- Neonatology Department, Fujian Maternity and Child Health Hospital College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, China
Metabolic syndrome (MetS) is a group of cardiometabolic risk factors with high prevalence in the adult population. To date, there is no consensus on the definition for MetS in children and adolescents despite the presence of well-established diagnostic criteria in adults. The etiology of MetS is associated with a complex interaction between genetic susceptibility and environmental factors, in which the modifiable environmental risk factors are considered to play an important role in this process. MetS is significantly associated with an increased risk of diabetes mellitus and cardiovascular diseases (CVDs). Thus, it is necessary to pay attention to the prevention of MetS in childhood and adulthood. Given the current epidemic of obesity in children and adolescents, there is an urgent need to provide adequate guidelines for the definition, screening, and treatment strategies of MetS in younger patients. In this narrative review, we provide some diagnostic criteria and epidemiological studies and highlight the pathogenesis and management of MetS.
Introduction
Metabolic syndrome (MetS) is a complex cluster of metabolic disorders characterized by disruptions in the metabolism of proteins, fats, and carbohydrates (1). It primarily includes central obesity, dyslipidemia, hypertension, and insulin resistance (IR) (2). In the past decades, MetS has been extensively studied in adult populations (3), however, our understanding of MetS in children and adolescents is still limited. Several large epidemiological cohort studies have demonstrated an association between MetS and cardiovascular outcomes in adults (4). These findings from adult studies, coupled with the rising prevalence of overweight among children and adolescents, have reignited interest in studying MetS in younger populations (5). As obesity-related MetS in childhood may persist into adulthood and is associated with cardiometabolic and psychosocial comorbidities, as well as premature death (6).
Patients with obesity usually present accumulation of free fatty acids (FFAs) in liver, adipocytes, skeletal muscle, and pancreas (7), which causes lipotoxicity in pancreatic β-cells and inhibition of insulin signaling in the liver and muscles, along with the eventual occurrence of IR (8). These patients show an increased risk of MetS and cardiovascular complications due to production of very low-density lipoprotein (VLDL) (9). Therefore, measurements with an aim to reduce the concentrations of cardiometabolic risk factors in children and adolescents can reduce the global burden of cardiovascular disease (CVD). This emphasizes the importance of MetS prevention in childhood. In this narrative review, we summarized the diagnostic criteria, epidemiology, pathophysiology, and treatment strategies of MetS in children and adolescents.
Definition of MetS
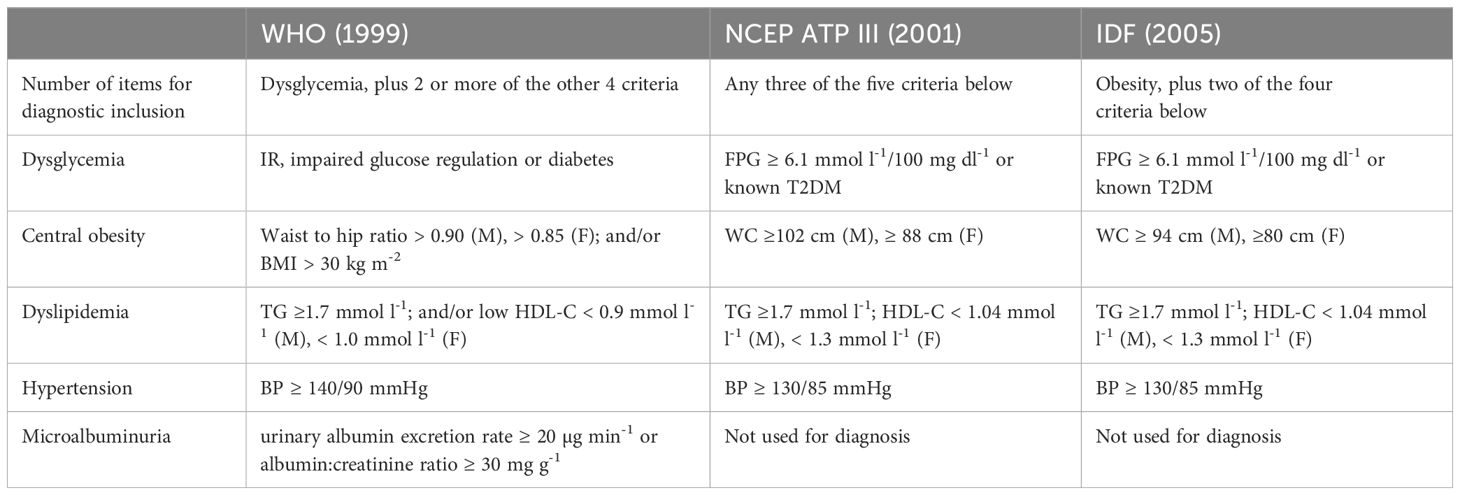
MetS is defined by a series of physiological, biochemical, clinical, and metabolic factors. In 1988, Gerald Reaven first used the term “Syndrome X” to describe a specific cluster of cardiometabolic risk factors (10), and then gave rise to the concept of MetS. Since then, this cluster of risk factors was represented by various names, including “Deadly Quartet” (11), “IR Syndrome” (12) and “Metabolic Abnormality Syndrome” or “Diabetes.” In 2001, the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATPIII) coined the term “MetS” and provided its definition (13). Subsequently, numerous organizations, including the World Health Organization (WHO) (14), the International Diabetes Federation (IDF), and the National Heart, Lung, and Blood Institute (NHLBI), issued their definitions. These definitions generally include aspects such as central obesity, hyperglycemia, hypercholesterolemia, low high-density lipoprotein cholesterol (HDL-C), and elevated blood pressure (BP) (Table 1). Although there are some similarities, these definitions differ in their threshold values for biochemical parameters and the targeted populations.
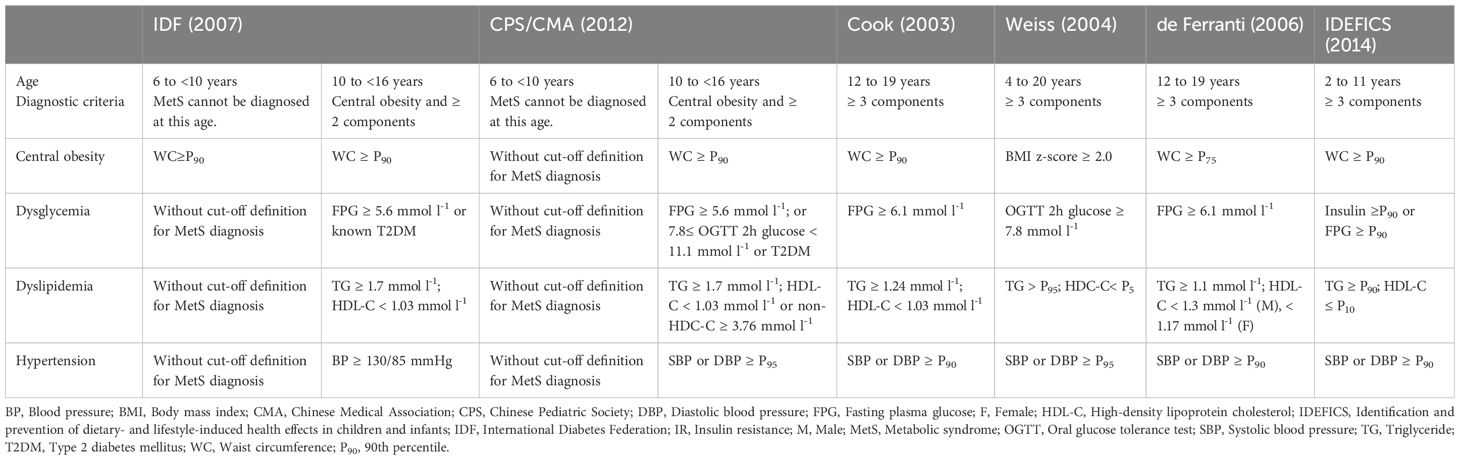
To the best of our knowledge, the definition of adult MetS cannot be simply used in children and adolescents, as the body size and proportions show a significant change with age. There are also remarkable changes in the fat distribution, insulin sensitivity of muscle and liver, and insulin release between adolescents and adults (15). Even in children and adolescents, there is no consensus on the definition of MetS. Its diagnosis requires assessment of waist circumference (WC), BP, lipids, and glucose (Table 2).
In 2003, Cook et al. assessed adolescents aged 12–19 years based on the NCEP/ATP-III definition, using modified criteria that included a WC above the 90th percentile (P90), BP above the limits set by the National Blood Pressure Education Program, lipid levels exceeding the pediatric thresholds set by the NCEP, and glucose levels above adult values (16). In 2004, body mass index (BMI) was adopted as a basis by Weiss et al. even though abdominal obesity may vary by race (17). Two years later, de Ferranti et al. proposed a definition similar to Cook’s but with lower thresholds for WC and lipid levels, which may result in a higher prevalence of MetS (18). Shortly thereafter, the IDF introduced a new definition based on its adult criteria. They categorized children into different age groups. For children aged 6–10 years, metabolic and BP variables were not well-defined, and only WC was evaluated. For children aged 10 years or more, MetS could be diagnosed with abdominal obesity and the presence of two or more clinical features (e.g. elevated TGs, low HDL-C, hypertension, or elevated glucose). For children aged 16 years or more, the IDF adult criteria were used (19). In this new definition, WC percentiles were used instead of absolute values to account for differences in child development and racial background. In 2014, European researchers proposed a definition of MetS for prepubertal children (ages 2-11) in the identification and prevention of dietary- and lifestyle-induced health effects in children and infants (IDEFICS) study. This definition addressed the limitations of previous pediatric definitions and the need for early diagnosis (20). The criteria included obesity (WC ≥ P90), TGs ≥ P90, HDL-C ≤ 10th percentile [P10], BP (systolic blood pressure [SBP] or diastolic blood pressure [DBP] ≥ P90), and glucose (insulin ≥ P90 or fasting plasma glucose [FPG] ≥ P90). Percentiles were used as references, better compensating for differences in child development and racial background.
In 2012, China adopted a definition of MetS for children and adolescents based on the IDF and ATP III criteria, established through consensus by experts from the Chinese Pediatric Society (CPS) of the Chinese Medical Association (CMA) (21). For children aged ≥10 years, central obesity is a prerequisite for MetS, defined as a WC ≥ P90 for age and sex, along with at least two of the following factors: hyperglycemia, hypertension, low HDL-C or high non-HDL-C, and hypertriglyceridemia. For children aged 6–10 years, whose physiological characteristics change rapidly, the diagnosis of MetS is still a challenge, and multiple CVD risk factors (e.g. obesity, hypertension, lipid metabolism disorders, and hyperglycemia) should be noted. Early intervention is recommended for children in this age group who exhibit multiple metabolic abnormalities. The definition proposed by CPS/CMA is similar to the IDF adolescent version but differs in certain thresholds and assessment items. The method for determining central obesity is different from the IDF’s obesity rate assessed by WC ≥ P90. Instead, it uses the waist-to-height ratio (WHtR), with a threshold of 0.48 for boys and 0.46 for girls (22).
