- 1School of Biomedical Sciences, Faculty of Health, Queensland University of Technology, Brisbane, QLD, Australia
- 2Centre for Immunology and Infection Control, Queensland University of Technology, Brisbane, QLD, Australia
- 3Centre for Vision and Eye Research, Queensland University of Technology, Brisbane, QLD, Australia
- 4Queensland Diabetes and Endocrine Centre, Mater Hospital Brisbane, Brisbane, QLD, Australia
- 5Mater Research Institute – University of Queensland, Faculty of Medicine, University of Queensland, Brisbane, QLD, Australia
- 6School of Clinical Medicine, Faculty of Health, Queensland University of Technology, Brisbane, QLD, Australia
- 7Queensland Eye Institute, Brisbane, QLD, Australia
Background: As the global prevalence of diabetes mellitus reaches epidemic proportions, research into new therapeutic targets that address the underlying pathomechanisms of the disease is essential. Recent studies have elucidated the fundamental role of intestinal metabolic pathways in human health and disease processes and yet, the underlying cause of metabolic dysregulation in diabetes is largely unknown. Therefore, this systematic review aimed to identify the intestinal metabolomic profiles associated with gestational diabetes mellitus, type 1 diabetes mellitus, pre-diabetes mellitus, and type 2 diabetes mellitus.
Methods: A systematic review of databases and grey literature repositories identified primary literature published between 2005 and 2022, that investigated patterns of human- and microbial-derived metabolite concentration in individuals with diabetes.
Results: Data extracted from thirty-four eligible studies revealed 272 metabolites that were associated with diabetes diseases; the majority correlated with incidence of type 2 diabetes mellitus only. Inter-study discrepancies were reported based on the biospecimen type used in metabolomic analyses, namely blood, stool, or urine.
Conclusion: The results of this review emphasise the paucity of research investigating gestational and type 1 diabetes mellitus intestinal metabolic perturbations. Furthermore, the potential for inter-study bias in downstream metabolomic analyses based on sample type warrants further investigation.
Introduction
The metabolites produced by enterocytes and commensal gut microbes directly regulate host immune responses and have the potential to contribute to metabolic inflammation (1). In healthy individuals, the metabolites contributed by eubiotic gut microbiota confer a plethora of benefits to the host, including nutrient synthesis and metabolism, inhibition of pathogen colonisation, regulation of host gene expression and metabolism, and regulation of the gut-immune axis (2–5). In contrast, the metabolites contributed by the dysbiotic gut microbiota promote pathogen colonisation and virulence, and dysregulate host physiology through modulation of intestinal mechanics and disturbed immune homeostasis (6). Through a multifaceted process of immunological crosstalk, the microbial metabolites released by dysbiotic microbiota act as immune-modulators that alter intestinal immune function and regulate the niches of microbe colonisation in the intestine; thereby creating conditions that favour the stabilisation of the dysbiotic configuration and inflammatory milieu (7, 8). It is therefore not surprising that an increasing volume of literature has implicated the metabolome of the gut microbiota in the development of diabetes and a range of metabolic disorders (9, 10).
However, discrepancies exist with respect to identifying the core diabetes-associated metabolites, and whether these metabolites help or hinder diabetic aetiology (11–13). Additionally, much of the current metabolomics research targets processes specific to type 2 diabetes mellitus (T2DM) only, given that T2DM accounts for approximately 90% of all diabetes diagnoses (4, 14–16). Therefore, research that also profiles the microbial and metabolomic profiles in individuals with gestational diabetes mellitus (GDM), and type 1 diabetes mellitus (T1DM) conditions is urgently needed to improve our understanding of diabetes pathophysiology.
To date, no systematic review has synthesised published intestinal metabolomic signature data encompassing all types of DM. In addressing this, we conducted this systematic review to collate, and recapitulate the main findings of studies that have investigated intestinal metabolite profiles in the context of GDM, T1DM, prediabetes (PreDM), and T2DM conditions. Furthermore, this review analysed the effect of study methods on metabolomic findings, by reviewing study findings in light of the collection techniques, and downstream analysis tools employed by researchers. This review ultimately aimed to synthesise and critically review existing literature so future diabetes research can be directed to the identification of pathophysiologically important metabolite markers.
Methods
Systematic review protocol
This systematic review was conducted in accordance with the core methods and processes outlined by the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) 2020 Statement and the Cochrane Collaboration Handbook for Systematic Reviews of Interventions (17, 18).
Eligibility criteria
This review included primary research that profiled the concentration of human- and microbial-derived metabolites in human cohorts with DM. Articles were not excluded based on the type of diabetes disease reported (PreDM, T1DM, T2DM, or GDM); provided the study only characterised the gut in a human population. Studies that characterised the gut metabolome of animals were excluded, based on the established anatomical, metabolic, and physiological differences between animal and human models (19–21).
Articles that were published over a seventeen-year period were screened (January 1, 2005, to June 30, 2022). This search period ensured research published prior to, and over the course of, the National Institutes of Health Human Microbiome Project (2007-2016) was screened for inclusion (22). Additionally, this commencement date correlates with the establishment of the HMDB and METLIN databases; the first standardised repositories of metabolomic data (23, 24). To ensure this review investigated the gut metabolome as the independent variable in the context of diabetes disease, only studies that reported on gut-associated metabolites were eligible for inclusion. That is, (i) metabolites that are utilised or produced as either intermediate or end-products of metabolic processes by human enterocytes or intestinal microbiota, (ii) reported in HMDB as originating from an intestinal biological location (whether directly from the intestinal organ, or from non-excretory fluid of faeces), or (iii) cited in existing literature as having a direct link with the gastrointestinal system. Only primary research literature was included for review; therefore, meta-analyses, review articles, systematic reviews, and commentaries were excluded, in addition to those that implemented an intervention (dietary, surgical, lifestyle) as part of the experimental methods. Similarly, only articles that were published (or translated for publication) in English were considered for inclusion in this systematic review.
Information sources and database searches
Five electronic white literature databases were accessed for article screening; namely PubMed, Scopus, Excerpta Medica database (EMBASE), Web of Science, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL). To ensure a comprehensive review of all existing primary literature was conducted, four grey literature databases were also consulted, spanning both National and International literature repositories; namely ProQuest Dissertations and Theses Global, Analysis and Policy Observatory (APO), Clinical trials.gov, and the International Network of Agencies for Health Technology Assessment Database (INAHTA).
Databases were searched using key search terms included in the National Library of Medicine’s (NLM) Medical Subject Headings (MeSH) Index. In doing so, searches exploited current indexing in databases such as PubMed, ClinicalTrials.gov, and Embase that are modelled on, or indexed according to, MeSH classifications. As depicted in Figure 1, search strings were compiled from combinations of five main groups of search terms; (‘diabetes’ or ‘diabetic’); (‘impaired fasting glucose’ or ‘impaired glucose tolerance’); (‘gut’ or ‘intestine’ or ‘intestinal’ or ‘gastrointestine’ or ‘gastrointestinal’ or ‘bowel’); (metabolite’ or ‘metabolites’ or ‘metabolomic’ or ‘metabolomics’ or ‘metabolome’ or ‘metabolomes’); (‘spectroscopy’ or ‘spectroscopic’ or ‘spectrometry’ or ‘spectrometric’).
