- 1Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Tamil Nadu, Kattankulathur, India
- 2Department of Microbiology, SRM Medical College Hospital and Research Centre (SRMMCH&RC), SRM Institute of Science and Technology, Kattankulathur, India
- 3Department of Oral Biology, School of Dental Medicine, University at Buffalo, Buffalo, NY, United States
- 4School of Chemical Engineering, Yeungnam University, Gyeongsan, Republic of Korea
- 5Department of Food Science, Institute of Postharvest Technology and Food Sciences, Agricultural Research Organization (ARO), Volcani Institute, Rishon LeZion, Israel
Vulvovaginal candidiasis (VVC) is an infection caused by Candida albicans that presents an escalating threat to humans. Lactobacilli may play a critical role in maintaining microbiome balance in the gut and vagina as well as limiting fungal colonization, including C. albicans. Certain Lactobacilli, classified as nomadic groups is gaining immense popularity in antifungal defense due to its unique morphological adaptations. One significant adaptation is the V-shaped cell chaining observed under low pH conditions governed by the LuxS-mediated quorum-sensing system. This structural adaptation potentiates altered secondary metabolite secretion. These geometric forms are not solely survival responses but reflect a structurally coordinated strategy that enhances both antibiofilm and antihyphal activities. In this perspective, we argue that morphology-driven transitions identify nomadic Lactobacilli as a promising frontier in probiotic therapy. By shifting from conventional probiotic formulations to structured microbial interventions, we propose the development of novel sustainable therapeutics for anticandidal therapy.
1 Etiology of vulvovaginal candidiasis
Vulvovaginal candidiasis (VVC) is a common and recurrent infection despite the availability of antifungal therapies. One major reason is the ability of Candida albicans to form biofilms, which enhances its resistance to treatment and immune elimination (Intra et al., 2022). Fluconazole is widely used, but reduced efficacy against biofilms is a major concern (Intra et al., 2022). While newer antifungals like ibrexafungerp and oteseconazole are alternatives, their long-term activity against biofilm-driven infections is still under evaluation (Intra et al., 2022). Misdiagnosis and frequent use of non-prescription antifungals lead to a delay in proper treatment, leading to recurrent episodes (Duar et al., 2017). These disadvantages raise an important question: can we develop simpler strategies to improve current therapies?
2 Nomadic Lactobacilli to tackle VVC
Lactobacilli belong to a genus of Gram-positive, rod-shaped lactic acid bacteria that actively compete with C. albicans in the vaginal microbiome (Intra et al., 2022). These bacteria are broadly classified into free-living, host-adapted, and nomadic variants (Duar et al., 2017). Among these, certain species, particularly the nomadic variants (Lactiplantibacillus plantarum, Lacticaseibacillus rhamnosus, and Lacticaseibacillus casei), seem promising for antibiofilm therapy (Rajasekharan and Shemesh, 2022). The nomadic lifestyle of Lactobacillii refers to their ability to survive and adapt across different environments, rather than being localized to a singular habitat. These bacteria can shift between various environmental habitats such as soil, plant surfaces, aquatic environments, fermented foods, the oral cavity and the gastrointestinal tract (Duar et al., 2017). They maintain their genomic and metabolic flexibility that allows them to reshape their phenotype according to the habitat. The current thinking in the field is that the geometrical structuring facilitates the nomadic lifestyle, leading to successful adaptation of Lactobacilli to different environmental niches, though the mechanistic understanding of structural changes enabling their nomadic lifestyle remains an active area of research. L. plantarum is one such nomadic variant that was recently shown to demonstrate multifaceted cell structures such as cone-shaped colonies, sacrifice-for-survival bundles, and V-shaped cell chaining in response to acidic pH conditions (Figure 1a) (Rajasekharan and Shemesh, 2022; Venugopal et al., 2025). Findings reveal that in acidic environment, mimicking the vaginal niche, L. plantarum transitions are linked to LuxS quorum-sensing pathways (Venugopal et al., 2025). This structural state coincides with modified metabolite secretion and raises questions about whether morphology-driven metabolic shifts enhance its antifungal capacity. This is particularly relevant in the context of VVC, a common mucosal infection affecting up to 75% of women during their reproductive years (Sobel, 2007; Intra et al., 2022). C. albicans, the primary causative agent, often forms drug-resistant biofilms and evades host immunity (Zeise et al., 2021; Poon and Hui, 2023). While conventional antifungals face increasing resistance, probiotics, particularly those containing Lactobacilli, offer an emerging alternative by restoring microbial balance, inhibiting Candida adhesion, biofilm formation and modulating immune responses (Table 1). Clinical studies have shown that species like L. plantarum underscoring their therapeutic value (Bertarello et al., 2024). We propose that structural shifts in L. plantarum, especially the formation of V-shaped structures alleviate symptoms and reduce recurrence in VVC-infected cells, which may be a functional adaptation for enhanced antifungal activity. This explores how the morphology-metabolite interplay could influence the next generation of probiotic design to target Candida biofilms and overcome antifungal resistance.
