- 1Shandong Institute of Parasitic Diseases, Shandong First Medical University & Shandong Academy of Medical Sciences, Jining, China
- 2School of Public Health, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, China
- 3Department of Pathogenic Biology, Jining Medical University, Jining, China
Introduction: Ticks are ectoparasitic blood-sucking arthropods. As key disease vectors, the pathogens transmitted by ticks pose significant threats to livestock and global public health.
Methods: We searched the Web of Science and Scopus databases for global literature on ticks published between 2015 and 2024. Using VOSviewer and CiteSpace software, we conducted bibliometric and visualization analyses of the national, institutional, journal, author, keyword, and reference data from the relevant literature. The aim was to assess the characteristics of global tick-related scientific research, identify research hotspots, and explore future trends in this field.
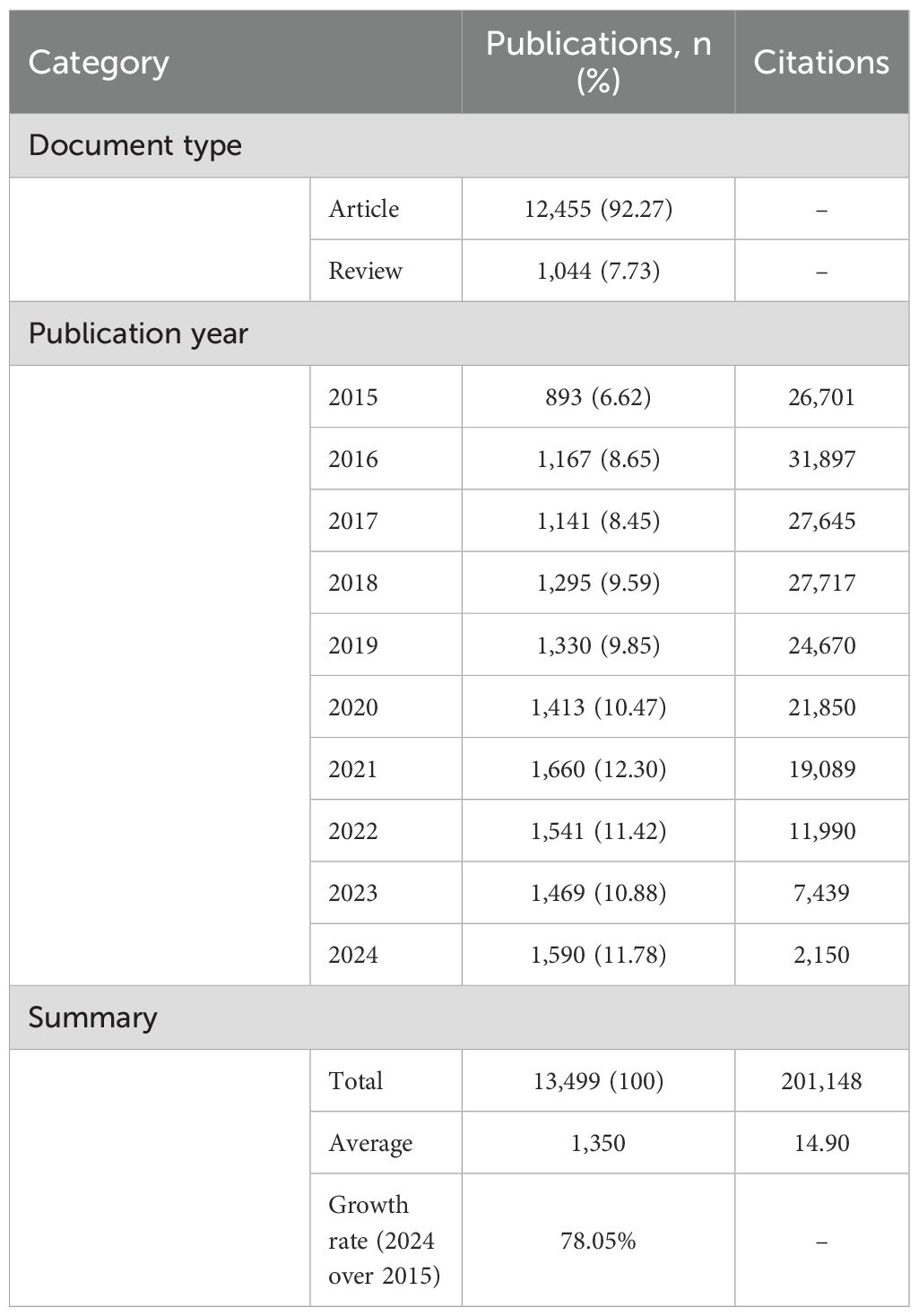
Results: The study comprised 13,499 valid articles. The United States led with 30.85% of the articles, followed by China (10.46%) and Brazil (9.99%). Marcelo B. Labruna, a Brazilian author, demonstrated the highest productivity. The institution with the most articles was Universidade de São Paulo, and the journal Ticks and Tick-borne Diseases had the largest number of publications. Keywords related to tick-borne diseases and pathogens, such as “Lyme disease”, “tick-borne encephalitis and tick-borne encephalitis virus”, “Borrelia burgdorferi”, and “Rickettsia”, appeared relatively often, while keywords such as “One Health” and “antimicrobial resistance” have emerged in recent years.
Discussion: The study of ticks and the diseases they transmit, as well as the pathogens they carry, has always been a focus for researchers worldwide. Under global climate change, the diversity of tick-borne pathogens is expanding, as evidenced by their increased geographical distribution patterns. Therefore, research is increasingly moving toward multidisciplinary and multi-sectoral approaches, aiming to safeguard the environment and to protect the health of humans and livestock through the establishment of systematic tick control systems.
Introduction
Ticks are obligate ectoparasites that feed on the blood of a wide variety of vertebrates, including amphibians, reptiles, birds, and mammals (de La Fuente and Kocan, 2006). Ticks are vectors of numerous pathogens that cause human and animal diseases, including Lyme disease, tick-borne encephalitis (TBE), anaplasmosis, babesiosis, and others (Estrada-Peña and Jongejan, 1999; Parola and Raoult, 2001; de La Fuente and Kocan, 2006). The global dissemination of tick-borne diseases (TBDs) represents an increasing challenge to public health, given the worldwide proliferation of tick populations (Madison-Antenucci et al., 2020). The costs of these diseases are considerable, not only in terms of human health but also in losses to livestock (Jongejan and Uilenberg, 1994; Brites-Neto et al., 2015), with annual estimates ranging from $13.9 billion to $18.7 billion globally (De Clercq et al., 2012; Sungirai et al., 2018). Recent studies have shown that changes in climate, land use, and human activities have precipitated shifts in tick distributions, thereby augmenting the prevalence of TBDs (Gray et al., 2009; Estrada-Peña et al., 2012; Semenza et al., 2022). As a result, the research community has increased its focus on the ecology, transmission dynamics, and control of ticks and their pathogens (de La Fuente et al., 2023). However, despite a growing body of literature, there is a lack of systematic integration and analysis that provides a comprehensive global overview of trends, hotspots, and emerging themes in tick research.
Bibliometrics is a field of study that utilizes statistical methods to evaluate the current state of research and forecast future trends in academic scholarship (Broadus, 1987; Ninkov et al., 2022). One of the primary objectives of this discipline is to provide both quantitative and qualitative analyses of the structure, impact, and dynamics of academic communication (Ninkov et al., 2022; Hassan and Duarte, 2024). Software tools such as VOSviewer and CiteSpace enable the aggregation, processing, analysis, and visualization of data related to research publications, citations, keywords, and related concepts. These tools enable the examination of research trends, influence, and collaboration, providing valuable insights into the evolution of research subjects and facilitating the identification of emerging research domains.
The objective of this study was to conduct a global bibliometric analysis of research related to ticks published between 2015 and 2024. Using advanced tools such as VOSviewer (van Eck and Waltman, 2010, 2014) and CiteSpace (Chen, 2004, 2006), we identified pivotal research themes by mapping knowledge structures and highlighting the most significant developments in tick research. By analyzing publication trends, author collaboration networks, and the co-occurrence of keywords, this study provides valuable insights into the current state and emerging areas of tick-related research. The results will contribute to strategic decision-making in the prevention and management of TBDs, particularly in the context of a constantly evolving global landscape.
In addressing the current state of tick research, this study sought to answer the following questions:
1. What advancements have been made in tick research over the past decade?
2. What are the prevailing research trends and hotspots in tick-related studies?
3. What are the future directions for tick research, and where do the greatest opportunities for advancing knowledge in this field lie?
Materials and methods
Database selection
The bibliometric methodology employed in this study referred to previously published research (Öztürk et al., 2024). WoSCC is esteemed for its rigorously curated content and strong coverage of high-impact, foundational literature, making it a cornerstone for citation analysis. Conversely, Scopus offers more extensive coverage of emerging and regional journals, thereby ensuring a broader and more inclusive perspective of the scientific landscape. Thus, these databases were deemed appropriate for searching studies relevant to tick research and obtaining a comprehensive review of the extant literature in this field.
Search strategy
The data for this study were retrieved from the Science Citation Index Expanded (SCI-Expanded) of the WoSCC and Scopus databases, covering the period from March to April 2025. A topic search was made using the keywords “tick” or “ticks.” Papers with these words in the title, abstract, author keywords, or keywords plus were mined. Articles published in English between January 1, 2015, and December 31, 2024 were included, excluding preprints or predictive data. We only selected the publication types of “Article” and “Review.” Ultimately, a total of 13,751 records from the WoSCC and 14,981 records from Scopus databases were obtained.
Inclusion and exclusion criteria
Specific inclusion and exclusion criteria were employed in this study to ensure the selection of relevant literature. We focused on articles and reviews, particularly original articles, to ensure the inclusion of significant research findings. The inclusion criteria limited the selection to studies published in English and indexed in the Web of Science and Scopus databases. Meeting abstracts, proceedings papers, editorial material, letters, lectures, and duplicate literature were excluded.
Quality management and data extraction
To ensure methodological rigor, this study implemented a set of stringent quality controls. The data extraction process was initiated through a systematic screening of article titles and abstracts. Subsequently, each selected publication was evaluated against the predetermined inclusion and exclusion criteria. Full bibliographic records and cited reference data were then extracted for analytical processing. A high citation index (H-index) was obtained from the WoSCC, and journal impact factors (IF) were obtained from the 2023 edition of the Journal Citation Reports (Clarivate Analytics, Philadelphia, PA, USA).
Data analysis
Microsoft Excel 2021 was used to analyze the data after manual quality control. The “EndNote desktop” data format from WoSCC and the “RIS” data format from Scopus were imported into EndNote X9.1 for deduplicate deduplication. The deduplicated data were then saved as text files with the filename “download_*.txt.” Subsequently, the data were imported into VOSviewer 1.6.20 (Leiden University, Leiden, The Netherlands) and CiteSpace 6.2.R6 (Drexel University, Philadelphia, PA, USA) for bibliometric and visual analyses. VOSviewer was used to generate knowledge maps of the contributing countries/regions, institutions, influential authors and journals, co-cited references, and keyword co-occurrences. The CiteSpace application was used to extract keywords and references from the literature that exhibited the strongest citation bursts. The parameters were as follows: (1) Timespan: 2015–2024 (Slice Length = 1); (2) Selection Criteria: g-index (k = 25); (3) Pruning: Pathfinder + pruning the merged network. Figure 1 illustrates the flow diagram of the literature search and analysis. This study did not involve human experiments and hence did not need ethical approval.
