- 1Department of Prosthetics, Affiliated Stomatology Hospital of Jinzhou Medical University, Jinzhou, China
- 2Collaborative Innovation Center for Health Promotion of Children and Adolescents of Jinzhou Medical University, Jinzhou, China
- 3Department of Pedodontics, Affiliated Stomatology Hospital of Jinzhou Medical University, Jinzhou, China
- 4School and Hospital of Stomatology, China Medical University, Shenyang, China
Introduction: This study investigates how psychological factors influence the comorbidity of dental caries and obesity in adolescents through the oral-gut-brain axis. Adolescence is a critical period for both physical and psychological development, yet dental caries and obesity are prevalent issues that can negatively impact mental health. The study aims to provide insights into the underlying mechanisms and potential prevention and treatment strategies.
Methods: An epidemiological survey was conducted on 1,024 students aged 12–15 from Beizhen No. 1 Junior High School. A total of 90 adolescents were selected for biosample research. The methods used included 16S rRNA gene sequencing, untargeted metabolomics, and SourceTracker analysis to examine oral and gut microbiota and metabolite concentrations.
Results: Significant differences in oral and gut microbiota and metabolite concentrations were found among adolescents with different health statuses. Adolescents with caries and obesity showed distinct microbial profiles compared to healthy controls. The study also identified potential oral and gut microbial biomarkers associated with psychological disorders. SourceTracker analysis revealed a higher rate of ectopic colonization of oral microbiota in the intestines of adolescents with caries and obesity.
Discussion: The study highlights the roles of the oral-gut and oral-brain axes in the comorbidity of dental caries and obesity among adolescents. The findings suggest that oral and gut microbiota play crucial roles in disease progression, and their imbalances may affect mental health through the oral-gut-brain axis. The results provide a theoretical foundation for developing integrated intervention strategies targeting both oral and systemic health.
1 Introduction
Adolescence represents a critical developmental window for both physical and psychological maturation; yet the concurrent “dual epidemic” of dental caries and obesity now threatens the health of this generation. The World Health Organization (WHO) 2022 report documents a 50–70% prevalence of permanent-tooth caries among 12- to 15-year-olds (Lobstein et al., 2015). According to the World Obesity Atlas 2023 and the 2025 Global Burden of Disease study in The Lancet, obesity prevalence among boys is projected to rise from 10% in 2020 to 20% by 2035, and among girls from 8% to 18% over the same period. The mental health landscape is equally alarming: UNICEF/WHO. State of the World’s Children 2021: On My Mind—Promoting, Protecting and Caring for Children’s Mental Health indicates that approximately 13–14% of children and adolescents worldwide suffer from psychological disorders, with prevalence rates for anxiety and depression increasing from 3.6% and 1.1% in 10- to 14-year-olds to 4.6% and 2.8% in 15- to 19-year-olds, respectively.
Dental caries, obesity, and psychological disorders do not occur in isolation; rather, they constitute a “networked disease complex” that reciprocally amplifies risk. Poor oral health undermines self-esteem and self-confidence, thereby precipitating anxiety and depression (Kisely et al., 2015). Conversely, individuals with compromised mental health—including severe mental illness, mood disorders, and eating disorders—often neglect oral hygiene (Kisely et al., 2016), which elevates the incidence of dental caries (Folayan et al., 2018). Emotional eating and stress-induced bingeing increase the intake of energy-dense foods, promoting obesity (Folayan et al., 2018). Obesity, in turn, reduces salivary flow and lowers oral pH, thereby enhancing caries susceptibility (Milaneschi et al., 2019). Carious lesions impair masticatory efficiency and nutrient absorption, further disrupting weight control (Chala et al., 2017).
Over the past five years, three converging “sub-axes” have provided the most recent empirical foundation for an integrated “oral–gut–brain axis.”Oral–gut axis: A systematic review by Yamazaki et al. demonstrated that periodontal pathogens—most notably Porphyromonas gingivalis—can be continuously swallowed with saliva, breach the intestinal barrier, and trigger local dysbiosis and systemic inflammation, thereby establishing the first direct mechanistic link between periodontitis and gut microbial imbalance (Yamazaki, 2023)Oral–brain axis: Bowland and Weyrich introduced the conceptual framework of the “oral–microbiome–brain axis,” emphasizing its relevance to neuropsychiatric disorders (Bowland and Weyrich, 2022). Dominy et al. identified P. gingivalis and its gingipains in post-mortem Alzheimer’s disease (AD) brain tissue, providing direct pathogenic evidence linking oral infection to neurodegeneration (Dominy et al., 2019).Gut–brain axis: Duan and Wu summarized how gut microbiota modulate serotonin (5-HT), brain-derived neurotrophic factor (BDNF), and microglial activity via short-chain fatty acids and tryptophan metabolites, thereby influencing the pathogenesis of depression and Parkinson’s disease (Duan and Wu, 2024). Furthermore, Zhang et al. reported that small-molecule inhibitors targeting oral pathogens can attenuate cerebral β-amyloid deposition, underscoring the therapeutic potential of cross-axis interventions (Zhang et al., 2023).
In summary, dental caries, obesity, and psychological disorders manifest as a syndemic among adolescents, and the emerging concept of the oral–gut–brain axis offers a unifying theoretical framework for elucidating their shared mechanistic underpinnings and for developing integrated intervention strategies (Figure 1).
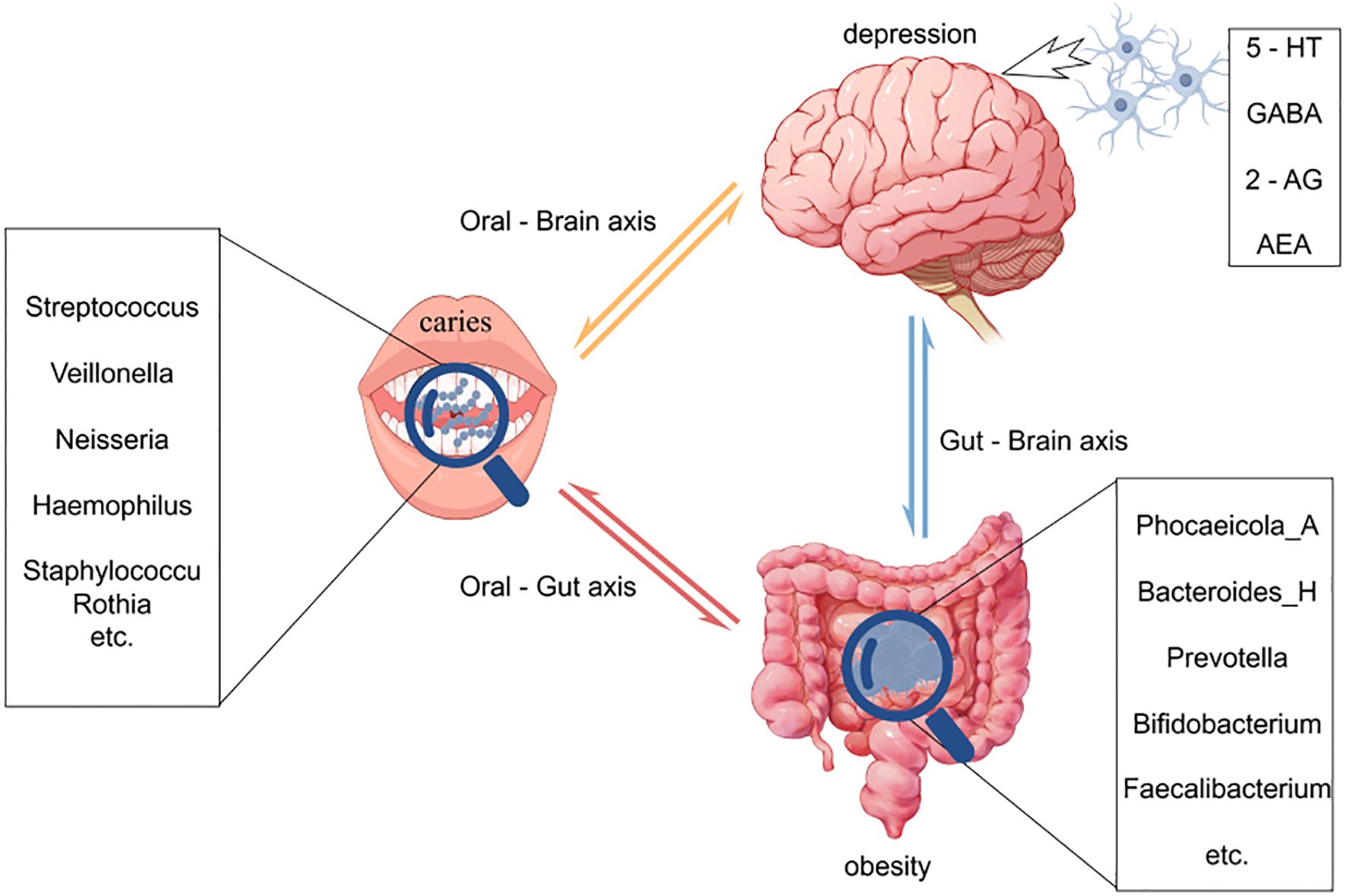
Figure 1. This figure illustrates the intricate interactions among the oral, gut, and brain axes and their effects on health. Oral microbiota directly associate with brain function through the oral-brain axis, potentially influencing the onset of neuropsychiatric disorders such as depression. Concurrently, oral microbes impact the gut microbiome via the oral-gut axis, subsequently modulating brain function and behavior through the gut-brain axis. Imbalances in oral microbiota (e.g., caries) and gut microbiota (e.g., changes associated with obesity) may affect the levels of neurotransmitters through these axes, thereby playing a role in the development of diseases like depression. This model underscores the dynamic connections among the oral, gut, and brain, highlighting their significance in overall health.
2 Materials and methods
2.1 Survey subjects
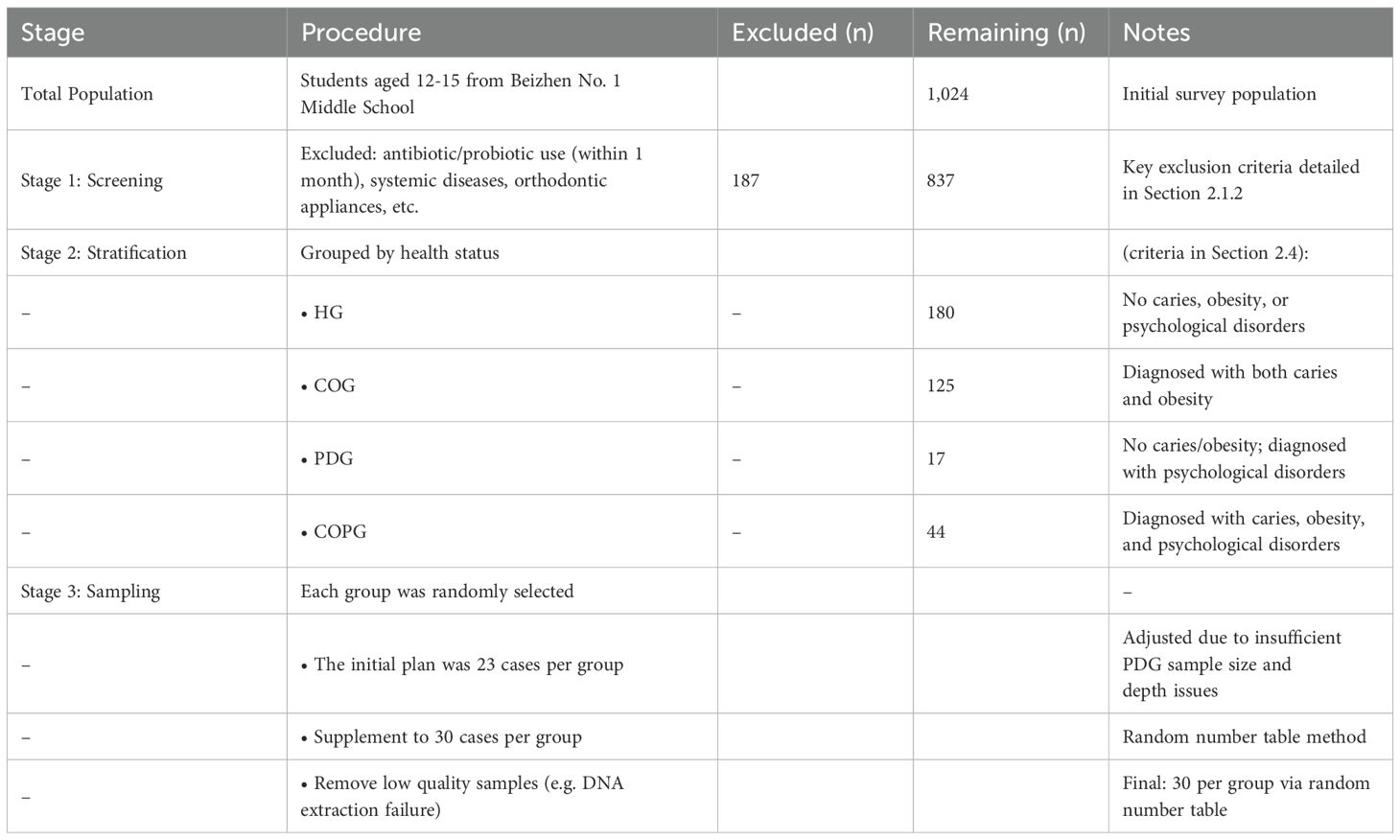
In September 2024, an epidemiological survey was conducted on 1024 students from the First Junior High School in Beizhen City, Jinzhou City. After questionnaire surveys, physical parameter measurements, and oral examinations, a total of 90 adolescents aged 12–15 were included in the biological sample study.
2.1.1 Sampling strategy and sample size calculation
This study employed a cross-sectional design with stratified cluster sampling. Fieldwork was conducted in the Beizhen district of Jinzhou City in September 2024. After selecting one fixed-point junior high school, we first stratified students by grade and then randomly selected intact classes within each stratum as primary sampling units.
Sample size was calculated according to the standard formula for cross-sectional surveys, N=K × Q/P, where K=400 when the permissible error is set at 10%. Using the most recent National Oral Health Epidemiological Survey (NOHES), the caries prevalence (P) for 12- and 15-year-olds was reported to be 38.5% and 44.4%, respectively; the larger value (44.4%) was adopted to yield a conservative estimate. This produced a minimum required sample of approximately 501 participants per group. To account for the design effect inherent in cluster sampling and an anticipated 10% non-response rate, the final sample was expanded to 1,024 adolescents, ensuring adequate statistical power and representativeness of the findings.
2.1.2 Inclusion criteria for biological sample subjects
All subjects had not taken antibiotics within 1 month before the examination, had no systemic diseases, no congenital diseases or other diseases (oral mucosal diseases), and did not wear orthodontic appliances in the mouth. This study was approved by the Ethics Committee (Approval Number: JYFELL202409). The children examined obtained the permission of their parents or guardians and signed the informed consent form. A total of 90 participants were enrolled for biological sample collection in this study. The inclusion method is presented in the following Table 1:
2.2 Research methods
2.2.1 Questionnaire survey
The demographic characteristics of the survey subjects, including the educational level of the parents of the survey subjects, the frequency of tooth brushing, the frequency of sweets and beverages, were collected through one-on-one questionnaire surveys. The International General Health Questionnaire-12 (GHQ-12) was used to assess the mental health status of students.
2.2.2 Physical parameter measurement
Trained medical examiners measured the height, weight, and waist circumference. The height was measured using a metal column height meter, the weight was measured using an electronic weighing scale, and the waist circumference was measured using a non-elastic soft ruler with a minimum scale of 1 mm. The weight was accurate to 0.01 kg, and the height and waist circumference were accurate to 0.1 cm. They were recorded separately, and the body mass index of each survey subject was calculated.
2.2.3 Dental caries examination
Using the methods and standards published by the World Health Organization, professional dentists used disposable oral instruments under a unified artificial light source, used probes and flat mirrors, and used a combination of visual and probing methods to examine and evaluate the dental health status of the survey subjects. A special recorder checked and recorded the caries, loss, and filling of each tooth.
