- 1Department of Internal Medicine, First Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin, China
- 2Graduate School of Heilongjiang University of Chinese Medicine, Harbin, China
- 3Department of Internal Medicine, Fourth Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin, China
- 4Department of Gynecology, The First Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, China
Menopause is an age-related loss of ovarian function. As a woman enters menopause, the estrogen produced by her ovaries decreases, which will adversely affect women’s health. The symptoms related to menopause are related to the imbalance of gut microbiota. Studies have shown that the diversity of gut microbiota after menopause is lower than that before menopause, and the weakening of microbial decomposition will lead to the decrease of circulating estrogen, gradually resulting in disorders of lipid metabolism, cognitive decline, osteoporosis and other diseases. Gut microbiota play a key role in regulating estrogen levels. By secreting β-glucuronidase, it increases the reabsorption of estrogen in the enterohepatic circulation and mediates phytoestrogen metabolism, regulates estrogen homeostasis in the host and affects disease development and prognosis. Therefore, the gut microbiota is an overall regulator of women’s estrogen status during menopause and an untapped new area for improving women’s postmenopausal health. Changing the gut microbiota through specific prebiotics, probiotics, etc., and then affecting estrogen levels provides exciting opportunities for future therapeutic applications.
1 Introduction
Menopause is a special stage in a woman’s life when she transitions from childbearing to old age (1). During this period, as ovarian function gradually declines, estrogen levels fluctuate and eventually decline, leading to a series of physiological and psychological changes (2), and some symptoms that affect the quality of daily life (3). More seriously, menopause is also a risk factor for a number of diseases, such as metabolic diseases, cardiovascular diseases, osteoporosis, anxiety, depression, dementia and even cancer, which begin to appear and develop during menopause (4, 5). With the large number of menopausal women around the world, improving the quality of life of menopausal women and the prevention and treatment of menopause-related symptoms requires more attention. Hormone therapy (HT) has been widely used in the past and is an effective method to relieve menopausal symptoms (6). However, the clinical use of HT has become controversial with some experimental studies showing an increase in cardiovascular risk with combined estrogen-progestin use (7). Therefore, the choice of clinical treatment must be individualized, assessing patient risks and benefits (8). In addition to hormone replacement therapy, there are also non-hormonal drugs that can be used to relieve menopausal symptoms (9). More clinical research is needed to develop other new therapies to minimize future health risks for menopausal women.
The gut microbiota is closely related to women’s health. Together with the host, the gut microbiota forms a large, complex and dynamically changing micro-ecosystem that influences many physiological functions of the host by regulating the host’s immune response, maintaining the intestinal barrier function and resisting the invasion of pathogens (10–12). The gut microbiota is also affected by menopause. A meta-analysis systematically identified differences in the gut flora of premenopausal and postmenopausal women (13). A metagenome-wide association study showed that Firmicutes and Roseburia spp. are depleted, while Bacteroidetes and the toluene-producing genus Tolumonas are overrepresented in fecal samples from postmenopausal women (14). During the perimenopausal period, the relative abundance of beneficial bacteria such as Lactobacillus and Bifidobacteria is markedly reduced while that of harmful bacteria such as Enterobacter is increased in women (15). A large-scale survey of menopause and the gut microbiome suggests that the diversity of the gut microbiome is lower after menopause (16).
The effects of menopause on the gut microbiota are associated with a decrease in estrogen, and there is growing evidence that the gut microbiota and estrogen are bi-directionally regulated, with the gut microbiota being influenced by estrogen, and in turn, the gut microbiota significantly influencing estrogen levels (17). Studies have shown that estrogen supplementation during menopause slows the progression of atherosclerosis and corrects lipid metabolism disorders by regulating the abundance of gut microbiota (18). The gut microbiota regulates estrogen by secreting β-glucuronidase and participates in the metabolism of estrogen in the blood (14). Therefore, gut microbiota may be a therapeutic target for reducing risk in menopausal women.
The aim of this article is to elucidate the interactions that exist between menopause-estrogen-gut microbiota, to recognize the potential benefits of gut microbiota on menopause, and to identify and develop modulators targeting the gut microbiota to modulate estrogen, which may be useful in the treatment and prevention of menopause-related diseases and have a significant role in alleviating symptoms, improving quality of life and reducing mortality in menopausal women.
2 Decreased estrogen levels cause symptoms associated with menopause
For menopausal women, many symptoms and signs related to menopause are caused by a lack of estrogen production, which brings a series of physiological and psychological challenges. Estrogen is an important sex hormone, mainly synthesized from cholesterol as the matrix, approximately 90% secreted by the ovaries, and a small amount produced by the adrenal gland and adipose tissue. 17-βestradiol (E2) is the main estrogen in the body and the most biologically active. Estrogen mainly exerts related biological effects by binding to estrogen receptor (ER) and plays a key role in regulating many physiological processes in the human body (Figure 1) (19). During a woman’s reproductive years, the average level of total estrogen is 100–250 pg/mL, however, circulating concentrations of E2 decline to 10 pg/mL after menopause (20). Therefore, estrogen reduction and gut microbiota imbalance interact, leading to the occurrence and progression of many diseases during menopause.
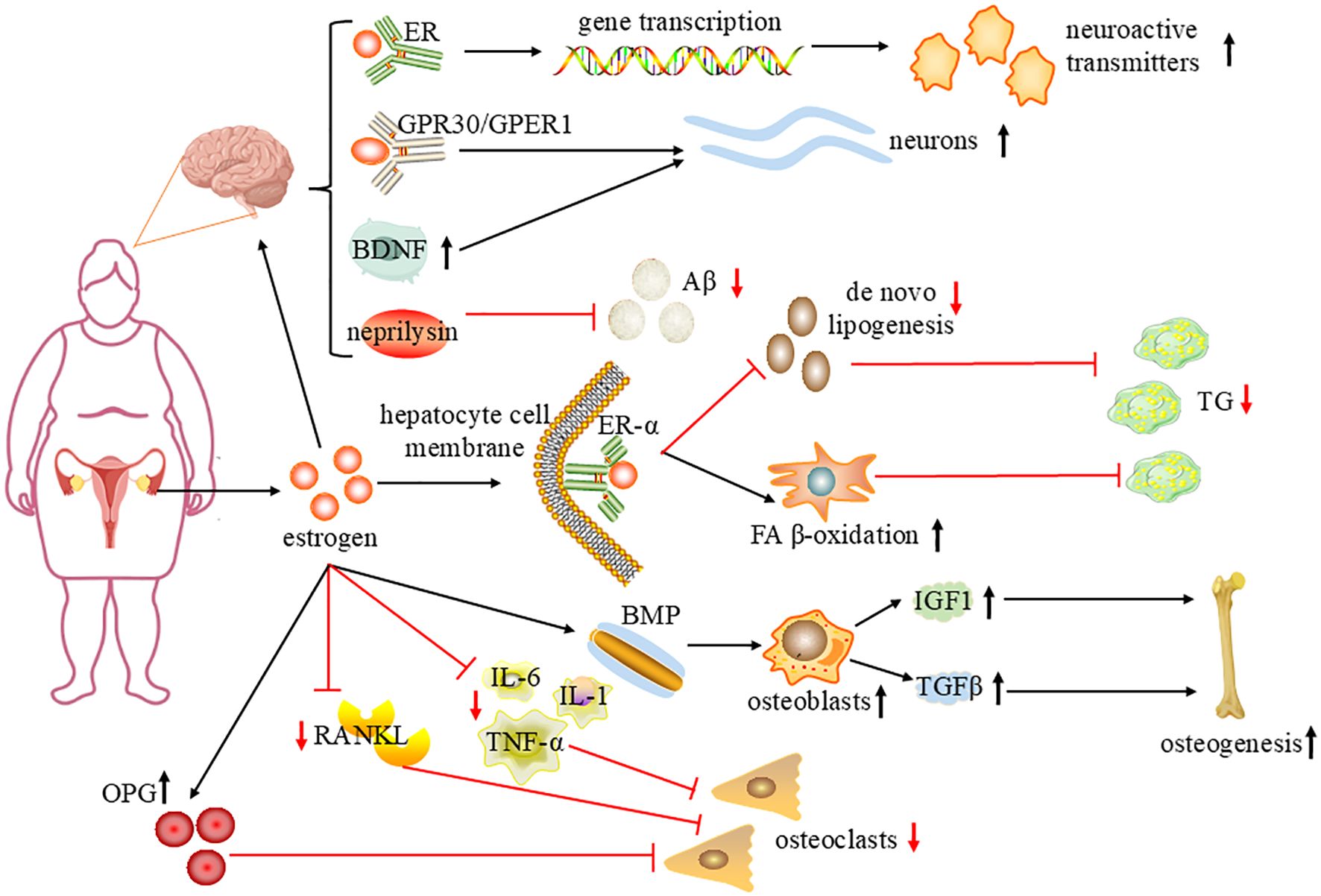
Figure 1. The mechanism of estrogen improving brain function, regulating lipid metabolism and alleviating osteoporosis. Estrogen is mainly secreted by the ovaries, In hepatocytes, estrogen binds to ERα, increasing FAβ-oxidation in mitochondria, reducing de novo lipogenesis, and reducing liver TG accumulation. Estrogen regulates gene transcription by interacting with nuclear receptors; activates different intracellular signal cascades through GPR30/GPER1 and regulates BDNF expression, protecting neurons; and regulates Neprilysin to degrade beta amyloid peptide (Aβ) and improve cognitive performance. Estrogen upregulates the bone morphogenetic protein (BMP) signaling pathway, stimulates osteoblasts to produce insulin-like growth factor I (IGF1) and transforming growth factor-β(TGFβ), promotes bone formation and remodeling; inhibits the expression of RANKL and IL-1, IL-6, TNF-α, promotes osteoprotegerin (OPG), thereby inhibiting the formation of osteoclasts. sharp arrows(→),stimulate; blunt arrows(⊥),inhibit; ↑, increase; ↓, decrease.
