- 1Department of Endocrinology and Metabolism, Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
- 2Department of Endocrinology, The Affiliated Taian City Central Hospital of Qingdao University, Tai’an, Shandong, China
Diabetes mellitus, a condition that significantly elevates the incidence and mortality risks associated with cardiovascular diseases, exacerbates the disease burden in China. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have garnered considerable attention, as they not only regulate blood glucose but also play a vital role in safeguarding the cardiovascular system. Recent research shows that metabolic reprogramming is a key mechanism for the cardioprotective effects of GLP-1RAs. GLP-1RAs can achieve metabolic reprogramming by regulating fatty acid, glucose, and ketone body metabolism, as well as mitochondrial function. This process optimizes cardiac energy metabolism, alleviates oxidative stress, and reduces the risk of cardiovascular diseases. This review provides a comprehensive summary of the energy metabolism under normal cardiac conditions and the metabolic reprogramming involved in diabetes-related heart disease. The potential applications and challenges of targeted metabolic reprogramming in the cardioprotective effects of GLP-1RAs are further discussed.
1 Introduction
With the advancement of the social economy and alterations in lifestyle patterns, chronic metabolic disorders like obesity and diabetes have emerged as major public health issues (1, 2). The International Diabetes Federation (IDF) data shows that by 2021, approximately 537 million adults worldwide suffered from diabetes, accounting for nearly one-tenth of the global adult population (3). In China, the number of diabetes patients exceeds 140 million, and it is projected to reach 174 million by 2045 (3). These patients commonly exhibit one or multiple components of metabolic syndrome. Such components are closely linked to a high incidence of cardiovascular disease (CVD) and heart failure (HF), resulting in elevated mortality rates and soaring healthcare costs (4, 5).
Type 2 diabetes mellitus (T2DM) is typically characterized by a gradual decline in β-cell function, often rooted in insulin resistance (6). Currently, the management of diabetes primarily relies on lifestyle modifications and pharmacological interventions to maintain optimal blood glucose levels, thereby preventing or delaying the onset of diabetes-related complications (7, 8).
Metabolic reprogramming represents the process wherein an organism modifies its energy metabolism pathways, such as transitioning from glucose metabolism to fatty acid oxidation or other metabolic routes, in response to environmental alterations or disease conditions. This adjustment is to meet new physiological needs (9). Emerging research has indicated that metabolic reprogramming serves as one of the key mechanisms through which GLP-1RAs confer their cardioprotective effects (10, 11). In the context of GLP-1RAs, metabolic reprogramming involves modulating energy metabolism and regulating cardiac function and pathological remodeling through various mechanisms (12, 13). This strategic approach helps prevent or slow the development of cardiovascular complications associated with diabetes. These effects may further support the cardio protection conferred by GLP-1RAs, a property that has been increasingly recognized in recent decades.
In this review, we will summarize the energy metabolism under normal cardiac conditions and the metabolic reprogramming involved in diabetes-related heart disease. Distinguished from prior reviews, we focus specifically on elucidating the role of GLP-1RAs in modulating cardiac metabolism and function through the lens of metabolic reprogramming. We explore the novel mechanisms by which GLP-1RAs confer cardiovascular protection in diabetic patients, offering fresh insights into their therapeutic potential. Our goal is to provide a broader and more nuanced perspective for future research on GLP-1RA-based therapeutics, potentially paving the way for innovative approaches to managing cardiometabolic diseases.
2 Clinical evidence for cardioprotective effects of GLP-1RAs
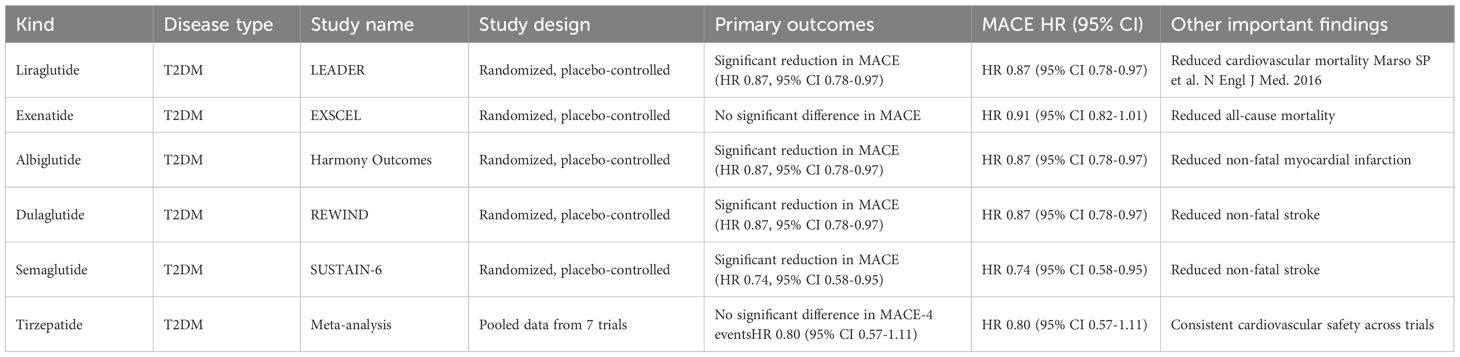
In the process of literature screening for this study, strict inclusion criteria were followed to ensure research quality and relevance to the research theme: In terms of study type, priority was given to randomized controlled trials (RCTs), prospective cohort studies, systematic reviews. In terms of study subjects, the focus was on patients with T2DM, T2DM patients complicated with cardiovascular diseases, or animal models of diabetes-related heart diseases. Moreover, in terms of outcome indicators, the selection centered on "cardioprotection". Cardiovascular-related indicators included the incidence of major adverse cardiovascular events (MACE), cardiac function indicators, and the progression of atherosclerotic lesions.
A search on PubMed with the keywords "Cardiovascular" and "GLP-1 receptor agonists" for the five-year period spanning from 2020 to 2025 retrieved 2,712 items. Existing evidence indicates that GLP-1RAs have demonstrated remarkable cardioprotective effects (14–19). Cardiovascular outcome trials have indicated that GLP-1RAs can decrease the primary composite outcome of first-time major adverse cardiovascular events (MACE) among diabetes patients (20). A systematic review and meta-analysis of randomized controlled trials demonstrated that GLP-1RAs were linked to a significant decline in the MACE incidence(hazard ratio [HR]: 0.86; 95% confidence interval [CI]: 0.79-0.94; I.: 0% (21). Since 2016, numerous cardiovascular outcome studies have revealed that GLP-1RAs can efficiently prevent cardiovascular events such as acute myocardial infarction and stroke, and also lower related mortality (22). Consequently, current guidelines suggest the use of GLP-1RAs for patients with a history of atherosclerotic vascular disease (23). Preclinical research has shown that GLP-1RAs can decelerate the development and progression of atherosclerotic lesions by stabilizing and reducing plaque vulnerability (24). GLP-1RAs have also been beneficial in both heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) (25). For instance, a prospective trial reported that semaglutide alleviated heart-failure-related symptoms and physical limitations in HFpEF patients (26). However, more research is required to determine whether semaglutide can reduce clinical heart -failure events in this patient group (27).
Clinical trials have shown that GLP-1RAs have a variety of effects, which are presented in Table 1. For example, liraglutide might reduce the occurrence rate of myocardial infarction in high-risk T2DM patients and enhance the clinical outcomes of myocardial infarction (28). Regarding heart failure, liraglutide can remarkably improve the left-ventricular diastolic function, indicating its potential in the treatment of T2DM (29). Tirzepatide, a dual GLP-1/GIP receptor agonist that has been approved for controlling blood sugar in T2DM, has emerging evidence suggesting that it is superior to GLP-1RAs in terms of glycemic control and weight loss (30, 31). In the SURPASS-4 trial, tirzepatide significantly decreased blood pressure, body weight, and HbA1c, and its dual-receptor agonism improved lipid profiles, increased insulin secretion, reduced inflammation, and promoted endothelial integrity (32). A recent randomized and double-blind trial revealed that, for adults with poorly controlled T2DM, oral semaglutide at total doses of 25 mg and 50 mg was more effective than the 14-mg total dose in reducing HbA1c and body weight (33). Nevertheless, whether the cardioprotective effect of GLP-1RAs follows a similar dose-dependent pattern still requires further investigation. This is because the primary and key secondary endpoints of this trial were limited to glycemic control and body weight reduction, with no assessment of cardiovascular outcomes that are used to define cardioprotection. Second, long-term data on the cardioprotective efficacy of high-dose oral semaglutide remain lacking.
In summary, GLP-1RAs have significant cardioprotective effects in HFrEF, HFpEF and other clinical settings. Their benefits go beyond glycemic control, suggesting potential as a key treatment for cardiovascular diseases. Future research should focus on clarifying their cardioprotective mechanisms and exploring applications in other cardiovascular conditions.
