- 1Department of Traditional Chinese Medicine, Jinan Maternity and Child Care Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 2M. Kandiah Faculty of Medicine and Health Sciences, Universiti Tunku Abdul Rahman, Kajang, Selangor, Malaysia
- 3School of Public Health, Jilin Medical University, Jilin, Jilin, China
- 4Department of Child Health, Jinan Maternity and Child Care Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
Purpose: Viral infections elicit different forms of host cell death. Indeed, pathways of programmed cell death (PCD) have emerged as central events in the pathogenesis of various viruses. Regulating PCD is also a critical factor in the pathogenesis of respiratory syncytial virus (RSV) infection. This review systematically summarizes the mechanisms and pathological significance of the main PCD pathways related to RSV infection, and aims to deepen the understanding of RSV regulation of PCD. These findings may provide a new insights and potential therapeutic strategies for the precise prevention and treatment of RSV-related diseases.
Methodology: This review provides a comprehensive overview of the historical development of different forms of PCD. A systematic review was conducted across major academic databases, including Elsevier, PubMed, Springer, Google Scholar, and Web of Science, to collect studies related to RSV and PCD, published between the inception of each database and September 2025. The collected studies were then categorized and organized according to PCD type and affected cell type.
Results: In RSV infection, there are a total of five types of PCD identified, including apoptosis, necroptosis, pyroptosis, NETosis, and ferroptosis. Among these, apoptosis is the most frequently regulated form of cell death during RSV infection. A variety of cell types undergo different forms of PCD during RSV infection, including airway epithelial cells, macrophages, neutrophils, dendritic cells (DCs), lymphocytes and neuronal cells. Notably, PCD is related to airway epithelial cells, which is the most common type of PCD.
Conclusions: PCD serves as a central link in the interaction between RSV infection and the host cell. Different PCD pathways (apoptosis, necroptosis, pyroptosis, NETosis, and ferroptosis) play a dual role in RSV pathogenesis; however, the complex relationship between RSV and PCD remains unclear. Further studies are warranted to explore new forms of PCD in RSV infection, as well as the complex relationship between PCD and RSV structure, the cross-regulatory mechanisms between different PCDs, and the variability of PCD in different cell types. Targeted intervention strategies based on PCD pathways may provide new targets and treatment options for RSV-related diseases.
1 Background
The global pandemic of infectious diseases made humanity aware of the devastating threat posed by respiratory viruses. While long standing viral diseases (with the exception of smallpox) remain endemic, a new viral diseases have emerged intermittently. These diseases not only inflict significant harm on individuals but also impose substantial burdens on families, healthcare systems, and societies, resulting in considerable economic losses (He et al., 2025). Respiratory syncytial virus (RSV), one of the most common respiratory viral pathogens worldwide, was discovered in 1956 by Dr. Robert Chanock after its isolation from children with upper respiratory tract infection (URTI) (Casadevall et al., 2025). RSV causes acute respiratory tract illness (ARTI) in individuals of all age groups and is responsible for seasonal outbreaks of respiratory illness worldwide (Kuang et al., 2024).
The outcome of a viral infection in the host is determined by diverse cellular responses, including abortive, productive, latent, and destructive infections (Rex et al., 2022). These cellular responses generated, whether the infection remains limited, establishes persistence, or leads to an extensive cytopathic damage. The viral-host interaction should be further elucidated to facilitate the development of novel virus control methods (Yuan et al., 2023). Virus-induced cell death has been thought of as a double edged sword in the inhibition or exacerbation of viral infections (Wang et al., 2022). Cell death is a fundamental physiological process in all organisms, which includes accidental cell death (ACD) and programmed cell death (PCD). In their seminal paper of 1972, Kerr, Wyllie and Currie first collated and defined the distinct morphological features of PCD in different contexts (Kerr et al., 1972). PCD plays a central role in regulating animal development and tissue homeostasis and is also implicated in a broad range of human diseases (Rex et al., 2022). In the setting of viral infection, the primary purpose of PCD is thought to be the restriction of replication and dissemination by depriving viruses of the resources needed to propagate infection. However, previous researchers has revealed that PCD signaling shapes the host response to infection in complex ways (Angel and Daniels, 2022). Multiple viruses, including RSV, have been shown to manipulate various forms of PCD, thereby promoting their replication and persistent infection. Therefore, elucidating on how virus regulates these PCD pathways is crucial to understand its pathogenic mechanisms and may reveal novel therapeutic targets.
The core of RSV pathogenicity lies in the dynamic interplay between viral infection and host defense: on the one hand, the host eliminates infected cells via PCD to restrict viral spread; on the other hand, RSV selectively activates/inhibits specific PCD pathways, thereby triggering excessive inflammation and tissue damage, and severe consequences such as bronchiolitis and asthma sensitization. A growing number of studies have shown that PCD is closely related to RSV infection. In this review, we systematically review the distinct modalities of cell death triggered by RSV infection, clarifying their molecular underpinnings to illuminate viral pathogenesis and identify actionable therapeutic targets.
2 Principle of RSV
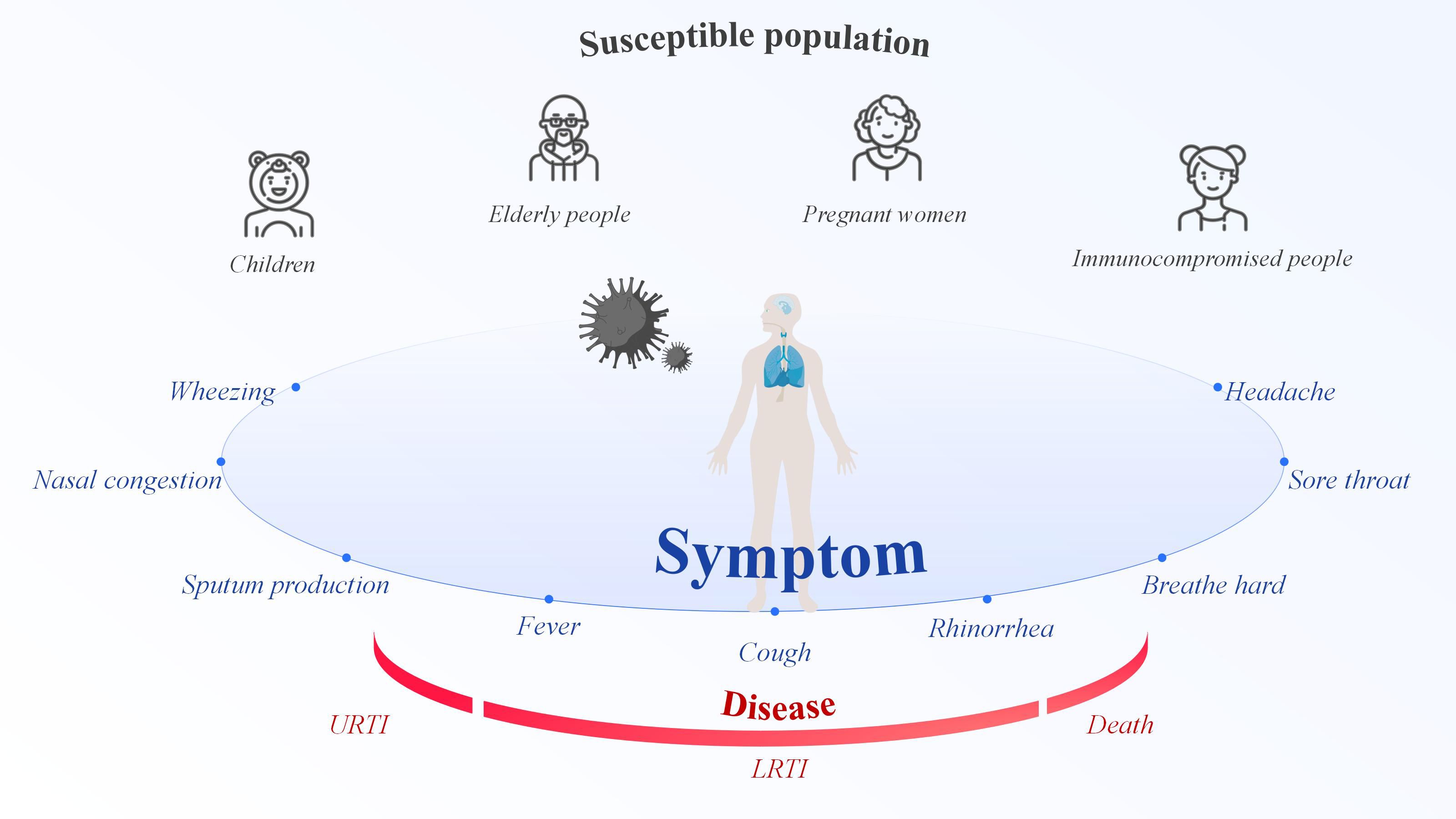
RSV is an enveloped, negative-sense, single-stranded RNA virus belonging to the Orthopneumovirus genus of the Pneumoviridae family, order Mononegavirales. RSV first replicates in the nasal turbinates and then propagates to the bronchioles, where increased mucus production, impaired ciliary action, and sloughed epithelial cells obstruct in the lumen. This obstruction results in trapped air, causes alveolar collapse after absorption (Brüssow, 2025). As an RNA virus transmitted via droplets, RSV can infect individuals of all ages, placing everyone at risk of infection (Wildenbeest et al., 2024). However, children, the elderly, pregnant women, and people with weakened immune systems are at the highest risk of RSV infection (Figure 1). In 2019, there were 33 million RSV-associated acute lower respiratory infection episodes, 3.6 million hospital admissions, 26,300 in-hospital deaths, and 101,400 overall deaths in children aged 0–60 months, which encompasses the period from birth until the fifth birthday (Li et al., 2022). Infants who are premature, immunocompromised, or who have congenital heart disease, congenital lung disease, or congenital airway defects are most vulnerable to severe RSV-related conditions (Dominoni et al., 2024). RSV is the second leading cause of hospitalization from respiratory infections in the elderly (Barahimi et al., 2023). RSV constitutes a significant cause of respiratory illness and mortality among older adults, that is expanding with considerable impact on healthcare systems worldwide (Duan et al., 2024). For example, In the United States (US), RSV is responsible for an estimated 6,000~10,000 annual deaths among older adults (Kim and Choi, 2024). In the past, little was known about the impact of RSV infection during pregnancy, but this issue has gradually received attention in recent years. Pregnant individuals undergo substantial physiologic and immunologic changes over the course of their pregnancy, increasing their risk for respiratory virus infections (Rumfelt et al., 2025). RSV infection during pregnancy can be clinically severe and may be accompanied by pregnancy complications (Hause et al., 2021). The estimated incidence of prenatal RSV infection is reported to be 2.1 cases per 1,000 person-months, or 26.0 cases per 1,000 person-years (Kenmoe et al., 2023), indicating a considerable disease burden. In addition, RSV infection is significantly associated with immunocompromised populations (such as high-risk allogeneic hematopoietic stem cell transplant and solid organ transplant recipients), resulting in higher morbidity and mortality in this group (Koval and Gonzalez, 2024). Furthermore, natural immunity to RSV is incomplete, and reinfection occurs throughout life (Mazur et al., 2023). As a seasonal disease, RSV infection peaks when temperatures decrease in temperate zones and humidity increases in tropical regions (Chi and Chung, 2023). In temperate regions, RSV epidemics typically last for 2~5 months, with the peak commonly occurring during winter. In the tropics, RSV seasonality varies and have longer durations when compare to temperate regions, however the peak typically aligns with the rainy season (Guo et al., 2024). However, these patterns are not static; in fact, the seasonal prevalence of RSV is changing. For example, in the US, the seasonal circulation of RSV was disrupted during the coronavirus disease 2019 (COVID-19) pandemic. Non-pharmaceutical interventions reduced respiratory virus transmission, leading to an accumulation of susceptible individuals and large epidemics with a typical seasonality (Hamid et al., 2023). The seasonality of respiratory virus epidemics is driven by a complex interplay of environmental and host factors, such as temperature, humidity, sunlight, vitamin levels, and behavior (Moriyama et al., 2020). Human behavior is the key to RSV infection; avoiding crowded places is the best option, and this also applies to most respiratory viruses. Most RSV infections are mild and self-limiting, typically present as mild respiratory illness. Clinical cases shown insufficient to differentiate RSV from other acute respiratory infections (Wildenbeest et al., 2024). The clinical presentation of RSV range from a mild cold to a serious respiratory illness with complications compare to those caused by influenza and other respiratory viruses (Nguyen-Van-Tam et al., 2022). RSV symptoms include rhinorrhea, sore throat, nasal congestion, wheezing, headache, fever, cough, breath hard, and sputum production. The most commonly reported RSV signs and symptoms in high-risk and immunocompromised adult patients were primarily lower respiratory tract infection (LRTI) manifestations (Colosia et al., 2023). For a certain number of people who are at risk of more severe respiratory disease, RSV infection might cause pneumonia or even death.