2.1 Lipid metabolism disorder
Lipid metabolism disorder refers to the abnormal increase or decrease of plasma lipids caused by various reasons and is a common pathological manifestation of menopausal women (21). It may induce stroke, coronary heart disease and other diseases, and is an important risk factor for cardiovascular disease, which seriously affects the health of menopausal women (22).
Ovariectomy was found to lead to elevated serum Low density lipoprotein cholesterol (LDL-C), excessive storage of glycogen and lipids in hepatocytes and alteration of the gut microbiota in female mice, demonstrating the important role of estrogens in the maintenance of glycolipid metabolism homeostasis (23). The liver is a central organ in the regulation of lipid and glucose metabolism, and ERα is the major receptor subtype in the liver. In hepatocytes, estrogen binds to ERα, increasing FA β-oxidation in the mitochondria and decreasing de novo lipogenesis, thereby reducing hepatic triglyceride(TG) accumulation (24). Estrogen has an important protective effect on adipose tissue distribution and fat metabolism. The decrease of estrogen level in women during menopause causes changes in the expression of key enzymes and genes in the process of lipid synthesis and decomposition, resulting in the increase of TG and LDL-C in serum and the decrease of high-density lipoprotein cholesterol (25, 26). In addition, it can also cause fat redistribution, which in turn leads to the rapid accumulation of visceral fat, forming central obesity (27). With the increase of visceral fat content, free fatty acids will also increase due to the excessive breakdown of fat, which leads to insulin resistance, triggering metabolic diseases (28). It has been demonstrated that estrogen deficiency after ovariectomy leads to reduced lipolysis and increased non-esterified fatty acid in white adipose tissue and brown adipose tissue, resulting in reduced fatty acid oxidation in the liver of ovariectomized(OVX) rats, which in turn leads to hepatic steatosis (29). The results of a cell-based study suggest that E2 directly activates SREBP2 gene expression, leading to excessive cholesterol accumulation and increased risk of cardiovascular disease (30).
Therefore, menopause is therefore a critical period during which alterations in lipid metabolism should be understood in order to reduce the risk of associated diseases caused by dyslipidemia.
2.2 Cognition impairment
Cognition impairment increases as menopause progresses, and the vast majority of women with dementia are postmenopausal (31). A study has confirmed that menopause impairs cerebrovascular function, which in turn leads to more widespread cognitive impairment in a mouse model of vascular contributions to cognitive impairment and dementia (32). Results of a cross-sectional study of older women in rural northern China show that earlier menopause is associated with poor cognitive performance and significantly increases the risk of mild cognitive impairment and dementia, particularly Alzheimer’s disease (AD), dementia with Lewy bodies and vascular dementia (33).
Cognitive decline is a consequence of reduced estrogen (34). J. Hu et al. found that higher blood levels of E2 were associated with lower rates of cognitive impairment in southeastern China (35). Other studies found that E2 levels and the expression of ERα, ERβ and GPER in OVX mice were significantly reduced, and there was a significant correlation with dyslipidemia and cognitive impairment, E2 supplementation or lipid lowering is an effective method to ameliorate postmenopausal hyperlipidemia induced hippocampal damage and cognitive impairment by upregulating ERs (36). Song X et al. study finds earlier use of oral contraceptives and hormone replacement therapy at menopause is associated with reduced risk of cognitive impairment (37).
Estrogen has neuroprotective effects, ranging from classical nuclear to non-classical membrane-mediated actions, and it affects the brain through complex cellular mechanisms. In the classic mechanism, estrogen regulates gene transcription by interacting with nuclear receptors, regulating the synthesis, release and metabolism of many neuroactive transmitters and the expression of their receptors. The nonclassical estrogen action is probably mediated by receptors integrated or associated with the cell membrane and by the activation of distinct intracellular signaling cascades through the high-affinity membrane-associated G protein-coupled estrogen receptor GPR30/GPER1, protecting neurons from excitotoxins and free radicals (38, 39). Estrogen can improve mitochondrial function (40). It has been shown that chronic ovariectomy reduces oxygen consumption, ATP production rates and mitochondrial membrane potential in NADH-associated respiration in Wistar adult female rats’hippocampal mitochondria (41). High levels of β-amyloid peptide (Aβ) in brain tissue are a risk factor for AD.17β-estradiol promotes Aβ degradation by regulating the expression of neprilysin, which may be a key factor in improving cognitive performance in menopausal women (42). Estrogen improves cerebral blood flow and provides beneficial clinical effects in cognitive functioning (43).
Overall, estrogen has been a major focus in the field of hormonal cognition as it prevents cognitive impairment by modulating neurotransmitters, protecting mitochondrial function, reducing Aβ formation, and increasing cerebral blood flow.
2.3 Emotional disorder
More and more research shows that menopausal women can be associated with emotional problems, such as anxiety and depression (44). A cross-sectional assessment of depression and anxiety in perimenopausal and menopausal women showed that 21.9% of women had moderate anxiety and 24.76% were clinically depressed (45). Women with emotion regulation disorders such as anxiety, depression and stress are more likely to develop severe menopausal symptoms that affect quality of life (46).
Estrogen has a significant effect on emotional disorders (47). Estrogen has the ability to modulate the release of neurotransmitters such as 5-hydroxytryptamine, noradrenaline, and dopamine, which is a key mechanism for antidepressant effects (48). brain-derived neurotrophic factor (BDNF) is a neurotrophic factor that is essential for maintaining brain function, there is a strong association between reduced levels of BDNF and the development of depression, and estrogen regulates BDNF expression, which promotes neurogenesis, synaptic plasticity, and neuronal survival and contributes to emotion improvement (49). Estrogen can also affect depressive behavior by regulating the disturbance of gut microbiota. Enterococcus in the stool of patients with Major depressive disorder (MDD) were significantly increased compared with that of healthy control group, which is related to pro-inflammation (50). Pathophysiological processes such as inflammation and immune activation, oxidative stress, and neurotransmitter synthesis in emotional disorders can be altered by regulating the gut microbiota (51). In addition, immune imbalance caused by declining estrogen levels during menopause is a new focus of research on menopausal depression, estrogen deficiency disrupts immune homeostasis through the ERα/ERβ/Gper-associated NLRP3/NF-κ B signaling pathway, leading to elevated levels of inflammatory cytokines, resulting in disruption of the blood-brain barrier, neurotransmitter dysfunction, impaired BDNF synthesis, and reduced neuroplasticity (52). Estrogen inhibits the production and release of inflammatory molecules in the body and protects nerve cell integrity by reducing inflammatory responses (47).
Estrogen regulates emotion by promoting the release of neurotransmitters, upregulating BDNF expression, maintaining the balance of the gut microbiota, and suppressing inflammatory factors. However, more clinical research is needed to clarify the best way to use estrogen therapy to relieve menopausal emotional disorders.
