- 1Department of Laboratory Medicine, the Second Affiliated Hospital of Xuzhou Medical University, Xuzhou, China
- 2Department of Laboratory Medicine, The General Hospital of Xuzhou Mining Group, Xuzhou, China
- 3Department of Laboratory Medicine, Chongqing General Hospital, Chongqing, China
- 4Department of Laboratory Medicine, Affiliated Hospital of Xuzhou Medical University, Xuzhou, China
- 5School of Medical Technology, Xuzhou Medical University, Xuzhou, China
- 6The Center for Clinical Research and Transformation of Pathogen Diagnosis, Xuzhou Medical University, Xuzhou, China
Background: Repeat urine testing is key for the management of candiduria. Current urine testing methods are time-consuming. This study presents an assay coupling PCR with matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (MS), termed MALDI-TOF nucleic acid MS (MALDI-TOF NAMS), for rapid detection of five medically important Candida species (Candida albicans, Candida tropicalis, Candida glabrata, Candida parapsilosis and Candida krusei) in urine.
Methods: Species-specific primers and probes targeting the internal transcribed spacer (ITS) regions the five Candida species were designed, followed by PCR amplification, single-base extension, and MALDI-TOF MS analysis. The sensitivity assessment was conducted using Candida suspensions and simulated candiduria samples. DNA from bacteria and other fungi was tested to analyse the specificity. To evaluate multiplex detection capability, all five Candida species were simultaneously tested in a single-tube reaction. Clinical performance was validated using collected urine samples, with results compared with urine culture.
Results: The developed assay demonstrated a limit of detection (LoD) ranging from 101 CFU/mL to 103 CFU/mL across different Candida species, with no cross-reactivity against common uropathogens. The assay allowed simultaneous detection of multiple Candida species in a single tube. The diagnostic sensitivity of our developed method was 100% using urine culture as the gold standard, with a diagnostic specificity of 98.7%. The entire detection process could be completed within 5 hours.
Conclusion: The novel MALDI-TOF NAMS assay enables rapid detection of five Candida species in urine with validated performance. The assay supports clinical candiduria management and is adaptable for broader Candida species detection.
1 Introduction
Candiduria is defined as the presence of Candida species in the urine. Candiduria is commonly observed among hospitalized patients, particularly those admitted to intensive care units (ICUs) (He et al., 2021b). Additional risk factors include older age, female sex, diabetes mellitus, recent antibiotic use, urinary tract obstruction or instrumentation (e.g., surgery), and indwelling urinary catheterization (Kauffman, 2014; He et al., 2021b). Candida albicans was the most common infectious agent causing candiduria, accounting for 50% to 70% of isolates (Alvarez-Lerma et al., 2003; Bougnoux et al., 2008; Kauffman, 2014). Besides, there was a shift towards non-albicans Candida, such as Candida glabrata, Candida tropicalis, Candida parapsilosis and Candida krusei (Gharaghani et al., 2018; Aghili et al., 2020).
The detection of Candida species from urine poses a clinical challenge, as its significance varies from clinically irrelevant contamination or benign colonization to pathogenic urinary tract infections (UTI), each requiring distinct management approaches (Kauffman, 2014; Alfouzan and Dhar, 2017; García-Agudo et al., 2018; He et al., 2021b). As an example, in cases of suspected Candida UTI, further species identification is clinically imperative because many isolates of Candida glabrata and all isolates of Candida krusei are resistant to the first-line antifungal agent (Dias, 2020). Repeat urine testing serves as a critical step to determining the origin of candiduria (Kauffman, 2014; García-Agudo et al., 2018). For instance, an initial repeat clean-catch urine test was suggested to rule out contamination, and a repeat urine test after correcting the predisposing factors (e.g. stopping antibiotics, removing catheter) to assess efficacy (Kauffman, 2014). At present, urine culture is the predominant method used for repeat candiduria testing. However, the process is time-consuming and results are usually not available for several days, making timely management of candiduria difficult.
Molecular biology-based pathogen detection techniques offer potential solutions to this limitation. Polymerase chain reaction (PCR) and its derived techniques, such as quantitative real-time PCR (qPCR), are widely employed for the rapid detection of certain Candida from clinical samples (Lima et al., 2017; Walchak et al., 2020; Hernández-Carreón et al., 2021). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is another clinically widely adopted technique for pathogen identification, requiring prior culture. The MALDI-TOF nucleic acid MS (MALDI-TOF NAMS) an emerging analytical technique that integrates PCR amplification with MALDI-TOF MS detection. It mainly includes four procedures: (1) locus-specific PCR reaction; (2) shrimp alkaline phosphatase (SAP) treatment to inactivate dNTPs; (3) probe extension reaction; (4) detection of the probe by MS. The probe, an oligonucleotide primer, anneals the sequence in amplified target loci and extends with a single complementary base; the mass signal of the extended probe could be depicted as a peaks at specific position in the spectrum, enabling the culture-independent microorganism identification (Gabriel et al., 2009). Shuai et al. have developed a multiplex MALDI-TOF NAMS method which enables fourteen porcine viruses to be identified at the same time (Shuai et al., 2024). Moreover, the technique has been employed for both species identification within the Mycobacterium tuberculosis complex (MTBC) and rapid diagnosis of drug-resistant pulmonary tuberculosis through simultaneous analysis of multiple single nucleotide polymorphisms (SNPs) (Bouakaze et al., 2011; Shi et al., 2025). However, the clinical application of MALDI-TOF NAMS for the detection of fungal pathogens, particularly Candida species, has not yet reported in the literature.
Currently, the ribosomal RNA (rRNA) coding genes (rDNA) have been widely recognized as optimal target loci for pathogen detection and identification using PCR-based molecular tools. The rDNA sequences demonstrate significant evolutionary conservation among diverse Candida species (Raja et al., 2017). In our previous study, a universal primers/probe system targeting the 5.8S rDNA region was successfully developed, allowing accurate quantification of a wide range of Candida species (Guo et al., 2016). In contrast, the internal transcribed spacer (ITS) regions flanking the rDNA exhibit the higher variation, and are useful for species-level identification (Raja et al., 2017).
Thus, in this study, we developed a MALDI-TOF NAMS assay with specific primers/probe systems targeting ITS regions, allowing the detection of five common Candida species in urine samples, including Candida albicans, Candida tropicalis, Candida glabrata, Candida parapsilosis and Candida krusei. Our method not only provide laboratory basis for the optimal management of candiduria, but also lays the groundwork for the future development of a multiplex detection platform targeting more Candida species.
2 Materials and methods
2.1 Microbial strains and urine specimens
Strains used in this study were classified as reference strains and clinical isolates. Two reference Candida species (Candida albicans BNCC337321, Candida tropicalis BNCC334135) were were purchased from BeNa Culture Collection (BNCC), while the other three (Candida glabrata ATCC90030, Candida parapsilosis ATCC22019, Candida krusei ATCC6258) were obtained from the American Type Culture Collection (ATCC). Clinical strains were recovered from Department of Clinical Microbiology of the Affiliated Hospital of Xuzhou Medical University, including the prevalent pathogens in UTI (Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, Enterococcus faecalis, Enterococcus faecium, Proteus mirabilis, Pseudomonas aeruginosa and Staphylococcus aureus) and other isolated fungi (Aspergillus flavus, Aspergillus fumigatus, Cryptococcus neoformans, Meyerozyma guilliermondii, Trichosporon asahii, Candida rugosa, Candida metapsilosis, Candida orthopsilosis and Candida dubliniensis).
Whole blood and clean-catch midstream urine specimens were collected from healthy volunteers at Xuzhou Medical University’s Health Examination Center, with the urine specimens confirmed pathogen-free by culture for the simulated candiduria study. To evaluate clinical performance, urine specimens were collected from hospitalized patients between June and September 2024. The study protocol was approved by the Institutional Review Board of the Affiliated Hospital of Xuzhou Medical University (Approval No.: XYFY2024-KL178-01).
2.2 Sample preparation
Reference strains of Candida were inoculated onto Sabouraud dextrose agar (SDA) plates and cultured at 28°C for 48h. The isolated colonies were suspended in sterile double-distilled water, and the concentration was adjusted using an improved Neubauer hemocytometer. Ten-fold serial dilution was performed to obtain suspensions ranging from 106 CFU/mL to 100 CFU/mL for limit of detection (LoD) determination. In addition, 100 μL serial dilutions (107 CFU/mL to 101 CFU/mL) of each Candida species were spiked with 900 μL sterile urine from the same healthy volunteer to simulate candiduria (106 CFU/mL to 100 CFU/mL). For specificity assessment, the sterile double-distilled water were spiked with clinical isolates of bacteria and fungi, as described in “1. Microbial strains and urine specimens”.
2.3 DNA extraction
The prepared samples or clinical specimens were centrifuged at 12,000 rpm for 5min, and the supernatant was discarded. The pellet was resuspended in 100 μL of nucleic acid extraction buffer (Hybribio, China, Cat. No. HBRT-23) and gently mixed. DNA was then released using a thermal shock method, which involved three cycles of heating at 100°Cfor 5min followed by freezing at -80°Cfor 5min. After centrifugation, the supernatant containing crude DNA extract was collected for downstream analysis. Human genomic DNA was extracted from the whole blood samples according to the manufacturer’s instructions of the commercial kit (TIANGEN, China, Cat. No. DP348-02).
