- 1Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Respiratory and Critical Care Medicine, Taizhou Hospital, Taizhou, China
- 3Department of Respiratory and Critical Care Medicine, The Affiliated Hospital, Shaoxing University, Shaoxing, China
- 4Department of Respiratory and Critical Care Medicine, Shaoxing Central Hospital, Shaoxing, China
Introduction: Invasive aspergillosis (IA) is a severe fungal infection. Metagenomic Next Generation Sequencing (mNGS) is abroad and highly sensitive pathogen detection method that can accurately differentiate fungi to the species, and even subspecies level.
Methods: To explore the value of plasma mNGSs in the diagnosis of invasive aspergillosis, a retrospective analysis was conducted on the clinical data of 334 patients with findings of Aspergillus spp. From mNGS from plasma at 4 hospitals, Zhejiang, from February 2021 to December 2022. The study analyzed risk factors, clinical manifestations, imaging features, microbiological results, and treatment outcomes of patients with Aspergillus infection.
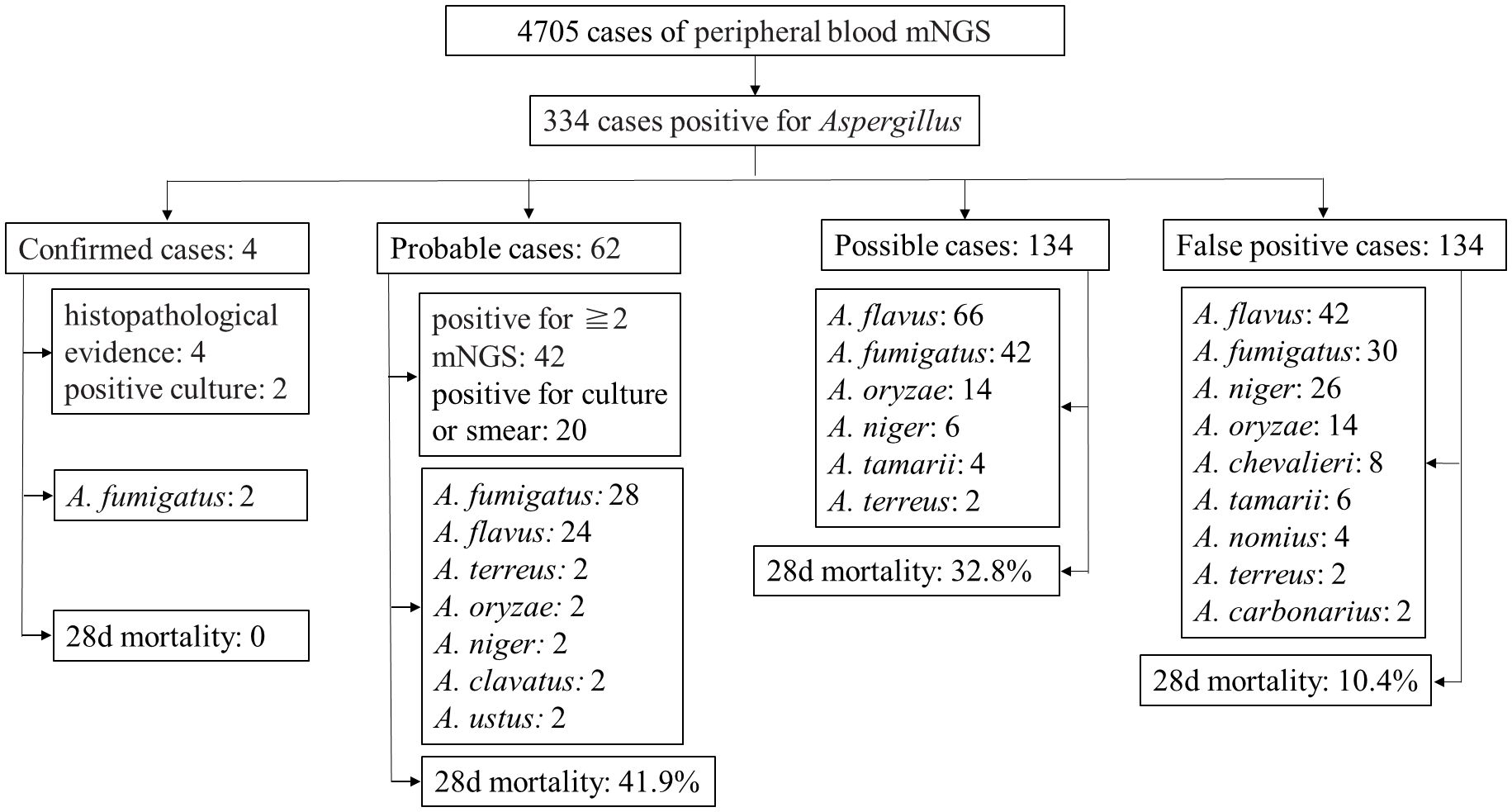
Results and discussion: According to the diagnostic criteria for IA, among the 334 patients, there were 4 confirmed cases, 62 probable cases, 134 possible cases, and 134 false-positive cases. All 196 probable and possible cases exhibited risk factors, clinical manifestations, imaging features, and treatment outcomes consistent with Aspergillus infection. In 18 out of the 62 probable cases, the same Aspergillus nucleic acid was found in 2–4 peripheral blood mNGS samples collected at intervals of 17 days. The remaining 134 patients had detectable Aspergillus in plasma mNGS but lacked high-risk factors and clinical characteristics of Aspergillus infection, and there was a lack of other microbiological evidence, determined as false positives. Among the cases included in this study, the positive predictive value of plasma mNGS for diagnosing invasive aspergillosis was 59.9%. Plasma mNGS detection has significant reference value for diagnosing IA. However, comprehensive judgment should still be made in conjunction with clinical features.