3 Normal cardiac energy metabolism and abnormal cardiac energy metabolism
3.1 Energy metabolism of the normal heart
Under normal physiological conditions, the heart generates adenosine triphosphate (ATP) through mitochondrial oxidative phosphorylation, utilizing fatty acids oxidation (FAO), glucose, lactic acid, ketone bodies, and amino acids (AA) (34, 35). This process is crucial for meeting the heart’s energy requirements. Among these energy sources, FAO is the main contributor, providing 50-70% of the ATP for muscle contraction (34, 36, 37). In normal cardiac metabolism, the heart favors fatty acids for ATP production because they are much more efficient than glucose (38). For instance, the complete oxidation of 1 mole of a 20-carbon fatty acid generates approximately 134 moles of ATP, while 1 mole of glucose only yields about 30 moles of ATP (39). Although fatty acid oxidation demands more oxygen, under aerobic conditions, its ATP-generating efficiency is considerably higher (40). Therefore, fatty acids are the preferred substrate for ATP production in a healthy heart. Glucose also plays an essential role. In anaerobic conditions, glucose undergoes glycolysis to form lactate, generating 2 ATP per molecule. In aerobic conditions, 94-97% of pyruvate enters the mitochondria for the tricarboxylic acid (TCA) cycle, and only 3-6% is converted into lactate (41). Additionally, lactic acid contributes to cardiac energy metabolism. During fasting, it can account for up to 2.8% of the ATP production in the human heart (42, 43). Recent research has revealed that under specific circumstances, lactic acid can even become the dominant supplier of pyruvate for the heart, highlighting its importance (44). Recent studies have shown that under certain conditions, lactic acid can even be the primary source of pyruvate for the heart, emphasizing its significance (45–47). Finally, amino acid oxidation, particularly of branched-chain amino acids (BCAAs), is a minor source of ATP, contributing less than 2% (34, 48, 49). This adaptability allows the heart to regulate the utilization of different energy substrates according to its needs, maintaining normal cardiac function and ensuring a continuous supply of ATP (34, 50).
3.2 Abnormal cardiac energy metabolism
In the state of diabetes, the heart’s metabolic processes experience substantial alterations because of a changed metabolic environment marked by hyperglycemia, hyperlipidemia, and insulin resistance (51). FAO becomes less efficient in terms of energy production and causes lipotoxicity (52). This leads to the build-up of lipid intermediates such as long-chain acyl-CoAs, acylcarnitines, ceramides, diacylglycerols, and triacylglycerols within cardiomyocytes (52). These intermediate substances interfere with mitochondrial function, cause oxidative stress, and initiate apoptosis. Moreover, insulin resistance impairs glucose uptake and utilization, further disrupting the heart’s energy metabolism (53). This metabolic imbalance makes the inefficiencies related to fatty acid oxidation even worse and contributes to overall metabolic disorder in the hearts of diabetic patients (54). Collectively, these factors result in a decrease in cardiac efficiency and an increase in oxidative stress. Eventually, they promote the development of heart failure in diabetic individuals.
In HF, the heart loses its metabolic adaptability, which throws energy metabolism into disarray. It has difficulty generating sufficient ATP, much like an engine running out of fuel (55, 56). The heart’s ability to alternate among fatty acids, glucose, and lactate as energy sources is compromised, unable to meet the high-energy requirements. The most prominent metabolic alterations in HF are a reduction in the utilization of FAO and ATP production (57–59). The capacity for fatty acid oxidation declines. Firstly, as heart failure advances, the myocardium’s capability to oxidize fatty acids diminishes (60). Secondly, the genes that code for key proteins involved in fatty acid oxidation and their regulatory factors are inhibitedOn one hand, as heart failure progresses, the myocardium’s ability to oxidize fatty acids weakens (34). When the heart switches from depending mainly on FAO to using more glucose and ketone bodies, it might further damage the myocardium.
In HF, when mitochondrial oxidative metabolism and ATP synthesis decrease, it is frequently offset by an augmented glycolytic response (61). During this compensatory process, the expression of the GLUT1 glucose transporter protein, which is a glycolytic intermediate, is upregulated (62). Simultaneously, the activity of phosphofructokinase-1 (PFK-1) rises, and the overall glycolytic flux also increases (62). However, the relatively small energy increment from glycolysis is not enough to completely counteract the cardiac dysfunction caused by energy deficiency (62). This situation might be regulated by the overexpression of mitochondrial ATPase inhibitor 1 (ATPIF1) (34, 63). It is worth noting that in cases where HF occurs concurrently with diabetes, glucose oxidation does not show an upward trend. Instead, there is an increase in anaerobic glycolysis, while aerobic glycolysis decreases (56).
Mitochondrial malfunction plays a crucial role in cardiac metabolic remodeling. It is characterized by elevated oxidative stress, disturbed calcium balance, abnormal mitochondrial dynamics, and irregular mitophagy (34). High reactive oxygen species (ROS) in cardiomyocytes cause lipid peroxidation, mitochondrial DNA damage, antioxidant depletion, and less ATP production (64). Disrupted calcium homeostasis impairs metabolic enzyme activity and activates cell-death pathways (65). Altered mitochondrial dynamics with more fission and less fusion lead to fragmented networks and lower metabolic efficiency. Dysregulated mitophagy accumulates damaged mitochondria (66).
When fuel metabolism is disrupted and physiological stress occurs, alternative energy sources like ketone bodies can become essential for meeting the heart’s energy demands (67). Nevertheless, high levels of ketone bodies have been associated with an increased mortality risk (68). In metabolic disorders, BCAAs often exhibit elevated concentrations (69). The buildup of BCAAs may lead to cardiac enlargement and contribute to the progression of hypertension and coronary heart disease (70, 71). Human epidemiological research has mainly shown an association between higher plasma BCAA levels in HF and unfavorable outcomes (72). The energy metabolism of normal and abnormal hearts is illustrated in Figure 1. The flexible utilization of energy substrates by the normal heart is central to maintaining cardiac function. However, pathological conditions such as diabetes mellitus disrupt this balance. In contrast, GLP-1RAs can regulate the aforementioned key metabolic processes to help impaired hearts restore an energy metabolism pattern approaching normality, with the specific regulatory mechanisms to be elaborated in the section on the mechanism of action of GLP-1RAs.
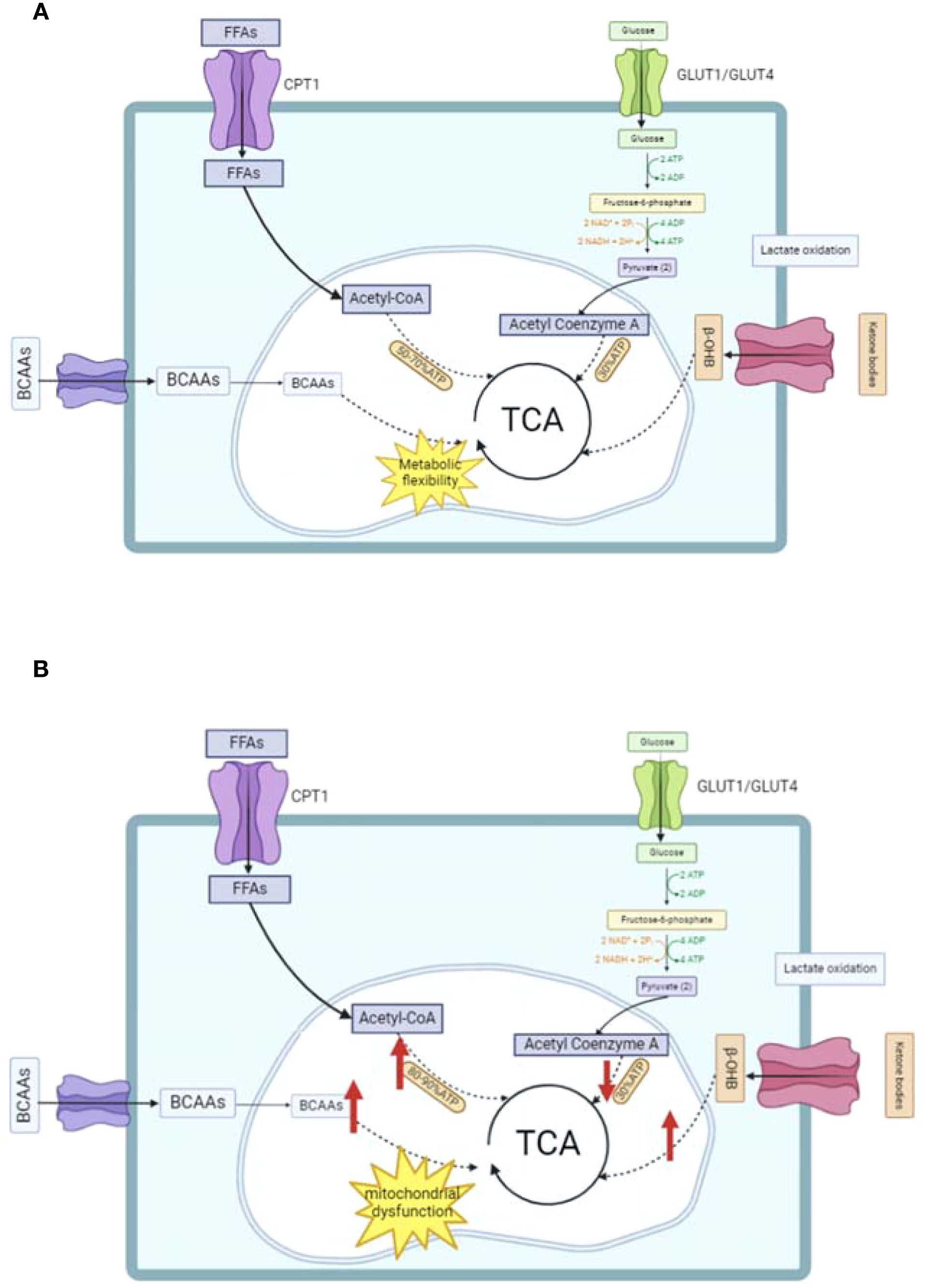
Figure 1. Schematic of energy metabolism in normal and abnormal hearts. (A) Normal cardiac energy metabolism: Illustrates the primary energy substrates (free fatty acids [FFAs], glucose, branched-chain amino acids [BCAAs]) being transported via proteins (e.g., CPT1, GLUT1/GLUT4) and integrated into the tricarboxylic acid (TCA) cycle for ATP production. (B) Abnormal cardiac energy metabolism: Highlights impaired fatty acid oxidation, disrupted mitochondrial function, and altered substrate utilization (e.g., increased anaerobic glycolysis) observed in conditions like diabetes or heart failure. TCA, tricarboxylic acid cycle; BCAAs, branched-chain amino acids; FFAs, free fatty acids.
