- 1Department of Otolaryngology-Head and Neck Surgery, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan
- 2Department of Infectious Diseases and Laboratory Medicine, Kanazawa University, Kanazawa, Ishikawa, Japan
- 3Sendai Sta. North Gate ENT. Clinic, Sendai, Miyagi, Japan
- 4Center for Otologic Surgery, Sen-en Rifu Hospital, Sendai, Miyagi, Japan
- 5Department of Otolaryngology-Head and Neck Surgery, Iwate Medical University, School of Medicine, Shiwa, Iwate, Japan
Introduction: Haemophilus influenzae and Streptococcus pneumoniae are two of the major pathogens responsible for pediatric rhinosinusitis. Rising antimicrobial resistance (AMR) and pneumococcal serotype replacement have complicated treatment decisions. This study aimed to investigate bacterial distribution and AMR patterns in nasal discharge samples from children at a Japanese otolaryngology clinic.
Methods: We conducted a retrospective study at an otolaryngology clinic in Sendai, Japan, from February 2023 to March 2025. A total of 2009 nasal discharge specimens were analyzed. Bacterial identification and antimicrobial susceptibility testing were performed according to Clinical and Laboratory Standards Institute guidelines. H. influenzae and S. pneumoniae were phenotypically classified and stratified by age (0-2, 3-5, and 6-13 years). Age-group comparisons were performed using Fisher’s exact test with Holm correction.
Results: Pathogens were detected in 1862 samples (92.7%). The most frequently isolated organisms were Moraxella catarrhalis (30.9%), H. influenzae (23.0%), and S. pneumoniae (20.6%). Among the 697 H. influenzae isolates, 44.8% were ampicillin-resistant, and 31.3% of all isolates were β-lactamase-negative ampicillin-resistant (BLNAR) strains. Some BLNAR strains exhibited reduced susceptibility to amoxicillin–clavulanic acid (MIC90 = 8 μg/mL). Cefotaxime, cefditoren, and levofloxacin remained highly active. Among the 625 S. pneumoniae isolates, 66.6% were penicillin-susceptible, 31.0% were intermediate, and 2.4% were resistant; resistance to clarithromycin was observed in 84.3% of isolates. The prevalence of Staphylococcus aureus increased with age, with 25% of isolates in the 6-13-year group identified as methicillin-resistant.
Conclusion: H. influenzae and S. pneumoniae remain key pathogens in pediatric rhinosinusitis and exhibit high AMR rates. Age-specific trends, including increased methicillin-resistant S. aureus in older children, should guide empiric therapy. Ongoing AMR surveillance and culture-based management are essential.
1 Introduction
Rhinosinusitis and related otorhinolaryngologic infections are common in both children and adults, often developing as bacterial complications of viral upper respiratory tract infections. The causative pathogens of rhinosinusitis differ between pediatric and adult populations. In children, Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis are most frequently implicated (Sawada and Matsubara, 2021). Over the past two decades, the emergence and spread of antimicrobial resistance among otorhinolaryngologic pathogens has become a major public health concern. In Japan, the prevalence of penicillin-resistant S. pneumoniae (PRSP) and ampicillin-resistant H. influenzae—particularly β-lactamase-negative ampicillin-resistant (BLNAR) strains—increased significantly during the 2000s (Suzuki et al., 2020). To prevent severe pneumococcal infections, Japan introduced the 7-valent pneumococcal conjugate vaccine (PCV7) in April 2013, followed by the 13-valent vaccine (PCV13) in November of the same year. Although the prevalence of vaccine-type strains declined sharply, non-vaccine, multidrug-resistant serotypes such as 23A, 15A, and 35B have since emerged as dominant (Kawaguchiya et al., 2024). To address this, newer conjugate vaccines—PCV15 (approved for children in June 2023) and PCV20 (approved in March 2024)—have been introduced and are expected to enhance serotype coverage. In contrast, no widely used vaccine exists for non-typeable H. influenzae, making antimicrobial stewardship critical for managing resistance. National surveillance data show that the prevalence of BLNAR strains remains high, at approximately 40-50% (Yamada et al., 2020).
Thus, otorhinolaryngologic infections, particularly rhinosinusitis, represent an especially important disease group in children. Among them, acute otitis media (AOM) is one of the most common pediatric infections and often develops as a complication of viral upper respiratory tract infections. By school age, approximately 80-90% of children experience otitis media, with S. pneumoniae, non-typeable H. influenzae, and M. catarrhalis being the predominant causative pathogens. The pathogenesis of AOM shares several mechanisms with rhinosinusitis, most notably contiguous mucosal inflammation and Eustachian tube dysfunction. Furthermore, because the nasopharynx and middle ear are anatomically connected via the Eustachian tube and harbor overlapping bacterial pathogens, a strong clinical and microbiological association between the two diseases has been demonstrated (Xu et al., 2019; Silva et al., 2021).
In response, the 2024 revision of the Japanese Pediatric AOM Guidelines recommended high-dose amoxicillin as the first-line therapy, and the position of watchful waiting was reiterated as a strategy to reduce the selective pressure for antimicrobial resistance (The Japanese Society for Pediatric Otorhinolaryngology, 2024). Nevertheless, despite these vaccination and guideline-based interventions, up-to-date epidemiological data from routine clinical settings remain scarce.
Therefore, we conducted this study using nasal discharge culture results collected from an otolaryngology clinic in Sendai between 2022 and 2025 to clarify the distribution of bacterial species and recent trends in antimicrobial susceptibility in the context of new vaccine implementation and evolving clinical practice guidelines.
2 Materials and methods
2.1 Study design and setting
We conducted a retrospective observational study using microbiological data from nasal discharge cultures and clinical information from patients treated at the Sendai sta. North Gate ENT. Clinic in Miyagi Prefecture, Japan. This clinic provides outpatient otolaryngologic care to patients of all ages. The study period spanned from February 2023 to March 2025. All nasal discharge specimens submitted for bacterial culture during this period were included in the analysis. These specimens were typically collected from patients presenting with symptoms of rhinosinusitis or persistent rhinorrhea suspected to be of bacterial origin. Patients who underwent fungal cultures only or viral testing only were excluded. Clinical information, including age and sex, was extracted from the patients’ medical records. When multiple specimens were collected from the same patient during the study period, each specimen was treated as an independent observational unit. Additionally, if the same bacterial species was repeatedly isolated from a single patient, each isolate was considered a separate episode if it was determined to represent a distinct infectious event, such as a recurrence. The primary outcomes of this study were the distribution of bacterial species isolated and the proportion of isolates exhibiting key antimicrobial resistance phenotypes.
