTZ, no comparisons survived FDR correction. However, nominal differences (unadjusted p < 0.05) were observed: BTZ had higher abundances of Bacteroides (q = 0.34), Collinsella (q = 0.36), and Pantoea (q = 0.51), while HTZ had higher abundances of Erysipelatoclostridium (q = 0.25). These trends suggest potential compositional distinctions that merit exploration in larger cohorts (Figure 7).
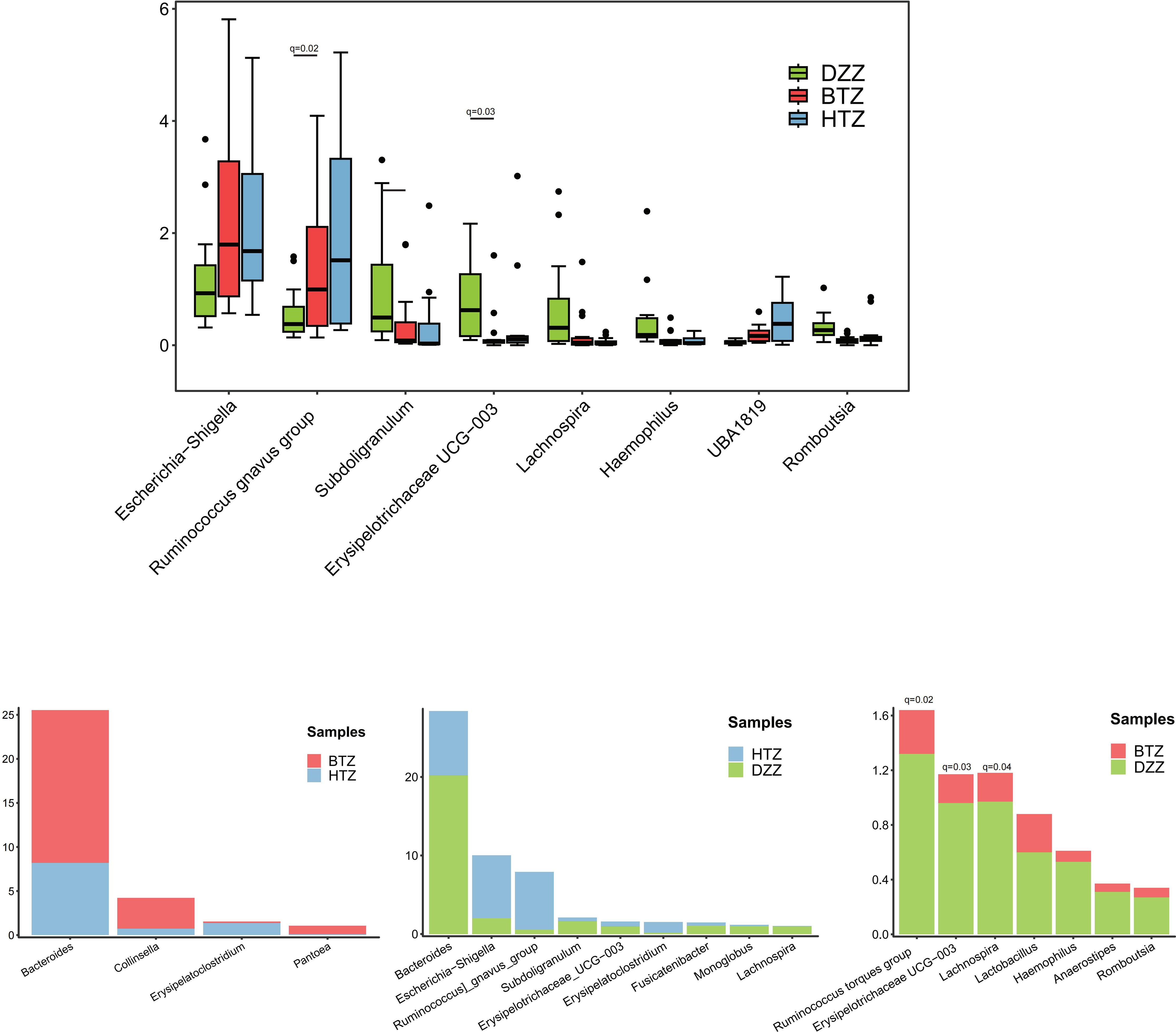
Figure 7. Comparison of microbial composition at the genus level. Bray-Curtis clustering revealed that HTZ and BTZ groups were more similar in genus-level microbial composition, clearly separated from the DZZ group. Compared to DZZ (controls), HTZ/BTZ showed significantly lower Subdoligranulum, Lachnospira, Romboutsia, Haemophilus, Erysipelotrichaceae UCG-003 and nominally higher UBA1819, Escherichia-Shigella, Ruminococcus gnavus group, though only changes in UBA1819 remained significant after FDR correction (q < 0.05). HTZ: nominally higher Erysipelatoclostridium/Ruminococcus gnavus group vs. DZZ; BTZ: significantly lower Romboutsia/Erysipelotrichaceae UCG-003/Haemophilus vs. DZZ. HTZ vs. BTZ: BTZ had nominally higher Bacteroides, Collinsella, Pantoea; HTZ had nominally higher Erysipelatoclostridium.
