- 1Center for Pharmacological and Botanical Studies (CEFYBO-UBA-CONICET), Medical Faculty, Buenos Aires University, Buenos Aires, Argentina
- 2Shenzhen Key Laboratory of Reproductive Immunology for Peri-implantation, Shenzhen Zhongshan Institute for Reproduction and Genetics, Fertility Center, Shenzhen Zhongshan Urology Hospital, Shenzhen, China
- 3Centro Integrativo de Biología Y Química Aplicada. Universidad Bernardo O’Higgins, Santiago, Chile
Systemic lupus erythematosus is a debilitating autoimmune disease characterized by uncontrolled activation of adaptive immunity, particularly B cells, which predominantly affects women in a 9 to 1 ratio compared to men. This stark sex disparity strongly suggests a role for female sex hormones in the disease’s onset and progression. Indeed, it is widely recognized that estradiol not only enhances the survival of autoreactive B cells but also stimulates the production of autoantibodies associated with systemic lupus erythematosus, such as anti-nuclear antibodies and anti-dsDNA antibodies. Clinical manifestations of systemic lupus erythematosus typically emerge after puberty and persist throughout reproductive life. Furthermore, symptoms often exacerbate during the premenstrual period and pregnancy, as increased levels of estradiol can contribute to disease flares. Despite being fertile, women with lupus face a heightened risk of pregnancy-related complications, including pregnancy loss and stillbirth, which significantly surpass the rates observed in the healthy population. Therefore, this review aims to summarize and discuss the existing literature on the influence of female sex hormones on B-cell activation in patients with systemic lupus erythematosus, with a particular emphasis on their impact on pregnancy loss.
1 Introduction
Recurrent pregnancy loss (RPL) is a distressing pregnancy disorder experienced by ~2.5% of women trying to conceive. It is defined as the spontaneous demise of two or more clinically recognized pregnancies before the fetus reaches viability; RPL includes embryonic and fetal losses from the time of conception until 24 weeks of gestation (1, 2).
Autoimmune disorders have been included along with chromosomal errors, anatomical uterine defects, and endometrial dysfunction as the most common etiologies linked to RPL (3). Indeed, certain features commonly associated with autoimmune diseases, such as inappropriate complement activation (4–6) and the prevalence of specific autoantibodies (4, 7–11) show strong associations with RPL.
Furthermore, systemic autoimmune diseases, including systemic lupus erythematosus (SLE), have been identified as significant risk factors for RPL, similar to other autoimmune conditions (12).
SLE is a chronic autoimmune disease that predominantly affects women of reproductive age compared to men and has the potential to affect any organ in the body (13–15). The intricate clinical presentation and pathogenesis of SLE make its definition exceptionally challenging. According to the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR), the classification criteria for SLE consist of a mandatory entry criterion of positive anti-nuclear antibodies (ANAs) at least once, followed by additive weighted criteria grouped into seven clinical domains, namely, constitutional, hematologic, neuropsychiatric, mucocutaneous, serosal, musculoskeletal, and renal, and three immunological domains: antiphospholipid antibodies (aPLs), complement proteins, and SLE-specific antibodies (16). ANAs are a group of autoantibodies that target components of the cell nucleus and can bind to proteins, nucleic acids, and protein–nucleic acid complexes (17).
From an immunological perspective, the intricate interplay of environmental, genetic, and hormonal factors results in dysregulation and abnormal activation of the innate and adaptive immune system. This leads to the generation of pathogenic autoantibodies, such as ANAs, anti-double-stranded DNA antibodies (anti-dsDNA), and aPLs, as well as the deposition of immune complexes, ultimately causing tissue damage (18, 19).
Moreover, the impact of ANAs (20) and the presence of various types of aPLs (21) significantly varies between women with RPL and autoimmune diseases, in comparison to those without autoimmunity (22). Indeed, the rate of pregnancy loss among patients with SLE is substantially higher compared to the general healthy population (3). Furthermore, the stage of SLE that the patient is in at the moment of becoming pregnant, including disease activity and renal involvement, not only impacts the health status of the mother but may also influence fetal and neonatal outcomes (23, 24). In this regard, several studies have found that increased serum levels of IL-6, IL-10, and INF-α in patients with SLE are associated with disease activity (25, 26). Regarding disease activity at the time of conception, numerous prospective studies have recently shown that women with inactive SLE generally experience minimal flares during pregnancy, while those with active SLE face an elevated risk of adverse maternal and fetal outcomes (27–29). These findings are consistent with previous reports, indicating that the rate of live births is lower in patients with clinically active SLE in the 6 months before conception compared to those with inactive disease prior to conception (30). Furthermore, RPL among women with SLE appears to be linked to a higher rate of fetal death, which is associated with the presence of aPLs (31, 32). Furthermore, it is well established that newborn babies born to mothers with SLE can develop neonatal lupus, a rare condition that is not a form of SLE, but rather a condition that affects the newborn due to the transfer of maternal autoantibodies across the placenta during pregnancy (33).
Considering the sex and age predisposition of SLE, female sex hormones are undeniably involved in the pathogenesis of the disease (34). Studies conducted on SLE-prone mice using gonadectomy/hormone deprivation and hormone supplementation have consistently confirmed this association, revealing that estrogen exacerbates the disease, while its removal ameliorates the disease in female subjects [reviewed in (35)]. In the context of pregnancy, the increase in female sex hormone levels may influence or potentiate the abnormal function of the immune cells in patients with SLE, thereby exacerbating the disease symptoms and leading to pregnancy complications, including RPL (36, 37).
Considering all the evidence, the objective of this review is to examine the current state of knowledge regarding the impact of preexisting SLE on the development of RPL, with particular focus on the role of female sex hormones in B cell activation and autoantibody production.