2.3 Diagnostic criteria
2.3.1 Psychological disorder
The GHQ-12 questionnaire consisted of 12 questions, using the Likert 4-point scoring method, with options ranging from 1 point “never” to 4 points “often”. The score ranged from 12 to 48, and the higher the score, the lower the mental health level. A total score > 27 indicated poor mental health.
2.3.2 Dental caries
(1) Caries-free: No signs of caries and no fillings due to caries.
(2) Dental Caries: The pits or smooth surfaces of the teeth have softening at the bottom, potential damage to the enamel, or softening of the walls, which are divided into pit and fissure caries and smooth surface caries.
(3) Filled with Caries: Teeth with permanent fillings and one or more new caries.
(4) Filled without Caries: Teeth with one or more permanent fillings and no other caries.
(5) Missing due to Caries: Teeth lost or extracted due to caries.
2.3.3 Obesity
(1) BMI(Body Mass Index): According to the Comprehensive Evaluation of the Development Level of Children and Adolescents (GB/T 31178-2014) standard (Comprehensive evaluation of children and adolescents development, n.d.), children and adolescents are divided into thin, normal, overweight, and obese. When the BMI of the investigator is less than the “normal” threshold point of the corresponding gender and age group, it is considered thin; when the BMI is less than the “overweight” threshold point of the corresponding gender and age group, it is considered normal; when the BMI is greater than or equal to the “overweight” threshold point of the corresponding gender and age group, it is considered overweight or obese. (BMI=Weight (kg)/[Height (m)]^2).
(2) Waist Circumference: According to the Screening Threshold for High Waist Circumference in Children and Adolescents aged 7–18 Years (WS/T 611-2018) (20180704145130574.pdf), the P90 of waist circumference in different gender and age groups is used as the judgment standard for central obesity.
2.4 Biological sample collection
All samples were divided into four groups, with 10 samples taken from each group
1. Healthy Group (HG)
2. Caries and Obesity Group (COG)
3. Psychological Disorders Group (PDG)
4. Caries, Obesity and Psychological Disorders Group (COPG)
2.5 Statistical methods
IBM SPSS Statistics 26.0 statistical software was used for data processing and analysis. Quantitative data that conformed to a normal distribution were described using (x¯ ± s), and the t-test was used for comparison between two groups, and analysis of variance was used for comparison between multiple groups. Qualitative data were described using the number of cases (%). The significance level α was set to 0.05 unless otherwise specified.
A one-way analysis of variance (ANOVA) was performed for each demographic variable (age, sex, BMI, etc.). Table 2 presents the ANOVA results with the total psychological score as the dependent variable and caries status, BMI, age, sex, and comorbidity as independent variables. Participants with caries (n=205) exhibited significantly higher psychological scores than their caries-free counterparts (n=819, p < 0.001). A statistically significant age difference was also observed between the two groups (p=0.009). In contrast, neither BMI (22.15 ± 5.19 vs. 21.68 ± 5.14) nor sex distribution (1.51 ± 0.50 vs. 1.53 ± 0.50) differed significantly (p > 0.05). Notably, the caries–obesity comorbidity showed the most pronounced between-group difference (p < 0.001), indicating that the co-occurrence of caries and obesity may exert an additive effect on psychological distress.
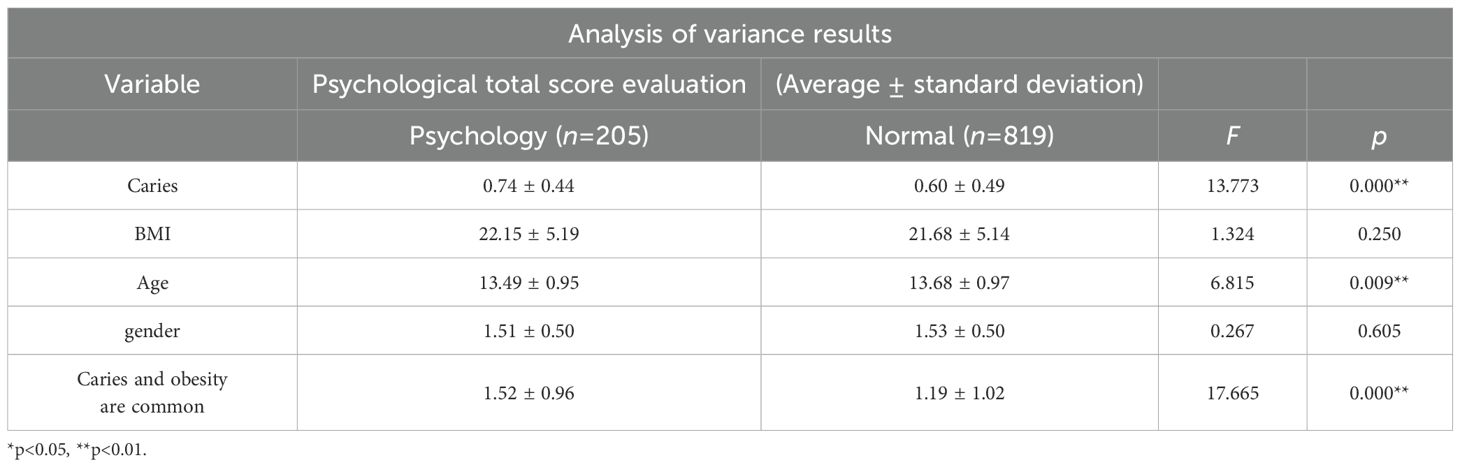
Table 2. The aforementioned demographic variables—age, sex, BMI, and psychological status—will be included as covariates in subsequent microbiota analyses to control for their potential confounding effects on community structure.
3 Results
3.1 Basic characteristics
A total of 1,024 students were enrolled, comprising 483 (47.17%) males and 541 (52.83%) females. The majority were of Manchu ethnicity (n=813, 79.47%), followed by Han (n=197, 19.26%), Mongolian (n=8, 0.78%), and other ethnicities (n=5, 0.49%). Age distribution peaked at 13–14 years, accounting for 33.01% and 34.96%, respectively, with fewer participants in other age brackets. The overall caries detection rate was 62.50% (n=640). Regarding body-mass index (BMI), 499 participants (48.73%) were classified as having normal weight, 292 (28.52%) as obese, 83 (8.11%) as overweight, and 150 (14.65%) as underweight. Psychological assessment revealed that 819 individuals (79.98%) exhibited normal mental health status, whereas 205 (20.02%) presented with psychological or behavioral disorders (Table 3).
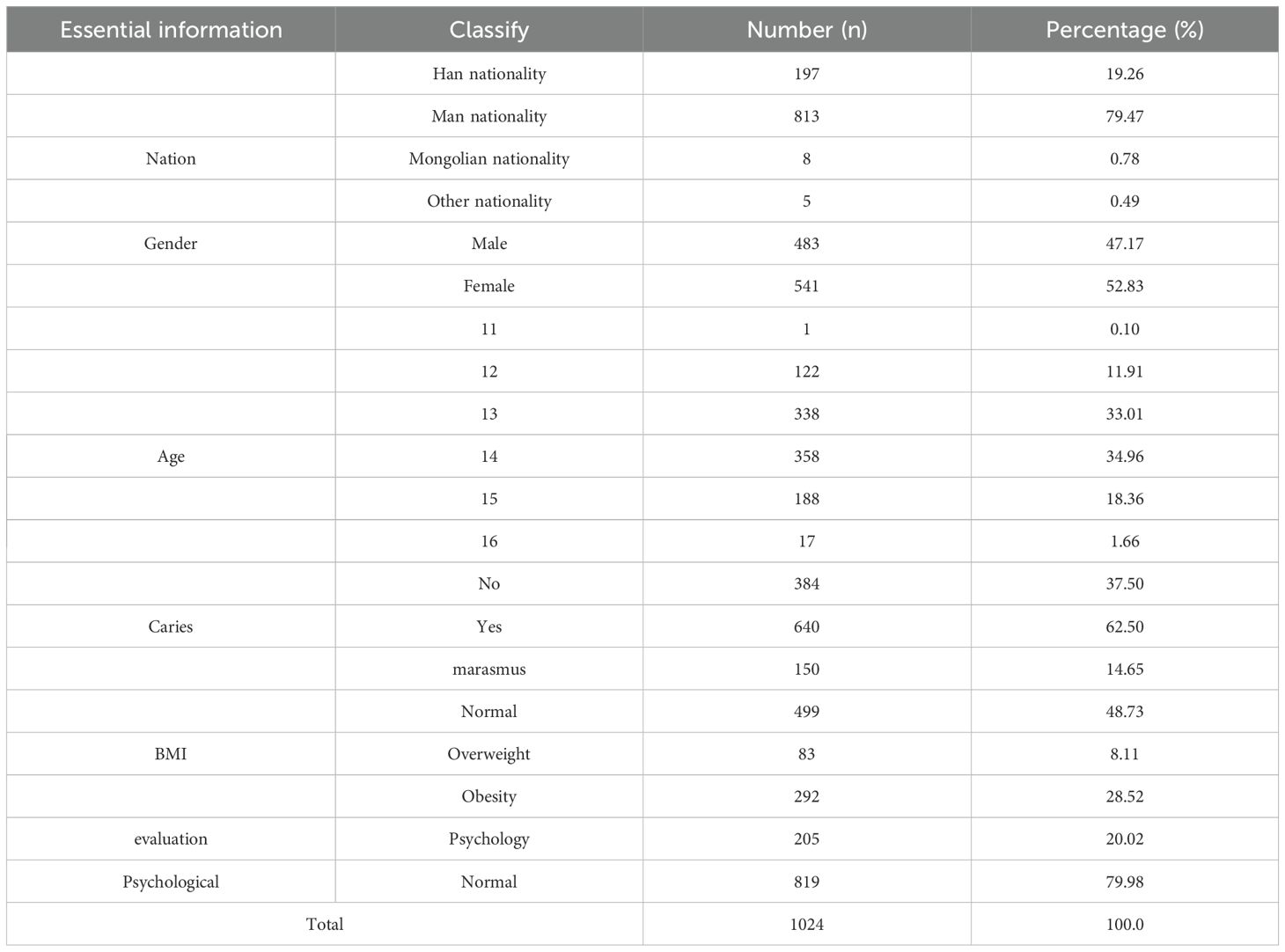
Table 3. The aforementioned demographic variables—age, sex, BMI, and psychological status—will be included as covariates in subsequent microbiota analyses to control for their potential confounding effects on community structure.
3.2 Demographic characteristics of participants providing biological samples
Of the 1,024 adolescents aged 12–15 years initially surveyed, four groups were established for biological sampling: the Healthy Group (HG, n=180), the Caries-Obesity Group (COG, n=125), the Psychological Disorders Group (PDG, n=17), and the Comorbid Caries-Obesity-Psychological Disorders Group (COPG, n=44). Mean age ranged from 13.30 to 14.59 years across the groups. BMI analyses revealed that the COG (29.11 kg/m²) and COPG (28.37 kg/m²) exhibited significantly higher values than the HG (19.45 kg/m²) and PDG (19.84 kg/m²), underscoring the co-occurrence of obesity and dental caries. Psychological assessment via the GHQ-12 (higher scores indicate poorer mental health) showed that the PDG (29.53) and COPG (28.95) displayed comparable and markedly elevated scores relative to the HG (17.73) and COG (18.60), thereby validating the diagnostic criteria used to define psychological morbidity in the grouping protocol (Table 4).
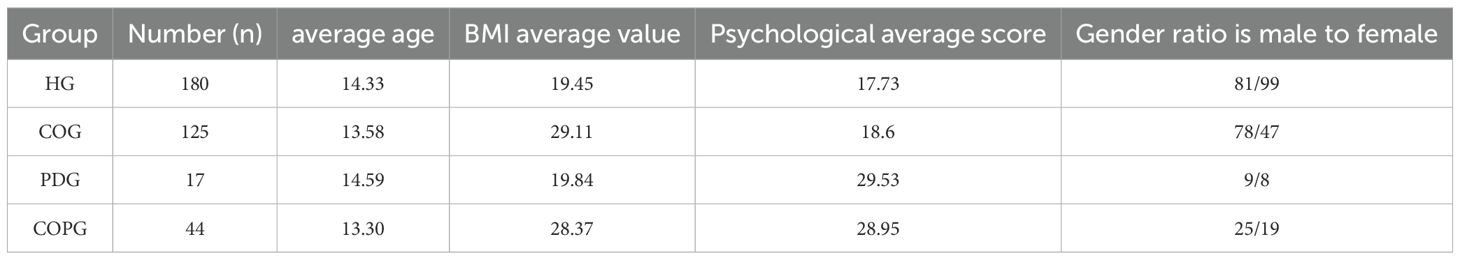
Table 4. Comparison of average age, BMI, psychological scores, and gender ratios across four groups: Healthy (HG), Caries-Obesity (COG), Psychological Disorders (PDG), and Comorbid Caries-Obesity-Psychological Disorders (COPG).
3.3 Sequencing information
All microbiota analyses were adjusted for age, sex, BMI, and psychological score to ensure that observed microbial differences are attributable to disease status rather than demographic characteristics.
90 samples were divided into three groups, all samples were sequenced by Illumina platform, the results are as follows:
Saliva: Among them, all samples in HG, COG and COPG group contained 68543 ASVs (Amplicon Sequence Variant): 354 ASVs in HG and COG group; 192 ASVs in HG and COPG group; 210 ASVs in COG and COPG group; ASVs 2982 unique to HG group; ASVs 5407 unique to COG group; and ASVs 2371 unique to COPG group (Figure 2A).
Feces: All samples in HG, COG and COPG group contained 49126 ASVs: 116 ASVs in HG and COG group; 88 ASVs in HG and COPG group; 161 ASVs in COG and COPG group; ASVs 1753 unique to HG group; ASVs 2926 unique to COG group; ASVs 2372 unique to COPG group (Figure 2B).
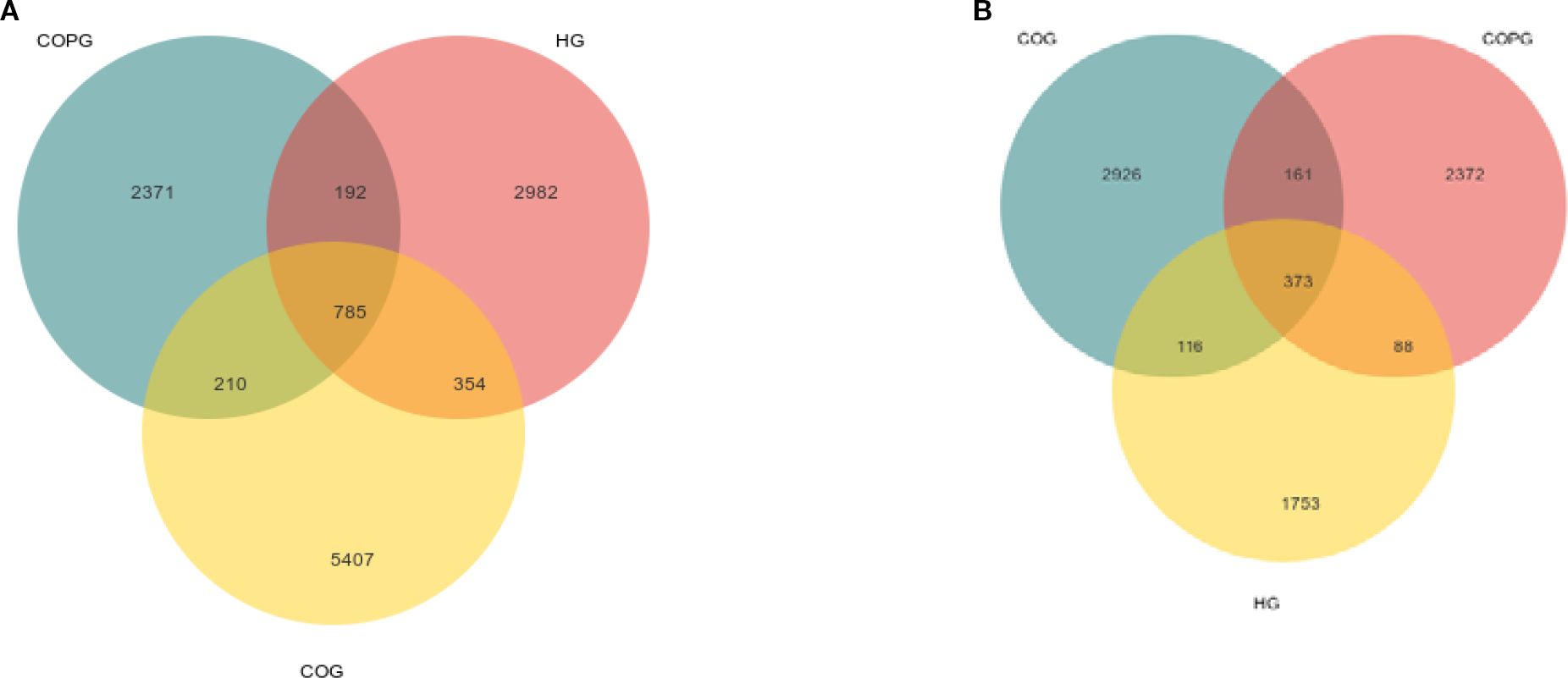
Figure 2. (A, B) Venn diagram illustrating the overlap and unique classifications among COG, HG, and COPG.
