- 1Stomatology Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2School of Stomatology, Zhengzhou University, Zhengzhou, China
- 3Department of Stomatology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 4Department of Orthopedics, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
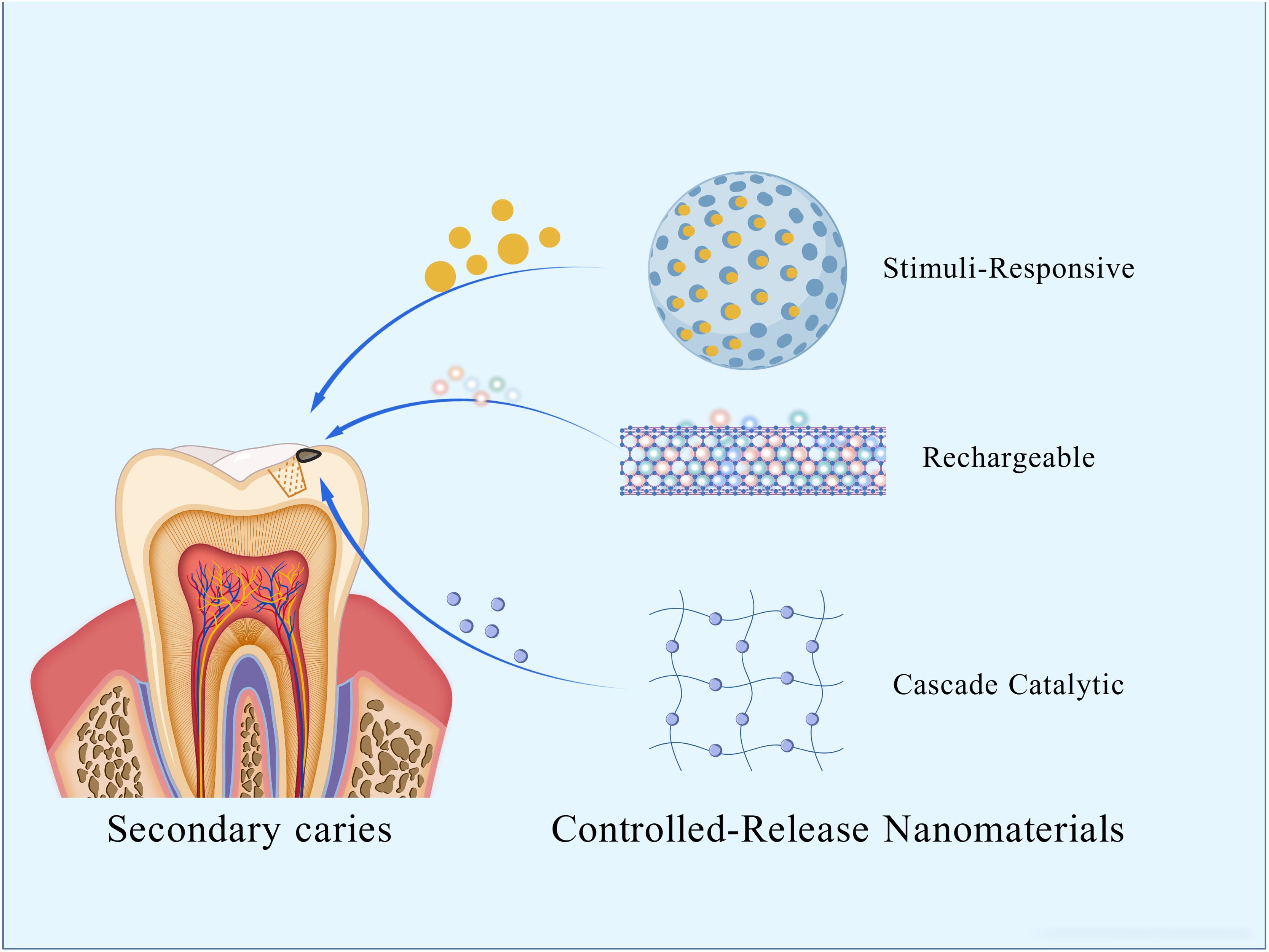
Secondary caries is a leading cause of restoration failure. Inhibiting caries through antimicrobial efficacy is essential for extending the restoration’s service life. Antimicrobial agents have been incorporated into restorative materials for decades. Based on their mechanism of antimicrobial action, these materials are classified as either releasing or non-releasing types. However, the simple release strategy is often insufficient for long-term caries prevention, as it lacks the precision, durability, and adaptability now required. This necessitates the development of next-generation systems that can provide a controlled, sustained, and targeted antimicrobial activity. To this end, this review focuses on advanced, controlled-release antimicrobial strategies, exploring the design of novel nanomaterials, their functional efficacy, and the mechanisms of their representative antimicrobial agents.
1 Introduction
Secondary caries is a common reason associated with failed restorations. Factors such as polymerization shrinkage of composite resin materials, mechanical stress, and adhesive degradation can lead to microgaps forming between the restoration and the tooth structure (Alizadeh Oskoee et al., 2017). The polymerization shrinkage of composite resins, combined with mechanical stress and adhesive degradation, can create micro-gaps at the restoration-tooth interface (Bowen et al., 2018). These marginal defects allow acid-producing bacteria to penetrate, metabolizing carbohydrates into a localized acidic microenvironment. Once established, this process of secondary caries accelerates the loss of tooth structure and elevates the risk that pulp treatment will be required (Schwendicke et al., 2021). While modern restorative materials can meet functional restoration needs, they cannot actively address bacterial infections at vulnerable margins or demineralization issues.
Early efforts to extend the service life of dental composites centered on enhancing their mechanical properties and minimizing polymerization shrinkage (Zhang et al., 2015; Balhaddad et al., 2021). Advances in nanotechnology have spurred interest in multifunctional composites, marking a pivotal shift from passive to active strategies in preventing secondary caries. Antimicrobial composites reduce bacterial adhesion via antimicrobial release or contact-killing, preventing biofilm formation (Jiao et al., 2019; Xue et al., 2020). Composites designed for remineralization can provide ion sources: calcium and phosphate to compensate for structural damage caused by demineralization in early-stage caries (Besinis et al., 2014). Traditional antimicrobial agents or remineralization ions are often released linearly or uncontrolled, resulting in short release durations and uncontrollable release quantities (Sivakumar et al., 2014). However, with the continuous development of nanotechnology, this issue has been effectively addressed, enabling precise control over the release of antimicrobial agents and remineralization ions (Rostami et al., 2025). By carefully designing the structure of nanomaterials to respond to specific stimuli, targeted release can be achieved (Wang et al., 2024). Rechargeable nanomaterials allow for the repeated release of bioactive agents, while sophisticated cascade nanoreactors enable a synergistic, multi-step process of antibacterial action and remineralization within the caries microenvironment. Crucially, nanoparticles preserve the mechanical strength of restorative materials while introducing new functionalities (Amin et al., 2025).
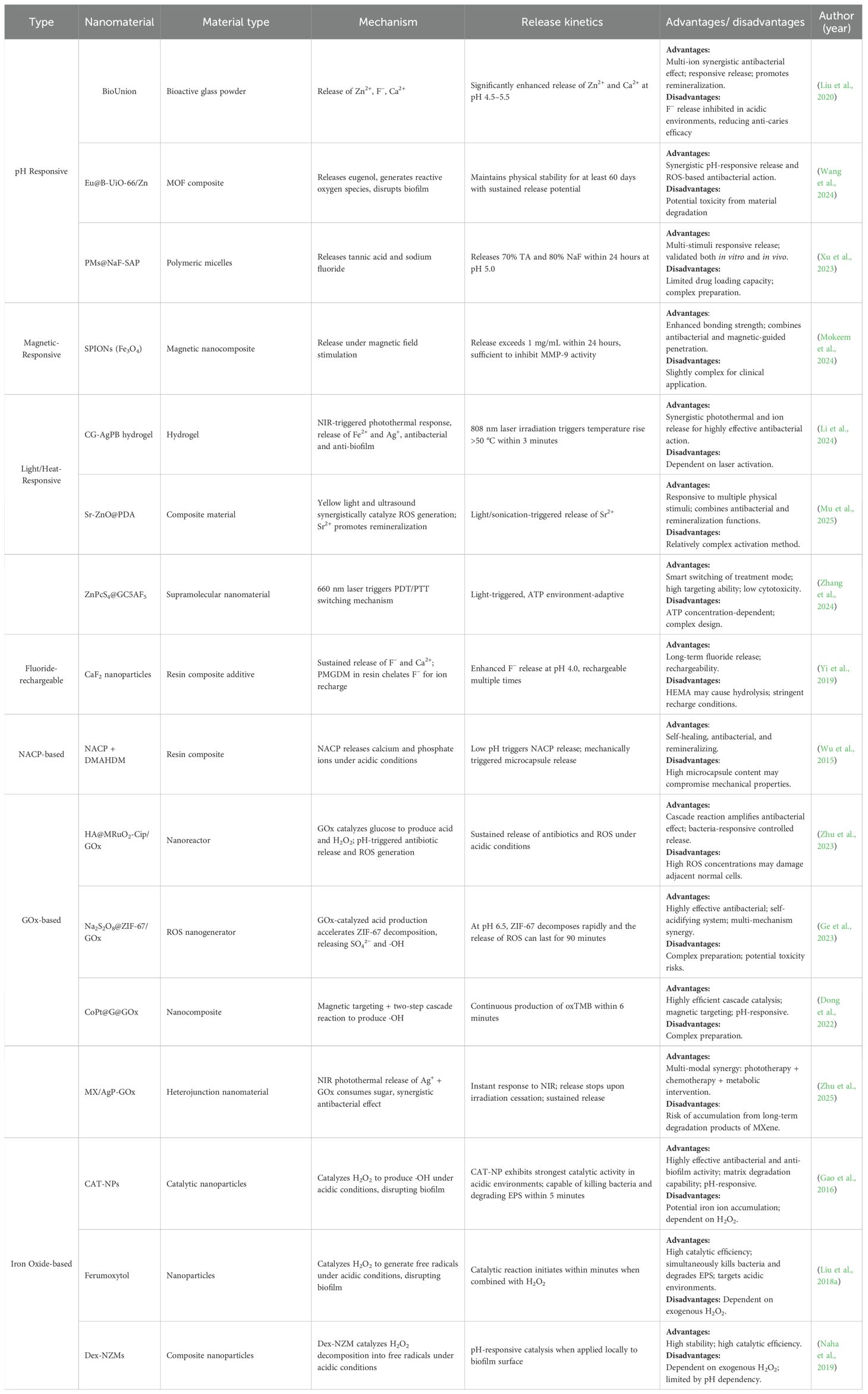
Therefore, this paper reviews the latest progress of controlled-release antimicrobial nanomaterials (as summarized in Table 1) in preventing and treating secondary caries from the perspective of the strategy mechanism.
2 Stimuli-responsive strategy
Incorporating antimicrobial nano-ions into resin can address bacterial infections that cause secondary caries while maintaining the mechanical strength of the restorative material. Traditional antimicrobial agents often compromise the mechanical properties of resins (Ibrahim et al., 2020). In contrast, nano-antimicrobial agents can effectively kill bacteria at lower concentrations with their high specific surface area and extremely small volume (Sowmya et al., 2024). The nano-structure enriches the antimicrobial mechanisms, including contact killing, inducing cellular oxidative stress, and interfering with metabolism (Jowkar et al., 2025), making it less prone to bacterial resistance—a global concern. Initially, nano-antimicrobial agents faced the challenge of the burst effect: the early release of large amounts of antimicrobial agents caused local drug concentrations to rise, leading to significant cellular toxicity and a sharp decline in antimicrobial efficacy later on (de Jesus et al., 2024).