2 Pregnancy in patients with systemic lupus erythematosus: the impact on recurrent pregnancy loss
As mentioned earlier, SLE predominantly affects women during their reproductive age, when individuals may seek to become pregnant. However, while fertility is generally preserved in women with SLE, pregnancy in these patients can be associated with adverse maternal and fetal outcomes, including RPL (38). In a recent meta-analysis of pregnancy studies published from 2017 to 2019, it was shown that patients with SLE had markedly increased risk of stillbirth (risk ratio (RR) 16.49, 95% CI 2.95 to 92.13; p =0 .001) and fetal loss (RR 7.55, 95% CI 4.75 to 11.99; p = 0.00001) compared to healthy pregnant women (39). Despite substantial declines in rates of pregnancy loss among patients with SLE in recent years, they remain higher compared to the healthy population (40). Indeed, approximately 20% of pregnancies in patients with SLE result in miscarriages (40).
Several biomarkers have been investigated as potential predictors of pregnancy complications in women with SLE. Notably, aPLs, including anticardiolipin antibodies and lupus anticoagulants, have been associated with obstetric complications such as RPL, recurrent implantation failure, pre-eclampsia, and preterm birth (41, 42). Additionally, research has shown that low levels of complement proteins, such as C3 and C4 during the first trimester are associated with an increased risk of pregnancy loss (43) in patients with SLE.
Although the causes behind poor pregnancy outcomes in patients with SLE are diverse, there is a general consensus that active disease, characterized by the activation of autoreactive B cells and production of autoantibodies, at the time of conception and during pregnancy significantly impacts maternal and fetal outcomes (38). This is not surprising, given that a successful pregnancy relies on a precisely regulated balance between maternal immune activation and immune tolerance (44). Any disruptions or imbalances in this delicate equilibrium can lead to pregnancy loss. Conversely, during pregnancy, an increase in the levels of female sex hormones can promote B cell autoreactivity and exacerbate the symptoms of SLE, creating a negative feedback loop. This phenomenon leads to the activation of various immune mechanisms, which can not only worsen the symptoms of SLE but also contribute to pregnancy loss. Therefore, the hormonal regulation of B cell activation during SLE and its implication in pregnancy loss will be discussed in greater detail below.
3 The impact of female sex hormones on B cell activation in patients with systemic lupus erythematosus
B cells are essential components of the adaptive immune system responsible for antibody production. They can be classified into marginal zone (MZ), B1, and B2 B cells based on their phenotype, localization, and functionality (45). While T cell activation relies on antigen presentation by antigen-presenting cells (APCs), B cells, on the other hand, can directly interact with antigens through their receptor (B cell receptor, BCR) (46). However, apart from the signal provided by antigens through BCR, B cells require a second signal for proper activation, which can be delivered by toll-like receptors (TLRs), BAFF-R, or BCR cross-linking in the case of MZ and B1 B cells (47). On the other hand, upon antigen recognition, B2 B cells migrate to the germinal center, where they receive a second signal from follicular T-helper (Tfh) cells. Subsequently, they mature into either antibody-producing plasma cells or memory B cells.
Female sex hormones play a significant role in the development and activity of the immune system (48). Both innate and adaptive immune cells bear receptors for sex hormones and respond to hormonal cues (49). Women display higher frequencies of B cells (50) along with enhanced B cell survival, maturation, and class switching. They also demonstrate greater antibody responses and higher basal levels of immunoglobulins (Igs) compared to men (51), suggesting the involvement of female sex hormones in controlling diverse B cell functions. Indeed, estrogen has been shown to reduce the production of B cell precursors, impair B cell tolerance, and increase the activation and survival of autoreactive B cells (52, 53). While B cells express both estrogen receptor (ER) α and β, it is ERα that predominantly regulates BCR signal strength (54). Elevated levels of estrogen and ERα engagement result in reduced BCR signal strength and modulation of survival regulators such as Bcl-2, CD22, and SH2-containing protein tyrosine phosphatase (SHP)-2, thereby suppressing apoptosis (52). Moreover, elevated estrogen levels result in increased serum BAFF levels, which, together with reduced BCR signal strength, promote the survival of autoreactive B cells that would otherwise be eliminated from the naive repertoire. Consequently, these autoreactive B cells gain entry into the mature B cell pool (55, 56). In such circumstances, heightened estrogen stimulation on B cells triggers a breakdown of tolerance and uncontrolled proliferation and enhances the survival of high-affinity DNA-reactive B cells, which may potentially lead to autoimmunity (54).
A significant proportion of autoreactive B cells originates from the B2 B cell pool, which requires second signals provided by follicular T helper cells to complete their activation. The significance of these pathways in promoting autoantibody production has been demonstrated in genetically modified lupus-prone mice and using blocking antibodies against various costimulatory molecules, such as inducible costimulatory ligand (ICOS-L) and CD40 ligand. Consequently, T helper cells play a crucial role in the development and progression of SLE disease (57). Furthermore, Tfh cells not only express estrogen receptors, but it has also been demonstrated that estradiol promotes the expansion of Tfh cells and, consequently, enhances the humoral immune response (58). Therefore, in the context of SLE, estradiol appears to exert its effects on the Tfh/B2 B cell axis, promoting the development and survival of autoreactive B cells.
