- 1Department of Neurology, University of Buffalo Jacobs School of Medicine and Biomedical Sciences, Buffalo, NY, United States
- 2Dysautonomia Clinic, Williamsville, NY, United States
- 3University at Buffalo Jacobs School of Medicine and Biomedical Sciences, Buffalo, NY, United States
Postural orthostatic tachycardia syndrome (POTS), neurocardiogenic syncope, and orthostatic hypotension are the most common autonomic disorders encountered in clinical practice. The autoimmune etiology and association of these conditions with systemic autoimmune and inflammatory disorders, autonomic neuropathy, and post-acute infectious syndromes, including Long COVID, suggest that immunotherapies should be considered as a therapeutic option, at least in a subset of patients. However, the treatment of common autonomic disorders has traditionally included pharmacologic and non-pharmacologic symptomatic therapies as the standard approach. Unfortunately, these symptomatic therapies have been of limited or insufficient efficacy to meaningfully improve functional status or result in recovery, especially in patients with severe symptoms. Case reports, case series, and clinical experience suggest that intravenous and subcutaneous immunoglobulin, as well as other immunologic therapies (such as plasmapheresis, corticosteroids, and rituximab), may be effective in some patients with severe POTS and other common autonomic disorders who are refractory to standard therapies. In this narrative review, we summarize the literature available on the topic of immunotherapies for POTS, other common autonomic disorders, and Long COVID. We also highlight the need for large, multicenter, placebo-controlled trials of immunoglobulin, plasmapheresis, intermittent corticosteroids, and other repurposed immunotherapies in patients with common autonomic disorders who have significant functional impairment.
1 Introduction
Postural orthostatic tachycardia syndrome (POTS), one of the most common disorders affecting the autonomic nervous system, is a disabling condition with no U.S. Food and Drug Administration (FDA)-approved treatment. Neurocardiogenic syncope, orthostatic hypotension, inappropriate sinus tachycardia, and post-COVID dysautonomia are other common autonomic disorders (OCADs) frequently encountered in clinical practice. The treatment of these conditions traditionally includes non-pharmacologic and pharmacologic regimens consisting of symptomatic treatment, which is currently accepted as the standard of care. However, for many patients with POTS and OCADs, these symptomatic therapies have been of limited and often insufficient efficacy, resulting in significant improvement or recovery. Case reports, case series, and clinical experience suggest that immunotherapies and immunomodulating agents may present potentially effective therapeutic options for some patients with standard treatment-refractory POTS and OCADs. In this narrative review, we discuss the available literature on the use of immunotherapies in POTS and OCADs, including post-COVID dysautonomia as part of Long COVID, and we discuss the complexities, challenges, and future direction of immunologic therapies as treatments for the underlying autoimmune and immune-mediated etiologies of these disorders.
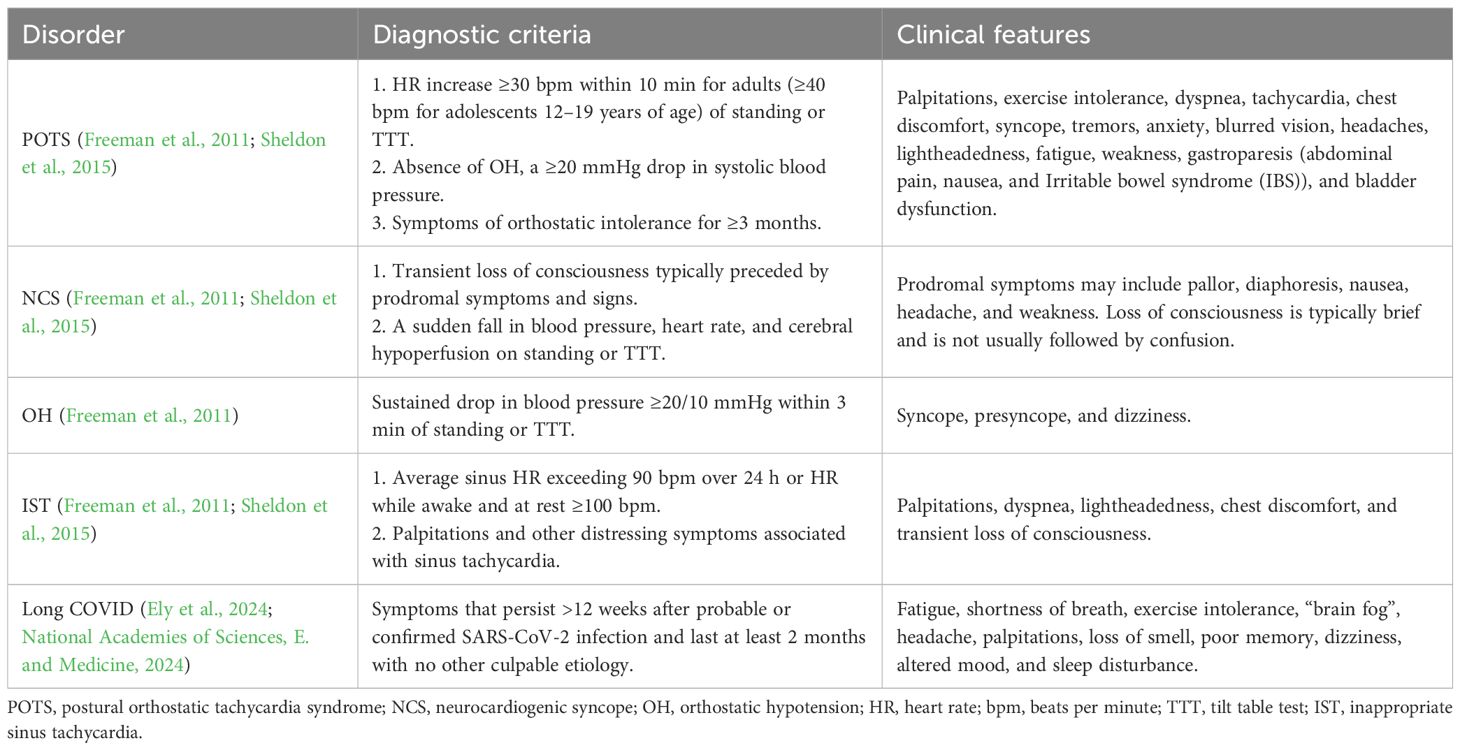
1.1 Postural orthostatic tachycardia syndrome
POTS is a chronic disorder of the autonomic nervous system characterized by orthostatic tachycardia, which is defined as an increase in heart rate by ≥30 bpm in adults and ≥40 bpm in adolescents 12–19 years old, from supine to standing position, associated with orthostatic symptoms that last for at least 3 months (Freeman et al., 2011; Sheldon et al., 2015) (Table 1). Although it is defined by postural tachycardia, the clinical features of POTS are numerous and include dizziness, headache, fatigue, nausea, generalized weakness, and sleep disturbances (Low et al., 1995; Thieben et al., 2007). The pathophysiologic mechanisms of POTS are also numerous and diverse, including autoimmunity, hypovolemia, hyperadrenergic state, cerebral hypoperfusion, and small fiber neuropathy (Low et al., 1995; Thieben et al., 2007; Shaw et al., 2019). The onset of POTS may be sudden or insidious and can follow various triggers, such as infection, puberty, pregnancy, vaccinations, surgery, concussion, and injury (Shaw et al., 2019). Importantly, patients with POTS have diminished quality of life and functional impairment similar to patients with congestive heart failure and chronic obstructive pulmonary disease, with greater than 50% of patients unable to maintain employment (Benrud-Larson et al., 2002; Bourne et al., 2021).
Prior to the COVID-19 pandemic, POTS was estimated to affect approximately 0.2%–1% of the US population (1–3 million people) (Vernino et al., 2021). After the COVID-19 pandemic, the incidence of POTS was found to have increased 15-fold due to POTS and autonomic dysfunction being common manifestations of Long COVID (Dulal et al., 2025). POTS predominantly affects women of reproductive age, ages of 15–25 (Vernino et al., 2021), but men are also becoming increasingly affected due to post-COVID POTS. Common comorbidities include migraines (at least 40%), gastrointestinal disorders (at least 30%), small fiber neuropathy (at least 50%), Ehlers–Danlos syndrome and hypermobility spectrum disorders (HSDs) (at least 30%), autoimmune disorders (at least 20%), myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) (at least 20%), and mast cell activation syndrome (at least 20%) (Shaw et al., 2019).
There are no FDA-approved therapies for POTS, but a commonly accepted therapeutic approach to POTS consists of non-pharmacologic and pharmacologic treatment options. Pharmacotherapy includes first-line medications such as beta-blockers, which decrease resting and postural tachycardia by reducing sympathetic overactivity; fludrocortisone, a mineralocorticoid that augments retention of water and sodium and expands plasma volume; midodrine, which is an alpha-1 agonist that causes vasoconstriction and increased peripheral resistance; and pyridostigmine, a parasympathetic nervous system enhancer (Raj et al., 2022; Grubb and Grubb, 2023).
1.2 Neurocardiogenic syncope
Neurocardiogenic syncope (NCS) (also known as vasovagal syncope or neurally mediated syncope) is defined as a sudden fall in blood pressure, heart rate, and cerebral hypoperfusion on standing or a tilt table test (Freeman et al., 2011; Sheldon et al., 2015) (Table 1). It is usually of rapid onset and short duration and may be preceded by prodromal symptoms, such as pallor, diaphoresis, nausea, headache, and weakness. The loss of consciousness is typically brief and is not usually followed by confusion. NCS can occur after various triggers, including standing, pain, dehydration, heat, and the sight of blood. This form of syncope is common, with 42% of women and 32% of men experiencing at least one episode by age 60. Although when NCS occurs occasionally it is benign, recurrent and frequent NCS can greatly impair quality of life (Sheldon et al., 2015). One common mechanism of syncope involves ineffective reflex response, where baroreceptors fail to perceive drops in venous return upon standing or pathologic vasodilation is triggered. The resulting hypotension causes loss of consciousness and has often been observed together with vagally mediated bradycardia. Recurrent episodes of syncope often involve sympathetic nervous system dysfunction (Sheldon et al., 2015). While autoimmunity is typically not considered the cause of NCS in otherwise healthy individuals, when recurrent NCS occurs in the context of post-acute infectious syndromes, autoimmune disorders, or neurologic conditions, including autonomic neuropathy, autoimmune and immune-mediated etiologies should be considered.
Diagnosis is based primarily on clinical history, and a tilt table test can be utilized when the origin of syncope is unclear, although it can only point toward a susceptibility to vasovagal syncope and cannot definitively diagnose the condition (Sheldon et al., 2015). Similar to the treatment of POTS, the treatment of NCS involves increased fluid and salt intake, education about counterpressure maneuvers to be performed when prodromal symptoms occur, and wearing compression garments. For those with recurrent episodes with significant impact on daily functioning, medical management can include a trial of midodrine, fludrocortisone, beta-blockers, or selective serotonin reuptake inhibitors (SSRIs), while pacemaker implantation can be considered in treatment-refractory patients with severe and disabling NCS with a predominant cardioinhibitory component (Gampa and Upadhyay, 2018).