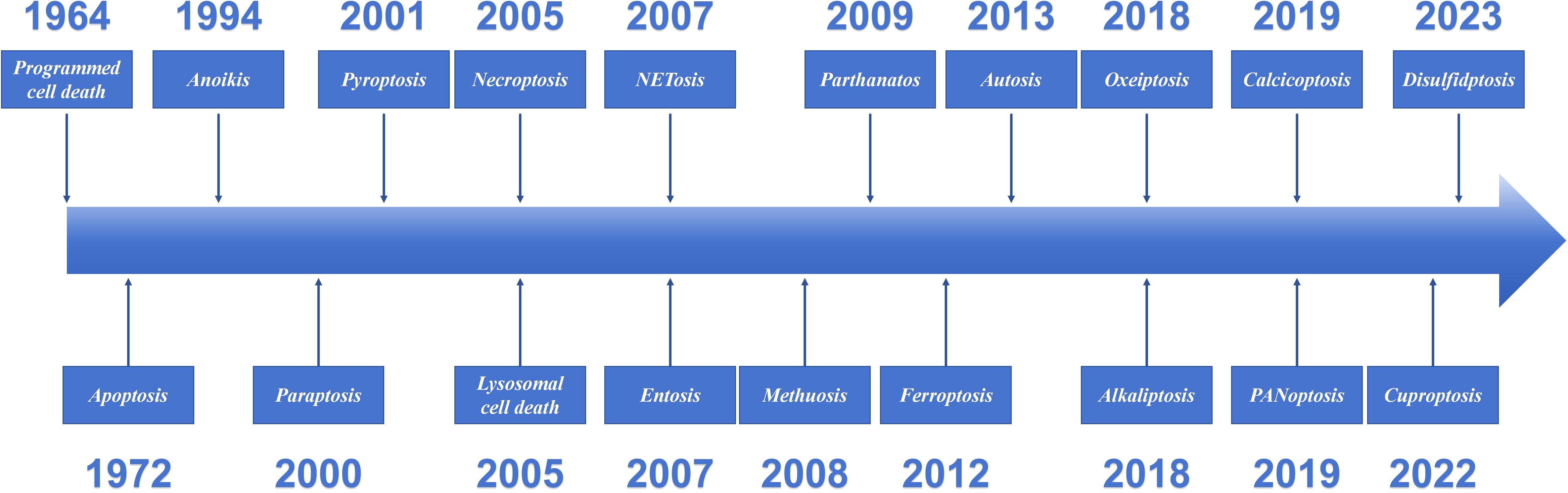
3 The 60-years of programmed cell death
Cell death is a fundamental physiological process in all living organisms. Since 1960s and 1970s, systematic studies of cell death have begun, making it one of the most rapidly developing areas in biomedicine (Kopeina and Zhivotovsky, 2022). PCD has existed since the earliest stages of cellular evolution. An adult is estimated to lose approximately 1011 cells per day through PCD (Newton et al., 2024). However, the phenomenon was not formally summarized until 1964, when R. Lockshin and C. Williams coined the term “Programmed cell death” (Lockshin and Williams, 1964). Over 60 years after its discovery, PCD has emerged as a cornerstone of biomedical research. The significance of apoptosis in the life sciences has been established. In fact, its role and potential value have not yet been fully elucidated. In 2002, The Nobel Prize in Physiology or Medicine was awarded jointly to Sydney Brenner, H. Robert Horvitz and John E. Sulston “for their discoveries concerning genetic regulation of organ development and programmed cell death”. PCD is a cell-initiated, gene-regulated death process, and the discovery and definition of its different forms have gradually become clearer with the progress of research. This review introduced the definitions and characteristics of the main types of PCD identified to date (Figure 2).
3.1 Apoptosis
The discovery of apoptosis was a fundamental hallmark in the study of cell death and expanded our understanding of various types of cell death (Park et al., 2023). In 1972, John F. Kerr, Andrew H. Wyllie and A. R. Currie coined the term “apoptosis” to differentiate naturally occurring developmental cell death from the necrosis that results from acute tissue injury (Kerr et al., 1972). Caspase family proteases have long been regarded as the “executioners” of cell death, initiating an irreversible apoptotic cascade upon activation, akin to a domino effect (Li et al., 2025). Apoptosis is carried out by intracellular caspase-3 and caspase-7, which cleave diverse intracellular substrates, leading to cell shrinkage, chromatin fragmentation, membrane blebbing, and breakdown into membrane-wrapped vesicles called apoptotic bodies, which is also a morphological characteristic of apoptotic cells (Ai et al., 2024). Apoptosis is a complex interplay of two main signaling pathways: the intrinsic (mitochondrial) and the extrinsic (death receptor). The extrinsic signaling through death receptors that leads to the formation of the death-inducing signaling complex (DISC), and intrinsic signaling mainly through mitochondria leads to the formation of the apoptosome. Formation of the DISC or apoptosome, respectively, activates initiator and common effector caspases that execute the apoptosis process. The key genes and pathways involved in apoptosis have been increasingly clarified after more than 50 years. The value of apoptosis in explaining the pathological mechanisms of various diseases has been further explored, supporting the development of new therapeutic strategies. Indeed, apoptosis has revolutionized our understanding of cell death and its importance in various physiological and pathological conditions (Capela E Silva and Rodrigues, 2023).
3.2 Anoikis
Anoikis was coined by Frisch and Francis in 1994 (Frisch and Francis, 1994). The term was borrowed from the Greek words meaning “the state of being without a home”. Anoikis, also known as matrix detachment-induced apoptosis or detachment-induced cell death, is directly induced by a disruption in cell-cell attachments or cell-extracellular matrix (ECM) attachments (Wang et al., 2024). The disruption of cytosolic connectors leads to intracellular cytoskeletal and signaling pathway alterations, ultimately activating caspases and initiating PCD, which is an outside-in process that mediated by a caspase cascade reaction (Dai et al., 2023). Anoikis is a distinct form of apoptosis that cannot be simply categorized as traditional apoptosis due to its unique initiation process (Mei et al., 2024). Anoikis maintains tissue homeostasis by eliminating cells that are detached or misplaced due to physiological or pathological conditions.
3.3 Paraptosis
Paraptosis is a distinct form of PCD characterized by massive cytoplasmic vacuolation involving the dilation of endoplasmic reticulum (ER) and/or mitochondria. Paraptosis is caspase-independent and lacks the typical morphological changes of apoptosis (Li et al., 2022). Membrane blebbing, chromatin condensation, and nuclear fragmentation—characteristic morphological hallmarks of apoptosis—are absent in the process of paraptosis. It is regulated by several signaling pathways, for instance, those associated with ER stress, calcium overload, oxidative stress, and specific cascades. Paraptosis is a distinct form of non-apoptotic cell death gaining attention for its potential role in cancer therapy and resistance (Kunst et al., 2024). As a novel type of PCD, paraptosis was first identified by Sperandio in 2000 (Sperandio et al., 2000). Despite its extensive and long research history in pathophysiology, the incidence of Paraptosis is likely underestimated due to insufficient understanding of its biochemical mechanisms and a lack of specific biomarkers.
3.4 Pyroptosis
Pyroptosis is a lytic and inflammatory type of PCD that is triggered by inflammasomes and executed by gasdermin proteins. Pyroptosis is characterized by the activation of inflammatory caspases (mainly caspase-1, 4, 5, 11) and cleavage of various members of the Gasdermin family to form membrane perforation components (Wei et al., 2022). Cell swelling, membrane perforation, and release of cellular contents are its major cell morphological changes. In fact, pyroptosis in infected macrophages was discovered in early 1992 but mistakenly classified as apoptosis (Liu et al., 2024). However, the term “pyroptosis” was first proposed by Cookson and Brennan in 2001 to describe this PCD type. Pyroptosis from the Greek roots pyro, relating to fire or fever, and ptosis (to-sis) to denote a falling, to describe pro-inflammatory programmed cell death (Cookson and Brennan, 2001). In terms of physiology, pyroptosis plays a critical role in host defense against pathogen infection (Rao et al., 2022). As the sole form of PCD that intimately couples cell death with an intense inflammatory response, pyroptosis possesses unique value.
3.5 Lysosomal cell death
Lysosomal cell death (LCD) is a form of PCD triggered by increased lysosomal membrane permeabilization (LMP), leading to the cytoplasmic release of lysosomal enzymes and subsequent execution of the cell death cascade. In 1955, Christian de Duve discovered lysosomes—a breakthrough that inaugurated a new era in cellular physiology and pathophysiology. He was awarded the Nobel Prize in Physiology or Medicine in 1974 for his discovery and characterization of lysosomes as cellular ‘recycling bins’. The concept of LCD was first proposed by Christian de Duve (Aits and Jäättelä, 2013). There is much controversy exist when the term “lysosomal cell death” was first proposed. However, it was actually first proposed in 2005, by Kroemer et al., who discussed it as an independent form of PCD (Kroemer and Jäättelä, 2005).
3.6 Necroptosis
Necroptosis, as an independent term, was first proposed by Degterev et al. in 2005 (Degterev et al., 2005). Necroptosis is a form of programmed inflammatory cell death, characterized by distinct morphological and biochemical hallmarks, including cell membrane rupture, organelle swelling, cytoplasmic and nuclear disintegration, cellular contents leakage, and release of damage-associated molecular patterns (DAMPs), accompanied by the inflammatory responses (Zhu and Wu, 2024). Necroptosis differs from apoptosis and other forms of PCD because it does not rely on caspase activity. Necroptosis is mediated by the necrosome, which consists of mixed lineage kinase domain-like protein (MLKL), receptor-interacting protein kinase 1 (RIPK1), and RIPK3 (Ye et al., 2023).
3.7 Entosis
Entosis is one of the known types of vacuole-dependent, non-apoptotic PCD (Gaptulbarova et al., 2024). In 2007, entosis was proposed by Overholtzer M (Overholtzer et al., 2007), which was derived from the Greek word that means “inside” or “within”. Entosis is a form of PCD in which one cell inserts itself into a neighboring cell, leading to the eventual death of the invading cell. This creates a characteristic cell-in-cell (CIC) pattern, a phenomenon observed as early as 1891 by Steinhaus in tumor samples (Kianfar et al., 2022). During entosis, the inner cell actively invades the host cell, a process driven by the activation of Rho proteins, the formation of adhesion bonds, and actomyosin filaments. Once the phenomenon happened, the inner cell is enclosed within a double-membraned entotic vacuole, featuring a large space between the membranes (Mlynarczuk-Bialy et al., 2020). The four possible fates in entosis are: death of the inner cell, death of the outer cell, death of both cells, or survival of both cells.
3.8 NETosis
“NETosis” is a term first introduced by Steinberg and Grinstein in 2007 to describe the process of programmed neutrophil death associated with the formation of neutrophil extracellular traps (NETs) (Steinberg and Grinstein, 2007). NETosis is characterized by neutrophil cell death accompanied by the release of NETs, auto-inflammatory factors, and antigens. NETs are extracellular web-like structures composed of decondensed DNA decorated with cytosolic and granule proteins. Since it was initially shown that NET formation is accompanied by cell death, this process was termed NETosis (Vorobjeva and Chernyak, 2020). As the most typical and predominant form of cell death in neutrophils, NETosis is the only type of PCD that fights pathogens by releasing NETs.
3.9 Methuosis
Methuosis is one of the most recent additions to the list of nonapoptotic cell death phenotypes. The most prominent attribute in cells undergoing this form of death is the accumulation of large fluid-filled cytoplasmic vacuoles that originate from macropinosomes (Maltese and Overmeyer, 2014). Methuosis is a process that lacks the classic morphological features of apoptosis. In 2008, Overmeyer et al. discovered that treatment of human glioblastoma cells with a small molecule called MIPP ([3-(2-methyl-1H-indol-3-yl)-1H-pyrrolo[3,2-b]pyridine]) induced a unique type of cell death, coined the term “methuosis” (from the Greek word methuo, which means to drink to intoxication) (Overmeyer et al., 2008). Methuosis, as a novel, protease-independent form of cell death that expands the known diversity of cell death networks.
3.10 Parthanatos
Parthanatos, also known as PARP-1 dependent cell death, named in 2009 by Ted Dawson and Valina Dawson, who defined the term “Parthanatos” after Thanatos, the personification of death in Greek mythology (David, 2009). Its core mechanism involves hyperactivation of poly (ADP-ribose) polymerase-1 (PARP1). The excessive consumption of NAD+ and ATP subsequently leads to cellular energy crisis. Concurrently, the accumulation of poly (ADP-ribose) (PAR) polymers facilitates the nuclear translocation of apoptosis-inducing factor (AIF), culminating in massive DNA fragmentation and cell demise (Fang et al., 2025). Therefore, the main characteristics of Parthanatos are considered to be PARP1 hyperactivation, PAR polymer accumulation, mitochondrial depolarization, and AIF nuclear translocation, distinct from other cell death pathways, Parthanatos is caspase-independent and does not require Bax or apoptotic activating factor-1 (Apaf-1) (Yang et al., 2024).