3.4 Relative abundance of species at the phylum level
3.4.1 Saliva
The top five most abundant phyla in the saliva were selected, namely Firmicutes, Proteobacteria, Bacteroidota, and Actinobacteriota (Figure 3A). Compared with the HG group, the COG group exhibited higher relative abundance of Firmicutes, Bacteroidota, and Actinobacteriota, while the relative abundance of Proteobacteria was lower. In comparison with the COG group, the COPG group showed higher relative abundance of Firmicutes and Proteobacteria, and lower relative abundance of Bacteroidota and Actinobacteriota.
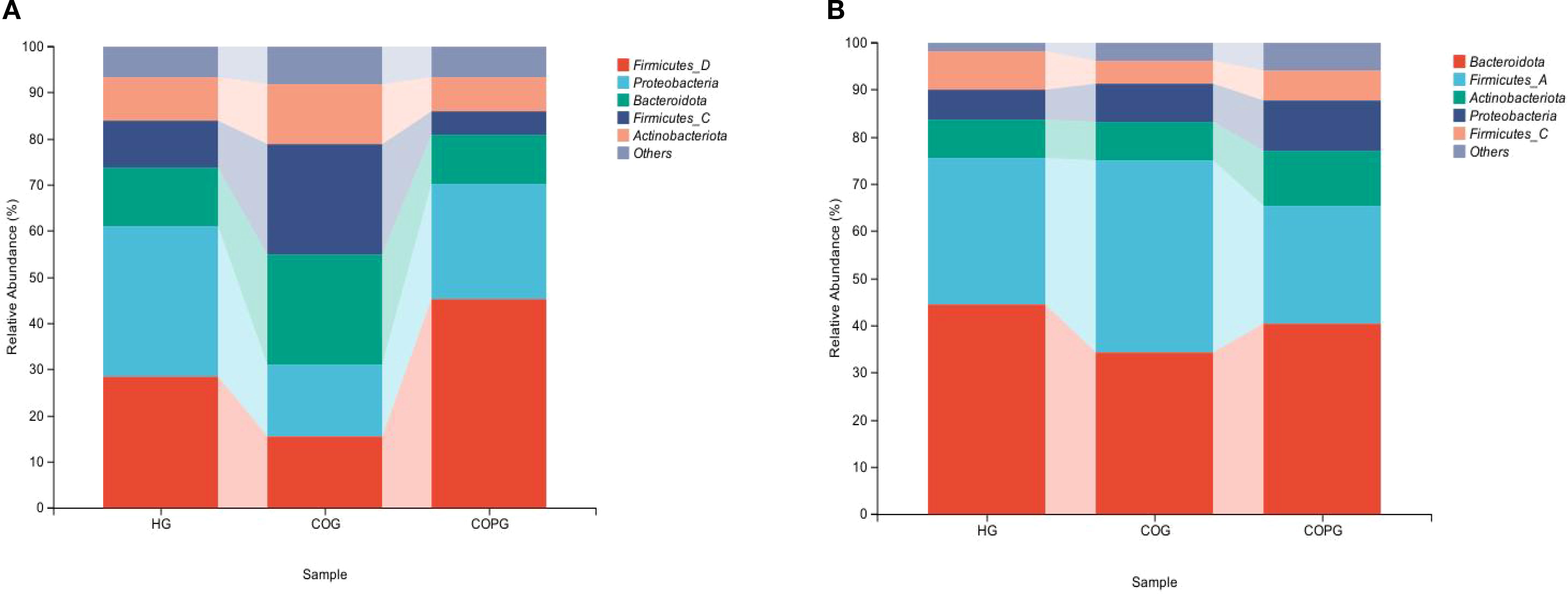
Figure 3. (A, B) Relative abundance of bacterial phyla across different samples at the phylum level.
3.4.2 Feces
The top five most abundant phyla in fecal samples were selected, namely Bacteroidota, Firmicutes, Actinobacteriota, and Proteobacteria, and a bar chart of their relative abundance was generated (Figure 3B). Compared with the HG group, the COG group exhibited higher relative abundance of Firmicutes and Proteobacteria, while the relative abundance of Bacteroidota and Actinobacteriota was lower. In comparison with the COG group, the COPG group showed higher relative abundance of Bacteroidota, Actinobacteriota, and Proteobacteria, and lower relative abundance of Firmicutes.
3.5 Relative abundance of species at the genus level
3.5.1 Saliva
The most abundant genera were selected based on their relative abundance, and a bar chart of their abundance was generated (Figure 4A). Compared with the HG group, the COG group exhibited higher relative abundance of Veillonella, Prevotella, and Rothia, while the relative abundance of Streptococcus, Neisseria, Staphylococcus, and Haemophilus was lower. In comparison with the COG group, the COPG group showed higher relative abundance of Streptococcus, Neisseria, Staphylococcus, and Haemophilus, and lower relative abundance of Veillonella and Prevotella.
3.5.2 Feces
The most abundant genera in fecal samples were selected based on their relative abundance, and a bar chart of their abundance was generated (Figure 4B). Compared with the HG group, the COG group exhibited higher relative abundance of Prevotella, Faecalibacterium, and Bifidobacterium, while the relative abundance of Phocaeicola and Megamonas was lower. In comparison with the COG group, the COPG group showed higher relative abundance of Bacteroides and Bifidobacterium, and lower relative abundance of Phocaeicola, Prevotella, and Faecalibacterium.
3.6 α-diversity analysis
α-diversity is an analysis of microbial diversity in community ecology, reflecting the distribution and differences of microbial communities (Miller et al., 2016).
Oral microbiota α-diversity analysis (Figure 5A) revealed that the Simpson index in the COG group was significantly higher than that in the HG and COPG groups (P < 0.05). The Pielou_c and Shannon indices in the COPG group were significantly lower than those in the HG and COG groups (P < 0.05). Additionally, the Faith_pd index in the HG group was significantly lower than that in the COG and COPG groups (P < 0.05).
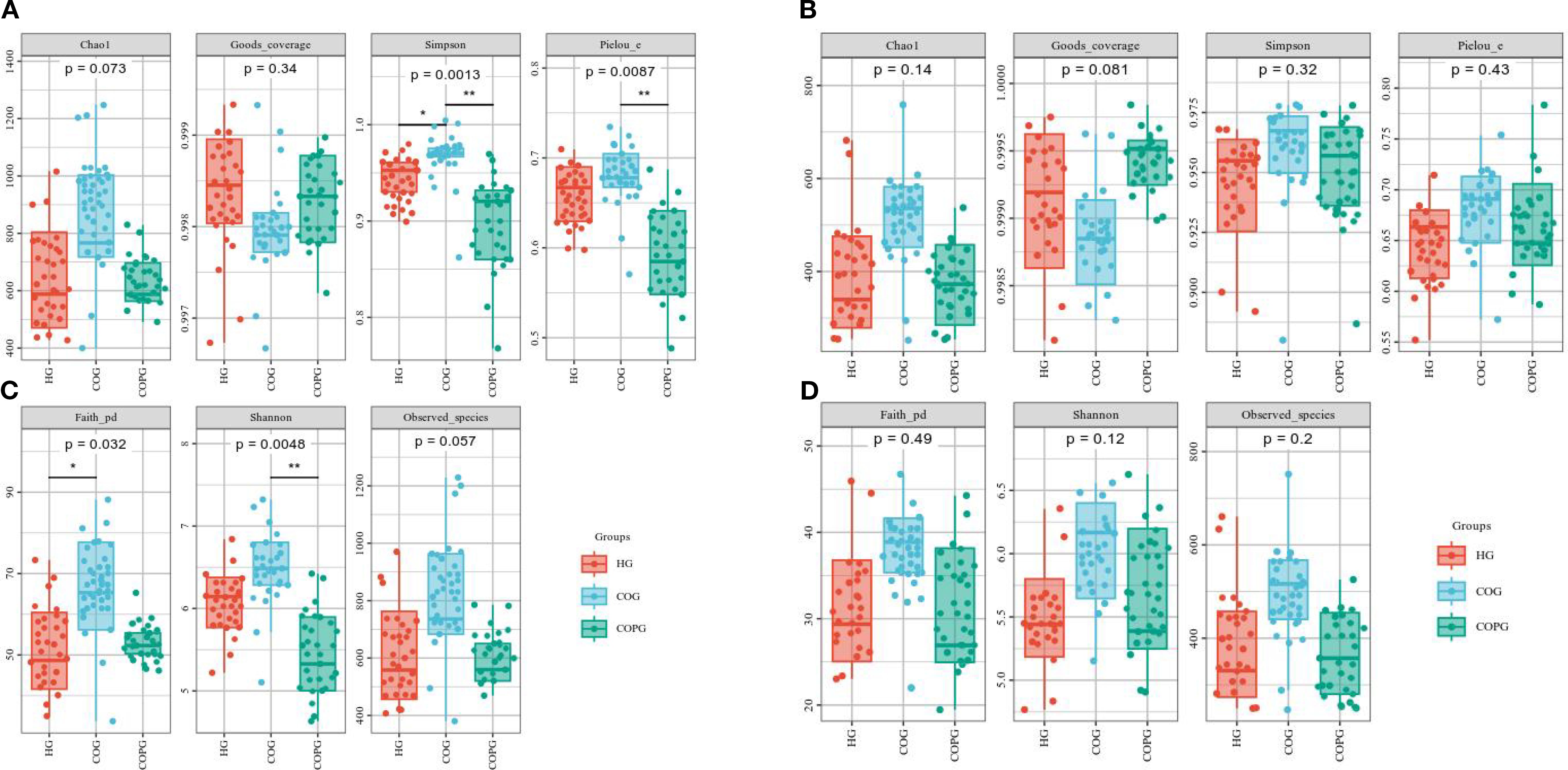
Figure 5. (A–D) Alpha-diversity analysis of oral and gut microbiota across different groups. All p-values were adjusted using the Benjamini–Hochberg FDR method; q < 0.05 was considered significant.
Gut microbiota α-diversity analysis (Figure 5B) showed that the Chao1, Simpson, and Observed species indices in the HG group were lower than those in the COG and COPG groups, although the differences were not statistically significant (P > 0.05). This indicates that there were no significant differences in the species diversity and richness of the gut microbiota among the groups.
3.7 β-diversity analysis
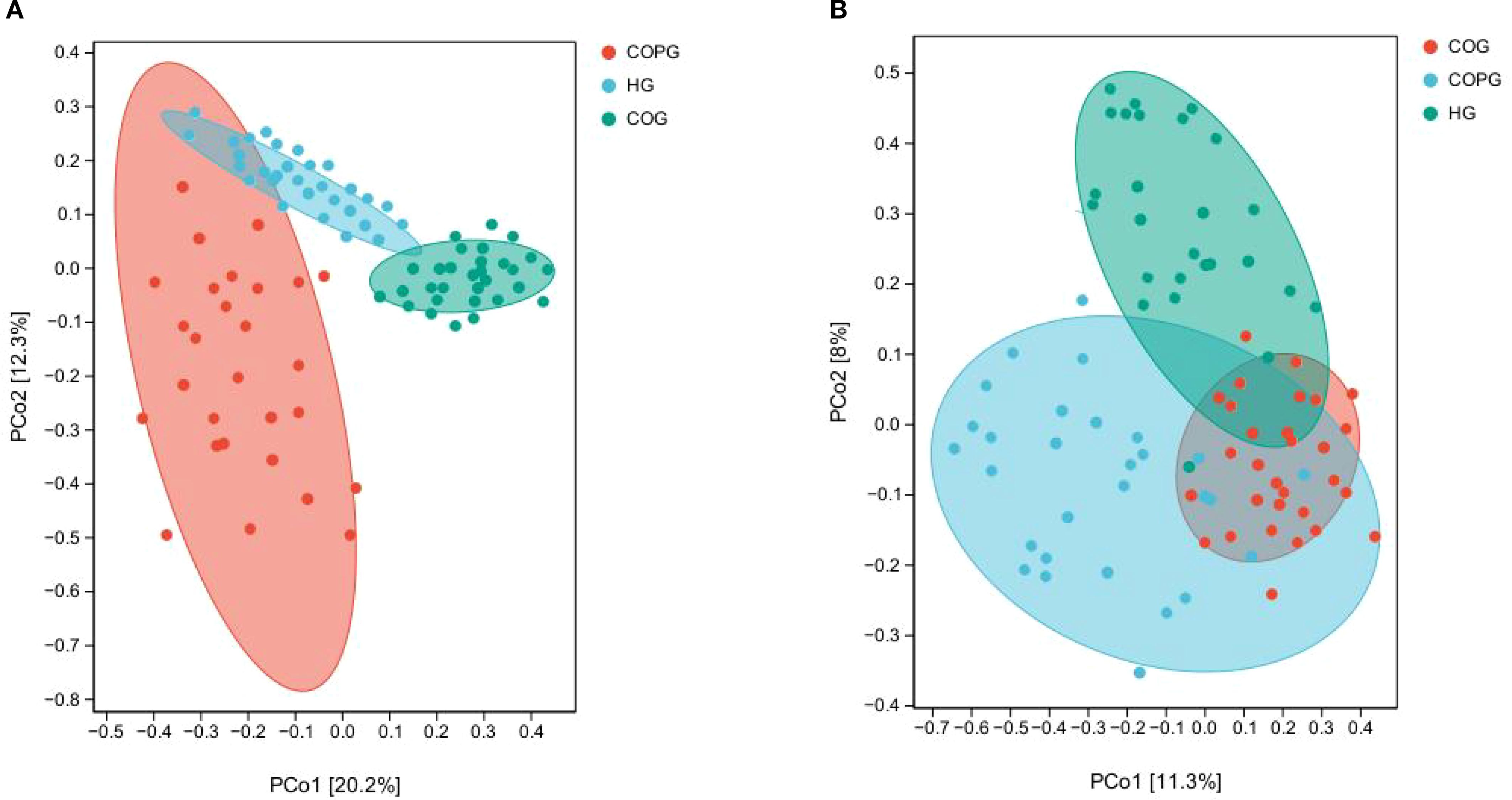
β-diversity is used to measure the similarity or difference among communities of different samples (Paudel et al., 2022).
The β-diversity differences among groups in saliva samples were assessed using Principal Coordinates Analysis (PCoA) methods (Figures 6A, B). To account for multiple comparisons, the p-values were adjusted using the Benjamini–Hochberg False Discovery Rate (FDR) method. The adjusted results indicated that the differences in microbial composition among the three groups (saliva and fecal samples) were significantly greater than the within-group differences, with all adjusted p-values being statistically significant (q < 0.05). This suggests that the oral and gut microbiota of healthy individuals, adolescents with dental caries and obesity, and those with psychological disorders exhibit distinct similarities and differences.
