- 1Department of Orthopedics, The First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
- 2First School of Clinical Medicine, The First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
- 3Department of Pathology, The First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
Osteoporosis is a common age-related bone metabolic disorder that significantly affects skeletal health, especially in aging populations. With global demographic shifts, the rising prevalence and disability burden of osteoporosis has placed increasing pressure on healthcare systems, making it a key area of research. A crucial factor in osteoporotic progression is the aging of mesenchymal stem cells (MSCs), which weakens bone regeneration through multiple mechanisms, including reduced osteogenic differentiation, heightened oxidative stress, chronic inflammation, and disrupted bone homeostasis. This review explores the intricate relationship between MSCs aging and osteoporosis development, focusing on key processes such as cell cycle arrest, telomere shortening, epigenetic changes, and osteogenic marker expression dysregulation. We also examine potential therapeutic strategies aimed at alleviating MSCs aging, including stem cell-based treatments, senolytic agents, inhibitors targeting the senescence-associated secretory phenotype, and biomaterial-assisted approaches such as extracellular vesicles and stimuli-responsive hydrogels. This review aims to provide insights into developing precise therapeutic strategies to restore MSCs function and slow bone loss. Furthermore, we discuss interdisciplinary approaches that link molecular mechanisms to practical applications, offering a broader perspective on addressing osteoporosis in aging societies.
1 Introduction
Aging is an irreversible physiological process in living organisms, characterized by a gradual decline in physiological functions, which leads to tissue damage and dysfunction. Aging is not only a natural biological phenomenon but also a fundamental cause of many chronic degenerative diseases, including cancer, diabetes, osteoporosis, and Alzheimer’s disease (1). At the macroscopic level, aging is characterized by a progressive decline in cellular proliferation and repair capacity, increasing susceptibility to disease and physiological deterioration. The mechanisms of aging are multifaceted, among which cellular senescence serves as a central contributor (2). Hallmark features of cellular senescence include reduced proliferative capacity, metabolic dysregulation, and heightened inflammatory responses. Notably, stem cell senescence, as a pivotal aspect of aging, exerts a more profound impact on the entire organism than other cell types. Stem cell senescence not only limits tissue regeneration and repair but also exacerbates multiple pathological changes associated with aging. Consequently, delaying stem cell senescence has emerged as a crucial research focus in the field of anti-aging.
Cellular senescence is closely linked to various age-related diseases, particularly playing a key role in bone metabolic disorders such as osteoporosis. With increasing age, the proliferative capacity of mesenchymal stem cells (MSCs) declines, especially the osteogenic differentiation potential of bone marrow-derived mesenchymal stem cells (BMSCs), leading to decreased bone density and deterioration of bone microarchitecture. The aging of MSCs directly results in impaired bone tissue regeneration, which constitutes one of the primary pathological mechanisms of primary osteoporosis (3, 4). Moreover, MSCs senescence is accompanied by an increased secretion of pro-inflammatory factors, further exacerbating osteoporosis progression (5). Therefore, investigating the relationship between MSCs senescence and osteoporosis not only provides deeper insights into the pathophysiology of osteoporosis but also offers a novel theoretical foundation for its prevention and treatment. This review comprehensively examines the relationship between MSCs senescence and osteoporosis, elaborates on the specific mechanisms underlying MSCs aging in osteoporosis pathogenesis, and summarizes current therapeutic strategies and the application of emerging pharmacological interventions. The aim is to provide a more comprehensive perspective and guidance for future research and therapeutic advancements in osteoporosis.
2 The mechanism and influencing factors of stem cell aging exacerbating osteoporosis
Stem cell senescence serves as a pivotal driver of osteoporotic pathogenesis. During cellular aging, the expression of osteogenic markers, such as Runx2 and Osterix, decreases (6, 7), and coincides with elevated oxidative stress (8), inflammatory microenvironment imbalance, and bone marrow microcirculatory dysfunction. These synergistic perturbations collectively suppress osteoblast differentiation and bone formation, thereby perturbing bone remodeling equilibrium and accelerating osteoporotic progression.
2.1 Stem Cell Senescence Suppresses Osteogenic Markers and Accelerates Osteoporosis
Stem cell senescence critically impairs osteogenic marker expression and directly drives osteoporotic progression. MSCs, a class of pluripotent progenitor cells, are widely distributed in bone marrow (9), adipose tissue (10), umbilical cord blood (11), and dental pulp (12), with the capacity to differentiate into osteoblasts, chondrocytes, and adipocytes, thereby holding significant potential in regenerative medicine and tissue engineering (13). Under the regulation of bone morphogenetic protein (BMP) and Wnt/β-catenin signaling, MSCs sequentially differentiate into osteoprogenitor cells (14, 15), mature osteoblasts, and ultimately functional osteocytes (16). Osteoblasts, the principal synthetic cells of the bone matrix, are regulated by core osteogenic markers including Runx2 (17), Osterix (7, 17), osteocalcin, and alkaline phosphatase (18, 19). However, senescent MSCs exhibit marked downregulation of these key factors, accompanied by diminished osteogenic differentiation capacity (18). Accumulating evidence demonstrates that both mRNA and protein levels of Runx2 and Osterix are reduced in senescent MSCs, correlating with alterations in intracellular signalling pathways (20). When the activity of BMP and Wnt/β-catenin signalling pathways is reduced, the production and quality of related proteins (e.g., Smad proteins, Wnt proteins) are reduced, which directly affects the activation of osteogenic genes, leading to insufficient synthesis and mineralisation of bone matrix, thus accelerating the development of osteoporosis. Collectively, MSCs senescence exacerbates osteoporosis via suppression of osteogenic differentiation, highlighting therapeutic opportunities to target this axis.
2.2 The interaction between stem cell aging and oxidative stress exacerbates osteoporosis, and regulating antioxidants can improve the condition.
The crosstalk between stem cell senescence and oxidative stress represents a pivotal contributor to osteoporotic pathogenesis. Oxidative stress arises from an imbalance between intracellular reactive oxygen species (ROS) production and antioxidant defense systems, culminating in macromolecular damage and cellular dysfunction. Mechanistically, excessive ROS activate DNA repair mechanisms, upregulate senescence-associated molecules (e.g., p53, p21), and induce stem cell senescence by triggering DNA damage, protein denaturation, and cell membrane disruption, ultimately promoting apoptosis (21). Furthermore, ROS amplifies local inflammatory responses through enhanced secretion of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), further promotion of stem cell senescence (22). Senescent stem cells exhibit markedly reduced antioxidant enzyme activity, with superoxide dismutase (23), catalase (24), and glutathione peroxidase (25) levels declining significantly compared to their non-senescent counterparts. This enzymatic impairment compromises ROS scavenging capacity, leading to intracellular oxidative stress accumulation, which exacerbates oxidative damage and functional degradation (25, 26). Moreover, recent studies suggest that in addition to MSCs, osteocyte senescence also contributes to bone homeostasis dysregulation. According to Frost’s mechanostat theory (27), osteocytes sense mechanical loading and regulate bone architecture to maintain mechanical integrity. However, aging leads to osteocyte senescence, which impairs their mechanosensitivity and downstream signaling pathways. This results in reduced bone adaptability to mechanical stress, decreased bone strength, and increased fracture risk (28). Consequently, in the pathological process of osteoporosis, a vicious circle is formed between oxidative stress and stem cell senescence: oxidative stress accelerates stem cell senescence by damaging key intracellular molecules and cellular structures; whereas senescent stem cells accelerate the senescence process by decreasing the antioxidant capacity and causing further accumulation of ROS. This vicious circle significantly reduces the self-renewal capacity and multidirectional differentiation potential of stem cells, affecting the regenerative capacity of tissues (29, 30). Therapeutic targeting of this axis demonstrates translational potential. By reducing ROS accumulation and restoring stem cell antioxidant capacity, cellular senescence can be attenuated, thereby promoting bone health. Current evidence indicates that antioxidant supplementation or specific antioxidant therapies significantly reduce oxidative stress levels and ameliorate clinical manifestations of osteoporosis. Thus, developing novel strategies to disrupt the senescence-oxidative stress interaction offers critical insights for osteoporosis prevention and treatment.
2.3 Inflammatory microenvironment accelerates the aging of BMSCs, enhances the risk of osteoporosis, and regulating inflammation can slow down the condition.
The relationship between the inflammatory microenvironment and the aging of BMSCs has become a prominent research focus in recent years. The inflammatory environment plays a pivotal role in accelerating BMSCs senescence, thereby promoting the development of osteoporosis. Inflammatory factors, including cytokines (e.g., TNF-α, interleukin-1, IL-6), chemokines (e.g., monocyte chemoattractant protein-1, CXCL-8), and prostaglandins (e.g., PGE2) (32–34, 216), contribute to the inflammatory response through distinct mechanisms. Cytokines regulate immune cell activity to amplify inflammation, chemokines recruit leukocytes to sustain inflammatory responses, and prostaglandins act as lipid signaling molecules involved in inflammation and nociception. Anti-inflammatory factors such as interleukin-10 and transforming growth factor-β counteract inflammation by suppressing pro-inflammatory cytokine production and maintaining inflammatory homeostasis. In aging or disease states, chronic inflammation accelerates BMSCs senescence, exacerbating osteoporosis. Senescent MSCs secrete pro-inflammatory factors (e.g., TNF-α, IL-6) that perpetuate local inflammation while suppressing their proliferative and osteogenic potential, thereby impairing bone repair and remodeling (36, 217). The inflammatory response promotes MSCs senescence through two primary mechanisms: on the one hand, persistent secretion of inflammatory factors elevates ROS levels, impairing stem cell function and promoting senescence (37); on the other hand, the inflammatory microenvironment inhibits osteogenesis and enhances bone resorption by modulating interactions between MSCs and osteoblasts, bone-resorbing cells (38). This process not only compromises bone health but also disrupts bone marrow microenvironment homeostasis, exacerbating osteoporotic manifestations (39). Additionally, senescent MSCs exhibit a senescence-associated secretory phenotype (SASP), characterized by increased inflammatory burden, reduced osteoblast function, and accelerated bone density loss (40, 41). Thus, the inflammatory microenvironment not only accelerates BMSCs aging but also directly elevates osteoporosis risk.
Modulating the inflammatory response represents a promising therapeutic strategy. By inhibiting pro-inflammatory factors (e.g., TNF-α, IL-6) or activating anti-inflammatory pathways (e.g., Transforming Growth Factor-beta, interleukin-10), it is possible to attenuate MSCs senescence and restore their differentiation and self-renewal capacity (42, 43). Anti-inflammatory treatments reduce SASP production, suppress bone marrow inflammation, and improve bone mineral density (BMD) while lowering fracture risk (44, 45). These findings highlight the potential of targeting the inflammatory microenvironment to alleviate osteoporosis and delay BMSCs aging, providing a theoretical foundation and practical framework for clinical osteoporosis management.
2.4 Impaired bone marrow microcirculation function is a key mechanism that promotes stem cell aging and the progression of osteoporosis.
Alterations in bone marrow microcirculation, particularly reduced vascularization, are critical contributors to stem cell senescence and osteoporosis (46). Bone marrow microcirculation comprises a network of microvessels, including micro arterioles, microbes, and capillaries, which supply oxygen and nutrients to bone marrow stem cells while removing metabolic waste, thereby maintaining local environmental stability (47). However, with aging or in osteoporotic conditions, both the quantity and quality of blood vessels decline, leading to diminished microcirculatory function, local hypoxia, insufficient nutrient supply, and reduced angiogenic capacity. These changes directly impair stem cell function, particularly in BMSCs (48). Specifically, senescent BMSCs exhibit reduced self-renewal and differentiation capacities and are more prone to entering a senescent state under hypoxic and nutrient-deficient conditions (49). Vascular endothelial growth factor (VEGF) is a key regulator of bone marrow microcirculation and angiogenesis (50). VEGF promotes neovascularization by stimulating endothelial cell proliferation and migration, ensuring adequate nutrient and oxygen supply to BMSCs (51). However, VEGF levels decline with aging, accompanied by microcirculatory dysfunction, leading to BMSCs functional decline and exacerbating osteoporotic progression (52). Mechanistically, VEGF binds to its receptor VEGFR, activating downstream signaling pathways such as PI3K/Akt and MAPK, which regulate cell proliferation, survival, and angiogenesis (53, 54). For instance, PI3K/Akt activation promotes cell survival and inhibits apoptosis, while also enhancing cell proliferation through the regulation of cell cycle-related factors (e.g., Cyclin D1) (55). Similarly, MAPK activation further modulates cell proliferation, migration, and angiogenesis. The decline in VEGF expression and function weakens anti-apoptotic effects, reduces angiogenesis, and deteriorates microcirculation, thereby accelerating osteoporosis (56, 57). Therefore, maintaining bone marrow microcirculation function, enhancing angiogenesis, and improving microcirculatory efficiency represent promising strategies to mitigate stem cell senescence and osteoporotic progression, ultimately promoting bone health.
