- 1Department of Pediatrics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2International Medical College, Chongqing Medical University, Chongqing, China
Adipose tissue is an endocrine organ that signals energy status to the hypothalamic–pituitary–gonadal axis to regulate reproductive function. Notably, in mammals, adipose tissue biology—adipose tissue expansion and body fat distribution—is closely linked to the onset of puberty. Some studies showed that early adipose tissue development continues into childhood or adulthood, indicating its potential impact on reproductive function. Factors such as maternal obesity, childhood body mass index gain, and adolescent obesity significantly contribute to early puberty onset and negative reproductive events including menstrual irregularity, polycystic ovary syndrome, and male infertility. However, the connection between adipose tissue development before adulthood (prenatal stage and childhood) and reproductive function has not yet been fully investigated and reviewed. In this study, we present a comprehensive review of hormonal and inherent dimorphisms on adipose tissue development; there is a novel discussion about the link between adipose tissue expansion tracking throughout early life stages and reproductive disorders. Our study aims to elucidate how adipocyte development during critical periods of life can affect future reproductive health from sexual maturation to fertility and points to the clinical significance of further unlocking the underlying mechanism and weight management. As such, early prevention and long-term management for weight control might be considered as effective measures to mitigate obesity-induced reproductive comorbidities.
1 Introduction
Adipose tissue (AT) participates in a wide range of physiological and pathological processes such as energy metabolism, inflammation, reproduction function, and several types of cancers. Obesity, literally the excess of white adipose tissue (WAT), is a global concern of metabolic health that contributes to earlier pubertal development, impaired fertility, and polycystic ovary syndrome (PCOS) (1–3). With the rates of overweight and obesity increasingly growing in children, an increasing number of studies have revealed the prevalence of obesity-associated reproductive disorders in children and adolescents. Substantial studies have explored the connection among AT, puberty, and gonadal function. AT development begins in prenatal life and infancy, and tracks into childhood; it influences sexual maturation (4–7). Furthermore, childhood obesity is associated with precocious puberty, hyperandrogenism, suppression of gonadotropins, and the development of PCOS (8–11). This review discusses hormonal and inherent dimorphisms on AT development and provides a broad description of AT development during fundamental life stages and its role in puberty and later reproductive function.
2 The characteristics of adipose tissue
In mammals, AT primarily consists of WAT and brown AT (BAT). BAT plays a thermogenic role and is essential for maintaining body temperature. As an endocrine organ, it is considered as a metabolic pool for glucose, lipid, and branched-chain amino acids, and is related to metabolic diseases such as overweight and obesity (12). In humans, BAT mainly exists in fetuses and newborns to enhance neonatal survival, but decreases shortly after birth (13). WAT stores excess energy in the form of triglyceride and secretes various hormones that regulate energy metabolism. WAT is generally classified as subcutaneous AT (SAT) and visceral AT (VAT). SAT depots are mainly the abdominal, gluteal, and femoral types, but VAT, the so-called intraabdominal fat depot, is associated with internal organs. Women generally have more fat accumulation than men. Men accumulate more fat in the upper body (central obesity), which is associated with the development of cardiovascular disease, insulin resistance, and type 2 diabetes mellitus (14–16). Women accumulate more in the lower body (peripheral obesity), which protects against metabolic disorders (14).
AT development is a dynamic and proliferative process involving adipogenesis and remodeling through differentiated adipocyte precursor cells (APCs). The expansion of fat mass can occur through an increase in the average size of fat cells and/or number of adipocytes. In adults, enlarged fat cells are a key feature of increased fat storage in fat depots, whereas in children with obesity, there is a significant increase in both adipocyte number and cell size (17–19). From 6 months to 1 year of age, cell size increased to adult levels and then decreased until 2 years of age, during which larger adipocytes contributed to an increase in fat depots. After 2 years of age, cell size did not change significantly until early adolescence age (19). Adipocyte number significantly increases only after age 10. In contrast, significant increases in cell number and size in obese children were observed throughout all ages (19). Overall, the changes in adipocytes for childhood obesity are characterized by adipocyte hypertrophy and hyperplasia.
3 The hormonal and inherent dimorphisms on adipose tissue development during puberty
Puberty is another important period for the hypercellularity of AT. For mammalian females, body fat increases during puberty onset and is predominantly localized in gluteofemoral fat depots, which is profoundly associated with the start of menarche (20, 21). For men, they also gain body fat during puberty, but lean mass mostly increases before a growth spurt to deplete body fat (22). This makes AT less remarkable on male pubertal development; however, it is still important in male puberty initiation (23). Sex differences exist in body fat distribution during puberty. In men, an android shape, which is mainly fat in the abdominal area, develops principally during this period. In women, fat is centered on the hips and remains gynecoid during puberty.
Sex steroid hormones (estrogen and androgen) are considered to be the main cause of sex differences in fat distribution. The mechanism underlying the sex- and depot-specific fat distribution remains poorly understood. Gonadal hormones, including estrogen (E2), progesterone, and androgen, have their receptors expressed in both VAT and SAT depots. In women, SAT has higher expressions of estrogen receptors (ERs) and progesterone receptors (PRs) than androgen receptors (ARs). Estrogen promotes subcutaneous fat depot only after sexual maturation in women (24). Moreover, its receptor ERα signaling in women leads to subcutaneous fat accrual and the reduction of visceral adipocyte mass, but ERβ may inhibit the effect of E2 on adipocytes after sex maturation (25–27). In both female and male mouse models, epididymal, perirenal, and inguinal WAT weighed more in ERα knockout (αERKO) mice than in their wild-type control (28). Moreover, androgen and adrenal steroids such as dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEAS), and some subtypes of 17-β hydroxysteroid dehydrogenase (HSD) isoenzymes are also associated with body fat distribution in women. Visceral adiposities were positively associated with omental 20α-HSD level and 3α-HSD-3 level (29). However, the association between plasma androgen levels and visceral fat accumulation is not always consistent.
As in female AT, ER is also expressed in male AT. Estrogen treatment decreased adipocyte size in male rats (30). αERKO male mice showed increased WAT, especially in epididymal, perirenal, and inguinal depots (28). In men, visceral fat accumulation is inversely associated with circulating testosterone (cT) levels and sex hormone-binding globulin (SHGB) (31–33), and testosterone treatment decreases abdominal subcutaneous and gluteal depots in female-to-male transsexuals (34). Additionally, androgen receptor knockout (ARKO) mice develop late-onset visceral adiposity and total fat mass (35–37).