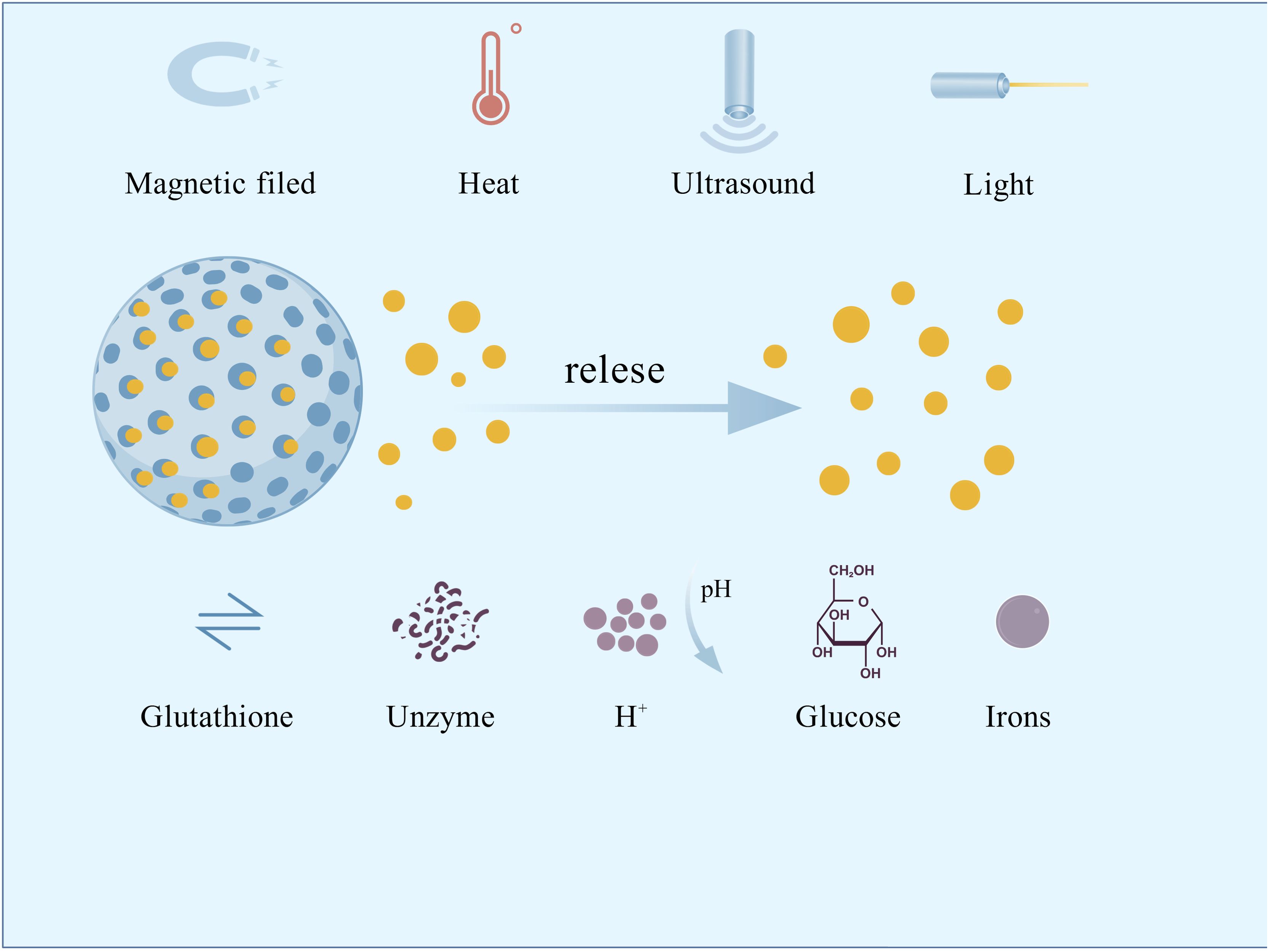
Additionally, concerns arose regarding the accumulation of metal nanoparticles in the body, their potential to induce inflammation, and the risk of breaching the blood-brain barrier (Wang et al., 2023; Yang et al., 2024). Controlled-release nano-antimicrobial materials, which can precisely respond to changes in the oral microenvironment to release antimicrobial agents, have emerged as a highly promising strategy to address this challenge. Common stimulants include exogenous magnetic fields, ultrasound, light, temperature, endogenous glutathione, enzymes, acids, glucose, ions, etc. (Figure 1). Light, temperature, and acid stimulants are commonly used to develop oral nanomedicines. Research on pH responsiveness to the special environment created by microbial acid production is the most prevalent.
2.1 pH-responsive
Dental caries-causing bacteria, such as Streptococcus mutans (S. mutans), produce organic acids (lactic acid and acetic acid) through glycolysis. This metabolic process lowers the pH at the interface between the filling material and the tooth structure, ultimately accelerating the dissolution of hydroxyapatite (Sangha et al., 2024).
The mechanism of acid-responsive nanomedicines involves three points: (1) protonation or ionization of functional groups. For example, amino groups (-NH2) protonate at low pH values to form -NH3+, releasing encapsulated antimicrobial agents; (2) cleavage of acid-labile bonds. Aldehyde bonds in specific mesoporous silica nanoparticles readily hydrolyze in acidic environments, enabling rapid drug release; (3) conformational changes in polymers. When pH drops below 5.5, carboxyl groups (–COOH) protonate, causing polymer contraction, which compresses internal pores and expels molecules (Meng et al., 2024; Boyuklieva et al., 2025; Tapponi et al., 2025). Their nanoscale dimensions further enhance biofilm penetration, ensuring efficient drug delivery. When functionalized with fluoride ions (F-), these systems exhibit synergistic antimicrobial-remineralizing dual functionality, presenting a promising strategy for secondary caries prevention and therapy (Qi et al., 2025). Currently, acid-stimuli-responsive nanomaterials are the most extensively studied. Below, three typical acid-responsive materials are introduced: BioUnion, Eugenol, and Tannic acid.
2.1.1 BioUnion
BioUnion filler is a novel bioactive glass powder (Xia et al., 2025), that addresses the issue of declining fluoride release over time observed in traditional glass ionomer cements (GICs) (Dcruz et al., 2024; Da et al., 2025). It composed of silicon dioxide (SiO2), zinc oxide (ZnO), calcium oxide (CaO), and a fluoride compound. Its particles can be incorporated into dental materials, releasing Zn²+ (combats oral bacteria and reduces dentin demineralization), F− (inhibits demineralization), and Ca2+ (enhances remineralization) ions to prevent secondary caries (Fallahzadeh et al., 2022; Piszko et al., 2025). It also exhibits pH-dependent selective release of Zn2+/Ca2+ (Liu et al., 2020).In bovine dentin subjected to 4-week pH-cycling, the GICs-Bio group (Control group: GICs) demonstrated superior inhibition of demineralization and promotion of remineralization. In vitro salivary titration assembly revealed that BioUnion™-incorporated GIC provides higher initial fluoride release and a more sustained release profile under simulated oral conditions (Htet et al., 2025).
2.1.2 Eugenol
Whereas BioUnio’s strategy relies on antibacterial action and remineralization, eugenol acts primarily through pathogen inhibition and pulp inflammation modulation to prevent secondary caries (Nazemisalman et al., 2024; Patil et al., 2025). Innovative nano-delivery systems now enhance its efficacy by improving solubility and enabling controlled release.
These systems retain Eugenol’s functionality while achieving slow, sustained drug release, and can be triggered to release in response to specific environmental stimuli, thereby avoiding the cytotoxicity associated with long-term high-concentration release, improving biosafety (Khan et al., 2019; Montoya et al., 2023). Specific applications include the Eu@B-UiO-66/Zn system, based on eugenol-loaded B-UiO-66 MOF complexed with Zn2+, which achieves pH-responsive release via a phenolic hydroxyl-Zn2+ cage-like network where the released Eugenol generates reactive oxygen species (ROS) to disrupt biofilms (Wang et al., 2024); nanoencapsulation significantly enhances eugenol stability, for example up to 60 days (Vilela et al., 2023);acid-triggered nanoparticles modified with casein and based on hydroxyapatite/calcium carbonate that adhere to dentin and release Eugenol under low pH conditions (Sereda et al., 2023); synthetic eugenol derivatives containing polymerizable methacrylate groups, such as EgMA, enabling participation in resin free-radical polymerization, which maintain antibacterial activity against Gram-positive(G+) and Gram-negative(G-) bacteria while avoiding detrimental effects on resin polymerization and strength (Almaroof et al., 2016).
2.1.3 Tannic acid
Nanotechnology not only overcomes the limitations of natural active substances but also endows them with new functionalities. Tannic acid (TA) is a water-soluble natural polyphenol extracted from plants with antibacterial properties (Yamauchi et al., 1989; Sileika et al., 2013). When combined with phenylboronic acid (PBA), it forms a borate ester bond that can break down and release functional molecules under pH changes caused by bacteria (Springsteen and Wang, 2002). Using nano-polymer micelles (PM) with a shell-core structure as a scaffold, Sodium fluoride (NaF), tannic acid, and salivary acquired peptides (SAP)were loaded to produce multifunctional smart-release nano-antimicrobial materials PMs@NaF-SAP. At pH 5.0, nearly 70% of TA was released from PMs@NaF -SAP, while only approximately 40% was released at pH 7.4. Additionally, the more acidic the pH, the faster the NaF release rate; at pH 5.0, approximately 80% of NaF is released within 24 hours, and its antibacterial efficacy at this pH value is significantly stronger than that of the chlorhexidine (CHX) control group. As determined, PMs@NaF-SAP exhibits excellent demineralization inhibition and remineralization promotion capabilities, with relatively smooth tooth surfaces. Results from a caries-prone rodent model indicate that PMs@NaF-SAP treatment reduces the incidence and severity of lesions on smooth and fissure surfaces, effectively preventing the development of caries in vivo (Xu et al., 2023).
2.2 Magnetic field-responsive
Magnetically responsive materials, activated by an external magnetic field, enable the stimulation or targeting of specific objects using materials containing magnetic substances (Menezes-Silva et al., 2021). The magnetic field can adjust ion distribution within the material, enhancing the marginal sealing of restorative materials and reducing microleakage. Under its guidance, nanoparticles can penetrate dentinal tubules effectively, improving bonding strength.
Superparamagnetic iron oxide nanoparticles (SPIONs) exhibit non-magnetic behavior without an external magnetic field. However, when subjected to an external field, they respond rapidly to Lorentz forces, enabling their movement or directional alignment under magnetic guidance (Hu et al., 2017). In dental adhesives, SPIONs (Fe3O4) guided by a magnetic field can infiltrate the micropores of dentin more effectively, leading to enhanced bond strength. This process generates mild thermal effects that can inhibit bacterial growth (Garcia et al., 2021). Ferroferric Oxide (Fe3O4) is a type of SPION. Constructing a core-shell structure with Fe3O4 cores and mesoporous silica shells allows for encapsulating antimicrobial agents like CHX and quaternary ammonium silane. This system achieved an inhibition rate of over 78% against S. mutans biofilms and maintained antibacterial activity even after 6 months of artificial aging, with no significant cytotoxicity observed (Mokeem et al., 2024). Targeted, sustained antimicrobial delivery to the critical interface prolongs restoration longevity.