2.2 Microbiological analysis
Nasal discharge specimens were submitted to the microbiology laboratory of BML Inc., a commercial facility located in Saitama Prefecture, Japan, where they were processed using standard bacterial culture techniques. Samples were inoculated onto appropriate media (e.g., blood agar, chocolate agar) and incubated under conditions suitable for both aerobic and facultative organisms. Bacterial identification was primarily performed using conventional methods, including colony morphology, hemolysis patterns, Gram staining, and biochemical testing. S. pneumoniae was identified based on α-hemolytic colonies, characteristic morphology, and optochin susceptibility. H. influenzae was identified by its colony appearance and its requirement for both X and V growth factors. Staphylococcus aureus was confirmed based on colony morphology and with automated identification systems such as the WalkAway® DXM1096 system (Beckman Coulter, Brea, CA, USA) and the VITEK® 2 XL (blue) system (bioMérieux, Marcy-l’Étoile, France). When conventional or automated methods were insufficient, mass spectrometry–based platforms such as the VITEK® MS system (bioMérieux) or MALDI Biotyper Sirius system (Bruker Daltonics, Bremen, Germany) were used for definitive identification. Isolates considered part of the normal commensal flora (e.g., coagulase-negative staphylococci, Corynebacterium spp.) were excluded as causative pathogens. When more than one potential pathogen was isolated from a specimen, each isolate was included in the frequency analysis.
Antimicrobial susceptibility testing was performed using the broth microdilution method with either the WalkAway® DXM1096 system (Beckman Coulter, Brea, CA, USA) or the VITEK® 2 XL (blue) system (bioMérieux, Marcy-l’Étoile, France), in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines (M100-S26 and 29th edition) (Clinical and Laboratory Standards Institute, 2019). Minimum inhibitory concentrations (MICs) and susceptibility breakpoints were determined for H. influenzae and S. pneumoniae. For S. pneumoniae, the antimicrobial agents tested included penicillin G, amoxicillin–clavulanic acid (2:1 ratio), cefotaxime, cefditoren, levofloxacin, clarithromycin, vancomycin, and meropenem. For H. influenzae, the agents tested were ampicillin, amoxicillin–clavulanic acid (2:1 ratio), cefotaxime, cefditoren, levofloxacin, clarithromycin, and meropenem. S. pneumoniae isolates were categorized based on CLSI susceptibility breakpoints for oral penicillin V in non-meningitis respiratory infections as follows: susceptible (MIC ≤ 0.06 μg/mL), intermediate (MIC = 0.12-1 μg/mL), and resistant (MIC ≥ 2 μg/mL). In this study, isolates interpreted as intermediate or resistant were considered penicillin-nonsusceptible S. pneumoniae. H. influenzae isolates were classified into five categories according to CLSI criteria (Clinical and Laboratory Standards Institute, 2019): β-lactamase–nonproducing ampicillin-susceptible (BLNAS; MIC of ampicillin ≤ 1 μg/mL), β-lactamase–nonproducing ampicillin-intermediately resistant (low-BLNAR; MIC of ampicillin = 2 μg/mL), β-lactamase–nonproducing ampicillin-resistant (BLNAR; MIC of ampicillin ≥ 4 μg/mL), β-lactamase–producing ampicillin-resistant (BLPAR; MIC of amoxicillin–clavulanic acid ≤4 mg/L), β-lactamase–producing amoxicillin–clavulanic acid–resistant (BLPACR; MIC of amoxicillin–clavulanic acid ≥8 mg/L). β-lactamase production was confirmed using the nitrocefin test (Showa Chemical, Tokyo, Japan). S. aureus isolates were defined as methicillin-resistant S. aureus (MRSA) if they exhibited resistance to either oxacillin or cefoxitin.
2.3 Statistical analysis
The results were expressed as medians with interquartile ranges or as proportions relative to the total number of patients or isolates. Differences in the distribution of bacterial species among the three age groups (0-2, 3-5, and 6-13 years) were analyzed using Fisher’s exact test for categorical variables. When significant differences were observed, pairwise comparisons between age groups were conducted using the Steel–Dwass test. p-values were adjusted for multiple comparisons using the Holm method. All statistical analyses were performed using GraphPad Prism version 10 (GraphPad Software, San Diego, CA, USA).
2.4 Ethical considerations
This study was conducted in accordance with the principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Biological Research Involving Human Subjects issued by the Japanese government. The study protocol was reviewed and approved by the Institutional Review Board of Sen-en Rifu Hospital (Approval No. 20250408). Given the retrospective nature of the study and the use of de-identified culture data, the requirement for informed consent was waived by the Institutional Review Board.
3 Results
3.1 Patient and sample characteristics
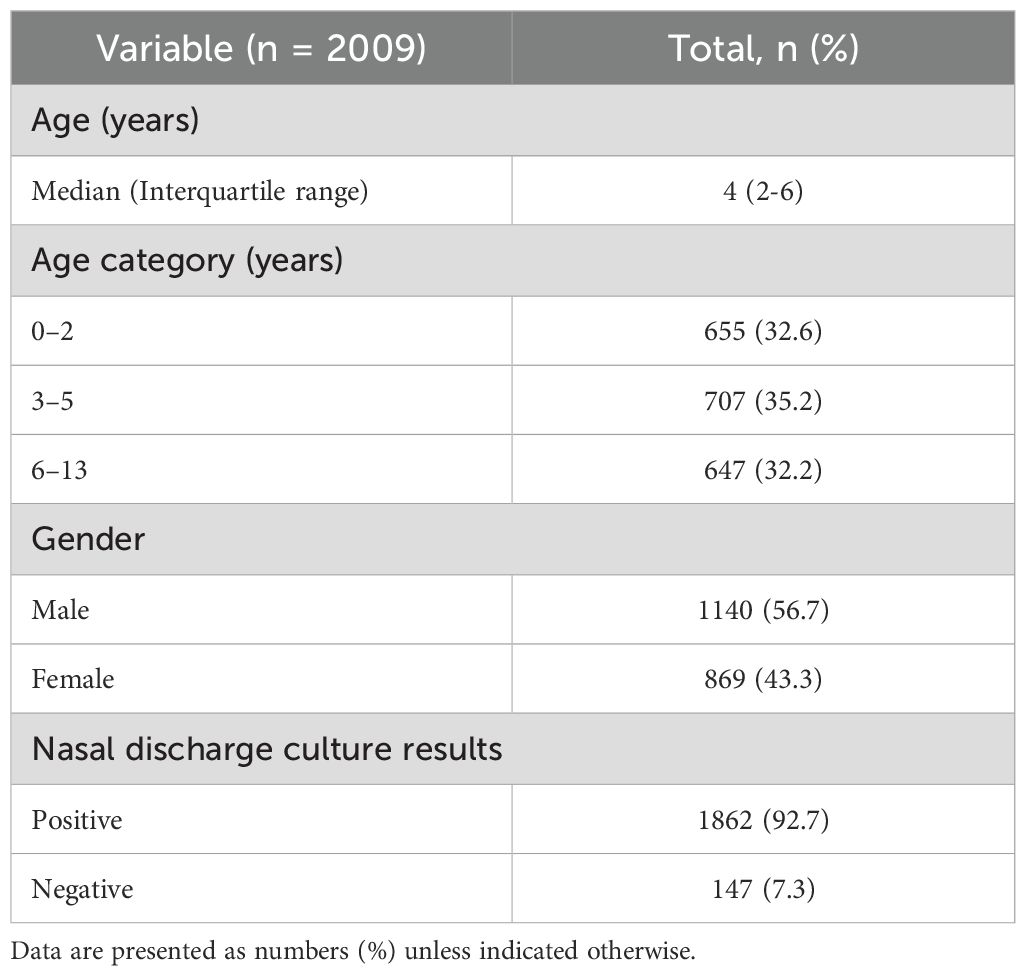
Between February 2023 and March 2025, a total of 2009 nasal discharge specimens were collected. The demographic and clinical characteristics of patients who underwent nasal discharge culture are summarized in Table 1. The median age was 4 years (interquartile range: 2-6 years). Most patients were young children, with 32.6% aged 0-2 years, 35.2% aged 3-5 years, and 32.2% aged 6-13 years. There was a slight male predominance: 1140 males (56.7%) and 869 females (43.3%).
