- 1Department of Biochemistry and Microbiology, University of Victoria, Victoria, BC, Canada
- 2Department of Medicine, Division of Allergy & Infectious Diseases, University of Washington, Seattle, WA, United States
Syphilis, caused by the extracellular bacterium Treponema pallidum ssp. pallidum, is a multi-stage and systemic infection that is lifelong in the absence of treatment. Two host cell types that frequently encounter T. pallidum during infection are endothelial cells and macrophages; treponemes disseminate through the vasculature and cross the blood–brain and placental barriers by traversing endothelial cell barriers, and macrophages are known to be critical for clearance of T. pallidum. Despite the importance of macrophages in treponemal clearance and endothelial cells in treponemal dissemination, a comprehensive understanding of the cytokines secreted by T. pallidum-exposed macrophages in the presence and absence of endothelial cells has not yet been achieved. To address this knowledge gap, we conducted time-course cytokine secretion profiling of macrophage-differentiated THP-1 cells alone and in co-culture with human brain microvascular endothelial cells. These experiments revealed reduced IL-8 secretion and increased secretion of RANTES, soluble ICAM-1, IL-1β, MCP-1, GM-CSF, TNF, and IL-6 in T. pallidum-exposed macrophage monocultures and macrophage-endothelial cell co-cultures compared to the same culture conditions in the absence of T. pallidum. These investigations enhance our understanding of macrophage-mediated, T. pallidum-focused innate immune responses occurring at endothelial sites. Further, this study provides insight into pro-inflammatory mechanisms elicited after exposure to this pathogen that may contribute to endothelial junction disruption, T. pallidum dissemination, and syphilis symptoms.
1 Introduction
Infectious syphilis, caused by the sexually transmitted bacterium Treponema pallidum subsp. pallidum, is a multi-stage disease with a global prevalence of 49.7 million cases (Chen et al., 2023) and an annual incidence of 8 million cases (World Health Organization, 2024). Global congenital syphilis estimates are 661,000 cases per year, with an estimated 355,000 adverse birth outcomes (Korenromp et al., 2019). With syphilis cases reaching a 20-year high (Spiteri et al., 2019; Aho et al., 2022; Chen et al., 2023; National Overview of STDs, 2021, 2023), an enhanced understanding of T. pallidum pathogenesis, and the resulting host response to infection, is needed to inform syphilis vaccine development.
Syphilis presents with a diverse array of symptoms that occur during distinct clinical stages of disease. These include the development of a characteristic chancre at the initial site of infection (primary stage), followed by a disseminated rash and general malaise (secondary stage). The infection then enters an asymptomatic latent phase, which may persist for an individual’s lifetime. Approximately one-third of individuals develop symptoms associated with the tertiary stage of disease, which can include severe central nervous system or cardiovascular complications (LaFond and Lukehart, 2006). The symptoms of syphilis are thought to arise from the host’s immune response to the presence of T. pallidum, highlighting the dichotomy of a beneficial inflammatory immune response which aids in T. pallidum clearance and a detrimental inflammatory response that contributes to bacterial dissemination, tissue damage, and disease (LaFond and Lukehart, 2006; Carlson et al., 2011).
Previous investigations have shown that T. pallidum clearance from local sites of infection is accomplished by a Th (T helper cell) 1-mediated delayed-type hypersensitivity (DTH) immune response (Carlson et al., 2011), mediated by interferon (IFN)-γ-activated macrophages and the process of opsonophagocytosis (Lukehart and Miller, 1978; Baker-Zander and Lukehart, 1992; Hawley et al., 2017). Although this response results in local clearance of the majority of T. pallidum, a subpopulation escapes immune clearance and disseminates via the bloodstream to distal tissue sites (Lukehart et al., 1992; Leader et al., 2003; Giacani et al., 2010; Reid et al., 2014). These observations emphasize the central role of macrophages in the immune response to T. pallidum.
Endothelial cells, positioned at the inner lining of the bloodstream, are among the first host cell types engaged by invading pathogens and are important mediators of innate immune activation. Endothelial cells contribute to immunity by secreting cytokines to recruit immune cells to sites of infection, and express cell adhesion molecules that enable leukocyte binding and subsequent leukocyte extravasation (Lemichez et al., 2010; Amersfoort et al., 2022). Treponema pallidum disseminates systemically via the bloodstream, traversing endothelial cell-cell junctions via both paracellular and transcellular migration (Wu et al., 2017; Lithgow et al., 2021). Adhesion of T. pallidum to the endothelium, an interaction critical for treponemal extravasation, is mediated by binding of T. pallidum vascular adhesins to extracellular matrix (ECM) components and endothelial cell receptors (Cameron, 2003; Cameron et al., 2004; Brinkman et al., 2008; Lithgow et al., 2020). Endothelial cells are activated by T. pallidum exposure, resulting in increased expression of cell adhesion molecules (Riley et al., 1992). Additionally, endothelial cells exposed to T. pallidum display altered expression of proteins involved in endothelial ECM organization/composition and cell death signaling. Further dysregulation includes increased secretion of pro-inflammatory junction-disrupting cytokines and growth factors such as vascular endothelial growth factor (VEGF), interleukin (IL)-6, and IL-8, as well as decreased secretion of monocyte chemoattractant protein 1 (MCP-1) (Waugh et al., 2023). Endothelial immune and ECM signaling responses have been shown to have downstream effects on monocyte and macrophage activity (Amersfoort et al., 2022). Similarly, macrophages can have reciprocal influence on endothelial function, including remodeling the endothelial ECM, promoting angiogenesis, and inducing endothelial cell death through the secretion of growth factors, cytokines, ECM components, and proteolytic enzymes (Bergers et al., 2000; Baer et al., 2013; Tomlin and Piccinini, 2018).
Despite evidence demonstrating the importance of both macrophages and endothelial cells during T. pallidum infection, the molecular outcomes of T. pallidum interaction with these cell types are incompletely understood (Lukehart and Miller, 1978; Baker-Zander and Lukehart, 1992; Hawley et al., 2017; Wu et al., 2017; Lithgow et al., 2021). In particular, the way T. pallidum affects macrophage-endothelial cell immune signaling remains unexplored. In the current study we address this knowledge gap by characterizing cytokine secretion of macrophages, singularly and in co-culture with endothelial cells, during T. pallidum exposure. This study enhances understanding of the interactions between T. pallidum, macrophages, and endothelial cells, providing novel insight into host immune responses that contribute to T. pallidum dissemination, immune signaling, and symptoms of syphilis.
2 Methods
2.1 Treponema pallidum culture and sample preparation
In vitro cultures of T. pallidum with Sf1Ep cells were grown as previously described (Edmondson and Norris, 2021). To maintain the integrity of T. pallidum outer membrane proteins, treponemes were dissociated from Sf1Ep cells by incubation with trypsin-free dissociation media for 30 minutes at 34 °C in 1.5% O2, 5% CO2, and 93.5% N2 (Edmondson and Norris, 2021). To prepare Viable Treponema pallidum (VTP) samples (Waugh et al., 2023), Sf1Ep cells were removed by centrifugation at 220 x g, and T. pallidum in the supernatant were quantified by darkfield microscopy (Nikon Eclipse E600; Nikon Canada, Mississauga, Ontario) using a Petroff-Hauser counting chamber (Hauser Scientific, Horsham, PA) and diluted to achieve a multiplicity of infection (MOI) of 30 in 24-well tissue culture plates (Avantor, Radnor, PA). The infection extract control (IEC) was prepared by removing T. pallidum via syringe filtration of the diluted T. pallidum supernatant three times through a 0.22μm PES membrane (Avantor) (Waugh et al., 2023). Successful removal of T. pallidum via filtration was confirmed by darkfield microscopy, with no T. pallidum organisms observed in the filtered extract.
