- 1Department of Healthy Examination, The Second Affiliated Hospital, Guangzhou Medical University, Guangzhou, Guangdong, China
- 2Department of Clinical Laboratory, The Second Affiliated Hospital, Guangzhou Medical University, Guangzhou, Guangdong, China
- 3Department of Laboratory Medicine, Jieyang People’s Hospital, Jieyang, Guangdong, China
Background: The Hepatic Steatosis Index (HSI) is a simple screening tool for adults with non-alcoholic fatty liver disease (NAFLD). While lipid and glucose metabolism are closely interrelated, few studies have examined the association between HSI and impaired fasting glucose (IFG). This study aims to investigate the relationship between HSI and IFG risk in a large Chinese cohort.
Methods: This retrospective cohort study analyzed health examination data collected from 2010 to 2016 across 11 cities in China by the Rich Healthcare Group. Multivariable Cox regression and restricted cubic spline (RCS) analyses were used to evaluate the association between baseline HSI and IFG. Subgroup analyses were conducted to assess the robustness of the findings.
Results: A total of 75,911 participants with a mean age of 40.9 ± 12.1 years were included, among whom 9,908 (13.1%) developed IFG. After adjusting for potential confounders, each one-unit increase in baseline HSI was associated with a 5% higher risk of IFG (HR=1.05, 95%CI). RCS analysis revealed that the increase of risk plateaued when HSI exceeded 35.31. Subgroup analyses demonstrated the stability of these findings.
Conclusion: Elevated baseline HSI is a significant risk factor for IFG in Chinese adults. These findings highlight the potential utility of HSI in identifying individuals at risk of glucose dysregulation.
1 Introduction
Diabetes, a chronic metabolic disorder, has emerged as a significant global health challenge, with its prevalence increasing nearly fourfold over the past three decades. Approximately 463 million adults worldwide are affected, representing 9.3% of the population aged 20-79 (1). In China, the prevalence has risen dramatically from 0.67% in 1980 to 11.2% in recent years (2). Among diabetes cases, type 2 diabetes mellitus (T2DM) constitutes 90%, featuring high prevalence, low control rates, and numerous complications. Identifying high-risk populations and screening for risk factors are indispensable for early prevention.
Prediabetes, encompassing IFG and impaired glucose tolerance (IGT), represents an early phase of T2DM. Affecting 10.6% of the global population and 35.2% of Chinese adults (2), making it a critical target for prevention, as approximately 60% of diabetes cases progress from this stage within five years (3). Interventions such as lifestyle modifications and pharmacotherapy can reverse prediabetes and reduce the risk of developing T2DM (4). NAFLD is a liver manifestation of metabolic syndrome, affecting 25% of the global population. The prevalence rate in China has soared from 15% in 2003 to 32.1% in 2025 (5). NAFLD disrupts glucose metabolism through several key mechanisms: Hepatic insulin resistance develops primarily through lipid accumulation-induced impairment of IRS phosphorylation, while concurrent mitochondrial dysfunction activates the ROS-JNK pathway, further disrupting insulin signaling. These metabolic disturbances are compounded by adipokine imbalance, creating a pro-inflammatory milieu. Clinically, NAFLD patients show significantly higher prediabetes risk with 5% liver fat increase correlating to 8% β-cell function decline (HOMA-β). This bidirectional relationship is reinforced by hyperinsulinemia-driven activation of SREBP-1c mediated lipogenesis, thereby perpetuating a self-sustaining cycle of metabolic dysfunction (6).
Hepatic steatosis, closely associated with T2DM and metabolic syndrome, has garnered considerable attention. The HSI, a non-invasive tool integrating BMI and liver enzyme levels, effectively screens for NAFLD (7, 8). HSI has also been correlated with metabolic disorders, including T2DM (9), gestational diabetes (10), and diabetic complications (11). Nevertheless, its role in predicting prediabetes, particularly IFG, remains ambiguous.
This multicenter retrospective cohort study investigates the relationship between HSI and the risk of IFG in individuals with normal baseline fasting glucose levels. By addressing this knowledge gap, the study provides valuable insights into how variations in HSI influence the development of IFG and informs strategies for diabetes prevention.
2 Materials and methods
2.1 Data source
This study utilized raw data from a cohort of 211,833 individuals, originally collected by Chen et al. (12). The dataset includes comprehensive medical records of participants undergoing health evaluations. Data were obtained from the Dryad Digital Repository (DRYAD) database (https://datadryad.org/stash/dataset/doi:10.5061/dryad.ft8750v). Ethical approval for data collection was granted by the National Center for Health Statistics Institutional Review Board, and all procedures adhered to the Declaration of Helsinki. As the dataset is anonymized, informed consent was not required.
2.2 Study population
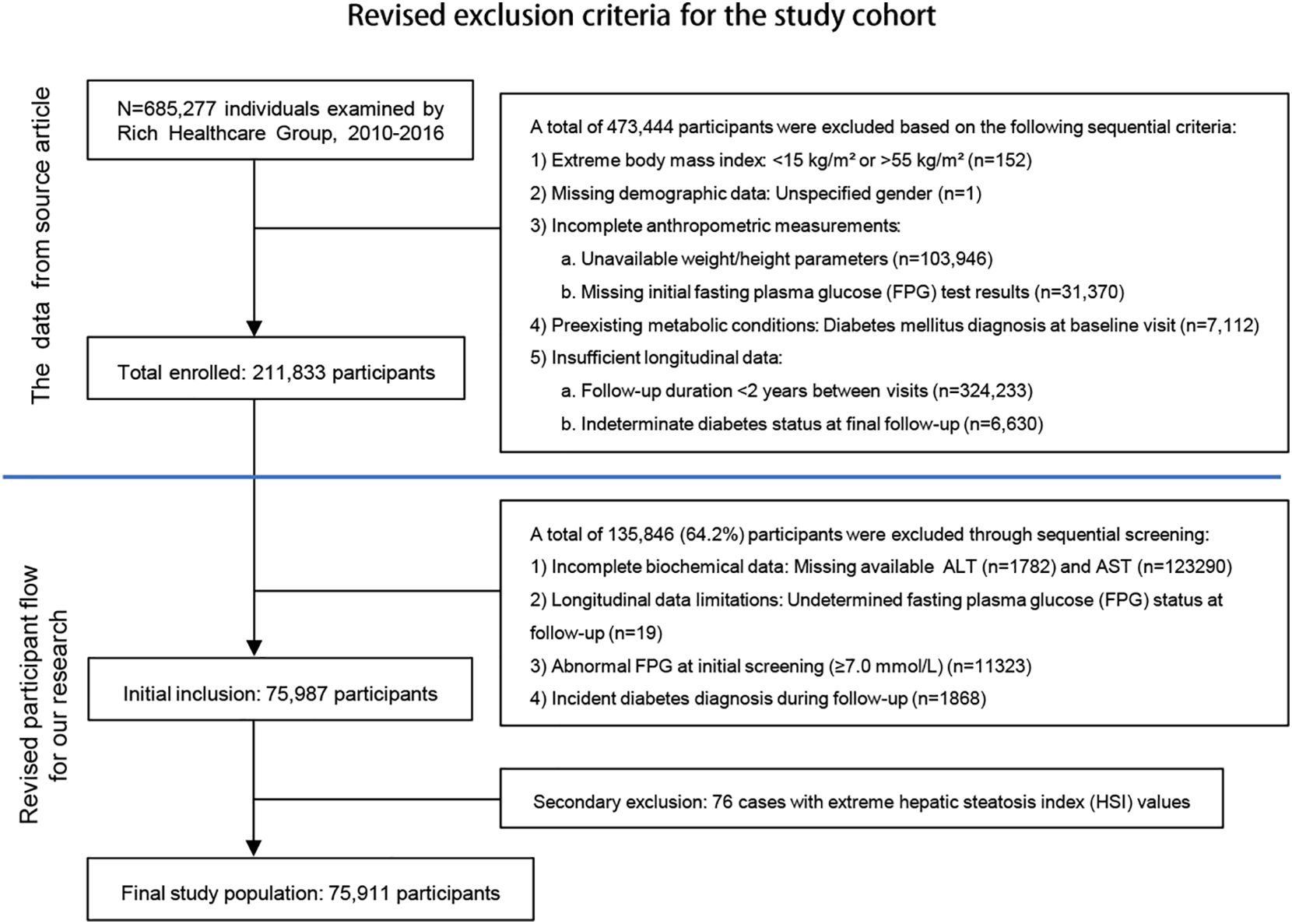
This study analyzed data from 685,277 adults (≥20 years) without diabetes history in the Rich Healthcare Group’s 2010–2016 database (12), applying rigorous exclusion criteria to yield 75,911 participants (Figure 1). Missing continuous variables were handled via multiple imputation (5 iterations) after confirming missingness was completely at random (Little’s MCAR test P = 0.12), this approach remains vulnerable to violations if unmeasured confounders affected missingness. The complete-case analysis for categorical variables risks selection bias when missingness correlates with outcomes, and the limited imputation cycles may inadequately model complex biomedical relationships. Additionally, the 6-year observational period may not account for evolving diagnostic standards or measurement protocols. For the limitations of the above scheme, sensitivity analysis will be used subsequently to verify the robustness of the results. The data cleaning protocol and refine solution complied with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (13).