2.4 Design of the primers and probes
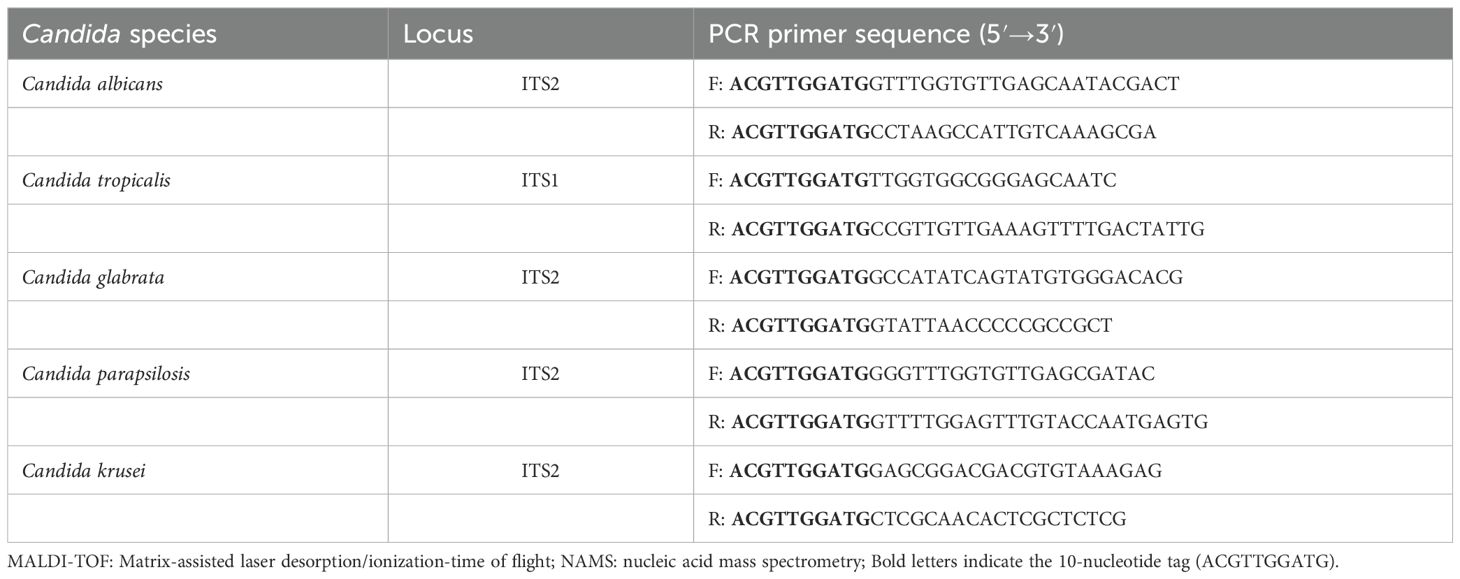
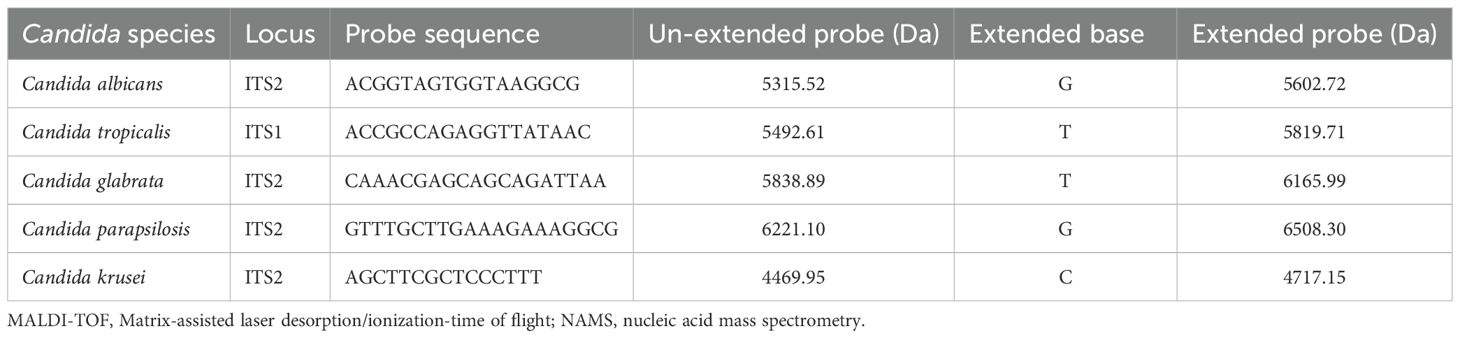
The rDNA sequences of different strains from the five Candida species were downloaded from the Nucleotide database in NCBI, with the requirement that all entries include complete and uninterrupted ITS1 and ITS2 regions. After importing the sequences into DNAMAN for multiple alignment, we retained the conserved regions in ITS1 or ITS2 that showed no variation within species. The selected segments were subsequently loaded into Primer Premier 6 software for the design of primers and probes. This mass-based detection method requires a minimum 30 Da mass difference between between probes and their extension products. In addition, to prevent detection interference from primers, each PCR primer required 5’-terminal incorporation of a 10-nucleotide tag (ACGTTGGATG). The sequences of the primers and probes were given in Tables 1, 2.
2.5 PCR reaction
The PCR was performed using the primers described in Section 4 (“Design of the Primers and Probes”) prior to MS analysis. For the PCR reaction, 5 μL purified DNA extract was mixed with 3μL double-distilled water, 1.5μL each of the forward and reverse primers (0.5μM) (Sangon, China), 7 μL reaction buffer and 2μL enzyme (from Zybio MALDI-TOF NAMS preparation kit, China, Cat. No. 01.09.67.01.04.01). The program was run as follows: (1) UNG digestion at 50°C for 2min to prevent carryover contamination; (2) pre-denaturation at 95°C for 5min; (3) 45 cycles of denaturation at 95°C for 10 s, annealing at 58°C for 15 s, and extension at 72°C for 20 s; (4) a final extension at 72°C for 3min. The products were purified using the TIANquick Mini Purification Kit (TIANGEN, China; Cat. No. DP203).
2.6 Single-base extension reaction
To dephosphorylate residual dNTPs, 2 µL of shrimp alkaline phosphatase (SAP) and 1µL of reaction buffer (Zybio, China, Cat. No. 01.09.67.01.04.01) were added to the purified PCR products. The mixture was incubated at 37°C for 30 minutes, followed by 85°C for 5 minutes.
For the single-base extension reaction, the products were mixed with 1.5 μL probes (as mentioned in the Section 4), 1.5 μL double-distilled water, 2μL extension buffer and 2 μL extension enzyme (Zybio, China, Cat. No. 01.09.67.01.04.01). After initial denaturation (95°C, 30s), 40 cycles were performed, each comprising: (1) denaturation at 95°C for 5s; (2) 5 iterations of annealing/extension (55°C/80°C, 5s each). Then, the final extension was conducted at 72°C for 3min.
2.7 MALDI-TOF MS analysis
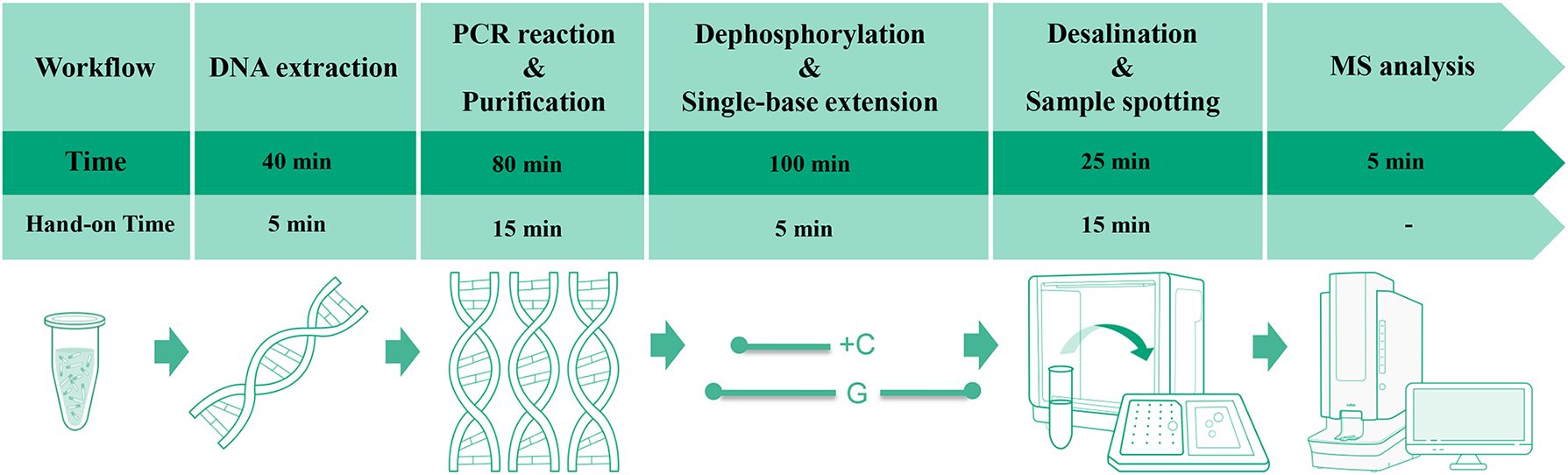
Following the extension reaction, desalting was performed by adding 40 μL double-distilled water and 25 mg disposable resin to each tube. After brief centrifugation, the mixture was vortexed for 10min and then centrifuged. A 20 μL aliquot of the supernatant was spotted onto a matrix-precoated MALDI target plate (Zybio, China, Cat. No. 01.09.67.01.04.01), air-dried, and analyzed by MS (Zybio, China, EXS2600). The complete workflow and time distribution are shown in Figure 1.
