- 1Department of Human Pathology of Adulthood and Childhood, University of Messina, Messina, Italy
- 2Neonatology Unit, Department of Medicine and Surgery, Pietro Barilla Children’s Hospital, University of Parma, Parma, Italy
- 3Department of Surgical Sciences, Obstetrics and Gynecology Unit, University of Parma, Parma, Italy
- 4Department of Medicine and Surgery, University of Parma, Parma, Italy
- 5Department of Chemistry “Ugo Schiff”, University of Florence, Sesto Fiorentino, Italy
- 6Department of Biomolecular Sciences, University of Urbino Carlo Bo, Urbino, Italy
- 7Clinical and Epidemiological Research Unit, University Hospital of Parma, Parma, Italy
- 8Unit of Paediatrics, University Hospital of Parma, Parma, Italy
To date, no shared guidelines have been approved for the diagnosis and management of low bone mineral density (BMD), especially in early infancy. Therefore, there is an increasing demand for new methodologies to allow the assessment of bone health status in this specific cohort, which is exposed to several risk factors (e.g. maternal vitamin D deficiency, pregnancy-associated diseases, preterm birth and comorbidities, low birth weight, intrauterine growth restriction). Currently, the assessment of BMD in newborn and infants relies mainly on serum and urinary biochemical markers, in association with several technologies to measure bone mineral content, such as dual-energy X-ray absorptiometry (DXA) and quantitative ultrasound (QUS) being traditionally used, despite many limitations. More recently, Radiofrequency Echographic Multi-Spectrometry (REMS) emerged as a promising tool in clinical practice for screening and monitoring BMD. Due to the radiation-free technology, an extremely ease of use, low costs, an excellent degree of sensitivity, specificity, and reproducibility, REMS technology has proven to be the gold standard technique in sensitive populations such as pregnant women, newborns and infants, allowing mass extended screening strategies. However, to date no validate cut-off reference for REMS in paediatric age are available. Future longitudinal studies on REMS methodology are needed to build reference standards and new shared algorithms, combining biochemical and instrumental data, for the diagnosis, management and treatment of decreased BMD before and after birth.
1 Introduction
The term “bone health” usually refers to bone’s strength, expressed as fracture resistance and measured by bone mineral reserve (1, 2). However, bone health appears to be more complex, including also all the intrinsic and extrinsic factors that may contribute to it, already during prenatal life. As a result, bone health relies on a sophisticated interplay between biological, genetics, metabolic, hormonal, environmental, mechanical, and nutritional factors, whose intricate interaction starts in the early stages of intrauterine life, continuing throughout childhood, and peaking between the second and third decade of life, when “peak bone mass” is reached (3). Indeed, it is well known that the main factors determining bone health exert their influence since prenatal age and mainly during the third trimester of pregnancy (Figure 1), when up to 80% of fetal bone mineral accumulation occurs, and a