2.4 Osteoporosis
Recent research has increasingly emphasized the importance of the gut microbiota in maintaining bone homeostasis (53). A study on the gut microbiota of postmenopausal women with osteoporosis in Shanghai, China, showed that the content of four bacteria genera, Roseburia, Clostridia_UCG.014, Agathsium and Dialister, were lower in the osteoporosis group than in the normal control group. They are all microorganisms with potential anti-osteoporosis properties (54). Osteoporosis (OP) is a systemic bone disease characterized by low bone mineral density (BMD) and deterioration of bone structure, leading to reduced bone strength and thus increased susceptibility to fracture (55). OP is more common in postmenopausal women, and menopausal estrogen deficiency is a major risk factor for postmenopausal osteoporosis (56). The cellular component of bone includes osteocytes, osteoblasts, and osteoclasts, each playing essential roles in bone integrity and remodeling (57). Decreased estrogen results in increased osteoclast differentiation and activation, accelerated bone resorption over the rate of formation and rapid bone loss, making bone brittle and prone to fracture (58).
Estrogen regulates the bone morphogenetic protein (BMP) signaling pathway, which is crucial for osteoblast differentiation and bone formation. In addition, estrogen stimulates osteoblasts to produce insulin-like growth factor I (IGF1) and transforming growth factor-β (TGF-β), further promoting bone formation and remodeling (59). Estrogen inhibits the expression of the receptor activator of NF-kB ligand (RANKL), a key factor in the activation of osteoclasts, and promotes osteoprotegerin (OPG) production. OPG acts as a decoy receptor for RANKL and directly inhibits osteoclast formation (60). In addition, estrogen inhibits cytokines such as interleukin-1(IL-1), interleukin-6(IL-6), and tumor necrosis factor-α(TNF-α), which indirectly inhibits osteoclast differentiation (61). Estrogen deficiency increases apoptosis of osteoblasts and also alters the mechanical responsiveness of MLO-Y4 osteoblasts, ultimately reducing osteoblast differentiation and function (62).
Overall, estrogen is a key factor in maintaining the health of menopausal women, and estrogen deficiency can lead to the development of a variety of menopause-related diseases. How to regulate hormonal changes during menopause and provide the body with the necessary hormones is a major area of research to alleviate menopausal diseases. Recent research suggests that the gut microbiota may play a key role in this.
3 Gut microbe-estrogen axis: gut microbiota regulates estrogen levels
The relationship between estrogen and gut microbes is an expanding area of research that may lead to new therapeutic options for a variety of conditions associated with estrogen deficiency during menopause. A growing body of research suggests that the relationship between estrogen and the gut microbiota is bidirectional (63).
The intestinal protective effect of estrogen reduces intestinal permeability by up-regulating the expression of tight junction proteins (64). Inhibit the NF-κ B pathway, reduce pro-inflammatory cytokines, reduce intestinal inflammation, promote the abundance of beneficial bacteria, and maintain intestinal homeostasis (65). Estrogen levels can affect the diversity of gut microbiota, it has been shown that the lack of ER β induces a dysregulation of the intestinal ecology, leading to anxiety and depression-like behaviors in mice (66). The abundance of Aggregatibacter segnis, Bifidobacterium animalis and Acinetobacter guillouiae, which are associated with sex hormone levels, were all found to be decreased in the gut microbes of patients with menopausal syndrome (67). The experimental results showed that supplementing a small amount Low dose brain estrogen can maintain the diversity of intestinal microorganisms in estrogen-deficient rats (68).
Gut microbiota participate in the estrogen metabolic cycle through β-glucuronidase and can also convert phytoestrogens into estrogen analogues to regulate estrogen levels (Figure 2). See the following text for details.
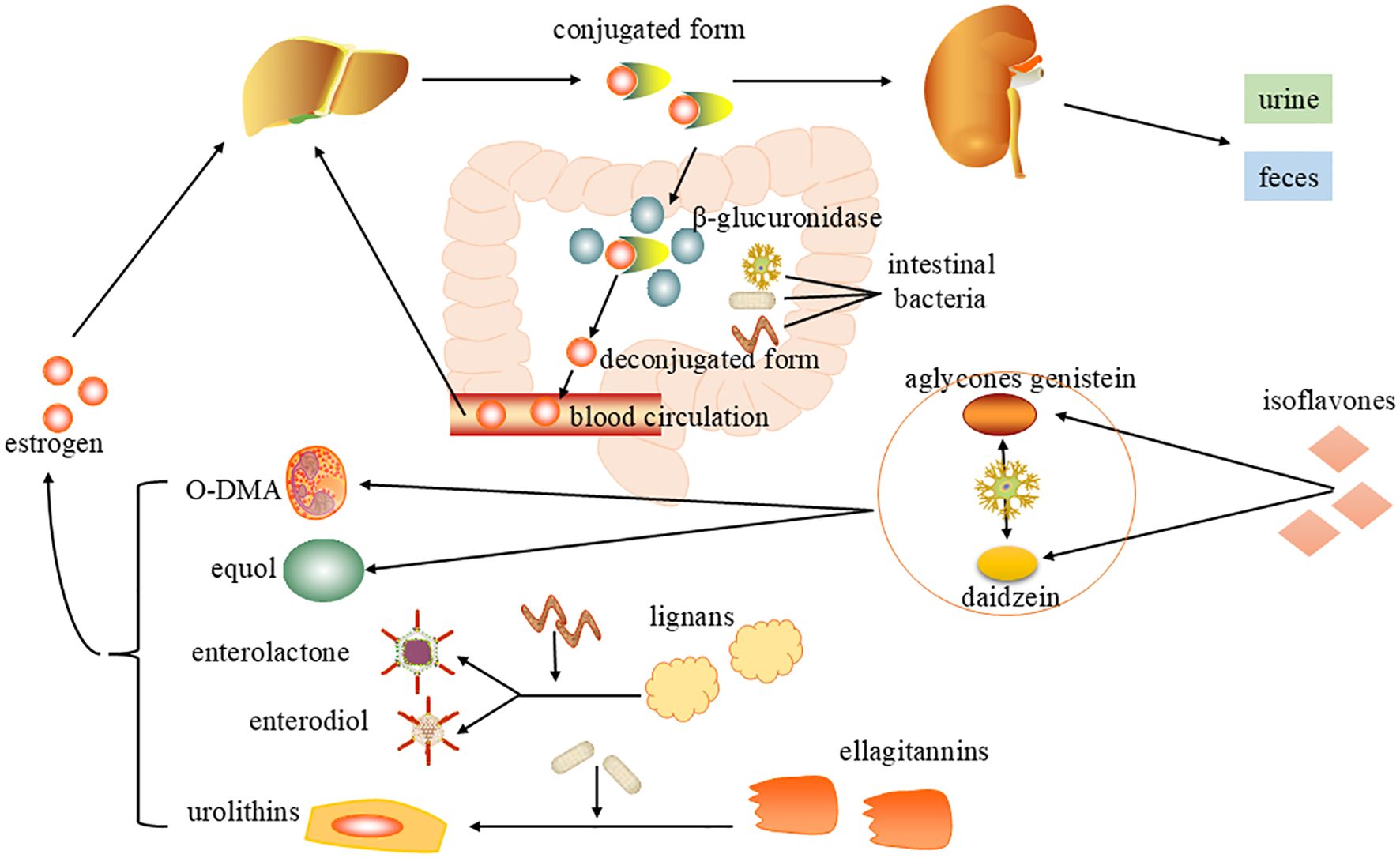
Figure 2. Mechanisms of gut microbiota regulation of estrogen levels. After metabolism by the liver, estrogen enters the intestine in bile as an inactive conjugated form and is metabolized by β-glucuronidase (gmGUS) secreted by intestinal microorganisms to an active deconjugated form, which is reabsorbed through the intestinal mucosa into the blood circulation and returned to the liver. Gut microbes can also convert phytoestrogens into estrogen analogues. Through bacterial metabolism, isoflavones can be transformed into equol and O-desmethylangolensin (O-DMA). Lignans are converted into enterolactone(ENL) and enterdiol(END). Ellagitannins transform a series of compounds called urolithins, and the bioavailability and biological activity of these microbial-derived compounds have both been enhanced.
