- 1Clinical and Experimental Endocrinology, Department of Chronic Diseases and Metabolism, KU Leuven, Leuven, Belgium
- 2Department of Nutrition and Movement Sciences, Research Institute of Nutrition and Translational Research in Metabolism (NUTRIM), Maastricht University, Maastricht, Netherlands
- 3Department of Abdominal Surgery, University Hospitals, Leuven, Belgium
- 4Department of Endocrinology, University Hospitals Leuven, Leuven, Belgium
A Correction on
Pharmacotherapy for obesity: are we ready to select, tailor and combine pharmacotherapy to achieve more ambitious goals?
By Steenackers N, Toumazia J, Deleus E, Mertens A, Lannoo M, Pazmino S, van Laar ADE, Van der Schueren B and Vangoitsenhoven R (2025) Front. Endocrinol. 16:1569468. doi: 10.3389/fendo.2025.1569468
The figure captions were in the wrong order in the PDF version of this paper. Specifically, Figure 1 was intended to be Figure 3. Its caption was at Figure 2. Figure 2 was intended to be Figure 1. Its caption was at Figure 3. Figure 3 was intended to be Figure 2. Its caption was at Figure 1. The corrected captions of Figures 1–3 appear below.
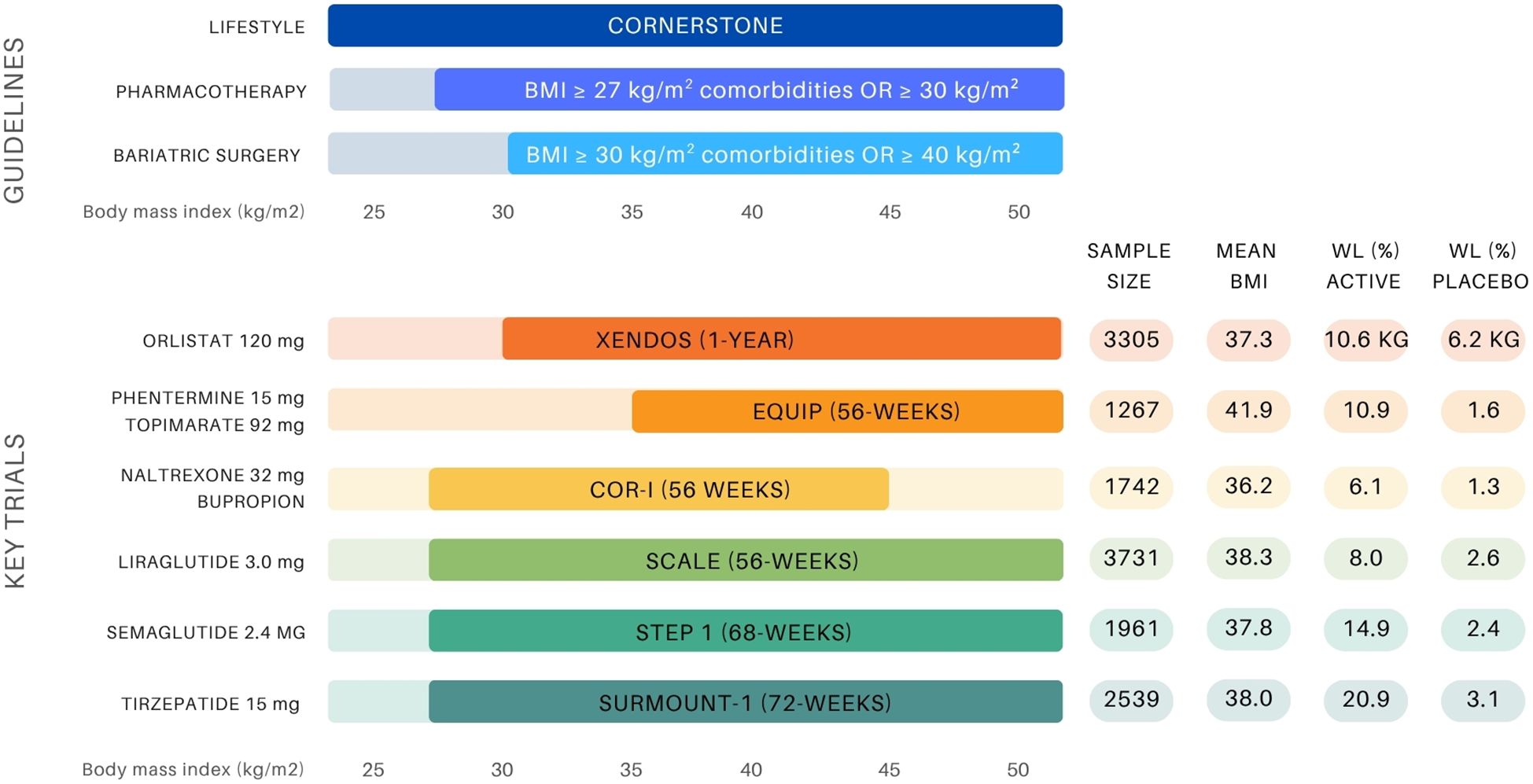
Figure 1. Overview of BMI-based treatment recommendations for obesity management and landmark anti-obesity drug trials (10, 11, 14, 19-21, 23, 25, 34, 35). The upper panel presents guideline thresholds for lifestyle intervention, pharmacotherapy (BMI ≥27 kg/m² with comorbidities or ≥30 kg/m²), and bariatric surgery (BMI ≥35–40 kg/m² depending on comorbidity status). The lower panel displays key clinical trials supporting the approval of six anti-obesity agents, indicating sample size, mean baseline BMI (entire group or active group), and weight loss outcomes (%WL unless otherwise stated) in both the active and placebo arms.
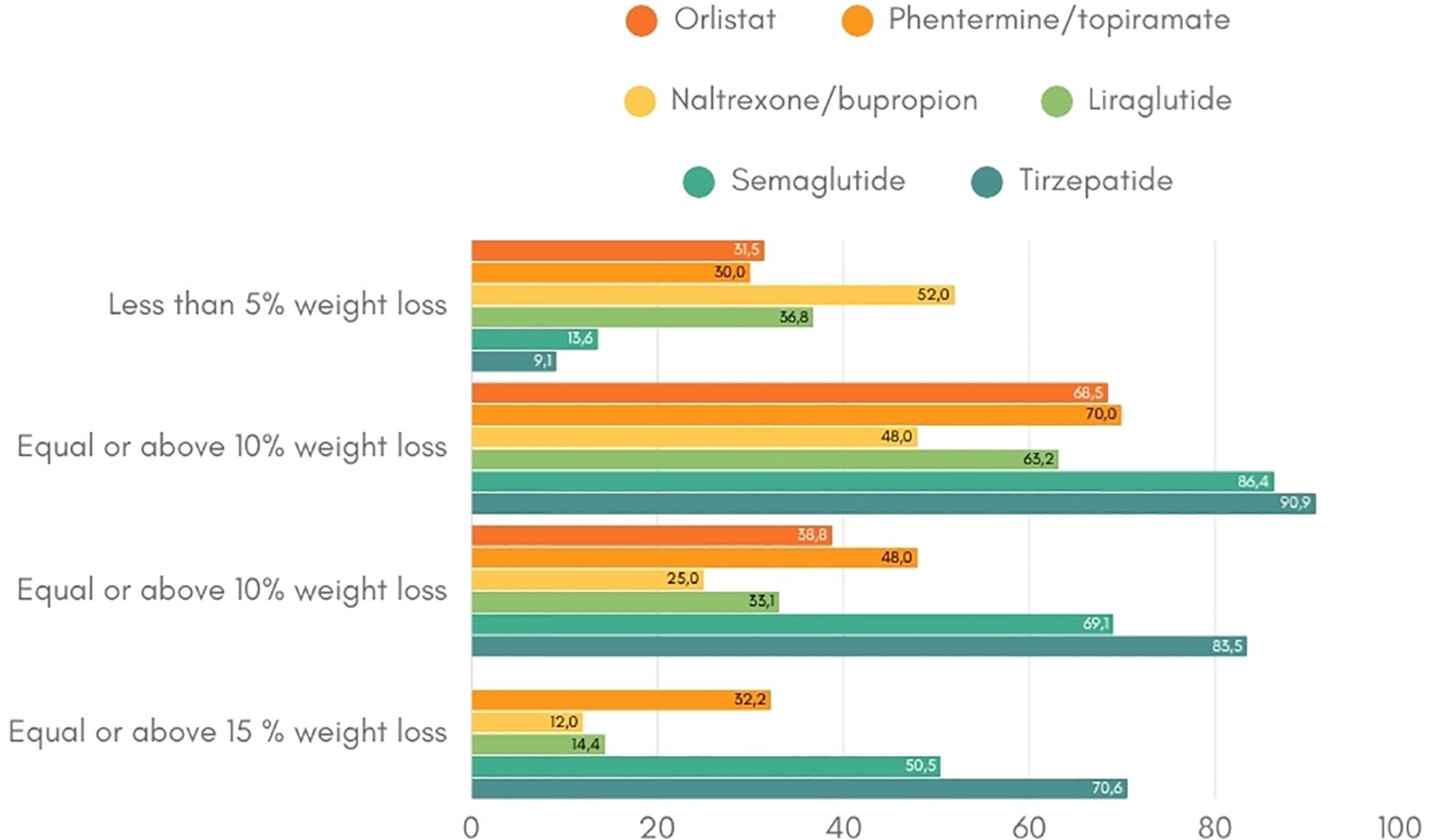
Figure 2. Weight loss response per anti-obesity drug (19-21, 23, 25, 34, 35). This figure indicates the percentage of patients that achieve the weight loss targets of at least 5%, 10% and 15% per anti-obesity medication.
