- 1Lund University, Lund, Sweden
- 2Division of Internal Medicine, Skåne University Hospital, Lund, Sweden
Gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone, and luteinizing hormone orchestrate the reproduction cycle and regulate the sex steroid secretion from the gonads. In mammals, GnRH1 is secreted as a hormone from the hypothalamus, whereas both GnRH1 and GnRH2 are present as neurotransmitters/peptides in various tissues, where the peptides exert many different effects. mRNA coding for GnRH1 and GnRH2 have been described in the human gastrointestinal tract, and GnRH has been found in both submucosal and myenteric neurons. mRNA coding for GnRH and the fully expressed peptide have been found in rat enteric neurons by some researchers but not by others. mRNA coding for GnRH receptors, but not the fully expressed receptor, has been found in one rat study. GnRH influences gastrointestinal motility and secretion. GnRH analogs are clinically used in the treatment of sex hormone-dependent diseases, i.e., endometriosis and malignancies, and as pretreatment for in vitro fertilization. Reduced numbers of enteric neurons and IgM antibodies against GnRH and progonadoliberin-2 (precursor of GnRH2) have been observed after such treatment, with the clinical picture of gastrointestinal dysmotility. Similarly, a rat model of enteric neurodegeneration has been developed after administration of the GnRH analog buserelin. Serum IgM antibodies against GnRH1, progonadoliberin-2, and GnRH receptors have been described in patients with signs and symptoms of gastrointestinal dysmotility and/or autonomic dysfunction, such as irritable bowel syndrome, enteric dysmotility, diabetes mellitus, and primary Sjögren’s syndrome. Thus, apart from regulation of reproduction and sex hormone secretion, GnRH also constitutes a part of enteric nervous system (ENS) and its functions during physiological and pathological conditions. This review aimed to describe the role of GnRH in the ENS.
Introduction
Gonadotropin-releasing hormone (GnRH) is secreted in a pulsatile fashion from hypothalamic neurons into the portal circulation, where GnRH receptors on the anterior pituitary are activated with subsequent secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (1, 2). FSH and LH target the gonads and regulate the secretion of steroid hormones (3). Since GnRH is secreted into the portal circulation and has a half-life of a few minutes, the hormone levels cannot be analyzed in peripheral blood (2). Instead, measurements of FSH and LH levels in blood are used to estimate the hypothalamic–pituitary function. In vertebrates, 23 native decapeptides of GnRH exist. Changes of amino acids in molecular positions 5–8 differ the decapeptides from each other (4). In mammals, two types of GnRH have been found: GnRH1 and GnRH2. GnRH1 is secreted from the hypothalamus, whereas both types are present in several organs and tissues of the body, e.g., neural tissue, where they exert neuroendocrine, paracrine, and autocrine functions in the central and peripheral nervous system (4). The GnRH receptor is a G-protein-coupled receptor with seven transmembrane domains (5). Although several different receptors are described, only the GnRH1 receptor is expressed in mammals (3). Both GnRH1 and GnRH2 act through the GnRH1 receptor (4).
Organization of the Enteric Nervous System (ENS)
The autonomic nervous system is divided into three parts called the sympathetic nervous system, the parasympathetic nervous system, and the ENS (6). The ENS consists of more than 100 million neurons, which are as many neurons as in the spinal cord. The ENS has the ability to control gastrointestinal function independent of brain and spinal cord (7). It is organized in microcircuits, with interneurons and intrinsic afferent neurons, which can initiate reflexes. All kinds of neurotransmitters in the central nervous system (CNS) can be detected in the ENS (7). Nevertheless, 90% of vagal neurons are afferent, suggesting that the brain is mostly a receiver of information (8). The greatest efferent traffic from CNS to the gastrointestinal tract is to the most proximal and most distal parts of the tract, e.g., regulating functions such as mastication and swallowing (7). There is a great evidence that pathophysiological mechanisms in the CNS could also affect the ENS in a similar manner (9).
The ENS consists of two plexus: the myenteric nervous plexus, situated in-between the longitudinal and circular muscle layers, and the submucosal plexus, situated deep in the submucosa. The submucosal plexus mainly regulates the sensory and secretory functions of the gut, whereas the myenteric plexus mainly regulates the motility (7). Both plexus contain excitatory and inhibitory neurotransmitters (7). The submucosal plexus is by unknown reasons less often affected by neurological diseases (9).
Expression of GnRH and GnRH Receptors in the ENS
Gonadotropin-releasing hormone receptor mRNA was initially described in rat myenteric neurons (10). Later, mRNAs and the fully expressed peptide of GnRH were found in both submucosal and myenteric nerve plexus, whereas GnRH receptors only were found in parasympathetic ganglion cells in rat digestive tract (11). However, neither GnRH nor GnRH receptor could be detected by immunocytochemistry in rat gastrointestinal tract in vivo by another research group (12, 13). mRNA for both GnRH1 and GnRH2 could be detected by polymerase chain reaction (PCR) in the human gastrointestinal tract, whereas the GnRH receptor could neither be detected by PCR nor by immunocytochemistry (12) (Table 1). The cellular localization of GnRH has been described in about half of the submucosal and myenteric neurons along the entire human gastrointestinal tract (14, 15).
