- 1National Clinical Research Center for Laboratory Medicine, Department of Laboratory Medicine, The First Hospital of China Medical University, Shenyang, China
- 2Department of Laboratory Medicine, The First Hospital of China Medical University, Shenyang, China
Candida auris is an invasive fungal pathogen recognized globally as a significant health threat due to its marked resistance to multiple classes of antifungal agents, including azoles, echinocandins, and polyenes. The associated high morbidity and mortality rates present considerable public health challenges. Research efforts have largely focused on understanding the molecular mechanisms of antifungal resistance and developing alternative therapies to counteract this issue. This review summarizes current advances in the identification of natural antifungal compounds, the development of novel synthetic agents, biological antifungals, nanotechnology-based approaches, combination therapies, and photodynamic treatments. Notably, several synthetic compounds such as rezafungin and fosmanogepix are in clinical trials for C. auris infections. Biological antifungals, including monoclonal antibodies, vaccines, and peptides, have shown the capacity to enhance host immune responses and reduce mortality in murine models. Combination therapies have proven particularly valuable for overcoming resistance by exploiting synergistic effects and broadening antimicrobial coverage. Despite these promising developments, majority of studies have been conducted in vitro, with a relative lack of in vivo or human research. Therefore, further investigation is needed to validate the efficacy and safety of these alternative antifungal strategies for the treatment of drug-resistant C. auris infections.
1 Introduction
Candida species remain a leading cause of opportunistic fungal infections, with mortality rates exceeding 40% in invasive candidiasis (Pfaller and Diekema, 2007; Chen et al., 2021). A comprehensive global review estimates that between 250,000 and 700,000 cases of candidemia and invasive candidiasis occur annually worldwide. The rates of candidemia in hospital settings demonstrate a consistent prevalence, with incidence rates in recent years ranging from approximately 0.022% to 0.029% of hospitalized patients (Dai et al., 2025; Mallick et al., 2025). The species of Candida most frequently identified is C. albicans; however, non-albicans species are increasingly observed. Candida infections are prevalent among critically ill and hospitalized patients, significantly contributing to global morbidity and mortality (Cornely et al., 2025; Khan et al., 2025; Mallick et al., 2025). Candida auris is a highly concerning fungus within this genus due to its notable antifungal resistance. This organism was first isolated from the ear canal of a hospitalized patient in Japan in 2009, and since that time, it has disseminated globally (Satoh et al., 2009; Eix and Nett, 2025). Under normal circumstances, C. auris is part of the human skin flora without causing infection. However, C. auris can induce bloodstream infections that may lead to invasive diseases, particularly due to the presence of medical devices and catheters, a compromised immune system, and prolonged stays in an intensive care unit (Adams et al., 2018; Park et al., 2019; Shastri et al., 2020). According to data from the Centers for Disease Control and Prevention (CDC), there were over 2,377 reported clinical cases of C. auris in the United States, with new clinical cases increasing to 4,515 in 2023 (Hayes, 2024; Bhargava et al., 2025; Mallick et al., 2025). In a five-year continuous study conducted from 2017 to 2022 in United States hospitals, the mortality rate associated with 192 cases of C. auris infection was as high as 34%. In comparison, reported mortality rates in hospitals in Europe, Pakistan, and India were 41.4%, 62.6%, and 19.6%, respectively (Chakrabarti et al., 2015; Ruiz-Gaitan et al., 2018; Sayeed et al., 2020). Moreover, in hospital mortality rates associated with C. auris bloodstream infections have been reported to range from 30% to 72% across various studies (Cortegiani et al., 2018; Chowdhary et al., 2023). In addition to the significantly high mortality rate associated with C. auris infections, another critical factor of concern is the organism’s drug resistance. According to data from the CDC, the tentative minimum inhibitory concentration (MIC) breakpoints (in μg/ml) have been established as follows: fluconazole ≥ 32, amphotericin B (AmB) ≥ 2, anidulafungin ≥ 4, caspofungin ≥ 2, and micafungin ≥ 4 (CDC, 2024). According to the antifungal susceptibility testing data obtained from the SENTRY Antifungal Surveillance Program, which collected 78 C. auris isolates from various anatomical sites of patients across different countries, it was found that 82.1% of the strains exhibited resistance to fluconazole, 17.9% demonstrated resistance to AmB, and 1.3% was resistant to caspofungin. This extensive study indicates that fluconazole resistance is highly prevalent on a global scale, while resistance to other antifungal agents, such as AmB and echinocandins, is also observed, albeit at a lower frequency (Castanheira et al., 2024). Clinical strains that exhibit resistance to three or even four classes of antifungal agents are referred to as pan-resistant C. auris (Ostrowsky et al., 2020; Jacobs et al., 2022). The CDC has classified C. auris as an urgent threat due to its rapid dissemination and frequent resistance to antifungal treatments. While resistance to echinocandins remains relatively uncommon, it is on the rise and poses significant concern. The emergence of resistance to multiple antifungal agents complicates treatment strategies and raises substantial public health issues (CDC, 2025). The recent emergence of pan-resistant C. auris strains has highlighted significant deficiencies in conventional antifungal therapies that target ergosterol biosynthesis (azoles), cell membrane integrity (polyenes), and β-glucan synthesis (echinocandins) (Forsberg et al., 2019; Lima et al., 2019; Vila et al., 2020; Vitiello et al., 2023). This therapeutic crisis has prompted the exploration of alternative strategies, including natural phenolic compounds, engineered nanoparticles, and monoclonal antibodies. Briefly, over 90% of C. albicans biofilm was inhibited by the combination of a curcumin derivative (a natural phenolic compound) and fluconazole. Most antifungal drugs have poor solubility, and engineered nanoparticles (NPs) feature a hydrophilic outer membrane and a hydrophobic core to encapsulate these poorly soluble drugs (Wu et al., 2025). Additionally, engineered NPs reduce cytotoxicity and enhance the antifungal efficacy of antifungal drugs. Maciel-Magalhaes et al. demonstrated that encapsulating amphotericin B (AmB) in polycaprolactone (PCL) and polylactic acid (PLA) polymeric NPs results in lower adverse effects compared to the free (unencapsulated) drug. This encapsulation protects non-target tissues from exposure to high concentrations of free AmB, thereby decreasing cytotoxicity and renal side effects while maintaining antifungal efficacy (Maciel-Magalhaes et al., 2025). Furthermore, research conducted by Seth et al. in 2024 highlighted that an engineered nanoformulation of AmB improved drug targetability and bioavailability, leading to increased antifungal efficacy with significantly reduced toxicity compared to traditional formulations (Seth et al., 2024). Additionally, combination therapies that leverage Food and Drug Administration (FDA)-approved antifungals alongside novel adjuvants show promise in overcoming resistance mechanisms through synergistic action. This review systematically evaluates these emerging strategies in terms of mechanistic innovation, preclinical efficacy, and clinical feasibility.
2 Novel antifungal drugs and strategies
2.1 Natural compounds as antifungal agents
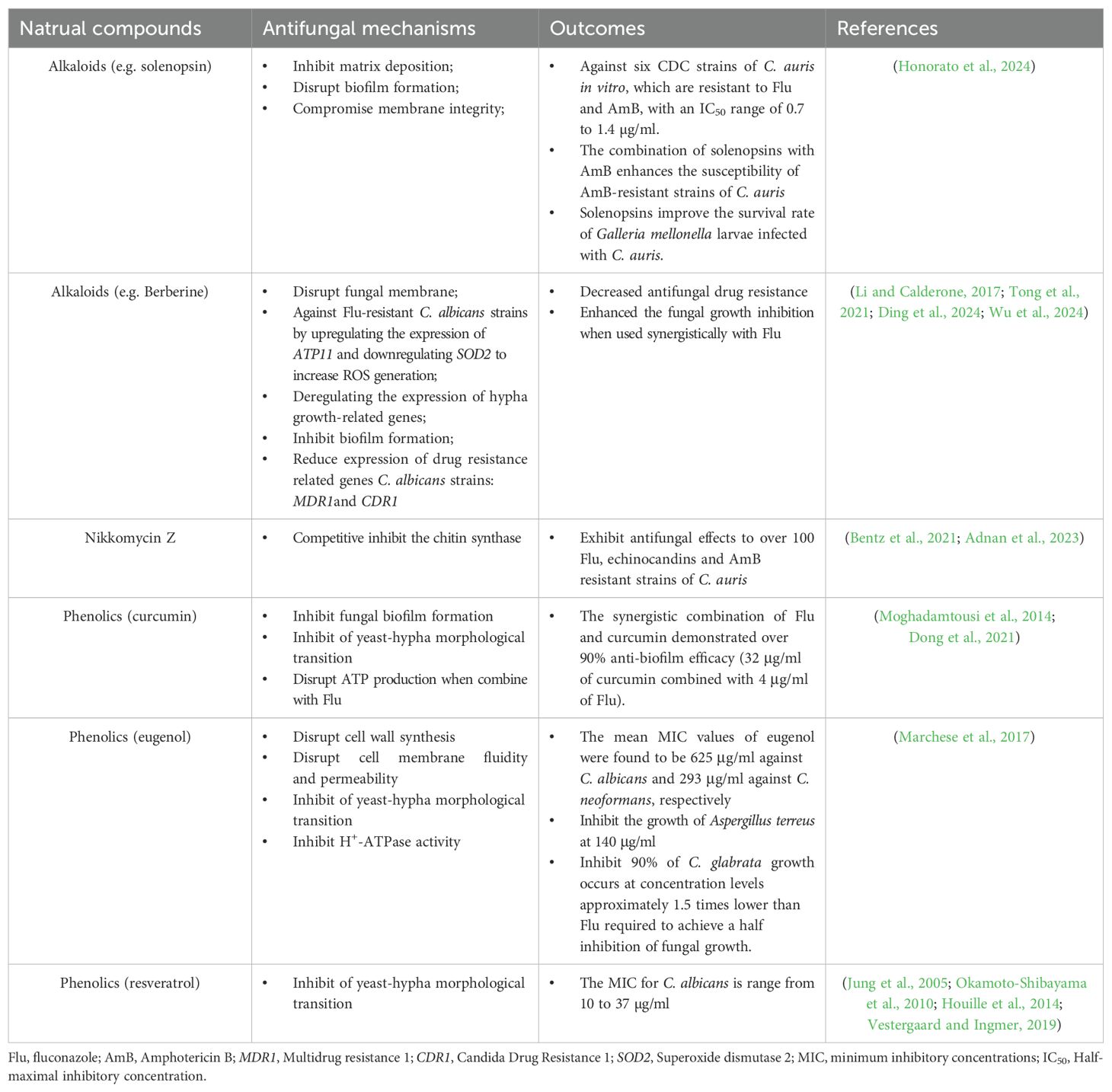
Natural products have long been a rich source of antifungal agents. Recent studies have identified novel compounds, such as terpenoids and alkaloids, with potent antifungal activity (Zacchino et al., 2017; Ganeshkumar et al., 2023; Honorato et al., 2024) (Table 1). A recent study demonstrated that a derivative of the natural compound nikkomycin Z exhibited strong inhibitory effects against C. auris and other resistant strains (Bentz et al., 2021). Although the inhibition of this competitive chitin synthase inhibitor (which hinders fungal cell wall synthesis) has only been tested in vitro, it offers a novel antifungal agent against Candida species (Adnan et al., 2023). Berberine is an alkaloid extracted from Berberis vulgaris that exhibits broad-spectrum antifungal activities through mechanisms such as membrane disruption, reactive oxygen species (ROS) generation, biofilm inhibition, and mitochondrial dysfunction (Li and Calderone, 2017; Ding et al., 2024). Recent studies have demonstrated the efficacy of this natural compound in targeting drug-resistant Candida species, with berberine showing significant potential in overcoming multidrug resistance in C. albicans by inhibiting the expression of the efflux pump MDR1 gene (Tong et al., 2021). Additionally, berberine displays remarkable antifungal efficacy against fluconazole-resistant C. albicans by upregulating the expression of the ATP11 gene and downregulating SOD2, which leads to increased ROS generation (Huang et al., 2022). By downregulating the expression of efflux pump genes CDR1 and MDR, as well as hypha growth-related genes, berberine significantly enhances the antifungal effects of fluconazole when used synergistically with this antifungal agent (Wu et al., 2024). Although berberine has been studied for its antifungal efficacy against various Candida species, such as C. albicans and C. glabrata, its effectiveness against C. auris has not been fully established (Xie et al., 2020; Gupta et al., 2023). This antifungal agent may hold great promise for treating multidrug-resistant strains of C. auris (Liu et al., 2020). Another significant family of natural compounds that demonstrate antifungal effects against drug-resistant strains is phenolics, which includes curcumin (found in turmeric), eugenol (derived from clove oil), and resveratrol (present in grapes) (Moghadamtousi et al., 2014; Marchese et al., 2017; Vestergaard and Ingmer, 2019). Interestingly, these three natural compounds exhibit distinct actions against fungi. Curcumin inhibits fungal biofilm formation, eugenol disrupts cell wall synthesis, and resveratrol interferes with inhibiting of yeast-hyphae morphological transition (Okamoto-Shibayama et al., 2010; Kerekes et al., 2013; Houille et al., 2014; Marchese et al., 2017; Dong et al., 2021; Lima et al., 2025). Although the antibacterial and antiviral properties of resveratrol have been established, its antifungal effects require further elucidation, as several studies have yielded conflicting results (Collado-Gonzalez et al., 2012). Furthermore, the molecular mechanisms underlying its antifungal activity remain inadequately investigated. Additionally, Jin’s laboratory identified a derivative of curcumin (Compound 4) that exhibits significant inhibition of biofilm formation and the yeast-to-hypha morphological transition. Furthermore, the synergistic combination of fluconazole and Compound 4 demonstrated over 90% anti-biofilm efficacy (32 μg/ml of Compound 4 combined with 4 μg/ml of fluconazole). Additionally, the use of these two antifungal agents inhibited nearly 90% of ATP production (Dong et al., 2021). Although this study focuses on fluconazole-resistant C. albicans, it offers a novel approach for utilizing curcumin to inhibit biofilm formation in C. auris. These findings underscore the diverse mechanisms and therapeutic potential of natural products—including terpenoids, alkaloids, phenolics, and chitin synthase inhibitors—as promising antifungal candidates against drug-resistant fungal pathogens.
