- Department of Endocrinology, the First Medical Center of Chinese PLA General Hospital, Beijing, China
Thyroid-associated ophthalmopathy (TAO), a sight-threatening ocular condition intricately associated with autoimmune thyroid diseases, is the most common orbital disorder among adults. Accurate assessment of TAO is crucial for effective clinical management. However, the current evaluation system is hindered by significant subjectivity and a lack of standardized objective criteria, thereby complicating the pursuit of precise and individualized treatment strategies. Imaging techniques are integral to the clinical management of TAO, as they provide detailed anatomical visualization of the orbit and reflect underlying pathophysiological changes. This article reviews the applications of three prevalent imaging modalities—ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI)—in the diagnosis and management of TAO. We examine their respective advantages, limitations, and roles in disease diagnosis, staging, and evaluation of therapeutic efficacy, with the aim of providing a scientific basis for the optimization of clinical practice.
1 Introduction
Thyroid-associated ophthalmopathy (TAO) is an orbital disorder closely related to autoimmune thyroid diseases. The clinical presentation of TAO is multifaceted and varied, predominantly encompassing proptosis, eyelid retraction, and diplopia, among other symptoms. In severe instances, the condition may advance to dysthyroid optic neuropathy (DON). These manifestations not only alter patients’ physical appearance but also have detrimental psychological and social repercussions, thereby substantially diminishing their quality of life (1). A precise evaluation of the activity and severity of TAO is essential for formulating rational and effective therapeutic strategies.
Currently, the clinical evaluation of TAO predominantly employs assessments such as the Clinical Activity Score (CAS), NOSPECS severity classification, the European Group on Graves’ Orbitopathy (EUGOGO) severity classification, and the Graves’ Ophthalmopathy Quality of Life (GO-QoL) questionnaire for disease staging and grading (2). Nonetheless, these existing methodologies largely rely on subjective clinical symptoms and physical signs, which present certain limitations regarding accuracy and objectivity. Consequently, there is a pressing need for a more objective and precise assessment approach to enhance the diagnosis and management of TAO.
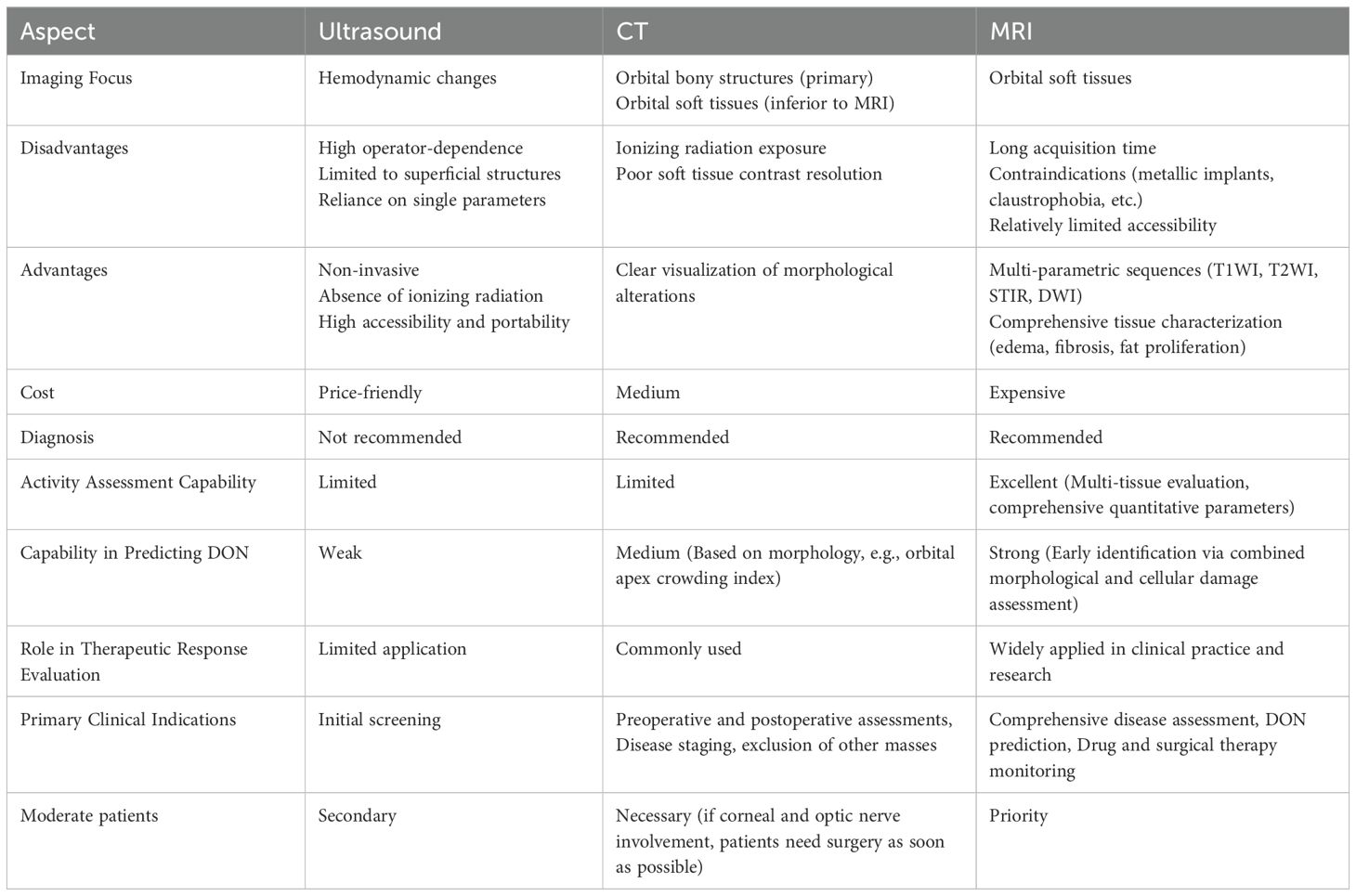
Imaging examinations offer a range of qualitative and quantitative indicators that can more objectively and comprehensively reflect alterations in the orbital structures and physiological conditions of TAO patients. While ultrasonography is primarily utilized to assess TAO by monitoring orbital hemodynamic changes, it is limited in its ability to visualize deeper structures, such as the extraocular muscles and the optic nerve. Computed tomography (CT) is excellent for measuring exophthalmometric values and the structure of extraocular muscles quantitatively. However, CT involves ionizing radiation and is not effective at visualizing soft tissue inflammation. On the other hand, magnetic resonance imaging (MRI) is a non-invasive method with high resolution for soft tissues, offering clear images of orbital structures. The multi-parametric imaging capabilities of MRI provide unique benefits for evaluating TAO. Imaging methods, especially MRI, are crucial for accurately assessing TAO, offering more dependable evidence for personalized diagnosis and treatment. The comparison of these techniques are summarized in Table 1. In the following, the applications of three imaging methods in TAO are reviewed in detail.