2.8 Method validation
Performance verification of the established method was undertaken as follows: (1) control verification: purified DNA mixtures from five Candida species served as positive controls to verify single-base extension products generated by each probe. Sterile water and human genomic DNA were used as blank and negative controls, respectively, to confirm the absence of non-specific extension peaks; (2) sensitivity assessment: each concentration of the serially diluted Candida suspensions and simulated candiduria samples was analyzed in 10 replicates. The lowest concentration demonstrating a detection rate >95% and a signal-to-noise ratio (S/N) >3 was defined as the LoD; (3) specificity analysis: genomic DNA from bacteria and other fungi (listed in “1. Microbial strains and urine specimens”) was tested to validate probe specificity; (4) multiplex detection capacity evaluation: DNA extracts from single or five Candida species were simultaneously tested against all five probes in a single-tube reaction, and the extension profiles of each probe were observed; (5) clinical validation: urine specimens collected from clinical settings were analyzed using our assay, and the results were compared with those of conventional urine culture.
3 Results
3.1 The probe peaks in blank and negative controls
Firstly, the species-specific probes targeting five Candida species were directly analyzed by MALDI-TOF MS without prior PCR amplification or single-base extension. Figure 2A showed five well-resolved characteristic peaks corresponding to the respective probes at their predicted m/z values, demonstrating excellent mass resolution without any observable signal overlap or cross-interference. Then, the blank control and negative control were analyzed using the MALDI-TOF NAMS protocol. Only a single peak was observed in each mass spectragram of the five Candida species (Figures 2B–F), indicating that their probes were not extended.
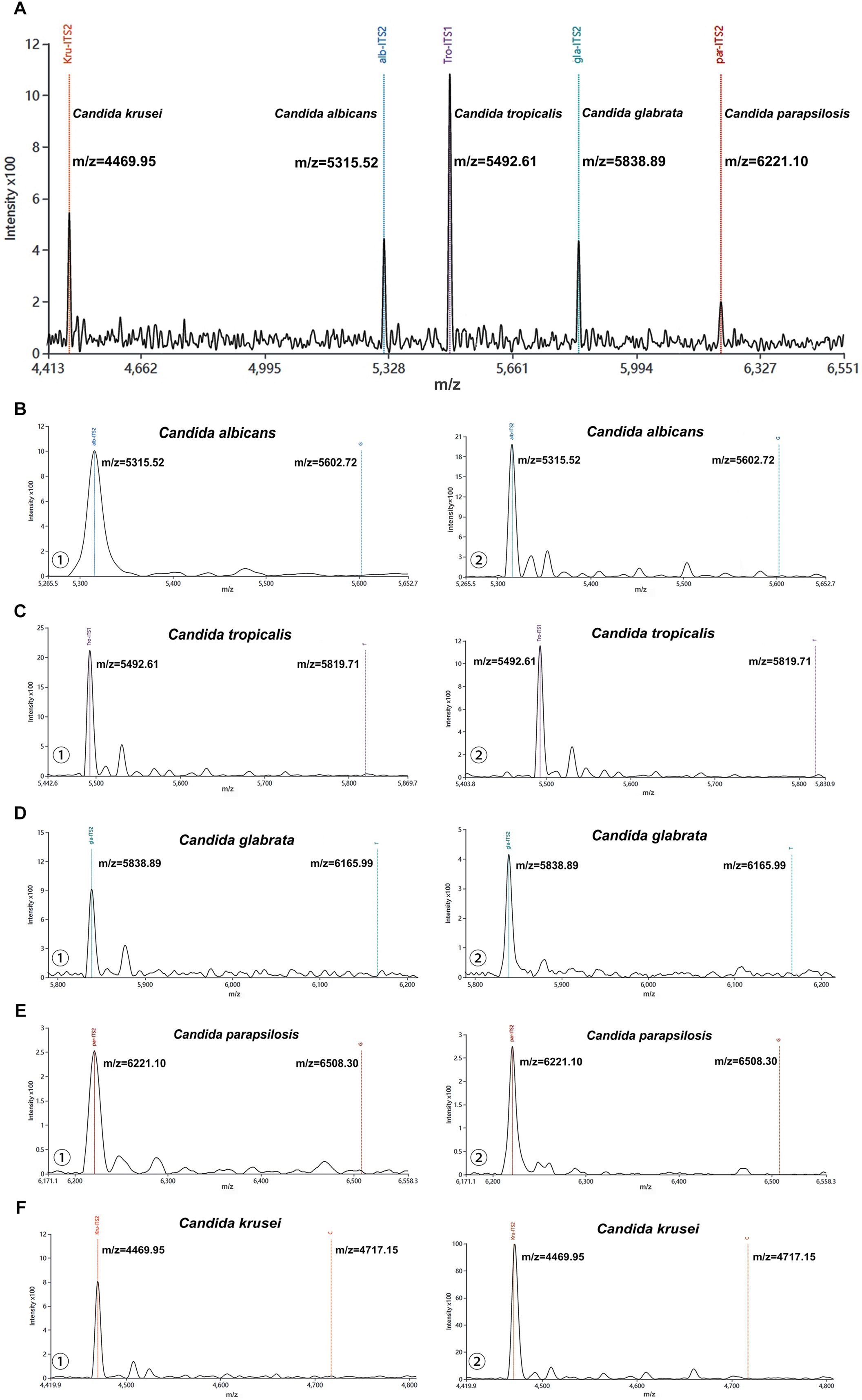
Figure 2. Characterization of probes from five Candida species using MALDI-TOF NAMS assay. (A) The peak profile of unextended probes from five Candida species. (B-F) Mass spectra of ① negative and ② blank controls detected by probes from five Candida species.
3.2 The sensitivity and specificity of the MALDI-TOF NAMS assay
The MALDI-TOF NAMS detection platform demonstrated 100% detection rates (10/10) for the five Candida suspensions at concentrations ranging from 101 CFU/mL to 106 CFU/mL. At 100 CFU/mL, the detection rates varied among species: Candida albicans (80%, 8/10), Candida tropicalis (60%, 6/10), Candida glabrata (50%, 5/10), while Candida parapsilosis and Candida krusei showed lower detection rates (30%, 3/10 each). Based on a S/N threshold of >3, the LoD for each species were determined as follows: Candida albicans, Candida tropicalis, and Candida glabrata exhibited an LoD of 101 CFU/mL; Candida parapsilosis had an LoD of 102 CFU/mL; and Candida krusei required a higher concentration (103 CFU/mL) for reliable detection. Details could be seen in Figures 3A–E.
