- 1Department of Preventive Dentistry, School of Dentistry, Jeonbuk National University, Jeonju, Republic of Korea
- 2Department of Oral Pathology, School of Dentistry, Jeonbuk National University, Jeonju, Republic of Korea
- 3Institute of Oral Bioscience, Jeonbuk National University, Jeonju, Republic of Korea
Introduction: The increased consumption of refined carbohydrates, particularly sucrose, has contributed to metabolic disorders and oral diseases such as dental caries by promoting dysbiotic biofilm formation and reducing microbial diversity. Allulose, a rare sugar with physicochemical properties similar to sucrose, has been suggested to offer metabolic health benefits; however, its impact on oral biofilm ecology remains unclear.
Methods: We evaluated the cariogenic potential of allulose using a multi-tiered in vitro platform consisting of single-species planktonic and biofilm models, a dual-species biofilm model involving Streptococcus mutans (pathogen) and Streptococcus oralis (commensal), and a saliva-derived microcosm biofilm model. Key virulence indicators, including bacterial growth, acid production, biofilm biomass, exopolysaccharide (EPS) synthesis, and microbial community composition, were quantitatively assessed.
Results: Compared to sucrose, glucose, and fructose, allulose supported reduced bacterial growth and acid production, showing a profile similar to non-fermentable sugar alcohols such as xylitol and erythritol. Biofilms developed under allulose conditions lacked the dense EPS-enmeshed microcolonies and dome-shaped architecture characteristic of sucrose-induced S. mutans-dominant biofilms. In the saliva-derived microcosm model, allulose-treated biofilms maintained higher microbial diversity and preserved health-compatible genera such as Neisseria, Haemophilus, Veillonella, and Granulicatella.
Discussion: These findings demonstrate that allulose supports lower bacterial virulence activity and minimal biofilm formation compared to common dietary sugars while preserving microbial diversity. This highlights its potential as a non-cariogenic sugar alternative with microbiome-conscious benefits and provides ecological insight into how allulose may modulate oral biofilm structure and function.
Introduction
The evolution of human diets has been accompanied by an increased refined carbohydrate consumption, which has increased metabolic disorders, such as obesity and diabetes, as well as oral health issues, such as dental caries. The habitual intake of refined sugars, particularly sucrose, promotes oral biofilm formation by reducing microbial diversity and fostering pathogenic microorganisms (Bowen et al., 2018). Streptococcus mutans is widely recognized as a primary etiological agent owing to its strong biofilm-forming ability and acidogenic properties (Palmer et al., 2010; Hajishengallis et al., 2017).
Among the fermentable dietary sugars, sucrose plays a crucial role in caries development (Forssten et al., 2010; Sheiham and James, 2015; Benahmed et al., 2021), significantly contributing to dental biofilm accumulation and pathogenicity (Leme et al., 2006; Bowen et al., 2018). It serves as a key substrate for the synthesis of exopolysaccharides (EPS), specifically glucans, catalyzed by glucosyltransferases (Gtfs) secreted by S. mutans. These EPS glucans facilitate bacterial adhesion to tooth surfaces, promote biofilm accumulation, and enhance the structural stability of biofilms (Bowen and Koo, 2011; Klein et al., 2015; Kim et al., 2020, 2022). Additionally, water-insoluble glucans trap nutrients and sugars within biofilms, creating an environment conducive to bacterial proliferation.
During sucrose metabolism, S. mutans produces acidic metabolites, which reduce the biofilm pH and accelerate enamel demineralization and dental caries progression (Bowen et al., 2018). The metabolic activity of S. mutans within dental plaques is central to dental caries development (Parisotto et al., 2010; Durso et al., 2014; Zhang et al., 2022). Although other microorganisms within the biofilm can also be considered cariogenic, S. mutans possesses several potential characteristics such as rapid dietary carbohydrate transport and fermentation, acidic byproduct production, extracellular and intracellular polysaccharide synthesis, and stress-responsive carbohydrate metabolism (Banas, 2004; Beighton, 2005; Kanasi et al., 2010).
Considering the critical role of dietary sugars in EPS synthesis and biofilm development, efforts to mitigate dental caries have primarily focused on reducing sugar consumption and enhancing oral hygiene practices (Philip et al., 2018). However, sugar reduction alone is not always feasible owing to dietary habits and lifestyle choices. Consequently, developing non-cariogenic sugar substitutes has attracted attention as an alternative approach. Non-nutritive sweeteners, including synthetic and naturally occurring compounds such as sugar alcohols (polyols), have been widely studied as they interfere with bacterial metabolism and inhibit biofilm formation (Milgrom et al., 2012; Rice et al., 2020; Staszczyk et al., 2020). Despite their potential, the effectiveness of these sugar alternatives varies, causing gastrointestinal side effects and offering limited long-term benefits (Mäkinen, 2016).
D-Allulose, a naturally occurring monosaccharide classified as a rare sugar, is found in small amounts in maple syrup, dried figs or raisins, and brown sugar. It is a fructose epimer via enzymatic treatment with epimerases, with 70% sweetness of sucrose and minimal caloric content (Hu et al., 2021). Clinical studies have suggested that allulose consumption positively influences metabolic health, including improved plasma glucose control, insulin regulation, and weight management, showing benefits in healthy populations and individuals with type 2 diabetes (Hasturk, 2018; Document, 2019; Daniel et al., 2022; Lee et al., 2022). In addition, it has been generally recognized as safe (GRAS) by the United States Food and Drug Administration (FDA), allowing its use as a food ingredient (FDA, 2023).
Clinical and controlled feeding studies have shown that allulose is not efficiently metabolized in mammalian systems (Iida et al., 2010; Iwasaki et al., 2018), but its effects on oral microbial ecology—including biofilm interactions—remain poorly explored. Despite the promising attributes of allulose, few studies have explored its effects on oral biofilm formation and microbial diversity. In this study, the effects of dietary carbohydrates on oral microbial cariogenicity were assessed across various ecological models using a multi-tiered platform (Figure 1), including single-species planktonic and biofilm, dual-species models involving S. mutans and S. oralis, and saliva-derived microcosm biofilm experiments with increasing complexity. Each model was used to assess key virulence parameters, including bacterial growth, glycolytic pH drop, biofilm biomass, EPS synthesis, and microbial community composition. The cariogenic potential of allulose has been systematically compared to that of conventional fermentable sugars, such as sucrose, glucose, and fructose (Benahmed et al., 2021), as well as with that of non-fermentable sugar alcohols, such as xylitol and erythritol (Razak et al., 2017; Jeong et al., 2024). Using clinically relevant oral biofilm models, including a hydroxyapatite disc model that mimics the enamel surface and a saliva-based model simulating the oral microbiome, this study provides novel insights into the ecological impact of allulose and highlights its potential as a preventive strategy against dental caries.
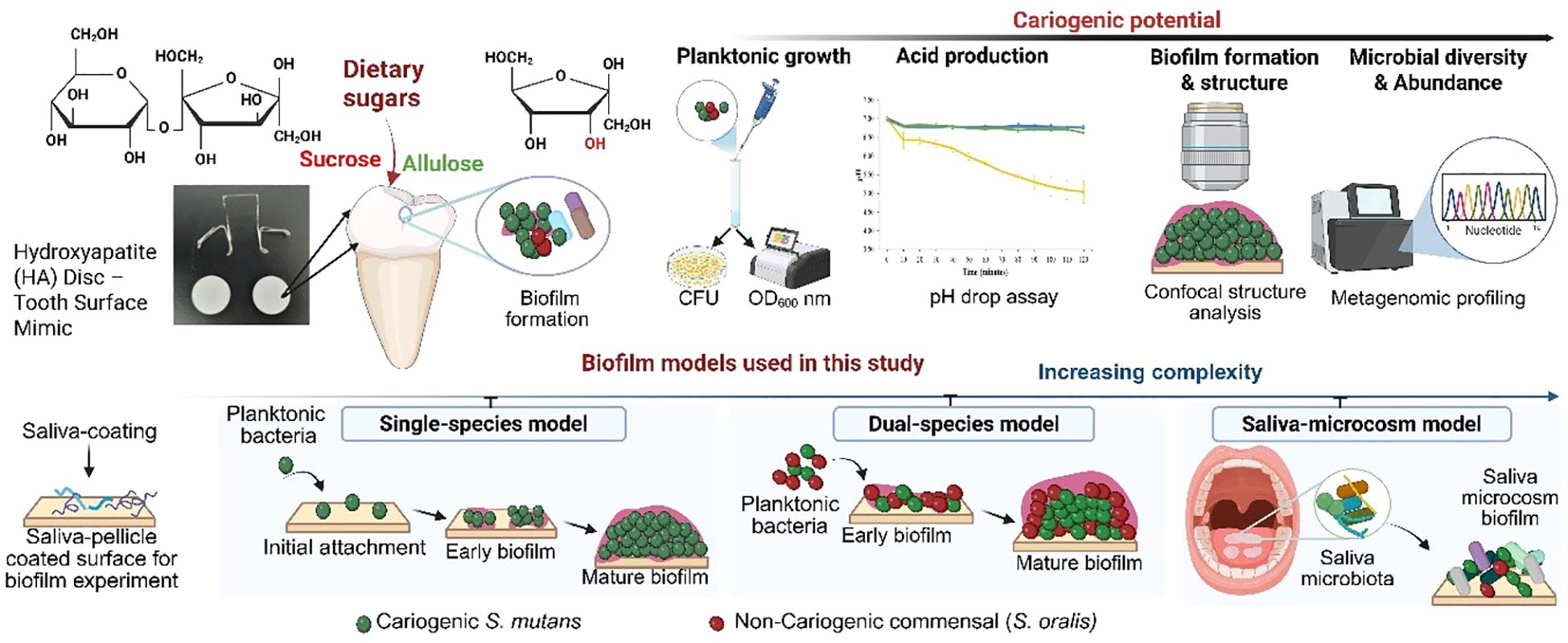
Figure 1. Schematic representation of the overall experimental approach to assess the cariogenic potential of dietary sugars using biofilm models of increasing complexity. The study evaluates the impact of sucrose and allulose on Streptococcus mutans biofilm formation and oral microbial communities. The biofilm models progress in complexity: single-species (S. mutans), dual-species (S. mutans and Streptococcus oralis), and a saliva-derived microcosm model that simulates the natural oral environment. This comprehensive approach provides mechanistic insights into the role of dietary sugars in biofilm development and microbial dysbiosis. Figure created using BioRender.
Materials and methods
Bacterial strains and culture conditions
S. mutans UA159 (an established cariogenic dental pathogen and well-characterized EPS producer) was used to generate single- and multi-species biofilms. For inoculum preparation, S. mutans was cultured to the mid-exponential phase [optical density at 600 nm (OD600) approximately 1.0] in ultrafiltered (10-kDa molecular-mass cutoff membrane; Millipore, MA, USA) tryptone–yeast extract broth [UFTYE; 2.5% tryptone and 1.5% yeast extract (BD Biosciences, San Jose, CA, USA)] with 1% (w/v) glucose at 37°C under 5% CO2 as previously described (Kim et al., 2017).
