- Placental Cell Biology Group, National Institute of Environmental Health Sciences, National Institutes of Health, Durham, NC, United States
The placenta is a dynamic endocrine organ that plays a crucial role in fetal development by secreting a diverse array of peptide hormones that regulate maternal and fetal physiology. These hormones, including human chorionic gonadotropin (hCG), human placental lactogen (hPL), and placental growth hormone (hPGH), among others, are essential for pregnancy maintenance, fetal growth, and metabolic adaptation. Dysregulation of the secretory machinery and the levels of these hormones in circulation is associated with a myriad of pregnancy-related disorders. Despite their significance, the mechanisms governing their intracellular trafficking and secretion remain incompletely understood. This review synthesizes current knowledge on the secretion pathways of placental hormones, highlighting the interplay between constitutive and regulated secretion, and the challenges in defining these mechanisms due to the unique structure of the syncytiotrophoblast. We also discuss how emerging technologies, such as 2D and 3D placental models and advanced protein trafficking assays, can provide deeper insights into the regulation of placental hormone secretion. Understanding these processes will not only enhance our knowledge of placental biology but also provide new avenues for diagnosing and treating pregnancy-related disorders.
Introduction
The placenta is credited for its importance in mammalian in utero development. As the first and largest fetal organ to develop, the placenta is the medium through which oxygen and nutrients are provided to the embryo alongside other metabolic and endocrinologic functions (1–4). Disorders in human placental development can have consequences in the health of the fetus and pregnant host that are lifelong. Common diseases during pregnancy, such as preeclampsia and intrauterine growth restriction, are attributed to defects in placental development and secretion (5, 6). The effects of fetal development on the predisposition to health complications in adulthood have already been proposed, according to the fetal origins hypothesis (7).
In order to assure the proper execution of the fetal development program, the placenta secretes a variety of hormones throughout the entire pregnancy. These hormones are mostly proteins and are produced and secreted through the secretory pathway (8–10). A compartmentalized cell maintains correct folding, processing, and delivery of all proteins entering and exiting the secretory cells dynamically. Placental hormones are mostly produced by a special cellular structure called syncytiotrophoblast. This multinucleated epithelium layer emerges in direct contact with the blood of the pregnant host as a result of regulated cell-cell fusion (11). The complexity of this cellular scenario makes it difficult to study intracellular trafficking events, including hormone secretion. Accordingly, dysregulation of such pathways has significant effects on placenta function and development.
Despite its long-lasting contribution to health and disease, both within and beyond the scope of reproduction, the human placenta remains largely overlooked in scientific research and its complexities are not fully understood. Ethical and practical implications for achieving a human placental model creates additional obstacles in research. The purpose of this review is to outline key components of placental development with a focus on protein trafficking based on published work. The compilation of existing knowledge is short of extensive, given the ongoing gaps in placenta research. We also encourage an open discussion on the future of placental research through emerging technologies, such as 2D and 3D lab-generated models, that can illuminate placental development and offer new tools to interrogate cellular mechanisms in greater detail.
Placenta development
The origins of the human placenta can be traced to the trophectoderm, visible around day 5 post-fertilization. The trophectoderm is the external layer of the blastocyst and has a region in contact with the inner cell mass (12) (Figure 1A). This polar side of the trophectoderm, proximal to the inner cell mass, adheres to the uterine epithelium during embryo implantation. The trophectoderm subsequently transforms into the trophoblast and initiates the implantation process around day 6 post-fertilization (13).
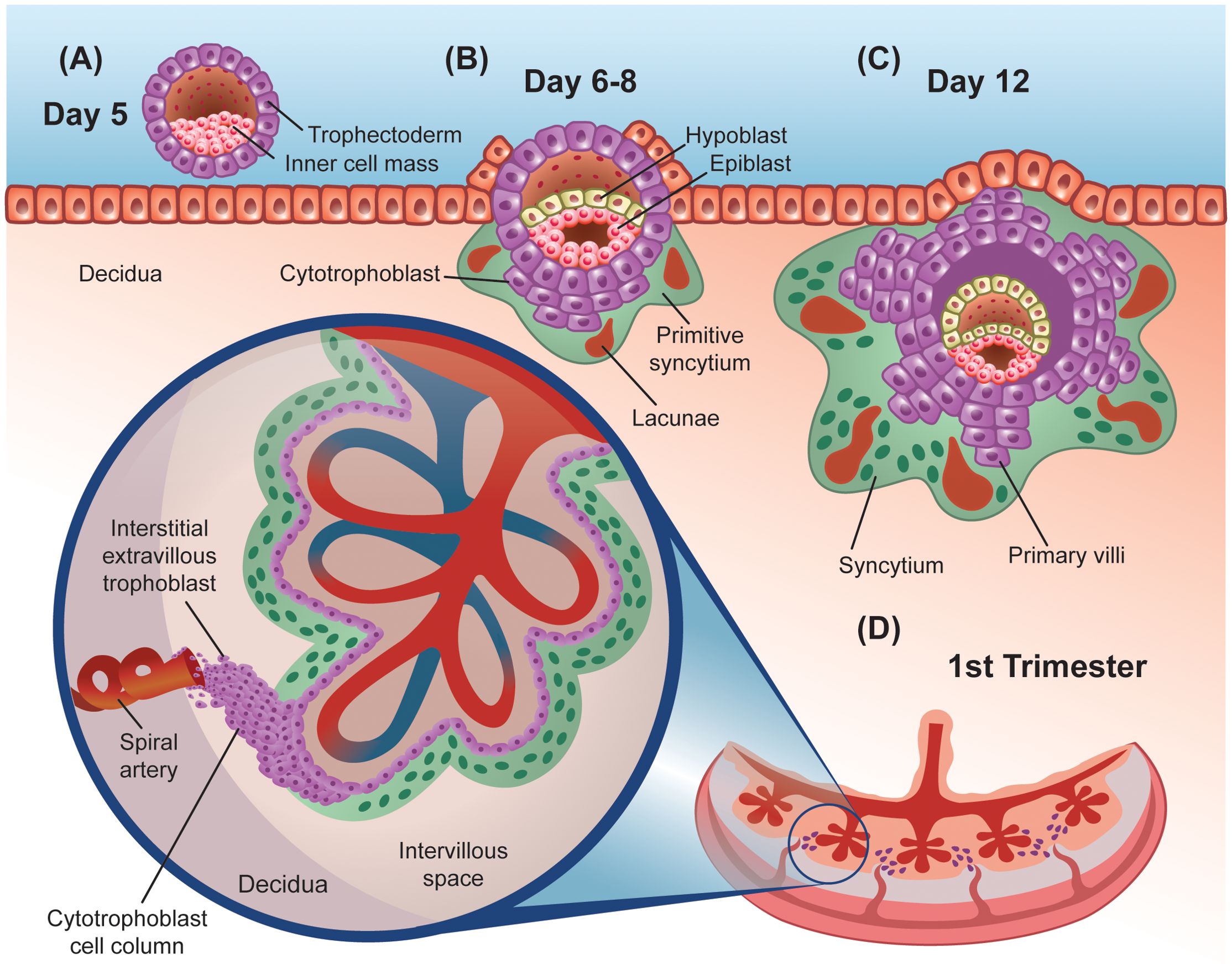
Figure 1. Graphic representation of early stages of human placenta development. (A) Preimplantation embryo, showing the external trophectoderm cell layer that protects the inner cell mass or embryo proper, in close contact with a layer of uterine epithelial cells that rest on top of the decidua. (B, C) Early post-implantation embryo, showing the primitive syncytium surrounded by lacunae and primary villi made out of cytotrophoblasts. (D) First trimester fully developed placenta. Inset shows the detailed cellular architecture of an anchoring villus. The cytotrophoblast cell column keeps this villus attached to the decidua and provides the interstitial extravillous trophoblasts that invade nearby spiral arteries, plugging them first and remodeling them later in pregnancy.
Trophoblastic cells proliferate throughout the invasion of the uterine epithelium, forming a dual layer structure. The inner layer is comprised of mono-nucleated cytotrophoblast cells (CTB) that are initially shielded from the host tissue by a primitive, external syncytiotrophoblast (STB) (Figure 1B). The STB is a continuous multi-nucleated structure that forms from the fusion of neighboring CTBs (11). Across the surface of the STB, microvilli function as a region of contact between the placenta and the pregnant host and continue to expand until term (14). More broadly and later in development, the STB is regarded as the primary site for nutrient and gas exchange and noted for its endocrine function (15, 16).
The lacunar stage occurs between days 8 and 13 post-fertilization and is characterized by the appearance of fluid-filled masses within the primitive STB that grow to form lacunae (Figure 1C). Trabeculae, which are bands of the STB, separate the lacunae from one another (12, 17). The formation of the lacunar system divides the trophoblast layer into three sublayers: a primary chorionic plate, the lacunar system, and the trophoblastic shell (18). The STB continues to invade the uterus into the endometrium which transforms into decidual tissue and completely engulfs the blastocyst by day 12 post-conception (19, 20). Specifically, the decidual tissue beneath the blastocyst, and subsequently placenta, is termed the decidua basalis (12). Decidual cells are derived from the proliferation and differentiation of endometrial stromal cells during early implantation (18). Trophoblastic proliferation exhibits higher density on the side of the blastocyst that implanted (implantation pole), therefore this site will eventually give rise to the placenta (18).
Around day 13, the underlying CTB cells proliferate to form projections that extend through the STB into the trabeculae and invade the lacunar system (Figure 1C). This marks the start of the villous stage, happening between days 13 and 28 post-fertilization. Proliferation and branching of the trophoblast lead to the formation of primary villous trees, with the lacunae functioning as the intervillous space. The invasive nature of the trophoblast also causes the release of erythrocytes from maternal capillaries into the lacunae (18). Mesenchymal cells emerge around day 14 and proliferate along the interior lining of the cytotrophoblast cells (18). CTB cells continue to grow to form a continuous, shell structure around the villi that separate it from the decidua around day 15, with some CTB cells invading the decidua as extravillous trophoblasts (EVTs).
Mesenchymal cells from the embryo grow through the villous core, invading the primary villi, to form secondary villi (21). Between days 18 and 20, fetal capillaries emerge in the mesenchyme, resulting in the establishment of tertiary villi upon the cross sectioning of fetal capillaries in the villous stroma (22, 23) (Figure 1D). Fetal circulation will be separated from maternal blood via the placental barrier which consists of a continuous STB lining the intervillous space, a layer of villous CTB cells, a basal lamina (made of mostly laminin, collagen, and fibronectin), connective tissue, and fetal endothelium (18). At the start of the fifth week, the framework of the placenta is established. Vascular remodeling is also apparent near the end of the first trimester.
In summary, throughout the first month of pregnancy, the intricate process of placental formation unfolds, with the CTBs serving as a perpetual stem cell constantly regenerating the STB and monitoring fetal needs given the proximity to the fetal circulation. The STB responds to their environment via developmentally timed endocytosis and secretion of a myriad of hormones, and the EVTs are active in remodeling and regulating host blood flow. Altogether, these three main trophoblast cell types orchestrate the formation and function of the placenta for the entire pregnancy period.
Placental protein hormones
More than a passive physical barrier, the placenta is a highly active endocrine organ. Placental cells release large quantities of hormones in the host bloodstream throughout pregnancy that are vital for gestational success (Figure 2). Placental hormones are predominantly made by the STB, although CTBs and EVTs are also observed to have a role in hormone production (24, 25). The trafficking of these placental hormones can be an important indicator of pregnancy-related diseases. This section explores the major protein hormones secreted by the placenta, including their cellular origin, functions, and mechanisms in particular physiological pathways (Table 1).
