- 1Institute of Animal Health, Guangdong Academy of Agricultural Sciences, Guangzhou, China
- 2Key Laboratory for Prevention and Control of Avian Influenza and Other Major Poultry Diseases, Ministry of Agriculture and Rural Affairs, Guangzhou, China
- 3Guangdong Province Key Laboratory of Livestock Disease Prevention, Guangzhou, China
- 4Department of Animal Science, College of Biology and Food, Shangqiu Normal University, Shangqiu, China
- 5Zhejiang Hisun Animal Healthcare Products Co., Ltd., Guangzhou, China
- 6National S&T Innovation Center for Modern Agricultural Industry, Guangzhou, China
Over the past few decades, battery industry and electronic equipment have undergone explosive growth, but the heavy metal waste generated has led to significant global ecological and public health challenges. Currently, increasing evidences have confirmed the detrimental effects of heavy metal exposure on animal reproduction, immunity, and metabolism. However, research focused on the impacts of battery leakage on the gut microbiota remain scarce. Thus, this study aims to investigate the detrimental effects of battery on gut microbiota in chickens. Results revealed that battery exposure can lead to a significant increase in spleen index and a significant decrease in thymus index in chickens. Furthermore, battery exposure can significantly increase serum ALT, AST and MDA levels, and while concurrently reducing levels of GSH-Px and SOD. Battery exposure also cause a significant reduction in the gut microbial alpha diversity, accompanied by significant alterations in taxonomic composition. Bacterial taxonomic analysis indicated that the relative abundances of 1 phyla and 4 genera increased dramatically, while the relative abundance of 3 phylum and 115 genera decreased significantly during battery exposure. In conclusion, this study suggests that battery exposure leads to gut microbial dysbiosis and affect antioxidant ability in chickens. The significant alterations of gut microbiota may represent one of the mechanisms through which battery exerts its intestinal and renal toxicity. Given the context of battery pollutant leakage and inadequate recycling supervision, this study contributes to providing impetus for environmental protection agencies and organizations worldwide to enhance the recycling of battery waste.
Introduction
Batteries, including lithium-ion, zinc-manganese, and nickel-chromium types, serve as the primary power sources for various mobile electronic devices such as smartphones and computers (Xiao et al., 2021). Moreover, they are also recognized as highly eco-toxic contaminants. Over the past few decades, the widespread use and rapid advancement of mobile electronic devices have led to explosive growth in the battery industry. According to statistics, the global lithium-ion battery market size reached approximately USD 29.86 billion in 2017, with continued gradual growth. China is one of the major battery consumers, with over 25 billion waste lithium-ion batteries generated in 2020, amounting to nearly 500,000 tons of production. Unfortunately, a substantial portion of these waste batteries cannot be effectively recycled, resulting in their release into the ecological environment and contributing to severe global environmental issues (Xie et al., 2017; Gottesfeld et al., 2018). Surveys indicate that the growth rate of battery waste was as high as 8% in 2018, with projections suggesting a further increase of 18% to 30% by 2030. Although some waste battery recycling technologies have emerged, the management and control of waste batteries remain severely constrained due to inadequate institutional support for recycling facilities (Zhu et al., 2017; Urrutia-Goyes et al., 2018). Consequently, most batteries are ineffectively managed, often disposed of through deep burial, composting, or incineration. Battery waste predominantly contains heavy metals, organic solvents, and plastic fragments, which can accumulate in water and soil, leading to significant biosafety and ecological concerns. Furthermore, battery waste is resistant to rapid degradation and may bioaccumulate in aquatic organisms, insects, and plants, thereby posing risks to food safety and animal health through the food chain (Debrah et al., 2024). Despite the rapid growth of the new battery industry, the potential risks that battery waste poses to animal health remain inadequately addressed.
The intestine serves as the primary channel for food intake and harbors approximately 100 trillion microorganisms, including bacteria, fungi, and viruses (Biagi et al., 2017; Tong et al., 2022). These microorganisms, namely the gut microbiota, play a crucial role in host health and various physiological functions by establishing a complex symbiotic relationship with the host (Huang et al., 2020; Passos and Chaturvedi, 2021). Research has demonstrated that the gut microbiota can metabolize carbohydrates from food to produce short-chain fatty acids (SCFAs), vitamins, and antimicrobial peptides, which positively regulate resistance to pathogenic bacterial infections and enhance growth and development (Mehmood et al., 2023; Nogal et al., 2021). Furthermore, the gut microbiota has also been shown to be involved in the maturation of the immune system, the maintenance of intestinal barrier function, and bone development (Jabeen et al., 2023; Yan et al., 2022; Wang et al., 2023). Numerous studies have indicated that the stability of the gut microbiota is closely linked to the maintenance of host health and various complex physiological functions (Zhang et al., 2023b). Conversely, the gut microbial dysbiosis can adversely affect host health. Typically, the gut microbiota is influenced by a variety of internal and external factors, resulting in dynamic changes (Celorrio et al., 2021; Chen et al., 2025a). Notably, external factors such as antibiotics, heavy metals, and microplastics can significantly impact the composition and structure of the gut microbiota, potentially inducing gut microbial dysbiosis (Jubair et al., 2018; Liu et al., 2022). This imbalance not only disrupts intestinal function but also extends its detrimental effects beyond the gastrointestinal system, leading to systemic consequences (Jiang et al., 2019). Studies have shown that the gut microbial dysbiosis can increase intestinal permeability, subsequently contributing to the development of diseases such as enteritis, diarrhea, and colitis (Shen et al., 2022; Wang et al., 2024; Xie et al., 2024). Recent studies focusing on gut microbial dysbiosis have also revealed its important role in diseases such as diabetes, Parkinson’s syndrome, allergies, and obesity. Consequently, any factor that disrupts the homeostasis of gut microbiota warrants special attention (Wu et al., 2020).
Chickens have increasingly become a significant source of protein for humans, attributed to their rapid growth rate and nutritional value (Li et al., 2025; Wu et al., 2024). According to statistics, the total global chicken production in 2024 is 103.046 million tons, with a market size estimated at approximately US$217.75 billion. In the same year, China’s chicken production is approximately 15 million tons, representing 14.56% of the global total and ranking second worldwide. Furthermore, it is predicted that China’s per capita chicken consumption will reach 15.2 kilograms in 2025, while Hong Kong’s per capita consumption is projected to be as high as 55.52 kilograms. As a major producer and consumer of chickens, the production and health of chickens in China are closely linked to the lives of residents. Previous research indicated that exposure to waste batteries can significantly impact the liver and kidneys of mice (Liao et al., 2022). Similarly, Wang et al. (2022) have demonstrated the toxic effects of extracts from waste batteries on zebrafish. However, there is a paucity of studies investigating the effects of used battery pollutant leakage on chickens health. Therefore, this study aims to explore the impacts of waste battery exposure on the gut microbiota and antioxidant ability of chickens.
Materials and methods
Experimental design and sample acquisition
In this study, 40 healthy AA chickens of similar body weight were procured from a commercial hatchery. The chickens were housed collectively for three days to minimize the impact of stress reactions on the experiment. Subsequently, they were randomly divided into two groups: control group (CON) and battery exposure group (EXE), each consisting of 20 chickens. The chickens were maintained in a standard environment with controlled temperature (from 33°C~35°C during the first week, gradually decreased to 29°C at the end of the second week), humidity (60%~65%), and lighting (23 h/1h light/dark cycle) to ensure optimal growth conditions. Adequate diet and drinking water were provided to all chickens throughout the duration of the experiment. Notably, battery waste was added to the water (230 mg/L) of the experimental group to induce battery poisoning. In addition, the main components of battery waste include cobalt, nickel, manganese, iron, copper, calcium, sodium and zinc. During the experiment, feed intake, average daily weight gain, and changes in body weight were recorded to compare the effects of battery exposure on the growth performance of chickens. The experimental period was based on previous studies (Liao et al., 2022). After 28 days, all chickens were euthanize using pentobarbital (25 mg/kg) (Hou et al., 2025). The serum, spleen, bursa of Fabricius, and thymus were collected to assess changes in immune organ indices. Concurrently, the cecal contents of the chickens were immediately collected and snap-frozen in liquid nitrogen for amplicon sequencing.
Biochemical assays
Serum biochemical indicators including ALT, AST, and T-AOC, GSH-Px, CAT, SOD and MDA were measured using commercial kits in accordance with established methodologies from previous studies (Liao et al., 2022).
DNA extraction and amplicon sequencing
Amplicon sequencing of the gut microbiota was conducted following previous established protocols (Hou et al., 2025; Peng et al., 2024). Briefly, DNA from cecal contents was extracted in accordance with the kit instructions. After assessing the DNA quality, qualified DNA was amplified using primers (338F: ACTCCTACGGGAGGCAGCA and 806R: GGACTACHVGGGTWTCTAAT). The PCR reaction system and conditions were established based on prior studies (Hou et al., 2025; Peng et al., 2024). Following PCR, the amplified products were purified, quality assessed, and quantified to prepare sequencing libraries. The constructed libraries underwent an initial quality check, and only those with concentrations exceeding 2 nM were sequenced using the Illumina NovaSeq 6000 platform. Given that the raw data from high-throughput sequencing contained unqualified sequences, a series of processing steps, including quality filtering and DADA2 denoising, were implemented to obtain valid sequences. Briefly, quality screening and primer elimination of original data were conducted to achieve clean reads devoid of defective, short, or mismatched sequences utilizing Trimmomatic (v0.33) and Cutadapt software (1.9.1). The resulting clean reads were then spliced and subjected to a secondary filter based on the length of the spliced sequences utilizing Usearch software (v10). Subsequently, chimera sequences in the raw data were identified and removed using UCHIME software (v4.2) to yield effective reads. The valid sequences from each sample were clustered into OTUs based on 97% sequence similarity. Additionally, the alpha diversity index of the gut microbiota was calculated based on the number of OTUs in each sample. Simultaneously, the PCoA diagram was also generated to visualize the structure of the gut microbiota. The data were presented as Mean ± standard deviation (SD). Metastats and LEfSe analyses were employed to identify differential taxa associated with battery exposure, using a significance threshold of P < 0.05 or LDA > 4.
Results
Effects of battery exposure on growth performance and organ indices in chickens
Body weight changes and organ indices of chicken in each group are present in Figure 1. Results revealed that battery exposure had no effect on the body weight gain, average daily weight gain, average daily feed intake and food conversion ratio of the chickens (Figures 1A-D). Organ index analysis revealed that exposure to batteries did not affect the bursa index of broiler chickens, but it significantly increased the spleen index and markedly decreased the thymus index (P < 0.05) (Figures 1E-G).
