- Department of Cardiology, Taizhou Central Hospital (Taizhou University Hospital), Taizhou, China
Calcific aortic valve disease (CAVD) is a progressive disease, of which the 2-year mortality is >50% for symptomatic aortic valve stenosis unless transcatheter aortic valve replacement (TAVR) or surgical aortic valve replacement (SAVR) is performed promptly. The prevalence of diabetes among CAVD has increased rapidly in recent years. The combination of diabetes with its cardio-renal and metabolic comorbidities, such as hypertension, hyperlipidemia, chronic kidney disease, and ageing, accelerated the progression of CAVD and increased the subsequent needs for aortic valve replacement. Clinical data regarding the impact of diabetes on outcomes of patients undergoing TAVR or SAVR have exhibited inconsistent results. Compared with non-diabetes, the short-term mortality after TAVR was not significant in diabetes, while the mid-term mortality differed from different cohorts. Although there were worse mid-term and long-term mortalities after SAVR in diabetes, the short-term mortality in diabetes was disputable. As for complications, there were common worse manifestations with coronary heart disease, acute kidney injury, heart failure, and systemic inflammatory response syndrome in diabetes undergoing TAVR or SAVR. Moreover, diabetes was one of the risk factors for deterioration of bioprosthetic aortic valves, leading to increased long-term mortality. Based on the efficacy for CAVD and atherosclerotic cardiovascular disease, glucose-lowering medications might have potential to inhibit deterioration of bioprosthetic aortic valves independent of glucose control.
1 Introduction
Calcific aortic valve disease (CAVD) is a progressive disease that has been occurring with rapidly increasing morbidity because of the ageing of the population (1). With the progression of aortic valve stenosis (AVS), the extent of cardiac damage gradually increased from left ventricle to right ventricle (2). Although mortality did not increase when AVS was asymptomatic, the 2-year mortality was more than 50% for patients with symptomatic stenosis (3, 4). Emerging evidence has indicated that CAVD was an active and regulable pathological process in which the risk factors, such as diabetes, hypertension, and hyperlipidemia, were similar to those of other cardiovascular diseases (5, 6). However, lipid-lowering therapy with atorvastatin, simvastatin and ezetimibe, or rosuvastatin did not prevent the progression of CAVD (7–9). There are currently no effective pharmacotherapies to retard or reverse the progression of CAVD. Transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) are the only effective treatments for end-stage CAVD.
Patients with diabetes were at increased risk of developing various cardiovascular diseases, including coronary artery disease (CAD), stroke, peripheral artery diseases, and CAVD (10). According to large-scale retrospective observations worldwide, the prevalence of diabetes in CAVD ranged from 11.4% to 31.6%, and increased by almost 50% in the recent decade (11–14). It was worth noting that the prevalence of diabetes in CAVD undergoing TAVR or SAVR also increased rapidly in recent years (13–16). Diabetes stood as a major risk factor for developing hypertension, hyperlipidemia, chronic kidney disease (CKD), and ageing (17), which were all associated with the initiation of CAVD (Figure 1) (18). The combination of diabetes with these cardio-renal and metabolic comorbidities accelerated the progression of CAVD and increased the subsequent needs for aortic valve replacement (AVR). The underlying mechanism of diabetes and its comorbidities involved endothelial dysfunction, immune cell infiltration, oxidative stress, lipid retention, as well as subsequent osteogenic and myofibroblastic differentiation of valvular interstitial cells (VICs) and eventual calcification (18, 19). Once fibrosis and calcification of the aortic valve initiated, the majority of patients developed AVS progressively (20).
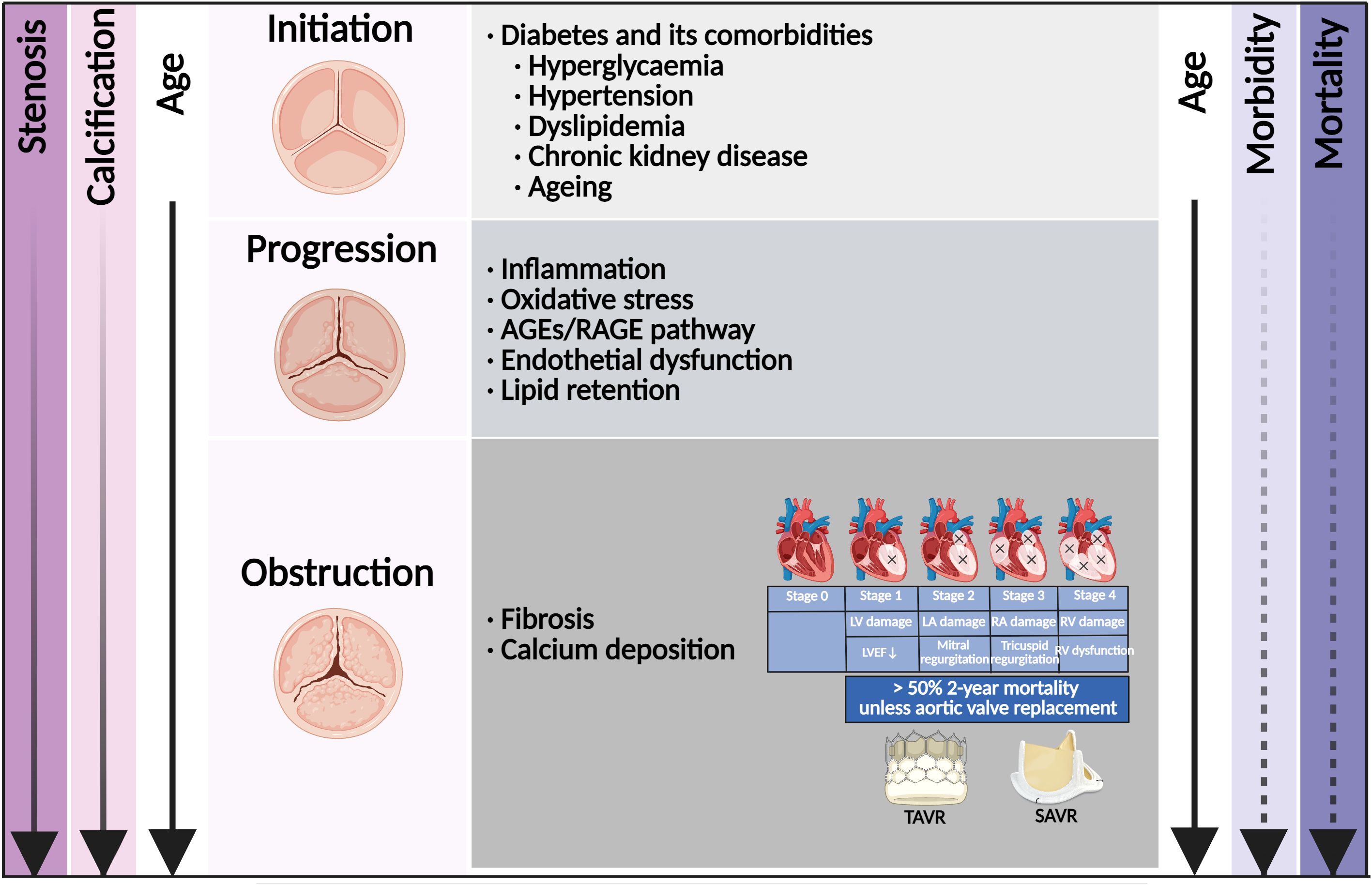
Figure 1. Risk factor and time course of diabetes concomitant to calcific aortic valve disease. Shown is the relationship among disease stage, risk factor, molecular link, valve anatomy, stage of cardiac damage, and the age of the patient. The morbidity of aortic valve stenosis (dashed line) increased rapidly with age. Once in symptomatic stenosis, the mortality of aortic valve stenosis (solid line) increased rapidly. Once with cardiac damage, aortic valve replacement was the only effective treatment. AGEs, advanced glycation end products; RAGE, receptor for AGEs; LV, left ventricle; LA, left atrium; RV, right ventricle; RA, right atrium; LVEF, left ventricular ejection fraction; TAVR, transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement.
Diabetes was generally considered as an adverse factor in patients with cardiovascular diseases needing surgical or invasive interventions. The impact of diabetes on AVR manifested with various complications, such as CAD, acute kidney injury (AKI), heart failure, and systemic inflammatory response syndrome (SIRS). However, clinical data regarding the impact of diabetes on outcomes of patients undergoing AVR have exhibited inconsistent results. The short-term mortality after SAVR was significantly lower in diabetes according to the Spanish National Hospital Discharge Database, while another Spanish study found no difference in short-term mortality between diabetes and non-diabetes patients (14, 21). Diabetes was found as a risk factor for mid-term mortality after TAVR in a meta-analysis including 64 studies (22). However, according to the VARC-2 criteria, diabetic patients did not have increased mid-term mortality after TAVR compared with non-diabetic patients (23). There is currently no article that has summarized the influence of diabetes on clinical outcomes after AVR.
In this review, we present the prevalence of diabetes in CAVD. Then, we discuss the underlying mechanisms of diabetes and its comorbidities in CAVD. Most importantly, we discuss the controversies of clinical outcomes in diabetic patients undergoing TAVR or SAVR. Finally, we summarize updated knowledge about the influence of diabetes on the deterioration of bioprosthetic aortic valve (BAV).
2 The prevalence of diabetes in CAVD
With the ageing of the population, the incidence rate of diabetes concomitant to AVS has increased rapidly by years (Table 1). The CURRENT AS registry, enrolling 3,815 consecutive patients with CAVD in Japan between 2003 and 2011, showed that 11.4% of patients had concomitant diabetes (11). The multi-central, prospective, observational PRIMID AS study, conducted in 10 hospitals in the United Kingdom between 2012 and 2014, showed that 14.4% of patients with moderate to severe AVS had concomitant diabetes (12). Another study, performed in the Swedish population-based cohort study, showed that the prevalence of diabetes was 15.8% in severe AVS (24). In recent decades, the incidence of AVR increased rapidly in CAVD with diabetes. A retrospective analysis of patients with SAVR between 1987 and 2016 in Netherlands revealed that the prevalence of diabetes has increased from 7.8% to 17.9% over three decades (15). Likewise, the retrospective data from Spanish cohorts (2001–2015) showed that the prevalence of diabetes increased significantly from 16.7% to 23.5% in patients undergoing SAVR (14). The MedPAR file, reporting trends in demographic characteristics associated with isolated AVR in the United States, showed that the prevalence of diabetes increased from 19.7% to 31.6% between 2009 and 2015 in the SAVR cohort and increased from 34.2% to 36.8% between 2012 and 2015 in the TAVR cohort (13). According to the data from the National Inpatient Sample between 2012 and 2017, hospitalizations of diabetes undergoing TAVR increased from 0.97 to 7.68/100,000 adults (16). In the Danish nationwide registers, the prevalence of diabetes undergoing TAVR significantly increased from 14.2% in 2008–2010 to 19.4% in 2017–2018 (25). Overall, with the increased prevalence of diabetes undergoing AVR in recent years, the influences of diabetes on clinical outcomes after TAVR or SAVR have been getting more and more attention.
3 Risk factor and molecular link for CAVD in diabetes
3.1 Hyperglycemia
Diabetes is a chronic disease characterized by hyperglycemia and manifested by various cardiovascular diseases. Recent research has found that a hyperglycemia-simulating environment attenuated experimentally induced osteogenic differentiation of cultured human VICs (26). To further mimic the events of aortic valve tissue in diabetic conditions, chronic hyperglycemia was assessed in valvular endothelial cells (VECs) and VICs via a gelatin methacrylate 3-dimension model (27). The gene expressions of MCP-1 and IL-1β were increased both in VECs and VICs after high-glucose treatment for 14 days, exhibiting changes of extracellular matrix (ECM) remodeling and inflammation (27). However, in another dynamic three-dimension aortic valve leaflet model using a software-governed bioreactor system with controlled pulsatile flow, hyperglycemia did not exhibit any impact on fibrosis or calcification on the aortic valve (28). In vivo, LDLR-/-ApoB100/100 mice fed with diabetogenic diet had higher incidence of aortic valve incrassation and stenosis in comparison with normal chow (29). This controversial relationship between hyperglycemia and VIC osteogenesis indicated that there were other complicated mechanisms of diabetes on developing CAVD beyond sole hyperglycemia. Exposure to hyperglycemia in diabetes rapidly accelerated circulating advanced glycation end product (AGE) formation (30). Extracellular AGEs modified global tissue structure and function by binding to the receptor for AGEs (RAGE) (31). In a study on 76 patients undergoing AVR, both the plasma and valvular levels of AGEs were increased in patients with diabetes (32). Overexpression of valvular AGEs was associated with increased mean transvalvular pressure gradient, and overexpression of plasma AGEs was associated with aortic valve area and max transvalvular pressure gradient (32). In an animal model of CAVD, RAGE deficiency attenuated morphometric infiltration, calcification, and AGE accumulation in the aortic valve (33). In vivo, the knockdown of RAGE in high-cholesterol diet-fed ApoE-/- mice attenuated the expression of RUNX2 mRNA via NF-κB/ATF4/CHOP pathway (Figure 2 Panel 5) (34).
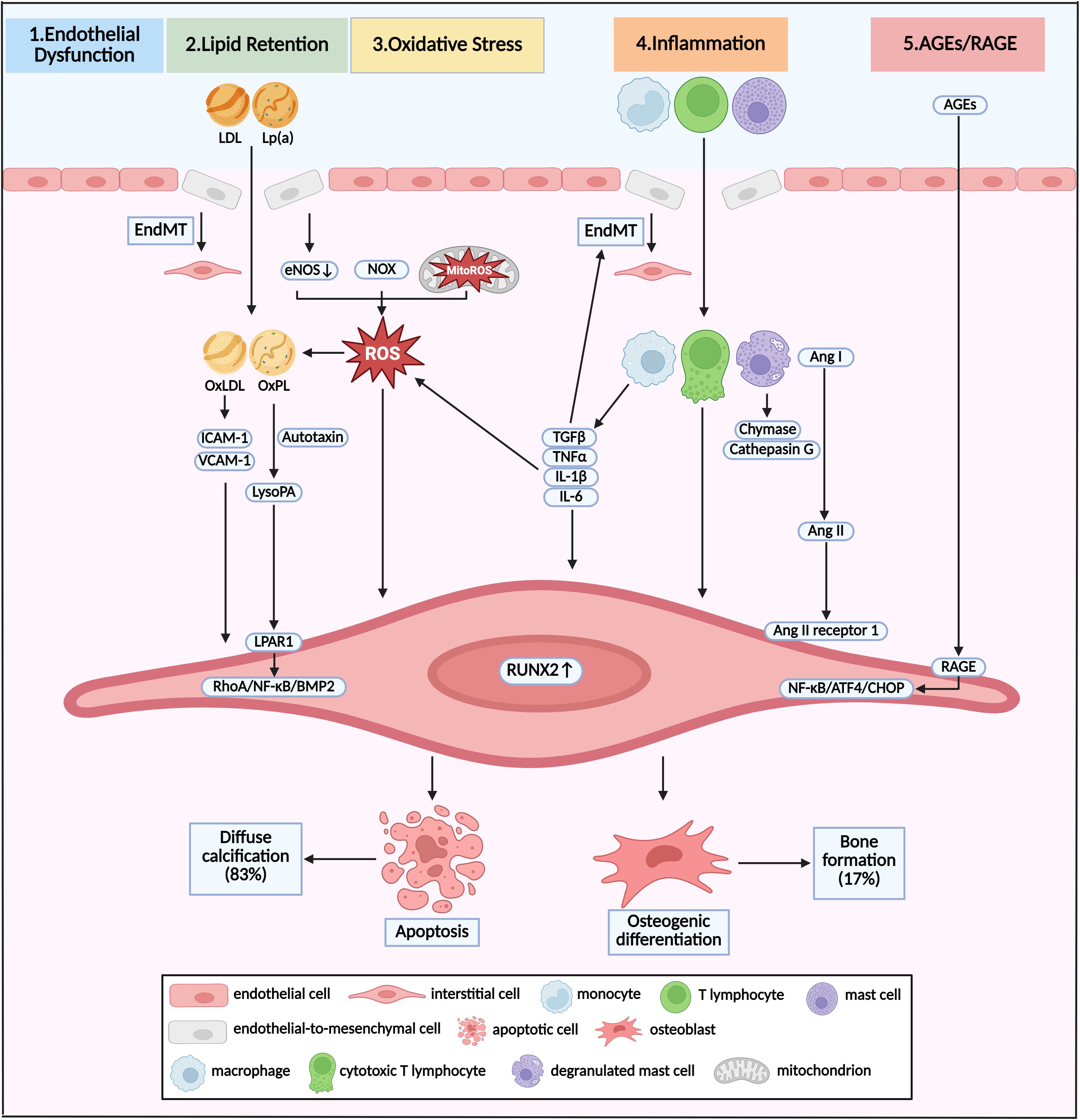
Figure 2. Pathway and molecular link between diabetes and calcific aortic valve disease. Pathological mechanism of initiation and progression of CAVD in diabetes was shown as crosstalk of various pathways. Different stimuli induced EndMT and broke the endothelial barrier, resulting in infiltration of lipoproteins and immune cells. This infiltration was accompanied by the overproduction of ROS via dysregulation of eNOS, and accumulation of NOX and MitoROS. Oxidative stress could promote the formation of OxLDL and OxPL, which induced osteogenic differentiation of VICs, and eventual bone formation. Apoptosis of VICs and subsequent diffuse calcification were induced by infiltrated macrophages, T lymphocytes and mast cells via direct interaction, activation of Ang II, and secretion TGFβ, TNFα, IL-1β, and IL-6. Increased circulating AGEs induced the pro-osteogenic reprogramming via RAGE/NF-κB/ATF4/CHOP pathway. Diffuse calcification accounted for approximately 83% of all calcification deposits, while bone formation accounted for the other 17%. LDL, low-density lipoprotein; Lp(a), lipoprotein (a); EndMT, endothelial-to-mesenchymal transition; ROS, reactive oxygen species; eNOS, endothelial nitric oxide synthase; NOX, nicotinamide adenine dinucleotide phosphate oxidase, MitoROS, mitochondria-generated ROS; OxLDL, oxidized LDL; OxPL, oxidized phospholipids; Ang, angiotensin.