3.11 Ferroptosis
Ferroptosis is caused by the accumulation of iron and lipid peroxidation, it was first observed in cells treated with erastin, a small-molecule inducer discovered by Dolma et al. in 2003 (Dolma et al., 2003). Ferroptotic cells lack of classic apoptosis features like chromatin condensation, apoptotic bodies, autophagosome formation, or nuclear structural changes. However, mitochondria exhibit unique characteristics, including reduced volume, loss of structural integrity, increased membrane density, outer membrane rupture, and decreased or absent cristae (Shen et al., 2025). The concept of this distinct form of regulated, iron-dependent cell death driven by lipid peroxidation, along with the term “Ferroptosis” was later introduced by Dixon et al. in 2012 (Dixon et al., 2012).
3.12 Autosis
In 2013, Liu and Levine identified a novel form of autophagy-induced cell death, termed “autosis”. They found that the selective overactivation of autophagy results in cell death with unique morphological features distinct from those of apoptosis or necrosis (Liu et al., 2013). Before this concept was proposed, the relationship between autophagy and PCD was often considered to be contradictory. Autotic cells are characterized by a dramatic initial increase in cytoplasmic autophagic and empty vesicles, followed by later-stage changes including electron-dense mitochondria, ballooning of the perinuclear space due to the separation of nuclear membranes, and concavity of the nucleus (Schwartz, 2021). Autosis is uniquely characterized by its direct dependence on the overactivation and execution of the autophagic process.
3.13 Alkaliptosis
Acid-base homeostasis is essential for normal physiology, cellular metabolism, and function. Alkaliptosis is defined by the lethal rise in intracellular pH, regulated by the interplay of ion channels and transporters in intracellular and extracellular pathways. Compared to other forms of RCD, the induction of alkaliptosis does not involve the commonly recognized cell death effectors, such as caspases and MLKL (Chen et al., 2023). As a type of pH-dependent non-apoptotic PCD, alkaliptosis was first identified by Tang et al. in 2018 in pancreatic ductal adenocarcinoma (PDAC) cells (Song et al., 2018).
3.14 Oxeiptosis
Oxeiptosis is a novel caspase-independent PCD pathway with apoptotic-like features, first proposed as an academic concept by Holze et al. in 2018 (Holze et al., 2018). Reactive oxygen species (ROS) play essential roles in all forms of cell death. ROS-induced cell death includes apoptosis, necroptosis, ferroptosis and autosis (Villalpando-Rodriguez and Gibson, 2021). However, Oxeiptosis operates in a non- inflammatorily, independently of caspases, and is induced by ROS or ROS-generating. Distinguished from other cell death pathways, oxeiptosis shown a unique damage-causing factors pivotal genes, and Kelch-like ECH-associated protein-1 (KEAP1)/Phosphoglycerate mutase family member 5 (PGAM5)/apoptosis-inducing factor mitochondrial associated 1(AIFM1) signaling pathways (Chen et al., 2024). Pathologically high ROS cause oxidative stress, which damages macromolecules (proteins, lipids, DNA) and cellular organelles. This ultimately compromises cellular integrity and leads to apoptosis-like cell death.
3.15 PANoptosis
PANoptosis is a unique inflammatory cell death, representing the convergence of apoptosis, pyroptosis, and necroptosis, and plays a crucial role in regulating cell death and immune responses (Gao et al., 2024). Pyroptosis, apoptosis, and necroptosis, three previously considered separate and independent PCD pathways, actually exhibit mechanistic overlap and extensive, multifaceted crosstalk. Crosstalk among pyroptosis, apoptosis, and necroptosis was systematically reported as early as 2016 (Kuriakose et al., 2016). However, the concept of “PANoptosis” was not proposed until 2019 by R K Subbarao Malireddi et al (Malireddi et al., 2019). The emergence of the concept of PANoptosis has provided a novel perspective for cell death research, drawing increasing attention to the crosstalk mechanisms underlying PCD.
3.16 Calcicoptosis
Disordered cytosolic calcium [Ca2+](c) signaling plays a critical role in cell death (Bhosale et al., 2015). The concept of calcicoptosis as a new type of cell death was recently proposed by Zhang et al. in 2019 (Zhang et al., 2019). The calcicoptosis is a novel form of cell death characterized by calcium-induced damage and oxidative stress, triggered by Ca2+ over-loading that leads to mitochondrial dysfunction with excess ROS generation. Under oxidative stress, abnormal function of cellular calcium channels impairs Ca2+ concentration modulation, which subsequently causes cell membrane mineralization by Ca3(PO4)2 precipitation (Guo et al., 2022). Calcicoptosis is proposed as a distinct form of PCD, different from other cell types, suggesting cell death induced by calcium.
3.17 Cuproptosis
Cuproptosis is a recently discovered form of mitochondria-related cell death triggered by excess Cu2+. In 2022, Tsvetkov et al. published a landmark study in Science, systematically unraveling and naming its molecular mechanism (Tsvetkov et al., 2022). Cuproptosis, a metal-dependent pathway of cell death, is triggered by excessive copper exposure and subsequent proteotoxic stress. The mechanism involves intracellular copper targeting and binding to lipoylated components in the tricarboxylic acid (TCA) cycle. Aggregation of these copper-bound lipoylated mitochondrial proteins, along with the subsequent reduction in Fe–S (iron–sulfur) clusters, ultimately induces proteotoxic stress and cuproptosis (Chen et al., 2022).
3.18 Disulfidptosis
Disulfidptosis, as a new form of PCD triggered by disulfide stress, is characterized by the collapse of cytoskeleton proteins and F-actin due to the intracellular accumulation of disulfides (Zheng et al., 2023). J Chen and B Gan found that the aberrant accumulation of intracellular disulfides in Solute carrier family 7 member 11 (SLC7A11) - cells under high glucose starvation induces a previously uncharacterized type of cell death, which they term “Disulfidptosis” in 2023 (Liu et al., 2023).
For example, “zinc-induced cell death” currently lacks a formally designated independent cell death mode or academic term within the mainstream classification system (Vitale et al., 2023; Galluzzi et al., 2018) published by the Nomenclature Committee on Cell Death (NCCD). As for now, the term “zinc death” has not been formally proposed in a single paper. Rather, it is a literal translation or simplified expression used by researchers to describe “zinc-induced cell death.” It has not yet been formally recognized as a distinct cell death mode, unlike ferroptosis, necroptosis, and cuproptosis. The expanding landscape of PCD, with its diverse molecular mechanisms and profound implications for disease outcomes, offers a critical theoretical and research framework for understanding how viral infections precisely orchestrate cell death pathways to control viral replication and associated tissue damage.
4 Programmed cell death in RSV infection
In response to viral infection, many cells undergo apoptosis, resulting in a decrease in the release of progeny virus (Rex et al., 2022). Viruses adapt to changes in cell death through various strategies and exploit cell death pathways to facilitate infection and associated tissue damage. The interaction between the virus and cell death is a dynamic interplay between “host clearance” and “viral escape”. Data on RSV and PCD were sourced from key databases, including Elsevier, PubMed, Springer, Google Scholar and Web of Science, covering the period from the inception of the relevant databases to September 2025. Apoptosis, necroptosis, pyroptosis, NETosis and ferroptosis have all been reported in RSV infection (Figure 3). This review summarizes current understanding of how RSV regulates PCD and its target cells (Table 1).
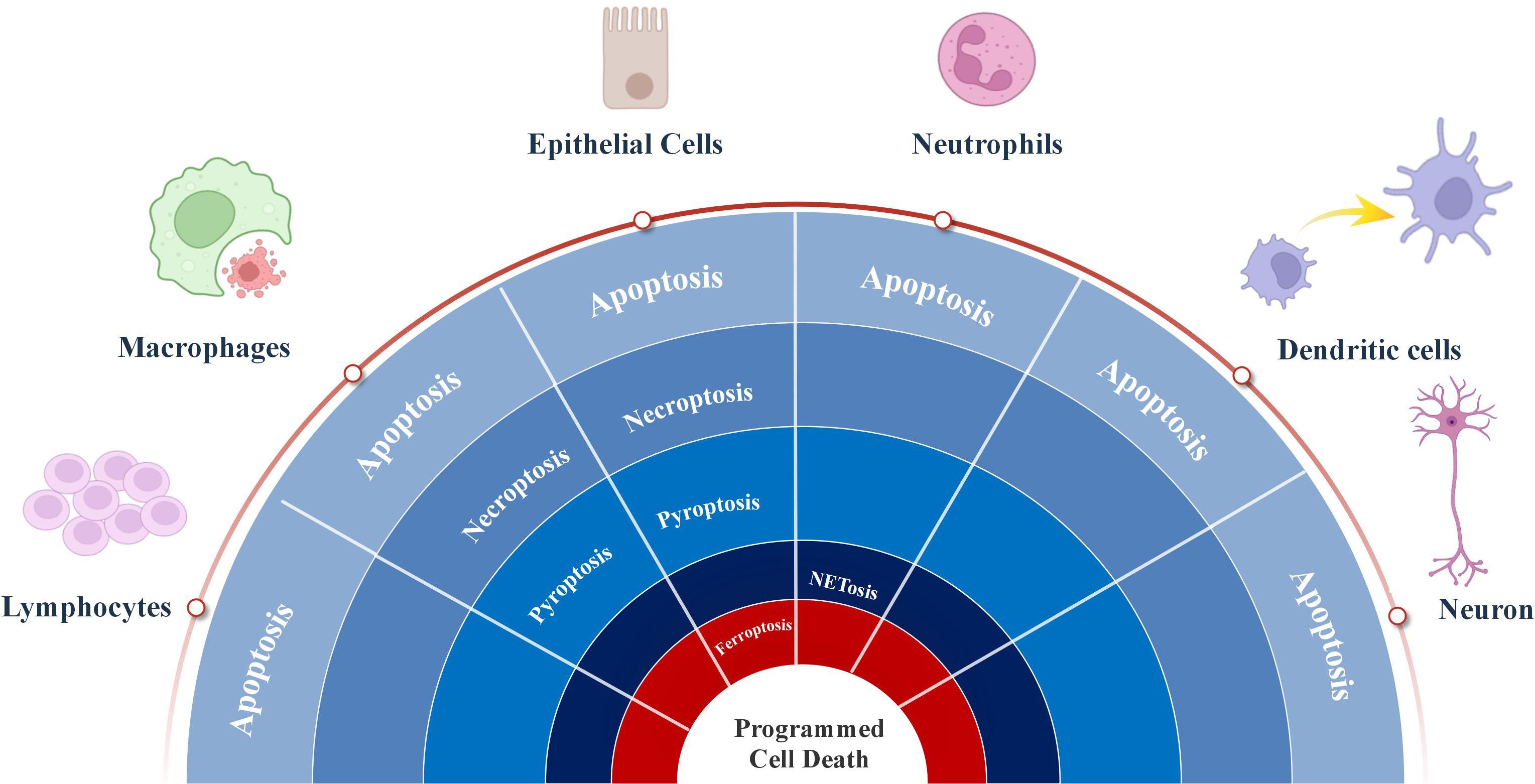
Figure 3. The roles of process of programmed cell death regulated by respiratory syncytial virus in multiple types cells.
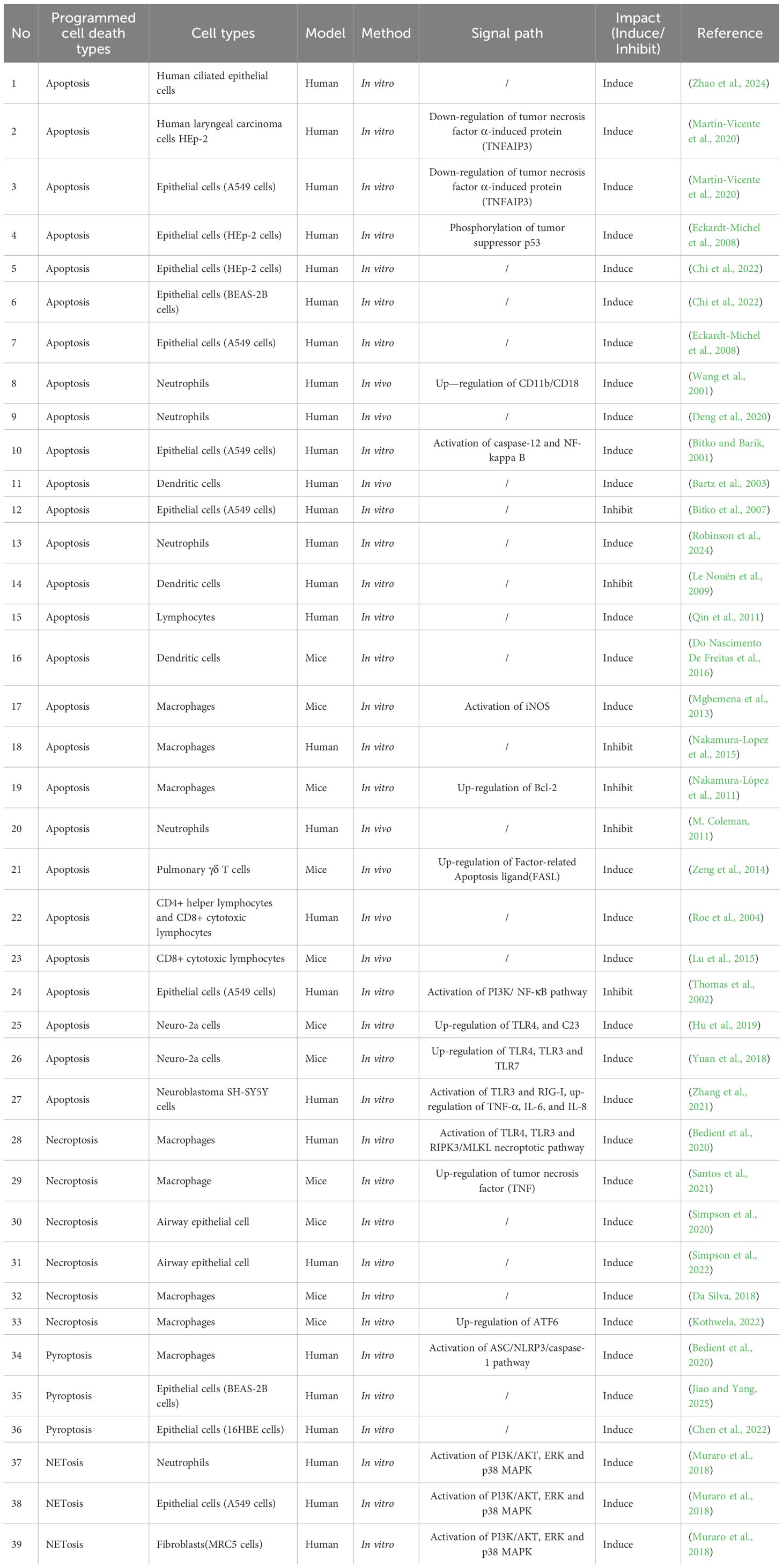
Table 1. Respiratory syncytial virus regulates programmed cell death: forms, target cells, and mechanisms.