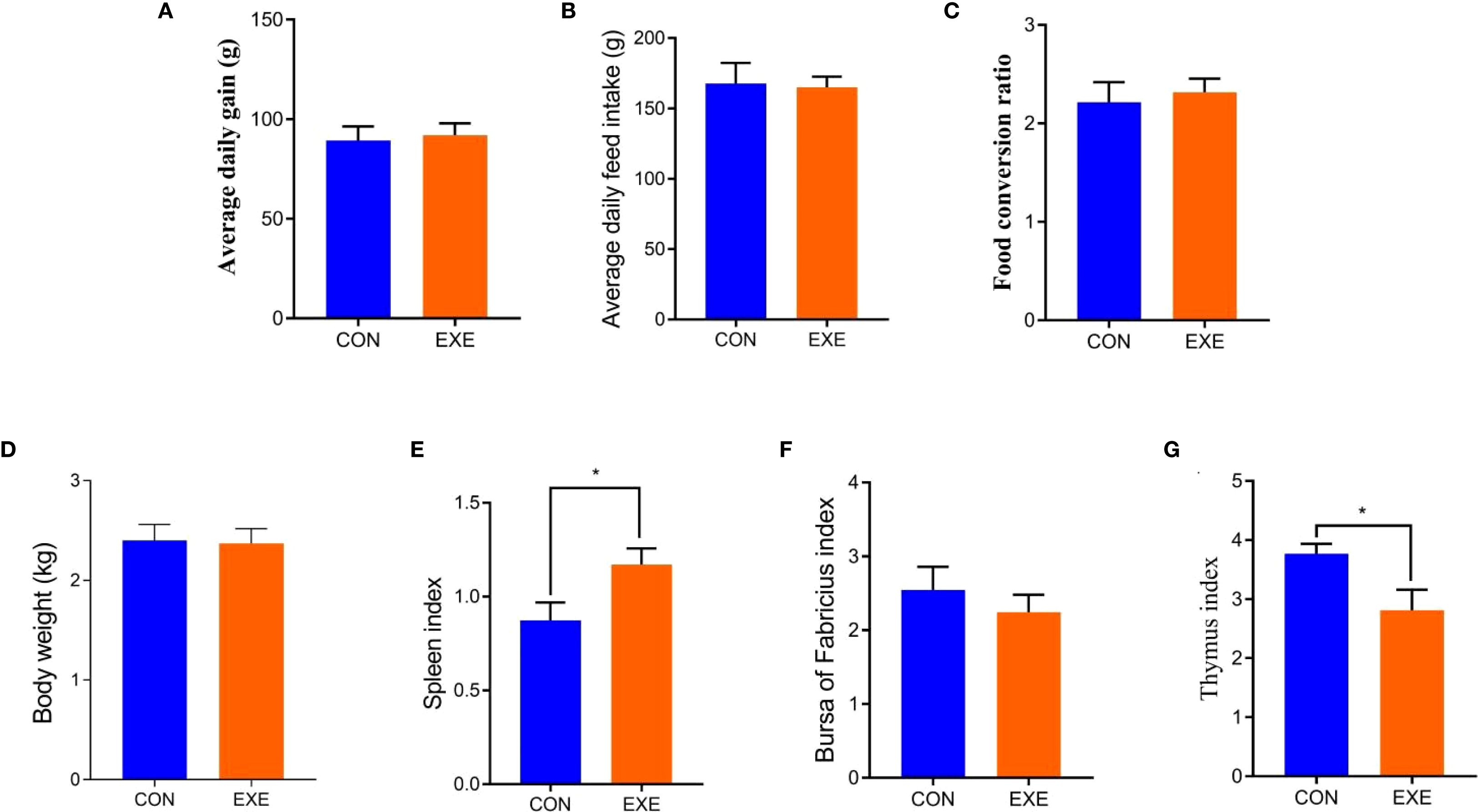
Figure 1. Effects of battery exposure on growth performance-related parameters in broiler chickens. (A) average daily gain; (B) average daily feed intake; (C) food conversion ratio; (D) Body weight; (E) spleen index; (F) bursa of fabricius index, (G) thymus index. The data was expressed as mean ± SD. *P < 0.05.
Effects of battery exposure on serum biochemical indices in chickens
Serum biochemical analysis showed that the levels of ALT and AST in the EXE were higher than those in the CON (P < 0.05) (Figures 2A, B). Additionally, we also observed that battery exposure dramatically reduced the antioxidant ability of chicken, characterized by increased levels of MDA and decreased levels of SOD, and GSH-Px (P < 0.05 or P < 0.01) (Figures 2C-E). Notably, battery exposure had no significant effect on the levels of CAT and T-AOC (Figures 2F, G).
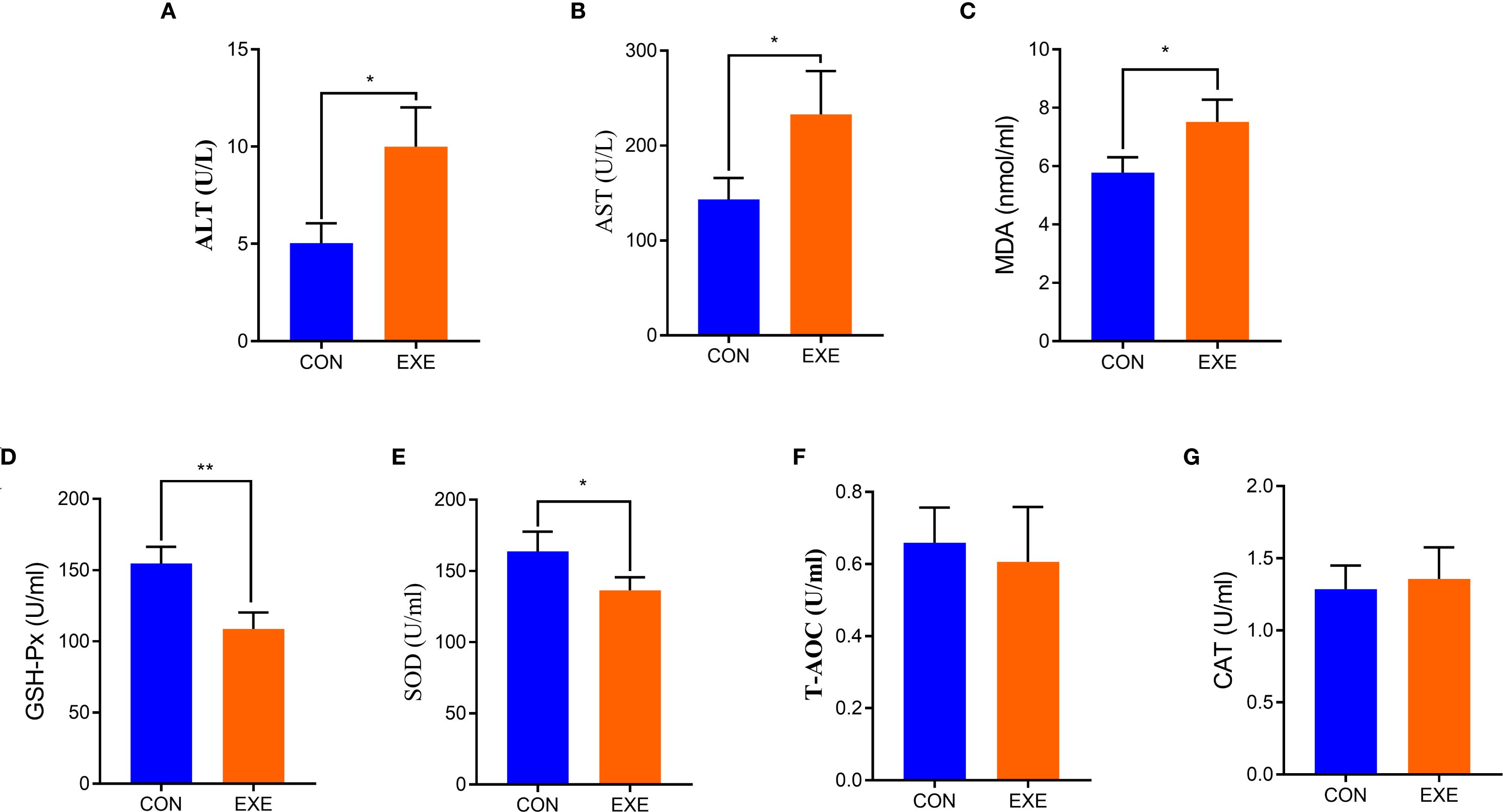
Figure 2. Effects of battery exposure on serum biochemical parameters in broiler chickens. (A) ALT; (B) AST; (C) MDA; (D) GSH-Px; (E) SOD; (F) T-AOC, (G) CAT. The data was expressed as mean ± SD. *P < 0.05, **P < 0.01.
Sequence analysis
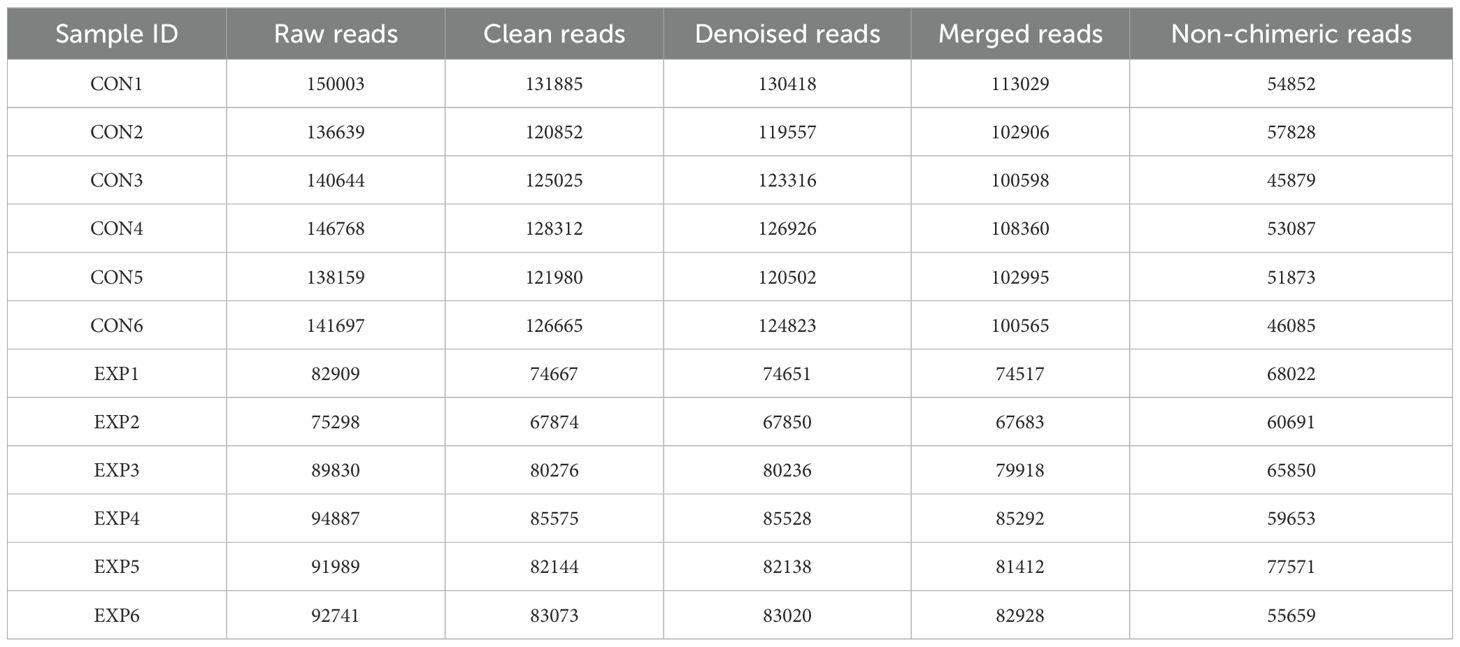
In this study, microbiome sequencing was performed on samples collected from both the CON and EXE groups, generating a total of 1,381,564 (CON=853,910, EXE=527,654) raw sequences. After removing questionable sequences, 697,050 (CON=309,604, EXE=387,446) valid sequences were identified, yielding a qualification rate of over 50% (Table 1). These valid sequences were subsequently clustered into 15,692 OTUs, with 15,449 and 349 OTUs identified in the CON and EXE, respectively (Figures 3A, B). Additionally, 106 OTUs were shared between both groups, accounting for 0.67% of the total OTUs (Figure 3C). The individual OTU counts in the CON and EXE were 15,343 and 243, respectively, which represented 98.45% and 2.22% of their respective OTU totals. Importantly, the rarefaction curve analysis, which was employed to assess sequencing depth, revealed that the curves reached saturation, indicating that the sequencing depth was adequate (Figures 3D, E).
