- 1Clinical and Experimental Endocrinology, Department of Chronic Diseases and Metabolism, KU Leuven, Leuven, Belgium
- 2Department of Nutrition and Movement Sciences, Research Institute of Nutrition and Translational Research in Metabolism (NUTRIM), Maastricht University, Maastricht, Netherlands
- 3Department of Abdominal Surgery, University Hospitals, Leuven, Belgium
- 4Department of Endocrinology, University Hospitals Leuven, Leuven, Belgium
Recent advancements in obesity pharmacotherapy have seen the approval of novel agents, like glucagon-like peptide-1 receptor agonist and dual agonists, offering unprecedented efficacy for obesity management. However, treatment outcomes remain highly variable, necessitating a more personalized approach to pharmacotherapy tailored to individual profiles. This review evaluates the current landscape of obesity pharmacotherapy, while exploring factors influencing variability in treatment response including early response predictors, genetic markers, and physiological traits. Additionally, the potential of combining treatment modalities and some emerging drugs are highlighted. Finally, a stepwise algorithm is proposed for personalized obesity treatment, integrating comorbidities, phenotypes, and responses to medication, paving the way for more effective and efficient obesity management.
Introduction
Obesity is a chronic disease with significant public health implications (1, 2). Characterized by an excessive accumulation of fat that impairs health, obesity increases the risk of developing serious comorbidities, such as cardiovascular disease, type 2 diabetes, musculoskeletal disorders, certain cancers, and psychological disorders (3, 4). The physical health consequences together with the stigma surrounding obesity negatively impacts the quality of life and reduces life expectancy (5). Economically, obesity poses an important financial burden, accounting for approximately 7% of the European healthcare budgets, and an estimated € 70 billion annually in the European Union, including healthcare costs and lost productivity (6). Furthermore, the effects of obesity surpass individual health and societal costs by influencing future generations through potential epigenetic changes (7).
Despite the increasing prevalence and predictions suggesting that a quarter of the world’s population will suffer from obesity by 2035 (8), a detailed understanding of the disease’s pathophysiology and management remains limited (1, 9). Although once thought, the origin of obesity is much more complex than simply an accumulation of excess calories and sedentary behavior (1, 9). A dynamic interplay of environmental, genetic, psychological, and physiological factors can lead to a central dysregulation of energy balance, causing a tendency to promote weight gain and impeding weight loss (1, 9). This complexity underscores the need for a paradigm shift from solely focusing on weight loss to managing a healthy energy homeostasis.
Addressing obesity is challenging but not insurmountable as several effective treatments are available. Lifestyle modification, including medical nutrition therapy and increased physical activity, is the key pillar in the treatment of obesity (10, 11). While these approaches might achieve the desired weight loss of at least 5%, sustaining the loss can be challenging (12). Metabolic/bariatric surgery has for a long time been the only weight loss intervention with long-term weight loss (on average 30%) and associated health benefits (13). However, the widespread application is affected by limited accessibility, resource requirements, potential complications, and a patients resistance to undergo a surgical procedure (14, 15). With lessons learned from bariatric surgery, a great deal of research has been devoted towards the development of pharmacotherapy as a non-invasive, safe and effective treatment alternative for obesity treatment (16). After decades of weight loss medication with poor safety profiles, such as with sibutramine or rimonabant, recent progress in pharmacotherapy has led to the approval of a new generation of anti-obesity medications, starting with liraglutide (once-daily) followed by semaglutide (once-weekly), by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) (Table 1) (17). These therapies, recommended for individuals with a body mass index (BMI) ≥ 30 kg/m² or for individuals with a BMI ≥ 27 kg/m2 and obesity-related comorbidities such as hypertension, type 2 diabetes and sleep apnea, mark the beginning of a new era in obesity treatment (11). Medications like semaglutide have achieved double digit weight loss percentages (>10%), previously seen only with surgical interventions. However, large interindividual variability in weight loss outcomes highlights the complexity of obesity and its treatment (18). This heterogeneity underscores the need for a more personalized approach to pharmacotherapy, as “one-size-fits-all” strategies are unlikely to succeed. Currently, there are no guidelines to assist clinicians in selecting pharmacotherapy tailored to a patient’s clinical profile. This review provides an overview of available pharmacotherapies, identifies knowledge gaps in treatment guidelines, and propose strategies for personalized patient selection, sequence and combination approaches to improve treatment outcomes.
Search strategy and selection criteria
References for this review were identified through searches of PubMed for articles published from January, 1947, to May 2025. A combination of search terms was applied pertinent to the topic of obesity pharmacotherapy including “obesity pharmacotherapy”, “GLP-1 receptor agonist”, “semaglutide”, “tirzepatide”, “liraglutide”, “naltrexone/bupropion”, “phentermine/topiramate”, “orlistat”, “retatrutide”, “survodutide”, “weight loss medications”, “Body Mass Index”, “BMI”, “weight management”, “weight loss” and “obesity”. Articles were screened based on their relevance for the current review. Reference list cited in those articles were reviewed to identify if some studies have not been captured. Only articles published in English were included.
Currently approved pharmacotherapy
Body mass index (BMI) thresholds for obesity interventions vary depending on the treatment modality, as outlined in Figure 1. Pharmacotherapy is indicated for patients with a BMI ≥30 kg/m² or ≥27 kg/m² with comorbidities. Currently, the FDA has approved six medications, and the EMA has approved five medications that are available for chronic weight management (Table 1). The approvals are based on robust clinical trial data demonstrating their efficacy and safety with indications typically targeting a specific BMI category (10, 11, 19–27).
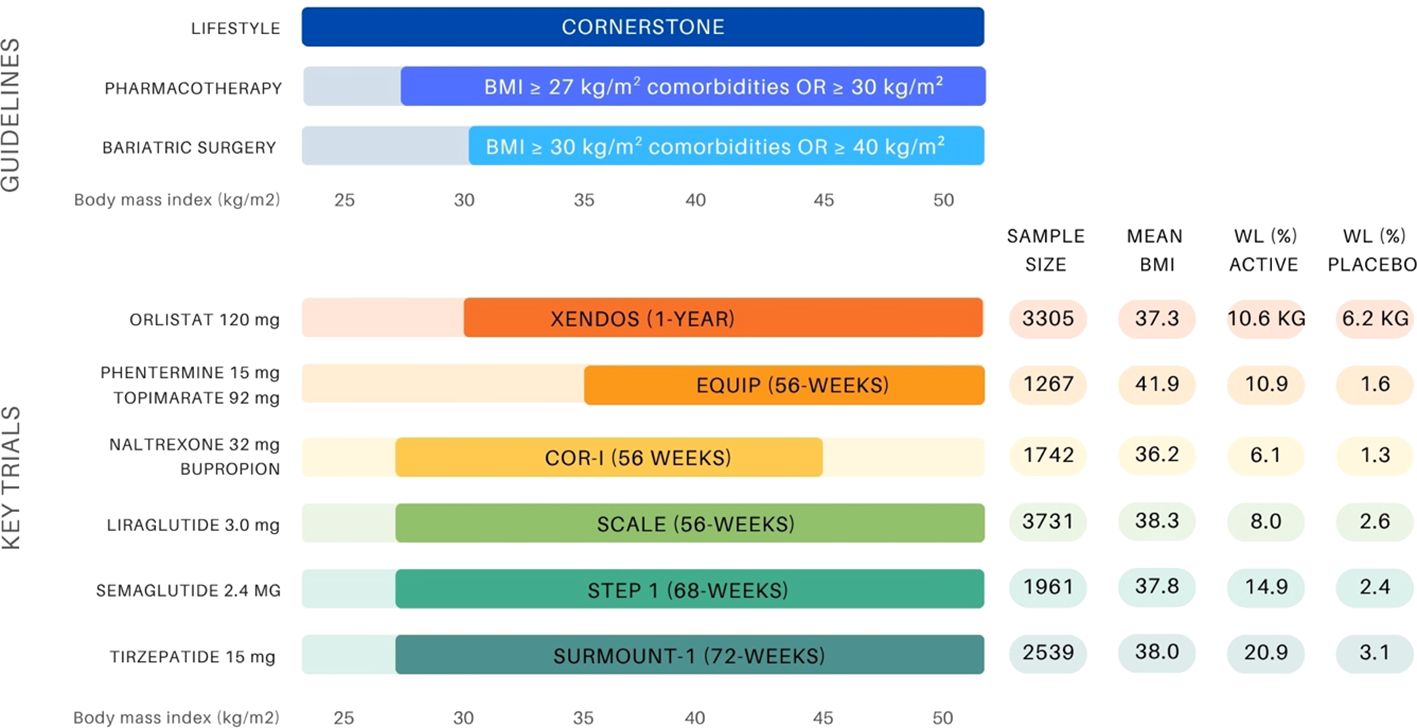
Figure 1. Overview of BMI-based treatment recommendations for obesity management and landmark anti-obesity drug trials (10, 11, 14, 19–21, 23, 25, 34, 35). The upper panel presents guideline thresholds for lifestyle intervention, pharmacotherapy (BMI ≥27 kg/m² with comorbidities or ≥30 kg/m²), and bariatric surgery (BMI ≥35–40 kg/m² depending on comorbidity status). The lower panel displays key clinical trials supporting the approval of six anti-obesity agents, indicating sample size, mean baseline BMI (entire group or active group), and weight loss outcomes (%WL unless otherwise stated) in both the active and placebo arms.
Orlistat, the earliest approved anti-obesity medication, remains available (Brand name ‘Xenical’ produced by Roche; Brand name ‘Alli’ produced by GlaxoSmithkline). It acts as a gastric and pancreatic lipase inhibitor, reducing the absorption of dietary fat by preventing the lipase-catalyzed breakdown in the gastrointestinal tract, leading to weight loss (22, 28). While it has been widely used, the gastrointestinal side effects including steatorrhea and malabsorption often lead to treatment discontinuation due to their (socially) intolerable nature (28). The next approved drugs include the centrally acting agents, namely naltrexone extended release (ER)/bupropion ER (Brand name ‘Contrave’ produced by Orexigen Therapeutics Inc, brand name ‘Mysimba’ in Europe) and phentermine/topiramate ER (Brand name ‘Qsymia’ produced by Vivus, Inc). The first combination includes naltrexone, an opioid receptor antagonist, traditionally used to treat alcohol/opioid dependence and bupropion, a norepinephrine and dopamine reuptake inhibitor, indicated as antidepressant/smoking cessation aid (29). The second combination includes phentermine, a sympathomimetic amine, suppresses appetite that is potentiated by topiramate, which modulates satiety through GABA receptor activation (30). Both therapies achieve similar weight loss results as orlistat, but these combinations might induce other side effects like nausea, irritability, depression, suicidal ideation and cardiovascular complications (i.e. phentermine), limiting their suitability for certain patient populations (29, 30). Initially developed for type 2 diabetes management, two incretin analogues have been approved that were previously indicated for diabetes management, namely liraglutide (Brand name ‘Saxenda’ produced by Novo Nordisk) and semaglutide (Brand name ‘Wegovy’ produced by Novo Nordisk). By stimulating the glucagon-like-peptide-1 (GLP-1) receptor, they stimulate insulin secretion, suppress appetite, increase satiety and slow gastric emptying, thereby reducing calorie intake and potentially changing food preferences (31). Although both drugs operate through the same mechanism, the more recently approved semaglutide has a much larger weight loss effect (20).