3 Mechanisms and modulators of MSCs aging
3.1 Intrinsic drivers of stem cell senescence
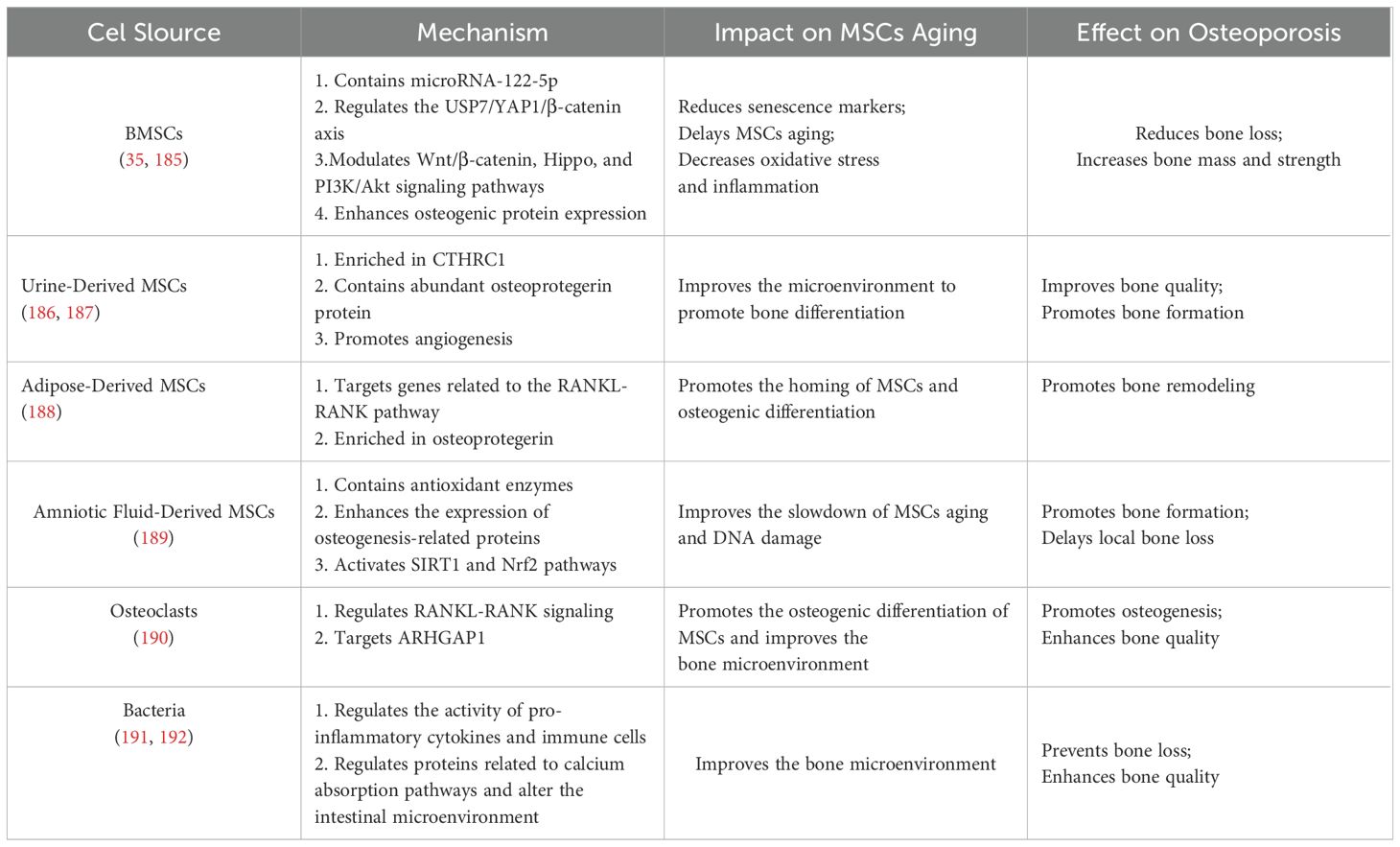
3.1.1 Cell cycle dysregulation and stem cell senescence
The stem cell cycle, encompassing the G1, S, G2, and M phases, is fundamental to cell proliferation, repair, and differentiation. Regulation of the stem cell cycle involves cyclins, cyclin-dependent kinase (CDK), and CDK inhibitors (e.g., p21, p15, p53) (58, 59). Among these phases, G1 and G2 are particularly critical in cellular senescence, as DNA damage during these phases can induce cell cycle arrest and impair cellular function.
During the G1 phase, cells synthesize proteins necessary for DNA replication and continued growth. Stem cell aging is often associated with G1 phase arrest, which inhibits cell proliferation (60). DNA damage accumulates in stem cells with age due to endogenous metabolic stress and exogenous factors (e.g., ultraviolet light, chemical toxins), and is detected and repaired through the DNA damage response pathway (61). DNA damage response activation primarily depends on p53 (62), p21, and p15 (59), which inhibit CDK activity or increase CDK inhibitor levels to arrest the cell cycle in the G1 phase (63). Specifically, p53 upregulates p21 to prevent cells from entering the S phase, ensuring DNA repair; if repair fails, cells undergo senescence or apoptosis (64, 65). p15 restricts cell proliferation and promotes senescence by inhibiting CDK4/6 (66). Additionally, p21 maintains the non-phosphorylated state of Rb proteins, inhibits E2F activity, and impairs MSCs self-renewal and differentiation (59). These changes deplete the MSCs pool, reduce osteogenic capacity, and accelerate osteoporotic progression.
In the G2 phase, cells ensure accurate genetic material transmission through DNA damage detection, a critical quality control checkpoint. Senescent MSCs exhibit impaired G2 phase regulation, preventing entry into the M phase and compromising regenerative capacity. Unrepaired DNA damage (e.g., double-strand breaks) triggers G2 phase arrest, which typically occurs shortly after DNA damage and persists if repair fails. Activation of the p53 pathway induces p21 expression and inhibits CDK1, preventing M phase entry and maintaining G2 phase arrest (67, 68). Chk1 and Chk2 further inhibit Cdc25 phosphatase activity, downregulating CDK1 and preventing M phase transition (69). Persistent DNA damage response activation due to DNA damage accumulation leads to permanent G2 phase arrest, reducing MSCs numbers and tissue repair capacity, ultimately contributing to osteoporosis (70, 71).
The S phase involves DNA synthesis and replication, while the M phase encompasses cell division. During aging, the S phase is frequently disrupted by DNA damage or oxidative stress. When damage exceeds stem cell repair capacity, repair failure occurs, upregulating proteins such as p52 and p21. These proteins regulate cell cycle checkpoints, halting cells in the G1 or G2 phase to prevent replication of damaged DNA (72, 73). In the M phase, senescent stem cells often exhibit division errors (e.g., chromosomal abnormalities or unequal division) due to unrepaired DNA damage, resulting in M phase arrest or aberrant division (74, 75). In summary, the S and M phases regulate cell proliferation and differentiation through DNA damage and abnormal cell division, respectively. Therefore, cell cycle impairment is a central mechanism underlying stem cell senescence and osteoporosis. Strategies to repair DNA damage and modulate key regulators such as p53, p21, and p15 may offer novel approaches to slowing osteoporotic progression (57). Stem cell cycle dysregulation is presented in Figure 1.
3.1.2 The impact of telomere alterations on cellular aging
Telomeres are protective structures at chromosome ends, composed of TTAGGG repeat sequences and associated proteins that form t-loops to prevent chromosomal misrecognition or degradation (76, 77). Telomere shortening and dysfunction are critical contributors to cellular senescence and related diseases (78, 79). Due to the inherent limitations of DNA replication, tens of telomere base pairs are lost with each cell cycle (80, 81), leading to progressive telomere attrition. Although human embryonic stem cells and tumor cells can delay aging through telomerase expression (82, 83), most human somatic cells lack active telomerase and cannot maintain telomere length (84). This results in critically short and dysfunctional telomeres in some cells (85). Extremely short telomeres trigger DNA damage signaling and telomere dysfunction (86, 87, 213), leading to cell cycle arrest and increased susceptibility to DNA damage as telomeres shorten (89, 90). Dysfunctional telomeres also induce cellular senescence, particularly affecting rapidly dividing or regenerating tissues. Additionally, SIRT1 plays a key role in telomerase regulation, and its inhibition reduces telomerase activity, accelerating MSCs senescence and contributing to osteoporosis (91, 92).
Telomere-associated disorders, which promote stem cell senescence, are classified into two categories: primary and secondary telomere diseases (86, 93). Primary telomere diseases result from mutations in telomerase maintenance genes (e.g., DKC1, hTERC, or hTERT), impairing telomerase activity, accelerating telomere shortening, and promoting MSCs senescence (89). This leads to a reduction in pre-osteoblast differentiation, contributing to osteoporosis. Secondary telomere diseases arise from mutations in DNA repair or structural proteins, with environmental factors and certain diseases also causing telomere damage (86, 94). Patients with these disorders exhibit premature cellular senescence, telomere aberrations, or random telomere loss (86, 95), reducing the number of differentiated osteoblasts and osteocytes, thereby promoting osteoporosis. Telomeric alterations drive stem cell senescence: From molecular erosion to functional decline (see Figure 1).
3.2 Regulatory and Facilitating Mechanisms of MSCs Senescence
3.2.1 Transcription and transcriptional regulation abnormalities in mscs senescence
In MSCs senescence, transcription and its regulatory network become widely dysregulated, encompassing both upstream epigenetic modifications that influence transcriptional activity and downstream disturbances in mRNA processing and modifications. Together, these alterations disrupt the stability and plasticity of gene expression in MSCs, progressively driving them into a senescent state.
During senescence, MSCs undergo epigenetic modifications, including histone modifications, DNA methylation, and chromatin remodeling, which are closely linked to transcriptional regulation (96, 97). Histone modifications regulate senescence by modulating the transcriptional activity of DNA regions associated with the cell cycle (98). Generally, histone acetylation and methylation promote transcription, while phosphorylation and ubiquitination tend to inhibit it (99). Defects in histone deacetylase upregulate the histone demethylase JMJD3 and indirectly downregulate polycomb group genes via the RB/E2F pathway, leading to p16^INK4A transcription activation and H3K27me3 demethylation, thereby promoting MSCs senescence (97, 100). Additionally, SIRT6 maintains genomic integrity and prevents cellular senescence by deacetylating H3K9, H3K18, and H3K56, regulating transcription factor recruitment and promoting repressive heterochromatin structures (31, 101). DNA methylation profiles are also associated with MSCs senescence. DNA methyltransferase-catalyzed DNA methylation typically suppresses transcription (31). Age-associated methylation changes, particularly H3K9me-promoted hypermethylation of p16^INK4A, are key features of epigenetic senescence in MSCs (31, 102). Furthermore, hypermethylation of key osteogenic transcription factors, such as Hox and Runx2, reduces their expression, impairing osteogenesis and accelerating osteoporosis (103, 104). Chromatin remodeling also plays a critical role in transcriptional regulation during senescence. For example, Brg1-mediated SWI/SNF chromatin remodeling maintains MSCs transcriptional activity, and its loss facilitates DNA methyltransferase recruitment to the Nanog promoter, repressing transcription and accelerating senescence (105). Notably, endogenous hormonal fluctuations—particularly the decline in estrogen levels during menopause—serve as important disruptors of the epigenetic regulatory network in MSCs (106). Studies have shown that estrogen, via its receptor ERα, interacts synergistically with the epigenetic regulator EZH2 to modulate the expression of adipogenic transcription factors in MSCs and to maintain H3K27me3 levels at their promoters (219). This interaction governs MSCs lineage commitment and functional homeostasis (219). In postmenopausal conditions, estrogen deficiency disrupts this regulation, contributing to senescence-associated phenotypic alterations in MSCs, impairing osteogenesis, and thereby accelerating osteoporosis progression (219).
mRNA processing and modifications are closely linked to MSCs senescence. The transcription rate of RNA polymerase II increases with age, but proper exon splicing depends on optimal transcription rates. Excessive transcription rates may lead to exon skipping and intron retention (107, 108). Since RNA polymerase II elongation is regulated by factors such as SPT5, PAF1C, SPT6, and SEC, targeting these elongation factors or increasing histone gene expression may represent novel strategies to delay senescence (109). Transcription factors also contribute to MSCs senescence. For instance, NF-κB activation triggers the SASP, promoting inflammation and accelerating senescence (110, 111). Downregulation of FOXO1 accelerates MSCs senescence and alters the transcriptome (112, 214). mRNA modifications, particularly m6A methylation, are critical in MSCs senescence. Enhanced m6A levels in senescent MSCs are mitigated by ALKBH5 through its m6A demethylation activity (113, 115). METTL3, an RNA-modifying enzyme, stabilizes MIS12 transcripts via m6A modification, attenuating MSCs senescence (115, 116). RNA-binding proteins, such as HuR, regulate mRNA stability through selective splicing, 5’-end capping, and 3’-end polyadenylation. HuR delays MSCs senescence by stabilizing SIRT1 mRNA, but its levels decline with senescence, impairing cellular function (117). Despite these insights, the precise mechanisms by which transcriptional dysregulation drives MSCs senescence remain unclear and warrant further investigation.
3.2.2 Non-coding RNA-mediated post-transcriptional regulation in mscs senescence
Non-coding RNAs, including long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) (2), play critical roles in cellular processes. During senescence, the miRNA expression profile of MSCs is markedly altered in response to oxidative stress, inflammatory microenvironments, epigenetic modifications, and metabolic dysregulation, thereby affecting cellular function by targeting key senescence-associated genes. Among these, miRNAs regulate aging by selectively binding to the 3’-untranslated region of target mRNAs, inhibiting translation or promoting mRNA degradation (118, 119). For instance, miR-504, miR-125b, miR-25, and miR-30d delay senescence by directly binding to p53 mRNA and suppressing p53 protein expression (120). Conversely, miR-192, miR-194 and miR-605 stabilize p53 by targeting MDM2, leading to cell cycle arrest and senescence induction (120). The cell cycle regulator p21 is targeted by multiple miRNAs, including the miR-106b family, miR-130b, and miR-302a, which inhibit p21 expression to maintain cell cycle progression and delay senescence (118). miR-195 promotes senescence by targeting SIRT1 and TERT, inhibiting telomere elongation and enhancing p53 signaling (121). Additionally, miR-486-5p accelerates MSCs senescence by binding to the 3’-untranslated region of the SIRT1 gene, suppressing osteogenic and adipogenic differentiation (30). In the context of p16^INK4a suppression, miR-24 inhibits its translation, maintaining cell cycle progression and delaying senescence (30, 122). Furthermore, miR-26b, miR-181a, miR-210, and miR-424 target polycomb repressive complex proteins (e.g., CBX7, EED, EZH2, and Suz12), inducing p16 upregulation and accelerating senescence (123). lncRNAs also regulate cellular senescence by competitively binding to miRNAs, modulating the translational efficiency of target mRNAs (124). For example, Xist lncRNA competitively binds to miR-19a-3p, inhibiting its activity and thereby impairing osteogenic differentiation of BMSCs while accelerating senescence (125). Similarly, lincRNA-p21, induced by p53, increases ROS levels and accelerates MSCs senescence by regulating p21 transcription and inhibiting the Wnt pathway (126). Moreover, during menopause, declining estrogen levels lead to increased oxidative stress and elevated pro-inflammatory cytokines such as TNF-α and IL-6. These inflammatory signals influence transcription factors that markedly reshape miRNA expression profiles, disrupting the balance between osteoblasts and osteoclasts. This imbalance results in reduced bone formation and enhanced bone resorption. For instance, the accumulation of ROS has been shown to upregulate miR-141, which suppresses the expression of osteogenic proteins and thereby exacerbates osteoporosis progression (127). In summary, the regulation of senescence-related genes such as p53, p21, SIRT1, and p16^INK4a by miRNAs and lncRNAs influences cell cycle progression, proliferation, differentiation, and senescence. Modulating the expression of these ncRNAs may provide novel strategies to delay MSCs senescence and enhance their therapeutic potential. Transcriptional regulation in MSCs senescence: epigenetic programming and mRNA processing networks (see Figure 1).