2 Application of ultrasonography in TAO
Color Doppler Imaging (CDI), a safe and non-invasive tool, allows real-time evaluation of hemodynamic features. In patients with active TAO, inflammation can cause endothelial cell damage (3), which inhibits vasoconstriction, leading to the dilation of orbital blood vessels and increased blood flow. Ultrasonography evaluation of the blood flow characteristics in the superior ophthalmic vein and the ophthalmic artery provides critical evidence for the diagnosis and assessment of TAO.
Research indicates that enlargement of the extraocular muscles in TAO patients may compress the orbital veins, resulting in decreased maximum blood flow velocity and volume in the superior ophthalmic vein compared to normal controls (4, 5). Notably, a minimum blood flow velocity of 3.99 cm/s in the superior ophthalmic vein serves as a significant marker for distinguishing between active and inactive TAO, with a diagnostic sensitivity of 91.2% and specificity of 81.2% (4). Furthermore, severe orbital venous stasis is strongly associated with the onset of DON, highlighting the potential utility of assessing superior ophthalmic vein blood flow in predicting the development of DON. In terms of arterial blood flow, the end-diastolic velocity of the ophthalmic artery in patients with TAO is significantly elevated compared to normal controls (4, 6), while the ophthalmic artery resistance index is reduced relative to normal controls (4). This resistance index serves as an indicator for evaluating changes in the condition before and after surgical treatment (7).
Although ultrasonography offers advantages such as rapidity, non-invasiveness, and cost-effectiveness, its accuracy and generalizability in clinical applications are constrained by factors such as vascular diameter, resistance, impedance, compliance, equipment performance, and patient cooperation.
3 Application of CT in TAO
CT has recognized advantages in bone structure imaging. It is an important evaluation method for patients with TAO before undergoing orbital decompression surgery, and provides a key basis for the operator to formulate the operation plan and determine the decompression range. In addition, with the development of imaging technology, CT has been gradually applied to the quantitative measurement of soft tissues such as extraocular muscles, intra-orbital fat tissue and lacrimal glands, providing objective indicators for diagnosis and assessment.
3.1 Application of CT in quantitative measurement of TAO
The extensive utilization of CT in evaluating TAO has introduced a novel approach for quantifying exophthalmos. In comparison to the conventional Hertel exophthalmometer, CT-based exophthalmometry not only broadens the scope of applicability but also exhibits notable advantages in terms of accuracy and objectivity (8–10). Nevertheless, the potential risk of ionizing radiation associated with CT constrains its extensive use in exophthalmos measurement. Quantitative assessments derived from CT imaging also facilitate the identification of potential imaging biomarkers and their association with clinical characteristics (11). Research indicates a positive correlation between the volume of extraocular muscles and the severity of TAO, with a marked increase observed progressively from healthy individuals to those with mild and severe TAO (12). Moreover, parameters associated with extraocular muscles (EOMs) exhibit significant correlations with visual function impairment and ocular motility deficits in patients with TAO (13). The quantitative assessment of EOMs and adipose tissue using CT is essential for the classification of TAO. Clinically, fat-dominant TAO is typically characterized by upper eyelid retraction, lower eyelid retraction, and exophthalmos, while muscle-dominant TAO is primarily associated with restricted ocular motility, diplopia, and strabismus (14). These distinct subtypes of TAO may be linked to different differentiation mechanisms of orbital fibroblasts (15).
3.2 Application of CT in diagnosis and prediction of TAO
The benefits of quantitative measurements using CT are further evidenced in the diagnostic and prognostic capabilities for TAO. Studies suggest that hypertrophy of the retro-orbicularis oculi fat and sub-orbicularis oculi fat may possess significant diagnostic value (16). Furthermore, volume-related parameters demonstrate high precision in predicting the severity of TAO, with an accuracy rate of 0.838 and an area under the curve (AUC) of 0.929 (12). Regression models developed based on parameters such as the total volume of extraocular muscles, lacrimal gland volume, intraorbital fat volume, and lacrimal gland density also exhibit significant diagnostic value in evaluating TAO activity (17). An observational study comprising 50 control subjects and 50 patients diagnosed with TAO has demonstrated that the extraocular muscle index is a reliable predictor of the overall inflammatory status in patients with TAO (18).
3.3 Application of CT in diagnosis of DON
DON is one of the most severe complications of TAO, and its clinical diagnosis lacks a standardized criteria, primarily relying on nonspecific symptoms such as vision loss, visual field defects, and color vision impairment (19, 20). Although the precise mechanism of DON is not fully elucidated, it is currently believed that mechanical compression of the optic nerve by orbital tissues, including extraocular muscles and intraorbital fat, is a major contributing factor. Statistics indicate that over 90% of DON cases are associated with optic nerve compression due to extraocular muscle hypertrophy (21). Consequently, improving the accuracy of early DON diagnosis is a clinical priority. CT imaging parameters are crucial in diagnosing DON. Current research highlights that optic nerve crowding (P<0.001) and intracranial fat prolapse (P<0.05) serve as independent risk factors for concurrent optic neuropathy in patients with TAO (22). Traditionally, the Barrett index has been utilized as a diagnostic indicator, demonstrating a sensitivity of 79% and a specificity of 72% (23). Among the volumes of individual extraocular muscles, the medial rectus volume emerges as the most robust predictor of DON, with a sensitivity of 73.7% and a specificity of 86.7% (24). The volumetric orbital apex crowding index (VACI) exhibits superior diagnostic efficacy, with a sensitivity of 92%, specificity of 86%, and an accuracy of 88% (25). Furthermore, the combined use of VACI and thyrotropin receptor antibodies (TRAb) levels may enhance the predictive accuracy for DON (26). A retrospective study further illustrated the exceptional diagnostic efficacy for detecting DON through the application of machine-learning radiomics analysis on CT scans (27).