Currently, naltrexone/bupropion and phentermine/topiramate are the only approved combination therapies, designed to synergistically target two distinct pathways to induce weight loss (26). However, dual GLP1 and glucose-dependent insulinotropic peptide (GIP) receptor agonists are refining the pharmacological landscape. These novel pharmacological strategies, such as tirzepatide, were initially developed to address glycemic management, but significantly impact weight, metabolic status and cardiovascular outcomes. By activating two complementary pathways, tirzepatide offers greater efficacy than GLP-1 receptor agonists alone (23). Being FDA-approved for diabetes management first in 2022 (Brand name ‘Mounjaro’ produced by Eli Lilly), tirzepatide received subsequent FDA approval under a Fast Track designation in 2023 (Brand name ‘Zepbound’ produced by Eli Lily) (32). Clinical trials have demonstrated weight reductions averaging between 15-20% depending on dosage, outcomes comparable to the outcomes historically only achieved with bariatric surgery (16). While these medications demonstrate significant efficacy, long-term data on cardiovascular outcomes and mortality remains limited for most (33). Although GLP-1 receptor agonists have shown favorable cardiovascular profiles with reductions in major adverse cardiovascular events, robust evidence linking these therapies to reduced all-cause mortality is still under investigation. Future research must focus on addressing these gaps to fully establish the role of pharmacotherapy in improving long-term complications and survival rates.
(Early) responders and non-responders for weight loss
The variability in response to anti-obesity interventions remains a significant and poorly understood aspect of weight management (40). Across all obesity treatment modalities – lifestyle interventions, pharmacotherapy, and bariatric surgery – patients can be classified into either ‘responders’ or ‘non-responders’ based on their weight loss outcomes following treatment. The definition of a ‘responder’ varies slightly between clinical studies but generally refers to a weight loss of 3-5% within the first three to six months of treatment (34, 41–44). This heterogeneity in treatment responses suggest the existence of underlying determinants – both biological and environmental – that influence efficacy among patients (Figure 2). Notably, non-response rates vary across pharmacotherapy ranging from 9 to 52%, with naltrexone/bupropion having the highest proportion of non-responders. In contrast, the newer incretin-based therapies, such as GLP-1 receptor agonist and dual GLP-1/GIP receptor agonists, show lower non-response rates. For instance, preliminary findings from the phase 2 trial of retatrutide, a triple hormone receptor agonist, reported a 100% response rate of more than 5% weight loss for the dosages of 8 or 12 mg weekly over 48 weeks (45). This reflects the improved efficacy and response rates associated with these newer drug classes. Real-world data will have to proof the durability in treatment response and persistence in drug adherence.
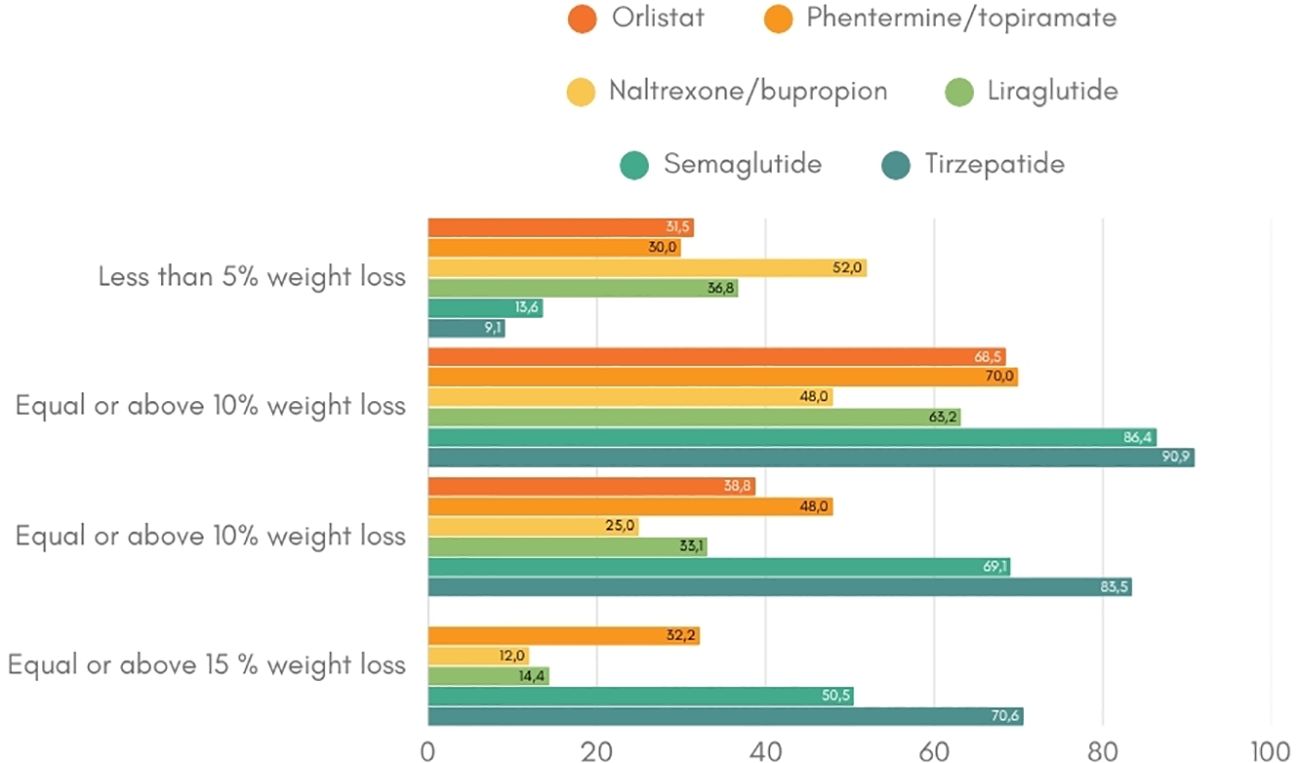
Figure 2. Weight loss response per anti-obesity drug (19–21, 23, 25, 34, 35). This figure indicates the percentage of patients that achieve the weight loss targets of at least 5%, 10% and 15% per anti-obesity medication.
To date, little is known about specific predictive factors for treatment response, especially for pharmacotherapy (40). However, one well-established predictor of long-term weight loss is an early response to treatment (34, 41, 42). Substantial weight loss during the initial phase of treatment correlates with greater weight loss after 12 months, while minimal early weight loss signals a poor likelihood of response over time (34, 41, 42). This principle is integrated in the European guidelines for obesity treatment, which recommend discontinuing anti-obesity medication if patients lose less than 5% of their weight (or less than 3% in patients with diabetes) within the first three months of treatment (11). Patients who meet or exceed the threshold are considered ‘early responders’, while those who do not are considered ‘non-responders’, warranting a change in therapeutic approach. While early weight loss is a valuable predictor, it does not fully explain the factors determining individual response to treatment. Continued research is needed to improve response prediction and optimize personalized pharmacotherapy.
Research has indicated that the presence of type 2 diabetes significantly impacts weight loss response (46). Over the past three decades, while glycemic control in diabetes has steadily improved, the average BMI of patients with diabetes has also increased (47). Research using older generations of anti-obesity medication indicated that individuals with both obesity and diabetes faced greater challenges in achieving weight loss (46). A post-hoc analysis of pooled data from the SCALE Obesity and Prediabetes and SCALE Diabetes trial demonstrated notable differences in the proportion of ‘early responders’ and ‘early non-responders’ to liraglutide. Over one-third of patients with type 2 diabetes were classified as non-responders compared to less than a quarter of patients without diabetes (without type 2 diabetes: 77.3% responders and 22.7% non-responders; with type 2 diabetes: 62.7% responders and 37.3% non-responders) (42). Furthermore, among responders, the weight reduction was generally lower for patients with type 2 diabetes compared to those without diabetes (-8.5% versus -10.8 after 56 weeks). Although the full mechanism underlying this difference remains unclear, several contributing factors have been proposed. Both insulin-resistance, which hampers fat oxidation, and the use of certain antidiabetic medications like insulin and sulfonylurea promote weight gain and might counteract the effects of anti-obesity medication (48–50). Moreover, intensive glycemic control itself has been linked with weight gain (51).
However, the development of newer drugs like tirzepatide offer hope for improved outcomes (52, 53). The SURMOUNT-2 trial, which investigated tirzepatide for obesity treatment in people with type 2 diabetes, reported a mean body weight reduction of 12.8% and 14.7% for weekly doses of 10 and 15 mg, respectively, after 72 weeks (53). This represents the highest weight reduction achieved in a phase 3 trial for this population to date (53, 54). These results suggest that dual agonists are promising obesity treatment options for managing obesity in patients with diabetes. In addition, the current EASD guidelines include bariatric surgery as a treatment option, and weight management is increasingly recognized as a key factor in the choice of antidiabetic medication within the choice of treatment (55, 56).
To explore the role of genetic predictors, recent research pooled data from seven major lifestyle intervention trials (NUGENOB, DIOGenes, Look AHEAD, Diabetes Prevention Program, Diabetes Prevention Study, DIETFITS, PREDIMED-PLUS). By doing so, a polygenic score comprising 59 single nucleotide polymorphisms was identified to partially explain variability in weight loss among white participants, expressed through a composite measure of waist circumference, waist-to-hip ratio and BMI. However, this was not observed in African American participants. Although the effect was small and clinically significant, the findings highlight the potential role of genetic variants associated with central adiposity in obesity management. Further investigation is required to determine whether this polygenic score influence pharmacotherapy outcomes (57).
Regarding currently available anti-obesity medication (Table 1), more research on genetic influences on treatment response is emerging. For instance, a Taq1A polymorphism related to striatal dopamine D2 receptor density has been shown to affect the weight loss response to naltrexone/bupropion (43). This pilot study found that patients carrying the A1+ allele were more likely to respond and achieve higher weight loss (A1+ genotype: 5.9 ± 3.2%; A1-genotype: 4.2 ± 4.2%; P=0.03) (43). Although the sample size was rather small, these findings present an interesting perspective for advancing personalized medicine. Similarly, variability in weight loss responses to GLP-1 receptor agonists has raised interest in pharmacogenomic studies in people with type 2 diabetes. A genome-wide analysis revealed that variation in HbA1C reduction with GLP-1 receptor agonist treatment was associated with a common genetic variation in GLP1R (rs6923761G→A (Gly168Ser)) and a rare variant in ARRB1 (rs140226575G→A (Thr370Met)) (58). Moreover, patients with type 2 diabetes carrying the variant allele (A) of the rs6923761 GLP-1 R polymorphism demonstrated greater reductions in BMI, weight, and fat mass with liraglutide treatment (59). While these findings were obtained using type 2 diabetes treatment, it raises the possibility that variants in genes encoding GLP-1 receptor might affect weight loss response in people with obesity alone.
