- 1Infectious Disease Center, Guangzhou Eighth People’s Hospital, Guangzhou Medical University, Guangzhou, Guangdong, China
- 2Guangzhou Medical Research Institute of Infectious Diseases, Guangzhou Eighth People’s Hospital, Guangzhou Medical University, Guangzhou, Guangdong, China
- 3Institute of Infectious Disease, Guangzhou Eighth People’s Hospital, Guangzhou Medical University, Guangzhou, Guangdong, China
- 4Guangzhou Key Laboratory of Clinical Pathogen Research for Infectious Diseases, Guangzhou Medical University, Guangzhou, Guangdong, China
Background: HIV-1 mutation V179D/E can confer potential low-level resistance to multiple non-nucleoside reverse transcriptase inhibitors (NNRTIs), and its detection rate has increased in recent years. However, its effect on antiretroviral therapy (ART) outcomes remains unclear.
Methods: This study included people living with HIV-1 (PLWH) with only V179D/E mutation detected by pre-treatment drug resistance (PDR) testing at Guangzhou Eight People’s Hospital between January 2018 and December 2022. Two control groups were matched 1:1:1 using propensity score matching (PSM): a PDR-negative group and an NNRTI-DR group with low-level or higher NNRTI resistance. Virological and immunological outcomes were compared over 3 years. Logistic regression analyzed virological failure (VF) risk factors in the V179D/E group and assessed acquired drug resistance (ADR).
Results: Among 6021 patients tested, the detection rate of V179D/E was 14.8%. After exclusions, 626 patients were included in this study. Additionally, 195 patients met the criteria for the NNRTI-DR group. After 1:1:1 PSM, the baseline characteristics were balanced across the three groups. In 1 year, the V179D/E group showed lower virological suppression and higher VF than the PDR-negative group, with no significant difference from the NNRTI-DR group. Differences disappeared by years 2 and 3. In the V179D/E group, NNRTI-based regimens increased VF risk, while baseline CD4+ T cell counts >200 cells/μL were protective. Among 37 patients with VF tested for ADR in the V179D/E group, 54.1% developed new mutations, 85.0% of whom were on efavirenz (EFV)-based regimens.
Conclusions: V179D/E is highly prevalent among ART-naïve PLWH in Guangzhou and may impair early virological response to NNRTI-based regimens, particularly EFV-based regimens while increasing ADR risk.
1 Introduction
HIV-1 exhibits a remarkably high mutation rate and extensive genetic diversity, which facilitates the emergence of drug-resistant variants and poses a significant threat to the long-term efficacy of antiretroviral therapy (ART). According to the World Health Organization (WHO) guidelines (World Health Organization, 2019), HIV drug resistance is classified into acquired drug resistance (ADR), transmitted drug resistance (TDR), and pre-treatment drug resistance (PDR). PDR refers to the presence of resistance-associated mutations detected at the initiation or re-initiation of ART in individuals with no prior ART exposure or only limited exposure to antiretroviral (ARV) drugs.
Among the various classes of ARVs, non-nucleoside reverse transcriptase inhibitors (NNRTIs) are particularly prone to the development of drug resistance because of their low genetic barrier (Steegen et al., 2017; Taffa et al., 2018). Globally, the prevalence of NNRTI-associated PDR has increased significantly since 2001, with several countries reporting rates exceeding 10% among individuals initiating ART (World Health Organization, 2019). Despite this trend, NNRTIs remain widely used in first-line treatment regimens (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2024; European AIDS Clinical Society Guidelines, 2024), underscoring the importance of continued surveillance of NNRTI-associated resistance.
HIV drug resistance is commonly evaluated using the Stanford HIV Drug Resistance Database algorithm in clinical practice (HIVDB Genotypic Resistance Test Interpretation System, 2019), which categorizes resistance into five levels: susceptible, potential low-level resistance, low-level resistance, intermediate-level resistance, and high-level resistance. Among these, low-level resistance and higher are generally considered clinically significant and may lead to virological failure (VF) (Jespersen et al., 2018), whereas the clinical impact of potential low-level resistance remains unclear.
V179D and V179E are two accessory mutations associated with NNRTI resistance, both of which can confer potential low-level resistance to multiple NNRTIs. V179D is a polymorphic mutation that can act synergistically with the K103R mutation, significantly enhancing resistance to efavirenz (EFV) and nevirapine (NVP). V179E is a non-polymorphic mutation, although both exhibit similar resistance profiles under drug pressure. In recent years, the prevalence of V179D/E has continued to increase (Li et al., 2016). In our previous study, we found that the overall prevalence of PDR in Guangzhou, China, was 7.4%, with NNRTI resistance being the most common. Among these, V179D/E mutation was the most frequently detected, with a prevalence of 17.8% (Lv et al., 2024).
Preliminary studies have suggested that people living with HIV-1 (PLWH) harboring V179D/E may exhibit poorer virological suppression (VS) and immune reconstitution when treated with EFV-based regimens, whereas regimens based on protease inhibitors (PIs) or integrase strand transfer inhibitors (INSTIs) tend to yield better outcomes (Wang et al., 2023). However, evidence regarding the long-term clinical impact of V179D/E remains limited. Therefore, this study aimed to assess the prevalence of V179D/E among ART-naïve patients with HIV-1 in Guangzhou, China, and to investigate their influence on antiretroviral treatment outcomes, with the goal of informing the optimization of initial ART regimens and resistance management strategies.