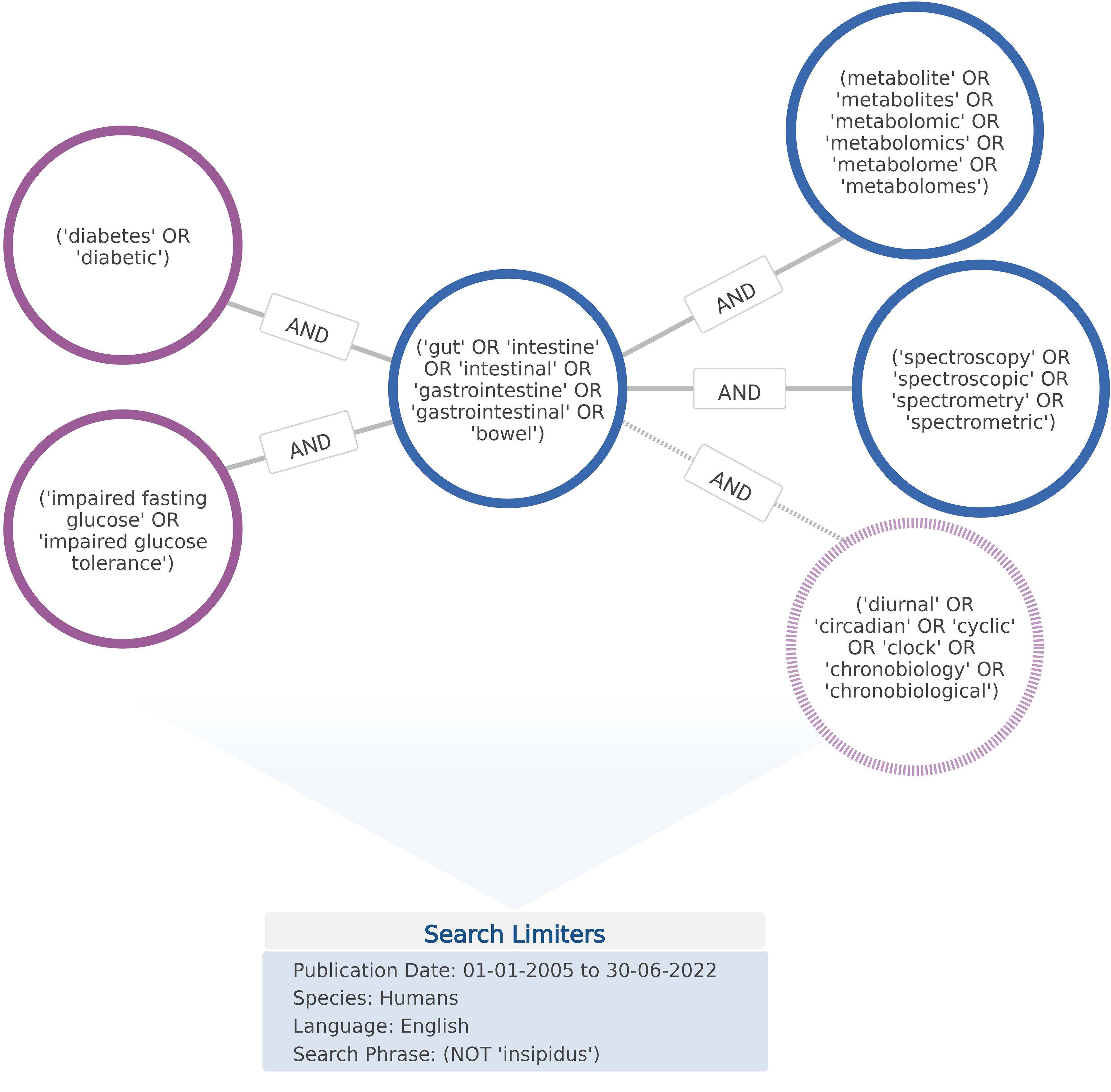
Figure 1. Terms and operators comprising search string queries applied to database and grey literature repository searches. Figure developed using BioRender software.
Search strategies were applied consistently across all five databases and all four grey literature repositories, with database-specific filters, limiters and expanders applied, as required. To ensure method repeatability, a comprehensive record of the inputs and outputs of every literature search was assembled in Microsoft Excel (v. 2019). Results from every targeted search were labelled and indexed into Smart Groups in referencing software, Endnote X9. Using both the ‘Find Duplicates’ function and manual screening, all duplicate records were then identified and excluded from the library, as per the PRISMA Statement process (17).
Data collection and synthesis of results
At the completion of screening, one reviewer (KG) extracted specific data from the included articles and compiled these items into a Microsoft Excel (v. 2019) spreadsheet. The data items extracted from each article were: study and cohort descriptors (bibliographical details, diabetes disease investigated, experimental study design, cohorts and respective sizes, any inclusion/exclusion criteria applied to the study cohorts, and type of biological samples used for metabolome analysis), and analysis descriptors (overall metabolomics analysis approach, and metabolomics technique/s employed).
In addition to these data, the key metabolome data from each article was extracted and tabulated according to the metabolites that every publication reported as being significantly associated with a diabetes disease. Publication findings were synthesised according to whether metabolites were reported as being increased or decreased in cohorts with diabetes; in addition to the number of times each metabolite appeared in individual publications. This summation of metabolite count data highlights which targets warrant further investigation as potential diabetes biomarkers.
All metabolites identified by articles in this systematic review were annotated with the relevant HMDB primary accession number (25); to avoid simultaneous use of synonymous metabolites. Where a reported metabolite could not be matched to a HMDB entry, it was instead annotated with the compound identifier (CID) assigned by the NLM PubChem database. Furthermore, if the reported target did not match a specific HMDB entry (i.e., the target corresponds to a mixture of isomers or chemical species), then the HMDB ID of the most common compound variant was provided as an example.
To ensure consistency in reporting, metabolite targets from different publications were consolidated under a single compound name if listed as synonyms within the HMDB. Additionally, in keeping with the focus of this systematic review, only data pertaining to gut-derived metabolites were extracted from publications; that is, those metabolites known to originate from an intestinal biological location (whether directly from the intestinal organ, the gut microbiota, or from non-excretory fluid of faeces) or cited as having a direct link with the gastrointestinal system. Moreover, only metabolites identified using metabolomics tools are reported (i.e., not with commercially available laboratory assays including, for example, ELISAs). This ensured standardisation in the findings between different methodologies.
Risk of bias in individual studies
Despite targeting a similar panel of gut-derived metabolites, significant methodological differences exist between research articles that investigate a facet of the intestinal metagenome. These differences extend from biological specimen collection techniques, to the methods and instruments used for downstream analyses. In addressing these discrepancies, this article aimed to evaluate if the employed methods of the included articles significantly impacted the metabolomic outputs reported. To achieve this, metabolomic findings were reported according to the type of biological specimen used in metabolomic analyses, and also based on the diabetes disease against which the findings were reported. Within these groups, Chi-Square tests of association were conducted to determine if the levels of any key gut-associated metabolites were significantly increased or decreased relative to (i) any diabetes disease type (i.e., T1DM, GDM, PreDM, or T2DM), or (ii) the reported sample collection method (i.e., blood, stool, or urine). These Chi-Square tests were conducted using R software, with Post-hoc pairwise tests conducted using the chisq.posthoc.test package to confirm significance of correlations between individual variables.
Results
The search strategy originally yielded 3,099 records. After removal of duplicate records, 2,042 records were screened by title and abstract and 1,953 studies were excluded having not met the defined inclusion criteria. The remaining eighty-nine full text articles were screened; from which, a total of thirty-four articles were included as part of the systematic review. In summarising this, Figure 2 depicts the study workflow for this review and details the number of articles included and excluded at each stage of the identification and screening processes.
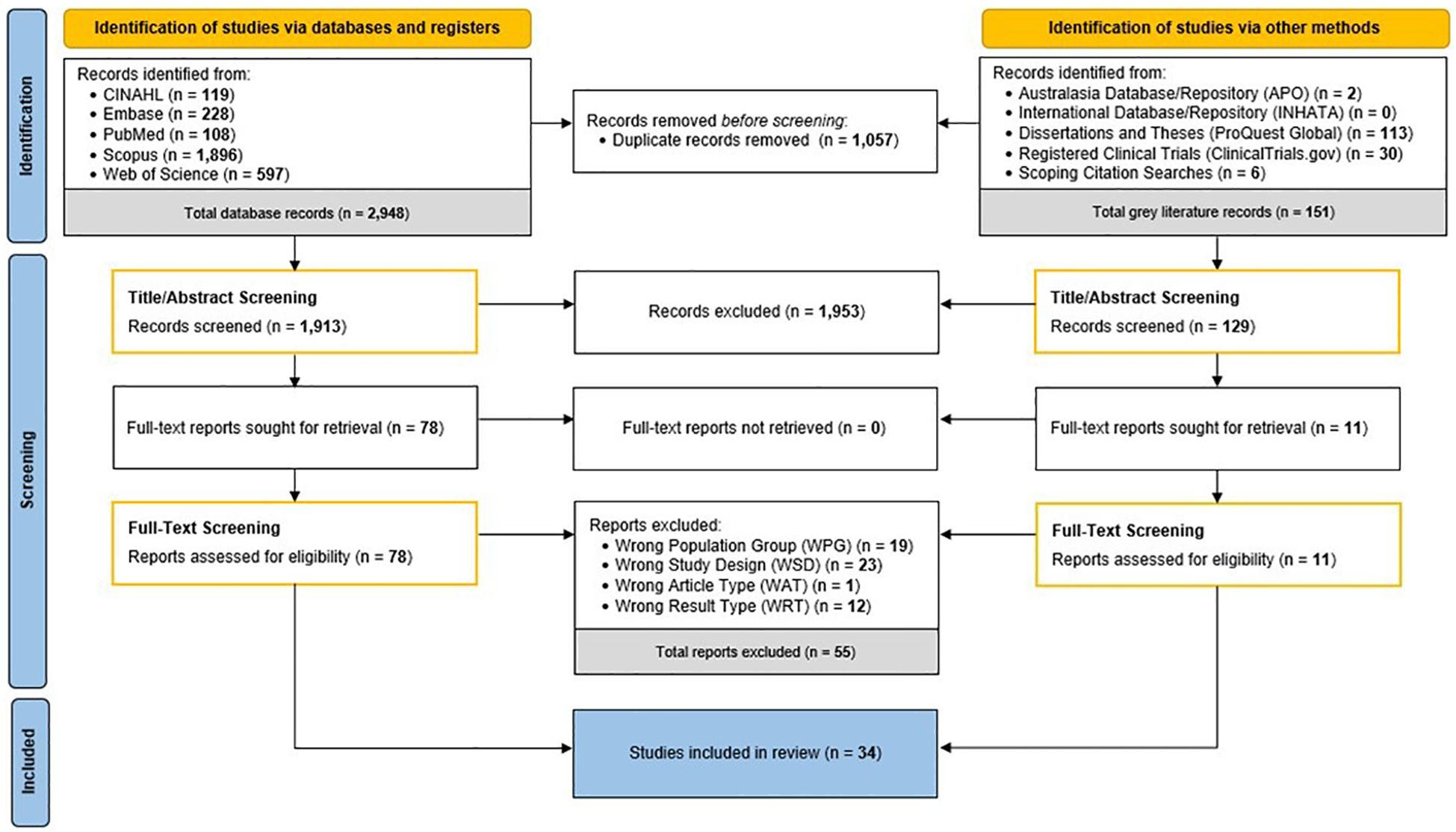
Figure 2. Workflow of systematic review identification, screening, and inclusion processes. APO, Analysis Policy Observatory; INHATA, International Network of Agencies for Health Technology Assessment; WAT, wrong article type; WPG, wrong population group; WRT, wrong result type; WSD, wrong study design. Schematic adapted from PRISMA 2020 Flow Diagram (17).
