- 1Organ Transplantation Clinical Medical Center of Xiamen University, Department of General Surgery, Xiang’an Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, China
- 2Organ Transplantation Institute of Xiamen University, Xiamen Human Organ Transplantation Quality Control Center, Xiamen Key Laboratory of Regeneration Medicine, Xiamen, China
- 3Fujian Provincial Key Laboratory of Organ and Tissue Regeneration, School of Medicine, Xiamen University, Xiamen, China
Liver transplantation (LT) is one of the most effective treatments for end-stage liver disease, as evidenced by a 1-year survival rate of approximately 90% and a 5-year survival rate exceeding 70%. Bacterial infections not only are major complications affecting the quality of life and graft function of LT patients but also constitute the primary causes of morbidity and mortality in this population. Additionally, the rejection response following LT increases the need for postoperative immunosuppressive therapy, and because of the complexity of the immune response in both donors and recipients, LT recipients are more susceptible to bacterial infections than other postoperative patients are. Reports indicate that gram-negative bacteria (such as Enterobacter, Klebsiella, and Pseudomonas) and gram-positive bacteria (such as Staphylococcus and Enterococcus) are common pathogens causing infections after LT. In particular, LT patients are prone to infection with multidrug-resistant (MDR) bacteria, which further complicates infection management. New detection technologies (such as digital droplet PCR, high-resolution melting, surface-enhanced Raman spectroscopy, and cell-mediated immunity) are highly sensitive in the early identification of drug-resistant bacteria and assessment of graft damage. Combining perioperative antibiotic and nonantibiotic therapy can help prevent infections and improve patient prognosis. Currently, effective precautionary warning systems are still lacking internationally, and issues such as dysbiosis caused by broad-spectrum antibiotics and overreliance on traditional methods for infection diagnosis and treatment need to be urgently addressed. This article reviews the relevant literature on the epidemiology and causes of post-LT bacterial infections and new diagnostic and treatment methods to provide a reference for the clinical prediction and prevention of such infections.
Introduction
Bacterial infection after liver transplantation (LT) is a significant factor leading to patient mortality, particularly in the early postoperative period (Lacaille, 2012; Vilarinho and Lifton, 2012; Pedersen and Seetharam, 2014; Weiss and Thabut, 2019). The primary pathogens involved in post-LT infections include gram-negative bacteria such as Pseudomonas aeruginosa and Escherichia coli, as well as gram-positive bacteria such as Staphylococcus aureus and Enterococcus spp (del Pozo, 2008; Martin-Gandul et al., 2015; Hand and Patel, 2016; Idossa and Simonetto, 2017). In recent years, the misuse of antibiotics has led to the emergence of multidrug-resistant (MDR) organisms, which are closely associated with increased mortality rates in patients with post-LT infections (Kusne et al., 1988; Patel and Paya, 1997; Singh et al., 2004; Reid et al., 2009; van Delden, 2014; Righi, 2018). The unique characteristics of LT patients, including preoperative comorbidities, the complexity of the surgical procedure, postoperative immunosuppressive therapy, and frequent hospitalizations, increase the likelihood of bacterial infections (Bennett et al., 2015). The aim of this review is to explore the current status of research on the primary sites, risk factors, detection methods, infection mechanism and prevention and treatment strategies for bacterial infections after LT.
Current status of bacterial infections
Pathogen profile
Research indicates that 40% to 89% of LT patients experience varying degrees of infection within the first year after LT (Rayes et al., 2005; van Delden, 2014; Martin-Gandul et al., 2015; Diaz et al., 2024; Zuccaro et al., 2024). Among these, bacterial infections are the most common, accounting for 30% to 60% of all cases (Fishman, 2007; Gelb and Feng, 2009; Kawecki et al., 2009; Baganate et al., 2018; Righi, 2018; Ferrarese et al., 2024; Wu et al., 2024). During the first month after LT, most bacterial infections are related to prolonged surgical duration, massive blood loss during surgery, and the induction of immunosuppression (IS) at high doses (Shen et al., 2007; Shinde and Kapoor, 2024). From the second to the sixth months after LT, bacterial infections are frequently multifactorial in that they include immunosuppressive therapy; persistent infections from the perioperative period, such as Clostridium difficile colitis; residual pneumonia; technical issues (e.g., anastomotic leaks, empyema, cholangitis, and infected hematoma) (Fishman and Rubin, 1998; Fishman, 2007; Fishman, 2017); the cumulative effect of preoperative colonization by opportunistic pathogens; and maintenance IS with periodic modification on the basis of graft function. Bacterial infections occurring more than six months post-transplantation are associated with environmental exposure, late biliary complications, graft function, reactivation of hepatitis viruses, maintenance of low-key IS levels, and community exposure (Cervera et al., 2011).
The pathogens present after LT vary across different geographic regions. In North America (Safdar et al., 2004; Hellinger et al., 2009; Reid et al., 2009; Lee et al., 2011b; Shoji et al., 2015; Viehman et al., 2016; Anesi et al., 2018; Rolak et al., 2024), Enterococcus spp. are the most frequently isolated pathogens (31–42%), followed by E. coli (15–24%). In Europe (Moreno et al., 2007; Garcia Prado et al., 2008; Bert et al., 2010; Karvellas et al., 2011; Beranger et al., 2020; Hrenczuk et al., 2020; Rasmussen et al., 2021; Kremer et al., 2022; Neofytos et al., 2023; Martin-Mateos et al., 2024), gram-positive bacteria, such as Enterococcus spp., S. aureus, and methicillin-resistant coagulase-negative staphylococci (MRCNS), are present in the early period after transplantation, whereas gram-negative bacteria, such as Enterobacter spp., become predominant later.
In South America (Freire et al., 2013; Santoro-Lopes and de Gouvea, 2014; Oliveira et al., 2019; Freire et al., 2021; Lemos et al., 2024), Klebsiella pneumoniae is the most common pathogen that causes surgical site infections (SSIs), which may be related to inappropriate postoperative antibiotic treatment and insufficient nosocomial infection prevention measures.
In contrast, Asian (Avkan-Oguz et al., 2015; Aktas et al., 2019; Pouladfar et al., 2019; Jafarpour et al., 2020; Selimoglu et al., 2020) LT patients exhibit a higher rate of gram-negative bacterial infections caused by Acinetobacter spp. and Klebsiella spp. and a higher rate of multidrug resistance. In China and South Korea (Shi et al., 2009; Kim et al., 2022; Zhao et al., 2022; Liu et al., 2023; Tu et al., 2023; Wu et al., 2023; Choi et al., 2024; Sun et al., 2024), Enterococcus faecium is the dominant gram-positive bacterium, and K. pneumoniae (17/90, 18.9%) is the most common gram-negative bacterium detected in LT patients. In Japan (Iida et al., 2010), the most common pathogen is S. aureus, “methicillin-resistant S. aureus” (MRSA), followed by Klebsiella spp. and MRCNS. In the Philippines (Abad et al., 2017), Enterococcus spp. are the pathogens most frequently identified. Among all the detected bacteria, Staphylococcus spp. (34.3%) and methicillin-resistant MRCNS (43.2%) are the dominant species and multidrug-resistant organisms, respectively.
In Oceania (Tun et al., 2024), the most common microorganisms isolated from SSIs are MRCNS, followed by Enterococcus spp. and Corynebacterium.
In Africa, gram-negative bacteria are predominant, with P. aeruginosa being the most common species (Mukhtar et al., 2014; Montasser et al., 2017), followed by Klebsiella spp. Among the gram-positive bacteria, MRSA is predominant (Figure 1).
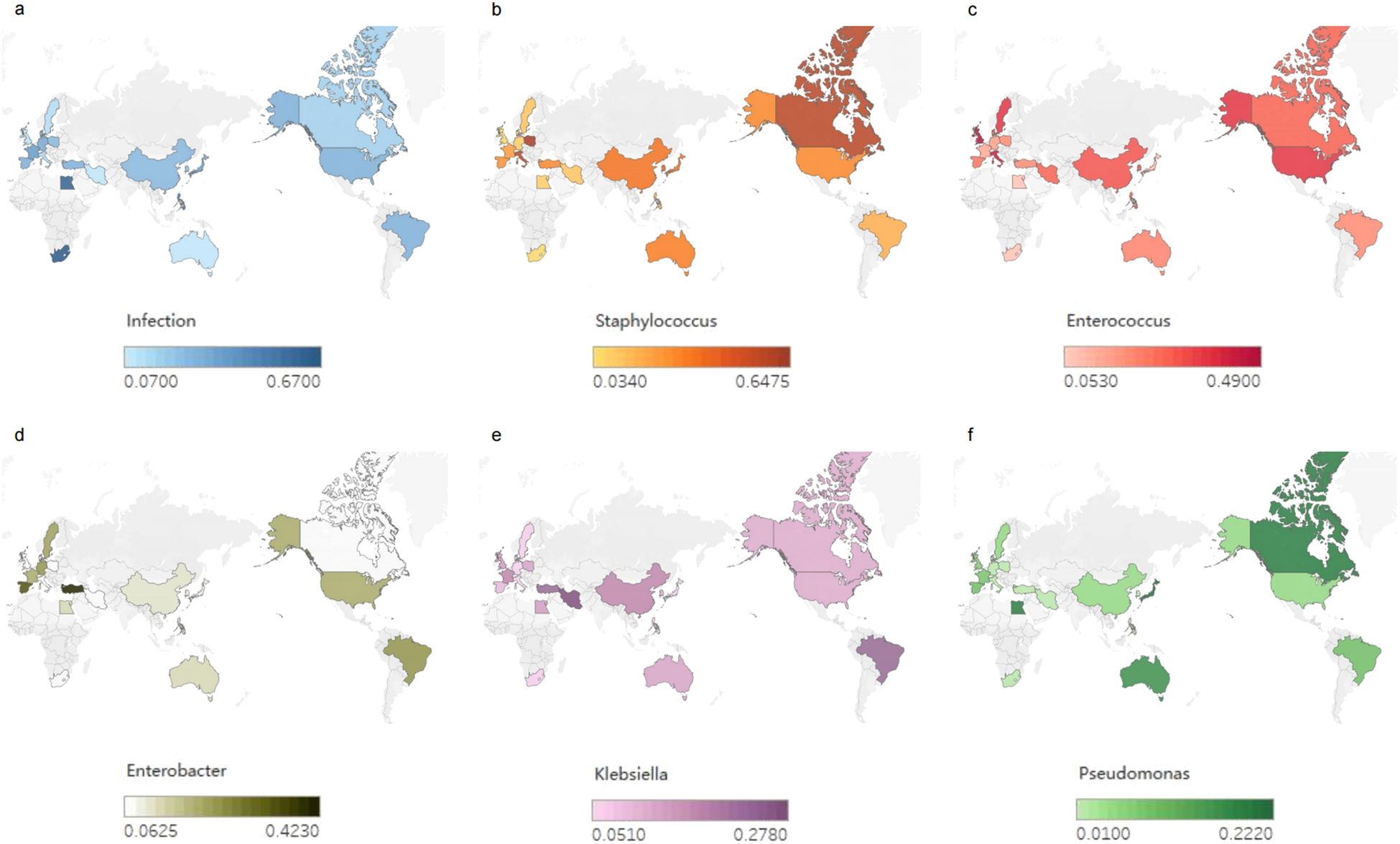
Figure 1. Global bacterial infections after liver transplantation (LT). (a) Global incidence of bacterial infections after LT. (b-f) Global distribution of infection rates of Staphylococcus, Enterococci, Enterobacteriaceae, Klebsiella and Pseudomonas after LT.
Overall, infections caused by gram-negative bacteria, including E. coli, are relatively common, with the incidence of these infections increasing (Hand and Patel, 2016; Resino et al., 2016; Anesi et al., 2018; Jafarpour et al., 2020).
Notably, gram-negative bacteria were the predominant pathogens present during the first few decades after the advent of LT (Kusne et al., 1988; del Pozo, 2008; Martin-Gandul et al., 2015; Hand and Patel, 2016; Kritikos and Manuel, 2016; Idossa and Simonetto, 2017). Gram-positive bacteria, particularly S. aureus (Singh et al., 2000; Torre-Cisneros et al., 2002) and “vancomycin-resistant Enterococci” (VRE) (Newell et al., 1998), emerged as increasingly common pathogens in the mid-1990s because of technical surgical advances and catheter-related bloodstream infections (BSIs) (Singh et al., 1997). Despite the ongoing evolution of transplantation practices, gram-negative bacilli have re-emerged as the predominant bacteria causing infections after LT, with a notable increase in infections caused by MDR gram-negative bacilli (Singh et al., 2004; Garcia Prado et al., 2008; Oriol et al., 2017).
MDR
In recent years, MDR has increased the difficulty of managing post-LT infections (Santoro-Lopes and de Gouvea, 2014; Hand and Patel, 2016; Righi, 2018). The proportion of MDR bacteria increased from less than 10% in 1998 to 23% in 2010 (Fernandez et al., 2012), and in the past five years, the infection rate has further increased to 14.3% to 47% (Freire et al., 2021; Brigati et al., 2023). Santoro-Lopes (Santoro-Lopes and de Gouvea, 2014) reviewed MDR pathogens that are prevalent in global LT centers and highlighted the close association between MDR bacterial infections and higher mortality rates. Studies indicate that gram-negative bacilli are more common and exhibit greater resistance (Zhong et al., 2012; Santoro-Lopes and de Gouvea, 2014), with postoperative E. coli and K. pneumoniae strains causing postoperative infections producing extended-spectrum beta-lactamases at rates of 59.4% and 62.1%, respectively (Zhou et al., 2006; Romero and Razonable, 2011). P. aeruginosa is resistant to broad-spectrum antibiotics such as cefepime, piperacillin/tazobactam, and imipenem at rates of 38%, 27%, and 14% (Mulugeta et al., 2017), respectively. Common MDR bacteria include VRE, MRSA, and “carbapenem-resistant Enterobacteriaceae” (CRE) (Kritikos and Manuel, 2016; Resino et al., 2016). The respective colonization rates, infection rates, and mortality rates are shown in Table 1 (Santoro-Lopes and de Gouvea, 2014; Idossa and Simonetto, 2017), illustrating the growing challenges associated with managing immunosuppressed patients. These strains can cause complications such as poor wound healing and pulmonary, abdominal, urinary tract, and bloodstream infections in 20%-40% of patients postoperatively and can even cause sepsis (Kim, 2014; Santoro-Lopes and de Gouvea, 2014).
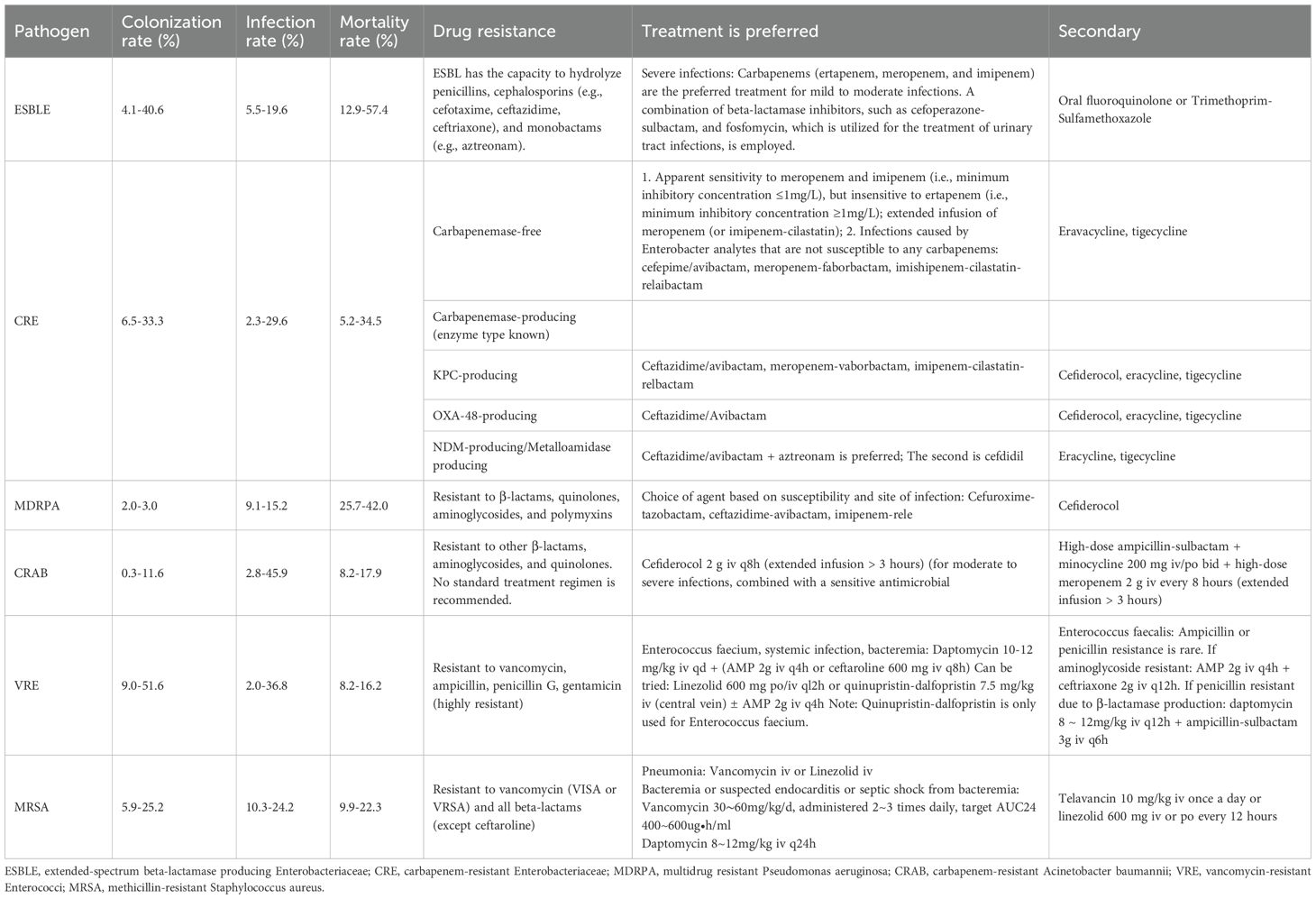
Table 1. Multidrug-resistant bacteria after liver transplantation (LT): colonization rate, infection rate, mortality rate, drug resistance and treatment.
CRE
Since the initial identification of CRE approximately 15 years ago, the prevalence of CRE has increased worldwide. Owing to the clinical impact of numerous hospital outbreaks, especially in highly complex environments and intensive care unit (ICU) settings, CRE colonization and infections have represented some of the most investigated types of infections in transplant patients over the last decade. The overall crude mortality rate range for CRE infections in liver transplant recipients spans from a lower limit of 19.2% for 30-day mortality to a higher limit of 71% to 78% for overall mortality. In specific clinical settings such as intensive care units (ICUs), the mortality rate can rise as high as 82% (Kalpoe et al., 2012; Mularoni et al., 2019; Chen Y. et al., 2020; Liu et al., 2022). The emergence of CRE is related to multiple mechanisms, including carbapenemase production, efflux pump hyperexpression, and porin inactivation (Eichenberger and Thaden, 2019; Garcia-Bustos et al., 2022). Carbapenemases are a heterogeneous group of β-lactamases that hydrolyze carbapenems (Lynch et al., 2021; Dolci et al., 2023).
Epidemiological studies of CRE colonization and infection in LT recipients are summarized in Table 1. A steady increase in infections due to CRE has been documented over the past decade (Chen et al., 2021; Miller and Arias, 2024; Chang et al., 2025).