2.2 Novel synthetic antifungal compounds
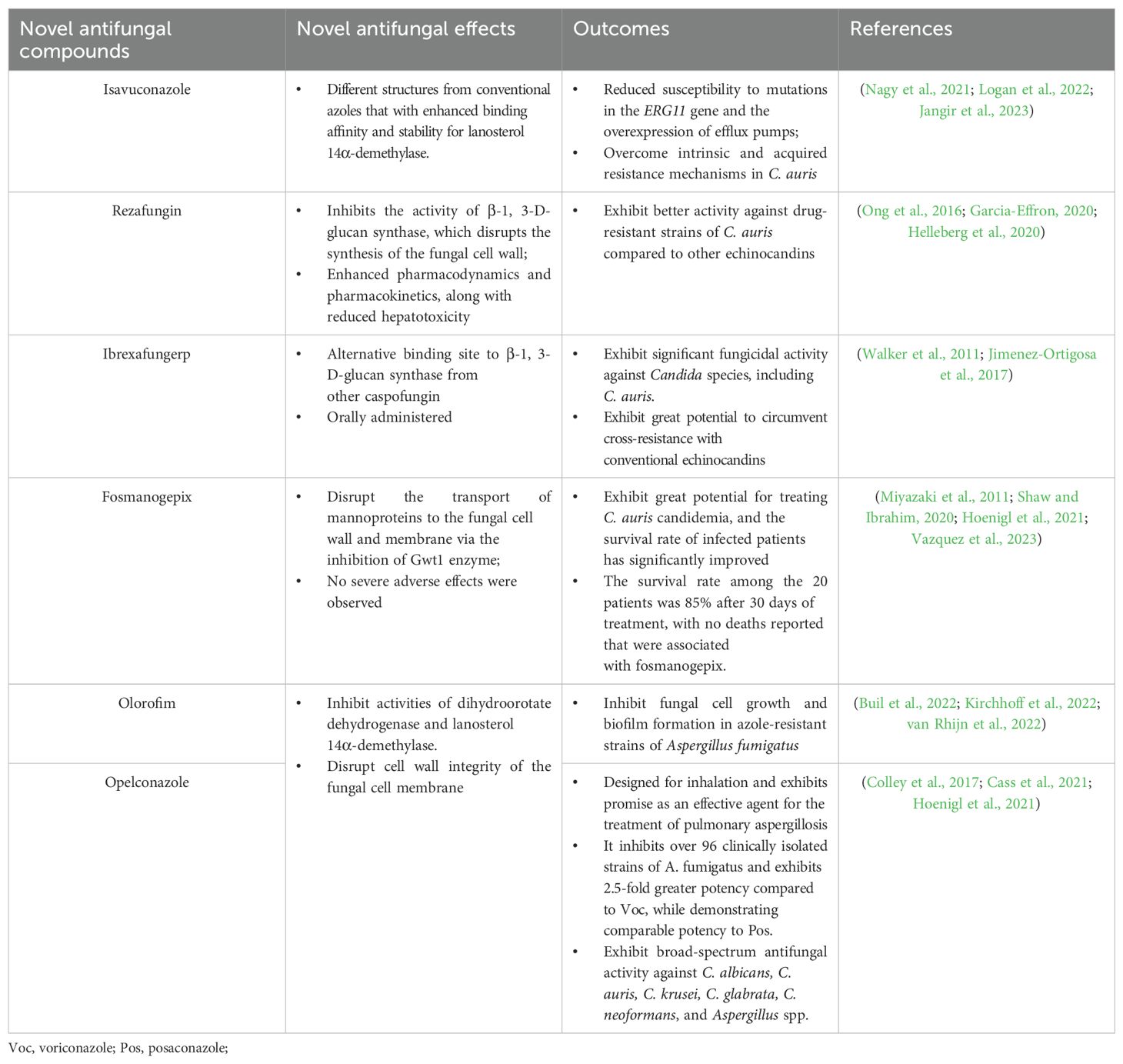
Synthetic antifungal compounds, such as azoles, polyenes, and echinocandins, are widely used to combat fungal infections. However, the prolonged administration and misuse of these drugs can lead to significant drug resistance and side effects, resulting in severe consequences. Therefore, the primary objective of the next generation of antifungal drugs is to maintain the efficacy of fungal eradication while minimizing the development of resistance as much as possible (Table 2). For example, the newer triazole, isavuconazole, is currently being investigated for its potential to overcome resistance mechanisms in C. auris (Nagy et al., 2021). Isavuconazole demonstrates the ability to overcome drug resistance in C. auris through mechanisms that are distinct from those of conventional azoles, such as fluconazole. Specifically, the unique molecular structure of isavuconazole contributes to its broader spectrum of binding affinity for lanosterol 14α-demethylase and enhances its stability, resulting in reduced susceptibility to mutations in the ERG11 gene and the overexpression of efflux pumps. These characteristics enable isavuconazole to effectively address both intrinsic and acquired resistance mechanisms in C. auris, positioning it as a promising alternative in cases where conventional antifungal agents exhibit diminished efficacy (Logan et al., 2022; Jangir et al., 2023). Moreover, there are several antifungal drugs are under investigation to overcome conventional drug resistance, including rezafungin, ibrexafungerp, fosmanogepix, olorofim, and opelconazole (Hoenigl et al., 2021; Espinel-Ingroff and Wiederhold, 2024). Rezafungin is classified to the member of echinocandins and under two phase III trials (ReSTORE, NCT03667690 and ReSPECT, NCT04368559). Rezafungin inhibits the activity of β-1, 3-D-glucan synthase, which disrupts the synthesis of the fungal cell wall (Garcia-Effron, 2020). In comparison to conventional echinocandins, such as caspofungin, rezafungin exhibits enhanced pharmacodynamics and pharmacokinetics, along with reduced hepatotoxicity (Ong et al., 2016). In in vitro tests, the results of the minimum inhibitory concentrations (MIC) demonstrated that rezafungin exhibited better activity against drug-resistant strains of C. auris compared to other echinocandins (Helleberg et al., 2020). Ibrexafungerp is a novel echinocandin that acts as a glucan synthase inhibitor and can be administered orally. This antifungal drug exhibits significant fungicidal activity against Candida species, including C. auris. Because the binding sites of ibrexafungerp on β-1, 3-D-glucan synthase are distinct from those of caspofungin, this antifungal agent shows great potential to circumvent cross-resistance with conventional echinocandins (Walker et al., 2011; Jimenez-Ortigosa et al., 2017). Fosmanogepix is another novel synthetic antifungal agent that is converted into the active form, manogepix, once administered to humans (Shaw and Ibrahim, 2020). This antifungal agent aims to inhibit the Gwt1 enzyme, which hinders the transport of mannoproteins to the fungal cell wall and membrane, thereby causing a loss of stability in fungal cells (Miyazaki et al., 2011). Clinically, this antifungal agent exhibits great potential for treating C. auris candidemia, and the survival rate of infected patients has significantly improved (Hoenigl et al., 2021). A multicenter, open-label, single-arm Phase 2 clinical trial was conducted in the United States in 2023. A total of 20 patients were recruited, and the survival rate reached 85% following 30 days of treatment, with no reported deaths related to fosmanogepix. Furthermore, no severe adverse effects were observed, indicating that this antifungal agent demonstrates promising safety and efficacy in the treatment of candidemia caused by the multidrug-resistant pathogen C. auris (Vazquez et al., 2023). The other two novel antifungal drugs are olorofim and opelconazole, which act as inhibitors of dihydroorotate dehydrogenase and lanosterol 14α-demethylase. These enzymes are crucial for maintaining the integrity of the fungal cell membrane; their inhibition ultimately leads to the suppression of fungal cell growth. Olorofim and opelconazole are newly developed antifungal drugs that belong to the dihydroorotate dehydrogenase enzyme inhibitor and triazole families, respectively. They have been optimized for oral and inhalation administration to treat infections caused by Aspergillus species (Kriegl et al., 2024). Additionally, several studies have demonstrated that olorofim possesses a strong ability to treat azole-resistant strains of Aspergillus fumigatus by inhibiting fungal cell growth and biofilm formation (Buil et al., 2022; Kirchhoff et al., 2022; van Rhijn et al., 2022). Thus, the research findings may provide novel strategies and perspectives for treating drug-resistant strains of C. auris. In conclusion, the development of next-generation antifungal agents, such as isavuconazole, rezafungin, and ibrexafungerp represents a critical strategy for balancing therapeutic effectiveness with the reduction of resistance development in challenging pathogens such as C. auris and A. fumigatus.