The fact that 90% of patients with SLE are women clearly highlights a strong sex bias in this autoimmune disease. Several hypotheses have been proposed to explain this phenomenon, with the influence of female sex hormones being the most widely accepted (59). In this regard, it is known that the clinical manifestation of the disease typically appears after puberty, affecting women between the ages of 20 to 50, a period during which levels of estradiol and progesterone significantly rise (59). The strongest evidence supporting the role of female sex hormones in SLE comes from the observation that patients with SLE experience disease exacerbation during the premenstrual period and in pregnancy (35, 59). Interestingly, a case report demonstrated that administering cross-gender hormones to a transgender female resulted in lupus nephritis, and the withdrawal of estradiol supplementation upon admission prevented the worsening of symptoms. This provides further support for the role of estradiol in driving SLE (60). Animal studies also provide support for the role of estrogen in SLE. Ovariectomized lupus-prone mice showed ameliorated disease, while estrogen supplementation in castrated male mice worsened the symptoms [reviewed in (35)]. Moreover, targeted deletion of ERα specifically in B cells has been shown to reduce the production of pathogenic autoantibodies and the development of nephritis in lupus-prone mice (61). Additionally, tamoxifen treatment significantly reduced autoantibody production and improved the course of SLE in SLE-prone mice (62).
In pregnant SLE patients, estrogen levels and ERα expression not only mediate the increase in anti-dsDNA but also alter the B-cell repertoire, leading to the expansion of autoreactive clones (63, 64). As a result, hormone levels during pregnancy have a substantial impact on the function of autoreactive B cells, intensifying SLE symptoms and contributing to adverse pregnancy outcomes, including RPL (36, 37). In fact, E2 has been demonstrated to decrease B-cell lymphopoiesis in the bone marrow at the pro–B-cell stages in mice and to alter transitional 2 (T2) B cell maturation, both during pregnancy and in patients with SLE (53, 65). Under SLE conditions, elevated BAFF levels and reduced BCR signal strength can lead to the maturation of transitional B cells into a marginal zone (MZ) B cell expansion. Under specific conditions, marginal zone (MZ) B cells can serve as precursors of unswitched memory B cells without T cell help (66). It has been previously demonstrated that during pregnancy, there is a bias toward the development of marginal zone (MZ) B cells (67). This, along with the abnormal differentiation of unswitched memory B cells observed in patients with SLE (68) may pose a risk to the successful development of pregnancy in patients with SLE. In fact, an increase in unswitched memory B cells is observed in patients with a history of RPL and obstetric complications (69, 70).
Therefore, the presence of autoreactive B cells, along with increased B cell activation and autoantibody production in patients with SLE, poses significant challenges when it comes to achieving a full-term pregnancy.
4 B-cell activation and autoantibody production in lupus: impact on pregnancy well-being
Upon activation, B cells undergo a series of tightly regulated processes that culminate in the differentiation of highly specialized cells capable of producing antibodies, as well as memory B cells (45). In addition to antibodies, activated B cells can produce a wide range of cytokines, especially when their activation goes through their BCR together with BAFF-R (71). Signaling through the BAFF-R activates several downstream pathways, including NF-KB, ERK, and MAPK, which regulate the survival functions of immature, transitional, and mature B cells (72, 73). Interestingly, it has been demonstrated that B cells from pregnant women show downregulation of transcripts associated with these pathways (74) along with reduced levels of BAFF in serum as pregnancy progresses (75), suggesting that B cells are less susceptible to being activated during pregnancy. Indeed, a transcriptomic analysis performed on B cells isolated from pregnant mice confirmed that several B cell activation pathways, including BCR, TLR, and BAFF-R, are significantly diminished compared to B cells from non-pregnant control animals (44). Furthermore, a study by Valeff et al. (44) found that B cells isolated from pregnant women in the first trimester of pregnancy produced significantly lower levels of inflammatory cytokines when activated through their BCR and TLRs compared to B cells from non-pregnant women, reinforcing the notion of B cells being less susceptible to activation, at least during the early stages of pregnancy.
In the context of SLE, aberrant B-cell activation plays a significant role in the pathogenesis of the disease. Dysregulation of BCR and BAFF-R pathways are common and dominant factors involved in this aberrant B-cell activation (76). Furthermore, patients with SLE exhibit elevated levels of BAFF in their serum (77–79), strongly indicating the involvement of the BAFF-R pathway in B cells as a key component of SLE pathology. Indeed, mice overexpressing BAFF develop a lupus-like disease characterized by the production of ANAs and anti-dsDNA (80).
In the context of pregnancy, while the production of natural and protective antibodies is related to pregnancy success (81, 82), the presence of autoantibodies is associated with RPL (8). There is growing evidence suggesting that ANAs can play a role in both early pregnancy complications, such as embryo implantation, and pregnancy loss (83). While low titers of ANAs are common in healthy women, those with RPL often exhibit high titers of ANAs (>1:160) (83). Moreover, ANAs have been suggested to have a direct effect on the quality and development of oocytes and embryos, leading to reduced implantation rates (84). In the fetal-maternal interface, ANAs can induce the precipitation of immune complexes, attributed to elevated C3 levels, resulting in T cell activation and increased production of inflammatory cytokine (IFN-α), which in turn stimulates the humoral immune response (85, 86). Complement activation rapidly increases the production of the pro-inflammatory cytokine TNF, which in turn recruits inflammatory cells into the placenta, ultimately contributing to pregnancy loss (87).
It is well known that imbalances toward a pro-inflammatory milieu are associated with poor pregnancy outcomes (88). Moreover, the TNF/IL-10 ratio in serum is used as an indicator or predictor of pregnancy loss (89). In line with this, the production of IL-10 by B cells is considered essential for successful pregnancies (90). Interestingly, in patients with SLE, there is a significant decrease in IL-10 production by B cells (91). Even though, the elevated serum levels of IL-10 observed in pregnant women with SLE compared to controls (25) would be an advantage in normal pregnancy conditions, the immunosuppressive and anti-inflammatory effects of this cytokine are impaired in patients with SLE compared to healthy individuals (92).
Therefore, it is reasonable to speculate that uncontrolled B cell activation in patients with SLE during gestation may lead to the production of pro-inflammatory cytokines and harmful antibodies, which could potentially compromise the well-being of the pregnancy.