4.1 Apoptosis
Apoptosis is the most prevalent form of cell death in RSV infection. It was also the first type of PCD to be documented, both in general and specifically within the context of RSV research. Apoptosis is the key mechanism in RSV pathogenesis with its ability to subvert the apoptotic pathway in host cells. RSV infects and alters the apoptotic fate of a wide range of cells, from structural epithelial cells to defensive immune cells like macrophages and T-cells. This targeted manipulation of cell death is a cornerstone of RSV infection and propagation.
4.1.1 Epithelial cells
Epithelial cells are primarily perceived as a physical barrier and mediator of mucociliary clearance. Apart from its physical barrier function, the epithelial cells play a central role in innate and adaptive immune responses (Dorscheid et al., 2025). As early as before 2000, a large number of studies had confirmed the complex connection between RSV infection of respiratory epithelial cells and apoptosis (O’Donnell et al., 1999). Airway epithelial cells are the main target cells of RSV (Glaser et al., 2019). Apoptosis of lung epithelial cells serves as a major pathogenic event in the pathogenesis of RSV-induced lung injury. Furthermore, this contributes to the loss of the alveolar capillary barrier, thereby increasing lung permeability and resulting in lung edema (Van Den Berg et al., 2015).
A549 cells, a tumor cell line with properties of normal airway epithelial cells, are a widely recognized in vitro cell model for RSV infection. This model shows (Rajan et al., 2022). Nuclear factor kappa-B (NF-κB) has the ability to directly modulate both pro-survival and apoptotic responses (Sherekar et al., 2023). It is also a downstream component of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B(PKB/AKT)pathway. The prevention of apoptosis in airway epithelial cells may serve to preserve host cell integrity until the virus’s replication phase is completed. RSV inhibits A549 apoptosis by activates NF-κB through a PI3K-dependent pathway (Thomas et al., 2002). However, the necrosis and destruction of ciliated epithelial cells is one of the characteristics of RSV bronchiolitis (Guo-Parke et al., 2013). The apoptosis of RSV-infected ciliated cells promotes virus spread and the ongoing process of apoptotic in extruded ciliated cells provides a time window for these cells to spread RSV (Zhao et al., 2024). Both inhibition and promotion of epithelial cell apoptosis occur, but are differentially phase: inhibition of apoptosis in the early stages and promotion of apoptosis in the later stages. The flow cytometry expression showed that RSV infection did not induce apoptosis in A549 cells at 24 h postinfection (Singh et al., 2007). More experiments are needed to confirm the precise timing of cell apoptosis, and it seems unlikely that in vitro studies alone can fully address the need.
As an epithelial cell line, HEp-2 cell line was obtained in 1951 from a patient presenting with a laryngeal carcinoma (Gorphe, 2019). The BEAS-2B cell line is a widely used immortalized but non-tumorigenic human cell line established from normal human bronchial epithelium obtained from a non-cancerous individual by Curtis C. Harris’ group in 1988 (Han et al., 2020). RSV infection leads to an increased inflammatory symptoms and apoptosis in the HEp-2 cells and BEAS-2B cells through mechanisms involving ROS generation (Chi et al., 2022). RSV-induced overproduction of ROS is the central mechanism that activates the epidermal growth factor receptor (EGFR) pathway, and promotes the release of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, IL-18).
4.1.2 Immune cells
Macrophages play a key role in the early innate immune and inflammatory responses to viral pathogens and act as the main cells that participate in RSV infection. Macrophages are polarized during RSV infection, forming two macrophage phenotypes termed as M1-like and M2-like macrophages, involved in the modulation of inflammatory responses (Churiso et al., 2022). The inducible nitric oxide synthase(iNOS) expression/activity is required for optimal apoptosis during RSV infection, and RSV induces macrophage apoptosis via activating iNOS (Mgbemena et al., 2013). The apoptosis involves caspase-dependent and caspase-independent pathways and occurs via intrinsic and extrinsic pathways. Although viral genome encodes 11 viral proteins, RSV phosphor-protein (P) shares a 52% homology with the caspase-8 death domain. RSV P-protein impairs extrinsic apoptosis pathway in a macrophage-like cell line persistently infected with RSV (Nakamura-Lopez et al., 2015). RSV suppresses macrophage apoptosis through Bcl-2 upregulation and manipulation of the intrinsic pathway, mediated by enhanced mitochondrial respiration and the sequestration of pro-apoptotic proteins to stabilize the mitochondrial membrane (Nakamura-López et al., 2011).
Neutrophils are the predominant airway leukocytes in RSV infections and their products are likely to play an important role in viral infection (Wang et al., 2001). In fact, neutrophil apoptosis has already occurred in the early stages of RSV infection. At 72 hours, post-RSV infection, neutrophil exposure led to an increase in the numbers of apoptotic neutrophils in vivo (Deng et al., 2020). The apoptosis of neutrophils in RSV infection showed age-related differences, greater numbers of infant neutrophils became apoptotic when compared with adult (Robinson et al., 2024).
Dendritic cells (DCs) are a type of professional antigen-presenting cell. Immature DCs have strong migratory capacity; mature DCs can effectively activate naive T cells and initiate the body’s adaptive immune response in a timely and appropriate manner. RSV induced increased apoptosis in immature DCs. It is conceivable that early apoptosis of RSV-infected DCs could prevent efficient T-cell activation and could account for suppression of cell-mediated immunity (Bartz et al., 2003). RSV can have a negative influence on DCs, causing their apoptosis, which prevents active presentation of foreign antigens and T cell activation. Apoptosis also occurs in mature DCs, and RSV-infected bone marrow dendritic cells (BMDCs) show increased apoptotic cell death in mature DCs (Do Nascimento De Freitas et al., 2016).
Lymphocytes are cells with immune recognition function. RSV can infect human lymphocytes during the immune response to viral challenge (Roberts and O’Banion, 2025). RSV infection induces significant lymphocyte proliferation and accelerated apoptosis (Qin et al., 2011). CD4+ helper lymphocytes and CD8+ cytotoxic lymphocytes are key players in the immune response against viral infection. In RSV infection, both CD4+ and CD8+ lymphocytes are primed to undergo apoptosis (Roe et al., 2004; Lu et al., 2015). γδ T cells develop in the thymus from progenitor T cells originating from bone marrow hematopoietic stem cells (Hu et al., 2023). In an Ovalbumin (OVA)-sensitized murine model, RSV infection influences FasL-mediated apoptosis of pulmonary γδ T cells. This effect partly suppresses the subsequent development of OVA-induced allergic responses (Zeng et al., 2014).
4.1.3 Other cells
Growing evidence links RSV infection to neurological manifestations, such as febrile seizures, encephalopathy, and less frequently, encephalitis or meningoencephalitis (Stravoravdi et al., 2025). Neuro-2a(N2a) cells, a rapidly growing mouse neuroblastoma cell line, were derived from a spontaneous tumor in an albino strain A mouse. RSV prolifically entered and infected N2a neuronal cells, leading to the modulated expression of Toll-like receptor 4 (TLR4) and C23, as well as of TLR3, TLR7, and their downstream inflammatory factors, suggesting a direct induction of RSV-associated encephalopathy in infants by the infection of neuronal cells (Yuan et al., 2018; Hu et al., 2019). The SH-SY5Y human neuroblastoma cell line, like the N2a cell line, allows the study of the nervous system-related diseases. RSV infection of microglia induces SY5Y neuronal injury and apoptosis via the release of inflammatory cytokines (TNF-α, IL-6, and IL-8) and the upregulation of TLR3 and RIG-I expression (Zhang et al., 2021).
4.2 Necroptosis
Necroptosis has been implicated as a critical cell death pathway in virus-infected cells (Cotsmire et al., 2021). RSV has been shown to trigger necroptosis in an infected lungs. Necroptosis blockade may represent a therapeutic strategy for a range of lung pathologies with viral etiologies (Gautam et al., 2024).
RSV infection is a complicated process, several cell types are implicated in the disease progression. As the primary barrier against respiratory pathogens, the airway epithelium can be bypassed by viruses via various means, with their most straightforward approach being the direct infection of epithelial cells. RSV infection readily induces necroptosis in RSV-infected airway epithelial cells. This is detrimental to viral clearance and accentuates immunopathology and the ensuing propensity to develop asthma (Harker and Snelgrove, 2020). RSV infection of airway epithelial cells induces phosphorylation of necroptosis proteins prior to the induction of cell death. RSV infection induced an “early” (6 hpi) and “late” (24 hpi) phosphorylation of receptor-interacting protein kinase 3 (RIPK1)/receptor-interacting protein kinase 3 (RIPK3)/mixed-lineage kinase domain-like (MLKL). However, necroptotic cell death was restricted to the late phase (Simpson et al., 2022). Inhibition of necroptosis may be a viable strategy to limit the severity of viral bronchiolitis and break its nexus with asthma (Simpson et al., 2020).
Macrophages may exacerbate the severity of RSV-induced disease, and necroptosis is closely associated with this process. One of the key factors driving exaggerated lung inflammation during RSV infection is the necroptosis of RSV-infected immune cells, particularly macrophages. The RIPK1-dependent necrotic cell death was termed “Necroptosis”. Despite morphological similarities, it is distinct from necrosis, which is an accidental cell death caused by irreversible cellular injury. RIPK3 and MLKL are the key downstream molecules of RIPK1, with MLKL serving as the ultimate executor of necroptosis (Makuch et al., 2024). During RSV infection of macrophages, viral replication promotes tumor necrosis factor (TNF) production and secretion; TNF then binds to TNF receptor 1 (TNFR1), triggering RIPK1/RIPK3 phosphorylation, subsequent MLKL activation, and ultimately leading to macrophage necroptosis (Santos et al., 2021). Previous studies have confirmed that macrophages undergo necroptosis during RSV infection, a process that depends on RIPK3 and MLKL (Da Silva, 2018; Bedient et al., 2020). RIPK3 forms a complex called the necrosome, which then recruits and phosphorylates MLKL. This triggers MLKL oligomerization, enabling the formation of pore-like structures in the plasma membrane that execute necroptosis (Shi et al., 2023). Persistent or aberrant ER stress induces cell injury (e.g., apoptosis, necroptosis), whereas activating transcription factor 6 (ATF6)—acting as a transcription factor—induces ER chaperone proteins (Li et al., 2023). Notably, the ATF6-dependent ER stress pathway plays a positive role in RSV-mediated necroptosis of macrophages, and this effect is exerted via the induction and activation of ATF6 protein in RSV-infected macrophages (Kothwela, 2022).
4.3 Pyroptosis
Pyroptosis plays a pivotal role in eradicating intracellular pathogen replication niches and modulating the immune system via the release of danger signals. Pyroptosis is an important innate immune response that is critical in fighting infections (Wei et al., 2022). Viruses precisely activate or inhibit host pyroptotic pathways, thereby evading immune surveillance, promoting dissemination, and establishing persistent infections (Zhang et al., 2025). Previous studies confirm that RSV infection induces severe inflammation and lung tissue damage via pyroptosis. The Nod-like receptor protein containing pyrin 3(NLRP3) inflammasome-induced pyroptosis pathway contributes to enhanced lung immunopathology during RSV infection and promotes the development of RSV-associated asthma (Lin and Porto, 2025). The NLRP3 inflammasome leads to caspase-1 activation and the cleavage of different cellular proteins, including pro-interleukin (IL)-1β and pro-IL-18, as well as gasdermin D (GSDMD). The resulting GSDMD N-terminal fragment oligomerizes in the plasma membrane, forming GSDMD pores, inducing large ruptures in the membrane, thereby leading to pyroptosis (Schachter et al., 2025).