2 Methods
2.1 Study design and participants
This study was a retrospective cohort study. The study population consisted of PLWH who underwent PDR testing at Guangzhou Eighth People’s Hospital, Guangzhou Medical University (GZ8H), between January 2018 and December 2021, and were found to carry only a single V179D or V179E mutation. Inclusion criteria were as follows: 1) confirmed diagnosis of HIV-1; 2) detection of V179D or V179E as the only mutation in PDR, with no other drug resistance mutations (DRMs) identified; and 3) initiation and continuous receipt of ART at GZ8H, with complete follow-up data for a minimum of 3 years. Patients were excluded if they were lost to follow-up, died, discontinued ART, or were transferred to other medical institutions during the follow-up period.
To assess the impact of V179D/E on virological and immunological outcomes over a 3-year follow-up period, two control groups were selected from the same study period. The PDR-negative group included patients in whom no DRMs were identified in PDR, while the NNRTI-DR group included patients who were found to exhibit low-level resistance or higher to NNRTIs only, with full susceptibility to all other classes of ARVs. The three groups were matched in a 1:1:1 ratio using propensity score matching (PSM). Matching variables included sex, age, baseline CD4+ T cell count, and initial ART regimen to minimize baseline differences. Baseline viral load (VL) was not included due to a high rate of missing data.
2.2 Data collection and definitions
Demographic and clinical data for the three study groups were obtained from the National Free Antiretroviral Treatment Database for Disease Control and Prevention. The collected baseline information included age, sex, route of HIV transmission, WHO clinical stage, baseline CD4+ T cell count, baseline plasma VL, and initial ART regimen. Additionally, CD4+ T cell counts and VL measurements were collected annually during the three-year follow-up period for all three groups. For patients in the V179D/E group, any changes in ART regimen during the follow-up period were also recorded.
VS was defined as a plasma VL of <50 copies/mL after at least 24 weeks of ART. VF was defined as plasma VL persistently >200 copies/mL 6 months after the initiation or adjustment of treatment, or virological rebound, defined as the recurrence of VL >200 copies/mL after full VS has been achieved (Acquired Immunodeficiency Syndrome Professional Group et al., 2024).
2.3 Analysis of HIV-1 subtype and drug resistance
Plasma samples were subjected to RNA extraction and reverse transcription, followed by nested polymerase chain reaction (PCR) amplification of the HIV-1 pol gene fragment, which includes the entire protease region and the first 240 amino acids of the reverse transcriptase region (corresponding to HXB2 positions 2253 to 3318), as previously described (Lv et al., 2024). The PCR products were subsequently analyzed using Sanger sequencing. Sequence data were assembled and edited using Sequencer version 5.0 and manually corrected with BioEdit version 7.2.6.1. HIV-1 subtypes were determined using the HIV-1 BLAST and the COMET online platform. Sequences were submitted to the Stanford HIV Drug Resistance Database to identify DRMs and assess ARV susceptibilities. The degree of drug resistance was classified into five categories: susceptible, potential low-level, low-level, intermediate-level, and high-level. Drug resistance was defined as low-level or higher in this study.
2.4 Statistical analysis
Continuous variables were presented as medians with interquartile range (IQR), while categorical variables were summarized as frequencies and percentages. Group comparisons of categorical variables were conducted using the Pearson chi-square test, and continuous variables were analyzed using the Wilcoxon rank-sum test for nonparametric analysis. To identify risk factors associated with VF in the V179D/E group, univariable and multivariable logistic regression models were constructed, and odds ratio (OR), adjusted OR (aOR), and corresponding 95% confidence interval (CI) were reported. All statistical tests were two-sided, and a P-value of <0.05 was considered statistically significant. PSM was performed using R software (version 4.4.1, R Core Team, Vienna, Austria). All other statistical analyses were conducted using SPSS software (IBM SPSS Statistics, version 26.0).
2.5 Ethical statement
This study was approved by the ethics committee of Guangzhou Eighth People’s Hospital, Guangzhou Medical University (GZ8H) (approval number: 202033166). Each participant signed a written consent form.
3 Results
3.1 Prevalence of V179D/E mutations and baseline characteristics of carriers
In total, 6021 patients underwent PDR testing during the study period. Among them, 14.8% (893/6021) were found to carry either V179D or V179E. Specifically, the detection rate of V179D was 4.3% (259/6021), while that of V179E was 10.5% (634/6021). After excluding patients who had discontinued ART, were lost to follow-up, or were referred elsewhere, 626 patients were included in the final analysis.