1 Introduction
Invasive aspergillosis (IA) is a severe fungal infection caused by Aspergillus, often occurring in patients with severe immunodeficiency, such as those with hematological malignancies, hematopoietic stem cell transplantation, solid organ transplantation, etc. Mortality is high in patients who do not receive early and appropriate antifungal treatment (Yerbanga et al., 2023). There is an increasing number of reports of IA in critically ill patients in the intensive care unit, especially in those with risk factors such as corticosteroid use, chronic obstructive pulmonary disease, liver failure, malnutrition, chronic kidney disease, diabetes, severe viral pneumonia (such as Influenza, COVID-19), etc (Bassetti et al., 2021).
Timely and accurate diagnosis with effective treatment is a key factor in improving prognosis and reducing unreasonable use of antibiotics. The definitive diagnosis of Aspergillus infection relies on microscopic examination or culture. Microbiological evidence for the clinical diagnosis of aspergillosis mainly includes microscopic examination, cultures, Aspergillus galactomannan, or nucleic acid detection. However, the positive rate of culture for clinical samples is low. In 2020, Aspergillus PCR (Molecular Diagnostic Assays) was included in the definition of invasive aspergillosis, requiring two positive results to provide sufficient specificity for confirmation of the diagnosis (Koehler et al., 2021). Metagenomic Next Generation Sequencing (mNGS) is a broad and highly sensitive pathogen detection method that can accurately differentiate fungi to the species, and even subspecies level. This technology has the potential to improve the sensitivity of detection for invasive fungal infections (Jenks et al., 2023). Therefore, we aim to explore the diagnostic value of plasma mNGS in Aspergillus infections. Here, we retrospectively analyzed the clinical data of 167 patients with detectable Aspergillus spp. in plasma mNGS at 4 hospitals, Zhejiang, from February 2021 to December 2022, and the findings are summarized as follows.
2 Objectives and methods
2.1 Study subjects
A total of 4705 patients underwent peripheral blood mNGS testing at 4 hospitals, Zhejiang, between 01/02/2021 and 30/12/2022. Patients who were found to have detectable nucleic acid of the Aspergillus genus were included. Clinical comprehensive diagnoses of invasive aspergillosis or non-infection were made by three senior experts in respiratory medicine, infectious diseases, and radiology. This decision was reached through discussions with the patient’s primary medical team, considering clinical symptoms, laboratory results, imaging studies, microbiology, pathology examinations, as well as treatment, prognosis, etc. Cases with incomplete clinical data, individuals under 18 years old, pregnant or postpartum women, and cases of non-hospitalized patients were excluded.
2.2 Relevant definitions
IA is categorized into confirmed, probable and possible diagnosis. Cases included in this study had at least one instance of blood mNGS detecting Aspergillus, upon which the diagnosis was based. The diagnostic criteria followed the guidelines for invasive fungal diseases jointly published by the European Organization for Research on Treatment of Cancer (EORTC) and the Mycoses Study Group Education and Research Consortium (MSGERC) in 2020 (Pappas et al., 2021). Patients were categorized into confirmed, probable, and possible diagnoses of IA based on these diagnostic criteria.
Confirmed Diagnosis: Criteria: Pathological findings from surgical or biopsy tissue at the infection site confirm Aspergillus infection.
Probable Diagnosis: Host factors, clinical symptoms or signs, and microbiological criteria consistent with Aspergillus infection are required. The microbiological criteria for clinical diagnosis are as follows: Aspergillus identified by smear or culture from specimens at the infection site, and detection of the same Aspergillus species in at least two mNGS tests (including blood or specimens from the infection site).
Possible Diagnosis: Host factors and clinical features consistent with Aspergillus infection. Host factors include hematological malignancies, solid organ and hematopoietic stem cell transplantation (HSCT), chemotherapy or immunotherapy for solid tumors, granulocyte deficiency, prolonged use of corticosteroids (average minimum dose exceeding 0.3 mg/kg/day for more than 3 weeks of prednisone or equivalent), ICU critically ill patients, chronic obstructive pulmonary disease, diabetes, liver failure, renal failure, etc (Gaffney et al., 2023).
2.3 Testing for Aspergillus spp.
The diagnostic methods for Aspergillus spp. include fungal culture, calcofluor white stain for fungi, galactomannan (GM) antigen test. The GM antigen was detected using a Platelia Aspergillus EIA kit (Bio-Rad, Marnes-la-Coquette, France), according to the manufacturer’s instructions. Both positive and negative controls were included in each assay. A result with an index value of >0.5 (serum) or 0.8 (BALF) in duplicate tests was considered positive.
2.4 Plasma mNGS testing
The testing protocol, experimental parameters, quality control, and test result reporting of the plasma mNGS testing in our laboratory are described by Han et al. (7) Briefly, peripheral venous blood samples were collected and sent to the clinical laboratory as soon as possible. Plasma was separated under the centrifugal parameters of 500 g for 5 min at 4°C. The separated plasma is used to extract cell free DNA (cfDNA) and cell free RNA (cfRNA) and libraries were constructed. The size distribution was measured and the concentration of the libraries was quantified. Library pools were then loaded onto the Illumina Nextseq CN500 sequencer for 50 cycles of single-end sequencing (SE-50), generating approximately 20 million reads for each library. Microbial reads were aligned to the database using Burrows-Wheeler Aligner software which contains a proprietary curated database consisting of more than 20,000 microbial reference genomes. Most mNGS results returned within 36 hours.