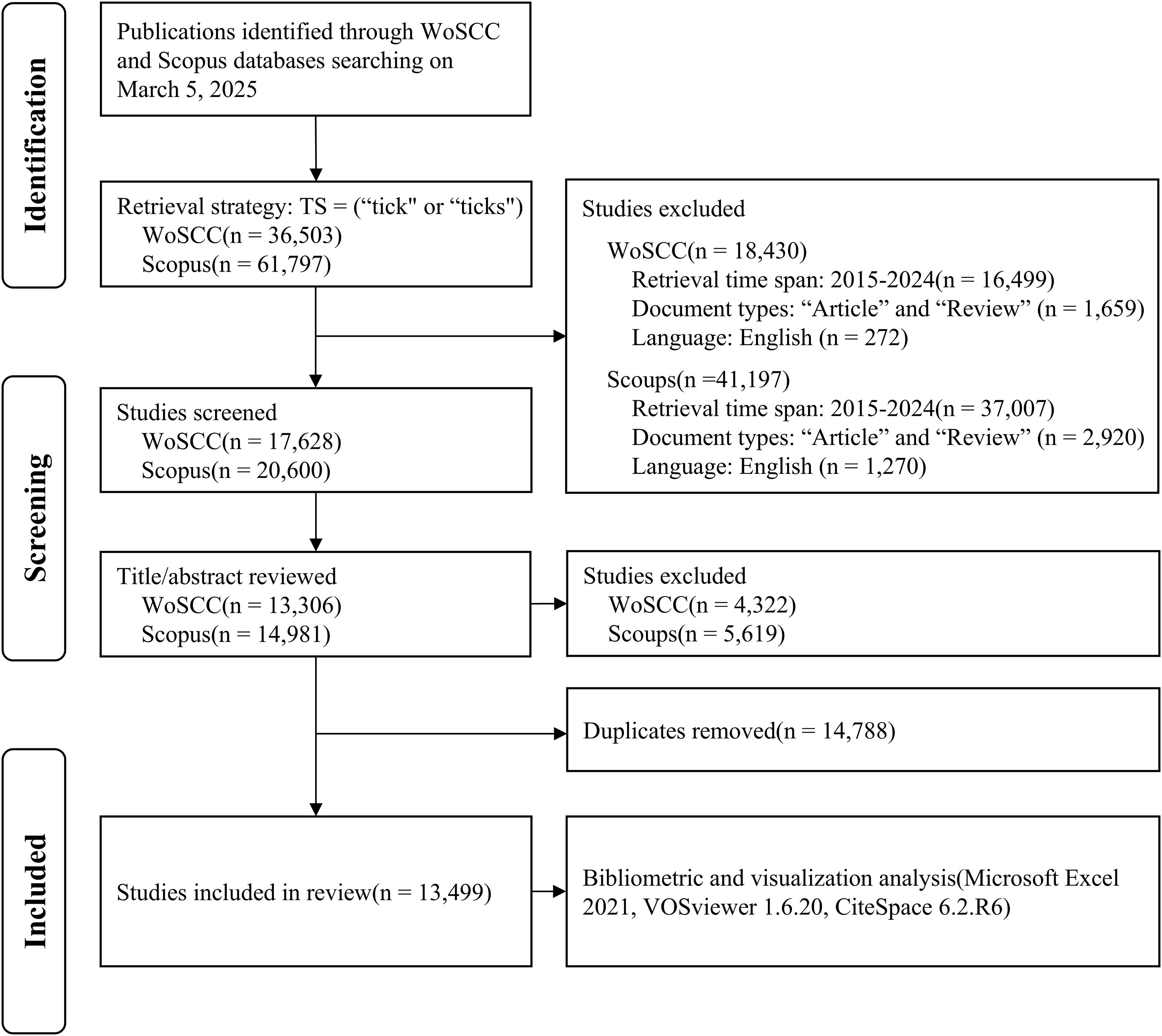
Figure 1. Flow diagram of the retrieval strategy and analysis. The process includes identification, screening, and inclusion phases. Initially, 36,503 publications from WoSCC and 61,797 from Scopus are identified. After excluding irrelevant studies and duplicates, 13,499 studies are included in the review. The exclusion criteria involve timeframe, document types, and language. Tools used for bibliometric and visualization analysis include Microsoft Excel 2021, VOSviewer 1.6.20, and CiteSpace 6.2.R6. WoSCC, Web of Science Core Collection.
Results
Trends in publication and citation
The final analysis encompassed a total of 13,499 publications, comprising 12,455 research papers and 1,044 review articles (Table 1). According to the search strategy, the number of annual publications steadily increased, from 893 in 2015 to 1,590 in 2024. This indicates a significant escalation in research activity, with a 78.05% increase over the 10-year period. The maximum number of papers observed was 1,660 in 2021. However, after reaching a peak in 2016, the number of citations declined year by year, with a particularly pronounced drop observed after 2020. This indicates that the pace of publication could be outpacing the citation potential of each paper. This trend could be attributed to the phenomenon of “citation lag,” where it takes several years for research to be widely cited and recognized in the academic community (Wang, 2013). As of the retrieval date, these publications had been cited 201,148 times, averaging 14.90 citations per publication (Figure 2).
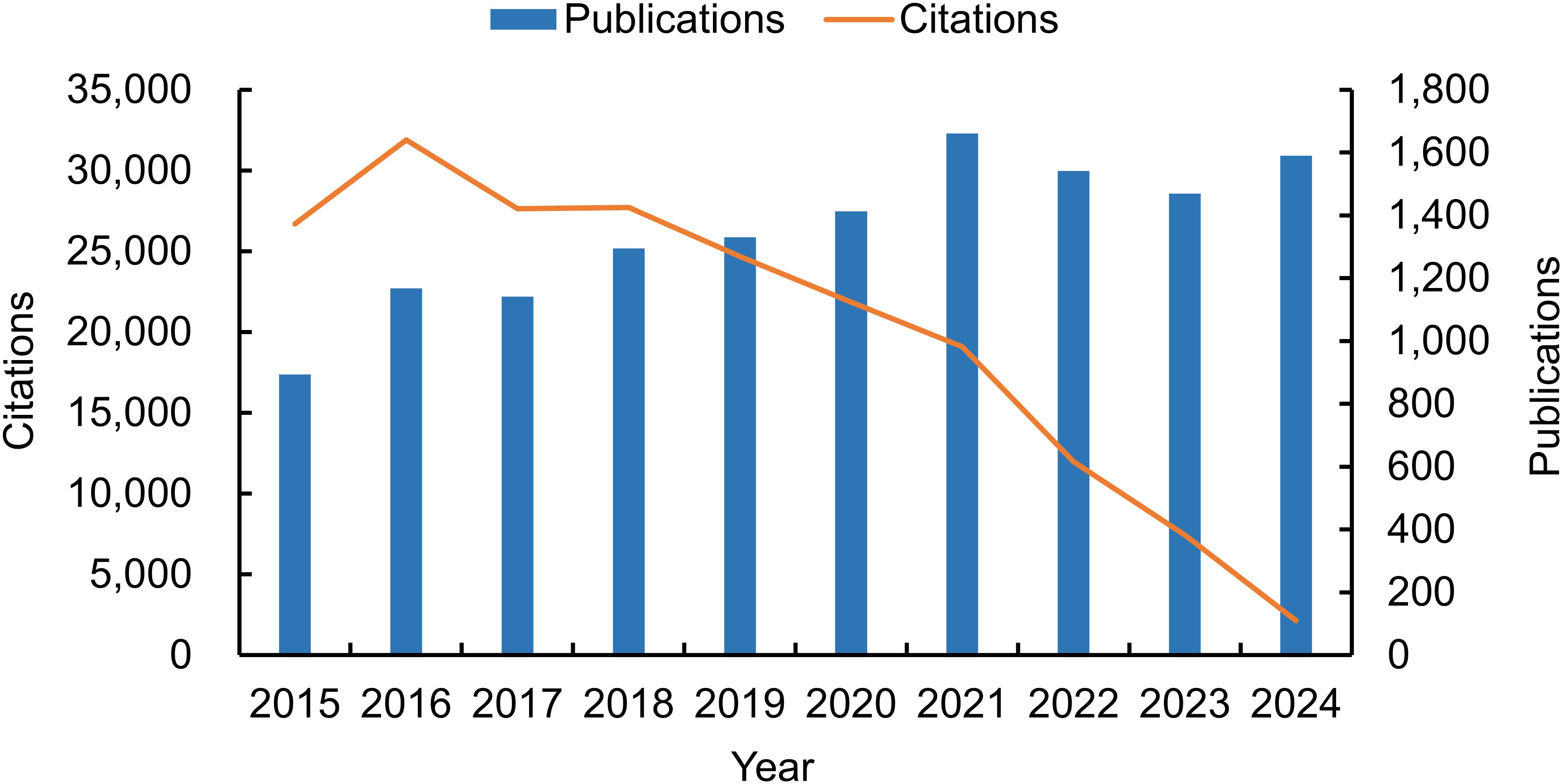
Figure 2. Global trends in annual publications and citations related to tick research from 2015 to 2024.
Countries/regions and institutions
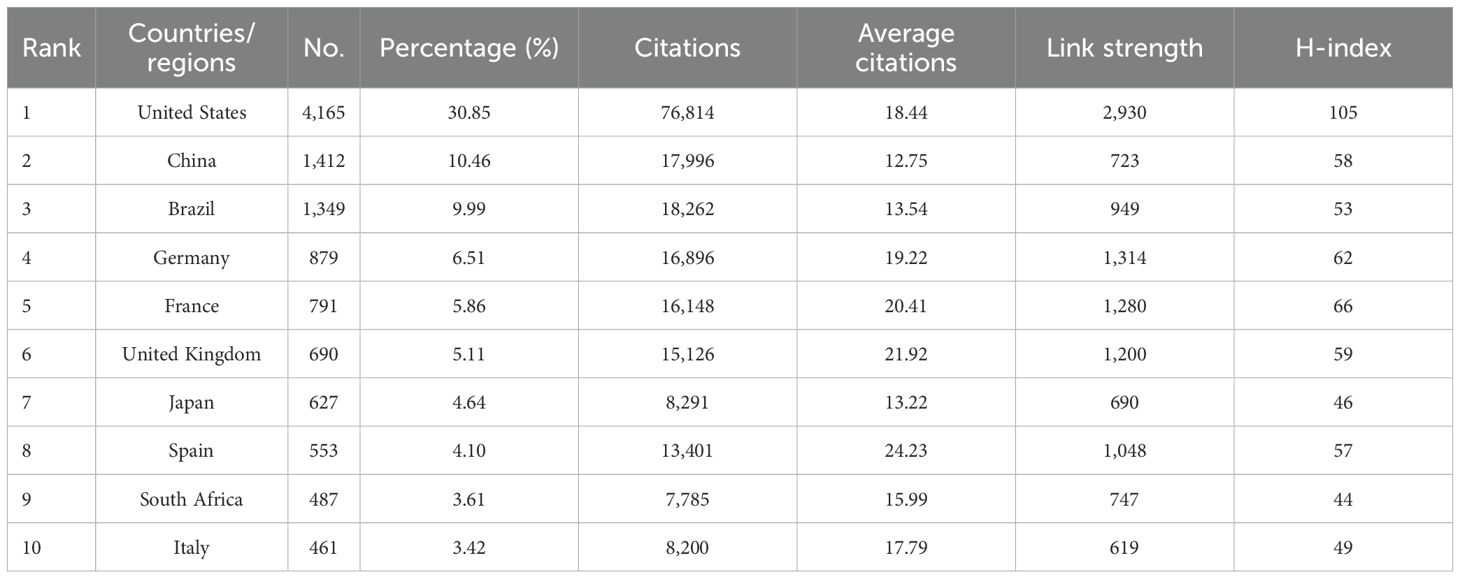
VOSviewer bibliometric analysis identified 166 countries and regions, as well as 10,850 institutions, engaged in tick-related research during the period 2015–2024. Table 2; Figure 3A list the 10 most productive countries and regions. The United States dominated the scholarly output (4,165 publications), accounting for approximately 30.85% of the total publications. China ranked second (1,412 publications), followed by Brazil (1,349), Germany (879), and France (791). The citation analysis further established U.S. leadership, with 76,814 citations, accounting for 38.62% of the total citations among the top 10 nations. To map international collaboration networks, co-authorship relationships were visualized through VOSviewer (Figure 3B), revealing the United States as the central hub and indicating its pivotal role in facilitating global research exchange.