The key cohort and analysis descriptors from the thirty-four reviewed records are summarised in Table 1 according to the type of diabetes disease targeted; detailing the location, experimental design, sample type and associated storage conditions, study cohorts, and metabolomics methods incorporated.
Table 2 lists the gut-associated metabolites reported as being significantly associated with diabetes. In this table, metabolites are reported alongside the sample type/s used in sourcing analysis, and colour-coded depending on whether the target was found to be increased or decreased in DM cohorts relative to non-diabetes (ND) control cohorts. Additionally, all 272 metabolites are listed with the corresponding HMDB primary accession number, to facilitate cross-referencing with the chemical repository.
Figure 3 demonstrates the proportions of reviewed studies that investigated each DM disease (inner ring), and, within each disease type, the proportion of studies that incorporated blood, urine and/or stool samples in metabolomic analyses (outer ring). The relative proportions depicted in Figure 3 are based on the number of metabolites (targets listed in Table 2) each study reported as being significantly associated with DM.
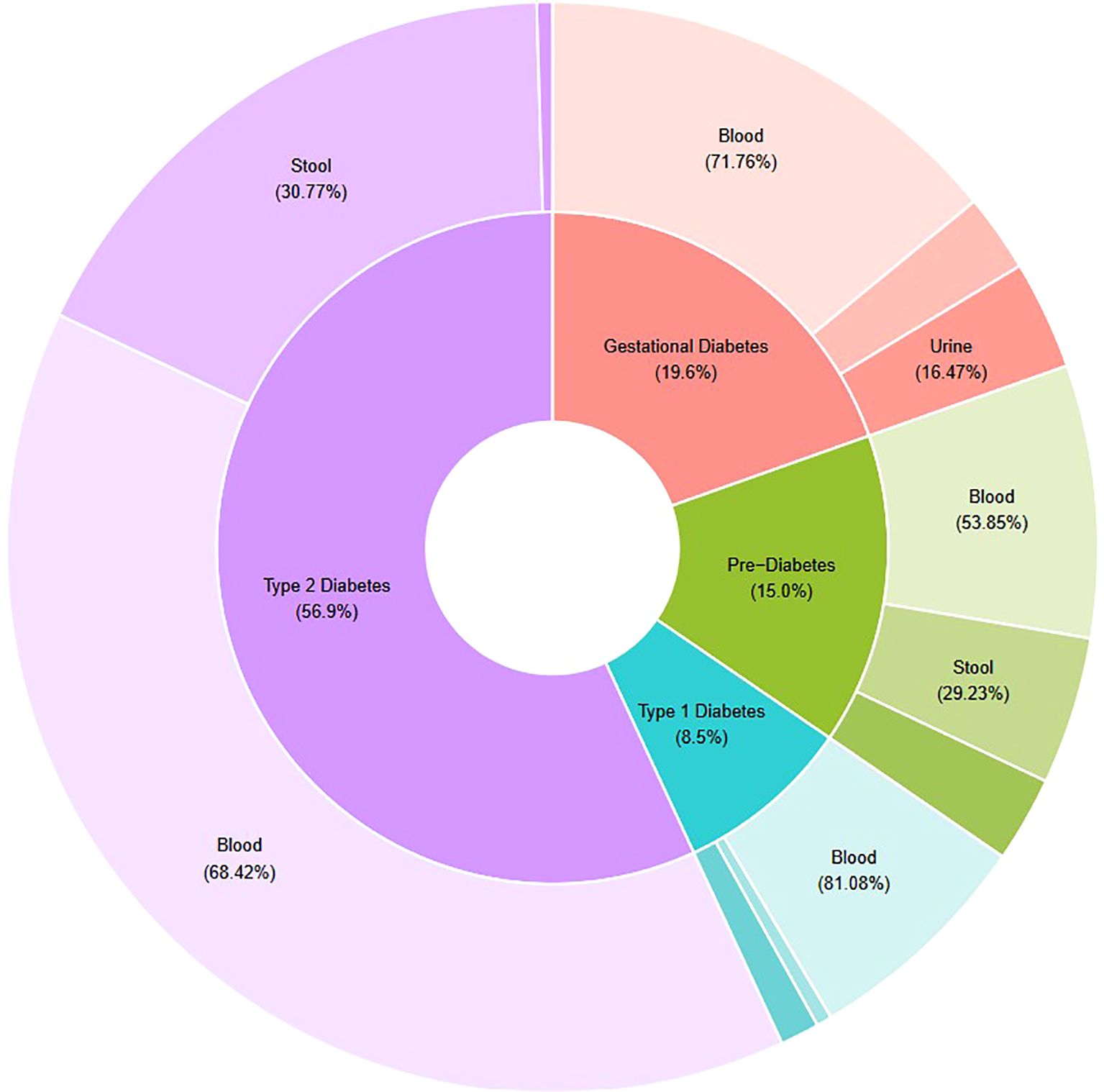
Figure 3. Schematic representing research targets of thirty-four studies analysed in this systematic review. Relative proportions based on counts of metabolites considered significantly different between cohorts depending on diabetes disease type (inner ring) and biological sample type (outer ring). Figure developed using R Studio software.
Summary of cohort descriptors
Thirty-four articles were analysed in this systematic review. The concentration of gut metabolites in individuals with T1DM was investigated in four studies (26–29); seven studies investigated GDM cohorts (30–36); eight studies investigated PreDM, or PreDM and T2DM cohorts (2, 37–43); and fifteen studies investigated T2DM cohorts only (44–58). Given the pre-determined exclusion criteria for this systematic review, all thirty-four studies incorporated an observational experimental design, with no interventional input. Cohort sizes varied significantly between studies depending on study objectives and stringency of inclusion/exclusion criteria. As depicted in Figure 3, across all diabetes diseases, the majority of metabolomic findings were obtained from blood components (plasma and/or serum). However, as outlined in Table 1, eleven studies analysed two types of biological samples (plasma and urine, plasma and stool, plasma and serum, stool and urine, or stool and serum).
Summary of analysis descriptors
Of the thirty-four studies reviewed, fifteen employed targeted metabolomic approaches; the majority of which targeted bile acids (BA), short-chain fatty acids (SCFA), or trimethylamine N-oxide (TMAO) and precursor metabolites. Fourteen articles incorporated untargeted metabolomic analyses, while an additional five studies combined targeted and untargeted metabolomic approaches. The details of each metabolomic method are outlined in Table 1. Briefly, mass spectrometry (MS) analyses were the most widely employed method; specifically, liquid chromatography-mass spectrometry configurations were employed in thirty-seven analyses (75.5%), gas chromatography-mass spectrometry methods were utilised in nine articles (18.3%), while capillary electrophoresis-mass spectrometry was used in a single study (2.5%). Nuclear magnetic resonance (NMR) metabolomic analyses were incorporated in five analyses (12.8%). Differential mobility spectroscopy and high-performance liquid chromatography with ultra-violet spectroscopy were each utilised in one analysis (2.5% per method).