3.4 Application of CT in efficacy evaluation of TAO
The condition of TAO can be assessed through changes observed in exophthalmos and parameters related to the extraocular muscles and orbital fat, as measured by CT. This imaging modality has become increasingly utilized for evaluating treatment efficacy and optimizing therapeutic strategies (28–31). For example, the diameter of the extraocular muscles and the muscle diameter index may serve as predictors for the effect of extraocular muscle contraction following retrobulbar glucocorticoid injections (32). This highlights the significant clinical importance of quantitative CT analysis in the personalized treatment of TAO.
In conclusion, CT was utilized to assess alterations in orbital structure, thereby providing essential support in the diagnosis of TAO and its complications, and offering a reliable foundation for clinical decision-making.
4 Application of MRI in TAO
In comparison to ultrasound and CT, MRI offers unparalleled value in both clinical research and practical applications related to TAO. This is attributed to its superior soft tissue resolution, multiparametric imaging capabilities, and lack of ionizing radiation. MRI facilitates precise visualization of morphological changes in structures such as extraocular muscles, adipose tissue, lacrimal glands, and the optic nerve. Additionally, it allows for a comprehensive evaluation of pathological characteristics in affected tissues through multi-sequence and multiparametric imaging techniques (33). These advantages position MRI as an objective foundation for accurate diagnosis, assessment of disease severity, and monitoring of therapeutic interventions in the management of TAO.
4.1 Multimodal application of MRI in the evaluation of extraocular muscles in TAO
Multimodal MRI technology offers a distinctive approach for the comprehensive evaluation of extraocular muscles involvement in TAO by integrating structural, functional, and quantitative imaging modalities. This technology not only diagnoses and assesses disease severity through structural parameters such as extraocular muscles cross-sectional area and volume (34), but also facilitates the monitoring of disease progression via functional imaging and quantitative analyses. While conventional T2-weighted imaging (T2WI) does not reveal significant differences in extraocular muscles hyperintensity between active and inactive TAO patients (35, 36), the signal intensity ratio of extraocular muscles to ipsilateral temporal muscle [SIR (EOM/temporalis)] or SIR of EOM to ipsilateral cerebral white matter demonstrates a correlation with the CAS, thereby providing additional evidence for disease staging (37–39).
Dynamic contrast-enhanced MRI (DCE-MRI) is utilized to quantify tissue microcirculation, with its parameters serving as reliable biomarkers for evaluating disease activity (40). Research indicates distinct microcirculatory characteristics in extraocular muscles across different stages of TAO (41, 42). Several DCE-MRI parameters exhibit significant correlations with the CAS and demonstrate positive predictive values of approximately 90% for disease activity (35). Hu’s study further substantiates the clinical utility of DCE-MRI in monitoring TAO (43). Diffusion-weighted imaging (DWI) assesses restricted water diffusion in extraocular muscles via the apparent diffusion coefficient (ADC), facilitating the early detection of inflammatory changes during asymptomatic phases (44). Patients with TAO show significantly elevated mean ADC values in extraocular muscles compared to healthy controls (45), with ADC values in the medial, inferior, and lateral rectus muscles positively correlating with inflammatory subscores in the VISA classification system (46). Nonetheless, the association between ADC values and CAS remains a subject of debate in the literature (44, 45). Diffusion tensor imaging (DTI) serves as an important instrument in evaluating the disease activity associated with TAO (47).
Quantitative imaging modalities, such as T2 mapping, facilitate the sensitive detection of early extraocular muscle involvement (48). T1 mapping further elucidates fat infiltration patterns that are strongly correlated with refractory diplopia, demonstrating high diagnostic accuracy (AUC=0.89) in patients with TAO who have a history of diplopia resolution (49). The extracellular volume (ECV) functions as an objective quantitative biomarker for evaluating the extent of extraocular muscle fibrosis (50).
The integrative application of multimodal MRI technologies significantly enhances diagnostic precision and dynamic monitoring capabilities for TAO-related extraocular muscle pathology, thereby refining the comprehensive MRI-based evaluation framework for the pathological progression of TAO.
4.2 Applications of MRI in the assessment of orbital adipose tissue and exophthalmos in TAO
Orbital fibroblasts exhibit the potential for adipogenic differentiation, and the subsequent expansion of adipose tissue volume is strongly correlated with the presence of exophthalmos. Quantitative MRI analyses have demonstrated that both total orbital adipose tissue volume (r=0.70, P=0.0006) and anterior orbital adipose tissue volume (r=0.64, P=0.0023) show significantly stronger correlations with the severity of exophthalmos compared to extraocular muscle volume (r=0.58, P=0.008). This suggests that adipose hyperplasia is a key factor in the progression of proptosis (51). Consequently, therapies targeting adipose metabolic pathways may represent promising new strategies for mitigating proptosis in patients with TAO. Nevertheless, no significant associations were found between orbital adipose volume and variables such as patient age, disease duration, or CAS (52).
4.3 Applications of MRI in the assessment of lacrimal gland in TAO
Patients with TAO often exhibit symptoms indicative of lacrimal gland dysfunction, such as dry eyes and conjunctival hyperemia. Recent research has increasingly identified the lacrimal gland as a significant target organ in TAO. The expression of thyroid-stimulating hormone receptor (TSHR) by lacrimal gland acinar cells suggests that autoimmune responses in TAO may lead to damage of the lacrimal gland (53). Studies have demonstrated that patients with TAO not only experience altered tear composition (54–56), but also exhibit increased lacrimal gland volumes (57, 58). Although approximately 30% of TAO patients present with lacrimal gland enlargement (59), no relevant morphological parameters of lacrimal gland have been identified as effective in differentiating between active and inactive TAO (57, 60, 61). Further investigations have shown that the extent of lacrimal gland protrusion serves as a critical imaging marker for differentiating disease stages in TAO. Patients with active TAO exhibit significantly greater lacrimal gland protrusion compared to those with inactive disease, with a linear correlation observed with TRAb levels (62). Moreover, the degree of lacrimal gland prolapse positively correlates with the CAS, proptosis, and extraocular muscle volume, providing an additional indicator for assessing TAO activity (63).