3.2 Diabetic complications
3.2.1 Hypertension
Hypertension was the most common complication of diabetes, and 40–60% patients with diabetes would develop abnormal blood pressure or hypertension sooner or later (35). Patients with diabetes developed increased arterial resistance caused by vascular remodeling and increased circulating volume caused by hyperglycemia, both of which elevated blood pressure (36). Approximately 70% of the patients with AVS had concomitant hypertension (37). In a large-scale clinical observation involving a 5.4-million population without known valvular heart disease, long-term exposure to elevated blood pressure (median follow-up of 9.2 years) was associated with an increased risk of AVS (38). Specifically, each 20-mmHg increase in systolic blood pressure was associated with a 41% higher risk of AVS (38). VECs were the first cells to be affected by hemodynamic changes. There were pieces of evidence finding that hypertension could accelerate the progression of CAVD by hemodynamic flow disturbance, which could cause mechanical damage to the VECs, especially on the aortic side (39, 40). Under exposure to circulating stimulants, VECs could differentiate into mesenchymal valve progenitor cells, a precursor of VICs, in a process called endothelial-to-mesenchymal transition (EndMT) (19). A relatively high rate of EndMT led to a destruction of the endothelial barrier due to the loss of adherent junction (Figure 2 Panel 1) (41). Lymphocyte and macrophage infiltrated into the aortic valve through a destroyed endothelial layer and secreted various procalcific and proinflammatory cytokines (42). TGFβ and IL-1β would in turn stimulate the EndMT of VECs (43, 44). When those pathological factors constantly existed in the aortic valve, endothelial-derived VICs could differentiate into the osteoblastic phenotype (19). Once the osteogenesis has been launched, RUNX2 served as the marker of calcification. The current hypotheses suggested that the activation of RUNX2 involved activated STAT3 or STAT5, which, due to activating inflammatory signaling, translocated into the nucleus and bound onto STAT binding sites in the promoter region of RUNX2, leading to the recruitment of additional transcription factors, co-transcription factors, and chromatin remodelers (45, 46).
3.2.2 Hyperlipidemia
Abnormal lipids metabolism was one of the major comorbidities in diabetes, which has been recognized as a hallmark in the early stage of CAVD and could be detected long before calcium deposits by PET-CT (47). A genome-wide meta-analysis of 11.6 million variants in 10 cohorts, involving 653,867 European ancestry participants, supported a causal contribution of lipoprotein (a) (Lp(a)), apolipoprotein B, and low-density lipoprotein (LDL) to AVS (48). In the Global Lipids Genetics Consortium, which included 188,577 participants, the odds ratio for developing AVS per unit increase in lipid parameter was 1.52 for LDL (49), indicating that LDL-lowering medication might be effective in prevention of CAVD. However, three large-scale randomized clinical trials (RCT) failed to illustrate any significant benefit of LDL-lowering medication with statins on the prevention of AVS (7–9), indicating that further studies were needed to seek the association of other lipid indexes and CAVD. Accumulation of reactive oxygen species (ROS) promoted the transformation of LDL to oxidized LDL (OxLDL) and the transformation of Lp(a) to oxidized phospholipids (OxPL), which have been proven to facilitate the osteogenic differentiation of VIC in vitro study (Figure 2 Panel 2) (50, 51). OxLDL increased the expression of cell adhesion molecules, including ICAM-1 and VCAM-1, which consequently promoted RUNX2 expression and calcific remodeling in the aortic valve (52, 53). Liquid chromatography–tandem mass spectrometry demonstrated that lysophosphatidic acid (LysoPA), the decomposition production of OxPL catalyzed by autotaxin, was overexpressed in calcified leaflets in comparison with normal leaflets (54). Further study has shown that OxPL and LysoPA could accelerate the osteogenic differentiation of VICs by binding to LysoPA receptor 1 (LPAR1), levels of which were also increased in calcified leaflets (55). Inhibition of LPAR1 decelerated the progression of AVS and calcium deposits in the aortic valve (56). LPAR1 could instigate a pro-calcific gene program in VICs via RhoA/NF-κB/BMP2 activation (56).
3.2.3 Chronic kidney disease
As one of the major complications of diabetes, approximately 30–40% of patients with diabetes developed diabetic nephropathy eventually (57), which progressively caused CKD and was associated with increased mortality (58). The prevalence of CAVD ranged from 28% to 85% in patients with CKD (59). Even in stage 2 and 3 CKD, >30% of the patients were found to have detectable aortic and/or mitral valve calcification (60). Moreover, functional AVS was found in 9.5% of patients with CKD in comparison with 3.5% of the general population (61). Even when taking account of age, race, sex, diabetes, and hypertension, patients with CKD had a 1.2- to 1.3-fold increased risk of CAVD (61). Multiple mediators in CKD, including hyperphosphatemia, calcium–phosphate product, parathyroid hormone, and systematic inflammation, have been identified as risk factors of calcium deposition in the aortic valve (62). By conducting single-cell RNA sequencing with aortic valve leaflets excised from CAVD patients, VICs (72.64%) accounted for a major proportion among all cell types, followed by monocytic cells (19.52%), lymphocytes (6.23%), VECs (1.28%), and mast cells (0.33%) (63). Immunohistochemistry staining showed chronic immune cells infiltration on calcified valve leaflets, involving CD68+ macrophages, CD3+ T lymphocytes, and mast cells, while only few unactivated immune cells were exhibited in healthy valve leaflets (Figure 2 Panel 4) (64, 65). Monocytes and macrophages activated the RUNX2 overexpression in VICs through secretion of TGFβ, TNFα, IL-1β, and IL-6 (66). Moreover, the release of extracellular vesicles from macrophages induced diffuse calcification due to the release of apoptotic bodies by VIC apoptosis (67). Likewise, the infiltrated CD8+ cytotoxic lymphocytes in diseased aortic valve induced apoptosis of VICs by direct interaction (68). The bulk of mast cells have been activated to degranulate and release chymase and cathepsin G in the calcified aortic valve, which both could convert angiotensin I to angiotensin II (69). Notably, exposure of VICs to angiotensin II promoted the expression of RUNX2, by binding to the angiotensin II receptor 1 (70). Accordingly, exposure of ApoE-/- mice to high-dose angiotensin II contributed to myofibroblastic differentiation of VICs and eventual aortic valve leaflet thickening (71).
3.2.4 Ageing
Ageing was a powerful independent risk factor for degenerative aortic valve disease. Diabetes was closely associated with ageing and was a major risk factor for ageing-associated cardiovascular diseases (72). Aortic valve calcification used to be viewed as a degenerative process, where calcification was thought to be the consequence of physiological ageing (5). Interestingly, a renewed characterization of the ageing aortic valve has emerged in recent years. Many prior studies that examined “healthy” elderly valves actually involved valve leaflets with calcification (73, 74), meaning that those data could not be used to define the characteristics of a normal, ageing valve. That was because the majority of samples in these references had come from individuals over 60 years old, and approaches and techniques have significantly evolved. Furthermore, while CAVD was considered a disease of old people, recent demographics showed that CAVD could be detected in the twenties, especially in those suffering from bicuspid aortic valve (75). Thus, diabetes-related pathological ageing probably participated more in the onset and development of CAVD compared with physiological ageing. Accelerated oxidative stress was a common factor in ageing and diabetes (76). Three major sources of ROS were uncoupled nitric oxide synthases (NOS), reduced nicotinamide adenine dinucleotide phosphate oxidase (NOX), and mitochondria-generated ROS (MitoROS) (77). Exposure of VECs to exogenous TNFα and H2O2 promoted endothelial NOS (eNOS) uncoupling, leading to increases in endogenous superoxide and H2O2 levels, which could promote ECM remodeling and calcium deposition in aortic valve (Figure 2 Panel 3) (78). Recent evidence has demonstrated that isoform specific NOX-derived ROS might be involved in the development of CAVD. Intense NOX2 accumulation was found in VIC osteogenesis and in calcified regions of aortic valve leaflets (79). In hypercholesteremic mice, the mRNA level of NOX2 was increased in harvested valve leaflets, while no change was observed for NOX4 (80). Mitochondrion was a critical organelle responsible for both ROS production via end product from oxidative phosphorylation and ROS elimination via mitochondrial superoxide dismutase-mediated dismutation of superoxide (81). Loss of mitochondria was found in aortic valve leaflets excised from patients with CAVD and LDLR-/- mice fed with high-cholesterol chow (82). In cultured human VICs, treatment with Lp(a) promoted VIC osteogenic differentiation accompanied by MitoROS production (83). Our recent study revealed that both β-glycerophosphate acid and TGFβ treatment stimulated MitoROS production and RUNX2 expression in VICs, which was accompanied by decreased mitochondrial biogenesis and mitochondrial dysfunction (82).
3.3 Multifactorial interactions
Apart from direct effects of diabetes (e.g., hyperglycemia, AGEs/RAGE pathway, oxidative stress, EndMT, and inflammation), there were multifactorial interactions between those diabetic complications (e.g., hypertension, hyperlipidemia, CKD, ageing), which participated jointly in the initiation and progression of CAVD. Hypertension-induced hemodynamic shear stress on the aortic side induced endothelial dysfunction and hampered barrier function, which exacerbated lipid deposition in an aortic valve under a hyperlipidemic condition (84). CKD caused hypertension through an interplay of factors, including water–sodium retention, renin–angiotensin system overactivation, and endothelial dysfunction (85), which were common pathological factors of CAVD. Hyperglycemia represented a key cellular stress in the kidney by altering cellular metabolism in endothelial cells and podocytes (86). Thereafter, increased oxidative stress and activation of inflammatory pathways caused progressive kidney function decline and fibrosis (86). Hyperlipidemia was associated with low-grade systemic inflammation, which might lead to insulin resistance, insulin deficiency, and consequent hyperglycemia (87). As two of the subsets of metabolic syndrome, hyperlipidemia and hyperglycemia jointly promoted CAVD via oxidative stress and chronic inflammation (84).
4 Diabetes in transcatheter aortic valve replacement
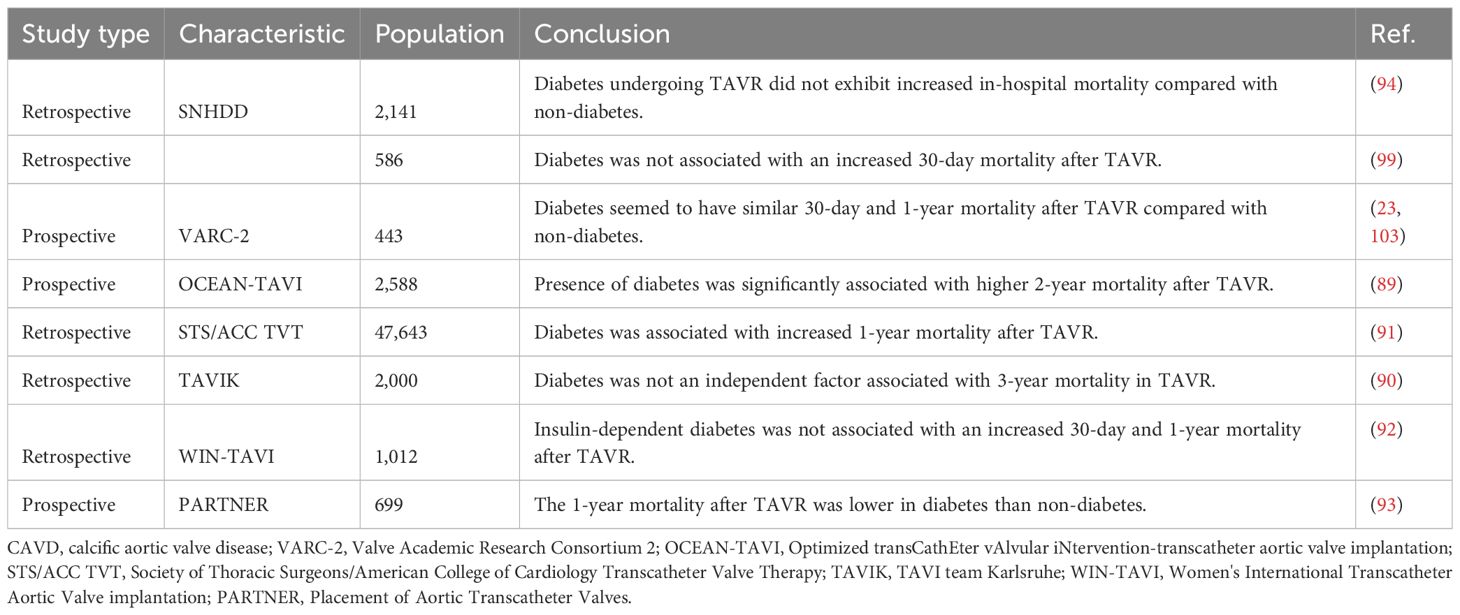
TAVR was a percutaneous treatment option for symptomatic severe aortic valve stenosis, especially among patients at high surgical risk (88). The prevalence of diabetes was up to a third of cases in TAVR patients (89–91). However, the association between diabetes and outcomes after TAVR procedure remained controversial (Table 2). Some studies described similar rates of complications (90, 92), while others reported higher (89, 91) or even lower 1-year mortality rates (93, 94). This controversy was represented in mortality risk prediction scores, which were based on data from surgical patients. STS-PROM score included diabetes as a risk factor (95). However, logistic EuroSCORE did not include diabetes as a risk factor (96), whereas EuroSCORE II only included insulin-treated diabetes (97). EuroSCORE II was the most accurate risk score with slight underestimation of actual mortality, whereas STS-PROM score and logistic EuroSCORE overestimated observed mortality (98). A meta-analysis including 64 studies with a total of 38,686 patients found that diabetes was associated with increased 1-year mortality after TAVR (22). Moreover, although insulin-treated diabetes was not associated with adverse outcome compared with orally treated diabetes, elevated HbA1c levels might be associated with increased mortality during long-term follow-up (99). Stress hyperglycemia ratio has recently been recognized as an accurate biomarker that represented true hyperglycemia status (100). In a prospective single-center study with a median follow-up of 3.9 years, stress hyperglycemia ratio was linearly associated with all-cause mortality and cardiovascular mortality in patients undergoing TAVR (101). However, according to the VARC-2 criteria, there were no significant differences of 30-day and 2-year mortality in patients undergoing TAVR between diabetes and non-diabetes (23, 99). Moreover, in a propensity matched analysis of multicentral registry including data from >12,000 patients undergoing transfemoral TAVR, diabetes was not associated with worse outcomes within the first year after TAVR, which underscored the safety of TAVR treatment in diabetes patients (98). Similarly, according to the data from the Spanish cohorts, diabetic patients undergoing TAVR did not exhibit increased in-hospital mortality compared with non‐diabetic patients (94). Further study has found some relationship between diabetes and TAVR in a specific cohort. An observational study of all consecutive patients treated with a transfemoral TAVR in a single-center cohort revealed that male patients with diabetes had significantly higher 3-year mortality compared with males without diabetes and there was no difference in 3-year mortality for female patients with and without diabetes, indicating gender-dependent association between diabetes and mortality after TAVR (102). Nevertheless, another observational study demonstrated that no influences of diabetes presence on the risk of 30-day and 1-year mortality after adjustment for age and gender (103). Anyway, in a post-hoc analysis of the PARTNER trial, diabetic patients were noted to have decreased 1-year mortality when treated with TAVR compared to SAVR (93). Overall, the short-term mortality after TAVR was not significant between diabetes and non-diabetes, while the mid-term mortality remained controversial. Taken together, these observations might tend to favor a transcatheter approach when either approach would be a reasonable option, particularly in those with diabetes. There is a need for RCT and large cohorts with long-term follow-up of diabetes vs. non-diabetes in patients undergoing TAVR (Figure 3).
