- 1School of Medicine, University of Electronic Science and Technology, Chengdu, China
- 2Department of Laboratory Medicine and Sichuan Provincial Key Laboratory for Human Disease Gene Study, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Bone turnover markers (BTMs) are biomedical indicators used to assess the bone metabolism processes reflecting the activity of osteoblasts and osteoclasts. During childhood and adolescence, bone metabolism is highly active, leading to distinct levels and trends of BTMs compared with those of adults. BTMs correlate significantly with age, gender and environmental factors, making them valuable for evaluating bone health and developmental trajectories in pediatric populations. Due to the non-invasive characters and dynamic monitoring capabilities, BTMs are increasingly employed in research and clinical practice. Preliminary observations propose that BTMs demonstrate clinical utility in predicting fracture risk, enabling early diagnosis of osteoporosis and rickets, and monitoring therapeutic efficacy. However, Tracability of BTM measurement results and limited pediatric reference intervals remain critical challenges. Further research is needed to expand our understanding of the their mechanisms and optimize clinical applications. This article reviews the physiological and pathological states in children, discusses the current dilemmas of clinical application, and highlights the future research prospects.
1 Introduction
Bone is a vital structural and metabolic organ, providing mechanical support and participating in mineral homeostasis. As a dynamic tissue, bone undergoes continuous remodeling through two counterbalanced processes: osteoblast-mediated formation and osteoclast-driven resorption (1). Clinically, dual-energy X-ray absorptiometry (DXA) and quantitative computed tomography (QCT) are gold-standard techniques for assessing bone mineral density (BMD) and content due to their high accuracy and rapid results (2). However, their utility in longitudinal bone metabolism monitoring is constrained by radiation exposure risks and cost limitations. In pediatric populations, these limitations are compounded by reduced measurement precision, primarily due to motion artifacts and patient noncompliance during imaging (1, 2). BTMs metabolite or enzyme released during bone remodeling—reflect real-time osteoblast or osteoclast activity. These minimally invasive biomarkers enable dynamic monitoring of systemic bone metabolism (3, 4), contrasting with bone mineral content (BMC) and bone mineral density (BMD), which provide only a static assessment of bone mass (5).
Pediatric bone metabolism differs significantly from adults, integrating both developmental growth and remodeling processes. Studies indicate that pediatric BTMs exhibit 5- to 20-fold higher concentrations compared to adults (6, 7). As dual-purpose indicators, BTMs not only evaluate bone metabolic status but also serve as proxies for tracking growth velocity and maturation patterns. These attributes position BTMs as essential tools for early diagnosis, disease classification, and therapeutic surveillance in pediatric growth disorders (2, 3, 8).
2 Literature search and selection criteria
A comprehensive search was conducted by using keywords and MeSH terms to identify studies related to BTMs in children and adolescents. The keywords ‘children and adolescents’ and ‘bone turnover marker’ were employed, along with specific disorders and specific markers such as ‘osteocalcin’, ‘type I procollagen N-terminal propeptide’, ‘N-terminal cross-linked terminal peptide’, and ‘C-terminal cross-linked terminal peptide’. The search was carried out across multiple databases, including Web of Science, Google Scholar, and PubMed. Studies were included if they focused on the measurement, interpretation, reference ranges, or clinical utility of BTMs in children and adolescents (aged 0–18 years). Original research articles, systematic reviews, meta-analyses, and relevant clinical guidelines were prioritized. Animal studies, case reports, articles not in English, and studies exclusively in adults were excluded.
3 Characteristics of children’s bone metabolism
Bone mass accrual predominantly occurs during childhood developmental stages, with accelerated deposition observed in early childhood and adolescence periods characterized by rapid skeletal growth and critical mineralization windows (5, 9, 10). During childhood and adolescence, BTMs primarily reflect growth plate activity, while bone modeling processes remain active. Pediatric bone remodeling rates are approximately threefold higher than those in adults (11). Elevated BTM levels in children and adolescents reflect heightened remodeling activity, a physiological adaptation to mechanical loading during growth. Notably, BTMs demonstrate an inverse correlation with bone mineral density (BMD), suggesting a predominance of formation over resorption (12). Longitudinal studies indicate that approximately 90% of peak bone mass (PBM) is attained by early adulthood, reaching maximal density during Tanner stage III pubertal development, stabilizing thereafter, and declining progressively with advancing age (12, 13). Given that childhood skeletal development establishes a critical foundation for lifelong bone health, dynamic monitoring of bone metabolism and maturation patterns during this period is increasingly recognized as essential.
4 Bone turnover markers
Bone turnover markers are non-invasive biomarkers, obtained from blood or urine samples, that provide a dynamic assessment of bone metabolism (14). These biomarkers originate from the coupled activities of osteoblasts and osteoclasts. During the formative phase, osteoblasts orchestrate type I collagen biosynthesis and secretion of non-collagenous regulatory proteins, including osteocalcin (OC) and bone-specific alkaline phosphatase (BALP). Proteolytic cleavage of procollagen during extracellular matrix maturation generates quantifiable fragments such as procollagen type I N-terminal propeptide (PINP), serving as specific surrogates for osteoblast activity. In contrast, osteoclast-driven resorption involves enzymatic degradation of the collagenous matrix, liberating degradation byproducts like C-terminal telopeptide of type I collagen (CTX) and N-terminal telopeptide (NTX), which directly correlate with osteoclast functional status (15) (Figure 1). Historically, urinary BTMs were widely utilized due to their direct association with renal excretion of bone resorption byproducts, but due to the difficulty of collecting urine samples and the fact that BTMs values are limited by creatinine levels, serum or plasma samples are now preferred by laboratories to test for BTMs, which are easier to process and have a high degree of stability (2, 16). In 2010, the International Osteoporosis Foundation (IOF) and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) established serum PINP and β-CTX as reference biomarkers for bone metabolism dynamics, a designation later formalized by the IFCC Bone Marker Standards Working Group to recognize PINP and β-CTX as the gold-standard biomarkers for bone formation and resorption respectively (2, 14). These designations have solidified BTMs as essential clinical tools for diagnosing metabolic bone disorders, monitoring therapeutic responses, and evaluating treatment adherence. In pediatric populations, serum levels of BTMs rise markedly during puberty, correlating strongly with growth velocity, peak bone mass accrual, and biological sex (Figure 2). Consequently, BTMs provide sensitive, dynamic indices for tracking remodeling processes and detecting early bone mass abnormalities (8, 19).