Sweetener supplementation
The effects of different sweeteners on S. mutans biofilm formation and microbial dynamics were evaluated using a panel of common dietary sugars, such as sucrose, glucose, and fructose (Sigma-Aldrich, Saint Louis, MO, USA), and sugar substitutes, such as allulose (Samyang Co., Seongnam, Korea), xylitol (Sigma-Aldrich), and erythritol (Samyang Co.). Each sweetener was freshly prepared at 1% (w/v) final concentration in sterile UFTYE medium based on previous studies demonstrating its physiological relevance in stimulating dietary sugar exposure in the oral cavity (Benahmed et al., 2021). The UFTYE medium (without additional sugars) was used for the baseline comparison, and basal culture medium was used for both planktonic and biofilm models. To replicate normal dietary sugar exposure and oral clearance patterns, the medium was replaced twice daily (at 19 h and 28 h).
Planktonic growth assay
To assess the cariogenic potential of each sweetener, planktonic growth kinetics and acid production by S. mutans were evaluated in UFTYE medium supplemented with 1% glucose, fructose, allulose, xylitol, or erythritol. UFTYE medium without additional sugars served as a blank control. Growth was monitored by measuring the OD600 at 30 min intervals for 24 h.
Glycolytic pH drop assay in planktonic cells
Glycolytic acid production by S. mutans was assessed using a pH drop assay as previously described (Jeon et al., 2011; Jung et al., 2022). Briefly, planktonic cultures were harvested, washed once, and resuspended in salt solution (50 mM KCl + 1 mM MgCl2). The suspension pH was adjusted to 7.0 using 0.2 M KOH. Each sweetener was added at 1% (w/v) final concentration and the pH was recorded at 10 min intervals over 120 min using a calibrated glass pH electrode (Orion 3-Star, Thermo Scientific, Waltham, MA, USA).
Gene expression analysis by quantitative real-time polymerase chain reaction
RNA was extracted and purified using protocols optimized for in vitro biofilm formation (He et al., 2017). Total RNA was isolated and treated with on-column DNase using a RNeasy Micro kit (Qiagen, Valencia, CA, USA). The RNAs were further treated with a second DNase I (Turbo DNase, Applied Biosystems/Ambion) and purified using the Qiagen RNeasy MinElute Cleanup Kit (Qiagen). Complementary DNA (cDNA) was synthesized from 0.5 µg purified RNA using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA) (Cai et al., 2018). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the Applied Biosystems StepOne Real-Time PCR system with gene-specific primers targeting gtfB, gtfC, gtfD, ftf, dexA, pdhA, adhE, ldh, atpD, and 16S rRNA as previously described (Jeon et al., 2009; He et al., 2017; Cai et al., 2018). Gene expression was analyzed using the comparative ΔΔCt method, normalizing each target gene to 16S rRNA as the internal reference.
Single-species biofilm model
To replicate the smooth surfaces of the pellicle-coated tooth, biofilms were formed on saliva-coated hydroxyapatite (sHA) disc (surface area: 2.7 ± 0.2 cm2) vertically suspended in 24-well plates using a specifically designed wire specimen holder (Xiao et al., 2012; Kim et al., 2018). Filter-sterilized human whole saliva was collected from healthy donors as previously described (Koo et al., 2000). Hydroxyapatite (HA) discs were immersed in cell-free saliva for 1 h to stimulate salivary pellicle formation. The discs were then vertically suspended in a 24-well plate using custom-made holders; inoculated with S. mutans (105 colony-forming unit (CFU)/mL; mid-exponential growth phase) in 2.8 mL UFTYE medium supplemented with 1% (w/v) sucrose, glucose, fructose, allulose, xylitol, or erythritol; and incubated at 37°C under 5% CO2. The inoculum size reflected the typical S. mutans levels in the saliva of caries-active individuals (Ren et al., 2022). The sweetener-containing media were changed at 19 h and 28 h to stimulate eating (meal-like) episodes under continuous sugar exposure. Biofilms were harvested and analyzed at 19, 23, and 43 h post-incubation. Sucrose served as the control (cariogenic reference) for head-to-head comparison.
Acidogenicity of pre-formed biofilms
To assess the glycolytic activity, a pH drop assay was performed on pre-formed biofilms cultivated on sHA discs. S. mutans biofilms were grown for 43 h in UFTYE medium supplemented with 1% (w/v) sucrose. At 43 h, the discs were transferred to fresh solutions containing 1% (w/v) sweetener, and pH was recorded at 10-min intervals over 120 min to monitor acid production. The initial rate of acid production, which is considered the best indicator of the acid production capacity of the biofilm, was determined from the pH values.
Dual-species biofilm model
A dual-species biofilm model was developed using the cariogenic pathogen S. mutans UA159 and oral commensal S. oralis KCTC 13038 [originated from ATCC 35037; obtained from Korean Collection for Type Cultures (KCTC), Jeongeup, Korea]. Bacterial suspensions were prepared and mixed to obtain final inoculum concentrations of 105 and 107 CFU/mL for S. mutans and S. oralis, respectively. Consistent with the ecological plaque hypothesis, this mixed inoculum was cultured in UFTYE medium containing 0.1% (w/v) sucrose (cariogenic reference) for 19 h to establish an initial colonization community. The discs were then transferred to UFTYE containing 1% sucrose to stimulate a cariogenic challenge at 19 h. The culture medium was changed at 28 h, and the biofilms were harvested at 43 h to determine the viable bacterial count, expressed as CFU per biofilm, using blood agar plating.
Saliva-derived microcosm biofilm model
To simulate a clinically relevant oral microenvironment, a saliva-derived microcosm biofilm model was established using sHA discs (Liu et al., 2023) with slight modifications. The saliva-originated microbial consortium was centrifuged at 3,000× g for 10 min to remove the host cells, and the salivary microbiome (saliva collected from healthy individuals, as qualified S. mutans absence) was inoculated for initial binding (1 h). Next, approximately 105 CFU/mL S. mutans was inoculated into UFTYE medium containing 1% (w/v) sucrose or allulose, or UFTYE medium without additional sugars (blank).
Biofilm imaging using confocal microscopy
The biofilms formed under each condition were examined using confocal microscopy. The bacterial cells were stained with 2.5 μM SYTO 9 green-fluorescent nucleic acid stain (485/498 nm; Molecular Probes Inc., Eugene, OR, USA), while EPS was labeled with 1 μM Alexa Fluor 647–dextran conjugate (647/668 nm; Molecular Probes Inc.) The 3D biofilm architecture was acquired using a C2+ confocal microscope (Nikon, Tokyo, Japan) with 20× (0.75 numerical aperture (NA)). NIS-Elements software version 5.21 (Nikon) was used to create 3D renderings to visualize the biofilm architecture (Jung et al., 2022).
Metagenome profiling of saliva-derived microcosm biofilms
Microcosm biofilm samples were collected from the sHA discs and eluted in phosphate-buffered saline (PBS). Genomic DNA was extracted using the FastDNA® Spin Kit for Soil (MP Biomedicals, USA) and quantified using a BioTek Epoch™ spectrophotometer. DNA quality was verified using 1% agarose gel electrophoresis. The V3–V4 region of the bacterial 16S rRNA gene was amplified using the universal primers 341F and 805R with overhang Illumina adapter sequences following the Nextera™ consensus design. Polymerase chain reaction (PCR) amplification was conducted in two steps: the first round of target amplification and the second round of indexing. The first PCR included 25 cycles using Takara Ex Taq polymerase, and the second PCR consisted of eight indexing cycles. Libraries were purified using AMPure XP beads (Beckman Coulter) and quantified using the Quant-iT PicoGreen dsDNA Assay Kit. Library quality was assessed using an Agilent 2100 Bioanalyzer, and sequencing was performed using the Illumina MiSeq platform with the MiSeq Reagent Kit v2 (500 cycles). Chimeric sequences were detected and removed using the UCHIME method and embedded in the EzBioCloud database (Yoon et al., 2017). Downstream analysis included alpha- and beta-diversity metrics (e.g., Shannon index and Bray–Curtis distance) and relative abundance profiling across taxonomic ranks. The 16S rRNA gene sequences are available in the NCBI Sequence Read Archive (BioProject accession number: PRJNA1269248).
Statistical analysis
All data are presented as mean ± standard deviation (SD). For comparisons involving multiple groups against a single control, a one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test was applied. Interspecies CFU comparisons were analyzed using non-parametric Kruskal–Wallis tests with Dunn’s post-hoc correction. For gene expression analysis, ΔCt values were evaluated using one-way ANOVA followed by Holm–Šídák’s multiple comparisons test. Statistical significance was set at p<0.05. Analyses were performed using GraphPad Prism version 10.4.0 (GraphPad Software, San Diego, CA, USA).
Results and discussion
Planktonic growth and acid production of S. mutans in response to various sweeteners
To evaluate allulose with fermentable sugars and non-fermentable polyols in a controlled setting, the initial phase of the cariogenic evaluation platform concentrated on a single-species model using the key cariogenic pathogen S. mutans (Hajishengallis et al., 2017; Kim et al., 2020). To assess how different sweeteners affect the planktonic growth kinetics (OD600) and the acid production (pH drop assay), S. mutans was cultured in a UFTYE defined medium supplemented with 1% (w/v) sweetener. Basal medium UFTYE (blank) is a complex medium that contains low-molecular-weight nutrients (<10 kDa). The minimal growth observed in the UFTYE medium without additional sugars likely reflects the utilization of these residual nutrients. The growth curves showed that glucose and fructose supported robust bacterial growth with an extended exponential phase, reaching the stationary phase (OD600: approximately 1.0, Figure 2A). These observations align with previous findings that fermentable carbohydrates serve as a preferred and rapidly metabolizable energy source that fuels bacterial proliferation (Jurakova et al., 2023). Moreover, these sugars induced a steep pH drop, with the final pH value dropping to 4.20 ± 0.04 within 30 min, indicating high acid production from glycolytic fermentation (Figure 2B).