Magnetic field-responsive materials provide superior spatiotemporal control and deep-tissue activation capabilities but at the cost of requiring external hardware. Acid-responsive materials are simpler, more autonomous, and highly biocompatible, but their action is less precise and confined to acidic micro-environments.
2.3 Light/heat responsive
For clinical ease-of-use, photothermal-responsive systems are ideal, as they allow therapeutic agents to be activated on-demand with a straightforward light source. Traditional nanomaterials exert antibacterial effects by releasing metal ions such as Ag+, Cu2+, and Zn2+, which disrupt bacterial cell membranes and interfere with enzymatic activities (Xiu et al., 2011; Fan et al., 2021). However, their “burst release” mechanism leads to short therapeutic duration and lacks precise control (Jiang et al., 2023). Photo-responsive nanomaterials based on photodynamic therapy (PDT) offer a novel approach for preventing and managing secondary caries. PDT involves three key components: oxygen, an excitation light source, and a photosensitizer (PS). When exposed to light of specific wavelengths, the PS generates toxic ROS. These ROS possess strong oxidizing power and high reactivity, thereby inducing rapid lipid peroxidation in bacteria (Pereira et al., 2013; Fiegler-Rudol et al., 2025).
Photothermal therapy (PTT) and PDT are two complementary and promising phototherapeutic approaches. Silver-ion-doped Prussian Blue (AgPB) nanoparticles were encapsulated in cationic guar gum (CG) to form an antibacterial PTT hydrogel, CG-AgPB, exhibiting a photothermal conversion efficiency of 34.4%. Upon exposure to an 808 nm laser at a power density of 0.4 W cm², the hydrogel surpassed 50 °C within 3 minutes, synchronized with the release of Ag+ ions from interstitial sites of the AgPB lattice, thereby inhibiting both individual oral cariogenic bacteria and their biofilms. In vivo, CG-AgPB-mediated PTT significantly reduced cariogenic bacteria to less than 1% of the original load in a rat caries model (Li et al., 2024). The laser power and irradiation duration can be precisely modulated to confine the therapeutic thermal zone, thereby minimizing collateral damage to adjacent healthy tissues (Alumutairi et al., 2020).
A polydopamine (PDA)-coated strontium-doped zinc oxide composite (Sr-ZnO@PDA) responds to yellow light and ultrasound. Synergistic piezophotocatalysis generates ROS, destroying bacterial cell membrane structures and decomposing dental surface pigments. Additionally, strontium ions (Sr2+) released from SZ@PDA promote remineralization of enamel and dentin, repairing damaged tooth tissues (Mu et al., 2025). Another supramolecular nanoformulation achieves highly efficient inhibition of S. mutans biofilms through an adaptive PDT/PTT switching mechanism. Guanidinium groups on the material surface specifically bind to negatively charged moieties such as lipoteichoic acid and ATP on the S. mutans cell membrane, enabling targeted accumulation. Upon 660 nm laser irradiation, synergistic PTT and PDT effects are observed. In low-ATP environments (planktonic bacteria), the photosensitizer ZnPcS4 exists as monomers that predominantly generate ROS via PDT, disrupting bacterial membrane integrity. In high-ATP environments (biofilms), ZnPcS4 forms H-aggregates that chiefly produce heat through PTT for bactericidal action, circumventing the limited ROS penetration issue. Experiments also confirmed the material’s low cytotoxicity; compared to the markedly demineralized control group, enamel remained notably intact in a rat dental caries model (Zhang Y. et al., 2024).
This section highlights the promise of stimuli-responsive nanomedicines for combating secondary caries. pH-responsive systems offer high clinical relevance by leveraging the cariogenic microenvironment to trigger targeted drug release and demonstrate synergistic antibacterial-remineralization effects. In comparison, external field-responsive strategies provide superior spatiotemporal control over treatment, enabling on-demand activation. Yet they face significant practical limitations. For light-responsive systems in particular, limited tissue penetration depth remains a major constraint. In morphologically complex teeth such as molars, light may be unable to effectively reach lesions in proximal or deep dentinal areas, resulting in incomplete treatment. Similarly, magnetic-responsive approaches require externally applied devices, which may complicate clinical integration and routine use.
3 Rechargeable strategy
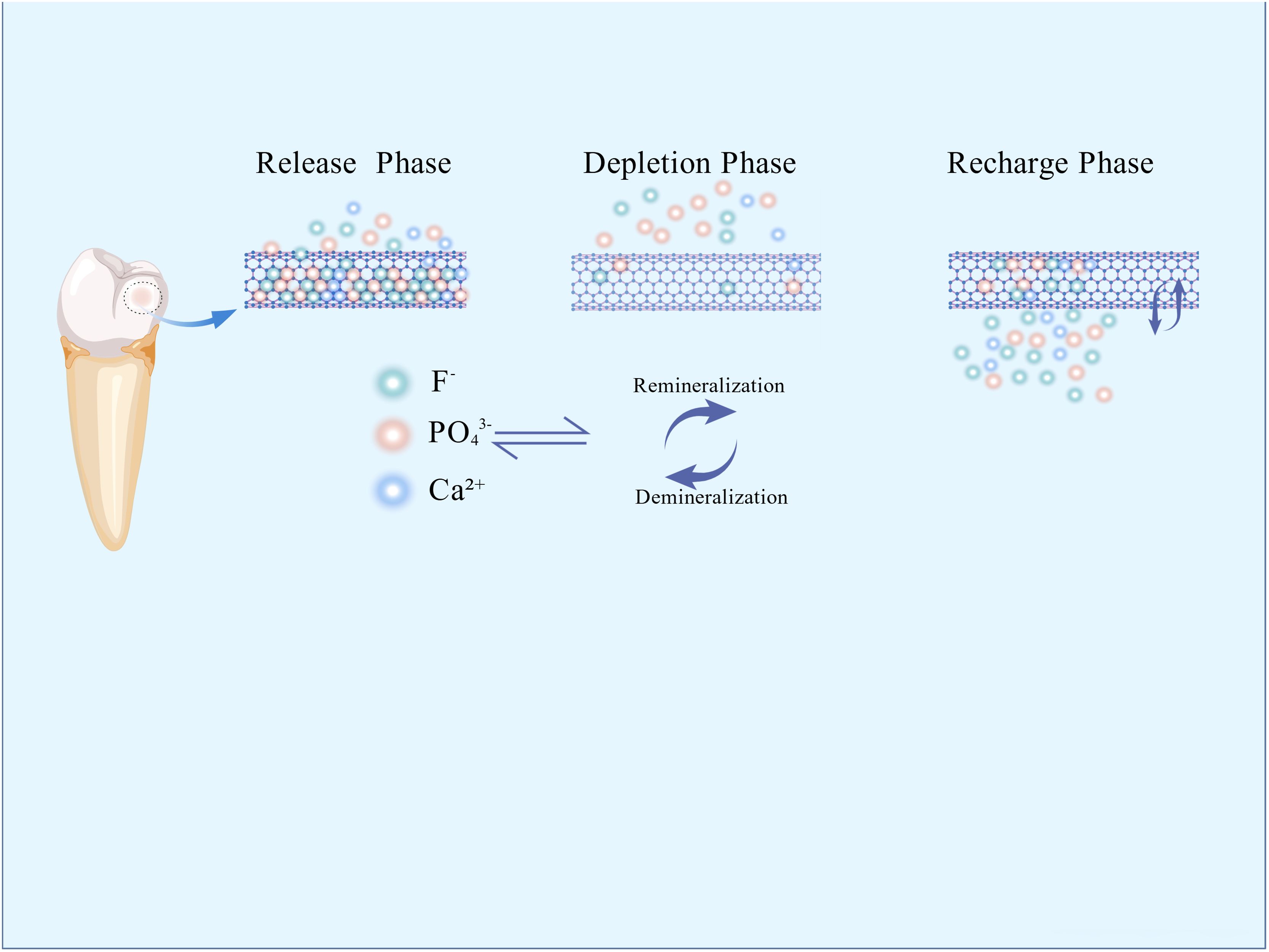
Interventions for secondary caries primarily encompass antibacterial approaches and remineralization. For years, researchers have incorporated remineralizing ions into restorative materials. Fluoride ions(F-) facilitate the deposition of calcium ions (Ca2+) and phosphorus ions (PO4³−) within the tooth structure, forming more acid-resistant fluorapatite (Ca5(PO4) 3F), while also buffering the acidic environment created by bacteria. Sodium fluoride nanoparticles have reduced secondary caries at restoration margins by releasing F- and Ca2+ (Albelasy et al., 2023). However, the short duration of effective ion release from these materials, relative to the expected lifespan of restorations, remains a critical challenge. To address this, a rechargeable strategy has been proposed to prolong ion release (Kelić et al., 2023). This approach centers on replenishing the diminishing active ions within the restorative material through exogenous ion exchange, restoring its remineralizing potential. There are three phases: 1) Release phase: Soluble active ions (F−/Ca2+) are released from the material, promoting remineralization; 2) Depletion phase: Continued ion release depletes surface reservoirs, leading to declining ion concentration and diminished remineralization capacity; 3) Recharging phase: The material is exposed to an exogenous high-concentration ion solution. Driven by concentration gradients or ion exchange, new ions diffuse into the inorganic matrix/microporous structure, achieving reloading (Puttipanampai et al., 2025) (Figure 2).
3.1 Fluoride
As widely documented, fluoride combats dental caries through three mechanisms: inhibiting demineralization, promoting remineralization, and suppressing bacterial metabolism (Monjarás-Ávila et al., 2025). To address the transient fluoride release from existing restorative materials, rechargeable fluoride nanomaterials offer a new way for managing secondary caries.
Calcium fluoride (CaF2) nanoparticles (average 58 nm), synthesized via spray-drying and incorporated into resin matrices, exhibit excellent biocompatibility and significantly enhanced F− release capacity that remains stable over repeated recharging cycles (Yi et al., 2019). These materials demonstrate high pH sensitivity, where acidic conditions trigger a surge in fluoride release, particularly pronounced in systems containing CaF2 or fluorohydroxyapatite under cariogenic pH (4.5-5.5) generated by bacterial metabolism (Morawska-Wilk et al., 2025). Studies confirm that ion release (Ca2+, F−, PO4³−) at acidic pH (4.5-5.5) is markedly higher than at neutral pH (6.5), a trend sustained even after recharging (Venkataiah et al., 2025). This pH-responsive sustained release is critical in resin-based restorations: it enhances remineralization within cariogenic microenvironments and extends restoration longevity. Both silane- and methacrylate-based composites effectively serve as rechargeable fluoride carriers (Madhyastha et al., 2025). The synergy between acid-triggered release and on-demand recharging holds significant promise for combating secondary caries.
3.2 Nanoamorphous calcium phosphate
Amorphous calcium phosphate (ACP) possesses a non-crystalline structure and exhibits high ionic release activity (Degli Esposti and Iafisco, 2022). Nanotechnology has effectively addressed the challenge of uncontrolled ion release from traditional ACP. Under acidic conditions, NACP rapidly releases significant amounts of calcium (Ca2+) and phosphate (PO4³−) ions, while maintaining structural stability with minimal ion release in neutral or alkaline environments (Liang et al., 2019).