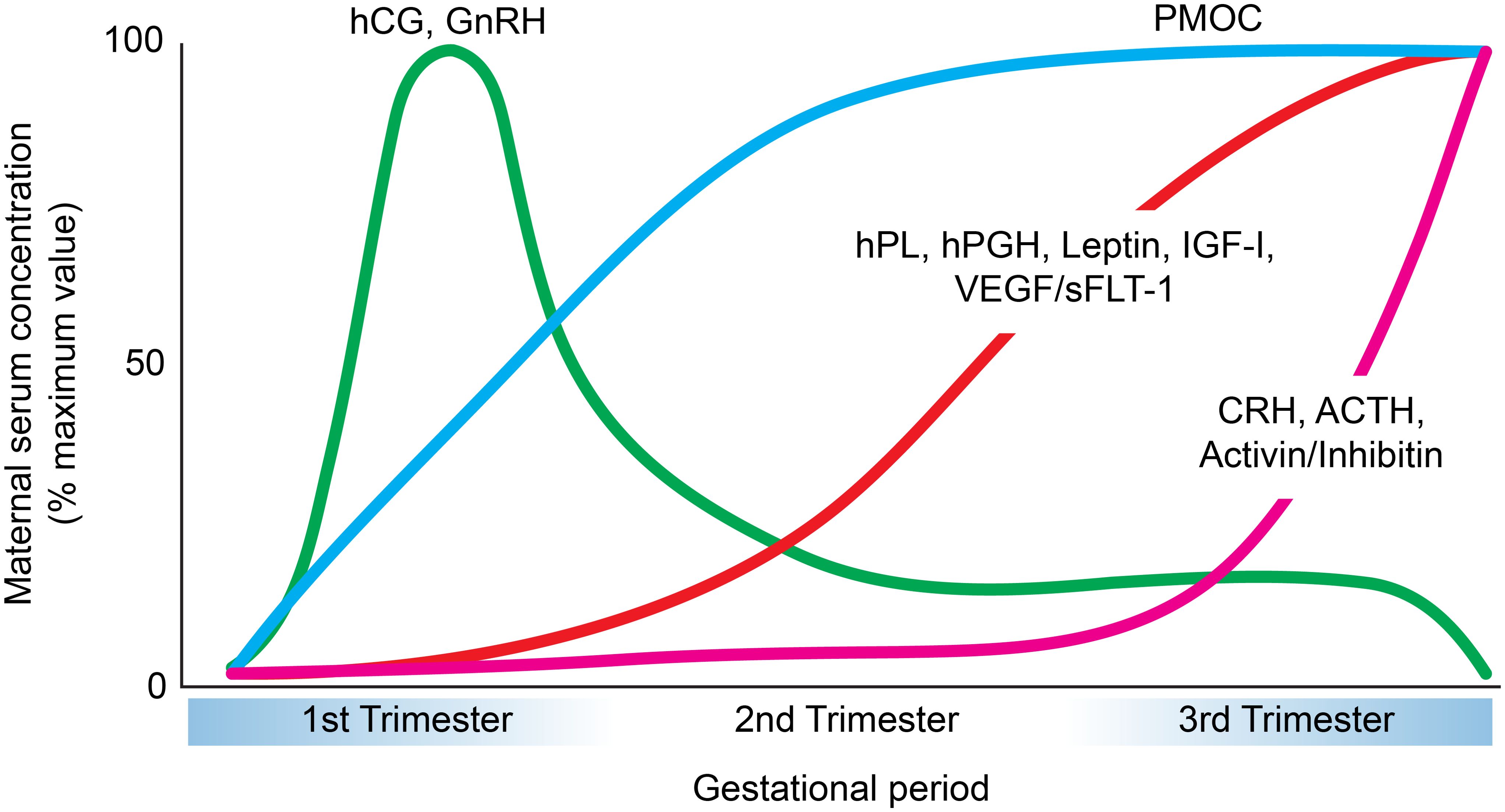
Figure 2. Temporal patterns of major placental peptide hormone secretion across pregnancy. Graphical representation of the maternal serum concentrations (expressed as a percentage of maximum value) of key placental hormones during gestation. Human chorionic gonadotropin (hCG) and gonadotropin-releasing hormone (GnRH) peak during the first trimester and decline thereafter (104, 250). Placental growth hormone (hPGH), human placental lactogen (hPL), leptin, insulin-like growth factor-I (IGF-I), and vascular endothelial growth factor/soluble fms-like tyrosine kinase-1 (VEGF/sFLT-1) progressively increase during the second and third trimesters (250–256). Proopiomelanocortin (PMOC) shows a steady rise throughout pregnancy that plateus during the second trimester (257). Finally, corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and activin/inhibin demonstrate a marked elevation toward late gestation (258–261).
hCG
Human chorionic gonadotropin (hCG) is a heterodimeric glycoprotein with two subunits, hCG-α and hCG-β. This hormone is primarily generated by the STB and may possibly be synthesized by EVTs as well (26). The levels of hCG rapidly increase during the first trimester, with peak concentration near week 10 of pregnancy. Between 12 to 16 weeks, however, hCG levels gradually decline to a fifth of the peak concentration until term (27) (Figure 2).
This hormone binds to a G-protein coupled receptor that is also receptive to luteinizing hormone (LH). The LH-hCG receptor is expressed predominantly in gonadal and uterine cells (28), as well as placental STB, CTBs and EVTs (29, 30). Binding of hCG to its LH-hCG receptor leads to the activation of adenylyl cyclase and phospholipase C. Stimulation of adenylyl cyclase results in higher intracellular concentrations of cyclic adenosine monophosphate (cAMP), whereas phospholipase C subsequently generates inositol phosphates and increases intracellular calcium levels (31).
The recognition and maintenance of pregnancy is mediated by hCG. Binding of hCG to LH receptors on ovarian corpus luteum (CL) cells prevents luteolysis and prolongs CL function. In doing so, hCG promotes progesterone production via the CL that is necessary for averting menstruation and establishing pregnancy (32). It is also proposed that hCG facilitates the fusion of trophoblast cells, particularly the differentiation of CTBs into the STB. This is made possible by the increase in cAMP which initiates the protein kinase A pathway and activates downstream proteins, such as glial cells missing transcripton factor 1 (GCM1) (33). Targets of the GCM1 protein include the endogenous retroviral protein syncytin-1, which has direct involvement in CTB fusion (34). Notably, there is an upregulation of placental proteins specific for the STB, such as the beta-subunit of hCG (hCG-β) after syncytialization (33). The hCG hormone also enhances trophoblast invasion through increasing CTB secretion of matrix metalloproteinases MMP-2 and MMP-9 and decreasing the levels of their TIMP inhibitors TIMP-1, TIMP-2, and TIMP-3 (35). These effects are achieved by the hCG-mediated activation of ERK and AKT signaling pathways (36). Additionally, hyperglycosylated hCG, an hCG variant, binds and antagonizes TFGβ receptors which allows for the tumor-like invasion of trophoblast cells into the uterus (37). The secretion of hCG is enhanced by the cAMP-PKA and ERK pathways alongside other hormones like GnRH, leptin, and activin (38–42). Hormones such as inhibin and progesterone are noted for their antagonistic effects on hCG secretion (42, 43). Other observed functions characterize hCG as an angiogenic factor contributing to placental and uterine vascularization (44–46). Additionally, hCG plays a role in promoting vascular endothelial growth factor (VEGF) production by the STB, facilitating umbilical cord growth (47–49), and promoting fetal organ development, a suggestion made after hCG/LH receptors were found in fetal organs that are not present in adult organs (37). Given the importance of hCG, defects in expression of this hormone can have dire outcomes like miscarriage and preeclampsia (50–52).
hPL
Human placental lactogen (hPL) is a polypeptide hormone primarily generated by the STB, but it can also be synthesized by EVTs (25). This hormone is initially detected 5–10 days after implantation and becomes visible in host plasma near the 6th week of pregnancy. The levels of hPL increase consistently and reach a peak at 30 weeks (Figure 2). Interestingly, hPL concentration is positively correlated with the placental mass and number of growing fetuses, suggesting a role for hPL on placental and fetal growth (53).
This hormone binds to the human prolactin receptor (PRLR) as well as the human growth hormone receptor (GHR), although with lower affinity to the latter (54). Prolactin receptors are widely expressed across various tissues, including the mammary gland, ovary, and pancreas (55, 56). The functions of hPL pertain mostly to carbohydrate and lipid metabolism. It has been observed that hPL reduces insulin sensitivity of the host during pregnancy, while promoting glucose uptake, oxidation, and storage as glycogen (53). Additionally, hPL increases the rates of lipolysis in vitro as well as the plasma concentrations of fatty acids, ketones, and glycerol in vivo, all of which can support fetal development (53). Furthermore, hPL contributes to the formation of pancreatic islets and upregulates insulin secretion in the host (57, 58). Studies also suggest a role for hPL in breast epithelial cell proliferation of the host, presumably in preparation for lactation (59). With respect to the fetus, hPL is noted to encourage fetal anabolism by stimulating DNA synthesis, amino acid uptake, and IGF-I production (60). Despite its relatively lower concentration in the fetal circulation compared to the host, hPL is speculated to play a part in fetal pancreatic development, although it is unknown whether this hormone regulates fetal islets similar to maternal islets (53, 61, 62). Abnormalities in hPL secretion are associated with low fetal birth weight, perinatal maternal mood, and pregnancy loss (63–65). Molecules that stimulate hPL release include cAMP, insulin, and growth hormone releasing hormone (66–68). Somatostatin has been associated with inhibitory effects on hPL secretion due to its expression levels during pregnancy that are inverse to that of hPL (69).
hPGH
Human placental growth hormone (hPGH) is a polypeptide made by the STB, however it can also be produced by EVTs (70). This hormone is distinguished from its pituitary human growth hormone (hGH) counterpart (secreted by the somatotropic cells of the anterior pituitary gland) based on a difference of 13 amino acids (71). It appears that hPGH emerges in host circulation around the second half of pregnancy and begins to dominate over pituitary growth hormone, continuing to rise until term (72) (Figure 2).
This hormone binds to the hGH receptor (GHR), abundantly expressed in liver cells, as well as the prolactin receptor (54, 73). The function of hPGH has been linked to promoting maternal IGF-I synthesis and insulin resistance (74, 75). Moreover, hPGH exerts metabolic effects through enhancing gluconeogenesis and lipolysis, thus allowing for higher nutrient availability to the fetoplacental unit (71). The combined effects of hPGH on IGF-I production and host metabolism are correlated with fetal development (76). Additionally, hPGH increases the invasion of EVTs, directly contributing to the growth of the placenta (70). Reductions in hPGH expression may hinder IGF-I production and indirectly cause low fetal birth weight, whereas association of hPGH levels to preeclampsia is conflicting (77). The secretion of the hPGH hormone is promoted by cAMP and inhibited by glucose, leptin, and insulin (71, 78).
Leptin
Leptin is a peptide hormone largely produced by the STB and EVTs (79, 80). The concentration of leptin increases dramatically during the first and second trimester, peaking near week 28, and then declines rapidly after parturition (81, 82) (Figure 2).
Leptin binds to the long-form Leptin receptor LepRb, highly expressed in cells of the hypothalamus and placenta, and activates the ERK and JAK2-STAT5 pathways (83, 84). This hormone increases the secretion of hCG and simultaneously inhibits the synthesis of progesterone and hPGH (41, 78). Leptin also enhances CTB proliferation through upregulating cyclin-D1 and inhibiting apoptosis to advance cell cycle progression to the G2/M stage (85). Furthermore, leptin promotes trophoblast invasion by inducing the expression of MMP-2 and MMP-9 (80). Leptin function has also been linked to placental angiogenesis, leading to the formation of capillary-like tubes in vitro (86). Additionally, leptin was found to have catabolic effects on the host, including lipolysis and the reduction of placental triglyceride and cholesterol levels (87). It has also been demonstrated that leptin increases host-derived amino acid transport, thereby contributing to the growth of the fetus (88). Dysregulation of leptin expression is related to pathologies such as gestational diabetes, recurrent miscarriage, preeclampsia, and intrauterine fetal growth restriction (89–94) Leptin production is enhanced by the cAMP, ERK, and PKA/PKC pathways (95, 96). Molecules such as hCG and insulin also promote leptin secretion (97, 98). Hormones such as hPL and progesterone antagonize the release of leptin (99).
GnRH
Gonadotropin hormone-releasing hormone (GnRH) is secreted in two forms by the placenta, GnRH-I and GnRH-II. Placental peptide GnRH-I is immunologically and biochemically equivalent to that of the hypothalamus, with the exception of differences in the 5’-untranslated region of the gene (100, 101). GnRH-I is produced by all the trophoblasts in the placenta. On the other hand, GnRH-II is expressed by CTBs and EVTs. GnRH-I and GnRH-II are both identified in first trimester placentas, although only GnRH-I is detected at term (102) (Figure 2).
The mechanisms of chorionic GnRH are yet to be clearly defined. Previous studies report that placental GnRH stimulates hCG secretion from the STB after binding to the type 1 GnRH receptor localized to the STB and CTBs (103, 104). It has been proposed that GnRH-stimulated hCG secretion is a receptor-mediated process (105). Parallel to hCG levels, placental GnRH receptors were observed to peak at week 9 of gestation, prior to declining between weeks 12–20 and later becoming undetectable at term (106). In addition, chorionic GnRH contributes to trophoblast invasion through regulation of MMP-2 and MMP-9 in primary EVTs (107, 108). Evidently, GnRH function corresponds to the establishment and maintenance of pregnancy and disruption of placental GnRH expression or receptor activity can lead to unfavorable pregnancy outcomes including pregnancy loss, stillbirth, and low fetal birth weight (109–111). The secretion of chorionic GnRH is upregulated by molecules such as cAMP and activin (112).
Activin/Inhibin
Activins and inhibins are dimeric glycoproteins that are characterized for their regulatory nature. More specifically, inhibins are heterodimers with α and β subunits, and prevent the release of FSH from the pituitary system (113). Activins can be homo or heterodimers of β-inhibin and exhibit opposing effects for that of inhibin by functioning as a stimulant for the release of FSH (113, 114). Activin A and Inhibins A and B are predominantly synthesized by the STB, although they can also be generated by the CTB (115, 116). These molecules are observed to have rising concentrations during pregnancy (117) (Figure 2).
Activin A binds to type II activin receptors, ActRIIA and ActRIIB, located mainly on the STB of the placenta, among other cells in the pituitary, hypothalamus, or gonads (118–121). Activins facilitate CTB differentiation into the STB and EVT and also promote trophoblast invasion (122–124). With its shared β-subunit, inhibins competitively bind to activin receptors and serve as regulators for activin (125). Inhibin reverses the effects of activin by suppressing GnRH activity, which then results in a decrease in hCG production (42). Disruptions in the secretion of activins and inhibins have been linked to pathologies such as spontaneous or pre-term labor as well as preeclampsia and gestational diabetes (126–129).
Inhibin secretion is promoted by molecules such as GnRH, hCG, and cAMP (130). The production of inhibin is downregulated by Activin A through a feedback loop (131). The release of activin is upregulated by CRH (132).
CRH
Corticotropin-releasing hormone (CRH) is a peptide generated mainly by the STB (133, 134). Chorionic CRH is identical to CRH produced in the hypothalamus and is detected in low levels between weeks 7-19, with rising concentrations during weeks 35–40 of gestation (135–138) (Figure 2). Placental CRH binds to receptors CRH-R1 and CRH-R2, located primarily in the pituitary and central nervous system as well as placental and fetal membranes (139, 140). Competition with CRH binding protein (CRH-BP), which has greater affinity to the CRH receptor, renders CRH in host circulation as inactive for most of gestation. CRH-BP levels decrease in the final weeks of pregnancy and coincides with the increase in CRH bioavailability and activity, marking CRH as a signal for parturition (141). The functions of CRH encompass increasing intracellular cAMP and enhancing the synthesis of both proopiomelanocortin (POMC) and its derivative adrenocorticotropic hormone (ACTH) in the placenta (142, 143). Additionally, CRH stimulates placental secretion of prostaglandins, possibly contributing to myometrial contractions at term (144). Moreover, CRH facilitates steroid production by fetal adrenals, which may further incite parturition (145).