2.3 Biological antifungals
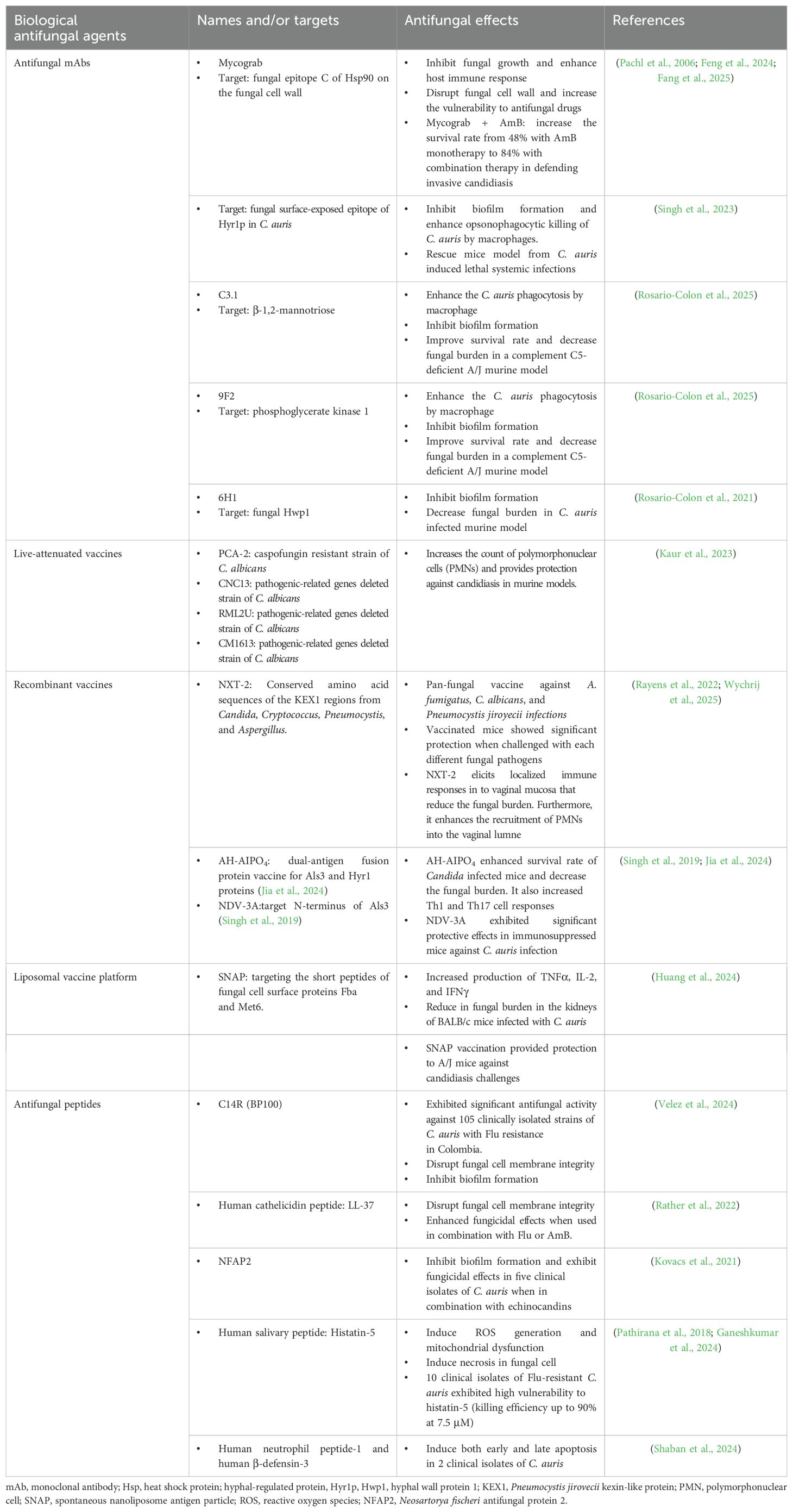
Biological antifungals, such as monoclonal antibodies (mAbs), vaccines and antifungal peptides, are emerging as novel therapeutic options (Table 3). Nowadays, fungal heat shock proteins (Hsp), particularly Hsp90, are critical for the fungal stress response and survival. They are also important targets for antifungal monoclonal antibodies (mAbs).The exposed or secreted forms of Hsp90 are recognized by these mAbs, leading to the disruption of protein function, which impedes fungal growth and enhances the host immune response (Fang et al., 2025). Antifungal mAbs, such as Mycograb, are always administered alongside conventional antifungal agents like AmB. Mycograb specifically recognizes and binds to the epitope C of Hsp90 on the fungal cell wall, thereby disrupting its function, which is critical for the fungal stress response and survival, particularly under antifungal drug pressure. When used in combination with AmB, Mycograb enhances its fungicidal effects. Additionally, the presence of Mycograb supports fungal clearance by the immune system and reduces the side effects induced by AmB due to the limited drug dosage (Feng et al., 2024). The combination of Mycograb and AmB significantly decreased the mortality rate in patients with invasive candidiasis caused by Candida infections. This is evidenced by an increase in the survival rate from 48% with AmB monotherapy to 84% with combination therapy (Pachl et al., 2006). Relying on silico protein modeling and analysis, Ashraf S. Ibrahim’s research team identified a highly immunogenic and surface-exposed epitope in C. auris (hyphal-regulated protein, Hyr1p). The monoclonal antibody (mAb) was able to recognize this conserved protein across several clinical isolates of C. auris, preventing biofilm formation and enhancing opsonophagocytic killing of C. auris by macrophages. The Hyr1p-mAb significantly reduced the fungal burden and mortality rate in mice infected with lethal C. auris (Singh et al., 2023). Other identified antifungal mAbs that demonstrate significant fungal clearance in C. auris-infected mice target β-1, 2-mannotriose, hyphal wall protein 1 (Hwp1), and phosphoglycerate kinase 1 (Rosario-Colon et al., 2021, 2025). These antifungal mAbs represent a promising therapeutic option against drug-resistant strains of C. auris.
Another immunology-based therapy for the treatment of fungal infections is vaccination, which encompasses live-attenuated vaccines, pan-fungal recombinant vaccines, subunit vaccines, and conjugate vaccines (Kaur et al., 2023). The live-attenuated vaccines utilize genetically modified strains of C. albicans to elicit host immune protection. For example, PCA-2 is a C. albicans strain that exhibits resistance to caspofungin; its administration significantly increases the count of polymorphonuclear cells (PMNs) and provides protection against candidiasis in murine models. Other live-attenuated vaccine candidates include the CNC13, RML2U, and CM1613 strains of C. albicans, which have had pathogenic-related genes deleted (Kaur et al., 2023). NXT-2 serves as a representative example of a pan-fungal recombinant vaccine, which has been developed to provide significant immune protection against invasive candidiasis. The underlying principle of pan-fungal recombinant vaccines is to target common proteins found in various fungal pathogens. For instance, mannoproteins and β-glucans are essential for maintaining cell wall integrity and are present in numerous fungal species, making them attractive targets for the design of pan-fungal vaccines. The design of NXT-2 is based on the conserved amino acid sequences of the KEX1 (kexin-like protein) regions from Candida, Cryptococcus, Pneumocystis and Aspergillus (Rayens et al., 2022). In the murine model of vulvovaginal candidiasis, NXT-2 elicits localized immune responses in the vaginal mucosa that reduce the fungal burden. Furthermore, it enhances the recruitment of PMNs into the vaginal lumen (Wychrij et al., 2025). Other recombinant vaccines associated with C. albicans target two virulent proteins, Als3 and Hyr1, which are responsible for adhesion, biofilm formation, and evasion of the host immune response. The targeting of both adhesion and immune evasion through this dual-antigen fusion protein vaccine effectively prevents Candida infection and dissemination (Jia et al., 2024). The pathogenic role of Als3 has been identified in C. auris, analogous to its role in C. albicans. The N-terminus of Als3 has been developed as a vaccine (named as NDV-3A), which has demonstrated significant protective effects in immunosuppressed mice against C. auris infection (Singh et al., 2019). In 2024, Xin’s laboratory developed a vaccine against Candida by targeting the fungal cell surface proteins Fba and Met6. Short peptides derived from these two proteins were administered to a murine model using a liposomal vaccine platform known as spontaneous nanoliposome antigen particle (SNAP). The activation of SNAP was enhanced by the interaction between cobalt porphyrin phospholipid encapsulated in liposomes and three histidine residues located at the N-terminus of the synthetic short peptide immunogens. Mice immunized with SNAP-Fba+Met6 exhibited increased production of TNFα, IL-2, and IFNγ, and demonstrated a significant reduction in fungal burden in the kidneys of BALB/c mice infected with C. auris. Additionally, the SNAP vaccination provided protection to A/J mice against candidiasis challenges (Huang et al., 2024). The determination of targets, specifically fungal proteins and epitopes, for the design of vaccines is a fundamental step, as illustrated by the aforementioned examples. A research team from Germany has developed a methodology to identify novel CD4+ T cell epitopes with potential vaccine applications against C. auris infection. This approach utilizes the analysis of genomic databases, evolutionary information, and reverses vaccinology techniques. The team screened proteins encoded by the C. auris genome and identified several promising vaccine candidates. Furthermore, this methodology enables the exclusion of highly mutated or substituted epitopes that could compromise the efficacy of the vaccine (Gupta et al., 2022). It is essential to recognize that this approach is predominantly reliant on bioinformatics analysis; consequently, candidate vaccines must undergo evaluation through animal experimentation. Currently, vaccines specifically designed to target C. auris are less common than those developed for C. albicans. In vivo experiments and preclinical studies are still insufficient; thus, the evaluation of protective vaccines for C. auris continues to be a subject of active discussion and research.
In addition to mAbs and vaccines, small cationic peptides are also classified as biological agents that demonstrate innate antifungal activity through various mechanisms. These mechanisms include membrane disruption, inhibition of β-glucan synthase leading to cell wall disruption, induction of mitochondrial dysfunction, and the generation of reactive oxygen species. Currently, C14R, LL-37, NFAP2, and histatin-5 are well-studied antifungal peptides to against Candida infections (Fernandez de Ullivarri et al., 2020; Struyfs et al., 2021; Perez-Rodriguez et al., 2022; Velez et al., 2024). To address the challenge posed by multidrug-resistant isolates of C. auris, the research team of Firacative demonstrated that an analogue peptide of BP100, known as C14R, exhibits significant antifungal activity against 105 clinically isolated strains of C. auris that are resistant to fluconazole in Colombia. This activity is attributed to the peptide’s ability to disrupt cell membrane integrity and inhibit biofilm formation. The mean MIC values for these isolated strains were found to be 5.34 μg/ml (Velez et al., 2024). The human cathelicidin LL-37 peptide, similar to C14R, disrupts the fungal cell membrane, leading to rapid cell death and cytoplasmic leakage. Additionally, this peptide exhibits enhanced antifungal effects when used in combination with fluconazole or AmB (Rather et al., 2022). Furthermore, in contrast to the anti-Candida properties exhibited by C14R and LL-37, Dermaseptin demonstrates efficacy against four strains of C. auris by the stress and apoptosis. This peptide exhibits a strong antifungal activity with a MIC of 15.62 μg/ml (Wani et al., 2024). Neosartorya fischeri antifungal protein 2 (NFAP2) is a cysteine-rich, cationic peptide that exhibits significant anti-Candida activity. In conjunction with echinocandins, NFAP2 effectively inhibits biofilm formation in five clinical isolates of C. auris (Kovacs et al., 2021). Histatin-5, a human salivary peptide, has been identified as a potent anti-Candida auris agent. This peptide enters fungal cells through energy-dependent mechanisms, allowing it to bind to mitochondria. This interaction results in ATP leakage and the subsequent production of ROS, ultimately leading to cell death (Ganeshkumar et al., 2024). In the article published by Ruvini et al. in 2018, ten clinical isolates of C. auris were treated with histatin-5. The majority of the clinical strains, including those resistant to fluconazole, exhibited high susceptibility to histatin-5, with a killing efficiency of up to 90% at a concentration of 7.5 μM (Pathirana et al., 2018). Additionally, a study investigated the antifungal properties of human neutrophil peptide-1, human β-defensin-3, and histatin-5 against two clinical strains of C. auris. The results from FITC-Annexin V/PI staining and terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) analysis indicated that human neutrophil peptide-1 and human β-defensin-3 induce both early and late apoptosis, whereas histatin-5 appears to trigger necrosis. However, it is important to note that this study was limited to only two clinical isolates and did not include in vivo experiments; thus, the full potential of these antifungal agents against C. auris may not be completely elucidated (Shaban et al., 2024). Although mAB, vaccines, and antifungal peptides have been extensively tested against various Candida species, the existing body of published studies indicates that their therapeutic efficacy against pan-resistant C. auris strains remains insufficient. Thus, further intensive in vivo and clinical studies are required to evaluate their fungicidal effects.