In conclusion, maintaining a balanced B-cell activation is essential for a successful pregnancy. In women with preexisting SLE, hormonal changes may disrupt this balance, leading to the production of inflammatory cytokines and autoantibodies. This dysregulation can exacerbate disease symptoms and contribute to pregnancy complications, including RPL. Therefore, understanding the impact of B-cell activation and its relationship with hormonal changes during gestation is crucial for managing SLE and optimizing pregnancy outcomes (Figure 1).
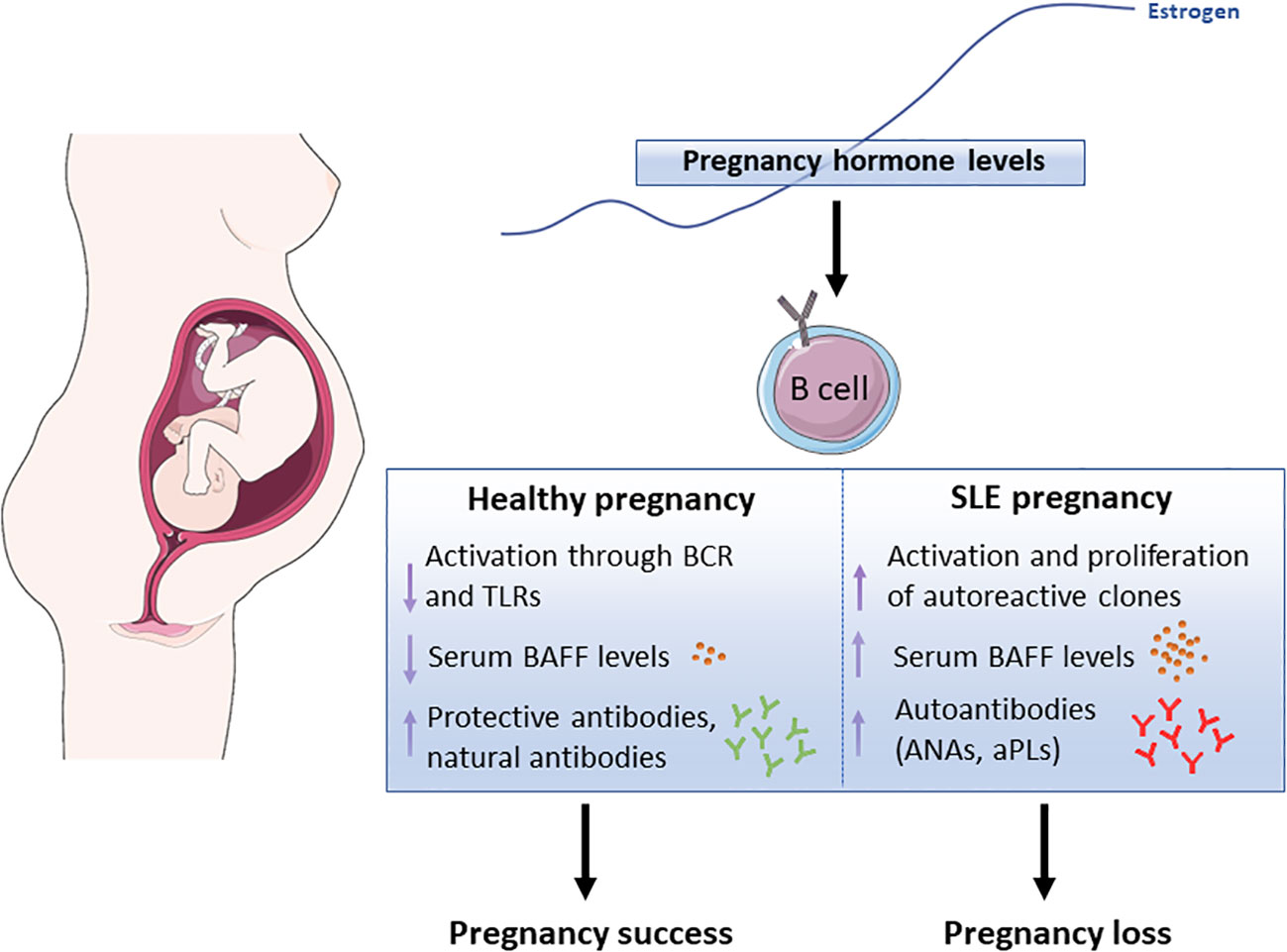
Figure 1 Schematic representation showing the potential effect of pregnancy-associated hormones, in particular estrogen, in B cell functions both, in healthy pregnancy and lupus pregnancy. BCR (B cell receptor), TLR (Toll-Like Receptor), BAFF (B Cell-Activating Factor), ANAs (anti-Nuclear Antibodies), aPLs (anti-Phospholipid Antibodies). The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/)
Author contributions
NV designed, drafted, and revised the work. MV designed and drafted the work. LD drafted and revised the work. FJ designed, drafted, supervised, and revised the work. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by a grant from Agencia Nacional de Promoción Cientıfíca y Tecnológica (PICT-2020-00393) to FJ and Natural Science Foundation of Guangdong Province-General Programme, China (2022A1515010650) to LD.