2.3 Variables
Demographic data collected included age, sex, family history of diabetes, smoking status, and alcohol consumption. Physical examinations measured height, weight, and blood pressure, while laboratory tests assessed fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), blood urea nitrogen (BUN), creatinine clearance rate (CCR), aspartate aminotransferase (AST), and alanine aminotransferase (ALT). BMI was calculated as weight (kg) divided by height squared (m²). HSI was calculated as follows: HSI = 8 × (ALT/AST ratio) + BMI (+2 for females) (7).
2.4 Definitions
According to the American Diabetes Association (ADA) diagnostic criteria, IFG is characterized by FPG levels ranging from 5.6 mmol/L (inclusive) to below 7.0 mmol/L, in the absence of self-reported diabetes. This biochemical range specifically identifies individuals with prediabetic metabolic dysregulation (14). For study purposes, IFG onset time was operationally defined as the first detection date meeting diagnostic criteria during active surveillance (2010–2016), requiring at least two health examinations with >2-year intervals. Diabetes diagnosis required either FPG ≥ 7.0 mmol/L or self-reported diabetes history, creating mutually exclusive diagnostic categories that facilitated clear metabolic status classification.
2.5 Statistical analysis
Continuous variables were described as mean ± standard deviation for normally distributed data or median (interquartile range) for non-normally distributed data, while categorical variables were expressed as frequencies (percentages). Group differences were assessed using ANOVA for normally distributed continuous variables, Kruskal-Wallis tests for non-normally distributed variables, and chi-square tests for categorical variables.
Cox regression was selected to model time-to-event data for IFG risk factors, given its capacity to handle censored observations and provide hazard ratio estimates (15). Three adjustment models were implemented: 1) crude; 2) age/sex-adjusted; 3) fully-adjusted for clinical/laboratory/behavioral confounders (SBP, DBP, lipids, renal function, smoking/alcohol history, and diabetes family history). RCS with four knots (5th, 35th, 65th, 95th percentiles) were applied to evaluate potential non-linear HSI-IFG relationships, complemented by two-segment logistic regression with bootstrap-derived inflection points.
Subgroup analyses were conducted to explore effect modification across different groups, including age (<65 or ≥65 years) (16), sex, BMI (<24, 24-28, or ≥28 kg/m²) (17), family history of diabetes, alcohol consumption (current, former, or never), and smoking status (current, former, or never). Statistical analyses were performed using R 4.2.2 (http://www.R-project.org) and Free Statistics Analysis Platform 1.9.2 (http://www.clinicalscientists.cn/freestatistics), with two-sided P-values <0.05 considered statistically significant. As this study was based on existing data, sample size calculations were not performed.
3 Result
3.1 Baseline characteristics
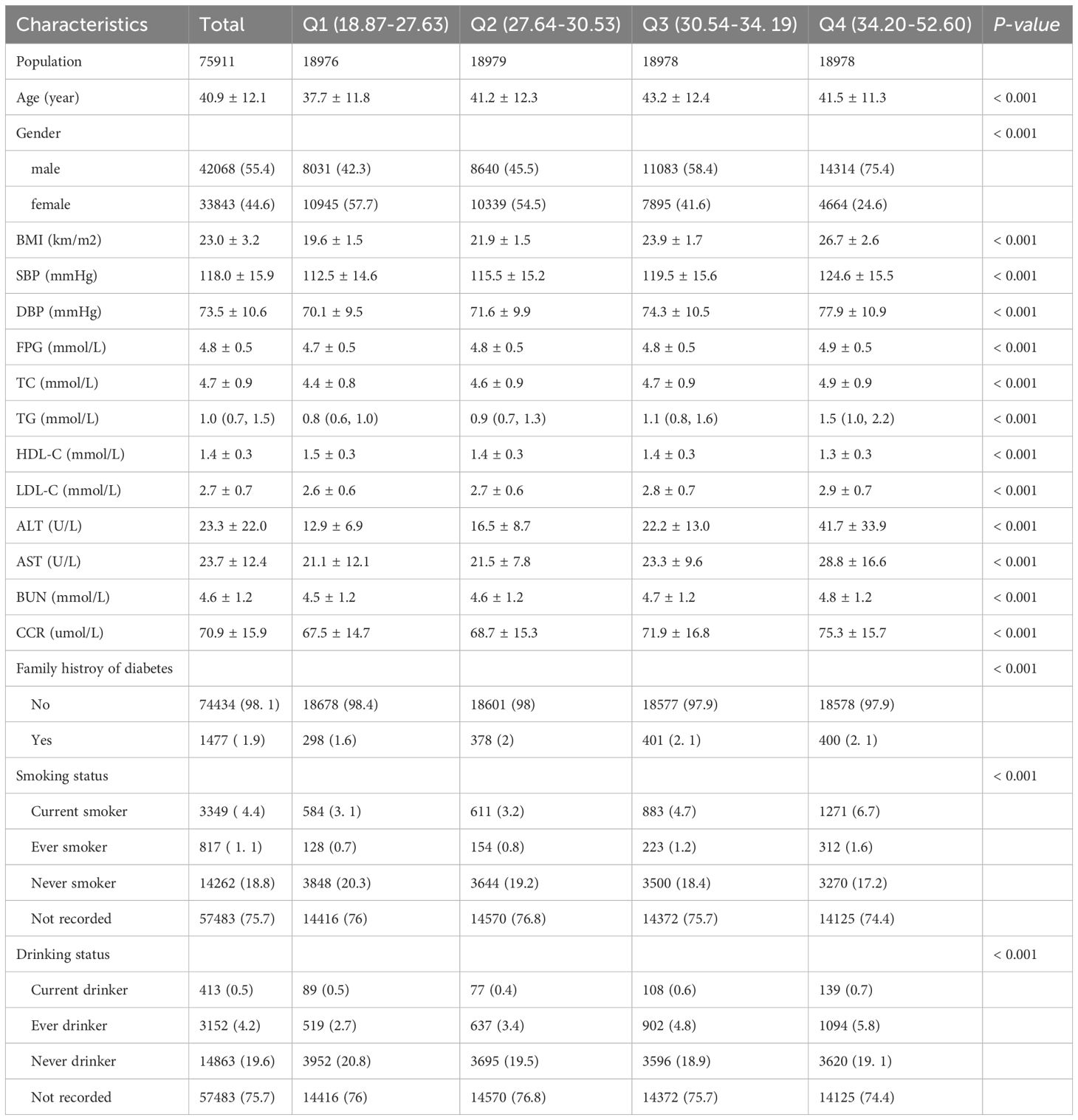
This study included 75,911 participants with an average age of 40.9 ± 12.1 years. Among them, 33,843 (44.6%) were female, and 42,068 (55.4%) were male. Participants were divided into four quartiles based on HSI: Quartile 1 (Q1: 18.87–27.63), Quartile 2 (Q2: 27.64–30.53), Quartile 3 (Q3: 30.54–34.19), and Quartile 4 (Q4: 34.20–52.60). Compared to the other three groups, Quartile 4 exhibited significantly higher BMI, SBP, DBP, FPG, TC, TG, LDL-C, AST, ALT, BUN, and CCR levels. Additionally, the proportions of males, smokers, and alcohol consumers were higher in Quartile 4. Age, HDL-C levels, and family history of diabetes also differed significantly among the quartiles (P < 0.001) (Table 1).