4 Mechanism of action of GLP-1RAs especially the metabolic reprogramming perspective
GLP-1RAs are hormones secreted by intestinal L cells in the ileum and colon following nutrient intake (73). Their synthesis occurs through the proteolytic processing of the proglucagon precursor by various prohormone convertases (74). These agents play a critical role in regulating postprandial glucose levels by enhancing glucose-dependent insulin secretion, a mechanism that ensures precise control of blood sugar following meals.
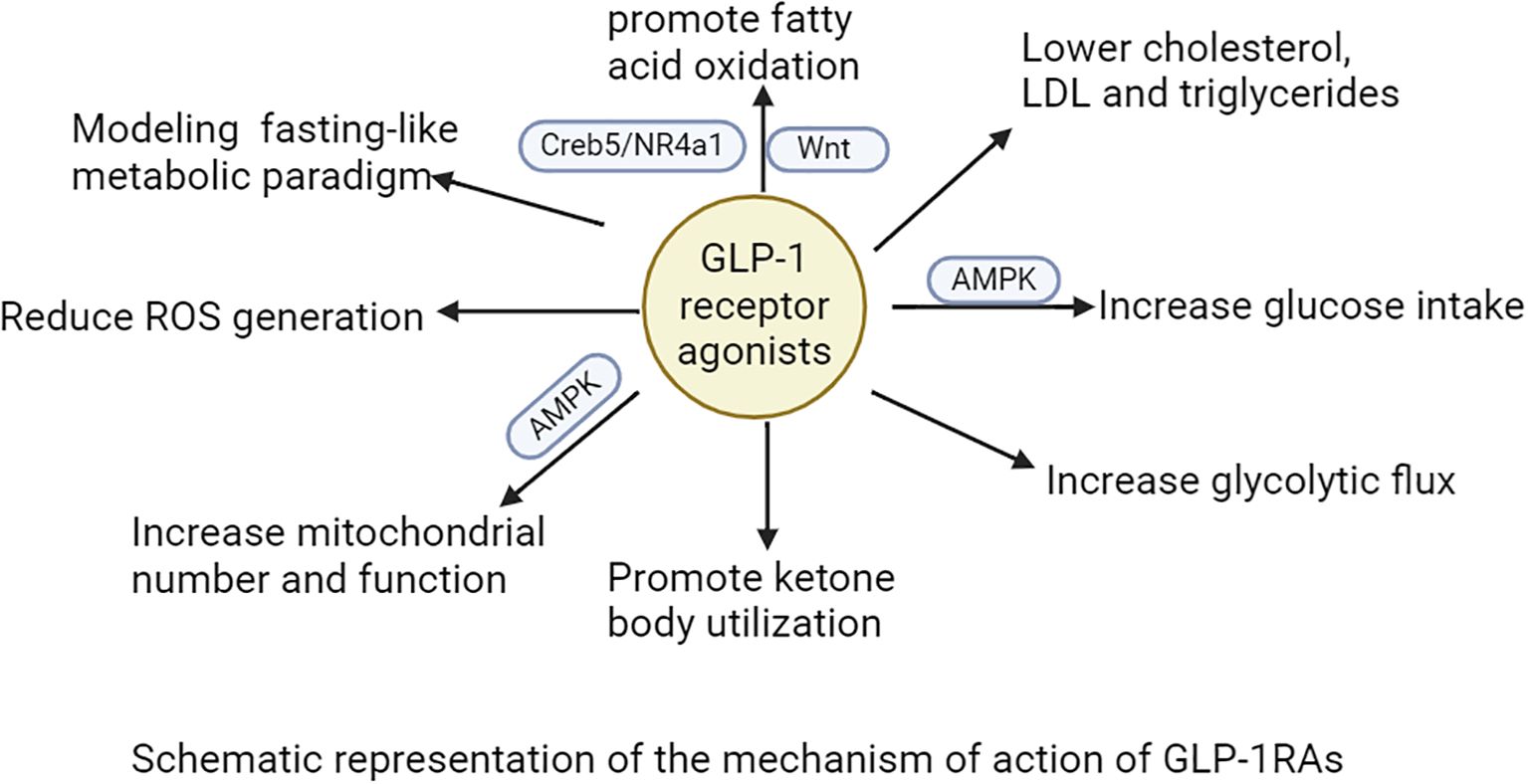
Accumulating clinical evidence indicates that GLP-1RAs mediate their cardioprotective actions largely via metabolic reprogramming (75, 76). This mechanism involves the modulation of fatty acid, glucose, and ketone body metabolism, mitochondrial function, as well as anti-inflammatory and antioxidant processes (77–80). Elevated plasma fatty acid levels, being associated with an increased risk of HF, can give rise to lipotoxicity (81). Such lipotoxicity induces cardiotoxic effects through bioactive sphingolipids like ceramides and diacylglycerols (DAGs) (82). GLP-1RAs mitigate these adverse impacts by enhancing fatty acid oxidation via the Creb5/NR4a1 signaling axis, thereby reducing mitochondrial damage, lipid accumulation, and ATP deficiency (77, 78, 83). This metabolic regulation diminishes lipotoxic stress and optimizes cardiac energy utilization, conferring direct cardioprotective benefits (83). Additionally, GLP-1RAs reduce levels of cholesterol, low-density lipoprotein (LDL), and triglycerides, thereby decreasing the likelihood of cardiovascular events (84–86). Preclinical investigations have shown that these agents downregulate proprotein convertase subtilisin/kexin type 9 (PCSK9) expression, upregulate low-density lipoprotein receptor (LDLR) levels, and suppress postprandial secretion of triglycerides and chylomicrons (87, 88). For example, exendin-4 lowers very-low-density lipoprotein cholesterol (VLDL-C) and LDL-C in animal models by reducing hepatic sterol regulatory element-binding protein 2 (SREBP2) levels and cholesterol absorption (88). Tapolutide has been shown to decrease total cholesterol, LDL-C, triglycerides, and hepatic steatosis (84). Tirzepatide further attenuates lipopolysaccharide (LPS)-induced left ventricular remodeling and dysfunction by inhibiting the TLR4/NF-κB/NLRP3 inflammatory pathway (89). Collectively, these actions improve lipid profiles and alleviate lipotoxic burdens on the myocardium, contributing to the comprehensive cardioprotective effects of GLP-1RAs.