Incorporating nano-sized NACP alongside dimethylaminohexadecyl methacrylate (DMAHDM) nanoparticles into resin-based materials creates a dual-functional composite. The Ca2+ and PO4³− ions released from NACP neutralize acids produced by cariogenic bacteria, disrupting the acidic environment conducive to demineralization and promoting remineralization. Simultaneously, the DMAHDM component exerts a potent direct antibacterial effect (Wu et al., 2015; Sales-Junior et al., 2025). Furthermore, animal models have demonstrated that these composites induce minimal pulp irritation and stimulate tertiary dentin formation (Li et al., 2014). In an in vitro saliva-derived biofilm secondary caries model, it inhibits the growth of cariogenic bacteria at the resin margin and has no negative impact on tooth enamel hardness (Zhou et al., 2020).
NACP has also been successfully integrated into dental adhesives, often with monobasic calcium phosphate dihydrate (MCPM), to enhance their remineralization and antibacterial capabilities (Liu et al., 2018a). Despite finite ion release duration (typically months), rechargeable NACP nanocomposites overcome this limitation via periodic calcium/pHospHate solution immersion. Both rechargeable NACP and NACP-DMAHDM variants retain flexural strength and elastic modulus comparable to commercial counterparts. They significantly inhibit biofilm metabolic activity and lactic acid production, while drastically reducing biofilm colony-forming units (CFUs). A single recharge sustains effective ion release for up to 42 days (Al-Dulaijan et al., 2018). Crucially, the release profile remains stable over multiple recharge cycles, demonstrating long-term ion release and remineralization potential. This recharge strategy also shows significant anti-caries efficacy in adhesives and sealants (Ibrahim et al., 2020). For instance, a novel self-healing dental adhesive incorporating poly(urea-formaldehyde) (PUF) microcapsules, DMAHDM, and NACP achieved a 67% crack-healing efficiency without compromising dentin bond strength. Concurrently, it reduced biofilm CFUs by four orders of magnitude, confirming its potent antibacterial properties (Wu et al., 2019).
This rechargeable strategy substantially bridges the translational gap: Recharging requires mere minutes per session, with a single treatment sustaining caries-preventive efficacy for up to six months. Crucially, at-home recharge via mouth rinse empowers patient autonomy. It establishes a clinically viable protocol for long-term dynamic management of secondary caries.
Rechargeable resin-based restoratives can markedly extend the functional lifespan of dental restorations through their replenishable ion-release mechanism, simultaneously enhancing remineralization and antimicrobial efficacy. In acidic environments, these materials exhibit intelligent, pH-responsive ionic discharge while maintaining favorable biocompatibility and mechanical stability, offering a novel long-term dynamic management strategy for recurrent caries.
Nevertheless, recent investigations have highlighted several shortcomings. First, although CaF2 and NACP nanoparticles are intrinsically cytocompatible, high fluoride loads or DMAHDM quaternary ammonium antimicrobials still display concentration-dependent cytotoxicity in 3-D spheroid cultures or human dental-pulp stem-cell assays, compromising the biosafety of the pulp and surrounding soft tissues (Fei et al., 2024). Second, even advanced CAD/CAM resin composites undergo inevitable deterioration in flexural strength and surface hardness after prolonged exposure to the moist oral milieu, primarily attributable to water sorption and hydrolytic degradation (Wendler et al., 2021). Third, fluoride-releasing materials experience significant deterioration of nanohardness, elastic modulus, and surface roughness when challenged by acidic beverages (Güner and Köse, 2024). Fourth, no consensus exists on the concentration, pH, or contact time of “recharge mouthwashes”; regulatory agencies have yet to establish in vitro–in vivo correlation guidelines, and published clinical trials are underpowered with follow-up periods < 2 years, precluding reliable assessment of long-term marginal integrity and secondary-caries incidence (Zhang J. et al., 2024).
4 Cascade catalytic nanoreactor
Integrating nanotechnology, catalytic chemistry, and biomedicine has led to the development of cascade catalytic nanoreactors, representing an advanced strategy for preventing and treating oral infectious diseases. Inspired by intracellular multi-enzyme cascade reactions, researchers initially attempted to construct artificial cascade systems by encapsulating natural enzymes within porous synthetic materials, including metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and hydrogen-bonded organic frameworks (HOFs) (Lykourinou et al., 2011; Huang et al., 2022). However, these early systems suffered from poor enzyme stability, low reaction efficiency, and inadequate controllability (Meghwanshi et al., 2020). Subsequent advancements replaced natural enzymes with inorganic nanocatalysts (Fe3O4 or Au nanoparticles), effectively resolving stability issues (Li et al., 2015; Kwon et al., 2021; Ma et al., 2024). Further innovation incorporated stimuli-responsive materials (ultrasound or pH-activated components) to achieve precisely controlled activation (Chen and Li, 2020; Li et al., 2022). Self-supplying substrate systems were engineered to overcome dependence on external reactants (Figure 3). Consequently, application scenarios have expanded beyond tumor therapy to encompass anti-biofilm interventions.
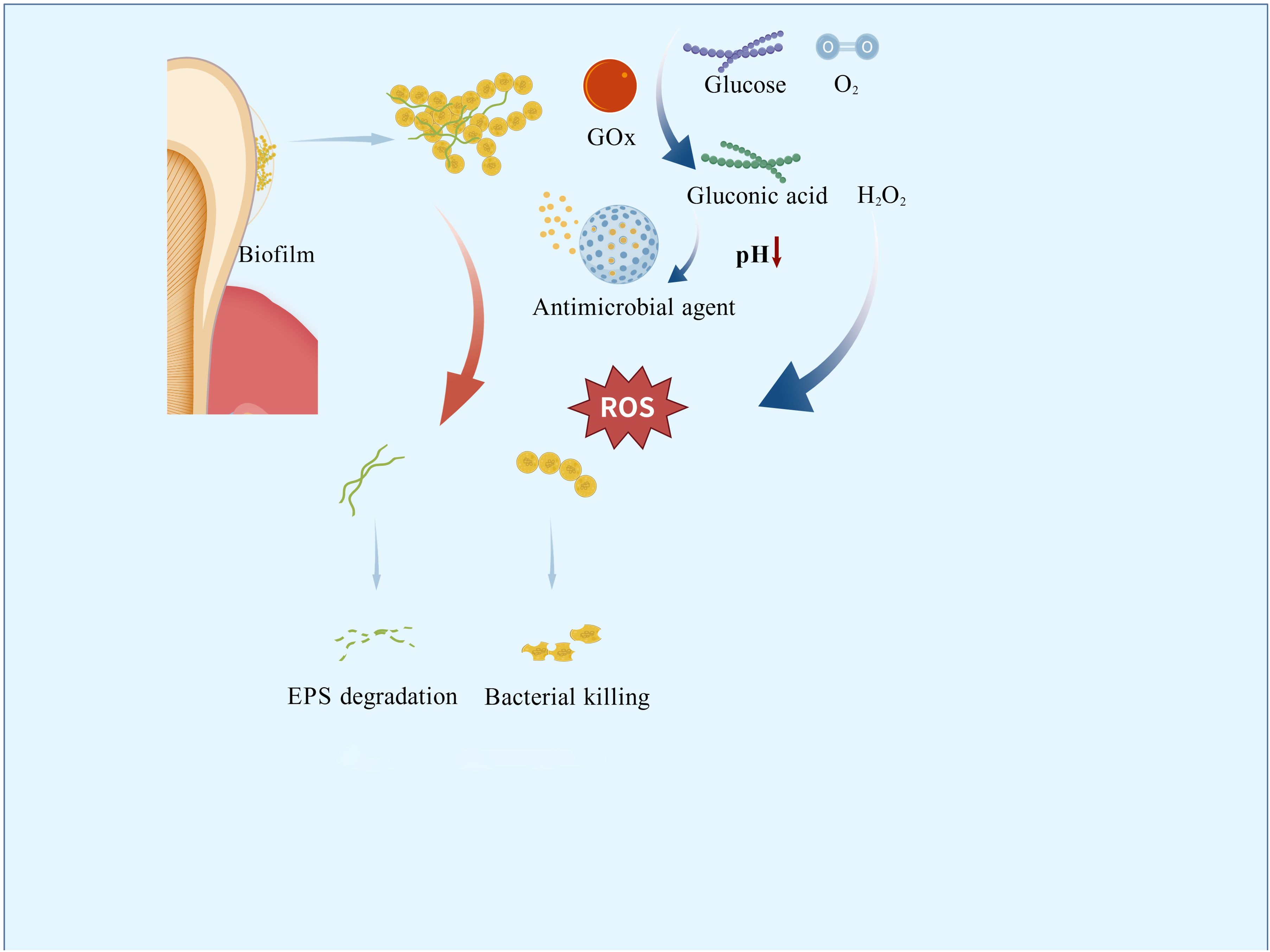
4.1 Glucose oxidase
GOx exhibits catalytic properties and bioactivities uniquely suited to the cariogenic microenvironment: it oxidizes glucose and oxygen into gluconic acid and hydrogen peroxide (Dubey et al., 2017). This reaction directly depletes free glucose, competing with S. mutans for metabolic substrates and suppressing bacterial growth and acid production (Senol et al., 2007; Yuan et al., 2015). The in situ-generated hydrogen peroxide reduces glucosyltransferase activity, diminishing viscous glucan synthesis and disrupting biofilm formation mechanisms (Zhang et al., 2021). Concurrently, gluconic acid production triggers pH-responsive drug release (Wang et al., 2022). GOx is not used in isolation. It is integrated into nanocomposites that incorporate additional targeting strategies (such as magnetic guidance, pH-responsive release, or photothermal activation) which enhance spatial precision and biological specificity. GOx does not target specific bacterial species through molecular recognition, but rather functionally discriminates against cariogenic pathogens. These targeted mechanisms establish a novel paradigm for constructing multimodal antimicrobial systems.
The greatest advantage of cascade nanoreactors lies in their ability to integrate and leverage distinct molecular functions. The HA@MRuO2–Cip/GOx composite (composed of hydroxyapatite, HA; mesoporous ruthenium dioxide, MRuO2; ciprofloxacin, Cip; and GOx) incorporates a mesoporous RuO2 core co-loaded with Cip and GOx. In vitro, the composite disrupts biofilms by cleaving extracellular DNA, thereby enhancing the bactericidal efficacy of Cip against planktonic bacteria. Under acidic conditions, it not only sustains ROS generation but also allows controlled release of antibiotics (Zhu et al., 2023).
Similarly leveraging acidic conditions for antibacterial activity, Na2S2O8@ZIF-67/GOx(composed of sodium persulfate, Na2S2O8; zeolitic imidazolate framework-67, ZIF-67; and GOx) functions as a novel ROS nanogenerator. GOx-mediated catalysis in acidic microenvironments produces H2O2 and gluconic acid, which further reduces pH to accelerate ZIF-67 decomposition and Na2S2O8 release. This process yields highly toxic sulfate (SO42−) and hydroxyl (-OH) radicals, effectively suppressing bacterial proliferation and biofilm formation (Ge et al., 2023).