In the United States, infections due to CR-K. pneumoniae constitute up to 2.5% of the total infections (Wilson et al., 2017). As of 2020, most Northern and Western European countries have CR-K. pneumoniae infection rates of <1%. However, significantly higher rates have been reported in Southern and Eastern Europe, with rates of CR-K. pneumoniae infection exceeding 50% in Belarus, Georgia, Greece, Moldova, Russia and Ukraine. From 2013 - 2016, the incidence of CR-K. pneumoniae in Latin America ranged from 0.8 to 12.7%, and that in the Asia–Pacific region ranged from 0 to 9.3% (Castanheira et al., 2019).
Risk factors for acquiring CRE infection include prior CRE colonization, exposure to broad-spectrum antimicrobials, admission to the ICU, mechanical ventilation, prolonged hospital stay and indwelling urinary catheters (Palacios-Baena et al., 2021).
Fortunately, the treatment landscape for CRE is changing owing to the availability of newer β-lactam–β-lactamase inhibitor (BL–BLI) combinations (ceftazidime–avibactam, meropenem–vaborbactam, imipenem–cilastatin–relebactam) that target class A serine β-lactamases (including ESBLs and carbapenemases), AmpC enzymes and, in the case of avibactam, some class D OXA-48-like enzymes; the likely introduction of broad-spectrum BL–BLI combinations that inhibit or have activity against strains producing common class B metallo-β-lactamases (cefepime–taniborbactam, avibactam–aztreonam and cefepime–zidebactam, among others); and the introduction of the siderophore cephalosporin cefiderocol.
Retrospective clinical data suggest that the newer BL–BLI combinations offer a significant therapeutic advantage over polymyxin-based regimens, with lower 30-day in-hospital mortality (9% versus 32%, when comparing ceftazidime–avibactam and colistin for CRE infection) (van Duin et al., 2018; Lenhard et al., 2019). Similarly, the 30 - day mortality rates for BSIs due to CRE were reported to be 38–45%, largely before the introduction of these agents, but more recent clinical data show lower rates of mortality (34%) (Gutierrez-Gutierrez et al., 2017; Falcone et al., 2020; Perovic et al., 2020; Wang M. et al., 2022). This finding suggests that clinical outcomes for CRE infections may improve with the use of novel therapeutics.
Bacterial infections at different sites
Bacterial infections commonly affect the surgical site (peritoneal cavity), lungs, bloodstream, biliary tract, urinary tract, and central nervous system after LT (Paya and Hermans, 1989; Blair and Kusne, 2005; Kawecki et al., 2009; Romero and Razonable, 2011). The increasing prevalence of MDR infections has further complicated the treatment of SSIs (Hand and Patel, 2016) (Figure 2).
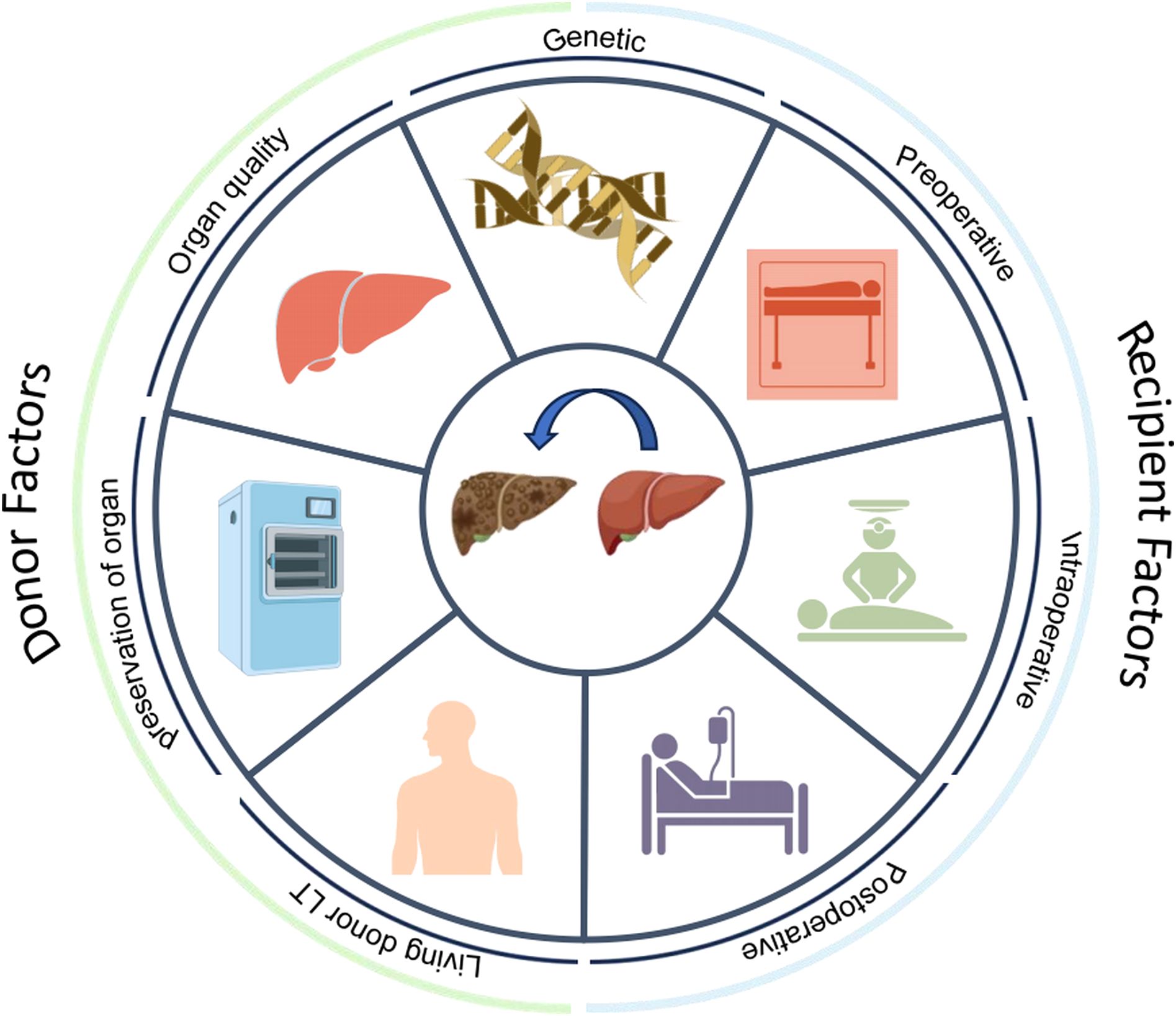
Figure 2. Primary sites and distribution of major pathogenic bacteria infections after liver transplantation (LT).
SSIs
The overall incidence of SSIs following LT ranges from 18% to 37%. Specifically, the incidence of incisional infections is between 9% and 21.5%, that of cholangitis ranges from 6% to 18%, that of peritonitis ranges from 6.3% to 9%, and that of intra-abdominal abscesses ranges from 4% to 12.9%. Severe abdominal infections are commonly caused by E. coli (accounting for approximately 40% of total infections) or a mixture of E. coli and other bacteria (Winterbottom and Jenkins, 2017; Oliveira et al., 2019; Hrenczuk et al., 2020; Schreiber et al., 2025). Enterococcus spp. (accounting for approximately 25% of total infections) and S. aureus (accounting for approximately 15% of total infections) (Meije et al., 2014) are also predominant pathogens.
Compared with patients who undergo other abdominal surgeries, LT patients are more susceptible to severe abdominal infections (Cai et al., 2023; Rodriguez-Fernandez et al., 2024). The mortality rate of sepsis due to SSIs can reach 20%-30% (Santoro-Lopes and de Gouvea, 2014). The primary causes include postoperative immunosuppression (Hashimoto et al., 2008; Kim, 2014; Pouladfar et al., 2017), prolonged peritoneal exposure during LT, gut barrier dysfunction leading to bacterial translocation, large surgical wounds causing abdominal blood seepage and accumulation, rupture of the hollow viscera due to resection of diseased liver tissue, and biliary anastomotic complications inducing abdominal fluid accumulation and bile leakage. Furthermore, the vicious cycle of low blood volume and fluid accumulation during LT can lead to acute abdominal compartment syndrome, causing a rapid increase in intra-abdominal pressure, exacerbating symptoms of abdominal infection, and further worsening the patient’s condition (Soong et al., 2012; Unal et al., 2013).
Respiratory tract infections
Pulmonary infections rank second only to abdominal infections, with incidence ranging from 10% to 20% (Golfieri et al., 2000; Santoro-Lopes and de Gouvea, 2014; Angarita et al., 2017). Early postoperative bacterial pneumonia in LT patients is predominantly hospital-acquired, accounting for 50% to 75% of all pulmonary infections (Kim, 2014). The main pathogens causing bacterial pneumonia include P. aeruginosa (approximately 30%) (Angarita et al., 2017; Yamazhan et al., 2020), Streptococcus pneumoniae (approximately 20%), and S. aureus (approximately 15%), and other bacteria, as well as E. coli, K. pneumoniae, and A. baumannii (Angarita et al., 2017). Community-acquired pneumonia is most common more than 6 months after transplantation (Idossa and Simonetto, 2017), with the common pathogens including S. pneumoniae, Haemophilus influenzae, and Legionella spp (Angarita et al., 2017; Idossa and Simonetto, 2017; Dendle et al., 2018).
The main independent risk factors for postoperative pneumonia include prolonged mechanical ventilation, an extended ICU stay, postoperative pleural effusion (Shen et al., 2007), and the use of corticosteroids for acute rejection treatment (Mermel and Maki, 1990; Wu et al., 2019). The primary complication of pulmonary infection following LT is acute respiratory distress syndrome, which is a major factor contributing to the increased risk of postoperative mortality (Kim, 2014).
BSIs
BSIs are typically associated with multiple factors, including prolonged central venous catheterization (Kim, 2014), postoperative biliary obstruction, vascular complications (Patel and Huprikar, 2012; Kim et al., 2013; Righi, 2018), and reoperation after transplantation (Chueiri Neto et al., 2019).
The incidence of BSI following LT ranges from 5% to 37% (Righi, 2018; Heldman et al., 2019). Compared with patients who undergo kidney, heart, or lung transplantation, LT recipients have a greater incidence of BSIs (Kim, 2014; Righi, 2018). Recent studies have revealed that gram-negative bacteria are more commonly responsible for bacteremia (Bert et al., 2010; Kritikos and Manuel, 2016; Idossa and Simonetto, 2017; Righi, 2018), with Enterobacteriaceae being the predominant pathogens.
BSIs not only prolong the length of hospital stay but also serve as predictors of the mortality and long-term survival in some LT patients (Wade et al., 1995; van Hoek et al., 2012; Meije et al., 2014; van Delden, 2014). The mortality rate can reach 40% when bacteremia is complicated by sepsis (Kawecki et al., 2014), and it can reach 50% in patients with septic shock (Kim, 2014; Pedersen and Seetharam, 2014).
Biliary tract infections
BTIs have become one of the most common complications after LT, with an incidence rate of up to 10%-30% (Nemes et al., 2015; Wu et al., 2022). In the early post-LT period, gram-negative bacteria are the primary pathogens causing BTIs, with E. coli and P. aeruginosa being dominant. However, in the late post-transplantation period, gram-positive bacteria, particularly E. faecalis and E. faecium, become more prevalent (van Delden, 2014).
The vulnerability of the biliary system makes it prone to complications such as strictures or bile leaks, which are key factors in the development of BTIs. Post-LT biliary strictures can be anastomotic or nonanastomotic, with the latter being more common and often associated with ischemic injury (Seehofer et al., 2013). Additionally, Roux-en-Y biliary–intestinal anastomosis, a type of biliary reconstruction procedure, is associated with a greater risk of biliary tract infection than end-to-end anastomosis (Yamamoto et al., 2015). T-tube drainage or surgical catheterization during the transplant procedure may also increase the risk of infection, especially when biliary drainage is poor or catheterization is prolonged (Wojcicki et al., 2008).
The mortality rate due to BTIs after LT ranges from 15% to 40% (Cockbain et al., 2010; Kim, 2014; Resino et al., 2016; Wu et al., 2022). Patients with primary biliary cholangitis and primary sclerosing cholangitis have a greater risk of BTI after LT, as evidenced by a significantly increased postoperative mortality rate of 20-40% (Mayo Moldes et al., 2005; Fagiuoli et al., 2014).
Urinary tract infections
The incidence of UTIs following LT typically ranges from 12.9% to 36.8% (Pouladfar et al., 2017; Jafarpour et al., 2020; Yamazhan et al., 2020). The pathogens responsible for post-LT UTIs are predominantly gram-negative bacteria (63.3%), with E. coli being the most common pathogen, accounting for 30.4% of all infections (Kim, 2014; Meije et al., 2014; Pouladfar et al., 2017). K. pneumoniae (19.1%) and A. baumannii (11.4%) are also significant pathogens (Kawecki et al., 2011). Among gram-positive bacteria, Enterococcus spp., particularly vancomycin-resistant Enterococcus spp., account for 17.6% of all gram-positive bacterial infections (Kawecki et al., 2011; Kim, 2014).
Catheter use during surgery and prolonged postoperative catheterization are significant risk factors for UTIs. Catheters can serve as conduits for bacterial entry infections. Additionally, owing to their shorter urethra, female patients are at greater risk of bacterial entry into the bladder, thereby increasing the risk of infection (Vidal et al., 2012; Tawab et al., 2017). Moreover, patients with a high body mass index may experience increased intra-abdominal pressure, which can contribute to the development of postoperative infections (Pouladfar et al., 2017).
UTIs in LT patients can significantly increase the risk of sepsis and renal failure, thereby increasing hospitalization and mortality rates (Rice and Safdar, 2009; Santoro-Lopes and de Gouvea, 2014; Ferrarese et al., 2018; Jafarpour et al., 2020).
Central nervous system infections
Although CNS infections are less common (5% to 10%), they can be severe in LT patients because of the immunosuppressed state of these patients (Campagna et al., 2010; Vizzini et al., 2011; Weiss and Thabut, 2019). The initial signs of a CNS infection may be masked in these patients (Amodio et al., 2007). The mortality rate for CNS infections is extremely high (40–100%), significantly impacting patient prognosis (Feltracco et al., 2010). In post-LT CNS infections, while fungi and viruses are common pathogens, bacterial infections remain a major source of concern (Amodio et al., 2007; Feltracco et al., 2010; Weiss and Thabut, 2019). Particularly in the early postoperative period, bacterial infections are often related to the surgical technique used. Feltracco et al. noted that bacterial infections are closely associated with postoperative intra-abdominal infections, BSIs, and catheter-related infections. Common bacteria include gram-positive organisms such as S. aureus (Feltracco et al., 2010) and Enterococcus spp., as well as gram-negative bacteria such as E. coli (Saner et al., 2007) and P. aeruginosa (Feltracco et al., 2010). These bacteria can invade through catheters, surgical sites, or the respiratory tract and are then disseminated to the CNS. Listeria monocytogenes is often transmitted through contaminated food sources such as milk, cheese, undercooked meat, and vegetables (Sartor et al., 2015). In the later stages of transplantation, meningitis can also be secondary to Mycobacterium tuberculosis infection (Weiss and Thabut, 2019).
Risk factors
Clinical aspects
Donor factors
The incidence of infection in donors can reach 19.2% (Nanni Costa et al., 2008). The spectrum of donor-derived infections after LT was dominated by gram-negative bacteria, among which K. pneumoniae, E. coli, and P. aeruginosa were the most common pathogens. It is important to acknowledge the substantial impact of the prevalence of carbapenem-resistant strains on clinical prognosis, particularly in the context of carbapenem-resistant K. pneumoniae, carbapenem-resistant P. aeruginosa, and “carbapenem-resistant Acinetobacter baumannii” (CRAB). Furthermore, MRSA has emerged as a significant pathogen in cases of donor-derived infection. The infection of the aforementioned MDR bacteria not only increases the difficulty of anti-infective treatment but also affects the survival rate of patients (Altman et al., 2014; Giani et al., 2014; Miller et al., 2015; Lewis and Sifri, 2016; Ye et al., 2017; Tong et al., 2020; Song ATW. et al., 2024) (Figure 3).
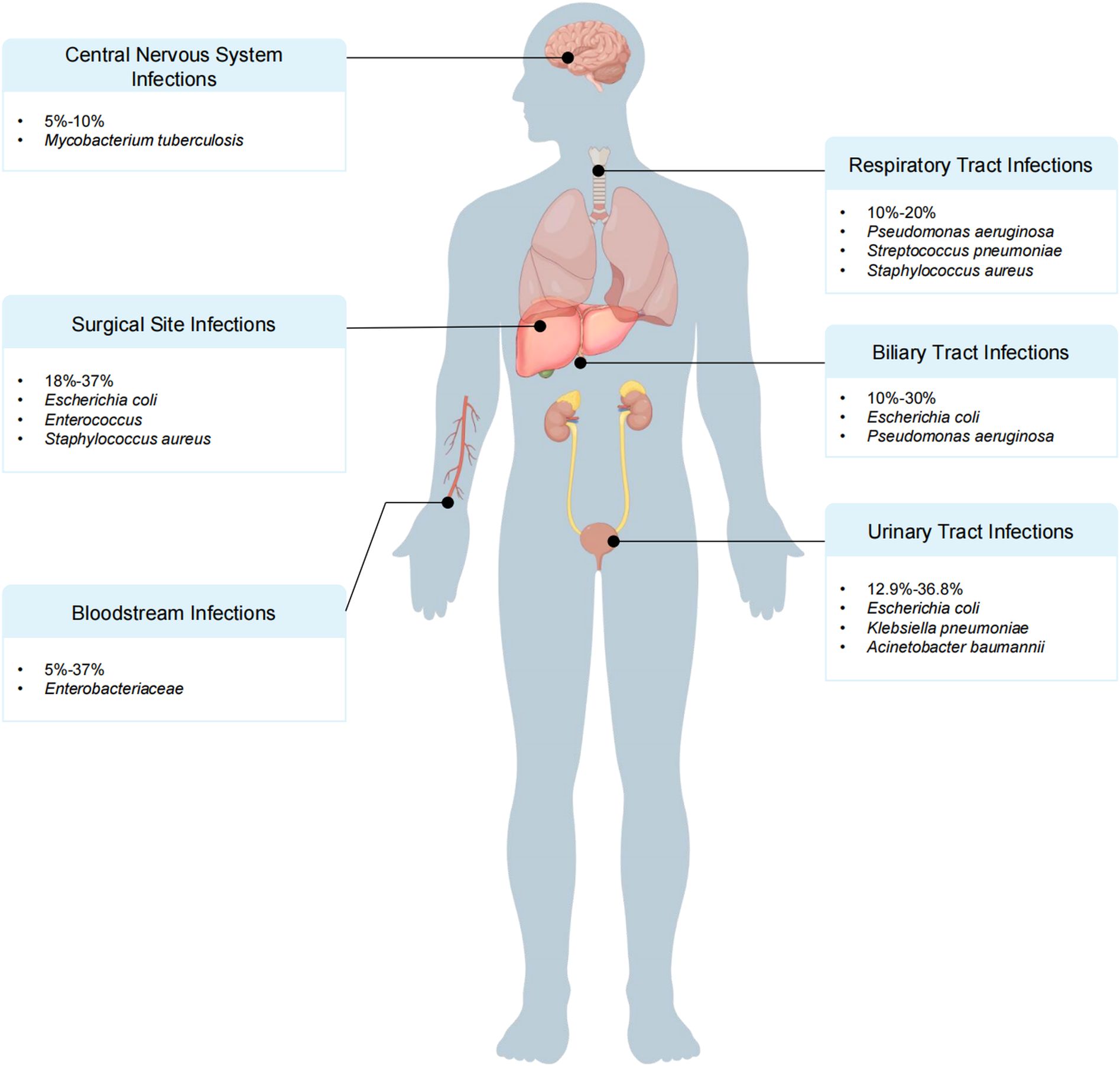
Figure 3. Donor and recipient risk factors for bacterial infection after liver transplantation (LT).