progressive expansion of bone volume takes place through an increase in trabecular thickness and cortical architecture under the control of mineral availability (4). Therefore, most of the information on factors affecting bone health in early childhood comes from studies carried on premature infants. Since the great majority of bone mineralization occurs during the third trimester, preterm birth represents per se a risk factor for decreased bone mass: the sudden interruption of transplacental transport of calcium and phosphate due to premature birth and the co-occurrence of conditions such as sepsis, necrotizing enterocolitis, cholestasis, bronchopulmonary dysplasia, etc., as well as low birth weight (less than 1500 g) or intrauterine growth restriction (IUGR) make premature babies more at risk of developing reduced bone mineralization in later life (5).
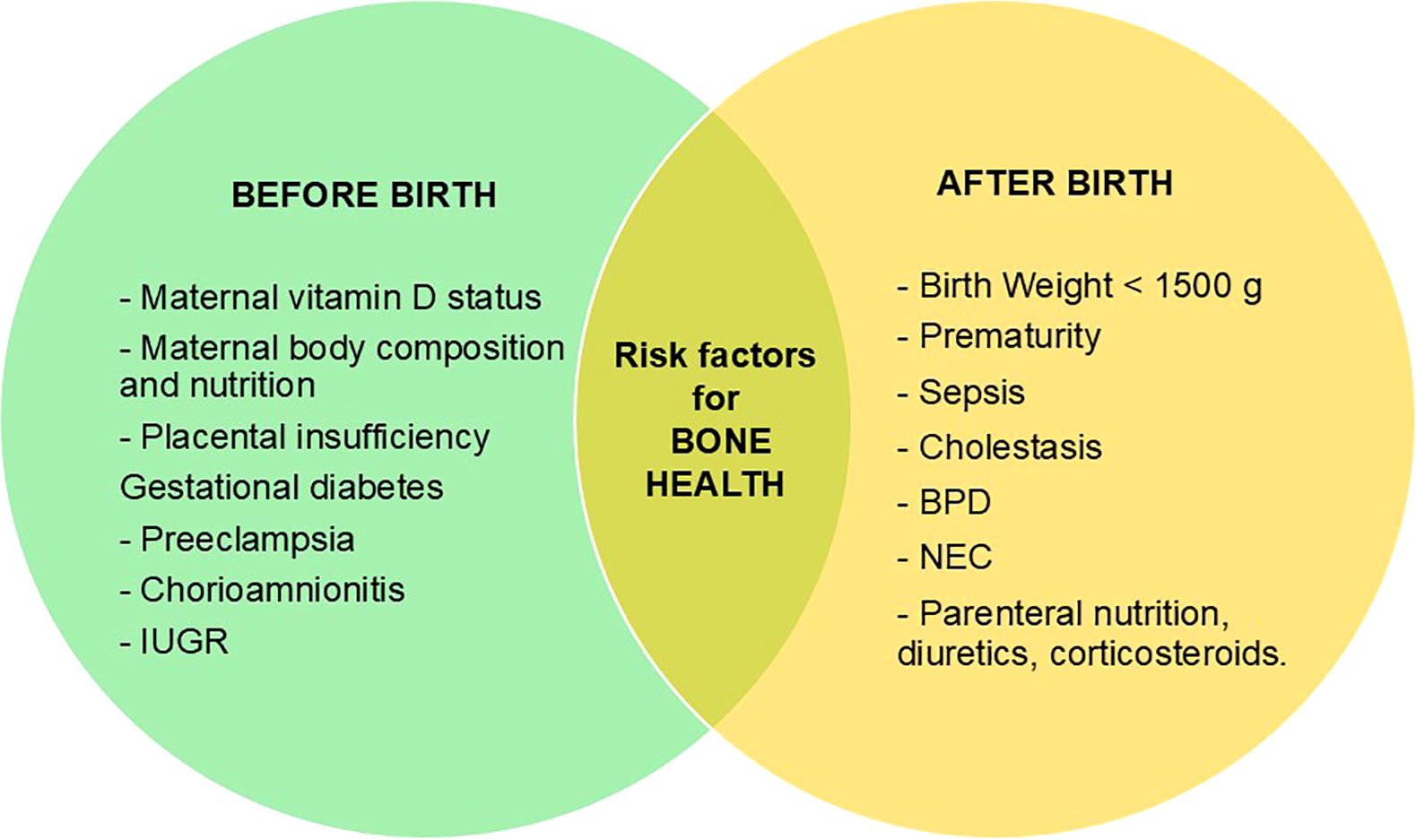
Figure 1. Factors influencing negatively bone health status before and after birth. IUGR, intrauterine growth restriction; BPD, Bronchopulmonary dysplasia; NEC, Necrotizing enterocolitis.
In recent years, expanding knowledge of fetal bone development has led to the identification of a growing number of endocrine and non-endocrine factors that play a key role in ensuring bone health.
The role of leptin, cytokines, oxidative stress (OS) and endocrine disrupting chemicals (EDCs) on bone formation and resorption is emerging, in addition to the pregnancy specific regulation of calcium-phoshate metabolism and the well-known influence of cortisol, GH/IGF-1 axis and vitamin D status. In particular, cytokines (IL-1, IL-6 and TNF-α) and oxidative stress exert their negative effects by impairing osteoblast differentiation and bone remodeling in favor of resorption. Likewise, high plasma levels of EDCs, such as poly- and perfluoroalkyl substances (PFAS), in first 1000 days of life are associated with lower bone mineral density (BMD) SDS at age of 3 years (5–8). Furthermore, these substances seem to have long-term effects on bone health through multiple epigenetic mechanisms and gene expression modulation. In addition, maternal issues during pregnancy, such as vitamin D deficiency, impaired body composition and nutrition, pregnancy-related diseases, have been widely reported as influencing fetal bone mass and BMD and peak bone mass in adulthood, even though the pathogenic mechanisms of fetal endocrine programming is not yet completely understood (9–13).
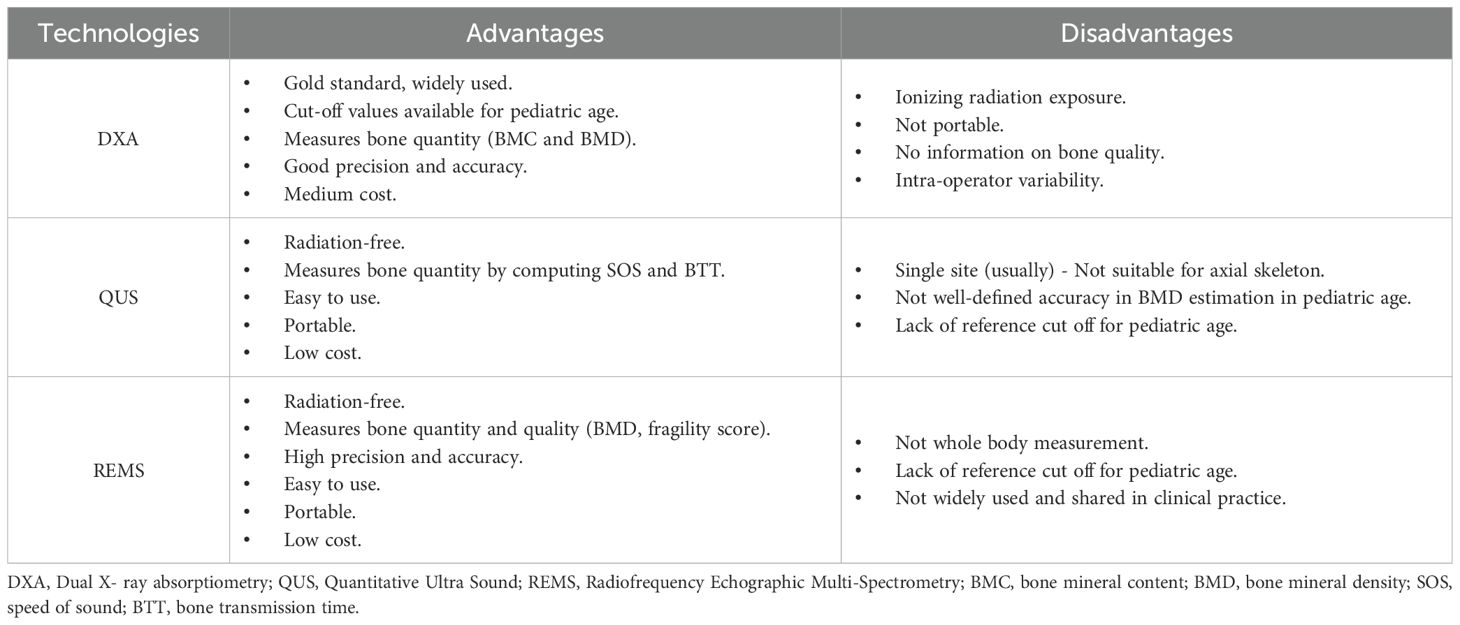
It is therefore quite necessary to implement effective strategies for bone health prevention, starting with the identification of the categories of patients most at risk, including the application of new technologies that could be non-invasive, easily, and longitudinally performed, suitable and applicable in early childhood, to assess any changes in bone health status (14, 15). Over the years, several technologies have been proposed to measure bone mineral content, including dual-energy X-ray absorptiometry (DXA) and quantitative ultrasound (QUS), which are traditionally used (16–20). Most recently, Radiofrequency Echographic Multi-Spectrometry (REMS) has emerged as a promising tool in clinical practice for screening and monitoring BMD.