2.4 Nanotechnology-based antifungal strategies
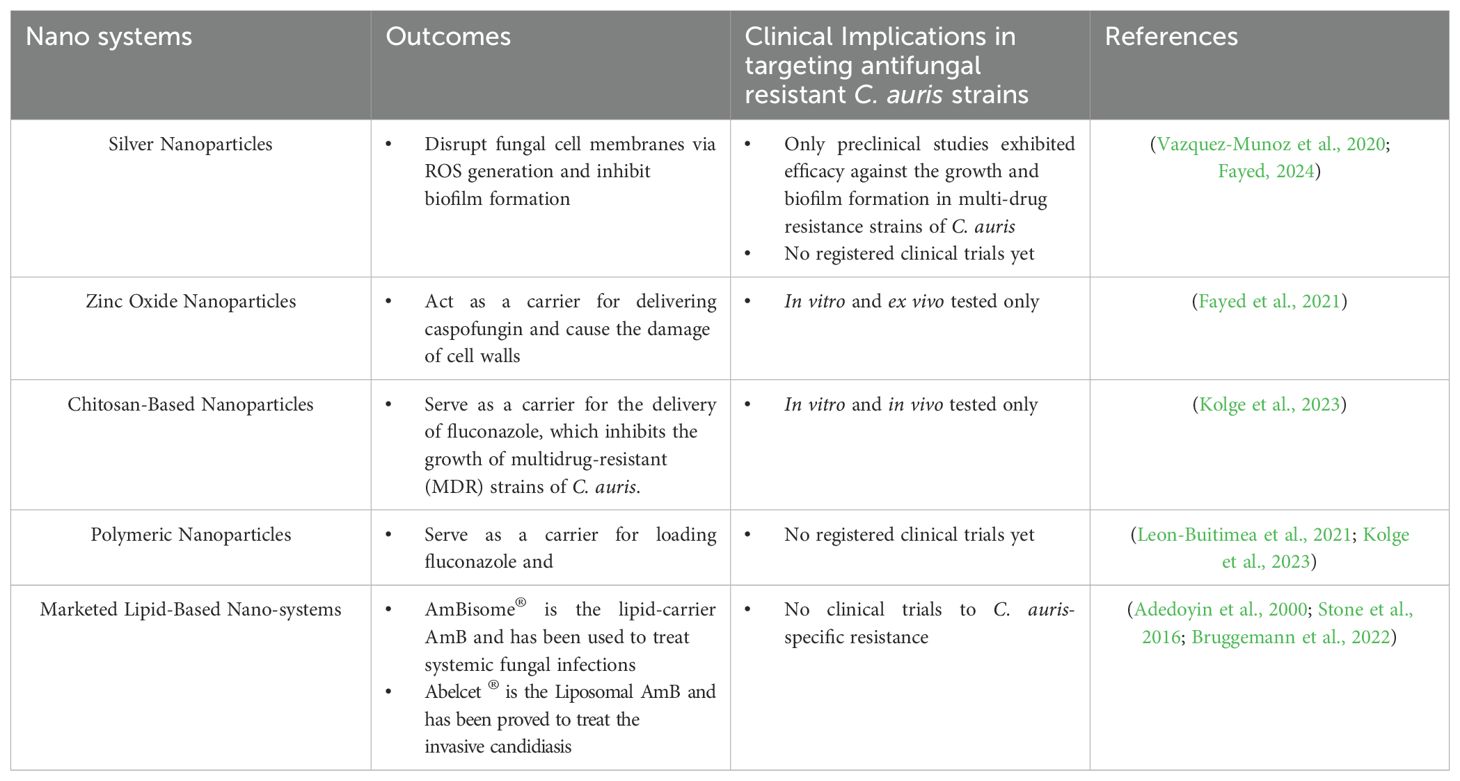
Nanotechnology has revolutionized the delivery of antifungal agents. The limited solubility of antifungal agents, such as fluconazole, which is only slightly soluble in water, significantly restricts their clinical applications due to their poor hydrophilic characteristics. As previously mentioned, various nanoparticles have been developed to carry or encapsulate antifungal drugs, thereby enhancing their bioavailability and antifungal efficacy. For instance, orally administered AmB nanoformulations from Martina Biopharma Inc. have undergone a Phase I clinical trial, demonstrating no severe side effects at oral doses of 200, 400, and 800 mg over a two-week period. These oral AmB nanoformulations have been evaluated for their ability to eliminate Cryptococcus from cerebrospinal fluid (CSF) in HIV patients and to treat vulvovaginal candidiasis (VVC) at dosages of 200 and 400 mg. However, the antifungal efficacy of these nanoformulations against C. auris and their final approval for clinical use may still necessitate further intensive research (Fairuz et al., 2022). Lipid-based nanoparticles, such as AmBisome®, are extensively utilized for intravenous administration in the treatment of infections caused by Cryptococcus, Aspergillus, and Candida species. According to the recommendations from the CDC, patients should receive a dosage of 5 mg/kg intravenously once daily for the treatment of C. auris infection, particularly in cases where there is a lack of response to echinocandin therapy or in instances of persistent fungemia lasting more than five days. Additionally, recent advances include the use of lipid nanoparticles and metal-organic frameworks (MOFs) to enhance the bioavailability and efficacy of antifungal drugs were summarized in Table 4. Targeting C. auris, nanoparticle delivery systems include lipid-based, and metal-based; both of these systems exhibited enhanced antifungal efficacy, particularly in inhibiting biofilm formation (Sutar et al., 2022; Fayed, 2024). Interestingly, one study demonstrated that silver nanoparticles exhibited fungistatic activity even without the incorporation of antifungal drugs (AlJindan and AlEraky, 2022). Moreover, zinc oxide nanoparticles serving as carriers for caspofungin demonstrated enhanced antifungal efficacy against caspofungin-resistant strains of C. auris (Fayed et al., 2021). Additionally, chitosan–PLGA (polylactide-co-glycolide) loaded with fluconazole exhibited improved drug encapsulation and prolonged release of azoles. The formulation of fluconazole loaded in chitosan-PLGA demonstrates a 64-fold increase in antifungal efficacy compared to fluconazole alone. Moreover, this fluconazole-chitosan-PLGA system showed significantly enhanced antifungal efficacy against fluconazole-resistant C. auris and C. albicans in both in vitro and in vivo models by inhibiting biofilm formation. Furthermore, the nephrotoxicity and hepatotoxicity associated with this nanoparticle-based delivery system are negligible in these nano-drug delivery systems (Kolge et al., 2023). Another critical nano-system that combats fungal infections is the lipid-based system, commonly referred to as liposomes (Marena et al., 2024). This system demonstrates significant advantages in the sustained release of drugs and enhanced bioavailability of antifungal agents. Consequently, lipid-based nano-systems have received approval from the FDA for the treatment of invasive candidiasis and aspergillosis, specifically the formulations AmBisome®, and Abelcet®, which contain amphotericin B (AmB) (Adedoyin et al., 2000; Stone et al., 2016; Bruggemann et al., 2022). Furthermore, the aforementioned marketed liposomal AmB formulations demonstrate properties that mitigate the nephrotoxicity associated with AmB in patients (Olson et al., 2008). Although these FDA-approved lipid-based nanosystems demonstrate significant antifungal efficacy against Candida and Aspergillus species, evidence regarding their fungicidal effects on drug-resistant strains of C. auris remains lacking.
2.5 Combination therapies
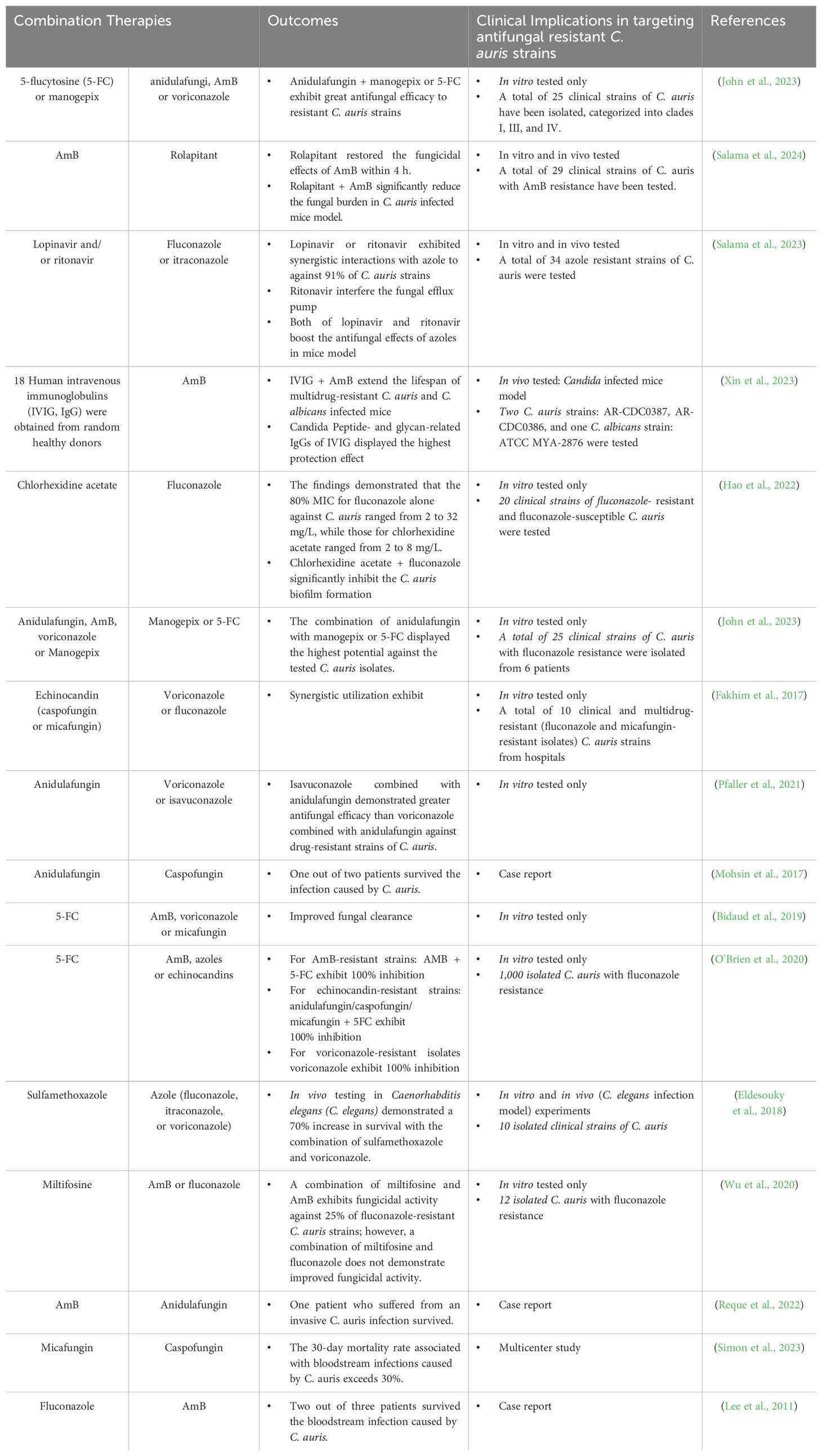
Combination therapies utilize various FDA-approved antifungal drugs to treat fungal infections, demonstrating significant efficacy in addressing resistant fungal strains (Table 5). For resistant C. auris, combination therapies have shown promise in overcoming antifungal resistance, and several clinical studies have reported positive outcomes from the combination of azoles and echinocandins (Griffith and Danziger, 2020). One study demonstrated the effectiveness of combining new drugs (diphenyl diselenide and nikkomycin Z) with currently used antifungals against C. auris. These combination therapies significantly inhibited the growth of C. auris. However, the monotherapy of these two new drugs exhibited less antifungal efficacy (Poester et al., 2022). A research team from Brown University discovered that benzodiazepines can improve the effectiveness of fluconazole and reestablish the sensitivity of azole-resistant C. albicans isolates. Notably, the exclusive application of this small molecule did not exhibit any toxic effects on fungal cells. Although this study focuses exclusively on C. albicans and does not employ animal infection models, it proposes a potential method for the synergistic use of small molecules to enhance the antifungal efficacy of traditional antifungal agents (Alabi et al., 2023). Furthermore, in addition to the combinations of two or more antifungal agents, a research team from the United States demonstrated that the synergistic use of AmB and an antiemetic drug exhibits significant anti-C. auris activities by enhancing the fungicidal activity of AmB in a very short time. They screened over 2,600 FDA-approved drugs and clinical compounds to identify rolapitant as a promoting agent that enhances antifungal effects by inducing oxidative stress, interfering with ATP production, and compromising the mitochondrial functions of C. auris (Salama et al., 2024). The same team identified that the combination of HIV protease inhibitors (lopinavir and ritonavir) with azoles (e.g., fluconazole and itraconazole) significantly reduces the burden of resistant strains of C. auris in mice (Salama et al., 2023).