A pilot study investigating GLP-1 receptor genes in patients with obesity has provided further insight (60). This study investigated two polymorphisms – rs6923761 (p.Gly168Ser) and rs10305420 (p.Pro7Leu) – in 57 women with obesity and polycystic ovarian syndrome (PCOS). Significant distinctions were found between responders and non (or poor) responders with responders achieving greater weight loss of (7.38 ± 1.74 kg vs 2.11 ± 2.17kg, respectively). Furthermore, individuals carrying at least one rs10305420 allele experienced significantly less weight loss compared to those with wild-type alleles (60). While those with at least one rs6923761 allele had superior outcomes, consistent with the findings from type 2 diabetes studies (58, 59). Despite these promising associations, the clinical application of pharmacogenomics in obesity pharmacotherapy remains premature. Most findings to date stem from pilot studies with limited replication across independent cohorts. For now, insufficient data limits the current clinical utility.
Beyond type 2 diabetes and genetic variants, some physiological markers have been identified as predictors of weight loss response to anti-obesity medication. For example, gastric emptying half-time has been shown to correlate with weight loss outcomes. Delayed gastric emptying at five weeks is related with greater weight loss at 16 weeks of liraglutide treatment (61). Additionally, a proof-of-concept randomized controlled study found that baseline food intake was associated with the weight loss response at two weeks of treatment with central acting phentermine/topiramate treatment (62). Overall, weight loss response appears to be a multifactorial phenomenon influences by biological, environmental and psychosocial components, many of which remain to be fully elucidated.
Combining lines of treatment
To date, evidence supporting add-on therapy for obesity remains limited and rather speculative. A few trials explored the additional effect of adding pharmacotherapy with other weight loss management strategies, such as lifestyle interventions and metabolic surgery. However, most studies have focused on specific patient subgroups, limiting the generalizability of the findings to the broader population of individuals with obesity. In the GRAVITAS randomized controlled trial, liraglutide has been studied as an add-on therapy for patients with persistent type 2 diabetes following bariatric surgery (63). While the primary objective was the change in HbA1c levels, secondary endpoints included weight loss. The study demonstrated significant additional weight loss of -4.23 kg compared to the control group after 26 weeks, irrespective of the type of bariatric surgery. However, the trial only included patients with both type 2 diabetes and obesity, leaving questions about its applicability to the general population with obesity. In 2019, Wharton et al. conducted an observational study to investigate add-on liraglutide 3.0 mg therapy for insufficient weight loss or weight regain after bariatric surgery, irrespective of diabetes presence (64). The results showed average weight loss of -5.5% ± 6.2% with liraglutide independent of the type of surgery performed. However, the lack of control group limits the strength of these findings to some extent. A prospective, randomized controlled trial is needed to confirm liraglutide’s efficacy as adjunct therapy after bariatric surgery.
Liraglutide has also been studied in combination with a very low calory diet in the SCALE maintenance study (65). In this randomized controlled trial, patients who already lost equal or more than 5% of their initial weight during a low-calorie diet run-in were randomly assigned to liraglutide or placebo. The liraglutide group not only maintained their weight loss better (81.4% versus 48.9%) but also achieved additional weight loss compared to the control group, with 50.5% versus 21.8% reaching at least 5% weight loss, respectively. Furthermore, improvements in certain cardiovascular risk factors were observed (65). However, since the trial only included a subgroup of patients who responded well to an initial low-calorie diet, it remains unclear whether liraglutide with diet would benefit patients who do not respond to dietary interventions alone. Additionally, the study by Lundgren et al. examined the effect of liraglutide with exercise on weight loss (66). The findings revealed that combining pharmacotherapy with exercise resulted in superior weight loss compared to either of the interventions alone. Moreover, post-treatment analysis demonstrated improved weight loss maintenance with combination therapy after treatment termination (67). Significantly more patients who underwent both supervised exercise and pharmacotherapy maintained at least 10% weight loss one year after treatment cessation (45%), compared to those who received only exercise (29%), only liraglutide (16%) or placebo (10%) (67).
Together, these studies suggest that a combinative approach to obesity management may improve outcomes both in terms of the magnitude of weight loss and its long-term maintenance. However, the current body of evidence remains insufficient to draw definitive conclusions. Larger, well-controlled add-on trials are necessary to substantiate the benefits of combining pharmacotherapy with other treatment modalities. In addition to combining pharmacotherapy with lifestyle or surgical interventions, there is potential benefit of combining different pharmacotherapies to target multiple pathways or organs. Existing combination therapies, such as phentermine/topiramate and naltrexone/bupropion, as well as the dual agonist tirzepatide, represent steps in this direction. However, there is a lack of evidence for using multiple pharmacotherapies simultaneously, as studies investigating this approach are currently unavailable.
Tailor treatment to each patient
Obesity is a heterogenous disease with multifactorial origins, a diverse patient population, and significant variability in treatment response. Despite this complexity, current treatments predominantly focus on BMI thresholds with little consideration for individual phenotypes or response variability (10, 11). However, BMI alone is insufficient to describe or predict health status or mortality risk in individuals (68). The Edmonton Obesity Staging System, which accounts for comorbidities and functional status, offers better mortality predictions without relying on BMI or adiposity (68). This underscores the need for stratified treatment strategies tailored to patient profiles. Given its epidemiology, its diverse presentations, and suboptimal treatment outcomes for some patients, it is becoming evident that a ‘one-size-fits-all’ approach is inadequate when discussing obesity treatment (69, 70). Precision medicine, which incorporates genetics, environment, metabolites and other individual factors, holds promise for optimizing treatment outcomes (71, 72). A clustering approach – using comorbidities, or phenotypes, – offers a pragmatic first step towards tailoring treatment (73, 74). By aligning choices of anti-obesity drug with their specific mechanism of action towards patient specific factors, better treatment responses can be achieved (75).
Comorbidities
BMI is currently the primary criterion for prescribing weight loss medications with thresholds of ≥30 kg/m² for general obesity and ≥27 kg/m² for those with comorbidities. However, tailoring therapy based on the specific comorbidities can maximize therapeutic benefits rather than addressing each condition separately (10, 11). Evidence suggest that certain anti-obesity medications not only target the excess weight but also address underlying comorbidities (74).
Type 2 diabetes, a common obesity-related comorbidity, significantly improves with weight loss of at least 15% (76). GLP-1 receptor agonists - liraglutide, semaglutide and tirzepatide - were initially developed as antidiabetic agents due to their ability to stimulate insulin secretion in a glucose-dependent manner (77–79). The LEAD trial (liraglutide) and the SUSTAIN trial (semaglutide) demonstrated that both drugs effectively reduce body weight, glycemia and HbA1c in patients with type 2 diabetes (77, 78). Tirzepatide has shown superior glycemic control, reducing fasting glucose by 15 to 20 mg/dL and HbA1C by 1% more than semaglutide and insulin Glargine (80–82). In the SURMOUNT-1 trial, tirzepatide not only produced substantial and sustained weight loss over 176 weeks in individuals with obesity and prediabetes, but also significantly delayed the onset of type 2 diabetes compared to placebo. The incidence of type 2 diabetes was markedly lower in the tirzepatide groups versus placebo at week 176 (1.3% vs. 13.3%; HR: 0.07 (95% CI: 0.0 to 0.1), P<0.001), and this benefit persisted after a 17-week off-treatment period (HR: 0.12 (95% CI: 0.1 to 0.2), P<0.001) (83). These findings support tirzepatide’s potential to serve not only as a weight loss agent, but also as a preventive strategy for type 2 diabetes in high-risk populations. Beyond these, naltrexone/bupropion significantly lowered HbA1c levels in patients with obesity and type 2 diabetes in the COR-DM trial, although no significant changes were observed in fasting glucose or insulin (84).
Cardiovascular disease is another major obesity-related comorbidity. Evidence regarding naltrexone/bupropion’s cardiovascular effects remain inconclusive, following publication of the results for the prematurely terminated LIGHT outcomes study. The trial was halted after the original commercial sponsor inappropriately released preliminary findings of a confidential early analysis. While the 25% interim analysis suggested a potential reduction in MACE (HR: 0.59; 95% CI: 0.39-0.90), subsequent data did not confirm this benefit. The 50% analysis showed a neutral effect (HR: 0.88; 99.7% CI: 0.57–1.34) (85). In contrast, GLP-1 agonists demonstrated protective cardiovascular effects (86–89). The SELECT trial, published in 2023, was the first to demonstrate that semaglutide 2.4 mg reduces the risk of major cardiovascular events (MACE) by 20% in patients with obesity without diabetes (86). This establishes semaglutide as the first weight-loss medication to show cardiovascular benefit independent of glycemic effects (86). In contrast, earlier trials like the LEADER (liraglutide) and SUSTAIN-6 (semaglutide 1.0 mg) trial enrolled people with type 2 diabetes and high cardiovascular risk. Both trials showed significant reductions in the incidence of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke (87, 90). PIONEER-6, evaluating oral semaglutide, confirmed non-inferiority for cardiovascular outcomes in patients with type 2 diabetes (88). In patients with obesity and heart failure with a preserved ejection fraction included in the STEP-HFpEF trial, semaglutide significantly reduced symptoms, improved physical limitations, and enhanced exercise functions compared to placebo (89). The cardioprotective effect of GLP-1 agonists are likely mediated by reductions in visceral adipose tissue (88). Tirzepatide has shown remarkable promise in improving cardiovascular outcomes in patients with obesity and heart failure with preserved ejection fraction (HFpEF). In the SUMMIT trial (91), Tirzepatide significantly reduced the risk of a composite endpoint of cardiovascular death or worsening heart failure events compared to placebo. Death from cardiovascular causes or a worsening heart-failure event occurred in 9.9% of the tirzepatide group versus 15.3% of the placebo group. Beyond reducing adverse cardiovascular outcomes, tirzepatide led to substantial improvements in health status, as reflected by a 6.9-point greater increase in the Kansas City Cardiomyopathy Questionnaire clinical summary score over 52 weeks. Patients also experienced significant weight loss (−13.9% vs. −2.2%) and a marked reduction in systemic inflammation, as indicated by decreased high-sensitivity C-reactive protein levels. These findings highlight tirzepatide’s multifaceted cardiometabolic benefits, suggesting that it may be a transformative therapy for managing both obesity and HFpEF by targeting excess adiposity, systemic inflammation, and cardiovascular risk (91). Despite these promising results, a limitation in the field remains the lack of long-term cardiovascular outcomes data for newer agents. To address this, the SURMOUNT-MMO trial is currently underway, investigating tirzepatide’s effects on cardiovascular morbidity and mortality in adults with obesity, irrespective of diabetes status (92). Similarly, SYNCHRONIZE-CVOT (survodutide), a glucagon/GLP-1 receptor co-agonist, is being evaluated in a large-scale randomized controlled trial to assess its cardiovascular safety and efficacy in people with obesity (93). These trials will be pivotal in establishing the long-term benefits and safety of next-generation anti-obesity medications beyond weight loss alone.
