- 1School of Basic Medical Sciences, Nanchang University, Nanchang, China
- 2Clinical Medical Experimental Center, Nanchang University, Nanchang, China
- 3Jiangxi Provincial Key Laboratory of Reproductive Physiology and Pathology, Nanchang University, Nanchang, China
As emerging organic contaminants, per- and polyfluoroalkyl substances (PFASs) have aroused worldwide concern due to their environmental persistence, ubiquitous presence, bioaccumulation, and potential toxicity. It has been demonstrated that PFASs can accumulate in human body and cause multiple adverse health outcomes. Notably, PFASs have been detected in the semen of human, posing a potential hazard to male fecundity. This article reviews the evidence about the toxic effects of exposure to PFASs on male reproduction, focusing on the sperm quality. Epidemiological studies showed that PFASs, such as perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), were adversely associated with the semen parameters in humans, including sperm count, morphology and motility. Experimental results also confirmed that PFAS exposure led to testicular and epididymal damage, therefore impairing spermatogenesis and sperm quality. The mechanisms of reproductive toxicity of PFASs may be involved in blood-testosterone barrier destruction, testicular apoptosis, testosterone synthesis disorder, and membrane lipid composition alteration, oxidative stress and Ca2+ influx in sperm. In conclusion, this review highlighted the potential threat of exposure to PFASs to human spermatozoa.
Introduction
The decline in human fertility rates has drawn considerable concern (1, 2). Accumulating evidence suggests that human semen quality has decreased worldwide over the past few decades (3–9). Although the causative factors remain to be fully discovered, exposure to environmental pollutants is considered to be a major contributor for impaired male fecundity (2, 10–12). Per- and polyfluoroalkyl substances (PFASs) are a family of fluorinated synthetic chemicals that have been extensively used in industry and consumer products since the 1950s. As emerging persistent organic contaminants, PFASs are extremely resistant to environmental degradation and metabolic clearance due to their unique and stable physicochemical properties (13). Consequently, these compounds are ubiquitous and persistent in the environment and accumulate in the food chain (14, 15), posing a serious threat to ecological and human health worldwide. A variety of PFASs have been detected in the semen of human (16–19), implying a potential hazard of PFASs to male fecundity. Hence, this review briefly summarizes the epidemiological and experimental evidence regarding the toxic effects of PFAS exposure on male reproduction, focusing on sperm quality.
Human exposure to PFASs
Accumulating evidence has revealed that humans are universally exposed to PFASs (20–24). The intake of polluted food and drinking water, inhalation of indoor air and dust, and dermal contact are claimed as the major routes of human exposure to PFASs (14, 25–27). Perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) are the most predominant and frequently detected PFASs in human blood (28). Their serum half-lives were estimated to be 3.8 and 5.4 years in human body, respectively (29). In human plasma, PFASs primarily bind to albumin and are transferred through the body (30). Epidemiological investigations have indicated a possible association between PFAS exposure and adverse health outcomes, such as liver function abnormality (31), glucose homeostasis disturbance (32), dyslipidemia (33), cardiovascular diseases (34), fetal growth restriction (35), and bone mineral density reduction (36).