3.1 Gut microbiota increases estrogen reabsorption in enterohepatic circulation
Circulating estrogens are highly regulated by symbiotic bacterial activity, the human gut microbiota regulates estrogen metabolism through the “estrobolome” that is a collection of bacterial genes that encode enzymes like β-glucuronidases and β-glucosidases. These enzymes increase the reabsorption of active free estrogens into the bloodstream in the enterohepatic circulation, affecting circulating levels and are important mediators of gut microbiota-host interactions (69). Estrogens are metabolized mainly in the liver, forming biologically inactive conjugated form that are excreted in the bile and eventually enter the intestine, where they are partly excreted in the feces and urine. The enzyme β-glucuronidase (gm GUS) secreted by gut microbiota metabolizes estrogen from its conjugated form to its unconjugated form, restores its activity, and is reabsorbed through the intestinal mucosa into the blood circulation and back to the liver, a process known as the enterohepatic circulation of estrogen (70). Both the increased number of bacteria with “estrobolome” and the increased activity of these gene-encoding enzymes can accelerate the early dissociation and hydroxylation of estrogens in the intestine so that the free estrogens could increase significantly in enterohepatic circulation and maintain at a physiological level (71). Conversely, if the gut microbiota is imbalanced and microbial diversity is reduced, β-glucuronidase activity is reduced and the enterohepatic circulation is compromised, leading to a reduction in circulating estrogens (72). A randomized controlled trial demonstrated that supplementation with a probiotic formula with β-glucuronidase activity regulated serum estrogen levels in healthy postmenopausal women compared to a placebo group, setting the stage for future use of probiotics in the postmenopausal population (73).
3.2 Conversion of phytoestrogens to estrogen analogues by gut microbes
Phytoestrogens are a class of plant produced polyphenolic compounds with diphenolic structure. Its structure is similar to that of the major estrogen 17-βestradiol, which binds to the estrogen receptor and has estrogen agonist and estrogen antagonist effects (74). When estrogen levels decrease, these plants increase, offering a gentle boost to maintain hormonal harmony. In situations with high estrogen concentrations, phytoestrogens work by blocking stronger estrogens from binding to receptors and keeping things in check. Common sources of phytoestrogens include soybeans, flaxseed, grains, fruits, and vegetables, which are synthesized through the phenylpropanoid pathway and subsequently transformed into diverse chemical structures through specific enzymes (75). Phytoestrogens are classified into seven groups: Isoflavones, flavones, flavanones, chalcones, coumestanes, lignanes and stilbenes. Among them, isoflavones, lignans, and coumestans are the main bioactive types (76).
Phytoestrogens have been shown to have a variety of health benefits for humans such as antioxidants, neuroprotection, immune system enhancement, cardiovascular protection and more (77). A study of older adults in southern Italy found that higher intake of phytoestrogens, particularly isoflavones, was associated with better cognitive performance (78). Phytoestrogens can also improve bone density, alleviate menopausal vascular relaxation symptoms, and have a certain therapeutic effect on menopausal related diseases (79). Despite the various health benefits, the bioavailability of phytoestrogens in the human body is low, as most of the ingested phytoestrogens are not absorbed in the small intestine, and the effects of phytoestrogens on organisms are largely mediated by the gut microbiota, which are metabolized by gut bacteria to modulate phytoestrogen activity and increase their bioavailability (80).
Plant isoflavones are the most famous of all phytoestrogens and have attracted attention due to their health properties, isoflavones are transformed in first place into the aglycones genistein and daidzein. Daidzein can be metabolized by a few bacteria like Adlercreutzia equolifaciens, Eggerthella sp. YY7918, Lactococcus garvieae, Slackia equolifaciens, Slackia isoflavoniconvertens, Slackia spp. into equol and O-desmethylangolensin (O-DMA) (81). Lignans are not easily absorbed by the intestine and must be metabolized into enterolactone(ENL) and enterdiol(END) by gut microbiota such as Clostridiumsaccharogumia, Eggerthella lenta and Blautia producta, which are called enterolignans, before they can enter the body and play their roles (82). Ellagitannins and its hydrolyzed product ellagic acid are difficult to be absorbed by the blood, gut bacteria gradually metabolize it by means of lactonering cleavage, decarboxylation and dehydroxylation reactions, which lead to the formation of a series of compounds named urolithins (83). These microbe-derived compounds have improved bioavailability and bioactivity with higher estrogenic/anti-estrogenic, antioxidant, anti-inflammatory and anti-tumor activities (84). However, it should be noted that phytoestrogens can also have some side effects, such as allergic reactions in some people, so moderate intake is crucial (85).
The interaction between gut microbiota and phytoestrogens is bidirectional. Phytoestrogens are metabolized by the gut microbiota on the one hand, and on the other hand, the metabolites formed regulate and reshape the gut microbial composition by altering the diversity and abundance of bacteria (86). In summary, the gut microbiota plays a very important role in determining the absorption, metabolism, distribution and excretion of ingested phytoestrogens and their metabolites (87).
There is growing evidence that the gut microbiota plays an important role in estrogen regulation, which may be an untapped new area of women’s health before and after menopause (73). Therefore, a better understanding of the changes in the gut microbiota during menopause and the development of specific probiotic strains to modulate the gut microbiota, which in turn affects estrogen levels, could be a potential therapeutic approach to counteracting the changes in estrogen during the menopausal transition and improving women’s health.
4 Clinical implications of gut microbiota modulation in menopause-associated disorders
The gut microbiota consists of trillions of complex and dynamic microorganisms, and the pathogenesis of many human diseases may be related to the ‘dysbiosis’ of the gut microbiota, which is manifested by a decrease in beneficial bacteria, an increase in harmful bacteria, and a loss of compositional and functional diversity (88). Therefore, gut microbes have been considered as possible therapeutic targets to address menopause-related diseases. Probiotic supplementation may be a viable and safe strategy for the treatment of menopause-related disorders. In particular, oral probiotic formulations—especially those including Lactobacillus ssp. casei, helveticus, rhamnosus and reuteri, may have multiple beneficial effects on health (89). Besides, dietary interventions, fecal microbiota transplantation (FMT), exercise or drugs, have been progressively applied in clinical or preclinical studies (90).
4.1 Probiotic
4.1.1 Improve estrogen activity and adjust lipid metabolism disorder
Probiotics multiply the gut and produce beneficial gut bacterial metabolites such as short-chain fatty acids (SCFAs), restoring the normal functional activity of the gut microbiota, improving glycolipid metabolism and increasing serum 17β-estradiol concentrations (89). 17β-estradiol can be activated by PI3K/AKT signaling mediated by ERβ and GPR30 to alleviate postmenopausal dyslipidemia (91), and also upregulates the ERα/SIRT1/PGC-1α signaling pathway, protecting mitochondrial function and preventing lipoatrophy (92). In one study, supplementation with the probiotic B. longum 15M1, as well as a combination of Lactobacillus plantarum 30M5 and a diet of soy isoflavones (SIFs), both alleviated lipid metabolism disorders during menopause (93). It has been demonstrated that butyric acid supplementation restores PPARα activity in high fat diet fed rats, which enhances fatty acid β-oxidation, inhibits lipid synthesis, and down-regulates nuclear factor κB pathways and inflammation, alleviating hepatic steatosis (94).
4.1.2 Regulating neurotransmitters to alleviate emotional disorders
The study showed that the degradation of estradiol by gut microbes containing 3β-hydroxysteroid leads to a decrease in serum estradiol levels, which leads to depression in female mice, revealing that gut microbes may be a new intervention target for depression (95). Bifidobacterium and Lactobacillus have been shown to improve emotion disorders such as anxiety, depression and stress in animal studies (96). Increased levels of glutamate and N-acetyl aspartate in the brains of mice treated with L. rhamnosus JB-1 provide direct evidence that probiotic treatment modulates neurotransmitter concentrations to influence brain activity (97). Oral administration of a probiotic formulation, consisting of L. helveticus R0052 and B. longum R0175, over 30 days was shown to improve mood in generally healthy volunteers (96). Lactobacillus Calmette-Guerin (CP2305) is a paraprobiotic that has been shown to improve psychological symptoms specific to menopausal women in a controlled clinical trial (98).
4.1.3 Increase beneficial gut bacteria and inhibit bone loss
The regulation of bone metabolism by probiotics and prebiotics is gradually becoming a hot research topic. Prevotella histicola prevents estrogen deficiency-induced bone loss via the gut-bone axis in postmenopausal women and OVX mice, promising as a therapeutic target for osteoporosis treatment (99).Prebiotics are the food components that are fermented by gut microbiota, resistant to gastric acid and hydrolytic enzymes, but not digested and absorbed by the intestine, and prebiotics can also selectively modulate the activity of one or more beneficial gut microbiota to hos (100).Inulin-type prebiotics can increase the number of beneficial bacteria in the intestine and promote the release of organic acids, thus reducing the pH value of intestine, promoting the absorption of minerals, and inhibiting the bone loss (101).