3.8 Analysis of microbial species differences
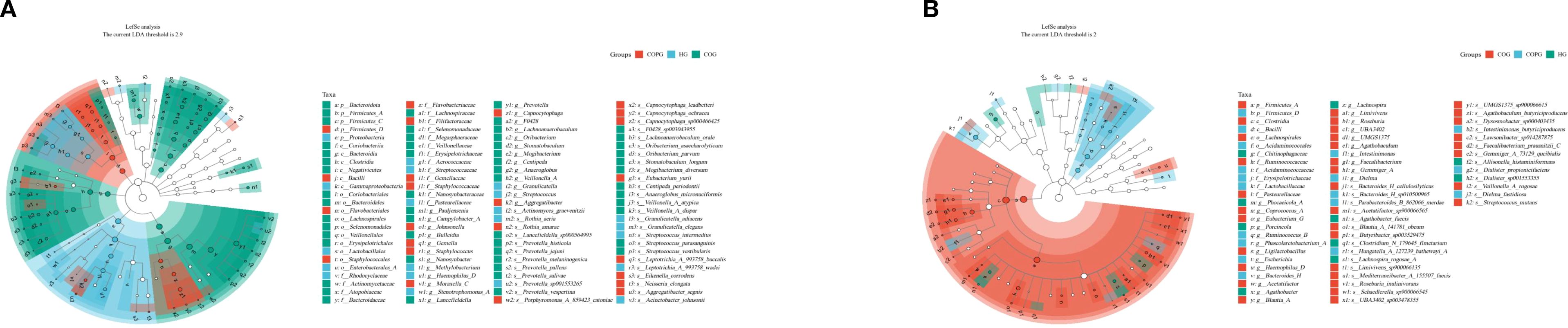
LEFSe analysis (Figures 7A, B) was conducted to validate the differences in microbial species and identify potential oral and gut microbial biomarkers. To control for multiple comparisons, the p-values were adjusted using the Benjamini–Hochberg False Discovery Rate (FDR) method. Compared among the three groups, 114 species in the oral microbiota and 63 species in the gut microbiota exhibited significant differences (LDA > 2, q < 0.05).
In the oral microbiota, the HG group showed higher relative abundance of Neisseria and Porphyromonas. In contrast, the COG group exhibited significantly increased relative abundance of Fusobacterium, Rothia, and Prevotella. The COPG group had higher relative abundance of Streptococcus and Veillonella compared to the other groups.
In the gut microbiota, the HG group exhibited significantly higher relative abundance of Bacilli and Erysipelotrichaceae. The COG group had higher relative abundance of Firmicutes and Clostridia. These differentially abundant microbial taxa may play unique functional roles within their respective groups.
3.9 SourceTracker analysis
To further investigate whether there is ectopic colonization of oral microbiota in the intestines of patients with dental caries and obesity, we employed the SourceTracker method for analysis(Figure 8A, B). The results demonstrated that nearly 44.88% of the Prevotella in the intestines of our experimental subjects originated from ectopic colonization of oral microbiota, which was higher than the nearly 100% result observed in the healthy population in this study.
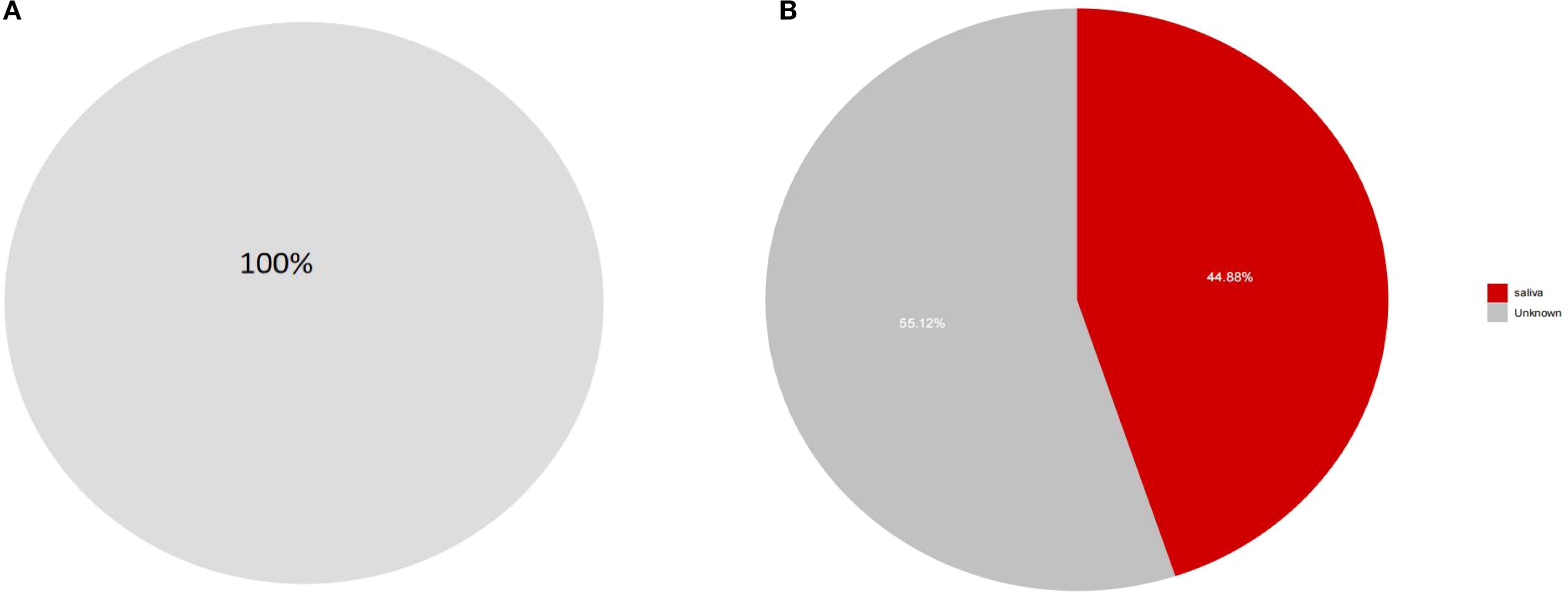
Figure 8. (A, B) SourceTracker analysis of ectopic colonization of oral microbiota in the intestines. These findings suggest that patients with dental caries and obesity have a higher rate of ectopic colonization of oral microbiota in their intestines compared to healthy individuals, which may have implications for understanding the microbial imbalances associated with these conditions.
3.10 Untargeted metabolomics analysis
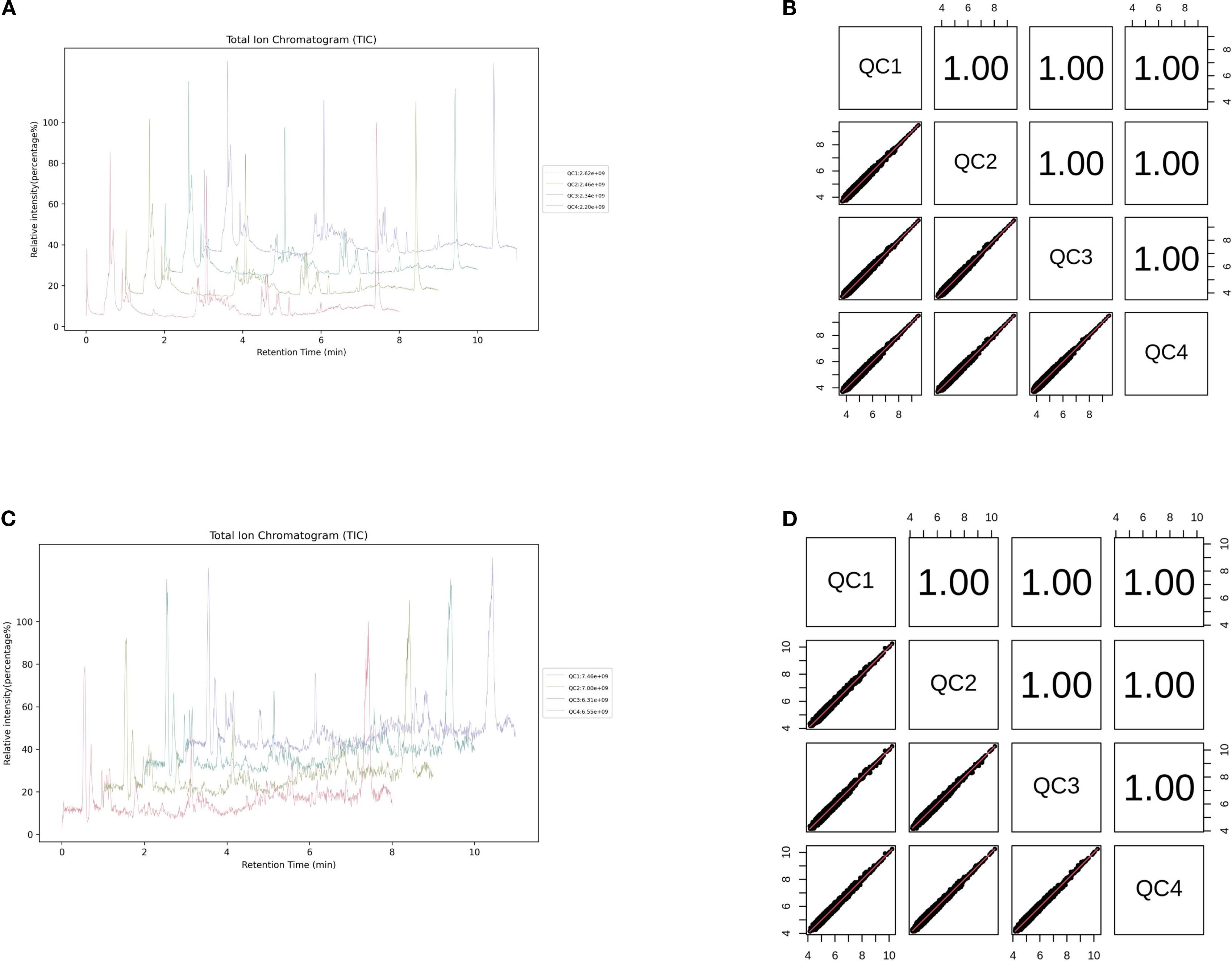
This study conducted a comprehensive identification and quantitative analysis of metabolites in intestinal samples using untargeted metabolomics. Quality control (QC) analysis ensured the reliability and stability of the experimental data. The total ion chromatogram (TIC) of QC samples showed that the response intensity and retention time of each chromatographic peak were largely overlapping, indicating minimal variation caused by instrument error. The Pearson correlation coefficients between QC samples were all greater than 0.9, demonstrating excellent experimental reproducibility (Figures 9A–D).
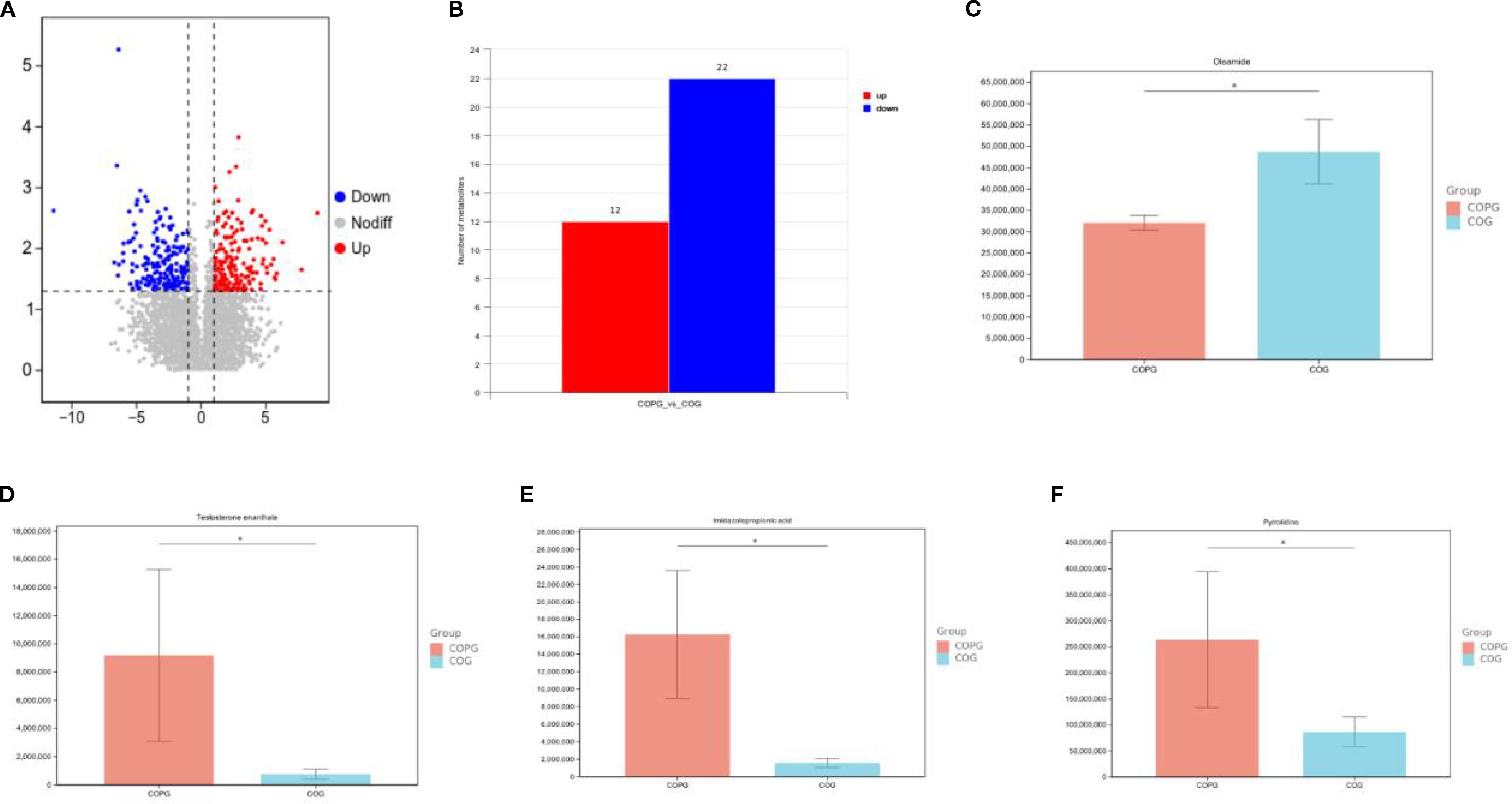
In the fecal sample comparison between the COG and COPG groups, significant differences in metabolite profiles were observed, with 12 metabolites upregulated and 22 downregulated in the COPG group compared to the COG group. To account for multiple comparisons, the p-values were adjusted using the Benjamini–Hochberg False Discovery Rate (FDR) method. Oleamide was found to be significantly lower in the COPG group than in the COG group (q < 0.05). In contrast, testosterone enanthate, imidazolepropionic acid, and pyrrolidine were significantly elevated in the COPG group (q < 0.05) (Figures 10A–F).