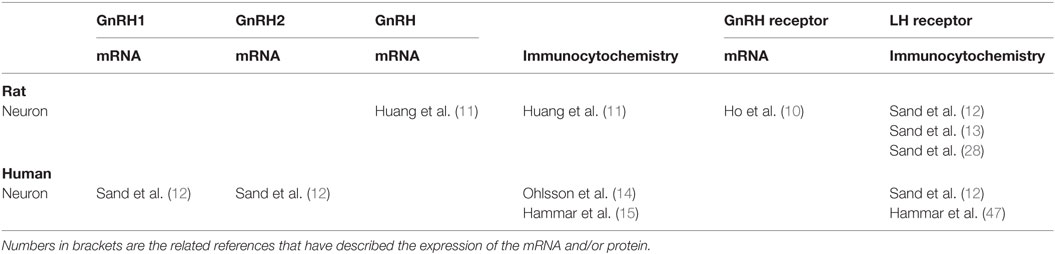
Table 1. The expression of gonadotropin-releasing hormone (GnRH), GnRH receptor, and luteinizing hormone (LH) receptor in the gastrointestinal tract in rat and humans.
Effects of GnRH or GnRH Analogs on the Function of the ENS
Gonadotropin-releasing hormone and its analog alarelin have been shown to inhibit gastric secretion and gastrin release in rat and dog (Table 2) (16, 17). The mechanisms behind the inhibition seems to be mediated both through direct actions on the parietal cells and by inhibition of the vagus nerve (16, 17). When studying jejunal motility in rats, migrating myoelectric complexes (MMCs) were frequently found during fasted state, albeit more seldom postprandially. After ovariectomy, low-dose treatment of the GnRH agonist leuprolide rendered typical fed-state patterns without MMCs. High-dose treatment of leuprolide inhibited the fed-state pattern and MMCs occurred at a frequency similar to fasted control rats (Table 2). Thus, reproductive hormones have significant effects on gastrointestinal motility (18). However, another GnRH analog could not inhibit the substance P-induced contractions of isolated guinea pig ileum, as it could inhibit substance P-induced elevation of arterial blood pressure (19). Thus, the analog may act as a substance P receptor antagonist in CNS which can inhibit the sympathetic vasomotor outflow, but without effect on peripheral substance P receptors (19).
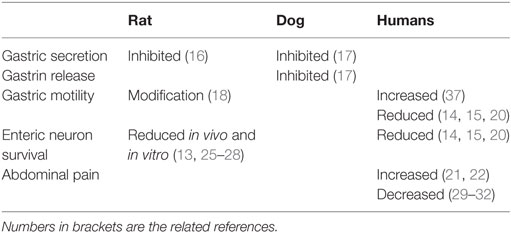
Table 2. The function of gonadotropin-releasing hormone in the gastrointestinal tract in rat and humans.
GnRH Analog-Induced Enteric Neurodegeneration in Humans
Pharmacologic treatment with GnRH analogs of endometriosis and pretreatment of in vitro fertilization (IVF) has induced severe, gastrointestinal dysmotility in some women (Table 2) (14, 15, 20). Histopathological examination of the patients have revealed a reduced total amount of enteric neurons and a reduced percentage of GnRH-expressing enteric neurons, along with serum IgM antibodies against GnRH1 and/or progonadoliberin-2 (14, 15, 20). Polymorphism in the LH receptor was common in the women who developed severe dysmotility after GnRH treatment (20).
When examining consecutive patients at an infertility clinic, treatment with buserelin led to significantly more symptoms of constipation, nausea and vomiting, impaired psychological well-being, and negative influence of intestinal symptoms on daily life, and a tendency to increased abdominal pain and bloating, compared with prior treatment (21). Five years after the start of the treatment, the patients had increased abdominal pain and better psychological well-being compared with prior IVF treatment. Fifteen percent had developed irritable bowel syndrome (IBS), or had exacerbated symptoms, but none had developed severe dysmotility (21).
In a cohort of women with endometriosis (n = 109), patients with a history of GnRH treatment had more severe abdominal pain than patients who had never been treated with GnRH analogs (22). Antibody development seems not to be obligate after GnRH treatment and occurred only in patients developing complications to the treatment (23, 24).