GLP-1RAs improve glucose uptake in cardiomyocytes through dual mechanisms: triggering AMPK activation to facilitate GLUT4 translocation to the cell membrane and regulating the insulin signaling pathway to upregulate GLUT4 expression (79, 80). These agents further optimize glucose utilization by activating glycolytic enzymes such as hexokinase and phosphofructokinase, thereby enhancing glycolytic flux (90). GLP-1RAs also alleviate high-sugar-induced dysfunction in endothelial progenitor cells through the SDF-1β/CXCR7-AMPK/p38-MAPK/IL-6 signaling axis (91). During ischemia or periods of high energy demand, GLP-1RAs enhance ketone body utilization, providing additional energy for cardiomyocytes (92). This process is vital for reducing oxidative stress and damage during myocardial ischemia-reperfusion injury. In terms of mitochondrial function, GLP-1RAs act through multiple pathways: first, stimulating mitochondrial biogenesis via the AMPK signaling pathway to boost both the number and functionality of mitochondria (93). Second, regulating mitochondrial dynamics to decrease fragmentation and optimize morphological and functional integrity (94). Finally, GLP-1RAs decrease ROS to ease oxidative stress and shield mitochondria (94). Their anti-inflammatory and antioxidant properties further contribute to mitigating cardiac injury and improving overall cardiovascular health. Proteomic studies in T2DM patients show that liraglutide treatment enhances cardiac-metabolic profiles by modulating 72 key proteins involved in acute-phase responses, chronic inflammation, and oxidative stress-changes that may improve heart health outcomes (95). Additionally, GLP-1RAs therapy increases circulating vascular progenitor cell content while reducing proinflammatory granulocyte precursor levels, representing an additional mechanism underlying their cardioprotective effects (96).
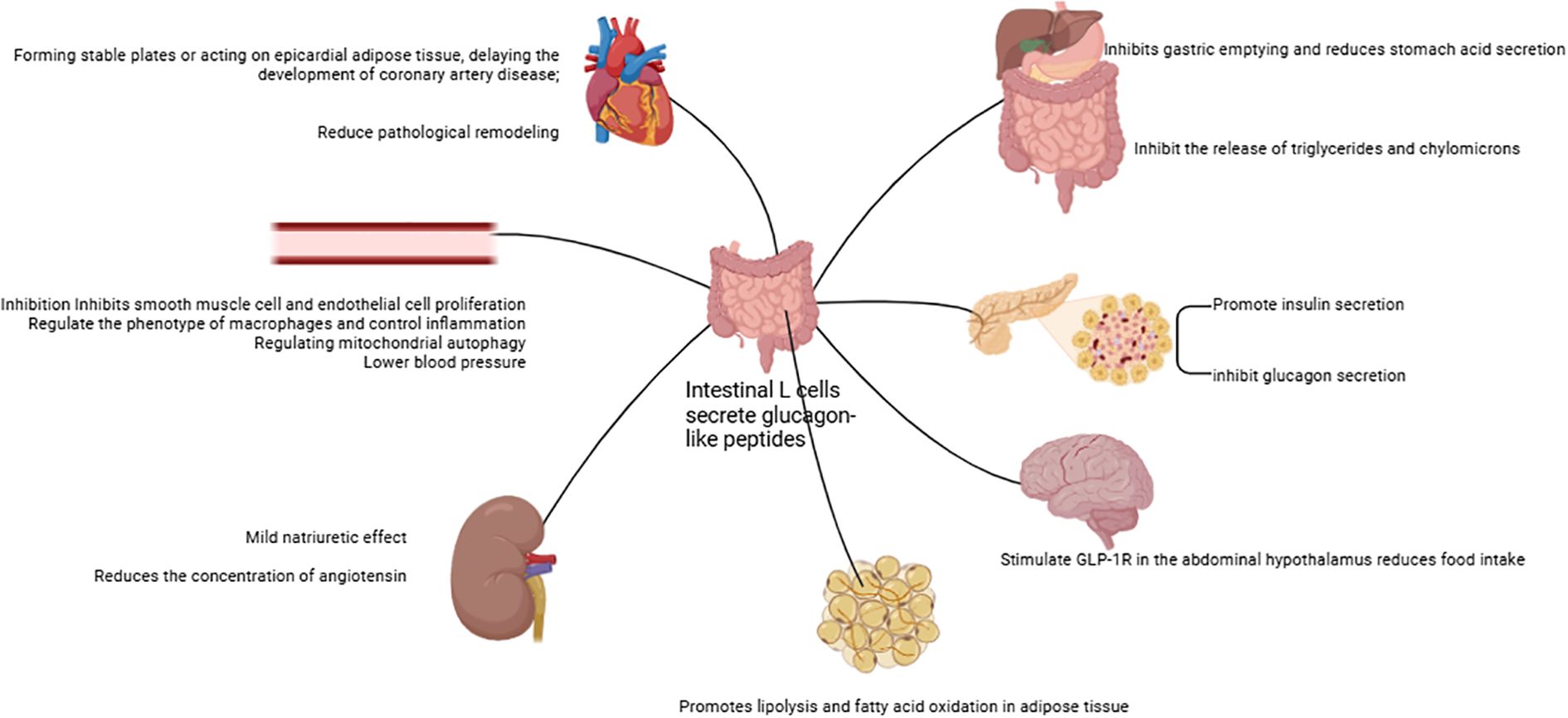
The glucagon-like peptide-1 receptor (GLP-1R) is ubiquitously present across multiple bodily tissues, including the pancreas, lungs, kidneys, central nervous system, cardiovascular system, gastrointestinal tract, as well as skin and vagus nerves (its tissue distribution is illustrated in Figure 2) (97). By binding to these receptors, GLP-1RAs induce calorie expenditure through mechanisms that mimic a fasting-mimicking metabolic state. This adaptive pattern triggers systemic adjustments in energy metabolism, encompassing glucose homeostasis, hormonal secretion, energy substrate utilization, and energy expenditure regulation.
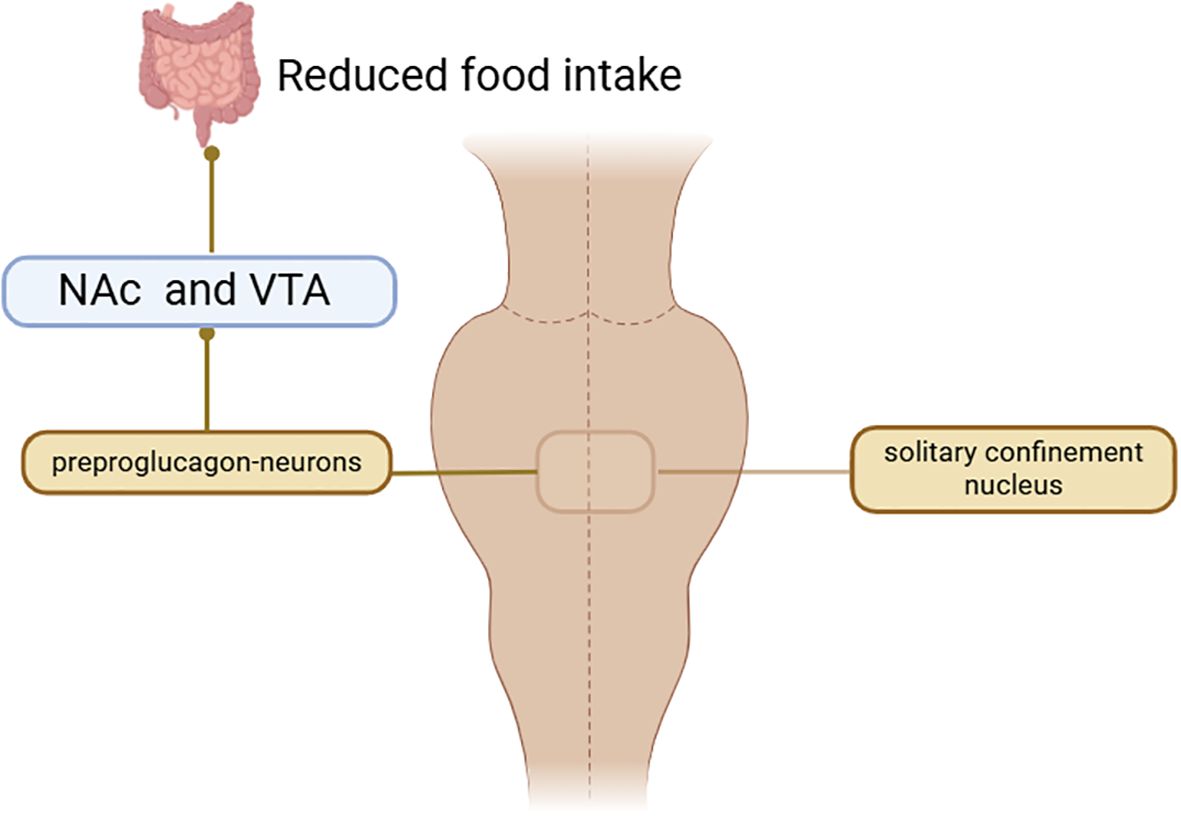
Within the central nervous system, GLP-1RAs function to reduce hunger sensations, suppress appetite, lower caloric intake, amplify satiety, and facilitate better management of eating behaviors (98–102). Activation of GLP-1R in the hypothalamic paraventricular nucleus (PVN) triggers an appetite-suppressing response through neural pathways involving corticotropin-releasing hormone (CRH) excitatory neurons (103, 104). Preclinical studies have indicated that GLP-1RAs require AMPK inhibition to exert their anorectic effects (105). AMPK is a nutrient and glucose sensor in the hypothalamus that is affected by substances such as blood glucose、intracellular energy levels, leptin、GHrelin releasing peptide, and MT-2136 (106–109). This regulatory mechanism is central to how GLP-1RAs control energy intake, as outlined in the primary pathways for food intake inhibition shown in Figure 3. Beyond central nervous system actions, the weight-loss effects of GLP-1 analogs also involve peripheral metabolic adaptations. These agonists can facilitate the transformation of visceral white adipose tissue (WAT) into brown adipose tissue (BAT), thereby stimulating BAT thermogenesis through sympathetic nervous system activation to enhance energy expenditure (110–112). The comprehensive mechanism of action for GLP-1RAs is depicted in Figure 4. And GLP-1RAs exert cardioprotective effects through metabolic reprogramming is illustrated in Figure 5.