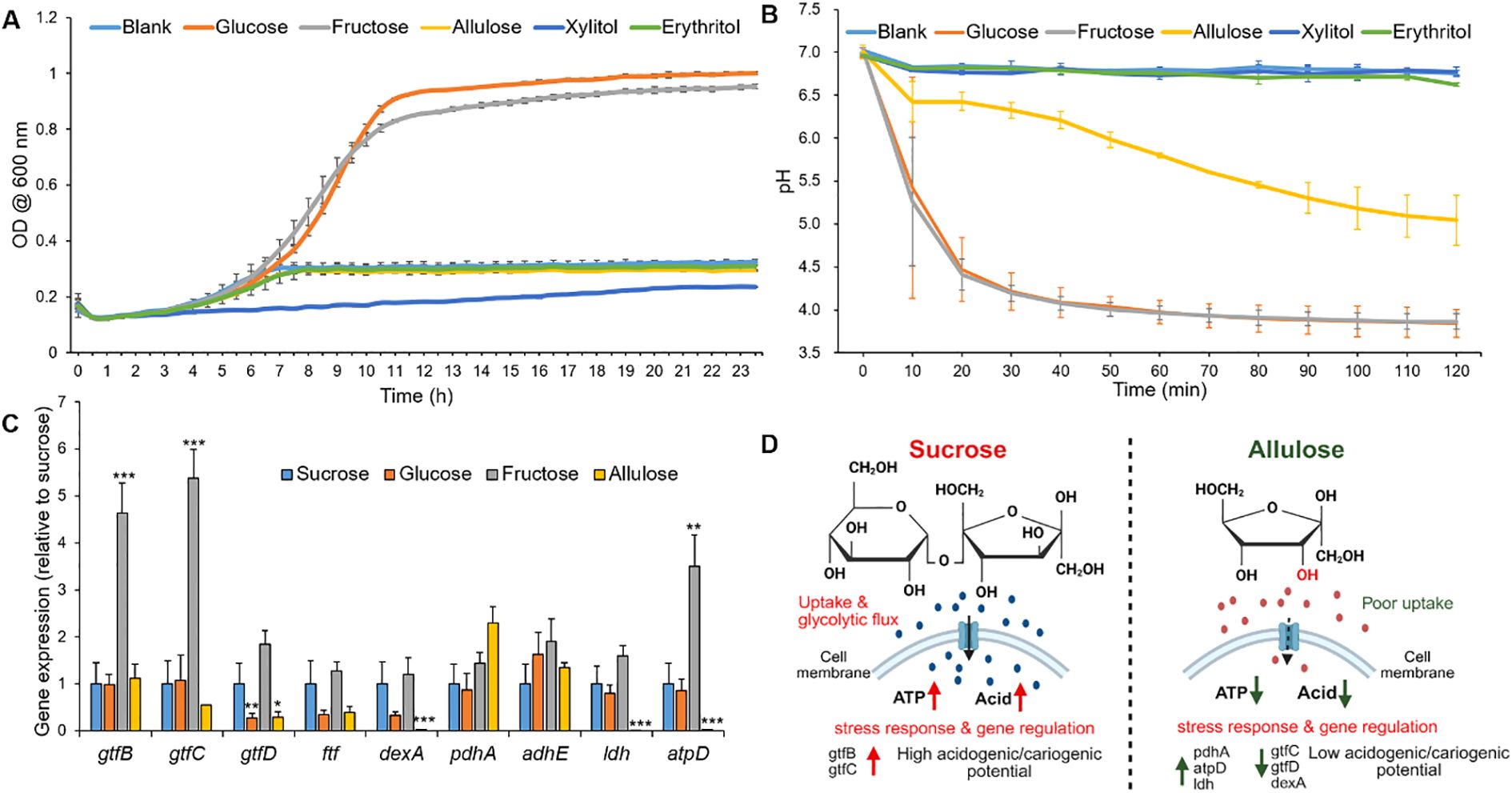
Figure 2. Bacterial growth curve, glycolytic pH drop, and relative gene expression of Streptococcus mutans in response to different sweeteners. (A) Growth curve of S. mutans measured as optical density at 600 nm (OD600) over time in a UFTYE medium supplemented with 1% (w/v) glucose, fructose, allulose, xylitol, or erythritol. The UFTYE medium without any sugar supplementation was used as a blank control. (B) pH drop assay of S. mutans in the presence of different sweeteners. Different sweeteners were added to 50 mM KCl + 1 mM MgCl2 solution (pH=7) to obtain a concentration of 1% (w/v), and pH changes were assessed over 120 min. The data are presented as mean ± standard deviation (n=3). (C) Relative gene expression of S. mutans planktonic cells in response to different sweeteners at 19 h. Bar graph shows the relative expression levels of key genes associated with biofilm formation (gtfB, gtfC, gtfD, ftf), extracellular matrix (dexA), energy metabolism (pdhA, adhE, atpD), and acid production (ldh) in S. mutans cultured in UFTYE medium supplemented with 1% (w/v) sucrose, glucose, fructose, or allulose. Xylitol and erythritol were excluded from gene expression analysis due to minimal growth, which precluded reliable RNA extraction. Data represent mean ± standard deviation from biological replicates (n=6). Only statistically significant differences compared to the sucrose group are indicated with asterisks: *p<0.05; **p<0.01; ***p<0.001. All other comparisons were not significant (ns). (D) Conceptual working model summarizing observed phenotypes under sucrose versus allulose, including bacterial growth, glycolytic pH drop, EPS production, and gene expression profiles. The schematic is illustrative and created using BioRender.
Conversely, although allulose had a structural resemblance to fructose, it did not promote exponential growth and exhibited only marginal acidification (Figures 2A, B). The growth in the presence of allulose supplementation remained constant at OD600 approximately 0.3, and the pH remained above 5.0 (critical pH for enamel demineralization) over 120 min with a reduction in acid production by 99% compared to that in the presence of sucrose, indicating the absence of key metabolic pathways for effectively metabolizing allulose. This growth profile highlights that, under the conditions tested, allulose supported minimal bacterial growth and acid production compared to conventional sugars. Xylitol and erythritol also failed to support exponential growth and acid production, establishing their roles as non-fermentable sugar alcohols (Figures 2A, B) (Mäkinen, 2010; Jeong et al., 2024).
Dynamics of cariogenicity-associated genes in response to allulose and other fermentable sugars
To elucidate the molecular basis underlying the observed differences in planktonic growth and acid production, we examined the expression of key virulence genes in 19 h-old S. mutans cells cultured in the presence of different dietary sweeteners (Figure 2C). Planktonic gene expression is important because it may indicate the ability of free cells to colonize a pre-formed biofilm or a new surface (Durso et al., 2014). The cariogenicity of S. mutans is closely linked to its ability to synthesize extracellular glucans and produce acids via carbohydrate fermentation.
Glucosyltransferase (Gtf catalyzes EPS synthesis and forms a protective scaffold that supports biofilm integrity under external stress (Wang et al., 2018). Specifically, gtfB and gtfC are associated with insoluble and soluble glucan production, whereas gtfD contributes to soluble glucan production (Zhao et al., 2014). In our study, gtfB and gtfC expression were significantly upregulated in the presence of fructose than in the presence of sucrose (p<0.0001), consistent with prior findings on fructose-mediated EPS-related gene induction (Shemesh et al., 2006). In contrast, gtfD was significantly downregulated in the presence of allulose (p<0.05), which may limit the primer availability for initial EPS synthesis, thereby impairing biofilm formation.
Notably, gtfB expression remained unaffected by allulose exposure. Since environmental stress often triggers gtfB upregulation to promote adhesion and glucan synthesis (Zhang et al., 2022), its stable expression in the presence of allulose may represent a compensatory mechanism for surface attachment in nutrient-limited or metabolically inactive states. It is important to note that transcriptional levels may not always correspond to enzymatic activity because post-transcriptional regulation can modulate the final protein function (Zhang et al., 2021).
Unlike gtf genes, ftf expression, which is involved in fructan synthesis, remained unchanged in the presence of all sweeteners, suggesting a limited role of fructan-mediated EPS under these experimental conditions. Interestingly, the significant downregulation of dexA, which encodes the dextranase responsible for glucan degradation during biofilm remodeling, suggests that, under allulose conditions, EPS synthesis and biofilm maturation pathways were less active compared to fermentable sugars (Igarashi et al., 2002).
pdhA, which encodes a component of the pyruvate dehydrogenase complex, was upregulated in the presence of allulose. This enzyme links glycolysis to the tricarboxylic acid (TCA) cycle by converting pyruvate into acetyl-CoA, suggesting a shift toward alternative energy metabolism in response to inefficient allulose fermentation. This metabolic reprogramming was consistent with previous findings under carbohydrate-limited conditions (Lemos and Burne, 2008), indicating that under allulose conditions, S. mutans exhibited a low-metabolic, low-virulence-like state.
In terms of acidogenicity, the expression of ldh, which encodes lactate dehydrogenase, a key enzyme in lactic acid production, was significantly lower in the presence of allulose than in the presence of sucrose (p<0.001), correlating with the reduced acid production. Similarly, atpD, encoding the β-subunit of the F1F0–ATPase complex responsible for proton extrusion under acidic conditions, was highly expressed in the presence of fructose (p<0.01), reflecting increased acid stress. Conversely, its significant downregulation in the presence of allulose (p<0.001) supports the notion that allulose imposes minimal acidogenic stress, further reinforcing its low cariogenic potential.
Taken together, the gene expression profiling and phenotypic data on growth and acid production support a less-cariogenic profile for allulose under the tested conditions (Figure 2D). Its inability to activate key virulence pathways, including those involved in EPS synthesis (gtfD), acid production (ldh), and acid tolerance (atpD), aligns it with the properties of non-cariogenic sugar alcohols, such as xylitol and erythritol (Mäkinen, 2011; Milgrom et al., 2012; Runnel et al., 2013; De Cock, 2018).
Modulation of S. mutans biofilm formation and EPS synthesis by sweeteners
To assess the effects of dietary sugars on biofilm development and EPS production, which are key indicators of cariogenic potential, we employed a sHA disc model to simulate a pellicle-formed tooth surface (Figure 3A). S. mutans was incubated for 43 h in the presence of 1% (w/v) various sweeteners, and the mature biofilm biomass was quantified in terms of CFU and dry weight (Figures 3B, C). The three-dimensional (3D) architecture of the biofilm, consisting of both bacterial cells and EPS, was visualized using confocal microscopy (Figure 3D).
