- 1College of Life and Health Sciences, Northeastern University, Shenyang, China
- 2School of Life Science and Bio-pharmaceutics, Shenyang Pharmaceutical University, Shenyang, China
Fungal infections pose a significant threat to human health, particularly in immunocompromised individuals, driving a sustained increase in the demand for effective antifungal agents. These agents can be classified into several categories based on their mechanisms of action and chemical structures, including inhibitors of sterol synthesis, cell wall synthesis, DNA synthesis, and cell membrane function. Each class exerts its antifungal effects through distinct molecular pathways that disrupt fungal cell growth and reproduction. However, the clinical utility of current antifungal therapies is hindered by challenges such as the emergence of drug resistance, limited antifungal spectra, and adverse side effects. Consequently, the development of safe and efficacious antifungal agents remains a pressing need. This review provides a comprehensive overview of the classification and molecular mechanisms of antifungal drugs, discusses the current challenges in antifungal therapy, and explores potential strategies for future drug development, aiming to inform and advance antifungal research and treatment.
1 Introduction
The incidence of fungal infections has significantly increased in recent years, ranging from mild allergic reactions to potentially life-threatening invasive fungal diseases (IFDs) (Kronstad et al., 2011; Stop neglecting fungi, 2017; Almeida et al., 2019; Fisher and Denning, 2023; Denning, 2024). Globally, over one billion people worldwide are affected by fungal infections each year, among whom more than 6.55 million suffer from fungal diseases that threaten their lives immediately (Denning, 2024). Such infections not only seriously damage the quality of life of patients, but also impose a heavy burden on the global healthcare system (Bongomin et al., 2017; Denning, 2024; Iliev et al., 2024).
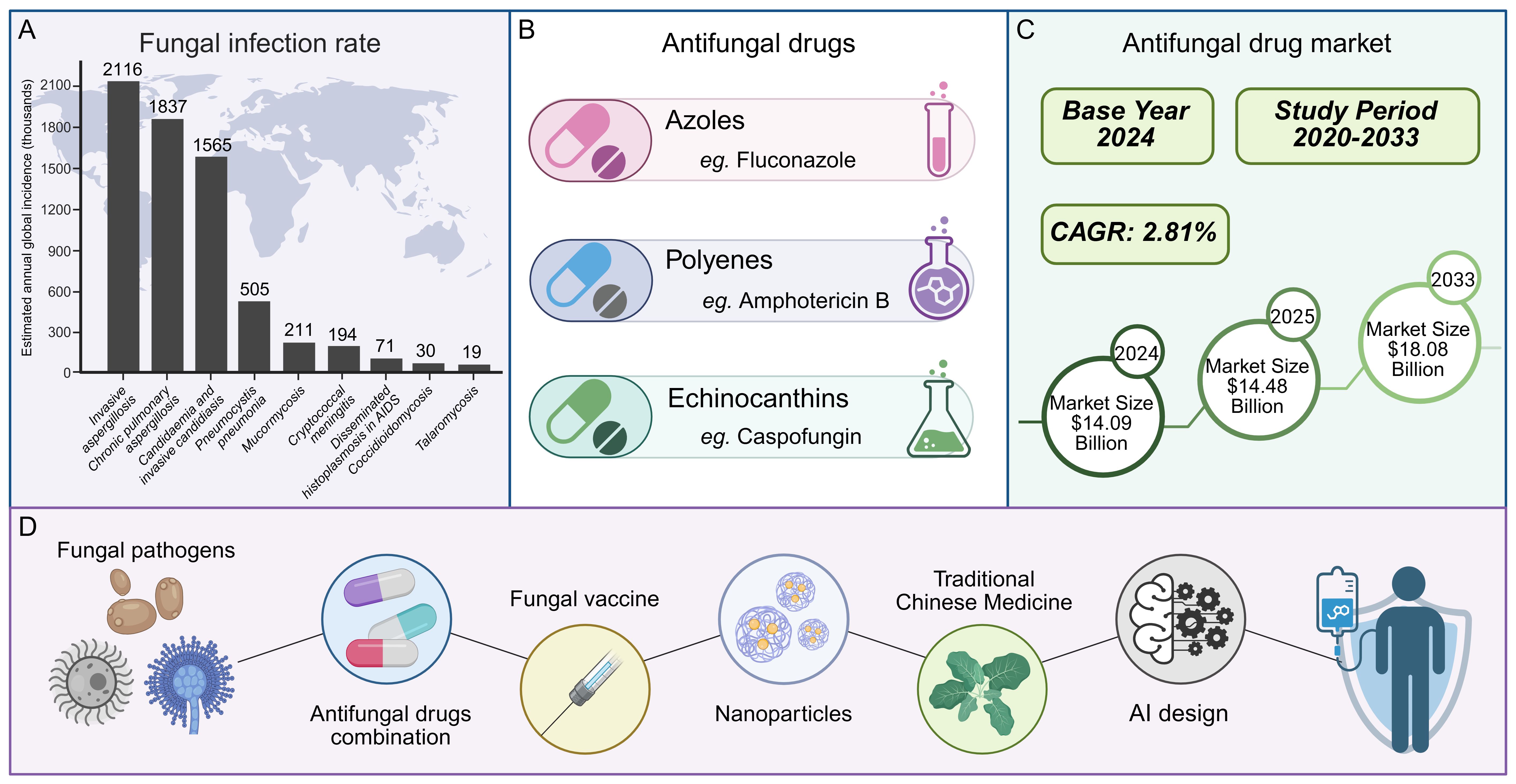
In the battle against human pathogenic fungi, antifungal drugs have emerged as indispensable tools. Currently, the treatment of invasive fungal infections relies exclusively on three main classes of antifungal drugs: polyenes, azoles, and echinocandins (Perfect, 2017). Consequently, the development of resistance by pathogenic fungi to any one of these drug classes would drastically narrow the range of available clinical treatment options. From a market perspective, the demand for antifungal drugs continues to rise: The market is expected to expand at a compound annual growth rate (CAGR) of 2.81% from 2020 to 2033, with a size of 14.09 billion US dollars in 2024, expected to rise to 14.48 billion US dollars in 2025, and reach 18.08 billion US dollars by 2033 (Figure 1) (Insights, 2025).However, the development and application of antifungal drugs face numerous challenges (Fisher et al., 2018; Iyer et al., 2021). Notably, with the increasing clinical utilization of antifungal drugs, the problem of fungal drug resistance has become increasingly prominent (Fisher et al., 2018; Vitiello et al., 2023; Iliev et al., 2024). The resistance rates of some fungi to existing antifungal drugs have risen significantly, directly resulting in treatment failures (Fisher et al., 2022; Antimicrobial resistance: a silent pandemic, 2024). Moreover, current drugs may suffer from inadequate selectivity for specific fungal species and significant side effects, which limits their widespread clinical use (Puumala et al., 2024).
With advancements in molecular biology and medicinal chemistry, research on antifungal drugs has been continuously progressing. New strategies for the development of antifungal drugs have gradually become a research hotspot, including the targeting of novel biological pathways, the combination of antifungal drugs, the development of fungal vaccines, the creation of innovative therapeutics (such as small-molecule peptides and nanoparticles), the extraction of active ingredients from traditional Chinese medicine, and the utilization of artificial intelligence in drug design (Figure 1) (Wall and Lopez-Ribot, 2020; Wu et al., 2023; Zhu et al., 2023; Saini et al., 2025). These studies offer new insights for the future development of antifungal medications. This review aims to systematically discuss the classification, molecular mechanisms, and drug development strategies in fungal treatment, providing valuable references for researchers and clinicians in related fields. Additionally, we will explore the current challenges in antifungal drug research and future directions, with the goal of promoting innovation and progress in the field of antifungal therapy.
This infographic outlines the landscape of fungal infections and antifungal efforts. Globally, fungal infections affect 6.55 million yearly, with rates climbing, as shown by the varied incidence of different fungal diseases (A, data are derived from Denning, D. W., Lancet Infect Dis, 2024 (Denning, 2024)). Antifungal drugs exist in classes like azoles, polyenes, and echinocandins, yet drug resistance complicates treatment (B). The market for these drugs, growing at a 2.81% CAGR from 2020–2033, was $14.09 billion in 2024, projected to reach $14.48 billion in 2025 and $18.08 billion by 2033 (C). To tackle challenges, new approaches are underway: combining antifungal drugs, developing fungal vaccines, creating innovative medications (including small - molecule peptides and nanoparticles), extracting actives from traditional Chinese medicine, and leveraging AI for drug design—all aiming to improve fungal infection management (D).
2 Major invasive fungal pathogens and their drug resistance status
Fungi represent a major biological kingdom with an extensive evolutionary history, comprising an estimated global diversity of over 5 million species, among which more than one million have been formally identified (Schueffler and Anke, 2014; Hawksworth and Lücking, 2017). While many fungi play beneficial roles in medicine (e.g., Ganoderma lucidum, Poria cocos), agriculture (e.g., mycorrhizal fungi enhancing plant nutrient uptake), and the food industry, a considerable number also pose serious threats to human health, agricultural productivity, and ecosystem stability. Notably, fungi have become one of the most formidable targets in anti-infective therapy (Lange, 2014; Liu et al., 2024; Cargill et al., 2025; Guo et al., 2025; Lei et al., 2025; Moora et al., 2025).
According to the World Health Organization (WHO), approximately 6.5 million cases of invasive fungal infections occur globally each year, resulting in an estimated 3.8 million deaths, of which 68% (around 2.5 million) are directly attributed to fungal diseases (Organization, W. H., 2022; Lockhart et al., 2023; Denning, 2024). Despite this, the threat of fungal pathogens to human health has long been underestimated. Regardless of economic development status, the incidence of invasive fungal diseases is steadily increasing, exerting a profound impact on public health (Seagle et al., 2021; Denning, 2024; Thompson et al., 2024a). Since 2016, Aspergillus fumigatus (associated with a mortality rate of 50%–90%), Cryptococcus neoformans (20%–70%), and Candida albicans (20%–40%) have been recognized as the leading causes of life-threatening fungal infections (Vandeputte et al., 2012; Han et al., 2016). In recognition of their clinical significance and growing resistance profiles, the WHO has designated these pathogens, along with Candida auris, as part of the “Critical Priority Group” in its Fungal Priority Pathogens List (Lockhart et al., 2023). Additionally, a growing number of emerging and re-emerging fungal pathogens, such as species of Fusarium, Mucorales, Histoplasma capsulatum, and Sporothrix, pose significant threats to immunocompromised populations. Although these pathogens are less prevalent globally, their morbidity and mortality may be substantially underestimated due to limited surveillance, low prioritization in public health frameworks, and geographically restricted endemicity. These pathogens often exhibit intrinsic or acquired resistance to antifungal agents, severely limiting treatment options. For example, Fusarium solani and F. oxysporum are intrinsically resistant to multiple antifungals, including azoles and echinocandins, making infections exceedingly difficult to treat (Dignani and Anaissie, 2004; Lortholary et al., 2010; Wiederhold et al., 2010; Demonchy et al., 2024). Although mucormycosis is rare globally, its incidence in India is approximately 80 times higher than elsewhere (Prakash and Chakrabarti, 2019; Skiada et al., 2020; Kottarathil et al., 2023). Histoplasma capsulatum is endemic to tropical and subtropical regions of the Americas (Teixeira Mde et al., 2016; Woods, 2016; Araúz and Papineni, 2021), while Sporothrix species (e.g., S. schenckii, S. brasiliensis, S. globosa) are closely linked to zoonotic outbreaks, particularly in China and Brazil (Alvarez et al., 2022; Rodrigues et al., 2022). The resistance profiles and regionally concentrated disease burdens of these fungi underscore the need for intensified mycological research.
It is noteworthy that fungal pathogens affecting plants and wildlife also pose significant global threats and offer unique insights into human fungal diseases. For instance, Magnaporthe oryzae (rice blast disease) and Fusarium oxysporum (vascular wilt in crops) are major plant pathogens that threaten global food security and cause substantial economic losses (Nalley L et al., 2016; Dita M et al., 2018). In the wildlife domain, Batrachochytrium dendrobatidis and B. salamandrivorans have been implicated in catastrophic amphibian population declines worldwide, while Pseudogymnoascus destructans is the causative agent of white-nose syndrome, which has led to mass mortality in North American bat populations (Drees KP et al., 2017; Grogan LF et al., 2018). Studies on these non-human pathogenic fungi have shed light on cross-species virulence mechanisms and provided valuable models for understanding fungal pathogenesis, host interaction, and immune evasion in humans. However, in the face of the rising burden of fungal infections, current treatment strategies remain inadequate.
Despite recent progress in antifungal therapy and the advancement of several novel agents into clinical trials, the rapid emergence of antifungal resistance has emerged as a critical barrier to effective treatment and is now recognized by the World Health Organization as one of the top ten global public health threats (Ferdinand et al., 2023; Vitiello et al., 2023). Currently available antifungal drugs are frequently associated with significant toxicity, pronounced side effects, narrow spectra of activity, and a high propensity for inducing resistance (Albrecht, 2019; Szymański et al., 2022; Zhou et al., 2022; Cavassin et al., 2024; Puumala et al., 2024; Gu et al., 2025). These limitations are particularly critical in the management of multidrug-resistant (MDR) and pan-drug-resistant (PDR) fungal infections, where therapeutic options remain extremely limited. These growing challenges underscore the urgent need for the development of antifungal agents with novel mechanisms of action, improved specificity, and reduced toxicity, alongside the identification of new molecular targets and therapeutic strategies to mitigate the escalating global burden of fungal diseases.
2.1 Candida
Candida species are major fungal pathogens, particularly in immunocompromised individuals, where infections are associated with high mortality, up to 45% despite antifungal therapy (Chowdhary et al., 2023; Robbins and Cowen, 2025). With the widespread use of antifungal agents, resistance among Candida species has become increasingly concerning, especially with the emergence of multidrug-resistant strains such as Candida auris, now recognized as a global health threat (Clancy and Nguyen, 2016; Lockhart et al., 2017; Rhodes and Fisher, 2019).
Among Candida species, C. albicans remains the most prevalent, followed by C. parapsilosis, C. glabrata and C. tropicalis. These non-albicans species are showing rising resistance, particularly to azoles like fluconazole, complicating treatment and increasing the risk of clonal outbreaks (Lass-Flörl et al., 2024; Khan et al., 2025). A 10-year study at Duke University (2001–2010) reported an increase in C. glabrata resistance to echinocandins from 4.9% to 12.3%, and fluconazole resistance rates from 18% to 30% (Alexander et al., 2013). In Europe, from 2016 to 2022, echinocandin resistance in 15,400 C. glabrata isolates ranged from 1.5% to 12% (Rodríguez-Cerdeira et al., 2025). Globally, fluconazole resistance in C. parapsilosis had exceeded 10% before 2019. Since 2020, echinocandin and multidrug-resistant C. parapsilosis strains have been increasingly reported (Daneshnia et al., 2023). A multicenter study across 11 hospitals in China (over three years, 1,072 non-albicans Candida isolates) found that C. tropicalis exhibited a 7.1% resistance rate to both fluconazole and voriconazole, while C. glabrata showed 14.3% resistance to fluconazole and 11.6% cross-resistance to voriconazole (Xiao et al., 2015). Candida auris has drawn considerable attention due to its high level of multidrug resistance, with fluconazole resistance detected in 70%-90% of isolates (Navalkele et al., 2017; Ostrowsky et al., 2020). Its tolerance to 10% NaCl and quaternary ammonium disinfectants enhances its persistence in healthcare settings (Ahmad and Alfouzan, 2021) (Supplementary File 1). Most notably, unlike other Candida species, C. auris displays a remarkable ability to colonize abiotic surfaces (Santana et al., 2023). This trait facilitates its presence on a wide range of medical equipment, including catheters, ventilators, and surgical tools, contributing to nosocomial transmission (Haq et al., 2024). Skin colonization by C. auris is a known risk factor for bloodstream infections (BSIs), with approximately 5%-10% of colonized individuals developing fungemia (Lyman et al., 2023) (Figure 2).
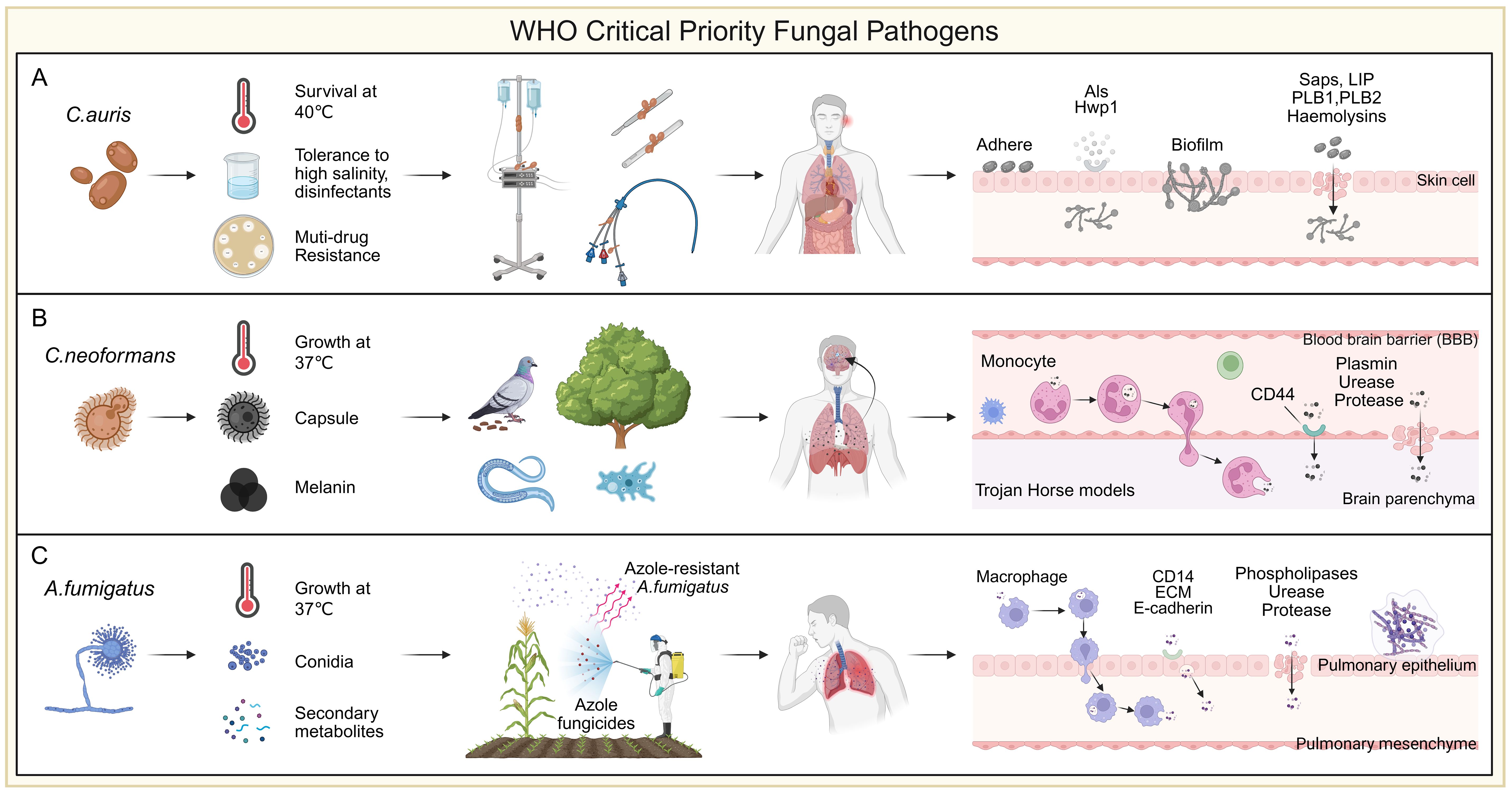
Figure 2. WHO critical priority fungal pathogens. (A) Candida auris is a multidrug-resistant fungus that can survive at 40 °C, tolerate high-salt conditions, and resist common disinfectants. A key risk factor for infection is its ability to adhere to abiotic surfaces, particularly medical devices such as ventilator tubes, central lines, feeding tubes, and urinary catheters. Its strong biofilm-forming capacity on these surfaces and skin promotes persistent colonization and increases the risk of invasive infection. Initial adhesion is mainly mediated by the Als family and Hwp1, while tissue invasion involves enzymes such as Saps, Plb1/2, Lip, and hemolysins. (B) Cryptococcus species produce classical virulence factors such as capsule and melanin, and exhibit thermotolerance at 37 °C. Interactions with environmental hosts like pigeons and amoebae have contributed to their resistance to heat and phagocytosis. C. neoformans displays notable neurotropism, disseminating from the lungs and crossing the blood-brain barrier (BBB) to cause meningoencephalitis. Translocation occurs via endothelial transcytosis or a “Trojan horse” mechanism mediated by monocytes. Inhaled spores are taken up by circulating monocytes, transported to the brain, and released into the parenchyma. Fungal cells also attach to the endothelium via CD44 to cross into tissue. To facilitate CNS invasion, C. neoformans secretes enzymes such as fibrinolysin, urease, and proteases that degrade host barriers. (C) Aspergillus fumigatus is a widespread environmental mold, with optimal growth at 37 °C. Triazole fungicides, structurally similar to medical azoles, can select for resistant strains in the environment. Inhalation of such strains by susceptible individuals may lead to azole-resistant infections. Humans inhale 100-1,000 conidia daily, typically cleared by the mucociliary system and alveolar macrophages. In immunocompromised hosts, conidia may evade clearance, germinate, and cause invasive disease. Surviving conidia can cross epithelial barriers via macrophage-mediated translocation or direct epithelial uptake involving CD14, ECM, and E-cadherin. Secreted phospholipases, ureases, and proteases further disrupt epithelial integrity, promoting tissue invasion.
In recent years, Candida-related breakthrough BSIs have emerged as complex clinical challenges, often occurring despite standard antifungal therapy. These infections are mainly attributed to C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis (Zhai et al., 2020; Puerta-Alcalde et al., 2023). Recent reviews indicate that such breakthrough infections are strongly associated with antifungal resistance (Kontoyiannis et al., 2010; Fisher et al., 2019; Jenks et al., 2020). Notably, beyond classical resistance mechanisms, heterogeneous resistance has been implicated in C. parapsilosis breakthrough infections during echinocandin prophylaxis, suggesting more nuanced resistance dynamics (Zhai et al., 2024).
2.2 Cryptococcus
Cryptococcosis is a life-threatening invasive fungal infection primarily caused by Cryptococcus neoformans and Cryptococcus gattii, accounting for approximately 152,000 new cases and 110,000 deaths annually, predominantly among immunocompromised individuals such as HIV/AIDS patients and organ transplant recipients (Chayakulkeeree and Perfect, 2006; Kwon-Chung et al., 2014). Cryptococcus species are widely distributed in nature, and their environmental adaptation strategies have facilitated the evolution of traits enabling human infection. Natural hosts such as pigeons, amoebae, and nematodes have contributed to the development of thermotolerance and resistance to phagocytosis in Cryptococcus (May et al., 2016). Notably, C. neoformans exhibits pronounced neurotropism and frequently invades the central nervous system (Chen et al., 2022). Both C. neoformans and C. gattii disseminate from the lungs and cross the blood-brain barrier (BBB) to cause meningoencephalitis (Kronstad et al., 2011) (Figure 2). Fungal cells penetrate the BBB either through transcytosis across endothelial cells lining cerebral vessels or via a “Trojan horse” mechanism involving carriage within phagocytes, ultimately leading to life-threatening meningoencephalitis (Kronstad et al., 2011). Globally, nearly 250,000 cases occur each year, and without timely intervention, the mortality rate approaches 100% (Iyer et al., 2021). Current treatment options are limited to three major classes of antifungal agents. Azoles, especially fluconazole, are widely used for consolidation and maintenance therapy. However, resistance to fluconazole in emerging Cryptococcus strains is on the rise (Ngan et al., 2022). Echinocandins are largely ineffective against Cryptococcus, and the efficacy of other agents is often compromised by host toxicity or fungal resistance (Denning, 2003) (Supplementary File 1). Amphotericin B (AmB), the only approved fungicidal agent for cryptococcosis, targets ergosterol in the fungal membrane and remains a cornerstone of induction therapy. However, despite its effectiveness, AmB often fails to achieve complete fungal clearance, and relapses are common (Guidelines for The Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection, 2018). Its clinical utility is further limited by severe toxicity and restricted availability due to economic and logistical barriers (Perfect et al., 2010). Recent studies highlight the role of drug tolerance and persistence in cryptococcosis (Chen et al., 2024; Ke et al., 2024). Unlike genetic resistance, these phenotypes enable fungal cells to survive high concentrations of antifungals without a measurable increase in minimum inhibitory concentration (MIC), contributing to chronic and relapsing infections (Berman and Krysan, 2020). For example, during pulmonary infection, Cryptococcus can enter a quiescent state that confers high tolerance to AmB (Ke et al., 2024). Upon CNS invasion, activation of the glucose repression regulator Mig1 has been linked to enhanced AmB tolerance (Chen et al., 2024). These mechanisms significantly reduce AmB efficacy in animal models of cryptococcal meningitis. A growing hypothesis proposes that drug tolerance and persistence may precede and facilitate the development of stable resistance, as observed in bacterial pathogens. Despite their clinical implications, these non-classical mechanisms of antifungal failure remain underexplored and warrant further investigation to inform new therapeutic strategies.
2.3 Aspergillus
Invasive aspergillosis (IA) is one of the most common fungal infections in immunocompromised hosts, including invasive pulmonary aspergillosis, sinusitis, disseminated aspergillosis, and infections affecting individual organs (Schauwvlieghe et al., 2018). In the European Union alone, more than 2.25 million people suffer from infections caused by Aspergillus (Prevention, E. C. f. D. & Control, 2013). Unfortunately, recent studies have reported global emerging resistance to azole antifungals in clinical and environmental isolates (Barber et al., 2021; Rhodes et al., 2022) (Supplementary File 1). Azole resistance in A. fumigatus can arise via two main routes. In the clinical setting, prolonged azole exposure in patients undergoing antifungal therapy may lead to the selection of resistant strains that persist despite treatment and continue to cause infection (Howard et al., 2009). Alternatively, in the external environment, A. fumigatus strains residing on decaying plant material may be exposed to azole-based agricultural fungicides, which share structural and functional similarities with medical azoles (Prigitano et al., 2019; Schoustra et al., 2019; Pontes et al., 2020). This environmental route has been increasingly recognized as a major driver of resistance development (Snelders et al., 2009; Verweij et al., 2020). Notably, these resistance mechanisms have been shown to spread globally via horticultural products, particularly plant bulbs, and the airborne dissemination of conidia is uncontrollable (Dunne et al., 2017). Humans inhale an estimated 100-1,000 Aspergillus conidia daily, most of which are cleared by the mucociliary system of airway epithelial cells and resident alveolar macrophages (van de Veerdonk et al., 2017). However, in immunocompromised individuals, conidia that escape clearance can persist, germinate, and initiate invasive infection (Margalit and Kavanagh, 2015; van de Veerdonk et al., 2017) (Figure 2).
Effective treatment measures for IA include optimized prevention, timely diagnosis, and early antifungal therapy, which may also involve immunomodulation and surgery. The development of new antifungal drugs for aspergillosis includes Re-zafungin (CD101-IV), a novel echinocandin with unique pharmacokinetic properties that allows for weekly dosing and shows effective in vitro and in vivo activity against multiple Aspergillus species (Sandison et al., 2017; Wiederhold et al., 2018); Fosmanogepix (E1210/APX001), a broad-spectrum antifungal agent with a novel mechanism of action (inhibition of fungal glycosylphosphatidylinositol-insulin glucose biosynthesis), which has shown efficacy in IA animal models (Shaw and Ibrahim, 2020); Ibrexafungerp (SCY-078), a new class of unique glucan synthase inhibitors (triterpene compounds) (Rivero-Menendez et al., 2021); and Olorofim (F901318), a broad-spectrum antifungal agent with fungal-specific inhibition (Maertens et al., 2025). Voriconazole, isavuconazole, and posaconazole are substrates and inhibitors of CYP3A4 (Townsend et al., 2017). Long-term use of carbamazepine, phenytoin, and rifampin can significantly reduce the steady-state plasma concentrations of these drugs, leading to treatment failure. Additionally, voriconazole is also a substrate and inhibitor of CYP2C19, and glucocorticoids (CYP2C19 inducers) and CYP2C19 gene polymorphisms can influence its metabolism (Tian et al., 2021). Genomic epidemiology methods further suggest a potential link between the increasing clinical incidence of azole-resistant IA and the increasingly widespread presence of azole-resistant genotypes in environmental isolates (Cao et al., 2021).
3 Common antifungal drugs and associated resistance mechanisms
Common antifungal drugs include polyenes, azoles, allylamines, morpholines, and echinocandins, all of which function as antimetabolic agents targeting essential fungal structures or biosynthetic pathways (Fisher et al., 2022; Vitiello et al., 2023). Their mechanisms of action are summarized in Figure 3, and the classification, molecular targets, and known resistance mechanisms are listed in Table 1. Fungal responses to antifungal agents typically fall into three categories: resistance, tolerance, and persistence. Additionally, many intracellular or latent fungal cells can enter a dormant state, resulting in downregulation of drug targets, reduced membrane permeability, and decreased susceptibility to treatment (Arastehfar et al., 2023). These adaptations significantly limit the efficacy of antifungal therapy. For example, phagocytosis of fungal cells can be influenced by macrophage surface receptors interacting with fungal ligands, affecting drug access and immune clearance (Uribe-Querol and Rosales, 2020). Clinically, antifungal resistance has become an emerging challenge with both spatial and temporal dimensions (Fisher et al., 2018; Lockhart et al., 2023). Notable examples include resistant variants of Aspergillus fumigatus and Candida auris, a multidrug-resistant species that has spread globally (Chowdhary and Meis, 2018; Rhodes and Fisher, 2019). The vast diversity of fungal species and the evolutionary pressures driving resistance highlight the unpredictable nature of future fungal threats. Therefore, continuous surveillance and rapid response are critical to mitigating the growing burden of antifungal resistance.