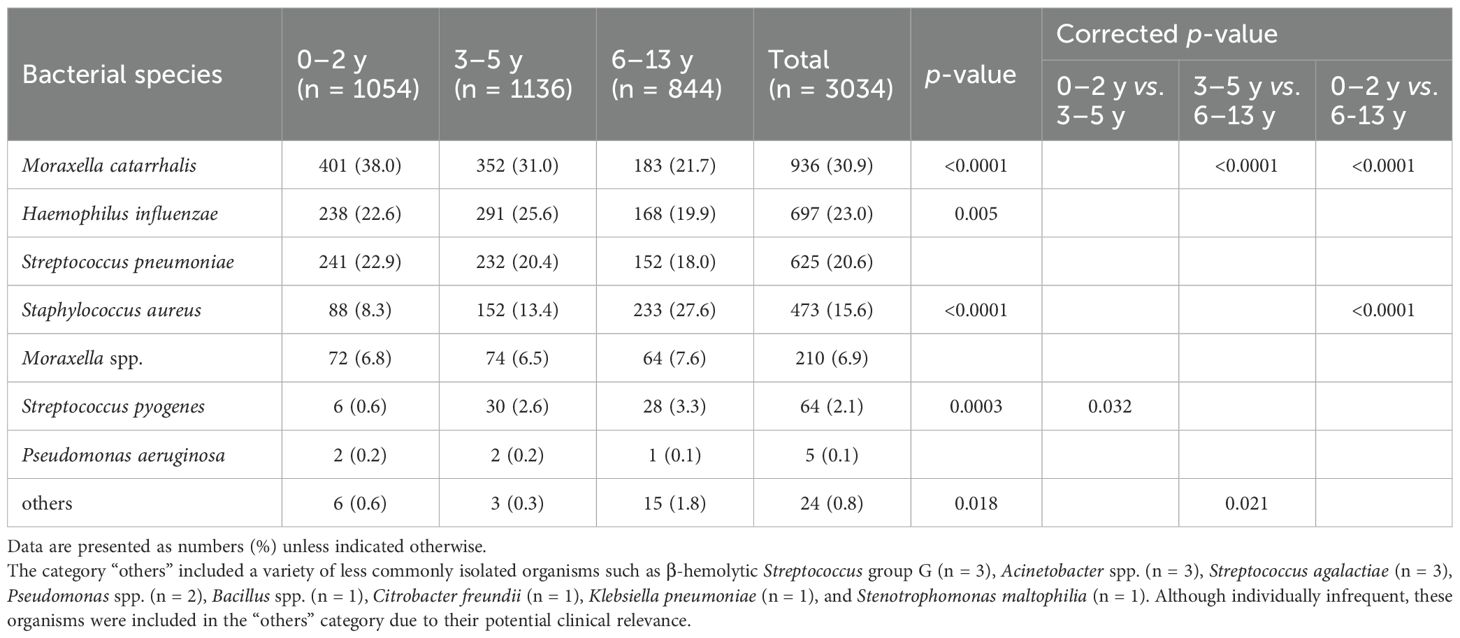
Of the 2009 specimens, 1862 (92.7%) yielded one or more bacterial species with pathogenic potential, while 147 (7.3%) were either culture-negative or grew only normal nasal flora. In total, 3034 bacterial isolates were identified. The most frequently identified organism was M. catarrhalis (936 isolates, 30.9%), followed by H. influenzae (697 isolates, 23.0%) and S. pneumoniae (625 isolates, 20.6%). S. aureus accounted for 473 isolates (15.6%), other Moraxella species for 210 isolates (6.9%), and Streptococcus pyogenes for 64 isolates (2.1%). Pseudomonas aeruginosa was detected in 5 isolates (0.2%).
3.2 Antimicrobial susceptibility patterns of H. influenzae and S. pneumoniae
The antimicrobial susceptibility profiles of H. influenzae (n = 697) and S. pneumoniae (n = 625) are summarized in Table 2. Among H. influenzae isolates, 44.8% were classified as ampicillin-resistant, comprising 31.3% BLNAR, 12.5% BLPAR, and 1.0% BLPACR strains. Among BLNAR isolates, 29.4% were resistant to amoxicillin–clavulanic acid (MIC90 = 8 μg/mL). Cefotaxime and cefditoren remained highly effective, with over 90% of isolates susceptible regardless of resistance phenotype. Levofloxacin demonstrated activity against virtually all isolates, while clarithromycin non-susceptibility was more variable, observed in 17.4% of BLNAR, 21.8% of BLPAR, and 28.6% of BLPACR strains. Meropenem showed relatively potent activity against all isolates (MIC90 ≤ 0.5 μg/mL). Among S. pneumoniae isolates, penicillin-susceptible strains (PSSP) accounted for 66.6%, penicillin-intermediate strains (PISP) for 31.0%, and PRSP for 2.4%. Almost all PSSP and PISP strains were fully susceptible to amoxicillin-clavulanate, cefotaxime, and cefditoren. However, among PRSP isolates, reduced susceptibility to cefditoren was observed (MIC90 = 1 μg/mL; 33.3% intermediate). Notably, 84.3% of all S. pneumoniae isolates were resistant to clarithromycin, with resistance particularly high among PISP (97.0%) and PRSP (100%) strains. In contrast, levofloxacin and vancomycin retained high activity, with susceptibility rates exceeding 95%.