2.6 Photodynamic-based therapies
Photodynamic therapy (PDT) has been widely used in cancer treatment since the 1970sand demonstrated significant efficacy in the treatment of superficial fungal infections, particularly against Candida infections. Research conducted by Hamblin et al. has focused on the application of PDT for the treatment of cutaneous infections caused by C. albicans. The study identified that the combination of methylene blue and red light (630 nm, 90 seconds per session, 4.5 J/cm²) is highly effective in reducing the fungal burden on the skin in murine models by 90%. Furthermore, this approach exhibited 100% efficacy in preventing C. albicans infections, positioning it as a rapid, non-invasive treatment strategy. Methylene blue serves as a critical photosensitizer in this synergistic approach to combat cutaneous fungal infections (Dai et al., 2011). Recently, due to the increasing prevalence of fungal infections and the rising incidence of antifungal drug-resistant cases, several research groups have sought to utilize PDT to treat C. auris infections (Bapat and Nobile, 2021). Bapat et al. investigated the antifungal effects of blue light alone, red light in conjunction with a photosensitizer, and green light combined with a photosensitizer. The results indicated that blue light alone exhibited the most significant inhibitory effect, reducing biofilm formation by 77% and disrupting mature biofilms by 57% after a 24-hour exposure, in comparison to the other two light treatments. Furthermore, the combination of blue light with photosensitizers, such as toluidine blue O, enhanced biofilm inhibition by an additional 7-22%. In the cellular and molecular level, PDT works by damaging various molecules within fungal cells through the stimulation of ROS generation. Unlike conventional antifungal agents, which target specific molecules, PDT minimizes the risk of inducing drug resistance. For instance, one study introduced the cage-modified hypocrellin as an innovative antifungal compound that demonstrates significant effectiveness against multidrug-resistant Candida species. This efficacy is underpinned by a well-defined mechanism of action, offering a promising therapeutic strategy for tackling resistant fungal infections (Liu et al., 2022). Although PDT has the potential to combat drug-resistant C. auris strains, several limitations must be acknowledged. These include the limited tissue penetration depth of activating light wavelengths, reduced efficacy against biofilm-embedded fungi due to impaired diffusion of photosensitizers, oxygen dependency in hypoxic infection sites, potential photo-toxicity to host tissues, and insufficient residual activity against fungal regrowth following treatment. These factors necessitate repeated applications, complicating clinical implementation.
3 Future directions
Despite significant progress in the development of alternative antifungal strategies for Candida auris, several critical gaps and challenges persist. The intricate nature of antifungal resistance mechanisms, combined with the organism’s capacity to thrive in various clinical settings, underscores the need for a comprehensive research agenda. Addressing the disparity between promising laboratory results and effective clinical applications will necessitate innovation in both scientific and translational domains. Several avenues for future research can be identified: 1. Application of artificial intelligence (AI) and machine learning (ML) methods for the selection of novel antifungal agents; 2. Addressing challenges in clinical translation; and 3. Enhancement of resistance monitoring and diagnostics.
AI has undergone rapid development over the past decade, significantly influencing various fields worldwide. ML, a subset of AI, employs statistical models and algorithms to analyze data. The application of AI and ML methodologies is anticipated to accelerate the discovery of novel antifungal targets and the optimization of antifungal drugs (Fu et al., 2021; Thorn and Xu, 2025). However, the transition of alternative therapeutic agents from laboratory research to clinical application is hindered by challenges such as in vivo toxicity, pharmacokinetics, formulation stability, and the regulatory approval processes for novel compounds and delivery systems. There is an urgent need for more comprehensive preclinical evaluations and the development of optimized formulation strategies. Additionally, proactive engagement with regulatory agencies is essential to clarify the approval pathways for emerging classes of antifungal agents. The development and implementation of rapid, real-time diagnostic tools for resistance profiling in clinical isolates are critical. The establishment of enhanced surveillance models will facilitate the early detection of resistance trends and support tailored therapeutic strategies (Cornely et al., 2025).
In conclusion, addressing the drug resistance of C. auris necessitates sustained innovative research into alternative therapeutic options, as well as decisive measures to overcome translational, diagnostic, and regulatory challenges. By synthesizing new scientific knowledge with cutting-edge technologies and collaborative frameworks, the field is strategically positioned to achieve significant advancements in combating this emerging threat.
4 Conclusion
Lethal infections caused by fungal pathogens pose a significant threat to human health, and the issues of antifungal resistance and the limitations of conventional antifungal therapies should not be overlooked. Among the most dangerous fungi, Candida auris has been associated with rising mortality and incidence rates worldwide. Many clinical isolates of C. auris exhibit natural resistance to antifungal drugs, including azoles, polyenes, and echinocandins. Consequently, numerous studies are focused on developing alternative antifungal strategies, which include the creation of novel antifungal drugs, the identification of natural antifungal agents, the design of nanotechnology-based drug delivery systems, and the exploration of combination therapies, among others. In this review, we have highlighted the antifungal mechanisms and advancements associated with alternative therapies. Natural antifungal compounds demonstrate fungistatic effects against various multi-drug resistant clinical strains of C. auris, suggesting that not only chemically synthesized compounds can be classified as antifungal agents. Novel synthetic antifungal compounds, such as isavuconazole, possess distinct structures that interact with lanosterol 14α-demethylase and inhibit the expression of efflux pumps in C. auris. Fosmanogepix treats candidemia by inhibiting the Gwt1 enzyme, thereby disrupting the transport of mannoproteins within the fungal cell wall and membrane. These innovative synthetic antifungal agents effectively circumvent conventional drug targets, thereby overcoming drug resistance. The antifungal mechanisms of biological agents, vaccines, peptides, and monoclonal antibodies (mAbs) rely on the host immune system to eliminate C. auris strains. Combination therapeutic strategies are frequently employed in antifungal treatments, as synergistic administration and dosage restriction of antifungal agents can reduce toxicity, enhance antifungal efficacy, and particularly mitigate the development of acquired drug resistance. Nanotechnology-based delivery systems aim to improve the bioavailability of antifungal drugs, significantly reducing the required dosage and the emergence of drug resistance. Finally, while photodynamic therapies utilize physical methods to directly eradicate C. auris and prevent drug resistance, the antifungal efficacy of these approaches for invasive candidemia requires further investigation.
While these strategies have shown promising fungistatic and fungicidal effects against clinically isolated multidrug-resistant C. auris strains, the potential antifungal effects must be rigorously tested in animal models and human subjects. Additionally, the pharmacodynamics and pharmacokinetics of these antifungal agents should be evaluated, and any potential side effects must be identified. Consequently, with the rising incidence of infections, the increased consumption of antifungal medications, and the growing issue of antifungal drug resistance, the development of alternative antifungal therapies remains an emerging area of research.
Author contributions
WD: Conceptualization, Investigation, Writing – original draft. QW: Writing – original draft. MZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by the National Key Technologies R&D Program provided by Ministry of Science and Technology of the People’s Republic of China (Project Grant # 2022YFC3602300, Sub-project Grant # 2022YFC3602302).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams, E., Quinn, M., Tsay, S., Poirot, E., Chaturvedi, S., Southwick, K., et al. (2018). Candida auris in healthcare facilities, new york, USA 2013-2017. Emerg. Infect. Dis. 24, 1816–1824. doi: 10.3201/eid2410.180649
Adedoyin, A., Swenson, C. E., Bolcsak, L. E., Hellmann, A., Radowska, D., Horwith, G., et al. (2000). A pharmacokinetic study of amphotericin B lipid complex injection (Abelcet) in patients with definite or probable systemic fungal infections. Antimicrob. Agents Chemother. 44, 2900–2902. doi: 10.1128/AAC.44.10.2900-2902.2000
Adnan, A., Borman, A. M., Toth, Z., Forgacs, L., Kovacs, R., Balazsi, D., et al. (2023). In Vitro Killing Activities of Anidulafungin and Micafungin with and without Nikkomycin Z against Four Candida auris Clades. Pharmaceutics 15 (5), 1365. doi: 10.3390/pharmaceutics15051365
Alabi, P. E., Gautier, C., Murphy, T. P., Gu, X., Lepas, M., Aimanianda, V., et al. (2023). Small molecules restore azole activity against drug-tolerant and drug-resistant Candida isolates. mBio 14, e0047923. doi: 10.1128/mbio.00479-23
AlJindan, R. and AlEraky, D. M. (2022). Silver Nanoparticles: A Promising Antifungal Agent against the Growth and Biofilm Formation of the Emergent Candida auris. J. Fungi (Basel) 8 (7), 744. doi: 10.3390/jof8070744
Bapat, P. S. and Nobile, C. J. (2021). Photodynamic therapy is effective against candida auris biofilms. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.713092
Bentz, M. L., Nunnally, N., Lockhart, S. R., Sexton, D. J., and Berkow, E. L. (2021). Antifungal activity of nikkomycin Z against Candida auris. J. Antimicrob. Chemother. 76, 1495–1497. doi: 10.1093/jac/dkab052
Bhargava, A., Klamer, K., Sharma, M., Ortiz, D., and Saravolatz, L. (2025). Candida auris: A continuing threat. Microorganisms 13 (3), 652. doi: 10.3390/microorganisms13030652
Bidaud, A. L., Botterel, F., Chowdhary, A., and Dannaoui, E. (2019). In vitro antifungal combination of flucytosine with amphotericin B, voriconazole, or micafungin against Candida auris shows no antagonism. Antimicrob. Agents Chemother. 63 (12). doi: 10.1128/AAC.01393-19
Bruggemann, R. J., Jensen, G. M., and Lass-Florl, C. (2022). Liposomal amphotericin B-the past. J. Antimicrob. Chemother. 77, ii3–ii10. doi: 10.1093/jac/dkac351
Buil, J. B., Oliver, J. D., Law, D., Baltussen, T., Zoll, J., Hokken, M. W. J., et al. (2022). Resistance profiling of Aspergillus fumigatus to olorofim indicates absence of intrinsic resistance and unveils the molecular mechanisms of acquired olorofim resistance. Emerg. Microbes Infect. 11, 703–714. doi: 10.1080/22221751.2022.2034485
Cass, L., Murray, A., Davis, A., Woodward, K., Albayaty, M., Ito, K., et al. (2021). Safety and nonclinical and clinical pharmacokinetics of PC945, a novel inhaled triazole antifungal agent. Pharmacol. Res. Perspect. 9, e00690. doi: 10.1002/prp2.690
Castanheira, M., Deshpande, L. M., Rhomberg, P. R., and Carvalhaes, C. G. (2024). Recent increase in Candida auris frequency in the SENTRY surveillance program: antifungal activity and genotypic characterization. Antimicrob. Agents Chemother. 68, e0057024. doi: 10.1128/aac.00570-24
CDC (2024). Antifungal Susceptibility Testing for C. auris. Available online at: https://www.cdc.gov/candida-auris/hcp/laboratories/antifungal-susceptibility-testing.html (Accessed April 23 2024).