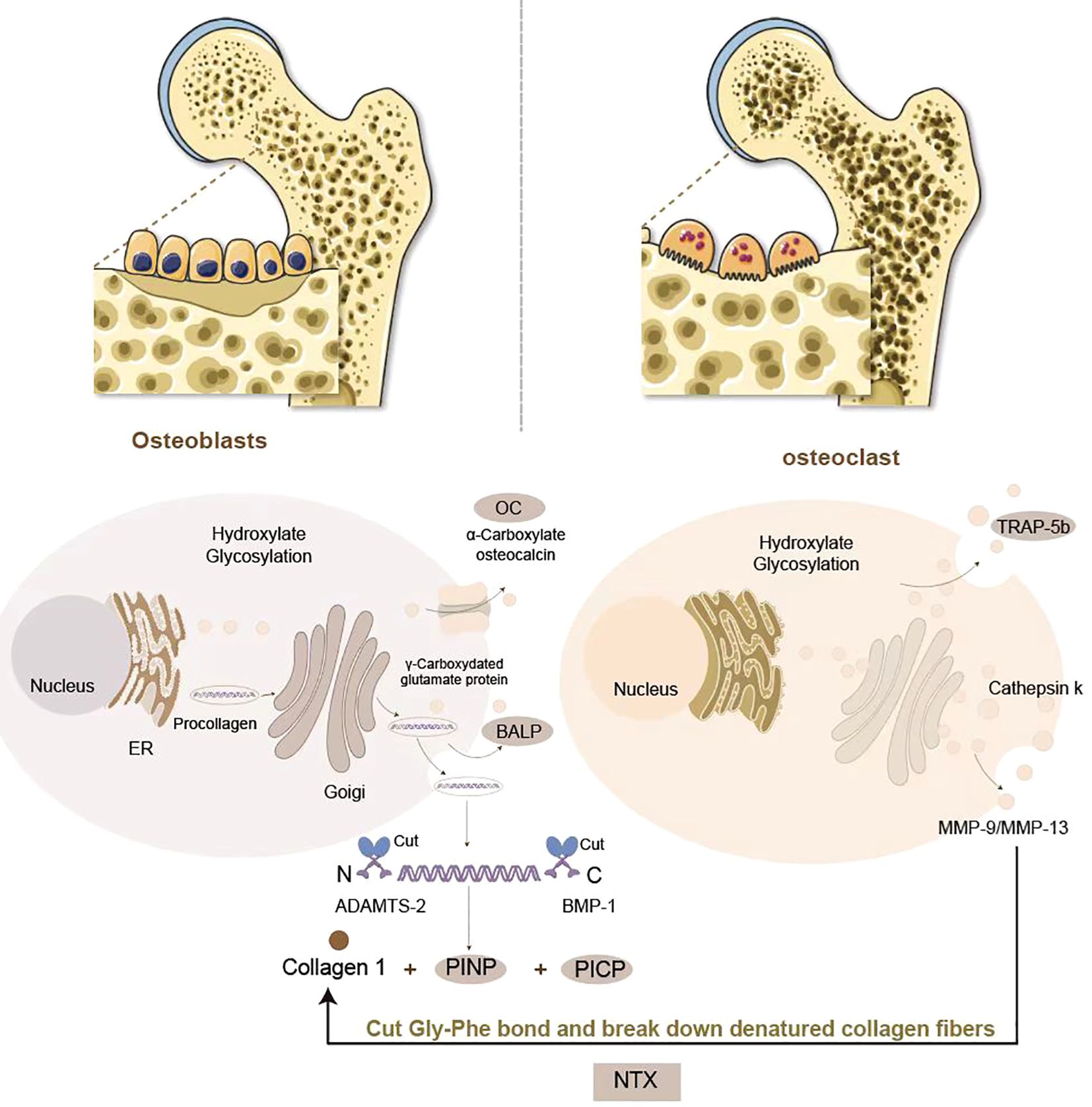
Figure 1. Generation of BTMs in bone formation and resorption: Osteoblasts orchestrate bone formation through type I collagen biosynthesis. PINP and PICP are produced by procollagen digestion, which serve as biomarkers of osteoblastic activity. Osteoblasts secrete BALP to facilitate hydroxyapatite deposition and OC to regulate mineralization. Osteoclasts mediate bone resorption via collagenolytic degradation. Upon attachment to the bone matrix, osteoclasts secrete tartrate-resistant acid phosphatase 5b (TRAP-5b) and create an acidic microenvironment through vacuolar H+-ATPases, activating cathepsin K and matrix metalloproteinases (MMP-9/MMP-13). These enzymes cleave the Gly-Phe bond in collagen’s triple helix, releasing NTX and CTX as biomarkers of osteoclastic activity.
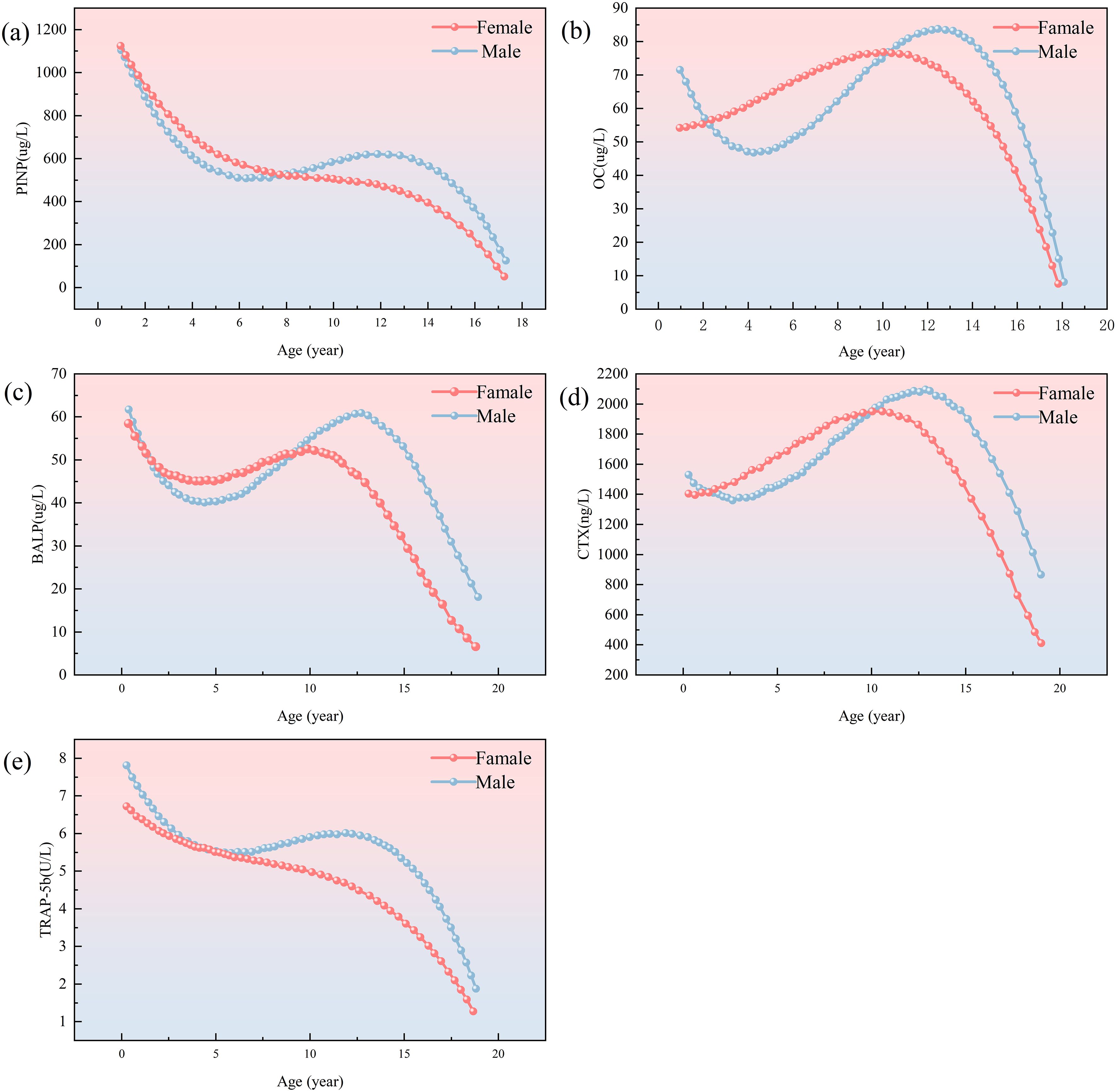
Figure 2. Trend charts for bone formation markers PINP, BALP, OC, CTX and TRAP-5b in male (blue dotted line) and female (red dotted line). Graph (a) shows PINP levels, decreasing with age for both genders. Graph (b) shows OCwith a peak in childhood. Graph (c) depicts BALP with similar trends. Graph (d) showsCTX levels peaking around age 15. Graph (e) illustrates TRACP-5b levels, decreasing asage increases. Modified and cited from Choi, J. S et al. (2019) (17), Rauchenzauner, M et al. (2007) (18).