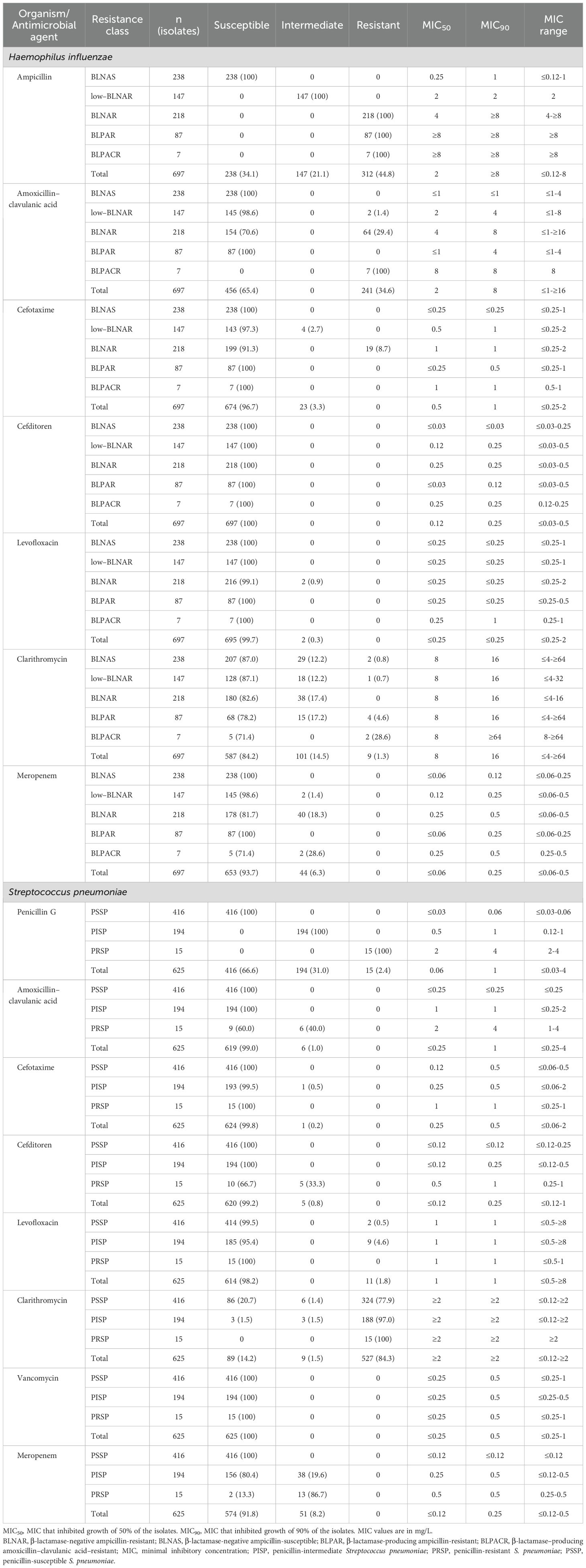
Table 2. Antimicrobial susceptibility profiles of Haemophilus influenzae and Streptococcus pneumoniae.
3.3 Age-specific distribution of bacterial isolates and antimicrobial susceptibility of H. influenzae and S. pneumoniae
The antimicrobial susceptibility profiles of H. influenzae and S. pneumoniae varied significantly by age group (Table 3). Among the 625 S. pneumoniae isolates, PSSP accounted for 54.8% in the 0-2-year age group, 69.8% in the 3-5-year group, and 80.3% in the 6-13-year group, showing a significant increasing trend with age (p < 0.0001). After multiple comparisons correction, significant differences were observed between the 3-5 and 6-13-year groups (p = 0.0002), and between the 0-2 and 6-13-year groups (p < 0.0001). Conversely, PISP were more frequently detected in younger children, accounting for 41.5% in the 0-2-year group, 28.5% in the 3-5-year group, and 18.4% in the 6-13-year group (p < 0.0001). Adjusted p-values confirmed significant differences between the 3-5 and 6-13-year groups (p = 0.013), as well as between the 0-2 and 6-13-year groups (p = 0.0007). PRSP were infrequently detected across all age groups. In contrast, no significant age-related differences were observed in the antimicrobial susceptibility categories of H. influenzae.
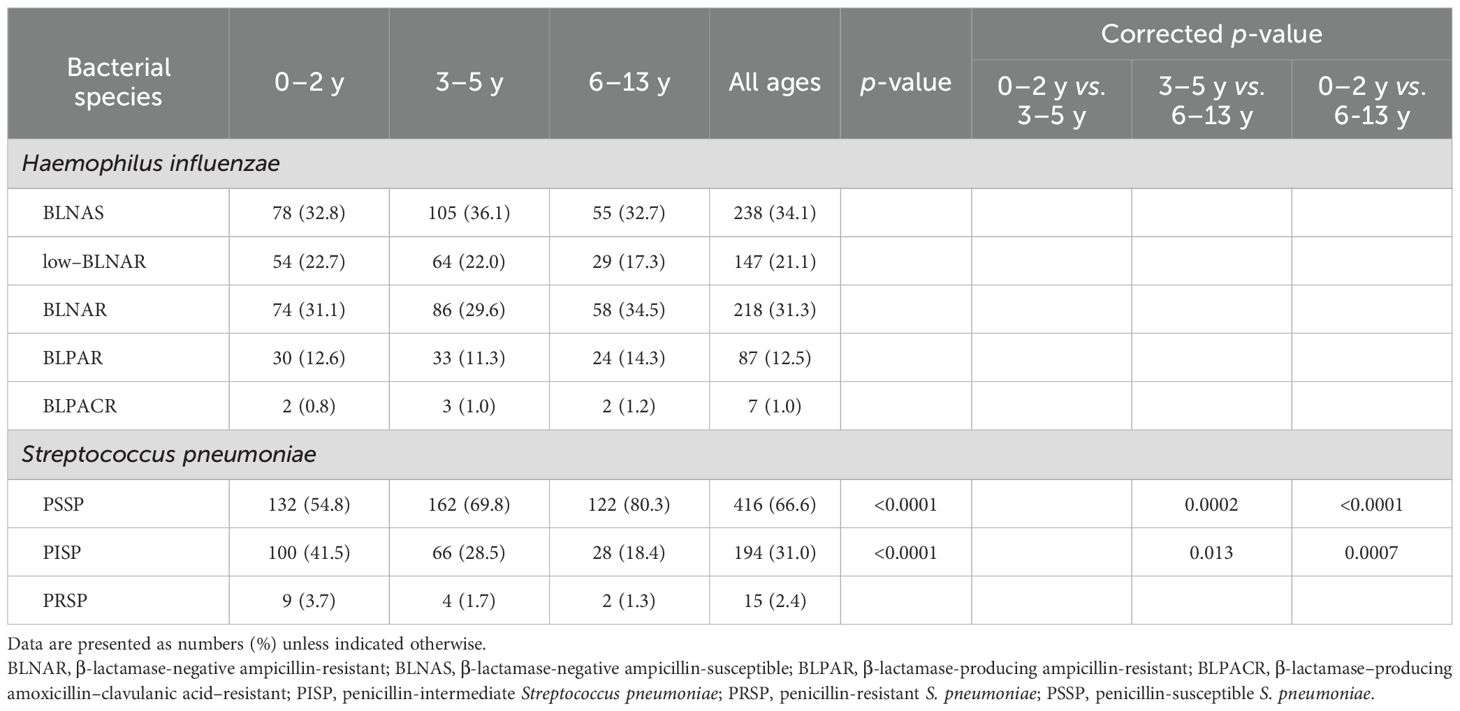
Table 3. Age distribution of Haemophilus influenzae and Streptococcus pneumoniae Isolates by antimicrobial susceptibility classification.