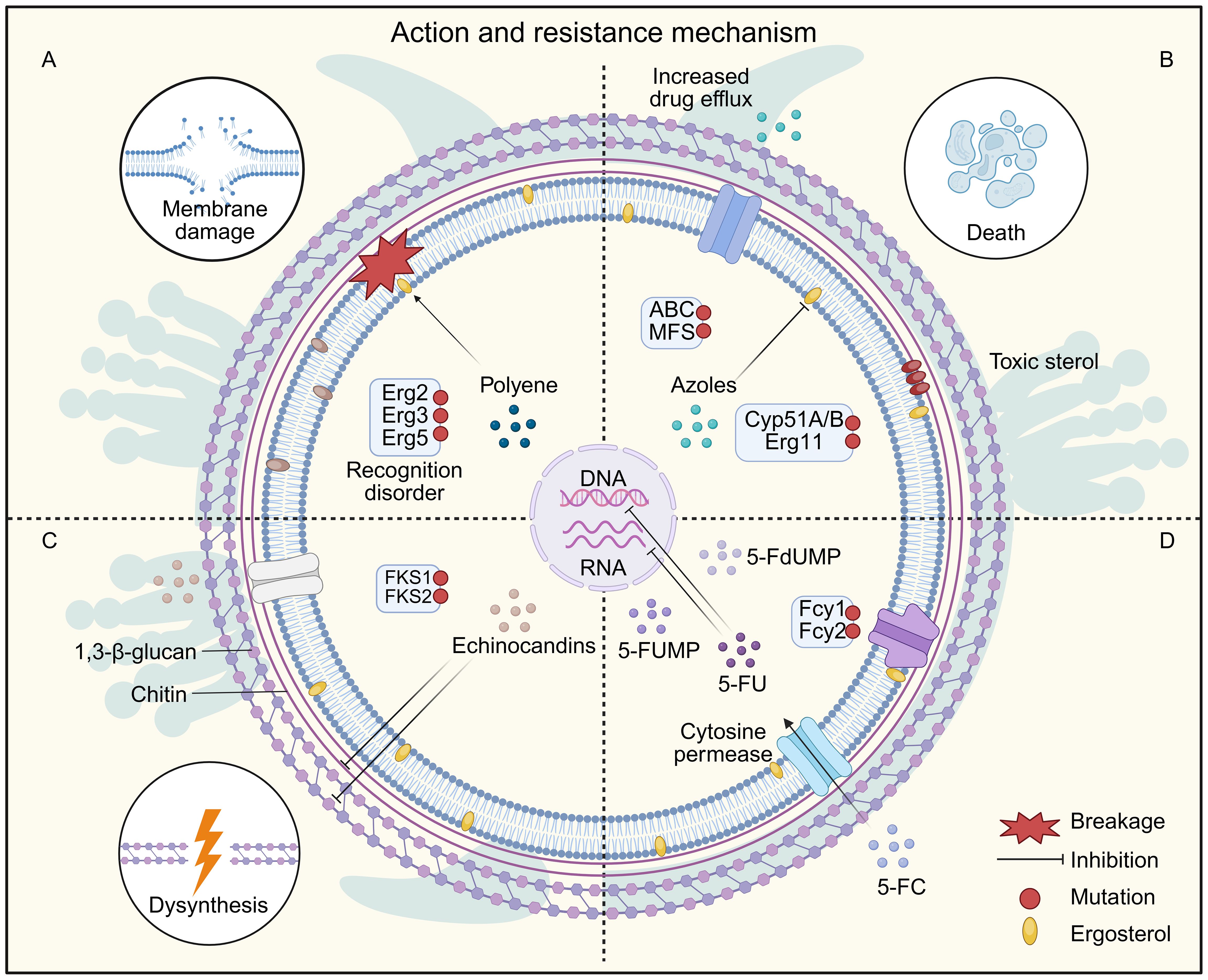
Figure 3. Fungal Resistance Mechanisms of Common Antifungal Drugs. (A) Polyene drugs cause cell death by inhibiting ergosterol synthesis and forming ion pores on the cell membrane. Resistance to polyenes is mainly caused by mutations in ergosterol biosynthesis genes, which deplete the target ergosterol, leading to the production of alternative sterols that do not interact with polyenes. (B) Azole drugs exert their antibacterial activity by inhibiting lanosterol 14-α-demethylase (encoded by ERG11 in Candida and CYP51A/B in Aspergillus), and blocking the ergosterol synthesis and accumulation of toxic sterols. Resistance to azole drugs occurs mainly through mutations in the drug target, resulting in reduced drug-binding affinity, and also through overexpression of the drug target through the transcriptional activator Upc2. In addition, overexpression of ABC/MFS efflux pumps is also involved in drug resistance. (C) Echinocandin prevents 1,3-β-D-glucan and chitin biosynthesis by inhibiting 1,3-β-glucan synthase and chitin synthase, thereby causing loss of cell wall integrity and cell wall stress. Resistance to echinocandin mainly involves mutations in the genes encoding the drug target FKS. (D) 5-FC enters the cell by cytosine permease, interferes with RNA and DNA synthesis after conversion to 5-FU by cytosine deaminase. 5-FU is converted to 5-FdUMP, thereby inhibiting thymidylate synthesis and downstream DNA biosynthesis. 5-FU is also converted to 5-FUMP by UPRT, inhibiting RNA interference to translate proteins. Resistance to 5-FC mainly involves mutations in the cytosine permeases Fcy1 and Fcy2.
3.1 Mechanisms of resistance to cell wall-targeting antifungals: echinocandins
Antifungal agents targeting the fungal cell wall exert their effects by disrupting the biosynthesis of essential structural components. Among them, echinocandins, such as caspofungin, are the most clinically advanced class. These agents are cyclic hexapeptides with lipid side chains that inhibit 1,3-β-D-glucan synthase, an enzyme critical for glucan synthesis and fungal cell wall integrity (Chen et al., 2011). Echinocandins exhibit fungicidal activity against Candida species and fungistatic activity against Aspergillus spp. However, they have limited or no efficacy against certain emerging Candida species, such as Candida auris and Candida parapsilosis, highlighting a growing concern regarding their spectrum of activity (Roemer and Krysan, 2014). Other antifungal agents, including polyoxins and nikkomycins, act as chitin synthase inhibitors (Li et al., 2011). These compounds are structural analogs of chitin synthase substrates and competitively inhibit enzyme activity, thereby disrupting chitin biosynthesis and impairing fungal cell wall construction (Jackson et al., 2013). Although promising in vitro, their clinical application remains limited, and further development is required to evaluate their therapeutic potential. The primary mechanism of resistance to echinocandins involves point mutations in the FKS1 and FKS2 genes, which encode subunits of 1,3-β-D-glucan synthase (Deane, 2023; Hu et al., 2023; Li J. et al., 2025; Zajac et al., 2025). These mutations reduce drug binding affinity and are associated with treatment failure in invasive candidiasis (Schikora-Tamarit and Gabaldón, 2024; ElFeky et al., 2025; Zajac et al., 2025).
3.2 Mechanisms of resistance to membrane-targeting antifungals: polyenes and azoles
3.2.1 Polyene antifungal antibiotics interact with cell membrane sterols
Polyene antifungal agents—such as nystatin, AMB, and natamycin—are broad-spectrum drugs commonly used to treat opportunistic fungal infections caused by Candida, Cryptococcus, Aspergillus, and Lentinus species (Carolus et al., 2020; Tugume et al., 2023; Lass-Flörl et al., 2024; Vishwakarma M and Soni, 2024). AMB exerts its antifungal activity through a dual mechanism. Their primary mode of action involves binding to ergosterol, a key sterol component of fungal cell membranes, leading to the formation of pores that disrupt membrane integrity and cause leakage of intracellular ions such as Na+, K+, H+, and Cl-, ultimately inhibiting fungal growth (Gray et al., 2012; Wang et al., 2021; Lee et al., 2023). In addition to membrane disruption, AMB induces an oxidative burst by promoting the generation of reactive oxygen species (ROS) within fungal cells. This ROS production, linked to mitochondrial respiratory chain dysfunction, leads to oxidative damage of critical cellular components including membranes, mitochondria, proteins, and DNA (Mesa-Arango et al., 2014; Singh et al., 2017). The combined effects of ionic imbalance and elevated ROS levels cause multiple deleterious impacts culminating in fungal cell death (Phillips et al., 2003; Sangalli-Leite et al., 2011; Mesa-Arango et al., 2014). Unlike protein-based targets, ergosterol is not gene-encoded, making polyene resistance relatively rare. However, when resistance does occur, such as in Candida albicans or Aspergillus, it is typically associated with mutations in the ergosterol biosynthesis pathway, particularly in genes such as ERG2, ERG3, ERG5 and ERG11 (Bhattacharya et al., 2018). These mutations lead to altered membrane sterol composition, including depletion or substitution of ergosterol, reducing drug binding and effectiveness (Cannon et al., 2009; Jensen et al., 2015). Despite their potency, polyenes, especially AMB, are associated with significant toxicity, including nephrotoxicity, infusion-related reactions (e.g., fever, chills), and venous irritation at the injection site (Deray, 2002; Kagan et al., 2012; Lakhani et al., 2019; Wang et al., 2021). Moreover, their intravenous administration requirement limits outpatient and long-term use (Gray et al., 2012).
3.2.2 Azole antifungal agents can inhibit cytochrome P450
Azoles are a major class of antifungal drugs characterized by a five-membered heterocyclic ring. Clinically relevant azoles include fluconazole, clotrimazole, miconazole, and ketoconazole, which are widely used for treating mucosal and cutaneous candidiasis as well as dermatophytosis, particularly in immunocompromised patients (Shapiro et al., 2011; Roemer and Krysan, 2014). Azoles exert their antifungal effect by inhibiting lanosterol 14-α-demethylase (Erg11), a cytochrome P450-dependent enzyme essential for ergosterol biosynthesis, thereby disrupting cell membrane integrity and function (Roemer and Krysan, 2014). However, azoles are less effective for aspergillosis or systemic yeast infections, and long-term use may result in hepatotoxicity, though they are generally better tolerated than polyenes (Benitez and Carver, 2019). Resistance to azoles is increasingly reported and involves several mechanisms: Overexpression of efflux pumps, including members of the ABC transporter superfamily and major facilitator superfamily (MFS); Point mutations or overexpression of ERG11, reducing azole binding to the target enzyme (Lee et al., 2021). These resistance mechanisms reduce intracellular drug accumulation and undermine treatment efficacy, posing a major challenge in the clinical management of fungal infections.
3.3 Mechanism of resistance to fungal nucleic acid synthesis: 5-fluorocytosine
Among antifungal antimetabolites, 5-fluorocytosine (5-FC) is the most prominent example. As a fluorinated analog of cytosine, it enters fungal cells and is converted intracellularly to 5-fluorouracil (5-FU), which inhibits DNA and RNA synthesis, thereby exerting antifungal effects (Lee et al., 2021). Despite its clinical utility, 5-FC is prone to rapid resistance development when used as monotherapy. The primary resistance mechanisms include mutations in FCY2, encoding cytosine permease (which mediates drug uptake), and mutations in FCY1, encoding cytosine deaminase (required for 5-FC activation) (Longley et al., 2003; Després et al., 2022). These mutations impair drug entry or metabolic conversion, leading to treatment failure. To enhance efficacy and reduce the risk of resistance, 5-FC is typically used in combination with AMB, especially in the treatment of cryptococcal meningitis (Bennett et al., 1979; Saag et al., 2000; Billmyre et al., 2020). This combination has demonstrated synergistic effects and remains a cornerstone of therapy for invasive cryptococcosis. Additionally, it shows activity against Candida albicans and certain saprophytic fungi (Hope et al., 2004; Papon et al., 2007).
4 A decade of progress: novel antifungal drugs targeting resistance
The main mechanism of antifungal action lies in inhibiting essential molecules, such as ergosterol (azole class) or 1,3-β-D-glucan (echinocandins), or by binding to ergosterol (polyene class), leading to the formation of pores and altering the integrity and permeability of the cell membrane, thereby affecting the membrane or fungal cell wall.
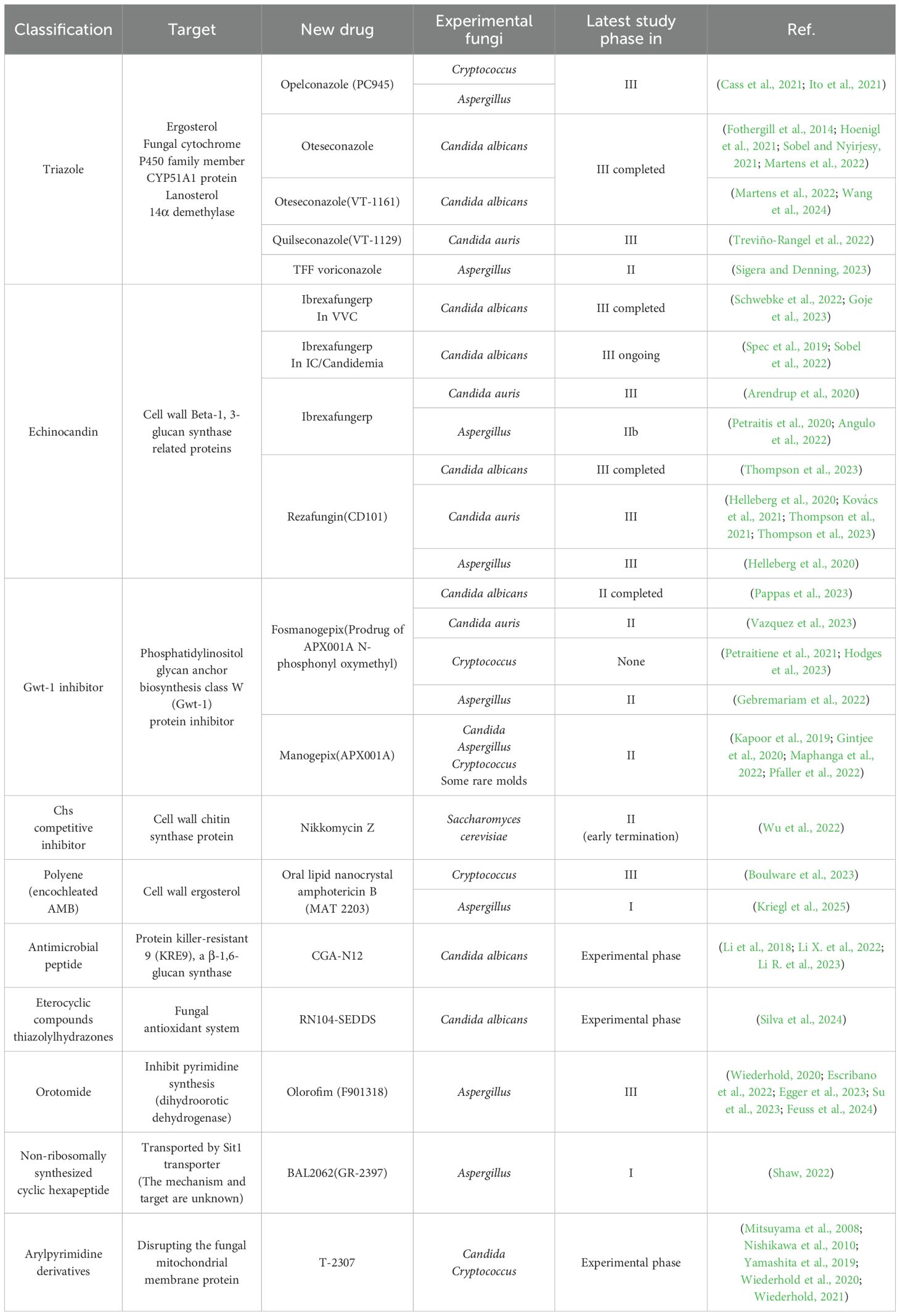
Despite the limited number of targets and the emergence of resistance, which pose challenges for antifungal therapy, new drugs such as Ibrexafungerp (formerly known as SCY-078), Rezafungin, Fosmanogepix and Olorofim have shown promising clinical efficacy (Arendrup et al., 2020; Wiederhold, 2020; Kovács et al., 2021; Thompson et al., 2021; Thompson et al., 2023; Vazquez et al., 2023; Feuss et al., 2024). Currently, marketed antifungal drugs have undergone extensive structural modifications and modifications. After thorough safety and efficacy evaluations, along with in vivo and in vitro model studies, the most promising antifungal compounds in preclinical and clinical development include novel triazoles, glucan synthase inhibitors, and small-molecule polypeptides. We have collected the latest 10 years’ developments in the development of novel antifungal drugs targeting four common pathogenic fungi: Candida albicans, Candida auris, Cryptococcus, and Aspergillus (Table 1).
4.1 Antifungal drugs targeting the cell wall
Recent advances in antifungal therapy have introduced new agents targeting the fungal cell wall, notably Ibrexafungerp and Rezafungin, both of which have shown promising results in clinical trials against Candida albicans, Candida auris, and Aspergillus spp (Spec et al., 2019; Arendrup et al., 2020; Petraitis et al., 2020; Angulo et al., 2022; Schwebke et al., 2022; Sobel et al., 2022; Goje et al., 2023; Thompson et al., 2023). These agents represent novel approaches in overcoming limitations of traditional antifungals, particularly in addressing resistance and improving patient compliance. Ibrexafungerp (formerly SCY-078/MK-3118, brand name Brexafemme) is a first-in-class oral β-(1,3)-D-glucan synthase inhibitor (GSI) and a fourth-generation triterpenoid antifungal. It offers a broad-spectrum activity against multiple Candida species, including strains resistant to azoles and echinocandins, as well as activity against Aspergillus, Penicillium variotii, and some rare dimorphic fungi (Spec et al., 2019; Arendrup et al., 2020; Petraitis et al., 2020; Angulo et al., 2022; Sobel et al., 2022). Preclinical studies also suggest potential efficacy against Pneumocystis jirovecii (El Ayoubi et al., 2024). Its dual route of administration, oral and intravenous, provides dosing flexibility, which is particularly beneficial for long-term outpatient management. Additionally, Ibrexafungerp maintains efficacy against Candida strains harboring FKS mutations, reducing the risk of cross-resistance with echinocandins (Nunnally et al., 2019). Its high protein-binding capacity and preferential accumulation in vaginal tissues make it especially suitable for treating vulvovaginal candidiasis (Schwebke et al., 2022; Goje et al., 2023; Kow et al., 2025). However, it has limited activity against Mucorales and Fusarium spp., and its relatively low oral bioavailability (~50%) may impact systemic efficacy (Lamoth and Alexander, 2015). Long-term safety data are also still being collected. Rezafungin (formerly CD101) is a next-generation echinocandin with structural modifications that confer a long half-life (~133 hours), enabling once-weekly intravenous administration. This feature greatly enhances treatment convenience and adherence, especially in outpatient or maintenance settings. FDA-approved for the treatment of candidemia and invasive candidiasis, Rezafungin demonstrates strong efficacy against Candida spp., including C. auris, with a well-documented safety profile (Thompson et al., 2024b). However, its spectrum of activity is narrower than that of Ibrexafungerp, with limited data on efficacy against molds or rare fungal pathogens (Fung and Shirley, 2025). Additionally, its intravenous-only formulation restricts its use in home-based care or resource-limited settings. There is currently a lack of clinical data for infections such as endocarditis, osteomyelitis, and meningitis caused by Candida. Another promising agent is Fosmanogepix, a first-in-class Gwt1 enzyme inhibitor that targets glycosylphosphatidylinositol (GPI) anchor biosynthesis, a process essential for fungal cell wall integrity, adhesion, and virulence (Wiederhold, 2020; Escribano et al., 2022; Egger et al., 2023; Vazquez et al., 2023; Feuss et al., 2024). By inhibiting Gwt1, Fosmanogepix disrupts the anchoring of mannoproteins on the fungal surface, impairing growth and pathogenicity through a mechanism distinct from azoles and echinocandins, thereby reducing the likelihood of cross-resistance (Pappas et al., 2023). It exhibits potent in vitro activity against a broad range of pathogens, including Candida spp. (except C. krusei), C. auris, Aspergillus spp., and even Mucorales, a group notoriously resistant to conventional antifungals (Petraitiene et al., 2021; Hodges et al., 2023; Vazquez et al., 2023). Fosmanogepix also shows favorable pharmacokinetic properties, with >90% oral bioavailability unaffected by food intake and a half-life of approximately 2.5 days. Its ability to penetrate sanctuary sites such as the central nervous system and eyes further enhances its therapeutic potential (Hodges et al., 2024). Currently in Phase III trials, Fosmanogepix has demonstrated efficacy in early studies for invasive candidiasis and is considered a promising candidate for drug-resistant or difficult-to-treat infections. Together, these emerging agents represent a significant shift in antifungal therapy, expanding treatment options beyond traditional mechanisms, addressing current resistance gaps, and offering improved pharmacological and patient-centered profiles. Future research should continue to evaluate these novel agents in diverse clinical settings and against emerging fungal threats.
4.2 Antifungal drugs targeting the cell membrane
Among antifungal agents targeting the fungal cell membrane, Oteseconazole (VT-1161) has emerged as a promising novel triazole, with recent clinical and regulatory progress. It has successfully completed Phase III clinical trials and has been approved by the U.S. FDA for the treatment of recurrent vulvovaginal candidiasis (RVVC) (Fothergill et al., 2014; Hoenigl et al., 2021; Sobel and Nyirjesy, 2021; Martens et al., 2022; Wiederhold, 2022). Mechanistically, Oteseconazole, like other triazoles, inhibits 14α-demethylase in the fungal cytochrome P450 system, thereby disrupting ergosterol biosynthesis, an essential component of fungal cell membranes (Hoy, 2022). This inhibition compromises membrane integrity, alters permeability, and leads to leakage of intracellular contents, ultimately impairing fungal growth and replication. Compared to existing azoles, Oteseconazole demonstrates enhanced antifungal activity, including efficacy against azole-resistant strains of Candida and Aspergillus spp. It exhibits favorable pharmacokinetics, including a suitable half-life, wide tissue distribution, and effective tissue penetration, critical for eradicating infection at various anatomical sites. In preclinical and clinical studies, it has shown superior in vitro activity and consistent therapeutic effects in animal models. Clinically, Oteseconazole has shown significant benefits in RVVC, achieving symptom relief, fungal burden reduction, and lower recurrence rates compared to standard therapies (Sobel and Nyirjesy, 2021; Martens et al., 2022). Its less frequent dosing also improves patient compliance, making it a potentially superior alternative to traditional azoles in this indication. In terms of safety, clinical trials indicate good tolerability, with mild and transient adverse effects and a low incidence of serious reactions. However, like other azoles, Oteseconazole inhibits human cytochrome P450 enzymes, posing a potential risk of drug, drug interactions, which remains a limitation, especially in patients receiving multiple medications.
Although azoles remain central to antifungal therapy due to their broad spectrum and oral availability, the risk of resistance, drug interactions, and incomplete eradication calls for continued refinement. Oteseconazole provides a valuable step forward, but long-term studies are still needed to fully assess its safety, effectiveness across populations, and utility in additional indications beyond RVVC.
4.3 Antifungal drugs targeting organelles
Mitochondria are essential organelles in fungi, playing central roles in energy metabolism, the respiratory chain, redox homeostasis, and various biosynthetic pathways. Disruption of mitochondrial function has emerged as a promising antifungal strategy, with several novel compounds demonstrating efficacy through mitochondrial targeting. One such agent is Olorofim, a dihydroorotate dehydrogenase inhibitor currently in advanced clinical development, which impairs mitochondrial pyrimidine biosynthesis, leading to defective DNA synthesis and subsequent fungal cell death (Oliver et al., 2016). Another example is CGA-N12, a synthetic antimicrobial peptide that exerts potent antifungal effects against Candida albicans by inducing reactive oxygen species (ROS) accumulation and collapsing the mitochondrial membrane potential, ultimately triggering apoptosis (Li et al., 2018). ATI-2307 (T-2307), an aromatic amidine compound, represents a novel class of mitochondrial respiratory chain inhibitors (Yamashita et al., 2019). It selectively targets fungal mitochondrial complexes, blocking electron transport and disrupting the proton gradient across the inner membrane. This results in membrane potential dissipation, ATP synthase inhibition, and energy depletion, culminating in fungal growth arrest and cell death (Nishikawa et al., 2010). ATI-2307 has demonstrated strong in vitro activity against various Candida species, with preliminary evidence suggesting potential efficacy against other pathogenic fungi, including Rhizopus arrhizus, Mucor racemosus, Scedosporium spp., and Trichosporon asahii, although further studies are warranted. In addition to synthetic compounds, several natural products have shown mitochondrial-targeting antifungal activity. For instance, berberine selectively accumulates in fungal mitochondria, disrupts membrane potential, and binds subunits of complex I; it also interacts with the Mdr1p efflux pump, potentially reversing azole resistance in C. albicans (Tong et al., 2021). Other plant-derived compounds, such as citronellal and perillaldehyde, induce ROS overproduction, resulting in mitochondrial and DNA damage (Tian et al., 2016; Chen et al., 2020; Trindade et al., 2022; Venancio et al., 2025). Moreover, cyclooxygenase inhibitors, which suppress prostaglandin E2 synthesis, have been shown to reduce fungal biofilm formation, highlighting their potential as adjunctive agents in antifungal therapy (Abdelmegeed and Shaaban, 2013).
4.4 Antifungal drugs targeting metabolic pathways and enzymes
Fungal metabolism involves a wide array of biochemical pathways essential for growth, survival, and virulence, including N-acetylglucosamine utilization, trehalose metabolism, lipid biosynthesis, energy production, and intracellular transport (Pan et al., 2018; Wijnants et al., 2022). These metabolic processes present attractive targets for antifungal drug development due to their indispensable roles in fungal physiology and pathogenesis (Ramakrishnan et al., 2016). A growing number of intracellular enzymes have been identified as potential antifungal targets. For instance, AMP-17, a novel antifungal peptide, interferes with several critical metabolic pathways in Candida albicans, including oxidative phosphorylation, RNA degradation, and fatty acid metabolism, effectively suppressing fungal growth (Yang et al., 2022). Similarly, ApoB-derived peptides exhibit antifungal properties primarily by compromising cell membrane integrity in C. albicans (Dell'Olmo et al., 2021). Another promising compound, α-erythromycin myrrh (α-red myrrh), inhibits Δ;24-sterol methyltransferase, a key enzyme in ergosterol biosynthesis encoded by ERG6. This inhibition reduces ergosterol content in a dose-dependent manner, disrupts membrane integrity, and inhibits fungal proliferation (Jahanshiri et al., 2017). Notably, α-red myrrh may also exert indirect effects on fungal gene expression by modulating host signaling pathways such as NF-κB, p38, and JNK, which influence ERG6 regulation, suggesting a multifaceted mechanism of action (Jahanshiri et al., 2017). In addition to these, several novel enzyme inhibitors have shown promise. (S)-2-amino-4-oxo-5-hydroxyvaleric acid (RI-331), a homoserine dehydrogenase inhibitor, acts through an enzyme-assisted suicide mechanism by irreversibly binding to and inactivating Hom6p, an enzyme involved in amino acid biosynthesis, ultimately leading to fungal cell death (Yamaki et al., 1992). Likewise, RI-331 exhibits selective antifungal activity against C. albicans, C. tropicalis, and C. glabrata, but not against Aspergillus species (Jacques et al., 2003; Kuplińska and Rząd, 2021). Importantly, some fungal-specific enzymes offer high target selectivity with minimal risk to the host. For example, class II fructose-1,6-bisphosphate aldolase (FBA-II) is found exclusively in fungi and is absent in animals and higher plants, making it an ideal candidate for developing targeted antifungal agents with reduced off-target toxicity (Han et al., 2017; Wen et al., 2022).
4.5 Antifungal drugs targeting iron transporters
Iron is essential for fungal growth and pathogenicity, but the host limits its availability to prevent infection (Choi and Bessman, 2025). This has led to the concept of iron hijacking as a novel antifungal strategy. One promising approach is disrupting siderophore (ferrifer) biosynthesis, which fungi rely on to acquire iron (Balhara et al., 2014; Choi and Bessman, 2025). Inhibitors targeting enzymes such as adenosine phosphate transferase, non-ribosomal peptide synthase (NRPS), polyketide synthase, and NRPS-independent siderophore synthases impair microbial iron uptake and enhance host-mediated clearance (Petrik et al., 2012; Leal et al., 2013; Süssmuth and Mainz, 2017; Qiao et al., 2023; Zhang L. et al., 2023).
Natural products are also being explored. Celastrol, derived from Tripterygium wilfordii, inhibits the flavin-dependent monooxygenase FerA, essential for siderophore synthesis in Aspergillus fumigatus (Sun et al., 2019). This inhibition disrupts L-ornithine hydroxylation, a critical step in siderophore production, resulting in iron starvation and suppressed fungal growth (Martín Del Campo et al., 2016; FA et al., 2025). This highlights celastrol as both a potential therapeutic and a new antifungal target. Another strategy involves siderophore-drug conjugates, which improve delivery of antifungal agents by hijacking the fungal iron uptake system. Conjugates like ferrimycin combine a siderophore with antifungal drugs (e.g., triazoles, echinocandins, or polyenes) (Lakshminarayanan et al., 2024). These agents specifically bind fungal iron transporters, enhancing drug targeting and reducing toxicity to host cells, thus improving both efficacy and safety. A leading compound in this class is GR-2397 (also known as ASP2397 or VL-2397), a cyclic hexapeptide developed by Gravitas Therapeutics (Nakamura et al., 2017; Kovanda et al., 2019; Shaw, 2022). It enters fungal cells via the SIT1 transporter, which is absent in humans, ensuring fungal specificity (Dietl et al., 2019; Nakamura et al., 2019). GR-2397 has shown rapid fungicidal activity in murine aspergillosis models, and Phase 1 clinical trials demonstrated it is safe and well-tolerated (Rubino et al.,; Arendrup et al., 2016; Mammen Mammen et al., 2019). Recognized by the FDA as a Qualified Infectious Disease Product (QIDP), orphan drug, and Fast Track agent, GR-2397 is set to enter Phase 2 trials in 2025. Finally, competitive iron chelators like lactoferrin and gallium reduce fungal biofilm formation by replacing iron (Fernandes and Carter, 2017; Bastos et al., 2019; Fernandes et al., 2020; Li F. et al., 2022). Biofilm thinning enhances the treatment of mucosal infections and complements conventional antifungal therapy. Overall, targeting iron acquisition mechanisms represents a powerful, fungus-specific therapeutic direction with multiple avenues for innovation.
4.6 Antifungal drugs targeting antioxidant defense systems
During infection, fungal pathogens are continuously exposed to oxidative stress generated by the host immune response (Yaakoub et al., 2022). To survive and establish infection, they have developed a robust antioxidant defense system, including catalases, superoxide dismutases (SODs), glutathione peroxidases (GPxs), and peroxiredoxins (Leal et al., 2012; Arastehfar et al., 2023). These enzymes work synergistically to eliminate reactive oxygen species (ROS) and maintain redox homeostasis. Recent studies have highlighted their critical roles in fungal virulence and identified them as promising targets for antifungal therapy (Lionakis et al., 2023).
Amphotericin B (AMB), a widely used antifungal, can broadly induce reactive oxygen species (ROS) accumulation across 44 fungal species, including Candida albicans, C. parapsilosis, C. glabrata, C. tropicalis, C. krusei, C.neoformans, and C. gattii (Mesa-Arango et al., 2014). This oxidative stress is accompanied by the upregulation of genes encoding antioxidant proteins (Mesa-Arango et al., 2014). Correspondingly, AMB-resistant isolates often exhibit elevated catalase levels (Mesa-Arango et al., 2014). In C. glabrata, however, fluconazole-resistant strains harboring the Pdr1 P927L mutation show reduced catalase expression (Vermitsky and Edlind, 2004; Edlind and Katiyar, 2022). Similarly, in Candida auris, fluconazole resistance is associated with an adaptive trade-off: fluconazole-susceptible isolates display enhanced resistance to oxidative stress, whereas the majority (94.5%) of fluconazole-resistant strains exhibit reduced oxidative tolerance (Das et al., 2024). suggesting that catalase functions differently depending on the antifungal class involved.
Several compounds that target fungal antioxidant defenses have shown synergistic effects with existing antifungals. Cyclams, macrocyclic polyamines with antimicrobial activity, have demonstrated antifungal potential. For example, the cyclam salt H4[H2(4-CF3PhCH2)2Cyclam]Cl4 inhibits C. albicans biofilm formation and catalase activity, suppresses morphological transition, and reduces melanin production in C. neoformans (Cerqueira et al., 2024). SODs are also emerging attractive targets. Inhibitors such as N,N′-diethyldithiocarbamate (DDC) and ammonium tetrathiomolybdate (ATM) impair C. albicans biofilm formation and sensitize it to AMB (Seneviratne et al., 2008; De Brucker et al., 2013). Natural dihydroxybenzaldehydes (DHBAs), including 2,3- and 2,5-DHBA, inhibit SOD and glutathione reductase in Candida and Cryptococcus species, enhancing AMB efficacy (Kim et al., 2012b). Benzaldehyde and its analogs (e.g., trans-cinnamaldehyde, o-vanillin) inhibit filamentous fungi like Aspergillus fumigatus and act as chemosensitizers against C. albicans and Candida auris when combined with AMB, fluconazole, or itraconazole (Faria et al., 2011; Kim et al., 2011b).Phenolic compounds with redox-modulating activity also enhance antifungal action. Thymol (THY) disrupts fungal redox and ion homeostasis and synergizes with itraconazole against A. fumigatus (Kim et al., 2012a). Co-administration of THY with AMB sensitizes yeast pathogens including C. albicans, C. tropicalis, and C. neoformans (Kim et al., 2012a). Similarly, salicylaldehyde shows comparable effects (Kim et al., 2011a). Antioxidant defenses are equally critical in plant-pathogenic fungi (Park and Son, 2024). Phytic acid inhibits Fusarium oxysporum by compromising membrane integrity and suppressing antioxidant enzyme activity such as superoxide dismutase (SOD) and catalase (Li N. et al., 2023). Dehydroabietic acid (DHA), derived from rosin, inhibits the growth of multiple plant pathogens (such as Alternaria alternata, Botrytis cinerea, Valsa mali, Pestalotiopsis neglecta, and F. oxysporum) and reduces the activity of SOD, catalase, and peroxidase in Alternaria alternata (Chen et al., 2025).