Summary of metabolomic findings
Analysis of metabolomic findings revealed a total of 272 metabolites, across thirty-eight chemical classes and sub-classes, determined to be significantly associated with incidence of diabetes. Amino acid compounds were the most widely identified across all diabetes diseases; whilst only single metabolite targets were identified from several smaller classes of organoheterocyclic compounds (such as lactones and dihydrofuran compounds). The metabolites most widely cited as elevated in the reviewed studies were amino acids, leucine, and isoleucine. In contrast, levels of the sphingolipid metabolite, sphingomyelin, were most consistently observed to be decreased, with three articles reporting down-regulation in T2DM cohorts, relative to non-diabetes controls. As Table 2 depicts, consistent reporting of increased or decreased metabolite levels was predominantly observed in T2DM cohorts only; across other diabetes diseases, reported metabolites were only supported by single findings. This also reflects the trend depicted in Figure 3, in which the largest metabolite profiles were obtained from studies of T2DM (59.22%).
Analysis of the core metabolome shared between all the diseases revealed four metabolites to be significantly associated with T1DM, GDM, PreDM and T2DM cohorts (Figure 4). Of these, the alpha-hydroxy acid, 2-hydroxybutyric acid and the branched-chain amino acid (BCAA), valine, were determined to be consistently elevated. Whilst the amino acid, alanine, and BCAAs, leucine/isoleucine were also reported across all disease types, discrepancies existed between studies as to whether these compounds were increased or decreased in T1DM cohorts. In addition to these core metabolites, a further eleven targets were identified as being shared across three of the diabetes diseases (Figure 4). These included glucose, mannose, 3-hydroxybutyric acid, palmitic acid, and ketoisocaproic acid which were all reported as being significantly elevated across three different diabetes disease types. Tyrosine, tryptophan, palmitoleic acid, phosphatidylcholines, phosphatidylcholines, and triglycerides were also reported across three diabetes diseases, but findings of increased or decreased levels were discrepant between articles, depending on the sample type used in downstream metabolomic analyses. Pathways involved in the biosynthesis of BCAAs valine, leucine and isoleucine were determined to have the highest enrichment ratio and strongest enrichment p-value; followed by pathways involved in the biosynthesis of phenylalanine, tyrosine and tryptophan, and unsaturated fatty acids (Figure 5). Biosynthesis of the alpha-amino acid, tryptophan, is a highly enriched metabolic pathway (Figure 5), and the metabolite is also shown to share a high number of metabolic relationships within the network (degree = 96, betweenness = 10279.40).
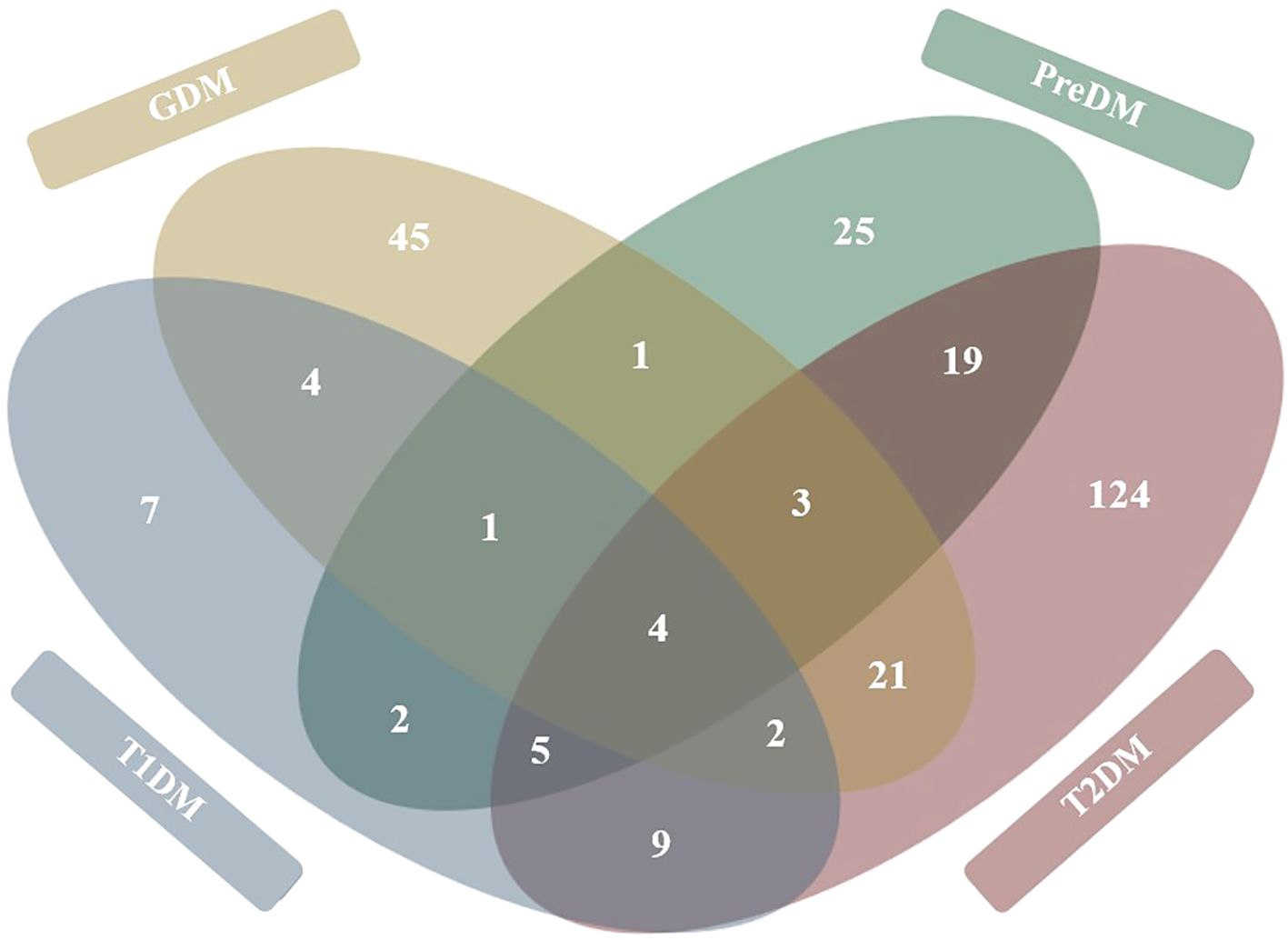
Figure 4. Venn diagram representation of metabolites significantly associated with diabetes mellitus diseases. Figure represents 272 total metabolites reported as being significantly associated (either positively or negatively) with each of the four diabetes disease types. Metabolites shared between diabetes diseases are tallied in overlapping circles. Figure developed based on findings of thirty-four primary research articles reviewed in systematic review.