In addition to being a serious comorbidity, obstructive sleep apnea syndrome (OSAS) exerts a negative influence on weight loss capacity by impairing muscle energy metabolism, reducing exercise capacity, and altering ghrelin levels that regulate hunger (94). The SCALE sleep study demonstrated that liraglutide significantly improves OSAS, by reducing the apnea-hypopnea index by 12.2 apnea events per hour compared to 6.1 events per hour in the control group (95). This effect may be linked to a reduced GLP1 receptor response observed in individuals with OSAS. In the SURMOUNT-OSA phase 3 trials, tirzepatide markedly reduced the apnea–hypopnea index by up to 29.3 events per hour over 52 weeks compared to minimal reductions with placebo in people with obesity treated with positive airway pressure (estimated treatment difference: 23.8 events per hour (95% CI: −29.6 to −17.9), P<0.001). This improvement was accompanied by substantial weight loss, alongside significant decreases in hypoxic burden, high-sensitivity C-reactive protein levels, and systolic blood pressure. Additionally, patients reported better sleep quality and reduced sleep-related impairment, which in turn can impact weight gain (96). These findings highlight dual benefit in effectively managing obesity-driven sleep apnea, offering a promising pharmacological alternative to traditional mechanical therapies such as positive airway pressure.
Obesity is a significant contributor to reduced fertility and infertility in women (97–100). Women with obesity typically experience poorer reproductive outcomes including longer time to conception, and an increased risk of miscarriage (97–101). One of the leading causes of infertility in women of reproductive age is polycystic ovary syndrome (PCOS), a condition often exacerbated by obesity (97). Weight reduction plays a crucial role in managing infertility and other PCOS-related symptoms, such as hyperlipidemia, hyperandrogenism, decreased insulin sensitivity, and hypertension (102–104). Evidence suggest that PCOS pathogenesis may be linked to alterations in GLP-1 receptors, supporting the use of GLP-1 receptor agonists as a potential treatment in these cases (105, 106). A randomized controlled trial involving women with PCOS and obesity demonstrated that treatment with liraglutide significantly improved reproductive markers including increased sex hormone-binding globulin, decreased free testosterone and ovarian volume, and improved bleeding ratio (107). Although evidence on pregnancy outcomes with GLP-1 receptor agonists is still emerging, two studies offer promising insights. In one trial, women randomized to receive either Exenatide or Metformin, followed by Metformin alone, achieved a natural pregnancy rate over twice as high in the exenatide group compared to the metformin group (43.60% vs 18.70%) (108). Similarly, a small pilot study showed that combining liraglutide with Metformin before conception significantly increased in vitro fertilization pregnancy rates compared to Metformin alone (109).
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a prevalent metabolic disorder that significantly increases the risk of developing cardiovascular disease, type 2 diabetes, chronic kidney disease, liver cirrhosis, and both intra and extrahepatic cancers (110). Addressing MASLD is critical in managing obesity-related complications due to its strong association with metabolic dysfunction. Evidence from a pooled post hoc analysis suggests that naltrexone/bupropion may positively influence liver health by improving the liver fibrosis index and reducing alanine aminotransferase levels (111). However, further research is needed to confirm these findings. GLP-1 receptor agonists might be effective in both reducing cardiovascular disease, and hepatic health (112). Specifically, all three approved GLP-1 agonists - liraglutide, semaglutide, and Exenatide – have shown significant reductions in liver fat content (113–115). The LEAN study highlighted liraglutide’s impact on liver fibrosis progression, where 9% of patients treated with liraglutide experienced fibrosis progression compared to 36% in the placebo group (113). A detailed overview of pharmacological strategies targeting MASLD in the context of obesity management is provided elsewhere (116).
In addition, GLP-1 receptor agonists are effective in managing reduced satiety a common issue in patients with obesity (61). These medications prolong satiety by acting on the central nervous system, and delaying gastric emptying, a physiological response of which the magnitude may serve as predictive marker for future weight loss (61, 117).
Depression and obesity are highly intertwined with each condition increasing the risk of developing the other (118). For patients with coexisting depression and obesity, naltrexone/bupropion can be considered first-line pharmacotherapy due to the antidepressant properties of bupropion, which functions as a noradrenalin and dopamine reuptake inhibitor (119). Supporting this approach, a post hoc analysis of clinical trials indicated that patients with obesity treated with naltrexone/bupropion exhibited lower rates of depression (119). Moreover, some antidepressants and antipsychotic medications are associated with weight gain, potentially worsening obesity. In such cases, transitioning to bupropion either alone or in combination with naltrexone could be a suitable option for managing both mood disorders and obesity, provided this approach is agreed upon with the treating psychiatrist or general practitioner.
Smoking addiction is another critical condition that exacerbates obesity-related health risks, significantly increasing the likelihood of developing or reinforcing cardiovascular disease, type 2 diabetes, and chronic obstructive pulmonary disease (120). Addressing smoking cessation is essential in comprehensive obesity management (120). Notably, bupropion was originally approved for smoking cessation, making naltrexone/bupropion a good choice for patients dealing with both obesity and nicotine dependence (121). In addition, naltrexone/bupropion effectively targets emotional eating due to its dual action on mood regulation, appetite control, and craving suppression (73, 122–125). This combination has shown to reduce the frequency and magnitude of food cravings by reducing the central food reward effect to food stimuli (26, 35). Moreover, a combination of liraglutide and intensive behavioral therapy significantly improved binge eating behaviors and eating disorder psychopathology at 24 weeks, but these benefits attenuated over time (126).
Beyond managing common comorbidities, there is emerging evidence that anti-obesity medications may also improve conditions less frequently associated with obesity such as neurodegenerative diseases and osteoarthritis. Among these, GLP-1 receptor agonists have gained particular attention for its potential neuroprotective effects that may offer therapeutic benefits in Parkinson’s disease (125). This is currently being investigated in a phase 2 clinical trial (127).
Obesity phenotypes
Beyond BMI and comorbidities, a more nuanced classification of obesity can be achieved by considering a patient’s metabolic profile, behavioral traits, and physiological characteristics. Recognizing this complexity, Acosta et al. proposed a phenotypic classification system that stratifies patients into four different subtypes driven by specific pathophysiological and behavioral mechanisms, aiming to tailor therapy accordingly (73). In their study, patients were evaluated based on multiple parameters including body composition, resting energy expenditure, satiety, satiation, eating behavior, emotional affect, and physical activity. From this comprehensive assessment, four obesity phenotypes were identified including hungry brain characterized by abnormal satiation, emotional hunger characterized by hedonic eating, hungry gut characterized by abnormal satiety, and slow burn characterized by a decreased metabolic rate. Pharmacological treatments were selected per phenotype, targeting the main driving factor (phentermine/topiramate for hungry brain, naltrexone/bupropion for emotional hunger, liraglutide for hungry gut, and phentermine for slow burn) (73). This phenotype-driven strategy demonstrated promising results. Patients, who received treatment tailored to their phenotype, achieved significantly greater weight loss after 12 months compared to those receiving standard care (15.9% vs 9.0%, respectively) (73). The concept of this phenotype-driven strategy is rather new. Not all patients fit neatly into one phenotype, and additional undiscovered factors may further refine this model (128). Despite these limitations, phenotype-based treatment holds substantial potential for improving patient outcomes by increasing treatment efficacy, sustainability of weight loss, and optimized use of pharmacotherapy.
Beyond tailoring pharmacotherapy to comorbidities and phenotypes, clinicians must also account for body composition changes that extend beyond fat mass. While the efficacy of anti-obesity medications is relatively well documented, emerging evidence indicates potential adverse effects on muscle and bone mass. GLP-1 receptor agonists, in particular, may lead to disproportionate loss of lean mass, especially in older adults (129). This has implications for frailty, fracture risk, and long-term functional status. A secondary analysis of a randomized clinical trial further supports these concerns, showing that liraglutide treatment alone significantly reduced hip and lumbar spine bone mineral density (BMD) compared with exercise or placebo. However, the combination of liraglutide with moderate- to vigorous-intensity exercise preserved BMD at all clinically relevant sites (66). Until more data are available, clinicians are advised to monitor muscle mass and functional performance especially in at-risk populations.
Future prospects – dose titration and personalized nutrition
Emerging research highlights several promising avenues for enhancing the personalization and effectiveness of obesity pharmacotherapy. While the standard recommended dose of liraglutide is 3.0 mg daily, research suggests that maximum dosage is not always necessary to achieve the same amount of weight loss (130). A retrospective study evaluating real-world liraglutide use implemented weekly up-titration up to 3.0 mg/day (130). However, many patients were unable to tolerate the full dose due to side effects and maintained using lower dose of 1.2, 1.8 or 2.4 mg/day. Surprisingly, patients on these lower doses experienced weight loss comparable to those on the full dose of 3.0 mg, with average weight reductions of 7.4, 7.8, 9.0 and 8.0 kg for dosages of 1.2, 1.8, 2.4 and 3.0 mg, respectively (130). This observation implies that not all patients need to take on the highest dose to have the beneficial effects of liraglutide. Some individuals may have a heightened sensitivity to liraglutide’s effects, potentially due to genetic variations in the GLP-1 receptor or other unknown factors. These findings emphasize the need for individualized dose titration to identify the minimal effective dose per patient, maximizing therapeutic outcomes while minimizing side effects and reducing medication costs. Dose titration can be a new form of tailored treatment, serving as a valuable strategy in precision medicine. However, as this evidence is currently limited to liraglutide, further research is warranted to explore wither similar dose-response variability exists for other anti-obesity medications. Additionally, studies are needed to identify predictive factors that can guide clinicians in tailoring dosages to individual patient profiles.
Another promising option for individualizing obesity is the integration of personalized nutrition. A study by Zeevi et al. demonstrated that tailoring dietary recommendations to individual patients significantly improved postprandial glycemic responses in individuals with diabetes (131). This approach utilized multifaceted data, including blood biomarkers, CGM-derived features, gut microbiome composition, anthropometric measurements, food intake records, and lifestyle questionnaires to develop customized dietary plans (131). Due to the interrelatedness of obesity and type 2 diabetes, a similar strategy in obesity management could enhance the effectiveness of lifestyle interventions. While precision nutrition holds significant potential, its detailed exploration is beyond the scope of this review and has been reviewed elsewhere (132).
Expanding on earlier discussions, genetic profiling holds potential for advancing personalized obesity treatment. Variations in genes encoding drug targets, such as GLP-1 receptor polymorphisms, have been linked in clinical research to differential responses to medications like liraglutide and naltrexone/bupropion’s. Incorporating pharmacogenomic testing could enable clinicians to predict which patients will respond best to specific medications, allowing for more effective and individualized treatment strategies. For now, insufficient data limits its current clinical utility.