3.2.3 Protein homeostasis imbalance in MSCs senescence
Protein homeostasis, the process of maintaining proper protein folding, function, and clearance of misfolded proteins, is critical for cellular health (128, 129). In MSCs, protein homeostasis imbalance is a key driver of aging. Protein homeostasis imbalance impairs MSCs function by promoting the accumulation of misfolded proteins and disrupting their degradation, which in turn triggers oxidative stress and inflammatory responses. These disturbances form a vicious cycle that ultimately accelerates cellular senescence. This imbalance arises from two major factors: diminished molecular chaperone function and impaired protein degradation systems. Molecular chaperones maintain protein homeostasis by assisting protein folding, preventing misfolding and aggregation, and promoting the degradation of damaged proteins (130). However, chaperone function declines with aging. For example, Hsp70 upregulation is regulated by HSF1, but reduced HSF1 activity in senescent cells limits chaperone protein expression (131, 132), leading to protein homeostasis imbalance (133). This imbalance compromises MSCs function and promotes senescence. The protein degradation systems, including the ubiquitin-proteasome system (UPS) and autophagy, also deteriorate with age. Senescence reduces UPS function, characterized by decreased proteasomal subunit expression, assembly defects, and reduced ubiquitination levels, primarily due to increased deubiquitinating enzyme activity and diminished ubiquitin-conjugating enzyme activity (134). UPS dysfunction impairs the clearance of misfolded proteins, leading to protein aggregation and functional disruption, thereby accelerating MSCs senescence (135, 136). Autophagy is significantly impaired in osteoporotic conditions (137). For instance, sustained activation of mTORC1, a major negative regulator of autophagy, inhibits autophagic activity and disrupts protein homeostasis (138, 139). Additionally, lysosomal pH, enzyme activity, and membrane fusion capacity decline with age, further impairing protein degradation and causing metabolic imbalance (140). In aged BMSCs, reduced expression of autophagy-related proteins (e.g., Atg7, Beclin1, and P62) hinders the clearance of dysfunctional mitochondria and damaged proteins, elevating ROS levels and increasing DNA damage. These changes accelerate BMSCs senescence and promote osteoporosis (141). Furthermore, the age-related decline in cellular antioxidant defenses increases ROS levels and reduces ATP synthesis (8). These factors not only damage protein structure and function but also inhibit protein degradation systems (142, 143), creating a vicious cycle of mitochondrial damage and ROS accumulation that further accelerates MSCs senescence (8). In summary, protein homeostasis imbalance drives MSCs senescence through multiple mechanisms, including impaired autophagy, UPS dysfunction, and oxidative stress, contributing to the development and progression of age-related diseases such as osteoporosis. Proteostatic control of aging: translational fidelity and protein quality surveillance (see Figure 1).
Figure 1. The Aging Mechanism of Stem Cells (Created in https://BioRender.com).
4 Innovative and effective therapies for osteoporosis
4.1 Stem cell therapy in osteoporosis treatment
Decreased osteogenic capacity triggered by senescence of BMSCs leads to decreased BMD (144, 145), and supplementation of MSCs and induction of bone tissue regeneration can effectively improve osteoporosis (146). Since Bab et al. first demonstrated the osteogenic potential of BMSCs in 1998 (147), stem cell therapy has shown promising results in various osteoporosis animal models. The therapeutic mechanisms include: 1. MSCs Nesting role: Adipose-derived stem cells injected via the tail vein can target bone tissues, and aspirin enhances this homing effect, improving bone loss in ovariectomized rats (148). CXCR4-transfected BMSCs increase vertebral bone density and biomechanical properties in rats (149), highlighting the role of MSCs homing in promoting osteogenesis. 2.Direct osteogenic differentiation: Overexpression of Mettl3 in BMSCs enhances osteogenic differentiation and prevents osteoporosis in ovariectomized mice, while its deficiency leads to reduced bone mass and bone marrow adiposity. Younger MSCs exhibit greater osteogenic capacity (150). 3. Regulation of osteoclast function: MSCs inhibit osteoclast differentiation by secreting osteoprotegerin (151). Additionally, BMSCs suppress osteoclastogenesis through WNT-1 expression, maintaining bone mass balance (215). 4. BMSCs promote angiogenesis, enhancing osteogenesis and bone repair: Zhang et al. demonstrated that BMSCs improve osteogenesis and repair bone defects in rats (218). Similarly, Jia et al. found that local injection of exosomes in a rat tibial bone defect model enhances endothelial cell migration, accelerates angiogenesis, and facilitates bone repair (154). However, direct evidence of MSCs-mediated bone repair through angiogenesis in vivo remains lacking and requires further investigation. In addition, utilizing stem cells as a novel source of osteoblasts and guiding their differentiation through the creation or exploitation of appropriate mechanical environments to promote bone formation and restore skeletal mechanostasis may represent an emerging strategy in stem cell-based therapy (155).
Despite advancements in clinical trials of stem cell therapies, limitations such as small sample sizes, short study durations, and inconclusive results persist. Therefore, additional clinical data are needed to confirm the efficacy of stem cell therapy in osteoporosis and optimize treatment protocols for clinical translation (see Figure 2).
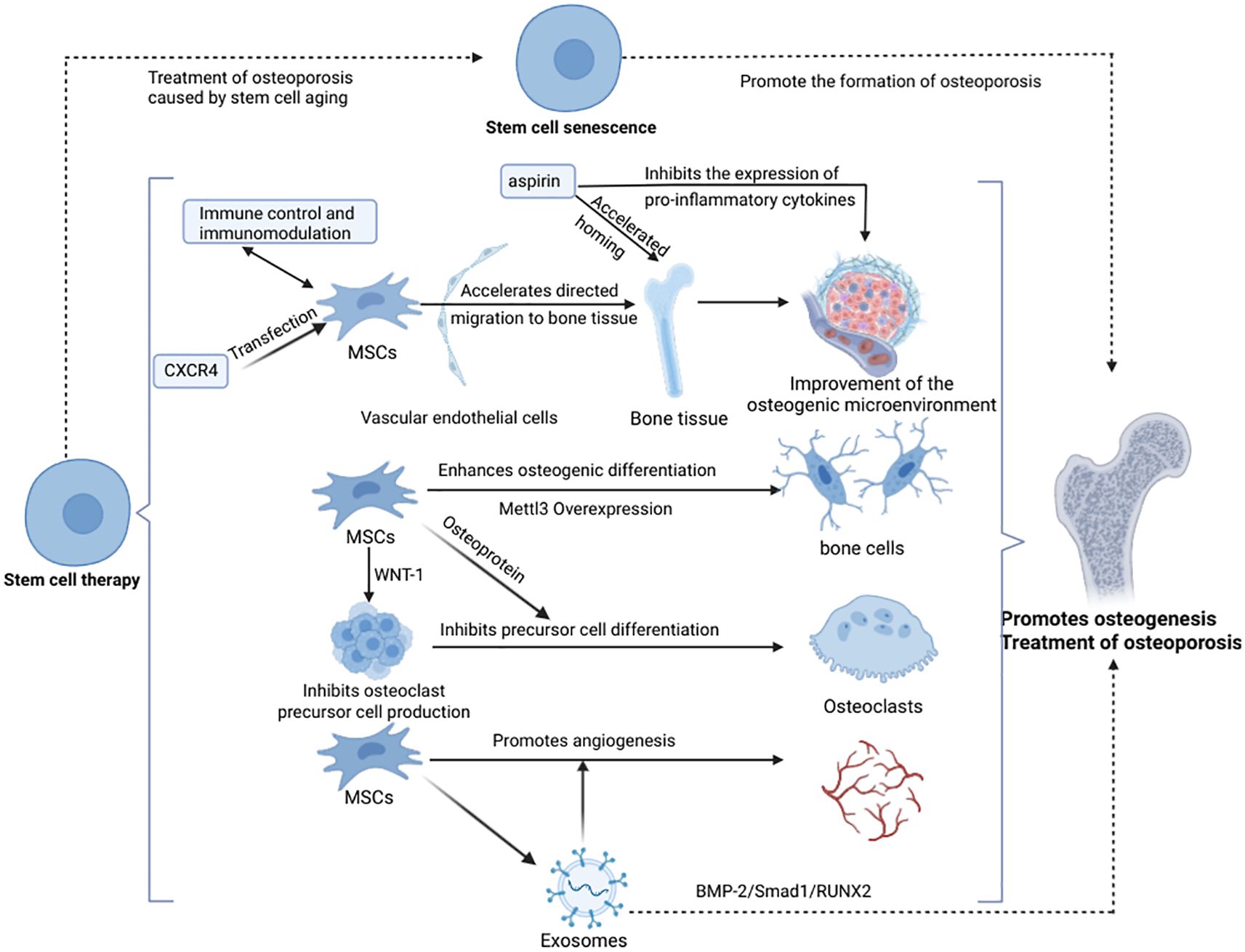
Figure 2. Stem cell therapy promotes osteogenesis and treats osteoporosis (Created in https://BioRender.com).
4.2 Research progress on drug and small molecule therapies targeting the mechanism of MSCs senescence in the treatment of osteoporosis
Modulating aging-related signaling pathways, restoring impaired cellular function, and selectively eliminating senescent cells through targeted drugs and small molecules have emerged as promising strategies to reverse MSCs senescence, representing a current research hotspot in the treatment of osteoporosis. Among these, SASP inhibitors mitigate the aging process of MSCs by reducing the secretion of inflammatory cytokines and SASP factors. Compounds such as rapamycin, melatonin, resveratrol, metformin, and ferulic acid have demonstrated notable SASP-inhibitory effects, primarily through suppression of the mTOR signaling pathway or activation of AMPK signaling. These mechanisms help to attenuate chronic inflammation during MSCs senescence, thereby showing therapeutic potential in age-related osteoporosis (96, 141, 152). The targets and mechanisms of these SASP inhibitors are summarized in Table 1 (see Table 1). In addition to SASP inhibition, a group of emerging therapeutic strategies—including senolytic agents, small molecule-targeting compounds, and antioxidants—have shown broad potential in delaying MSCs senescence and promoting bone regeneration. Senolytic agents, such as dasatinib and quercetin, can selectively eliminate senescent MSCs accumulated in tissues, thereby restoring regenerative capacity and slowing the progression of osteoporosis (96, 168, 169). Furthermore, small molecule-targeting compounds such as fibroblast growth factor 21 and insulin-like growth factor 1 can activate downstream signaling pathways to enhance MSCs proliferation, differentiation, and resistance to apoptosis. In parallel, antioxidant agents such as nicotinamide and its precursor nicotinamide adenine dinucleotide mitigate ROS generation and oxidative stress, thereby slowing MSCs aging and contributing positively to osteoporosis therapy (170, 171). Although some of these agents were identified decades ago, their potential in reversing MSCs senescence and treating osteoporosis has only recently been elucidated. A comprehensive summary of these interventions is presented in Table 2 (see Table 2). While many of the aforementioned agents remain in the preclinical or early research phase, accumulating evidence supports their therapeutic promise in managing osteoporosis. Future studies are warranted to further validate their efficacy and safety, with the aim of developing novel treatment options for patients with aging-related bone disorders.