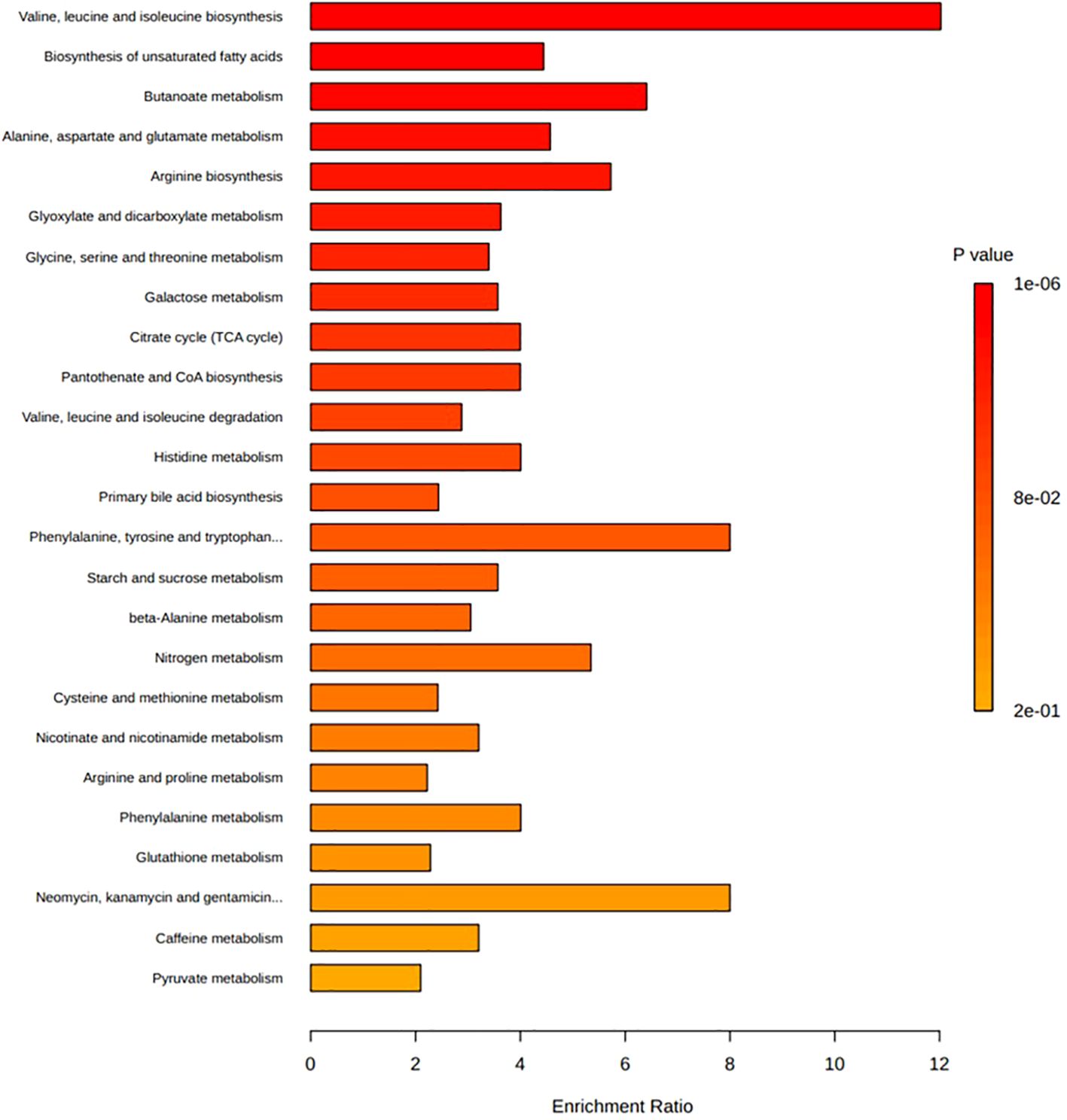
Figure 5. Enrichment ratio and p-value from enrichment analyses of the top twenty-five pathways associated with those metabolites reported as being significantly associated with diabetes diseases. Bar lengths represent enrichment ratio, calculated by the number of hits divided by the expected number of hits within each pathway. Enrichment analysis conducted on metabolites determined to be significantly associated with incidence of diabetes disease (Table 2) and those with primary accession numbers indexed in the HMDB library. Enriched metabolic pathways are ordered according to decreasing p-value. Metabolite pathways defined based on KEGG human metabolic pathways library. Enrichment analysis conducted and visualised by MetaboAnalyst program.
Figure 5 depicts the enrichment ratios and p-values from enrichment analyses conducted on the metabolites reported as being significantly associated with the incidence of T1DM, GDM, PreDM and T2DM (Table 2). Bar plot depicts the top twenty-five metabolite pathways (according to calculated p-value) enriched in diabetes disease, based on the gut-associated metabolites collated in this systematic review. Data and corresponding metabolite-metabolite network for this enrichment analysis are presented in Supplementary Table S1.
Across all four diabetes diseases, discrepancies were reported between articles as to the concentrations of metabolites in the same, and different sample types. For example, fatty acids butyrate, heptadecenoic acid, and myristic acid were reported as both increased and decreased in blood samples collected from T2DM cohorts (relative to respective non-diabetes cohorts); but were also reported to be elevated in stool samples from T2DM cohorts. Further exemplifying this discrepancy; the concentration of key bile acids glycodeoxycholic acid, glycocholic acid, cholic acid and chenodeoxycholic acid were reported to be elevated in blood samples but decreased in stool samples collected from T2DM cohorts. The concentrations of four metabolites was dependent on carbon configuration, isomer structure or metabolite species: namely, ceramide, tridecanoic acid, and phosphatidylethanolamine and triglyceride compounds.
Specimen type used in studies of diabetes correlates with disease type and metabolite regulation findings
Chi-Square tests of association revealed evidence of a significant correlation between the type of specimen used in metabolomic analysis, and whether metabolite levels were reported as being increased or decreased (p = 3.5e-8). Incorporation of stool samples was significantly associated with identification of decreased metabolite levels; whilst the opposite was true of studies that utilised blood samples, as this specimen type was significantly associated with the identification of increased metabolite levels.
Further Chi-Square testing revealed a significant correlation between the type of diabetes and specimen used in metabolomic analyses (p = 1.4e-9). Post-hoc testing of this correlation revealed significant associations between two groups; GDM studies identified metabolites more from urine biospecimens, while T2DM studies identified metabolites more from stool and less from urine samples. Finally, statistical analyses revealed no significant association between type of diabetes disease and whether metabolite levels were reported as being increased or decreased.
Discussion
Metabolic disorders, particularly T1DM, GDM and T2DM, are physiologically distinct diseases, with very different causes, treatments, and outcomes. As such, the aim of this review was not to directly compare these aetiologies, but to collectively synthesise published metabolomics data and identify common disease targets; thereby maximising the future research potential of identified biomarkers. In achieving this, our review identified a core metabolomic signature that is common to all DM diseases. The four metabolites that comprise this shared diabetes metabolome are the alpha hydroxy acid, 2-hydoxybutyric acid, the amino acid, alanine, and BCAAs, valine, and the leucine/isoleucine isomer system (Table 2, Figure 4). As a by-product of protein metabolism, 2-hydroxybutyric acid has been positively correlated to the impairment of pancreatic β-cells (59) as a well-established early hallmark of insulin sensitivity, and resistance (60). Corroborating this, the findings of our review showed the metabolite to be elevated in blood samples taken from T1DM (26), GDM (61, 62), PreDM (2), and T2DM (48) cohorts. Similarly, concentrations of valine were significantly higher across all DM diseases in blood (2, 27, 40, 42, 55), stool (63), and urine (29); an expected finding given valine is widely associated with the incidence of oxidative stress, decreased insulin secretion, and high glucose levels (64). In contrast to 2-hydroxybutyric acid and valine, the role of leucine and isoleucine in DM is controversial. Some studies report that, alongside valine, the BCAAs have a detrimental effect on human metabolic health by decreasing insulin secretion; and even linking isoleucine with increased T2DM risk (65). However, other studies report that the metabolite acutely stimulates insulin production and plays an important role in ameliorating adiposity and maintaining glucose homeostasis (66, 67). In our review, the concentration of leucine and isoleucine were found to be elevated in the blood (2, 27, 34, 40, 42, 51, 55, 58) and urine (29) of individuals with DM, whilst leucine was reported to be decreased in T1DM cohorts (26). These contradictory findings reiterate the need for future studies that provide clarity around the regulatory profiles of these BCAAs in different biological sample types, and across the spectrum of diabetes diseases.
The majority of studies analysed in this review characterised the human intestinal metabolome associated with T2DM (Figures 3, 4). The focus of research on T2DM is commensurate with its high prevalence (86.4% of Australasian DM diagnoses (2, 27, 34, 40, 42, 51, 55, 58)). Our review has drawn attention to the unmet need for GDM- and T1DM-specific metabolomic research, given the distinct pathophysiological processes associated with the development of DM diseases. Our review has also highlighted the importance of appropriate interpretation of GDM studies; ensuring any significant findings are drawn from comparisons of appropriate disease and control cohorts. This is best exemplified in the findings of Ivanovova et al. (2021), who demonstrated significant differences in the levels of multiple SCFAs between GDM and non-pregnant cohorts, but not between GDM and pregnant-non-GDM cohorts. For future studies in the field of GDM-research, this highlights the importance of incorporating proper phenotype controls in study designs. Doing so will ensure conclusions can be drawn regarding the impact of GDM, and not pregnancy itself; a period that is already well-known to involve significant changes to the female gut microbiota and metabolome (33).
Our findings reinforce that biological sample type has a significant impact on metabolomic findings. This is best exemplified by comparing independent findings published by Zhou et al. (2019, 2020) (68, 69); which indicated that the levels of the same BA and SCFA metabolite targets were elevated in serum yet decreased in stool collected from the same participant cohorts. Overall, our review identified twenty-seven metabolites that were both increased and decreased, depending on whether blood, urine or stool was used in metabolomic analyses (Table 2). BAs were the most frequently discrepantly reported chemical species; with decreased levels in stool samples, yet increased levels in blood samples from DM cohorts. A site-specific difference is expected given only an estimated 5% of primary and secondary BAs are excreted in stool, while up to 95% are reabsorbed by the terminal ileum and transported back to the liver via portal circulation (62). However, literature reports that diabetes initiates changes in BA metabolism and composition (70, 71), and is accompanied by an increase in BA stool excretion; which does not support the decreased levels seen in stool profiles reported in the reviewed studies. Such discrepancies highlight the potential bias that sample type and collection methods may impose on metabolomic findings and emphasise that researchers should consider the inherent advantages and disadvantages of each sample type when designing studies that target human- and microbial-derived metabolites in the gut.
