- Department of Biological Sciences, Sam Houston State University, Huntsville, TX, United States
Galleria mellonella larva have served as a simple, cost-effective model for studying innate immunity and Campylobacter jejuni infection. The model commonly employs an acute, high-dose septic infection via hemocoel injection, with observable endpoints of death and melanization. Studies using G. mellonella have provided insights into C. jejuni virulence factors, including the capsule, transcriptional regulators, outer membrane vesicles, and a T6SS. It has revealed signals for virulence, such as pancreatic amylase and growth temperature, and also allowed for comparisons between C. jejuni strains and across multiple species in the genus. Limitations include the use of high bacterial doses that may obscure the role of specific virulence factors, lack of accounting for larval size variations, and unclear connection to the human anaerobic, microbially-rich gut environment. Future development of this model could allow oral infections for exploring pathogen-microbiome interactions and further assessing mechanisms of this important pathogen.
Introduction
Campylobacter is the leading cause of bacterial foodborne gastroenteritis worldwide. While there are 50 valid species in this genus of Gram-negative curved rods (Parte et al., 2020), C. jejuni and C. coli are responsible for more than 90% of human infections (Tikhomirova et al., 2024). Typically, humans have self-limiting gastroenteritis, with a fever and vomiting phase of one to three days, then abdominal pain with watery or bloody diarrhea for an additional three to seven days (Kim et al., 2021). Antibiotic treatment is uncommon, reserved for immunocompromised patients, and typically straightforward, although fluoroquinolone resistance is increasing (Sproston et al., 2018; Whelan et al., 2019). However, rare but chronic post-infection complications can occur, including Guillain-Barre syndrome and reactive arthritis. These complications are likely due to antigen cross-reactivity (Finsterer, 2022) between human antigens and C. jejuni unusual glycomodifications on the capsular polysaccharides, lipooligosaccharides (LOS), and flagella (Omole et al., 2024). There are two subspecies within C. jejuni, which are Cj subsp. jejuni and Cj subsp. doylei, which is rare and linked to human cases of gastroenteritis that escape the gut and lead to bacteremia (Miller et al., 2007). This review will focus on Cj subsp. jejuni, as none of the 24 studies discussed here included Cj subsp. doylei. Campylobacter has some major differences compared to other common enterics, such as Salmonella or Enterobacter. It is microaerobic (preferring ~5% O2), has a comparatively small genome, different virulence factors, and a relatively low infective dose.
Many virulence models have been developed for C. jejuni, none being a close match to the human clinical presentation. The models include human intestinal epithelial cells in culture (for adhesion and invasion) (Harrer et al., 2019), ferrets (Nemelka et al., 2009), infant rabbits (Shang et al., 2016), pigs (Rath et al., 2022), Drosophila (Myles et al., 2024), antibiotic pre-treated mice (Yu et al., 2016), primates (Crofts et al., 2018), Acanthamoeba (Shagieva et al., 2021), and in limited studies even human volunteers (Crofts et al., 2018). Since chickens are a major reservoir and source for human infections, chick colonization (Davis and DiRita, 2008) is also an important system, although not modeling human infections.
Galleria as a model
Larva of Galleria mellonella, the greater wax moth or the honeycomb moth, have been used as a simple, inexpensive model for interactions of innate host defense with pathogens (Wojda et al., 2020). Studies with 11 different bacterial pathogens, including C. jejuni, are summarized in a 2021 review (Ménard et al., 2021). Immune defenses in the body cavity include antimicrobial peptides, lysozyme, six types of hemocytes, and melanization. Methods typically involve infection of the larva with death as an endpoint. The immune reaction of melanization is also observable, as it is easily visualized as darkening. Melanization can be recorded in more detail by pattern (Hesketh-Best et al., 2021) or in a scoring system (Mehat et al., 2018; Emery et al., 2021; Ménard et al., 2021). Hemolymph can be isolated by squeezing from the larva’s body, then used to microscopically examine bacterial presence in fluids or hemocytes. Tissues can be isolated and examined similarly. In addition to pathogenesis, larva have been used to examine antimicrobials, probiotics, and phage therapy (Ménard et al., 2021). G. mellonella are one of the few insect models that allow for incubation at mammalian body temperature of 37°C. With developments in genetic manipulation, genomics, and microbiomes, this system will continue to expand in utility for infection biology. Gene knockdown is already available (Dutta et al., 2021). No review of the Galleria model with Campylobacter has been published, although a book chapter in 2017 focused on methods did include a brief evaluation of the field (Askoura and Stintzi, 2017).