3.2 Tendency of IFG Across HSI quartiles
Over a mean follow-up period of 3.02 years, 9,908 participants (13.1%) developed into IFG. The Kaplan-Meier analysis disclosed significant differences in the risk of IFG across the HSI quartiles (P < 0.05), with a decreasing probability of maintaining normal glucose levels as the HSI increased (Figure 2).
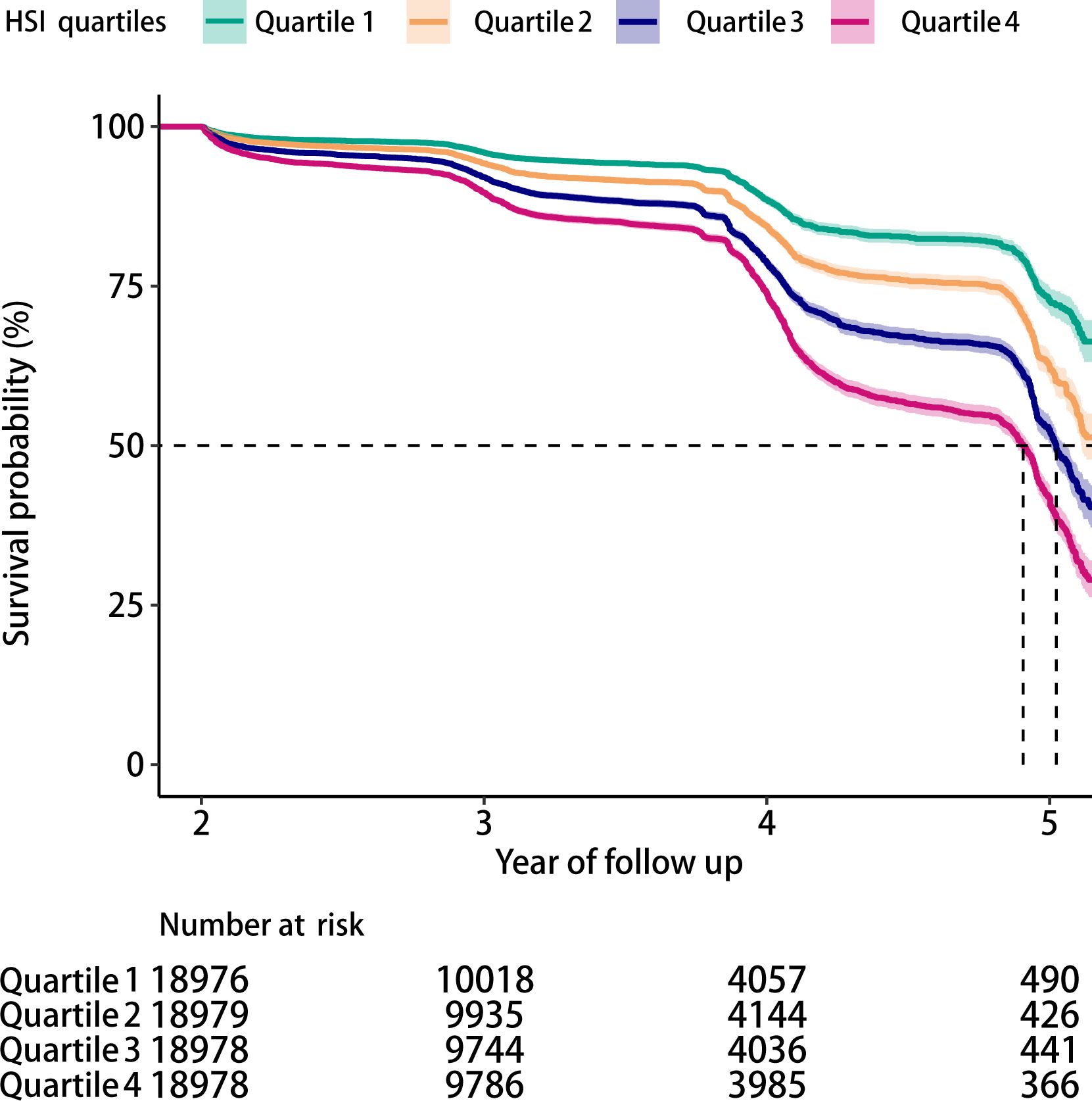
Figure 2. Kaplan-Meier survival curves showing the incidence of IFG across HSI quartiles (log-rank test, P < 0.05).