Effect of PFASs on human sperm quality and quantity
Sperm concentration and count
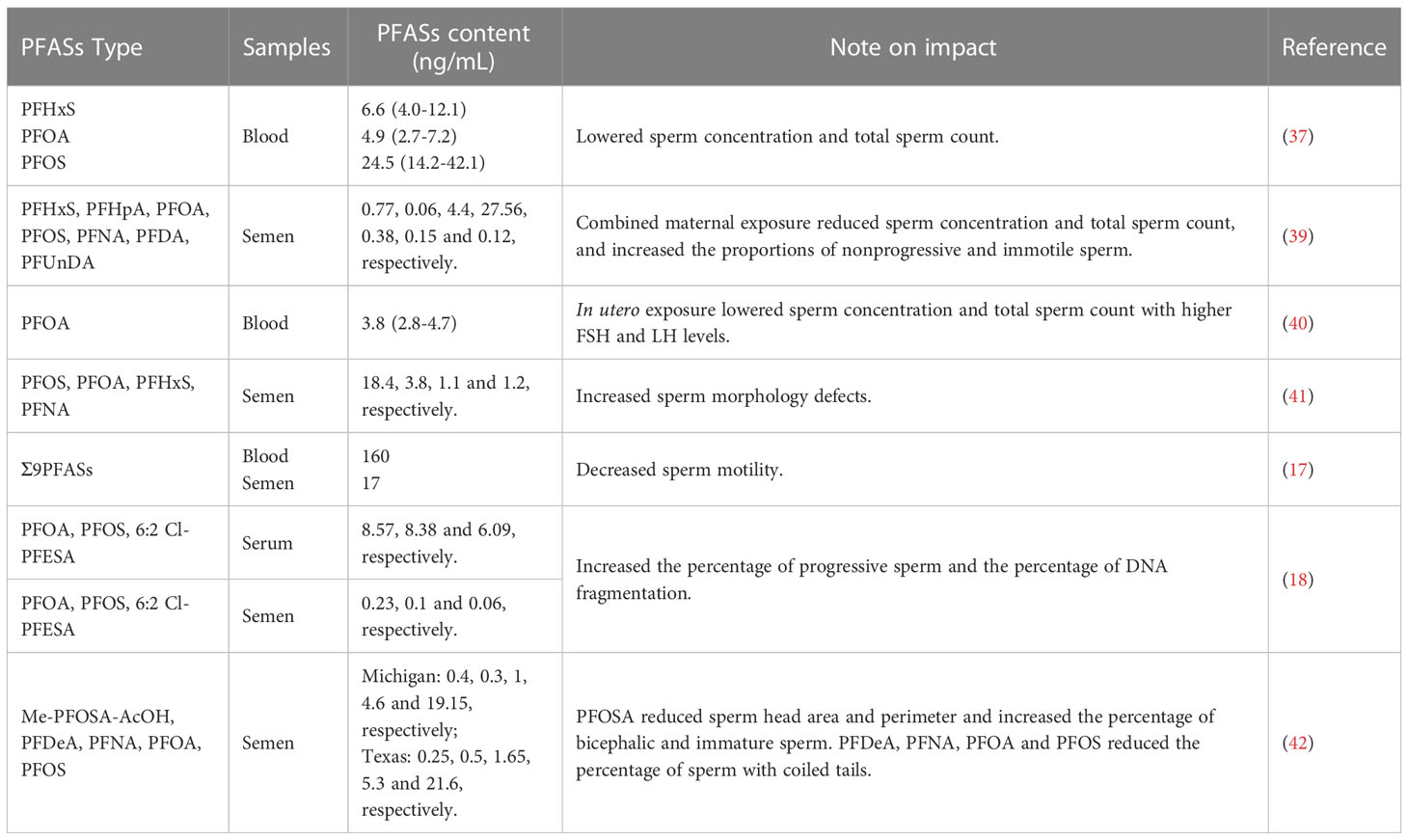
In a previous investigate on 105 Danish males from the general population, sperm concentration and total sperm count showed a reduced tendency in those with high PFAS levels, although not at statistically significant levels (37). Similarly, a nonsignificant decrease was observed in 212 young men from the PFASs-polluted Veneto region (38). However, a recent investigation on 864 young males from the general Danish population showed that maternal exposure to PFASs was linked to lower sperm concentration and total sperm count (39). A multivariable linear regression analysis on 169 male offspring also demonstrated that in utero exposure to PFOA was related to lower sperm concentration and total sperm count (40).
Morphology
Sperm morphology is an important determinant of semen quality and male fertility. Epidemiological studies have shown that exposure to high levels of PFASs are associated with a lower percentage of morphologically normal spermatozoa (37, 38). An investigation on the partners of pregnant women from arctic and European populations showed a 35% decrease in the proportion of sperm with normal morphology in those with the highest PFOS exposure relative to those with the lowest exposure (41). Furthermore, a Longitudinal Investigation of Fertility and the Environment (LIFE) study reported that perfluorooctane sulfonamide (PFOSA) was related to smaller sperm head area and perimeter and a higher percentage of bicephalic and immature sperm, and PFOA, PFOS, perfluorodecanoate (PFDeA) and perfluorononanoate (PFNA) were associated with a lower percentage of sperm with coiled tails (42).
Motility
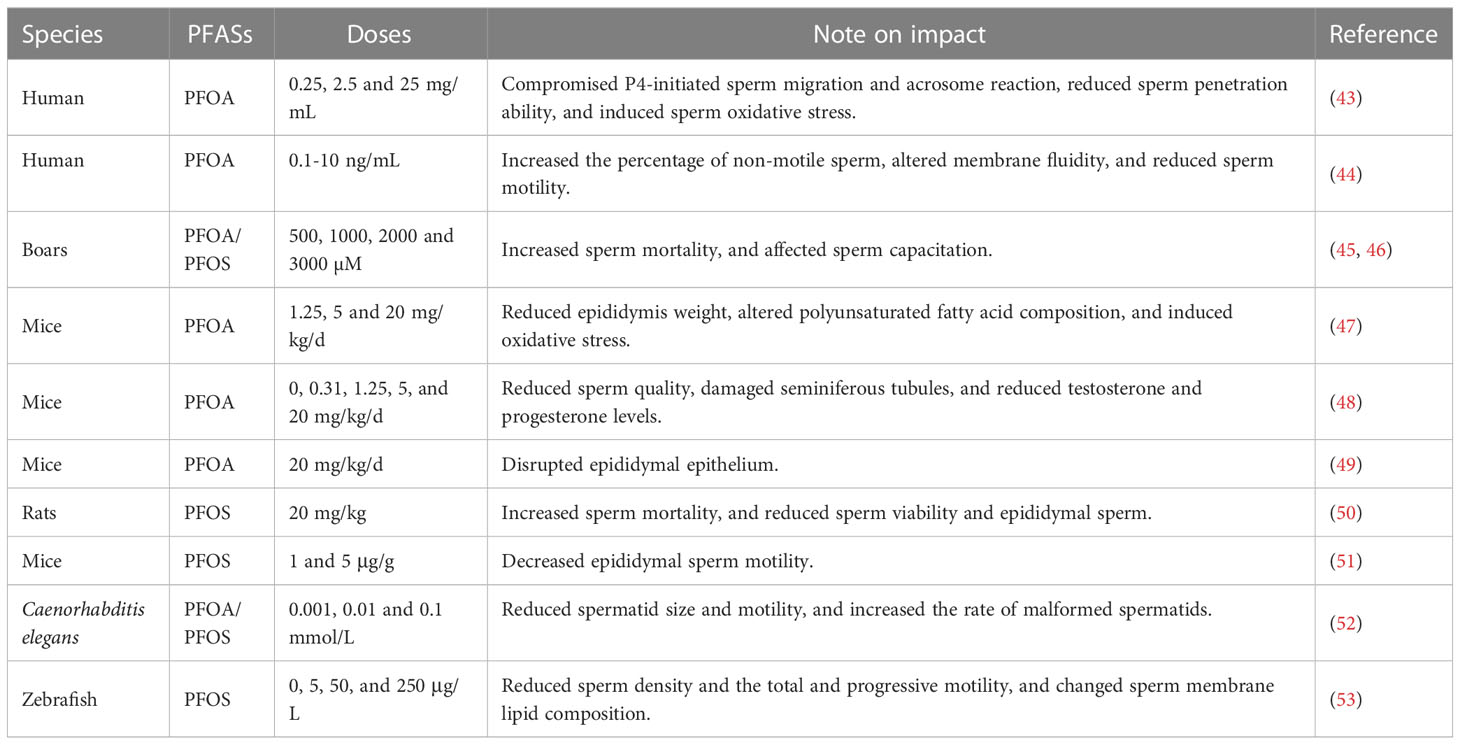
Sperm motility is a decisive factor for male fecundity. In the population of the Pearl River Delta region in China, a significantly negative correlation was observed between sperm motility and PFASs in semen (17). Consistent with this finding, in a cross-sectional study, seminal PFOS, PFOA and emerging chlorinated polyfluorinated ether sulfonate (6:2 Cl-PFESA) were associated with a decline in the percentage of progressive sperm and an elevation in the percentage of DNA fragmentation (18). In addition, maternal exposure to PFASs was also linked to a higher percentage of immotile and nonprogressive sperm in the young adulthood (39). In our previous study, in vitro exposure to PFOA conspicuously impaired the capability of human sperm to penetrate artificial cervical mucus (43). Similarly, in vitro incubation with PFOA led to a remarkable reduction in progressive motility in human sperm (44).
Capacitation, acrosome reaction and hyperactivation
Sperm capacitation, acrosome reaction and hyperactivation are essential prerequisites for the fertilization of oocyte. However, epidemiological data regarding the effects of PFASs on these processes are scarce. Our previous study showed that incubation with PFOA in vitro compromised progesterone-induced acrosome reaction and viscous medium penetration in human sperm (43). Similarly, in vitro exposure to PFOA and PFOS decreased the number of capacitated spermatozoa and hindered progesterone-induced acrosomal reaction in boar spermatozoa (45, 46).