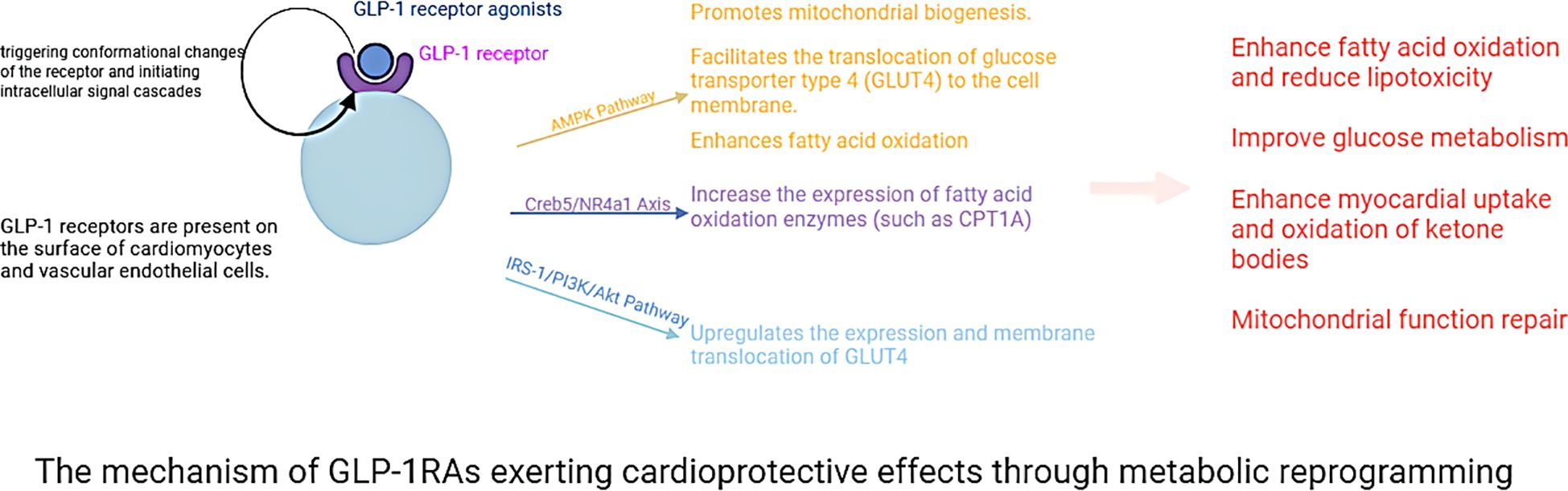
Figure 5. The mechanism of GLP-1RAs exerting cardioprotective effects through metabolic reprogramming.
5 Conclusion
Diabetes mellitus imposes a heavy burden on public health worldwide, particularly due to its close association with increased incidence and mortality of cardiovascular diseases. Clinical evidence consistently demonstrates that GLP-1RAs exert cardioprotective effects, including a significant reduction in the risk of MACE, alleviation of heart failure-related symptoms, and delay in the progression of atherosclerotic lesions. For instance, semaglutide alleviates physical limitations in HFpEF patients, while tirzepatide reduces the composite endpoint of cardiovascular death or worsening HF in obese patients with HFpEF-underscoring their broad utility in cardiometabolic disease management. The notable cardioprotective benefits of GLP-1RAs have spurred exploration into their mechanistic actions beyond glycemic control. Recent investigations into their broad influences on glucose, lipid, and protein metabolism have provided fresh insights into deciphering the advantageous cardioprotective effects of this drug class in T2DM-related cardiovascular disorders. Intriguingly, GLP-1RAs initiate systemic metabolic reprogramming that emulates a fasting-like state to regulate metabolic processes and energy balance. This reprogramming entails enhanced glucose utilization, optimized lipid metabolic pathways, and improved protein homeostasis-all of which likely contribute to their cardiorenal protective actions. Specifically, GLP-1RAs boost fatty acid oxidation, facilitate glucose uptake and utilization, enhance mitochondrial function, and increase ketone body utilization. Collectively, these mechanisms optimize cardiac energy metabolism, mitigate oxidative stress, and support overall cardioprotection. Further research is needed to resolve uncertainties regarding the specific metabolic alterations and to achieve a comprehensive understanding of how GLP-1RAs affect metabolism and the underlying molecular pathways. Future studies should assess whether the metabolic reprogramming induced by GLP-1RAs exhibits dose-dependent characteristics and employ bibliometric approaches to investigate the temporal patterns of these metabolic changes.
Author contributions
XuW: Writing – original draft, Writing – review & editing. MQ: Writing – review & editing, Writing – original draft. LLY: Writing – review & editing, Writing – original draft. LBY: Writing – review & editing, Writing – original draft. XiW: Writing – review & editing, Writing – original draft. FZ: Writing – review & editing, Writing – original draft. YC: Writing – original draft, Writing – review & editing. DW: Writing – review & editing, Writing – original draft. YW: Writing – review & editing, Writing – original draft. WL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Science and Technology Project of the Shandong Geriatrics Society (LKJGG2024Z003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lu Y, Zhang H, Lu J, Ding Q, Li X, Wang X, et al. Prevalence of dyslipidemia and availability of lipid-lowering medications among primary health care settings in China. JAMA Netw Open. (2021) 4:e2127573. doi: 10.1001/jamanetworkopen.2021.27573
2. Russo MP, Grande-Ratti MF, Burgos MA, Molaro AA, and Bonella MB. Prevalence of diabetes, epidemiological characteristics and vascular complications. Arch Cardiol Mex. (2023) 93:30–6. doi: 10.24875/acm.21000410
3. Magliano DJ, Boyko EJ, and committee IDFDAtes. Idf Diabetes Atlas. Idf Diabetes Atlas. Vol. 2021. . Brussels: International Diabetes Federation© International Diabetes Federation (2021).