Overall, the definition proposed by the IDF is the most effective and widely used in clinical practice. Due to significant variations in metabolic and physiological characteristics based on age and sex during the growth and development of children and adolescents, as well as notable differences in dietary habits and lifestyles across countries and regions, there is no consistent definition of MetS in children. We then identify common mechanisms to facilitate the establishment of a comprehensive and accurate definition and diagnostic criteria for MetS in children and adolescents.
Epidemiology
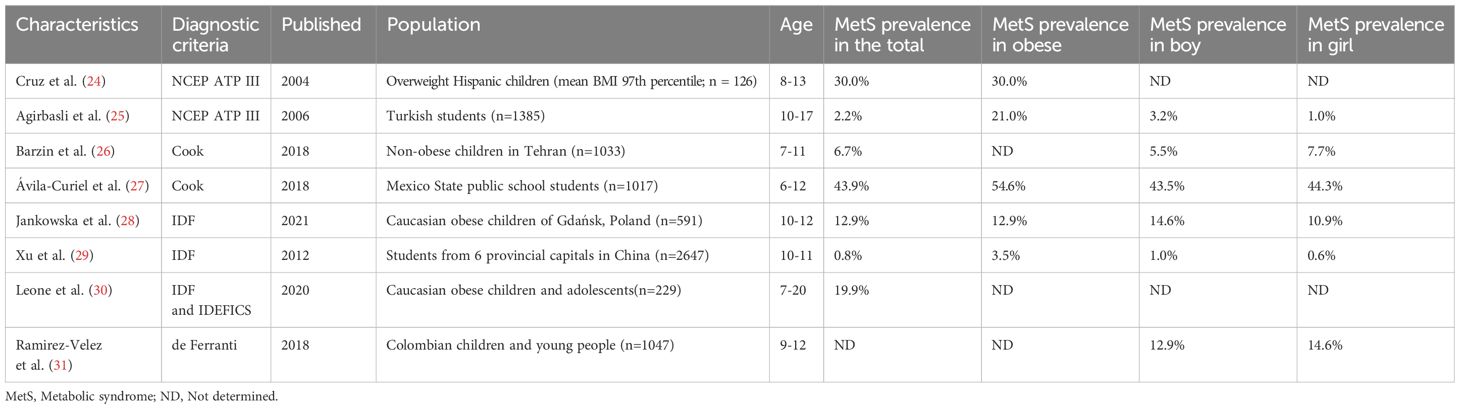
It is estimated that approximately 39% of the global population is facing challenges of overweight, and the prevalence of overweight conditions is gradually increasing among children and adolescents (23). MetS is a complex disease that has been extensively studied in the adult population, but information on the prevalence in pediatric population is still limited (3). The epidemiology of MetS varies greatly between nations, and the prevalence is mainly associated with the diagnostic criteria, obesity rates, and race (Table 3) (24–31).
To date, there is still no consensus on the diagnosis of MetS in children, and the cutoff values are in a huge difference that yields to various prevalence (32). Take the IDF criteria as an example, the Spanish study found that the prevalence of MetS varied from 2.5% in adolescents with a mean age of 13 years to 19.6% in children and adolescents aged 5–19 years (33). However, the results are not consistent when using different diagnostic criteria to the same population. Based on the NCEP/ATP III diagnostic criteria, Peña-Espinoza et al. found that the prevalence of MetS in children aged 9–12 years was 21.1%, 15.5% using the IDF criteria, 13.8% using the Cook criteria, and 45.9% using the De Ferranti criteria (34). Serrano et al. reported a prevalence of 9.5% for MetS in children aged 6–10 years using the NCEP/ATP III criteria and 8% using the IDF criteria (35).
The overall prevalence of MetS in children is relatively low, while that in overweight adolescents shows a 4–8 fold increase (36). The prevalence of MetS in European pediatric populations ranges from 1.44% to 55.8% (37). In a previous study, the global prevalence of MetS in 2020 was estimated at 2.8% in children and 4.8% in adolescents (38). A comprehensive review of 85 studies mostly using IDF, ATP III, and WHO criteria concluded that the median prevalence of MetS in the general population was 3.3% (ranging from 0% to 19.2%), 11.9% in overweight children (ranging from 2.8% to 29.3%), and 29.2% in obese populations (ranging from 10% to 66%) (39). In a systematic review, Sharma et al. reported that the prevalence of MetS in children and adolescents was 3.4% in normal-weight groups and 29% in obese groups (36). In 2012, China adopted the NCEP-ATP III diagnostic criteria, adjusted for age- and sex-specific WC and BP. In a study performed in Jiangsu Province, the prevalence of MetS was 5.1% among children and adolescents aged 7–17 years, 5.9% among those aged 13–17 years, and the prevalence of MetS in obese populations showed 40.2-fold increase compared to normal-weight peers (40). This highlights that the obesity rate within a study population is directly related to the prevalence of MetS.
Generally, ethnicity has been reported to be closely associated with the prevalence of MetS. Globally, the prevalence of MetS was higher in the following regions or ethnic groups. In the Middle East especially the Iran showed a prevalence of 7.6% according to IDF standards (41), 9.8% in the United Arab Emirates (38), and 20.6% in the Saudi Arabia based on to de Ferranti’s standards (42). In Europe, the prevalence of MetS in Spain was 9.9% (38). In North America, the United States showed a prevalence of 5.4% according to IDF standards (43), 10.1% according to Ford et al. and 12.3% in Mexico (38, 44). In South America, Chile showed a prevalence of 9.5% according to IDF definition (38, 45). In the United States, Miller et al. reported that the prevalence of MetS varied across different ethnic groups, with Hispanic adolescents showed the highest rate of 14.6%, followed by non-Hispanic whites (9.8%) and non-Hispanic blacks (5.2%) (44). This was consistent with the latest report from the US NHANES population (46). Some studies have found that despite the high obesity rate among African American adolescents (23.6%), their prevalence of MetS is relatively low (47). These findings suggest that the impact of obesity on MetS may vary by ethnicity. In summary, there are differences in the prevalence of MetS among different ethnic groups, but there is no consistent pattern.
Risk factors and pathophysiology
Genetic factors
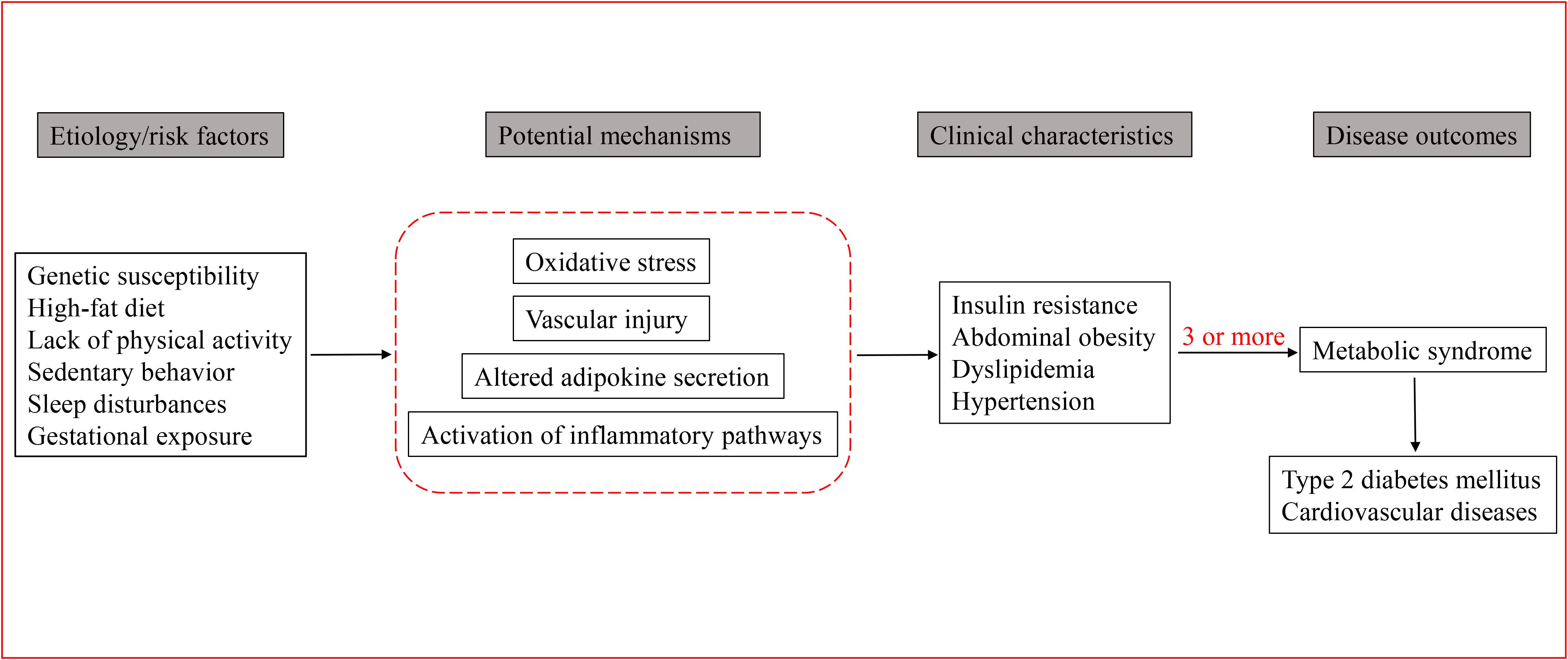
MetS is the result of a complex interaction between genetic and environmental factors (48). Figure 1 depicts the MetS developmental process, involving genetic factors, oxidative stress, and inflammation pathways. Currently, great attention has been paid to the association between genes and individual components of MetS in children and adolescents, such as the relationship between certain genes and obesity, lipid levels, or IR.
The Fat Mass and Obesity-Associated (FTO) gene, located on chromosome 16, plays a critical role in body weight regulation and energy balance (49). The A/A phenotype of the risk allele rs9939609 (T/A) is closely associated with the development of obesity, a correlation observed in children as well (50). Among the polymorphisms of FTO, rs9939609 is the most widely studied locus, and increasing evidence suggest that it plays a central role in the development of MetS (51, 52). Almén et al. studied the genome-wide DNA methylation profiles of prepubescent girls with different variants of the rs9939609 polymorphism and identified 20 differentially methylated obesity-related loci (53). These findings suggest that increased FTO transcription in carriers of the A allele of rs9939609 may contribute to the higher risk of MetS. The latest study has updated the evidence, and some scholars have found that rs8050136 on the FTO is most strongly associated with MetS in children (52). However, the exact mechanisms by these single nucleotide polymorphisms (SNPs) increase the risk of MetS in children remain unclear. In a study, the author speculated that FTO variants could interfere with the methylation status of FTO target mRNAs and other non-coding RNAs, leading to an imbalance in energy intake and expenditure (54). Besides, the A allele of rs9939609 is associated with increased appetite and reduced satiety (55), resulting in increased energy intake in children. The leptin sensitivity showed reduction in the individuals with obesity, resulting in ineffective satiety responses and excessive hunger (56). Furthermore, energy expenditure may also involve in the association between FTO polymorphisms and MetS components. This helps to explain the fact that children carrying A allele are significantly associated with reduced physical activity.