Aim of this narrative review is to summarize the state of the art on technologies currently available for the assessment of bone health in the early infancy, focusing on new emergent methodologies for early identification, stratification, and management of osteopenia in this specific cohort of patients.
2 The main technologies to assess bone health in early infancy
2.1 Dual x-ray absorptiometry and x-ray
Dual X- ray absorptiometry (DXA) is a speed, precise, safety, relatively low-cost technique and it has been considered recently as the gold standard for the evaluation of bone density parameters.Since its introduction in clinical practice, DXA scans have been performed both in infants and children, and numerous research studies validated its precision and accuracy (16, 21, 22).
Two DXA parameters, BMC (bone mineral content) and BMD, provide informations on the state of bone health through the analysis of different X-ray absorption by the bone, subtracting soft tissue components.
In clinical practice, the reference parameter is Z-score, defining the number of standard deviations of the patient’s bone density with respect to a reference population of the same age, gender and ethnicity.
Due to the rapid growth characterizing the early age of life, the information about BMD in children under 3 years of age is mainly obtained through the evaluation of whole-body measurements, while the posterior anterior lumbar spine scans are less frequently used under 5 years of age. Although both the sites have been validated by current recommendations of the International Society for Clinical Densitometry (ISCD) (23), areal BMD measurement should not be used routinely in infants (difficulty to place the babies in appropriate positioning, scarce uniformity of bones in the three dimensions secondary to the rapid growth process) (24).
There are some important limitations to the use of DXA in early infancy: in addition to limited availability secondary to cumulative radiation exposure, the accuracy of DXA is also affected by technical and operator variability, with significant variation in the parameters reported for a subject due to different skills and software used for analysis. In addition, variations in height, skeletal size and shape and the amount of soft tissue that occurs during the rapid growth of infants may limit the comparative evaluation of DXA scans at various ages. In children, DXA BMD measurements are influenced by height, so bone mineral apparent density, and height-for-age Z score are used and recommended to reduce the confounding effect of short stature on spine bone density (16, 25–27).
Overall, X-rays have limited application in assessing bone status. According to the literature, X-rays could be used to identify significant signs of osteoporosis or bone fractures, but they are not suitable for early diagnosis: some forms of osteoporosis with bone loss <20-40% may not be evident with this technique, and significant degrees of demineralization or fractures may be absent at an early stage (28). Despite this, the Koo score (29) is still used to describe the radiological features of metabolic bone disease (MBD) in premature infants.
2.2 Quantitative ultrasound
Quantitative Ultra Sound (QUS) is an non-invasive, unexpensive, portable and radiation-free method to assess bone density in children, especially for very young pediatric populations, where the use of traditional techniques, such as DXA may be less appropriate considering the exposition to ionizing radiation (30). It assesses both BMC and quantitative properties of bone (cortical thickness, microarchitecture, and elasticity), providing comprehensive information on “bone strength” through the evaluation of two parameters: speed of sound (SOS) and bone transmission time (BTT), depending on the velocity or attenuation of the ultrasound waves through the bone tissue (29, 31). QUS could be used in the assessment of bone mineral status in both preterm infants and children and appropriately in the evaluation of MBD. Most QUS devices are designed to be positioned only on a single skeletal site (e.g. proximal phalanges of the hand, heel, radius and/or tibia), but a multisite QUS device is also available, with different probes on one or more skeletal sites, which in children are usually the tibia (midshaft) and radius (distal third) (20, 32–34).
Althoug QUS devices are suitable for pediatric patients, there are currently insufficient data to determine whether this technique is equivalent to DXA in providing an estimate of bone health, and the limited information available from comparing BMD measured with QUS and DXA has shown conflicting results.
Furthermore, the absence of reliable reference values for pediatric age, the impossibility to using it for the axial skeleton, and technological diversity among QUS devices, both in terms of measurements sites and bone parameters, represent a major problem in the widespread use of QUS in clinical practice (35–38).
2.3 Radiofrequency echographic multi spectrometry
The most recently validated instrumental technique to measure bone mineral status is known as Radiofrequency Echographic Multi-Spectrometry (REMS). REMS is a non-invasive radiation-free methodology, based on the use of ultrasound (39–41).
In adults, REMS technology enables the assessment of axial BMD by a rapid ultrasound scan of lumbar vertebrae (80 s scan) and femoral neck (40 s scan), which represents central anatomical reference sites (42).