2.2 Mammalian cell culture
THP-1 cells (Cedarlane, Burlington, ON) were grown in RPMI-1640 medium (Cedarlane) supplemented with 10% fetal bovine serum (Corning) and 0.05mM 2-mercaptoethanol (henceforth referred to as ‘THP-1 complete medium’). THP-1 cells were differentiated to achieve a phenotype similar to monocyte-derived macrophages by incubation with 25ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, St. Louis, MO, USA) for 48 hours at 37 °C, 5% CO2, followed by a recovery period of 24 hours in fresh THP-1 complete media. Brain microvascular endothelial cells (hCMEC/d3, Cedarlane) were cultured as previously described (Waugh et al., 2023).
THP-1 cells were plated at a density of 2.4 x 105 cells per well in 24-well tissue culture plates (Avantor) and differentiated into macrophages as described above. Macrophages were then exposed in triplicate to either VTP at a MOI of 30, IEC, or basal media (TpCM2, Edmondson and Norris, 2021) for 5, 12, 24, and 48 hours at 37°C, 1.5% O2, 5% CO2, 93.5% N2. At each timepoint, supernatant sample collection and T. pallidum viability confirmation were performed as previously detailed (Waugh et al., 2023). THP-1 and endothelial cell viability was assessed at 24 and 48 hours by trypan blue staining, with no significant difference in cell viability observed between treatment conditions (data not shown).
2.3 Macrophage and endothelial cell co-culture
hCMEC/d3 endothelial cells were passaged and resuspended in RPMI-1640 medium at a concentration of 2.4 x 105 cells/mL. Similarly, macrophages were lifted and resuspended at a concentration of 2.4 x 105 cells/mL in RPMI-1640 medium. The suspensions of THP-1 cells and HMBECs were then mixed to achieve a THP-1:HMBEC ratio of 1:1, plated in 24-well tissue culture plates at a density of 2.4 x 105 cells/well, and incubated overnight at 37° in 5% CO2 to allow for adherence. Following overnight growth, the RPMI-1640 medium was removed, and cell cultures were exposed to VTP, IEC, or basal TpCM2, with supernatant samples collected at 5, 12, 24, and 48 hours post-exposure.
2.4 Cytokine analysis by cytometric bead arrays
Secretion of the cytokines IL-1β, IL-6, IL-8, IL-10, VEGF, Monocyte Chemoattractant Protein-1 (MCP-1), Regulated upon Activation, Normal T cell Expressed and Secreted (RANTES; CCL5), Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), Tumor Necrosis Factor (TNF), and soluble Intracellular Adhesion Molecule 1 (sICAM-1) was quantified in supernatant samples using cytometric bead array flex sets (BD Biosciences, San Jose, California, USA) according to the manufacturer’s recommendations. For measurement of IL-6, RANTES, and TNF, culture supernatant was diluted 1:10 to ensure the measured cytokine concentrations fell within the standard curve for quantitation; for all other cytokines, neat culture supernatant was used for the assay. Raw data was acquired on a CytoFlex Flow Cytometer (Beckman Coulter, Mississauga, ON) and analyzed using CytExpert software (Beckman Coulter). Statistical analysis was performed using GraphPad Prism 9 by applying one-way ANOVA tests followed by Dunnett’s multiple comparisons to compare the mean of samples exposed to VTP to samples exposed to IEC and basal media. P-values ≤ 0.05 were considered statistically significant.
4 Results
4.1 Treponema pallidum exposure increased secretion of TNF, IL-1β, and IL-6 from macrophages and macrophage-endothelial co-cultures
We measured temporal cytokine secretion from macrophage-differentiated THP-1 cells exposed to T. pallidum, referred to here as “monoculture”, and macrophage-differentiated THP-1 cells in a 1:1 ratio with hCMEC/d3 human brain microvascular endothelial cells (HBMECs) exposed to T. pallidum, referred to here as “co-culture”. Cultures were exposed to either viable T. pallidum (VTP), infection extract control [IEC; to control for background T. pallidum culture components (Waugh et al., 2023)], or basal culture media.
Secretion of the pro-inflammatory cytokines TNF, IL-1β, and IL-6 were elevated in both the VTP-exposed monoculture and co-culture treatments, in comparison to the IEC and basal media control treatments (Figure 1, Supplementary Figures S1–S3). Additionally, TNF secretion increased over time in the VTP-exposed monocultures whereas the VTP-exposed co-cultures displayed decreased TNF secretion over time (Figure 1, Supplementary Figure S1). Secretion of IL-1β increased over time in both the VTP-exposed mono- and co-cultures (Figure 1, Supplementary Figure S2), as did secretion of IL-6 (Figure 1, Supplementary Figure S3). Finally, we observed minimal alteration in secretion of these cytokines in cultures exposed to the IEC and basal media controls, indicating that the secretion of these cytokines is specific to the presence of T. pallidum.