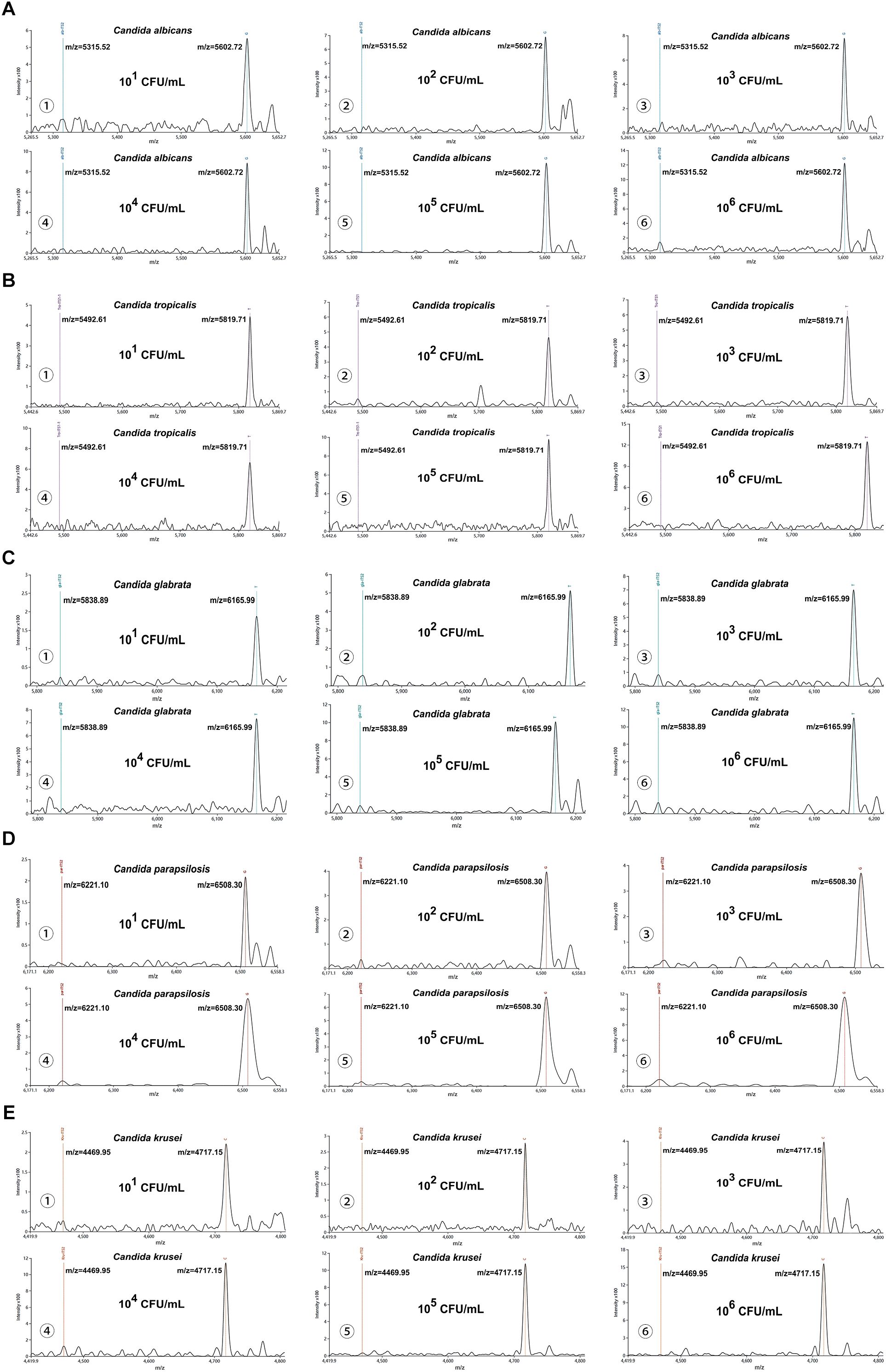
Figure 3. Analytical sensitivity of the MALDI-TOF NAMS assay. (A–E) Mass spectra of serial dilutions (106 to 101 CFU/mL) of Candida suspensions detected with corresponding species-specific probes.
Besides, our platform achieved 100% detection rates (10/10) for simulated candiduria samples across concentrations ranging from 101 to 106 CFU/mL. At the lowest tested concentration (100 CFU/mL), detection rates were observed: Candida albicans (70%, 7/10), Candida tropicalis and Candida glabrata (50%, 5/10 each), Candida parapsilosis (40%, 4/10), and Candida krusei (30%, 3/10). Using a S/N threshold of >3 as the criterion for reliable detection, the LoD for simulated candiduria were established as follows: 101 CFU/mL for Candida albicans, 102 CFU/mL for Candida tropicalis, Candida glabrata, and Candida parapsilosis, and 103 CFU/mL for Candida krusei (Figures 4A–E).
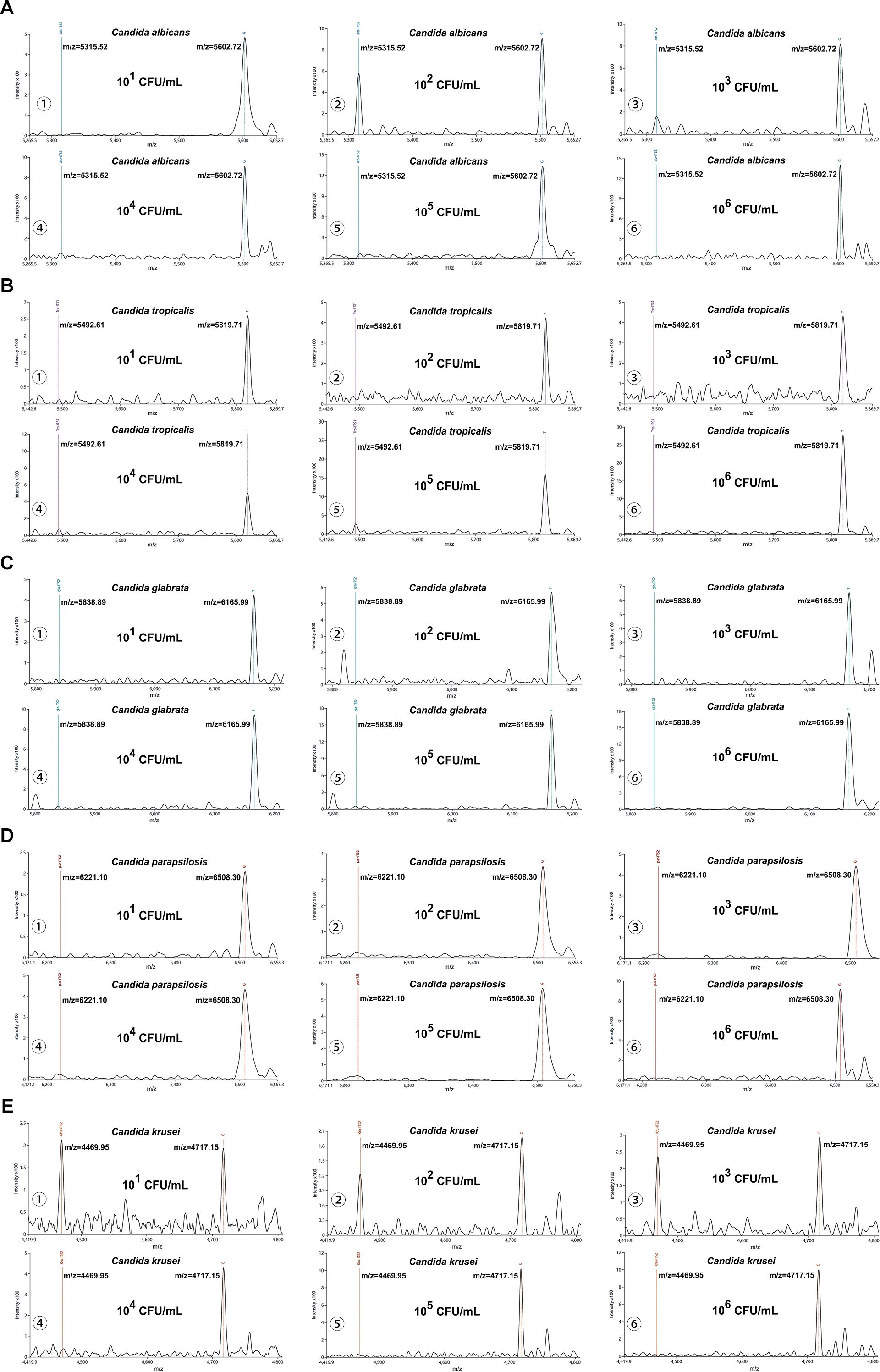
Figure 4. Interference testing of the MALDI-TOF NAMS assay. (A–E) Mass spectra of simulated candiduria samples containing Candida species at concentrations from from 106 to 101 CFU/mL detected with species-specific probes.
To evaluate the specificity of our methodology, genomic DNA from eight bacterial strains and nine non-target fungal species was individually analyzed as templates. Results indicated the presence of only non-extended probe peaks, confirming the high specificity of our detection system for the five targeted Candida species without any cross-reactivity with other non-target pathogens.
3.3 The multiplex MALDI-TOF NAMS assay
To evaluate the multiplex detecition capacity of our detection system, we employed a multiplex assay in which the amplified products derived from certain Candida species were hybridized with five probes in a single tube. As illustrated in Figure 3A, the probe specific for Candida albicans successfully extended, generating a characteristic peak that was clearly distinguishable from those non-extended probes. Similarly, probes for remaining four Candida species demonstrated selective single nucleotide extension, generating their identifiable peaks respectively (Figure 5A). Moreover, when the amplicons from five Candida strains were mixed with all species-specific probes in a single reaction, the characteristic peaks corresponding to each extended probe were unambiguously identifiable (Figure 5B). These results demonstrate that the developed detection system enables the simultaneous identification of at least five clinically relevant Candida species, providing a robust basis for candiduria analysis in the clinical settings.