Cholesteryl ester transfer protein (CETP) promotes the exchange of cholesteryl esters from HDL or LDL to TG-rich lipoproteins, resulting in reduced HDL-C concentration and generation of small-sized LDL particles (57). The CETP gene, located on chromosome 16 encoding the CETP protein, is reported to show a close link with the pathogenesis of MetS. (58). For instance, rs708272 was closely associated with increased HDL and reduced TG levels (52). In addition, a significant interaction was reported between the rs11774572 polymorphism and CETP-TaqIB (59), but the mechanism is still not well defined. This SNP is located between the GATA binding protein 4 (GATA4) and retinitis pigmentosa 1 (RP1) genes, which play a key role in cholesterol metabolism. GATA4 encodes a transcription factor that mediates the transport of cholesterol and phytosterols and inhibits their abnormal accumulation (60). Variants in the RP1 gene alter the lipoprotein phenotype by changing plasma TG and HDL-C concentrations, leading to hypertriglyceridemia (61). Therefore, the potential linkage disequilibrium between rs11774572 and functional mutations in these two genes may help to define the roles of CETP in MetS.
Furthermore, the rs662799 on the Apolipoprotein A5 (APOA5) gene is associated with high TG levels in both adults and children, as it could inhibit the activation of lipoprotein lipase (59). Similarly, a GWAS study in Korean population also revealed significant or suggestive loci for MetS in APOA5 (62). In a study performed in Mexican population, the most commonly associated signal for TG was rs651821 in APOA5, followed by rs180326 in BUD13 (63).
Studies have focused on the link between Caucasian and Asian adolescents and the TCF7L2 in the pathogenesis of MetS (64). There is evidence that carriers of the TCF7L2 rs7903146 risk allele have higher fasting insulin concentrations, impaired insulin sensitivity, and greater IR compared to CC homozygotes (65). TCF7L2 gene polymorphisms increase the risk of T2DM by altering its gene expression, disrupting glucose homeostasis, impairing insulin secretion, and weakening insulin sensitivity (66). In addition, increased nut intake may reduce the risk of MetS in the T risk allele of TCF7L2 rs7903146 and rs12255372 variants (67).
The prevalence of MetS in children and adolescents may be controlled by later intervention of environmental factors when genetic variation cannot be modified (68). There are indeed studies designed to investigate the association of genes with a single MetS disease, however, they did not take the fact that most genetic loci have pleiotropic effects on multiple MetS components into consideration. Therefore, it is appropriate to evaluate the effect of each SNP on MetS risk.
Early life exposures
Early life exposures, primarily maternal behaviors during pregnancy, may contribute to the early development of MetS. Susceptibility to MetS begins before birth, as obesity during pregnancy and associated gestational conditions (e.g. gestational diabetes, hypertension, and hyperlipidemia) increase the risk of obesity and metabolic disorders in offspring (69). Therefore, the pre-pregnancy and perinatal periods provide women and their offspring with a unique opportunity to modify short-term and long-term risks. A large number of observational studies have found that maternal obesity, hypertension, and hyperglycemia during pregnancy increase the risk of MetS in offspring (70).
Increased maternal glucose levels are associated with a higher incidence of obesity in newborns (71). When examining the gene expression profiles in placentas from women with gestational diabetes and those with normal glucose tolerance, researchers found an upregulation of genes related to lipid metabolism. This indicated that lipids might serve as a nutritional source contributing to increased neonatal obesity (72, 73). Consistently, Boney et al. reported that the offsprings of obese women had a higher likelihood of being obese at age 11, along with 2.0-fold increase in the risk of MetS (74).
The progenitor cells and adipocyte populations in subcutaneous adipose tissue have been formed in fetus, laying the foundation for an individual’s future fat distribution and metabolic health (75). This means that the “set point” for obesity has been determined in utero, and the intrauterine environment plays a crucial role in the development of MetS. Adverse intrauterine conditions such as obesity or diabetes accelerate the fat accumulation of fetal white adipose tissue (WAT) and disrupt its normal developmental trajectory (76). Furthermore, a high-sugar and high-fat condition promotes the stem cells to differentiate into adipocytes, and leads to a premature terminal differentiation process (77), which increases the susceptibility of offspring to obesity, limits the plasticity of WAT, and reduces its ability to adapt and regulate energy metabolism (78). In line with this, multiple rodent-based studies supported the long-term effects of maternal diet-induced obesity on the metabolic health of offspring. The offsprings have an increase in visceral adipose tissue volume, accompanied by a significant increase in the number and volume of fat cells, as well as significant IR (78).
It is worth noting that evidence has shown that epigenetics and prenatal programming might have influenced fetal/neonatal development, leading to MetS. The epigenome is dynamic and can change in response to factors such as nutrient availability and weight loss (79, 80). Intrauterine nutrition and environmental exposures may have permanently altered gene expression in offspring through epigenetic mechanisms, thereby changing the structure and function of cells and organs and resulting in metabolic abnormalities. This was well demonstrated in monozygotic twins, where offspring exhibited different DNA methylation and histone acetylation patterns (81). Epigenetic regulation of gene transcription is partly mediated through DNA methylation (82–84), which is particularly dynamic during embryogenesis. As embryonic development progresses, DNA methylation gradually increases, leading to differentiation and organ formation (85). This would promote the adipogenesis, while histone lysine methylation (H3K4) and acetylation (AcH3) can regulate adipocyte differentiation (86, 87). Higher pro-opiomelanocortin (POMC) methylation level in umbilical cord blood is associated with hyperinsulinemia in children, which may serve as a marker for future MetS (88). Epigenetic modifications such as DNA methylation and histone acetylation (89) are involved in the development and differentiation of pancreatic β-cells (90, 91). In the presence of poor intrauterine environment, pancreatic β-cells may undergo fetal developmental programming, resulting in a decrease in pancreatic β-cells number and/or dysfunction. All these may increase the risk of long-term metabolic complications in offspring.
Environmental factors
SNPs are established at conception, while environmental factors such as diet and lifestyle influence the baseline during growth. This raises the question of which variable is the “cause” and which is the “moderator,” a common issue in studies of gene-environment interactions. Environmental factors that contribute to MetS in children include sedentary behavior, high-fat diets, insufficient sleep, and systemic or tissue inflammation. Figure 2 depicts potential risk factors and mechanisms of MetS pathophysiology in children and adolescents. Epidemiological data indicates that sedentary lifestyles, lack of physical activity, and high-fat diets are key contributors to energy imbalance, closely linked to the prevalence of childhood obesity and metabolic disorders such as IR (92). Thus, we hypothesize that MetS begins with obesity but requires IR to progress to MetS in children, which was consistent with the hypothesis proposed by Weiss (17).
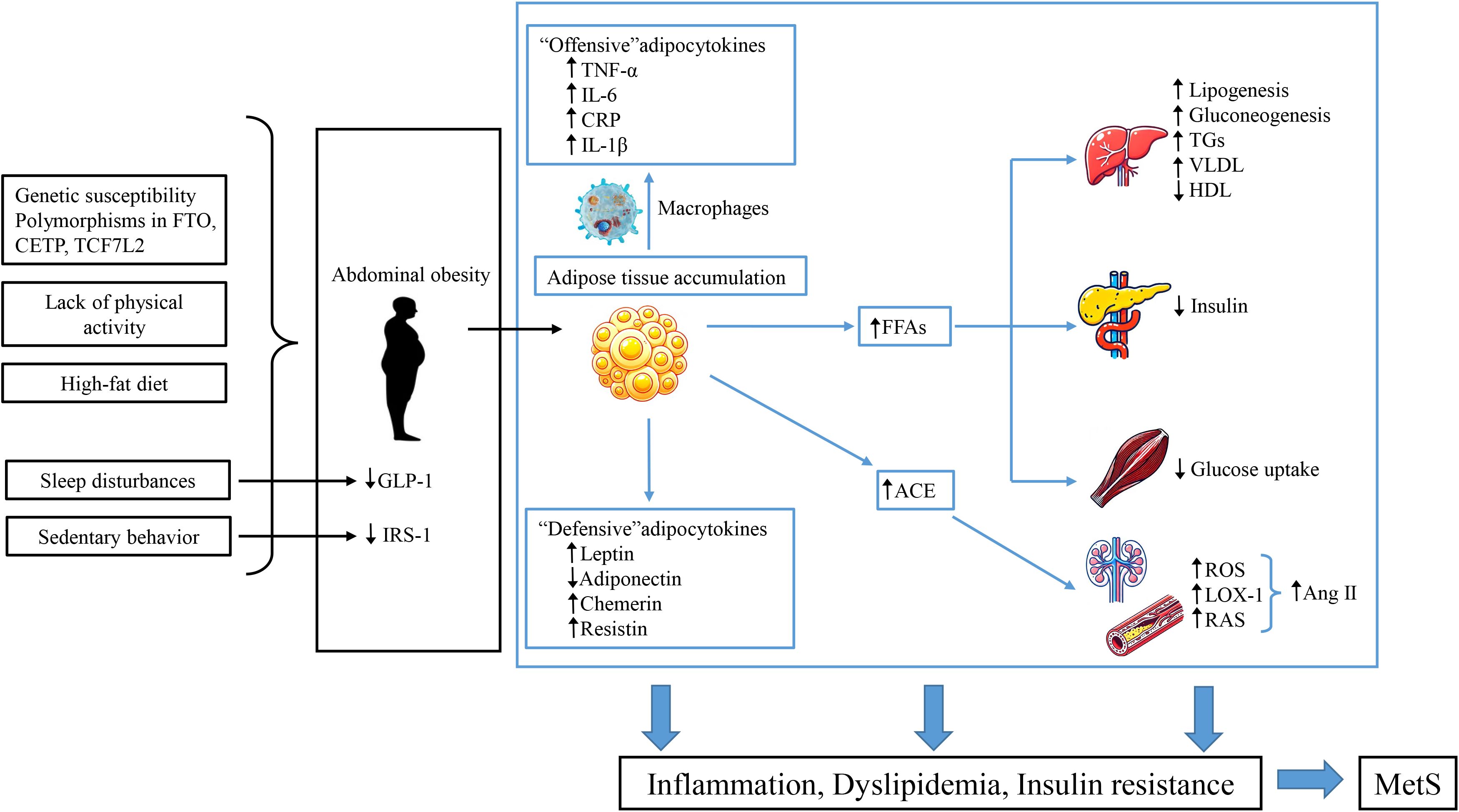
Figure 2. MetS pathogenesis in children and adolescents. This figure describes the potential risk factors and mechanisms underlying the pathophysiology of MetS in pediatric populations. Genetic susceptibility and unhealthy lifestyles contribute to central obesity, leading to an imbalance between “aggressive” adipokines produced by adipose tissue and macrophages and the dysfunction of “defensive” adipokines. This imbalance increases immune-inflammatory responses and promotes obesity-related metabolic disorders. The activation of adipose tissue leads to the production of angiotensin II (Ang II) peptides through angiotensin-converting enzyme (ACE), increasing OS and upregulating the expression of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), inducing endothelial dysfunction. Additionally, the increased secretion of FFAs from adipose tissue leads to reduced sensitivity in insulin-responsive organs. This cascade ultimately results in IR, dyslipidemia, and hypertension, significantly increasing the risk of MetS, T2DM, and CVD.