Experimental evidence for toxicities of PFASs to sperm
Numerous studies have confirmed the male reproductive toxicities of PFASs in rodents. It has been shown that PFOA exposure causes epididymis injury and reduces epididymal sperm count in mice (47–49). Furthermore, sperm motility and progressiveness were remarkably compromised and teratospermia rate was significantly elevated in PFOA-treated mice (48). Correspondingly, rats exposed to PFOS also displayed a prominent decline in epididymal sperm count and sperm viability and motility, concomitant with a notable increase in the percentage of morphological abnormalities of head, mid-piece and tail of sperm (50). In another study, PFOS exposure at 5 mg/kg did not affect the number of sperm or the percentage of motile sperm, but significantly reduced the motility of sperm reflected by decreased curvilinear, straight-line and average path velocity in mice (51). In addition, exposure to PFOA and PFOS induced male reproductive toxicity in Caenorhabditis elegans, leading to a reduction in spermatid size and motility and an increase in sperm malformation rate (52). Chronic exposure to PFOS also decreased sperm density and compromised the total and progressive motility in zebrafish (53).
Discussion
Due to the environmental persistence and pervasive presence, there is growing concern regarding the toxicities of PFASs to male fertility. Numerous studies have suggested that PFASs induce testicular toxicity. For example, exposure to PFOA repressed the expression of blood-testis barrier (BTB) proteins and increased TNFα content and p-p38/p38 MAPK ratio in mouse testis and cultured Sertoli cells (54). Similarly, hexafluoropropylene oxides and PFOS disturbed BTB by activating p38 MAPK/MMP9 pathway (55, 56). These results indicate that p38 MAPK signaling may contribute to PFASs-induced BTB disruption. Proteomic profile analysis also indicated that PFOA treatment altered blood-testis barrier remodeling in mouse testis (57). Furthermore, PFOS exposure promoted the generation of reactive oxygen species (ROS) and suppressed the activities of antioxidases in the testes, thereby impairing testicular physiology and spermatogenesis in rats (50). Oral PFOA administration resulted in the destruction of the seminiferous epithelium, induced oxidative stress, inhibited NRF2-mediated antioxidant response, and led to apoptosis in the testis of mice (49, 58). However, studies found that supplement with flavonoids rutin and pachypodol attenuated testicular damage caused by PFOA and PFOS through alleviating oxidative stress, respectively (49, 50). Additionally, maternal exposure to PFOA reduced serum testosterone levels, disrupted testis development, damaged testicular structure, and caused testicular apoptosis in the offspring mice (59, 60). These results suggest that exposure to PFASs can result in the disruption of testicular structure and function, which may be partly responsible for PFASs-caused reduction of sperm count. Moreover, antioxidative intervention with flavonoids may be a promising strategy for preventing and rescuing PFASs-induced spermatogenic impairment.