4.1 Bone formation markers
4.1.1 PINP/PICP
Procollagen type I N-terminal propeptide (PINP) and C-terminal propeptide (PICP) are enzymatically cleaved fragments derived from the C-terminal domain of type I collagen during osteoblast-mediated biosynthesis. These peptides serve as specific biomarkers of bone formation, reflecting collagen I synthesis rates and osteoblast activity (3). Because PINP resistant to freeze-thaw cycles, it is easier and more economical to measure than PICP, it is more widely used in clinical practice (20, 21). The Bone Marker Criteria Working Group of the Osteoporosis Foundation and the International Federation of Clinical Chemistry and Laboratory Medicine have recommended the use of serum PINP as a reference marker for evaluating bone formation (17, 22). Significant differences in PINP between age and sex have been observed in many studies. Serum concentrations of PINP peak during infancy, decline intermittently through childhood, and diverge markedly during puberty. Adolescent males exhibit sustained PINP elevation correlating with delayed peak height velocity, whereas females demonstrate progressive declines post-menarche. These patterns mirror sex-dimorphic endocrine regulation of skeletal maturation and growth plate dynamics (23, 24).
4.1.2 ALP/BALP
Bone-specific alkaline phosphatase (BALP), a tissue-specific isoform of alkaline phosphatase (ALP), is exclusively synthesized by osteoblasts and serves as a specific biomarker of osteoblast activity. While total ALP exhibits limited diagnostic specificity due to its expression in hepatobiliary and intestinal tissues, BALP demonstrates superior specificity for skeletal pathology in pediatric populations, particularly given the low incidence of hepatic comorbidities in children (25, 26). Notably, BALP quantification is minimally confounded by renal dysfunction, establishing its utility as the preferred biomarker for bone metabolism assessment in hemodialysis patients (27). During growth periods, BALP and PINP exhibit parallel trajectories, peaking during early-to-mid puberty with strong correlations to height velocity. Sexual dimorphism is evident: adolescent males display higher peak BALP and PINP concentrations occurring later than females, reflecting androgen-driven growth plate maturation (18, 28, 29).
4.1.3 OC
OC (osteocalcin), a 49-amino acid γ-carboxyglutamic acid-containing protein secreted by osteoblasts, regulates calcium deposition and hydroxyapatite crystallization within the bone matrix (3). OC increases with age, peaking at 11–13 years of age in boys and 9–12 years of age in girls. Unlike BALP and PINP, the pubertal peak of OC may be lower in boys than in girls (17). Unlike PINP and BALP which reflect collagen synthesis and mineralization phases respectively, OC demonstrates dual functionality: while classically categorized as a formation marker, emerging evidence implicates OC in coupled bone turnover through paracrine modulation of osteoblast-osteoclast cross-talk (30, 31). Controversy persists regarding OC’s primary role, with some studies proposing its classification as a turnover biomarker given its homeostatic regulation of formation-resorption equilibrium independent of mineralization (17, 32). Methodologically, OC exhibits pronounced diurnal variation, with the highest concentration in the morning, necessitating standardized morning phlebotomy to ensure analytical reliability (17, 33).
4.2 Bone resorption markers
4.2.1 NTX/CTX
N-terminal telopeptide (NTX) and C-terminal telopeptide (CTX) of type I collagen are osteoclast-derived proteolytic fragments released during collagen I degradation—the primary collagenous component of bone matrix. These telopeptides serve as specific biomarkers for quantifying bone resorption rates and metabolic turnover (34). While NTX is predominantly urinary-excreted, CTX exists in both serum and urine. Due to its ease of measurement, CTX is commonly measured in clinical settings. By specifically targeting β-isomerized aspartic acid residues within the C-terminal telopeptide region of type I collagen, β-CTX immunoassays have emerged as the gold-standard clinical methodology due to their enhanced epitope specificity and consistently superior analytical performance demonstrated in cross-laboratory validation studies (3, 34). CTX expression is relatively stable and slightly increased in childhood, peaks in early adolescence, and subsequently decreases (35, 36). serum β-CTX levels exhibit gradual prepubertal increases in both sexes, followed by sexual dimorphism during puberty. Females show stabilization post-menarche correlating with earlier growth plate closure, whereas males maintain ascending β-CTX levels until late puberty, reflecting prolonged endochondral ossification (34, 36). Studies have shown that CTX is more susceptible to diet, circadian patterns and renal function than PINP. Data suggest that CTX levels are inaccurately increased in patients with significant renal impairment and may be five times higher in hemodialysis patients. Standardized morning fasting sampling is therefore recommended to minimize pre-analytical variability (14, 37).
4.2.2 TRAP-5b
Tartrate-resistant acid phosphatase (TRAP), a lysosomal enzyme secreted by osteoclasts and monocyte-macrophage lineage cells, exists as two isoforms: TRAP-5a and TRAP-5b. TRAP-5b, being osteoclast-specific, serves as a robust biomarker for quantifying osteoclast numbers and bone resorption activity (38). TRAP-5b exhibits childhood-specific patterns, with a maximum value at birth followed by an accelerated and sustained decline during puberty (18). Unlike CTX, TRAP-5b directly correlates with osteoclast population size and is less affected by renal function, so TRAP-5b can be used to measure bone resorption in patients with impaired renal function (14, 39).
5 Clinical applications
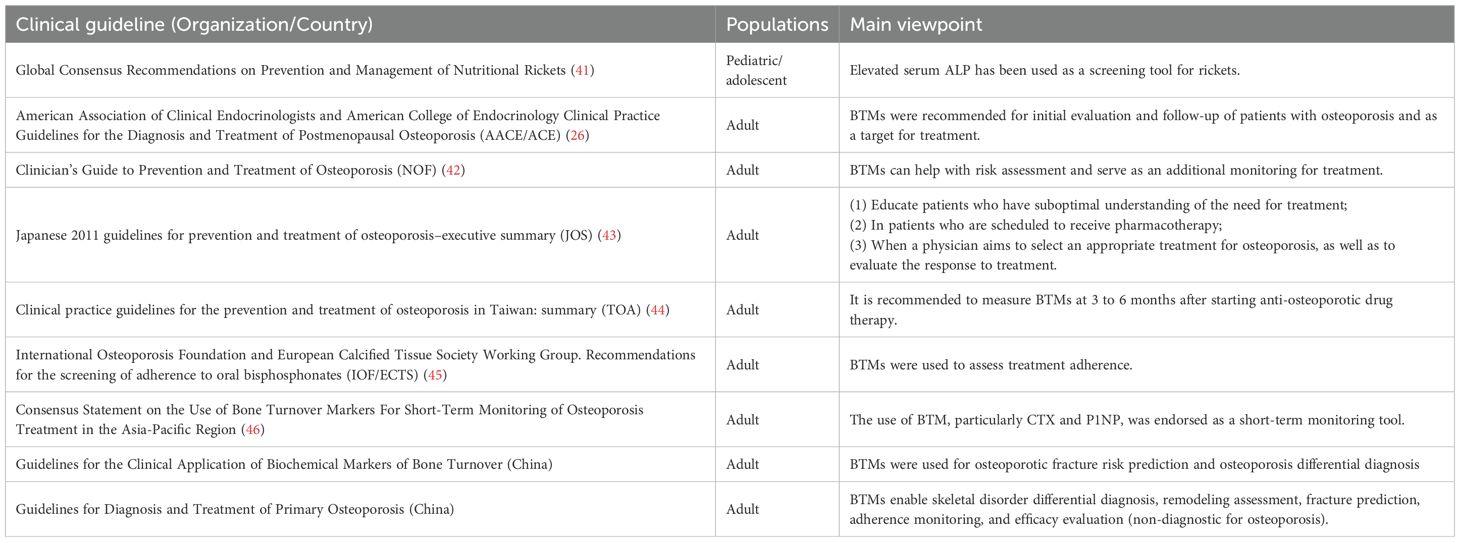
Bone metabolism markers are mostly used to assess bone health and bone metabolism status, and the most clinically used BTMs are PINP and CTX, which reflect bone formation and bone resorption respectively (2, 14, 40). BTMs play an important role in the assessment of bone metabolism-related disorders and the monitoring of bone metabolism modulators therapy. Table 1 summarized clinical guidelines and consensus for BTM use in bone disorders.