1.3 Orthostatic hypotension
Orthostatic hypotension (OH), defined as a reduction in blood pressure ≥20/10 mmHg that occurs within 3 min of standing or during a head tilt test, is often associated with symptoms commonly related to cerebral hypoperfusion, such as lightheadedness, dizziness, presyncope, or syncope (Freeman et al., 2011) (Table 1). OH can be associated with non-neurogenic causes (such as volume depletion or medication side effects) and neurogenic causes (such as senescence, neuropathic disorders, or neurodegenerative diseases). Medications, including vasodilators, nitrates, diuretics, phenothiazines, neuroleptics and antidepressants, can result in OH as a side effect (Medow et al., 2008). The severity of blood pressure reduction may also be influenced by the time of day, food ingestion, prolonged exposure to heat, fever, and alcohol consumption (Freeman et al., 2011). OH most often presents in the elderly, specifically one in five adults older than 60, and patients with neurodegenerative disorders (Freeman et al., 2011; Saedon et al., 2020). However, when OH occurs in the context of systemic autoimmune disorders, post-acute infectious syndromes, or neurologic disorders (such as autoimmune autonomic neuropathy or ganglionopathy), autoimmune and immune-mediated etiologies should be considered.
Mild cases of OH are commonly managed by discontinuing hypotensive medications and lifestyle changes, such as increasing water intake, avoiding alcohol, dietary changes, use of abdominal binders or leg stockings, and head-up tilt sleeping. The pharmacologic treatment approach for OH for patients with persistent symptoms is similar to that for patients with POTS and includes sympathomimetic agents (midodrine, yohimbine, vasopressin agonists, and clonidine), fludrocortisone, erythropoietin, pyridostigmine, selective serotonin reuptake inhibitors, and other medications (non-steroidal anti-inflammatory drugs (NSAIDs), antihistamines, caffeine, hydralazine, and ergotamine). Droxidopa, a norepinephrine precursor medication with combined central and peripheral alpha and beta agonist effects, was approved by the FDA for OH in 2014. It is indicated for the treatment of neurogenic OH and has shown improved symptoms and blood pressure elevation in four placebo-controlled randomized controlled trials (RCTs) (Brignole et al., 2018).
1.4 Inappropriate sinus tachycardia
Inappropriate sinus tachycardia (IST) is a chronic syndrome defined as an unexplained sinus heart rate of ≥100 bpm at rest or >90 bpm on average for 24 hours without orthostatic changes (Sheldon et al., 2015) (Table 1). IST may be associated with debilitating clinical symptoms, most often palpitations, and commonly occurs in women between the ages of 15 and 45. The pathophysiology of IST involves various proposed mechanisms, including an imbalance between sympathetic and parasympathetic inputs, accelerated intrinsic sinus node rate due to a deficient function of the acetylcholine and adenosine-sensitive potassium channels, and impaired baroreflex control (Ahmed et al., 2022). Since sinus tachycardia can be caused by various factors (including electrolyte abnormalities, dehydration, and hormonal abnormalities), these causes should be ruled out, and cardiac monitoring (such as an event monitor or an implantable loop recorder) should be used to correlate symptoms with heart rates (Ahmed et al., 2022). A 10-min stand test or a tilt table test can be used to distinguish IST from POTS, OH, and NCS (Olshansky and Sullivan, 2019), but sometimes, a patient may have more than one autonomic disorder, such as both POTS and IST.
The treatment of IST includes medications that reduce heart rate and symptoms, such as ivabradine (an If channel antagonist), beta-blockers, and calcium channel blockers. The combination of beta-blockers and ivabradine may be considered for ongoing management in some patients with IST (Olshansky and Sullivan, 2019). Sinus node modification, surgical ablation, and sympathetic denervation are not typically recommended as a part of routine care for patients with IST (Rodriguez-Manero et al., 2017).
1.5 Long COVID
Long COVID describes the health consequences of COVID-19 that persist beyond the initial infection. The World Health Organization defines post-COVID-19 conditions as symptoms that persist more than 12 weeks after probable or confirmed SARS-CoV-2 infection, which last at least 2 months and have no alternative explanations (Post COVID-19 condition (Long COVID), 2022). Similarly, the 2024 National Academies of Sciences, Engineering, and Medicine consensus defines Long COVID as “an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems” (National Academies of Sciences, E. and Medicine, 2024; Ely et al.., 2024). Long COVID can follow either asymptomatic or symptomatic SARS-CoV-2 infection, and the current diagnosis is entirely clinical (National Academies of Sciences, E. and Medicine, 2024), given that there are no reliable and validated biomarkers available to clinicians at this time. A Long COVID Household Pulse Survey showed that the rate of Long COVID is nearly 7% of all adults—roughly 17 million people—as of March 2024 (Statistics, N.C.f.H, 2024). In another study in 2023, the National Health Interview Survey, 8.4% of adults in the USA reported ever having Long COVID, and 3.6% reported currently having Long COVID (Vahratian et al., 2024).
The pathophysiology of Long COVID is multifactorial but frequently involves autonomic dysfunction, including symptoms and signs such as palpitations, orthostatic intolerance, labile blood pressure, fatigue, headaches, and “brain fog” (Larsen et al., 2021). Consequently, many patients with Long COVID have POTS or OCADs (Blitshteyn and Whitelaw, 2021; Davenport et al., 2024), with nearly 70% of patients having a high autonomic symptom burden (Larsen et al., 2022). Autoimmune, inflammatory, and immune dysregulations are identified as other major pathophysiologic mechanisms of Long COVID, which, together with autonomic dysfunction, closely parallel the pathophysiology of POTS and OCADs. Increased prevalence of elevated serum autoimmune and inflammatory markers has been reported in patients with both POTS and Long COVID (El-Rhermoul et al., 2023), and neuroinflammation at the brainstem, specifically at the dorsolateral inferior medulla, has been suggested as a potential central nervous system localization for POTS and Long COVID (Blitshteyn, 2025). Moreover, consensus guidelines on the assessment and treatment of post-COVID autonomic dysfunction have been developed using non-pharmacologic and pharmacologic treatment options similar to POTS and OCADs unrelated to COVID-19 (Blitshteyn et al., 2022).
2 Autoimmunity
2.1 Autoimmune markers in POTS and other common autonomic disorders
The pathophysiology of POTS has been deemed largely heterogeneous and traditionally classified as neuropathic, hypovolemic, and hyperadrenergic (Low et al., 2009). In the past decade, however, investigators zeroed in on autoimmunity as one of the major mechanisms. Patients with POTS were found to have a higher prevalence of various non-specific autoimmune markers, including antinuclear antibodies and comorbid autoimmune disorders, than the general population (Blitshteyn, 2015). More specifically to the autonomic nervous system, ganglionic N-type and P/Q-type acetylcholine receptor antibodies, alpha 1, beta 1, and beta 2 adrenergic antibodies, muscarinic M2 and M4 antibodies, angiotensin II type 1 receptor antibodies, and opioid-like 1 receptor antibodies have been identified in patients with POTS and OCADs (Thieben et al., 2007; Li et al., 2014; Watari et al., 2018; Yu et al., 2018; Gunning et al., 2019; Kharraziha et al., 2020). Many of these antibodies have also been identified in patients with chronic fatigue syndrome, small fiber neuropathy, complex regional pain syndromes, and cardiovascular disorders—conditions that have overlapping clinical features with POTS.
2.2 Comorbidity with undifferentiated connective tissue disease
POTS and OCADs are commonly comorbid with other autoimmune disorders, with the most common being Hashimoto’s thyroiditis (Blitshteyn, 2015). Their association with Sjögren’s syndrome, antiphospholipid syndrome, and celiac disease has also been reported (Schofield et al., 2014; Penny et al., 2016; Mannan and Pain, 2023). In addition, many patients with autonomic dysfunction, small fiber neuropathy, and positive autoimmune or inflammatory markers are diagnosed with undifferentiated connective tissue disease (UCTD) when they do not meet the diagnostic criteria of defined autoimmune disorders, such as systemic lupus erythematosus, mixed connective tissue disease, Sjögren’s syndrome, systemic sclerosis, polymyositis, dermatomyositis, or rheumatoid arthritis. In clinical practice, the presence of undifferentiated connective tissue disease can be common.
Like POTS, UCTD predominantly affects women of reproductive age and is thought to be heterogeneous in mechanisms and presentations. UCTD is caused by an autoimmune etiology and may precede the onset of lupus or another defined classical autoimmune disease. UCTD includes the following diagnostic criteria: 1) clinical presentation suggestive of a defined connective tissue disease, but not meeting its criteria; 2) positive serological markers on two separate occasions, including positive antinuclear antibody marker; and 3) the duration of symptoms is at least 3 years (Mosca et al., 1999).
Positive serological markers are essential in the diagnostic criteria for UCTD and should include routine screening tests, such as complete blood count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum creatinine, urinalysis with microscopic analysis, rheumatoid factor (RF), antinuclear antibodies (ANAs), anti-Ro/SSA/anti-SSB antibodies, and anti-U1-RNP (Marwa and Anjum, 2025). Treatment typically includes symptomatic management with non-steroidal anti-inflammatory medications, such as ibuprofen, naproxen, and celecoxib; corticosteroids, such as prednisone, methylprednisolone, and hydrocortisone; calcium channel blockers, such as diltiazem and nifedipine; and immunomodulatory therapy with an anti-malarial drug, hydroxychloroquine. In more severe cases, immunosuppressive medications, such as methotrexate and azathioprine, can be used, especially when there is evidence of significant organ damage or involvement (Rubio and Kyttaris, 2023). Further research is needed to elucidate whether POTS and OCADs with positive autoimmune markers represent a sizable subset of patients with UCTD, what longitudinal monitoring is required in this subset, and whether early intervention with treatment (such as hydroxychloroquine or low-dose naltrexone) can alter the natural history and potentially prevent further progression of the disease process.
2.3 Association with autonomic neuropathy
POTS and OCADs can often occur as part of, or in the context of, autonomic neuropathy. Experts who originally described POTS have considered it to be a limited or restricted form of autonomic neuropathy (Schondorf and Low, 1993; Vernino et al., 2008). Approximately half of patients with POTS have a length-dependent distribution (Low et al., 1994; Low et al., 2009) with distal postganglionic sudomotor denervation demonstrated by the quantitative sudomotor axon reflex test (QSART) or the thermoregulatory sweat test (Low, 1993). These tests commonly reveal sudomotor denervation in the feet and toes: adrenergic impairment in the lower extremity can be seen in neuropathic POTS as impaired norepinephrine spillover in the leg, while the arm response remains normal (Jacob et al., 2000). However, a non-length-dependent or patchy distribution of small fiber neuropathy can also occur, especially in conjunction with systemic autoimmune disorders (Gemignani et al., 2022). Autoimmune and immune-mediated etiologies have been suggested as among the major underlying mechanisms in autonomic neuropathy, with immunotherapy being recommended as the first-line treatment (Gavrilova et al., 2022; Maier et al., 2022; Gendre, 2024; Nakane et al., 2024).
2.4 Autoimmunity in Long COVID
Autoimmunity has been implicated as one of the major mechanisms of Long COVID, leading to a higher risk, overall incidence, and range of autoimmune conditions after SARS-CoV-2 infection (Sharma and Bayry, 2023). A variety of antibodies have been linked to Long COVID, including autoantibodies to inflammatory cytokines such as IgG to IL-2, D8B, thyroglobulin, and IFNδ (Rojas et al., 2022; El-Rhermoul et al., 2023; Peluso and Deeks, 2024). These autoantibodies have been associated with anti-SARS-CoV-2 IgG antibodies (Rojas et al., 2022; El-Rhermoul et al., 2023; Peluso and Deeks, 2024). G protein-coupled receptor antibodies, including against alpha- and beta-adrenergic antibodies and muscarinic antibodies, previously identified in patients with POTS, as well as autoantibodies to antinuclear and extractable nuclear antigens, have also been found in patients with Long COVID (Wallukat et al., 2021; El-Rhermoul et al., 2023; Son et al., 2023). The pro-inflammatory mediators, non-specific antibodies, and antibodies important to the function of the autonomic nervous system are thought to be implicated in the development of post-COVID autonomic disorders, such as POTS and OCADs (El-Rhermoul et al., 2023).