3.3 Risk factors for IFG
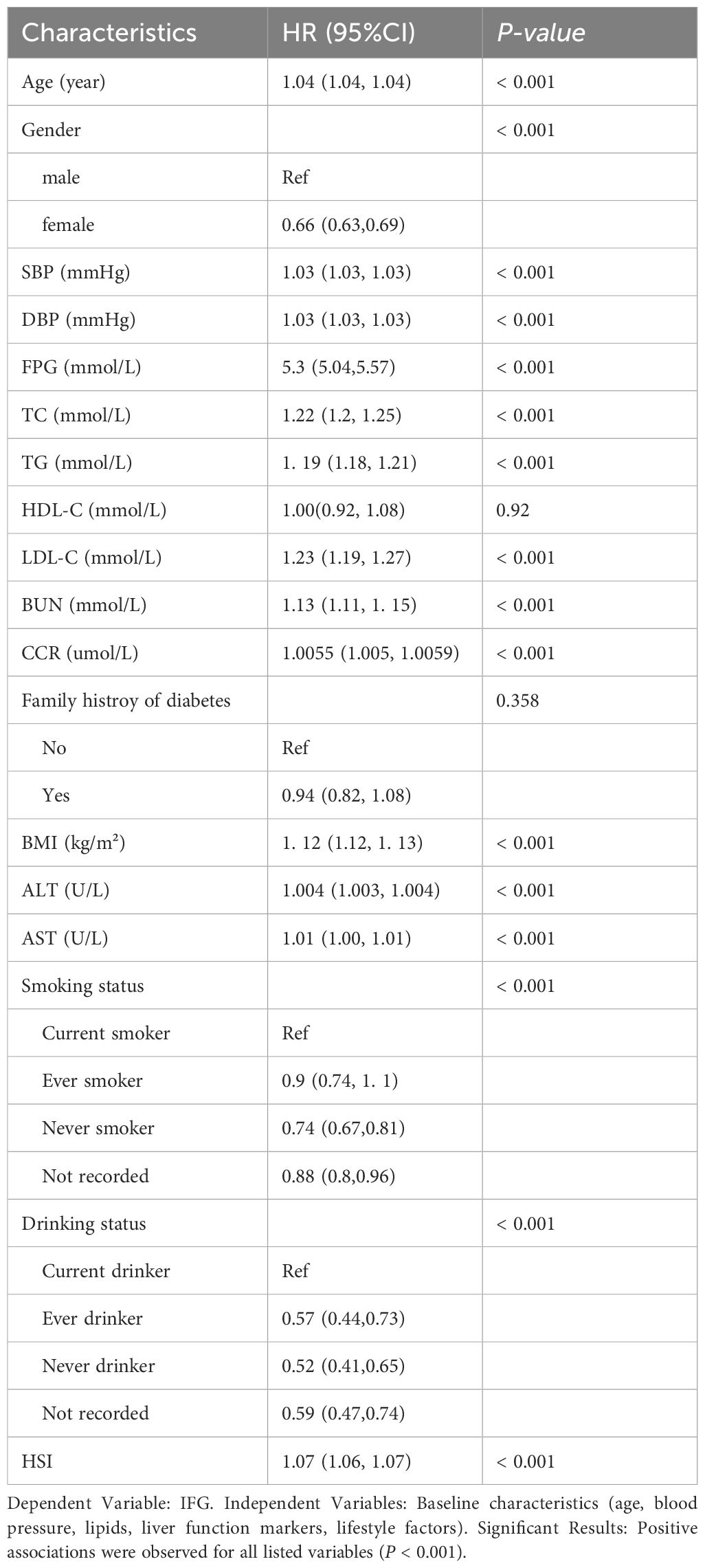
Our univariate Cox regression analysis revealed significant associations between multiple baseline variables and incident IFG risk (all P < 0.001). Key metabolic parameters including age, blood pressure (SBP/DBP), lipid profiles (TC/TG/LDL-C), renal function markers (BUN/CCR), liver enzymes (AST/ALT), and anthropometric measures (BMI/FPG) demonstrated positive correlations with IFG development. Notably, male sex and modifiable lifestyle factors (smoking/alcohol consumption) also emerged as independent risk determinants. These results collectively illustrate the complex interplay between physiological markers and behavioral patterns in fasting glucose dysregulation, as comprehensively detailed in Table 2. The multifactorial pathogenesis of IFG underscores the necessity for integrated prevention strategies targeting both metabolic abnormalities and health behaviors.
3.4 Association between HSI and IFG
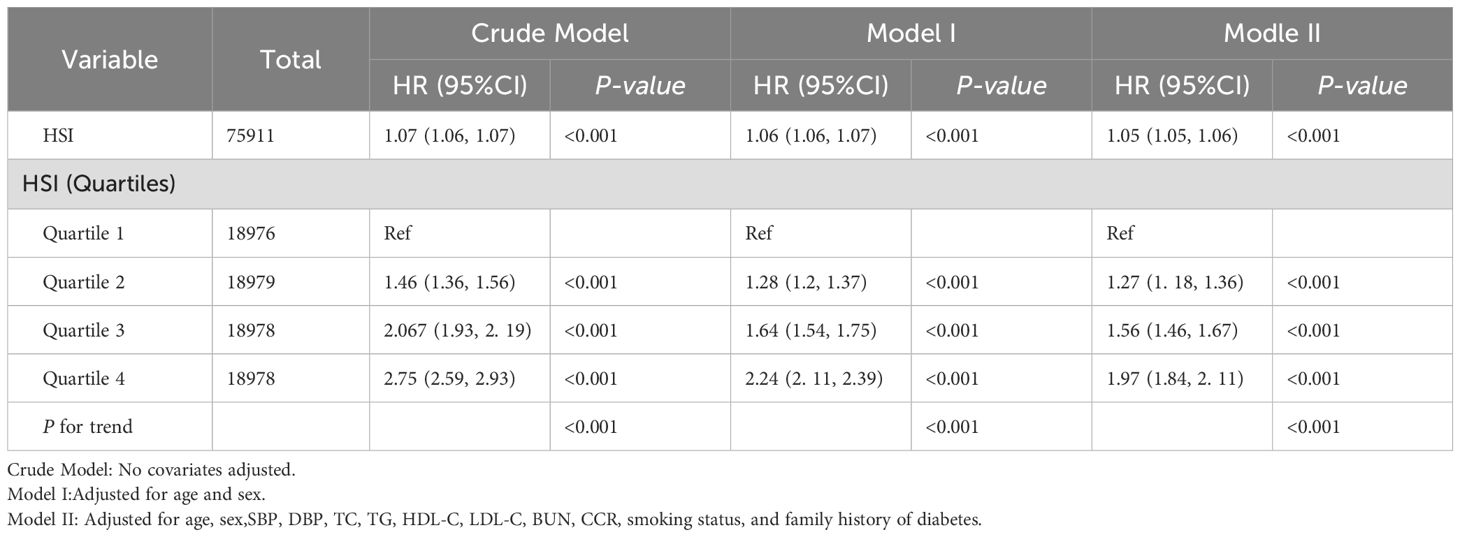
Given the intermittent follow-up design (median duration=3.02 years) precluding precise determination of IFG onset timing, we employed multivariable Cox regression to evaluate HSI-IFG risk associations (Table 3). The analysis revealed dose-dependent relationships: each unit increase in hepatic steatosis index (HSI) conferred a 5% elevated IFG risk (HR=1.05, 95%CI=1.05-1.06, P<0.001) in fully adjusted models, with quartile-based categorical analysis demonstrating consistent positive gradients. In pursuit of the stability of the Cox regression conclusion, complementary logistic regression assessing HSI-IFG prevalence associations was conducted. Crucially, both methodologies yielded concordant results: 1) equivalent magnitude of effect (OR=1.05 vs HR=1.05 per HSI unit); 2) identical statistical significance thresholds (P<0.001); 3) preserved quartile-response patterns (Supplementary Table S1). This rigorous analytical verification through complementary survival and cross-sectional frameworks substantiates the validity of our core findings regarding HSI’s predictive utility for IFG risk.
3.5 The nonlinear relationship between HSI and IFG
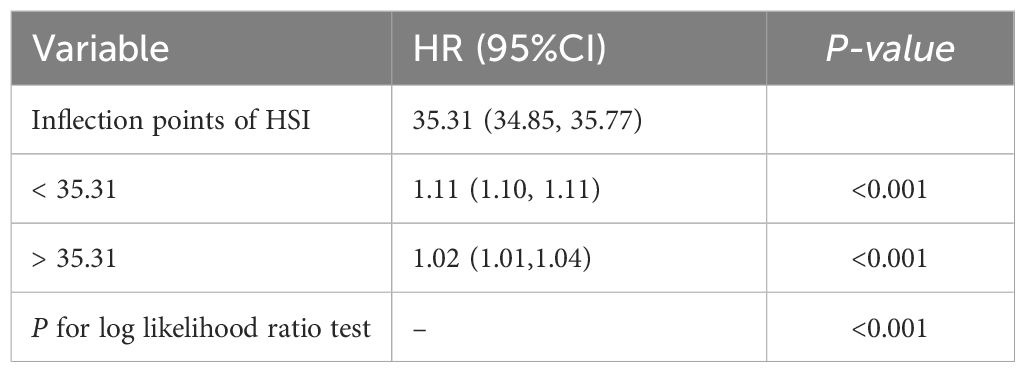
RCS analysis further demonstrated a non-linear relationship between HSI and IFG risk (P < 0.001) (Figure 3). Below an HSI threshold of 35.31, each 1-unit increase in HSI was associated with an 11% higher risk of IFG (HR = 1.11; 95% CI: 1.10–1.11). Above this threshold, the risk increased plateaued (Table 4).