Urocortins are a subclass of CRH peptides that are primarily made by the STB and, to a lesser extent, CTBs (146). Urocortins bind to CRH receptors and play a role in placental vasodilation in addition to shared effects of increasing cAMP concentrations and promoting POMC (see below) and ACTH secretion by trophoblast cells (147–149).
Improper release of CRH has been associated with adverse pregnancy outcomes such as pre-term labor, hypertension, impaired uterine artery blood flow, and preeclampsia (150–153). CRH secretion is upregulated by glucocorticoids and prostaglandins and obstructed by progesterone (144, 154).
POMC
Proopiomelanocortin (POMC) is a glycoprotein precursor to ACTH with proposed synthesis by the STB (155, 156). Although undetected in normal subjects, POMC is readily identified by the third month of gestation for pregnant individuals (Figure 2). This is attributed to differences in the processing of POMC between the pituitary and the placenta, as the latter secretes bulk quantities of the intact, uncleaved hormone (157). POMC levels gradually increase throughout pregnancy and are positively correlated with CRH concentration, however demonstrate no relationship with ACTH or cortisol levels (157). The functional significance of placental ACTH and other POMC-derivatives remains largely unspecified.
IGF-I and IGF-II
Insulin-like growth factors IGF-I and IGF-II are produced by the placenta and play a critical role in fetal development (158). IGF-I is secreted by the STB and CTBs throughout pregnancy (Figure 2). On the other hand, IGF-II is generated by CTBs and EVTs during the first trimester (159).
These peptides bind to tyrosine kinase receptor IGF-IR present in all placental cell types, while IGF-II can also bind to the IGF-II and insulin receptors (160, 161). IGFs stimulate trophoblast invasion and are also involved in the proliferation of CTBs via the PI3K and MAPK pathways (162–164). Moreover, IGFs contribute to fetal development by facilitating amino acid transport across the placenta (165). These molecules are regulated by IGF binding proteins that limit their availability to interact with IGF receptors (166). Alterations to placental IGF expression demonstrate consequences of restricted placental and fetal growth, and are implicated to have a pathological role in preeclampsia and gestational diabetes (158, 166, 167).
VEGF
Vascular endothelial growth factors (VEGFs) are a family of proteins that are characterized for their angiogenic properties. During gestation, VEGF-A and placental growth factor (PlGF) are secreted from the STB (168) (Figure 2). These molecules bind to placental tyrosine kinase receptors KDR and FLT-1, and are downregulated by association with the soluble form of FLT-1 (sFLT-1) (169). The functions of VEGFs are strongly linked to vascular endothelial cell proliferation and angiogenesis through effects of increased vasodilation and vascular permeability (170–172). Angiogenesis is further promoted via the antiapoptotic support VEGFs provide placental epithelial cells (173). VEGFs also influence the remodeling of host spiral arteries that is critical for gestational success (174, 175). Complications with VEGF/PlGF secretion can be indicative of pathologies like preeclampsia (176).
How does secretion of placental peptide hormones happen?
Transport of secreted proteins in eukaryote cells requires specialized membrane and cytosolic machinery of the secretory and endo-lysosomal pathways (8–10) (Figure 3). Most of the conventional secretory proteins are synthesized in the endoplasmic reticulum (ER) lumen via N-terminal signal sequence recruitment of ribosomes. Here, proteins fold and some acquire complex glycans that are further processed once the protein is exported to the Golgi apparatus. From there, proteins are sorted and trafficked to the cell surface via membrane-bound vesicles or tubular carriers (177–179). This rapid delivery of cargo to the extracellular space is described as constitutive secretion and operates in all cell types (180). Specialized cells such as lactotrophs of the anterior pituitary gland or β-cells in the pancreas, for example, produce large amounts of specific proteins that are stored in granules (secretory granules – SGs) at high concentration before being exocytosed upon stimulation. The formation, maturation, and release of these SGs require very specific trafficking and signaling regulation (181–184). Regulated secretory proteins contained in SGs include hormones, neuropeptides, enzymes, and extracellular components of mucus.
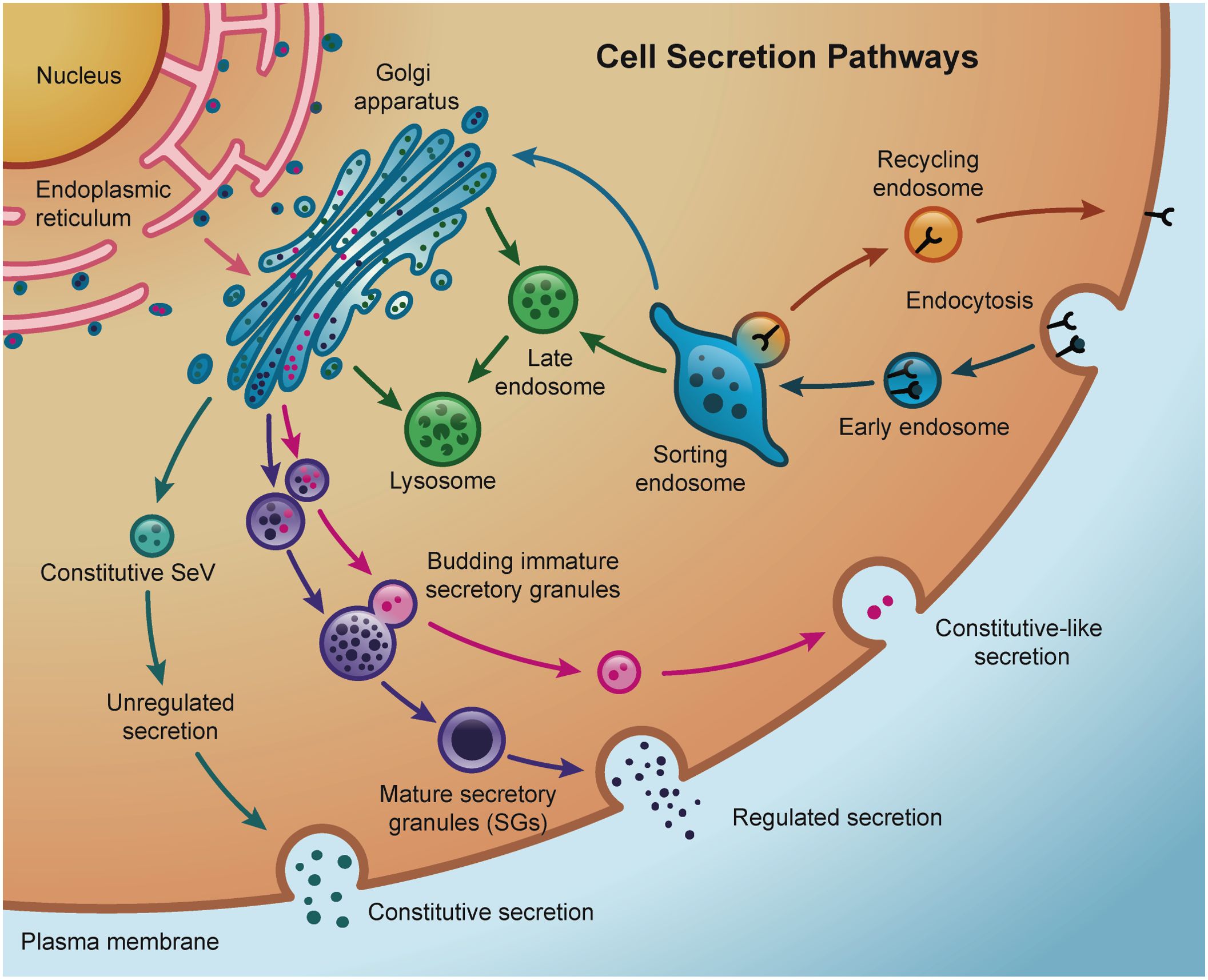
Figure 3. Schematic representation of the main secretion pathways in a cell. Active endocytosis and recycling through sorting endosomes allows the cell the control of cargo internalization and the reutilization of certain receptors depending on the intracellular demands. Cargo and receptors no longer required by the cell are degraded inside late endosomes and lysosomes, that receive lysosomal-specific degradative enzymes via direct trafficking from the Golgi or indirectly from the plasma membrane. Constitutive secretion brings Golgi newly synthesized in secretory vesicles (SeV) or recycled cargo directly back to the plasma membrane. In contrast, regulated secretion involves the packaging of cargo into highly-regulated secretory granules (SGs). Sorting of specific cargo from immature secretory granules constitutes constitutive-like secretion pathways.
Although presented as black-and-white alternative pathways, both constitutive and regulated secretion are not mutually exclusive, and a degree of overlapping could be observed in different systems (185, 186). For example, studies in acinar cells of the salivary glands (187) and pancreatic islets (188–191) have shown a constitutive-like pathway through endosomes where minor regulated secretion from immature SGs takes place under basal (i.e., no stimulus) conditions. Most of secreted proteins from salivary cells follow an exocrine, regulated pathway into the saliva but constitutive secretion is also observed (192, 193). Similarly, mucin secretion from goblet cells in the airways utilizes both constitutively and regulated secretion pathways (194). Additionally, lysosome-related organelles (LROs) are found and used to store and secrete proteins in many specialized cells different from exocrine cells (195–197). The biogenesis of many LROs involves common components of the endolysosomal and regulated secretory pathways, such as small GTPases, molecular motors, and sorting adaptor proteins and complexes (198).
The placenta, as a unique endocrine organ of temporary function, offers a particularly interesting system of study of protein secretion. As presented above, the placenta secretes a myriad of protein hormones and peptides. The most abundant ones (hCG and hPL) have high homology to pituitary hormones (hCG: 82% with LH (199); hPL: 85% with GH but 22% with hPRL (200)) which are shown to be secreted in SGs. Structurally, both hormones are very different from each other (Table 1), and they are secreted at different rates during pregnancy (37, 201) (Figure 2). How these two hormones are secreted by the placenta has been a controversial topic of discussion during early placental research.
Contemporary to the revolutionary discoveries of George Palade and colleagues by extensive use of ultrastructural characterization of the intracellular membranous system in specialized secretory cells (202), many researchers dug into similar approaches to uncover the localization of placental hormones. Initial studies using both first trimester and term placenta in resin embedded sections (203–205) showed a wide range of thin, smooth membrane-surrounded dense granules within or near the Golgi apparatus that were different from lipid droplets. Larger granules were observed near the apical face of the STB while smaller ones more dispersed around the cytosol, suggesting the former are originated from homotypic fusion between the latter. Although sparse and initially mistakenly as vesicles packed with steroid hormones, the existence of such SG-like structures was very evident. It was even suggested that the small SGs bud off the limiting membrane of the large vesicles in a mechanism that we would associate nowadays with constitutive-like biogenesis (206). A study using cryosections of human term placentas and HRP-conjugated antibodies to improve detection of hCG confirmed infrequent granules and a strong immunoreactivity in the plasma membrane of the STB (207), a stain that could not be observed later by others using similar approaches (208). The simultaneous establishment of the first human hormone-producing trophoblastic cell line (209), the choriocarcinoma BeWo cell line, allowed the interrogation of SG production and secretion in vitro. Results from investigations using this model were, however, not consistent with regulated secretion of hCG: long incubation times with secretagogues was required to stimulate secretion (210), ionophore treatment did not trigger exocytosis (211), incubation with high concentrations of K+ or cytoskeleton drugs did not affect secretion (212, 213), and insignificant amounts of the hormone was observed in stored form (214). Later, similar results were obtained in other choriocarcinoma cell lines (JAR and JEG-3) (215–217). However, more studies using intact chorionic villi and different immunolabeling approaches (218–221) followed up and confirmed the presence of plentiful large bodies resembling large SGs reactive for hCG. One study demonstrated that these abundant granules in first trimester placentas are rich in iron (222) suggesting the possibility of LRO-like characteristics.
Using the pioneer method of isolation of primary trophoblasts from midterm and term placenta by Hall et al. (223), Hochberg, Bick, and Perlman started exploring similar questions than the ones described above but for the secretion of hPL (68, 224–226). These studies agree with the previously mentioned reports for hCG secretion where a constitutive type of secretion is favored. Perhaps differently from the studies with hCG, these authors explicitly recognized the presence of a small fraction of hPL that could be secreted from a prestored pool (68). Using term placenta explants, it was initially shown that hPL does not follow a regulated secretion behavior when K+ and Ca2+ levels are manipulated (227), but such results were contradicted later (228, 229) suggesting then the possibility of a mixed mechanism of secretion. Not surprisingly, immunoelectron microscopy experiments using human placenta tissue showed robust hCG and hPL in small, medium, and large granules in the STB (230, 231), highlighting the differences between ex vivo tissue phenotypes and cells in culture. Conversely, systematic studies by the group of Boime and colleagues, demonstrated that although some of the hCG is stored in SGs and secreted by regulated mechanisms, the vast majority of the hormone is released constitutively (232). But similar studies for hPL (and the many other secreted placental hormones) are still lacking and extrapolation of these results might not be appropriate given the different structural characteristics to hCG, leading to still a larger open question: how is hormonal secretion truly regulated in the placenta?
Conclusions and future directions
The placenta has the remarkable task of coordinating the secretion of numerous substances using a very specialized and unique cellular structure: the STB. Although poorly characterized at the cellular level, the STB is constantly replenished with cytosol, membranes, and nuclei from the underlying CTB layer, perhaps as a mechanism to cope with this intense secretory duty in a local-specific fashion. It is still a mystery how this giant multinucleated structure manages to secrete, at different developmental milestones, different hormones that control its own and both the maternal and fetal fates during the entire pregnancy. The placenta undergoes continuous, sex-specific changes throughout gestation in response to the dynamic maternal-fetal environment to support healthy fetal development. From implantation to parturition, the placenta continuously senses and adapts to maternal and fetal signals, acting as a hormonally active organ uniquely present during pregnancy.