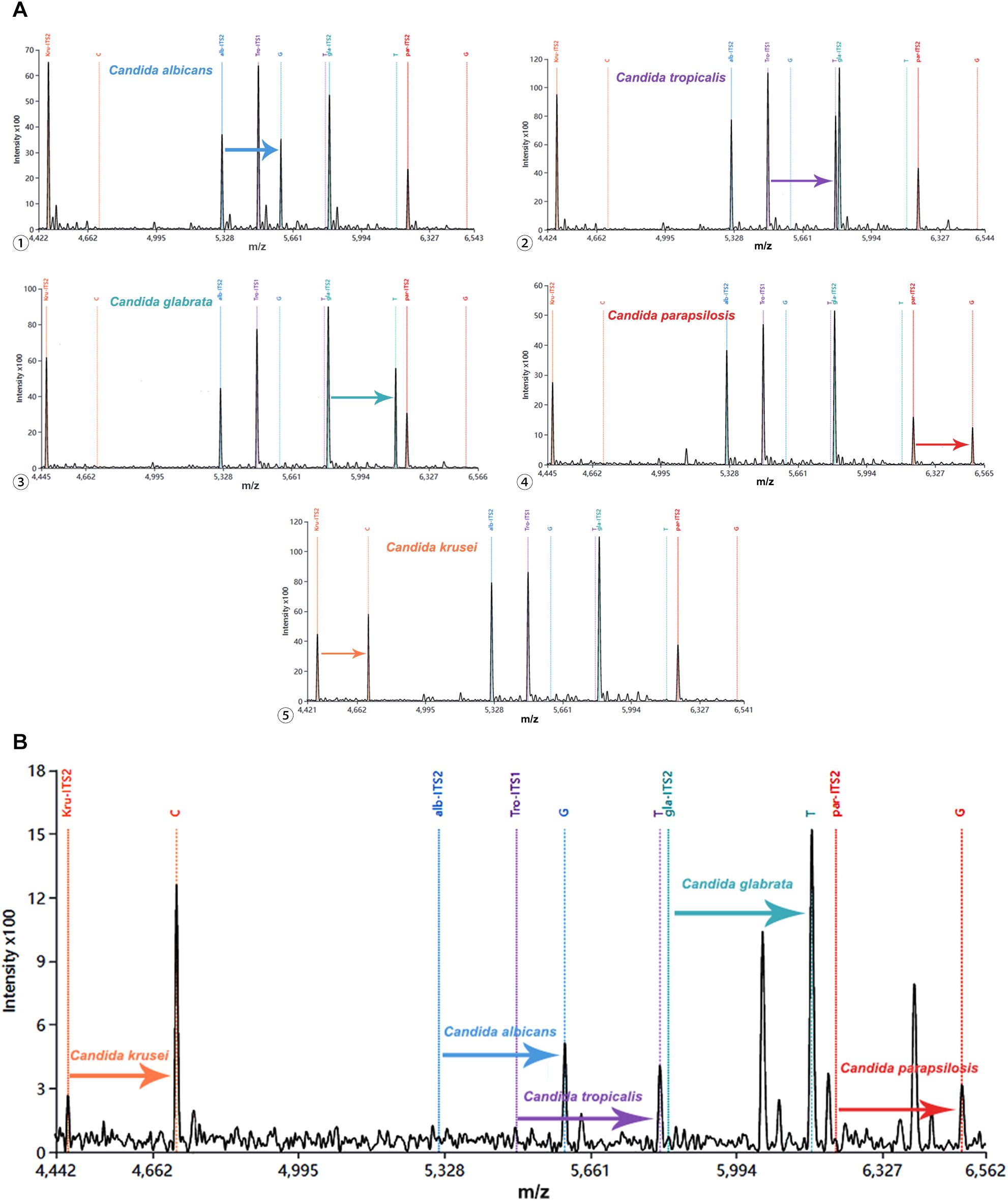
Figure 5. Multiple detection of five Candida species using MALDI-TOF NAMS assay. (A) Individual detection of each species: ① Candida albicans, ② Candida tropicalis, ③ Candida glabrata, ④ Candida parapsilosis and ⑤ Candida krusei; (B) Simultaneous detection of all five species in a mixed sample. MALDI-TOF, Matrix-assisted laser desorption/ionization-time of flight; NAMS, nucleic acid mass spectrometry.
3.4 Performance evaluation of MALDI-TOF NAMS using clinical urine samples
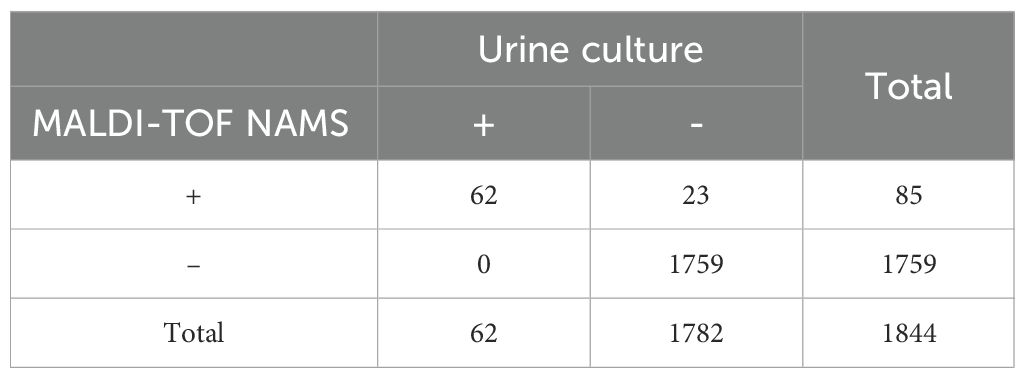
A total of 1,844 urine samples from patients were test using urine culture and our developed method. Urine culture showed that 31 specimens were positive for Candida albicans, 20 for Candida tropicalis, 9 for Candida glabrata, 1 for Candida parapsilosis and 1 for Candida krusei, with the mean (standard deviation, SD) turnaround time of 2812.3 (1012.6) minuets.
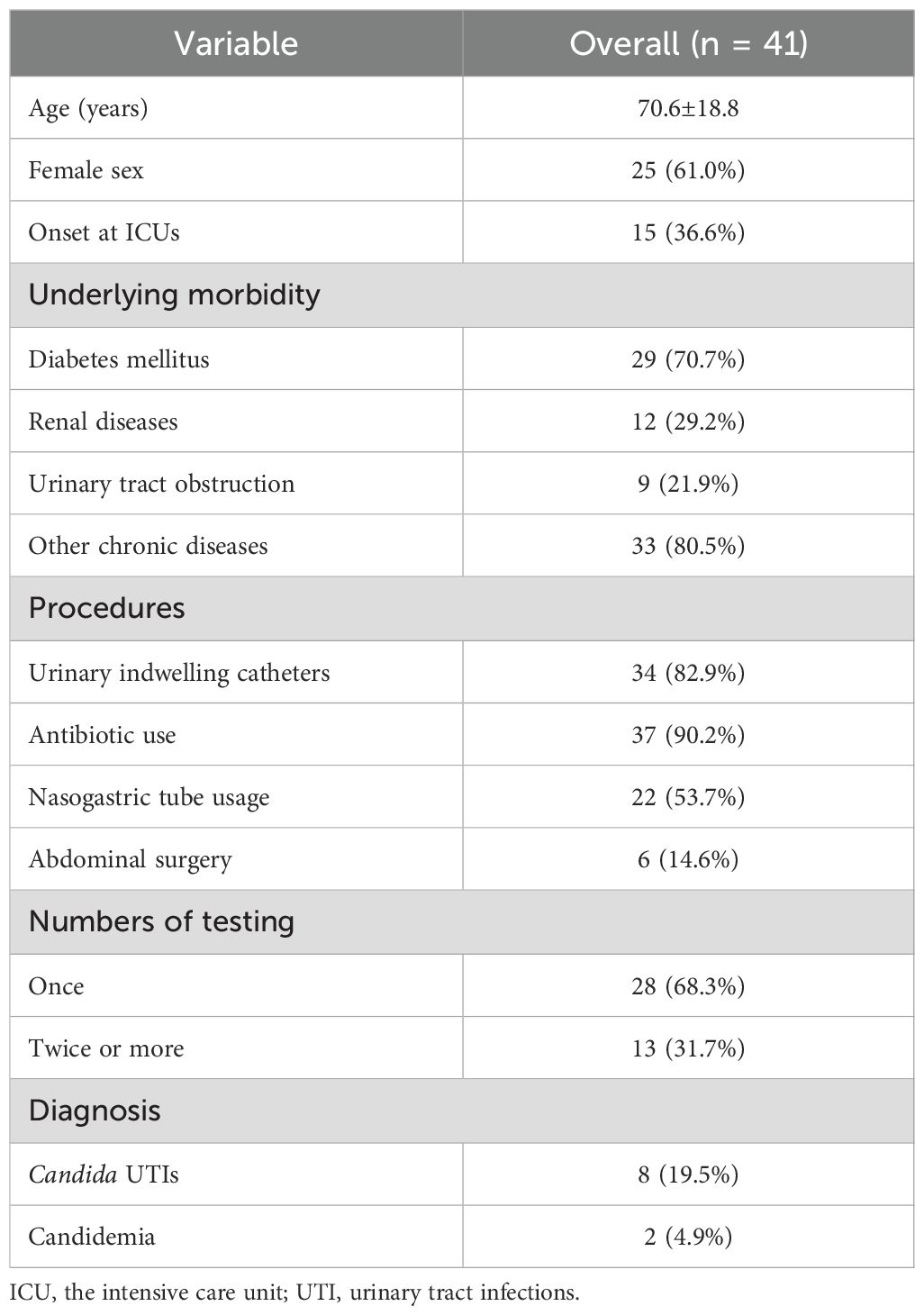
The 62 urine specimens with Candida were obtained from 41 patients with a mean (SD) age of 70.6 (18.8) years. Among them, 61.0% (n=25) were female. The majority of the patients were admitted to the ICU (36.6%, n=15) and internal medicine departments (53.7%, n=22), while the remaining patients were distributed in the surgery-urology department (7.3%, n=3) and burn unit (2.4%, n=1). The past histories of the patients were prevalent, with diabetes mellitus (70.7%), renal diseases (29.2%) and urinary tract obstruction (21.9%) being the most common. Additionally, 33 (80.5%) patients had other chronic conditions, particularly hypertension, coronary heart disease and cerebral infarction. Most patients underwent various procedures. Urinary catheterization was performed in 82.9% (34/41) of cases, antibiotic therapy was administered to 90.2% (37/41) of patients, while nasogastric tube insertion and abdominal surgery were required in 53.7% (22/41) and 14.6% (6/41) of cases, respectively.