2.5 Data collection
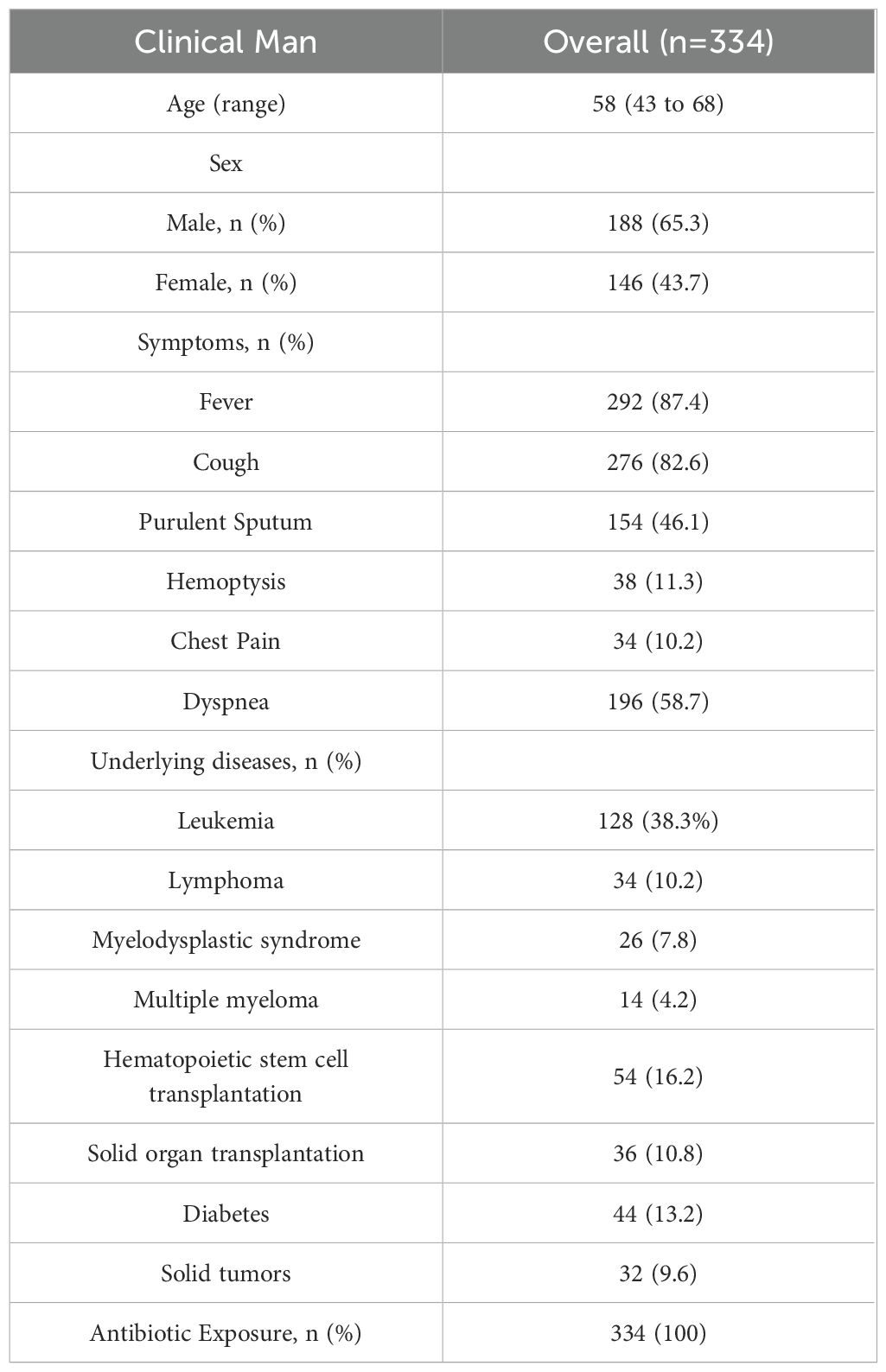
Demographic information, risk factors, underlying diseases, use of immunosuppressive drugs, steroids, chemotherapy drugs in the month preceding onset, clinical symptoms, laboratory examinations, imaging data, as well as treatment and prognosis, etc., were collected for the 334 included patients.
2.6 Comparison of clinical characteristics between IA and contaminated patients
Statistical comparisons were made between patients with positive blood mNGS for IA and contaminated patients. General conditions, risk factors, clinical manifestations, and prognosis were compared.
2.7 Data processing
SPSS 27.0 software (IBM Corporation, Chicago, Illinois, USA) was used for data analysis. Continuous variables conforming to a normal distribution were expressed as mean ± standard deviation (x ± s), and an independent sample t-test was used for intergroup comparison of measurement data. Non-normally distributed continuous variables were expressed as median and interquartile range, and the Mann-Whitney U test was used for comparison. Count data were compared using the chi-square test or Fisher’s exact test. A P value < 0.05 indicated a statistically significant difference.
3 Results
3.1 General characteristics
A total of 334 patients were included, comprising 188 males (56.3%) and 146 females (43.7%), with a median age of 58 years (range 43 to 68). Underlying diseases included hematological malignancies in 202 cases, with 128 cases of leukemia (38.3%), 34 cases of lymphoma (10.2%), 26 cases of myelodysplastic syndrome (7.8%), and 14 cases of multiple myeloma (4.2%). There were 54 cases post-hematopoietic stem cell transplantation (16.2%), 36 cases post-solid organ transplantation (10.8%), and 44 cases of diabetes (13.2%). Other underlying conditions included 32 cases of solid tumors (9.6%), among others, Table 1. Overall clinical and pathogen diagnosis of the recruited patients are showed in Figure 1.
3.2 Confirmed cases
There were 4 patients with confirmed invasive pulmonary aspergillosis based on histopathological evidence. There were 2 men and 2 women, 2 patients with lung transplantation, 2 patients with hematological malignancies, 1 patient with diabetes and 1 patient with bronchiectasis. Four patients were further confirmed to have Aspergillus infection through bronchoscopy biopsy and histopathological examination. In addition, both patients were confirmed to have Aspergillus infection in bronchoalveolar lavage fluid culture and mNGS testing. The Aspergillus infection in all 4 patients improved after treatment.