5.1 Bone metabolic diseases
5.1.1 Primary osteoporosis
Osteoporosis was previously recognized as a disease of old age, but is now of great concern in children and adolescents. The major determinant of lifetime risk for osteoporosis is the magnitude of peak bone mass attained in early adulthood, suggesting that bone health in childhood has a significant impact on lifetime risk for osteoporosis and osteoporotic fractures (35, 47). Osteoporosis in children is usually categorized into hereditary primary osteoporosis and secondary osteoporosis caused by systemic disease or drug use. The most common form of primary childhood osteoporosis is impaired osteogenesis, which is primarily associated with mutations in genes involved in the synthesis or post-transcriptional modification of type I collagen (48, 49). The International Society for Clinical Densitometry (ISCD) diagnostic criteria (2013 revision) mandate: (1) ≥1 vertebral compression fracture(s) without underlying focal pathology or high-impact trauma, or (2) low bone mineral density (BMD; Z-score <−2.0) plus significant fracture history (≥2 long bone fractures before age 10 or ≥3 before age 18) (48, 49).
Although not included in osteoporosis diagnostic criteria, BTMs are clinically essential for monitoring treatment response. BTMs are pivotal for monitoring antiresorptive therapy response, with bisphosphonate treatment typically reducing β-CTX by 50-80% within 2 months and PINP by 40-60% within 6 months (14). Multiple international guidelines recognize bone turnover markers (BTMs) as tools for initial osteoporosis assessment and treatment monitoring (Table 1). Their superior clinical responsiveness supports therapeutic decision-making. Consequently, the International Osteoporosis Foundation (IOF) and European Calcified Tissue Society (ECTS) recommend BTMs to detect suboptimal treatment adherence (45). International consensus further endorses BTM evaluation of bone remodeling status before initiating anti-osteoporosis therapy, facilitating early assessment of treatment adherence and responsiveness within months of commencement, thereby guiding ongoing therapeutic decisions (46). Specifically, IOF/ECTS advise baseline PINP or β-CTX measurement prior to oral bisphosphonate initiation, with reassessment at 3 months verifying reductions exceeding the least significant change (LSC) (50). Clinically, Denosumab is often used to treat osteoporosis, with transition to bisphosphonate therapy at the end of denosumab treatment. Within days of denosumab treatment, CTX decreases to near undetectable levels and PINP reaches its lowest value within 3–6 months. Without timely transition to bisphosphonate therapy after the final denosumab injection, BTMs rise rapidly, which may be associated with rebound compression fractures. The Endocrine Society therefore recommends the use of BTMs to guide the transition to denosumab therapy. And there are data from studies suggesting that patients who receive PINP monitoring are more likely to start oral bisphosphonate therapy after ending denosumab therapy (14, 51). However, additional studies are needed to validate the clinical usefulness of BTMs, establish standardized reference ranges, and determine the optimal timing and frequency of monitoring.
5.1.2 Rickets
Rickets, a metabolic bone disorder predominantly affecting children and adolescents, arises from vitamin D deficiency or aberrant vitamin D metabolism. Insufficient vitamin D impairs intestinal calcium and phosphate absorption, leading to hypocalcemia and hypophosphatemia that disrupt normal bone mineralization45. In hypophosphatemic rickets, bone formation markers such as OC and ALP may be elevated showing an increase in bone formation stimulation, while a decrease in ALP activity is a good indicator of treatment efficacy. Conversely, bone resorption markers such as NTX may be elevated, reflecting accelerated bone resorption and the extent of bone destruction (52, 53). These dynamic BTM profiles enable quantitative assessment of disease severity and treatment efficacy in rickets management.
5.1.3 Primary hyperparathyroidism
Primary hyperparathyroidism (PHPT) characterized by autonomous overproduction of parathyroid hormone (PTH) from one or more parathyroid glands. Studies have shown that serum markers of bone formation, such as BALP, are often elevated in patients with PHPT, whereas bone resorption markers may not be significantly increased. Early biochemical intervention is critical to mitigate progressive bone loss and osteoporosis risk. Serial BTM monitoring, combined with DXA and BMD assessment, facilitates personalized management to prevent fragility fractures (54, 55). Parathyroidectomy (PTX) normalizes BTMs, with β-CTX restoration to physiological levels indicating successful resolution of PTH-driven bone resorption (55).
PHPT associated with multiple parathyroid gland hyperplasia and/or adenomas (syndromic type) is a multiple endocrine tumor type 1 (MEN 1), the most common endocrinopathy, which is an inherited disorder. Elevated PTH begins in late childhood, adolescence, or early adulthood and may negatively affect the normal acquisition of peak bone mass. It was found that in untreated patients with MEN1-associated PHPT, serum bone alkaline phosphatase (BALP) levels were significantly elevated, showing higher osteoblastic activity. In contrast, in patients with sporadic PHPT, BALP levels were within the normal range. In addition, it was observed that serum PTH, calcium ion, total calcium and BALP levels were significantly reduced in patients with MEN1-associated PHPT and sporadic PHPT after PTX. This suggests that PTX restores normal levels of serum BTMs and reduces bone resorption activity (56). Thus, integrative BTM and BMD evaluation optimizes therapeutic decision-making in PHPT management.
5.1.4 Juvenile idiopathic arthritis
Juvenile idiopathic arthritis (JIA) is a disease associated with imbalances in bone metabolism. Studies demonstrate that BTMs correlate with disease activity and therapeutic response in JIA, BALP levels reflect inflammatory osteoblast activation (57), while increased β-CTX concentrations are associated with adiposity and hyperleptinemia (58, 59). Concomitant reductions in BMD further underscore the elevated osteoporosis and fracture risk in this population (57).
Current data suggest that JIA-associated bone loss arises from a resorption-formation imbalance, driven by chronic inflammation and metabolic disturbances. While BTM profiling provides actionable insights into skeletal health and treatment efficacy, the precise mechanisms linking JIA pathophysiology to bone remodeling remain incompletely elucidated. Targeted interventions to preserve bone mass in JIA, particularly those addressing inflammatory and leptin-mediated pathways, require further translational and clinical validation.