Several studies have suggested that there might be a link between high-fat diets, dietary fatty acids, and the risk of MetS (93, 94). The main components of the Western diet mainly include meat products, sugary drinks, junk food, refined grains, candy, and ultra-processed foods. A large amount of saturated fatty acids (SFA) and carbohydrates in these foods have been shown to be directly associated with an increased risk of MetS in children and adolescents (94–96). Dietary fatty acid is an important environmental factor, and excessive exposure has played a critical role in the development of MetS (97). Epidemiological and cohort studies have shown that SFA has an adverse effect on insulin sensitivity, promoting the development of diabetes (98). The Nurses’ Health Study found that higher SFA intake and a lower polyunsaturated to saturated fat (P:S) ratio were associated with an increased risk of CVD in women with T2DM (99). A cohort study indicated that the Western dietary pattern was linked to a high-risk metabolic cluster (100). In contrast, a Mediterranean dietary pattern or a higher Healthy Eating Index score, containing more grains, vegetables, fruits, milk, and meat/meat alternatives, was associated with the reduced prevalence of MetS (95). A recent study showed that dietary polyunsaturated fats (PUFA) modulated the genetic effect of TCF7L2 rs7903146 polymorphism on postprandial dyslipidemia (101). Therefore, adopting such a dietary pattern early in life could reduce the risk of MetS in children and adolescents.
There may be a possible threshold for the accumulation of fat in abdominal compartments and insulin-responsive tissues. Upon exceeding such threshold, the accumulation of lipids in these areas would be detrimental (102). Increased consumption of fructose and branched-chain amino acids, along with increased intracardiac lipid metabolites, lead to the serine phosphorylation of insulin receptor substrate-1 (IRS-1) (103). This results in defective skeletal muscle glucose uptake, along with decreased hepatic glycogen synthesis, and suppression of gluconeogenesis, which ultimately decrease hepatic insulin sensitivity (104). These changes further exacerbate IR and set the stage for obesity-related metabolic disorders.
Insulin sensitivity in adipose tissues decreases in children with obesity (105). Lipid deposition in muscle and liver also increases in those with obesity (106). Macrophage infiltration of subcutaneous and intraperitoneal fat depots induces local and systemic subclinical inflammation, which is closely related to poor lipid partitioning in obese adolescents (107). In addition, skeletal muscle precedes liver, followed by enterogenic circulating glucose to the liver. The liver responds to increased glucose flux by increasing de novo fat processes, which leads to increased intrahepatic fat, circulating fatty acids and TGs (108). Meanwhile, macrophage infiltration causes IR in adipose tissue, resulting in increase of lipolysis and decrease of lipogenesis (109). As a result, hepatic FFA flux increases, leading to enhanced TG synthesis and systemic hyperlipidemia (110).
MetS children are also accompanied by systemic and tissue inflammation, as evidenced by elevated levels of inflammatory cytokines, including interleukin-6 (IL-6), IL-18, and C-reactive protein (CRP) (111). Obesity and IR induce systemic oxidative stress (OS) that activates downstream inflammatory cascades, which accelerates the development of MetS (112). Excess OS was associated with increased adipogenesis and body fat mass, potentially linked to the overexpression of NADPH oxidase 4 (NOX4) and downregulation of AMP-activated protein kinase (AMPK) in adipocytes (113). Several key inflammatory markers have been reported to involve in obesity-induced inflammatory responses, including CRP, IL-6, and tumor necrosis factor-α (TNF-α) (114, 115). IL-6 plays a regulatory role in fat and glucose metabolism and can promote IR. In obesity, IL-6 is released from visceral adipocytes into the portal vein and directly acts on the liver to induce the production of CRP (116). In addition, IL-6 can increase the risk of thrombosis, and lead to atherosclerosis, inflammation and dysfunction of the vascular wall by activating the local renin-angiotensin system (RAS) pathway and promoting the expression of vascular cell adhesion molecules (117). TNF-α is mainly produced by macrophages in local adipose tissue, and its levels is proportional to the mass of adipose tissue and is closely related to IR (118). It weakens insulin metabolism through serine phosphorylation and inactivation of the insulin signaling pathway, and further exacerbates IR by increasing circulating FFA levels (119, 120). Obese children exhibit higher TNF-α levels than lean controls, which is associated with reduced LDL-C and increased TGs (121). In the future, more studies are required to investigate the feasibility of these inflammatory markers in the management of MetS and its complications.
Insufficient sleep profoundly impacts energy balance and overall metabolic health, thereby increasing the risk of obesity in adolescents. In an observational study involving 240 American adolescents, subjects slept for less than 8 hours on weekdays consumed a significantly higher percentage of calories from fat compared to those who slept for 8 hours or more (122). This indicated that insufficient sleep may lead to unhealthy eating habits and imbalance of metabolic health. Sleep disorders, including insufficient sleep, poor sleep quality, insomnia, and obstructive sleep apnea, lead to increased cortisol secretion by the adrenal cortex (123), which trigger increased calorie intake and excessive fat storage (124). Additionally, the severity of obstructive sleep apnea was correlated with higher cortisol levels, which can disrupt the normal response of glucagon-like peptide-1 (GLP-1) (110). Sleep disturbances can disrupt circadian rhythm, affecting GLP-1 production and glucose metabolism (125). In children, sleep reduction was closely associated with elevated fasting insulin concentrations, increased risk of IR, and decreased insulin sensitivity (126). These findings highlight the importance of adequate sleep in maintaining healthy metabolic function and preventing the occurrence of MetS in adolescents.
Prevention and treatment
Prevention
The previous section highlighted a range of risk factors for MetS. Beyond genetic factors, many of these risks are modifiable targets for preventive measures. From the perspective of childhood development, it appears essential to promote healthy nutrition and maintain normal body weight among adults of childbearing age, particularly considering the potential early exposure to these risks during pregnancy.
Breastfeeding has been confirmed as a protective factor against MetS. A systematic review involving 11 studies, 7 studies revealed a protective role of breastfeeding and MetS, particularly breastfeeding lasted for 6 months or longer (127). Besides, breastfeeding for more than 90 days significantly reduced the risk of MetS (128). Breastfeeding plays a protective role in preventing obesity in a dose-dependent manner (129). Additionally, breastfeeding for at least 3 months is associated with reduced risk of obesity, smaller WC, and fewer MetS-related complications in childhood and adolescence (130). Moreover, breastfeeding is linked to reduced risks of high cholesterol, hypertension, DM, glucose intolerance, and IR in adulthood (130, 131). Furthermore, breastfeeding can prevent prediabetes and MetS in offspring, regardless of GDM status, underscoring the importance of breastfeeding (132).
Breastfeeding helps to prevent obesity through the modulation of liver-hypothalamic communication and metabolism (133). Bioactive factors in breast milk, such as insulin, insulin-like growth factor-1 (IGF-1), and leptin, promote lean body weight and enhance appetite signaling (134, 135). This “positive programming” of nutrition and hormones may have profound implications for preventing MetS and related diseases. Other healthy lifestyle choices, including a balanced diet and regular exercise, are vital for avoiding MetS. Healthy dietary habits include consuming plenty of fruits, vegetables, and dietary fiber while reducing the intake of carbonated drinks and foods high in sugar, fat, and sodium (136). Taken together, the combination of breastfeeding and a healthy lifestyle will lay a solid foundation for improving the health of children and adolescents.
Treatment
The progression from a healthy state to obesity, IR, and eventually to the development of MetS is consistently associated with an imbalance between energy intake and expenditure. By the time MetS manifests, this energy imbalance has often been present for an extended period. Therefore, the primary goal of intervention is to reduce energy intake while increasing energy expenditure. Unfortunately, it is challenging to motivate pediatric patients to change unhealthy lifestyles, as many children and adolescents have become accustomed to a comfortable yet suboptimal way of living. Effective methods include motivational psychological interviews to explore the motivations of adolescents, assessing their willingness to change (137). To the best of our knowledge, multidisciplinary and family-based lifestyle education program supplemented with psychological support is recommended for the treatment and prevention of MetS. Thus, psychological adjustment is the first step in treating MetS. Subsequently, developing individualized treatment plans based on patient characteristics can enhance adherence to therapeutic regimens among adolescents (137). For younger children, the emphasis was on combining breastfeeding with a balanced diet, along with adequate sleep.
Lifestyle modifications
Basically, all successful treatment plans include interventions to reduce calorie intake and increase physical activity. According to recommendations from the American Academy of Pediatrics (AAP), the American Heart Association (AHA), and the WHO, the core of dietary intervention for children and adolescents is to increase the intake of vegetables and fruits while reducing the intake of sugar and saturated fat (138). The Chinese Society of Pediatrics recommends that children and adolescents should maintain food diversity in their diet, pay attention to the combination of meat and vegetables, and the combination of coarse and fine, and ensure the intake of fish, meat, milk, beans, and vegetables. The energy supply ratios of protein, fat, and carbohydrates are 12%-14%, 25%-30%, and 55%-65%, respectively (21). In terms of managing MetS, studies have shown that children and adolescents who adopt a Mediterranean diet, which mainly includes vegetables, fruits, fish, whole grains, beans, and olive oil, have significantly improved BMI, blood sugar, and blood lipid levels, especially in individuals with obesity or high risk of MetS (139). In addition, randomized controlled trial (RCT) have shown that reducing the intake of sugary drinks has a positive effect on weight management, thereby indirectly reducing the risk of MetS (140). For the blood lipid management, dietary adjustments, such as reducing the intake of simple carbohydrates (e.g. sugar and refined flour), can help control the phenotype of high TGs and low HDL-C. In contrast, increasing the intake of monounsaturated and PUFA can reduce TG levels and increase HDL-C levels (141). Additionally, whole grain intake is closely related to enhanced insulin sensitivity and reduced BMI in adolescents. In particular, dietary fiber intake can effectively reduce postprandial blood sugar fluctuations and has significant benefits for insulin sensitivity, obesity, and pancreatic function (142, 143). In terms of BP management, a meta-analysis of 10 RCTs showed that moderate reduction of salt intake can significantly reduce both SBP and DBP in children and adolescents (144). All these confirm that reasonable dietary adjustments are crucial to the long-term metabolic health of children and adolescents.