Epithelial cells are structural cells that maintain the stability of lung function and serve as an important battlefield for RSV infection. The human bronchial epithelial cell line, 16HBE14o- (16HBE), is widely used as a model for respiratory epithelial diseases and barrier function (Callaghan et al., 2020). RSV can induce 16HBE cell pyroptosis, thereby promoting viral replication and spread (Chen et al., 2022). BEAS-2B cells are immortalized cells derived from normal human bronchial epithelial cell. Electron microscopy was used to assess morphology changes associated with RSV-induced pyroptosis in BEAS-2B cells, including mitochondrial swelling and nuclear disruption. RSV infection elevates the expression of pyroptosis markers, such as NLRP3, apoptosis-associated speck-like protein(ASC), and cleaved caspase-1 (Jiao and Yang, 2025). Macrophage pyroptosis plays a critical role in virus infected pathophysiology. The caspase-1 dependent pyroptosis pathway, specifically the ASC/NLRP3/caspase-1 axis, serves as a key mechanism promoting lytic cell death in RSV-infected macrophages, while infection-generated ROS positively regulate this lytic cell death (Bedient et al., 2020).
4.4 others
NETosis is a special form of PCD with remarkable specificity. Neutrophils are one of the host’s first defensive measures against viral infection. Neutrophils employ three major defensive mechanisms, including phagocytosis, degranulation and NETosis (Sebina and Phipps, 2020). NETs can capture and kill RSV, contributing to host defense. Extensive neutrophil accumulation in the lungs is a distinct feature of severe RSV-LRTD, and these activated neutrophils release NETs (Cortjens et al., 2016). Neutrophils recognize the fusion protein of RSV via TLR4, subsequently releasing NETs (Hong et al., 2022). ROS are essential for NET formation or NETosis, excess ROS produced during neutrophil activation induces NETosis by inducing extensive DNA damage, and the subsequent DNA repair pathway, leading to chromatin decondensation. RSV can induce classic ROS-dependent NETosis in neutrophils (Muraro et al., 2018).
Numerous studies have demonstrated that ferroptosis plays a significant regulatory role in various respiratory infectious diseases (Hong et al., 2025). RSV-infected mice exhibit increased secretion of the pro-inflammatory chemokines CCL5 and CCL3, elevated mitochondrial iron content, and upregulated expression of 12/15-lipoxygenase (12/15-LOX, which catalyzes the deoxygenation of polyunsaturated fatty acids [PUFAs]). All these changes correlate with increased activation of the 12/15-LOX signaling pathway (Salimi et al., 2017). The cellular damage observed following RSV infection is mediated by the activation of ferroptosis, and these findings indicate that RSV acts as a trigger for ferroptosis (Kombe Kombe et al., 2024).
5 Discussion
5.1 Current status and research gaps in RSV-regulated PCD
PCD is considered a key player in a variety of cellular processes that help to regulate tissue growth, embryogenesis, cell turnover, immune response, and other biological processes (Kari et al., 2022). Although the forms of cell death are becoming increasingly diverse and studied, existing evidence suggests that RSV-induced PCD consists of only 5 forms: apoptosis, necroptosis, pyroptosis, NETosis, and ferroptosis. Given the diversity of cell death forms, it is reasonable to assume that many viral strategies to promote/inhibit cell death remain unexplored. Compared with the related research on other viral infections and PCD, there are still many gaps in the research on RSV and PCD. For example, like RSV, Severe Acute Respiratory Syndrome Coronavirus 2(SARS-CoV-2) coronavirus infection also modulates multiple PCDs, such as necroptosis, apoptosis, pyroptosis (Liang et al., 2024), NETosis (Zhu et al., 2022), ferroptosis (Wang et al., 2022). However, it can also regulate lysosomal cell death (Sun et al., 2022), PANoptosis (Yang et al., 2025) and cuproptosis (Wang et al., 2024). These PCD forms have not been reported in RSV studies. Related research on other viruses regulating host cell death can also provide new research ideas and references for RSV intervention in cell death. As a virus with a long history, the complex relationship between RSV and cell death can also provide reference for emerging viruses.
5.2 RSV viral proteins induce and inhibit PCD
Induction or inhibition are two distinct relationships between RSV and PCD, their dynamics remain a unresolved question. Different PCD forms, cell types, and stages of infection will lead to different RSV induction/inhibition interventions. However, the relatively stable structure of RSV seems to provide some theoretical basis for this issue. For example, the inhibition/induction of apoptotic mechanism of RSV seems to be related to viral proteins. The RSV genome encodes 11 viral proteins, including viral membrane glycoproteins (G), fusion proteins (F), and small hydrophobic proteins (SH). The crucial function of the SH protein is the inhibition of apoptosis through the suppression of TNF-alpha signaling, leading to the attenuation of caspase-8 activity (Okura et al., 2024). Furthermore, the RSV P-protein impairs the extrinsic apoptosis pathway in a macrophage-like cell line persistently infected with RSV (Nakamura-Lopez et al., 2015). The nonstructural NS proteins of RSV suppress premature apoptosis of the infected cell by a mechanism that is independent of suppression of interferon pathway by these proteins (Bitko et al., 2007). F protein expression can induce caspase-dependent cell death, suggesting its role as a potent inducer of apoptosis in RSV-infected cells (Faghirabadi et al., 2024). However, the binding forms and dominance of these proteins across different PCDs and different cells types remain largely unknown.
5.3 Crosstalk between cell death pathways in RSV infection
Studies over the years have revealed the distinctive molecular mechanisms and functional consequences of several key cell death pathways in RSV infection. Whether for host cells or viruses, PCD is a double-edged sword, playing the roles of both “protector” and “executioner”. Crosstalk is an unavoidable phenomenon in cell death research. Multiple cell death pathways, including apoptosis, necroptosis, pyroptosis, ferroptosis, and autosis, does not function in isolation but rather form a highly interconnected regulatory network. They frequently share the same signaling molecules, upstream triggers, and downstream effectors. RSV infection is a clear example; for example, RSV can induce the classical ROS-dependent NETosis through necroptosis pathways activation (Muraro et al., 2018). The crosstalk mechanisms between different forms of cell death, such as apoptosis and necroptosis (Rojas-Rivera et al., 2024), necroptosis and pyroptosis (Frank and Vince, 2019), and apoptosis and ferroptosis (Sun et al., 2021), have been reported. However, similar studies are rarely reported in RSV infection. The specific molecular interactions and crosstalk relationships among these PCDs in RSV infection are still relatively limited, and a systematic understanding has not yet been formed.
5.4 Different cell-specific PCDs in RSV infection
RSV induces distinct PCD patterns across various cell types, reflecting cell-specific functions in host defense and viral pathogenesis. Epithelial cells, the primary targets of RSV, exhibit stage-dependent apoptosis: early infection inhibits apoptosis to support viral replication, whereas late infection promotes apoptosis for viral spread. Macrophages show dual apoptotic regulation during the RSV infected process. NETosis is a program for formation of NETs, and both NETosis and apoptosis can occur in RSV-infected neutrophils. These differences arise from cell-specific functions and RSV’s tailored manipulation of PCD pathways. RSV primarily infects the respiratory epithelium links to neurologic sequelae with proof of previous studies (Pollard et al., 2024). PCD of neuronal cells confirms the mechanism of RSV nerve damage and provides a reference for Virus Infections in the nervous system, but the number of related studies is still insufficient.
6 Conclusion
In conclusion, the systematically review on previous studies revealed the diverse effects of RSV infection on PCD in host cells, showing on how RSV induces PCD, leads to tissue damage, inflammation, and severe disease, while suppressing PCD to evade physical and immune clearance. Although the details and mechanisms of RSV-induced PCD in different host cells remain incompletely understood, regulating PCD has been shown to be a crucial weapon in the battle between RSV and host cells. Further research is recommended to discover the detailed relationship between RSV and host cell-associated PCD and identify effective strategies to regulate dysregulated PCD, providing insights for RSV prevention and treatment.
Author contributions
PY: Writing – review & editing, Methodology, Writing – original draft. CM: Writing – review & editing. CL: Writing – review & editing, Resources, Funding acquisition.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors sincerely thank Ms. Heng Yee Jia for her valuable guidance and insightful suggestions regarding the language expression of this study. We also thank the editors and reviewers for their valuable help in refining this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviation
12/15-LOX, 12/15-Lipoxygenase; ACD, Accidental Cell Death; ATF6, Activating Transcription Factor 6; AECs, Airway Epithelial Cells; ASC, Apoptosis-Associated Speck-like Protein; BMDCs, Bone Marrow Dendritic Cells; COVID-19, Coronavirus Disease 2019; DCs, Dendritic Cells; DISC, Death-inducing signaling complex; EGFR, Epidermal growth factor receptor; ER, Endoplasmic Reticulum; iNOS, Inducible Nitric Oxide Synthase; MLKL, Mixed-Lineage Kinase Domain-Like; NETs, Neutrophil Extracellular Traps; NLRP3, Nod-like Receptor Protein 3; NF-κB, Nuclear Factor Kappa-B; OVA, Ovalbumin; PI3K, Phosphatidylinositol 3-Kinase; PUFAs, Polyunsaturated Fatty Acids; PCD, Programmed Cell Death; AKT, Protein Kinase B; ROS, Reactive Oxygen Species; RIPK, Receptor-Interacting Protein Kinase; RSV, Respiratory Syncytial Virus; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; TNFR1, TNF Receptor 1; TLR, Toll-like Receptor; TNF, Tumor Necrosis Factor.
References
Ai, Y., Meng, Y., Yan, B., Zhou, Q., and Wang, X. (2024). The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol. Cell 84, 170–179. doi: 10.1016/j.molcel.2023.11.040
Aits, S. and Jäättelä, M. (2013). Lysosomal cell death at a glance. J. Cell Sci. 126, 1905–1912. doi: 10.1242/jcs.091181
Angel, J. P. and Daniels, B. P. (2022). Paradoxical roles for programmed cell death signaling during viral infection of the central nervous system. Curr. Opin. Neurobiol. 77, 102629. doi: 10.1016/j.conb.2022.102629
Barahimi, E., Azad, M. H., Hesarooeyeh, Z. G., Hafshejani, N. H., Defaee, S., and Seddighi, N. (2023). Late diagnosis of respiratory syncytial virus and influenza co-infection during coronavirus disease 2019 pandemic: a case report. J. Med. Case Rep. 17, 437. doi: 10.1186/s13256-023-04187-3
Bartz, H., Rkel, O. T., Hoffjan, S., Rothoeft, T., Gonschorek, A., and Schauer, U. (2003). Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-c in naõÈve T cells. Immunology 109, 49–57. doi: 10.1046/j.1365-2567.2003.01629.x
Bedient, L., Pokharel, S. M., Chiok, K. R., Mohanty, I., Beach, S. S., Miura, T. A., et al. (2020). Lytic cell death mechanisms in human respiratory syncytial virus-infected macrophages: roles of pyroptosis and necroptosis. Viruses 12, 932. doi: 10.3390/v12090932
Bhosale, G., Sharpe, J. A., Sundier, S. Y., and Duchen, M. R. (2015). Calcium signaling as a mediator of cell energy demand and a trigger to cell death. Ann. New York Acad. Sci. 1350, 107–116. doi: 10.1111/nyas.12885
Bitko, V. and Barik, S. (2001). An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J. Cell. Biochem. 80, 441–454. doi: 10.1002/1097-4644(20010301)80:3%253C441::AID-JCB170%253E3.0.CO;2-C
Bitko, V., Shulyayeva, O., Mazumder, B., Musiyenko, A., Ramaswamy, M., Look, D. C., et al. (2007). Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF-κB-dependent, interferon-independent mechanism and facilitate virus growth. J. Virol. 81, 1786–1795. doi: 10.1128/JVI.01420-06
Brüssow, H. (2025). Respiratory syncytial virus: health burden, disease prevention, and treatment—recent progress and lessons learned. microLife 6, uqaf003. doi: 10.1093/femsml/uqaf003
Callaghan, P. J., Ferrick, B., Rybakovsky, E., Thomas, S., and Mullin, J. M. (2020). Epithelial barrier function properties of the 16HBE14o- human bronchial epithelial cell culture model. Bioscience Rep. 40, BSR20201532. doi: 10.1042/BSR20201532
Capela E Silva, F. and Rodrigues, C. M. P. (2023). Apoptosis—50 years after its discovery. Biomedicines 11, 1196. doi: 10.3390/biomedicines11041196
Casadevall, A., Roane, P. R., Shenk, T., and Roizman, B. (2025). The Story behind the Science: On the discovery of respiratory syncytial virus. mBio 16, e03074–e03024. doi: 10.1128/mbio.03074-24
Chen, F., Kang, R., Liu, J., and Tang, D. (2023). Mechanisms of alkaliptosis. Front. Cell Dev. Biol. 11. doi: 10.3389/fcell.2023.1213995
Chen, K.-Q., Wang, S.-Z., Lei, H.-B., and Liu, X. (2024). Mini-review: research and progress of oxeiptosis in diseases. Front. Cell Dev. Biol. 12. doi: 10.3389/fcell.2024.1428250
Chen, L., Min, J., and Wang, F. (2022). Copper homeostasis and cuproptosis in health and disease. Sig Transduct Target Ther. 7, 378. doi: 10.1038/s41392-022-01229-y
Chen, Y., Zhou, Y., Wang, Q., Chen, J., Chen, H., Xie, H., et al. (2022). Conciliatory anti-allergic decoction attenuates pyroptosis in RSV-infected asthmatic mice and lipopolysaccharide (LPS)-induced 16HBE cells by inhibiting TLR3/NLRP3/NF-κB/IRF3 signaling pathway. J. Immunol. Res. 2022, 1–16. doi: 10.1155/2022/1800401
Chi, H. and Chung, C.-H. (2023). Respiratory syncytial virus outbreak in infants and young children during COVID-19 pandemic in Taiwan. Children 10, 629. doi: 10.3390/children10040629
Chi, L., Shan, Y., and Cui, Z. (2022). N-Acetyl-L-Cysteine Protects Airway Epithelial Cells during Respiratory Syncytial Virus Infection against Mucin Synthesis, Oxidative Stress, and Inflammatory Response and Inhibits HSPA6 Expression. Analytical Cell. Pathol. 2022, 1–9. doi: 10.1155/2022/4846336
Churiso, G., Husen, G., Bulbula, D., and Abebe, L. (2022). Immunity cell responses to RSV and the role of antiviral inhibitors: A systematic review. IDR Volume 15, 7413–7430. doi: 10.2147/IDR.S387479
Colosia, A., Costello, J., McQuarrie, K., Kato, K., and Bertzos, K. (2023). Systematic literature review of the signs and symptoms of respiratory syncytial virus. Influenza Resp. Viruses 17, e13100. doi: 10.1111/irv.13100
Cookson, B. T. and Brennan, M. A. (2001). Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114. doi: 10.1016/S0966-842X(00)01944-2
Cortjens, B., De Boer, O. J., De Jong, R., Antonis, A. F., Sabogal Piñeros, Y. S., Lutter, R., et al. (2016). Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 238, 401–411. doi: 10.1002/path.4660
Cotsmire, S. M., Szczerba, M., and Jacobs, B. L. (2021). “Detecting necroptosis in virus-infected cells,” in Viruses as therapeutics, methods in molecular biology. Ed. Lucas, A. R. (Springer US, New York, NY), 199–216. doi: 10.1007/978-1-0716-1012-1_11
Dai, Y., Zhang, X., Ou, Y., Zou, L., Zhang, D., Yang, Q., et al. (2023). Anoikis resistance—-protagonists of breast cancer cells survive and metastasize after ECM detachment. Cell Commun. Signal 21, 190. doi: 10.1186/s12964-023-01183-4
Da Silva, A. G. (2018). Avaliação da expressão de RIPK3 e de MLKL durante a necroptose induzida pelo vírus sincicial respiratório em macrófagos. Trabalho de conclusão de curso (Graduação). Porto Alegre: Universidade Federal do Rio Grande do Sul. Available online at: http://hdl.handle.net/10183/236717.