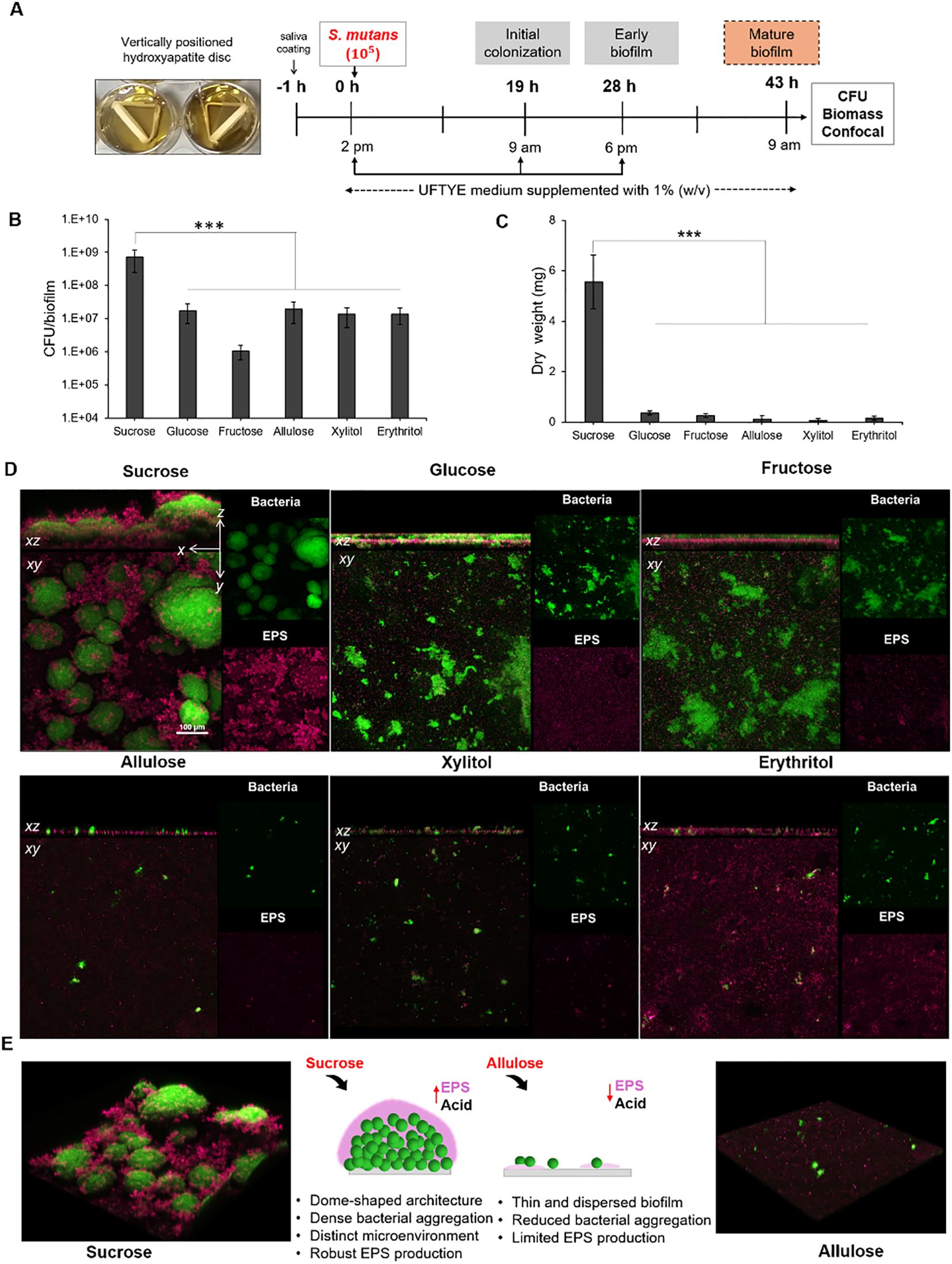
Figure 3. Experimental workflow and microbiological analysis of Streptococcus mutans biofilm architecture at 43 h. (A) Experimental design for S. mutans biofilm formation on the saliva-coated hydroxyapatite (sHA) discs. Biofilms were cultured in UFTYE media supplemented with 1% (w/v) sweetener at 37°C and 5% CO2. The culture medium was changed twice daily (at 19 h and 28 h). Biofilm quantification in terms of (B) colony-forming units (CFU)/biofilm and (C) dry weight. (D) Confocal images showing the structural and spatial organization of 43-h-old S. mutans biofilm grown in the presence of various sweeteners. (E) Representative 3D confocal microscopy images showing biofilm formation in the presence of sucrose (left) and allulose (right). Green represents bacterial cells, while magenta indicates the extracellular polysaccharide (EPS) matrix. The data are presented as mean ± standard deviation (n=5). One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test was used to compare each treatment group with the sucrose control. ***p<0.0001.
Quantitative data (Figures 3A, B) showed that sucrose yielded significantly higher CFU counts and dry biofilm weights (p<0.001) than all the other sugars. This further validates its established role as a high-carbohydrate sugar that enhances bacterial cell attachment and EPS production (Banas and Vickerman, 2003; Duarte et al., 2008; Razak et al., 2017). The dense, well-defined, dome-shaped microcolonies embedded in the EPS matrix under sucrose supplementation (Figures 3D, E) were consistent with those reported in previous reports on the sucrose-driven upregulation of Gtf exoenzymes, which are crucial for water-insoluble glucan synthesis and robust biofilm development. In contrast, supplementation with other fermentable sugars, such as glucose and fructose, resulted in comparatively less biomass with a dispersed cell–EPS arrangement (Figure 3D). These results indicate that, although these monosaccharides are fermentable, they cannot replace the dual role of sucrose as a fuel for acid production and a key substrate for water-insoluble EPS biosynthesis.
Biofilms developed in the presence of allulose showed significantly reduced CFU counts and dry weights, similar to those developed in the presence of xylitol and erythritol (Figures 3A, B). Confocal imaging further showed that biofilms formed under allulose conditions lacked the dense, matrix-rich structures typical of sucrose-induced S. mutans biofilms (Figures 3D, E). The initial inhibition of biofilm formation in the presence of allulose can be attributed to reduced planktonic growth compared to that in the presence of fermentable sugars, correlating with the gene expression data showing a more complicated adaptive response. The upregulation of genes associated with metabolic stress and compensatory energy pathways (pdhA and adhE, respectively; Figure 2C) indicates that S. mutans may activate adaptive strategies under nutrient-limited conditions rather than simply undergoing growth arrest. These results suggest that biofilms under allulose conditions were characterized by reduced biomass, less EPS, and altered metabolic programming compared to fermentable sugars.
Taken together, the results of the single-species S. mutans biofilm provide consistent evidence that, under the conditions tested, allulose exhibits fewer cariogenic phenotypes compared to fermentable sugars and shows functional similarities to previously reported sugar alcohols (Miyasawa‐Hori et al., 2006; Park et al., 2014). Our study further showed that biofilm architecture and EPS accumulation are the main indicators of cariogenic potential, and the sHA biofilm model is pertinent for evaluating dietary sugar under near-physiological conditions.
Acidogenic potential of sweeteners in mature S. mutans biofilm
Assessing how pre-formed oral biofilms respond to altered sugar exposure is necessary to comprehend how dietary sugar variations affect the cariogenic behavior of established biofilms. This was evaluated by a glycolytic pH drop assay using a 43 h-old S. mutans biofilm grown on tooth mimetics (Figure 4). To mimic the dietary changes, biofilms grown in the presence of 1% sucrose were exposed to various fermentable and non-fermentable sweeteners, and the pH drop was monitored for 120 min. The results show that sucrose supports the highest H+ ion release (1.95 µM/min) and reaches a pH of 4.5 within 20 min of exposure, which further establishes its role as a fuel for both bacterial metabolisms, followed by acid production and EPS-associated proton accumulation (Duarte et al., 2008; Razak et al., 2017; Jurakova et al., 2023). The acidogenic profile of fructose was similar to that of sucrose than that of glucose, which is consistent with earlier findings on the expression of fructose-induced virulence factors in S. mutans (Shemesh et al., 2006).
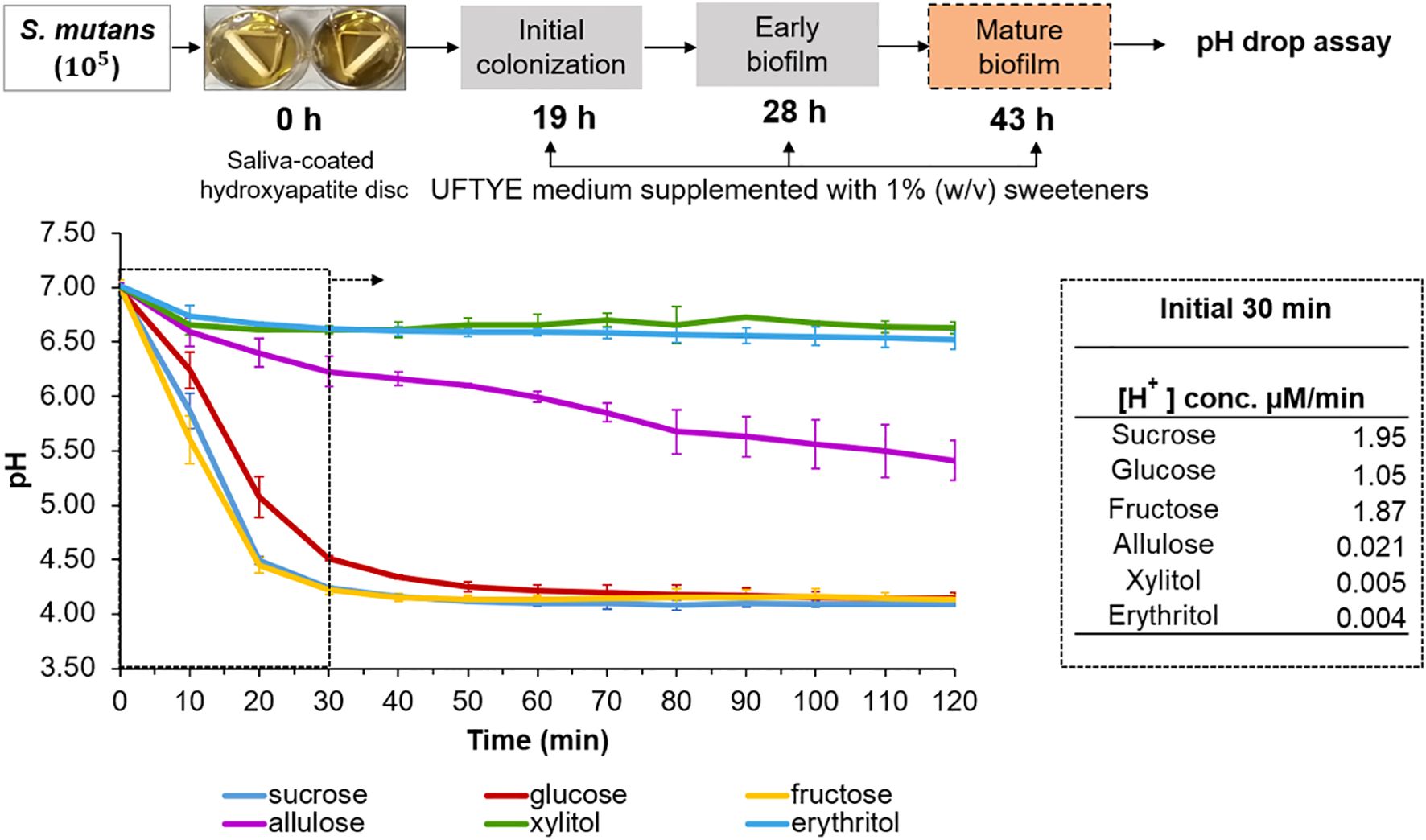
Figure 4. pH drop assay with pre-formed Streptococcus mutans biofilm in the presence of different sweeteners. The top panel illustrates the experimental design where S. mutans biofilms were grown on saliva-coated hydroxyapatite (sHA) discs in UFTYE medium supplemented with 1% (w/v) sucrose. The pH drop assay was performed at 43 h in the presence of different sweeteners. The bottom panel presents the pH drop over time, showing acid production from bacterial metabolism. Data are presented as the mean of two biologically different experiments.
Conversely, allulose-, xylitol-, or erythritol-supplemented biofilms maintained near-neutral pH throughout the assay period, with 98%, 99%, and 99% reduction in acid production, respectively, within 30 min compared to the sucrose-supplemented biofilm (Figure 4). This result confirms that they serve as poorly fermentable substrates for S. mutans metabolism.
Although the oral cavity generally maintains a near-neutral pH, localized acidification from dietary sugars can promote enamel demineralization, favor acid-tolerant microbiota proliferation, and disrupt biofilm homeostasis (Marsh, 1994; Welin-Neilands and Svensater, 2007; Ikäläinen et al., 2024). Poor oral hygiene may exacerbate these effects by allowing the aciduric biofilms to mature and persist (Sälzer et al., 2020). In this context, alternative sweeteners, such as allulose, may help suppress acid accumulation, even in 3D-structured biofilms.