A lack of physical activity is associated with a higher risk of MetS, as indicated by a higher MetS z-score (145). Regular physical activity helps improve lipid profiles by reducing LDL and TG concentrations along with increasing of HDL (146). Exercise also enhances the clearance of plasma TGs and promotes the formation of HDL particles, leading to positive effects on lipid metabolism (147). Physical activity significantly improves insulin sensitivity, reduces IR and significantly lowers fasting insulin levels (148). Also, exercise offers benefits to vascular health, including improvement of endothelial function, reduction of SBP and DBP, decrease of abdominal fat, and triggering the anti-inflammatory responses (149). The most effective exercise interventions should last at least 12 weeks, with sessions conducted three or more times per week, with each lasting 60 minutes or longer (150, 151). Consequently, regular and appropriate physical activity is one of the key factors in preventing MetS.
As individually oriented obesity prevention strategies are not adequate in addressing the obesity epidemic, more attention has been paid on the shift towards environment- or community-based prevention measurement, which promotes healthier lifestyles by altering the social environment. In a perspective of public health, more attempts should be made on community health programs, along with school-based physical activity initiatives and promote healthy eating styles.
Pharmacotherapy
Lifestyle modifications remain the primary approach for the prevention and treatment of childhood obesity and MetS, however, pharmacological and surgical interventions become necessary adjuncts in some extreme cases (152). Currently, there are no specific guidelines for pharmacological treatment of dyslipidemia related to MetS in children. For children and adolescents with severe lipid abnormalities, the use of statins to lower LDL-C has been shown to delay arterial damage. This treatment is typically recommended only for children aged 10 years and older (141). These children had fasting LDL-C levels persistently >190 mg/dL, or LDL-C levels >160 mg/dL along with a significant family history of early-onset CVD or two or more additional risk factors (153). In addition, GLP-1 analogs, such as liraglutide, have demonstrated long-term efficacy in treating obesity in adults. A small-scale trial of another GLP-1 medication, exenatide, has also shown potential efficacy and safety in treating severe obesity in adolescents (154). For children at high risk of IR, pharmacological treatment may be unavoidable. In a recent double-blind randomized trial, obese adolescents aged 12 to 19 years who were treated with metformin for 6 months showed significant improvements in glucose tolerance and fasting insulin levels (155, 156). In the setting of severe obesity, bariatric surgery is considered the most effective treatment, which can significantly reduce the weight of children and adolescents and improve related health risks, such as sleep apnea and T2DM. However, potential complications after surgery, such as malabsorption of vitamin D, calcium, and phosphorus, also need to be carefully considered (157). At present, drug treatment of metabolic syndrome in children in China is still in its infancy. and doctors should consider multiple factors before prescribing anti-obesity drugs, such as gender, age, drug contraindications, personal and family willingness, and cost. For children and adolescents with severe obesity or metabolic disorders, a comprehensive treatment strategy that combining drug and surgical intervention may be the key point to achieve the best health outcomes.
Challenges to MetS in children and adolescents
A lack of awareness of MetS remains the biggest challenge for the management of MetS in children and adolescents. In a meta-analysis, 50.7% of parents underestimated the weight of their overweight/obese children (158). In fact, a chubby infant or child is often seen as a sign of good health and care in developing countries experience long periods of economic underdevelopment and material scarcity. The belief that “chubby kids are healthy” leads to delayed diagnosis and treatment of obesity in children. Fortunately, more and more attention has been paid to childhood obesity that has been shown to be linked to the development of MetS and CVD in adulthood (159). Autopsy studies have revealed that multiple cardiovascular risk factors are associated with early stages of coronary atherosclerosis (160). We assume that a high incidence of MetS among overweight adolescents, coupled with the rising prevalence of childhood obesity, could lead to a disproportionate increase in CVD in adulthood.
Diagnostic thresholds, whether based on percentiles or absolute numbers, need to be established based on objective disease endpoints to be meaningful. Moreover, these thresholds may need to be adjusted according to age or pubertal stage as children grow. Given the differing risk profiles across various ethnic groups, it is unclear whether the same standards should apply to different racial groups. Thus, any pediatric definition of MetS must be rigorously evaluated, which is a complex and challenging medical issue (161).
Summary
The diagnosis and treatment of MetS is still a challenge in children and adolescents. Standardized diagnostic criteria and treatment protocols are urgently required to guide clinical practice. The prevalence, diagnosis, and treatment of MetS show a huge variance due to differences in economy among different countries and populations. The ideal treatment approach involves a collaborative effort between families, schools, and society. We should focus on improving dietary habits, increasing physical activity, reducing sedentary behavior, and enhancing energy expenditure in children. Given the complexity of MetS in children and adolescents, a multidisciplinary and multi-sectoral approach is necessary.
Author contributions
BZ: Formal Analysis, Investigation, Writing – original draft. HS: Investigation, Writing – review & editing. WC: Investigation, Writing – review & editing. BY: Investigation, Writing – review & editing. WX: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Fujian Provincial Natural Science Foundation of China (Grant No.: 2023J011225 and 2022J02049), and Startup Fund for Scientific Research, Fujian Medical University (Grant No.: 2020QH1194).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Laclaustra M, Corella D, and Ordovas JM. Metabolic syndrome pathophysiology: the role of adipose tissue. Nutr Metab Cardiovasc Dis. (2007) 17:125–39. doi: 10.1016/j.numecd.2006.10.005
2. Christian Flemming GM, Bussler S, Körner A, and Kiess W. Definition and early diagnosis of metabolic syndrome in children. J Pediatr Endocrinol Metab. (2020) 33:821–33. doi: 10.1515/jpem-2019-0552
3. Kassi E, Pervanidou P, Kaltsas G, and Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. (2011) 9:48. doi: 10.1186/1741-7015-9-48
4. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. (2010) 56:1113–32. doi: 10.1016/j.jacc.2010.05.034
5. Jebeile H, Kelly AS, O’Malley G, and Baur LA. Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. (2022) 10:351–65. doi: 10.1016/s2213-8587(22)00047-x
6. Pulgarón ER. Childhood obesity: a review of increased risk for physical and psychological comorbidities. Clin Ther. (2013) 35:A18–32. doi: 10.1016/j.clinthera.2012.12.014
7. Jakubiak GK, Osadnik K, Lejawa M, Osadnik T, Goławski M, Lewandowski P, et al. Obesity and insulin resistance” Is the component of the metabolic syndrome most strongly associated with oxidative stress. Antioxidants (Basel). (2021) 11:79. doi: 10.3390/antiox11010079
8. Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. (2022) 23:786. doi: 10.3390/ijms23020786
9. Magge SN, Goodman E, and Armstrong SC. The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics. (2017) 140:e20171603. doi: 10.1542/peds.2017-1603
10. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. (1988) 37:1595–607. doi: 10.2337/diab.37.12.1595
11. Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. (1989) 149:1514–20. doi: 10.1001/archinte.149.7.1514
12. Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, and Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes. (1992) 41:715–22. doi: 10.2337/diab.41.6.715
13. Cleeman JI. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III). Jama. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
14. Organization WH. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. World health organization (1999).
15. Chiarelli F and Mohn A. Early diagnosis of metabolic syndrome in children. Lancet Child Adolesc Health. (2017) 1:86–8. doi: 10.1016/s2352-4642(17)30043-3
16. Cook S, Weitzman M, Auinger P, Nguyen M, and Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. (2003) 157:821–7. doi: 10.1001/archpedi.157.8.821
17. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. New Engl J Med. (2004) 350:2362–74. doi: 10.1056/NEJMoa031049
18. de Ferranti SD, Gauvreau K, Ludwig DS, Newburger JW, and Rifai N. Inflammation and changes in metabolic syndrome abnormalities in US adolescents: findings from the 1988–1994 and 1999–2000 National Health and Nutrition Examination Surveys. Clin Chem. (2006) 52:1325–30. doi: 10.1373/clinchem.2006.067181
19. Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents. Lancet. (2007) 369:2059–61. doi: 10.1016/s0140-6736(07)60958-1
20. Ahrens W, Moreno LA, Mårild S, Molnár D, Siani A, De Henauw S, et al. Metabolic syndrome in young children: definitions and results of the IDEFICS study. Int J Obes (Lond). (2014) 38 Suppl 2:S4–14. doi: 10.1038/ijo.2014.130
21. Subspecialty Group of Endocrinologic HaMD, The Society of Pediatrics, Chinese Medical Association, Subspecialty Group of Cardiology, The Society of Pediatrics, Chinese Medical Association, and Subspecialty Groups of Child Health Care, The Society of Pediatrics, Chinese Medical Association. The definition of metabolic syndrome and prophylaxis and treatment proposal in Chinese children and adolescents. Chin J Pediatr. (2012) 50:420–2. doi: 10.37601cma.j.issn.0578-1310.2012,06.005
22. Liang L, Fu JF, and Du JB. Significance of exploring the definition of metabolic syndrome in Chinese children and adolescents. Zhonghua Er Ke Za Zhi. (2012) 50:401–4. doi: 10.3760/errnj.issrr.0578-1310.2012.06.001
23. Organization WH. World health organization obesity and overweight. (Puska P, Nishida C, Porter D. World Health Organization) (2011).
24. Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, and Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. (2004) 89:108–13. doi: 10.1210/jc.2003-031188
25. Agirbasli M, Cakir S, Ozme S, and Ciliv G. Metabolic syndrome in Turkish children and adolescents. Metabolism. (2006) 55:1002–6. doi: 10.1016/j.metabol.2006.03.009
26. Barzin M, Aryannezhad S, Serahati S, Beikyazdi A, Azizi F, Valizadeh M, et al. Incidence of obesity and its predictors in children and adolescents in 10 years of follow up: Tehran lipid and glucose study (TLGS). BMC Pediatr. (2018) 18:245. doi: 10.1186/s12887-018-1224-6
27. Ávila-Curiel A, Galindo-Gómez C, Juárez-Martínez L, and Osorio-Victoria ML. Metabolic syndrome in children aged 6 to 12 years with obesity in public schools of seven municipalities in the State of Mexico. Salud Publica Mex. (2018) 60:395–403. doi: 10.21149/8470
28. Jankowska A, Brzeziński M, Romanowicz-Sołtyszewska A, and Szlagatys Sidorkiewicz A. Metabolic syndrome in obese children-clinical prevalence and risk factors. Int J Environ Res Public Health. (2021) 18:106. doi: 10.3390/ijerph18031060
29. Xu H, Li Y, Liu A, Zhang Q, Hu X, Fang H, et al. Prevalence of the metabolic syndrome among children from six cities of China. BMC Public Health. (2012) 12:13. doi: 10.1186/1471-2458-12-13
30. Leone A, Vizzuso S, Brambilla P, Mameli C, Ravella S, De Amicis R, et al. Evaluation of different adiposity indices and association with metabolic syndrome risk in obese children: is there a winner? Int J Mol Sci. (2020) 21:4083. doi: 10.3390/ijms21114083
31. Ramírez-Vélez R, Correa-Bautista JE, Carrillo HA, González-Jiménez E, Schmidt-RioValle J, Correa-Rodríguez M, et al. Tri-ponderal mass index vs. Fat mass/height³ as a screening tool for metabolic syndrome prediction in Colombian children and young people. Nutrients. (2018) 10:412. doi: 10.3390/nu10040412
32. Tropeano A, Corica D, Li Pomi A, Pepe G, Morabito LA, Curatola SL, et al. The metabolic syndrome in pediatrics: do we have a reliable definition? A systematic review. Eur J Endocrinol. (2021) 185:265–78. doi: 10.1530/eje-21-0238
33. Wang J, Perona JS, Schmidt-RioValle J, Chen Y, Jing J, and González-Jiménez E. Metabolic syndrome and its associated early-life factors among chinese and spanish adolescents: A pilot study. Nutrients. (2019) 11:1568. doi: 10.3390/nu11071568
34. Peña-Espinoza BI, Granados-Silvestre M, Sánchez-Pozos K, Ortiz-López MG, and Menjivar M. Metabolic syndrome in Mexican children: Low effectiveness of diagnostic definitions. Endocrinol Diabetes Nutr. (2017) 64:369–76. doi: 10.1016/j.endinu.2017.04.004
35. Serrano N, Villa-Roel C, Gamboa-Delgado EM, Barrera JG, and Quintero-Lesmes DC. Early evaluation of the metabolic syndrome in Bucaramanga, Colombia. Transl Pediatr. (2019) 8:363–70. doi: 10.21037/tp.2019.04.04
36. Sharma V, Coleman S, Nixon J, Sharples L, Hamilton-Shield J, Rutter H, et al. A systematic review and meta-analysis estimating the population prevalence of comorbidities in children and adolescents aged 5 to 18 years. Obes Rev. (2019) 20:1341–9. doi: 10.1111/obr.12904
37. Orsini F, D’Ambrosio F, Scardigno A, Ricciardi R, and Calabrò GE. Epidemiological impact of metabolic syndrome in overweight and obese european children and adolescents: A systematic literature review. Nutrients. (2023) 15:3895. doi: 10.3390/nu15183895
38. Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, Nkeck JR, Nyaga UF, Ngouo AT, et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: a systematic review and modelling analysis. Lancet Child Adolesc Health. (2022) 6:158–70. doi: 10.1016/s2352-4642(21)00374-6
39. Friend A, Craig L, and Turner S. The prevalence of metabolic syndrome in children: a systematic review of the literature. Metab Syndr Relat Disord. (2013) 11:71–80. doi: 10.1089/met.2012.0122
40. Zhang JX, Tian T, Wang YY, Xie W, Zhu QR, and Dai Y. Prevalence of metabolic syndrome among children and adolescentsaged 7–17 years in Jiangsu Province, 2016-2017. Pract Preventive Med. (2022) 29:916–9. doi: 10.3969/j.issn.1006-3110.2022.08.005
41. Ahmadi N, Sadr SM, Mohammadi MR, Mirzaei M, Mehrparvar AH, Yassini Ardekani SM, et al. Prevalence of abdominal obesity and metabolic syndrome in children and adolescents: A community based cross-sectional study. Iran J Public Health. (2020) 49:360–8. doi: 10.18502/ijph.v49i2.3106
42. Amer OE, Sabico S, Khattak MNK, Alnaami AM, Aljohani NJ, Alfawaz H, et al. Increasing prevalence of pediatric metabolic syndrome and its components among arab youth: A time-series study from 2010-2019. Children (Basel). (2021) 8:1129. doi: 10.3390/children8121129
43. Rodríguez LA, Madsen KA, Cotterman C, and Lustig RH. Added sugar intake and metabolic syndrome in US adolescents: cross-sectional analysis of the National Health and Nutrition Examination Survey 2005-2012. Public Health Nutr. (2016) 19:2424–34. doi: 10.1017/s1368980016000057
44. Miller JM, Kaylor MB, Johannsson M, Bay C, and Churilla JR. Prevalence of metabolic syndrome and individual criterion in US adolescents: 2001–2010 National Health and Nutrition Examination Survey. Metab Syndr Relat Disord. (2014) 12:527–32. doi: 10.1089/met.2014.0055
45. Burrows R, Correa-Burrows P, Reyes M, Blanco E, Albala C, and Gahagan S. High cardiometabolic risk in healthy Chilean adolescents: associations with anthropometric, biological and lifestyle factors. Public Health Nutr. (2016) 19:486–93. doi: 10.1017/s1368980015001585
46. Dong B, Arnold LW, Peng Y, and Wang Z. Ethnic differences in cardiometabolic risk among adolescents across the waist-height ratio spectrum: National Health and Nutrition Examination Surveys (NHANES). Int J Cardiol. (2016) 222:622–8. doi: 10.1016/j.ijcard.2016.07.169
47. Chen W, Bao W, Begum S, Elkasabany A, Srinivasan SR, and Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects: the Bogalusa Heart Study. Diabetes. (2000) 49:1042–8. doi: 10.2337/diabetes.49.6.1042
48. Ramos-Lopez O, Milagro FI, Riezu-Boj JI, and Martinez JA. Epigenetic signatures underlying inflammation: An interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflammation Res. (2021) 70:29–49. doi: 10.1007/s00011-020-01425-y
49. Szkup M, Owczarek AJ, Schneider-Matyka D, Brodowski J, Łój B, and Grochans E. Associations between the components of metabolic syndrome and the polymorphisms in the peroxisome proliferator-activated receptor gamma (PPAR-γ), the fat mass and obesity-associated (FTO), and the melanocortin-4 receptor (MC4R) genes. Aging (Albany NY). (2018) 10:72–82. doi: 10.18632/aging.101360
50. Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. (2007) 316:889–94. doi: 10.1126/science.1141634
51. Sikhayeva N, Iskakova A, Saigi-Morgui N, Zholdybaeva E, Eap CB, and Ramanculov E. Association between 28 single nucleotide polymorphisms and type 2 diabetes mellitus in the Kazakh population: a case-control study. BMC Med Genet. (2017) 18:76. doi: 10.1186/s12881-017-0443-2
52. Nagrani R, Foraita R, Gianfagna F, Iacoviello L, Marild S, Michels N, et al. Common genetic variation in obesity, lipid transfer genes and risk of Metabolic Syndrome: Results from IDEFICS/I.Family study and meta-analysis. Sci Rep. (2020) 10:7189. doi: 10.1038/s41598-020-64031-2
53. Almén MS, Jacobsson JA, Moschonis G, Benedict C, Chrousos GP, Fredriksson R, et al. Genome wide analysis reveals association of a FTO gene variant with epigenetic changes. Genomics. (2012) 99:132–7. doi: 10.1016/j.ygeno.2011.12.007
54. Song Y, Wade H, Zhang B, Xu W, Wu R, Li S, et al. Polymorphisms of fat mass and obesity-associated gene in the pathogenesis of child and adolescent metabolic syndrome. Nutrients. (2023) 15:2643. doi: 10.3390/nu15122643
55. Emond JA, Tovar A, Li Z, Lansigan RK, and Gilbert-Diamond D. FTO genotype and weight status among preadolescents: Assessing the mediating effects of obesogenic appetitive traits. Appetite. (2017) 117:321–9. doi: 10.1016/j.appet.2017.07.009
56. Perakakis N, Farr OM, and Mantzoros CS. Leptin in leanness and obesity: JACC state-of-the-art review. J Am Coll Cardiol. (2021) 77:745–60. doi: 10.1016/j.jacc.2020.11.069
57. Junyent M, Lee YC, Smith CE, Arnett DK, Tsai MY, Kabagambe EK, et al. The effect of a novel intergenic polymorphism (rs11774572) on HDL-cholesterol concentrations depends on TaqIB polymorphism in the cholesterol ester transfer protein gene. Nutr Metab Cardiovasc Dis. (2010) 20:34–40. doi: 10.1016/j.numecd.2009.02.010
58. Povel CM, Boer JM, Reiling E, and Feskens EJ. Genetic variants and the metabolic syndrome: a systematic review. Obes Rev. (2011) 12:952–67. doi: 10.1111/j.1467-789X.2011.00907.x
59. Xu C, Bai R, Zhang D, Li Z, Zhu H, Lai M, et al. Effects of APOA5 -1131T>C (rs662799) on fasting plasma lipids and risk of metabolic syndrome: evidence from a case-control study in China and a meta-analysis. PLoS One. (2013) 8:e56216. doi: 10.1371/journal.pone.0056216
60. Bideyan L, López Rodríguez M, Priest C, Kennelly JP, Gao Y, Ferrari A, et al. Hepatic GATA4 regulates cholesterol and triglyceride homeostasis in collaboration with LXRs. Genes Dev. (2022) 36:1129–44. doi: 10.1101/gad.350145.122
61. Kwiterovich PO and Byrne KH. Diagnosis and treatment of dyslipoproteinemias in children and adolescents. In: Pediatric Endocrinology: A Practical Clinical Guide, 2nd ed. (2013). p. 537–66.