All results incorporated in this systematic review were based on findings from non-invasive, or minimally invasive sample types including stool, urine, and blood components (plasma/serum). Across all four DM diseases, blood was the most utilised sample, with stool the second most frequently utilised in T2DM and PreDM studies, and urine the second most utilised in T1DM and GDM studies (Figure 3). The incorporation of urine sample collection from T1DM and GDM cohorts is pragmatic in the clinical context given the often routine requirement for these patient groups to submit samples for urine ketone, protein and albumin testing, and hence easier recruitment to research studies. However, the discrepancies reported here, and in studies such as that of Deng, Xu, Shen, et al. (2023) (48), underline the importance of careful selection of sample types to ensure results provide the most accurate representation of the metabolome at the target organ site. Researchers may also consider the use of other sample types to analyse host-gut interactions, such as saliva, exhaled breath, or invasive sample types, such as tissue biopsies (26).
This systematic review also reported discrepant findings in the levels of twenty-six metabolites between studies that incorporated the same sample type in downstream metabolomic analyses (Table 2). These findings highlight the need for guidelines that standardise collection methods for biological samples used in metabolomic analyses. This could include best practice recommendations for sample collection times (such as first/second morning urine sampling), the preferred use of single-batch consumables to prevent inter-batch variability generating inconsistent artefacts, and recommendations to reduce contamination in tissue collection (for example, using protected specimen brush techniques in mucosal-luminal sampling) (68, 69). Enforcing the standardisation of these collection and preparation methods will work to limit inter-sample variability and maintain the biological and metabolic integrity of samples consistently across study cohorts (68).
In addition to sample type selection, pre-analytical procedures are well known to influence microbiome and metabolome readouts. In particular, collection and storage methods have a well-established impact on the accuracy and precision of downstream metabolomic results (65, 72), and metabolite extraction technique/s are critical to either preventing or causing biases in results (73). The majority of studies included in this review reported sample handling procedures in line with best practice guidelines (74) (Table 1). However, given the different sample types, chemical targets, metabolomic approaches and MS or NMR instrumentation used in each of the thirty-four reviewed studies (Table 1), the pre-treatment extraction and derivisation processes varied widely, and may explain discrepancies between studies. Therefore, while sample preparation methods have been comprehensively reviewed in the literature (29), further comparative analyses are required to elucidate inter-method and inter-platform biases.
Much of the research reviewed in this study reported differential concentrations of targeted metabolites; however, it is not made clear whether these markers were differentially increased or decreased between cohorts. Additional discrepancies also exist in the reporting of metabolite marker regulation; further confusing the role these metabolites play as either a ‘cause or cure’ to diabetes. This iterates the need for standardised reporting in future clinical metabolomics publications. Potential measures for implementation may include standardised reporting of fold changes between cohorts, as well as cross-referencing and reporting targets alongside primary accession codes linked to a publicly accessible database, such as HMDB, KEGG, PubChem, ChEBI, or UniProt. Doing so will prevent chemically synonymous compounds being misreported as novel targets and maximise the research potential of future studies in the metabolomics field.
A limitation of this review is the comparison of metabolite concentrations on a dichotomous scale; increased or decreased concentrations depending on healthy or diabetes status. Performing a quantitative comparison of metabolomic concentrations across the various study cohorts falls outside the scope of this systematic review and warrants further study. Therefore, further meta-analysis of the metabolomics data synthesised here is required to quantitatively compare metabolite concentrations; taking into consideration the inter-method effects of metabolomics tools, platform-specific biases, and the array of analytical pipelines consulted by researchers. Future statistical analysis should also consider controlling for participant baseline characteristics that vary from study to study; for instance, age, sex, duration of diabetes, race, genetic background, diet/lifestyle factors, or the use of any antidiabetic treatments or known microbiome-altering medications and supplements. Furthermore, the results of this review do not account for metabolome changes over time, or those stemming from diabetes-associated comorbidities (such as diabetic nephropathy, neuropathy, or retinopathy) which are known to further perturb the gut microbiota and metabolism (44, 45). It would therefore be pertinent for future studies to consider the effects attributable to the incidence and stage of comorbidities, by sub-stratifying participant cohorts accordingly. The results of our review may also be considered in conjunction with future microbiome-metabolome correlative analyses that incorporate tools such as 16S ribosomal RNA gene sequencing or whole genome shotgun sequencing. Alternatively, publicly available datasets, such as that curated by Muller, Algavi, and Borenstein (2022) (75) from faecal samples, provide fully processed, and benchmarked microbiome-metabolome integration tools to enable correlative analyses. Linking metabolomic outputs to microbial profiles is a key future research target, as it will provide a deeper insight into the functional capacity of the microbiota.
Conclusions
In conclusion, this systematic review analysed the findings of thirty-four articles investigating the intestinal metabolome associated with the incidence of T1DM, GDM, PreDM, and T2DM. Extracted data mapped the concentrations of 272 intestinal metabolites across thirty-eight chemical classes and sub-classes. To date, the majority of diabetes metabolome research has been conducted in T2DM cohorts; and while the experimental, and clinical importance of these studies is undeniable, this review highlights a bias in the research foci that has left GDM and T1DM diseases understudied. Our review also described a novel comparison of metabolite levels between blood, stool, and urine samples in DM cohorts. This is significant given few studies have investigated the potential biasing effect of sample type on the downstream identification of primary and secondary metabolites. The results of this work, and of other newly emerging research in the field (64), urge caution in directly inferring associations between the stool metabolome and the incidence of systemic diseases, such as diabetes. Overall, the key metabolites identified in this review warrant further investigation as potential diagnostic biomarkers or targets in the treatment of DM.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
KG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. SD: Supervision, Writing – review & editing. ST: Validation, Writing – review & editing. BF: Supervision, Writing – review & editing. FH: Supervision, Writing – review & editing. EP: Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors acknowledge the formal analysis conducted by Dr Leah South from the QUT School of Mathematical Sciences, as well as the input of Peter Sondergeld from the Faculty of Health Library Services in the development of methods employed in this review. Additionally, the authors acknowledge the feedback provided by Dr Helen Barrett in the development of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1559638/full#supplementary-material
References
1. Hirata S and Kunisawa J. Gut microbiome, metabolome, and allergic diseases. Allergol Int. (2017) 66:523–8. doi: 10.1016/j.alit.2017.06.008
2. Menni C, Fauman E, Erte I, Perry JR, Kastenmüller G, Shin SY, et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. (2013) 62:4270–6. doi: 10.2337/db13-0570
3. Rooks MG and Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. (2016) 16:341–52. doi: 10.1038/nri.2016.42
4. Savolainen O, Fagerberg B, Vendelbo Lind M, Sandberg AS, Ross AB, and Bergström G. Biomarkers for predicting type 2 diabetes development-can metabolomics improve on existing biomarkers? PloS One. (2017) 12:e0177738–e0177738. doi: 10.1371/journal.pone.0177738
5. Tirosh A, Calay ES, Tuncman G, Claiborn KC, Inouye KE, Eguchi K, et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci Transl Med. (2019) 11:eaav0120. doi: 10.1126/scitranslmed.aav0120
6. Wu H-J and Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. (2012) 3:4–14. doi: 10.4161/gmic.19320
7. Magliano DJ, Islam RM, Barr ELM, Gregg EW, Pavkov ME, Harding JL, et al. Trends in incidence of total or type 2 diabetes: systematic review. BMJ. (2019) 366:l5003. doi: 10.1136/bmj.l5003
8. Mearns H, Otiku PK, Shelton M, Kredo T, Kagina BM, and Schmidt B-M. Screening strategies for adults with type 2 diabetes mellitus: a systematic review protocol. Syst Rev. (2020) 9:156. doi: 10.1186/s13643-020-01417-3
9. Arneth B, Arneth R, and Shams M. Metabolomics of type 1 and type 2 diabetes. Int J Mol Sci. (2019) 20:2467. doi: 10.3390/ijms20102467
10. Zierer J, Jackson MA, Kastenmüller G, Mangino M, Long T, Telenti A, et al. The fecal metabolome as a functional readout of the gut microbiome. Nat Gene. (2018) 50:790–5. doi: 10.1038/s41588-018-0135-7
11. Bao X, Borné Y, Johnson L, Muhammad IF, Persson M, Niu K, et al. Comparing the inflammatory profiles for incidence of diabetes mellitus and cardiovascular diseases: a prospective study exploring the ‘common soil’ hypothesis. Cardiovasc Diabetol. (2018) 17:87. doi: 10.1186/s12933-018-0733-9
12. Ferreira-Divino LF, Suvitaival T, Rotbain Curovic V, Tofte N, Trošt K, Mattila IM, et al. Circulating metabolites and molecular lipid species are associated with future cardiovascular morbidity and mortality in type 1 diabetes. Cardiovasc Diabetol. (2022) 21:135. doi: 10.1186/s12933-022-01568-8
13. De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, and Foti DP. Type 2 diabetes mellitus and cardiovascular disease: genetic and epigenetic links. Front Endocrinol (Lausanne). (2018) 9:2. doi: 10.3389/fendo.2018.00002
14. Ng TW, Khan AA, and Meikle PJ. Investigating the pathogenesis and risk of Type 2 diabetes: clinical applications of metabolomics. Clin Lipidol. (2012) 7:641–59. doi: 10.2217/clp.12.75
15. Herrema H and Niess JH. Intestinal microbial metabolites in human metabolism and type 2 diabetes. Diabetologia. (2020) 63:2533–47. doi: 10.1007/s00125-020-05268-4