3.3.3 LEfSe analysis
The histogram of LDA values showed that 61 differential taxa (LDA score > 3, unadjusted p < 0.05) were observed among the HTZ, BTZ, and DZZ groups. However, after FDR correction, none of these taxa reached the threshold of statistical significance (q > 0.05). The LDA results thus represent unadjusted potential biomarkers that require validation in independent studies. Specifically, 15 differential taxa, including Ruminococcus gnavus group, Bacilli, Erysipelotrichales, and Erysipelatoclostridiaceae, had higher abundances in the HTZ group (unadjusted p < 0.05). Forty differential taxa, such as Bacteroidota, Bacteroidia, Subdoligranulum, Eubacterium eligens group, and Lachnospira unclassified, were more abundant in the intestinal microbiota of healthy individuals (unadjusted p < 0.05). Nine differential taxa, including Escherichia-Shigella, Collinsella unclassified, Bacteroides uniformis, and Collinsella, had higher abundances in the BTZ group (unadjusted p < 0.05). The Cladogram showed the significantly different taxa and their evolutionary branch relationships among the three groups. LEfSe analysis between HTZ and BTZ showed that 13 differential microbiota, including Bacilli (q = 0.31), Erysipelotrichales (q = 0.06), Erysipelatoclostridiaceae (q = 0.16), Burkholderiaceae (q = 0.16), and Ralstonia (q = 0.29), had nominally higher abundances (unadjusted p < 0.05) in the HTZ group, while 11 differential microbiota, such as Bacteroidaceae (q = 0.22), Collinsella (q = 0.36), Pantoea (q = 0.50), and Erwiniaceae (q = 0.35), had nominally higher abundances (unadjusted p < 0.05) in the BTZ group. The cladogram illustrates these potential taxonomic discriminators and their evolutionary relationships between HTZ and BTZ, though their statistical robustness is limited (Figure 8).
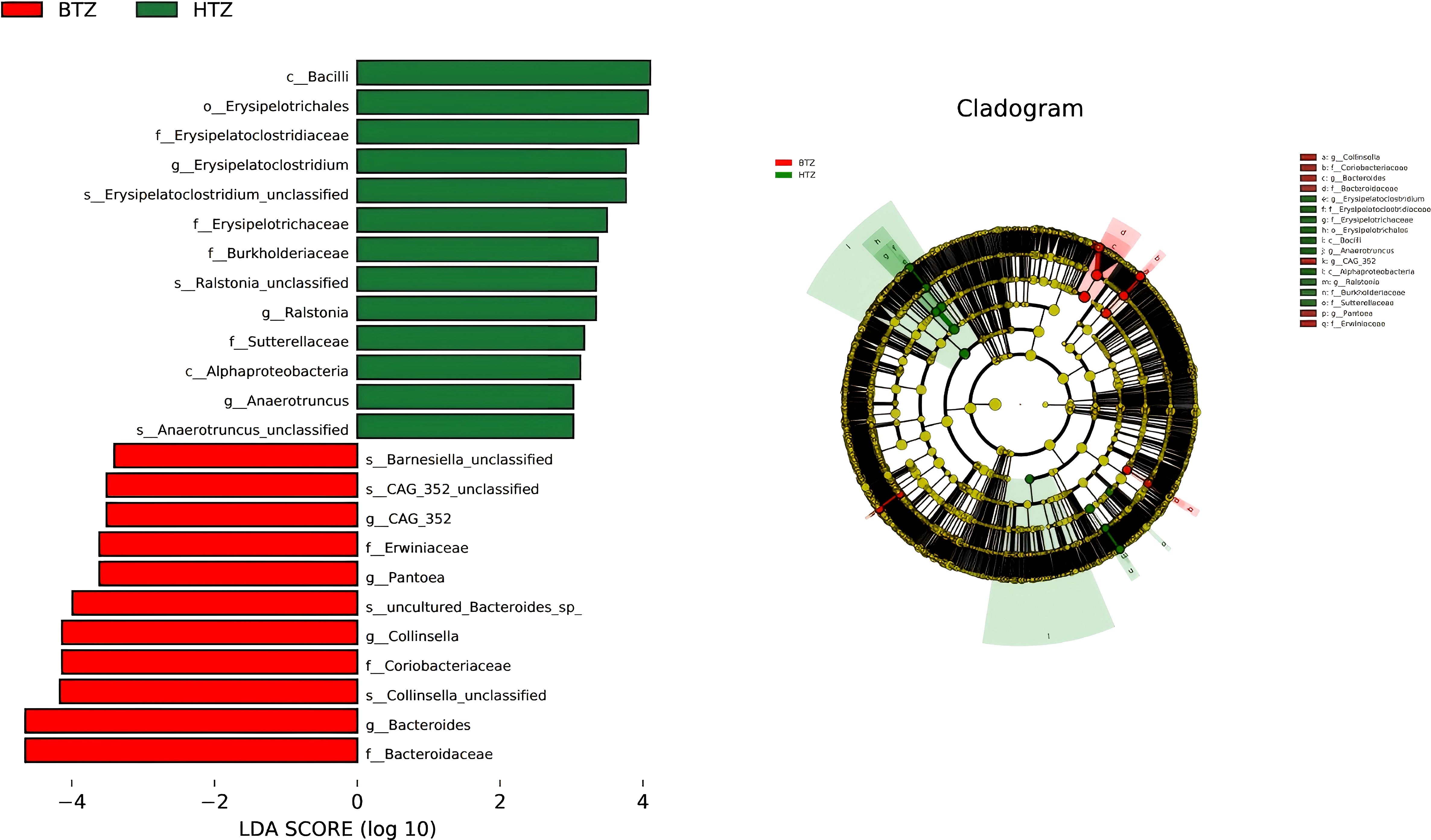
Figure 8. LEfSe analysis. LDA histogram (LDA > 3) and cladogram. Results are based on unadjusted p-values and represent potential biomarkers requiring further validation. HTZ-enriched taxa: Bacilli, Erysipelotrichales, Erysipelatoclostridiaceae, Burkholderiaceae, Ralstonia. BTZ-enriched taxa: Bacteroidaceae, Collinsella, Pantoea, Erwiniaceae. Cladogram shows taxonomic relationships of key discriminators.