Also operating under external energy stimulation, the nanozyme CoPt@G@GOx (composed of cobalt-platinum alloy, CoPt; graphene, G; and GOx) employs a two-step cascade reaction to generate potent -OH radicals in acidic environments, achieving 4-fold enhanced catalytic efficiency in disrupting S. mutans biofilms compared to simple mixtures. Its magnetic CoPt@grapHene core enables precise targeting of carious lesions via external magnetic fields, minimizing off-target effects (Dong et al., 2022). TiO2-GOx(composed of titanium dioxide, TiO2; and GOx)exhibits high catalytic activity in glucose-rich environments under UV irradiation, significantly amplifying ROS production (Kim et al., 2019).
Incorporating both photothermal and biochemical antibacterial mechanisms, MX/AgP-Gox (composed of MXene, MX; silver nanoparticles, AgNPs; and GOx) is a cascade bio-heterojunction (HJ) engineered to target both dental-plaque biofilms’ chemical and biological constituents. Under near-infrared (NIR) irradiation, the HJ rapidly heats up to generate photothermal effects while exploiting H2O2 to burst-produce massive ROS, achieving highly efficient phototherapy. Even in darkness, the bactericidal action of Ag+ ions and glucose depletion mediated by GOx act synergistically to suppress bacteria, ensuring long-lasting anti-caries efficacy after light withdrawal. Rat caries models further confirmed that the material inhibits enamel demineralization and exhibits excellent biocompatibility (Zhu et al., 2025).
4.2 Iron oxide
Catalytic iron oxide nanoparticles (CAT-NPs) mimic natural peroxidases through their intrinsic catalytic activity. Under acidic conditions, they catalyze H2O2 decomposition into hydroxyl radicals (-OH), disrupting bacterial membranes, degrading intracellular macromolecules, and breaking down extracellular polymeric substances (EPS) within biofilms. In vitro studies demonstrate that CAT-NPs reduce apatite demineralization at acidic pH. Topical application combined with H2O2 effectively suppresses caries initiation and progression without adverse effects on oral mucosal tissues in vivo (Gao et al., 2016).
Ferumoxytol is a Food and Drug Administration (FDA)-approved nanoparticle formulation used for magnetic resonance imaging and the treatment of iron deficiency (Dósa et al., 2011). Ferumoxytol can penetrate the interior of biofilms and, under acidic conditions, catalyze the production of reactive radicals from H2O2. These radicals disrupt bacterial cell membranes and degrade extracellular polysaccharide matrices, killing the bacteria. In ex vivo biofilm models, its combination with low concentrations of H2O2 effectively inhibits biofilm accumulation on natural teeth and prevents enamel demineralization (Liu et al., 2018b). Notably, it enables pathogenic biofilm detection via colorimetric responses, establishing a theranostic platform for caries management (Liu et al., 2021). Dextran-coated iron oxide nanoparticles (Dex-NZMs) maintain catalytic efficiency while enhancing stability. These particles exhibit potent catalytic activity at acidic pH, enabling biofilm-specific targeting. Dex-NZMs prevent severe carious lesions in murine caries models while demonstrating excellent biosafety (Naha et al., 2019).
Cascade-catalytic nanoreactors that emulate natural enzymatic cascades have recently emerged as promising therapeutics for oral infectious diseases. They efficiently generate ROS, respond precisely to the pathological microenvironment, supply their own substrates, and exert multimodal synergistic antimicrobial effects. Collectively, these attributes substantially enhance biofilm eradication while minimizing collateral damage to healthy tissues.
Nevertheless, clinical translation remains hindered by several unresolved limitations. Catalytic activity is prone to attenuation in the complex oral milieu owing to protein adsorption, pH fluctuations, and variable ionic strength, leading to suboptimal durability and limited functional persistence (Ming et al., 2020). Moreover, the fidelity of cascade activation is still imperfect; off-target triggering or adventitious side-reactions may compromise therapeutic predictability and patient safety (Zhang et al., 2018). It constitute an innovative therapeutic platform, yet scalable manufacturing, reproducible quality control, and comprehensive regulatory frameworks are still in their infancy.
5 Opportunities and challenges in clinical translation
The heterogeneity of biofilms and the presence of salivary proteins pose challenges for oral antibacterial materials. The former has long posed a significant challenge to antimicrobial materials: the oral biofilm forms a complex three-dimensional structure. The EPS matrix acts like a “fortress wall” that blocks the penetration of antimicrobial molecules (Zhao et al., 2023). Gradients of oxygen and nutrients from the surface to the deeper layers lead to altered bacterial metabolic activity, with dormant bacteria in the deep layers exhibiting stronger drug tolerance (Liu et al., 2024).
Controlled-release nano-antimicrobial materials show promising performance in addressing biofilm heterogeneity. Physically, their nanoscale structure provides high surface energy, enhancing adhesion to negatively charged bacterial biofilms and enabling penetration through the EPS matrix to deliver antimicrobial agents directly to dormant bacteria in the deeper layers (Han et al., 2022). Moreover, the released antimicrobial molecules can disrupt bacterial membrane potential, inhibit enzymatic activity, and promote ROS generation (Sahli et al., 2022). Finally, through controlled-release mechanisms, these materials maintain effective bactericidal concentrations at the infection site, preventing exposure to sublethal doses and thereby suppressing the enrichment of drug-resistant mutants. These advantages make them highly effective against biofilms.
However, salivary proteins present a double-edged sword. Within hours after cleaning, teeth become coated with a layer of salivary proteins, which can isolate antibacterial materials from bacteria, thereby reducing antimicrobial efficiency and long-term efficacy (Heo et al., 2013). At the same time, this protein layer facilitates bacterial adhesion. However, current nano-antibacterial materials often fail to account for this effect. How to resist salivary protein adhesion through surface modification of nanomaterials is thus an important issue. Research should also be conducted under conditions that simulate the oral environment to more accurately evaluate the practical antibacterial performance of these materials. Rather than focusing solely on avoiding salivary proteins, it would be more beneficial to explore how to leverage them to enhance material functionality.
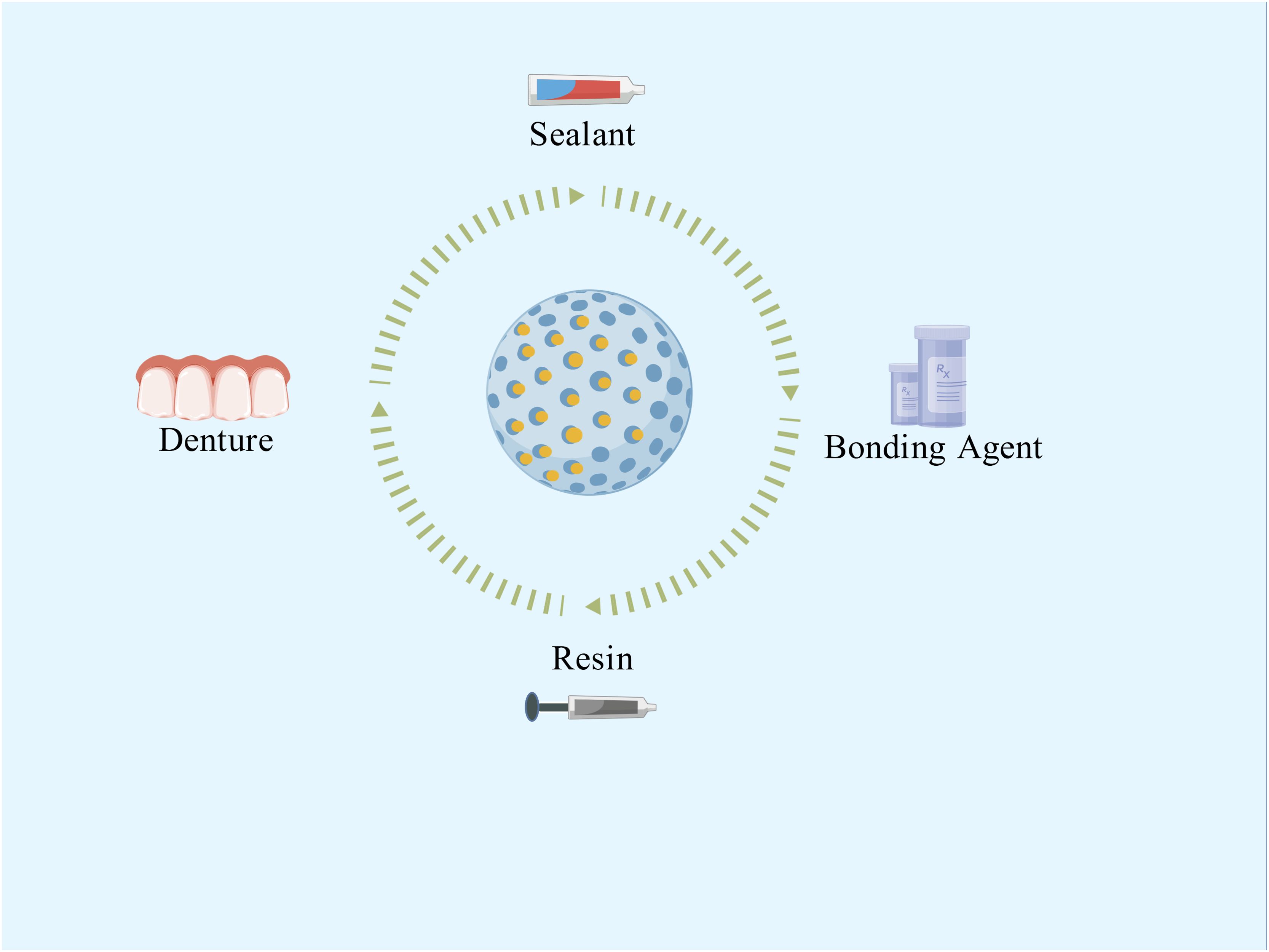
In addition to the two aforementioned oral-specific environmental resistance factors, the clinical translation of nanomaterials currently faces several challenges:(1) the long-term biosafety, biocompatibility, and functional durability of these nanomaterials within the oral cavity require rigorous assessment in live animal models and, ultimately, clinical trials (Sajithkumar et al., 2025). Many studies discussed in this article are primarily in vitro. Not only in the dental field, one of the biggest current challenges for nanomaterials is the lack of long-term in vivo experiments. While a few studies have employed rodent caries models to show efficacy in reducing lesion severity, these are often short-term and lack evaluation of critical endpoints; (2) the regulatory landscape for nano-enabled medical products remains fragmented. Harmonized characterization standards and quality-control guidelines are urgently required. Critical parameters—including particle-size distribution, surface charge, and degradation products—must be quantified through validated, standardized assays to guarantee batch-to-batch consistency and clinical reproducibility (Rodríguez-Gómez et al., 2025). (3) Since some dental materials need to meet aesthetic requirements, the coloration issue of certain metal nanoparticles also cannot be ignored. Despite these challenges, the unique properties of controlled-release nano-antimicrobial materials have led to their exploration in a wide range of dental applications. These applications aim to leverage their sustained and targeted antibacterial action to improve the longevity and success of various dental treatments and restorative procedures (Figure 4).
6 Conclusion
This paper systematically reviews the latest mechanistic advances in controlled-release nanomaterials for preventing secondary caries. Stimulus-responsive systems have evolved from single pH triggers to multi-stimulus synergistic activation, rechargeable nanocarriers have graduated from one-off ion reservoirs to “stimulus–response–re-supply” cycles, and cascade nanoplates now seamlessly integrate antibacterial, acid-neutralizing, and remineralizing functions for precise modulation of the cariogenic microenvironment.