Hormones in the hypothalamic-pituitary-gonadal axis are important regulators in the reproductive process. Intratesticular testosterone plays an important role in sperm number and sperm motility, and abnormal testosterone generation impairs spermatogenesis in humans (61). PFASs have been identified to act as endocrine disruptors affecting male reproductive health. A cross-sectional study reported that higher serum levels of PFASs are negatively associated with testosterone concentrations among male adolescents (62). The negative association between serum PFOS and testosterone levels was also observed in healthy young Danish men (63). In laboratorial experiments, PFOS exposure disrupted the hypothalamic-pituitary-testis axis activity (64, 65), and reduced luteinizing hormone, follicle-stimulating hormone and testosterone levels in adult male rats (50). Furthermore, both PFOA and PFOS significantly decreased the expression of steroidogenic enzymes and the concentrations of testosterone in male mice (48, 66), and the mechanisms may be involved in developmental inhibition, oxidative stress and apoptosis in Leydig cells (67–70). Inversely, low-dose PFOA stimulated steroid hormone synthesis by enhancing fatty acid metabolism and steroidogenic activation in Leydig cells (71). These results suggested that exposure to PFASs disordered testosterone biosynthesis, which may be correlated with the impaired semen quality.
Normal sperm function is crucial for male fertility. Some epidemiological and experimental investigations also showed a negative association between PFAS exposure and semen parameters, such as sperm count, morphology and motility, implying that PFASs have an adverse influence on sperm quality (Tables 1 and 2). Nevertheless, the toxicological mechanisms remain largely unelucidated. Rodent studies demonstrated that PFOA could accumulate in the epididymis and cause morphological change in epididymal epithelium (47, 49), suggesting that the epididymis is a potential target and PFOA may exert direct toxicity to spermatozoa. Lu et al. (47) found that PFOA exposure activated AKT/AMPK signaling pathway, altered polyunsaturated fatty acid composition, and triggered oxidative stress in the epididymis of mice (47). Furthermore, in vitro treatment with PFOA augmented ROS production and reduced sperm viability (47). The study implied that oxidative stress and membrane polyunsaturated fatty acid alteration may be involved in PFOA-induced sperm toxicity. Moreover, PFOA incubation resulted in accumulation in sperm membrane, and perturbed plasma membrane fluidity, mitochondrial respiratory activity and electrochemical potential, indicating that PFOA impacts human sperm motility by plasma membrane disruption (44). Our previous study showed that in vitro PFOA exposure compromised the penetration ability of human spermatozoa by inducing oxidative stress, evoking CatSper-mediated Ca2+ influx, and compromising progesterone-induced response (43). Testicular transcriptome profiling revealed that PFOS exposure led to alterations in microtubule-based movement, microtubule motor activity, cilium movement, cytoskeleton and spermatid development (51). In addition, PFOS altered sperm membrane lipid composition reflected by elevated ratio of cholesterol to phospholipids in male zebrafish (53), suggesting that PFOS may sperm function through disrupting membrane fluidity. These findings may help explain the abnormalities in sperm morphology and motility caused by PFASs.
Conclusions
We have summarized the toxicological effects of PFASs on sperm using recent epidemiological and experimental data. Increasing evidence suggests that exposure to PFASs is adversely associated with sperm quality, and their mechanisms of toxicity might be involved in testicular and epididymal damage, testosterone synthesis disorder, and oxidative stress, membrane lipid composition alteration and Ca2+ influx in sperm. However, due to the limited number of epidemiological studies, the reproductive health risk of human exposure to PFASs, especially their emerging alternatives, needs further investigation.