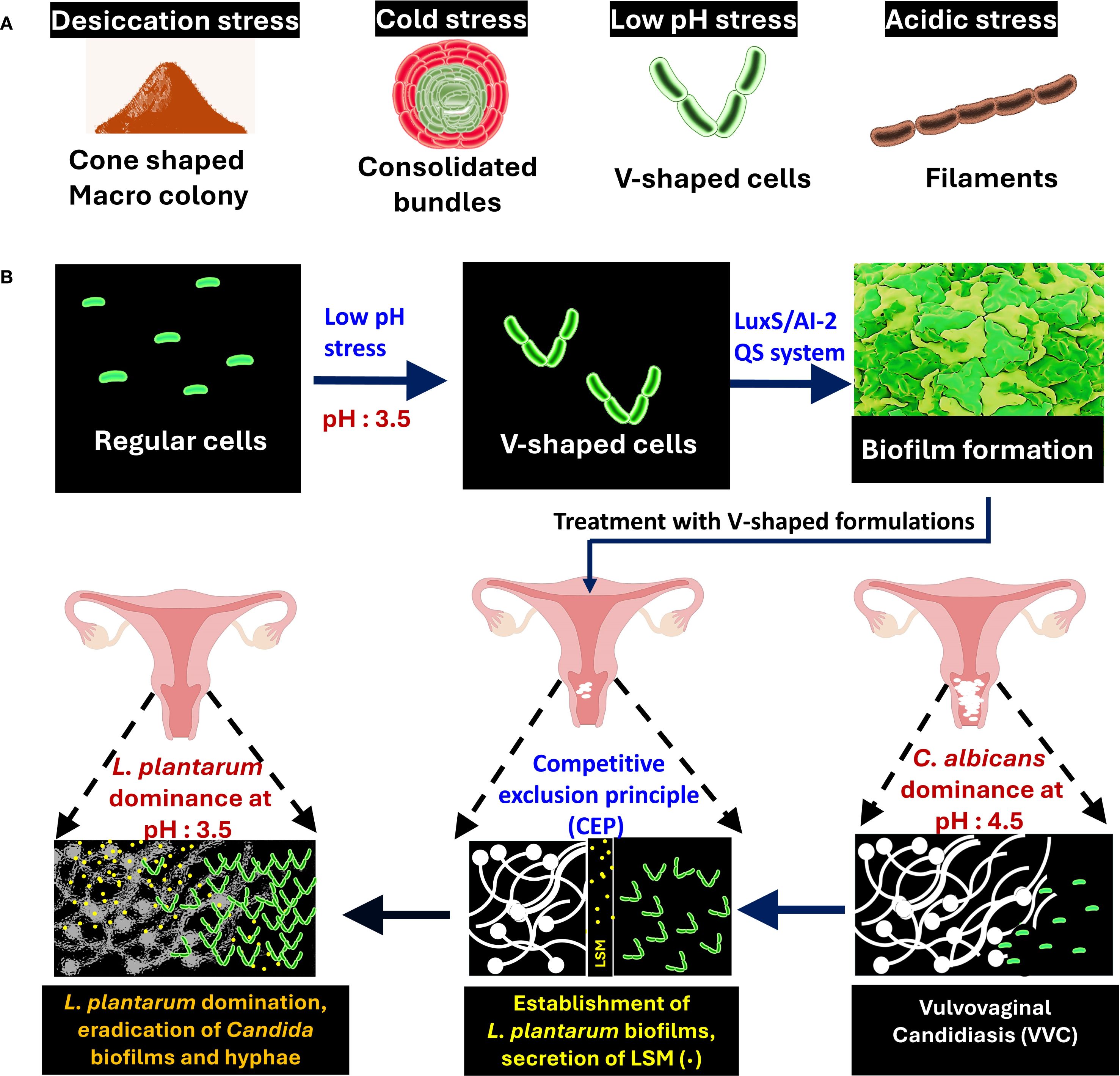
Figure 1. Morphological adaptation and protective role of Lactiplantibacillus plantarum in fighting Candida albicans biofilms in the vaginal microbiome. (A) Multicellular structures of L. plantarum formed under different stress conditions: desiccation (cone-shaped colonies), cold (bundles), low pH (V-shaped cells), and acidic stress (filaments). (B) L. plantarum transitions from regular cells to V-shaped cells under low pH stress, leading to biofilm formation via the LuxS/AI-2 Quorum Sensing (QS) system. Treatment with V-shaped formulations results in L. plantarum dominance, the competitive exclusion of Candida species, and the subsequent alleviation of Vulvovaginal Candidiasis (VVC).
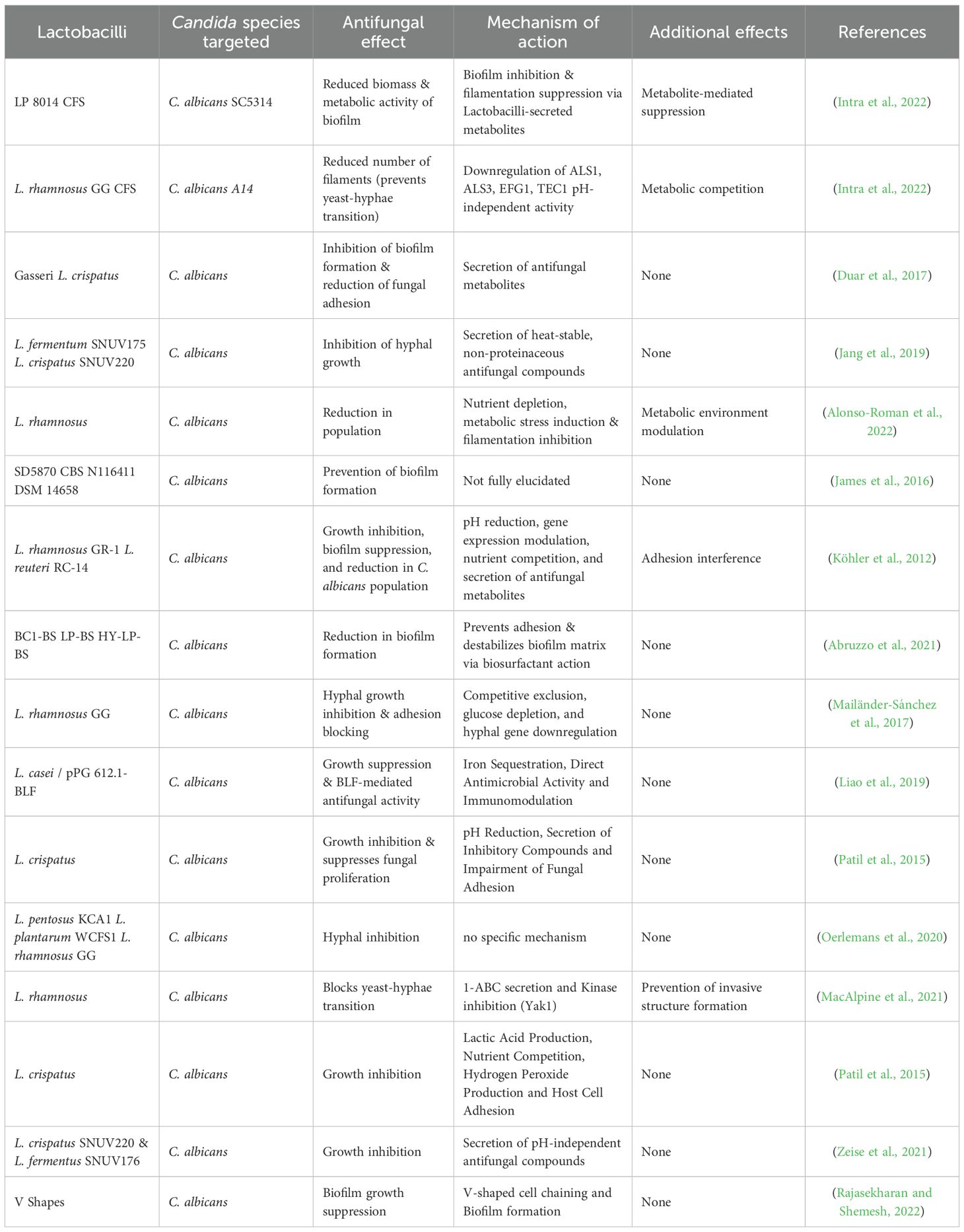
Table 1. Comparative analysis of antifungal properties of Lactobacilli against Candida albicans biofilms.
3 The V-shaped structuring