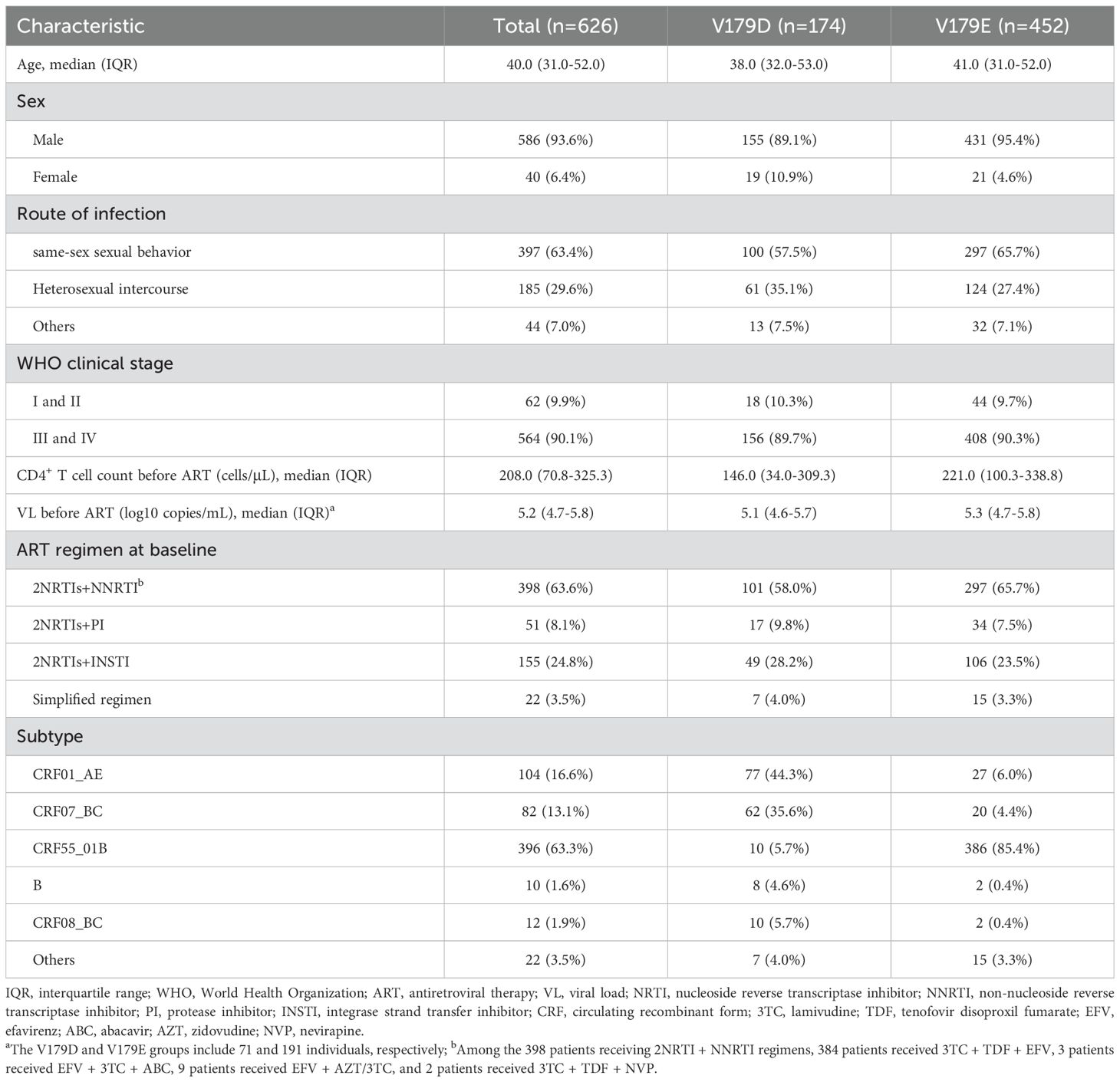
The median age of the included patients was 40.0 years (IQR: 31.0–52.0), with males accounting for 93.6% (586/626). The predominant route of transmission was same-sex sexual behavior, accounting for 63.4% (397/626) of the cohort. Most patients (90.1%, 564/626) were classified as WHO clinical stage III or IV. The median baseline CD4+ T cell count was 208.0 cells/μL (IQR: 70.8–325.3), and the median baseline VL was 5.2 log10 copies/mL (IQR: 4.7–5.8). At baseline, 63.6% (398/626) of the patients received an ART regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) plus an NNRTI, of which 99.5% (396/398) received an EFV-based regimen. Notably, 85.4% (386/452) of those with the V179E mutation harbored CRF55_01B. For specific details, refer to Table 1.
3.2 Comparison of virologic and immunologic responses among the V179D/E, PDR-negative, and NNRTI-DR groups, including V179D and V179E subgroup analysis
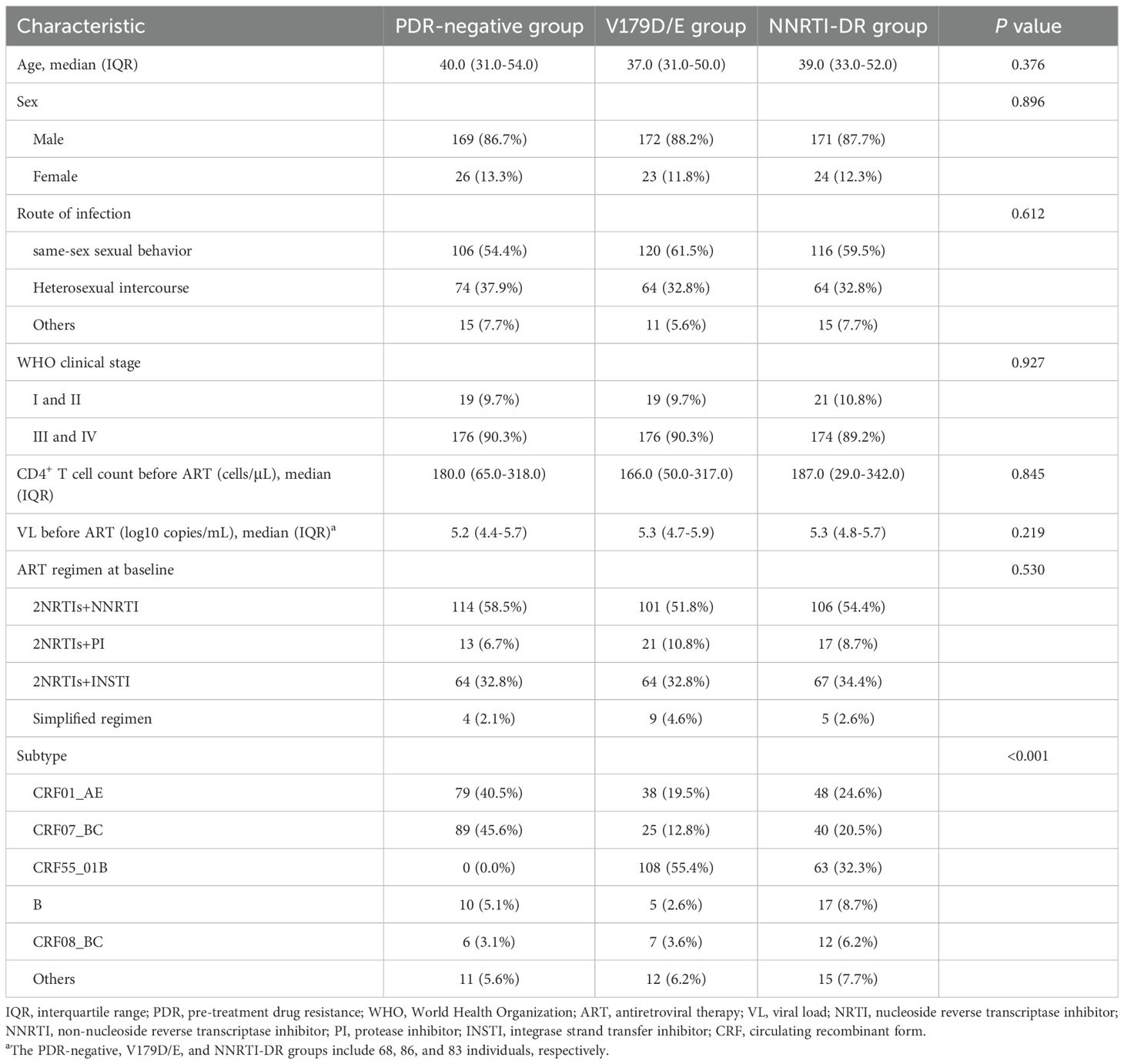
During the study period, 275 patients met the inclusion criteria for the NNRTI-DR group. After excluding 80 patients due to loss to follow-up, ART interruption, or referral, 195 patients were included. The specific NNRTI-associated mutations and corresponding resistance levels in these patients are shown in Supplementary Tables S1 and S2, respectively. Following 1:1:1 PSM, the baseline characteristics of the three groups are shown in Table 2. After matching, there were no statistically significant differences between the groups in terms of sex, age, transmission route, WHO clinical stage, baseline VL, baseline CD4+ T cell count, or initial ART regimen.