Functional imaging modalities are instrumental in assessing disease activity in TAO. DTI has revealed significant variations in the ADC values and fractional anisotropy (FA) of the lacrimal gland when comparing TAO patients to healthy controls, as well as between active and inactive TAO subgroups (64). The combination of lacrimal gland T2 mapping values with clinical parameters has the potential to optimize the CAS system (65). Furthermore, quantitative MRI parameters are capable of detecting fat infiltration and fibrosis within the lacrimal gland. Wu et al. demonstrated that certain inflammatory and fibrosis-related markers in the lacrimal gland are markedly elevated in TAO patients relative to those with Graves’ disease, facilitating the differentiation between TAO and Graves’ disease (66). These findings offer novel insights into the pathological mechanisms underlying TAO and provide a foundation for the development of personalized therapeutic strategies.
4.4 Multimodal application of MRI in the evaluation of DON
MRI exhibits significant technical advantages in the evaluation of DON, offering essential imaging support for early diagnosis and precise therapeutic intervention. The Dixon- T2WI technique effectively suppresses the interference of orbital fat signals, thereby enhancing the clarity of optic nerve visualization and minimizing artifacts, demonstrating superior sensitivity and specificity compared to regular MRI sequences (67). Functional imaging provides further insights into the pathological mechanisms underlying DON. Reduced ADC values in the optic nerve indicate secondary ischemic alterations due to mechanical compression (68). DTI reveals significantly increased axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD) (P=0.003-0.033), along with decreased FA (P=0.018), quantitatively indicating disruption of optic nerve axonal integrity and glial cell damage. Additionally, DTI demonstrates robust diagnostic performance, with an AUC of 0.801 (69). Another study also verified the above view (70).
The integration of multimodal MRI has markedly improved diagnostic accuracy. Specifically, the SIR of the optic nerve 3mm posterior to the globe and the orbital apex extraocular muscle index have been identified as independent risk predictors for DON. A combined model incorporating these parameters exhibited excellent diagnostic performance, with an AUC of 0.943 (71), thereby providing an objective basis for the early identification of high-risk patients. Additionally, studies have indicated potential microstructural alterations within the visual pathway of patients with DON. Further integration of orbital MRI with diffusion kurtosis imaging (DKI) for the assessment of the intracranial visual pathway could significantly enhance comprehensive diagnostic capabilities (68, 72). These investigations not only advance the understanding of the mechanisms underlying optic nerve injury but also offer critical references for the early detection and intervention of optic neuropathies.
4.5 Application of MRI in efficacy evaluation of TAO
MRI has been extensively utilized in clinical and scientific research to assess therapeutic efficacy. MRI-based assessments of morphological metrics in extraocular muscles and adipose tissue serve as valuable tools for evaluating treatment responsiveness (73). The study further corroborated the effectiveness of corticosteroid therapy in conjunction with orbital radiotherapy in Asian patients with active moderate-to-severe TAO (73). Moreover, the SIR of the levator palpebrae muscle has shown predictive utility for the efficacy of triamcinolone acetonide injections, achieving a sensitivity of 87.5%, specificity of 66.7%, and an AUC of 0.840 (74). The combination of extraocular muscle SIR with serum lipid metabolism parameters may enhance the prediction of responses to glucocorticoid (GC) therapy in patients with active and moderate-to-severe TAO (75). A retrospective study has also identified that the percentage change in SIR(EOM/temporalis)MAX following tocilizumab treatment may serve as a predictive indicator for the necessity of surgical intervention in patients with hormone-resistant DON (76). Additionally, DCE-MRI parameters reveal significant distinctions between responders and non-responders to GC therapy in active TAO, providing crucial insights into the effects of TAO-related microcirculatory alterations on treatment outcomes (43, 77). Despite the limited sample size in some studies, these research findings offer a scientific foundation for developing individualized treatment strategies. Given the characteristics of MRI, which include multiple sequences and parameters, MRI holds greater significance than CT in optimizing the therapeutic efficacy of TAO.
5 Conclusion
Imaging modalities are integral to the disease assessment, therapeutic monitoring, and early diagnosis of DON in TAO. Techniques such as ultrasound, CT, and MRI each offer distinct advantages in assessing hemodynamic changes, extraocular muscles, adipose tissue, and the optic nerve. MRI, in particular, not only provides detailed visualization of orbital soft tissue structures through conventional sequences but also utilizes functional imaging techniques and quantitative analyses to evaluate pathological changes such as tissue edema, fat infiltration, and fibrosis. This capability facilitates the identification of diverse imaging biomarkers crucial for disease staging and treatment monitoring. Recent advancements in radiomics have markedly advanced research in the diagnosis and management of TAO (78–80). Additionally, emerging neuroimaging studies have revealed altered functional activity in brain regions, including the insular cortex, inferior temporal gyrus, and superior frontal gyrus, in patients with TAO. These changes may be associated with visual function and peripheral immune status (81–84). These findings provide a foundation for further exploration of the mechanisms underlying TAO and DON.
Nevertheless, the clinical application of imaging technologies encounters several challenges. The prolonged duration of MRI scans, coupled with their high cost, restricts their accessibility for primary care and dynamic monitoring purposes. Additionally, certain functional imaging parameters necessitate validation in larger cohorts. Future research should prioritize the comprehensive exploration and validation of imaging techniques within larger populations to fully realize their potential in the early diagnosis of TAO, personalized treatment, and mechanistic investigations. This approach aims to ultimately offer comprehensive and effective support for clinical practice.