Several lines of evidence suggested that local androgen metabolism in AT could affect body fat distribution. DHEA, an adrenal precursor to the formation of active steroids, has been found to be negatively associated with abdominal fat accumulation in men (38). Peripheral androgen metabolites (PAMs), such as 5α-androstane-3α and 17β-diol glucuronide (3α-diol-G), are positively associated with visceral fat accumulation (39). Steroid-inactivating enzymes, such as the aldoketoreductase 1C (AKR1C) family, which are mostly responsible for androstenedione (DHT) inactivation, are highly expressed in AT and are positively related to omental adipocyte size and visceral fat accumulation (29, 40–42). From the evidence above, local androgens might play a more significant role in fat accumulation than circulating androgens in men.
Some androgen-metabolizing enzymes are identified in AT and might indirectly contribute to depot differences. 5α-Reductase type 1 (SRD5A1), an androgen-metabolizing enzyme, and its metabolites increased with obesity in humans (43–45). Other steroid-metabolizing enzymes, such as 3β-hydroxysteroid dehydrogenase (HSD) type 1; 17β-HSD types 2, 3, 7 and 12; and 17α-hydroxylase, have also been detected in AT, but their role in fat depots remains unknown. Furthermore, evidence has shown an association between gonadotropin levels and adipocytes. Li et al. discovered that gonadotropin-releasing hormone receptors (GnRHRs) are expressed in human adipocytes, and the activation of GnRHR could increase the cell number of preadipocytes and the accumulation of lipid droplets by inhibiting AMPK pathways (46). Thus, gonadal hormones, steroid hormones, and their metabolizing enzymes are likely to participate in adipocyte differentiation. Regional variation and intra-adipocyte hormone metabolism might explain the heterogeneity of hormonal effects on fat distribution. The roles of sex hormones, their receptors, and steroid-metabolizing enzymes in adipogenesis and adipolysis within fat depots are concluded in Figure 1.
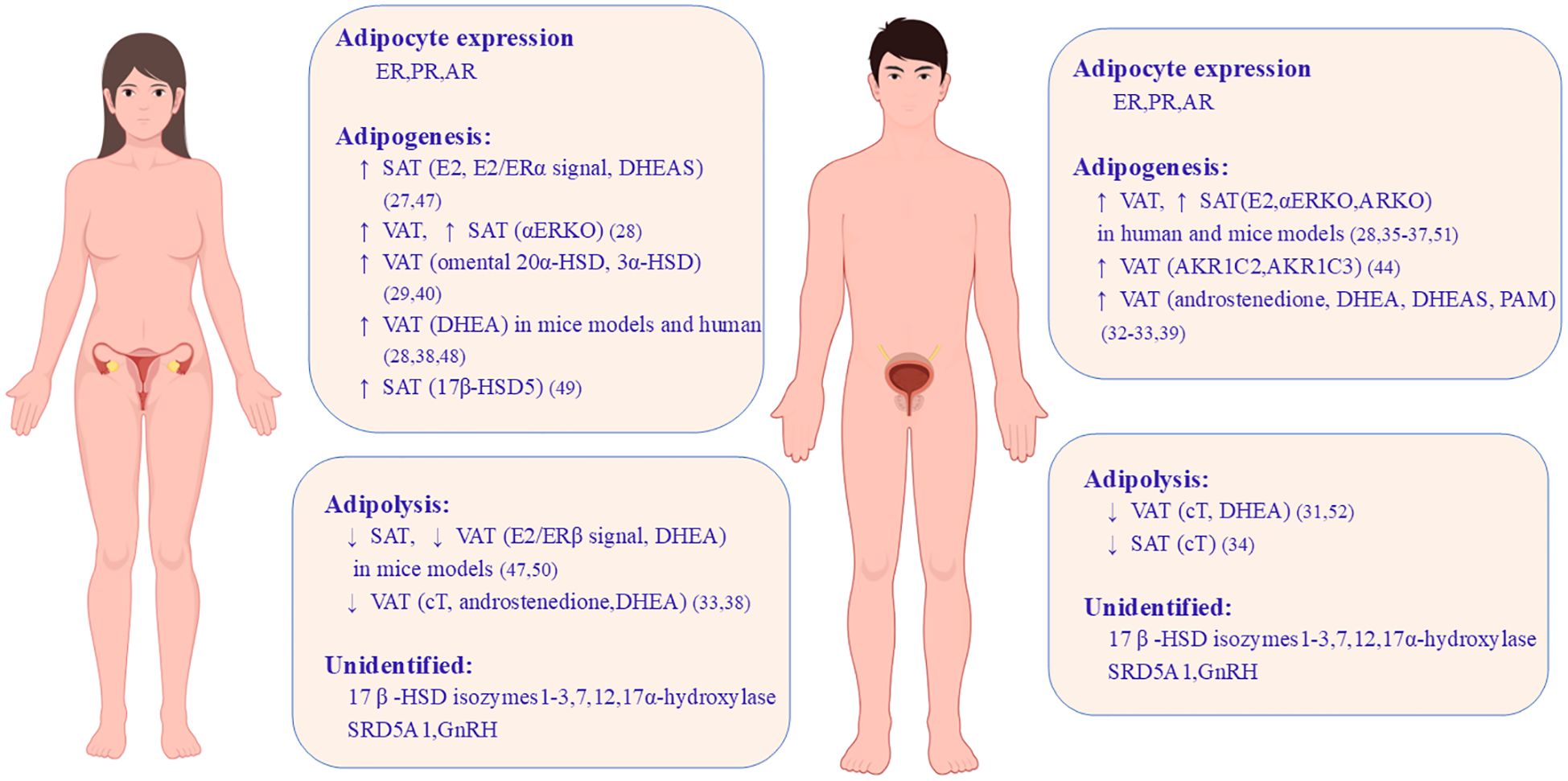
Figure 1. Sex differences caused by gonadal hormones and steroid-metabolizing enzymes on adipose tissue development. Both women and men have ER, PR, and AR expressed in adipocytes but their role in adipogenesis and adipolysis shows sex differences and depot differences. This image shows the roles of sex hormones, their cognate receptors, and steroid-metabolizing enzymes in VAT and SAT in mice models and human. ↑, increase; ↓, decrease; ER, estrogen receptor; E2, estrogen; PR, progesterone receptor; AR, androgen receptor; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; ERKO, estrogen receptor knockout; ARKO, androgen receptor knockout; HSD, hydroxysteroid dehydrogenase; cT, circulating testosterone; SRD5A1, 5α-reductase type 1; AKR1C2/AKR1C3, aldoketoreductase 1C family; PAM, peripheral androgen metabolites (47–52).