16. Goyal R and Jialal I. Diabetes Mellitus Type 2. Treasure Island (FL: StatPearls Publishing (2020).
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
18. Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, and Welch VA eds. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane. Chichester (UK): John Wiley & Sons (2022).
19. Turner PV. The role of the gut microbiota on animal model reproducibility. Anim Models Exp Med. (2018) 1:109–15. doi: 10.1002/ame2.12022
20. Nguyen TL, Vieira-Silva S, Liston A, and Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech. (2015) 8:1–16. doi: 10.1242/dmm.017400
21. Krych L, Hansen CHF, Hansen AK, van den Berg FWJ, and Nielsen DS. Quantitatively Different, yet Qualitatively Alike: A Meta-Analysis of the Mouse Core Gut Microbiome with a View towards the Human Gut Microbiome. PloS One. (2013) 8:e62578. doi: 10.1371/journal.pone.0062578
22. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, and Gordon JI. The human microbiome project. Nature. (2007) 449:804–10. doi: 10.1038/nature06244
23. Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, et al. HMDB: the human metabolome database. Nucleic Acids Res. (2007) 35:D521–526. doi: 10.1093/nar/gkl923
24. Smith CA, Maille GO, Want EJ, Qin C, Trauger SA, Brandon TR, et al. METLIN: A metabolite mass spectral database. Ther Drug Monit. (2005) 27:747–51. doi: 10.1097/01.ftd.0000179845.53213.39
25. Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. PubChem 2023 update. Nucleic Acids Res. (2022) 51:D1373–80. doi: 10.1093/nar/gkac956
26. Winther SA, Henriksen P, Vogt JK, Hansen TH, Ahonen L, Suvitaival T, et al. Gut microbiota profile and selected plasma metabolites in type 1 diabetes without and with stratification by albuminuria. Diabetologia. (2020) 63:2713–24. doi: 10.1007/s00125-020-05260-y
27. Kostic AD, Gevers D, Siljander H, Vatanen T, Hyotylainen T, Hamalainen AM, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. (2015) 17:260–73. doi: 10.1016/j.chom.2015.01.001
28. de Groot PF, Belzer C, Aydin Ö, Levin E, Levels JH, Aalvink S, et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PloS One. (2017) 12:e0188475. doi: 10.1371/journal.pone.0188475
29. Balderas C, Ruperez FJ, Ibanez E, Senorans J, Guerrero-Fernandez J, Casado IG, et al. Plasma and urine metabolic fingerprinting of type 1 diabetic children. Electrophoresis. (2013) 34:2882–90. doi: 10.1002/elps.201300062
30. Wang X, Liu HL, Li YF, Huang S, Zhang L, Cao CY, et al. Altered gut bacterial and metabolic signatures and their interaction in gestational diabetes mellitus. Gut Microbes. (2020) 12:1–13. doi: 10.1080/19490976.2020.1840765
31. Pinto J, Almeida LM, Martins AS, Duarte D, Barros AS, Gahano E, et al. Prediction of gestational diabetes through NMR metabolomics of maternal blood. J Proteome Res. (2015) 14:2696–706. doi: 10.1021/acs.jproteome.5b00260
32. Liang SF, Hou ZQ, Li X, Wang J, Cai LJ, Zhang RP, et al. The fecal metabolome is associated with gestational diabetes mellitus. Rsc Advances. (2019) 9:29973–9. doi: 10.1039/c9ra05569j
33. Ivanovová E, Piskláková B, Friedecká J, Krystyník O, Friedecký D, and Karásek D. Plasma short-chain fatty acids and their derivatives in women with gestational diabetes mellitus. Separations. (2021) 8:188. doi: 10.3390/separations8100188
34. Hou W, Meng X, Zhao A, Zhao W, Pan J, Tang J, et al. Development of multimarker diagnostic models from metabolomics analysis for gestational diabetes mellitus (GDM). Mol Cell Proteomics. (2018) 17:431–41. doi: 10.1074/mcp.RA117.000121
35. Gao YJ, Chen HM, Li JL, Ren SJ, Yang ZL, Zhou YP, et al. Alterations of gut microbiota-derived metabolites in gestational diabetes mellitus and clinical significance. J Clin Lab Analysis. (2022) 36:e24333. doi: 10.1002/jcla.24333
36. Dong LN, Han LN, Duan T, Lin SM, Li JG, and Liu XJ. Integrated microbiome-metabolome analysis reveals novel associations between fecal microbiota and hyperglycemia-related changes of plasma metabolome in gestational diabetes mellitus. Rsc Advances. (2020) 10:2027–36. doi: 10.1039/c9ra07799e
37. Zhou W, Sailani MR, Contrepois K, Zhou Y, Ahadi S, Leopold SR, et al. Longitudinal multi-omics of host–microbe dynamics in prediabetes. Nature. (2019) 569:663–71. doi: 10.1038/s41586-019-1236-x
38. Zhong H, Fang C, Fan Y, Lu Y, Wen B, Ren H, et al. Lipidomic profiling reveals distinct differences in plasma lipid composition in healthy, prediabetic, and type 2 diabetic individuals. GigaScience. (2017) 6:1–12. doi: 10.1093/gigascience/gix036
39. Zhao XJ, Fritsche J, Wang JS, Chen J, Rittig K, Schmitt-Kopplin P, et al. Metabonomic fingerprints of fasting plasma and spot urine reveal human pre-diabetic metabolic traits. Metabolomics. (2010) 6:362–74. doi: 10.1007/s11306-010-0203-1
40. Org E, Blum Y, Kasela S, Mehrabian M, Kuusisto J, Kangas AJ, et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol. (2017) 18:70. doi: 10.1186/s13059-017-1194-2
41. Nuli R, Azhati J, Cai JX, Kadeer A, Zhang B, and Mohemaiti P. Metagenomics and faecal metabolomics integrative analysis towards the impaired glucose regulation and type 2 diabetes in uyghur-related omics. J Diabetes Res. (2019) 2019:2893041. doi: 10.1155/2019/2893041
42. Gaike AH, Paul D, Bhute S, Dhotre DP, Pande P, Upadhyaya S, et al. The gut microbial diversity of newly diagnosed diabetics but not of prediabetics is significantly different from that of healthy nondiabetics. Msystems. (2020) 5:e00578-19. doi: 10.1128/mSystems.00578-19
43. Molinaro A, Bel Lassen P, Henricsson M, Wu H, Adriouch S, Belda E, et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat Commun. (2020) 11:5881. doi: 10.1038/s41467-020-19589-w
44. Zhou ZX, Zheng Z, Xiong XJ, Chen X, Peng JY, Yao H, et al. Gut microbiota composition and fecal metabolic profiling in patients with diabetic retinopathy. Front Cell Dev Biol. (2021) 9:732204. doi: 10.3389/fcell.2021.732204
45. Zhong C, Dai Z, Chai L, Wu L, Li J, Guo W, et al. The change of gut microbiota-derived short-chain fatty acids in diabetic kidney disease. J Clin Lab Analysis. (2021) 35:e24062. doi: 10.1002/jcla.24062
46. Zhao LJ, Lou HX, Peng Y, Chen SH, Zhang YL, and Li XB. Comprehensive relationships between gut microbiome and faecal metabolome in individuals with type 2 diabetes and its complications. Endocrine. (2019) 66:526–37. doi: 10.1007/s12020-019-02103-8
47. Zhao L, Lou H, Peng Y, Chen S, Fan L, and Li X. Elevated levels of circulating short-chain fatty acids and bile acids in type 2 diabetes are linked to gut barrier disruption and disordered gut microbiota. Diabetes Res Clin Practice. (2020) 169:108418. doi: 10.1016/j.diabres.2020.108418