3.3 Probable cases
There were 62 cases, including 32males, aged 21-95 (56.1 ± 16.7) years. Among them, 58 cases were clinically diagnosed with invasive pulmonary aspergillosis, 4 case with disseminated invasive aspergillosis, and 4 case with urinary system Aspergillus infection. Underlying diseases included 44 cases of leukemia, 4 cases of lymphoma, 4 cases of myelodysplastic syndrome, 2 case of multiple myeloma, 10 cases after solid organ transplantation, 12 cases after hematopoietic stem cell transplantation, 4 cases of diabetes, 2 case of chronic obstructive pulmonary disease, and 6 cases of solid tumors. Nine patients had the same genus Aspergillus detected in 2–4 peripheral blood mNGS tests with an interval of 16 (10, 61) days. 20 cases were positive in culture and smear. 24 cases had the same genus of Aspergillus detected by using mNGS in sputum, lavage fluid, or cerebrospinal fluid. Among the 62 cases, there were 28 cases of A. fumigatus, 24 cases of A. flavus, 2 case of A. terreus, 2 case of A. oryzae, 2 case of A. niger, 2 case of A. clavatus, and 2 case of A. ustus. Pulmonary CT of 58 IPA patients showed imaging features consistent with Aspergillus infection, including nodular shadows, patchy shadows, halo signs, cavities, consolidation, etc. After active treatment, 36 cases improved, and 26 cases died, 28d mortality: 41.9%.
3.4 Possible cases
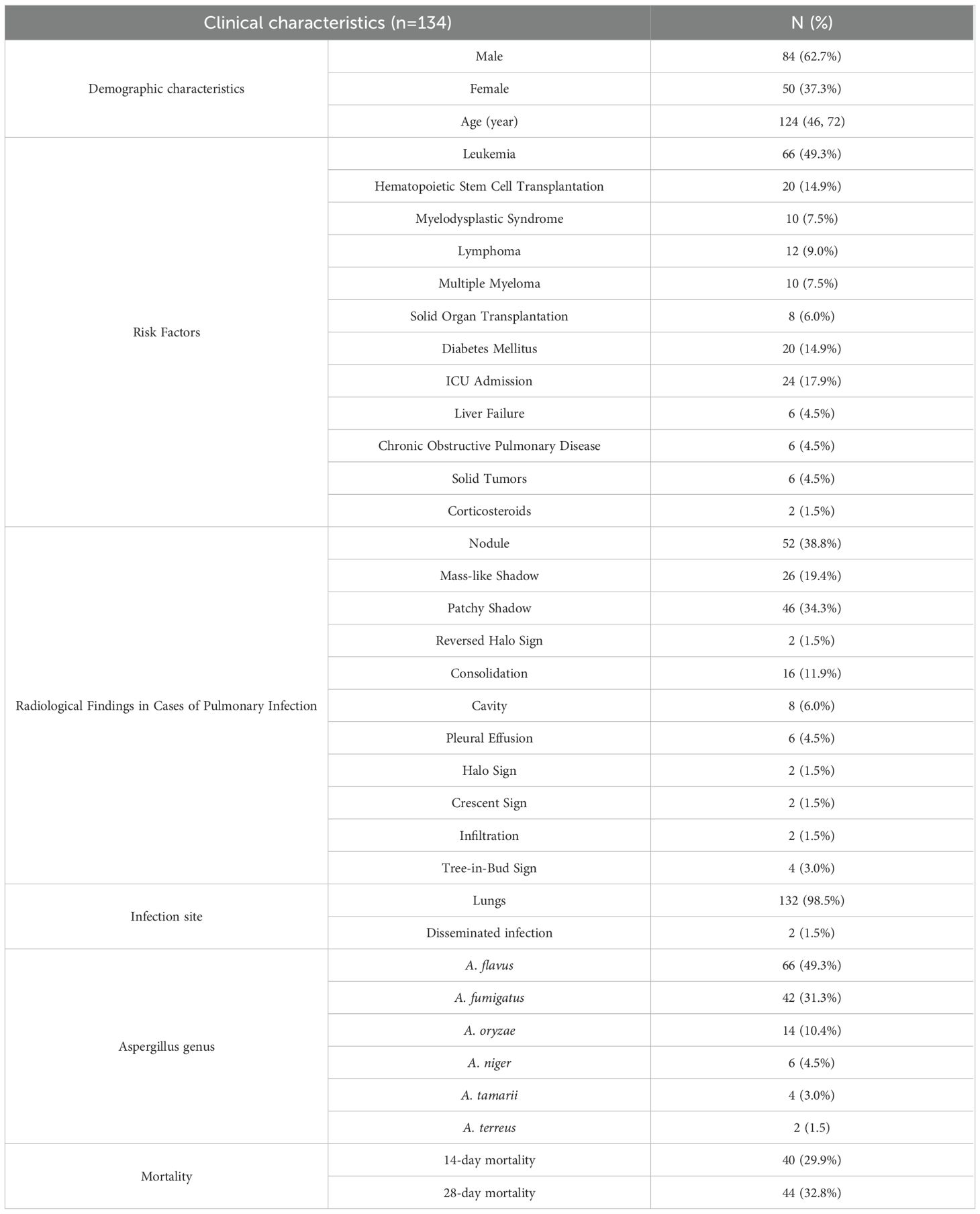
There were 134 suspected cases, including 84 males (62.7%). These patients, in addition to one instance of blood mNGS detecting Aspergillus, also presented with both the risk factors for Aspergillus infection and clinical features consistent with Aspergillus infection. Clinical characteristics are detailed in Table 2.