Except for gonadal hormones, sex chromosomes might inherently determine sex differences in adipocyte biology. Gonadectomized mice with XX versus XY gain more weight and adiposity, particularly inguinal WAT, indicating the effect of X chromosome on weight gain independent of gonadal steroids (53). These genetic studies show that an increased number of X chromosomes, rather than the Y chromosome, leads to differences in adiposity. The presence of two X chromosomes (XX and XXY mice with gonadectomy) led to higher body weight/fat than one X chromosome (XY and XO mice with gonadectomy), while the presence of the Y chromosome did not have an effect (53). Thus, these results implicated the X chromosome gene as a direct cause of sex differences in fat distribution.
Sexually dimorphic genes and epigenetic modification in gene expressions have been identified as contributing factors in AT biology. A review of these genes can be found in references (54–56). In conclusion, sex differences in body fat distribution during puberty are not solely determined by the secretion of gonadal hormones, but rather by a more complex interplay of depot-specific adipocyte differentiation, hormonal signaling, and genetic modifications.
4 The association between adipose tissue during early life stage and puberty
The development and expansion of AT begins in the fetus and extends throughout the lifespan. Rapid fat accrual occurs during the late prenatal period and infancy. Studies have indicated that adiposity in prenatal life and infancy tracks into childhood, and is associated with childhood obesity and body composition (4, 5, 57–59). Thus, the link between adiposity during this period and puberty has drawn much attention. Clinical studies have shown that early weight gain in infancy and the trajectories of body mass index (BMI) percentage during early childhood predict younger ages at menarche and the onset of breast development (7, 60–63). In a retrospective longitudinal study, the timing of menarche and thelarche showed an inverse relationship with the change in z-score from birth weight (64). Furthermore, restricted fetal growth and prenatal maternal fat also affect pubertal development. Girls born small-for-gestational age (SGA) reach all pubertal markers at an earlier mean age than those born appropriate-for-gestational age (AGA), except for breast development, while boys born SGA and large-for-gestational age (LGA) achieved puberty earlier than those born AGA (6).
Maternal obesity, even before pregnancy, is associated with earlier pubertal development in offspring (65–72). The mechanism linking maternal fat to offspring puberty remains to be elucidated. In rat models, maternal high-fat diet during the early postnatal period induced increased Kiss1 expression in the ARC and early puberty onset in female offspring (73). A study conducted by Lam et al. found that MC3R, expressed in KNDy neurons of the hypothalamic arcuate nucleus, could be activated as an intermediary signaling pathway to relay nutritional status to childhood growth and the timing of puberty (74). Increased endogenous estradiol in the progeny of obese rats is associated with precocious puberty and altered follicular development in adulthood (75). These findings underscore the importance of AT development during the prenatal period and childhood in determining their susceptibility to early pubertal maturation (Figure 2).
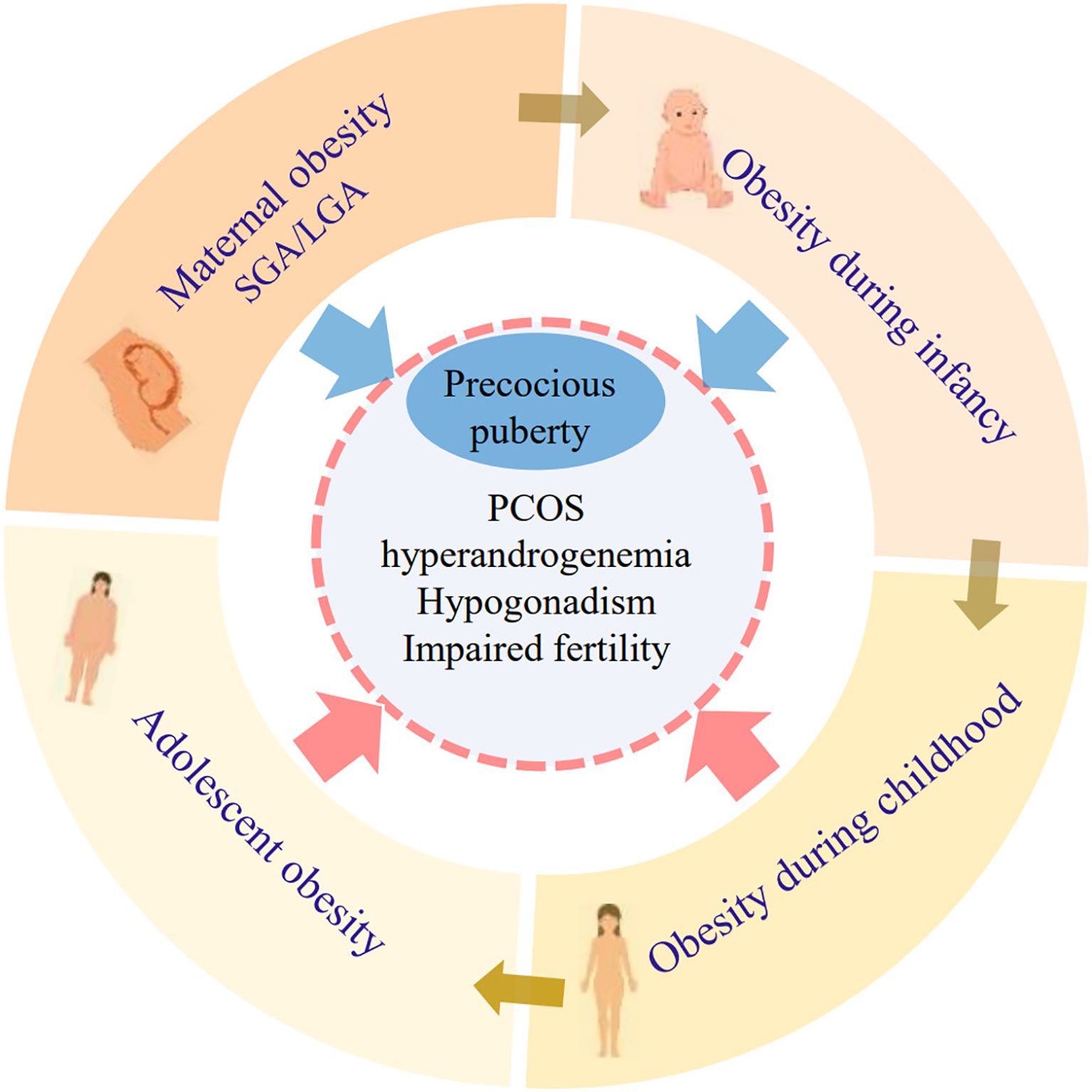
Figure 2. The connection between obesity during critical life stages and reproductive disorders. Adiposity at each developmental stage prior to puberty onset might last toward the next life stages. Perinatal factors (maternal obesity, SGA, LGA, and infant obesity) are closely related to precocious puberty. Obesity during childhood and adolescence is related to high risks of multiple reproductive disorders such as PCOS, hyperandrogenemia, hypogonadism, and impaired fertility. SGA, small for gestational age; LGA, large for gestational age; PCOS, polycystic ovary syndrome.