Author contributions
ZXX: Methodology, Conceptualization, Writing – review & editing, Writing – original draft. ZX: Conceptualization, Writing – review & editing. ZL: Project administration, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cockerham KP, Padnick-Silver L, Stuertz N, Francis-Sedlak M, and Holt RJ. Quality of life in patients with chronic thyroid eye disease in the United States. Ophthalmol Ther. (2021) 10:975–87. doi: 10.1007/s40123-021-00385-8
2. Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. (2021) 185:G43–67. doi: 10.1530/EJE-21-0479
3. Morikawa Y, Morikawa A, and Makino I. Relationship of thyroid states and serum thrombomodulin (TM) levels in patients with Graves’ disease: TM, a possible new marker of the peripheral activity of thyroid hormones. J Clin Endocrinol Metab. (1993) 76:609–14. doi: 10.1210/jcem.76.3.7680353
4. Jamshidian-Tehrani M, Nabavi A, Kasaee A, Hasanpoor N, Elhami E, Sharif-Kashani S, et al. Color Doppler imaging in thyroid eye disease and its correlation to disease activity. Orbit. (2019) 38:440–5. doi: 10.1080/01676830.2018.1556704
5. Somer D. Color doppler imaging of superior ophthalmic vein in thyroid-associated eye disease. Japanese J Ophthalmol. (2002) 46:341–5. doi: 10.1016/S0021-5155(02)00485-9
6. Alp MN. Color Doppler imaging of the orbital vasculature in Graves’ disease with computed tomographic correlation. Br J Ophthalmol. (2000) 84:1027–30. doi: 10.1136/bjo.84.9.1027
7. Pérez-López M, Sales-Sanz M, Rebolleda G, Casas-Llera P, González-Gordaliza C, Jarrín E, et al. Retrobulbar ocular blood flow changes after orbital decompression in graves’ Ophthalmopathy measured by color doppler imaging. Invest Ophthalmol Vis Sci. (2011) 52:5612. doi: 10.1167/iovs.10-6907
8. Bingham CM, Sivak-Callcott JA, Gurka MJ, Nguyen J, Hogg JP, Feldon SE, et al. Axial globe position measurement. Ophthalmic Plast Reconstructive Surg. (2016) 32:106–12. doi: 10.1097/IOP.0000000000000437
9. Choi KJ and Lee MJ. Comparison of exophthalmos measurements: Hertel exophthalmometer versus orbital parameters in 2-dimensional computed tomography. Can J Ophthalmol. (2018) 53:384–90. doi: 10.1016/j.jcjo.2017.10.015
10. Guo J, Qian J, and Yuan Y. Computed tomography measurements as a standard of exophthalmos? Two-dimensional versus three-dimensional techniques. Curr Eye Res. (2018) 43:647–53. doi: 10.1080/02713683.2018.1431285
11. Watanabe EM, Cavazzana RY, Ribeiro DDAM, Brandão LC, Haddad AV, Corrente JE, et al. Morphometric analysis of extraocular muscles and proptosis by computed tomography in Graves’ orbitopathy. Radiol Bras. (2024) 57:e20240040en. doi: 10.1590/0100-3984.2024.0040-en
12. Alkhadrawi AM, Lin LY, Langarica SA, Kim K, Ha SK, Lee NG, et al. Deep-learning based automated segmentation and quantitative volumetric analysis of orbital muscle and fat for diagnosis of thyroid eye disease. Invest Ophthalmol Vis Sci. (2024) 65:6. doi: 10.1167/iovs.65.5.6
13. Chaganti S, Mundy K, DeLisi MP, Nelson KM, Harrigan RL, Galloway RL, et al. Assessment of orbital computed tomography (CT) imaging biomarkers in patients with thyroid eye disease. J Digit Imaging. (2019) 32:987–94. doi: 10.1007/s10278-019-00195-2
14. Kim HC, Yoon SW, and Lew H. Usefulness of the ratio of orbital fat to total orbit area in mild-to-moderate thyroid-associated ophthalmopathy. BJR. (2015) 88:20150164. doi: 10.1259/bjr.20150164
15. Xiong C, Ren Z, Li X, Jin Q, Wang S, Gan P, et al. Orbital computed tomography imaging characteristics of thyroid-associated ophthalmopathy. Sci Rep. (2024) 14:28960. doi: 10.1038/s41598-024-76624-2
16. Thornton IL, Clark J, Sokol JA, Hite M, and Nunery WR. Radiographic evidence of prominent retro and suborbicularis oculi fat in thyroid-associated orbitopathy. Orbit. (2016) 35:35–8. doi: 10.3109/01676830.2015.1099689
17. Byun JS, Moon NJ, and Lee JK. Quantitative analysis of orbital soft tissues on computed tomography to assess the activity of thyroid-associated orbitopathy. Graefes Arch Clin Exp Ophthalmol. (2017) 255:413–20. doi: 10.1007/s00417-016-3538-0
18. Wu F, Huang J, Wang M, Qian Z, Wang Y, and Fang W. Extraocular muscle index as a novel indicator of inflammatory condition in graves’ ophthalmopathy patients. Front Endocrinol. (2025) 16:1594828. doi: 10.3389/fendo.2025.1594828
19. Saeed P, Tavakoli Rad S, and Bisschop PHLT. Dysthyroid optic neuropathy. Ophthalmic Plast Reconstructive Surg. (2018) 34:S60–7. doi: 10.1097/IOP.0000000000001146
20. Blandford AD, Zhang D, Chundury RV, and Perry JD. Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol. (2017) 12:111–21. doi: 10.1080/17469899.2017.1276444
21. Dolman PJ. Dysthyroid optic neuropathy: evaluation and management. J Endocrinol Invest. (2021) 44:421–9. doi: 10.1007/s40618-020-01361-y
22. Giaconi JA, Kazim M, Rho T, and Pfaff C. CT scan evidence of dysthyroid optic neuropathy. Ophthalmic Plast Reconstructive Surg. (2002) 18:177–82. doi: 10.1097/00002341-200205000-00005
23. Monteiro MLR, Gonçalves ACP, Silva CTM, Moura JP, Ribeiro CS, and Gebrim EMMS. Diagnostic ability of Barrett’s index to detect dysthyroid optic neuropathy using multidetector computed tomography. Clinics. (2008) 63:301–6. doi: 10.1590/S1807-59322008000300003