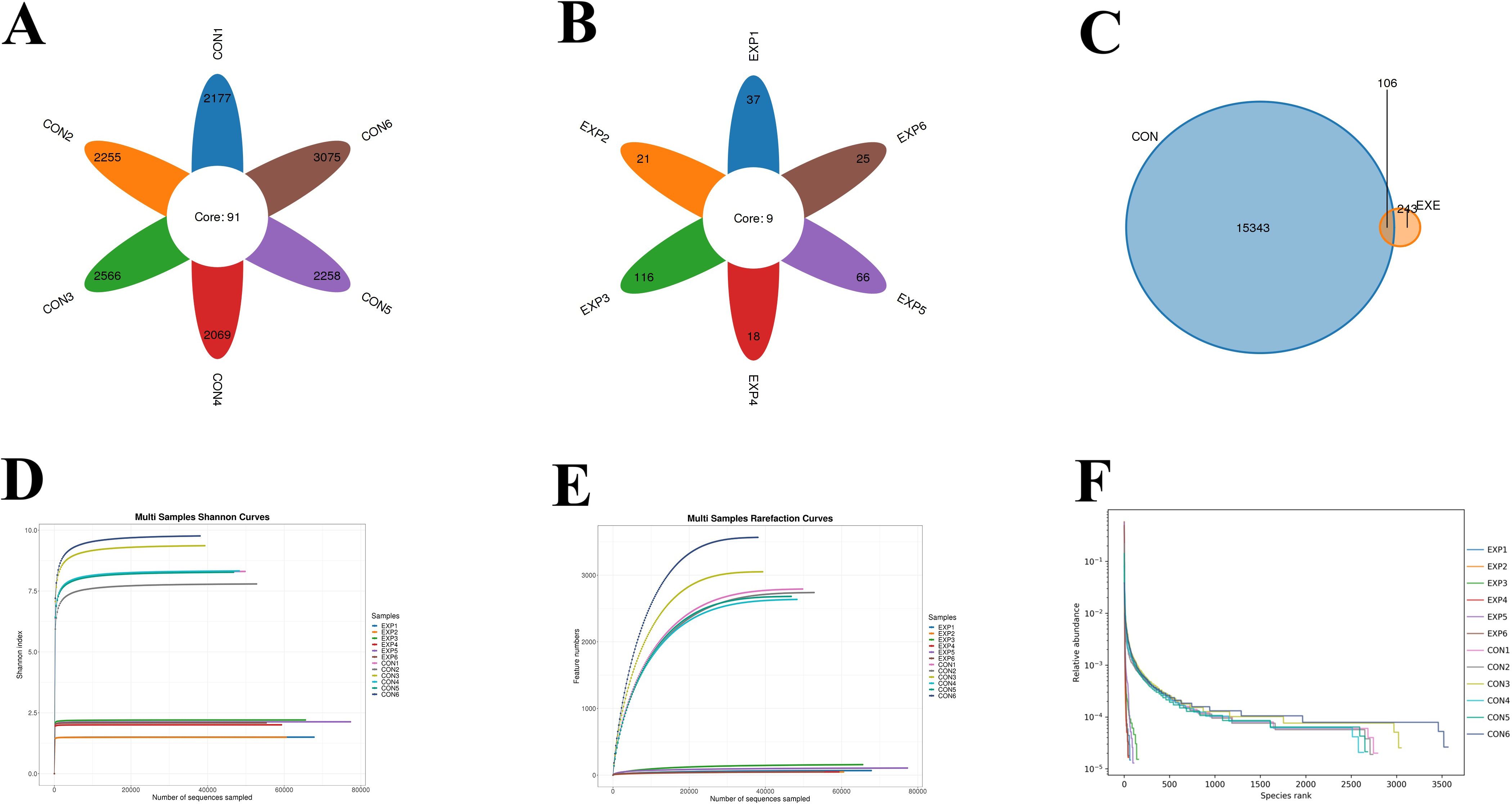
Figure 3. Effects of battery exposure on the diversity of gut microbiota. (A-C) The number of OTUs generated from valid sequences. (D-F) Feasibility analysis of the results obtained from gut microbiota sequencing.
Battery exposure changed the gut microbial diversity
To further compare the differences in gut microbial diversity between the CON and EXE, we calculated the alpha diversity index based on the abundance of OTUs in each sample. The results indicated that the average values of the ACE index were 2920.75 for the CON and 84.52 for the EXE, while the average Chao1 index values were 2924.76 and 86.08, respectively. Furthermore, the average values of Shannon index for the CON and EXE was 8.63 and 1.90, while and the average values of Simpson index was 0.97 and 0.63, respectively. Statistical comparisons revealed that battery exposure significantly reduced the gut microbial Chao1 (2924.76 ± 144.26 vs. 86.08 ± 19.39, P < 0.001) and ACE (2920.75 ± 144.15 vs. 84.52 ± 18.59, P < 0.001), Simpson (9.07± .0.72 vs. 0.63±0.025, P < 0.001) and Shannon (8.63±0.30 vs. 1.90 ± 0.13, P < 0.001) indices (Figures 4A-D). These findings suggested that battery exposure significantly decreased the abundance and diversity of gut microbiota. Additionally, beta diversity analysis based on the PCoA scatter plot demonstrated that samples from the CON and EXE were significantly separated, indicating that battery exposure significantly alter the structure of the gut microbiota (Figures 4E, F).
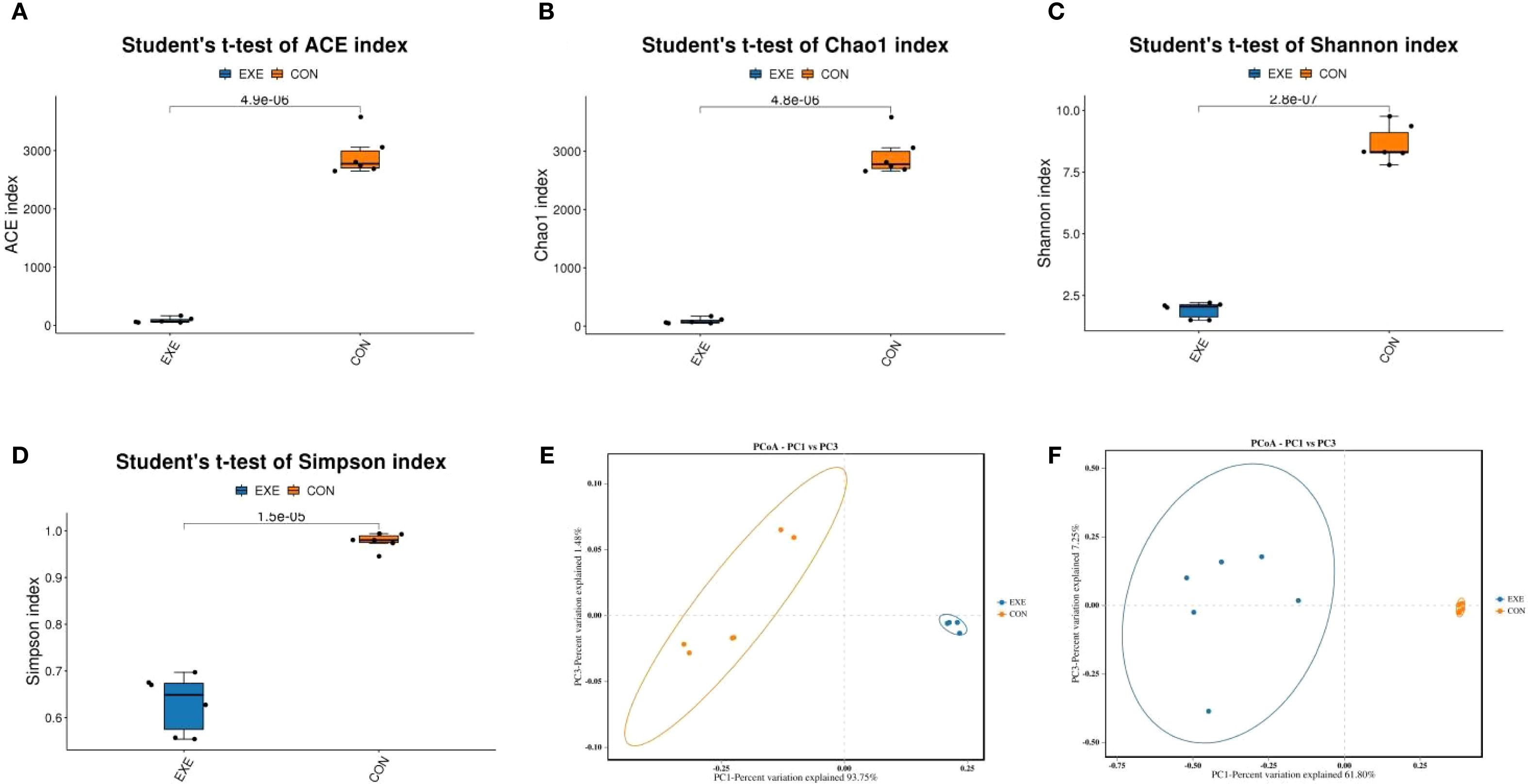
Figure 4. Effects of battery exposure on the diversity of gut microbiota in broiler chickens. (A-D) The ACE, Chao1, Shannon and Simpson indices were used for comparing the diversity and abundance. (E, F) PCoA scatter plots were generated to visualize the differences in the structure of gut microbiota. The data was expressed as mean ± SD.
Battery exposure changed the gut microbial composition
At the phylum level, Firmicutes (98.68%), Actinobacteriota (1.186%), Proteobacteria (0.057%) and Bacteroidota (0.037%) were identified as the most preponderant in the CON. As for the EXE, Firmicutes (99.27%), Proteobacteria (0.40%), unclassified_Bacteria (0.064%) and Bacteroidota (0.059%) constituted a significant proportion (Figure 5A). Other phyla such as Verrucomicrobiota (0.0047%, 0.0012%), Acidobacteriota (0.0025%, 0.0033%), unclassified_Archaea (0.00%, 0.0031%) and Aenigmarchaeota (0.00%, 0.0038%) in CON and EXE were recognized in low ratios. At the genus level, the Ruminococcus_torques_group (18.93%, 0.047%), Limosilactobacillus (17.30%, 57.39%) and Lactobacillus (6.54%, 41.50%) were abundantly present in the CON and EXE (Figure 5B). The abundance of more bacterial genera in the CON and EXE can also be displayed through cluster heat maps (Figure 6).