The donor factors primarily involve the quality, maintenance and preservation of organs. Livers from donors with moderate-to-severe fatty liver disease (Xie et al., 2024) and from those of advanced age may increase the risk of postoperative infection in recipients (van Hoek et al., 2012; Reddy et al., 2017). Additionally, a history of diabetes and liver dysfunction in the donor can also affect the rate of bacterial infections after LT. Urrunaga et al. conducted an analysis of the UNOS database and reported that all cohorts of older donors over 50 years of age presented increased risks of morbidity and mortality from sepsis (Lee et al., 2023). An analysis involving 129 patients revealed that donor age was associated with the infection incidence, with an average donor age of 36 years in the infection group and 30.5 years in the infection-free group (Gayowski et al., 1998).
With respect to the quality of organ maintenance and preservation, donors who have spent three or more days (Corman Dincer et al., 2019) in the ICU, who require vasoactive drugs to maintain blood pressure, who have undergone cardiopulmonary resuscitation, or who have experienced prolonged cold/hot ischemia are at a significantly greater risk of infection (Grossi et al., 2009; Ison and Nalesnik, 2011; van Hoek et al., 2012; Reddy et al., 2017; Wu et al., 2022).
Among these factors, living-donor LT results in greater susceptibility (11% vs. 4%) (Freise et al., 2008; Samstein et al., 2016) to biliary infections than does deceased-donor LT, which is attributable to the unique procedure. First, small donor grafts may lead to postoperative liver dysfunction with prolonged cholestasis and coagulopathy. Second, biliary leakage from the cut surface of the graft may subsequently lead to biloma formation, which may lead to secondary infection. Third, the surgical procedure for living-donor LT is technically more challenging and contributes to a higher incidence of complications such as biliary strictures (Kiuchi et al., 1999; Tucker and Heaton, 2005; Liu et al., 2006). Enterococcus spp. were the most frequently isolated pathogens (31%), followed by E. coli (15%) (Kim et al., 2008; Li C. et al., 2012; Pedersen and Seetharam, 2014; Abad et al., 2017).
Recipient factors
Preoperative factors
Preoperative risk factors primarily include the patient’s underlying disease and immunity status. Studies have shown that comorbidities such as cirrhosis, diabetes, chronic kidney disease, and a high MELD score (>20) increase the risk of postoperative infection (Pouladfar et al., 2017; Eklou et al., 2022). A history of surgery, severe obesity or cachexia, preoperative mechanical ventilation, and infection or antibiotic treatment within two months prior to surgery can also increase the risk of postoperative infection (Kim, 2014; Ying et al., 2020; Wu et al., 2022). These conditions can lead to a weaker immune response, thereby increasing the incidence of postoperative bacterial infections (Avkan-Oguz et al., 2013).
Intraoperative factors
Prolonged surgery duration (>8 hours), significant blood loss (>1000 ml), and extensive blood transfusion (erythrocytes > 6 U) (Shen et al., 2007; Li C. et al., 2012; Wu et al., 2019) are significantly associated with postoperative infections. These factors can lead to immunosuppression and tissue hypoxia (Velusamy et al., 2020), as well as gut flora translocation, and prolonged surgical exposure increases the opportunity for bacterial entry. Additionally, intraoperative management directly affects postoperative infections, with procedures such as common bile duct jejunostomy being risk factors for infection (Rayes et al., 2005; Pedersen and Seetharam, 2014; Anesi et al., 2018).
Biliary reconstruction remains a major point of controversy, particularly in living donor LT. The major concerns are early leakage and late stricture at the anastomotic site, which are associated with technical, anatomical, or microcirculatory considerations. Early biliary complications may lead to a fatal outcome in recipients who are critically ill or who receive relatively small grafts, and these conditions themselves may increase the risk of complications (Braun et al., 2021).
Postoperative factors
Patients are in an immunosuppressed state after LT. Factors such as an ICU stay of 9 days or more (Li C. et al., 2012; Laici et al., 2018; Jafarpour et al., 2020), the use of broad-spectrum antibiotics (Zhong et al., 2012), continuous positive pressure ventilation for more than 12 hours (Shen et al., 2007), prolonged catheterization (including specific endotracheal intubation, deep venous cannulation, and an abdominal drainage tube), and postoperative reoperation can expose patients to infection sources for extended periods, ultimately increasing the risk of infection (Wade et al., 1995; Avkan-Oguz et al., 2013; Selimoglu et al., 2020) (Figure 3).
Genetic factors
Genetics influence a recipient’s postoperative immune status and play a crucial role in susceptibility to bacterial infections following LT. Numerous studies have revealed that polymorphisms in the Toll-like receptor 2 (TLR-2) (Lee et al., 2011a), Toll-like receptor 9 (TLR-9) (Tsujimoto et al., 2006; Carrera-Silva et al., 2008; El-Bendary et al., 2020), mannose binding lectin 2 (MBL2), ficolin 2 (FCN2), and mannan-binding lectin serine protease 2 (MASP2) genes in donors and recipients (de Rooij et al., 2010; de Rooij et al., 2011; Igarashi et al., 2014; Debette-Gratien et al., 2016), the SLCO1B1 rs4149015 AA genotype in recipients (Guo et al., 2025), as well as interleukin-10 (IL-10) polymorphisms, may affect the expression levels of these genes and thereby alter the patient susceptibility to bacterial infections (Chuang et al., 2009; Shi et al., 2017; Dai et al., 2020).
The polymorphism of the C7 rs6876739 gene in donors is associated with the risk of early bacterial infections post-LT (Shen et al., 2012; Zhong et al., 2016). This phenomenon is related to the C7 protein and the generation of the membrane attack complex, which plays a role in antimicrobial processes by activating the NLRP3 inflammasome and releasing IL-1β (Shen et al., 2012; Zhong et al., 2016; Wu et al., 2019). Additionally, IL-18 stimulates the production of interferon-γ by natural killer cells and T cells to impact the occurrence of postoperative infection. One of its primary functions is to stimulate the production of interferon-γ by natural killer cells and T cells, which is crucial for the regulation of innate and adaptive immune responses (Kinoshita et al., 2004; Ghose et al., 2011; Ono et al., 2012). In experiments with a mouse burn model, IL-18 knockout mice presented a 35% reduction in infection rates (Kinoshita et al., 2006). Research has confirmed that the variation in the IL-18 rs1946518 genotype is significantly associated with an increased infection risk (Shi et al., 2017).
Pannexin-1 (Panx1) has been reported to be a key immunoregulatory target in post-transplantation infections. Panx1 is a transmembrane protein that allows signaling molecules such as extracellular adenosine triphosphate to be released from inside the cell to the extracellular space, thereby activating various downstream signaling pathways, including those associated with P2 receptors, and regulating inflammatory responses, apoptosis, and immune responses (Li et al., 2021). Through 64 eQTL data analyses and 200 independent sample validations, the donor liver Panx1 rs79475114 CC genotype was identified as an independent risk factor for MRSA infection post-LT. Additionally, a mouse LT model of MRSA infection post-LT confirmed that donor liver Panx1-mediated adenosine triphosphate release acts on the hepatocyte membrane P2X2 receptor, recruiting neutrophils and M1 macrophages to exert an anti-MRSA infection effect (Li et al., 2021). Furthermore, in patients with sepsis caused by gram-negative bacteria, Panx1 expression in the donor liver was significantly reduced, leading to decreased IL-33 release through the inhibition of the P2X7/NLRP3 signaling pathway, which reduced the proliferation and differentiation capacity of ST2+ Treg cells, increasing the incidence and mortality of sepsis after LT (Wang P. et al., 2022). Notably, Panx1 not only plays a role in the early stage of acute bacterial infections but also participates in the long-term immune surveillance mechanism of the liver. In certain chronic infection scenarios, such as peritoneal infections resulting from bacterial translocation in the gut, the Panx1-regulated adenosine triphosphate–P2X pathway is crucial for maintaining local immune homeostasis (del Pozo, 2008). This finding indicates that the absence of Panx1 significantly increases the risk of post-LT infections, particularly drug-resistant bacterial infections.
Diagnostic approaches
Diagnosing infections in transplant recipients is more challenging than in individuals with normal immune function, as the symptoms and signs of infection are often ignored. Additionally, transplant recipients may exhibit noninfectious causes of fever, such as allograft rejection, necessitating further exclusion.
Traditional diagnostic methods
Common diagnostic methods include imaging examination and microbiological testing.
High-resolution computed tomography (HRCT), magnetic resonance imaging (MRI) and other imaging studies can effectively aid in diagnosing peritoneal abscesses, BTIs, pulmonary infections, urinary tract obstructions, and CNS infections as potential primary infection sources (Feltracco et al., 2010; Vizzini et al., 2011; Qin et al., 2012; Kim et al., 2013). Additionally, magnetic resonance cholangiopancreatography is widely used to detect biliary strictures and leaks and allows noninvasive evaluation of the anatomy and function of the biliary system (Wojcicki et al., 2008).
Blood or body fluid cultures remain the gold standard for diagnosing infections. Blood cultures can be used to determine the causative pathogens of BSIs (Bert et al., 2010; Kawecki et al., 2011; Abad et al., 2017). For patients diagnosed with or highly suspected of having a BTI, bile cultures are used to identify the causative pathogens. Urine cultures are the primary method for diagnosing UTIs, allowing the detection of pathogenic species (Kawecki et al., 2011; Pouladfar et al., 2017). Cerebrospinal fluid analysis, including bacterial, fungal, and viral cultures of cerebrospinal fluid, is key in diagnosing CNS infections, and susceptibility testing is key for selecting appropriate antibiotics (Guo et al., 2022).
Novel diagnostic techniques
In recent years, with the advancement of molecular biological technologies, an increasing number of novel diagnostic methods have been applied to detect bacterial infections after LT. These techniques include digital droplet PCR (ddPCR), real-time PCR combined with high-resolution melting (HRM) curve analysis, nanoprobes with surface-enhanced Raman spectroscopy (SERS), and cell-mediated immunity.
ddPCR technology
ddPCR involves dividing the sample into tens of thousands of microdroplets, with individual PCR amplification being performed for each droplet. The principle involves precise detection of low-abundance DNA sequences, making it particularly suitable for the early detection of low-level bacterial infections. Compared with traditional PCR, ddPCR offers more precise quantitative assessment (Caviglia et al., 2018; Liu et al., 2024). Mirna et al. used ddPCR to detect and quantify A. baumannii in mini-BAL fluid and, by comparing colonization data, quantitative culture results, and different generations of PCR, showed that ddPCR is more sensitive than other molecular techniques (Giselle Moreira et al., 2024).
Although ddPCR provides absolute quantification, theoretically reducing interlaboratory variability, variability still exists. Factors ranging from differences in nucleic acid extraction methods to variations in primer-probe design, as well as the stability of droplet generation processes, can all influence the final results (Ramanan and Razonable, 2013). A major barrier to the adoption of ddPCR technology is its economic cost. Both the initial equipment investment for ddPCR systems (such as Bio-Rad QX200 and QIAGEN QIAcuity) and the reagent and consumable expenses per test typically exceed those of established qPCR workflows (Ai et al., 2024). Although some literature suggests ddPCR may offer potential cost-effectiveness advantages (Kojabad et al., 2021), the prevailing view in clinical adoption is that its high cost remains the primary barrier to widespread implementation (Ai et al., 2024).
High-resolution melting curve analysis
Real-time PCR combined with high-resolution melting curve analysis utilizes the melting characteristics of 16S rRNA gene amplification products to distinguish between various bacterial species precisely. This technique has shown significant advantages in the rapid diagnosis of peritoneal infections and BTIs, particularly in the case of acute infection requiring rapid intervention (Cheng et al., 2006; Hu et al., 2014). This method has partially replaced traditional culture methods for improved detection efficiency, but it requires high-precision instruments. Future optimization through algorithm improvements could increase the data processing speed, facilitating larger-scale clinical applications.
Although the reagent costs for HRM are relatively low, this technology requires a real-time quantitative PCR instrument equipped with high-resolution melting functionality and specialized analysis software (Verweij and Stensvold, 2014). More importantly, accurately interpreting subtle differences in melting curves requires extensive experience and well-established interpretation criteria—qualities not readily available in all clinical laboratories (Xu et al., 2021).
Nanoprobes and SERS detection
Nanoprobe technology achieves ultrasensitive detection of bacteria through SERS signals (Kearns et al., 2017; Cialla-May et al., 2024). Gao et al. introduced a SERS nanoprobe, AMD@HA, which utilizes localized surface plasmon resonance for photothermal therapy and enhanced Raman signals, providing a sensitive and noninvasive diagnostic tool for the rapid identification and eradication of MDR bacteria (Gao et al., 2024). Currently, SERS is still in the promotional stage in clinical settings, but its specificity and stability are evident. In the future, by improving the specificity of the nanoprobe structure, SERS could become an important high-throughput detection tool in clinical practice.
The entire workflow of SERS, from sample pretreatment and synthesis of nanoscale substrates to spectral acquisition and data post-processing, severely lacks standardized operating procedures (Tadesse et al., 2020). SERS spectra are typically highly complex and contain a wealth of information, often requiring sophisticated machine learning or chemometric algorithms for pattern recognition and interpretation. This makes it difficult to directly compare results across different laboratories (Tadesse et al., 2020). The lack of universally accepted standardized protocols and certified reference materials poses a significant barrier to obtaining regulatory approval and advancing into clinical applications (Cialla-May et al., 2024). Although the cost of precious metal raw materials (gold, silver) required for a single test may not be high, the cost of manufacturing highly uniform, reproducible nanostructures using advanced technologies such as photolithography is extremely high (Pelaz et al., 2017). How to economically scale up this production from the laboratory level to commercial, clinical-grade scale remains an unresolved challenge to this day (Pelaz et al., 2017).
Microbial cfDNA sequencing technology
cfDNA of microbial origin is released into the host’s body fluids during infection with pathogenic microorganisms (Muller et al., 2024). Free microbial DNA can be extracted from blood or body fluids and sequenced via next-generation sequencing (NGS), which can be used for the early detection of all known and unknown bacterial infections. Li et al. proposed a new multiplex PCR assay, MeltArray, which uses intact microbial cells as the source of genomic DNA (gDNA). The MeltArray assay showed a sensitivity of 93.8%, specificity of 98.6%, positive predictive value of 99.7%, and negative predictive value of 75.0%. In comparison, a cross-sectional study on the diagnostic value and clinical application of mNGS in post-liver-transplant infections reported sensitivity of 22.22%, specificity of 89.28%, positive predictive value of 66.67%, and negative predictive value of 54.35% (Zhao et al., 2022). They concluded that the MeltArray assay can be used as a rapid and reliable tool for the direct identification of pathogens in BSIs (Song J. et al., 2024). Furthermore, microbial cfDNA sequencing technology is characterized by its noninvasiveness, high sensitivity, and specificity, making it particularly suitable for detecting pathogens that are difficult to culture using traditional methods (Hennis et al., 2024).
The per-test cost of mNGS is extremely high, typically reaching thousands of dollars, which constitutes a major barrier to its routine application and a significant factor requiring careful consideration in hospital budgets (Azar et al., 2022). Although studies indicate that mNGS may reduce overall healthcare costs by shortening fever duration and optimizing antibiotic use (Jablonska et al., 2025), securing stable reimbursement from health insurance remains a challenge, significantly limiting its clinical accessibility. Currently, the analytical workflow and microbial reference databases used in mNGS lack standardized protocols, potentially leading to discrepancies in analysis results for the same sample across different laboratories (Tan et al., 2024).
Cell-mediated immunity
A novel global cell-mediated immunity assay (QuantiFERON Monitor [QFM], Qiagen) that measures plasma interferon-gamma (IFN-γ) levels after stimulation of whole blood with a combination of antigens designed to stimulate both the innate and adaptive arms of the immune system has been developed. A previous study using this assay revealed that plasma IFN-γ levels in liver transplant candidates and recipients were significantly lower than those in healthy control subjects (Sood et al., 2014). Kumar et al. performed a prospective observational cohort study in which a global immune monitoring assay was used to predict infectious complications during the first year post-transplantation. They reported that the assay was broadly predictive of the likelihood of subsequent infectious complications, with patients with low IFN-γ values being at the highest risk of subsequent infection. The best predictive value was observed at the 6-month time point, although predictive utility was observed at all 3 time points (Mian et al., 2018).
CMI testing is typically technically complex, labor-intensive, and costly. It often requires the use of fresh whole blood samples processed within a short timeframe after collection, placing high demands on laboratory technical capabilities and logistics (Chung et al., 2016). This limits the accessibility of CMI monitoring primarily to large transplant centers equipped with specialized immunology laboratories, making routine monitoring difficult in smaller hospitals or outpatient settings (Chung et al., 2016).
Prevention and treatment
Measures to prevent and treat bacterial infections following LT are categorized primarily into perioperative antibiotic use and nonantibiotic preventive measures, both of which play crucial roles in the management of these infections.
Perioperative antibiotic use
Microbial contamination of the donor organ preservation solution should be managed through the following steps: 1. Sterile sampling and testing—the storage solution should be subjected to NGS and cultured for bacteria immediately at the time of organ acquisition. 2. Pollution treatment—if the preservation solution tests positive, specific antibiotics (such as carbapenems or tigecycline for gram-negative bacteria) should be selected for local lavage of the donor organs according to the drug sensitivity results. The donor liver should be soaked with a storage solution containing antibiotics (e.g., a UW solution containing vancomycin and fluconazole) for 30 minutes to reduce the pathogen load (Theodoropoulos et al., 2021; Lentine et al., 2023; Pouch et al., 2025).
Nearly all LT procedures require the prophylactic administration of broad-spectrum antimicrobial agents (Resino et al., 2016; Lakomkin and Hadjipanayis, 2021; Williams et al., 2021; Steffani et al., 2024). For the prevention and treatment of SSIs, measures should primarily target gram-negative bacilli. High-risk recipients should also be protected against gram-positive cocci and fungi (Kim, 2014). Combination therapy with beta-lactams and beta-lactamase inhibitors, and if necessary, vancomycin, can be used (Chueiri Neto et al., 2019). The choice of antibiotics should be adjusted on the basis of the recipient’s allergy history, pathogen identification results, and renal function (Li H. et al., 2012; Kim, 2014; van Delden, 2014). The typical duration of prophylactic antibiotic administration is 24–72 hours post-surgery (Hashimoto et al., 2007; Hashimoto et al., 2008; Hashimoto et al., 2009). For transplants from high-risk donors (such as those colonized with MDR bacteria), the recipient should receive 7–14 days of targeted anti-infection treatment after surgery. Additionally, oral administration of rifaximin has been shown to be effective at preventing post-LT infections (Neff et al., 2010; Sun et al., 2012; Salehi et al., 2019; Campos-Varela et al., 2022).