Degterev, A., Huang, Z., Boyce, M., Li, Y., Jagtap, P., Mizushima, N., et al. (2005). Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119. doi: 10.1038/nchembio711
Deng, Y., Herbert, J. A., Robinson, E., Ren, L., Smyth, R. L., and Smith, C. M. (2020). Neutrophil-airway epithelial interactions result in increased epithelial damage and viral clearance during respiratory syncytial virus infection. J. Virol. 94, e02161–e02119. doi: 10.1128/JVI.02161-19
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. doi: 10.1016/j.cell.2012.03.042
Dolma, S., Lessnick, S. L., Hahn, W. C., and Stockwell, B. R. (2003). Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3, 285–296. doi: 10.1016/S1535-6108(03)00050-3
Dominoni, M., Gardella, B., and Spinillo, A. (2024). Respiratory syncytial virus in pregnancy: an obstetrics view. Pediatr. Rep. 16, 921–924. doi: 10.3390/pediatric16040078
Do Nascimento De Freitas, D., Gassen, R. B., Fazolo, T., and Souza, A. P. D. D. (2016). Rapamycin increases RSV RNA levels and survival of RSV-infected dendritic cell depending on T cell contact. Toxicol. Vitro 36, 114–119. doi: 10.1016/j.tiv.2016.07.016
Dorscheid, D., Gauvreau, G. M., Georas, S. N., Hiemstra, P. S., Varricchi, G., Lambrecht, B. N., et al. (2025). Airway epithelial cells as drivers of severe asthma pathogenesis. Mucosal Immunol. 18, 524–536. doi: 10.1016/j.mucimm.2025.03.003
Duan, Y., Liu, Z., Zang, N., Cong, B., Shi, Y., Xu, L., et al. (2024). Landscape of respiratory syncytial virus. Chin. Med. J. 137, 2953–2978. doi: 10.1097/CM9.0000000000003354
Eckardt-Michel, J., Lorek, M., Baxmann, D., Grunwald, T., Keil, G. M., and Zimmer, G. (2008). The fusion protein of respiratory syncytial virus triggers p53-dependent apoptosis. J. Virol. 82, 3236–3249. doi: 10.1128/JVI.01887-07
Faghirabadi, F., Abuei, H., Malekzadeh, M. H., Mojiri, A., and Farhadi, A. (2024). Intracellular delivery of antiviral shRNA using penetratin-based complexes effectively inhibits respiratory syncytial virus replication and host cell apoptosis. Virol. J. 21, 235. doi: 10.1186/s12985-024-02519-3
Fang, Q., Li, Y., Wang, Y., Mu, N., Ma, H., and Yu, L. (2025). Parthanatos: A redox-dependent cell death pathway in cardiovascular disease and myocardial aging. Pathol. - Res. Pract. 274, 156178. doi: 10.1016/j.prp.2025.156178
Frank, D. and Vince, J. E. (2019). Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ 26, 99–114. doi: 10.1038/s41418-018-0212-6
Frisch, S. and Francis, H. (1994). Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124, 619–626. doi: 10.1083/jcb.124.4.619
Galluzzi, L., Vitale, I., Aaronson, S. A., Abrams, J. M., Adam, D., Agostinis, P., et al. (2018). Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25, 486–541. doi: 10.1038/s41418-017-0012-4
Gao, J., Xiong, A., Liu, J., Li, X., Wang, J., Zhang, L., et al. (2024). PANoptosis: bridging apoptosis, pyroptosis, and necroptosis in cancer progression and treatment. Cancer Gene Ther. 31, 970–983. doi: 10.1038/s41417-024-00765-9
Gaptulbarova, K.А., Tsydenova, I. A., Dolgasheva, D. S., Kravtsova, E. A., Ibragimova, M. K., Vtorushin, S. V., et al. (2024). Mechanisms and significance of entosis for tumour growth and progression. Cell Death Discov. 10, 109. doi: 10.1038/s41420-024-01877-9
Gautam, A., Boyd, D. F., Nikhar, S., Zhang, T., Siokas, I., Van De Velde, L.-A., et al. (2024). Necroptosis blockade prevents lung injury in severe influenza. Nature 628, 835–843. doi: 10.1038/s41586-024-07265-8
Glaser, L., Coulter, P. J., Shields, M., Touzelet, O., Power, U. F., and Broadbent, L. (2019). Airway epithelial derived cytokines and chemokines and their role in the immune response to respiratory syncytial virus infection. Pathogens 8, 106. doi: 10.3390/pathogens8030106
Gorphe, P. (2019). A comprehensive review of Hep-2 cell line in translational research for laryngeal cancer. Am. J. Cancer Res. 9, 644–649.
Guo, L., Deng, S., Sun, S., Wang, X., and Li, Y. (2024). Respiratory syncytial virus seasonality, transmission zones, and implications for seasonal prevention strategy in China: a systematic analysis. Lancet Global Health 12, e1005–e1016. doi: 10.1016/S2214-109X(24)00090-1
Guo, S., Li, Z., Feng, J., Xiong, W., Yang, J., Lu, X., et al. (2022). Cycloacceleration of ferroptosis and calcicoptosis for magnetic resonance imaging-guided colorectal cancer therapy. Nano Today 47, 101663. doi: 10.1016/j.nantod.2022.101663
Guo-Parke, H., Canning, P., Douglas, I., Villenave, R., Heaney, L. G., Coyle, P. V., et al. (2013). Relative respiratory syncytial virus cytopathogenesis in upper and lower respiratory tract epithelium. Am. J. Respir. Crit. Care Med. 188, 842–851. doi: 10.1164/rccm.201304-0750OC
Hamid, S., Winn, A., Parikh, R., Jones, J. M., McMorrow, M., Prill, M. M., et al. (2023). Seasonality of respiratory syncytial virus — United states 2017–2023. MMWR Morb. Mortal. Wkly. Rep. 72, 355–361. doi: 10.15585/mmwr.mm7214a1
Han, X., Na, T., Wu, T., and Yuan, B.-Z. (2020). Human lung epithelial BEAS-2B cells exhibit characteristics of mesenchymal stem cells. PloS One 15, e0227174. doi: 10.1371/journal.pone.0227174
Harker, J. A. and Snelgrove, R. J. (2020). A not-so-good way to die? Respiratory syncytial virus–induced necroptotic cell death promotes inflammation and type 2–mediated pathology. Am. J. Respir. Crit. Care Med. 201, 1321–1323. doi: 10.1164/rccm.202003-0533ED
Hause, A. M., Panagiotakopoulos, L., Weintraub, E. S., Sy, L. S., Glenn, S. C., Tseng, H.-F., et al. (2021). Adverse outcomes in pregnant women hospitalized with respiratory syncytial virus infection: A case series. Clin. Infect. Dis. 72, 138–140. doi: 10.1093/cid/ciaa668
He, M., He, C.-Q., and Ding, N.-Z. (2025). Human viruses: An ever-increasing list. Virology 604, 110445. doi: 10.1016/j.virol.2025.110445
Holze, C., Michaudel, C., Mackowiak, C., Haas, D. A., Benda, C., Hubel, P., et al. (2018). Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 19, 130–140. doi: 10.1038/s41590-017-0013-y
Hong, L., Chen, X., Liu, Y., Liang, H., Zhao, Y., and Guo, P. (2025). The relationship between ferroptosis and respiratory infectious diseases: a novel landscape for therapeutic approach. Front. Immunol. 16. doi: 10.3389/fimmu.2025.1550968
Hong, W., Yang, J., Zou, J., Bi, Z., He, C., Lei, H., et al. (2022). Histones released by NETosis enhance the infectivity of SARS-CoV-2 by bridging the spike protein subunit 2 and sialic acid on host cells. Cell Mol. Immunol. 19, 577–587. doi: 10.1038/s41423-022-00845-6
Hu, Y., Hu, Q., Li, Y., Lu, L., Xiang, Z., Yin, Z., et al. (2023). γδ T cells: origin and fate, subsets, diseases and immunotherapy. Sig Transduct Target Ther. 8, 434. doi: 10.1038/s41392-023-01653-8
Hu, T., Yu, H., Lu, M., Yuan, X., Wu, X., Qiu, H., et al. (2019). TLR4 and nucleolin influence cell injury, apoptosis and inflammatory factor expression in respiratory syncytial virus-infected N2a neuronal cells. J. Cell. Biochem. 120, 16206–16218. doi: 10.1002/jcb.28902
Jiao, Y. and Yang, R. (2025). Berberine alleviates respiratory syncytial virus (RSV)-induced pediatric bronchiolitis and fibrosis via suppressing the HMGB1/TLR4/NF-κB pathway. Microbiol. Spectr. 13, e00900–e00925. doi: 10.1128/spectrum.00900-25
Kari, S., Subramanian, K., Altomonte, I. A., Murugesan, A., Yli-Harja, O., and Kandhavelu, M. (2022). Programmed cell death detection methods: a systematic review and a categorical comparison. Apoptosis 27, 482–508. doi: 10.1007/s10495-022-01735-y
Kenmoe, S., Chu, H. Y., Dawood, F. S., Milucky, J., and Kittikraisak, W. (2023). Burden of respiratory syncytial virus–associated acute respiratory infections during pregnancy. J. Infect. Dis. 229(Supplement_1), jiad449. doi: 10.1093/infdis/jiad449
Kerr, J. F. R., Wyllie, A. H., and Currie, A. R. (1972). Apoptosis: A basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer 26, 239–257. doi: 10.1038/bjc.1972.33
Kianfar, M., Balcerak, A., Chmielarczyk, M., Tarnowski, L., and Grzybowska, E. A. (2022). Cell death by entosis: triggers, molecular mechanisms and clinical significance. IJMS 23, 4985. doi: 10.3390/ijms23094985
Kim, T. and Choi, S.-H. (2024). Epidemiology and disease burden of respiratory syncytial virus infection in adults. Infect. Chemother. 56, 1. doi: 10.3947/ic.2024.0011
Kombe Kombe, A. J., Fotoohabadi, L., Nanduri, R., Gerasimova, Y., Daskou, M., Gain, C., et al. (2024). The role of the nrf2 pathway in airway tissue damage due to viral respiratory infections. IJMS 25, 7042. doi: 10.3390/ijms25137042
Kopeina, G. S. and Zhivotovsky, B. (2022). Programmed cell death: Past, present and future. Biochem. Biophys. Res. Commun. 633, 55–58. doi: 10.1016/j.bbrc.2022.09.022
Kothwela, V. K. (2022). Respiratory Syncytial Virus Infection Induces Necroptotic Lytic Cell Death Via Activation Of Endoplasmic Reticulum Stress Pathways (Washington, DC: Washington State University).