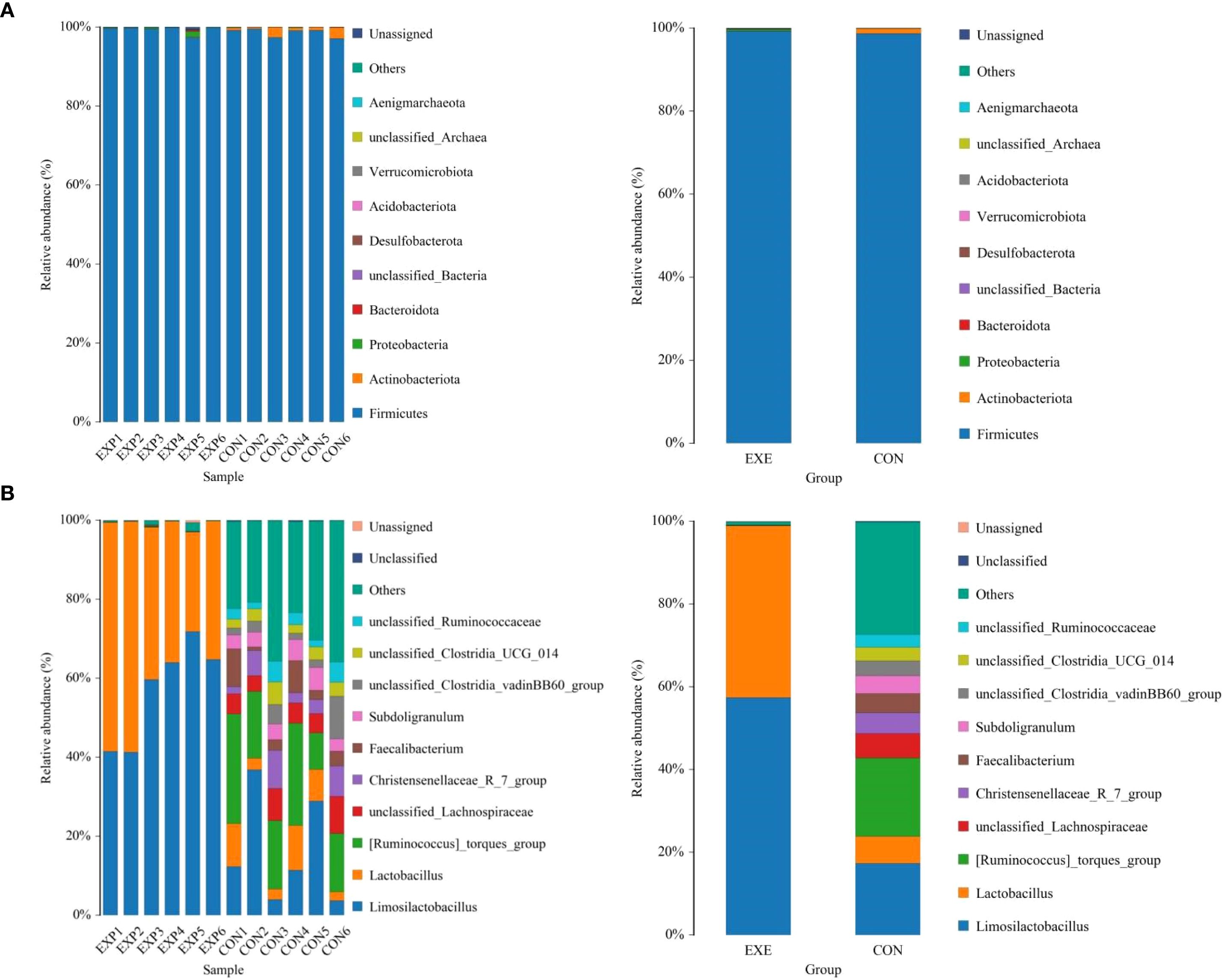
Figure 5. Effects of battery leakage on gut microbiota of broiler chickens. (A) phyla leval. (B) genus level. Each bar represents the average relative abundance of each bacterial taxon within a group.
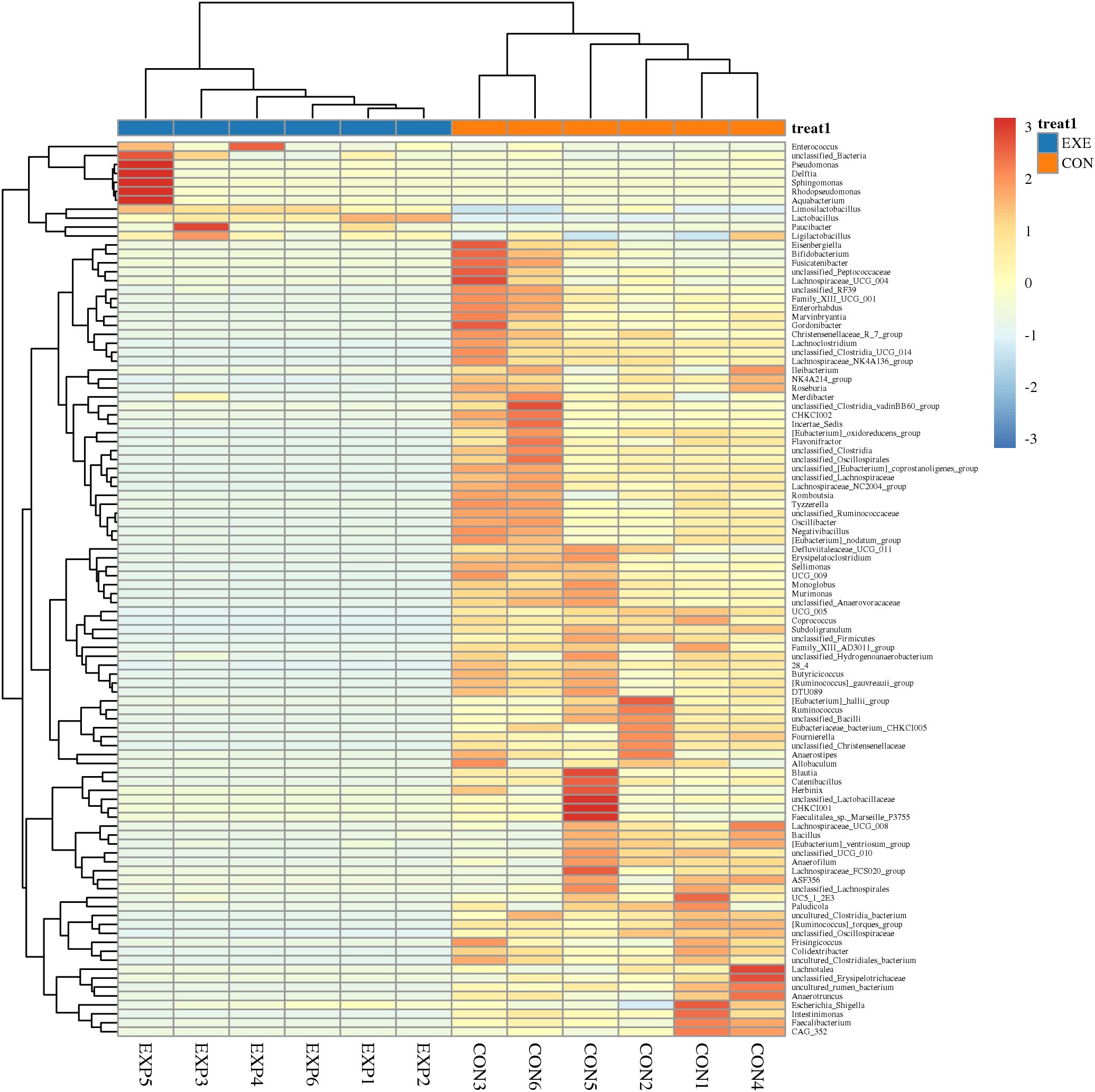
Figure 6. Cluster heat map analysis of gut microbiota. The relative abundance of gut microbiota is positively correlated with color depth. The values in the heat map represent the square-root-transformed relative abundance of each bacterial genus. The intensity of color in the heat map corresponds to the square-root-transformed values of the bacterial genera, with the legend located in the upper right corner of the figure.
Metastats and LEfSe analysis was used for identifying differential taxa associated with battery exposure. At the phylum level, Fusobacteriota was dramatically more preponderant in the EXE than in the CON, while the Actinobacteriota, Desulfobacterota and Cyanobacteria were lower (Figure 7A). Moreover, a total of 119 genera were found to be dramatically different between EXE and CON. Among them, the proportions of 4 bacterial genera (Mycoplasma, uncultured_Acidobacteria_bacteriu m, Limosilactobacillus and Lactobacillus) dramatically increased, while the relative richness of 115 bacterial genera (Coprococcus, Lachnoclostridium, Lachnospiraceae_NK4A136_group, Murimonas, Sporobacter, Subdoligranulum, Ruminococcus_torques_group, Anaerofustis, Butyricicoccus, Colidextribacter, Lachnospiraceae_NC2004_group, Monoglobus, Ruminococcus_gauvreauii_group, Fournierella, Sellimonas, Oscillibacter, Candidatus_Soleaferrea, Alistipes, Flavonifractor, Defluviitaleaceae_UCG_011, Intestinimonas, Negativibacillus, Christensenellaceae_R_7_group, Marvinbryantia, Ruminococcus, Roseburia, Catenibacillus, Romboutsia, Erysipelatoclostridium, Lactonifactor, Anaerofilum, Enterorhabdus, Gordonibacter, Tyzzerella, Frisingicoccus, Anaerostipes, Faecalibacterium, Incertae_Sedis, Paludicola, Bacillus, Anaerotruncus, Lachnospiraceae_UCG_008, Allobaculum, Blautia, Ileibacterium, Lachnospiraceae_UCG_010, Lachnospiraceae_UCG_006, Lachnospiraceae_FCS020_group, Acetitomaculum, Bacillaceae_bacterium_BM62, Faecalibaculum, Lachnotalea, Bifidobacterium, etc.) significantly decrease during battery exposure (Figure 7B). Notably, battery exposure even resulted in 68 bacterial genera (Coprococcus, Lachnospiraceae_NK4A136_group, Sporobacter, Anaerofustis, Lachnospiraceae_NC2004_group, Defluviitaleaceae_UCG_011, Intestinimonas, Catenibacillus, Romboutsia, Lactonifactor, Anaerofilum, Enterorhabdus, Gordonibacter, Tyzzerella, Frisingicoccus, Anaerotruncus and Lachnospiraceae_UCG_008, etc.) cannot be recognized in the gut microbiota. Moreover, LEfSe analysis indicated that the CON was significantly enriched for Ruminococcus:torques_group, unclassified_Lachnospiraceae, Faecalibacterium, Subdoligranulum, unclassified_Clostridia_vadinBB60_group, unclassified_Clostridia, unclassified_Ruminococcaceae, unclassified_Clostridia_UCG_014 and Lachnoclostridium, while the EXE indicated a significantly higher abundances of Limosilactobacillus, Lactobacillus and Paucibacter (Figure 8).