Figure 3. Clinical outcome and complication of diabetes in transcatheter aortic valve replacement. Compared with non-diabetes, the impact of diabetes on clinical outcomes and various complications after TAVR.
4.1 TAVR procedure
The risk factors of CAVD, including diabetes, hypertension, and CKD, were more involved in the disease development in patients with tricuspid aortic valves than those with bicuspid aortic valves (104). In asymptomatic moderate-to-severe AVS, patients with tricuspid aortic valves were older and had higher proportion of diabetes, compared with bicuspid aortic valves (104). However, the in-hospital mortality did not differ between transfemoral and transapical access in diabetes undergoing TAVR (105). Transfemoral access was associated with a higher incidence of vascular complications and permanent pacemaker implantation (PPI) implantation than transapical access (105). Especially, transfemoral access for TAVR was associated with higher mortality, acute stroke, AKI, hemodialysis, and percutaneous coronary intervention (PCI) in complicated diabetes with diabetes-related complications than in non-complicated diabetes (105). Although balloon pre-dilatation was not associated with device success or any post-procedural complications in TAVR procedure, there was less diabetes in pre-dilatation group (106). There were overall comparable outcomes between balloon-expandable and self-expanding valve for TAVR (107). In a subgroup analysis of in-hospital mortality, there was no significant difference concerning the type of valve in diabetes (108).
4.2 Obesity
The “abnormality” in in-hospital and short-term mortality in diabetes after TAVR might be attributed to diabetes-related obesity. The relationship between body mass index (BMI) and cardiovascular risk prediction was recognized as an “obesity paradox”. On the one hand, obesity has long been established as a risk factor for atherosclerotic cardiovascular disease (ASCVD) (109). On the other hand, increased BMI was found as a protective factor in patients undergoing cardiovascular surgery or intervention (110, 111). Similarly, in comparison with normal-weight patients, patients who were overweight or had obesity had a lower incidence of 30-day mortality, 1-year mortality, and long-term mortality after TAVR (112). Obesity was one of the most common concomitant statuses in patients with diabetes. In patients undergoing TAVR, increased BMI was associated with increased rate of diabetes at baseline (113). However, obesity has been identified as an independent risk factor for vascular complications, PPI, and AKI in patients undergoing TAVR (114, 115). The association between obesity and other post-TAVR complications, including cerebrovascular events, new-onset atrial fibrillation, and myocardial infarction, has received emerging research attention. Therefore, the nuanced influence of obesity on TAVR outcomes necessitated deeper exploration, particularly considering the unique physiological and metabolic profiles inherent to individuals with obesity.
4.3 Coronary artery disease
CAD and CAVD were frequently concomitant due to similar risk factors, such as diabetes. About 25% of TAVR recipients have undergone PCI before TAVR in real-world TAVR registries (116). However, coronary artery bypass grafting (CABG) at the time of SAVR has been considered the gold standard in such patients (117). More and more studies pointed to TAVR+PCI as an alternative method (118). Patients with prior CABG had higher rate of diabetes (119), which might be due to higher rate of three-vessel CAD and higher SYNTAX score in diabetes. As for prior PCI, diabetes was associated with increased risk of major adverse cardiovascular events (MACE) (120). However, in stable CAD with diabetes, completeness of PCI either staged or concomitantly with TAVR was similar to undergoing TAVR without PCI concerning the all-cause death and MACE (121). As for acute coronary syndrome after TAVR, the concomitance with diabetes was associated with a higher rate of early mortality among patients undergoing urgent or emergent PCI (122, 123).
4.4 Acute kidney injury
Adjusted multivariate Cox regression analyses found that AKI was associated with increased risk of long-term mortality after TAVR (23). A meta-analysis of 64 studies showed that diabetes was associated with increased AKI after TAVR (22). Similarly, based on the data from 410 patients undergoing TAVR, kidney function improvement after TAVR was lower in diabetes than that in non-diabetes (124, 125). Among patients with end-stage renal disease, diabetes was one of the predictors of dialysis and readmission after TAVR (126–128). Diabetes was known to be a cause and prognostic factor for patients on dialysis. The influence of diabetes on kidney function after TAVR was mainly dependent on the baseline glomerular filtration rate and prior blood glucose control. Concomitance with diabetic nephropathy, especially with end-stage renal disease, might be associated with worse outcome after TAVR in diabetic patients.
4.5 Heart failure
In TAVR, early findings showed that heart-failure-related death and sudden cardiac death accounted for approximately a third of total deaths and two-thirds of cardiac related deaths (129, 130). Among TAVRs with a newer-generation device including the self-expandable Evolut R/Pro/Pro+ valve and balloon-expandable SAPIEN S3/ULTRA valve, advanced heart-failure-related deaths accounted for 11.6% of total deaths and sudden cardiac death accounted for 7.5% of total deaths (131). Diabetes was independently associated with an increased risk of sudden cardiac death in TAVR (131). The several possible mechanisms were silent myocardial ischemia, QT interval prolongation, diabetic cardiomyopathy, increased arrhythmogenic potential, and hypercoagulability (132). Patients with diabetes were at an increased risk of hospitalization for heart failure at 1-year after TAVR (133, 134). Most of the patients obtained myocardial recovery after TAVR due to decreased cardiac afterload. However, post-TAVR left ventricular ejection fraction recovery was impaired in patients with diabetes (135, 136). Diabetes was associated with elevated left ventricular filling pressure and prior right ventricular dysfunction (137, 138), resulting in more severe heart failure symptoms and more loop diuretic therapy after TAVR (139). These residual myocardial injuries might explain the inferior manifestation of heart failure. The underlying pathophysiologic mechanisms might include changes in vascular homeostasis with diminished nitric oxide and increased ROS levels due to prolonged hyperglycemia, insulin resistance, and hyperinsulinemia, which activated pro-inflammatory pathways that resulted in the progression of atherothrombosis and dysfunction of the myocardium (140). Moreover, diabetes was associated with chronic and new-onset heart failure through neurohormonal dysregulation inducing cardiac fibrosis and decreasing cardiac efficiency (133).
4.6 Stroke
Post-procedural stroke was a devastating complication after TAVR and was associated with decreased long-term survival and reduced quality of life (141, 142). The national SWENTRY registry identified diabetes as one of the pre-disposing factors for stroke after TAVR (143). Up to 70% of the stroke was presented with clinically silent stroke or peri-procedural silent brain infarcts, which might be due to subclinical leaflet thrombosis (144). Meta-regression found that diabetes was associated with increased risk of silent brain infarcts (145). In prospective RETORIC trial, diabetes was an independent predictor of event of subclinical leaflet thrombosis (146). This should not seem surprising, as diabetes was independently linked to a higher risk of cerebrovascular disease, thereby reducing the insult threshold required for an ischemic event.
4.7 Permanent pacemaker implantation
Intraventricular conduction abnormalities, particularly high-degree atrioventricular block, requiring PPI were one of the major complications after TAVR procedure (147). New-onset left bundle branch block and diabetes independently predicted high-degree atrioventricular block requiring PPI after TAVR and helped to identify patients at risk (148–150). Moreover, post-TAVR PPI was associated with hospitalization of heart failure and myocardial infarction (150). However, there was still a literature finding diabetes as a negative predictor of PPI following TAVR (151). This contradictory result might be affected by confounders not included in the multivariate analysis, such as size of implanted valve and diabetic status.
4.8 Systemic inflammatory response syndrome
Previous work has reported that approximately one-third of patients developed an acute inflammatory response within 48 hours after TAVR (152). Therein, severe SIRS developed in approximately 6% of patients undergoing TAVR (153). This SIRS was manifested with significantly elevated levels of inflammatory cytokines (such as IL-6 or IL-8) and C-reactive protein (CRP). The occurrence of SIRS was shown to be a strong predictor of mortality in patients undergoing TAVR (153, 154). Previous study found that the presence of diabetes, increased baseline high sensitivity CRP, and low baseline Th2 cell counts were multivariate predictors of death after TAVR (155). Another study found elevated fasting glucose and CRP level as predictors of increased all-cause mortality after TAVR (156). As mentioned above, diabetes was associated with systematic inflammation in patients with CAVD. Plasma proteomics analysis of the biomarker cohort revealed that IL‐1 receptors, GDF15, and cathepsin D were significantly elevated and that pathways related to inflammatory response were enriched in diabetic CAVD patients (157). Overall, the chronic systemic inflammation status in diabetes would be activated to some extent in TAVR procedure, and the evocative severe SIRS was associated with the mortality.
4.9 Bleeding
In order to prevent postoperative thrombosis and delay artificial valve dysfunction, dual antiplatelet therapy was routinely required after TAVR procedure. Long-term bleeding after TAVR was associated with an increased risk of subsequent mortality (158). Previous study found that patients with high bleeding risk after TAVR were more frequently presented with diabetes compared with those with lower bleeding risk (159). Moreover, there were some research studies finding that patients with diabetes had a higher risk of major bleedings compared with those without diabetes after TAVR (91, 160, 161). Nevertheless, there was another research finding no significant differences in major bleeding after TAVR between diabetes and non-diabetes (22). Baseline diabetes was not associated with baseline platelet reactivity levels in TAVR procedure (162). Similarly, there were biases against the role of diabetes on bleeding in other cardiovascular diseases (163). At present, there are no differences in the antiplatelet therapy strategy following TAVR between diabetes and non-diabetes. The absolute benefits of antiplatelet therapy were largely counterbalanced by the bleeding hazard.
5 Diabetes in surgical aortic valve replacement
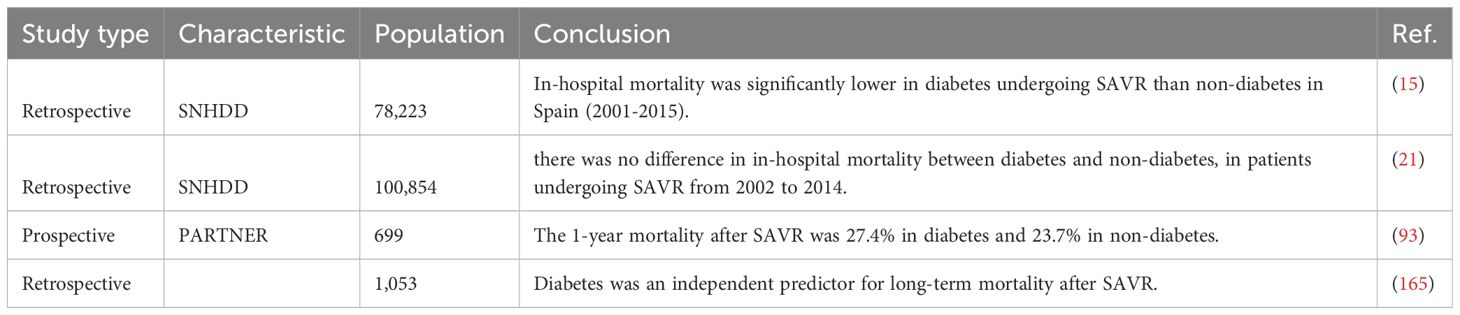
In recent decades, the incidence of bioprosthetic SAVR increased significantly in those with diabetes, which might partly be attributed to an increased prevalence of CAVD and an increased proportion of diseased patients diagnosed as such with ageing of population (14, 164). According to a retrospective study from Spanish cohorts, in‐hospital mortality was significantly lower in diabetes undergoing bioprosthetic SAVR than non-diabetes, which might be multifactorial (Table 3) (14). The impact of diabetes on short-term mortality after SAVR still remained controversial; diabetes has been found to be significantly and consistently associated to higher in-hospital mortality in a huge Spanish population after MACE, and there was no difference in 30-day mortality between diabetes and non-diabetes (21). However, the impact of diabetes on mid-term and long-term mortality after SAVR was consistent in several clinical studies. Long-term (5-year and 10-year) mortality was significantly higher in diabetes after SAVR compared with non-diabetes (19.4% vs. 12.9% and 30.3% vs. 23.5%) (165). A post-hoc analysis of the PARTNER trial, stratified according to the diabetes status of patients randomly assigned to undergo SAVR, revealed a 1-year mortality rate of 27.4% in diabetes and 23.7% in non-diabetes (93). This association was stronger among insulin-treated diabetes. In-hospital and long-term mortality rates were higher in the subgroup of insulin-treated diabetes compared with the subgroup of non-insulin treated diabetes (165). Insulin-treated diabetes had more comorbidities than non-insulin-treated diabetes and was prone to more revascularization procedures (166). Therefore, the results of mortality after SAVR in diabetes were partially dependent on the baseline diabetic status. Overall, the mid-term and long-term mortality rates after SAVR were higher in patients with diabetes than non-diabetes, while the short-term mortality remained controversial. There is a need for RCT and large cohorts with long-term follow-up of diabetes vs. non-diabetes in patients undergoing SAVR (Figure 4).
Figure 4. Clinical outcome and complication of diabetes in surgical aortic valve replacement. Compared with non-diabetes, the impact of diabetes on clinical outcomes and various complications after SAVR.
5.1 Obesity
Obesity was more prevalent in diabetes undergoing SAVR, which might have contributed to the decrease in in‐hospital mortality (167). The obesity paradox also indeed existed within the realm of SAVR. Post-SAVR complications such as myocardial infarction, stroke, reoperation rates, AKI, new renal failure, requirement of dialysis, and postoperative bleeding were either more frequent in patients with higher BMI or equivalent to their normal BMI counterparts (168, 169). Therefore, the role of obesity in SAVR was disputable, regarding the integrated consideration of mortality and postoperative complications. As obesity was a worldwide problem and surgical techniques were advancing, identification of the underlying causes of the obesity paradox was essential to providing optimal care for patients of all body sizes undergoing SAVR.
5.2 Coronary artery disease
Recent retrospective studies revealed that concomitant SAVR and CABG were associated with a significantly higher in-hospital mortality, while there was no additional mid-term or long-term survival risk compared with isolated SAVR (170–172). In patients undergoing concomitant SAVR and CABG, diabetes was associated with 30-day, 180-day, and long-term mortality (170, 173). No RCT focused on the long-term survival of performing a concomitant CABG with SAVR are currently available. Long-term mortality was higher in diabetes vs. non-diabetes, and especially in insulin- vs. non-insulin-treated diabetes regardless of undergoing PCI or CABG (174). Although the long-term mortality was not different in diabetes treated either with PCI or CABG, lower mortality was observed in CABG in the cohort with three-vessel CAD and high SYNTAX score (175). Based on the potential organ-protective and anti-inflammatory effects, a randomized, placebo-controlled clinical trial for efficacy of glucagon-like peptide-1 (GLP-1) receptor agonist exenatide and restrictive versus liberal oxygen supply in patients undergoing CABG with/without SAVR is in progress (176). The researchers intended to determine whether glucose-lowering medication could improve the outcomes in such high-risk patients.