Progress in understanding placental secretory pathways has been limited by restricted access to early gestation tissue, species differences between humans and model organisms, and the limitations of in vitro systems in replicating key physiological processes. We showed a clear example of the challenges faced by the field to study the intracellular trafficking of the main placental hormones: hCG and hPL. Through the research highlighted here, early and term placenta tissue are substantially different, not only in developmental terms but also in secretory capacities. The early placenta expresses high amounts of hCG while the term placenta favors the production of hPL (Figure 2). Both hormones have been found to be constitutively secreted, but a portion has been found in SG-like structures that we still have not fully characterized. While useful for research, trophoblast cell lines and primary cells often fail to fully replicate hormone synthesis, glycosylation, and trafficking due to limitations in their derivation and differentiation. Limited focus on the secretory mechanisms of other protein hormones in the literature has slowed progress in resolving key questions about placental hormone secretion.
With the advent of novel trophoblast models in 2D and 3D (233, 234) in combination with modern techniques for following protein trafficking inside the cells (for example: combining vesicle relocalization with spatial proteomics (235); photoactivation assays (236); probabilistic density maps (237); RUSH trafficking assays (238); transient CRISPR KO technology (239)) we have the opportunity to enter a new chapter in the interrogation of secretory pathways in the placenta. With these advancements, it is essential to evaluate both the strengths and limitations of emerging technologies in the study of placental hormone biology. Human trophoblast organoid models have marked a significant step forward, offering 3D systems that closely mimic placental morphology and endocrine function in vitro (240–242). These cultures can secrete physiologically relevant levels of hormones and support long-term experimentation. However, they primarily model early pregnancy, lack full in vivo architectural complexity, including complete syncytialization and maternal–fetal interface features, and current gene editing tools remain underdeveloped in these systems. Notably, many of the hormones discussed in this review have yet to be investigated in these model systems, highlighting a critical gap in validation and mechanistic understanding within the field. Similarly, advanced live-cell imaging methods—such as confocal, super-resolution, and lattice light-sheet microscopy—enable real-time tracking of vesicle trafficking and hormone secretion at high resolution (243–246). While these techniques are powerful, their application to placental tissues will require extensive optimization and access to specialized equipment, especially in thicker or more heterogeneous samples. Taken together, each technology provides a unique perspective, but a truly comprehensive understanding of placental cell biology will require integrative approaches that combine spatial, temporal, and functional data across multiple platforms.
Fields studying other secretory organs have faced similar challenges of fitting all the phenotypes into one or another model of secretion (193, 247, 248) and galvanized into mixed models that contemplate intermediate or completely new alternatives. Nonetheless, whether in SGs, vesicles, or both, many questions about placental hormone trafficking remain open. What is the machinery that regulates sorting and secretion in the morphologically complex syncytiotrophoblast? Are all the peptide hormones sorted together to the plasma membrane or do they arrive in different carriers? Given the fetal-maternal interface structure of the placenta, how is the polarized sorting controlled? What about the rest of the neglected placental secretome? It is our wish that the next generation of cell biology tools applied to the placenta and trophoblast fields can stimulate the development of specialized approaches and accelerate the discovery in this particularly challenging but equally exciting organ.
Author contributions
SA: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. MP: Conceptualization, Data curation, Visualization, Writing – original draft, Writing – review & editing. CG: Conceptualization, Data curation, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Intramural Program of NIEHS (ZIA ES103370-01).
Acknowledgments
We thank members of the Guardia’s lab for helpful discussions, and Paul Windsor from the NIEHS Office of Communications and Public Liaison for assistance with figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rangel Cenzi J, Albuquerque C, and Keutenedjian Mady CE. Phenomenological and thermodynamic model of gas exchanges in the placenta during pregnancy: A case study of intoxication of carbon monoxide. Int J Environ Res Public Health. (2019) 16(21):1–16. doi: 10.3390/ijerph16214138
2. Caniggia I, Winter J, Lye SJ, and Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. (2000) 21 Suppl A:S25–30. doi: 10.1053/plac.1999.0522
3. Burton GJ, Watson AL, Hempstock J, Skepper JN, and Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab. (2002) 87:2954–9. doi: 10.1210/jcem.87.6.8563
4. Rygaard K, Revol A, Esquivel-Escobedo D, Beck BL, and Barrera-Saldaña HA. Absence of human placental lactogen and placental growth hormone (HGH-V) during pregnancy: PCR analysis of the deletion. Hum Genet. (1998) 102:87–92. doi: 10.1007/s004390050658
5. Brosens I, Pijnenborg R, Vercruysse L, and Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. (2011) 204:193–201. doi: 10.1016/j.ajog.2010.08.009
6. Lindberg BS and Nilsson BA. Human placental lactogen (Hpl) levels in abnormal pregnancies. BJOG: Int J Obstetrics Gynaecol. (1973) 80:1046–53. doi: 10.1111/j.1471-0528.1973.tb02978.x
7. Langley-Evans SC and McMullen S. Developmental origins of adult disease. Med Princ Pract. (2010) 19:87–98. doi: 10.1159/000273066
8. Rothman JE. Lasker Basic Medical Research Award. The machinery and principles of vesicle transport in the cell. Nat Med. (2002) 8:1059–62. doi: 10.1038/nm770
9. Bonifacino JS and Glick BS. The mechanisms of vesicle budding and fusion. Cell. (2004) 116:153–66. doi: 10.1016/s0092-8674(03)01079-1
10. Tokarev AA, Alfonso A, and Segev N. Overview of intracellular compartments and trafficking pathways. In: Segev N, editor. Trafficking inside cells: pathways, mechanisms and regulation. Springer New York, New York, NY (2009). p. 3–14.
11. Teasdale F and Jean-Jacques G. Morphometric evaluation of the microvillous surface enlargement factor in the human placenta from mid-gestation to term. Placenta. (1985) 6:375–81. doi: 10.1016/s0143-4004(85)80014-x
12. Baergen RN. Manual of pathology of the human placenta. Manual of Pathology of the Human Placenta In: 2nd ed. Springer, New York, NY (2011). p. 544.
13. Kidder GM and Watson AJ. Roles of Na,K-ATPase in early development and trophectoderm differentiation. Semin Nephrol. (2005) 25:352–5. doi: 10.1016/j.semnephrol.2005.03.011
14. Teasdale F and Jean-Jacques G. Morphometry of the microvillous membrane of the human placenta in maternal diabetes mellitus. Placenta. (1986) 7:81–8. doi: 10.1016/s0143-4004(86)80020-0
15. Dicke JM and Henderson GI. Placental amino acid uptake in normal and complicated pregnancies. Am J Med Sci. (1988) 295:223–7. doi: 10.1097/00000441-198803000-00012
16. Ericsson A, Hamark B, Jansson N, Johansson BR, Powell TL, and Jansson T. Hormonal regulation of glucose and system A amino acid transport in first trimester placental villous fragments. Am J Physiol Regul Integr Comp Physiol. (2005) 288:R656–62. doi: 10.1152/ajpregu.00407.2004
17. Hertig AT, Rock J, and Adams EC. A description of 34 human ova within the first 17 days of development. Am J Anat. (1956) 98:435–93. doi: 10.1002/aja.1000980306
18. Benirschke K and Kaufmann P. Pathology of the human placenta. 4th ed. New York, NY: Springer-Verlag (2000). p. 950.
19. Enders AC and Schlafke S. Cytological aspects of trophoblast-uterine interaction in early implantation. Am J Anat. (1969) 125:1–29. doi: 10.1002/aja.1001250102
20. Enders AC and Schlafke S. Implantation in the ferret: epithelial penetration. Am J Anat. (1972) 133:291–315. doi: 10.1002/aja.1001330305
21. Wislocki GB and Streeter GL. On the placentation of the macaque (Macaca mulatta): from the time of implantation until the formation of the definitive placenta. Washington, D.C.: Carnegie Institution of Washington (1938).
22. King BF. Ultrastructural differentiation of stromal and vascular components in early macaque placental villi. Am J Anat. (1987) 178:30–44. doi: 10.1002/aja.1001780105
23. Demir R, Kaufmann P, Castellucci M, Erbengi T, and Kotowski A. Fetal vasculogenesis and angiogenesis in human placental villi. Acta Anat (Basel). (1989) 136:190–203. doi: 10.1159/000146886
24. Shih I-M. Chapter 16 - gestational trophoblastic lesions. Gynecologic Pathology In: Nucci MR, Oliva E, and Goldblum JR, editors. Gynecologic Pathology, Edinburgh: Churchill Livingstone (2009). p. 645–65.
25. Tarrade A, Lai Kuen R, Malassiné A, Tricottet V, Blain P, Vidaud M, et al. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab Investigation. (2001) 81:1199–211. doi: 10.1038/labinvest.3780334
26. Handschuh K, Guibourdenche J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, et al. Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-gamma. Endocrinology. (2007) 148:5011–9. doi: 10.1210/en.2007-0286
27. Cole LA, Kardana A, Park SY, and Braunstein GD. The deactivation of hCG by nicking and dissociation. J Clin Endocrinol Metab. (1993) 76:704–10. doi: 10.1210/jcem.76.3.8445030
28. Ziecik AJ, Derecka-Reszka K, and Rzucidło SJ. Extragonadal gonadotropin receptors, their distribution and function. J Physiol Pharmacol. (1992) 43:33–49.
29. Pidoux G, Gerbaud P, Tsatsaris V, Marpeau O, Ferreira F, Meduri G, et al. Biochemical characterization and modulation of LH/CG—receptor during human trophoblast differentiation. J Cell Physiol. (2007) 212:26–35. doi: 10.1002/jcp.20995
30. Tao YX, Lei ZM, Hofmann GE, and Rao CV. Human intermediate trophoblasts express chorionic gonadotropin/luteinizing hormone receptor gene. Biol Reprod. (1995) 53:899–904. doi: 10.1095/biolreprod53.4.899
31. Gudermann T, Birnbaumer M, and Birnbaumer L. Evidence for dual coupling of the murine luteinizing hormone receptor to adenylyl cyclase and phosphoinositide breakdown and Ca2+ mobilization. Studies with the cloned murine luteinizing hormone receptor expressed in L cells. J Biol Chem. (1992) 267:4479–88. doi: 10.1016/S0021-9258(18)42858-X
32. Hanson FW, Powell JE, and Stevens VC. Effects of HCG and human pituitary LH on steroid secretion and functional life of the human corpus luteum. J Clin Endocrinol Metab. (1971) 32:211–5. doi: 10.1210/jcem-32-2-211
33. Orendi K, Gauster M, Moser G, Meiri H, and Huppertz B. The choriocarcinoma cell line BeWo: syncytial fusion and expression of syncytium-specific proteins. Reproduction. (2010) 140:759–66. doi: 10.1530/rep-10-0221
34. Mi S, Lee X, X-p Li, GM V, Finnerty H, Racie L, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. (2000) 403:785–9. doi: 10.1038/35001608
35. Fluhr H, Bischof-Islami D, Krenzer S, Licht P, Bischof P, and Zygmunt M. Human chorionic gonadotropin stimulates matrix metalloproteinases-2 and -9 in cytotrophoblastic cells and decreases tissue inhibitor of metalloproteinases-1, -2, and -3 in decidualized endometrial stromal cells. Fertility Sterility. (2008) 90:1390–5. doi: 10.1016/j.fertnstert.2007.08.023
36. Prast J, Saleh L, Husslein H, Sonderegger S, Helmer H, and Knöfler M. Human chorionic gonadotropin stimulates trophoblast invasion through extracellularly regulated kinase and AKT signaling. Endocrinology. (2008) 149:979–87. doi: 10.1210/en.2007-1282
37. Cole LA. hCG, the wonder of today’s science. Reprod Biol Endocrinol. (2012) 10:24. doi: 10.1186/1477-7827-10-24
38. Islami D, Chardonnens D, Campana A, and Bischof P. Comparison of the effects of GnRH-I and GnRH-II on HCG synthesis and secretion by first trimester trophoblast. Mol Hum Reproduction. (2001) 7:3–9. doi: 10.1093/molehr/7.1.3