Virulence factors
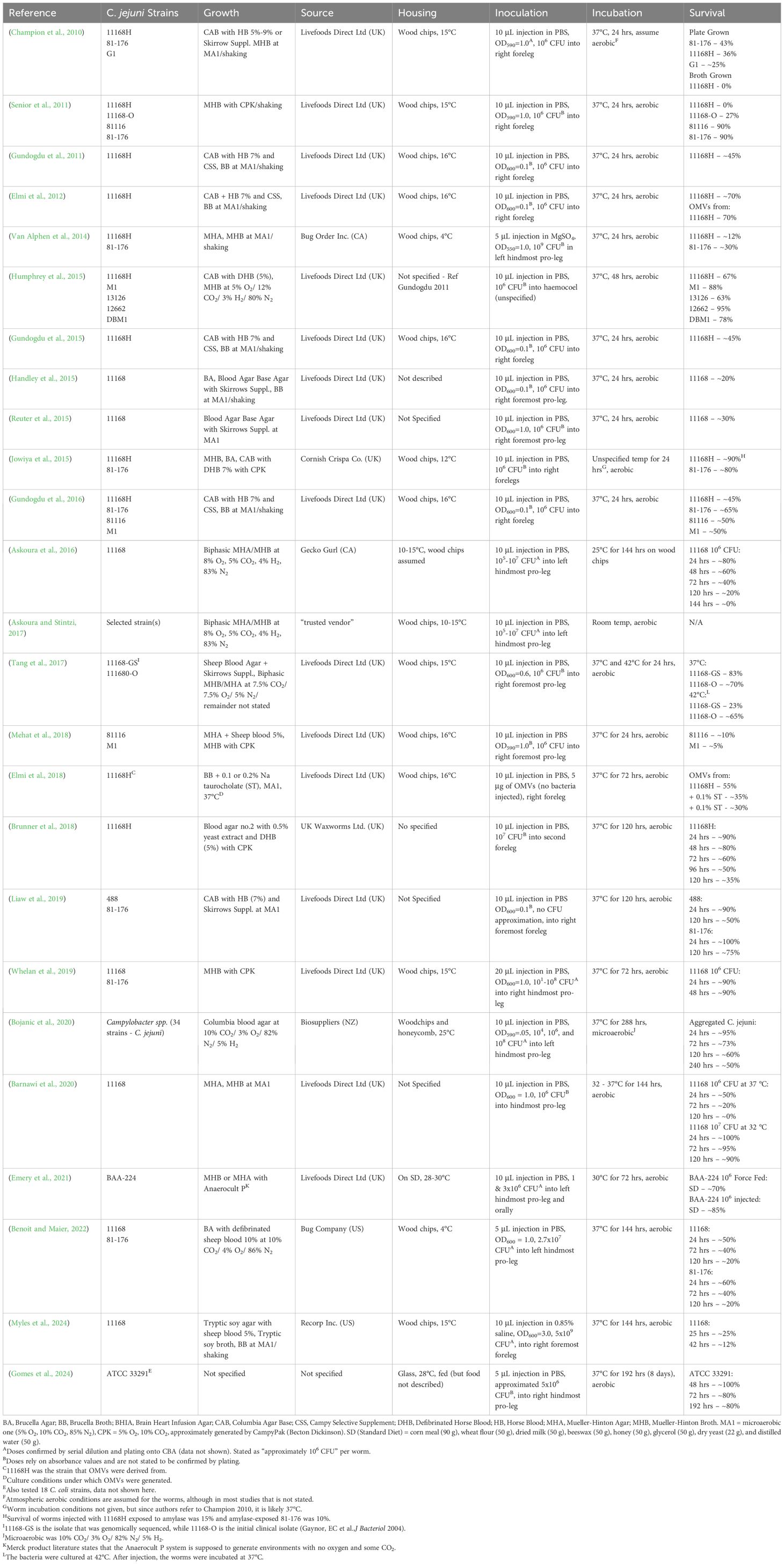
The earliest publication using Galleria with Campylobacter was in 2010 (Champion et al., 2010), which shaped the field as far as experimental methods and approaches. This is an acute, high dose, septic infection model, intended to examine innate host defenses. A major defense mechanism in larva is hemocytes, which are phagocytes analogous to neutrophils in humans (Wojda et al., 2020). Since a hallmark of human Campylobacter infections is gut inflammation (Omole et al., 2024), and neutrophil infiltration into tissues is a major inflammatory marker, bacteria-phagocyte interactions are important to examine. However, how well a disseminated infection (bacteria are injected into the larval body cavity) predicts the outcome of a human or chicken gut infection remains to be seen, as tissues are sterile yet the gut is a microbiologically dense, competitive environment. The infective dose of C. jejuni in humans is approximately 1000 bacteria, while the larva are typically given 105 to 107 CFU. Given that larva body weights are 150–300 mg and a typical adult human body weight is 62 kg, relatively that is more than a million-fold higher dose. A generic description of the larva killing assay (see Table 1 for more details) is: Campylobacter is grown under microaerobic conditions on plates (Columbia blood or MHA) or broth (Brucella or MHB) and suspended in PBS, with bacterial concentration approximated using OD (adjusted to 0.1 or 1.0). Larva maintained at lower temperatures (10 to 17°C) and starved for 2-8d are injected with 10 μL of the bacterial suspension, typically near the right foreleg, then transferred to 37°C and observed for darkening (the immune melanization response) and death, which is often rapid (24 hrs or less). Groups of ten larva are used for different conditions or strains, often in triplicate (so total n=30 for each condition). In Champion’s foundational study (Champion et al., 2010), three strains (11168H, G1, and 81-176) displayed equivalent killing (~70% at 24 hrs and 37°C) when grown on Columbia blood agar plates. Interestingly, strain 11168H grown in MHB killed all infected larva by 24 hrs (data from other two strains not shown). Perhaps the physiological state of the bacteria is different (such as flagella being used more in liquid versus the plate), or the suspension method from plates may expose the bacteria to more oxygen than when from broth. The larva are in a stressed state, since they have been starved for up to a week, and experienced a major temperature shift just after infection (from 15°C to 37°C). However, uninfected controls were included and none died in the 24 hr observation period. The authors of this publication chose to display survival of the larva at only one timepoint with column graphs, each column representing one group. This choice has been replicated often in the field as the dominant visualization of the killing assay. Note, with other animal challenge studies, the standard choice is Kaplan-Meier survival curves. The C. jejuni strain most commonly used for infection of G. mellonella is NCTC 11168 (and its hypermotile derivative, 11168H), see Table 1. While 11168 is referred to as the type strain in some publications (Pascoe et al., 2019; Whelan et al., 2019), the ATCC lists the Cj subsp. jejuni type strain as the bovine isolate CIP 702, also known as NCTC 11351 or ATCC 33560 (Campylobacter jejuni subsp. jejuni (Jones et al.) Steele and Owen, 2016).