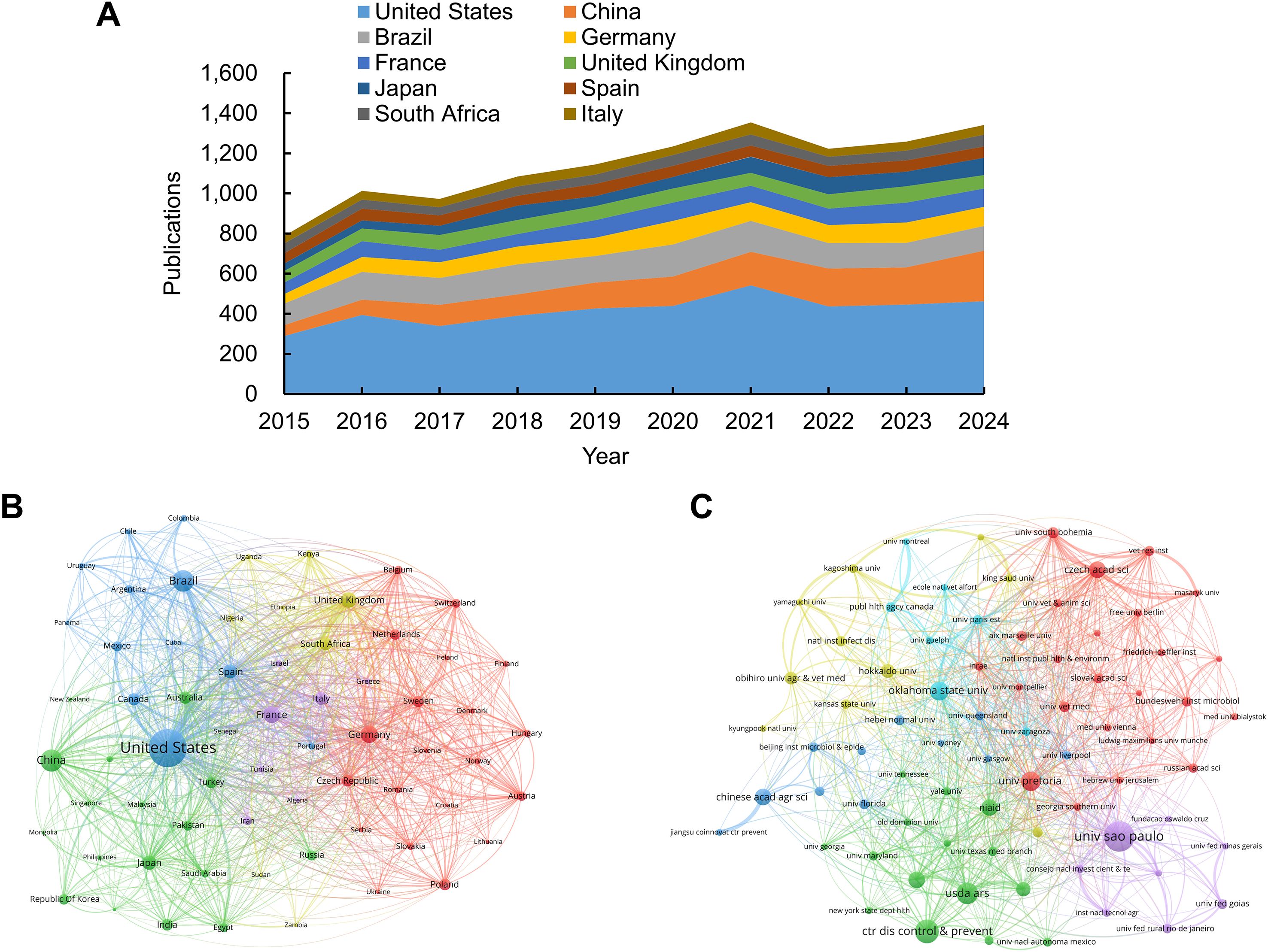
Figure 3. Visualization maps of countries/regions and institutions in tick research. (A) Trend in annual publication counts among the top 10 countries/regions from 2015 to 2024. (B) Co-authorship map of countries/regions involved in tick research. Node size corresponds to publication volume (range: 37–4,165); line thickness indicates collaboration strength (range: 47–2,930). (C) Co-authorship map of institutions involved in tick research. Node size corresponds to publication volume (range: 68–418); line thickness indicates collaboration strength (range: 12–351).
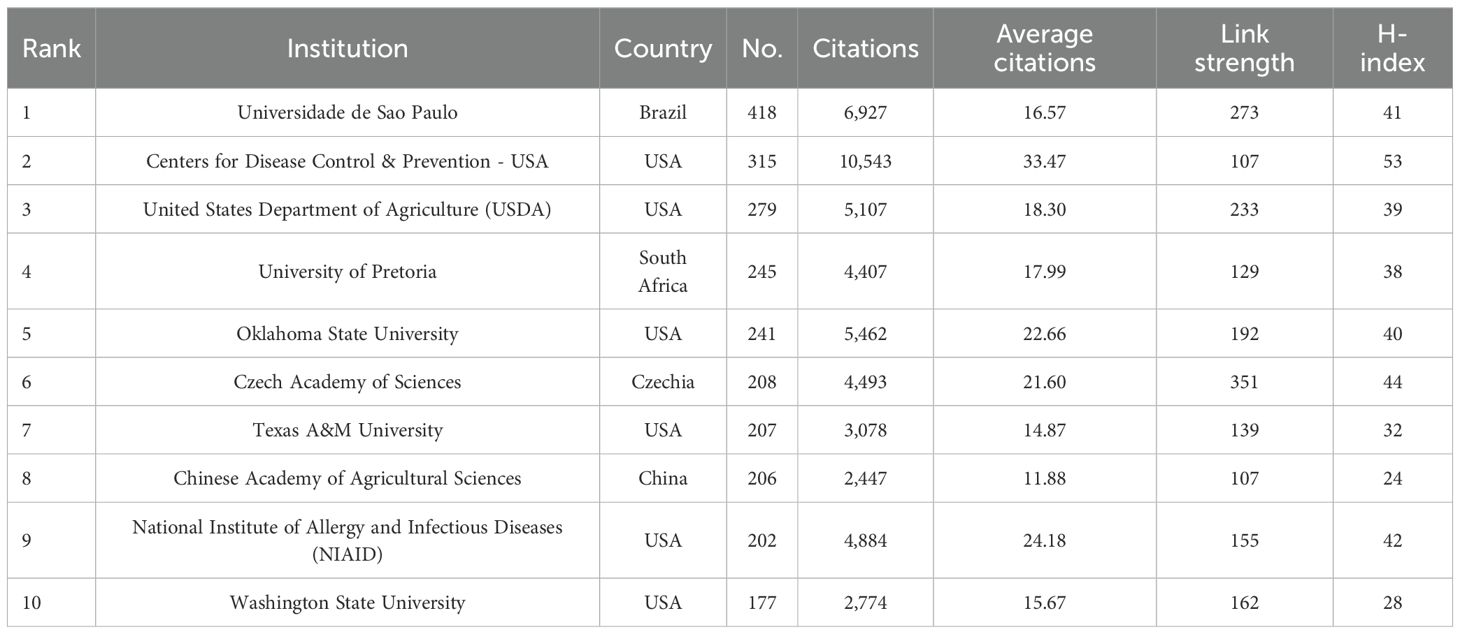
The top 10 institutions that have published the largest number of papers are listed in Table 3. Six of the top 10 are in the United States, representing more than half of the total. Brazil, South Africa, the Czech Republic, and China each had one institution. The Universidade de São Paulo was the leading institution in terms of publications, with 418 papers, followed by the Centers for Disease Control & Prevention (CDC) in the United States, which published 315 papers, and the United States Department of Agriculture (USDA), which published 279 papers. These institutions are at the forefront of tick research, reflecting the central roles of Brazil and the United States in advancing the field. The CDC was ranked first in terms of citation performance, with 10,543 citations and an average of 33.47 citations per article. Figure 3C illustrates the active collaborative relationships between these institutions, highlighting their significance in this field.
Authors and co-cited authors
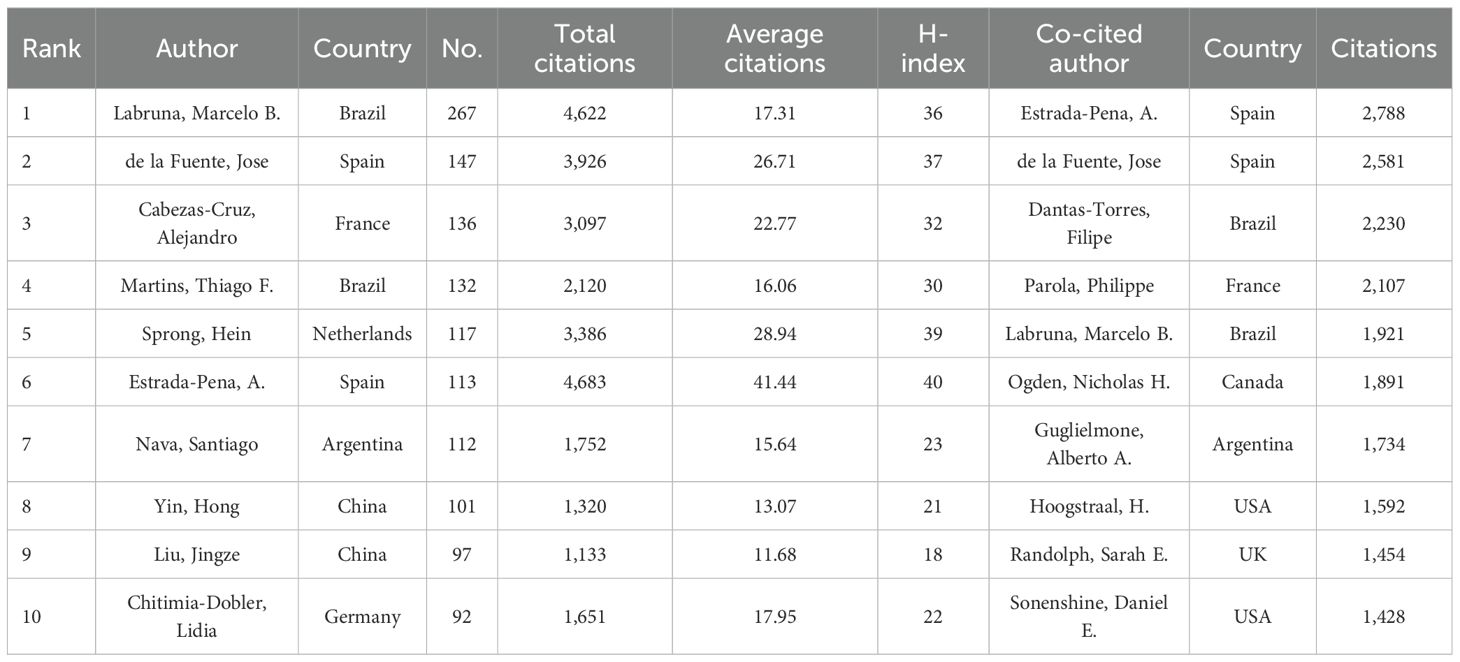
According to the most recent tally, 45,027 authors have published academic papers related to research on ticks. Table 4 lists the 10 authors who demonstrated the most prolific publication performance over the past decade. The author with the most publications is Marcelo B. Labruna (267), followed by José de la Fuente (147), Alejandro Cabezas-Cruz (136), and Thiago F. Martins (132). Notably, although Estrada-Peña from Spain ranked sixth in terms of publication count, he had the highest average citation per article at 41.44, the highest among all authors. José de la Fuente, meanwhile, ranks second in both publication volume and co-citation, indicating that his output was not only more prolific but also of higher quality and greater influence. Figure 4 illustrates the co-authorship and co-citation network analysis of prominent authors. Authors with higher publication output are typically associated with larger collaboration networks.