3.5 Contaminated cases
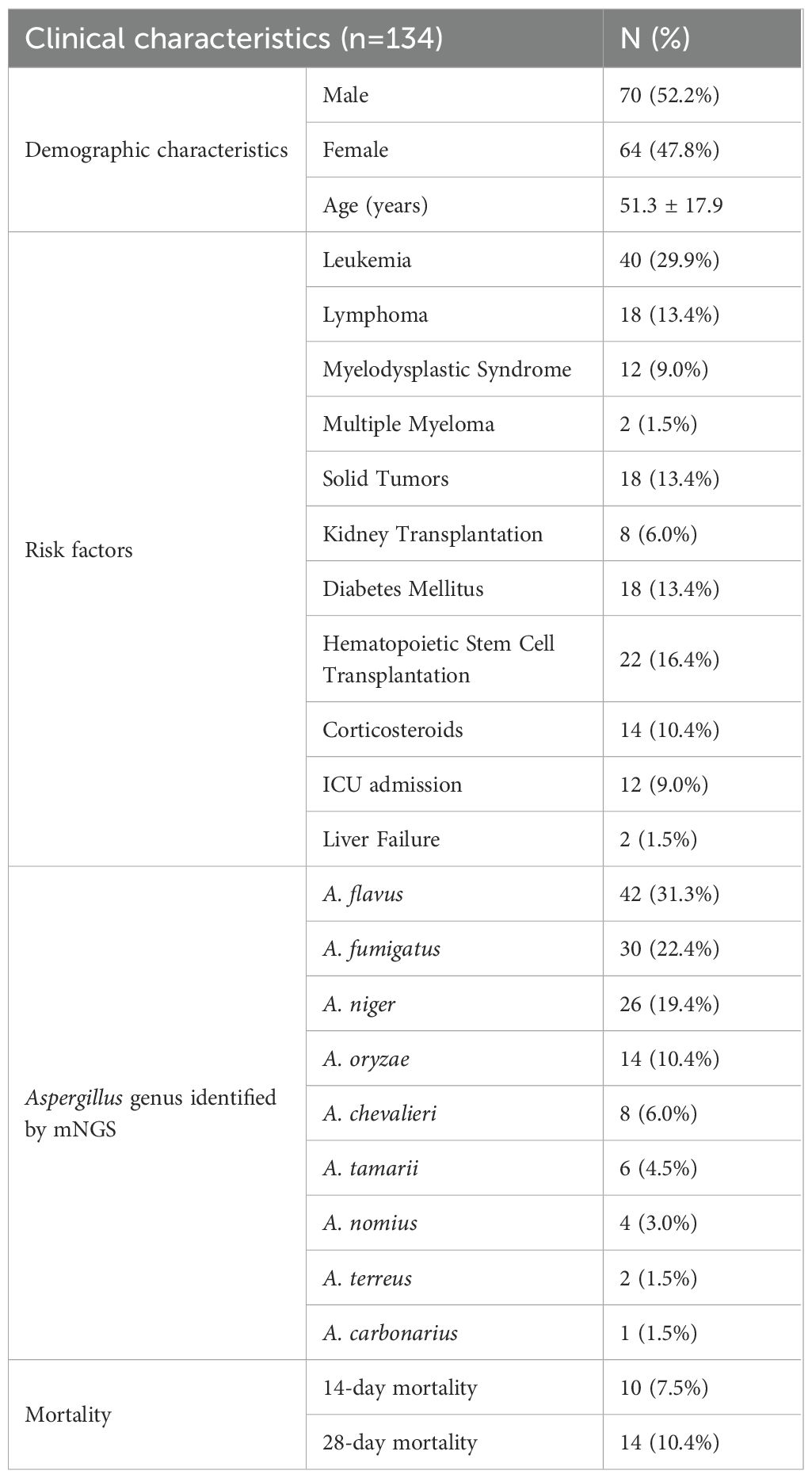
There were 134 contaminated cases, including 70 males (52.2%). The age ranged from 18 to 90 years (mean ± SD, 51.3 ± 17.9). Clinical characteristics are detailed in Table 3.
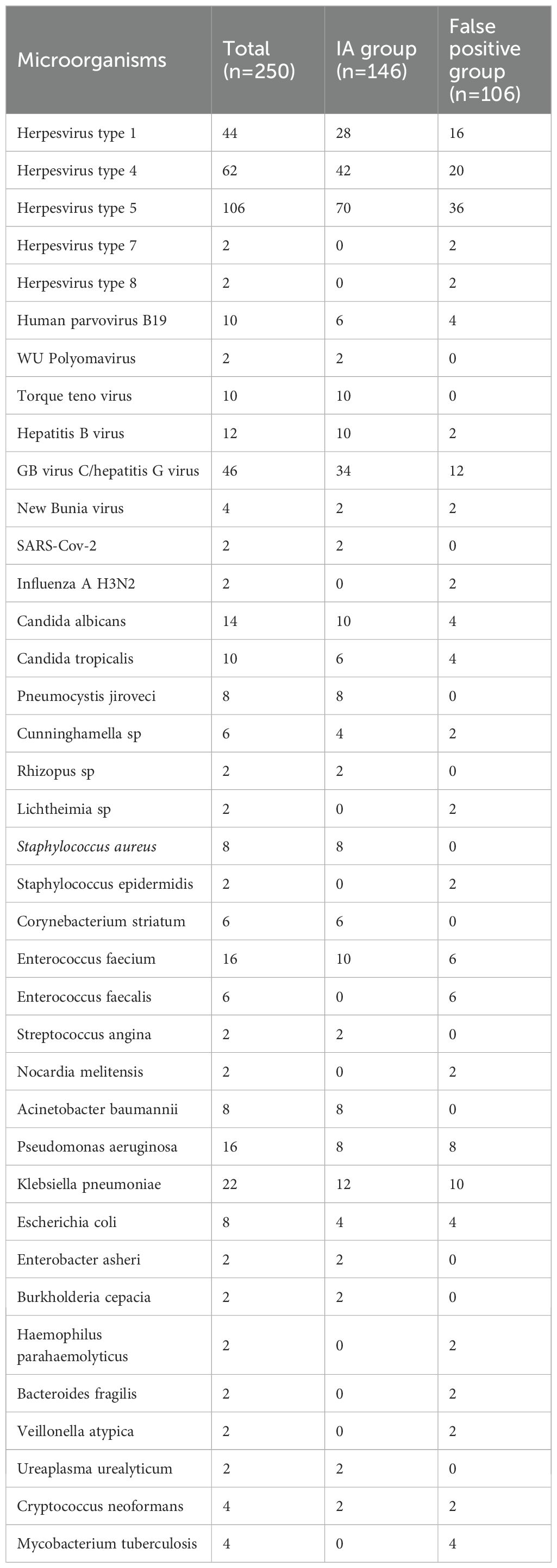
3.6 Other microorganisms except Aspergillus spp. detected in plasma mNGS of 167 patients
Only Aspergillus was detected in the plasma of 84 patients, and other microorganisms were detected in the plasma of 250 patients. The combined detected microorganisms are listed in Table 4. In IA group and false positive group, there was no significant difference in the detection of pathogens other than Aspergillus spp.