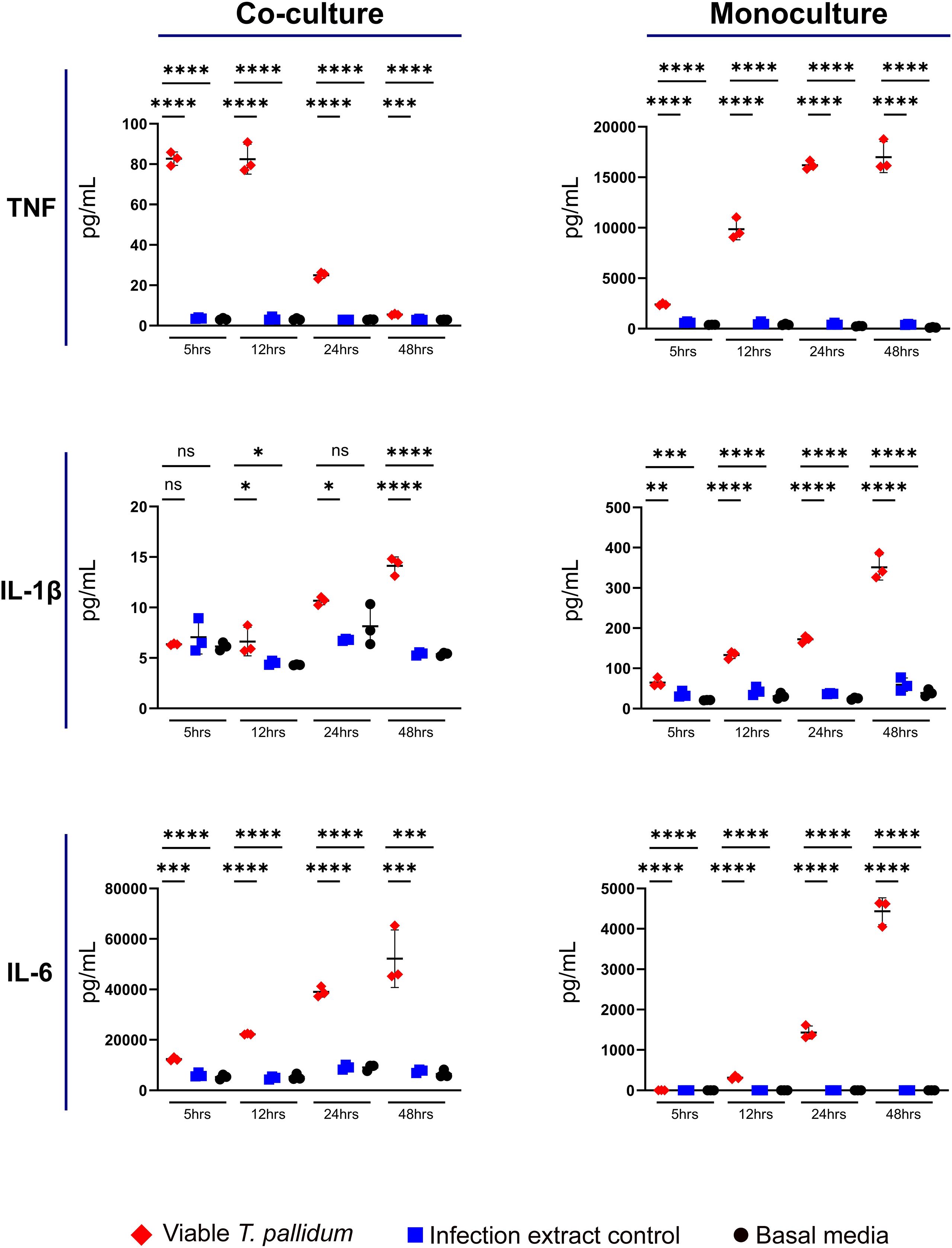
Figure 1. Supernatant concentrations of TNF, IL-1β, and IL-6 from macrophage-differentiated THP-1 cells in monoculture, or 1:1 co-culture with HMBECs, during exposure to T. pallidum (VTP) at an MOI of 30, infection extract control (IEC), or basal media for 5, 12, 24, or 48 hours. Data for each cytokine is representative of three experimental repeats; each replicate is shown in the Supplementary Material. Each timepoint represents a biological replicate, defined as an independent tissue culture well. The mean with standard deviation is shown. Statistical analysis was completed using a one-way ANOVA followed by Dunnetts multiple comparison. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Note the different Y-axis scales between co-cultures and monocultures.
4.2 Secretion of VEGF, RANTES and soluble ICAM-1 is increased during T. pallidum exposure
We observed increased secretion of RANTES (CCL5) and sICAM-1 in both mono- and co-cultures exposed to VTP versus the control treatments, with secretion of VEGF modestly increased under co-culture conditions exposed to VTP (Figure 2; Supplementary Figures S4–S6). Analysis of these cytokines indicated variable levels of secretion in response to IEC-exposed cultures, indicating a non-T. pallidum specific response to components arising from the T. pallidum culture system. We observed RANTES secretion to be increased in VTP-exposed mono- and co-cultures (Figure 2; Supplementary Figure S5), with RANTES secretion increased over time under both culture conditions (Figure 2; Supplementary Figure S5). Secretion of sICAM-1 followed a similar trend, where mono- and co-cultures exposed to VTP secreted significantly greater sICAM-1 in comparison to control conditions, and where the secretion of sICAM-1 increased over time in VTP-exposed cultures (Figure 2; Supplementary Figure S6). Collectively, these data indicate that RANTES, and sICAM-1 are induced by T. pallidum during contact with macrophages and macrophage-endothelial co-cultures. Similar to our previous studies (Waugh et al., 2023), these data indicate the importance of including proper controls when studying T. pallidum-host interactions.
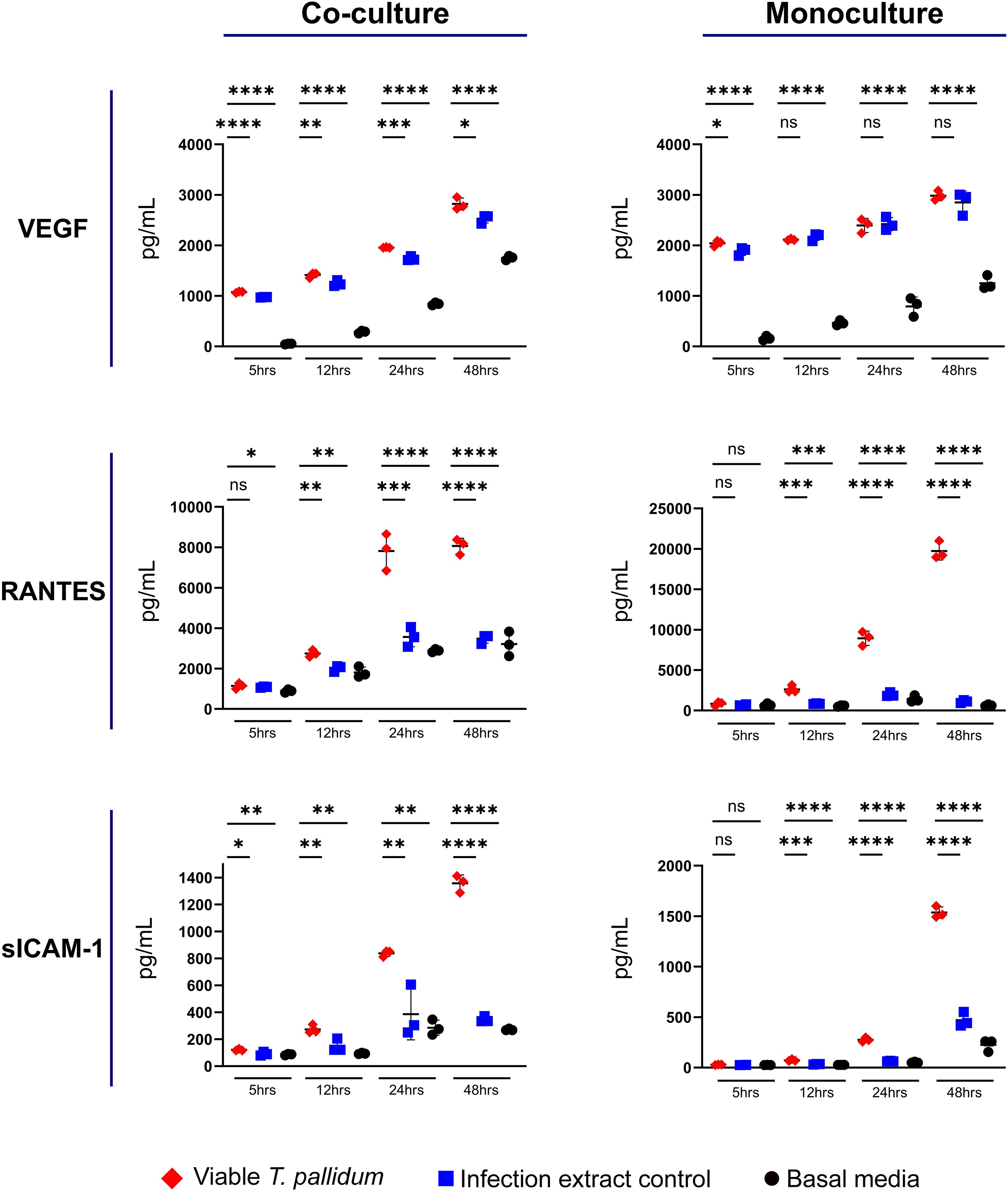
Figure 2. Supernatant concentrations of VEGF, RANTES, and sICAM-1 from macrophage-differentiated THP-1 cells in monoculture, or 1:1 co-culture with HMBECs, during exposure to T. pallidum (VTP) at an MOI of 30, infection extract control (IEC), or basal media for 5, 12, 24, or 48 hours. Data for each cytokine is representative of three experimental repeats; each replicate is shown in the Supplementary Material. Each timepoint represents a biological replicate, defined as an independent tissue culture well. The mean with standard deviation is shown. Statistical analysis was completed using a one-way ANOVA followed by Dunnetts multiple comparison. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Note the different Y-axis scales between co-cultures and monocultures.