Author contributions
ZS and YW: Writing-original draft preparation, writing-review and editing. BW and SD: Writing-review & editing. FZ and ZF: Data collection, conceptualization. YY: Writing-review & editing, conceptualization. DZ: Supervision, writing-review and editing, funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (82171606).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chang X, Xue Y, Li J, Zou L, Tang M. Potential health impact of environmental micro- and nanoplastics pollution. J Appl Toxicol (2020) 40:4–15. doi: 10.1002/jat.3915
2. Skakkebaek NE, Lindahl-Jacobsen R, Levine H, Andersson AM, Jorgensen N, Main KM, et al. Environmental factors in declining human fertility. Nat Rev Endocrinol (2022) 18:139–57. doi: 10.1038/s41574-021-00598-8
3. Rosa-Villagran L, Barrera N, Montes J, Riso C, Sapiro R. Decline of semen quality over the last 30 years in Uruguay. Basic Clin Androl (2021) 31:8. doi: 10.1186/s12610-021-00128-6
4. Siqueira S, Ropelle AC, Nascimento JAA, Fazano FAT, Bahamondes LG, Gabiatti JR, et al. Changes in seminal parameters among Brazilian men between 1995 and 2018. Sci Rep (2020) 10:6430. doi: 10.1038/s41598-020-63468-9
5. Bahri H, Ben Khalifa M, Ben Rhouma M, Abidi Z, Abbassi E, Ben Rhouma K, et al. Decline in semen quality of north African men: a retrospective study of 20,958 sperm analyses of men from different north African countries tested in Tunisia over a period of 6 years (2013-2018). Ann Hum Biol (2021) 48:350–9. doi: 10.1080/03014460.2021.1957501
6. Centola GM, Blanchard A, Demick J, Li S, Eisenberg ML. Decline in sperm count and motility in young adult men from 2003 to 2013: observations from a U.S. sperm bank. Andrology (2016) 4:270–6. doi: 10.1111/andr.12149
7. Punjani N, Alawamlh OA, Kim SJ, Salter CA, Wald G, Feliciano M, et al. Changes in semen analysis over time: A temporal trend analysis of 20 years of subfertile non-azoospermic men. World J Mens Health (2022) 40:e46. doi: 10.5534/wjmh.210201
8. Huang C, Li B, Xu K, Liu D, Hu J, Yang Y, et al. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil Steril (2017) 107:83–8 e2. doi: 10.1016/j.fertnstert.2016.09.035
9. Mishra P, Negi MPS, Srivastava M, Singh K, Rajender S. Decline in seminal quality in Indian men over the last 37 years. Reprod Biol Endocrinol (2018) 16:103. doi: 10.1186/s12958-018-0425-z
10. Krzastek SC, Farhi J, Gray M, Smith RP. Impact of environmental toxin exposure on male fertility potential. Transl Androl Urol (2020) 9:2797–813. doi: 10.21037/tau-20-685
11. Green MP, Harvey AJ, Finger BJ, Tarulli GA. Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period. Environ Res (2021) 194:110694. doi: 10.1016/j.envres.2020.110694
12. Pizzol D, Foresta C, Garolla A, Demurtas J, Trott M, Bertoldo A, et al. Pollutants and sperm quality: a systematic review and meta-analysis. Environ Sci pollut Res Int (2021) 28:4095–103. doi: 10.1007/s11356-020-11589-z
13. Cousins IT, DeWitt JC, Gluge J, Goldenman G, Herzke D, Lohmann R, et al. The high persistence of PFAS is sufficient for their management as a chemical class. Environ Sci Process Impacts (2020) 22:2307–12. doi: 10.1039/d0em00355g
14. Panieri E, Baralic K, Djukic-Cosic D, Buha Djordjevic A, Saso L. PFAS molecules: A major concern for the human health and the environment. Toxics (2022) 10:44. doi: 10.3390/toxics10020044
15. Evich MG, Davis MJB, McCord JP, Acrey B, Awkerman JA, Knappe DRU, et al. Per- and polyfluoroalkyl substances in the environment. Science (2022) 375:eabg9065. doi: 10.1126/science.abg9065
16. Guruge KS, Taniyasu S, Yamashita N, Wijeratna S, Mohotti KM, Seneviratne HR, et al. Perfluorinated organic compounds in human blood serum and seminal plasma: a study of urban and rural tea worker populations in Sri Lanka. J Environ Monit (2005) 7:371–7. doi: 10.1039/b412532k
17. Song X, Tang S, Zhu H, Chen Z, Zang Z, Zhang Y, et al. Biomonitoring PFAAs in blood and semen samples: Investigation of a potential link between PFAAs exposure and semen mobility in China. Environ Int (2018) 113:50–4. doi: 10.1016/j.envint.2018.01.010
18. Pan Y, Cui Q, Wang J, Sheng N, Jing J, Yao B, et al. Profiles of emerging and legacy per-/Polyfluoroalkyl substances in matched serum and semen samples: New implications for human semen quality. Environ Health Perspect (2019) 127:127005. doi: 10.1289/EHP4431