Buserelin-Induced Enteric Neurodegeneration in Rat
In rat, GnRH-induced enteric neuropathy has been developed after four repeated treatment sessions of buserelin, one session consisting of 5 days of 20 µg daily subcutaneous injections with 3 weeks of recovery (Table 2). This rendered a 50% reduction of both submucosal and myenteric neurons throughout the gastrointestinal tract, although most pronounced in myenteric neurons, and more pronounced distally than proximally in the gastrointestinal tract (13). Signs of ganglionitis were observed (25). Raised serum levels of estradiol, synchronization of the hormonal cycle, and thickened uterine muscle layer point to elevated FSH and LH secretions behind the neurotoxicity (13, 26). Furthermore, a reduced relative number of LH receptor-containing neurons were observed, preceded by increased expression of activated caspase-3 (13). Subclassification of neuron populations in colon showed increased relative numbers of neurons expressing cortiocotropin-releasing factor (CRF) in submucosal neurons and an absolute increased amount of CRF-containing myenteric neurons (27), whereas the relative numbers of neurons expressing calcitonin gene-related peptide, cocaine- and amphetamine-related transcript, galanin, gastrin-releasing peptide, neuropeptide Y, nitric oxide synthase, substance P, vasoactive intestinal peptide, and vesicular acetylcholine transporter were unaffected (26).
An in vitro study failed to show any effects on rat enteric neuron survival by the GnRH analog buserelin or by continuous LH stimulation. Instead, intermittent stimulation by a LH analog (lutrotroptin alpha) led to reduced neuronal survival (Table 2) (28).
Effects of GnRH on Abdominal Symptoms
In a randomized, double-blind, placebo-controlled study of patients with moderate to severe functional bowel disease, continuous treatment with leuprolide during 12 weeks improved symptoms of nausea, vomiting, bloating, abdominal pain, early satiety, and overall gastrointestinal symptoms (Table 2) (29). Continued treatment for 1 year led to even more significant improvements of the symptoms (30). A multicenter study could confirm a significant and persistent improvement in nausea and abdominal pain (31). Leuprolide treatment also improved all gastrointestinal symptoms and quality of life in women with menstrual cycle-related IBS (32).
Two hypotheses to the improved effect by leuprolide on gastrointestinal symptoms in functional bowel disorders have been described (29–32). First, GnRH binds to specific GnRH receptors on the pituitary and controls the secretion of gonadotropins (1). Both LH and ovarian products, such as progesterone and human chorionic gonadotropin (hCG), are neural antagonists of gastrointestinal motility (33, 34). By continuous stimulation of leuprolide, the hypothalamic–pituitary–gonadal axis is downmodulated and the secretion of gonadotropins and gonadal products are inhibited (3, 35). Second, by acting on GnRH receptors on myenteric neurons (10), leuprolide is an effective neural modulator through regulating the voltage-gated calcium channels and the endoplasmic reticulum calcium pump, resulting in the movement and control of intracellular and extracellular calcium (36). However, this assumption is dependent on the presence of fully expressed GnRH receptors in the ENS, which has never been demonstrated at the moment in rat or humans (10–12). Still, peripheral leuprolide restored gastrointestinal motor function both in a transplanted woman who developed chronic intestinal pseudo-obstruction after a virus infection (37) and in female ovariectomized rats (18), whereas administration of the same drug into the intraventricular system of the rat brain had no effect (38).
Antibody Formation Against GnRH and Gonadotropins
An enzyme-linked immunosorbent assay has been developed to measure GnRH antibodies in serum (14, 21, 39–41). IgM antibodies against GnRH1 have been found in patients with diabetes mellitus, gastrointestinal dysmotility, IBS, posterior laryngitis, and primary Sjögren’s syndrome, independent of treatment with GnRH analogs, in contrast to patients with celiac disease, inflammatory bowel disease, microscopic colitis, and sclerodermia, who express antibodies to the same extent as controls (39–45). IgM antibodies against GnRH receptors have been found in patients with dysmotility, IBS, and primary Sjögren’s syndrome (44, 45), and IgM antibodies against progonadoliberin-2, the precursor of GnRH2 (46), have been found in patients with diabetes mellitus, dysmotility, and IBS (45). Measurements over time showed that the antibody titer in serum was high after each buserelin administration, and the titer was then lowered after some time (14). All patients with reduced number of GnRH-containing enteric neurons displayed IgM antibodies against GnRH1 in serum, independent of GnRH treatment (15).
Discussion
Gonadotropin-releasing hormone has been found in enteric neurons in both rat and humans by several scientists (11–15). The expression of GnRH receptor is more uncertain, since only one article has described the presence of mRNA for the receptor in rat ENS (10), and no one has demonstrated the fully expressed receptor in submucosal or myenteric plexus of ENS. On the contrary, GnRH receptors have been found in parasympathetic ganglion cells outside the ENS in rat gastrointestinal tract (11), sites not examined in humans. The described effects of GnRH on the ENS are modulation of gastrointestinal motility and secretion (16–19). GnRH treatment has led to enteric neuron death in both rat in vivo and in vitro trials and in human in vivo trials (13–15, 20, 26–28). IgM antibodies against GnRH1, progonadoliberin-2, and GnRH receptors may occur in a subgroup of patients with functional bowel disorders and dysmotility, both in idiopathic forms and when associated with diabetes mellitus, posterior laryngitis, primary Sjögren’s syndrome, or GnRH treatment (39, 40, 42–45).