24. Berger M, Matlach J, Pitz S, Berres M, Axmacher F, Kahaly GJ, et al. Imaging of the medial rectus muscle predicts the development of optic neuropathy in thyroid eye disease. Sci Rep. (2022) 12:6259. doi: 10.1038/s41598-022-10043-z
25. Pieroni Gonçalves AC, Silva LN, Gebrim EMMS, Matayoshi S, and Monteiro MLR. Predicting dysthyroid optic neuropathy using computed tomography volumetric analyses of orbital structures. Clinics. (2012) 67:891–6. doi: 10.6061/clinics/2012(08)06
26. Deng Z, Chen L, Tan J, Wang S, Liu D, Wang J, et al. Combination model of thyrotrophin receptor antibody and volumetric orbital apex crowding index as an indicator of dysthyroid optic neuropathy. Dis Markers. (2021) 2021:1–7. doi: 10.1155/2021/9964232
27. Ma L, Jiang X, Yang X, Wang M, Hou Z, Zhang J, et al. CT-based machine learning radiomics analysis to diagnose dysthyroid optic neuropathy. Semin Ophthalmol. (2025) 1–7. doi: 10.1080/08820538.2025.2463948
28. Kim M, Chang JH, and Lee NK. Quantitative analysis of extraocular muscle volume and exophthalmos reduction after radiation therapy to treat Graves’ ophthalmopathy: A pilot study. Eur J Ophthalmol. (2021) 31:340–5. doi: 10.1177/1120672119873841
29. Kim JW, Han SH, Son BJ, Rim TH, Keum KC, and Yoon JS. Efficacy of combined orbital radiation and systemic steroids in the management of Graves’ orbitopathy. Graefes Arch Clin Exp Ophthalmol. (2016) 254:991–8. doi: 10.1007/s00417-016-3280-7
30. Reshef ER, Marsiglia M, Bouhadjer K, Chiou CA, O’Brien-Coon D, Reinshagen KL, et al. Reduction in extraocular muscle cross-sectional area and correlation with extraocular motility and diplopia following teprotumumab for thyroid eye disease. Ophthalmic Plast Reconstructive Surg. (2023) 39:433–39. doi: 10.1097/IOP.0000000000002337
31. La Rocca M, Leonardi B, Lo Greco M, Marano G, Milazzotto R, Liardo R, et al. Orbital radiotherapy for graves’ Ophthalmopathy: single institutional experience of efficacy and safety. Diseases. (2025) 13:61. doi: 10.3390/diseases13020061
32. Yang J, Chen J, Shi B, You Y, Pi X, Zhao G, et al. Effects of various extraocular muscle enlargement patterns on muscle diameter index in graves ophthalmopathy patients: a retrospective cohort study. Sci Rep. (2023) 13:16939. doi: 10.1038/s41598-023-43942-w
33. Čivrný J, Karhanová M, Hübnerová P, Schovánek J, and Heřman M. MRI in the assessment of thyroid-associated orbitopathy activity. Clin Radiol. (2022) 77:925–34. doi: 10.1016/j.crad.2022.08.124
34. Su Y, Liu X, Fang S, Huang Y, Li Y, Zhong S, et al. Age-related difference in extraocular muscles and its relation to clinical manifestations in an ethnically homogenous group of patients with Graves’ orbitopathy. Graefes Arch Clin Exp Ophthalmol. (2022) 260:583–9. doi: 10.1007/s00417-021-05377-9
35. Jiang H, Wang Z, Xian J, Li J, Chen Q, and Ai L. Evaluation of rectus extraocular muscles using dynamic contrast-enhanced MR imaging in patients with Graves’ ophthalmopathy for assessment of disease activity. Acta Radiol. (2012) 53:87–94. doi: 10.1258/ar.2011.110431
36. Sillaire I, Ravel A, Dalens H, Garcier JM, and Boyer L. Graves’ophthalmopathy: usefulness of T2 weighted muscle signal intensity. J Radiol. (2003) 84:139–42.
37. Kirsch EC, Kaim AH, De Oliveira MG, and Von Arx G. Correlation of signal intensity ratio on orbital MRI-TIRM and clinical activity score as a possible predictor of therapy response in Graves’ orbitopathy—a pilot study at 1.5 T. Neuroradiology. (2010) 52:91–7. doi: 10.1007/s00234-009-0590-z
38. Ge Q, Zhang X, Wang L, Fan Y, Huang Q, Yao N, et al. Quantitative evaluation of activity of thyroid-associated Ophthalmopathy using short-tau inversion recovery (STIR) sequence. BMC Endocr Disord. (2021) 21:226. doi: 10.1186/s12902-021-00895-3
39. Sun AL, Peng R, and Hao P. The value of signal intensity ratios of orbital tissue to white matter of orbital MRI in evaluating Graves’ orbitopathy. Int Ophthalmol. (2024) 45:14. doi: 10.1007/s10792-024-03385-2
40. Hodgson RJ, O’Connor P, and Moots R. MRI of rheumatoid arthritis image quantitation for the assessment of disease activity, progression and response to therapy. Rheumatology. (2008) 47:13–21. doi: 10.1093/rheumatology/kem250
41. Cakirer S, Cakirer D, Basak M, Durmaz S, Altuntas Y, and Yigit U. Evaluation of extraocular muscles in the edematous phase of graves ophthalmopathy on contrast-enhanced fat-suppressed magnetic resonance imaging. J Comput Assisted Tomography. (2004) 28:80–6. doi: 10.1097/00004728-200401000-00013
42. Taoka T, Sakamoto M, Nakagawa H, Fukusumi A, Iwasaki S, Taoka K, et al. Evaluation of extraocular muscles using dynamic contrast enhanced MRI in patients with chronic thyroid orbitopathy. J Comput Assisted Tomography. (2005) 29:115–20. doi: 10.1097/01.rct.0000146112.56194.24
43. Hu H, Pu X-Y, Zhou J, Jiang W-H, Wu Q, Lu J-L, et al. Determining disease activity and glucocorticoid response in thyroid-associated ophthalmopathy: preliminary study using dynamic contrast-enhanced MRI. Korean J Radiol. (2024) 25:1070. doi: 10.3348/kjr.2024.0335
44. Politi LS, Godi C, Cammarata G, Ambrosi A, Iadanza A, Lanzi R, et al. Magnetic resonance imaging with diffusion-weighted imaging in the evaluation of thyroid-associated orbitopathy: getting below the tip of the iceberg. Eur Radiol. (2014) 24:1118–26. doi: 10.1007/s00330-014-3103-3