CDC (2025). Antimicrobial Resistance Facts and Stats. Available online at: https://www.cdc.gov/antimicrobial-resistance/data-research/facts-stats/index.html (Accessed February 4, 2025).
Chakrabarti, A., Sood, P., Rudramurthy, S. M., Chen, S., Kaur, H., Capoor, M., et al. (2015). Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 41, 285–295. doi: 10.1007/s00134-014-3603-2
Chen, S. C., Perfect, J., Colombo, A. L., Cornely, O. A., Groll, A. H., Seidel, D., et al. (2021). Global guideline for the diagnosis and management of rare yeast infections: an initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 21, e375–e386. doi: 10.1016/S1473-3099(21)00203-6
Chowdhary, A., Jain, K., and Chauhan, N. (2023). Candida auris genetics and emergence. Annu. Rev. Microbiol. 77, 583–602. doi: 10.1146/annurev-micro-032521-015858
Collado-Gonzalez, M., Guirao-Abad, J. P., Sanchez-Fresneda, R., Belchi-Navarro, S., and Arguelles, J. C. (2012). Resveratrol lacks antifungal activity against Candida albicans. World J. Microbiol. Biotechnol. 28, 2441–2446. doi: 10.1007/s11274-012-1042-1
Colley, T., Alanio, A., Kelly, S. L., Sehra, G., Kizawa, Y., Warrilow, A. G. S., et al. (2017). In vitro and in vivo antifungal profile of a novel and long-acting inhaled azole, PC945, on aspergillus fumigatus infection. Antimicrob. Agents Chemother. 61 (5). doi: 10.1128/AAC.02280-16
Cornely, O. A., Sprute, R., Bassetti, M., Chen, S. C., Groll, A. H., Kurzai, O., et al. (2025). Global guideline for the diagnosis and management of candidiasis: an initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 25, e280–e293. doi: 10.1016/S1473-3099(24)00749-7
Cortegiani, A., Misseri, G., Fasciana, T., Giammanco, A., Giarratano, A., and Chowdhary, A. (2018). Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 6, 69. doi: 10.1186/s40560-018-0342-4
Dai, T., Bil de Arce, V. J., Tegos, G. P., and Hamblin, M. R. (2011). Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrob. Agents Chemother. 55, 5710–5717. doi: 10.1128/AAC.05404-11
Dai, Z., Lan, X., Cai, M., Liao, Y., Zhang, J., Ye, N., et al. (2025). Nineteen years retrospective analysis of epidemiology, antifungal resistance and a nomogram model for 30-day mortality in nosocomial candidemia patients. Front. Cell Infect. Microbiol. 15. doi: 10.3389/fcimb.2025.1504866
Ding, J., Yan, Z., Peng, L., Li, J., Yang, F., and Zheng, D. (2024). Inhibitory effects of berberine on fungal growth, biofilm formation, virulence, and drug resistance as an antifungal drug and adjuvant with prospects for future applications. World J. Microbiol. Biotechnol. 41, 5. doi: 10.1007/s11274-024-04223-4
Dong, H. H., Wang, Y. H., Peng, X. M., Zhou, H. Y., Zhao, F., Jiang, Y. Y., et al. (2021). Synergistic antifungal effects of curcumin derivatives as fungal biofilm inhibitors with fluconazole. Chem. Biol. Drug Des. 97, 1079–1088. doi: 10.1111/cbdd.13827
Eix, E. F. and Nett, J. E. (2025). Candida auris: epidemiology and antifungal strategy. Annu. Rev. Med. 76, 57–67. doi: 10.1146/annurev-med-061523-021233
Eldesouky, H. E., Li, X., Abutaleb, N. S., Mohammad, H., and Seleem, M. N. (2018). Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. Int. J. Antimicrob. Agents 52, 754–761. doi: 10.1016/j.ijantimicag.2018.08.016
Espinel-Ingroff, A. and Wiederhold, N. P. (2024). A Mini-Review of In Vitro Data for Candida Species, Including C. auris, Isolated during Clinical Trials of Three New Antifungals: Fosmanogepix, Ibrexafungerp, and Rezafungin. J. Fungi (Basel) 10 (5). doi: 10.3390/jof10050362
Fairuz, S., Nair, R. S., and Billa, N. (2022). Orally administered amphotericin B nanoformulations: physical properties of nanoparticle carriers on bioavailability and clinical relevance. Pharmaceutics 14 (9). doi: 10.3390/pharmaceutics14091823
Fakhim, H., Chowdhary, A., Prakash, A., Vaezi, A., Dannaoui, E., Meis, J. F., et al. (2017). In Vitro Interactions of Echinocandins with Triazoles against Multidrug-Resistant Candida auris. Antimicrob. Agents Chemother. 61 (11). doi: 10.1128/AAC.01056-17
Fang, T., Lu, H., and Jiang, Y. (2025). Extracellular fungal Hsp90 represents a promising therapeutic target for combating fungal infections. Eur. J. Pharm. Sci. 207, 107041. doi: 10.1016/j.ejps.2025.107041
Fayed, B. (2024). Nanoparticles in the battle against Candida auris biofilms: current advances and future prospects. Drug Delivery Transl. Res. 15, 1496–1512. doi: 10.1007/s13346-024-01749-w
Fayed, B., Jayakumar, M. N., and Soliman, S. S. M. (2021). Caspofungin-resistance in Candida auris is cell wall-dependent phenotype and potential prevention by zinc oxide nanoparticles. Med. Mycol. 59, 1243–1256. doi: 10.1093/mmy/myab059
Feng, Z., Lu, H., and Jiang, Y. (2024). Promising immunotherapeutic targets for treating candidiasis. Front. Cell Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1339501
Fernandez de Ullivarri, M., Arbulu, S., Garcia-Gutierrez, E., and Cotter, P. D. (2020). Antifungal peptides as therapeutic agents. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00105
Forsberg, K., Woodworth, K., Walters, M., Berkow, E. L., Jackson, B., Chiller, T., et al. (2019). Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 57, 1–12. doi: 10.1093/mmy/myy054
Fu, C., Zhang, X., Veri, A. O., Iyer, K. R., Lash, E., Xue, A., et al. (2021). Leveraging machine learning essentiality predictions and chemogenomic interactions to identify antifungal targets. Nat. Commun. 12, 6497. doi: 10.1038/s41467-021-26850-3
Ganeshkumar, A., Goncale, J. C., Rajaram, R., and Junqueira, J. C. (2023). Anti-candidal marine natural products: A review. J. Fungi (Basel) 9 (8). doi: 10.3390/jof9080800
Ganeshkumar, A., Muthuselvam, M., Lima, P. M. N., Rajaram, R., and Junqueira, J. C. (2024). Current perspectives of antifungal therapy: A special focus on candida auris. J. Fungi (Basel) 10 (6). doi: 10.3390/jof10060408
Garcia-Effron, G. (2020). Rezafungin-mechanisms of action, susceptibility and resistance: similarities and differences with the other echinocandins. J. Fungi (Basel) 6 (4). doi: 10.3390/jof6040262
Griffith, N. and Danziger, L. (2020). Candida auris urinary tract infections and possible treatment. Antibiot. (Basel) 9 (12). doi: 10.3390/antibiotics9120898
Gupta, P., Gupta, H., Tripathi, S., and Poluri, K. M. (2023). Biochemical and metabolomic insights into antifungal mechanism of berberine against Candida glabrata. Appl. Microbiol. Biotechnol. 107, 6085–6102. doi: 10.1007/s00253-023-12714-x
Gupta, S. K., Osmanoglu, O., Minocha, R., Bandi, S. R., Bencurova, E., Srivastava, M., et al. (2022). Genome-wide scan for potential CD4+ T-cell vaccine candidates in Candida auris by exploiting reverse vaccinology and evolutionary information. Front. Med. (Lausanne) 9. doi: 10.3389/fmed.2022.1008527
Hao, W., Wang, Y., Xi, Y., Yang, Z., Zhang, H., and Ge, X. (2022). Activity of chlorhexidine acetate in combination with fluconazole against suspensions and biofilms of Candida auris. J. Infect. Chemother. 28, 29–34. doi: 10.1016/j.jiac.2021.09.018
Hayes, J. F. (2024). Candida auris: epidemiology update and a review of strategies to prevent spread. J. Clin. Med. 13 (22). doi: 10.3390/jcm13226675
Helleberg, M., Jorgensen, K. M., Hare, R. K., Datcu, R., Chowdhary, A., and Arendrup, M. C. (2020). Rezafungin In Vitro Activity against Contemporary Nordic Clinical Candida Isolates and Candida auris Determined by the EUCAST Reference Method. Antimicrob. Agents Chemother. 64 (4). doi: 10.1128/AAC.02438-19
Hoenigl, M., Sprute, R., Egger, M., Arastehfar, A., Cornely, O. A., Krause, R., et al. (2021). The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 81, 1703–1729. doi: 10.1007/s40265-021-01611-0
Honorato, L., Artunduaga Bonilla, J. J., Ribeiro da Silva, L., Kornetz, J., Zamith-Miranda, D., Valdez, A. F., et al. (2024). Alkaloids solenopsins from fire ants display in vitro and in vivo activity against the yeast Candida auris. Virulence 15, 2413329. doi: 10.1080/21505594.2024.2413329
Houille, B., Papon, N., Boudesocque, L., Bourdeaud, E., Besseau, S., Courdavault, V., et al. (2014). Antifungal activity of resveratrol derivatives against Candida species. J. Nat. Prod. 77, 1658–1662. doi: 10.1021/np5002576
Huang, W. C., Eberle, K., Colon, J. R., Lovell, J. F., and Xin, H. (2024). Liposomal Fba and Met6 peptide vaccination protects mice from disseminated candidiasis. mSphere 9 (6), e0018924. doi: 10.1128/msphere.00189-24
Huang, X., Zheng, D., Yong, J., and Li, Y. (2022). Antifungal activity and potential mechanism of berberine hydrochloride against fluconazole-resistant Candida albicans. J. Med. Microbiol. 71. doi: 10.1099/jmm.0.001542
Jacobs, S. E., Jacobs, J. L., Dennis, E. K., Taimur, S., Rana, M., Patel, D., et al. (2022). Candida auris pan-drug-resistant to four classes of antifungal agents. Antimicrob. Agents Chemother. 66, e0005322. doi: 10.1128/aac.00053-22
Jangir, P., Kalra, S., Tanwar, S., and Bari, V. K. (2023). Azole resistance in Candida auris: mechanisms and combinatorial therapy. APMIS 131, 442–462. doi: 10.1111/apm.13336
Jia, K., Zhang, Y., Jiang, M., Cui, M., Wang, J., Zhang, J., et al. (2024). Dual-antigen fusion protein vaccination induces protective immunity against Candida albicans infection in mice. Hum. Vaccin. Immunother. 20, 2406065. doi: 10.1080/21645515.2024.2406065