The GnRH analogs stimulate the anterior pituitary rendering elevated LH secretion with stimulation of the LH receptors and ensuing elevated steroidal sex hormone secretion (1–3, 13, 26). Since the GnRH receptor has not been found in human gastrointestinal tract, the harmful effects evoked on the gastrointestinal tract could be mediated by LH receptors, which are found in the gastrointestinal tract in humans and rat (12, 13, 28, 47), and are downregulated after GnRH stimulation (13). Both LH and hCG exert their effects through LH receptors. As well, LH, hCG, and progesterone are known to reduce gastrointestinal motility (33, 34), which could explain the reduced symptom burden after continuous GnRH stimulation due to downregulated secretion of gonadotropins and sex steroids (1, 2, 29–32). The effects evoked by LH receptor stimulation seem to be mediated through cAMP/protein kinase A. Furthermore, LH stimulation leads to a change in gene transcripts coding for steroidogenic enzymes, cytoskeletal proteins, in addition to signaling molecules coding for pro- and antiapoptotic processes (48). A downregulation of LH receptors is therefore accompanied by decreased apoptosis (49), which was reflected by the increased relative number of activated caspase-3 immunoreactive enteric neurons prior to the neuronal loss in the GnRH-induced rat model of neuropathy (13). In vitro trials on enteric rat neurons confirmed this theory, with a reduced neuron survival only after intermittent stimulation with the LH analog lutrotropin alpha, and not with GnRH analogs (28).
In vitro fertilization treatments leads to repeated unphysiological LH stimulation, which may be the cause of severe dysmotility observed in some women with a polymorphism in the LH receptor (20). The LH receptor is present in both genital organs and the gastrointestinal tract (12, 13, 28, 47, 50) and could be a plausible explanation to the observed association in women between dysfunction of the digestive tract and diseases of genital organs (18, 51, 52). As much as 50% loss of enteric neurons were accompanied with mostly normal gastrointestinal function (26, 27), suggesting a huge reserve capacity of the ENS. Thus, full-thickness biopsies are mandatory to examine the effects of GnRH treatment on the ENS (13–15, 26, 27) and to differ functional bowel symptoms from enteric dysmotility.
The fact that some research groups have been able to demonstrate GnRH and its receptor mRNA in the rat ENS (10, 11), while not found by others (12, 13), may have several reasons. The native GnRH receptor could not be found in adult rat neurons from the superior cervical ganglion (53). However, after microinjection of cRNA coding for the human GnRH receptor, the expression of the protein could be demonstrated (53). Thus, the expression of GnRH receptors in the neural tissue may vary. Although the GnRH receptor has not been able to demonstrate, it can still be present in the ENS. In addition to a central stimulation by GnRH administration, GnRH may thus also exert peripheral effects, direct on the ENS. In rat hypothalamus, a cross talk between N-methyl-d-aspartate and adrenergic neurotransmission has been demonstrated in the regulation of the hypothalamic GnRH gene expression (54). Due to release of nitric oxide from endothelial cells, the vascular endothelium is involved in the release of neurohormones from the median eminence (55). Theoretically, similar cross talks and mediators from the epithelial cells may be present in the ENS, which has not been examined at all regarding the release and regulation of GnRH and LH and their receptors. Thus, we are in the beginning of this research field, and experimental in vivo and in vitro trials are necessary to further determine the expression and function of these peptides in the ENS and digestive tract. If GnRH receptors are present in the ENS, the same mechanisms may be involved in the GnRH-induced response in ENS as in the gonadotrophs, e.g., activation of cAMP, cGMP, phospholipases, and calcium channels (3). It has been found that subjects with autonomic dysfunction had an abnormal hypothalamic gonadotrophin secretion (56). The mechanisms involved in the regulation of GnRH, LH, and their receptors in subjects with dysfunction of the ENS have not been determined to date.
Although chronic GnRH treatment may improve abdominal symptoms, these analogs are not used in the clinical setting, due to the risk of menopausal symptoms and development of osteoporosis in long-term treatment (57). During the last years, stimulation with GnRH agonists has been replaced by administration of GnRH antagonists in the IVF setting, to prevent the LH surges and thereby to reduce the side effects (2). The observation of more abdominal pain in endometriosis patients with GnRH treatment may reflect that GnRH analogs in this treatment group induce enteric neuropathy with ensuing abdominal pain, apart from endometriosis pain (22). Even if the GnRH analog-treated endometriosis patients are the patients with most severe disease and pain, and the elevated pain in this group could reflect more severe disease, GnRH treatment seems not to be efficient to reduce pain.