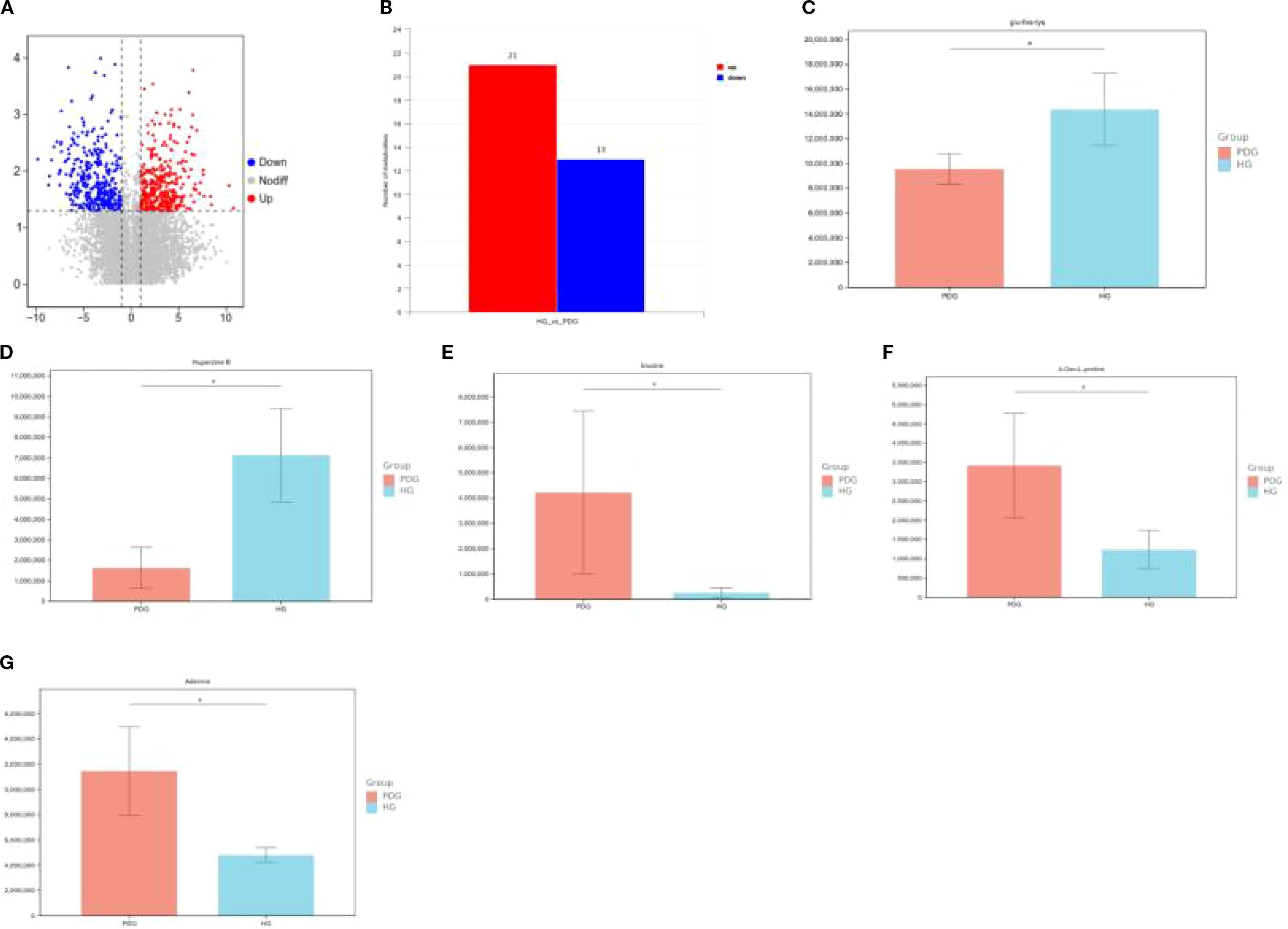
In the comparison of saliva samples between the HG and PDG groups, significant differences in metabolite profiles were observed, with 21 metabolites upregulated and 13 downregulated in the PDG group compared to the HG group. To account for multiple comparisons, the p-values were adjusted using the Benjamini–Hochberg False Discovery Rate (FDR) method. Specifically, Glu-His-Lys (glutamine-histidine-lysine) and Huperzine B were found to be significantly lower in the PDG group than in the HG group (q < 0.05). In contrast, brucine, 4-oxo-L-proline, and adenine were significantly higher in the PDG group compared to the HG group (q < 0.05) (Figures 11A–G).
3.11 16S rRNA gene sequencing combined with non-targeted metagenomics analysis
In the integrated analysis of 16S rRNA gene sequencing and non-targeted metabolomics between the dental caries-obesity group and the dental caries-obesity-mental disorder group, a connection between microorganisms and metabolites associated with psychological issues was identified:
4 Discussion
4.1 Oral microbiome characteristics of the adolescent caries-obesity-psychological disorder triad
The oral cavity, as the initial part of the digestive tract communicating with the external environment, serves as the entry point for microorganisms into the human body, including bacteria, fungi, viruses, mycoplasmas, and other biological entities. The oral microbiota represents one of the most diverse and unique microbial communities in the human body, constituting the second most complex microbial ecosystem after the digestive system microbiota (Al Bataineh et al., 2022). Saliva samples, being readily obtainable, effectively represent microbial community information from various oral sites (Leake et al., 2016; Soriano-Lerma et al., 2020) and are widely utilized in oral microbial ecology research (Kaczor-Urbanowicz et al., 2017; Borkent et al., 2020). Next-generation sequencing (NGS) technology has emerged as a powerful tool for understanding the oral microbiome in health and disease states (Johansson et al., 2016; Al-Hebshi et al., 2019). By targeting one or more hypervariable regions of the 16S rRNA gene, this method characterizes microbial communities, as these variable regions serve as effective markers for identifying bacterial taxa in samples. In recent years, this approach has been extensively applied in studies investigating cariogenic microbial communities (Magana et al., 2018), enhancing our understanding of microbial factors associated with dental caries. Dental caries arises from the interplay among oral microorganisms, host factors, dietary components, and temporal influences (Pitts et al., 2021; Song et al., 2024), with oral microbiota playing a pivotal role in caries initiation and progression. While the potential connections between caries and systemic health remain underexplored (Chapple et al., 2017), current evidence suggests a stronger association between obesity and caries compared to other systemic conditions (Sabharwal et al., 2021). Our research group has previously demonstrated a positive correlation between caries prevalence and obesity. Obesity, a complex metabolic disease, has been linked to the oral microbiota. Recent studies have uncovered potential connections between them. The oral microbiota in obese individuals has been shown to change, which may promote systemic inflammation and metabolic disruption. The oral microbiota can influence the host’s metabolism through various mechanisms, such as producing short-chain fatty acids (SCFAs) and regulating immune responses. A 2025 study investigated the effect of orally administered probiotics on weight and found that certain probiotics could significantly reduce the weight and visceral fat levels of obese patients, indicating that the oral microbiota may play a role in weight management (Guo et al., 2025). Moreover, studies have shown that the composition of the oral microbiota can affect the gut microbiota, which in turn impacts metabolic health. For example, specific oral bacteria, such as Prevotella, have been associated with obesity-related changes in the gut microbiota. These findings highlight the importance of considering the oral microbiota in research on obesity and its related metabolic diseases. Concurrently, mental health issues exhibit high prevalence among adolescents (Gulliver et al., 2010; Guo et al., 2025), affecting approximately 10–20% of children and adolescents worldwide (Kieling et al., 2011; Aarons et al., 2019). The role of the oral microbiome in mental health has recently been appreciated within the proposed oral-brain axis. A 2024 study published in Nature found that periodontitis, a chronic bacterial infection, is associated with mental disorders such as depression and anxiety (Malan-Müller et al., 2024). This suggests the existence of an “oral-brain axis” and highlights the potential role of the oral microbiome in mental health.
In the oral microbiota of this study, adolescents with caries and obesity showed distinct microbial profiles compared to healthy controls at the phylum level: higher relative abundances of Firmicutes, Bacteroidota, and Actinobacteriota, and lower relative abundance of Proteobacteria, consistent with findings by Agnello et al (Advances in research on oral microecology and caries prevention, n.d.; Agnello et al., 2017; De Lemos et al., 2024). At the genus level, increased relative abundances of Veillonella, Prevotella, and Rothia were observed, while Streptococcus, Neisseria, Staphylococcus, and Haemophilus exhibited reduced abundances. Multiple studies (Mashima and Nakazawa, 2014; Do et al., 2015; Liu et al., 2020; Korona-Glowniak et al., 2021)have identified strong associations of Veillonella, Prevotella, and Streptococcus with dental caries. Notably, statistical analyses of Prevotella abundance in prior studies revealed its dominance in Western populations’ oral microbiota, with high-fiber diets and obesity linked to its enrichment (Tett et al., 2021). The reduced relative abundance of Streptococcus in the COG group may be attributed to the altered dietary habits and lifestyle in obese individuals, which may lead to changes in saliva composition, such as variations in nutrient content, and alterations in saliva flow rate. These changes may create an environment unfavorable for the growth and colonization of Streptococcus, thereby reducing its relative abundance. While some studies associate Neisseria with caries (Zheng et al., 2018), others report its prominence in caries-free groups (Richards et al., 2017). Baker et al (Baker et al., 2021), using next-generation whole-genome sequencing to compare salivary microbiota in American children (>3 years old) with and without caries, observed higher abundances of Neisseria and Haemophilus in caries-free groups, aligning with the findings of this study.
In adolescents with psychological disorders compared to psychologically normal individuals at the phylum level, Firmicutes and Proteobacteria exhibited higher relative abundances, while Bacteroidota and Actinobacteriota showed lower abundances. The reduced abundance of Actinobacteriota was associated with anxiety states and cortisol levels in adolescents (Simpson et al., 2020), potentially serving as a marker of hypothalamic-pituitary-adrenal (HPA) axis hyperactivity, a common feature in the pathophysiology of depression. At the genus level, Streptococcus, Neisseria, Staphylococcus, and Haemophilus displayed higher relative abundances, whereas Veillonella and Prevotella demonstrated lower abundances. The observed associations between these microbial taxa and psychological disorders align with findings from studies by Yolken R et al (Prato et al., 2021; Wingfield et al., 2021; Yolken et al., 2021; Al Bataineh et al., 2022).
The results of alpha diversity analysis align with findings from Liu et al (Liu et al., 2019), a phenomenon potentially associated with oral microbial dysbiosis. According to the “ecological plaque hypothesis,” oral microbiota maintains equilibrium under healthy conditions, but alterations in the oral environment disrupt this balance, driving the transition of symbiotic microbial communities into pathogenic biofilms, ultimately leading to dysbiosis and caries development (Advances in research on oral microecology and caries prevention, n.d.). Beta diversity analysis revealed significant differences in oral microbiota between healthy individuals and adolescents with caries-obesity or psychological disorders, underscoring the critical role of oral microbial communities in both oral and systemic health. Concurrently, LefSe results demonstrated distinct compositional differences in salivary microbial communities across groups, likely attributable to specific oral health statuses or lifestyle factors within each cohort. In the COPG group, enriched Streptococcus and Veillonella genera—early colonizers and typical symbionts in the oral cavity—jointly participate in the formation of early-stage biofilms. Extensive research indicates that dysbiosis of Streptococcus and Veillonella is not only closely linked to oral diseases such as caries and periodontitis but may also breach gastrointestinal barriers for distal colonization, emerging as novel potential biomarkers for predicting the onset, progression, and prognosis of various systemic diseases.
4.2 Characteristics of the gut microbiome in adolescents with the caries-obesity-psychological disorders triad
The gut microbiota is one of the most complex and diverse microbial communities in the human body, with its diversity even surpassing that of the oral microbiota, making it the most diverse among human microbial communities (Li et al., 2024). These microbes, including bacteria, fungi, viruses, and archaea, form a complex ecosystem that plays a crucial role in human digestion, immunity, and metabolism. Notably, B. acidifaciens, typically associated with the gut microbiota, has also been found to play a role in the oral microbiota of children with severe dental caries, indicating a possible link between gut and oral microbiota in the development of dental caries (Eriksen et al., 2024). Another study highlighted that children with poor oral hygiene and high caries rates showed an increased abundance of specific bacterial phyla and genera, which may disrupt the balance of the gut microbiota and affect systemic health (Ribeiro and Paster, 2023). Numerous studies comparing the gut microbiota of overweight and normal-weight children through 16S rRNA gene sequencing have found significant differences in microbial diversity and composition between the two groups (Pan and Jiao, 2024). Moreover, there is also a certain relationship between the gut microbiota and mood disorders, with research indicating that the relative abundance of certain bacteria changes in individuals with depression (Marano et al., 2025).
In the gut microbiota at the phylum level, adolescents with caries and obesity exhibited an elevated Firmicutes/Bacteroidota (F/B) ratio—characterized by increased Firmicutes and decreased Bacteroidota —a critical alteration implicated in the pathogenesis of obesity and related metabolic complications (Gallardo-Becerra et al., 2020). Concurrently, the reduced relative abundance of Actinobacteriota aligns with findings from Rocío et al (Quiroga et al., 2020), further supporting the strong association between gut microbial shifts and the caries-obesity phenotype. Notably, adolescents with caries, obesity, and psychological disorders demonstrated distinct microbial profiles compared to their psychologically normal counterparts with obesity: lower Firmicutes abundance and higher relative abundances of Bacteroidota, Actinobacteriota, and Proteobacteria, consistent with studies by Huang, Plaza-Díaz J, and colleagues (Huang et al., 2018; Jiang et al., 2018; Plaza-Díaz et al., 2019).
At the genus level, adolescents with caries and obesity showed higher relative abundances of Prevotella, Faecalibacterium, and Bifidobacterium, in agreement with findings by Moran-Ramos S et al (Moran-Ramos et al., 2023), alongside reduced abundances of Faecalibacterium and Megamonas. In contrast, those with psychological disorders displayed elevated Bacteroides and Bifidobacterium abundances but lower Faecalibacterium, Prevotella, and Faecalibacterium levels. These observations align with prior studies reporting reduced Prevotella abundance during depressive states (Simpson et al., 2021) and diminished Faecalibacterium levels in individuals with anxiety, depression, bipolar disorder, psychosis, and schizophrenia (Nikolova et al., 2021).
Alpha Diversity Analysis: The observed differences lacked statistical significance, which partially aligns with findings from (Pinart et al., 2021). This meta-analysis incorporated 32 cross-sectional studies evaluating gut microbial composition in obese and non-obese adults via high-throughput sequencing. Among these, 7 out of 22 studies reported no significant differences in alpha diversity. This phenomenon may be attributed to similarities in dietary patterns and habits among study participants, leading to consistent nutrient availability for gut microbiota. Such uniformity likely provides comparable ecological niches for microbial taxa, promoting stable microbial community diversity and minimizing alpha diversity variations.
4.3 Microbiota-gut-brain axis
Communication between the gut and brain is bidirectional, involving multiple pathways such as neural, endocrine, and immune mechanisms (Figure 12). The microbiota and its derived metabolites act as critical regulators in gut-brain signaling, leading to the concept of the microbiota-gut-brain (MGB) axis (Cryan and Dinan, 2012). The gut-brain axis, a bidirectional communication system between the gut and the central nervous system mediated via neural, endocrine, and immune pathways, has emerged as a pivotal framework for studying interactions between gut microbes and neurological functions in recent years (Cryan and Dinan, 2012).