The basic principles of this technology consist in a combination between radiofrequencies signals and ultrasound imaging, acquired by a transducer. Simultaneous acquisition of radiofrequencies (native unfiltered ultrasound signals) allows to obtain all available information about the site studied, resulting in more precise and complete acquisition than other conventional ultrasound-based approaches.
The unfiltered radiofrequency signals acquired are then processed by a fully automatic algorithm, transformed into a specific spectrum of the patient, and compared with previously established reference spectral models matched by gender, age and BMI of healthy and osteoporotic bones (39, 43).
Starting from a simple and fast ultrasound scan, this approach allows to obtain quantitatively and qualitatively relevant information about bone health status. Indeed, in addition to quantitative parameters provided by DXA examination, REMS technology provides also a measure of bone quality through the Fragility score, a system validated to estimate 5-years prediction of fracture risk (44).
The 5-year follow-up study by Pisani et al. showed that REMS fragility score to be superior to the only BMD in fracture risk prediction for femur and spine, thanks to the additional information conveyed by REMS technology (45).
Di Paola et al. (46) compared REMS methodology with DXA for osteoporosis diagnosis, enhancing a satisfactory accuracy and precision. Interestingly, the high level of precision of REMS indicates a low intra-operator variability, which represents one of the main advantages of this technology.
Likewise, the REMS methodology showed a specificity and sensitivity (90.4% and 95.5%, respectively) comparable to DXA at femoral neck evaluation. Furthermore, studies in both Caucasian and Japanese subjects recently enhanced a potentially more accurate measure of REMS BMD versus DXA BMD, thanks to the possibility to automatically ignore artefacts due to calcifications, osteophytes, fractures, etc. (47–49).
Notably, the non-ionizing radiation technology and the high rate of reproducibility of REMS examinations makes this technique suitable for regular monitoring of BMD, both in primary prevention and in tracking therapeutic responses. Moreover, the extremely ease of use, the portability of the device, and the lower costs allow REMS methodology to be successfully employed in several healthcare settings, minimizing operator-dependent bias (41).
Accordingly, the several advantages of REMS support the use of this technology as a valuable alternative to DXA and QUS in bone health evaluation, especially in sensitive populations, such as the foetus and the newborn, which enable to safely fulfill extended mass screening strategies. However, the use of REMS is only partially known and shared in clinical practice to date, especially in early infancy. De Gennaro et al. validated REMS methodology in pregnant women, suggesting REMS as the new gold standard for the evaluation of the BMD in this specific cohort (37, 40, 50).
Data on the use of REMS in the newborns are extremely scarce and consequently reference models and population-based data are still lacking. In this regard, Perrone et al. proposed an algorithm which emphasizes the use of REMS during prenatal and postnatal life, in presence of maternal and fetal risk factors. This model is based on the association of echographic data with serum and urinary markers of bone metabolism to determine bone mineral status (51). Very recently, the same authors developed a pioneering study protocol to evaluate and standardize REMS BMD from intrauterine to extrauterine life. It consists in a multicenter clinical trial - currently ongoing- and included 200 mother-newborn dyads, with REMS follow-up planned until 12 months of age (52). Of course, to get an accurate and precise measurement in newborn and infants, it could be advisable to hold the baby still during the scanning by using immobilisation devices, parents, and/or staff, and to make repeated scans of the same site. Indeed, due to its safe and easy use, REMS technology could contribute to improve the knowledge of bone health before and after birth, thus allowing effective prevention strategies and stratification of the risk of fractures, with valuable insights for both obstetric and neonatal care (e.g type of delivery, type of intervention for the shoulder dystocia, specific programs for newborns with low bone mineral status).
3 Discussion
The accumulation of “bone mass”, which is a determining factor in bone strength and fracture risk, takes place during a delicate “time window” that begins during intrauterine life, and extends from childhood to early adulthood, representing an important period for achieving maximum growth and development of bone mineral tissue. Currently, bone health assessment cannot be separated from the analysis of serum and urinary biochemical markers, whose levels are reliable indicators of bone health and turnover, useful in identifying conditions associated with decreased BMD. However, there are still significant limitations for early diagnosis of MBD, even in at-risk categories.
In recent years, most research has focused on identifying screening strategies to measure bone mineral status in targeted populations, known to be more exposed to risk factors for osteopenia, such as premature birth, low or very low birth weight, IUGR, comorbidities of prematurity, total parenteral nutrition, maternal vitamin D deficiency, and several pregnancy-associated diseases (e.g., gestational diabetes). However, to date there are no shared guidelines or universal consensus for the diagnosis and management of MBD, particularly in early childhood.
In addition to already known pathological conditions, there are other factors that appear to influence bone health and strength, thus modulating lifetime risk of osteoporosis, such as the recently discovered epigenetic effects of fetal programming, OS and EDCs, also in full term healthy babies (51, 53–55).
The measurement of BMD in early infants is a controversial issue, due to the limitation of current diagnostic techniques in detecting early markers and/or signs of MBD, which is usually diagnosed in advanced stages, when there is a consistent lack of the expected mineralization for age. To date there is no universally accepted method for a large-scale screening of bone health, mainly because most techniques used for BMD measurement require ionizing radiation, instrumental dimensions are often inadequate for infants, and the time required to motion artifacts represent an unresolved issue. Nevertheless, there is an urgent need for non-invasive screening programs for the implementation of prevention strategies and early identification of BMD alterations (11–14, 51, 56, 57).
Currently, DXA and QUS are traditionally used, despite several limitations (Table 1). Although DXA remains the gold standard technique for evaluating bone health, the issue of radiation exposure and the rapid changes in skeletal size may limit its application in early age. The use of QUS has been implemented in recent years, probably due to its accessibility and safety, but its validity in measuring BMD in early childhood is still a matter of debate. The lack of universal QUS threshold values and validated reference cut-offs, the differences in ROIs and bone properties measured, and high percentage of classification errors compared to DXA scans make these techniques non-interchangeable in assessing the bone status of children (36, 58–60).