In the first year, the VS rate in the V179D/E group was significantly lower than that in the PDR-negative group (87.7% vs. 95.6%, P = 0.007) but higher than that in the NNRTI-DR group (87.7% vs. 82.8%, P = 0.238), although the latter difference was not statistically significant. Regarding VF, the V179D/E group had a higher rate than the PDR-negative group (7.8% vs. 1.6%, P = 0.006) but a lower rate than the NNRTI-DR group (7.8% vs. 14.0%, P = 0.066), with no statistically significant difference. These differences largely disappeared in the second and third years of follow-up (Figure 1A). In addition, CD4+ T cell count trajectories over the follow-up period were similar across the three groups, with no statistically significant differences (Figure 1B).

Figure 1. Longitudinal virological and immunological comparisons among three groups over a 3-year follow-up period. (A) Proportions of VS and VF patients in three group at years 1, 2, and 3. (B) Median CD4+ T cell counts in three group at years 1, 2, and 3. PDR, pre-treatment drug resistance; NNRT, non-nucleoside reverse transcriptase inhibitor; VL, viral load; VS, virological suppression; VF, virological failure. ** represents P < 0.01, and *** represents P < 0.001.
The virological outcomes were comparable between the V179D and V179E groups (Supplementary Figure S1A), with a slightly higher suppression rate in the V179E group; however, the difference was not statistically significant. Regarding immunologic outcomes (Supplementary Figure S1B), the baseline CD4+ T cell count was significantly higher in the V179E group than in the V179D group (221.0 [IQR: 100.3–338.8] vs. 146.0 [IQR: 34.0–309.3], P <0.001). However, this difference gradually diminished during the follow-up, with no statistically significant differences observed at subsequent time points.