Combining antioxidant-targeting agents with cell wall synthesis inhibitors has shown synergistic efficacy in model organisms, supporting combination therapies. However, due to the redundancy within antioxidant systems, complete inhibition via single targets remains challenging, and drug specificity must be optimized to avoid host toxicity. Targeting fungal redox homeostasis thus represents a promising strategy to overcome antifungal resistance.
4.7 Antifungal vaccines
Fungal vaccines offer a proactive strategy to prevent or control infections by stimulating the host immune system (Levitz and Golenbock, 2012). As antifungal resistance increases and limits the efficacy of conventional drugs, vaccines are emerging as promising adjuncts or alternatives (Dan and Levitz, 2006; Nami et al., 2019). Unlike single-target antifungals, vaccines trigger both T cell–mediated and antibody-based responses, enabling multi-pathway pathogen clearance (Cutler et al., 2007; Lionakis et al., 2023). These immune mechanisms are less susceptible to resistance mechanisms such as mutations, efflux pumps, and biofilm formation, making vaccines especially valuable against drug-resistant strains (Cassone, 2013; Sahu et al., 2022).
Several fungal vaccines have shown efficacy in preclinical studies, with some advancing to early clinical trials. Two recombinant Candida vaccines, PEV7 and NDV-3A, demonstrated safety and immunogenicity in Phase I trials. PEV7, which incorporates a truncated C. albicans Sap2 protein into influenza virosomes, protected rats from infection and induced memory B cell responses in human volunteers (De Bernardis et al., 2012; De Bernardis et al., 2018). A Sap2 vaccine derived from C. parapsilosis also conferred cross-species protection in C. tropicalis-infected mice (Shukla and Rohatgi, 2020). NDV-3A, based on the N-terminal region of C. albicans Als3 adhesin, elicited broad immunity and showed efficacy against recurrent vulvovaginal and oropharyngeal candidiasis (Spellberg et al., 2005; Spellberg et al., 2006). A Phase II study reported reduced recurrence of vulvovaginal candidiasis in women under 40 over 12 months (Edwards et al., 2018). NDV-3A also prevented kidney dissemination and catheter colonization in murine models, inhibited C. auris biofilm formation, enhanced macrophage phagocytosis, and improved micafungin efficacy (Alqarihi et al., 2019; Singh et al., 2019).
Targeting fungal cell wall polysaccharides is another promising strategy. These components activate complement and are recognized by receptors like Dectin-1, driving robust immune responses (Levitz, 2010). Vaccines against Cryptococcus neoformans capsule have been explored for over 40 years. Capsule-specific antibodies against the Cryptococcus neoformans improves survival, reduces fungal burden, and promotes granuloma formation in infected mice, limiting disease progression (Graybill et al., 1981; Dromer et al., 1987; Mukherjee et al., 1993b; Mukherjee et al., 1993a). These antibodies also enhance the efficacy of antifungals such as amphotericin B, fluconazole, and flucytosine, demonstrating synergistic effects in animal models and in vitro assays (Gordon and Lapa, 1964; Dromer and Charreire, 1991; Mukherjee et al., 1995; Feldmesser et al., 1996; Monari et al., 1999). The monoclonal antibody 18B7 completed Phase I trials in cryptococcal meningitis patients (Larsen et al., 2005). β-glucan conjugate vaccines (Levitz et al., 2015), such as Lam-CRM, a β-glucan–diphtheria toxin conjugate, and branched oligo-β-glucans, protect against systemic Candida and Aspergillus infections by enhancing phagocytosis and prolonging survival (Torosantucci et al., 2005; Bromuro et al., 2010; Liao et al., 2016). β-glucan particles (GPs), derived from Saccharomyces cerevisiae, also serve as effective antigen delivery systems and adjuvants (Huang et al., 2010; De Smet et al., 2013). Whole glucan particles (WGPs) conjugated with BSA have demonstrated protection against systemic aspergillosis and coccidioidomycosis (Clemons et al., 2014a; Clemons et al., 2015).
Pan-fungal vaccines can provide cross-protection against multiple fungal pathogens. Heat-killed S. cerevisiae (HKY) protects mice from infections caused by Aspergillus, Coccidioides, Candida, Cryptococcus, Rhizopus, and Pneumocystis (Stevens et al., 2011; Liu et al., 2012; Clemons et al., 2014b; Luo et al., 2014; Majumder et al., 2014; Martinez et al., 2017), and induces strong Th1 immune responses and antibodies against β-glucans and mannans (Liu et al., 2011). The peptide vaccine NXT-2 targets pathogens like Candida, Aspergillus, and Pneumocystis, shows protection in mice and primates (Rayens et al., 2021; Rayens et al., 2022; Wychrij et al., 2025). Deletion of the F-box protein Fbp1 in C. neoformans, part of the SCF(Fbp1) E3 ligase, triggers strong Th1-mediated immunity (Masso-Silva et al., 2018). Remarkably, heat-killed fbp1Δ cells confer cross-protection against diverse fungal pathogens, including C. neoformans, C. gattii, Aspergillus fumigatus, and Candida albicans, even in CD4+ T cell-deficient hosts, supporting their potential as a broad-spectrum vaccine (Wang et al., 2019).
mRNA-lipid nanoparticle (LNP) vaccines represent a novel platform. Fungal-targeted nanoconstructs (FTNx), using antisense oligonucleotides against FKS1 and CHS3, can inhibit fungal cell wall synthesis genes, reduce fungal burden and improve survival in murine candidiasis models, exhibit broad-spectrum antifungal activity, including against clinical isolates of Candida auris (Chung et al., 2025). Additionally, CDA1-LNP, an mRNA-LNP vaccine effective against cryptococcosis in mice, has been shown to protect the majority of vaccinated animals from lethal infection (Li et al., 2025a).
Despite promising advances, challenges remain. Fungal similarity to human cells, immune evasion, and antigen variability complicate vaccine development. Practical hurdles such as storage, delivery, and competition with antifungals further limit progress (Oliveira et al., 2021; Loh and Lam, 2023). Future efforts will benefit from interdisciplinary approaches, novel platforms, and deeper insight into host–fungus interactions.
5 New strategies for drug development
The emergence of resistance from the prolonged or monotherapeutic use of traditional antifungal agents, combined with the low success rate of new drug development, continues to hinder effective treatment of clinical fungal infections. Although repurposing existing drugs offers a cost-effective and time-efficient alternative for identifying new therapies, their effective concentrations—measured as the half-maximal inhibitory concentration (IC50)—often exceed the maximum safe plasma levels in humans, creating a major barrier to clinical application. Promisingly, combination therapy using drugs with different mechanisms of action can significantly lower the required dose of each agent, thereby reducing toxicity and limiting the likelihood of resistance development during treatment. At the same time, the development of novel antifungal agents remains a major research focus. Among recent advances, some efforts have centered on creating new formulations of existing effective drugs, including nanoparticle-based delivery systems, which improve drug solubility, bioavailability, and tissue targeting. Furthermore, novel small-molecule compounds such as cinnamaldehyde have shown antifungal activity through mechanisms like membrane disruption (Shreaz et al., 2016). In parallel, antimicrobial peptides targeting fungal-specific enzymes, such as chitin synthase, along with other innovative small molecules, are also under active investigation (Figure 4) (Ran et al., 2024).
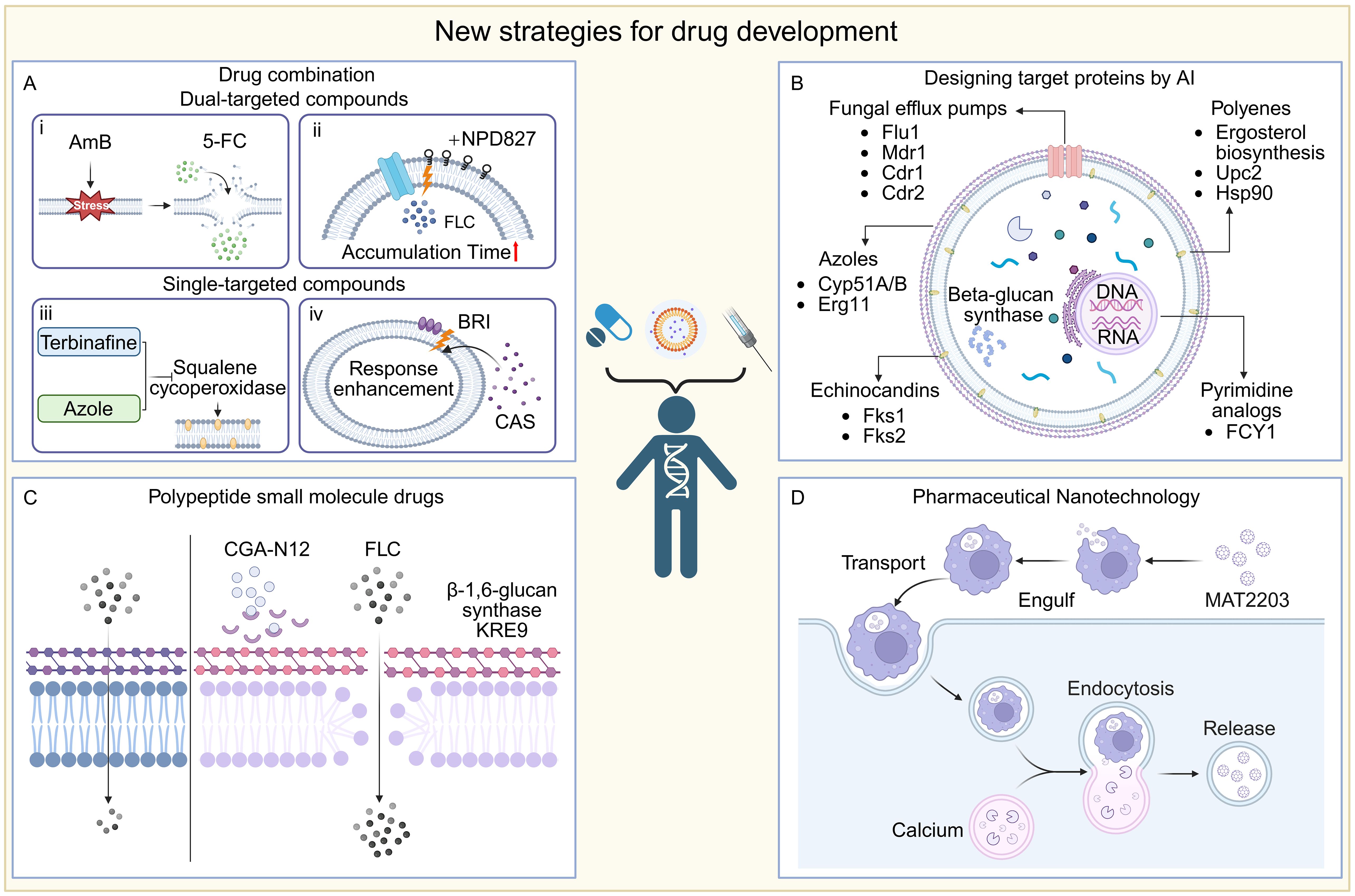
Figure 4. New strategies for drug development. (A) Combination: i) AMB disrupts fungal cell membrane integrity, thereby increasing intracellular 5-FC concentration and enhancing drug bioavailability; ii) Insertion of NPD827 into the cell membrane disrupts the efflux pump action and increases the accumulation time of the drug; iii) Squalene cycloperoxidase could be inhibited by terbinafine and azols, resulting in a dual inhibition of ergosterol biosynthesis; iv) The small molecule compound BRI enhances drug responsiveness to fungi by affecting the cell wall. (B) AI design: This figure shows the important target protein sites for the binding of drug small molecule compounds. (C) CGA-N12 inhibited the KRE9 target in β-1, 6-glucan synthase, disrupting the structural integrity of the fungal cell wall and improving the drug availability. (D) Pharmaceutical nanotechnology: an oral formulation of lipid nanocrystals, MAT2203, in which targeted cells (e.g., macrophages) swallow these nanocrystals and deliver them to the site of infection, where lower intracellular calcium concentrations trigger a nanocrystal-release mechanism that allows the drug to be released directly into the cell interior.
5.1 Drug combination therapy
Combination therapy has gained importance in managing infectious diseases, especially amid rising antifungal resistance (Johnson and Perfect, 2007; Spitzer et al., 2017; Lee et al., 2021). It offers key benefits such as reducing resistance development, improving efficacy at lower doses, shortening treatment duration, and lessening toxicities like amphotericin B-associated nephrotoxicity (Bicanic et al., 2015; Tyers and Wright, 2019). By targeting multiple fungal pathways simultaneously, combinations can yield synergistic or additive effects, enhancing clinical outcomes.
The standard of care for cryptococcal meningitis demonstrates this approach, with AmB plus flucytosine (5-FC) or fluconazole as preferred regimens. A 7-day course of AmB (1 mg/kg/day) with 5-FC (100 mg/kg/day) yields the lowest 10-week mortality (24.2%), with 5-FC outperforming fluconazole (Day et al., 2013; Molloy et al., 2018). Liposomal AmB reduces toxicity and prolongs CNS exposure (Stone et al., 2016). A recent trial found that a single high-dose liposomal AmB with 5-FC and fluconazole reduced mortality and halved adverse events compared to WHO recommendations (Jarvis et al., 2022). In invasive aspergillosis (IA), azole–echinocandin combinations improve fungicidal activity and survival (Paulussen et al., 2014; Marr et al., 2015). with meta-analyses confirming benefits in salvage therapy (Panackal et al., 2014). Rising azole-resistant Aspergillus fumigatus underscores the need for novel agents, including ibrexafungerp, fosmanogepix, and olorofim, now in clinical trials.
Beyond conventional antifungal combinations, a host defense peptide mimetic has emerged as promising enhancers of existing antifungal agents. Brilacidin (BRI), a synthetic small molecule that recapitulates the amphipathic architecture of HDPs, augments caspofungin (CAS) activity against Aspergillus fumigatus, Candida albicans, Candida auris, and CAS-resistant Cryptococcus isolates (Dos Reis et al., 2023; Diehl et al., 2024; Dos Reis et al., 2024). Additionally, BRI potentiates azole efficacy by disrupting fungal cell wall integrity pathways and perturbing membrane potential (Dos Reis et al., 2023). These observations underscore the potential of BRI as an adjunctive therapy for recalcitrant fungal infections.
Another promising strategy involves combining antifungal agents with non-traditional pharmacological compounds. Heat shock protein 90 (Hsp90) acts as a central regulator of fungal stress responses, modulating resistance, morphogenesis, and virulence factor expression (Cowen et al., 2009). Inhibiting Hsp90 markedly reduces resistance to azoles and echinocandins, restoring susceptibility (Cowen and Lindquist, 2005; Singh et al., 2009; Lamoth et al., 2016). Radicicol and geldanamycin, both Hsp90 inhibitors, enhance azole efficacy by disrupting membrane integrity and capsule formation, impairing Hsp90 mitochondrial localization, and increasing reactive oxygen species in fungal pathogens (Cordeiro et al., 2016; Xiong et al., 2024). The echinocandin micafungin is also active against Candida parapsilosis isolates from neonates (da Silva et al., 2024). High-throughput screening identified clofazimine as a broad-spectrum synergist with fluconazole, caspofungin, and AmB, and others, enhancing activity against Candida albicans, Cryptococcus neoformans, and additional fungal pathogens (Robbins et al., 2015).
Immunomodulator-based combination strategies aim to both enhance host antifungal defenses and directly kill the pathogen, representing a frontier in antifungal therapy (Pirofski and Casadevall, 2006). Immunomodulatory combinations aim to boost host defenses while targeting the pathogen. Examples include the lectin pCramoll plus fluconazole, which improved survival and reduced fungal burden in Cryptococcus gattii–infected mice (Jandú et al., 2017); interferon-γ with AmB, which decreased cryptococcal CNS infections; and macrophage colony-stimulating factor with fluconazole, which enhanced macrophage fungicidal activity (Brummer and Stevens, 1994).
In summary, the growing array of antifungal combination therapies plays a vital role in overcoming resistance, enhancing efficacy, and expanding treatment options against invasive fungal infections. These approaches, from conventional drug combinations to novel immunomodulatory and non-antifungal partnerships, represent a promising advance in antifungal therapeutics.
5.2 Drug repurposing strategies
Drug repurposing, which involves applying approved or known safe drugs to entirely new therapeutic areas, aims to significantly shorten the research and development cycle, reduce costs, and quickly address clinical challenges caused by drug-resistant fungi (Perfect, 2017; Farha and Brown, 2019; Zhang et al., 2021; Tuci et al., 2025). In recent years, many non-traditional antifungal drugs have demonstrated the potential to inhibit and even kill invasive fungi (Farha and Brown, 2019). Their mechanisms of action are rich and diverse, covering aspects such as interfering with cell wall/membrane synthesis, inhibiting virulence factors, disrupting energy metabolism, and regulating fungal signaling pathways (Lu et al., 2024; Zhen et al., 2024).
Antibacterial agents display notable broad-spectrum antifungal activity. They may be used alone or synergistically with antifungals to alter gene expression related to adhesion, mycelial or biofilm formation, reduce extracellular polysaccharides, decrease surface hydrophobicity, or inhibit efflux pumps. For instance, tobramycin combined with amphotericin B or voriconazole shows synergistic enhancement of Fusarium cell wall and membrane permeability (80% and 76% synergy, respectively) (Pozzebon Venturini et al., 2016). Minocycline inhibits Aspergillus spp. and Fusarium spp. (MIC 0.125–4 μg/mL) and potentiates multiple antifungal drugs (Gao et al., 2020). Polymyxin B binds to anionic membrane lipids (MIC100 8–256 μg/mL) and, when combined with fluconazole, disrupts membranes of Fusarium, Cryptococcus neoformans, Rhizopus oryzae, and Aspergillus fumigatus. Animal models confirm antibacterial–antifungal synergy: β-lactams, colistin, and quinolones enhance activity against Candida and Aspergillus when combined with existing antifungals (Keçeli et al., 2014; Fernández-Rivero et al., 2017; Mohamadzadeh et al., 2020).
Immunosuppressive agents with intrinsic antifungal activity are emerging as candidates for drug repurposing, including calcineurin inhibitors (e.g., cyclosporine, pimecrolimus, tacrolimus/FK506), mTOR inhibitors (e.g., rapamycin), antimetabolites (e.g., mizoribine [MZP], mycophenolic acid [MPA]), and glucocorticoids. Inosine monophosphate dehydrogenase (IMPDH), the rate-limiting enzyme in de novo guanine nucleotide biosynthesis, has gained particular attention (Hackstein and Thomson, 2004; Pail et al., 2025; Qin et al., 2025; Tufail et al., 2025). Benzo[b]thiophene-1,1-dioxide, an IMPDH inhibitor, markedly attenuates or abolishes the virulence of emerging Cryptococcus isolates and can exhibit fungicidal activity (Kummari et al., 2018). Other IMPDH inhibitors, including MPA and MZP, show potent activity against Candida albicans and Cryptococcus spp. by disrupting GTP biosynthesis (Kummari et al., 2018). Ribavirin, an antiviral with IMPDH-inhibitory properties, demonstrates in vitro and in vivo efficacy against C. albicans, alone or in combination with azoles, potentially via vacuolar dysfunction and reduced extracellular phospholipase activity (Yousfi et al., 2019; Zhang et al., 2020). Collectively, these data highlight IMPDH as a promising antifungal target, warranting further mechanistic and preclinical evaluation.
Statins were initially known as lipid-lowering and cholesterol-lowering drugs. Statins inhibit HMG-CoA reductase, decreasing ergosterol synthesis and impairing biofilms in Candida, Aspergillus, and zygomycetes, often synergizing with fluconazole (Macreadie et al., 2006; Callegari et al., 2010; Brilhante et al., 2015). Antiarrhythmics (e.g., verapamil, amiodarone) disrupt calcium homeostasis and efflux pumps, enhancing azole efficacy (da Silva et al., 2013; Yu et al., 2013; Yu et al., 2014; Homa et al., 2017; Alnajjar et al., 2018; Zeng et al., 2019). Antipsychotics (e.g., chlorpromazine, haloperidol derivatives) alter membrane structure or inhibit calmodulin, potentiating antifungal agents (Stylianou et al., 2014; Rossato et al., 2016; Holbrook et al., 2017; Kim et al., 2019). Antidepressants (e.g., fluoxetine, sertraline) damage membranes or inhibit virulence factors, active even against resistant fungi (Gu et al., 2016; Treviño-Rangel et al., 2019; Gowri et al., 2020; Jiang L. et al., 2020). Non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin and ibuprofen inhibit prostaglandin synthesis, induce reactive oxygen species (ROS) accumulation, and disrupt membrane integrity, leading to fungal death (Ogundeji et al., 2016). Ibuprofen additionally shows anti-spore activity (median MIC 256 μg/mL) and synergizes with amphotericin B, itraconazole, or terbinafine (Borba-Santos et al., 2021). Diclofenac sodium downregulates genes linked to RNA transport and cell cycle in Aspergillus fumigatus, reducing mycelial formation (Nargesi and Rezaie, 2018).
Antiparasitic drugs also show antifungal potential. Salicylanilide oxyclozanide has shown activity against Candida albicans, including azole- and echinocandin-resistant strains, by disrupting mitochondrial oxidative phosphorylation, collapsing membrane potential, and impairing utilization of non-fermentable carbon sources (Pic et al., 2019). The antimalarial chloroquine, in macrophages infected with Cryptococcus, induces fungal death via iron complex formation and inhibits thiamine transporter activity in Saccharomyces cerevisiae, linked to glucose metabolism (Huang et al., 2012). In azole-resistant C. albicans with abnormal ergosterol synthesis, chloroquine also disrupts morphogenesis (Shinde et al., 2013). Auranofin, an anti-rheumatic drug, inhibits inflammatory pathways and shows broad antifungal activity, including against Aspergillus fumigatus, Apiospora montagnei, and Apiospora siamensis, as well as biofilm inhibition (Thangamani et al., 2016).
Overall, drug repurposing leverages the multi-target potential of existing agents, expands the antifungal arsenal against resistant pathogens, and provides a theoretical foundation for developing combination therapy and novel antifungal strategies (Zhang et al., 2021). However, clinical translation requires thorough pharmacokinetic profiling and large-scale trials to validate efficacy and safety.
5.3 Popular target proteins related to fungal resistance
Fungal resistance represents a critical challenge in clinical mycology and antifungal drug development. The identification of resistance-related proteins as therapeutic targets is pivotal for guiding the rational design of antifungal agents. Drug targets are typically macromolecules, such as proteins or nucleic acids, that interact specifically with therapeutic compounds, mediating pharmacological effects or enabling targeted delivery. With the integration of bioinformatics resources, such as complete fungal proteomes from UniProtKB and domain data from Pfam, novel targets can be systematically identified. Furthermore, artificial intelligence (AI) enhances the predictive capacity for small molecule, protein interactions, facilitating the discovery of potent antifungal candidates (Li et al., 2025c; Yin et al., 2025). Resistance mechanisms vary with the mode of action (MOA) of antifungal drugs. In azoles, resistance is commonly attributed to overexpression of efflux pumps (particularly in Candida) and alterations in the sterol biosynthesis pathway (Lee et al., 2021). In Aspergillus fumigatus, Cyp51A point mutations and promoter insertions are major contributors (Garcia-Rubio et al., 2018; Roundtree et al., 2020). In Cryptococcus neoformans, chromosomal aneuploidy and hypermutations drive target overexpression and efflux (Iyer et al., 2021; Zhang et al., 2024). Polyenes act by binding ergosterol and disrupting membrane integrity; resistance results from mutations in ergosterol biosynthetic genes. For instance, Candida albicans exhibits resistance via ERG3 deletion and upregulation of ERG5, ERG6, and ERG25 (Yu et al., 2012; Zhou et al., 2018). Transcription factors such as Upc2, which regulate ergosterol biosynthesis, have emerged as critical resistance determinants (Yang et al., 2015; Tan et al., 2022). Heat shock protein Hsp90, involved in stress adaptation, also contributes to antifungal resistance (Wei et al., 2024). Echinocandin resistance is primarily driven by mutations in FKS1, while drug-induced cell wall stress can activate tolerance pathways such as the Ca²+/calcineurin and Hsp90/mTOR signaling cascades (Perlin, 2007; Walker et al., 2010; Hou et al., 2019; Tian et al., 2024). Pyrimidine analogs like 5-fluorocytosine inhibit nucleic acid synthesis, with resistance arising from FCY1 mutations (Chang et al., 2021). Additional targets include efflux pumps (e.g., Flu1, Mdr1, Cdr1, Cdr2), kinases and transcription factors (e.g., Snf1, Skr1), signaling proteins (e.g., Hog1, calcineurin), ribosomal proteins (e.g., S3, S6, L4), resistance regulators (e.g., Pdr1), and phosphodiesterases (e.g., Pde1, Pde2) (Day et al., 2018; Usher and Haynes, 2019; Shivarathri et al., 2020; Zgadzay et al., 2022; Kim et al., 2023; Lee et al., 2023; Engle and Kumar, 2024). These proteins offer a theoretical basis for targeted antifungal development. The integration of AI with bioinformatics, de novo protein design, and synthetic biology holds promise for precision therapeutics addressing fungal resistance.
5.4 Synthetic peptide drugs
Antimicrobial peptides (AMPs) are crucial components of the body’s defense system, exhibiting broad-spectrum antimicrobial activity against various pathogens (Pasupuleti et al., 2012). Peptide-based small-molecule drugs have shown potential in antifungal therapy, such as echinocandins and defensin-derived peptides. These peptide molecules possess several key characteristics, including disrupting cell membrane integrity, inhibiting DNA and protein synthesis, and interfering with cellular metabolic processes and cell wall biosynthesis (Buda De Cesare et al., 2020). Due to their unique mechanism of action, antimicrobial peptides are emerging as potential candidates for the control of drug-resistant fungi. For example, antimicrobial peptides designed based on chromogranin A (CGA) have demonstrated excellent antimicrobial performance. CGA is a protein widely distributed in neurons, and its N-terminal 65–76 amino acid sequence (CGA-N12) has been identified as an antimicrobial peptide with activity (Li et al., 2018; Li X. et al., 2022; Li R. et al., 2023). The uniqueness of CGA-N12 lies in its binding target, KRE9, a highly specific β-1,6-glucan synthase for Candida albicans. By inhibiting KRE9 activity, CGA-N12 disrupts the structural integrity of the fungal cell wall, effectively inhibiting fungal growth and reproduction (Li et al., 2018; Li X. et al., 2022). In terms of antifungal activity, CGA-N12 has shown stronger inhibitory effects compared to the traditional antifungal drug fluconazole (Li R. et al., 2023). This difference suggests that CGA-N12, as a novel antimicrobial peptide, has significant potential for future antifungal treatments.
However, peptide small-molecule drugs have various limitations. In terms of effectiveness, there are issues with drug stability and delivery, such as susceptibility to proteases and low oral bioavailability. In terms of target selection and host toxicity, the homology with eukaryotic organisms can interfere with host function, and drug penetration efficiency is insufficient. Additionally, in pharmacokinetics, many drugs have short half-lives, which result in high production costs, strict storage conditions, and poor accessibility. Some drugs also have immunogenicity and allergy risks. Moreover, their antifungal spectrum is narrow, making it difficult to address mixed infections or fungal morphological transitions (Wang et al., 2022; Luo et al., 2023).
Future research directions include structural optimization, new target development, innovations in delivery technologies, and combination therapy strategies. Breakthroughs in these areas require deep interdisciplinary collaboration between structural biology, synthetic chemistry, and clinical needs, balancing efficacy, safety, and accessibility to fill the gaps in antifungal therapy.
5.5 Nanotechnology for antifungal drugs
Changing the drug formulation is one of the commonly used and effective methods in drug optimization, significantly improving bioavailability, reducing adverse reactions, and enhancing therapeutic outcomes. For example, RN104 (2- [2-(cyclohexyl methylene) hydrazinyl]-4-phenylthiazole) is a thiazole hydrazone derivative with significant antifungal activity. However, due to its low solubility in physiological pH conditions, the oral bioavailability of RN104 is suboptimal (Silva et al., 2020). To overcome this issue, researchers designed a self-emulsifying drug delivery system (SEDDS) based on RN104 to improve its pharmacokinetic properties and oral bioavailability. In pharmacokinetic studies in mice, RN104-SEDDS significantly increased its oral bioavailability by 2133% compared to free RN104, enhancing its bioactivity (Silva et al., 2024).
Another classic example is AMB, a potent antifungal drug that, when administered intravenously, can cause severe adverse effects, including kidney, liver, and cardiovascular damage, as well as anemia and electrolyte imbalances (Jarvis et al., 2022). To reduce these side effects, a new oral formulation of Amphotericin B, lipid nanocrystal Amphotericin B (MAT2203), has been developed as an alternative to intravenous administration (Gu et al., 2024; Kriegl et al., 2025). The mechanism of action involves targeting cells, such as macrophages, which engulf these lipid nanocrystals and transport them to the infection site. At the infection site, the low intracellular calcium concentration triggers the release mechanism of the nanocrystals, allowing the drug to be directly released inside the cells, thereby avoiding systemic tissue damage caused by Amphotericin B (Boulware et al., 2023). This demonstrates how improvements in drug formulations play a critical role in antifungal drug development, enhancing drug efficacy while significantly reducing adverse effects. Looking ahead, the development of new antifungal drug formulations will undoubtedly bring more possibilities and greater expectations for the treatment of fungal infections.
5.6 Antifungal compounds of traditional Chinese medicine will become a new star
Approximately 80% of antibiotics currently used in clinical practice are derived from natural products, underscoring the value of natural sources in drug discovery (Newman et al., 2003). Traditional Chinese Medicine (TCM), with a long history of treating infectious diseases, has recently gained renewed interest for its potential in combating fungal infections. Increasing evidence suggests that various components of traditional Chinese herbs exhibit significant antifungal activity, functioning through diverse and often complementary mechanisms (Jiang B-C. et al., 2020). TCM-derived compounds have been shown to exert antifungal effects through several pathways, notably by targeting the fungal cell wall and cell membrane, as well as interfering with key metabolic and regulatory processes (Figure 5) (Zhang C-W. et al., 2023; Zou et al., 2023).