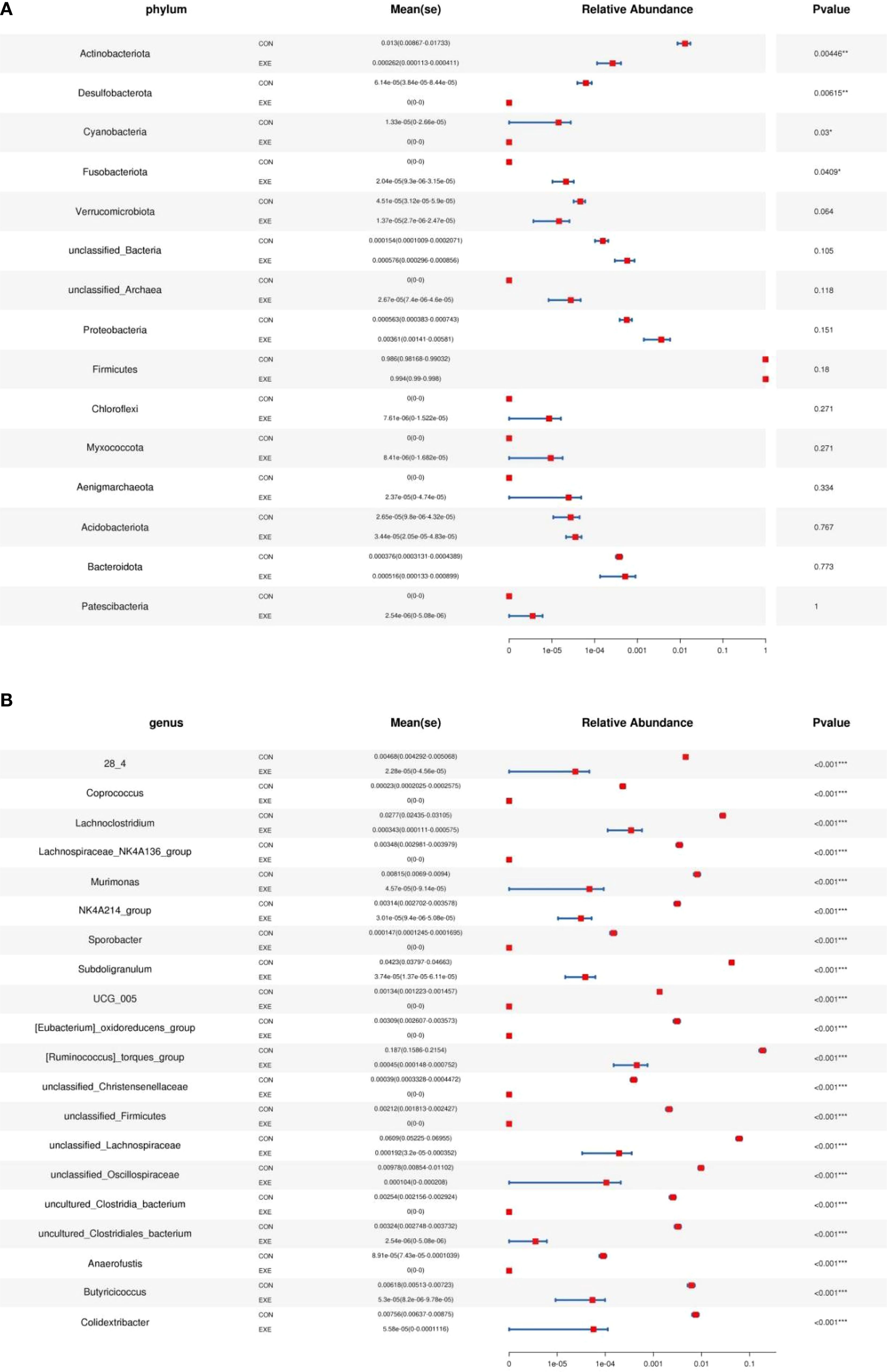
Figure 7. Metastats analysis was used to identify differential taxa at the phylum (A) and genus (B) levels. The data was expressed as mean ± SD. **P<0.01, ***P<0.001.
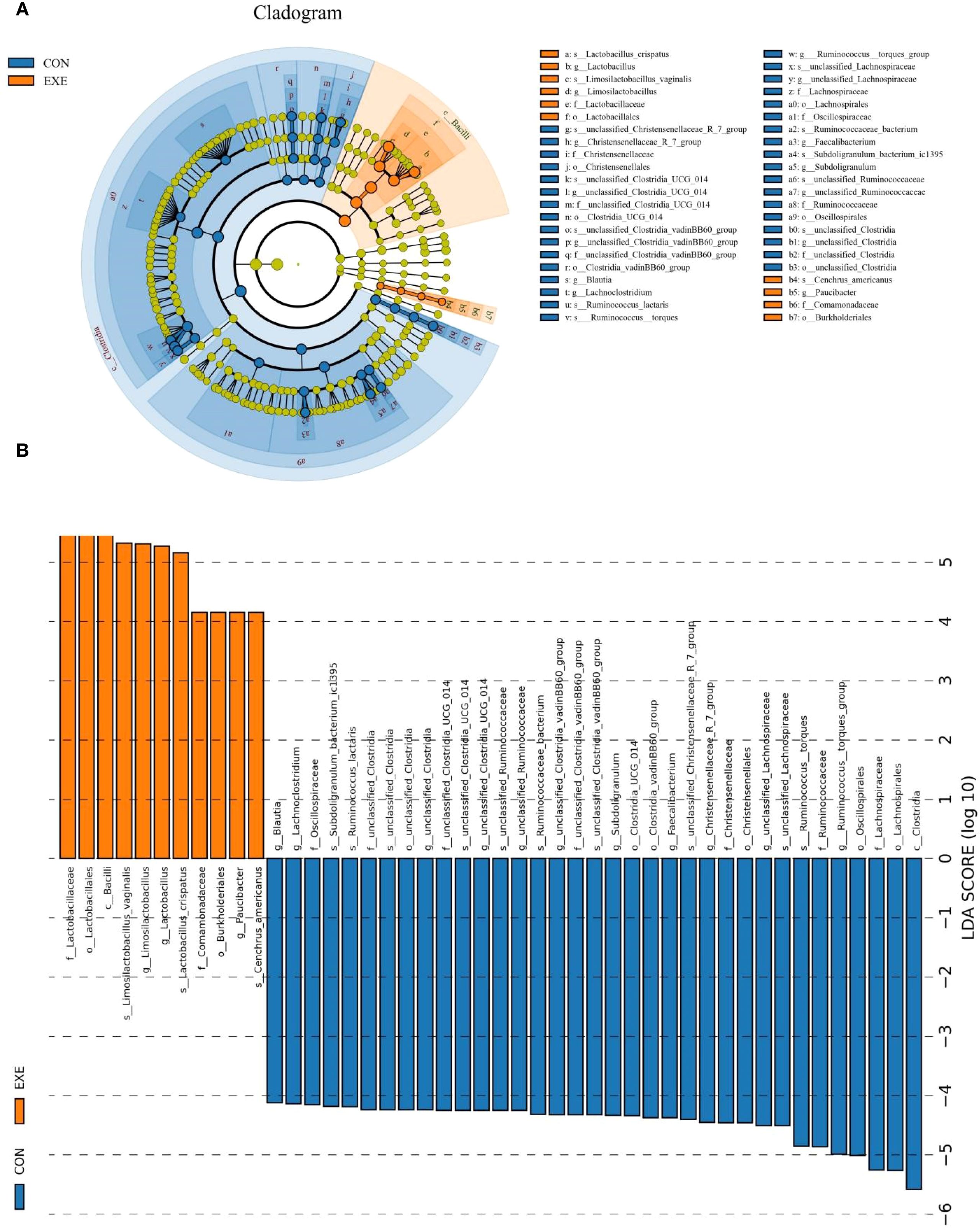
Figure 8. The differential taxa at phyla and genus levels were visualized by LEfSe analysis. (A) Evolutionary relationships of different species at different taxonomic levels. (B) LDA values ≥ 4 were set as the identification criteria for differential bacteria.
Correlation network analysis
Butyricicoccus showed a positive association with Blautia (0.99), Murimonas (0.96) and unclassified_Clostridia_UCG_014 (0.94). Intestinimonas was positively associated with Anaerotruncus (0.99), Faecalibacterium (0.95), Colidextribacter (0.94) and Flavonifractor (0.94) (Figure 9). Monoglobus was positively associated with unclassified_Clostridia_UCG_014 (0.97), Blautia (0.97), Butyricicoccus (0.97), Murimonas (0.95), 28_4 (0.95) and Christensenellaceae_R_7_group (0.94). Lachnospiraceae_NC2004_group was positively associated with Oscillibacter (0.97), unclassified_Ruminococcaceae (0.94), CHKCI002 (0.96), and unclassified_Lachnospiraceae (0.95). Flavonifractor was positively closely related to Oscillibacter (0.96), Faecalibacterium (0.95), CHKCI002 (0.94) and Colidextribacter (0.94).
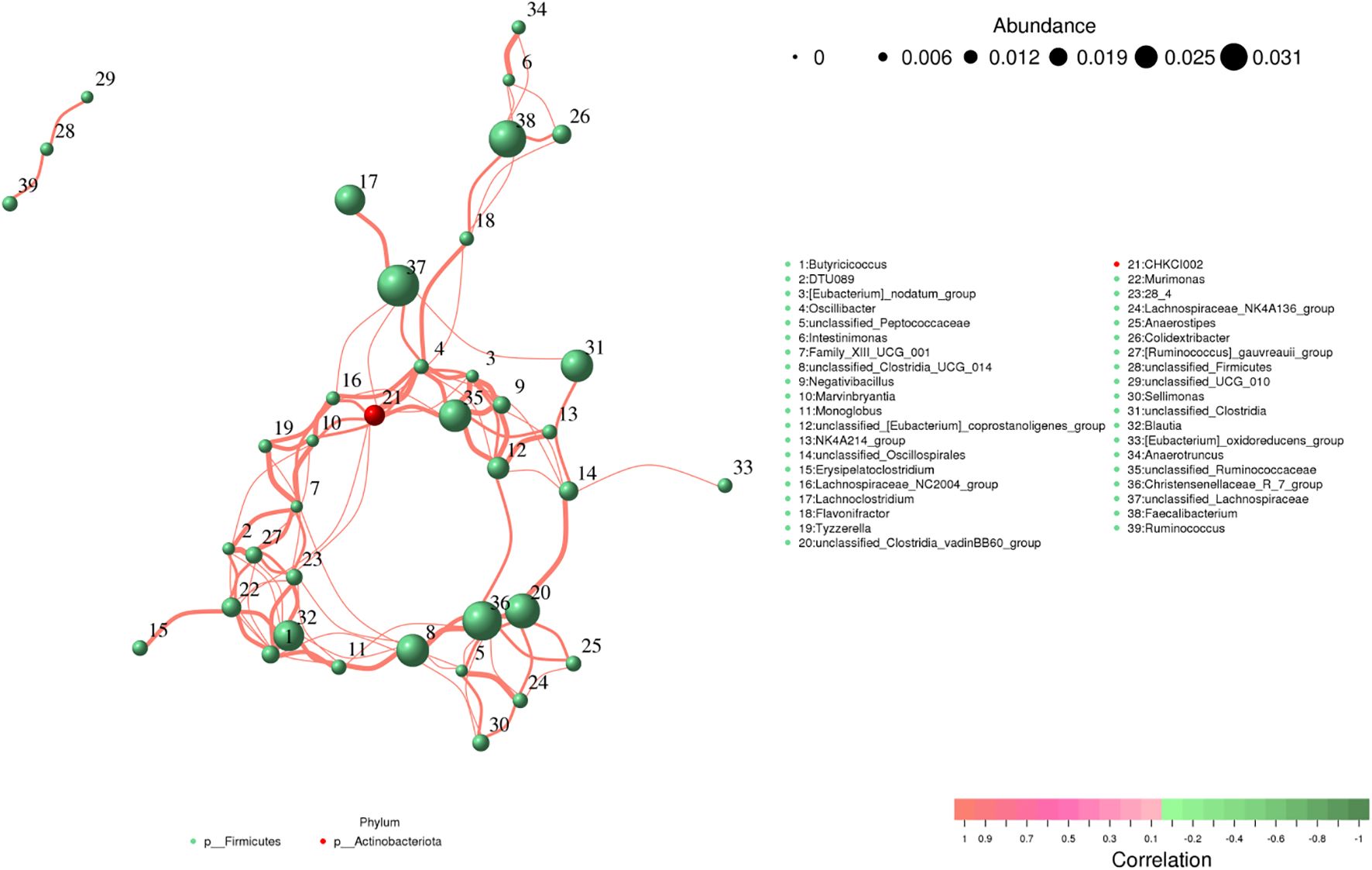
Figure 9. Correlation analysis of intestinal flora. The correlation between bacteria is shown by line segments. The thickness of the lines indicates the strength of the correlations, where orange lines represent positive correlations.