Developing an algorithm for personalized obesity treatment
To achieve more precise and effective obesity management tailored to individual patient’s needs, the integration of various clinical and behavioral factors into a structured treatment pathway is essential. For this approach to be practical for clinicians, a streamlined, evidence-based guiding tool is required. A useful starting point is to draw parallels between obesity and other chronic, multifactorial diseases such as type 2 diabetes and arterial hypertension (133, 134). These conditions share complex etiologies and require long-term management strategies. In the management of type 2 diabetes, the American Diabetes Association and the European Association for the Study of Diabetes emphasize a holistic patient-centered approach that considers comorbidities like obesity, chronic kidney disease, hypertension and cardiovascular disease when selecting treatments (56). This paradigm could serve as a model for obesity care, moving towards a personalized, stepwise treatment plan similar to established hypertension guidelines, where first-line treatments are selected based on patient profiles and therapeutic needs (135). However, a major challenge in translating such frameworks to obesity lies in the lack of universally accepted, objective stratifiers limiting precise classification. Therefore, we emphasize a risk-factor-based escalation model, where comorbidities, treatment response, and functional limitations guide progression through treatment steps. Inspired by this approach and integrating factors previously discussed, we propose a preliminary algorithm for obesity management. Although this model does not fully encompass all aspects of precision medicine, it serves as a practical foundation for individualized treatment. Each transition in our proposed algorithm is grounded in trial evidence, guideline thresholds, and comparative safety/efficacy profiles. The first step involves stratifying patients based on their predominant obesity phenotype, while recognizing overlap and individual variability. These are partially informed by the subtypes identified by Acosta et al. and partially by the health of the patient (73) (Figure 3). This distinction classifies patients into two dominant categories: a metabolic phenotype, characterized by comorbidities such as type 2 diabetes, hypertension, obstructive sleep apnea, and polycystic ovary syndrome), and a central/behavioral phenotype, dominated by behavioral and psychological factors such as increased cravings, emotional eating, smoking addiction, and depression. Regardless of phenotype, lifestyle modification, including dietary changes and increased physical activity, remains the foundational treatment (10, 11). Based on their clinical profile, patients can receive gut hormone-based therapy (i.e. semaglutide, liraglutide, or tirzepatide) or central acting therapy (i.e. naltrexone/bupropion, or phentermine/topiramate) as a first-choice treatment. Patients with metabolic risk factors or established comorbidities benefit most from gut hormone-based therapies (62, 87, 88, 95, 97, 136). Although most evidence supports semaglutide and liraglutide, tirzepatide, as a dual GLP-1 and GIP receptor agonist, likely offers similar benefits, though further research is warranted (137). Patients struggling with obesity and concurrent smoking addiction, depression, or emotional eating, should begin treatment with centrally acting medication such as naltrexone/bupropion or phentermine/topiramate (119, 121).
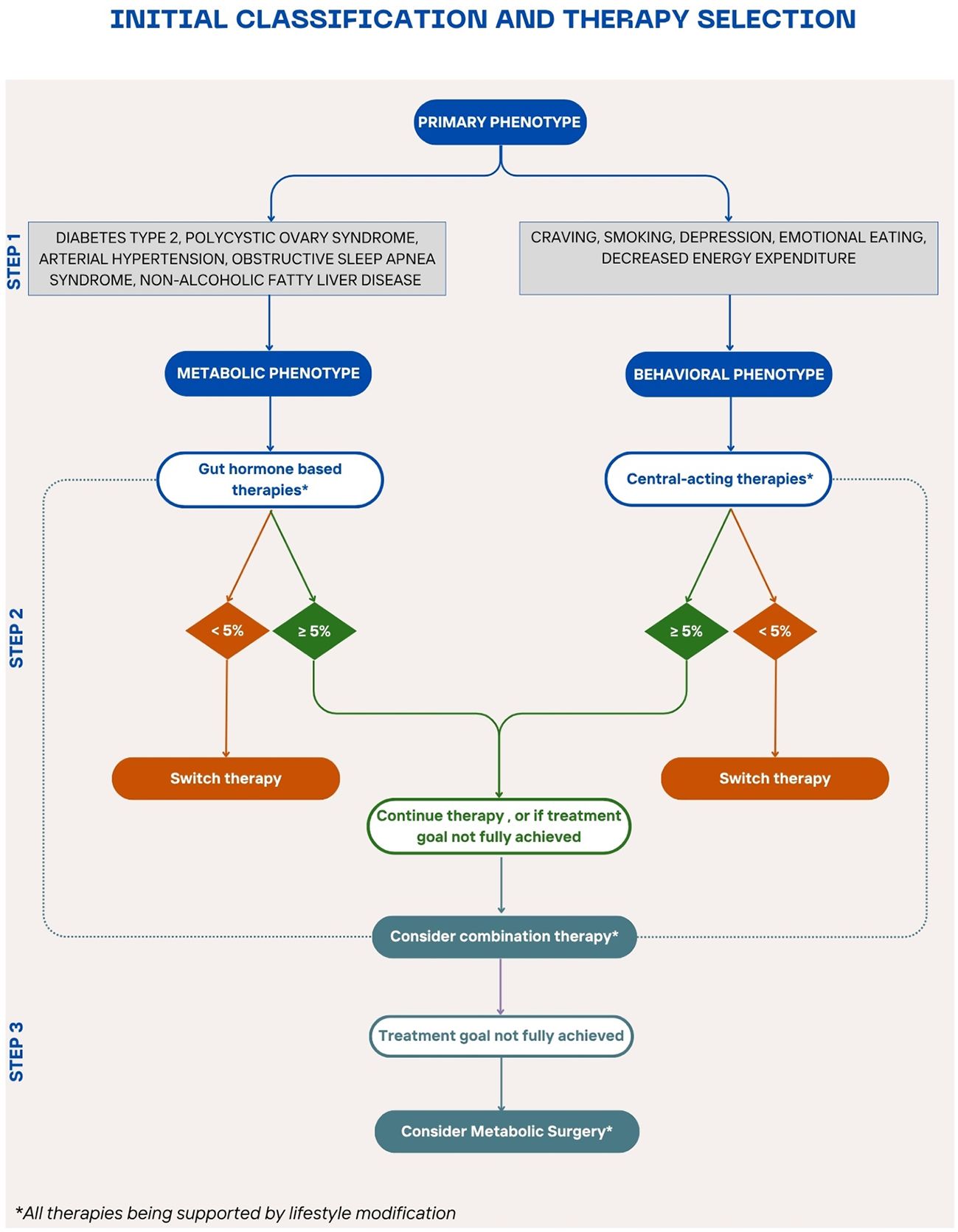
Figure 3. Stepwise algorithm for personalized obesity pharmacotherapy A proposed treatment algorithm integrating patient phenotypes, comorbidities, and treatment responses to guide personalized obesity management for patients who do not meet the criteria for metabolic surgery or have contraindications or not open to this option. The algorithm begins with lifestyle modification as the foundation, followed by phenotype-driven pharmacotherapy selection—gut hormone-based therapy for metabolic comorbidities and centrally acting therapy for behavioral/psychological factors. Treatment response is evaluated after 3 months to determine the need for therapy adjustment, combination treatment, or escalation to bariatric surgery for non-responders .
When initiating pharmacotherapy, patients should be closely monitored to assess early response to pharmacotherapy. Dose titration is critical as some patients may be more sensitive to lower dosages and can achieve effective weight loss without reaching the maximum recommended dose (130). After three months, evaluate if the patient has achieved a weight loss of 5% or more in non-diabetic patients, or 3% or more in diabetic patients in line with the European guidelines for obesity management (11). Responders should continue their current therapy, while non-responders should transition to an alternative pharmacotherapy or initiate combination therapy. While combination pharmacotherapy is promising, there are currently no formal guidelines, and robust clinical trials are needed to validate this strategy. If weight loss goals remain unmet after trying alternative or combination pharmacotherapy, escalation to more invasive treatments such as metabolic bariatric surgery should be considered. Bariatric procedures have demonstrated superior long-term weight loss outcomes but are typically reserved for patients with severe obesity or those who do not achieve clinically relevant improvements with less invasive interventions. Overall, our algorithm is intended not as a rigid decision tree, but as a flexible, risk-stratified framework that supports clinical reasoning and individualized care. Undoubtedly, further clinical research is essential to refine this model, validate predictive markers of treatment response, and establish clear guidelines for combination therapies.
Future treatments and remaining research gaps
Ongoing research in obesity pharmacotherapy continues to yield promising developments, with two treatments particularly relevant for their potential impact. Retatrutide, a triple agonist targeting the GLP-1, GIP, and glucagon receptors, represents a significant advancement beyond dual agonists like tirzepatide. In phase II trials, retatrutide demonstrated a mean weight loss of 24.2% at 48 weeks at the highest dosage (12 mg daily), compared to a 2.1% reduction in the placebo group, exceeding the impressive results achieved by tirzepatide (45, 138). Its safety profile thus far aligns with other approved incretin-based therapies, suggesting it could be a transformative option in the future for obesity management (136).
In addition to retatrutide, a growing pipeline of emerging compounds includes other multi-agonists such as Survodutide (GLP-1/glucagon co-agonist), and Cagrilintide (a long-acting amylin analogue), all currently being evaluated in advanced clinical trials (139). While each agent targets different hormonal pathways, these candidates reflect a broader strategy to enhance efficacy and address the limitations of existing therapies. Despite the efficacy of incretin-based therapies, their reliance on subcutaneous administration might limit convenience for some patients. The development of oral formulations addresses this challenge. An oral form of semaglutide, Rybelsus, is approved for diabetes but requires administration 30 minutes before meals for efficacy (140, 141). An oral GLP-1 receptor agonist, orfoglipron, has demonstrated promising results in phase II trials for obesity, with weight loss ranging from 9.4% to 14.7% over 36 weeks, comparable to other injectable GLP-1 analogues (142). While tirzepatide already exists as an incretin-based drug with superior weight loss outcomes, an oral formulation like orfoglipron could may increase treatment acceptability and adherence. A detailed overview of emerging pharmacotherapies is provided elsewhere (139).
Despite the substantial progress in obesity pharmacotherapy, several critical gaps persist. First, the long-term safety, and effects on mortality remain incompletely established for some agents. Second, post-marketing data reveal that real-world adherence and persistence are lower than in clinical trials, often due to gastrointestinal side effects, treatment fatigue, or cost-related barriers (143–145). Third, additional real-world effectiveness data are urgently needed to complement trial findings and assess outcomes in more diverse populations (146). In addition, real-world data on cost-effectiveness and optimal treatment duration are limited. For instance, one study reported that although tirzepatide would avert 45 609 obesity cases (95% uncertainty interval (UI): 45 092 - 46 126) per 100 000 individuals and semaglutide would avert 32 087 cases (95% UI: 31 292 - 32 882) per 100 000 individuals, their respective incremental cost-effectiveness ratios in the United States were $197 023 per QALY and $467 676 per QALY in the US. To reach the $100 000/QALY threshold, the current net prices would need to be reduced by 30.5% for tirzepatide and 81.9% for semaglutide. This raises questions regarding long-term economic sustainability of these therapies, if prices remain unaltered (147). Fourth, head-to-head comparative effectiveness studies remain scarce, which limits the available evidence to guide clinical decisions on optimal treatment sequencing, switching between therapies, and the potential benefits or risks of combination therap. Fifth, regulatory and reimbursement varies across countries and regions, affecting its widespread implementation (148). Finally, the integration of pharmacogenomics into treatment guidelines is not yet feasible due to insufficient clinical validation, although early data are promising. Addressing these unanswered questions should be a priority for future research.
Conclusion
Obesity remains an increasing health problem worldwide. Its treatment goes on to be a complex issue, with a variety of factors influencing treatment outcomes. While metabolic bariatric surgery has for a long term been the only effective weight loss intervention for morbid obesity, newly developing pharmacotherapy are reaching comparable results, offering both an alternative and a complementary approach. Still, outcomes of treatment remain variable. While previous reviews have highlighted the promise of obesity pharmacotherapy, we outline different factors that influence patients’ response to treatment, explore the options of added benefits by using medication specifically selected and targeted to patients’ comorbidities and their personal phenotype. Furthermore, this review uniquely proposes a stepwise, phenotype-driven treatment algorithm and synthesizes evidence on dose titration, genetic markers of drug response, and combination strategies to advance individualized care. While more research is needed to strengthen this type of approach, it can be a step towards more effective and efficient obesity treatment.