3.7 Comparison of clinical characteristics between patients with invasive aspergillosis and contaminated cases
A comparison of clinical data between 200 cases of blood mNGS-positive invasive aspergillosis and 134 contaminated cases revealed that the infection group had a higher incidence of lung imaging consolidation, masses, and cavities. There was no statistically significant difference in the read counts of Aspergillus between the infection group and the false positive group. Patients in the infection group had lower platelet counts, higher CRP levels, more critical cases, and poorer prognosis. Table 5.
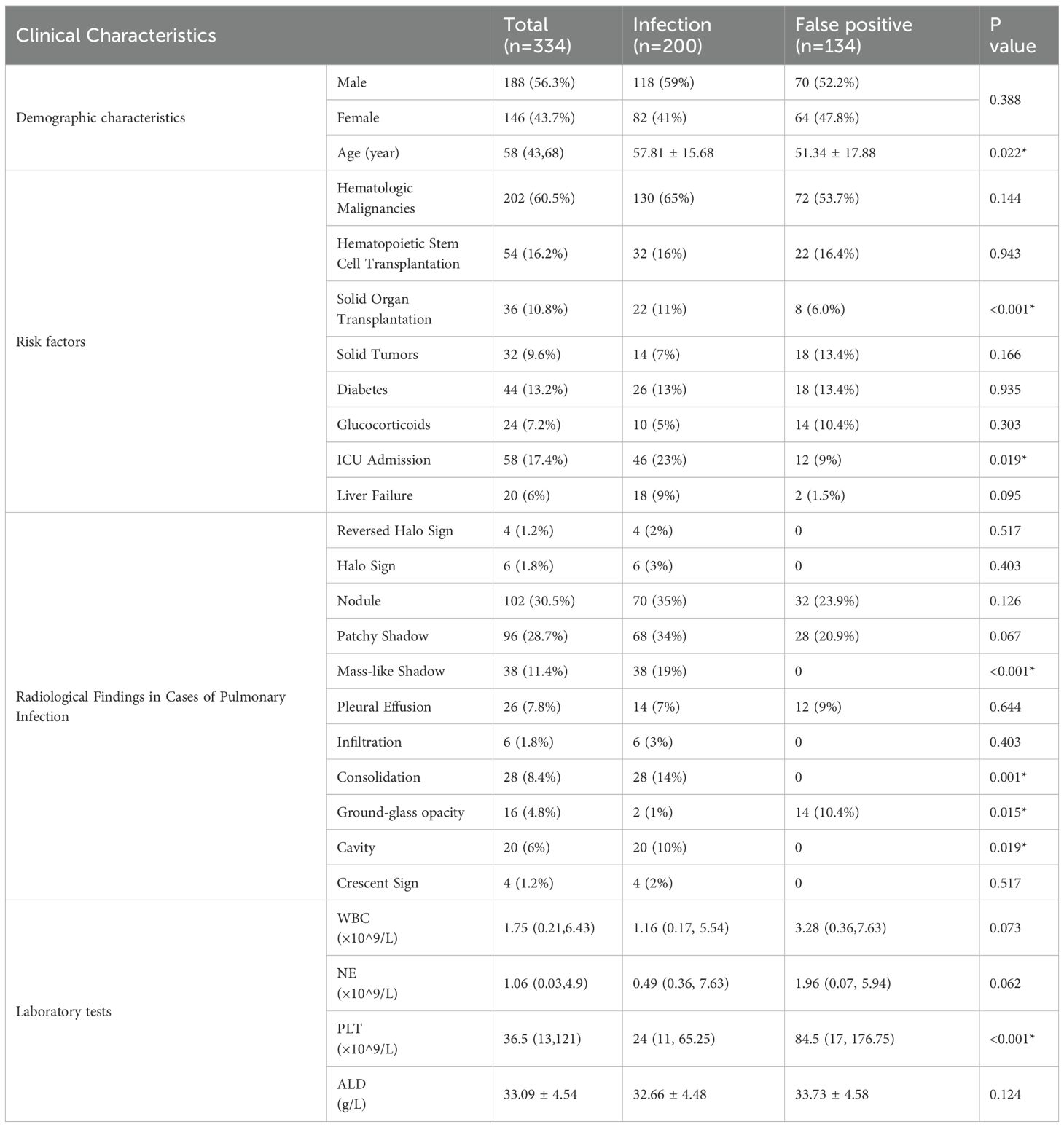
Table 5. Comparison of clinical characteristics between the Aspergillus infection and false positive group.
4 Discussion
Aspergillus, a ubiquitous saprophytic fungal pathogen, comprises over 250 species (Han et al., 2023), with A. fumigatus and A. flavus being the most common. Aspergillus produces abundant conidia, with each conidiophore generating thousands of conidia. These conidia, characterized by a small diameter (2 to 3 μm), can reach the alveoli when released into the atmosphere (Tegelaar and Wösten, 2017; Latgé and Chamilos, 2019). Environmental surveys indicate that humans inhale hundreds of Aspergillus conidia daily (Tanaka et al., 2015). Consequently, the lungs are the most common site for Aspergillus infections, with invasive pulmonary aspergillosis accounting for 98% of the 100 cases of Aspergillus infection in this study.