Battery exposure changed the intestinal function
In the KEGG functional prediction analysis, the EXE had significantly higher relative abundances of carbohydrate metabolism, lipid metabolism, amino acid metabolism, nucleotide metabolism, metabolism of terpenoids and polyketides, xenobiotics biodegradation and metabolism, metabolism of other amino acids, glycan biosynthesis and metabolism, translation, drug resistance: Antimicrobial, transcription, replication and repair, endocrine system, signaling molecules and interaction, cell growth and death, excretory system and immune diseases, whereas metabolism of cofactors and vitamins, energy metabolism, biosynthesis of other secondary metabolites, global and overview maps, signal transduction, cell motility, folding, sorting and degradation, transport and catabolism, aging, immune system, environmental adaptation, endocrine and metabolic diseases, digestive system and neurodegenerative diseases were observed to be more abundant in the CON (Figure 10A). As for the COG functional prediction analysis, the relative proportions of nucleotide transport and metabolism, lipid transport and metabolism, translation, ribosomal structure and biogenesis, replication, recombination and repair, cell wall/membrane/envelope biogenesis, posttranslational modification, protein turnover, chaperones, general function prediction only, function unknown and intracellular trafficking, secretion, and vesicular transport in the EXE was significantly higher than that in the CON, whereas the relative proportions of energy production and conversion, amino acid transport and metabolism, coenzyme transport and metabolism, transcription, cell motility, inorganic ion transport and metabolism, signal transduction mechanisms, defense mechanisms and cytoskeleton was lower (Figure 10B).
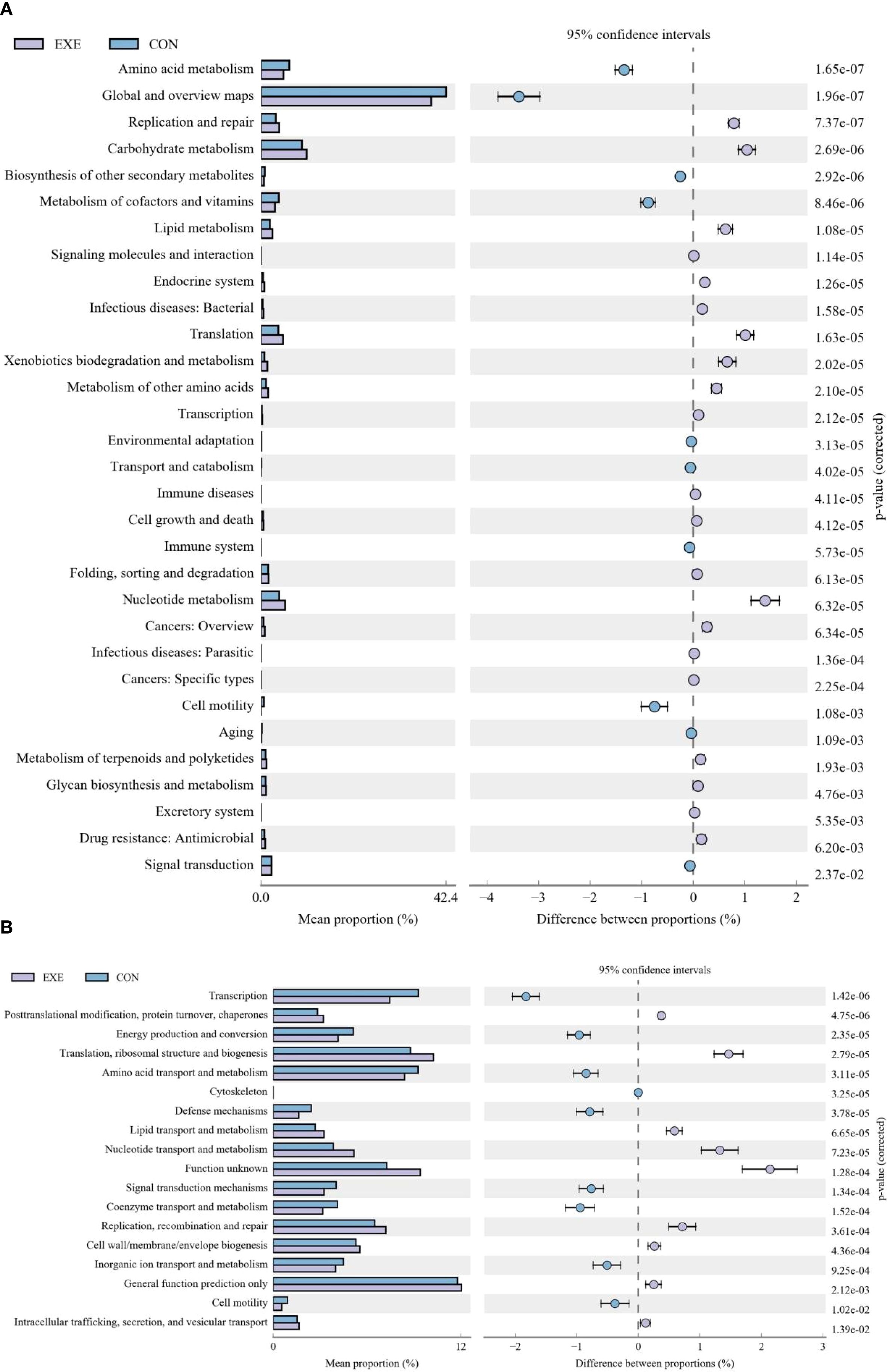
Figure 10. Effects of battery exposure on intestinal function in broiler chickens analyzed by KEGG (A) and COG (B).
Discussion
As a crucial energy carrier, battery play an irreplaceable role in various sectors, including industry, agriculture, and daily life (Wang et al., 2009). However, the recycling and reuse of battery remain significant challenges that cannot be overlooked. Ineffective recycling of discarded battery may lead to the release of toxic components into the ecological environment, ultimately compromising environmental health and food safety (Gottesfeld et al., 2018). Currently, the environmental pollution and increased governance costs associated with battery waste leakage have garnered the attention of numerous countries and institutions. Furthermore, the threat posed by battery pollution to public health and livestock production has also attracted widespread concern. Several studies have indicated the detrimental effects of batteries on the health of zebrafish and mice (Liao et al., 2022; Wang et al., 2022c). Previous studies mainly focused on model animals, with limited research conducted on the effects of battery exposure on chickens. In this study, we investigated the potential toxic effects of battery leakage on broiler chickens.
The diversity index of the gut microbiota serves as a crucial indicator of gut microbiota homeostasis and is influenced by various factors (Wen et al., 2024; Chen et al., 2025b). Generally, the gut microbial diversity index correlates positively with microbial plasticity and metabolic capacity (Laitinen and Mokkala, 2019). Conversely, gut microbial diversity is recognized as a significant marker for several diseases (Li et al., 2016). For instance, a reduction in gut microbial diversity has been closely linked to the development of chronic diseases, including colitis, diabetes, and Alzheimer’s disease (Kathania et al., 2020; Cheng et al., 2023). In this study, we observed a significant decrease in the diversity and abundance of the gut microbiota in chickens exposed to battery, indicating gut microbial dysbiosis (Sun et al., 2022; Wang et al., 2022b). Notably, previous research has also revealed the adverse effects of battery exposure on the gut microbiota in mice. Numerous research suggests that gut microbial dysbiosis can adversely affect host health and intestinal function. For instance, gut microbial dysbiosis may result in increased intestinal permeability, allowing endotoxins to traverse the intestinal barrier and enter the bloodstream, resulting in systemic effects. In this study, we found significant changes in the spleen, thymus, and antioxidant-related indices in chickens following battery exposure. The spleen is a crucial lymphoid organ in the host, performing multiple functions, including immunity, blood storage, hematopoiesis, and blood filtration. Additionally, the spleen maintains blood health and the host defense capabilities by removing aging red blood cells, storing platelets, and producing immune cells (Rosado et al., 2018). The thymus serves as an important lymphatic and endocrine organ, with core functions that include T cell differentiation and maturation, immune regulation, and endocrine effects (Lewis et al., 2019; Du et al., 2020). Therefore, battery exposure may influence the immune function of chickens by impacting spleen and thymus indices. Furthermore, gut microbial dysbiosis is closely linked to the immune system. However, whether exposure to batteries can affect host immunity through alterations in gut microbioat requires further investigation.