4.2 Herbal extracts
Some herbal extracts can also act on the gut microbiota, further exerting pharmacological effects. Radix angelica dahuricae (RAD) is a well-known traditional Chinese medicine that can attenuate estrogen deficiency-induced dyslipidemia, improve TC and TG levels, and reduce hepatic TNF-α, IL-6 and IL-1β gene expression in OVX rats by modulating the composition of the gut microbiota and bile acid signal (102). Trifolium pratense ethanolic extract (TPEE) improves gut microbiota composition in OVX rats, mainly including Firmicutes and Bacteroidetes. The abundance of Bacteroidetes in the gut helps maintain healthy blood cholesterol levels. TPEE also increases the abundance of Lactococcus sp. and converts biochanin A and formononetin into equol, thereby enhancing estrogen activity. TPEE-treated OVX rats showed significant reduction in TG and LDL levels (103).
However, it should be noted that the effectiveness of these extracts is mostly reflected in animal experiments, the lack of human studies to further verify the differences between animals and humans in the physiological structure and metabolic pathways, etc. In the future, more clinical trials are needed to validate the effectiveness and safety of these extracts on the human body before they can be further applied to clinical treatment.
4.3 Adjust exercise and diet patterns
Exercise has the ability to alter the composition and function of the gut microbiota. Studies in animal models suggest that wheel running exercise training enhances gut microbial abundance and has multiple beneficial effects on the gut microbiota, and may be a promising strategy for the treatment of cognitive impairment in menopause (104).
Adjusting dietary patterns is a relatively effective and healthy option for intervening in the gut microbiota and has significant modulatory effects on bone metabolism (105).Higher dietary protein intake increases the abundance and diversity of the gut microbiota, whereas fat-rich diets may promote bile secretion, have a detrimental effect on bacterial cell membranes, and play a negative role in the metabolic regulation of osteoporosis (106). Therefore, dietary intervention may become a more economical, effective and simple way to reduce side effects in the future.
4.4 Other intervention measures
The phytoestrogen Secoisolariciresinol diglucoside (SDG) promotes the production of the gut microbial metabolites END and ENL, inhibits cerebral Aβ deposition, activates GPER to enhance the CREB/BDNF signaling pathway and suppresses the neuroinflammatory response to ameliorate cognitive deficits (107). Supplementation with dimethyl itaconate (DI) improved changes in the gut microbiota of mice fed a high-fat diet, increasing the abundance of bacteria that produce propionic acid and butyric acid, thereby improving cognitive function (108).
Fecal microbiota transplantation (FMT) has been used exploratively in osteoporosis control studies in recent years and is a promising therapeutic option for osteoporosis (109). The results showed that FMT lasting 8 weeks prevented OVX-induced bone loss by optimizing the composition and abundance of gut microbiota, increasing SCFA levels, and suppressing the release of pro-osteoclastogenic cytokines (110).
In summary, The role of the gut microbiota is not limited to the gut, but extends even to the liver through the gut-liver axis to regulate metabolism (111), to the central nervous system through the gut-brain axis (112), and to the bone metabolism and maintenance of bone health through the gut-bone axis (113). Therefore, interventions such as ingestion of probiotics and prebiotics, adjustment of diet and exercise patterns, and FMT can improve the composition and abundance of gut microbiota and related metabolites to varying degrees, which may provide new ideas for the prevention and treatment of menopausal diseases.
5 Conclusion
There is growing evidence that gut microbiota and estrogen have complex bidirectional effects that have significant implications for menopausal women’s health. The decrease of estrogen level in menopause will affect the composition and function of gut microbes, and lead to lipid metabolism disorders, cognitive disorders, emotional disorders and osteoporosis, which seriously affect the quality of life. Gut microbiota also has a regulatory effect on estrogen levels, and this paper takes the intervention of gut microbiota as an entry point, and summarizes a variety of methods that can regulate the gut microbiota in the clinic, including supplementation of probiotics and prebiotics, herbal extracts, dietary interventions, and FMT, which have demonstrated satisfactory results in alleviating symptoms associated with menopause. Research on the gut microbiota provides new insights and treatments to improve the health of menopausal women, and has the potential to bring new benefits to postmenopausal women’s health.
Author contributions
HW: Writing – original draft. FS: Writing – review & editing. LZ: Writing – original draft. WZ: Writing – review & editing. BM: Writing – review & editing. SW: Writing – review & editing. XF: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82174421, 81973894), Project of the Administration of Traditional Chinese Medicine of Heilongjiang Province (ZHY2023-125).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gatenby C and Simpson P. Menopause: Physiology, definitions, and symptoms. Best Pract Res Clin Endocrinol Metab. (2024) 38:101855. doi: 10.1016/j.beem.2023.101855
2. Davis SR, Pinkerton J, Santoro N, and Simoncini T. Menopause—Biology, consequences, supportive care, and therapeutic options. Cell. (2023) 186:4038–58. doi: 10.1016/j.cell.2023.08.016
3. Lobo RA and Gompel A. Management of menopause: a view towards prevention. Lancet Diabetes Endocrinol. (2022) 10:457–70. doi: 10.1016/S2213-8587(21)00269-2
4. Mishra GD, Davies MC, Hillman S, Chung H-F, Roy S, Maclaran K, et al. Optimising health after early menopause. Lancet. (2024) 403:958–68. doi: 10.1016/S0140-6736(23)02800-3
5. Hickey M, LaCroix AZ, Doust J, Mishra GD, Sivakami M, Garlick D, et al. An empowerment model for managing menopause. Lancet. (2024) 403:947–57. doi: 10.1016/S0140-6736(23)02799-X
6. Crandall CJ, Mehta JM, and Manson JE. Management of menopausal symptoms: A review. JAMA. (2023) 329:405–20. doi: 10.1001/jama.2022.24140
7. Cho L, Kaunitz AM, Faubion SS, Hayes SN, Lau ES, Pristera N, et al. Rethinking menopausal hormone therapy: for whom, what, when, and how long? Circulation. (2023) 147:597–610. doi: 10.1161/CIRCULATIONAHA.122.061559
8. Flores VA, Pal L, and Manson JE. Hormone therapy in menopause: concepts, controversies, and approach to treatment. Endocrine Rev. (2021) 42:720–52. doi: 10.1210/endrev/bnab011
9. Madsen TE, Sobel T, Negash S, Shrout Allen T, Stefanick ML, Manson JE, et al. A review of hormone and non-hormonal therapy options for the treatment of menopause. Int J Women’s Health. (2023) 15:825–36. doi: 10.2147/IJWH.S379808
10. Fassarella M, Blaak EE, Penders J, Nauta A, Smidt H, and Zoetendal EG. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut. (2021) 70:595–605. doi: 10.1136/gutjnl-2020-321747
11. Yu LW, Agirman G, and Hsiao EY. The gut microbiome as a regulator of the neuroimmune landscape. Annu Rev Immunol. (2022) 40:143–67. doi: 10.1146/annurev-immunol-101320-014237
12. Zhang Y, Wang H, Sang Y, Liu M, Wang Q, Yang H, et al. Gut microbiota in health and disease: advances and future prospects. MedComm. (2024) 5:e70012. doi: 10.1002/mco2.70012