Koval, C. E. and Gonzalez, B. E. (2024). RSV in transplant and immunocompromised patients. CCJM 91, S34–S41. doi: 10.3949/ccjm.91.s1.06
Kroemer, G. and Jäättelä, M. (2005). Lysosomes and autophagy in cell death control. Nat. Rev. Cancer 5, 886–897. doi: 10.1038/nrc1738
Kuang, L., Xu, T., Wang, C., Xie, J., Zhang, Y., Guo, M., et al. (2024). Changes in the epidemiological patterns of respiratory syncytial virus and human metapneumovirus infection among pediatric patients and their correlation with severe cases: a long-term retrospective study. Front. Cell. Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1435294
Kunst, C., Tümen, D., Ernst, M., Tews, H. C., Müller, M., and Gülow, K. (2024). Paraptosis—A distinct pathway to cell death. IJMS 25, 11478. doi: 10.3390/ijms252111478
Kuriakose, T., Man, S. M., Subbarao Malireddi, R. K., Karki, R., Kesavardhana, S., Place, D. E., et al. (2016). ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 1. doi: 10.1126/sciimmunol.aag2045
Le Nouën, C., Munir, S., Losq, S., Winter, C. C., McCarty, T., Stephany, D. A., et al. (2009). Infection and maturation of monocyte-derived human dendritic cells by human respiratory syncytial virus, human metapneumovirus, and human parainfluenza virus type 3. Virology 385, 169–182. doi: 10.1016/j.virol.2008.11.043
Li, Y., Wang, X., Blau, D. M., Caballero, M. T., Feikin, D. R., Gill, C. J., et al. (2022). Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 399, 2047–2064. doi: 10.1016/S0140-6736(22)00478-0
Li, X., Wang, J., Li, Y., He, W., Cheng, Q.-J., Liu, X., et al. (2023). The gp130/STAT3-endoplasmic reticulum stress axis regulates hepatocyte necroptosis in acute liver injury. Croat Med. J. 64, 149–163. doi: 10.3325/cmj.2023.64.149
Li, J., Zhai, Q., Zhang, W., Chen, Y., Wang, C., Deng, X., et al. (2025). The multifunctionality of the caspase family of proteases: A new perspective beyond cell death. Mol. Aspects Med. 106, 101411. doi: 10.1016/j.mam.2025.101411
Li, G.-N., Zhao, X.-J., Wang, Z., Luo, M.-S., Shi, S.-N., Yan, D.-M., et al. (2022). Elaiophylin triggers paraptosis and preferentially kills ovarian cancer drug-resistant cells by inducing MAPK hyperactivation. Sig Transduct Target Ther. 7, 317. doi: 10.1038/s41392-022-01131-7
Liang, K., Barnett, K. C., Hsu, M., Chou, W.-C., Bais, S. S., Riebe, K., et al. (2024). Initiator cell death event induced by SARS-CoV-2 in the human airway epithelium. Sci. Immunol. 9, eadn0178. doi: 10.1126/sciimmunol.adn0178
Lin, R. and Porto, B. N. (2025). Pyroptosis in respiratory virus infections: A narrative review of mechanisms, pathophysiology, and potential therapeutic interventions. Microorganisms 13, 2109. doi: 10.3390/microorganisms13092109
Liu, X., Nie, L., Zhang, Y., Yan, Y., Wang, C., Colic, M., et al. (2023). Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat. Cell Biol. 25, 404–414. doi: 10.1038/s41556-023-01091-2
Liu, Y., Pan, R., Ouyang, Y., Gu, W., Xiao, T., Yang, H., et al. (2024). Pyroptosis in health and disease: mechanisms, regulation and clinical perspective. Sig Transduct Target Ther. 9, 245. doi: 10.1038/s41392-024-01958-2
Liu, Y., Shoji-Kawata, S., Sumpter, R. M., Wei, Y., Ginet, V., Zhang, L., et al. (2013). Autosis is a Na+,K+ -ATPase–regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia–ischemia. Proc. Natl. Acad. Sci. U.S.A. 110, 20364–20371. doi: 10.1073/pnas.1319661110
Lockshin, R. A. and Williams, C. M. (1964). Programmed cell death—II. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J. Insect Physiol. 10, 643–649. doi: 10.1016/0022-1910(64)90034-4
Lu, X., McCoy, K. S., Xu, J., Hu, W., Chen, H., Jiang, K., et al. (2015). Galectin-9 ameliorates respiratory syncytial virus-induced pulmonary immunopathology through regulating the balance between Th17 and regulatory T cells. Virus Res. 195, 162–171. doi: 10.1016/j.virusres.2014.10.011
Makuch, M., Stepanechko, M., and Bzowska, M. (2024). The dance of macrophage death: the interplay between the inevitable and the microenvironment. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1330461
Malireddi, R. K. S., Kesavardhana, S., and Kanneganti, T.-D. (2019). ZBP1 and TAK1: master regulators of NLRP3 inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis). Front. Cell. Infect. Microbiol. 9. doi: 10.3389/fcimb.2019.00406
Maltese, W. A. and Overmeyer, J. H. (2014). Methuosis. Am. J. Pathol. 184, 1630–1642. doi: 10.1016/j.ajpath.2014.02.028
Martín-Vicente, M., González-Sanz, R., Cuesta, I., Monzón, S., Resino, S., and Martínez, I. (2020). Downregulation of A20 expression increases the immune response and apoptosis and reduces virus production in cells infected by the human respiratory syncytial virus. Vaccines 8, 100. doi: 10.3390/vaccines8010100
Mazur, N. I., Terstappen, J., Baral, R., Bardají, A., Beutels, P., Buchholz, U. J., et al. (2023). Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect. Dis. 23, e2–e21. doi: 10.1016/S1473-3099(22)00291-2
M. Coleman, C. (2011). The anti-apoptotic effect of respiratory syncytial virus on human peripheral blood neutrophils is mediated by a monocyte derived soluble factor. TOVJ 5, 114–123. doi: 10.2174/1874357901105010114
Mei, J., Jiang, X., Tian, H., Rong, D., Song, J., Wang, L., et al. (2024). Anoikis in cell fate, physiopathology, and therapeutic interventions. MedComm 5, e718. doi: 10.1002/mco2.718
Mgbemena, V., Segovia, J., Chang, T.-H., and Bose, S. (2013). KLF6 and iNOS regulates apoptosis during respiratory syncytial virus infection. Cell. Immunol. 283, 1–7. doi: 10.1016/j.cellimm.2013.06.002
Mlynarczuk-Bialy, I., Dziuba, I., Sarnecka, A., Platos, E., Kowalczyk, M., Pels, K. K., et al. (2020). Entosis: from cell biology to clinical cancer pathology. Cancers 12, 2481. doi: 10.3390/cancers12092481
Moriyama, M., Hugentobler, W. J., and Iwasaki, A. (2020). Seasonality of respiratory viral infections. Annu. Rev. Virol. 7, 83–101. doi: 10.1146/annurev-virology-012420-022445
Muraro, S. P., De Souza, G. F., Gallo, S. W., Da Silva, B. K., De Oliveira, S. D., Vinolo, M. A. R., et al. (2018). Respiratory Syncytial Virus induces the classical ROS-dependent NETosis through PAD-4 and necroptosis pathways activation. Sci. Rep. 8, 14166. doi: 10.1038/s41598-018-32576-y
Nakamura-Lopez, Y., Villegas-Sepúlveda, N., and Gómez, B. (2015). RSV P-protein impairs extrinsic apoptosis pathway in a macrophage-like cell line persistently infected with respiratory syncytial virus. Virus Res. 204, 82–87. doi: 10.1016/j.virusres.2015.04.018
Nakamura-López, Y., Villegas-Sepúlveda, N., Sarmiento-Silva, R. E., and Gómez, B. (2011). Intrinsic apoptotic pathway is subverted in mouse macrophages persistently infected by RSV. Virus Res. 158, 98–107. doi: 10.1016/j.virusres.2011.03.016
Newton, K., Strasser, A., Kayagaki, N., and Dixit, V. M. (2024). Cell death. Cell 187, 235–256. doi: 10.1016/j.cell.2023.11.044
Nguyen-Van-Tam, J. S., O’Leary, M., Martin, E. T., Heijnen, E., Callendret, B., Fleischhackl, R., et al. (2022). Burden of respiratory syncytial virus infection in older and high-risk adults: a systematic review and meta-analysis of the evidence from developed countries. Eur. Respir. Rev. 31, 220105. doi: 10.1183/16000617.0105-2022
O’Donnell, D. R., Milligan, L., and Stark, J. M. (1999). Induction of CD95 (Fas) and apoptosis in respiratory epithelial cell cultures following respiratory syncytial virus infection. Virology 257, 198–207. doi: 10.1006/viro.1999.9650
Okura, T., Takahashi, T., Kameya, T., Mizukoshi, F., Nakai, Y., Kakizaki, M., et al. (2024). MARCH8 restricts RSV replication by promoting cellular apoptosis through ubiquitin-mediated proteolysis of viral SH protein. Viruses 16, 1935. doi: 10.3390/v16121935
Overholtzer, M., Mailleux, A. A., Mouneimne, G., Normand, G., Schnitt, S. J., King, R. W., et al. (2007). A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 131, 966–979. doi: 10.1016/j.cell.2007.10.040
Overmeyer, J. H., Kaul, A., Johnson, E. E., and Maltese, W. A. (2008). Active ras triggers death in glioblastoma cells through hyperstimulation of macropinocytosis. Mol. Cancer Res. 6, 965–977. doi: 10.1158/1541-7786.MCR-07-2036
Park, W., Wei, S., Kim, B.-S., Kim, B., Bae, S.-J., Chae, Y. C., et al. (2023). Diversity and complexity of cell death: a historical review. Exp. Mol. Med. 55, 1573–1594. doi: 10.1038/s12276-023-01078-x
Pollard, K. J., Traina-Dorge, V., Medearis, S. M., Bosak, A., Bix, G. J., Moore, M. J., et al. (2024). Respiratory syncytial virus infects peripheral and spinal nerves and induces chemokine-mediated neuropathy. J. Infect. Dis. 230, 467–479. doi: 10.1093/infdis/jiad596
Qin, L., Hu, C., Feng, J., and Xia, Q. (2011). Activation of lymphocytes induced by bronchial epithelial cells with prolonged RSV infection. PloS One 6, e27113. doi: 10.1371/journal.pone.0027113
Rajan, A., Piedra, F.-A., Aideyan, L., McBride, T., Robertson, M., Johnson, H. L., et al. (2022). Multiple respiratory syncytial virus (RSV) strains infecting HEp-2 and A549 cells reveal cell line-dependent differences in resistance to RSV infection. J. Virol. 96, e01904–e01921. doi: 10.1128/jvi.01904-21
Rao, Z., Zhu, Y., Yang, P., Chen, Z., Xia, Y., Qiao, C., et al. (2022). Pyroptosis in inflammatory diseases and cancer. Theranostics 12, 4310–4329. doi: 10.7150/thno.71086
Rex, D. A. B., Keshava Prasad, T. S., and Kandasamy, R. K. (2022). Revisiting regulated cell death responses in viral infections. IJMS 23, 7023. doi: 10.3390/ijms23137023
Roberts, N. J. and O’Banion, M. K. (2025). The differential early responses of human leukocytes to influenza virus and respiratory syncytial virus. Pathogens 14, 974. doi: 10.3390/pathogens14100974
Robinson, E., Sawhney, S., Cortina-Borja, M., David, A. L., Smith, C. M., and Smyth, R. L. (2024). Neutrophil responses to RSV infection show differences between infant and adult neutrophils. Thorax 79, 545–552. doi: 10.1136/thorax-2023-220081
Roe, M. F. E., Bloxham, D. M., White, D. K., Ross-Russell, R. I., Tasker, R. T. C., and O’Donnell, D. R. (2004). Lymphocyte apoptosis in acute respiratory syncytial virus bronchiolitis. Clin. Exp. Immunol. 137, 139–145. doi: 10.1111/j.1365-2249.2004.02512.x
Rojas-Rivera, D., Beltrán, S., Muñoz-Carvajal, F., Ahumada-Montalva, P., Abarzúa, L., Gomez, L., et al. (2024). The autophagy protein RUBCNL/PACER represses RIPK1 kinase-dependent apoptosis and necroptosis. Autophagy 20, 2444–2459. doi: 10.1080/15548627.2024.2367923