3.4 Predictive functional analysis
The species’ functions in the gut microbiota of both the groups were predicted and analyzed based on the amplified sequencing data. Using t-test for differential analysis with Benjamini-Hochberg correction for multiple testing, PICRUSt2-based functional prediction of the intestinal microbiota in MHD patients showed that compared to BTZ, HTZ had significantly enhanced microbial functions in cardiac muscle contraction (q = 0.05), beta-lactam resistance (q = 0.05), non-homologous end-joining (q = 0.04), Parkinson’s disease (q = 0.03), and D-arginine and D-ornithine metabolism (q = 0.01), while significantly decreased functions in cellular antigens (q = 0.02) and cell division (q = 0.01) (Figure 9).
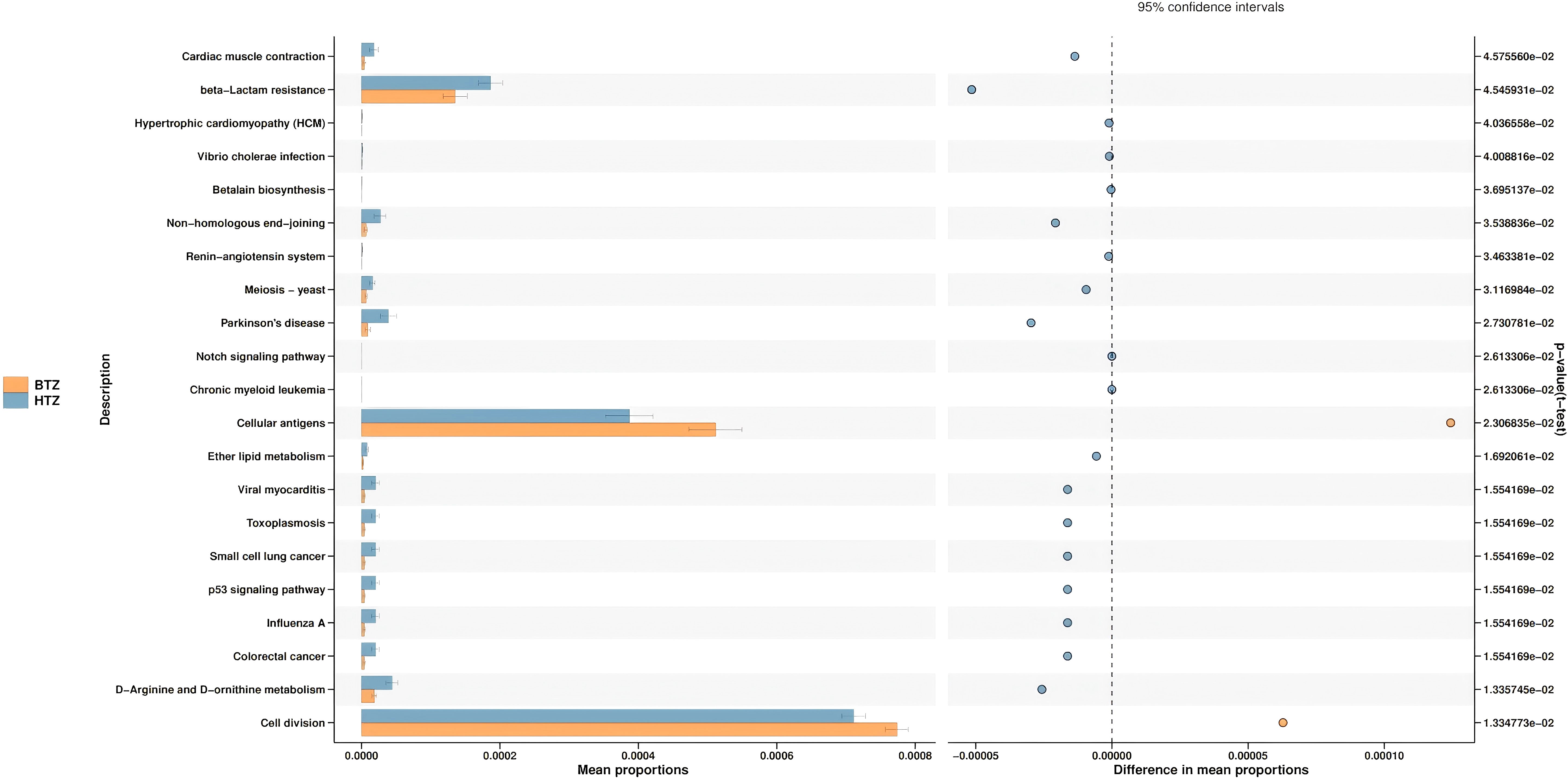
Figure 9. Predictive functional analysis. Key functional differences between HTZ and BTZ. HTZ showed enhanced pathways in cardiac muscle contraction, beta-lactam resistance, and Parkinson’s disease-related metabolism. Reduced functions in HTZ included cellular antigen processes and cell division.
4 Discussion
In this study, we found that tongue coating thickness in hemodialysis patients is associated with distinct differences in gut microbiota composition, diversity, and predicted function. Both thick tongue coating (HTZ) and thin tongue coating (BTZ) groups exhibited gut dysbiosis compared to healthy controls (DZZ), but they also differed from each other in specific microbial features. While several taxonomic comparisons between patient groups (HTZ vs. BTZ) did not retain statistical significance after rigorous multiple testing correction, the persistence of nominal differences (unadjusted p < 0.05) and consistent trends across analyses suggest potential, albeit subtle, distinctions in their gut microbial ecosystems that align with the tongue coating phenotype.