5 The association of adipose tissue during childhood and adolescence and puberty
Puberty is a critical process of sexual maturation and is characterized by a growth spurt. Prior to puberty, there are minimal sex differences in body composition, but differences in AT development become more apparent from puberty. Sexual dimorphism in regional fat patterning emerges, with girls exhibiting less waist and more hip fat than boys from puberty to early adulthood (76–78). Several studies have established a link between childhood adiposity and puberty. Childhood obesity is closely associated with earlier sexual maturation in girls (79, 80), whereas the relationship between obesity and pubertal timing in boys remains controversial (79, 81). A growing number of studies have shown that obesity in boys is also associated with early puberty onset (80, 82–86). The onset and progression of puberty in boys are positively related to weight and BMI (87). In a body composition analysis study, boys with high-level percentage of body fat (BFP) had an increased risk of earlier pubertal onset (88). These results might be attributable to the accurate assessment of testicular volume using a Prader orchidometer and BMI z-score or body composition metrics as indicators of body fat. Despite the link between adiposity during childhood and pubertal development, there is a lack of data on the link between body composition in children aged 2 to 5 years and the onset of puberty. This gap is largely due to the lack of age-specific body composition evaluation and a longer follow-up period.
6 Adipose tissue before puberty and adulthood reproductive outcomes
Overweight or obesity during childhood and adolescence is associated with impaired reproductive functions. In girls, obesity, especially central obesity, is associated with a high risk of menstrual irregularity and PCOS (10, 89–92). Weight loss is associated with improvement in PCOS symptoms. Among prepubertal girls, BMI is significantly and positively associated with free testosterone, and obese girls have a high risk of hyperandrogenemia. A cohort study recruiting individuals followed from birth to age 50 years (93) indicated that, in early childhood (age 3–6 years), there was no significant association between underweight, overweight, or obesity and any fertility outcomes, but obesity at age 11–15 years was associated with a higher risk of decreased fecundability and childlessness in adulthood, independent of PCOS.
Unlike girls, there is less evidence about the direct correlation between AT before puberty and male reproductive dysfunction. In male infertility, obesity is associated with disrupted spermatogenesis, reduced semen quality, and erectile dysfunction (94, 95). Several studies have reported a link between hypogonadism and obesity in adolescent men (96, 97). However, it remains unclear whether reproductive impairments related to obesity begin during childhood or adolescence. Genetic obesity syndromes offer new insight that early-onset obesity is presumably a contributing factor for reproductive impairments as obesity syndromes (e.g., Prader–Willi syndrome, Cohen syndrome, and Bardet–Biedl syndrome) often present with intractable obesity at an early age as well as hypogonadism and irregular menses (9, 98). The relationship between obesity during critical life stages and reproductive disorders is detailed in Figure 2. Conversely, Ramlau-Hansen et al. found that prepubertal BMI was not significantly associated with semen quality (99). The inconsistency might result from the lack of longitudinal evaluation of BMI change, not excluding the possibility of weight loss before semen collection. Little evidence from rat models has explored the effects of prepubertal obesity on gonadal function. In a rat model, prepubertal obesity resulted in a reduced number of Leydig cells, the decreased expressions of steroidogenic acute regulatory protein (StAR), and compromised ovarian oxidative stress and DNA repair (100, 101). These preliminary data provide a possibility that inconspicuous lesions exist in reproductive systems in the context of exposure to early-onset overweight or obesity.
7 Discussion
AT distribution is shaped by gonadal hormones, steroid-metabolizing enzymes, and genetic modification during puberty; AT development during early life stages has a close relationship with puberty timing and later reproductive function. Substantial evidence has shown that obesity (maternal obesity or obesity during infancy, childhood, and adolescence) contributes to high risks of precocious puberty, PCOS, and impaired fertility. Thus, weight management during early life stages should receive more attention, as it might be effective to improve reproductive outcomes in young adults. However, the mechanisms underlying sexual dimorphism in fat distribution, particularly the role of gonadal and steroid hormones in mediating depot-specific variations across distinct adipose depots, remain to be fully elucidated. In clinical practice, the early identification of obesity-associated reproductive dysfunction remains unclear, and there is still lack of evidence to assess the long-term impact of childhood obesity—whether transient or persistent—on adult reproductive outcomes. Future studies should focus on clarifying the mechanism whereby adiposity during early life stages leads to impaired reproductive function. Longitudinal cohort studies are needed to assess the effectiveness of weight management interventions in improving reproductive outcomes during young adulthood.