3.4 Age-specific distribution of bacterial isolates
As shown in Table 4, the distribution of bacterial species varied markedly by patient age group. M. catarrhalis was the most frequently isolated species overall, with particularly high prevalence among younger children. Its detection rate decreased significantly with age: 38.0% in the 0-2-year group, 31.0% in the 3-5-year group, and 21.7% in the 6-13-year group (p < 0.0001). Post hoc analysis with correction for multiple comparisons revealed significant differences between the 3-5 and 6-13-year groups, as well as between the 0-2 and 6-13-year groups (both corrected p < 0.0001). H. influenzae was the second most frequently detected species, with detection rates of 22.6%, 25.6%, and 19.9% in the 0-2, 3-5, and 6-13-year groups, respectively. Although a decreasing trend with age was observed, the overall difference among groups was statistically significant (p = 0.005). However, no significant differences were found between any of the groups after correction. S. pneumoniae showed a similar trend, with detection rates of 22.9% in the 0-2-year group, 20.4% in the 3-5-year group, and 18.0% in the 6-13-year group; however, these differences were not statistically significant. In contrast, S. aureus showed the opposite trend, with detection rates increasing with age: 8.3% in the 0-2-year group, 13.4% in the 3-5-year group, and 27.6% in the 6-13-year group. These differences were statistically significant (p < 0.0001), and post hoc comparisons revealed a significant difference between the 0-2 and 6-13-year groups (corrected p < 0.0001). S. pyogenes was infrequently detected but showed a slight age-related increase, with rates of 0.6% in the 0–2-year group, 2.6% in the 3–5-year group, and 3.3% in the 6–13-year group. The overall difference among groups was statistically significant (p = 0.0003), with a significant difference observed between the 0-2 and 3-5-year groups (corrected p = 0.032).
4 Discussion
4.1 Patient background and specimen characteristics
This study analyzed 2009 nasal discharge specimens collected at otorhinolaryngology outpatient clinics across Japan between 2023 and 2025. Consistent with the epidemiological trends of otorhinolaryngologic infections, the majority of patients were children aged 5 years or younger (0-2 years: 32.6%; 3-5 years: 35.2%; 6-13 years: 32.2%). The bacterial culture positivity rate was high, at 92.7%, indicating that specimens were appropriately collected from patients with suspected bacterial infections.
The most frequently isolated pathogens were M. catarrhalis, H. influenzae, and S. pneumoniae, which together accounted for 74.5% of all isolates. This distribution aligns with previous studies conducted in pediatric populations in Japan. For example, one earlier report found that H. influenzae and S. pneumoniae were detected in approximately 45% and 32% of pediatric acute maxillary sinusitis cases, respectively (Sawada and Matsubara, 2021), which is generally consistent with the detection rates observed in the present study. Similar bacterial profiles have also been reported in other studies of AOM and rhinosinusitis among Japanese children (Suzuki et al., 2020).
In the present study, the proportion of M. catarrhalis isolates was higher than that reported in previous studies. Several factors may account for this finding. First, the elevated detection rate may reflect the characteristics of the study population. This investigation was conducted in otolaryngology outpatient clinics and included children with relatively mild upper respiratory symptoms. In contrast, many prior studies focused on hospitalized patients or those with more severe infections (Hultman Dennison et al., 2019). In such cases, more invasive pathogens such as S. pneumoniae and H. influenzae tend to predominate, whereas in milder, community-based cases, colonizing organisms like M. catarrhalis may be more frequently detected. Second, the use of nasal discharge specimens may have contributed to the higher isolation rate of M. catarrhalis. This organism is a well-known colonizer of the upper respiratory tract and is more frequently identified in nasal secretions than in samples such as middle ear fluid or sinus aspirates (Conway et al., 2023). Thus, some of the M. catarrhalis isolates identified in this study may represent colonization rather than true pathogenic involvement in sinusitis. Nonetheless, M. catarrhalis is also recognized as a potential pathogen in pediatric rhinosinusitis, and clinical interpretation should take into account the patient’s symptoms and the severity of illness when evaluating its significance.
4.2 Antimicrobial susceptibility of H. influenzae and S. pneumoniae
One of the major concerns in this study was the high prevalence of antimicrobial resistance among community-acquired isolates. Among S. pneumoniae isolates, PISP accounted for approximately 31.0%, and PRSP for 2.4%. Resistance to clarithromycin was also high at 84.3% overall, with particularly elevated rates among penicillin-non-susceptible strains. These findings are largely consistent with previous nationwide multicenter studies in Japan, which reported that approximately 49.9% of pneumococcal strains from children were penicillin-non-susceptible and over 80% were resistant to macrolides (Kawaguchiya et al., 2024). This resistance trend is likely attributable to the clonal expansion of non-PCV13 serotypes such as 15A, 23A, and 35B, as supported by multiple surveillance studies (Kawaguchiya et al., 2017; Schellenberg et al., 2025). Although serotyping was not performed in this study, based on the timing of sample collection and observed resistance patterns, these non-vaccine serotypes were likely predominant. The introduction of PCV15 in 2023 and PCV20 in 2024 is expected to alter the serotype distribution and antimicrobial resistance patterns of S. pneumoniae. However, serotypes 15A and 23A, which are associated with multidrug-resistant community-acquired infections, are not included in PCV20, and infections caused by these serotypes are expected to persist. Similar trends involving emerging serotypes not covered by existing vaccines have also been reported in Western countries (Hoshino et al., 2013; Sheppard et al., 2016), suggesting a shared global challenge. Regarding H. influenzae, 44.8% of isolates were resistant to ampicillin, with 31.3% classified as BLNAR, 12.5% as BLPAR, and 1.0% as BLPACR. This distribution is generally consistent with domestic reports from the early 2010s (Barberán et al., 2012). Although the predominance of BLNAR strains has shown a slight improving trend, it still persists, and given the absence of a vaccine targeting non-typeable H. influenzae, appropriate antimicrobial stewardship remains the only effective strategy for control.