45. Kilicarslan R, Alkan A, Ilhan MM, Yetis H, Aralasmak A, and Tasan E. Graves’ ophthalmopathy: the role of diffusion-weighted imaging in detecting involvement of extraocular muscles in early period of disease. BJR. (2015) 88:20140677. doi: 10.1259/bjr.20140677
46. Singh M, Rana N, Ahuja C, Gupta P, and Zadeng Z. Correlation of clinical and radiological scores for evaluation of activity in patients having thyroid-associated orbitopathy: A prospective observational study. Indian J Ophthalmol. (2024) 72:844–8. doi: 10.4103/IJO.IJO_1702_23
47. Tang CY, Huang Q, Liang L, Zhang MQ, Zheng XY, and Long J. Application of apparent diffusion coefficient of extraocular muscles from diffusion tensor imaging scanning in the assessment of disease activity of thyroid eye disease. BMC Endocr Disord. (2024) 24:276. doi: 10.1186/s12902-024-01818-8
48. Liu P, Chen L, Wang Q, Luo B, Su H, Yuan G, et al. Histogram analysis of T2 mapping for detecting early involvement of extraocular muscles in patients with thyroid-associated ophthalmopathy. Sci Rep. (2020) 10:19445. doi: 10.1038/s41598-020-76341-6
49. Matsuzawa K, Izawa S, Kato A, Fukaya K, Matsumoto K, Okura T, et al. Low signal intensities of MRI T1 mapping predict refractory diplopia in Graves’ ophthalmopathy. Clin Endocrinol. (2020) 92:536–44. doi: 10.1111/cen.14178
50. Ma R, Geng Y, Gan L, Peng Z, Cheng J, Guo J, et al. Quantitative T1 mapping MRI for the assessment of extraocular muscle fibrosis in thyroid-associated ophthalmopathy. Endocrine. (2022) 75:456–64. doi: 10.1007/s12020-021-02873-0
51. Nishida Y, Tian S, Isberg B, Hayashi O, Tallstedt L, and Lennerstrand G. Significance of orbital fatty tissue for exophthalmos in thyroid-associated ophthalmopathy. Graefe’s Arch Clin Exp Ophthalmol. (2002) 240:515–20. doi: 10.1007/s00417-002-0498-3
52. Kaichi Y, Tanitame K, Terada H, Itakura H, Ohno H, Yoneda M, et al. Thyroid-associated orbitopathy: quantitative evaluation of the orbital fat volume and edema using IDEAL-FSE. Eur J Radiol Open. (2019) 6:182–6. doi: 10.1016/j.ejro.2019.05.003
53. Eckstein AK, Finkenrath A, Heiligenhaus A, Renzing-Köhler K, Esser J, Krüger C, et al. Dry eye syndrome in thyroid-associated ophthalmopathy: lacrimal expression of TSH receptor suggests involvement of TSHR-specific autoantibodies. Acta Ophthalmologica Scandinavica. (2004) 82:291–7. doi: 10.1111/j.1395-3907.2004.00268.x
54. Aass C, Norheim I, Eriksen EF, Børnick EC, Thorsby PM, and Pepaj M. Comparative proteomic analysis of tear fluid in Graves’ disease with and without orbitopathy. Clin Endocrinol. (2016) 85:805–12. doi: 10.1111/cen.13122
55. Shu X, Zeng C, Zhu Y, Chen Y, Huang X, and Wei R. Screening of pathologically significant diagnostic biomarkers in tears of thyroid eye disease based on bioinformatic analysis and machine learning. Front Cell Dev Biol. (2024) 12:1486170. doi: 10.3389/fcell.2024.1486170
56. Shi T-T, Zhao R-X, Xin Z, Hou Z-J, Wang H, Xie R-R, et al. Tear-derived exosomal biomarkers of Graves’ ophthalmopathy. Front Immunol. (2022) 13:1088606. doi: 10.3389/fimmu.2022.1088606
57. Bingham CM, Harris MA, Realini T, Nguyen J, Hogg JP, and Sivak-Callcott JA. Calculated computed tomography volumes of lacrimal glands and comparison to clinical findings in patients with thyroid eye disease. Ophthalmic Plast Reconstructive Surg. (2014) 30:116–8. doi: 10.1097/IOP.0000000000000015
58. Huh H-D, Kim J-H, Kim S-J, Yoo J-M, and Seo S-W. The change of lacrimal gland volume in Korean patients with thyroid-associated ophthalmopathy. Korean J Ophthalmol. (2016) 30:319. doi: 10.3341/kjo.2016.30.5.319
59. Rana K and Caltabiano C. Lacrimal gland enlargement in thyroid eye disease. Int Ophthalmol. (2024) 44:43. doi: 10.1007/s10792-024-03352-x
60. Harris MA, Realini T, Hogg JP, and Sivak-Callcott JA. CT dimensions of the lacrimal gland in graves orbitopathy. Ophthalmic Plast Reconstructive Surg. (2012) 28:69–72. doi: 10.1097/IOP.0b013e31823c4a3a
61. Hu H, Xu X-Q, Wu F-Y, Chen H-H, Su G-Y, Shen J, et al. Diagnosis and stage of Graves’ ophthalmopathy: Efficacy of quantitative measurements of the lacrimal gland based on 3-T magnetic resonance imaging. Exp Ther Med. (2016) 12:725–9. doi: 10.3892/etm.2016.3389
62. Gagliardo C, Radellini S, Morreale Bubella R, Falanga G, Richiusa P, Vadalà M, et al. Lacrimal gland herniation in Graves ophthalmopathy: a simple and useful MRI biomarker of disease activity. Eur Radiol. (2020) 30:2138–41. doi: 10.1007/s00330-019-06570-5
63. Gao Y, Chang Q, Li Y, Zhang H, Hou Z, Zhang Z, et al. Correlation between extent of lacrimal gland prolapse and clinical features of thyroid-associated ophthalmopathy: a retrospective observational study. BMC Ophthalmol. (2022) 22:66. doi: 10.1186/s12886-022-02270-9
64. Chen L, Hu H, Chen W, Wu Q, Zhou J, Chen H-H, et al. Usefulness of readout-segmented EPI-based diffusion tensor imaging of lacrimal gland for detection and disease staging in thyroid-associated ophthalmopathy. BMC Ophthalmol. (2021) 21:281. doi: 10.1186/s12886-021-02044-9
65. Jiang M, Song X, Zhang H, Tao X, Yang G, Wang Y, et al. The combination of T2-mapping value of lacrimal gland and clinical indicators can improve the stage prediction of Graves’ ophthalmopathy compared to clinical activity scores. Endocrine. (2022) 78:321–8. doi: 10.1007/s12020-022-03167-9