62. Jeong SW, Chung M, Park SJ, Cho SB, and Hong KW. Genome-wide association study of metabolic syndrome in koreans. Genomics Inform. (Totowa, NJ: Humana Press) (2014) 12:187–94. doi: 10.5808/gi.2014.12.4.187
63. Weissglas-Volkov D, Aguilar-Salinas CA, Nikkola E, Deere KA, Cruz-Bautista I, Arellano-Campos O, et al. Genomic study in Mexicans identifies a new locus for triglycerides and refines European lipid loci. J Med Genet. (2013) 50:298–308. doi: 10.1136/jmedgenet-2012-101461
64. Liu PH, Chang YC, Jiang YD, Chen WJ, Chang TJ, Kuo SS, et al. Genetic variants of TCF7L2 are associated with insulin resistance and related metabolic phenotypes in Taiwanese adolescents and Caucasian young adults. J Clin Endocrinol Metab. (2009) 94:3575–82. doi: 10.1210/jc.2009-0609
65. Phillips CM, Goumidi L, Bertrais S, Field MR, McManus R, Hercberg S, et al. Dietary saturated fat, gender and genetic variation at the TCF7L2 locus predict the development of metabolic syndrome. J Nutr Biochem. (2012) 23:239–44. doi: 10.1016/j.jnutbio.2010.11.020
66. Ferreira MC, da Silva MER, Fukui RT, Arruda-Marques MDC, and Dos Santos RF. TCF7L2 correlation in both insulin secretion and postprandial insulin sensitivity. Diabetol Metab Syndrome. (2018) 10:1–7. doi: 10.1186/s13098-018-0338-1
67. Hosseinpour-Niazi S, Bakhshi B, Zahedi AS, Akbarzadeh M, Daneshpour MS, Mirmiran P, et al. TCF7L2 polymorphisms, nut consumption, and the risk of metabolic syndrome: a prospective population based study. Nutr Metab (Lond). (2021) 18:10. doi: 10.1186/s12986-021-00542-7
68. Wang J, Kuusisto J, Vänttinen M, Kuulasmaa T, Lindström J, Tuomilehto J, et al. Variants of transcription factor 7-like 2 (TCF7L2) gene predict conversion to type 2 diabetes in the Finnish Diabetes Prevention Study and are associated with impaired glucose regulation and impaired insulin secretion. Diabetologia. (2007) 50:1192–200. doi: 10.1007/s00125-007-0656-6
69. Herring SJ and Oken E. Obesity and diabetes in mothers and their children: can we stop the intergenerational cycle? Curr Diabetes Rep. (2011) 11:20–7. doi: 10.1007/s11892-010-0156-9
70. Scheidl TB, Brightwell AL, Easson SH, and Thompson JA. Maternal obesity and programming of metabolic syndrome in the offspring: searching for mechanisms in the adipocyte progenitor pool. BMC Med. (2023) 21:50. doi: 10.1186/s12916-023-02730-z
71. Group. HSCR. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. (2009) 58:453–9. doi: 10.2337/db08-1112
72. Lu S, Wang J, Kakongoma N, Hua W, Xu J, Wang Y, et al. DNA methylation and expression profiles of placenta and umbilical cord blood reveal the characteristics of gestational diabetes mellitus patients and offspring. Clin Epigenet. (2022) 14:69. doi: 10.1186/s13148-022-01289-5
73. Radaelli T, Varastehpour A, Catalano P, and Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. (2003) 52:2951–8. doi: 10.2337/diabetes.52.12.2951
74. Boney CM, Verma A, Tucker R, and Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. (2005) 115:e290–6. doi: 10.1542/peds.2004-1808
75. Holtrup B, Church CD, Berry R, Colman L, Jeffery E, Bober J, et al. Puberty is an important developmental period for the establishment of adipose tissue mass and metabolic homeostasis. Adipocyte. (2017) 6:224–33. doi: 10.1080/21623945.2017.1349042
76. Orsso CE, Colin-Ramirez E, Field CJ, Madsen KL, Prado CM, and Haqq AM. Adipose tissue development and expansion from the womb to adolescence: an overview. Nutrients. (2020) 12:2735. doi: 10.3390/nu12092735
77. Muhlhausler BS, Duffield JA, and McMillen IC. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-gamma, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology. (2007) 148:878–85. doi: 10.1210/en.2006-1115
78. Mikolajczak A, Sallam NA, Singh RD, Scheidl TB, Walsh EJ, Larion S, et al. Accelerated developmental adipogenesis programs adipose tissue dysfunction and cardiometabolic risk in offspring born to dams with metabolic dysfunction. Am J Physiol Endocrinol Metab. (2021) 321:E581–e91. doi: 10.1152/ajpendo.00229.2021
79. McGee SL and Hargreaves M. Exercise and skeletal muscle glucose transporter 4 expression: molecular mechanisms. Clin Exp Pharmacol Physiol. (2006) 33:395–9. doi: 10.1111/j.1440-1681.2006.04362.x
80. Moleres A, Campión J, Milagro FI, Marcos A, Campoy C, Garagorri JM, et al. Differential DNA methylation patterns between high and low responders to a weight loss intervention in overweight or obese adolescents: the EVASYON study. FASEB J. (2013) 27:2504–12. doi: 10.1096/fj.12-215566
81. Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. (2005) 102:10604–9. doi: 10.1073/pnas.0500398102
82. Wu Y-L, Lin Z-J, Li C-C, Lin X, Shan S-K, Guo B, et al. Epigenetic regulation in metabolic diseases: mechanisms and advances in clinical study. Signal Transduction Targeted Ther. (2023) 8:98. doi: 10.1038/s41392-023-01333-7
83. Morselli M and Dieci G. Epigenetic regulation of human non-coding RNA gene transcription. Biochem Soc Trans. (2022) 50:723–36. doi: 10.1042/BST20210860
84. Robertson KD and Wolffe AP. DNA methylation in health and disease. Nat Rev Genet. (2000) 1:11–9. doi: 10.1038/35049533
85. Weaver JR, Susiarjo M, and Bartolomei MS. Imprinting and epigenetic changes in the early embryo. Mamm Genome. (2009) 20:532–43. doi: 10.1007/s00335-009-9225-2
86. Musri MM and Párrizas M. Epigenetic regulation of adipogenesis. Curr Opin Clin Nutr Metab Care. (2012) 15:342–9. doi: 10.1097/MCO.0b013e3283546fba
87. Ge K. Epigenetic regulation of adipogenesis by histone methylation. Biochim Biophys Acta. (2012) 1819:727–32. doi: 10.1016/j.bbagrm.2011.12.008
88. Yoo JY, Lee S, Lee HA, Park H, Park YJ, Ha EH, et al. Can proopiomelanocortin methylation be used as an early predictor of metabolic syndrome? Diabetes Care. (2014) 37:734–9. doi: 10.2337/dc13-1012
89. Komariah K, Manalu W, Kiranadi B, Winarto A, Handharyani E, and Roeslan MO. Valproic acid exposure of pregnant rats during organogenesis disturbs pancreas development in insulin synthesis and secretion of the offspring. Toxicol Res. (2018) 34:173–82. doi: 10.5487/tr.2018.34.2.173
90. Alejandro EU, Gregg B, Wallen T, Kumusoglu D, Meister D, Chen A, et al. Maternal diet-induced microRNAs and mTOR underlie β cell dysfunction in offspring. J Clin Invest. (2014) 124:4395–410. doi: 10.1172/jci74237
91. Bernstein D, Golson ML, and Kaestner KH. Epigenetic control of β-cell function and failure. Diabetes Res Clin Pract. (2017) 123:24–36. doi: 10.1016/j.diabres.2016.11.009
92. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8
93. Julibert A, Bibiloni MDM, and Tur JA. Dietary fat intake and metabolic syndrome in adults: A systematic review. Nutr Metab Cardiovasc Dis. (2019) 29:887–905. doi: 10.1016/j.numecd.2019.05.055
94. Slimani N, Deharveng G, Southgate DA, Biessy C, Chajès V, van Bakel MM, et al. Contribution of highly industrially processed foods to the nutrient intakes and patterns of middle-aged populations in the European Prospective Investigation into Cancer and Nutrition study. Eur J Clin Nutr. (2009) 63 Suppl 4:S206–25. doi: 10.1038/ejcn.2009.82
95. Akbarzadeh Z, Nourian M, Hovsepian S, and Kelishadi R. Dietary patterns and metabolic syndrome in children and adolescents: a systematic review. J Pediatr Rev. (2018) 6:2–13. doi: 10.5812/jpr.11656.
96. Mente A, de Koning L, Shannon HS, and Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. (2009) 169:659–69. doi: 10.1001/archinternmed.2009.38
97. Kabagambe EK, Tsai MY, Hopkins PN, Ordovas JM, Peacock JM, Borecki IB, et al. Erythrocyte fatty acid composition and the metabolic syndrome: a National Heart, Lung, and Blood Institute GOLDN study. Clin Chem. (2008) 54:154–62. doi: 10.1373/clinchem.2007.095059
98. Warensjö E, Risérus U, and Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. (2005) 48:1999–2005. doi: 10.1007/s00125-005-1897-x
99. Tanasescu M, Cho E, Manson JE, and Hu FB. Dietary fat and cholesterol and the risk of cardiovascular disease among women with type 2 diabetes. Am J Clin Nutr. (2004) 79:999–1005. doi: 10.1093/ajcn/79.6.999
100. Ambrosini GL, Huang RC, Mori TA, Hands BP, O’Sullivan TA, de Klerk NH, et al. Dietary patterns and markers for the metabolic syndrome in Australian adolescents. Nutr Metab Cardiovasc Dis. (2010) 20:274–83. doi: 10.1016/j.numecd.2009.03.024
101. Warodomwichit D, Arnett DK, Kabagambe EK, Tsai MY, Hixson JE, Straka RJ, et al. Polyunsaturated fatty acids modulate the effect of TCF7L2 gene variants on postprandial lipemia. J Nutr. (2009) 139:439–46. doi: 10.3945/jn.108.096461
102. Weiss R, Taksali SE, Dufour S, Yeckel CW, Papademetris X, Cline G, et al. The “obese insulin-sensitive” adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab. (2005) 90:3731–7. doi: 10.1210/jc.2004-2305
103. Petersen MC and Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. (2018) 98:2133–223. doi: 10.1152/physrev.00063.2017
104. Zhou M, Shao J, Wu CY, Shu L, Dong W, Liu Y, et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes. (2019) 68:1730–46. doi: 10.2337/db18-0927
105. Hershkop K, Besor O, Santoro N, Pierpont B, Caprio S, and Weiss R. Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J Clin Endocrinol Metab. (2016) 101:2423–31. doi: 10.1210/jc.2016-1376
106. Jin X, Qiu T, Li L, Yu R, Chen X, Li C, et al. Pathophysiology of obesity and its associated diseases. Acta Pharm Sin B. (2023) 13:2403–24. doi: 10.1016/j.apsb.2023.01.012
107. Lê KA, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-κB stress pathway. Diabetes. (2011) 60:2802–9. doi: 10.2337/db10-1263
108. Hijmans BS, Grefhorst A, Oosterveer MH, and Groen AK. Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Biochimie. (2014) 96:121–9. doi: 10.1016/j.biochi.2013.06.007
109. Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, and Smith U. Insulin resistance and impaired adipogenesis. Trends Endocrinol Metab. (2015) 26:193–200. doi: 10.1016/j.tem.2015.01.006
110. Alves-Bezerra M and Cohen DE. Triglyceride metabolism in the liver. Compr Physiol. (2017) 8:1–8. doi: 10.1002/cphy.c170012
111. Hertiš Petek T, Petek T, Močnik M, and Marčun Varda N. Systemic inflammation, oxidative stress and cardiovascular health in children and adolescents: a systematic review. Antioxidants. (2022) 11:894. doi: 10.3390/antiox11050894
112. Hotamisligil GS. Inflammation and metabolic disorders. Nature. (2006) 444:860–7. doi: 10.1038/nature05485
113. Shin SK, Cho HW, Song SE, Im SS, Bae JH, and Song DK. Oxidative stress resulting from the removal of endogenous catalase induces obesity by promoting hyperplasia and hypertrophy of white adipocytes. Redox Biol. (2020) 37:101749. doi: 10.1016/j.redox.2020.101749
114. Kirichenko TV, Markina YV, Bogatyreva AI, Tolstik TV, Varaeva YR, and Starodubova AV. The role of adipokines in inflammatory mechanisms of obesity. Int J Mol Sci. (2022) 23:14982. doi: 10.3390/ijms232314982
115. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, and Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. (2003) 112:1796–808. doi: 10.1172/jci19246
116. Gugliucci A. Biomarkers of dysfunctional visceral fat. Adv Clin Chem. (2022) 109:1–30. doi: 10.1016/bs.acc.2022.03.001
117. Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, et al. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. (2004) 94:534–41. doi: 10.1161/01.Res.0000115557.25127.8d
118. Engin A. Endothelial dysfunction in obesity and therapeutic targets. In: Obesity and Lipotoxicity (2024). (Obesity and Lipotoxicity. Advances in Experimental Medicine and Biology) p. 489–538.