Jimenez-Ortigosa, C., Perez, W. B., Angulo, D., Borroto-Esoda, K., and Perlin, D. S. (2017). De Novo Acquisition of Resistance to SCY-078 in Candida glabrata Involves FKS Mutations That both Overlap and Are Distinct from Those Conferring Echinocandin Resistance. Antimicrob. Agents Chemother. 61 (9). doi: 10.1128/AAC.00833-17
John, L. L. H., Thomson, D. D., Bicanic, T., Hoenigl, M., Brown, A. J. P., Harrison, T. S., et al. (2023). Heightened Efficacy of Anidulafungin When Used in Combination with Manogepix or 5-Flucytosine against Candida auris In Vitro. Antimicrob. Agents Chemother. 67, e0164522. doi: 10.1128/aac.01645-22
Jung, H. J., Hwang, I. A., Sung, W. S., Kang, H., Kang, B. S., Seu, Y. B., et al. (2005). Fungicidal effect of resveratrol on human infectious fungi. Arch. Pharm. Res. 28, 557–560. doi: 10.1007/BF02977758
Kaur, G., Chawla, S., Kumar, P., and Singh, R. (2023). Advancing vaccine strategies against candida infections: exploring new frontiers. Vaccines (Basel) 11 (11). doi: 10.3390/vaccines11111658
Kerekes, E. B., Deak, E., Tako, M., Tserennadmid, R., Petkovits, T., Vagvolgyi, C., et al. (2013). Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 115, 933–942. doi: 10.1111/jam.12289
Khan, S., Cai, L., Bilal, H., Khan, M. N., Fang, W., Zhang, D., et al. (2025). An 11-Year retrospective analysis of candidiasis epidemiology, risk factors, and antifungal susceptibility in a tertiary care hospital in China. Sci. Rep. 15, 7240. doi: 10.1038/s41598-025-92100-x
Kirchhoff, L., Dittmer, S., Furnica, D. T., Buer, J., Steinmann, E., Rath, P. M., et al. (2022). Inhibition of azole-resistant Aspergillus fumigatus biofilm at various formation stages by antifungal drugs, including olorofim. J. Antimicrob. Chemother. 77, 1645–1654. doi: 10.1093/jac/dkac062
Kolge, H., Patil, G., Jadhav, S., and Ghormade, V. (2023). A pH-tuned chitosan-PLGA nanocarrier for fluconazole delivery reduces toxicity and improves efficacy against resistant Candida. Int. J. Biol. Macromol. 227, 453–461. doi: 10.1016/j.ijbiomac.2022.12.139
Kovacs, R., Nagy, F., Toth, Z., Forgacs, L., Toth, L., Varadi, G., et al. (2021). The Neosartorya fischeri Antifungal Protein 2 (NFAP2): A New Potential Weapon against Multidrug-Resistant Candida auris Biofilms. Int. J. Mol. Sci. 22 (2). doi: 10.3390/ijms22020771
Kriegl, L., Egger, M., Boyer, J., Hoenigl, M., and Krause, R. (2024). New treatment options for critically important WHO fungal priority pathogens. Clin. Microbiol. Infect. 31 (6), 922–930. doi: 10.1016/j.cmi.2024.03.006
Lee, W. G., Shin, J. H., Uh, Y., Kang, M. G., Kim, S. H., Park, K. H., et al. (2011). First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 49, 3139–3142. doi: 10.1128/JCM.00319-11
Leon-Buitimea, A., Garza-Cervantes, J. A., Gallegos-Alvarado, D. Y., Osorio-Concepcion, M., and Morones-Ramirez, J. R. (2021). Nanomaterial-based antifungal therapies to combat fungal diseases aspergillosis, coccidioidomycosis, mucormycosis, and candidiasis. Pathogens 10 (10). doi: 10.3390/pathogens10101303
Li, D. and Calderone, R. (2017). Exploiting mitochondria as targets for the development of new antifungals. Virulence 8, 159–168. doi: 10.1080/21505594.2016.1188235
Lima, S. L., Colombo, A. L., and de Almeida Junior, J. N. (2019). Fungal cell wall: emerging antifungals and drug resistance. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02573
Lima, E. M. F., de Almeida, F. A., and Pinto, U. M. (2025). Exploring the antivirulence potential of phenolic compounds to inhibit quorum sensing in Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 41, 32. doi: 10.1007/s11274-025-04255-4
Liu, X., Fang, R., Feng, R., Li, Q., Su, M., Hou, C., et al. (2022). Cage-modified hypocrellin against multidrug-resistant Candida spp. with unprecedented activity in light-triggered combinational photodynamic therapy. Drug Resist. Update 65, 100887. doi: 10.1016/j.drup.2022.100887
Liu, J., Li, Q., Wang, C., Shao, J., Wang, T., Wu, D., et al. (2020). Antifungal evaluation of traditional herbal monomers and their potential for inducing cell wall remodeling in Candida albicans and Candida auris. Biofouling 36, 319–331. doi: 10.1080/08927014.2020.1759559
Logan, A., Wolfe, A., and Williamson, J. C. (2022). Antifungal resistance and the role of new therapeutic agents. Curr. Infect. Dis. Rep. 24, 105–116. doi: 10.1007/s11908-022-00782-5
Maciel-Magalhaes, M., Medeiros, R. J., Guedes, N., Brito, T. M., Souza, G. F., Canabarro, B. R., et al. (2025). Amphotericin B encapsulation in polymeric nanoparticles: toxicity insights via cells and zebrafish embryo testing. Pharmaceutics 17 (1). doi: 10.3390/pharmaceutics17010116
Mallick, D. C., Kaushik, N., Goyal, L., Mallick, L., and Singh, P. (2025). A comprehensive review of candidemia and invasive candidiasis in adults: focus on the emerging multidrug-resistant fungus candida auris. Diseases 13 (4). doi: 10.3390/diseases13040093
Marchese, A., Barbieri, R., Coppo, E., Orhan, I. E., Daglia, M., Nabavi, S. F., et al. (2017). Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 43, 668–689. doi: 10.1080/1040841X.2017.1295225
Marena, G. D., Ruiz-Gaitan, A., Bauab, T. M., and Chorilli, M. (2024). Improving antifungal lipid-based drug delivery against Candida: a review. Expert Opin. Drug Delivery 12 (6), 1–15. doi: 10.1080/17425247.2024.2421402
Miyazaki, M., Horii, T., Hata, K., Watanabe, N. A., Nakamoto, K., Tanaka, K., et al. (2011). In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents Chemother. 55, 4652–4658. doi: 10.1128/AAC.00291-11
Moghadamtousi, S. Z., Kadir, H. A., Hassandarvish, P., Tajik, H., Abubakar, S., and Zandi, K. (2014). A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed. Res. Int. 2014, 186864. doi: 10.1155/2014/186864
Mohsin, J., Hagen, F., Al-Balushi, Z. A. M., de Hoog, G. S., Chowdhary, A., Meis, J. F., et al. (2017). The first cases of Candida auris candidaemia in Oman. Mycoses 60, 569–575. doi: 10.1111/myc.12647
Nagy, F., Toth, Z., Nyikos, F., Forgacs, L., Jakab, A., Borman, A. M., et al. (2021). In vitro and in vivo interaction of caspofungin with isavuconazole against Candida auris planktonic cells and biofilms. Med. Mycol. 59, 1015–1023. doi: 10.1093/mmy/myab032
O’Brien, B., Chaturvedi, S., and Chaturvedi, V. (2020). In Vitro Evaluation of Antifungal Drug Combinations against Multidrug-Resistant Candida auris Isolates from New York Outbreak. Antimicrob. Agents Chemother. 64 (4). doi: 10.1128/AAC.02195-19
Okamoto-Shibayama, K., Sato, Y., and Azuma, T. (2010). Resveratrol impaired the morphological transition of Candida albicans under various hyphae-inducing conditions. J. Microbiol. Biotechnol. 20, 942–945. doi: 10.4014/jmb.0911.11014
Olson, J. A., Adler-Moore, J. P., Jensen, G. M., Schwartz, J., Dignani, M. C., and Proffitt, R. T. (2008). Comparison of the physicochemical, antifungal, and toxic properties of two liposomal amphotericin B products. Antimicrob. Agents Chemother. 52, 259–268. doi: 10.1128/AAC.00870-07
Ong, V., Hough, G., Schlosser, M., Bartizal, K., Balkovec, J. M., James, K. D., et al. (2016). Preclinical evaluation of the stability, safety, and efficacy of CD101, a novel echinocandin. Antimicrob. Agents Chemother. 60, 6872–6879. doi: 10.1128/AAC.00701-16
Ostrowsky, B., Greenko, J., Adams, E., Quinn, M., O’Brien, B., Chaturvedi, V., et al. (2020). Candida auris isolates resistant to three classes of antifungal medications - new yor. MMWR Morb. Mortal. Wkly Rep. 69, 6–9. doi: 10.15585/mmwr.mm6901a2
Pachl, J., Svoboda, P., Jacobs, F., Vandewoude, K., van der Hoven, B., Spronk, P., et al. (2006). A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin. Infect. Dis. 42, 1404–1413. doi: 10.1086/503428
Park, J. Y., Bradley, N., Brooks, S., Burney, S., and Wassner, C. (2019). Management of Patients with Candida auris Fungemia at Community Hospital, Brooklyn, New York, USA 2016-2018(1). Emerg. Infect. Dis. 25, 601–602. doi: 10.3201/eid2503.180927
Pathirana, R. U., Friedman, J., Norris, H. L., Salvatori, O., McCall, A. D., Kay, J., et al. (2018). Fluconazole-resistant candida auris is susceptible to salivary histatin 5 killing and to intrinsic host defenses. Antimicrob. Agents Chemother. 62 (2). doi: 10.1128/AAC.01872-17
Perez-Rodriguez, A., Eraso, E., Quindos, G., and Mateo, E. (2022). Antimicrobial peptides with anti-candida activity. Int. J. Mol. Sci. 23 (16). doi: 10.3390/ijms23169264
Pfaller, M. A. and Diekema, D. J. (2007). Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20, 133–163. doi: 10.1128/CMR.00029-06
Pfaller, M. A., Messer, S. A., Deshpande, L. M., Rhomberg, P. R., Utt, E. A., and Castanheira, M. (2021). Evaluation of Synergistic Activity of Isavuconazole or Voriconazole plus Anidulafungin and the Occurrence and Genetic Characterization of Candida auris Detected in a Surveillance Program. Antimicrob. Agents Chemother. 65 (4). doi: 10.1128/AAC.02031-20
Poester, V. R., Munhoz, L. S., Benelli, J. L., Melo, A. M., Al-Hatmi, A. M. S., Larwood, D. J., et al. (2022). Initial Results of the International Efforts in Screening New Agents against Candida auris. J. Fungi (Basel) 8 (8). doi: 10.3390/jof8080771
Rather, I. A., Sabir, J. S. M., Asseri, A. H., and Ali, S. (2022). Antifungal activity of human cathelicidin LL-37, a membrane disrupting peptide, by triggering oxidative stress and cell cycle arrest in candida auris. J. Fungi (Basel) 8 (2). doi: 10.3390/jof8020204