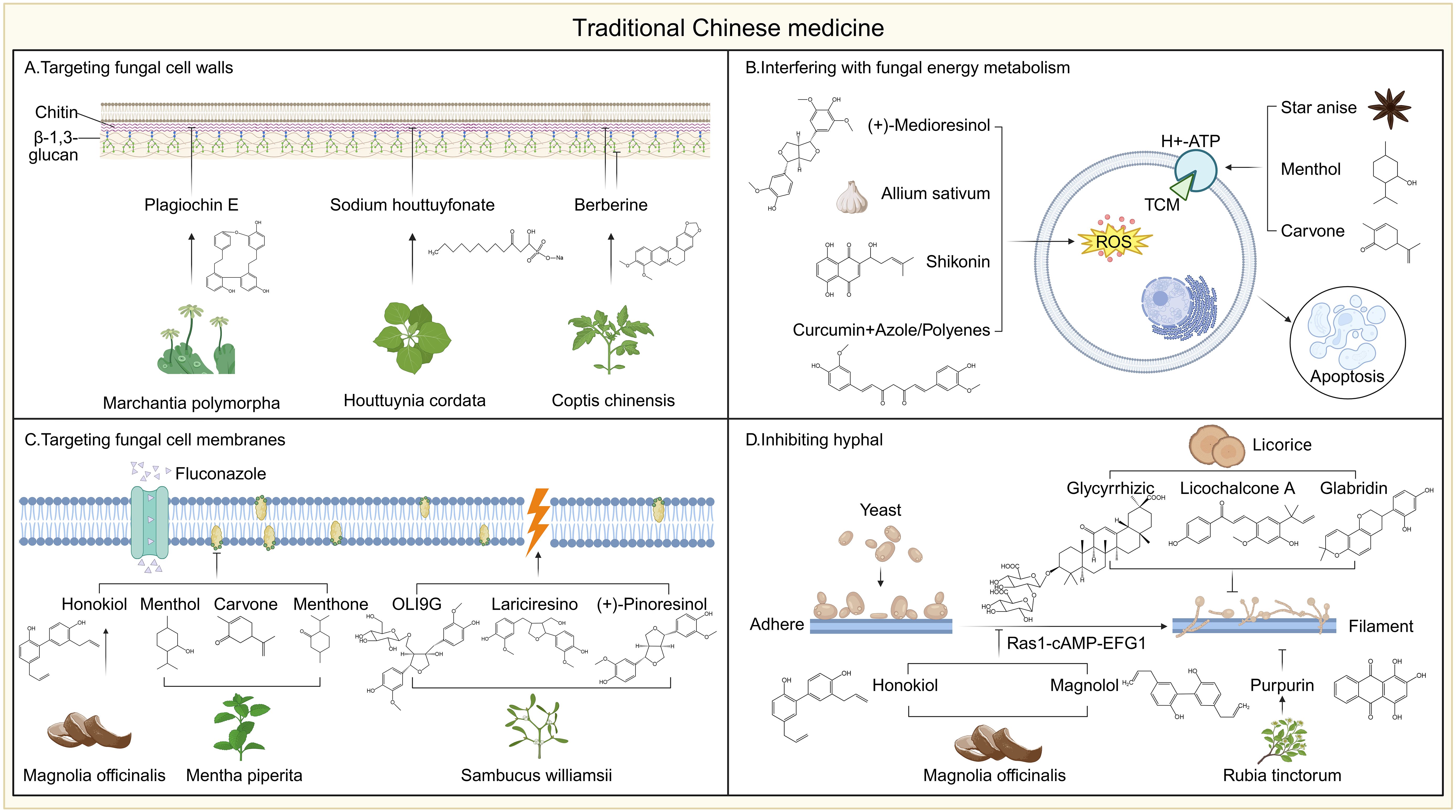
Figure 5. Mechanism of action of traditional Chinese medicine. (A) Plagiochin E from Marchantia polymorpha inhibited chitin synthesis, Sodium houttuydata from Houttuynia cordata regulated gene expression related to β-1,3-glucan synthesis, and berberine from Coptis chinensis promoted cell wall components exposure. (B) Constituents affecting cell metabolism such as star anise and carvone extracts caused cytosolic acidification by inhibiting H+-ATPase, (+)-medioresinol, isolated from Sambucus williamsii, shikonin and Allium sativum (garlic) extracts caused oxidative stress by inducing the accumulation of reactive oxygen species, while curcumin synergistically enhanced the ROS generation ability of other antifungal agents. (C) Membrane-targeting components such as honokiol from Magnolia and carvone, menthol and menthone from Mentha piperita destroy membrane integrity by reducing ergosterol content, while OLI9, lariciresinol, and (+)-pinoresinol from Sambucus williamsii directly disrupt the membrane structure. (D) Honokiol and magnolol from Magnolia blocked hyphal formation by inhibiting the Ras1-cAMP-EFG1 signaling pathway, and licorice-derived compounds such as licochalcone A, glabridin, and glycyrrhizic acid impair fungal growth and morphogenesis. Licochalcone A, Purpurin from Rubia tinctorum inhibit fungal morphological development and biofilm formation.
The fungal cell wall is essential for maintaining cellular integrity and morphology, and its absence in mammalian cells makes it an ideal antifungal target. Several traditional Chinese medicine (TCM)-derived compounds have been shown to disrupt fungal cell wall synthesis or compromise its structural stability. For instance, Plagiochin E, a macrocyclic bis(bibenzyl) compound isolated from Marchantia polymorpha, exhibits antifungal activity by targeting chitin synthesis. It significantly downregulates the expression of CHS1 and alters the expression profile of other chitin synthase genes, leading to impaired chitin biosynthesis and subsequent cell wall damage in Candida albicans (Guo et al., 2008; Wu et al., 2008). Sodium houttuyfonate (SH), a sulfur-containing compound derived from Houttuynia cordata, has also shown promising effects in combination with fluconazole against C. albicans, particularly in resistant strains. SH significantly reduced MIC values when used with fluconazole and exhibited strong synergy (FICI < 0.5). Gene expression analysis revealed that SH modulates the expression of genes involved in β-1,3-glucan synthesis and transport, including upregulation of ZAP1, ADH5, XOG1, and FKS1, suggesting its potential mechanism involves enhancing cell wall-targeted antifungal responses (Shao et al., 2017; Liu et al., 2021; Cheng et al., 2023). Berberine, an isoquinoline alkaloid from Coptis chinensis, disrupts Candida albicans cell-wall architecture by increasing surface exposure of β-glucans and chitin, thereby weakening the barrier and heightening susceptibility to immune attack and antifungal agents (Shi et al., 2017; Liu et al., 2020; Huang et al., 2021). Beyond this remodeling effect, berberine hydrochloride down-regulates the efflux-pump gene CDR1, reducing fluconazole extrusion and further sensitizing resistant strains (Zhu et al., 2014). These actions are magnified when berberine is paired with sodium houttuyfonate, which up-regulates β-1,3-glucan synthesis and transport genes, producing marked synergism with fluconazole against recalcitrant C. albicans (Tong et al., 2021).
Traditional Chinese medicine (TCM), derived compounds exhibit potent antifungal activity through multiple mechanisms, notably by targeting the fungal cell membrane and its associated components. Ergosterol, a key sterol unique to fungal membranes, is a primary target. For instance, honokiol, a biphenolic compound from Magnolia officinalis, has been shown to significantly reduce ergosterol levels in Candida albicans, thereby compromising membrane integrity and exerting direct antifungal effects (Sun LM. et al., 2015; Sun et al., 2017). Furthermore, honokiol enhances the efficacy of fluconazole by diminishing the impact of membrane transport proteins, thus reducing drug efflux and increasing intracellular drug accumulation (Jin et al., 2010). Similarly, essential oil components from Mentha piperita, including carvone, menthol, and menthone, have been reported to inhibit fungal growth by decreasing ergosterol content in the cell membrane (Samber et al., 2015; Giménez-Santamarina et al., 2022; Hudz et al., 2023). Extracts from Sambucus williamsii, such as (−)-olivil-9′-O-β-D-glucopyranoside, lariciresinol, and (+)-pinoresinol, also display membrane-disruptive properties; these compounds depolarize the fungal membrane and increase its permeability, ultimately leading to cell death (Hwang et al., 2010; Hwang et al., 2011; Choi et al., 2013). Beyond direct disruption of membrane lipids, some TCM compounds interfere with membrane-bound proteins and enzymes essential for fungal viability. For example, star anise and peppermint-derived constituents (carvone, menthol, and menthone) inhibit the activity of plasma membrane H+-ATPase, causing cytoplasmic acidification and cell death (Edris and Farrag, 2003; Baazeem et al., 2025; Nordin et al., 2025). Additionally, eugenol impairs nutrient uptake by inhibiting amino acid permeases such as Tat1p and Gap1p, further suppressing yeast growth (Darvishi et al., 2013; Nisar et al., 2021). These findings highlight the multifaceted strategies by which TCM compounds compromise fungal membrane function, offering promising avenues for antifungal development, particularly against drug-resistant strains.
TCM components can also exert antifungal effects by inhibiting hyphal and biofilm formation. Several natural compounds have been reported to inhibit both the hyphal morphogenesis and biofilm formation of Candida albicans through various mechanisms. For example, two newly identified lignans from Magnolia, magnolol and honokiol, suppress the Ras1–cAMP–EFG1 signaling pathway to inhibit hyphal transition (Sun L. et al., 2015). Similarly, licorice-derived compounds such as licochalcone A, glabridin, and glycyrrhizic acid impair fungal growth and morphogenesis (Seleem et al., 2016). Purpurin from Rubia tinctorum also demonstrates comparable antifungal activity (Messier and Grenier, 2011; Tsang et al., 2012). In addition, licochalcone A, purpurin, magnolol, and honokiol have been shown to further inhibit biofilm formation (Tsang et al., 2012; Sun L. et al., 2015; Seleem et al., 2016). Given the structural complexity of fungal biofilms, their disruption is particularly valuable. Cinnamaldehyde, derived from cinnamon, significantly impairs biofilm development by Candida species (Pires et al., 2011; Khan and Ahmad, 2012). Likewise, berberine, isolated from Coptis chinensis and Hydrastis canadensis, exhibits notable biofilm-inhibitory effects, especially in combination with miconazole (Wei et al., 2011). The treatment with Paeonol shows effective antifungal and antibiofilm activity against biofilms containing Candida albicans and/or Cryptococcus spp (Qian et al., 2022). Other natural compounds, including curcumin, thymol, and eugenol, have also been reported to interfere with biofilm formation (Braga et al., 2008; Miladi et al., 2017).
Several Traditional Chinese Medicine (TCM) compounds exert antifungal activity by disrupting fungal energy metabolism, primarily through the induction of oxidative stress and mitochondrial dysfunction. For example, (+)-medioresinol, isolated from Sambucus williamsii, promotes the accumulation of reactive oxygen species (ROS) and induces cell cycle arrest, ultimately triggering apoptosis in fungal cells (Hwang et al., 2012). Similarly, baicalin interferes with mitochondrial enzyme activity and cell cycle progression, leading to programmed cell death (Yang et al., 2014). Shikonin has also been shown to increase endogenous ROS levels and impair mitochondrial function, thereby enhancing its antifungal efficacy (Miao et al., 2012). Thymol, a monoterpenoid phenol, suppresses the expression of genes involved in the tricarboxylic acid (TCA) cycle, resulting in diminished energy production and inhibited growth of Fusarium species (Zhang et al., 2018). Consistent with these mechanisms, Allium sativum (garlic) induces oxidative stress by elevating intracellular ROS levels, further suppressing fungal proliferation (Lemar et al., 2005). Notably, curcumin, a polyphenolic compound derived from turmeric, has been reported to synergize with azoles and polyenes to amplify ROS generation in Candida albicans, leading to mitochondrial damage, apoptosis, and enhanced antifungal activity (Sharma et al., 2010b; Sharma et al., 2010a). These findings underscore the diverse mechanisms by which TCM-derived compounds interfere with fungal energy homeostasis and highlight their potential as valuable resources for the development of next-generation antifungal therapeutics.
5.7 AI in developing new molecules with antifungal activity
Artificial intelligence (AI) has emerged as a transformative tool in antifungal drug discovery, particularly for identifying and optimizing novel therapeutic agents. By leveraging large-scale biological, chemical, and clinical datasets, AI-driven platforms can accurately predict drug-target interactions, prioritize lead compounds, and optimize molecular structures (Jović and Šmuc, 2020; Zhou et al., 2025). Machine learning (ML) models trained on physicochemical properties of known antifungals effectively distinguish active from inactive compounds, streamlining virtual screening and substantially reducing the candidate space (Das et al., 2021; Randall et al., 2024). Furthermore, ML approaches play a pivotal role in predicting resistance-conferring mutations, while advances such as AlphaFold enable high-precision structural modeling for the identification of antifungal targets (Elfmann and Stülke, 2025; Li et al., 2025c).
The integration of deep generative models and molecular dynamics simulations has further accelerated antifungal discovery. Deep learning, based virtual screening is widely used to predict binding affinities of compounds targeting fungal proteins such as β-(1,3)-D-glucan synthase (Fks1), lanosterol 14α-demethylase (Erg11), and chitin synthase, and other essential genes involved fungal growth. For instance, one study used chemical descriptors to develop a machine learning model targeting Candida albicans FKS1, achieving 96.72% classification accuracy (Gao et al., 2022). Another study applied deep learning and molecular docking to screen 1,930 FDA-approved drugs against C. albicans dihydrofolate reductase, identifying paritaprevir, lumacaftor, and rifampin as promising inhibitors (Joshi et al., 2022). Addressing the rising incidence of azole-resistant Aspergillus fumigatus, researchers also developed novel inhibitors targeting AfDHODH, a key enzyme in pyrimidine biosynthesis, yielded 13 candidate molecules, with two demonstrating sub-100 μM activity in vitro (Li K. et al., 2025).
AI has also significantly advanced the discovery and design of antifungal peptides (AFPs) (Kavousi et al., 2020; Szymczak et al., 2025). DL-QSARES, a framework integrating deep learning with quantitative structure–activity relationship-based empirical screening, enabled de novo design of 49 AFPs, with AFP-13 showing potent activity against C. albicans and therapeutic efficacy in animal models (Yin et al., 2025). Other studies using diffusion models and molecular dynamics identified 25 peptides with antifungal properties, among which AMP-29 showed activity against C. glabrata (Wang Y. et al., 2025). Advanced AI-platforms like EvoGradient, BroadAMP-GPT, and AMPSphere have demonstrated success in designing antimicrobial peptides with high hit rates against resistant pathogens. EvoGradient used oral microbiome data to generate 32 peptides, all active against at least one ESKAPE pathogen and effective in mouse wound models (Wang B. et al., 2025). BroadAMP-GPT combined AI generation, filtering, and experimental validation, yielding a 57% hit rate against ESKAPE pathogens (Li et al., 2025b). AMPSphere screened over 150,000 genomes to predict 860,000 peptides; among 100 tested, 63 showed antibacterial activity via membrane disruption (Santos-Júnior et al., 2024).
Systems biology has further enhanced AI applications. The integration of comprehensive databases, including genomic, proteomic, and transcriptomic resources, can greatly accelerate the discovery of novel antifungal targets and the identification of genetic variants that confer drug resistance. The first genome-scale metabolic model (GSMM) iRV973 for Candida auris predicted growth under various nutrient conditions and identified 50 conserved, serum-essential enzymes—some as novel, non-homologous drug targets (Viana et al., 2023). In C. albicans, machine learning and chemogenetic interaction analysis enabled screening of ~6,500 genes, expanding the GRACE (gene replacement and conditional expression) database by 866 entries and revealing 149 fungus-specific essential genes (Fu et al., 2021). This led to the discovery of NP-BTA, a N-pyridinyl-β-thienyl-acrylamide derivative, was discovered to inhibit the essential enzyme glutaminyl-tRNA synthetase (Gln4), representing a novel antifungal mechanism of action (Fu et al., 2021). Recent advances include a predictive machine learning framework utilizing as few as ten genomic variant features to accurately classify isolates as heteroresistant or susceptible, enabling rapid detection of micafungin heteroresistance in C. parapsilosis (Zhai et al., 2024). Similarly, an ML-driven multi-omics integration approach, combining transcriptomic and genomic variation data, has effectively pinpointed highly multidrug-adapted traits within the Cryptococcus gattii species complex (Fan et al., 2024). Another study used ML on whole-genome annotations and Erg11 sequences to predict antifungal resistance profiles in yeasts, achieving 75% accuracy for fluconazole resistance and identifying novel resistance-associated residues (Harrison et al., 2025).
The advent of AlphaFold has transformed predictions of antifungal target protein structures and drug–binding interfaces. Leveraging AlphaFold, the structure of Fks1, the β-1,3-glucan synthase targeted by echinocandins, was resolved, revealing resistance-associated mutations, catalytic mechanisms, and the GTP-dependent conformational activation by Rho1 (Hu et al., 2023; Li J. et al., 2025). Furthermore, Simulations of the Erg11–Ncp1 interaction identified residues V234, F235, and L238 in Erg11 as critical for complex stability; disruption of this interface was predicted to sensitize Candida albicans to azoles via increased protein misfolding (Li J. et al., 2025). High-throughput deep mutational scanning, informed by AlphaFold, further pinpointed residues in CaErg11 that alter azole binding and resistance (Bédard et al., 2024). Coupling AlphaFold with FoldX identified conserved, non–active site mutations in Fcy1, the 5-fluorocytosine target enzyme, that destabilize the protein, constituting a major mechanism of 5-FC resistance (Després et al., 2022).
In summary, AI technologies, including machine learning, deep learning, and natural language processing—offer powerful solutions to combat antifungal resistance. From drug repurposing and target prediction to peptide design and personalized strategies, AI is poised to drive the next generation of antifungal therapeutics.
6 The complex mechanisms behind the failure of fungal treatment
Antifungal treatment failure is a growing clinical challenge, driven not only by pharmacological limitations but also by intrinsic fungal traits. Key factors such as biofilm formation, metabolic flexibility, genetic plasticity, and evolving resistance mechanisms significantly influence therapeutic outcomes (Fisher et al., 2022; Lockhart et al., 2023). Fungi like Candida and Aspergillus form drug-resistant biofilms, where extracellular matrices limit antifungal penetration and induce dormant “persister” cells (Morelli et al., 2021; Kaur and Nobile, 2023). Under nutrient stress, fungi reprogram metabolism and produce antioxidant enzymes, reducing susceptibility to oxidative agents like AMB (Ke et al., 2024). Additionally, some species, such as Candida auris, acquire resistance genes from the environment and modify host conditions to favor survival (Akinbobola et al., 2023). Genetic exchanges and epigenetic modifications further contribute to rapid resistance development (Meng et al., 2024; Zajac et al., 2025; Zhang et al., 2025). Clinical isolates show considerable genomic diversity, including chromosomal duplications and non-classical mutations, complicating treatment strategies (Priest et al., 2022; Rhodes et al., 2022; Ning et al., 2024; Zhou et al., 2024; Brassington et al., 2025). Traditional laboratory strains no longer reflect real-world pathogen behavior, highlighting the need to incorporate contemporary clinical isolates into research. Beyond genetic resistance, fungi exhibit heterogeneous resistance and drug tolerance (Chen et al., 2024; Zhai et al., 2024). Subpopulations may survive high drug concentrations through stress response pathways, while dormant phenotypes persist within host niches like macrophages (Arastehfar et al., 2023). These non-genetic adaptations often precede the emergence of stable, heritable resistance. The rise of multidrug-resistant strains, such as Candida auris and Rhodosporidiobolus fluvialis, underscores the urgency for new therapeutic approaches (Jackson et al., 2019; Huang et al., 2024). Environmental changes, including climate warming, may drive the emergence of such pathogens by promoting stress adaptation and thermal tolerance (Hofer, 2019; Zhai et al., 2021; Du Toit, 2023; Bottery and Denning, 2024). To address these challenges, antifungal development must shift toward targeting tolerance mechanisms, exploring niche-specific strategies, and expanding surveillance of emerging environmental and clinical pathogens. Future advances will depend on integrating fungal biology with ecological and genomic insights to stay ahead of rapidly adapting fungal threats.
7 Conclusion and future outlook
Antifungal drug research is advancing rapidly, driven by the urgent need to improve therapeutic outcomes and combat emerging drug resistance. Current efforts span a wide range of strategies—from structural optimization and target-specific drug design to the development of advanced delivery platforms. A successful response to antifungal resistance demands a multidisciplinary approach that integrates innovative pharmacology, biotechnology, and systems-level understanding of host-pathogen interactions.
One promising avenue lies in the development of antifungal peptide-based therapeutics. Structural optimization through biomimetic engineering, such as modifying host defense peptides or incorporating non-natural amino acids, can enhance stability, membrane permeability, and antifungal activity (Ting et al., 2020). Targeting fungal-specific biological pathways, such as chitin synthesis or virulence factor secretion, offers opportunities for designing highly selective and less toxic agents (Chung et al., 2025). Advances in drug delivery technologies, including liposomes, exosomes, and cell-penetrating peptides (CPPs), further improve bioavailability and tissue targeting (Zhu et al., 2023). Moreover, combination therapies involving immune modulators (e.g., IFN-γ) or antibiofilm agents may synergistically enhance efficacy and delay the onset of resistance (Scriven et al., 2017). The integration of artificial intelligence (AI), synthetic biology, and de novo protein design is redefining antifungal drug discovery (Liu et al., 2025). AI-driven computational modeling enables the rational design of antifungal agents and the prediction of resistance mutations, facilitating precision therapeutics. Coupling pathogen biology with host immunology and computational tools can help navigate the complexity of fungal infections and guide the development of targeted interventions. In parallel, technological innovations in pharmaceutical sciences are reshaping antifungal delivery strategies. Nanomaterials, such as mesoporous zinc oxide, are being explored for topical applications, while smart nano-delivery systems targeting the fungal microenvironment represent a promising frontier (Bayat et al., 2022; Thapliyal et al., 2025). These approaches may prolong the efficacy of existing antifungal drugs and accelerate the discovery of new compounds. Improving early diagnostic and monitoring capabilities is equally vital. Rapid detection tools and biomarker-based diagnostic kits can enable timely intervention, bridging prevention and treatment. Emerging data from proteomics and genomic studies suggest that the development of super-resistant fungal strains is associated with critical genomic transitions (Burrack et al., 2022; Lockhart et al., 2023; de Moraes and Ferreira-Pereira, 2024; Joste et al., 2025). Notably, these transitions may involve abrupt conformational changes in key target proteins. Integrating bioinformatics and protein structural biology to monitor such changes could facilitate earlier and more effective interventions.
In summary, the future of antifungal therapy hinges on cross-disciplinary innovation. Emphasis on targeted drug design, advanced delivery platforms, AI-driven prediction models, and early diagnostic tools will be essential in addressing the escalating challenge of antifungal resistance. Moving forward, a proactive approach that couple’s prevention with precision therapy will be critical to shifting the paradigm from treatment to long-term control of fungal diseases.
Author contributions
YJL: Investigation, Writing – review & editing, Writing – original draft. YL: Writing – review & editing, Writing – original draft. YJ: Writing – original draft. YY: Writing – original draft. WN: Writing – review & editing. WZ: Writing – review & editing. LT: Investigation, Writing – review & editing, Funding acquisition, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (NSFC No. 82304588), the Fundamental Research Funds for the Education fund item of Liaoning province (Grant No. JYTQN2023323), Fundamental Research Funds for the Shenyang Science and Technology item (Grant No. RC231149), and the project for Shenyang Pharmaceutical University (Grant No. YQ202305), Fundamental Research Funds for the Liaoning Province Doctoral Startup Project (Grant No. 2024-BS-074), the Basic Scientific Research Fund of Northeastern University of China (N25LPY044).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1662442/full#supplementary-material
References
Abdelmegeed, E. and Shaaban, M. I. (2013). Cyclooxygenase inhibitors reduce biofilm formation and yeast-hypha conversion of fluconazole resistant Candida albicans. J. Microbiol. 51, 598–604. doi: 10.1007/s12275-013-3052-6
Ahmad, S. and Alfouzan, W. (2021). Candida auris: epidemiology, diagnosis, pathogenesis, antifungal susceptibility, and infection control measures to combat the spread of infections in healthcare facilities. Microorganisms 9, 807. doi: 10.3390/microorganisms9040807
Akinbobola, A. B., Kean, R., Hanifi, S. M. A., and Quilliam, R. S. (2023). Environmental reservoirs of the drug-resistant pathogenic yeast Candida auris. PloS Pathog. 19, e1011268. doi: 10.1371/journal.ppat.1011268
Albrecht, W. (2019). Highlight report: hepatotoxicity of triazole fungicides. Arch. Toxicol. 93, 3037–3038. doi: 10.1007/s00204-019-02555-x
Alexander, B. D., Johnson, M. D., Pfeiffer, C. D., Jiménez-Ortigosa, C., Catania, J., Booker, R., et al. (2013). Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 56, 1724–1732. doi: 10.1093/cid/cit136
Almeida, F., Rodrigues, M. L., and Coelho, C. (2019). The still underestimated problem of fungal diseases worldwide. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.00214
Alnajjar, L. M., Bulatova, N. R., and Darwish, R. M. (2018). Evaluation of four calcium channel blockers as fluconazole resistance inhibitors in Candida glabrata. J. Global antimicrobial. resistance 14, 185–189. doi: 10.1016/j.jgar.2018.04.004
Alqarihi, A., Singh, S., Edwards, J. E., Jr., Ibrahim, A. S., and Uppuluri, P. (2019). NDV-3A vaccination prevents C. albicans colonization of jugular vein catheters in mice. Sci. Rep. 9, 6194. doi: 10.1038/s41598-019-42517-y
Alvarez, C. M., Oliveira, M. M. E., and Pires, R. H. (2022). Sporotrichosis: A review of a neglected disease in the last 50 years in Brazil. Microorganisms 10, 2152. doi: 10.3390/microorganisms10112152
Angulo, D. A., Alexander, B., Rautemaa-Richardson, R., Alastruey-Izquierdo, A., Hoenigl, M., Ibrahim, A. S., et al. (2022). Ibrexafungerp, a novel triterpenoid antifungal in development for the treatment of mold infections. J. Fungi (Basel) 8, 1121. doi: 10.3390/jof8111121
Arastehfar, A., Daneshnia, F., Cabrera, N., Penalva-Lopez, S., Sarathy, J., Zimmerman, M., et al. (2023). Macrophage internalization creates a multidrug-tolerant fungal persister reservoir and facilitates the emergence of drug resistance. Nat. Commun. 14, 1183. doi: 10.1038/s41467-023-36882-6
Araúz, A. B. and Papineni, P. (2021). Histoplasmosis. Infect. Dis. Clin. North Am. 35, 471–491. doi: 10.1016/j.idc.2021.03.011
Arendrup, M. C., Jensen, R. H., and Cuenca-Estrella, M. (2016). In Vitro Activity of ASP2397 against Aspergillus Isolates with or without Acquired Azole Resistance Mechanisms. Antimicrob. Agents Chemother. 60, 532–536. doi: 10.1128/aac.02336-15
Arendrup, M. C., Jørgensen, K. M., Hare, R. K., and Chowdhary, A. (2020). In Vitro Activity of Ibrexafungerp (SCY-078) against Candida auris Isolates as Determined by EUCAST Methodology and Comparison with Activity against C. albicans and C. glabrata and with the Activities of Six Comparator Agents. Antimicrob. Agents Chemother. 64, e02136–02119. doi: 10.1128/aac.02136-19
(2024). Antimicrobial resistance: a silent pandemic. Nat. Commun. 15, 6198. doi: 10.1038/s41467-024-50457-z
(2018). Guidelines for The Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection (Geneva, Switzerland: World Health Organization).
Baazeem, A., Hassan, M. M., Alqurashi, A. S., Al Seberi, H., Al-Harthi, H. F., Al-Said, H. M., et al. (2025). Peppermint and tarragon essential oils encapsulated in copper nanoparticles: A novel approach to sustainable fungal pathogen management in industrial crops. Ind. Crops Products 232, 121310. doi: 10.1016/j.indcrop.2025.121310
Balhara, M., Ruhil, S., Kumar, M., Dhankhar, S., and Chhillar, A. K. (2014). An anti-Aspergillus protein from Escherichia coli DH5α: putative inhibitor of siderophore biosynthesis in Aspergillus fumigatus. Mycoses 57, 153–162. doi: 10.1111/myc.12119
Barber, A. E., Sae-Ong, T., Kang, K., Seelbinder, B., Li, J., Walther, G., et al. (2021). Aspergillus fumigatus pan-genome analysis identifies genetic variants associated with human infection. Nat. Microbiol. 6, 1526–1536. doi: 10.1038/s41564-021-00993-x
Bastos, R. W., Rossato, L., Valero, C., Lagrou, K., Colombo, A. L., Goldman, G. H., et al. (2019). Potential of gallium as an antifungal agent. Front. Cell Infect. Microbiol. 9. doi: 10.3389/fcimb.2019.00414
Bayat, F., Mehryab, F., Akhlaghi, S., and Haeri, A. (2022). Emerging Nanomaterials and Nano-Based Drug Delivery Approaches to Combat Antimicrobial Resistance. Eds. Saravanan, M., Barabadi, H., Mostafavi, E., and Webster, T. (Amsterdam, The Netherlands: Elsevier), 179–232.