Biofilm formation is a key survival strategy for microbes in mucosal environments. In the vaginal niche, L. plantarum typically dominates through acidification and spatial exclusion, but not all species are equally effective against C. albicans. The environment provides signals such as acid stress, bile salts, osmotic stress, temperature fluctuations and nutrient availability that can have a direct impact on the morphology of Lactobacilli. The V-shaped structuring can be formed predominantly in the vaginal environment, where relatively high acidity may facilitate such cellular transformation. The structured cells possess enhanced biochemical defense mechanisms that effectively inhibit hyphal extension and biofilms, as was shown in a C. elegans model (Rajasekharan and Shemesh, 2022). This V-shaped structuring correlates with increased surface adherence and more cohesive biofilm formation compared to other known shapes, allowing it to physically outcompete C. albicans establishment (Rajasekharan and Shemesh, 2022). (Figure 1b). In our experimental models, V-shaped L. plantarum cells effectively inhibited yeast virulence in in vitro and in vivo nematode models (Rajasekharan and Shemesh, 2022). This suggests that the V-shaped morphology is not a passive stress response, but rather an adaptive strategy for enhanced mucosal colonization and spatial exclusion of fungal pathogens. The role of Candida in shaping Lactobacilli morphology remains unclear, but it is conceivable that the dysbiosis caused by the colonization of Candida may indeed be related to its possible impact on Lactobacilli morphology. Further studies are warranted to elucidate possible role during microbial competition and antagonistic interactions. Figure 1b illustrates how the geometrical structuring may lead to physical suppression of Candida colonization through dense probiotic biofilms.