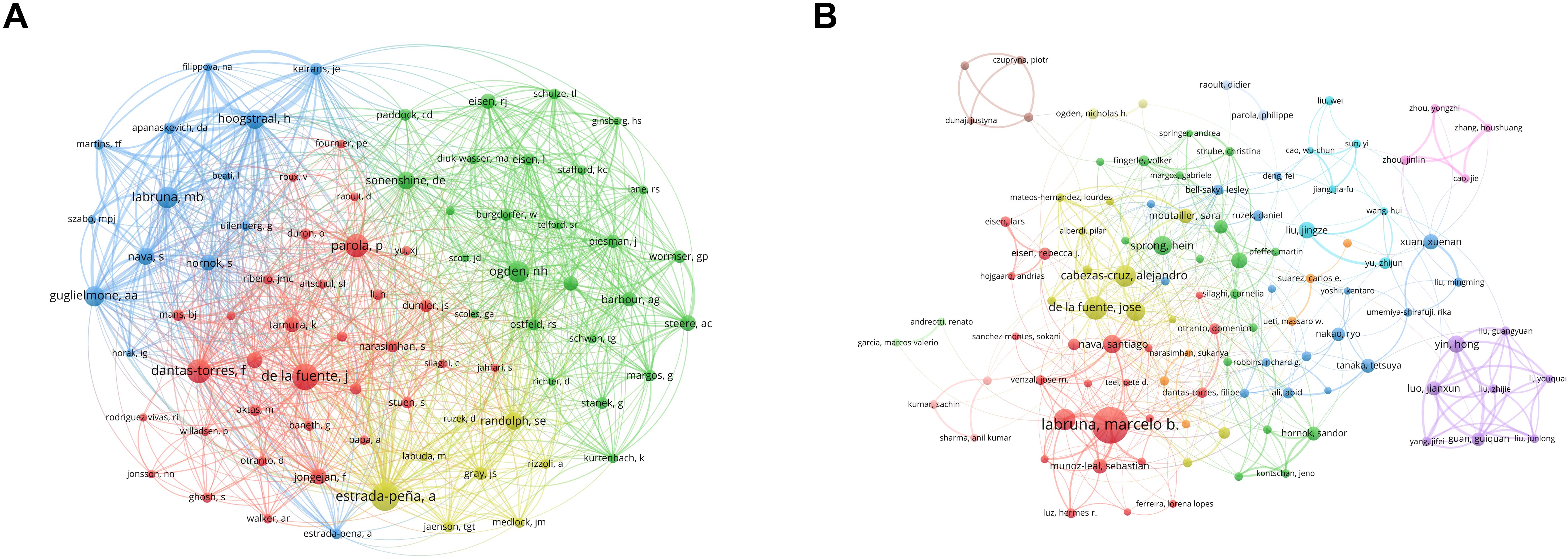
Figure 4. Visualization maps of co-authorship and co-citation involved in tick research. (A) Author co-citation network. Node size corresponds to citation frequency (range: 455–2,788); line thickness indicates co-citation strength (range: 1,102–42,128). (B) Author co-authorship network. Node size represents publication volume (range: 35–267); line thickness signifies collaboration intensity (range: 3–374).
Journals and co-cited journals
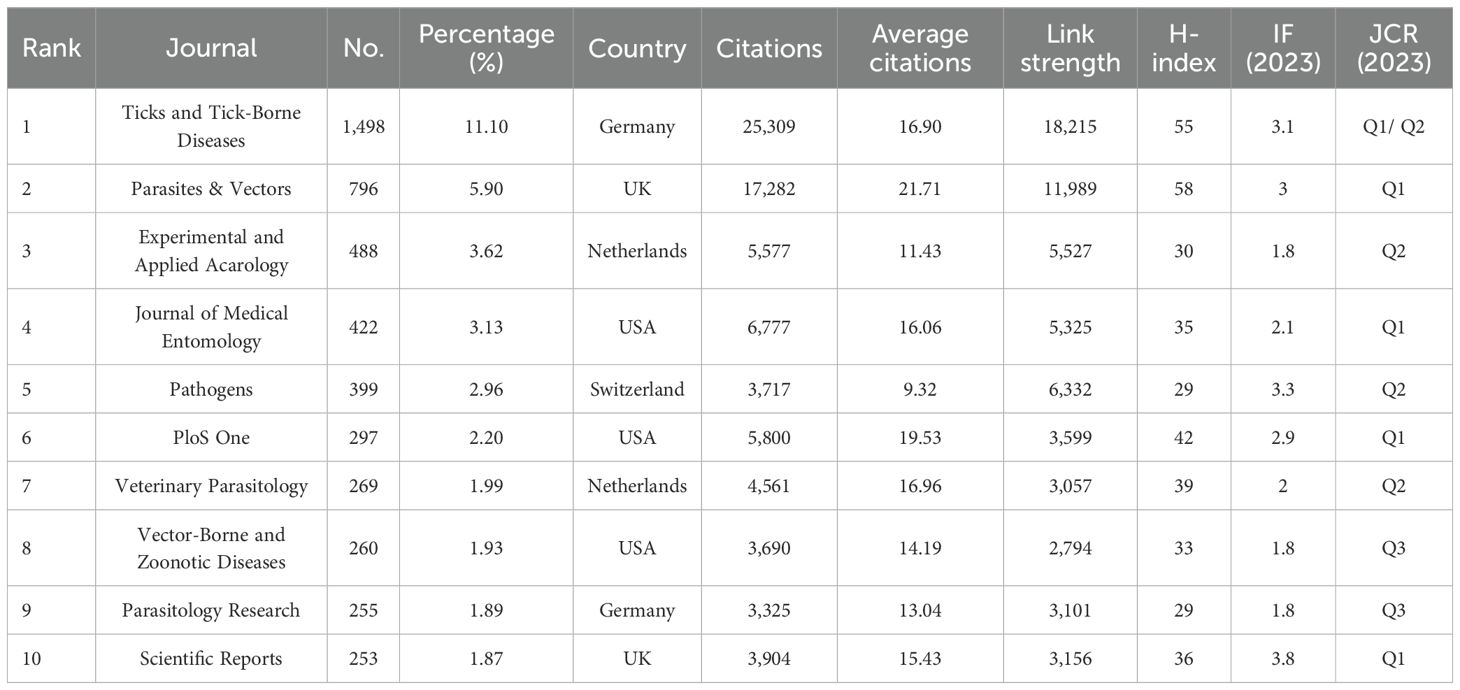
There were 1,220 journals that published research related to tick research during the past decade. We constructed a network visualization map that showed a close collaboration between leading journals (Figure 5A). Table 5 ranks the top 10 journals with the most published articles. Ticks and Tick-Borne Diseases is the most prolific publication, with 1,498 publications and 25,309 citations, demonstrating its central role in disseminating knowledge in this field. According to the 2023 Journal Citation Report (JCR), the majority of these journals are distributed within the Quartile 1 (Q1) or Quartile 2 (Q2) range.
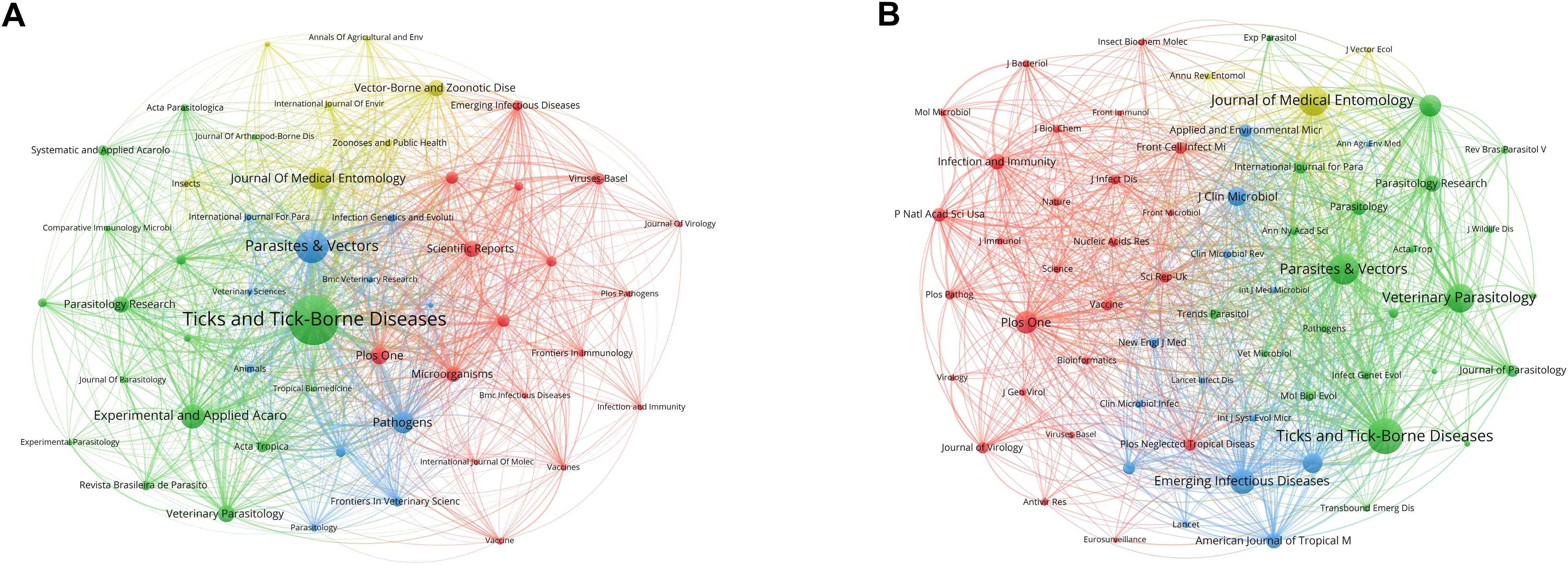
Figure 5. Visualization maps of relevant journals from 2015 to 2024. (A) Network visualization map of journals’ co-occurrence on tick research. Node size represents publication volume (range: 46–1498); line thickness indicates collaboration strength (range: 196–18,215). (B) Network visualization map of journal co-citation analysis on tick research. The sizes of the nodes represent the citation frequency (range: 1,543–29,376), and the lines between two nodes indicate that both were cited by the same journal.
The frequency of co-citation is a significant metric for assessing the impact of a journal. The co-citation analysis showed that 66 journals were co-cited over 1500 times (Figure 5B). The most co-cited journal was Ticks and Tick-Borne Diseases (29,376 times), followed by Parasites & Vectors (22,554 times). The patterns of co-citation reveal a collaborative ecosystem within parasitology and acarology research, characterized by interdisciplinary research that integrates tick biology, vector control, and disease ecology.