4.3 Secretion of monocyte recruiting and activating cytokines is increased during T. pallidum exposure
We observed that VTP exposure increased secretion of MCP-1 and GM-CSF in both the mono- and co-cultures (Figure 3; Supplementary Figures S7, S8). In the monocultures, significant induction of MCP-1 during VTP exposure over controls was not observed until the 24-hour timepoint, indicating the delayed induction of this cytokine by macrophages alone. In the co-cultures, MCP-1 secretion was significantly increased in VTP-exposed samples versus the controls by the 5 and 12 hour timepoints (Figure 3; Supplementary Figure S7). Secretion of GM-CSF demonstrated similar trends, where VTP-exposed cultures increased secretion of GM-CSF over time (Figure 3; Supplementary Figure S8). These data demonstrate that T. pallidum induces MCP-1 and GM-CSF secretion in both macrophage monocultures and macrophage-endothelial co-cultures, an induction that increases over time in both culture conditions.
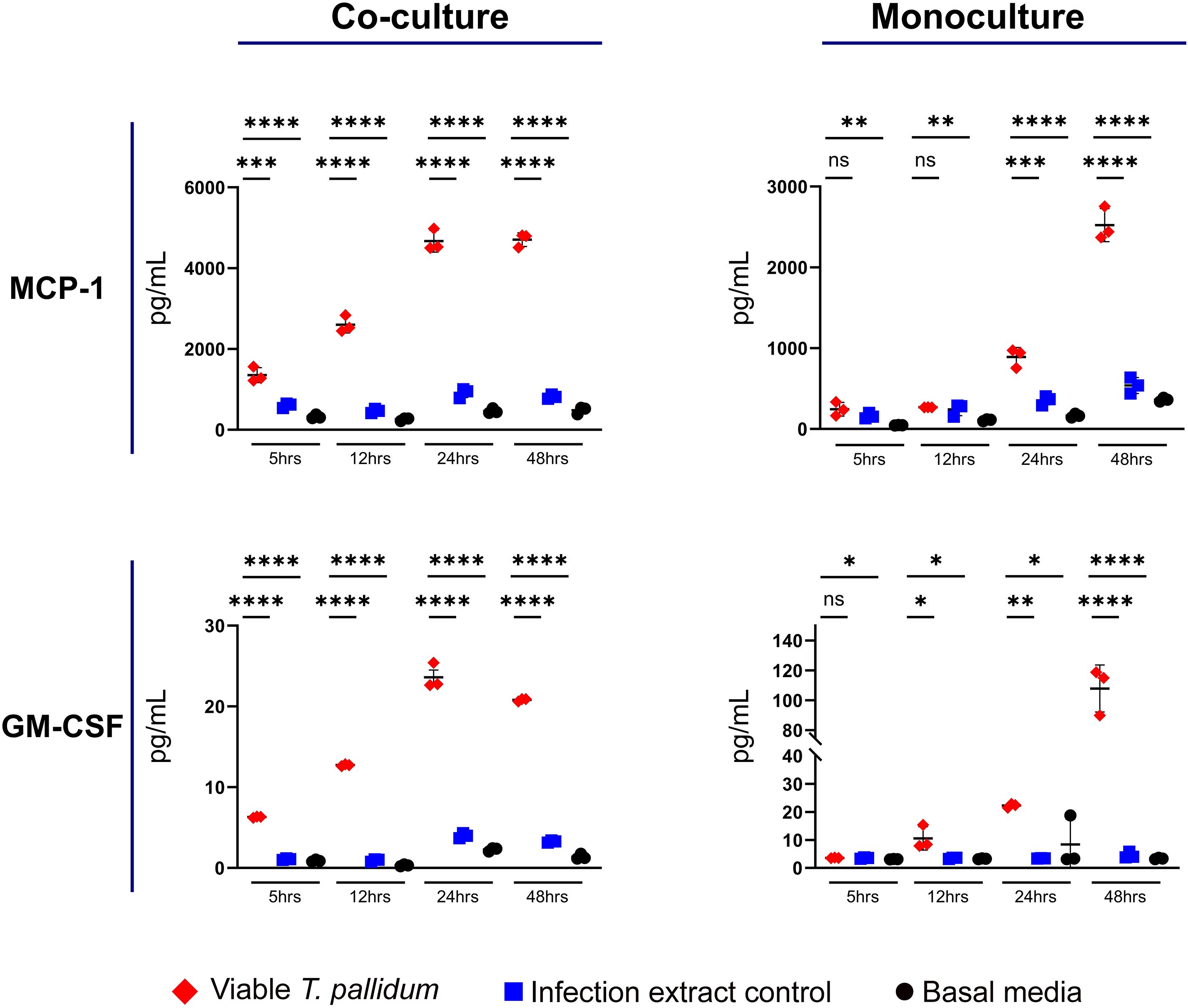
Figure 3. Supernatant concentrations of MCP-1 and GM-CSF from macrophage-differentiated THP-1 cells in monoculture, or 1:1 co-culture with HMBECs, during exposure to T. pallidum (VTP) at an MOI of 30, infection extract control (IEC), or basal media for 5, 12, 24, or 48 hours. Data for each cytokine is representative of three experimental repeats; each replicate is shown in the Supplementary Material. Each timepoint represents a biological replicate, defined as an independent tissue culture well. The mean with standard deviation is shown. Statistical analysis was completed using a one-way ANOVA followed by Dunnetts multiple comparison. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Note the different Y-axis scales between co-cultures and monocultures.
4.4 IL-8 secretion is decreased in macrophages and macrophage-endothelial co-cultures exposed to T. pallidum
We observed that VTP-exposed mono- and co-cultures had reduced secretion of IL-8 relative to both the basal media and IEC control exposures (Figure 4; Supplementary Figure S9). In both the mono- and co-culture systems, the basal media treatment resulted in the greatest secretion of IL-8 (Figure 4; Supplementary Figure S9). These data show that both T. pallidum and components found in the T. pallidum culture system decrease IL-8 secretion in our culture systems, with the presence of viable T. pallidum reducing IL-8 secretion to the greatest extent.