5.2 Endocrine diseases
5.2.1 Obesity
Pediatric obesity is associated with suppressed bone turnover, particularly in females, as evidenced by reduced OC, NTX, and OC/NTX ratios. This hypometabolic state may arise from dual mechanisms: (1) impaired osteoblast differentiation or activity due to adipocyte-dominated marrow microenvironmental remodeling; and (2) chronic low-grade inflammation with elevated TNF-α, IL-6 inhibiting Wnt/β-catenin signaling pathways critical for osteogenesis (36). The adipokine imbalance characteristic of obesity, notably hyperleptinemia, further exacerbates skeletal metabolism dysregulation through central nervous system-mediated suppression of bone formation (60).Although these mechanisms collectively suggest reduced bone strength and compromised peak bone mass accrual in obese adolescents, causal relationships remain contentious. Prospective cohort studies are needed to clarify whether obesity-induced bone turnover suppression independently predicts long-term fracture risk or merely associates with established osteoporosis risk factors.
5.2.2 Diabetes
Type 1 diabetes mellitus (T1DM) exerts deleterious effects on pediatric bone health through multifactorial pathways. Chronic hyperglycemia disrupts calcium-vitamin D homeostasis, induces advanced glycation end-product (AGE) accumulation in bone collagen, promotes marrow adiposity via osteoblast-adipocyte transdifferentiation, and exacerbates oxidative stress—collectively impairing osteoblastogenesis while enhancing osteoclastic activity (61). Evidence indicates that T1DM children exhibit suppressed bone remodeling, characterized by reduced bone formation markers (e.g., PINP) and elevated resorption markers (e.g., β-CTX), predisposing them to low BMD and fragility fractures (61, 62).
While optimized glycemic control may attenuate these metabolic perturbations, the precise molecular mechanisms underlying diabetic osteopathy, particularly AGE-RAGE axis activation and Wnt/β-catenin pathway inhibition, require further elucidation. Targeted therapeutic strategies combining glycemia management with bone anabolic agents represent a promising avenue for mitigating skeletal complications in pediatric T1DM.
5.3 Tumors
Acute lymphoblastic leukemia (ALL), the most prevalent pediatric malignancy, has achieved 5-year survival rates exceeding 90% through modern therapeutic protocols. However, skeletal changes observed at the time of diagnosis and during treatment negatively impact the skeletal health of patients, including osteolysis, sclerosis, and osteoporosis. The occurrence of these skeletal changes is largely attributed to the disease itself as well as the effects of intensive treatment regimens (e.g., methotrexate and glucocorticoids) on the skeleton (63). It has been found that pediatric ALL survivors continue to face decreased bone density after treatment, but there is a lack of clarity about the timing of the onset of bone loss, recovery, and long-term prognosis. The causes of decreased bone density may be multifactorial, including the disease itself, concurrent infections, malnutrition, decreased physical activity and abnormal vitamin D metabolism, as well as the therapeutic drugs and radiation therapy used (10, 63). Biochemical evidence demonstrates suppressed bone turnover during treatment, characterized by decreased ALP, PICP, and elevated β-CTX indicative of impaired formation and enhanced resorption (64). These alterations underscore the need for targeted skeletal surveillance in ALL survivorship care.
Bone tumors are characterized by pathological disruption of bone remodeling homeostasis, manifesting as imbalanced osteogenic-osteoclastic coupling. In osteosarcoma, BALP levels are consistently elevated compared to benign bone lesions, with meta-analyses demonstrating 3- to 5-fold higher concentrations (65). BALP quantification exhibits particular diagnostic value in radiologically equivocal cases, achieving 82% sensitivity and 91% specificity for differentiating osteosarcoma from benign tumors and osseous metastases in multicenter studies (66). Current consensus guidelines therefore recommend BALP as an adjunctive diagnostic tool rather than a standalone biomarker, requiring integration with imaging and histopathological findings for definitive diagnosis (65).
6 Discussion
Although BTMs aid in assessing pediatric bone health, predicting bone loss, and monitoring therapeutic efficacy, their clinical utility is limited by inherent biological variability and methodological differences, necessitating careful interpretation (67). Physiological variability arises from modifiable and non-modifiable factors. Modifiable factors include age, sex, ethnicity, and medication use, underscoring the significant clinical value of establishing pediatric-specific BTM reference intervals (RIs) (68). However, developing robust RIs requires large pediatric cohorts and addresses complex ethical considerations. Per CLSI EP28-A3 guidelines, establishing pediatric RIs mandates stratification by sex and narrow age intervals (e.g., 1–2 years), with each subgroup requiring more than 120 reference individuals to ensure statistical validity and clinical relevance. Furthermore, RIs require periodic reassessment to account for evolving laboratory methodologies (3, 35). Among non-modifiable factors, circadian rhythm exerts the strongest influence, followed by exercise intensity, seasonal variation, and dietary status (37, 67). Consequently, standardized morning fasting blood collection is recommended to enhance measurement consistency (69).
In terms of methodological differences, the clinical application of BTMs faces challenges in terms of standardization and measurement accuracy (70). Currently, the commonly used detection methods for BTMs are enzyme-linked immunosorbent assay (ELISA) and chemiluminescence immunoassay (CLIA) (6, 71). Different manufacturers use their own testing procedures and primary calibrators to assign values to their company’s products, and different laboratories use different measurement methods and units, making it difficult to compare and interpret results. Table 2 summarizes the pediatric reference ranges in different manufacturers. To address the issues of traceability and standardization of bone turnover marker measurements, it is possible to develop a reference measurement system and produce standard materials. By standardizing BTM measurements, researchers can ensure that the obtained results reflect the true bone turnover status, enabling more accurate diagnosis and monitoring of treatment (72–75). In this regard, the International Osteoporosis Foundation (IOF) and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) have convened the IOF-IFCC Bone Marker Standards Working Group for further research. Through developing commutable international reference materials, conducting methodological comparability studies, and standardizing detection protocols, our consortium has markedly improved the reliability and clinical utility of BTMs (76, 77). Consequently, significant age- and sex-dependent variations in BTMs among pediatric and adolescent populations will gain greater clinical relevance through future advances in reference interval establishment and methodological standardization. This progress will enhance BTM utility for growth monitoring, disease diagnosis, and therapeutic evaluation in these cohorts.
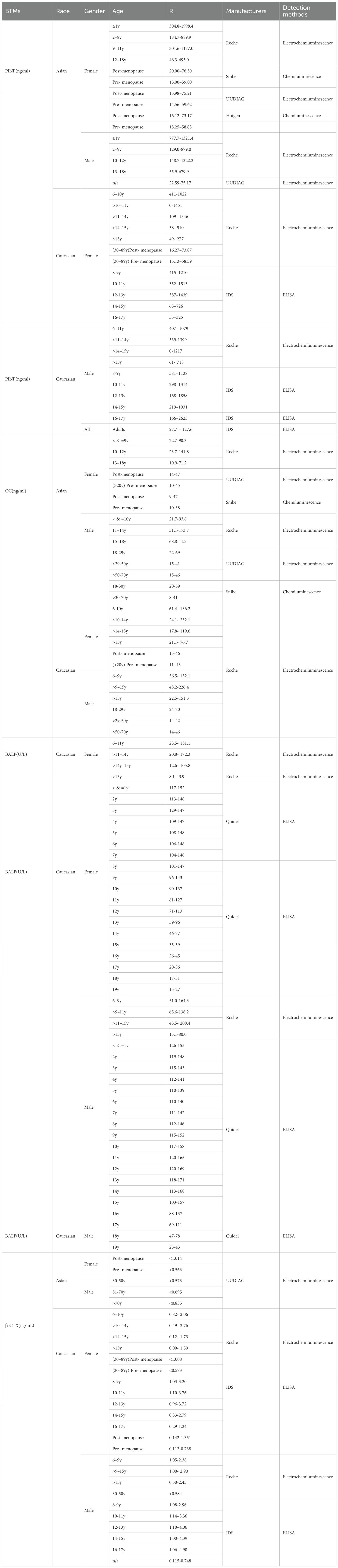
Table 2. Sudies providing the reference intervals (RIs) of BTMs in different race, gender, ages and manufacturers.