3.3 Analysis of risk factors for VF in the V179D/E group
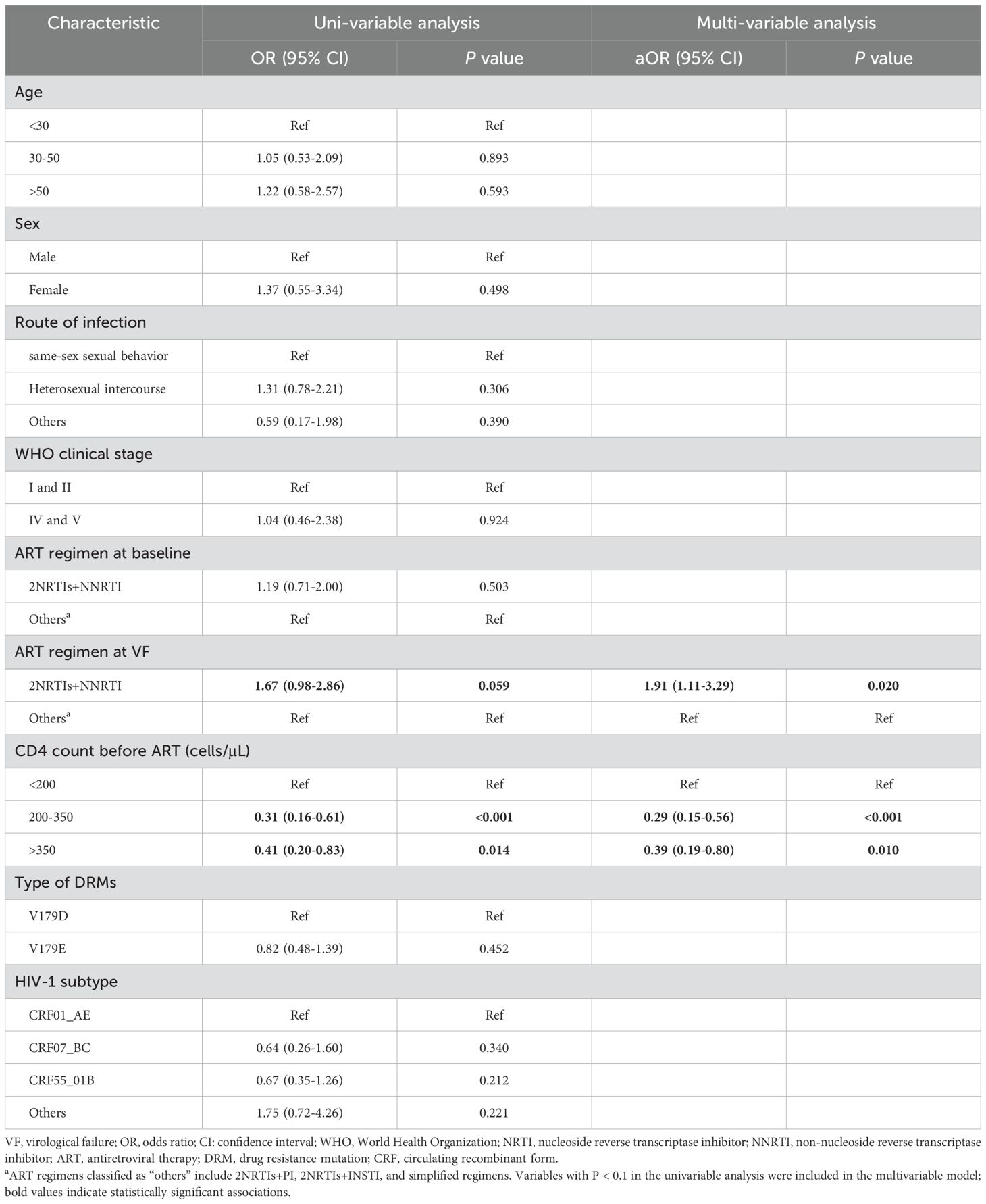
A total of 73 individuals (73/626, 11.7%) in the V179D/E group experienced VF during follow-up. A comparison of the clinical characteristics of patients with and without VF is presented in Supplementary Table S3. Multivariate logistic regression analysis identified the use of 2NRTIs+NNRTI as a risk factor for VF (aOR = 1.91, P = 0.020). The specific NNRTI-based ART regimens used by patients are provided in Supplementary Table S4. Compared to a baseline CD4+ T cell count of <200 cells/μL, baseline CD4+ T cell counts of 200–350 cells/μL (aOR = 0.29, P < 0.001) and >350 cells/μL (aOR = 0.39, P = 0.010) were both significantly associated with a reduced risk of VF. For more detailed information, please refer to Table 3.
3.4 ADR profiles in the V179D/E group
Of the 73 patients in the V179D/E group who experienced VF, 55 underwent ADR testing. Of these, 18 failed to be amplified, whereas the remaining 37 were successfully amplified. In addition to V179D/E, other new DRMs were identified in 20 patients (Figure 2A). These mutations were associated with resistance to both NRTIs and NNRTIs. Among the NRTI resistance mutations, M184V/I was the most common (85.0%), followed by K65R (40.0%). Among the NNRTI resistance mutations, V106M was the most frequently detected (50.0%), followed by K103N (30.0%). Figure 2B shows the levels of resistance to the various ARVs. All patients showed intermediate- to high-level resistance to emtricitabine (FTC) and lamivudine (3TC), 60% exhibited low-level resistance to tenofovir disoproxil fumarate (TDF), and 90% had high-level resistance to EFV and NVP. Notably, 85.0% (17/20) of the patients who developed new resistance mutations received a baseline ART regimen of 2NRTIs+NNRTI (3TC+TDF+EFV).