Bédard, C., Gagnon-Arsenault, I., Boisvert, J., Plante, S., Dubé, A. K., Pageau, A., et al. (2024). Most azole resistance mutations in the Candida albicans drug target confer cross-resistance without intrinsic fitness cost. Nat. Microbiol. 9, 3025–3040. doi: 10.1038/s41564-024-01819-2
Benitez, L. L. and Carver, P. L. (2019). Adverse effects associated with long-term administration of azole antifungal agents. Drugs 79, 833–853. doi: 10.1007/s40265-019-01127-8
Bennett, J. E., Dismukes, W. E., Duma, R. J., Medoff, G., Sande, M. A., Gallis, H., et al. (1979). A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis. N Engl. J. Med. 301, 126–131. doi: 10.1056/nejm197907193010303
Berman, J. and Krysan, D. J. (2020). Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 18, 319–331. doi: 10.1038/s41579-019-0322-2
Bhattacharya, S., Esquivel, B. D., and White, T. C. (2018). Overexpression or deletion of ergosterol biosynthesis genes alters doubling time, response to stress agents, and drug susceptibility in saccharomyces cerevisiae. mBio 9, e01291–01218. doi: 10.1128/mBio.01291-18
Bicanic, T., Bottomley, C., Loyse, A., Brouwer, A. E., Muzoora, C., Taseera, K., et al. (2015). Toxicity of amphotericin B deoxycholate-based induction therapy in patients with HIV-associated cryptococcal meningitis. Antimicrob. Agents Chemother. 59, 7224–7231. doi: 10.1128/aac.01698-15
Billmyre, R. B., Applen Clancey, S., Li, L. X., Doering, T. L., and Heitman, J. (2020). 5-fluorocytosine resistance is associated with hypermutation and alterations in capsule biosynthesis in Cryptococcus. Nat. Commun. 11, 127. doi: 10.1038/s41467-019-13890-z
Bongomin, F., Gago, S., Oladele, R. O., and Denning, D. W. (2017). Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi (Basel) 3, 57. doi: 10.3390/jof3040057
Borba-Santos, L. P., Nucci, M., Ferreira-Pereira, A., and Rozental, S. (2021). Anti-Sporothrix activity of ibuprofen combined with antifungal. Braz. J. microbiol.: [publication Braz. Soc. Microbiology] 52, 101–106. doi: 10.1007/s42770-020-00327-9
Bottery, M. J. and Denning, D. W. (2024). Body heat drives antifungal resistance. Nat. Microbiol. 9, 1638–1639. doi: 10.1038/s41564-024-01738-2
Boulware, D. R., Atukunda, M., Kagimu, E., Musubire, A. K., Akampurira, A., Tugume, L., et al. (2023). Oral lipid nanocrystal amphotericin B for cryptococcal meningitis: A randomized clinical trial. Clin. Infect. Dis. 77, 1659–1667. doi: 10.1093/cid/ciad440
Braga, P. C., Culici, M., Alfieri, M., and Dal Sasso, M. (2008). Thymol inhibits Candida albicans biofilm formation and mature biofilm. Int. J. Antimicrobial. Agents 31, 472–477. doi: 10.1016/j.ijantimicag.2007.12.013
Brassington, P. J. T., Klefisch, F. R., Graf, B., Pfüller, R., Kurzai, O., Walther, G., et al. (2025). Genomic reconstruction of an azole-resistant Candida parapsilosis outbreak and the creation of a multi-locus sequence typing scheme: a retrospective observational and genomic epidemiology study. Lancet Microbe 6, 100949. doi: 10.1016/j.lanmic.2024.07.012
Brilhante, R. S., Caetano, E. P., Oliveira, J. S., Castelo-Branco Dde, S., Souza, E. R., Alencar, L. P., et al. (2015). Simvastatin inhibits planktonic cells and biofilms of Candida and Cryptococcus species. Braz. J. Infect. Dis. 19, 459–465. doi: 10.1016/j.bjid.2015.06.001
Bromuro, C., Romano, M., Chiani, P., Berti, F., Tontini, M., Proietti, D., et al. (2010). Beta-glucan-CRM197 conjugates as candidates antifungal vaccines. Vaccine 28, 2615–2623. doi: 10.1016/j.vaccine.2010.01.012
Brummer, E. and Stevens, D. A. (1994). Macrophage colony-stimulating factor induction of enhanced macrophage anticryptococcal activity: synergy with fluconazole for killing. J. Infect. Dis. 170, 173–179. doi: 10.1093/infdis/170.1.173
Buda De Cesare, G., Cristy, S. A., Garsin, D. A., and Lorenz, M. C. (2020). Antimicrobial peptides: a new frontier in antifungal therapy. mBio 11, e02123–20. doi: 10.1128/mBio.02123-20
Burrack, L. S., Todd, R. T., Soisangwan, N., Wiederhold, N. P., and Selmecki, A. (2022). Genomic Diversity across Candida auris Clinical Isolates Shapes Rapid Development of Antifungal Resistance In Vitro and In Vivo. mBio 13, e0084222. doi: 10.1128/mbio.00842-22
Callegari, S., McKinnon, R. A., Andrews, S., and de Barros Lopes, M. A. (2010). Atorvastatin-induced cell toxicity in yeast is linked to disruption of protein isoprenylation. FEMS yeast Res. 10, 188–198. doi: 10.1111/j.1567-1364.2009.00593.x
Cannon, R. D., Lamping, E., Holmes, A. R., Niimi, K., Baret, P. V., Keniya, M. V., et al. (2009). Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 22, 291–321. doi: 10.1128/CMR.00051-08
Cao, D., Wang, F., Yu, S., Dong, S., Wu, R., Cui, N., et al. (2021). Prevalence of azole-resistant aspergillus fumigatus is highly associated with azole fungicide residues in the fields. Environ. Sci. Technol. 55, 3041–3049. doi: 10.1021/acs.est.0c03958
Cargill, R. I. M., Shimizu, T. S., Kiers, E. T., and Kokkoris, V. (2025). Cellular anatomy of arbuscular mycorrhizal fungi. Curr. Biol. 35, R545–r562. doi: 10.1016/j.cub.2025.03.053
Carolus, H., Pierson, S., Lagrou, K., and Van Dijck, P. (2020). Amphotericin B and other polyenes-discovery, clinical use, mode of action and drug resistance. J. Fungi (Basel) 6, 321. doi: 10.3390/jof6040321
Cass, L., Murray, A., Davis, A., Woodward, K., Albayaty, M., Ito, K., et al. (2021). Safety and nonclinical and clinical pharmacokinetics of PC945, a novel inhaled triazole antifungal agent. Pharmacol. Res. Perspect. 9, e00690. doi: 10.1002/prp2.690
Cassone, A. (2013). Development of vaccines for Candida albicans: fighting a skilled transformer. Nat. Rev. Microbiol. 11, 884–891. doi: 10.1038/nrmicro3156
Cavassin, F. B., Magri, M. M. C., Vidal, J. E., de Moraes Costa Carlesse, F. A., Falci, D. R., Baú-Carneiro, J. L., et al. (2024). Effectiveness, tolerability, and safety of different amphotericin B formulations in invasive fungal infections: A multicenter, retrospective, observational study. Clin. Ther. 46, 322–337. doi: 10.1016/j.clinthera.2024.01.011
Cerqueira, F., Medeiros, R., Lopes, I., Campos, C., Ferraz, M. P., Silva, F., et al. (2024). A Cyclam Salt as an Antifungal Agent: Interference with Candida spp. and Cryptococcus neoformans Mechanisms of Virulence. Antibiotics (Basel) 13, 222. doi: 10.3390/antibiotics13030222
Chang, Y. C., Lamichhane, A. K., Cai, H., Walter, P. J., Bennett, J. E., Kwon-Chung, K. J., et al. (2021). Moderate levels of 5-fluorocytosine cause the emergence of high frequency resistance in cryptococci. Nat. Commun. 12, 3418. doi: 10.1038/s41467-021-23745-1
Chayakulkeeree, M. and Perfect, J. R. (2006). Cryptococcosis. Infect. Dis. Clin. North Am. 20, 507–544, v-vi. doi: 10.1016/j.idc.2006.07.001
Chen, L., Qu, S., Yang, K., Liu, M., Li, Y. X., Keller, N. P., et al. (2020). Perillaldehyde: A promising antifungal agent to treat oropharyngeal candidiasis. Biochem. Pharmacol. 180, 114201. doi: 10.1016/j.bcp.2020.114201
Chen, L., Tian, X., Zhang, L., Wang, W., Hu, P., Ma, Z., et al. (2024). Brain glucose induces tolerance of Cryptococcus neoformans to amphotericin B during meningitis. Nat. Microbiol. 9, 346–358. doi: 10.1038/s41564-023-01561-1
Chen, Y. Z., Zhang, Y. D., Chen, C., Sa, Q. E., Yang, J., Zhang, G. C., et al. (2025). The antifungal activity and mechanism of dehydroabietic acid against alternaria alternata causing poplar leaf spot. J. Fungi (Basel) 11, 265. doi: 10.3390/jof11040265
Chen, Y., Shi, Z. W., Strickland, A. B., and Shi, M. (2022). Cryptococcus neoformans infection in the central nervous system: the battle between host and pathogen. J. Fungi (Basel) 8, 1069. doi: 10.3390/jof8101069
Chen, S. C., Slavin, M. A., and Sorrell, T. C. (2011). Echinocandin antifungal drugs in fungal infections: a comparison. Drugs 71, 11–41. doi: 10.2165/11585270-000000000-00000
Cheng, T., Xu, C., Wu, D., Yan, G., Wang, C., Wang, T., et al. (2023). Sodium houttuyfonate derived from Houttuynia cordata Thunb improves intestinal malfunction via maintaining gut microflora stability in Candida albicans overgrowth aggravated ulcerative colitis. Food Funct. 14, 1072–1086. doi: 10.1039/d2fo02369e
Choi, G. and Bessman, N. J. (2025). Iron at the crossroads of host–microbiome interactions in health and disease. Nat. Microbiol. 10, 1282–1293. doi: 10.1038/s41564-025-02001-y
Choi, H., Lee, J., Chang, Y. S., Woo, E. R., and Lee, D. G. (2013). Isolation of (-)-olivil-9'-O-β-d-glucopyranoside from Sambucus williamsii and its antifungal effects with membrane-disruptive action. Biochim. Biophys. Acta 1828, 2002–2006. doi: 10.1016/j.bbamem.2013.04.023
Chowdhary, A., Jain, K., and Chauhan, N. (2023). Candida auris genetics and emergence. Annu. Rev. Microbiol. 77, 583–602. doi: 10.1146/annurev-micro-032521-015858
Chowdhary, A. and Meis, J. F. (2018). Emergence of azole resistant Aspergillus fumigatus and One Health: time to implement environmental stewardship. Environ. Microbiol. 20, 1299–1301. doi: 10.1111/1462-2920.14055
Chung, J. Y., Hong, Y. K., Jeon, E., Yang, S., Park, A., Weissleder, R., et al. (2025). Effective treatment of systemic candidiasis by synergistic targeting of cell wall synthesis. Nat. Commun. 16, 5532. doi: 10.1038/s41467-025-60684-7
Clancy, C. J. and Nguyen, M. H. (2016). Emergence of candida auris: an international call to arms. Clin. Infect. Dis. 64, 141–143. doi: 10.1093/cid/ciw696
Clemons, K. V., Danielson, M. E., Michel, K. S., Liu, M., Ottoson, N. C., Leonardo, S. M., et al. (2014a). Whole glucan particles as a vaccine against murine aspergillosis. J. Med. Microbiol. 63, 1750–1759. doi: 10.1099/jmm.0.079681-0
Clemons, K. V., Martinez, M., Chen, V., Liu, M., Yoon, H. J., Stevens, D. A., et al. (2014b). Protection against experimental aspergillosis by heat-killed yeast is not antibody dependent. Med. Mycol 52, 422–426. doi: 10.1093/mmy/myt015
Clemons, K. V., Antonysamy, M. A., Danielson, M. E., Michel, K. S., Martinez, M., Chen, V., et al. (2015). Whole glucan particles as a vaccine against systemic coccidioidomycosis. J. Med. Microbiol. 64, 1237–1243. doi: 10.1099/jmm.0.000138
Cordeiro, R. A., Evangelista, A. J. J., Serpa, R., Marques, F. J. F., Melo, C. V. S., Oliveira, J. S., et al. (2016). Inhibition of heat-shock protein 90 enhances the susceptibility to antifungals and reduces the virulence of Cryptococcus neoformans/Cryptococcus gattii species complex. Microbiol. (Reading) 162, 309–317. doi: 10.1099/mic.0.000222
Cowen, L. E., Singh, S. D., Köhler, J. R., Collins, C., Zaas, A. K., Schell, W. A., et al. (2009). Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl. Acad. Sci. U.S.A. 106, 2818–2823. doi: 10.1073/pnas.0813394106
Cowen, L. E. and Lindquist, S. (2005). Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309, 2185–2189. doi: 10.1126/science.1118370
Cutler, J. E., Deepe, G. S., Jr., and Klein, B. S. (2007). Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev. Microbiol. 5, 13–28. doi: 10.1038/nrmicro1537
Dan, J. M. and Levitz, S. M. (2006). Prospects for development of vaccines against fungal diseases. Drug Resist. Update 9, 105–110. doi: 10.1016/j.drup.2006.05.004
Daneshnia, F., de Almeida Júnior, J. N., Ilkit, M., Lombardi, L., Perry, A. M., Gao, M., et al. (2023). Worldwide emergence of fluconazole-resistant Candida parapsilosis: current framework and future research roadmap. Lancet Microbe 4, e470–e480. doi: 10.1016/s2666-5247(23)00067-8
Darvishi, E., Omidi, M., Bushehri, A. A., Golshani, A., and Smith, M. L. (2013). The antifungal eugenol perturbs dual aromatic and branched-chain amino acid permeases in the cytoplasmic membrane of yeast. PloS One 8, e76028. doi: 10.1371/journal.pone.0076028
Das, P., Sercu, T., Wadhawan, K., Padhi, I., Gehrmann, S., Cipcigan, F., et al. (2021). Accelerated antimicrobial discovery via deep generative models and molecular dynamics simulations. Nat. BioMed. Eng. 5, 613–623. doi: 10.1038/s41551-021-00689-x
Das, S., Singh, S., Tawde, Y., Dutta, T. K., Rudramurthy, S. M., Kaur, H., et al. (2024). Comparative fitness trade-offs associated with azole resistance in Candida auris clinical isolates. Heliyon 10, e32386. doi: 10.1016/j.heliyon.2024.e32386
da Silva, C. R., de Andrade Neto, J. B., Sidrim, J. J., Angelo, M. R., Magalhães, H. I., Cavalcanti, B. C., et al. (2013). Synergistic effects of amiodarone and fluconazole on Candida tropicalis resistant to fluconazole. Antimicrobial. Agents chemother. 57, 1691–1700. doi: 10.1128/aac.00966-12
da Silva, C. M., de Lima Neto, R. G., de Carvalho, A. M. R., Macêdo, D. P. C., de Azevedo Melo, A. S., Neves, R. P., et al. (2024). Taxonomy of Candida parapsilosis complex isolated from neonates and the role of Hsp90 inhibitors to enhanced the antifungal activity of micafungin. Lett. Appl. Microbiol. 77, ovae044. doi: 10.1093/lambio/ovae044
Day, J. N., et al. (2013). Combination antifungal therapy for cryptococcal meningitis. N Engl. J. Med. 368, 1291–1302. doi: 10.1056/NEJMoa1110404
Day, A. M., McNiff, M. M., da Silva Dantas, A., Gow, N. A. R., and Quinn, J. (2018). Hog1 regulates stress tolerance and virulence in the emerging fungal pathogen candida auris. mSphere 3, e00506–18. doi: 10.1128/mSphere.00506-18
Deane, C. (2023). Fungal FKS in focus. Nat. Chem. Biol. 19, 536–536. doi: 10.1038/s41589-023-01332-3
De Bernardis, F., Amacker, M., Arancia, S., Sandini, S., Gremion, C., Zurbriggen, R., et al. (2012). A virosomal vaccine against candidal vaginitis: immunogenicity, efficacy and safety profile in animal models. Vaccine 30, 4490–4498. doi: 10.1016/j.vaccine.2012.04.069
De Bernardis, F., Graziani, S., Tirelli, F., and Antonopoulou, S. (2018). Candida vaginitis: virulence, host response and vaccine prospects. Med. Mycol 56, 26–31. doi: 10.1093/mmy/myx139
De Brucker, K., Bink, A., Meert, E., Cammue, B. P., and Thevissen, K. (2013). Potentiation of antibiofilm activity of amphotericin B by superoxide dismutase inhibition. Oxid. Med. Cell Longev 2013, 704654. doi: 10.1155/2013/704654
Dell'Olmo, E., Gaglione, R., Cesaro, A., Cafaro, V., Teertstra, W. R., de Cock, H., et al. (2021). Host defense peptides identified in human apolipoprotein B as promising antifungal agents. Appl. Microbiol. Biotechnol. 105, 1953–1964. doi: 10.1007/s00253-021-11114-3
Demonchy, J., Biard, L., Clere-Jehl, R., Wallet, F., Mokart, D., Moreau, A. S., et al. (2024). Multicenter retrospective study of invasive fusariosis in intensive care units, France. Emerg. Infect. Dis. 30, 215–224. doi: 10.3201/eid3002.231221
de Moraes, D. C. and Ferreira-Pereira, A. (2024). Multidrug-resistant fungi. J. Fungi (Basel) 10, 686. doi: 10.3390/jof10100686
Denning, D. W. (2003). Echinocandin antifungal drugs. Lancet 362, 1142–1151. doi: 10.1016/s0140-6736(03)14472-8
Denning, D. W. (2024). Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 24, e428–e438. doi: 10.1016/s1473-3099(23)00692-8
Deray, G. (2002). Amphotericin B nephrotoxicity. J. Antimicrob. Chemother. 49 Suppl 1, 37–41. doi: 10.1093/jac/49.suppl_1.37
De Smet, R., Demoor, T., Verschuere, S., Dullaers, M., Ostroff, G. R., Leclercq, G., et al. (2013). β-Glucan microparticles are good candidates for mucosal antigen delivery in oral vaccination. J. Control Release 172, 671–678. doi: 10.1016/j.jconrel.2013.09.007
Després, P. C., Cisneros, A. F., Alexander, E. M. M., Sonigara, R., Gagné-Thivierge, C., Dubé, A. K., et al. (2022). Asymmetrical dose responses shape the evolutionary trade-off between antifungal resistance and nutrient use. Nat. Ecol. Evol. 6, 1501–1515. doi: 10.1038/s41559-022-01846-4
Diehl, C., Pinzan, C. F., de Castro, P. A., Delbaje, E., García Carnero, L. C., Sánchez-León, E., et al. (2024). Brilacidin, a novel antifungal agent against Cryptococcus neoformans. mBio 15, e0103124. doi: 10.1128/mbio.01031-24
Dietl, A. M., Misslinger, M., Aguiar, M. M., Ivashov, V., Teis, D., Pfister, J., et al. (2019). The siderophore transporter sit1 determines susceptibility to the antifungal VL-2397. Antimicrob. Agents Chemother. 63, e00807–19. doi: 10.1128/aac.00807-19
Dignani, M. C. and Anaissie, E. (2004). Human fusariosis. Clin. Microbiol. Infect. 10 Suppl 1, 67–75. doi: 10.1111/j.1470-9465.2004.00845.x
Dita M, B. M., Heck, D., Mizubuti, E. S. G., and Staver, C. P. (2018). Fusarium wilt of banana: current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01468
Dos Reis, T. F., Diehl, C., Pinzan, C. F., de Castro, P. A., and Goldman, G. H. (2024). Brilacidin, a host defense peptide mimetic, potentiates ibrexafungerp antifungal activity against the human pathogenic fungus Aspergillus fumigatus. Microbiol. Spectr. 12, e0088824. doi: 10.1128/spectrum.00888-24
Dos Reis, T. F., de Castro, P. A., Bastos, R. W., Pinzan, C. F., Souza, P. F. N., Ackloo, S., et al. (2023). A host defense peptide mimetic, brilacidin, potentiates caspofungin antifungal activity against human pathogenic fungi. Nat. Commun. 14, 2052. doi: 10.1038/s41467-023-37573-y
Drees KP, L. J., Puechmaille, S. J., Parise, K. L., Wibbelt, G., Hoyt, J. R., Sun, K., et al. (2017). Phylogenetics of a fungal invasion: origins and widespread dispersal of white-nose syndrome. mBio 8, e01941-01917. doi: 10.1128/mBio.01941-17
Dromer, F. and Charreire, J. (1991). Improved amphotericin B activity by a monoclonal anti-Cryptococcus neoformans antibody: study during murine cryptococcosis and mechanisms of action. J. Infect. Dis. 163, 1114–1120. doi: 10.1093/infdis/163.5.1114
Dromer, F., Charreire, J., Contrepois, A., Carbon, C., and Yeni, P. (1987). Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect. Immun. 55, 749–752. doi: 10.1128/iai.55.3.749-752.1987
Dunne, K., Hagen, F., Pomeroy, N., Meis, J. F., and Rogers, T. R. (2017). Intercountry transfer of triazole-resistant aspergillus fumigatus on plant bulbs. Clin. Infect. Dis. 65, 147–149. doi: 10.1093/cid/cix257
Du Toit, A. (2023). Sticky candida auris. Nat. Rev. Microbiol. 21, 770–770. doi: 10.1038/s41579-023-00986-z
Edlind, T. and Katiyar, S. (2022). Intrinsically high resistance of candida glabrata to hydrogen peroxide and its reversal in a fluconazole-resistant mutant. Antimicrob. Agents Chemother. 66, e0072122. doi: 10.1128/aac.00721-22
Edris, A. E. and Farrag, E. S. (2003). Antifungal activity of peppermint and sweet basil essential oils and their major aroma constituents on some plant pathogenic fungi from the vapor phase. Nahrung 47, 117–121. doi: 10.1002/food.200390021
Edwards, J. E., Schwartz, M. M., Schmidt, C. S., Sobel, J. D., Nyirjesy, P., Schodel, F., et al. (2018). A fungal immunotherapeutic vaccine (NDV-3A) for treatment of recurrent vulvovaginal candidiasis-A phase 2 randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 66, 1928–1936. doi: 10.1093/cid/ciy185
Egger, M., Bellmann, R., Krause, R., Boyer, J., Jakšić, D., Hoenigl, M., et al. (2023). Salvage treatment for invasive aspergillosis and mucormycosis: challenges, recommendations and future considerations. Infect. Drug Resist. 16, 2167–2178. doi: 10.2147/idr.S372546
El Ayoubi, L. W., Allaw, F., Moussa, E., and Kanj, S. S. (2024). Ibrexafungerp: A narrative overview. Curr. Res. Microb. Sci. 6, 100245. doi: 10.1016/j.crmicr.2024.100245
ElFeky, D. S., El-Wakil, D. M., Mwafy, M. M., Atia, M. M. A., and Gohar, N. M. (2025). Comparative evaluation of antifungal susceptibility testing methods of invasive Candida species and detection of FKS genes mutations in caspofungin intermediate and resistant isolates. BMC Infect. Dis. 25, 114. doi: 10.1186/s12879-024-10435-8
Elfmann, C. and Stülke, J. (2025). Cutting-edge tools for structural biology: bringing AlphaFold to the people. Trends Microbiol. 33, 819–822. doi: 10.1016/j.tim.2025.03.014
Engle, K. and Kumar, G. (2024). Tackling multi-drug resistant fungi by efflux pump inhibitors. Biochem. Pharmacol. 226, 116400. doi: 10.1016/j.bcp.2024.116400
Escribano, P., Gómez, A., Reigadas, E., Muñoz, P., and Guinea, J. (2022). In vitro activity of olorofim against Aspergillus fumigatus sensu lato clinical isolates: activity is retained against isolates showing resistance to azoles and/or amphotericin B. Clin. Microbiol. Infect. 28, 1291.e1297–1291.e1210. doi: 10.1016/j.cmi.2022.05.013
FA, A. Y., Abbas, H. A., Ibrahim, T. M., Mansour, B., Awan, Z. A., Al-Rabia, M. W., et al. (2025). Celastrol boosts fluconazole efficacy against vaginal candidiasis: in vitro and in vivo evidence. AMB Express 15, 18. doi: 10.1186/s13568-025-01824-6
Fan, X., Chen, L., Chen, M., Zhang, N., Chang, H., He, M., et al. (2024). Pan-omics-based characterization and prediction of highly multidrug-adapted strains from an outbreak fungal species complex. Innovation (Camb) 5, 100681. doi: 10.1016/j.xinn.2024.100681
Farha, M. A. and Brown, E. D. (2019). Drug repurposing for antimicrobial discovery. Nat. Microbiol. 4, 565–577. doi: 10.1038/s41564-019-0357-1
Faria, N. C., Kim, J. H., Gonçalves, L. A., Martins Mde, L., Chan, K. L., Campbell, B. C., et al. (2011). Enhanced activity of antifungal drugs using natural phenolics against yeast strains of Candida and Cryptococcus. Lett. Appl. Microbiol. 52, 506–513. doi: 10.1111/j.1472-765X.2011.03032.x
Feldmesser, M., Mukherjee, J., and Casadevall, A. (1996). Combination of 5-flucytosine and capsule-binding monoclonal antibody in the treatment of murine Cryptococcus neoformans infections and in vitro. J. Antimicrob. Chemother. 37, 617–622. doi: 10.1093/jac/37.3.617
Ferdinand, A. S., Coppo, M. J. C., Howden, B. P., and Browning, G. F. (2023). Tackling antimicrobial resistance by integrating One Health and the Sustainable Development Goals. BMC Global Public Health 1, 11. doi: 10.1186/s44263-023-00003-8
Fernandes, K. E. and Carter, D. A. (2017). The antifungal activity of lactoferrin and its derived peptides: mechanisms of action and synergy with drugs against fungal pathogens. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00002
Fernandes, K. E., Weeks, K., and Carter, D. A. (2020). Lactoferrin is broadly active against yeasts and highly synergistic with amphotericin B. Antimicrob. Agents Chemother. 64, e02284–19. doi: 10.1128/aac.02284-19
Fernandes, K. E. and Carter, D. A. (2017). Activity of Amphotericin B and Anidulafungin Combined with Rifampicin, Clarithromycin, Ethylenediaminetetraacetic Acid, N-Acetylcysteine, and Farnesol against Candida tropicalis Biofilms. J. fungi (Basel Switzerland) 3, 16. doi: 10.3390/jof3010016
Feuss, A., Bougnoux, M. E., and Dannaoui, E. (2024). The role of olorofim in the treatment of filamentous fungal infections: A review of in vitro and in vivo studies. J. Fungi (Basel) 10, 345. doi: 10.3390/jof10050345
Fisher, M. C. and Denning, D. W. (2023). The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 21, 211–212. doi: 10.1038/s41579-023-00861-x
Fisher, B. T., Zaoutis, T., Dvorak, C. C., Nieder, M., Zerr, D., Wingard, J. R., et al. (2019). Effect of caspofungin vs fluconazole prophylaxis on invasive fungal disease among children and young adults with acute myeloid leukemia: A randomized clinical trial. Jama 322, 1673–1681. doi: 10.1001/jama.2019.15702
Fisher, M. C., Alastruey-Izquierdo, A., Berman, J., Bicanic, T., Bignell, E. M., Bowyer, P., et al. (2022). Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 20, 557–571. doi: 10.1038/s41579-022-00720-1
Fisher, M. C., Hawkins, N. J., Sanglard, D., and Gurr, S. J. (2018). Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360, 739–742. doi: 10.1126/science.aap7999
Fothergill, A. W., Sutton, D. A., McCarthy, D. I., and Wiederhold, N. P. (2014). Impact of new antifungal breakpoints on antifungal resistance in Candida species. J. Clin. Microbiol. 52, 994–997. doi: 10.1128/jcm.03044-13
Fu, C., Zhang, X., Veri, A. O., Iyer, K. R., Lash, E., Xue, A., et al. (2021). Leveraging machine learning essentiality predictions and chemogenomic interactions to identify antifungal targets. Nat. Commun. 12, 6497. doi: 10.1038/s41467-021-26850-3
Fung, S. and Shirley, M. (2025). Rezafungin: A review in invasive candidiasis. Drugs 85, 415–423. doi: 10.1007/s40265-024-02134-0
Gao, A., Kouznetsova, V. L., and Tsigelny, I. F. (2022). Machine-learning-based virtual screening to repurpose drugs for treatment of Candida albicans infection. Mycoses 65, 794–805. doi: 10.1111/myc.13475
Gao, L., Sun, Y., Yuan, M., Li, M., and Zeng, T. (2020). In vitro and in vivo study on the synergistic effect of minocycline and azoles against pathogenic fungi. Antimicrobial. Agents chemother. 64, e00290–20. doi: 10.1128/aac.00290-20
Garcia-Rubio, R., Alcazar-Fuoli, L., Monteiro, M. C., Monzon, S., Cuesta, I., Pelaez, T., et al. (2018). Insight into the Significance of Aspergillus fumigatus cyp51A Polymorphisms. Antimicrob. Agents Chemother. 62, e00241–18. doi: 10.1128/aac.00241-18
Gebremariam, T., Gu, Y., Alkhazraji, S., Youssef, E., Shaw, K. J., Ibrahim, A. S., et al. (2022). The combination treatment of fosmanogepix and liposomal amphotericin B is superior to monotherapy in treating experimental invasive mold infections. Antimicrob. Agents Chemother. 66, e0038022. doi: 10.1128/aac.00380-22
Giménez-Santamarina, S., Llorens-Molina, J. A., Sempere-Ferre, F., Santamarina, C., Roselló, J., Santamarina, M. P., et al. (2022). Chemical composition of essential oils of three Mentha species and their antifungal activity against selected phytopathogenic and post-harvest fungi. All Life 15, 64–73. doi: 10.1080/26895293.2021.2022007
Gintjee, T. J., Donnelley, M. A., and Thompson, G. R., 3rd (2020). Aspiring antifungals: review of current antifungal pipeline developments. J. Fungi (Basel) 6, 28. doi: 10.3390/jof6010028
Goje, O., Sobel, R., Nyirjesy, P., Goldstein, S. R., Spitzer, M., Faught, B., et al. (2023). Oral ibrexafungerp for vulvovaginal candidiasis treatment: an analysis of VANISH 303 and VANISH 306. J. Womens Health (Larchmt) 32, 178–186. doi: 10.1089/jwh.2022.0132
Gordon, M. A. and Lapa, E. (1964). Serum protein enhancement of antibiotic therapy in cryptococcosis. J. Infect. Dis. 114, 373–377. doi: 10.1093/infdis/114.4.373
Gowri, M., Jayashree, B., Jeyakanthan, J., and Girija, E. K. (2020). Sertraline as a promising antifungal agent: inhibition of growth and biofilm of Candida auris with special focus on the mechanism of action in vitro. J. Appl. Microbiol. 128, 426–437. doi: 10.1111/jam.14490
Gray, K. C., Palacios, D. S., Dailey, I., Endo, M. M., Uno, B. E., Wilcock, B. C., et al. (2012). Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. U.S.A. 109, 2234–2239. doi: 10.1073/pnas.1117280109
Graybill, J. R., Hague, M., and Drutz, D. J. (1981). Passive immunization in murine cryptococcosis. Sabouraudia 19, 237–244. doi: 10.1080/00362178185380411
Grogan LF, R. J., Berger, L., Skerratt, L. F., Scheele, B. C., Castley, J. G., Newell, D. A., et al. (2018). Review of the amphibian immune response to chytridiomycosis, and future directions. Front. Immunol. 9. doi: 10.3389/fimmu.2018.02536
Gu, Y., Gebremariam, T., Alkhazraji, S., Youssef, E., El-Gamal, S., Matkovits, T., et al. (2024). Efficacy of an oral lipid nanocrystal formulation of amphotericin B (MAT2203) in the neutropenic mouse model of pulmonary mucormycosis. Antimicrob. Agents Chemother. 68, e0154023. doi: 10.1128/aac.01540-23
Gu, J., Luo, Y., Liang, M., Fan, Y., Zhang, X., Ji, G., et al. (2025). A novel framework for industrial pesticide effluent assessment: Integrating chemical screening, multi-endpoint responses and literature-based validation. J. Hazard Mater 490, 137830. doi: 10.1016/j.jhazmat.2025.137830
Gu, W., Guo, D., Zhang, L., Xu, D., and Sun, S. (2016). The Synergistic Effect of Azoles and Fluoxetine against Resistant Candida albicans Strains Is Attributed to Attenuating Fungal Virulence. Antimicrobial. Agents chemother. 60, 6179–6188. doi: 10.1128/aac.03046-15
Guo, Y., Xu, M., Zhang, J., Ma, Z., Cui, J., Zhao, L., et al. (2025). Refined regulation of polysaccharide biosynthesis in edible and medicinal fungi: From pathways to production. Carbohydr Polym 358, 123560. doi: 10.1016/j.carbpol.2025.123560
Guo, X. L., Leng, P., Yang, Y., Yu, L. G., Lou, H. X., and Plagiochin, E. (2008). a botanic-derived phenolic compound, reverses fungal resistance to fluconazole relating to the efflux pump. J. Appl. Microbiol. 104, 831–838. doi: 10.1111/j.1365-2672.2007.03617.x
Hackstein, H. and Thomson, A. W. (2004). Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat. Rev. Immunol. 4, 24–34. doi: 10.1038/nri1256
Han, X. Y., Zhong, Y. F., Li, S. B., Liang, G. C., Zhou, G., Wang, X. K., et al. (2016). Synthesis, characterization and antifungal evaluation of novel thiochromanone derivatives containing indole skeleton. Chem. Pharm. Bull. (Tokyo) 64, 1411–1416. doi: 10.1248/cpb.c16-00366
Han, X., Zhu, X., Hong, Z., Wei, L., Ren, Y., Wan, F., et al. (2017). Structure-based rational design of novel inhibitors against fructose-1,6-bisphosphate aldolase from candida albicans. J. Chem. Inf Model. 57, 1426–1438. doi: 10.1021/acs.jcim.6b00763
Haq, M. F., Pearlmutter, B. S., Cadnum, J. L., and Donskey, C. J. (2024). Efficacy of 23 commonly used liquid disinfectants against Candida auris isolates from the 4 major clades. Infect. Control Hosp Epidemiol. 45, 127–131. doi: 10.1017/ice.2023.157
Harrison, M. C., Rinker, D. C., LaBella, A. L., Opulente, D. A., Wolters, J. F., Zhou, X., et al. (2025). Machine learning identifies novel signatures of antifungal drug resistance in Saccharomycotina yeasts. bioRxiv. doi: 10.1101/2025.05.09.653161
Hawksworth, D. L. and Lücking, R. (2017). Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 5. doi: 10.1128/microbiolspec.FUNK-0052-2016
Helleberg, M., Jørgensen, K. M., Hare, R. K., Datcu, R., Chowdhary, A., Arendrup, M. C., et al. (2020). Rezafungin In Vitro Activity against Contemporary Nordic Clinical Candida Isolates and Candida auris Determined by the EUCAST Reference Method. Antimicrob. Agents Chemother. 64, e02438–19. doi: 10.1128/aac.02438-19
Hodges, M. R., Ople, E., Wedel, P., Shaw, K. J., Jakate, A., Kramer, W. G., et al. (2023). Safety and pharmacokinetics of intravenous and oral fosmanogepix, a first-in-class antifungal agent, in healthy volunteers. Antimicrob. Agents Chemother. 67, e0162322. doi: 10.1128/aac.01623-22
Hodges, M. R., Ople, E., Evans, P., Pantophlet, A. J., Richardson, J., Williams, D., et al. (2024). A phase 1 open label study to assess the human mass balance and metabolite profile of (14)C-fosmanogepix, a novel Gwt-1 inhibitor in healthy male participants. Antimicrob. Agents Chemother. 68, e0027324. doi: 10.1128/aac.00273-24
Hoenigl, M., Sprute, R., Egger, M., Arastehfar, A., Cornely, O. A., Krause, R., et al. (2021). The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 81, 1703–1729. doi: 10.1007/s40265-021-01611-0
Hofer, U. (2019). Candida auris’ potential link to climate change. Nat. Rev. Microbiol. 17, 588–588. doi: 10.1038/s41579-019-0254-x
Holbrook, S. Y. L., Garzan, A., Dennis, E. K., Shrestha, S. K., and Garneau-Tsodikova, S. (2017). Repurposing antipsychotic drugs into antifungal agents: Synergistic combinations of azoles and bromperidol derivatives in the treatment of various fungal infections. Eur. J. medicinal Chem. 139, 12–21. doi: 10.1016/j.ejmech.2017.07.030
Homa, M., Hegedűs, K., Fülöp, Á., Wolfárt, V., Kadaikunnan, S., Khaled, J. M., et al. (2017). In vitro activity of calcium channel blockers in combination with conventional antifungal agents against clinically important filamentous fungi. Acta biologica Hungarica 68, 334–344. doi: 10.1556/018.68.2017.3.10
Hope, W. W., Tabernero, L., Denning, D. W., and Anderson, M. J. (2004). Molecular mechanisms of primary resistance to flucytosine in Candida albicans. Antimicrob. Agents Chemother. 48, 4377–4386. doi: 10.1128/aac.48.11.4377-4386.2004
Hou, X., Healey, K. R., Shor, E., Kordalewska, M., Ortigosa, C. J., Paderu, P., et al. (2019). Novel FKS1 and FKS2 modifications in a high-level echinocandin resistant clinical isolate of Candida glabrata. Emerg. Microbes Infect. 8, 1619–1625. doi: 10.1080/22221751.2019.1684209
Howard, S. J., Cerar, D., Anderson, M. J., Albarrag, A., Fisher, M. C., Pasqualotto, A. C., et al. (2009). Frequency and evolution of Azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15, 1068–1076. doi: 10.3201/eid1507.090043
Hoy, S. M. (2022). Oteseconazole: first approval. Drugs 82, 1017–1023. doi: 10.1007/s40265-022-01734-y
Hu, X., Yang, P., Chai, C., Liu, J., Sun, H., Wu, Y., et al. (2023). Structural and mechanistic insights into fungal β-1,3-glucan synthase FKS1. Nature 616, 190–198. doi: 10.1038/s41586-023-05856-5
Huang, Z., Srinivasan, S., Zhang, J., Chen, K., Li, Y., Li, W., et al. (2012). Discovering thiamine transporters as targets of chloroquine using a novel functional genomics strategy. PloS Genet. 8, e1003083. doi: 10.1371/journal.pgen.1003083
Huang, X., Yi, Y., Yong, J., Sun, J., Song, Z., Li, D., et al. (2021). Inhibitory effect of berberine hydrochloride against Candida albicans and the role of the HOG-MAPK pathway. J. Antibiot. (Tokyo) 74, 807–816. doi: 10.1038/s41429-021-00463-w
Huang, J., Hu, P., Ye, L., Shen, Z., Chen, X., Liu, F., et al. (2024). Pan-drug resistance and hypervirulence in a human fungal pathogen are enabled by mutagenesis induced by mammalian body temperature. Nat. Microbiol. 9, 1686–1699. doi: 10.1038/s41564-024-01720-ys
Huang, H., Ostroff, G. R., Lee, C. K., Specht, C. A., and Levitz, S. M. (2010). Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. mBio 1, e00164–10. doi: 10.1128/mBio.00164-10
Hudz, N., Kobylinska, L., Pokajewicz, K., Horčinová Sedláčková, V., Fedin, R., Voloshyn, M., et al. (2023). Mentha piperita: essential oil and extracts, their biological activities, and perspectives on the development of new medicinal and cosmetic products. Molecules 28, 7444. doi: 10.3390/molecules28217444
Hwang, B., Cho, J., Hwang, I. S., Jin, H. G., Woo, E. R., Lee, D. G., et al. (2011). Antifungal activity of lariciresinol derived from Sambucus williamsii and their membrane-active mechanisms in Candida albicans. Biochem. Biophys. Res. Commun. 410, 489–493. doi: 10.1016/j.bbrc.2011.06.004
Hwang, J. H., Hwang, I. S., Liu, Q. H., Woo, E. R., and Lee, D. G. (2012). (+)-Medioresinol leads to intracellular ROS accumulation and mitochondria-mediated apoptotic cell death in Candida albicans. Biochimie 94, 1784–1793. doi: 10.1016/j.biochi.2012.04.010
Hwang, B., Lee, J., Liu, Q. H., Woo, E. R., and Lee, D. G. (2010). Antifungal effect of (+)-pinoresinol isolated from Sambucus williamsii. Molecules 15, 3507–3516. doi: 10.3390/molecules15053507
Iliev, I. D., Brown, G. D., Bacher, P., Gaffen, S. L., Heitman, J., Klein, B. S., et al. (2024). Focus on fungi. Cell 187, 5121–5127. doi: 10.1016/j.cell.2024.08.016
Insights, G. G. (2025). Anti-fungal drugs market. Available online at: https://www.globalgrowthinsights.com/zh/market-reports/anti-fungal-drugs-market-108784 (Accessed August 11, 2025).