The MIC data in this study served as a key indicator for characterizing phenotypic resistance. For S. pneumoniae, the MIC50 and MIC90 for penicillin G were 0.06 μg/mL and 1 μg/mL, respectively, with PRSP strains exhibiting MICs exceeding 2 μg/mL. Cefotaxime and cefditoren were highly effective, with MIC90 values of 0.5 μg/mL and 0.25 μg/mL, respectively. However, PRSP strains demonstrated reduced susceptibility to cefditoren (MIC90 = 1 μg/mL), consistent with previous reports (Brook et al., 1994). For macrolides, the MIC90 for clarithromycin exceeded 2 μg/mL, indicating the presence of highly resistant strains, in alignment with nationwide surveillance data (Kawaguchiya et al., 2024). In contrast, levofloxacin and vancomycin exhibited MICs well below clinical breakpoints, supporting their potential utility in refractory cases, as highlighted in earlier studies (Lambert et al., 2025). For H. influenzae, the MIC50 and MIC90 for ampicillin were 2 μg/mL and ≥8 μg/mL, respectively, reflecting the high prevalence of BLNAR and BLPAR strains. Elevated MICs for amoxicillin–clavulanic acid were also observed among BLNAR isolates (MIC90 = 8 μg/mL). In contrast, third-generation cephalosporins such as cefotaxime and cefditoren maintained favorable activity, with MIC90 values of 1 μg/mL and 0.25 μg/mL, respectively. Clarithromycin MICs reached ≥64 mg/mL in some isolates, indicating limited efficacy of macrolides. Although meropenem showed a noticeable proportion of intermediate isolates among low-BLNAR, BLNAR, and BLPACR strains, there was no marked increase in MIC values (MIC90 0.25 mg/mL), supporting its role as a reliable therapeutic option in complicated or resistant cases. These MIC distributions corroborate observed phenotypic resistance patterns and underscore the clinical importance of selecting antimicrobials based on in vitro susceptibility, particularly in regions such as Japan, where resistant pathogens are highly prevalent.
Notably, compared with earlier reports from the 2010s, the proportion of BLNAR strains, while still high, appears to have declined slightly. For example, a study conducted between 2013 and 2016 reported that BLNAR strains accounted for over 50% of pediatric isolates, whereas the present study suggests a modest reduction. This shift may reflect the sustained impact of improved antimicrobial stewardship and reduced selective pressure following the 2013 revision of the pediatric AOM guidelines. Moreover, the 2024 guideline revision recommends high-dose amoxicillin and careful observation for mild cases, which may further influence future resistance trends (The Japanese Society for Pediatric Otorhinolaryngology, 2024). Indeed, countries such as the Netherlands and Sweden have reported decreased resistance rates following similar changes in antibiotic use policies (Takeuchi et al., 2017; Eriksen et al., 2021), and comparable benefits may also be anticipated in Japan.
In this study, the overall susceptibility of S. pneumoniae and H. influenzae to levofloxacin was not exceptionally high, with MIC90 values of 1 μg/mL and ≤0.25 μg/mL, respectively. However, a small number of isolates exhibited elevated MICs, raising concerns about the potential emergence of resistance with increasing pediatric use of fluoroquinolones. Since 2010, tosufloxacin has been approved as an oral fluoroquinolone for children in Japan and has been used in limited cases (Takeuchi et al., 2021). Although quinolone resistance among respiratory pathogens remains uncommon, H. influenzae strains harboring mutations in the gyrA and parC genes have been reported, indicating early warning signs of emerging resistance (Kovács et al., 2021). Furthermore, between 2023 and 2024, Japan experienced supply shortages of key β-lactam antibiotics such as amoxicillin and cefdinir, temporarily limiting access to first-line agents. As a result, increased use of fluoroquinolones was reported in some clinical settings, potentially heightening selective pressure for resistance. While fluoroquinolones remain clinically effective at present, their therapeutic margin is narrow, underscoring the need for continued surveillance. From an antimicrobial stewardship perspective, the use of fluoroquinolones in children should be restricted to specific indications—such as β-lactam allergy or infections caused by treatment-refractory pathogens—in accordance with current pediatric infectious disease guidelines (The Japanese Society for Pediatric Otorhinolaryngology, 2024).
It is also necessary to discuss the appropriateness of macrolide monotherapy in pediatric sinusitis. Although macrolides have historically been used as empirical therapy, their clinical efficacy against the major causative pathogens of sinusitis is limited. S. pneumoniae, one of the principal pathogens, frequently exhibits macrolide resistance, and recent surveillance data from Japan have reported resistance rates exceeding 80% (Nagai et al., 2014). Moreover, macrolides generally exert bacteriostatic rather than bactericidal activity, which may be insufficient for pathogen eradication in infections with a high bacterial burden. For these reasons, both international and domestic guidelines recommend β-lactam antibiotics as the first-line therapy, reserving macrolides only for patients with severe β-lactam allergy. Therefore, macrolide monotherapy should be avoided as an empirical treatment for pediatric sinusitis.
4.3 Age-specific distribution of bacteria
Age-stratified analysis revealed clear differences in the distribution of bacterial species. M. catarrhalis, H. influenzae, and S. pneumoniae were predominantly detected in children aged ≤5 years, reaffirming their roles as major pathogens in pediatric rhinosinusitis. In contrast, the detection frequency of S. aureus increased with age, reaching 27.6% in the 6–13-year age group. This trend may be associated with factors such as a higher prevalence of chronic rhinosinusitis during adolescence and age-related changes in the nasal microbiota (Kishita et al., 2023). Additionally, 22% of the S. aureus isolates were identified as MRSA, most of which are presumed to be community-acquired strains. MRSA remains a clinically significant pathogen in otorhinolaryngology, and national surveillance data have consistently reported its involvement in community-acquired infections (Suzuki et al., 2020; Silva-Costa et al., 2024). Furthermore, MRSA involvement in refractory rhinosinusitis among both children and adults is reportedly increasing (Lambert et al., 2025). In such difficult-to-treat cases, culture-based diagnosis and susceptibility-guided antimicrobial therapy are essential for effective management.