19. Cui Q, Pan Y, Wang J, Liu H, Yao B, Dai J. Exposure to per- and polyfluoroalkyl substances (PFASs) in serum versus semen and their association with male reproductive hormones. Environ Pollut (2020) 266:115330. doi: 10.1016/j.envpol.2020.115330
20. Calafat AM, Kato K, Hubbard K, Jia T, Botelho JC, Wong LY. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: Paired serum-urine data from the 2013-2014 national health and nutrition examination survey. Environ Int (2019) 131:105048. doi: 10.1016/j.envint.2019.105048
21. Gockener B, Weber T, Rudel H, Bucking M, Kolossa-Gehring M. Human biomonitoring of per- and polyfluoroalkyl substances in German blood plasma samples from 1982 to 2019. Environ Int (2020) 145:106123. doi: 10.1016/j.envint.2020.106123
22. Liu D, Tang B, Nie S, Zhao N, He L, Cui J, et al. Distribution of per- and poly-fluoroalkyl substances and their precursors in human blood. J Hazard Mater (2023) 441:129908. doi: 10.1016/j.jhazmat.2022.129908
23. Wang B, Chen Q, Shen L, Zhao S, Pang W, Zhang J. Perfluoroalkyl and polyfluoroalkyl substances in cord blood of newborns in shanghai, China: Implications for risk assessment. Environ Int (2016) 97:7–14. doi: 10.1016/j.envint.2016.10.008
24. Richterova D, Govarts E, Fabelova L, Rausova K, Rodriguez Martin L, Gilles L, et al. PFAS levels and determinants of variability in exposure in European teenagers - results from the HBM4EU aligned studies (2014-2021). Int J Hyg Environ Health (2022) 247:114057. doi: 10.1016/j.ijheh.2022.114057
25. Roth K, Imran Z, Liu W, Petriello MC. Diet as an exposure source and mediator of per- and polyfluoroalkyl substance (PFAS) toxicity. Front Toxicol (2020) 2:601149. doi: 10.3389/ftox.2020.601149
26. Calvert L, Green MP, De Iuliis GN, Dun MD, Turner BD, Clarke BO, et al. Assessment of the emerging threat posed by perfluoroalkyl and polyfluoroalkyl substances to Male reproduction in humans. Front Endocrinol (Lausanne) (2021) 12:799043. doi: 10.3389/fendo.2021.799043
27. Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol (2019) 29:131–47. doi: 10.1038/s41370-018-0094-1
28. Jian JM, Chen D, Han FJ, Guo Y, Zeng L, Lu X, et al. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci Total Environ (2018) 636:1058–69. doi: 10.1016/j.scitotenv.2018.04.380
29. Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect (2007) 115:1298–305. doi: 10.1289/ehp.10009
30. Forsthuber M, Kaiser AM, Granitzer S, Hassl I, Hengstschlager M, Stangl H, et al. Albumin is the major carrier protein for PFOS, PFOA, PFHxS, PFNA and PFDA in human plasma. Environ Int (2020) 137:105324. doi: 10.1016/j.envint.2019.105324
31. Liu JJ, Cui XX, Tan YW, Dong PX, Ou YQ, Li QQ, et al. Per- and perfluoroalkyl substances alternatives, mixtures and liver function in adults: A community-based population study in China. Environ Int (2022) 163:107179. doi: 10.1016/j.envint.2022.107179
32. Yu G, Jin M, Huang Y, Aimuzi R, Zheng T, Nian M, et al. Environmental exposure to perfluoroalkyl substances in early pregnancy, maternal glucose homeostasis and the risk of gestational diabetes: A prospective cohort study. Environ Int (2021) 156:106621. doi: 10.1016/j.envint.2021.106621
33. Averina M, Brox J, Huber S, Furberg AS. Exposure to perfluoroalkyl substances (PFAS) and dyslipidemia, hypertension and obesity in adolescents. the fit futures study. Environ Res (2021) 195:110740. doi: 10.1016/j.envres.2021.110740
34. Feng X, Long G, Zeng G, Zhang Q, Song B, Wu KH. Association of increased risk of cardiovascular diseases with higher levels of perfluoroalkylated substances in the serum of adults. Environ Sci Pollut Res Int (2022) 29:89081–92. doi: 10.1007/s11356-022-22021-z
35. Chang CJ, Barr DB, Ryan PB, Panuwet P, Smarr MM, Liu K, et al. Per- and polyfluoroalkyl substance (PFAS) exposure, maternal metabolomic perturbation, and fetal growth in African American women: A meet-in-the-middle approach. Environ Int (2022) 158:106964. doi: 10.1016/j.envint.2021.106964
36. Carwile JL, Seshasayee SM, Ahrens KA, Hauser R, Driban JB, Rosen CJ, et al. Serum PFAS and urinary phthalate biomarker concentrations and bone mineral density in 12-19 year olds: 2011-2016 NHANES. J Clin Endocrinol Metab (2022) 107:e3343–e52. doi: 10.1210/clinem/dgac228
37. Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebaek NE, Jorgensen N. Do perfluoroalkyl compounds impair human semen quality? Environ Health Perspect (2009) 117:923–7. doi: 10.1289/ehp.0800517