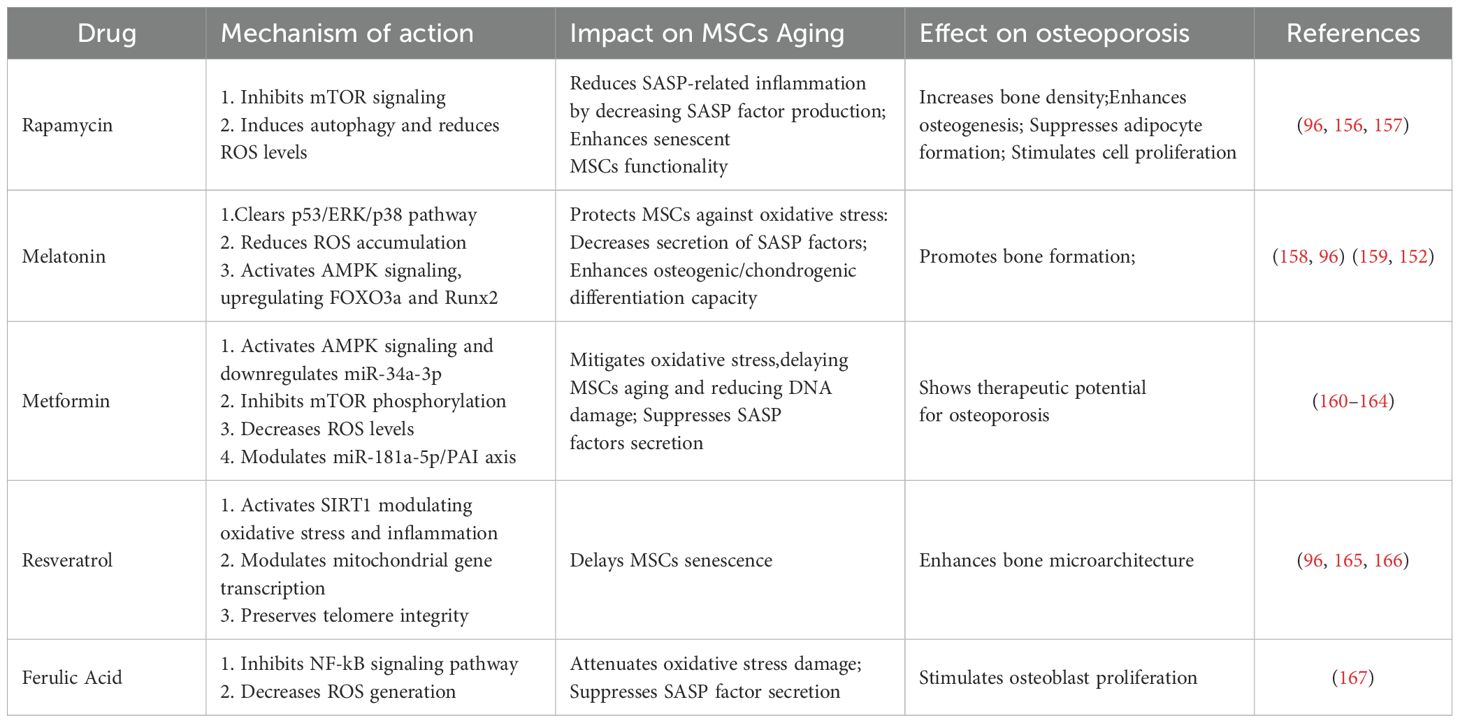
Table 1. Targets and mechanisms of SASP inhibitors in reversing MSCs aging and treating osteoporosis.
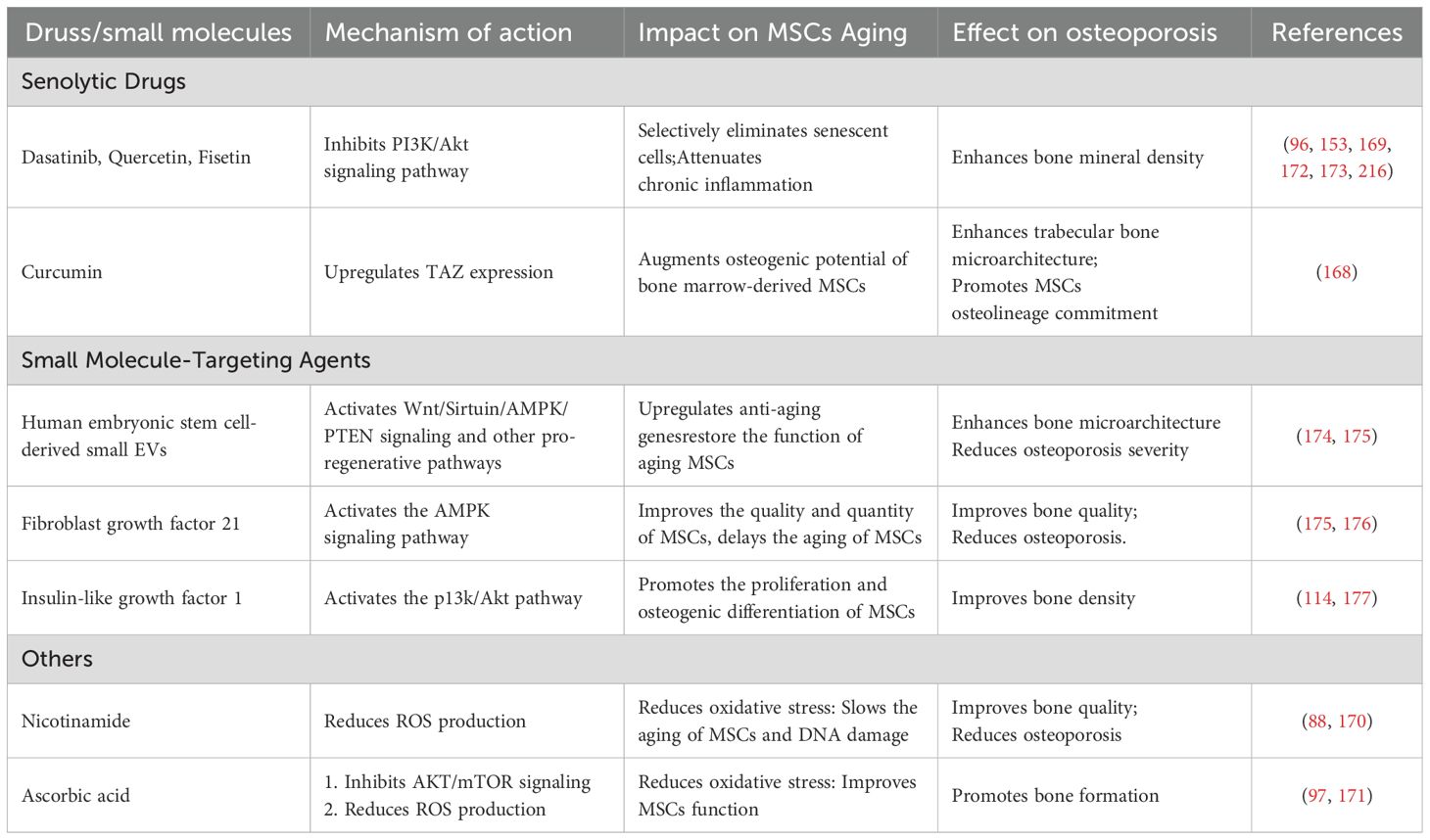
Table 2. Advances in senolytics and other small molecule-targeting agents for reversing MSCs senescence and treating osteoporosis.
4.3 The application of novel therapies using hydrogels and extracellular vesicles in the treatment of osteoporosis
The development of osteoporosis is closely linked to the disruption of bone tissue structure. Actively improving the microenvironment and tissue structure creates more favorable conditions for stem cell survival. In bone tissue engineering, hydrogels have been employed as scaffolds to address osteoporotic bone defects, enhance the bone microenvironment, promote osteogenic differentiation of MSCs, and delay cellular aging. Notably, stimulus-responsive hydrogels, which can modulate mechanical properties, shape, and drug release in response to triggers such as temperature, pH, electromagnetic radiation, magnetic fields, or biological factors, have become a prominent focus in bone-enabling research (178–180). For example, Ye et al. developed a thermo-responsive injectable hydrogel (MnO2@Pol/HA) that supports osteogenic differentiation of BMSCs by scavenging ROS and modulating macrophage polarization (181). Tang et al. designed a dual-network hydrogel (GelMA/ALN-OSA) that responds to pH changes to maintain stable drug concentrations and promote bone regeneration (182). Zhou et al. developed an electrochemical deposition-constructed hydrogel (Mg@PEG-PLGA) that scavenges ROS by releasing H2 through hydrolysis reactions, improves the bone microenvironment, and promotes osteogenic differentiation of MSCs (183). However, further optimization of the hydrogel’s biocompatibility, mechanical properties, and biodegradability is necessary to ensure synergistic effects with the bone regeneration process (184).
Recent studies have also highlighted the potential of EVs, including exosomes, due to their unique nanostructures, stable drug-carrying capacity, and excellent biocompatibility. EVs can improve the bone microenvironment, delay MSCs aging, and have become a key area of interest in osteoporosis research. Liu et al. used synthetic biology to integrate BMP-2 and CXCR4 onto the surface of bacterial EVs to deliver BMP-2, activating osteogenic signaling and significantly enhancing bone density and strength while inhibiting adipogenesis, thereby improving osteoporosis (112). EVs from various sources have demonstrated promising applications in osteoporosis treatment through multiple mechanisms (see Table 3). Additionally, the combination of EVs with hydrogels has shown significant potential in bone regeneration. Ding et al. developed a gelatin/ECM composite scaffold loaded with apoptotic vesicles derived from adipose-derived MSCs under hypoxic conditions. This scaffold significantly promoted osteochondral defect repair in rat osteoarthritis by enhancing stem cell proliferation, migration, and cartilage-forming differentiation, as well as promoting macrophage M2 polarization (193). Guo et al. developed a GEL-OCS/MBGN composite hydrogel loaded with EVs, which significantly promoted bone defect repair in rats by enhancing osteogenic differentiation of BMSCs through modulation of the miR-19b/WWP1 axis (194). Despite these advancements, further in-depth studies and clinical validation are needed to fully realize the potential of EVs and hydrogels in osteoporosis therapy.
5 Conclusion and outlook
5.1 The key connection and influence mechanism between MSCs aging and osteoporosis
The aging of MSCs plays a critical role in the pathogenesis of osteoporosis. Studies have demonstrated that the osteogenic capacity and self-renewal potential of MSCs decline significantly with age, a phenomenon closely associated with reduced expression of osteogenic markers, elevated oxidative stress levels, and increased inflammatory responses (195–197). Specifically, DNA damage accumulated in MSCs during aging activates cell cycle regulatory pathways, particularly the p53-p21 pathway, leading to cell cycle arrest (198). This arrest not only impedes normal cell proliferation and differentiation but also directly affects the repair and regenerative capacity of bone tissue, thereby accelerating the progression of osteoporosis (199). Therefore, a deeper understanding of the specific mechanisms underlying MSCs senescence can help elucidate the pathophysiological characteristics of osteoporosis and provide more targeted intervention strategies (200).
5.2 Prevention and treatment of osteoporosis caused by MSCs aging
Given the strong link between MSCs aging and osteoporosis, interventions targeting the aging process of MSCs are of paramount importance. Existing studies have shown that lifestyle modifications (e.g., moderate exercise, a balanced diet, smoking cessation, and alcohol restriction) and the development of novel drugs can effectively delay MSCs aging, thereby reducing the risk of osteoporosis (201, 202). For instance, physical inactivity has been associated with a significant decline in BMD, and studies have found that older women who engage in regular exercise exhibit significantly higher BMD compared to those who are sedentary (203). Additionally, smoking interferes with calcium absorption and increases oxidative stress, damaging bone cells and accelerating bone loss (204, 205). Excessive alcohol consumption not only inhibits osteoblast activity but may also indirectly affect bone metabolism through liver damage (206, 207). Thus, improving lifestyle habits not only helps delay MSCs aging but also promotes overall bone health and slows the progression of osteoporosis. Therapeutic strategies targeting oxidative stress and inflammation may also offer new avenues for osteoporosis prevention and treatment (127, 196, 208).
5.3 Directions for future research
Future research should focus on exploring the mechanisms of MSCs aging and its role in osteoporosis at the cellular and molecular levels. In particular, in-depth studies on the interactions between cell cycle regulation, oxidative stress, and inflammatory responses are needed to identify potential intervention targets (209). Additionally, the development of novel drugs and intervention strategies, such as gene editing technologies or small molecule compounds, may become effective means to delay MSCs aging. In terms of clinical applications, the focus should be on evaluating the efficacy and safety of these interventions in diverse populations (e.g., the elderly and individuals at high risk for osteoporosis), aiming to provide more effective solutions for osteoporosis prevention and treatment (210–212). These studies will not only deepen our understanding of the impact of aging on bone health but also provide new theoretical foundations and practical guidance for the prevention and treatment of related diseases.
Author contributions
YS-T: Supervision, Conceptualization, Writing – review & editing. YL-T: Visualization, Writing – original draft. JY-W: Writing – original draft. XY-L: Writing – original draft. QS: Writing – review & editing. YH-W: Conceptualization, Writing – review & editing. WZ-W: Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by The National Natural Science Foundation of China (82460428), Yunnan Provincial Department of Science and Technology-Kunming Medical University Joint Special Fund for Basic Research General Program (202501AY070001-166),the 76th Batch of the China Postdoctoral Science Foundation General Funding-Regional Special Support Program (2024MD763983), Yunnan Health Training Project of High Level talents (H-2024026), Youth Project of Yunnan Basic Research Programme of Yunnan Provincial Department of Science and Technology (202401AU070046), Teachers’ Project of Scientific Research Fund of Yunnan Provincial Department of Education - Special Project on Basic Research for Young Talents (2024J0180), The fifth batch of 535 young academic backbone training subjects of the First Affiliated Hospital of Kunming Medical University (2025535Q08), 2024 Yunnan Province Colorful Cloud Postdoctoral Program Innovation Project, Education and Teaching Research Project of the First Affiliated Hospital of Kunming Medical University (2024 JY-17), Kunming Medical University Student Innovation and Entrepreneurship Training Program Project (2024CYD013、2024CYD00、2024CYD020、2024CYD025、2024CYD082).
Acknowledgments
We thank the Department of Pathophysiology, Kunming Medical University for academic support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMSCs, Bone marrow-derived mesenchymal stem cells; BMP, bone morphogenetic protein; BMD, bone mineral density; CDK, cyclin-dependent kinase; EVs, extracellular vesicles; IL-6, interleukin-6; lncRNAs, long non-coding RNAs; MSCs, mesenchymal stem cells; miRNAs, MicroRNAs; ROS, reactive oxygen species; SASP, senescence-associated secretory phenotype; TNF-α, tumor necrosis factor-α; UPS, ubiquitin-proteasome system; VEGF, vascular endothelial growth factor.