Capsule
Capsular polysaccharide structures exhibit unusually wide variety between C. jejuni strains, and the serotypes are often due to phase-variable modifications such as ethanolamine, O-methyl, and other modifications (Van Alphen et al., 2014). Gene knockout mutants lacking transferase activity for adding the O-methyl phosphoramidate (MeOPN) modification to the capsule exhibited the same lethality (~70-80% killing at 24 hrs) as the respective wt strains (81–176 and 11168H, which were also statistically indistinguishable), while the acapsular mutant (ΔkpsM in 81-176) had only 20% killing. However, mutants which cannot synthesize the MeOPN (knockout of cj1415 in 81–176 or cj1416 in 11168H) did have lower (~40%) larva killing. The authors proposed that since transferase mutants still contain MeOPN modified sugars while the biosynthetic mutants do not, this may account for differences. However, pure MeOPN monomers or purified capsule displayed no larva killing. Alternatively, the biosynthetic mutants may contain less capsule overall. Note that p-values were given for larva killing assays, but the statistical test was not specified, yet it was likely an unpaired t-test, as that was used in other assays. As this test assumes equal variance between groups and normal distribution of data, it may not be the appropriate test for this data. In comparison, Champion et al. (Champion et al., 2010) had 100% killing by 11168H and ~10% by Δ1416, while van Alphen et al. (Van Alphen et al., 2014) had 70% killing by 11168H and 30% by Δ1416 mutant. The van Alphen study had a different source of larva (Bug Order Inc, Alberta vs Livefood, UK for Champion), held larva at 4 °C for up to a week before infection, had 5 μL injections, and uniquely suspended bacteria in 10 mM MgSO4 (pH not given) rather than PBS. Champion determined doses by plating and estimated 106 bacteria per animal, while van Alphen estimated doses at 5 x 106 per larva. Methodological differences such as these limit direct comparisons across studies. In a later study also focused on the capsule (Myles et al., 2024), four gene knockout mutants disrupted in adding hexoses to the capsule structures displayed equivalent killing in larva to the wt 11168 (~80% dead at 17hrs). However, the dose was extremely high (5x107 per worm), killing was abnormally rapid, the saline negative control larva had ~20% death, and the acapsular mutant ΔkpsM also had equivalent killing. This massive dose may have simply overwhelmed the host defenses or killed them via chemical toxicity (i.e. LOS); note, a heat-killed bacterial control examining that possibility was not reported.
Transcriptional regulators
Five different studies have examined transcriptional regulators related to Campylobacter virulence with larva. Knockout of the cj1556 regulator gene (rrpB) reduced larva killing from ~55% with the wt 11168H to ~35% with the mutant (Gundogdu et al., 2011). While it was stated that the p-value was less than 0.05 (actual value not given), the Student’s t-test was used (limitations same as above). Also, the methods state that survival was recorded in 24hr intervals, but the total observation time was not given. Knockout of the related regulator gene rrpA reduced killing at 24hrs from ~55% with 11168H to ~25% with the mutant (Gundogdu et al., 2015). Surprisingly, the double mutant was not statistically different from wt in killing (the authors suggest compensatory regulation but don’t have transcriptional data). Also, knockouts in the oxidative defense genes katA, ahpC, and sodB had apparently reduced killing, but not statistically significant. In a related study, rrpA deletion in 81–176 also had reduced killing, but deletion in strain 81116 was not significantly different from the wt (Gundogdu et al., 2016), suggesting the genetic background is important. While deletion of the perR (cj0332) regulator, which represses expression of several oxidative stress genes, increased aerotolerance, it did not affect killing of larva (Handley et al., 2015). Deletion of the iron uptake regulator fur in strain 11168 also reduced larva killing significantly (LD50 increased from 3.1 x 105 for wt to 2.6 x 106 for the mutant) (Askoura et al., 2016).
Outer membrane vesicles
Proteins in outer membrane vesicles (OMVs) from C. jejuni 11168H were identified (Elmi et al., 2012). Injection of an OMV preparation containing 5 μg of total protein killed larva similarly (~30%) as a dose of live bacteria (~106 CFU). A lower dose (0.5 μg) killed less and heat-treated OMVs had no killing at any dose, suggesting the killing is not due to LOS content. In a second study from the same group (Elmi et al., 2018), the same 5 μg protein dose of 11168H OMVs killed more larva (45%), and when the OMVs came from bacteria grown with taurocholate (shown to have increased protease activities), killed ~70%. Given the same conditions stated in the methods sections of those two studies, the reason for the difference is unclear.