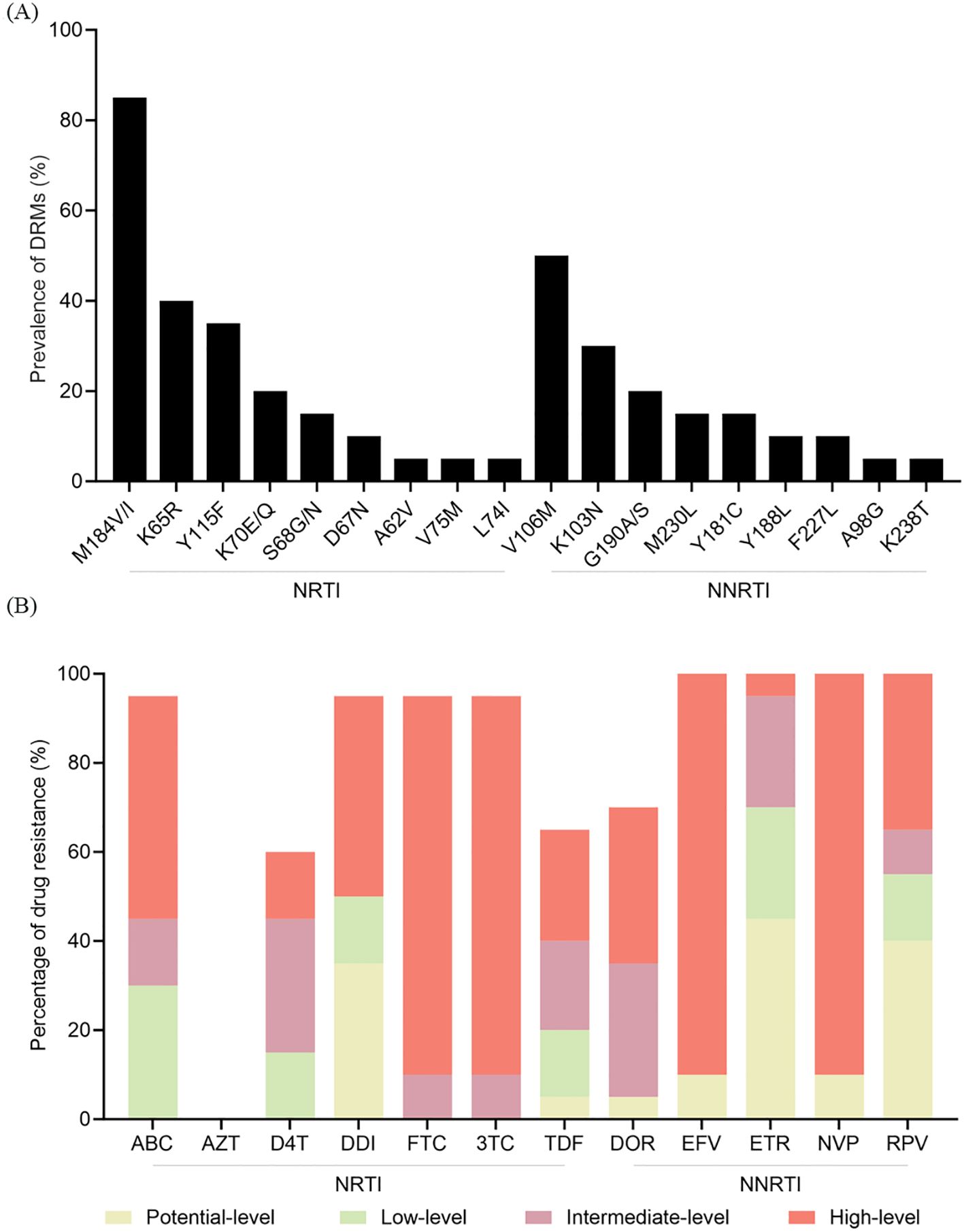
Figure 2. ADR profiles in the V179D/E group. (A) Newly emerged mutations identified during follow-up. (B) Cumulative drug resistance to various ARVs. NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; DRM, drug resistance mutation; ABC, abacavir; AZT, zidovudine; D4T, stavudine; DDI, didanosine; FTC, emtricitabine; 3TC, lamivudine; TDF, tenofovir disoproxil fumarate; DOR, doravirine; EFV, efavirenz; ETR, etravirine; NVP, nevirapine; RPV, rilpivirine; ADR, antiretroviral therapy; ARV, antiretroviral.
4 Discussion
In recent years, the prevalence of PDR has steadily increased, particularly in low- and middle-income countries (Inzaule et al., 2019). Studies have indicated that this trend is primarily driven by NNRTI-associated mutations (Su et al., 2025), among which V179D/E is the most frequently observed (Li et al., 2016; Zhang et al., 2021). Our previous study yielded similar results (Lv et al., 2024). In this study, the overall prevalence of V179D/E was 14.8%, which is similar to that reported in Shanghai, China (Zhang et al., 2024). V179E (10.5%) was much more common than V179D (4.3%), and 85.4% of V179E cases were linked to the CRF55_01B subtype, supporting earlier findings that V179E mainly circulates within this lineage (Liu et al., 2020; Lan et al., 2020).
V179D/E is polymorphic NNRTI-associated mutation that typically confer potential low levels of resistance. However, some studies have reported that patients with this mutation show poorer virological and immunological responses to EFV-based regimens than to PI- or INSTI-based therapies (Wang et al., 2023). Conversely, other studies have suggested that although V179D/E reduces NNRTI susceptibility in vitro (Vink et al., 2016), it does not significantly affect the clinical efficacy of NNRTI-based regimens or CD4+ T cell count recovery. Thus, the clinical significance of the V179D/E mutation remains controversial. Further studies are required to clarify their effects on first-line treatment efficacy, especially NNRTI-based regimens, which are crucial for optimizing therapy in ART-naïve individuals.
To assess the effect of V179D/E on treatment outcomes, two control groups were included: patients without PDR mutations (PDR-negative group) and patients with low or high NNRTI resistance (NNRTI-DR group). To minimize baseline differences that could influence outcomes, PSM was applied to control for sex, age, baseline CD4+ T-cell count, and initial ART regimen. In contrast, baseline VL was excluded because of missing data. In the first year of ART, the V179D/E group showed significantly lower virological efficacy than the PDR-negative group. Although the outcomes were slightly better than those of the NNRTI-DR group, the difference was not significant. No significant differences were observed between the three groups during the second and third years. These findings suggested that V179D/E may impair early virological responses. However, unlike previous studies (Wang et al., 2023), no significant differences in CD4+ T cell count recovery were observed, indicating a limited effect on immune reconstitution.
We further analyzed the risk factors for VF in the V179D/E group and found that the use of NNRTI-based regimens significantly increased the risk of VF. This finding is consistent with previous studies (Wang et al., 2023), indicating that although V179D/E confers only potential low-level resistance, it may still reduce the treatment efficacy of NNRTIs. Additionally, our results indicated that a baseline CD4+ T cell count above 200 cells/µL was associated with a lower risk of VF. These results underscore the need for caution when using NNRTI-based regimens in patients with V179D/E, especially those with CD4+ T cell counts below 200 cells/µL.