Almost half of the patients (n=19, 46.3%) failed to receive any form of clinical management after the presence of candiduria. Urine culture was only repeated in 31.7% (n=13) of the patients with candiduria. Except for that, bladder irrigation represented the most frequently performed medical intervention (n=9, 22.0%), followed by catheter replacement (n=6, 14.6%) and antibiotic discontinuation (n=3, 7.3%). Only one patient (2.4%) had his urinary catheter removed. Eight patient (19.5%) were finally diagnosed as Candida UTI and administered antifungal therapy. Moreover, 2 patient (4.9%) progressed to candidemia. The relevant data were presented in Table 3.
Our species-specific probes successfully identified all Candida to the species level in the 62 candiduria samples. Besides, extension peaks were also detected in 23 urine specimens that were culture-negative for Candida, of which 10 corresponded to Candida albicans, 12 to Candida tropicalis, and 1 to Candida glabrata. Consequently, the diagnostic sensitivity our developed method was 100.0% when using urine culture as the gold standard, and the diagnostic specificity was 98.7%. Table 4 provides a comprehensive summary of the clinical evaluation data.
4 Discussion
In the present study, we established a novel multiplex MALDI-TOF NAMS assay for the simultaneous identification of five prevalent Candida species associated with candiduria. The developed assay demonstrated a LoD ranging from 101 CFU/mL to 103 CFU/mL across different Candida species, with no observed cross-reactivity against a panel of UTI pathogens and commonly encountered fungi. The diagnostic sensitivity of our developed method was 100% using urine culture as the gold standard, with the diagnostic specificity of 98.7%. The entire detection process could be completed within 5 hours, since our assay circumvented the need for conventional incubation procedures of urine specimens.
The prevalence of candiduria differs across patient populations. Colodner et al. reported that the occurrence of community-acquired candiduria was 0.14%, while the prevalence of candiduria from outpatients and inpatients was 0.77% (Colodner et al., 2008). Among catheterized hospitalized patients, the incidence of candiduria rose to 19.49% (Padawer et al., 2015). In ICU settings, the proportion of patients with candiduria reached was as high as 22.00% (Alvarez-Lerma et al., 2003). Our study population encompassed general ward patients, catheterized patients, and ICU patients, placing our findings within the range of results reported in previous studies (62/1844, 3.36%). In a regional healthcare complex comprising three hostipals providing tertiary general care services, Castellano-Sánchez et al. reported that the isolation rate of Candida spp. from urine culture was 2.72% (Castellano-Sánchez et al., 2025), which was similar to our result.
Candiduria often receives insufficient clinical consideration, leading to neglected management in routine practice. Most patients with candiduria receive no medical intervention with a range of 36.3% to 81.4% (Colodner et al., 2008; Padawer et al., 2015; He et al., 2021a), and our finding (46.3%) also falls within this range. According to the Infectious Diseases Society of America (IDSA) guidelines, at least one clean-catch repeat urine culture is required to rule out contamination, even when candiduria is detected in an asymptomatic non-catheterized patient (Pappas et al., 2016). In high-risk candiduria cases, follow-up urine testing should be performed after addressing the underlying predisposing conditions (Kauffman, 2014). It is the urine culture’s laborious process that somewhat impacts clinical decisions on candiduria management. In our study cohort, five patients were discharged on the day following candiduria detection, precluding clinicians from awaiting repeat culture results and consequently resulting in no medical intervention.
PCR-based molecular methods enable rapid detection of candiduria and identification of specific Candida species. Hernández-Carreón et al. introduced a fast endpoint PCR method to reliably identify Candida glabrata in urine specimens (Hernández-Carreón et al., 2021). To rapidly and directly detect Candida auris from urine samples, Walchak et al. developed a real-time PCR assay utilizing specific primers and probes (Walchak et al., 2020). Few common isolates in candiduria could be identified through the PCR-RFLP analysis with MspI restriction enzyme digestion (Ortiz et al., 2018; Fazeli et al., 2019). However, conventional PCR methods were limited in the number of pathogens they could simultaneously detect within a single panel (Zhang et al., 2021). In addition, their insufficient resolution hindered the accurate differentiation of closely related species, such as Candida albicans and Candida dubliniensis (Ortiz et al., 2018).
Compared with other traditional PCR-based assays, our method employed MS to analyze amplified DNA fragments, thereby achieving high resolution and multiplex detection capability. The assay discriminates closely related Candida species (e.g., Candida albicans vs. Candida dubliniensis; Candida parapsilosis vs. Candida orthopsilosis/metapsilosis) down to single-base differences, while allowing simultaneous detection of multiple Candida species in a single tube. Moreover, unlike conventional MALDI-TOF MS for proteins, our MS assay targeted PCR products, enabling culture-independent identification of five clinically relevant Candida species directly in urine within 5 hours (Figure 1).
The developed platform demonstrated high sensitivity (101 CFU/mL to 103 CFU/mL), meeting the clinical diagnostic requirements for UTI. When combining recombinase polymerase amplification (RPA) with lateral flow strips (LFS) for the detection of Candida glabrata and Candida tropicalis, the sensitivity was limited to 104 CFU/mL and 9940 CFU/mL, respectively (Wang et al., 2022; Wang et al., 2022). Zhao et al. reported a lower LoD of 10 CFU/50 µL (200 CFU/mL) for Candida krusei using the same technology; however, this finding lacked validation through replicate testing (Zhao et al., 2022). Of note, the current method showed higher sensitivity for suspensions than for simulated candiduria samples when detecting Candida glabrata, Candida tropicalis, and Candida parapsilosis, which may be attributed to matrix effects inherent in clinical specimens (Munch et al., 2019). There are some advantages of our methodology that we believe differentiate it from commercial kits (Table 5). Firstly, commercial kits may not always be readily accessible in certain regions, particularly in resource-limited settings. In contrast, our in-house designed panel is more adaptable to local needs, easier to produce, and more cost-effective. Moreover, our methodology offers the flexibility to tailor the panel to clinical requirements, focusing on Candida species that are most prevalent in the local population, such as Candida albicans, Candida glabrata and Candida tropicalis. This adaptability enhances the clinical utility of our method, particularly in settings where these species are the primary concern.
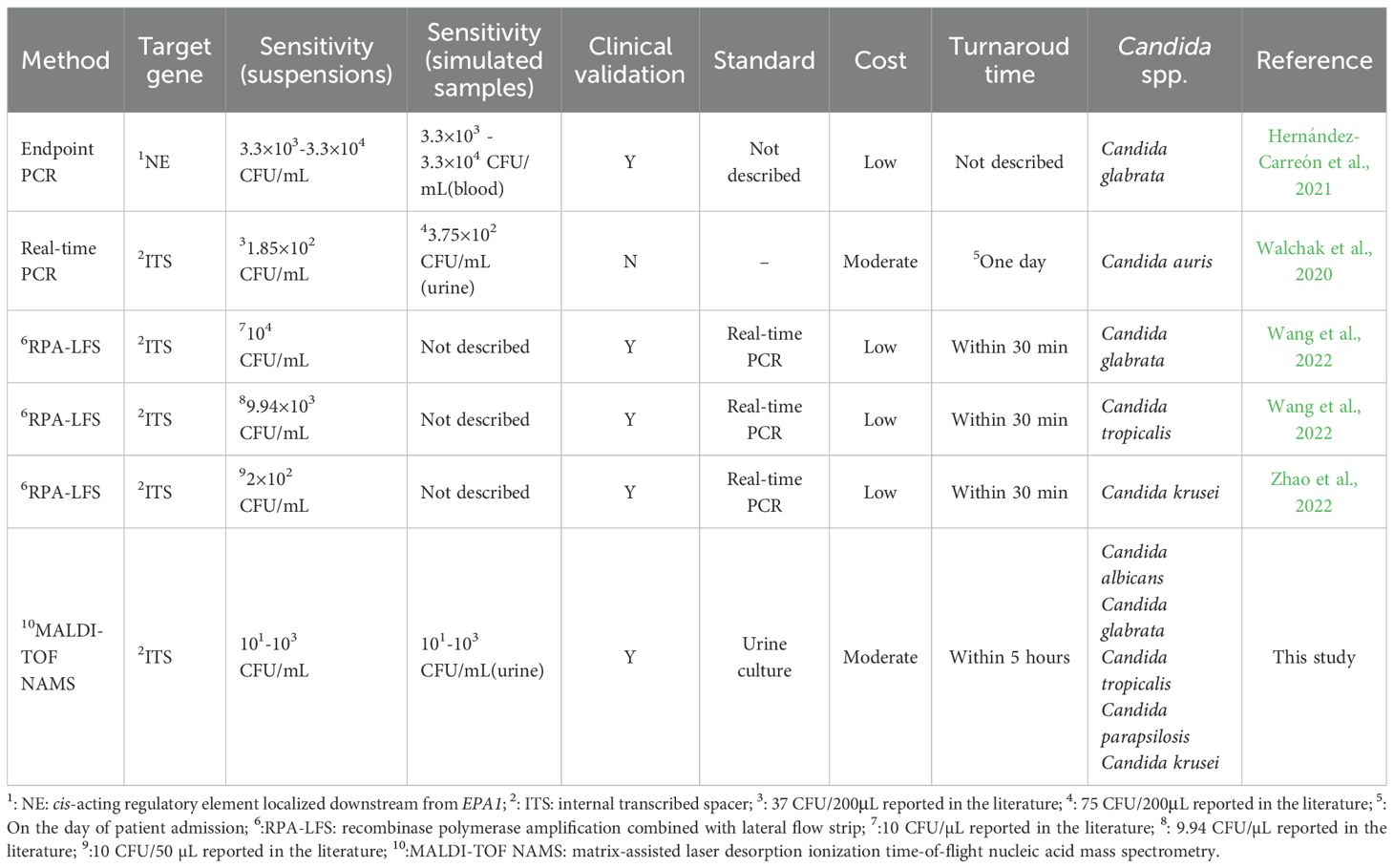
Table 5. A head-to-head comparison of the reported molecular methods in the literature for the candiduria detection.