5.3 Acute kidney injury
AKI was another serious complication after SAVR and held increased mortality. Diabetes was significantly associated with the development of AKI after SAVR (177, 178). Insulin-dependent diabetes was one of the predictors for post-SAVR renal failure with hemodialysis (179). Kidney recovery after SAVR was more frequent than AKI, which was associated with improved secondary clinical outcomes (180). Diabetes was a negative predictor of kidney recovery after SAVR (125), indicating less reversible kidney injury in diabetes. Compared with patients undergoing SAVR without hemodialysis, patients with chronic renal failure on hemodialysis had diabetes more frequently (181). Moreover, concomitance with diabetes was associated with increased 30-day mortality in chronic dialysis patients after SAVR (182). The conclusions in findings might be related to differences in patients’ controlled hyperglycemia, use of medications, and prior glomerular filtration rate, as diabetes was associated with ischemia and kidney injury.
5.4 Heart failure
Although SAVR resulted in significant improvements of pre-existing myocardial impairments, concomitance with diabetes exhibited more residual changes in myocardial structure, contractile function, and blood flow (135, 183). It might be due to more cumulative myocardial injuries in diabetes prior to SAVR and persistent systematic diabetic toxicity after replacement. Therefore, diabetes was one of the independent risk factors of rehospitalization for heart failure after SAVR (184). The initiation and development of myocardial structural/functional abnormalities leading to heart failure in diabetes included multiple mechanisms, which remained incompletely elucidated (185). Nevertheless, they encompassed common systemic factors with CAVD, including hyperglycemia, insulin resistance, excessive production of AGEs, and activation of the renin–angiotensin–aldosterone system (186). Therefore, further studies are needed to explore the potential efficacy of glucose-lowering medications in alleviating both diabetic myocardial and aortic valve injuries.
5.5 Patient–prosthesis mismatch
The concept of patient–prosthetic mismatch (PPM) after SAVR referred to the clinical situation in which a normally functioning prosthetic valve did not allow an adequate cardiac output without an excessive gradient across the aortic valve. The prevalence of moderate PPM ranged from 20% to 70% and that of severe PPM ranged from 2% to 20%, respectively (187). Clinical outcomes of patients with mild and moderate PPM were not significantly different to those without PPM, while severe PPM was associated with increased mid-term and long-term mortality after SAVR (188). Diabetes was one of the predictors of the prevalence of PPM in patients undergoing SAVR (189, 190). Diabetes was associated with the occurrence of mild and moderate PPM but did not have a significant effect on the occurrence of severe PPM (191). As PPM was only marginally associated with survival, it was not related to risk of reoperation after SAVR (192).
5.6 Systemic inflammatory response syndrome
SIRS developed in 11% of SAVR patients and was associated with a higher mortality after SAVR (153). Although diabetes was not associated with an increased risk of SIRS, severe SIRS had a greater effect on mortality in diabetes (153). Proteomics analysis of plasma from CAVD with diabetes found that IL-1 receptors, GDF15, and cathepsin D as well as the pathways associated with inflammation were significantly elevated (157). This systemic pro-inflammatory response might account for the worse clinical outcomes in diabetes undergoing SAVR.
5.7 Infective endocarditis
According to the latest 10-year outcomes of the NOTION trial, the rate of infective endocarditis was similar for both TAVR and SAVR (7.2% vs. 7.4%) (193). Once endocarditis occurred, the all-cause mortality increased rapidly, especially in the SAVR cohort (194, 195). Among patients with endocarditis, the rate of all-cause 1-year or 5-year mortality was higher in the SAVR group than that in the TAVR group (195). In the analysis of pooled statistics of three large RCT, patients with endocarditis after SAVR had diabetes more frequently at baseline than those without endocarditis (195). However, in the PARTNER 1 and PARTNER 2 trials, concomitance with diabetes was not associated with the occurrence of endocarditis after SAVR (194). The baseline clinical characteristics of patient population in different study, including age, previous CABG, and other valvular heart diseases, were disparate. The difference in those factors might account for the differences observed. Further RCT are needed to elucidate whether concomitance with diabetes is associated with the occurrence of endocarditis after SAVR and related mortality.
6 Diabetes in bioprosthetic aortic valve deterioration
6.1 Bioprosthetic aortic valve deterioration after AVR
Structural valve deterioration (SVD), manifested with leaflet calcification or fibrosis, was one of the pivotal factors limiting the durability of BAV and the prognosis after transcatheter or surgical replacement. Non-structural bioprosthetic valve dysfunction (BVD), defined as any abnormality not intrinsic to the aortic valve, included PPM and paravalvular regurgitation, which occurred at the time of SAVR or TAVR procedure and existed persistently during follow-up, while SVD developed progressively during follow-up. The durability of BAV is becoming a critical problem of TAVR, as this procedure is now considered for younger and lower-risk populations with longer life expectancy (196). In a propensity-matched analysis of intermediate-risk patients (PARTNER 2 and PARTNER 2A), the incidence of SVD at 5 years was 3.9% in TAVR with balloon-expandable SAPIEN 3 vs. 3.5% in SAVR (197). In the PARTNER 3 trial, the incidence of SVD at 5 years was 4.2% in TAVR with SAPIEN 3 vs. 3.8% in SAVR among low-risk patients (196). As for self-expanding CoreValve or Evolut, the CoreValve US High Risk Pivotal and SURTAVI trials found a lower rate of SVD in intermediate- or high-risk patients undergoing self-expanding TAVR vs. SAVR at 5 years (1.82% in TAVR vs. 2.67% in SAVR) (198). The only long-term NOTION trial revealed similar results in comparison between TAVR with CoreValve and SAVR (12.5% in TAVR vs. 13.9% in SAVR) (193). The CHOICE trial compared the first or second generations of SAPIEN with CoreValve in high-risk patients and found superior valve hemodynamic performance for self-expanding valves with lower rate of SVD at 5 years (199). The SMART trial also found that the self-expanding valve was superior to the balloon-expandable valve in the aspect of SVD at 1-year among patients with small aortic annulus (200). The superior performance of the self-expanding valve in SVD might be due to the supra-annular design with better hemodynamic properties. Large RCT with long follow-up are thus needed to compare the durability of different TAVR prostheses, especially among low-risk populations.
6.2 Bioprosthetic aortic valve deterioration in diabetes
Although deterioration of BAV has long been considered as a passive degenerative process, emerging studies revealed that active and potentially modifiable mechanisms might also participate in the fibrocalcific process of BAV (201). SVD shared common risk factors and similar pathological process with CAVD. One of the crucial risk factors that have been associated with SVD following TAVR or SAVR was diabetes (197, 198, 201, 202). In the PARTNER 2 trial, diabetes was associated with SVD at 5 years in the SAPIEN 3 TAVR cohort (197). In the prospective study of SVD after SAVR, univariate and multivariate Cox regression analyses found diabetes as one of the risk factors for deterioration of BAV and all-cause mortality (201). In another retrospective study, diabetes was associated with hemodynamic deterioration of BAV, especially at early years (202). Thus, concomitance with diabetes accelerated the deterioration of BAV and restricted the durability of BAV after TAVR or SAVR. The underlying mechanisms of SVD in diabetic conditions might include inflammation, oxidative stress, lipid retention, endothelial dysfunction, and AGEs/RAGE, which were similar to the molecular mechanisms of CAVD concomitant to diabetes as mentioned above. Diabetes was one of the predictors for the prevalence of PPM (189, 190), which in turn caused BVD after AVR. Bioprosthetic valve endocarditis was often associated with morphologic and hemodynamic valve deterioration and might thus lead to SVD (203). One of the prevalent predisposing conditions of prosthetic valve endocarditis was diabetes (204). The retrospective cohort study, conducted in five German cardiac surgery centers, multivariable analyzed 3,143 patients (73.1%) undergoing surgery for native valve endocarditis and 1,157 patients (26.9%) for prosthetic valve endocarditis (205). Patients with prosthetic valve endocarditis presented with higher proportion of diabetes than native valve endocarditis (205). Transcatheter valve-in-valve implantation was an alternative option in inoperable or high-risk patients with severe SVD (206). Age and diabetes were identified as independent predictors of all-cause 30-day mortality in patients with transcatheter valve-in-valve implantation (207). Currently, most of the clinically available BAVs are fabricated from glutaraldehyde-treated heterogeneous aortic valves or bovine/porcine pericardium (208). Previous studies have indicated superior hemodynamic characteristics in bovine pericardial valves compared to porcine valves (209, 210). Moreover, bovine valves were associated with better survival than porcine valves in diabetes (211). However, to date, no vitro or vivo experiments has specifically investigated the influences of diabetes on different bioprosthetic pericardial valves. Diabetes and its complications jointly participated in the deterioration of BAV, leading to increased long-term mortality and various complications after AVR.
6.3 Glucose-lowering medications in bioprosthetic aortic valve deterioration
Once SVD developed into bioprosthetic valve failure, the death rate increased rapidly except in cases of transcatheter valve-in-valve implantation or transthoracic reoperation (203). However, there are currently no medications to prevent or reverse the progression of BAV deterioration. The peroxisome proliferator-activated receptor γ (PPARγ) was a nuclear receptor that participated in various physiological processes as a transcriptional regulator (212). Activation of PPARγ by specific agonists, thiazolidinediones such as pioglitazone, has been widely used for lowering glucose via insulin-sensitizing and pancreatic β-cell preserving effects (213). Apart from glucose-lowering effect, PPARγ agonists have also been found to have anti-atherogenic and anti-inflammatory effects via regulating the expression of related genes (214, 215). Single-cell RNA sequencing analysis found conservation of PPARγ in non-calcified human aortic valve leaflets and activated PPARγ pathway in CD36-positive VECs in hyperlipidemic mice (216). Many vitro and vivo studies have revealed the anti-calcification effect of PPARγ agonists in the degeneration of native aortic valves (30, 80). Moreover, its effect on the deterioration of BAV was assessed in various rat models. In the streptozotocin-induced diabetic rats, pioglitazone led to an inhibition of BAV deterioration, manifested with a lower expression of chondro-osteogenic genes and calcium deposits (217). In the guide wire injury-induced AVS rats as well as hypercholesterolemic and obese rats, systemic PPARγ activation inhibited inflammation and calcification in heterologous aortic valve conduits and seemed to inhibit functional impairment of the implanted aortic valve (218, 219). Overall, based on the protective effects for CAVD and BAV deterioration in vitro and vivo, PPARγ agonists are currently one of the promising glucose-lowering medications to inhibit deterioration of BAV probably independent of glucose control (Figure 5).
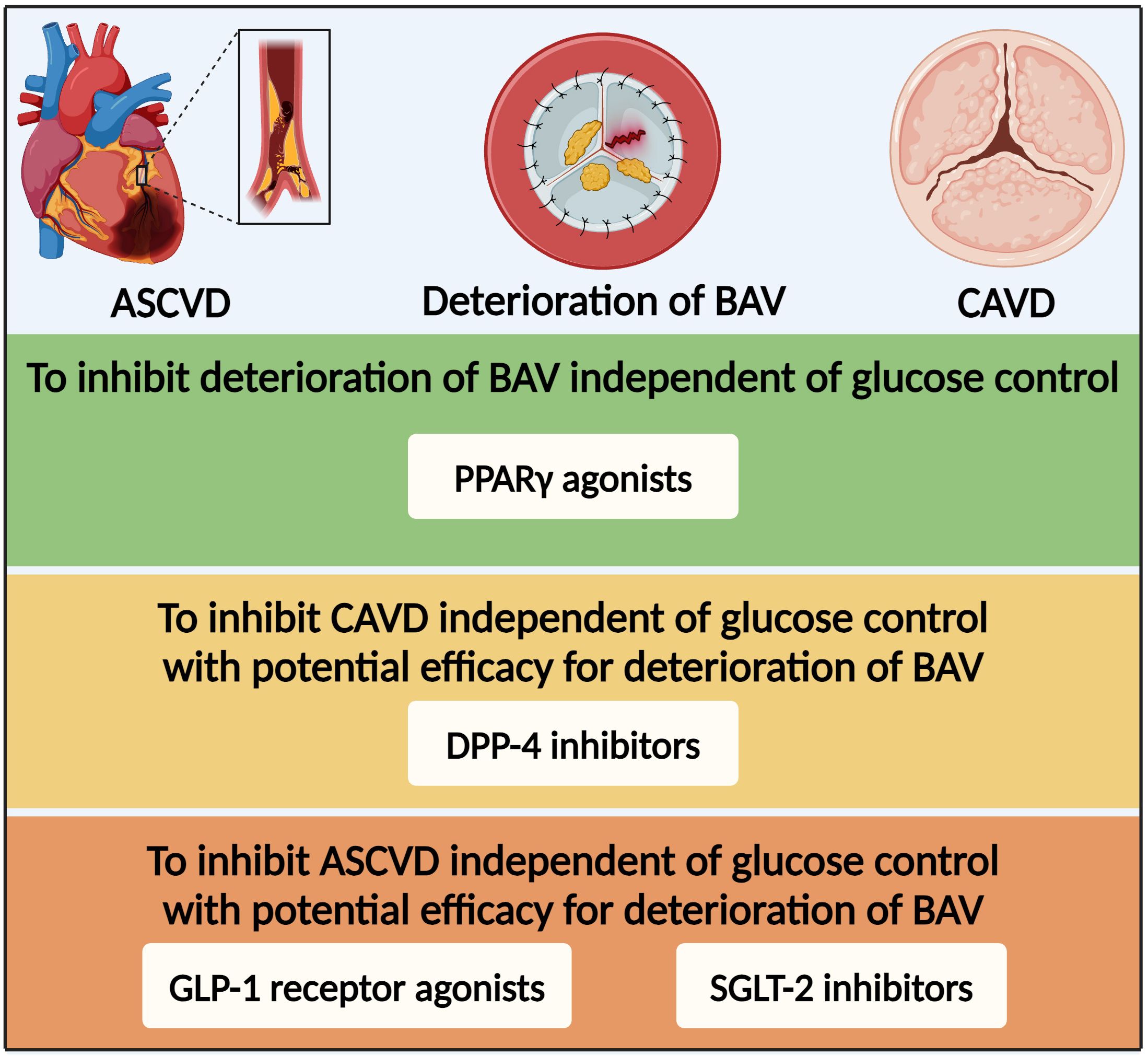
Figure 5. Glucose-lowering medication option for patients with diabetes and deterioration of bioprosthetic aortic valve based on the efficacy for CAVD and ASCVD. ASCVD, atherosclerotic cardiovascular disease; BAV, bioprosthetic aortic valve; PPARγ, peroxisome proliferator-activated receptor γ; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; SGLT-2, sodium–glucose co-transporter-2.
Dipeptidyl peptidase-4 (DPP-4) inhibitors have been widely applied to treat diabetes via inhibiting degradation of GLP-1 to regulate insulin secretion (220). DPP-4 was widely expressed in the cardiovascular tissues and participated in the physiopathologic process of various cardiovascular diseases (221). Although multiple large-scale clinical studies have demonstrated statistical non-inferiority but not superiority for the DPP-4 inhibitors in the primary MACE endpoint (222, 223), recent studies have found that DPP-4 inhibitors showed benefits on various cardiovascular diseases, such as hypertension, CAD, and CAVD (224). In vitro, DPP-4 upregulation by nitric oxide deprivation-dependent NF-κB activation resulted in osteogenic differentiation of VICs (225). In vivo, DPP-4 inhibitors markedly reduced calcification of aortic valve in eNOS-deficient mice and rabbit fed with cholesterol-enriched diet and vitamin D (225, 226). DPP-4 inhibitors suppressed CAVD by alleviating inflammation, fibrosis, and calcification (225–227). Overall, DPP-4 inhibitors might be able to inhibit CAVD independent of glucose control with potential efficacy for deterioration of BAV.
Over the last decade, the results of numerous large cardiovascular outcome trials in patients with diabetes at high cardiovascular risk with novel glucose-lowering medications, such as sodium-glucose co-transporter-2 (SGLT-2) inhibitors and GLP-1 receptor agonists, have substantially offered more available medications, resulting in brand new evidence-based medical therapy for the management of this population (10). Based on the cardiovascular benefits independent of glucose control, SGLT-2 and GLP-1 receptor agonist inhibitors might be the promising medications with potential efficacy for CAVD or even deterioration of BAV. Thus, further experiments and large-scale clinical studies are essential for verification of the effects of glucose-lowering medications on deterioration of BAV.