4 Morphotype-driven regulation of Lactobacilli-secreted metabolites
Although numerous antimicrobial compounds of Lactobacilli are well established, how they relate to structural morphology remains unexplored. Based on the results discussed in Section 3, we found that L. plantarum under acidic stress exhibits a distinct metabolic shift associated with the previously described chaining morphology. Notably, this morphotype displayed increased secretion of metabolites of cyclic dipeptides, which are stable and interfere with the maturation of biofilms (Narasimulu et al., 2024). These compounds were more abundant in the V-shaped state compared to rod-shaped cells, indicating that the structural adaptation also enhances metabolite secretion. This observation proposes a novel concept in probiotic design; morphological states may directly influence the potency and spectrum of antimicrobial metabolites. Notably, formulated V-shaped cells hold significant potential to be made into probiotic products, thus merging the structural advantages with practical formulating benefits. Such structure-informed probiotic strategies could yield targeted solutions for recurrent Candida infections. In the following section, we detail the intracellular targets of these metabolites and how they plausibly disrupt C. albicans biofilm formation (Figure 2).
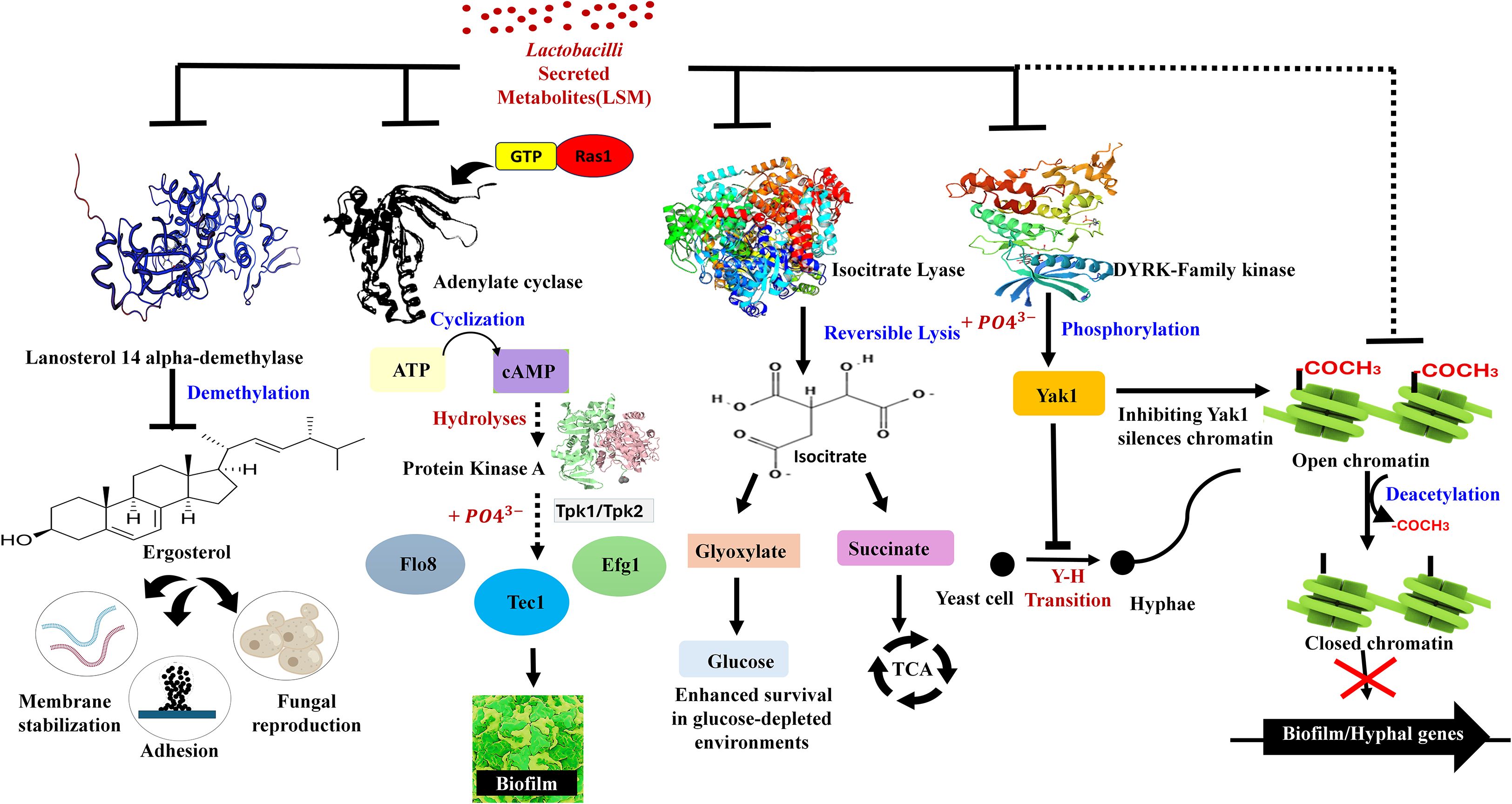
Figure 2. Inhibitory mechanisms of Lactobacilli-secreted metabolites (LSMs) on biofilm and hyphal regulatory pathways in Candida albicans. The schematic diagram illustrates the plausible pathways by which LSM might inhibit fungal pathogenesis (biofilm and hyphae). These pathways include Ras1/cAMP signaling, ergosterol biosynthesis, glyoxylate metabolism, DYRK/Yak1 and chromatin regulation.