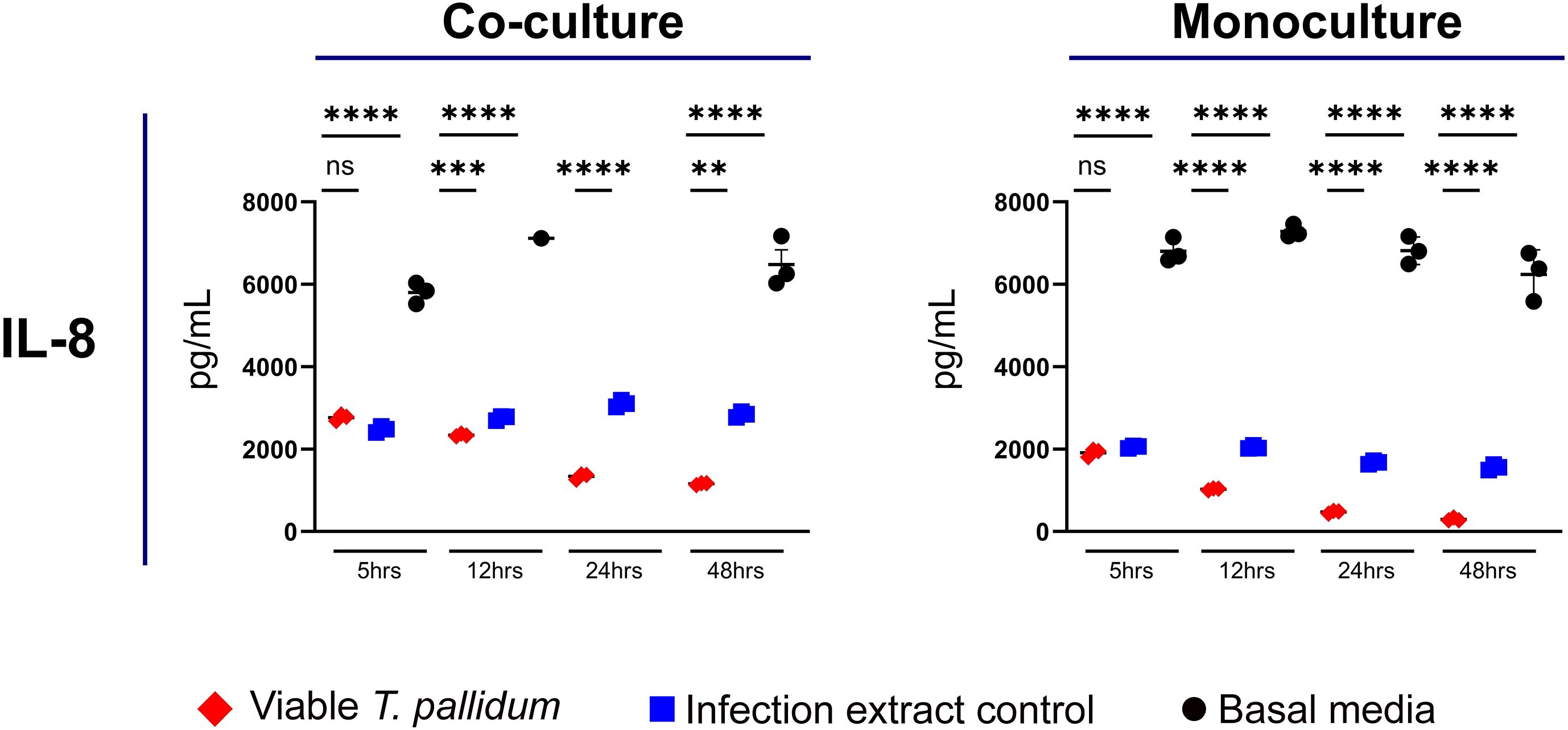
Figure 4. Supernatant concentrations of IL-8 from macrophage-differentiated THP-1 cells in monoculture, or 1:1 co-culture with HMBECs, during exposure to T. pallidum (VTP) at a MOI of 30, infection extract control (IEC), or basal media for 5, 12, 24, or 48 hours. Data for each cytokine in representative of three experimental repeats; each replicate is shown in the Supplementary Material. Each timepoint represents a biological replicate, defined as an independent tissue culture well. The mean with standard deviation is shown. For the basal media-exposed co-cultures, at the 12 hour timepoint two replicates were above the upper limit of quantitation, and at 24 hours all replicates were above the upper limit of quantitation, therefore these datapoints are not shown in the Figure. Statistical analysis was completed using a one-way ANOVA followed by Dunnetts multiple comparison. **p < 0.01, ***p < 0.001, ****p < 0.0001.
5 Discussion
Endothelial cells and macrophages are important cell types that T. pallidum encounters during all stages of infection. Endothelial cells assist in directing innate immune responses, including through recruiting and enabling extravasation of leukocytes (Amersfoort et al., 2022). Further, endothelial cells form the vascular barrier through which T. pallidum traverses during bacterial dissemination to distant locales in the body. Macrophages are an important contributor to clearance of T. pallidum from sites of infection, and both endothelial cells and macrophages undergo bidirectional signaling to influence function and activity in the other cell type. Endothelial-macrophage interactions are involved in regulating vascular homeostasis, angiogenesis, innate immunity, and in bridging adaptive and innate immune responses (Baer et al., 2013; Amersfoort et al., 2022). Despite this diverse array of interactions and functional relevance to T. pallidum infection, understanding of how these two infection-relevant cell types respond on a molecular level to T. pallidum exposure is incomplete. Here we begin to address this knowledge gap by profiling the secretion of a panel of ten cytokines from macrophage-differentiated THP-1 cells in monoculture, and in 1:1 co-culture with human brain microvascular endothelial cells during exposure to viable T. pallidum.
In the current study we observed increased secretion of the pro-inflammatory cytokines TNF, IL-1β, and IL-6 in mono- and co-culture conditions in response to T. pallidum. Previous investigations reported increased TNF and IL-6 secretion in human macrophages (Hawley et al., 2017) and HBMECS (Waugh et al., 2023) during T. pallidum exposure, findings which are supported by this study, thus identifying these cytokines as important regulators of both the endothelial and macrophage innate immune response to T. pallidum. At endothelial sites, IL-6, TNF, and IL-1β downregulate tight junction proteins and promotes junctional relaxation, which may enhance T. pallidum dissemination (Paul et al., 2003; O’Carroll et al., 2015; Blecharz-Lang et al., 2018). Previous studies investigating macrophage cytokine secretion did not report increased IL-1β secretion (Hawley et al., 2017), whereas in the current study IL-1β secretion was increased in T. pallidum-exposed macrophage mono- and co-cultures. The variation in these findings may result from different macrophage sources (Primary vs. THP-1), methods of macrophage activation (IFN-γ vs. PMA), or sampling collection times (8 hours vs. 5–48 hours) (Hawley et al., 2017). Also of interest, in the current study a temporal trend of decreased TNF secretion was observed in co-culture conditions, while increasing TNF secretion was observed over time in monoculture conditions. The reason for this divergent result is not currently understood, but may suggest that endothelial cells have a moderating effect on TNF secretion from macrophages when exposed to T. pallidum.