We evaluated ADR in patients from the V179D/E group who experienced VF and found that 54.1% (20/37) developed new DRMs. The most common NRTI-associated mutation was M184V/I, which causes high-level resistance to FTC and 3TC, followed by K65R, which confers intermediate-level resistance to TDF and abacavir (ABC). For NNRTIs, V106M was the most frequent, followed by K103N, both of which are linked to high-level resistance to EFV and NVP. Notably, seventeen of the 20 patients who developed new DRMs were on NNRTI-based regimens. All 17 of these patients were treated with the regimen 3TC+TDF+EFV, which likely explains why these specific mutations occurred. This suggests that continuing NNRTI therapy in the presence of V179D/E may not only reduce the initial virological response but also increase the risk of further resistance, complicating future treatment.
This study had some limitations. First, as this was a single-center retrospective study, the findings may not be widely applicable and require confirmation in larger multicenter studies. Second, drug resistance testing in this study was conducted using Sanger sequencing, which has a detection threshold of approximately 15%–20%, limiting the ability to detect low-frequency variants (Capina et al., 2020). As these mutations may still contribute to VF (Stella-Ascariz et al., 2017; Milne et al., 2019), their potential impact on treatment outcomes cannot be excluded. Additionally, as 99.5% of patients in the V179D/E group were treated with an EFV-based regimen, the conclusions may be more applicable to this treatment regimen, and the effect of other regimens could not be adequately assessed due to the small number of patients on other regimens.
5 Conclusions
Our findings indicated that the V179D/E mutation has a relatively high prevalence in ART-naïve patients with HIV-1 in Guangzhou, China. This may reduce the early virological response to NNRTI-based regimens, especially in patients treated with an EFV-based regimen, and increase the risk of acquired resistance mutations, especially in patients with low baseline CD4+ T cell counts.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the ethics committee of Guangzhou Eighth People’s Hospital, Guangzhou Medical University (GZ8H) (approval number: 202033166). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SL: Writing – review & editing, Writing – original draft, Data curation. YL: Data curation, Methodology, Writing – review & editing. QL: Writing – review & editing, Methodology, Data curation. XL: Writing – review & editing, Data curation, Methodology. JL: Data curation, Formal Analysis, Writing – review & editing. CW: Writing – review & editing, Formal Analysis, Data curation, Supervision. YhL: Methodology, Writing – review & editing, Funding acquisition. JC: Funding acquisition, Writing – original draft, Resources. XC: Project administration, Resources, Writing – original draft. WC: Supervision, Writing – review & editing, Investigation. XT: Writing – review & editing, Investigation, Conceptualization. LL: Investigation, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Key Research and Development Program of China (2024YFC2311100), Guangdong Province Basic and Applied Basic Research Fund (2024A1515013281), Science and Technology Project of Guangzhou (2024A03J0879, 2024A03J0876, 2025A03J3863) and the Key Medical Discipline of Guangzhou (Infectious Diseases, 2025-2027).
Acknowledgments
We sincerely thank the hospital staff for their support in recruitment, sample collection, follow-up, and data processing. We are grateful to all participants for their contributions to this study. We also thank the BioBank of Guangzhou Eighth People’s Hospital for providing biosamples and related services. The study participants were enrolled from a clinical cohort study (NCT04667026).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1691715/full#supplementary-material
Supplementary Figure S1 | Virological and immunological comparisons among V179D and V179E groups over a 3-year follow-up period. (A) Proportions of VS and VF patients in the two groups at years 1, 2, and 3. (B) Median CD4+ T cell counts in the two groups at years 1, 2, and 3. VL, viral load; VS, virological suppression; VF, virological failure. * represents P < 0.05.
References
Acquired Immunodeficiency Syndrome Professional Group, Society of Infectious Diseases, Chinese Medical Association, and Chinese Center for Disease Control and Prevention (2024). Chinese guidelines for the diagnosis and treatment of human immunodeficiency virus infection/acquired immunodeficiency syndrome, (2024 edition). Chin. Med. J. (Engl). 137, 2654–2680. doi: 10.1097/CM9.0000000000003383
Capina, R., Li, K., Kearney, L., Vandamme, A. M., Harrigan, P. R., and Van Laethem, K. (2020). Quality control of next-generation sequencing-based HIV-1 drug resistance data in clinical laboratory information systems framework. Viruses. 12, 645. doi: 10.3390/v12060645
European AIDS Clinical Society Guidelines. (2024). European guidelines for clinical management and treatment of HIV-1-infected adults in Europe, version 12.1. Available online at: https://eacs.sanfordguide.com (Accessed August 20, 2025).
HIVDB Genotypic Resistance Test (GRT) Interpretation System (2019). Available online at: https://cms.hivdb.org/prod/downloads/release-notes/genotypic-resistance-test-interpretation.systemoct.2019.pdf (Accessed August 20, 2025).