References
1. Tjempakasari A, Suroto H, and Santoso D. Mesenchymal stem cell senescence and osteogenesis. Medicina (Kaunas). (2021) 58:(1). doi: 10.3390/medicina58010061
2. López-Otín C, Blasco MA, Partridge L, Serrano M, and Kroemer G. Hallmarks of aging: An expanding universe. Cell. (2023) 186:243–78. doi: 10.1016/j.cell.2022.11.001
3. Chen J, Kuang S, Cen J, Zhang Y, Shen Z, Qin W, et al. Multiomics profiling reveals VDR as a central regulator of mesenchymal stem cell senescence with a known association with osteoporosis after high-fat diet exposure. Int J Oral Sci. (2024) 16:41. doi: 10.1038/s41368-024-00309-9
4. Huo S, Tang X, Chen W, Gan D, Guo H, Yao Q, et al. Epigenetic regulations of cellular senescence in osteoporosis. Ageing Res Rev. (2024) 99:102235. doi: 10.1016/j.arr.2024.102235
5. Zhivodernikov IV, Kirichenko TV, Markina YV, Postnov AY, and Markin AM. Molecular and cellular mechanisms of osteoporosis. Int J Mol Sci. (2023) 24:(21). doi: 10.3390/ijms242115772
6. Komori T. Whole aspect of runx2 functions in skeletal development. Int J Mol Sci. (2022) 23:(10). doi: 10.3390/ijms23105776
7. Ren L, Zeng F, Deng J, Bai Y, Chen K, Chen L, et al. Inflammatory osteoclasts-derived exosomes promote bone formation by selectively transferring lncRNA LIOCE into osteoblasts to interact with and stabilize Osterix. FASEB J. (2022) 36:e22115. doi: 10.1096/fj.202101106RR
8. Riegger J, Schoppa A, Ruths L, Haffner-Luntzer M, and Ignatius A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: a narrative review. Cell Mol Biol Lett. (2023) 28:76. doi: 10.1186/s11658-023-00489-y
9. Vizoso FJ, Eiro N, Cid S, Schneider J, and Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. (2017) 18:(9). doi: 10.3390/ijms18091852
10. Qin Y, Ge G, Yang P, Wang L, Qiao Y, Pan G, et al. An update on adipose-derived stem cells for regenerative medicine: where challenge meets opportunity. Adv Sci (Weinh). (2023) 10:e2207334. doi: 10.1002/advs.202207334
11. Zhu Y, Huang C, Zheng L, Li Q, Ge J, Geng S, et al. Safety and efficacy of umbilical cord tissue-derived mesenchymal stem cells in the treatment of patients with aging frailty: a phase I/II randomized, double-blind, placebo-controlled study. Stem Cell Res Ther. (2024) 15:122. doi: 10.1186/s13287-024-03707-2
12. Mattei V and Delle Monache S. Dental pulp stem cells (DPSCs) and tissue regeneration: mechanisms mediated by direct, paracrine, or autocrine effects. Biomedicines. (2023) 11:(2). doi: 10.3390/biomedicines11020386
13. Yamanaka S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. (2020) 27:523–31. doi: 10.1016/j.stem.2020.09.014
14. Adamička M, Adamičková A, Danišovič L, Gažová A, and Kyselovič J. Pharmacological approaches and regeneration of bone defects with dental pulp stem cells. Stem Cells Int. (2021) 2021:1–7. doi: 10.1155/2021/4593322
15. Zhang Y, Zhao Y, Xie Z, Li M, Liu Y, and Tu X. Activating wnt/β-catenin signaling in osteocytes promotes osteogenic differentiation of BMSCs through BMP-7. Int J Mol Sci. (2022) 23:(24). doi: 10.3390/ijms232416045
16. Wang X, Tian Y, Liang X, Yin C, Huai Y, Zhao Y, et al. Bergamottin promotes osteoblast differentiation and bone formation via activating the Wnt/β-catenin signaling pathway. Food Funct. (2022) 13:2913–24. doi: 10.1039/d1fo02755g
17. Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, et al. Glucose uptake and runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell. (2015) 161:1576–91. doi: 10.1016/j.cell.2015.05.029
18. Diemar SS, Møllehave LT, Quardon N, Lylloff L, Thuesen BH, Linneberg A, et al. Effects of age and sex on osteocalcin and bone-specific alkaline phosphatase-reference intervals and confounders for two bone formation markers. Arch Osteoporos. (2020) 15:26. doi: 10.1007/s11657-020-00715-6
19. Diemar SS, Lylloff L, Rønne MS, Møllehave LT, Heidemann M, Thuesen BH, et al. Reference intervals in Danish children and adolescents for bone turnover markers carboxy-terminal cross-linked telopeptide of type I collagen (β-CTX), pro-collagen type I N-terminal propeptide (PINP), osteocalcin (OC) and bone-specific alkaline phosphatase (bone ALP). Bone. (2021) 146:115879. doi: 10.1016/j.bone.2021.115879
20. Chen G, Deng C, and Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. (2012) 8:272–88. doi: 10.7150/ijbs.2929
21. Sies H and Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. (2020) 21:363–83. doi: 10.1038/s41580-020-0230-3
22. Guo S, Fu L, Yin C, Shao W, Sun Q, Chen L, et al. ROS-induced gingival fibroblast senescence: implications in exacerbating inflammatory responses in periodontal disease. Inflammation. (2024) 47:1918–35. doi: 10.1007/s10753-024-02014-5
23. Zhao H, Zhang R, Yan X, and Fan K. Superoxide dismutase nanozymes: an emerging star for anti-oxidation. J Mater Chem B. (2021) 9:6939–57. doi: 10.1039/d1tb00720c
24. Baker A, Lin CC, Lett C, Karpinska B, Wright MH, and Foyer CH. Catalase: A critical node in the regulation of cell fate. Free Radic Biol Med. (2023) 199:56–66. doi: 10.1016/j.freeradbiomed.2023.02.009
25. Flohé L, Toppo S, and Orian L. The glutathione peroxidase family: Discoveries and mechanism. Free Radic Biol Med. (2022) 187:113–22. doi: 10.1016/j.freeradbiomed.2022.05.003
26. Kimball JS, Johnson JP, and Carlson DA. Oxidative stress and osteoporosis. J Bone Joint Surg Am. (2021) 103:1451–61. doi: 10.2106/jbjs.20.00989
27. Castoldi NM, Lagzouli A, Pickering E, Meakin L, Cooper DML, Delisser P, et al. Reverse engineering Frost’s mechanostat model in mouse tibia: Insights from combined PTH and mechanical loading. Bone. (2025) 197:117491. doi: 10.1016/j.bone.2025.117491
28. Cui J, Shibata Y, Zhu T, Zhou J, and Zhang J. Osteocytes in bone aging: Advances, challenges, and future perspectives. Ageing Res Rev. (2022) 77:101608. doi: 10.1016/j.arr.2022.101608
29. Waheed TO, Hahn O, Sridharan K, Mörke C, Kamp G, and Peters K. Oxidative stress response in adipose tissue-derived mesenchymal stem/stromal cells. Int J Mol Sci. (2022) 23:(21). doi: 10.3390/ijms232113435
30. Mi L, Hu J, Li N, Gao J, Huo R, Peng X, et al. The mechanism of stem cell aging. Stem Cell Rev Rep. (2022) 18:1281–93. doi: 10.1007/s12015-021-10317-5
31. Wang R, Wang Y, Zhu L, Liu Y, and Li W. Epigenetic regulation in mesenchymal stem cell aging and differentiation and osteoporosis. Stem Cells Int. (2020) 2020:8836258. doi: 10.1155/2020/8836258
32. Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. (2021) 33:127–48. doi: 10.1093/intimm/dxaa078
33. Molnar V, Matišić V, Kodvanj I, Bjelica R, Jeleč Ž, Hudetz D, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci. (2021) 22:9208. doi: 10.3390/ijms22179208
34. Wautier JL and Wautier MP. Pro- and anti-inflammatory prostaglandins and cytokines in humans: A mini review. Int J Mol Sci. (2023) 24:(11). doi: 10.3390/ijms24119647
35. Wang X, Zou C, Hou C, Bian Z, Jiang W, Li M, et al. Extracellular vesicles from bone marrow mesenchymal stem cells alleviate osteoporosis in mice through USP7-mediated YAP1 protein stability and the Wnt/β-catenin pathway. Biochem Pharmacol. (2023) 217:115829. doi: 10.1016/j.bcp.2023.115829
36. Xu L, Wang Y, Wang J, Zhai J, Ren L, and Zhu G. Radiation-induced osteocyte senescence alters bone marrow mesenchymal stem cell differentiation potential via paracrine signaling. Int J Mol Sci. (2021) 22:(17). doi: 10.3390/ijms22179323
37. Almalki WH and Almujri SS. Aging, ROS, and cellular senescence: a trilogy in the progression of liver fibrosis. Biogerontology. (2024) 26:10. doi: 10.1007/s10522-024-10153-3
38. Kushioka J, Chow SK, Toya M, Tsubosaka M, Shen H, Gao Q, et al. Bone regeneration in inflammation with aging and cell-based immunomodulatory therapy. Inflammation Regener. (2023) 43:29. doi: 10.1186/s41232-023-00279-1
39. Ambrosi TH, Marecic O, McArdle A, Sinha R, Gulati GS, Tong X, et al. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature. (2021) 597:256–62. doi: 10.1038/s41586-021-03795-7
40. Farr JN, Kaur J, Doolittle ML, and Khosla S. Osteocyte cellular senescence. Curr Osteoporos Rep. (2020) 18:559–67. doi: 10.1007/s11914-020-00619-x
41. Chandra A and Rajawat J. Skeletal aging and osteoporosis: mechanisms and therapeutics. Int J Mol Sci. (2021) 22:(7). doi: 10.3390/ijms22073553
42. Widjaja AA, Lim WW, Viswanathan S, Chothani S, Corden B, Dasan CM, et al. Inhibition of IL-11 signalling extends mammalian healthspan and lifespan. Nature. (2024) 632:157–65. doi: 10.1038/s41586-024-07701-9
43. Han D, Gong H, Wei Y, Xu Y, Zhou X, Wang Z, et al. Hesperidin inhibits lung fibroblast senescence via IL-6/STAT3 signaling pathway to suppress pulmonary fibrosis. Phytomedicine. (2023) 112:154680. doi: 10.1016/j.phymed.2023.154680
44. Lagoumtzi SM and Chondrogianni N. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic Biol Med. (2021) 171:169–90. doi: 10.1016/j.freeradbiomed.2021.05.003
45. Khosla S, Farr JN, Tchkonia T, and Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. (2020) 16:263–75. doi: 10.1038/s41574-020-0335-y
46. Li Y, Hao W, Guan J, Li B, Meng L, Sun S, et al. Relationship between indices of circulating blood cells and bone homeostasis in osteoporosis. Front Endocrinol (Lausanne). (2022) 13:965290. doi: 10.3389/fendo.2022.965290
47. Prisby RD. Bone marrow microvasculature. Compr Physiol. (2020) 10:1009–46. doi: 10.1002/cphy.c190009
48. Neag G, Finlay M, and Naylor AJ. The cellular choreography of osteoblast angiotropism in bone development and homeostasis. Int J Mol Sci. (2021) 22:(14). doi: 10.3390/ijms22147253
49. Xu Y, Chang L, Chen Y, Dan Z, Zhou L, Tang J, et al. USP26 combats age-related declines in self-renewal and multipotent differentiation of BMSC by maintaining mitochondrial homeostasis. Adv Sci (Weinh). (2024) 11:e2406428. doi: 10.1002/advs.202406428
50. Ahmad A and Nawaz MI. Molecular mechanism of VEGF and its role in pathological angiogenesis. J Cell Biochem. (2022) 123:1938–65. doi: 10.1002/jcb.30344
51. Kang F, Yi Q, Gu P, Dong Y, Zhang Z, Zhang L, et al. Controlled growth factor delivery system with osteogenic-angiogenic coupling effect for bone regeneration. J Orthop Translat. (2021) 31:110–25. doi: 10.1016/j.jot.2021.11.004
52. Keller-Baruch J, Forgetta V, Manousaki D, Zhou S, and Richards JB. Genetically decreased circulating vascular endothelial growth factor and osteoporosis outcomes: A mendelian randomization study. J Bone Miner Res. (2020) 35:649–56. doi: 10.1002/jbmr.3937
53. Ning W, Li S, Yang W, Yang B, Xin C, Ping X, et al. Blocking exosomal miRNA-153-3p derived from bone marrow mesenchymal stem cells ameliorates hypoxia-induced myocardial and microvascular damage by targeting the ANGPT1-mediated VEGF/PI3k/Akt/eNOS pathway. Cell Signal. (2021) 77:109812. doi: 10.1016/j.cellsig.2020.109812
54. Liu H, Huang B, Xue S, Tsang LL, Zhang X, Li G, et al. Functional crosstalk between mTORC1/p70S6K pathway and heterochromatin organization in stress-induced senescence of MSCs. Stem Cell Res Ther. (2020) 11:279. doi: 10.1186/s13287-020-01798-1
55. Sun K, Luo J, Guo J, Yao X, Jing X, and Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis Cartilage. (2020) 28:400–9. doi: 10.1016/j.joca.2020.02.027
56. Grunewald M, Kumar S, Sharife H, Volinsky E, Gileles-Hillel A, Licht T, et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science. (2021) 373:(6554). doi: 10.1126/science.abc8479
57. Siddiqui S, Mahdi AA, and Arshad M. Genistein contributes to cell cycle progression and regulates oxidative stress in primary culture of osteoblasts along with osteoclasts attenuation. BMC Complement Med Ther. (2020) 20:277. doi: 10.1186/s12906-020-03065-5