Other virulence factors
Deletion of capC, a virulence factor of unknown function found in the outer membrane, reduced killing of larva by both strain 8116 and M1 (Mehat et al., 2018). A type six secretion system (T6SS), which are phage-related structures that inject effoctor proteins into neighboring cells, may play a role in C. jejuni virulence. Deletion of a critical factor (tssD) in T6SS from the genome of clinical strain 488 reduced killing of larva (Liaw et al., 2019). Additionally, a clinical isolate lacking the T6SS gene cluster (strain 81-176) exhibited lower killing than the wt 488, although not statistically significant. The role of T6SS during human infections remains unclear, as a screen in 366 isolates showed the gene cluster in only 4.7% of the strains, none of them from human sources (Siddiqui et al., 2015). Isolates resistant to nalidixic acid or ciprofloxacin (three strains for each antibiotic) were generated in vitro, and displayed increased killing (i.e., LD50 for wt 11168 at 24 hrs of 10x7.59 ± 0.02 CFU/larva while resistant strains had LD50 values ranging from 10x6.60 to 6.01) (Whelan et al., 2019). In vitro growth in MHB was reported to be the same, although instead of growth curves, the OD600 was shown at one time point only. Interestingly, the mutants had decreased motility, increased biofilm formation, and increased adherence to HT29 epithelial cells. This suggests these properties may be related to larva killing. A related study reported no fitness cost for fluoroquinolone resistance during a competition assay for chicken colonization (Zhang et al., 2003) while another reported resistant C. jejuni strains were less competitive in food and chicken gut (Zeitouni and Kempf, 2011). These conflicting results suggest that genetic background or other factors may control how mutations in the target gyrase gene affect bacterial fitness.
Virulence signals
Growth of two strains of C. jejuni (11168H and 81-176) on MHA in the presence of pancreatic amylase dramatically increased larva killing at 24hrs, from 10-20% without treatment to 90% with (Jowiya et al., 2015). Treated bacteria generate an extracellular dextran, have enhanced biofilm formation, and have increased adhesion and invasion of Caco-2 cells. Controls of larva injected with amylase or dextran were not reported.
Temperature
While Campylobacter causes gut inflammation in mammals (37°C body temperature), chickens colonized with the bacteria have few overt effects (42°C body temperature), thus temperature is investigated as a potentially important factor in virulence regulation. Proteomic analysis was used to identify proteins differentially regulated between these two temperatures (Tang et al., 2017). Strain 11168 was more lethal to larva when coming from culture at 42°C (~80% killing at 24 hrs) compared to 37°C (~20% killing). This is interesting given the lower pathogenicity of Campylobacter in chickens, suggesting factors other than temperature are important. Strain 11168 displays much less larva killing at 32°C compared to 37°C (Barnawi et al., 2020). For comparison, the minimal growth temperature determined for strains 104 and 33560 was 32 and 31°C, respectively (Hazeleger et al., 1998), while the optimal growth range for Galleria is 25-33°C. This study (Barnawi et al., 2020) also interestingly suggested that the ability to tolerate zinc may be important in larva, as a mutant which continues expression of zinc exporter czcD (normally repressed at 32°C) kills more larva than the wild-type at 32°C. The combination of the nickel chelator DMG (dimethylglyoxime) with copper was found to kill C. jejuni in vitro, with an MBC value versus 11168 of 8 μM for copper in the presence of 4 mM DMG in MHB (Benoit and Maier, 2022). This effect may be restricted to Campylobacter, as the growth of four related genera (Klebsiella, Escherichia, Salmonella, and Acinetobacter) were unaffected by the combination (DMG concentrations up to 5 mM and copper up to 250 μM). Additionally, larva injected with a DMG/copper (10 mM/1mM) combination two hours before bacterial injection had decreased and delayed killing with both 1168 and 81-176. However, no copper only or DMG only control was included. Inclusion of DMG/copper in drinking water also decreased colonization of chickens by 11168. Further investigation into this potential new therapeutic is warranted.
Species and strain comparisons
The ease of the Galleria model allows virulence comparisons across many different strains or species quickly and with relatively high numbers of animals. Sixty-seven C. jejuni isolates from humans, chickens, and other animals were screened for larva killing (Senior et al., 2011). While data for individual strains was not shown, larva survival was shown when infected with strains placed into six MLST groupings, although only one pairwise comparison (ST21, least killing, vs ST257, most killing) was statistically different. However, screening of six strains of C. jejuni in larva revealed the highest killing rate (~35% at 48hrs) by strain 13126, a poultry isolate, also type ST21 (Humphrey et al., 2015). Given the genetic diversity even within MLST groupings, this is not surprising. Four clinical isolates of C. concisus had less larva killing than C. jejuni 1168H (Brunner et al., 2018). In a more extensive study, 22 strains of C. upsaliensis and 13 strains of C. helveticus (both mainly isolated from cats and dogs) had less killing of larva that 34 C. jejuni strains (Bojanić et al., 2020). Incubation of infected larva was uniquely done under microaerobic conditions, where about 10% of uninfected control larva died after 10d. Three of 18 strains of C. coli killed fewer larva than the C. jejuni reference strain, while the other 15 strains caused higher mortality. (Gomes et al., 2024) However, the reference strain was clinical isolate ATCC 33291, not the commonly used 11168, so how it compares to other studies is unclear.