More recently, REMS has been proposed as an innovative ultrasound-based technology with valuable insights in several clinical settings. Over the last years, studies carried out in adulthood underlined that REMS is a promising and ductile methodology, relying on a specificity and sensitivity highly comparable to DEXA at femoral neck evaluation, together with a satisfactory degree of accuracy and precision (42, 46, 47). Moreover, when compared to other densitometric techniques, REMS technology showed a potential superiority, providing not only traditional quantitative parameters (BMD, T-score and Z-score), but also qualitative estimation of bone quality through the Fragility score (44, 45). These features, in addition with a high rate of reproducibility, make REMS BMD measurements suitable for short-term therapeutic monitoring, overcoming the temporal limits existing for other densitometric techniques, which typically require a minimum interval of at least 1 year between two scans. Due to the non-ionizing radiation methodology, an extremely ease of use, a high rate of reproducibility, and low costs, REMS appears to be the elective technology for BMD screening, even in sensitive populations such as in pregnancy and childhood.
Despite these advantages, there are some limitations in the application of REMS methodology on a large scale of patients, including the impossibility to obtain “whole body” measurements, which could be useful in early childhood because of the rapid growth of body. Above all, reference limit values for measurements of BMD with REMS in early childhood is still missing. The lack of information about the distribution of BMD in newborns limits the application of this methodology, but preliminary and encouraging data from ongoing research studies may support the validity of BMD Z-score measurement in a single site to evaluate bone status (52).
In the future, the integration of REMS in early MBD screening could have important insights, taking into account the actual absence of a technique giving complete information on bone mineral quality in newborns and infants. Of course, the identification of early physiological and non-physiological variations of bone structure through REMS may have long-term implications for lifelong skeletal health, thus offering unique information not always accessible via traditionally used imaging techniques.
In conclusion, innovative, non-invasive and ductile technologies, such as REMS methodology, would open new scenarios to significantly improve neonatal/pediatric care with screening strategies for bone health assessment, resulting in a potential reduction in MBD. and risk of long-term fractures.
Future longitudinal studies on this issue are needed to allow the building of new shared algorithms and dedicated software, combining biochemical and instrumental data, for the diagnosis, management and treatment of decreased BMD in early infancy.
Author contributions
GP: Methodology, Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. LM: Methodology, Conceptualization, Writing – review & editing, Data curation, Writing – original draft, Investigation. DC: Investigation, Writing – review & editing, Data curation, Visualization. TA: Data curation, Writing – review & editing, Investigation, Visualization. VB: Visualization, Data curation, Writing – review & editing, Investigation. ES: Writing – review & editing, Visualization, Data curation, Investigation. CP: Investigation, Validation, Data curation, Writing – review & editing. AD’A: Investigation, Writing – review & editing, Visualization, Data curation. FG: Writing – review & editing, Investigation, Visualization, Data curation. A-MS: Visualization, Data curation, Investigation, Writing – review & editing. AP: Investigation, Writing – review & editing, Data curation, Visualization. MA: Supervision, Writing – review & editing, Validation. SC: Investigation, Data curation, Validation, Supervision, Writing – review & editing. SB: Writing – review & editing, Data curation, Investigation. GM: Data curation, Supervision, Methodology, Writing – review & editing. MP: Supervision, Data curation, Validation, Investigation, Writing – review & editing. CC: Visualization, Validation, Writing – review & editing, Supervision. TG: Validation, Writing – review & editing, Visualization. M-ES: Validation, Writing – review & editing, Conceptualization, Supervision, Methodology. SP: Project administration, Validation, Conceptualization, Methodology, Supervision, Writing – review & editing, Investigation. MW: Investigation, Methodology, Validation, Conceptualization, Writing – review & editing, Supervision, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the PNNR project n. PNRR-MAD-2022-12376819 (CUP H43C22001220006) within the National Recovery and Resilience Plan (PNRR, M6/C2_CALL 2022 Italian Ministry of Health funded by the European Union -NextGenerationEU).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bachrach LK. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. (2001) 12:22–8. doi: 10.1016/S1043-2760(00)00336-2
2. Baronio F and Baptista F. Editorial: Bone health and development in children and adolescents. Front Endocrinol (Lausanne). (2022) 12:13. doi: 10.3389/fendo.2022.1101403
3. Masztalerz-Kozubek D, Zielinska-Pukos MA, and Hamulka J. Maternal diet, nutritional status, and birth-related factors influencing offspring’s bone mineral density: A narrative review of observational, cohort, and randomized controlled trials. Nutrients. (2021) 13:2302. doi: 10.3390/nu13072302
4. Karpen HE. Mineral homeostasis and effects on bone mineralization in the preterm neonate. Clin Perinatol. (2018) 45:129–41. doi: 10.1016/j.clp.2017.11.005
5. Upadhyay J, Farr OM, and Mantzoros CS. The role of leptin in regulating bone metabolism. Metabolism. (2015) 64:105–13. doi: 10.1016/j.metabol.2014.10.021
6. Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. (2000) 141:3956–64. doi: 10.1210/endo.141.11.7739
7. Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, and Reszka AA M-CSF. TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. (2003) 10:1165–77. doi: 10.1038/sj.cdd.4401285