Studies in perimenopausal women demonstrate a clear association between elevated BTMs and cortical/trabecular bone loss, with particularly strong correlations observed at the spine (37). However, comparable evidence linking BTMs to bone mass changes remains scarce for male and pediatric populations. Future research should establish BTM-bone mass relationships across demographics and identify optimal markers for early diagnosis and treatment monitoring. Complementary approaches, such as Bone Balance Index (BBI) studies, demonstrate utility for predicting bone loss in perimenopausal populations (78). Future efforts should focus on integrating BTMs with other clinical indicators such as bone densitometry to enhance the accuracy and efficiency of diagnosis and monitoring. Combining these different measures can improve the sensitivity and specificity of diagnosis and monitoring, allowing for earlier detection of bone-related disorders and more effective treatment strategies (14). Furthermore, more efforts should integrate combining proteomic BTM profiles with genomic signatures to unravel individualized bone remodeling dynamics. This will be beneficial in bridging the gap between mechanistic insights and clinical translation.
Author contributions
YW: Writing – original draft, Software, Investigation. HH: Methodology, Writing – review & editing. YH: Project administration, Writing – review & editing, Resources.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work is supported by the Chengdu Municipal Bureau of Science and Technology program (2022-YF05-01613-SN) and Science and Technology Department of Sichuan Province program (2023NSFSC0033).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang Y, Zhang J, Huang X, Yu X, Li Y, Yu F, et al. Variation of bone turnover markers in childhood and adolescence. Int J Clin Practice. (2023) 2023:5537182. doi: 10.1155/2023/5537182
2. Zhang Y, Huang X, Li C, Zhang J, Yu X, Li Y, et al. Broad application prospects of bone turnover markers in pediatrics. J Clin Lab analysis. (2022) 36:e24656. doi: 10.1002/jcla.24656
3. Ladang A, Rauch F, Delvin E, and Cavalier E. Bone turnover markers in children: from laboratory challenges to clinical interpretation. Calcified Tissue Int. (2023) 112:218–32. doi: 10.1007/s00223-022-00964-2
4. Cao B, Liu M, Luo Q, Wang Q, Liu M, Liang X, et al. The effect of BMI, age, gender, and pubertal stage on bone turnover markers in Chinese children and adolescents. Front Endocrinology. (2022) 13:880418. doi: 10.3389/fendo.2022.880418
5. Gracia-Marco L, Ortega FB, Jiménez-Pavón D, Rodríguez G, Valtueña J, Díaz-Marténez ÁE, et al. Contribution of bone turnover markers to bone mass in pubertal boys and girls. J Pediat Endocrin Metab. (2011) 24(11-12):971–4. doi: 10.1515/JPEM.2011.326
6. Schini M, Vilaca T, Gossiel F, Salam S, and Eastell R. Bone turnover markers: basic biology to clinical applications. Endocrine Rev. (2023) 44:417–73. doi: 10.1210/endrev/bnac031
7. Mora S, Pitukcheewanont P, Kaufman FR, Nelson JC, and Gilsanz V. Biochemical markers of bone turnover and the volume and the density of bone in children at different stages of sexual development. J Bone mineral Res. (1999) 14:1664–71. doi: 10.1359/jbmr.1999.14.10.1664
8. Zhang J, Gao R, Jiang Y, Zhang Y, Liu C, Yu F, et al. Novel serological biomarker models composed of bone turnover markers, vitamin D, and estradiol and their auxiliary diagnostic value in girls with idiopathic central precocious puberty. Bone. (2022) 154:116221. doi: 10.1016/j.bone.2021.116221
9. Tuchman S, Thayu M, Shults J, Zemel BS, Burnham JM, and Leonard MB. Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease. J pediatrics. (2008) 153:484–90. e2. doi: 10.1016/j.jpeds.2008.04.028
10. Alikasifoglu A, Yetgin S, Cetin M, Tuncer M, Gumruk F, Gurgey A, et al. Bone mineral density and serum bone turnover markers in survivors of childhood acute lymphoblastic leukemia: Comparison of megadose methylprednisolone and conventional-dose prednisolone treatments. Am J hematology. (2005) 80:113–8. doi: 10.1002/ajh.20438
11. Parfitt AM, Travers R, Rauch F, and Glorieux FH. Structural and cellular changes during bone growth in healthy children. Bone. (2000) 27:487–94. doi: 10.1016/S8756-3282(00)00353-7
12. Bayer M. Reference values of osteocalcin and procollagen type I N-propeptide plasma levels in a healthy Central European population aged 0–18 years. Osteoporosis Int. (2014) 25:729–36. doi: 10.1007/s00198-013-2485-4
13. Eastell R and Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes endocrinology. (2017) 5:908–23. doi: 10.1016/S2213-8587(17)30184-5
14. Jain S. Role of bone turnover markers in osteoporosis therapy. Endocrinol Metab Clinics. (2021) 50:223–37. doi: 10.1016/j.ecl.2021.03.007
15. Bellissimo MP, Roberts JL, Jones DP, Liu KH, Taibl KR, Uppal K, et al. Metabolomic associations with serum bone turnover markers. Nutrients. (2020) 12:3161. doi: 10.3390/nu12103161
16. Jürimäe J. Interpretation and application of bone turnover markers in children and adolescents. Curr Opin pediatrics. (2010) 22:494–500. doi: 10.1097/MOP.0b013e32833b0b9e
17. Choi JS, Park I, Lee SJ, Ju HJ, Lee H, and Kim J. Serum procollagen type I N-terminal propeptide and osteocalcin levels in Korean children and adolescents. Yonsei Med J. (2019) 60:1174–80. doi: 10.3349/ymj.2019.60.12.1174
18. Rauchenzauner M, Schmid A, Heinz-Erian P, Kapelari K, Falkensammer G, Griesmacher A, et al. Sex-and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. (2007) 92:443–9. doi: 10.1210/jc.2006-1706
19. Van Coeverden S, Netelenbos J, De Ridder C, Roos J, Popp-Snijders C, and Delemarre-van de Waal H. Bone metabolism markers and bone mass in healthy pubertal boys and girls. Clin endocrinology. (2002) 57:107–16. doi: 10.1046/j.1365-2265.2002.01573.x
20. Bauer D, Krege J, Lane N, Leary E, Libanati C, Miller P, et al. National Bone Health Alliance Bone Turnover Marker Project: current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporosis Int. (2012) 23:2425–33. doi: 10.1007/s00198-012-2049-z
21. Vasikaran S, Cooper C, Eastell R, Griesmacher A, Morris HA, Trenti T, et al. International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med (CCLM). (2011) 49:1271–4. doi: 10.1515/CCLM.2011.602
22. Qvist P, Christgau S, Pedersen BJ, Schlemmer A, and Christiansen C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone. (2002) 31:57–61. doi: 10.1016/S8756-3282(02)00791-3
23. Yilmaz D, Ersoy B, Bilgin E, Gümüşer G, Onur E, and Pinar ED. Bone mineral density in girls and boys at different pubertal stages: relation with gonadal steroids, bone formation markers, and growth parameters. J Bone mineral Metab. (2005) 23:476–82. doi: 10.1007/s00774-005-0631-6
24. Szulc P, Seeman E, and Delmas P. Biochemical measurements of bone turnover in children and adolescents. Osteoporosis Int. (2000) 11:281–94. doi: 10.1007/s001980070116
25. Van Hoof VO and De Broe ME. Interpretation and clinical significance of alkaline phosphatase isoenzyme patterns. Crit Rev Clin Lab Sci. (1994) 31:197–293. doi: 10.3109/10408369409084677
26. Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, et al. American association of clinical endocrinologists and american college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis — 2016–executive summary. Endocrine Practice. (2016) 22:1111–8. doi: 10.4158/EP161435.ESGL