Summary and future directions
The most prolific authors in this field have been Nick Dorrell (7 publications, 6 as senior author), Brendan Wren (9 publications), Abdi Elmi and Ozan Gundogdu (6 publications each), and Mona Bajaj-Elliott and Arnould van Vliet (4 publications each). The dominant strategy of high dose, acute, disseminated infection in Galleria has helped reveal the role of multiple virulence factors, compare virulence across strains/species, and begin to understand the relationship of temperature and infection. When examined, the results of the larva killing assays generally line up with those from chick colonization or cultured cell invasion. The lack of killed bacterial cell controls is a limitation in most studies, given the rather high lethality (~50% by day 4) of heat-killed cells (Bojanić et al., 2020). Since high bacterial doses are injected into larva, some of the lethality may be coming from bacterial products such as LOS or cell wall materials, which is a confounding factor when comparing across studies. Another potential confounding factor is that larva sizes are not described in these studies and is probably varying, and larva weight has been shown to affect lethal dose with Staphylococcus (Hesketh-Best et al., 2021). A third issue is that the infected larva are incubated under normal atmospheric oxygen levels, and while that may be appropriate to model extra-intestinal tissue infections in humans, Campylobacter by far remains in the anaerobic gut in both humans and chickens. Further, Campylobacter was much more lethal to larva incubated under microaerobic compared to aerobic conditions (Bojanić et al., 2020), which deserves more study. In most protocols, larva are held at 10-15°C before bacterial injection, then at 37°C after. How this affects larva immunocompetency is unclear, but temperature shifts of larva can increase hemocyte density, expression of immune genes, and resistance to killing by Candida (Mowlds and Kavanagh, 2008). However, starvation (for 7d) can lower hemocyte count and increase susceptibility of larva to Candida (Banville et al., 2012). One conflict in the current literature is whether larva can be incubated at 42°C, as one study reports such data (Senior et al., 2011) (however, study does not show any control larva data at 42°C) while another reports that larva cannot survive at that temperature (Champion et al., 2010). In our hands and others (Vertyporokh et al., 2015; Liu et al., 2019), the larva cannot survive at 42°C, so perhaps larva from different sources have varying temperature tolerances. In future studies, the oral route of inoculation, called force feeding, may be more revealing than the injection route. Oral would allow for examining Campylobacter interactions with the gut microbiome and with food, determining crossing of the gut barrier and invasion into tissues, and initial analysis of potential probiotics. In one study that included force feeding and body injection (Emery et al., 2021), the oral dose had higher lethality (25% killing at 24 hrs) than the injection method (10% killing). However, comparison with other studies is unclear, as the larva incubation temperature was lower (30°C), the BAA-224 Campylobacter strain was unique, and larva were fed previously, while commonly they are starved. Applying oral infection studies would necessitate a more thorough determination of the larva gut microbiome and how it is affected by experimental manipulations, like incubation at lowered temperature or starvation. Finally, direct comparisons of strains in larva with other models, such as chickens (Humphrey et al., 2015), will help validate the Galleria system.
Author contributions
TP: Writing – review & editing, Formal Analysis, Supervision, Writing – original draft, Methodology, Conceptualization. SC: Methodology, Writing – review & editing, Writing – original draft, Formal Analysis. AK: Formal Analysis, Writing – review & editing, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Askoura, M., Sarvan, S., Couture, J. F., and Stintzi, A. (2016). The Campylobacter jejuni ferric uptake regulator promotes acid survival and cross-protection against oxidative stress. Infect. Immun. 84, 1287–1300. doi: 10.1128/iai.01377-15?download=true
Askoura, M. and Stintzi, A. (2017). “Using Galleria mellonella as an infection model for Campylobacter jejuni Pathogenesis,” in Methods in Molecular Biology (Totowa, New Jersey: Humana Press Inc), 163–169.
Campylobacter jejuni subsp. jejuni (Jones et al.) Steele and Owen (ATCC). (2016). Available online at: https://www.atcc.org/products/33560 (Accessed August 15, 2025).