Cocited reference and reference bursts
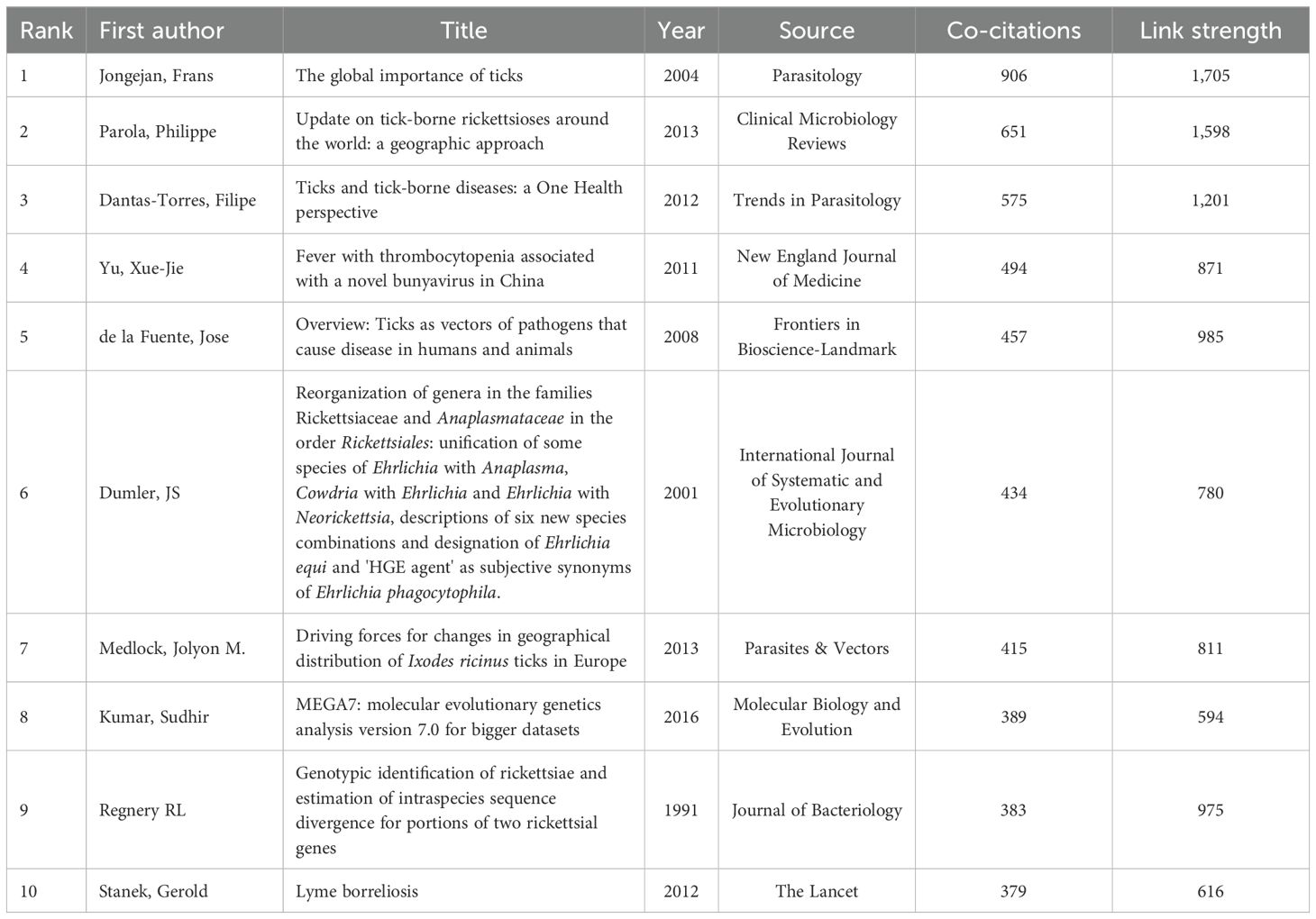
Co-citation analyses are employed within the field of scholarship to measure the degree of association between references within a specific research domain. VOSviewer was employed to generate a network map of the references co-cited more than 200 times (Figure 6A). The 10 references with the highest number of co-citations in tick research are listed in Table 6. The study published in Parasitology by Frans Jongejan in 2004 entitled “The global importance of ticks” was the most co-cited article (906 times) in this field (Jongejan and Uilenberg, 2004).
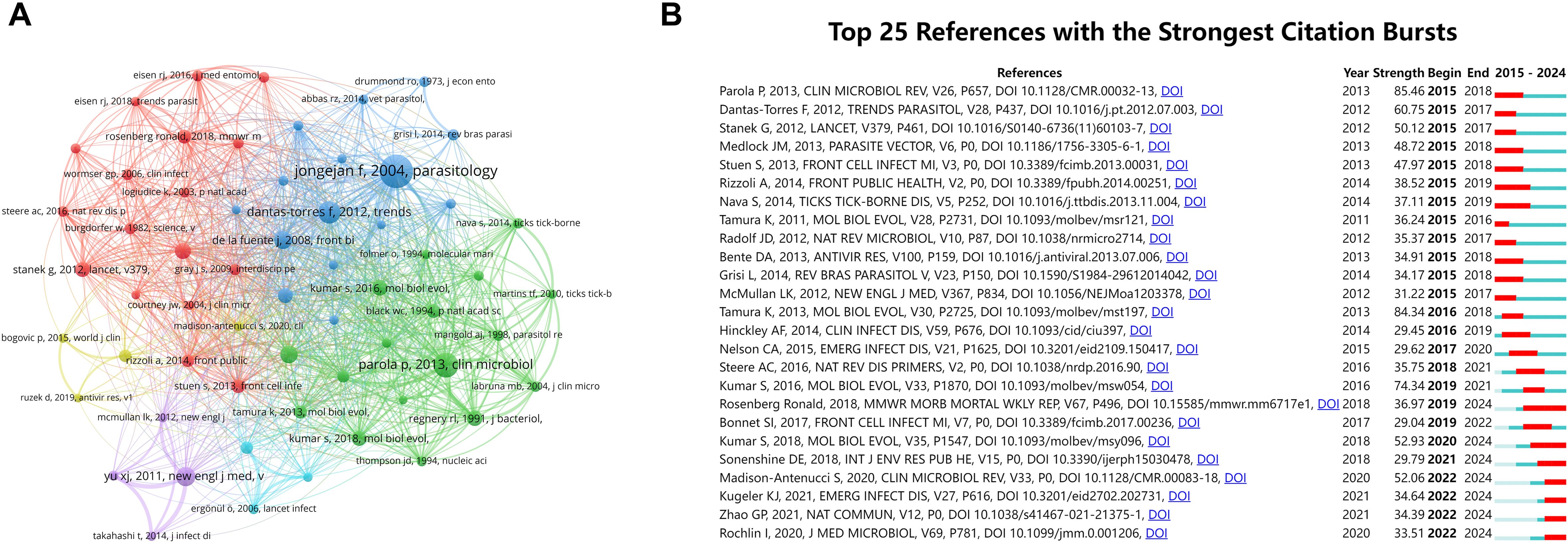
Figure 6. Co-citation networks and high-impact reference dynamics in tick research. (A) Co-citation network map of references on ticks. Node size corresponds to citation frequency (range: 202–906); line thickness indicates co-citation strength (range: 437–1,705) (B) Top 25 references with the highest burst activity in tick research (generated by CiteSpace). The blue bars indicate the time interval, and the red bars indicate the active time.
Using CiteSpace software, we examined the references that showed the strongest citation bursts (Figure 6B). Among the references with a significant surge in citations, the articles with the highest values were Parola et al. (2013); Tamura et al. (2013), and Kumar et al. (2016). The rapid evolution of Molecular Evolutionary Genetics Analysis (MEGA) underscores the need for researchers to undertake comparative analyses of DNA and protein sequences from substantial datasets. Notably, the most prominent citation surge corresponds to the second most co-cited article, with an intensity of 85.46. The majority of high-burst articles were concentrated in publications from 2012 to 2016, while more recent publications (post-2020) exhibited relatively lower burstiness, although the trend has persisted, indicating an ongoing evolution of research hotspots.
Keywords analysis and burst research
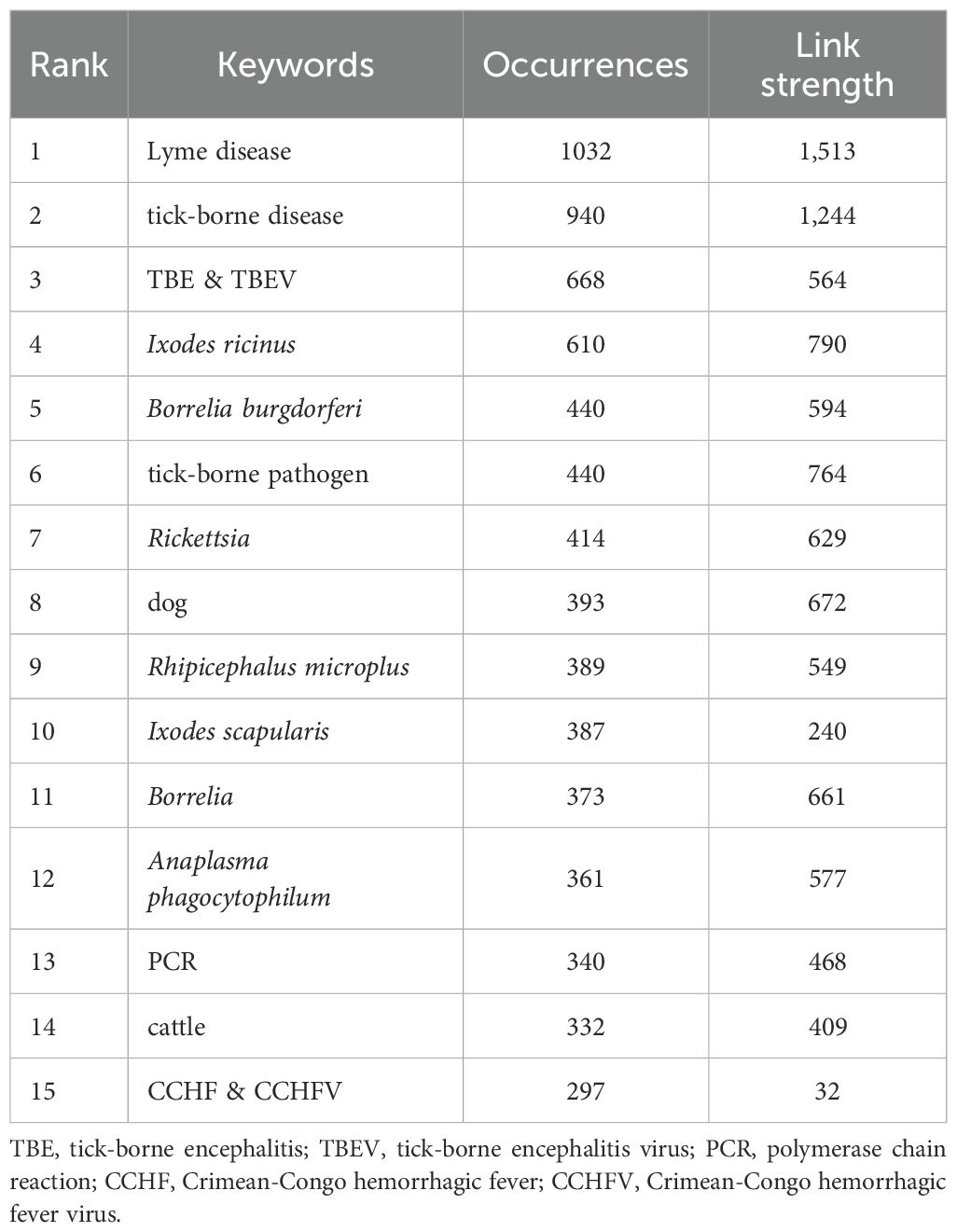
The keyword analysis revealed the research hotspots and scientific trends of ticks. A total of 16,977 keywords were obtained, and a subsequent analysis revealed 55 words that occurred more than 100 times (Figure 7A). The identified keywords could be divided into five clusters: (1) research on TBDs and pathogens, including terms such as Lyme disease, TBE, Borrelia burgdorferi, Babesia, Rickettsia and Anaplasma phagocytophilum (Supplementary Figure S1); (2) research on tick species and vectors, including Ixodes ricinus, I. scapularis, Rhipicephalus microplus, Haemaphysalis longicornis and Amblyomma americanum (Supplementary Figure S2); (3) research on surveillance, epidemiology, and risk factors related to TBDs, such as zoonosis and climate change; (4) research on diagnosing TBDs, including terms related to methods such as the polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA), and (5) research on vaccine development and control measures related to TBDs, including terms such as vaccine, acaricide, and resistance. Table 7 presents a summary of the 15 most frequent keywords, with the majority of top-ranked terms involving TBDs and pathogens.