5 Biofilm-based targets of Lactobacilli-secreted metabolites
We examine the proposed role of morphology-driven metabolite adaptation on how Lactobacilli-secreted metabolites (LSMs) interfere with intracellular pathways that regulate the formation and survival of C. albicans biofilms. Several LSMs have been shown to inhibit adenylate cyclase, which can disrupt the RAS-cAMP-MAPK signaling cascade and downstream regulators such as tpk1, efg1, flo8, and tec1, which are crucial transcription factors that drive hyphal transition and biofilm maturation (Alonso-Roman et al., 2022). LSMs, such as pyruvate and oxaloacetate, may interfere with metabolism for competitive inhibition of isocitrate lyase, which inhibits the glyoxylate cycle and C. albicans ability to grow in nutrient-poor mucosal environments. Other targets include lanosterol 14α-demethylase, which disrupts ergosterol synthesis and destabilizes fungal membranes. Compounds like 1-acetyl-β-carboline (1-ABC) directly inhibit Yak1, a kinase critical for morphogenesis and biofilm development (MacAlpine et al., 2021). Additionally, sodium butyrate, a short-chain fatty acid and histone deacetylase inhibitor (HDACi), impairs fungal gene expression by altering chromatin organization and structure (Poon and Hui, 2023). These proposed mechanisms are illustrated in Figure 2, which integrates our hypothesis with known fungal regulatory pathways. Increased biofilm inhibitory activity of V-shaped L. plantarum may not only come from competitive exclusion but also from a specialized LSM profile that precisely targets fungal virulence factors. Understanding these molecular interactions will open a path toward next-generation probiotic therapies for persistent biofilm-associated infections such as recurrent VVC.
6 Concluding remarks and future perspectives
Our investigation indicates that structural adaptation of L. plantarum, more especially, its transition to V-shaped cells in vaginal-like acidic environments, may have a major impact on the synthesis of antifungal metabolites. This presents an intriguing hypothesis that cell morphology is a functional state that improves probiotic competitiveness against C. albicans. A promising bioactive profile for preventing biofilm-associated infections might be attributed to cyclic dipeptides and other specialized LSMs, including Prolyl-arginine, Valyl-threonine, and Valyl-glutamine (Narasimulu et al., 2024). Future research should focus on characterizing these metabolic pathways and understanding how they interface with fungal signaling and host immunity. Unlocking the regulatory controls behind such transitions could lead to novel bioengineered probiotic therapies designed not just by strain selection, but by regulating growth conditions and structure to maximize antifungal efficacy. This structure-guided approach may open a new path to address drug resistance and design more robust microbiome-based interventions for Biofilm-associated infection. While our research highlights the structural and metabolic role of L. plantarum, other nomadic Lactobacilli such as L. rhamnosus and L. casei remain largely unexplored and warrants further studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