Author contributions
XN: Writing – original draft, Formal Analysis, Investigation. QH: Validation, Writing – review & editing. DG: Visualization, Writing – original draft. YZ: Methodology, Resources, Writing – original draft. YL: Methodology, Resources, Writing – original draft. XL: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We are grateful to all staff members of our research team for their opinions and support in completing this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Calcaterra V, Magenes VC, Hruby C, Siccardo F, Mari A, Cordaro E, et al. Links between childhood obesity, high-fat diet, and central precocious puberty. Children (Basel). (2023) 10(2):241. doi: 10.3390/children10020241
2. Ibáñez L and de Zegher F. Adolescent PCOS: a postpubertal central obesity syndrome. Trends Mol Med. (2023) 29:354–63. doi: 10.1016/j.molmed.2023.02.006
3. Incedal Irgat S and Bakirhan H. The effect of obesity on human reproductive health and foetal life. Hum Fertil (Camb). (2022) 25:860–71. doi: 10.1080/14647273.2021.1928774
4. Koontz MB, Gunzler DD, Presley L, and Catalano PM. Longitudinal changes in infant body composition: association with childhood obesity. Pediatr Obes. (2014) 9:e141–4. doi: 10.1111/ijpo.2014.9.issue-6
5. Ong KK, Emmett P, Northstone K, Golding J, Rogers I, Ness AR, et al. Infancy weight gain predicts childhood body fat and age at menarche in girls. J Clin Endocrinol Metab. (2009) 94:1527–32. doi: 10.1210/jc.2008-2489
6. Hvidt JJ, Brix N, Ernst A, Lauridsen LLB, and Ramlau-Hansen CH. Size at birth, infant growth, and age at pubertal development in boys and girls. Clin Epidemiol. (2019) 11:873–83. doi: 10.2147/CLEP.S217388
7. Bleil ME, Appelhans BM, Gregorich SE, Thomas AS, Hiatt RA, Roisman GI, et al. Patterns of early life weight gain and female onset of puberty. J Endocr Soc. (2021) 5:bvab165. doi: 10.1210/jendso/bvab165
8. McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. (2007) 92:430–6. doi: 10.1210/jc.2006-2002
9. Elliott V, Waldrop SW, Wiromrat P, Carreau AM, and Green MC. The interaction of obesity and reproductive function in adolescents. Semin Reprod Med. (2022) 40:53–68. doi: 10.1055/s-0042-1744495
10. Anderson AD, Solorzano CM, and McCartney CR. Childhood obesity and its impact on the development of adolescent PCOS. Semin Reprod Med. (2014) 32:202–13. doi: 10.1055/s-0034-1371092
11. Shi L, Jiang Z, and Zhang L. Childhood obesity and central precocious puberty. Front Endocrinol (Lausanne). (2022) 13:1056871. doi: 10.3389/fendo.2022.1056871
12. Yin X, Chen Y, Ruze R, Xu R, Song J, Wang C, et al. The evolving view of thermogenic fat and its implications in cancer and metabolic diseases. Signal Transduct Target Ther. (2022) 7:324. doi: 10.1038/s41392-022-01178-6
13. Cannon B and Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. (2004) 84:277–359. doi: 10.1152/physrev.00015.2003
14. Lee MJ, Wu Y, and Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. (2013) 34:1–11. doi: 10.1016/j.mam.2012.10.001
15. Laharrague P and Casteilla L. The emergence of adipocytes. Endocr Dev. (2010) 19:21–30. doi: 10.1159/000316894
16. Cox AJ, West NP, and Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. (2015) 3:207–15. doi: 10.1016/S2213-8587(14)70134-2
17. Björntorp P. Effects of age, sex, and clinical conditions on adipose tissue cellularity in man. Metabolism. (1974) 23:1091–102. doi: 10.1016/0026-0495(74)90076-6
18. Hirsch J and Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. (1976) 5:299–311. doi: 10.1016/S0300-595X(76)80023-0
19. Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, and Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest. (1979) 63:239–46. doi: 10.1172/JCI109295
20. de Ridder CM, Thijssen JH, Bruning PF, Van den Brande JL, Zonderland ML, and Erich WB. Body fat mass, body fat distribution, and pubertal development: a longitudinal study of physical and hormonal sexual maturation of girls. J Clin Endocrinol Metab. (1992) 75:442–6. doi: 10.1210/jcem.75.2.1639945
21. Lassek WD and Gaulin SJ. Brief communication: menarche is related to fat distribution. Am J Phys Anthropol. (2007) 133:1147–51. doi: 10.1002/ajpa.v133:4
22. Riumallo J and Durnin JV. Changes in body composition in adolescent boys. Eur J Clin Nutr. (1988) 42:107–12.
23. Siervogel RM, Demerath EW, Schubert C, Remsberg KE, Chumlea WC, Sun S, et al. Puberty and body composition. Horm Res. (2003) 60:36–45. doi: 10.1159/000071224
24. Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly YM, Rudling M, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. (2000) 278:640–5. doi: 10.1006/bbrc.2000.3827
25. Hewitt KN, Pratis K, Jones ME, and Simpson ER. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. (2004) 145:1842–8. doi: 10.1210/en.2003-1369
26. Gu Q, Korach KS, and Moss RL. Rapid action of 17beta-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology. (1999) 140:660–6. doi: 10.1210/endo.140.2.6500
27. Cooke PS and Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood). (2004) 229:1127–35. doi: 10.1177/153537020422901107
28. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, and Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U.S.A. (2000) 97:12729–34. doi: 10.1073/pnas.97.23.12729
29. Blouin K, Blanchette S, Richard C, Dupont P, Luu-The V, and Tchernof A. Expression and activity of steroid aldoketoreductases 1C in omental adipose tissue are positive correlates of adiposity in women. Am J Physiol Endocrinol Metab. (2005) 288:E398–404. doi: 10.1152/ajpendo.00312.2004
30. Pedersen SB, Børglum JD, Eriksen EF, and Richelsen B. Nuclear estradiol binding in rat adipocytes. Regional variations and regulatory influences of hormones. Biochim Biophys Acta. (1991) 1093:80–6. doi: 10.1016/0167-4889(91)90141-J
31. Gapstur SM, Gann PH, Kopp P, Colangelo L, Longcope C, and Liu K. Serum androgen concentrations in young men: a longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol Biomarkers Prev. (2002) 11:1041–7.
32. Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, et al. Contribution of body fatness and adipose tissue distribution to the age variation in plasma steroid hormone concentrations in men: the HERITAGE Family Study. J Clin Endocrinol Metab. (2000) 85:1026–31. doi: 10.1210/jcem.85.3.6427
33. Garaulet M, Pérex-Llamas F, Fuente T, Zamora S, and Tebar FJ. Anthropometric, computed tomography and fat cell data in an obese population: relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. Eur J Endocrinol. (2000) 143:657–66. doi: 10.1530/eje.0.1430657
34. Elbers JM, de Jong S, Teerlink T, Asscheman H, Seidell JC, and Gooren LJ. Changes in fat cell size and in vitro lipolytic activity of abdominal and gluteal adipocytes after a one-year cross-sex hormone administration in transsexuals. Metabolism. (1999) 48:1371–7. doi: 10.1016/S0026-0495(99)90146-4
35. Sato T, Matsumoto T, Yamada T, Watanabe T, Kawano H, and Kato S. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem Biophys Res Commun. (2003) 300:167–71. doi: 10.1016/S0006-291X(02)02774-2
36. Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, et al. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. (2005) 54:1000–8. doi: 10.2337/diabetes.54.4.1000
37. Rubinow KB, Wang S, den Hartigh LJ, Subramanian S, Morton GJ, Buaas FW, et al. Hematopoietic androgen receptor deficiency promotes visceral fat deposition in male mice without impairing glucose homeostasis. Andrology. (2015) 3:787–96. doi: 10.1111/andr.2015.3.issue-4
38. Tchernof A and Labrie F. Dehydroepiandrosterone, obesity and cardiovascular disease risk: a review of human studies. Eur J Endocrinol. (2004) 151:1–14. doi: 10.1530/eje.0.1510001
39. Tchernof A, Labrie F, Bélanger A, Prud’homme D, Bouchard C, Tremblay A, et al. Androstane-3alpha,17beta-diol glucuronide as a steroid correlate of visceral obesity in men. J Clin Endocrinol Metab. (1997) 82:1528–34. doi: 10.1210/jcem.82.5.3924;97
40. Blanchette S, Blouin K, Richard C, Dupont P, Luu-The V, and Tchernof A. Expression and activity of 20alpha-hydroxysteroid dehydrogenase (AKR1C1) in abdominal subcutaneous and omental adipose tissue in women. J Clin Endocrinol Metab. (2005) 90:264–70. doi: 10.1210/jc.2004-0583
41. Blouin K, Richard C, Bélanger C, Dupont P, Daris M, Laberge P, et al. Local androgen inactivation in abdominal visceral adipose tissue. J Clin Endocrinol Metab. (2003) 88:5944–50. doi: 10.1210/jc.2003-030535
42. Blouin K, Richard C, Brochu G, Hould FS, Lebel S, Marceau S, et al. Androgen inactivation and steroid-converting enzyme expression in abdominal adipose tissue in men. J Endocrinol. (2006) 191:637–49. doi: 10.1677/joe.1.06365
43. Russell DW and Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. (1994) 63:25–61. doi: 10.1146/annurev.bi.63.070194.000325
44. Wake DJ, Strand M, Rask E, Westerbacka J, Livingstone DE, Soderberg S, et al. Intra-adipose sex steroid metabolism and body fat distribution in idiopathic human obesity. Clin Endocrinol (Oxf). (2007) 66:440–6. doi: 10.1111/j.1365-2265.2007.02755.x
45. Andrew R, Phillips DI, and Walker BR. Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab. (1998) 83:1806–9. doi: 10.1210/jcem.83.5.4951
46. Li X, Zhang X, Shen Z, Chen Z, Wang H, and Zhang X. GnRH receptor mediates lipid storage in female adipocytes via AMPK pathway. Int J Med Sci. (2022) 19:1442–50. doi: 10.7150/ijms.74335
47. De Pergola G, Zamboni M, Sciaraffia M, Turcato E, Pannacciulli N, Armellini F, et al. Body fat accumulation is possibly responsible for lower dehydroepiandrosterone circulating levels in premenopausal obese women. Int J Obes Relat Metab Disord. (1996) 20:1105–10.
48. Barrett-Connor E and Ferrara A. Dehydroepiandrosterone, dehydroepiandrosterone sulfate, obesity, waist-hip ratio, and noninsulin-dependent diabetes in postmenopausal women: the Rancho Bernardo Study. J Clin Endocrinol Metab. (1996) 81:59–64. doi: 10.1210/jcem.81.1.8550794
49. Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, and Arlt W. Androgen generation in adipose tissue in women with simple obesity–a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5. J Endocrinol. (2004) 183:331–42. doi: 10.1677/joe.1.05762
50. Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, et al. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): a potential role for estrogen receptor beta (ERbeta). Horm Metab Res. (2002) 34:758–63. doi: 10.1055/s-2002-38259
51. Lapauw B, Taes Y, Simoens S, Van Caenegem E, Weyers S, Goemaere S, et al. Body composition, volumetric and areal bone parameters in male-to-female transsexual persons. Bone. (2008) 43:1016–21. doi: 10.1016/j.bone.2008.09.001
52. Tchernof A, Després JP, Bélanger A, Dupont A, Prud’homme D, Moorjani S, et al. Reduced testosterone and adrenal C19 steroid levels in obese men. Metabolism. (1995) 44:513–9. doi: 10.1016/0026-0495(95)90060-8
53. Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, et al. The number of x chromosomes causes sex differences in adiposity in mice. PloS Genet. (2012) 8:e1002709. doi: 10.1371/journal.pgen.1002709
54. Yang X, SChadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. (2006) 16:995–1004. doi: 10.1101/gr.5217506
55. Xie H, Lim B, and Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes. (2009) 58:1050–7. doi: 10.2337/db08-1299