However, the oral pH landscape is constantly perturbed by diet, saliva and microbial metabolism, making it difficult for materials to respond specifically to acid niches generated solely by cariogenic bacteria; The layer-by-layer fabrication of cascade structures still relies on complex processes, which limits large-scale production and cost control; and unified characterization protocols together with long-term biosafety regulation for nanomaterials are still absent. These challenges are compounded by patient compliance issues, as complex or expensive preventive measures may lead to poor adherence, reducing real-world effectiveness. Therefore, overcoming these technical and economic barriers while ensuring user-friendly and affordable solutions, which is essential to advancing effective caries management.
Author contributions
YW: Visualization, Writing – original draft, Writing – review & editing. XD: Investigation, Writing – original draft, Writing – review & editing. YJ: Investigation, Writing – review & editing. LQ: Investigation, Writing – review & editing. FL: Funding acquisition, Investigation, Writing – review & editing. YC: Funding acquisition, Validation, Writing – review & editing. SW: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Henan Provincial Medical Science and Technology Research Plan Provincial Key Project (No. SBGJ202102162); Henan Provincial Department of Education Key Scientific Research Project of Higher Education Institutions (No. 22A320006); Joint Construction Project of Henan Medical Science and Technology Research and Development Program (No. LHGJ20220353).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albelasy, E. H., Chen, R., Fok, A., Montasser, M., Hamama, H. H., Mahmoud, S. H., et al. (2023). Inhibition of caries around restoration by ion-releasing restorative materials: an in vitro optical coherence tomography and micro-computed tomography evaluation. Materials (Basel) 16 (16), 5558. doi: 10.3390/ma16165558
Al-Dulaijan, Y. A., Cheng, L., Weir, M. D., Melo, M. A. S., Liu, H., Oates, T. W., et al. (2018). Novel rechargeable calcium phosphate nanocomposite with antibacterial activity to suppress biofilm acids and dental caries. J. Dent. 72, 44–52. doi: 10.1016/j.jdent.2018.03.003
Alizadeh Oskoee, P., Pournaghi Azar, F., Jafari Navimipour, E., Ebrahimi Chaharom, M. E., Naser Alavi, F., and Salari, A. (2017). The effect of repeated preheating of dimethacrylate and silorane-based composite resins on marginal gap of class V restorations. J. Dent. Res. Dent. Clin. Dent. Prospects 11, 36–42. doi: 10.15171/joddd.2017.007
Almaroof, A., Niazi, S. A., Rojo, L., Mannocci, F., and Deb, S. (2016). Influence of a polymerizable eugenol derivative on the antibacterial activity and wettability of a resin composite for intracanal post cementation and core build-up restoration. Dent. Mater 32, 929–939. doi: 10.1016/j.dental.2016.04.001
Alumutairi, L., Yu, B., Filka, M., Nayfach, J., and Kim, M. H. (2020). Mild magnetic nanoparticle hyperthermia enhances the susceptibility of Staphylococcus aureus biofilm to antibiotics. Int. J. Hyperthermia 37, 66–75. doi: 10.1080/02656736.2019.1707886
Amin, F., Moin, S. F., Kumar, N., Asghar, M. A., Mahmood, S. J., and Palma, P. J. (2025). The impact of zirconium oxide nanoparticles on the mechanical and physical properties of glass ionomer dental materials. Int. J. Mol. Sci. 26 (11), 5382. doi: 10.3390/ijms26115382
Balhaddad, A. A., Garcia, I. M., Mokeem, L., Alsahafi, R., Collares, F. M., and Sampaio de Melo, M. A. (2021). Metal oxide nanoparticles and nanotubes: ultrasmall nanostructures to engineer antibacterial and improved dental adhesives and composites. Bioengineering (Basel) 8 (10), 146. doi: 10.3390/bioengineering8100146
Besinis, A., van Noort, R., and Martin, N. (2014). Remineralization potential of fully demineralized dentin infiltrated with silica and hydroxyapatite nanoparticles. Dent. Mater 30, 249–262. doi: 10.1016/j.dental.2013.11.014
Bowen, W. H., Burne, R. A., Wu, H., and Koo, H. (2018). Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 26, 229–242. doi: 10.1016/j.tim.2017.09.008
Boyuklieva, R., Zahariev, N., Simeonov, P., Penkov, D., and Katsarov, P. (2025). Next-generation drug delivery for neurotherapeutics: the promise of stimuli-triggered nanocarriers. Biomedicines 13 (6), 1464. doi: 10.3390/biomedicines13061464
Chen, D. and Li, J. (2020). Ultrasmall Au nanoclusters for bioanalytical and biomedical applications: the undisclosed and neglected roles of ligands in determining the nanoclusters’ catalytic activities. Nanoscale Horiz 5, 1355–1367. doi: 10.1039/D0NH00207K
Da, Y., He, T., Ma, X., Yang, R., Lin, Y., Yang, Q., et al. (2025). Probing into the triggering effects of zinc presence on the mineral formation, hydration evolution, and mechanical properties of fluorine-bearing clinker. Langmuir 41, 11417–11427. doi: 10.1021/acs.langmuir.5c00302
Dcruz, M. M., Tapashetti, S., Naik, B., Shah, M. A., Mogi, P., and Horatti, P. (2024). Comparative evaluation of fluoride release profiles in new glass ionomer cements and conventional type II GIC: Implications for cariostatic efficacy. Bioinformation 20, 2009–2014. doi: 10.6026/9732063002002009
Degli Esposti, L. and Iafisco, M. (2022). Amorphous calcium phosphate, the lack of order is an abundance of possibilities. Biomater Biosyst. 5, 100037. doi: 10.1016/j.bbiosy.2021.100037
de Jesus, R. A., de Assis, G. C., de Oliveira, R. J., Costa, J. A. S., da Silva, C. M. P., Iqbal, H. M. N., et al. (2024). Metal/metal oxide nanoparticles: A revolution in the biosynthesis and medical applications. Nano-Structures Nano-Objects 37, 101071. doi: 10.1016/j.nanoso.2023.101071
Dong, Q., Li, Z., Xu, J., Yuan, Q., Chen, L., and Chen, Z. (2022). Versatile graphitic nanozymes for magneto actuated cascade reaction-enhanced treatment of S. mutans biofilms. Nano Res. 15, 9800–9808. doi: 10.1007/s12274-022-4258-x
Dósa, E., Tuladhar, S., Muldoon, L. L., Hamilton, B. E., Rooney, W. D., and Neuwelt, E. A. (2011). MRI using ferumoxytol improves the visualization of central nervous system vascular malformations. Stroke 42, 1581–1588. doi: 10.1161/STROKEAHA.110.607994
Dubey, M. K., Zehra, A., Aamir, M., Meena, M., Ahirwal, L., Singh, S., et al. (2017). Improvement strategies, cost effective production, and potential applications of fungal glucose oxidase (GOD): current updates. Front. Microbiol. 8, 1032. doi: 10.3389/fmicb.2017.01032
Fallahzadeh, F., Heidari, S., Najafi, F., Hajihasani, M., Noshiri, N., and Nazari, N. F. (2022). Efficacy of a novel bioactive glass-polymer composite for enamel remineralization following erosive challenge. Int. J. Dent. 2022, 6539671. doi: 10.1155/2022/6539671
Fan, X., Yahia, L., and Sacher, E. (2021). Antimicrobial properties of the ag, cu nanoparticle system. Biol. (Basel) 10 (2), 137. doi: 10.3390/biology10020137
Fei, X., Li, Y., Zhang, Q., Tian, C., Li, Y., Dong, Q., et al. (2024). Novel pit and fissure sealant with nano-CaF(2) and antibacterial monomer: Fluoride recharge, microleakage, sealing ability and cytotoxicity. Dent. Mater J. 43, 346–358. doi: 10.4012/dmj.2023-166
Fiegler-Rudol, J., Kapłon, K., Kotucha, K., Moś, M., Skaba, D., Kawczyk-Krupka, A., et al. (2025). Hypocrellin-mediated PDT: A systematic review of its efficacy, applications, and outcomes. Int. J. Mol. Sci. 26 (9), 4038. doi: 10.3390/ijms26094038
Gao, L., Liu, Y., Kim, D., Li, Y., Hwang, G., Naha, P. C., et al. (2016). Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 101, 272–284. doi: 10.1016/j.biomaterials.2016.05.051
Garcia, I. M., Balhaddad, A. A., Lan, Y., Simionato, A., Ibrahim, M. S., Weir, M. D., et al. (2021). Magnetic motion of superparamagnetic iron oxide nanoparticles- loaded dental adhesives: physicochemical/biological properties, and dentin bonding performance studied through the tooth pulpal pressure model. Acta Biomater 134, 337–347. doi: 10.1016/j.actbio.2021.07.031
Ge, G., Wu, L., Zhang, F., Wang, T., Han, L., Kong, X., et al. (2023). Na(2)S(2)O(4)@Co-metal organic framework (ZIF-67) @glucose oxidase for biofilm-infecting wound healing with immune activation. Int. J. Biol. Macromol 226, 1533–1546. doi: 10.1016/j.ijbiomac.2022.11.265
Güner, Z. and Köse, H. D. (2024). Evaluation of nanohardness, elastic modulus, and surface roughness of fluoride-releasing tooth colored restorative materials. J. Clin. Pediatr. Dent. 48, 131–137. doi: 10.22514/jocpd.2024.112
Han, X., Lou, Q., Feng, F., Xu, G., Hong, S., Yao, L., et al. (2022). Spatiotemporal release of reactive oxygen species and NO for overcoming biofilm heterogeneity. Angew Chem. Int. Ed Engl. 61, e202202559. doi: 10.1002/anie.202202559
Heo, S. M., Ruhl, S., and Scannapieco, F. A. (2013). Implications of salivary protein binding to commensal and pathogenic bacteria. J. Oral. Biosci. 55, 169–174. doi: 10.1016/j.job.2013.06.004
Htet, K., Hiraishi, N., Sanon, K., Ubolsaard, P., Sone, K. P., and Shimada, Y. (2025). Effect of zinc-releasing glass ionomer cement on preventing dentin demineralization. J. Dent. 156, 105718. doi: 10.1016/j.jdent.2025.105718
Hu, P., Kang, L., Chang, T., Yang, F., Wang, H., Zhang, Y., et al. (2017). High saturation magnetization Fe3O4 nanoparticles prepared by one-step reduction method in autoclave. J. Alloys Compounds 728, 88–92. doi: 10.1016/j.jallcom.2017.08.290
Huang, S., Chen, G., and Ouyang, G. (2022). Confining enzymes in porous organic frameworks: from synthetic strategy and characterization to healthcare applications. Chem. Soc. Rev. 51, 6824–6863. doi: 10.1039/D1CS01011E