Author contributions
NS: Writing – original draft, Writing – review & editing. JT: Writing – original draft, Writing – review & editing. ED: Writing – review & editing. AM: Writing – review & editing. ML: Writing – review & editing. SP: Writing – review & editing. AV: Writing – review & editing. BV: Writing – review & editing. RV: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fendo.2025.1656611.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Heymsfield SB and Wadden TA. Mechanisms, pathophysiology, and management of obesity. New Engl J Med. (2017) 376:254–66. doi: 10.1056/NEJMra1514009
2. World Health Organization. Obesity and overweight. Geneva: World Health Organization (2024). Fact sheet. Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
4. Lung T, Jan S, Tan EJ, Killedar A, and Hayes A. Impact of overweight, obesity and severe obesity on life expectancy of Australian adults. Int J Obes. (2019) 43(4):782–9. doi: 10.1038/s41366-018-0210-2
5. Wu Y-K and Berry DC. Impact of weight stigma on physiological and psychological health outcomes for overweight and obese adults: A systematic review. J Advanced Nursing. (2018) 74:1030–42. doi: 10.1111/jan.2018.74.issue-5
6. Moulac M, Aouati O, Carletti G, Pelsy F, van den Bos S, Kirk SFL, et al. Current challenges and opportunities for addressing obesity. Study requested by the Subcommittee on Public Health (SANT). Luxembourg: European Parliament, Policy Department for Economic, Scientific and Quality of Life Policies (2024). Available online at: https://www.europarl.europa.eu/RegData/etudes/STUD/2024/754218/IPOL_STU(2024)754218_EN.pdf.
7. King SE and Skinner MK. Epigenetic transgenerational inheritance of obesity susceptibility. Trends Endocrinol Metab. (2020) 31:478–94. doi: 10.1016/j.tem.2020.02.009
8. Okunogbe A, Nugent R, Spencer G, Powis J, Ralston J, Wilding J, et al. Economic impacts of overweight and obesity: current and future estimates for 161 countries. World Obesity Atlas (2022) 7(9):e009773. doi: 10.1136/bmjgh-2022-009773
9. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:1. doi: 10.1038/s41574-019-0176-8
10. Mechanick J, Pessah- Pollack R, Camacho P, Correa R, Figaro M, Garber J, et al. American association of clinical endocrinologists and american college of endocrinology protocol for standardized production of clinical practice guidelines, algorithms, and checklists – 2017 update. Endocrine Practice. (2017) 23:1006–21. doi: 10.4158/EP171866.GL
11. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European guidelines for obesity management in adults. Obes Facts. (2015) 8:402–24. doi: 10.1159/000442721
12. Kheniser K, Saxon D, and Kashyap S. Long-term weight loss strategies for obesity. J Clin Endocrinol Metab. (2021) 106(7):1854–66. doi: 10.1210/clinem/dgab091
13. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes — 5-year outcomes. New Engl J Med. (2017) 376:641–51. doi: 10.1056/NEJMoa1600869
14. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Internal Med. (2012) 273(3):219–34. doi: 10.1111/joim.12012
15. Phillips B and Shikora S. The history of metabolic and bariatric surgery: Development of standards for patient safety and efficacy. Metabolism. (2018) 79:97–107. doi: 10.1016/j.metabol.2017.12.010
16. Albaugh VL, He Y, Münzberg H, Morrison CD, Yu S, and Berthoud H-R. Regulation of body weight: Lessons learned from bariatric surgery. Mol Metab. (2023) 68:101517. doi: 10.1016/j.molmet.2022.101517
17. Raj P and Majumdar S. Drug treatments for obesity: Orlistat, sibutramine, and rimonabant. Lancet. (2007) 369:71–7. doi: 10.1016/S0140-6736(07)60033-6
18. Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: A systematic review and meta-analysis. JAMA. (2016) 315:2424–34. doi: 10.1001/jama.2016.7602
19. Di Lorenzo N, Antoniou SA, Batterham RL, Busetto L, Godoroja D, Iossa A, et al. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg Endoscopy. (2020) 34:2332–58. doi: 10.1007/s00464-020-07555-y
20. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. New Engl J Med. (2021) 384:989–1002. doi: 10.1056/NEJMoa2032183
21. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New Engl J Med. (2015) 373:11–22. doi: 10.1056/NEJMoa1411892
22. Sjöström L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HPF, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet. (1998) 352:167–72. doi: 10.1016/S0140-6736(97)11509-4
23. Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. New Engl J Med. (2022) 387:205–16. doi: 10.1056/NEJMoa2206038
24. Gadde K, Allison D, and Ryan D. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. (2011) 377(9774):1341–52. doi: 10.1016/S0140-6736(11)60205-5
25. Allison D, Gadde K, Garvey W, Peterson C, Schwiers M, Najarian T, et al. Controlled-release phentermine/topiramate in severely obese adults: A randomized controlled trial (EQUIP). Obes (Silver Spring Md). (2011) 20(2):330–42. doi: 10.1038/oby.2011.330
26. Apovian C, Aronne L, Rubino D, Still C, Wyatt H, Burns C, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obes (Silver Spring Md). (2013) 21(5):935–43. doi: 10.1002/oby.20309
27. Wadden T, West D, Delahanty L, Jakicic J, Rejeski J, Williamson D, et al. The look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obes (Silver Spring Md). (2006) 14:737–52. doi: 10.1038/oby.2006.84
28. Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, and Elisaf MS. Orlistat-associated adverse effects and drug interactions. Drug Safety. (2008) 31:53–65. doi: 10.2165/00002018-200831010-00005
29. Billes SK, Sinnayah P, and Cowley MA. Naltrexone/bupropion for obesity: An investigational combination pharmacotherapy for weight loss. Pharmacol Res. (2014) 84:1–11. doi: 10.1016/j.phrs.2014.04.004
30. Smith SM, Meyer M, and Trinkley KE. Phentermine/topiramate for the treatment of obesity. Ann Pharmacotherapy. (2013) 47:340–9. doi: 10.1345/aph.1R501
31. Andersen A, Lund A, Knop FK, and Vilsboll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol. (2018) 14:390–403. doi: 10.1038/s41574-018-0016-2
32. Eli Lilly and Company. FDA approves Lilly’s Zepbound™ (tirzepatide) for chronic weight management. Lilly Investor Relations. (2023). Available online at: https://investor.lilly.com/news-releases/news-release-details/fda-approves-lillys-zepboundtm-tirzepatide-chronic-weight.
33. Liu L, Li Z, Ye W, Peng P, Wang Y, Wan L, et al. Safety and effects of anti-obesity medications on weight loss, cardiometabolic, and psychological outcomes in people living with overweight or obesity: a systematic review and meta-analysis. EClinicalMedicine. (2025) 79:103020. doi: 10.1016/j.eclinm.2024.103020
34. Rissanen A, Lean M, Rössner S, Segal K, and Sjöström L. Predictive value of early weight loss in obesity management with orlistat: An evidence-based assessment of prescribing guidelines. Int J Obes related Metab Disord. (2003) 27:103–9. doi: 10.1038/sj.ijo.0802165
35. Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2010) 376:595–605. doi: 10.1016/S0140-6736(10)60888-4
36. European Medicines Agency. Xenical. (London: European Medicines Agency) (2018). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/xenical.
37. Garvey W, Ryan D, Look M, Gadde K, Allison D, Peterson C, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): A randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. (2011) 95:297–308. doi: 10.3945/ajcn.111.024927
39. European Medicines Agency. Mounjaro - opinion on variation to marketing authorisation. textbar European Medicines Agency.
40. Bray GA and Ryan DH. Evidence-based weight loss interventions: Individualized treatment options to maximize patient outcomes. Diabetes Obes Metab. (2021) 23:50–62. doi: 10.1111/dom.v23.S1
41. Fujioka K, Plodkowski R, O’Neil P, Gilder K, Walsh B, and Greenway F. The relationship between early weight loss and weight loss at 1 year with naltrexone ER/bupropion ER combination therapy. Int J Obes. (2005) . 2016:40. doi: 10.1038/ijo.2016.67
42. Fujioka K, O’Neil P, Davies M, Greenway F, Lau D, Claudius B, et al. Early Weight Loss with Liraglutide 3.0 mg Predicts 1-Year Weight Loss and is Associated with Improvements in Clinical Markers: Liraglutide 3.0 mg: Early Response, 1-Year Outcomes. Obesity. (2016) 24:2278–88. doi: 10.1002/oby.v24.11
43. Mullally J, Chung W, Leduc C, Reid T, Febres G, Holleran S, et al. Weight loss response to naltrexone/bupropion is modulated by the taq1A genetic variant near DRD2 (rs1800497): A pilot study. Diabetes Obes Metab. (2020) 23:850–3. doi: 10.1111/dom.14267
44. Kyriakidou A, Kyriazou A, Koufakis T, Vasilopoulos Y, Grammatiki M, Tsekmekidou X, et al. Clinical and genetic predictors of glycemic control and weight loss response to liraglutide in patients with type 2 diabetes. J Personalized Med. (2022) 12:424. doi: 10.3390/jpm12030424
45. Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, et al. Triple–hormone-receptor agonist retatrutide for obesity — A phase 2 trial. New Engl J Med. (2023) 389:514–26. doi: 10.1056/NEJMoa2301972
46. Kahan S and Fujioka K. Obesity pharmacotherapy in patients with type 2 diabetes. Diabetes Spectr. (2017) 30:250–7. doi: 10.2337/ds17-0044
47. Hu S, Lin C, Cai X, Li Z, Lv F, Yang W, et al. Trends in baseline HbA1c and body-mass index in randomised placebo-controlled trials of type 2 diabetes from 1987 to 2022: a systematic review and meta-analysis. eClinicalMedicine. (2023) 57:101868. doi: 10.1016/j.eclinm.2023.101868
48. Russell-Jones D and Khan R. Insulin-associated weight gain in diabetes – causes, effects and coping strategies. Diabetes Obes Metab. (2007) 9:799–812. doi: 10.1111/j.1463-1326.2006.00686.x
49. Apovian CM, Okemah J, and O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. (2019) 36:44–58. doi: 10.1007/s12325-018-0824-8
50. Galgani J and Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes (2005). (2009) 32 Suppl 7:S109–19. doi: 10.1038/ijo.2008.246
51. Bagg W, Plank LD, Gamble G, Drury PL, Sharpe N, and Braatvedt GD. The effects of intensive glycaemic control on body composition in patients with type 2 diabetes. Diabetes Obes Metab. (2001) 3:410–6. doi: 10.1046/j.1463-1326.2001.00153.x
52. Lilly’s tirzepatide achieved up to 15.7% weight loss in adults with obesity or overweight and type 2 diabetes in SURMOUNT-2. Eli Lilly and Company.