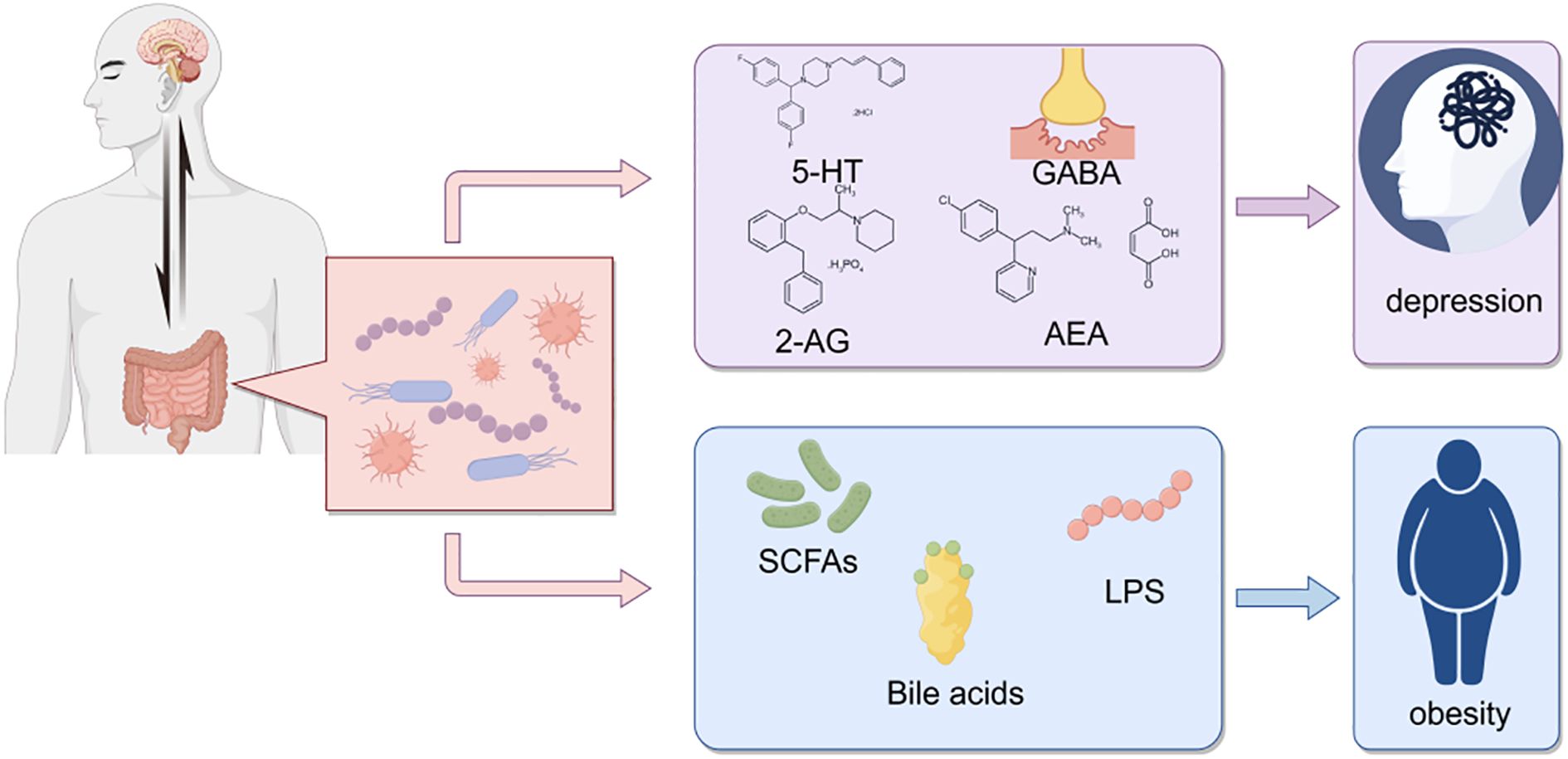
Figure 12. Interactions between gut microbiota and host health. This schematic diagram illustrates the complex interactions between gut microbiota and host health, highlighting the potential roles of various microbial metabolites in influencing neurological and metabolic conditions.
Analysis of fecal samples from the COG and COPG groups revealed significant alterations in multiple metabolite levels, which may exert broad effects on brain function via the gut-brain axis. Oleamide, which exhibits antidepressant-like properties in rat models, modulates neurotransmitter systems (e.g., 5-HT and GABA), and its decreased levels may impair mood regulation (Boger et al., 1998; Ge et al., 2015). Additionally, a Japanese study reported psychological changes, such as emotional instability, in some transgender males undergoing gender-affirming hormone therapy with testosterone enanthate, suggesting its potential influence on mood regulation via neuroendocrine pathways (Kirisawa et al., 2021). Imidazolepropionic acid, a histidine-derived microbial metabolite, may participate in neurological regulation by affecting neuroinflammation and neuronal function in the brain (Caradonna et al., 2024). Pyrrolidine, through its substituted derivatives, enhances 5-HT1A receptor potency by approximately 12-fold, indicating its potential role in modulating neurotransmitter system activity via 5-HT1A receptor regulation (Warren et al., 2024).
These findings further support the hypothesis that gut microbial metabolites may regulate brain function via the HPA axis (Foster and McVey Neufeld, 2013) and influence neuroinflammation, neurotransmitter systems, and mood regulation through the gut-brain axis, thereby exerting broad impacts on cerebral function. The results provide novel insights into the interactions between gut microbiota and the nervous system, suggesting that microbial metabolites could serve as potential therapeutic targets for future neuropsychiatric interventions.
Gln-Trp (Glutamine-Tryptophan): Tryptophan, a precursor to the neurotransmitter serotonin (5-hydroxytryptamine), is crucial for regulating emotions, sleep, and appetite. Glutamine, involved in various metabolic processes and important in the nervous system, associates with psychological factors through substance metabolism and neurotransmitter synthesis.
(2Z)-2-Benzylidenesuccinic Acid: As a phthalate compound, it is prevalent in daily life and may disrupt the endocrine system. Some studies indicate that exposure to phthalates is linked to neurological abnormalities, anxiety, and depression. A U.S. study measured phthalate metabolites in the urine of 153 pregnant women and found that higher mono-isobutyl phthalate levels in boys’ urine were associated with increased scores for inattention, rule-breaking, aggression, and conduct problems. Higher MBzP levels correlated with elevated scores for defiant behavior and conduct problems in boys.
Gln-Trp was positively correlated with Pseudoxanthomonas, suggesting Pseudoxanthomonas might influence psychological disorders. Conversely, Limivivens, Paraprevotella, Acetatifactor, and Eubacterium showed negative correlations, indicating their abundance might link to psychological issues. Notably, Paraprevotella, part of the Prevotellaceae family, warrants further attention.
4.4 Oral-brain axis
The mechanisms linking oral microbiota and the brain (termed the “oral-brain axis”) remain largely unexplored (Yano et al., 2015; Valles-Colomer et al., 2019). However, recent studies have proposed several potential routes through which oral bacteria may reach the brain and influence neuroimmune activity and inflammation (Olsen and Hicks, 2020). For instance, routine dental procedures such as flossing, brushing, and professional cleanings may introduce oral bacteria into the bloodstream, causing bacteremia (Olsen, 2008). Certain microbes might subsequently traverse the blood-brain barrier. Additionally, altered blood-brain barrier permeability could expose the brain to bacterial metabolites, triggering inflammatory responses that disrupt central nervous system function and contribute to psychological disorders such as anxiety and depression (Figure 13).
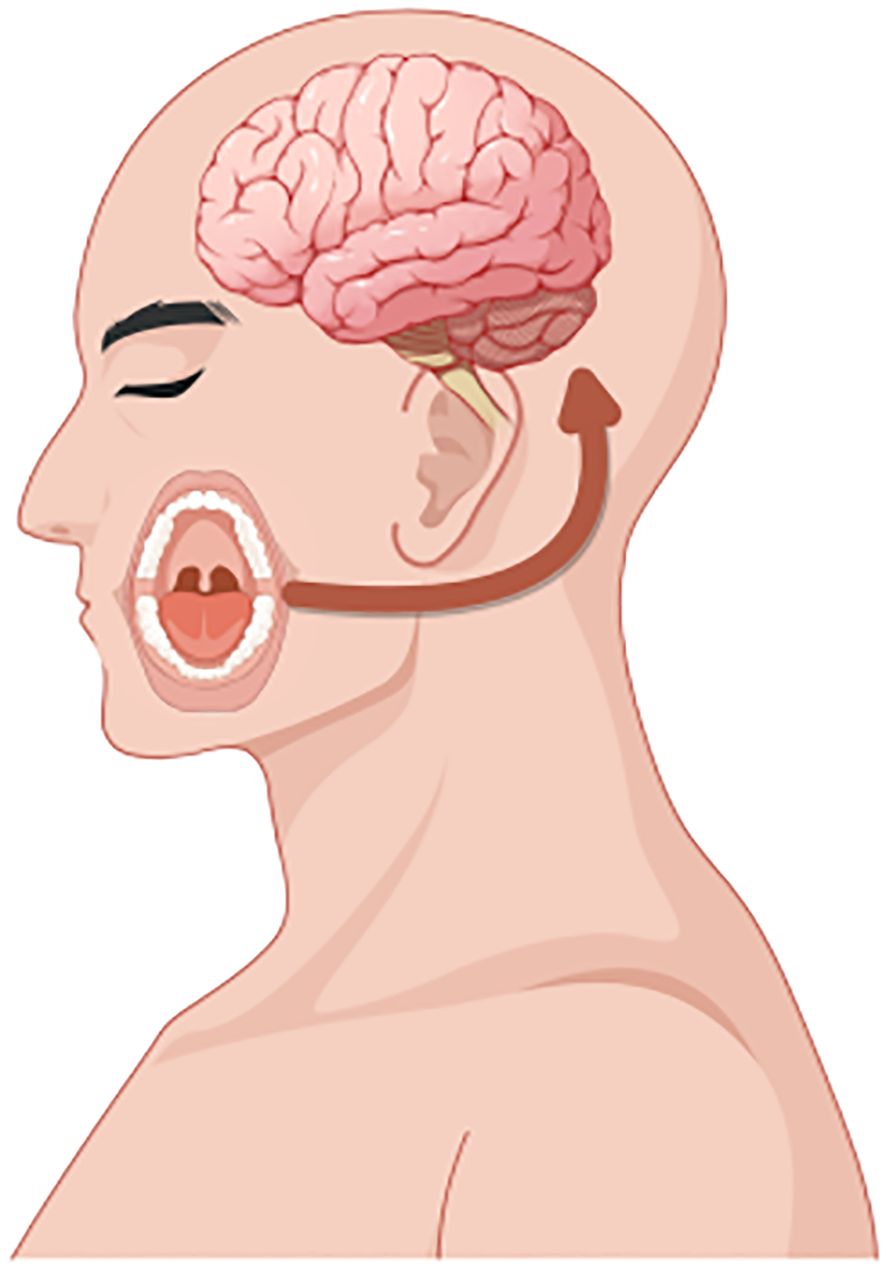
Figure 13. Schematic representation of the oral-brain axis. This schematic serves as a conceptual framework for understanding the complex interactions that may occur between oral microbiota, oral health conditions, and brain health, including the potential impact on cognitive functions and neurological disorders.
Analysis of differential metabolites in salivary samples from the HG and PDG groups revealed significant alterations in multiple metabolite levels. Notably, the glutamine-histidine-lysine (Glu-His-Lys) tripeptide, composed of three amino acids, exhibited notable changes. Glutamate (Glu), a critical excitatory neurotransmitter in the brain, is essential for normal cognitive functions such as learning, memory, and attention. However, imbalanced glutamate levels are strongly associated with the pathogenesis of psychiatric disorders (The structure, function and role in neuropsychiatric disorders of glutamate transporters, n.d.), where excessive concentrations may contribute to depression, anxiety, and schizophrenia. Magnetic resonance spectroscopy (MRS) studies further highlight glutamate’s potential role in conditions like depression and schizophrenia through quantitative measurements of its cerebral concentrations (The role of the glutamate system in mental disorders: Based on magnetic resonance spectroscopy imaging technology, n.d.). Histidine (His), a precursor of histamine, serves as both a neurotransmitter and neuromodulator in the brain, regulating sleep-wake cycles, feeding behavior, and learning (Ma et al., 2023). Clinical and experimental studies have elucidated histamine’s role in neuropsychiatric disorders (Cheng et al., 2021). Specifically, histamine binds to inhibitory receptors on murine serotonergic neurons, directly suppressing serotonin (5-hydroxytryptamine, 5-HT) release—a key molecule in mood regulation (Hersey et al., 2021). Reduced serotonin levels are closely linked to adverse psychological states such as depression and anxiety. Lysine (Lys), an essential amino acid involved in protein synthesis, also contributes to serotonin production (Yang et al., 2018). By enhancing serotonin availability, lysine may modulate mood regulation and potentially alleviate anxiety symptoms. Additionally, Huperzine B—a natural alkaloid derived from Huperzia species—exhibits potent acetylcholinesterase (AChE) inhibitory activity, demonstrating therapeutic potential for Alzheimer’s disease (Knölker, 2013). This suggests its applicability in managing psychological disorders associated with cholinergic dysfunction, such as Alzheimer’s disease.
Brucine intoxication may increase neuroexcitability, leading to muscle spasms and seizures, symptoms potentially linked to psychological states such as anxiety and tension (Lu et al., 2020). Direct associations between 4-Oxo-L-proline and psychological disorders remain understudied; however, its parent compound, L-proline, has been extensively investigated for its role in the nervous system and the relationship between its metabolic abnormalities and psychiatric conditions. Elevated concentrations of L-proline derivatives may exert toxic effects on the nervous system, such as inducing seizures or impairing normal neuronal function (Das et al., 2025). Studies have highlighted the role of proline metabolism in neuronal activity, suggesting that its dysregulation may contribute to neurological dysfunction (Yao and Han, 2022), thereby increasing susceptibility to neuropsychiatric disorders, including depression and anxiety. Adenine, a key component of purine metabolism, produces adenosine as a metabolite that plays a vital regulatory role in the nervous system. Research indicates that adenosine and its receptors (particularly the A2A subtype) are critically involved in mood regulation and mood disorders. In individuals with depression, adenosine metabolism may be disrupted, and the activity of its receptors (e.g., A2A) is closely associated with emotional behaviors (Qi et al., 2025).
In summary, the interaction between oral microbiota and the brain (termed the “oral-brain axis”) may play a significant role in mental health. Oral bacteria and their metabolites can influence the central nervous system through multiple pathways, including direct penetration of the blood-brain barrier, activation of immune responses, or signal transmission via neural routes. Dysbiosis of the oral microbiota is closely associated with psychiatric disorders such as anxiety and depression, highlighting oral health as a critical determinant of mental well-being. These insights not only deepen our understanding of the oral-brain axis but may also inform novel strategies for clinical interventions targeting psychiatric conditions.
4.5 Oral-gut axis
The oral-gut barrier, primarily shaped by the physical distance between the oral cavity and gut as well as differences in their chemical environments, allows each niche to harbor distinct microbial communities (Paudel et al., 2022). A substantial number of oral bacteria are swallowed in saliva, but the low pH of gastric acid creates harsh chemical conditions that prevent many oral bacteria from colonizing the gut (Figure 14). However, various factors, including disease, medication, and aging, may facilitate ectopic colonization of oral bacteria in the gut (Park et al., 2021). Oral bacteria can translocate to the gut and alter gut microbiota composition. The linkage between oral pathogenic bacteria and gut microbiota may be mediated via the “oral-gut axis,” wherein oral bacteria migrate through saliva swallowing from the oropharynx or oral digestive sites and ectopically colonize the gut, triggering inflammatory responses (MaChado et al., 2021). This process may disrupt the normal equilibrium of gut microbiota, leading to gut microbial dysbiosis (Bao et al., 2022; Wu et al., 2022). Additionally, these translocated bacteria may modify oral microbial composition during oral transit before entering the gastrointestinal tract. These findings suggest a bidirectional communication between altered microbial communities in the oral and gut ecosystems, influencing both niches (Park et al., 2021).