Inzaule, S. C., Hamers, R. L., Doherty, M., Shafer, R. W., Bertagnolio, S., and Rinke de Wit, T. F. (2019). Curbing the rise of HIV drug resistance in low-income and middle-income countries: the role of dolutegravir-containing regimens. Lancet Infect. Dis. 19, e246–e252. doi: 10.1016/s1473-3099(18)30710-2
Jespersen, S., Hønge, B. L., Krarup, H., Medstrand, P., Sørensen, A., Medina, C., et al. (2018). Protease inhibitors or NNRTIs as first-line HIV-1 treatment in west africa (PIONA): A randomized controlled trial. J. Acquir. Immune Defic. Syndr. 79, 386–393. doi: 10.1097/qai.0000000000001820
Lan, Y., Xin, R., Cai, W., Deng, X., Li, L., Li, F., et al. (2020). Characteristics of drug resistance in HIV-1 CRF55_01B from ART-experienced patients in Guangdong, China. J. Antimicrob. Chemother. 75, 1925–1931. doi: 10.1093/jac/dkaa116
Li, X., Xue, Y., Lin, Y., Gai, J., Zhang, L., Cheng, H., et al. (2016). Evolutionary dynamics and complicated genetic transmission network patterns of HIV-1 CRF01_AE among MSM in shanghai, China. Sci. Rep. 6, 34729. doi: 10.1038/srep34729
Liu, Y., Li, H., Wang, X., Han, J., Jia, L., Li, T., et al. (2020). Natural presence of V179E and rising prevalence of E138G in HIV-1 reverse transcriptase in CRF55_01B viruses. Infect. Genet. Evol. 77, 104098. doi: 10.1016/j.meegid.2019.104098
Lv, S., Lan, Y., He, Y., Li, Q., Ling, X., Li, J., et al. (2024). Pretreatment drug resistance among people living with HIV from 2018 to 2022 in Guangzhou, China. J. Med. Virol. 96, e29937. doi: 10.1002/jmv.29937
Milne, R. S., Silverman, R. A., Beck, I. A., Mckernan-Mullin, J., Deng, W., Sibley, T. R., et al. (2019). Minority and majority pretreatment HIV-1 drug resistance associated with failure of first-line nonnucleoside reverse-transcriptase inhibitor antiretroviral therapy in Kenyan women. AIDS. 33, 941–951. doi: 10.1097/qad.0000000000002134
Panel on Antiretroviral Guidelines for Adults and Adolescents (2024). Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of health and human services. Available online at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv (Accessed August 20, 2025).
Steegen, K., Bronze, M., Papathanasopoulos, M. A., van Zyl, G., Goedhals, D., Variava, E., et al. (2017). HIV-1 antiretroviral drug resistance patterns in patients failing NNRTI-based treatment: results from a national survey in South Africa. J. Antimicrob. Chemother. 72, 210–219. doi: 10.1093/jac/dkw358
Stella-Ascariz, N., Arribas, J. R., Paredes, R., and Li, J. Z. (2017). The role of HIV-1 drug-resistant minority variants in treatment failure. J. Infect. Dis. 216, S847–S850. doi: 10.1093/infdis/jix430
Su, Q., Li, Y., Huang, T., Wei, L., He, J., Huang, Y., et al. (2025). The prevalence of pretreatment drug resistance and transmission networks among newly diagnosed HIV-1-infected individuals in nanning, guangxi, China. Pathogens. 14 (4), 336. doi: 10.3390/pathogens14040336
Taffa, N., Roscoe, C., Sawadogo, S., De Klerk, M., Baughman, A. L., Wolkon, A., et al. (2018). Pretreatment HIV drug resistance among adults initiating ART in Namibia. J. Antimicrob. Chemother. 73, 3137–3142. doi: 10.1093/jac/dky278
Vink, J., McFaul, K., Bradshaw, D., and Nelson, M. (2016). Does the presence of a mutation at position V179 impact on virological outcome in patients receiving antiretroviral medication? J. Infect. 72, 632–633. doi: 10.1016/j.jinf.2016.03.003
Wang, Z., Zhang, M., Wang, J., Liu, L., Chen, J., Zhang, R., et al. (2023). Efficacy of efavirenz-based regimen in antiretroviral-naïve patients with HIV-1 V179D/E mutations in shanghai, China. Infect. Dis. Ther. 12, 245–255. doi: 10.1007/s40121-022-00723-8
World Health Organization (2019). HIV drug resistance report 2019. Available online at: https://www.who.int/publications/i/item/WHO-CDS-HIV-19.21 (Accessed August 20, 2025).
Zhang, Y., Luo, Y., Li, Y., Zhang, Y., Wu, W., Peng, H., et al. (2021). Genetic diversity, complicated recombination, and deteriorating drug resistance among HIV-1-infected individuals in wuhan, China. AIDS Res. Hum. Retroviruses 37, 246–251. doi: 10.1089/aid.2020.0142
Keywords: HIV-1, pre-treatment drug resistance, mutation, V179D, V179E, efficacy
Citation: Lv S, Lan Y, Li Q, Ling X, Li J, Wen C, Li Y, Chen J, Chen X, Cai W, Tang X and Li L (2025) Impact of V179D/E mutations on antiretroviral therapy outcomes in people living with HIV-1: a 3-year retrospective study. Front. Cell. Infect. Microbiol. 15:1691715. doi: 10.3389/fcimb.2025.1691715
Received: 24 August 2025; Accepted: 20 November 2025; Revised: 03 November 2025;
Published: 04 December 2025.
Edited by:
Raphael Zozimus Sangeda, Muhimbili University of Health and Allied Sciences, TanzaniaReviewed by:
Shivanjali Shankaran, Rush University Medical Center, United StatesTülin Demir, Ahi Evran Universitesi Tip Fakultesi, Türkiye
Copyright © 2025 Lv, Lan, Li, Ling, Li, Wen, Li, Chen, Chen, Cai, Tang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linghua Li, bGxoZWxpemFAMTI2LmNvbQ==; Xiaoping Tang, dGFuZ3hwQGd6aG11LmVkdS5jbg==; Weiping Cai, Z3o4aGN3cEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Shiyun Lv1,2†
Shiyun Lv1,2† Yun Lan
Yun Lan Xuemei Ling
Xuemei Ling Weiping Cai
Weiping Cai Xiaoping Tang
Xiaoping Tang Linghua Li
Linghua Li