Banville, N., Browne, N., and Kavanagh, K. (2012). Effect of nutrient deprivation on the susceptibility of Galleria mellonella larvae to infection. Virulence 3, 497–503. doi: 10.4161/viru.21972?download=true
Barnawi, H., Masri, N., Hussain, N., Al-Lawati, B., Mayasari, E., Gulbicka, A., et al. (2020). RNA-based thermoregulation of a Campylobacter jejuni zinc resistance determinant. PloS Pathog. 16, e1009008. doi: 10.1371/journal.ppat.1009008
Benoit, S. L. and Maier, R. J. (2022). Copper toxicity towards Campylobacter jejuni is enhanced by the nickel chelator dimethylglyoxime. Metallomics 14. doi: 10.1093/mtomcs/mfab076
Bojanić, K., Acke, E., Roe, W. D., Marshall, J. C., Cornelius, A. J., Biggs, P. J., et al. (2020). Comparison of the pathogenic potential of campylobacter jejuni, C. Upsaliensis and c. helveticus and limitations of using larvae of galleria mellonella as an infection model. Pathogens. 9, 1–15. doi: 10.3390/pathogens9090713
Brunner, C., John, C. M., Phillips, N. J., Alber, D. G., Gemmell, M. R., Hansen, R., et al. (2018). Novel Campylobacter concisus lipooligosaccharide is a determinant of inflammatory potential and virulence. J. Lipid Res. 59, 1893–1905. doi: 10.1194/jlr.M085860
Champion, O. L., Karlyshev, A. V., Senior, N. J., Woodward, M., La Ragione, R., Howard, S. L., et al. (2010). Insect infection model for Campylobacter jejuni reveals that O-methyl phosphoramidate has insecticidal activity. J. Infect. Diseases. 201, 776–782. doi: 10.1086/650494
Crofts, A. A., Poly, F. M., Ewing, C. P., Kuroiwa, J. M., Rimmer, J. E., Harro, C., et al. (2018). Campylobacter jejuni transcriptional and genetic adaptation during human infection. Nat. Microbiol. 3, 494–502. Available online at: https://www.nature.com/articles/s41564-018-0133-7.
Davis, L. and DiRita, V. (2008). Experimental chick colonization by Campylobacter jejuni. Nat. Rev. Microbiol. 5, 665–679. doi: 10.1002/9780471729259.mc08a03s11
Dutta, T. K., Veeresh, A., Mathur, C., Phani, V., Mandal, A., Sagar, D., et al. (2021). The induced knockdown of GmCAD receptor protein encoding gene in Galleria mellonella decreased the insect susceptibility to a Photorhabdus akhurstii oral toxin. Virulence 12, 2957. doi: 10.1080/21505594.2021.2006996
Elmi, A., Watson, E., Sandu, P., Gundogdu, O., Mills, D. C., Inglis, N. F., et al. (2012). Campylobacter jejuni outer membrane vesicles play an important role in bacterial interactions with human intestinal epithelial cells. Infect. Immun. 80, 4089–4098. doi: 10.1128/iai.00161-12?download=true
Elmi, A., Dorey, A., Watson, E., Jagatia, H., Inglis, N. F., Gundogdu, O., et al. (2018). The bile salt sodium taurocholate induces Campylobacter jejuni outer membrane vesicle production and increases OMV-associated proteolytic activity. Cell Microbiol. 20, e12814. doi: 10.1111/cmi.12814
Emery, H., Butt, T. M., and Coates, C. J. (2021). Nutraceutical intervention protects against bacterial and chemical-induced gastrotoxicity in a non-mammalian model, Galleria mellonella. Food Chem. Toxicol. 154. doi: 10.1016/j.fct.2021.112354
Finsterer, J. (2022). Triggers of guillain–barré Syndrome: campylobacter jejuni predominates. Int. J. Mol. Sci. 23. MDPI. doi: 10.3390/ijms232214222
Gomes, C. N., Felice, A. G., Pereira G do, N., Ceballos, V. A. S., Soares, S. D. C., Tonani, L., et al. (2024). Comparative genomics and virulence potential of Campylobacter coli strains isolated from different sources over 25 years in Brazil. BMC Microbiol. 24, 1–16. doi: 10.1186/s12866-024-03642-5
Gundogdu, O., da Silva, D. T., Mohammad, B., Elmi, A., Mills, D. C., Wren, B. W., et al. (2015). The Campylobacter jejuni MarR-like transcriptional regulators RrpA and RrpB both influence bacterial responses to oxidative and aerobic stresses. Front. Microbiol. 6. doi: 10.3389/fmicb.2015.00724
Gundogdu, O., da Silva, D. T., Mohammad, B., Elmi, A., Wren, B. W., van Vliet, A. H. M., et al. (2016). The Campylobacter jejuni oxidative stress regulator RrpB is associated with a genomic hypervariable region and altered oxidative stress resistance. Front. Microbiol. 7, 227256. Available online at: www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2016.02117/full.