39. Knöfler M, Saleh L, Strohmer H, Husslein P, and Wolschek MF. Cyclic AMP- and differentiation-dependent regulation of the proximal αHCG gene promoter in term villous trophoblasts. Mol Hum Reproduction. (1999) 5:573–80. doi: 10.1093/molehr/5.6.573
40. Daoud G, Amyot M, Rassart E, Masse A, Simoneau L, and Lafond J. ERK1/2 and p38 regulate trophoblasts differentiation in human term placenta. J Physiol. (2005) 566:409–23. doi: 10.1113/jphysiol.2005.089326
41. Cameo P, Bischof P, and Calvo JC. Effect of leptin on progesterone, human chorionic gonadotropin, and interleukin-6 secretion by human term trophoblast cells in culture. Biol Reproduction. (2003) 68:472–7. doi: 10.1095/biolreprod.102.006122
42. Petraglia F, Vaughan J, and Vale W. Inhibin and activin modulate the release of gonadotropin-releasing hormone, human chorionic gonadotropin, and progesterone from cultured human placental cells. Proc Natl Acad Sci. (1989) 86:5114–7. doi: 10.1073/pnas.86.13.5114
43. Szilágyi A, Benz R, and Rossmanith WG. Human chorionic gonadotropin secretion from the early human placenta: In vitro regulation by progesterone and its antagonist. Gynecological Endocrinol. (1993) 7:241–50. doi: 10.3109/09513599309152508
44. Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Münstedt K, Rao CV, et al. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. (2002) 87:5290–6. doi: 10.1210/jc.2002-020642
45. Herr F, Baal N, Reisinger K, Lorenz A, McKinnon T, Preissner KT, et al. HCG in the regulation of placental angiogenesis. Results of an in vitro study. Placenta. (2007) 28 Suppl A:S85–93. doi: 10.1016/j.placenta.2007.02.002
46. Berndt S, Blacher S, Perrier d’Hauterive S, Thiry M, Tsampalas M, Cruz A, et al. Chorionic gonadotropin stimulation of angiogenesis and pericyte recruitment. J Clin Endocrinol Metab. (2009) 94:4567–74. doi: 10.1210/jc.2009-0443
47. Rao CV, Li X, Toth P, Lei ZM, and Cook VD. Novel expression of functional human chorionic gonadotropin/luteinizing hormone receptor gene in human umbilical cords. J Clin Endocrinol Metab. (1993) 77:1706–14. doi: 10.1210/jcem.77.6.8263161
48. FerrÉ MS, Lawrence CC, and Jaffe RB. Role of hCG in regulation of the fetal zone of the human fetal adrenal gland*. J Clin Endocrinol Metab. (1978) 46:834–7. doi: 10.1210/jcem-46-5-834
49. Islami D, Bischof P, and Chardonnens D. Modulation of placental vascular endothelial growth factor by leptin and hCG. Mol Hum Reprod. (2003) 9:395–8. doi: 10.1093/molehr/gag053
50. Kovalevskaya G, Birken S, Kakuma T, Ozaki N, Sauer M, Lindheim S, et al. Differential expression of human chorionic gonadotropin (hCG) glycosylation isoforms in failing and continuing pregnancies: preliminary characterization of the hyperglycosylated hCG epitope. J Endocrinol. (2002) 172:497–506. doi: 10.1677/joe.0.1720497
51. Skogler J, Moberg T, Tancredi L, Styrmisdóttir L, Hedayati E, Alarcon-Ruiz CA, et al. Association between human chorionic gonadotropin (hCG) levels and adverse pregnancy outcomes: A systematic review and meta-analysis. Pregnancy Hypertens. (2023) 34:124–37. doi: 10.1016/j.preghy.2023.11.003
52. Peris M, Crompton K, Shepherd DA, and Amor DJ. The association between human chorionic gonadotropin and adverse pregnancy outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol. (2024) 230:118–84. doi: 10.1016/j.ajog.2023.08.007
53. Handwerger S and Freemark M. The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J Pediatr Endocrinol Metab. (2000) 13:343–56. doi: 10.1515/JPEM.2000.13.4.343
54. Lowman HB, Cunningham BC, and Wells JA. Mutational analysis and protein engineering of receptor-binding determinants in human placental lactogen. J Biol Chem. (1991) 266:10982–8. doi: 10.1016/S0021-9258(18)99116-7
55. Sakai S, Kohmoto K, and Johke T. A receptor site for prolactin in lactating mouse mammary tissues. Endocrinol Jpn. (1975) 22:379–87. doi: 10.1507/endocrj1954.22.379
56. Nagano M and Kelly PA. Tissue distribution and regulation of rat prolactin receptor gene expression. Quantitative analysis by polymerase chain reaction. J Biol Chem. (1994) 269:13337–45. doi: 10.1016/S0021-9258(17)36838-2
57. Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, et al. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology. (1993) 132:879–87. doi: 10.1210/en.132.2.879
58. Lombardo MF, De Angelis F, Bova L, Bartolini B, Bertuzzi F, Nano R, et al. Human placental lactogen (hPL-A) activates signaling pathways linked to cell survival and improves insulin secretion in human pancreatic islets. Islets. (2011) 3:250–8. doi: 10.4161/isl.3.5.16900
59. Polgar N, Fogelgren B, Shipley JM, and Csiszar K. Lysyl oxidase interacts with hormone placental lactogen and synergistically promotes breast epithelial cell proliferation and migration*. J Biol Chem. (2007) 282:3262–72. doi: 10.1074/jbc.M609407200
60. Hill DJ, Crace CJ, Strain AJ, and Milner RD. Regulation of amino acid uptake and deoxyribonucleic acid synthesis in isolated human fetal fibroblasts and myoblasts: effect of human placental lactogen, somatomedin-C, multiplication-stimulating activity, and insulin. J Clin Endocrinol Metab. (1986) 62:753–60. doi: 10.1210/jcem-62-4-753
61. Swenne I, Hill DJ, Strain AJ, and Milner RD. Effects of human placental lactogen and growth hormone on the production of insulin and somatomedin C/insulin-like growth factor I by human fetal pancreas in tissue culture. J Endocrinol. (1987) 113:297–303. doi: 10.1677/joe.0.1130297
62. Mohan R, Baumann D, and Alejandro EU. Fetal undernutrition, placental insufficiency, and pancreatic β-cell development programming in utero. Am J Physiology-Regulatory Integr Comp Physiol. (2018) 315:R867–R78. doi: 10.1152/ajpregu.00072.2018
63. Rassie K, Giri R, Joham AE, Teede H, and Mousa A. Human placental lactogen in relation to maternal metabolic health and fetal outcomes: A systematic review and meta-analysis. Int J Mol Sci. (2022) 23(24):1–27. doi: 10.3390/ijms232415621
64. Sumption LA, Garay SM, and John RM. Low serum placental lactogen at term is associated with postnatal symptoms of depression and anxiety in women delivering female infants. Psychoneuroendocrinology. (2020) 116:104655. doi: 10.1016/j.psyneuen.2020.104655
65. Whittaker PG, Schreiber CA, and Sammel MD. Gestational hormone trajectories and early pregnancy failure: a reassessment. Reprod Biol Endocrinol. (2018) 16:95. doi: 10.1186/s12958-018-0415-1
66. Harman I, Costello A, Sane A, and Handwerger S. Cyclic adenosine-3′,5′-monophosphate stimulates the acute release of placental lactogen from human trophoblast cells. Endocrinology. (1987) 121:59–63. doi: 10.1210/endo-121-1-59
67. Hochberg Z, Perlman R, Brandes JM, and Benderli A. INSULIN REGULATES PLACENTAL LACTOGEN AND ESTRADIOL SECRETION BY CULTURED HUMAN TERM TROPHOBLAST. J Clin Endocrinol Metab. (1983) 57:1311–3. doi: 10.1210/jcem-57-6-1311
68. Hochberg Z, Bick T, and Perlman R. Two pathways of placental lactogen secretion by cultured human trophoblast. Biochem Med Metab Biol. (1988) 39:111–6. doi: 10.1016/0885-4505(88)90065-5
69. Kumasaka T, Nishi N, Yaoi Y, Kido Y, Saito M, Okayasu I, et al. Demonstration of immunoreactive somatostatin-like substance in villi and decidua in early pregnancy. Am J Obstetrics Gynecol. (1979) 134:39–44. doi: 10.1016/0002-9378(79)90793-2
70. Lacroix M-C, Guibourdenche J, Fournier T, Laurendeau I, Igout A, Goffin V, et al. Stimulation of human trophoblast invasion by placental growth hormone. Endocrinology. (2005) 146:2434–44. doi: 10.1210/en.2004-1550
71. Alsat E, Guibourdenche J, Couturier A, and Evain-Brion D. Physiological role of human placental growth hormone. Mol Cell Endocrinol. (1998) 140:121–7. doi: 10.1016/S0303-7207(98)00040-9
72. Frankenne F, Closset J, Gomez F, Scippo ML, Smal J, and Hennen G. The physiology of growth hormones (GHs) in pregnant women and partial characterization of the placental GH variant*. J Clin Endocrinol Metab. (1988) 66:1171–80. doi: 10.1210/jcem-66-6-1171
73. Goodyer CG, Figueiredo RMO, Krackovitch S, De Souza Li L, Manalo JA, and Zogopoulos G. Characterization of the growth hormone receptor in human dermal fibroblasts and liver during development. Am J Physiology-Endocrinol Metab. (2001) 281:E1213–E20. doi: 10.1152/ajpendo.2001.281.6.E1213
74. Caufriez A, Frankenne F, Hennen G, and Copinschi G. Regulation of maternal IGF-I by placental GH in normal and abnormal human pregnancies. Am J Physiology-Endocrinol Metab. (1993) 265:E572–E7. doi: 10.1152/ajpendo.1993.265.4.E572
75. Barbour LA, Shao J, Qiao L, Pulawa LK, Jensen DR, Bartke A, et al. Human placental growth hormone causes severe insulin resistance in transgenic mice. Am J Obstetrics Gynecol. (2002) 186:512–7. doi: 10.1067/mob.2002.121256
76. Chellakooty M, Vangsgaard K, Larsen T, Scheike T, Falck-Larsen J, Legarth J, et al. A longitudinal study of intrauterine growth and the placental growth hormone (GH)-insulin-like growth factor I axis in maternal circulation: association between placental GH and fetal growth. J Clin Endocrinol Metab. (2004) 89:384–91. doi: 10.1210/jc.2003-030282
77. Velegrakis A, Sfakiotaki M, and Sifakis S. Human placental growth hormone in normal and abnormal fetal growth. BioMed Rep. (2017) 7:115–22. doi: 10.3892/br.2017.930
78. Zeck W, Widberg C, Maylin E, Desoye G, Lang U, McIntyre D, et al. Regulation of placental growth hormone secretion in a human trophoblast model—The effects of hormones and adipokines. Pediatr Res. (2008) 63:353–7. doi: 10.1203/01.pdr.0000304935.19183.07
79. Henson MC, Swan KF, and O’Neil JS. Expression of placental leptin and leptin receptor transcripts in early pregnancy and at term. Obstetrics Gynecol. (1998) 92:1020–8. doi: 10.1016/S0029-7844(98)00299-3
80. Castellucci M, De Matteis R, Meisser A, Cancello R, Monsurrò V, Islami D, et al. Leptin modulates extracellular matrix molecules and metalloproteinases: Possible implications for trophoblast invasion. Mol Hum Reproduction. (2000) 6:951–8. doi: 10.1093/molehr/6.10.951
81. Sivan E, Whittaker PG, Sinha D, Homko CJ, Lin M, Reece EA, et al. Leptin in human pregnancy: The relationship with gestational hormones. Am J Obstetrics Gynecol. (1998) 179:1128–32. doi: 10.1016/S0002-9378(98)70118-8
82. Stock SM, Sande EM, and Bremme KA. Leptin levels vary significantly during the menstrual cycle, pregnancy, and in vitro fertilization treatment: possible relation to estradiol. Fertility Sterility. (1999) 72:657–62. doi: 10.1016/S0015-0282(99)00321-0
83. Allison MB and Myers MG. Connecting leptin signaling to biological function. J Endocrinol. (2014) 223:T25–35. doi: 10.1530/JOE-14-0404
84. Vaisse C, Halaas JL, Horvath CM, Dernell JE Jr., Stoffel M, and Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. (1996) 14:95–7. doi: 10.1038/ng0996-95
85. Magariños MP, Sánchez-Margalet V, Kotler M, Calvo JC, and Varone CL. Leptin promotes cell proliferation and survival of trophoblastic cells1. Biol Reproduction. (2007) 76:203–10. doi: 10.1095/biolreprod.106.051391
86. Bouloumié A, Drexler HCA, Lafontan M, and Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. (1998) 83:1059–66. doi: 10.1161/01.RES.83.10.1059
87. White V, González E, Capobianco E, Pustovrh C, Martínez N, Higa R, et al. Leptin modulates nitric oxide production and lipid metabolism in human placenta. Reproduction Fertility Dev. (2006) 18:425–32. doi: 10.1071/RD05105
88. Jansson N, Greenwood SL, Johansson BR, Powell TL, and Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab. (2003) 88:1205–11. doi: 10.1210/jc.2002-021332
89. Kautzky-Willer A, Pacini G, Tura A, Bieglmayer C, Schneider B, Ludvik B, et al. Increased plasma leptin in gestational diabetes. Diabetologia. (2001) 44:164–72. doi: 10.1007/s001250051595
90. Laird SM, Quinton ND, Anstie B, Li TC, and Blakemore AI. Leptin and leptin-binding activity in women with recurrent miscarriage: correlation with pregnancy outcome. Hum Reprod. (2001) 16:2008–13. doi: 10.1093/humrep/16.9.2008
91. McCarthy JF, Misra DN, and Roberts JM. Maternal plasma leptin is increased in preeclampsia and positively correlates with fetal cord concentration. Am J Obstet Gynecol. (1999) 180:731–6. doi: 10.1016/s0002-9378(99)70280-2
92. Sooranna SR, Ward S, and Bajoria R. Fetal leptin influences birth weight in twins with discordant growth. Pediatr Res. (2001) 49:667–72. doi: 10.1203/00006450-200105000-00010