Ito, K., Kizawa, Y., Kimura, G., Nishimoto, Y., Daly, L., Knowles, I., et al. (2021). Relationship between anti-fungal effects and lung exposure of PC945, a novel inhaled antifungal agent, in Aspergillus fumigatus infected mice: Pulmonary PK-PD analysis of anti-fungal PC945. Eur. J. Pharm. Sci. 163, 105878. doi: 10.1016/j.ejps.2021.105878
Iyer, K. R., Revie, N. M., Fu, C., Robbins, N., and Cowen, L. E. (2021). Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat. Rev. Microbiol. 19, 454–466. doi: 10.1038/s41579-021-00511-0
Jackson, B. R., Chow, N., Forsberg, K., Litvintseva, A. P., Lockhart, S. R., Welsh, R., et al. (2019). On the origins of a species: what might explain the rise of candida auris? J. Fungi (Basel) 5, 58. doi: 10.3390/jof5030058
Jackson, K. E., Pogula, P. K., and Patterson, S. E. (2013). Polyoxin and nikkomycin analogs: recent design and synthesis of novel peptidyl nucleosides. Heterocyclic Commun. 19, 375–386. doi: 10.1515/hc-2013-0141
Jacques, S. L., Mirza, I. A., Ejim, L., Koteva, K., Hughes, D. W., Green, K., et al. (2003). Enzyme-assisted suicide: molecular basis for the antifungal activity of 5-hydroxy-4-oxonorvaline by potent inhibition of homoserine dehydrogenase. Chem. Biol. 10, 989–995. doi: 10.1016/j.chembiol.2003.09.015
Jahanshiri, Z., Shams-Ghahfarokhi, M., Asghari-Paskiabi, F., Saghiri, R., and Razzaghi-Abyaneh, M. (2017). α-Bisabolol inhibits Aspergillus fumigatus Af239 growth via affecting microsomal Δ;(24)-sterol methyltransferase as a crucial enzyme in ergosterol biosynthesis pathway. World J. Microbiol. Biotechnol. 33, 55. doi: 10.1007/s11274-017-2214-9
Jandú, J. J., Costa, M. C., Santos, J. R. A., Andrade, F. M., Magalhães, T. F., Silva, M. V., et al. (2017). Treatment with pCramoll Alone and in Combination with Fluconazole Provides Therapeutic Benefits in C. gattii Infected Mice. Front. Cell Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00211
Jarvis, J. N., Lawrence, D. S., Meya, D. B., Kagimu, E., Kasibante, J., Mpoza, E., et al. (2022). Single-dose liposomal amphotericin B treatment for cryptococcal meningitis. N Engl. J. Med. 386, 1109–1120. doi: 10.1056/NEJMoa2111904
Jenks, J. D., Cornely, O. A., Chen, S. C., Thompson, G. R., 3rd, and Hoenigl, M. (2020). Breakthrough invasive fungal infections: Who is at risk? Mycoses 63, 1021–1032. doi: 10.1111/myc.13148
Jensen, R. H., Astvad, K. M., Silva, L. V., Sanglard, D., Jørgensen, R., Nielsen, K. F., et al. (2015). Stepwise emergence of azole, echinocandin and amphotericin B multidrug resistance in vivo in Candida albicans orchestrated by multiple genetic alterations. J. Antimicrob. Chemother. 70, 2551–2555. doi: 10.1093/jac/dkv140
Jiang, B. C., Shen, J. Y., Wu, J., Lu, R. Y., Zheng, W., Dong, J. X., et al. (2020). In vitro and in vivo evaluation of the antifungal activity of fluoxetine combined with antifungals against Candida albicans biofilms and oral candidiasis. Biofouling 36, 537–548. doi: 10.1080/08927014.2020.1777401
Jiang, L., Zheng, L., Sun, K. A., Zhou, P., Xu, R., Gu, J., et al. (2020). In vitro antifungal activity of 163 extracts from traditional Chinese medicine herbs. Eur. J. Integr. Med. 39, 101213. doi: 10.1016/j.eujim.2020.101213
Jin, J., Guo, N., Zhang, J., Ding, Y., Tang, X., Liang, J., et al. (2010). The synergy of honokiol and fluconazole against clinical isolates of azole-resistant Candida albicans. Lett. Appl. Microbiol. 51, 351–357. doi: 10.1111/j.1472-765X.2010.02900.x
Johnson, M. D. and Perfect, J. R. (2007). Combination antifungal therapy: what can and should we expect? Bone Marrow Transplant. 40, 297–306. doi: 10.1038/sj.bmt.1705687
Joshi, T., Pundir, H., and Chandra, S. (2022). Deep-learning based repurposing of FDA-approved drugs against Candida albicans dihydrofolate reductase and molecular dynamics study. J. Biomol. Struct. Dyn 40, 8420–8436. doi: 10.1080/07391102.2021.1911851
Joste, V., Delouis, M., Mouhajir, A., Gera Denis-Petit, S., Moënne-Locoz, P., Kernéis, S., et al. (2025). Genomic investigation of an antifungal-resistant Aspergillus fumigatus outbreak in a French hospital. Med. Mycol 63. doi: 10.1093/mmy/myaf012
Jović, O. and Šmuc, T. (2020). Combined machine learning and molecular modelling workflow for the recognition of potentially novel fungicides. Molecules 25, 2198. doi: 10.3390/molecules25092198
Kagan, S., Ickowicz, D., Shmuel, M., Altschuler, Y., Sionov, E., Pitusi, M., et al. (2012). Toxicity mechanisms of amphotericin B and its neutralization by conjugation with arabinogalactan. Antimicrob. Agents Chemother. 56, 5603–5611. doi: 10.1128/aac.00612-12
Kapoor, M., Moloney, M., Soltow, Q. A., Pillar, C. M., and Shaw, K. J. (2019). Evaluation of resistance development to the gwt1 inhibitor manogepix (APX001A) in candida species. Antimicrob. Agents Chemother. 64, e01387–19. doi: 10.1128/aac.01387-19
Kaur, J. and Nobile, C. J. (2023). Antifungal drug-resistance mechanisms in Candida biofilms. Curr. Opin. Microbiol. 71, 102237. doi: 10.1016/j.mib.2022.102237
Kavousi, K., Bagheri, M., Behrouzi, S., Vafadar, S., Atanaki, F. F., Lotfabadi, B. T., et al. (2020). IAMPE: NMR-assisted computational prediction of antimicrobial peptides. J. Chem. Inf. modeling 60, 4691–4701. doi: 10.1021/acs.jcim.0c00841
Ke, W., Xie, Y., Chen, Y., Ding, H., Ye, L., Qiu, H., et al. (2024). Fungicide-tolerant persister formation during cryptococcal pulmonary infection. Cell Host Microbe 32, 276–289.e277. doi: 10.1016/j.chom.2023.12.012
Keçeli, S. A., Willke, A., Tamer, G. S., Boral, O. B., Sonmez, N., Cağatay, P., et al. (2014). Interaction between caspofungin or voriconazole and cefoperazone-sulbactam or piperacillin-tazobactam by in vitro and in vivo methods. APMIS: Acta pathologica microbiologica immunologica Scandinavica 122, 412–417. doi: 10.1111/apm.12159
Khan, M. S. and Ahmad, I. (2012). Antibiofilm activity of certain phytocompounds and their synergy with fluconazole against Candida albicans biofilms. J. Antimicrob. Chemother. 67, 618–621. doi: 10.1093/jac/dkr512
Khan, S., Cai, L., Bilal, H., Khan, M. N., Fang, W., Zhang, D., et al. (2025). An 11-Year retrospective analysis of candidiasis epidemiology, risk factors, and antifungal susceptibility in a tertiary care hospital in China. Sci. Rep-Uk 15, 7240. doi: 10.1038/s41598-025-92100-x
Kim, J. H., Campbell, B. C., Mahoney, N., Chan, K. L., and Molyneux, R. J. (2011a). Chemosensitization of aflatoxigenic fungi to antimycin A and strobilurin using salicylaldehyde, a volatile natural compound targeting cellular antioxidation system. Mycopathologia 171, 291–298. doi: 10.1007/s11046-010-9356-8
Kim, J. H., Chan, K. L., Faria, N. C., Martins Mde, L., and Campbell, B. C. (2012a). Targeting the oxidative stress response system of fungi with redox-potent chemosensitizing agents. Front. Microbiol. 3. doi: 10.3389/fmicb.2012.00088
Kim, J. H., Chan, K. L., Mahoney, N., and Campbell, B. C. (2011b). Antifungal activity of redox-active benzaldehydes that target cellular antioxidation. Ann. Clin. Microbiol. Antimicrob. 10, 23. doi: 10.1186/1476-0711-10-23
Kim, J. H., Chan, K. L., Cheng, L. W., Tell, L. A., Byrne, B. A., Clothier, K., et al. (2019). High efficiency drug repurposing design for new antifungal agents. Methods Protoc. 2, 31. doi: 10.3390/mps2020031
Kim, J. H., Faria, N. C., Martins Mde, L., Chan, K. L., and Campbell, B. C. (2012b). Enhancement of antimycotic activity of amphotericin B by targeting the oxidative stress response of Candida and cryptococcus with natural dihydroxybenzaldehydes. Front. Microbiol. 3. doi: 10.3389/fmicb.2012.00261
Kim, J. S., Lee, K. T., and Bahn, Y. S. (2023). Deciphering the regulatory mechanisms of the cAMP/protein kinase A pathway and their roles in the pathogenicity of Candida auris. Microbiol. Spectr. 11, e0215223. doi: 10.1128/spectrum.02152-23
Kontoyiannis, D. P., Marr, K. A., Park, B. J., Alexander, B. D., Anaissie, E. J., Walsh, T. J., et al. (2010). Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 50, 1091–1100. doi: 10.1086/651263
Kottarathil, M., Thayanidhi, P., Sathyamurthy, P., and Jyoti Kindo, A. (2023). Rise of mucormycosis during the COVID-19 pandemic and the challenges faced. Curr. Med. Mycol 9, 44–55. doi: 10.18502/cmm.2023.345032.1400
Kovács, R., Tóth, Z., Locke, J. B., Forgács, L., Kardos, G., Nagy, F., et al. (2021). Comparison of In Vitro Killing Activity of Rezafungin, Anidulafungin, Caspofungin, and Micafungin against Four Candida auris Clades in RPMI-1640 in the Absence and Presence of Human Serum. Microorganisms 9, 863. doi: 10.3390/microorganisms9040863
Kovanda, L. L., Sullivan, S. M., Smith, L. R., Desai, A. V., Bonate, P. L., Hope, W. W., et al. (2019). Population pharmacokinetic modeling of VL-2397, a novel systemic antifungal agent: analysis of a single- and multiple-ascending-dose study in healthy subjects. Antimicrob. Agents Chemother. 63, e00163–19. doi: 10.1128/aac.00163-19
Kow, C. S., Ramachandram, D. S., Hasan, S. S., and Thiruchelvam, K. (2025). Ibrexafungerp for the treatment of vulvovaginal candidiasis: A systematic review and meta-analysis of randomized placebo-controlled trials. J. Mycol Med. 35, 101534. doi: 10.1016/j.mycmed.2025.101534
Kriegl, L., Egger, M., Boyer, J., Hoenigl, M., and Krause, R. (2025). New treatment options for critically important WHO fungal priority pathogens. Clin. Microbiol. Infect. 31, 922–930. doi: 10.1016/j.cmi.2024.03.006
Kronstad, J. W., Attarian, R., Cadieux, B., Choi, J., D'Souza, C. A., Griffiths, E. J., et al. (2011). Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat. Rev. Microbiol. 9, 193–203. doi: 10.1038/nrmicro2522
Kummari, L. K., Butler, M. S., Furlong, E., Blundell, R., Nouwens, A., Silva, A. B., et al. (2018). Antifungal benzo[b]thiophene 1,1-dioxide IMPDH inhibitors exhibit pan-assay interference (PAINS) profiles. Bioorg. Med. Chem. 26, 5408–5419. doi: 10.1016/j.bmc.2018.09.004
Kuplińska, A. and Rząd, K. (2021). Molecular targets for antifungals in amino acid and protein biosynthetic pathways. Amino Acids 53, 961–991. doi: 10.1007/s00726-021-03007-6
Kwon-Chung, K. J., Fraser, J. A., Doering, T. L., Wang, Z., Janbon, G., Idnurm, A., et al. (2014). Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb. Perspect. Med. 4, a019760. doi: 10.1101/cshperspect.a019760
Lakhani, P., Patil, A., and Majumdar, S. (2019). Challenges in the polyene- and azole-based pharmacotherapy of ocular fungal infections. J. Ocul. Pharmacol. Ther. 35, 6–22. doi: 10.1089/jop.2018.0089
Lakshminarayanan, K., Murugan, D., Venkatesan, J., Vasanthakumari Thirumalaiswamy, H., Gadais, C., Rangasamy, L., et al. (2024). Siderophore-conjugated antifungals: A strategy to potentially cure fungal infections. ACS Infect. Dis. 10, 2448–2466. doi: 10.1021/acsinfecdis.4c00046
Lamoth, F. and Alexander, B. D. (2015). Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non-Aspergillus mold isolates. Antimicrob. Agents Chemother. 59, 4308–4311. doi: 10.1128/aac.00234-15
Lamoth, F., Juvvadi, P. R., and Steinbach, W. J. (2016). Heat shock protein 90 (Hsp90): A novel antifungal target against Aspergillus fumigatus. Crit. Rev. Microbiol. 42, 310–321. doi: 10.3109/1040841x.2014.947239
Lange, L. (2014). The importance of fungi and mycology for addressing major global challenges*. Ima Fungus 5, 463–471. doi: 10.5598/imafungus.2014.05.02.10
Larsen, R. A., Pappas, P. G., Perfect, J., Aberg, J. A., Casadevall, A., Cloud, G. A., et al. (2005). Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother. 49, 952–958. doi: 10.1128/aac.49.3.952-958.2005
Lass-Flörl, C., Kanj, S. S., Govender, N. P., Thompson, G. R., Ostrosky- Zeichner, L., Govrins, M. A., et al. (2024). Invasive candidiasis. Nat. Rev. Dis. Primers 10, 20. doi: 10.1038/s41572-024-00503-3
Leal, S. M., Vareechon, C., Cowden, S., Cobb, B. A., Latgé, J. P., Momany, M., et al. (2012). Fungal antioxidant pathways promote survival against neutrophils during infection. J. Clin. Invest. 122, 2482–2498. doi: 10.1172/jci63239
Leal, S. M., Roy, S., Vareechon, C., Carrion, S., Clark, H., Lopez-Berges, M. S., et al. (2013). Targeting iron acquisition blocks infection with the fungal pathogens Aspergillus fumigatus and Fusarium oxysporum. PloS Pathog. 9, e1003436. doi: 10.1371/journal.ppat.1003436
Lee, Y., Puumala, E., Robbins, N., and Cowen, L. E. (2021). Antifungal drug resistance: molecular mechanisms in candida albicans and beyond. Chem. Rev. 121, 3390–3411. doi: 10.1021/acs.chemrev.0c00199
Lee, Y., Robbins, N., and Cowen, L. E. (2023). Molecular mechanisms governing antifungal drug resistance. NPJ Antimicrob. Resist. 1, 5. doi: 10.1038/s44259-023-00007-2
Lei, J., et al. (2025). A multidimensional perspective on Poria cocos, an ancient fungal traditional Chinese medicine. J. Ethnopharmacol. 348, 119869. doi: 10.1016/j.jep.2025.119869
Lemar, K. M., Passa, O., Aon, M. A., Cortassa, S., Müller, C. T., Plummer, S., et al. (2005). Allyl alcohol and garlic (Allium sativum) extract produce oxidative stress in Candida albicans. Microbiol. (Reading) 151, 3257–3265. doi: 10.1099/mic.0.28095-0
Levitz, S. M. (2010). Innate recognition of fungal cell walls. PloS Pathog. 6, e1000758. doi: 10.1371/journal.ppat.1000758
Levitz, S. M. and Golenbock, D. T. (2012). Beyond empiricism: informing vaccine development through innate immunity research. Cell 148, 1284–1292. doi: 10.1016/j.cell.2012.02.012
Levitz, S. M., Huang, H., Ostroff, G. R., and Specht, C. A. (2015). Exploiting fungal cell wall components in vaccines. Semin. Immunopathol. 37, 199–207. doi: 10.1007/s00281-014-0460-6
Li, Y., Ambati, S., Meagher, R. B., and Lin, X. (2025a). Developing mRNA lipid nanoparticle vaccine effective for cryptococcosis in a murine model. NPJ Vaccines 10, 24. doi: 10.1038/s41541-025-01079-z
Li, R., Zhang, R., Yang, Y., Wang, X., Yi, Y., Fan, P., et al. (2018). CGA-N12, a peptide derived from chromogranin A, promotes apoptosis of Candida tropicalis by attenuating mitochondrial functions. Biochem. J. 475, 1385–1396. doi: 10.1042/BCJ20170894
Li, X., Hu, Q., Lin, Q., Luo, J., Xu, J., Chen, L., et al. (2022). Inhibition of Candida albicans in vivo and in vitro by antimicrobial peptides chromogranin A-N12 through microRNA-155/suppressor of cytokine signaling 1 axis. Bioengineered 13, 2513–2524. doi: 10.1080/21655979.2021.2017680
Li, R., Wu, J., He, F., Xu, Q., Yin, K., Li, S., et al. (2023). Rational design, synthesis, antifungal evaluation and docking studies of antifungal peptide CGA-N12 analogues based on the target CtKRE9. Bioorg. Chem. 132, 106355. doi: 10.1016/j.bioorg.2023.106355
Li, N., Wu, Y. X., Zhang, Y. D., Wang, S. R., Zhang, G. C., Yang, J., et al. (2023). Phytic acid is a new substitutable plant-derived antifungal agent for the seedling blight of Pinus sylvestris var. mongolica caused by Fusarium oxysporum. Pestic Biochem. Physiol. 191, 105341. doi: 10.1016/j.pestbp.2023.105341
Li, J., et al. (2025). Cryo-EM structure of the β-1,3-glucan synthase FKS1-Rho1 complex. Nat. Commun. 16, 2054. doi: 10.1038/s41467-025-57152-7
Li, Y., et al. (2025b). BroadAMP-GPT: AI-Driven generation of broad-spectrum antimicrobial peptides for combating multidrug-resistant ESKAPE pathogens. Gut Microbes 17, 2523811. doi: 10.1080/19490976.2025.2523811
Li, J., Li, L., Tian, Y., Niu, G., and Tan, H. (2011). Hybrid antibiotics with the nikkomycin nucleoside and polyoxin peptidyl moieties. Metab. Eng. 13, 336–344. doi: 10.1016/j.ymben.2011.01.002
Li, F., Liu, F., Huang, K., and Yang, S. (2022). Advancement of gallium and gallium-based compounds as antimicrobial agents. Front. Bioeng. Biotechnol. 10. doi: 10.3389/fbioe.2022.827960
Li, Y., Qiao, Y., Ma, Y., Xue, P., and Ding, C. (2025c). AI in fungal drug development: opportunities, challenges, and future outlook. Front. Cell Infect. Microbiol. 15. doi: 10.3389/fcimb.2025.1610743
Li, K., Xia, W., and Zhang, J. Z. H. (2025). Discovery of Novel Inhibitors of Aspergillus fumigatus DHODH via Virtual Screening, MD Simulation, and In Vitro Activity Assay. Molecules 30, 2607. doi: 10.3390/molecules30122607
Liao, G., Zhou, Z., Liao, J., Zu, L., Wu, Q., Guo, Z., et al. (2016). 6-O-branched oligo-β-glucan-based antifungal glycoconjugate vaccines. ACS Infect. Dis. 2, 123–131. doi: 10.1021/acsinfecdis.5b00104
Lionakis, M. S., Drummond, R. A., and Hohl, T. M. (2023). Immune responses to human fungal pathogens and therapeutic prospects. Nat. Rev. Immunol. 23, 433–452. doi: 10.1038/s41577-022-00826-w
Liu, M., Clemons, K. V., Bigos, M., Medovarska, I., Brummer, E., Stevens, D. A., et al. (2011). Immune responses induced by heat killed Saccharomyces cerevisiae: a vaccine against fungal infection. Vaccine 29, 1745–1753. doi: 10.1016/j.vaccine.2010.12.119
Liu, M., Clemons, K. V., Johansen, M. E., Martinez, M., Chen, V., Stevens, D. A., et al. (2012). Saccharomyces as a vaccine against systemic candidiasis. Immunol. Invest. 41, 847–855. doi: 10.3109/08820139.2012.692418
Liu, J., Li, Q., Wang, C., Shao, J., Wang, T., Wu, D., et al. (2020). Antifungal evaluation of traditional herbal monomers and their potential for inducing cell wall remodeling in Candida albicans and Candida auris. Biofouling 36, 319–331. doi: 10.1080/08927014.2020.1759559
Liu, X., Zhong, L., Xie, J., Sui, Y., Li, G., Ma, Z., et al. (2021). Sodium houttuyfonate: A review of its antimicrobial, anti-inflammatory and cardiovascular protective effects. Eur. J. Pharmacol. 902, 174110. doi: 10.1016/j.ejphar.2021.174110
Liu, J., Zhang, B., Wang, L., Li, S., Long, Q., Xiao, X., et al. (2024). Bioactive components, pharmacological properties and underlying mechanism of Ganoderma lucidum spore oil: A review. Chin. Herb Med. 16, 375–391. doi: 10.1016/j.chmed.2023.09.007
Liu, H., Song, Z., Zhang, Y., Wu, B., Chen, D., Zhou, Z., et al. (2025). De novo design of self-assembling peptides with antimicrobial activity guided by deep learning. Nat. Mater. 24, 1295–1306. doi: 10.1038/s41563-025-02164-3
Lockhart, S. R., Etienne, K. A., Vallabhaneni, S., Farooqi, J., Chowdhary, A., Govender, N. P., et al (2023). The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat. Rev. Microbiol. 21, 818–832. doi: 10.1038/s41579-023-00960-9
Lockhart, S. R., et al. (2017). Simultaneous emergence of multidrug-resistant candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 64, 134–140. doi: 10.1093/cid/ciw691
Loh, J. T. and Lam, K. P. (2023). Fungal infections: Immune defense, immunotherapies and vaccines. Adv. Drug Delivery Rev. 196, 114775. doi: 10.1016/j.addr.2023.114775
Longley, D. B., Harkin, D. P., and Johnston, P. G. (2003). 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer 3, 330–338. doi: 10.1038/nrc1074
Lortholary, O., Obenga, G., Biswas, P., Caillot, D., Chachaty, E., Bienvenu, A. L., et al. (2010). International retrospective analysis of 73 cases of invasive fusariosis treated with voriconazole. Antimicrob. Agents Chemother. 54, 4446–4450. doi: 10.1128/aac.00286-10
Lu, X., Wang, R., Yu, Y., Wei, J., Xu, Y., Zhou, L., et al. (2024). Drug repurposing of ACT001 to discover novel promising sulfide prodrugs with improved safety and potent activity for neutrophil-mediated antifungal immunotherapy. J. Med. Chem. 67, 5783–5799. doi: 10.1021/acs.jmedchem.3c02453
Luo, X., Chen, H., Song, Y., Qin, Z., Xu, L., He, N., et al. (2023). Advancements, challenges and future perspectives on peptide-based drugs: Focus on antimicrobial peptides. Eur. J. Pharm. Sci. 181, 106363. doi: 10.1016/j.ejps.2022.106363
Luo, G., Gebremariam, T., Clemons, K. V., Stevens, D. A., and Ibrahim, A. S. (2014). Heat-killed yeast protects diabetic ketoacidotic-steroid treated mice from pulmonary mucormycosis. Vaccine 32, 3573–3576. doi: 10.1016/j.vaccine.2014.04.086
Lyman, M., Forsberg, K., Sexton, D. J., Chow, N. A., Lockhart, S. R., Jackson, B. R., et al. (2023). Worsening spread of candida auris in the United States, 2019 to 2021. Ann. Intern. Med. 176, 489–495. doi: 10.7326/m22-3469
Macreadie, I. G., Johnson, G., Schlosser, T., and Macreadie, P. I. (2006). Growth inhibition of Candida species and Aspergillus fumigatus by statins. FEMS Microbiol. Lett. 262, 9–13. doi: 10.1111/j.1574-6968.2006.00370.x
Maertens, J. A., Thompson, G. R., Spec, A., Donovan, F. M., Hammond, S. P., Bruns, A. H. W., et al. (2025). Olorofim for the treatment of invasive fungal diseases in patients with few or no therapeutic options: a single-arm, open-label, phase 2b study. Lancet Infect. Dis. S1473-3099(25)00224-5. doi: 10.1016/s1473-3099(25)00224-5
Majumder, T., Liu, M., Chen, V., Martinez, M., Alvarado, D., Clemons, K. V., et al. (2014). Killed Saccharomyces cerevisiae protects against lethal challenge of Cryptococcus grubii. Mycopathologia 178, 189–195. doi: 10.1007/s11046-014-9798-5
Mammen, M. P., Armas, D., Hughes, F. H., Hopkins, A. M., Fisher, C. L., Resch, P. A., et al. (2019). First-in-human phase 1 study to assess safety, tolerability, and pharmacokinetics of a novel antifungal drug, VL-2397, in healthy adults. Antimicrob. Agents Ch 63, e00969–19. doi: 10.1128/AAC.00969-19
Maphanga, T. G., Mpembe, R. S., Naicker, S. D., and Govender, N. P. (2022). In Vitro Antifungal Activity of Manogepix and Other Antifungal Agents against South African Candida auris Isolates from Bloodstream Infections. Microbiol. Spectr. 10, e0171721. doi: 10.1128/spectrum.01717-21
Margalit, A. and Kavanagh, K. (2015). The innate immune response to Aspergillus fumigatus at the alveolar surface. FEMS Microbiol. Rev. 39, 670–687. doi: 10.1093/femsre/fuv018
Marr, K. A., Schlamm, H. T., Herbrecht, R., Rottinghaus, S. T., Bow, E. J., Cornely, O. A., et al. (2015). Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann. Intern. Med. 162, 81–89. doi: 10.7326/m13-2508
Martens, M. G., Maximos, B., Degenhardt, T., Person, K., Curelop, S., Ghannoum, M., et al. (2022). Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am. J. Obstet Gynecol. 227, 880.e881–880.e811. doi: 10.1016/j.ajog.2022.07.023
Martín Del Campo, J. S., Vogelaar, N., Tolani, K., Kizjakina, K., Harich, K., Sobrado, P., et al. (2016). Inhibition of the flavin-dependent monooxygenase siderophore A (SidA) blocks siderophore biosynthesis and aspergillus fumigatus growth. ACS Chem. Biol. 11, 3035–3042. doi: 10.1021/acschembio.6b00666
Martinez, M., Clemons, K. V., and Stevens, D. A. (2017). Heat-killed yeast as a pan-fungal vaccine. Methods Mol. Biol. 1625, 23–30. doi: 10.1007/978-1-4939-7104-6_2
Masso-Silva, J., Espinosa, V., Liu, T. B., Wang, Y., Xue, C., Rivera, A., et al. (2018). The F-box protein fbp1 shapes the immunogenic potential of cryptococcus neoformans. mBio 9, e01828–17. doi: 10.1128/mBio.01828-17
May, R. C., Stone, N. R., Wiesner, D. L., Bicanic, T., and Nielsen, K. (2016). Cryptococcus: from environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 14, 106–117. doi: 10.1038/nrmicro.2015.6
Meng, Y., Ni, Y., Li, Z., Jiang, T., Sun, T., Li, Y., et al. (2024). Interplay between acetylation and ubiquitination of imitation switch chromatin remodeler Isw1 confers multidrug resistance in Cryptococcus neoformans. Elife 13, e85728. doi: 10.7554/eLife.85728
Mesa-Arango, A. C., Trevijano-Contador, N., Román, E., Sánchez-Fresneda, R., Casas, C., Herrero, E., et al. (2014). The production of reactive oxygen species is a universal action mechanism of Amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 58, 6627–6638. doi: 10.1128/aac.03570-14
Messier, C. and Grenier, D. (2011). Effect of licorice compounds licochalcone A, glabridin and glycyrrhizic acid on growth and virulence properties of Candida albicans. Mycoses 54, e801–e806. doi: 10.1111/j.1439-0507.2011.02028.x
Miao, H., Zhao, L., Li, C., Shang, Q., Lu, H., Fu, Z., et al. (2012). Inhibitory effect of Shikonin on Candida albicans growth. Biol. Pharm. Bull. 35, 1956–1963. doi: 10.1248/bpb.b12-00338
Miladi, H., Zmantar, T., Kouidhi, B., Chaabouni, Y., Mahdouani, K., Bakhrouf, A., et al. (2017). Use of carvacrol, thymol, and eugenol for biofilm eradication and resistance modifying susceptibility of Salmonella enterica serovar Typhimurium strains to nalidixic acid. Microb. Pathogenesis 104, 56–63. doi: 10.1016/j.micpath.2017.01.012
Mitsuyama, J., Nomura, N., Hashimoto, K., Yamada, E., Nishikawa, H., Kaeriyama, M., et al. (2008). In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob. Agents Chemother. 52, 1318–1324. doi: 10.1128/aac.01159-07
Mohamadzadeh, M., Zarei, M., and Vessal, M. (2020). Synthesis, in vitro biological evaluation and in silico molecular docking studies of novel β-lactam-anthraquinone hybrids. Bioorganic Chem. 95, 103515. doi: 10.1016/j.bioorg.2019.103515
Molloy, S. F., Kanyama, C., Heyderman, R. S., Loyse, A., Kouanfack, C., Chanda, D., et al. (2018). Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl. J. Med. 378, 1004–1017. doi: 10.1056/NEJMoa1710922
Monari, C., Casadevall, A., Retini, C., Baldelli, F., Bistoni, F., Vecchiarelli, A., et al. (1999). Antibody to capsular polysaccharide enhances the function of neutrophils from patients with AIDS against Cryptococcus neoformans. Aids 13, 653–660. doi: 10.1097/00002030-199904160-00005
Moora, M., Davison, J., Kohout, P., and Zobel, M. (2025). The importance of the plant mycorrhizal collaboration niche across scales. Nat. Rev. Biodiversity 1, 262–273. doi: 10.1038/s44358-025-00030-3
Morelli, K. A., Kerkaert, J. D., and Cramer, R. A. (2021). Aspergillus fumigatus biofilms: Toward understanding how growth as a multicellular network increases antifungal resistance and disease progression. PloS Pathog. 17, e1009794. doi: 10.1371/journal.ppat.1009794
Mukherjee, J., Casadevall, A., and Scharff, M. D. (1993a). Molecular characterization of the humoral responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J. Exp. Med. 177, 1105–1116. doi: 10.1084/jem.177.4.1105
Mukherjee, J., Feldmesser, M., Scharff, M. D., and Casadevall, A. (1995). Monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan enhance fluconazole efficacy. Antimicrob. Agents Chemother. 39, 1398–1405. doi: 10.1128/aac.39.7.1398
Mukherjee, J., Pirofski, L. A., Scharff, M. D., and Casadevall, A. (1993b). Antibody-mediated protection in mice with lethal intracerebral Cryptococcus neoformans infection. Proc. Natl. Acad. Sci. U.S.A. 90, 3636–3640. doi: 10.1073/pnas.90.8.3636
Nakamura, I., Yoshimura, S., Masaki, T., Takase, S., Ohsumi, K., Hashimoto, M., et al. (2017). ASP2397: a novel antifungal agent produced by Acremonium persicinum MF-347833. J. Antibiot. (Tokyo) 70, 45–51. doi: 10.1038/ja.2016.107
Nakamura, I., Ohsumi, K., Takeda, S., Katsumata, K., Matsumoto, S., Akamatsu, S., et al. (2019). ASP2397 Is a Novel Natural Compound That Exhibits Rapid and Potent Fungicidal Activity against Aspergillus Species through a Specific Transporter. Antimicrob. Agents Chemother. 63, e02689–18. doi: 10.1128/aac.02689-18
Nalley L, T. F., Durand-Morat, A., Shew, A., and Thoma, G. (2016). Economic and environmental impact of rice blast pathogen (Magnaporthe oryzae) alleviation in the United States. PloS One 11, e0167295. doi: 10.1371/journal.pone.0167295
Nami, S., Mohammadi, R., Vakili, M., Khezripour, K., Mirzaei, H., Morovati, H., et al. (2019). Fungal vaccines, mechanism of actions and immunology: A comprehensive review. BioMed. Pharmacother. 109, 333–344. doi: 10.1016/j.biopha.2018.10.075
Nargesi, S. and Rezaie, S. (2018). Investigation an antifungal activity of diclofenac sodium against hyphae formation in aspergillus fumigatus with attention to the expression of ef-1 gene. Iranian J. Public Health 47, 770–772.