Rumfelt, K. E., Englund, J. A., and Kachikis, A. (2025). The burden, pathogenesis, clinical outcomes, and treatment of common respiratory virus infections during pregnancy. Women’s Health 21. doi: 10.1177/17455057251338501
Salimi, V., Ramezani, A., Mirzaei, H., Tahamtan, A., Faghihloo, E., Rezaei, F., et al. (2017). Evaluation of the expression level of 12/15 lipoxygenase and the related inflammatory factors (CCL5, CCL3) in respiratory syncytial virus infection in mice model. Microbial Pathogenesis 109, 209–213. doi: 10.1016/j.micpath.2017.05.045
Santos, L. D., Antunes, K. H., Muraro, S. P., De Souza, G. F., Da Silva, A. G., Felipe, J. D. S., et al. (2021). TNF-mediated alveolar macrophage necroptosis drives disease pathogenesis during respiratory syncytial virus infection. Eur. Respir. J. 57, 2003764. doi: 10.1183/13993003.03764-2020
Schachter, J., Guijarro, A., Angosto-Bazarra, D., Pinilla, M., Hurtado-Navarro, L., Meunier, E., et al. (2025). Gasdermin D mediates a fast transient release of ATP after NLRP3 inflammasome activation before ninjurin 1-induced lytic cell death. Cell Rep. 44, 115233. doi: 10.1016/j.celrep.2025.115233
Schwartz, L. M. (2021). Autophagic cell death during development – ancient and mysterious. Front. Cell Dev. Biol. 9. doi: 10.3389/fcell.2021.656370
Sebina, I. and Phipps, S. (2020). The contribution of neutrophils to the pathogenesis of RSV bronchiolitis. Viruses 12, 808. doi: 10.3390/v12080808
Shen, G., Liu, J., Wang, Y., Deng, Z., and Deng, F. (2025). Ferroptosis in cancer and inflammatory diseases: mechanisms and therapeutic implications. MedComm 6, e70349. doi: 10.1002/mco2.70349
Sherekar, S., Todankar, C. S., and Viswanathan, G. A. (2023). Modulating the dynamics of NFκB and PI3K enhances the ensemble-level TNFR1 signaling mediated apoptotic response. NPJ Syst. Biol. Appl. 9, 57. doi: 10.1038/s41540-023-00318-0
Shi, F., Yuan, L., Wong, T., Li, Q., Li, Y., Xu, R., et al. (2023). Dimethyl fumarate inhibits necroptosis and alleviates systemic inflammatory response syndrome by blocking the RIPK1-RIPK3-MLKL axis. Pharmacol. Res. 189, 106697. doi: 10.1016/j.phrs.2023.106697
Simpson, J., Loh, Z., Ullah, M. A., Lynch, J. P., Werder, R. B., Collinson, N., et al. (2020). Respiratory syncytial virus infection promotes necroptosis and HMGB1 release by airway epithelial cells. Am. J. Respir. Crit. Care Med. 201, 1358–1371. doi: 10.1164/rccm.201906-1149OC
Simpson, J., Spann, K. M., and Phipps, S. (2022). MLKL regulates rapid cell death-independent HMGB1 release in RSV infected airway epithelial cells. Front. Cell Dev. Biol. 10. doi: 10.3389/fcell.2022.890389
Singh, D., McCann, K. L., and Imani, F. (2007). MAPK and heat shock protein 27 activation are associated with respiratory syncytial virus induction of human bronchial epithelial monolayer disruption. Am. J. Physiology-Lung Cell. Mol. Physiol. 293, L436–L445. doi: 10.1152/ajplung.00097.2007
Song, X., Zhu, S., Xie, Y., Liu, J., Sun, L., Zeng, D., et al. (2018). JTC801 induces pH-dependent death specifically in cancer cells and slows growth of tumors in mice. Gastroenterology 154, 1480–1493. doi: 10.1053/j.gastro.2017.12.004
Sperandio, S., De Belle, I., and Bredesen, D. E. (2000). An alternative, nonapoptotic form of programmed cell death. Proc. Natl. Acad. Sci. U.S.A. 97, 14376–14381. doi: 10.1073/pnas.97.26.14376
Steinberg, B. E. and Grinstein, S. (2007). Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci. STKE 2007. doi: 10.1126/stke.3792007pe11
Stravoravdi, A., Topalidou, X., and Papazisis, G. (2025). Neurological complications associated with Respiratory Syncytial Virus Infections: a scoping review of prospective clinical trials conducted in populations up to 17 years of age. Pathogens 14, 503. doi: 10.3390/pathogens14050503
Sun, Y., Qiao, Y., Liu, Y., Zhou, J., Wang, X., Zheng, H., et al. (2021). ent-Kaurane diterpenoids induce apoptosis and ferroptosis through targeting redox resetting to overcome cisplatin resistance. Redox Biol. 43, 101977. doi: 10.1016/j.redox.2021.101977
Sun, X., Yu, J., Wong, S. H., Chan, M. T. V., Zhang, L., and Wu, W. K. K. (2022). SARS-CoV-2 targets the lysosome to mediate airway inflammatory cell death. Autophagy 18, 2246–2248. doi: 10.1080/15548627.2021.2021496
Thomas, K. W., Monick, M. M., Staber, J. M., Yarovinsky, T., Carter, A. B., and Hunninghake, G. W. (2002). Respiratory syncytial virus inhibits apoptosis and induces NF-κB activity through a phosphatidylinositol 3-kinase-dependent pathway. J. Biol. Chem. 277, 492–501. doi: 10.1074/jbc.M108107200
Tsvetkov, P., Coy, S., Petrova, B., Dreishpoon, M., Verma, A., Abdusamad, M., et al. (2022). Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 375, 1254–1261. doi: 10.1126/science.abf0529
Van Den Berg, E., Bal, S. M., Kuipers, M. T., Matute-Bello, G., Lutter, R., Bos, A. P., et al. (2015). The caspase inhibitor zVAD increases lung inflammation in pneumovirus infection in mice. Physiol. Rep. 3, e12332. doi: 10.14814/phy2.12332
Villalpando-Rodriguez, G. E. and Gibson, S. B. (2021). Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat. Oxid. Med. Cell. Longevity 2021, 9912436. doi: 10.1155/2021/9912436
Vitale, I., Pietrocola, F., Guilbaud, E., Aaronson, S. A., Abrams, J. M., Adam, D., et al. (2023). Apoptotic cell death in disease—Current understanding of the NCCD 2023. Cell Death Differ 30, 1097–1154. doi: 10.1038/s41418-023-01153-w
Vorobjeva, N. V. and Chernyak, B. V. (2020). NETosis: molecular mechanisms, role in physiology and pathology. Biochem. Moscow 85, 1178–1190. doi: 10.1134/S0006297920100065
Wang, Y., Cheng, S., Fleishman, J. S., Chen, J., Tang, H., Chen, Z.-S., et al. (2024). Targeting anoikis resistance as a strategy for cancer therapy. Drug Resistance Updates 75, 101099. doi: 10.1016/j.drup.2024.101099
Wang, D., Deng, X., Li, S., and Sana, S. R. G. L. (2024). Impact of SARS-CoV-2 infection on immune cell cuproptosis in patients with lung adenocarcinoma via glutamine regulation. Int. Immunopharmacol. 140, 112912. doi: 10.1016/j.intimp.2024.112912
Wang, M., Joshua, B., Jin, N., Du, S., and Li, C. (2022). Ferroptosis in viral infection: the unexplored possibility. Acta Pharmacol. Sin. 43, 1905–1915. doi: 10.1038/s41401-021-00814-1
Wang, B., Shen, W.-B., Yang, P., and Turan, S. (2022). SARS-CoV-2 infection induces activation of ferroptosis in human placenta. Front. Cell Dev. Biol. 10. doi: 10.3389/fcell.2022.1022747
Wang, S.-Z., Smith, P. K., Lovejoy, M., Bowden, J. J., Alpers, J. H., and Forsyth, K. D. (2001). The apoptosis of neutrophils is accelerated in respiratory syncytial virus (RSV)-induced bronchiolitis. Clin. Exp. Immunol. 114, 49–54. doi: 10.1046/j.1365-2249.1998.00681.x
Wei, S., Feng, M., and Zhang, S. (2022). Molecular characteristics of cell pyroptosis and its inhibitors: A review of activation, regulation, and inhibitors. IJMS 23, 16115. doi: 10.3390/ijms232416115
Wildenbeest, J. G., Lowe, D. M., Standing, J. F., and Butler, C. C. (2024). Respiratory syncytial virus infections in adults: a narrative review. Lancet Respir. Med. 12, 822–836. doi: 10.1016/S2213-2600(24)00255-8
Yang, L., Guttman, L., Dawson, V. L., and Dawson, T. M. (2024). Parthanatos: Mechanisms, modulation, and therapeutic prospects in neurodegenerative disease and stroke. Biochem. Pharmacol. 228, 116174. doi: 10.1016/j.bcp.2024.116174
Yang, B., Hu, A., Wang, T., Chen, X., Ma, C., Yang, X., et al. (2025). SARS-CoV-2 infection induces ZBP1-dependent PANoptosis in bystander cells. Proc. Natl. Acad. Sci. U.S.A. 122, e2500208122. doi: 10.1073/pnas.2500208122
Ye, K., Chen, Z., and Xu, Y. (2023). The double-edged functions of necroptosis. Cell Death Dis. 14, 163. doi: 10.1038/s41419-023-05691-6
Yuan, X., Hu, T., He, H., Qiu, H., Wu, X., Chen, J., et al. (2018). Respiratory syncytial virus prolifically infects N2a neuronal cells, leading to TLR4 and nucleolin protein modulations and RSV F protein co-localization with TLR4 and nucleolin. J. BioMed. Sci. 25, 13. doi: 10.1186/s12929-018-0416-6
Yuan, C., Ma, Z., Xie, J., Li, W., Su, L., Zhang, G., et al. (2023). The role of cell death in SARS-CoV-2 infection. Sig Transduct Target Ther. 8, 357. doi: 10.1038/s41392-023-01580-8
Zeng, S., Wu, J., Liu, J., Qi, F., Kimura, Y., Cao, Y., et al. (2014). Infection with respiratory syncytial virus influences FasL-mediated apoptosis of pulmonary γδ T cells in a murine model of allergen sensitization. J. Asthma 51, 360–365. doi: 10.3109/02770903.2013.878954
Zhang, M., Song, R., Liu, Y., Yi, Z., Meng, X., Zhang, J., et al. (2019). Calcium-overload-mediated tumor therapy by calcium peroxide nanoparticles. Chem 5, 2171–2182. doi: 10.1016/j.chempr.2019.06.003
Zhang, X.-Y., Zhang, X.-C., Yu, H.-Y., Wang, Y., Chen, J., Wang, Y., et al. (2021). Respiratory syncytial virus infection of microglia exacerbates SH-SY5Y neuronal cell injury by inducing the secretion of inflammatory cytokines: A Transwell in vitro study. Iranian J. Basic Med. Sci. 24. doi: 10.22038/ijbms.2020.49193.11263
Zhang, Y., Zhao, D., Wang, T., Li, P., Yu, D., Gao, H., et al. (2025). Pyroptosis, a double-edged sword during pathogen infection: a review. Cell Death Discov. 11, 289. doi: 10.1038/s41420-025-02579-6
Zhao, C., Bai, Y., Wang, W., Amonkar, G. M., Mou, H., Olejnik, J., et al. (2024). Activation of STAT3-mediated ciliated cell survival protects against severe infection by respiratory syncytial virus. J. Clin. Invest. 134, e183978. doi: 10.1172/JCI183978
Zheng, T., Liu, Q., Xing, F., Zeng, C., and Wang, W. (2023). Disulfidptosis: a new form of programmed cell death. J. Exp. Clin. Cancer Res. 42, 137. doi: 10.1186/s13046-023-02712-2
Zhu, Z., Shi, J., Li, L., Wang, J., Zhao, Y., and Ma, H. (2022). Therapy targets SARS-coV-2 infection-induced cell death. Front. Immunol. 13. doi: 10.3389/fimmu.2022.870216
Keywords: respiratory syncytial virus, programmed cell death, apoptosis, necroptosis, pyroptosis, NETosis, ferroptosis
Citation: Yao P, Ma C and Liu C (2025) Programmed cell death in human respiratory syncytial virus infection. Front. Cell. Infect. Microbiol. 15:1719352. doi: 10.3389/fcimb.2025.1719352
Received: 06 October 2025; Accepted: 11 November 2025; Revised: 05 November 2025;
Published: 28 November 2025.
Edited by:
Gustavo Ramirez-Martínez, National Institute of Respiratory Diseases (INER), MexicoReviewed by:
Li Xing, Shanxi University, ChinaRushikesh Deshpande, University of Pittsburgh, United States
Copyright © 2025 Yao, Ma and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Liu, d2hzbGMyMDAyQDEyNi5jb20=
 Pengyu Yao
Pengyu Yao Chang Ma
Chang Ma Chao Liu
Chao Liu