93. Yan B, Yu Y, Lin M, Li Z, Wang L, Huang P, et al. High, but stable, trend in the prevalence of gestational diabetes mellitus: A population-based study in Xiamen, China. J Diabetes Investig. (2019) 10:1358–64. doi: 10.1111/jdi.13039
94. Teppa RJ, Ness RB, Crombleholme WR, and Roberts JM. Free leptin is increased in normal pregnancy and further increased in preeclampsia. Metabolism. (2000) 49:1043–8. doi: 10.1053/meta.2000.7707
95. Maymó JL, Pérez Pérez A, Dueñas JL, Calvo JC, Sánchez-Margalet Vc, and Varone CL. Regulation of placental leptin expression by cyclic adenosine 5′-monophosphate involves cross talk between protein kinase A and mitogen-activated protein kinase signaling pathways. Endocrinology. (2010) 151:3738–51. doi: 10.1210/en.2010-0064
96. Yura S, Sagawa N, Ogawa Y, Masuzaki H, Mise H, Matsumoto T, et al. Augmentation of leptin synthesis and secretion through activation of protein kinases A and C in cultured human trophoblastic cells. J Clin Endocrinol Metab. (1998) 83:3609–14. doi: 10.1210/jc.83.10.3609
97. Ge YC, Li JN, Ni XT, Guo CM, Wang WS, Duan T, et al. Cross talk between cAMP and p38 MAPK pathways in the induction of leptin by hCG in human placental syncytiotrophoblasts. Reproduction. (2011) 142:369–75. doi: 10.1530/REP-11-0053
98. Pérez-Pérez A, Maymó J, Gambino Y, Guadix P, Dueñas JL, Varone C, et al. Insulin enhances leptin expression in human trophoblastic cells. Biol Reprod. (2013) 89(1):1–8. doi: 10.1095/biolreprod.113.109348
99. Coya R, Martul P, Algorta J, Aniel-Quiroga MA, Busturia MA, and Señarís R. Progesterone and human placental lactogen inhibit leptin secretion on cultured trophoblast cells from human placentas at term. Gynecological Endocrinol. (2005) 21:27–32. doi: 10.1080/09513590500099305
100. Radovick S, Wondisford FE, Nakayama Y, Yamada M, Cutler GB Jr., and Weintraub BD. Isolation and characterization of the human gonadotropin-releasing hormone gene in the hypothalamus and placenta. Mol Endocrinol. (1990) 4:476–80. doi: 10.1210/mend-4-3-476
101. Khodr GS and Siler-Khodr TM. Placental luteinizing hormone-releasing factor and its synthesis. Science. (1980) 207:315–7. doi: 10.1126/science.6985750
102. Chou C-S, Beristain AG, MacCalman CD, and Leung PCK. Cellular localization of gonadotropin-releasing hormone (GnRH) I and gnRH II in first-trimester human placenta and decidua. J Clin Endocrinol Metab. (2004) 89:1459–66. doi: 10.1210/jc.2003-031636
103. Wolfahrt S, Kleine B, and Rossmanith WG. Detection of gonadotrophin releasing hormone and its receptor mRNA in human placental trophoblasts using in-situ reverse transcription-polymerase chain reaction. Mol Hum Reproduction. (1998) 4:999–1006. doi: 10.1093/molehr/4.10.999
104. Siler-Khodr TM, Khodr GS, and Valenzuela G. Immunoreactive gonadotropin-releasing hormone level in maternal circulation throughout pregnancy. Am J Obstet Gynecol. (1984) 150:376–9. doi: 10.1016/s0002-9378(84)80142-8
105. Cheng CK and Leung PCK. Molecular biology of gonadotropin-releasing hormone (GnRH)-I, gnRH-II, and their receptors in humans. Endocrine Rev. (2005) 26:283–306. doi: 10.1210/er.2003-0039
106. Lin LS, Roberts VJ, and Yen SS. Expression of human gonadotropin-releasing hormone receptor gene in the placenta and its functional relationship to human chorionic gonadotropin secretion. J Clin Endocrinol Metab. (1995) 80:580–5. doi: 10.1210/jcem.80.2.7852524
107. Liu J, Maccalman CD, Wang YL, and Leung PC. Promotion of human trophoblasts invasion by gonadotropin-releasing hormone (GnRH) I and GnRH II via distinct signaling pathways. Mol Endocrinol. (2009) 23:1014–21. doi: 10.1210/me.2008-0451
108. Chou CS, Tai CJ, MacCalman CD, and Leung PC. Dose-dependent effects of gonadotropin releasing hormone on matrix metalloproteinase (MMP)-2, and MMP-9 and tissue specific inhibitor of metalloproteinases-1 messenger ribonucleic acid levels in human decidual Stromal cells in vitro. J Clin Endocrinol Metab. (2003) 88:680–8. doi: 10.1210/jc.2002-021277
109. Kang IS, Kuehl TJ, and Siler-Khodr TM. Effect of treatment with gonadotropin-releasing hormone analogues on pregnancy outcome in the baboon**Supported by grant 83058A from the World Health Organization, Geneva, Switzerland, and by grant HD-10202 from the National Institutes of Child Health and Human Development, Center for the study of Reproduction RIA Core, Bethesda, Maryland. Fertil Steril (1989) 52(5):846–53. doi: 10.1016/S0015-0282(16)53051-9
110. Sridaran R, Lee MA, Haynes L, Srivastava RK, Ghose M, Sridaran G, et al. GnRH action on luteal steroidogenesis during pregnancy. Steroids. (1999) 64:618–23. doi: 10.1016/s0039-128x(99)00042-2
111. Tug N, Uslu U, Cumbul A, Eyuboglu S, Cam C, Karateke A, et al. Effects of the gonadotropin-releasing hormone antagonist cetrorelix in the early postimplantation period on rat pregnancy. Eur J Obstet Gynecol Reprod Biol. (2011) 155:166–70. doi: 10.1016/j.ejogrb.2010.12.015
112. Penn AA. 13 - endocrine and paracrine function of the human placenta. In: Polin RA, Abman SH, Rowitch DH, Benitz WE, and Fox WW, editors. Fetal and neonatal physiology, Fifth Edition. Philadelphia, PA: Elsevier (2017). p. 134–44.e4.
113. Ling N, Ying SY, Ueno N, Esch F, Denoroy L, and Guillemin R. Isolation and partial characterization of a Mr 32,000 protein with inhibin activity from porcine follicular fluid. Proc Natl Acad Sci. (1985) 82:7217–21. doi: 10.1073/pnas.82.21.7217
114. Vale W, Rivier J, Vaughan J, McClintock R, Corrigan A, Woo W, et al. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. (1986) 321:776–9. doi: 10.1038/321776a0
115. Petraglia F, Sawchenko P, Lim AT, Rivier J, and Vale W. Localization, secretion, and action of inhibin in human placenta. Science. (1987) 237:187–9. doi: 10.1126/science.3299703
116. Petraglia F. Inhibin, activin and follistatin in the human placenta—a new family of regulatory proteins. Placenta. (1997) 18:3–8. doi: 10.1016/S0143-4004(97)90065-5
117. Fowler PA, Evans LW, Groome NP, Templeton A, and Knight PG. A longitudinal study of maternal serum inhibin-A inhibin-B, activin-A activin-AB, pro-αC and follistatin during pregnancy. Hum Reproduction. (1998) 13:3530–6. doi: 10.1093/humrep/13.12.3530
118. Greenwald J, Groppe J, Gray P, Wiater E, Kwiatkowski W, Vale W, et al. The BMP7/actRII extracellular domain complex provides new insights into the cooperative nature of receptor assembly. Mol Cell. (2003) 11:605–17. doi: 10.1016/S1097-2765(03)00094-7
119. Schneider-Kolsky ME, Manuelpillai U, Waldron K, Dole A, and Wallace EM. The distribution of activin and activin receptors in gestational tissues across human pregnancy and during labour. Placenta. (2002) 23:294–302. doi: 10.1053/plac.2002.0787
120. Matzuk MM, Kumar TR, and Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. (1995) 374:356–60. doi: 10.1038/374356a0
121. Ma X, Reyna A, Mani SK, Matzuk MM, and Kumar TR. Impaired male sexual behavior in activin receptor type II knockout mice1. Biol Reproduction. (2005) 73:1182–90. doi: 10.1095/biolreprod.105.043794
122. Barber CV, Yo JH, Rahman RA, Wallace EM, Palmer KR, and Marshall SA. Activin A and pathologies of pregnancy: a review. Placenta. (2023) 136:35–41. doi: 10.1016/j.placenta.2023.03.008
123. Caniggia I, Lye SJ, and Cross JC. Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology. (1997) 138:3976–86. doi: 10.1210/endo.138.9.5403
124. Li Y, Klausen C, Zhu H, and Leung PCK. Activin A increases human trophoblast invasion by inducing SNAIL-mediated MMP2 up-regulation through ALK4. J Clin Endocrinol Metab. (2015) 100:E1415–E27. doi: 10.1210/jc.2015-2134
125. Mathews LS and Vale WW. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. (1991) 65:973–82. doi: 10.1016/0092-8674(91)90549-E
126. Petraglia F, Di Blasio AM, Florio P, Gallo R, Genazzani AR, Woodruff TK, et al. High levels of fetal membrane activin βA and activin receptor IIB mRNAs and augmented concentration of amniotic fluid activin A in women in term or preterm labor. J Endocrinol. (1997) 154:95–101. doi: 10.1677/joe.0.1540095
127. Rosenberg VA, Buhimschi IA, Dulay AT, Abdel-Razeq SS, Oliver EA, Duzyj CM, et al. Modulation of amniotic fluid activin-A and inhibin-A in women with preterm premature rupture of the membranes and infection-induced preterm birth. Am J Reprod Immunol. (2012) 67:122–31. doi: 10.1111/j.1600-0897.2011.01074.x
128. Muttukrishna S, Knight PG, Groome NP, Redman CW, and Ledger WL. Activin A and inhibin A as possible endocrine markers for pre-eclampsia. Lancet. (1997) 349:1285–8. doi: 10.1016/s0140-6736(96)09264-1
129. Yue CY, Zhang CY, Ni YH, and Ying CM. Are serum levels of inhibin A in second trimester predictors of adverse pregnancy outcome? PloS One. (2020) 15:e0232634. doi: 10.1371/journal.pone.0232634
130. Keelan J, Song Y, and France JT. Comparative regulation of inhibin, activin and human chorionic gonadotropin production by placental trophoblast cells in culture. Placenta. (1994) 15:803–18. doi: 10.1016/S0143-4004(05)80183-3
131. Qu J and Thomas K. Regulation of inhibin secretion in human placental cell culture by epidermal growth factor, transforming growth factors, and activin. J Clin Endocrinol Metab. (1993) 77:925–31. doi: 10.1210/jcem.77.4.8408467
132. Reis FM, Luisi S, Florio P, Degrassi A, and Petraglia F. Corticotropin-releasing factor, urocortin and endothelin-1 stimulate activin A release from cultured human placental cells. Placenta. (2002) 23:522–5. doi: 10.1053/plac.2002.0831
133. Riley SC, Walton JC, Herlick JM, and Challis JRG. The localization and distribution of corticotropin-releasing hormone in the human placenta and fetal membranes throughout gestation*. J Clin Endocrinol Metab. (1991) 72:1001–7. doi: 10.1210/jcem-72-5-1001
134. Challis JR, Matthews SG, Van Meir C, and Ramirez MM. Current topic: the placental corticotrophin-releasing hormone-adrenocorticotrophin axis. Placenta. (1995) 16:481–502. doi: 10.1016/s0143-4004(05)80001-3
135. Frim DM, Emanuel RL, Robinson BG, Smas CM, Adler GK, and Majzoub JA. Characterization and gestational regulation of corticotropin-releasing hormone messenger RNA in human placenta. J Clin Invest. (1988) 82:287–92. doi: 10.1172/jci113585
136. McLean M and Smith R. Corticotrophin-releasing hormone and human parturition. Reproduction. (2001) 121:493–501. doi: 10.1530/rep.0.1210493
137. Smith R, Mesiano S, and McGrath S. Hormone trajectories leading to human birth. Regul Pept. (2002) 108:159–64. doi: 10.1016/s0167-0115(02)00105-2
138. King BR, Smith R, and Nicholson RC. The regulation of human corticotrophin-releasing hormone gene expression in the placenta. Peptides. (2001) 22:1941–7. doi: 10.1016/s0196-9781(01)00486-7
139. Perrin MH and Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann New York Acad Sci. (1999) 885:312–28. doi: 10.1111/j.1749-6632.1999.tb08687.x
140. Florio P, Franchini A, Reis FM, Pezzani I, Ottaviani E, and Petraglia F. Human placenta, chorion, amnion and decidua express different variants of corticotropin-releasing factor receptor messenger RNA. Placenta. (2000) 21:32–7. doi: 10.1053/plac.1999.0461
141. McLean M, Bisits A, Davies J, Woods R, Lowry P, and Smith R. A placental clock controlling the length of human pregnancy. Nat Med. (1995) 1:460–3. doi: 10.1038/nm0595-460
142. Sandman CA and Glynn LM. Corticotropin-releasing hormone (CRH) programs the fetal and maternal brain. Future Neurol. (2009) 4:257–61. doi: 10.2217/fnl.09.8
143. Petraglia F, Sawchenko PE, Rivier J, and Vale W. Evidence for local stimulation of ACTH secretion by corticotropin-releasing factor in human placenta. Nature. (1987) 328:717–9. doi: 10.1038/328717a0
144. Jones SA and Challis JRG. Local stimulation of prostagiandin production by corttcotropin-releasing hormone in human fetal membranes and placenta. Biochem Biophys Res Communications. (1989) 159:192–9. doi: 10.1016/0006-291X(89)92422-4
145. De Bonis M, Torricelli M, Severi FM, Luisi S, De Leo V, and Petraglia F. Neuroendocrine aspects of placenta and pregnancy. Gynecol Endocrinol. (2012) 28 Suppl 1:22–6. doi: 10.3109/09513590.2012.651933
146. Petraglia F, Florio P, Gallo R, Simoncini T, Saviozzi M, Di Blasio AM, et al. Human placenta and fetal membranes express human urocortin mRNA and peptide. J Clin Endocrinol Metab. (1996) 81:3807–10. doi: 10.1210/jcem.81.10.8855842
147. Leitch IM, Boura AL, Botti C, Read MA, Walters WA, and Smith R. Vasodilator actions of urocortin and related peptides in the human perfused placenta in vitro. J Clin Endocrinol Metab. (1998) 83:4510–3. doi: 10.1210/jcem.83.12.5356