The study period coincided with the post– coronavirus disease 2019 pandemic era, during which mask-wearing and the suppression of respiratory viral infections may have temporarily influenced the dynamics of bacterial infections. Indeed, an increase in outpatient visits and nasal discharge cultures was observed after the pandemic, possibly reflecting a return to typical seasonal patterns of respiratory infections. A similar “post-pandemic rebound” in bacterial infections following the resurgence of viral illnesses has also been reported in European pediatric respiratory surveillance studies (Silva et al., 2021). Furthermore, the increased detection rate of MRSA in adolescents may be related to cumulative antibiotic exposure and alterations in nasal flora associated with changes in hygiene practices. These age-related differences in pathogen distribution provide important insights for empirical antimicrobial selection. In particular, in older children or in cases of recurrent or treatment-resistant rhinosinusitis, MRSA should be considered, and individualized treatment based on culture and susceptibility testing is recommended if β-lactam therapy fails. To support and refine such treatment strategies, continued region-specific microbiological surveillance is critically important.
5 Limitations and future directions
This study demonstrated that H. influenzae and S. pneumoniae remain the predominant pathogens in pediatric chronic rhinosinusitis, while the clinical significance of S. aureus, particularly MRSA, is increasing in older children. Given the high resistance rates of S. pneumoniae to penicillin and macrolides, empirical treatment with macrolide monotherapy is not recommended. In contrast, amoxicillin–clavulanic acid remains a reasonable treatment option due to its effectiveness against H. influenzae, M. catarrhalis, and many strains of S. pneumoniae. Furthermore, in older children and in cases of recurrent or refractory rhinosinusitis, the potential involvement of S. aureus—especially MRSA—should be taken into consideration.
The limitations of this study include its single-center design, the absence of serotyping or genotyping, and the lack of clinical differentiation between acute and chronic cases. In addition, the absence of data on prior antibiotic exposure—which may represent a potential confounding factor for resistance rates—the lower specificity of nasal discharge cultures compared with sinus aspirate cultures in reflecting true infection, and the lack of information on adherence to treatment guidelines should also be acknowledged as limitations of this study. Nevertheless, the findings are consistent with existing national surveillance data and are considered to have a reasonable degree of generalizability. Looking ahead, multicenter molecular epidemiological studies are needed to evaluate the impact of the introduction of PCV15 and PCV20, as well as adherence to pediatric clinical guidelines, on trends in antimicrobial resistance. In addition, a comprehensive assessment of host factors, underlying medical conditions, and vaccination history will be essential for further advancing clinical understanding and management.
6 Conclusion
This study provides timely and clinically relevant data on the microbiological profile of pediatric acute and chronic rhinosinusitis during a transitional period marked by the introduction of new vaccines and revisions to antibiotic prescribing guidelines. Notably, the predominance of H. influenzae and S. pneumoniae in young children, along with the increasing clinical relevance of S. aureus in older children, offers practical insights for age-specific empirical therapy. Furthermore, the high prevalence of penicillin- and macrolide-resistant S. pneumoniae, as well as persistent ampicillin resistance in H. influenzae, highlights the importance of appropriate antibiotic use. Clinically, these findings support rational antibiotic selection in pediatric otorhinolaryngologic care, advocate for culture-based management in recurrent or refractory cases, and emphasize the need to consider MRSA in adolescents and cases of chronic rhinosinusitis. Ongoing microbiological surveillance remains essential for tracking resistance trends and guiding regionally appropriate treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Sen−en Rifu Hospital (Approval No. 20250408). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because written informed consent was waived due to the retrospective nature of the study and use of de−identified data.
Author contributions
SK: Investigation, Conceptualization, Data curation, Writing – review & editing, Writing – original draft, Formal analysis, Methodology. TK: Supervision, Conceptualization, Data curation, Writing – review & editing. KN: Writing – review & editing. YN: Writing – review & editing. RI: Formal analysis, Software, Writing – review & editing. HK: Writing – review & editing. YK: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the clinical staff of Sendai sta. North Gate ENT Clinic for their cooperation in specimen collection and BML Inc. for help with microbiological testing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barberán, J., Aguilar, L., and Giménez, M.-J. (2012). Update on the clinical utility and optimal use of cefditoren. Int. J. Gen. Med. 5, 455–464. doi: 10.2147/IJGM.S25989
Brook, I., Thompson, D. H., and Frazier, E. H. (1994). Microbiology and management of chronic maxillary sinusitis. Arch. Otolaryngol Head Neck Surg. 120, 1317–1320. doi: 10.1001/archotol.1994.01880360015003
Clinical and Laboratory Standards Institute (2019). “Performance standards for antimicrobial susceptibility testing,” in CLSI supplement M100, 29th (CLSI, Wayne, PA).