8. van Beijsterveldt IALP, Dorrepaal DJ, van Zelst BD, van den Berg SAA, and Hokken-Koelega ACS. Poly- and perfluoroalkyl substances (PFAS) in the first 1000 days reduce linear growth, lean body mass and bone mineral density at age 3 years. Clin Nutr. (2025) 50:175–82. doi: 10.1016/j.clnu.2025.05.017
9. Berendsen AD and Olsen BR. Bone development. Bone. (2015) 80:14–8. doi: 10.1016/j.bone.2015.04.035
10. Olsen BR, Reginato AM, and Wang W. Bone development. Annu Rev Cell Dev Biol. (2000) 16:191–220. doi: 10.1146/annurev.cellbio.16.1.191
11. Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, et al. Neonatal bone mass: Influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res. (2001) 16:1694–703. doi: 10.1359/jbmr.2001.16.9.1694
12. Lanham SA, Roberts C, Perry MJ, Cooper C, and Oreffo RO. Intrauterine programming of bone. Part 2: Alteration of skeletal structure. Osteoporos Int. (2008) 19:157–67. doi: 10.1007/s00198-007-0448-3
13. Rustico SE, Calabria AC, and Garber SJ. Metabolic bone disease of prematurity. J Clin Transl Endocrinol. (2014) 1:85–91. doi: 10.1016/j.jcte.2014.06.004
14. Wang J, Zhao Q, Chen B, Sun J, Huang J, Meng J, et al. Risk factors for metabolic bone disease of prematurity: A meta-analysis. PloS One. (2022) 17:e0269180. doi: 10.1371/journal.pone.0269180
15. Sopher AB and Fennoy I. Oberfield SE An update on childhood bone health: mineral accrual, assessment and treatment. Curr Opin Endocrinol Diabetes Obes. (2015) . 22:35–40. doi: 10.1097/MED.0000000000000124
16. Rack B, Lochmüller EM, Janni W, Lipowsky G, Engelsberger I, Friese K, et al. Ultrasound for the assessment of bone quality in preterm and term infants. J Perinatol. (2012) 32:218–26. doi: 10.1038/jp.2011.82
17. Gilsanz V. Bone density in children: a review of the available techniques and indications. Eur J Radiol. (1998) 26:177–82. doi: 10.1016/s0720-048x(97)00093-4
18. Bouxsein ML and Radloff SE. Quantitative ultrasound of the calcaneus reflects the mechanical properties of calcaneal trabecular bone. J Bone Miner Res. (1997) 12:839–46. doi: 10.1359/jbmr.1997.12.5.839
19. Fricke O, Tutlewski B, Schwahn B, and Schoenau E. Speed of sound: relation to geometriccharacteristics of bone in children, adolescents, and adults. J Pediatr. (2005) 146:764–8. doi: 10.1016/j.jpeds.2005.01.038
20. Baroncelli GI. Quantitative ultrasound methods to assess bone mineral status in children: technical characteristics, performance, and clinical application. Pediatr Res. (2008) 63:220–8. doi: 10.1203/PDR.0b013e318163a286
21. Ramot R, Kachhawa G, Kulshreshtha V, Varshney S, Sankar MJ, Devasenathipathy K, et al. Bone mass in newborns assessed by DXA – A systematic review and meta-analysis. Ind J End Metab. (2019) 23:198–209. doi: 10.4103/ijem.IJEM_681_18
22. Larry A and Henwood MJ. Pediatric DXA: technique and interpretation. Pediatr Radiol. (2007) 37:21–31. doi: 10.1007/s00247-006-0153-y
23. Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, et al. International Society for Clinical Densitometry. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: The revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. (2014) 17:225–42. doi: 10.1016/j.jocd.2014.01.003
24. Shalof H, Dimitri P, Shuweihdi F, and Offiah AC. Which skeletal imaging modality is best for assessing bone health in children and young adults compared to DXA? A systematic review and meta-analysis. Bone. (2021) 150:116013. doi: 10.1016/j.bone.2021.116013
25. Blake GM and Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J. (2007) 83:509–17. doi: 10.1136/pgmj.2007.057505
26. van Beijsterveldt IALP, Beunders VAA, Bijlsma A, Vermeulen MJ, Joosten KFM, and Hokken-Koelega ACS. Body composition assessment by air-displacement plethysmography compared to dual-energy X-ray absorptiometry in full-term and preterm aged three to five years. J Clin Med. (2022) 11:1604. doi: 10.3390/jcm11061604
27. Di Iorgi N, Maruca K, Patti G, and Mora S. Update on bone density measurements and their interpretation in children and adolescents. Best Pr Res Clin Endocrinol Metab. (2022) 32:477–98. doi: 10.1016/j.beem.2018.06.002
28. Faienza MF, D’Amato E, Natale MP, Grano M, Chiarito M, Brunetti G, et al. Metabolic bone disease of prematurity: diagnosis and management. Front Pediatr. (2019) 12:143. doi: 10.3389/fped.2019.00143
29. Koo WW, Gupta JM, Nayanar VV, Wilkinson M, and Posen S. Skeletal changes in preterm infants. Arch Dis Child. (1982) 57:447–52. doi: 10.1136/adc.57.6.447
30. Baroncelli G, Federico G, Vignolo M, Valerio G, del Puente A, Maghnie M, et al. Cross-sectional reference data for phalangeal quantitative ultrasound from early childhood to young-adulthood according to gender, age, skeletal growth, and pubertal development. Bone. (2006) 39:159–73. doi: 10.1016/j.bone.2005.12.010
31. de Lange A, Maaskant JM, and van Weissenbruch MM. Is quantitative ultrasound a measure for metabolic bone disease in preterm-born infants? A prospective subcohort study. Eur J Pediatr. (2021) 180:3009–17. doi: 10.1007/s00431-021-04081-4
32. De Terlizzi F, Battista S, Cavani F, Canè V, and Cadossi R. Influence of bone tissue density and elasticity on ultrasound propagation: An in vitro study. J Bone Miner Res. (2000) 15:2458–66. doi: 10.1359/jbmr.2000.15.12.2458
33. Gonnelli S, Montagnani A, Gennari L, Martini S, Merlotti D, Cepollaro C, et al. Feasibility of quantitative ultrasound measurements on the humerus of newborn infants for the assessment of the skeletal status. Osteoporos Int. (2004) 15:541–6. doi: 10.1007/s00198-003-1558-1
34. Ritschl E, Wehmeijer K, DeTerlizzi F, Wipfler E, Cadossi R, Douma D, et al. Assessment of skeletal development in preterm and term infants by quantitative ultrasound. Pediatr Res. (2005) 58:341–6. doi: 10.1203/01.PDR.0000169996.25179.EC
35. Tong L, Gopal-Kothandapani JS, and Offiah AC. Feasibility of quantitative ultrasonography for the detection of metabolic bone disease in preterm infants - systematic review. Pediatr Radiol. (2018) 48:1537–49. doi: 10.1007/s00247-018-4161-5
36. Chong KH, Poh BK, Jamil NA, Kamaruddin NA, and Deurenberg P. Radial quantitative ultrasound and dual energy x-ray absorptiometry: intermethod agreement for bone status assessment in children. BioMed Res Int. (2015) 232876. doi: 10.1155/2015/232876. Erratum in: Biomed Res Int. (2015) 2015:318739. doi: 10.1155/2015/318739.