However, the misuse of antibiotics can lead to the emergence of MDR organisms, posing challenges to antibiotic prophylaxis strategies (Patel and Huprikar, 2012; Fagiuoli et al., 2014; van Delden, 2014). Once MDR infection has been diagnosed, empiric treatment must be started directly to reduce complications and mortality. The treatment of MDR infections should be guided by the type of pathogen and susceptibility testing, with a minimum treatment duration of 2–3 weeks (Bert et al., 2010; Kim, 2014). For Enterobacteriaceae strains that produce broad-spectrum lactamases, carbapenems are preferred, followed by lactam/lactamase inhibitors or cephalosporins. For CRE, ceftazidime-avibactam and tigecycline as monotherapy or combination therapy, or polymyxin-based combination therapy, can be considered. For metallo-beta-lactamase-producing CRE, ceftazidime-avibactam combined with aztreonam is recommended. For CRPA, combination therapy with ceftazidime–avibactam, polymyxin, or antipseudomonal beta-lactams can be used. Combination therapy with sulbactam and its derivatives, tigecycline, or polymyxin may be considered as a secondary choice. For MRSA and methicillin-resistant MRCNS, vancomycin, linezolid, daptomycin, or teicoplanin can be chosen for treatment (Silva et al., 2018). Table 1 summarizes the antibiotics and the conditions for which each is used (Horcajada et al., 2019; Tamma et al., 2019; Heil et al., 2021; Tamma et al., 2021; Antimicrobial Resistance C, 2022; Castanheira et al., 2023; Meschiari et al., 2024; Miller and Arias, 2024; Tamma et al., 2024).
Nonantibiotic preventive measures
Selective decontamination of the intestine
SID is another widely used preventive measure that reduces the abundance of gram-negative bacteria in the intestine via the use of antibiotics that do not kill anaerobes, thereby reducing the incidence of infections caused by these strains. Resino et al. investigated the effectiveness of SID in preventing postoperative infections and reported that it significantly reduced the occurrence of gram-negative bacterial infections (Rayes et al., 2005). However, it may increase the risk of gram-positive infections, especially enterococcal infections (Resino et al., 2016). By selectively removing gram-negative bacteria from the intestine, SID lowers the risk of these pathogens translocating to the bloodstream or surgical wounds, thus reducing the likelihood of related infections. The advantage of this method is that it maintains the presence of anaerobes, thereby preserving the balance of the gut microbiota (Resino et al., 2016).
Probiotics and prebiotics
Early enteral nutrition and the administration of probiotics to modulate the gut flora have been shown to reduce the incidence of infections and effectively shorten the length of ICU stay and overall hospitalization time (Wang et al., 2015; Annavajhala et al., 2019; Ancona et al., 2021; Cooper et al., 2022). Probiotics primarily exert their clinical effects by maintaining gut barrier function, promoting the colonization by beneficial bacteria, and competitively excluding harmful bacteria. Beneficial bacteria produce metabolites such as short-chain fatty acids, which enhance the intestinal epithelial barrier and inhibit the growth of pathogenic bacteria while also regulating the host’s immune response and reducing the release of inflammatory factors (Zhang et al., 2013; Sawas et al., 2015; Kahn et al., 2020). Commonly used probiotics include Bifidobacterium spp. and Lactobacillus spp., as well as synbiotics, which are combinations of prebiotics and probiotics. These strains have been shown in multiple clinical studies to improve the gut microecology posttransplantation, reducing the colonization and translocation of pathogenic bacteria (Rayes et al., 2005; Zhang et al., 2013; Kahn et al., 2020; Cooper et al., 2022; Yoshiya et al., 2025).
Improvements in surgical techniques and postoperative management
Precise surgical techniques and meticulous postoperative management are crucial (Kim et al., 2019). Shorter operative times and less trauma during intraoperative procedures reduce the incidence of postoperative infections. Optimizing the surgical procedure can effectively reduce the chance of infection.
Several aspects should be considered for patient management when managing patients after LT. First, strict fluid volume control and maintenance of the lower central venous pressure are essential to prevent pulmonary edema and cardiac insufficiency while protecting liver and kidney function and avoiding infections at other sites, such as catheter-related infections, especially in patients with severe abdominal infections. Effective removal of the source of infection, such as the timely removal of central venous catheters, endotracheal tubes, and urinary catheters, is crucial for infection control (Patel and Huprikar, 2012; Zhong et al., 2012; Meije et al., 2014; Pedersen and Seetharam, 2014; Righi, 2018; Kim et al., 2021). Additionally, management should begin at the primary site of infection. For example, surgical intervention is often necessary to remove the source of infection in patients with intra-abdominal infections (Shoji et al., 2015). However, a focus on necrotic tissue and strict adherence to the principles of damage control are important. Removal of all the purulent necrotic tissue in the abdomen is not necessary, as this procedure can increase the absorption of bacteria and toxins into the bloodstream and exacerbate dysfunction of the respiratory, circulatory, and other systems. Open exploration of the lower abdomen should be avoided to prevent further contamination of the peritoneum. If a patient develops sepsis, intensive care and the use of vasopressors to maintain blood pressure are typically needed (Kawecki et al., 2009). Furthermore, for biliary infections caused by structural issues such as biliary stricture or bile leakage, ERCP, percutaneous transhepatic biliary drainage interventional therapy, or even surgical intervention may be necessary to relieve the obstruction, repair the biliary system, or remove infected bile (Nemes et al., 2015).
Finally, the use of immunosuppressants should be adjusted according to the infection status to help the body restore its own immune defense function (Wu et al., 2019).
Immunosuppressive agents
Currently, the tacrolimus-based immunosuppressive regimen is widely used in clinical practice (Tolou Ghamari and Palizban, 2023). International recommendations suggest minimizing antirejection therapy for organ transplantation (Thomson et al., 2020). Studies have shown that maintaining the tacrolimus dose at a dose of 4–10 ng/ml can prevent acute rejection and reduce the occurrence of side effects such as infections (Shi et al., 2023). When an infection occurs, therapeutic drug monitoring is needed, and dynamic adjustments should be made in combination with the severity of the infection. If the infection continues to progress, the dosage can be further reduced, or administration can be temporarily discontinued (≤ 7 days).
Owing to the dual effects of mycophenolate mofetil (MMF) on bone marrow suppression and immunosuppression, multiple clinical studies have confirmed that discontinuing MMF during severe infections can improve infection control and that short-term discontinuation (e.g., 7–14 days) does not significantly increase the risk of rejection. Therefore, we recommend the early withdrawal of MMF when an infection occurs and a gradual increase in the dosage after the pathogen is cleared. Moreover, the lymphocyte counts and the levels of inflammatory markers (such as white blood cells, C-reactive protein and procalcitonin) should be monitored to avoid excessive immunosuppression.
Currently, most centers use antirejection regimens without corticosteroids or with low-dose corticosteroids (Massarollo et al., 1998; Shepshelovich et al., 2019; Trezeguet Renatti et al., 2024). Most studies suggest gradually reducing the steroid dose during severe infections rather than sudden withdrawal. For example, in LT patients with severe infections, the methylprednisolone dose can be gradually reduced to physiological levels (e.g., 5–10 mg/d hydrocortisone) to avoid adrenal insufficiency and the inflammatory cytokine storm caused by the sudden withdrawal of immunosuppression. The pharmaceutical agents employed in the treatment of this condition are listed in Table 2 (Chen F. et al., 2020; Harada et al., 2020; Marx et al., 2020; Ueno et al., 2020; Wang et al., 2020; Yamada et al., 2020; Zegarska et al., 2020; Deppermann et al., 2021; Friman et al., 2021; Gilad et al., 2021; Tustumi et al., 2021; Udomkarnjananun et al., 2021; Vnucak et al., 2021; Campos-Varela et al., 2022; Cinar and Bulbuloglu, 2022; Kniepeiss et al., 2022; Rabbani et al., 2022; Wei et al., 2022; Mathew and Philips, 2023; Park et al., 2023; Amjad et al., 2024; Diaz et al., 2024; Ntobe-Bunkete and Lemaitre, 2024; Tharanon et al., 2024).
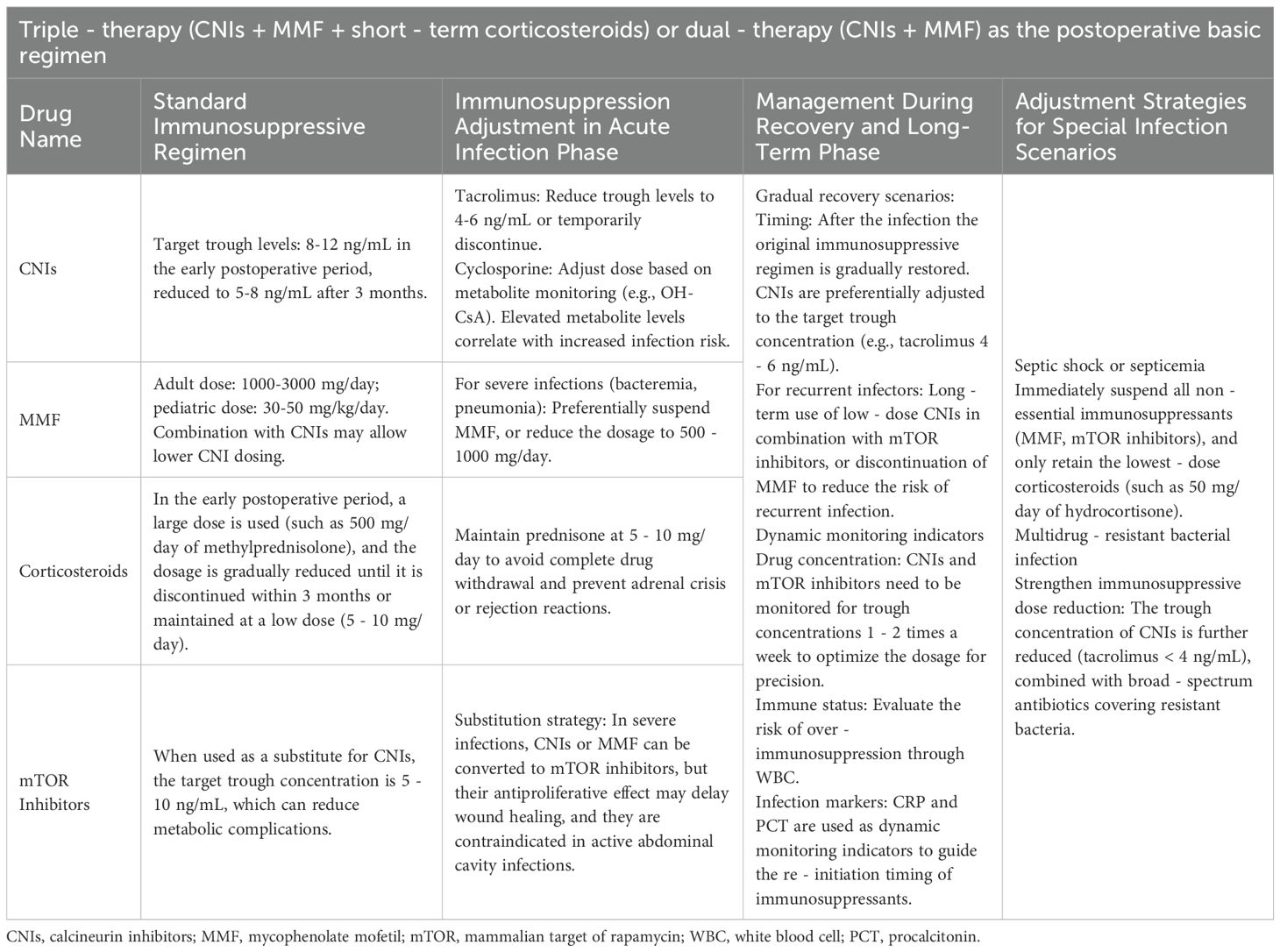
Table 2. Adjusting immunosuppressive medicines for bacterial infections after liver transplantation (LT).
Conclusions
The management of MDR bacterial infections following LT presents significant challenges, especially since the current literature lacks standardized control and prevention protocols (Zhong et al., 2012; Kim, 2014). Future approaches may include the combination of broad-spectrum antibiotics with personalized medication regimens and isolation measures for high-risk patients to reduce the transmission of MDR organisms. Currently, no comprehensive strategies are available for managing postoperative infections at diverse sites, particularly the abdomen, biliary tract, and urinary tract. Large-scale studies are needed to develop a multistage, site-specific comprehensive management strategy to optimize preoperative, intraoperative, and postoperative prevention and treatment processes (Nafady-Hego et al., 2011; Taddei et al., 2023). Additionally, while bacterial infections are major postoperative complications, strategies involving the rational use of antibiotics and selective gut decontamination (Resino et al., 2016), along with variability in environments across centers, hinder the development of standard practices. In future studies, researchers should integrate multidimensional factors to further explore infection mechanisms and refine prevention and management strategies.
Author contributions
HL: Resources, Supervision, Writing – review & editing. JG: Writing – review & editing, Methodology, Investigation, Writing – original draft, Data curation. YW: Writing – review & editing, Methodology. JM: Writing – original draft, Investigation. YX: Methodology, Writing – original draft. BS: Writing – original draft, Supervision, Methodology. WA: Writing – original draft, Methodology. JW: Writing – review & editing, Supervision, Resources.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82370669, 82201964), the Natural Science Fund for Distinguished Young Scholars of Fujian Province (2023J06058), the Project of Middle-Aged and Young Key Personnel Training Program of Fujian Province Health Commission (2023GGB03), the Major Science and Technology Innovation Project of Fujian Province (2023Y9269), the Natural Science Foundation of Fujian Province (2023J01239), the Guiding Project of Fujian Provincial Department of Science and Technology (2023D034), the Natural Science and Technology Major Project of Xiamen (3502Z20231034), and the Natural Science Foundation of Xiamen (3502Z202373107, 3502Z202373106, 3502Z202372092, 3502Z20227283, 3502Z20227122), Scientific Research Foundation of State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory (2024XAKJ0102013).
Acknowledgments
We are grateful to Zhihai, Peng Professor, for his help with the clinical experience of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abad, C. L., Lahr, B. D., and Razonable, R. R. (2017). Epidemiology and risk factors for infection after living donor liver transplantation. Liver. Transpl. 23, 465–477. doi: 10.1002/lt.24739
Ai, L., Zhao, Y., Tan, C., Bai, L., Huang, G., Wang, R., et al. (2024). Development of a droplet digital PCR assay for the detection of BK polyomavirus. Microbiol. Spectr. 12, e0108924. doi: 10.1128/spectrum.01089-24
Aktas, A., Kayaalp, C., Gunes, O., Gokler, C., Uylas, U., Cicek, E., et al. (2019). Surgical site infection and risk factors following right lobe living donor liver transplantation in adults: A single-center prospective cohort study. Transpl. Infect. Dis. 21, e13176. doi: 10.1111/tid.13176
Altman, D. R., Sebra, R., Hand, J., Attie, O., Deikus, G., Carpini, K. W., et al. (2014). Transmission of methicillin-resistant Staphylococcus aureus via deceased donor liver transplantation confirmed by whole genome sequencing. Am. J. Transplant. 14, 2640–2644. doi: 10.1111/ajt.12897
Amjad, W., Hamaad Rahman, S., Schiano, T. D., and Jafri, S. M. (2024). Epidemiology and management of infections in liver transplant recipients. Surg. Infect. (Larchmt). 25, 272–290. doi: 10.1089/sur.2023.346
Amodio, P., Biancardi, A., Montagnese, S., Angeli, P., Iannizzi, P., Cillo, U., et al. (2007). Neurological complications after orthotopic liver transplantation. Dig. Liver. Dis. 39, 740–747. doi: 10.1016/j.dld.2007.05.004
Ancona, G., Alagna, L., Lombardi, A., Palomba, E., Castelli, V., Renisi, G., et al. (2021). The interplay between gut microbiota and the immune system in liver transplant recipients and its role in infections. Infect. Immun. 89, e0037621. doi: 10.1128/IAI.00376-21
Anesi, J. A., Blumberg, E. A., and Abbo, L. M. (2018). Perioperative antibiotic prophylaxis to prevent surgical site infections in solid organ transplantation. Transplantation 102, 21–34. doi: 10.1097/TP.0000000000001848
Angarita, S. A. K., Russell, T. A., and Kaldas, F. M. (2017). Pneumonia after liver transplantation. Curr. Opin. Organ Transplant. 22, 328–335. doi: 10.1097/MOT.0000000000000427
Annavajhala, M. K., Gomez-Simmonds, A., Macesic, N., Sullivan, S. B., Kress, A., Khan, S. D., et al. (2019). Colonizing multidrug-resistant bacteria and the longitudinal evolution of the intestinal microbiome after liver transplantation. Nat. Commun. 10, 4715. doi: 10.1038/s41467-019-12633-4
Antimicrobial Resistance C (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655. doi: 10.1016/S0140-6736(21)02724-0
Avkan-Oguz, V., Ozkardesler, S., Unek, T., Ozbilgin, M., Akan, M., Firuzan, E., et al. (2013). Risk factors for early bacterial infections in liver transplantation. Transplant. Proc. 45, 993–997. doi: 10.1016/j.transproceed.2013.02.067
Avkan-Oguz, V., Unek, T., Firuzan, E., Ozbilgin, M., Egeli, T., Bacakoglu, A., et al. (2015). Bacterial pathogens isolated in liver transplant recipients with surgical site infection and antibiotic treatment. Transplant. Proc. 47, 1495–1498. doi: 10.1016/j.transproceed.2015.04.047
Azar, M. M., Turbett, S., Gaston, D., Gitman, M., Razonable, R., Koo, S., et al. (2022). A consensus conference to define the utility of advanced infectious disease diagnostics in solid organ transplant recipients. Am. J. Transplant. 22, 3150–3169. doi: 10.1111/ajt.17147
Baganate, F., Beal, E. W., Tumin, D., Azoulay, D., Mumtaz, K., Black, S. M., et al. (2018). Early mortality after liver transplantation: Defining the course and the cause. Surgery 164, 694–704. doi: 10.1016/j.surg.2018.04.039
Bennett, J. E., Dolin, R., and Blaser, M. J. (2015). Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Eighth edition (Philadelphia, PA: Elsevier/Saunders).