Gundogdu, O., Mills, D. C., Elmi, A., Martin, M. J., Wren, B. W., and Dorrell, N. (2011). The Campylobacter jejuni transcriptional regulator Cj1556 plays a role in the oxidative and aerobic stress response and is important for bacterial survival in vivo. J. Bacteriol. 193, 4238–4249. doi: 10.1128/JB.05189-11
Handley, R. A., Mulholland, F., Reuter, M., Ramachandran, V. K., Musk, H., Clissold, L., et al. (2015). PerR controls oxidative stress defence and aerotolerance but not motility-associated phenotypes of campylobacter jejuni. Microbiol. (United Kingdom) 161, 1524–1536. doi: 10.1099/mic.0.000109
Harrer, A., Bücker, R., Boehm, M., Zarzecka, U., Tegtmeyer, N., Sticht, H., et al. (2019). Campylobacter jejuni enters gut epithelial cells and impairs intestinal barrier function through cleavage of occludin by serine protease HtrA. Gut Pathog. 11, 1–16. doi: 10.1186/s13099-019-0283-z
Hazeleger, W. C., Wouters, J. A., Rombouts, F. M., and Abee, T. (1998). Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl. Environ. Microbiol. 64, 3917–3922. doi: 10.1128/aem.64.10.3917-3922.1998?download=true
Hesketh-Best, P. J., Mouritzen, M. V., Shandley-Edwards, K., Billington, R. A., and Upton, M. (2021). Galleria mellonella larvae exhibit a weight-dependent lethal median dose when infected with methicillin-resistant Staphylococcus aureus. Pathog. Dis. 79. doi: 10.1093/femspd/ftab003
Humphrey, S., Lacharme-Lora, L., Chaloner, G., Gibbs, K., Humphrey, T., Williams, N., et al. (2015). Heterogeneity in the infection biology of campylobacter jejuni isolates in three infection models reveals an invasive and virulent phenotype in a ST21 isolate from poultry. PloS One 10, e0141182. doi: 10.1371/journal.pone.0141182
Jowiya, W., Brunner, K., Abouelhadid, S., Hussain, H. A., Nair, S. P., Sadiq, S., et al. (2015). Pancreatic amylase is an environmental signal for regulation of biofilm formation and host interaction in Campylobacter jejuni. Infect. Immun. 83, 4884–4895. doi: 10.1128/iai.01064-15?download=true
Kim, S. H., Chelliah, R., Ramakrishnan, S. R., Perumal, A. S., Bang, W. S., Rubab, M., et al. (2021). Review on stress tolerance in campylobacter jejuni. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.596570
Liaw, J., Hong, G., Davies, C., Elmi, A., Sima, F., Stratakos, A., et al. (2019). The campylobacter jejuni type VI secretion system enhances the oxidative stress response and host colonization. Front. Microbiol. 10, 462614. doi: 10.3389/fmicb.2019.02864
Liu, M., Huang, M., Huang, L., Biville, F., Zhu, D., Wang, M., et al. (2019). New perspectives on galleria mellonella larvae as a host model using riemerella anatipestifer as a proof of concept. Infect. Immun. 87. doi: 10.1128/iai.00072-19?download=true
Mehat, J. W., Park, S. F., van Vliet, A. H. M., and La Ragione, R. M. (2018). CapC, a novel autotransporter and virulence factor of campylobacter jejuni. Applied Environmental Microbiol. 84, e01032-18. doi: 10.1128/AEM.01032-18
Ménard, G., Rouillon, A., Cattoir, V., and Donnio, P. Y. (2021). Galleria mellonella as a suitable model of bacterial infection: past, present and future. Front. Cell. Infection Microbiol. 11. Frontiers Media S.A. doi: 10.3389/fcimb.2021.782733
Miller, W. G., Parker, C. T., Heath, S., and Lastovica, A. J. (2007). Identification of genomic differences between Campylobacter jejuni subsp. jejuni and C. jejuni subsp. doylei at the nap locus leads to the development of a C. jejuni subspeciation multiplex PCR method. BMC Microbiol. 7. doi: 10.1186/1471-2180-7-11
Mowlds, P. and Kavanagh, K. (2008). Effect of pre-incubation temperature on susceptibility of Galleria mellonella larvae to infection by Candida albicans. Mycopathologia 165, 5–12. doi: 10.1007/s11046-007-9069-9
Myles, M., Barnawi, H., Mahmoudpour, M., Shlimon, S., Chang, A., Zimmermann, D., et al. (2024). Effect of the polysaccharide capsule and its heptose on the resistance of Campylobacter jejuni to innate immune defenses. Microbiologyopen 13. doi: 10.1002/mbo3.1400
Nemelka, K. W., Brown, A. W., Wallace, S. M., Jones, E., Asher, L. V., Pattarini, D., et al. (2009). Immune Response to and Histopathology of Campylobacter jejuni Infection in Ferrets (Mustela putorius furo). Comp. Med. 59, 363. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC2779212/.
Omole, Z., Dorrell, N., Elmi, A., Nasher, F., Gundogdu, O., and Wren, B. W. (2024). Pathogenicity and virulence of Campylobacter jejuni: What do we really know? Virulence 15. Taylor and Francis Ltd. doi: 10.1080/21505594.2024.2436060
Parte, A. C., Sardà Carbasse, J., Meier-Kolthoff, J. P., Reimer, L. C., and Göker, M. (2020). List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 70, 5607–5612. doi: 10.1099/ijsem.0.004332
Pascoe, B., Williams, L. K., Calland, J. K., Meric, G., Hitchings, M. D., Dyer, M., et al. (2019). Domestication of campylobacter jejuni NCTC 11168. Microb. Genom 5. doi: 10.1099/mgen.0.000279
Rath, A., Rautenschlein, S., Rzeznitzeck, J., Lalk, M., Methling, K., Rychlik, I., et al. (2022). Investigation on the colonisation of Campylobacter strains in the pig intestine depending on available metabolites. Comp. Immunol. Microbiol. Infect. Dis. 88. doi: 10.1016/j.cimid.2022.101865
Reuter, M., Periago, P. M., Mulholland, F., Brown, H. L., and van Vliet, A. H. M. (2015). A PAS domain-containing regulator controls flagella-flagella interactions in Campylobacter jejuni. Front. Microbiol. 6, 770. doi: 10.3389/fmicb.2015.00770
Senior, N. J., Bagnall, M. C., Champion, O. L., Reynolds, S. E., La Ragione, R. M., Woodward, M. J., et al. (2011). Galleria mellonella as an infection model for Campylobacter jejuni virulence. J. Med. Microbiol. 60, 661–669. doi: 10.1099/jmm.0.026658-0
Shagieva, E., Demnerova, K., and Michova, H. (2021). Waterborne Isolates of Campylobacter jejuni Are Able to Develop Aerotolerance, Survive Exposure to Low Temperature, and Interact With Acanthamoeba polyphaga. Front. Microbiol. 12, 730858. doi: 10.3389/fmicb.2021.730858
Shang, Y., Ren, F., Song, Z., Li, Q., Zhou, X., Wang, X., et al. (2016). Insights into Campylobacter jejuni colonization and enteritis using a novel infant rabbit model. Sci. Rep. 6, 1–12. Available online at: https://www.nature.com/articles/srep28737.