LP: Writing – original draft, Investigation, Visualization, Data curation. HH: Investigation, Writing – original draft, Visualization, Data curation. LK: Writing – review & editing. AS: Writing – review & editing. JL: Writing – review & editing. MS: Supervision, Writing – original draft, Writing – review & editing. SR: Conceptualization, Writing – original draft, Supervision, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by Anusandhan National Research Foundation (ANRF), Government of India, ANRF/ECRG/2024/001532/LS and Selective Excellence Research Initiative (SERI) No. SRMIST/R/AR(A)/SERI2024/174/06 awarded to SKR by ANRF and SRMIST respectively. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors acknowledge the support provided by the management of SRM Institute of Science and Technology (SRMIST). Financial support rendered to HCH by SRMIST as part of her PhD programme is also gratefully acknowledged.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abruzzo, A., Giordani, B., Parolin, C., De Gregorio, P. R., Foschi, C., Cerchiara, T., et al. (2021). Lactobacillus crispatus BC1 biosurfactant delivered by hyalurosomes: an advanced strategy to counteract candida biofilm. Antibiotics (Basel) 10. doi: 10.3390/antibiotics10010033
Alonso-Roman, R., Last, A., Mirhakkak, M. H., Sprague, J. L., Möller, L., Großmann, P., et al. (2022). Lactobacillus rhamnosus colonisation antagonizes Candida albicans by forcing metabolic adaptations that compromise pathogenicity. Nat. Commun. 13, 3192. doi: 10.1038/s41467-022-30661-5
Bertarello, C., Savio, D., Morelli, L., Bouzalov, S., Davidova, D., and Bonetti, A. (2024). Efficacy and safety of Lactobacillus plantarum P 17630 strain soft vaginal capsule in vaginal candidiasis: a randomized non-inferiority clinical trial. Eur. Rev. Med. Pharmacol. Sci. 28, 384–391. doi: 10.26355/eurrev_202401_34927
Duar, R. M., Lin, X. B., Zheng, J., Martino, M. E., Grenier, T., Pérez-Muñoz, M. E., et al. (2017). Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol. Rev. 41, S27–s48. doi: 10.1093/femsre/fux030
Intra, J., Sala, M. R., Brambilla, P., Carcione, D., and Leoni, V. (2022). Prevalence and species distribution of microorganisms isolated among non-pregnant women affected by vulvovaginal candidiasis: A retrospective study over a 20 year-period. J. Mycol Med. 32, 101278. doi: 10.1016/j.mycmed.2022.101278
James, K. M., MacDonald, K. W., Chanyi, R. M., Cadieux, P. A., and Burton, J. P. (2016). Inhibition of Candida albicans biofilm formation and modulation of gene expression by probiotic cells and supernatant. J. Med. Microbiol. 65, 328–336. doi: 10.1099/jmm.0.000226
Jang, S. J., Lee, K., Kwon, B., You, H. J., and Ko, G. (2019). Vaginal lactobacilli inhibit growth and hyphae formation of Candida albicans. Sci. Rep. 9, 8121. doi: 10.1038/s41598-019-44579-4
Köhler, G. A., Assefa, S., and Reid, G. (2012). Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect. Dis. Obstet Gynecol 2012, 636474. doi: 10.1155/2012/636474
Liao, H., Liu, S., Wang, H., Su, H., and Liu, Z. (2019). Enhanced antifungal activity of bovine lactoferrin-producing probiotic Lactobacillus casei in the murine model of vulvovaginal candidiasis. BMC Microbiol. 19, 7. doi: 10.1186/s12866-018-1370-x
MacAlpine, J., Daniel-Ivad, M., Liu, Z., Yano, J., Revie, N. M., Todd, R. T., et al. (2021). A small molecule produced by Lactobacillus species blocks Candida albicans filamentation by inhibiting a DYRK1-family kinase. Nat. Commun. 12, 6151. doi: 10.1038/s41467-021-26390-w