Modulation of interspecies balance in a dual-species biofilm model by sweeteners
Microbial interactions play crucial roles in the modulation of health and disease. Cooperative and competitive interactions among oral pathogens and commensals, as well as their composition, are modulated by ecological factors, such as nutrients and pH (Marsh, 2003). In the second phase of our cariogenic assessment platform, we evaluated the impact of dietary sugar (as it is the major nutrient source) on the dual-species biofilm model using S. mutans as the oral pathogen and S. oralis as the commensal bacterium (Figure 5A). The results show that supplementation with 1% (w/v) sucrose, glucose, or fructose selectively enriched S. mutans over S. oralis (p<0.001, Figure 5B). S. mutans enrichment in the presence of fermentable sugars such as sucrose, glucose, and fructose is driven by its robust metabolic and ecological adaptations. S. mutans can produce acid and survive in low-acid conditions because of its highly efficient fermentable sugar uptake system and virulence-associated gene expression. Particularly, sucrose can serve as both a substrate and precursor for EPS matrix synthesis for dense, acidic, and robust biofilm formation (Koo et al., 2010). Previous reports have shown that S. mutans can compete with commensal species, such as S. oralis, and dominate biofilms through bacteriocin production and a quorum-sensing mechanism (Li et al., 2002; Kreth et al., 2005). Conversely, S. oralis is an early colonizer associated with healthy oral biofilms, sensitive to acidic conditions, and lacks EPS production capacity, which makes it vulnerable to sugar-rich and acidic environments. This clearly shows the ecological imbalance that can shift the biofilm into a dysbiotic state with enhanced cariogenic potential.
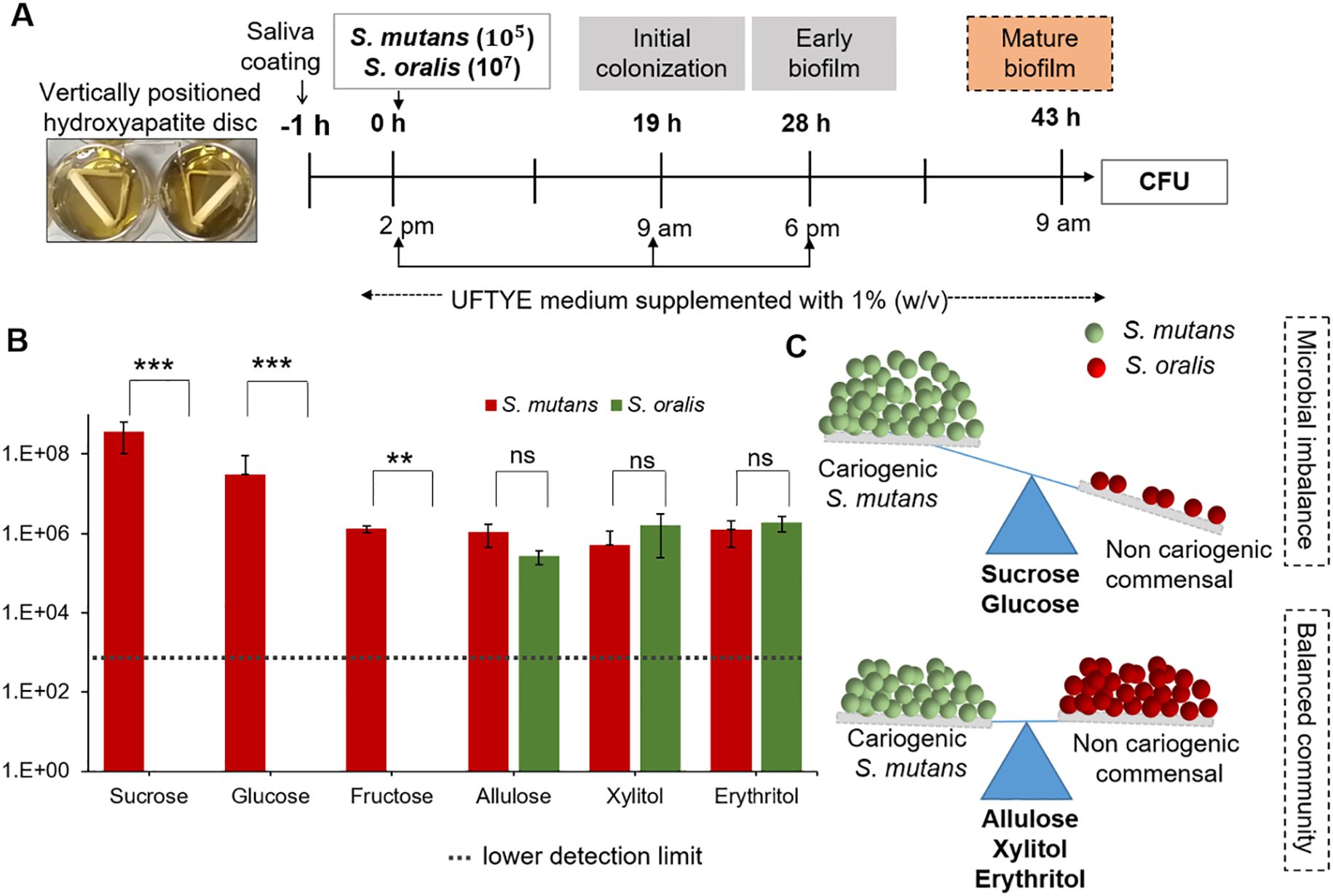
Figure 5. Impact of different sweeteners on Streptococcus mutans and Streptococcus oralis interspecies biofilm formation. (A) Experimental design for interspecies biofilm formation of S. mutans and S. oralis saliva-coated hydroxyapatite (sHA) discs. The biofilms were cultured in UFTYE medium supplemented with 1% (w/v) sweetener at 37°C and 5% CO2. (B) Quantification of S. mutans and S. oralis colony-forming units (CFU) in 43 h-old biofilms grown in the presence of different sweeteners. (C) Schematic representation of microbial balance shift induced by different sweeteners, illustrating the impact of sugar supplementation on the dominance of S. mutans (cariogenic) and S. oralis (commensal species). Data are presented as mean ± standard deviation (n=8). Pairwise comparisons between S. mutans and S. oralis in the presence of each sweetener were performed using Dunn’s multiple comparisons test following Kruskal–Wallis analysis. ***p<0.0001; **p<0.001; ns, not significant.
In contrast, allulose, xylitol, and erythritol maintained a balanced microbial composition, with no significant overgrowth of S. mutans toward S. oralis (Figure 5B). Previous studies have shown that the balance of S. oralis abundance in oral biofilms can be beneficial for dental health because these commensals secrete hydrogen peroxide, which can interfere with S. mutans growth and colonization (Kim et al., 2022). These findings align with the ecological plaque hypothesis, indicating the importance of non- or less-fermentable sugars such as allulose in reducing acid production and suppressing the overgrowth of acid-tolerant species over that of sensitive commensal bacteria in oral biofilms (Marsh, 1994, 2003). Therefore, oral microbial homeostasis and cariogenic biofilm formation control depend on the preservation of the balance between S. mutans and S. oralis (Figure 5C).
Ecological modulation in saliva-derived multi-species microcosm biofilm model by allulose
Next, we used HA discs coated with pooled whole saliva to stimulate a clinically relevant polymicrobial environment and support early colonization by native microbes. The ecological response to allulose was evaluated under caries-promoting conditions by supplementation with 1% (w/v) sucrose, followed by adding S. mutans, and compared with that of the no-added-carbohydrate saliva control. Changes in biofilm structure and community profile were examined in early (19 h) and mature (43 h) biofilms (Figure 6A).
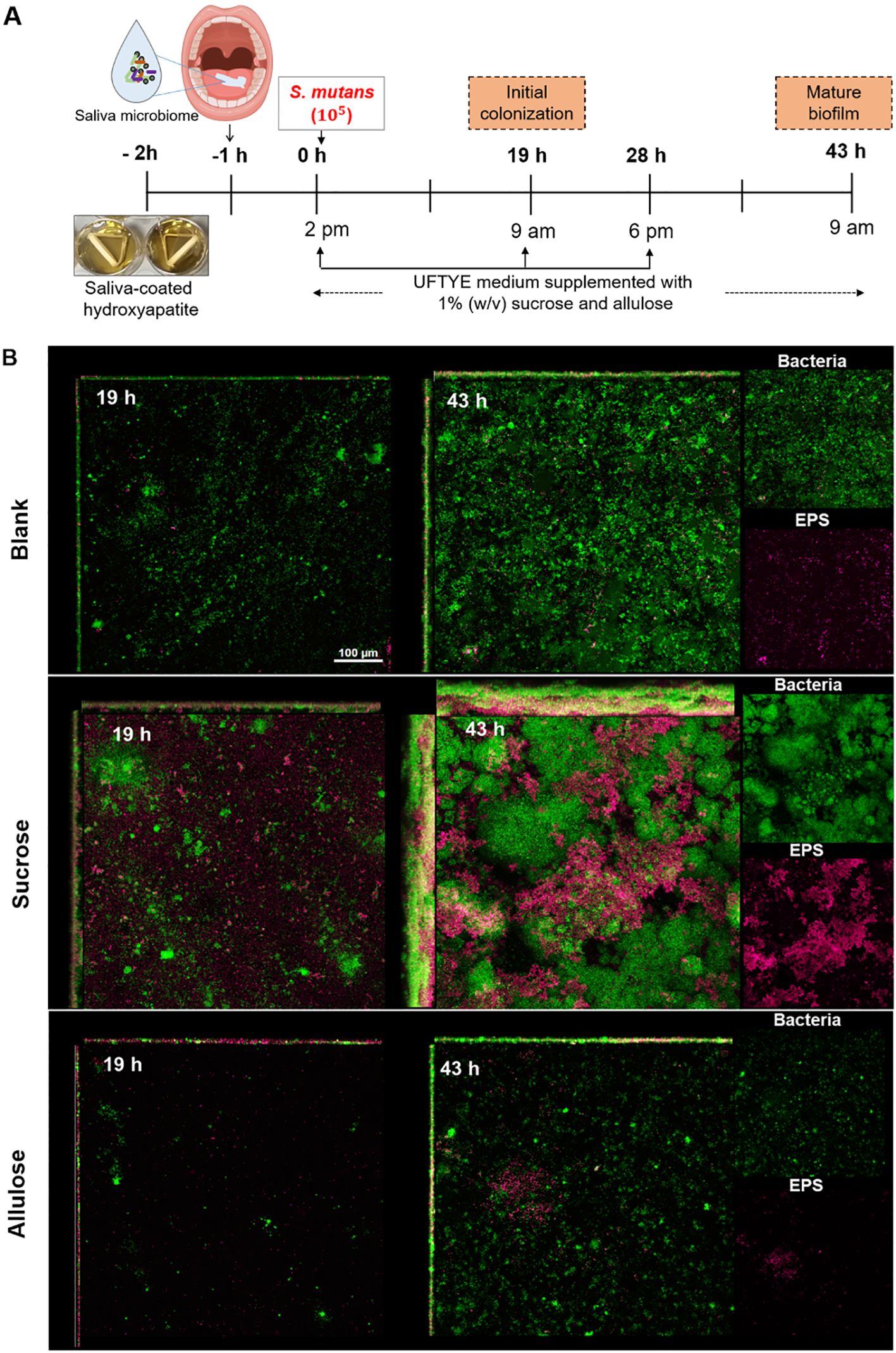
Figure 6. Biofilm development and extracellular polysaccharide (EPS) production in a saliva-derived microcosm model in the presence of 1% (w/v) sucrose or allulose. (A) Schematic representation of an ex vivo microcosm biofilm model using saliva-coated hydroxyapatite (sHA) discs. The discs were first incubated with the saliva from healthy donors for 1 h to allow initial microbial colonization, followed by incubation in UFTYE media supplemented with 1% (w/v) sucrose or allulose and inoculated with S. mutans. UFTYE media without any sweetener supplementation served as the blank control. (B) Confocal laser scanning microscopy images of biofilms grown at 19 and 43 h. Green and magenta represent bacterial cells and the extracellular polysaccharides (EPS), respectively.