We also observed increased secretion of VEGF, RANTES, and sICAM-1 in VTP-exposed mono- and co-cultures, relative to the controls, with concentrations increasing over time. Previous studies identified increased secretion and expression of VEGF in HMBECs exposed to T. pallidum (Waugh et al., 2023; Waugh et al., 2025), and VEGF is increased in secondary syphilis lesions (Macaron et al., 2003). However, our study revealed that IEC and basal media exposures also induced significant and variable secretion of these cytokines. These data suggest that secreted T. pallidum components and/or materials resulting from Sf1Ep cell culture influence secretion of these cytokines. Further, prior studies have shown that T. pallidum-exposed macrophages release exosomes that act upon endothelial cells to promote secretion of ICAM-1, Vascular Cell Adhesion Protein 1 (VCAM-1), VEGF, and IL-8 (Xu et al., 2019). Increased levels of VEGF have been observed in angiogenic cutaneous syphilis lesions, suggesting a role for VEGF in remodeling endothelial and tissue sites during infection (Macaron et al., 2003).
Our observation in the current study that macrophages secrete RANTES in response to T. pallidum exposure is of interest considering the involvement of RANTES in recruitment of leukocytes to the vasculature and arrest of monocytes and macrophages at endothelial sites (Baltus et al., 2003). Importantly, RANTES increases blood-brain barrier permeability and endothelial dysfunction (Terao et al., 2008). Therefore, by inference, the high levels of RANTES secretion in VTP-exposed mono- and co-cultures observed in the current study is expected to contribute to endothelial barrier dysfunction. This inflammatory milieu may also enhance T. pallidum traversal across endothelial barriers, including the blood-brain barrier. Previous reports show that T. pallidum and T. pallidum-derived proteins induce expression of the RANTES receptor, C-C chemokine receptor type 5 (CCR5), in peripheral blood mononuclear cells (Sellati et al., 2000). Interestingly, increased expression of both RANTES and CCR5 facilitate the pathogenesis of Human Immunodeficiency Virus (HIV) due to the role of CCR5 as a HIV co-receptor on both macrophages and T cells (Gornalusse et al., 2015). Treponema pallidum-induced secretion of RANTES may facilitate the development of a favorable endothelial microenvironment for HIV to persist and infect both T cells and macrophages in individuals infected with T. pallidum, offering a potential explanation for the frequent incidence of syphilis-HIV co-infections.
sICAM-1 is an inflammatory biomarker expressed as a spliced isoform of membrane-bound ICAM-1 and created as a product of proteolytic cleavage of ICAM-1 (King et al., 1995; Bui et al., 2020). sICAM-1 is released by activated endothelial cells and macrophages, particularly in response to IL-6 and TNF stimulation (Bui et al., 2020). Increased secretion of sICAM-1 is implicated in multiple inflammatory diseases of infectious and non-infectious origin (Bui et al., 2020), including sepsis (Hedetoft et al., 2021). Further, increased sICAM-1 secretion has been shown to promote angiogenesis, enhance endothelial motility, and inhibit leukocyte-endothelial interactions (Rieckmann et al., 1995), with increased serum concentrations of sICAM-1 documented in blood-cerebrospinal barrier dysfunction and acute bacterial meningitis (Rieckmann et al., 1993). Thus, the consequence of increased sICAM-1 in the context of a syphilis infection may include attenuating endothelial-macrophage interactions and contributing to an inflammatory response that disrupts endothelial barrier integrity. Other neuroinvasive bacteria, including Streptococcus suis, have been shown to induce secretion of sICAM-1 (Grenier and Bodet, 2008), suggesting possible convergent mechanisms of endothelial barrier disruption between T. pallidum and other neuroinvasive pathogens.
Induction of secretion of MCP-1 and GM-CSF was observed in both VTP-exposed mono- and co-cultures, a situation which would assist with recruiting and activating monocytes at sites of infection. Conversely, previously we have shown reduced MCP-1 secretion in IL-32γ-activated macrophages exposed to T. pallidum-derived proteins (Houston et al., 2022) and brain endothelial cells exposed to T. pallidum (Waugh et al., 2023). These divergent results highlight the importance of environmental context, with immune responses to T. pallidum anticipated to be influenced by the presence of co-localized immune cells and direct pathogen-host cell engagement.
In this study mono- and co-cultures exposed to T. pallidum had significantly reduced IL-8 secretion compared to controls, while our previous investigations found increased IL-8 secretion by T. pallidum-exposed brain endothelial cells cultured in the absence of macrophages (Waugh et al., 2023). Similarly, IL-8 secretion was observed to increase in our previous investigation of IL-32γ-activated macrophages exposed to T. pallidum proteins (Houston et al., 2022), as well as a prior study conducted by Pozzobon et al. investigating human umbilical vein endothelial cells exposed to a recombinant T. pallidum protein (Pozzobon et al., 2016). These data suggest that the extent of expression of this cytokine can be influenced by multiple factors, including direct T. pallidum engagement rather than the use of recombinant T. pallidum proteins, the methodology used for macrophage activation, and the presence of additional host cell types.
Here we report time course secretion profiles of THP-1 macrophages alone and in 1:1 co-culture with HMBECs during exposure to T. pallidum. To our knowledge, this is the first study to explore in vitro macrophage-endothelial co-cultures in the presence of T. pallidum. While our study expands knowledge on host responses to T. pallidum beyond conventional single host cell conditions, it has limitations. These include the use of PMA to activate THP-1 cells, which results in differentiation of macrophages into an M0 lineage (Tedesco et al., 2018). This macrophage phenotype may exhibit non-biologically relevant effects, exemplified by the observed high baseline secretion of IL-8 observed in the current study and previously reported in PMA-treated THP-1 cells (Kämpfer et al., 2017). An additional limitation is that equal numbers of endothelial cells and macrophages were seeded in the co-culture conditions, thus resulting in a higher number of macrophages seeded in the monoculture conditions. These experimental conditions were required to ensure sufficient cell numbers in the plate wells, and consistent total cell numbers between conditions. Therefore, in this study we did not make direct comparisons of cytokine secretions between monocultures and co-cultures. Further, our in vitro model will not provide a comprehensive readout of the immune response to T. pallidum, since during an in vivo infection additional cell types and inflammatory mediators are present. Also, in our experimental design we are unable to exclude secreted T. pallidum components and products from lysed treponemes in our infection extract control, which may affect the interpretation of our results. However, despite these limitations the studies presented here expand on previous findings detailing increased secretion of IL-6 and TNF (Hawley et al., 2017) and report the secretion of RANTES, sICAM-1, IL-1β, GM-CSF, and MCP-1 in macrophages and macrophage-endothelial co-cultures during T. pallidum exposure. These findings further our understanding of temporal cytokine responses to T. pallidum and identify immune signaling factors induced during the host immune response to T. pallidum infection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
SW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. LR: Conceptualization, Formal Analysis, Investigation, Writing – review & editing. CC: Conceptualization, Formal Analysis, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants R37AI051334, U19AI144133, and U01AI182035 (CC) from the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH), as well as awards from Open Philanthropy (52345) and CIHR (506704 and 471857) to CC. SW is the recipient of a CIHR Canada Graduate Scholarship-Doctoral (CGS-D).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1681813/full#supplementary-material
References
Aho, J., Lybeck, C., Tetteh, A., Issa, C., Kouyoumdjian, F., Wong, J., et al. (2022). Rising syphilis rates in Canada, 2011–2020. Can. Commun. Dis. Rep. 48, 52–60. doi: 10.14745/ccdr.v48i23a01
Amersfoort, J., Eelen, G., and Carmeliet, P. (2022). Immunomodulation by endothelial cells — partnering up with the immune system? Nat. Rev. Immunol. 22, 576–588. doi: 10.1038/s41577-022-00694-4
(2023). National Overview of STDs, 2021. Available online at: https://www.cdc.gov/std/statistics/2021/overview.htm (Accessed August 10, 2023).