148. Tu H, Kastin AJ, and Pan W. Corticotropin-releasing hormone receptor (CRHR)1 and CRHR2 are both trafficking and signaling receptors for urocortin. Mol Endocrinol. (2007) 21:700–11. doi: 10.1210/me.2005-0503
149. Petraglia F, Florio P, Benedetto C, Marozio L, Di Blasio AM, Ticconi C, et al. Urocortin stimulates placental adrenocorticotropin and prostaglandin release and myometrial contractility in vitro. J Clin Endocrinol Metab. (1999) 84:1420–3. doi: 10.1210/jcem.84.4.5585
150. Holzman C, Jetton J, Siler-Khodr T, Fisher R, and Rip T. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstetrics Gynecol. (2001) 97:657–63. doi: 10.1016/S0029-7844(00)01209-6
151. Petraglia F, Florio P, Benedetto C, Gallo C, Woods RJ, Genazzani AR, et al. High levels of corticotropin-releasing factor (CRF) are inversely correlated with low levels of maternal CRF-binding protein in pregnant women with pregnancy-induced hypertension. J Clin Endocrinol Metab. (1996) 81:852–6. doi: 10.1210/jcem.81.2.8636316
152. Florio P, Calonaci G, Severi FM, Torricelli M, Bocchi C, Fiore G, et al. Reduced maternal plasma urocortin concentrations and impaired uterine artery blood flow at human mid pregnancy. J Soc Gynecologic Investigation. (2005) 12:191–4. doi: 10.1016/j.jsgi.2004.11.002
153. Karteris E, Vatish M, Hillhouse EW, and Grammatopoulos DK. Preeclampsia is associated with impaired regulation of the placental nitric oxide-cyclic guanosine monophosphate pathway by corticotropin-releasing hormone (CRH) and CRH-related peptides. J Clin Endocrinol Metab. (2005) 90:3680–7. doi: 10.1210/jc.2004-2210
154. Korebrits C, Yu DHT, Ramirez MM, Marinoni E, Bocking AD, and Challis JRG. Antenatal glucocorticoid administration increases corticotrophin-releasing hormone in maternal plasma. BJOG: Int J Obstetrics Gynaecol. (1998) 105:556–61. doi: 10.1111/j.1471-0528.1998.tb10158.x
155. Chen C-LC, Chang C-C, Krieger DT, and Bardin CW. Expression and regulation of proopiomelanocortin-like gene in the ovary and placenta: comparison with the testis*. Endocrinology. (1986) 118:2382–9. doi: 10.1210/endo-118-6-2382
156. Grigorakis SI, Anastasiou E, Dai K, Souvatzoglou A, and Alevizaki M. Three mRNA transcripts of the proopiomelanocortin gene in human placenta at term. Eur J Endocrinol. (2000) 142:533–6. doi: 10.1530/eje.0.1420533
157. Raffin-Sanson M-L, Massias JF, Ankotche A, Coste J, De Keyzer Y, Oliver C, et al. High precursor level in maternal blood results from the alternate mode of proopiomelanocortin processing in human placenta. Clin Endocrinol. (1999) 50:85–94. doi: 10.1046/j.1365-2265.1999.00612.x
158. Constância M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. (2002) 417:945–8. doi: 10.1038/nature00819
159. Hiden U, Glitzner E, Hartmann M, and Desoye G. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. J Anat. (2009) 215:60–8. doi: 10.1111/j.1469-7580.2008.01035.x
160. Jones JI and Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions*. Endocrine Rev. (1995) 16:3–34. doi: 10.1210/edrv-16-1-3
161. Holmes R, Porter H, Newcomb P, Holly JM, and Soothill P. An immunohistochemical study of type I insulin-like growth factor receptors in the placentae of pregnancies with appropriately grown or growth restricted fetuses. Placenta. (1999) 20:325–30. doi: 10.1053/plac.1998.0387
162. Forbes K, Westwood M, Baker PN, and Aplin JD. Insulin-like growth factor I and II regulate the life cycle of trophoblast in the developing human placenta. Am J Physiol Cell Physiol. (2008) 294:C1313–22. doi: 10.1152/ajpcell.00035.2008
163. Hamilton GS, Lysiak JJ, Han VK, and Lala PK. Autocrine-paracrine regulation of human trophoblast invasiveness by insulin-like growth factor (IGF)-II and IGF-binding protein (IGFBP)-1. Exp Cell Res. (1998) 244:147–56. doi: 10.1006/excr.1998.4195
164. Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, et al. Regulation of neuronal survival by the serine-threonine protein kinase akt. Science. (1997) 275:661–5. doi: 10.1126/science.275.5300.661
165. Karl PI. Insulin-like growth factor-1 stimulates amino acid uptake by the cultured human placental trophoblast. J Cell Physiol. (1995) 165:83–8. doi: 10.1002/jcp.1041650111
166. Crossey PA, Pillai CC, and Miell JP. Altered placental development and intrauterine growth restriction in IGF binding protein-1 transgenic mice. J Clin Invest. (2002) 110:411–8. doi: 10.1172/jci10077
167. Koukoura O, Sifakis S, Soufla G, Zaravinos A, Apostolidou S, Jones A, et al. Loss of imprinting and aberrant methylation of IGF2 in placentas from pregnancies complicated with fetal growth restriction. Int J Mol Med. (2011) 28:481–7. doi: 10.3892/ijmm.2011.754
168. Ahmed A, Li XF, Dunk C, Whittle MJ, Rushton DI, and Rollason T. Colocalisation of vascular endothelial growth factor and its flt-1 receptor in human placenta. Growth Factors. (1995) 12:235–43. doi: 10.3109/08977199509036883
169. Saito T, Takeda N, Amiya E, Nakao T, Abe H, Semba H, et al. VEGF-A induces its negative regulator, soluble form of VEGFR-1, by modulating its alternative splicing. FEBS Letters. (2013) 587:2179–85. doi: 10.1016/j.febslet.2013.05.038
170. Drake CJ, LaRue A, Ferrara N, and Little CD. VEGF regulates cell behavior during vasculogenesis. Dev Biol. (2000) 224:178–88. doi: 10.1006/dbio.2000.9744
171. Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. (2002) 8:831–40. doi: 10.1038/nm731
172. Dvorak HF, Brown LF, Detmar M, and Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. (1995) 146:1029–39. doi: 10.1007/978-3-642-59953-8_6
173. Zhou Y, Bellingard V, Feng K-T, McMaster M, and Fisher SJ. Human cytotrophoblasts promote endothelial survival and vascular remodeling through secretion of Ang2, PlGF, and VEGF-C. Dev Biol. (2003) 263:114–25. doi: 10.1016/S0012-1606(03)00449-4
174. Torry DS and Torry RJ. Angiogenesis and the expression of vascular endothelial growth factor in endometrium and placenta. Am J Reprod Immunol. (1997) 37:21–9. doi: 10.1111/j.1600-0897.1997.tb00189.x
175. Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, and Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. (1996) 149:293–305.
176. Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. (2002) 160:1405–23. doi: 10.1016/S0002-9440(10)62567-9
177. Chen Y, Gershlick DC, Park SY, and Bonifacino JS. Segregation in the Golgi complex precedes export of endolysosomal proteins in distinct transport carriers. J Cell Biol. (2017) 216:4141–51. doi: 10.1083/jcb.201707172
178. De Matteis MA and Luini A. Exiting the golgi complex. Nat Rev Mol Cell Biol. (2008) 9:273–84. doi: 10.1038/nrm2378
179. Rodriguez-Boulan E, Kreitzer G, and Müsch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. (2005) 6:233–47. doi: 10.1038/nrm1593
180. Stalder D and Gershlick DC. Direct trafficking pathways from the Golgi apparatus to the plasma membrane. Semin Cell Dev Biol. (2020) 107:112–25. doi: 10.1016/j.semcdb.2020.04.001
181. Dannies PS. Protein hormone storage in secretory granules: mechanisms for concentration and sorting. Endocr Rev. (1999) 20:3–21. doi: 10.1210/edrv.20.1.0354
182. Kim T, Gondre-Lewis MC, Arnaoutova I, and Loh YP. Dense-core secretory granule biogenesis. Physiol (Bethesda). (2006) 21:124–33. doi: 10.1152/physiol.00043.2005
183. Park JJ and Loh YP. How peptide hormone vesicles are transported to the secretion site for exocytosis. Mol Endocrinol. (2008) 22:2583–95. doi: 10.1210/me.2008-0209
184. Bowman GR, Cowan AT, and Turkewitz AP. Biogenesis of dense-core secretory granules. Trafficking Inside Cells. (2009), 183–209. doi: 10.1007/978-0-387-93877-6_10
185. Ma CJ, Burgess J, and Brill JA. Maturing secretory granules: Where secretory and endocytic pathways converge. Adv Biol Regul. (2021) 80:100807. doi: 10.1016/j.jbior.2021.100807
186. Beznoussenko GV, Pavelka M, Mironov AA, Mironov AA, and Pavelka M, editors. The Golgie Apparatus. Springer Vienna, Vienna (2008). p. 475–84.
187. Huang AY, Castle AM, Hinton BT, and Castle JD. Resting (basal) secretion of proteins is provided by the minor regulated and constitutive-like pathways and not granule exocytosis in parotid acinar cells. J Biol Chem. (2001) 276:22296–306. doi: 10.1074/jbc.M100211200
188. Turner MD and Arvan P. Protein traffic from the secretory pathway to the endosomal system in pancreatic beta-cells. J Biol Chem. (2000) 275:14025–30. doi: 10.1074/jbc.275.19.14025
189. Arvan P, Kuliawat R, Prabakaran D, Zavacki AM, Elahi D, Wang S, et al. Protein discharge from immature secretory granules displays both regulated and constitutive characteristics. J Biol Chem. (1991) 266:14171–4. doi: 10.1016/S0021-9258(18)98661-8
190. Kuliawat R, Klumperman J, Ludwig T, and Arvan P. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta-cells. J Cell Biol. (1997) 137:595–608. doi: 10.1083/jcb.137.3.595
191. Klumperman J, Kuliawat R, Griffith JM, Geuze HJ, and Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J Cell Biol. (1998) 141:359–71. doi: 10.1083/jcb.141.2.359
192. Kagami H, O’Connell BC, and Baum BJ. Evidence for the systemic delivery of a transgene product from salivary glands. Hum Gene Ther. (1996) 7:2177–84. doi: 10.1089/hum.1996.7.17-2177
193. Isenman L, Liebow C, and Rothman S. The endocrine secretion of mammalian digestive enzymes by exocrine glands. Am J Physiol. (1999) 276:E223–32. doi: 10.1152/ajpendo.1999.276.2.E223
194. Zhu Y, Abdullah LH, Doyle SP, Nguyen K, Ribeiro CM, Vasquez PA, et al. Baseline goblet cell mucin secretion in the airways exceeds stimulated secretion over extended time periods, and is sensitive to shear stress and intracellular mucin stores. PloS One. (2015) 10:e0127267. doi: 10.1371/journal.pone.0127267
195. Dell’Angelica EC, Mullins C, Caplan S, and Bonifacino JS. Lysosome-related organelles. FASEB J. (2000) 14:1265–78. doi: 10.1096/fasebj.14.10.1265
196. Luzio JP, Hackmann Y, Dieckmann NM, and Griffiths GM. The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb Perspect Biol. (2014) 6:a016840. doi: 10.1101/cshperspect.a016840
197. Delevoye C, Marks MS, and Raposo G. Lysosome-related organelles as functional adaptations of the endolysosomal system. Curr Opin Cell Biol. (2019) 59:147–58. doi: 10.1016/j.ceb.2019.05.003
198. Bowman SL, Bi-Karchin J, Le L, and Marks MS. The road to lysosome-related organelles: Insights from Hermansky-Pudlak syndrome and other rare diseases. Traffic. (2019) 20:404–35. doi: 10.1111/tra.12646
199. Bulun SE. CHAPTER 17 - physiology and pathology of the female reproductive axis. In: Melmed S, Polonsky KS, Larsen PR, and Kronenberg HM, editors. Williams textbook of endocrinology, Twelfth Edition. W.B. Saunders, Philadelphia (2011). p. 581–660.
200. Brooks CL. Molecular mechanisms of prolactin and its receptor. Endocr Rev. (2012) 33:504–25. doi: 10.1210/er.2011-1040
201. Grumbach MM, Kaplan SL, Sciarra JJ, and Burr IM. Chorionic growth hormone-prolactin (CGP): secretion, disposition, biologic activity in man, and postulated function as the “growth hormone” of the 2d half of pregnancy. Ann N Y Acad Sci. (1968) 148:501–31. doi: 10.1111/j.1749-6632.1968.tb20372.x
202. Palade G. Intracellular aspects of the process of protein synthesis. Science. (1975) 189:347–58. doi: 10.1126/science.1096303
203. Rhodin JAG and Terzakis J. The ultrastructure of the human full-term placenta. J Ultrastructure Res. (1962) 6:88–106. doi: 10.1016/S0022-5320(62)90063-1
204. Terzakis JA. The ultrastructure of normal human first trimester placenta. J Ultrastructure Res. (1963) 9:268–84. doi: 10.1016/S0022-5320(63)80007-6
205. Yoshida Y. Glycogen formation in the cytotrophoblast of human placenta in early pregnancy, as revealed by electron microscopy. Exp Cell Res. (1964) 34:293–304. doi: 10.1016/0014-4827(64)90365-9
206. Tighe JR, Garrod PR, and Curran RC. The trophoblast of the human chorionic villus. J Pathol Bacteriol. (1967) 93:559–67. doi: 10.1002/path.1700930217
207. Dreskin RB, Spicer SS, and Greene WB. ULTRASTRUCTURAL LOCALIZATION OF CHORIONIC GONADOTROPIN IN HUMAN TERM PLACENTA. J Histochem Cytochem. (1970) 18:862–74. doi: 10.1177/18.12.862
208. Morinaga S, Haga N, and Sasano N. Ultrastructural localization of hCG in the placenta and in testicular germ cell tumors. Virchows Arch B Cell Pathol Incl Mol Pathol. (1984) 45:397–406. doi: 10.1007/bf02889882