Ibrahim, M. S., Balhaddad, A. A., Garcia, I. M., Collares, F. M., Weir, M. D., Xu, H. H. K., et al. (2020). pH-responsive calcium and phosphate-ion releasing antibacterial sealants on carious enamel lesions in vitro. J. Dent. 97, 103323. doi: 10.1016/j.jdent.2020.103323
Jiang, T., Su, W., Li, Y., Jiang, M., Zhang, Y., Xian, C. J., et al. (2023). Research progress on nanomaterials for tissue engineering in oral diseases. J. Funct. Biomater 14(8), 404. doi: 10.3390/jfb14080404
Jiao, Y., Tay, F. R., Niu, L. N., and Chen, J. H. (2019). Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral. Sci. 11, 28. doi: 10.1038/s41368-019-0062-1
Jowkar, Z., Askarzadeh, S., Hamidi, S. A., Fattah, Z., and Moaddeli, A. (2025). Assessment of the antimicrobial properties of mesoporous zinc oxide nanoparticles against streptococcus mutans: an in vitro investigation. Int. J. Dentistry 2025, 4438269. doi: 10.1155/ijod/4438269
Kelić, M., Kilić, D., Kelić, K., Šutej, I., Par, M., Peroš, K., et al. (2023). The fluoride ion release from ion-releasing dental materials after surface loading by topical treatment with sodium fluoride gel. J. Funct. Biomater 14(2), 102. doi: 10.3390/jfb14020102
Khan, I., Saeed, K., and Khan, I. (2019). Nanoparticles: Properties, applications and toxicities. Arabian J. Chem. 12, 908–931. doi: 10.1016/j.arabjc.2017.05.011
Kim, B. C., Jeong, E., Kim, E., and Hong, S. W. (2019). Bio-organic–inorganic hybrid photocatalyst, TiO2 and glucose oxidase composite for enhancing antibacterial performance in aqueous environments. Appl. Catalysis B: Environ. 242, 194–201. doi: 10.1016/j.apcatb.2018.09.102
Kwon, T., Kumari, N., Kumar, A., Lim, J., Son, C. Y., and Lee, I. S. (2021). Au/pt-egg-in-nest nanomotor for glucose-powered catalytic motion and enhanced molecular transport to living cells. Angew Chem. Int. Ed Engl. 60, 17579–17586. doi: 10.1002/anie.202103827
Li, K., Chen, C., Chen, C., Wang, Y., Wei, Z., Pan, W., et al. (2015). Magnetosomes extracted from Magnetospirillum magneticum strain AMB-1 showed enhanced peroxidase-like activity under visible-light irradiation. Enzyme Microb. Technol. 72, 72–78. doi: 10.1016/j.enzmictec.2015.02.009
Li, R., Landfester, K., and Ferguson, C. T. J. (2022). Temperature- and pH-responsive polymeric photocatalysts for enhanced control and recovery. Angew Chem. Int. Ed Engl. 61, e202211132. doi: 10.1002/anie.202211132
Li, S., Li, Q., Zhang, H., Li, F., Hu, J., Qian, J., et al. (2024). Dental caries management with antibacterial silver-doped pRussian blue hydrogel by the combined effects of photothermal response and ion discharge. ACS Appl. Mater Interfaces 16, 28172–28183. doi: 10.1021/acsami.4c04302
Li, F., Wang, P., Weir, M. D., Fouad, A. F., and Xu, H. H. (2014). Evaluation of antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomater 10, 2804–2813. doi: 10.1016/j.actbio.2014.02.033
Liang, K., Wang, S., Tao, S., Xiao, S., Zhou, H., Wang, P., et al. (2019). Dental remineralization via poly(amido amine) and restorative materials containing calcium phosphate nanoparticles. Int. J. Oral. Sci. 11, 15. doi: 10.1038/s41368-019-0048-z
Liu, Y., Huang, Y., Kim, D., Ren, Z., Oh, M. J., Cormode, D. P., et al. (2021). Ferumoxytol nanoparticles target biofilms causing tooth decay in the human mouth. Nano Lett. 21, 9442–9449. doi: 10.1021/acs.nanolett.1c02702
Liu, Y., Kohno, T., Tsuboi, R., Kitagawa, H., and Imazato, S. (2020). Acidity-induced release of zinc ion from BioUnion(TM) filler and its inhibitory effects against Streptococcus mutans. Dent. Mater J. 39, 547–553. doi: 10.4012/dmj.2019-061
Liu, Y., Naha, P. C., Hwang, G., Kim, D., Huang, Y., Simon-Soro, A., et al. (2018b). Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat. Commun. 9, 2920. doi: 10.1038/s41467-018-05342-x
Liu, H. Y., Prentice, E. L., and Webber, M. A. (2024). Mechanisms of antimicrobial resistance in biofilms. NPJ Antimicrob. Resist. 2, 27. doi: 10.1038/s44259-024-00046-3
Liu, Y., Zhang, L., Niu, L. N., Yu, T., Xu, H. H. K., Weir, M. D., et al. (2018a). Antibacterial and remineralizing orthodontic adhesive containing quaternary ammonium resin monomer and amorphous calcium phosphate nanoparticles. J. Dent. 72, 53–63. doi: 10.1016/j.jdent.2018.03.004
Lykourinou, V., Chen, Y., Wang, X. S., Meng, L., Hoang, T., Ming, L. J., et al. (2011). Immobilization of MP-11 into a mesoporous metal-organic framework, MP-11@mesoMOF: a new platform for enzymatic catalysis. J. Am. Chem. Soc. 133, 10382–10385. doi: 10.1021/ja2038003
Ma, L., Zheng, J. J., Zhou, N., Zhang, R., Fang, L., Yang, Y., et al. (2024). A natural biogenic nanozyme for scavenging superoxide radicals. Nat. Commun. 15, 233. doi: 10.1038/s41467-023-44463-w
Madhyastha, P. S., Naik, D. G., Natarajan, S., and Vinodhini, R. S. (2025). Influence of time interval, temperature, and storage condition on fluoride release and recharge from silorane-based restorative materials. Dent. J. (Basel) 13 (5), 197. doi: 10.3390/dj13050197
Meghwanshi, G. K., Kaur, N., Verma, S., Dabi, N. K., Vashishtha, A., Charan, P. D., et al. (2020). Enzymes for pharmaceutical and therapeutic applications. Biotechnol. Appl. Biochem. 67, 586–601. doi: 10.1002/bab.1919
Menezes-Silva, R., Velasco, S. R. M., BRESCIANi, E., Bastos, R. D. S., and Navarro, M. F. L. (2021). A prospective and randomized clinical trial evaluating the effectiveness of ART restorations with high-viscosity glass-ionomer cement versus conventional restorations with resin composite in Class II cavities of permanent teeth: two-year follow-up. J. Appl. Oral. Sci. 29, e20200609. doi: 10.1590/1678-7757-2020-0609
Meng, W., Huang, L., Guo, J., Xin, Q., Liu, J., and Hu, Y. (2024). Innovative nanomedicine delivery: targeting tumor microenvironment to defeat drug resistance. Pharmaceutics 16(12), 1549. doi: 10.3390/pharmaceutics16121549
Ming, J., Zhu, T., Yang, W., Shi, Y., Huang, D., Li, J., et al. (2020). Pd@Pt-GOx/HA as a novel enzymatic cascade nanoreactor for high-efficiency starving-enhanced chemodynamic cancer therapy. ACS Appl. Mater Interfaces 12, 51249–51262. doi: 10.1021/acsami.0c15211
Mokeem, L. S., Martini Garcia, I., Balhaddad, A. A., Lan, Y., Seifu, D., Weir, M. D., et al. (2024). Multifunctional dental adhesives formulated with silane-coated magnetic fe(3)O(4)@m-siO(2) core-shell particles to counteract adhesive interfacial breakdown. ACS Appl. Mater Interfaces 16, 2120–2139. doi: 10.1021/acsami.3c15157
Monjarás-Ávila, A. J., Hardan, L., Cuevas-Suárez, C. E., Alonso, N. V. Z., Fernández-Barrera, M., Moussa, C., et al. (2025). Systematic review and meta-analysis of remineralizing agents: outcomes on white spot lesions. Bioengineering (Basel) 12 (1), 93. doi: 10.3390/bioengineering12010093
Montoya, C., Roldan, L., Yu, M., Valliani, S., Ta, C., Yang, M., et al. (2023). Smart dental materials for antimicrobial applications. Bioact Mater 24, 1–19. doi: 10.1016/j.bioactmat.2022.12.002
Morawska-Wilk, A., Kensy, J., Kiryk, S., Kotela, A., Kiryk, J., Michalak, M., et al. (2025). Evaluation of factors influencing fluoride release from dental nanocomposite materials: A systematic review. Nanomaterials (Basel) 15 (9), 651. doi: 10.3390/nano15090651
Mu, Y., Wang, Y., Huang, L., Weng, Z., Zhong, T., Yu, S., et al. (2025). Yellow light and ultrasound Dual-responsive strontium-doped zinc oxide composites for dental caries prevention and remineralization. Bioact Mater 47, 403–416. doi: 10.1016/j.bioactmat.2025.01.029
Naha, P. C., Liu, Y., Hwang, G., Huang, Y., Gubara, S., Jonnakuti, V., et al. (2019). Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and pH-activated biofilm disruption. ACS Nano 13, 4960–4971. doi: 10.1021/acsnano.8b08702
Nazemisalman, B., Niaz, S., Darvish, S., Notash, A., Ramazani, A., and Luchian, I. (2024). The antibacterial properties of a reinforced zinc oxide eugenol combined with cloisite 5A nanoclay: an in-vitro study. J. Funct. Biomater 15 (7), 198. doi: 10.3390/jfb15070198
Patil, P., Gupta, A., Kishlay, K., Rathaur, S., Vaidya, N., Sharma, M., et al. (2025). Comparative Evaluation of the Antimicrobial Efficacy of Endodontic Sealers Against Staphylococcus aureus and Streptococcus mutans: An In Vitro Study. Cureus 17, e80435. doi: 10.7759/cureus.80435
Pereira, C. A., Costa, A. C., Carreira, C. M., Junqueira, J. C., and Jorge, A. O. (2013). Photodynamic inactivation of Streptococcus mutans and Streptococcus sanguinis biofilms in vitro. Lasers Med. Sci. 28, 859–864. doi: 10.1007/s10103-012-1175-3
Piszko, P. J., Piszko, A., Kiryk, S., Kiryk, J., Kensy, J., Michalak, M., et al. (2025). Fluoride release from two commercially available dental fluoride gels-in vitro study. Gels 11(2), 135. doi: 10.3390/gels11020135
Puttipanampai, O., Panpisut, P., and Sitthisettapong, T. (2025). Assessment of fluoride-releasing materials in remineralization of adjacent demineralized enamel. Appl. Sci. 15 (4), 2077. doi: 10.3390/app15042077
Qi, J., Si, C., Liu, H., Li, H., Kong, C., Wang, Y., et al. (2025). Advances of metal-based nanomaterials in the prevention and treatment of oral infections. Adv. Healthc Mater 14, e2500416. doi: 10.1002/adhm.202500416
Rodríguez-Gómez, F. D., Monferrer, D., Penon, O., and Rivera-Gil, P. (2025). Regulatory pathways and guidelines for nanotechnology-enabled health products: a comparative review of EU and US frameworks. Front. Med. (Lausanne) 12, 1544393. doi: 10.3389/fmed.2025.1544393
Rostami, A., Molabashi, V., Ganji, S., Moosavi, S. P., Koushki, A., Fathi-Karkan, S., et al. (2025). Chlorhexidine loaded nanomaterials for dental plaque control: enhanced antibacterial activity and biocompatibility. BioMed. Microdevices 27, 28. doi: 10.1007/s10544-025-00755-0