4. Nakamura K, Miyoshi T, Yoshida M, Akagi S, Saito Y, Ejiri K, et al. Pathophysiology and treatment of diabetic cardiomyopathy and heart failure in patients with diabetes mellitus. Int J Mol Sci. (2022) 23:3587. doi: 10.3390/ijms23073587
5. Ma CX, Ma XN, Guan CH, Li YD, Mauricio D, and Fu SB. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. (2022) 21:74. doi: 10.1186/s12933-022-01516-6
6. Yong J, Johnson JD, Arvan P, Han J, and Kaufman RJ. Therapeutic opportunities for pancreatic B-cell er stress in diabetes mellitus. Nat Rev Endocrinol. (2021) 17:455–67. doi: 10.1038/s41574-021-00510-4
7. Artasensi A, Pedretti A, Vistoli G, and Fumagalli L. Type 2 diabetes mellitus: A review of multi-target drugs. Molecules. (2020) 25:1987. doi: 10.3390/molecules25081987
8. Padhi S, Nayak AK, and Behera A. Type ii diabetes mellitus: A review on recent drug based therapeutics. BioMed Pharmacother. (2020) 131:110708. doi: 10.1016/j.biopha.2020.110708
9. Chakraborty S, Balan M, Sabarwal A, Choueiri TK, and Pal S. Metabolic reprogramming in renal cancer: events of a metabolic disease. Biochim Biophys Acta Rev Cancer. (2021) 1876:188559. doi: 10.1016/j.bbcan.2021.188559
10. Li Y, Sha Z, and Peng H. Metabolic reprogramming in kidney diseases: evidence and therapeutic opportunities. Int J Nephrol. (2021) 2021:5497346. doi: 10.1155/2021/5497346
11. Li Z, Lu S, and Li X. The role of metabolic reprogramming in tubular epithelial cells during the progression of acute kidney injury. Cell Mol Life Sci. (2021) 78:5731–41. doi: 10.1007/s00018-021-03892-w
12. Hamal S, Cherukuri L, Shaikh K, Kinninger A, Doshi J, Birudaraju D, et al. Effect of semaglutide on coronary atherosclerosis progression in patients with type ii diabetes: rationale and design of the semaglutide treatment on coronary progression trial. Coron Artery Dis. (2020) 31:306–14. doi: 10.1097/mca.0000000000000830
13. Silver HJ, Olson D, Mayfield D, Wright P, Nian H, Mashayekhi M, et al. Effect of the glucagon-like peptide-1 receptor agonist liraglutide, compared to caloric restriction, on appetite, dietary intake, body fat distribution and cardiometabolic biomarkers: A randomized trial in adults with obesity and prediabetes. Diabetes Obes Metab. (2023) 25:2340–50. doi: 10.1111/dom.15113
14. Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, et al. Sodium-glucose cotransporter protein-2 (Sglt-2) inhibitors and glucagon-like peptide-1 (Glp-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. Bmj. (2021) 372:m4573. doi: 10.1136/bmj.m4573
15. McGuire DK, Busui RP, Deanfield J, Inzucchi SE, Mann JFE, Marx N, et al. Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: design and baseline characteristics of soul, a randomized trial. Diabetes Obes Metab. (2023) 25:1932–41. doi: 10.1111/dom.15058
16. Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with glp-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. (2021) 9:653–62. doi: 10.1016/s2213-8587(21)00203-5
17. Riley DR, Essa H, Austin P, Preston F, Kargbo I, Ibarburu GH, et al. All-cause mortality and cardiovascular outcomes with sodium-glucose co-transporter 2 inhibitors, glucagon-like peptide-1 receptor agonists and with combination therapy in people with type 2 diabetes. Diabetes Obes Metab. (2023) 25:2897–909. doi: 10.1111/dom.15185
18. Gourdy P, Darmon P, Dievart F, Halimi JM, and Guerci B. Combining glucagon-like peptide-1 receptor agonists (Glp-1ras) and sodium-glucose cotransporter-2 inhibitors (Sglt2is) in patients with type 2 diabetes mellitus (T2dm). Cardiovasc Diabetol. (2023) 22:79. doi: 10.1186/s12933-023-01798-4
19. Scheen AJ. Underuse of glp-1 receptor agonists in the management of type 2 diabetes despite a favorable benefit-safety profile. Expert Opin Drug Saf. (2024) 23:797–810. doi: 10.1080/14740338.2024.2354885
20. Valensi P. Evidence of a bi-directional relationship between heart failure and diabetes: A strategy for the detection of glucose abnormalities and diabetes prevention in patients with heart failure. Cardiovasc Diabetol. (2024) 23:354. doi: 10.1186/s12933-024-02436-3
21. Lavalle-Cobo A, Masson W, Lobo M, Masson G, and Molinero G. Glucagon-like peptide-1 receptor agonists and cardioprotective benefit in patients with type 2 diabetes without baseline metformin: A systematic review and update meta-analysis. High Blood Press Cardiovasc Prev. (2021) 28:605–12. doi: 10.1007/s40292-021-00479-1
22. Nauck MA, Quast DR, Wefers J, and Meier JJ. Glp-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. (2021) 46:101102. doi: 10.1016/j.molmet.2020.101102
23. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 Esc guidelines on diabetes, pre-Diabetes, and cardiovascular diseases developed in collaboration with the easd. Eur Heart J. (2020) 41:255–323. doi: 10.1093/eurheartj/ehz486
24. Rakipovski G, Rolin B, Nøhr J, Klewe I, Frederiksen KS, Augustin R, et al. The Glp-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis In apoe(-/-) and Ldlr(-/-) Mice By a mechanism That Includes Inflammatory Pathways. JACC Basic Transl Sci. (2018) 3:844–57. doi: 10.1016/j.jacbts.2018.09.004
25. Deanfield J, Verma S, Scirica BM, Kahn SE, Emerson SS, Ryan D, et al. Semaglutide and cardiovascular outcomes in patients with obesity and prevalent heart failure: A prespecified analysis of the select trial. Lancet. (2024) 404:773–86. doi: 10.1016/s0140-6736(24)01498-3
26. Verma S, Butler J, Borlaug BA, Davies M, Kitzman DW, Shah SJ, et al. Efficacy of Semaglutide by Sex in Obesity-Related Heart failure with Preserved ejection fraction: Step-Hfpef Trials. J Am Coll Cardiol. (2024) 84:773–85. doi: 10.1016/j.jacc.2024.06.001
27. Petrie MC, Borlaug BA, Butler J, Davies MJ, Kitzman DW, Shah SJ, et al. Semaglutide and Nt-Probnp in Obesity-Related Hfpef: Insights from the step-Hfpef program. J Am Coll Cardiol. (2024) 84:27–40. doi: 10.1016/j.jacc.2024.04.022
28. Marso SP, Nauck MA, Monk Fries T, Rasmussen S, Treppendahl MB, and Buse JB. Myocardial infarction subtypes in patients with type 2 diabetes mellitus and the effect of liraglutide therapy (from the leader trial). Am J Cardiol. (2018) 121:1467–70. doi: 10.1016/j.amjcard.2018.02.030
29. Ida S, Kaneko R, Imataka K, Okubo K, Shirakura Y, Azuma K, et al. Effects of oral antidiabetic drugs and glucagon-like peptide-1 receptor agonists on left ventricular diastolic function in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. Heart Fail Rev. (2021) 26:1151–8. doi: 10.1007/s10741-020-09936-w
30. Andraos J, Muhar H, and Smith SR. Beyond glycemia: comparing tirzepatide to glp-1 analogues. Rev Endocr Metab Disord. (2023) 24:1089–101. doi: 10.1007/s11154-023-09825-1
31. Nesti L and Trico D. Cardioprotective effects of glucagon-like peptide 1 receptor agonists in heart failure: myth or truth? World J Diabetes. (2024) 15:818–22. doi: 10.4239/wjd.v15.i5.818
32. Sardar MB, Nadeem ZA, and Babar M. Tirzepatide: A novel cardiovascular protective agent in type 2 diabetes mellitus and obesity. Curr Probl Cardiol. (2024) 49:102489. doi: 10.1016/j.cpcardiol.2024.102489
33. Aroda VR, Aberle J, Bardtrum L, Christiansen E, Knop FK, Gabery S, et al. Efficacy and safety of once-daily oral semaglutide 25 mg and 50 mg compared with 14 mg in adults with type 2 diabetes (Pioneer plus): A multicentre, randomised, phase 3b trial. Lancet. (2023) 402:693–704. doi: 10.1016/s0140-6736(23)01127-3
34. Lopaschuk GD, Karwi QG, Tian R, Wende AR, and Abel ED. Cardiac energy metabolism in heart failure. Circ Res. (2021) 128:1487–513. doi: 10.1161/circresaha.121.318241
35. Chen Z, Jin ZX, Cai J, Li R, Deng KQ, Ji YX, et al. Energy substrate metabolism and oxidative stress in metabolic cardiomyopathy. J Mol Med (Berl). (2022) 100:1721–39. doi: 10.1007/s00109-022-02269-1
36. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, and Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. (2010) 90:207–58. doi: 10.1152/physrev.00015.2009
37. Ketema EB and Lopaschuk GD. Post-translational acetylation control of cardiac energy metabolism. Front Cardiovasc Med. (2021) 8:723996. doi: 10.3389/fcvm.2021.723996
38. Allard MF, Schönekess BO, Henning SL, English DR, and Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to atp production in hypertrophied hearts. Am J Physiol. (1994) 267:H742–50. doi: 10.1152/ajpheart.1994.267.2.H742