58. Arora M, Moser J, Hoffman TE, Watts LP, Min M, Musteanu M, et al. Rapid adaptation to CDK2 inhibition exposes intrinsic cell-cycle plasticity. Cell. (2023) 186:2628–2643.e21. doi: 10.1016/j.cell.2023.05.013
59. Engeland K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. (2022) 29:946–60. doi: 10.1038/s41418-022-00988-z
60. Wang Z. Regulation of cell cycle progression by growth factor-induced cell signaling. Cells. (2021) 10:(12). doi: 10.3390/cells10123327
61. Kumari R and Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. (2021) 9:645593. doi: 10.3389/fcell.2021.645593
62. Sheekey E and Narita M. p53 in senescence - it’s a marathon, not a sprint. FEBS J. (2023) 290:1212–20. doi: 10.1111/febs.16325
63. Knudsen ES, Witkiewicz AK, and Rubin SM. Cancer takes many paths through G1/S. Trends Cell Biol. (2024) 34:636–45. doi: 10.1016/j.tcb.2023.10.007
64. He P, Li Z, Xu F, Ru G, Huang Y, Lin E, et al. AMPK Activity Contributes to G2 Arrest and DNA Damage Decrease via p53/p21 Pathways in Oxidatively Damaged Mouse Zygotes. Front Cell Dev Biol. (2020) 8:539485. doi: 10.3389/fcell.2020.539485
65. Li YL, Gan XL, Zhu RP, Wang X, Liao DF, Jin J, et al. Anticancer activity of platinum (II) complex with 2-benzoylpyridine by induction of DNA damage, S-phase arrest, and apoptosis. Anticancer Agents Med Chem. (2020) 20:504–17. doi: 10.2174/1871520619666191112114340
66. De Braekeleer M, Douet-Guilbert N, and De Braekeleer E. Prognostic impact of p15 gene aberrations in acute leukemia. Leuk Lymphoma. (2017) 58:257–65. doi: 10.1080/10428194.2016.1201574
67. Manohar S, Estrada ME, Uliana F, Vuina K, Alvarez PM, de Bruin RAM, et al. Genome homeostasis defects drive enlarged cells into senescence. Mol Cell. (2023) 83:4032–4046.e6. doi: 10.1016/j.molcel.2023.10.018
68. Tran AP, Tralie CJ, Reyes J, Moosmüller C, Belkhatir Z, Kevrekidis IG, et al. Long-term p21 and p53 dynamics regulate the frequency of mitosis events and cell cycle arrest following radiation damage. Cell Death Differ. (2023) 30:660–72. doi: 10.1038/s41418-022-01069-x
69. Bulavin DV, Amundson SA, and Fornace AJ. p38 and Chk1 kinases: different conductors for the G(2)/M checkpoint symphony. Curr Opin Genet Dev. (2002) 12:92–7. doi: 10.1016/s0959-437x(01)00270-2
70. Garyn CM, Bover O, Murray JW, Ma J, Salas-Briceno K, Ross SR, et al. G2 arrest primes hematopoietic stem cells for megakaryopoiesis. Cell Rep. (2024) 43:114388. doi: 10.1016/j.celrep.2024.114388
71. Tian RC, Zhang RY, and Ma CF. Rejuvenation of bone marrow mesenchymal stem cells: mechanisms and their application in senile osteoporosis treatment. Biomolecules. (2025) 15:(2). doi: 10.3390/biom15020276
72. Mansilla SF, de la Vega MB, Calzetta NL, Siri SO, and Gottifredi V. CDK-independent and PCNA-dependent functions of p21 in DNA replication. Genes (Basel). (2020) 11:(6). doi: 10.3390/genes11060593
73. Hume S, Grou CP, Lascaux P, D’Angiolella V, Legrand AJ, Ramadan K, et al. The NUCKS1-SKP2-p21/p27 axis controls S phase entry. Nat Commun. (2021) 12:6959. doi: 10.1038/s41467-021-27124-8
74. Matthews HK, Bertoli C, and de Bruin RAM. Cell cycle control in cancer. Nat Rev Mol Cell Biol. (2022) 23:74–88. doi: 10.1038/s41580-021-00404-3
75. Zhu H, Rao Z, Yuan S, You J, Hong C, He Q, et al. One therapeutic approach for triple-negative breast cancer: Checkpoint kinase 1 inhibitor AZD7762 combination with neoadjuvant carboplatin. Eur J Pharmacol. (2021) 908:174366. doi: 10.1016/j.ejphar.2021.174366
76. Zanella E and Doksani Y. In the loop: unusual DNA structures at telomeric repeats and their impact on telomere function. Cold Spring Harb Perspect Biol. (2025). doi: 10.1101/cshperspect.a041694
77. Revy P, Kannengiesser C, and Bertuch AA. Genetics of human telomere biology disorders. Nat Rev Genet. (2023) 24:86–108. doi: 10.1038/s41576-022-00527-z
78. Gao X, Yu X, Zhang C, Wang Y, Sun Y, Sun H, et al. Telomeres and mitochondrial metabolism: implications for cellular senescence and age-related diseases. Stem Cell Rev Rep. (2022) 18:2315–27. doi: 10.1007/s12015-022-10370-8
79. Eppard M, Passos JF, and Victorelli S. Telomeres, cellular senescence, and aging: past and future. Biogerontology. (2024) 25:329–39. doi: 10.1007/s10522-023-10085-4
80. Beauséjour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. (2003) 22:4212–22. doi: 10.1093/emboj/cdg417
81. Gao J and Pickett HA. Targeting telomeres: advances in telomere maintenance mechanism-specific cancer therapies. Nat Rev Cancer. (2022) 22:515–32. doi: 10.1038/s41568-022-00490-1
82. Zhou Z, Li Y, Xu H, Xie X, He Z, Lin S, et al. An inducible CRISPR/Cas9 screen identifies DTX2 as a transcriptional regulator of human telomerase. iScience. (2022) 25:103813. doi: 10.1016/j.isci.2022.103813
83. Zou Y, Tong HJ, Li M, Tan KS, and Cao T. Telomere length is regulated by FGF-2 in human embryonic stem cells and affects the life span of its differentiated progenies. Biogerontology. (2017) 18:69–84. doi: 10.1007/s10522-016-9662-8
84. Lupatov AY and Yarygin KN. Telomeres and telomerase in the control of stem cells. Biomedicines. (2022) 10:(10). doi: 10.3390/biomedicines10102335
85. López-Otín C, Blasco MA, Partridge L, Serrano M, and Kroemer G. The hallmarks of aging. Cell. (2013) 153:1194–217. doi: 10.1016/j.cell.2013.05.039
86. Opresko PL and Shay JW. Telomere-associated aging disorders. Ageing Res Rev. (2017) 33:52–66. doi: 10.1016/j.arr.2016.05.009
87. Liu S, Nong W, Ji L, Zhuge X, Wei H, Luo M, et al. The regulatory feedback of inflammatory signaling and telomere/telomerase complex dysfunction in chronic inflammatory diseases. Exp Gerontol. (2023) 174:112132. doi: 10.1016/j.exger.2023.112132
88. Li J, Zhang J, Xue Q, Liu B, Qin R, Li Y, et al. Pyrroloquinoline quinone alleviates natural aging-related osteoporosis via a novel MCM3-Keap1-Nrf2 axis-mediated stress response and Fbn1 upregulation. Aging Cell. (2023) 22:e13912. doi: 10.1111/acel.13912
89. Aubert G and Lansdorp PM. Telomeres and aging. Physiol Rev. (2008) 88:557–79. doi: 10.1152/physrev.00026.2007
90. Huang X, Huang L, Lu J, Cheng L, Wu D, Li L, et al. The relationship between telomere length and aging-related diseases. Clin Exp Med. (2025) 25:72. doi: 10.1007/s10238-025-01608-z
91. Mojiri A, Walther BK, Jiang C, Matrone G, Holgate R, Xu Q, et al. Telomerase therapy reverses vascular senescence and extends lifespan in progeria mice. Eur Heart J. (2021) 42:4352–69. doi: 10.1093/eurheartj/ehab547
92. Zheng X, Wang Q, Xie Z, and Li J. The elevated level of IL-1α in the bone marrow of aged mice leads to MSC senescence partly by down-regulating Bmi-1. Exp Gerontol. (2021) 148:111313. doi: 10.1016/j.exger.2021.111313
93. Shay JW and Wright WE. Telomeres and telomerase: three decades of progress. Nat Rev Genet. (2019) 20:299–309. doi: 10.1038/s41576-019-0099-1
94. Holohan B, Wright WE, and Shay JW. Cell biology of disease: Telomeropathies: an emerging spectrum disorder. J Cell Biol. (2014) 205:289–99. doi: 10.1083/jcb.201401012
95. Armanios M and Blackburn EH. The telomere syndromes. Nat Rev Genet. (2012) 13:693–704. doi: 10.1038/nrg3246
96. Al-Azab M, Safi M, Idiiatullina E, Al-Shaebi F, and Zaky MY. Aging of mesenchymal stem cell: machinery, markers, and strategies of fighting. Cell Mol Biol Lett. (2022) 27:69. doi: 10.1186/s11658-022-00366-0
97. Weng Z, Wang Y, Ouchi T, Liu H, Qiao X, Wu C, et al. Mesenchymal stem/stromal cell senescence: hallmarks, mechanisms, and combating strategies. Stem Cells Transl Med. (2022) 11:356–71. doi: 10.1093/stcltm/szac004
98. Sun Y, Zhang H, Qiu T, Liao L, and Su X. Epigenetic regulation of mesenchymal stem cell aging through histone modifications. Genes Dis. (2023) 10:2443–56. doi: 10.1016/j.gendis.2022.10.030
99. Portela A and Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. (2010) 28:1057–68. doi: 10.1038/nbt.1685
100. Ding Y, Yao Y, Gong X, Zhuo Q, Chen J, Tian M, et al. JMJD3: a critical epigenetic regulator in stem cell fate. Cell Commun Signal. (2021) 19:72. doi: 10.1186/s12964-021-00753-8
101. O’Callaghan C and Vassilopoulos A. Sirtuins at the crossroads of stemness, aging, and cancer. Aging Cell. (2017) 16:1208–18. doi: 10.1111/acel.12685
102. Luo C, Hajkova P, and Ecker JR. Dynamic DNA methylation: In the right place at the right time. Science. (2018) 361:1336–40. doi: 10.1126/science.aat6806
103. Shan Y, Wang L, Li G, Shen G, Zhang P, and Xu Y. Methylation of bone SOST impairs SP7, RUNX2, and ERα transactivation in patients with postmenopausal osteoporosis. Biochem Cell Biol. (2019) 97:369–74. doi: 10.1139/bcb-2018-0170
104. Bentivegna A, Roversi G, Riva G, Paoletta L, Redaelli S, Miloso M, et al. The effect of culture on human bone marrow mesenchymal stem cells: focus on DNA methylation profiles. Stem Cells Int. (2016) 2016:5656701. doi: 10.1155/2016/5656701
105. Smith N, Shirazi S, Cakouros D, and Gronthos S. Impact of environmental and epigenetic changes on mesenchymal stem cells during aging. Int J Mol Sci. (2023) 24:(7). doi: 10.3390/ijms24076499
106. Meyer MB, Benkusky NA, Sen B, Rubin J, and Pike JW. Epigenetic plasticity drives adipogenic and osteogenic differentiation of marrow-derived mesenchymal stem cells. J Biol Chem. (2016) 291:17829–47. doi: 10.1074/jbc.M116.736538
107. Debès C, Papadakis A, Grönke S, Karalay Ö, Tain LS, Mizi A, et al. Ageing-associated changes in transcriptional elongation influence longevity. Nature. (2023) 616:814–21. doi: 10.1038/s41586-023-05922-y
108. Bhadra M, Howell P, Dutta S, Heintz C, and Mair WB. Alternative splicing in aging and longevity. Hum Genet. (2020) 139:357–69. doi: 10.1007/s00439-019-02094-6
109. Aoi Y and Shilatifard A. Transcriptional elongation control in developmental gene expression, aging, and disease. Mol Cell. (2023) 83:3972–99. doi: 10.1016/j.molcel.2023.10.004
110. Yoon DS, Lee KM, Choi Y, Ko EA, Lee NH, Cho S, et al. TLR4 downregulation by the RNA-binding protein PUM1 alleviates cellular aging and osteoarthritis. Cell Death Differ. (2022) 29:1364–78. doi: 10.1038/s41418-021-00925-6
111. Wu KK. Control of mesenchymal stromal cell senescence by tryptophan metabolites. Int J Mol Sci. (2021) 22:(2). doi: 10.3390/ijms22020697
112. Liu H, Song P, Zhang H, Zhou F, Ji N, Wang M, et al. Synthetic biology-based bacterial extracellular vesicles displaying BMP-2 and CXCR4 to ameliorate osteoporosis. J Extracell Vesicles. (2024) 13:e12429. doi: 10.1002/jev2.12429
113. Ye G, Li J, Yu W, Xie Z, Zheng G, Liu W, et al. ALKBH5 facilitates CYP1B1 mRNA degradation via m6A demethylation to alleviate MSC senescence and osteoarthritis progression. Exp Mol Med. (2023) 55:1743–56. doi: 10.1038/s12276-023-01059-0
114. Wu L, Zhang G, Guo C, and Pan Y. Intracellular Ca(2+) signaling mediates IGF-1-induced osteogenic differentiation in bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. (2020) 527:200–6. doi: 10.1016/j.bbrc.2020.04.048
115. Wu Z, Shi Y, Lu M, Song M, Yu Z, Wang J, et al. METTL3 counteracts premature aging via m6A-dependent stabilization of MIS12 mRNA. Nucleic Acids Res. (2020) 48:11083–96. doi: 10.1093/nar/gkaa816
116. Zheng J, Lu Y, Lin Y, Si S, Guo B, Zhao X, et al. Epitranscriptomic modifications in mesenchymal stem cell differentiation: advances, mechanistic insights, and beyond. Cell Death Differ. (2024) 31:9–27. doi: 10.1038/s41418-023-01238-6