13. Yang M, Wen S, Zhang J, Peng J, Shen X, Xu L, et al. Systematic Review and Meta-analysis: Changes of Gut Microbiota before and after Menopause. Dis Markers. (2022) 2022:1–10. doi: 10.1155/2022/3767373
14. Zhao H, Chen J, Li X, Sun Q, Qin P, and Wang Q. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota. FEBS Letters. (2019) 593:2655–64. doi: 10.1002/1873-3468.13527
15. Xie X, Song J, Wu Y, Li M, Guo W, Li S, et al. Study on gut microbiota and metabolomics in postmenopausal women. BMC Women’s Health. (2024) 24:608. doi: 10.1186/s12905-024-03448-7
16. Peters BA, Lin J, Qi Q, Usyk M, Isasi CR, Mossavar-Rahmani Y, et al. Menopause is associated with an altered gut microbiome and estrobolome, with implications for adverse cardiometabolic risk in the hispanic community health study/study of latinos. mSystems. (2022) 7:e0027322. doi: 10.1128/msystems.00273-22
17. Santos-Marcos JA, Rangel-Zuñiga OA, Jimenez-Lucena R, Quintana-Navarro GM, Garcia-Carpintero S, Malagon MM, et al. Influence of gender and menopausal status on gut microbiota. Maturitas. (2018) 116:43–53. doi: 10.1016/j.maturitas.2018.07.008
18. Meng Q, Ma M, Zhang W, Bi Y, Cheng P, Yu X, et al. The gut microbiota during the progression of atherosclerosis in the perimenopausal period shows specific compositional changes and significant correlations with circulating lipid metabolites. Gut Microbes. (2021) 13:1–27. doi: 10.1080/19490976.2021.1880220
19. Mahboobifard F, Pourgholami MH, Jorjani M, Dargahi L, Amiri M, Sadeghi S, et al. Estrogen as a key regulator of energy homeostasis and metabolic health. Biomedicine Pharmacotherapy. (2022) 156:113808. doi: 10.1016/j.biopha.2022.113808
20. Cervellati C and Bergamini CM. Oxidative damage and the pathogenesis of menopause related disturbances and diseases. Clin Chem Lab Med. (2016) 54:739–53. doi: 10.1515/cclm-2015-0807
21. Torosyan N, Visrodia P, Torbati T, Minissian MB, and Shufelt CL. Dyslipidemia in midlife women: Approach and considerations during the menopausal transition. Maturitas. (2022) 166:14–20. doi: 10.1016/j.maturitas.2022.08.001
22. Conway-O’Donnell CK and Chesler NC. The stronger sex, until menopause: understanding the impact of estrogen loss on heart function. Am J Physiol Heart Circ Physiol. (2022) 323:H128–H9. doi: 10.1152/ajpheart.00270.2022
23. Lei Z, Wu H, Yang Y, Hu Q, Lei Y, Liu W, et al. Ovariectomy impaired hepatic glucose and lipid homeostasis and altered the gut microbiota in mice with different diets. Front Endocrinology. (2021) 12:708838. doi: 10.3389/fendo.2021.708838
24. Polyzos SA and Goulis DG. Menopause and metabolic dysfunction-associated steatotic liver disease. Maturitas. (2024) 186:108024. doi: 10.1016/j.maturitas.2024.108024
25. Fröhlich E and Wahl R. Insight into potential interactions of thyroid hormones, sex hormones and their stimulating hormones in the development of non-alcoholic fatty liver disease. Metabolites. (2022) 12:718. doi: 10.3390/metabo12080718
26. Ko S-H and Kim H-S. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients. (2020) 12:202. doi: 10.3390/nu12010202
27. Mao L, Wang L, Bennett S, Xu J, and Zou J. Effects of follicle-stimulating hormone on fat metabolism and cognitive impairment in women during menopause. Front Physiol. (2022) 13:1043237. doi: 10.3389/fphys.2022.1043237
28. Ko S-H and Jung Y. Energy metabolism changes and dysregulated lipid metabolism in postmenopausal women. Nutrients. (2021) 13:4556. doi: 10.3390/nu13124556
29. Malinská H, Hüttl M, Miklánková D, Trnovská J, Zapletalová I, Poruba M, et al. Ovariectomy-induced hepatic lipid and cytochrome P450 dysmetabolism precedes serum dyslipidemia. Int J Mol Sci. (2021) 22:4527. doi: 10.3390/ijms22094527
30. Meng Y and Zong L. Estrogen stimulates SREBP2 expression in hepatic cell lines via an estrogen response element in the SREBP2 promoter. Cell Mol Biol Letters. (2019) 24:65. doi: 10.1186/s11658-019-0194-5
31. Sochocka M, Karska J, Pszczołowska M, Ochnik M, Fułek M, Fułek K, et al. Cognitive decline in early and premature menopause. Int J Mol Sci. (2023) 24:6566. doi: 10.3390/ijms24076566
32. Gannon OJ, Naik JS, Riccio D, Mansour FM, Abi-Ghanem C, Salinero AE, et al. Menopause causes metabolic and cognitive impairments in a chronic cerebral hypoperfusion model of vascular contributions to cognitive impairment and dementia. Biol Sex Differences. (2023) 14:34. doi: 10.1186/s13293-023-00518-7
33. Xi H, Gan J, Liu S, Wang F, Chen Z, Wang X-D, et al. Reproductive factors and cognitive impairment in natural menopausal women: A cross-sectional study. Front Endocrinology. (2022) 13:893901. doi: 10.3389/fendo.2022.893901
34. Cipriano GL, Mazzon E, and Anchesi I. Estrogen receptors: A new frontier in Alzheimer’s disease therapy. Int J Mol Sci. (2024) 25:9077. doi: 10.3390/ijms25169077
35. Hu J, Chu K, Song Y, Chatooah ND, Ying Q, Ma L, et al. Higher level of circulating estradiol is associated with lower frequency of cognitive impairment in Southeast China. Gynecological Endocrinology. (2017) 33:840–4. doi: 10.1080/09513590.2017.1320379
36. Meng Q, Chao Y, Zhang S, Ding X, Feng H, Zhang C, et al. Attenuation of estrogen and its receptors in the post-menopausal stage exacerbates dyslipidemia and leads to cognitive impairment. Mol Brain. (2023) 16:80. doi: 10.1186/s13041-023-01068-0
37. Song X, Wu J, Zhou Y, Feng L, Yuan J-M, Pan A, et al. Reproductive and hormonal factors and risk of cognitive impairment among Singapore Chinese women. Am J Obstetrics Gynecology. (2020) 223:410.e1–.e23. doi: 10.1016/j.ajog.2020.02.032
38. Conde DM, Verdade RC, Valadares ALR, Mella LFB, Pedro AO, and Costa-Paiva L. Menopause and cognitive impairment: A narrative review of current knowledge. World J Psychiatry. (2021) 11:412–28. doi: 10.5498/wjp.v11.i8.412
39. Bustamante-Barrientos FA, Méndez-Ruette M, Ortloff A, Luz-Crawford P, Rivera FJ, Figueroa CD, et al. The impact of estrogen and estrogen-like molecules in neurogenesis and neurodegeneration: beneficial or harmful? Front Cell Neurosci. (2021) 15:636176. doi: 10.3389/fncel.2021.636176
40. Zhao W, Hou Y, Zhang Q, Yu H, Meng M, Zhang H, et al. Estrogen receptor β exerts neuroprotective effects by fine-tuning mitochondrial homeostasis through NRF1/PGC-1α. Neurochemistry Int. (2023) 171:105636. doi: 10.1016/j.neuint.2023.105636
41. Zárate S, Astiz M, Magnani N, Imsen M, Merino F, Álvarez S, et al. Hormone deprivation alters mitochondrial function and lipid profile in the hippocampus. J Endocrinol. (2017) 233:1–14. doi: 10.1530/JOE-16-0451
42. Liang K, Yang L, Yin C, Xiao Z, Zhang J, Liu Y, et al. Estrogen stimulates degradation of β-amyloid peptide by up-regulating neprilysin. J Biol Chem. (2010) 285:935–42. doi: 10.1074/jbc.M109.051664
43. Barnes JN and Charkoudian N. Integrative cardiovascular control in women: Regulation of blood pressure, body temperature, and cerebrovascular responsiveness. FASEB J. (2021) 35:e21143. doi: 10.1096/fj.202001387R
44. Kuck MJ and Hogervorst E. Stress, depression, and anxiety: psychological complaints across menopausal stages. Front Psychiatry. (2024) 15:1323743. doi: 10.3389/fpsyt.2024.1323743
45. Nagda AL, Datar MC, Naphade NM, and Shetty JV. A cross-sectional assessment of depression, anxiety, and cognition in perimenopausal and menopausal women. J Midlife Health. (2023) 14:117–22. doi: 10.4103/jmh.jmh_34_23