3 Immunotherapies
3.1 Immunologic therapies and ongoing clinical trials for POTS and other common autonomic disorders
3.1.1 Immunoglobulin
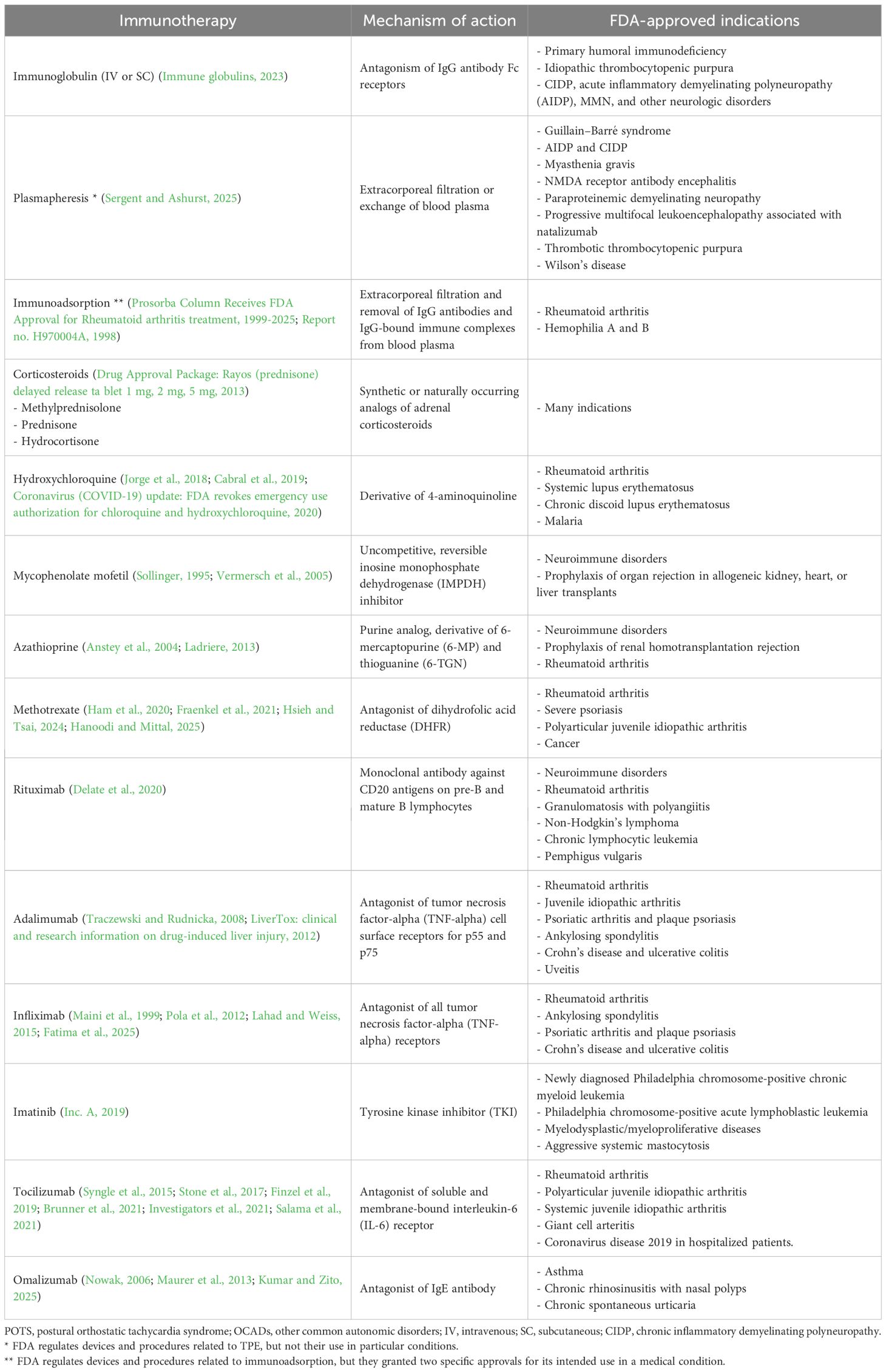
Intravenous immunoglobulin (IVIG) or subcutaneous immunoglobulin (SCIG) comes from a concentrate of pooled immunoglobulins derived from 1,000 to 100,000 healthy donors and serves as an immunomodulating therapy that can neutralize autoantibodies, reduce cellular immunity, and decrease endothelial inflammation by increasing IgG levels in the bloodstream (Danieli et al., 2025). Immunoglobulins play a vital role in humoral adaptive immunity, and therefore, IVIG reflects a collective exposure of the donor population to their environment and can be expected to contain various antibodies of multiple specificities against a broad spectrum of infectious agents (bacterial, viral, and others), self-antigens, and anti-idiotype antibodies. The composition of IVIG products closely corresponds to that of immunoglobulins in normal human plasma, especially IgG (along with its subclasses), IgA, traces of other Igs, cytokines, and soluble receptors (Perez et al., 2017).
IVIG has been indicated as a replacement therapy in immunodeficiencies, as an immunomodulatory and anti-inflammatory therapy for immunomodulation in hematological and organ-specific autoimmune disorders, and as an anti-inflammatory in rheumatic inflammatory conditions and infectious neurologic disorders. It has also been utilized as a hyperimmune therapy against specific infectious agents (Perez et al., 2017).
Given its widespread use in neurologic conditions [such as Guillain–Barré syndrome, chronic inflammatory demyelinating polyneuropathy (CIDP), acute disseminated encephalomyelitis (ADEM), multifocal motor neuropathy (MMN), dermatomyositis, and myasthenia gravis], IVIG has also been used successfully in treating less common peripheral neuropathies, such as autoimmune autonomic ganglionopathy (AAG) and autoimmune autonomic neuropathy (AAN) (Gibbons et al., 2008; Dalakas, 2021). To this end, a trial of IVIG or SCIG seems reasonable in POTS—a restricted form of AAN—and OCADs, especially in patients with comorbid small fiber neuropathy (SFN), UCTD, or systemic autoimmune disorder.
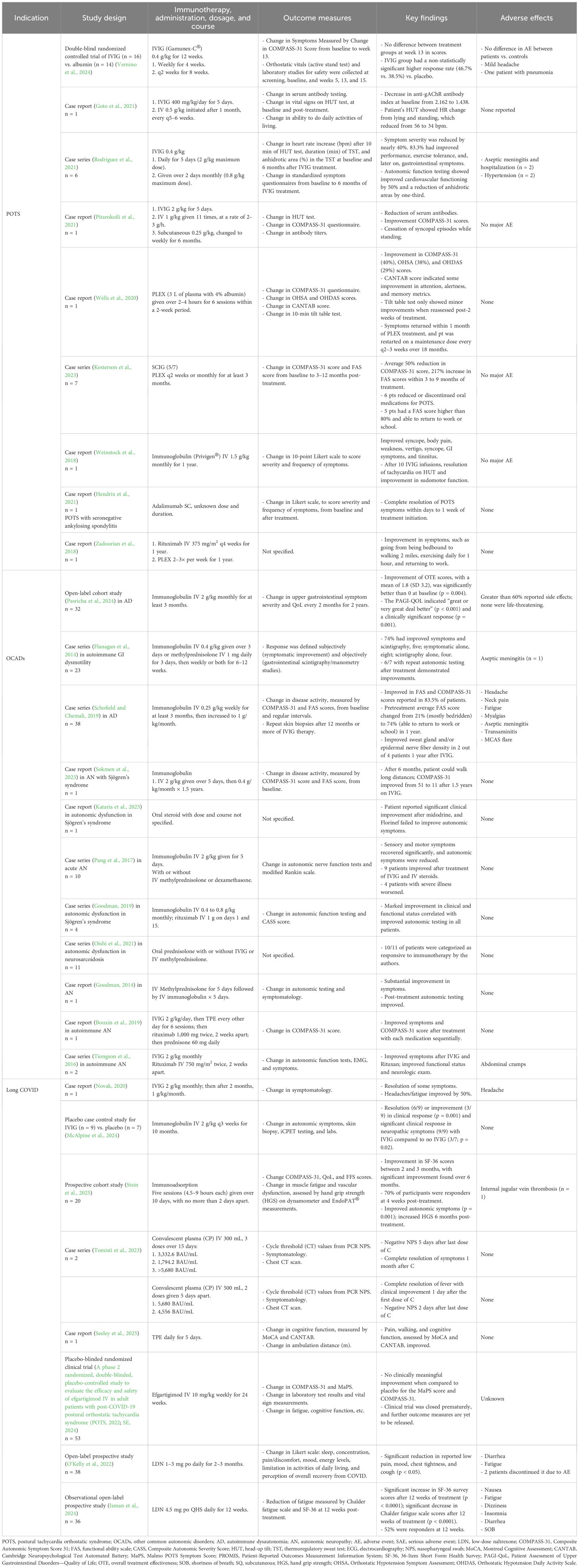
Over the past decade, case reports and case series describing the benefits of IVIG in POTS and OCADs have been accumulating. All reported reduced autonomic symptoms, orthostatic intolerance, fatigue, functional impairment, and lowered antibody titers when available. Similar findings were observed in other case reports of IVIG or SCIG in patients with OCADs (Table 2). Importantly, these reports suggest that IVIG and SCIG are well-tolerated without significant serious adverse events, although side effects, including post-infusion headache and flu-like symptoms, were common. Slower infusion rates with pretreatment with IV saline, antihistamines, and anti-inflammatories may mitigate these side effects and improve tolerability (Guo et al., 2018).
Recently, a small randomized controlled study found no significant benefit of 16 patients treated with IVIG vs. 14 patients treated with albumin with autoimmune POTS despite a trend toward a higher response rate in the IVIG-treated group (Vernino et al., 2024). However, the true benefit of IVIG may not have been captured, as the study was underpowered, used lower IVIG doses than those for autoimmune disorders, was of short duration, and had other major limitations (Chemali et al., 2024). Further research with large, multicenter, randomized controlled trials of longer duration and addressing major limitations is needed to provide a comprehensive and objective assessment of the efficacy of IVIG in patients with POTS (Chemali et al., 2024).
3.1.2 Plasma exchange
Therapeutic plasma exchange (TPE), also known as plasmapheresis, is a technique that rapidly removes circulating autoantibodies and other humoral factors from the vascular compartment and has been used as the first effective acute treatment for neurologic disorders, such as Guillain–Barré syndrome and myasthenia gravis, before intravenous immunoglobulin became available (Osman et al., 2020). It is still used when IVIG is not available or ineffective in a variety of neuroimmune disorders, including CIDP and autoimmune encephalitis (Osman et al., 2020). Isolated cases of a total of five patients with severe POTS have been described in scientific literature; their POTS symptoms improved significantly with TPE, with patients being able to return to work and other daily activities, such as walking and exercising (Zadourian et al., 2018; Wells et al., 2020; Kesterson et al., 2023) (Table 2). Despite no significant adverse events reported, further studies are necessary to determine the efficacy and safety of TPE in patients with severe POTS and OCADs.