Baer, C., Squadrito, M. L., Iruela-Arispe, M. L., and De Palma, M. (2013). Reciprocal interactions between endothelial cells and macrophages in angiogenic vascular niches. Exp. Cell Res. 319, 1626–1634. doi: 10.1016/j.yexcr.2013.03.026
Baker-Zander, S. A. and Lukehart, S. A. (1992). Macrophage-mediated killing of opsonized Treponema pallidum. J. Infect. Dis. 165, 69–74. doi: 10.1093/infdis/165.1.69
Baltus, T., Weber, K. S. C., Johnson, Z., Proudfoot, A. E. I., and Weber, C. (2003). Oligomerization of RANTES is required for CCR1-mediated arrest but not CCR5-mediated transmigration of leukocytes on inflamed endothelium. Blood 102, 1985–1988. doi: 10.1182/blood-2003-04-1175
Bergers, G., Brekken, R., McMahon, G., Vu, T. H., Itoh, T., Tamaki, K., et al. (2000). Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2, 737–744. doi: 10.1038/35036374
Blecharz-Lang, K. G., Wagner, J., Fries, A., Nieminen-Kelhä, M., Rösner, J., Schneider, U. C., et al. (2018). Interleukin 6-mediated endothelial barrier disturbances can be attenuated by blockade of the IL6 receptor expressed in brain microvascular endothelial cells. Transl. Stroke Res. 9, 631–642. doi: 10.1007/s12975-018-0614-2
Brinkman, M. B., McGill, M. A., Pettersson, J., Rogers, A., Matějková, P., Šmajs, D., et al. (2008). A novel Treponema pallidum antigen, TP0136, is an outer membrane protein that binds human fibronectin. Infect. Immun. 76, 1848–1857. doi: 10.1128/IAI.01424-07
Bui, T. M., Wiesolek, H. L., and Sumagin, R. (2020). ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 108, 787–799. doi: 10.1002/JLB.2MR0220-549R
Cameron, C. E. (2003). Identification of a Treponema pallidum laminin-binding protein. Infect. Immun. 71, 2525–2533. doi: 10.1128/IAI.71.5.2525-2533.2003
Cameron, C. E., Brown, E. L., Kuroiwa, J. M. Y., Schnapp, L. M., and Brouwer, N. L. (2004). Treponema pallidum fibronectin-binding proteins. J. Bacteriol 186, 7019–7022. doi: 10.1128/JB.186.20.7019-7022.2004
Carlson, J. A., Dabiri, G., Cribier, B., and Sell, S. (2011). The immunopathobiology of syphilis: the manifestations and course of syphilis are determined by the level of delayed-type hypersensitivity. Am. J. Dermatopathol 33, 433–460. doi: 10.1097/DAD.0b013e3181e8b587
Chen, T., Wan, B., Wang, M., Lin, S., Wu, Y., and Huang, J. (2023). Evaluating the global, regional, and national impact of syphilis: results from the global burden of disease study 2019. Sci. Rep. 13, 11386. doi: 10.1038/s41598-023-38294-4
Edmondson, D. G. and Norris, S. J. (2021). In vitro cultivation of the syphilis spirochete Treponema pallidum. Curr. Protoc. 1, e44. doi: 10.1002/cpz1.44
Giacani, L., Molini, B. J., Kim, E. Y., Godornes, B. C., Leader, B. T., Tantalo, L. C., et al. (2010). Antigenic Variation in Treponema pallidum: TprK Sequence Diversity Accumulates in Response to Immune Pressure during Experimental Syphilis. J. Immunol. 184, 3822–3829. doi: 10.4049/jimmunol.0902788
Gornalusse, G. G., Mummidi, S., Gaitan, A. A., Jimenez, F., Ramsuran, V., Picton, A., et al. (2015). Epigenetic mechanisms, T-cell activation, and CCR5 genetics interact to regulate T-cell expression of CCR5, the major HIV-1 coreceptor. Proc. Natl. Acad. Sci. 112, E4762–E4771. doi: 10.1073/pnas.1423228112
Grenier, D. and Bodet, C. (2008). Streptococcus suis stimulates ICAM-1 shedding from microvascular endothelial cells. FEMS Immunol. Med. Microbiol. 54, 271–276. doi: 10.1111/j.1574-695X.2008.00476.x
Hawley, K. L., Cruz, A. R., Benjamin, S. J., La Vake, C. J., Cervantes, J. L., LeDoyt, M., et al. (2017). IFNγ Enhances CD64-potentiated phagocytosis of Treponema pallidum opsonized with human syphilitic serum by human macrophages. Front. Immunol. 8. doi: 10.3389/fimmu.2017.01227
Hedetoft, M., Moser, C., Jensen, PØ, Vinkel, J., and Hyldegaard, O. (2021). Soluble ICAM-1 is modulated by hyperbaric oxygen treatment and correlates with disease severity and mortality in patients with necrotizing soft-tissue infection. J. Appl. Physiol. 130, 729–736. doi: 10.1152/japplphysiol.00844.2020
Houston, S., Schovanek, E., Conway, K. M. E., Mustafa, S., Gomez, A., Ramaswamy, R., et al. (2022). Identification and functional characterization of peptides with antimicrobial activity from the syphilis spirochete, Treponema pallidum. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.888525
Kämpfer, A. A. M., Urbán, P., Gioria, S., Kanase, N., Stone, V., and Kinsner-Ovaskainen, A. (2017). Development of an in vitro co-culture model to mimic the human intestine in healthy and diseased state. Toxicol. In Vitro 45, 31–43. doi: 10.1016/j.tiv.2017.08.011
King, P. D., Sandberg, E. T., Selvakumar, A., Fang, P., Beaudet, A. L., and Dupont, B. (1995). Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative RNA splicing. J. Immunol. 154, 6080–6093. doi: 10.4049/jimmunol.154.11.6080
Korenromp, E. L., Rowley, J., Alonso, M., Mello, M. B., Wijesooriya, N. S., Mahiané, S. G., et al. (2019). Global burden of maternal and congenital syphilis and associated adverse birth outcomes—Estimates for 2016 and progress since 2012. PLoS One 14, e0211720. doi: 10.1371/journal.pone.0211720
LaFond, R. E. and Lukehart, S. A. (2006). Biological basis for syphilis. Clin. Microbiol. Rev. 19, 29–49. doi: 10.1128/CMR.19.1.29-49.2006
Leader, B. T., Hevner, K., Molini, B. J., Barrett, L. K., Van Voorhis, W. C., and Lukehart, S. A. (2003). Antibody responses elicited against the Treponema pallidum repeat proteins differ during infection with different isolates of Treponema pallidum subsp. pallidum. Infect. Immun. 71, 6054–6057. doi: 10.1128/IAI.71.10.6054-6057.2003
Lemichez, E., Lecuit, M., Nassif, X., and Bourdoulous, S. (2010). Breaking the wall: targeting of the endothelium by pathogenic bacteria. Nat. Rev. Microbiol. 8, 93–104. doi: 10.1038/nrmicro2269
Lithgow, K. V., Church, B., Gomez, A., Tsao, E., Houston, S., Swayne, L. A., et al. (2020). Identification of the neuroinvasive pathogen host target, LamR, as an endothelial receptor for the Treponema pallidum adhesin Tp0751. mSphere 5, e00195–e00120. doi: 10.1128/mSphere.00195-20