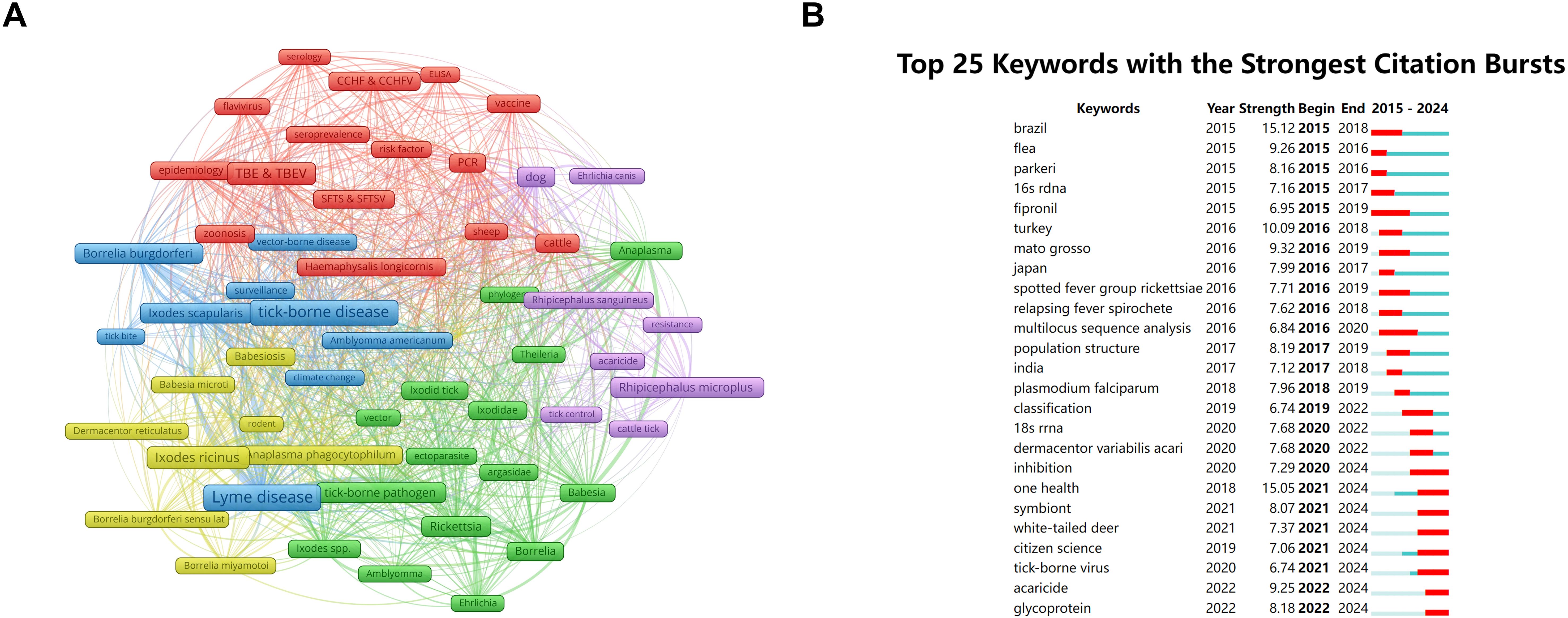
Figure 7. Keywords co-occurrence network and burst dynamic in tick research. (A) Network visualization map of keywords co-occurrence in tick research. Node size corresponds to keyword frequency (range: 104–1,062); line thickness indicates co-occurrence strength (range: 71–1,513). (B) Top 25 keywords with the strongest citation bursts in tick research (generated by CiteSpace). The blue bars indicate the time interval, and the red bars indicate the active time.
The CiteSpace software generated keywords with the strongest citation bursts (Figure 7B), reflecting the research frontiers and trends. The term “Brazil” exhibited the earliest citation burst, with a strength of 15.12, highlighting the geographic significance of this nation in TBDs research. Notably, while “One Health”—an interdisciplinary approach that integrates the health of humans, animals, and their shared environment—appeared in the literature as early as 2018, its citation surge peaked during 2021–2024, with a strength of 15.05, likely attributable to accelerated multidisciplinary collaborations in recent years (Zinsstag et al., 2011).
Discussion
Studies have shown that the prevalence of TBDs and the associated risks to human and animal health have been steadily increasing (Estrada-Peña et al., 2012; Madison-Antenucci et al., 2020). Despite concerted efforts at eradication, ticks and the pathogens they disseminate persist, constituting a serious threat to human and animal health on a global scale (Jongejan and Uilenberg, 1994; de La Fuente and Kocan, 2006; Semenza et al., 2022). As the volume of related literature increases, it becomes increasingly challenging to track emerging research trends. Bibliometric analyses offer a valuable means to comprehend the growth, development, and dissemination of knowledge in tick research, with the aim of capturing emerging trends and predicting future research directions (Thompson and Walker, 2015; Kraus et al., 2024; Öztürk et al., 2024).
The present study employed a bibliometric analysis to assess global tick research, analyzing publication data to identify research hotspots and trends. A comprehensive analysis of 13,499 articles published over the past decade revealed a significant upward trend in the number of publications, suggesting a growing focus on tick research. However, this growth has not been mirrored by an increase in the number of citations. The disparity between publications and citations suggests a delay in the recognition of newer research; this is expected as the academic community processes and integrates findings over time. The top five countries accounted for more than 60% of the publications. The United States has assumed a prominent position in tick research, exhibiting a higher volume of publications compared to other nations. This may be attributed to the extensive history of tick research in the United States and the high prevalence of TBDs (Beard et al., 2019; Kugeler et al., 2021), which is exemplified by the seminal article on Babesia bigemina published by Smith and Kilborne in 1893 (Smith and Kilborne, 1893). China has exhibited the most rapid growth in tick research, which can be attributed to the rising frequency of new TBDs and the growing burden of various existing tick-borne illnesses (Wu et al., 2025). Marcelo B. Labruna from the University of São Paulo ranked first in the number of publications, and Estrada-Pena from the University of Zaragoza ranked first among co-cited authors.
Over the past decade, research on TBDs and pathogens has remained at the forefront of the field. Important TBDs, such as Lyme disease (caused by B. burgdorferi), TBE (caused by TBEV), Rocky Mountain spotted fever (caused by spotted fever group rickettsiae), and babesiosis (caused by protozoan parasites within the genus Babesia spp.), have been extensively studied. Numerous epidemiological surveys and molecular studies have revealed the transmission routes, pathogenesis, and genetic diversity of pathogens responsible for these classic TBDs. At the same time, global warming has led to a significant expansion of the geographic distribution of ticks, while the fragmentation of wildlife habitats and the acceleration of international trade and tourism have led to the emergence of new TBDs. Human granulocytic anaplasmosis (HGA) is a well-documented tick-borne zoonosis. The initial report occurred in the United States in 1990, and the disease was confirmed in Europe in 1997 (Zhang et al., 2008; Nepveu-Traversy et al., 2024). The geographic distribution of the causative agent, the phagocytic A. phagocytophilum, and its primary vector, the castor tick, has been expanding, encompassing nearly the entirety of continental Europe and the Atlantic Ocean (Stuen et al., 2013). Crimean-Congo hemorrhagic fever virus (CCHFV) was originally endemic to Africa, Asia, the Middle East, and Southeastern Europe; in recent years, the emergence of cases of indigenous transmission in European countries such as Spain suggests that the disease has spread to temperate regions (Shahhosseini et al., 2021). Severe fever with thrombocytopenia syndrome (SFTS) is caused by a novel Bandavirus (Dabie bandavirus), and it is now endemic in many East Asian countries since it was first reported in China in 2009 (Yu et al., 2011; Kim et al., 2024). However, the virus’s complete life cycle in nature, its animal host spectrum, and its tick-mediated transmission mechanism have not been fully elucidated, and there is no commercial vaccine or standardized treatment regimen for the virus (Nepveu-Traversy et al., 2024). In addition, other pathogens have emerged, such as Kyasanur forest disease virus (the case range increased dramatically in 2005), Heartland virus (discovered in Missouri, USA in 2009), Jingmen tick virus (discovered in China in 2010), Alongshan virus (first described in Northeast China in 2017), and others (Qin et al., 2014; Bosco-Lauth et al., 2015; Gurav et al., 2018; Wang et al., 2019). The geographical range of classic tick-borne pathogens is expanding, and new pathogens are emerging. These factors have contributed to the complexity of public health prevention and control, presenting novel challenges related to early detection, molecular typing, and specific treatment.
Concurrently, advancements in pathogen detection methodologies have facilitated substantial progress in tick research. Initially, conventional methods such as microscopic examination, culture isolation, and serological identification were utilized (Tokarz and Lipkin, 2021). However, these techniques have limitations in detecting low-abundance or novel pathogens. The advent of PCR technology has enabled researchers to screen tick samples for specific pathogens in a more cost-effective, time-efficient, and productive manner (Telford et al., 1997; Sparagano et al., 1999). This was followed by the advent of next-generation sequencing (NGS), which enabled the detection of pathogens to be expanded beyond the confines of a single target. This development facilitated macro-genomics analyses, leading to the concurrent identification of multiple pathogens and their co-existence in larger samples (Carpi et al., 2011; Andreotti et al., 2011; Trout Fryxell and DeBruyn, 2016). This technological innovation has precipitated a shift in tick research from single-target detection to a large-scale, multi-target surveillance model. This has led to significant improvement in the ability to understand disease transmission trends and the feasibility of developing early warning systems. Since then, a new trend in TBDs research has emerged, thanks to the development of advanced genomic tools, including NGS. Researchers have gained a deeper level of comprehension regarding the tick microbiome and endosymbionts within ticks, as well as their functions in tick biology, including immune regulation and the transmission of pathogens (Narasimhan and Fikrig, 2015; Gurfield et al., 2017). These endophytic bacteria have important implications for tick development, survival, and ability as pathogen vectors (Hussain et al., 2022; Kolo and Raghavan, 2023). Through deeper excavation of the tick microbiome interaction network, researchers have been able to analyze the complex linkages between ticks, symbiotic bacteria, and pathogens and provide a basis for future strategies based on intervening with symbiotic bacteria to block pathogen transmission.
The analysis of keywords revealed several noteworthy patterns and emerging hotspots in the field. Between 2015 and 2018, keywords such as “Brazil” (burst intensity: 15.12) and “Turkey” (10.09) experienced frequent bursts, reflecting a geographical shift in research focus. Brazil’s prominent position is closely linked to ecological disturbances caused by the development of the rainforest. Tropical rainforest fragmentation has forced the migration of tick hosts to human-populated areas and the consequent spread of tick-borne pathogens such as Rickettsia rickettsii to human settlements, thereby increasing the risk of human exposure to ticks and the diseases they transmit (Szabó et al., 2009; Dantas-Torres et al., 2019; Fonseca et al., 2020; Fornazari et al., 2021). This trend was further exacerbated by the 2015 Zika virus outbreak in May 2015 (Campos et al., 2015), and the ensuing global public health crisis spurred international collaboration in vector-borne disease research (including TBDs), positioning South America as a new hotspot for emerging research. As tick research expands on a global scale, patterns of collaboration across geographic and disciplinary boundaries are becoming clear. Although the concept of “One Health” was introduced in the literature many years ago (Nzietchueng et al., 2023), its burst period was delayed until 2021–2024 (burst intensity 15.05), likely related to the frequent occurrence of new TBDs worldwide. The global pandemic exposed the vulnerability of the human–animal–environment interface and accelerated the development of interdisciplinary collaborative mechanisms (Sprong et al., 2018). This has led to a growing recognition that pathogen ecology and disease management require an integrated approach. It is imperative to expand the scope of research from ticks and the pathogens they carry to encompass the complex interactions between humans, animals, and the ecosystem. This necessitates policy adjustments and technological innovations on a global scale (Kading et al., 2018). This transition marks a pivotal advancement in the domain of tick research, with a shift from conventional disease surveillance toward a more integrated and systematic approach. This trend has laid the groundwork for the development of a collaborative control system that incorporates human medicine, veterinary science, and environmental science.