37. Cannalire G, Biasucci G, Bertolini L, Patianna V, Petraroli M, Pilloni S, et al. Osteoporosis and bone fragility in children: diagnostic and treatment strategies. J Clin Med. (2024) 13:4951. doi: 10.3390/jcm13164951
38. Shalof H, Dimitri P, Shuweihdi F, and Offiah AC. Which skeletal imaging modality is best for assessing bone health in children and young adults compared to DXA? A systematic review and meta-analysis. Bone. (2021) 150:116013. doi: 10.1016/j.bone.2021.116013
39. Diez-Perez A, Brandi ML, Al-Daghri N, Branco JC, Bruyère O, Cavalli L, et al. Radiofrequency echographic multi-spectrometry for the in-vivo assessment of bone strength: State of the art-outcomes of an expert consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Aging Clin Exp Res. (2019) 31:1375–89. doi: 10.1007/s40520-019-01294-4
40. Degennaro VA, Brandi ML, Cagninelli G, Casciaro S, Ciardo D, Conversano F, et al. First assessment of bone mineral density in healthy pregnant women by means of Radiofrequency Echographic Multi Spectrometry (REMS) technology. Eur J Obstet Gynecol Reprod Biol. (2021) 263:44–9. doi: 10.1016/j.ejogrb.2021.06.014
41. Fuggle NR, Reginster JY, Al-Daghri N, Bruyere O, Burlet N, Campusano C, et al. Radiofrequency echographic multi spectrometry (REMS) in the diagnosis and management of osteoporosis: state of the art. Aging Clin Exp Res. (2024) 36:135. doi: 10.1007/s40520-024-02784-w
42. Conversano F, Franchini R, Greco A, Soloperto G, Chiriacò F, Casciaro E, et al. A novel ultrasound methodology for estimating spine mineral density. Ultrasound Med Biol. (2015) 41:281–300. doi: 10.1016/j.ultrasmedbio.2014.08.017
43. Casciaro S, Conversano F, Pisani P, and Muratore M. New perspectives in echographic diagnosis of osteoporosis on hip and spine. Clin cases Miner Bone Metab. (2015) 12:142–50. doi: 10.11138/ccmbm/2015.12.2.142
44. Greco A, Pisani P, Conversano F, Soloperto G, Renna MD, Muratore M, et al. Ultrasound fragility score: an innovative approach for the assessment of bone fragility. Measur. (2017) 101:236–42. doi: 10.1016/j.measurement.2016.01.033
45. Pisani P, Conversano F, Muratore M, Adami G, Brandi ML, Caffarelli C, et al. Fragility Score: a REMS-based indicator for the prediction of incident fragility fractures at 5 years. Aging Clin Exp Res. (2023) 35:763–73. doi: 10.1007/s40520-023-02358-2
46. Di Paola M, Gatti D, Viapiana O, Cianferotti L, Cavalli L, Caffarelli C, et al. Radiofrequency echographic multispectrometry compared with dual X-ray absorptiometry for osteoporosis diagnosis on lumbar spine and femoral neck. Osteoporos Int. (2019) 30:391–402. doi: 10.1007/s00198-018-4686-3
47. Cortet B, Dennison E, Diez-Perez A, Locquet M, Muratore M, Nogués X, et al. Radiofrequency Echographic Multi Spectrometry (REMS) for the diagnosis of osteoporosis in a European multicenter clinical context. Bone. (2021) 143:115786. doi: 10.1016/j.bone.2020.115786
48. Caffarelli C, Tomai Pitinca MD, Al Refaie A, De Vita M, Catapano S, and Gonnelli S. Could radiofrequency echographic multispectrometry (REMS) overcome the overestimation in BMD by dual-energy X-ray absorptiometry (DXA) at the lumbar spine? BMC Musculoskelet Disord. (2022) 23:469. doi: 10.1186/s12891-022-05430-6
49. Ishizu H, Shimizu T, Sakamoto Y, Toyama F, Kitahara K, Takayama H, et al. Radiofrequency echographic multispectrometry (REMS) can overcome the effects of structural internal artifacts and evaluate bone fragility accurately. Calcif Tissue Int. (2024) 114:246–54. doi: 10.1007/s00223-023-01167-z
50. Adami G, Arioli G, Bianchi G, Brandi ML, Caffarelli C, Cianferotti L, et al. Radiofrequency echographic multi spectrometry for the prediction of incident fragility fractures: A 5-year follow-up study. Bone. (2020) 134:115297. doi: 10.1016/j.bone.2020.115297
51. Perrone S, Caporilli C, Grassi F, Ferrocino M, Biagi E, Dell’Orto V, et al. Prenatal and neonatal bone health: updated review on early identification of newborns at high risk for osteopenia. Nutrients. (2023) 15:3515. doi: 10.3390/nu15163515