7 Conclusions
In recent years, the prevalence of diabetes has increased rapidly in patients with CAVD. Except for individual effects of diabetes (e.g., hyperglycemia, AGEs/RAGE pathway, oxidative stress, EndMT, and inflammation), there were multifactorial interactions between those diabetic complications (e.g., hypertension, hyperlipidemia, CKD, ageing), which mutually took part in the initiation and development of CAVD. The prevalence of diabetes in patients undergoing TAVR or SAVR has also increased along with the progression of CAVD. However, clinical outcomes and postoperative complications in diabetes after TAVR or SAVR remained controversial. Compared with non-diabetes, the short-term mortality after TAVR was not significant in diabetes, while the mid-term mortality remained disputable. The mid-term and long-term mortality rates after SAVR were higher in patients with diabetes than non-diabetes, while the short-term mortality remained disputable. There were common worse manifestations with CAD, AKI, heart failure, and SIRS in diabetes undergoing TAVR or SAVR, compared with non-diabetes. The role of diabetes-related obesity paradox in TAVR or SAVR remains disputable. There is a need for RCT and large cohorts with long-term follow-up of diabetes vs. non-diabetes in patients undergoing TAVR or SAVR. Moreover, diabetes and its complications also jointly participated in the deterioration of BAV, leading to increased long-term mortality and various postoperative complications after TAVR or SAVR. Based on the efficacy for CAVD and BAV deterioration in vitro and vivo, PPARγ agonists might be the promising glucose-lowering medication to inhibit BAV deterioration independent of glucose control.
Author contributions
FL: Conceptualization, Writing – original draft. HC: Formal Analysis, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Taizhou Science and Technology Plan (grant number: 1901ky41, H. C.) and Taizhou University Medical Plan (grant number: Z2024FSXY02, H. C.).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Otto CM and Prendergast B. Aortic-valve stenosis–from patients at risk to severe valve obstruction. N Engl J Med. (2014) 371:744–56. doi: 10.1056/NEJMra1313875
2. Généreux P, Pibarot P, Redfors B, Bax JJ, Zhao Y, Makkar RR, et al. Evolution and prognostic impact of cardiac damage after aortic valve replacement. J Am Coll Cardiol. (2022) 80:783–800. doi: 10.1016/j.jacc.2022.05.006
3. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. (2010) 363:1597–607. doi: 10.1056/NEJMoa1008232
4. Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG, et al. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. (2020) 382:799–809. doi: 10.1056/NEJMoa1910555
5. Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. (2011) 124:1783–91. doi: 10.1161/circulationaha.110.006767
6. Yan AT, Koh M, Chan KK, Guo H, Alter DA, Austin PC, et al. Association between cardiovascular risk factors and aortic stenosis: the CANHEART aortic stenosis study. J Am Coll Cardiol. (2017) 69:1523–32. doi: 10.1016/j.jacc.2017.01.025
7. Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. (2005) 352:2389–97. doi: 10.1056/NEJMoa043876
8. Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. (2008) 359:1343–56. doi: 10.1056/NEJMoa0804602
9. Chan KL, Teo K, Dumesnil JG, Ni A, and Tam J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. (2010) 121:306–14. doi: 10.1161/circulationaha.109.900027
10. Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, et al. ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. (20232023) 44:4043–140. doi: 10.1093/eurheartj/ehad192
11. Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, et al. Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. (2015) 66:2827–38. doi: 10.1016/j.jacc.2015.10.001
12. Singh A, Greenwood JP, Berry C, Dawson DK, Hogrefe K, Kelly DJ, et al. Comparison of exercise testing and CMR measured myocardial perfusion reserve for predicting outcome in asymptomatic aortic stenosis: the PRognostic Importance of MIcrovascular Dysfunction in Aortic Stenosis (PRIMID AS) Study. Eur Heart J. (2017) 38:1222–9. doi: 10.1093/eurheartj/ehx001
13. Culler SD, Cohen DJ, Brown PP, Kugelmass AD, Reynolds MR, Ambrose K, et al. Trends in aortic valve replacement procedures between 2009 and 2015: has transcatheter aortic valve replacement made a difference? Ann Thorac Surg. (2018) 105:1137–43. doi: 10.1016/j.athoracsur.2017.10.057
14. López-de-Andrés A, Perez-Farinos N, de Miguel-Díez J, Hernández-Barrera V, Méndez-Bailón M, de Miguel-Yanes JM, et al. Impact of type 2 diabetes mellitus in the utilization and in-hospital outcomes of surgical aortic valve replacement in Spain (2001-2015). Cardiovasc Diabetol. (2018) 17:135. doi: 10.1186/s12933-018-0780-2
15. Çelik M, Durko AP, Bekkers JA, Oei FBS, Mahtab EAF, and Bogers A. Outcomes of surgical aortic valve replacement over three decades. J Thorac Cardiovasc Surg. (2022) 164:1742–1751.e1748. doi: 10.1016/j.jtcvs.2021.04.064
16. Khan S, Dargham S, Al Suwaidi J, Jneid H, and Abi Khalil C. Trends and outcomes of aortic valve replacement in patients with diabetes in the US. Front Cardiovasc Med. (2022) 9:844068. doi: 10.3389/fcvm.2022.844068
17. Tomic D, Shaw JE, and Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. (2022) 18:525–39. doi: 10.1038/s41574-022-00690-7
18. Moncla LM, Briend M, Bossé Y, and Mathieu P. Calcific aortic valve disease: mechanisms, prevention and treatment. Nat Rev Cardiol. (2023) 20:546–59. doi: 10.1038/s41569-023-00845-7
19. Goody PR, Hosen MR, Christmann D, Niepmann ST, Zietzer A, Adam M, et al. Aortic valve stenosis: from basic mechanisms to novel therapeutic targets. Arterioscler Thromb Vasc Biol. (2020) 40:885–900. doi: 10.1161/atvbaha.119.313067
20. Novaro GM, Katz R, Aviles RJ, Gottdiener JS, Cushman M, Psaty BM, et al. Clinical factors, but not C-reactive protein, predict progression of calcific aortic-valve disease: the Cardiovascular Health Study. J Am Coll Cardiol. (2007) 50:1992–8. doi: 10.1016/j.jacc.2007.07.064
21. de Miguel-Yanes JM, Jiménez-García R, Hernández-Barrera V, Méndez-Bailón M, de Miguel-Díez J, and Lopez-de-Andrés A. Impact of type 2 diabetes mellitus on in-hospital-mortality after major cardiovascular events in Spain (2002-2014). Cardiovasc Diabetol. (2017) 16:126. doi: 10.1186/s12933-017-0609-4
22. Mina GS, Gill P, Soliman D, Reddy P, and Dominic P. Diabetes mellitus is associated with increased acute kidney injury and 1-year mortality after transcatheter aortic valve replacement: A meta-analysis. Clin Cardiol. (2017) 40:726–31. doi: 10.1002/clc.22723
23. Berkovitch A, Segev A, Barbash I, Grossman Y, Maor E, Erez A, et al. Clinical impact of diabetes mellitus in patients undergoing transcatheter aortic valve replacement. Cardiovasc Diabetol. (2015) 14:131. doi: 10.1186/s12933-015-0291-3
24. Ljungberg J, Johansson B, Engström KG, Albertsson E, Holmer P, Norberg M, et al. Traditional cardiovascular risk factors and their relation to future surgery for valvular heart disease or ascending aortic disease: A case-referent study. J Am Heart Assoc. (2017). doi: 10.1161/jaha.116.005133
25. Strange JE, Sindet-Pedersen C, Gislason GH, Torp-Pedersen C, Kragholm KH, Lundahl C, et al. Temporal trends in utilization of transcatheter aortic valve replacement and patient characteristics: A nationwide study. Am Heart J. (2022) 243:140–6. doi: 10.1016/j.ahj.2021.09.010
26. Zabirnyk A, Evensen D, Kvitting JE, Kaljusto ML, Stensløkken KO, and Vaage J. Hyperglycemia-simulating environment attenuated experimentally induced calcification in cultured human aortic valve interstitial cells. Scand Cardiovasc J. (2024) 58:2353070. doi: 10.1080/14017431.2024.2353070
27. Ciortan L, Macarie RD, Cecoltan S, Vadana M, Tucureanu MM, Mihaila AC, et al. Chronic high glucose concentration induces inflammatory and remodeling changes in valvular endothelial cells and valvular interstitial cells in a gelatin methacrylate 3D model of the human aortic valve. Polymers (Basel). (2020) 12. doi: 10.3390/polym12122786
28. Selig JI, Boulgaropoulos J, Niazy N, Ouwens DM, Preuß K, Horn P, et al. Crosstalk of diabetic conditions with static versus dynamic flow environment-impact on aortic valve remodeling. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22136976
29. Scatena M, Jackson MF, Speer MY, Leaf EM, Wallingford MC, and Giachelli CM. Increased Calcific Aortic Valve Disease in response to a diabetogenic, procalcific diet in the LDLr(-/-)ApoB(100/100) mouse model. Cardiovasc Pathol. (2018) 34:28–37. doi: 10.1016/j.carpath.2018.02.002
30. Li F, Cai Z, Chen F, Shi X, Zhang Q, Chen S, et al. Pioglitazone attenuates progression of aortic valve calcification via down-regulating receptor for advanced glycation end products. Basic Res Cardiol. (2012) 107:306. doi: 10.1007/s00395-012-0306-0
31. Kopytek M, Mazur P, Ząbczyk M, Undas A, and Natorska J. Diabetes concomitant to aortic stenosis is associated with increased expression of NF-κB and more pronounced valve calcification. Diabetologia. (2021) 64:2562–74. doi: 10.1007/s00125-021-05545-w
32. Kopytek M, Ząbczyk M, Mazur P, Undas A, and Natorska J. Accumulation of advanced glycation end products (AGEs) is associated with the severity of aortic stenosis in patients with concomitant type 2 diabetes. Cardiovasc Diabetol. (2020) 19:92. doi: 10.1186/s12933-020-01068-7
33. Hofmann B, Yakobus Y, Indrasari M, Nass N, Santos AN, Kraus FB, et al. RAGE influences the development of aortic valve stenosis in mice on a high fat diet. Exp Gerontol. (2014) 59:13–20. doi: 10.1016/j.exger.2014.05.001
34. Wang B, Cai Z, Liu B, Liu Z, Zhou X, Dong N, et al. RAGE deficiency alleviates aortic valve calcification in ApoE(-/-) mice via the inhibition of endoplasmic reticulum stress. Biochim Biophys Acta Mol Basis Dis. (2017) 1863:781–92. doi: 10.1016/j.bbadis.2016.12.012
35. Lee SW, Kim HC, Lee JM, Yun YM, Lee JY, and Suh I. Association between changes in systolic blood pressure and incident diabetes in a community-based cohort study in Korea. Hypertens Res. (2017) 40:710–6. doi: 10.1038/hr.2017.21
36. Ohishi M. Hypertension with diabetes mellitus: physiology and pathology. Hypertens Res. (2018) 41:389–93. doi: 10.1038/s41440-018-0034-4
37. Linhartová K, Filipovský J, Cerbák R, Sterbáková G, Hanisová I, and Beránek V. Severe aortic stenosis and its association with hypertension: analysis of clinical and echocardiographic parameters. Blood Press. (2007) 16:122–8. doi: 10.1080/08037050701343241
38. Rahimi K, Mohseni H, Kiran A, Tran J, Nazarzadeh M, Rahimian F, et al. Elevated blood pressure and risk of aortic valve disease: a cohort analysis of 5.4 million UK adults. Eur Heart J. (2018) 39:3596–603. doi: 10.1093/eurheartj/ehy486
39. Bermejo J. The effects of hypertension on aortic valve stenosis. Heart. (2005) 91:280–2. doi: 10.1136/hrt.2004.041749
40. Lindman BR and Otto CM. Time to treat hypertension in patients with aortic stenosis. Circulation. (2013) 128:1281–3. doi: 10.1161/circulationaha.113.005275
41. Timmerman LA, Grego-Bessa J, Raya A, Bertrán E, Pérez-Pomares JM, Díez J, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. (2004) 18:99–115. doi: 10.1101/gad.276304
42. Basile C, Fucile I, Lembo M, Manzi MV, Ilardi F, Franzone A, et al. Arterial hypertension in aortic valve stenosis: A critical update. J Clin Med. (2021) 10. doi: 10.3390/jcm10235553
43. Kaden JJ, Dempfle CE, Grobholz R, Tran HT, Kiliç R, Sarikoç A, et al. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. (2003) 170:205–11. doi: 10.1016/s0021-9150(03)00284-3
44. Satta J, Melkko J, Pöllänen R, Tuukkanen J, Pääkkö P, Ohtonen P, et al. Progression of human aortic valve stenosis is associated with tenascin-C expression. J Am Coll Cardiol. (2002) 39:96–101. doi: 10.1016/s0735-1097(01)01705-3
45. Kurozumi A, Nakano K, Yamagata K, Okada Y, Nakayamada S, and Tanaka Y. IL-6 and sIL-6R induces STAT3-dependent differentiation of human VSMCs into osteoblast-like cells through JMJD2B-mediated histone demethylation of RUNX2. Bone. (2019) 124:53–61. doi: 10.1016/j.bone.2019.04.006
46. Cuevas RA, Hortells L, Chu CC, Wong R, Crane A, Boufford CK, et al. Non-canonical TERT activity initiates osteogenesis in calcific aortic valve disease. Circ Res. (2025) 136:403–21. doi: 10.1161/circresaha.122.321889
47. Abdelbaky A, Corsini E, Figueroa AL, Subramanian S, Fontanez S, Emami H, et al. Early aortic valve inflammation precedes calcification: a longitudinal FDG-PET/CT study. Atherosclerosis. (2015) 238:165–72. doi: 10.1016/j.atherosclerosis.2014.11.026
48. Yu Chen H, Dina C, Small AM, Shaffer CM, Levinson RT, Helgadóttir A, et al. Dyslipidemia, inflammation, calcification, and adiposity in aortic stenosis: a genome-wide study. Eur Heart J. (2023) 44:1927–39. doi: 10.1093/eurheartj/ehad142
49. Nazarzadeh M, Pinho-Gomes AC, Bidel Z, Dehghan A, Canoy D, Hassaine A, et al. Plasma lipids and risk of aortic valve stenosis: a Mendelian randomization study. Eur Heart J. (2020) 41:3913–20. doi: 10.1093/eurheartj/ehaa070
50. Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. (1997) 17:680–7. doi: 10.1161/01.atv.17.4.680
51. Tsimikas S and Witztum JL. The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr Opin Lipidol. (2008) 19:369–77. doi: 10.1097/MOL.0b013e328308b622
52. Côté C, Pibarot P, Després JP, Mohty D, Cartier A, Arsenault BJ, et al. Association between circulating oxidised low-density lipoprotein and fibrocalcific remodelling of the aortic valve in aortic stenosis. Heart. (2008) 94:1175–80. doi: 10.1136/hrt.2007.125740
53. Que X, Hung MY, Yeang C, Gonen A, Prohaska TA, Sun X, et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature. (2018) 558:301–6. doi: 10.1038/s41586-018-0198-8
54. Saga H, Ohhata A, Hayashi A, Katoh M, Maeda T, Mizuno H, et al. A novel highly potent autotaxin/ENPP2 inhibitor produces prolonged decreases in plasma lysophosphatidic acid formation in vivo and regulates urethral tension. PloS One. (2014) 9:e93230. doi: 10.1371/journal.pone.0093230
55. Torzewski M, Ravandi A, Yeang C, Edel A, Bhindi R, Kath S, et al. Lipoprotein(a) associated molecules are prominent components in plasma and valve leaflets in calcific aortic valve stenosis. JACC Basic Transl Sci. (2017) 2:229–40. doi: 10.1016/j.jacbts.2017.02.004
56. Nsaibia MJ, Boulanger MC, Bouchareb R, Mkannez G, Le Quang K, Hadji F, et al. OxLDL-derived lysophosphatidic acid promotes the progression of aortic valve stenosis through a LPAR1-RhoA-NF-κB pathway. Cardiovasc Res. (2017) 113:1351–63. doi: 10.1093/cvr/cvx089
57. Selby NM and Taal MW. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. (2020) 22 Suppl 1:3–15. doi: 10.1111/dom.14007
58. Jin Q, Liu T, Qiao Y, Liu D, Yang L, Mao H, et al. Oxidative stress and inflammation in diabetic nephropathy: role of polyphenols. Front Immunol. (2023) 14:1185317. doi: 10.3389/fimmu.2023.1185317
59. Rattazzi M, Bertacco E, Del Vecchio A, Puato M, Faggin E, and Pauletto P. Aortic valve calcification in chronic kidney disease. Nephrol Dial Transplant. (2013) 28:2968–76. doi: 10.1093/ndt/gft310
60. Hensen LCR, Mahdiui ME, van Rosendael AR, Smit JM, Jukema JW, Bax JJ, et al. Prevalence and prognostic implications of mitral and aortic valve calcium in patients with chronic kidney disease. Am J Cardiol. (2018) 122:1732–7. doi: 10.1016/j.amjcard.2018.08.009
61. Samad Z, Sivak JA, Phelan M, Schulte PJ, Patel U, and Velazquez EJ. Prevalence and outcomes of left-sided valvular heart disease associated with chronic kidney disease. J Am Heart Assoc. (2017) 6. doi: 10.1161/jaha.117.006044
62. Yutzey KE, Demer LL, Body SC, Huggins GS, Towler DA, Giachelli CM, et al. Calcific aortic valve disease: a consensus summary from the Alliance of Investigators on Calcific Aortic Valve Disease. Arterioscler Thromb Vasc Biol. (2014) 34:2387–93. doi: 10.1161/atvbaha.114.302523
63. Lyu T, Liu Y, Li B, Xu R, Guo J, and Zhu D. Single-cell transcriptomics reveals cellular heterogeneity and macrophage-to-mesenchymal transition in bicuspid calcific aortic valve disease. Biol Direct. (2023) 18:35. doi: 10.1186/s13062-023-00390-w
64. Otto CM, Kuusisto J, Reichenbach DD, Gown AM, and O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. (1994) 90:844–53. doi: 10.1161/01.cir.90.2.844
65. Šteiner I, Stejskal V, and Žáček P. Mast cells in calcific aortic stenosis. Pathol Res Pract. (2018) 214:163–8. doi: 10.1016/j.prp.2017.07.016
66. Isoda K, Matsuki T, Kondo H, Iwakura Y, and Ohsuzu F. Deficiency of interleukin-1 receptor antagonist induces aortic valve disease in BALB/c mice. Arterioscler Thromb Vasc Biol. (2010) 30:708–15. doi: 10.1161/atvbaha.109.201749
67. Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, and Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. (2000) 87:1055–62. doi: 10.1161/01.res.87.11.1055
68. Coté N, Mahmut A, Bosse Y, Couture C, Pagé S, Trahan S, et al. Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation. (2013) 36:573–81. doi: 10.1007/s10753-012-9579-6
69. Helske S, Lindstedt KA, Laine M, Mäyränpää M, Werkkala K, Lommi J, et al. Induction of local angiotensin II-producing systems in stenotic aortic valves. J Am Coll Cardiol. (2004) 44:1859–66. doi: 10.1016/j.jacc.2004.07.054
70. Katwa LC, Ratajska A, Cleutjens JP, Sun Y, Zhou G, Lee SJ, et al. Angiotensin converting enzyme and kininase-II-like activities in cultured valvular interstitial cells of the rat heart. Cardiovasc Res. (1995) 29:57–64.