Acknowledgments
We especially thank the personal staff from the CEFYBO: Patricia Fernandez, María Alejandra Veron, María Cristian Lincon, Alberto Capriolo, and Alcira Mazziotti.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Andersen A-MN. Maternal age and fetal loss: population based register linkage study. BMJ (2000) 320(7251):1708–12. doi: 10.1136/bmj.320.7251.1708
2. Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open (2018) 2018(2):hoy004. doi: 10.1093/hropen/hoy004
3. Fausett MB, Branch DW. Autoimmunity and pregnancy loss. Semin Reprod Med (2000) 18(04):379–92. doi: 10.1055/s-2000-13728
4. Ohmura K, Oku K, Kitaori T, Amengual O, Hisada R, Kanda M, et al. Pathogenic roles of anti-C1q antibodies in recurrent pregnancy loss. Clin Immunol (2019) 203:37–44. doi: 10.1016/j.clim.2019.04.005
5. Amari Chinchilla K, Vijayan M, Taveras Garcia B, Jim B. Complement-mediated disorders in pregnancy. Adv Chronic Kidney Dis (2020) 27(2):155–64. doi: 10.1053/j.ackd.2020.01.002
6. Girardi G, Lingo JJ, Fleming SD, Regal JF. Essential role of complement in pregnancy: from implantation to parturition and beyond. Front Immunol (2020) 11:1681. doi: 10.3389/fimmu.2020.01681
7. Gleicher N, El-Roeiy A, Confino E, Friberg J. Reproductive failure because of autoantibodies: Unexplained infertility and pregnancy wastage. Am J Obstetrics Gynecol (1989) 160(6):1376–85. doi: 10.1016/0002-9378(89)90858-2
8. D’Ippolito S, Ticconi C, Tersigni C, Garofalo S, Martino C, Lanzone A, et al. The pathogenic role of autoantibodies in recurrent pregnancy loss. Am J Reprod Immunol (2020) 83(1):0–3. doi: 10.1111/aji.13200
9. Edelman PH, Rouquette AM, Verdy E, Elias A, Cabane J, Cornet D, et al. Autoimmunity, fetal losses, lupus anticoagulant: beginning of systemic lupus erythematosus or new autoimmune entity with gynaeco-obstetrical expression? Hum Reprod (1986) 1(5):295–7. doi: 10.1093/oxfordjournals.humrep.a136408
10. Aoki K, Dudkiewicz AB, Matsuura E, Novotny M, Kaherlein G, Gleicher N. Clinical significance of β2-glycoprotein I-dependent anticardiolipin antibodies in the reproductive autoimmune failure syndrome: Correlation with conventional antiphospholipid antibody detection systems. Am J Obstetrics Gynecol (1995) 172(3):926–31. doi: 10.1016/0002-9378(95)90023-3
11. Harger JH, Rabin BS, Marchese SG. The prognostic value of antinuclear antibodies in women with recurrent pregnancy losses: a prospective controlled study. Obstetrics Gynecol (1989) 73(3 Pt 1):419–24.
12. Gao R, Zeng X, Qin L. Systemic autoimmune diseases and recurrent pregnancy loss: research progress in diagnosis and treatment. Chin Med J (2021) 134(17):2140–2. doi: 10.1097/CM9.0000000000001691
13. Tedeschi SK, Bermas B, Costenbader KH. Sexual disparities in the incidence and course of SLE and RA. Clin Immunol (2013) 149(2):211–8. doi: 10.1016/j.clim.2013.03.003
14. Moulton VR, Suarez-Fueyo A, Meidan E, Li H, Mizui M, Tsokos GC. Pathogenesis of human systemic lupus erythematosus: A cellular perspective. Trends Mol Med (2017) 23(7):615–35. doi: 10.1016/j.molmed.2017.05.006
15. Tsokos GC. Mechanisms of disease systemic lupus erythematosus. N Engl J Med (2011) 22(1):2110–21. doi: 10.1056/NEJMra1100359
16. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 european league against rheumatism/american college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol (Hoboken N.J.) (2019) 71(9):1400–12. doi: 10.1002/art.40930
17. Pisetsky DS. The immunopathogenesis and immunopathology of systemic lupus erythematosus. In Schur P., Massarotti E. (eds) Lupus Erythematosus (New York, NY: Springer) (2012), 13–26. doi: 10.1007/978-1-4614-1189-5_2
18. Choi J, Kim ST, Craft J. The pathogenesis of systemic lupus erythematosus—an update. Curr Opin Immunol (2012) 24(6):651–7. doi: 10.1016/j.coi.2012.10.004
19. Mohan C, Putterman C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat Rev Nephrol (2015) 11(6):329–41. doi: 10.1038/nrneph.2015.33
20. Kwak-Kim J, Skariah A, Wu L, Salazar D, Sung N, Ota K. Humoral and cellular autoimmunity in women with recurrent pregnancy losses and repeated implantation failures: A possible role of vitamin D. Autoimmun Rev (2016) 15(10):943–7. doi: 10.1016/J.AUTREV.2016.07.015
21. Xu J, Chen D, Duan X, Li L, Tang Y, Peng B. The association between antiphospholipid antibodies and late fetal loss: A systematic review and meta-analysis. Acta Obstetricia Gynecologica Scandinavica (2019) 98(12,):1523–33. doi: 10.1111/aogs.13665
22. Mekinian A, Alijotas-Reig J, Carrat F, Costedoat-Chalumeau N, Ruffatti A, Lazzaroni MG, et al. Refractory obstetrical antiphospholipid syndrome: Features, treatment, and outcome in a European multicenter retrospective study. Autoimmun Rev (2017) 16(7):730–4. doi: 10.1016/j.autrev.2017.05.006