39. Goodwin GW, Taylor CS, and Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. (1998) 273:29530–9. doi: 10.1074/jbc.273.45.29530
40. Actis Dato V, Lange S, and Cho Y. Metabolic flexibility of the heart: the role of fatty acid metabolism in health, heart failure, and cardiometabolic diseases. Int J Mol Sci. (2024) 25:1211. doi: 10.3390/ijms25021211
41. Ng YH, Koay YC, Marques FZ, Kaye DM, and O’Sullivan JF. Leveraging metabolism for better outcomes in heart failure. Cardiovasc Res. (2024) 25:1211. doi: 10.1093/cvr/cvae216
42. Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. (2020) 370:364–8. doi: 10.1126/science.abc8861
43. Zhao Y, Zhou Y, Xiao M, Huang Y, Qi M, Kong Z, et al. Impaired glucose tolerance is associated with enhanced postprandial pancreatic polypeptide secretion. J Diabetes. (2022) 14:334–44. doi: 10.1111/1753-0407.13268
44. Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, et al. Glucose feeds the tca cycle via circulating lactate. Nature. (2017) 551:115–8. doi: 10.1038/nature24057
45. Cotter DG, Schugar RC, and Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. (2013) 304:H1060–76. doi: 10.1152/ajpheart.00646.2012
46. Karwi QG, Biswas D, Pulinilkunnil T, and Lopaschuk GD. Myocardial ketones metabolism in heart failure. J Card Fail. (2020) 26:998–1005. doi: 10.1016/j.cardfail.2020.04.005
47. Matsuura TR, Puchalska P, Crawford PA, and Kelly DP. Ketones and the heart: metabolic principles and therapeutic implications. Circ Res. (2023) 132:882–98. doi: 10.1161/circresaha.123.321872
48. Karwi QG and Lopaschuk GD. Branched-chain amino acid metabolism in the failing heart. Cardiovasc Drugs Ther. (2023) 37:413–20. doi: 10.1007/s10557-022-07320-4
49. Uddin GM, Karwi QG, Pherwani S, Gopal K, Wagg CS, Biswas D, et al. Deletion of bcatm increases insulin-stimulated glucose oxidation in the heart. Metabolism. (2021) 124:154871. doi: 10.1016/j.metabol.2021.154871
50. Glatz JFC, Nabben M, Young ME, Schulze PC, Taegtmeyer H, and Luiken J. Re-balancing cellular energy substrate metabolism to mend the failing heart. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165579. doi: 10.1016/j.bbadis.2019.165579
51. Rajbhandari J, Fernandez CJ, Agarwal M, Yeap BXY, and Pappachan JM. Diabetic heart disease: A clinical update. World J Diabetes. (2021) 12:383–406. doi: 10.4239/wjd.v12.i4.383
52. Wang H, Shen M, Shu X, Guo B, Jia T, Feng J, et al. Cardiac metabolism, reprogramming, and diseases. J Cardiovasc Transl Res. (2024) 17:71–84. doi: 10.1007/s12265-023-10432-3
53. Kosmas CE, Bousvarou MD, Kostara CE, Papakonstantinou EJ, Salamou E, and Guzman E. Insulin resistance and cardiovascular disease. J Int Med Res. (2023) 51:3000605231164548. doi: 10.1177/03000605231164548
54. Ritchie RH and Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. (2020) 126:1501–25. doi: 10.1161/circresaha.120.315913
55. Karwi QG, Uddin GM, Ho KL, and Lopaschuk GD. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med. (2018) 5:68. doi: 10.3389/fcvm.2018.00068
56. Da Dalt L, Cabodevilla AG, Goldberg IJ, and Norata GD. Cardiac lipid metabolism, mitochondrial function, and heart failure. Cardiovasc Res. (2023) 119:1905–14. doi: 10.1093/cvr/cvad100
57. Aubert G, Vega RB, and Kelly DP. Perturbations in the gene regulatory pathways controlling mitochondrial energy production in the failing heart. Biochim Biophys Acta. (2013) 1833:840–7. doi: 10.1016/j.bbamcr.2012.08.015
58. Barger PM and Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci. (1999) 318:36–42. doi: 10.1097/00000441-199907000-00006
59. Schwartz B, Gjini P, Gopal DM, and Fetterman JL. Inefficient batteries in heart failure: metabolic bottlenecks disrupting the mitochondrial ecosystem. JACC Basic Transl Sci. (2022) 7:1161–79. doi: 10.1016/j.jacbts.2022.03.017
60. Lahey R, Wang X, Carley AN, and Lewandowski ED. Dietary fat supply to failing hearts determines dynamic lipid signaling for nuclear receptor activation and oxidation of stored triglyceride. Circulation. (2014) 130:1790–9. doi: 10.1161/circulationaha.114.011687
61. Napoli C, Benincasa G, Donatelli F, and Ambrosio G. Precision medicine in distinct heart failure phenotypes: focus on clinical epigenetics. Am Heart J. (2020) 224:113–28. doi: 10.1016/j.ahj.2020.03.007
62. Chen S, Zou Y, Song C, Cao K, Cai K, Wu Y, et al. The role of glycolytic metabolic pathways in cardiovascular disease and potential therapeutic approaches. Basic Res Cardiol. (2023) 118:48. doi: 10.1007/s00395-023-01018-w
63. Zhou B, Caudal A, Tang X, Chavez JD, McMillen TS, Keller A, et al. Upregulation of mitochondrial atpase inhibitory factor 1 (Atpif1) mediates increased glycolysis in mouse hearts. J Clin Invest. (2022) 132. doi: 10.1172/jci155333
64. Tsutsui H, Kinugawa S, and Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. (2011) 301:H2181–90. doi: 10.1152/ajpheart.00554.2011
65. O’Rourke B, Ashok D, and Liu T. Mitochondrial ca(2+) in heart failure: not enough or too much? J Mol Cell Cardiol. (2021) 151:126–34. doi: 10.1016/j.yjmcc.2020.11.014
66. Chaanine AH, Joyce LD, Stulak JM, Maltais S, Joyce DL, Dearani JA, et al. Mitochondrial morphology, dynamics, and function in human pressure overload or ischemic heart disease with preserved or reduced ejection fraction. Circ Heart Fail. (2019) 12:e005131. doi: 10.1161/circheartfailure.118.005131
67. Caudal A, Tang X, Chavez JD, Keller A, Mohr JP, Bakhtina AA, et al. Mitochondrial interactome quantitation reveals structural changes in metabolic machinery in the failing murine heart. Nat Cardiovasc Res. (2022) 1:855–66. doi: 10.1038/s44161-022-00127-4
68. Oyetoro RO, Conners KM, Joo J, Turecamo S, Sampson M, Wolska A, et al. Circulating ketone bodies and mortality in heart failure: A community cohort study. Front Cardiovasc Med. (2024) 11:1293901. doi: 10.3389/fcvm.2024.1293901
69. Walejko JM, Christopher BA, Crown SB, Zhang GF, Pickar-Oliver A, Yoneshiro T, et al. Branched-chain A-ketoacids are preferentially reaminated and activate protein synthesis in the heart. Nat Commun. (2021) 12:1680. doi: 10.1038/s41467-021-21962-2
70. Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol. (2019) 18:86. doi: 10.1186/s12933-019-0892-3
71. Dziedzic M, Józefczuk E, Guzik TJ, and Siedlinski M. Interplay between plasma glycine and branched-chain amino acids contributes to the development of hypertension and coronary heart disease. Hypertension. (2024) 81:1320–31. doi: 10.1161/hypertensionaha.123.22649
72. McGarrah RW and White PJ. Branched-chain amino acids in cardiovascular disease. Nat Rev Cardiol. (2023) 20:77–89. doi: 10.1038/s41569-022-00760-3
73. Trujillo JM, Nuffer W, and Smith BA. Glp-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. (2021) 12:2042018821997320. doi: 10.1177/2042018821997320
74. Smith NK, Hackett TA, Galli A, and Flynn CR. Glp-1: molecular mechanisms and outcomes of a complex signaling system. Neurochem Int. (2019) 128:94–105. doi: 10.1016/j.neuint.2019.04.010
75. Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. (2023) 389:2221–32. doi: 10.1056/NEJMoa2307563
76. Kuthati Y, Davuluri VNG, and Wong CS. Therapeutic effects of Glp-1 receptor agonists and Dpp-4 inhibitors in neuropathic pain: mechanisms and clinical implications. Biomolecules. (2025) 15(5). doi: 10.3390/biom15050622
77. Shimabukuro M. Cardiac adiposity and global cardiometabolic risk: new concept and clinical implication. Circ J. (2009) 73:27–34. doi: 10.1253/circj.cj-08-1012
78. Capone F, Sotomayor-Flores C, Bode D, Wang R, Rodolico D, Strocchi S, et al. Cardiac metabolism in hfpef: from fuel to signalling. Cardiovasc Res. (2023) 118:3556–75. doi: 10.1093/cvr/cvac166
79. Kim SM, Kwon EJ, Oh JY, Kim HS, Park S, Jang G, et al. Ampk activation by glycogen expenditure primes the exit of naïve pluripotency. EMBO Rep. (2025) 26(6):1504–27. doi: 10.1038/s44319-025-00384-x
80. Westermeier F and Fisman EZ. Glucagon like peptide-1 (Glp-1) agonists and cardiometabolic protection: historical development and future challenges. Cardiovasc Diabetol. (2025) 24:44. doi: 10.1186/s12933-025-02608-9
81. Djoussé L, Benkeser D, Arnold A, Kizer JR, Zieman SJ, Lemaitre RN, et al. Plasma free fatty acids and risk of heart failure: the cardiovascular health study. Circ Heart Fail. (2013) 6:964–9. doi: 10.1161/circheartfailure.113.000521