Siddiqui, F., Champion, O., Akram, M., Studholme, D., Eqani, S.A.M.A.S., Wren, B. W., et al. (2015). Molecular detection identified a type six secretion system in Campylobacter jejuni from various sources but not from human cases. J. Appl. Microbiol. 118, 1191–1198. doi: 10.1111/jam.12748
Sproston, E. L., Wimalarathna, H. M. L., and Sheppard, S. K. (2018). Trends in fluoroquinolone resistance in Campylobacter. Microb. Genom 4. doi: 10.1099/mgen.0.000198
Tang, Y., Cawthraw, S., Bagnall, M. C., Gielbert, A. J., Woodward, M. J., Petrovska, L., et al. (2017). Identification of temperature regulated factors of Campylobacter jejuni and their potential roles in virulence. AIMS Microbiol. 3, 885–898. doi: 10.3934/microbiol.2017.4.885
Tikhomirova, A., McNabb, E. R., Petterlin, L., Bellamy, G. L., Lin, K. H., Santoso, C. A., et al. (2024). Campylobacter jejuni virulence factors: update on emerging issues and trends. J. BioMed. Sci. 31. doi: 10.1186/s12929-024-01033-6
Van Alphen, L. B., Wenzel, C. Q., Richards, M. R., Fodor, C., Ashmus, R. A., Stahl, M., et al. (2014). Biological roles of the O-methyl phosphoramidate capsule modification in Campylobacter jejuni. PloS One 9. doi: 10.1371/journal.pone.0087051
Vertyporokh, L., Taszłow, P., Samorek-Pieróg, M., and Wojda, I. (2015). Short-term heat shock affects the course of immune response in Galleria mellonella naturally infected with the entomopathogenic fungus Beauveria bassiana. J. Invertebr Pathol. 130, 42–51. Available online at: https://www.sciencedirect.com/science/article/abs/pii/S0022201115001196.
Whelan, M. V. X., Ardill, L., Koide, K., Nakajima, C., Suzuki, Y., Simpson, J. C., et al. (2019). Acquisition of fluoroquinolone resistance leads to increased biofilm formation and pathogenicity in Campylobacter jejuni. Sci. Rep. 9. doi: 10.1038/s41598-019-54620-1
Wojda, I., Staniec, B., Sułek, M., and Kordaczuk, J. (2020). The greater wax moth Galleria mellonella: Biology and use in immune studies. Pathog. Dis. 78. Oxford University Press. doi: 10.1093/femspd/ftaa057
Yu, Z. T., Nanthakumar, N. N., and Newburg, D. S. (2016). The Human Milk Oligosaccharide 2′-Fucosyllactose Quenches Campylobacter jejuni–Induced Inflammation in Human Epithelial Cells HEp-2 and HT-29 and in Mouse Intestinal Mucosa. J. Nutr. 146, 1980–1990. Available online at: https://www.sciencedirect.com/science/article/pii/S0022316623007332.
Zeitouni, S. and Kempf, I. (2011). Fitness cost of fluoroquinolone resistance in campylobacter coli and campylobacter jejuni. Microbial Drug Resistance 17, 171–179. doi: 10.1089/mdr.2010.0139?download=true
Zhang, Q., Lin, J., and Pereira, S. (2003). Fluoroquinolone-resistant Campylobacter in animal reservoirs: dynamics of development, resistance mechanisms and ecological fitness. Anim. Health Res. Rev. 4, 63–72. Available online at: https://www.cambridge.org/core/journals/animal-health-research-reviews/article/abs/fluoroquinoloneresistant-campylobacter-in-animal-reservoirs-dynamics-ofdevelopment-resistance-mechanisms-and-ecological-fitness/0EFFEFFE0E3046FCB247DA8CCD5DBBC6.
Keywords: Campylobacter jejuni, Galleria mellonella, virulence, infection model, capsule
Citation: Primm TP, Cannon SR and Karki AB (2025) Small worms big discoveries: Galleria mellonella as a model for Campylobacter jejuni infection. Front. Cell. Infect. Microbiol. 15:1686074. doi: 10.3389/fcimb.2025.1686074
Received: 14 August 2025; Accepted: 08 September 2025;
Published: 30 September 2025.
Edited by:
Percy Schröttner, Technische Universität Dresden, GermanyReviewed by:
William T. Doerrler, Louisiana State University, United StatesCopyright © 2025 Primm, Cannon and Karki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Todd P. Primm, dHByaW1tQHNoc3UuZWR1
 Todd P. Primm
Todd P. Primm Samuel R. Cannon
Samuel R. Cannon Anand B. Karki
Anand B. Karki