Confocal imaging revealed significant architectural differences between the biofilms grown under sucrose and allulose supplementation. Early biofilm maturation, in terms of significant bacterial adhesion and EPS deposition, was observed in sucrose-supplemented biofilms at 19 h (Figure 6B). In contrast, the saliva-only control (without sugar addition) showed moderate bacterial attachment with little or no EPS, whereas the allulose-supplemented biofilms showed sparse bacterial colonization with minimal EPS deposition. Minimal baseline growth or biofilm may have resulted from trace amounts of sugars in the UFTYE medium; however, all groups were cultured under the same basal medium to ensure comparability. Upon extended incubation for 43 h, sucrose induced the development of dense dome-shaped microcolonies with a robust EPS matrix. The allulose-supplemented biofilm maintained a reduced biofilm structure with minimal bacteria and EPS, suggesting that biofilms under allulose conditions remained thinner and less structured over time compared to sucrose-treated biofilms; as expected, biofilms developed in the saliva-only group remained less organized and thinner.
Following confocal imaging, diversity analysis showed that the bacterial community structure was distinct among the groups (Figure 7). Alpha-diversity, in terms of the Shannon index, which is a measure of both richness and evenness (Kitikidou et al., 2024), was significantly lower in the sucrose-supplemented biofilm than in the no-sugar-added control and allulose-supplemented biofilms in early and mature stages, respectively (Figures 7A, B; p=2.8E−2). Interestingly, allulose maintained the highest Shannon diversity index compared to the other two sweeteners at both 19 and 43 h. This suggests the ecological neutrality of allulose under the tested conditions by maintaining or promoting microbial diversity. Beta-diversity analysis (principal coordinate analysis based on the Bray–Curtis distance) revealed distinct clustering of microbial communities by treatment and time point. At both early (19 h) and mature (43 h) stages, sucrose-treated biofilms showed strong separation from both the allulose-supplemented and no-sugar-added control biofilms, indicating a marked shift in microbial community composition toward a dysbiotic state. In contrast, allulose-treated communities clustered closer to the no-sugar-added control communities, suggesting the maintenance of a more health-compatible microbial community (Figure 7C). Sucrose significantly reduced community diversity by selectively promoting the growth of acidogenic and aciduric Streptococcus and Lactobacillus species. In contrast, the relative abundance was maintained or increased under allulose supplementation compared to that without any supplementation (Figure 7A).
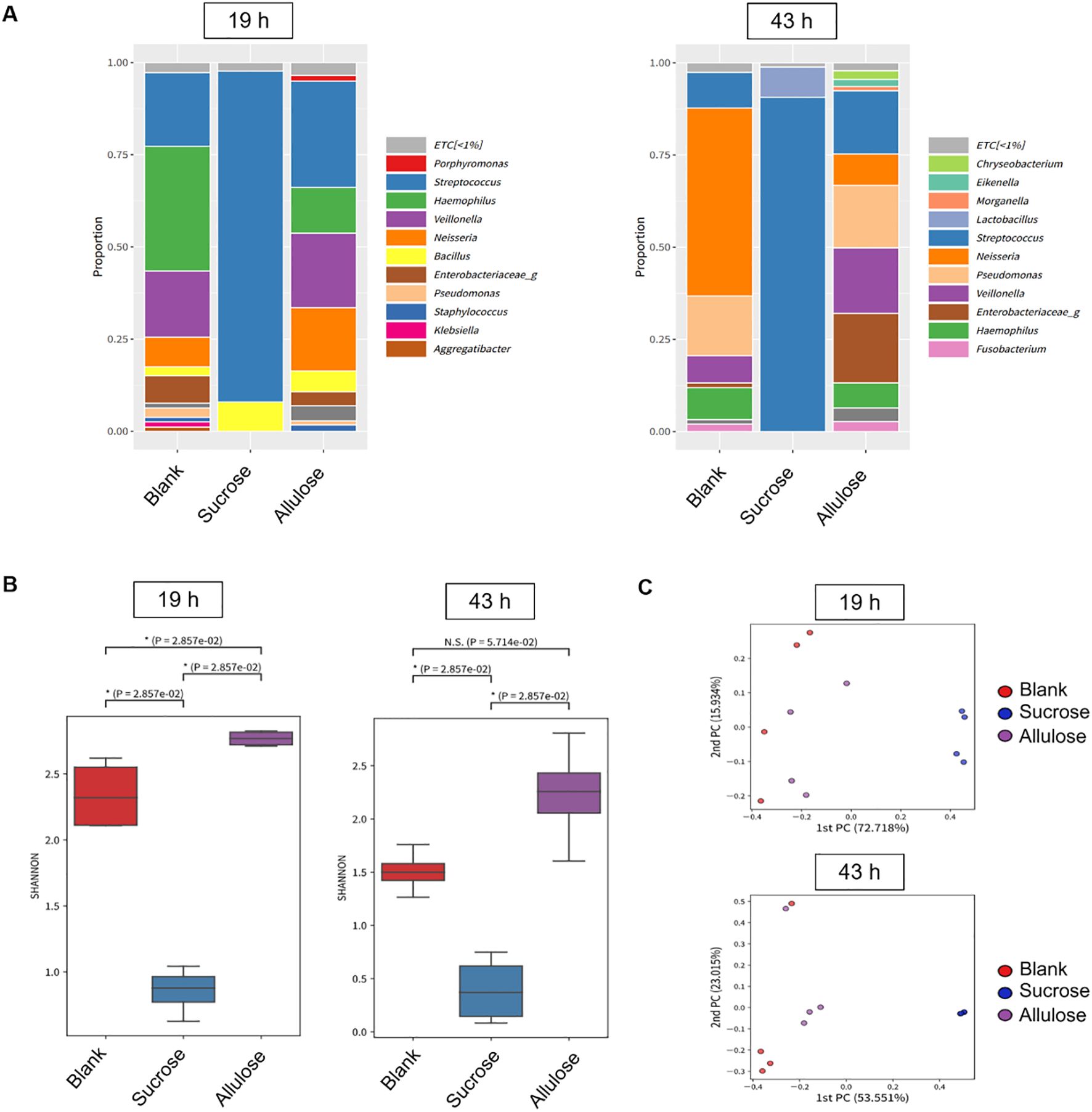
Figure 7. Microbial diversity and community structure in biofilm grown in the presence of 1% (w/v) sucrose or allulose. (A) Relative abundance of bacterial genera in 19 (left) and 43 h (right) biofilms grown on saliva-coated hydroxyapatite (sHA) discs with 1% (w/v) sucrose, allulose, or no sugar (blank). (B) Alpha-diversity (Shannon index) of microbial communities among treatment groups at 19 and 43 h. (C) Principal coordinate analysis (PCoA) of beta-diversity (Bray–Curtis distance) across treatment groups.
Biofilm development is a multistep process that begins with the attachment of early colonizers via specific or non-specific interactions with pellicle-coated surfaces (Marsh and Bradshaw, 1995; Dang and Lovell, 2000; Rickard et al., 2003). Favorable conditions promote microcolony formation by primary colonizers, subsequently facilitating secondary (late) colonizers to adhere primarily through co-aggregation (Rickard et al., 2003). Genera such as Streptococcus, Actinomyces, Haemophilus, Veillonella, and Neisseria are well-established early colonizers (Ritz, 1967; Foster and Kolenbrander, 2004; Dige et al., 2009; Huang et al., 2011). In this study, the presence of all these genera (except Actinomyces) in the allulose-treated and blank (saliva-origin microbiome) groups supported the establishment of a health-associated early biofilm community. Veillonella species convert the lactic acid produced by fermentative bacteria, such as Streptococcus sp., into weak acids, which may reduce the enamel demineralization rate (Geddes, 1972; Mikx and Van der Hoeven, 1975). The enrichment and maintenance of Veillonella sp. in the allulose-treated group than in the no-sugar-added group confirmed a more balanced community with metabolic cooperation (Figure 7A).
Additionally, the presence of Neisseria, Haemophilus, Granulicatella, Fusobacterium, and Veillonella further supports the establishment of a healthy nitrate-reducing biofilm with a beneficial role in maintaining oral homeostasis (Hyde et al., 2014). Notably, Fusobacterium plays a key bridging role in plaque development, promoting co-aggregation and supporting anaerobic colonizers (Bradshaw et al., 1998; Kolenbrander et al., 1999; Rickard et al., 2003). Hence, its presence in the allulose-supplemented group suggests a diverse microbial community structure, where microbial connectivity and balance were preserved (Figures 7A–C).
Altogether, our microbial community analysis data align with the “ecological plaque hypothesis,” which explains that environmental shifts owing to acid production from dietary sugar fermentation can select acid-tolerating species such as Streptococcus and Lactobacillus, developing dysbiotic, caries-associated biofilms. By dealing with these detrimental and virulent factors, the allulose-treated microcosms retained microbial interactions, community diversity, and spatial organization, which are key characteristics of health-compatible biofilms.
In this study, we used a multi-tiered platform to investigate the ecological and functional effects of allulose on oral biofilm development. Using a biologically relevant model that included single-species planktonic, biofilm, dual-species biofilm, and saliva-derived microcosms, we systematically showed that allulose does not support the key virulence traits typically promoted by commonly used fermentable sugars such as sucrose, fructose, and glucose. Allulose consistently resulted in lower growth, acidogenicity, and EPS matrix formation compared to fermentable sugars, while preserving the ecological equilibrium of commensal bacteria (S. oralis). Additionally, it preserved many health-compatible taxa such as Neisseria, Haemophilus, Granulicatella, and Veillonella and maintained high alpha-diversity, indicating microbial equilibrium and ecological resilience. The follow-up experiment will apply shotgun metagenomics to identify species-level community shifts and functional gene analyses to link taxonomic shifts to cariogenic traits.
The overall findings of our study indicate that allulose is a microbiome-friendly sugar substitute that may limit cariogenic shifts under in vitro conditions. While promising, the translational potential of allulose as an alternative to established cariostatic agents, such as xylitol and erythritol, warrants further investigation.