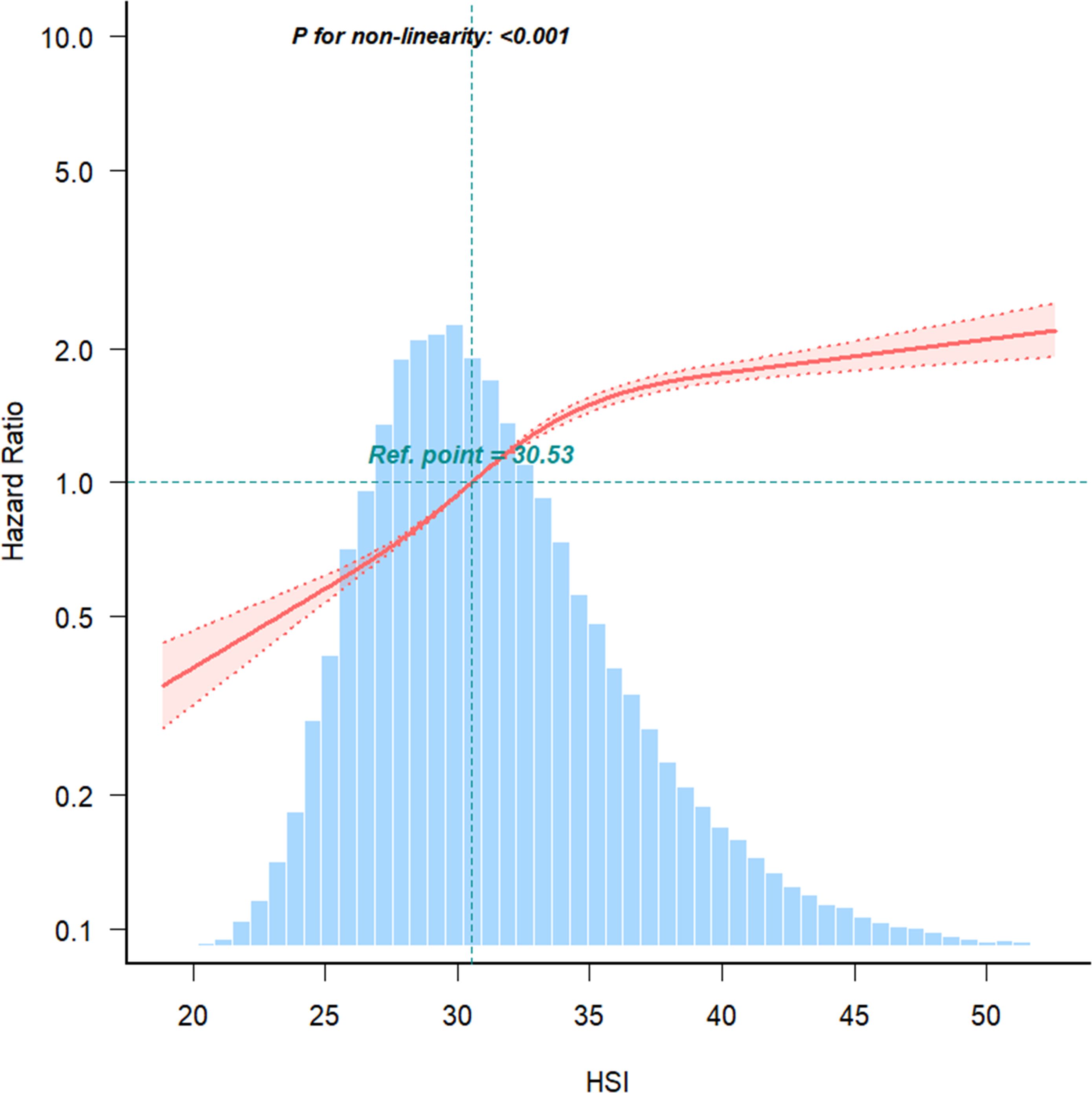
Figure 3. The nonlinear relationship between the relationship between HSI and IFG risk, with density plots of HSI distribution.
3.6 Subgroup analysis
Subgroup analyses were conducted to verify the robustness of the findings across different strata, including sex, age, blood pressure, BMI, family history of diabetes, smoking status, and alcohol consumption. In all subgroups, HSI remained positively associated with IFG risk. The association was stronger among female participants, aged <65 years and with normal blood pressure (Figure 4). These results consistently indicate that HSI is an independent risk factor for IFG.
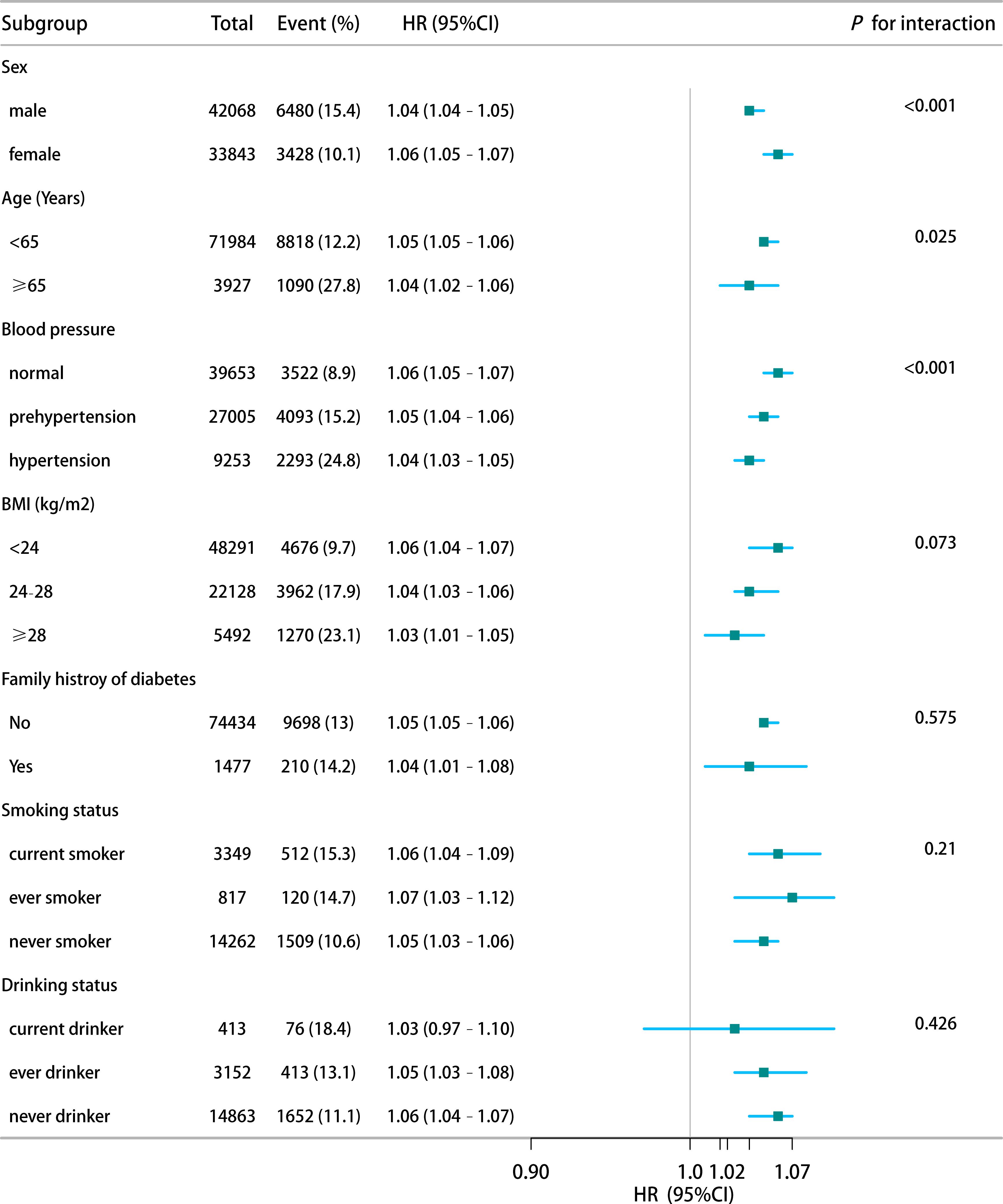
Figure 4. Stratified analysis of HSI-IFG risk associations by demographic and clinical characteristics.