In clinical validation, our assay successfully identified Candida to species level in all culture-positive urine specimens. 23 NAMS positive results were obtained in 1782 culture-negative specimens. The diagnostic specificity aligns with established literature reports, ranging from 93.0% to 99.0% (Eshoo et al., 2010; Bacconi et al., 2014; Desmet et al., 2016). Among the 23 culture-negative but NAMS-positive specimens, more than half (12) were from patients who had received antifungal therapy. The antifungal treatment may have eradicated the Candida cells, leading to negative urine cultures (Facchini et al., 2024), but the DNA of the non-viable Candida remained in the urine, resulting in positive PCR-based detection. In 4 culture-negative/NAMS-positive cases, subsequent urine cultures from the same patients later grew Candida, matching the species identified by MALDI-TOF NAMS. This may be related to the lower sensitivity of culture methods (Clancy and Nguyen, 2013). As the disease progresses, Candida load in the urinary tract increases, resulting in positive cultures in follow-up samples. The remaining patients most likely represented temporary Candida presence in the urine (Wellinghausen et al., 2009). The low Candida load may have been cleared by the host immune system, making urine cultures negative, but the released DNA can still be detected by PCR-based methods. Nevertheless, the possibility of a false-positive PCR result cannot be completely ruled out (Wellinghausen et al., 2009). Candida is a common commensal organism in the human body, particularly colonizing the oral cavity, gastrointestinal tract, and skin (Romo and Kumamoto, 2020). During specimen collection, Candida from the skin may contaminate the urine container. While low-level Candida loads may not yield positive cultures due to insufficient microbial growth or viability, highly sensitive PCR assays could still amplify trace DNA derived from contaminants.
Besides, it should be noted that the method established in this study presents several other limitations. Firstly, The current assay mainly focuses on the five most prevalent Candida species in the clinical setting. However, the MALDI-TOF NAMS platform is inherently scalable and can be readily expanded to include additional probes for emerging or clinically relevant Candida species, such as Candida auris, Candida dubliniensis and Candida lusitaniae. The detection principle of MALDI-TOF NAMS is based on mass differences among nucleic acid extension products (Gabriel et al., 2009). Therefore, in theory, there is no fundamental limitation on the number of targets that can be detected simultaneously, provided that the probes are well-designed to avoid mass overlap and non-specific interactions. For instance, a recent study successfully applied MALDI-TOF NAMS to simultaneously detect 14 porcine viruses in a single assay, demonstrating the feasibility of high-plex detection in complex samples (Shuai et al., 2024). This supports the adaptability of our platform for future inclusion of additional Candida species as needed. Secondly, while the current assay is specifically validated for urine samples, the underlying MALDI-TOF NAMS holds considerable potential for adaptation to other clinical specimens, such as blood or respiratory samples. Preliminary studies on various clinical samples are needed to assess the assay’s performance, as different specimen matrices may impact detection. Additionally, Candida auris, which has emerged as a significant global pathogen with multidrug resistance, is not currently included in the assay, but could be considered for future inclusion for surveillance purposes. Expanding the assay’s scope would enhance its utility in broader clinical settings. Finally, the performance of the multiplex assay remains to be systematically further validated.
5 Conclusion
In this study, we developed a novel MALDI-TOF NAMS assay for the rapid identification of five clinically prevalent Candida species (Candida albicans, Candida tropicalis, Candida glabrata, Candida parapsilosis, and Candida krusei) in urine samples. The assay demonstrated superior sensitivity, excellent specificity, and high concordance rate with urine culture results. Its multiplex detection capability and streamlined workflow address critical limitations of conventional methods, offering a robust tool to differentiate contamination, colonization or UTI for timely candiduria management in high-risk populations.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was approved by the Institutional Review Board of the Affiliated Hospital of Xuzhou Medical University (Approval No.: XYFY2024-KL178-01) .The studies were conducted in accordance with thelocal legislation and institutional requirements. The participantsprovided their written informed consent to participate in this study. Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
YG: Investigation, Funding acquisition, Conceptualization, Writing – original draft. YC: Software, Formal analysis, Writing – original draft, Methodology, Validation. JS: Resources, Writing – review & editing, Data curation. HZ: Validation, Formal analysis, Methodology, Writing – review & editing, Software. HK: Supervision, Writing – review & editing, Project administration, Funding acquisition. YX: Funding acquisition, Project administration, Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82402722), the China Postdoctoral Science Foundation (No. 2023M732975), the Xuzhou Science and Technology Program (General Program of Medicine and Health in 2023) (No. KC23265) and the Innovation and Entrepreneurship Program of Xuzhou Medical University Science and Technology Park (No.: CXCYZX2024004).
Acknowledgments
The authors would like to express their sincere gratitude to Mr. Shulong Zhao from the Department of Laboratory Medicine, Affiliated Hospital of Xuzhou Medical University, and Zybio Co., Ltd. for their valuable technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aghili, S. R., Abastabar, M., Soleimani, A., Haghani, I., and Azizi, S. (2020). High prevalence of asymptomatic nosocomial candiduria due to Candida glabrata among hospitalized patients with heart failure: a matter of some concern? Curr. Med. Mycol 6, 1–8. doi: 10.18502/cmm.6.4.5327
Alfouzan, W. A. and Dhar, R. (2017). Candiduria: Evidence-based approach to management, are we there yet? J. Mycol Med. 27, 293–302. doi: 10.1016/j.mycmed.2017.04.005
Alvarez-Lerma, F., Nolla-Salas, J., León, C., Palomar, M., Jordá, R., Carrasco, N., et al. (2003). Candiduria in critically ill patients admitted to intensive care medical units. Intensive Care Med. 29, 1069–1076. doi: 10.1007/s00134-003-1807-y
Bacconi, A., Richmond, G. S., Baroldi, M. A., Laffler, T. G., Blyn, L. B., Carolan, H. E., et al. (2014). Improved sensitivity for molecular detection of bacterial and Candida infections in blood. J. Clin. Microbiol. 52, 3164–3174. doi: 10.1128/JCM.00801-14
Bouakaze, C., Keyser, C., Gonzalez, A., Sougakoff, W., Veziris, N., Dabernat, H., et al. (2011). Matrix-assisted laser desorption ionization-time of flight mass spectrometry-based single nucleotide polymorphism genotyping assay using iPLEX gold technology for identification of Mycobacterium tuberculosis complex species and lineages. J. Clin. Microbiol. 49, 3292–3299. doi: 10.1128/JCM.00744-11
Bougnoux, M. E., Kac, G., Aegerter, P., d’Enfert, C., and Fagon, J. Y. (2008). Candidemia and candiduria in critically ill patients admitted to intensive care units in France: incidence, molecular diversity, management and outcome. Intensive Care Med. 34, 292–299. doi: 10.1007/s00134-007-0865-y
Castellano-Sánchez, L., Rosales-Castillo, A., Marcos-Rodríguez, R., Olvera-Porcel, M. C., Navarro-Marí, J. M., and Gutiérrez-Fernández, J. (2025). Microbiological relevance of candida in urine cultures. J Fungi (Basel) 11(7):483–492. doi: 10.3390/jof11070483
Clancy, C. J. and Nguyen, M. H. (2013). Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 56, 1284–1292. doi: 10.1093/cid/cit006
Colodner, R., Nuri, Y., Chazan, B., and Raz, R. (2008). Community-acquired and hospital-acquired candiduria: comparison of prevalence and clinical characteristics. Eur. J. Clin. Microbiol. Infect. Dis. 27, 301–305. doi: 10.1007/s10096-007-0438-6
Desmet, S., Maertens, J., Bueselinck, K., and Lagrou, K. (2016). Broad-range PCR coupled with electrospray ionization time of flight mass spectrometry for detection of bacteremia and fungemia in patients with neutropenic fever. J. Clin. Microbiol. 54, 2513–2520. doi: 10.1128/JCM.01066-16
Dias, V. (2020). Candida species in the urinary tract: is it a fungal infection or not? Future Microbiol. 15, 81–83. doi: 10.2217/fmb-2019-0262
Eshoo, M. W., Crowder, C. D., Li, H., Matthews, H. E., Meng, S., Sefers, S. E., et al. (2010). Detection and identification of Ehrlichia species in blood by use of PCR and electrospray ionization mass spectrometry. J. Clin. Microbiol. 48, 472–478. doi: 10.1128/JCM.01669-09
Facchini, N., Wernli, L., Rieken, M., Bonkat, G., Wirz, D., and Braissant, O. (2024). Again and again-survival of Candida albicans in urine containing antifungals. Pharmaceutics 16(5)605–616. doi: 10.3390/pharmaceutics16050605
Fazeli, A., Kordbacheh, P., Nazari, A., Daie Ghazvini, R., Mirhendi, H., Safara, M., et al. (2019). Candiduria in hospitalized patients and identification of isolated candida species by morphological and molecular methods in Ilam, Iran. Iran J. Public Health 48, 156–161.