A Venn diagram of ASVs illustrated that while all three groups shared a core of 667 ASVs, each patient group had unique taxa, with HTZ showing the highest total ASV count (2970) compared to BTZ (2487) and DZZ (2964). This indicates that HTZ patients harbored a broader range of microbial species (including possibly opportunistic taxa) relative to BTZ. Correspondingly, α-diversity measures (Observed species, Shannon, Simpson indices) were significantly lower in BTZ than in healthy controls, whereas HTZ showed intermediate diversity. DZZ exhibited greater species richness and evenness than BTZ (q < 0.05), while no significant difference was noted between HTZ and BTZ in these indices. These results suggest that BTZ patients have a more depleted gut microbiome diversity compared to healthy individuals, whereas HTZ patients, despite severe illness, did not experience the same loss of diversity. However, β-diversity analysis revealed that both HTZ and BTZ groups cluster closely together and separate from DZZ (Figure 4). Although α-diversity indices showed no statistically significant difference between HTZ and DZZ, this likely reflects preserved overall richness and evenness in some HD patients with thick coatings. However, our β-diversity and taxonomic analyses revealed clear compositional shifts between these two groups, including the nominal enrichment of pathogenic genera in HTZ. This suggests that tongue coating phenotype in HD patients corresponds more strongly with microbiota structure than with global diversity metrics. The PCoA plots showed only partial overlap between patient and control samples, with HTZ and BTZ microbiotas more similar to each other than either is to healthy controls. This indicates that both patient groups share a common dysbiotic footprint characteristic of maintenance hemodialysis, distinct from the normal gut microbiome, even though HTZ and BTZ differ in certain nuances.
When comparing gut microbiota composition, we observed broad shifts at multiple taxonomic levels associated with tongue coating thickness. At the phylum level, the gut communities of all groups were dominated by Firmicutes, Bacteroidota, Actinobacteriota, and Proteobacteria, consistent with a typical human gut profile. Nonetheless, several lower-abundance phyla showed significant differences between groups (Figure 6). At the phylum level, the nominal increases in Patescibacteria, Deferribacterota, Planctomycetota, and Bdellovibrionota in HTZ versus BTZ, though not statistically robust after correction, are intriguing. These phyla are often minor constituents or considered to be associated with specific ecological niches or dysbiotic states. Their potential elevation in HTZ could hint at a more disrupted or specialized microbial community deserving of further scrutiny. In contrast, healthy controls showed statistically significant higher abundances (q < 0.05) of Cyanobacteria (likely reflecting dietary plant chloroplasts or commensal Cyanobacterial lineages) and Deinococcota than BTZ. The BTZ group’s near absence of these taxa may be due to dietary differences or a generally reduced microbial complexity in patients. Additionally, the major phylum Bacteroidota was nominally depleted in HTZ compared to healthy controls. This aligns with the genus-level finding of reduced Bacteroides and points toward a potential loss of important commensal, fiber-degrading bacteria in thick-coated patients. Taken together, these phylum-level changes suggest that HTZ microbiomes are skewed toward uncommon or potentially pathogenic phyla, whereas BTZ microbiomes show loss of some normally occurring phyla found in healthy guts.
At the genus level, dysbiosis patterns were evident in both patient groups relative to controls, as well as between HTZ and BTZ themselves. The overall top ten genera in all groups included Bacteroides, Bifidobacterium, Faecalibacterium, Escherichia-Shigella, Akkermansia, Ruminococcus gnavus group, Streptococcus, Megamonas, Klebsiella, and Ruminococcus (torques group), indicating that many core gut genera are present across the spectrum of health and disease. However, many beneficial genera were significantly under-represented in both HTZ and BTZ patients compared to healthy DZZ, consistent with the known impact of uremia on the gut microbiome (Simões-Silva et al., 2018). Specifically, both patient groups showed significant reductions in SCFA-producing and fiber-fermenting bacteria such as Subdoligranulum, Lachnospira, Romboutsia, Haemophilus and Erysipelotrichaceae UCG-003 compared to controls (q < 0.05). Concurrently, opportunistic or proteolytic genera were elevated: for example, UBA1819, Escherichia-Shigella and the Ruminococcus gnavus group were nominally higher in both HTZ and BTZ vs. DZZ, though only changes in UBA1819 remained significant after FDR correction (q < 0.05). These shifts mirror hallmark features of CKD-related gut dysbiosis - a loss of commensals that produce beneficial metabolites (like butyrate) and an overgrowth of bacteria that produce endotoxins and uremic toxins (Beau et al., 2025). Indeed, Escherichia-Shigella (an Enterobacteriaceae member) can generate endotoxin and indoxyl sulfate precursors (Hayashi et al., 2018; Yu et al., 2025), while R. gnavus is known for mucin degradation and pro-inflammatory polysaccharide production (Crost et al., 2016); their increase in both HTZ and BTZ underscores a gut environment inclined toward inflammation and toxin production in MHD patients. Notably, some differences with healthy controls were unique to each patient subgroup. HTZ patients showed a nominal increase in Erysipelatoclostridium (genera in the Erysipelotrichaceae family) and Ruminococcus gnavus group compared to DZZ, suggesting that certain pro-inflammatory or putrefactive taxa may preferentially expand when the tongue coating is thick. Meanwhile, BTZ patients had a greater loss of Romboutsia, Erysipelotrichaceae UCG-003, and Haemophilus compared to DZZ; Romboutsia is a beneficial anaerobe that can utilize lactate and, through cross-feeding interactions with other microbes, promote butyrate production (Chen et al., 2025)- indicating that even thin-coated patients suffer a deficit of key commensals relative to healthy individuals. Thus, a thin tongue coating should not be equated with a normal microbiome; rather, it represents a milder dysbiosis where some harmful changes are present but perhaps to a lesser degree than in thick-coated patients.