38. Di Nisio A, Sabovic I, Valente U, Tescari S, Rocca MS, Guidolin D, et al. Endocrine disruption of androgenic activity by perfluoroalkyl substances: Clinical and experimental evidence. J Clin Endocrinol Metab (2019) 104:1259–71. doi: 10.1210/jc.2018-01855
39. Haervig KK, Petersen KU, Hougaard KS, Lindh C, Ramlau-Hansen CH, Toft G, et al. Maternal exposure to per- and polyfluoroalkyl substances (PFAS) and Male reproductive function in young adulthood: Combined exposure to seven PFAS. Environ Health Perspect (2022) 130:107001. doi: 10.1289/EHP10285
40. Vested A, Ramlau-Hansen CH, Olsen SF, Bonde JP, Kristensen SL, Halldorsson TI, et al. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ Health Perspect (2013) 121:453–8. doi: 10.1289/ehp.1205118
41. Toft G, Jonsson BA, Lindh CH, Giwercman A, Spano M, Heederik D, et al. Exposure to perfluorinated compounds and human semen quality in Arctic and European populations. Hum Reprod (2012) 27:2532–40. doi: 10.1093/humrep/des185
42. Louis GM, Chen Z, Schisterman EF, Kim S, Sweeney AM, Sundaram R, et al. Perfluorochemicals and human semen quality: the LIFE study. Environ Health Perspect (2015) 123:57–63. doi: 10.1289/ehp.1307621
43. Yuan Y, Ding X, Cheng Y, Kang H, Luo T, Zhang X, et al. PFOA evokes extracellular Ca2+ influx and compromises progesterone-induced response in human sperm. Chemosphere (2020) 241:125074. doi: 10.1016/j.chemosphere.2019.125074
44. Sabovic I, Cosci I, De Toni L, Ferramosca A, Stornaiuolo M, Di Nisio A, et al. Perfluoro-octanoic acid impairs sperm motility through the alteration of plasma membrane. J Endocrinol Invest (2020) 43:641–52. doi: 10.1007/s40618-019-01152-0
45. Ortiz-Sanchez PB, Roa-Espitia AL, Fierro R, Lopez-Torres AS, Jimenez-Morales I, Oseguera-Lopez I, et al. Perfluorooctane sulfonate and perfluorooctanoic acid induce plasma membrane dysfunction in boar spermatozoa during in vitro capacitation. Reprod Toxicol (2022) 110:85–96. doi: 10.1016/j.reprotox.2022.03.013
46. Oseguera-Lopez I, Perez-Cerezales S, Ortiz-Sanchez PB, Mondragon-Payne O, Sanchez-Sanchez R, Jimenez-Morales I, et al. Perfluorooctane sulfonate (PFOS) and perfluorohexane sulfonate (PFHxS) alters protein phosphorylation, increase ROS levels and DNA fragmentation during In vitro capacitation of boar spermatozoa. Anim (Basel) (2020) 10:1934. doi: 10.3390/ani10101934
47. Lu Y, Pan Y, Sheng N, Zhao AZ, Dai J. Perfluorooctanoic acid exposure alters polyunsaturated fatty acid composition, induces oxidative stress and activates the AKT/AMPK pathway in mouse epididymis. Chemosphere (2016) 158:143–53. doi: 10.1016/j.chemosphere.2016.05.071
48. Zhang H, Lu Y, Luo B, Yan S, Guo X, Dai J. Proteomic analysis of mouse testis reveals perfluorooctanoic acid-induced reproductive dysfunction via direct disturbance of testicular steroidogenic machinery. J Proteome Res (2014) 13:3370–85. doi: 10.1021/pr500228d
49. Ma X, Ren X, Zhang X, Griffin N, Liu H, Wang L. Rutin ameliorates perfluorooctanoic acid-induced testicular injury in mice by reducing oxidative stress and improving lipid metabolism. Drug Chem Toxicol (2022) 13:1–12. doi: 10.1080/01480545.2022.2145483
50. Umar Ijaz M, Rauf A, Mustafa S, Ahmed H, Ashraf A, Al-Ghanim K, et al. Pachypodol attenuates perfluorooctane sulphonate-induced testicular damage by reducing oxidative stress. Saudi J Biol Sci (2022) 29:1380–5. doi: 10.1016/j.sjbs.2021.12.012
51. Li Z, Lin Z, Ji S, Lai KP, Wan HT, Wong CKC, et al. Perfluorooctanesulfonic acid exposure altered hypothalamic metabolism and disturbed male fecundity. Sci Total Environ (2022) 844:156881. doi: 10.1016/j.scitotenv.2022.156881
52. Yin J, Jian Z, Zhu G, Yu X, Pu Y, Yin L, et al. Male Reproductive toxicity involved in spermatogenesis induced by perfluorooctane sulfonate and perfluorooctanoic acid in caenorhabditis elegans. Environ Sci Pollut Res Int (2021) 28:1443–53. doi: 10.1007/s11356-020-10530-8
53. Wang M, Chen J, Lin K, Chen Y, Hu W, Tanguay RL, et al. Chronic zebrafish PFOS exposure alters sex ratio and maternal related effects in F1 offspring. Environ Toxicol Chem (2011) 30:2073–80. doi: 10.1002/etc.594
54. Lu Y, Luo B, Li J, Dai J. Perfluorooctanoic acid disrupts the blood-testis barrier and activates the TNFα/p38 MAPK signaling pathway in vivo and in vitro. Arch Toxicol (2016) 90:971–83. doi: 10.1007/s00204-015-1492-y