Rayens, E., Rabacal, W., Willems, H. M. E., Kirton, G. M., Barber, J. P., Mousa, J. J., et al. (2022). Immunogenicity and protective efficacy of a pan-fungal vaccine in preclinical models of aspergillosis, candidiasis, and pneumocystosis. PNAS Nexus 1, pgac248. doi: 10.1093/pnasnexus/pgac248
Reque, J., Arlandis, R., Panizo, N., Pascual, M. J., and Perez-Alba, A. (2022). Candida auris Invasive Infection after Kidney Transplantation. Case Rep. Nephrol. 2022, 6007607. doi: 10.1155/2022/6007607
Rosario-Colon, J., Eberle, K., Adams, A., Courville, E., and Xin, H. (2021). Candida Cell-Surface-Specific Monoclonal Antibodies Protect Mice against Candida auris Invasive Infection. Int. J. Mol. Sci. 22 (11). doi: 10.3390/ijms22116162
Rosario-Colon, J., Eberle, K., and Xin, H. (2025). Monoclonal antibodies targeting Candida disrupt biofilms and inhibit growth across global clinical isolates. iScience 28, 112459. doi: 10.1016/j.isci.2025.112459
Ruiz-Gaitan, A., Moret, A. M., Tasias-Pitarch, M., Aleixandre-Lopez, A. I., Martinez-Morel, H., Calabuig, E., et al. (2018). An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 61, 498–505. doi: 10.1111/myc.12781
Salama, E. A., Eldesouky, H. E., Elgammal, Y., Abutaleb, N. S., and Seleem, M. N. (2023). Lopinavir and ritonavir act synergistically with azoles against Candida auris in vitro and in a mouse model of disseminated candidiasis. Int. J. Antimicrob. Agents 62, 106906. doi: 10.1016/j.ijantimicag.2023.106906
Salama, E. A., Elgammal, Y., Utturkar, S. M., Lanman, N. A., Hazbun, T. R., and Seleem, M. N. (2024). Overcoming amphotericin B resistance in Candida auris using the antiemetic drug rolapitant. Antimicrob. Agents Chemother. 68, e0055624. doi: 10.1128/aac.00556-24
Satoh, K., Makimura, K., Hasumi, Y., Nishiyama, Y., Uchida, K., and Yamaguchi, H. (2009). Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 53, 41–44. doi: 10.1111/j.1348-0421.2008.00083.x
Sayeed, M. A., Farooqi, J., Jabeen, K., and Mahmood, S. F. (2020). Comparison of risk factors and outcomes of Candida auris candidemia with non-Candida auris candidemia: A retrospective study from Pakistan. Med. Mycol. 58, 721–729. doi: 10.1093/mmy/myz112
Seth, R., Meena, A., Gosai, A., Imam, M. W., Meena, R., and Luqman, S. (2024). Novel nanoformulation for enhanced amphotericin B efficacy and sustained release using vetiver root cellulose nanofibers against Candida albicans. Int. J. Biol. Macromol. 282, 136555. doi: 10.1016/j.ijbiomac.2024.136555
Shaban, S., Patel, M., and Ahmad, A. (2024). Antifungal activity of human antimicrobial peptides targeting apoptosis in Candida auris. J. Med. Microbiol. 73 (5). doi: 10.1099/jmm.0.001835
Shastri, P. S., Shankarnarayan, S. A., Oberoi, J., Rudramurthy, S. M., Wattal, C., and Chakrabarti, A. (2020). Candida auris candidaemia in an intensive care unit - Prospective observational study to evaluate epidemiology, risk factors, and outcome. J. Crit. Care 57, 42–48. doi: 10.1016/j.jcrc.2020.01.004
Shaw, K. J. and Ibrahim, A. S. (2020). Fosmanogepix: A review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J. Fungi (Basel) 6 (4). doi: 10.3390/jof6040239
Simon, S. P., Li, R., Silver, M., Andrade, J., Tharian, B., Fu, L., et al. (2023). Comparative outcomes of candida auris bloodstream infections: A multicenter retrospective case-control study. Clin. Infect. Dis. 76, e1436–e1443. doi: 10.1093/cid/ciac735
Singh, S., Barbarino, A., Youssef, E. G., Coleman, D., Gebremariam, T., and Ibrahim, A. S. (2023). Protective Efficacy of Anti-Hyr1p Monoclonal Antibody against Systemic Candidiasis Due to Multi-Drug-Resistant Candida auris. J. Fungi (Basel) 9 (1). doi: 10.3390/jof9010103
Singh, S., Uppuluri, P., Mamouei, Z., Alqarihi, A., Elhassan, H., French, S., et al. (2019). The NDV-3A vaccine protects mice from multidrug resistant Candida auris infection. PloS Pathog. 15, e1007460. doi: 10.1371/journal.ppat.1007460
Stone, N. R., Bicanic, T., Salim, R., and Hope, W. (2016). Liposomal amphotericin B (AmBisome((R))): A review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 76, 485–500. doi: 10.1007/s40265-016-0538-7
Struyfs, C., Cammue, B. P. A., and Thevissen, K. (2021). Membrane-interacting antifungal peptides. Front. Cell Dev. Biol. 9. doi: 10.3389/fcell.2021.649875
Sutar, Y., Nabeela, S., Singh, S., Alqarihi, A., Solis, N., Ghebremariam, T., et al. (2022). Niclosamide-loaded nanoparticles disrupt Candida biofilms and protect mice from mucosal candidiasis. PloS Biol. 20, e3001762. doi: 10.1371/journal.pbio.3001762
Thorn, V. and Xu, J. (2025). From patterns to prediction: machine learning and antifungal resistance biomarker discovery. Can. J. Microbiol. 71, 1–13. doi: 10.1139/cjm-2024-0248
Tong, Y., Zhang, J., Sun, N., Wang, X. M., Wei, Q., Zhang, Y., et al. (2021). Berberine reverses multidrug resistance in Candida albicans by hijacking the drug efflux pump Mdr1p. Sci. Bull. (Beijing) 66, 1895–1905. doi: 10.1016/j.scib.2020.12.035
van Rhijn, N., Hemmings, S., Storer, I. S. R., Valero, C., Bin Shuraym, H., Goldman, G. H., et al. (2022). Antagonism of the azoles to olorofim and cross-resistance are governed by linked transcriptional networks in aspergillus fumigatus. mBio 13, e0221522. doi: 10.1128/mbio.02215-22
Vazquez, J. A., Pappas, P. G., Boffard, K., Paruk, F., Bien, P. A., Tawadrous, M., et al. (2023). Clinical efficacy and safety of a novel antifungal, fosmanogepix, in patients with candidemia caused by candida auris: results from a phase 2 trial. Antimicrob. Agents Chemother. 67, e0141922. doi: 10.1128/aac.01419-22
Vazquez-Munoz, R., Lopez, F. D., and Lopez-Ribot, J. L. (2020). Silver nanoantibiotics display strong antifungal activity against the emergent multidrug-resistant yeast candida auris under both planktonic and biofilm growing conditions. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.01673
Velez, N., Argel, A., Kissmann, A. K., Alpizar-Pedraza, D., Escandon, P., Rosenau, F., et al. (2024). Pore-forming peptide C14R exhibits potent antifungal activity against clinical isolates of Candida albicans and Candida auris. Front. Cell Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1389020
Vestergaard, M. and Ingmer, H. (2019). Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 53, 716–723. doi: 10.1016/j.ijantimicag.2019.02.015
Vila, T., Sultan, A. S., Montelongo-Jauregui, D., and Jabra-Rizk, M. A. (2020). Candida auris: a fungus with identity crisis. Pathog. Dis. 78 (4), ftaa034. doi: 10.1093/femspd/ftaa034
Vitiello, A., Ferrara, F., Boccellino, M., Ponzo, A., Cimmino, C., Comberiati, E., et al. (2023). Antifungal drug resistance: an emergent health threat. Biomedicines 11 (4). doi: 10.3390/biomedicines11041063
Walker, S. S., Xu, Y., Triantafyllou, I., Waldman, M. F., Mendrick, C., Brown, N., et al. (2011). Discovery of a novel class of orally active antifungal beta-1,3-D-glucan synthase inhibitors. Antimicrob. Agents Chemother. 55, 5099–5106. doi: 10.1128/AAC.00432-11
Wani, M. Y., Srivastava, V., Saleh, T. S., Al-Bogami, A. S., Aqlan, F. M., and Ahmad, A. (2024). Regulation of oxidative stress enzymes in Candida auris by Dermaseptin: potential implications for antifungal drug discovery. RSC Adv. 14, 36886–36894. doi: 10.1039/d4ra06392a
Wu, Q., Cen, F., Xie, Y., Ning, X., Wang, J., Lin, Z., et al. (2025). Nanoparticle-based antifungal therapies innovations mechanisms and future prospects. PeerJ 13, e19199. doi: 10.7717/peerj.19199
Wu, S., Jia, W., Lu, Y., Jiang, H., Huang, C., Tang, S., et al. (2024). Mechanism and bioinformatics analysis of the effect of berberine-enhanced fluconazole against drug-resistant Candida albicans. BMC Microbiol. 24, 196. doi: 10.1186/s12866-024-03334-0
Wu, Y., Totten, M., Memon, W., Ying, C., and Zhang, S. X. (2020). In vitro antifungal susceptibility of the emerging multidrug-resistant pathogen candida auris to miltefosine alone and in combination with amphotericin B. Antimicrob. Agents Chemother. 64 (2). doi: 10.1128/AAC.02063-19
Wychrij, D. A., Chapman, T. I., Rayens, E., Rabacal, W., Willems, H. M. E., Oworae, K. O., et al. (2025). Protective efficacy of the pan-fungal vaccine NXT-2 against vulvovaginal candidiasis in a murine model. NPJ Vaccines 10, 112. doi: 10.1038/s41541-025-01171-4
Xie, Y., Liu, X., and Zhou, P. (2020). In vitro Antifungal Effects of Berberine Against Candida spp. In Planktonic and Biofilm Conditions. Drug Des. Devel Ther. 14, 87–101. doi: 10.2147/DDDT.S230857
Xin, H., Rosario-Colon, J. A., and Eberle, K. (2023). Novel Intravenous Immunoglobulin Therapy for the Prevention and Treatment of Candida auris and Candida albicans Disseminated Candidiasis. mSphere 8, e0058422. doi: 10.1128/msphere.00584-22
Keywords: antifungal resistance, Candida auris, alternative antifungal therapies, natural compounds, nanotechnology delivery, combination therapies, photodynamic therapy
Citation: Du W, Wang Q and Zhao M (2025) Innovative antifungal strategies to combat drug-resistant Candida auris: recent advances and clinical implications. Front. Cell. Infect. Microbiol. 15:1641373. doi: 10.3389/fcimb.2025.1641373
Received: 05 June 2025; Accepted: 14 July 2025;
Published: 31 July 2025.
Edited by:
Yuanwei Zhang, Nanjing Normal University, ChinaReviewed by:
Estela Ruiz-Baca, Juárez University of the State of Durango, MexicoShiori Kitaya, Kanazawa University Hospital, Japan
Copyright © 2025 Du, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Zhao, bWluemhhb0BjbXUuZWR1LmNu
†These authors have contributed equally to this work
 Wei Du
Wei Du Qihui Wang
Qihui Wang Min Zhao
Min Zhao