Sahli, C., Moya, S. E., Lomas, J. S., Gravier-Pelletier, C., Briandet, R., and Hémadi, M. (2022). Recent advances in nanotechnology for eradicating bacterial biofilm. Theranostics 12, 2383–2405. doi: 10.7150/thno.67296
Sajithkumar, A., Shenoy, M., Vinod, K. R., and Nadakkavukkaran, D. (2025). Nanotechnology applications in oral pathology: A scoping review. J. Oral. Maxillofac. Pathol. 29, 127–136. doi: 10.4103/jomfp.jomfp_187_24
Sales-Junior, R. A., de Bessa, M. S., Oliveira, F. J. D., Barbosa, B. F. S., Santos, K. S., Owen, M., et al. (2025). Multifaceted characterization of antibacterial resin composites: A scoping review on efficacy, properties, and in vivo performance. Jpn Dent. Sci. Rev. 61, 112–137. doi: 10.1016/j.jdsr.2025.05.003
Sangha, J. S., Barrett, P., Curtis, T. P., Métris, A., Jakubovics, N. S., and Ofiteru, I. D. (2024). Effects of glucose and lactate on Streptococcus mutans abundance in a novel multispecies oral biofilm model. Microbiol. Spectr. 12, e0371323. doi: 10.1128/spectrum.03713-23
Schwendicke, F., Walsh, T., Lamont, T., Al-Yaseen, W., Bjørndal, L., Clarkson, J. E., et al. (2021). Interventions for treating cavitated or dentine carious lesions. Cochrane Database Syst. Rev. 7, Cd013039. doi: 10.1002/14651858.CD013039.pub2
Senol, G., Kirakli, C., and Halilçolar, H. (2007). In vitro antibacterial activities of oral care products against ventilator-associated pneumonia pathogens. Am. J. Infect. Control 35, 531–535. doi: 10.1016/j.ajic.2006.10.016
Sereda, G., Ahammadullah, A., Wijewantha, N., and Solano, Y. A. (2023). Acid-triggered release of eugenol and fluoride by desensitizing macro- and nanoparticles. J. Funct. Biomater 14 (1), 42. doi: 10.3390/jfb14010042
Sileika, T. S., Barrett, D. G., Zhang, R., Lau, K. H., and Messersmith, P. B. (2013). Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew Chem. Int. Ed Engl. 52, 10766–10770. doi: 10.1002/anie.201304922
Sivakumar, I., Arunachalam, K. S., Sajjan, S., Ramaraju, A. V., Rao, B., and Kamaraj, B. (2014). Incorporation of antimicrobial macromolecules in acrylic denture base resins: a research composition and update. J. Prosthodont 23, 284–290. doi: 10.1111/jopr.12105
Sowmya, R., Karthick Raja Namasivayam, S., and Krithika Shree, S. (2024). A critical review on nano-selenium based materials: synthesis, biomedicine applications and biocompatibility assessment. J. Inorganic Organometallic Polymers Materials 34, 3037–3055. doi: 10.1007/s10904-023-02959-4
Springsteen, G. and Wang, B. (2002). A detailed examination of boronic acid–diol complexation. Tetrahedron 58, 5291–5300. doi: 10.1016/S0040-4020(02)00489-1
Tapponi, S., Yusuf, A., Alsaafin, F., and Hussain, Z. (2025). Breaking barriers with pH-responsive nanocarriers: a new frontier in precision oncology. Int. J. Pharm. 682, 125931. doi: 10.1016/j.ijpharm.2025.125931
Venkataiah, V. S., Krithikadatta, J., Teja, K. V., Mehta, D., and Doble, M. (2025). Ion release dynamics of bioactive resin cement under variable pH conditions. Front. Oral. Health 6, 1564838. doi: 10.3389/froh.2025.1564838
Vilela, A., Ferreira, L., Biscaia, P., da Silva, K., Beltrame, F., Camargo, G., et al. (2023). Preparation, characterization and stability study of eugenol-loaded eudragit RS100 nanocapsules for dental sensitivity reduction. Braz. Arch. Biol. Technol. 66. doi: 10.1590/1678-4324-ssbfar-2023230300
Wang, T., Dong, D., Chen, T., Zhu, J., Wang, S., Wen, W., et al. (2022). Acidity-responsive cascade nanoreactor based on metal-nanozyme and glucose oxidase combination for starving and photothermal-enhanced chemodynamic antibacterial therapy. Chem. Eng. J. 446, 137172. doi: 10.1016/j.cej.2022.137172
Wang, J., Li, L., Hu, X., Zhou, L., and Hu, J. (2024). pH-responsive on-demand release of eugenol from metal-organic frameworks for synergistic bacterial killing. Dalton Trans. 53, 2826–2832. doi: 10.1039/D3DT04216B
Wang, Y., Liu, C., Ren, Y., Song, J., Fan, K., Gao, L., et al. (2024). Nanomaterial-based strategies for attenuating T-cell-mediated immunodepression in stroke patients: advancing research perspectives. Int. J. Nanomedicine 19, 5793–5812. doi: 10.2147/IJN.S456632
Wang, K., Wang, S., Yin, J., Yang, Q., Yu, Y., and Chen, L. (2023). Long-term application of silver nanoparticles in dental restoration materials: potential toxic injury to the CNS. J. Mater Sci. Mater Med. 34, 52. doi: 10.1007/s10856-023-06753-z
Wendler, M., Stenger, A., Ripper, J., Priewich, E., Belli, R., and Lohbauer, U. (2021). Mechanical degradation of contemporary CAD/CAM resin composite materials after water ageing. Dent. Mater 37, 1156–1167. doi: 10.1016/j.dental.2021.04.002
Wu, J., Weir, M. D., Melo, M. A., and Xu, H. H. (2015). Development of novel self-healing and antibacterial dental composite containing calcium phosphate nanoparticles. J. Dent. 43, 317–326. doi: 10.1016/j.jdent.2015.01.009
Wu, J., Xie, X., Zhou, H., Tay, F. R., Weir, M. D., Melo, M. A. S., et al. (2019). Development of a new class of self-healing and therapeutic dental resins. Polymer Degradation Stability 163, 87–99. doi: 10.1016/j.polymdegradstab.2019.02.024
Xia, Y., Yang, L., Xu, S., Xia, Y., Peng, L., Wu, Y., et al. (2025). Trapping effect of surface deficient cocrystal synergizes with bimetallic nanoparticles against bacterial infection in wounds. J. Colloid Interface Sci. 695, 137805. doi: 10.1016/j.jcis.2025.137805
Xiu, Z. M., Ma, J., and Alvarez, P. J. (2011). Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ. Sci. Technol. 45, 9003–9008. doi: 10.1021/es201918f
Xu, Y., You, Y., Yi, L., Wu, X., Zhao, Y., Yu, J., et al. (2023). Dental plaque-inspired versatile nanosystem for caries prevention and tooth restoration. Bioact Mater 20, 418–433. doi: 10.1016/j.bioactmat.2022.06.010
Xue, J., Wang, J., Feng, D., Huang, H., and Wang, M. (2020). Application of antimicrobial polymers in the development of dental resin composite. Molecules 25(20), 4738. doi: 10.3390/molecules25204738
Yamauchi, M., Nigauri, A., Yamamoto, K., Nakazato, G., Kawano, J., and Kimura, K. (1989). Antibacterial actions of denture base resin on oral bacteria. Nihon Hotetsu Shika Gakkai Zasshi 33, 571–576. doi: 10.2186/jjps.33.571
Yang, H., Niu, S., Guo, M., and Xue, Y. (2024). Molecular mechanisms of silver nanoparticle-induced neurotoxic injury and new perspectives for its neurotoxicity studies: A critical review. Environ. pollut. 362, 124934. doi: 10.1016/j.envpol.2024.124934
Yi, J., Weir, M. D., Melo, M. A. S., Li, T., Lynch, C. D., Oates, T. W., et al. (2019). Novel rechargeable nano-CaF(2) orthodontic cement with high levels of long-term fluoride release. J. Dent. 90, 103214. doi: 10.1016/j.jdent.2019.103214
Yuan, H., Bai, H., Liu, L., Lv, F., and Wang, S. (2015). A glucose-powered antimicrobial system using organic-inorganic assembled network materials. Chem. Commun. (Camb) 51, 722–724. doi: 10.1039/C4CC07533A
Zhang, N., Chen, C., Melo, M. A., Bai, Y. X., Cheng, L., and Xu, H. H. (2015). A novel protein-repellent dental composite containing 2-methacryloyloxyethyl phosphorylcholine. Int. J. Oral. Sci. 7, 103–109. doi: 10.1038/ijos.2014.77
Zhang, Y., Jiang, Z. T., Wang, Y., Wang, H. Y., Hong, S., Li, W., et al. (2024). A supramolecular nanoformulation with adaptive photothermal/photodynamic transformation for preventing dental caries. ACS Nano 18, 27340–27357. doi: 10.1021/acsnano.4c06051
Zhang, Q., Ma, Q., Wang, Y., Wu, H., and Zou, J. (2021). Molecular mechanisms of inhibiting glucosyltransferases for biofilm formation in Streptococcus mutans. Int. J. Oral. Sci. 13, 30. doi: 10.1038/s41368-021-00137-1
Zhang, Y., Tsitkov, S., and Hess, H. (2018). Complex dynamics in a two-enzyme reaction network with substrate competition. Nat. Catalysis 1, 276–281. doi: 10.1038/s41929-018-0053-1
Zhang, J., Yang, Y., Chen, Y., Chen, X., Li, A., Wang, J., et al. (2024). A review of new generation of dental restorative resin composites with antibacterial, remineralizing and self-healing capabilities. Discov. Nano 19, 189. doi: 10.1186/s11671-024-04151-0
Zhao, A., Sun, J., and Liu, Y. (2023). Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell Infect. Microbiol. 13, 1137947. doi: 10.3389/fcimb.2023.1137947
Zhou, W., Peng, X., Zhou, X., Bonavente, A., Weir, M. D., Melo, M. A. S., et al. (2020). Novel nanocomposite inhibiting caries at the enamel restoration margins in an in vitro saliva-derived biofilm secondary caries model. Int. J. Mol. Sci. 21(17), 6369. doi: 10.3390/ijms21176369
Zhu, X., Guo, J., Yang, Y., and Liu, J. (2023). Macrophage polarization induced by bacteria-responsive antibiotic-loaded nanozymes for multidrug resistance-bacterial infections management. Small 19, e2204928. doi: 10.1002/smll.202204928
Keywords: antimicrobial, nanomaterials, controlled-release, stimulus-responsive, rechargeable, cascade catalytic nanoreactor, secondary caries
Citation: Wang Y, Du X, Jia Y, Qin L, Liu F, Cai Y and Wang S (2025) Recent progress in antimicrobial strategies of controlled-release nanomaterials for secondary caries. Front. Cell. Infect. Microbiol. 15:1669643. doi: 10.3389/fcimb.2025.1669643
Received: 20 July 2025; Accepted: 08 September 2025;
Published: 01 October 2025.
Edited by:
Xuelian Huang, University of Washington, United StatesReviewed by:
Chengcheng Liu, Sichuan University, ChinaSanthiyagu Prakash, Tamil Nadu Fisheries University, India
Yuncong Li, Xi’an Jiaotong University School of Stomotology, China
Copyright © 2025 Wang, Du, Jia, Qin, Liu, Cai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Liu, bGl1ZmVpZGVudGlzdEAxNjMuY29t; Yingchun Cai, ODE1NTYwMjg5QHFxLmNvbQ==; Suping Wang, d2FuZ3N1cGluZ2RlbnRAMTYzLmNvbQ==
†These authors share first authorship
 Yiyi Wang1,2†
Yiyi Wang1,2† Fei Liu
Fei Liu Suping Wang
Suping Wang