53. Garvey WT, Frias JP, Jastreboff AM, le Roux CW, Sattar N, Aizenberg D, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. (2023) 402:613–26. doi: 10.1016/S0140-6736(23)01200-X
54. Frandsen CS and Madsbad S. SURMOUNT-2: new advances for treating obese type 2 diabetes with tirzepatide. Lancet. (2023) 402:586–8. doi: 10.1016/S0140-6736(23)01292-8
55. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. (2018) 61:2461–98. doi: 10.1007/s00125-018-4729-5
56. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. (2022) 65:1925–66. doi: 10.1007/s00125-022-05787-2
57. McCaffery JM, Jablonski KA, Pan Q, Astrup A, Revsbech Christiansen M, Corella D, et al. Genetic predictors of change in waist circumference and waist-to-hip ratio with lifestyle intervention: the trans-NIH consortium for genetics of weight loss response to lifestyle intervention. Diabetes. (2022) 71:669–76. doi: 10.2337/db21-0741
58. Dawed AY, Mari A, Brown A, McDonald TJ, Li L, Wang S, et al. Pharmacogenomics of GLP-1 receptor agonists: a genome-wide analysis of observational data and large randomised controlled trials. Lancet Diabetes Endocrinology. (2023) 11:33–41. doi: 10.1016/S2213-8587(22)00340-0
59. de Luis DA, Diaz Soto G, Izaola O, and Romero E. Evaluation of weight loss and metabolic changes in diabetic patients treated with liraglutide, effect of RS 6923761 gene variant of glucagon-like peptide 1 receptor. J Diabetes Complications. (2015) 29:595–8. doi: 10.1016/j.jdiacomp.2015.02.010
60. Janez A. Genetic variability in GLP-1 receptor is associated with inter-individual differences in weight lowering potential of liraglutide in obese women with PCOS: a pilot study. Eur J Clin Pharmacol. (2015). doi: 10.1007/s00228-015-1868-1
61. Halawi H, Khemani D, Eckert D, O’Neill J, Kadouh H, Grothe K, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatology. (2017) 2:890–9. doi: 10.1016/S2468-1253(17)30285-6
62. Acosta A, Camilleri M, Shin A, Vazquez-Roque MI, Iturrino J, Burton D, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology. (2015) 148:537–46.e4. doi: 10.1053/j.gastro.2014.11.020
63. Miras A, Pevida BE, Aldhwayan M, Kamocka A, McGlone ER, Al-Najim W, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. (2019) 7:549–59. doi: 10.1016/S2213-8587(19)30157-3
64. Wharton S, Kuk JL, Luszczynski M, Kamran E, and Christensen RAG. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post-bariatric surgery. Clin Obes. (2019) 9:e12323. doi: 10.1111/cob.12338
65. Wadden T, Hollander P, Klein S, Niswender K, Woo V, Hale P, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: The SCALE Maintenance randomized study. Int J Obes. (2005) . 2013:39. doi: 10.1038/ijo.2013.120
66. Lundgren JR, Janus C, Jensen SBK, Juhl CR, Olsen LM, Christensen RM, et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. New Engl J Med. (2021) 384:1719–30. doi: 10.1056/NEJMoa2028198
67. Jensen SBK, Blond MB, Sandsdal RM, Olsen LM, Juhl CR, Lundgren JR, et al. Healthy weight loss maintenance with exercise, GLP-1 receptor agonist, or both combined followed by one year without treatment: a post-treatment analysis of a randomised placebo-controlled trial. eClinicalMedicine. (2024) 69:1719–30. doi: 10.1016/j.eclinm.2024.102475
68. Raj P, Pajewski N, Allison D, and Sharma A. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ. (2011) 183:E1059–66. doi: 10.1503/cmaj.110387
69. Field A, Camargo C, and Ogino S. The merits of subtyping obesity one size does not fit all. JAMA. (2013) 310:2147–8.
70. Michelle K and Neha P. Towards a personalised approach for obesity treatment: one size does not fit all. Heart. (2021) 107:1526. doi: 10.1136/heartjnl-2021-319726
71. A M and Acosta A. Precision medicine and obesity. Gastroenterol Clinics North America. (2021) 50:127–139. doi: 10.1016/j.gtc.2020.10.005
72. Collins F and Varmus H. A new initiative on precision medicine. New Engl J Med. (2015) 372:793–5. doi: 10.1056/NEJMp1500523
73. Acosta A, Camilleri M, Abu Dayyeh B, Calderon G, Gonzalez D, McRae A, et al. Selection of antiobesity medications based on phenotypes enhances weight loss: A pragmatic trial in an obesity clinic. Obesity. (2021) 29:662–71. doi: 10.1002/oby.23120
74. Guglielmi V, Bettini S, Sbraccia P, Busetto L, Pellegrini M, Yumuk V, et al. Beyond weight loss: added benefits could guide the choice of anti-obesity medications. Curr Obes Rep. (2023) 12(2):127–46. doi: 10.1007/s13679-023-00502-7
75. Roberts CA, Christiansen P, and Halford JCG. Tailoring pharmacotherapy to specific eating behaviours in obesity: Can recommendations for personalised therapy be made from the current data? Acta Diabetologica. (2017) 54:715–25. doi: 10.1007/s00592-017-0994-x
76. Lingvay I, Sumithran P, Cohen RV, and le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. (2022) 399:394–405. doi: 10.1016/S0140-6736(21)01919-X
77. Sorli C, Harashima S-I, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinology. (2017) 5:251–60. doi: 10.1016/S2213-8587(17)30013-X
78. Madsbad S. Liraglutide effect and action in diabetes (LEADTM) trial. Expert Rev Endocrinol Metab. (2009) 4:119–29. doi: 10.1586/17446651.4.2.119
79. Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. (2021) 398:143–55. doi: 10.1016/S0140-6736(21)01324-6
80. Ludvik B, Giorgino F, Jódar E, Frias JP, Fernández Landóo L, Brown K, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. (2021) 398:583–98. doi: 10.1016/S0140-6736(21)01443-4
81. Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. (2021) 398:1811–24. doi: 10.1016/S0140-6736(21)02188-7
82. Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. New Engl J Med. (2021) 385:503–15. doi: 10.1056/NEJMoa2107519
83. Jastreboff AM, le Roux CW, Stefanski A, Aronne LJ, Halpern B, Wharton S, et al. Tirzepatide for obesity treatment and diabetes prevention. N Engl J Med. (2025) 392:958–71. doi: 10.1056/NEJMoa2410819
84. Hollander P, Gupta A, Plodkowski R, Greenway F, Bays H, Burns C, et al. Effects of naltrexone sustained- release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. (2013) 36:4022–9. doi: 10.2337/dc13-0234
85. Nissen S, Wolski K, Prcela L, Wadden T, Buse J, Bakris G, et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors. JAMA. (2016) 315:990. doi: 10.1001/jama.2016.1558
86. Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. New Engl J Med. (2023) 389(24):2221–32. doi: 10.1056/NEJMoa2307563
87. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. New Engl J Med. (2016) 375:311–22. doi: 10.1056/NEJMoa1603827
88. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New Engl J Med. (2019) 381:841–51. doi: 10.1056/NEJMoa1901118
89. Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. New Engl J Med. (2023) 389:1069–84. doi: 10.1056/NEJMoa2306963
90. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2016) 375:1834–44. doi: 10.1056/NEJMoa1607141
91. Packer M, Zile MR, Kramer CM, Baum SJ, Litwin SE, Menon V, et al. Tirzepatide for heart failure with preserved ejection fraction and obesity. N Engl J Med. (2025) 392:427–37. doi: 10.1056/NEJMoa2410027
92. Abdul Wahab R and le Roux CW. A review of the evidence on cardiovascular outcomes from obesity treatment. Obes Pillars. (2023) 7:100071. doi: 10.1016/j.obpill.2023.100071
93. Kosiborod MN, Platz E, Wharton S, le Roux CW, Brueckmann M, Ajaz Hussain S, et al. Survodutide for the treatment of obesity: rationale and design of the SYNCHRONIZE cardiovascular outcomes trial. JACC Heart Fail. (2024) 12:2101–9. doi: 10.1016/j.jchf.2024.09.004
94. Sevencan B, Steenackers N, van Laar ADE, Lucio SP, Buyse B, Kalkanis A, et al. Evaluating the potential of metabolic drugs in obstructive sleep apnea and obesity: a narrative review. J Clin Sleep Med. (2025) 21. doi: 10.5664/jcsm.11682
95. Blackman A, Foster G, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: The SCALE Sleep Apnea randomized clinical trial. Int J Obes. (2005) . 2016:40. doi: 10.1038/ijo.2016.52
96. Malhotra A, Bednarik J, Chakladar S, Dunn JP, Weaver T, Grunstein R, et al. Tirzepatide for the treatment of obstructive sleep apnea: Rationale, design, and sample baseline characteristics of the SURMOUNT -OSA phase 3 trial. Contemp Clin Trials. (2024) 141:107516. doi: 10.1016/j.cct.2024.107516
97. Cena H, Chiovato L, and Nappi RE. Obesity, polycystic ovary syndrome, and infertility: A new avenue for GLP-1 receptor agonists. J Clin Endocrinol Metab. (2020) 105:e2695–e709. doi: 10.1210/clinem/dgaa285
98. Talmor A and Dunphy B. Female obesity and infertility. Obes Gynaecology. (2015) 29:498–506. doi: 10.1016/j.bpobgyn.2014.10.014
99. Ramlau-Hansen C, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TIA, and Olsen J. Subfecundity in overweight and obese couples. Hum Reprod (Oxford England). (2007) 22:1634–7. doi: 10.1093/humrep/dem035
100. Steeg J, Steures P, Eijkemans M, Habbema J, Hompes P, Burggraaff J, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod (Oxford England). (2008) 23:324–8. doi: 10.1093/humrep/dem371
101. Metwally M, Ong KJ, Ledger WL, and Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertility Sterility. (2008) 90:714–26. doi: 10.1016/j.fertnstert.2007.07.1290
102. Thomson R, Buckley J, Noakes M, Clifton P, Norman R, and Brinkworth G. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2008) 93:3373–80. doi: 10.1210/jc.2008-0751
103. Haqq L, McFarlane J, Dieberg G, and Smart N. Lifestyle intervention and endocrine profile in polycystic ovarian syndrome:a meta-analysis. Endocrine connections. (2014) 3:36–46. doi: 10.1530/EC-14-0010
104. Lim SS, Hutchison SK, Van Ryswyk E, Norman RJ, Teede HJ, and Moran LJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database systematic Rev. (2019) 3:CD007506. doi: 10.1002/14651858.CD007506.pub4
105. Vrbikova J, Hill M, Bendlova B, Grimmichova T, Dvorakova K, Vondra K, et al. Incretin levels in polycystic ovary syndrome. Eur J Endocrinol. (2008) 159:121–7. doi: 10.1530/EJE-08-0097
106. Ozgen Saydam B and Yildiz B. Gut-brain axis and metabolism in polycystic ovary syndrome. Curr Pharm design. (2016) 22:5572–87. doi: 10.2174/1381612822666160715143933
107. Nylander M, Frøssing S, Clausen H, Kistorp C, Faber J, and Skouby S. Effects of liraglutide on ovarian dysfunction in polycystic ovary syndrome: A randomized clinical trial. Reprod BioMedicine Online. (2017) 35:121–7. doi: 10.1016/j.rbmo.2017.03.023
108. Liu X, Zhang Y, Zheng S, Lin R, Y-j X, Chen H, et al. Efficacy of Exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome. Clin Endocrinol. (2017) 87):767–74. doi: 10.1111/cen.2017.87.issue-6
109. Salamun V, Jensterle Sever M, Janez A, and Vrtacnik Bokal E. Liraglutide increases IVF pregnancy rates in obese PCOS women with poor response to first-line reproductive treatments: a pilot randomized study. Eur J Endocrinol. (2018) 179:EJE–18. doi: 10.1530/EJE-18-0175
110. Targher G, Tilg H, and Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatology. (2021) 6:578–88. doi: 10.1016/S2468-1253(21)00020-0
111. Bajaj HS, Burrows M, Blavignac J, Paron E, Camacho F, Gould E, et al. Extended-release naltrexone/bupropion and liver health: Pooled, post hoc analysis from four randomized controlled trials. Diabetes Obes Metab. (2021) 23:861–5. doi: 10.1111/dom.14284
112. Mantovani A, Byrne CD, and Targher G. Efficacy of peroxisome proliferator-activated receptor agonists, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors for treatment of non-alcoholic fatty liver disease: a systematic review. Lancet Gastroenterol Hepatology. (2022) 7:367–78. doi: 10.1016/S2468-1253(21)00261-2
113. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. (2016) 387:679–90. doi: 10.1016/S0140-6736(15)00803-X
114. Gastaldelli A, Cusi K, Landó L, Bray R, Brouwers B, and Rodríguez A. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. (2022) 10:393–406. doi: 10.1016/S2213-8587(22)00070-5
115. Flint A, Andersen G, Hockings P, Johansson L, Morsing A, Palle M, et al. Randomised clinical trial: Semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with nonalcoholic fatty liver disease assessed by magnetic resonance imaging. Alimentary Pharmacol Ther. (2021) 54:1150–61. doi: 10.1111/apt.16608
116. Kokkorakis M, Muzurovic E, Volcansek S, Chakhtoura M, Hill MA, Mikhailidis DP, et al. Steatotic liver disease: pathophysiology and emerging pharmacotherapies. Pharmacol Rev. (2024) 76:454–99. doi: 10.1124/pharmrev.123.001087
117. Halawi H, Camilleri M, Acosta A, Vazquez-Roque M, Oduyebo I, Burton D, et al. Relationship of gastric emptying or accommodation with satiation, satiety, and postprandial symptoms in health. Am J Physiology-Gastrointestinal Liver Physiol. (2017) 313:G442–G7. doi: 10.1152/ajpgi.00190.2017
118. Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. (2006) 63:824–30. doi: 10.1001/archpsyc.63.7.824
119. Pi-Sunyer X, Apovian C, McElroy S, Dunayevich E, Acevedo L, and Greenway F. Psychiatric adverse events and effects on mood with prolonged-release naltrexone/bupropion combination therapy: a pooled analysis. Int J Obes. (2019) 43:2085–94. doi: 10.1038/s41366-018-0302-z
120. Maritz G and Mutemwa M. Tobacco smoking: patterns, health consequencesfor adults, and the long-term health of the offspring. Global J Health science. (2012) 4:62–75. doi: 10.5539/gjhs.v4n4p62