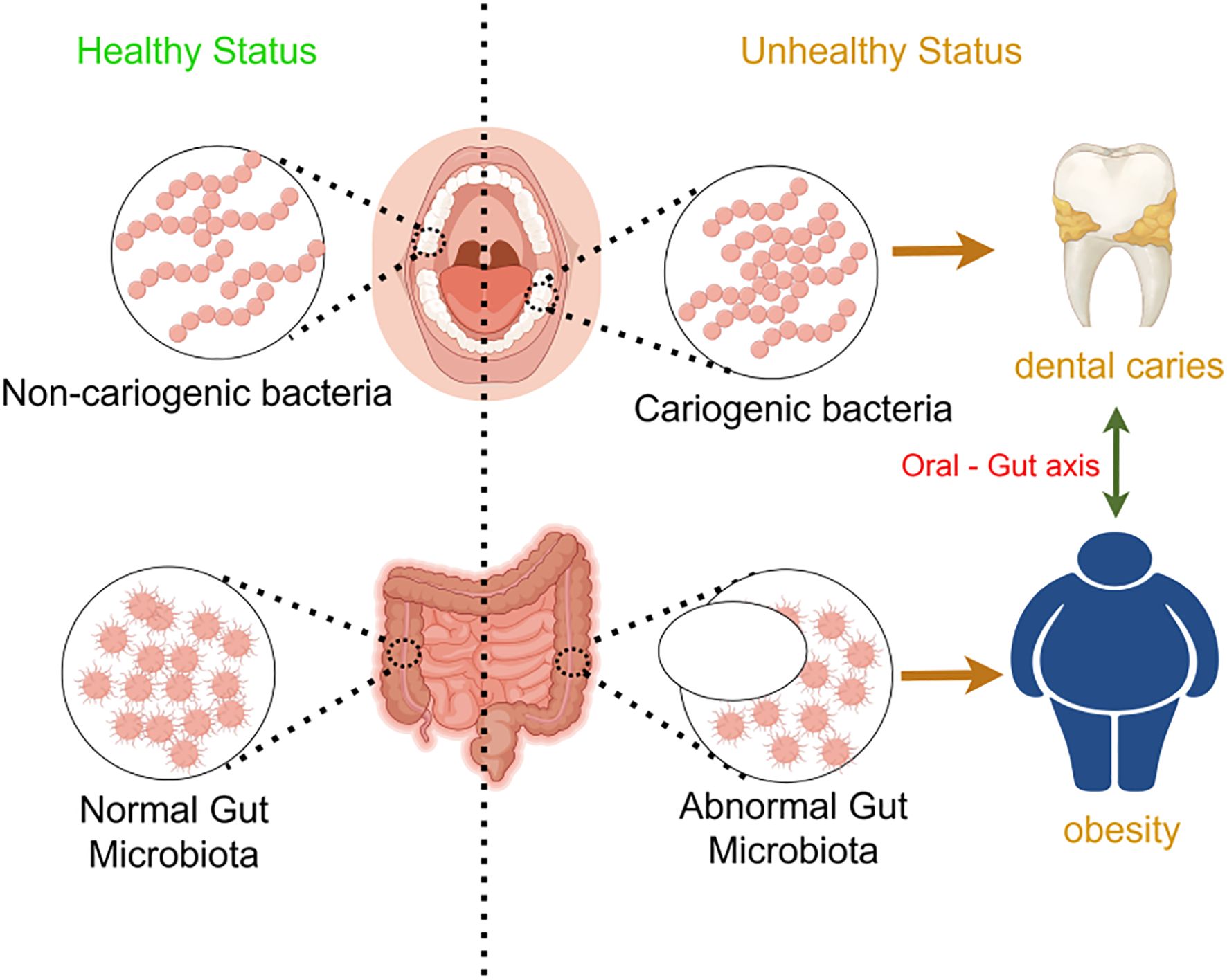
Figure 14. Schematic representation of the oral-gut axis and its impact on health. This schematic highlights the importance of maintaining a healthy oral and gut microbiota to prevent dental diseases and metabolic disorders, emphasizing the role of the oral-gut axis in overall health.
In this experiment, SourceTracker analysis targeting the genus Prevotella was performed on samples from the HG and COG groups. The results showed that the transmission effect in the COG group was significantly greater than that in the HG group, further confirming the role of the “oral-gut axis” in disease development. Specifically, the migration of Prevotella from the oral cavity to the gut was more pronounced in the COG group, indicating that in the comorbidity of dental caries and obesity, the oral-gut barrier is altered, making it easier for oral bacteria to colonize the gut. Prevotella is a common oral bacterium, Gram-negative and anaerobic, and is widely found in the human oral cavity, gastrointestinal tract, and urogenital tract (Research progress on Prevotella copri in the gut and its association with inflammatory diseases, n.d.). Studies have shown that Prevotella can migrate from the oral cavity to the gut via the oral-gut axis and colonize the gut, potentially triggering inflammatory responses, disrupting gut barrier function, and leading to systemic inflammation and metabolic disorders (Yamazaki and Kamada, 2024). This migration and colonization may affect the host’s health through various mechanisms.
5 Conclusion
This study explored the impact of psychological factors on adolescent caries-obesity comorbidity from the “oral-gut-brain axis,” yielding key conclusions:
1. A complex interrelation exists among adolescent caries, obesity, and psychological disorders, forming a health problem network.
2. Oral and gut microbiota play crucial roles in the development of these conditions. 16S rRNA sequencing revealed significant differences in oral and gut microbiota at phylum and genus levels among adolescents in different health states, with microbial diversity and composition closely linked to health.
3. Non-targeted metabolomics showed significant metabolite changes between groups, suggesting oral and gut microbial metabolites may influence brain function via the “gut-brain axis” and “oral-brain axis,” potentially impacting neuropsychiatric diseases.
4. Source tracker analysis confirmed the “oral-gut axis” role in disease, with oral bacteria from caries-obesity patients colonizing the gut and influencing its microbiota, contributing to disease development.
Data availability statement
The data presented in the study are deposited in the NCBI repository, accession number PRJNA1305107\MTBLS12873.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Scientific Research Department, Affiliated Stomatology Hospital of Jinzhou Medical University, Jinzhou City, Liaoning Province, China. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
MW: Formal Analysis, Visualization, Software, Project administration, Writing – original draft, Data curation, Investigation, Conceptualization, Methodology, Validation, Supervision, Writing – review & editing. XY: Data curation, Writing – review & editing, Investigation, Formal Analysis, Validation. YL: Formal Analysis, Software, Conceptualization, Investigation, Writing – review & editing. MJ: Writing – review & editing, Project administration, Formal Analysis. BC: Methodology, Writing – review & editing, Validation. WY: Conceptualization, Writing – review & editing, Methodology. NM: Project administration, Writing – review & editing, Validation. SH: Writing – review & editing, Visualization. CW: Supervision, Methodology, Writing – review & editing, Resources, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was financially supported by Science and technology innovation team of Liaoning Education Department (LJ222410160037).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
20180704145130574.pdf (n.d.). Available at: http://www.nhc.gov.cn/ewebeditor/uploadfile/2018/07/20180704145130574.pdf (Accessed April 9, 2025).
Aarons, G. A., Seijo, C., Green, A. E., Moullin, J. C., Hasson, H., Von Thiele Schwarz, U., et al. (2019). Fostering international collaboration in implementation science and research: a concept mapping exploratory study. BMC Res. Notes 12, 778. doi: 10.1186/s13104-019-4800-4
Agnello, M., Marques, J., Cen, L., Mittermuller, B., Huang, A., Chaichanasakul Tran, N., et al. (2017). Microbiome associated with severe caries in Canadian first nations children. J. Dent. Res. 96, 1378–1385. doi: 10.1177/0022034517718819
Al Bataineh, M. T., Künstner, A., Dash, N. R., Abdulsalam, R. M., Al-Kayyali, R. Z.A., Adi, M. B., et al. (2022). Altered composition of the oral microbiota in depression among cigarette smokers: A pilot study. Front. Psychiatry 13, 902433. doi: 10.3389/fpsyt.2022.902433
Al-Hebshi, N. N., Baraniya, D., Chen, T., Hill, J., Puri, S., Tellez, M., et al. (2019). Metagenome sequencing-based strain-level and functional characterization of supragingival microbiome associated with dental caries in children. J. Oral. Microbiol. 11, 1557986. doi: 10.1080/20002297.2018.1557986
Comprehensive evaluation of children and adolescents development (n.d.). Available at: http://www.nhc.gov.cn/wjw/pqt/201504/3661756c241b46329dbc6ad73eba0bd1.shtml(Accessed April 9, 2025).
Advances in research on oral microecology and caries prevention (n.d.). Available at: https://kns.cnki.net/kcms2/article/abstract?v=uXGtp3S0eCAk7If9PQMD_dpfCkp5XSGuoHnlsBE_lghMarzv5ViR8_XgfDt0623zyHhm0_8GoKPf9n0zHoBBW8_A8k-cq9WBekOSZlYjcaOEgMjKKpTVTMa9K3UpizNV8i1pv0579GXs46ptZWtAe961lOXFt6Ksm-_hszKs10ntQSWm-7qQGA==&uniplatform=NZKPT&language=CHS(Accessed April 9, 2025).
Study on the structure of oral microbiota in children with dental caries based on high-throughput sequencing technology (n.d.). Available at: https://kns.cnki.net/kcms2/article/abstract?v=uXGtp3S0eCA_ngkbmBHWeiDLSuYztaJVhcKWIBhajUb1CHLd-QKJgk8nfEY7bIA9yPW2zXRiYHpABq6kqdz207bRclFHjnBnmjOlhz2cGjuC3V3ns2-CZRJwFevHf8PAsIFoNGJMdQoNdWnsVrFTSo_lhStXYb6uxd0MddFYJrjCrk-YiJzVag==&uniplatform=NZKPT&language=CHS(Accessed April 9, 2025).
Research progress on Prevotella copri in the gut and its association with inflammatory diseases (n.d.). Available at: https://kns.cnki.net/kcms2/article/abstract?v=uXGtp3S0eCD2d-Jr6xJ5xOoRPPsw3-dOovvDmynaXTqJ3CkRpkCacPQA25QgQcbg3ijHzqEvWF-h1usFoTzxZhpgDiF064JVHyP2b28NYYlriWjXN7lReO8soi1MS0amdMZCJ1dIAOSxgmJzTZpKn0rmL4LaFJMSoWRysUNkyJs7uJovRdZyiA==&uniplatform=NZKPT&language=CHS(Accessed April 9, 2025).
The role of the glutamate system in mental disorders: Based on magnetic resonance spectroscopy imaging technology (n.d.). Available at: https://kns.cnki.net/kcms2/article/abstract?v=uXGtp3S0eCDb-Aoz-Yw1kKTLwNfApQtUzPrC2AE9ySYsewJdzag6MTTNoaL0SkQCrCAQTOeObwwumhyLm-aoDr4d1IrjDyMmUxgfyChOX3Dfr-ej9HyLSPVOH_EoY-U2PrDRIt3xxyeW4_3fztdrH1l8Xfi3FnbdWQlbydNx1ThxByT5-0gteg==&uniplatform=NZKPT&language=CHS (Accessed April 9, 2025).
The structure, function and role in neuropsychiatric disorders of glutamate transporters (n.d.). Available at: https://kns.cnki.net/kcms2/article/abstract?v=uXGtp3S0eCD1caR2fN9YQrPzoIs5-En0Pkh8vVpPtqjO_XuB_9iao-vvHdszR2fgE-2vZUzTrMY9mzmu-uC9j1laYTQ8AdxjjxVUGuvcAT3Kpls4CbOLdxK1Hya_SXQmEHTMwKautezUpocr_BuZqS8hYwT7Ixpe2_v6x-sRlNWaOMjJMarRcA==&uniplatform=NZKPT&language=CHS (Accessed April 9, 2025).
Baker, J. L., Morton, J. T., Dinis, M., Alvarez, R., Tran, N. C., Knight, R., et al. (2021). Deep metagenomics examines the oral microbiome during dental caries, revealing novel taxa and co-occurrences with host molecules. Genome Res. 31, 64–74. doi: 10.1101/gr.265645.120
Bao, J., Li, L., Zhang, Y., Wang, M., Chen, F., Ge, S., et al. (2022). Periodontitis may induce gut microbiota dysbiosis via salivary microbiota. Int. J. Oral. Sci. 14, 32. doi: 10.1038/s41368-022-00183-3
Boger, D. L., Henriksen, S. J., and Cravatt, B. F. (1998). Oleamide: an endogenous sleep-inducing lipid and prototypical member of a new class of biological signaling molecules. Curr. Pharm. Des. 4, 303–314. doi: 10.2174/138161280404221010152220
Borkent, D., Reardon, R. J. M., McLACHLAN, G., Glendinning, L., and Dixon, P. M. (2020). A microbiome analysis of equine peripheral dental caries using next generation sequencing. Equine Veterinary J. 52, 67–75. doi: 10.1111/evj.13126
Bowland, G. B. and Weyrich, L. S. (2022). The oral-microbiome-brain axis and neuropsychiatric disorders: an anthropological perspective. Front. Psychiatry 13, 810008. doi: 10.3389/fpsyt.2022.810008
Caradonna, E., Nemni, R., Bifone, A., Gandolfo, P., Costantino, L., Giordano, L., et al. (2024). The brain–gut axis, an important player in alzheimer and Parkinson disease: A narrative review. JCM 13, 4130. doi: 10.3390/jcm13144130
Chala, S., El Aidouni, M., Abouqal, R., and Abdallaoui, F. (2017). U-shaped association between untreated caries and body mass index in adults at Rabat dental University hospital, Morocco: cross sectional study. BMC Res. Notes 10, 5. doi: 10.1186/s13104-016-2356-0
Chapple, I. L. C., Bouchard, P., Cagetti, M. G., Campus, G., Carra, M., Cocco, F., et al. (2017). Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clinic Periodontol. 44, S42. doi: 10.1111/jcpe.12685
Cheng, L., Liu, J., and Chen, Z. (2021). The histaminergic system in neuropsychiatric disorders. Biomolecules 11, 1345. doi: 10.3390/biom11091345
Cryan, J. F. and Dinan, T. G. (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712. doi: 10.1038/nrn3346
Das, A., Gauthier-Coles, G., Bröer, S., and Rae, C. D. (2025). l-proline alters energy metabolism in brain cortical tissue slices. Neurochem. Res. 50, 16. doi: 10.1007/s11064-024-04262-1
De Lemos, G. M., Resende, C. M.M., Campello, C. P., Ribeiro, I. S., Mendes, A. K., De Lima, E. L.S., et al. (2024). Is oral microbiota associated with overweight and obesity in children and adolescents? A systematic review. Crit. Rev. Food Sci. Nutr. 64, 4275–4285. doi: 10.1080/10408398.2022.2140330
Do, T., Sheehy, E. C., Mulli, T., Hughes, F., and Beighton, D. (2015). Transcriptomic analysis of three Veillonella spp. present in carious dentine and in the saliva of caries-free individuals. Front. Cell. Infect. Microbiol. 5.