56. Trajkovski M, Ahmed K, Esau CC, and Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol. (2012) 14:1330–5. doi: 10.1038/ncb2612
57. Hao G, Wang X, Treiber FA, Harshfield G, Kapuku G, and Su S. Body mass index trajectories in childhood is predictive of cardiovascular risk: results from the 23-year longitudinal Georgia Stress and Heart study. Int J Obes (Lond). (2018) 42:923–5. doi: 10.1038/ijo.2017.244
58. Chomtho S, Wells JC, Williams JE, Davies PS, Lucas A, and Fewtrell MS. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr. (2008) 87:1776–84. doi: 10.1093/ajcn/87.6.1776
59. Admassu B, Ritz C, Wells JCK, Girma T, Andersen GS, Belachew T, et al. Accretion of fat-free mass rather than fat mass in infancy is positively associated with linear growth in childhood. J Nutr. (2018) 148:607–15. doi: 10.1093/jn/nxy003
60. Maisonet M, Christensen KY, Rubin C, Holmes A, Flanders WD, Heron J, et al. Role of prenatal characteristics and early growth on pubertal attainment of British girls. Pediatrics. (2010) 126:e591–600. doi: 10.1542/peds.2009-2636
61. Wang Y, Dinse GE, and Rogan WJ. Birth weight, early weight gain and pubertal maturation: a longitudinal study. Pediatr Obes. (2012) 7:101–9. doi: 10.1111/j.2047-6310.2011.00022.x
62. Juul F, Chang VW, Brar P, and Parekh N. Birth weight, early life weight gain and age at menarche: a systematic review of longitudinal studies. Obes Rev. (2017) 18:1272–88. doi: 10.1111/obr.v18.11
63. Wei J, Liu S, Cheng Y, Yang W, Zhu Z, and Zeng L. Association of infant physical development and rapid growth with pubertal onset among girls in rural China. JAMA Netw Open. (2021) 4:e216831. doi: 10.1001/jamanetworkopen.2021.6831
64. Ferrari V, Stefanucci S, Ferrari M, Ciofi D, and Stagi S. Retrospective longitudinal analysis of the effects of postnatal weight gain on the timing and tempo of puberty and menarche in a cohort of Italian girls. Ital J Pediatr. (2022) 48:20. doi: 10.1186/s13052-022-01222-9
65. Connor KL, Vickers MH, Beltrand J, Meaney MJ, and Sloboda DM. Nature, nurture or nutrition? Impact of maternal nutrition on maternal care, offspring development and reproductive function. J Physiol. (2012) 590:2167–80. doi: 10.1113/jphysiol.2011.223305
66. Sloboda DM, Howie GJ, Pleasants A, Gluckman PD, and Vickers MH. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PloS One. (2009) 4:e6744. doi: 10.1371/journal.pone.0006744
67. Keim SA, Branum AM, Klebanoff MA, and Zemel BS. Maternal body mass index and daughters’ age at menarche. Epidemiology. (2009) 20:677–81. doi: 10.1097/EDE.0b013e3181b093ce
68. Windham GC, Zhang L, Longnecker MP, and Klebanoff M. Maternal smoking, demographic and lifestyle factors in relation to daughter’s age at menarche. Paediatr Perinat Epidemiol. (2008) 22:551–61. doi: 10.1111/j.1365-3016.2008.00948.x
69. Brix N, Ernst A, Lauridsen LLB, Arah OA, Nohr EA, Olsen J, et al. Maternal pre-pregnancy obesity and timing of puberty in sons and daughters: a population-based cohort study. Int J Epidemiol. (2019) 48:1684–94. doi: 10.1093/ije/dyz125
70. Hounsgaard ML, Håkonsen LB, Vested A, Thulstrup AM, Olsen J, Bonde JP, et al. Maternal pre-pregnancy body mass index and pubertal development among sons. Andrology. (2014) 2:198–204. doi: 10.1111/j.2047-2927.2013.00171.x
71. Zhou J, Zhang F, Zhang S, Li P, Qin X, Yang M, et al. Maternal pre-pregnancy body mass index, gestational weight gain, and pubertal timing in daughters: A systematic review and meta-analysis of cohort studies. Obes Rev. (2022) 23:e13418. doi: 10.1111/obr.13418
72. Yang Z, Feng G, Gao X, Yan X, Li Y, Wang Y, et al. Maternal adiposity and perinatal and offspring outcomes: an umbrella review. Nat Hum Behav. (2024) 8:2406–22. doi: 10.1038/s41562-024-01994-6
73. Takumi K, Shimada K, Iijima N, and Ozawa H. Maternal high-fat diet during lactation increases Kiss1 mRNA expression in the arcuate nucleus at weaning and advances puberty onset in female rats. Neurosci Res. (2015) 100:21–8. doi: 10.1016/j.neures.2015.06.004
74. Lam BYH, Williamson A, Finer S, Day FR, Tadross JA, Gonçalves Soares A, et al. MC3R links nutritional state to childhood growth and the timing of puberty. Nature. (2021) 599:436–41. doi: 10.1038/s41586-021-04088-9
75. Ambrosetti V, Guerra M, Ramírez LA, Reyes A, Álvarez D, Olguín S, et al. Increase in endogenous estradiol in the progeny of obese rats is associated with precocious puberty and altered follicular development in adulthood. Endocrine. (2016) 53:258–70. doi: 10.1007/s12020-016-0858-0
76. Taylor RW, Grant AM, Williams SM, and Goulding A. Sex differences in regional body fat distribution from pre- to postpuberty. Obes (Silver Spring). (2010) 18:1410–6. doi: 10.1038/oby.2009.399
77. Lemieux S, Prud’homme D, Bouchard C, Tremblay A, and Després JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. (1993) 58:463–7. doi: 10.1093/ajcn/58.4.463
78. Blouin K, Boivin A, and Tchernof A. Androgens and body fat distribution. J Steroid Biochem Mol Biol. (2008) 108:272–80. doi: 10.1016/j.jsbmb.2007.09.001
79. Crocker MK, Stern EA, Sedaka NM, Shomaker LB, Brady SM, Ali AH, et al. Sexual dimorphisms in the associations of BMI and body fat with indices of pubertal development in girls and boys. J Clin Endocrinol Metab. (2014) 99:E1519–29. doi: 10.1210/jc.2014-1384