3.1.3 Biologic immunotherapies
Biologic therapies in POTS and OCAD cases have not been explored in-depth but may be a good option to explore in patients with severe symptoms. Rituximab, an anti-CD20 monoclonal antibody, could be of benefit in autoimmune autonomic disorders, as it targets B cells that are created by the adaptive immune system and are responsible for autoantibody production. There are limited data on its use in POTS and OCADs; however, it has been utilized in select cases with other autoimmune neurologic conditions with autonomic involvement (Hollenbeck et al., 2011; Bouxin et al., 2019). Currently, rituximab use has been reported in one POTS patient and three OCAD patients (Tiongson et al., 2016; Zadourian et al., 2018; Goodman, 2019). All patients reported autonomic symptomatic resolution, with two demonstrating absence or a decrease in autoimmune antibodies post-treatment.
Adalimumab is a monoclonal antibody against tumor necrosis factor-alpha (TNF-α), a pro-inflammatory cytokine made by the innate immune system, that is responsible for regulating inflammation, cell differentiation, and tissue destruction. It is approved by the FDA for the treatment of rheumatoid arthritis, inflammatory bowel disease, and other autoimmune and inflammatory disorders. One case report described the use of adalimumab in a patient with POTS and seronegative ankylosing spondylitis, which led to complete symptom resolution of POTS symptoms within 1 week of the induction dose and no adverse effects (Hendrix et al., 2021) (Table 2).
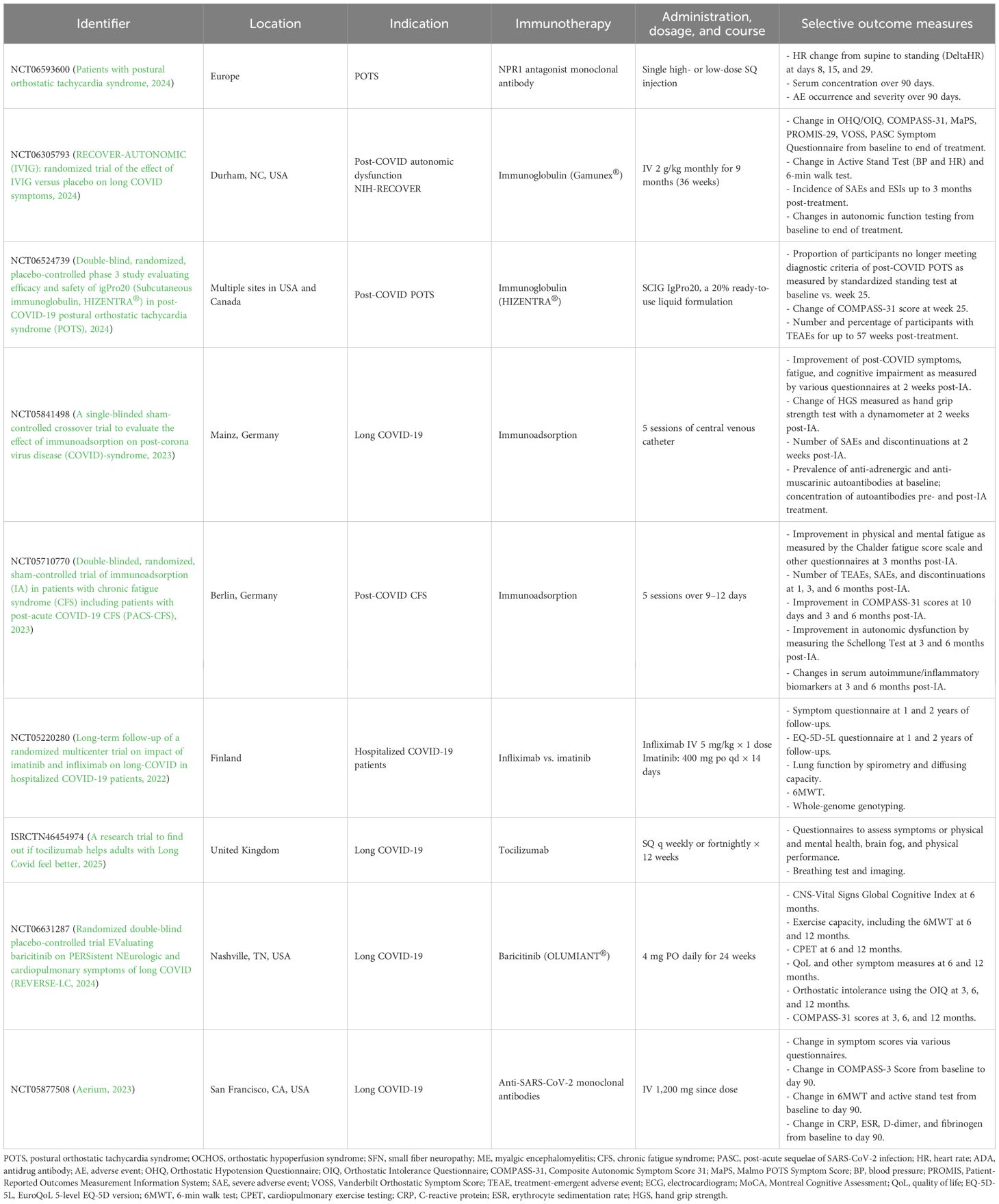
Tocilizumab is an IL-6 receptor antagonist that activates the JAK/STAT3 pathway and regulates inflammation, B-cell activation, and autoantibody production. Although it has been used in neurologic and autoimmune disorders, such as neuromyelitis optica spectrum disorder (Du et al., 2021) and rheumatoid arthritis (Syngle et al., 2015), it has yet to be explored in POTS and OCADs. Currently, the application of biologic therapies in POTS and OCADs remains extremely limited, primarily due to the inaccessibility of these agents, high cost, and potential for adverse effects, but future pharmaceutical research and investment in clinical trials are warranted to assess their full therapeutic potential. Notably, there is one phase II double-blind placebo-controlled clinical trial investigating a novel monoclonal antibody against natriuretic peptide receptor 1 that began recruiting POTS patients in late 2024 (Patients with postural orthostatic tachycardia syndrome, 2024) (Table 3).
3.1.4 Other traditional immunomodulators
Although immunomodulating therapies have not been typically included in the standard pharmacologic approaches for POTS and OCADs, these treatment options have been gaining utility, especially in the context of comorbid UCTD, systemic autoimmune disorders, and Long COVID. These pharmacotherapies include oral, IV, and subcutaneous (SQ) corticosteroids, low-dose naltrexone, and immunosuppressants, such as hydroxychloroquine. These medications may be attractive, as they have more established safety profiles, clinical familiarity, and easier accessibility through insurance coverage compared to other immunologic therapies. Corticosteroids are effective in reducing inflammation and autoimmunity and have been used for decades for acute exacerbation of multiple sclerosis, neuromyelitis optica, myasthenia gravis, and others. They have been reported for treatment of autonomic dysfunction either as monotherapy or in combination with other immunotherapies in patients with neurologic Sjögren’s syndrome and autonomic neuropathy associated with neurosarcoidosis (Flanagan et al., 2014; Goodman, 2014; Pang et al., 2017; Oishi et al., 2021; Kataria et al., 2023). Improvement with corticosteroids has been observed in these small case series; however, long-term use is not recommended due to significant steroid-induced side effects, including long-term risk of diabetes, osteoporosis, hypertension, and Cushing’s syndrome (Buchman, 2001).
Naltrexone is a potent mu-opioid receptor antagonist at high doses, primarily used to prevent relapse in opioid use disorder. Below 5 mg, low-dose naltrexone (LDN) acts as a glial modulator, inhibits Toll-like-receptor-4 (TLR-4), and only partly antagonizes opioid receptors. Its anti-TLR-4 effects inhibit proinflammatory cytokine production, while its partial opioid receptor downregulation signals for increased opioid production and can downregulate the immune system in POTS and OCADs (Li et al., 2018; Trofimovitch and Baumrucker, 2019). There are no clinical trials on the use of LDN in POTS and OCADs, with only one case report documenting beneficial LDN use in POTS (Weinstock et al., 2018) (Table 2). Clinical experience suggests that many patients report improvement in chronic pain, chronic fatigue, and mast cell-related symptoms with the use of LDN.
Antimetabolite immunosuppressants, such as mycophenolate mofetil, azathioprine, or Hydroxychloroquine, could also be of potential therapeutic benefit in autoimmune POTS and OCADs, but the use of these medications in patients with POTS and OCADs has not been investigated. Anecdotal reports of patients with POTS and OCADs and comorbid autoimmune disorders, such as UCTD and Sjögren’s syndrome, suggest that there may be potential benefits in this subset of patients.
3.2 Immunologic therapies and ongoing clinical trials for Long COVID
Immunotherapies documented in Long COVID case reports and cohort studies include IVIG, immunoadsorption, convalescent plasma (CP), TPE, and LDN. Due to their proposed therapeutic role in autoimmune POTS and OCADs, these therapies could be considered potential therapeutic options for Long COVID-associated dysautonomia, but their use is extremely limited due to a lack of access and insurance coverage.
Three case reports have documented the utility of IVIG, TPE, and CP treatments in Long COVID. Novak reported improvement in headache and fatigue, with complete symptom resolution of all other symptoms (Novak, 2020). Minor adverse effects, such as headaches, were alleviated by dose down-titration. Tomisti et al. treated two patients with CP who reported complete symptom resolution within 1 month after their final treatment dose and reported no side effects (Tomisti et al., 2023). Lastly, Seeley et al. treated one patient with TPE who reported improved cognitive function, peripheral pain, and ambulation capacity from 5 to 12 m (Seeley et al., 2025). They also did not report side effects (Table 2).
Four prospective studies, although limited in sample size, have demonstrated clinical improvements in Long COVID and post-COVID syndromes following treatment with LDN (n = 38), immunoadsorption (n = 20), and immunoglobulin (n = 9) (O’Kelly et al., 2022; McAlpine et al., 2024; Stein et al., 2025). O’Kelly et al. conducted an open-label prospective study with 38 patients receiving 1 mg of LDN, assessing improved outcomes by self-reported questionnaires (O’Kelly et al., 2022). They found the biggest effect of symptom reduction in joint pain. Additionally, Isman et al. investigated LDN in an open-label prospective study with 36 Long COVID subjects over 12 weeks. They reported significant improvements in the patient’s quality of life and fatigue, measured by their 36-Item Short Form Health Survey (SF-36) and CFS scores. Approximately half of their participants were identified as clinical responders (Isman et al., 2024) (Table 2).