Navalkele, B. D., Revankar, S., and Chandrasekar, P. (2017). Candida auris: a worrisome, globally emerging pathogen. Expert Rev. Anti Infect. Ther. 15, 819–827. doi: 10.1080/14787210.2017.1364992
Newman, D. J., Cragg, G. M., and Snader, K. M. (2003). Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod 66, 1022–1037. doi: 10.1021/np030096l
Ngan, N. T. T., Flower, B., and Day, J. N. (2022). Treatment of cryptococcal meningitis: how have we got here and where are we going? Drugs 82, 1237–1249. doi: 10.1007/s40265-022-01757-5
Ning, Y., Dai, R., Zhang, L., Xu, Y., and Xiao, M. (2024). Copy number variants of ERG11: mechanism of azole resistance in Candida parapsilosis. Lancet Microbe 5, e10. doi: 10.1016/s2666-5247(23)00294-x
Nisar, M. F., Khadim, M., Rafiq, M., Chen, J., Yang, Y., Wan, C. C., et al. (2021). Pharmacological properties and health benefits of eugenol: A comprehensive review. Oxid. Med. Cell Longev 2021, 2497354. doi: 10.1155/2021/2497354
Nishikawa, H., Yamada, E., Shibata, T., Uchihashi, S., Fan, H., Hayakawa, H., et al. (2010). Uptake of T-2307, a novel arylamidine, in Candida albicans. J. Antimicrob. Chemother. 65, 1681–1687. doi: 10.1093/jac/dkq177
Nordin, S., Mohd Esah, E., and Mahror, N. (2025). Antifungal activity of cinnamon, clove, and star anise essential oils against Aspergillus flavus and Aspergillus Niger, and their potential application in chilli paste to inhibit fungal growth. Food Biosci. 66, 106217. doi: 10.1016/j.fbio.2025.106217
Nunnally, N. S., Etienne, K. A., Angulo, D., Lockhart, S. R., and Berkow, E. L. (2019). In Vitro Activity of Ibrexafungerp, a Novel Glucan Synthase Inhibitor against Candida glabrata Isolates with FKS Mutations. Antimicrob. Agents Chemother. 63, e01692–19. doi: 10.1128/aac.01692-19
Ogundeji, A. O., Pohl, C. H., and Sebolai, O. M. (2016). Repurposing of aspirin and ibuprofen as candidate anti-cryptococcus drugs. Antimicrobial. Agents chemother. 60, 4799–4808. doi: 10.1128/aac.02810-15
Oliveira, L. V. N., Wang, R., Specht, C. A., and Levitz, S. M. (2021). Vaccines for human fungal diseases: close but still a long way to go. NPJ Vaccines 6, 33. doi: 10.1038/s41541-021-00294-8
Oliver, J. D., Sibley, G. E. M., Beckmann, N., Dobb, K. S., Slater, M. J., McEntee, L., et al. (2016). F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc. Natl. Acad. Sci. U.S.A. 113, 12809–12814. doi: 10.1073/pnas.1608304113
Ostrowsky, B., Greenko, J., Adams, E., Quinn, M., O'Brien, B., Chaturvedi, V., et al. (2020). Candida auris isolates resistant to three classes of antifungal medications - New York, 2019. MMWR Morb Mortal Wkly Rep. 69, 6–9. doi: 10.15585/mmwr.mm6901a2
Pail, O., Lin, M. J., Anagnostou, T., Brown, B. D., and Brody, J. D. (2025). Cancer vaccines and the future of immunotherapy. Lancet 406, 189–202. doi: 10.1016/s0140-6736(25)00553-7
Pan, J., Hu, C., and Yu, J. H. (2018). Lipid biosynthesis as an antifungal target. J. Fungi (Basel) 4, 50. doi: 10.3390/jof4020050
Panackal, A. A., Parisini, E., and Proschan, M. (2014). Salvage combination antifungal therapy for acute invasive aspergillosis may improve outcomes: a systematic review and meta-analysis. Int. J. Infect. Dis. 28, 80–94. doi: 10.1016/j.ijid.2014.07.007
Papon, N., Noël, T., Florent, M., Gibot-Leclerc, S., Jean, D., Chastin, C., et al. (2007). Molecular mechanism of flucytosine resistance in Candida lusitaniae: contribution of the FCY2, FCY1, and FUR1 genes to 5-fluorouracil and fluconazole cross-resistance. Antimicrob. Agents Chemother. 51, 369–371. doi: 10.1128/aac.00824-06
Pappas, P. G., Vazquez, J. A., Oren, I., Rahav, G., Aoun, M., Bulpa, P., et al. (2023). Clinical safety and efficacy of novel antifungal, fosmanogepix, for the treatment of candidemia: results from a Phase 2 trial. J. Antimicrob. Chemother. 78, 2471–2480. doi: 10.1093/jac/dkad256
Park, J. and Son, H. (2024). Antioxidant systems of plant pathogenic fungi: functions in oxidative stress response and their regulatory mechanisms. Plant Pathol. J. 40, 235–250. doi: 10.5423/ppj.Rw.01.2024.0001
Pasupuleti, M., Schmidtchen, A., and Malmsten, M. (2012). Antimicrobial peptides: key components of the innate immune system. Crit. Rev. Biotechnol. 32, 143–171. doi: 10.3109/07388551.2011.594423
Paulussen, C., Boulet, G. A., Cos, P., Delputte, P., and Maes, L. J. (2014). Animal models of invasive aspergillosis for drug discovery. Drug Discov. Today 19, 1380–1386. doi: 10.1016/j.drudis.2014.06.006
Perfect, J. R. (2017). The antifungal pipeline: a reality check. Nat. Rev. Drug Discov. 16, 603–616. doi: 10.1038/nrd.2017.46
Perfect, J. R., Dismukes, W. E., Dromer, F., Goldman, D. L., Graybill, J. R., Hamill, R. J., et al. (2010). Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 50, 291–322. doi: 10.1086/649858
Perlin, D. S. (2007). Resistance to echinocandin-class antifungal drugs. Drug Resist. Update 10, 121–130. doi: 10.1016/j.drup.2007.04.002
Petraitiene, R., Petraitis, V., Maung, B. B. W., Mansbach, R. S., Hodges, M. R., Finkelman, M. A., et al. (2021). Efficacy and pharmacokinetics of fosmanogepix (APX001) in the treatment of candida endophthalmitis and hematogenous meningoencephalitis in nonneutropenic rabbits. Antimicrob. Agents Chemother. 65, e01795-20. doi: 10.1128/aac.01795-20
Petraitis, V., Petraitiene, R., Katragkou, A., Maung, B. B. W., Naing, E., Kavaliauskas, P., et al. (2020). Combination therapy with ibrexafungerp (Formerly SCY-078), a first-in-class triterpenoid inhibitor of (1→3)-β-d-glucan synthesis, and isavuconazole for treatment of experimental invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 64, e02429–19. doi: 10.1128/aac.02429-19
Petrik, M., Franssen, G. M., Haas, H., Laverman, P., Hörtnagl, C., Schrettl, M., et al. (2012). Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur. J. Nucl. Med. Mol. Imaging 39, 1175–1183. doi: 10.1007/s00259-012-2110-3
Pfaller, M. A., Huband, M. D., Rhomberg, P. R., Bien, P. A., and Castanheira, M. (2022). Activities of Manogepix and Comparators against 1,435 Recent Fungal Isolates Collected during an International Surveillance Program (2020). Antimicrob. Agents Chemother. 66, e0102822. doi: 10.1128/aac.01028-22
Phillips, A. J., Sudbery, I., and Ramsdale, M. (2003). Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc. Natl. Acad. Sci. U.S.A. 100, 14327–14332. doi: 10.1073/pnas.2332326100
Pic, E., Burgain, A., and Sellam, A. (2019). Repurposing the anthelminthic salicylanilide oxyclozanide against susceptible and clinical resistant Candida albicans strains. Med. mycol. 57, 387–390. doi: 10.1093/mmy/myy027
Pires, R. H., Montanari, L. B., Martins, C. H., Zaia, J. E., Almeida, A. M., Matsumoto, M. T., et al. (2011). Anticandidal efficacy of cinnamon oil against planktonic and biofilm cultures of Candida parapsilosis and Candida orthopsilosis. Mycopathologia 172, 453–464. doi: 10.1007/s11046-011-9448-0
Pirofski, L. A. and Casadevall, A. (2006). Immunomodulators as an antimicrobial tool. Curr. Opin. Microbiol. 9, 489–495. doi: 10.1016/j.mib.2006.08.004
Pontes, L., Beraquet, C. A. G., Arai, T., Pigolli, G. L., Lyra, L., Watanabe, A., et al. (2020). Aspergillus fumigatus Clinical Isolates Carrying CYP51A with TR34/L98H/S297T/F495I Substitutions Detected after Four-Year Retrospective Azole Resistance Screening in Brazil. Antimicrob. Agents Chemother. 64, e02059–19. doi: 10.1128/aac.02059-19
Pozzebon Venturini, T., Rossato, L., Chassot, F., Tairine Keller, J., Baldissera Piasentin, F., Morais Santurio, J., et al. (2016). In vitro synergistic combinations of pentamidine, polymyxin B, tigecycline and tobramycin with antifungal agents against Fusarium spp. J. Med. Microbiol. 65, 770–774. doi: 10.1099/jmm.0.000301
Prakash, H. and Chakrabarti, A. (2019). Global epidemiology of mucormycosis. J. Fungi 5, 26. doi: 10.3390/jof5010026
Prevention, E. C. f. D. & Control (2013). Risk assessment on the impact of environmental usage of traizoles on the development and spread of resistance to medical triazoles in Aspergillus species (Solna, Sweden: European Centre for Disease Prevention and Control).
Priest, S. J., Yadav, V., Roth, C., Dahlmann, T. A., Kuck, U., Magwene, P. M., et al. (2022). Uncontrolled transposition following RNAi loss causes hypermutation and antifungal drug resistance in clinical isolates of Cryptococcus neoformans. Nat. Microbiol. 7, 1239–1251. doi: 10.1038/s41564-022-01183-z
Prigitano, A., Esposto, M. C., Romanò, L., Auxilia, F., and Tortorano, A. M. (2019). Azole-resistant Aspergillus fumigatus in the Italian environment. J. Glob. Antimicrob. Resist. 16, 220–224. doi: 10.1016/j.jgar.2018.10.017
Puerta-Alcalde, P., Monzó-Gallo, P., Aguilar-Guisado, M., Ramos, J. C., Laporte-Amargós, J., Machado, M., et al. (2023). Breakthrough invasive fungal infection among patients with hematologic Malignancies: A national, prospective, and multicenter study. J. Infect. 87, 46–53. doi: 10.1016/j.jinf.2023.05.005
Puumala, E., Fallah, S., Robbins, N., and Cowen, L. E. (2024). Advancements and challenges in antifungal therapeutic development. Clin. Microbiol. Rev. 37, e0014223. doi: 10.1128/cmr.00142-23
Qian, W., Li, X., Liu, Q., Lu, J., Wang, T., Zhang, Q., et al. (2022). Antifungal and Antibiofilm Efficacy of Paeonol Treatment Against Biofilms Comprising Candida albicans and/or Cryptococcus neoformans. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.884793
Qiao, L., Dong, Y., Zhou, H., and Cui, H. (2023). Effect of post-polyketide synthase modification groups on property and activity of polyene macrolides. Antibiotics (Basel) 12, 119. doi: 10.3390/antibiotics12010119
Qin, H., Zhou, Z., Shi, R., Mai, Y., Xu, Y., Peng, F., et al. (2025). Insights into next-generation immunotherapy designs and tools: molecular mechanisms and therapeutic prospects. J. Hematol. Oncol. 18, 62. doi: 10.1186/s13045-025-01701-6
Ramakrishnan, J., Rathore, S. S., and Raman, T. (2016). Review on fungal enzyme inhibitors – potential drug targets to manage human fungal infections. RSC Adv. 6, 42387–42401. doi: 10.1039/C6RA01577H
Ran, Y., Shehzadi, K., Liang, J. H., and Yu, M. J. (2024). Rationally designed novel antimicrobial peptides targeting chitin synthase for combating soybean phytophthora blight. Int. J. Mol. Sci. 25, 3512. doi: 10.3390/ijms25063512
Randall, J. R., Vieira, L. C., Wilke, C. O., and Davies, B. W. (2024). Deep mutational scanning and machine learning for the analysis of antimicrobial-peptide features driving membrane selectivity. Nat. BioMed. Eng. 8, 842–853. doi: 10.1038/s41551-024-01243-1
Rayens, E., Rabacal, W., Kang, S. E., Celia, B. N., Momany, M., Norris, K. A., et al. (2021). Vaccine-induced protection in two murine models of invasive pulmonary aspergillosis. Front. Immunol. 12. doi: 10.3389/fimmu.2021.670578
Rayens, E., Rabacal, W., Willems, H. M. E., Kirton, G. M., Barber, J. P., Mousa, J. J., et al. (2022). Immunogenicity and protective efficacy of a pan-fungal vaccine in preclinical models of aspergillosis, candidiasis, and pneumocystosis. PNAS Nexus 1, pgac248. doi: 10.1093/pnasnexus/pgac248
Rhodes, J., Abdolrasouli, A., Dunne, K., Sewell, T. R., Zhang, Y., Ballard, E., et al. (2022). Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment. Nat. Microbiol. 7, 663–674. doi: 10.1038/s41564-022-01091-2
Rhodes, J. and Fisher, M. C. (2019). Global epidemiology of emerging Candida auris. Curr. Opin. Microbiol. 52, 84–89. doi: 10.1016/j.mib.2019.05.008
Rivero-Menendez, O., Soto-Debran, J. C., Cuenca-Estrella, M., and Alastruey-Izquierdo, A. (2021). In vitro activity of ibrexafungerp against a collection of clinical isolates of aspergillus, including cryptic species and cyp51A mutants, using EUCAST and CLSI methodologies. J. Fungi (Basel) 7, 232. doi: 10.3390/jof7030232
Robbins, N. and Cowen, L. E. (2025). Candida. Curr. Biol. 35, R522–R526. doi: 10.1016/j.cub.2025.01.063
Robbins, N., Spitzer, M., Yu, T., Cerone, R. P., Averette, A. K., Bahn, Y. S., et al. (2015). An antifungal combination matrix identifies a rich pool of adjuvant molecules that enhance drug activity against diverse fungal pathogens. Cell Rep. 13, 1481–1492. doi: 10.1016/j.celrep.2015.10.018
Rodrigues, A. M., Hagen, F., and de Camargo, Z. P. (2022). A spotlight on sporothrix and sporotrichosis. Mycopathologia 187, 407–411. doi: 10.1007/s11046-022-00642-9
Rodríguez-Cerdeira, C., Pinto-Almazán, R., Saunte, D. M. L., Hay, R., Szepietowski, J. C., Moreno-Coutiño, G., et al. (2025). Virulence and resistance factors of Nakaseomyces glabratus (formerly known as Candida glabrata) in Europe: A systematic review. J. Eur. Acad. Dermatol. Venereol. 39, 377–388. doi: 10.1111/jdv.20273
Roemer, T. and Krysan, D. J. (2014). Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb. Perspect. Med. 4, a019703. doi: 10.1101/cshperspect.a019703
Rossato, L., Loreto, É. S., Zanette, R. A., Chassot, F., Santurio, J. M., Alves, S. H., et al. (2016). In vitro synergistic effects of chlorpromazine and sertraline in combination with amphotericin B against Cryptococcus neoformans var. grubii. Folia microbiologica 61, 399–403. doi: 10.1007/s12223-016-0449-8
Roundtree, M. T., Juvvadi, P. R., Shwab, E. K., Cole, D. C., and Steinbach, W. J. (2020). Aspergillus fumigatus cyp51A and cyp51B proteins are compensatory in function and localize differentially in response to antifungals and cell wall inhibitors. Antimicrob. Agents Chemother. 64, e00735–20. doi: 10.1128/aac.00735-20
Rubino, C. M., Smith, L. R., Mammen, M. P., Hopkins, A. M., Lakota, E. A., Sullivan, S. M., et al. (2017) Open Forum Infectious Diseases (Oxford, England, UK: Oxford University Press US), S473–S473.
Saag, M. S., Graybill, R. J., Larsen, R. A., Pappas, P. G., Perfect, J. R., Powderly, W. G., et al. (2000). Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin. Infect. Dis. 30, 710–718. doi: 10.1086/313757
Sahu, S. R., Bose, S., Singh, M., Kumari, P., Dutta, A., Utkalaja, B. G., et al. (2022). Vaccines against candidiasis: Status, challenges and emerging opportunity. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.1002406
Saini, V., Safwan, S. M., Mehta, D., Das, E. E., and Bajaj, A. (2025). Recent advances in the development of antifungal agents: beyond azoles, polyenes, and echinocandins. ACS Infect. Dis. 11, 1271–1295. doi: 10.1021/acsinfecdis.4c00828
Samber, N., Khan, A., Varma, A., and Manzoor, N. (2015). Synergistic anti-candidal activity and mode of action of Mentha piperita essential oil and its major components. Pharm. Biol. 53, 1496–1504. doi: 10.3109/13880209.2014.989623
Sandison, T., Ong, V., Lee, J., and Thye, D. (2017). Safety and pharmacokinetics of CD101 IV, a novel echinocandin, in healthy adults. Antimicrob. Agents Chemother. 61, a019703. doi: 10.1128/aac.01627-16
Sangalli-Leite, F., Scorzoni, L., Mesa-Arango, A. C., Casas, C., Herrero, E., Gianinni, M. J., et al. (2011). Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect. 13, 457–467. doi: 10.1016/j.micinf.2011.01.015
Santana, D. J., Anku, J. A. E., Zhao, G., Zarnowski, R., Johnson, C. J., Hautau, H., et al. (2023). A Candida auris-specific adhesin, Scf1, governs surface association, colonization, and virulence. Science 381, 1461–1467. doi: 10.1126/science.adf8972
Santos-Júnior, C. D., Torres, M. D. T., Duan, Y., Rodríguez Del Río, Á., Schmidt, T. S. B., Chong, H., et al. (2024). Discovery of antimicrobial peptides in the global microbiome with machine learning. Cell 187, 3761–3778.e3716. doi: 10.1016/j.cell.2024.05.013
Schauwvlieghe, A., Rijnders, B. J. A., Philips, N., Verwijs, R., Vanderbeke, L., Van Tienen, C., et al. (2018). Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir. Med. 6, 782–792. doi: 10.1016/s2213-2600(18)30274-1
Schikora-Tamarit, M.À. and Gabaldón, T. (2024). Recent gene selection and drug resistance underscore clinical adaptation across Candida species. Nat. Microbiol. 9, 284–307. doi: 10.1038/s41564-023-01547-z
Schoustra, S. E., Debets, A. J. M., Rijs, A., Zhang, J., Snelders, E., Leendertse, P. C., et al. (2019). Environmental hotspots for azole resistance selection of aspergillus fumigatus, the Netherlands. Emerg. Infect. Dis. 25, 1347–1353. doi: 10.3201/eid2507.181625
Schueffler, A. and Anke, T. (2014). Fungal natural products in research and development. Nat. Prod Rep. 31, 1425–1448. doi: 10.1039/c4np00060a
Schwebke, J. R., Sobel, R., Gersten, J. K., Sussman, S. A., Lederman, S. N., Jacobs, M. A., et al. (2022). Ibrexafungerp versus placebo for vulvovaginal candidiasis treatment: A phase 3, randomized, controlled superiority trial (VANISH 303). Clin. Infect. Dis. 74, 1979–1985. doi: 10.1093/cid/ciab750
Scriven, J. E., Tenforde, M. W., Levitz, S. M., and Jarvis, J. N. (2017). Modulating host immune responses to fight invasive fungal infections. Curr. Opin. Microbiol. 40, 95–103. doi: 10.1016/j.mib.2017.10.018
Seagle, E. E., Williams, S. L., and Chiller, T. M. (2021). Recent trends in the epidemiology of fungal infections. Infect. Dis. Clin. North Am. 35, 237–260. doi: 10.1016/j.idc.2021.03.001
Seleem, D., Benso, B., Noguti, J., Pardi, V., and Murata, R. M. (2016). In Vitro and In Vivo Antifungal Activity of Lichochalcone-A against Candida albicans Biofilms. PloS One 11, e0157188. doi: 10.1371/journal.pone.0157188
Seneviratne, C. J., Wang, Y., Jin, L., Abiko, Y., and Samaranayake, L. P. (2008). Candida albicans biofilm formation is associated with increased anti-oxidative capacities. Proteomics 8, 2936–2947. doi: 10.1002/pmic.200701097
Shao, J., Cui, Y., Zhang, M., Wang, T., Wu, D., Wang, C., et al. (2017). Synergistic in vitro activity of sodium houttuyfonate with fluconazole against clinical Candida albicans strains under planktonic growing conditions. Pharm. Biol. 55, 355–359. doi: 10.1080/13880209.2016.1237977
Shapiro, R. S., Robbins, N., and Cowen, L. E. (2011). Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 75, 213–267. doi: 10.1128/MMBR.00045-10
Sharma, M., Manoharlal, R., Negi, A. S., and Prasad, R. (2010a). Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res. 10, 570–578. doi: 10.1111/j.1567-1364.2010.00637.x
Sharma, M., Manoharlal, R., Puri, N., and Prasad, R. (2010b). Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci. Rep. 30, 391–404. doi: 10.1042/bsr20090151
Shaw, K. J. (2022). GR-2397: review of the novel siderophore-like antifungal agent for the treatment of invasive aspergillosis. J. Fungi (Basel) 8, 909. doi: 10.3390/jof8090909
Shaw, K. J. and Ibrahim, A. S. (2020). Fosmanogepix: A review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J. Fungi (Basel) 6, 239. doi: 10.3390/jof6040239
Shi, G., Shao, J., Wang, T., Wu, D., and Wang, C. (2017). Mechanism of berberine-mediated fluconazole-susceptibility enhancement in clinical fluconazole-resistant Candida tropicalis isolates. BioMed. Pharmacother. 93, 709–712. doi: 10.1016/j.biopha.2017.06.106
Shinde, R. B., Rajput, S. B., Raut, J. S., and Karuppayil, S. M. (2013). An in vitro repositioning study reveals antifungal potential of chloroquine to inhibit growth and morphogenesis in Candida albicans. J. Gen. Appl. Microbiol. 59, 167–170. doi: 10.2323/jgam.59.167
Shivarathri, R., Jenull, S., Stoiber, A., Chauhan, M., Mazumdar, R., Singh, A., et al. (2020). The two-component response regulator ssk1 and the mitogen-activated protein kinase hog1 control antifungal drug resistance and cell wall architecture of candida auris. mSphere 5, e00973-20. doi: 10.1128/mSphere.00973-20
Shreaz, S., Wani, W. A., Behbehani, J. M., Raja, V., Irshad, M., Karched, M., et al. (2016). Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 112, 116–131. doi: 10.1016/j.fitote.2016.05.016
Shukla, M. and Rohatgi, S. (2020). Vaccination with Secreted Aspartyl Proteinase 2 Protein from Candida parapsilosis Can Enhance Survival of Mice during C. tropicalis-Mediated Systemic Candidiasis. Infect. Immun. 88, e00312–20. doi: 10.1128/iai.00312-20
Sigera, L. S. M. and Denning, D. W. (2023). Invasive aspergillosis after renal transplantation. J. Fungi (Basel) 9, 255. doi: 10.3390/jof9020255
Silva, I. R., Braga, A. V., Gloria, M. B. A., Machado, R. R., César, I. C., Oliveira, R. B., et al. (2020). Preclinical pharmacokinetic study of a new thiazolyl hydrazone derivative with antifungal activity in mice plasma by LC-MS/MS. J. Chromatogr. B Analyt Technol. BioMed. Life Sci. 1149, 122180. doi: 10.1016/j.jchromb.2020.122180
Silva, I. R., Souza, M., Machado, R. R., Oliveira, R. B., Leite, E. A., César, I. D. C., et al. (2024). Enhancing oral bioavailability of an antifungal thiazolylhydrazone derivative: Development and characterization of a self-emulsifying drug delivery system. Int. J. Pharm. 655, 124011. doi: 10.1016/j.ijpharm.2024.124011
Singh, S. D., Robbins, N., Zaas, A. K., Schell, W. A., Perfect, J. R., Cowen, L. E., et al. (2009). Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PloS Pathog. 5, e1000532. doi: 10.1371/journal.ppat.1000532
Singh, K., Ali, V., Pratap Singh, K., Gupta, P., Suman, S. S., Ghosh, A. K., et al. (2017). Deciphering the interplay between cysteine synthase and thiol cascade proteins in modulating Amphotericin B resistance and survival of Leishmania donovani under oxidative stress. Redox Biol. 12, 350–366. doi: 10.1016/j.redox.2017.03.004
Singh, S., Uppuluri, P., Mamouei, Z., Alqarihi, A., Elhassan, H., French, S., et al. (2019). The NDV-3A vaccine protects mice from multidrug resistant Candida auris infection. PloS Pathog. 15, e1007460. doi: 10.1371/journal.ppat.1007460
Skiada, A., Pavleas, I., and Drogari-Apiranthitou, M. (2020). Epidemiology and diagnosis of mucormycosis: an update. J. Fungi (Basel). 6, 265. doi: 10.3390/jof6040265
Snelders, E., Huis In Veld, R. A., Rijs, A. J., Kema, G. H., Melchers, W. J., Verweij, P. E., et al. (2009). Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microbiol. 75, 4053–4057. doi: 10.1128/aem.00231-09
Sobel, R., Nyirjesy, P., Ghannoum, M. A., Delchev, D. A., Azie, N. E., Angulo, D., et al. (2022). Efficacy and safety of oral ibrexafungerp for the treatment of acute vulvovaginal candidiasis: a global phase 3, randomized, placebo-controlled superiority study (VANISH 306). Bjog 129, 412–420. doi: 10.1111/1471-0528.16972
Sobel, J. D. and Nyirjesy, P. (2021). Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 16, 1453–1461. doi: 10.2217/fmb-2021-0173
Spec, A., Pullman, J., Thompson, G. R., Powderly, W. G., Tobin, E. H., Vazquez, J., et al. (2019). MSG-10: a Phase 2 study of oral ibrexafungerp (SCY-078) following initial echinocandin therapy in non-neutropenic patients with invasive candidiasis. J. Antimicrob. Chemother. 74, 3056–3062. doi: 10.1093/jac/dkz277
Spellberg, B. J., Ibrahim, A. S., Avenissian, V., Filler, S. G., Myers, C. L., Fu, Y., et al. (2005). The anti-Candida albicans vaccine composed of the recombinant N terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice. Infect. Immun. 73, 6191–6193. doi: 10.1128/iai.73.9.6191-6193.2005
Spellberg, B. J., Ibrahim, A. S., Avanesian, V., Fu, Y., Myers, C., Phan, Q. T., et al. (2006). Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J. Infect. Dis. 194, 256–260. doi: 10.1086/504691
Spitzer, M., Robbins, N., and Wright, G. D. (2017). Combinatorial strategies for combating invasive fungal infections. Virulence 8, 169–185. doi: 10.1080/21505594.2016.1196300
Stevens, D. A., Clemons, K. V., and Liu, M. (2011). Developing a vaccine against aspergillosis. Med. Mycol 49 Suppl 1, S170–S176. doi: 10.3109/13693786.2010.497775
Stone, N. R., Bicanic, T., Salim, R., Hope, W., and Liposomal Amphotericin, B. (2016). (AmBisome(®)): A review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 76, 485–500. doi: 10.1007/s40265-016-0538-7
Stylianou, M., Kulesskiy, E., Lopes, J. P., Granlund, M., Wennerberg, K., Urban, C. F., et al. (2014). Antifungal application of nonantifungal drugs. Antimicrobial. Agents chemother. 58, 1055–1062. doi: 10.1128/aac.01087-13
Su, H., Zhu, M., Tsui, C. K., van der Lee, H., Tehupeiory-Kooreman, M., Zoll, J., et al. (2023). Potency of olorofim (F901318) compared to contemporary antifungal agents against clinical Aspergillus fumigatus isolates, and review of azole resistance phenotype and genotype epidemiology in China. Antimicrob. Agents Chemother. 65, e02546–20. doi: 10.1128/aac.02546-20
Sun, Q., Li, C., Lin, J., Peng, X., Wang, Q., Jiang, N., et al. (2019). Celastrol ameliorates Aspergillus fumigatus keratitis via inhibiting LOX-1. Int. Immunopharmacol. 70, 101–109. doi: 10.1016/j.intimp.2019.02.017
Sun, L., Liao, K., Hang, C., and Wang, D. (2017). Honokiol induces reactive oxygen species-mediated apoptosis in Candida albicans through mitochondrial dysfunction. PloS One 12, e0172228. doi: 10.1371/journal.pone.0172228
Sun, L. M., Liao, K., Liang, S., Yu, P. H., and Wang, D. Y. (2015). Synergistic activity of magnolol with azoles and its possible antifungal mechanism against Candida albicans. J. Appl. Microbiol. 118, 826–838. doi: 10.1111/jam.12737
Sun, L., Liao, K., and Wang, D. (2015). Effects of magnolol and honokiol on adhesion, yeast-hyphal transition, and formation of biofilm by Candida albicans. PloS One 10, e0117695. doi: 10.1371/journal.pone.0117695
Süssmuth, R. D. and Mainz, A. (2017). Nonribosomal peptide synthesis-principles and prospects. Angew Chem. Int. Ed Engl. 56, 3770–3821. doi: 10.1002/anie.201609079
Szymański, M., Chmielewska, S., Czyżewska, U., Malinowska, M., and Tylicki, A. (2022). Echinocandins - structure, mechanism of action and use in antifungal therapy. J. Enzyme Inhib Med. Chem. 37, 876–894. doi: 10.1080/14756366.2022.2050224
Szymczak, P., Zarzecki, W., Wang, J., Duan, Y., Wang, J., Coelho, L. P., et al. (2025). AI-driven antimicrobial peptide discovery: mining and generation. Acc Chem. Res. 58, 1831–1846. doi: 10.1021/acs.accounts.0c00594
Tan, L., Chen, L., Yang, H., Jin, B., Kim, G., Im, Y. J., et al. (2022). Structural basis for activation of fungal sterol receptor Upc2 and azole resistance. Nat. Chem. Biol. 18, 1253–1262. doi: 10.1038/s41589-022-01117-0
Teixeira Mde, M., Patané, J. S., Taylor, M. L., Gómez, B. L., Theodoro, R. C., de Hoog, S., et al. (2016). Worldwide phylogenetic distributions and population dynamics of the genus histoplasma. PloS Negl. Trop. Dis. 10, e0004732. doi: 10.1371/journal.pntd.0004732
Thangamani, S., Mohammad, H., Abushahba, M. F., Sobreira, T. J., Hedrick, V. E., Paul, L. N., et al. (2016). Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci. Rep. 6, 22571. doi: 10.1038/srep22571
Thapliyal, P., Rautela, I., Rayal, R., Sudhanshu Verma, R., and Sharma, M. D. (2025). Navigating Endophytic Research for Next-Generation Therapeutics. Eds. Dwibedi, V., Kapoor, N., Gambhir, L., and Kajale, S. (Cambridge, MA, USA: Academic Press), 355–380.