Beranger, A., Capito, C., Lacaille, F., Ferroni, A., Bouazza, N., Girard, M., et al. (2020). Early bacterial infections after pediatric liver transplantation in the era of multidrug-resistant bacteria: nine-year single-center retrospective experience. Pediatr. Infect. Dis. J. 39, e169–ee75. doi: 10.1097/INF.0000000000002662
Bert, F., Larroque, B., Paugam-Burtz, C., Janny, S., Durand, F., Dondero, F., et al. (2010). Microbial epidemiology and outcome of bloodstream infections in liver transplant recipients: an analysis of 259 episodes. Liver. Transpl. 16, 393–401. doi: 10.1002/lt.21991
Blair, J. E. and Kusne, S. (2005). Bacterial, mycobacterial, and protozoal infections after liver transplantation–part I. Liver. Transpl. 11, 1452–1459. doi: 10.1002/lt.20624
Braun, H. J., Grab, J. D., Dodge, J. L., Syed, S. M., Roll, G. R., Schwab, M. P., et al. (2021). Retransplantation after living donor liver transplantation: data from the adult to adult living donor liver transplantation study. Transplantation 105, 1297–1302. doi: 10.1097/TP.0000000000003361
Brigati, E., Bandera, A., Consonni, D., Grancini, A., Maggi, U., Piconi, S., et al. (2023). Surgical site infections in liver transplantation in the era of multidrug-resistant bacteria. Minerva. Surg. 78, 345–354. doi: 10.23736/S2724-5691.22.09807-0
Cai, X., Yan, H., Zhang, W., Zhao, W., Zhang, L., Wang, X., et al. (2023). Intra-abdominal infection after tumor surgery: tigecycline combined with beta-lactam antibiotics versus tigecycline alone. BMC Cancer 23, 682. doi: 10.1186/s12885-023-11169-7
Campagna, F., Biancardi, A., Cillo, U., Gatta, A., and Amodio, P. (2010). Neurocognitive-neurological complications of liver transplantation: a review. Metab. Brain Dis. 25, 115–124. doi: 10.1007/s11011-010-9183-0
Campos-Varela, I., Blumberg, E. A., Giorgio, P., Kotton, C. N., Saliba, F., Wey, E. Q., et al. (2022). What is the optimal antimicrobial prophylaxis to prevent postoperative infectious complications after liver transplantation? A systematic review of the literature and expert panel recommendations. Clin. Transplant. 36, e14631. doi: 10.1111/ctr.14631
Carrera-Silva, E. A., Cano, R. C., Guinazu, N., Aoki, M. P., Pellegrini, A., and Gea, S. (2008). TLR2, TLR4 and TLR9 are differentially modulated in liver lethally injured from BALB/c and C57BL/6 mice during Trypanosoma cruzi acute infection. Mol. Immunol. 45, 3580–3588. doi: 10.1016/j.molimm.2008.05.004
Castanheira, M., Deshpande, L. M., Mendes, R. E., Canton, R., Sader, H. S., and Jones, R. N. (2019). Variations in the occurrence of resistance phenotypes and carbapenemase genes among enterobacteriaceae isolates in 20 years of the SENTRY antimicrobial surveillance program. Open Forum Infect. Dis. 6, S23–S33. doi: 10.1093/ofid/ofy347
Castanheira, M., Kimbrough, J. H., DeVries, S., Mendes, R. E., and Sader, H. S. (2023). Trends of beta-Lactamase Occurrence Among Escherichia coli and Klebsiella pneumoniae in United States Hospitals During a 5-Year Period and Activity of Antimicrobial Agents Against Isolates Stratified by beta-Lactamase Type. Open Forum Infect. Dis. 10, ofad038. doi: 10.1093/ofid/ofad038
Caviglia, G. P., Abate, M. L., Tandoi, F., Ciancio, A., Amoroso, A., Salizzoni, M., et al. (2018). Quantitation of HBV cccDNA in anti-HBc-positive liver donors by droplet digital PCR: A new tool to detect occult infection. J. Hepatol. 69, 301–307. doi: 10.1016/j.jhep.2018.03.021
Cervera, C., Fernandez-Ruiz, M., Valledor, A., Linares, L., Anton, A., Angeles Marcos, M., et al. (2011). Epidemiology and risk factors for late infection in solid organ transplant recipients. Transpl. Infect. Dis. 13, 598–607. doi: 10.1111/j.1399-3062.2011.00646.x
Chang, J., Peng, X., Wang, X., and Zhang, Z. (2025). In vitro Evaluation of the Antibacterial Activity of the Combination of Avibactam and beta-Lactams Against Highly Virulent Carbapenem-Resistant Klebsiella pneumoniae. Infect. Drug Resist. 18, 2137–2152. doi: 10.2147/IDR.S515858
Chen, F., Pang, X. Y., Shen, C., Han, L. Z., Deng, Y. X., Chen, X. S., et al. (2020). High mortality associated with gram-negative bacterial bloodstream infection in liver transplant recipients undergoing immunosuppression reduction. World J. Gastroenterol. 26, 7191–7203. doi: 10.3748/wjg.v26.i45.7191
Chen, J., Tian, S., Nian, H., Wang, R., Li, F., Jiang, N., et al. (2021). Carbapenem-resistant Enterobacter cloacae complex in a tertiary Hospital in Northeast China, 2010-2019. BMC Infect. Dis. 21, 611. doi: 10.1186/s12879-021-06250-0
Chen, Y., Wang, W. L., Zhang, W., Zhang, Y. T., Tang, S. X., Wu, P. P., et al. (2020). Risk factors and outcomes of carbapenem-resistant enterobacteriaceae infection after liver transplantation: A retrospective study in a chinese population. Infect. Drug Resist. 13, 4039–4045. doi: 10.2147/IDR.S278084
Cheng, J. C., Huang, C. L., Lin, C. C., Chen, C. C., Chang, Y. C., Chang, S. S., et al. (2006). Rapid detection and identification of clinically important bacteria by high-resolution melting analysis after broad-range ribosomal RNA real-time PCR. Clin. Chem. 52, 1997–2004. doi: 10.1373/clinchem.2006.069286
Choi, M. C., Min, E. K., Yim, S. H., Kim, D. G., Lee, J. G., Joo, D. J., et al. (2024). High number of plasma exchanges increases the risk of bacterial infection in ABO-incompatible living donor liver transplantation. Transplantation 108, 1760–1768. doi: 10.1097/TP.0000000000004883
Chuang, J. Y., Yang, S. S., Lu, Y. T., Hsieh, Y. Y., Chen, C. Y., Chang, S. C., et al. (2009). IL-10 promoter gene polymorphisms and sustained response to combination therapy in Taiwanese chronic hepatitis C patients. Dig. Liver. Dis. 41, 424–430. doi: 10.1016/j.dld.2008.09.017
Chueiri Neto, F., Emidio, L. A., Perales, S. R., Stucchi, R. S. B., Dragosavac, D., Falcao, A. L. E., et al. (2019). Bloodstream infections in early postsurgery liver transplant: an analysis of 401 patients over 10 years. Transplant. Proc. 51, 1972–1977. doi: 10.1016/j.transproceed.2019.03.040
Chung, P. H., Chan, S. C., Chan, K. L., Chan, Y. S., Kwok, J. S., and Lo, C. M. (2016). Lowered immune cell function in liver recipients recovered from posttransplant lymphoproliferative disease who developed graft tolerance. Transplant. Direct. 2, e66. doi: 10.1097/TXD.0000000000000577
Cialla-May, D., Bonifacio, A., Bocklitz, T., Markin, A., Markina, N., Fornasaro, S., et al. (2024). Biomedical SERS - the current state and future trends. Chem. Soc. Rev. 53, 8957–8979. doi: 10.1039/D4CS00090K
Cinar, F. and Bulbuloglu, S. (2022). The effect of adherence to immunosuppressant therapy on gastrointestinal complications after liver transplantation. Transpl. Immunol. 71, 101554. doi: 10.1016/j.trim.2022.101554
Cockbain, A. J., Goldsmith, P. J., Gouda, M., Attia, M., Pollard, S. G., Lodge, J. P., et al. (2010). The impact of postoperative infection on long-term outcomes in liver transplantation. Transplant. Proc. 42, 4181–4183. doi: 10.1016/j.transproceed.2010.09.026
Cooper, T. E., Scholes-Robertson, N., Craig, J. C., Hawley, C. M., Howell, M., Johnson, D. W., et al. (2022). Synbiotics, prebiotics and probiotics for solid organ transplant recipients. Cochrane Database Syst. Rev. 9, CD014804. doi: 10.1002/14651858.CD014804.pub2
Corman Dincer, P., Tore Altun, G., Birtan, D., Arslantas, R., Sarici Mert, N., Ozdemir, I., et al. (2019). Incidence and risk factors for systemic infection in deceased donors. Transplant. Proc. 51, 2195–2197. doi: 10.1016/j.transproceed.2019.03.054
Dai, L., Su, B., Liu, A., Zhang, H., Wu, H., Zhang, T., et al. (2020). Adverse events in Chinese human immunodeficiency virus (HIV) patients receiving first line antiretroviral therapy. BMC Infect. Dis. 20, 158. doi: 10.1186/s12879-020-4878-2
Debette-Gratien, M., Woillard, J. B., Picard, N., Sebagh, M., Loustaud-Ratti, V., Sautereau, D., et al. (2016). Influence of donor and recipient CYP3A4, CYP3A5, and ABCB1 genotypes on clinical outcomes and nephrotoxicity in liver transplant recipients. Transplantation 100, 2129–2137. doi: 10.1097/TP.0000000000001394
del Pozo, J. L. (2008). Update and actual trends on bacterial infections following liver transplantation. World J. Gastroenterol. 14, 4977–4983. doi: 10.3748/wjg.14.4977
Dendle, C., Stuart, R. L., Mulley, W. R., and Holdsworth, S. R. (2018). Pneumococcal vaccination in adult solid organ transplant recipients: A review of current evidence. Vaccine 36, 6253–6261. doi: 10.1016/j.vaccine.2018.08.069
Deppermann, C., Peiseler, M., Zindel, J., Zbytnuik, L., Lee, W. Y., Pasini, E., et al. (2021). Tacrolimus impairs kupffer cell capacity to control bacteremia: why transplant recipients are susceptible to infection. Hepatology 73, 1967–1984. doi: 10.1002/hep.31499
de Rooij, B. J., van der Beek, M. T., van Hoek, B., Vossen, A. C., Rogier Ten Hove, W., Roos, A., et al. (2011). Mannose-binding lectin and ficolin-2 gene polymorphisms predispose to cytomegalovirus (re)infection after orthotopic liver transplantation. J. Hepatol. 55, 800–807. doi: 10.1016/j.jhep.2011.01.039
de Rooij, B. J., van Hoek, B., ten Hove, W. R., Roos, A., Bouwman, L. H., Schaapherder, A. F., et al. (2010). Lectin complement pathway gene profile of donor and recipient determine the risk of bacterial infections after orthotopic liver transplantation. Hepatology 52, 1100–1110. doi: 10.1002/hep.23782
Diaz, L. A., Villalon, A., Ochoa, G., Garcia, S., Severino, N., Ayares, G., et al. (2024). Updates in general management and frequent complications following adult liver transplant. Rev. Med. Chil. 152, 704–717. doi: 10.4067/s0034-98872024000600704
Dolci, G., Burastero, G. J., Paglia, F., Cervo, A., Meschiari, M., Guaraldi, G., et al. (2023). Epidemiology and prevention of early infections by multi-drug-resistant organisms in adults undergoing liver transplant: A narrative review. Microorganisms 11. doi: 10.3390/microorganisms11061606
Eichenberger, E. M. and Thaden, J. T. (2019). Epidemiology and mechanisms of resistance of extensively drug resistant gram-negative bacteria. Antibiot. (Basel). 8. doi: 10.3390/antibiotics8020037
Eklou, N. D., Janigen, B. M., Pisarski, P., Walz, G., and Schneider, J. (2022). Evaluation of deceased donor kidney transplantation in the eurotransplant senior program in comparison to standard allocation. Ann. Transplant. 27, e936514. doi: 10.12659/AOT.936514
El-Bendary, M., Naemattalah, M., Yassen, A., Mousa, N., Elhammady, D., Sultan, A. M., et al. (2020). Interrelationship between Toll-like receptors and infection after orthotopic liver transplantation. World J. Transplant. 10, 162–172. doi: 10.5500/wjt.v10.i6.162
Fagiuoli, S., Colli, A., Bruno, R., Craxi, A., Gaeta, G. B., Grossi, P., et al. (2014). Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J. Hepatol. 60, 1075–1089. doi: 10.1016/j.jhep.2013.12.021
Falcone, M., Bassetti, M., Tiseo, G., Giordano, C., Nencini, E., Russo, A., et al. (2020). Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit. Care 24, 29. doi: 10.1186/s13054-020-2742-9
Feltracco, P., Barbieri, S., Furnari, M., Milevoj, M., Rizzi, S., Galligioni, H., et al. (2010). Central nervous system infectious complications early after liver transplantation. Transplant. Proc. 42, 1216–1222. doi: 10.1016/j.transproceed.2010.03.108
Fernandez, J., Acevedo, J., Castro, M., Garcia, O., de Lope, C. R., Roca, D., et al. (2012). Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology 55, 1551–1561. doi: 10.1002/hep.25532
Ferrarese, A., Senzolo, M., Sasset, L., Bassi, D., Cillo, U., and Burra, P. (2024). Multidrug-resistant bacterial infections in the liver transplant setting. Updates. Surg. 76, 2521–2529. doi: 10.1007/s13304-024-01903-6
Ferrarese, A., Zanetto, A., Becchetti, C., Sciarrone, S. S., Shalaby, S., Germani, G., et al. (2018). Management of bacterial infection in the liver transplant candidate. World J. Hepatol. 10, 222–230. doi: 10.4254/wjh.v10.i2.222
Fishman, J. A. (2007). Infection in solid-organ transplant recipients. N. Engl. J. Med. 357, 2601–2614. doi: 10.1056/NEJMra064928
Fishman, J. A. (2017). Infection in organ transplantation. Am. J. Transplant. 17, 856–879. doi: 10.1111/ajt.14208
Fishman, J. A. and Rubin, R. H. (1998). Infection in organ-transplant recipients. N. Engl. J. Med. 338, 1741–1751. doi: 10.1056/NEJM199806113382407
Freire, M. P., Soares Oshiro, I. C., Bonazzi, P. R., Guimaraes, T., Ramos Figueira, E. R., Bacchella, T., et al. (2013). Surgical site infections in liver transplant recipients in the model for end-stage liver disease era: an analysis of the epidemiology, risk factors, and outcomes. Liver. Transpl. 19, 1011–1019. doi: 10.1002/lt.23682
Freire, M. P., Song, A. T. W., Oshiro, I. C. V., Andraus, W., D'Albuquerque, L. A. C., and Abdala, E. (2021). Surgical site infection after liver transplantation in the era of multidrug-resistant bacteria: what new risks should be considered? Diagn. Microbiol. Infect. Dis. 99, 115220. doi: 10.1016/j.diagmicrobio.2020.115220
Freise, C. E., Gillespie, B. W., Koffron, A. J., Lok, A. S., Pruett, T. L., Emond, J. C., et al. (2008). Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am. J. Transplant. 8, 2569–2579. doi: 10.1111/j.1600-6143.2008.02440.x
Friman, S., Tisone, G., Nevens, F., Lehner, F., Santaniello, W., Bechstein, W. O., et al. (2021). Long-term, prolonged-release tacrolimus-based immunosuppression in de novo liver transplant recipients: 5-year prospective follow-up of patients in the DIAMOND study. Transplant. Direct. 7, e722. doi: 10.1097/TXD.0000000000001166
Gao, Q., Liu, R., Wu, Y., Wang, F., and Wu, X. (2024). Versatile self-assembled near-infrared SERS nanoprobes for multidrug-resistant bacterial infection-specific surveillance and therapy. Acta Biomater. 189, 559–573. doi: 10.1016/j.actbio.2024.09.054
Garcia-Bustos, V., Cabanero-Navalon, M. D., and Salavert Lleti, M. (2022). Resistance to beta-lactams in Gram-negative bacilli: relevance and potential therapeutic alternatives. Rev. Esp. Quimioter. 35 Suppl 2, 1–15. doi: 10.37201/req/s02.01.2022
Garcia Prado, M. E., Matia, E. C., Ciuro, F. P., Diez-Canedo, J. S., Sousa Martin, J. M., Porras Lopez, F. M., et al. (2008). Surgical site infection in liver transplant recipients: impact of the type of perioperative prophylaxis. Transplantation 85, 1849–1854. doi: 10.1097/TP.0b013e3181735407
Gayowski, T., Marino, I. R., Singh, N., Doyle, H., Wagener, M., Fung, J. J., et al. (1998). Orthotopic liver transplantation in high-risk patients: risk factors associated with mortality and infectious morbidity. Transplantation 65, 499–504. doi: 10.1097/00007890-199802270-00008
Gelb, B. and Feng, S. (2009). Management of the liver transplant patient. Expert Rev. Gastroenterol. Hepatol. 3, 631–647. doi: 10.1586/egh.09.58
Ghose, P., Ali, A. Q., Fang, R., Forbes, D., Ballard, B., and Ismail, N. (2011). The interaction between IL-18 and IL-18 receptor limits the magnitude of protective immunity and enhances pathogenic responses following infection with intracellular bacteria. J. Immunol. 187, 1333–1346. doi: 10.4049/jimmunol.1100092
Giani, T., Conte, V., Mandala, S., D'Andrea, M. M., Luzzaro, F., Conaldi, P. G., et al. (2014). Cross-infection of solid organ transplant recipients by a multidrug-resistant Klebsiella pneumoniae isolate producing the OXA-48 carbapenemase, likely derived from a multiorgan donor. J. Clin. Microbiol. 52, 2702–2705. doi: 10.1128/JCM.00511-14
Gilad, O., Rabinowich, L., Levy, S., Gotlieb, N., Lubezky, N., Goykhman, Y., et al. (2021). Metabolic and renal effects of mammalian target of rapamycin inhibitors treatment after liver transplantation: real-life single-center experience. Transplant. Proc. 53, 221–227. doi: 10.1016/j.transproceed.2020.05.021
Giselle Moreira, M., Guimaraes Oliveira, A. G., Ul Haq, I., Pinheiro de Oliveira, T. F., Alonazi, W. B., Fonseca Junior, A. A., et al. (2024). Droplet digital PCR for acinetobacter baumannii diagnosis in bronchoalveolar lavage samples from patients with ventilator-associated pneumonia. Antibiot. (Basel). 13. doi: 10.3390/antibiotics13090878
Golfieri, R., Giampalma, E., Morselli Labate, A. M., d'Arienzo, P., Jovine, E., Grazi, G. L., et al. (2000). Pulmonary complications of liver transplantation: radiological appearance and statistical evaluation of risk factors in 300 cases. Eur. Radiol. 10, 1169–1183. doi: 10.1007/s003309900268
Grossi, P. A., Fishman, J. A., and Practice ASTIDCo (2009). Donor-derived infections in solid organ transplant recipients. Am. J. Transplant. 9 Suppl 4, S19–S26. doi: 10.1111/j.1600-6143.2009.02889.x
Guo, J., Su, Z., Zhong, J., Li, L., An, W., Shi, B., et al. (2025). Early bacterial pathogen distribution and risk factors for infections after liver transplantation: retrospective cohort study. Int. J. Infect. Dis. 156, 107907. doi: 10.1016/j.ijid.2025.107907