Due to the invasiveness and high mortality of Aspergillus infections, it is crucial for clinicians to rapidly and efficiently identify Aspergillus and initiate effective treatment. Microscopic examination and culture form the basis for diagnosing Aspergillus infections but lack sensitivity. Blood cultures have a low positivity rate and are prone to contamination, limiting their diagnostic value. Histopathological examination relies on invasive methods to obtain infected tissue, often constrained by critically ill patients, low platelet counts, or coagulation disorders. Currently, our challenge lies in the absence of non-invasive, rapid, and reliable diagnostic methods.
With the advent of more powerful methods such as novel PCR testing, mNGS, nanotechnology-based tools, and artificial intelligence-based models, the landscape of fungal diagnosis is continuously evolving in a positive direction. Molecular diagnostic methods, being rapid, effective, and non-invasive, allow for convenient sampling from blood, urine, sputum, and other specimens. The International Fungal PCR Initiative is actively working to incorporate fungal PCR diagnostics into the European Organization for Research on Treatment of Cancer (EORTC) and the Mycoses Study Group Education and Research Consortium (MSG) guidelines. Notably, Aspergillus PCR diagnostics have been successfully integrated into the EORTC/MSG guidelines for the diagnosis of aspergillosis. Aspergillus PCR exhibits high sensitivity, reaching up to 96% in routine blood samples, with excellent specificity, often reaching 100% in certain studies (Herbrecht et al., 2005). mNGS allows unbiased detection of various pathogens, including Aspergillus, with a short detection period. It enables identification at the species level and is less influenced by prior antifungal therapy (Cornely et al., 2014; Millon et al., 2016). For challenging severe cases suspected of Aspergillus infection, specimens such as blood and bronchoalveolar lavage fluid can be subjected to mNGS testing (Cornely et al., 2019). This study found that the positive predictive value of blood mNGS in diagnosing invasive aspergillosis was 59.9%, emphasizing the need for careful result interpretation and the importance of combining clinical features while actively seeking additional evidence of Aspergillus infection.
Our retrospective study failed to answer the sensitivity of plasma mNGS in the diagnosis of IA. According to the multiplex PCR detection results of Aspergillus reported in the literature (Herbrecht et al., 2005), the specificity of multiplex PCR in the diagnosis of IA is better than that of mNGS in this study. Multiplex PCR will be cheaper and shorter detection time, which has certain advantages. However, most of the patients enrolled in this study are immune deficiency patients such as hematological tumors, and the proportion of mixed infections is high. mNGS has the ability to detect multiple pathogens at the same time, which also has advantages.
Among the patients included in this study, 202 had hematologic malignancies, and 58 were admitted to the ICU, all with severely compromised immune function, making blood mNGS testing a crucial diagnostic method in this patient population where invasive sampling may not be feasible.
In our daily living environment, the concentration of Aspergillus spores typically ranges from 1 to 100 spores/m3, reaching up to 108 spores/m3 in certain specific environments (Tegelaar and Wösten, 2017). Therefore, isolating Aspergillus species from respiratory cultures of asymptomatic individuals without evidence of invasive or allergic disease is quite common (Mortensen et al., 2011; Máiz et al., 2015; Nguyen et al., 2015). The proportion of Aspergillus DNA found in lung biopsy specimens from healthy adults is reported to be 37% (Denning et al., 2011). This study observed a false-positive rate of 40.1% in the mNGS detection of Aspergillus, which may be attributed to environmental contamination during sampling, transportation, and the testing process.
Patients with neutropenia are prone to invasive aspergillosis. In this study, both the infection and contamination groups primarily had underlying hematologic diseases, resulting in varying degrees of neutropenia. However, there was no statistically significant difference in white blood cell count and neutrophil count between the infection and contamination groups.
This study has several limitations. Firstly, being a single-center study, the enrolled patients were predominantly characterized by hematologic diseases, and the site of Aspergillus infection was mostly limited to the lungs (98%), indicating a lack of diversity. Secondly, as a retrospective study, it could not evaluate the sensitivity and specificity of mNGS in diagnosing Aspergillus infection Thirdly, the study included only two confirmed cases, while there were 67 possible cases, which may introduce bias in assessing the diagnostic efficacy of mNGS for Aspergillus infection. Future research should focus on prospective, multicenter studies with a larger sample size to further evaluate the efficacy of peripheral blood mNGS in diagnosing invasive aspergillosis.
5 Conclusion
Peripheral blood mNGS exhibits a high positive predictive value in the diagnosis of invasive aspergillosis. It can be employed in critically ill patients, aiding in the earlier diagnosis and initiation of treatment when combined with high-risk factors and clinical characteristics.
Data availability statement
The original contributions presented in the study are publicly available. The mNGS datasets have been submitted to NCBI Sequence Read Archive (SRA) under the Bioproject accession number PRJNA1140277.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine, and informed consent had been waived. Ethics approval number: IIT20221282A. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective, observational study.