A placebo-controlled clinical trial was conducted for efgartigimod in 53 patients with post-COVID POTS, but preliminary outcomes showed no benefit of efgartigimod compared to placebo (A phase 2 randomized, double-blinded, placebo-controlled study to evaluate the efficacy and safety of efgartigimod IV in adult patients with post-COVID-19 postural orthostatic tachycardia syndrome (POTS, 2022). The clinical trial was stopped in 2024, and its outcome data have yet to be released (SE, 2024) (Table 2). Currently, eight immunotherapy clinical trials are ongoing for Long COVID and post-COVID autonomic disorders. These clinical trials are investigating IVIG, immunoadsorption, infliximab compared to imatinib, tocilizumab, baricitinib, and an anti-SARS-CoV-2 monoclonal antibody therapy. Four clinical trials are being held in North America (the USA and Canada), including one as part of the NIH-RECOVER autonomic study, with the other trials taking place in Germany, Finland, and the United Kingdom (Long-term follow-up of a randomized multicenter trial on impact of imatinib and infliximab on long-COVID in hospitalized COVID-19 patients, 2022; Aerium, 2023; A single-blinded sham-controlled crossover trial to evaluate the effect of immunoadsorption on post-corona virus disease (COVID)-syndrome, 2023; Double-blinded, randomized, sham-controlled trial of immunoadsorption (IA) in patients with chronic fatigue syndrome (CFS) including patients with post-acute COVID-19 CFS (PACS-CFS), 2023; Double-blind, randomized, placebo-controlled phase 3 study evaluating efficacy and safety of igPro20 (Subcutaneous immunoglobulin, HIZENTRA®) in post-COVID-19 postural orthostatic tachycardia syndrome (POTS), 2024; Randomized double-blind placebo-controlled trial EValuating baricitinib on PERSistent NEurologic and cardiopulmonary symptoms of long COVID (REVERSE-LC, 2024; RECOVER-AUTONOMIC (IVIG): randomized trial of the effect of IVIG versus placebo on long COVID symptoms, 2024) (Table 3).
3.3 Immunologic therapies for ME/CFS
ME/CFS has overlapping clinical features with POTS, OCADs, and Long COVID and is therefore relevant to this review. A number of immunologic therapies have been studied in ME/CFS, including IVIG, SCIG, and IgG depletion by immunoadsorption (McAlpine et al., 2024; Sjogren et al., 2024; Stein et al., 2025). Four double-blind placebo-controlled RCTs of IVIG for ME/CFS were conducted in the 1990s: one study reported that immunoglobulin is effective in a “significant number of patients”, and another reported that IVIG “is unlikely to be of clinical benefit in CFS” (Lloyd et al., 1990; Peterson et al., 1990). The third study reported a beneficial effect of IVIG in adolescent patients, but a fourth trial reported that IVIG was ineffective (Rowe, 1997; Vollmer-Conna et al., 1997). Despite these conflicting results from clinical trials, some authors believe that IVIG presents a potentially curative treatment for a proportion of patients with ME/CFS and that further randomized controlled trials should be conducted with urgency, especially since many patients with Long COVID met the criteria for ME/CFS (Brownlie and Speight, 2021).
More recently, in a case–control study of patients with post-COVID SFN who had comorbid ME/CFS, IVIG administered to nine patients resulted in decreased allodynia and neuropathic symptoms compared to patients who were not treated with IVIG (McAlpine et al., 2024). Subcutaneous low-dose immunoglobulin therapy has also been shown to be effective in 17 patients with ME/CFS (Sjogren et al., 2024). In a cohort of 20 patients, immunoadsorption was used to remove select immunoglobulins and autoantibodies from plasma, which led to symptomatic improvement in some patients (Stein et al., 2025). Further research involving more robust, controlled study designs with larger sample sizes is needed to elucidate the efficacy of these immunologic therapies for the treatment of ME/CFS.
3.4 Potential immunologic therapies for POTS, other common autonomic disorders, and Long COVID
Since POTS, OCADs, and Long COVID have been increasingly linked to autoimmunity and immune system dysregulation, new and repurposed immunologic therapies present a potentially effective treatment option and should be explored in future clinical trials. These therapies may be used either as a last resort in patients who failed standard non-pharmacologic and pharmacologic therapies or as a first-line treatment in patients with POTS and OCADs of suspected autoimmune or inflammatory etiologies, or comorbid SFN, UCTD, and other systemic autoimmune disorders. Many immunologic therapies have already been approved for other indications that could have the potential to treat POTS and OCADs, including immunoglobulin, plasmapheresis, immunoadsorption, corticosteroids, hydroxychloroquine, mycophenolate, azathioprine, methotrexate, monoclonal antibody treatments, and various receptor inhibitors (Table 4). Availability and accessibility of these immunotherapies to patients with POTS, OCADs, and Long COVID may present a potentially effective treatment option and prevent future disability incurred as a result of progressive disease course.
4 Future direction
Although immunomodulating therapies appear to be beneficial in at least a subset of patients with POTS and OCADs, the next step is to invest in large, multicenter, placebo-controlled trials of immunoglobulin, plasmapheresis, intermittent corticosteroids, and other repurposed immunologic therapies. However, these trials may be more difficult to execute than similar trials for patients with immune-mediated peripheral neuropathies, multiple sclerosis, myasthenia gravis, and other autoimmune disorders. The reasons for these complexities are multifaceted. First, the heterogeneity of the patient population, diverse pathophysiology and autoantibodies, and a lack of a precise unifying biomarker underlying POTS and dysautonomia in general can make it difficult to interpret and generalize the outcomes. Second, the 30-bpm heart rate elevation as a diagnostic criterion for POTS may not be a good marker to assess treatment outcome, as this change in heart rate is highly variable and imprecise. Moreover, there is a lack of established inclusion criteria for patients with presumed autoimmune POTS. Additionally, comorbidity with small fiber neuropathy, UCTD, and autonomic neuropathy, which are predominantly driven by autoimmune and inflammatory etiologies, needs to be considered. Furthermore, the effect of saline and albumin as comparators needs to be examined, as these agents may not be truly placebo and may have significant blood volume and some immunologic effects (Chemali et al., 2024). Another difficulty is the high prevalence of patients with allergies and sensitivities to medications, excipients, and preservatives among patients with POTS; therefore, patients may require individualized and modified trial protocols. Immunotherapy dose, duration, and cross-over timelines also need to be evaluated, given that at least 3–6 months of treatment may be required to see the full effect and that at least 6 months may be needed for the effect of immunotherapy to dissipate. Moreover, the optimal timing of immunotherapy initiation relative to disease onset needs to be determined. It is possible that starting immunotherapy sooner rather than later in the disease course would yield better efficacy and treatment outcomes than starting it at any point in the disease course. Finally, validated questionnaires to assess autonomic symptom burden, fatigue, functional abilities, and quality of life should be used as primary outcomes, and objective heart rate and blood pressure responses should be used as secondary outcomes because there is a high rate of discrepancy and variability between symptom severity and vital signs. Despite these challenges, however, we believe that conducting large, well-designed clinical trials of immunotherapies is a priority for patients with POTS and OCADs, including those with post-COVID onset.
5 Conclusion
Combining the limited data outlined in this review, the current and future clinical trials, and our clinical experience, we conclude that immunologic therapies present an important and, potentially, very effective therapeutic option for patients with POTS, OCADs, and Long COVID. To this end, we believe that patients with severe POTS, OCADs, and Long COVID should have access to a variety of therapeutic options involving immunomodulation, including a 3–6-month trial of IVIG, SCIG, or plasmapheresis—therapies that are already available to patients with demyelinating neuropathies, autonomic neuropathy, autoimmune autonomic ganglionopathy, and other neurologic and autoimmune disorders.
Author contributions
SB: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. GF: Data curation, Investigation, Writing – original draft, Writing – review & editing. AS: Data curation, Investigation, Writing – original draft, Writing – review & editing. MH: Data curation, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
SB serves as a paid consultant for CSL Behring. SB also serves on the NIH-RECOVER-TLC Neurological Agents Committee as a non-paid member.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aerium, T. (Ed.) (2023). An exploratory, randomized, double-blind placebo-controlled study to assess the safety of an anti-SARS-coV-2 monoclonal antibody and response to treatment in individuals with long COVID (outSMART-LC) (C. Patient-Led Research and F. PolyBio Research). San Francisco, USA, Clinicaltrials.gov https://clinicaltrials.gov/study/NCT05877508 (Accessed February 24, 2025).
Ahmed, A., Pothineni, N. V. K., Charate, R., Garg, J., Elbey, M., and de Asmundis, C. (2022). Inappropriate sinus tachycardia: etiology, pathophysiology, and management: JACC review topic of the week. J. Am. Coll. Cardiol. 79, 2450–2462. doi: 10.1016/j.jacc.2022.04.019
Anstey, A. V., et al. (2004). Guidelines for prescribing azathioprine in dermatology. Br. J. Dermatol. 151, 1123–1132. doi: 10.1111/j.1365-2133.2004.06323.x
(2022). Post COVID-19 condition (Long COVID). Available online at: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (Accessed February 16, 2025).
(2024). “A phase 2 double-blind placebo-controlled single-dose study of pharmacodynamics, pharmacokinetics, safety, and tolerability of REGN7544, an NPR1 antagonist monoclonal antibody,” in Patients with postural orthostatic tachycardia syndrome Multi-site (USA, Canada): Clinicaltrials.gov. (Accessed February 24, 2025 ).
(2022). A phase 2 randomized, double-blinded, placebo-controlled study to evaluate the efficacy and safety of efgartigimod IV in adult patients with post-COVID-19 postural orthostatic tachycardia syndrome (POTS (L. Iqvia Pty).
(2022). Long-term follow-up of a randomized multicenter trial on impact of imatinib and infliximab on long-COVID in hospitalized COVID-19 patients (H. University of).
(2023). Double-blinded, randomized, sham-controlled trial of immunoadsorption (IA) in patients with chronic fatigue syndrome (CFS) including patients with post-acute COVID-19 CFS (PACS-CFS) Berlin, Germany: Clinicaltrials.gov.
(2023). A single-blinded sham-controlled crossover trial to evaluate the effect of immunoadsorption on post-corona virus disease (COVID)-syndrome (Mainz, Germany: Clinicaltrials.gov).
(2024). RECOVER-AUTONOMIC (IVIG): randomized trial of the effect of IVIG versus placebo on long COVID symptoms Durham, USA: Clinicaltrials.gov.
(2024). Double-blind, randomized, placebo-controlled phase 3 study evaluating efficacy and safety of igPro20 (Subcutaneous immunoglobulin, HIZENTRA®) in post-COVID-19 postural orthostatic tachycardia syndrome (POTS) Multi-site (USA, Canada): Clinicaltrials.gov.
(2024). Randomized double-blind placebo-controlled trial EValuating baricitinib on PERSistent NEurologic and cardiopulmonary symptoms of long COVID (REVERSE-LC (H. National Institutes of and A. National Institute on, Editors).
(2025). A research trial to find out if tocilizumab helps adults with Long Covid feel better United Kingdom: ISRCTN (International Standard Randomised Controlled Trial Number). doi: 10.1186/ISRCTN46454974
(2023). Immune globulins. Available online at: https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/immune-globulins (Accessed February 24, 2025).
(1999-2025). Prosorba Column Receives FDA Approval for Rheumatoid arthritis treatment. Available online at: https://www.hopkinsarthritis.org/arthritis-news/prosorba-column-receives-fda-approval-for-rheumatoid-arthritis-treatment/?utm_source=chatgpt.com (Accessed February 24, 2025).
(2013). Drug Approval Package: Rayos (prednisone) delayed release ta blet 1 mg, 2 mg, 5 mg. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202020_rayos_toc.cfm (Accessed February 24, 2025).
(2020). Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine (U.S. Food and Drug Administration).
(2012). “Adalimumab,” in LiverTox: clinical and research information on drug-induced liver injury(Bethesda (MD). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK548542/.