121. Warner C and Shoaib M. How does bupropion work as a smoking cessation aid? Addict Biol. (2005) 10:219–31.
122. Acosta A, Streett S, Kroh M, Cheskin L, Saunders K, Kurian M, et al. White paper AGA: POWER — Practice guide on obesity and weight management, education and resources. Clin Gastroenterol Hepatol. (2017) 15:631-649.e10. doi: 10.1016/j.cgh.2016.10.023
123. Wang G-J, Zhao J, Tomasi D, Kojori ES, Wang R, Wiers CE, et al. Effect of combined naltrexone and bupropion therapy on the brain’s functional connectivity. Int J Obes. (2018) 42:1890–9. doi: 10.1038/s41366-018-0040-2
124. Rebello CJ and Greenway FL. Reward-induced eating: therapeutic approaches to addressing food cravings. Adv Ther. (2016) 33:1853–66. doi: 10.1007/s12325-016-0414-6
125. Kim DS, Choi H-I, Wang Y, Luo Y, Hoffer BJ, and Greig NH. A new treatment strategy for parkinson’s disease through the gut–brain axis: the glucagon-like peptide-1 receptor pathway. Cell Transplantation. (2017) 26:1560–71. doi: 10.1177/0963689717721234
126. Chao AM, Wadden TA, Walsh OA, Gruber KA, Alamuddin N, Berkowitz RI, et al. Effects of liraglutide and behavioral weight loss on food cravings, eating behaviors, and eating disorder psychopathology. Obesity. (2019) 27:2005–10. doi: 10.1002/oby.v27.12
127. Tagliati M. A Phase II, Randomized, Double-blinded, Placebo-controlled Trial of Liraglutide in Parkinson’s Disease. Bethesda, MD: U.S: Clinical trial registration (2022).
128. Zandvakili I, Pulaski M, and Pickett-Blakely O. A phenotypic approach to obesity treatment. Nutr Clin Practice. (2023) 38:959–75. doi: 10.1002/ncp.11013
129. Stefanakis K, Kokkorakis M, and Mantzoros CS. The impact of weight loss on fat-free mass, muscle, bone and hematopoiesis health: Implications for emerging pharmacotherapies aiming at fat reduction and lean mass preservation. Metabolism. (2024) 161:156057. doi: 10.1016/j.metabol.2024.156057
130. Trenson L, Trenson S, van Nes F, Moyson C, Lannoo M, Deleus E, et al. Liraglutide for weight management in the real world: significant weight loss even if the maximal daily dose is not achieved. Obes Facts. (2021) 15:83–9. doi: 10.1159/000520217
131. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized nutrition by prediction of glycemic responses. Cell. (2015) 163:1079–94. doi: 10.1016/j.cell.2015.11.001
132. Ulusoy-Gezer HG and Rakicioglu N. The Future of Obesity Management through Precision Nutrition: Putting the Individual at the Center. Curr Nutr Rep. (2024) 13:455–77. doi: 10.1007/s13668-024-00550-y
133. Waeber B and Brunner H. The multifactorial nature of hypertension: The greatest challenge for its treatment? J hypertension Supplement. (2001) 19:S9–16.
134. Kaul K, Tarr JM, Ahmad SI, Kohner EM, and Chibber R. Introduction to diabetes mellitus. In: Ahmad SI, editor. Diabetes: An Old Disease, a New Insight. Springer New York, New York, NY (2013). p. 1–11.
135. Zhang Z-Y, Yu Y-L, Asayama K, Hansen TW, Maestre GE, and Staessen JA. Starting antihypertensive drug treatment with combination therapy. Hypertension. (2021) 77:788–98. doi: 10.1161/HYPERTENSIONAHA.120.12858
136. Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. (2021) 397:971–84. doi: 10.1016/S0140-6736(21)00213-0
137. Tall Bull S, Nuffer W, and Trujillo JM. Tirzepatide: A novel, first-in-class, dual GIP/GLP-1 receptor agonist. J Diabetes its Complications. (2022) 36:108332. doi: 10.1016/j.jdiacomp.2022.108332
138. Rosenstock J, Frias J, Jastreboff AM, Du Y, Lou J, Gurbuz S, et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet. (2023) 402:529–44. doi: 10.1016/S0140-6736(23)01053-X
139. Kokkorakis M, Chakhtoura M, Rhayem C, Al Rifai J, Ghezzawi M, Valenzuela-Vallejo L, et al. Emerging pharmacotherapies for obesity: A systematic review. Pharmacol Rev. (2025) 77:100002. doi: 10.1124/pharmrev.123.001045
140. Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. (2019) 42:1724–32. doi: 10.2337/dc19-0749
141. Saxena AR, Frias JP, Brown LS, Gorman DN, Vasas S, Tsamandouras N, et al. Efficacy and safety of oral small molecule glucagon-like peptide 1 receptor agonist danuglipron for glycemic control among patients with type 2 diabetes: A randomized clinical trial. JAMA Netw Open. (2023) 6:e2314493. doi: 10.1001/jamanetworkopen.2023.14493
142. Wharton S, Blevins T, Connery L, Rosenstock J, Raha S, Liu R, et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. New Engl J Med. (2023) 389:877–88. doi: 10.1056/NEJMoa2302392
143. Gleason PP, Urick BY, Marshall LZ, Friedlander N, Qiu Y, and Leslie RS. Real-world persistence and adherence to glucagon-like peptide-1 receptor agonists among obese commercially insured adults without diabetes. J Manag Care Spec Pharm. (2024) 30:860–7. doi: 10.18553/jmcp.2024.23332
144. Sharma AM, Birney S, Crotty M, Finer N, Segal-Lieberman G, Vazquez-Velazquez V, et al. Determinants of adherence to obesity medication: A narrative review. Obes Rev. (2025) 26:e13885. doi: 10.1111/obr.13885
145. Do D, Lee T, Peasah SK, Good CB, Inneh A, and Patel U. GLP-1 receptor agonist discontinuation among patients with obesity and/or type 2 diabetes. JAMA Netw Open. (2024) 7:e2413172. doi: 10.1001/jamanetworkopen.2024.13172
146. Kim C, Ross JS, Jastreboff AM, Meeker D, Freedman H, Krumholz HM, et al. Uptake of and disparities in semaglutide and tirzepatide prescribing for obesity in the US. JAMA. (2025) 29:e254735. doi: 10.1001/jama.2025.4735
147. Hwang JH, Laiteerapong N, Huang ES, and Kim DD. Lifetime health effects and cost-effectiveness of tirzepatide and semaglutide in US adults. JAMA Health Forum. (2025) 6:e245586. doi: 10.1001/jamahealthforum.2024.5586
Keywords: obesity, obesity pharmacotherapy, precision medicine, personalized treatment, combination therapy, weight management, weight loss
Citation: Steenackers N, Toumassian J, Deleus E, Mertens A, Lannoo M, Pazmino S, van Laar ADE, Van der Schueren B and Vangoitsenhoven R (2025) Pharmacotherapy for obesity: are we ready to select, tailor and combine pharmacotherapy to achieve more ambitious goals? Front. Endocrinol. 16:1569468. doi: 10.3389/fendo.2025.1569468
Received: 31 January 2025; Accepted: 27 May 2025;
Published: 18 June 2025; Corrected: 02 October 2025.
Edited by:
Chanan Meydan, Rabin Medical Center, IsraelReviewed by:
Michail Kokkorakis, Harvard Medical School, United StatesDanny Sugimoto, Cedar Crosse Research Center, United States
Copyright © 2025 Steenackers, Toumassian, Deleus, Mertens, Lannoo, Pazmino, van Laar, Van der Schueren and Vangoitsenhoven. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roman Vangoitsenhoven, cm9tYW4udmFuZ29pdHNlbmhvdmVuQHV6bGV1dmVuLmJl
 Nele Steenackers
Nele Steenackers Julia Toumassian1
Julia Toumassian1 Ellen Deleus
Ellen Deleus Bart Van der Schueren
Bart Van der Schueren Roman Vangoitsenhoven
Roman Vangoitsenhoven