46. Mueller SC, De Franceschi M, Brzozowska J, Herman AM, Ninghetto M, Burnat K, et al. An influence of menopausal symptoms on mental health, emotion perception, and quality of life: a multi-faceted approach. Qual Life Res. (2024) 33:1925–35. doi: 10.1007/s11136-024-03641-z
47. Sun Q, Li G, Zhao F, Dong M, Xie W, Liu Q, et al. Role of estrogen in treatment of female depression. Aging (Albany NY). (2024) 16:3021–42. doi: 10.18632/aging.205507
48. Gava G, Orsili I, Alvisi S, Mancini I, Seracchioli R, and Meriggiola MC. Cognition, mood and sleep in menopausal transition: the role of menopause hormone therapy. Medicina. (2019) 55:668. doi: 10.3390/medicina55100668
49. Turek J and Gąsior Ł. Estrogen fluctuations during the menopausal transition are a risk factor for depressive disorders. Pharmacol Reports. (2023) 75:32–43. doi: 10.1007/s43440-022-00444-2
50. Xie Z, Huang J, Sun G, He S, Luo Z, Zhang L, et al. Integrated multi-omics analysis reveals gut microbiota dysbiosis and systemic disturbance in major depressive disorder. Psychiatry Res. (2024) 334:115804. doi: 10.1016/j.psychres.2024.115804
51. Mörkl S, Butler MI, and Lackner S. Advances in the gut microbiome and mood disorders. Curr Opin Psychiatry. (2023) 36:1–7. doi: 10.1097/YCO.0000000000000829
52. Zhang Y, Tan X, and Tang C. Estrogen-immuno-neuromodulation disorders in menopausal depression. J Neuroinflammation. (2024) 21:159. doi: 10.1186/s12974-024-03152-1
53. Li S, Mao Y, Zhou F, Yang H, Shi Q, and Meng B. Gut microbiome and osteoporosis. Bone Joint Res. (2020) 9:524–30. doi: 10.1302/2046-3758.98.BJR-2020-0089.R1
54. Ji J, Gu Z, Li N, Dong X, Wang X, Yao Q, et al. Gut microbiota alterations in postmenopausal women with osteoporosis and osteopenia from Shanghai, China. PeerJ. (2024) 12:e17416. doi: 10.7717/peerj.17416
55. Adejuyigbe B, Kallini J, Chiou D, and Kallini JR. Osteoporosis: molecular pathology, diagnostics, and therapeutics. Int J Mol Sci. (2023) 24:14583. doi: 10.3390/ijms241914583
56. Walker MD, Solomon CG, and Shane E. Postmenopausal osteoporosis. New Engl J Medicine. (2023) 389:1979–91. doi: 10.1056/NEJMcp2307353
57. Song S, Guo Y, Yang Y, and Fu D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol Ther. (2022) 237:108168. doi: 10.1016/j.pharmthera.2022.108168
58. Ding W, Hua F, and Ding K. Gut microbiome and osteoporosis. Aging disease. (2020) 11:438–47. doi: 10.14336/AD.2019.0523
59. Lu L and Tian L. Postmenopausal osteoporosis coexisting with sarcopenia: the role and mechanisms of estrogen. J Endocrinol. (2023) 259:e230116. doi: 10.1530/JOE-23-0116
60. Kverka M and Stepan JJ. Associations among estrogens, the gut microbiome and osteoporosis. Curr Osteoporosis Reports. (2024) 23:2. doi: 10.1007/s11914-024-00896-w
61. Cheng C-H, Chen L-R, and Chen K-H. Osteoporosis due to hormone imbalance: an overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. Int J Mol Sci. (2022) 23:1376. doi: 10.3390/ijms23031376
62. Deepak V, Kayastha P, and McNamara LM. Estrogen deficiency attenuates fluid flow-induced [Ca2+]ioscillations and mechanoresponsiveness of MLO-Y4 osteocytes. FASEB J. (2017) 31:3027–39. doi: 10.1096/fj.201601280R
63. Peters B, Santoro N, Kaplan R, and Qi Q. Spotlight on the gut microbiome in menopause: current insights. Int J Women’s Health. (2022) 14:1059–72. doi: 10.2147/IJWH.S340491
64. Collins FL, Rios-Arce ND, Atkinson S, Bierhalter H, Schoenherr D, Bazil JN, et al. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol Reports. (2017) 5:e13263. doi: 10.14814/phy2.13263
65. Xiang X, Palasuberniam P, and Pare R. The role of estrogen across multiple disease mechanisms. Curr Issues Mol Biol. (2024) 46:8170–96. doi: 10.3390/cimb46080483
66. Ma Y, Liu T, Li X, Kong A, Xiao R, Xie R, et al. Estrogen receptor β deficiency impairs gut microbiota: a possible mechanism of IBD-induced anxiety-like behavior. Microbiome. (2022) 10:160. doi: 10.1186/s40168-022-01356-2
67. Liu Y, Zhou Y, Mao T, Huang Y, Liang J, Zhu M, et al. The relationship between menopausal syndrome and gut microbes. BMC Women’s Health. (2022) 22:437. doi: 10.1186/s12905-022-02029-w
68. Park S, Kim DS, Kang ES, Kim DB, and Kang S. Low-dose brain estrogen prevents menopausal syndrome while maintaining the diversity of the gut microbiomes in estrogen-deficient rats. Am J Physiology-Endocrinology Metab. (2018) 315:E99–E109. doi: 10.1152/ajpendo.00005.2018
69. Ervin SM, Li H, Lim L, Roberts LR, Liang X, Mani S, et al. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem. (2019) 294:18586–99. doi: 10.1074/jbc.RA119.010950
70. Cross T-WL, Simpson AMR, Lin C-Y, Hottmann NM, Bhatt AP, Pellock SJ, et al. Gut microbiome responds to alteration in female sex hormone status and exacerbates metabolic dysfunction. Gut Microbes. (2024) 16:2295429. doi: 10.1080/19490976.2023.2295429
71. Hu S, Ding Q, Zhang W, Kang M, Ma J, and Zhao L. Gut microbial beta-glucuronidase: a vital regulator in female estrogen metabolism. Gut Microbes. (2023) 15:2236749. doi: 10.1080/19490976.2023.2236749
72. Huang F, Cao Y, Liang J, Tang R, Wu S, Zhang P, et al. The influence of the gut microbiome on ovarian aging. Gut Microbes. (2024) 16:2295394. doi: 10.1080/19490976.2023.2295394
73. Honda S, Tominaga Y, Espadaler-Mazo J, Huedo P, Aguiló M, Perez M, et al. Supplementation with a probiotic formula having β-glucuronidase activity modulates serum estrogen levels in healthy peri- and postmenopausal women. J Medicinal Food. (2024) 27:720–7. doi: 10.1089/jmf.2023.k.0320
74. Ceccarelli I, Bioletti L, Peparini S, Solomita E, Ricci C, Casini I, et al. Estrogens and phytoestrogens in body functions. Neurosci Biobehavioral Rev. (2022) 132:648–63. doi: 10.1016/j.neubiorev.2021.12.007
75. Chavda VP, Chaudhari AZ, Balar PC, Gholap A, and Vora LK. Phytoestrogens: Chemistry, potential health benefits, and their medicinal importance. Phytotherapy Res. (2024) 38:3060–79. doi: 10.1002/ptr.v38.6
76. Patra S, Gorai S, Pal S, Ghosh K, Pradhan S, and Chakrabarti S. A review on phytoestrogens: Current status and future direction. Phytotherapy Res. (2023) 37:3097–120. doi: 10.1002/ptr.v37.7
77. Canivenc-Lavier M-C and Bennetau-Pelissero C. Phytoestrogens and health effects. Nutrients. (2023) 15:317. doi: 10.3390/nu15020317
78. Giampieri F, Godos J, Caruso G, Owczarek M, Jurek J, Castellano S, et al. Dietary phytoestrogen intake and cognitive status in southern italian older adults. Biomolecules. (2022) 12:760. doi: 10.3390/biom12060760
79. Rowe IJ and Baber RJ. The effects of phytoestrogens on postmenopausal health. Climacteric. (2021) 24:57–63. doi: 10.1080/13697137.2020.1863356
80. Petrine JCP and Del Bianco-Borges B. The influence of phytoestrogens on different physiological and pathological processes: An overview. Phytotherapy Res. (2020) 35:180–97. doi: 10.1002/ptr.v35.1
81. Alqudah S and Claesen J. Mechanisms of gut bacterial metabolism of dietary polyphenols into bioactive compounds. Gut Microbes. (2024) 16:2426614. doi: 10.1080/19490976.2024.2426614
82. Baldi S, Tristán Asensi M, Pallecchi M, Sofi F, Bartolucci G, and Amedei A. Interplay between lignans and gut microbiota: nutritional, functional and methodological aspects. Molecules. (2023) 28:343. doi: 10.3390/molecules28010343
83. Peirotén Á, Bravo D, and Landete JM. Bacterial metabolism as responsible of beneficial effects of phytoestrogens on human health. Crit Rev Food Sci Nutrition. (2019) 60:1922–37. doi: 10.1080/10408398.2019.1622505
84. Landete JM, Arqués J, Medina M, Gaya P, de Las Rivas B, and Muñoz R. Bioactivation of phytoestrogens: intestinal bacteria and health. Crit Rev Food Sci Nutrition. (2016) 56:1826–43. doi: 10.1080/10408398.2013.789823