Author contributions
XW: Data curation, Writing – original draft, Formal analysis. WZ: Methodology, Formal analysis, Data curation, Writing – original draft. LX: Investigation, Formal analysis, Writing – original draft, Data curation. WW: Methodology, Formal analysis, Data curation, Writing – original draft. JZ: Conceptualization, Supervision, Writing – review & editing, Data curation. HZ: Supervision, Writing – review & editing, Conceptualization, Funding acquisition, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by research grants, including the Key R&D Plan of the Ministry of Science and Technology of China (2022YFC2504502), the Research and Development Program of Zhejiang Province (2023C03068), the National Natural Science Foundation of China (82272338). The funders had no involvement in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1656233/full#supplementary-material
References
Bassetti, M., Azoulay, E., Kullberg, B. J., Ruhnke, M., Shoham, S., Vazquez, J., et al. (2021). EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the intensive care unit working group. Clin. Infect. Dis. 12, S121–S127. doi: 10.1093/cid/ciaa1751
Cornely, O. A., Alastruey-Izquierdo, A., Arenz, D., Chen, S. C. A., Dannaoui, E., Hochhegger, B., et al. (2019). Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 19, e405–e421. doi: 10.1016/S1473-3099(19)30312-3
Cornely, O. A., Arikan-Akdagli, S., Dannaoui, E., Groll, A. H., Lagrou, K., Chakrabarti, A., et al. (2014). ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin. Microbiol. Infect. 20, 5–26. doi: 10.1111/1469-0691.12371
Denning, D. W., Park, S., Lass-Florl, C., Fraczek, M. G., Kirwan, M., Gore, R., et al. (2011). High-frequency triazole resistance found In nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin. Infect. Dis. 52, 1123–1129. doi: 10.1093/cid/cir179
Gaffney, S., Kelly, D. M., Rameli, P. M., Kelleher, E., and Martin-Loeches, I. (2023). Invasive pulmonary aspergillosis in the intensive care unit: current challenges and best practices. APMIS. 131, 654–667. doi: 10.1111/apm.13316
Han, D., Yu, F., Zhang, D., Yang, Q., Xie, M., Yuan, L., et al. (2023). The real-world clinical impact of plasma mNGS testing: an observational study. Microbiol. Spectr. 11(2), e0398322. doi: 10.1128/spectrum.03983-22
Herbrecht, R., Moghaddam, A., Mahmal, L., Natarajan-Ame, S., Fornecker, L. M., Letscher-Bru, V., et al. (2005). Invasive aspergillosis in the hematologic and immunologic patient: new findings and key questions in leukemia. Med. Mycol. 43, S239–S242. doi: 10.1080/13693780400025161
Jenks, J. D., White, P. L., Kidd, S. E., Goshia, T., Fraley, S. I., Hoenigl, M., et al. (2023). An update on current and novel molecular diagnostics for the diagnosis of invasive fungal infections. Expert Rev. Mol. Diagn. 23, 1135–1152. doi: 10.1080/14737159.2023.2267977
Koehler, P., Bassetti, M., Chakrabarti, A., Chen, S. C. A., Colombo, A. L., Hoenigl, M., et al. (2021). Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 21, e149–e162. doi: 10.1016/S1473-3099(20)30847-1
Latgé, J. P. and Chamilos, G. (2019). Aspergillus fumigatus and aspergillosis in 2019. Clin. Microbiol. Rev. 33, e00140–e00118. doi: 10.1128/CMR.00140-18
Máiz, L., Vendrell, M., Olveira, C., Girón, R., Nieto, R., Martínez-García, M. Á., et al. (2015). Prevalence and factors associated with isolation of Aspergillus and Candida from sputum in patients with non-cystic fibrosis bronchiectasis. Respiration. 89, 396–403. doi: 10.1159/000381289
Millon, L., Herbrecht, R., Grenouillet, F., Morio, F., Alanio, A., Letscher-Bru, V., et al. (2016). Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin. Microbiol. Infect. 22, 810.e1–810.e8. doi: 10.1016/j.cmi.2015.12.006
Mortensen, K. L., Johansen, H. K., Fuursted, K., Knudsen, J. D., Gahrn-Hansen, B., Jensen, R. H., et al. (2011). A prospective survey of Aspergillus spp. in respiratory tract samples: prevalence, clinical impact and antifungal susceptibility. Eur. J. Clin. Microbiol. Infect. Dis. 30, 1355–1363. doi: 10.1007/s10096-011-1229-7
Nguyen, L. D., Viscogliosi, E., and Delhaes, L. (2015). The lung mycobiome: an emerging field of the human respiratory microbiome. Front. Microbiol. 6. doi: 10.3389/fmicb.2015.00089
Pappas, P. G., Chen, S. C., and Donnelly, J. P. (2021). The evidence supporting the revised EORTC/MSGERC definitions for invasive fungal infections. Clin. Infect. Dis. 72, S77–S78. doi: 10.1093/cid/ciaa1765
Tanaka, R. J., Boon, N. J., Vrcelj, K., Nguyen, A., Vinci, C., Armstrong-James, D., et al. (2015). In silico modeling of spore inhalation reveals fungal persistence following low dose exposure. Sci. Rep. 5, 13958. doi: 10.1038/srep13958
Tegelaar, M. and Wösten, H. A. B. (2017). Functional distinction of hyphal compartments. Sci. Rep. 7, 6039. doi: 10.1038/s41598-017-06422-6
Yerbanga, I. W., Nakanabo Diallo, S., Rouamba, T., Denis, O., Rodriguez-Villalobos, H., Montesinos, I., et al. (2023). A systematic review of epidemiology, risk factors, diagnosis, antifungal resistance, and management of invasive aspergillosis in Africa. J. Mycol. Med. 33, 101328. doi: 10.1016/j.mycmed.2022.101328
Keywords: plasma, MNGs, aspergillus, invasive aspergillosis, microbial cell free-DNA
Citation: Wu X, Wang W, Xu L, Zhou W, Zhou J and Zhou H (2025) Value of plasma metagenomic next-generation sequencing for the diagnosis of invasive aspergillosis: a multicenter-center retrospective study. Front. Cell. Infect. Microbiol. 15:1656233. doi: 10.3389/fcimb.2025.1656233
Received: 29 June 2025; Accepted: 30 October 2025;
Published: 21 November 2025.
Edited by:
Christoph Gabler, Free University of Berlin, GermanyReviewed by:
Mohamed Tarek Badr, Freiburg University Medical Center, GermanyMine Aydin Kurç, Tekirdag Namik Kemal University, Türkiye
Copyright © 2025 Wu, Wang, Xu, Zhou, Zhou and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianying Zhou, emp5aHpAemp1LmVkdS5jbg==; Hua Zhou, emhvdWh1YTFAemp1LmVkdS5jbg==
†These authors have contributed equally to this work
 Xiaomai Wu
Xiaomai Wu Wei Wang3†
Wei Wang3† Hua Zhou
Hua Zhou