66. Wu D, Zhu H, Hong S, Li B, Zou M, Ma X, et al. Utility of multi-parametric quantitative magnetic resonance imaging of the lacrimal gland for diagnosing and staging Graves’ ophthalmopathy. Eur J Radiol. (2021) 141:109815. doi: 10.1016/j.ejrad.2021.109815
67. Ollitrault A, Charbonneau F, Herdan M-L, Bergès O, Zuber K, Giovansili L, et al. Dixon-T2WI magnetic resonance imaging at 3 tesla outperforms conventional imaging for thyroid eye disease. Eur Radiol. (2021) 31:5198–205. doi: 10.1007/s00330-020-07540-y
68. Hu H, Zhou J, Jiang W-H, Wu Q, Pu X-Y, Liu H, et al. Diagnosis of dysthyroid optic neuropathy: combined value of orbital MRI and intracranial visual pathway diffusion kurtosis imaging. Eur Radiol. (2024) 34:5401–11. doi: 10.1007/s00330-024-10615-9
69. Liu P, Luo B, Zhai L, Wu H-Y, Wang Q-X, Yuan G, et al. Multi-parametric diffusion tensor imaging of the optic nerve for detection of dysthyroid optic neuropathy in patients with thyroid-associated ophthalmopathy. Front Endocrinol. (2022) 13:851143. doi: 10.3389/fendo.2022.851143
70. Moledina M, Lee V, Bhatia K, Madani G, Feeney C, George N, et al. Radiological activity score (RAS)—MRI characteristics in dysthyroid optic neuropathy in a multi-ethnic thyroid eye disease population. Clin Endocrinol. (2025) 103:385–95. doi: 10.1111/cen.15272
71. Song C, Luo Y, Huang W, Duan Y, Deng X, Chen H, et al. Extraocular muscle volume index at the orbital apex with optic neuritis: a combined parameter for diagnosis of dysthyroid optic neuropathy. Eur Radiol. (2023) 33:9203–12. doi: 10.1007/s00330-023-09848-x
72. Zhou J, Liu J, Lu J-L, Pu X-Y, Chen H-H, Liu H, et al. White-matter alterations in dysthyroid optic neuropathy: a diffusion kurtosis imaging study using tract-based spatial statistics. Jpn J Radiol. (2024) 43:603–11. doi: 10.1007/s11604-024-01710-4
73. Xu L, Li L, Xie C, Guan M, and Xue Y. Thickness of extraocular muscle and orbital fat in MRI predicts response to glucocorticoid therapy in Graves’ Ophthalmopathy. Int J Endocrinol. (2017) 2017:1–8. doi: 10.1155/2017/3196059
74. Duan M, Xu D-D, Zhou H-L, Fang H-Y, Meng W, Wang Y-N, et al. Triamcinolone acetonide injection in the treatment of upper eyelid retraction in Graves’ ophthalmopathy evaluated by 3.0 Tesla magnetic resonance imaging. Indian J Ophthalmol. (2022) 70:1736–41. doi: 10.4103/ijo.IJO_2228_21
75. Zhang H, Hu H, Wang Y, Duan X, Chen L, Zhou J, et al. Predicting glucocorticoid effectiveness in thyroid eye disease: combined value from serological lipid metabolism and an orbital MRI parameter. Eur Thyroid J. (2024) 13:e230109. doi: 10.1530/ETJ-23-0109
76. Shu W, Yan M, Gan L, Li L, Tao H, Peng Z, et al. Key indicators for guiding tocilizumab therapy to prevent orbital decompression surgery in hormone-resistant dysthyroid optic neuropathy. Front Immunol. (2025) 16:1556742. doi: 10.3389/fimmu.2025.1556742
77. Xia D, Chew SP, Zhang H, Li R, Sun J, Li Y, et al. Contrast-enhanced orbital MRI for activity assessment and treatment response prediction in thyroid eye disease. Eur J Radiol. (2025) 188:112136. doi: 10.1016/j.ejrad.2025.112136
78. Ma Z-C, Lin J-Y, Li S-K, Liu H-J, and Zhang Y-Q. Deep learning methods for diagnosis of Graves’ ophthalmopathy using magnetic resonance imaging. Quant Imaging Med Surg. (2024) 14:5099–108. doi: 10.21037/qims-24-80
79. Lin C, Song X, Li L, Li Y, Jiang M, Sun R, et al. Detection of active and inactive phases of thyroid-associated ophthalmopathy using deep convolutional neural network. BMC Ophthalmol. (2021) 21:39. doi: 10.1186/s12886-020-01783-5
80. Zhang H, Jiang M, Chan HC, Zhang H, Xu J, Liu Y, et al. Whole-orbit radiomics: machine learning-based multi- and fused- region radiomics signatures for intravenous glucocorticoid response prediction in thyroid eye disease. J Transl Med. (2024) 22:56. doi: 10.1186/s12967-023-04792-2
81. Zhang H, Liu Y, Xia D, Jiang M, Li Y, Sun J, et al. The insular cortex is not insular in thyroid eye disease: neuroimaging revelations of central–peripheral system interaction. J Neuroinflamm. (2024) 21:51. doi: 10.1186/s12974-024-03044-4
82. Zhang H, Liu Y, Jiang M, Xia D, Peng Y, Zhu L, et al. Optic nerve compression associated with visual cortex functional alteration in dysthyroid optic neuropathy: A combined orbital and brain imaging study. CNS Neurosci Ther. (2024) 30:e14820. doi: 10.1111/cns.14820
83. Zhang H, Liu Y, Jiang M, Shen F, Zhu T, Xia D, et al. Immune-related visual dysfunction in thyroid eye disease: a combined orbital and brain neuroimaging study. Eur Radiol. (2023) 34:4516–26. doi: 10.1007/s00330-023-10309-8
Keywords: thyroid-associated ophthalmopathy, ultrasonography, computed tomography, magnetic resonance imaging, clinical management
Citation: Xu Z, Xue Z and Lyu Z (2025) Advancements in imaging research in thyroid-associated ophthalmopathy. Front. Endocrinol. 16:1601556. doi: 10.3389/fendo.2025.1601556
Received: 28 March 2025; Accepted: 26 August 2025;
Published: 08 September 2025.
Edited by:
Dhiraj Kumar, National Eye Institute (NIH), United StatesReviewed by:
Christopher Charles Glisson, Michigan State University, United StatesRudolf Gesztelyi, University of Debrecen, Hungary
Copyright © 2025 Xu, Xue and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaohui Lyu, bWV0YWJvbGlzbTMwMUAxMjYuY29t
 Zuxing Xu
Zuxing Xu Zhe Xue
Zhe Xue Zhaohui Lyu
Zhaohui Lyu