Guo, Y., Zhu, Z., Cai, W., Tao, S., and Yin, D. (2022). Intracerebral opportunistic infections caused by immunosuppressants after orthotopic liver transplantation: Report of two cases and literature review. Front. Immunol. 13, 1003254. doi: 10.3389/fimmu.2022.1003254
Gutierrez-Gutierrez, B., Salamanca, E., de Cueto, M., Hsueh, P. R., Viale, P., Pano-Pardo, J. R., et al. (2017). Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect. Dis. 17, 726–734. doi: 10.1016/S1473-3099(17)30228-1
Hand, J. and Patel, G. (2016). Multidrug-resistant organisms in liver transplant: Mitigating risk and managing infections. Liver. Transpl. 22, 1143–1153. doi: 10.1002/lt.24486
Harada, N., Yoshizumi, T., Yoshiya, S., Takeishi, K., Toshima, T., Itoh, S., et al. (2020). Use of mycophenolate mofetil suspension as part of induction therapy after living-donor liver transplant. Exp. Clin. Transplant. 18, 485–490. doi: 10.6002/ect.2020.0041
Hashimoto, M., Sugawara, Y., Tamura, S., Kaneko, J., Matsui, Y., Moriya, K., et al. (2007). Impact of new methicillin-resistant Staphylococcus aureus carriage postoperatively after living donor liver transplantation. Transplant. Proc. 39, 3271–3275. doi: 10.1016/j.transproceed.2007.09.035
Hashimoto, M., Sugawara, Y., Tamura, S., Kaneko, J., Matsui, Y., Togashi, J., et al. (2008). Bloodstream infection after living donor liver transplantation. Scand. J. Infect. Dis. 40, 509–516. doi: 10.1080/00365540701824116
Hashimoto, M., Sugawara, Y., Tamura, S., Kaneko, J., Matsui, Y., Kokudo, N., et al. (2009). Pseudomonas aeruginosa infection after living-donor liver transplantation in adults. Transpl. Infect. Dis. 11, 11–19. doi: 10.1111/j.1399-3062.2008.00341.x
Heil, E. L., Bork, J. T., Abbo, L. M., Barlam, T. F., Cosgrove, S. E., Davis, A., et al. (2021). Optimizing the management of uncomplicated gram-negative bloodstream infections: consensus guidance using a modified delphi process. Open Forum Infect. Dis. 8, ofab434. doi: 10.1093/ofid/ofab434
Heldman, M. R., Ngo, S., Dorschner, P. B., Helfrich, M., and Ison, M. G. (2019). Pre- and post-transplant bacterial infections in liver transplant recipients. Transpl. Infect. Dis. 21, e13152. doi: 10.1111/tid.13152
Hellinger, W. C., Crook, J. E., Heckman, M. G., Diehl, N. N., Shalev, J. A., Zubair, A. C., et al. (2009). Surgical site infection after liver transplantation: risk factors and association with graft loss or death. Transplantation 87, 1387–1393. doi: 10.1097/TP.0b013e3181a25133
Hennis, R., Raynor, M. A., Hock, R. A., Yousaf, M., Allen, J. C., Heh, E., et al. (2024). Use of cell-free DNA testing to diagnose infective endocarditis in a patient with negative blood cultures. Cureus 16, e72191. doi: 10.7759/cureus.72191
Horcajada, J. P., Montero, M., Oliver, A., Sorli, L., Luque, S., Gomez-Zorrilla, S., et al. (2019). Epidemiology and treatment of multidrug-resistant and extensively drug-resistant pseudomonas aeruginosa infections. Clin. Microbiol. Rev. 32. doi: 10.1128/CMR.00031-19
Hrenczuk, M., Biedrzycka, A., Lagiewska, B., Kosieradzki, M., and Malkowski, P. (2020). Surgical site infections in liver transplant patients: A single-center experience. Transplant. Proc. 52, 2497–2502. doi: 10.1016/j.transproceed.2020.02.093
Hu, S., Li, G., Li, H., Liu, X., Niu, J., Quan, S., et al. (2014). Rapid detection of isoniazid resistance in Mycobacterium tuberculosis isolates by use of real-time-PCR-based melting curve analysis. J. Clin. Microbiol. 52, 1644–1652. doi: 10.1128/JCM.03395-13
Idossa, D. W. and Simonetto, D. A. (2017). Infectious complications and Malignancies arising after liver transplantation. Anesthesiol. Clin. 35, 381–393. doi: 10.1016/j.anclin.2017.04.002
Igarashi, H., Hirano, H., Yahagi, A., Saika, T., and Ishihara, K. (2014). Anti-apoptotic roles for the mutant p53R248Q through suppression of p53-regulated apoptosis-inducing protein 1 in the RA-derived fibroblast-like synoviocyte cell line MH7A. Clin. Immunol. 150, 12–21. doi: 10.1016/j.clim.2013.10.013
Iida, T., Kaido, T., Yagi, S., Yoshizawa, A., Hata, K., Mizumoto, M., et al. (2010). Posttransplant bacteremia in adult living donor liver transplant recipients. Liver. Transpl. 16, 1379–1385. doi: 10.1002/lt.22165
Ison, M. G. and Nalesnik, M. A. (2011). An update on donor-derived disease transmission in organ transplantation. Am. J. Transplant. 11, 1123–1130. doi: 10.1111/j.1600-6143.2011.03493.x
Jablonska, A., Sadkowski, A., Richert-Przygonska, M., and Styczynski, J. (2025). Next-generation sequencing for infectious disease diagnostics in pediatric patients with Malignancies or after hematopoietic cell transplantation: A systematic review. J. Clin. Med. 14. doi: 10.3390/jcm14186444
Jafarpour, Z., Pouladfar, G., Malek Hosseini, S. A., Firoozifar, M., and Jafari, P. (2020). Bacterial infections in the early period after liver transplantation in adults: A prospective single-center cohort study. Microbiol. Immunol. 64, 407–415. doi: 10.1111/1348-0421.12785
Kahn, J., Pregartner, G., and Schemmer, P. (2020). Effects of both pro- and synbiotics in liver surgery and transplantation with special focus on the gut-liver axis-A systematic review and meta-analysis. Nutrients 12. doi: 10.3390/nu12082461
Kalpoe, J. S., Sonnenberg, E., Factor, S. H., del Rio Martin, J., Schiano, T., Patel, G., et al. (2012). Mortality associated with carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver. Transpl. 18, 468–474. doi: 10.1002/lt.23374
Karvellas, C. J., McPhail, M., Pink, F., Asthana, S., Muiesan, P., Heaton, N., et al. (2011). Bloodstream infection after elective liver transplantation is associated with increased mortality in patients with cirrhosis. J. Crit. Care 26, 468–474. doi: 10.1016/j.jcrc.2010.12.018
Kawecki, D., Chmura, A., Pacholczyk, M., Lagiewska, B., Adadynski, L., Wasiak, D., et al. (2009). Bacterial infections in the early period after liver transplantation: etiological agents and their susceptibility. Med. Sci. Monit. 15, CR628–CR637. doi: 10.1586/egh.09.58
Kawecki, D., Pacholczyk, M., Lagiewska, B., Adadynski, L., Lisik, W., Sawicka-Grzelak, A., et al. (2011). Urinary tract infections in the early posttransplant period after liver transplantation: etiologic agents and their susceptibility. Transplant. Proc. 43, 3052–3054. doi: 10.1016/j.transproceed.2011.09.003
Kawecki, D., Pacholczyk, M., Lagiewska, B., Sawicka-Grzelak, A., Durlik, M., Mlynarczyk, G., et al. (2014). Bacterial and fungal infections in the early post-transplantation period after liver transplantation: etiologic agents and their susceptibility. Transplant. Proc. 46, 2777–2781. doi: 10.1016/j.transproceed.2014.08.031
Kearns, H., Goodacre, R., Jamieson, L. E., Graham, D., and Faulds, K. (2017). SERS detection of multiple antimicrobial-resistant pathogens using nanosensors. Anal. Chem. 89, 12666–12673. doi: 10.1021/acs.analchem.7b02653
Kim, S. I. (2014). Bacterial infection after liver transplantation. World J. Gastroenterol. 20, 6211–6220. doi: 10.3748/wjg.v20.i20.6211
Kim, Y. E., Choi, H. J., Lee, H. J., Oh, H. J., Ahn, M. K., Oh, S. H., et al. (2022). Assessment of pathogens and risk factors associated with bloodstream infection in the year after pediatric liver transplantation. World J. Gastroenterol. 28, 1159–1171. doi: 10.3748/wjg.v28.i11.1159
Kim, W., Kim, S., Oh, J., Jeong, Y. J., Rhu, J., Kim, K. S., et al. (2019). Incidence and risk factors for herpes zoster after adult liver transplantation. Ann. Surg. Treat Res. 96, 95–99. doi: 10.4174/astr.2019.96.2.95
Kim, Y. J., Kim, S. I., Wie, S. H., Kim, Y. R., Hur, J. A., Choi, J. Y., et al. (2008). Infectious complications in living-donor liver transplant recipients: a 9-year single-center experience. Transpl. Infect. Dis. 10, 316–324. doi: 10.1111/j.1399-3062.2008.00315.x
Kim, S. H., Mun, S. J., Ko, J. H., Huh, K., Cho, S. Y., Kang, C. I., et al. (2021). Poor outcomes of early recurrent post-transplant bloodstream infection in living-donor liver transplant recipients. Eur. J. Clin. Microbiol. Infect. Dis. 40, 771–778. doi: 10.1007/s10096-020-04074-5
Kim, H. K., Park, Y. K., Wang, H. J., Kim, B. W., Shin, S. Y., Lim, S. K., et al. (2013). Epidemiology and clinical features of post-transplant bloodstream infection: an analysis of 222 consecutive liver transplant recipients. Infect. Chemother. 45, 315–324. doi: 10.3947/ic.2013.45.3.315
Kinoshita, M., Seki, S., Ono, S., Shinomiya, N., and Hiraide, H. (2004). Paradoxical effect of IL-18 therapy on the severe and mild Escherichia coli infections in burn-injured mice. Ann. Surg. 240, 313–320. doi: 10.1097/01.sla.0000133354.44709.28
Kinoshita, M., Shinomiya, N., Ono, S., Tsujimoto, H., Kawabata, T., Matsumoto, A., et al. (2006). Restoration of natural IgM production from liver B cells by exogenous IL-18 improves the survival of burn-injured mice infected with Pseudomonas aeruginosa. J. Immunol. 177, 4627–4635. doi: 10.4049/jimmunol.177.7.4627
Kiuchi, T., Kasahara, M., Uryuhara, K., Inomata, Y., Uemoto, S., Asonuma, K., et al. (1999). Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation 67, 321–327. doi: 10.1097/00007890-199901270-00024
Kniepeiss, D., Rosenkranz, A. R., Fickert, P., and Schemmer, P. (2022). Update: Immunosuppression in organ transplantation. Dtsch. Med. Wochenschr. 147, 1199–1212. doi: 10.3748/wjg.v26.i45.7191
Kojabad, A. A., Farzanehpour, M., Galeh, H. E. G., Dorostkar, R., Jafarpour, A., Bolandian, M., et al. (2021). Droplet digital PCR of viral DNA/RNA, current progress, challenges, and future perspectives. J. Med. Virol. 93, 4182–4197. doi: 10.1002/jmv.26846
Kremer, W. M., Gairing, S. J., Kaps, L., Ismail, E., Kalampoka, V., Hilscher, M., et al. (2022). Characteristics of bacterial infections and prevalence of multidrug-resistant bacteria in hospitalized patients with liver cirrhosis in Germany. Ann. Hepatol. 27, 100719. doi: 10.1016/j.aohep.2022.100719
Kritikos, A. and Manuel, O. (2016). Bloodstream infections after solid-organ transplantation. Virulence 7, 329–340. doi: 10.1080/21505594.2016.1139279
Kusne, S., Dummer, J. S., Singh, N., Iwatsuki, S., Makowka, L., Esquivel, C., et al. (1988). Infections after liver transplantation. An analysis of 101 consecutive cases. Med. (Baltimore). 67, 132–143. doi: 10.1097/00005792-198803000-00006
Lacaille, F. (2012). Liver transplantation and liver cell transplantation. Clin. Res. Hepatol. Gastroenterol. 36, 304–307. doi: 10.1016/j.clinre.2012.03.029
Laici, C., Gamberini, L., Bardi, T., Siniscalchi, A., Reggiani, M. L. B., and Faenza, S. (2018). Early infections in the intensive care unit after liver transplantation-etiology and risk factors: A single-center experience. Transpl. Infect. Dis. 20, e12834. doi: 10.1111/tid.12834
Lakomkin, N. and Hadjipanayis, C. G. (2021). The role of prophylactic intraventricular antibiotics in reducing the incidence of infection and revision surgery in pediatric patients undergoing shunt placement. Neurosurgery 88, 301–305. doi: 10.1093/neuros/nyaa413
Lee, S. O., Brown, R. A., Kang, S. H., Abdel-Massih, R. C., and Razonable, R. R. (2011a). Toll-like receptor 2 polymorphism and Gram-positive bacterial infections after liver transplantation. Liver. Transpl. 17, 1081–1088. doi: 10.1002/lt.22327
Lee, S. O., Kang, S. H., Abdel-Massih, R. C., Brown, R. A., and Razonable, R. R. (2011b). Spectrum of early-onset and late-onset bacteremias after liver transplantation: implications for management. Liver. Transpl. 17, 733–741. doi: 10.1002/lt.22296
Lee, D. U., Ponder, R., Lee, K. J., Chou, H., Lee, K., Jung, D., et al. (2023). The prognostic relationship between donor age and infectious risk in liver transplant patients with nonalcoholic steatohepatitis: Analysis of UNOS database. Dig. Liver. Dis. 55, 751–762. doi: 10.1016/j.dld.2023.01.160
Lemos, G. T., Terrabuio, D. R. B., Nunes, N. N., Song, A. T. W., Oshiro, I. C. V., D'Albuquerque, L. A. C., et al. (2024). Pre-transplant multidrug-resistant infections in liver transplant recipients-epidemiology and impact on transplantation outcome. Clin. Transplant. 38, e15173. doi: 10.1111/ctr.15173
Lenhard, J. R., Bulman, Z. P., Tsuji, B. T., and Kaye, K. S. (2019). Shifting gears: the future of polymyxin antibiotics. Antibiot. (Basel). 8. doi: 10.3390/antibiotics8020042
Lentine, K. L., Smith, J. M., Miller, J. M., Bradbrook, K., Larkin, L., Weiss, S., et al. (2023). OPTN/SRTR 2021 annual data report: kidney. Am. J. Transplant. 23, S21–S120. doi: 10.1016/j.ajt.2023.02.004
Lewis, J. D. and Sifri, C. D. (2016). Multidrug-resistant bacterial donor-derived infections in solid organ transplantation. Curr. Infect. Dis. Rep. 18, 18. doi: 10.1007/s11908-016-0526-9
Li, C., Wen, T. F., Mi, K., Wang, C., Yan, L. N., and Li, B. (2012). Analysis of infections in the first 3-month after living donor liver transplantation. World J. Gastroenterol. 18, 1975–1980. doi: 10.3748/wjg.v18.i16.1975
Li, H., Yu, D. L., Ren, L., Zhong, L., Peng, Z. H., and Teng, M. J. (2012). Analysis of Gram-positive bacterial infection in patients following liver transplantation. Chin. Med. J. (Engl). 125, 2417–2421.
Li, H., Yu, X., Shi, B., Zhang, K., Yuan, L., Liu, X., et al. (2021). Reduced pannexin 1-IL-33 axis function in donor livers increases risk of MRSA infection in liver transplant recipients. Sci. Transl. Med. 13. doi: 10.1126/scitranslmed.aaz6169
Liu, C. L., Fan, S. T., Lo, C. M., Wei, W. I., Chan, S. C., Yong, B. H., et al. (2006). Operative outcomes of adult-to-adult right lobe live donor liver transplantation: a comparative study with cadaveric whole-graft liver transplantation in a single center. Ann. Surg. 243, 404–410. doi: 10.1097/01.sla.0000201544.36473.a2
Liu, S., Huang, X., Zhang, S., Han, H., Qin, W., Wang, J., et al. (2024). Droplet digital polymerase chain reaction (ddPCR) for bloodstream infections: A meta-analysis. J. Infect. 89, 106329. doi: 10.1016/j.jinf.2024.106329
Liu, M., Li, C., Liu, J., and Wan, Q. (2023). Risk factors of early bacterial infection and analysis of bacterial composition, distribution and drug susceptibility after cadaveric liver transplantation. Ann. Clin. Microbiol. Antimicrob. 22, 63. doi: 10.1186/s12941-023-00616-9
Liu, N., Yang, G., Dang, Y., Liu, X., Chen, M., Dai, F., et al. (2022). Epidemic, risk factors of carbapenem-resistant Klebsiella pneumoniae infection and its effect on the early prognosis of liver transplantation. Front. Cell Infect. Microbiol. 12, 976408. doi: 10.3389/fcimb.2022.976408
Lynch, J. P., 3rd, Clark, N. M., and Zhanel, G. G. (2021). Escalating antimicrobial resistance among Enterobacteriaceae: focus on carbapenemases. Expert Opin. Pharmacother. 22, 1455–1473. doi: 10.1080/14656566.2021.1904891
Martin-Gandul, C., Mueller, N. J., Pascual, M., and Manuel, O. (2015). The impact of infection on chronic allograft dysfunction and allograft survival after solid organ transplantation. Am. J. Transplant. 15, 3024–3040. doi: 10.1111/ajt.13486
Martin-Mateos, R., Martinez-Arenas, L., Carvalho-Gomes, A., Aceituno, L., Cadahia, V., Salcedo, M., et al. (2024). Multidrug-resistant bacterial infections after liver transplantation: Prevalence, impact, and risk factors. J. Hepatol. 80, 904–912. doi: 10.1016/j.jhep.2024.02.023
Marx, S., Adam, C., Mihm, J., Weyrich, M., Sester, U., and Sester, M. (2020). A polyclonal immune function assay allows dose-dependent characterization of immunosuppressive drug effects but has limited clinical utility for predicting infection on an individual basis. Front. Immunol. 11, 916. doi: 10.3389/fimmu.2020.00916
Massarollo, P. C., Mies, S., Abdala, E., Leitao, R. M., and Raia, S. (1998). Immunosuppression withdrawal for treatment of severe infections in liver transplantation. Transplant. Proc. 30, 1472–1474. doi: 10.1016/S0041-1345(98)00321-2
Mathew, J. S. and Philips, C. A. (2023). Drug interactions and safe prescription writing for liver transplant recipients. J. Clin. Exp. Hepatol. 13, 869–877. doi: 10.1016/j.jceh.2023.03.011
Mayo Moldes, M., Galan Torres, J., Moreno Gazquez, A., Llacer Borras, E., and Moreno Puigdollers, I. (2005). Prevalence and risk factors for early postoperative infection after liver transplantation. Rev. Esp. Anestesiol. Reanim. 52, 200–207.
Meije, Y., Piersimoni, C., Torre-Cisneros, J., Dilektasli, A. G., and Aguado, J. M. (2014). Hosts ESGoIiC. Mycobacterial infections in solid organ transplant recipients. Clin. Microbiol. Infect. 20 Suppl 7, 89–101. doi: 10.1111/1469-0691.12641
Mermel, L. A. and Maki, D. G. (1990). Bacterial pneumonia in solid organ transplantation. Semin. Respir. Infect. 5, 10–29.