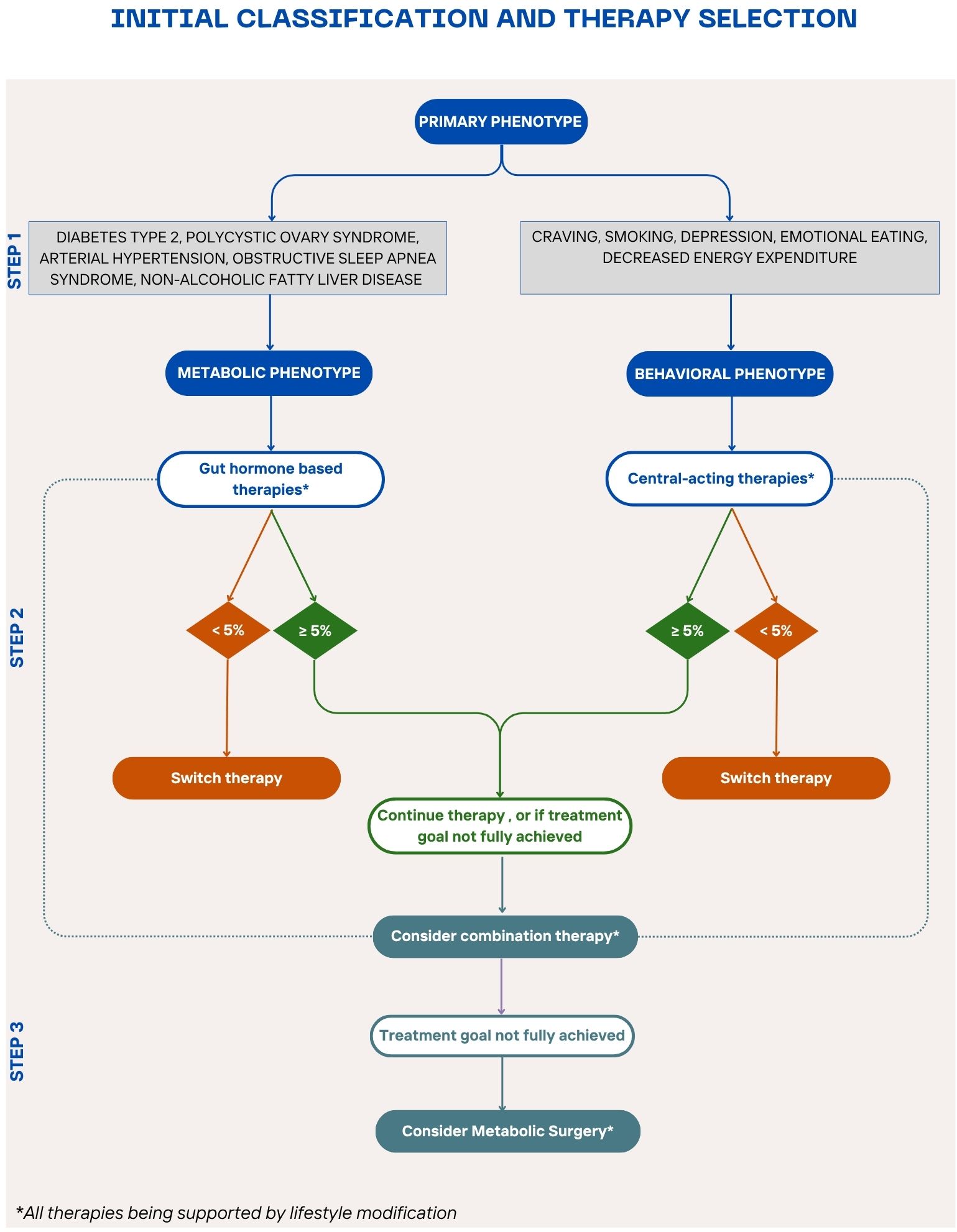
Figure 3. Stepwise algorithm for personalized obesity pharmacotherapy A proposed treatment algorithm integrating patient phenotypes, comorbidities, and treatment responses to guide personalized obesity management for patients who do not meet the criteria for metabolic surgery or have contraindications or not open to this option. The algorithm begins with lifestyle modification as the foundation, followed by phenotype-driven pharmacotherapy selection—gut hormone-based therapy for metabolic comorbidities and centrally acting therapy for behavioral/psychological factors. Treatment response is evaluated after 3 months to determine the need for therapy adjustment, combination treatment, or escalation to bariatric surgery for non-responders.
The original version of this article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: obesity, obesity pharmacotherapy, precision medicine, personalized treatment, combination therapy, weight management, weight loss
Citation: Steenackers N, Toumassian J, Deleus E, Mertens A, Lannoo M, Pazmino S, van Laar ADE, Van der Schueren B and Vangoitsenhoven R (2025) Correction: Pharmacotherapy for obesity: are we ready to select, tailor and combine pharmacotherapy to achieve more ambitious goals? Front. Endocrinol. 16:1656611. doi: 10.3389/fendo.2025.1656611
Received: 30 June 2025; Accepted: 20 August 2025;
Published: 02 October 2025.
Approved by:
Frontiers Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2025 Steenackers, Toumassian, Deleus, Mertens, Lannoo, Pazmino, van Laar, Van der Schueren and Vangoitsenhoven. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roman Vangoitsenhoven, cm9tYW4udmFuZ29pdHNlbmhvdmVuQHV6bGV1dmVuLmJl
 Nele Steenackers
Nele Steenackers Julia Toumassian1
Julia Toumassian1 Ellen Deleus
Ellen Deleus Bart Van der Schueren
Bart Van der Schueren Roman Vangoitsenhoven
Roman Vangoitsenhoven