Direct comparison between HTZ and BTZ groups revealed a subset of genera that differentiate the two dysbiotic states (Figure 7). At the genus level, the pattern of dysbiosis was more clearly evident when comparing both patient groups to healthy controls, with several changes, such as the increase in Escherichia-Shigella and decrease in Erysipelotrichaceae UCG-003, surviving multiple testing correction. The direct comparison between HTZ and BTZ yielded nominal differences that, while requiring cautious interpretation, paint a biologically plausible picture. For instance, the trend of higher Bacteroides in BTZ and higher Erysipelatoclostridium in HTZ is consistent with the notion that a thin coating might be associated with a relatively better-preserved capacity for fiber fermentation, while a thick coating might indicate a shift towards pro-inflammatory or putrefactive taxa, but these specific HTZ vs BTZ contrasts were nominal and require validation in larger cohorts. The LEfSe analysis, though comprised of unadjusted p-values, further supports this narrative by identifying coherent groups of taxa (e.g., Erysipelotrichales and Burkholderiaceae in HTZ; Bacteroidaceae and Erwiniaceae in BTZ) that differentiate the two groups (Figure 8). The convergence of these trends from different analytical approaches strengthens the hypothesis that tongue coating reflects meaningful, albeit subtle, variations in the gut microbiome of MHD patients.
The functional potential of the gut microbiota, inferred via PICRUSt2 analysis, also differed between HTZ and BTZ, though in subtler ways (Figure 9). However, these functional predictions are derived from 16S rRNA gene data and are inherently speculative. They lack the resolution of direct functional profiling methods (e.g., metagenomics or metabolomics) and should be interpreted with caution, particularly in disease-altered microbiomes such as ESRD where taxonomic-functional relationships may be disrupted. Notably, HTZ showed higher representation of gene pathways related to cardiovascular diseases and infections compared to BTZ. For instance, pathways such as “Cardiac muscle contraction”, “Hypertrophic cardiomyopathy”, and infectious disease pathways (e.g., “Vibrio cholerae infection”) were enriched in the HTZ microbiome, whereas these were less prominent in BTZ. HTZ also had higher predicted abundances of pathways involved in various metabolic diseases (“disease metabolism”). These enriched functions suggest that the HTZ-associated microbiota may contribute to a more pro-inflammatory or pathological metabolic milieu – for example, harboring more genes related to toxin production or host-interactive pathways that could influence cardiac and immune function. In contrast, certain fundamental cellular processes were relatively under-represented in HTZ versus BTZ. Our data indicate that pathways involved in basic cellular functions (for example, DNA repair mechanisms like non-homologous end joining, and cell division-related pathways) were predicted to be reduced in HTZ. One interpretation is that the microbial community in HTZ might be skewed away from maintaining normal cellular homeostasis and towards stress or virulence-associated metabolism, perhaps reflecting a community under or causing host stress. It is important to emphasize that these functional predictions are hypothetical – they provide hints that HTZ microbiomes could be functionally more disruptive (e.g., contributing to cardiovascular risk or infections), whereas BTZ microbiomes might retain relatively more “basic” functionality – but they need experimental validation.
The observed differences in gut microbiota between thick and thin tongue coating patients beg the question of mechanism and causality. One plausible explanation involves the oral-gut microbial axis. Tongue coating is essentially an oral microbial biofilm; patients with thick coatings likely harbor an overgrowth of oral microbes (including anaerobes and possibly yeast or others) on the tongue surface (Guo et al., 2025). These microbes, or their metabolites, can be continuously swallowed and thus influence the gut microbiota composition downstream. Indeed, our team’s previous work showed significant differences in the oral microbiota between HTZ and BTZ patients. For example, we found higher abundance of certain oral genera (like Prevotella and Megasphaera) in thick-coated patients. It has been reported that the oral and gut microbiomes can mirror each other in specific ways – microbes prevalent in the tongue coating often appear in the gut, and changes in oral microbiota can accompany changes in gut microbiota in various diseases (Deng et al., 2024). Previous studies have documented correlations such as the relative abundance of Prevotella being positively linked between tongue coating and feces in healthy individuals (Guo et al., 2022). In disease states (e.g., diarrhea-predominant IBSor MAFLD), shifts in tongue-coating microbiota parallel alterations in the gut community (Lu et al., 2022; Tang et al., 2023). Moreover, systemic illnesses (like COVID-19 or schizophrenia) are known to perturb both oral and gut microbiomes simultaneously, and translocation of oral bacteria to the gut tends to increase with age or immune compromise (Murray et al., 2021; Jin et al., 2022; Ghorbani, 2023; Zhang et al., 2023). Given this context, it is reasonable to speculate that a thick tongue coating could be contributing to gut dysbiosis by serving as a reservoir for microbes that colonize or influence the intestinal environment. For instance, Erysipelatoclostridium and other Erysipelotrichaceae found enriched in HTZ gut are also known inhabitants of the oral cavity; an overgrowth on the tongue could seed the gut continually. Likewise, the presence of Ralstonia or Pantoea might originate from environmental exposure in the oral cavity (water, food) that, under normal conditions, would not persist in the gut but in an altered host environment they manage to survive. Additionally, from a Traditional Chinese Medicine (TCM) perspective, a thick tongue coating is thought to indicate internal “dampness” or stagnation in the gastrointestinal system (Guo et al., 2025). Biomedically, this concept may translate to slower gut motility, altered pH, or retention of food residues - conditions that can foster microbial overgrowth. It’s conceivable that HTZ patients have underlying GI functional differences (such as more frequent constipation or delayed transit) which could allow certain bacteria to proliferate excessively, whereas BTZ patients might have relatively faster transit or less substrate accumulation limiting microbial expansion. We did not specifically measure motility or oral-gut transmission in this study, so these mechanisms remain speculative. Nevertheless, the oral-gut axis hypothesis is supported by our finding that many of the taxa distinguishing HTZ and BTZ are not independent random occurrences but belong to known oral-related or dysbiosis-associated groups. Future studies integrating oral microbiome sequencing and gut transit measurements could elucidate this causal chain more clearly.