Importantly, this research also indicated that battery exposure cause distinct changes in some functional bacteria, which may be critical for host health and intestinal homeostasis. Notably, most of these quantitatively decreased bacteria (Coprococcus, Lachnospiraceae_NK4A136_group, Ruminococcus_torques_group, Lachnospiraceae_NC2004_group, Ruminococcus_gauvreauii_group, Oscillibacter, Alistipes, Intestinimonas, Ruminococcus, Roseburia, Romboutsia, Faecalibacterium, Bacillus, Lachnospiraceae_UCG_008, Blautia, Lachnospiraceae_UCG_010, Lachnospiraceae_UCG_006, Lachnospiraceae_FCS020_group, Acetitomaculum and Bifidobacterium, etc.) in the battery exposure group were deemed as intestinal beneficial bacteria, implying that the current intestinal environment is not conducive to the survival of these bacteria. Faecalibacterium possesses numerous important physiological functions and plays a crucial role in gut-host interactions. Previous research indicated that Faecalibacterium is negatively correlated with type 2 diabetes, non-alcoholic fatty liver disease (NAFLD), and obesity (Ganesan et al., 2018). Conversely, supplementation with Faecalibacterium can reduce plasma endotoxin (LPS) concentrations and maintain gut microbial balance, thereby alleviating conditions such as atherosclerosis, colitis, and NAFLD (Xu et al., 2020; Safarzadeh et al., 2025). The Lachnospiraceae is widely distributed throughout the animal gastrointestinal tract and is essential for energy metabolism, immune regulation, and disease progression (Zhang et al., 2023a). Ruminococcus effectively breaks down complex polysaccharides, including resistant starch and plant cell walls, by secreting cellulases and hemicellulases, which enhances host energy utilization (Pal et al., 2021). Furthermore, abnormal levels of Ruminococcus have been closely associated with obesity, diabetes, and enteritis (Nie et al., 2025). Previous studies indicated that Butyricicoccus can ferment dietary fiber to produce butyrate via the Acetyl-CoA pathway, playing a critical role in maintaining the intestinal barrier and combating inflammation (Boesmans et al., 2018). Christensenellaceae has been linked to host metabolic health, participating in the degradation of complex polysaccharides and maintaining gut microbiota balance (Waters and Ley, 2019). Flavonifractor, a gram-negative anaerobic bacterium, exhibits anti-inflammatory properties and protects the intestinal barrier (Mikami et al., 2020). Additionally, the abundance of Flavonifractor is significantly reduced in patients with irritable bowel syndrome, inflammatory bowel disease, and type 2 diabetes. Bifidobacterium, a core beneficial gut bacterium, regulates various physiological functions, including immune homeostasis, energy metabolism, and neuroendocrine function, through microbiota-host interactions (Gavzy et al., 2023). Research indicates that Bifidobacterium not only preserves intestinal barrier integrity by competitively inhibiting pathogen colonization but also directly modulates host epigenetic modifications via the secretion of active substances such as exopolysaccharides (Meng et al., 2024). Furthermore, Bifidobacterium specifically recognizes Toll-like receptor 2 (TLR2) on dendritic cells, initiating downstream signaling pathways and significantly enhancing the secretion of the anti-inflammatory cytokine IL-10 (Akkerman et al., 2025). Recent investigations have also highlighted the potential of Bifidobacterium in alleviating intestinal inflammation, obesity, and diabetes (Zhang et al., 2025). Numerous studies have shown that Bacillus produces broad-spectrum natural antimicrobial substances that not only directly inhibit the growth of pathogenic bacteria but also disrupt their cell membrane structures (Choyam et al., 2021). Additionally, Bacillus can enhance host immunity by increasing macrophage phagocytosis, the secretion of anti-inflammatory factors, and immunoglobulin levels (Wang et al., 2022a). In the livestock industry, Bacillus is widely utilized as a new, green feed additive to maintain livestock health and promote growth performance by balancing gut microbiota, enhancing intestinal immunity, and secreting digestive enzymes (Chen et al., 2021; Yu et al., 2024). Notably, battery exposure also led to a significant reduction in some bacteria (Bifidobacterium, Bacillus, Flavonifractor, Christensenellaceae, Acetitomaculum, Blautia, Roseburia, Intestinimonas, Coprococcus, Lachnospiraceae_NK4A136_group, Lachnospiraceae_NC2004_group, Lachnospiraceae_UCG_010, Lachnospiraceae_UCG_006, Lachnospiraceae_FCS020_group, Ruminococcus_torques_group, Ruminococcus_gauvreauii_group, Flavonifractor, Oscillibacter, Alistipes, Faecalibacterium and Butyricicoccus) that produce SCFAs. SCFAs, recognized as pivotal signaling molecules in the interaction between gut microbiota and the host, have garnered significant attention in recent years due to their multifaceted roles in maintaining intestinal homeostasis and modulating host metabolism and immunity (Jadhav et al., 2022; Ma et al., 2022a). For instance, SCFAs can exert systemic effects on the host’s nervous, endocrine, and cardiovascular systems by mediating the activation of G protein-coupled receptors and inhibiting histone deacetylases (Lu et al., 2016; Yang et al., 2018). Furthermore, diminished levels of SCFAs can compromise the integrity of the intestinal barrier, causing endotoxemia and chronic inflammation, which may promote insulin resistance and fat accumulation, ultimately resulting in obesity and type 2 diabetes (Ma et al., 2022b). Notably, SCFAs also play a crucial role in autoimmune diseases such as rheumatoid arthritis and inflammatory bowel disease by regulating the balance between Th17 and Treg cells (Sun et al., 2017). Interestingly, previous studies have also demonstrated the effects of battery exposure on the gut microbiota of mice, which is accompanied by a significant reduction in beneficial bacteria (Liao et al., 2022). These findings highlight the negative impact of battery exposure on the host’s intestinal homeostasis.
Conclusion
In conclusion, this study investigated the negative effects of battery exposure on gut microbiota in chickens. Results indicated that battery exposure can lead to gut microbial dysbiosis, primarily characterized by decreased microbial diversity and altered microbial composition. Furthermore, battery exposure also results in abnormalities in immune organ indices and serum biochemical markers. This study provides detailed and novel insights into the interplay between gut microbiota and host health under battery exposure conditions. Additionally, it expands the understanding of the toxic effects of battery, revealing that gut microbial dysbiosis may be significant mechanisms through which battery exerts its toxic effects. Future research should focus on the potential role of gut microbiota in monitoring poisoning and treating toxic diseases in animals.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by ethics committee of the Shangqiu Normal University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XQ: Writing – original draft. SS: Writing – review & editing. GX: Writing – review & editing. SW: Writing – review & editing. SL: Writing – review & editing. CY: Writing – review & editing. XW: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study was supported by Research project of Guangdong Province Science and Technology Project (2023B1212060040).
Conflict of interest
Author SW was employed by Zhejiang Hisun Animal Healthcare Products Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akkerman, R., Oerlemans, M., Ferrari, M., Fernandez-Lainez, C., Walvoort, M., and de Vos, P. (2025). Exopolysaccharides from bifidobacterium longum subsp. Infantis and bifidobacterium adolescentis modulate toll-like receptor signaling. Carbohydr. Polym. 349, 123017. doi: 10.1016/j.carbpol.2024.123017
Biagi, E., Rampelli, S., Turroni, S., Quercia, S., Candela, M., and Brigidi, P. (2017). The gut microbiota of centenarians: signatures of longevity in the gut microbiota profile. Mech. Ageing. Dev. 165, 180–184. doi: 10.1016/j.mad.2016.12.013
Boesmans, L., Valles-Colomer, M., Wang, J., Eeckhaut, V., Falony, G., Ducatelle, R., et al. (2018). Butyrate producers as potential next-generation probiotics: safety assessment of the administration of butyricicoccus pullicaecorum to healthy volunteers. mSystems 3, e00094-18. doi: 10.1128/mSystems.00094-18
Celorrio, M., Abellanas, M. A., Rhodes, J., Goodwin, V., Moritz, J., Vadivelu, S., et al. (2021). Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis. Acta Neuropathol. Commun. 9, 40. doi: 10.1186/s40478-021-01137-2
Chen, M., Liao, Z., Yang, Z., Li, Y., Ma, L., and Hu, J. (2025a). Diversity analysis of oral and gut microbiota in osteoporotic rats. PLoS One 20, e320063. doi: 10.1371/journal.pone.0320063
Chen, J., Lv, Z., Cheng, Z., Wang, T., Li, P., Wu, A., et al. (2021). Bacillus amyloliquefaciens b10 inhibits aflatoxin b1-induced cecal inflammation in mice by regulating their intestinal flora. Food. Chem. Toxicol. 156, 112438. doi: 10.1016/j.fct.2021.112438
Chen, Q. Z., Shang, J. M., Jiang, Y. Q., Yang, Y., Zang, C. X., Ma, J. W., et al. (2025b). Gut microbial dysbiosis aggravated parkinson-like pathology induced by mptp/probenecid. Physiol. Behav. 299, 115008. doi: 10.1016/j.physbeh.2025.115008
Cheng, H., Zhang, D., Wu, J., Liu, J., Tan, Y., Feng, W., et al. (2023). Atractylodes macrocephala koidz. Volatile oil relieves acute ulcerative colitis via regulating gut microbiota and gut microbiota metabolism. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1127785
Choyam, S., Jain, P. M., and Kammara, R. (2021). Characterization of a potent new-generation antimicrobial peptide of bacillus. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.710741
Debrah, J. K., Teye, G. K., and Dinis, M. (2024). Factors influencing management of dry cell battery waste: a case of greater accra region in Ghana. Environ. Monit. Assess. 196, 1181. doi: 10.1007/s10661-024-13297-4
Du, Z., Wang, Q., Huang, X., Yi, S., Mei, S., Yuan, G., et al. (2020). Effect of berberine on spleen transcriptome and gut microbiota composition in experimental autoimmune uveitis. Int. Immunopharmacol. 81, 106270. doi: 10.1016/j.intimp.2020.106270
Ganesan, K., Chung, S. K., Vanamala, J., and Xu, B. (2018). Causal relationship between diet-induced gut microbiota changes and diabetes: a novel strategy to transplant faecalibacterium prausnitzii in preventing diabetes. Int. J. Mol. Sci. 19, 3720. doi: 10.3390/ijms19123720
Gavzy, S. J., Kensiski, A., Lee, Z. L., Mongodin, E. F., Ma, B., and Bromberg, J. S. (2023). Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 15, 2291164. doi: 10.1080/19490976.2023.2291164
Gottesfeld, P., Were, F. H., Adogame, L., Gharbi, S., San, D., Nota, M. M., et al. (2018). Soil contamination from lead battery manufacturing and recycling in seven african countries. Environ. Res. 161, 609–614. doi: 10.1016/j.envres.2017.11.055
Hou, J., Wu, P., Cai, J., Xia, B., Lei, Y., Huang, C., et al. (2025). Gut microbiota dysbiosis amplifies thiram hepatotoxicity via a mitochondrial-autophagy-apoptosis nexus orchestrated by the gut-liver axis. Cell. Signal. 136, 112104. doi: 10.1016/j.cellsig.2025.112104
Huang, Q., Yu, F., Liao, D., and Xia, J. (2020). Microbiota-immune system interactions in human neurological disorders. CNS Neurol. Disord.-Drug Targets 19, 509–526. doi: 10.2174/1871527319666200726222138
Jabeen, Z., Bukhari, S., Malik, S., Hussain, G., and Kamal, S. (2023). Improved gut microbiota escalates muscle function rehabilitation and ameliorates oxidative stress following mechanically induced peripheral nerve injury in mice. Pak. Vet. J. 43, 707–713. doi: 10.29261/pakvetj/2023.098