27. Cavalier E, Delanaye P, Collette J, Krzesinski J-M, and Chapelle J-P. Evaluation of different bone markers in hemodialyzed patients. Clinica chimica Acta. (2006) 371:107–11. doi: 10.1016/j.cca.2006.02.029
28. Ladang A, Rousselle O, Huyghebaert L, Bekaert A-C, Kovacs S, Le Goff C, et al. Parathormone, bone alkaline phosphatase and 25-hydroxyvitamin D status in a large cohort of 1200 children and teenagers. Acta Clinica Belgica. (2022) 77:4–9. doi: 10.1080/17843286.2020.1769285
29. Fischer D-C, Mischek A, Wolf S, Rahn A, Salweski B, Kundt G, et al. Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase. Ann Clin Biochem. (2012) 49:546–53. doi: 10.1258/acb.2012.011274
30. Christo K, Prabhakaran R, Lamparello B, Cord J, Miller KK, Goldstein MA, et al. Bone metabolism in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects. Pediatrics. (2008) 121:1127–36. doi: 10.1542/peds.2007-2392
31. Zürcher S, Borter N, Kränzlin M, Neyer P, Meyer U, Rizzoli R, et al. Relationship between bone mineral content and bone turnover markers, sex hormones and calciotropic hormones in pre-and early pubertal children. Osteoporosis Int. (2020) 31:335–49. doi: 10.1007/s00198-019-05180-7
32. Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. (1996) 382:448–52. doi: 10.1038/382448a0
33. van der Spoel E, Oei N, Cachucho R, Roelfsema F, Berbee JF, Blauw GJ, et al. The 24-hour serum profiles of bone markers in healthy older men and women. Bone. (2019) 120:61–9. doi: 10.1016/j.bone.2018.10.002
34. Garnero P, Ferreras M, Karsdal M, Nicamhlaoibh R, Risteli J, Borel O, et al. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Mineral Res. (2003) 18:859–67. doi: 10.1359/jbmr.2003.18.5.859
35. Diemar SS, Lylloff L, Rønne MS, Møllehave LT, Heidemann M, Thuesen BH, et al. Reference intervals in Danish children and adolescents for bone turnover markers carboxy-terminal cross-linked telopeptide of type I collagen (β-CTX), pro-collagen type I N-terminal propeptide (PINP), osteocalcin (OC) and bone-specific alkaline phosphatase (bone ALP). Bone. (2021) 146:115879. doi: 10.1016/j.bone.2021.115879
36. Geserick M, Vogel M, Eckelt F, Schlingmann M, Hiemisch A, Baber R, et al. Children and adolescents with obesity have reduced serum bone turnover markers and 25-hydroxyvitamin D but increased parathyroid hormone concentrations–results derived from new pediatric reference ranges. Bone. (2020) 132:115124. doi: 10.1016/j.bone.2019.115124
37. Greenblatt MB, Tsai JN, and Wein MN. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin Chem. (2017) 63:464–74. doi: 10.1373/clinchem.2016.259085
38. Nowak Z, Konieczna M, and Wańkowicz Z. Tartrate-resistant acid phosphatase–TRAP 5b–as a novel marker of bone resorption in patients with irreversible renal failure treated with dialysis. Polski Merkuriusz Lekarski: Organ Polskiego Towarzystwa Lekarskiego. (2004) 17:138–41.
39. Cavalier E. Bone markers and chronic kidney diseases. J Lab Precis Med. (2018) 3. doi: 10.21037/jlpm.2018.07.03
40. Banica T, Vandewalle S, Zmierczak H-G, Goemaere S, De Buyser S, Fiers T, et al. The relationship between circulating hormone levels, bone turnover markers and skeletal development in healthy boys differs according to maturation stage. Bone. (2022) 158:116368. doi: 10.1016/j.bone.2022.116368
41. Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. (2016) 101:394–415. doi: 10.1210/jc.2015-2175
42. Cosman F, de Beur SJ, LeBoff M, Lewiecki E, Tanner B, Randall S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporosis Int. (2014) 25:2359–81. doi: 10.1007/s00198-014-2794-2
43. Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis–executive summary. Springer Open Choice. (2012) 7:3–20. doi: 10.1007/s11657-012-0109-9
44. Hwang J-S, Chan D-C, Chen J-F, Cheng T-T, Wu C-H, Soong Y-K, et al. Clinical practice guidelines for the prevention and treatment of osteoporosis in Taiwan: summary. J Bone mineral Metab. (2014) 32:10–6. doi: 10.1007/s00774-013-0495-0
45. Diez-Perez A, Naylor KE, Abrahamsen B, Agnusdei D, Brandi ML, Cooper C, et al. International osteoporosis foundation and european calcified tissue society working group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporosis Int. (2017) 28:767–74. doi: 10.1007/s00198-017-3906-6
46. Wu C-H, Chang Y-F, Chen C-H, Lewiecki EM, Wüster C, Reid I, et al. Consensus statement on the use of bone turnover markers for short-term monitoring of osteoporosis treatment in the Asia-Pacific region. J Clin Densitometry. (2021) 24:3–13. doi: 10.1016/j.jocd.2019.03.004
47. Grover M and Bachrach LK. Osteoporosis in children with chronic illnesses: diagnosis, monitoring, and treatment. Curr Osteoporosis Rep. (2017) 15:271–82. doi: 10.1007/s11914-017-0371-2
48. Rauch F, Plotkin H, DiMeglio L, Engelbert RH, Henderson RC, Munns C, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2007 Pediatric Official Positions. J Clin Densitometry. (2008) 11:22–8. doi: 10.1016/j.jocd.2007.12.003
49. Gordon CM, Leonard MB, and Zemel BS. 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin densitometry. (2014) 17:219–24. doi: 10.1016/j.jocd.2014.01.007
50. Davis S, Simpson E, Hamilton J, Martyn-St James M, Rawdin A, Wong R, et al. Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: a systematic review and economic evaluation. Health Technol Assess (Winchester England). (2020) 24:1. doi: 10.3310/hta24290
51. Mattia L, Davis S, Mark-Wagstaff C, Abrahamsen B, Peel N, Eastell R, et al. Utility of PINP to monitor osteoporosis treatment in primary care, the POSE study (PINP and Osteoporosis in Sheffield Evaluation). Bone. (2022) 158:116347. doi: 10.1016/j.bone.2022.116347
52. Colares Neto G, Pereira RMR, Alvarenga J, Takayama L, Funari M, and Martin R. Evaluation of bone mineral density and microarchitectural parameters by DXA and HR-pQCT in 37 children and adults with X-linked hypophosphatemic rickets. Osteoporosis Int. (2017) 28:1685–92. doi: 10.1007/s00198-017-3949-8
53. Michałus I, Łupińska A, Woch I, Wieczorek-Szukała K, Chlebna-Sokół D, and Lewiński A. Bone turnover markers and bone mineral density in children with hypophosphatemic rickets. J Clin Med. (2022) 11:4622. doi: 10.3390/jcm11154622
54. Ni W, Yuan Y, Chu X, Chen G, Han X, Li J, et al. Bone turnover markers in response to ultrasound-guided microwave ablation for primary hyperparathyroidism. Front Endocrinology. (2021) 12:782050. doi: 10.3389/fendo.2021.782050
55. Cortet B, Cortet C, Blanckaert F, Racadot A, d'Herbomez M, Marchandise X, et al. Bone ultrasonometry and turnover markers in primary hyperparathyroidism. Calcified Tissue Int. (2000) 66:11–5. doi: 10.1007/s002230050004
56. Marini F, Giusti F, Cioppi F, Maraghelli D, Cavalli T, Tonelli F, et al. Bone and Mineral Metabolism Phenotypes in MEN1-Related and Sporadic Primary Hyperparathyroidism, before and after Parathyroidectomy. Cells. (2021) 10:1895. doi: 10.3390/cells10081895
57. Janicka-Szczepaniak M, Orczyk K, Szymbor K, Chlebna-Sokół D, and Smolewska E. Is it possible to predict a risk of osteoporosis in patients with juvenile idiopathic arthritis? A study of serum levels of bone turnover markers. Acta Biochim Polonica. (2018) 65:297–302. doi: 10.18388/abp.2017_2561
58. Górska A, Urban M, Bartnicka M, Żelazowska-Rutkowska B, and Wysocka J. Bone mineral metabolism in children with juvenile idiopathic arthritis–preliminary report. Ortopedia Traumatologia Rehabilitacja. (2008) 10:54–62.