85. Tjeerdsma AM, van Hunsel FPAM, van de Koppel S, Ekhart C, Vitalone A, and Woerdenbag HJ. Analysis of safety concerns on herbal products with assumed phytoestrogenic activity. Pharmaceuticals. (2023) 16:1137. doi: 10.3390/ph16081137
86. Seyed Hameed AS, Rawat PS, Meng X, and Liu W. Biotransformation of dietary phytoestrogens by gut microbes: A review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotechnol Advances. (2020) 43:107576. doi: 10.1016/j.biotechadv.2020.107576
87. Farhat EK, Sher EK, Džidić-Krivić A, Banjari I, and Sher F. Functional biotransformation of phytoestrogens by gut microbiota with impact on cancer treatment. J Nutr Biochem. (2023) 118:109368. doi: 10.1016/j.jnutbio.2023.109368
88. Brettle H, Tran V, Drummond GR, Franks AE, Petrovski S, Vinh A, et al. Sex hormones, intestinal inflammation, and the gut microbiome: Major influencers of the sexual dimorphisms in obesity. Front Immunol. (2022) 13:971048. doi: 10.3389/fimmu.2022.971048
89. Barrea L, Verde L, Auriemma RS, Vetrani C, Cataldi M, Frias-Toral E, et al. Probiotics and prebiotics: any role in menopause-related diseases? Curr Nutr Rep. (2023) 12:83–97. doi: 10.1007/s13668-023-00462-3
90. Koneru S, Thiruvadi V, and Ramesh M. Gut microbiome and its clinical implications: exploring the key players in human health. Curr Opin Infect Diseases. (2023) 36:353–9. doi: 10.1097/QCO.0000000000000958
91. Meng Q, Li J, Chao Y, Bi Y, Zhang W, Zhang Y, et al. β-estradiol adjusts intestinal function via ERβ and GPR30 mediated PI3K/AKT signaling activation to alleviate postmenopausal dyslipidemia. Biochem Pharmacol. (2020) 180:114134. doi: 10.1016/j.bcp.2020.114134
92. Tian Y, Hong X, Xie Y, Guo Z, and Yu Q. 17β-estradiol (E2) upregulates the ERα/SIRT1/PGC-1α Signaling pathway and protects mitochondrial function to prevent bilateral oophorectomy (OVX)-induced nonalcoholic fatty liver disease (NAFLD). Antioxidants. (2023) 12:2100. doi: 10.3390/antiox12122100
93. Chen Q, Wang B, Wang S, Qian X, Li X, Zhao J, et al. Modulation of the gut microbiota structure with probiotics and isoflavone alleviates metabolic disorder in ovariectomized mice. Nutrients. (2021) 13:1793. doi: 10.3390/nu13061793
94. Alpini GD, Liu L, Fu Q, Li T, Shao K, Zhu X, et al. Gut microbiota and butyrate contribute to nonalcoholic fatty liver disease in premenopause due to estrogen deficiency. PloS One. (2022) 17:e0262855. doi: 10.1371/journal.pone.0262855
95. Li D, Sun T, Tong Y, Le J, Yao Q, Tao J, et al. Gut-microbiome-expressed 3β-hydroxysteroid dehydrogenase degrades estradiol and is linked to depression in premenopausal females. Cell Metab. (2023) 35:685–94.e5. doi: 10.1016/j.cmet.2023.02.017
96. Kim N, Yun M, Oh YJ, and Choi H-J. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. J Microbiology. (2018) 56:172–82. doi: 10.1007/s12275-018-8032-4
97. Janik R, Thomason LAM, Stanisz AM, Forsythe P, Bienenstock J, and Stanisz GJ. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. NeuroImage. (2016) 125:988–95. doi: 10.1016/j.neuroimage.2015.11.018
98. Sawada D, Sugawara T, Hirota T, and Nakamura Y. Effects of lactobacillus gasseri CP2305 on mild menopausal symptoms in middle-aged women. Nutrients. (2022) 14:1695. doi: 10.3390/nu14091695
99. Wang Z, Chen K, Wu C, Chen J, Pan H, Liu Y, et al. An emerging role of Prevotella histicola on estrogen deficiency–induced bone loss through the gut microbiota–bone axis in postmenopausal women and in ovariectomized mice. Am J Clin Nutrition. (2021) 114:1304–13. doi: 10.1093/ajcn/nqab194
100. Al-Habsi N, Al-Khalili M, Haque SA, Elias M, Olqi NA, and Al Uraimi T. Health benefits of prebiotics, probiotics, synbiotics, and postbiotics. Nutrients. (2024) 16:3955. doi: 10.3390/nu16223955
101. Hughes RL, Alvarado DA, Swanson KS, and Holscher HD. The prebiotic potential of inulin-type fructans: A systematic review. Adv Nutrition. (2022) 13:492–529. doi: 10.1093/advances/nmab119
102. Chen L, Liu Y, Tang Z, Song Z, Cao F, Shi X, et al. Radix Angelica dahuricae extract ameliorates oestrogen deficiency-induced dyslipidaemia in ovariectomized (OVX) rats by modulating the gut microbiota and bile acid signalling. Phytomedicine. (2022) 107:154440. doi: 10.1016/j.phymed.2022.154440
103. Quah Y, Park N-H, Lee E-B, Lee K-J, Yi-Le JC, Ali MS, et al. Trifolium pratense ethanolic extract alters the gut microbiota composition and regulates serum lipid profile in the ovariectomized rats. BMC Complementary Med Therapies. (2022) 22:5. doi: 10.1186/s12906-021-03486-w
104. Sun Y, Baptista LC, Roberts LM, Jumbo-Lucioni P, McMahon LL, Buford TW, et al. The gut microbiome as a therapeutic target for cognitive impairment. J Gerontol A Biol Sci Med Sci. (2020) 75:1242–50. doi: 10.1093/gerona/glz281
105. Seely KD, Kotelko CA, Douglas H, Bealer B, and Brooks AE. The human gut microbiota: A key mediator of osteoporosis and osteogenesis. Int J Mol Sci. (2021) 22:9452. doi: 10.3390/ijms22179452
106. Zhang Y-W, Song P-R, Wang S-C, Liu H, Shi Z-M, and Su J-C. Diets intervene osteoporosis via gut-bone axis. Gut Microbes. (2024) 16:2295432. doi: 10.1080/19490976.2023.2295432
107. Jia M, Ning F, Wen J, Wang X, Chen J, Hu J, et al. Secoisolariciresinol diglucoside attenuates neuroinflammation and cognitive impairment in female Alzheimer’s disease mice via modulating gut microbiota metabolism and GPER/CREB/BDNF pathway. J Neuroinflammation. (2024) 21:201. doi: 10.1186/s12974-024-03195-4
108. Pan W, Zhao J, Wu J, Xu D, Meng X, Jiang P, et al. Dimethyl itaconate ameliorates cognitive impairment induced by a high-fat diet via the gut-brain axis in mice. Microbiome. (2023) 11:30. doi: 10.1186/s40168-023-01471-8
109. Zhang Y-W, Cao M-M, Li Y-J, Zhang R-L, Wu M-T, Yu Q, et al. Fecal microbiota transplantation as a promising treatment option for osteoporosis. J Bone Mineral Metab. (2022) 40:874–89. doi: 10.1007/s00774-022-01375-x
110. Zhang Y-W, Cao M-M, Li Y-J, Lu P-P, Dai G-C, Zhang M, et al. Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function. J Orthopaedic Translation. (2022) 37:46–60. doi: 10.1016/j.jot.2022.08.003
111. Santos-Marcos JA, Mora-Ortiz M, Tena-Sempere M, Lopez-Miranda J, and Camargo A. Interaction between gut microbiota and sex hormones and their relation to sexual dimorphism in metabolic diseases. Biol Sex Differences. (2023) 14:4. doi: 10.1186/s13293-023-00490-2
112. Nakhal MM, Yassin LK, Alyaqoubi R, Saeed S, Alderei A, Alhammadi A, et al. The microbiota–gut–brain axis and neurological disorders: A comprehensive review. Life. (2024) 14:1234. doi: 10.3390/life14101234
Keywords: gut microbiota, menopause, estrogen, lipid metabolism disorder, cognition impairment, clinical application
Citation: Wang H, Shi F, Zheng L, Zhou W, Mi B, Wu S and Feng X (2025) Gut microbiota has the potential to improve health of menopausal women by regulating estrogen. Front. Endocrinol. 16:1562332. doi: 10.3389/fendo.2025.1562332
Received: 17 January 2025; Accepted: 19 May 2025;
Published: 09 June 2025.
Edited by:
Victoria Ceperuelo-Mallafré, University of Rovira i Virgili, SpainReviewed by:
Valentina Caputi, University College Cork, IrelandJulang Li, University of Guelph, Canada
Copyright © 2025 Wang, Shi, Zheng, Zhou, Mi, Wu and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoling Feng, ZG9jdG9yZnhsQDE2My5jb20=
†These authors share first authorship
 Haiqiang Wang
Haiqiang Wang Fan Shi
Fan Shi Lihong Zheng
Lihong Zheng Wenhui Zhou2
Wenhui Zhou2 Siyu Wu
Siyu Wu Xiaoling Feng
Xiaoling Feng