Benrud-Larson, L. M., Dewar, M. S., Sandroni, P., Rummans, T. A., Haythornthwaite, J. A., and Low, P. A. (2002). Quality of life in patients with postural tachycardia syndrome. Mayo Clin. Proc. 77, 531–537. doi: 10.4065/77.6.531
Blitshteyn, S. (2025). Neuroinflammation at the dorsolateral inferior medulla: A possible central nervous system localization for POTS and long COVID. Biomedicines 13. doi: 10.3390/biomedicines13010166
Blitshteyn, S. (2015). Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS). Lupus 24, 1364–1369. doi: 10.1177/0961203315587566
Blitshteyn, S., Whiteson, J. H., Abramoff, B., Azola, A., Bartels, M. N., Bhavaraju-Sanka, R., et al. (2022). Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of autonomic dysfunction in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R 14, 1270–1291. doi: 10.1002/pmrj.12894
Blitshteyn, S. and Whitelaw, S. (2021). Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol. Res. 69, 205–211. doi: 10.1007/s12026-021-09185-5
Bourne, K. M., Chew, D. S., Stiles, L. E., Shaw, B. H., Shibao, C. A., Okamoto, L. E., et al. (2021). Postural orthostatic tachycardia syndrome is associated with significant employment and economic loss. J. Intern. Med. 290, 203–212. doi: 10.1111/joim.13245
Bouxin, M., Schvartz, B., Mestrallet, S., Debrumetz, A., Hentzien, M., Tabary, T., et al. (2019). Rituximab treatment in seronegative autoimmune autonomic neuropathy and autoimmune autonomic ganglionopathy: Case-report and literature review. J. Neuroimmunol 326, 28–32. doi: 10.1016/j.jneuroim.2018.11.009
Brignole, M., Moya, A., de Lange, F. J., Deharo, J. C., Elliott, P. M., Fanciulli, A., et al. (2018). 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 39, 1883–1948. doi: 10.1093/eurheartj/ehy037
Brownlie, H. and Speight, N. (2021). Back to the future? Immunoglobulin therapy for myalgic encephalomyelitis/chronic fatigue syndrome. Healthcare (Basel) 9, 1546. doi: 10.3390/healthcare9111546
Brunner, H. I., Ruperto, N., Zuber, Z., Cuttica, R., Keltsev, V., Xavier, R. M., et al. (2021). Efficacy and safety of tocilizumab for polyarticular-course juvenile idiopathic arthritis in the open-label two-year extension of a phase III trial. Arthritis Rheumatol 73, 530–541. doi: 10.1002/art.41528
Buchman, A. L. (2001). Side effects of corticosteroid therapy. J. Clin. Gastroenterol. 33, 289–294. doi: 10.1097/00004836-200110000-00006
Cabral, R. T. S., Klumb, E. M., Couto, M., and Carneiro, S. (2019). Evaluation of toxic retinopathy caused by antimalarial medications with spectral domain optical coherence tomography. Arq Bras. Oftalmol 82, 12–17. doi: 10.5935/0004-2749.20190002
Chemali, K. R., Blitshteyn, S., Perez, J. A., and Schofield, J. (2024). iSTAND trial of IVIG in POTS: a step in the right direction, but more studies are needed. Clin. Auton Res. 35, 335–337. doi: 10.1007/s10286-024-01087-4
Dalakas, M. C. (2021). Update on intravenous immunoglobulin in neurology: modulating neuro-autoimmunity, evolving factors on efficacy and dosing and challenges on stopping chronic IVIg therapy. Neurotherapeutics 18, 2397–2418. doi: 10.1007/s13311-021-01108-4
Danieli, M. G., Antonelli, E., Gammeri, L., Longhi, E., Cozzi, M. F., Palmeri, D., et al. (2025). Intravenous immunoglobulin as a therapy for autoimmune conditions. Autoimmun Rev. 24, 103710. doi: 10.1016/j.autrev.2024.103710
Davenport, T. E., Blitshteyn, S., Clague-Baker, N., Davies-Payne, D., Treisman, G. J., and Tyson, S. F. (2024). Long COVID is not a functional neurologic disorder. J. Pers. Med. 14, 799. doi: 10.3390/jpm14080799
Delate, T., Hansen, M. L., Gutierrez, A. C., and Le, K. N. (2020). Indications for rituximab use in an integrated health care delivery system. J. Manag Care Spec Pharm. 26, 832–838. doi: 10.18553/jmcp.2020.26.7.832
Du, C., Zeng, P., Han, J. R., Zhang, T. X., Jia, D., Shi, F. D., et al. (2021). Early initiation of tocilizumab treatment against moderate-to-severe myelitis in neuromyelitis optica spectrum disorder. Front. Immunol. 12, 660230. doi: 10.3389/fimmu.2021.660230
Dulal, D., Maraey, A., Elsharnoby, H., Chacko, P., and Grubb, B. (2025). Impact of COVID-19 Pandemic on the incidence and prevalence of postural orthostatic tachycardia syndrome. Eur. Heart J. Qual Care Clin. Outcomes. 11, 698–704. doi: 10.1093/ehjqcco/qcae111
El-Rhermoul, F. Z., Fedorowski, A., Eardley, P., Taraborrelli, P., Panagopoulos, D., Sutton, R., et al. (2023). Autoimmunity in long covid and POTS. Oxf Open Immunol. 4, iqad002. doi: 10.1093/oxfimm/iqad002
Ely, E. W., Brown, L. M., and Fineberg, H. V. (2024). Long covid defined. N Engl. J. Med. 391, 1746–1753. doi: 10.1056/NEJMsb2408466
Fatima, R., Bittar, K., and Aziz, M. (2025). “Infliximab,” in StatPearls (Treasure Island (FL): StatPearls [Internet]).
Finzel, S., Kraus, S., Figueiredo, C. P., Regensburger, A., Kocijan, R., Rech, J., et al. (2019). Comparison of the effects of tocilizumab monotherapy and adalimumab in combination with methotrexate on bone erosion repair in rheumatoid arthritis. Ann. Rheum Dis. 78, 1186–1191. doi: 10.1136/annrheumdis-2018-214894
Flanagan, E. P., Saito, Y. A., Lennon, V. A., McKeon, A., Fealey, R. D., Szarka, L. A., et al. (2014). Immunotherapy trial as diagnostic test in evaluating patients with presumed autoimmune gastrointestinal dysmotility. Neurogastroenterol Motil. 26, 1285–1297. doi: 10.1111/nmo.12391
Fraenkel, L., Bathon, J. M., England, B. R., St Clair, E. W., Arayssi, T., Carandang, K., et al. (2021). American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. (Hoboken) 73 (7), 924–939. doi: 10.1002/acr.24596
Freeman, R., Wieling, W., Axelrod, F. B., Benditt, D. G., Benarroch, E., Biaggioni, I., et al. (2011). Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton Res. 21, 69–72. doi: 10.1007/s10286-011-0119-5
Gampa, A. and Upadhyay, G. A. (2018). Treatment of neurocardiogenic syncope: from conservative to cutting-edge. J. Innov. Card Rhythm. Manag 9, 3221–3231. doi: 10.19102/icrm.2018.090702
Gavrilova, N., Kamaeva, E., Ignatova, M., Ryabkova, V., Lukashenko, M., Soprun, L., et al. (2022). Intravenouse immunoglobuline in dysautonomia. Clin. Immunol. 240, 109039. doi: 10.1016/j.clim.2022.109039
Gemignani, F., Bellanova, M. F., Saccani, E., and Pavesi, G. (2022). Non-length-dependent small fiber neuropathy: Not a matter of stockings and gloves. Muscle Nerve 65, 10–28. doi: 10.1002/mus.27379
Gendre, T., Lefaucheur, J. P., Nordine, T., Baba-Amer, Y., Authier, F. J., Devaux, J., et al. (2024). Characterizing acute-onset small fiber neuropathy. . Neurol. Neuroimmunol Neuroinflamm 11, e200195. doi: 10.1212/NXI.0000000000200195
Gibbons, C. H., Vernino, S. A., and Freeman, R. (2008). Combined immunomodulatory therapy in autoimmune autonomic ganglionopathy. Arch. Neurol. 65, 213–217. doi: 10.1001/archneurol.2007.60
Goodman, B. (2014). Immunoresponsive postinfectious autonomic neuropathy. Am. J. Ther. 21, e120–e123. doi: 10.1097/MJT.0b013e31825e6068
Goodman, B. (2019). Immunoresponsive autonomic neuropathy in sjogren syndrome-case series and literature review. Am. J. Ther. 26, e66–e71. doi: 10.1097/MJT.0000000000000583
Goto, Y., Sunami, Y., Sugaya, K., Nakane, S., and Takahashi, K. (2021). A case of chronic postural tachycardia syndrome with positive anti-ganglionic acetylcholine receptor (gAChR) antibody. Rinsho Shinkeigaku 61, 547–551. doi: 10.5692/clinicalneurol.cn-001598
Grubb, A. F. and Grubb, B. (2023). Postural orthostatic tachycardia syndrome: New concepts in pathophysiology and management. Trends Cardiovasc. Med. 33, 65–69. doi: 10.1016/j.tcm.2021.10.007
Gunning, W. T., 3rd, Kvale, H., Kramer, P. M., Karabin, B. L., and Grubb, B. P. (2019). Postural orthostatic tachycardia syndrome is associated with elevated G-protein coupled receptor autoantibodies. J. Am. Heart Assoc. 8, e013602. doi: 10.1161/JAHA.119.013602
Guo, Y., Tian, X., Wang, X., and Xiao, Z. (2018). Adverse effects of immunoglobulin therapy. Front. Immunol. 9, 1299. doi: 10.3389/fimmu.2018.01299
Ham, J. C., van Meerten, E., Fiets, W. E., Beerepoot, L. V., Jeurissen, F. J. F., Slingerland, M., et al. (2020). Methotrexate plus or minus cetuximab as first-line treatment in a recurrent or metastatic (R/M) squamous cell carcinoma population of the head and neck (SCCHN), unfit for cisplatin combination treatment, a phase Ib-randomized phase II study Commence. Head Neck 42, 828–838. doi: 10.1002/hed.26053
Hanoodi, M. and Mittal, M. (2025). “Methotrexate,” StatPearls [Internet] (StatPearls, Treasure Island (FL).
Hendrix, A., Nesheiwat, Z., Towheed, A., Brar, V., and Grubb, B. P. (2021). Adalimumab as a potential treatment for postural orthostatic tachycardia syndrome. HeartRhythm Case Rep. 7, 56–58. doi: 10.1016/j.hrcr.2020.11.003
Hollenbeck, R., Black, B. K., Peltier, A. C., Biaggioni, I., Robertson, D., Winton, E. F., et al. (2011). Long-term treatment with rituximab of autoimmune autonomic ganglionopathy in a patient with lymphoma. Arch. Neurol. 68, 372–375. doi: 10.1001/archneurol.2010.289
Hsieh, T. S. and Tsai, T. F. (2024). Combination of methotrexate with oral disease-modifying antirheumatic drugs in psoriatic arthritis: a systematic review. Immunotherapy 16, 115–130. doi: 10.2217/imt-2023-0139
Inc. A (2019). Label: IMATINIB MESYLATE 400 MG- imatinib mesylate tab let, film coated. Available online at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7b7cc194-29e4-4484-a364-a1ac7d7d6cf5.