59. Markula-Patjas KP, Ivaska KK, Pekkinen M, Andersson S, Moilanen E, Viljakainen HT, et al. High adiposity and serum leptin accompanied by altered bone turnover markers in severe juvenile idiopathic arthritis. J Rheumatol. (2014) 41:2474–81. doi: 10.3899/jrheum.131107
60. Matusik P, Olszanecka-Glinianowicz M, Chudek J, and Małecka-Tendera E. Bone turnover markers in the obese children–relation to gender, body composition and leptin level. Pediatr Endocrinol Diabetes Metab. (2015) 21:154–61. doi: 10.18544/PEDM-21.04.0037
61. El Amrousy D, El-Afify D, and Shabana A. Relationship between bone turnover markers and oxidative stress in children with type 1 diabetes mellitus. Pediatr Res. (2021) 89:878–81. doi: 10.1038/s41390-020-01197-5
62. Jaworski M, Wierzbicka E, Czekuć-Kryśkiewicz E, Płudowski P, Kobylińska M, and Szalecki M. Bone density, geometry, and mass by peripheral quantitative computed tomography and bone turnover markers in children with diabetes mellitus type 1. J Diabetes Res. (2022) 2022:9261512. doi: 10.1155/2022/9261512
63. Delvin E, Alos N, Rauch F, Marcil V, Morel S, Boisvert M, et al. Vitamin D nutritional status and bone turnover markers in childhood acute lymphoblastic leukemia survivors: A PETALE study. Clin Nutr. (2019) 38:912–9. doi: 10.1016/j.clnu.2018.02.006
64. Crofton PM, Ahmed SF, Wade JC, Elmlinger MW, Ranke MB, Kelnar CJ, et al. Bone turnover and growth during and after continuing chemotherapy in children with acute lymphoblastic leukemia. Pediatr Res. (2000) 48:490–6. doi: 10.1203/00006450-200010000-00012
65. Wang J, Pei F, Tu C, Zhang H, and Qiu X. Serum bone turnover markers in patients with primary bone tumors. Oncology. (2008) 72:338–42. doi: 10.1159/000113063
66. Du W-X, Duan S-F, Chen J-J, Huang J-F, Yin L-M, and Tong P-J. Serum bone-specific alkaline phosphatase as a biomarker for osseous metastases in patients with Malignant carcinomas: a systematic review and meta-analysis. J Cancer Res Ther. (2014) 10:C140–C3. doi: 10.4103/0973-1482.145842
67. Glendenning P. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards: osteoporos int. Osteoporosis international. (2011) 22:391–420. doi: 10.1007/s00198-010-1501-1
68. Hannon R and Eastell R. Preanalytical variability of biochemical markers of bone turnover. Osteoporosis Int. (2000) 11:S30–44. doi: 10.1007/s001980070004
69. Szulc P and Delmas P. Biochemical markers of bone turnover: potential use in the investigation and management of postmenopausal osteoporosis. Osteoporosis Int. (2008) 19:1683–704. doi: 10.1007/s00198-008-0660-9
70. Vasikaran S, Eastell R, Bruyere O, Foldes A, Garnero P, Griesmacher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporosis Int. (2011) 22:391–420. doi: 10.1007/s00198-010-1501-1
71. Garnero P, Borel O, and Delmas PD. Evaluation of a fully automated serum assay for C-terminal cross-linking telopeptide of type I collagen in osteoporosis. Clin Chem. (2001) 47:694–702. doi: 10.1093/clinchem/47.4.694
72. Müller MM. Implementation of reference systems in laboratory medicine. Clin Chem. (2000) 46:1907–9. doi: 10.1093/clinchem/46.12.1907
73. Panteghini M. Traceability, reference systems and result comparability. Clin Biochemist Rev. (2007) 28:97.
74. Morris HA. Traceability and standardization of immunoassays: a major challenge. Clin Biochem. (2009) 42:241–5. doi: 10.1016/j.clinbiochem.2008.09.005
75. Panteghini M. Traceability as a unique tool to improve standardization in laboratory medicine. Clin Biochem. (2009) 42:236–40. doi: 10.1016/j.clinbiochem.2008.09.098
76. Bhattoa HP, Cavalier E, Eastell R, Heijboer AC, Jørgensen NR, Makris K, et al. Analytical considerations and plans to standardize or harmonize assays for the reference bone turnover markers PINP and β-CTX in blood. Clinica Chimica Acta. (2021) 515:16–20. doi: 10.1016/j.cca.2020.12.023
77. Cavalier E, Eastell R, Jørgensen NR, Makris K, Tournis S, Vasikaran SD, et al. A multicenter study to evaluate harmonization of assays for C-terminal telopeptides of type I collagen ([béta]-CTX): a report from the IFCC-IOF committee for bone metabolism (C-BM). Calcified Tissue Int. (2021) 108.6(2021):785–97. doi: 10.1007/s00223-021-00816-5
Keywords: bone turnover markers, children and adolescents, bone metabolism, pediatric bone health, pediatric developmental disorders
Citation: Wang Y, Hu H and Huang Y (2025) Advances in the application of bone turnover markers for pediatric growth and developmental disorders: a review. Front. Endocrinol. 16:1615712. doi: 10.3389/fendo.2025.1615712
Received: 21 April 2025; Accepted: 25 August 2025;
Published: 10 September 2025.
Edited by:
Sally Radovick, The State University of New Jersey, United StatesReviewed by:
Francesca Arfuso, University of Messina, ItalyShangfu Li, Third Affiliated Hospital of Sun Yat-sen University, China
Copyright © 2025 Wang, Hu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Honghua Hu, aGhoMDgyNTQwMDA2M0AxNjMuY29t; Yi Huang, aHd1YW5neWlAMTI2LmNvbQ==
 Yuxin Wang1,2
Yuxin Wang1,2 Yi Huang
Yi Huang