From a clinical standpoint, our findings carry potential practical significance. Tongue coating is a simple, noninvasive clinical observation that could serve as a surrogate marker for gut microbiota status in hemodialysis patients. If a thick tongue coating correlates with a greater degree of gut dysbiosis - characterized by higher loads of pro-inflammatory bacteria and altered metabolic potential - it might alert clinicians to patients who are at risk of GI complications or systemic inflammation stemming from the gut. Conversely, a thin tongue coating, while not indicating a normal microbiota, might correspond to a relatively milder dysbiosis. This concept aligns with the tenets of TCM, but here we provide scientific evidence that tongue appearance does reflect internal micro-ecology. Tongue coating thickness could thus be used as a screening tool or adjunct in evaluating patient health, supporting the notion of it as a “window” into the gut microbiome (Zhu et al., 2025). For example, an MHD patient presenting with a notably thick, greasy tongue coating might benefit from early interventions targeting the gut flora - such as prebiotic fiber supplementation, probiotics, or diet modifications (e.g. increasing fermentable fiber or reducing protein load to curb proteolytic bacteria). In contrast, a patient with a thin coating might be monitored for loss of beneficial microbes and could benefit from strategies to increase microbial diversity (such as a more plant-rich diet or specific commensal probiotics).
5 Limitations and future directions
While this study provides novel insights into the microbiota-tongue coating relationship, several limitations warrant consideration. First, the cross-sectional design precludes causal inference, necessitating longitudinal studies to track microbiota dynamics and clinical outcomes. Second, the functional predictions are based on computational modeling rather than direct experimental validation, requiring follow-up in vitro/in vivo studies to mechanistically validate pathway alterations. Third, the sample size, while sufficient to detect larger effects against healthy controls, may have limited the power to detect more subtle but biologically relevant differences between the HTZ and BTZ groups after stringent multiple testing correction. The consistent trends observed (unadjusted p < 0.05) despite the lack of FDR significance highlight the need for larger, specifically powered studies to confirm or refute these potential associations. Such inferences are limited by the incomplete representation of gene functions in reference databases and may not accurately reflect the true metabolic potential of complex or dysbiotic microbiomes, such as those in ESRD patients. Caution is warranted when interpreting KEGG pathway enrichments as direct indicators of disease mechanisms. Lastly, the generalizability of findings may be limited by the specific HD patient population studied, emphasizing the need for multi-center cohorts with diverse ethnic and dietary backgrounds. Fourth, we did not further stratify healthy controls (DZZ) by tongue coating thickness, as none of them exhibited a thick coating (TCT ≥ 24) or a thin coating (TCT ≤ 13). All healthy individuals had moderate TCT scores (14–23), representing physiologically normal variation. This limited stratification helps avoid overinterpretation but may restrict the exploration of subtle oral–gut microbiome associations in non-HD populations. Future studies with larger healthy cohorts are warranted to determine whether similar associations hold in the general population. Future research should prioritize integrative approaches combining metagenomics, metabolomics, and proteomics to unravel the complex interactions between microbiota, host metabolism, and tongue coating physiology. Additionally, interventional trials evaluating probiotics, prebiotics, or fecal microbiota transplantation (FMT) in HTZ patients could establish whether microbial reconstitution alleviates clinical symptoms and improves long-term outcomes in HD.
6 Conclusion
This study reveals that tongue coating thickness in maintenance hemodialysis patients reflects distinct subtypes of gut microbiota dysbiosis. Thick coatings are associated with pro-inflammatory microbial features, while thin coatings indicate lower diversity yet partial preservation of commensals. These patterns suggest that tongue appearance may serve as a visible indicator of internal microbial states, offering clinically relevant insights into systemic health. Integrating traditional tongue diagnosis with microbiome profiling could support personalized strategies to improve gastrointestinal and metabolic outcomes in dialysis care.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1267578.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of Hangzhou Hospital of TCM. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YW: Writing – original draft. XYZ: Writing – original draft. MW: Writing – original draft. BL: Writing – original draft. JW: Writing – original draft. SPC: Writing – original draft. AZ: Writing – original draft. MH: Writing – original draft. YZ: Writing – original draft. HL: Writing – review & editing. FZ: Writing – review & editing. SLC: Writing – original draft. XZ: Writing – original draft. LZ: Writing – original draft. JL: Writing – original draft. RL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported in part by Grants-in-Aid from Zhejiang Provincial Traditional Chinese Medicine Science and Technology Plan (project 2021ZB204; 2023ZL110; 2024ZL110 and 2025ZL440), and by Zhejiang Province 2022 national famous old Chinese medicine experts create city studio construction project (CaiFeng Zhu), and the Medical Scientific Research Foundation of Zhejiang Province, China (Grant number: 2024KY1374).