4 Discussion
This large-scale retrospective cohort study employing biennial follow-up design (median 3.02 years) systematically examined the association between HSI and IFG. Our analysis revealed a novel nonlinear dose-response relationship with an inflection point at HSI=35.31: each unit increase below this threshold elevated IFG risk by 11%, while risk escalation attenuated to 5% per unit above this critical value. Notably, this association demonstrated significant interaction in women aged < 65 years. Methodologically, we implemented Cox proportional hazards models to accommodate irregular follow-up intervals, defining event time as first IFG detection and censoring date as last follow-up for non-converters (18). This approach outperformed logistic regression by effectively integrating heterogeneous follow-up durations and addressing right-censoring (19). Kaplan-Meier analysis corroborated the inverse relationship between HSI quartiles and normoglycemia maintenance probability (log-rank P<0.001), showing remarkable consistency with Cox model stratification. Standardized biochemical protocols coupled with sensitivity analyses ensured robustness against metabolic parameter measurement variability.
Prediabetes, characterized by IFG and/or IGT, carries a high risk of progression to T2DM. Global data indicate that 16% of IFG cases and 21% of IGT cases progress to T2DM within five years (20), and over 70% within a decade (21). The IFG incidence of 13.1% observed in our study exceeds prior reports, possibly due to differences in inclusion criteria and overlapping cases of simple or mixed IFG, as OGTT 2-hour glucose levels (<7.8 mmol/L) were not separately analyzed. Mechanistically, IFG primarily results from hepatic insulin resistance and β-cell dysfunction, whereas IGT is driven by peripheral insulin resistance, particularly in skeletal muscle (22), explaining the stronger correlation between HSI and IFG compared to IGT.
The relationship between HSI and prediabetes is non-linear, with the risk increase slowing when HSI exceeds 35.31. This phenomenon may be attributed to compensatory mechanisms, a biological saturation point, or the staged nature of clinical and pathological changes associated with hepatic fat accumulation (23). HSI has been closely linked to hepatic insulin resistance (24), which itself is influenced by various factors, including genetics (25), lifestyle (26), and metabolic conditions (27). When HSI surpasses 35.31, the slower risk increase for IFG may reflect the activation of compensatory mechanisms. Studies in high-fat diet-induced insulin resistance models in canines have demonstrated that compensatory hyperinsulinemia can occur independently of elevated blood glucose (28). Thus, beyond a certain threshold of HSI, more severe insulin resistance might be required to further elevate IFG risk. Pathologically, excessive hepatic fat accumulation disrupts insulin signaling, contributing to insulin resistance and increased glucose production (29). At HSI levels above 35.31, the liver may reach a biological saturation point, reducing its sensitivity to additional fat accumulation and insulin resistance.
The stronger association between HSI and IFG observed in individuals younger than 65 years may be due to the presence of multiple glucose-regulating factors in older adults, such as chronic inflammation (30) and reduced muscle mass (31), which diminish the relative contribution of HSI to IFG. Additionally, age-related factors, such as altered fat functionality and redistribution, complicate the impact of hepatic fat on glucose regulation (32). In younger individuals, where hepatic metabolic function and sensitivity to glucose-lipid regulation are relatively higher, hepatic fat accumulation likely exerts a more direct influence on fasting glucose regulation, resulting in a stronger correlation.
Among women, the association between HSI and IFG is more pronounced, possibly due to the regulatory role of estrogen on glucose metabolism. Hepatic fat accumulation may inhibit estrogen receptors (33), diminishing estrogen’s protective effects on insulin sensitivity (34). This interaction could accelerate the development of IFG in women, making changes in hepatic fat more significantly linked to fasting glucose dysregulation.
We further identified key demographic and metabolic factors associated with IFG risk, including male sex, obesity, hypertension, dyslipidemia, elevated transaminase levels, smoking, and alcohol consumption. These findings align with prior research highlighting obesity and chronic low-grade inflammation as drivers of insulin resistance and glucose dysregulation (35, 36). Obesity-related changes, such as altered gut microbiota and adipokine imbalance, exacerbate insulin resistance, leading to prediabetes. Effective interventions, including diet, aerobic exercise, and resistance training, have been shown to reverse prediabetes and normalize glucose levels (37, 38).
Liver function emerges as a pivotal factor in glucose regulation, with studies consistently linking elevated ALT, AST, and GGT levels to prediabetes and T2DM risk (39). Liver fat accumulation, a key feature of hepatic steatosis, impairs hepatic insulin sensitivity and triggers systemic inflammation, contributing to glucose dysregulation (40, 41). In our study, HSI, which incorporates ALT, AST, and BMI, was a strong predictor of IFG risk, particularly in younger individuals. This suggests that HSI reflects early hepatic metabolic disturbances that precede systemic glucose abnormalities.
While Wu et al. recently evaluated HSI’s predictive value for glycemic transitions (regression to normoglycemia vs diabetes progression) in high-risk populations with baseline IFG (42), our study extends this investigation to initially normoglycemic individuals. We demonstrate that elevated HSI predicts IFG development (5% increased risk per unit, HR=1.05), identifying a clinically actionable threshold (HSI=35.31) where risk escalation plateaus. Utilizing routinely measured parameters (age, sex, liver function, BMI) (43), HSI emerges as a cost-effective screening tool for population-level diabetes prevention strategies (44). Complementing prior research focused on high-risk management (42) our findings-supported by large-scale validation, simplified risk quantification, and direct primary prevention applications-provide critical insights for early-stage intervention in public health frameworks.
Our study has notable limitations requiring consideration. First, while incorporating representative samples from 11 cities, we could not fully account for regional heterogeneity in dietary patterns (45) and socioeconomic status (46) - factors potentially influencing IFG progression through microbiota-mediated metabolic regulation (47) and psychosocial pathways (48). Second, although excluding baseline diabetes and incident cases, undocumented glucose-altering medications initiated during the mean 3.02-year follow-up period may confound natural dysglycemia progression (49). Third, the retrospective design’s reliance solely on fasting glucose measurements excluded individuals with isolated impaired glucose tolerance, while the absence of oral glucose tolerance tests (OGTT) and hemoglobin A1c (HbA1c) data constrained comprehensive metabolic evaluation. Finally, similar to most single-cohort studies (50), the generalizability of our identified HSI threshold (35.31) requires validation across diverse populations to establish population-specific risk stratification criteria. Future investigations should integrate regional electronic health records for medication tracking, incorporate multi-omics metabolic assessments, and conduct multi-cohort analyses with social determinant evaluations to advance precision prevention strategies.