We acknowledge the limitations of this platform, particularly the inability of the continuous sugar exposure model to accurately mimic the dynamic nature of dietary intake. Future research should aim to improve the ecological relevance of cariogenic evaluations by incorporating a feast–famine strategy. In addition, future research should evaluate the composition of plaques, the dynamics of salivary pH, and the incidence of caries over time to validate these in vitro results using in vivo models, including clinical trials. Ecological engineering of oral microbiota may also be clarified by further studies on the interactions of allulose with fluoride, oral prebiotics, or probiotics. Overall, this study establishes a strong foundation for developing next-generation microbiome-conscious sugar alternatives that support oral and systemic health.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, PRJNA1269248.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Jeonbuk National University (IRB ref. JBNU 2021-12-008-003). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SH: Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. KR: Formal analysis, Validation, Visualization, Writing – original draft. SP: Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. JL: Investigation, Methodology, Writing – review & editing. HJ: Investigation, Methodology, Validation, Writing – review & editing. JK: Conceptualization, Methodology, Resources, Writing – review & editing. DK: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Visualization, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported in part by the National Research Foundation (NRF) [grant number RS-2021-NR061314 to DK] and the Bio & Medical Technology Development Program of NRF [grant number RS-2022-NR067350 to DK], funded by the Korean government (MIST).
Acknowledgments
High-purity allulose and erythritol were provided by Samyang Co. (Seongnam, Korea).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that Generative AI was used in the creation of this manuscript. The author(s) used ChatGPT in order to improve the language and readability of the manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Banas, J. A. (2004). Virulence properties of Streptococcus mutans. Front. Biosci. 9, 1267–1277. doi: 10.2741/1305
Banas, J. and Vickerman, M. (2003). Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral. Biol. Med. 14, 89–99. doi: 10.1177/154411130301400203
Beighton, D. (2005). The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent. Oral. Epidemiol. 33, 248–255. doi: 10.1111/j.1600-0528.2005.00232.x
Benahmed, A. G., Gasmi, A., Dadar, M., Arshad, M., and Bjørklund, G. (2021). The role of sugar-rich diet and salivary proteins in dental plaque formation and oral health. J. Oral. Biosci. 63, 134–141. doi: 10.1016/j.job.2021.01.007
Bowen, W. H., Burne, R. A., Wu, H., and Koo, H. (2018). Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 26, 229–242. doi: 10.1016/j.tim.2017.09.008
Bowen, W. and Koo, H. (2011). Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 45, 69–86. doi: 10.1159/000324598
Bradshaw, D. J., Marsh, P. D., Watson, G. K., and Allison, C. (1998). Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect. Immun. 66, 4729–4732. doi: 10.1128/IAI.66.10.4729-4732.1998
Cai, J.-N., Jung, J.-E., Lee, M.-H., Choi, H.-M., and Jeon, J. G. (2018). Sucrose challenges to Streptococcus mutans biofilms and the curve fitting for the biofilm changes. FEMS Microbiol. Ecol. 94, fiy091. doi: 10.1093/femsec/fiy091
Dang, H. and Lovell, C. R. (2000). Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes. Appl. Environ. Microbiol. 66, 467–475. doi: 10.1128/AEM.66.2.467-475.2000
Daniel, H., Hauner, H., Hornef, M., and Clavel, T. (2022). Allulose in human diet: the knowns and the unknowns. Br. J. Nutr. 128, 172–178. doi: 10.1017/S0007114521003172
De Cock, P. (2018). Erythritol functional roles in oral-systemic health. Adv. Dent. Res. 29, 104–109. doi: 10.1177/0022034517736499
Dige, I., Nyengaard, J. R., Kilian, M., and Nyvad, B. (2009). Application of stereological principles for quantification of bacteria in intact dental biofilms. Oral. Microbiol. Immunol. 24, 69–75. doi: 10.1111/j.1399-302X.2008.00482.x
Document, G. (2019). The Declaration of Allulose and Calories from Allulose on Nutrition and Supplement Facts Labels: Guidance for Industry (MD, USA: Center for Food Safety and Applied Nutrition College Park).
Duarte, S., Klein, M., Aires, C., Cury, J., Bowen, W., and Koo, H. (2008). Influences of starch and sucrose on Streptococcus mutans biofilms. Oral. Microbiol. Immunol. 23, 206–212. doi: 10.1111/j.1399-302X.2007.00412.x
Durso, S. C., Vieira, L., Cruz, J., Azevedo, C., Rodrigues, P., and Simionato, M. R. L. (2014). Sucrose substitutes affect the cariogenic potential of Streptococcus mutans biofilms. Caries Res. 48, 214–222. doi: 10.1159/000354410
Forssten, S. D., Björklund, M., and Ouwehand, A. C. (2010). Streptococcus mutans, caries and simulation models. Nutrients 2, 290–298. doi: 10.3390/nu2030290
Foster, J. S. and Kolenbrander, P. E. (2004). Development of a multispecies oral bacterial community in a saliva-conditioned flow cell. Appl. Environ. Microbiol. 70, 4340–4348. doi: 10.1128/AEM.70.7.4340-4348.2004
Geddes, D. A. (1972). The production of l (+) and d (–) lactic acid and volatile acids by human dental plaque and the effect of plaque buffering and acidic strength on pH. Arch. Oral. Biol. 17, 537–545. doi: 10.1016/0003-9969(72)90069-6
Hajishengallis, E., Parsaei, Y., Klein, M. I., and Koo, H. (2017). Advances in the microbial etiology and pathogenesis of early childhood caries. Mol. Oral. Microbiol. 32, 24–34. doi: 10.1111/omi.12152
Hasturk, H. (2018). A clinical study on the effect of allulose, a low-calorie sugar, on in vivo dental plaque pH (Boston, MA, USA: Forsyth Institute).
He, J., Kim, D., Zhou, X., Ahn, S.-J., Burne, R. A., Richards, V. P., et al. (2017). RNA-Seq reveals enhanced sugar metabolism in Streptococcus mutans co-cultured with Candida albicans within mixed-species biofilms. Front. Microbiol. 8, 1036. doi: 10.3389/fmicb.2017.01036
Hu, M., Li, M., Jiang, B., and Zhang, T. (2021). Bioproduction of D-allulose: Properties, applications, purification, and future perspectives. Compr. Rev. Food Sci. Food Saf. 20, 6012–6026. doi: 10.1111/1541-4337.12859
Huang, R., Li, M., and Gregory, R. L. (2011). Bacterial interactions in dental biofilm. Virulence 2, 435–444. doi: 10.4161/viru.2.5.16140
Hyde, E. R., Andrade, F., Vaksman, Z., Parthasarathy, K., Jiang, H., Parthasarathy, D. K., et al. (2014). Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PloS One 9, e88645. doi: 10.1371/journal.pone.0088645
Igarashi, T., Morisaki, H., Yamamoto, A., and Goto, N. (2002). An essential amino acid residue for catalytic activity of the dextranase of Streptococcus mutans. Oral. Microbiol. Immunol. 17, 193–196. doi: 10.1034/j.1399-302X.2002.170310.x
Iida, T., Hayashi, N., Yamada, T., Yoshikawa, Y., Miyazato, S., Kishimoto, Y., et al. (2010). Failure of d-psicose absorbed in the small intestine to metabolize into energy and its low large intestinal fermentability in humans. Metabolism 59, 206–214. doi: 10.1016/j.metabol.2009.07.018
Ikäläinen, H., Guzman, C., Saari, M., Söderling, E., and Loimaranta, V. (2024). Real-time acid production and extracellular matrix formation in mature biofilms of three Streptococcus mutans strains with special reference to xylitol. Biofilm 8, 100219. doi: 10.1016/j.bioflm.2024.100219
Iwasaki, Y., Sendo, M., Dezaki, K., Hira, T., Sato, T., Nakata, M., et al. (2018). GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D-allulose. Nat. Commun. 9, 113. doi: 10.1038/s41467-017-02488-y
Jeon, J. G., Klein, M. I., Xiao, J., Gregoire, S., Rosalen, P. L., and Koo, H. (2009). Influences of naturally occurring agents in combination with fluoride on gene expression and structural organization of Streptococcus mutans in biofilms. BMC Microbiol. 9, 1–10. doi: 10.1186/1471-2180-9-228
Jeon, J. G., Pandit, S., Xiao, J., Gregoire, S., Falsetta, M. L., Klein, M. I., et al. (2011). Influences of trans-trans farnesol, a membrane-targeting sesquiterpenoid, on Streptococcus mutans physiology and survival within mixed-species oral biofilms. Int. J. Oral. Sci. 3, 98–106. doi: 10.4248/IJOS11038
Jeong, G.-J., Khan, F., Tabassum, N., and Kim, Y.-M. (2024). Alteration of oral microbial biofilms by sweeteners. Biofilm 7, 100171. doi: 10.1016/j.bioflm.2023.100171
Jung, H.-Y., Cai, J.-N., Yoo, S. C., Kim, S.-H., Jeon, J.-G., and Kim, D. (2022). Collagen peptide in a combinatorial treatment with Lactobacillus rhamnosus inhibits the cariogenic properties of Streptococcus mutans: An in vitro study. Int. J. Mol. Sci. 23, 1860. doi: 10.3390/ijms23031860
Jurakova, V., Farková, V., Kucera, J., Dadakova, K., Zapletalova, M., Paskova, K., et al. (2023). Gene expression and metabolic activity of Streptococcus mutans during exposure to dietary carbohydrates glucose, sucrose, lactose, and xylitol. Mol. Oral. Microbiol. 38, 424–441. doi: 10.1111/omi.12428
Kanasi, E., Johansson, I., Lu, S. C., Kressin, N. R., Nunn, M. E., Kent, R., Jr., et al. (2010). Microbial risk markers for childhood caries in pediatricians’ offices. J. Dent. Res. 89, 378–383. doi: 10.1177/0022034509360010
Kim, D., Barraza, J. P., Arthur, R. A., Hara, A., Lewis, K., Liu, Y., et al. (2020). Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc. Natl. Acad. Sci. U.S.A. 117, 12375–12386. doi: 10.1073/pnas.1919099117
Kim, D., Ito, T., Hara, A., Li, Y., Kreth, J., and Koo, H. (2022). Antagonistic interactions by a high H2O2-producing commensal streptococcus modulate caries development by Streptococcus mutans. Mol. Oral. Microbiol. 37, 244–255. doi: 10.1111/omi.12394
Kim, D., Liu, Y., Benhamou, R. I., Sanchez, H., Simón-Soro, Á., Li, Y., et al. (2018). Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 12, 1427–1442. doi: 10.1038/s41396-018-0113-1
Kim, D., Sengupta, A., Niepa, T. H., Lee, B.-H., Weljie, A., Freitas-Blanco, V. S., et al. (2017). Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci. Rep. 7, 41332. doi: 10.1038/srep41332
Kitikidou, K., Milios, E., Stampoulidis, A., Pipinis, E., and Radoglou, K. (2024). Using biodiversity indices effectively: considerations for forest management. Ecologies 5, 42–51. doi: 10.3390/ecologies5010003
Klein, M. I., Hwang, G., Santos, P. H., Campanella, O. H., and Koo, H. (2015). Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front. Cell. Infect. Microbiol. 5, 10. doi: 10.3389/fcimb.2015.00010
Kolenbrander, P. E., Andersen, R. N., Clemans, D. L., Whittaker, C. J., and Klier, C. M. (1999). Potential role of functionally similar coaggregation mediators in bacterial succession. Dental plaque revisited: Oral. biofilms Health Dis. 171-186.