Mailänder-Sánchez, D., Braunsdorf, C., Grumaz, C., Müller, C., Lorenz, S., Stevens, P., et al. (2017). Antifungal defense of probiotic Lactobacillus rhamnosus GG is mediated by blocking adhesion and nutrient depletion. PloS One 12, e0184438. doi: 10.1371/journal.pone.0184438
Narasimulu, J., Baburajan, N., Saravanan, T. S., Raorane, C. J., Vaidyanathan, V. K., Ravichandran, V., et al. (2024). Dipeptides from Lactiplantibacillus plantarum limit Pseudomonas aeruginosa pathogenesis. J. Appl. Microbiol. 135. doi: 10.1093/jambio/lxae285
Oerlemans, E. F. M., Bellen, G., Claes, I., Henkens, T., Allonsius, C. N., Wittouck, S., et al. (2020). Impact of a lactobacilli-containing gel on vulvovaginal candidosis and the vaginal microbiome. Sci. Rep. 10, 7976. doi: 10.1038/s41598-020-64705-x
Patil, S., Rao, R. S., Majumdar, B., and Anil, S. (2015). Clinical appearance of oral candida infection and therapeutic strategies. Front. Microbiol. 6, 1391. doi: 10.3389/fmicb.2015.01391
Poon, Y. and Hui, M. (2023). Inhibitory effect of lactobacilli supernatants on biofilm and filamentation of Candida albicans, Candida tropicalis, and Candida parapsilosis. Front. Microbiol. 14, 1105949. doi: 10.3389/fmicb.2023.1105949
Rajasekharan, S. K. and Shemesh, M. (2022). Spatiotemporal bio-shielding of bacteria through consolidated geometrical structuring. NPJ Biofilms Microbiomes. 8, 37. doi: 10.1038/s41522-022-00302-2
Sobel, J. D. (2007). Vulvovaginal candidosis. Lancet 369, 1961–1971. doi: 10.1016/S0140-6736(07)60917-9
Venugopal, A., Sionov, R. V., Kroupitski, Y., Steinberg, D., and Shemesh, M. (2025). The V-Shaped Structuring Regulated via the LuxS-Dependent Quorum-Sensing Pathway Is Associated With Lactiplantibacillus plantarum Survivability in Acidic Environments. Food Frontiers. 6, 801–818. doi: 10.1002/fft2.514
Zeise, K. D., Woods, R. J., and Huffnagle, G. B. (2021). Interplay between candida albicans and lactic acid bacteria in the gastrointestinal tract: impact on colonization resistance, microbial carriage, opportunistic infection, and host immunity. Clin. Microbiol. Rev. 34, e0032320. doi: 10.1128/CMR.00323-20
Keywords: cellular heterogeneity, V-shaped structure, probiotic biofilm, Lactobacilli, C. albicans, nomadic
Citation: Paul LS, Hameed HC, K. V. L, Sharma A, Lee J, Shemesh M and Rajasekharan SK (2025) Nomadic Lactobacilli as cell factory for antibiofilm therapy. Front. Cell. Infect. Microbiol. 15:1668573. doi: 10.3389/fcimb.2025.1668573
Received: 18 July 2025; Accepted: 23 September 2025;
Published: 16 October 2025.
Edited by:
Carolina Henritta Pohl, University of the Free State, South AfricaReviewed by:
Anna Dongari-Bagtzoglou, University of Connecticut Health Center, United StatesWinschau Van Zyl, University of the Free State, South Africa
Copyright © 2025 Paul, Hameed, K. V., Sharma, Lee, Shemesh and Rajasekharan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satish Kumar Rajasekharan, c2F0aXNoa3IyQHNybWlzdC5lZHUuaW4=; Moshe Shemesh, bW9zaGVzaEB2b2xjYW5pLmFncmkuZ292Lmls
†These authors have contributed equally to this work
 Linette Shoby Paul1†
Linette Shoby Paul1† Ashu Sharma
Ashu Sharma Jintae Lee
Jintae Lee Satish Kumar Rajasekharan
Satish Kumar Rajasekharan