Antibodies against neuronal tissue have previously been described secondary to gut dysmotility (58). GnRH antibodies may represent neuronal damage in a subgroup of patients and may not be causal, since GnRH analogs per se did not induce serum antibody expression in humans or rat (13, 21, 26), and antibodies were present also in patients without previous GnRH treatment (14, 15, 20, 39, 40, 42–45). The absence of GnRH antibodies in rats may depend on lack of GnRH expression in rat enteric neurons (13, 26), or very small amounts of the peptide (10, 11). GnRH1 and GnRH2 are present in both the central and peripheral nervous system (3, 4, 12, 46). Autonomic neuropathy and gastrointestinal complaints are common in patients with diabetes mellitus and primary Sjögren’s syndrome (42, 44) and autonomic neuropathy, depression, and affective disorders are common in patients with functional bowel diseases and gastrointestinal dysmotility (58–61). Theoretically, antibodies against GnRH may be secondary to either a central neuronal damage or a peripheral neuronal damage (39, 40, 42, 44, 45).
Conclusion
Gonadotropin-releasing hormone has been found in the human ENS in repeated examinations. GnRH has been found in rat ENS in some studies, although not reproducible by others. Fully expressed GnRH receptors have never been found in rat or human ENS. GnRH modulates gastrointestinal motility and secretion. Treatment with GnRH analogs may induce enteric neurodegeneration in both rat and human ENS. LH receptor activation is the postulated target of the effects observed, since LH receptors are described on enteric neurons and enteric rat neuronal survival was decreased after intermittent in vitro stimulation of the LH receptor. Autoantibodies against GnRH and its receptor are found in a subgroup of patients with disturbances from the gastrointestinal tract and/or autonomic nervous system, independent of treatment with GnRH analogs.
Altogether, the knowledge about GnRH expression and function in the gastrointestinal tract suggests a role for GnRH on the ENS, but the field is rudimentary studied. The key point in this research field is to study the local effect of GnRH in the gastrointestinal tract and the communication between GnRH and LH receptors. Future research should include a search for fully expressed GnRH receptors in rat and human ENS. Further, a controlled study comparing the effect of GnRH analogs compared with GnRH antagonists on neuron survival should be performed in vitro and in vivo. Another alternative is to compare the effects on the ENS by sole GnRH analogs and GnRH analogs in combination with LH receptor antagonists. Both cell culture trials with enteric neurons and organ bath experiments are needed to characterize the route of effects by GnRH on the ENS, and the effects evoked.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ELISA, enzyme-linked immunosorbent assay; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; hCG, human chorionic gonadotropin; IBS, irritable bowel syndrome; IVF, in vitro fertilization; LH, luteinizing hormone; MMC, migrating myoelectric complex; PCR, polymerase chain reaction.
References
1. Hazum E, Conn PM. Molecular mechanism of gonadotropin releasing hormone (GnRH) action. I. The GnRH receptor. Endocr Rev (1998) 9:379–86. doi: 10.1210/edrv-9-4-379
2. Conn PM, Crowley WF Jr. Gonadotropin-releasing hormone and its analogues. N Engl J Med (1991) 324:93–103. doi:10.1056/NEJM199101103240205
3. Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol (2009) 30:10–29. doi:10.1016/j.yfrne.2008.07.001
4. Millar RP. GnRHs and GnRH receptors. Anim Reprod Sci (2005) 88:5–28. doi:10.1016/j.anireprosci.2005.05.032
5. White RB, Eisen JA, Kasten TL, Fernald RD. Second gene for gonadotropin-releasing hormone in humans. Proc Natl Acad Sci U S A (1998) 95:305–9. doi:10.1073/pnas.95.1.305
6. Langley JN. The Autonomic Nervous System, Part 1 (1921). Cornell Univ. Library. Cambridge: Cambridge University Press (2010).
7. Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol (2014) 817:39–71. doi:10.1007/978-1-4939-0897-4_3
8. Forsythe P, Bienestcok J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol (2014) 817:115–33. doi:10.1007/978-1-4939-0897-4_5
9. Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol (2016) 1:517–28. doi:10.1038/nrgastro.2016.107
10. Ho J, Nagel G, Mathias JR, Clench MH, Fan X, Kalmaz GD, et al. Presence of gonadotropin-releasing hormone (GnRH) receptor mRNA in rat myenteric plexus cells. Comp Biochem Physiol (1996) 113:817–21. doi:10.1016/0305-0491(95)02114-0
11. Huang W, Yao B, Sun L, Pu R, Wang L, Zang R. Immunohistochemical and in situ hybridization studies of gonadotropin releasing hormone (GnRH) and its receptor in rat digestive tract. Life Sci (2001) 68:1727–34. doi:10.1016/S0024-3205(01)00968-7
12. Sand E, Bergvall M, Ekblad E, D’Amato M, Ohlsson B. Expression and distribution of GnRH, LH, and FSH and their receptors in gastrointestinal tract of man and rat. Regul Pept (2013) 187:24–8. doi:10.1016/j.regpep.2013.09.002
13. Sand E, Voss U, Hammar O, Nordin Fredrikson G, Alm R, Ohlsson B, et al. Gonadotropin-releasing hormone analog buserelin causes neuronal loss in rat gastrointestinal tract. Cell Tissue Res (2013) 351:521–34. doi:10.1007/s00441-012-1534-1
14. Ohlsson B, Veress B, Janciauskiene S, Montgomery A, Haglund M, Ohlsson B. Chronic intestinal pseudo-obstruction due to buserelin-induced formation of anti-GnRH antibodies. Gastroenterology (2007) 132:45–51. doi:10.1053/j.gastro.2006.10.036
15. Hammar O, Ohlsson B, Veress B, Nordin Fredrikson G, Alm R, Montgomery A. Depletion of enteric gonadotropin-releasing hormone is found in a few patients suffering from severe gastrointestinal dysmotility. Scand J Gastroenterol (2012) 47:1165–73. doi:10.3109/00365521.2012.706826
16. Chen L, Sun XD, Zhao J, Yang AG, Huang W. Distribution, cloning and sequencing of GnRH, its receptor, and effects of gastric acid secretion of GnRH analogue in gastric parietal cells of rats. Life Sci (2005) 76:1351–65. doi:10.1016/j.lfs.2004.10.005
17. Soldani G, Del Tacca M, Bambini G, Polloni A, Bernardini C, Martinotti E, et al. Effects of gonadotropin-releasing hormone (GnRH) on gastric secretion and gastrin release in the dog. J Endocrinol Invest (1982) 5:393–6. doi:10.1007/BF03350539
18. Khanna R, Browne RM, Heiner AD, Clench MH, Mathias JR. Leuprolide acetate affects intestinal motility in female rats before and after ovariectomy. Am J Physiol (1992) 262:G185–90.
19. Takano Y, Sawyer WB, Sanders NL, Loewy AD. LH-RH analogue acts as substance P antagonist by inhibiting spinal cord vasomotor responses. Brain Res (1985) 337:357–61. doi:10.1016/0006-8993(85)90075-7
20. Cordeddu L, Bergvall M, Sand E, Roth B, Papadaki E, Li L, et al. Severe, gastrointestinal dysmotility developed after treatment with gonadotropin-releasing hormone analogs. Scand J Gastroenterol (2015) 50:291–9. doi:10.3109/00365521.2014.958098
21. Hammar O, Roth B, Bengtsson M, Mandl T, Ohlsson B. Autoantibodies and gastrointestinal symptoms in infertile women in relation to in vitro fertilization. BMC Pregnancy Childbirth (2013) 13:201. doi:10.1186/1471-2393-13-201
22. Ek M, Roth B, Ekström P, Valentin L, Ohlsson B. Gastrointestinal symptoms among patients with endometriosis. A case-cohort study. BMC Womens Health (2015) 15:59. doi:10.1186/s12905-015-0213-2
23. Lindner J, McNeil LW, Marney S, Conway M, Rivier J, Vale W, et al. Characterization of human anti-luteinizing hormone-releasing hormone (LRH) antibodies in the serum of a patient with isolated gonadotropin deficiency treated with synthetic LRH. J Clin Endocrinol Metab (1981) 52:267–70. doi:10.1210/jcem-52-2-267
24. Holland FJ, Fishman L, Costigan DC, Luna L, Leeder S. Pharmacokinetic characteristics of the gonadotropin-releasing hormone analog d-Ser(TBU)-6EA-10 luteinizing hormone-releasing hormone (buserelin) after subcutaneous and intranasal administration in children with central precocious puberty. J Clin Endocrinol Metab (1986) 63:1065–70. doi:10.1210/jcem-63-5-1065
25. Ohlsson B, Sand E, Veress B. Ganglioneuritits is common in rats with enteric neuropathy due to buserelin treatment. Regul Pept (2014) 19(0–191):43–5. doi:10.1016/j.regpep.2014.03.005
26. Sand E, Roth B, Weström B, Bonn P, Ekblad E, Ohlsson B. Structural and functional consequences after buserelin-induced enteric neuropathy in rat. BMC Gastroenterol (2014) 14:209. doi:10.1186/s12876-014-0209-7