Gabriel, S., Ziaugra, L., and Tabbaa, D. (2009). SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr. Protoc. Hum. Genet. Chapter 2, Unit 2.12. doi: 10.1002/0471142905.hg0212s60
García-Agudo, L., Rodríguez-Iglesias, M., and Carranza-González, R. (2018). Approach of clinicians to candiduria and related outcome in the elderly. J. Mycol Med. 28, 428–432. doi: 10.1016/j.mycmed.2018.05.011
Gharaghani, M., Taghipour, S., Halvaeezadeh, M., and Mahmoudabadi, A. Z. (2018). Candiduria; a review article with specific data from Iran. Turk J. Urol 44, 445–452. doi: 10.5152/tud.2018.54069
Guo, Y., Yang, J. X., and Liang, G. W. (2016). A Real-Time PCR Assay Based on 5.8S rRNA Gene (5.8S rDNA) for Rapid Detection of Candida from Whole Blood Samples. Mycopathologia 181, 405–413. doi: 10.1007/s11046-015-9977-z
He, Z., Huo, X., Lei, D., Zhao, H., Jia, K., and Wang, F. (2021a). Management of candiduria in hospitalized patients: a single-center study on the implementation of IDSA guidelines and factors affecting clinical decisions. Eur. J. Clin. Microbiol. Infect. Dis. 40, 59–65. doi: 10.1007/s10096-020-03999-1
He, Z., Su, C., Bi, Y., Cheng, Y., Lei, D., and Wang, F. (2021b). Evaluation of a novel laboratory candiduria screening protocol in the intensive care unit. Infect. Drug Resist. 14, 489–496. doi: 10.2147/IDR.S289885
Hernández-Carreón, O., Hernández-Howell, C., Hernández-Hernández, G., Herrera-Basurto, M. S., González-Gómez, B. E., Gutiérrez-Escobedo, G., et al. (2021). Highly specific and rapid molecular detection of Candida glabrata in clinical samples. Braz. J. Microbiol. 52, 1733–1744. doi: 10.1007/s42770-021-00584-2
Kauffman, C. A. (2014). Diagnosis and management of fungal urinary tract infection. Infect. Dis. Clin. North Am. 28, 61–74. doi: 10.1016/j.idc.2013.09.004
Lima, G. M. E., Nunes, M. O., Chang, M. R., Tsujisaki, R. A. S., Nunes, J. O., Taira, C. L., et al. (2017). Identification and antifungal susceptibility of Candida species isolated from the urine of patients in a university hospital in Brazil. Rev. Inst Med. Trop. Sao Paulo 59, e75. doi: 10.1590/s1678-9946201759075
Munch, M. M., Chambers, L. C., Manhart, L. E., Domogala, D., Lopez, A., Fredricks, D. N., et al. (2019). Optimizing bacterial DNA extraction in urine. PloS One 14, e0222962. doi: 10.1371/journal.pone.0222962
Ortiz, B., Pérez-Alemán, E., Galo, C., and Fontecha, G. (2018). Molecular identification of Candida species from urinary infections in Honduras. Rev. Iberoam Micol 35, 73–77. doi: 10.1016/j.riam.2017.07.003
Padawer, D., Pastukh, N., Nitzan, O., Labay, K., Aharon, I., Brodsky, D., et al. (2015). Catheter-associated candiduria: Risk factors, medical interventions, and antifungal susceptibility. Am. J. Infect. Control 43, e19–e22. doi: 10.1016/j.ajic.2015.03.013
Pappas, P. G., Kauffman, C. A., Andes, D. R., Clancy, C. J., Marr, K. A., Ostrosky-Zeichner, L., et al. (2016). Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 62, e1–50. doi: 10.1093/cid/civ933
Raja, H. A., Miller, A. N., Pearce, C. J., and Oberlies, N. H. (2017). Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod 80, 756–770. doi: 10.1021/acs.jnatprod.6b01085
Romo, J. A. and Kumamoto, C. A. (2020). On commensalism of. Candida. J. Fungi (Basel) 6(1), 16–29. doi: 10.3390/jof6010016
Shi, H. M., Wang, Z. K., Wu, H., Li, J. K., Mo, Y. Y., Liu, X., et al. (2025). Clinical application of time-of-flight mass spectrometry nucleic acid detection technology in diagnosis of drug-resistant pulmonary tuberculosis. Diagn. Microbiol. Infect. Dis. 111, 116686. doi: 10.1016/j.diagmicrobio.2025.116686
Shuai, J., Song, S., Wang, Z., Zeng, R., Han, X., and Zhang, X. (2024). MALDI-TOF nucleic acid mass spectrometry for simultaneously detection of fourteen porcine viruses and its application. J. Virol. Methods 329, 114990. doi: 10.1016/j.jviromet.2024.114990
Walchak, R. C., Buckwalter, S. P., Zinsmaster, N. M., Henn, K. M., Johnson, K. M., Koelsch, J. M., et al. (2020). Candida auris direct detection from surveillance swabs, blood, and urine using a laboratory-Developed PCR method. J. Fungi (Basel) 6(4):224–239. doi: 10.3390/jof6040224
Wang, K., Huo, L., Li, Y., Zhu, L., Wang, Y., and Wang, L. (2022). Establishment of a rapid diagnosis method for Candida glabrata based on the ITS2 gene using recombinase polymerase amplification combined with lateral flow strips. Front. Cell Infect. Microbiol. 12, 953302. doi: 10.3389/fcimb.2022.953302
Wang, L., Xu, A., Zhou, P., Zhao, M., Xu, C., Wang, Y., et al. (2022). Rapid detection of Candida tropicalis in clinical samples from different sources using RPA-LFS. Front. Cell Infect. Microbiol. 12, 898186. doi: 10.3389/fcimb.2022.898186
Wellinghausen, N., Siegel, D., Winter, J., and Gebert, S. (2009). Rapid diagnosis of candidaemia by real-time PCR detection of Candida DNA in blood samples. J. Med. Microbiol. 58, 1106–1111. doi: 10.1099/jmm.0.007906-0
Zhang, H., Yan, Z., Wang, X., Gaňová, M., Chang, H., Laššáková, S., et al. (2021). Determination of advantages and limitations of qPCR duplexing in a single fluorescent channel. ACS Omega 6, 22292–22300. doi: 10.1021/acsomega.1c02971
Keywords: Candida, urine, MALDI-TOF NAMS, ITS, candiduria
Citation: Guo Y, Chen Y, Sun J, Zhu H, Kang H and Xu Y (2025) A MALDI-TOF nucleic acid mass spectrometry assay for rapid detection of five Candida species in urine. Front. Cell. Infect. Microbiol. 15:1682711. doi: 10.3389/fcimb.2025.1682711
Received: 09 August 2025; Accepted: 24 October 2025;
Published: 18 November 2025.
Edited by:
Rodolfo García-Contreras, National Autonomous University of Mexico, MexicoReviewed by:
Ayse Kalkanci, Gazi University, TürkiyeCaleb Perez, Postgraduate Unit, National Autonomous University of Mexico, Mexico
Copyright © 2025 Guo, Chen, Sun, Zhu, Kang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiquan Kang, aHFrODExMDI5QDE2My5jb20=; Yinhai Xu, ZG9sbWd1b3lpQHh6aG11LmVkdS5jbg==
†These authors share first authorship
‡These authors have contributed equally to this work
 Yi Guo
Yi Guo Yan Chen3†
Yan Chen3† Jingfang Sun
Jingfang Sun Haiquan Kang
Haiquan Kang Yinhai Xu
Yinhai Xu