23. Branch DW. Immunologic disease and fetal death. Clin Obstetrics Gynecol (1987) 30(2):295–311. doi: 10.1097/00003081-198706000-00009
24. Petri M. Pregnancy and systemic lupus erythematosus. Best Pract Res Clin Obstetrics Gynaecol (2020) 64:24–30. doi: 10.1016/j.bpobgyn.2019.09.002
25. Björkander S, Bremme K, Persson J-O, van Vollenhoven RF, Sverremark-Ekström E, Holmlund U. Pregnancy-associated inflammatory markers are elevated in pregnant women with systemic lupus erythematosus. Cytokine (2012) 59(2):392–9. doi: 10.1016/j.cyto.2012.04.046
26. Ruchakorn N, Ngamjanyaporn P, Suangtamai T, Kafaksom T, Polpanumas C, Petpisit V, et al. Performance of cytokine models in predicting SLE activity. Arthritis Res Ther (2019) 21(1):287. doi: 10.1186/s13075-019-2029-1
27. Petri M, Howard D, Repke J. Frequency of lupus flare in pregnancy. The Hopkins Lupus Pregnancy Center experience. Arthritis Rheumatism (1991) 34(12):1538–45. doi: 10.1002/art.1780341210
28. Clowse MEB, Magder LS, Witter F, Petri M. The impact of increased lupus activity on obstetric outcomes. Arthritis Rheumatism (2005) 52(2):514–21. doi: 10.1002/art.20864
29. Liu J, Zhao Y, Song Y, Zhang W, Bian X, Yang J, et al. Pregnancy in women with systemic lupus erythematosus: a retrospective study of 111 pregnancies in Chinese women. J Maternal-Fetal Neonatal Med (2012) 25(3):261–6. doi: 10.3109/14767058.2011.572310
30. Hayslett JP, Lynn RI. Effect of pregnancy in patients with lupus nephropathy. Kidney Int (1980) 18(2):207–20. doi: 10.1038/ki.1980.129
31. Lockshin MD, Druzin ML, Goei S, Qamar T, Magid MS, Jovanovic L, et al. Antibody to cardiolipin as a predictor of fetal distress or death in pregnant patients with systemic lupus erythematosus. New Engl J Med (1985) 313(3):152–6. doi: 10.1056/NEJM198507183130304
32. Ogasawara M, Aoki K, Hayashi Y. A prospective study on pregnancy risk of antiphospholipid antibodies in association with systemic lupus erythematosus. J Reprod Immunol (1995) 28(2):159–64. doi: 10.1016/0165-0378(94)00912-Q
33. Gryka-Marton M, Szukiewicz D, Teliga-Czajkowska J, Olesinska M. An overview of neonatal lupus with anti-ro characteristics. Int J Mol Sci (2021) 22(17):9281. doi: 10.3390/ijms22179281
34. Sachdeva R, Pal R. A pregnancy hormone-cell death link promotes enhanced lupus-specific immunological effects. Front Immunol (2022) 13:1051779. doi: 10.3389/fimmu.2022.1051779
35. Bose M, Jefferies C. Sex bias in systemic lupus erythematosus: a molecular insight. Immunometabolism (2022) 4(3):e00004. doi: 10.1097/IN9.0000000000000004
36. Peart E, Clowse MEB. Systemic lupus erythematosus and pregnancy outcomes: An update and review of the literature. Curr Opin Rheumatol (2014) 26(2):118–23. doi: 10.1097/BOR.0000000000000030
37. Bundhun PK, Soogund MZS, Huang F. Impact of systemic lupus erythematosus on maternal and fetal outcomes following pregnancy: A meta-analysis of studies published between years 2001–2016. J Autoimmun (2017) 79:17–27. doi: 10.1016/j.jaut.2017.02.009
38. Singh AG, Chowdhary VR. Pregnancy-related issues in women with systemic lupus erythematosus. Int J Rheumatic Dis (2015) 18(2):172–81. doi: 10.1111/1756-185X.12524
39. He WR, Wei H. Maternal and fetal complications associated with systemic lupus erythematosus. Medicine (2020) 99(16):e19797. doi: 10.1097/MD.0000000000019797
40. Clark CA, Spitzer KA, Laskin CA. Decrease in pregnancy loss rates in patients with systemic lupus erythematosus over a 40-year period. J Rheumatol (2005) 32(9):1709–12.
41. Buyon JP, Kim MY, Guerra MM, Laskin CA, Petri M, Lockshin MD, et al. Predictors of pregnancy outcomes in patients with lupus. Ann Internal Med (2015) 163(3):153–63. doi: 10.7326/M14-2235
42. Mekinian A, Cohen J, Alijotas-Reig J, Carbillon L, Nicaise-Roland P, Kayem G, et al. Unexplained recurrent miscarriage and recurrent implantation failure: is there a place for immunomodulation? Am J Reprod Immunol (2016) 76(1):8–28. doi: 10.1111/aji.12493
43. Mankee A, Petri M, Magder LS. Lupus anticoagulant, disease activity and low complement in the first trimester are predictive of pregnancy loss. Lupus Sci Med (2015) 2(1):e000095. doi: 10.1136/lupus-2015-000095
44. Valeff N, Muzzio DO, Matzner F, Dibo M, Golchert J, Homuth G, et al. B cells acquire a unique and differential transcriptomic profile during pregnancy. Genomics (2021) 113(4):2614–22. doi: 10.1016/j.ygeno.2021.06.016
45. Lebien TW, Tedder TF. B lymphocytes: How they develop and function. Blood (2008) 112(5):1570–80. doi: 10.1182/blood-2008-02-078071
46. Rastogi I, Jeon D, Moseman JE, Muralidhar A, Potluri HK, McNeel DG. Role of B cells as antigen presenting cells. Front Immunol (2022) 13:954936. doi: 10.3389/fimmu.2022.954936
47. Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol (2013) 13(2):118–32. doi: 10.1038/nri3383
48. Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol (2003) 38(1):13–22. doi: 10.1016/S0928-8244(03)00202-5
49. Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol (2018) 9:2279(OCT). doi: 10.3389/fimmu.2018.02279
50. Abdullah M, Chai P-S, Chong M-Y, Tohit ERM, Ramasamy R, Pei CP, et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol (2012) 272(2):214–9. doi: 10.1016/j.cellimm.2011.10.009
51. Dodd KC, Menon M. Sex bias in lymphocytes: Implications for autoimmune diseases. Front Immunol (2022) 13:945762(November). doi: 10.3389/fimmu.2022.945762
52. Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest (2002) 109(12):1625–33. doi: 10.1172/jci14873
53. Grimaldi CM, Hill L, Xu X, Peeva E, Diamond B. Hormonal modulation of B cell development and repertoire selection. Mol Immunol (2005) 42(7):811–20. doi: 10.1016/j.molimm.2004.05.014
54. Hill L, Jeganathan V, Chinnasamy P, Grimaldi C, Diamond B. Differential roles of estrogen receptors α and β in control of B-cell maturation and selection. Mol Med (2011) 17(3–4):211–20. doi: 10.2119/molmed.2010.00172
55. Bynoe MS, Grimaldi CM, Diamond B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naïve B cells. Proc Natl Acad Sci (2000) 97(6):2703–8. doi: 10.1073/pnas.040577497
56. Grimaldi CM, Jeganathan V, Diamond B. Hormonal regulation of B cell development: 17β-estradiol impairs negative selection of high-affinity DNA-reactive B cells at more than one developmental checkpoint. J Immunol (2006) 176(5):2703–10. doi: 10.4049/jimmunol.176.5.2703
57. Tenbrock K, Rauen T. T cell dysregulation in SLE. Clin Immunol (2022) 239:109031. doi: 10.1016/j.clim.2022.109031
58. Monteiro C, Kasahara T, Sacramento PM, Dias A, Leite S, Silva VG, et al. Human pregnancy levels of estrogen and progesterone contribute to humoral immunity by activating TFH /B cell axis. Eur J Immunol (2021) 51(1):167–79. doi: 10.1002/eji.202048658
59. Tsokos GC. Systemic lupus erythematosus. New Engl J Med (2011) 365(22):2110–21. doi: 10.1056/NEJMra1100359
60. Hill BG, Hodge B, Misischia R. Lupus nephritis in a transgender woman on cross-sex hormone therapy: a case for the role of oestrogen in systemic lupus erythematosus. Lupus (2020) 29(13):1807–10. doi: 10.1177/0961203320946372
61. Tabor DE, Gould KA. Estrogen receptor alpha promotes lupus in (NZB×NZW)F1 mice in a B cell intrinsic manner. Clin Immunol (2017) 174(3):41–52. doi: 10.1016/j.clim.2016.10.011
62. Sthoeger ZM, Zinger H, Mozes E. Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZBxNZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Ann Rheumatic Dis (2003) 62(4):341–6. doi: 10.1136/ard.62.4.341
63. Doria A, Iaccarino L, Sarzi-Puttini P, Ghirardello A, Zampieri S, Arienti S, et al. Estrogens in pregnancy and systemic lupus erythematosus. Ann New York Acad Sci (2006) 1069(1):247–56. doi: 10.1196/annals.1351.022
64. Cohen-Solal JFG, Jeganathan V, Hill L, Kawabata D, Rodriguez-Pinto D, Grimaldi C, et al. Hormonal regulation of B-cell function and systemic lupus erythematosus. Lupus (2008) 17(6):528–32. doi: 10.1177/0961203308089402
65. Medina KL, Kincade PW. Pregnancy-related steroids are potential negative regulators of B lymphopoiesis. Proc Nati. Acad Sci USA (1994) 91(12):5382–6. doi: 10.1073/pnas.91.12.5382
66. Sanz I, Wei C, Jenks SA, Cashman KS, Tipton C, Woodruff MC, et al. Challenges and opportunities for consistent classification of human B cell and plasma cell populations. Front Immunol (2019) 10:2458(OCT). doi: 10.3389/fimmu.2019.02458
67. Muzzio DO, Soldati R, Ehrhardt J, Utpatel K, Evert M, Zenclussen AC, et al. B cell development undergoes profound modifications and adaptations during pregnancy in mice1. Biol Reprod (2014) 91(5):1–11. doi: 10.1095/biolreprod.114.122366
68. Canny SP, Jackson SW. B cells in systemic lupus erythematosus: from disease mechanisms to targeted therapies. Rheumatic Dis Clinics North America (2021) 47(3):395–413. doi: 10.1016/j.rdc.2021.04.006
69. Carbone J, Sarmiento E, Gallego A, Lanio N, Navarro J, García S, et al. Peripheral blood T- and B-cell immunophenotypic abnormalities in selected women with unexplained recurrent miscarriage. J Reprod Immunol (2016) 113:50–3. doi: 10.1016/j.jri.2015.11.003
70. Ângelo-Dias M, Martins C, Dias SS, Borrego LM, Lima J. Association of B cells with idiopathic recurrent pregnancy loss: A systematic review and meta-analysis. Int J Mol Sci (2022) 23(23):15200. doi: 10.3390/ijms232315200
71. Hoffman W, Lakkis FG, Chalasani G. B cells, antibodies, and more. Clin J Am Soc Nephrol (2016) 11(1):137–54. doi: 10.2215/CJN.09430915
72. Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol (2009) 183(6):3561–7. doi: 10.4049/jimmunol.0800933
73. Smulski CR, Eibel H. BAFF and BAFF-receptor in B cell selection and survival. Front Immunol (2018) 9:2285(OCT). doi: 10.3389/fimmu.2018.02285
74. Chen D, Wang W, Wu L, Liang L, Wang S, Cheng Y, et al. Single-cell atlas of peripheral blood mononuclear cells from pregnant women. Clin Trans Med (2022) 12(5):e821. doi: 10.1002/ctm2.821