117. Boreikaitė V and Passmore LA. 3’-end processing of eukaryotic mRNA: machinery, regulation, and impact on gene expression. Annu Rev Biochem. (2023) 92:199–225. doi: 10.1146/annurev-biochem-052521-012445
118. Potter ML, Hill WD, Isales CM, Hamrick MW, and Fulzele S. MicroRNAs are critical regulators of senescence and aging in mesenchymal stem cells. Bone. (2021) 142:115679. doi: 10.1016/j.bone.2020.115679
119. Saul D and Kosinsky RL. Epigenetics of aging and aging-associated diseases. Int J Mol Sci. (2021) 22:(1). doi: 10.3390/ijms22010401
120. Suh N. MicroRNA controls of cellular senescence. BMB Rep. (2018) 51:493–9. doi: 10.5483/BMBRep.2018.51.10.209
121. Okada M, Kim HW, Matsu-ura K, Wang YG, Xu M, and Ashraf M. Abrogation of age-induced microRNA-195 rejuvenates the senescent mesenchymal stem cells by reactivating telomerase. Stem Cells. (2016) 34:148–59. doi: 10.1002/stem.2211
122. Soriano-Arroquia A, Gostage J, Xia Q, Bardell D, McCormick R, McCloskey E, et al. miR-24 and its target gene Prdx6 regulate viability and senescence of myogenic progenitors during aging. Aging Cell. (2021) 20:e13475. doi: 10.1111/acel.13475
123. Bischof O and Martínez-Zamudio RI. MicroRNAs and lncRNAs in senescence: A re-view. IUBMB Life. (2015) 67:255–67. doi: 10.1002/iub.1373
124. Ghafouri-Fard S, Abak A, Talebi SF, Shoorei H, Branicki W, Taheri M, et al. Role of miRNA and lncRNAs in organ fibrosis and aging. BioMed Pharmacother. (2021) 143:112132. doi: 10.1016/j.biopha.2021.112132
125. Xie ZY, Wang P, Wu YF, and Shen HY. Long non-coding RNA: The functional regulator of mesenchymal stem cells. World J Stem Cells. (2019) 11:167–79. doi: 10.4252/wjsc.v11.i3.167
126. Cai J, Qi H, Yao K, Yao Y, Jing D, Liao W, et al. Non-coding RNAs steering the senescence-related progress, properties, and application of mesenchymal stem cells. Front Cell Dev Biol. (2021) 9:650431. doi: 10.3389/fcell.2021.650431
127. Iantomasi T, Romagnoli C, Palmini G, Donati S, Falsetti I, Miglietta F, et al. Oxidative stress and inflammation in osteoporosis: molecular mechanisms involved and the relationship with microRNAs. Int J Mol Sci. (2023) 24:(4). doi: 10.3390/ijms24043772
128. Ma C, Yu R, Li J, Chao J, and Liu P. Targeting proteostasis network in osteoporosis: Pathological mechanisms and therapeutic implications. Ageing Res Rev. (2023) 90:102024. doi: 10.1016/j.arr.2023.102024
129. Ottens F, Franz A, and Hoppe T. Build-UPS and break-downs: metabolism impacts on proteostasis and aging. Cell Death Differ. (2021) 28:505–21. doi: 10.1038/s41418-020-00682-y
130. Binder MJ and Pedley AM. The roles of molecular chaperones in regulating cell metabolism. FEBS Lett. (2023) 597:1681–701. doi: 10.1002/1873-3468.14682
131. Margulis B, Tsimokha A, Zubova S, and Guzhova I. Molecular chaperones and proteolytic machineries regulate protein homeostasis in aging cells. Cells. (2020) 9:(5). doi: 10.3390/cells9051308
132. Gomez CR. Role of heat shock proteins in aging and chronic inflammatory diseases. Geroscience. (2021) 43:2515–32. doi: 10.1007/s11357-021-00394-2
133. Lazaro-Pena MI, Ward ZC, Yang S, Strohm A, Merrill AK, Soto CA, et al. HSF-1: guardian of the proteome through integration of longevity signals to the proteostatic network. Front Aging. (2022) 3:861686. doi: 10.3389/fragi.2022.861686
134. Koyuncu S, Loureiro R, Lee HJ, Wagle P, Krueger M, and Vilchez D. Rewiring of the ubiquitinated proteome determines ageing in C. elegans. Nature. (2021) 596:285–90. doi: 10.1038/s41586-021-03781-z
135. Korovila I, Hugo M, Castro JP, Weber D, Höhn A, Grune T, et al. Proteostasis, oxidative stress and aging. Redox Biol. (2017) 13:550–67. doi: 10.1016/j.redox.2017.07.008
136. Stekel Z, Sheng Y, and Zhang W. The multifaceted role of the ubiquitin proteasome system in pathogenesis and diseases. Biomolecules. (2022) 12:(7). doi: 10.3390/biom12070925
137. Chen R, Yang C, Yang F, Yang A, Xiao H, Peng B, et al. Targeting the mTOR-autophagy axis: unveiling therapeutic potentials in osteoporosis. Biomolecules. (2024) 14:(11). doi: 10.3390/biom14111452
138. Carroll B and Korolchuk VI. Dysregulation of mTORC1/autophagy axis in senescence. Aging (Albany NY). (2017) 9:1851–2. doi: 10.18632/aging.101277
139. Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: A mini-review. Gerontology. (2018) 64:127–34. doi: 10.1159/000484629
140. Roger L, Tomas F, and Gire V. Mechanisms and regulation of cellular senescence. Int J Mol Sci. (2021) 22:(23). doi: 10.3390/ijms222313173
141. Ma Y, Qi M, An Y, Zhang L, Yang R, Doro DH, et al. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell. (2018) 17:(1). doi: 10.1111/acel.12709
142. Höhn A, Weber D, Jung T, Ott C, Hugo M, Kochlik B, et al. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. (2017) 11:482–501. doi: 10.1016/j.redox.2016.12.001
143. Gong B, Radulovic M, Figueiredo-Pereira ME, and Cardozo C. The ubiquitin-proteasome system: potential therapeutic targets for alzheimer’s disease and spinal cord injury. Front Mol Neurosci. (2016) 9:4. doi: 10.3389/fnmol.2016.00004
144. Wang ZX, Lin X, Cao J, Liu YW, Luo ZW, Rao SS, et al. Young osteocyte-derived extracellular vesicles facilitate osteogenesis by transferring tropomyosin-1. J Nanobiotechnology. (2024) 22:208. doi: 10.1186/s12951-024-02367-x
145. Ma Y, Wang S, Wang H, Chen X, Shuai Y, Wang H, et al. Mesenchymal stem cells and dental implant osseointegration during aging: from mechanisms to therapy. Stem Cell Res Ther. (2023) 14:382. doi: 10.1186/s13287-023-03611-1
146. Zupan J, Strazar K, Kocijan R, Nau T, Grillari J, and Marolt Presen D. Age-related alterations and senescence of mesenchymal stromal cells: Implications for regenerative treatments of bones and joints. Mech Ageing Dev. (2021) 198:111539. doi: 10.1016/j.mad.2021.111539
147. Caplan AI, Reuben D, and Haynesworth SE. Cell-based tissue engineering therapies: the influence of whole body physiology. Adv Drug Delivery Rev. (1998) 33:3–14. doi: 10.1016/s0169-409x(98)00016-7
148. Liu H, Li W, Liu Y, Zhang X, and Zhou Y. Co-administration of aspirin and allogeneic adipose-derived stromal cells attenuates bone loss in ovariectomized rats through the anti-inflammatory and chemotactic abilities of aspirin. Stem Cell Res Ther. (2015) 6:200. doi: 10.1186/s13287-015-0195-x
149. Sanghani A, Osagie-Clouard L, Samizadeh S, Coathup MJ, Kalia P, Di Silvio L, et al. CXCR4 has the potential to enhance bone formation in osteopenic rats. Tissue Eng Part A. (2018) 24:1775–83. doi: 10.1089/ten.TEA.2018.0121
150. Wu Y, Xie L, Wang M, Xiong Q, Guo Y, Liang Y, et al. Mettl3-mediated m(6)A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nat Commun. (2018) 9:4772. doi: 10.1038/s41467-018-06898-4
151. Abe T, Sumi K, Kunimatsu R, Oki N, Tsuka Y, Nakajima K, et al. The effect of mesenchymal stem cells on osteoclast precursor cell differentiation. J Oral Sci. (2019) 61:30–5. doi: 10.2334/josnusd.17-0315
152. Wang B, Wen H, Smith W, Hao D, He B, and Kong L. Regulation effects of melatonin on bone marrow mesenchymal stem cell differentiation. J Cell Physiol. (2019) 234:1008–15. doi: 10.1002/jcp.27090
153. Zhang D, Yu K, Yang J, Xie S, Yang J, and Tan L. Senolytic controls bone marrow mesenchymal stem cells fate improving bone formation. Am J Transl Res. (2020) 12:3078–88.
154. Jia Y, Zhu Y, Qiu S, Xu J, and Chai Y. Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res Ther. (2019) 10:12. doi: 10.1186/s13287-018-1115-7
155. Hughes JM and Petit MA. Biological underpinnings of Frost’s mechanostat thresholds: the important role of osteocytes. J Musculoskelet Neuronal Interact. (2010) 10:128–35.
156. Liu C, He L, Wang J, Wang Q, Sun C, Li Y, et al. Anti-angiogenic effect of Shikonin in rheumatoid arthritis by downregulating PI3K/AKT and MAPKs signaling pathways. J Ethnopharmacol. (2020) 260:113039. doi: 10.1016/j.jep.2020.113039
157. Sheppard AJ, Delgado K, Barfield AM, Xu Q, Massey PA, Dong Y, et al. Rapamycin inhibits senescence and improves immunomodulatory function of mesenchymal stem cells through IL-8 and TGF-β Signaling. Stem Cell Rev Rep. (2024) 20:816–26. doi: 10.1007/s12015-024-10682-x
158. Lee S, Le NH, and Kang D. Melatonin alleviates oxidative stress-inhibited osteogenesis of human bone marrow-derived mesenchymal stem cells through AMPK activation. Int J Med Sci. (2018) 15:1083–91. doi: 10.7150/ijms.26314
159. Shuai Y, Liao L, Su X, Yu Y, Shao B, Jing H, et al. Melatonin treatment improves mesenchymal stem cells therapy by preserving stemness during long-term in vitro expansion. Theranostics. (2016) 6:1899–917. doi: 10.7150/thno.15412
160. Acar MB, Ayaz-Güner Ş, Gunaydin Z, Karakukcu M, Peluso G, Di Bernardo G, et al. Proteomic and biological analysis of the effects of metformin senomorphics on the mesenchymal stromal cells. Front Bioeng Biotechnol. (2021) 9:730813. doi: 10.3389/fbioe.2021.730813
161. Bai L, Zhang X, Han Z, Yang X, and Hao Y. Inject able porous microspheres for articular cartilage regeneration through in situ stem cell recruitment and macrophage polarization. Acta Biomater. (2024) 185:429–40. doi: 10.1016/j.actbio.2024.07.007
162. Hong G, Zhou Y, Yang S, Yan S, Lu J, Xu B, et al. Metformin acts on miR-181a-5p/PAI-1 axis in stem cells providing new strategies for improving age-related osteogenic differentiation decline. Stem Cells. (2024) 42:1055–69. doi: 10.1093/stmcls/sxae057
163. Kim H, Yu MR, Lee H, Kwon SH, Jeon JS, Han DC, et al. Metformin inhibits chronic kidney disease-induced DNA damage and senescence of mesenchymal stem cells. Aging Cell. (2021) 20:e13317. doi: 10.1111/acel.13317
164. Smieszek A, Tomaszewski KA, Kornicka K, and Marycz K. Metformin promotes osteogenic differentiation of adipose-derived stromal cells and exerts pro-osteogenic effect stimulating bone regeneration. J Clin Med. (2018) 7:(12). doi: 10.3390/jcm7120482
165. Hu C and Li L. The application of resveratrol to mesenchymal stromal cell-based regenerative medicine. Stem Cell Res Ther. (2019) 10:307. doi: 10.1186/s13287-019-1412-9
166. Lv YJ, Yang Y, Sui BD, Hu CH, Zhao P, Liao L, et al. Resveratrol counteracts bone loss via mitofilin-mediated osteogenic improvement of mesenchymal stem cells in senescence-accelerated mice. Theranostics. (2018) 8:2387–406. doi: 10.7150/thno.23620
167. Wagle S, Sim HJ, Bhattarai G, Choi KC, Kook SH, Lee JC, et al. Supplemental ferulic acid inhibits total body irradiation-mediated bone marrow damage, bone mass loss, stem cell senescence, and hematopoietic defect in mice by enhancing antioxidant defense systems. Antioxidants (Basel). (2021) 10:(8). doi: 10.3390/antiox10081209
168. Wang N, Li Z, Li S, Li Y, Gao L, Bao X, et al. Curculigoside ameliorates bone loss by influencing mesenchymal stem cell fate in aging mice. Front Cell Dev Biol. (2021) 9:767006. doi: 10.3389/fcell.2021.767006
169. Wong PF, Dharmani M, and Ramasamy TS. Senotherapeutics for mesenchymal stem cell senescence and rejuvenation. Drug Discov Today. (2023) 28:103424. doi: 10.1016/j.drudis.2022.103424
170. Ok JS, Song SB, and Hwang ES. Enhancement of replication and differentiation potential of human bone marrow stem cells by nicotinamide treatment. Int J Stem Cells. (2018) 11:13–25. doi: 10.15283/ijsc18033
171. Qu YN, Zhang L, Wang T, Zhang HY, Yang ZJ, Yuan FF, et al. Vitamin C treatment rescues prelamin A-induced premature senescence of subchondral bone mesenchymal stem cells. Stem Cells Int. (2020) 2020:3150716. doi: 10.1155/2020/3150716