80. Li W, Liu Q, Deng X, Chen Y, Yang B, Huang X, et al. Association of prepubertal obesity with pubertal development in Chinese girls and boys: A longitudinal study. Am J Hum Biol. (2018) 30:e23195. doi: 10.1002/ajhb.23195
81. Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. (2002) 110:903–10. doi: 10.1542/peds.110.5.903
82. Dai YL, Fu JF, Liang L, Gong CX, Xiong F, Luo FH, et al. Association between obesity and sexual maturation in Chinese children: a muticenter study. Int J Obes (Lond). (2014) 38:1312–6. doi: 10.1038/ijo.2014.116
83. Liu G, Guo J, Zhang X, Lu Y, Miao J, and Xue H. Obesity is a risk factor for central precocious puberty: a case-control study. BMC Pediatr. (2021) 21:509. doi: 10.1186/s12887-021-02936-1
84. Busch AS, Højgaard B, Hagen CP, and Teilmann G. Obesity is associated with earlier pubertal onset in boys. J Clin Endocrinol Metab. (2020) 105(4):dgz222. doi: 10.1210/clinem/dgz222
85. Brix N, Ernst A, Lauridsen LLB, Parner ET, Arah OA, Olsen J, et al. Childhood overweight and obesity and timing of puberty in boys and girls: cohort and sibling-matched analyses. Int J Epidemiol. (2020) 49:834–44. doi: 10.1093/ije/dyaa056
86. Huang JS, Gao C, Xiao WQ, Zhang XY, Zhong XW, Qin YQ, et al. Association of childhood obesity with pubertal development in boys: A systematic review and meta-analysis. Obes Rev. (2025) 26:e13869. doi: 10.1111/obr.13869
87. Tomova A, Robeva R, and Kumanov P. Influence of the body weight on the onset and progression of puberty in boys. J Pediatr Endocrinol Metab. (2015) 28:859–65. doi: 10.1515/jpem-2014-0363
88. Li Y, Ma T, Ma Y, Gao D, Chen L, Chen M, et al. Adiposity status, trajectories, and earlier puberty onset: results from a longitudinal cohort study. J Clin Endocrinol Metab. (2022) 107:2462–72. doi: 10.1210/clinem/dgac395
89. Lake JK, Power C, and Cole TJ. Women’s reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. (1997) 21:432–8. doi: 10.1038/sj.ijo.0800424
90. Ollila MM, Piltonen T, Puukka K, Ruokonen A, Järvelin MR, Tapanainen JS, et al. Weight gain and dyslipidemia in early adulthood associate with polycystic ovary syndrome: prospective cohort study. J Clin Endocrinol Metab. (2016) 101:739–47. doi: 10.1210/jc.2015-3543
91. Koivuaho E, Laru J, Ojaniemi M, Puukka K, Kettunen J, Tapanainen JS, et al. Age at adiposity rebound in childhood is associated with PCOS diagnosis and obesity in adulthood-longitudinal analysis of BMI data from birth to age 46 in cases of PCOS. Int J Obes (Lond). (2019) 43:1370–9. doi: 10.1038/s41366-019-0318-z
92. He Y, Tian J, Blizzard L, Oddy WH, Dwyer T, Bazzano LA, et al. Associations of childhood adiposity with menstrual irregularity and polycystic ovary syndrome in adulthood: the Childhood Determinants of Adult Health Study and the Bogalusa Heart Study. Hum Reprod. (2020) 35:1185–98. doi: 10.1093/humrep/deaa069
93. Laru J, Nedelec R, Koivuaho E, Ojaniemi M, Järvelin MR, Tapanainen JS, et al. BMI in childhood and adolescence is associated with impaired reproductive function-a population-based cohort study from birth to age 50 years. Hum Reprod. (2021) 36:2948–61. doi: 10.1093/humrep/deab164
94. Davidson LM, Millar K, Jones C, Fatum M, and Coward K. Deleterious effects of obesity upon the hormonal and molecular mechanisms controlling spermatogenesis and male fertility. Hum Fertil (Camb). (2015) 18:184–93. doi: 10.3109/14647273.2015.1070438
95. Leisegang K, Sengupta P, Agarwal A, and Henkel R. Obesity and male infertility: Mechanisms and management. Andrologia. (2021) 53:e13617. doi: 10.1111/and.13617
96. Dhindsa S, Ghanim H, Batra M, and Dandona P. Hypogonadotropic hypogonadism in men with diabesity. Diabetes Care. (2018) 41:1516–25. doi: 10.2337/dc17-2510
97. Mogri M, Dhindsa S, Quattrin T, Ghanim H, and Dandona P. Testosterone concentrations in young pubertal and post-pubertal obese males. Clin Endocrinol (Oxf). (2013) 78:593–9. doi: 10.1111/cen.2013.78.issue-4
98. Hammoud AO, Gibson M, Peterson CM, Hamilton BD, and Carrell DT. Obesity and male reproductive potential. J Androl. (2006) 27:619–26. doi: 10.2164/jandrol.106.000125
99. Ramlau-Hansen CH, Hansen M, Jensen CR, Olsen J, Bonde JP, and Thulstrup AM. Semen quality and reproductive hormones according to birthweight and body mass index in childhood and adult life: two decades of follow-up. Fertil Steril. (2010) 94:610–8. doi: 10.1016/j.fertnstert.2009.01.142
100. Wagner IV, Klöting N, Atanassova N, Savchuk I, Spröte C, Kiess W, et al. Prepubertal onset of obesity negatively impacts on testicular steroidogenesis in rats. Mol Cell Endocrinol. (2016) 437:154–62. doi: 10.1016/j.mce.2016.08.027
Keywords: adipose tissue, puberty, adipocyte development, early life stage, reproductive function
Citation: Ning X, Huang Q, Guo D, Zhou Y, Li Y and Li X (2025) The role of adipose tissue in puberty and reproductive health. Front. Endocrinol. 16:1543787. doi: 10.3389/fendo.2025.1543787
Received: 11 December 2024; Accepted: 23 May 2025;
Published: 17 June 2025.
Edited by:
Ana Paula Santos-Silva, Federal University of Rio de Janeiro, BrazilReviewed by:
Chiara Di Berardino, University of Teramo, ItalyBeatriz Alexandre-Santos, Fluminense Federal University, Brazil
Copyright © 2025 Ning, Huang, Guo, Zhou, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Li, MzE0NDQwODIwQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xin Ning
Xin Ning Qing Huang2†
Qing Huang2† Doudou Guo
Doudou Guo Xin Li
Xin Li