75. Stohl HE, Stohl W. Maternal and cord blood BAFF and APRIL levels during pregnancy. Am J Reprod Immunol (2023) 89(3):e13654. doi: 10.1111/aji.13654
76. Kang N, Liu X, You X, Sun W, Haneef K, Sun X, et al. Aberrant B-cell activation in systemic lupus erythematosus. Kidney Dis (2022) 8(6):437–45. doi: 10.1159/000527213
77. Stohl W, Metyas S, Tan S-M, Cheema GS, Oamar B, Xu D, et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: Longitudinal observations. Arthritis Rheumatism (2003) 48(12):3475–86. doi: 10.1002/art.11354
78. Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheumatism (2008) 58(8):2453–9. doi: 10.1002/art.23678
79. Vincent FB, Kandane-Rathnayake R, Koelmeyer R, Hoi AY, Harris J, Mackay F, et al. Analysis of serum B cell-activating factor from the tumor necrosis factor family (BAFF) and its soluble receptors in systemic lupus erythematosus. Clin Trans Immunol (2019) 8(4):e1047. doi: 10.1002/cti2.1047
80. Thorn M, Lewis RH, Mumbey-Wafula A, Kantrowitz S, Spatz LA. BAFF overexpression promotes anti-dsDNA B-cell maturation and antibody secretion. Cell Immunol (2010) 261(1):9–22. doi: 10.1016/j.cellimm.2009.10.004
81. Ziegler KB, Muzzio DO, Matzner F, Bommer I, Ventimiglia MS, Malinowsky K, et al. Human pregnancy is accompanied by modifications in B cell development and immunoglobulin profile. J Reprod Immunol (2018) 129(May):40–7. doi: 10.1016/j.jri.2018.07.003
82. Banjar S, Kadour E, Khoudja R, Ton-leclerc S, Beauchamp C, Beltempo M, et al. Intravenous immunoglobulin use in patients with unexplained recurrent pregnancy loss. Am J Reprod Immunol (2023) 90(2):e13737. doi: 10.1111/aji.13737
83. Liu T, Guo X, Liao Y, Liu Y, Zhu Y, Chen X. Correlation between the presence of antinuclear antibodies and recurrent pregnancy loss: A mini review. Front Endocrinol (2022) 13:873286. doi: 10.3389/fendo.2022.873286
84. Ying Y, Zhong Y, Zhou C, Xu Y, Wang Q, Li J, et al. Antinuclear antibodies predicts a poor IVF-ET outcome: impaired egg and embryo development and reduced pregnancy rate. Immunol Investigations (2012) 41(5):458–68. doi: 10.3109/08820139.2012.660266
85. Papadimitraki ED, Choulaki C, Koutala E, Bertsias G, Tsatsanis C, Gergianaki I, et al. Expansion of toll-like receptor 9–expressing B cells in active systemic lupus erythematosus: Implications for the induction and maintenance of the autoimmune process. Arthritis Rheumatism (2006) 54(11):3601–11. doi: 10.1002/art.22197
86. Zeng M, Wen P, Duan J. Association of antinuclear antibody with clinical outcome of patients undergoing in vitro fertilization/intracytoplasmic sperm injection treatment: A meta-analysis. Am J Reprod Immunol (2019) 82(3):e13158. doi: 10.1111/aji.13158
87. Girardi G. Complement inhibition keeps mothers calm and avoids fetal rejection. Immunol Investigations (2008) 37(5–6):645–59. doi: 10.1080/08820130802191615
88. Azizieh FY, Raghupathy RG. Tumor necrosis factor-α and pregnancy complications: A prospective study. Med Principles Pract (2015) 24(2):165–70. doi: 10.1159/000369363
89. Kaislasuo J, Simpson S, Petersen JF, Peng G, Aldo P, Lokkegaard E, et al. IL-10 to TNFα ratios throughout early first trimester can discriminate healthy pregnancies from pregnancy losses. Am J Reprod Immunol (2020) 83(1):e13195. doi: 10.1111/aji.13195
90. Danaii S, Ghorbani F, Ahmadi M, Abbaszadeh H, Koushaeian L, Soltani-Zangbar MS, et al. IL-10-producing B cells play important role in the pathogenesis of recurrent pregnancy loss. Int Immunopharmacol (2020) 87:106806. doi: 10.1016/j.intimp.2020.106806
91. Menon M, Blair PA, Isenberg DA, Mauri C. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity (2016) 44(3):683–97. doi: 10.1016/j.immuni.2016.02.012
Keywords: recurrent pregnancy loss, lupus, B cells, hormones, pregnancy
Citation: Valeff NJ, Ventimiglia MS, Diao L and Jensen F (2023) Lupus and recurrent pregnancy loss: the role of female sex hormones and B cells. Front. Endocrinol. 14:1233883. doi: 10.3389/fendo.2023.1233883
Received: 02 June 2023; Accepted: 08 September 2023;
Published: 03 October 2023.
Edited by:
Richard Ivell, University of Nottingham, United KingdomReviewed by:
Mai Shaker, National Research Centre, EgyptMaria Emilia Solano, University Medical Center Regensburg, Germany
Copyright © 2023 Valeff, Ventimiglia, Diao and Jensen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natalin Jimena Valeff, bmF0YWxpdmFsZWZmQGdtYWlsLmNvbQ==; Maria Silvia Ventimiglia, bWFzaXZlbnRAZ21haWwuY29t; Federico Jensen, ZmplbnNlbkB1bmFqLmVkdS5hcg==
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share senior authorship
 Natalin Jimena Valeff
Natalin Jimena Valeff Maria Silvia Ventimiglia
Maria Silvia Ventimiglia Lianghui Diao2‡
Lianghui Diao2‡ Federico Jensen
Federico Jensen