5 Conclusions
This study first reveals a nonlinear HSI-IFG association (plateau risk at HSI=35.31) in Chinese adults. Hepatic markers show early predictive value, particularly for younger populations, suggesting HSI’s utility as a cost-effective screening tool for targeted diabetes prevention in resource-limited settings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://datadryad.org/stash/dataset/doi:10.5061/dryad.ft8750v.
Ethics statement
The requirement of ethical approval was waived by Ethics Committee of the Second Affiliated Hospital of Guangzhou Medical University for the studies involving humans because As this was a retrospective observational study, informed consent was not required, and previous studies were ethically reviewed. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because As this was a retrospective observational study, informed consent was not required, and previous studies were ethically reviewed. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YC: Funding acquisition, Writing – original draft. JH: Data curation, Formal analysis, Investigation, Writing – original draft. SZ: Data curation, Investigation, Resources, Writing – original draft. YS: Data curation, Formal analysis, Funding acquisition, Investigation, Software, Validation, Writing – original draft. HJ: Project administration, Supervision, Visualization, Writing – review & editing. SL: Formal analysis, Visualization, Writing – original draft. JW: Conceptualization, Methodology, Writing – review & editing. SH: Conceptualization, Funding acquisition, Methodology, Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Guangdong Medical Science and Technology Research Fund Project [grant No. B2023049, and No. A2023165], and the Guangzhou Health Science and Technology Project [grant No. 20251A011074, and No. 20251A011073].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. ChatGPT was used solely for language polishing and did not contribute to any creative knowledge generation.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1556169/full#supplementary-material
References
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9(Th) edition. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
2. Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the american diabetes association: national cross sectional study. BMJ (Clinical Res ed). (2020) 369:m997. doi: 10.1136/bmj.m997
3. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2010) 33 Suppl 1:S62–9. doi: 10.2337/dc10-S062
4. Khan RMM, Chua ZJY, Tan JC, Yang Y, Liao Z, and Zhao Y. From pre-diabetes to diabetes: diagnosis, treatments and translational research. Medicina (Kaunas). (2019) 55(9):546. doi: 10.3390/medicina55090546
5. Hu R, Zhao Z, Xie L, Ma Z, Wu W, and Li S. Global, regional, and national burden of chronic kidney disease due to diabetes mellitus type 2 from 1990 to 2021, with projections to 2036: A systematic analysis for the global burden of disease study 2021. Front Med. (2025) 12:1531811. doi: 10.3389/fmed.2025.1531811
6. Saeed A, Ajmal HMS, Khan MUA, and Toor WT. (2024). Enhancing early detection of diabetes using machine learning algorithms, in: 2024 1st International Conference on Innovative Engineering Sciences and Technological Research (ICIESTR). Muscat, Oman. 1–6. doi: 10.1109/ICIESTR60916.2024.10798412
7. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Digestive liver disease: Off J Ital Soc Gastroenterol Ital Assoc Study Liver. (2010) 42:503–8. doi: 10.1016/j.dld.2009.08.002
8. Preveden T, Veres B, Ruzic M, Pete M, Bogic S, Kovacevic N, et al. Triglyceride-glucose index and hepatic steatosis index for the assessment of liver steatosis in hcv patients. Minerva Gastroenterol. (2023) 69:254–60. doi: 10.23736/s2724-5985.22.03168-0
9. Fennoun H, Mansouri SE, Tahiri M, Haraj NE, Aziz SE, Hadad F, et al. Interest of hepatic steatosis index (Hsi) in screening for metabolic steatopathy in patients with type 2 diabetes. Pan Afr Med J. (2020) 37:270. doi: 10.11604/pamj.2020.37.270.9087
10. Wu P, Wang Y, Ye Y, Yang X, Huang Y, Ye Y, et al. Liver biomarkers, lipid metabolites, and risk of gestational diabetes mellitus in a prospective study among chinese pregnant women. BMC Med. (2023) 21:150. doi: 10.1186/s12916-023-02818-6
11. Tripolino C, Irace C, Cutruzzolà A, Parise M, Barone M, Scicchitano C, et al. Hepatic steatosis index is associated with type 1 diabetes complications. Diabetes Metab syndrome obesity: Targets Ther. (2019) 12:2405–10. doi: 10.2147/dmso.S221969
12. Chen Y, Zhang XP, Yuan J, Cai B, Wang XL, Wu XL, et al. Association of body mass index and age with incident diabetes in chinese adults: A population-based cohort study. BMJ Open. (2018) 8:e021768. doi: 10.1136/bmjopen-2018-021768
13. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, and Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (Strobe) statement: guidelines for reporting observational studies. J Clin Epidemiol. (2008) 61:344–9. doi: 10.1016/j.jclinepi.2007.11.008
14. Egan AM, Wood-Wentz CM, Mohan S, Bailey KR, and Vella A. Baseline fasting glucose level, age, sex, and body mass index and the development of diabetes in us adults. JAMA network Open. (2025) 8:e2456067. doi: 10.1001/jamanetworkopen.2024.56067
15. Wang Y, Chen S, Yao T, Li D, Wang Y, Li Y, et al. Homocysteine as a risk factor for hypertension: A 2-year follow-up study. PloS One. (2014) 9:e108223. doi: 10.1371/journal.pone.0108223
16. Wei Y, Lin JL, Chen G, and Pei LJ. a cohort study of association between sleep duration and cognitive impairment in the elderly aged 65 years and older in China. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. (2022) 43:359–65. doi: 10.3760/cma.j.cn112338-20210410-00305
17. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American association of clinical endocrinologists and american college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocrine practice: Off J Am Coll Endocrinol Am Assoc Clin Endocrinologists. (2016) 22 Suppl 3:1–203. doi: 10.4158/ep161365.Gl
18. Chen X, Ding J, Shi Z, Bai K, Shi S, and Tian Q. Association of longitudinal trajectories of fasting plasma glucose with all-cause and cardiovascular mortality among a chinese older population: A retrospective cohort study. BMC Public Health. (2024) 24:1335. doi: 10.1186/s12889-024-18823-0
19. Wang T, Lu J, Shi L, Chen G, Xu M, Xu Y, et al. Association of insulin resistance and β-cell dysfunction with incident diabetes among adults in China: A nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol. (2020) 8:115–24. doi: 10.1016/s2213-8587(19)30425-5
20. Liu Y, Li J, Wu Y, Zhang H, Lv Q, Zhang Y, et al. Evidence from a systematic review and meta-analysis: classical impaired glucose tolerance should be divided into subgroups of isolated impaired glucose tolerance and impaired glucose tolerance combined with impaired fasting glucose, according to the risk of progression to diabetes. Front Endocrinol. (2022) 13:835460. doi: 10.3389/fendo.2022.835460