Meschiari, M., Asquier-Khati, A., Tiseo, G., Luque-Paz, D., Murri, R., Boutoille, D., et al. (2024). Treatment of infections caused by multidrug-resistant Gram-negative bacilli: A practical approach by the Italian (SIMIT) and French (SPILF) Societies of Infectious Diseases. Int. J. Antimicrob. Agents 64, 107186. doi: 10.1016/j.ijantimicag.2024.107186
Mian, M., Natori, Y., Ferreira, V., Selzner, N., Husain, S., Singer, L., et al. (2018). Evaluation of a novel global immunity assay to predict infection in organ transplant recipients. Clin. Infect. Dis. 66, 1392–1397. doi: 10.1093/cid/cix1008
Miller, W. R. and Arias, C. A. (2024). ESKAPE pathogens: antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nat. Rev. Microbiol. 22, 598–616. doi: 10.1038/s41579-024-01054-w
Miller, R., Covington, S., Taranto, S., Carrico, R., Ehsan, A., Friedman, B., et al. (2015). Communication gaps associated with donor-derived infections. Am. J. Transplant. 15, 259–264. doi: 10.1111/ajt.12978
Montasser, M. F., Abdelkader, N. A., Abdelhakam, S. M., Dabbous, H., Montasser, I. F., Massoud, Y. M., et al. (2017). Bacterial infections post-living-donor liver transplantation in Egyptian hepatitis C virus-cirrhotic patients: A single-center study. World J. Hepatol. 9, 896–904. doi: 10.4254/wjh.v9.i20.896
Moreno, A., Cervera, C., Gavalda, J., Rovira, M., de la Camara, R., Jarque, I., et al. (2007). Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am. J. Transplant. 7, 2579–2586. doi: 10.1111/j.1600-6143.2007.01964.x
Mukhtar, A., Abdelaal, A., Hussein, M., Dabous, H., Fawzy, I., Obayah, G., et al. (2014). Infection complications and pattern of bacterial resistance in living-donor liver transplantation: a multicenter epidemiologic study in Egypt. Transplant. Proc. 46, 1444–1447. doi: 10.1016/j.transproceed.2014.02.022
Mularoni, A., Martucci, G., Douradinha, B., Campanella, O., Hazen, B., Medaglia, A., et al. (2019). Epidemiology and successful containment of a carbapenem-resistant Enterobacteriaceae outbreak in a Southern Italian Transplant Institute. Transpl. Infect. Dis. 21, e13119. doi: 10.1111/tid.13119
Muller, J., Hartwig, C., Sonntag, M., Bitzer, L., Adelmann, C., Vainshtein, Y., et al. (2024). A novel approach for in vivo DNA footprinting using short double-stranded cell-free DNA from plasma. Genome Res. 34, 1185–1195. doi: 10.1101/gr.279326.124
Mulugeta, S. G., Veve, M. P., Jantz, A. S., Lanfranco, O. A., and Davis, S. L. (2017). Antipseudomonal drug exposure associated with MDR organisms in the liver and lung transplant population. Open Forum Infect. Dis. 4, S492–S49S. doi: 10.1093/ofid/ofx163.1269
Nafady-Hego, H., Elgendy, H., Moghazy, W. E., Fukuda, K., and Uemoto, S. (2011). Pattern of bacterial and fungal infections in the first 3 months after pediatric living donor liver transplantation: an 11-year single-center experience. Liver. Transpl. 17, 976–984. doi: 10.1002/lt.22278
Nanni Costa, A., Grossi, P., Gianelli Castiglione, A., Grigioni, W. F., and Italian Transplant Research N (2008). Quality and safety in the Italian donor evaluation process. Transplantation 85, S52–S56. doi: 10.1097/TP.0b013e31816c2f05
Neff, G., Zacharias, V., Kaiser, T. E., Gaddis, A., and Kemmer, N. (2010). Rifaximin for the treatment of recurrent Clostridium difficile infection after liver transplantation: A case series. Liver. Transpl. 16, 960–963. doi: 10.1002/lt.22092
Nemes, B., Gaman, G., and Doros, A. (2015). Biliary complications after liver transplantation. Expert Rev. Gastroenterol. Hepatol. 9, 447–466. doi: 10.1586/17474124.2015.967761
Neofytos, D., Stampf, S., Hoessly, L. D., D'Asaro, M., Tang, G. N., Boggian, K., et al. (2023). Bacteremia during the first year after solid organ transplantation: an epidemiological update. Open Forum Infect. Dis. 10, ofad247. doi: 10.1093/ofid/ofad247
Newell, K. A., Millis, J. M., Arnow, P. M., Bruce, D. S., Woodle, E. S., Cronin, D. C., et al. (1998). Incidence and outcome of infection by vancomycin-resistant Enterococcus following orthotopic liver transplantation. Transplantation 65, 439–442. doi: 10.1097/00007890-199802150-00027
Ntobe-Bunkete, B. and Lemaitre, F. (2024). Therapeutic drug monitoring in kidney and liver transplantation: current advances and future directions. Expert Rev. Clin. Pharmacol. 17, 505–514. doi: 10.1080/17512433.2024.2354276
Oliveira, R. A., Mancero, J. M. P., Faria, D. F., and Poveda, V. B. (2019). A retrospective cohort study of risk factors for surgical site infection following liver transplantation. Prog. Transplant. 29, 144–149. doi: 10.1177/1526924819835831
Ono, S., Obara, H., Takayanagi, A., Tanabe, M., Kawachi, S., Itano, O., et al. (2012). Suppressive effects of interleukin-18 on liver function in rat liver allografts. J. Surg. Res. 176, 293–300. doi: 10.1016/j.jss.2011.07.053
Oriol, I., Sabe, N., Simonetti, A. F., Llado, L., Manonelles, A., Gonzalez, J., et al. (2017). Changing trends in the aetiology, treatment and outcomes of bloodstream infection occurring in the first year after solid organ transplantation: a single-centre prospective cohort study. Transpl. Int. 30, 903–913. doi: 10.1111/tri.12984
Palacios-Baena, Z. R., Giannella, M., Manissero, D., Rodriguez-Bano, J., Viale, P., Lopes, S., et al. (2021). Risk factors for carbapenem-resistant Gram-negative bacterial infections: a systematic review. Clin. Microbiol. Infect. 27, 228–235. doi: 10.1016/j.cmi.2020.10.016
Park, J. I., Song, G. W., Ryu, J. H., Choi, S. T., Choi, N. G., Jung, B. H., et al. (2023). A multicenter, randomized, open-label study to compare the efficacy and safety of tacrolimus and corticosteroids in combination with or without mycophenolate mofetil in liver transplantation recipients infected with hepatitis B virus. Transplant. Proc. 55, 387–395. doi: 10.1016/j.transproceed.2023.01.013
Patel, G. and Huprikar, S. (2012). Infectious complications after orthotopic liver transplantation. Semin. Respir. Crit. Care Med. 33, 111–124. doi: 10.1055/s-0032-1301739
Patel, R. and Paya, C. V. (1997). Infections in solid-organ transplant recipients. Clin. Microbiol. Rev. 10, 86–124. doi: 10.1128/CMR.10.1.86
Paya, C. V. and Hermans, P. E. (1989). Bacterial infections after liver transplantation. Eur. J. Clin. Microbiol. Infect. Dis. 8, 499–504. doi: 10.1007/BF01967467
Pedersen, M. and Seetharam, A. (2014). Infections after orthotopic liver transplantation. J. Clin. Exp. Hepatol. 4, 347–360. doi: 10.1016/j.jceh.2014.07.004
Pelaz, B., Alexiou, C., Alvarez-Puebla, R. A., Alves, F., Andrews, A. M., Ashraf, S., et al. (2017). Diverse applications of nanomedicine. ACS Nano. 11, 2313–2381. doi: 10.1021/acsnano.6b06040
Perovic, O., Ismail, H., Quan, V., Bamford, C., Nana, T., Chibabhai, V., et al. (2020). Carbapenem-resistant Enterobacteriaceae in patients with bacteraemia at tertiary hospitals in South Africa, 2015 to 2018. Eur. J. Clin. Microbiol. Infect. Dis. 39, 1287–1294. doi: 10.1007/s10096-020-03845-4
Pouch, S. M., Anesi, J. A., Pruett, T., Harmon, M., Dionne, S. O., Hasz, R., et al. (2025). Deceased donor infectious diseases testing and antimicrobial use: surveys of organ procurement organizations and transplant professionals. Transpl. Infect. Dis. 27, e14407. doi: 10.1111/tid.14407
Pouladfar, G., Jafarpour, Z., Malek Hosseini, S. A., Firoozifar, M., Rasekh, R., Khosravifard, L., et al. (2017). Urinary tract infections among hospitalized adults in the early post-liver transplant period: prevalence, risk factors, causative agents, and microbial susceptibility. Exp. Clin. Transplant. 15, 190–193. doi: 10.6002/ect.mesot2016.P68
Pouladfar, G., Jafarpour, Z., Malek Hosseini, S. A., Firoozifar, M., Rasekh, R., and Khosravifard, L. (2019). Bacterial infections in pediatric patients during early post liver transplant period: A prospective study in Iran. Transpl. Infect. Dis. 21, e13001. doi: 10.1111/tid.13001
Qin, J., Xu, J., Dong, Y., Tang, W., Wu, B., An, Y., et al. (2012). High-resolution CT findings of pulmonary infections after orthotopic liver transplantation in 453 patients. Br. J. Radiol. 85, e959–e965. doi: 10.1259/bjr/26230943
Rabbani, A., Aliabbar, S., Nikeghbalian, S., Malek-Hosseini, A., and Baziboroun, M. (2022). Immunosuppressive regimens on conversion of cytomegalovirus infection to disease in liver transplant recipients. Caspian. J. Intern. Med. 13, 721–727. doi: 10.22088/cjim.13.4.721
Ramanan, P. and Razonable, R. R. (2013). Cytomegalovirus infections in solid organ transplantation: a review. Infect. Chemother. 45, 260–271. doi: 10.3947/ic.2013.45.3.260
Rasmussen, D. B., Moller, D. L., Knudsen, A. D., Rostved, A. A., Knudsen, J. D., Rasmussen, A., et al. (2021). Enterococcal infections the first year after liver transplantation-A prospective cohort study. Microorganisms 9. doi: 10.3390/microorganisms9081740
Rayes, N., Seehofer, D., Theruvath, T., Schiller, R. A., Langrehr, J. M., Jonas, S., et al. (2005). Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation–a randomized, double-blind trial. Am. J. Transplant. 5, 125–130. doi: 10.1111/j.1600-6143.2004.00649.x
Reddy, A., Zhang, J., Davis, N. S., Moffitt, A. B., Love, C. L., Waldrop, A., et al. (2017). Genetic and functional drivers of diffuse large B cell lymphoma. Cell 171, 481–94 e15. doi: 10.1016/j.cell.2017.09.027
Reid, G. E., Grim, S. A., Sankary, H., Benedetti, E., Oberholzer, J., and Clark, N. M. (2009). Early intra-abdominal infections associated with orthotopic liver transplantation. Transplantation 87, 1706–1711. doi: 10.1097/TP.0b013e3181a60338
Resino, E., San-Juan, R., and Aguado, J. M. (2016). Selective intestinal decontamination for the prevention of early bacterial infections after liver transplantation. World J. Gastroenterol. 22, 5950–5957. doi: 10.3748/wjg.v22.i26.5950
Rice, J. C. and Safdar, N. (2009). Practice ASTIDCo. Urinary tract infections in solid organ transplant recipients. Am. J. Transplant. 9 Suppl 4, S267–S272. doi: 10.1111/j.1600-6143.2009.02919.x
Righi, E. (2018). Management of bacterial and fungal infections in end stage liver disease and liver transplantation: Current options and future directions. World J. Gastroenterol. 24, 4311–4329. doi: 10.3748/wjg.v24.i38.4311
Rodriguez-Fernandez, M., Trigo-Rodriguez, M., Martinez-Baena, D., Herrero, R., Espindola-Gomez, R., Martinez Perez-Crespo, P., et al. (2024). Role of rectal colonization by third-generation cephalosporin-resistant Enterobacterales on the risk of surgical site infection after hepato-pancreato-biliary surgery. Microbiol. Spectr. 12, e0087824. doi: 10.1128/spectrum.00878-24
Rolak, S. C., Yetmar, Z. A., Lahr, B. D., Beam, E., Razi, S., Watt, K., et al. (2024). Risk factors for surgical-site infections after liver transplant: does perioperative antibiotic regimen matter? Transplantation 108, 1179–1188. doi: 10.1097/TP.0000000000004810
Romero, F. A. and Razonable, R. R. (2011). Infections in liver transplant recipients. World J. Hepatol. 3, 83–92. doi: 10.4254/wjh.v3.i4.83
Safdar, N., Said, A., Lucey, M. R., Knechtle, S. J., D'Alessandro, A., Musat, A., et al. (2004). Infected bilomas in liver transplant recipients: clinical features, optimal management, and risk factors for mortality. Clin. Infect. Dis. 39, 517–525. doi: 10.1086/422644
Salehi, S., Tranah, T. H., Lim, S., Heaton, N., Heneghan, M., Aluvihare, V., et al. (2019). Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all-cause admissions in patients on the liver transplant waiting list. Aliment. Pharmacol. Ther. 50, 435–441. doi: 10.1111/apt.15326
Samstein, B., Smith, A. R., Freise, C. E., Zimmerman, M. A., Baker, T., Olthoff, K. M., et al. (2016). Complications and their resolution in recipients of deceased and living donor liver transplants: findings from the A2ALL cohort study. Am. J. Transplant. 16, 594–602. doi: 10.1111/ajt.13479
Saner, F. H., Sotiropoulos, G. C., Gu, Y., Paul, A., Radtke, A., Gensicke, J., et al. (2007). Severe neurological events following liver transplantation. Arch. Med. Res. 38, 75–79. doi: 10.1016/j.arcmed.2006.07.005
Santoro-Lopes, G. and de Gouvea, E. F. (2014). Multidrug-resistant bacterial infections after liver transplantation: an ever-growing challenge. World J. Gastroenterol. 20, 6201–6210. doi: 10.3748/wjg.v20.i20.6201
Sartor, C., Gregoire, E., Albanese, J., and Fournier, P. E. (2015). Invasive Listeria monocytogenes infection after liver transplantation: a life-threatening condition. Lancet 385, 200. doi: 10.1016/S0140-6736(14)61831-6
Sawas, T., Al Halabi, S., Hernaez, R., Carey, W. D., and Cho, W. K. (2015). Patients receiving prebiotics and probiotics before liver transplantation develop fewer infections than controls: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 13, 1567–74 e3. quiz e143-4. doi: 10.1016/j.cgh.2015.05.027
Schreiber, P. W., Hoessly, L. D., Boggian, K., Neofytos, D., van Delden, C., Egli, A., et al. (2025). Surgical site infections, risk factors, and outcomes after liver transplant. JAMA Netw. Open 8, e251333. doi: 10.1001/jamanetworkopen.2025.1333
Seehofer, D., Eurich, D., Veltzke-Schlieker, W., and Neuhaus, P. (2013). Biliary complications after liver transplantation: old problems and new challenges. Am. J. Transplant. 13, 253–265. doi: 10.1111/ajt.12034
Selimoglu, M. A., Kaya, S., Gungor, S., Varol, F. I., Gozukara-Bag, H. G., and Yilmaz, S. (2020). Infection risk after paediatric liver transplantation. Turk. J. Pediatr. 62, 46–52. doi: 10.24953/turkjped.2020.01.007
Shen, Y. H., Fan, J., Zhou, J., Wu, Z. Q., Qiu, S. J., Huang, X. W., et al. (2007). Pulmonary infection and its risk factors after orthotopic liver transplantation. Zhonghua. Gan. Zang. Bing. Za. Zhi. 15, 833–836.