71. Fujisaka T, Hoshiga M, Hotchi J, Takeda Y, Jin D, Takai S, et al. Angiotensin II promotes aortic valve thickening independent of elevated blood pressure in apolipoprotein-E deficient mice. Atherosclerosis. (2013) 226:82–7. doi: 10.1016/j.atherosclerosis.2012.10.055
72. Beckman JA, Creager MA, and Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. (2002) 287:2570–81. doi: 10.1001/jama.287.19.2570
73. Sell S and Scully RE. Aging changes in the aortic and mitral valves. Histologic and histochemical studies, with observations on the pathogenesis of calcific aortic stenosis and calcification of the mitral annulus. Am J Pathol. (1965) 46:345–65.
74. Waller BF. The old-age heart: normal aging changes which can produce or mimic cardiac disease. Clin Cardiol. (1988) 11:513–7. doi: 10.1002/clc.4960110802
75. Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M, et al. Global epidemiology of valvular heart disease. Nat Rev Cardiol. (2021) 18:853–64. doi: 10.1038/s41569-021-00570-z
76. Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M, et al. Diabetic cardiovascular disease induced by oxidative stress. Int J Mol Sci. (2015) 16:25234–63. doi: 10.3390/ijms161025234
77. Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, and Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. (2018) 122:877–902. doi: 10.1161/circresaha.117.311401
78. Farrar EJ, Huntley GD, and Butcher J. Endothelial-derived oxidative stress drives myofibroblastic activation and calcification of the aortic valve. PloS One. (2015) 10:e0123257. doi: 10.1371/journal.pone.0123257
79. Liu H, Wang L, Pan Y, Wang X, Ding Y, Zhou C, et al. Celastrol alleviates aortic valve calcification via inhibition of NADPH oxidase 2 in valvular interstitial cells. JACC Basic Transl Sci. (2020) 5:35–49. doi: 10.1016/j.jacbts.2019.10.004
80. Chu Y, Lund DD, Weiss RM, Brooks RM, Doshi H, Hajj GP, et al. Pioglitazone attenuates valvular calcification induced by hypercholesterolemia. Arterioscler Thromb Vasc Biol. (2013) 33:523–32. doi: 10.1161/atvbaha.112.300794
81. Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg. (2018) 1859:940–50. doi: 10.1016/j.bbabio.2018.05.019
82. Liu F, Chen J, Hu W, Gao C, Zeng Z, Cheng S, et al. PTP1B inhibition improves mitochondrial dynamics to alleviate calcific aortic valve disease via regulating OPA1 homeostasis. JACC Basic Transl Sci. (2022) 7:697–712. doi: 10.1016/j.jacbts.2022.03.002
83. Yu B, Khan K, Hamid Q, Mardini A, Siddique A, Aguilar-Gonzalez LP, et al. Pathological significance of lipoprotein(a) in aortic valve stenosis. Atherosclerosis. (2018) 272:168–74. doi: 10.1016/j.atherosclerosis.2018.03.025
84. Kraler S, Blaser MC, Aikawa E, Camici GG, and Lüscher TF. Calcific aortic valve disease: from molecular and cellular mechanisms to medical therapy. Eur Heart J. (2022) 43:683–97. doi: 10.1093/eurheartj/ehab757
85. Gupta A, Nagaraju SP, Bhojaraja MV, Swaminathan SM, and Mohan PB. Hypertension in chronic kidney disease: an update on diagnosis and management. South Med J. (2023) 116:237–44. doi: 10.14423/smj.0000000000001516
86. Mohandes S, Doke T, Hu H, Mukhi D, Dhillon P, and Susztak K. Molecular pathways that drive diabetic kidney disease. J Clin Invest. (2023) 133. doi: 10.1172/jci165654
87. Masenga SK, Kabwe LS, Chakulya M, and Kirabo A. Mechanisms of oxidative stress in metabolic syndrome. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24097898
88. Sundt TM and Jneid H. Guideline update on indications for transcatheter aortic valve implantation based on the 2020 american college of cardiology/american heart association guidelines for management of valvular heart disease. JAMA Cardiol. (2021) 6:1088–9. doi: 10.1001/jamacardio.2021.2534
89. Matsumoto S, Ohno Y, Miyamoto J, Ikari Y, Tada N, Naganuma T, et al. Impact of diabetes mellitus on outcome after transcatheter aortic valve replacement: Identifying high-risk diabetic population from the OCEAN-TAVI registry. Catheter Cardiovasc Interv. (2021) 98:E1058–e1065. doi: 10.1002/ccd.29960
90. Tzamalis P, Herzberger V, Bergmann J, Wuerth A, Bramlage P, Schroefel H, et al. The association of diabetes mellitus treated with oral antidiabetic drugs and insulin with mortality after transcatheter valve implantation: a 3-year follow-up of the TAVIK registry. Cardiovasc Diabetol. (2019) 18:63. doi: 10.1186/s12933-019-0873-6
91. Abramowitz Y, Vemulapalli S, Chakravarty T, Li Z, Kapadia S, Holmes D, et al. Clinical impact of diabetes mellitus on outcomes after transcatheter aortic valve replacement: insights from the society of thoracic surgeons/american college of cardiology transcatheter valve therapy registry. Circ Cardiovasc Interv. (2017). doi: 10.1161/circinterventions.117.005417
92. Goel R, Sartori S, Cao D, Claessen BE, Baber U, Chandiramani R, et al. Impact of diabetes mellitus on female subjects undergoing transcatheter aortic valve implantation: Insights from the WIN-TAVI international registry. Int J Cardiol. (2021) 322:65–9. doi: 10.1016/j.ijcard.2020.08.035
93. Lindman BR, Pibarot P, Arnold SV, Suri RM, McAndrew TC, Maniar HS, et al. Transcatheter versus surgical aortic valve replacement in patients with diabetes and severe aortic stenosis at high risk for surgery: an analysis of the PARTNER Trial (Placement of Aortic Transcatheter Valve). J Am Coll Cardiol. (2014) 63:1090–9. doi: 10.1016/j.jacc.2013.10.057
94. Mendez-Bailon M, Lorenzo-Villalba N, Muñoz-Rivas N, de Miguel-Yanes JM, De Miguel-Diez J, Comín-Colet J, et al. Transcatheter aortic valve implantation and surgical aortic valve replacement among hospitalized patients with and without type 2 diabetes mellitus in Spain (2014-2015). Cardiovasc Diabetol. (2017) 16:144. doi: 10.1186/s12933-017-0631-6
95. Arsalan M, Weferling M, Hecker F, Filardo G, Kim WK, Pollock BD, et al. TAVI risk scoring using established versus new scoring systems: role of the new STS/ACC model. EuroIntervention. (2018) 13:1520–6. doi: 10.4244/eij-d-17-00421
96. Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, and Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. (1999) 16:9–13. doi: 10.1016/s1010-7940(99)00134-7
97. Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. EuroSCORE II. Eur J Cardiothorac Surg. (2012) 41:734–44. doi: 10.1093/ejcts/ezs043
98. van Nieuwkerk AC, Santos RB, Mata RB, Tchétché D, de Brito FS Jr., Barbanti M, et al. Diabetes mellitus in transfemoral transcatheter aortic valve implantation: a propensity matched analysis. Cardiovasc Diabetol. (2022) 21:246. doi: 10.1186/s12933-022-01654-x
99. Chorin E, Finkelstein A, Banai S, Aviram G, Barkagan M, Barak L, et al. Impact of diabetes mellitus and hemoglobin A1C on outcome after transcatheter aortic valve implantation. Am J Cardiol. (2015) 116:1898–903. doi: 10.1016/j.amjcard.2015.09.032
100. Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O’Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. (2015) 100:4490–7. doi: 10.1210/jc.2015-2660
101. Hu X, Feng D, Zhang Y, Wang C, Chen Y, Niu G, et al. Prognostic effect of stress hyperglycemia ratio on patients with severe aortic stenosis receiving transcatheter aortic valve replacement: a prospective cohort study. Cardiovasc Diabetol. (2024) 23:73. doi: 10.1186/s12933-024-02160-y
102. Linke A, Schlotter F, Haussig S, Woitek FJ, Stachel G, Adam J, et al. Gender-dependent association of diabetes mellitus with mortality in patients undergoing transcatheter aortic valve replacement. Clin Res Cardiol. (2019) 108:39–47. doi: 10.1007/s00392-018-1309-0
103. Tokarek T, Dziewierz A, Wiktorowicz A, Bagienski M, Rzeszutko L, Sorysz D, et al. Effect of diabetes mellitus on clinical outcomes and quality of life after transcatheter aortic valve implantation for severe aortic valve stenosis. Hellenic J Cardiol. (2018) 59:100–7. doi: 10.1016/j.hjc.2017.08.002
104. Sia CH, Ho JS, Chua JJ, Tan BY, Ngiam NJ, Chew N, et al. Comparison of clinical and echocardiographic features of asymptomatic patients with stenotic bicuspid versus tricuspid aortic valves. Am J Cardiol. (2020) 128:210–5. doi: 10.1016/j.amjcard.2020.05.008
105. Elbadawi A, Mohamed AH, Elgendy IY, Ogunbayo GO, Megaly M, Shahin HI, et al. Comparative outcomes of transapical versus transfemoral access for transcatheter aortic valve replacement in diabetics. Cardiol Ther. (2020) 9:107–18. doi: 10.1007/s40119-019-00155-5
106. Fink N, Segev A, Kornowski R, Finkelstein A, Assali A, Rozenbaum Z, et al. Balloon dilatation and outcome among patients undergoing trans-femoral aortic valve replacement. Int J Cardiol. (2017) 230:537–41. doi: 10.1016/j.ijcard.2016.12.062
107. Stachon P, Hehn P, Wolf D, Heidt T, Oettinger V, Zehender M, et al. In-hospital outcomes of self-expanding and balloon-expandable transcatheter heart valves in Germany. Clin Res Cardiol. (2021) 110:1977–82. doi: 10.1007/s00392-021-01928-6
108. Oettinger V, Hilgendorf I, Wolf D, Rilinger J, Maier A, Zehender M, et al. Comparing balloon-expandable and self-expanding transfemoral transcatheter aortic valve replacement based on subgroups in Germany 2019/2020. Clin Res Cardiol. (2024) 113:168–76. doi: 10.1007/s00392-023-02326-w
109. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900–000 adults: collaborative analyses of 57 prospective studies. Lancet. (2009) 373:1083–96. doi: 10.1016/s0140-6736(09)60318-4
110. Lancefield T, Clark DJ, Andrianopoulos N, Brennan AL, Reid CM, Johns J, et al. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter Australian registry. JACC Cardiovasc Interv. (2010) 3:660–8. doi: 10.1016/j.jcin.2010.03.018
111. Hastie CE, Padmanabhan S, Slack R, Pell AC, Oldroyd KG, Flapan AD, et al. Obesity paradox in a cohort of 4880 consecutive patients undergoing percutaneous coronary intervention. Eur Heart J. (2010) 31:222–6. doi: 10.1093/eurheartj/ehp317
112. Gupta R, Mahmoudi E, Behnoush AH, Khalaji A, Malik AH, Sood A, et al. Effect of BMI on patients undergoing transcatheter aortic valve implantation: A systematic review and meta-analysis. Prog Cardiovasc Dis. (2023) 78:58–66. doi: 10.1016/j.pcad.2022.12.006
113. Quine EJ, Dagan M, William J, Nanayakkara S, Dawson LP, Duffy SJ, et al. Long-term outcomes stratified by body mass index in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. (2020) 137:77–82. doi: 10.1016/j.amjcard.2020.09.039
114. Madanat L, Jabri A, Hanson ID, Khalili H, Rodés-Cabau J, Pilgrim T, et al. Obesity paradox in transcatheter aortic valve replacement. Curr Cardiol Rep. (2024) 26:1005–9. doi: 10.1007/s11886-024-02098-3
115. Seo J, Kharawala A, Borkowski P, Singh N, Akunor H, Nagraj S, et al. Obesity and transcatheter aortic valve replacement. J Cardiovasc Dev Dis. (2024) 11. doi: 10.3390/jcdd11060169
116. Faroux L, Guimaraes L, Wintzer-Wehekind J, Junquera L, Ferreira-Neto AN, Del Val D, et al. Coronary artery disease and transcatheter aortic valve replacement: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 74:362–72. doi: 10.1016/j.jacc.2019.06.012
117. Lund O, Nielsen TT, Pilegaard HK, Magnussen K, and Knudsen MA. The influence of coronary artery disease and bypass grafting on early and late survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. (1990) 100:327–37.