Koo, H., Vacca Smith, A. M., Bowen, W. H., Rosalen, P. L., Cury, J. A., and Park, Y. K. (2000). Effects of Apis mellifera propolis on the activities of streptococcal glucosyltransferases in solution and adsorbed onto saliva–coated hydroxyapatite. Caries Res. 34, 418–426. doi: 10.1159/000016617
Koo, H., Xiao, J., Klein, M., and Jeon, J. (2010). Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 192, 3024–3032. doi: 10.1128/JB.01649-09
Kreth, J., Merritt, J., Shi, W., and Qi, F. (2005). Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 187, 7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005
Lee, H.-Y., Lee, G.-H., Hoang, T.-H., Park, S.-A., Lee, J., Lim, J., et al. (2022). D-allulose ameliorates hyperglycemia through IRE1α sulfonation-RIDD-sirt1 decay axis in the skeletal muscle. Antioxid. Redox Signal. 37, 229–245. doi: 10.1089/ars.2021.0207
Leme, A. P., Koo, H., Bellato, C., Bedi, G., and Cury, J. (2006). The role of sucrose in cariogenic dental biofilm formation—new insight. J. Dent. Res. 85, 878–887. doi: 10.1177/154405910608501002
Lemos, J. A. and Burne, R. A. (2008). A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology 154, 3247–3255. doi: 10.1099/mic.0.2008/023770-0
Li, Y.-H., Tang, N., Aspiras, M. B., Lau, P. C., Lee, J. H., Ellen, R. P., et al. (2002). A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 184, 2699–2708. doi: 10.1128/JB.184.10.2699-2708.2002
Liu, Y., Daniel, S. G., Kim, H.-E., Koo, H., Korostoff, J., Teles, F., et al. (2023). Addition of cariogenic pathogens to complex oral microflora drives significant changes in biofilm compositions and functionalities. Microbiome 11, 123. doi: 10.1186/s40168-023-01561-7
Mäkinen, K. K. (2010). Sugar alcohols, caries incidence, and remineralization of caries lesions: a literature review. Int. J. Dent. 2010, 981072. doi: 10.1155/2010/981072
Mäkinen, K. K. (2011). Sugar alcohol sweeteners as alternatives to sugar with special consideration of xylitol. Med. Princ. Pract. 20, 303–320. doi: 10.1159/000324534
Mäkinen, K. K. (2016). Gastrointestinal disturbances associated with the consumption of sugar alcohols with special consideration of xylitol: scientific review and instructions for dentists and other health-care professionals. Int. J. Dent. 2016, 5967907. doi: 10.1155/2016/5967907
Marsh, P. D. (1994). Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 8, 263–271. doi: 10.1177/08959374940080022001
Marsh, P. D. (2003). Are dental diseases examples of ecological catastrophes? Microbiology 149, 279–294. doi: 10.1099/mic.0.26082-0
Marsh, P. D. and Bradshaw, D. J. (1995). Dental plaque as a biofilm. J. Ind. Microbiol. 15, 169–175. doi: 10.1007/BF01569822
Mikx, F. and van der Hoeven, J. (1975). Symbiosis of Streptococcus mutans and Veillonella alcalescens in mixed continuous cultures. Arch. Oral. Biol. 20, 407–410. doi: 10.1016/0003-9969(75)90224-1
Milgrom, P., Söderling, E., Nelson, S., Chi, D., and Nakai, Y. (2012). Clinical evidence for polyol efficacy. Adv. Dent. Res. 24, 112–116. doi: 10.1177/0022034512449467
Miyasawa-Hori, H., Aizawa, S., and Takahashi, N. (2006). Difference in the xylitol sensitivity of acid production among Streptococcus mutans strains and the biochemical mechanism. Oral. Microbiol. Immunol. 21, 201–205. doi: 10.1111/j.1399-302X.2006.00273.x
Palmer, C., Kent, R., Jr., Loo, C., Hughes, C., Stutius, E., Pradhan, N., et al. (2010). Diet and caries-associated bacteria in severe early childhood caries. J. Dent. Res. 89, 1224–1229. doi: 10.1177/0022034510376543
Parisotto, T. M., Steiner-Oliveira, C., Silva, C. M. S. E., Rodrigues, L. K. A., and Nobre-dos-Santos, M. (2010). Early childhood caries and mutans streptococci: a systematic review. Oral. Health Prev. Dent. 8(1), 59-70.
Park, Y.-N., Jeong, S.-S., Zeng, J., Kim, S.-H., Hong, S.-J., Ohk, S.-H., et al. (2014). Anti-cariogenic effects of erythritol on growth and adhesion of Streptococcus mutans. Food Sci. Biotechnol. 23, 1587–1591. doi: 10.1007/s10068-014-0215-0
Philip, N., Suneja, B., and Walsh, L. J. (2018). Ecological approaches to dental caries prevention: paradigm shift or shibboleth? Caries Res. 52, 153–165. doi: 10.1159/000484985
Razak, F. A., Baharuddin, B. A., Akbar, E. F. M., Norizan, A. H., Ibrahim, N. F., and Musa, M. Y. (2017). Alternative sweeteners influence the biomass of oral biofilm. Arch. Oral. Biol. 80, 180–184. doi: 10.1016/j.archoralbio.2017.04.014
Ren, Z., Jeckel, H., Simon-Soro, A., Xiang, Z., Liu, Y., Cavalcanti, I. M., et al. (2022). Interkingdom assemblages in human saliva display group-level surface mobility and disease-promoting emergent functions. Proc. Natl. Acad. Sci. U.S.A. 119, e2209699119. doi: 10.1073/pnas.2209699119
Rice, T., Zannini, E., K. Arendt, E., and Coffey, A. (2020). A review of polyols–biotechnological production, food applications, regulation, labeling and health effects. Crit. Rev. Food Sci. Nutr. 60, 2034–2051. doi: 10.1080/10408398.2019.1625859
Rickard, A. H., Gilbert, P., High, N. J., Kolenbrander, P. E., and Handley, P. S. (2003). Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 11, 94–100. doi: 10.1016/S0966-842X(02)00034-3
Ritz, H. (1967). Microbial population shifts in developing human dental plaque. Arch. Oral. Biol. 12, 1561–1568. doi: 10.1016/0003-9969(67)90190-2
Runnel, R., Mäkinen, K. K., Honkala, S., Olak, J., Mäkinen, P. L., Nõmmela, R., et al. (2013). Effect of three-year consumption of erythritol, xylitol and sorbitol candies on various plaque and salivary caries-related variables. J. Dent. 41, 1236–1244. doi: 10.1016/j.jdent.2013.09.007
Sälzer, S., Graetz, C., Dörfer, C. E., Slot, D. E., and van der Weijden, F. A. (2020). Contemporary practices for mechanical oral hygiene to prevent periodontal disease. Periodontol. 2000 84, 35–44. doi: 10.1111/prd.12332
Sheiham, A. and James, W. (2015). Diet and dental caries: the pivotal role of free sugars reemphasized. J. Dent. Res. 94, 1341–1347. doi: 10.1177/0022034515590377
Shemesh, M., Tam, A., Feldman, M., and Steinberg, D. (2006). Differential expression profiles of Streptococcus mutans ftf, gtf and vicR genes in the presence of dietary carbohydrates at early and late exponential growth phases. Carbohydr. Res. 341, 2090–2097. doi: 10.1016/j.carres.2006.05.010
Staszczyk, M., Jurczak, A., Magacz, M., Kościelniak, D., Gregorczyk-Maga, I., Jamka-Kasprzyk, M., et al. (2020). Effect of polyols and selected dental materials on the ability to create a cariogenic biofilm–on children caries-associated Streptococcus mutans isolates. Int. J. Environ. Res. Public Health 17, 3720. doi: 10.3390/ijerph17103720
US Food and Drug Administration (FDA) (2023). Generally Recognized as Safe (GRAS) Notice (GRN) No. 1029: D-psicose. Silver Spring, MD: U.S. Food and Drug Administration.
Wang, Y., Wang, X., Jiang, W., Wang, K., Luo, J., Li, W., et al. (2018). Antimicrobial peptide GH12 suppresses cariogenic virulence factors of Streptococcus mutans. J. Oral. Microbiol. 10, 1442089. doi: 10.1080/20002297.2018.1442089
Welin-Neilands, J. and Svensater, G. (2007). Acid tolerance of biofilm cells of Streptococcus mutans. Appl. Environ. Microbiol. 73, 5633–5638. doi: 10.1128/AEM.01049-07
Xiao, J., Klein, M. I., Falsetta, M. L., Lu, B., Delahunty, C. M., Yates, J. R., III, et al. (2012). The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PloS Pathog. 8, e1002623. doi: 10.1371/journal.ppat.1002623
Yoon, S.-H., Ha, S.-M., Kwon, S., Lim, J., Kim, Y., Seo, H., et al. (2017). Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 67, 1613–1617. doi: 10.1099/ijsem.0.001755
Zhang, Q., Ma, Q., Wang, Y., Wu, H., and Zou, J. (2021). Molecular mechanisms of inhibiting glucosyltransferases for biofilm formation in Streptococcus mutans. Int. J. Oral. Sci. 13, 30. doi: 10.1038/s41368-021-00137-1
Zhang, L., Shen, Y., Qiu, L., Yu, F., Hu, X., Wang, M., et al. (2022). The suppression effect of SCH-79797 on Streptococcus mutans biofilm formation. J. Oral. Microbiol. 14, 2061113. doi: 10.1080/20002297.2022.2061113
Keywords: oral biofilm, extracellular polymeric substances, dental caries, Streptococcus mutans, microcosm
Citation: Han S, Rajitha K, Park S, Lim J, Jung H-Y, Kim J and Kim D (2025) Unveiling the impact of allulose on oral microbiota and biofilm formation via a cariogenic potential assessment platform. Front. Cell. Infect. Microbiol. 15:1670139. doi: 10.3389/fcimb.2025.1670139
Received: 21 July 2025; Accepted: 06 October 2025;
Published: 20 October 2025.
Edited by:
Keke Zhang, Wenzhou Medical University, ChinaReviewed by:
Lin Zeng, University of Florida, United StatesNoura Ahmed, Salt Lake Community College, United States
Copyright © 2025 Han, Rajitha, Park, Lim, Jung, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junghyun Kim, ZHZtaHl1bkBqYm51LmFjLmty; Dongyeop Kim, YmlvZmlsbWtpbUBqYm51LmFjLmty
†These authors have contributed equally to this work and share first authorship
 Seunghun Han
Seunghun Han Kuthirakkal Rajitha
Kuthirakkal Rajitha Sungbin Park1†
Sungbin Park1† Junghyun Kim
Junghyun Kim Dongyeop Kim
Dongyeop Kim