27. Sand E, Lozinska L, Egecioglu E, Roth B, Weström B, Ekblad E, et al. Buserelin treatment to rats causes enteric neurodegeneration with moderate effects on CRF-immunoreactive neurons and Enterobacteriaceae in colon, acetylcholine-mediated small intestinal permeability in ileum, and stress behavior. BMC Res Notes (2015) 8:824. doi:10.1186/s13104-015-1800-x
28. Sand E, Voss U, Ohlsson B, Ekblad E. Luteinizing hormone receptors are expressed in rat myenteric neurons and mediate neuronal loss. Auton Neurosci (2015) 193:104–7. doi:10.1016/j.autneu.2015.10.001
29. Mathias JR, Clench MH, Reeves-Darby VG, Fox LM, Hsu PH, Roberts PH, et al. Effect of leuprolide acetate in patients with moderate to severe functional bowel disease. Double-blind, placebo-controlled study. Dig Dis Sci (1994) 39:1155–62. doi:10.1007/BF02093779
30. Mathias JR, Clench MH, Roberts PH, Reeves-Darby VG. Effect of leuprolide acetate in patients with functional bowel disease. Long-term follow-up after double-blind, placebo-controlled study. Dig Dis Sci (1994) 39:1163–70. doi:10.1007/BF02093779
31. Mathias JR, Clench MH, Abell TL, Koch KL, Lehman G, Robinson M, et al. Effect of leuprolide acetate in treatment of abdominal pain and nausea in premenopausal women with functional bowel disease: a double-blind, placebo-controlled, randomized study. Dig Dis Sci (1998) 43:1347–55. doi:10.1023/A:1018888631286
32. Palomba S, Orio F Jr, Manguso F, Russo T, Falbo A, Lombardi G, et al. Leuprolide acetate treatment with and without coadministration of tibolone in premenopausal women with menstrual cycle-related irritable bowel syndrome. Fertil Steril (2005) 83:1012–20. doi:10.1016/j.fertnstert.2004.12.007
33. Ducker TE, Boss JW, Altug SA, Mehrabian H, Dekeratry DR, Clench MH, et al. Luteinizing hormone and human chorionic gonadotropin fragment the migrating motor complex in rat small intestine. Neurogastroenterol Motil (1996) 8:95–100. doi:10.1111/j.1365-2982.1996.tb00249.x
34. Wang F, Zheng TZ, Li W, Qu SY, He DY. Action of progesterone on contractile activity of isolated gastric strips in rats. World J Gastroenterol (2003) 9:775–8. doi:10.3748/wjg.v9.i4.775
35. Rabin D, McNeil LW. Pituitary and gonadal desensitization after continuous luteinizing hormone-releasing infusion in normal females. J Clin Endocrinol Metab (1980) 51:873–6. doi:10.1210/jcem-51-4-873
36. Stojikovic SS, Tomic M. GnRH-induced calcium and current oscillations in gonadotrophs. Trends Endocrinol Metab (1996) 7:379–84. doi:10.1016/S1043-2760(96)00189-0
37. Mathias JR, Baskin GS, Reeves-Darby VG, Clench MH, Smith LL, Calhoon JH. Chronic intestinal pseudoobstruction in a patient with heart-lung transplant. Therapeutic effect of leuprolide acetate. Dig Dis Sci (1992) 37:1761–8. doi:10.1007/BF01299872
38. Heiner AM, Browne RM, Khanna R, Shinnick-Gallagher P, Callahan P, Clench MH, et al. Effect of centrally administered leuprolide acetate on myoelectric activity of the small intestine in rats (abstract). Gastroenterology (1998) 96:202.
39. Ohlsson B, Scheja A, Janciauskiene S, Mandl T. Functional bowel symptoms and GnRH antibodies: common findings in patients with primary Sjögren’s syndrome but not in systemic sclerosis. Scand J Rheumatol (2009) 38:391–3. doi:10.1080/03009740802709069
40. Ohlsson B, Sjöberg K, Alm R, Nordin Fredrikson G. Patients with irritable bowel syndrome and dysmotility express antibodies against gonadotropin-releasing hormone in serum. Neurogastroenterol Motil (2011) 23:1000–6. doi:10.1111/j.1365-2982.2011.01744.x
41. Roth B, Ohlsson B. Gastrointestinal symptoms and psychological well-being in patients with microscopic colitis. Scand J Gastroenterol (2013) 48:27–34. doi:10.3109/00365521.2012.741614
42. Berntorp K, Frid A, Alm R, Nordin Fredrikson G, Sjöberg K, Ohlsson B. Antibodies against gonadotropin-releasing hormone (GnRH) in patients with diabetes mellitus is associated with lower body weight and autonomic neuropathy. BMC Res Notes (2013) 6:329. doi:10.1186/1756-0500-6-329
43. Pendleton H, Alm R, Nordin Fredrikson G, Ohlsson B. Antibodies against gonadotropin-releasing hormone in patients with posterior laryngitis. Drug Target Insights (2013) 7:1–8. doi:10.4137/DTI.S10837
44. Mandl T, Roth B, Ohlsson B. Antibodies against GnRH and its receptor in patients with primary Sjögren’s syndrome. Scand J Rheumatol (2014) 43:338–48. doi:10.3109/03009742.2013.878388