82. Choi RH, Tatum SM, Symons JD, Summers SA, and Holland WL. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol. (2021) 18:701–11. doi: 10.1038/s41569-021-00536-1
83. Ma YL, Kong CY, Guo Z, Wang MY, Wang P, Liu FY, et al. Semaglutide ameliorates cardiac remodeling in male mice by optimizing energy substrate utilization through the creb5/nr4a1 axis. Nat Commun. (2024) 15:4757. doi: 10.1038/s41467-024-48970-2
84. Muzurović E and Mikhailidis DP. Impact of glucagon-like peptide 1 receptor agonists and sodium-glucose transport protein 2 inhibitors on blood pressure and lipid profile. Expert Opin Pharmacother. (2020) 21:2125–35. doi: 10.1080/14656566.2020.1795132
85. Liu H, Zhan YL, Luo GJ, Zou LL, Li Y, and Lu HY. Liraglutide and insulin have contrary effects on adipogenesis of human adipose-derived stem cells via wnt pathway. Diabetes Metab Syndr Obes. (2020) 13:3075–87. doi: 10.2147/dmso.S253097
86. Han Y, Liu W, Chen L, Xin X, Wang Q, Zhang X, et al. Effective oral delivery of exenatide-zn(2+) complex through distal ileum-targeted double layers nanocarriers modified with deoxycholic acid and glycocholic acid in diabetes therapy. Biomaterials. (2021) 275:120944. doi: 10.1016/j.biomaterials.2021.120944
87. Vergès B, Duvillard L, Pais de Barros JP, Bouillet B, Baillot-Rudoni S, Rouland A, et al. Liraglutide increases the catabolism of apolipoprotein B100-containing lipoproteins in patients with type 2 diabetes and reduces proprotein convertase subtilisin/kexin type 9 expression. Diabetes Care. (2021) 44:1027–37. doi: 10.2337/dc20-1843
88. Hori M, Hasegawa Y, Hayashi Y, Nakagami T, and Harada-Shiba M. Acute cholesterol-lowering effect of exendin-4 in ldlr(-/-) and C57bl/6j mice. J Atheroscler Thromb. (2023) 30:74–86. doi: 10.5551/jat.60921
89. Liu Q, Zhu J, Kong B, Shuai W, and Huang H. Tirzepatide attenuates lipopolysaccharide-induced left ventricular remodeling and dysfunction by inhibiting the tlr4/nf-kb/nlrp3 pathway. Int Immunopharmacol. (2023) 120:110311. doi: 10.1016/j.intimp.2023.110311
90. Liu X, Chen S, Liu X, Wu X, Jiang X, Li Y, et al. Enpp1 ameliorates mafld by regulating hepatocyte lipid metabolism through the ampk/pparα Signaling pathway. Cell Biosci. (2025) 15:22. doi: 10.1186/s13578-025-01364-3
91. Chen K, Jin HJ, Wu ZH, Zhang BF, Wu J, Huang ZY, et al. Glucagon-like peptide-1 receptor agonist exendin 4 ameliorates diabetes-associated vascular calcification by regulating mitophagy through the ampk signaling pathway. Mol Med. (2024) 30:58. doi: 10.1186/s10020-024-00817-8
92. Selvaraj S, Kelly DP, and Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation. (2020) 141:1800–12. doi: 10.1161/circulationaha.119.045033
93. Marin TL, Gongol B, Zhang F, Martin M, Johnson DA, Xiao H, et al. Ampk promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors dnmt1, rbbp7, and hat1. Sci Signal. (2017) 10. doi: 10.1126/scisignal.aaf7478
94. Xie S, Zhang M, Shi W, Xing Y, Huang Y, Fang WX, et al. Long-term activation of glucagon-like peptide-1 receptor by dulaglutide prevents diabetic heart failure and metabolic remodeling in type 2 diabetes. J Am Heart Assoc. (2022) 11:e026728. doi: 10.1161/jaha.122.026728
95. Ekhzaimy AA, Masood A, Benabdelkamel H, Elhassan T, Musambil M, and Alfadda AA. Plasma proteomics reveals an improved cardio-metabolic profile in patients with type 2 diabetes post-liraglutide treatment. Diabetes Vasc Dis Res. (2022) 19:14791641221094322. doi: 10.1177/14791641221094322
96. Park B, Krishnaraj A, Teoh H, Bakbak E, Dennis F, Quan A, et al. Glp-1ra therapy increases circulating vascular regenerative cell content in people living with type 2 diabetes. Am J Physiol Heart Circ Physiol. (2024) 327:H370–h6. doi: 10.1152/ajpheart.00257.2024
97. Ussher JR and Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol. (2023) 20:463–74. doi: 10.1038/s41569-023-00849-3
98. Zander M, Madsbad S, Madsen JL, and Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: A parallel-group study. Lancet. (2002) 359:824–30. doi: 10.1016/s0140-6736(02)07952-7
99. Meier JJ, Gethmann A, Götze O, Gallwitz B, Holst JJ, Schmidt WE, et al. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia. (2006) 49:452–8. doi: 10.1007/s00125-005-0126-y
100. Stefanou MI, Palaiodimou L, Theodorou A, Safouris A, Fischer U, Kelly PJ, et al. Risk of Major Adverse Cardiovascular Events and All-Cause Mortality under Treatment with Glp-1 Ras or the Dual Gip/Glp-1 Receptor Agonist Tirzepatide in Overweight or Obese Adults without Diabetes: A Systematic Review and Meta-Analysis. Ther Adv Neurol Disord. (2024) 17:17562864241281903. doi: 10.1177/17562864241281903
101. Burmeister MA, Ayala J, Drucker DJ, and Ayala JE. Central glucagon-like peptide 1 receptor-induced anorexia requires glucose metabolism-mediated suppression of ampk and is impaired by central fructose. Am J Physiol Endocrinol Metab. (2013) 304:E677–85. doi: 10.1152/ajpendo.00446.2012
102. Tinsley IC, Borner T, Swanson ML, Chepurny OG, Doebley SA, Kamat V, et al. Synthesis, optimization, and biological evaluation of corrinated conjugates of the glp-1r agonist exendin-4. J Med Chem. (2021) 64:3479–92. doi: 10.1021/acs.jmedchem.1c00185
103. Liu J, Conde K, Zhang P, Lilascharoen V, Xu Z, Lim BK, et al. Enhanced ampa receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron. (2017) 96:897–909.e5. doi: 10.1016/j.neuron.2017.09.042
104. Huang KP, Acosta AA, Ghidewon MY, McKnight AD, Almeida MS, Nyema NT, et al. Dissociable hindbrain glp1r circuits for satiety and aversion. Nature. (2024) 632:585–93. doi: 10.1038/s41586-024-07685-6
105. Kola B. Role of amp-activated protein kinase in the control of appetite. J Neuroendocrinol. (2008) 20:942–51. doi: 10.1111/j.1365-2826.2008.01745.x
106. Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, et al. Amp-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. (2004) 428:569–74. doi: 10.1038/nature02440
107. Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, et al. Amp-activated protein kinase plays a role in the control of food intake. J Biol Chem. (2004) 279:12005–8. doi: 10.1074/jbc.C300557200
108. Han SM, Namkoong C, Jang PG, Park IS, Hong SW, Katakami H, et al. Hypothalamic amp-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia. (2005) 48:2170–8. doi: 10.1007/s00125-005-1913-1
109. Mandal SK, Shrestha PK, Alenazi FSH, Shakya M, Alhamami H, and Briski KP. Role of hindbrain adenosine 5’-monophosphate-activated protein kinase (Ampk) in hypothalamic ampk and metabolic neuropeptide adaptation to recurring insulin-induced hypoglycemia in the male rat. Neuropeptides. (2017) 66:25–35. doi: 10.1016/j.npep.2017.08.001
110. Spezani R, Marcondes-de-Castro IA, Marinho TS, Reis-Barbosa PH, Cardoso LEM, Aguila MB, et al. Cotadutide improves brown adipose tissue thermogenesis in obese mice. Biochem Pharmacol. (2023) 217:115852. doi: 10.1016/j.bcp.2023.115852
111. Rossetti CL, Andrade IS, Fonte Boa LF, Neves MB, Fassarella LB, Bertasso IM, et al. Liraglutide prevents body and fat mass gain in ovariectomized wistar rats. Mol Cell Endocrinol. (2024) 594:112374. doi: 10.1016/j.mce.2024.112374
Keywords: metabolic reprogramming, glucagon-like peptide-1 receptor agonists, heart failure, cardiovascular system, diabetes
Citation: Wang X, Qi M, Yang L, Yang L, Wang X, Zhang F, Cui Y, Wang D, Wang Y and Lv W (2025) GLP-1 receptor agonists synergistic effects of metabolic reprogramming and cardioprotection. Front. Endocrinol. 16:1614726. doi: 10.3389/fendo.2025.1614726
Received: 19 April 2025; Accepted: 24 September 2025;
Published: 13 October 2025.
Edited by:
Marcia Hiriart, Universidad Nacional Autonoma de Mexico, MexicoReviewed by:
Absalon Gutierrez, University of Texas Health Science Center at Houston, United StatesMubashshir Ali, University of South Florida, United States
Copyright © 2025 Wang, Qi, Yang, Yang, Wang, Zhang, Cui, Wang, Wang and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenshan Lv, bHZ3ZW5zaGFuQDE2My5jb20=
†These authors have contributed equally to this work
 Xuan Wang
Xuan Wang Mengmeng Qi1†
Mengmeng Qi1† Lili Yang
Lili Yang Fang Zhang
Fang Zhang Yangang Wang
Yangang Wang Wenshan Lv
Wenshan Lv