Dominy, S. S., Lynch, C., Ermini, F., Benedyk, M., Marczyk, A., Konradi, A., et al. (2019). Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5, eaau3333. doi: 10.1126/sciadv.aau3333
Duan, Z.-M. and Wu, L.-F. (2024). Role of oral-gut-brain axis in psychiatric and neurological disorders. WCJD 32, 878–886. doi: 10.11569/wcjd.v32.i12.878
Eriksen, C., Boustedt, K., Sonne, S. B., Dahlgren, J., Kristiansen, K., Twetman, S., et al. (2024). Early life factors and oral microbial signatures define the risk of caries in a Swedish cohort of preschool children. Sci. Rep. 14, 8463. doi: 10.1038/s41598-024-59126-z
Folayan, M. O., Adeniyi, A. A., Oziegbe, E. O., Fatusi, A. O., and Harrison, A. (2018). Integrated oral, mental and sexual health management for adolescents: a call for professional collaboration. Int. J. Adolesc. Med. Health 30, 20160060. doi: 10.1515/ijamh-2016-0060
Foster, J. A. and McVey Neufeld, K.-A. (2013). Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 36, 305–312. doi: 10.1016/j.tins.2013.01.005
Gallardo-Becerra, L., Cornejo-Granados, F., García-López, R., Valdez-Lara, A., Bikel, S., Canizales-Quinteros, S., et al. (2020). Metatranscriptomic analysis to define the Secrebiome, and 16S rRNA profiling of the gut microbiome in obesity and metabolic syndrome of Mexican children. Microb. Cell Fact 19, 61. doi: 10.1186/s12934-020-01319-y
Ge, L., Zhu, M., Yang, J.-Y., Wang, F., Zhang, R., Zhang, J.-H., et al. (2015). Differential proteomic analysis of the anti-depressive effects of oleamide in a rat chronic mild stress model of depression. Pharmacol. Biochem. Behav. 131, 77–86. doi: 10.1016/j.pbb.2015.01.017
Gulliver, A., Griffiths, K. M., and Christensen, H. (2010). Perceived barriers and facilitators to mental health help-seeking in young people: a systematic review. BMC Psychiatry 10, 113. doi: 10.1186/1471-244X-10-113
Guo, M., Li, J., Zhang, L., Chen, C., Wei, Y., and Shen, Z. (2025). Effects of oral supplementation of probiotics on body weight and visceral fat in obese patients: a meta-analysis and systematic review. Sci. Rep. 15, 6355. doi: 10.1038/s41598-025-90820-8
Hersey, M., Samaranayake, S., Berger, S. N., Tavakoli, N., Mena, S., Nijhout, H. F., et al. (2021). Inflammation-induced histamine impairs the capacity of escitalopram to increase hippocampal extracellular serotonin. J. Neurosci. 41, 6564–6577. doi: 10.1523/JNEUROSCI.2618-20.2021
Huang, E., Kang, S., Park, H., Park, S., Ji, Y., and Holzapfel, W. H. (2018). Differences in anxiety levels of various murine models in relation to the gut microbiota composition. Biomedicines 6, 113. doi: 10.3390/biomedicines6040113
Jiang, H., Zhang, X., Yu, Z., Zhang, Z., Deng, M., Zhao, J., et al. (2018). Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 104, 130–136. doi: 10.1016/j.jpsychires.2018.07.007
Johansson, I., Witkowska, E., Kaveh, B., Lif Holgerson, P., and Tanner, A. C. R. (2016). The microbiome in populations with a low and high prevalence of caries. J. Dent. Res. 95, 80–86. doi: 10.1177/0022034515609554
Kaczor-Urbanowicz, K. E., Martin Carreras-Presas, C., Aro, K., Tu, M., Garcia-Godoy, F., and Wong, D. T. (2017). Saliva diagnostics–Current views and directions. Exp. Biol. Med. (Maywood) 242, 459–472. doi: 10.1177/1535370216681550
Kieling, C., Baker-Henningham, H., Belfer, M., Conti, G., Ertem, I., Omigbodun, O., et al. (2011). Child and adolescent mental health worldwide: evidence for action. Lancet 378, 1515–1525. doi: 10.1016/S0140-6736(11)60827-1
Kirisawa, T., Ichihara, K., Sakai, Y., Morooka, D., Iyoki, T., and Masumori, N. (2021). Physical and psychological effects of gender-affirming hormonal treatment using intramuscular testosterone enanthate in Japanese transgender men. Sexual Med. 9, 100306–100306. doi: 10.1016/j.esxm.2020.100306
Kisely, S., Baghaie, H., Lalloo, R., Siskind, D., and Johnson, N. W. (2015). A systematic review and meta-analysis of the association between poor oral health and severe mental illness. Psychosomatic Med. 77, 83–92. doi: 10.1097/PSY.0000000000000135
Kisely, S., Sawyer, E., Siskind, D., and Lalloo, R. (2016). The oral health of people with anxiety and depressive disorders–a systematic review and meta-analysis. J. Affect. Disord. 200, 119–132. doi: 10.1016/j.jad.2016.04.040
Korona-Glowniak, I., Piatek, D., Fornal, E., Lukowiak, A., Gerasymchuk, Y., Kedziora, A., et al. (2021). Patterns of oral microbiota in patients with apical periodontitis. JCM 10, 2707. doi: 10.3390/jcm10122707
Leake, S. L., Pagni, M., Falquet, L., Taroni, F., and Greub, G. (2016). The salivary microbiome for differentiating individuals: proof of principle. Microbes Infection 18, 399–405. doi: 10.1016/j.micinf.2016.03.011
Li, C., Stražar, M., Mohamed, A. M.T., Pacheco, J. A., Walker, R. L., Lebar, T., et al. (2024). Gut microbiome and metabolome profiling in Framingham heart study reveals cholesterol-metabolizing bacteria. Cell 187, 1834–1852.e19. doi: 10.1016/j.cell.2024.03.014
Liu, X.-X., Jiao, B., Liao, X.-X., Guo, L.-N., Yuan, Z.-H., Wang, X., et al. (2019). Analysis of salivary microbiome in patients with alzheimer’s disease. JAD 72, 633–640. doi: 10.3233/JAD-190587
Liu, G., Wu, C., Abrams, W. R., and Li, Y. (2020). Structural and functional characteristics of the microbiome in deep-dentin caries. J. Dent. Res. 99, 713–720. doi: 10.1177/0022034520913248
Lobstein, T., Jackson-Leach, R., Moodie, M. L., Hall, K. D., Gortmaker, S. L., Swinburn, B. A., et al. (2015). Child and adolescent obesity: part of a bigger picture. Lancet 385, 2510–2520. doi: 10.1016/S0140-6736(14)61746-3
Lu, L., Huang, R., Wu, Y., Jin, J.-M., Chen, H.-Z., Zhang, L.-J., et al. (2020). Brucine: A review of phytochemistry, pharmacology, and toxicology. Front. Pharmacol. 11, 377. doi: 10.3389/fphar.2020.00377
Ma, Q., Jiang, L., Chen, H., An, D., Ping, Y., Wang, Y., et al. (2023). Histamine H2 receptor deficit in glutamatergic neurons contributes to the pathogenesis of schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 120, e2207003120. doi: 10.1073/pnas.2207003120
MaChado, V., Botelho, J., Escalda, C., Hussain, S. B., Luthra, S., Mascarenhas, P., et al. (2021). Serum C-reactive protein and periodontitis: A systematic review and meta-analysis. Front. Immunol. 12, 706432. doi: 10.3389/fimmu.2021.706432
Magana, M., Sereti, C., Ioannidis, A., Mitchell, C. A., Ball, A. R., Magiorkinis, E., et al. (2018). Options and limitations in clinical investigation of bacterial biofilms. Clin. Microbiol. Rev. 31, e00084–e00016. doi: 10.1128/CMR.00084-16
Malan-Müller, S., Vidal, R., O’Shea, E., Montero, E., Figuero, E., Zorrilla, I., et al. (2024). Probing the oral-brain connection: oral microbiome patterns in a large community cohort with anxiety, depression, and trauma symptoms, and periodontal outcomes. Transl. Psychiatry 14, 419. doi: 10.1038/s41398-024-03122-4
Marano, G., Rossi, S., Sfratta, G., Traversi, G., Lisci, F. M., Anesini, M. B., et al. (2025). Gut microbiota: A new challenge in mood disorder research. Life 15, 593. doi: 10.3390/life15040593
Mashima, I. and Nakazawa, F. (2014). The influence of oral Veillonella species on biofilms formed by Streptococcus species. Anaerobe 28, 54–61. doi: 10.1016/j.anaerobe.2014.05.003
Milaneschi, Y., Simmons, W. K., Van Rossum, E. F. C., and Penninx, B. W. (2019). Depression and obesity: evidence of shared biological mechanisms. Mol. Psychiatry 24, 18–33. doi: 10.1038/s41380-018-0017-5
Miller, G. E., Engen, P. A., Gillevet, P. M., Shaikh, M., Sikaroodi, M., Forsyth, C. B., et al. (2016). Lower neighborhood socioeconomic status associated with reduced diversity of the colonic microbiota in healthy adults. PloS One 11, e0148952. doi: 10.1371/journal.pone.0148952
Moran-Ramos, S., Cerqueda‐García, D., López‐Contreras, B., Larrieta‐Carrasco, E., Villamil‐Ramírez, H., Molina‐Cruz, S., et al. (2023). A metagenomic study identifies a Prevotella copri enriched microbial profile associated with non-alcoholic steatohepatitis in subjects with obesity. J. Gastro Hepatol. 38, 791–799. doi: 10.1111/jgh.16147
Nikolova, V. L., Smith, M. R.B., Hall, L. J., Cleare, A. J., Stone, J. M., and Young, A. H. (2021). Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry 78, 1343. doi: 10.1001/jamapsychiatry.2021.2573
Olsen, I. (2008). Update on bacteraemia related to dental procedures. Transfusion Apheresis Sci. 39, 173–178. doi: 10.1016/j.transci.2008.06.008
Olsen, I. and Hicks, S. D. (2020). Oral microbiota and autism spectrum disorder (ASD). J. Oral. Microbiol. 12, 1702806. doi: 10.1080/20002297.2019.1702806
Pan, Y. and Jiao, F.-Y. (2024). Link between childhood obesity and gut microbiota. World J. Gastroenterol. 30, 3560–3563. doi: 10.3748/wjg.v30.i30.3560
Park, S.-Y., Hwang, B.-O., Lim, M., Ok, S.-H., Lee, S.-K., Chun, K.-S., et al. (2021). Oral–gut microbiome axis in gastrointestinal disease and cancer. Cancers 13, 2124.
Paudel, D., Uehara, O., Giri, S., Yoshida, K., Morikawa, T., Kitagawa, T., et al. (2022). Effect of psychological stress on the oral-gut microbiota and the potential oral-gut-brain axis. Japanese Dental Sci. Rev. 58, 365–375. doi: 10.1016/j.jdsr.2022.11.003
Pinart, M., Dötsch, A., Schlicht, K., Laudes, M., Bouwman, J., Forslund, S. K., et al. (2021). Gut microbiome composition in obese and non-obese persons: A systematic review and meta-analysis. Nutrients 14, 12. doi: 10.3390/nu14010012
Pitts, N. B., Twetman, S., Fisher, J., and Marsh, P. D. (2021). Understanding dental caries as a non-communicable disease. Br. Dent. J. 231, 749–753. doi: 10.1038/s41415-021-3775-4
Plaza-Díaz, J., Gómez-Fernández, A., Chueca, N., Torre-Aguilar, M. J.D.L., Gil, Á., Perez-Navero, J. L., et al. (2019). Autism spectrum disorder (ASD) with and without mental regression is associated with changes in the fecal microbiota. Nutrients 11, 337. doi: 10.3390/nu11020337
Prato, A., Gulisano, M., Scerbo, M., Barone, R., Vicario, C. M., and Rizzo, R. (2021). Diagnostic approach to pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): A narrative review of literature data. Front. Pediatr. 9, 746639. doi: 10.3389/fped.2021.746639
Qi, C., Yang, Y., Tang, P., Zheng, C., Li, X., Jiang, N., et al. (2025). Adenosine A2A receptor-mediated interactions between Th1+ T cells and the choroid plexus epithelium via IFN-γ signalling control T-Cell infiltration in experimental autoimmune encephalomyelitis. Cell Commun. Signal 23, 94. doi: 10.1186/s12964-025-02100-7
Quiroga, R., Nistal, E., Estébanez, B., Porras, D., Juárez-Fernández, M., Martínez-Flórez, S., et al. (2020). Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 52, 1048–1061. doi: 10.1038/s12276-020-0459-0
Ribeiro, A. A. and Paster, B. J. (2023). Dental caries and their microbiomes in children: what do we do now? J. Oral. Microbiol. 15, 2198433. doi: 10.1080/20002297.2023.2198433
Richards, V. P., Alvarez, A. J., Luce, A. R., Bedenbaugh, M., Mitchell, M. L., Burne, R. A., et al. (2017). Microbiomes of site-specific dental plaques from children with different caries status. Infect. Immun. 85, e00106–e00117. doi: 10.1128/IAI.00106-17
Sabharwal, A., Stellrecht, E., and Scannapieco, F. A. (2021). Associations between dental caries and systemic diseases: a scoping review. BMC Oral. Health 21, 472. doi: 10.1186/s12903-021-01803-w
Simpson, C. A., Adler, C., Du Plessis, M. R., Landau, E. R., Dashper, S. G., Reynolds, E. C., et al. (2020). Oral microbiome composition, but not diversity, is associated with adolescent anxiety and depression symptoms. Physiol. Behav. 226, 113126. doi: 10.1016/j.physbeh.2020.113126
Simpson, C. A., Diaz-Arteche, C., Eliby, D., Schwartz, O. S., Simmons, J. G., and Cowan, C. S.M. (2021). The gut microbiota in anxiety and depression–A systematic review. Clin. Psychol. Rev. 83, 101943. doi: 10.1016/j.cpr.2020.101943
Song, C., Liu, R., Fang, Y., Gu, H., and Wang, Y. (2024). Developing functional hydrogels for treatment of oral diseases. Smart Med. 3, e20240020. doi: 10.1002/SMMD.20240020
Soriano-Lerma, A., Pérez-Carrasco, V., Sánchez-Marañón, M., Ortiz-González, M., Sánchez-Martín, V., Gijón, J., et al. (2020). Influence of 16S rRNA target region on the outcome of microbiome studies in soil and saliva samples. Sci. Rep. 10, 13637. doi: 10.1038/s41598-020-70141-8
Tett, A., Pasolli, E., Masetti, G., Ercolini, D., and Segata, N. (2021). Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 19, 585–599. doi: 10.1038/s41579-021-00559-y
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632. doi: 10.1038/s41564-018-0337-x
Warren, A. L., Lankri, D., Cunningham, M. J., Serrano, I. C., Parise, L. F., Kruegel, A. C., et al. (2024). Structural pharmacology and therapeutic potential of 5-methoxytryptamines. Nature 630, 237–246. doi: 10.1038/s41586-024-07403-2
Wingfield, B., Lapsley, C., McDowell, A., Miliotis, G., McLafferty, M., O’Neill, S. M., et al. (2021). Variations in the oral microbiome are associated with depression in young adults. Sci. Rep. 11, 15009. doi: 10.1038/s41598-021-94498-6
Wu, L., Han, J., Nie, J.-Y., Deng, T., Li, C., Fang, C., et al. (2022). Alterations and correlations of gut microbiota and fecal metabolome characteristics in experimental periodontitis rats. Front. Microbiol. 13, 865191. doi: 10.3389/fmicb.2022.865191
Yamazaki, K. (2023). Oral-gut axis as a novel biological mechanism linking periodontal disease and systemic diseases: A review. Japanese Dental Sci. Rev. 59, 273–280. doi: 10.1016/j.jdsr.2023.08.003
Yamazaki, K. and Kamada, N. (2024). Exploring the oral-gut linkage: Interrelationship between oral and systemic diseases. Mucosal Immunol. 17, 147–153. doi: 10.1016/j.mucimm.2023.11.006
Yang, Q.-Q., Zhao, D.-S., Zhang, C.-Q., Wu, H.-Y., Li, Q.-F., Gu, M.-H., et al. (2018). A connection between lysine and serotonin metabolism in rice endosperm. Plant Physiol. 176, 1965–1980. doi: 10.1104/pp.17.01283
Yano, J. M., Yu, K., Donaldson, G. P., Shastri, G. G., Ann, P., Ma, L., et al. (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276. doi: 10.1016/j.cell.2015.02.047
Yao, Y. and Han, W. (2022). Proline metabolism in neurological and psychiatric disorders. Mol. Cells 45, 781–788. doi: 10.14348/molcells.2022.0115
Yolken, R., Prandovszky, E., Severance, E. G., Hatfield, G., and Dickerson, F. (2021). The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophr. Res. 234, 51–57. doi: 10.1016/j.schres.2020.03.010
Zhang, M., Mi, N., Ying, Z., Lin, X., and Jin, Y. (2023). Advances in the prevention and treatment of Alzheimer’s disease based on oral bacteria. Front. Psychiatry 14, 1291455. doi: 10.3389/fpsyt.2023.1291455
Keywords: oral-gut-brain axis, caries, obesity, adolescents, microbiota
Citation: Wang M, Yang X, Li Y, Jiang M, Chen B, Yang W, Ma N, He S and Wang C (2025) Exploring the influence of psychological factors on the comorbidity of dental caries and obesity in adolescents from the perspective of the oral-gut-brain axis. Front. Cell. Infect. Microbiol. 15:1659042. doi: 10.3389/fcimb.2025.1659042
Received: 03 July 2025; Accepted: 19 August 2025;
Published: 16 September 2025.
Edited by:
Soumyadev Sarkar, Arizona State University, United StatesReviewed by:
Maurice H. T. Ling, University of Newcastle, SingaporeEmiliana D’Angelo, Università della Campania Luigi Vanvitelli, Italy
Copyright © 2025 Wang, Yang, Li, Jiang, Chen, Yang, Ma, He and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengyue Wang, d2FuZ2N5QGp6bXUuZWR1LmNu
†These authors share first authorship
 Meng Wang1,2†
Meng Wang1,2† Nan Ma
Nan Ma Chengyue Wang
Chengyue Wang