55. Peng BX, Li F, Mortimer M, Xiao X, Ni Y, Lei Y, et al. Perfluorooctanoic acid alternatives hexafluoropropylene oxides exert male reproductive toxicity by disrupting blood-testis barrier. Sci Total Environ (2022) 846:157313. doi: 10.1016/j.scitotenv.2022.157313
56. Qiu L, Qian Y, Liu Z, Wang C, Qu J, Wang X, et al. Perfluorooctane sulfonate (PFOS) disrupts blood-testis barrier by down-regulating junction proteins via p38 MAPK/ATF2/MMP9 signaling pathway. Toxicology (2016) 373:1–12. doi: 10.1016/j.tox.2016.11.003
57. Lu Y, Wang J, Guo X, Yan S, Dai J. Perfluorooctanoic acid affects endocytosis involving clathrin light chain a and microRNA-133b-3p in mouse testes. Toxicol Appl Pharmacol (2017) 318:41–8. doi: 10.1016/j.taap.2017.01.014
58. Liu W, Yang B, Wu L, Zou W, Pan X, Zou T, et al. Involvement of NRF2 in perfluorooctanoic acid-induced testicular damage in Male mice. Biol Reprod (2015) 93:41. doi: 10.1095/biolreprod.115.128819
59. Bao J, Zhang Y, Zhang L, Wang X. Effects of maternal exposure to PFOA on testes of male offspring mice. Chemosphere (2021) 272:129585. doi: 10.1016/j.chemosphere.2021.129585
60. Song P, Li D, Wang X, Zhong X. Effects of perfluorooctanoic acid exposure during pregnancy on the reproduction and development of male offspring mice. Andrologia (2018) 50:e13059. doi: 10.1111/and.13059
61. Christin-Maitre S, Young J. Androgens and spermatogenesis. Ann Endocrinol (Paris) (2022) 83:155–8. doi: 10.1016/j.ando.2022.04.010
62. Zhou Y, Hu LW, Qian ZM, Chang JJ, King C, Paul G, et al. Association of perfluoroalkyl substances exposure with reproductive hormone levels in adolescents: By sex status. Environ Int (2016) 94:189–95. doi: 10.1016/j.envint.2016.05.018
63. Joensen UN, Veyrand B, Antignac JP, Blomberg Jensen M, Petersen JH, Marchand P, et al. PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Hum Reprod (2013) 28:599–608. doi: 10.1093/humrep/des425
64. Lopez-Doval S, Salgado R, Lafuente A. The expression of several reproductive hormone receptors can be modified by perfluorooctane sulfonate (PFOS) in adult male rats. Chemosphere (2016) 155:488–97. doi: 10.1016/j.chemosphere.2016.04.081
65. Lopez-Doval S, Salgado R, Pereiro N, Moyano R, Lafuente A. Perfluorooctane sulfonate effects on the reproductive axis in adult male rats. Environ Res (2014) 134:158–68. doi: 10.1016/j.envres.2014.07.006
66. Wan HT, Zhao YG, Wong MH, Lee KF, Yeung WS, Giesy JP, et al. Testicular signaling is the potential target of perfluorooctanesulfonate-mediated subfertility in male mice. Biol Reprod (2011) 84:1016–23. doi: 10.1095/biolreprod.110.089219
67. Zhang H, Lu H, Chen P, Chen X, Sun C, Ge RS, et al. Effects of gestational perfluorooctane sulfonate exposure on the developments of fetal and adult leydig cells in F1 males. Environ Pollut (2020) 262:114241. doi: 10.1016/j.envpol.2020.114241
68. Lu H, Zhang H, Gao J, Li Z, Bao S, Chen X, et al. Effects of perfluorooctanoic acid on stem leydig cell functions in the rat. Environ Pollut (2019) 250:206–15. doi: 10.1016/j.envpol.2019.03.120
69. Li L, Li X, Chen X, Chen Y, Liu J, Chen F, et al. Perfluorooctane sulfonate impairs rat leydig cell development during puberty. Chemosphere (2018) 190:43–53. doi: 10.1016/j.chemosphere.2017.09.116
70. Zhang DY, Xu XL, Shen XY, Ruan Q, Hu WL. Analysis of apoptosis induced by perfluorooctane sulfonates (PFOS) in mouse leydig cells in vitro. Toxicol Mech Methods (2015) 25:21–5. doi: 10.3109/15376516.2014.971140
Keywords: per- and polyfluoroalkyl substances, reproductive toxicity, sperm, testosterone, male fecundity
Citation: Sun Z, Wen Y, Wang B, Deng S, Zhang F, Fu Z, Yuan Y and Zhang D (2023) Toxic effects of per- and polyfluoroalkyl substances on sperm: Epidemiological and experimental evidence. Front. Endocrinol. 14:1114463. doi: 10.3389/fendo.2023.1114463
Received: 02 December 2022; Accepted: 07 February 2023;
Published: 20 February 2023.
Edited by:
Katja Teerds, Wageningen University and Research, NetherlandsReviewed by:
Xiaoning Zhang, Nantong University, ChinaYuling Mi, Zhejiang University, China
Xinbao Ding, Cornell University, United States
Copyright © 2023 Sun, Wen, Wang, Deng, Zhang, Fu, Yuan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dalei Zhang, emhhbmdkYWxlaUBuY3UuZWR1LmNu
†These authors have contributed equally to this work
 Zhangbei Sun1†
Zhangbei Sun1† Yiqian Wen
Yiqian Wen Dalei Zhang
Dalei Zhang