In recent years, the keyword “acaricides” (burst intensity 9.25) has garnered increasing attention, indicating the urgency of addressing the global crisis in disease resistance. In recent decades, the widespread and intensive use of various acaricides has led to the development of resistance in many species (Wyk et al., 2016; Rodriguez-Vivas et al., 2018; Dzemo et al., 2022; De Rouck et al., 2023). Moreover, increasing evidence suggests that strategies heavily reliant on acaricides are not cost-effective, and that these chemicals have detrimental environmental impacts, including residual chemical residues in livestock products (Graf et al., 2004; Jeschke, 2021). This clearly contradicts the “One Health” concept. These issues highlight the limitations of chemical pest control and have motivated researchers to seek alternatives to conventional acaricides, accelerating the implementation of new technologies to address pesticide resistance. Host immunity has emerged as a promising alternative. In years past, researchers verified the potential of anti-tick vaccines in diminishing tick populations and curtailing the transmission of certain diseases (Canales et al., 1997; de La Fuente and Kocan, 2006). In recent years, a substantial body of research has emerged that attests to the effectiveness of various vaccines (de La Fuente and Ghosh, 2024; Nepveu-Traversy et al., 2024). However, challenges in tick vaccinology remain.
The issues associated with climate change, including rising surface temperatures and greenhouse gas emissions, are becoming increasingly severe. As is the case with numerous other arthropods, ticks are sensitive to climatic change. Studies have suggested that higher temperatures may cause shifts in the geographical range of certain tick species (Lindgren and Gustafson, 2001; Karbowiak, 2014; Clow et al., 2017). The expansion of tick habitats has resulted in an escalation in the prevalence of numerous TBDs (Lukan et al., 2010; Monaghan et al., 2015; Dahlgren et al., 2016). As a result, there is growing attention being given to tick control and vector management strategies, especially in the context of global public health crises. Encouragingly, citizen science has emerged as an efficient tool for generating large-scale datasets regarding tick distribution, complementing data collection efforts by researchers (Eisen and Eisen, 2021; Ballman et al., 2023).
The data presented herein were derived from the WoSCC and Scopus databases, thereby ensuring the accuracy and authenticity of the search results. However, despite these strengths, our study has certain limitations. First, although these databases are extensive, they may not encompass all relevant journals, particularly those with a regional focus or outside the mainstream academic sphere. Consequently, some pertinent research may have been overlooked. Second, our analysis is limited to articles and reviews, excluding other valuable sources such as conference proceedings or books, which may offer additional insights. Furthermore, excluding non-English articles may introduce language and regional biases, thereby limiting the representativeness of research from non-English-speaking regions. Finally, similar to any classical bibliometric analysis, our study is subject to the challenges of subjective bias during manual analysis, as well as potential errors in data cleaning and processing.
Conclusion
The publication of scholarly articles on tick research has exhibited a gradual upward trend over the past decade, and TBDs and their associated pathogens remain at the center of the research landscape. Global climate change has reshaped the tick’s ecological niche, manifested as an expanding geographic distribution and significantly longer active seasons. This has directly led to the emergence of new TBDs and pathogens worldwide, creating challenges in the diagnosis and treatment. The accelerated development of molecular diagnostic techniques has facilitated the identification of novel pathogens, a development that may also underlie the rising number of reports concerning new pathogens. At the same time, traditional tick control strategies are facing serious challenges. The sustainability of chemical insecticides is being questioned, and the development of vaccines against ticks or pathogens is rapidly emerging as a promising alternative control option due to the potential specificity and environmental benefits of vaccines. In conclusion, these visualized data reveal the evolution of tick research, transitioning from a macroscopic to a microscopic perspective, from singular to multifaceted approaches, and from local to integrative frameworks. In the future, tick research will continue to evolve, becoming more interdisciplinarily, with increasing collaboration between various fields, including environmental science, molecular biology, and public health. Subsequent research efforts will prioritize genetic studies, innovative vector control methodologies, and the One Health framework to mitigate the international burden of TBDs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HS: Formal analysis, Methodology, Data curation, Conceptualization, Writing – original draft, Visualization, Resources, Investigation, Writing – review & editing. MG: Validation, Supervision, Writing – review & editing, Funding acquisition, Project administration. LL: Supervision, Writing – review & editing, Resources, Visualization. ZS: Validation, Writing – review & editing, Investigation, Supervision, Funding acquisition. BZ: Funding acquisition, Validation, Resources, Writing – review & editing, Project administration, Methodology, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study was financially supported by the National Natural Science Foundation of China (Grant No.81902096); the Innovation Project of Shandong Academy of Medical Sciences; Joint Innovation Team for Clinical & Basic Research(202407).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1697791/full#supplementary-material
Supplementary Figure 1 | Top 20 tick-borne diseases (A) and tick-borne pathogens (B) in the relevant research literature.
Supplementary Figure 2 | Top 20 tick species in terms of number of relevant research literature.
Abbreviations
CDC, Centers for Disease Control & Prevention; NGS, next-generation sequencing; PCR, polymerase chain reaction; TBE, tick-borne encephalitis; TBDs, tick-borne diseases; WoSCC, Web of Science Core Collection.
References
Andreotti, R., Pérez De León, A. A., Dowd, S. E., Guerrero, F. D., Bendele, K. G., and Scoles, G. A. (2011). Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol. 11, 6. doi: 10.1186/1471-2180-11-6
Ballman, E. S., Leahy, J. E., Sponarski, C. C., Galli, M. G., and Gardner, A. M. (2023). A citizen science approach to investigate the distribution, abundance, and pathogen infection of vector ticks through active surveillance. Ticks Tick Borne Dis. 14, 102144. doi: 10.1016/j.ttbdis.2023.102144
Beard, C. B., Visser, S. N., and Petersen, L. R. (2019). The need for a national strategy to address vector-borne disease threats in the United States. J. Med. Entomol. 56, 1199–1203. doi: 10.1093/jme/tjz074
Bosco-Lauth, A. M., Panella, N. A., Root, J. J., Gidlewski, T., Lash, R. R., Harmon, J. R., et al. (2015). Serological investigation of heartland virus (Bunyaviridae: Phlebovirus) exposure in wild and domestic animals adjacent to human case sites in Missouri 2012-2013. Am. J. Trop. Med. Hyg. 92, 1163–1167. doi: 10.4269/ajtmh.14-0702
Brites-Neto, J., Duarte, K. M. R., and Martins, T. F. (2015). Tick-borne infections in human and animal population worldwide. Vet. World 8, 301–315. doi: 10.14202/vetworld.2015.301-315
Broadus, R. N. (1987). Toward a definition of “bibliometrics. Scientometrics 12, 373–379. doi: 10.1007/BF02016680
Campos, G. S., Bandeira, A. C., and Sardi, S. I. (2015). Zika virus outbreak, Bahia, Brazil. Emerg. Infect. Dis. 21, 1885–1886. doi: 10.3201/eid2110.150847
Canales, M., Enríquez, A., Ramos, E., Cabrera, D., Dandie, H., Soto, A., et al. (1997). Large-scale production in Pichia pastoris of the recombinant vaccine GavacTM against cattle tick. Vaccine 15, 414–422. doi: 10.1016/s0264-410x(96)00192-2
Carpi, G., Cagnacci, F., Wittekindt, N. E., Zhao, F., Qi, J., Tomsho, L. P., et al. (2011). Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PloS One 6, e25604. doi: 10.1371/journal.pone.0025604
Chen, C. (2004). Searching for intellectual turning points: progressive knowledge domain visualization. Proc. Natl. Acad. Sci. U. S. A. 101 Suppl 1, 5303–5310. doi: 10.1073/pnas.0307513100
Chen, C. (2006). CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc Inf. Sci. Technol. 57, 359–377. doi: 10.1002/asi.20317
Clow, K. M., Leighton, P. A., Ogden, N. H., Lindsay, L. R., Michel, P., Pearl, D. L., et al. (2017). Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PloS One 12, e0189393. doi: 10.1371/journal.pone.0189393
Dahlgren, F. S., Paddock, C. D., Springer, Y. P., Eisen, R. J., and Behravesh, C. B. (2016). Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am. J. Trop. Med. Hyg. 94, 35–42. doi: 10.4269/ajtmh.15-0580
Dantas-Torres, F., Melo, M. F., Sales, K. G. D. S., Da Silva, F. J., Figueredo, L. A., and Labruna, M. B. (2019). Phenology of Amblyomma sculptum in a degraded area of Atlantic rainforest in north-eastern Brazil. Ticks Tick Borne Dis. 10, 101263. doi: 10.1016/j.ttbdis.2019.07.007
De Clercq, E. M., Vanwambeke, S. O., Sungirai, M., Adehan, S., Lokossou, R., and Madder, M. (2012). Geographic distribution of the invasive cattle tick Rhipicephalus microplus, a country-wide survey in Benin. Exp. Appl. Acarol. 58, 441–452. doi: 10.1007/s10493-012-9587-0
de La Fuente, J., Estrada-Peña, A., Rafael, M., Almazán, C., Bermúdez, S., Abdelbaset, A. E., et al. (2023). Perception of ticks and tick-borne diseases worldwide. Pathogens 12, 1258. doi: 10.3390/pathogens12101258
de La Fuente, J. and Ghosh, S. (2024). Evolution of tick vaccinology. Parasitology 151, 1045–1052. doi: 10.1017/S003118202400043X
de La Fuente, J. and Kocan, K. M. (2006). Strategies for development of vaccines for control of ixodid tick species. Parasite Immunol. 28, 275–283. doi: 10.1111/j.1365-3024.2006.00828.x
De Rouck, S., İnak, E., Dermauw, W., and Van Leeuwen, T. (2023). A review of the molecular mechanisms of acaricide resistance in mites and ticks. Insect Biochem. Mol. Biol. 159, 103981. doi: 10.1016/j.ibmb.2023.103981
Dzemo, W. D., Thekisoe, O., and Vudriko, P. (2022). Development of acaricide resistance in tick populations of cattle: A systematic review and meta-analysis. Heliyon 8, e08718. doi: 10.1016/j.heliyon.2022.e08718
Eisen, L. and Eisen, R. J. (2021). Benefits and drawbacks of citizen science to complement traditional data gathering approaches for medically important hard ticks (Acari: Ixodidae) in the United States. J. Med. Entomol. 58, 1–9. doi: 10.1093/jme/tjaa165
Estrada-Peña, A., Ayllón, N., and de la Fuente, J. (2012). Impact of climate trends on tick-borne pathogen transmission. Front. Physiol. 3. doi: 10.3389/fphys.2012.00064
Estrada-Peña, A. and Jongejan, F. (1999). Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acarol. 23, 685–715. doi: 10.1023/a:1006241108739
Fonseca, M. S., Bahiense, T. C., Silva, A. A. B., Onofrio, V. C., Barral, T. D., Souza, B. M. P., et al. (2020). Ticks and associated pathogens from rescued wild animals in rainforest fragments of northeastern Brazil. Front. Vet. Sci. 7. doi: 10.3389/fvets.2020.00177
Fornazari, F., Freirias, C. D., Lima, H. C., Rodrigues, M. M., Langoni, H., and Teixeira, C. R. (2021). A new focus of Brazilian spotted fever in the central-west region of São Paulo state, Brazil. Rev. Soc Bras. Med. Trop. 54, e0391–e2020. doi: 10.1590/0037-8682-0391-2020
Graf, J. F., Gogolewski, R., Leach-Bing, N., Sabatini, G. A., Molento, M. B., Bordin, E. L., et al. (2004). Tick control: an industry point of view. Parasitology 129 Suppl, S427–S442. doi: 10.1017/s0031182004006079
Gray, J. S., Dautel, H., Estrada-Peña, A., Kahl, O., and Lindgren, E. (2009). Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 593232. doi: 10.1155/2009/593232
Gurav, Y. K., Yadav, P. D., Gokhale, M. D., Chiplunkar, T. R., Vishwanathan, R., Patil, D. Y., et al. (2018). Kyasanur forest disease prevalence in western ghats proven and confirmed by recent outbreak in Maharashtra, Indi. Vector Borne Zoonotic Dis. 18, 164–172. doi: 10.1089/vbz.2017.2129
Gurfield, N., Grewal, S., Cua, L. S., Torres, P. J., and Kelley, S. T. (2017). Endosymbiont interference and microbial diversity of the Pacific coast tick, Dermacentor occidentalis, in San Diego County, California. PeerJ 5, e3202. doi: 10.7717/peerj.3202
Hassan, W. and Duarte, A. E. (2024). Bibliometric analysis: a few suggestions. Curr. Probl. Cardiol. 49, 102640. doi: 10.1016/j.cpcardiol.2024.102640
Hussain, S., Perveen, N., Hussain, A., Song, B., Aziz, M. U., Zeb, J., et al. (2022). The symbiotic continuum within ticks: opportunities for disease control. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.854803
Jeschke, P. (2021). Status and outlook for acaricide and insecticide discovery. Pest Manage. Sci. 77, 64–76. doi: 10.1002/ps.6084
Jongejan, F. and Uilenberg, G. (1994). Ticks and control methods. Rev. Sci. Tech. 13, 1201–1226. doi: 10.20506/rst.13.4.818
Jongejan, F. and Uilenberg, G. (2004). The global importance of ticks. Parasitology 129 Suppl, S3–14. doi: 10.1017/s0031182004005967
Kading, R. C., Golnar, A. J., Hamer, S. A., and Hamer, G. L. (2018). Advanced surveillance and preparedness to meet a new era of invasive vectors and emerging vector-borne diseases. PloS Negl. Trop. Dis. 12, e0006761. doi: 10.1371/journal.pntd.0006761
Karbowiak, G. (2014). The occurrence of the Dermacentor reticulatus tick – its expansion to new areas and possible causes. Ann. Parasitol. 60, 37–47.