Conway, I. O., Kurs-Lasky, M., and Shaikh, N. (2023). Moraxella catarrhalis infrequently cultured from middle ear fluid of children with acute otitis media. Pediatr. Infect. Dis. J. 42, e416–e417. doi: 10.1097/INF.0000000000004028
Eriksen, J., Björkman, I., Röing, M., Essack, S. Y., and Stålsby Lundborg, C. (2021). Exploring the one health perspective in Sweden’s policies for containing antibiotic resistance. Antibiotics (Basel). 10, 526. doi: 10.3390/antibiotics10050526
Hoshino, T., Sato, Y., Toyonaga, Y., Hanaki, H., and Sunakawa, K. (2013). Drug-Resistant Pathogen Surveillance Group in Pediatric Infectious Disease. Nationwide survey of the development of drug resistance in the pediatric field in 2007 and 2010: drug sensitivity of Haemophilus influenzae in Japan (second report). J. Infect. Chemother. 19, 495–503. doi: 10.1007/s10156-013-0591-z
Hultman Dennison, S., Schollin Ask, L., Eriksson, M., Granath, A., Hertting, O., Bennet, R., et al. (2019). Serious complications due to acute rhinosinusitis in children up to five years old in Stockholm, Sweden-Still a challenge in the pneumococcal conjugate vaccine era. Int. J. Pediatr. Otorhinolaryngol. 121, 50–54. doi: 10.1016/j.ijporl.2019.02.034
Kawaguchiya, M., Urushibara, N., Aung, M. S., Ohashi, N., Tsutida, S., Kurashita, K., et al. (2024). Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolated from children in Japan, 2023. New Microbes New Infect. 62, 101513. doi: 10.1016/j.nmni.2024.101513
Kawaguchiya, M., Urushibara, N., and Kobayashi, N. (2017). Multidrug resistance in non-PCV13 serotypes of Streptococcus pneumoniae in northern Japan, 2014. Microb. Drug Resist. 23, 206–214. doi: 10.1089/mdr.2016.0054
Kishita, M., Matsumura, Y., Yamamoto, M., Nagao, M., Takemura, M., Sumi, M., et al. (2023). Increase in the frequency of community-acquired methicillin-resistant Staphylococcus aureus clones among inpatients of acute care hospitals in the Kyoto and Shiga regions, Japan. J. Infect. Chemother. 29, 458–463. doi: 10.1016/j.jiac.2023.01.013
Kovács, E., Sahin-Tóth, J., Tóthpál, A., van der Linden, M., Tirczka, T., and Dobay, O. (2021). Co-carriage of Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis among three different age categories of children in Hungary. PloS One 16, e0248082. doi: 10.1371/journal.pone.0248082
Lambert, M., Veldkamp, R., Weesie, Y., Lambooij, A., Cals, J. W. L., Taxis, K., et al. (2025). Adherence to antibiotic prescribing guidelines in Dutch primary care: an analysis of national prescription data on ear and respiratory tract symptoms and conditions among 384 general practices. Fam Pract. 42, cmaf031. doi: 10.1093/fampra/cmaf031
Nagai, K., Kimura, O., Domon, H., Maekawa, T., Yonezawa, D., and Terao, Y. (20142019). Antimicrobial susceptibility of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis clinical isolates from children with acute otitis media in Japan from 2014 to 2017. J. Infect. Chemother. 25, 229–232. doi: 10.1016/j.jiac.2018.08.018
Sawada, S. and Matsubara, S. (2021). Microbiology of acute maxillary sinusitis in children. Laryngoscope. 131, E2705–E2711. doi: 10.1002/lary.29564
Schellenberg, J. J., Adam, H. J., Baxter, M. R., Karlowsky, J. A., Golden, A. R., Martin, I., et al. (2025). Comparing serotype coverage of pneumococcal vaccines with PCV21 (V116), a new 21-valent conjugate pneumococcal vaccine, and the epidemiology of its eight unique Streptococcus pneumoniae serotypes (15A, 15C, 16F, 23A, 23B, 24F, 31 and 35B) causing invasive pneumococcal disease in adult patients in Canada: SAVE study, 2018-21. J. Antimicrob. Chemother. 80, 1377–1385. doi: 10.1093/jac/dkaf085
Sheppard, C., Fry, N. K., Mushtaq, S., Woodford, N., Reynolds, R., Janes, R., et al. (2016). Rise of multidrug-resistant non-vaccine serotype 15A Streptococcus pneumoniae in the United Kingdom, 2001 to 2014. Euro Surveill. 21, 30423. doi: 10.2807/1560-7917.ES.2016.21.50.30423
Silva, M. D., Lima, A., Marçal, N., Dias, L., Gama, M., and Sillankorva, S. (2021). Identification of the bacterial pathogens in children with otitis media: a study in the northwestern Portuguese district of Braga. Microorganisms. 10, 54. doi: 10.3390/microorganisms10010054
Silva-Costa, C., Gomes-Silva, J., Pinho, M., Friães, A., Subtil-Limpo, F., Ramirez, M., et al. (2024). Rebound of pediatric invasive pneumococcal disease in Portugal after the COVID - 19 pandemic was not associated with significant serotype changes. J. Infect. 89, 106242. doi: 10.1016/j.jinf.2024.106242
Suzuki, K., Kurono, Y., Ikeda, K., Hotomi, M., Yano, H., Watanabe, A., et al. (2020). The seventh nationwide surveillance of six otorhinolaryngological infectious diseases and the antimicrobial susceptibility patterns of the isolated pathogens in Japan. J. Infect. Chemother. 26, 890–899. doi: 10.1016/j.jiac.2020.05.020
Takeuchi, N., Ohkusu, M., Hoshino, T., Naito, S., Takaya, A., Yamamoto, T., et al. (2017). Emergence of quinolone-resistant strains in Streptococcus pneumoniae isolated from paediatric patients since the approval of oral fluoroquinolones in Japan. J. Infect. Chemother. 23, 218–223. doi: 10.1016/j.jiac.2016.12.012
Takeuchi, N., Ohkusu, M., Hoshino, T., Yamamoto, S., Segawa, S., Murata, S., et al. (2021). Emergence of Haemophilus influenzae with low susceptibility to quinolones isolated from pediatric patients in Japan. J. Infect. Chemother. 27, 1020–1026. doi: 10.1016/j.jiac.2021.02.022
The Japanese Society for Pediatric Otorhinolaryngology (2024). Clinical practice guidelines for acute otitis media in children. Available online at: https://www.otology.gr.jp/common/pdf/guideline_otitis2024.pdf (Accessed July 8, 2025).
Xu, Q., Gill, S., Xu, L., Gonzalez, E., and Pichichero, M. E. (2019). Comparative analysis of microbiome in nasopharynx and middle ear in young children with acute otitis media. Front. Genet. 10. doi: 10.3389/fgene.2019.01176
Keywords: pediatric rhinosinusitis, antimicrobial resistance, Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus
Citation: Kitaya S, Kikuchi T, Nomura K, Nomura Y, Ikeda R, Kanamori H and Katori Y (2025) Epidemiology and antimicrobial resistance of pathogens in pediatric sinus infections: a retrospective study at a Japanese otolaryngology clinic (2023–2025). Front. Cell. Infect. Microbiol. 15:1662544. doi: 10.3389/fcimb.2025.1662544
Received: 31 July 2025; Accepted: 19 August 2025;
Published: 15 September 2025.
Edited by:
Muhammad Usman Munir, Jouf University, Saudi ArabiaReviewed by:
Tauqeer Hussain Mallhi, Jouf University, Saudi ArabiaVlademir Cantarelli, Federal University of Health Sciences of Porto Alegre, Brazil
Copyright © 2025 Kitaya, Kikuchi, Nomura, Nomura, Ikeda, Kanamori and Katori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiori Kitaya, c2hpb3JpLmtpdGF5YS5iN0B0b2hva3UuYWMuanA=
 Shiori Kitaya
Shiori Kitaya Toshiaki Kikuchi3
Toshiaki Kikuchi3 Ryoukichi Ikeda
Ryoukichi Ikeda Hajime Kanamori
Hajime Kanamori