172. Rizzo MG, Best TM, Huard J, Philippon M, Hornicek F, Duan Z, et al. Therapeutic perspectives for inflammation and senescence in osteoarthritis using mesenchymal stem cells, mesenchymal stem cell-derived extracellular vesicles and senolytic agents. Cells. (2023) 12:(10). doi: 10.3390/cells12101421
173. Zhou Y, Xin X, Wang L, Wang B, Chen L, Liu O, et al. Senolytics improve bone forming potential of bone marrow mesenchymal stem cells from aged mice. NPJ Regener Med. (2021) 6:34. doi: 10.1038/s41536-021-00145-z
174. Gong L, Chen B, Zhang J, Sun Y, Yuan J, Niu X, et al. Human ESC-sEVs alleviate age-related bone loss by rejuvenating senescent bone marrow-derived mesenchymal stem cells. J Extracell Vesicles. (2020) 9:1800971. doi: 10.1080/20013078.2020.1800971
175. Jiang X, Li W, Ge L, and Lu M. Mesenchymal stem cell senescence during aging: from mechanisms to rejuvenation strategies. Aging Dis. (2023) 14:1651–76. doi: 10.14336/ad.2023.0208
176. Li X, Hong Y, He H, Jiang G, You W, Liang X, et al. FGF21 mediates mesenchymal stem cell senescence via regulation of mitochondrial dynamics. Oxid Med Cell Longev. (2019) 2019:4915149. doi: 10.1155/2019/4915149
177. Chen CY, Tseng KY, Lai YL, Chen YS, Lin FH, and Lin S. Overexpression of insulin-like growth factor 1 enhanced the osteogenic capability of aging bone marrow mesenchymal stem cells. Theranostics. (2017) 7:1598–611. doi: 10.7150/thno.16637
178. Yang K, Wu Z, Zhang K, Weir MD, Xu HHK, Cheng L, et al. Unlocking the potential of stimuli-responsive biomaterials for bone regeneration. Front Pharmacol. (2024) 15:1437457. doi: 10.3389/fphar.2024.1437457
179. Ho TC, Chang CC, Chan HP, Chung TW, Shu CW, Chuang KP, et al. Hydrogels: properties and applications in biomedicine. Molecules. (2022) 27:(9). doi: 10.3390/molecules27092902
180. Zhang S, Lv R, Zhang Z, Wang Z, and Jin Z. Advancements in hydrogel-based embolic agents: Categorized by therapeutic mechanisms. Cancer Med. (2024) 13:e70183. doi: 10.1002/cam4.70183
181. Ye Y, Zhong H, Huang S, Lai W, Huang Y, Sun C, et al. Reactive oxygen species scavenging hydrogel regulates stem cell behavior and promotes bone healing in osteoporosis. Tissue Eng Regener Med. (2023) 20:981–92. doi: 10.1007/s13770-023-00561-w
182. Tang G, Zhu L, Wang W, Zuo D, Shi C, Yu X, et al. Alendronate-functionalized double network hydrogel scaffolds for effective osteogenesis. Front Chem. (2022) 10:977419. doi: 10.3389/fchem.2022.977419
183. Zhou H, He Z, Cao Y, Chu L, Liang B, Yu K, et al. An injectab le magnesium-loaded hydrogel releases hydrogen to promote osteoporotic bone repair via ROS scavenging and immunomodulation. Theranostics. (2024) 14:3739–59. doi: 10.7150/thno.97412
184. Ding Q, Zhang S, Liu X, Zhao Y, Yang J, Chai G, et al. Hydrogel tissue bioengineered scaffolds in bone repair: A review. Molecules. (2023) 28:(20). doi: 10.3390/molecules28207039
185. Yang Y, Yuan L, Cao H, Guo J, Zhou X, and Zeng Z. Application and molecular mechanisms of extracellular vesicles derived from mesenchymal stem cells in osteoporosis. Curr Issues Mol Biol. (2022) 44:6346–67. doi: 10.3390/cimb44120433
186. Chen CY, Rao SS, Ren L, Hu XK, Tan YJ, Hu Y, et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics. (2018) 8:1607–23. doi: 10.7150/thno.22958
187. Chen CY, Rao SS, Tan YJ, Luo MJ, Hu XK, Yin H, et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. (2019) 7:18. doi: 10.1038/s41413-019-0056-9
188. Lee KS, Lee J, Kim HK, Yeom SH, Woo CH, Jung YJ, et al. Extracellular vesicles from adipose tissue-derived stem cells alleviate osteoporosis through osteoprotegerin and miR-21-5p. J Extracell Vesicles. (2021) 10:e12152. doi: 10.1002/jev2.12152
189. Gatti M, Beretti F, Zavatti M, Bertucci E, Luz SR, Palumbo C, et al. Amniotic fluid stem cell‑derived extracellular vesicles counteract steroid‑induced osteoporosis in vitro. Int J Mol Sci. (2021) 22(1):1–18. doi: 10.3390/ijms22010038
190. Yuan FL, Wu QY, Miao ZN, Xu MH, Xu RS, Jiang DL, et al. Osteoclast-derived extracellular vesicles: novel regulators of osteoclastogenesis and osteoclast-osteoblasts communication in bone remodeling. Front Physiol. (2018) 9:628. doi: 10.3389/fphys.2018.00628
191. Liu JH, Chen CY, Liu ZZ, Luo ZW, Rao SS, Jin L, et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci (Weinh). (2021) 8:2004831. doi: 10.1002/advs.202004831
192. Xu X, Jia X, Mo L, Liu C, Zheng L, Yuan Q, et al. Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res. (2017) 5:17046. doi: 10.1038/boneres.2017.46
193. Ding Z, Yan Z, Yuan X, Tian G, Wu J, Fu L, et al. Apoptotic extracellular vesicles derived from hypoxia-preconditioned mesenchymal stem cells within a modified gelatine hydrogel promote osteochondral regeneration by enhancing stem cell activity and regulating immunity. J Nanobiotechnology. (2024) 22:74. doi: 10.1186/s12951-024-02333-7
194. Guo R, Wu C, Liu F, Dong T, and Zhang T. Biomimetic composite hydrogel promotes new bone formation in rat bone defects through regulation of miR-19b-3p/WWP1 axis by loaded extracellular vesicles. J Nanobiotechnology. (2023) 21:459. doi: 10.1186/s12951-023-02201-w
195. Huang C and Wang Y. Downregulation of METTL14 improves postmenopausal osteoporosis via IGF2BP1 dependent posttranscriptional silencing of SMAD1. Cell Death Dis. (2022) 13:919. doi: 10.1038/s41419-022-05362-y
196. Zhang C, Li H, Li J, Hu J, Yang K, and Tao L. Oxidative stress: A common pathological state in a high-risk population for osteoporosis. BioMed Pharmacother. (2023) 163:114834. doi: 10.1016/j.biopha.2023.114834
197. Gao Y, Zou Y, Sokolowskei D, Xing X, Tower RJ, Lai Z, et al. Nr4a1 enhances Wnt4 transcription to promote mesenchymal stem cell osteogenesis and alleviates inflammation-inhibited bone regeneration. Mol Ther. (2024) 32:1479–96. doi: 10.1016/j.ymthe.2024.02.034
198. Mehdizadeh R, Madjid Ansari A, Forouzesh F, Shahriari F, Shariatpanahi SP, Salaritabar A, et al. P53 status, and G2/M cell cycle arrest, are determining factors in cell-death induction mediated by ELF-EMF in glioblastoma. Sci Rep. (2023) 13:10845. doi: 10.1038/s41598-023-38021-z
199. Zou YC, Gao K, Cao BT, He XL, Zheng W, Wang XF, et al. Syringin protects high glucose-induced BMSC injury, cell senescence, and osteoporosis by inhibiting JAK2/STAT3 signaling. J Appl BioMed. (2024) 22:197–207. doi: 10.32725/jab.2024.021
200. He F, Luo S, Liu S, Wan S, Li J, Chen J, et al. Zanthoxylum bungeanum seed oil inhibits RANKL-induced osteoclastogenesis by suppressing ERK/c-JUN/NFATc1 pathway and regulating cell cycle arrest in RAW264.7 cells. J Ethnopharmacol. (2022) 289:115094. doi: 10.1016/j.jep.2022.115094
201. Martel J, Ojcius DM, and Young JD. Lifestyle interventions to delay senescence. BioMed J. (2024) 47:100676. doi: 10.1016/j.bj.2023.100676
202. LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. (2022) 33:2049–102. doi: 10.1007/s00198-021-05900-y
203. Brooke-Wavell K, Skelton DA, Barker KL, Clark EM, De Biase S, Arnold S, et al. Strong, steady and straight: UK consensus statement on physical activity and exercise for osteoporosis. Br J Sports Med. (2022) 56:837–46. doi: 10.1136/bjsports-2021-104634
204. Hou W, Chen S, Zhu C, Gu Y, Zhu L, and Zhou Z. Associations between smoke exposure and osteoporosis or osteopenia in a US NHANES population of elderly individuals. Front Endocrinol (Lausanne). (2023) 14:1074574. doi: 10.3389/fendo.2023.1074574
205. Jing Z, Li Y, Zhang H, Chen T, Yu J, Xu X, et al. Tobacco toxins induce osteoporosis through ferroptosis. Redox Biol. (2023) 67:102922. doi: 10.1016/j.redox.2023.102922
206. Luo Z, Liu Y, Liu Y, Chen H, Shi S, and Liu Y. Cellular and molecular mechanisms of alcohol-induced osteopenia. Cell Mol Life Sci. (2017) 74:4443–53. doi: 10.1007/s00018-017-2585-y
207. Huang Q, Guo J, Zhao H, Zheng Y, and Zhang Y. The associations of alcoholic liver disease and nonalcoholic fatty liver disease with bone mineral density and the mediation of serum 25-Hydroxyvitamin D: A bidirectional and two-step Mendelian randomization. PloS One. (2023) 18:e0292881. doi: 10.1371/journal.pone.0292881
208. Chotiyarnwong P and McCloskey EV. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol. (2020) 16:437–47. doi: 10.1038/s41574-020-0341-0
209. Siraj Y, Galderisi U, and Alessio N. Senescence induces fundamental changes in the secretome of mesenchymal stromal cells (MSCs): implications for the therapeutic use of MSCs and their derivates. Front Bioeng Biotechnol. (2023) 11:1148761. doi: 10.3389/fbioe.2023.1148761
210. Zhang JY, Zhong YH, Chen LM, Zhuo XL, Zhao LJ, and Wang YT. Recent advance of small-molecule drugs for clinical treatment of osteoporosis: A review. Eur J Med Chem. (2023) 259:115654. doi: 10.1016/j.ejmech.2023.115654
211. Suda M, Paul KH, Tripathi U, Minamino T, Tchkonia T, and Kirkland JL. Targeting cell senescence and senolytics: novel interventions for age-related endocrine dysfunction. Endocr Rev. (2024) 45:655–75. doi: 10.1210/endrev/bnae010
212. Oh WT, Yang YS, Xie J, Ma H, Kim JM, Park KH, et al. WNT-modulating gene silencers as a gene therapy for osteoporosis, bone fracture, and critical-sized bone defects. Mol Ther. (2023) 31:435–53. doi: 10.1016/j.ymthe.2022.09.018
213. Li S, Liu Z, Zhang J, and Li L. Links between telomere dysfunction and hallmarks of aging. Mutat Res Genet Toxicol Environ Mutagen. (2023) 888:503617. doi: 10.1016/j.mrgentox.2023.503617
214. Liu F, Lu Y, Wang X, Sun S, Pan H, Wang M, et al. Identification of FOXO1 as a geroprotector in human synovium through single-nucleus transcriptomic profiling. Protein Cell. (2024) 15:441–59. doi: 10.1093/procel/pwad060
215. Wang F, Tarkkonen K, Nieminen-Pihala V, Nagano K, Majidi RA, Puolakkainen T, et al. Mesenchymal cell-derived juxtacrine wnt1 signaling regulates osteoblast activity and osteoclast differentiation. J Bone Miner Res. (2019) 34:1129–42. doi: 10.1002/jbmr.3680
216. Wang Y, Che L, Chen X, He Z, Song D, Yuan Y, et al. Repurpose dasatinib and quercetin: Targeting senescent cells ameliorates postmenopausal osteoporosis and rejuvenates bone regeneration. Bioact Mater. (2023) 25:13–28. doi: 10.1016/j.bioactmat.2023.01.009
217. Wang Y, Che M, Xin J, Zheng Z, Li J, and Zhang S. The role of IL-1β and TNF-α in intervertebral disc degeneration. BioMed Pharmacother. (2020) 131:110660. doi: 10.1016/j.biopha.2020.110660
218. Zhang L, Jiao G, Ren S, Zhang X, Li C, Wu W, et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. (2020) 11:38. doi: 10.1186/s13287-020-1562-9
Keywords: mesenchymal stem cells, aging, osteoporosis cellular senescence, bone regeneration, senescence-associated secretory phenotype, oxidative Stress
Citation: Tong Y, Tu Y, Wang J, Liu X, Su Q, Wang Y and Wang W (2025) Mechanisms and therapeutic strategies linking mesenchymal stem cells senescence to osteoporosis. Front. Endocrinol. 16:1625806. doi: 10.3389/fendo.2025.1625806
Received: 09 May 2025; Accepted: 30 June 2025;
Published: 21 July 2025.
Edited by:
Bingdong Sui, Air Force Medical University, ChinaReviewed by:
Gustavo Roberto Cointry, National University of Rosario, ArgentinaLingwei Huang, Binzhou Medical University, China
Copyright © 2025 Tong, Tu, Wang, Liu, Su, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanghao Wang, MTA1NzY1MDIzMUBxcS5jb20=; Weizhou Wang, d2FuZ3dlaXpob3VAa21tdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yashuang Tong
Yashuang Tong Yulin Tu
Yulin Tu Jingying Wang
Jingying Wang Xiuyu Liu
Xiuyu Liu Qian Su
Qian Su Yanghao Wang
Yanghao Wang Weizhou Wang
Weizhou Wang