Lithgow, K. V., Tsao, E., Schovanek, E., Gomez, A., Swayne, L. A., and Cameron, C. E. (2021). Treponema pallidum disrupts VE-cadherin intercellular junctions and traverses endothelial barriers using a cholesterol-dependent mechanism. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.691731
Lukehart, S. A. and Miller, J. N. (1978). Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J. Immunol. 121, 2014–2024. doi: 10.4049/jimmunol.121.5.2014
Lukehart, S. A., Shaffer, J. M., and Baker-Zander, S. A. (1992). A subpopulation of Treponema pallidum is resistant to phagocytosis: possible mechanism of persistence. J. Infect. Dis. 166, 1449–1453. doi: 10.1093/infdis/166.6.1449
Macaron, N. C., Cohen, C., Chen, S. C., and Arbiser, J. L. (2003). Cutaneous lesions of secondary syphilis are highly angiogenic. J. Am. Acad. Dermatol. 48, 878–881. doi: 10.1067/mjd.2003.504
O’Carroll, S. J., Kho, D. T., Wiltshire, R., Nelson, V., Rotimi, O., Johnson, R., et al. (2015). Pro-inflammatory TNFα and IL-1β differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J. Neuroinflamm. 12:131. doi: 10.1186/s12974-015-0346-0
Paul, R., Koedel, U., Winkler, F., Kieseier, B. C., Fontana, A., Kopf, M., et al. (2003). Lack of IL-6 augments inflammatory response but decreases vascular permeability in bacterial meningitis. Brain J. Neurol. 126, 1873–1882. doi: 10.1093/brain/awg171
Pozzobon, T., Facchinello, N., Bossi, F., Capitani, N., Benagiano, M., Di Benedetto, G., et al. (2016). Treponema pallidum (syphilis) antigen TpF1 induces angiogenesis through the activation of the IL-8 pathway. Sci. Rep. 6, 18785. doi: 10.1038/srep18785
Reid, T. B., Molini, B. J., Fernandez, M. C., and Lukehart, S. A. (2014). Antigenic variation of tprK facilitates development of secondary syphilis. Infect. Immun. 82, 4959–4967. doi: 10.1128/iai.02236-14
Rieckmann, P., Michel, U., Albrecht, M., Brück, W., Wöckel, L., and Felgenhauer, K. (1995). Soluble forms of intercellular adhesion molecule-1 (ICAM-1) block lymphocyte attachment to cerebral endothelial cells. J. Neuroimmunol 60, 9–15. doi: 10.1016/0165-5728(95)00047-6
Rieckmann, P., Nünke, K., Burchhardt, M., Albrecht, M., Wiltfang, J., Ulrich, M., et al. (1993). Soluble intercellular adhesion molecule-1 in cerebrospinal fluid: an indicator for the inflammatory impairment of the blood-cerebrospinal fluid barrier. J. Neuroimmunol 47, 133–140. doi: 10.1016/0165-5728(93)90023-r
Riley, B. S., Oppenheimer-Marks, N., Hansen, E. J., Radolf, J. D., and Norgard, M. V. (1992). Virulent Treponema pallidum activates human vascular endothelial cells. J. Infect. Dis. 165, 484–493. doi: 10.1093/infdis/165.3.484
Sellati, T. J., Wilkinson, D. A., Sheffield, J. S., Koup, R. A., Radolf, J. D., and Norgard, M. V. (2000). Virulent Treponema pallidum, lipoprotein, and synthetic lipopeptides induce CCR5 on human monocytes and enhance their susceptibility to infection by human immunodeficiency virus type 1. J. Infect. Dis. 181, 283–293. doi: 10.1086/315209
Spiteri, G., Unemo, M., Mårdh, O., and Amato-Gauci, A. J. (2019). The resurgence of syphilis in high-income countries in the 2000s: a focus on Europe. Epidemiol. Infect. 147, e143. doi: 10.1017/S0950268819000281
Tedesco, S., De Majo, F., Kim, J., Trenti, A., Trevisi, L., Fadini, G. P., et al. (2018). Convenience versus biological significance: are PMA-differentiated THP-1 cells a reliable substitute for blood-derived macrophages when studying in vitro polarization? Front. Pharmacol. 9. doi: 10.3389/fphar.2018.00071
Terao, S., Yilmaz, G., Stokes, K. Y., Russell, J., Ishikawa, M., Kawase, T., et al. (2008). Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation and tissue injury following focal ischemia-reperfusion. Stroke J. Cereb Circ. 39, 2560–2570. doi: 10.1161/STROKEAHA.107.513150
Tomlin, H. and Piccinini, A. M. (2018). A complex interplay between the extracellular matrix and the innate immune response to microbial pathogens. Immunology 155, 186–201. doi: 10.1111/imm.12972
Waugh, S., Goodyear, M. C., Gomez, A., Ranasinghe, A., Lithgow, K. V., Falsafi, R., et al. (2025). Time-course transcriptomics reveals the impact of Treponema pallidum on microvascular endothelial cell function and phenotype. Front. Microbiol. 16. doi: 10.3389/fmicb.2025.1649738
Waugh, S., Ranasinghe, A., Gomez, A., Houston, S., Lithgow, K. V., Eshghi, A., et al. (2023). Syphilis and the host: multi-omic analysis of host cellular responses to Treponema pallidum provides novel insight into syphilis pathogenesis. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1254342
World Health Organization (2024). Implementing the global health sector strategies on HIV, viral hepatitis and sexually transmitted infections, 2022–2030 |report on progress and gaps 2024. Available online at: https://www.who.int/publications/i/item/9789240094925 (Accessed September 5, 2024).
Wu, F., Zhang, J.-P., and Wang, Q.-Q. (2017). Scanning electron microscopy of the adhesion of Treponema pallidum subspecies pallidum (Nichol strain) to human brain microvascular endothelial cells. vitro. J. Eur. Acad. Dermatol. Venereol JEADV 31, e221–e223. doi: 10.1111/jdv.13984
Keywords: syphilis, immunology, pathogenesis, macrophages, endothelial cells, Treponema pallidum
Citation: Waugh S, Ranasinghe A, Reynolds LA and Cameron CE (2025) Treponema pallidum induces pro-inflammatory cytokine secretion in macrophages and macrophage-endothelial co-cultures. Front. Cell. Infect. Microbiol. 15:1681813. doi: 10.3389/fcimb.2025.1681813
Received: 07 August 2025; Accepted: 06 October 2025;
Published: 17 October 2025.
Edited by:
Artem Rogovsky, Michigan State University, United StatesReviewed by:
Timothy Casselli, University of South Alabama, United StatesLinda Grillova, Wellcome Sanger Institute (WSI), United Kingdom
Copyright © 2025 Waugh, Ranasinghe, Reynolds and Cameron. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caroline E. Cameron, Y2Fyb2NAdXZpYy5jYQ==
†These authors share first authorship
 Sean Waugh
Sean Waugh Akash Ranasinghe
Akash Ranasinghe Lisa A. Reynolds
Lisa A. Reynolds Caroline E. Cameron
Caroline E. Cameron