21. DeJesus RS, Breitkopf CR, Rutten LJ, Jacobson DJ, Wilson PM, and Sauver JS. Incidence rate of prediabetes progression to diabetes: modeling an optimum target group for intervention. Popul Health Manag. (2017) 20:216–23. doi: 10.1089/pop.2016.0067
22. Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. . Diabetes. (2009) 58:773–95. doi: 10.2337/db09-9028
23. Lee E, Korf H, and Vidal-Puig A. An adipocentric perspective on the development and progression of non-alcoholic fatty liver disease. J Hepatol. (2023) 78:1048–62. doi: 10.1016/j.jhep.2023.01.024
24. Ye J, Lin Y, Shao C, Sun Y, Feng S, and Zhong B. Comparisons of insulin resistance- and steatosis-based scores in monitoring metabolic associated fatty liver disease treatment response. Ann Nutr Metab. (2023) 79:448–59. doi: 10.1159/000530531
25. James DE, Stöckli J, and Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol. (2021) 22:751–71. doi: 10.1038/s41580-021-00390-6
26. Thomsen MN, Skytte MJ, Samkani A, Carl MH, Weber P, Astrup A, et al. Dietary carbohydrate restriction augments weight loss-induced improvements in glycaemic control and liver fat in individuals with type 2 diabetes: A randomised controlled trial. Diabetologia. (2022) 65:506–17. doi: 10.1007/s00125-021-05628-8
27. Hilton C, Sabaratnam R, Drakesmith H, and Karpe F. Iron, glucose and fat metabolism and obesity: an intertwined relationship. Int J Obes (2005). (2023) 47:554–63. doi: 10.1038/s41366-023-01299-0
28. Ader M and Bergman RN. Hyperinsulinemic compensation for insulin resistance occurs independent of elevated glycemia in male dogs. Endocrinology. (2021) 162(9):bqab119. doi: 10.1210/endocr/bqab119
29. Taylor R, Al-Mrabeh A, and Sattar N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. (2019) 7:726–36. doi: 10.1016/s2213-8587(19)30076-2
30. Baechle JJ, Chen N, Makhijani P, Winer S, Furman D, and Winer DA. Chronic inflammation and the hallmarks of aging. Mol Metab. (2023) 74:101755. doi: 10.1016/j.molmet.2023.101755
31. Perez K, Ciotlos S, McGirr J, Limbad C, Doi R, Nederveen JP, et al. Single nuclei profiling identifies cell specific markers of skeletal muscle aging, frailty, and senescence. Aging. (2022) 14:9393–422. doi: 10.18632/aging.204435
32. Ou MY, Zhang H, Tan PC, Zhou SB, and Li QF. Adipose tissue aging: mechanisms and therapeutic implications. Cell Death Dis. (2022) 13:300. doi: 10.1038/s41419-022-04752-6
33. Wang S, Yao H, Ding L, Gao Y, Wang P, and Xue Y. Effects of high-glucose and high-fat condition on estrogen receptor- and sexual precocity-related genes in gt1–7 cells. Med Sci monitor: Int Med J Exp Clin Res. (2020) 26:e922860. doi: 10.12659/msm.922860
34. Mahboobifard F, Pourgholami MH, Jorjani M, Dargahi L, Amiri M, Sadeghi S, et al. Estrogen as a key regulator of energy homeostasis and metabolic health. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2022) 156:113808. doi: 10.1016/j.biopha.2022.113808
35. Fang H, EL RR, and Schertzer JD. Obesity promotes a leaky gut, inflammation and pre-diabetes by lowering gut microbiota that metabolise ethanolamine. Gut. (2023) 72:1809–11. doi: 10.1136/gutjnl-2023-329815
36. Szukiewicz D. Molecular mechanisms for the vicious cycle between insulin resistance and the inflammatory response in obesity. Int J Mol Sci. (2023) 24(12):9818. doi: 10.3390/ijms24129818
37. Magkos F, Hjorth MF, and Astrup A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. (2020) 16:545–55. doi: 10.1038/s41574-020-0381-5
38. Luo X, Wang Z, Li B, Zhang X, and Li X. Effect of resistance vs. Aerobic exercise in pre-diabetes: an rct. Trials. (2023) 24:110. doi: 10.1186/s13063-023-07116-3
39. Sakharkar P and Deb S. Examining liver function in adults with diabetes in the United States. J Pharm Pharm sciences: Publ Can Soc Pharm Sciences Societe Can Des Sci Pharm. (2021) 24:317–28. doi: 10.18433/jpps31851
40. Gastaldelli A. Fatty liver disease: the hepatic manifestation of metabolic syndrome. Hypertension research: Off J Japanese Soc Hypertension. (2010) 33:546–7. doi: 10.1038/hr.2010.60
41. Chait A and den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. (2020) 7:22. doi: 10.3389/fcvm.2020.00022
42. Wu J, Chen Q, Zou JZ, Chen YY, Chen HH, Lin YY, et al. Association between hepatic steatosis index and glucose status conversion from impaired fasting glucose base on chinese adults: A cohort study from real-world. Eur J Med Res. (2025) 30:100. doi: 10.1186/s40001-025-02354-4
43. Choe EK, Shivakumar M, Lee SM, Verma A, and Kim D. Dissecting the clinical relevance of polygenic risk score for obesity-a cross-sectional, longitudinal analysis. Int J Obes (2005). (2022) 46:1686–93. doi: 10.1038/s41366-022-01168-2
44. Wang D, Chen Z, Wu Y, Ren J, Shen D, Hu G, et al. Association between two novel anthropometric measures and type 2 diabetes in a chinese population. Diabetes Obes Metab. (2024) 26:3238–47. doi: 10.1111/dom.15651
45. Gomez-Delgado F, Katsiki N, Lopez-Miranda J, and Perez-Martinez P. Dietary habits, lipoprotein metabolism and cardiovascular disease: from individual foods to dietary patterns. Crit Rev Food Sci Nutr. (2021) 61:1651–69. doi: 10.1080/10408398.2020.1764487
46. Williams ED, Magliano DJ, Zimmet PZ, Kavanagh AM, Stevenson CE, Oldenburg BF, et al. Area-level socioeconomic status and incidence of abnormal glucose metabolism: the Australian diabetes, obesity and lifestyle (Ausdiab) study. Diabetes Care. (2012) 35:1455–61. doi: 10.2337/dc11-1410
47. Yin P, Zhang C, Du T, Yi S, Yu L, Tian F, et al. Meta-analysis reveals different functional characteristics of human gut bifidobacteria associated with habitual diet. Food Res Int (Ottawa Ont). (2023) 170:112981. doi: 10.1016/j.foodres.2023.112981
48. Peng W, Lin S, Chen B, Bai X, Wu C, Zhang X, et al. Area-level socioeconomic inequalities in mortality in China: A nationwide cohort study based on the Chinaheart project. Lancet Public Health. (2024) 9:e1014–e24. doi: 10.1016/s2468-2667(24)00154-3
49. Labandeira CM, Fraga-Bau A, Arias Ron D, Alvarez-Rodriguez E, Vicente-Alba P, Lago-Garma J, et al. Parkinson’s disease and diabetes mellitus: common mechanisms and treatment repurposing. Neural regeneration Res. (2022) 17:1652–8. doi: 10.4103/1673-5374.332122
Keywords: impaired fasting glucose, hepatic steatosis index, retrospective cohort study, risk factors, Chinese adults
Citation: Chen Y, Han J, Zhao S, Shi Y, Jia H, Lu S, Wu J and Huang S (2025) Association between hepatic steatosis index and impaired fasting glucose: a multicenter retrospective cohort study in China. Front. Endocrinol. 16:1556169. doi: 10.3389/fendo.2025.1556169
Received: 06 January 2025; Accepted: 26 May 2025;
Published: 09 June 2025.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Guangda He, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaAlejandro Nieto Dominguez, Cook County Health and Hospitals System, United States
Copyright © 2025 Chen, Han, Zhao, Shi, Jia, Lu, Wu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sicong Huang, MjAxODY4MjAwNUBnemhtdS5lZHUuY24=; Juan Wu, NjI1MTU2OTIzQHFxLmNvbQ==
†These authors have contributed equally to this work
 Yimei Chen1†
Yimei Chen1† Juan Wu
Juan Wu Sicong Huang
Sicong Huang