118. Del Portillo JH, Farjat-Pasos J, Galhardo A, Avvedimento M, Mas-Peiro S, Mengi S, et al. Aortic stenosis with coronary artery disease: SAVR or TAVR-when and how? Can J Cardiol. (2024) 40:218–34. doi: 10.1016/j.cjca.2023.09.023
119. Yamashita Y, Sicouri S, Baudo M, Dokollari A, Rodriguez R, Gnall EM, et al. Impact of prior coronary artery bypass grafting and coronary lesion complexity on outcomes of transcatheter aortic valve replacement for severe aortic stenosis. Coron Artery Dis. (2024). doi: 10.1097/mca.0000000000001386
120. Faroux L, Campelo-Parada F, Munoz-Garcia E, Nombela-Franco L, Fischer Q, Donaint P, et al. Procedural characteristics and late outcomes of percutaneous coronary intervention in the workup pre-TAVR. JACC Cardiovasc Interv. (2020) 13:2601–13. doi: 10.1016/j.jcin.2020.07.009
121. Costa G, Pilgrim T, Amat Santos IJ, De Backer O, Kim WK, Barbosa Ribeiro H, et al. Management of myocardial revascularization in patients with stable coronary artery disease undergoing transcatheter aortic valve implantation. Circ Cardiovasc Interv. (2022) 15:e012417. doi: 10.1161/circinterventions.122.012417
122. Kim WK, Pellegrini C, Ludwig S, Möllmann H, Leuschner F, Makkar R, et al. Feasibility of coronary access in patients with acute coronary syndrome and previous TAVR. JACC Cardiovasc Interv. (2021) 14:1578–90. doi: 10.1016/j.jcin.2021.05.007
123. Mentias A, Desai MY, Saad M, Horwitz PA, Rossen JD, Panaich S, et al. Incidence and outcomes of acute coronary syndrome after transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2020) 13:938–50. doi: 10.1016/j.jcin.2019.11.027
124. Fan J, Yu C, Ren K, Lin W, Ng S, Chen Z, et al. Kidney function change after transcatheter aortic valve replacement in patients with diabetes and/or hypertension. J Zhejiang Univ Sci B. (2021) 22:241–7. doi: 10.1631/jzus.B2000431
125. Lahoud R, Butzel DW, Parsee A, Huang YL, Solomon RJ, DeVries JT, et al. Acute kidney recovery in patients who underwent transcatheter versus surgical aortic valve replacement (from the northern new england cardiovascular disease study group). Am J Cardiol. (2020) 125:788–94. doi: 10.1016/j.amjcard.2019.11.024
126. Ogami T, Kurlansky P, Takayama H, Ning Y, Ali ZA, Nazif TM, et al. Long-term outcomes of transcatheter aortic valve replacement in patients with end-stage renal disease. J Am Heart Assoc. (2021) 10:e019930. doi: 10.1161/jaha.120.019930
127. Pasceri V, Pelliccia F, Mehran R, Dangas G, Porto I, Radico F, et al. Risk score for prediction of dialysis after transcatheter aortic valve replacement. J Am Heart Assoc. (2024) 13:e032955. doi: 10.1161/jaha.123.032955
128. Park DY, An S, Hanna JM, Wang SY, Cruz-Solbes AS, Kochar A, et al. Readmission rates and risk factors for readmission after transcatheter aortic valve replacement in patients with end-stage renal disease. PloS One. (2022) 17:e0276394. doi: 10.1371/journal.pone.0276394
129. Svensson LG, Blackstone EH, Rajeswaran J, Brozzi N, Leon MB, Smith CR, et al. Comprehensive analysis of mortality among patients undergoing TAVR: results of the PARTNER trial. J Am Coll Cardiol. (2014) 64:158–68. doi: 10.1016/j.jacc.2013.08.1666
130. Urena M, Webb JG, Eltchaninoff H, Muñoz-García AJ, Bouleti C, Tamburino C, et al. Late cardiac death in patients undergoing transcatheter aortic valve replacement: incidence and predictors of advanced heart failure and sudden cardiac death. J Am Coll Cardiol. (2015) 65:437–48. doi: 10.1016/j.jacc.2014.11.027
131. Mesnier J, Ternacle J, Cheema AN, Campelo-Parada F, Urena M, Veiga-Fernandez G, et al. Cardiac death after transcatheter aortic valve replacement with contemporary devices. JACC Cardiovasc Interv. (2023) 16:2277–90. doi: 10.1016/j.jcin.2023.07.015
132. Lynge TH, Svane J, Pedersen-Bjergaard U, Gislason G, Torp-Pedersen C, Banner J, et al. Sudden cardiac death among persons with diabetes aged 1–49 years: a 10-year nationwide study of 14 294 deaths in Denmark. Eur Heart J. (2020) 41:2699–706. doi: 10.1093/eurheartj/ehz891
133. Auffret V, Bakhti A, Leurent G, Bedossa M, Tomasi J, Belhaj Soulami R, et al. Determinants and impact of heart failure readmission following transcatheter aortic valve replacement. Circ Cardiovasc Interv. (2020) 13:e008959. doi: 10.1161/circinterventions.120.008959
134. Park JJ. Epidemiology, pathophysiology, diagnosis and treatment of heart failure in diabetes. Diabetes Metab J. (2021) 45:146–57. doi: 10.4093/dmj.2020.0282
135. Jex N, Greenwood JP, Cubbon RM, Rider OJ, Chowdhary A, Thirunavukarasu S, et al. Association between type 2 diabetes and changes in myocardial structure, contractile function, energetics, and blood flow before and after aortic valve replacement in patients with severe aortic stenosis. Circulation. (2023) 148:1138–53. doi: 10.1161/circulationaha.122.063444
136. Kolte D, Bhardwaj B, Lu M, Alu MC, Passeri JJ, Inglessis I, et al. Association between early left ventricular ejection fraction improvement after transcatheter aortic valve replacement and 5-year clinical outcomes. JAMA Cardiol. (2022) 7:934–44. doi: 10.1001/jamacardio.2022.2222
137. Nair RM, Chawla S, Verma B, Kumar S, Abou Hassan O, Ghimire B, et al. Impact of elevated left ventricular filling pressure on long-term outcomes after transcatheter aortic valve replacement. Open Heart. (2022) 9. doi: 10.1136/openhrt-2022-002015
138. Durand E, Sacri C, Levesque T, Tron C, Barbe T, Hemery T, et al. Incidence, predictive factors, and prognostic impact of right ventricular dysfunction before transcatheter aortic valve implantation. Am J Cardiol. (2021) 161:63–9. doi: 10.1016/j.amjcard.2021.08.068
139. Cantey EP, Chang KY, Blair JEA, Brummel K, Sweis RN, Pham DT, et al. Impact of loop diuretic use on outcomes following transcatheter aortic valve implantation. Am J Cardiol. (2020) 131:67–73. doi: 10.1016/j.amjcard.2020.06.033
140. Yasmin F, Aamir M, Moeed A, Iqbal K, Iqbal A, Asghar MS, et al. Causes and determinants of heart failure readmissions post transcutaneous aortic valve replacement: A systematic review and meta-analysis. Curr Probl Cardiol. (2023) 48:101428. doi: 10.1016/j.cpcardiol.2022.101428
141. Kapadia SR, Huded CP, Kodali SK, Svensson LG, Tuzcu EM, Baron SJ, et al. Stroke after surgical versus transfemoral transcatheter aortic valve replacement in the PARTNER trial. J Am Coll Cardiol. (2018) 72:2415–26. doi: 10.1016/j.jacc.2018.08.2172
142. Werner N, Zeymer U, Schneider S, Bauer T, Gerckens U, Linke A, et al. Incidence and clinical impact of stroke complicating transcatheter aortic valve implantation: results from the german TAVI registry. Catheter Cardiovasc Interv. (2016) 88:644–53. doi: 10.1002/ccd.26612
143. Bjursten H, Norrving B, and Ragnarsson S. Late stroke after transcatheter aortic valve replacement: a nationwide study. Sci Rep. (2021) 11:9593. doi: 10.1038/s41598-021-89217-0
144. Indja B, Woldendorp K, Vallely MP, and Grieve SM. Silent brain infarcts following cardiac procedures: A systematic review and meta-analysis. J Am Heart Assoc. (2019) 8:e010920. doi: 10.1161/jaha.118.010920
145. Woldendorp K, Indja B, Bannon PG, Fanning JP, Plunkett BT, and Grieve SM. Silent brain infarcts and early cognitive outcomes after transcatheter aortic valve implantation: a systematic review and meta-analysis. Eur Heart J. (2021) 42:1004–15. doi: 10.1093/eurheartj/ehab002
146. Szilveszter B, Oren D, Molnár L, Apor A, Nagy AI, Molnár A, et al. Subclinical leaflet thrombosis is associated with impaired reverse remodelling after transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging. (2020) 21:1144–51. doi: 10.1093/ehjci/jez256
147. Urena M, Mok M, Serra V, Dumont E, Nombela-Franco L, DeLarochellière R, et al. Predictive factors and long-term clinical consequences of persistent left bundle branch block following transcatheter aortic valve implantation with a balloon-expandable valve. J Am Coll Cardiol. (2012) 60:1743–52. doi: 10.1016/j.jacc.2012.07.035
148. Pavlicek V, Mahfoud F, Bubel K, Fries P, Ewen S, Böhm M, et al. Prediction of conduction disturbances in patients undergoing transcatheter aortic valve replacement. Clin Res Cardiol. (2023) 112:677–90. doi: 10.1007/s00392-023-02160-0
149. Prajapathi S and Pradhan A. Predictors of permanent pacemaker implantation following transcatheter aortic valve replacement-the search is still on! World J Cardiol. (2024) 16:104–8. doi: 10.4330/wjc.v16.i3.104
150. Nwaedozie S, Zhang H, Najjar Mojarrab J, Sharma P, Yeung P, Umukoro P, et al. Novel predictors of permanent pacemaker implantation following transcatheter aortic valve replacement. World J Cardiol. (2023) 15:582–98. doi: 10.4330/wjc.v15.i11.582
151. Rajah FT, Alaamiri AA, Mahmoodurrahman M, Alhowaish TS, Aldosari SF, Hussain AO, et al. Incidence, predictors, and clinical outcomes of permanent pacemaker insertion following transcatheter aortic valve implantation in an Arab population. J Interv Card Electrophysiol. (2022) 63:545–54. doi: 10.1007/s10840-021-01039-2
152. Schwietz T, Behjati S, Gafoor S, Seeger F, Doss M, Sievert H, et al. Occurrence and prognostic impact of systemic inflammatory response syndrome in transfemoral and transapical aortic valve implantation with balloon- and self-expandable valves. EuroIntervention. (2015) 10:1468–73. doi: 10.4244/eijy14m06_05
153. Lindman BR, Goldstein JS, Nassif ME, Zajarias A, Novak E, Tibrewala A, et al. Systemic inflammatory response syndrome after transcatheter or surgical aortic valve replacement. Heart. (2015) 101:537–45. doi: 10.1136/heartjnl-2014-307057
154. Sinning JM, Scheer AC, Adenauer V, Ghanem A, Hammerstingl C, Schueler R, et al. Systemic inflammatory response syndrome predicts increased mortality in patients after transcatheter aortic valve implantation. Eur Heart J. (2012) 33:1459–68. doi: 10.1093/eurheartj/ehs002
155. Hoffmann J, Mas-Peiro S, Berkowitsch A, Boeckling F, Rasper T, Pieszko K, et al. Inflammatory signatures are associated with increased mortality after transfemoral transcatheter aortic valve implantation. ESC Heart Fail. (2020) 7:2597–610. doi: 10.1002/ehf2.12837
156. Dekany G, Keresztes K, Bartos VP, Csenteri O, Gharehdaghi S, Horvath G, et al. Elevated fasting glucose and C-reactive protein levels predict increased all-cause mortality after elective transcatheter aortic valve implantation. Life (Basel). (2022). doi: 10.3390/life13010054
157. Lee HJ, Park CS, Lee S, Park JB, Kim HK, Park SJ, et al. Systemic proinflammatory-profibrotic response in aortic stenosis patients with diabetes and its relationship with myocardial remodeling and clinical outcome. Cardiovasc Diabetol. (2023) 22:30. doi: 10.1186/s12933-023-01763-1
158. Li SX, Patel NK, Flannery LD, Cigarroa RJ, Shaqdan AW, Erickson P, et al. Impact of bleeding after transcatheter aortic valve replacement in patients with chronic kidney disease. Catheter Cardiovasc Interv. (2021) 97:E172–e178. doi: 10.1002/ccd.28989
159. Garot P, Neylon A, Morice MC, Tamburino C, Bleiziffer S, Thiele H, et al. Bleeding risk differences after TAVR according to the ARC-HBR criteria: insights from SCOPE 2. EuroIntervention. (2022) 18:503–13. doi: 10.4244/eij-d-21-01048
160. Goel R, Power D, Tchetche D, Chandiramani R, Guedeney P, Claessen BE, et al. Impact of diabetes mellitus on short term vascular complications after TAVR: Results from the BRAVO-3 randomized trial. Int J Cardiol. (2019) 297:22–9. doi: 10.1016/j.ijcard.2019.09.063
161. Lunardi M, Pighi M, Banning A, Reimers B, Castriota F, Tomai F, et al. Vascular complications after transcatheter aortic valve implantation: treatment modalities and long-term clinical impact. Eur J Cardiothorac Surg. (2022) 61:934–41. doi: 10.1093/ejcts/ezab499
162. Trejo-Velasco B, Tello-Montoliu A, Cruz-González I, Moreno R, Baz-Alonso JA, Salvadores PJ, et al. Impact of comorbidities and antiplatelet regimen on platelet reactivity levels in patients undergoing transcatheter aortic valve implantation. J Cardiovasc Pharmacol. (2021) 78:463–73. doi: 10.1097/fjc.0000000000001075
163. Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. (2018) 379:1529–39. doi: 10.1056/NEJMoa1804988
164. Dunning J, Gao H, Chambers J, Moat N, Murphy G, Pagano D, et al. Aortic valve surgery: marked increases in volume and significant decreases in mechanical valve use–an analysis of 41,227 patients over 5 years from the Society for Cardiothoracic Surgery in Great Britain and Ireland National database. J Thorac Cardiovasc Surg. (2011) 142:776–782.e773. doi: 10.1016/j.jtcvs.2011.04.048
165. Ram E, Kogan A, Levin S, Fisman EZ, Tenenbaum A, Raanani E, et al. Type 2 diabetes mellitus increases long-term mortality risk after isolated surgical aortic valve replacement. Cardiovasc Diabetol. (2019) 18:31. doi: 10.1186/s12933-019-0836-y
166. Bundhun PK, Li N, and Chen MH. Adverse cardiovascular outcomes between insulin-treated and non-insulin treated diabetic patients after percutaneous coronary intervention: a systematic review and meta-analysis. Cardiovasc Diabetol. (2015) 14:135. doi: 10.1186/s12933-015-0300-6
167. Lavie CJ, McAuley PA, Church TS, Milani RV, and Blair SN. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. (2014) 63:1345–54. doi: 10.1016/j.jacc.2014.01.022
168. Carnethon MR and Khan SS. An apparent obesity paradox in cardiac surgery. Circulation. (2017) 135:864–6. doi: 10.1161/circulationaha.117.026856
169. El-Andari R, Bozso SJ, Kang JJH, Bedard AMA, Adams C, Wang W, et al. Heart valve surgery and the obesity paradox: A systematic review. Clin Obes. (2022) 12:e12506. doi: 10.1111/cob.12506
170. Masraf H, Sef D, Chin SL, Hunduma G, Trkulja V, Miskolczi S, et al. Long-Term Survival among Octogenarians Undergoing Aortic Valve Replacement with or without Simultaneous Coronary Artery Bypass Grafting: A 22-Year Tertiary Single-Center Experience. J Clin Med. (2023). doi: 10.3390/jcm12144841
171. D’Alessandro S, Tuttolomondo D, Singh G, Hernandez-Vaquero D, Pattuzzi C, Gallingani A, et al. The early and long-term outcomes of coronary artery bypass grafting added to aortic valve replacement compared to isolated aortic valve replacement in elderly patients: a systematic review and meta-analysis. Heart Vessels. (2022) 37:1647–61. doi: 10.1007/s00380-022-02073-4