Kim, D., Lai, C. J., Cha, I., and Jung, J. U. (2024). Current progress of severe fever with thrombocytopenia syndrome virus (SFTSV) vaccine development. Viruses 16, 128. doi: 10.3390/v16010128
Kolo, A. O. and Raghavan, R. (2023). Impact of endosymbionts on tick physiology and fitness. Parasitology 150, 859–865. doi: 10.1017/S0031182023000793
Kraus, S., Bouncken, R. B., and Yela Aránega, A. (2024). The burgeoning role of literature review articles in management research: an introduction and outlook. Rev. Manage. Sci. 18, 299–314. doi: 10.1007/s11846-024-00729-1
Kugeler, K. J., Schwartz, A. M., Delorey, M. J., Mead, P. S., and Hinckley, A. F. (2021). Estimating the frequency of Lyme disease diagnoses, United States 2010-2018. Emerg. Infect. Dis. 27, 616–619. doi: 10.3201/eid2702.202731
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Lindgren, E. and Gustafson, R. (2001). Tick-borne encephalitis in Sweden and climate change. Lancet. 358, 16–18. doi: 10.1016/S0140-6736(00)05250-8
Lukan, M., Bullova, E., and Petko, B. (2010). Climate warming and tick-borne encephalitis, Slovakia. Emerg. Infect. Dis. 16, 524–526. doi: 10.3201/eid1603.081364
Madison-Antenucci, S., Kramer, L. D., Gebhardt, L. L., and Kauffman, E. (2020). Emerging tick-borne diseases. Clin. Microbiol. Rev. 33, e00083–e00018. doi: 10.1128/CMR.00083-18
Monaghan, A. J., Moore, S. M., Sampson, K. M., Beard, C. B., and Eisen, R. J. (2015). Climate change influences on the annual onset of Lyme disease in the United States. Ticks Tick Borne Dis. 6, 615–622. doi: 10.1016/j.ttbdis.2015.05.005
Narasimhan, S. and Fikrig, E. (2015). Tick microbiome: the force within. Trends Parasitol. 31, 315–323. doi: 10.1016/j.pt.2015.03.010
Nepveu-Traversy, M. E., Fausther-Bovendo, H., and Babuadze, G. G. (2024). Human tick-borne diseases and advances in anti-tick vaccine approaches: a comprehensive review. Vaccines (Basel) 12, 141. doi: 10.3390/vaccines12020141
Ninkov, A., Frank, J. R., and Maggio, L. A. (2022). Bibliometrics: methods for studying academic publishing. Perspect. Med. Educ. 11, 173–176. doi: 10.1007/s40037-021-00695-4
Nzietchueng, S., Kitua, A., Nyatanyi, T., and Rwego, I. B. (2023). Facilitating implementation of the one health approach: a definition of a one health intervention. One Health 16, 100491. doi: 10.1016/j.onehlt.2023.100491
Öztürk, O., Kocaman, R., and Kanbach, D. K. (2024). How to design bibliometric research: an overview and a framework proposal. Rev. Manage. Sci. 18, 3333–3361. doi: 10.1007/s11846-024-00738-0
Parola, P., Paddock, C. D., Socolovschi, C., Labruna, M. B., Mediannikov, O., and Kernif, T. (2013). Update on tick-borne rickettsioses around the world: a geographic approach. Clin. Microbiol. Rev. 26, 657–702. doi: 10.1128/CMR.00032-13
Parola, P. and Raoult, D. (2001). Tick-borne bacterial diseases emerging in Europe. Clin. Microbiol. Infect. 7, 80–83. doi: 10.1046/j.1469-0691.2001.00200.x
Qin, X. C., Shi, M., Tian, J. H., Lin, X. D., Gao, D. Y., He, J. R., et al. (2014). A tick-borne segmented RNA virus contains genome segments derived from unsegmented viral ancestors. Proc. Natl. Acad. Sci. U. S. A. 111, 6744–6749. doi: 10.1073/pnas.1324194111
Rodriguez-Vivas, R. I., Jonsson, N. N., and Bhushan, C. (2018). Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res. 117, 3–29. doi: 10.1007/s00436-017-5677-6
Semenza, J. C., Rocklöv, J., and Ebi, K. L. (2022). Climate change and cascading risks from infectious disease. Infect. Dis. Ther. 11, 1371–1390. doi: 10.1007/s40121-022-00647-3
Shahhosseini, N., Wong, G., Babuadze, G., Camp, J. V., Ergonul, O., Kobinger, G. P., et al. (2021). Crimean-Congo hemorrhagic fever virus in Asia, Africa and Europe. Microorganisms 9, 1907. doi: 10.3390/microorganisms9091907
Smith, T. and Kilborne, F. L. (1893). Investigations into the nature, causation, and prevention of Texas or southern cattle fever (Washington, Government Printing Office: US Department of Agriculture, Bureau of Animal Industry).
Sparagano, O. A., Allsopp, M. T., Mank, R. A., Rijpkema, S. G. T., Figueroa, J. V., and Jongejan, F. (1999). Molecular detection of pathogen DNA in ticks (Acari: Ixodidae): a review. Exp. Appl. Acarol. 23, 929–960. doi: 10.1023/a:1006313803979
Sprong, H., Azagi, T., Hoornstra, D., Nijhof, A. M., Knorr, S., Baarsma, M. E., et al. (2018). Control of Lyme borreliosis and other Ixodes ricinus-borne diseases. Parasitol. Vectors 11, 145. doi: 10.1186/s13071-018-2744-5
Stuen, S., Granquist, E. G., and Silaghi, C. (2013). Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 3. doi: 10.3389/fcimb.2013.00031
Sungirai, M., Baron, S., Moyo, D. Z., De Clercq, P., Maritz-Olivier, C., and Madder, M. (2018). Genotyping acaricide resistance profiles of Rhipicephalus microplus tick populations from communal land areas of Zimbabwe. Ticks Tick Borne Dis. 9, 2–9. doi: 10.1016/j.ttbdis.2017.10.017
Szabó, M. P. J., Labruna, M. B., Garcia, M. V., Pinter, A., Castagnolli, K. C., Pacheco, R. C., et al. (2009). Ecological aspects of the free-living ticks (Acari: Ixodidae) on animal trails within Atlantic rainforest in south-eastern Brazil. Ann. Trop. Med. Parasitol. 103, 57–72. doi: 10.1179/136485909X384956
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Telford, S. R., Armstrong, P. M., Katavolos, P., Foppa, I., Garcia, A. S., Wilson, M. L., et al. (1997). A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg. Infect. Dis. 3, 165–170. doi: 10.3201/eid0302.970209
Thompson, D. F. and Walker, C. K. (2015). A descriptive and historical review of bibliometrics with applications to medical sciences. Pharmacotherapy 35, 551–559. doi: 10.1002/phar.1586
Tokarz, R. and Lipkin, W. I. (2021). Discovery and surveillance of tick-borne pathogens. J. Med. Entomol. 58, 1525–1535. doi: 10.1093/jme/tjaa269
Trout Fryxell, R. T. and DeBruyn, J. M. (2016). The microbiome of Ehrlichia-infected and uninfected Lone Star ticks (Amblyomma americanum). PloS One 11, e0146651. doi: 10.1371/journal.pone.0146651
van Eck, N. J. and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. doi: 10.1007/s11192-009-0146-3
van Eck, N. J. and Waltman, L. (2014). “Visualizing bibliometric networks,” in Measuring Scholarly Impact: Methods and Practice. Eds. Ding, Y., Rousseau, R., and Wolfram, D. (Springer International Publishing, Cham), 285–320.
Wang, J. (2013). Citation time window choice for research impact evaluation. Scientometrics 94, 851–872. doi: 10.1007/s11192-012-0775-9
Wang, Z. D., Wang, B., Wei, F., Han, S. Z., Zhang, L., Yang, Z. T., et al. (2019). A new segmented virus associated with human febrile illness in China. N. Engl. J. Med. 380, 2116–2125. doi: 10.1056/NEJMoa1805068
Wu, Y. F., Zhang, M. Z., Su, X. L., Wang, Y. F., Du, L. F., Cui, X. M., et al. (2025). Emerging tick-borne diseases in mainland China over the past decade: a systematic review and mapping analysis. Lancet Reg. Health West. Pac. 59, 101599. doi: 10.1016/j.lanwpc.2025.101599
Wyk, R. D. J., Baron, S., and Maritz-Olivier, C. (2016). An integrative approach to understanding pyrethroid resistance in Rhipicephalus microplus and R. decoloratus ticks. Ticks Tick Borne Dis. 7, 586–594. doi: 10.1016/j.ttbdis.2016.01.007
Yu, X. J., Liang, M. F., Zhang, S. Y., Liu, Y., Li, J. D., Sun, Y. L., et al. (2011). Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364, 1523–1532. doi: 10.1056/NEJMoa1010095
Zhang, L., Liu, Y., Ni, D., Li, Q., Yu, Y., Yu, X. J., et al. (2008). Nosocomial transmission of human granulocytic anaplasmosis in China. JAMA 300, 2263–2270. doi: 10.1001/jama.2008.626
Keywords: tick, tick-borne diseases, tick-borne pathogens, visual analysis, bibliometric analysis
Citation: Su H, Gong M, Liu L, Sheng Z and Zhang B (2025) Global trends in tick research: a comprehensive visualization and bibliometric study (2015–2024). Front. Cell. Infect. Microbiol. 15:1697791. doi: 10.3389/fcimb.2025.1697791
Received: 02 September 2025; Accepted: 17 October 2025;
Published: 28 October 2025.
Edited by:
He Zhang, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Yidong Fei, Jilin Agricultural Science and Technology College, ChinaShuangxing Li, National Institutes for Food and Drug Control, China
Copyright © 2025 Su, Gong, Liu, Sheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benguang Zhang, emhhbmdiZW5ndWFuZ0BzZGZtdS5lZHUuY24=; Zhaoan Sheng, c3phOTEzNUAxNjMuY29t; Lijuan Liu, bGl1bGlqdWFuQHNkZm11LmVkdS5jbg==
 Hangli Su
Hangli Su Maoqing Gong1
Maoqing Gong1 Zhaoan Sheng
Zhaoan Sheng Benguang Zhang
Benguang Zhang