209. Pattillo RA and Gey GO. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. (1968) 28:1231–6.
210. Pattillo RA and Hussa RO. Early stimulation of human chorionic gonadotropin secretion by dibutyryl cyclic AMP and theophylline in human Malignant trophoblast cells in vitro: inhibition by actinomycin D, α-amanitin, and cordycepin. Gynecologic Investigation. (1975) 6:365–77. doi: 10.1159/000301538
211. Hussa RO. Studies on human chorionic gonadotropin secretion: effects of EGTA, lanthanum, and the ionophore A23187. J Clin Endocrinol Metab. (1977) 44:520–29. doi: 10.1210/jcem-44-3-520
212. Hussa RO. Effects of antimicrotubule agents, potassium and inhibitors of energy production on hCG secretion. In Vitro. (1979) 15:237–45. doi: 10.1007/bf02618946
213. Sekiya S, Kaiho T, Iwasawa H, Inaba N, Shirotake S, and Takamizawa H. Effect of cytochalasin B on growth, Multinucleation and human chorionic gonadotropin secretion in a human choriocarcinoma cell line. Eur J Cancer Clin Oncol. (1984) 20:525–33. doi: 10.1016/0277-5379(84)90239-6
214. Hussa RO. Immunologic and physical characterization of human chorionic gonadotropin and its subunits in cultures of human Malignant trophoblast. J Clin Endocrinol Metab. (1977) 44:1154–62. doi: 10.1210/jcem-44-6-1154
215. Martell RE and Ruddon RW. Patterns of human chorionic gonadotropin expression in untreated and 8-bromoadenosine-treated JAR choriocarcinoma cells. Endocrinology. (1990) 126:2757–64. doi: 10.1210/endo-126-5-2757
216. Burnside J, Nagelberg SB, Lippman SS, and Weintraub BD. Differential regulation of hCG alpha and beta subunit mRNAs in JEG-3 choriocarcinoma cells by 8-bromo-cAMP. J Biol Chem. (1985) 260:12705–9. doi: 10.1016/S0021-9258(17)38931-7
217. Larry Jameson J, Jaffe RC, Leslie Gleason S, and Habener JF. Transcriptional regulation of chorionic gonadotropin α- and β-subunit gene expression by 8-bromo-adenosine 3′,5′-monophosphate*. Endocrinology. (1986) 119:2560–7. doi: 10.1210/endo-119-6-2560
218. Kasai K and Yoshida Y. MORPHOLOGICAL STUDY ON THE PRODUCTION AND SECRETION MECHANISM OF HUMAN CHORIONIC GONADOTROPIN (hCG). Acta Histochemica Cytochemica. (1979) 12:283–91. doi: 10.1267/ahc.12.283
219. Hamasaki K, Okamura Y, Ueda H, Kagawa H, and Fujimoto S. Immunocytochemistry of human chorionic gonadotropin in human chorionic villi. Am J Obstetrics Gynecol. (1987) 156:479–83. doi: 10.1016/0002-9378(87)90314-0
220. Morrish DW, Marusyk H, and Siy O. Demonstration of specific secretory granules for human chorionic gonadotropin in placenta. J Histochem Cytochem. (1987) 35:93–101. doi: 10.1177/35.1.2432115
221. Fukui Y, Ueda H, Hamasaki K, and Fujimoto S. Protein-secretory patterns of normal and abnormal human placentas with special reference to human chorionic gonadotropin. Acta Anat (Basel). (1991) 140:254–9. doi: 10.1159/000147065
222. Hamasaki K, Fujimoto S, Yamamoto K, and Okamura Y. Iron-containing granules in the syncytiotrophoblast of the human chorionic villi. Acta Anatomica. (1985) 121:194–6. doi: 10.1159/000145965
223. Hall CS, James TE, Goodyer C, Branchaud C, Guyda H, and Giroud CJ. Short term culture of human midterm and term placenta: parameters of hormonogenesis. Steroids. (1977) 30:569–80. doi: 10.1016/0039-128x(77)90101-5
224. Hochberg Z, Bick T, Perlman R, Lahav M, and Barzilai D. The modulation of placental lactogen secretion by calcium: studies with cultured human term trophoblast. Mol Cell Endocrinol. (1984) 37:359–62. doi: 10.1016/0303-7207(84)90106-0
225. Perlman R, Barzilai D, Bick T, and Hochberg Z. The effect of inhibitors on insulin regulation of hormone secretion by cultured human trophoblast. Mol Cell Endocrinol. (1985) 43:77–82. doi: 10.1016/0303-7207(85)90044-9
226. Hochberg Z, Perlman R, and Bick T. Interrelated calcium ion and cyclic AMP inhibition of placental lactogen secretion by cultured human term trophoblast. Acta Endocrinol (Copenh). (1987) 114:68–73. doi: 10.1530/acta.0.1140068
227. Choy VJ and Watkins WB. Effect of ionic environment on the release of human placental lactogen in vitro. J Endocrinol. (1976) 69:349–58. doi: 10.1677/joe.0.0690349
228. Meuris S, Polliotti B, Robyn C, and Lebrun P. Ca2+ entry through L-type voltage-sensitive Ca2+ channels stimulates the release of human chorionic gonadotrophin and placental lactogen by placental explants. Biochim Biophys Acta (BBA) - Mol Cell Res. (1994) 1220:101–6. doi: 10.1016/0167-4889(94)90124-4
229. Williams JL, Fyfe GK, Sibley CP, Baker PN, and Greenwood SL. K+ channel inhibition modulates the biochemical and morphological differentiation of human placental cytotrophoblast cells in vitro. Am J Physiol Regul Integr Comp Physiol. (2008) 295:R1204–13. doi: 10.1152/ajpregu.00193.2008
230. Fujimoto S, Hamasaki K, Ueda H, and Kagawa H. Immunoelectron microscope observations on secretion of human placental lactogen (hPL) in the human chorionic villi. Anat Rec. (1986) 216:68–72. doi: 10.1002/ar.1092160112
231. Hamasaki K, Doi Y, Yokoyama M, Kashimura M, and Fujimoto S. Immunoelectron microscopy of human chorionic gonadotropin (hCG) and human placental lactogen (hPL) in the syncytiotrophoblast of human placenta, clarifying the nature of the So-called large dense bodies. Med Electron Microscopy. (1993) 26:133–8. doi: 10.1007/BF02348039
232. Muyan M and Boime I. Secretion of chorionic gonadotropin from human trophoblasts. Placenta. (1997) 18:237–41. doi: 10.1016/S0143-4004(97)80056-2
233. Li X, Li Z-H, Wang Y-X, and Liu T-H. A comprehensive review of human trophoblast fusion models: recent developments and challenges. Cell Death Discov. (2023) 9:372. doi: 10.1038/s41420-023-01670-0
234. Liu X, Wang G, Huang H, Lv X, Si Y, Bai L, et al. Exploring maternal-fetal interface with in vitro placental and trophoblastic models. Front Cell Dev Biol. (2023) 11:1279227. doi: 10.3389/fcell.2023.1279227
235. Shin JJH, Crook OM, Borgeaud AC, Cattin-Ortolá J, Peak-Chew SY, Breckels LM, et al. Spatial proteomics defines the content of trafficking vesicles captured by golgin tethers. Nat Communications. (2020) 11:5987. doi: 10.1038/s41467-020-19840-4
236. Ecker M, Redpath GMI, Nicovich PR, and Rossy J. Quantitative visualization of endocytic trafficking through photoactivation of fluorescent proteins. Mol Biol Cell. (2021) 32:892–902. doi: 10.1091/mbc.E20-10-0669
237. Kristine Schauer TD, Gomes-Santos CS, and Goud B. Chapter 20 - studying intracellular trafficking pathways with probabilistic density maps. Methods Cell Biol. (2013) 118:325–43. doi: 10.1016/B978-0-12-417164-0.00020-3
238. Parchure A, Tian M, Stalder D, Boyer CK, Bearrows SC, Rohli KE, et al. Liquid-liquid phase separation facilitates the biogenesis of secretory storage granules. J Cell Biol. (2022) 221(12):1–23. doi: 10.1083/jcb.202206132
239. Pereira C, Stalder D, Anderson GSF, Shun-Shion AS, Houghton J, Antrobus R, et al. The exocyst complex is an essential component of the mammalian constitutive secretory pathway. J Cell Biol. (2023) 222(5):1–20. doi: 10.1083/jcb.202205137
240. Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature. (2018) 563:347–53. doi: 10.1038/s41586-018-0698-6
241. Haider S, Meinhardt G, Saleh L, Kunihs V, Gamperl M, Kaindl U, et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Reports. (2018) 11:537–51. doi: 10.1016/j.stemcr.2018.07.004
242. Yang L, Liang P, Yang H, and Coyne CB. Trophoblast organoids with physiological polarity model placental structure and function. J Cell Sci. (2024) 137(5):1–9. doi: 10.1242/jcs.261528
243. Schermelleh L, Ferrand A, Huser T, Eggeling C, Sauer M, Biehlmaier O, et al. Super-resolution microscopy demystified. Nat Cell Biol. (2019) 21:72–84. doi: 10.1038/s41556-018-0251-8
244. Tojima T, Miyashiro D, Kosugi Y, and Nakano A. Super-resolution live imaging of cargo traffic through the golgi apparatus in mammalian cells. Methods Mol Biol. (2023) 2557:127–40. doi: 10.1007/978-1-0716-2639-9_10
245. Baranov MV, Olea RA, and van den Bogaart G. Chasing uptake: super-resolution microscopy in endocytosis and phagocytosis. Trends Cell Biol. (2019) 29:727–39. doi: 10.1016/j.tcb.2019.05.006
246. Shah HP and Devergne O. Confocal and super-resolution imaging of polarized intracellular trafficking and secretion of basement membrane proteins during drosophila oogenesis. J Vis Exp. (2022) (183):1–37. doi: 10.3791/63778
247. Arvan P and Halban PA. Sorting ourselves out: seeking consensus on trafficking in the beta-cell. Traffic. (2004) 5:53–61. doi: 10.1111/j.1600-0854.2004.00152.x
248. Gorr SU, Venkatesh SG, and Darling DS. Parotid secretory granules: crossroads of secretory pathways and protein storage. J Dent Res. (2005) 84:500–9. doi: 10.1177/154405910508400604
249. Meng EC, Goddard TD, Pettersen EF, Couch GS, Pearson ZJ, Morris JH, et al. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. (2023) 32:e4792. doi: 10.1002/pro.4792
250. Mesiano S. Endocrinology of human pregnancy and fetal-placental neuroendocrine development. In: Strauss JF III RB, Dokras A, Williams CJ, and Williams Z, editors. Yen & Jaffe’s reproductive endocrinology, 9 ed. Elsevier, Philadelphia, PA (2024). p. 254–76.e9.
251. Fuglsang J, Skjaerbaek C, Espelund U, Frystyk J, Fisker S, Flyvbjerg A, et al. Ghrelin and its relationship to growth hormones during normal pregnancy. Clin Endocrinol (Oxf). (2005) 62:554–9. doi: 10.1111/j.1365-2265.2005.02257.x
252. Hardie L, Trayhurn P, Abramovich D, and Fowler P. Circulating leptin in women: a longitudinal study in the menstrual cycle and during pregnancy. Clin Endocrinol (Oxf). (1997) 47:101–6. doi: 10.1046/j.1365-2265.1997.2441017.x
253. Farias DR, Poston L, Franco-Sena AB, Moura da Silva AA, Pinto T, de Oliveira LC, et al. Maternal lipids and leptin concentrations are associated with large-for-gestational-age births: a prospective cohort study. Sci Rep. (2017) 7:804. doi: 10.1038/s41598-017-00941-y
254. Caron P, Buscail L, Beckers A, Estève JP, Igout A, Hennen G, et al. Expression of somatostatin receptor SST4 in human placenta and absence of octreotide effect on human placental growth hormone concentration during pregnancy. J Clin Endocrinol Metab. (1997) 82:3771–6. doi: 10.1210/jcem.82.11.4350
255. Hunter A, Aitkenhead M, Caldwell C, McCracken G, Wilson D, and McClure N. Serum levels of vascular endothelial growth factor in preeclamptic and normotensive pregnancy. Hypertension. (2000) 36:965–9. doi: 10.1161/01.hyp.36.6.965
256. Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. (2008) 21:9–23. doi: 10.1080/14767050701830480
257. Raffin-Sanson ML, Ferré F, Coste J, Oliver C, Cabrol D, and Bertagna X. Pro-opiomelanocortin in human pregnancy: evolution of maternal plasma levels, concentrations in cord blood, amniotic fluid and at the feto-maternal interface. Eur J Endocrinol. (2000) 142:53–9. doi: 10.1530/eje.0.1420053
258. Campbell EA, Linton EA, Wolfe CD, Scraggs PR, Jones MT, and Lowry PJ. Plasma corticotropin-releasing hormone concentrations during pregnancy and parturition. J Clin Endocrinol Metab. (1987) 64:1054–9. doi: 10.1210/jcem-64-5-1054
259. Carr BR, Parker CR Jr., Madden JD, MacDonald PC, and Porter JC. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am J Obstet Gynecol. (1981) 139:416–22. doi: 10.1016/0002-9378(81)90318-5
260. Lockwood GM, Muttukrishna S, and Ledger WL. Inhibins and activins in human ovulation, conception and pregnancy. Hum Reprod Update. (1998) 4:284–95. doi: 10.1093/humupd/4.3.284
Keywords: human placenta, peptide hormones, secretion, pregnancy, cell biology
Citation: Ahmadi SM, Perez ML and Guardia CM (2025) Secretion of placental peptide hormones: functions and trafficking. Front. Endocrinol. 16:1584303. doi: 10.3389/fendo.2025.1584303
Received: 27 February 2025; Accepted: 12 May 2025;
Published: 12 June 2025.
Edited by:
Giovanni Tossetta, Marche Polytechnic University, ItalyReviewed by:
Yingshi Ouyang, Magee-Womens Research Institute, United StatesKate Rassie, Monash University, Australia
Copyright © 2025 Ahmadi, Perez and Guardia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos M. Guardia, Y2FybG9zLmd1YXJkaWFAbmloLmdvdg==
 Sadia M. Ahmadi
Sadia M. Ahmadi Maira L. Perez
Maira L. Perez Carlos M. Guardia
Carlos M. Guardia