45. Roth B, Berntorp K, Ohlsson B. The expression of serum antibodies against gonadotropin-releasing hormone (GnRH1), progonadoliberin-2, luteinizing hormone (LH), and related receptors in patients with gastrointestinal dysfunction or diabetes mellitus. Drug Target Insights (2014) 8:45–50. doi:10.4137/DTI.S19352
46. Pawson AJ, Morgan K, Maudsley SR, Millar RP. Type II gonadotrophin-releasing hormone (GnRH-II) in reproductive biology. Reproduction (2003) 126:271–8. doi:10.1530/rep.0.1260271
47. Hammar O, Veress B, Montgomery A, Ohlsson B. Expression of luteinizing hormone receptor in the gastrointestinal tract in patients with and without dysmotility. Drug Target Insights (2012) 6:13–8. doi:10.4137/DTI.S9324
48. Sasson R, Rimon E, Dantes A, Cohen T, Shinder V, Land-Bracha A, et al. Gonadotrophin-induced gene regulation in human granulosa cells obtained from IVF patients. Modulation of steroidogenic genes, cytoskeletal genes and genes coding for apoptotic signalling and protein kinases. Mol Hum Reprod (2004) 10:299–311. doi:10.1093/molehr/gah041
49. Srivastava RK, Krishna A. Increased circulating leptin level inhibits folliculogenesis in vespertilionid bat, Scotophilus heathii. Mol Cell Endocrinol (2011) 337:24–35. doi:10.1016/j.mce.2011.01.017
50. Menon KM, Menon B. Structure, function and regulation of gonadotropin receptors – a perspective. Mol Cell Endocrinol (2012) 356:88–97. doi:10.1016/j.mce.2012.01.021
51. Mathias JR, Franklin R, Quast DC, Fraga N, Loftin CA, Yates L, et al. Relation of endometriosis and neuromuscular disease of the gastrointestinal tract: new insights. Fertil Steril (1998) 70:81–8. doi:10.1016/S0015-0282(98)00096-X
52. Gonenne J, Esfandyari T, Camilleri M, Burton DD, Stephens DA, Baxter KL, et al. Effect of female sex hormone supplementation and withdrawal on gastrointestinal and colonic transit in postmenopausal women. Neurogastroenterol Motil (2006) 18:911–8. doi:10.1111/j.1365-2982.2006.00808.x
53. Lewis DL, Ikeda SR. Inhibition of M-type K+ and N-type Ca2+ channels by the human gonadotropin-releasing hormone receptor heterologously expressed in adult neurons. Neuroendocrinology (1997) 66:235–45. doi:10.1159/000127244
54. Suh J, Song ES, Kim C, Yu MH, Kim K. Cross-talk between N-methyl-d-aspartate and adrenergic neurotransmission in the regulation of hypothalamic GnRH gene expression. Brain Res (1994) 645:36–40. doi:10.1016/0006-8993(94)91635-7
55. Prevot V, Rialas CM, Croix D, Salzet M, Dupouy JP, Poulain P, et al. Morphine and anandamide coupling to nitric oxide stimulates GnRH and CRF release from rat median eminence: neurovascular regulation. Brain Res (1998) 790:236–44. doi:10.1016/S0006-8993(98)00066-3
56. Williams TDM, Lightman SL, Johnson MR, Carmichael DJS, Bannister R. Selective defect in gonadotrophin secretion in patients with autonomic failure. Clin Endocrinol (1989) 30:285–92. doi:10.1111/j.1365-2265.1989.tb02237.x
57. DiVasta AD, Laufer MR. The use of gonadotropin releasing hormone analogues in adolescent and young patients with endometriosis. Curr Opin Obstet Gynecol (2013) 25:287–92. doi:10.1097/GCO.0b013e32836343eb
58. Kashyap P, Farrugia G. Enteric autoantibodies and gut motility disorders. Gastroenterol Clin North Am (2008) 37:397–410. doi:10.1016/j.gtc.2008.02.005
59. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol (2014) 6:71–80. doi:10.2147/CLEP.S40245
60. Mattsson T, Roos R, Sundkvist G, Valind S, Ohlsson B. Sympathetic nerve dysfunction is common in patients with chronic intestinal pseudo-obstruction. J Clin Gastroenterol (2008) 42:174–7. doi:10.1097/01.mcg.0000225649.54566.02
Keywords: enteric nervous system, enteric neurodegeneration, gonadotropin-releasing hormone, gonadotropin-releasing hormone receptor, gonadotropin-releasing hormone antibodies
Citation: Ohlsson B (2017) Gonadotropin-Releasing Hormone and Its Role in the Enteric Nervous System. Front. Endocrinol. 8:110. doi: 10.3389/fendo.2017.00110
Received: 16 April 2017; Accepted: 08 May 2017;
Published: 07 June 2017
Edited by:
Ivana Bjelobaba, University of Belgrade, SerbiaReviewed by:
Balachandar Nedumaran, University of Colorado Anschutz Medical Campus, United StatesHélène Volkoff, Memorial University of Newfoundland, Canada
Copyright: © 2017 Ohlsson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bodil Ohlsson, Ym9kaWwub2hsc3NvbkBtZWQubHUuc2U=
 Bodil Ohlsson
Bodil Ohlsson