Jadhav, V. V., Han, J., Fasina, Y., and Harrison, S. H. (2022). Connecting gut microbiomes and short chain fatty acids with the serotonergic system and behavior in gallus gallus and other avian species. Front. Physiol. 13. doi: 10.3389/fphys.2022.1035538
Jiang, J. W., Chen, X. H., Ren, Z., and Zheng, S. S. (2019). Gut microbial dysbiosis associates hepatocellular carcinoma via the gut-liver axis. Hepatob. Pancreatic. Dis. Int. 18, 19–27. doi: 10.1016/j.hbpd.2018.11.002
Jubair, W. K., Hendrickson, J. D., Severs, E. L., Schulz, H. M., Adhikari, S., Ir, D., et al. (2018). Modulation of inflammatory arthritis in mice by gut microbiota through mucosal inflammation and autoantibody generation. Arthritis Rheumatol. 70, 1220–1233. doi: 10.1002/art.40490
Kathania, M., Tsakem, E. L., Theiss, A. L., and Venuprasad, K. (2020). Gut microbiota contributes to spontaneous colitis in e3 ligase itch-deficient mice. J. Immunol. 204, 2277–2284. doi: 10.4049/jimmunol.1701478
Laitinen, K. and Mokkala, K. (2019). Overall dietary quality relates to gut microbiota diversity and abundance. Int. J. Mol. Sci. 20, 1835. doi: 10.3390/ijms20081835
Lewis, S. M., Williams, A., and Eisenbarth, S. C. (2019). Structure and function of the immune system in the spleen. Sci. Immunol. 4, 6085. doi: 10.1126/sciimmunol.aau6085
Li, Y., Li, C., Xu, W., Zhao, J., Liu, K., Liu, X., et al. (2025). Chondroitin sulfate reverses tibial dyschondroplasia, broiler chondrocyte proliferation and differentiation dysfunction via the CHST11/β-Catenin pathway. Int. J. Biol. Macromol. 315, 144488. doi: 10.1016/j.ijbiomac.2025.144488
Li, H., Qu, J., Li, T., Li, J., Lin, Q., and Li, X. (2016). Pika population density is associated with the composition and diversity of gut microbiota. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.00758
Liao, J., Liu, Y., Yi, J., Li, Y., Li, Q., Li, Y., et al. (2022). Gut microbiota disturbance exaggerates battery wastewater-induced hepatotoxicity through a gut-liver axis. Sci. Total Environ. 809, 152188. doi: 10.1016/j.scitotenv.2021.152188
Liu, Z., Jiang, Z., Zhang, Z., Liu, T., Fan, Y., Liu, T., et al. (2022). Bacillus coagulans in combination with chitooligosaccharides regulates gut microbiota and ameliorates the dss-induced colitis in mice. Microbiol. Spectr. 10, e64122. doi: 10.1128/spectrum.00641-22
Lu, Y., Fan, C., Li, P., Lu, Y., Chang, X., and Qi, K. (2016). Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating g protein-coupled receptors and gut microbiota. Sci. Rep. 6, 37589. doi: 10.1038/srep37589
Ma, J., Piao, X., Mahfuz, S., Long, S., and Wang, J. (2022a). The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 9, 159–174. doi: 10.1016/j.aninu.2021.09.012
Ma, J., Piao, X., Mahfuz, S., Long, S., and Wang, J. (2022b). The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 9, 159–174. doi: 10.1016/j.aninu.2021.09.012
Mehmood, A., Nawaz, M., Rabbani, M., and Mushtaq, M. (2023). Probiotic effect of Limosilactobacillus fermentum on growth performance and competitive exclusion of Salmonella gallinarum in poultry. Pak. Vet. J. 43, 659–664. doi: 10.29261/pakvetj/2023.103
Meng, Y., Zhao, M., Ma, Q., Hua, Q., Hu, J., Zhou, Q., et al. (2024). Bifidobacterium bifidum alleviates adenine-induced acute kidney injury in mice by improving intestinal barrier function. Food Funct. 15, 8030–8042. doi: 10.1039/d4fo02014f
Mikami, A., Ogita, T., Namai, F., Shigemori, S., Sato, T., and Shimosato, T. (2020). Oral administration of flavonifractor plautii attenuates inflammatory responses in obese adipose tissue. Mol. Biol. Rep. 47, 6717–6725. doi: 10.1007/s11033-020-05727-6
Nie, H. Y., Zhao, M. F., Wu, T. Y., Zou, M. J., Tang, Y. P., Wang, X. C., et al. (2025). Elevated mevalonolactone from ruminococcus torques contributes to metabolically unhealthy obesity development. J. Biol. Chem. 301, 110281. doi: 10.1016/j.jbc.2025.110281
Nogal, A., Valdes, A. M., and Menni, C. (2021). The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 13, 1–24. doi: 10.1080/19490976.2021.1897212
Pal, D., Naskar, M., Bera, A., and Mukhopadhyay, B. (2021). Chemical synthesis of the pentasaccharide repeating unit of the o-specific polysaccharide from ruminococcus gnavus. Carbohydr. Res. 507, 108384. doi: 10.1016/j.carres.2021.108384
Passos, G. A. and Chaturvedi, V. K. (2021). Preface special issue: microbiome-immune system interactions. Crit. Rev. Immunol. 41, v. doi: 10.1615/CritRevImmunol.2022043499
Peng, S., Xu, C., He, Q., Xu, J., Kiani, F., Choudhary, O., et al. (2024). Fucoidan alleviates intestine damage in mice induced by LPS via regulation of microbiota. Pak. Vet. J. 44, 517–525. doi: 10.29261/pakvetj/2024.190
Rosado, M. M., Aranburu, A., Scarsella, M., Cascioli, S., Giorda, E., Del, C. F., et al. (2018). Spleen development is modulated by neonatal gut microbiota. Immunol. Lett. 199, 1–15. doi: 10.1016/j.imlet.2018.04.010
Safarzadeh, H., Aliramezani, A., Rahimi, A., Yousefi, B., and Heidarzadeh, S. (2025). The advantages of faecalibacterium prasnitzii in gestational diabetes mellitus. Future Microbiol. 20, 695–706. doi: 10.1080/17460913.2025.2520699
Shen, L., Shen, Y., You, L., Zhang, Y., Su, Z., Peng, G., et al. (2022). Pueraria lobata polysaccharides alleviate neonatal calf diarrhea by modulating gut microbiota and metabolites. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.1024392
Sun, R., Niu, H., Sun, M., Miao, X., Jin, X., Xu, X., et al. (2022). Effects of bacillus subtilis natto jlcc513 on gut microbiota and intestinal barrier function in obese rats. J. Appl. Microbiol. 133, 3634–3644. doi: 10.1111/jam.15797
Sun, M., Wu, W., Liu, Z., and Cong, Y. (2017). Microbiota metabolite short chain fatty acids, gpcr, and inflammatory bowel diseases. J. Gastroenterol. 52, 1–8. doi: 10.1007/s00535-016-1242-9
Tong, S., Zhang, P., Cheng, Q., Chen, M., Chen, X., Wang, Z., et al. (2022). The role of gut microbiota in gout: is gut microbiota a potential target for gout treatment. Front. Cell. Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.1051682
Urrutia-Goyes, R., Argyraki, A., and Ornelas-Soto, N. (2018). Characterization of soil contamination by lead around a former battery factory by applying an analytical hybrid method. Environ. Monit. Assess. 190, 429. doi: 10.1007/s10661-018-6820-2
Wang, W. Q., Chen, H. H., Zhao, W. J., Fang, K. M., Sun, H. J., and Zhu, F. Y. (2022c). Ecotoxicological assessment of spent battery extract using zebrafish embryotoxicity test: a multi-biomarker approach. Chemosphere 287, 132120. doi: 10.1016/j.chemosphere.2021.132120
Wang, J., Ishfaq, M., Miao, Y., Liu, Z., Hao, M., Wang, C., et al. (2022a). Dietary administration of bacillus subtilis kc1 improves growth performance, immune response, heat stress tolerance, and disease resistance of broiler chickens. Poult. Sci. 101, 101693. doi: 10.1016/j.psj.2021.101693
Wang, F., Leung, A. O., Wu, S. C., Yang, M. S., and Wong, M. H. (2009). Chemical and ecotoxicological analyses of sediments and elutriates of contaminated rivers due to e-waste recycling activities using a diverse battery of bioassays. Environ. pollut. 157, 2082–2090. doi: 10.1016/j.envpol.2009.02.015
Wang, R., Yang, X., Liu, J., Zhong, F., Zhang, C., Chen, Y., et al. (2022b). Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat. Commun. 13, 2522. doi: 10.1038/s41467-022-30240-8
Wang, D., Zeng, J., Wujin, C., Ullah, Q., and Su, Z. (2024). Lactobacillus reuteri derived from horse alleviates escherichia coli-induced diarrhea by modulating gut microbiota. Microb. Pathog. 188, 106541. doi: 10.1016/j.micpath.2024.106541
Wang, Y., Zhang, X., Tang, G., Deng, P., Qin, Y., Han, J., et al. (2023). The causal relationship between gut microbiota and bone mineral density: a mendelian randomization study. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1268935
Waters, J. L. and Ley, R. E. (2019). The human gut bacteria christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 17, 83. doi: 10.1186/s12915-019-0699-4
Wen, M., Chen, S., Zhang, Y., Liu, Y., Tang, C., Zhang, J., et al. (2024). Diversity and host interaction of the gut microbiota in specific pathogen-free pigs. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1402807
Wu, S., Liu, K., Huang, X., Sun, Q., Wu, X., and Mehmood, K. (2024). Molecular mechanism of miR-203a targeting Runx2 to regulate thiram induced-chondrocyte development. Pestic. Biochem. Physiol. 200, 105817. doi: 10.1016/j.pestbp.2024.105817
Wu, H., Tremaroli, V., Schmidt, C., Lundqvist, A., Olsson, L. M., Kramer, M., et al. (2020). The gut microbiota in prediabetes and diabetes: a population-based cross-sectional study. Cell Metab. 32, 379–390. doi: 10.1016/j.cmet.2020.06.011
Xiao, J., Niu, B., and Xu, Z. (2021). Highly efficient selective recovery of lithium from spent lithium-ion batteries by thermal reduction with cheap ammonia reagent. J. Hazard. Mater. 418, 126319. doi: 10.1016/j.jhazmat.2021.126319
Xie, Q., Zhang, Y., Zhang, Z., Gong, S., Mo, Q., and Li., J. (2024). Characteristics and dynamic changes of gut microbiota in cats with colitis. Pak. Vet. J. 44, 414–422. doi: 10.29261/pakvetj/2024.175
Xie, Z., Zhang, X., Zhang, Z., and Zhou, Z. (2017). Metal-co(2) batteries on the road: co(2) from contamination gas to energy source. Adv. Mater. 29. doi: 10.1002/adma.201605891
Xu, J., Liang, R., Zhang, W., Tian, K., Li, J., Chen, X., et al. (2020). Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression. J. Diabetes 12, 224–236. doi: 10.1111/1753-0407.12986
Yan, Q., Cai, L., and Guo, W. (2022). New advances in improving bone health based on specific gut microbiota. Front. Cell. Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.821429
Yang, G., Chen, S., Deng, B., Tan, C., Deng, J., Zhu, G., et al. (2018). Implication of g protein-coupled receptor 43 in intestinal inflammation: a mini-review. Front. Immunol. 9. doi: 10.3389/fimmu.2018.01434
Yu, H., Rahman, A., Sadique, M., Batool, T., Imtiaz, B., Zaman, M., et al. (2024). Impact of Bacillus subtilis probiotic on growth performance, bone health, intestinal morphology, and cecal microbiota in cobb broiler chicks. Pak. Vet. J. 44, 1243–1248. doi: 10.29261/pakvetj/2024.254
Zhang, B., Qiu, J., Qu, Z., Xiao, R., Wang, L., Tian, P., et al. (2025). Bifidobacterium adolescentis fjssz23m10 modulates gut microbiota and metabolism to alleviate obesity through strain-specific genomic features. Food Funct. 16, 2415–2431. doi: 10.1039/d4fo06449f
Zhang, X., Yu, D., Wu, D., Gao, X., Shao, F., Zhao, M., et al. (2023a). Tissue-resident lachnospiraceae family bacteria protect against colorectal carcinogenesis by promoting tumor immune surveillance. Cell Host Microbe 31, 418–432. doi: 10.1016/j.chom.2023.01.013
Zhang, Z., Zhang, Z., Shu, H., Meng, Y., Lin, T., Ma, J., et al. (2023b). Association between gut microbiota and bone metabolism: insights from bibliometric analysis. Front. Physiol. 14. doi: 10.3389/fphys.2023.1156279
Keywords: battery, pollutant, gut microbiota, diversity, chicken
Citation: Qin X, Song S, Xiang G, Wang S, Luo S, Yang C and Wen X (2025) Battery pollutant leakage disrupts antioxidant ability and gut microbial homeostasis of chickens. Front. Cell. Infect. Microbiol. 15:1682969. doi: 10.3389/fcimb.2025.1682969
Received: 10 August 2025; Accepted: 12 September 2025;
Published: 17 October 2025.
Edited by:
Jianzhao Liao, South China Agricultural University, ChinaReviewed by:
Tariq Jamil, University of Veterinary and Animal Sciences, PakistanYanfang Lan, Wuhan Business University, China
Copyright © 2025 Qin, Song, Xiang, Wang, Luo, Yang and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Wen, d2VueGlhb2h1aUBnZGFhcy5jbg==
 Xinxi Qin
Xinxi Qin Shuai Song
Shuai Song Guoqing Xiang
Guoqing Xiang Shengmin Wang5
Shengmin Wang5 Shengjun Luo
Shengjun Luo Caijuan Yang
Caijuan Yang Xiaohui Wen
Xiaohui Wen