172. Sakakura R, Asai T, Suzuki T, Kinoshita T, Enomoto M, Kondo Y, et al. Outcomes after aortic valve replacement for aortic valve stenosis, with or without concomitant coronary artery bypass grafting. Gen Thorac Cardiovasc Surg. (2019) 67:510–7. doi: 10.1007/s11748-018-1053-4
173. Krey R, Jakob M, Karck M, Arif R, and Farag M. Male-female differences following concomitant coronary artery bypass grafting and aortic valve replacement surgery. ESC Heart Fail. (2024). doi: 10.1002/ehf2.14847
174. Boden WE, De Caterina R, and Taggart DP. Is there equivalence between PCI and CABG surgery in long-term survival of patients with diabetes? Importance of interpretation biases and biological plausibility. Eur Heart J. (2021) 43:68–70. doi: 10.1093/eurheartj/ehab445
175. Wang R, Serruys PW, Gao C, Hara H, Takahashi K, Ono M, et al. Ten-year all-cause death after percutaneous or surgical revascularization in diabetic patients with complex coronary artery disease. Eur Heart J. (2021) 43:56–67. doi: 10.1093/eurheartj/ehab441
176. Wiberg S, Kjaergaard J, Møgelvang R, Møller CH, Kandler K, Ravn H, et al. Efficacy of a glucagon-like peptide-1 agonist and restrictive versus liberal oxygen supply in patients undergoing coronary artery bypass grafting or aortic valve replacement: study protocol for a 2-by-2 factorial designed, randomised clinical trial. BMJ Open. (2021) 11:e052340. doi: 10.1136/bmjopen-2021-052340
177. Ibrahim KS, Kheirallah KA, Mayyas FA, and Alwaqfi NA. Predictors of acute kidney injury following surgical valve replacement. Thorac Cardiovasc Surg. (2021) 69:396–404. doi: 10.1055/s-0040-1710318
178. Bove T, Calabrò MG, Landoni G, Aletti G, Marino G, Crescenzi G, et al. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth. (2004) 18:442–5. doi: 10.1053/j.jvca.2004.05.021
179. Caceres Polo M, Thibault D, Thourani VH, Badhwar V, Xian Y, and Shemin RJ. Risk analysis and outcomes of postoperative renal failure after aortic valve surgery in the United States. Ann Thorac Surg. (2020) 109:1133–41. doi: 10.1016/j.athoracsur.2019.07.052
180. Azarbal A, Malenka DJ, Huang YL, Ross CS, Solomon RJ, DeVries JT, et al. Recovery of kidney dysfunction after transcatheter aortic valve implantation (from the northern new england cardiovascular disease study group). Am J Cardiol. (2019) 123:426–33. doi: 10.1016/j.amjcard.2018.10.042
181. Malik A, Longi F, Naeem A, Clemence JJ, Makkinejad A, Norton E, et al. Outcomes in patients with chronic renal failure on hemodialysis after aortic valve or root replacement. Semin Thorac Cardiovasc Surg. (2022) 34:880–8. doi: 10.1053/j.semtcvs.2021.05.019
182. Färber G, Bleiziffer S, Doenst T, Bon D, Böning A, Weiler H, et al. Transcatheter or surgical aortic valve implantation in chronic dialysis patients: a German Aortic Valve Registry analysis. Clin Res Cardiol. (2021) 110:357–67. doi: 10.1007/s00392-020-01717-7
183. Guzzetti E, Annabi MS, Ong G, Zenses AS, Dagenais F, Tastet L, et al. Impact of metabolic syndrome and/or diabetes mellitus on left ventricular mass and remodeling in patients with aortic stenosis before and after aortic valve replacement. Am J Cardiol. (2019) 123:123–31. doi: 10.1016/j.amjcard.2018.09.015
184. López-Martínez H, Vilalta V, Farjat-Pasos J, Ferrer-Sistach E, Mohammadi S, Escabia C, et al. Heart failure hospitalization following surgical or transcatheter aortic valve implantation in low-risk aortic stenosis. ESC Heart Fail. (2024). doi: 10.1002/ehf2.14887
185. Seferović PM and Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J. (2015) 36:1718–1727, 1727a-1727c. doi: 10.1093/eurheartj/ehv134
186. Seferović PM, Paulus WJ, Rosano G, Polovina M, Petrie MC, Jhund PS, et al. Diabetic myocardial disorder. A clinical consensus statement of the Heart Failure Association of the ESC and the ESC Working Group on Myocardial & Pericardial Diseases. Eur J Heart Fail. (2024). doi: 10.1002/ejhf.3347
187. Pibarot P and Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation. (2009) 119:1034–48. doi: 10.1161/circulationaha.108.778886
188. Chen J, Lin Y, Kang B, and Wang Z. Indexed effective orifice area is a significant predictor of higher mid- and long-term mortality rates following aortic valve replacement in patients with prosthesis-patient mismatch. Eur J Cardiothorac Surg. (2014) 45:234–40. doi: 10.1093/ejcts/ezt245
189. Dayan V, Vignolo G, Soca G, Paganini JJ, Brusich D, and Pibarot P. Predictors and outcomes of prosthesis-patient mismatch after aortic valve replacement. JACC Cardiovasc Imaging. (2016) 9:924–33. doi: 10.1016/j.jcmg.2015.10.026
190. Bilkhu R, Jahangiri M, and Otto CM. Patient-prosthesis mismatch following aortic valve replacement. Heart. (2019). doi: 10.1136/heartjnl-2018-313515
191. Gupta A, Aliter H, Theriault C, and Chedrawy E. Patient-prosthesis mismatch and surgical aortic valve replacement outcomes: Retrospective analysis of single-center surgical data. J Card Surg. (2021) 36:2805–15. doi: 10.1111/jocs.15658
192. Graser M, Bleiziffer S, Zittermann A, Mayr B, Sideris K, Puluca N, et al. Impact of patient-prosthesis mismatch on long-term outcomes after aortic valve replacement. Ann Thorac Surg. (2024). doi: 10.1016/j.athoracsur.2024.05.025
193. Thyregod HGH, Jørgensen TH, Ihlemann N, Steinbrüchel DA, Nissen H, Kjeldsen BJ, et al. Transcatheter or surgical aortic valve implantation: 10-year outcomes of the NOTION trial. Eur Heart J. (2024) 45:1116–24. doi: 10.1093/eurheartj/ehae043
194. Summers MR, Leon MB, Smith CR, Kodali SK, Thourani VH, Herrmann HC, et al. Prosthetic valve endocarditis after TAVR and SAVR: insights from the PARTNER trials. Circulation. (2019) 140:1984–94. doi: 10.1161/circulationaha.119.041399
195. Lanz J, Reardon MJ, Pilgrim T, Stortecky S, Deeb GM, Chetcuti S, et al. Incidence and outcomes of infective endocarditis after transcatheter or surgical aortic valve replacement. J Am Heart Assoc. (2021) 10:e020368. doi: 10.1161/jaha.120.020368
196. Blankenberg S, Seiffert M, Vonthein R, Baumgartner H, Bleiziffer S, Borger MA, et al. Transcatheter or surgical treatment of aortic-valve stenosis. N Engl J Med. (2024) 390:1572–83. doi: 10.1056/NEJMoa2400685
197. Pibarot P, Ternacle J, Jaber WA, Salaun E, Dahou A, Asch FM, et al. Structural deterioration of transcatheter versus surgical aortic valve bioprostheses in the PARTNER-2 trial. J Am Coll Cardiol. (2020) 76:1830–43. doi: 10.1016/j.jacc.2020.08.049
198. O’Hair D, Yakubov SJ, Grubb KJ, Oh JK, Ito S, Deeb GM, et al. Structural valve deterioration after self-expanding transcatheter or surgical aortic valve implantation in patients at intermediate or high risk. JAMA Cardiol. (2023) 8:111–9. doi: 10.1001/jamacardio.2022.4627
199. Abdel-Wahab M, Landt M, Neumann FJ, Massberg S, Frerker C, Kurz T, et al. 5-year outcomes after TAVR with balloon-expandable versus self-expanding valves: results from the CHOICE randomized clinical trial. JACC Cardiovasc Interv. (2020) 13:1071–82. doi: 10.1016/j.jcin.2019.12.026
200. Herrmann HC, Mehran R, Blackman DJ, Bailey S, Möllmann H, Abdel-Wahab M, et al. Self-expanding or balloon-expandable TAVR in patients with a small aortic annulus. N Engl J Med. (2024) 390:1959–71. doi: 10.1056/NEJMoa2312573
201. Salaun E, Mahjoub H, Girerd N, Dagenais F, Voisine P, Mohammadi S, et al. Rate, timing, correlates, and outcomes of hemodynamic valve deterioration after bioprosthetic surgical aortic valve replacement. Circulation. (2018) 138:971–85. doi: 10.1161/circulationaha.118.035150
202. Nitsche C, Kammerlander AA, Knechtelsdorfer K, Kraiger JA, Goliasch G, Dona C, et al. Determinants of bioprosthetic aortic valve degeneration. JACC Cardiovasc Imaging. (2020) 13:345–53. doi: 10.1016/j.jcmg.2019.01.027
203. Pibarot P, Herrmann HC, Wu C, Hahn RT, Otto CM, Abbas AE, et al. Standardized definitions for bioprosthetic valve dysfunction following aortic or mitral valve replacement: JACC state-of-the-art review. J Am Coll Cardiol. (2022) 80:545–61. doi: 10.1016/j.jacc.2022.06.002
204. Lee JH, Burner KD, Fealey ME, Edwards WD, Tazelaar HD, Orszulak TA, et al. Prosthetic valve endocarditis: clinicopathological correlates in 122 surgical specimens from 116 patients (1985-2004). Cardiovasc Pathol. (2011) 20:26–35. doi: 10.1016/j.carpath.2009.09.006
205. Weber C, Petrov G, Luehr M, Aubin H, Tugtekin SM, Borger MA, et al. Surgical results for prosthetic versus native valve endocarditis: A multicenter analysis. J Thorac Cardiovasc Surg. (2021) 161:609–619.e610. doi: 10.1016/j.jtcvs.2019.09.186
206. Gallo M, Dvir D, Demertzis S, Pedrazzini G, Berdajs D, and Ferrari E. Transcatheter valve-in-valve implantation for degenerated bioprosthetic aortic and mitral valves. Expert Rev Med Devices. (2016) 13:749–58. doi: 10.1080/17434440.2016.1207521
207. D’Onofrio A, Tarja E, Besola L, Luzi G, Agrifoglio M, Aiello M, et al. Early and midterm clinical and hemodynamic outcomes of transcatheter valve-in-valve implantation: results from a multicenter experience. Ann Thorac Surg. (2016) 102:1966–73. doi: 10.1016/j.athoracsur.2016.05.055
208. Williams DF, Bezuidenhout D, de Villiers J, Human P, and Zilla P. Long-term stability and biocompatibility of pericardial bioprosthetic heart valves. Front Cardiovasc Med. (2021) 8:728577. doi: 10.3389/fcvm.2021.728577
209. Walther T, Lehmann S, Falk V, Metz S, Doll N, Rastan A, et al. Prospectively randomized evaluation of stented xenograft hemodynamic function in the aortic position. Circulation. (2004). doi: 10.1161/01.Cir.0000138947.63799.89
210. Borger MA, Nette AF, Maganti M, and Feindel CM. Carpentier-Edwards Perimount Magna valve versus Medtronic Hancock II: a matched hemodynamic comparison. Ann Thorac Surg. (2007) 83:2054–8. doi: 10.1016/j.athoracsur.2007.02.062
211. Kim HR, Kim HJ, Kim S, Kim Y, Ahn JM, Kim JB, et al. Bovine pericardial versus porcine bioprosthetic aortic valves: A nationwide population-based cohort study in Korea. J Thorac Cardiovasc Surg. (2023). doi: 10.1016/j.jtcvs.2023.10.060
212. Lehrke M and Lazar MA. The many faces of PPARgamma. Cell. (2005) 123:993–9. doi: 10.1016/j.cell.2005.11.026
213. DeFronzo RA, Inzucchi S, Abdul-Ghani M, and Nissen SE. Pioglitazone: The forgotten, cost-effective cardioprotective drug for type 2 diabetes. Diabetes Vasc Dis Res. (2019) 16:133–43. doi: 10.1177/1479164118825376
214. Nakamura T, Yamamoto E, Kataoka K, Yamashita T, Tokutomi Y, Dong YF, et al. Beneficial effects of pioglitazone on hypertensive cardiovascular injury are enhanced by combination with candesartan. Hypertension. (2008) 51:296–301. doi: 10.1161/hypertensionaha.107.099044
215. Ricote M, Li AC, Willson TM, Kelly CJ, and Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. (1998) 391:79–82. doi: 10.1038/34178
216. Lee SH, Kim N, Kim M, Woo SH, Han I, Park J, et al. Single-cell transcriptomics reveal cellular diversity of aortic valve and the immunomodulation by PPARγ during hyperlipidemia. Nat Commun. (2022) 13:5461. doi: 10.1038/s41467-022-33202-2
217. Katahira S, Sugimura Y, Grupp S, Doepp R, Selig JI, Barth M, et al. PPAR-gamma activation may inhibit the in vivo degeneration of bioprosthetic aortic and aortic valve grafts under diabetic conditions. Int J Mol Sci. (2021). doi: 10.3390/ijms222011081
218. Assmann AK, Goschmer D, Sugimura Y, Chekhoeva A, Barth M, Assmann A, et al. A role for peroxisome proliferator-activated receptor gamma agonists in counteracting the degeneration of cardiovascular grafts. J Cardiovasc Pharmacol. (2021) 79:e103–15. doi: 10.1097/fjc.0000000000001150
219. Assmann AK, Winnicki V, Sugimura Y, Chekhoeva A, Barth M, Assmann A, et al. Impact of PPAR-gamma activation on the durability of biological heart valve prostheses in hypercholesterolaemic rats. Eur J Cardiothorac Surg. (2022). doi: 10.1093/ejcts/ezad005
220. Pratley RE and Salsali A. Inhibition of DPP-4: a new therapeutic approach for the treatment of type 2 diabetes. Curr Med Res Opin. (2007) 23:919–31. doi: 10.1185/030079906x162746
221. Bao W, Morimoto K, Hasegawa T, Sasaki N, Yamashita T, Hirata K, et al. Orally administered dipeptidyl peptidase-4 inhibitor (alogliptin) prevents abdominal aortic aneurysm formation through an antioxidant effect in rats. J Vasc Surg. (2014) 59:1098–108. doi: 10.1016/j.jvs.2013.04.048
222. Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. Jama. (2019) 321:69–79. doi: 10.1001/jama.2018.18269
223. Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. Jama. (2019) 322:1155–66. doi: 10.1001/jama.2019.13772
224. Scheen AJ. Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and SGLT-2 inhibitors. Circ Res. (2018) 122:1439–59. doi: 10.1161/circresaha.117.311588
225. Choi B, Lee S, Kim SM, Lee EJ, Lee SR, Kim DH, et al. Dipeptidyl peptidase-4 induces aortic valve calcification by inhibiting insulin-like growth factor-1 signaling in valvular interstitial cells. Circulation. (2017) 135:1935–50. doi: 10.1161/circulationaha.116.024270
226. Choi B, Kim EY, Kim JE, Oh S, Park SO, Kim SM, et al. Evogliptin suppresses calcific aortic valve disease by attenuating inflammation, fibrosis, and calcification. Cells. (2021). doi: 10.3390/cells10010057
Keywords: calcific aortic valve disease, diabetes, transcatheter aortic valve replacement, surgical aortic valve replacement, deterioration of bioprosthetic aortic valve
Citation: Liu F and Cai H (2025) Diabetes and calcific aortic valve disease: controversy of clinical outcomes in diabetes after aortic valve replacement. Front. Endocrinol. 16:1577762. doi: 10.3389/fendo.2025.1577762
Received: 16 February 2025; Accepted: 14 July 2025;
Published: 30 July 2025.
Edited by:
Morgan Salmon, University of Michigan, United StatesReviewed by:
Rolando A Cuevas, University of Pittsburgh, United StatesAnkit P. Laddha, University of Connecticut, United States
Copyright © 2025 Liu and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haipeng Cai, MTkxNzQ4MzMwNkBxcS5jb20=
 Feng Liu
Feng Liu Haipeng Cai
Haipeng Cai