52. Perrone S, Wasniewska M, Street ME, Beretta V, Scarpa E, Petrolini C, et al. Radiofrequency echographic multi-spectrometry for early bone health: The REMS-bone study protocol (Trial acronym: REMS-bone). Global Ped. (2025) 13:2667–0097:100261. doi: 10.1016/j.gpeds.2025.100261
53. Broy SB, Cauley JA, Lewiecki ME, Schousboe JT, Shepherd JA, and Leslie WD. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 1: hip geometry. J Clin Densitom. (2015) 18:287–308. doi: 10.1016/j.jocd.2015.06.005
54. Hovi P, Andersson S, Järvenpää AL, Eriksson JG, Strang-Karlsson S, Kajantie E, et al. Decreased bone mineral density in adults born with very low birth weight: a cohort study. PloS Med. (2009) 6:e1000135. doi: 10.1371/journal.pmed.1000135
55. Martínez-Mesa J, Restrepo-Méndez MC, González DA, Wehrmeister FC, Horta BL, Domingues MR, et al. Life-course evidence of birth weight effects on bone mass: systematic review and meta-analysis. Osteoporos Int. (2013) 24:7–18. doi: 10.1007/s00198-012-2114-7
56. Rauch F and Schoenau E. Skeletal development in premature infants: a review of bone physiology beyond nutritional aspects. Arch Dis Child Fetal Neonatal Ed. (2002) 86:F82–5. doi: 10.1136/fn.86.2.f82
57. Chinoy A, Mughal MZ, and Padidela R. Metabolic bone disease of prematurity: Causes, recognition, prevention, treatment and long-term consequences. Arch Dis Child Fetal Neonatal Ed. (2019) 104:F560–6. doi: 10.1136/archdischild-2018-316330
58. Khan KM, Sarafoglou K, Somani A, Frohnert B, and Miller BS. Can ultrasound be used to estimate bone mineral density in children with growth problems? Acta Paed. (2013) 102:e407–12. doi: 10.1111/apa.12314
59. Williams JE, Wilson CM, Biassoni L, Suri R, and Fewtrell MS. Dual energy x-ray absorptiometry and quantitative ultrasound are not interchangeable in diagnosing abnormal bones. Arch Dis Child. (2012) 97:822–4. doi: 10.1136/archdischild-2011-301326
60. Christoforidis A, Printza N, Gkogka C, Siomou E, Challa A, Kazantzidou E, et al. Comparative study of quantitative ultrasonography and dual-energy X-ray absorptiometry for evaluating renal osteodystrophy in children with chronic kidney disease. J Bone Min Metab. (2011) 29:321–7. doi: 10.1007/s00774-010-0220-1
Keywords: bone mineral density, early infancy, REMS technology, ultrasound, DXA
Citation: Pepe G, Morabito LA, Corica D, Aversa T, Beretta V, Scarpa E, Petrolini C, Dall’Asta A, Grassi F, Shulhai A-M, Papini AM, Albertini MC, Carloni S, Benedetti S, Maglietta G, Puntoni M, Caminiti C, Ghi T, Street ME, Perrone S and Wasniewska M (2025) Challenges in assessing bone health in early infancy: a narrative review of existing technologies. Front. Endocrinol. 16:1651094. doi: 10.3389/fendo.2025.1651094
Received: 20 June 2025; Accepted: 08 October 2025;
Published: 21 October 2025.
Edited by:
Fátima Baptista, Universidade de Lisboa, PortugalReviewed by:
Hsueh-Kuan Lu, National Taiwan University of Sport, TaiwanCopyright © 2025 Pepe, Morabito, Corica, Aversa, Beretta, Scarpa, Petrolini, Dall’Asta, Grassi, Shulhai, Papini, Albertini, Carloni, Benedetti, Maglietta, Puntoni, Caminiti, Ghi, Street, Perrone and Wasniewska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Domenico Corica, ZG9tZW5pY28uY29yaWNhQHVuaW1lLml0; Tommaso Aversa, dG9tbWFzby5hdmVyc2FAdW5pbWUuaXQ=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Giorgia Pepe
Giorgia Pepe Letteria Anna Morabito
Letteria Anna Morabito Domenico Corica1*
Domenico Corica1* Tommaso Aversa
Tommaso Aversa Chiara Petrolini
Chiara Petrolini Andrea Dall’Asta
Andrea Dall’Asta Anna-Mariia Shulhai
Anna-Mariia Shulhai Anna Maria Papini
Anna Maria Papini Maria Cristina Albertini
Maria Cristina Albertini Silvia Carloni
Silvia Carloni Giuseppe Maglietta
Giuseppe Maglietta Matteo Puntoni
Matteo Puntoni Caterina Caminiti
Caterina Caminiti Maria Elisabeth Street
Maria Elisabeth Street Serafina Perrone
Serafina Perrone Malgorzata Wasniewska
Malgorzata Wasniewska