119. Khalid M, Alkaabi J, Khan MA, and Adem A. Insulin signal transduction perturbations in insulin resistance. Int J Mol Sci. (2021) 22:8590. doi: 10.3390/ijms22168590
120. Hotamisligil GS, Murray DL, Choy LN, and Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. (1994) 91:4854–8. doi: 10.1073/pnas.91.11.4854
121. Galcheva SV, Iotova VM, Yotov YT, Bernasconi S, and Street ME. Circulating proinflammatory peptides related to abdominal adiposity and cardiometabolic risk factors in healthy prepubertal children. Eur J Endocrinol. (2011) 164:553–8. doi: 10.1530/eje-10-1124
122. Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, and Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep. (2010) 33:1201–9. doi: 10.1093/sleep/33.9.1201
123. Wang F, Liu H, Wan Y, Li J, Chen Y, Zheng J, et al. Sleep duration and overweight/obesity in preschool-aged children: A prospective study of up to 48,922 children of the jiaxing birth cohort. Sleep. (2016) 39:2013–9. doi: 10.5665/sleep.6234
124. Jansen EC, Burgess HJ, Chervin RD, Dolinoy DC, Téllez-Rojo MM, Cantoral A, et al. Sleep duration and timing are prospectively linked with insulin resistance during late adolescence. Obesity (Silver Spring). (2023) 31:912–22. doi: 10.1002/oby.23680
125. McKay NJ, Giorgianni NR, Czajka KE, Brzyski MG, Lewandowski CL, Hales ML, et al. Plasma levels of ghrelin and GLP-1, but not leptin or amylin, respond to a psychosocial stressor in women and men. Horm Behav. (2021) 134:105017. doi: 10.1016/j.yhbeh.2021.105017
126. Hjorth MF, Chaput JP, Damsgaard CT, Dalskov SM, Andersen R, Astrup A, et al. Low physical activity level and short sleep duration are associated with an increased cardio-metabolic risk profile: a longitudinal study in 8–11 year old Danish children. PLoS One. (2014) 9:e104677. doi: 10.1371/journal.pone.0104677
127. Wisnieski L, Kerver J, Holzman C, Todem D, and Margerison-Zilko C. Breastfeeding and risk of metabolic syndrome in children and adolescents: A systematic review. J Hum Lact. (2018) 34:515–25. doi: 10.1177/0890334417737038
128. Khuc K, Blanco E, Burrows R, Reyes M, Castillo M, Lozoff B, et al. Adolescent metabolic syndrome risk is increased with higher infancy weight gain and decreased with longer breast feeding. Int J Pediatr. (2012) 2012:478610. doi: 10.1155/2012/478610
129. González-Jiménez E, Montero-Alonso MA, Schmidt-RioValle J, García-García CJ, and Padez C. Metabolic syndrome in Spanish adolescents and its association with birth weight, breastfeeding duration, maternal smoking, and maternal obesity: a cross-sectional study. Eur J Nutr. (2015) 54:589–97. doi: 10.1007/s00394-014-0740-x
130. de Armas MG, Megías SM, Modino SC, Bolaños PI, Guardiola PD, and Alvarez TM. Importance of breastfeeding in the prevalence of metabolic syndrome and degree of childhood obesity. Endocrinol Nutr. (2009) 56:400–3. doi: 10.1016/s1575-0922(09)72709-3
131. Fagerberg B, Bondjers L, and Nilsson P. Low birth weight in combination with catch-up growth predicts the occurrence of the metabolic syndrome in men at late middle age: the Atherosclerosis and Insulin Resistance study. J Intern Med. (2004) 256:254–9. doi: 10.1111/j.1365-2796.2004.01361.x
132. Vandyousefi S, Goran MI, Gunderson EP, Khazaee E, Landry MJ, Ghaddar R, et al. Association of breastfeeding and gestational diabetes mellitus with the prevalence of prediabetes and the metabolic syndrome in offspring of Hispanic mothers. Pediatr Obes. (2019) 14:e12515. doi: 10.1111/ijpo.12515
133. Liu F, Lv D, Wang L, Feng X, Zhang R, Liu W, et al. Breastfeeding and overweight/obesity among children and adolescents: a cross-sectional study. BMC Pediatr. (2022) 22:347. doi: 10.1186/s12887-022-03394-z
134. Socha P, Grote V, Gruszfeld D, Janas R, Demmelmair H, Closa-Monasterolo R, et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: data from a randomized clinical trial. Am J Clin Nutr. (2011) 94:1776s–84s. doi: 10.3945/ajcn.110.000596
135. Valassi E, Scacchi M, and Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. (2008) 18:158–68. doi: 10.1016/j.numecd.2007.06.004
136. James J, Thomas P, Cavan D, and Kerr D. Preventing childhood obesity by reducing consumption of carbonated drinks: cluster randomised controlled trial. Bmj. (2004) 328:1237. doi: 10.1136/bmj.38077.458438.EE
137. Bean MK, Powell P, Quinoy A, Ingersoll K, Wickham EP 3rd, and Mazzeo SE. Motivational interviewing targeting diet and physical activity improves adherence to paediatric obesity treatment: results from the MI Values randomized controlled trial. Pediatr Obes. (2015) 10:118–25. doi: 10.1111/j.2047-6310.2014.226.x
138. Organization WH. Interim report of the commission on ending childhood obesity. (Geneva: World Health Organization) (2015).
139. Velázquez-López L, Santiago-Díaz G, Nava-Hernández J, Muñoz-Torres AV, Medina-Bravo P, and Torres-Tamayo M. Mediterranean-style diet reduces metabolic syndrome components in obese children and adolescents with obesity. BMC Pediatr. (2014) 14:175. doi: 10.1186/1471-2431-14-175
140. Scharf RJ and DeBoer MD. Sugar-sweetened beverages and children’s health. Annu Rev Public Health. (2016) 37:273–93. doi: 10.1146/annurev-publhealth-032315-021528
141. Pacifico L, Anania C, Martino F, Poggiogalle E, Chiarelli F, Arca M, et al. Management of metabolic syndrome in children and adolescents. Nutr Metab Cardiovasc Dis. (2011) 21:455–66. doi: 10.1016/j.numecd.2011.01.011
142. Steffen LM, Jacobs DR Jr., Murtaugh MA, Moran A, Steinberger J, Hong CP, et al. Whole grain intake is associated with lower body mass and greater insulin sensitivity among adolescents. Am J Epidemiol. (2003) 158:243–50. doi: 10.1093/aje/kwg146
143. Delzenne NM and Cani PD. A place for dietary fibre in the management of the metabolic syndrome. Curr Opin Clin Nutr Metab Care. (2005) 8:636–40. doi: 10.1097/01.mco.0000171124.06408.71
144. He FJ and MacGregor GA. Importance of salt in determining blood pressure in children: meta-analysis of controlled trials. Hypertension. (2006) 48:861–9. doi: 10.1161/01.HYP.0000245672.27270.4a
145. Ekelund U, Anderssen SA, Froberg K, Sardinha LB, Andersen LB, and Brage S. Independent associations of physical activity and cardiorespiratory fitness with metabolic risk factors in children: the European youth heart study. Diabetologia. (2007) 50:1832–40. doi: 10.1007/s00125-007-0762-5
146. Bremer AA, Auinger P, and Byrd RS. Relationship between insulin resistance-associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in US adolescents: findings from the 1999–2004 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. (2009) 163:328–35. doi: 10.1001/archpediatrics.2009.21
147. Franczyk B, Gluba-Brzózka A, Ciałkowska-Rysz A, Ławiński J, and Rysz J. The impact of aerobic exercise on HDL quantity and quality: a narrative review. Int J Mol Sci. (2023) 24:4653. doi: 10.3390/ijms24054653
148. Guinhouya BC, Samouda H, Zitouni D, Vilhelm C, and Hubert H. Evidence of the influence of physical activity on the metabolic syndrome and/or on insulin resistance in pediatric populations: a systematic review. Int J Pediatr Obes. (2011) 6:361–88. doi: 10.3109/17477166.2011.605896
149. Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, and Beghetti M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. (2009) 54:2396–406. doi: 10.1016/j.jacc.2009.08.030
150. Delayun CF. Patient-Specific and Program Outcome Evaluation of a Novel Pediatric Exercise Medicine Program. Canada: University of Toronto (2021).
151. Barutcu A, Ornek C, and Kozanoglu E. A growing problem in childhood and adolescence: Metabolic syndrome and its relationship with physical activity and fitness. Marmara Med J. (2023) 36:255–61. doi: 10.5472/marumj.1307990
152. Weihe P and Weihrauch-Blüher S. Metabolic syndrome in children and adolescents: diagnostic criteria, therapeutic options and perspectives. Curr Obes Rep. (2019) 8:472–9. doi: 10.1007/s13679-019-00357-x
153. Zappalla FR and Gidding SS. Lipid management in children. Endocrinol Metab Clin North Am. (2009) 38:171–83. doi: 10.1016/j.ecl.2008.11.006
154. Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. (2013) 167:355–60. doi: 10.1001/jamapediatrics.2013.1045
155. Sadeghi A, Mousavi SM, Mokhtari T, Parohan M, and Milajerdi A. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Childhood Obesity. (2020) 16:174–91. doi: 10.1089/chi.2019.0040
156. Freemark M and Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. (2001) 107:E55. doi: 10.1542/peds.107.4.e55
157. Inge TH, Miyano G, Bean J, Helmrath M, Courcoulas A, Harmon CM, et al. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics. (2009) 123:214–22. doi: 10.1542/peds.2008-0522
158. Lundahl A, Kidwell KM, and Nelson TD. Parental underestimates of child weight: a meta-analysis. Pediatrics. (2014) 133:e689–703. doi: 10.1542/peds.2013-2690
159. Detection NCEPEPo, Adults ToHBCi. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). The Program (2002).
160. McGill HC Jr., McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. (2002) 105:2712–8. doi: 10.1161/01.cir.0000018121.67607.ce
Keywords: metabolic syndrome, children, adolescents, diabetes mellitus, obesity, cardiovascular diseases, epidemiology
Citation: Zhang B, Shi H, Cai W, Yang B and Xiu W (2025) Metabolic syndrome in children and adolescents: definitions, epidemiology, pathophysiology, interventions, and challenges. Front. Endocrinol. 16:1512642. doi: 10.3389/fendo.2025.1512642
Received: 17 October 2024; Accepted: 14 May 2025;
Published: 11 June 2025.
Edited by:
Gianpaolo De Filippo, Hôpital Robert Debré, FranceReviewed by:
Anna-Mariia Shulhai, University of Parma, ItalyErkan Kozanoglu, Cukurova University, Türkiye
Copyright © 2025 Zhang, Shi, Cai, Yang and Xiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenlong Xiu, d2x4aXVAZmptdS5lZHUuY24=
 Baoquan Zhang
Baoquan Zhang Wenlong Xiu
Wenlong Xiu