Shen, Y., Zhang, J., Xu, X., Fu, J., and Li, J. (2012). Expression of complement component C7 and involvement in innate immune responses to bacteria in grass carp. Fish. Shellfish. Immunol. 33, 448–454. doi: 10.1016/j.fsi.2012.05.016
Shepshelovich, D., Tau, N., Green, H., Rozen-Zvi, B., Issaschar, A., Falcone, M., et al. (2019). Immunosuppression reduction in liver and kidney transplant recipients with suspected bacterial infection: A multinational survey. Transpl. Infect. Dis. 21, e13134. doi: 10.1111/tid.13134
Shi, S. H., Kong, H. S., Xu, J., Zhang, W. J., Jia, C. K., Wang, W. L., et al. (2009). Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transpl. Infect. Dis. 11, 405–412. doi: 10.1111/j.1399-3062.2009.00421.x
Shi, B., Liu, Y., Liu, D., Yuan, L., Guo, W., Wen, P., et al. (2023). Genotype-guided model significantly improves accuracy of tacrolimus initial dosing after liver transplantation. EClinicalMedicine 55, 101752. doi: 10.1016/j.eclinm.2022.101752
Shi, B. J., Yu, X. Y., Li, H., et al. (2017). Association between donor and recipient Interleukin-18 gene polymorphisms and the risk of infection after liver transplantation. Clin. Invest. Med. 40, E176–EE87. doi: 10.25011/cim.v40i5.28623
Shinde, A. S. and Kapoor, D. (2024). Infections after liver transplant -timeline, management and prevention. J. Clin. Exp. Hepatol. 14, 101316. doi: 10.1016/j.jceh.2023.101316
Shoji, K., Funaki, T., Kasahara, M., Sakamoto, S., Fukuda, A., Vaida, F., et al. (2015). Risk factors for bloodstream infection after living-donor liver transplantation in children. Pediatr. Infect. Dis. J. 34, 1063–1068. doi: 10.1097/INF.0000000000000811
Silva, J. T., Fernandez-Ruiz, M., and Aguado, J. M. (2018). Multidrug-resistant Gram-negative infection in solid organ transplant recipients: implications for outcome and treatment. Curr. Opin. Infect. Dis. 31, 499–505. doi: 10.1097/QCO.0000000000000488
Singh, N., Wagener, M. M., Obman, A., Cacciarelli, T. V., de Vera, M. E., Gayowski, T., et al. (1997). Invasive aspergillosis in liver transplant recipients in the 1990s. Transplantation 64, 716–720. doi: 10.1097/00007890-199709150-00009
Singh, N., Paterson, D. L., Chang, F. Y., Gayowski, T., Squier, C., Wagener, M. M., et al. (2000). Methicillin-resistant Staphylococcus aureus: the other emerging resistant gram-positive coccus among liver transplant recipients. Clin. Infect. Dis. 30, 322–327. doi: 10.1086/313658
Singh, N., Wagener, M. M., Obman, A., Cacciarelli, T. V., de Vera, M. E., and Gayowski, T. (2004). Bacteremias in liver transplant recipients: shift toward gram-negative bacteria as predominant pathogens. Liver. Transpl. 10, 844–849. doi: 10.1002/lt.20214
Song, J., Lin, S., Zhu, L., Lin, Y., An, W., Zhang, J., et al. (2024). Direct identification of pathogens via microbial cellular DNA in whole blood by MeltArray. Microb. Biotechnol. 17, e14380. doi: 10.1111/1751-7915.14380
Song, A. T. W., Yrbas, M. L. A., Pierrotti, L. C., Malan, R., Delfino, C., Pontes, D. F. S., et al. (2024). Global perspectives on donor-derived infections: Brazil and Argentina. Transpl. Infect. Dis. 26 Suppl 1, e14389. doi: 10.1111/tid.14389
Sood, S., Cundall, D., Yu, L., Miyamasu, M., Boyle, J. S., Ong, S. Y., et al. (2014). A novel biomarker of immune function and initial experience in a transplant population. Transplantation 97, e50–e51. doi: 10.1097/TP.0000000000000078
Soong, R. S., Chan, K. M., Chou, H. S., Wu, T. J., Lee, C. F., Wu, T. H., et al. (2012). The risk factors for early infection in adult living donor liver transplantation recipients. Transplant. Proc. 44, 784–786. doi: 10.1016/j.transproceed.2012.03.028
Steffani, M., Jager, C., Huser, N., Friess, H., Hartmann, D., Demir, I. E., et al. (2024). Postoperative prophylactic antibiotic therapy after pancreaticoduodenectomy in bile duct-stented patients reduces postoperative major complications. Surgery 176, 1162–1168. doi: 10.1016/j.surg.2024.03.025
Sun, X., Sun, X., Zhou, T., Li, P., Wang, B., Pan, Q., et al. (2024). Long-term outcomes and risk factors for early bacterial infection after pediatric liver transplantation: a prospective cohort study. Int. J. Surg. 110, 5452–5462. doi: 10.1097/JS9.0000000000001670
Sun, H. Y., Wagener, M., Cacciarelli, T. V., and Singh, N. (2012). Impact of rifaximin use for hepatic encephalopathy on the risk of early post-transplant infections in liver transplant recipients. Clin. Transplant. 26, 849–852. doi: 10.1111/j.1399-0012.2012.01619.x
Taddei, R., Riccardi, N., Tiseo, G., Galfo, V., and Biancofiore, G. (2023). Early intra-abdominal bacterial infections after orthotopic liver transplantation: A narrative review for clinicians. Antibiot. (Basel). 12. doi: 10.3390/antibiotics12081316
Tadesse, L. F., Safir, F., Ho, C. S., Hasbach, X., Khuri-Yakub, B. P., Jeffrey, S. S., et al. (2020). Toward rapid infectious disease diagnosis with advances in surface-enhanced Raman spectroscopy. J. Chem. Phys. 152, 240902. doi: 10.1063/1.5142767
Tamma, P. D., Heil, E. L., Justo, J. A., Mathers, A. J., Satlin, M. J., Bonomo, R. A., et al (2024). Infectious diseases society of america 2024 guidance on the treatment of antimicrobial-resistant gram-negative infections. Clin. Infect. Dis. doi: 10.1093/cid/ciae403
Tamma, P. D., Sharara, S. L., Pana, Z. D., Amoah, J., Fisher, S. L., Tekle, T., et al. (2019). Molecular epidemiology of ceftriaxone non-susceptible enterobacterales isolates in an academic medical center in the United States. Open Forum Infect. Dis. 6, ofz353. doi: 10.1093/ofid/ofz353
Tamma, P. D., Smith, T. T., Adebayo, A., Karaba, S. M., Jacobs, E., Wakefield, T., et al. (2021). Prevalence of bla(CTX-M) Genes in Gram-Negative Bloodstream Isolates across 66 Hospitals in the United States. J. Clin. Microbiol. 59. doi: 10.1128/JCM.00127-21
Tan, J. K., Servellita, V., Stryke, D., Kelly, E., Streithorst, J., Sumimoto, N., et al. (2024). Laboratory validation of a clinical metagenomic next-generation sequencing assay for respiratory virus detection and discovery. Nat. Commun. 15, 9016. doi: 10.1038/s41467-024-51470-y
Tawab, K. A., Gheith, O., Al Otaibi, T., Nampoory, N., Mansour, H., Halim, M. A., et al. (2017). Recurrent urinary tract infection among renal transplant recipients: risk factors and long-term outcome. Exp. Clin. Transplant. 15, 157–163. doi: 10.6002/ect.2016.0069
Tharanon, V., Iamrahong, P., Tuamsem, J., Choochaeam, K., Auamnoy, T., and Sobhonslidsuk, A. (2024). Hemoglobin and total bilirubin may affect tacrolimus clearance in liver transplant patient following the early postoperative period: a case report. J. Med. Case Rep. 18, 408. doi: 10.1186/s13256-024-04753-3
Theodoropoulos, N. M., Greenwald, M. A., Chin-Hong, P., and Ison, M. G. (2021). Testing deceased organ donors for infections: An organ procurement organization survey. Am. J. Transplant. 21, 1924–1930. doi: 10.1111/ajt.16552
Thomson, A. W., Vionnet, J., and Sanchez-Fueyo, A. (2020). Understanding, predicting and achieving liver transplant tolerance: from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 17, 719–739. doi: 10.1038/s41575-020-0334-4
Tolou Ghamari, Z. and Palizban, A. A. (2023). Tacrolimus pharmacotherapy: infectious complications and toxicity in organ transplant recipients; an updated review. Curr. Drug Res. Rev. doi: 10.2174/0125899775259326231212073240
Tong, L., Hu, X. G., Huang, F., Huang, S. W., Li, L. F., Tang, Z. X., et al. (2020). Clinical impacts and outcomes with possible donor-derived infection in infected donor liver transplantation: A single-center retrospective study in China. J. Infect. Dis. 221, S164–SS73. doi: 10.1093/infdis/jiz591
Torre-Cisneros, J., Herrero, C., Canas, E., Reguera, J. M., de la Mata, M., and Gomez-Bravo, M. A. (2002). High mortality related with Staphylococcus aureus bacteremia after liver transplantation. Eur. J. Clin. Microbiol. Infect. Dis. 21, 385–388. doi: 10.1007/s10096-002-0725-1
Trezeguet Renatti, G., Riva, N., Minetto, J., Reijenstein, H., Gole, M., Meza, V., et al. (2024). Feasibility of steroid-free tacrolimus-basiliximab immunosuppression in pediatric liver transplantation and predictors for steroid requirement. Liver. Transpl. 30, 61–71. doi: 10.1097/LVT.0000000000000216
Tsujimoto, H., Ono, S., Matsumoto, A., Kawabata, T., Kinoshita, M., Majima, T., et al. (2006). A critical role of CpG motifs in a murine peritonitis model by their binding to highly expressed toll-like receptor-9 on liver NKT cells. J. Hepatol. 45, 836–843. doi: 10.1016/j.jhep.2006.07.024
Tu, Z. H., Jin, P. B., Chen, D. Y., Chen, Z. Y., Li, Z. W., Zhang, B. S., et al. (2023). Infection evaluation in the early period after liver transplantation: A single-center exploration. Transpl. Infect. Dis. 25, e14002. doi: 10.1111/tid.14002
Tucker, O. N. and Heaton, N. (2005). The 'small for size' liver syndrome. Curr. Opin. Crit. Care 11, 150–155. doi: 10.1097/01.ccx.0000157080.11117.45
Tun, T., Marinelli, T., Liu, K., Strasser, S. I., Crawford, M., and Patanwala, A. E. (2024). Low rate of surgical site infections after liver transplantation: A 5-year retrospective cohort study. Transpl. Infect. Dis. 26, e14280. doi: 10.1111/tid.14280
Tustumi, F., Miranda Neto, A. A., Silveira Junior, S., Fernandes, F. A., Silva, M., Ernani, L., et al. (2021). Safety and effectiveness of mycophenolate mofetil associated with tacrolimus for liver transplantation immunosuppression: a systematic review and meta-analysis of randomized controlled trials. Clinics (Sao Paulo). 76, e2597. doi: 10.6061/clinics/2021/e2597
Udomkarnjananun, S., Francke, M. I., De Winter, B. C. M., Mulder, M. B., Baan, C. C., Metselaar, H. J., et al. (2021). Therapeutic drug monitoring of immunosuppressive drugs in hepatology and gastroenterology. Best Pract. Res. Clin. Gastroenterol. 54-55, 101756. doi: 10.1016/j.bpg.2021.101756
Ueno, T., Kodama, T., Noguchi, Y., Deguchi, K., Nomura, M., Saka, R., et al. (2020). Serum trough concentration and effects of mycophenolate mofetil based on pathologic findings in infants after liver transplantation. Transplant. Proc. 52, 1855–1857. doi: 10.1016/j.transproceed.2020.01.160
Unal, B., Gonultas, F., Aydin, C., Otan, E., Kayaalp, C., and Yilmaz, S. (2013). Hepatic artery thrombosis-related risk factors after living donor liver transplantation: single-center experience from Turkey. Transplant. Proc. 45, 974–977. doi: 10.1016/j.transproceed.2013.02.070
van Delden, C. (2014). Bacterial biliary tract infections in liver transplant recipients. Curr. Opin. Organ Transplant. 19, 223–228. doi: 10.1097/MOT.0000000000000083
van Duin, D., Lok, J. J., Earley, M., Cober, E., Richter, S. S., Perez, F., et al. (2018). Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant enterobacteriaceae. Clin. Infect. Dis. 66, 163–171. doi: 10.1093/cid/cix783
van Hoek, B., de Rooij, B. J., and Verspaget, H. W. (2012). Risk factors for infection after liver transplantation. Best Pract. Res. Clin. Gastroenterol. 26, 61–72. doi: 10.1016/j.bpg.2012.01.004
Velusamy, S., Tran, T., Mongkolrattanothai, T., Walker, H., McGee, L., and Beall, B. (2020). Expanded sequential quadriplex real-time polymerase chain reaction (PCR) for identifying pneumococcal serotypes, penicillin susceptibility, and resistance markers. Diagn. Microbiol. Infect. Dis. 97, 115037. doi: 10.1016/j.diagmicrobio.2020.115037
Verweij, J. J. and Stensvold, C. R. (2014). Molecular testing for clinical diagnosis and epidemiological investigations of intestinal parasitic infections. Clin. Microbiol. Rev. 27, 371–418. doi: 10.1128/CMR.00122-13
Vidal, E., Torre-Cisneros, J., Blanes, M., Montejo, M., Cervera, C., Aguado, J. M., et al. (2012). Bacterial urinary tract infection after solid organ transplantation in the RESITRA cohort. Transpl. Infect. Dis. 14, 595–603. doi: 10.1111/j.1399-3062.2012.00744.x
Viehman, J. A., Clancy, C. J., Clarke, L., Shields, R. K., Silveira, F. P., Kwak, E. J., et al. (2016). Surgical site infections after liver transplantation: emergence of multidrug-resistant bacteria and implications for prophylaxis and treatment strategies. Transplantation 100, 2107–2114. doi: 10.1097/TP.0000000000001356
Vilarinho, S. and Lifton, R. P. (2012). Liver transplantation: from inception to clinical practice. Cell 150, 1096–1099. doi: 10.1016/j.cell.2012.08.030
Vizzini, G., Asaro, M., Miraglia, R., Gruttadauria, S., Fili, D., D'Antoni, A., et al. (2011). Changing picture of central nervous system complications in liver transplant recipients. Liver. Transpl. 17, 1279–1285. doi: 10.1002/lt.22383
Vnucak, M., Granak, K., Skalova, P., Laca, L., Mokan, M., and Dedinska, I. (2021). Effect of mycophenolic acid and tacrolimus on the incidence of infectious complications after kidney transplantation. Int. Immunopharmacol. 98, 107908. doi: 10.1016/j.intimp.2021.107908
Wade, J. J., Rolando, N., Hayllar, K., Philpott-Howard, J., Casewell, M. W., and Williams, R. (1995). Bacterial and fungal infections after liver transplantation: an analysis of 284 patients. Hepatology 21, 1328–1336. doi: 10.1002/hep.1840210517
Wang, M., Earley, M., Chen, L., Hanson, B. M., Yu, Y., Liu, Z., et al. (2022). Clinical outcomes and bacterial characteristics of carbapenem-resistant Klebsiella pneumoniae complex among patients from different global regions (CRACKLE-2): a prospective, multicentre, cohort study. Lancet Infect. Dis. 22, 401–412. doi: 10.1016/S1473-3099(21)00399-6
Wang, P., Jiang, Z., Wang, C., Liu, X., Li, H., Xu, D., et al. (2020). Immune tolerance induction using cell-based strategies in liver transplantation: clinical perspectives. Front. Immunol. 11, 1723. doi: 10.3389/fimmu.2020.01723
Wang, P., Shi, B., Wang, C., Wang, Y., Que, W., Jiang, Z., et al. (2022). Hepatic pannexin-1 mediates ST2(+) regulatory T cells promoting resolution of inflammation in lipopolysaccharide-induced endotoxemia. Clin. Transl. Med. 12, e849. doi: 10.1002/ctm2.849
Wang, W., Xu, S., Ren, Z., Jiang, J., and Zheng, S. (2015). Gut microbiota and allogeneic transplantation. J. Transl. Med. 13, 275. doi: 10.1186/s12967-015-0640-8
Wei, Y., Wu, D., Chen, Y., Dong, C., Qi, J., Wu, Y., et al. (2022). Population pharmacokinetics of mycophenolate mofetil in pediatric patients early after liver transplantation. Front. Pharmacol. 13, 1002628. doi: 10.3389/fphar.2022.1002628
Weiss, N. and Thabut, D. (2019). Neurological complications occurring after liver transplantation: role of risk factors, hepatic encephalopathy, and acute (on chronic) brain injury. Liver. Transpl. 25, 469–487. doi: 10.1002/lt.25420
Williams, M. J., Carvalho Ribeiro do Valle, C., and Gyte, G. M. (2021). Different classes of antibiotics given to women routinely for preventing infection at caesarean section. Cochrane Database Syst. Rev. 3, CD008726. doi: 10.1002/14651858.CD008726.pub3
Wilson, B. M., El Chakhtoura, N. G., Patel, S., Saade, E., Donskey, C. J., Bonomo, R. A., et al. (2017). Carbapenem-resistant enterobacter cloacae in patients from the US veterans health administration, 2006-2015. Emerg. Infect. Dis. 23, 878–880. doi: 10.3201/eid2305.162034
Winterbottom, F. and Jenkins, M. (2017). Infections in the intensive care unit: posttransplant infections. Crit. Care Nurs. Clin. North Am. 29, 97–110. doi: 10.1016/j.cnc.2016.09.002
Wojcicki, M., Milkiewicz, P., and Silva, M. (2008). Biliary tract complications after liver transplantation: a review. Dig. Surg. 25, 245–257. doi: 10.1159/000144653
Wu, D. X., Hu, J. X., Wu, X. L., Han, J. N., Chang, K. Y., Quan, X. L., et al. (2024). Preoperative evidence-based practice for prevention of early postoperative infections in patients receiving a liver transplant. Ann. Transplant. 29, e943610. doi: 10.12659/AOT.943610
Wu, J., Lu, A. D., Zhang, L. P., Zuo, Y. X., and Jia, Y. P. (2019). Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Zhonghua. Xue. Ye. Xue. Za. Zhi. 40, 52–57.
Wu, X., Wu, L., Shu, L., Xie, C., and Wan, Q. (2023). Characteristics of Gram-positive cocci infection and the therapeutic effect after liver transplantation. Zhong. Nan. Da. Xue. Xue. Bao. Yi. Xue. Ban. 48, 707–715. doi: 10.11817/j.issn.1672-7347.2023.220631
Wu, X., Wu, L., and Wan, Q. (2022). Pathogen distribution and risk factors of bacterial and fungal infections after liver transplantation. Zhong. Nan. Da. Xue. Xue. Bao. Yi. Xue. Ban. 47, 1120–1128.
Xie, Z., Lin, X., Wang, Y., Chen, Z., Zeng, P., He, X., et al. (2024). Development and validation of a model for early survival prediction following liver transplantation based on donor and recipient characteristics. Ann. Med. 56, 2410404. doi: 10.1080/07853890.2024.2410404
Xu, S., Qiu, X., Hou, X., Zhou, H., Chen, D., Wang, X., et al. (2021). Direct detection of Corynebacterium striatum, Corynebacterium propinquum, and Corynebacterium simulans in sputum samples by high-resolution melt curve analysis. BMC Infect. Dis. 21, 21. doi: 10.1186/s12879-020-05633-z
Yamada, T., Zhang, M., and Masuda, S. (2020). Significance of ethnic factors in immunosuppressive therapy management after organ transplantation. Ther. Drug Monit. 42, 369–380. doi: 10.1097/FTD.0000000000000748
Yamamoto, M., Takakura, S., Iinuma, Y., Hotta, G., Matsumura, Y., Matsushima, A., et al. (2015). Changes in surgical site infections after living donor liver transplantation. PloS One 10, e0136559. doi: 10.1371/journal.pone.0136559
Yamazhan, T., Bulut Avsar, C., Zeytunlu, M., Tasbakan, M., Sertoz, R., Zeytinoglu, A., et al. (2020). Infections developing in patients undergoing liver transplantation: Recipients of living donors may be more prone to bacterial/fungal infections. Turk. J. Gastroenterol. 31, 894–901. doi: 10.5152/tjg.2020.19286
Ye, Q. F., Zhou, W., and Wan, Q. Q. (2017). Donor-derived infections among Chinese donation after cardiac death liver recipients. World J. Gastroenterol. 23, 5809–5816. doi: 10.3748/wjg.v23.i31.5809
Ying, Y., Li, R. D., Ai, J. W., Zhu, Y. M., Zhou, X., Qian, Y. Y., et al. (2020). Infection within 2 weeks before liver transplantation closely related to prognosis of posttransplant infection: A single-center retrospective observational study in China. Hepatobil. Pancreat. Dis. Int. 19, 358–364. doi: 10.1016/j.hbpd.2020.06.001
Yoshiya, S., Itoh, S., Toshima, T., Bekki, Y., Izumi, T., Iseda, N., et al. (2025). The impact of perioperative synbiotics treatment in living-donor liver transplantation after induction of early enteral nutrition. Surg. Today 55, 475–482. doi: 10.1007/s00595-024-02918-7
Zegarska, J., Hryniewiecka, E., Zochowska, D., Samborowska, E., Jazwiec, R., Dadlez, M., et al. (2020). Higher concentrations of cyclosporine metabolites in liver transplant recipients with a history of viral and bacterial infections. Transplant. Proc. 52, 2503–2506. doi: 10.1016/j.transproceed.2020.03.039
Zhang, Y., Chen, J., Wu, J., Chalson, H., Merigan, L., and Mitchell, A. (2013). Probiotic use in preventing postoperative infection in liver transplant patients. Hepatobil. Surg. Nutr. 2, 142–147. doi: 10.3978/j.issn.2304-3881.2013.06.05
Zhao, D., Guo, L., Lian, D., Gu, Y., Yan, X., Hu, H., et al. (2022). Diagnostic value and clinical application of mNGS for post-liver transplantation infection: A cross-sectional study with case reports. Front. Microbiol. 13, 919363. doi: 10.3389/fmicb.2022.919363
Zhong, L., Li, H., Li, Z., Shi, B., Wang, P., Wang, C., et al. (2016). C7 genotype of the donor may predict early bacterial infection after liver transplantation. Sci. Rep. 6, 24121. doi: 10.1038/srep24121
Zhong, L., Men, T. Y., Li, H., Peng, Z. H., Gu, Y., Ding, X., et al. (2012). Multidrug-resistant gram-negative bacterial infections after liver transplantation - spectrum and risk factors. J. Infect. 64, 299–310. doi: 10.1016/j.jinf.2011.12.005
Zhou, J. D., Guo, J. J., Zhang, Q., Chen, Y., Zhu, S. H., and Peng, H. Y. (2006). Drug resistance of infectious pathogens after liver transplantation. Hepatobil. Pancreat. Dis. Int. 5, 190–194.
Keywords: liver transplantation, bacterial infection, bacterial infections, post-liver transplantation, treatment
Citation: Guo J, Wang Y, Ma J, Xu Y, Shi B, An W, Wang J and Li H (2025) Post-liver transplantation bacterial infection: current status, prevention and treatment. Front. Cell. Infect. Microbiol. 15:1698937. doi: 10.3389/fcimb.2025.1698937
Received: 06 September 2025; Accepted: 30 October 2025;
Published: 25 November 2025.
Edited by:
Abdulellah Almohaya, King Abdulaziz Medical City in Riyadh, Saudi ArabiaReviewed by:
Li-Ying Sun, Capital Medical University, ChinaQiquan Wan, Central South University, China
Copyright © 2025 Guo, Wang, Ma, Xu, Shi, An, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Wang, d2FuZ2ppZWxqQHhtdS5lZHUuY24=; Hao Li, bGloYW82NjU2QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Jingjie Guo1,2,3†
Jingjie Guo1,2,3† Jingmiao Ma
Jingmiao Ma Hao Li
Hao Li