48. Vangipurapu J, Silva LF, Kuulasmaa T, Smith U, and Laakso M. Microbiota-related metabolites and the risk of type 2 diabetes. Diabetes Care. (2020) 43:1319–25. doi: 10.2337/dc19-2533
49. Therdtatha P, Song YY, Tanaka M, Mariyatun M, Almunifah M, Manurung NEP, et al. Gut microbiome of Indonesian adults associated with obesity and type 2 diabetes: A cross-sectional study in an Asian City, Yogyakarta. Microorganisms. (2021) 9:897. doi: 10.3390/microorganisms9050897
50. Tang WHW, Wang Z, Li XS, Fan Y, Li DS, Wu Y, et al. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. (2017) 63:297–306. doi: 10.1373/clinchem.2016.263640
51. Suhre K, Meisinger C, Doring A, Altmaier E, Belcredi P, Gieger C, et al. Metabolic footprint of diabetes: A multiplatform metabolomics study in an epidemiological setting. PloS One. (2010) 5:e13953. doi: 10.1371/journal.pone.0013953
52. Shan Z, Sun T, Huang H, Chen S, Chen L, Luo C, et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr. (2017) 106:888–94. doi: 10.3945/ajcn.117.157107
53. Schüssler-Fiorenza Rose SM, Contrepois K, Moneghetti KJ, Zhou W, Mishra T, Mataraso S, et al. A longitudinal big data approach for precision health. Nat Med. (2019) 25:792–804. doi: 10.1038/s41591-019-0414-6
54. Scheijen J, Hanssen NMJ, van de Waarenburg MPH, Jonkers D, Stehouwer CDA, and Schalkwijk CG. L(+) and D(-) lactate are increased in plasma and urine samples of type 2 diabetes as measured by a simultaneous quantification of L(+) and D(-) lactate by reversed-phase liquid chromatography tandem mass spectrometry. Exp Diabetes Res. (2012) 2012:234812. doi: 10.1155/2012/234812
55. Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BAH, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. (2016) 535:376–81. doi: 10.1038/nature18646
56. Menni C, Zhu JL, Le Roy CI, Mompeo O, Young K, Rebholz CM, et al. Serum metabolites reflecting gut microbiome alpha diversity predict type 2 diabetes. Gut Microbes. (2020) 11:1632–42. doi: 10.1080/19490976.2020.1778261
57. Liu W, Wang C, Xia Y, Xia W, Liu G, Ren C, et al. Elevated plasma trimethylamine-N-oxide levels are associated with diabetic retinopathy. Acta Diabetol. (2021) 58:221–9. doi: 10.1007/s00592-020-01610-9
58. Lalande C, Drouin-Chartier JP, Tremblay AJ, Couture P, and Veilleux A. Plasma biomarkers of small intestine adaptations in obesity-related metabolic alterations. Diabetol Metab Syndrome. (2020) 12:e13953. doi: 10.1186/s13098-020-00530-6
59. Sousa AP, Cunha DM, Franco C, Teixeira C, Gojon F, Baylina P, et al. Which role plays 2-hydroxybutyric acid on insulin resistance? Metabolites. (2021) 11:835. doi: 10.3390/metabo11120835
60. Syed Ikmal SI, Zaman Huri H, Vethakkan SR, and Wan Ahmad WA. Potential biomarkers of insulin resistance and atherosclerosis in type 2 diabetes mellitus patients with coronary artery disease. Int J Endocrinol. (2013) 2013:698567. doi: 10.1155/2013/698567
61. Prawitt J, Caron S, and Staels B. Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr Diabetes Rep. (2011) 11:160–6. doi: 10.1007/s11892-011-0187-x
62. Alberto González-Regueiro J, Moreno-Castañeda L, Uribe M, and Carlos Chávez-Tapia N. The role of bile acids in glucose metabolism and their relation with diabetes. Ann Hepatol. (2017) 16:S15–20. doi: 10.5604/01.3001.0010.5494
63. Feng J, Gong Z, Sun Z, Li J, Xu N, Thorne RF, et al. Microbiome and metabolic features of tissues and feces reveal diagnostic biomarkers for colorectal cancer. Front Microbiol. (2023) 14:1034325. doi: 10.3389/fmicb.2023.1034325
64. Deng K, Xu JJ, Shen L, Zhao H, Gou W, Xu F, et al. Comparison of fecal and blood metabolome reveals inconsistent associations of the gut microbiota with cardiometabolic diseases. Nat Commun. (2023) 14:571. doi: 10.1038/s41467-023-36256-y
65. Vangipurapu J, Stancáková A, Smith U, Kuusisto J, and Laakso M. Nine amino acids are associated with decreased insulin secretion and elevated glucose levels in a 7.4-year follow-up study of 5,181 finnish men. Diabetes. (2019) 68:1353–8. doi: 10.2337/db18-1076
66. Yang J, Chi Y, Burkhardt BR, Guan Y, and Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev. (2010) 68:270–9. doi: 10.1111/j.1753-4887.2010.00282.x
67. Bishop CA, Machate T, Henning T, Henkel J, Püschel G, Weber D, et al. Detrimental effects of branched-chain amino acids in glucose tolerance can be attributed to valine induced glucotoxicity in skeletal muscle. Nutr Diabetes. (2022) 12:20. doi: 10.1038/s41387-022-00200-8
68. González-Domínguez R, González-Domínguez Á, Sayago A, and Fernández-Recamales Á. Recommendations and best practices for standardizing the pre-analytical processing of blood and urine samples in metabolomics. Metabolites. (2020) 10:229. doi: 10.3390/metabo10060229
69. Tang Q, Jin G, Wang G, Liu T, Liu X, Wang B, et al. Current sampling methods for gut microbiota: A call for more precise devices. Front Cell Infect Microbiol. (2020) 10:151. doi: 10.3389/fcimb.2020.00151
70. Abrams JJ, Ginsberg H, and Grundy SM. Metabolism of cholesterol and plasma triglycerides in nonketotic diabetes mellitus. Diabetes. (1982) 31:903–10. doi: 10.2337/diab.31.10.903
71. Bennion LJ and Grundy SM. Effects of diabetes mellitus on cholesterol metabolism in man. New Engl J Med. (1977) 296:1365–71. doi: 10.1056/NEJM197706162962401
72. Liao X, Liu B, Qu H, Zhang L, Lu Y, Xu Y, et al. A high level of circulating valine is a biomarker for type 2 diabetes and associated with the hypoglycemic effect of sitagliptin. Mediators Inflamm. (2019) 2019:8247019. doi: 10.1155/2019/8247019
73. Saoi M and Britz-McKibbin P. New advances in tissue metabolomics: A review. Metabolites. (2021) 11:672. doi: 10.3390/metabo11100672
74. Chen MX, Wang SY, Kuo CH, and Tsai IL. Metabolome analysis for investigating host-gut microbiota interactions. J Formosan Med Assoc. (2019) 118:S10–22. doi: 10.1016/j.jfma.2018.09.007
Keywords: diabetes mellitus, metabolomics, systematic review, intestines, microbial metabolite
Citation: Gough KL, Dando SJ, Teasdale SL, Feigl B, Huygens F and Pelzer ES (2025) Gut-associated metabolites and diabetes pathology: a systematic review. Front. Endocrinol. 16:1559638. doi: 10.3389/fendo.2025.1559638
Received: 13 January 2025; Accepted: 01 May 2025;
Published: 21 May 2025.
Edited by:
Dinakaran Vasudevan, SKAN Research Trust, IndiaReviewed by:
Sylwia Dziegielewska-Gesiak, Medical University of Silesia, PolandYang Xiao, Mayo Clinic, United States
Copyright © 2025 Gough, Dando, Teasdale, Feigl, Huygens and Pelzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katelyn L. Gough, a2wuZ291Z2hAcXV0LmVkdS5hdQ==
 Katelyn L. Gough
Katelyn L. Gough Samantha J. Dando
Samantha J. Dando Stephanie L. Teasdale
Stephanie L. Teasdale Beatrix Feigl
Beatrix Feigl Flavia Huygens
Flavia Huygens Elise S. Pelzer
Elise S. Pelzer