Acknowledgments
The authors would like to express their gratitude to the Zhejiang Province TCM Science and Technology Program and Hangzhou Hospital of Traditional Chinese Medicine for their financial support of this study. The authors thank Hangzhou Lianchuan Biotechnology Company for technical support of this study. The authors thank Dr. Shaojie Zhou from Hangzhou Cosmos Wisdom for his technical support of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1640429/full#supplementary-material
References
Beau, A., Natividad, J., Benoit, B., Delerive, P., Duboux, S., Feng, Y., et al. (2025). A specifically designed multi-biotic reduces uremic toxin generation and improves kidney function. Gut Microbes 17, 2531202. doi: 10.1080/19490976.2025.2531202
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J., and Holmes, S. P. (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Chen, S., Ling, B., Liu, X., Liu, L., Feng, J., Zhang, J., et al. (2025). Structural characterization of β-glucan in Hericium erinaceus pretreated by steam explosion and its effects on human gut microbiota in vitro. Food Chem. 482, 144156. doi: 10.1016/j.foodchem.2025.144156
Crost, E. H., Tailford, L. E., Monestier, M., Swarbreck, D., Henrissat, B., Crossman, L. C., et al. (2016). The mucin-degradation strategy of Ruminococcus gnavus: The importance of intramolecular trans-sialidases. Gut Microbes 7, 302–312. doi: 10.1080/19490976.2016.1186334
Cui, G. Y., Rao, B. C., Zeng, Z. H., Wang, X. M., Ren, T., Wang, H. Y., et al. (2022). Characterization of oral and gut microbiome and plasma metabolomics in COVID-19 patients after 1-year follow-up. Mil Med. Res. 9, 32. doi: 10.1186/s40779-022-00387-y
Deng, J., Dai, S., Liu, S., Tu, L., Cui, J., Hu, X., et al. (2024). Application of tongue image characteristics and oral-gut microbiota in predicting pre-diabetes and type 2 diabetes with machine learning. Front. Cell Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1477638
Douglas, G. M., Maffei, V. J., Zaneveld, J. R., Yurgel, S. N., Brown, J. R., Taylor, C. M., et al. (2020). PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 38, 685–688. doi: 10.1038/s41587-020-0548-6
Ghorbani, M. (2023). Unveiling the human brain virome in brodmann area 46: novel insights into dysbiosis and its association with schizophrenia. Schizophr. Bull. Open 4, sgad029. doi: 10.1093/schizbullopen/sgad029
Glasel, J. A. (1995). Validity of nucleic acid purities monitored by 260nm/280nm absorbance ratios. Biotechniques 18, 62–63.
Guo, Y., He, J., Li, S., Zou, S., Zhang, H., Yang, X., et al. (2025). Warm and humid environment induces gut microbiota dysbiosis and bacterial translocation leading to inflammatory state and promotes proliferation and biofilm formation of certain bacteria, potentially causing sticky stool. BMC Microbiol. 25, 24. doi: 10.1186/s12866-024-03730-6
Guo, X. J., Jiang, T., Ma, X. X., Hu, X. J., Huang, J. B., Cui, L. T., et al. (2022). Relationships between diurnal changes of tongue coating microbiota and intestinal microbiota. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.813790
Hayashi, T., Yamashita, T., Watanabe, H., Kami, K., Yoshida, N., Tabata, T., et al. (2018). Gut microbiome and plasma microbiome-related metabolites in patients with decompensated and compensated heart failure. Circ. J. 83, 182–192. doi: 10.1253/circj.CJ-18-0468
Iwauchi, M., Horigome, A., Ishikawa, K., Mikuni, A., Nakano, M., Xiao, J. Z., et al. (2019). Relationship between oral and gut microbiota in elderly people. Immun. Inflammation Dis. 7, 229–236. doi: 10.1002/iid3.266
Jin, S., Wetzel, D., and Schirmer, M. (2022). Deciphering mechanisms and implications of bacterial translocation in human health and disease. Curr. Opin. Microbiol. 67, 102147. doi: 10.1016/j.mib.2022.102147
Karahan, D. and Şahin, İ. (2022). Comparison of gastrointestinal symptoms and findings in renal replacement therapy modalities. BMC Nephrol. 23, 261. doi: 10.1186/s12882-022-02893-6
Kelly, B. J., Gross, R., Bittinger, K., Sherrill-Mix, S., Lewis, J. D., Collman, R. G., et al. (2015). Power and sample-size estimation for microbiome studies using pairwise distances and PERMANOVA. Bioinformatics 31, 2461–2468. doi: 10.1093/bioinformatics/btv183
Kuskunov, T., Tilkiyan, E., Doykov, D., Boyanov, K., Bivolarska, A., and Hristov, B. (2023). The effect of synbiotic supplementation on uremic toxins, oxidative stress, and inflammation in hemodialysis patients-results of an uncontrolled prospective single-arm study. Med. (Kaunas) 59 (8), 1383. doi: 10.3390/medicina59081383
Ling, Z., Cheng, Y., Liu, X., Yan, X., Wu, L., Shao, L., et al. (2023). Altered oral microbiota and immune dysfunction in Chinese elderly patients with schizophrenia: a cross-sectional study. Transl. Psychiatry 13, 383. doi: 10.1038/s41398-023-02682-1
Lu, C., Zhu, H., Zhao, D., Zhang, J., Yang, K., Lv, Y., et al. (2022). Oral-gut microbiome analysis in patients with metabolic-associated fatty liver disease having different tongue image feature. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.787143
Matsumoto, T. and Sugano, M. (2013). 16S rRNA gene sequence analysis for bacterial identification in the clinical laboratory. Rinsho Byori 61, 1107–1115.