Remap-Cap Investigators, Gordon, A. C., Mouncey, P. R., Al-Beidh, F., Rowan, K. M., Nichol, A. D, et al. (2021). Interleukin-6 receptor antagonists in critically ill patients with covid-19. N Engl. J. Med. 384, 1491–1502. doi: 10.1056/NEJMoa2100433
Isman, A., Nyquist, A., Strecker, B., Harinath, G., Lee, V., Zhang, X., et al. (2024). Low-dose naltrexone and NAD+ for the treatment of patients with persistent fatigue symptoms after COVID-19. Brain Behav. Immun. Health 36, 100733. doi: 10.1016/j.bbih.2024.100733
Jacob, G., Costa, F., Shannon, J. R., Robertson, R. M., Wathen, M., Stein, M., et al. (2000). The neuropathic postural tachycardia syndrome. N Engl. J. Med. 343, 1008–1014. doi: 10.1056/NEJM200010053431404
Jorge, A., Ung, C., Young, L. H., Melles, R. B., and Choi, H. K. (2018). Hydroxychloroquine retinopathy - implications of research advances for rheumatology care. Nat. Rev. Rheumatol 14, 693–703. doi: 10.1038/s41584-018-0111-8
Kataria, R., Suja, L., Anil, A. A., and Senthil, N. (2023). Primary Sjogren’s syndrome presenting as an isolated severe autonomic dysfunction treated with steroids. BMJ Case Rep. 16, e256412. doi: 10.1136/bcr-2023-256412
Kesterson, K., Schofield, J., and Blitshteyn, S. (2023). Immunotherapy with subcutaneous immunoglobulin or plasmapheresis in patients with postural orthostatic tachycardia syndrome (POTS). J. Neurol. 270, 233–239. doi: 10.1007/s00415-022-11344-z
Kharraziha, I., Axelsson, J., Ricci, F., Di Martino, G., Persson, M., Sutton, R., et al. (2020). Serum activity against G protein-coupled receptors and severity of orthostatic symptoms in postural orthostatic tachycardia syndrome. J. Am. Heart Assoc. 9, e015989. doi: 10.1161/JAHA.120.015989
Kumar, C. and Zito, M. (2025). “Omalizumab,” StatPearls [Internet] (StatPearls, Treasure Island (FL).
Ladriere, M. (2013). Current indications of azathioprine in nephrology. Nephrol. Ther. 9, 8–12. doi: 10.1016/j.nephro.2012.08.002
Lahad, A. and Weiss, B. (2015). Current therapy of pediatric Crohn’s disease. World J. Gastrointest Pathophysiol 6, 33–42. doi: 10.4291/wjgp.v6.i2.33
Larsen, N. W., Stiles, L. E., Shaik, R., Schneider, L., Muppidi, S., Tsui, C. T., et al. (2022). Characterization of autonomic symptom burden in long COVID: A global survey of 2,314 adults. Front. Neurol. 13, 1012668. doi: 10.3389/fneur.2022.1012668
Larsen, N. W., Stiles, L. E., and Miglis, M. G. (2021). Preparing for the long-haul: Autonomic complications of COVID-19. Auton Neurosci. 235, 102841. doi: 10.1016/j.autneu.2021.102841
Li, H., Yu, X., Liles, C., Khan, M., Vanderlinde-Wood, M., Galloway, A., et al. (2014). Autoimmune basis for postural tachycardia syndrome. J. Am. Heart Assoc. 3, e000755. doi: 10.1161/JAHA.113.000755
Li, Z., You, Y., Griffin, N., Feng, J., and Shan, F. (2018). Low-dose naltrexone (LDN): A promising treatment in immune-related diseases and cancer therapy. Int. Immunopharmacol 61, 178–184. doi: 10.1016/j.intimp.2018.05.020
Lloyd, A., Hickie, I., Wakefield, D., Boughton, C., and Dwyer, J. (1990). A double-blind, placebo-controlled trial of intravenous immunoglobulin therapy in patients with chronic fatigue syndrome. Am. J. Med. 89, 561–568. doi: 10.1016/0002-9343(90)90173-B
Low, A. (1993). Autonomic nervous system function. J. Clin. Neurophysiol. 10, 14–27. doi: 10.1097/00004691-199301000-00003
Low, P. A., Opfer-Gehrking, T. L., Textor, S. C., Schondorf, R., Suarez, G. A., Fealey, R. D., et al. (1994). Comparison of the postural tachycardia syndrome (POTS) with orthostatic hypotension due to autonomic failure. J. Auton Nerv Syst. 50, 181–188. doi: 10.1016/0165-1838(94)90008-6
Low, P. A., Opfer-Gehrking, T. L., Textor, S. C., Benarroch, E. E., Shen, W. K., Schondorf, R., et al. (1995). Postural tachycardia syndrome (POTS). Neurology 45, S19–S25.
Low, A., Sandroni, P., Joyner, M., and Shen, W. K. (2009). Postural tachycardia syndrome (POTS). J. Cardiovasc. Electrophysiol 20, 352–358. doi: 10.1111/j.1540-8167.2008.01407.x
Maier, A., Kapfenberger, R., Katona, I., Weis, J., Schulz, J. B., and Rolke, R. (2022). Nonregional small fibre neuropathy in cases of autoimmune autonomic neuropathy. J. Neurol. 269, 6648–6654. doi: 10.1007/s00415-022-11340-3
Maini, R., St Clair, E. W., Breedveld, F., Furst, D., Kalden, J., Weisman, M., et al. (1999). Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Grou Lancet 354, 1932–1939. doi: 10.1016/S0140-6736(99)05246-0
Mannan, H. and Pain, C. M. (2023). Sex adjusted standardized prevalence ratios for celiac disease and other autoimmune diseases in patients with postural orthostatic tachycardia syndrome (POTS): A systematic review and meta-analysis. Heliyon 9, e12982. doi: 10.1016/j.heliyon.2023.e12982
Marwa, K. and Anjum, F. (2025). “Undifferentiated connective tissue disease,” in StatPearls(Treasure Island (FL): StatPearls [Internet]).
Maurer, M., Rosen, K., Hsieh, H. J., Saini, S., Grattan, C., Gimenez-Arnau, A., et al. (2013). Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl. J. Med. 368, 924–935. doi: 10.1056/NEJMoa1215372
McAlpine, L., Zubair, A. S., Joseph, P., and Spudich, S. (2024). Case-control study of individuals with small fiber neuropathy after COVID-19. Neurol. Neuroimmunol Neuroinflamm 11, e200244. doi: 10.1212/NXI.0000000000200244
Medow, M. S., Stewart, J. M., Sanyal, S., Mumtaz, A., Sica, D., and Frishman, W. H. (2008). Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol. Rev. 16, 4–20. doi: 10.1097/CRD.0b013e31815c8032
Mosca, M., Neri, R., and Bombardieri, S. (1999). Undifferentiated connective tissue diseases (UCTD): a review of the literature and a proposal for preliminary classification criteria. Clin. Exp. Rheumatol 17, 615–620.
Nakane, S., Koike, H., Hayashi, T., and Nakatsuji, Y. (2024). Autoimmune autonomic neuropathy: from pathogenesis to diagnosis. Int. J. Mol. Sci. 25, 2296. doi: 10.3390/ijms25042296
National Academies of Sciences, E. and Medicine (2024). A long COVID definition: A chronic, systemic disease state with profound consequences. Ed. Fineberg, H. V., et al (Washington, DC: The National Academies Press), 186.
Novak (2020). Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. eNeurologicalSci 21, 100276. doi: 10.1016/j.ensci.2020.100276
Nowak, D. (2006). Management of asthma with anti-immunoglobulin E: a review of clinical trials of omalizumab. Respir. Med. 100, 1907–1917. doi: 10.1016/j.rmed.2005.10.004
O’Kelly, B., Vidal, L., McHugh, T., Woo, J., Avramovic, G., and Lambert, J. S. (2022). Safety and efficacy of low dose naltrexone in a long covid cohort; an interventional pre-post study. Brain Behav. Immun. Health 24, 100485. doi: 10.1016/j.bbih.2022.100485
Oishi, M., Mukaino, A., Kunii, M., Saito, A., Arita, Y., Koike, H., et al. (2021). Association between neurosarcoidosis with autonomic dysfunction and anti-ganglionic acetylcholine receptor antibodies. J. Neurol. 268, 4265–4279. doi: 10.1007/s00415-021-10551-4
Olshansky, B. and Sullivan, R. M. (2019). Inappropriate sinus tachycardia. Europace 21, 194–207. doi: 10.1093/europace/euy128
Osman, C., Jennings, R., El-Ghariani, K., and Pinto, A. (2020). Plasma exchange in neurological disease. Pract. Neurol. 20, 92–99. doi: 10.1136/practneurol-2019-002336
Pang, L. Y., Ding, C. H., Wang, Y. Y., Liu, L. Y., Li, Q. J., and Zou, L. P. (2017). Acute autonomic neuropathy with severe gastrointestinal symptoms in children: a case series. BMC Neurol. 17, 164. doi: 10.1186/s12883-017-0943-x
Pasricha, P. J., McKnight, M., Villatoro, L., Barahona, G., Brinker, J., Hui, K., et al (2024). Joint Hypermobility, Autonomic dysfunction, gastrointestinal dysfunction, and autoimmune markers: clinical associations and response to intravenous immunoglobulin therapy. Am. J. Gastroenterol. 119, 2298–2306. doi: 10.14309/ajg.0000000000002910
Peluso, M. J. and Deeks, S. G. (2024). Mechanisms of long COVID and the path toward therapeutics. Cell 187, 5500–5529. doi: 10.1016/j.cell.2024.07.054
Penny, H. A., Aziz, I., Ferrar, M., Atkinson, J., Hoggard, N., Hadjivassiliou, M., et al. (2016). Is there a relationship between gluten sensitivity and postural tachycardia syndrome? Eur. J. Gastroenterol. Hepatol. 28, 1383–1387. doi: 10.1097/MEG.0000000000000740
Perez, E. E., Orange, J. S., Bonilla, F., Chinen, J., Chinn, I. K., Dorsey, M., et al. (2017). Update on the use of immunoglobulin in human disease: A review of evidence. J. Allergy Clin. Immunol. 139, S1–S46. doi: 10.1016/j.jaci.2016.09.023
Peterson, P. K., Shepard, J., Macres, M., Schenck, C., Crosson, J., Rechtman, D., et al. (1990). A controlled trial of intravenous immunoglobulin G in chronic fatigue syndrome. Am. J. Med. 89, 554–560. doi: 10.1016/0002-9343(90)90172-A
Pitarokoili, K., Maier, A., de Moya Rubio, E. C., Hahn, K., Wallukat, G., Athanasopoulos, D., et al. (2021). Maintenance therapy with subcutaneous immunoglobulin in a patient with immune-mediated neuropathic postural tachycardia syndrome. J. Transl. Autoimmun 4, 100112. doi: 10.1016/j.jtauto.2021.100112
Pola, S., Patel, D., Ramamoorthy, S., McLemore, E., Fahmy, M., Rivera-Nieves, J., et al. (2012). Strategies for the care of adults hospitalized for active ulcerative colitis. Clin. Gastroenterol. Hepatol. 10, 1315–1325.e4. doi: 10.1016/j.cgh.2012.07.006
Raj, S. R., Fedorowski, A., and Sheldon, R. S. (2022). Diagnosis and management of postural orthostatic tachycardia syndrome. CMAJ 194, E378–E385. doi: 10.1503/cmaj.211373
Rodriguez, B., Hoepner, R., Salmen, A., Kamber, N., and Z'Graggen, W. J. (2021). Immunomodulatory treatment in postural tachycardia syndrome: A case series. Eur. J. Neurol. 28, 1692–1697. doi: 10.1111/ene.14711
Rodriguez-Manero, M., Kreidieh, B., Al Rifai, M., Ibarra-Cortez, S., Schurmann, P., Alvarez, P. A., et al. (2017). Ablation of inappropriate sinus tachycardia: A systematic review of the literature. JACC Clin. Electrophysiol 3, 253–265. doi: 10.1016/j.jacep.2016.09.014
Rojas, M., Rodriguez, Y., Acosta-Ampudia, Y., Monsalve, D. M., Zhu, C., Li, Q. Z., et al. (2022). Autoimmunity is a hallmark of post-COVID syndrome. J. Transl. Med. 20, 129. doi: 10.1186/s12967-022-03328-4
Rowe, K. S. (1997). Double-blind randomized controlled trial to assess the efficacy of intravenous gammaglobulin for the management of chronic fatigue syndrome in adolescents. J. Psychiatr. Res. 31, 133–147. doi: 10.1016/S0022-3956(96)00047-7
Rubio, J. and Kyttaris, V. C. (2023). Undifferentiated connective tissue disease: comprehensive review. Curr. Rheumatol Rep. 25, 98–106. doi: 10.1007/s11926-023-01099-5
Saedon, N. I., Pin Tan, M., and Frith, J. (2020). The prevalence of orthostatic hypotension: A systematic review and meta-analysis. J. Gerontol A Biol. Sci. Med. Sci. 75, 117–122. doi: 10.1093/gerona/gly188
Salama, C., Han, J., Yau, L., Reiss, W. G., Kramer, B., Neidhart, J. D., et al. (2021). Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl. J. Med. 384, 20–30. doi: 10.1056/NEJMoa2030340
Schofield, J. R. and Chemali, K. R. (2019). Intravenous immunoglobulin therapy in refractory autoimmune dysautonomias: A retrospective analysis of 38 patients. Am. J. Ther. 26, 570–582. doi: 10.1097/MJT.0000000000000778
Schofield, J. R., Blitshteyn, S., Shoenfeld, Y., and Hughes, G. R. (2014). Postural tachycardia syndrome (POTS) and other autonomic disorders in antiphospholipid (Hughes) syndrome (APS). Lupus 23, 697–702. doi: 10.1177/0961203314524468
Schondorf, R. and Low, A. (1993). Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 43, 132–137. doi: 10.1212/WNL.43.1_Part_1.132
SE, A. (2024). argenx to unveil its ‘Vision 2030: Taking Breakthrough Science to 50,000 Patients’ during its Upcoming R&D Day on July 16, 2024 (Globe News Wire).