Thompson, G. R., Soriano, A., Skoutelis, A., Vazquez, J. A., Honore, P. M., Horcajada, J. P., et al. (2021). Rezafungin versus caspofungin in a phase 2, randomized, double-blind study for the treatment of candidemia and invasive candidiasis: the STRIVE trial. Clin. Infect. Dis. 73, e3647–e3655. doi: 10.1093/cid/ciaa1380
Thompson, G. R., Soriano, A., Cornely, O. A., Kullberg, B. J., Kollef, M., Vazquez, J., et al. (2023). Rezafungin versus caspofungin for treatment of candidemia and invasive candidiasis (ReSTORE): a multicenter, double-blind, double-dummy, randomized phase 3 trial. Lancet 401, 49–59. doi: 10.1016/s0140-6736(22)02324-8
Thompson, G. R., Chen, S. C., Alfouzan, W. A., Izumikawa, K., Colombo, A. L., Maertens, J., et al. (2024a). A global perspective of the changing epidemiology of invasive fungal disease and real-world experience with the use of isavuconazole. Med. Mycol 62, myae083. doi: 10.1093/mmy/myae083
Thompson, G. R., Soriano, A., Honore, P. M., Bassetti, M., Cornely, O. A., Kollef, M., et al. (2024b). Efficacy and safety of rezafungin and caspofungin in candidemia and invasive candidiasis: pooled data from two prospective randomized controlled trials. Lancet Infect. Dis. 24, 319–328. doi: 10.1016/s1473-3099(23)00551-0
Tian, J., Wang, Y., Lu, Z., Sun, C., Zhang, M., Zhu, A., et al. (2016). Perillaldehyde, a Promising Antifungal Agent Used in Food Preservation, Triggers Apoptosis through a Metacaspase-Dependent Pathway in Aspergillus flavus. J. Agric. Food Chem. 64, 7404–7413. doi: 10.1021/acs.jafc.6b03546
Tian, X., Zhang, C., Qin, Z., Wang, D., Yang, J., Zhang, X., et al. (2021). Impact of CYP2C19 phenotype and drug-drug interactions on voriconazole concentration in pediatric patients. Antimicrob. Agents Chemother. 65, e0020721. doi: 10.1128/aac.00207-21
Tian, S., Wu, Y., Li, H., Rong, C., Wu, N., Chu, Y., et al. (2024). Evolutionary accumulation of FKS1 mutations from clinical echinocandin-resistant Candida auris. Emerg. Microbes Infect. 13, 2377584. doi: 10.1080/22221751.2024.2377584
Ting, D. S. J., Beuerman, R. W., Dua, H. S., Lakshminarayanan, R., and Mohammed, I. (2020). Strategies in translating the therapeutic potentials of host defense peptides. Front. Immunol. 11. doi: 10.3389/fimmu.2020.00983
Tong, Y., Zhang, J., Sun, N., Wang, X. M., Wei, Q., Zhang, Y., et al. (2021). Berberine reverses multidrug resistance in Candida albicans by hijacking the drug efflux pump Mdr1p. Sci. Bull. (Beijing) 66, 1895–1905. doi: 10.1016/j.scib.2020.12.035
Torosantucci, A., Bromuro, C., Chiani, P., De Bernardis, F., Berti, F., Galli, C., et al. (2005). A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 202, 597–606. doi: 10.1084/jem.20050749
Townsend, R., Dietz, A., Hale, C., Akhtar, S., Kowalski, D., Lademacher, C., et al. (2017). Pharmacokinetic evaluation of CYP3A4-mediated drug-drug interactions of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethindrone in healthy adults. Clin. Pharmacol. Drug Dev. 6, 44–53. doi: 10.1002/cpdd.285
Treviño-Rangel, R. J., Villanueva-Lozano, H., Méndez-Galomo, K. S., Solís-Villegas, E. M., Becerril-García, M. A., Montoya, A. M., et al. (2019). In vivo evaluation of the antifungal activity of sertraline against Aspergillus fumigatus. J. antimicrobial. chemother. 74, 663–666. doi: 10.1093/jac/dky455
Treviño-Rangel, R. J., González, G. M., Montoya, A. M., Rojas, O. C., Elizondo-Zertuche, M., Álvarez-Villalobos, N. A., et al. (2022). Recent Antifungal Pipeline Developments against Candida auris: A Systematic Review. J. Fungi (Basel) 8, 1144. doi: 10.3390/jof8111144
Trindade, L. A., Cordeiro, L. V., de Figuerêdo Silva, D., Figueiredo, P. T. R., de Pontes, M. L. C., de Oliveira Lima, E., et al. (2022). The antifungal and antibiofilm activity of Cymbopogon nardus essential oil and citronellal on clinical strains of Candida albicans. Braz. J. Microbiol. 53, 1231–1240. doi: 10.1007/s42770-022-00740-2
Tsang, P. W., Bandara, H. M., and Fong, W. P. (2012). Purpurin suppresses Candida albicans biofilm formation and hyphal development. PloS One 7, e50866. doi: 10.1371/journal.pone.0050866
Tuci, S., Mercorelli, B., and Loregian, A. (2025). Antiviral drug repurposing: different approaches and the case of antifungal drugs. Pharmacol. Ther. 273, 108903. doi: 10.1016/j.pharmthera.2025.108903
Tufail, M., Jiang, C. H., and Li, N. (2025). Immune evasion in cancer: mechanisms and cutting-edge therapeutic approaches. Signal Transduct Target Ther. 10, 227. doi: 10.1038/s41392-025-02280-1
Tugume, L., Ssebambulidde, K., Kasibante, J., Ellis, J., Wake, R. M., Gakuru, J., et al. (2023). Cryptococcal meningitis. Nat. Rev. Dis. Primers 9, 62. doi: 10.1038/s41572-023-00472-z
Tyers, M. and Wright, G. D. (2019). Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 17, 141–155. doi: 10.1038/s41579-018-0141-x
Uribe-Querol, E. and Rosales, C. (2020). Phagocytosis: our current understanding of a universal biological process. Front. Immunol. 11. doi: 10.3389/fimmu.2020.01066
Usher, J. and Haynes, K. (2019). Attenuating the emergence of anti-fungal drug resistance by harnessing synthetic lethal interactions in a model organism. PloS Genet. 15, e1008259. doi: 10.1371/journal.pgen.1008259
Vandeputte, P., Ferrari, S., and Coste, A. T. (2012). Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012, 713687. doi: 10.1155/2012/713687
van de Veerdonk, F. L., Gresnigt, M. S., Romani, L., Netea, M. G., and Latgé, J. P. (2017). Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 15, 661–674. doi: 10.1038/nrmicro.2017.90
Vazquez, J. A., Pappas, P. G., Boffard, K., Paruk, F., Bien, P. A., Tawadrous, M., et al. (2023). Clinical efficacy and safety of a novel antifungal, fosmanogepix, in patients with candidemia caused by candida auris: results from a phase 2 trial. Antimicrob. Agents Chemother. 67, e0141922. doi: 10.1128/aac.01419-22
Venancio, A. N., Silva, M. J., Parreira, L. A., Júlio, A. A., Souza, G. R., Conceição Santos, M. F., et al. (2025). Citronellal: a natural aldehyde with important properties. Nat. Prod Res. 39, 1199–1212. doi: 10.1080/14786419.2024.2332949
Vermitsky, J. P. and Edlind, T. D. (2004). Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob. Agents Chemother. 48, 3773–3781. doi: 10.1128/aac.48.10.3773-3781.2004
Verweij, P. E., Lucas, J. A., Arendrup, M. C., Bowyer, P., Brinkmann, A. J. F., Denning, D. W., et al. (2020). The one health problem of azole resistance in Aspergillus fumigatus: current insights and future research agenda. Fungal Biol. Rev. 34, 202–214. doi: 10.1016/j.fbr.2020.10.003
Viana, R., Carreiro, T., Couceiro, D., Dias, O., Rocha, I., Teixeira, M. C., et al. (2023). Metabolic reconstruction of the human pathogen Candida auris: using a cross-species approach for drug target prediction. FEMS Yeast Res. 23, foad045. doi: 10.1093/femsyr/foad045
Vishwakarma M, H. T. and Soni, V. (2024). Update on fungal lipid biosynthesis inhibitors as antifungal agents. Microbiol. Res. 278, 127517. doi: 10.1016/j.micres.2023.127517
Vitiello, A., Ferrara, F., Boccellino, M., Ponzo, A., Cimmino, C., Comberiati, E., et al. (2023). Antifungal drug resistance: an emergent health threat. Biomedicines 11, 1063. doi: 10.3390/biomedicines11041063
Walker, L. A., Gow, N. A. R., and Munro, C. A. (2010). Fungal echinocandin resistance. Fungal Genet. Biol. 47, 117–126. doi: 10.1016/j.fgb.2009.09.003
Wall, G. and Lopez-Ribot, J. L. (2020). Screening repurposing libraries for identification of drugs with novel antifungal activity. Antimicrob. Agents Chemother. 64, e00924–20. doi: 10.1128/aac.00924-20
Wang, X., Mohammad, I. S., Fan, L., Zhao, Z., Nurunnabi, M., Sallam, M. A., et al. (2021). Delivery strategies of amphotericin B for invasive fungal infections. Acta Pharm. Sin. B 11, 2585–2604. doi: 10.1016/j.apsb.2021.04.010
Wang, L., Wang, N., Zhang, W., Cheng, X., Yan, Z., Shao, G., et al. (2022). Therapeutic peptides: current applications and future directions. Signal Transduct Target Ther. 7, 48. doi: 10.1038/s41392-022-00904-4
Wang, X., Chen, L., Ruan, H., Xiong, Z., Wang, W., Qiu, J., et al. (2024). Oteseconazole versus fluconazole for the treatment of severe vulvovaginal candidiasis: a multicenter, randomized, double-blinded, phase 3 trial. Antimicrob. Agents Chemother. 68, e0077823. doi: 10.1128/aac.00778-23
Wang, Y., Song, M., Liu, F., Liang, Z., Hong, R., Dong, Y., et al. (2025). Artificial intelligence using a latent diffusion model enables the generation of diverse and potent antimicrobial peptides. Sci. Adv. 11, eadp7171. doi: 10.1126/sciadv.adp7171
Wang, B., Lin, P., Zhong, Y., Tan, X., Shen, Y., Huang, Y., et al. (2025). Explainable deep learning and virtual evolution identifies antimicrobial peptides with activity against multidrug-resistant human pathogens. Nat. Microbiol. 10, 332–347. doi: 10.1038/s41564-024-01907-3
Wang, Y., Wang, K., Masso-Silva, J. A., Rivera, A., and Xue, C. (2019). A heat-killed cryptococcus mutant strain induces host protection against multiple invasive mycoses in a murine vaccine model. mBio 10, e02145–19. doi: 10.1128/mBio.02145-19
Wei, H., Zhang, Y., Jia, Y., Chen, X., Niu, T., Chatterjee, A., et al. (2024). Heat shock protein 90: biological functions, diseases, and therapeutic targets. MedComm (2020) 5, e470. doi: 10.1002/mco2.470
Wei, G. X., Xu, X., and Wu, C. D. (2011). In vitro synergism between berberine and miconazole against planktonic and biofilm Candida cultures. Arch. Oral. Biol. 56, 565–572. doi: 10.1016/j.archoralbio.2010.11.021
Wen, W., Cao, H., Huang, Y., Tu, J., Wan, C., Wan, J., et al. (2022). Structure-guided discovery of the novel covalent allosteric site and covalent inhibitors of fructose-1,6-bisphosphate aldolase to overcome the azole resistance of candidiasis. J. Med. Chem. 65, 2656–2674. doi: 10.1021/acs.jmedchem.1c02102
Wiederhold, N. P. (2020). Review of the novel investigational antifungal olorofim. J. Fungi (Basel) 6, 122. doi: 10.3390/jof6030122
Wiederhold, N. P. (2021). Review of T-2307, an investigational agent that causes collapse of fungal mitochondrial membrane potential. J. Fungi (Basel) 7, 130. doi: 10.3390/jof7020130
Wiederhold, N. P. (2022). Pharmacodynamics, mechanisms of action and resistance, and spectrum of activity of new antifungal agents. J. Fungi (Basel) 8, 857. doi: 10.3390/jof8080857
Wiederhold, N. P., Najvar, L. K., Jaramillo, R., Olivo, M., Patterson, H., Connell, A., et al. (2020). The Novel Arylamidine T-2307 Demonstrates In Vitro and In Vivo Activity against Candida auris. Antimicrob. Agents Chemother. 64, 3063–3067. doi: 10.1128/aac.02198-19
Wiederhold, N. P., Locke, J. B., Daruwala, P., and Bartizal, K. (2018). Rezafungin (CD101) demonstrates potent in vitro activity against Aspergillus, including azole-resistant Aspergillus fumigatus isolates and cryptic species. J. Antimicrob. Chemoth 73, 3063–3067. doi: 10.1093/jac/dky280
Wiederhold, N. P., Najvar, L. K., Bocanegra, R., Graybill, J. R., and Patterson, T. F. (2010). Efficacy of posaconazole as treatment and prophylaxis against Fusarium solani. Antimicrob. Agents Chemother. 54, 1055–1059. doi: 10.1128/aac.01445-09
Wijnants, S., Vreys, J., and Van Dijck, P. (2022). Interesting antifungal drug targets in the central metabolism of Candida albicans. Trends Pharmacol. Sci. 43, 69–79. doi: 10.1016/j.tips.2021.10.003
Woods, J. P. (2016). Revisiting old friends: Developments in understanding Histoplasma capsulatum pathogenesis. J. Microbiol. 54, 265–276. doi: 10.1007/s12275-016-6044-5
Wu, X. Z., Cheng, A. X., Sun, L. M., and Lou, H. X. (2008). Effect of plagiochin E, an antifungal macrocyclic bis(bibenzyl), on cell wall chitin synthesis in Candida albicans. Acta Pharmacol. Sin. 29, 1478–1485. doi: 10.1111/j.1745-7254.2008.00900.x
Wu, Y., Zhang, M., Yang, Y., Ding, X., Yang, P., Huang, K., et al. (2022). Structures and mechanism of chitin synthase and its inhibition by antifungal drug Nikkomycin Z. Cell Discov. 8, 129. doi: 10.1038/s41421-022-00495-y
Wu, S., Song, R., Liu, T., and Li, C. (2023). Antifungal therapy: Novel drug delivery strategies driven by new targets. Adv. Drug Delivery Rev. 199, 114967. doi: 10.1016/j.addr.2023.114967
Wychrij, D. A., Chapman, T. I., Rayens, E., Rabacal, W., Willems, H. M. E., Oworae, K. O., et al. (2025). Protective efficacy of the pan-fungal vaccine NXT-2 against vulvovaginal candidiasis in a murine model. NPJ Vaccines 10, 112. doi: 10.1038/s41541-025-01171-4
Xiao, M., Fan, X., Chen, S. C., Wang, H., Sun, Z. Y., Liao, K., et al. (2015). Antifungal susceptibilities of Candida glabrata species complex, Candida krusei, Candida parapsilosis species complex and Candida tropicalis causing invasive candidiasis in China: 3 year national surveillance. J. Antimicrob. Chemother. 70, 802–810. doi: 10.1093/jac/dku460
Xiong, J., Wang, L., Feng, Y., Zhen, C., Hang, S., Yu, J., et al. (2024). Geldanamycin confers fungicidal properties to azole by triggering the activation of succinate dehydrogenase. Life Sci. 348, 122699. doi: 10.1016/j.lfs.2024.122699
Yaakoub, H., Mina, S., Calenda, A., Bouchara, J. P., and Papon, N. (2022). Oxidative stress response pathways in fungi. Cell Mol. Life Sci. 79, 333. doi: 10.1007/s00018-022-04353-8
Yamaki, H., Yamaguchi, M., Tsuruo, T., and Yamaguchi, H. (1992). Mechanism of action of an antifungal antibiotic, RI-331, (S) 2-amino-4-oxo-5-hydroxypentanoic acid; kinetics of inactivation of homoserine dehydrogenase from Saccharomyces cerevisiae. J. Antibiot. (Tokyo) 45, 750–755. doi: 10.7164/antibiotics.45.750
Yamashita, K., Miyazaki, T., Fukuda, Y., Mitsuyama, J., Saijo, T., Shimamura, S., et al. (2019). The novel arylamidine T-2307 selectively disrupts yeast mitochondrial function by inhibiting respiratory chain complexes. Antimicrob. Agents Chemother. 63, e00374–19. doi: 10.1128/aac.00374-19
Yang, S., Fu, Y., Wu, X., Zhou, Z., Xu, J., Zeng, X., et al. (2014). Baicalin prevents Candida albicans infections via increasing its apoptosis rate. Biochem. Biophys. Res. Commun. 451, 36–41. doi: 10.1016/j.bbrc.2014.07.040
Yang, H., Tong, J., Lee, C. W., Ha, S., Eom, S. H., Im, Y. J., et al. (2015). Structural mechanism of ergosterol regulation by fungal sterol transcription factor Upc2. Nat. Commun. 6, 6129. doi: 10.1038/ncomms7129
Yang, L. B., Guo, G., Tian, Z. Q., Zhou, L. X., Zhu, L. J., Peng, J., et al. (2022). TMT-based quantitative proteomic analysis of the effects of novel antimicrobial peptide AMP-17 against Candida albicans. J. Proteomics 250, 104385. doi: 10.1016/j.jprot.2021.104385
Yin, K., Li, R., Zhang, S., Sun, Y., Huang, L., Jiang, M., et al. (2025). Deep learning combined with quantitative structure–Activity relationship accelerates de novo design of antifungal peptides. Adv. Sci. (Weinh) 12, e2412488. doi: 10.1002/advs.202412488
Yousfi, H., Cassagne, C., Ranque, S., Rolain, J. M., and Bittar, F. (2019). Repurposing of ribavirin as an adjunct therapy against invasive candida strains in an in vitro study. Antimicrobial. Agents chemother. 63, e00263–19. doi: 10.1128/aac.00263-19
Yu, Q., Ding, X., Xu, N., Cheng, X., Qian, K., Zhang, B., et al. (2013). In vitro activity of verapamil alone and in combination with fluconazole or tunicamycin against Candida albicans biofilms. Int. J. antimicrobial. Agents 41, 179–182. doi: 10.1016/j.ijantimicag.2012.10.009
Yu, Q., Ding, X., Zhang, B., Xu, N., Jia, C., Mao, J., et al. (2014). Inhibitory effect of verapamil on Candida albicans hyphal development, adhesion and gastrointestinal colonization. FEMS yeast Res. 14, 633–641. doi: 10.1111/1567-1364.12150
Yu, L. H., Wei, X., Ma, M., Chen, X. J., and Xu, S. B. (2012). Possible inhibitory molecular mechanism of farnesol on the development of fluconazole resistance in Candida albicans biofilm. Antimicrob. Agents Chemother. 56, 770–775. doi: 10.1128/aac.05290-11
Zajac, C., Scott, N. E., Kline, S., Erayil, S. E., and Selmecki, A. (2025). Hotspot gene conversion between FKS1 and FKS2 in echinocandin resistant Candida glabrata serial isolates. NPJ Antimicrobials Resistance 3, 31. doi: 10.1038/s44259-025-00102-6
Zeng, Q., Zhang, Z., Chen, P., Long, N., Lu, L., Sang, H., et al. (2019). In vitro and in vivo Efficacy of a Synergistic Combination of Itraconazole and Verapamil Against Aspergillus fumigatus. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.01266
Zgadzay, Y., Kolosova, O., Stetsenko, A., Wu, C., Bruchlen, D., Usachev, K., et al. (2022). E-site drug specificity of the human pathogen Candida albicans ribosome. Sci. Adv. 8, eabn1062. doi: 10.1126/sciadv.abn1062
Zhai, B., Ola, M., Rolling, T., Tosini, N. L., Joshowitz, S., Littmann, E. R., et al. (2020). High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis. Nat. Med. 26, 59–64. doi: 10.1038/s41591-019-0709-7
Zhai, B., Liao, C., Jaggavarapu, S., Tang, Y., Rolling, T., Ning, Y., et al. (2024). Antifungal heteroresistance causes prophylaxis failure and facilitates breakthrough Candida parapsilosis infections. Nat. Med. 30, 3163–3172. doi: 10.1038/s41591-024-03183-4
Zhai, B., Rolling, T., and Hohl, T. M. (2021). Exploring Candida auris in its habitat. Cell Host Microbe 29, 150–151. doi: 10.1016/j.chom.2021.01.010
Zhang, L., Wang, C., Chen, K., Zhong, W., Xu, Y., Molnár, I., et al. (2023). Engineering the biosynthesis of fungal nonribosomal peptides. Nat. Prod Rep. 40, 62–88. doi: 10.1039/d2np00036a
Zhang, C. W., Zhong, X. J., Zhao, Y. S., Rajoka, M. S. R., Hashmi, M. H., Zhai, P., et al. (2023). Antifungal natural products and their derivatives: A review of their activity and mechanism of actions. Pharmacol. Res. - Modern Chin. Med. 7, 100262. doi: 10.1016/j.prmcm.2023.100262
Zhang, Z. H., Sun, L. L., Fu, B. Q., Deng, J., Jia, C. L., Miao, M. X., et al. (2024). Aneuploidy underlies brefeldin A-induced antifungal drug resistance in Cryptococcus neoformans. Front. Cell Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1397724
Zhang, Y., Zeng, L., Huang, X., Wang, Y., Chen, G., Moses, M., et al. (2025). Targeting epigenetic regulators to overcome drug resistance in the emerging human fungal pathogen Candida auris. Nat. Commun. 16, 4668. doi: 10.1038/s41467-025-59898-6
Zhang, M., Ge, J., and Yu, X. (2018). Transcriptome Analysis Reveals the Mechanism of Fungicidal of Thymol Against Fusarium oxysporum f. sp. niveum. Curr. Microbiol. 75, 410–419. doi: 10.1007/s00284-017-1396-6
Zhang, Q., Liu, F., Zeng, M., Mao, Y., and Song, Z. (2021). Drug repurposing strategies in the development of potential antifungal agents. Appl. Microbiol. Biotechnol. 105, 5259–5279. doi: 10.1007/s00253-021-11407-7
Zhang, M., Yan, H., Lu, M., Wang, D., and Sun, S. (2020). Antifungal activity of ribavirin used alone or in combination with fluconazole against Candida albicans is mediated by reduced virulence. Int. J. antimicrobial. Agents 55, 105804. doi: 10.1016/j.ijantimicag.2019.09.008
Zhen, C., Wang, L., Feng, Y., Whiteway, M., Hang, S., Yu, J., et al. (2024). Otilonium bromide exhibits potent antifungal effects by blocking ergosterol plasma membrane localization and triggering cytotoxic autophagy in candida albicans. Adv. Sci. (Weinh) 11, e2406473. doi: 10.1002/advs.202406473
Zhou, Y., Liao, M., Zhu, C., Hu, Y., Tong, T., Peng, X., et al. (2018). ERG3 and ERG11 genes are critical for the pathogenesis of Candida albicans during the oral mucosal infection. Int. J. Oral. Sci. 10, 9. doi: 10.1038/s41368-018-0013-2
Zhou, Z. X., Yin, X. D., Zhang, Y., Shao, Q. H., Mao, X. Y., Hu, W. J., et al. (2022). Antifungal drugs and drug-induced liver injury: A real-world study leveraging the FDA adverse event reporting system database. Front. Pharmacol. 13. doi: 10.3389/fphar.2022.891336
Zhou, X., Hilk, A., Solis, N. V., Scott, N., Beach, A., Soisangwan, N., et al. (2024). Single-cell detection of copy number changes reveals dynamic mechanisms of adaptation to antifungals in Candida albicans. Nat. Microbiol. 9, 2923–2938. doi: 10.1038/s41564-024-01795-7
Zhou, Z., Shyti, I., Kim, J., and You, L. (2025). Predicting population dynamics of antimicrobial resistance using mechanistic modeling and machine learning. Adv. Drug Delivery Rev. 225, 115661. doi: 10.1016/j.addr.2025.115661
Zhu, S. L., Yan, L., Zhang, Y. X., Jiang, Z. H., Gao, P. H., Qiu, Y., et al. (2014). Berberine inhibits fluphenazine-induced up-regulation of CDR1 in Candida albicans. Biol. Pharm. Bull. 37, 268–273. doi: 10.1248/bpb.b13-00734
Zhu, P., Li, Y., Guo, T., Liu, S., Tancer, R. J., Hu, C., et al. (2023). New antifungal strategies: Drug combination and co-delivery. Adv. Drug Delivery Rev. 198, 114874. doi: 10.1016/j.addr.2023.114874
Keywords: fungal infections, antifungal resistance, antifungal agents, drug development, drug design
Citation: Li Y, Liu Y, Jiang Y, Yang Y, Ni W, Zhang W and Tan L (2025) New antifungal strategies and drug development against WHO critical priority fungal pathogens. Front. Cell. Infect. Microbiol. 15:1662442. doi: 10.3389/fcimb.2025.1662442
Received: 09 July 2025; Accepted: 18 August 2025;
Published: 25 September 2025.
Edited by:
Yuanwei Zhang, Nanjing Normal University, ChinaReviewed by:
Estela Ruiz-Baca, Juárez University of the State of Durango, MexicoAntonio Vitiello, Ministry of Health, Rome, Italy
Hu Pengjie, Chinese Academy of Sciences (CAS), China
Copyright © 2025 Li, Liu, Jiang, Yang, Ni, Zhang and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingchen Tan, dGFubGluZ2NoZW5AbWFpbC5uZXUuZWR1LmNu
 Yanjian Li
Yanjian Li Yang Liu2
Yang Liu2 Lingchen Tan
Lingchen Tan