McMurdie, P. J. and Holmes, S. (2013). phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS One 8, e61217. doi: 10.1371/journal.pone.0061217
Murray, N., Al Khalaf, S., Kaulmann, D., Lonergan, E., Cryan, J. F., Clarke, G., et al. (2021). Compositional and functional alterations in the oral and gut microbiota in patients with psychosis or schizophrenia: A systematic review. HRB Open Res. 4, 108. doi: 10.12688/hrbopenres.13416.1
Park, S. Y., Hwang, B. O., Lim, M., Ok, S. H., Lee, S. K., Chun, K. S., et al. (2021). Oral-gut microbiome axis in gastrointestinal disease and cancer. Cancers (Basel) 13 (7), 1748. doi: 10.3390/cancers13092124
Park, S. C., Jung, J., Kwon, Y. E., Baeg, S. I., Oh, D. J., Kim, D. H., et al. (2025). Constipation and risk of death and cardiovascular events in patients on hemodialysis. Kidney Res. Clin. Pract. 44, 155–163. doi: 10.23876/j.krcp.24.174
Parks, D. H. and Beiko, R. G. (2010). Identifying biologically relevant differences between metagenomic communities. Bioinformatics 26, 715–721. doi: 10.1093/bioinformatics/btq041
Regis, B., Passeri, L., Moreira, N. X., Borges, N. A., Ribeiro-Alves, M., and Mafra, D. (2025). Plasma trimethylamine N-oxide levels in nondialysis chronic kidney disease patients following meal challenge. Mol. Nutr. Food Res. 69 (14), e70121. doi: 10.1002/mnfr.70121
Sambrook, J. and Russell, D. W. (2006). Purification of nucleic acids by extraction with phenol:chloroform. CSH Protoc. 2006 (1), pdb.prot4455. doi: 10.1101/pdb.prot4455
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60. doi: 10.1186/gb-2011-12-6-r60
Shao, B., Nong, Y., Lin, Y., Meng, Y., Zhou, Y., Huang, M., et al. (2025). Study on the influence and mechanism of resveratrol on cognitive impairment in chronic kidney disease rats through regulating gut microbiota and the TLR4/NFκB pathway. J. Inflammation Res. 18, 6049–6060. doi: 10.2147/jir.S510867
Shimizu, T., Ueda, T., and Sakurai, K. (2007). New method for evaluation of tongue-coating status. J. Oral. Rehabil. 34, 442–447. doi: 10.1111/j.1365-2842.2007.01733.x
Simões-Silva, L., Araujo, R., Pestana, M., Soares-Silva, I., and Sampaio-Maia, B. (2018). The microbiome in chronic kidney disease patients undergoing hemodialysis and peritoneal dialysis. Pharmacol. Res. 130, 143–151. doi: 10.1016/j.phrs.2018.02.011
Simpson, R. J. (2010). CTAB-PAGE. Cold Spring Harb. Protoc. 2010, pdb.prot5412. doi: 10.1101/pdb.prot5412
Sulaiman, Y., Pacauskienė, I. M., Šadzevičienė, R., and Anuzyte, R. (2024). Oral and gut microbiota dysbiosis due to periodontitis: systemic implications and links to gastrointestinal cancer: A narrative review. Med. (Kaunas) 60 (9), 1416. doi: 10.3390/medicina60091416
Tang, B., Hu, Y., Chen, J., Su, C., Zhang, Q., and Huang, C. (2023). Oral and fecal microbiota in patients with diarrheal irritable bowel syndrome. Heliyon 9, e13114. doi: 10.1016/j.heliyon.2023.e13114
Velasquez, M. T., Centron, P., Barrows, I., Dwivedi, R., and Raj, D. S. (2018). Gut microbiota and cardiovascular uremic toxicities. Toxins (Basel) 10 (7), 287. doi: 10.3390/toxins10070287
Wang, X., Wen, P., Gao, F., Zhao, J., and Miao, S. (2025). Microbiota-friendly diet ameliorates hypoalbuminemia in chronic kidney disease: evidence from NHANES. Front. Immunol. 16. doi: 10.3389/fimmu.2025.1546031
Wen, T., Niu, G., Chen, T., Shen, Q., Yuan, J., and Liu, Y. X. (2023). The best practice for microbiome analysis using R. Protein Cell 14, 713–725. doi: 10.1093/procel/pwad024
Yu, W., Yu, S., Zhang, F., Xu, Q., Zhang, X., and Kong, J. (2025). Ultrasensitive electrochemical sensor for lipopolysaccharide detection catalyzed by 3,4,9,10-perylenetetracarboxylic diimide. Anal. Chim. Acta 1352, 343926. doi: 10.1016/j.aca.2025.343926
Zhang, A., Chen, S., Zhu, Y., Wu, M., Lu, B., Zhou, X., et al. (2024). Intestinal microbiome changes and mechanisms of maintenance hemodialysis patients with constipation. Front. Cell Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1495364
Zhang, D., Weng, S., Xia, C., Ren, Y., Liu, Z., Xu, Y., et al. (2023). Gastrointestinal symptoms of long COVID-19 related to the ectopic colonization of specific bacteria that move between the upper and lower alimentary tract and alterations in serum metabolites. BMC Med. 21, 264. doi: 10.1186/s12916-023-02972-x
Zhu, Y., Zeng, X., Zhang, A., Lu, B., Wu, M., Liu, H., et al. (2025). Correlation of tongue coating thickness with microinflammatory state and oral microbiome in maintenance hemodialysis patients. J. Oral. Microbiol. 17, 2488054. doi: 10.1080/20002297.2025.2488054
Keywords: hemodialysis, tongue coating, gut microbiota, oral-gut axis, gastrointestinal complications
Citation: Wang Y, Zeng X, Wu M, Lu B, Wang J, Chen S, Zhang A, Huang M, Zhu Y, Liu H, Zhu F, Chen S, Zhou X, Zhao L, Liu J and Lin R (2025) Thick vs. thin tongue coatings in hemodialysis patients: unveiling gut microbiome dysregulation and systemic health implications. Front. Cell. Infect. Microbiol. 15:1640429. doi: 10.3389/fcimb.2025.1640429
Received: 03 June 2025; Accepted: 15 September 2025;
Published: 29 September 2025.
Edited by:
Jingjie Du, Cornell University, United StatesReviewed by:
Tao Sun, Cornell University, United StatesYiwei Liu, National Human Genome Research Institute (NIH), United States
Copyright © 2025 Wang, Zeng, Wu, Lu, Wang, Chen, Zhang, Huang, Zhu, Liu, Zhu, Chen, Zhou, Zhao, Liu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Riyang Lin, bGluX3JpX3lhbmdAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yuqing Wang
Yuqing Wang Xueyan Zeng2†
Xueyan Zeng2† Mengqi Wu
Mengqi Wu Bin Lu
Bin Lu Aiping Zhang
Aiping Zhang Yanqin Zhu
Yanqin Zhu Hong Liu
Hong Liu Xin Zhou
Xin Zhou Riyang Lin
Riyang Lin