Seeley, M. C., Hooper, M., Tan, J., Wells, R., Gallagher, C., and Lau, D. H. (2025). Plasma exchange improves cognitive function in long-COVID-related postural orthostatic tachycardia syndrome and autoimmune neurological dysfunction. Am. J. Med. 138, 153–154. doi: 10.1016/j.amjmed.2023.01.043
Sergent, S. R. and Ashurst, J. V. (2025). “Plasmapheresis,” in StatPearls (Treasure Island (FL): StatPearls [Internet]).
Sharma, C. and Bayry, J. (2023). High risk of autoimmune diseases after COVID-19. Nat. Rev. Rheumatol 19, 399–400. doi: 10.1038/s41584-023-00964-y
Shaw, B. H., Stiles, L. E., Bourne, K., Green, E. A., Shibao, C. A., Okamoto, L. E., et al. (2019). The face of postural tachycardia syndrome - insights from a large cross-sectional online community-based survey. J. Intern. Med. 286, 438–448. doi: 10.1111/joim.12895
Sheldon, R. S., Grubb, B. P., Olshansky, B., Shen, W. K., Calkins, H., Brignole, M., et al. (2015). 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 12, e41–e63. doi: 10.1016/j.hrthm.2015.03.029
Sjogren, Bragee, B., and Britton, S. (2024). Successful subcutaneous immunoglobulin therapy in a case series of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Clin. Ther. 46, 597–600. doi: 10.1016/j.clinthera.2024.05.010
Sokmen, O., Temucin, C. M., Ayhan Seker, C., and Tan, E. (2023). Immunotherapy provides electrophysiological recovery and excellent clinical response in sjogren’s syndrome-linked quite severe autonomic neuropathy. Neurologist 28, 204–206. doi: 10.1097/NRL.0000000000000468
Sollinger, H. W. (1995). Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant. Mycophenolate Mofetil Study Grou Transplant. 60, 225–232. doi: 10.1097/00007890-199508000-00003
Son, K., Jamil, R., Chowdhury, A., Mukherjee, M., Venegas, C., Miyasaki, K., et al. (2023). Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur. Respir. J. 61, 2200970. doi: 10.1183/13993003.00970-2022
Statistics, N.C.f.H (2024). Long COVID, household pulse survey, 2022–2024. Available online at: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm (Accessed February 23, 2025).
Stein, E., Heindrich, C., Wittke, K., Kedor, C., Rust, R., Freitag, H., et al. (2025). Efficacy of repeated immunoadsorption in patients with post-COVID myalgic encephalomyelitis/chronic fatigue syndrome and elevated beta2-adrenergic receptor autoantibodies: a prospective cohort study. Lancet Reg. Health Eur. 49, 101161. doi: 10.1016/j.lanepe.2024.101161
Stone, J. H., Tuckwell, K., Dimonaco, S., Klearman, M., Aringer, M., Blockmans, D., et al. (2017). Trial of tocilizumab in giant-cell arteritis. N Engl. J. Med. 377, 317–328. doi: 10.1056/NEJMoa1613849
Syngle, A., Verma, I., and Krishan (2015). Interleukin-6 blockade improves autonomic dysfunction in rheumatoid arthritis. Acta Reumatol Port 40, 85–88.
Thieben, M. J., Sandroni, P., Sletten, D. M., Benrud-Larson, L. M., Fealey, R. D., Vernino, S., et al. (2007). Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin. Proc. 82, 308–313. doi: 10.1016/S0025-6196(11)61027-6
Tiongson, E., Pimentel, N., Ramos-Platt, L., and Jaradeh, S. (2016). Pediatric anti-ganglionic antibody positive autonomic neuropathy: clinical presentation and response to treatment. Pediatr. Neurol. 64, 72–76. doi: 10.1016/j.pediatrneurol.2016.06.007
Tomisti, L., Angelotti, F., Lenzi, M., Amadori, F., Sarteschi, G., Porcu, A., et al. (2023). Efficacy of convalescent plasma to treat long-standing COVID-19 in patients with B-cell depletion. Life (Basel) 13, 1266. doi: 10.3390/life13061266
Traczewski and Rudnicka, L. (2008). Adalimumab in dermatology. Br. J. Clin. Pharmacol. 66, 618–625. doi: 10.1111/j.1365-2125.2008.03263.x
Trofimovitch, D. and Baumrucker, S. J. (2019). Pharmacology update: low-dose naltrexone as a possible nonopioid modality for some chronic, nonmalignant pain syndromes. Am. J. Hosp Palliat Care 36, 907–912. doi: 10.1177/1049909119838974
Vahratian, A., Saydah, S., Bertolli, J., Unger, E. R., and Gregory, C. O. (2024). Prevalence of post-COVID-19 condition and activity-limiting post-COVID-19 condition among adults. JAMA Netw. Open 7, e2451151. doi: 10.1001/jamanetworkopen.2024.51151
Vermersch, Stojkovic, T., and de Seze, J. (2005). Mycophenolate mofetil and neurological diseases. Lupus 14 Suppl 1, s42–s45. doi: 10.1191/0961203305lu2117oa
Vernino, S., Hopkins, S., Bryarly, M., Hernandez, R. S., and Salter, A. (2008). Invited Article: Autonomic ganglia: target and novel therapeutic tool. Neurology 70, 1926–1932. doi: 10.1212/01.wnl.0000312280.44805.5d
Vernino, S., Bourne, K. M., Stiles, L. E., Grubb, B. P., Fedorowski, A., Stewart, J. M., et al. (2021). Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting - Part 1. Auton Neurosci. 235, 102828. doi: 10.1016/j.autneu.2021.102828
Vernino, S., Sandroni, P., Singer, W., and Low, P. A. (2024). Randomized controlled trial of intravenous immunoglobulin for autoimmune postural orthostatic tachycardia syndrome (iSTAND). Clin. Auton Res. 34, 153–163. doi: 10.1007/s10286-024-01020-9
Vollmer-Conna, U., Hickie, I., Hadzi-Pavlovic, D., Tymms, K., Wakefield, D., Dwyer, J., et al. (1997). Intravenous immunoglobulin is ineffective in the treatment of patients with chronic fatigue syndrome. Am. J. Med. 103, 38–43. doi: 10.1016/S0002-9343(97)90045-0
Wallukat, G., Hohberger, B., Wenzel, K., Furst, J., Schulze-Rothe, S., Wallukat, A., et al. (2021). Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun 4, 100100. doi: 10.1016/j.jtauto.2021.100100
Watari, M., Nakane, S., Mukaino, A., Nakajima, M., Mori, Y., Maeda, Y., et al. (2018). Autoimmune postural orthostatic tachycardia syndrome. Ann. Clin. Transl. Neurol. 5, 486–492. doi: 10.1002/acn3.524
Weinstock, L. B., Brook, J. B., Myers, T. L., and Goodman, B. (2018). Successful treatment of postural orthostatic tachycardia and mast cell activation syndromes using naltrexone, immunoglobulin and antibiotic treatment. BMJ Case Rep. 2018. doi: 10.1136/bcr-2017-221405
Wells, R., Hissaria, P., Elliott, A. D., Sanders, P., Page, A., Baumert, M., et al. (2020). Plasma exchange therapy in postural tachycardia syndrome: A novel long-term approach? Am. J. Med. 133, e157–e159. doi: 10.1016/j.amjmed.2019.10.016
Yu, X., Li, H., Murphy, T. A., Nuss, Z., Liles, J., Liles, C., et al. (2018). Angiotensin II type 1 receptor autoantibodies in postural tachycardia syndrome. J. Am. Heart Assoc. 7. doi: 10.1161/JAHA.117.008351
Keywords: postural orthostatic tachycardia syndrome, dysautonomia, autonomic disorders, immunotherapy, immunoglobulin, autoimmunity, therapeutics
Citation: Blitshteyn S, Funez-dePagnier G, Szombathy A and Hutchinson M (2025) Immunotherapies for postural orthostatic tachycardia syndrome, other common autonomic disorders, and Long COVID: current state and future direction. Front. Cell. Infect. Microbiol. 15:1647203. doi: 10.3389/fcimb.2025.1647203
Received: 15 June 2025; Accepted: 25 August 2025;
Published: 29 September 2025.
Edited by:
Hui-Qi Qu, Children’s Hospital of Philadelphia, United StatesReviewed by:
Viktor Hamrefors, Lund University, SwedenMichael Weintraub, New York Medical College, United States
Copyright © 2025 Blitshteyn, Funez-dePagnier, Szombathy and Hutchinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Svetlana Blitshteyn, c2IyNUBidWZmYWxvLmVkdQ==
 Svetlana Blitshteyn
Svetlana Blitshteyn Gabriela Funez-dePagnier
Gabriela Funez-dePagnier Anna Szombathy
Anna Szombathy Meagan Hutchinson3
Meagan Hutchinson3